Abstract
Background
This is an updated version of the original Cochrane Review published in Issue 10, 2014. There is a need to expand monotherapy options available to a clinician for the treatment of new focal or generalized seizures. A Cochrane systematic review for clobazam monotherapy is expected to define its place in the treatment of new‐onset or untreated seizures and highlight gaps in evidence.
Objectives
To evaluate the efficacy, effectiveness, tolerability and safety of clobazam as monotherapy in people with new‐onset focal or generalized seizures.
Search methods
For the latest update we searched the following databases on 19 March 2018: the Cochrane Register of Studies (CRS Web), which includes the Cochrane Epilepsy Group Specialized Register and the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid, 1946‐ ), BIOSIS Previews (1969‐ ), ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). There were no language restrictions.
Selection criteria
Randomized or quasi‐randomized controlled trials comparing clobazam monotherapy versus placebo or other anti‐seizure medication in people with two or more unprovoked seizures or single acute symptomatic seizure requiring short‐term continuous anti‐seizure medication, were eligible for inclusion.
Data collection and analysis
Primary outcome measure was time on allocated treatment (retention time), reflecting both efficacy and tolerability. Secondary outcomes included short‐ and long‐term effectiveness measures, tolerability, quality of life, and tolerance measures. Two authors independently extracted the data.
Main results
We identified three trials fulfilling the review criteria, which included 206 participants. None of the identified studies reported the preselected primary outcome measure. A meta‐analysis was not possible. Lack of detail regarding allocation concealment and a high risk of performance and detection bias in two studies prompted us to downgrade the quality of evidence (by using the GRADE approach) for some of our results due to risk of bias.
Regarding retention at 12 months, we detected no evidence of a statistically significant difference between clobazam and carbamazepine (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.61 to 1.12; low‐quality evidence). There was low‐quality evidence that clobazam led to better retention compared with phenytoin (RR 1.43, 95% CI 1.08 to 1.90). We could not determine whether participants receiving clobazam were found to be less likely to discontinue it due to adverse effects as compared to phenytoin (RR 0.10, 95% CI 0.01 to 1.65, low‐quality evidence).
Authors' conclusions
We found no advantage for clobazam over carbamazepine for retention at 12 months in drug‐naive children and a slight advantage of clobazam over phenytoin for retention at six months in adolescents and adults with neurocysticercosis in a single clinical trial each. At present, the available evidence is insufficient to inform clinical practice.
Plain language summary
Clobazam monotherapy for focal or generalized seizures
Review question
We reviewed the evidence for clobazam monotherapy for the treatment of new‐onset or untreated focal or generalized seizures.
Background
Epilepsy is characterized by an abnormal propensity for the brain to have seizures, which are discrete episodes of abnormal neuronal electrical activity. Seizures may arise from a particular part (focal seizures) or whole of the brain at once (generalized seizures). In this review we assessed clobazam as monotherapy (single‐drug treatment) for new‐onset or untreated focal or generalized onset seizures.
Study characteristics
Our searches identified three studies of low quality comparing clobazam with carbamazepine and phenytoin for monotherapy of seizures. They included a multi‐center study that compared the effectiveness of clobazam and carbamazepine monotherapy in 115 children with untreated epilepsy; a single‐center study comparing clobazam with phenytoin in 48 adolescents and adults with neurocysticercosis; and a single‐center study comparing clobazam with carbamazepine monotherapy in 43 children with benign childhood epilepsy with centrotemporal spikes (BCECTS). In clinical trials with anti‐seizure medications, effectiveness is conventionally measured by the time a person remains on the allocated treatment, which reflects that the person will stop taking medicine either if it is ineffective or if there are unacceptable adverse effects.
Key results
No significant advantage of clobazam over carbamazepine in people with previously untreated focal or generalized seizures was seen for retention at 12 months. A slight advantage of clobazam over phenytoin for retention at six months was noted in one study, which was limited to participants having seizures due to neurocysticercosis. No significant differences in proportion of BCECTS participants who achieved seizure freedom at four weeks, between four and 40 weeks, and during the last nine months of treatment between clobazam and carbamazepine were found. Also, no significant difference in the proportion of participants who achieved a 50% or greater reduction in their seizures at four weeks on clobazam versus carbamazepine was noted. However, participants randomized to clobazam achieved seizure control sooner than those treated with carbamazepine.
None of the studies measured the amount of time that participants remained on allocated treatment (retention time), or quality of life. We did assess data from the studies regarding the number of participants who remained on treatment at specific time points. However, we were unable to determine whether people with previously untreated focal or generalized seizures receiving clobazam were more likely to remain on treatment at 12 months than those given carbamazepine due to low numbers of participants in the study. One of the studies showed that social and academic outcomes improved in participants taking clobazam as well as carbamazepine; however, there was no comparative analysis.
Quality of evidence
At present, there is insufficient evidence to inform clinical practice regarding clobazam monotherapy in focal or generalized seizures. The quality of evidence for the outcomes was also affected by design issues and low sample‐size. There is a definite need for well‐designed, adequately powered, randomized controlled trials of clobazam monotherapy in people with new‐onset/untreated seizures.
The evidence is current to March 2018.
Summary of findings
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Arya 2014).
Description of the condition
Epilepsy is one of the most common disorders of the brain (Engel 2008). It accounts for 1% of the global burden of disease, a figure equivalent to breast cancer in women and lung cancer in men (Murray 1994).
An epileptic seizure is defined as a transient occurrence of signs or symptoms due to abnormal excessive or synchronous neuronal activity in the brain (Fisher 2005). Sometimes, a seizure can be a natural response of the normal brain to transient disturbances in function. Such seizures are referred to as provoked or acute symptomatic seizures. Epilepsy is a chronic condition of the brain characterized by an enduring propensity to generate epileptic seizures, and by the neurobiological, cognitive, psychological, and social consequences of this condition (Fisher 2005).
Worldwide, epilepsy affects 50 million people (Engel 2008). In high‐income countries, the age‐adjusted incidence of epilepsy ranges from 24 to 53 per 100,000 person‐years (Banerjee 2008). Total population studies reporting the incidence of a first diagnosis of unprovoked seizures provide estimates of incidence ranging from 26 to 70 per 100,000 person‐years (Banerjee 2008). It is likely that the incidence of epilepsy may be higher in low‐ and middle‐income countries than in high‐income countries, due to increased occurrence of perinatal insults and neuro‐infections.
Description of the intervention
Clobazam is a benzodiazepine anti‐seizure medication. It has been shown to be an effective anticonvulsant for monotherapy of previously untreated children with focal seizures, with a similar efficacy to phenytoin or carbamazepine. Clobazam is also efficacious when used as an add‐on treatment for refractory focal epilepsy in children and adults (Koeppen 1987; Schmidt 1986), and intermittent clobazam therapy may be useful for the treatment of febrile seizures (Rose 2005). The Canadian Clobazam Cooperative Group performed the largest single study, comprising 877 participants with epilepsy, both children and adults, with multiple types of seizures including atypical absence, myoclonic seizures, Lennox‐Gastaut syndrome, and generalized tonic‐clonic seizures (Canadian Clobazam Cooperative Group 1991). More than half the participants became seizure free or had a greater than 50% reduction in seizure frequency. Clobazam is thought to have fewer side effects than other benzodiazepines at equipotent doses (Schmidt 2008), although development of tolerance in 30% to 50% of people is an important disadvantage.
Clobazam is metabolized by hepatic microsomal enzymes. The main active metabolite is desmethylclobazam, also called norclobazam. Clobazam and its metabolite are slowly eliminated with a half‐life of 20 to 56 hours. Although it is only one‐fifth as potent as the parent compound, because the ratio of plasma levels of desmethylclobazam to those of the parent drug is about 10:1, and because the metabolite also has a longer half‐life, the anticonvulsant property may be more attributable to desmethylclobazam than to clobazam (Schmidt 2008).
How the intervention might work
Gamma amino butyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system. It binds to at least two classes of receptors, GABA‐A and GABA‐B, which are found on almost all cortical neurons (Macdonald 2002). Benzodiazepines act primarily as anticonvulsants by interacting with GABA‐A receptors at a specific binding site and allosterically modifying the receptor currents to enhance inhibition by increasing the frequency of receptor opening without significantly affecting the mean open time or conductance of the channel. It is now known that the GABA receptor is made up of many different subunits (α, β, γ, δ, ρ) with different subtypes in many combinations. GABA‐A receptors containing the α1 subunit are an important target for benzodiazepines (Rogawski 2004).
