Abstract
Background
Children with acute pneumonia may be vitamin D deficient. Clinical trials have found that prophylactic vitamin D supplementation decreases the risk of developing pneumonia in children. Data on the therapeutic effects of vitamin D in acute childhood pneumonia are limited.
Objectives
To evaluate the efficacy and safety of vitamin D supplementation as an adjunct to antibiotics for the treatment of acute childhood pneumonia.
Search methods
We searched CENTRAL (2017, Issue 7), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register; Ovid MEDLINE Epub Ahead of Print; In‐Process & Other Non‐Indexed Citations; Ovid MEDLINE Daily and Ovid MEDLINE (1946 to July Week 4, 2017); and Embase (2010 to 28 July 2017). We also searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on 28 July 2017. There were no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) including children (aged over one month and up to five years) hospitalised with acute community‐acquired pneumonia, as defined by the WHO acute respiratory infection guidelines, that compared vitamin D supplementation with control.
Data collection and analysis
Two review authors independently assessed studies for inclusion and extracted data. For dichotomous data, we extracted the number of participants experiencing the outcome and the total number of participants in each treatment group. For continuous data, we used the arithmetic mean and standard deviation (SD) for each treatment group together with numbers of participants in each group. We used standard methodological procedures expected by Cochrane.
Main results
We included seven RCTs conducted in low‐income countries that involved 1529 children (780 with pneumonia and 749 with severe or very severe pneumonia). Four studies used a single 100,000 IU dose of vitamin D₃ at the onset of illness or within 24 hours of hospital admission; two used a daily dose of oral vitamin D₃ (1000 IU for children aged up to one year and 2000 IU for children aged over one year) for five days; and one used a daily dose of oral vitamin D₃ (50,000 IU) for two days. One study reported microbiological and radiological diagnosis of pneumonia.
The effects of vitamin D on outcomes were inconclusive when compared with control: time to resolution of acute illness (hours) (mean difference (MD) ‐0.95, 95% confidence interval (CI) ‐6.14 to 4.24; 3 studies; 935 children; low‐quality evidence) mortality rate (risk ratio (RR) 0.97, 95% CI 0.06 to 15.28; 1 study; 193 children; very low‐quality evidence); duration of hospitalisation (MD 0.49, 95% CI ‐8.41 to 9.4; 4 studies; 835 children; very low‐quality evidence) and time to resolution of fever (MD 1.66, 95% CI ‐2.44 to 5.76; 4 studies; 584 children; very low‐quality evidence).
No major adverse events were reported.
The GRADE assessment found very low‐quality evidence (due to serious study limitations, inconsistencies, indirectness, and imprecision) for all outcomes except time to resolution of acute illness.
One study was funded by the New Zealand Aid Corporation; one study was funded by an institutional grant; and five studies were unfunded.
Authors' conclusions
We are uncertain as to whether vitamin D has an important effect on outcomes because the results were imprecise. No major adverse events were reported. We assessed the quality of the evidence as very low to low. Several trials are ongoing and may provide additional information.
Plain language summary
Is vitamin D an effective and safe addition to antibiotics to treat children with acute pneumonia?
Review question
We wanted to find out if vitamin D helps children with acute pneumonia who are also receiving antibiotic treatment get better faster.
Background
Pneumonia is an acute lower respiratory tract infection that affects the lungs. Treatment for pneumonia includes antibiotics, providing supplementary oxygen to air that is breathed in through a mask, and other supportive therapies. Vitamin D boosts immune defences and reduces excessive inflammation, effects that may help children recover from an acute episode of pneumonia.
Search date
The evidence is current to 28 July 2017.
Study characteristics
We included seven studies involving a total of 1529 children (780 with pneumonia (4 studies) and 749 with severe or very severe pneumonia (3 studies)) aged under 5 years from low‐income countries. In four studies, a single large dose of vitamin D was used either when the child joined the study or within 24 hours of admission to hospital; in two studies, vitamin D was used for five days; and in one study, vitamin D was used for two days. One study excluded children whose vitamin D levels were normal. One study reported the cause of children's pneumonia.
Study funding sources
One study was funded by the New Zealand Aid Corporation; one was funded by an institutional grant; and five studies were unfunded.
Key results
We are uncertain as to whether vitamin D has an important effect on outcomes due to the very‐low quality of the evidence. Vitamin D may slightly decrease the time taken to get better from acute pneumonia (by 60 minutes) and the risk of death, and Vitamin D may increase the length of time in hospital (by 30 minutes) and the time taken for fever to resolve (by 90 minutes). However, there was no significant difference between groups for these outcomes. No major adverse events were reported.
Quality of the evidence
The quality of the evidence was very low, except for time to resolution of acute illness, which we assessed as low quality. We identified problems with the study methods and reporting, resulting in lack of precision in the included studies.
Summary of findings
Summary of findings for the main comparison. Vitamin D compared with placebo for acute childhood pneumonia.
| Vitamin D compared with placebo for acute childhood pneumonia | ||||||
|
Patient or population: children with acute pneumonia Settings: hospital setting Intervention: oral vitamin D Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vitamin D | |||||
| Time to resolution of acute illness | Mean time in the control group ranged from 50.8 to 119.52 hours. | The mean time in the intervention group was 0.95 hours less (6.14 hours less to 4.24 hours more). | ‐ | 935 (3 studies) | ⊕⊕⊝⊝ Low1‐4 | 4 studies reported the outcome (data pooled from 3 studies). The appropriate effect measure for time‐to‐event data is hazard ratio. However, the studies reported this information as continuous data (1 study reported as a proportion). The MD was around 1 hour less for the vitamin D group. |
| Duration of hospitalisation | Mean duration in the control group ranged from 106.82 to 168 hours. | The mean duration in the intervention group was from 0.49 hours more (8.41 hours less to 9.4 hours more). | ‐ | 835 (4 studies) | ⊕⊝⊝⊝ Very low5‐8 | 5 studies reported the outcome (data pooled from 4 studies; mean and standard deviation calculated for 2 studies that reported the data as median). The MD was around half an hour more for the vitamin D group. |
| Mortality rate | 97 per 1000 | 94 per 1000 (6 to 1000) |
RR 0.97 (0.06 to 15.28) | 193 (1 study) |
⊕⊝⊝⊝ Very low9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1No serious study limitations: all studies had adequately concealed allocation and both participants and study staff were blinded. Considered at low risk of bias. 2No serious inconsistency: there was a low (I² = 36%) and non‐significant (P = 0.21) statistical heterogeneity. 3Serious indirectness: only studies from low‐income regions with high baseline prevalence of vitamin D deficiency were assessed in this comparison (the result cannot be generalised to all locations). The characteristics of the populations in the two studies also differed slightly (one study included children aged up to three years, and another included children aged up to five years). Downgraded by one level. 4Serious imprecision: the 95% CI around the pooled effect is wide and different in the included studies. The 95% CI includes no effect, and the upper or lower confidence limit crosses the minimal important difference for benefit (here the time to resolution of illness was 5.75 hours less to 3.97 hours more). Downgraded by one level. 5Serious study limitations: one study only was assessed as at unclear risk of bias for allocation concealment; blinding of both participants and study staff was considered as at unclear risk of bias. Downgraded by one level. 6No serious inconsistency: there was no statistical heterogeneity. 7Serious indirectness: only studies from low‐income regions with high baseline prevalence of vitamin D deficiency were assessed in this comparison (the result cannot be generalised to all situations). The characteristics of the populations in the two studies also differed slightly (one study included children aged from two months, and another included children aged from six months). Downgraded by one level. 8Serious imprecision: the 95% CI includes no effect, and the upper or lower confidence limit crosses the minimal important difference for benefit (here the duration of hospitalisation was 14.09 hours less to 2.64 hours more). Downgraded by one level. 9Data came from just one study: no serious study limitations or inconsistency, serious indirectness (downgraded by one level, lack of generalisability), serious imprecision (downgraded by one level, the 95% CI includes no effect).
Background
Description of the condition
Pneumonia is an acute lower respiratory tract infection that primarily affects the lungs. Microscopically, the alveoli are filled with exudative fluid (pus), which compromises breathing and gas exchange in the lungs (Scott 2012). Clinical manifestations of acute childhood pneumonia include cough, fever, breathing difficulty, and lower chest wall indrawing (Scott 2012). The most common aetiological agents responsible are bacteria and viruses (e.g. Streptococcus pneumoniae, Haemophilus influenzae, and respiratory syncytial virus).
Risk factors for acute childhood pneumonia include mixed feeding or non‐breastfeeding, under nutrition, indoor air pollution, prematurity, overcrowding, and no immunisation against measles (Rudan 2008). In 2010, of the 7.6 million deaths of children aged up to five years globally, 64% were due to infectious diseases (Liu 2012), and 18.3% (1396 million) were attributed to pneumonia (Liu 2012; Lozano 2011).
The management of acute childhood pneumonia includes antibiotics, oxygen, supportive therapy, and assisted ventilation (in severe cases) (Lassi 2014; Rojas‐Reyes 2006). Nutritional supplementation such as zinc, vitamin A, and vitamin C have been used, although results have been unfavourable (Chang 2006; Das 2012; Haider 2011a; Haider 2011b; Hemilä 2013; Wu 2005). Similarly, the use of over‐the‐counter medications (such as mucolytics and antitussives) have not shown beneficial effect (Chang 2014).
Description of the intervention
Vitamin D is a fat‐soluble vitamin that plays an important role in calcium and phosphorous homeostasis and bone mineralisation (Wagner 2008). There are two types: vitamin D₂ (ergocalciferol), which is derived from plants, and vitamin D₃ (cholecalciferol), which is derived from animals. Vitamin D is primarily synthesised in the skin (as vitamin D₃) after exposure to ultraviolet B radiation; less than 10% is derived from dietary sources (McAllister 2006; Misra 2008). The main form circulating in the blood is 25‐hydroxy‐vitamin D (25(OH)‐D) (synthesised in the liver), and the active or true hormonal form is 1,25‐dihydroxyvitamin D (1,25(OH)₂‐D or calcitriol) (synthesised in the kidneys) (Misra 2008; Walker 2009). The active form binds to the receptor present in the nucleus (vitamin D receptor, or VDR) before orchestrating a wide range of actions in the body (Walker 2009). Vitamin D receptor is present in cells of almost every organ system of the body, including immune cells. Due to a longer half‐life, measurement of the blood 25(OH)‐D level is the best available parameter to indicate the status of vitamin D in the body and is used to define the status of vitamin D in children as: sufficient (50 to 250 nmol/L), insufficient (37.5 to 50 nmol/L), deficient (≤ 37.5 nmol/L), severely deficient (≤ 12.5 nmol/L), and excessive (> 250 nmol/L) (Misra 2008).
