Abstract
Background
Dental pain can have a detrimental effect on quality of life. Symptomatic apical periodontitis and acute apical abscess are common causes of dental pain and arise from an inflamed or necrotic dental pulp, or infection of the pulpless root canal system. Clinical guidelines recommend that the first‐line treatment for teeth with these conditions should be removal of the source of inflammation or infection by local, operative measures, and that systemic antibiotics are currently only recommended for situations where there is evidence of spreading infection (cellulitis, lymph node involvement, diffuse swelling) or systemic involvement (fever, malaise). Despite this, there is evidence that dentists frequently prescribe antibiotics in the absence of these signs. There is concern that this could contribute to the development of antibiotic‐resistant bacterial colonies within both the individual and the community. This review is an update of the original version that was published in 2014.
Objectives
To evaluate the effects of systemic antibiotics provided with or without surgical intervention (such as extraction, incision and drainage of a swelling, or endodontic treatment), with or without analgesics, for symptomatic apical periodontitis and acute apical abscess in adults.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 26 February 2018), the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library (searched 26 February 2018), MEDLINE Ovid (1946 to 26 February 2018), Embase Ovid (1980 to 26 February 2018), and CINAHL EBSCO (1937 to 26 February 2018). The US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. A grey literature search was conducted using OpenGrey (to 26 February 2018) and ZETOC Conference Proceedings (1993 to 26 February 2018). No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
Randomised controlled trials of systemic antibiotics in adults with a clinical diagnosis of symptomatic apical periodontitis or acute apical abscess, with or without surgical intervention (considered in this situation to be extraction, incision and drainage or endodontic treatment) and with or without analgesics.
Data collection and analysis
Two authors screened the results of the searches against inclusion criteria, extracted data and assessed risk of bias independently and in duplicate. We calculated mean differences (MD) (standardised mean difference (SMD) when different scales were reported) and 95% confidence intervals (CI) for continuous data. A fixed‐effect model was used in the meta‐analysis as there were fewer than four studies. We contacted study authors to obtain missing information.
Main results
We included two trials in this review, with 62 participants included in the analyses. Both trials were conducted in university dental schools in the USA and compared the effects of oral penicillin V potassium (penicillin VK) versus a matched placebo when provided in conjunction with a surgical intervention (total or partial pulpectomy) and analgesics to adults with acute apical abscess or symptomatic necrotic tooth. The patients included in these trials had no signs of spreading infection or systemic involvement (fever, malaise). We assessed one study as having a high risk of bias and the other study as having unclear risk of bias.
The primary outcome variables reported in both studies were participant‐reported pain and swelling (one trial also reported participant‐reported percussion pain). One study reported the type and number of analgesics taken by participants. One study recorded the incidence of postoperative endodontic flare‐ups (people who returned with symptoms that necessitated further treatment). Adverse effects, as reported in one study, were diarrhoea (one participant, placebo group) and fatigue and reduced energy postoperatively (one participant, antibiotic group). Neither study reported quality of life measurements.
Objective 1: systemic antibiotics versus placebo with surgical intervention and analgesics for symptomatic apical periodontitis or acute apical abscess Two studies provided data for the comparison between systemic antibiotics (penicillin VK) and a matched placebo for adults with acute apical abscess or a symptomatic necrotic tooth when provided in conjunction with a surgical intervention. Participants in one study all underwent a total pulpectomy of the affected tooth, while participants in the other study had their tooth treated by either partial or total pulpectomy. Participants in both trials received oral analgesics. There were no statistically significant differences in participant‐reported measures of pain or swelling at any of the time points assessed within the review. The MD for pain (short ordinal numerical scale 0 to 3) was ‐0.03 (95% CI ‐0.53 to 0.47) at 24 hours; 0.32 (95% CI ‐0.22 to 0.86) at 48 hours; and 0.08 (95% CI ‐0.38 to 0.54) at 72 hours. The SMD for swelling was 0.27 (95% CI ‐0.23 to 0.78) at 24 hours; 0.04 (95% CI ‐0.47 to 0.55) at 48 hours; and 0.02 (95% CI ‐0.49 to 0.52) at 72 hours. The body of evidence was assessed as at very low quality.
Objective 2: systemic antibiotics without surgical intervention for adults with symptomatic apical periodontitis or acute apical abscess We found no studies that compared the effects of systemic antibiotics with a matched placebo delivered without a surgical intervention for symptomatic apical periodontitis or acute apical abscess in adults.
Authors' conclusions
There is very low‐quality evidence that is insufficient to determine the effects of systemic antibiotics on adults with symptomatic apical periodontitis or acute apical abscess.
Plain language summary
The effects of antibiotics on toothache caused by inflammation or infection at the root of the tooth in adults
This Cochrane Review has been produced to assess the effects of antibiotics on the pain and swelling experienced by adults in two conditions commonly responsible for causing dental pain. The review set out to assess the effects of taking antibiotics when provided with, or without, dental treatment.
Background
Dental pain is a common problem and can arise when the nerve within a tooth dies due to progressing decay or injury. Without treatment, bacteria can infect the dead tooth and cause a dental abscess, which can lead to swelling and spreading infection, which can occasionally be life threatening.
The recommended treatment for these forms of toothache is removal of the dead nerve and associated bacteria. This is usually done by extraction of the tooth or root canal treatment (a procedure where the nerve and pulp are removed and the inside of the tooth cleaned and sealed). Antibiotics are only recommended when there is severe infection that has spread from the tooth into the surrounding tissues. However, some dentists still routinely prescribe oral antibiotics to patients with acute dental conditions who have no signs of spreading infection, or without dental treatment to remove the infected material.
Use of antibiotics contributes to the development of antibiotic‐resistant bacteria. It is therefore important that antibiotics are only used when they are likely to result in benefit for the patient. Dentists prescribe approximately 8% to 10% of all primary care antibiotics in high‐income countries, and therefore it is important to ensure that dentists have good information about when antibiotics are likely to be beneficial for patients.
Study characteristics
The evidence on which this review is based was up‐to‐date as of 26 February 2018. We searched scientific databases and found two trials, with 62 participants included in the analysis. Both trials were conducted at dental schools in the USA and evaluated the use of oral antibiotics in the reduction of pain and swelling reported by adults after having the first stage of root canal treatment under local anaesthetic. The antibiotic used in both trials was penicillin VK and all participants also received painkillers.
Key results
The two studies included in the review reported that there were no clear differences in the pain or swelling reported by participants who received oral antibiotics compared with a placebo (a dummy treatment) when provided alongside the first stage of root canal treatment and painkillers. However, the studies were small and produced poor quality evidence, and therefore we cannot be certain if the results are correct. Neither study examined the effect of antibiotics on their own, without surgical dental treatment.
One trial reported side effects among participants: one person who received the placebo medication had diarrhoea and one person who received antibiotics experienced tiredness and reduced energy after their treatment.
Quality of evidence
We judged the quality of evidence to be very low. There is currently insufficient evidence to be able to determine the effects of antibiotics in these conditions.
Summary of findings
Summary of findings for the main comparison. Systemic antibiotics with a surgical intervention and analgesics for managing symptomatic apical periodontitis and acute apical abscess in adults.
| Systemic antibiotics with a surgical intervention and analgesics for managing symptomatic apical periodontitis and acute apical abscess in adults | ||||||
| Patient or population: adults with a symptomatic necrotic tooth or localised acute apical abscess (no signs of spreading infection or systemic involvement) Settings: university dental schools, USA Intervention: systemic antibiotics, partial or total pulpectomy and analgesics Comparison: matched placebo, partial or total pulpectomy and analgesics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Matched placebo, partial or total pulpectomy and analgesics | Systemic antibiotics, partial or total pulpectomy and analgesics | |||||
| Pain at 24 hours Short ordinal numerical scale. Scale from: 0 to 3 | The mean pain at 24 hours ranged across control groups from: 1.0 to 1.68 | The mean pain at 24 hours in the intervention groups was 0.03 lower (0.53 lower to 0.47 higher) | ‐ | 61 (2 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | ‐ |
| Pain at 48 hours Short ordinal numerical scale. Scale from: 0 to 3 | The mean pain at 48 hours ranged across control groups from: 0.8 to 0.95 | The mean pain at 48 hours in the intervention groups was 0.32 higher (0.22 lower to 0.86 higher) | ‐ | 61 (2 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | ‐ |
| Pain at 72 hours Short ordinal scale. Scale from: 0 to 3 | The mean pain at 72 hours ranged across control groups from: 0.3 to 0.82 | The mean pain at 72 hours in the intervention groups was 0.08 higher (0.38 lower to 0.54 higher) | ‐ | 61 (2 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | ‐ |
| Swelling at 24 hours Different short ordinal numerical scales | The mean swelling at 24 hours in the control groups was 0.594 | The mean swelling at 24 hours in the intervention groups was 0.27 standard deviations higher (0.23 lower to 0.78 higher) | ‐ | 62 (2 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | This converts back into a 36% increase (95% CI 31% decrease to 105% increase) of control mean for antibiotics (based on 1 study at unclear risk of bias) |
| Swelling at 48 hours Different short ordinal numerical scales | The mean swelling at 48 hours in the control groups was 0.734 | The mean swelling at 48 hours in the intervention groups was 0.04 standard deviations higher (0.47 lower to 0.55 higher) | ‐ | 61 (2 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | This converts back into a 4% increase (95% CI 49% decrease to 58% increase) of control mean for antibiotics (based on 1 study at unclear risk of bias) |
| Swelling at 72 hours Different short ordinal numerical scales | The mean swelling at 72 hours in the control groups was 0.594 | The mean swelling at 72 hours in the intervention groups was 0.02 standard deviations higher (0.49 lower to 0.52 higher) | ‐ | 61 (2 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | This converts back into a 2% increase (95% CI 55% decrease to 59% increase) of control mean for antibiotics (based on 1 study at unclear risk of bias) |
| Adverse effects | During the 3‐day follow‐up period in Fouad 1996, 1 participant in the placebo group reported diarrhoea and 1 participant in the antibiotic group reported fatigue and reduced energy postoperatively | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
11 study with high risk of bias and small group sizes. 21 study with unclear risk of bias and small group sizes. 3Different surgical interventions employed. Participants in 1 study had partial or total pulpectomy (there was no way of distinguishing between the 2 treatment modalities) while participants in the other all had total pulpectomy. 4Re‐expressed from the standardised mean difference into the short ordinal numerical scale used by Henry 2001. Results should be interpreted with caution since back‐translation of the effect size is based on the results of only 1 study.
Background
Description of the condition
Dental pain can have a detrimental effect on an individual's social functioning and quality of life (Pau 2005; Reisine 1995). In the UK Adult Dental Health Survey of 2009, 29% of individuals reported experiencing dental pain "occasionally" or "fairly/very often" during the preceding 12 months. The overall prevalence of dental pain among survey respondents was 9%, with higher values reported for younger individuals and those from lower socioeconomic groups (Steele 2011). Among adults presenting with acute dental conditions, approximately 16% will have symptomatic apical periodontitis and a further 20% will have an acute apical abscess (Cope 2016).
Apical periodontitis arises following injury to the pulpal tissues of a tooth caused by dental caries, tooth fracture, trauma, or iatrogenic damage. While the dental pulp can recover from reversible pulpitis resulting from a mild to moderate injury, persistent or extensive damage results in irreversible levels of inflammation within the pulpal tissues. Should this occur, an individual might experience symptoms of irreversible pulpitis. Without treatment, irreversibly inflamed teeth then undergo pulpal necrosis and bacterial colonisation of the root canal system (Abbott 2004; Bergenholtz 2010).
Apical periodontitis (also known as periapical periodontitis) is an inflammatory lesion of the periradicular tissues that arises principally due to the egress of irritants, such as bacteria and toxins, from an inflamed or necrotic pulp (Torabinejad 1994). Its evolutionary role is protective: to contain the root canal bacteria and prevent the spread of infection. While the vast majority of cases are asymptomatic, exacerbations of apical periodontitis can present as symptomatic apical periodontitis or an acute apical abscess(Bergenholtz 2010).
Symptomatic apical periodontitis can arise either from a formerly healthy tooth that has subsequently undergone pulpal breakdown or from a tooth with a previously asymptomatic apical periodontitis. It is characterised by a dull or throbbing pain that is exacerbated by biting. The affected tooth usually has a negative or delayed positive response to vitality testing and is often highly sensitive to percussive forces (Bergenholtz 2010).
