Abstract
Background
Shared decision making (SDM) is a process by which a healthcare choice is made by the patient, significant others, or both with one or more healthcare professionals. However, it has not yet been widely adopted in practice. This is the second update of this Cochrane review.
Objectives
To determine the effectiveness of interventions for increasing the use of SDM by healthcare professionals. We considered interventions targeting patients, interventions targeting healthcare professionals, and interventions targeting both.
Search methods
We searched CENTRAL, MEDLINE, Embase and five other databases on 15 June 2017. We also searched two clinical trials registries and proceedings of relevant conferences. We checked reference lists and contacted study authors to identify additional studies.
Selection criteria
Randomized and non‐randomized trials, controlled before‐after studies and interrupted time series studies evaluating interventions for increasing the use of SDM in which the primary outcomes were evaluated using observer‐based or patient‐reported measures.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
We used GRADE to assess the certainty of the evidence.
Main results
We included 87 studies (45,641 patients and 3113 healthcare professionals) conducted mainly in the USA, Germany, Canada and the Netherlands. Risk of bias was high or unclear for protection against contamination, low for differences in the baseline characteristics of patients, and unclear for other domains.
Forty‐four studies evaluated interventions targeting patients. They included decision aids, patient activation, question prompt lists and training for patients among others and were administered alone (single intervention) or in combination (multifaceted intervention). The certainty of the evidence was very low. It is uncertain if interventions targeting patients when compared with usual care increase SDM whether measured by observation (standardized mean difference (SMD) 0.54, 95% confidence interval (CI) ‐0.13 to 1.22; 4 studies; N = 424) or reported by patients (SMD 0.32, 95% CI 0.16 to 0.48; 9 studies; N = 1386; risk difference (RD) ‐0.09, 95% CI ‐0.19 to 0.01; 6 studies; N = 754), reduce decision regret (SMD ‐0.10, 95% CI ‐0.39 to 0.19; 1 study; N = 212), improve physical (SMD 0.00, 95% CI ‐0.36 to 0.36; 1 study; N = 116) or mental health‐related quality of life (QOL) (SMD 0.10, 95% CI ‐0.26 to 0.46; 1 study; N = 116), affect consultation length (SMD 0.10, 95% CI ‐0.39 to 0.58; 2 studies; N = 224) or cost (SMD 0.82, 95% CI 0.42 to 1.22; 1 study; N = 105).
It is uncertain if interventions targeting patients when compared with interventions of the same type increase SDM whether measured by observation (SMD 0.88, 95% CI 0.39 to 1.37; 3 studies; N = 271) or reported by patients (SMD 0.03, 95% CI ‐0.18 to 0.24; 11 studies; N = 1906); (RD 0.03, 95% CI ‐0.02 to 0.08; 10 studies; N = 2272); affect consultation length (SMD ‐0.65, 95% CI ‐1.29 to ‐0.00; 1 study; N = 39) or costs. No data were reported for decision regret, physical or mental health‐related QOL.
Fifteen studies evaluated interventions targeting healthcare professionals. They included educational meetings, educational material, educational outreach visits and reminders among others. The certainty of evidence is very low. It is uncertain if these interventions when compared with usual care increase SDM whether measured by observation (SMD 0.70, 95% CI 0.21 to 1.19; 6 studies; N = 479) or reported by patients (SMD 0.03, 95% CI ‐0.15 to 0.20; 5 studies; N = 5772); (RD 0.01, 95%C: ‐0.03 to 0.06; 2 studies; N = 6303); reduce decision regret (SMD 0.29, 95% CI 0.07 to 0.51; 1 study; N = 326), affect consultation length (SMD 0.51, 95% CI 0.21 to 0.81; 1 study, N = 175), cost (no data available) or physical health‐related QOL (SMD 0.16, 95% CI ‐0.05 to 0.36; 1 study; N = 359). Mental health‐related QOL may slightly improve (SMD 0.28, 95% CI 0.07 to 0.49; 1 study, N = 359; low‐certainty evidence).
It is uncertain if interventions targeting healthcare professionals compared to interventions of the same type increase SDM whether measured by observation (SMD ‐0.30, 95% CI ‐1.19 to 0.59; 1 study; N = 20) or reported by patients (SMD 0.24, 95% CI ‐0.10 to 0.58; 2 studies; N = 1459) as the certainty of the evidence is very low. There was insufficient information to determine the effect on decision regret, physical or mental health‐related QOL, consultation length or costs.
Twenty‐eight studies targeted both patients and healthcare professionals. The interventions used a combination of patient‐mediated and healthcare professional directed interventions. Based on low certainty evidence, it is uncertain whether these interventions, when compared with usual care, increase SDM whether measured by observation (SMD 1.10, 95% CI 0.42 to 1.79; 6 studies; N = 1270) or reported by patients (SMD 0.13, 95% CI ‐0.02 to 0.28; 7 studies; N = 1479); (RD ‐0.01, 95% CI ‐0.20 to 0.19; 2 studies; N = 266); improve physical (SMD 0.08, ‐0.37 to 0.54; 1 study; N = 75) or mental health‐related QOL (SMD 0.01, ‐0.44 to 0.46; 1 study; N = 75), affect consultation length (SMD 3.72, 95% CI 3.44 to 4.01; 1 study; N = 36) or costs (no data available) and may make little or no difference to decision regret (SMD 0.13, 95% CI ‐0.08 to 0.33; 1 study; low‐certainty evidence).
It is uncertain whether interventions targeting both patients and healthcare professionals compared to interventions of the same type increase SDM whether measured by observation (SMD ‐0.29, 95% CI ‐1.17 to 0.60; 1 study; N = 20); (RD ‐0.04, 95% CI ‐0.13 to 0.04; 1 study; N = 134) or reported by patients (SMD 0.00, 95% CI ‐0.32 to 0.32; 1 study; N = 150 ) as the certainty of the evidence was very low. There was insuffient information to determine the effects on decision regret, physical or mental health‐related quality of life, or consultation length or costs.
Authors' conclusions
It is uncertain whether any interventions for increasing the use of SDM by healthcare professionals are effective because the certainty of the evidence is low or very low.
Plain language summary
A review of activities to help healthcare professionals share decisions about care with their patients
What is the aim of this review?
Healthcare professionals often do not involve their patients in decision making about their care. With shared decision making, healthcare professionals inform patients about their choices and invite them to choose the option that reflects what is important to them, including the option not to proceed with treatment. Shared decision making is said to be desirable because patient involvement is accepted as a right and patients in general want more information about their health condition and prefer to take an active role in decisions about their health. The aim of this review was to find out if activities to increase shared decision making by healthcare professionals are effective or not. Examples of these activities are training programs, giving out leaflets, or email reminders. Cochrane researchers collected and analyzed all relevant studies to answer this question, and found 87 studies.
Key messages
A great variety of activities exist to increase shared decision making by healthcare professionals, but we cannot be confident about which of these activities work best because the certainty (or the confidence) of the evidence has been assessed as very low.
What was studied in the review?
Our review examined the 87 studies that tested what kind of activities work best to help healthcare professionals involve their patients more in decision making about their care. We also examined the effect of these activities on decision regret, physical or mental health‐related quality of life, length of the consultation, and cost.
The studies were so different that these activities were difficult to compare.
First, we divided the studies into ones that used outside observers to measure shared decision making and ones that used patients to measure shared decision making.
We then divided studies into ones that looked at activities a) for healthcare professionals only (e.g. training), b) for patients only (e.g. giving them a decision aid, which is a pamphlet explaining options and inviting them to think about their values and preferences), and c) for both healthcare professionals and patients (e.g. training plus a decision aid).
Finally, we subdivided each of these three categories into studies that compared the activity with usual care and studies that compared the activity with another activity.
We also looked at how certain the evidence was for our primary outcome (the extent to which healthcare professionals involve their patients more in decision making about their care) and secondary outcomes (decision regret, physical or mental health‐related quality of life, length of the consultation, and cost) of interest.
What are the main results of the review?
Forty‐four studies looked at activities for patients only, while 28 studies looked at activities for both healthcare professionals and patients, and 15 studies looked at activities for healthcare professionals only.
While studies in all three categories had tested many different activities to increase shared decision making by healthcare professionals, overall we cannot be confident in the effectiveness of these activities because the certainty of the evidence was weak. This is because there were many possible sources of error (e.g. not making sure the tested activities were not also provided to the comparison groups), and often poor reporting of results (i.e. not providing enough information to judge the quality of the evidence).
Although it was hard to come to any firm conclusions, we can say that compared to no activity at all, activities for healthcare professionals may slightly improve mental health‐related quality of life, but make little or no difference to physical health‐related quality of life (two studies). We can also say that activities targeting both healthcare professionals and patients may make little or no difference to decision regret (one study).
How up‐to‐date is this review?
We searched for studies published up to June 2017.
Summary of findings
Background
Description of the condition
There is increasing recognition of the ethical imperative to share important decisions with patients (Salzburg Global Seminar 2011). Shared decision making (SDM) can be defined as an interpersonal, interdependent process in which health professionals, patients and their caregivers relate to and influence each other as they collaborate in making decisions about a patient’s health (Charles 1997; Légaré 2011; Légaré 2013; Towle 1999). It is considered the crux of patient‐centered care (Weston 2001). Briefly, SDM depends on knowing and understanding the best available evidence about the risks and benefits across all available options while ensuring that the patient's values and preferences are taken into account (Charles 1997; Elwyn 1999; Towle 1999).