As a nutritional supplement, vitamin D is included in most multivitamins (doses vary from 50 IU to 1000 IU) as liquids, tablets, and capsules. Intramuscular injections may be used in special circumstances. A daily dose of 400 IU has been recommended for children and adolescents. The upper limit should not exceed 2000 IU per day in children aged over one year, and 1000 IU per day in infants aged up to one year. However, in deficient states, a daily dose of 1000 IU to 10,000 IU (depending on the child's age) can be used for two to three months to enable the serum vitamin D level to normalise and replenish stores (Misra 2008).
Vitamin D deficiency or insufficiency is prevalent in low‐income countries (Joshi 2014; Kelishadi 2014; Thacher 2012; Torun 2013; Zhang 2013), and also occurs in high‐income countries (Grant 2009; Mansbach 2009; Ward 2007). A 10‐year data series reported 126 cases of vitamin D deficient rickets in Australia (Robinson 2006), and a two‐year data series reported 104 cases in Canada (Ward 2005). In the USA and the UK, there are no definitive prevalence estimates for vitamin D deficient rickets. Historical prevalence estimates from the USA indicate increases of vitamin D deficient rickets from 65 instances between 1975 and 1985 rising to 166 instances between 1986 and 2003 (Weisberg 2004); there were 62 instances between 2004 and 2006 (McAllister 2006; Mylott 2004). Vitamin D deficient rickets remains a significant public health problem in other parts of the world (Joshi 2014; Wondale 2005).
Increased urbanisation, time spent indoors, use of sunscreen, increasing latitude, seasonal change (winter or rainy season), air pollution, prematurity, and malabsorptive disorders are associated with vitamin D deficiency (Misra 2008). Vitamin D deficiency substantially increases the risk of pneumonia among children aged up to five years (Muhe 1997; Najada 2004; Rehman 1994; Wayse 2004). Studies have also found a link between single nucleotide polymorphisms (SNPs) of genes related to the VDR, and susceptibility to respiratory syncytial virus infection (Janssen 2007). A trial that provided vitamin D supplements for children with pneumonia reported a reduction in recurrent episodes (Manaseki‐Holland 2010).
How the intervention might work
Vitamin D has been reported to boost mucosal immune defences and reduce excessive inflammation (Pfeffer 2012). The immune‐enhancing actions of vitamin D include induction of monocyte differentiation, inhibition of lymphocyte proliferation, stimulation of phagocytosis‐dependent and antibody‐dependent macrophages, and modulation of cytokine and antibody‐producing lymphocytes (Liu 2006; Raloff 2006; White 2008). Hydoxylation of vitamin D to the active form occurs in the kidneys by the enzyme 1‐alpha hydroxylase or CYP27B1; the same hydroxylation pathway also operates in other cells that participate in immune regulation (e.g. epithelial cells and monocyte‐macrophages) (Hewison 2011). In the airways (bronchial epithelial cells), 1‐alpha hydroxylase activity increases in response to any acute insult or inflammatory stimulus (Hansdottir 2008). Cathelicidin is an antimicrobial peptide whose expression is increased over immune cells in response to infection, and the whole cascade is vitamin D dependent (Gombart 2005; White 2008). As a result, the vitamin D/cathelicidin‐circuit is activated in acute respiratory tract infections, including pneumonia. Experimental models have shown that vitamin D dampens adaptive immune responses and modulates key elements of innate immune response in dendritic cells following S pneumoniae infection (Olliver 2013). Vitamin D also confers antiviral properties that affect the role of toll‐like receptors (TLR), particularly TLR7 (Alvarez‐Rodriguez 2012).
Some studies have shown that daily vitamin D supplementation has better therapeutic efficacy than large bolus doses for pneumonia, tuberculosis, and fracture in the elderly (Heaney 2012; Hollis 2011; Manaseki‐Holland 2012; Martineau 2012; Nielsen 2010). Clinical studies have shown that vitamin D at a higher dose could be immunosuppressive. Kimball 2011 investigated high‐dose vitamin D (10,000 IU per day) and found suppressed proliferative response of peripheral blood monocytes. This finding is supported by Manaseki‐Holland 2012. Large bolus doses (100,000 IU) of vitamin D four times per year in infants indicated a slightly increased risk of repeat episodes of all types of pneumonia and mortality (Manaseki‐Holland 2010). However, the preventive role of vitamin D in children using a single large bolus dose (100,000 IU) found reduced risk of clinically defined pneumonia by 13% up to three months after vitamin D treatment (Manaseki‐Holland 2010). This may mean that the immunological effectiveness of vitamin D is blunted at extremes of dosages (either very high or low therapeutic dose).
Why it is important to do this review
Pneumonia is a leading cause of global mortality in children aged up to five years; more than 90% of deaths occur in low‐income countries (Liu 2012; Lozano 2011). Although antibiotic therapy is the main treatment for pneumonia, irrational use has contributed to increasing resistance. In addition, accessibility to healthcare facilities is challenging for most children in low‐income countries. Vitamin D is a relatively simple intervention that may be a helpful addition to standard treatment for acute childhood pneumonia. Due to its low cost and ease of administration, vitamin D can be administered to children with acute pneumonia. Vitamin D, as an adjunct to antibiotics, may help to reduce childhood mortality from pneumonia. In a previous review, we found no role for oral vitamin D supplementation in children aged under five with acute pneumonia (Das 2013). This Cochrane Review includes updated searches to assist healthcare providers in making informed decisions about providing vitamin D for children with acute pneumonia.
Objectives
To evaluate the efficacy and safety of vitamin D supplementation as an adjunct to antibiotics for the treatment of acute childhood pneumonia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Children aged from one month up to five years, hospitalised with a clinical diagnosis of community‐acquired pneumonia (CAP). We defined pneumonia according to World Health Organization (WHO) acute respiratory infection guidelines (Scott 2012). We defined pneumonia in the presence of age‐specific tachypnoea (> 60 breaths per minute in children aged up to 2 months; > 50 breaths per minute in children aged from 2 months to 11 months; > 40 breaths per minute in children aged from 12 months to 60 months) with or without cough or fever. We defined severe pneumonia in the presence of age‐specific tachypnoea along with lower chest wall indrawing or any of the danger signs (cyanosis, inability to feed, lethargy). We excluded studies that included children with other debilitating diseases, severe wasting (weight for height z‐score below ‐3), asthma or other respiratory diseases, hospital‐acquired pneumonia and postoperative conditions.
Types of interventions
The intervention was commenced after the diagnosis of CAP had been made. The interventions consisted of treatment with vitamin D as an adjunct to antibiotics and other supportive measures. The trials compared vitamin D with placebo only. We considered any dose schedule (low versus high dose, daily dose versus bolus dose, any duration) and route (oral or injection) of vitamin D.
Types of outcome measures
Outcome measures frequently used to determine the clinical efficacy of any acute pneumonia treatment are time to resolution of illness, duration of hospitalisation, treatment failure, adverse events, or death.
Primary outcomes
Time to resolution of acute illness.
Duration of hospitalisation.
Secondary outcomes
-
Time to resolution of:
tachypnoea;
lower chest wall indrawing;
hypoxia (blood oxygen saturation < 95%);
fever;
inability to feed, or lethargy.
Treatment failure rate.
Mortality rate.
Adverse events (if any).
We defined the time to resolution of acute pneumonia as the time period to achieve the following parameters from initiation of treatment: respiratory rate less than the age‐specific cut‐offs, no lower chest indrawing, no danger signs or hypoxia, and ability to feed. These parameters were present for at least two consecutive days or 48 hours. We defined the duration of hospitalisation as the time period between study enrolment and discharge. We defined treatment failure as no reduction in tachypnoea over a 48‐hour period compared to that detected at enrolment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2017, Issue 7, part of the Cochrane Library (accessed 28 July 2017), which contains the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register; Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to July Week 4, 2017); and Embase (2010 to 28 July 2017).
We used the search strategy in Appendix 1 to search CENTRAL and MEDLINE. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011; Schünemann 2011). The search strategy was adapted to search Embase (Appendix 2).
Searching other resources
We searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en) on 28 July 2017. We searched the Journal of Orthomolecular Medicine (1986 to July 2017), which focuses on dietary supplements for illness. We contacted researchers in the relevant field to identify additional studies that may be eligible for inclusion. We also checked the reference lists of all studies identified.
Data collection and analysis
Selection of studies
Two review authors (RRD, SSN) independently assessed records for possible inclusion in the review. The review authors screened titles and abstracts to identify potentially relevant studies. When we could not ascertain relevance from the titles or abstracts, we retrieved the full text for assessment. Two review authors (RRD, SSN) independently assessed the eligibility of potentially relevant studies by evaluating the full‐text reports and completing eligibility forms designed in accordance with the specified inclusion criteria. Any differences in opinion were resolved through discussion or by consulting the third review author (MS) when necessary. We described excluded studies in Characteristics of excluded studies tables, along with reasons for their exclusion. In the case of conference abstracts, if additional data were not forthcoming, we used the information provided in the abstract. We contacted trial authors where we required clarification or additional information.
Data extraction and management
We extracted the following information from each study: author; year; location (country); setting (hospital or community); method of recruitment; inclusion criteria; unit of analysis; allocation ratio; risk of bias; participants (age, sex, sample size, pneumonia or severe pneumonia); intervention (dosage, duration, frequency, and co‐intervention if any); outcomes (outcome definition, valid unit of measurement, time points of collection and reporting); loss to follow‐up; and miscellaneous (key conclusions, references to other relevant trials, and additional data required). We designed and pilot tested the data extraction form prior to extraction of data.
Two review authors (RRD, SSN) independently extracted data from the studies. Any discrepancies were resolved through discussion with the third review author (MS). For dichotomous data (such as mortality and adverse events), we extracted the number of participants experiencing the condition and the total number of participants in each treatment group. For continuous data (such as time period and duration of illness), we used the arithmetic mean and standard deviation (SD) for each treatment group together with the number of participants in each group. If a standard error (SE) was reported, we converted it to an SD. If data were reported using geometric means, we recorded this information and extracted the SD on the log scale. If medians were used, we extracted the median and the range. If a 95% confidence interval (CI) was provided instead of a mean and SD for continuous data, we extracted the mean and SD from the 95% CI.
Assessment of risk of bias in included studies
Two review authors (RRD, SSN) independently assessed methodological quality using Cochrane's 'Risk of bias' tool (Higgins 2011). We assessed included studies based on the following six components: method of sequence generation; allocation concealment; blinding of participants (providers and outcome assessors); incomplete outcome data; selective outcome reporting; and other biases (including detection bias, e.g. differential effort to locate death records for the intervention and control groups). We presented findings in 'Risk of bias' tables with the review authors' judgement (low, high, or unclear risk of bias) and support for the judgement. We also reported the results in a 'Risk of bias' graph. We attempted to contact the trial authors for clarification where data were missing or we needed clarification about reporting. A third review author (MS) resolved any disagreements between the review authors. Further details about the 'Risk of bias' tool are provided in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We looked for sources of bias originating from differences between individual RCTs and cluster‐RCTs (e.g. the relationship between allocation concealment and recruitment bias may be greater in cluster‐RCTs).