It should be noted that in determining the health of pulpal tissues, the term 'vitality testing' is commonly used. True 'vitality' tests attempt to examine the presence of pulp blood flow, while 'sensibility' tests employ the use of thermal or electrical stimuli to elicit a response from innervated tissue (Chen 2009). Although neither can definitively indicate the health of the dental pulp, they remain useful diagnostic aids, commonly used in both clinical practice and scientific studies.
Acute apical abscesses develop in the presence of a pre‐existing apical periodontitis (Carrotte 2004). The persistent presence of infective material within the pulpless root canal system and around the apex of a tooth can lead to a massive influx of polymorphonuclear leukocytes into the periradicular tissues, leading to tissue liquefaction and pus formation (Bergenholtz 2010). Also known as a periapical, dentoalveolar, or alveolar abscess, an apical abscess is characterised by the accumulation of pus in the periradicular tissues and can present as either an acute or a chronic lesion. Individuals with acute apical abscesses typically complain of a rapid onset, spontaneous pain, tenderness of the tooth to pressure, pus formation, and swelling of associated tissues (Glickman 2009). Left untreated, the abscess may spread, resulting in a potentially serious head and neck infection accompanied by fever, malaise, and lymph node involvement (Abbott 2004). Since symptomatic apical periodontitis and acute apical abscess represent a continuum of the same disease process, it is appropriate to consider both conditions in this review (Sutherland 2004).
Description of the intervention
Clinical guidelines currently recommend that the first‐line treatment for teeth with either symptomatic apical periodontitis or an acute apical abscess is the removal of the source of inflammation or infection by local, operative measures (Glenny 2004; SDCEP 2016). This could involve extraction of the offending tooth or extirpation (removal) of the pulpal tissues, possibly in combination with the incision and drainage of any swelling present.
Systemic antibiotics are currently only recommended for situations where there is evidence of spreading infection (cellulitis, lymph node involvement, diffuse swelling) or systemic symptoms (fever, malaise) (Palmer 2015; SDCEP 2016). Despite this, there is evidence that antibiotics are often prescribed by dentists to patients with symptomatic apical periodontitis or acute apical abscess in the absence of these signs (Cope 2016; Germack 2017; Segura‐Egea 2010; Yingling 2002). In a study, 69% of individuals attending a British out‐of‐hours dental clinic with symptomatic apical periodontitis received a prescription for systemic antibiotics, many in the absence of a surgical intervention (Dailey 2001).
How the intervention might work
Doctors and dentists may prescribe systemic antibiotics to minimise the signs and symptoms of symptomatic apical periodontitis or acute apical abscess, and to treat or prevent the development of a serious orofacial swelling with systemic involvement. Antibiotics can be prescribed as an adjunctive or stand‐alone treatment. People prescribed antibiotics may be given analgesics at the same time.
Why it is important to do this review
There is international concern about the overuse of antibiotics and the emergence of antibiotic‐resistant bacterial strains (World Health Organization 2000). Since dentists prescribe approximately 8% to 10% of antibiotics dispensed in primary care in high‐income countries, it is important not to underestimate the potential contribution of the dental profession to the development of antibiotic resistance (Al‐Haroni 2007; Halling 2017; Holyfield 2009). The use of antibiotics in situations where their use is not indicated not only drives antibiotic resistance, it is a misuse of resources, increases the risk of potentially fatal anaphylactic reactions and exposes people to unnecessary side effects (Costelloe 2010; Gonzales 2001). Furthermore, antibiotic prescribing for common medical problems may increase patient expectations for antibiotics, leading to a vicious cycle of increased prescribing in order to meet expectations (Coenen 2006; Little 1997).
It is important that antibiotics are prescribed for patients only when they are likely to result in clinical benefit. If systemic antibiotics are effective in the treatment of symptomatic apical periodontitis or acute apical abscess then it is important that the nature of any benefits are quantified. However, if antibiotics are ineffective, patients are being unnecessarily exposed to harmful side effects and the increased possibility of developing antibiotic‐resistant bacterial colonies. Therefore, the objective of this review was to evaluate the effects of systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. This is an update of the original version that was published in 2014 (Cope 2014).
Objectives
To evaluate the effects of systemic antibiotics provided with or without surgical intervention (such as extraction, incision and drainage of a swelling, or endodontic treatment), with or without analgesics, for symptomatic apical periodontitis or acute apical abscess in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with parallel group design in the review. We excluded cluster RCTs.
Types of participants
Studies of adults (18 years of age or older), male or female, who presented with a single tooth with a clinical diagnosis of either symptomatic apical periodontitis or acute apical abscess.
Types of interventions
Active intervention
Administration of any systemic antibiotic (either oral or intravenous) at any dosage prescribed in the symptomatic phase of apical periodontitis or acute apical abscess with or without analgesics, and with or without surgical intervention (extraction, incision and drainage or endodontic treatment).
Control
Administration of a matched placebo prescribed in the symptomatic phase of apical periodontitis or acute apical abscess with or without analgesics, and with or without surgical intervention.
Types of outcome measures
Primary outcomes
Measures of participant‐reported pain and swelling, gauged on either a continuous scale, such as visual analogue scale (VAS), or using binary or dichotomous outcomes.
Clinician‐reported measures of infection, such as swelling, temperature, trismus (reduced mouth opening), regional lymphadenopathy or cellulitis. These outcomes may have be reported as continuous, categorical or dichotomous variables.
Secondary outcomes
Participant‐reported quality of life measures.
Type, dose and frequency of analgesics used.
Any adverse effects or harm (hypersensitivity or other reactions) attributed to antibiotics or analgesics, complications of surgical treatment or hospitalisations.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 26 February 2018);
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library (searched 26 February 2018);
MEDLINE Ovid (1946 to 26 February 2018);
Embase Ovid (1980 to 26 February 2018);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 26 February 2018);
OpenGrey (to 26 February 2018);
ZETOC Conference Proceedings (1993 to 26 February 2018).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6 (Lefebvre 2011).
The full search strategies used for each database can be found in Appendix 1.
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 26 February 2018);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 26 February 2018).
We checked the reference lists of all included and excluded studies to identify any further trials.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (Anwen L Cope (ALC) and Ivor G Chestnutt (IGC)) independently assessed the titles and abstracts (where available) of the articles identified by the search strategy and made decisions regarding eligibility. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomised. Full‐text versions were obtained for all articles being considered for inclusion, as were those with insufficient information in the title or abstract to make a clear decision. We resolved any disagreements by discussion. We excluded studies later found not to meet the inclusion criteria and recorded them in the Characteristics of excluded studies table.
Data extraction and management
We entered study details into the Characteristics of included studies table. ALC and IGC independently extracted the outcome data from the included studies using a standard data extraction form. The review authors discussed the results and resolved any disagreements. In cases where uncertainties persisted, we contacted the study authors for clarification.
We extracted the following characteristics of the studies.
Study methodology: study design, methods of allocation, method of randomisation, randomisation concealment, blinding, time of follow‐up, loss to follow‐up, country conducted in, number of centres, recruitment period and funding source.
Participants: sampling frame, diagnostic criteria, inclusion criteria, exclusion criteria, number of participants in each group, baseline group demographics and clinical diagnosis.
Intervention: type of antibiotic, dose, frequency and duration of course. Information about co‐interventions, for example, surgical treatment or analgesia.
Outcomes: primary outcomes at 24, 48 and 72 hours and 7 days, and secondary outcomes as previously described (see Primary outcomes; Secondary outcomes).
Assessment of risk of bias in included studies
Two review authors (ALC and IGC) independently assessed the risk of bias of the included studies and resolved any disagreements by discussion. We completed a 'Risk of bias' table for each included study following the recommended methods for assessing the risk of bias in studies included in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This was a two‐part tool addressing specific key domains including sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other bias. We tabulated relevant information describing what happened, as reported in the study or revealed by correspondence with the study authors, for each included study, along with a judgement of low, high or unclear risk of bias for each individual domain.
A summary assessment of the risk of bias of each included study was made as follows:
low risk of bias (plausible bias unlikely to seriously alter the results) if we assessed all key domains to be at low risk of bias;
unclear risk of bias (plausible bias that raises some doubt about the results) if we assessed one or more key domains as unclear;
high risk of bias (plausible bias that seriously weakens confidence in the results) if we assessed one or more key domains to be at high risk of bias.
We completed a 'Risk of bias' table for each included study. We also presented the results graphically.
Measures of treatment effect
For dichotomous outcomes, we expressed the estimate of effect of the intervention as risk ratios (RR) together with 95% confidence intervals (CI). For continuous outcomes (such as mean VAS scores), we reported mean differences (MD) (or standardised mean differences (SMD) when different scales measuring the same concept) and their corresponding 95% CI.
Unit of analysis issues
We anticipate that, by the nature of the outcome variables being recorded, studies included in future updates may involve repeat observations. Results from more than one time point for each study cannot be combined in a standard meta‐analysis without a unit‐of‐analysis error. Therefore, we assessed outcomes at 24, 48 and 72 hours and 7 days postoperatively, as the data allowed.
We included no clustered trials in the review.
Given the nature of the conditions and intervention under review, it is high unlikely any cross‐over trials will be suitable for inclusion in the future.
In updates, we will consider multi‐arm studies for inclusion in the review, in accordance with recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we will combine all relevant experimental groups and considered them as a single group and compared them with a combined group of all the control groups, if present.
Dealing with missing data
We contacted the original investigators in cases of missing data.
Assessment of heterogeneity
We planned to assess heterogeneity using the Chi2 test (P value < 0.10 regarded as statistically significant). For studies judged as clinically homogeneous, we test heterogeneity using the I2 statistic, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The I2 statistic describes the percentage of variability in effect estimates that is due to heterogeneity rather than sampling error. An I2 of 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may have substantial heterogeneity, and 75% to 100% studies has substantial heterogeneity.
Assessment of reporting biases
We examined within‐study selective outcome reporting as a part of the overall risk of bias assessment and contacted study authors for clarification.
If there had been at least 10 studies included in a meta‐analysis, we would have assessed between‐study reporting bias by creating a funnel plot of effect estimates against their standard errors. If we had found asymmetry of the funnel plot by inspection and confirmed this by statistical tests, we would have considered possible explanations and taken into account in the interpretation of the overall estimate of treatment effects.
Data synthesis
We only carried out meta‐analysis where studies of similar comparisons, reported similar outcomes, for people with similar clinical conditions. We combined MDs (or SMDs where studies had used different scales) for continuous outcomes, and combined RRs for dichotomous outcomes, using a fixed‐effect model if there were only two or three studies, or a random‐effects model if there were four or more studies.
Subgroup analysis and investigation of heterogeneity
We planned to investigate clinical heterogeneity by examining the following subgroups should sufficient data have been available.
Different antibiotic class (e.g. penicillins versus macrolides).
The effects of accompanying surgical intervention (extraction, incision and drainage or endodontic treatment).
Sensitivity analysis
Provided there were sufficient studies for each outcome and intervention, we had planned to undertake sensitivity analysis based on trials judged to be of low risk of bias.
Presentation of main results
We developed a 'Summary of findings' table for the primary outcomes of this review using GRADEPro software (GRADEpro GDT 2015), with the GRADE assessment of the quality of the body of evidence.
Results
Description of studies
Results of the search
After de‐duplication, the electronic searches conducted in 2013 yielded 625 references. We identified one additional trial by checking the bibliographies of the selected trials and reviews (Al‐Belasy 2003). After examination of the titles, and abstracts where available, we excluded 590 references from further analysis. We obtained full‐text copies of the remaining 36 trials, translated them where required, and subjected them to further evaluation. At this stage, we excluded 34 studies and recorded their characteristics (Characteristics of excluded studies).
After de‐duplication, the electronic searches conducted for the current update (February 2018) yielded an additional 190 references not included in the previously published version. We retrieved no additional citations from other sources. After examination of the titles and abstracts where available, we excluded all 190 references from further analysis (Figure 1).
1.