Although SDM represents a complex set of behaviors that must be achieved by both members of the patient‐healthcare professional dyad (LeBlanc 2009), it is possible to specify behaviors that both parties must adopt for SDM to occur in clinical practice (Frosch 2009; Légaré 2007a). A systematic review of SDM as a concept identified 161 definitions and summarized the key elements into one integrative model of SDM in medical encounters (Makoul 2006). This model identifies nine essential elements that can be translated into specific SDM‐related behaviors that healthcare professionals need to demonstrate during consultations with patients:
define and explain the healthcare problem,
present options,
discuss pros and cons (benefits, risks, costs),
clarify patient values and preferences,
discuss patient ability and self‐efficacy,
present what is known and make recommendations,
check and clarify the patient's understanding,
make or explicitly defer a decision, and
arrange follow‐up.
Description of the intervention
A variety of interventions have been designed to change healthcare professionals' behavior. Based on the Effective Practice and Organisation of Care (EPOC) taxonomy of interventions (EPOC 2015), these interventions aim at changing the performance of healthcare professionals through interactions with patients, or information provided by or to patients. Interventions may include, but are not limited to, the distribution of printed educational materials, educational meetings, audit and feedback, reminders, educational outreach visits and patient‐mediated interventions. In the context of SDM it is possible to identify three overarching categories of implementation intervention: 1) interventions targeting patients, 2) interventions targeting healthcare professionals, and 3) interventions targeting both.
How the intervention might work
Theoretical and empirical evidence about behavior change in healthcare professionals (Godin 2008) and complex behavior change frameworks (Michie 2009) allow us to make certain hypotheses regarding the mechanisms by which interventions might promote SDM. For example, the distribution of printed educational materials may improve professionals' attitudes to SDM by reinforcing their intention to engage in SDM (Giguère 2012). The training of professionals in SDM through educational meetings may increase professionals' perceptions of self‐efficacy, or their belief in their ability to succeed in a situation, which is one of the key determinants of behavior (Godin 2008). Patient‐mediated interventions could be a discussion with a nurse, a patient education program, or a decision aid, for example. Decision aids are tools (they can be pamphlets or online modules) that help patients become involved in decision making. They help patients clarify the decision that needs to be made, and give information about options and outcomes. They also invite patients to articulate their personal values and preferences regarding the options (Stacey 2017). In turn, the habits of healthcare professionals may change when patients themselves take the initiative to engage more in the decision‐making process, as this may increase health professionals’ knowledge and use of emerging evidence in their area of expertise (Brouwers 2010).
Regarding the association between SDM and patient outcomes, some authors have shown that communication between healthcare professionals and patients, including SDM, can lead to improved health outcomes in direct but also in indirect ways (Street 2009). Thus, according to an adapted conceptual framework linking clinician‐patient communication to health outcomes, SDM can have an impact on affective‐cognitive outcomes (e.g. knowledge, understanding, satisfaction, trust), behavioral outcomes (treatment decisions, adherence to recommended treatments and adoption of health behaviors), as well as health outcomes (e.g. quality of life, self‐rated health and biological measures of health) (Shay 2015).
Why it is important to do this review
Policy makers perceive SDM as desirable (Shafir 2012) because: a) patient involvement is accepted as a right (Straub 2008); b) patients in general want more information about their health condition and prefer to take an active role in decisions about their health (Alston 2012; Kiesler 2006); c) SDM may reduce the overuse of options not clearly associated with benefits for all and increase the use of options clearly associated with benefits for the vast majority of the concerned population (Mulley 2012); d) SDM may reduce unwarranted healthcare practice variations (Wennberg 2004); and e) SDM may foster the sustainability of the healthcare system by increasing patient ownership of their own health care (Coulter 2006).
Nonetheless, SDM has not yet been widely implemented in clinical practice. A systematic review of 33 studies using the Observing Patient Involvement in Decision Making instrument (OPTION) showed low levels of patient‐involving behaviors (Couët 2013). The rationale for this review of interventions for increasing use of SDM among healthcare professionals is to determine what kinds of intervention have been shown to increase patient‐involving behaviors among healthcare professionals.
This is the second update of a previously published Cochrane review. The review was first undertaken in 2010 (Légaré 2010) and updated in 2014 (Légaré 2014). As the demand for SDM training programs for healthcare professionals is increasing internationally (Diouf 2016), we considered a second update was important to keep abreast of developments.
Objectives
To determine the effectiveness of interventions for increasing the use of SDM by healthcare professionals. We considered interventions targeting patients, interventions targeting healthcare professionals, and interventions targeting both and compared them with usual care or other type of interventions by target group.
Methods
Criteria for considering studies for this review
Types of studies
This review considered:
randomized trials;
non‐randomized trials;
controlled before‐after studies (CBAs); and
interrupted time series (ITS) analyses.
To be included as a CBA, the Cochrane EPOC group (Effective Practice and Organisation of Care) requires the study to have a minimum of two intervention sites and two control sites. For ITS studies, there needs to be a clearly defined point in time when the intervention occurred and at least three data points before and three after the intervention (EPOC 2017).
Types of participants
Participants could be any healthcare professional (e.g. physicians, nurses, pharmacists, social workers), including professionals in training (for example, medical residents). We defined professionals as being licensed or registered to practice or, in the case of physicians in training, as having completed their basic pre‐licensure education. Participants could also be patients, including healthcare consumers and simulated patients. However, studies that included simulated patients were deemed eligible only if the outcome was observer‐reported.
Types of interventions
We included studies that evaluated an intervention designed to increase the use of SDM. Interventions were organized into three target categories using the EPOC taxonomy of interventions (EPOC 2015):
interventions targeting patients (for example, patient‐mediated interventions);
interventions targeting healthcare professionals (for example, distribution of printed educational material, educational meetings, audit and feedback, reminders and educational outreach visits);
interventions targeting both patients and healthcare professionals (for example, a patient‐mediated intervention combined with an intervention targeting healthcare professionals).
Patient decision aids were considered a patient‐mediated intervention since one of their purposes is to foster patient participation in decisions during the clinical encounter (Stacey 2017). Studies that evaluated patient‐mediated interventions (for example, patients' use of patient decision aids in preparation for or during their consultation with a healthcare professional) were considered only if these studies directly assessed the healthcare professional‐related outcome of interest, that is their use of SDM (see Types of outcome measures).
Types of outcome measures
Primary outcomes
Use of SDM, using objective observer‐based outcome measures (OBOMs) or patient‐reported outcome measures (PROMs). OBOMs are instruments used by a third observer to capture the decision‐making process during an encounter between a healthcare professional and a patient/family caregiver when facing health treatment or screening decisions. They are only used in the reporting of observable concepts (e.g. signs or behaviors). Unlike clinician‐reported outcome measures, OBOMs are reported by people (e.g. teachers or caregivers) who do not have professional training relevant to the measurement being made (Velentgas 2013). PROMs are instruments that collect information directly from patients. The measurement is recorded without amendment or interpretation by a clinician or other observer. The measurement can be recorded by the patient directly, or recorded by an interviewer, provided that the interviewer records the patient's response exactly (Velentgas 2013).
Secondary outcomes
Patient outcomes
Affective‐cognitive outcomes
Knowledge
Satisfaction (satisfaction with care, with the choice, with the decision‐making process, with the intervention, helpfulness of the intervention)
Decisional conflict
Decision regret
Patient‐clinician communication
Self‐efficacy
Empowerment
Behavioral outcomes
Match between preferred and actual level of participation in decision making
Match between preferred option and decision made
Adherence to decision made
Health outcomes
Health status (generic instrument types)
Health‐related quality of life (generic instrument types)
Anxiety
Depression
Stress
Distress
Process outcomes
Consultation length
Costs
Equity
Adverse effects (potential harms of interventions)
Search methods for identification of studies
Electronic searches
We searched for studies published up to 15 June 2017. Searches were not restricted by language. The following electronic databases were searched for primary studies.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5) in the Cochrane Library
Health Technology Assessment Database (HTA; 2016, Issue 4) in the Cochrane Library
NHS Economic Evaluation Database (NHSEED; 2015, Issue 2) in the Cochrane Library
PubMed
Embase Ovid (1974 to 14 June 2017)
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1980 to 15 June 2017)
PsycINFO Ovid (1967 to June Week 1 2017)
All search strategies used are provided in Appendix 1.
Searching other resources
Trial registries
We searched:
ClinicalTrials.gov, US National Institutes of Health (NIH) at http://clinicaltrials.gov/ (search performed in week 1, August 2017);
World Health Organization International Clinical Trials Registry Platform http://apps.who.int/trialsearch/ (search performed in week 1, August 2017).