The anti‐absence activity of benzodiazepines probably results from their ability to interfere with hypersynchronous activity in the thalamocortical circuitry. More specifically, benzodiazepines are thought to reduce the inhibitory output of the reticular neurons by effects on benzodiazepine‐sensitive α3‐containing GABA‐A receptors (Rogawski 2004). However, some of the clinical effects of benzodiazepines in experimental animals cannot be explained simply by their potentiation of GABA‐activated currents (Schmidt 2008), and benzodiazepines may act on targets other than the GABA‐A receptor. Through unknown mechanisms, benzodiazepines may produce elevations of GABA levels in the cerebrospinal fluid (Löscher 1987). At high concentrations, especially those that may be achieved in the treatment of status epilepticus, benzodiazepines may have significant effects on voltage‐gated sodium and, to a lesser extent, on calcium channels (Schmidt 2008).
Why it is important to do this review
The antiepileptic drug (AED) choices for monotherapy of new‐onset or untreated seizures has a fairly limited evidence base. The updated International League Against Epilepsy monotherapy guidelines has identified Level A evidence, according to their criteria, for only carbamazepine, phenytoin, levetiracetam and zonisamide for focal seizures in adults, and for ethosuximide and valproate in childhood absence epilepsy (Glauser 2013). This rigorous update also highlighted the lack of well‐designed clinical trials and appropriate evidence for monotherapy of several other patient groups with new‐onset or untreated epilepsy, who are candidates for monotherapy with AEDs.
Also, the majority of the conventional and some of the newer AEDs have important adverse effects in a significant proportion of people receiving them (Porter 2007), which precludes their use in certain patient groups. Hence, there is a continued need to assess the efficacy and safety of alternative newer AEDs for monotherapy of new‐onset or untreated epilepsy. A Cochrane Review of clobazam monotherapy would help to define its place in the monotherapy of new‐onset or untreated epilepsy and might help in increasing the number of available choices for treatment of these people.
Objectives
To evaluate the efficacy, effectiveness, tolerability and safety of clobazam as monotherapy in people with new‐onset focal or generalized seizures.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and quasi‐randomized controlled trials (QRCTs).
Studies may be double‐blinded, single‐blinded or unblinded.
Types of participants
Adults and children aged more than six months with two or more unprovoked seizures or a single acute symptomatic seizure.
Types of interventions
Intervention: clobazam monotherapy for at least three months continuously.
Comparator: placebo or any other anti‐seizure drug.
Types of outcome measures
Primary outcomes
Time on allocated treatment (retention time)
This is a combined outcome reflecting both efficacy and tolerability, as treatment may be withdrawn due to continued seizures, side effects or a combination of both. This is an outcome to which the participant makes a contribution, and is the primary outcome measure recommended by the Commission on Antiepileptic Drugs of the International League Against Epilepsy (Commission 1998).
Secondary outcomes
Short‐term effectiveness measure(s)
Seizure cessation within eight weeks of starting clobazam
A decrease in seizure burden of 50% or more (50% responder rate) within eight weeks of starting clobazam
Long‐term effectiveness measure(s)
The proportion of individuals who achieve six, 12 and 24 months' seizure freedom
Tolerability measure(s)
Proportion of individuals experiencing adverse events requiring medication withdrawal
Proportion of individuals experiencing any adverse events emerging during clobazam monotherapy (specific vigilance for sedation or drowsiness would be kept and data presented in each study would be summarized)
Quality‐of‐life measure(s)
Quality‐of‐life outcomes measured on any validated scale (commonly used, validated, health‐related quality‐of‐life scales used in epilepsy include: Quality of Life in Childhood Epilepsy Questionnaire (QOLCE) and Quality of Life in Epilepsy Inventory‐89 and 31 (QOLIE‐89/31))
Tolerance measure(s)
Data on the development of anti‐seizure drug tolerance or tachyphylaxis using the study definitions
Search methods for identification of studies
We carried out a comprehensive search to identify all eligible trials regardless of language, year of publication, or status of publication (published in peer‐reviewed journal, conference proceedings, thesis, or unpublished).
Electronic searches
Searches were run for the original review in July 2011 and subsequent searches were run in March 2013, April 2015, January 2017, and March 2018. For the latest update we searched the following databases, with no language restrictions:
Cochrane Register of Studies (CRS Web, 19 March 2018), which includes the Cochrane Epilepsy Group Specialized Register and the Cochrane Central Register of Controlled Trials (CENTRAL) using the search strategy outlined in Appendix 1.
MEDLINE (Ovid, 1946 to 19 March 2018) using the search strategy set out in Appendix 2.
BIOSIS Previews (1969 to 19 March 2018) using the search strategy outlined in Appendix 3.
ClinicalTrials.gov (19 March 2018) using the search strategy shown in Appendix 4.
World Health Organization International Clinical Trials Registry Platform (ICTRP, 19 March 2018) using the search strategy shown in Appendix 5.
Previously we also searched the Database of Abstracts of Reviews of Effectiveness (DARE) using the search strategy outlined in Appendix 6, but this is now a closed archive, so it has not been searched again.
Searching other resources
We checked the reference lists of identified trials for additional reports of relevant studies.
Data collection and analysis
Selection of studies
Two authors (RA, VA) scanned the results of the search and performed a preliminary screen rejecting publications that were obviously unsuitable and retaining publications in which interventions seemed relevant. Two authors (VA, SK) independently inspected the remaining references that were not rejected and obtained the full text of all possibly relevant studies. Two authors independently applied inclusion and exclusion criteria, resolving disagreements by discussion. No doubts about any trial's eligibility were raised. We scrutinized the trial reports according to dates, location of the trial and interventions, to ensure that multiple publications from the same trial are included only once. We recorded potentially relevant references that were excluded along with the reason for their exclusion.
Data extraction and management
For eligible studies, at least two of the authors (RA, VA, SK, NG) extracted the data using the pre‐designed agreed form, resolving discrepancies through discussion or by consulting the third author. We entered data into Review Manager software (RevMan 2014), and checked them for accuracy. We ensured accuracy by comparing data extracted independently by two of the authors (RA, VA, SK, NG) and resolved any discrepancies by discussion or by consultation with the third author.
Assessment of risk of bias in included studies
Two authors (from RA, VA, SK, NG) independently assessed the methodological quality of each study and risk of bias using The Cochrane Collaboration’s 'Risk of bias' tool (Higgins 2011a). Assessors were not blinded to the authors or source institution. The items included for appraisal were sequence generation for randomization, concealment of allocation, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. Each of these factors was categorized as low risk, high risk or unclear risk with a brief overview (Appendix 7).
Measures of treatment effect
We performed statistical analysis using RevMan 5 (RevMan 2014). Since data for each outcome were available from any one study only, we could not perform meta‐analysis.
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals (CI) for dichotomous outcomes.
Continuous data
For continuous variables, in future versions of the review, we will calculate difference in means or standardized mean differences (SMDs) along with 95% CIs. When two or more studies present their data derived from the same instrument of evaluation (with the same units of measurement), we will pool data as a mean difference (MD). Conversely, when primary studies express the same variables through different instruments (and different units of measurement) we will use the SMD.
Dealing with missing data
In future versions of the review, for missing data, we will attempt to contact the authors of original studies to obtain additional data. For all outcomes, we will carry out analyses on an intention‐to‐treat (ITT) basis as far as possible, that is, including all participants randomized in their original groups.
For missing continuous data, we will estimate standard deviations (SDs) from other available data such as standard errors (SEs), or we will impute the data using methods suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We will make no assumptions about loss to follow‐up for continuous data and we will base analyses on those individuals who completed the trial. We will perform ITT analyses where appropriate. We will perform a sensitivity analysis by calculating the treatment effect including and excluding the imputed data to see whether this would alter the outcome of the analysis. We will investigate the effect of drop‐outs and exclusions by conducting 'worst‐case' versus 'best‐case' scenario analyses. If there are any discrepancies in the number randomized and the number analyzed in each treatment group, we will calculate the percentage lost to follow‐up in each group and report the results. If drop‐outs exceed 10% for any trial, we shall assign the worse outcome to those lost to follow‐up for dichotomous outcomes and assess the impact of this in sensitivity analyses with the results of those completing the study. Where it is not possible to obtain missing data, we will record this in the data extraction form and report the information in the 'Risk of bias' table. For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
Assessment of heterogeneity
Although not relevant to the current version of the review, in future we will assess heterogeneity between pooled trials using the Chi² test in conjunction with the I² statistic (Higgins 2003), which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (Deeks 2011). We will consider a P value of less than 0.10 as statistically significant. If enough trials are identified, we will explore sources of heterogeneity using subgroup analyses. We will display results graphically using forest plots, and present a summary statistic if no major statistical heterogeneity (lack of overlap of confidence intervals on the forest plots) exists. We will consider values of I² of less than 25% to denote low heterogeneity, 50% or greater to be significant heterogeneity and 75% or greater to be substantial and major heterogeneity.