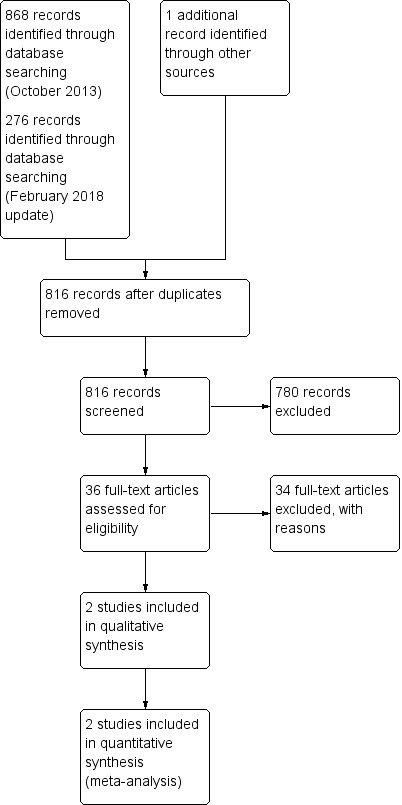
Study flow diagram.
Included studies
Two randomised controlled trials (RCTs) satisfied the inclusion criteria (Fouad 1996; Henry 2001). See Characteristics of included studies table for further details.
Characteristics of trial designs and settings
Both studies were of parallel group design, one had three arms (Fouad 1996), and the other had two arms (Henry 2001). Both studies were conducted at university dental schools in the USA and based at a single centre. One study was supported by a university research fund and the other did not declare funding sources. Neither study reported sample size calculations.
Characteristics of participants
We included 62 participants in the analysis for this review, with 21 people analysed in Fouad 1996, and 41 people analysed in Henry 2001. Both studies were conducted on otherwise healthy adults.
Participants in one study had a mean age of 36 years (standard deviation (SD) 13.7 years) and had a clinical diagnosis of acute apical abscess with pulpal necrosis, periapical pain or swelling, or both (Additional Table 2; Fouad 1996). Potential participants were excluded if their temperature was elevated (judged by investigators to be above 100 °F (37.8 °C) or if they had was malaise or fascial space involvement.
1. Baseline characteristics for penicillin and placebo trial arms (Fouad 1996).
| Trial arm | Penicillin (n = 13) | Placebo (n = 15) | P value |
| Gender | 4W:8Ma | 6W:7Mb | ‐ |
| Mean age in years (SD) | 34.92 (17.33) | 37.17 (9.40) | 0.696 |
| Mean baseline pain (SD) | 2.40 (1.08) | 2.00 (1.10) | 0.410 |
| Mean baseline swelling (SD) | 1.91 (1.51) | 2.00 (1.48) | 0.866 |
M: men; n: number in group; SD: standard deviation; W: women. Unpublished data from personal communication. aGender of 1 participant not recorded. bGender of 2 participants not recorded.
Participants in the other study had a mean age of 37 years (SD 16.5 years) in the penicillin arm and 38 years (SD 18.8 years) in the placebo arm (Additional Table 3; Henry 2001). All had a symptomatic necrotic tooth with a periapical radiolucency and no mucosal sinus tract (Henry 2001).
2. Baseline characteristics for penicillin and placebo trial arms (Henry 2001).
| Variable | Penicillin (n = 19) | Placebo (n = 22) | P value |
| Age in years (SD) | 37 (16.5) | 38 (18.8) | 0.884 |
| Gender | 10W:9M | 10W:12M | 0.647 |
| Weight in pounds (SD) | 172 (28.4) | 170 (41.3) | 0.874 |
| Estimated lesion area in mm (SD) | 14.0 (16.5) | 24.8 (22.6) | 0.105 |
| Median baseline pain (SD) | 2.00 (2.00) | 2.00 (1.00) | 0.463 |
| Median baseline percussion pain (SD) | 2.00 (2.00) | 2.00 (2.00) | 0.868 |
| Median baseline swelling (SD) | 1.00 (2.00) | 0 (1.00) | 0.097 |
M: men; n: number in group; SD: standard deviation; W: women.
One trial had more male participants (Fouad 1996) and the other had similar numbers of male and female participants (Henry 2001). There were no significant differences in the intra‐study baseline characteristics of participants (Additional Table 2; Additional Table 3).
Characteristics of intervention
Objective 1: systemic antibiotics versus a matched placebo provided in conjunction with a surgical intervention
In one trial, participants underwent total or partial pulpectomy under local anaesthesia with temporary restoration at the baseline visit (Fouad 1996). In the other trial, all participants underwent total pulpectomy with temporary restoration at the baseline visit (Henry 2001).
In the study by Fouad 1996, participants in the penicillin group received oral penicillin (phenoxymethyl) VK 1 g following treatment and then 500 mg, every 6 hours for 7 days. Participants in the placebo group received an oral matched placebo taken according to the same regimen. In the trial by Henry 2001, participants in the penicillin group received 500 mg oral penicillin VK tablets (Wyeth Laboratories, Philadelphia, PA) which they were instructed to take every 6 hours for 7 days. Participants in the placebo group received an oral matched placebo (lactose) taken according to the same regimen.
In one trial, all participants also received ibuprofen 600 mg immediately before treatment, on four occasions during the next 24 hours, and then as required (Fouad 1996). In the other trial, all participants received a bottle of ibuprofen 200 mg tablets (Advil, Whitehall Laboratories, New York, NY) with instructions to take two tablets every 4 to 6 hours as required. Each participant also received a labelled bottle of paracetamol (acetaminophen) with codeine (Tylenol #3, McNeil Consumer Products, Fort Washington, PA) with dosing instructions, to take if two ibuprofen did not relieve their discomfort. One participant was given Percocet (oxycodone plus paracetamol (acetaminophen)) instead (Henry 2001).
Objective 2: systemic antibiotics versus a matched placebo provided without a surgical intervention
We found no studies comparing systemic antibiotics versus a matched placebo provided without a surgical intervention.
Heterogeneity of interventions
There was heterogeneity with respect to the operative treatment, doses of antibiotics given to participants in the intervention arms and type, dose and frequency of analgesics provided to participants between the two studies.
Characteristics of the outcome measures
Primary outcomes
Both studies reported participant‐reported pain. Both utilised a short ordinal numerical scale graded from 0 to 3. In Fouad 1996, this score was determined by converting the value from a VAS on the post‐treatment card into a whole number rank. Pain was measured at the following data points:
6 hours, 12 hours, 24 hours, 48 hours and 72 hours (Fouad 1996);
day 1, day 2, day 3, day 4, day 5, day 6, day 7 (Henry 2001).
Both studies also reported participant‐reported swelling. In Henry 2001, investigators utilised a short ordinal numerical scale graded from 0 to 3. In Fouad 1996, increase or decrease in swelling compared with baseline was recorded on a short ordinal numerical scale graded from 0 to 4. Swelling was measured at the following data points:
6 hours, 12 hours, 24 hours, 48 hours and 72 hours (Fouad 1996);
day 1, day 2, day 3, day 4, day 5, day 6, day 7 (Henry 2001).
One study included percussion pain (Henry 2001). This was measured on a short ordinal numerical scale graded from 0 to 3.
One study included incidence of endodontic flare‐up (Fouad 1996). This was measured dichotomously and was clinician‐assessed based on the presence of: no relief or an increase in the severity of pain; no resolution or an increase in the size of swelling, fever, trismus or difficulty swallowing; signs of a drug allergy or any other abnormal symptoms.
Secondary outcomes
One study included the number and type of analgesics required (Henry 2001). In Fouad 1996, participants recorded whether they required additional analgesia; however, this information was not reported and was not available after contacting the investigators.
One study reported adverse effects (Fouad 1996).
Handling of data/data assumptions made in the review
For objective 1, we compared pain and swelling scores at 24, 48 and 72 hours and 7 days postoperatively. For the purposes of the analysis, we made the assumption that the data points from Henry 2001 (day 1, day 2 and day 3) were sufficiently analogous to those measure in Fouad 1996 to be combined.
Excluded studies
We excluded the majority of references as they were not RCTs. Other excluded studies did not report relevant health outcomes, had no placebo control or had other characteristics that did not satisfy the inclusion criteria (see Characteristics of excluded studies table).
Risk of bias in included studies
The review authors' judgements about each risk of bias item for each included study are given in Figure 2.
2.
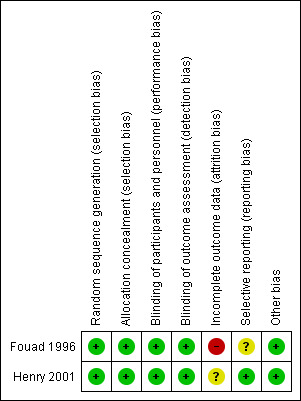
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation
We considered both studies to be at low risk of bias for random sequence generation.
Allocation concealment
We assessed both studies to have adequate concealment of allocation prior to assignment. In Fouad 1996, individuals enrolling participants into the trial were not aware of the upcoming allocation sequence; envelopes were sequentially numbered, opaque and sealed; envelopes for the penicillin and placebo groups were identical in appearance and weight and were only opened after being assigned to the participant. In Henry 2001, participants were given sequentially numbered drug containers of identical appearance in accordance with the randomisation sequence produced prior to the experiment.
Blinding
We judged both studies to have employed adequate measures to ensure that active and placebo tablets had identical appearance, and, therefore, we considered risk of performance bias to be low for both studies. Similarly, we considered both studies to have low risk of detection bias as blinding was unlikely to have been broken.
Incomplete outcome data
We considered Fouad 1996 to be at high risk of attrition bias. Rates of withdrawal were in excess of 20% in across groups, with higher rates of withdrawal from the placebo than the penicillin group. We judged differential attrition as likely to be related to treatment outcomes. In Henry 2001, we were unable to judge risk of bias due to insufficient reporting of relative attrition rates and reasons for withdrawal and, therefore, this risk for this domain is 'unclear'.
Selective reporting
We judged one study to be at unclear risk of reporting bias, as investigators did not report whether the need for additional analgesia differed between the two trial arms, although this information was collected on the post‐treatment card (Fouad 1996). There was no evidence of selective reporting within Henry 2001 and all expected outcomes were presented. We judged this study to be at low risk of reporting bias.
Other potential sources of bias
We judged both trials to be at low risk of other potential sources of bias.
Overall risk of bias
One study had high overall risk of bias (Fouad 1996), and one had unclear risk of bias (Henry 2001) (Figure 2).
Effects of interventions
See: Table 1
Objective 1: systemic antibiotics versus a matched placebo provided in conjunction with a surgical intervention
Two studies, one at unclear risk of bias (Henry 2001), and one at high risk of bias (Fouad 1996), provided data for this comparison. Both compared oral penicillin V potassium K (penicillin VK) against a matched placebo when provided alongside partial or total pulpectomy for adults with localised acute apical abscess or symptomatic necrotic tooth in otherwise healthy adults.
Primary outcomes
Pain
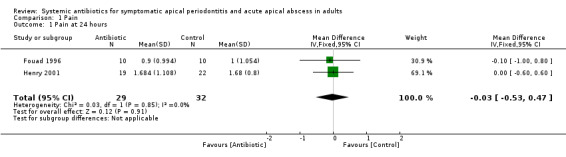
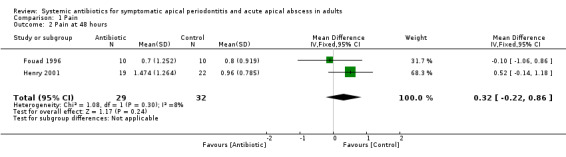
The analysis of participant‐reported pain at data points 24, 48 and 72 hours was based on data from two studies (61 participants), one at high risk of bias (Fouad 1996), and one at unclear risk of bias (Henry 2001). Analysis of the 7‐day time point was based on data from one study (41 participants) at unclear risk of bias (Henry 2001).
For the antibiotic group:
mean difference (MD) at 24 hours ‐0.03 (95% confidence interval (CI) ‐0.53 to 0.47);
MD at 48 hours 0.32 (95% CI ‐0.22 to 0.86);
MD at 72 hours 0.08 (95% CI ‐0.38 to 0.54);
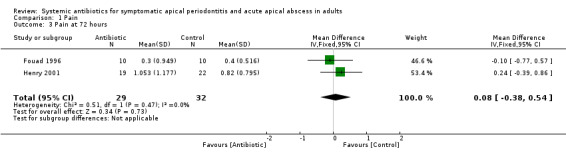
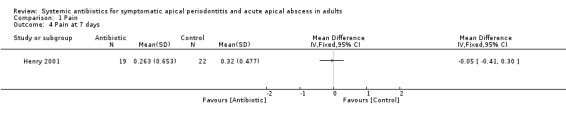
MD at 7 days ‐0.05 (95% CI ‐0.41 to 0.30, P value = 0.77).