Measures of treatment effect
We extracted and entered outcome data into Review Manager 5 software for statistical analysis (Review Manager 2014). We used the standard methods of the Cochrane Acute Respiratory Infections (ARI) Review Group to synthesise data. Outcomes with continuous data included time to resolution of acute illness, duration of hospitalisation, and duration of resolution of tachypnoea, chest retractions, hypoxia, fever, inability to feed or lethargy. For continuous data, we calculated mean differences (MD) with 95% CI to estimate the treatment effect. Where data were provided as medians and ranges, we presented these in a tabular form. Outcomes with dichotomous data included treatment failure rate, mortality rate, and adverse events rate. For dichotomous data, we reported the proportions of participants with these events, but were unable to calculate a pooled estimate of the treatment effect for each outcome using risk ratio (RR) with 95% CI due to insufficient numbers of included trials.
Unit of analysis issues
In studies randomising units other than individuals (i.e. clusters), results should be presented with controls for clustering (e.g. robust SEs or hierarchical linear models). We did not include any cluster‐randomised trials in our review, so we had no unit of analysis issues.
Dealing with missing data
We described missing data, including dropouts. Differential dropout rates can lead to biased estimates of the effect size, and bias may arise if the reasons for dropping out differ across groups. We reported reasons children dropped out of studies. If data were missing, or if reasons for dropping out were not reported, we contacted the trial authors for further information. We performed quantitative analysis on an intention‐to‐treat basis. When analyses were reported for completers as well as controlling for dropout (e.g. imputed using regression methods), we extracted the latter.
Assessment of heterogeneity
We assessed included studies for clinical, methodological, and statistical heterogeneity. We assessed clinical heterogeneity by comparing the distribution of important factors such as study participants, study setting, dose and duration of the intervention, and co‐interventions. We evaluated methodological heterogeneity on the basis of factors such as the method of sequence generation, allocation concealment, blinding of outcome assessment, and losses to follow‐up. We used the Chi² test for statistical heterogeneity between studies and considered P ≤ 0.10 as indicating significant heterogeneity. We used the I² statistic to assess the magnitude of heterogeneity (Higgins 2011). We considered an I² statistic above 50% to indicate problematic heterogeneity between studies and carefully considered the value of any pooled analysis.
Assessment of reporting biases
We undertook a comprehensive electronic search and searched trial registries to minimise the effects of publication bias. Had we identified 10 or more studies, we planned to create funnel plots of effect estimates against their standard errors (on a reversed scale) using Review Manager 5 (Review Manager 2014). However, we included only seven trials in the review.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2014). We performed meta‐analyses using a random‐effects model due to the presence of clinical heterogeneity. We did not include any cluster‐RCTs. We calculated overall effects using inverse variance methods. We presented results as the average treatment effect with 95% CI and Tau² and I² estimates.
'Summary of findings' table and GRADE
We created a 'Summary of findings' table using the following outcomes: time to resolution of acute illness, duration of hospitalisation, and mortality rate. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies which contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), employing GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary. Where it was not possible to carry out a meta‐analysis, we summarised the data for each trial.
Subgroup analysis and investigation of heterogeneity
If we identify more trials for inclusion in future updates of this review, we will attempt to conduct subgroup analyses and investigate sources and causes of heterogeneity. Possible subgroup analyses would involve different dose schedules of vitamin D (low or high dose, single bolus or daily dose, oral or parenteral route, duration < 5 or > 5 days); population (aged up to 1 year or 1 to 5 years, boys or girls); study setting (hospital or community, outpatient or inpatient, low‐, middle‐, or high‐income countries); aetiology (bacterial or viral pneumonia); and severity of illness (pneumonia, severe, or very severe pneumonia).
Sensitivity analysis
In future updates as more data become available, we will carry out sensitivity analysis to explore the effect of trial quality on results. This will involve analyses excluding studies at risk of bias (selection, performance, detection, attrition, or reporting) to assess for any substantive difference to the overall result. We will investigate the effects of the randomisation unit (individual versus cluster) on the outcomes. We will explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity, and the effects of any assumptions made such as the value of the intracluster correlation coefficient (ICC) used for cluster‐randomised trials. We will use primary outcomes in sensitivity analyses.
Results
Description of studies
Results of the search
Our searches in July 2017 identified 285 records, of which two were ongoing trials. After removal of 86 duplicate records, we screened 199 records by title and abstract. We excluded 191 records that did not match our inclusion criteria. We obtained eight full‐text studies for assessment. We included seven studies (Choudhary 2012; Dhungel 2015; Gupta 2016a; Manaseki‐Holland 2010; Rahmati 2016; Rajshekhar 2016; Somnath 2017), and excluded one study that did not meet our inclusion criteria (Zhou 2016). We identified two ongoing trials (NCT02054182; NCT02185196). See Figure 1.
1.
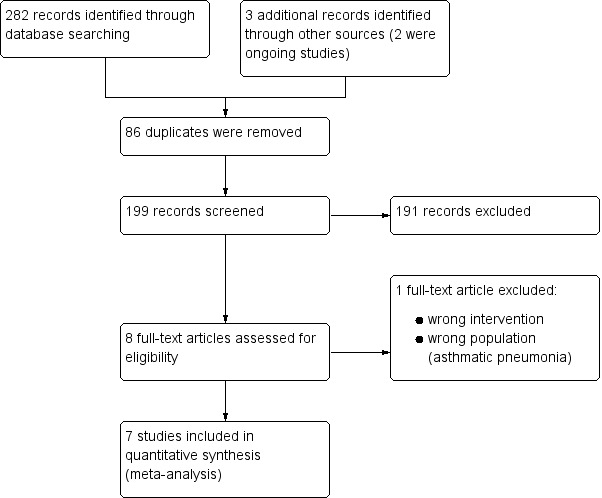
Study flow diagram.
Included studies
See Characteristics of included studies.
We included seven studies involving a total of 1529 children (780 with pneumonia and 749 with severe or very severe pneumonia) (Choudhary 2012; Dhungel 2015; Gupta 2016a; Manaseki‐Holland 2010; Rahmati 2016; Rajshekhar 2016; Somnath 2017). None of the included studies were conducted in high‐income countries. Childrens' characteristics, aetiological agents, and prevalence of vitamin D deficiency appeared to be very similar among studies. The studies differed in terms of inclusion criteria and dose and duration of vitamin D use. One study was funded by the New Zealand Aid Corporation; one was funded by an institutional grant; and five studies were unfunded.
Choudhary 2012 was a single‐centre study conducted in India. The study objective was to determine the role of oral vitamin D supplementation for resolution of severe pneumonia in children. The study enrolled 200 children (100/100 intervention/control) aged between two months and five years, who were hospitalised with WHO‐defined severe pneumonia. History of recurrent pneumonia was present in 30% of children in the vitamin D group and 33% of children in the control group. Five children had clinical evidence of rickets. A third of the children had wheezing at enrolment.
Children in the intervention arm received oral vitamin D₃ for five days at doses of 1000 IU for children aged up to one year and 2000 IU for children aged over one year. Children in the placebo arm received lactose alone. The children received antibiotics in accordance with the Indian Academy of Pediatrics (IAP) 2006 guidelines (IAP 2006). The guidelines recommend: infants aged up to three months: cefotaxime/ceftriaxone ± gentamicin/amikacin as first‐line therapy and co‐amoxyclav + gentamicin/amikacin as second‐line treatment; children aged from three months to five years: ampicillin/chloramphenicol or ampicillin + chloramphenicol or co‐amoxyclav as first‐line therapy and co‐amoxyclav or cefotaxime/ceftriaxone as second‐line treatment. In children with staphylococcal infection, adjuvant therapy with cloxacillin as first‐line therapy and vancomycin/teicoplanin as second‐line therapy are to be added to age‐specific treatment combinations.
Primary study outcomes assessed were time to resolution of severe pneumonia (absence of lower chest indrawing, hypoxia, cyanosis, lethargy and inability to feed). Secondary outcomes assessed were duration of hospitalisation, time to resolution of tachypnoea, chest retraction, and inability to feed.
Dhungel 2015 was a single‐centre study conducted in Pakistan. The study objective was to determine if supplementation of oral vitamin D₃ with antibiotics reduced duration of hospitalisation in children with pneumonia. The study enrolled 200 children (100/100, intervention/control) aged between two months and 60 months, who were hospitalised with WHO‐defined acute pneumonia. Among children randomised, severe pneumonia was present in 43%; the remainder had pneumonia. Children with very severe pneumonia and those with normal vitamin D levels were excluded. All children received antibiotics as per the hospital's policy (cefotaxime, ceftriaxone, ampiclox, clarithromycin, amikacin, vancomycin. The antibiotics were given as monotherapy or combination therapy).
Children in the intervention arm received a single dose (100,000 IU) of intramuscular vitamin D₃ within 24 hours of admission. Children in the control arm received antibiotics alone. Study outcomes assessed were duration of hospitalisation and repeat episodes of pneumonia.
Gupta 2016a was a single‐centre study conducted in India. The study objective was to determine the role of oral vitamin D supplementation for the treatment and prevention of pneumonia in children. The study enrolled 324 children (162/162, intervention/control) aged between six months and five years, who were hospitalised with WHO‐defined severe pneumonia. The prevalence of moderate malnutrition according to the WHO definition was 21.3%. The prevalence of anaemia (haemoglobin < 11 g/dL) was 82.4%; hypocalcaemia was 55.9%; hypophosphataemia was 31.4%. Vitamin D deficiency was present in 37.6% of children in the vitamin D group and 40.1% of children in the placebo group. Blood culture was positive in 8.6% (Staphylococcus aureus isolated in 8.3%). Around 90.1% of children had abnormal chest x‐ray findings (consolidation, bilateral patchy opacities and hyperinflation or minor infiltrates). Wheezing was present in 84.6% of children in the vitamin D group and 78.4% of children in the control group.
Children in the intervention arm received a single oral dose of vitamin D₃ of 100,000 IU on the day of enrolment. Children in the placebo arm received a product similarly prepared and given as a single dose on day of enrolment. The children received antibiotics according to IAP 2006 guidelines (IAP 2006) (as specified for the study by Choudhary 2012.
Primary study outcomes assessed were time to resolution of severe pneumonia (duration from enrolment to chest indrawing was no longer present and maintained for 24 hours) and proportion of children with recurrent pneumonia over the next six months. Secondary outcomes assessed were change in serum level 25(OH)‐D and parathyroid hormone after two weeks and three months of therapy; change in serum level of cathelicidin and immunoglobulins after two weeks of therapy; duration of hospitalisation; time to complete recovery from pneumonia (normalisation of respiratory rate), fever clearance time; and repeat episodes of pneumonia.