Assessment of reporting biases
As sufficient trials become available in future, we will attempt to assess publication bias by preparing a funnel plot. We will then perform a visual assessment of funnel plot asymmetry. We will perform exploratory analyses to investigate any suggestion of visual asymmetry in the funnel plots. Our searches for trials listed in clinical trial registers and trial protocols should help to avoid publication bias and to assess outcome selection bias. Where necessary, we will contact study authors in an attempt to establish a full data set or obtain reasons for the non‐reporting of certain outcomes.
Data synthesis
We entered quantitative data into RevMan 5 and analyzed the data using Cochrane MetaView (RevMan 2014). Pooled analyses were not feasible for the current version of the review. In future versions, we will use adjusted summary statistics if feasible, otherwise we will use unadjusted results.
For time‐to‐event data, we will pool hazard ratios (HR) using the generic inverse variance facility of RevMan 5.
For dichotomous outcomes, we will calculate and pool RRs for each study.
For continuous outcomes, we will pool MDs between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we will pool SMDs, as discussed above.
We will use a fixed‐effect meta‐analysis unless significant statistical or clinical heterogeneity are found, in which case we will use a random‐effects analysis if considered appropriate (DerSimonian 1986). If possible, we will synthesize studies making different comparisons using the methods described by Bucher 1997.
We assessed the quality of the body of the evidence using the GRADE approach (Higgins 2011a). Domains that determined the quality of the evidence were:
study design;
risk of bias;
inconsistency of results;
indirectness (non‐generalizability);
imprecision (insufficient data);
other factors (e.g. reporting bias).
We reduced the quality of the evidence by one level for each of the domains that we encountered. We considered all plausible confounding factors and their effects as a reason to reduce any claimed effect and dose‐response gradient. We defined the following levels of evidence.
High‐quality evidence: further research is very unlikely to change our confidence in the estimate of effect. There are consistent findings among 75% of RCTs with low risk of bias that are generalizable to the population in question. There are sufficient data, with narrow confidence intervals. There are no known or suspected reporting biases (all of the domains are met).
Moderate‐quality evidence: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate (one of the domains is not met).
Low‐quality evidence: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate (two of the domains are not met).
Very low‐quality evidence: we are very uncertain about the estimate (three of the domains are not met).
No evidence: no RCTs were identified that measured the outcome.
We also considered a number of factors to place the results into a larger clinical context: temporality, plausibility, strength of association, adverse events, and costs. Not all of these were applicable for the current review, but are likely to be useful as more studies for clobazam monotherapy become available.
Subgroup analysis and investigation of heterogeneity
Considerations of clinical diversity included assessment of differences in study location and setting, participant characteristics including co‐morbidities, characteristics of the trial interventions and of other care provided.
We intended to analyze studies according to differences in the following pre‐planned subgroups.
Age (children versus adults)
Seizure semiology (focal with or without secondary generalization versus primary generalized)
Seizure frequency prior to enrolment in the study
Pre‐treatment electroencephalographic (EEG) findings
Neuro‐imaging findings
In the present version of review, sufficient data for sub‐group analyses were not available.
Sensitivity analysis
Where possible, we intended to perform sensitivity analyses to explore the effects of various aspects of trial and review methodology, including the effects of missing data and whether allocation was concealed. If sufficient data were available, we intended to conduct sensitivity analyses to determine the impact of excluding studies with lower methodological quality, for example:
excluding trials at a high or unclear risk of bias;
excluding unpublished studies, these may not have been subjected to the peer review process and may have intrinsic bias issues;
excluding industry studies;
excluding trials that have not assessed compliance.
'Summary of findings' tables
We used the principles of the GRADE approach to assess the quality of the body of evidence associated with specific outcomes and to construct 'Summary of findings' tables (SoF) (Schünemann 2011). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
We identified 291 publications by the search of electronic databases and trial registers. Because of duplication, we removed 89 articles. After review of 202 titles and abstracts by two authors (VA, RA), we considered that 17 studies were potentially eligible for inclusion, out of which 14 were excluded because either they used clobazam only for short‐term intermittent treatment for acute symptomatic seizures (Bajaj 2005; Feely 1982a; Feely 1982b; Feely 1982c; Khosroshahi 2011; RamachandranNair 2005; Rose 2005), or they used clobazam as add‐on therapy instead of monotherapy (Figueroa 1984; Koeppen 1987; Vajda 1985; Yagi 1995; Yamatogi 1997). One study (Feely 1984), was found to be a case series, and was therefore excluded. Another study (Canadian Study Group 1999), was part of a larger multi‐center study on the efficacy of clobazam and analyzed neuropsychological measures in children receiving clobazam verus those receiving standard monotherapy. However, those randomized to receive clobazam also included children who had previously been treated unsuccessfully with carbamazepine or one or more other AEDs. This study was therefore excluded (Characteristics of excluded studies). Three trials (Andrade 2009; Canadian Study Group 1998; Kaushal 2006), with 163 individuals randomized to clobazam monotherapy or other anti‐seizure medication (carbamazepine or phenytoin) monotherapy were analyzed (See Figure 1).
1.
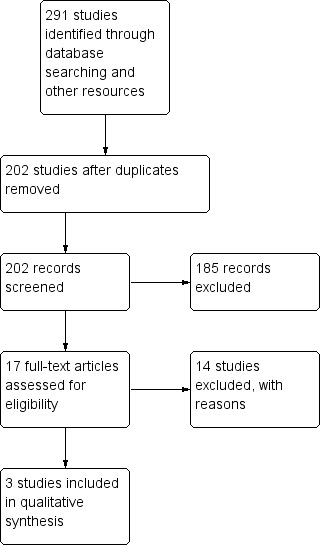
Study flow diagram (results from updated searches)
Included studies
This single‐center, randomized open‐label study compared clobazam versus carbamazepine in children with benign childhood epilepsy with centrotemporal spikes (BCECTS). The outcome measures included the number of participants with more than 50% reduction in seizures, number of participants who were free of seizures in the last nine months, and the average time it took to achieve seizure freedom in participants treated with clobazam versus those treated with carbamazepine. This study also examined academic performance, behavior, adherence to treatment, parents’ degree of satisfaction and side effect profiles.
This multi‐center study aimed to compare the effectiveness of clobazam, carbamazepine and phenytoin monotherapy in children with epilepsy. It had a randomized, double‐blind design with ITT analysis for the primary outcome measure of retention on the study medication for 12 months or discontinuation of the study medication for any reason, including adverse effects or inadequate seizure control. The study had three intervention arms: drug‐naive, previous failure with carbamazepine, and previous failure with other anti‐seizure medications. Only the first arm met the inclusion criteria for this review and was analyzed. This arm compared clobazam with standard treatment (carbamazepine) in drug‐naive participants with epilepsy, for a period of 12 months.
This single‐center, randomized, open‐label study compared clobazam versus phenytoin in adolescents and adults with solitary neurocysticercosis granuloma. The primary outcome measure was number of treatment failures in each group due to breakthrough seizures or adverse events during the first six months after initiation. This study had some methodologic limitations as mentioned in Risk of bias in included studies.
The studies are summarized in Characteristics of included studies.
Excluded studies
The reasons for exclusion are summarized in Characteristics of excluded studies.
Risk of bias in included studies
Two of the included studies were adequately randomized, using either the permuted block technique (Canadian Study Group 1998), or a computer‐generated random number list (Kaushal 2006). However, Andrade 2009 did not mention their method of random sequence generation. The review authors' judgements about the risks of bias are summarized in Figure 2 and Figure 3.
2.
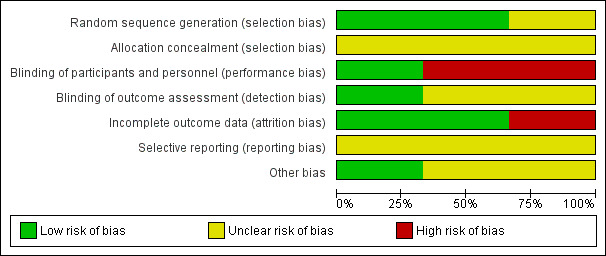
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
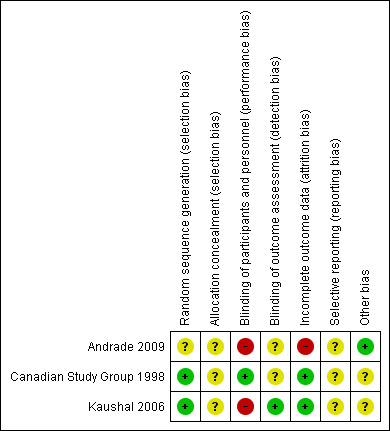
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
The allocation sequence concealment is not described in any of the studies.