Swelling
The analysis of participant‐reported swelling at data points 24 hours (61 participants), 48 hours (62 participants) and 72 hours (61 participants) was based on data from two studies, one at high risk of bias (Fouad 1996), and one at unclear risk of bias (Henry 2001). Analysis of 7‐day time point was based on data from one study at unclear risk of bias (Henry 2001). Standardised mean difference (SMD) was used to combine the different scales used for the 24‐, 48‐ and 72‐hour data points.
For the antibiotic group:
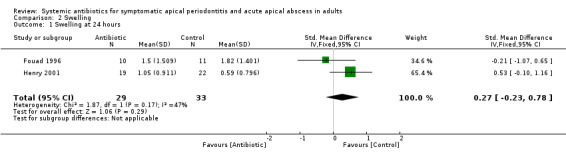
SMD at 24 hours 0.27 (95% CI ‐0.23 to 0.78). This converts back into a 36% increase (95% CI 31% decrease to 105% increase) of control mean for antibiotics. Re‐expressed from the SMD into the short ordinal numerical scale used by Henry 2001. Results should be interpreted with caution since back‐translation of the effect size was based on the results of only one study;
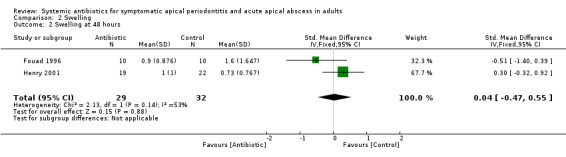
SMD at 48 hours 0.04 (95% CI ‐0.47 to 0.55). This converts back into a 4% increase (95% CI 49% decrease to 58% increase) of control mean for antibiotics. Re‐expressed from the SMD into the short ordinal numerical scale used by Henry 2001. Results should be interpreted with caution since back‐translation of the effect size was based on the results of only one study;
SMD at 72 hours 0.02 (95% CI ‐0.49 to 0.52). This converts back into a 2% increase (95% CI 55% decrease to 59% increase) of control mean for antibiotics. Re‐expressed from the SMD into the short ordinal numerical scale used by Henry 2001. Results should be interpreted with caution since back‐translation of the effect size was based on the results of only one study;
MD at 7 days 0.02 (95% CI ‐0.28 to 0.32, P value = 0.90).
Percussion pain
The analysis of participant‐reported percussion data at data points 24, 48 and 72 hours was based on data from one study (41 participants) at unclear risk of bias (Henry 2001).
For the antibiotic group:
MD at 24 hours ‐0.32 (95% CI ‐0.85 to 0.21, P value = 0.24);
MD at 48 hours 0.09 (95% CI ‐0.44 to 0.62, P value = 0.74);
MD at 72 hours 0.05 (95% CI ‐0.55 to 0.65, P value = 0.87);
MD at 7 days 0.06 (95% CI ‐0.29 to 0.41, P value = 0.73).
Endodontic flare‐up
The analysis of clinician‐assessed incidence of endodontic flare‐up over 3‐day follow‐up period was based on data from one study at high risk of bias (20 participants) (Fouad 1996).
For the antibiotic group:
risk ratio (RR) of endodontic flare‐up 0.27 (95% CI 0.01 to 4.90, P value = 0.37).
Secondary outcomes
Analgesics
The analysis of the number of analgesic tablets required during the 7‐day follow‐up period was based on data from one study (41 participants) at unclear risk of bias (Henry 2001).
For the antibiotic group:
MD for total number of ibuprofen tablets 1.58 (95% CI ‐4.55 to 7.71, P value = 0.62).
MD for total number of paracetamol (acetaminophen) with codeine tablets ‐0.31 (95% CI ‐3.94 to 3.32, P value = 0.87).
Adverse effects
During the 3‐day follow‐up period in Fouad 1996 (20 participants, high risk of bias), one participant in the placebo group reported diarrhoea and one participant in the antibiotic group reported fatigue and reduced energy postoperatively.
Objective 2: systemic antibiotics versus a matched placebo provided without a surgical intervention
We found no studies comparing systemic antibiotics versus a matched placebo provided without a surgical intervention.
Discussion
Summary of main results
The review process identified two studies suitable for inclusion, both of which assessed the effects of penicillin VK compared with a matched placebo in adults with localised apical abscess or a symptomatic necrotic tooth (no signs of spreading infection or systemic involvement) when provided in conjunction with partial or total pulpectomy conducted under local anaesthesia, and oral analgesics. There were no statistically significant differences in primary outcomes (participant‐reported pain, swelling or percussion pain or incidence of endodontic flare‐up) or secondary outcomes (analgesic use or incidence of adverse events) between participants who had received antibiotics and participants who had received a matched placebo. We considered this body of evidence (two studies, one at unclear risk of bias and one at high risk of bias) to be of very low quality and therefore the results should be interpreted with caution.
We found no studies that reported the effects of systemic antibiotics versus a matched placebo for symptomatic apical periodontitis when provided in conjunction with a surgical intervention. We found no studies that reported the effects of systemic antibiotics versus a matched placebo for symptomatic apical periodontitis or acute apical abscess when provided without a surgical intervention.
Overall completeness and applicability of evidence
We employed a comprehensive search strategy and we are confident that the majority of published trials are included in this review. We made efforts to identify all relevant studies and excluded no studies due to language.
The two included trials partially addressed the first of the two objectives (Fouad 1996; Henry 2001), which both investigated the effect of systemic antibiotics for acute apical abscess or symptomatic necrotic tooth provided in conjunction with total or partial pulpectomy in adults. However, there were no trials that assessed the effects of antibiotics for symptomatic apical periodontitis when used in conjunction with a surgical intervention. Furthermore, we found no trials assessing the second objective, which sought to compare antibiotics and a placebo for symptomatic apical periodontitis or acute apical abscess when provided without a surgical intervention.
The participants included in the two trials can be considered broadly representative of people who would consult a dentist due to an acute apical abscess or symptomatic necrotic tooth who do not have evidence of spreading infection or systemic involvement: participants came from a wide age range, were about equal gender mix and the majority had moderate pain at the baseline visit. However, both the trials excluded participants with co‐morbidities or who may have been immunocompromised. Therefore, the results of this review may not be generalisable to a group of people who may be at higher risk of infection. While future trials should endeavour to obtain a representative sample, it is unlikely to be feasible or ethical to conduct placebo‐controlled trials in these groups of people.
One trial excluded participants with signs of spreading infection and systemic involvement (Fouad 1996), and the other trial included only a small number of participants with evidence of severe infections at baseline (Henry 2001). Therefore, the results of this review may not be generalisable to people with severe swelling or other signs of spreading infection or systemic involvement.
Both of the included studies were conducted at university dental schools and, in both trials, endodontic treatment was completed by practitioners who either worked in the Department of Endodontics (Fouad 1996), or were senior endodontic graduate students (Henry 2001). It would be reasonable to consider that both groups of practitioners had endodontic skills in excess of those of an average primary care dentist. The specialist settings in which the trials were conducted were also unlikely to face the time constraints encountered in routine clinical practice. Therefore, the intervention provided within these studies may only have limited applicability to the treatment routinely provided at emergency appointments in general dental practice, where treatment decisions are often dictated by time pressures (Palmer 2000). Therefore, more trials in a primary care setting would enhance the evidence base for answering the questions posed by this review.
We found no trials assessing the effect of other surgical interventions, such as dental extraction, or incision and drainage of a swelling. Since dental extraction is a common treatment for both symptomatic apical periodontitis and acute apical abscess (Cope 2016), the effects of this intervention could be considered in future trials.
The outcomes reported by the two trials measured the harms as well as the benefits of interventions. This is important as antibiotics can have adverse effects such as hypersensitivity reactions, gastrointestinal upset, and the risk of development of antibiotic‐resistant bacterial colonies. Many of the outcome measures in the two included trials were participant‐centred, such as pain, percussion pain and swelling. Since both pain and discomfort are known to impact an individual's quality of life (Skevington 1998), future trials should also consider formally measuring oral health‐related quality of life outcomes to assess the beneficial and harmful effects of this intervention in more detail.
Quality of the evidence
The quality of the evidence, as summarised in Table 1 for the main comparison, was rated as very low.
Given the considerable number of antibiotics prescribed by dentists to adults with acute dental conditions and the problems associated with indiscriminate use of antibiotics, the paucity of high‐quality trials evaluating the effects of systemic antibiotics in the management of symptomatic apical periodontitis and acute apical abscess is disappointing. Only two studies met the inclusion criteria for this review; we judged one to be at high risk of bias and the other to be of unclear risk of bias. Both had methodological flaws with respect to attrition bias, and the overall quality of evidence was very low. Furthermore, small group sizes mean that both studies were likely to lack the statistical power to detect differences between intervention and placebo groups. Sample size calculations were not reported in either study. Therefore, caution should be exercised when interpreting the results presented in this review.
Potential biases in the review process
Two independent review authors extracted data and assessed the methodological quality of each study, minimising potential bias.
We are confident that the extensive literature search used in this review has captured relevant literature and minimised the likelihood that we missed any relevant trials. We applied no language or publication restrictions in our search.
In the event of incomplete or unclear reporting of trial data, we contacted the trial authors to obtain any unpublished data or clarification of results.
Despite these efforts, it must be acknowledged that there is a small possibility that there were additional studies (published and unpublished) that we did not identify. It is possible that additional literature searches, such as searching non‐English language databases and handsearching relevant journals, would have found additional studies.
Agreements and disagreements with other studies or reviews
Systematic reviews of the emergency management of acute apical periodontitis and acute apical abscess in the permanent dentition were published in 2003 (Matthews 2003; Sutherland 2003). These reviews had wider inclusion criteria and included trials of analgesics, local pharmacotherapeutics and surgical interventions in addition to antibiotic trials. Sutherland 2003 concluded that "the use of antibiotics in the management of AAP [acute apical periodontitis] is not recommended" and Matthews 2003 recommended that "the use of antibiotics in the management of localized AAA [acute apical abscess] over and above establishing drainage of the abscess, is not recommended".
Authors' conclusions
Implications for practice.
Based on the current available data, which are of very low quality, there was insufficient evidence to determine the effects of the administration of systemic antibiotics to adults with symptomatic apical periodontitis or acute apical abscess.
Since antibiotic use is recognised as a major contributor to antimicrobial resistance, dental professionals should be judicious in their use of these agents and should refer to evidence‐based best practice guidelines when managing people with acute dental conditions.
Implications for research.
Large‐scale, adequately powered and well‐designed randomised controlled trials are needed to clarify the effectiveness of systemic antibiotics in the treatment of symptomatic apical periodontitis and acute apical abscess. However, all future trials should be carefully designed to ensure the potential benefits of providing systemic antibiotics to participants outweigh risks associated with antibiotic usage, both adverse effects and the possible contribution to antibiotic resistance.
Future studies should consider both utilising validated participant‐ and clinician‐reported outcome measures, and report results according to CONSORT guidelines (www.consort‐statement.com/).
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2018 | New citation required but conclusions have not changed | Conclusions are the same. Change of review authors. |
| 26 February 2018 | New search has been performed | Searches updated. No additional eligible studies identified. |
Acknowledgements
The authors acknowledge the following help in the conduct of the review.
Anne Littlewood, Information Specialist of Cochrane Oral Health, who provided invaluable support in constructing and running the search strategies.
Mala Mann, Specialist Unit for Review Evidence, Cardiff University, who was involved in the original review. Mala drafted the protocol, screened search results, extracted the data, performed risk of bias assessment and was involved in writing the final report of the 2014 review.
Dr Rebecca Payle, Senior Lecturer in Medical Statistics at Cardiff University School of Dentistry, who gave advice on the statistical elements of the protocol.
Anwen L Cope would like to acknowledge the financial support received from a Clinical Research Time Award from Health and Care Wales.
The assistance of several colleagues who helped with translating articles during the selection of studies.