We also:
handsearched the proceedings of the International Conference on Shared Decision Making (from 2003 to 2017)(Appendix 2);
handsearched the proceedings of the annual North American meetings of the Society for Medical Decision Making (from 2004 to 2016) (Appendix 3); we intended to search the European Association for Communication in Healthcare (EACH) but were unable to obtain detailed information either online or in paper form);
reviewed reference lists of all included studies, relevant systematic reviews (Appendix 4) and primary studies (Appendix 5); and
contacted authors of relevant studies or reviews to clarify reported published information and to seek unpublished data.
Data collection and analysis
Selection of studies
Review author Rhéda Adekpedjou (RA), and graduate students Jessica Hébert (JH), Élodie Chenard (EC), Alexandrie Boucher (AB), Lionel Adisso (LA) ‐ (see Acknowledgements) independently screened each title and abstract to find studies that met the inclusion criteria. Studies were only selected if published in English or French. We retrieved full‐text copies of all studies that might be relevant or for which the inclusion criteria were not clear in the title or abstract. In this update, when more than one publication described the same study but each presented new and complementary data, we included them all. Any disagreements about selection were resolved by discussion with two review authors (ST, FL). For more details about study selection, see Figure 1.
1.
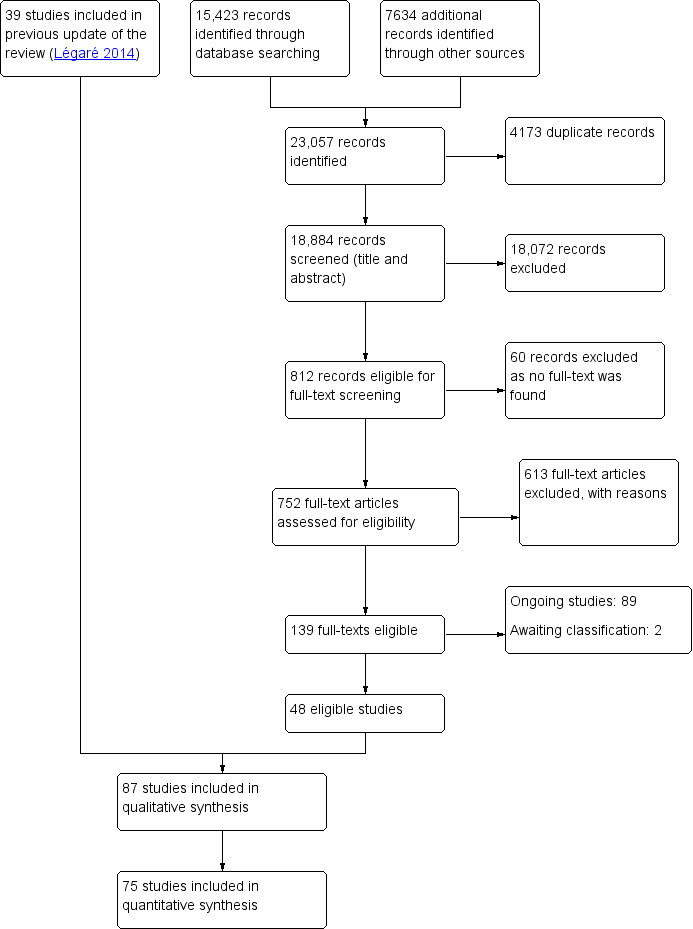
Flow diagram of Cochrane update on interventions for increasing the use of shared decision making by healthcare professionals (up to 15 June 2017)
Data extraction and management
To extract data, we designed a form derived from the EPOC Review Group data collection checklist (EPOC 2017b). At least two review authors (including RA and ST) independently extracted data from eligible studies. We reached consensus about discrepancies, and any disagreement was adjudicated by discussion among the review authors (FL, RA, DS, ST, JK, IDG, AL, MCP, RT, GE, NDB). We entered data into Review Manager Software (RevMan 5) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact the study authors of to ask them to provide further details.
In addition to EPOC's standardized data collection checklist, we extracted the following characteristics of the settings and interventions.
Level of care: primary or specialized care (as defined by the type of provider).
Setting of care: ambulatory or non‐ambulatory care (e.g. hospitalized patients in acute‐care or long‐term care facilities).
Conceptual or theoretical underpinnings of the intervention (i.e. study authors stated that the intervention was based on a theory or at least referred to a theory).
Barriers assessment (i.e. study authors stated that a barriers assessment was conducted and the intervention was designed to overcome identified barriers).
Number of components included in the intervention based on the EPOC taxonomy (when a barriers assessment was mentioned it was considered a component of the intervention).
Assessment of risk of bias in included studies
At least two review authors (including RA and ST) independently assessed the risk of bias in each included study using the criteria outlined in the suggested risk of bias criteria for EPOC reviews (EPOC 2017c) and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for ITS designs. For blinding, incomplete data, and baseline outcome measurement, we assessed the primary outcomes and secondary outcomes that were selected for inclusion in the 'Summary of findings' table (see below for details of selection process). Any disagreement was resolved through discussion with FL. Each risk of bias criterion was assessed as 'Low risk', 'High risk' or 'Unclear'. The 10 standard criteria as suggested for all randomized trials and CBA studies are listed below.
Random sequence generation (protection against selection bias)
Concealment of allocation (protection against selection bias)
Protection against contamination.
Blinded assessment (protection against detection bias)
Baseline outcome measurement
Patient baseline characteristics
Healthcare professional baseline characteristics
Selective reporting outcome
Incomplete data outcome
Other risk of bias.
Measures of treatment effect
We structured data analysis using statistical methods developed for EPOC by Grimshaw and colleagues (Grimshaw 2004). For each study, we reported results for categorical and continuous primary outcomes separately and in natural units. When included studies assessed SDM using an adaptation of the Control Preference Scale (Degner 1992), we dichotomized into SDM versus no SDM (Légaré 2012).
For categorical measures, we calculated the difference in risk between the intervention of interest and the control intervention. We calculated standardized mean difference (SMD) for continuous measures by dividing the mean score difference of the intervention and comparison groups in each study by the pooled estimate standard deviation for the two groups. When possible, for categorical and continuous outcomes, we constructed 95% confidence intervals (CIs) to compare groups before and after the intervention, according to the recommendations in RevMan 5. The absence of a '0' value in the CI indicated that the baselines differed or that the intervention had a positive effect compared to the control intervention or to usual care. When the baseline was different between the two groups, we used the size of the difference and its associated standard error to compare them. When there were not enough quantitative data available to make these calculations, we extracted a direct quote from the primary study on the effectiveness of the intervention and on confounding factors, if available. When no baseline was reported, we considered groups to be similar prior to the intervention.
For the analysis, we divided the studies into six comparison categories: 1) interventions targeting patients compared with usual care; 2) interventions targeting healthcare professionals compared with usual care; 3) interventions targeting both patients and healthcare professionals compared with usual care; 4) interventions targeting patients compared with other types of interventions targeting patients; 5) interventions targeting healthcare professionals compared with other types of interventions targeting healthcare professionals; 6) interventions targeting both patients and healthcare professionals compared with other types of interventions targeting both patients and healthcare professionals.
We performed a meta‐analysis if there were enough studies in each of the six comparison categories. When the study reported repeated measurements for an outcome for the same participants, only the measure closest in time to the consultation was kept in the meta‐analysis. When studies with more than two arms reported several comparisons of different outcomes or different interventions, we kept only the comparisons that most reduced the heterogeneity of the comparison group in the meta‐analysis. We considered a SMD of 0.2 as small, 0.5 as medium, and 0.8 as large (Cohen 1988).
Unit of analysis issues
We included cluster‐randomized trials in the analyses along with individually‐randomized trials. Comparisons that randomize or allocate clusters (groups of healthcare professionals or organizations) but do not account for clustering during the analysis have potential unit of analysis errors that can produce artificially significant P values and overly narrow CIs (Ukoumunne 1999). Therefore, when possible, we contacted study authors for missing information and attempted to re‐analyze studies with potential unit of analysis errors. When missing information was unavailable, we reported only the point estimate.
Assessment of heterogeneity
To explore heterogeneity, we designed tables that compared SMDs of the studies and their risk differences. We considered the following variables as potential sources of heterogeneity in the results of the included studies: type of intervention; characteristics of the intervention (e.g. duration); clinical setting (primary care versus specialized care); type of healthcare professional (physicians versus other healthcare professionals); level of training of healthcare professionals (e.g. in training versus in practice).
Data synthesis
We estimated a weighted intervention effect with 95% confidence intervals. For continuous measures, we used SMDs; for dichotomous outcomes, we calculated the risk difference. We analyzed all data with a random‐effects model because of the diverse nature of the studies being combined and then anticipated variability in the populations and interventions of the included studies. We summarized all of the results for the primary and selected secondary outcomes and rated the strength of evidence using GRADE (Andrews 2013), and then presented these results in the 'Summary of findings' tables (Higgins 2011). As the non‐randomized evidence has a high level of uncertainty and that there are few non‐randomized trials, we reported only the results of randomized trials in the Summary of findings' tables. For studies not included in the quantitative synthesis, we assessed how their results could have impacted the pooled estimate of the effect size regarding the direction of the effect (Appendix 6).