Blinding
Two studies provided information on blinding. One of the included studies was double‐blind and used a 'double dummy' technique (Canadian Study Group 1998), but the other was an open‐label trial (Kaushal 2006). The Canadian Study Group 1998 had two outcome assessors, one of whom was blinded, whereas the blinding of the outcome assessor is not mentioned in Kaushal 2006. This lack of blinding might have affected the assessment of subjective outcomes such as tolerability measures. Andrade 2009 did not provide details of blinding in outcome assessment.
Incomplete outcome data
The risk of attrition bias is assessed to be low, as all of the randomized participants were analyzed in all of the studies that is, the primary analysis was on an ITT basis.
Selective reporting
We could not assess reporting bias as there were no pre‐published protocols available for any of the studies.
Other potential sources of bias
The Canadian Study Group 1998 trial was funded by Hoechst‐Marion‐Roussel Canada, Inc, and the monitoring of data collection and entry was accomplished in this trial by Hoechst‐Marion‐Roussel Canada, Inc. Kaushal 2006 did not mention any sources of funding. However, this study initially estimated a sample size of 270 to detect a 10% difference in the primary outcome measure with a 90% confidence; but, they prematurely terminated the study with an actual sample of only 48 participants, due to relocation of a study investigator. Also in this study, outcome was assessed by the treating physician and a blinded research team member, but they do not mention how they reached a consensus in case of disagreement or discrepancy. Further, they do not report whether and how informed consent was obtained.Andrade 2009 do not mention any funding source. No other sources of bias that could have affected the results were noted.
Effects of interventions
Summary of findings for the main comparison. Clobazam versus carbamazepine for focal or generalized seizures.
| Clobazam versus carbamazepine for focal or generalized seizures | ||||||
| Patient or population: people with focal or generalized seizures Settings: 15 Canadian pediatric epilepsy centers (Canadian Study Group 1998); single center, outpatient clinic from Cuba (Andrade 2009) Intervention: clobazam versus carbamazepine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Carbamazepine | Clobazam | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 12 months Follow‐up: mean 12 months | Study population | RR 0.83 (0.61 to 1.12) | 115 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 654 per 1000 | 543 per 1000 (399 to 732) | |||||
| Seizure freedom at 4 weeks | 720 per 1000 | 611 per 1000 (396 to 950) | RR 0.85 (0.55 to 1.32) | 43 (1 study) |
low2,3,4 | |
| Seizure freedom 4‐40 weeks | 760 per 1000 | 722 per 1000 (502 to 1034) | RR 0.95 (0.66 to 1.36) | 43 (1 study) |
low2,3,4 | |
| 50% responder rate | 800 per 1000 | 944 per 1000 (752 to 1184) | RR 1.18 (0.94 to 1.48) | 43 (1 study) |
low2,3,4 | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
1Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of this outcome. Downgraded by 1. 2Only 1 study, hence no 'inconsistency' and no 'publication bias'. 3Wide CI, downgraded by 1.
4Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study.
Summary of findings 2. Clobazam versus phenytoin for focal or generalized seizures.
| Clobazam versus phenytoin for focal or generalized seizures | ||||||
|
Patient or population: drug‐naïve people with focal or generalized seizures with solitary cysticercus granuloma Settings: single Indian teaching hospital Intervention: clobazam Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Clobazam versus phenytoin | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 6 months (measured by the number of treatment failures in each group, which was defined as either breakthrough seizure or adverse effects requiring discontinuation or dose modification of study medication) Follow‐up: mean 6 months | Study population | RR 1.43 (1.08 to 1.9) | 48 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 667 per 1000 | 953 per 1000 (720 to 1267) | |||||
| Seizure freedom at 4 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Seizure freedom 4‐40 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| 50% responder rate | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Study population | RR 0.10 (0.01 to 1.65) | 48 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 222 per 1000 | 22 per 1000 (2 to 367) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
1Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of 'adverse effects requiring withdrawal' and hence 'retention time'. Downgraded by 1. 2Only 1 study, thus no 'inconsistency' and no 'publication bias'. 3Wide confidence interval and small sample size. Downgraded by 1.
Retention at 12 months
Data on 115 drug‐naive children were available for analysis of this outcome from one study only (Canadian Study Group 1998), in which 63 participants were randomized to clobazam and 52 to carbamazepine respectively. No statistically significant advantage of clobazam over carbamazepine was detected, with a risk ratio (RR) of 0.83, 95% CI (0.61 to 1.12) (Analysis 1.1; Figure 4). No data were available for any subgroup analysis.
1.1. Analysis.
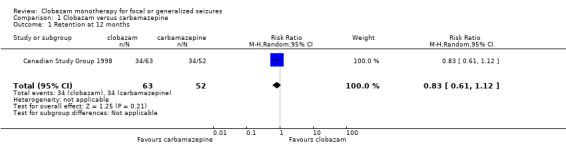
Comparison 1 Clobazam versus carbamazepine, Outcome 1 Retention at 12 months.
4.
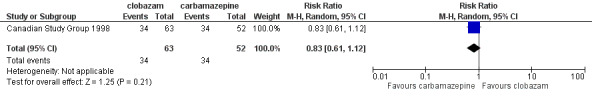
Forest plot of comparison: 2 clobazam vs carbamazepine, outcome: 2.1 retention at 12 months
Retention at six months
Data on 48 drug‐naive adolescents and adults with solitary cysticercus granuloma were available for analysis of this outcome from only one study (Kaushal 2006). The number of participants randomized to clobazam and phenytoin arm were 21 and 27 respectively. Twenty out of 21 participants randomized to receive clobazam remained on the study medication for six months, compared to 18 out of 27 in the phenytoin group (RR 1.43, 95% CI 1.08 to 1.90) (Analysis 2.1; Figure 5). No data were available for any subgroup analysis.
2.1. Analysis.

Comparison 2 Clobazam versus phenytoin, Outcome 1 Retention at 6 months.
5.

Forest plot of comparison: 1 clobazam versus phenytoin, outcome: 1.1 retention at 6 months
Seizure freedom at specified time intervals
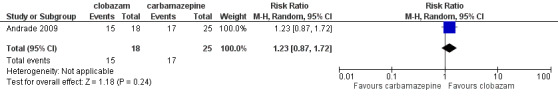
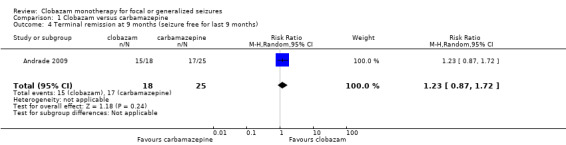
Data on 43 children were available for analysis of this outcome (Andrade 2009). Participants with BCECTS were evaluated for seizure freedom at four weeks, between four and 40 weeks, and during the last nine months of treatment, and no statistically significant differences were identified between those allocated to receive clobazam versus carbamazepine. Eleven out of 18 participants randomized to receive clobazam were seizure free at four weeks, compared to 18/25 in the carbamazepine group (RR 0.85, 95% CI 0.55 to 1.32 Analysis 1.2). Thirteen out of 18 participants on clobazam were seizure free during the four to‐40‐week interval, compared to 19/25 in the carbamazepine group (RR 0.95, 95% CI 0.66 to 1.36 Analysis 1.3). Lastly, 15/18 participants in clobazam group were seizure free during the last nine months of therapy, compared to 17/25 in the carbamazepine group (RR 1.23, 95% CI 0.87 to 1.72 Figure 6).
1.2. Analysis.

Comparison 1 Clobazam versus carbamazepine, Outcome 2 Seizure freedom at 4 weeks.
1.3. Analysis.
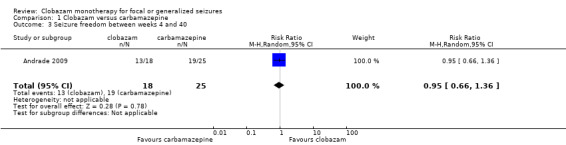
Comparison 1 Clobazam versus carbamazepine, Outcome 3 Seizure freedom between weeks 4 and 40.
6.
Forest plot of comparison: 1 clobazam versus carbamazepine, outcome: 1.4 terminal remission at 9 months (seizure free for last 9 months)
50% responder rate at four weeks
Andrade 2009 provided data on 43 children regarding this outcome. There was no statistically significant difference in the proportion of children whose seizure frequency decreased by at least 50% (50% responder rate) at four weeks between the clobazam and carbamazepine groups (Analysis 1.5). Seventeen out of 18 participants randomized to receive clobazam achieved a 50% or greater reduction in seizures at four weeks, compared to 20/25 in the carbamazepine group (RR 1.18, 95% CI 0.94 to 1.48).
1.5. Analysis.

Comparison 1 Clobazam versus carbamazepine, Outcome 5 50% responder rate at 4 weeks.