Appendices
Appendix 1. Search strategies
Cochrane Oral Health's Trials Register search strategy
From October 2013, searches of Cochrane Oral Health's Trials Register were conducted for this review using the Cochrane Register of Studies and the search strategy below:
1. ((antibiotic* or anti‐biotic* or "anti biotic*" or antibacterial* or anti‐bacterial* or "anti bacterial*" or antiinfect* or anti‐infect* or "anti infect*" or antimicrobial* or anti‐microbial* or "anti microbial*"):ti,ab) AND (INREGISTER) 2. ((penicillin* or amoxicillin or amoxycillin or co‐amoxiclav or ampicillin or erythromycin or clindamycin*):ti,ab) AND (INREGISTER) 3. ((doxycycline* or metronidazole or azithromycin or co‐amoxiclav or oxytetracycline or cefalexin or cephalexin or cefradine or cephradine or clarithromycin):ti,ab) AND (INREGISTER) 4. ((tetracycline or actimoxi or amoxicilline or amoxil or BRL‐2333 or clamoxyl or hydroxyampicillin or penamox or polymox or trimox or wymox or amoxi‐clav or amoxi‐clavulanate or augmentin or BRL‐25000):ti,ab) AND (INREGISTER) 5. ((clavulanate or clavulin or coamoxiclav or spektramox or synulox or phenoxymethylpenicillin or apocillin or beromycin or berromycin or betapen or fenoxymethylpenicillin or "Pen VK" or "v‐cillin K" or vegacillin or clont or danizol ):ti,ab) AND (INREGISTER) 6. ((trichazol* or trichapol or trivazol or satric or metrogyl or flagyl or gineflavir or metrodzhil or nidagyl or chlolincocin or chlorlincocin or cleocin or "dalacin c"):ti,ab) AND (INREGISTER) 7. (#1 or #2 or #3 or #4 or #5 or #6) AND (INREGISTER) 8. ((abscess* or periapical or peri‐apical or “peri apical”):ti,ab) AND (INREGISTER) 9. (#7 and #8) AND (INREGISTER)
A previous search of Cochrane Oral Health's Trials Register was conducted in June 2012, using the Procite software and the search strategy below:
((antibiotic* or anti‐biotic* or "anti biotic*" or antibacterial* or anti‐bacterial* or "anti bacterial*" or antiinfect* or anti‐infect* or "anti infect*" or antimicrobial* or anti‐microbial* or "anti microbial*" or penicillin* or amoxicillin or amoxycillin or co‐amoxiclav or ampicillin or erythromycin or clindamycin* or doxycycline* or metronidazole or azithromycin or co‐amoxiclav or oxytetracycline or cefalexin or cephalexin or cefradine or cephradine or clarithromycin or tetracycline or actimoxi or amoxicilline or amoxil or BRL‐2333 or clamoxyl or hydroxyampicillin or penamox or polymox or trimox or wymox or amoxi‐clav or amoxi‐clavulanate or augmentin or BRL‐25000 or clavulanate or clavulin or coamoxiclav or spektramox or synulox or phenoxymethylpenicillin or apocillin or beromycin or berromycin or betapen or fenoxymethylpenicillin or "Pen VK" or "v‐cillin K" or vegacillin or clont or danizol or trichazol* or trichapol or trivazol or satric or metrogyl or flagyl or gineflavir or metrodzhil or nidagyl or chlolincocin or chlorlincocin or cleocin or "dalacin c") AND abscess* or periapical or peri‐apical or "peri apical"))
Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Anti‐Infective Agents explode all trees #2 MeSH descriptor Penicillins explode all trees #3 (antibiotic* in All Text or anti‐biotic* in All Text or "anti biotic*" in All Text) #4 (antibacterial* in All Text or anti‐bacterial* in All Text or "anti bacterial*" in All Text) #5 (antiinfect* in All Text or anti‐infect* in All Text or "anti infect*" in All Text) #6 (antimicrobial* in All Text or anti‐microbial* in All Text or "anti microbial*" in All Text) #7 (penicillin* in All Text or amoxicillin in All Text or amoxycillin in All Text or co‐amoxiclav in All Text or ampicillin in All Text or erythromycin in All Text or clindamycin* in All Text or doxycycline* in All Text or metronidazole in All Text or azithromycin in All Text or co‐amoxiclav in All Text or oxytetracycline in All Text or cefalexin in All Text or cephalexin in All Text or cefradine in All Text or cephradine in All Text or clarithromycin in All Text or tetracycline in All Text) #8 (actimoxi in All Text or amoxicilline in All Text or amoxil in All Text or BRL‐2333 in All Text or clamoxyl in All Text or hydroxyampicillin in All Text or penamox in All Text or polymox in All Text or trimox in All Text or wymox in All Text or amoxi‐clav in All Text or amoxi‐clavulanate in All Text or augmentin in All Text or BRL‐25000 in All Text or clavulanate in All Text or clavulin in All Text or coamoxiclav in All Text or spektramox in All Text or synulox in All Text) #9 (phenoxymethylpenicillin in All Text or apocillin in All Text or beromycin in All Text or berromycin in All Text or betapen in All Text or fenoxymethylpenicillin in All Text or "Pen VK" in All Text or "v‐cillin K" in All Text or vegacillin in All Text) #10 (clont in All Text or danizol in All Text or trichazol* in All Text or trichapol in All Text or trivazol in All Text or satric in All Text or metrogyl in All Text or flagyl in All Text or gineflavir in All Text or metrodzhil in All Text or nidagyl in All Text) #11 (chlolincocin in All Text or chlorlincocin in All Text or cleocin in All Text or "dalacin c" in All Text) #12 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11) #13 MeSH descriptor Periapical diseases explode all trees #14 (dental* in All Text near/5 absces* in All Text) #15 ( (tooth in All Text near/5 absces* in All Text) or (teeth in All Text near/5 absces* in All Text) ) #16 ( (periapical in All Text near/5 absces* in All Text) or (peri‐apical in All Text near/5 absces* in All Text) or (apical in All Text near/5 absces* in All Text) ) #17 ( (periapical in All Text near/5 periodont* in All Text) or (peri‐apical in All Text near/5 periodont* in All Text) or (apical in All Text near/5 periodont* in All Text) ) #18 ( (periapical in All Text near/5 inflam* in All Text) or (peri‐apical in All Text near/5 inflam* in All Text) or (apical in All Text near/5 inflam* in All Text) ) #19 ( (periapical in All Text near/5 infect* in All Text) or (peri‐apical in All Text near/5 infect* in All Text) or (apical in All Text near/5 infect* in All Text) ) #20 ( (dentoalveol* in All Text near/5 absces* in All Text) or (dento‐alveol* in All Text near/5 absces* in All Text) or (alveol* in All Text near/5 absces* in All Text) ) #21 ( (periradicular in All Text near/5 absces* in All Text) or (peri‐radicular in All Text near/5 absces* in All Text) or (radicular in All Text near/5 absces* in All Text) ) #22 (#13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21) #23 (#12 and #22)
MEDLINE Ovid search strategy
1. exp Anti‐Infective Agents/ 2. exp Penicillins/ 3. (antibiotic$ or anti‐biotic$ or "anti biotic$").tw. 4. (antibacterial$ or anti‐bacterial$ or "anti bacterial$").tw. 5. (antiinfect$ or anti‐infect$ or "anti infect$").tw. 6. (antimicrobial$ or anti‐microbial$ or "anti microbial$").tw. 7. (penicillin$ or amox?cillin or co‐amoxiclav or ampicillin or erythromycin or clindamycin$ or doxycycline$ or metronidazole or azithromycin or co‐amoxiclav or oxytetracycline or cefalexin or cephalexin or cefradine or cephradine or clarithromycin or tetracycline).tw. 8. (actimoxi or amoxicilline or amoxil or BRL‐2333 or clamoxyl or hydroxyampicillin or penamox or polymox or trimox or wymox or amoxi‐clav or amoxi‐clavulanate or augmentin or BRL‐25000 or clavulanate or clavulin or coamoxiclav or spektramox or synulox).tw. 9. (phenoxymethylpenicillin or apocillin or beromycin or berromycin or betapen or fenoxymethylpenicillin or "Pen VK" or "v‐cillin K" or vegacillin).tw. 10. (clont or danizol or trichazol$ or trichapol or trivazol or satric or metrogyl or flagyl or gineflavir or metrodzhil or nidagyl).tw. 11. (chlolincocin or chlorlincocin or cleocin or "dalacin c").tw. 12. or/1‐11 13. exp Periapical diseases/ 14. (dental$ adj5 absces$).tw. 15. ((tooth or teeth) adj5 absces$).tw. 16. ((periapical adj5 absces$) or (peri‐apical adj5 absces$) or (apical adj5 absces$)).tw. 17. ((periapical adj5 periodont$) or (peri‐apical adj5 periodont$) or (apical adj5 periodont$)).tw. 18. ((periapical adj5 inflam$) or (peri‐apical adj5 inflam$) or (apical adj5 inflam$)).tw. 19. ((periapical adj5 infect$) or (peri‐apical adj5 infect$) or (apical adj5 infect$)).tw. 20. ((dentoalveol$ adj5 absces$) or (dento‐alveol$ adj5 absces$) or (alveol$ adj5 absces$)).tw. 21. ((periradicular adj5 absces$) or (peri‐radicular adj5 absces$) or (radicular adj5 absces$)).tw. 22. or/13‐21 23. 12 and 22
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011) (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Embase Ovid search strategy
1. exp Antiinfective agent/ 2. exp Penicillin derivate/ 3. (antibiotic$ or anti‐biotic$ or "anti biotic$").tw. 4. (antibacterial$ or anti‐bacterial$ or "anti bacterial$").tw. 5. (antiinfect$ or anti‐infect$ or "anti infect$").tw. 6. (antimicrobial$ or anti‐microbial$ or "anti microbial$").tw. 7. (penicillin$ or amox?cillin or co‐amoxiclav or ampicillin or erythromycin or clindamycin$ or doxycycline$ or metronidazole or azithromycin or co‐amoxiclav or oxytetracycline or cefalexin or cephalexin or cefradine or cephradine or clarithromycin or tetracycline).tw. 8. (actimoxi or amoxicilline or amoxil or BRL‐2333 or clamoxyl or hydroxyampicillin or penamox or polymox or trimox or wymox or amoxi‐clav or amoxi‐clavulanate or augmentin or BRL‐25000 or clavulanate or clavulin or coamoxiclav or spektramox or synulox).tw. 9. (phenoxymethylpenicillin or apocillin or beromycin or berromycin or betapen or fenoxymethylpenicillin or "Pen VK" or "v‐cillin K" or vegacillin).tw. 10. (clont or danizol or trichazol$ or trichapol or trivazol or satric or metrogyl or flagyl or gineflavir or metrodzhil or nidagyl).tw. 11. (chlolincocin or chlorlincocin or cleocin or "dalacin c").tw. 12. or/1‐11 13. exp Tooth periapical disease/ 14. (dental$ adj5 absces$).tw. 15. ((tooth or teeth) adj5 absces$).tw. 16. ((periapical adj5 absces$) or (peri‐apical adj5 absces$) or (apical adj5 absces$)).tw. 17. ((periapical adj5 periodont$) or (peri‐apical adj5 periodont$) or (apical adj5 periodont$)).tw. 18. ((periapical adj5 inflam$) or (peri‐apical adj5 inflam$) or (apical adj5 inflam$)).tw. 19. ((periapical adj5 infect$) or (peri‐apical adj5 infect$) or (apical adj5 infect$)).tw. 20. ((dentoalveol$ adj5 absces$) or (dento‐alveol$ adj5 absces$) or (alveol$ adj5 absces$)).tw. 21. ((periradicular adj5 absces$) or (peri‐radicular adj5 absces$) or (radicular adj5 absces$)).tw. 22. or/13‐21 23. 12 and 22
This subject search was linked to an adapted version of the Cochrane Centralised Search Project filter for identifying RCTs in Embase Ovid (see www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
CINAHL EBSCO search strategy
S1 (MH "Antiinfective Agents+") S2 (MH "Penicillins+") S3 (antibiotic* or anti‐biotic* or "anti biotic*") S4 (antibacterial* or anti‐bacterial* or "anti bacterial*") S5 (antiinfect* or anti‐infect* or "anti infect*") S6 (antimicrobial* or anti‐microbial* or "anti microbial*") S7 (penicillin* or amoxicillin or amoxycillin or co‐amoxiclav or ampicillin or erythromycin or clindamycin* or doxycycline* or metronidazole or azithromycin or co‐amoxiclav or oxytetracycline or cefalexin or cephalexin or cefradine or cephradine or clarithromycin or tetracycline) S8 (actimoxi or amoxicilline or amoxil or BRL‐2333 or clamoxyl or hydroxyampicillin or penamox or polymox or trimox or wymox or amoxi‐clav or amoxi‐clavulanate or augmentin or BRL‐25000 or clavulanate or clavulin or coamoxiclav or spektramox or synulox) S9 (phenoxymethylpenicillin or apocillin or beromycin or berromycin or betapen or fenoxymethylpenicillin or "Pen VK" or "v‐cillin K" or vegacillin) S10 (clont or danizol or trichazol* or trichapol or trivazol or satric or metrogyl or flagyl or gineflavir or metrodzhil or nidagyl) S11 (chlolincocin or chlorlincocin or cleocin or "dalacin c") S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S13 (MH "Periapical Diseases") S14 (dental* N5 absces*) S15 ((tooth N5 absces*) or (teeth N5 absces*)) S16 ((periapical N5 absces*) or (peri‐apical N5 absces*) or (apical N5 absces*)) S17 ((periapical N5 periodont*) or (peri‐apical N5 periodont*) or (apical N5 periodont*)) S18 ((periapical N5 inflam*) or (peri‐apical N5 inflam*) or (apical N5 inflam*)) S19 ((periapical N5 infect*) or (peri‐apical N5 infect*) or (apical N5 infect*)) S20 ((dentoalveol* N5 absces*) or (dento‐alveol* N5 absces*) or (alveol* N5 absces*)) S21 ((periradicular N5 absces*) or (peri‐radicular N5 absces*) or (radicular N5 absces*)) S22 S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 S23 S12 and S22