Manaseki‐Holland 2010 was a single‐centre study conducted in Afghanistan. The study objective was to determine if supplementation of oral vitamin D₃ with antibiotics reduced duration of illness in children with pneumonia. The study enrolled 453 children (224/229, intervention/control) aged between one month and 36 months with WHO‐defined acute pneumonia treated in outpatient and inpatient settings. Among children randomised, severe pneumonia was present in 17.4% of intervention group and 15.3% of placebo group children; the remaining children had pneumonia. The study excluded children with very severe pneumonia. All children received antibiotics as per the Integrated Management of Childhood Illness (IMCI) guidelines (children aged up to two months: intramuscular gentamicin and intramuscular benzylpenicillin; children aged from two months to five years: oral amoxicillin or intramuscular chloramphenicol (if unable to take orally)).
We included this study after reaching consensus on the following points: although Manaseki‐Holland 2010 did not comply with all predefined inclusion criteria (not all children were treated as inpatients), we decided to include the study because some study participants had pneumonia. We felt that exclusion of the study (which included children with pneumonia and severe pneumonia) may have introduced bias.
Children in the intervention arm received a single dose (100,000 IU) of oral vitamin D₃ at onset of pneumonia. Children in the placebo arm received olive oil alone. All children received antibiotics according to the national pneumonia treatment protocol based on IMCI guidelines.
Study outcomes assessed were time to resolution of pneumonia or recovery for 48 consecutive hours, treatment failure, and pneumonia recurrence.
Rahmati 2016 was a single‐centre study conducted in Iran. The study objective was to determine the role of oral vitamin D supplementation using the respiratory index of severity in children hospitalised with community‐acquired pneumonia. The study enrolled 100 children (50/50, intervention/placebo) aged between two months and six years who were hospitalised with pneumonia. Pneumonia was defined as the presence of fever, cough, audible findings in lungs (rale), respiratory distress symptoms, and chest x‐ray changes. No children had severe pneumonia.
Children in the intervention arm received oral vitamin D₃ 50,000 IU per day for two days. Children in the placebo arm received olive oil. Children may have received ceftriaxone as the only antibiotic. We contacted the study authors to confirm that all children received ceftriaxone, but received no response (Das 2017). Primary study outcomes assessed were duration of antibiotic therapy, hospitalisation, and duration of fever.
Rajshekhar 2016 was a single‐centre study conducted in India. The study objective was to determine the role of oral vitamin D supplementation for resolution of severe pneumonia. The study enrolled 96 children (48/48, intervention/control) aged between two months and five years who were hospitalised with WHO‐defined severe pneumonia. History of recurrent pneumonia was present in 35.4% of children in the vitamin D group and 39.6% of children in the control group.
Children in the intervention arm received oral vitamin D₃ for five days at doses of 1000 IU for children aged up to one year and 2000 IU for children aged over one year. Children in the placebo arm received lactose alone. The children received antibiotics as per the IAP 2012 guidelines (amoxycillin, amoxyclav, third‐generation parenteral cephalosporins, and macrolides) (Ghosh 2012).
Study outcomes assessed were time to resolution of severe pneumonia (absence of lower chest indrawing, hypoxia, cyanosis, and inability to feed).
Somnath 2017 was a single‐centre study conducted in India. The study objective was to determine the role of oral vitamin D supplementation on pneumonia in children. The study enrolled 156 children (78/78, intervention/placebo) aged between two months and five years who were hospitalised with WHO‐defined pneumonia. Very severe pneumonia was present in 12.8% (N = 10) of children in the vitamin D group and 14.3% (N = 11) of children in the control group.
Children in the intervention arm received a single dose of oral vitamin D₃ 100,000 IU administered on Day 1 of admission as cholecalciferol granules mixed with water or milk. Children in the placebo arm received standard therapy. The children received antibiotics according to practice guidelines at the study centre. The primary study outcome assessed was duration of hospitalisation, and secondary outcomes were time to resolution of fever, complications associated with pneumonia, need for transfer to the paediatric intensive care unit, mortality, and pneumonia recurrence in the next six months.
Excluded studies
We excluded one study (Zhou 2016). This study supplemented nutrients (vitamin A, vitamin D, zinc, and iron) for children with pneumonia who were nutritionally deficient for these vitamins and minerals. Moreover, the study included children with asthmatic pneumonia.
Risk of bias in included studies
We considered overall risk of bias to be low for four studies (Choudhary 2012; Gupta 2016a; Manaseki‐Holland 2010; Somnath 2017), and unclear for three (Dhungel 2015; Rahmati 2016; Rajshekhar 2016). See Figure 2 and Figure 3. We contacted the authors of studies by Rajshekhar 2016 and Rahmati 2016 respectively for clarification regarding methods of blinding and allocation concealment and clarification of interventions used (Das 2017; Rajshekhar 2016 [pers comm]).
2.
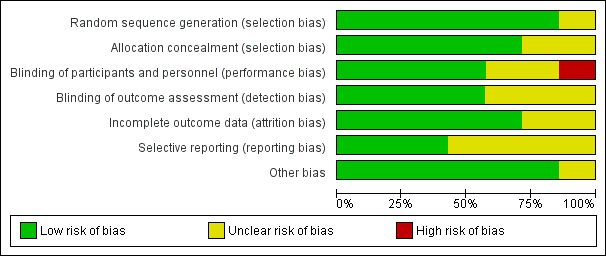
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
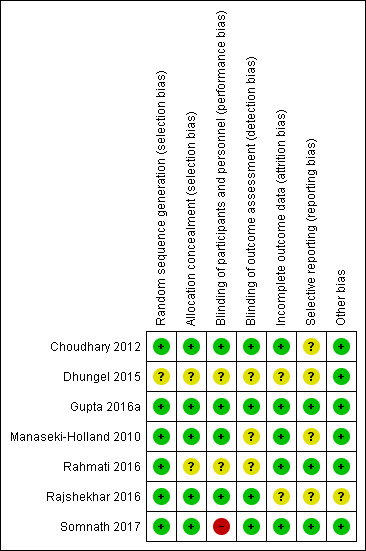
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five studies used sequentially numbered, sealed envelopes or codes for allocation of participants to the two groups and were assessed as at low risk of bias (Choudhary 2012; Gupta 2016a; Manaseki‐Holland 2010; Rajshekhar 2016; Somnath 2017). Two studies did not mention the method of allocation and were assessed as at unclear risk of bias (Dhungel 2015; Rahmati 2016).
Blinding
In four studies blinding of participants and carers was conducted, and it was unlikely that the blinding could have been broken as both interventions and placebos were similar in terms of appearance, taste, and colour (Choudhary 2012; Gupta 2016a; Manaseki‐Holland 2010; Rajshekhar 2016). In three studies the code key was opened only after administration of the intervention, data collection, follow‐up, and tabulation were completed, thus preventing detection bias (Choudhary 2012; Gupta 2016a; Rajshekhar 2016); these studies were assessed as at low risk of bias for this domain. Dhungel 2015 and Manaseki‐Holland 2010 did not describe methods to prevent detection bias and were assessed as at unclear risk of bias. Participants and personnel in Somnath 2017 were unblinded, presenting a high risk of performance bias, however outcome assessment was blinded, presenting a low risk of detection bias.
Incomplete outcome data
We assessed five studies as at low risk of bias for this domain (Choudhary 2012; Gupta 2016a; Manaseki‐Holland 2010; Rahmati 2016; Somnath 2017). Choudhary 2012 reported an attrition rate of 4.5%; Gupta 2016a reported an attrition rate of 4.63%; Manaseki‐Holland 2010 reported an attrition rate of 3%; Rahmati 2016 reported no attrition; and Somnath 2017 reported an attrition rate of 1.3% before outcomes were measured. Manaseki‐Holland 2010 and Gupta 2016a analysed data using the intention‐to‐treat principle. Dhungel 2015 and Rajshekhar 2016 did not provide this information and were assessed as at unclear risk of bias.
Selective reporting
We assessed three studies as at low risk of reporting bias, as they were registered in the clinical trial registry (Gupta 2016a; Rahmati 2016; Somnath 2017). For the remaining four studies, protocols were neither available nor were they registered in clinical trials registries, therefore these studies were assessed as at unclear risk of bias.
Other potential sources of bias
There were no other potential sources of bias in the included studies.
Effects of interventions
See: Table 1
Primary outcomes
1. Time to resolution of acute illness
Four studies reported time to resolution of acute illness (hours) (Choudhary 2012; Gupta 2016a; Manaseki‐Holland 2010; Rajshekhar 2016). Data were provided as mean and standard deviation (SD) in Manaseki‐Holland 2010 and Gupta 2016a, and as median and interquartile range (IQR) in Choudhary 2012. We contacted the corresponding author of Choudhary 2012 to request data in mean and SD, but these data were unavailable (Gupta 2016b). We therefore calculated the corresponding mean and SD as per the available statistical methods (Hozo 2005; Luo 2018; Wan 2014). The pooled result from three studies showed no significant difference for time to resolution of acute illness (mean difference (MD) ‐0.95, 95% confidence interval (CI) ‐6.14 to 4.24; N = 935; low‐quality evidence; Analysis 1.1) (Choudhary 2012; Gupta 2016a; Manaseki‐Holland 2010). Rajshekhar 2016 presented data as proportions for three time periods (24 hours, 24 to 48 hours, and > 48 hours); there was no difference between the vitamin D and control group in the time to resolution of severe pneumonia.
1.1. Analysis.
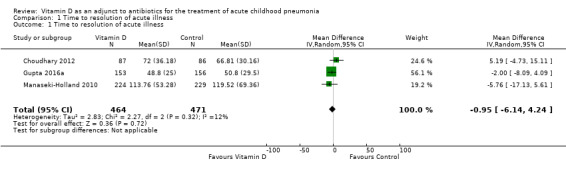
Comparison 1 Time to resolution of acute illness, Outcome 1 Time to resolution of acute illness.
2. Duration of hospitalisation
Five studies reported duration of hospitalisation (hours) (Choudhary 2012; Dhungel 2015; Gupta 2016a; Rahmati 2016; Somnath 2017). Data were provided as mean and SD in Dhungel 2015 and Gupta 2016a, and as median and IQR in Choudhary 2012 and Somnath 2017. We contacted the corresponding author of Choudhary 2012 to request data in mean and SD, but these data were unavailable (Gupta 2016b). We calculated the corresponding mean and SD as per the available statistical methods (Hozo 2005; Luo 2018; Wan 2014). In Rahmati 2016, the duration of hospitalisation was shorter in the intervention group compared to the control group (441.6 hours versus 535.2 hours; P < 0.05). Because the duration was too long for a pneumonia episode, we contacted the corresponding author to seek clarification, but did not receive a response (Das 2017). The pooled result from four studies (except Rahmati 2016) showed no significant difference in duration of hospitalisation (MD 0.49, 95% CI ‐8.41 to 9.40; N = 835; very low‐quality evidence; Analysis 2.1).