Time to achieve seizure freedom
Data on 43 children were available for this outcome (Andrade 2009). The mean time to achieve seizure freedom was 33.3 (± 45) days in participants randomized to clobazam (n = 18), compared to 48.2 (± 72.3) days in those allocated to receive carbamazepine (n = 25).
Adverse effects
Data on adverse effects were provided by two studies.
The first study compared clobazam with phenytoin in 48 adolescents and adults with symptomatic seizures due to solitary cysticercus granuloma (Kaushal 2006). Overall, six out of 27 participants in the phenytoin group discontinued study medication during the study period after experiencing adverse effects, compared to none in the 21 participants in the clobazam group (RR 0.10, 95% CI 0.01 to 1.65) (Analysis 2.3; Figure 6).The reported adverse effects included sedation, skin problems, allergic rash, tiredness, oral‐gingival problems, headache and weight gain (Analysis 2.4). The incidence of these adverse effects was not different between clobazam and phenytoin: sedation (RR 0.96, 95% CI 0.40 to 2.35), skin problems (RR 0.18, 95% CI 0.02 to 1.38), tiredness (RR 0.43, 95% CI 0.1 to 1.91), oral or gingival problems (RR 0.21, 95% CI 0.03 to 1.65), headache (RR 2.57, 95% CI 0.73 to 9.09) and weight gain (RR 11.45, 95% CI 0.65 to 201.6).
2.3. Analysis.
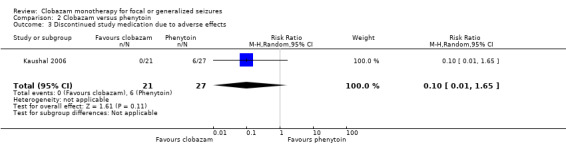
Comparison 2 Clobazam versus phenytoin, Outcome 3 Discontinued study medication due to adverse effects.
2.4. Analysis.

Comparison 2 Clobazam versus phenytoin, Outcome 4 Reported adverse effects.
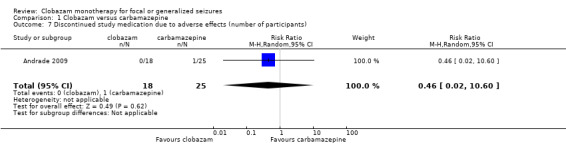
The second study compared clobazam with carbamazepine in 43 children with BCECTS (Andrade 2009). Overall, one participant out of 25 in the carbamazepine group discontinued the medication during the study period after experiencing adverse effects, compared to none in the 18 participants in the clobazam group. Reported adverse effects included vertigo, rash, headaches, somnolence/sedation, nausea/vomiting, diarrhea, tremors, and fatigue. The number of participants reporting adverse effects were not different between the clobazam and carbamazepine groups (RR 0.52, 95% CI 0.16 to 1.70, P = 0.26, Analysis 1.6).
1.6. Analysis.

Comparison 1 Clobazam versus carbamazepine, Outcome 6 Reported adverse effects (number of participants).
Discussion
Summary of main results
Three studies with a total of 206 participants met the inclusion criteria for this review (Andrade 2009; Canadian Study Group 1998; Kaushal 2006). Two of the included trials were adequately randomized, and overall, we judged them to be of low quality (Figure 3). None of the studies provided data for our primary outcome of time on allocated treatment, a clinically meaningful measure combining both efficacy and tolerability. No statistically significant advantage of clobazam over carbamazepine was detected in 115 participants in the Canadian Study Group 1998 trial (RR 0.83, 95% CI 0.61 to 1.12) for retention at 12 months. However, a slight advantage for clobazam over phenytoin (RR 1.43, 95% CI 1.08 to 1.90) was seen in 48 participants with single cysticercus granuloma in the brain for retention at six months (Kaushal 2006).
No significant differences were identified between clobazam and carbamazepine for seizure freedom at four weeks (RR 0.85, 95% CI 0.55 to 1.32), between four and 40 weeks (RR 0.95, 95% CI 0.66 to 1.36), and during the last nine months of treatment (RR 1.23, 95% CI 0.87 to 1.72). Also, no significant differences were noted for clobazam versus carbamazepine in 43 participants with BCECTS in the 50% responder rate (RR 1.18, 95% CI 0.94 to 1.48) at four weeks (Andrade 2009). However, participants randomized to clobazam achieved seizure control sooner than those treated with carbamazepine (Andrade 2009).
In one of the studies, people receiving clobazam were found to be less likely to discontinue it due to adverse effects compared to phenytoin (RR 0.10, 95% CI 0.01 to 1.65, Kaushal 2006). However, the wide confidence interval and potential for bias in the one open‐label study where this outcome could be analyzed, compromise the clinical relevance of this result.
In another study, no difference in the number of participants reporting adverse effects with clobazam or carbamazepine was noted, and the proportion of participants discontinuing treatment due to adverse effects was minimal and comparable between the two groups (Andrade 2009).
Overall completeness and applicability of evidence
An extensive, structured search found three trials fulfilling the inclusion criteria for this review. Overall, the result of this systematic review finds no evidence for the superiority of clobazam monotherapy compared to adequate comparator monotherapy for retention at 12 months, and only slight advantage for retention at six months, and time to achieve seizure freedom, in people with focal or generalized seizures. The analyzed outcomes were based on results from a single study in each case. In the absence of individual participant data, pooled analysis was not possible for the six months' retention outcome. The clinical applicability for 12 months' retention is limited by lack of data for focal versus generalized seizures, an important decision‐making factor in clinical practice. Similarly, the generalizability for six months' retention is not possible, due to a modest effect size and selected population consisting only of participants with solitary brain cysticercus granuloma.The modest advantage seen regarding time to achieve seizure freedom is also difficult to interpret, as far as clinical applicability and generalizability is concerned. This outcome was reported in only participants with BCECTS, and was not substantiated by sustaining seizure freedom over time, which is more important in clinical practice.
Quality of the evidence
The quality of evidence is low due to actual or possible biases in the included studies. None of the studies have described allocation concealment. The Canadian Study Group 1998 trial was funded by a pharmaceutical company, which also monitored data collection and entry. The Kaushal 2006 study was open‐label and certain issues were not addressed including informed consent, resolution of conflict in case of disagreement between two outcome assessors and discrepancy between CONSORT flow diagram and text/table regarding numbers randomized to each arm. The author having access to the study data did not reply, although the corresponding author had promised to do so. The Andrade 2009 study was also open‐label, and did not provide information about randomization and allocation concealment.
Potential biases in the review process
The eligibility for inclusion, data extraction and 'Risk of bias' assessment was carried out by two authors (VA and RA) independently, thereby reducing the chances of bias. No major potential biases in the review process are highlighted.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews analyzing clobazam monotherapy in focal or generalized seizures. However, the International League Against Epilepsy (ILAE) guideline for anti‐epileptic drug monotherapy (Glauser 2006), included the Canadian Study Group 1998 trial and categorized it as a class III study based on their criteria. Since the ILAE guideline analyzed outcomes based on participants' age and seizure type, the Canadian Study Group 1998 trial was considered uninformative as it fails to provide outcome data separately for focal and generalized seizures.
Authors' conclusions
Implications for practice.
This systematic review did not find sufficient evidence from randomized controlled trials to inform clinical practice meaningfully.
Implications for research.
Conventionally, clobazam has been used as an add‐on therapy especially in children with Lennox‐Gastaut syndrome and other difficult‐to‐treat epilepsies. Certain evidence gaps must be addressed to better define the role of clobazam as monotherapy in people with epilepsy.
There is a need for double‐blind randomized controlled trials comparing clobazam with other anti‐seizure drugs. These clinical trials must fulfil the criteria for class I studies as described in the recent update by International League Against Epilepsy (ILAE) (Glauser 2013). These prospective, randomized, controlled, clinical trials should be carried out in several representative patient samples with a primary outcome of efficacy or effectiveness and double‐blind treatment duration of at least 48 weeks without any forced study‐exit criteria. Either clobazam should have clear superiority over an adequate comparator, or a failed superiority study or non‐inferiority study should be analyzed in accordance with the criteria provided in the ILAE update (Glauser 2013).
As evidence becomes available for other drugs to be considered as acceptable monotherapies for focal or generalized seizures in clinical practice and as adequate comparators for clinical trials, studies should be designed to compare clobazam with these.
A sufficient sample size should be estimated to have adequate power to conduct subgroup analysis for different age groups and seizure types, and to compare adverse effect frequencies.
Future studies should include tolerability measures (treatment‐emergent adverse events), quality of life measures, and, tolerance measures.