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
dental abscess* AND antibiotic* dental abscess* AND penicillin* dental abscess* AND antibacterial* dental abscess* AND antimicrobial* dental abscess* AND antiinfect* periapical abscess* AND antibiotic* periapical abscess* AND penicillin* periapical abscess* AND antibacterial* periapical abscess* AND antimicrobial* periapical abscess* AND antiinfect*
World Health Organization International Clinical Trials Registry Platform search strategy
dental abscess AND antibiotic dental abscess AND penicillin dental abscess AND antibacterial dental abscess AND antimicrobial dental abscess AND antiinfectious periapical abscess AND antibiotic periapical abscess AND penicillin periapical abscess AND antibacterial periapical abscess AND antimicrobial periapical abscess AND antiinfectious
OpenGrey search strategy
dental abscess* AND antibiotic* dental abscess* AND penicillin* dental abscess* AND antibacterial* dental abscess* AND antimicrobial* dental abscess* AND antiinfect* periapical abscess* AND antibiotic* periapical abscess* AND penicillin* periapical abscess* AND antibacterial* periapical abscess* AND antimicrobial* periapical abscess* AND antiinfect*
ZETOC Conference Proceedings search strategy
dental abscess* AND antibiotic* dental abscess* AND penicillin* dental abscess* AND antibacterial* dental abscess* AND antimicrobial* dental abscess* AND antiinfect* periapical abscess* AND antibiotic* periapical abscess* AND penicillin* periapical abscess* AND antibacterial* periapical abscess* AND antimicrobial* periapical abscess* AND antiinfect*
Data and analyses
Comparison 1. Pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at 24 hours | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.53, 0.47] |
| 2 Pain at 48 hours | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.22, 0.86] |
| 3 Pain at 72 hours | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.38, 0.54] |
| 4 Pain at 7 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Pain, Outcome 1 Pain at 24 hours.
1.2. Analysis.
Comparison 1 Pain, Outcome 2 Pain at 48 hours.
1.3. Analysis.
Comparison 1 Pain, Outcome 3 Pain at 72 hours.
1.4. Analysis.
Comparison 1 Pain, Outcome 4 Pain at 7 days.
Comparison 2. Swelling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Swelling at 24 hours | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.23, 0.78] |
| 2 Swelling at 48 hours | 2 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.47, 0.55] |
| 3 Swelling at 72 hours | 2 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.49, 0.52] |
| 4 Swelling at 7 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Swelling, Outcome 1 Swelling at 24 hours.
2.2. Analysis.
Comparison 2 Swelling, Outcome 2 Swelling at 48 hours.
2.3. Analysis.
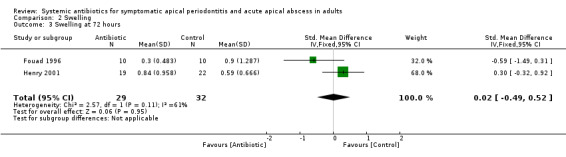
Comparison 2 Swelling, Outcome 3 Swelling at 72 hours.
2.4. Analysis.
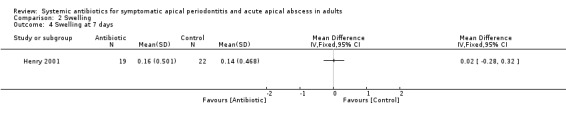
Comparison 2 Swelling, Outcome 4 Swelling at 7 days.
Comparison 3. Percussion pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percussion pain at 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Percussion pain at 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Percussion pain at 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Percussion pain at 7 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
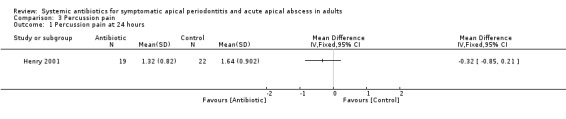
Comparison 3 Percussion pain, Outcome 1 Percussion pain at 24 hours.
3.2. Analysis.
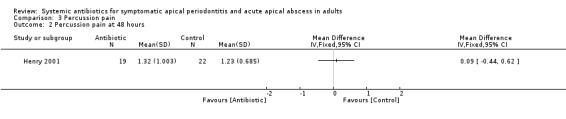
Comparison 3 Percussion pain, Outcome 2 Percussion pain at 48 hours.
3.3. Analysis.
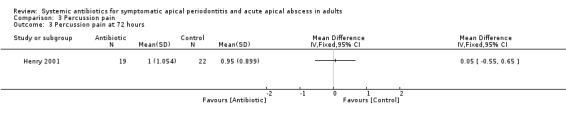
Comparison 3 Percussion pain, Outcome 3 Percussion pain at 72 hours.
3.4. Analysis.
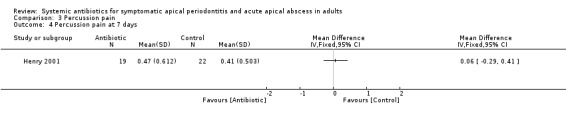
Comparison 3 Percussion pain, Outcome 4 Percussion pain at 7 days.
Comparison 4. Endodontic flare‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of endodontic flare‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
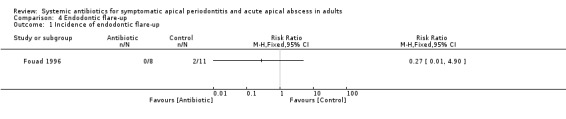
Comparison 4 Endodontic flare‐up, Outcome 1 Incidence of endodontic flare‐up.
Comparison 5. Analgesics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total number of ibuprofen tablets | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Total number of paracetamol (acetaminophen) with codeine tablets | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.
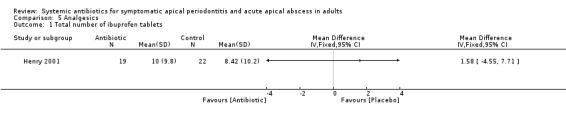
Comparison 5 Analgesics, Outcome 1 Total number of ibuprofen tablets.
5.2. Analysis.
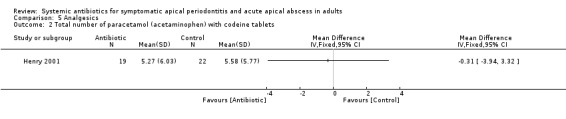
Comparison 5 Analgesics, Outcome 2 Total number of paracetamol (acetaminophen) with codeine tablets.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fouad 1996.
| Methods | RCT Number of centres: 1 (Department of Endodontics, University of Iowa, USA) Recruitment period: 3.5 years Design: parallel group 3‐arm RCT |
|
| Participants | Adults presenting for emergency treatment Group 1 (penicillin) Mean age 34.92 years (SD 17.33 years). Gender: 4 women, 8 men (1 gender not recorded). Mean baseline pain (SD): 2.40 (1.08). Mean baseline swelling (SD): 1.91 (1.51) Group 2 (placebo) Mean age 37.17 years (SD 9.40 years). Gender: 6 women, 7 men (2 gender not recorded). Mean baseline pain (SD): 2.00 (1.10). Mean baseline swelling (SD): 2.00 (1.48) Included participants had a clinical diagnosis of acute apical abscess with pulpal necrosis with periapical pain or swelling, or both Participants were excluded if they had: elevated temperature (above 37.8 ºC (100 ºF); malaise; fascial space involvement; allergy to penicillin or cephalosporins; diseases or medications compromising the immune system; renal failure or any other significant renal or hepatic impairment; people who had taken antibiotics in the 2‐week period prior to their visit; pregnant or lactating or taking oral contraceptives Number of participants at randomisation: group 1 = 13; group 2 = 15 Number of participants included in the analysis: group 1 = 10; group 2 = 11 |
|
| Interventions | Endodontic treatment: all participants had the affected tooth treated by total or partial pulpectomy on day 0. This involved delivery of local anaesthesia, assessment of the tooth, determination of working length, partial/total cleaning and shaping of the canals with copious irrigation with 2.6% sodium hypochlorite. Canals were dried and calcium hydroxide paste applied and the access cavity temporised with CavitTM (a light‐cured temporary sealing compound for temporary restoration of cavities) or IRM® (intermediate restorative material is a polymer‐reinforced zinc oxide‐eugenol composition restorative material designed for intermediate restorations). Some participants also underwent incision and drainage of a localised intraoral swelling, if judged to be clinically indicated Participants were then assigned to a trial arm: Group 1: oral penicillin (phenoxymethyl) VK 500 mg, 1 g after endodontic treatment followed by 500 mg 6‐hourly for 7 days Group 2: oral matched placebo taken according to the same regimen Group 3: neither medication group Analgesics: all participants received ibuprofen 600 mg immediately before treatment, 4 times daily for 24 hours and then as needed |
|
| Outcomes |
Primary outcomes Participants were required to complete a post‐treatment card recording their experiences up to 3 days postoperatively. This card was then returned to the authors via post. Pain was assessed using a VAS, which was then converted into a short ordinal numerical scale from 0 to 3: 0 indicated pain of no clinical significance; 1 = mild pain; 2 = moderate pain; 3 = severe pain. Postoperative swelling relief was recorded on a simple categorical scale ('no swelling', 'much less', 'slightly less', 'same', 'slightly more') with participants required to compare current levels of swelling to those they had experienced preoperatively. The categorical scale was then given scores from 0 to 4: 0 = no swelling; 1 = significant reduction in swelling; 2 = slight decrease in swelling; 3 = same size swelling as before; 4 = an increase in the size of swelling Incidence of flare‐up: measured dichotomously and was clinician‐assessed based on the presence of: no relief or an increase in the severity of pain; no resolution or an increase in the size of swelling, fever, trismus or difficulty swallowing; signs of a drug allergy or any other abnormal symptoms Secondary outcomes Incidence of participant‐reported side effects; type and frequency of additional analgesic medication |
|
| Notes | Funding source: not stated Sample size calculation: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician provided random numbers" (email from author) Comment: the participants appeared to be equally distributed between the penicillin and placebo groups |
| Allocation concealment (selection bias) | Low risk | Author confirmed that there was no way for the individual(s) enrolling participants into the trial to know the upcoming allocation sequence; study envelopes were sequentially numbered, opaque and sealed; and study envelopes were only opened once the participant was enrolled onto the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Author confirmed that the placebo looked exactly the same as the penicillin tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants recorded the outcome measures and were blinded to their group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Following randomisation, there were 13 participants in the penicillin group and 15 in the placebo group. 3 participants in each group did not return their post‐treatment card and were judged to have dropped out. A further 2 participants in the placebo group were withdrawn (1 at 6 hours and 1 at 24 hours) after returning with symptoms necessitating further treatment. The missing data related to these 2 participants was likely to be related to treatment outcomes (levels of pain or swelling, or both). Attrition for both arms of the trial was in excess of 20%, and was higher in the placebo than the penicillin group Furthermore, following personal communication with trial authors, it was identified that there was incomplete baseline data (age, gender, baseline pain or swelling) for 5 study participants across the 2 trial arms. Since the numbers of participants recruited to each group were low, baseline characteristics of these 5 individuals may have led to differences between the penicillin and placebo groups |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome measures reported, 1 secondary outcome (additional analgesia) not reported |
| Other bias | Low risk | No other sources of bias identified |
Henry 2001.