2.1. Analysis.
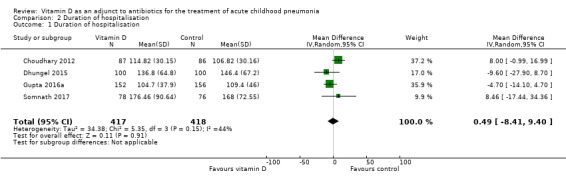
Comparison 2 Duration of hospitalisation, Outcome 1 Duration of hospitalisation.
Secondary outcomes
Choudhary 2012 reported the following outcomes for children with severe pneumonia as median and IQR. We contacted the corresponding author of Choudhary 2012 to request data in mean and SD, but these data were unavailable (Gupta 2016b). We therefore calculated the corresponding mean and SD as per the available statistical methods (Hozo 2005; Luo 2018; Wan 2014). We assessed the quality of the evidence for all outcomes as very low.
1a. Time to resolution of tachypnoea
The pooled result showed no significant difference in the time to resolution of tachypnoea (MD 4.93, 95% CI ‐6.15 to 16.01; N = 171; very low‐quality evidence; Analysis 3.1).
3.1. Analysis.
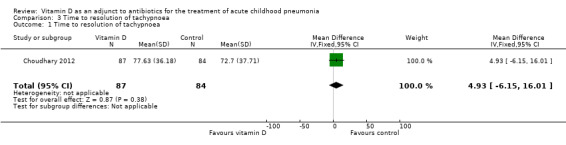
Comparison 3 Time to resolution of tachypnoea, Outcome 1 Time to resolution of tachypnoea.
1b. Time to resolution of lower chest wall indrawing
The time to resolution of lower chest indrawing was the same in both groups (MD 0.00, 95% CI ‐10.78 to 10.78; N = 173; very low‐quality evidence; Analysis 4.1).
4.1. Analysis.
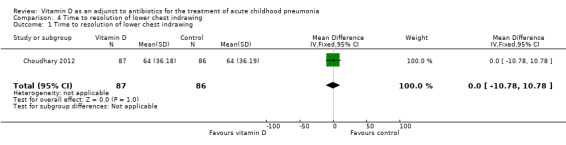
Comparison 4 Time to resolution of lower chest indrawing, Outcome 1 Time to resolution of lower chest indrawing.
1c. Time to resolution of hypoxia
The time to resolution of hypoxia was the same in both groups (MD 0.00, 95% CI ‐3.59 to 3.59; N = 173; very low‐quality evidence; Analysis 5.1).
5.1. Analysis.
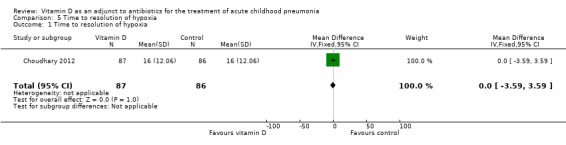
Comparison 5 Time to resolution of hypoxia, Outcome 1 Time to resolution of hypoxia.
1d. Time to resolution of fever
Four studies reported time to resolution of fever (hours) (Choudhary 2012; Gupta 2016a; Rahmati 2016; Somnath 2017). The pooled result showed no significant difference between groups in time to resolution of fever (MD 1.66, 95% CI ‐2.44 to 5.76; N = 584; very low‐quality evidence; Analysis 6.1).
6.1. Analysis.
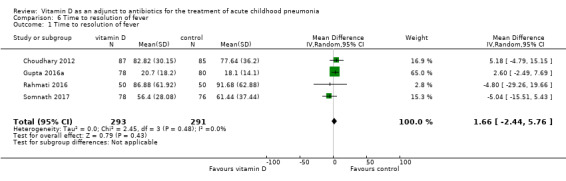
Comparison 6 Time to resolution of fever, Outcome 1 Time to resolution of fever.
1e. Time to resolution of inability to feed or lethargy
The pooled result showed a significantly lesser time to resolution of inability to feed or lethargy in the control group (MD 8.00, 95% CI 0.60 to 15.40; N = 173; very low‐quality evidence; Analysis 7.1).
7.1. Analysis.
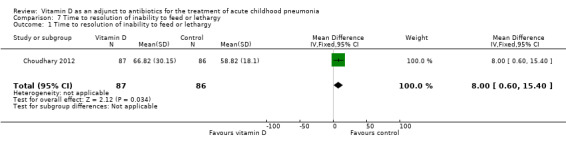
Comparison 7 Time to resolution of inability to feed or lethargy, Outcome 1 Time to resolution of inability to feed or lethargy.
2. Treatment failure rate
Although the failure rate after 48 hours of treatment was not specified, Choudhary 2012 reported that 23 (11.5%) children remained in the same condition, and four (2%) children had their condition worsen. There was no description of the groups or the time period. Given that data for this outcome were from a single study, we assessed the quality of evidence as very low.
3. Mortality rate
Two studies reported mortality (Choudhary 2012; Somnath 2017). Choudhary 2012 reported that two children died, one each in the vitamin D and placebo groups. There was no significant difference between intervention and placebo groups (risk ratio 0.97, 95% CI 0.06 to 15.28; N = 193; very low‐quality evidence; Analysis 8.1). Somnath 2017 reported no deaths in either group.
8.1. Analysis.
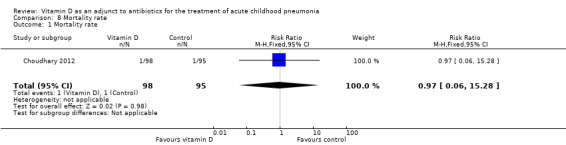
Comparison 8 Mortality rate, Outcome 1 Mortality rate.
Manaseki‐Holland 2010 reported three deaths (two in the vitamin D group and one in the placebo group) during the 90‐day follow‐up evaluation of repeated episodes of pneumonia. Because it was not clear if the children died during treatment of the index episode of pneumonia, we contacted the corresponding author (Manaseki‐Holland 2010 [pers comm]). It was confirmed that the children died during the 90‐day follow‐up period (not during the index episode). Accordingly, we did not include mortality data from Manaseki‐Holland 2010 in the analysis.
4. Adverse events
Choudhary 2012 reported two minor events: one child experienced vomiting and another had diarrhoea in the vitamin D group. Given that data for this outcome were from a single study, we assessed the quality of evidence as very low.
Discussion
Summary of main results
We included seven studies involving a total of 1529 children (780 with pneumonia and 749 with severe or very severe pneumonia) (Choudhary 2012; Dhungel 2015; Gupta 2016a; Manaseki‐Holland 2010; Rahmati 2016; Rajshekhar 2016; Somnath 2017). None of the included studies were conducted in high‐income countries. Childrens' characteristics, aetiological agents, and prevalence of vitamin D deficiency appeared to be very similar among studies. The studies differed in terms of inclusion criteria and dose and duration of vitamin D use. One study was funded by the New Zealand Aid Corporation; one was funded by an institutional grant; and five studies were unfunded.
With the exceptions of time to resolution of acute illness, duration of hospitalisation, time to resolution of fever, and mortality rate, results could not be pooled; all secondary outcomes were reported by Choudhary 2012. Gupta 2016a, Rahmati 2016, and Somnath 2017 provided data regarding duration of fever only. The appropriate effect measure for time‐to‐event data (time to resolution of acute illness) is hazard ratio, but the studies reported this information as continuous data. Except for Rahmati 2016, none of the studies reported vitamin D benefits for children with acute pneumonia. Dhungel 2015 and Manaseki‐Holland 2010 included children with both non‐severe and severe pneumonia, but neither study reported outcomes separately for children with severe pneumonia. Though mortality rate is an important outcome for policy decisions, this outcome was reported in only two studies (Choudhary 2012; Somnath 2017). See Table 1.
We questioned whether vitamin D was ineffective in treating children with acute pneumonia due to real therapeutic ineffectiveness of vitamin D, or improper dose or mode of administration, or type or severity of the underlying illness. These are important aspects needing investigation in future studies.
The included studies differed significantly in vitamin D dose. Two studies administered oral vitamin D in a total dose of 5000 IU to 10,000 IU according to the child's age, over five days (Choudhary 2012; Rajshekhar 2016). Four studies administered a single bolus dose of 100,000 IU either at the onset of illness or within 24 hours of admission (Dhungel 2015; Gupta 2016a; Manaseki‐Holland 2010; Somnath 2017). Rahmati 2016 administered 50,000 IU per day for two days. As discussed previously, a daily vitamin D supplementation has better therapeutic efficacy than large bolus doses for pneumonia, tuberculosis, and fracture in the elderly (Heaney 2012; Hollis 2011; Manaseki‐Holland 2012; Martineau 2012; Nielsen 2010). Clinical studies have shown that at a higher dose, vitamin D could be immunosuppressive, and the immunological effectiveness of vitamin D is blunted at extremes of dosages (either a very high or a low therapeutic dose) (Kimball 2011; Manaseki‐Holland 2010; Manaseki‐Holland 2012). This was also supported by the findings of Choudhary 2012, who used a daily dose of 1000 IU to 2000 IU per day of vitamin D, but did not document any benefit. In Choudhary 2012, a low dose might possibly have been used considering the high prevalence of vitamin D deficiency along with severe illness (severe pneumonia) in the studied population. The ideal situation would have been correlation with vitamin D levels measured before and after supplementation. Two studies reported vitamin D level both pre‐ and post supplementation, but failed to show any benefit of vitamin D (Gupta 2016a; Somnath 2017).
The National Institutes of Health defines no observed adverse effect level (2400 IU) and lowest observed adverse effect level (3800 IU) of vitamin D (NIH Vitamin D Fact Sheet). Future trials may consider use of higher doses with monitoring of toxicity and serum vitamin D level. It is premature to hypothesise about the real therapeutic ineffectiveness of vitamin D because clinical trials are ongoing and results are not yet available.
Although the included studies were conducted in low‐income countries (Afghanistan, Pakistan, Iran, and India), prevalence of malnutrition (moderate or grade II) was reported in only two studies (Dhungel 2015; Gupta 2016a). However, these two studies did not report the prevalence of malnutrition in either vitamin D or control groups, leading to difficulty in correlating the effect of vitamin D in malnourished children. This is an important aspect because underlying malnutrition may affect the state of immunity and hence blunt the vitamin D effect. Malnutrition also lowers serum vitamin D level. Furthermore, severe pneumonia may establish a systemic inflammatory response in the host that causes a decrease in the serum vitamin D level (Bang 2011). It would therefore be prudent to study the level of inflammatory markers along with vitamin D levels in children with non‐severe/severe pneumonia.
None of the included studies reported the aetiology of pneumonia. This is important for any differential therapeutic effect of vitamin D (if any) in bacterial or viral pneumonia. Because there is a seasonal variation in the predominance of aetiological agents (e.g. viruses dominating over bacteria in the rainy season), any trial conducted over a year would be meaningful. This is also important because vitamin D levels may decrease during winter and the rainy season due to less sun exposure. One study was conducted over a 29‐month period (Gupta 2016a); two were conducted over a 12‐month period (Rahmati 2016; Somnath 2017); two were conducted over a three‐month period (Dhungel 2015; Manaseki‐Holland 2010); and two did not mention the study duration (Choudhary 2012; Rajshekhar 2016).