What's new
| Date | Event | Description |
|---|---|---|
| 19 March 2018 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 19 March 2018 | New search has been performed | Searches were updated on 19 March 2018; one new study was included. |
History
Protocol first published: Issue 8, 2011 Review first published: Issue 10, 2014
| Date | Event | Description |
|---|---|---|
| 9 October 2014 | Amended | Figure 3 wasn't displaying correctly in the previously published version; this has now been rectified. |
Acknowledgements
We would like to acknowledge Cochrane Epilepsy for their support. Also, thanks to Ms. Rachael Kelly for assistance, Mr. Graham Chan and Ms. Alison Beamond for providing the search results, Dr. Yasuko Yamatogi for completing the data extraction form for their study in Japanese, and to Drs. Carol and Peter Camfield for offering their help to find the original participant data. Dr. Benedict Michael was a co‐author for the previous version of this systematic review and we acknowledge his input.
Appendices
Appendix 1. Cochrane Register of Studies search strategy
1. clobazam* or Aedon or Anxirloc or Castilium or Chlorepin or Clarmyl or Clobam or Clobamax or Clobator or Clofritis or Clopax or Clorepin or Frisium or Grifoclobam or Karidium or Lucium or Mystan or Noiafren or Onfi or Sederlona or Sentil or Urbadan or Urbanil or Urbanol or Urbanyl AND INREGISTER
2. >26/01/2017:CRSCREATED AND INREGISTER
3. #1 AND #2 AND INREGISTER
4. clobazam* or Aedon or Anxirloc or Castilium or Chlorepin or Clarmyl or Clobam or Clobamax or Clobator or Clofritis or Clopax or Clorepin or Frisium or Grifoclobam or Karidium or Lucium or Mystan or Noiafren or Onfi or Sederlona or Sentil or Urbadan or Urbanil or Urbanol or Urbanyl AND CENTRAL:TARGET
5. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
6. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
7. epilep* OR seizure* OR convuls* AND CENTRAL:TARGET
8. #5 OR #6 OR #7 AND CENTRAL:TARGET
9. #4 AND #8 AND CENTRAL:TARGET
10. ((adjunct* or "add‐on" or "add on" or adjuvant* or combination* or polytherap*) not (monotherap* or alone or singl*)):TI AND CENTRAL:TARGET
11. #9 NOT #10 AND CENTRAL:TARGET
12. >26/01/2017:CRSINCENTRAL AND CENTRAL:TARGET
13. #11 AND #12 AND CENTRAL:TARGET
14. #3 OR #13
Appendix 2. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2011.
1. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. exp Epilepsy/
8. exp Seizures/
9. (epilep$ or seizure$ or convuls$).tw.
10. 7 or 8 or 9
11. (clobazam or frisium or urbanol or onfi).tw.
12. 6 and 10 and 11
13. ((adjunct$ or "add‐on" or "add on" or adjuvant$ or combination$ or polytherap$) not (monotherap$ or alone or singl$)).ti.
14. 12 not 13
15. remove duplicates from 14
16. limit 15 to ed=20150416‐20180319
17. 15 not (1$ or 2$).ed.
18. 17 and (2015$ or 2016$ or 2017$ or 2018$).dt.
19. 16 or 18
Appendix 3. BIOSIS Previews search strategy
Indexes=BIOSIS Previews Timespan=2014‐2018
| #7 | #6 AND #5 |
| #6 | TS=((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind*) NEAR/2 (trial OR method OR procedure OR study)) |
| #5 | #3 NOT #4 |
| #4 | TI=(adjunct* OR "add‐on" OR "add on" OR adjuvant* OR combination* OR polytherap*) NOT TI=(monotherap* OR alone OR singl*) |
| #3 | #2 AND #1 |
| #2 | TOPIC: (clobazam or frisium or urbanol or onfi) |
| #1 | TOPIC: (epilep* OR "infantile spasm" OR seizure OR convuls* OR (syndrome NEAR/2 (aicardi OR angelman OR doose OR dravet OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR "unverricht lundborg" OR west)) OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency") NOTTITLE: (*eclampsia) |
Appendix 4. ClinicalTrials.gov search strategy
Epilepsy | Clobazam | First posted on or after 01/26/2017
Appendix 5. ICTRP search strategy
Condition: epilepsy AND Intervention: clobazam AND Date of registration 26/01/2017 to 19/03/2018
Appendix 6. DARE search strategy
1 (clobazam or Frisium or Urbanol or Onfi) IN DARE
2 MeSH DESCRIPTOR epilepsy EXPLODE ALL TREES IN DARE
3 MeSH DESCRIPTOR Seizures EXPLODE ALL TREES IN DARE
4 (epilep* or seizure* or convuls*) IN DARE
5 #2 OR #3 OR #4
6 #1 AND #5
7 (adjunct* OR "add‐on" OR "add on" OR adjuvant* OR combination* OR polytherap*):TI NOT (monotherap* or alone or singl*):TI IN DARE
8 #6 NOT #7
Appendix 7. Assessment of risk of bias
We recorded each of the following aspects and graded them as high risk, low risk or unclear risk.
Sequence generation
Was the allocation sequence adequately generated: e.g. coin toss, random number tables, computer generated, other?
Allocation concealment
Was allocation adequately concealed in a way that would not allow both the investigators and the participants to know or influence the intervention group before an eligible participant is entered into the study: e.g. central randomization, or sequentially numbered, opaque, sealed envelopes?
Incomplete outcome data
Were incomplete outcome data adequately addressed? Incomplete outcome data essentially include: attrition, exclusions and missing data. If any withdrawals occurred, were they described and reported by treatment group with reasons given? Whether or not there were clear explanations for withdrawals and drop‐outs in treatment groups was recorded. An example of an adequate method to address incomplete outcome data is the use of intention‐to‐treat analysis (ITT).
Selective outcome reporting
Are reports of the study free from suggestion of selective outcome reporting? This will be interpreted as no evidence that statistically non‐significant results might have been selectively withheld from publication: e.g. selective under‐reporting of data, or selective reporting of a subset of data.
Other sources of bias
Was the study apparently free of other problems that could put it at a high risk of bias: e.g. baseline imbalance, or the use of an insensitive instrument to measure outcomes?
Blinding
Details of blinding participants, personnel (surgeons) and outcome assessors were assessed. This will be recorded as: yes, no or not possible, or unclear.
Quality assessment/internal validity
Quality assessment criteria will be categorized as being at low, unclear or high risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Data and analyses
Comparison 1. Clobazam versus carbamazepine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention at 12 months | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.12] |
| 2 Seizure freedom at 4 weeks | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| 3 Seizure freedom between weeks 4 and 40 | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.36] |
| 4 Terminal remission at 9 months (seizure free for last 9 months) | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.87, 1.72] |
| 5 50% responder rate at 4 weeks | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 6 Reported adverse effects (number of participants) | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.70] |
| 7 Discontinued study medication due to adverse effects (number of participants) | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 10.60] |
1.4. Analysis.
Comparison 1 Clobazam versus carbamazepine, Outcome 4 Terminal remission at 9 months (seizure free for last 9 months).
1.7. Analysis.
Comparison 1 Clobazam versus carbamazepine, Outcome 7 Discontinued study medication due to adverse effects (number of participants).
Comparison 2. Clobazam versus phenytoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention at 6 months | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.08, 1.90] |
| 2 Breakthrough seizure(s) during study period | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.83] |
| 3 Discontinued study medication due to adverse effects | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.65] |
| 4 Reported adverse effects | 1 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.14] |
| 4.1 Sedation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.40, 2.35] |
| 4.2 Skin problems including allergic rash | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 4.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.10, 1.91] |
| 4.4 Oral or gingival problems | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.65] |
| 4.5 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.73, 9.09] |
| 4.6 Weight gain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 11.45 [0.65, 201.60] |
2.2. Analysis.
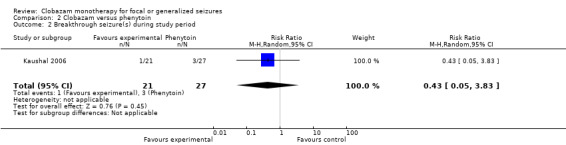
Comparison 2 Clobazam versus phenytoin, Outcome 2 Breakthrough seizure(s) during study period.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrade 2009.