'Summary of findings' tables
We evaluated the certainty of the evidence according to the GRADE guidelines (Guyatt 2011) and the methods described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We assessed primary and selected secondary outcomes in all six comparison categories. For each outcome, we rated conclusions as follows:
high: this research provides a very good indication of the likely effect; the likelihood that the effect will be substantially different is low;
moderate: this research provides a good indication of the likely effect; the likelihood that the effect will be substantially different is moderate;
low: this research provides some indication of the likely effect; however, the likelihood that it will be substantially different is high;
very low: this research does not provide a reliable indication of the likely effect; the likelihood that the effect will be substantially different is very high).
From starting score of certainty of evidence according to the study design, we downgraded the rating if one or more of the five following criteria were present: study limitation, indirect evidence, inconsistency, imprecision of the observed effect and publication bias. A review author (RA) and a graduate student (AB) independently assessed the certainty of the evidence and reached consensus in collaboration with FL.
As the use of SDM is the only primary outcome of this review, we assessed this outcome using the GRADE approach and included it in the 'Summary of findings' tables. We used the method proposed in EPOC Worksheets (EPOC 2017d) to determine which secondary outcomes should be assessed and included in the 'Summary of findings' tables. First, the study co‐authors generated a list of relevant secondary outcomes for the review. Then we independently selected outcomes important enough to be included in the 'Summary of findings' tables by rating them on a 9‐point scale ranging from 1 (not important) to 9 (critical), and came to a consensus. Then we calculated the median of the scores we had attributed to each secondary outcome and agreed to include all that scored above 7. The selected secondary outcomes were: decision regret, health‐related quality of life (mental and physical), consultation length and cost.
Subgroup analysis and investigation of heterogeneity
Analysis was pre‐defined using a subgroup analysis approach, and we did not combine data from observer‐based outcome measures (OBOMs) with patient‐reported outcome measures (PROMs) as they measured different concepts. In addition, within each comparison category, we explored how individually‐randomized trials compared to cluster‐randomized trials regarding our primary outcome when applicable. We further investigated heterogeneity by exploring how the study design (cluster‐randomized trials versus individually‐randomized trials) affected statistical heterogeneity (Higgins 2011).
Sensitivity analysis
No sensitivity analysis were performed.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We identified 23,057 new potentially relevant records and excluded 18,072 during abstract screening. We retrieved 752 full‐text publications for more detailed screening and excluded another 613 records based on the identified inclusion criteria. We were not able to find the full text of 60 records for assessment . We included 87 studies in the review, 48 of which were newly identified for this update (Figure 1).
Included studies
This update search added 48 new studies (Adarkwah 2016; Almario 2016; Ampe 2017; Barton 2016; Branda 2013; Causarano 2014; Cooper 2013; Cox 2017; Coylewright 2016; Davison 2002; Eggly 2017; Epstein 2017; Feng 2013; Fiks 2015; Hamann 2011; Hamann 2014; Hamann 2017; Härter 2015; Hess 2016; Jouni 2017; Kennedy 2013; Koerner 2014; Köpke 2014; Korteland 2017; LeBlanc 2015a; LeBlanc 2015b; Maclachlan 2016; Maindal 2014; Maranda 2014; Mathers 2012; Perestelo‐Perez 2016; Pickett 2012; Rise 2012; Sanders 2017; Schroy 2016; Sheridan 2012; Sheridan 2014; Smallwood 2017; Tai‐Seale 2016; Thomson 2007; Tinsel 2013; van der Krieke 2013; van Roosmalen 2004; van Tol‐Geerdink 2016; Vestala 2013; Warner 2015; Wilkes 2013; Wolderslund 2017) to the 39 original studies for a total of 87 studies.
We identified 89 ongoing studies through trial registration databases, proceedings of conferences and protocols published in electronic databases (see Characteristics of ongoing studies). For two studies, we were unable to decide whether to include them or not because not enough information was available (See Characteristics of studies awaiting classification).
All the studies were randomized trials except for four: three non‐randomized controlled trials (Almario 2016; Barton 2016; Deinzer 2009) and a controlled before‐after study (CBA) (Ampe 2017). Among the randomized trials, 21 were cluster‐randomized trials (Branda 2013; Cox 2017; Elwyn 2004; Epstein 2017; Feng 2013; Hamann 2007; Haskard 2008; Kennedy 2013; Koerner 2014; LeBlanc 2015a; Légaré 2012; Loh 2007; Mathers 2012; O'Cathain 2002; Perestelo‐Perez 2016; Sanders 2017; Tai‐Seale 2016; Tinsel 2013; van Roosmalen 2004; Wetzels 2005; Wilkes 2013).
Settings and participants
Of the 87 included studies, 44 evaluated interventions targeting patients, 15 evaluated interventions targeting health professionals, and 28 targeted both patients and health professionals. The four most represented countries were the USA (37 studies), Germany (15 studies) and Canada (eight studies) and the Netherlands (eight studies). There were two studies by international collaborations. The level of care was primary care in 44 studies, specialized care in 36 studies and both primary and specialized care in one study. In six studies, the level of care was unclear. In 49 studies, the healthcare professionals involved were licensed; in 16 studies they were licensed and in training; in 22 studies their level of training was unclear. The three most frequent clinical conditions studied were cancer (22 studies), cardiovascular diseases (14 studies) and psychiatric conditions (11 studies) (see Characteristics of included studies).
Target categories
Interventions targeting patients (44 studies)
Most of the 44 studies of interventions targeting patients were conducted in Europe or the USA (36 studies). There was one study from Africa. Specialized care was the most frequent care setting (22 studies), and all but eight studies were conducted in and recruited patients in an ambulatory setting. Studies varied greatly regarding the number of patients involved, ranging from 26 (Lalonde 2006) to 913 (Hess 2016). Most of the studies did not report the number of healthcare professionals involved. The most common clinical conditions were oncologic (14 studies), cardiovascular (eight studies) and psychiatric (six studies).
Interventions targeting healthcare professionals (15 studies)
The majority of the studies of interventions targeting healthcare professionals were conducted in Europe or the USA (14 studies). There was one study by an international collaboration. The care setting was mainly primary care (11 studies), with most of the participants recruited in ambulatory care (11 studies). Among the 12 studies that used non‐simulated patients, the number of patients involved ranged from 298 (Cox 2017) to 10,070 (O'Cathain 2002). Two studies did not report the number of patients involved, and three did not report the number of healthcare professionals involved. The clinical condition was different in every study.
Interventions targeting both patients and healthcare professionals (28 studies)
The majority of the 28 studies of interventions targeting both patients and healthcare professionals were conducted in Europe or the USA (27 studies). There was one study by an international collaboration. The most common care setting was primary care (16 studies), with most of the participants recruited in ambulatory care (23 studies). Among the 26 studies that used non‐simulated patients, a total of 12,078 patients were enrolled, with a minimum of 60 (Fiks 2015) and a maximum of 4349 (Wolderslund 2017). Twenty‐five studies reported participating healthcare professionals, ranging from 10 per study (Bieber 2006) to 156 per study (Haskard 2008).The most common clinical condition was cancer (seven studies), followed by cardiovascular diseases (four studies), psychiatric conditions (four studies) and type‐2 diabetes (four studies).
Characteristics of interventions and comparisons
Some studies reported more than one comparison. For such studies, we extracted only data for the comparisons that corresponded to one or more of the six comparison categories in our review. In each category of comparison, no study was counted twice for the analysis. For details, see Characteristics of included studies.
Interventions targeting patients
Twenty‐four studies compared interventions targeting patients with usual care (Almario 2016; Cooper 2011; Deen 2012; Eggly 2017; Hamann 2014; Haskard 2008; Korteland 2017; Krist 2007; Landrey 2012; LeBlanc 2015a; LeBlanc 2015b; Maclachlan 2016; Maranda 2014; Murray 2001; van Peperstraten 2010; Perestelo‐Perez 2016; Pickett 2012; Sheridan 2014; Tai‐Seale 2016; van der Krieke 2013; van Tol‐Geerdink 2016; Vestala 2013; Vodermaier 2009; Wolderslund 2017). All but one study compared patient‐mediated interventions to usual care. Patient‐mediated interventions included decision aids, patient activation, question prompt lists and training for patients. The interventions were administered alone (single interventions) or in combination (multifaceted intervention).
Twenty‐eight studies presented comparisons of interventions targeting patients with other interventions targeting patients ( Adarkwah 2016; Barton 2016; Butow 2004; Causarano 2014; Davison 1997; Davison 2002; Deen 2012; Deschamps 2004; Dolan 2002; Eggly 2017; Hamann 2011; Hamann 2017; Jouni 2017; Kasper 2008; Köpke 2014; Krist 2007; Lalonde 2006; Montori 2011; Nannenga 2009; Raynes‐Greenow 2010; Schroy 2011; Schroy 2016; Smallwood 2017; Stiggelbout 2008; Street 1995; Thomson 2007; van Roosmalen 2004; Wolderslund 2017). Of these, 18 studies compared a single intervention (these included decision aid, consultation preparation package, empowerment sessions, brochure, training of patients in shared decision making (SDM), interactive‐4‐hour education program, literacy‐appropriate medication guide) to another single intervention (these included decision aid, booklets, information packages, patient activation, pamphlets, cognitive training, 4‐hour Multiple Sclerosis specific stress management program, existing medication guide); 10 studies compared a multifaceted intervention (these included decision aid and patient activation, decision aid and literacy‐appropriate medication guide, decision aid and risk assessment tool, question prompt list and assistance of a communication coach) to a single intervention (these included decision aid, question prompt list, literacy‐appropriate medication guide, existing medication guide) and three studies compared a multifaceted intervention (these included decision aid and information booklet about immunotherapy, conventional risk and genetic risk information and decision aid) to another multifaceted intervention (these included decision aid and standard information package, conventional risk information and decision aid).