Viral pneumonia is commonly associated with wheezing. Manaseki‐Holland 2010 and Rahmati 2016 excluded children with wheezing, but almost a third of children in Choudhary 2012 and more than 80% of children in Gupta 2016a had wheezing. This finding was not reported by the other three included studies (Dhungel 2015; Rajshekhar 2016; Somnath 2017). In Choudhary 2012 and Rajshekhar 2016 almost a third of children in each study arm had recurrent pneumonia, which indicates some underlying immune dysfunction or other problem predisposing to recurrence. Vitamin D alone may not correct this, resulting in no observable clinical effect or benefit, or both. Outcome measures may also have been affected by the timing of vitamin D administration in the course of pneumonia (early versus late). Any information regarding the duration of illness prior to initiating vitamin D would be meaningful and should be included in future studies. Additionally, information regarding prior antibiotic use should be included because this can affect recovery from illness. Unfortunately, this information was not provided in any of the studies. Similarly, a large proportion of children needing second‐line or broader spectrum antibiotics due to severe illness would lead to a reduction in study power, making it difficult to detect any difference attributable to vitamin D.
Overall completeness and applicability of evidence
We included seven studies, but could pool results for three outcomes (time to resolution of illness, duration of hospitalisation, and mortality rate); most outcomes were reported in one study. None of the included studies showed any beneficial effect of vitamin D for children with acute pneumonia. Our results may not be applicable to children with acute pneumonia in high‐income countries, those with HIV or severe malnutrition, and neonates (aged up to a month). Two large ongoing trials may provide additional evidence.
Quality of the evidence
We considered the overall risk of bias to be low in four studies (Choudhary 2012; Gupta 2016a; Manaseki‐Holland 2010; Somnath 2017), and unclear in three studies (Dhungel 2015; Rahmati 2016; Rajshekhar 2016). The GRADE assessment of the evidence was very‐low quality for all outcomes except time to resolution of acute illness (low‐quality evidence). See Table 1. Our assessment of low quality was due to no serious study limitations (all studies had adequately concealed allocation and blinding), no serious inconsistency (low and non‐significant statistical heterogeneity), serious indirectness (because of non‐generalisation of study findings to all situations, and differing characteristics of the study population), and serious imprecision (wide 95% CI around the pooled effect, inclusion of no effect, and the upper or lower confidence limit crossed the minimal important difference for benefit). Our assessment of very low quality was due to serious study limitations (one study had unclear allocation concealment and blinding), no serious inconsistency (no statistical heterogeneity), serious indirectness (because of non‐generalisation of study findings to all situations, and differing characteristics of the study population), and serious imprecision (wide 95% CI around the pooled effect, inclusion of no effect, and the upper or lower confidence limit crossed the minimal important difference for benefit).
Potential biases in the review process
All included studies measured the effect of vitamin D in childhood pneumonia. There was potential to miss studies that may have measured pneumonia as an acute respiratory tract infection or lower respiratory tract infection as a secondary outcome, under the broader scope of childhood infection. We aimed to avoid this scenario by conducting a wide search and carefully assessing the relevance of each paper identified. We were unable to assess aetiological agents (bacterial or viral) to inform reporting of any beneficial effects of vitamin D as none of the included studies reported these data. Furthermore, because serum vitamin D levels were not measured, we could not suggest the dose‐response effect in children with acute pneumonia.
Agreements and disagreements with other studies or reviews
We published an earlier systematic review on this topic (Das 2013), and included two studies in this review (Choudhary 2012; Manaseki‐Holland 2010). The conclusions are the same.
Authors' conclusions
Implications for practice.
We are uncertain as to whether oral vitamin D supplementation as an adjunct for the treatment of children aged under five years with acute pneumonia has an important effect on outcomes. Low‐ to very low‐quality evidence was available from only seven studies. Future studies should focus on the limitations or weaknesses identified in this review so that good‐quality evidence can be generated.
Implications for research.
Future studies may consider use of a higher dosage schedule of vitamin D along with monitoring of toxicity and serum vitamin D level. Baseline and post‐treatment vitamin D levels should be measured to correlate the outcome measures with subclinical or clinical vitamin D deficiency. Prevalence of malnutrition should be reported in future trials, as this may affect the therapeutic effect of vitamin D independently of the illness.
Future studies should also report the level of inflammatory markers along with vitamin D levels in children affected with acute non‐severe or severe pneumonia. The aetiology (bacterial or viral or atypical) of pneumonia should be studied, and studies should ideally be conducted over a year to include all seasons, because vitamin D levels may be affected by seasonal change, as is the aetiology of pneumonia.
Any information regarding the duration of illness prior to initiation of therapy with vitamin D would be meaningful and should be included in future studies.
Information regarding prior antibiotic use should also be included because this can modify recovery from illness. This information should be included in the baseline data to enable comparison among studies.
Future studies should also include a subgroup of children with severe malnutrition or rickets or both and those with wheezing.
Acknowledgements
We acknowledge all the help and infrastructure provided by the All India Institute of Medical Sciences (AIIMS), Bhubaneswar and Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. We thank the following people for commenting on the draft protocol: Ann Fonfa, Denny John, Rakesh Lodha, Pankaj Shah, Viviana Rodriguez, and Michelle Guppy. We also wish to thank the following people for commenting on the draft review: Ruth Day, Janet Wale, Rakesh Lodha, Pankaj Shah, Mark Jones, and Michelle Guppy. Finally, we would also like to thank Mohamed Hassan and Sushil Agwan, medical students at Bond University, Australia, for checking the review for quality issues and consistency in reporting for the draft review.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy)
1 exp Pneumonia/ (75695) 2 (pneumon* or bronchpneumon* or pleuropneumon*).tw. (132976) 3 Respiratory Tract Infections/ (31796) 4 (lower respiratory tract infection* or lower respiratory infection* or lrti).tw. (5207) 5 or/1‐4 (189394) 6 exp Vitamin D/ (45162) 7 ("vitamin d" or "vit d").tw,nm. (44814) 8 ("vitamin d2" or "vitamin d3").tw,nm. (8508) 9 (ergocalciferol* or cholecalciferol* or calcitriol*).tw,nm. (24708) 10 or/6‐9 (59727) 11 5 and 10 (214)
Appendix 2. Embase (Elsevier) search strategy
| #13 | #11 AND #12 | 140 |
| #12 | 954414 | |
| #12.8 #12.3 NOT #12.7 954412 #12.7 #12.4 NOT #12.6 #12.6#12.4 AND #12.5 #12.5'human'/de AND [embase]/lim #12.4'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de AND [embase]/lim #12.3#12.1 OR #12.2 #12.2random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti AND [embase]/lim #12.1'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim |
||
| #11 | #5 AND #10 | 922 |
| #10 | #6 OR #7 OR #8 OR #9 | 96411 |
| #9 | ergocalciferol*:ab,ti OR cholecalciferol*:ab,ti OR calcitriol*:ab,ti AND [embase]/lim | 6851 |
| #8 | 'vitamin d2':ab,ti OR 'vitamin d3':ab,ti AND [embase]/lim | 3634 |
| #7 | 'vitamin d':ab,ti OR 'vit d':ab,ti AND [embase]/lim | 54129 |
| #6 | 'vitamin d'/exp AND [embase]/lim | 87300 |
| #5 | #1 OR #2 OR #3 OR #4 | 244145 |
| #4 | 'lower respiratory tract infection':ab,ti OR 'lower respiratory tract infections':ab,ti OR 'lower respiratory infection':ab,ti OR 'lower respiratory infections':ab,ti OR lrti:ab,ti AND [embase]/lim | 6451 |
| #3 | 'lower respiratory tract infection'/de AND [embase]/lim | 7631 |
| #2 | pneumon*:ab,ti OR pleuropneumon*:ab,ti OR bronchopneumon*:ab,ti AND [embase]/lim | 150505 |
| #1 | 'pneumonia'/exp AND [embase]/lim | 174841 |
Data and analyses
Comparison 1. Time to resolution of acute illness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of acute illness | 3 | 935 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐6.14, 4.24] |
Comparison 2. Duration of hospitalisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of hospitalisation | 4 | 835 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐8.41, 9.40] |
Comparison 3. Time to resolution of tachypnoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of tachypnoea | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | 4.93 [‐6.15, 16.01] |
Comparison 4. Time to resolution of lower chest indrawing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of lower chest indrawing | 1 | 173 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐10.78, 10.78] |
Comparison 5. Time to resolution of hypoxia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of hypoxia | 1 | 173 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.59, 3.59] |
Comparison 6. Time to resolution of fever.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of fever | 4 | 584 | Mean Difference (IV, Random, 95% CI) | 1.66 [‐2.44, 5.76] |
Comparison 7. Time to resolution of inability to feed or lethargy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of inability to feed or lethargy | 1 | 173 | Mean Difference (IV, Fixed, 95% CI) | 8.00 [0.60, 15.40] |
Comparison 8. Mortality rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality rate | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choudhary 2012.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Study duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding source: not funded by any funding agency Other: microbiological and radiological diagnosis of pneumonia was not done. Serum vitamin D₃ level was not measured. Contact with study authors: email: 24 April 2016. Data were reported in median and IQR; we requested data in mean and SD, but this information was unavailable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and caretaker was ensured and unlikely that the blinding could have been broken, as both the intervention and placebo looked alike in terms of appearance, taste, and colour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code key was opened only after the intervention, data collection, follow‐up, and tabulation were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.5% (9/200) children lost to follow‐up or died |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered, so this was unclear, but all outcomes proposed in the methods section were reported. |
| Other bias | Low risk | No other known risks of bias |
Dhungel 2015.