| Methods | Open‐label randomized controlled trial | |
| Participants |
Inclusion criteria: diagnosis of benign focal epilepsy of childhood with centro‐temporal spikes by ILAE criteria and EEG evidence; 6 or more seizures/month or "considerable parental concern" about seizure(s) Exclusion criteria: people with neurologic disorders other than benign focal epilepsy of childhood with centro‐temporal spikes; history of pseudoseizures; use of psychotropic drugs; metabolic disorders; active infection or malignancy; previous treatment with clobazam or carbamazepine in which therapy stopped because of adverse effects or a diagnosis that prevented randomization or continuation in the study No. randomized: clobazam 18, carbamazepine 25 Duration of trial: 96 weeks Follow‐up: at 1, 2, 3, 4, 5, 6, 20, 14, 18, 22, 26, 30, 34, 40, 48, 56, 64, 72, 80, 88, and 96 weeks Setting: single center, out‐patient clinic, from Cuba |
|
| Interventions | 1. Clobazam 1 mg/kg/d for the first week, increased by 0.25 mg/kg/d every week, until a maximum dose of 2 mg/kg/d is reached 2. Carbamazepine 10 mg/kg/d in 3 divided doses for the first week, increased by 5 mg/kg/d every week, until a maximum dose of 30 mg/kg/d is reached |
|
| Outcomes | 1. Number of participants that remained in each treatment group 2. Number of participants with more than 50% reduction in seizures after 4 weeks of treatment 3. Number of participants who were free of seizures after week 4, between weeks 4 to 40, and in the last 9 months of treatment 4. Average time in days it took to achieve seizure freedom 5. Number of adverse events 6. Performance on neuropsychological testing; academic performance; behavioral/conduct problems; grade of satisfaction of treatment from parents and teachers |
|
| Notes | 1. Retention time (although measured at pre‐specified time points) is mentioned as an outcome in methods section, but not provided in the results 2. Ethics committee approval: yes 3. Informed consent: yes 4. Sample size calculation: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not mentioned in study |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned in study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “open, controlled and randomized study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned in study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “The number of patients that completed the study was 21 (84%) in the group assigned carbamazepine and 17 (94.4%) in the group with clobazam.” |
| Selective reporting (reporting bias) | Unclear risk | Comment: no pre‐published protocol |
| Other bias | Low risk | No baseline imbalance between participants assigned to clobazam vs carbamazepine; no use of imprecise instruments for outcome measurements |
Canadian Study Group 1998.
| Methods | Double‐blind randomized controlled trial | |
| Participants |
Inclusion criteria: age 6 months‐17 years; diagnosis of epilepsy (≥ 2 unprovoked seizures) by a child neurologist; seizure types of focal, focal with secondary generalization, or primary generalized tonic clonic Exclusion criteria: seizure type: absence, atonic, tonic, or myoclonic seizures; progressive neurological disease; other serious chronic illness; previous history of poor drug compliance with anti‐epileptic drugs No. randomized: clobazam: 63; carbamazepine: 52 Duration of trial: 4 years Follow‐up: 3 and 6 weeks; 3, 6 and 12 months Setting: 15 Canadian pediatric epilepsy centers |
|
| Interventions | 1. Clobazam: aim 0.5 mg/kg/d; average maximum dose 0.6 mg/kg/d 2. Carbamazepine: aim 10 mg/kg/d; average maximum dose 14.8 mg/kg/d Study medication was introduced over 1‐3 weeks. Both were given as 2 daily doses |
|
| Outcomes | Not classified into primary and secondary. End point was defined as retention on the study medication for 12 months or discontinuation of the medication for any reason, including side effects or inadequate seizure control. | |
| Notes | 1. The study had 3 arms: a. drug‐naive b. previous failure with carbamazepine c. previous failure with other anti‐seizure medications Only the first arm is included for analysis in this review 2. Demographic characteristics Age range: this was not provided; instead the number of participants belonging to arbitrary age groups (< 6 years, 6 to ≤ 12 years and > 12 years) were given for all drug‐naïve participants together and not separately for those receiving clobazam versus carbamazepine Gender: Boys 66, distribution between groups is not stated 3. Ethics committee approval: yes 4. Informed consent: yes 5. Sample size calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Within each study arm, drug assignment was randomized using a permuted block technique with a depth of 6 for each study center" Comment: mentioned in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: mot mentioned in study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was double blind using a modified 'double dummy' technique" Comment: mentioned in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "For the primary analysis, all data were analyzed according to the intention‐to‐treat principle, including all randomized patients." Comment: mentioned in the study |
| Selective reporting (reporting bias) | Unclear risk | No pre‐published protocol |
| Other bias | Unclear risk | Funding from Hoechst‐Marion‐Roussel Canada, Inc. which also monitored the data collection and entry |
Kaushal 2006.
| Methods | Randomized open‐label trial | |
| Participants |
Inclusion criteria: age > 12 years; recent onset (< 2 weeks) of generalized seizures or focal seizures with or without secondary generalization; not already on antiepileptic drug treatment; imaging demonstrated a solitary cysticercus granuloma Exclusion criteria: concomitant serious systemic disorder; pregnant women; unwilling to comply with the follow‐up schedule; presenting in status epilepticus or seizure clusters (2 or more seizures in 24 h prior to presentation) No. randomized: clobazam 21, phenytoin 27 (see risk of bias table) Age range: phenytoin: 12‐66 years, clobazam: 12‐46 years Gender: Male participants 17 in phenytoin group, 14 in clobazam group Duration of trial: 14 months Follow‐up: 1, 2, 3 and 6 months Setting: Outpatient Neurology Clinic of an Indian teaching hospital |
|
| Interventions | 1. Clobazam 1 mg/kg oral loading followed by 0.5 mg/kg/d 2. Phenytoin 15 mg/kg oral loading in 3 divided doses over 24 h, followed by 0.5 mg/kg/d Both medications for at least 6 months None of the participants received any cysticidal treatment |
|
| Outcomes |
Primary: total number of treatment failures in each group during 6 months of follow‐up. Treatment failure was defined as breakthrough seizure or adverse effect that required discontinuation or modification of study medication either with increased dose or with additional anti‐seizure medication Secondary: frequency of adverse effects (not explicitly mentioned) |
|
| Notes | 1. Ethics committee approval: yes 2. Informed consent: not stated 3. Sample size calculation: yes (see risk of bias table) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized strictly according to computer‐generated random list" Comment: mentioned in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned in study, authors contacted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open labelled trial" Comment: mentioned in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " In addition to the treating physician, an independent research team member evaluated seizure control and adverse effects at follow‐up. He was blinded to the treatment arms for the duration of study" Comment: mentioned in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomized participants were analyzed |
| Selective reporting (reporting bias) | Unclear risk | No prepublished protocol |
| Other bias | Unclear risk | 1.Outcome was assessed by the treating physician and a blinded research team member, but they do not report how a consensus was reached in case of disagreement. 2. No report on whether and how informed consent was obtained 3. There is a discrepancy in the number of participants randomized to receive each study treatment. Whereas in the CONSORT flow chart 27 and 21 participants were reported to be respectively randomized to clobazam and phenytoin, these numbers were exactly reversed in the text and subsequent table comparing baseline characteristics and outcome measures 4. Estimated that a sample size of 270 was needed to detect a 10% difference in the primary outcome measure with a 90% confidence, however, study was terminated early with an actual sample of only 48 due to relocation of a study investigator |
EEG: electroencephalogram; ILAE: International League Against Epilepsy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bajaj 2005 | It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy |
| Canadian Study Group 1999 | The group randomized to receive clobazam also included children who have failed carbamazepine or discontinued one or more other AED |
| Feely 1982a | Used clobazam as a short‐term intermittent treatment |
| Feely 1982b | Used clobazam as a short‐term intermittent treatment |
| Feely 1982c | Uses clobazam as a short‐term intermittent treatment |
| Feely 1984 | Case series, not a randomized controlled clinical trial |
| Figueroa 1984 | Used clobazam as add‐on therapy (not monotherapy) |
| Khosroshahi 2011 | It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy |
| Koeppen 1987 | It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) |
| RamachandranNair 2005 | It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy |
| Rose 2005 | It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy |
| Vajda 1985 | It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) |
| Yagi 1995 | It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) |
| Yamatogi 1997 | It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) |
AED: anti‐epileptic drug
Differences between protocol and review
In 'Types of interventions', we decided to include studies with clobazam monotherapy given continuously for at least three months.
Contributions of authors
The review was conceived by RA and SKG. Data were extracted by VA, SKG, NG, and analyzed by SKG, RA and VA. RA and VA wrote the manuscript, which was reviewed by SKG and NG. All review authors have reviewed and approved the final version.
Sources of support
Internal sources
-
Cincinnati Children's Hospital Medical Center, USA.
Library, Biostatistics
External sources
-
National Institute for Health Research (NIHR), UK.