Four studies reported basing their intervention on a barriers assessment (Hamann 2011; Jouni 2017; Korteland 2017; van Peperstraten 2010).
Interventions targeting healthcare professionals
Fifteen studies compared interventions targeting the healthcare professionals with usual care ( Ampe 2017; Bernhard 2011; Cooper 2011; Cox 2017; Fossli 2011; Kennedy 2013; Koerner 2014; LeBlanc 2015b; Légaré 2012; O'Cathain 2002; Sanders 2017; Shepherd 2011; Stacey 2006; Tinsel 2013; Wilkes 2013). Of these, seven studies compared a single intervention (educational meeting, distribution of educational material, educational outreach visit, and reminder) to usual care and eight studies compared a multifaceted intervention (educational meeting and audit and feed‐back; educational meeting and distribution of educational material; educational meeting and audit and feedback and distribution of educational material; distribution of educational materials and educational meeting and audit and feedback and barriers assessment) to usual care.
Two studies compared an intervention targeting the healthcare professional (educational meeting, reminder) with one targeting the patient (decision aid, patient coaching by community health workers) (Cooper 2011; LeBlanc 2015b).
Three studies compared interventions targeting the healthcare professional with other interventions targeting the healthcare professional (Elwyn 2004; Feng 2013; Krones 2008 (ARRIBA‐Herz)). Of these, one study compared a multifaceted intervention (educational meeting and audit and feedback focusing on SDM skills) to another multifaceted intervention (educational meetings and audit and feedback focusing on risk communication skills), one study compared a single intervention (distribution of educational material) to another single intervention (distribution of educational material), and one study compared a multifaceted intervention (educational meeting, audit and feedback, distribution of educational material, and an educational outreach component) to a single intervention (educational meeting).
Four studies reported the performance of a barriers assessment and based their interventions on the identified barriers (Ampe 2017; Bernhard 2011; Murray 2010; Stacey 2006).
Interventions targeting both patients and healthcare professionals
Seventeen studies compared an intervention targeting patients and healthcare professionals with usual care (Branda 2013; Cooper 2011; Coylewright 2016; Epstein 2017; Hamann 2007; Härter 2015; Haskard 2008; Hess 2012; Hess 2016; Leighl 2011; Loh 2007; Mathers 2012; Murray 2010; Rise 2012; Tai‐Seale 2016; Wetzels 2005; Wilkes 2013). Of these, 11 studies presented interventions that used educational meetings and patient‐mediated interventions; one study presented a patient‐mediated intervention with educational outreach visits; one study presented an arm with an intervention that used a combination of a patient‐mediated intervention, distribution of educational material and educational meetings (Haskard 2008); one study presented an arm with a patient‐mediated intervention and a distribution of educational material (Wilkes 2013); one study presented an arm with a patient‐mediated intervention and a reminder; one study presented an arm with a combination of educational meeting, audit and feedback, distribution of educational material, educational outreach visit and barriers assessment; one study presented interventions that used educational meetings, patient‐mediated interventions and distribution of educational material.
Seven studies compared interventions targeting both patients and healthcare professionals with interventions targeting patients alone (Bieber 2006; Cooper 2011; Deinzer 2009; Mullan 2009; Sheridan 2012; Tai‐Seale 2016; Warner 2015). Of these, six studies compared educational meetings and patient‐mediated interventions with patient‐mediated interventions alone; one study compared interventions that used educational meetings, patient‐mediated interventions and distribution of educational material with patient‐mediated interventions alone.
Five studies compared interventions targeting both patients and healthcare professionals with interventions targeting healthcare professionals alone (Cooper 2011; Feng 2013; Fiks 2015; Maindal 2014; Roter 2012). Of these, two studies compared patient‐mediated interventions and the distribution of educational materials with the distribution of educational materials alone; two study compared educational meetings and patient‐mediated interventions with educational meetings alone; and one study compared patient‐mediated interventions and reminders with reminders alone.
Three studies compared an intervention targeting both patients and healthcare professionals with another intervention targeting both patients and healthcare professionals (Cooper 2013; Myers 2011; Tai‐Seale 2016). Of these, one study compared a multifaceted intervention including a patient‐mediated intervention, educational outreach visit, distribution of educational material and audit and feedback with another multifaceted intervention including a patient‐mediated intervention, educational outreach visit and distribution of educational material; one study compared a multifaceted intervention including a patient‐mediated intervention and reminders with another multifaceted intervention also including a patient‐mediated intervention and reminders; and one study compared a multifaceted intervention including educational meetings, patient‐mediated interventions and distribution of educational material with another multifaceted intervention including educational meetings, patient‐mediated interventions and distribution of educational material.
Three studies reported the performance of a barriers assessment and based its interventions on identified barriers (Cooper 2013; Coylewright 2016; Epstein 2017).
Conceptual framework
Thirty‐one out of the 87 studies included in this update used or referred to a conceptual framework. Six studies referred to the Ottawa Decision Support Framework (Causarano 2014; Murray 2010; Raynes‐Greenow 2010; Schroy 2011; Stacey 2006; van Peperstraten 2010); two studies referred to the RE‐AIM framework (Reach, Effectiveness, Adoption, Implementation, Maintenance) (Branda 2013; LeBlanc 2015a); two studies referred to the Four Habits model (Fossli 2011; Tai‐Seale 2016); and two studies referred to the UKMRC framework (Medical Research Council guidance) (Köpke 2014; Mathers 2012). Five studies used a conceptual model but did not describe it (Bernhard 2011; Butow 2004; Hamann 2011; Hamann 2014; Loh 2007). The 14 other studies each referred to a different conceptual model, including the 4E Model (Haskard 2008); the Empowerment Model by Conger and Kanungo (Davison 1997); the LEAPS (Listen, Educate, Assess, Partner and Support) framework (Roter 2012); the Markov Model (Stiggelbout 2008); the Model of Interpersonal Interaction (Elwyn 2004); the SWOT analysis (Strengths, Weaknesses, Opportunities and Threats) (Wetzels 2005); the Theory of Planned Behaviour (Légaré 2012); the Framework for Accountable Decision‐Making (FADM) (Maranda 2014); the Integrative Theory, Protection Motivation Theory and Self‐Determination Theory (Sheridan 2014); the WISE (Whole System Informing Self‐management Engagement) Model (Kennedy 2013); the NIH PROMIS framework (Almario 2016); the three‐step model for SDM (Ampe 2017); the Systems Engineering Initiative for Patient Safety (SEIPS) Model (Cox 2017); and the Bandura’s social cognitive theory of self‐efficacy (Maclachlan 2016) .
Outcome measures
Primary outcome (use of shared decision making)
Of 87 studies, 59 reported patient‐reported outcome measures (PROMs), 19 reported observer‐based outcome measures (OBOMs), and nine reported both OBOMs and PROMs. PROMs were used to measure patient or family caregiver’s self‐reported experiences of participating in the decision‐making process when facing health treatment or screening decisions. Among 68 studies using PROMs, 30 unique scales or subscales were used to measure the use of SDM from a patient'S perspective. In 29 studies, PROMs were the “perceived level of control in decision making” or “role assumed during the consultation” (adaptation of the Control Preference Scale (Degner 1992). Two other PROMs were the SDMQ‐9 (Kriston 2010), and the Patient Activation Measure (PAM) (Hibbard 2004; Hibbard 2005). Twenty‐seven additional unique scales or subscales were used in the studies analyzed. For more details, see Characteristics of included studies. Among the 28 studies that used OBOMs, 16 unique scales or subscales were used to measure the use of SDM from an observer‐based perspective. Study authors reporting observer‐based outcomes used the OPTION scale (Elwyn 2003) in 15 studies, and OPTION‐5 (Barr 2015) in two studies. Fourteen additional unique scales or subscales were used in the studies analyzed. For more details, see Characteristics of included studies.
Secondary outcomes
Study authors reported most on affective‐cognitive outcomes, followed by health outcomes, behavioral outcomes and process outcomes. Adverse events were seldom reported. None of the studies assessed distress or equity.
Excluded studies
After full‐text assessment of articles for eligibility, we initially excluded 613 articles. The reasons for exclusion were related to the design of the study, the type of participants, the type of outcome measure, the content of the intervention, and the language. Main reasons for exclusion of the 39 studies listed in Excluded studies are presented under Characteristics of excluded studies.
Risk of bias in included studies
Further details on the ratings and rationale for risk of bias are in the 'Risk of bias' tables in the Characteristics of included studies tables and displayed in Figure 2 and Figure 3. The 'Risk of bias' assessment reported there was based on the primary outcome only.
2.