| Methods | RCT Number of centres: 1 (The Ohio State University College of Dentistry, USA) Recruitment period: not stated Design: parallel group 3‐arm RCT |
|
| Participants | Adults presenting for emergency treatment Group 1 (penicillin) Mean age 37 years (SD 16.5 years). Gender: 10 women, 9 men. Median baseline pain (SD): 2.00 (2.00). Median baseline percussion pain (SD): 2.00 (2.00). Median baseline swelling (SD): 1.00 (2.00) Group 2 (placebo) Mean age 38 years (SD 18.8 years). Gender: 10 women, 12 men. Median baseline pain (SD): 2.00 (1.00). Median baseline percussion pain (SD): 2.00 (2.00). Median baseline swelling (SD): 0 (1.00) Included participants had a symptomatic necrotic tooth and actively had spontaneous pain. To be eligible the affected tooth had to test negative to an electric pulp test (Analytic Technology Corp, Redmond, WA) and ice; have a periapical radiolucency and not have had previous endodontic treatment. Included participants were in good health (as determined by written and verbal history), had not received antibiotics in the 30 days prior to enrolment to the trial and did not have a probable or actively draining sinus tract Number of participants at randomisation: not stated in paper, approximately 51 (from personal communication) Number of participants included in the analysis: group 1 = 19; group 2 = 22 |
|
| Interventions | Endodontic treatment: all participants underwent total pulpectomy of the affected tooth on day 0. Canals were prepared using a step‐back preparation and K‐type files (LD Caulk, Inc, Milford, DE) and irrigated with 2.62% hypochlorite. Following instrumentation, canals were dried and a temporary restoration placed (CavitTM (a light‐cured temporary sealing compound for temporary restoration of cavities)) Participants were then assigned to a trial arm Group 1: oral penicillin (phenoxymethyl) VK 500 mg, 6‐hourly for 7 days Group 2: oral matched placebo taken according to the same regimen Analgesics: all participants received a supply of ibuprofen and were advised to take 400 mg (2 x 200 mg tablets) every 4‐6 hours, as required. Each participant also received a labelled bottle of paracetamol (acetaminophen) with codeine (30 mg), which they were instructed to take 1 or 2 tablets every 4 hours only if 2 ibuprofen tablets did not relieve their discomfort |
|
| Outcomes |
Primary outcomes Participant‐reported pain, percussion pain and swelling experience at the baseline visit and upon rising for 7 days after treatment on categorical scales. Participants received a 7‐day diary to record postoperative symptoms upon rising each day. This was returned at the obturation appointment (typically the end of root canal treatment). Pain was assessed using a short ordinal numerical scale from 0 to 3: 0 = no pain; 1 = mild pain; 2 = moderate pain; 3 = severe pain. Participants used the same scale to rate pain to percussion (achieve by tapping the affected tooth with a finger). Swelling was assessed on a similar ordinal numerical scale from 0 to 3: 0 = no swelling; 1 = mild swelling, a mild puffiness that was not bothersome; 2 = moderate swelling that caused facial distortion and was bothersome; 3 = a severe swelling that caused serious facial distortion and was very bothersome Secondary outcomes The number and type of pain medication taken |
|
| Notes | Funding source: Graduate Endodontic Student Research Fund and Goldberg Memorial Fund, Graduate Endodontics, College of Dentistry, The Ohio State University Sample size calculation: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was assigned a 5‐digit random number from a random number table before the experiment" (email from author) |
| Allocation concealment (selection bias) | Low risk | Quote: "The study investigator only gave the labelled bottle to the subject without knowing the content because he only saw the random numbers not the assignment of the drug" (email from author) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each 500 mg gelatin capsule of either penicillin or placebo was identical in form. The 500 mg tablets of penicillin VK were ground into a powder and placed into clear gelatin capsules. The white powder of the lactose placebo was indistinguishable from the white powder of the penicillin tablets when viewed through the capsule" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome measures were participant‐assessed and it was highly unlikely blinding was broken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of relative attrition rates and reasons for withdrawal precludes judgement of the risk of bias |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias were identified |
RCT: randomised controlled trial; SD: standard deviation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achard 1967 | Not an RCT |
| Al‐Belasy 2003 | No placebo control |
| Alves 2000 | No placebo control |
| Angelini 1983 | Not an RCT |
| Anonymous 1968 | Not an RCT |
| Banoczy 1985 | Not an RCT |
| Baratieri 1968 | Not an RCT |
| Brabant 1968 | Not an RCT |
| Brennan 2006 | Not all participants met inclusion criteria for clinical diagnosis. No subgroup data presented |
| Citoler Gutierrez 1969 | Not an RCT |
| Cumming 1984 | Not an RCT |
| D'Atri 1973 | Not an RCT |
| Davis 1969 | Sample included children |
| De Vries 1974 | Not an RCT |
| Deffez 1992 | No placebo control |
| Diamantes‐Kepiotes 1974 | Intervention did not include a systemic antibiotic |
| Dolci 1982 | Not an RCT |
| Flood 1977 | Not an RCT |
| Gabka 1968 | Not an RCT |
| Groshikov 1970 | Not an RCT |
| Haapasalo 1986 | Not an RCT |
| Hood 1978 | Not an RCT |
| Hooley 1969 | Not an RCT |
| Khosla 1970 | Not an RCT |
| Krzywicki 1975 | Not an RCT |
| Lewis 1986 | No placebo control |
| Lin 2006 | Intervention did not include a systemic antibiotic |
| Lindeboom 2005 | Prevention study not fulfilling inclusion criteria |
| Lorber 1967 | Not an RCT |
| Matijevic 2009 | Sample included children |
| Nowakowska 1974 | Not an RCT |
| Oeda 1985 | No placebo control |
| Ranta 1988 | Not all participants met inclusion criteria for clinical diagnosis. No subgroup data presented |
| Re 1988 | No placebo control |
RCT: randomised controlled trial.
Differences between protocol and review
We have made a minor alteration to the objectives to specify the difference between antibiotics provided with a surgical intervention and those without.
We used a fixed‐effect model in the meta‐analysis not a random‐effects model as specified by the protocol. This was because fewer trials were suitable for inclusion than we initially anticipated.
Contributions of authors
Anwen L Cope initiated the review, drafted the protocol, screened search results, extracted the data, performed risk of bias assessment and wrote the final review.
Ivor G Chestnutt initiated the review, made amendments to the protocol, screened search results, reviewed data extraction and risk of bias assessment (2018 review), was the arbiter during study selection and data extraction (2014 review), and was involved in writing the final review.
Nick Francis and Fiona Wood initiated the review, made amendments to the protocol and were involved in writing the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Wales School for Primary Care Research, UK.
Financial support for this project was provided by the Wales School for Primary Care Research.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and the Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Health and Care Research Wales, UK.
Anwen L Cope was supported by a Clinical Research Time Award from Health and Care Research Wales.
Declarations of interest
Anwen L Cope, Nick Francis, Fiona Wood, Ivor G Chestnutt: no interests to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Fouad 1996 {published and unpublished data}
- Fouad AF, Rivera EM, Walton RE. Penicillin as a supplement in resolving the localized acute apical abscess. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 1996;81(5):590‐5. [DOI] [PubMed] [Google Scholar]
Henry 2001 {published and unpublished data}
- Henry M, Reader A, Beck M. Effect of penicillin on postoperative endodontic pain and swelling in symptomatic necrotic teeth. Journal of Endodontics 2001;27(2):117‐23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Achard 1967 {published data only}
- Achard GJ, Rouit M. Pulpo‐apical infection in the age of antibiotics. Information Dentaire 1967;49(19):1937‐49. [PubMed] [Google Scholar]
Al‐Belasy 2003 {published data only}
- Al‐Belasy FA, Hairam AR. The efficacy of azithromycin in the treatment of acute infraorbital space infection. Journal of Oral and Maxillofacial Surgery 2003;61(3):310‐6. [DOI] [PubMed] [Google Scholar]
Alves 2000 {published data only}
- Alves DF. Clinical avaliation of the competence of some antimicrobienics drugs on the treatment of the periapical abscess in evoluation. Journal of Dental Research 2000;79(5):1077 (Abs No A‐068). [Google Scholar]
Angelini 1983 {published data only}
- Angelini F, Ferrieres G, Lesous J. Clinical profile of a new bioprecursor. Revue d'Odonto‐Stomatologie 1983;12(5):387‐91. [PubMed] [Google Scholar]
Anonymous 1968 {published data only}
- Anonymous. Jaw abscesses. Nederlands Tijdschrift voor Geneeskunde 1968;112(32):1456‐7. [PubMed] [Google Scholar]
Banoczy 1985 {published data only}
- Banoczy J, Nyarasky I, Ferenczi I. Research on the effectiveness of metronidazole in the treatment of root infections. Chirurgien‐Dentiste de France 1985;55(277):37‐9. [PubMed] [Google Scholar]
Baratieri 1968 {published data only}
- Baratieri A. Present progress in biologic therapy of septic‐degenerative conditions of the dental pulp and of periapical affections. Revue Belge de Medecine Dentaire 1968;23(2):181‐98. [PubMed] [Google Scholar]
Brabant 1968 {published data only}
- Brabant H, Ketelbant R. Clinical studies of the use in stomatology of a new antibiotic: doxycycline. Revue de Stomatologie et de Chirurgie Maxillo‐Faciale 1968;69(1):43‐8. [PubMed] [Google Scholar]
Brennan 2006 {published data only}
- Brennan MT, Runyon MS, Batts JJ, Fox PC, Kent ML, Cox TL, et al. Odontogenic signs and symptoms as predictors of odontogenic infection: a clinical trial. Journal of the American Dental Association 2006;137(1):62‐6. [DOI] [PubMed] [Google Scholar]
Citoler Gutierrez 1969 {published data only}
- Citoler Gutierrez G. Clinical trial with the use of doxycycline in dentistry. Anales Españoles de Odontoestomatología 1969;28(5):372‐7. [PubMed] [Google Scholar]
Cumming 1984 {published data only}
- Cumming CG, Ross PW, Smith GF, Lough H, Moyes A. The use of cefadroxil in the treatment of acute orofacial infections. Journal of Dentistry 1984;12(3):247‐51. [DOI] [PubMed] [Google Scholar]
D'Atri 1973 {published data only}
- D'Atri G, Silvano G. Results with a new antibiotic (cephalexin) in cases of odontogenic inflammation. Minerva Stomatologica 1973;22(1):8‐17. [PubMed] [Google Scholar]
Davis 1969 {published data only}
- Davis WM Jr, Balcom JH 3rd. Lincomycin studies of drug absorption and efficacy. An evaluation by double‐blind technique in treatment of odontogenic infections. Oral Surgery, Oral Medicine, Oral Pathology 1969;27(5):688‐96. [DOI] [PubMed] [Google Scholar]
Deffez 1992 {published data only}
- Deffez JP, Scheimberg A, Rezvani Y. Multicenter double‐blind study of the efficacy and tolerance of roxithromycin versus erythromycin ethylsuccinate in acute orodental infection in adults. Diagnostic Microbiology and Infectious Disease 1992;15(4 Suppl):133S‐7S. [DOI] [PubMed] [Google Scholar]