| Methods |
Study design: randomised controlled trial Study duration: 3 months |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding source: not funded by any funding agency Other: microbiological diagnosis of pneumonia was not done. Only 4% children had normal chest x‐ray. Serum vitamin D₃ level was measured and those with normal level (> 20 ng/mL) were excluded. No report of adverse events or mortality. Contact with study authors: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered, so this was unclear, but all outcomes proposed in the methods section were reported. |
| Other bias | Low risk | No other known risks of bias |
Gupta 2016a.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Study duration: 29 months |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding source: not funded by any funding agency Other: blood culture was positive in 8.6% (Staphylococcus aureus isolated in 8.3%), and 90.1% of children abnormal chest x‐ray in the form of consolidation, bilateral patchy opacities, and hyperinflation or minor infiltrates. Contact with study authors: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and caretaker was ensured and unlikely that the blinding could have been broken, as both the intervention and placebo looked alike in terms of appearance, taste, and colour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code key was opened only after the intervention, data collection, follow‐up, and tabulation were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.63% (15/324) children lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The trial was registered. |
| Other bias | Low risk | No other known risks of bias |
Manaseki‐Holland 2010.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Study duration: 2 months |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding source: funded by the New Zealand Aid Corporation. The study medications were provided by the pharmacy department of Aga Khan University Hospital, Karachi. Other: microbiological and radiological diagnosis of pneumonia was not done. Serum vitamin D₃ level was not measured. The trial reported 3 deaths (2 in vitamin D group and 1 in placebo group) during the 90‐day follow‐up evaluation of repeat episodes of pneumonia. Contact with study authors: email: 4 May 2017. Because it was not clear whether the children died including during the treatment of the index episode of pneumonia, we contacted the corresponding author, and her opinion was that the children died during the 90‐day follow‐up (not during the index episode). Mortality data were therefore not used in the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence was generated in an Excel spreadsheet with no restrictions. |
| Allocation concealment (selection bias) | Low risk | Sealed codes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and caretaker was ensured and unlikely that the blinding could have been broken, as both the intervention and placebo looked alike in terms of appearance, taste, and colour. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% children lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered, so this was unclear, but all outcomes proposed in the methods section were reported. |
| Other bias | Low risk | No known other risks |
Rahmati 2016.
| Methods |
Study design: randomised controlled trial Study duration: 12 months |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Primary
|
|
| Notes |
Funding source: not funded by any funding agency Other: microbiological diagnosis of pneumonia was not done. Serum vitamin D₃ level was not measured. No report of adverse events or mortality. Contact with study authors: emails: 8 November 2017 and 15 November 2017. We requested that the authors clarify the outcomes on duration of antibiotic therapy and duration of hospitalisation as well as the reporting measure (day or hour) for duration of patient admission. No response received before 10 January 2018. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs |
| Selective reporting (reporting bias) | Low risk | The trial was registered. |
| Other bias | Low risk | No other known risks |
Rajshekhar 2016.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Study duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Primary
|
|
| Notes |
Funding source: not funded by any funding agency Other: microbiological and radiological diagnosis of pneumonia was not done. Serum vitamin D₃ level was not measured. Meyer Organics Private Ltd. was involved in the randomisation process, allotment of study and placebo medications. Contact with study authors: email: 3 June 2017. Information on study methodology was sought and the authors provided the same. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation by a third party |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelope was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and caretaker was ensured and unlikely that the blinding could have been broken, as both the intervention and placebo looked alike in terms of appearance, taste, and colour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code key was disclosed only after the intervention, data collection, follow‐up, and tabulations. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered, so this was unclear, but all outcomes proposed in the methods section were reported. |
| Other bias | Unclear risk | No other known risks |
Somnath 2017.
| Methods |
Study design: randomised controlled trial Study duration: 12 months |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding source: institute (JIPMER) Intramural Grant Other: microbiological diagnosis of pneumonia was not done. Only 7% of study population had normal serum, and 71% were deficient range. No report of adverse events or mortality Contact with study authors: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelope was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.3% (2/156) children lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The trial was registered. |
| Other bias | Low risk | No other known risks |
ALRI: acute lower respiratory tract infection f: female Hb: haemoglobin IAP: Indian Academy of Pediatrics Ig: immunoglobulin IQR: interquartile range m: male PTH: parathyroid hormone PICU: paediatric intensive care unit SD: standard deviation TB: tuberculosis 25(OH)‐D: 25‐hydroxy‐vitamin D WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Zhou 2016 | Multiple nutrients (iron, zinc, vitamin A, and vitamin D) were supplemented in children with nutrient deficiency/malnutrition and pneumonia. The trial included children with asthmatic pneumonia. |
Characteristics of ongoing studies [ordered by study ID]
NCT02054182.
| Trial name or title | Effect of vitamin D supplementation in young South African children hospitalised with acute lower respiratory infection |
| Methods | RCT |
| Participants | Children aged from 1 month to 5 years, admitted to hospital with acute lower respiratory tract infection, i.e. bronchiolitis or pneumonia or both |
| Interventions | Vitamin D: 2500 IU daily from enrolment until hospital discharge |
| Outcomes | Primary: change from baseline in modified Respiratory Distress Assessment Instrument score at hospital discharge between intervention and placebo groups Secondary: duration of hospitalisation |
| Starting date | February 2014 |
| Contact information | Winter‐Rose S Nkosi, Univeristy of Limpopo, Medunsa Campus, czawr@webmail.co.za |
| Notes | ClinicalTrials.gov identifier: NCT02054182 |
NCT02185196.
| Trial name or title | Vitamin D supplementation: impact on severe pneumonia among under 5 children |
| Methods | RCT |
| Participants | Children of either sex aged 3 to 59 months, with a clinical diagnosis of severe pneumonia with or without diarrhoea |
| Interventions | Vitamin D₃: 20,000 IU vitamin D₃ in children < 6 months; 50,000 IU in children 6 to 12 months; and 100,000 IU in children 13 to 59 months of age on first day and 10,000 IU for next 4 days Placebo: 20,000 IU Miglyol‐oil in children < 6 months; 50,000 IU in children 6 to 12 months; and 100,000 IU in children 13 to 59 months of age on first day and 10,000 IU for next 4 days |
| Outcomes | Primary: time taken for recovery from severe pneumonia Secondary: duration of hospitalisation, time taken for normalisation of temperature and respiratory rate, time taken for recovery from chest indrawing, time taken for oxygen saturation, feeding, and mental status to normalise, proportion of study children who will develop new episodes of pneumonia during the follow‐up period |
| Starting date | June 2014 |
| Contact information | Fahmida Chowdhury, International Centre for Diarrhoeal Disease Research, Bangladesh, fahmida_chow@icddrb.org |
| Notes | ClinicalTrials.gov identifier: NCT02185196 |
RCT: randomised controlled trial
Differences between protocol and review
In the protocol, we indicated that if we found cluster‐randomised trials for inclusion, we would adjust for clustering by intracluster correlation coefficient. However, we did not identify any cluster‐randomised trials for inclusion.
We also indicated that we would perform additional analyses (subgroup analysis, and sensitivity analysis). However, there were insufficient studies and data to inform conducting these analyses for this review.
Contributions of authors
Dr Rashmi Ranjan Das (RRD), Dr Meenu Singh (MS), and Dr Sushree Samiksha Naik (SSN) Conceiving the review: RRD. Co‐ordinating the review: RRD, SSN. Undertaking manual searches: RRD, SSN. Screening search results: RRD, SSN. Organising retrieval of papers: RRD, SSN. Screening retrieved papers against inclusion criteria: RRD, MS. Appraising quality of papers: RRD, MS. Abstracting data from papers: RRD, SSN. Writing to authors of papers for additional information: RRD, MS. Providing additional data about papers: RRD. Obtaining and screening data on unpublished studies: RRD, MS. Managing data for the review: RRD, SSN. Entering data into Review Manager 5: RRD, SSN. Calculating Review Manager 5 statistical data: RRD, MS. Performing other statistical analysis not using Review Manager 5: RRD, MS. Interpreting data: RRD, MS. Making statistical inferences: RRD, MS. Writing the review: RRD, SSN, MS. Performing previous work that served as the foundation of the present study: RRD, MS. Serving as guarantor for the review (one author): RRD. Taking responsibility for reading and checking the review before submission: RRD, MS, SSN.
Declarations of interest
Rashmi R Das: none known. Meenu Singh: none known. Sushree S Naik: none known.
New
References
References to studies included in this review
Choudhary 2012 {published data only}
- Choudhary N, Gupta P. Vitamin D supplementation for severe pneumonia ‐ a randomized controlled trial. Indian Pediatrics 2012;49(6):449‐54. [DOI] [PubMed] [Google Scholar]
Dhungel 2015 {published data only}
- Dhungel A, Alam MS. Efficacy of vitamin D in children with pneumonia: a randomized control trial study. Janaki Medical College Journal of Medical Sciences 2015;3(1):5‐13. [Google Scholar]
Gupta 2016a {published data only}
- Gupta P, Dewan P, Shah D, Sharma N, Bedi N, Kaur IR, et al. Vitamin D supplementation for treatment and prevention of pneumonia in under‐five children: a randomized double‐blind placebo controlled trial. Indian Pediatrics 2016;53(11):967‐76. [DOI] [PubMed] [Google Scholar]
Manaseki‐Holland 2010 {published data only}
- Manaseki‐Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Tropical Medicine and International Health 2010;15(10):1148‐55. [DOI] [PubMed] [Google Scholar]
Rahmati 2016 {published data only}
- Rahmati MB, Rezapour M, Shahvari SZ. The effects of vitamin D supplementation in respiratory index of severity in children (RISC) of hospitalized patients with community‐acquired pneumonia: a double‐blind randomized clinical trial. Acta HealthMedica 2016;1(3):60‐4. [Google Scholar]
Rajshekhar 2016 {published data only}
- Rajshekhar CS, Vanaki R, Badakali AV, Pol RR, Yelamali BC. Efficacy of vitamin D supplementation in the treatment of severe pneumonia in children aged less than five years. International Journal of Contemporary Pediatrics 2016;3(1):96‐9. [Google Scholar]
Somnath 2017 {published data only}
- Somnath SH, Biswal N, Chandrasekaran V, Jagdisan B, Bobby Z. Therapeutic effect of vitamin D in acute lower respiratory infection: a randomized controlled trial. Clinical Nutrition ESPEN 2017;20:24‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Zhou 2016 {published data only}
- Zhou W, Zuo X, Li J, Yu Z. Effects of nutrition intervention on the nutritional status and outcomes of pediatric patients with pneumonia. Minerva Pediatrica 2016;68(1):5‐10. [PubMed] [Google Scholar]
References to ongoing studies
NCT02054182 {published data only}
- NCT02054182. Effect of vitamin D supplementation in young South African children hospitalized with acute lower respiratory infection. clinicaltrials.gov/ct2/show/NCT02054182 (first received 18 January 2014).
NCT02185196 {published data only}
- NCT02185196. Vitamin D supplementation: impact on severe pneumonia among under‐five children. clinicaltrials.gov/ct2/show/NCT02185196 (first received 22 June 2014).
Additional references
Alvarez‐Rodriguez 2012
- Alvarez‐Rodriguez L, Lopez‐Hoyos M, Garcia‐Unzueta M, Amado JA, Cacho PM, Martinez‐Taboada VM. Age and low levels of circulating vitamin D are associated with impaired innate immune function. Journal of Leukocyte Biology 2012;91(5):829‐38. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 2011
- Bang UC, Novovic S, Andersen AM, Fenger M, Hansen MB, Jensen JE. Variations in serum 25‐hydroxy vitamin D during acute pancreatitis: an exploratory longitudinal study. Endocrine Research 2011;36(4):135‐41. [DOI] [PubMed] [Google Scholar]
Chang 2006
- Chang AB, Torzillo PJ, Boyce NC, White AV, Stewart PM, Wheaton GR, et al. Zinc and vitamin A supplementation in indigenous Australian children hospitalized with lower respiratory tract infection: a randomised controlled trial. Medical Journal of Australia 2006;184(3):107‐12. [DOI] [PubMed] [Google Scholar]
Chang 2014
- Chang CC, Cheng AC, Chang AB. Over‐the‐counter (OTC) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD006088.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2012
- Das RR, Singh M, Shafiq N. Short‐term therapeutic role of zinc in children < 5 years of age hospitalised for severe acute lower respiratory tract infection. Paediatric Respiratory Reviews 2012;13(3):184‐91. [DOI] [PubMed] [Google Scholar]
Das 2017
- Das R. Information needed regarding antibiotic therapy and hospitalisation [personal communication]. Email to: M Rezapour 8 November 2017.