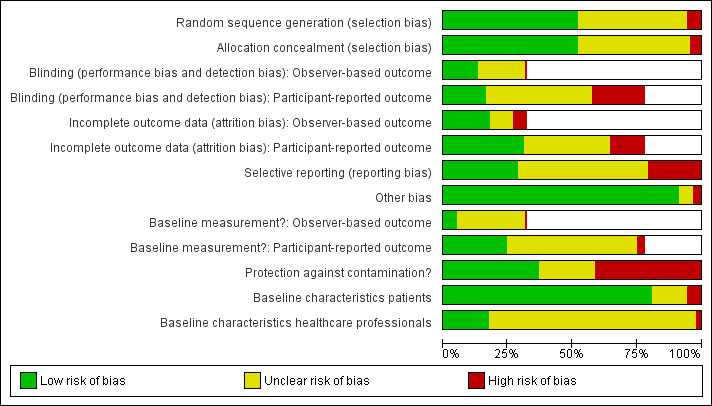
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
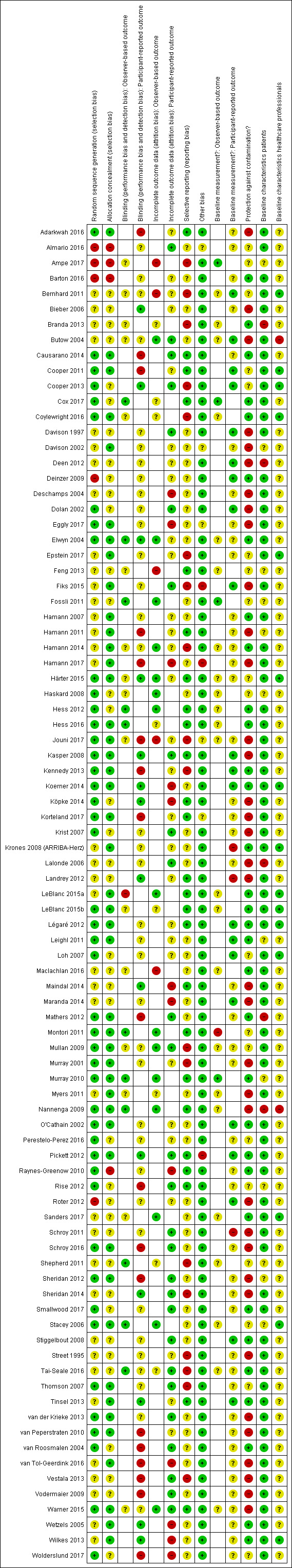
'Risk of bias' summary for each included study.
Allocation
Allocation concealment was rated as being at low risk of bias in 44 of 87 studies (51%), unclear risk of bias in 38 studies (44%) and high risk of bias in five studies (6%) .
Blinding
For assessing risk of detection bias in the 28 studies that used observer‐based outcome measures (OBOMs), blinding was rated as being at low risk of bias in 11 studies (39%), unclear risk in 16 studies (57%) and high risk in one study (4%). In the 68 studies that used patient‐reported outcome measures (PROMs), blinding was rated as being at low risk of bias in 14 studies (21%), unclear risk in 36 studies (53%) and high risk in 18 studies (26%).
Incomplete outcome data
Of the 28 studies that used OBOMs, incomplete outcome data were rated as being at low risk of bias in 15 studies (53%), unclear risk in eight studies (29%) and high risk in five studies (18%). Of the 68 studies that used PROMs, incomplete outcome data were rated as being at low risk of bias in 27 studies (40%), unclear risk in 29 studies (42%) and high risk in 12 studies (17%).
Selective reporting
For assessing risk of reporting bias, selective outcome reporting was rated as being at low risk of bias in 25 of 87 studies (29%), unclear risk in 44 studies (50%) and high risk in 18 studies (21%).
Other potential sources of bias
Among the 87 studies, in 79 studies other risks of bias were rated as low (91%), in five studies they were unclear (6%) and in three studies they were high (3%).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Interventions targeting patients compared to usual care or interventions of the same type for shared decision making.
| Interventions targeting patients compared to usual care or to interventions of the same type for shared decision making | ||||||
| Patient or population: patients, including healthcare consumers and simulated patients Settings: Australia, Canada, Germany, Namibia, Spain, Sweden, the Netherlands, UK, USA Interventions: interventions designed to improve shared decision making among healthcare professionals that target patients (for example, patient‐mediated interventions) Comparison: usual care or interventions of the same type | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk difference (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| [control] | [experimental] | |||||
| a‐ Intervention targeting patients compared to usual care | ||||||
| Shared decision making (observer based outcome measure (OBOM), continuous measures) (follow‐up: up to 6 months) | ‐ | SMD 0.54 higher (0.13 lower to 1.22 higher) | ‐ | 424 (4 randomized trials) | ⨁◯◯◯ VERY LOW a,b,c,d | Scales are: OPTION (0‐100) and RIAS. Higher score indicates more shared decision making use. One study was not included in the quantitative synthesis and was consistent with the pooled result |
| Shared decision making (patient reported outcome measure (PROM), continuous measures) (follow‐up: up to 3 years) | ‐ | SMD 0.32 higher (0.16 higher to 0.48 higher) | ‐ | 1386 (9 randomized) | ⨁◯◯◯ VERY LOW a,b,c | Scales are: Patient activation measure (0‐100), patient self‐advocacy (1‐5), COMRADE (0‐100), decision evaluation scale (1‐5), clinicians’ participatory decision making (1‐5), satisfaction with decision making process (0‐100), CollaboRATE (0‐100), patient role in treatment decision (1‐5). Higher score indicates more shared decision making use. One study was not included in the quantitative synthesis. It is unlikely that it would change the direction of the effect size estimate given that its sample size was not very large. |
| Shared decision making (PROM), dichotomous measures (follow‐up : up to 3 months) | Study population | ‐0.09 (‐0.19 to 0.01) | 754 (6 randomized trials) | ⨁◯◯◯ VERY LOW a,c,f | Three studies were not included in the quantitative synthesis.The first study did not support the pooled result but given that the pooled estimate of the effect size is in favor of the control group, it is likely that adding that study would move the pooled estimate of the effect size toward a null effect. The second study did not support the pooled result but given its very large sample size, it is likely that adding this study would move the pooled estimate of the effect size toward a positive effect. The third study supported the pooled result toward the null effect. | |
| 56 per 100 | 46 per 100 | |||||
| Low risk population | ||||||
| 33 per 100e | 33 per 100 | |||||
| High risk population | ||||||
| 88 per 100e | 60 per 100 | |||||
| Decision regret (follow‐up : 6 months) | ‐ | SMD 0.10 lower (0.39 lower to 0.19 higher) | ‐ | 212 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,h | Decision regret scale (0‐100). Higher score indicates more regret after decision |
| Health‐related quality of life (physical) (follow‐up: 3 months post‐operatively) | ‐ | SMD 0.00 (0.36 lower to 0.36 higher) | ‐ | 116 (1 randomized trial) | ⨁◯◯◯ VERY LOW c,g,h | Physical component scale of SF‐36 (0‐100). Higher score indicate better quality of life. |
| Health‐related quality of life (mental) (follow‐up: 3 months post‐operatively) | ‐ | SMD 0.10 higher (0.26 lower to 0.46 higher) | ‐ | 116 (1 randomized trial) | ⨁◯◯◯ VERY LOW c,g,h | Mental component scale of SF‐36 (0‐100). Higher score indicate better quality of life. |
| Consultation length (minutes) | ‐ | SMD 0.10 higher (0.39 lower to 0.58 higher) | ‐ | 224 (2 randomized trials) | ⨁◯◯◯ VERY LOW c,f,g,h | |
| Cost (£) | ‐ | SMD 0.82 higher (0.42 higher to 1.22 higher) | ‐ | 105 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,h | |
| b‐ Intervention targeting patients compared to intervention of the same type | ||||||
| Shared decision making (OBOM, continuous) (post‐visit) | ‐ | SMD 0.88 higher (0.39 higher to 1.37 higher) | ‐ | 271 (3 randomized trials) | ⨁◯◯◯ VERY LOW b,c,g,h | OPTION scale (0‐100). Higher score indicates more shared decision making use. Decision aid study increase the use of shared decision making compared to booklet or pamphlet |
| Shared decision making (PROM, continuous) (follow‐up: up to 6 months) | ‐ | SMD 0.03 higher (0.18 lower to 0.24 higher) | ‐ | 1906 (11 randomized trials) | ⨁◯◯◯ VERY LOW b,c,g,h | Scales are: Decision making subscale of the Modified Perceived involvement in care scale (4‐20), Patient Activation Measure (0‐100), 1‐item question on “who makes decisions about medical treatment” (1‐5), Satisfaction With Decision Making Process scale (12‐60), Problem‐Solving Decision‐Making Scale (1‐5), SDM‐Q9 (0‐100), patient role in treatment decision (1‐5), SDM‐Q (0‐11), Patient‐reported shared decision making (0‐4). Higher score indicates more shared decision making use. Two studies were not included in the quantitative synthesis but supported the pooled results. |
| Shared decision making (PROM, categorical or dichotomous) (follow‐up : up to 6 weeks) | Study population | 0.03 (‐0.02 to 0.08) | 2272 (10 randomized trials) | ⨁◯◯◯ VERY LOW a,c,f | Three studies were not included in the quantitative synthesis.Two of them were consistent with the pooled results, but the third reported an increase in the use of shared decision making for the intervention group. | |
| 38 per 100 | 40 per 100 | |||||
| Low risk population | ||||||
| 18 per 100e | 22 per 100 | |||||
| High risk population | ||||||
| 73 per 100e | 52 per 100 | |||||
| Decision regret | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Health‐related quality of life (physical) | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Health‐related quality of life (mental) | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Consultation length (minutes) | ‐ | SMD 0.65 lower (1.29 lower to 0.00 ) | ‐ | 39 (1 randomized trial) | ⨁◯◯◯ VERY LOW c,g,h | |
| Cost | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. We downgraded the certainty of evidence by two levels due to very serious limitations in the design (most of the studies are at high risk of bias (≥ 50%). Across studies, taking all low risk and unclear risk judgements together, there are ≥ 50% of unclear risk for our key domains) b. We downgraded the certainty of evidence by two level due to unexplained high heterogeneity (I2 ≥ 50% and P value for heterogeneity ≤ 0.05) c. We downgraded the certainty of evidence by one level due to indirectness of evidence (important difference in populations) d. We downgraded the certainty of evidence by two levels due to imprecision (insufficient number of participants for more than one study and large confidence interval) e. The low and high risk values are the two extreme percentages of events. f. We downgraded the certainty of evidence by one level due to small heterogeneity (I2 < 50% or I2 ≥ 50% and P value > 0.05) g. We downgraded the certainty of evidence by one level due to serious limitations in the design (most of the studies are at unclear risk of bias (≥ 50% of the studies are at unclear risk)) h. We downgraded the certainty of evidence by one level due to imprecision (insufficient number of participants for one study and/or large confidence interval) GRADE: Grading of Recommendations Assessment, Development and Evaluation; OBOM: Observer‐based outcome measures; PROMs: Patient‐reported outcome measures; RD: Risk difference; SMD: Standardized mean difference.