De Vries 1974 {published data only}
- Vries J, Francis LE. Penicillin substitutes ‐ erythromycin and clindamycin. Dental Journal ‐ Journal Dentaire 1974;40(4):295‐8. [PubMed] [Google Scholar]
Diamantes‐Kepiotes 1974 {published data only}
- Diamantes‐Kepiotes A, Sykaras SN. Clinical study on the effect of corticosteroid‐antibiotic compound in the prevention and treatment of acute apical periodontitis. Odontostomatologike Proodos 1974;28(1):26‐33. [PubMed] [Google Scholar]
Dolci 1982 {published data only}
- Dolci G, Ricca L, Vergnani M. Evaluation of the analgesic effect of fosfomycin in odontostomatological infections. Clinica Terapeutica 1982;102(1):59‐65. [PubMed] [Google Scholar]
Flood 1977 {published data only}
- Flood MK. Clinical evaluation of troleandomycin for dental abscesses. Acta Stomatologica Belgica 1977;74(1):49‐52. [PubMed] [Google Scholar]
Gabka 1968 {published data only}
- Gabka J. Antibacterial activity of lincomycin in diseases of the maxillofacial region. Arzneimittel‐Forschung 1968;18(11):1453‐7. [PubMed] [Google Scholar]
Groshikov 1970 {published data only}
- Groshikov MI. The treatment of apical periodontitis. Stomatologiia 1970;49(2):66‐70. [PubMed] [Google Scholar]
Haapasalo 1986 {published data only}
- Haapasalo M, Ranta H, Ranta K, Shah H. Black‐pigmented Bacteroides spp. in human apical periodontitis. Infection and Immunity 1986;53(1):149‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hood 1978 {published data only}
- Hood FJ. The place of metronidazole in the treatment of acute oro‐facial infection. Journal of Antimicrobial Chemotherapy 1978;4 Suppl C:71‐3. [DOI] [PubMed] [Google Scholar]
Hooley 1969 {published data only}
- Hooley JR. Cloxacillin in the treatment of dental infection. Oral Surgery, Oral Medicine, Oral Pathology 1969;28(4):485‐7. [DOI] [PubMed] [Google Scholar]
Khosla 1970 {published data only}
- Khosla VM. Lincomycin in oral surgery. Oral Surgery, Oral Medicine, Oral Pathology 1970;29(4):485‐90. [DOI] [PubMed] [Google Scholar]
Krzywicki 1975 {published data only}
- Krzywicki R, Olejnik A. Evaluation of oxyterracin in the treatment of odontogenic inflammatory conditions. Czasopismo Stomatologiczne 1975;28(9):921‐5. [PubMed] [Google Scholar]
Lewis 1986 {published data only}
- Lewis MA, McGowan DA, MacFarlane TW. Short‐course high‐dosage amoxycillin in the treatment of acute dento‐alveolar abscess. British Dental Journal 1986;161(8):299‐302. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin S, Levin L, Emodi O, Abu El‐Naaj E, Peled M. Etodolac versus dexamethasone effect in reduction of postoperative symptoms following surgical endodontic treatment: a double‐blind study. Oral Surgery, Oral Medicines, Oral Pathology, Oral Radiology, and Endodontics 2006;101:814‐7. [DOI] [PubMed] [Google Scholar]
Lindeboom 2005 {published data only}
- Lindeboom JA, Frenken JW, Valkenburg P, Akker HP. The role of preoperative prophylactic antibiotic administration in periapical endodontic surgery: a randomized, prospective double‐blind placebo‐controlled study. International Endodontic Journal 2005;38(12):877‐81. [DOI] [PubMed] [Google Scholar]
Lorber 1967 {published data only}
- Lorber CG. Antibiotics and sulfonamides in stomatology. Stoma 1967;20(2):110‐20. [PubMed] [Google Scholar]
Matijevic 2009 {published data only}
- Matijevic S, Lazic Z, Kuljic‐Kapulica N, Nonkovic Z. Empirical antimicrobial therapy of acute dentoalveolar abscess. Vojnosanitetski Pregled 2009;66(7):544‐50. [DOI] [PubMed] [Google Scholar]
Nowakowska 1974 {published data only}
- Nowakowska A, Olko S, Poniewska A. Oleflavit in the treatment of periodontal abscesses. Czasopismo Stomatologiczne 1974;27(10):1139‐41. [PubMed] [Google Scholar]
Oeda 1985 {published data only}
- Oeda N, Mimura T, Yoshimine S, Tanaka T, Kiwaki M. Use of bacampicillin in acute infections in the mouth. Shikai Tenbo ‐ Dental Outlook 1985;65(3):671‐7. [PubMed] [Google Scholar]
Ranta 1988 {published data only}
- Ranta H, Haapasalo M, Ranta K, Kontiainen S, Kerosuo E, Valtonen V, et al. Bacteriology of odontogenic apical periodontitis and effect of penicillin treatment. Scandinavian Journal of Infectious Diseases 1988;20(2):187‐92. [DOI] [PubMed] [Google Scholar]
Re 1988 {published data only}
- Re G, Barbero P, Briccarello MP, Manzon W, Zoccola GC. Oral amoxicillin 1g b.i.d vs 1g t.i.d. in the treatment of oral periodontitis. Randomized and double‐blind pilot trial. Minerva Stomatologica 1988;37(6):507‐9. [PubMed] [Google Scholar]
Additional references
Abbott 2004
- Abbott PV. Classification, diagnosis and clinical manifestations of apical periodontitis. Endodontic Topics 2004;8(1):36‐54. [Google Scholar]
Al‐Haroni 2007
- Al‐Haroni M, Skaug N. Incidence of antibiotic prescribing in dental practice in Norway and its contribution to national consumption. Journal of Antimicrobial Chemotherapy 2007;59(6):1161‐6. [DOI] [PubMed] [Google Scholar]
Bergenholtz 2010
- Bergenholtz G, Hörsted‐Bindslev P, Reit C, editor(s). Textbook of Endodontology. 2nd Edition. Chichester: Wiley‐Blackwell, 2010. [Google Scholar]
Carrotte 2004
- Carrotte P. Endodontics: part 3. Treatment of endodontic emergencies. British Dental Journal 2004;197(6):299‐305. [DOI] [PubMed] [Google Scholar]
Chen 2009
- Chen E, Abbott PV. Dental pulp testing: a review. International Journal of Dentistry 2009;2009:365785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coenen 2006
- Coenen S, Michiels B, Renard D, Denekens J, Royen P. Antibiotic prescribing for acute cough: the effect of perceived patient demand. British Journal of General Practice 2006;56(524):183‐90. [PMC free article] [PubMed] [Google Scholar]
Cope 2016
- Cope AL, Francis NA, Wood F, Chestnutt IG. Antibiotic prescribing in UK general dental practice: a cross‐sectional study. Community Dentistry and Oral Epidemiology 2016;44(2):145‐53. [DOI] [PubMed] [Google Scholar]
Costelloe 2010
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay A. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis. BMJ 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
Dailey 2001
- Dailey YM, Martin MV. Are antibiotics being used appropriately for emergency dental treatment?. British Dental Journal 2001;191(7):391‐3. [DOI] [PubMed] [Google Scholar]
Germack 2017
- Germack M, Sedgley CM, Sabbah W, Whitten B. Antibiotic use in 2016 by members of the American Association of Endodontists: report of a national survey. Journal of Endodontics 2017;43(10):1615‐22. [DOI] [PubMed] [Google Scholar]
Glenny 2004
- Glenny AM, Simpson T. Clinical practice guideline on emergency management of acute apical periodontitis (AAP) in adults. Evidence‐Based Dentistry 2004;5(1):7‐11. [DOI] [PubMed] [Google Scholar]
Glickman 2009
- Glickman GN. AAE Consensus Conference on Diagnostic Terminology: background and perspectives. Journal of Endodontics 2009;35(12):1619‐20. [DOI] [PubMed] [Google Scholar]
Gonzales 2001
- Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clinical Infectious Diseases 2001;33(6):757‐62. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 12 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Halling 2017
- Halling F, Neff A, Heyman P, Ziebart T. Trends in antibiotic prescribing by dental practitioners in Germany. Journal of Cranio‐Maxillo‐Facial Surgery 2017;45(11):1854‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holyfield 2009
- Holyfield G, Karki A. Review of prescribing by dentists in Wales. National Public Health Service for Wales, 2009. Available from www.1000livesplus.wales.nhs.uk/opendoc/179908.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Little 1997
- Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997;315(7104):350‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2003
- Matthews DC, Sutherland S, Basrani B. Emergency management of acute apical abscess in the permanent dentition: a systematic review of the literature. Journal of the Canadian Dental Association 2003;69(10):660. [PubMed] [Google Scholar]
Palmer 2000
- Palmer NA, Pealing R, Ireland RS, Martin MV. A study of therapeutic antibiotic prescribing in National Health Service general dental practice in England. British Dental Journal 2000;188(10):554‐8. [DOI] [PubMed] [Google Scholar]
Palmer 2015
- Palmer NOA, Longman L, Randall C, Pankhurst CL. Antimicrobial Prescribing for General Dental Practitioners. London: Faculty of General Dental Practice (UK), 2015. [Google Scholar]
Pau 2005
- Pau A, Croucher R, Marcenes W, Leung T. Development and validation of a dental pain‐screening questionnaire. Pain 2005;119(1‐3):75‐81. [DOI] [PubMed] [Google Scholar]
Reisine 1995
- Reisine S, Locker D. Social, psychological and economic impacts of oral conditions treatments. In: Cohen LK, Gift HC editor(s). Disease Prevention and Oral Health Promotion: Social‐Dental Sciences in Action. Copenhagen: Munksgaard, 1995:33‐71. [Google Scholar]
SDCEP 2016
- Scottish Dental Clinical Effectiveness Programme (SDCEP). Drug Prescribing for Dentistry: Dental Clinical Guidance. 3rd Edition. Dundee: Scottish Dental Clinical Effectiveness Programme, 2016. [DOI] [PubMed] [Google Scholar]
Segura‐Egea 2010
- Segura‐Egea JJ, Velasco‐Ortega E, Torres‐Lagares D, Velasco‐Ponferrada MC, Monsalve‐Guil L, Llamas‐Carreras JM. Pattern of antibiotic prescription in the management of endodontic infections amongst Spanish oral surgeons. International Endodontic Journal 2010;43(4):342‐50. [DOI] [PubMed] [Google Scholar]
Skevington 1998
- Skevington SM. Investigating the relationship between pain and discomfort and quality of life, using the WHOQOL. Pain 1998;76(3):395‐406. [DOI] [PubMed] [Google Scholar]
Steele 2011
- Steele J, Pitts N, Fuller E, Treasure E. 3: Urgent conditions ‐ a report from the Adult Dental Health Survey 2009. The Health and Social Care Information Centre, 2011. Available from files.digital.nhs.uk/publicationimport/pub01xxx/pub01086/adul‐dent‐heal‐surv‐summ‐them‐the3‐2009‐rep5.pdf.
Sutherland 2003
- Sutherland S, Matthews DC. Emergency management of acute apical periodontitis in the permanent dentition: a systematic review of the literature. Journal of the Canadian Dental Association 2003;69(3):160. [PubMed] [Google Scholar]
Sutherland 2004
- Sutherland SE, Matthews DC. Conducting systematic reviews and creating clinical practice guidelines in dentistry: lessons learned. Journal of the American Dental Association 2004;135(6):747‐53. [DOI] [PubMed] [Google Scholar]
Torabinejad 1994
- Torabinejad M. Mediators of acute and chronic periradicular lesions. Oral Surgery, Oral Medicine, and Oral Pathology 1994;78(4):511‐21. [DOI] [PubMed] [Google Scholar]
World Health Organization 2000
- World Health Organization. World Health Organization Report on Infectious Diseases 2000: Overcoming Antimicrobial Resistance. Geneva: World Health Organization, 2000. [Google Scholar]
Yingling 2002
- Yingling NM, Byrne BE, Hartwell GR. Antibiotic use by members of the American Association of Endodontists in the year 2000: report of a national survey. Journal of Endodontics 2002;28(5):396‐404. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cope 2012
- Cope A, Francis N, Wood F, Mann MK, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010136] [DOI] [PubMed] [Google Scholar]
Cope 2014
- Cope A, Francis N, Wood F, Mann MK, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database of Systematic Reviews 2014, Issue Issue 6. [DOI: 10.1002/14651858.CD010136.pub2] [DOI] [PubMed] [Google Scholar]