Ghosh 2012
- Ghosh G. Community acquired pneumonia ‐ management guidelines. Indian Journal of Practical Pediatrics 2012; Vol. 14, issue 3:258‐66.
Gombart 2005
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up‐regulated in myeloid cells by 1,25‐dihydroxy vitamin D₃. FASEB Journal 2005;19(9):1067‐77. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Version (accessed prior to 8 June 2017). Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Grant 2009
- Grant CC, Wall CR, Crengle S, Scragg R. Vitamin D deficiency in early childhood: prevalent in the sunny South Pacific. Public Health Nutrition 2009;12(10):1893‐901. [DOI] [PubMed] [Google Scholar]
Gupta 2016b
- Gupta P. Providing data reported in median (IQR) in our trial as mean (SD) [personal communication]. Email to: RR Das 24 April 2016.
Haider 2011a
- Haider BA, Lassi ZS, Ahmed A, Bhutta ZA. Zinc supplementation as an adjunct to antibiotics in the treatment of pneumonia in children 2 to 59 months of age. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007368.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 2011b
- Haider BA, Lassi ZS, Bhutta ZA. Short‐course versus long‐course antibiotic therapy for non‐severe community‐acquired pneumonia in children aged 2 months to 59 months. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD005976.pub2] [DOI] [PubMed] [Google Scholar]
Hansdottir 2008
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. Journal of Immunology 2008;181(10):7090‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heaney 2012
- Heaney RP. Vitamin D ‐ baseline status and effective dose. New England Journal of Medicine 2012;367(1):77‐8. [DOI] [PubMed] [Google Scholar]
Hemilä 2013
- Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD005532.pub3] [DOI] [PubMed] [Google Scholar]
Hewison 2011
- Hewison M. Antibacterial effects of vitamin D. Nature Reviews. Endocrinology 2011;7(6):337‐45. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hollis 2011
- Hollis BW. Short‐term and long‐term consequences and concerns regarding valid assessment of vitamin D deficiency: comparison of recent food supplementation and clinical guidance reports. Current Opinion in Clinical Nutrition and Metabolic Care 2011;14(6):598‐604. [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
IAP 2006
- Agarwal R, Singh V, Yewale V. RTI Facts. IAP consensus guidelines on rational management of respiratory tract infections in children. Mumbai: Indian Academy of Pediatrics (IAP); 2006 January to March.
Janssen 2007
- Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, Doornbos G, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. Journal of Infectious Diseases 2007;196(6):826‐34. [DOI] [PubMed] [Google Scholar]
Joshi 2014
- Joshi K, Bhatia V. Vitamin D deficiency in a tropical country ‐ treatment and prevention in children. Indian Journal of Pediatrics 2014;81(1):84‐9. [DOI] [PubMed] [Google Scholar]
Kelishadi 2014
- Kelishadi R, Ardalan G, Motlagh ME, Shariatinejad K, Heshmat R, Poursafa P, et al. National report on the association of serum vitamin D with cardiometabolic risk factors in the pediatric population of the Middle East and North Africa (MENA): the CASPIAN‐III Study. Nutrition 2014;30(1):33‐8. [DOI] [PubMed] [Google Scholar]
Kimball 2011
- Kimball S, Vieth R, Dosch HM, Bar‐Or A, Cheung R, Gagne D, et al. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. Journal of Clinical Endocrinology and Metabolism 2011;96(9):2826‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2014
- Lassi ZS, Kumar R, Das JK, Salam RA, Bhutta ZA. Antibiotic therapy versus no antibiotic therapy for children aged two to 59 months with WHO‐defined non‐severe pneumonia and wheeze. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD009576.pub2] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2006
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science 2006;311(5768):1770‐3. [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379(9832):2151‐61. [DOI] [PubMed] [Google Scholar]
Lozano 2011
- Lozano R, Wang H, Foreman KJ, Rajaratnam JK, Naghavi M, Marcus JR, et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet 2011;378(9797):1139‐65. [DOI] [PubMed] [Google Scholar]
Luo 2018
- Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Statistical Methods in Medical Research 2018;27(6):1785‐805. [DOI] [PubMed] [Google Scholar]
Manaseki‐Holland 2010 [pers comm]
- Manaseki‐Holland 2010. Mortality data reported in our trial [personal communication]. Email to: RR Das 4 May 2017.
Manaseki‐Holland 2012
- Manaseki‐Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012;379(9824):1419‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansbach 2009
- Mansbach JM, Ginde AA, Camargo CA Jr. Serum 25‐hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D?. Pediatrics 2009;124(5):1404‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martineau 2012
- Martineau AR. Bolus‐dose vitamin D and prevention of childhood pneumonia. Lancet 2012;379(9824):1373‐5. [DOI] [PubMed] [Google Scholar]
McAllister 2006
- McAllister JC, Lane AT, Buckingham BA. Vitamin D deficiency in the San Francisco Bay Area. Journal of Pediatric Endocrinology and Metabolism 2006;19(3):205‐8. [DOI] [PubMed] [Google Scholar]
Misra 2008
- Misra M, Pacaud D, Petryk A, Collett‐Solberg PF, Kappy M, Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;122(2):398‐417. [DOI] [PubMed] [Google Scholar]
Muhe 1997
- Muhe L, Lulseged S, Mason KE, Simoes EA. Case‐control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 1997;349(9068):1801‐4. [DOI] [PubMed] [Google Scholar]
Mylott 2004
- Mylott BM, Kump T, Bolton ML, Greenbaum LA. Rickets in the Dairy State. Wisconsin Medical Journal 2004;103(5):84‐7. [PubMed] [Google Scholar]
Najada 2004
- Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. Journal of Tropical Pediatrics 2004;50(6):364‐8. [DOI] [PubMed] [Google Scholar]
Nielsen 2010
- Nielsen NO, Skifte T, Andersson M, Wohlfahrt J, Søborg B, Koch A, et al. Both high and low serum vitamin D concentrations are associated with tuberculosis: a case‐control study in Greenland. British Journal of Nutrition 2010;104(10):1487‐91. [DOI] [PubMed] [Google Scholar]
NIH Vitamin D Fact Sheet
- National Institutes of Health (NIH). Office of Dietary Supplements. Vitamin D Fact Sheet for Health Professionals. ods.od.nih.gov/factsheets/VitaminD‐HealthProfessional/ (accessed prior to 8 June 2017).
Olliver 2013
- Olliver M, Spelmink L, Hiew J, Meyer‐Hoffert U, Henriques‐Normark B, Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. Journal of Infectious Diseases 2013;208(9):1474‐81. [DOI] [PubMed] [Google Scholar]
Pfeffer 2012
- Pfeffer PE, Hawrylowicz CM. Vitamin D and lung disease. Thorax 2012;67(11):1018‐20. [DOI] [PubMed] [Google Scholar]
Rajshekhar 2016 [pers comm]
- Rajshekhar C. Blinding and allocation concealment used in our trial [personal communication]. Email to: RR Das 3 June 2017.
Raloff 2006
- Raloff J. The antibiotic vitamin: deficiency in vitamin D may predispose people to infection. Science News 2006;170(20):312‐7. [Google Scholar]
Rehman 1994
- Rehman PK. Sub‐clinical rickets and recurrent infection. Journal of Tropical Pediatrics 1994;40(1):58. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2006
- Robinson PD, Högler W, Craig ME, Verge CF, Walker JL, Piper AC, et al. The re‐emerging burden of rickets: a decade of experience from Sydney. Archives of Disease in Childhood 2006;91(7):564‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rojas‐Reyes 2006
- Rojas‐Reyes MX, Granados Rugeles C. Oral antibiotics versus parenteral antibiotics for severe pneumonia in children. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004979.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudan 2008
- Rudan I, Boschi‐Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization 2008;86(5):408‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Scott 2012
- Scott JA, Wonodi C, Moïsi JC, Deloria‐Knoll M, DeLuca AN, Karron RA, et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the Pneumonia Etiology Research for Child Health study. Clinical Infectious Diseases 2012;54(Suppl 2):109‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thacher 2012
- Thacher TD, Fischer PR, Isichei CO, Zoakah AI, Pettifor JM. Prevention of nutritional rickets in Nigerian children with dietary calcium supplementation. Bone 2012;50(5):1074‐80. [DOI] [PubMed] [Google Scholar]
Torun 2013
- Torun E, Genç H, Gönüllü E, Akovalı B, Ozgen IT. The clinical and biochemical presentation of vitamin D deficiency and insufficiency in children and adolescents. Journal of Pediatric Endocrinology and Metabolism 2013;26(5‐6):469‐75. [DOI] [PubMed] [Google Scholar]
Wagner 2008
- Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122(5):1142‐52. [DOI] [PubMed] [Google Scholar]
Walker 2009
- Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatric Research 2009;65(5 Pt 2):R106‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2005
- Ward LM. Vitamin D deficiency in the 21st century: a persistent problem among Canadian infants and mothers. Canadian Medical Association Journal 2005;172(6):769‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2007
- Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D deficiency rickets among children in Canada. Canadian Medical Association Journal 2007;177(2):161‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wayse 2004
- Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. European Journal of Clinical Nutrition 2004;58(4):563‐7. [DOI] [PubMed] [Google Scholar]
Weisberg 2004
- Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. American Journal of Clinical Nutrition 2004;8(Suppl 6):1697‐705. [DOI] [PubMed] [Google Scholar]
White 2008
- White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infection and Immunity 2008;76(9):3837‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wondale 2005
- Wondale Y, Shiferaw F, Lulseged S. A systematic review of nutritional rickets in Ethiopia: status and prospects. Ethiopian Medical Journal 2005;43(3):203‐10. [PubMed] [Google Scholar]
Wu 2005
- Wu T, Ni J, Wei J. Vitamin A for non‐measles pneumonia in children. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003700.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition 2013;29(7‐8):953‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Das 2013
- Das RR, Singh M, Panigrahi I, Naik SS. Vitamin D supplementation for the treatment of acute childhood pneumonia: a systematic review. ISRN Pediatrics 2013;459160:eCollection 2013. [DOI: 10.1155/2013/459160] [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2015
- Das RR, Singh M, Naik SS. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD011597] [DOI] [Google Scholar]