Summary of findings 2. Interventions targeting healthcare professionals compared to usual care or interventions of the same type for shared decision making.
| Interventions targeting healthcare professionals compared to usual care or interventions of the same type for shared decision making | ||||||
| Patient or population: healthcare professionals responsible for patient care Settings: Australia, Austria, Belgium, Canada, Germany, the Netherlands, New Zealand, Norway, Switzerland, USA, UK Interventions: interventions designed to improve shared decision making among healthcare professionals that target healthcare professionals (for example, distribution of printed educational material, educational meetings, audit and feedback, reminders and educational outreach visits) Comparison: usual care or interventions of the same type | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk difference (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| [control] | [experimental] | |||||
| a‐ Intervention targeting healthcare professionals compared to usual care | ||||||
| Shared decision making (OBOM, continuous) (follow‐up: up to 3 months post‐intervention) | ‐ | SMD 0.70 higher (0.21 higher to 1.19 higher) | ‐ | 479 (6 randomized trials) | ⨁◯◯◯ VERY LOW a,b,c,d | Scales are: Fours Habits Coding Scheme (23‐115), OPTION (0‐100), Decision Support Analysis Tool (0‐100), Control Preference Scale (0‐4), and Family engagement (number of utterances or decision‐making events that families engaged). Higher score indicates more shared decision making use. Two studies were not included in the quantitative synthesis. The first study and one sub‐sample of the second study were consistent with the pooled result. The other sub‐sample of the second study reported no difference between the study groups. |
| Shared decision making (PROM, continuous) (follow‐up : up to 12 months) | ‐ | SMD 0.03 higher (0.15 lower to 0.20 higher) | ‐ | 5772 (5 randomized trials) | ⨁◯◯◯ VERY LOW a,b,c,d | Scales are: Physicians’ participatory decision making style (0‐4), short‐form healthcare climate questionnaire (0‐100), SDM‐Q9 (0‐100), and Overall PSA SDM perception (5‐20). Higher score indicates more shared decision making use. One study was not included in the quantitative synthesis and reported an increase in the use of shared decision making for the intervention group. |
| Shared decision making (PROM, categorical or dichotomous) (follow‐up: up to 8 weeks after delivery of pregnant women) | Study population | 0.01 (‐0.03 to 0.06) | 6303 (2 randomized trials) | ⨁◯◯◯ VERY LOW a,b,c | One study was not included in the quantitative synthesis and was consistent with the pooled results. | |
| 21 per 100 | 22 per 100 | |||||
| Low risk population | ||||||
| 19 per 100e | 17 per 100 | |||||
| High risk population | ||||||
| 36 per 100e | 45 per 100 | |||||
| Decision regret (follow‐up: 2 weeks) | ‐ | SMD 0.29 higher (0.07 higher to 0.51 higher) | ‐ | 326 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,d | Decision regret scale (0‐100). Higher score indicates more regret after decision. The slight effect observed on patient decisional regret was not clinically significant. |
| Health‐related quality of life (physical) (follow‐up: 2 weeks) | ‐ | SMD 0.16 higher (0.05 lower to 0.36 higher) | ‐ | 359 (1 randomized trial) | ⨁⨁◯◯ LOW a,c | Scale are : Physical scale of SF‐12 (0‐100) and SF12v2 (0‐100). Higher score indicate better quality of life. |
| Health‐related quality of life (mental) (follow‐up: 2 weeks) | ‐ | SMD 0.28 higher (0.07 to 0.49 higher) | ‐ | 359 (1 randomized trial) | ⨁⨁◯◯ LOW a,c | Scale are : Mental scale of SF‐12 (0‐100) and SF12v2 (0‐100). Higher score indicate better quality of life. |
| Consultation length (minutes) | ‐ | SMD 0.51 higher (0.21 higher to 0.81 higher) | ‐ | 175 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,d | |
| Cost | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| b‐ Intervention targeting healthcare professionals compared to intervention of the same type | ||||||
| Shared decision making (OBOM, continuous) (post‐visit) | ‐ | SMD 0.30 lower (1.19 lower to 0.59 higher) | ‐ | 20 (1 randomized trial) | ⨁◯◯◯ VERY LOW c,f | OPTION scale (0‐100). Higher score indicates more shared decision making use.
Intervention group included: education meeting (in shared decision‐making skill) + audit and feed‐back. Control group included: educational meeting (in risk communication skills) + audit and feed‐back. One study was not included in the quantitative synthesis but reported significant positive results. |
| Shared decision making (PROM, continuous) (follow‐up: up to 4 weeks) | ‐ | SMD 0.24 higher (0.10 lower to 0.58 higher) | ‐ | 1459 (2 randomized trials) | ⨁◯◯◯ VERY LOW a,b,c,d | Scales are: COMRADE (0‐100) and SDM‐Q. Higher score indicates more shared decision making use. In one study multifaceted intervention like educational meeting, audit and feedback, educational material and educational outreach visit increase the use of shared decision making compared to educational meeting alone (control group). In the second study, no differences were found between intervention and control groups. |
| Decision regret | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Health‐related quality of life (physical) | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Health‐related quality of life (mental) | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Consultation length | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Cost | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. We downgraded the certainty of evidence by one level due to serious limitations in the design (most of the studies are at unclear risk of bias (≥ 50% of the studies are at unclear risk)) b. We downgraded the certainty of evidence by two levels due to unexplained high heterogeneity (I2 ≥ 50% and P value for heterogeneity ≤ 0.05) c. We downgraded the certainty of evidence by one level due to indirectness of evidence (important difference in populations) d. We downgraded the certainty of evidence by one level due to imprecision (insufficient number of participants for one study and/or large confidence interval)
e. The low and high risk values are the two extreme percentages of events
f. We downgraded the certainty of evidence by two levels due to imprecision (insufficient number of participants for more than one study and large confidence interval) GRADE: Grading of Recommendations Assessment, Development and Evaluation; OBOM: Observer‐based outcome measures; PROMs: Patient‐reported outcome measures; RD: Risk difference; SMD: Standardized mean difference.