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Ravindra Arya receives research support (total 5% effort) from Pediatric Epilepsy Research Foundation, which is unrelated to the present work Vidhu Anand: none known Nisha Giridharan: none known Sushil K Garg: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andrade 2009 {published data only}
- Andrade R, García‐Espinosa A, Machado‐Rojas A, García‐González ME, Trápaga‐Quincoses O, Morales‐Chacón LM. A prospective, open, controlled and randomised study of clobazam versus carbamazepine in patients with frequent episodes of Rolandic epilepsy [Estudio prospectivo, abierto, controlado y aleatorizado de clobazam frente a carbamacepina en pacientes con crisis frecuentes de epilepsia Rolándica]. Revista de Neurologia 2009;49(11):581‐6. [PubMed] [Google Scholar]
Canadian Study Group 1998 {published data only}
- Canadian Study Group for Childhood Epilepsy. Clobazam has equivalent efficacy to carbamazepine and phenytoin as monotherapy for childhood epilepsy. Epilepsia 1998;39(9):952‐9. [PUBMED: 9738674] [DOI] [PubMed] [Google Scholar]
Kaushal 2006 {published data only}
- Kaushal S, Rani A, Chopra SC, Singh G. Safety and efficacy of clobazam versus phenytoin‐sodium in the antiepileptic drug treatment of solitary cysticercus granulomas. Neurology India 2006;54(2):157‐60. [PUBMED: 16804259] [PubMed] [Google Scholar]
References to studies excluded from this review
Bajaj 2005 {published data only}
- Bajaj AS, Bajaj BK, Puri V, Tayal G. Intermittent clobazam in febrile seizures: an Indian experience. Journal of Pediatric Neurology 2005;3(1):19‐23. [Google Scholar]
Canadian Study Group 1999 {published data only}
- Bawden HN, Camfield CS, Camfield PR, Cunningham C, Darwish H, Dooley JM, et al. The cognitive and behavioural effects of clobazam and standard monotherapy are comparable; Canadian Study Group for Childhood Epilepsy. Epilepsy Research 1999;33(2‐3):133‐43. [PUBMED: 10094425] [DOI] [PubMed] [Google Scholar]
Feely 1982a {published data only}
- Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy. A model for evaluating anticonvulsants. Lancet 1982;2(8289):71‐3. [PUBMED: 6123810] [DOI] [PubMed] [Google Scholar]
Feely 1982b {published data only}
- Feely M, Calvert R, Gibson J. Catamenial epilepsy as a model for testing a new anticonvulsant (clobazam). Irish Journal of Medical Science 1982;151(11):358‐9. [Google Scholar]
Feely 1982c {published data only}
- Feely M, Calvert R, Gibson J. Clobazam in the treatment of catamenial epilepsy. British Journal of Clinical Pharmacology 1982;13(2):273P‐4P. [Abstract: Proceedings of the British Pharmacological Society; 1981 Sept 16‐18; University of Oxford, Oxford, UK] [Google Scholar]
Feely 1984 {published data only}
- Feely M, Gibson J. Intermittent clobazam for catamenial epilepsy: tolerance avoided. Journal of Neurology, Neurosurgery, and Psychiatry 1984;47(12):1279‐82. [PUBMED: 6392481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueroa 1984 {published data only}
- Figueroa D, Adlerstein L, Manterola A. Clobazam in refractory epilepsies of children [Clobazam en epilepsias refractarias del nino]. Revista Chilena de Pediatria 1984;55(6):401‐5. [PUBMED: 6399391] [PubMed] [Google Scholar]
Khosroshahi 2011 {published data only}
- Khosroshahi N, Faramarzi F, Salamati P, Haghighi SM, Kamrani K. Diazepam versus clobazam for intermittent prophylaxis of febrile seizures. Indian Journal of Pediatrics 2011;78(1):38‐40. [PUBMED: 20890683] [DOI] [PubMed] [Google Scholar]
Koeppen 1987 {published data only}
- Koeppen D, Baruzzi A, Capozza M, Chauvel P, Courjon J, Favel P, et al. Clobazam in therapy‐resistant patients with partial epilepsy: a double‐blind placebo‐controlled crossover study. Epilepsia 1987;28(5):495‐506. [PUBMED: 3115770] [DOI] [PubMed] [Google Scholar]
RamachandranNair 2005 {published data only}
- Ramachandran Nair R, Parameswaran M, Girija AS. Intermittent oral prophylaxis in preventing recurrent febrile seizures, diazepam versus clobazam: a randomised study in south Indian rural children. Epilepsia 2005;46(Suppl 6):240. [Google Scholar]
Rose 2005 {published data only}
- Rose W, Kirubakaran C, Scott JX. Intermittent clobazam therapy in febrile seizures. Indian Journal of Pediatrics 2005;72(1):31‐3. [PUBMED: 15684445] [DOI] [PubMed] [Google Scholar]
Vajda 1985 {published data only}
- Vajda FJ, Bladin PF, Parsons BJ. Clinical experience with clobazam: a new 1,5 benzodiazepine in the treatment of refractory epilepsy. Clinical and Experimental Neurology 1985;21:177‐82. [PUBMED: 3843217] [PubMed] [Google Scholar]
Yagi 1995 {published data only}
- Yagi K, Takeda A, Kawai I. The clinical efficacy of nh‐15 (clobazam) for the treatment refractory epilepsy by the double‐blind comparative study with inactive placebo. Igakunoayumi 1995;174(3):229‐41. [Google Scholar]
Yamatogi 1997 {published data only}
- Yamatogi Y, Ohtahara S, Shigematsu H, Oguni H, Nakashima M. Single blind comparative study of clobazam (NH‐15) with clonazepam in intractable childhood epilepsies. Journal of the Japan Epilepsy Society 1997;15(2):110‐21. [Google Scholar]
Additional references
Banerjee 2008
- Banerjee PN, Hauser WA. Incidence and Prevalence. In: Engel Jr J, Pedley TA editor(s). Epilepsy. A Comprehensive Textbook. 2nd Edition. Vol. 1, Philadelphia: Lippincott Williams & Wilkins, 2008:45‐56. [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐ analysis of randomised controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Canadian Clobazam Cooperative Group 1991
- Canadian Clobazam Cooperative Group. Clobazam in treatment of refractory epilepsy: the Canadian experience. A retrospective study. Epilepsia 1991;32(3):407‐16. [PubMed] [Google Scholar]
Commission 1998
- The ILAE Commission on Antiepileptic Drugs. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 1998;39(7):799‐803. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Engel 2008
- Engel J Jr, Pedley TA. Introduction: What is Epilepsy?. In: Engel J Jr, Pedley TA editor(s). Epilepsy. A Comprehensive Textbook. 2nd Edition. Vol. 1, Philadelphia: Lippincott Williams & Wilkins, 2008:1‐7. [Google Scholar]
Fisher 2005
- Fisher RS, Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy. Definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46(4):470‐2. [DOI] [PubMed] [Google Scholar]
Glauser 2006
- Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE Treatment Guidelines: Evidence‐based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006;47(7):1094‐120. [DOI] [PubMed] [Google Scholar]
Glauser 2013
- Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kalviainen R, et al. for the ILAE subcommission of AED Guidelines. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013;54(3):551‐63. [PUBMED: 23350722] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Cochrane Collaboration.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Löscher 1987
- Löscher W, Schmidt D. Diazepam increases aminobutyric acid in human cerebrospinal fluid. Journal of Neurochemistry 1987;49:152‐7. [DOI] [PubMed] [Google Scholar]
Macdonald 2002
- Macdonald R. Benzodiazepines. Mechanism of Action. In: Levy R, Mattson R, Meldrum B, et al. editor(s). Antiepileptic Drugs. 5th Edition. Philadelphia: Lippincott, Williams & Wilkins, 2002:179‐86. [Google Scholar]
Murray 1994
- Murray CGL, Lopez AD. Global comparative assessment in the Health Sector; Disease Burden, Expenditures, and Intervention Packages. Geneva: WHO, 1994. [Google Scholar]
Porter 2007
- Porter RJ, Meldrum BS. Antiseizure Drugs. In: Katzung BG editor(s). Basic and Clinical Pharmacology. 10th Edition. Mc Graw Hill, 2007:374‐94. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogawski 2004
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nature Reviews Neuroscience 2004;5(1):1‐12. [DOI] [PubMed] [Google Scholar]
Schmidt 1986
- Schmidt D, Rohde M, Wolf P, Roeder‐Wanner U. Clobazam for refractory focal epilepsy. A controlled trial. Archives of Neurology 1986;43(8):824‐6. [DOI] [PubMed] [Google Scholar]
Schmidt 2008
- Schmidt D, Wilensky AJ. Benzodiazepines. In: Engel J Jr, Pedley TA editor(s). Epilepsy. A Comprehensive Textbook. 2nd Edition. Vol. 2, Philadelphia: Lippincott Williams & Wilkins, 2008:1531‐41. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chaper 12: Interpreting results and drawing conclusions. In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated September 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
References to other published versions of this review
Arya 2011
- Arya R, Kumar S, Michael B, Anand V. Clobazam monotherapy for partial onset or generalized onset seizures. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009258] [DOI] [PubMed] [Google Scholar]
Arya 2014
- Arya R, Anand V, Garg SK, Michael BD. Clobazam monotherapy for partial‐onset or generalized‐onset seizures. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD009258.pub2] [DOI] [PubMed] [Google Scholar]