Summary of findings 3. Interventions targeting healthcare professionals and patients compared to usual care or interventions of the same type for shared decision making.
| Interventions targeting healthcare professionals and patients compared to usual care or interventions of the same type for shared decision making | ||||||
| Patient or population: healthcare professionals and patients Settings: Australia, Canada, Denmark, Germany, Norway, the Netherlands, UK, USA Interventions: intervention designed to improve shared decision making among healthcare professionals that target both healthcare professionals and patients (for example, a patient‐mediated intervention combined with an intervention targeting healthcare professionals) Comparison: usual care or interventions of the same type | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk difference (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| [control] | [experimental] | |||||
| a‐ Interventions targeting healthcare professionals and patients compared to usual care | ||||||
| Shared decision making (OBOM, continuous) (follow‐up : up to 3 months) | ‐ | SMD 1.10 higher (0.42 higher to 1.79 higher) | ‐ | 1270 (6 randomized trials) | ⨁◯◯◯ VERY LOW a,b,c,d,e | OPTION scale (0‐100). Higher score indicates more shared decision making use. |
| Shared decision making (PROM, continuous) (follow‐up: up to 6 weeks) | ‐ | SMD 0.13 higher (0.02 lower to 0.28 higher) | ‐ | 1479 (7 randomized trials) | ⨁◯◯◯ VERY LOW a,c,f | Scales are: Physicians’ participatory decision making style (0‐4), SDM‐Q9 (0‐100), COMRADE (0‐100), Patient activation measure (0‐100), Overall PSA SDM perception (5‐20), CollaboRATE (0‐100), Healthcare Climate Questionnaire (0‐100). Higher score indicates more shared decision making use. Two studies were not included in the quantitative synthesis. One study reported an increase in the use of shared decision making for the intervention group and the second study did not report any differences between the study groups. |
| Shared decision making (PROM, categorical or dichotomous) (post‐visit) | Study population | ‐0.01 (‐0.20 to 0.19) | 266 (2 randomized trials) | ⨁◯◯◯ VERY LOW a,c,d,f | One study was not included in the quantitative synthesis and the results were consistent with the pooled results. | |
| 41 per 100 | 36 per 100 | |||||
| Low risk population | ||||||
| 36 per 100g | 27 per 100 | |||||
| High risk population | ||||||
| 48 per 100g | 58 per 100 | |||||
| Decision regret (follow‐up : 3 months) | ‐ | SMD 0.13 higher (0.08 lower to 0.33 higher) | ‐ | 369 (1 randomized trial) | ⨁⨁◯◯ LOW a,c | Decision regret scale (0‐100). Higher score indicates more regret after decision |
| Health‐related quality of life (physical) (follow‐up: 6 weeks) | ‐ | SMD 0.08 higher (0.37 lower to 0.54 higher) | ‐ | 75 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,d | Scale are : Physical scale of SF‐12 (0‐100) and SF12v2 (0‐100). Higher score indicate better quality of life. |
| Health‐related quality of life (mental) (follow‐up: 6 weeks) | ‐ | SMD 0.01 higher (0.44 lower to 0.46 higher) | ‐ | 75 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,d | Scale are : Mental scale of SF‐12 (0‐100) and SF12v2 (0‐100). Higher score indicate better quality of life. |
| Consultation length (minutes) | ‐ | SMD 3.72 higher (3.44 higher to 4.01 higher) | ‐ | 536 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,d | |
| Cost | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| b‐ Interventions targeting healthcare professionals and patients compared to intervention of the same type | ||||||
| Shared decision making (OBOM, continuous) (post‐visit) | ‐ | SMD 0.29 lower (1.17 lower to 0.6 higher) | ‐ | 20 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,d | OPTION scale (0‐100). Higher score indicates more shared decision making use. |
| Shared decision making (OBOM, categorical or dichotomous) (post‐visit) | Study population | ‐0.04 (‐0.13 to 0.04) | 134 (1 randomized trial) | ⨁◯◯◯ VERY LOW c,d,h | ||
| 8 per 100 | 4 per 100 | |||||
| Low risk population | ||||||
| N/A | N/A | |||||
| High risk population | ||||||
| N/A | N/A | |||||
| Shared decision making (PROM, continuous) (post‐visit) | ‐ | SMD 0.00 (0.32 lower to 0.32 higher) | ‐ | 150 (1 randomized trial) | ⨁◯◯◯ VERY LOW a,c,d | CollaboRATE (0‐100). Higher score indicates more shared decision making use. |
| Decision regret | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Health‐related quality of life (physical) | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Health‐related quality of life (mental) | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Consultation length | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| Cost | ‐ | ‐ | ‐ | ‐ | ‐ | No data available for this outcome |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. We downgraded the certainty of evidence by one level due to serious limitations in the design (most of the studies are at unclear risk of bias (≥ 50% of the studies are at unclear risk)) b. We downgraded the certainty of evidence by two levels due to unexplained high heterogeneity (I2 ≥ 50% and P value for heterogeneity ≤ 0.05) c. We downgraded the certainty of evidence by one level due to indirectness of evidence (important difference in populations) d. We downgraded the certainty of evidence by one level due to imprecision (insufficient number of participants for one study and/or large confidence interval) e. We downgraded the certainty of evidence by one level due to possible publication bias f. We downgraded the certainty of evidence by one level due to small heterogeneity (I2 < 50% or I2 ≥ 50% and P value > 0.05) g. The low and high risk values are the two extreme percentage of events h. We downgraded the certainty of evidence by two levels due to very serious limitations in the design (most of the studies are at high risk of bias (≥ 50%). Across studies, taking all low risk and unclear risk judgements together, there are ≥ 50% of unclear risk for our key domains. GRADE: Grading of Recommendations Assessment, Development and Evaluation; OBOM: Observer‐based outcome measures; PROMs: Patient‐reported outcome measures; RD: Risk difference; SMD: Standardized mean difference.
Please refer to Table 1, Table 2, Table 3, Data and analyses,Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15, Figure 4, Figure 5, Figure 6, Appendix 8, Appendix 9 and Appendix 6 for detailed results.
1. Effect of intervention on primary outcome: interventions targeting patients compared to usual care.
| Observer‐based outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | N | SMD | SMD (95% CI) | I2 | |
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | OPTION | 96 | 0.93 (0.50 to 1.36) | 0.54 (‐0.13 to 1.22) | 84% | X |
| Maclachlan 2016 | Patient‐mediated intervention (educational meeting for patient) | Usual care | RIAS for patients (Patient activation and engagement) | 289 | ‐0.05 (‐0.28 to 0.18) | X | ||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | OPTION (/100) | 19 | 1.23 (0.17 to 2.29) | x | ||
| Tai‐Seale 2016 | Patient‐mediated intervention (one‐page ASK handout) | Usual care | OPTION5 (/100) | 20 | 0.32 (‐0.56 to 1.21) | x | ||
| Observer‐based outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Observer‐based outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | Direct quote | ||||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Who made the decision in today's consultation | Intervention: Median (2) ‐ Range (1‐4); Control: Median (2) ‐ Range (1‐4) ‐ No difference | na | |||
| Haskard 2008 | Patient‐mediated intervention | Usual care | Physician informative and participatory | Unit of error analysis | na | |||
| Haskard 2008 | Patient‐mediated intervention | Usual care | Patient active | Unit of error analysis | na | |||
| Haskard 2008 | Patient‐mediated intervention | Usual care | Physician‐patient interaction | Unit of error analysis | na | |||
| Patient‐reported outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Deen 2012 | Patient‐mediated intervention (decision aid) | Usual care | Patient Activation Measure (PAM) | 138 | ‐0.07 (‐0.40 to 0.26) | 0.28 (0.13 to 0.44) | 57% | |
| Deen 2012 | Patient‐mediated intervention (Patient Activation) | Usual care | Patient Activation Measure (PAM) | 142 | 0.09 (‐0.24 to 0.41) | X | ||
| Deen 2012 | Patient‐mediated intervention (decision aid + Patient Activation) | Usual care | Patient Activation Measure (PAM) | 137 | 0.04 (‐0.29 to 0.38) | |||
| Maranda 2014 | Patient‐mediated intervention (patient activated intervention) | Usual care | Patient Activation Measure (PAM) | 132 | 0.12 (‐0.22 to 0.47) | x | ||
| Pickett 2012 | Patient‐mediated intervention (training for patients) | Usual care | Patient self‐advocacy (immediately after) | Pre: 428; Post: 342 | 0.20 (‐0.01 to 0.41) | x | ||
| Pickett 2012 | Patient‐mediated intervention (training for patients) | Usual care | Patient self‐advocacy (6 months after) | Pre: 428; Post: 318 | 0.02 (‐0.20 to 0.24) | |||
| van der Krieke 2013 | Patient‐mediated intervention (web‐based information and decision tool) | Usual care | COMRADE (communication) | 73 | 0.94 (0.46 to 1.43) | x | ||
| van Peperstraten 2010 | Patient‐mediated intervention (decision aid + support call) | Usual care | Decision Evaluation scale | 252 | 0.53 (0.28 to 0.78) | x | ||
| Cooper 2011 | Patient‐mediated intervention | Usual care | Participatory Decision making (PDM) | 83 | 0.21 (‐0.22 to 0.64) | x | ||
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Satisfaction with Decision making process (SDMP) | 153 | 0.50 (0.18 to 0.82) | X | ||
| Tai‐Seale 2016 | Patient‐mediated intervention (one‐page ASK handout) | Usual care | CollaboRATE (%) | 150 | 0.31 (‐0.01 to 0.63) | X | ||
| Almario 2016 | Patient‐mediated intervention (GI PROMIS) | Usual care | SDMQ‐9 | 303 | 0.02 (‐0.22 to 0.25) | X | ||
| Eggly 2017 | Patient‐mediated intervention (QPL‐only) | Usual care | Patient role in treatment decision | 59 | 0.05 (‐0.46 to 0.56) | x | ||
| Eggly 2017 | Patient‐mediated intervention (QPL‐plus‐Coach) | Usual care | Patient role in treatment decision | 55 | ‐0.14 (‐0.67 to 0.39) | |||
| Patient‐reported outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | N | RD | RD (95% CI) | I2 | |
| Krist 2007 | Patient‐mediated intervention (decision aid brochure) | Usual care | Modified Control Preference Scale | 237 | 0.00 (‐0.14 to 0.14) | ‐0.09 (‐0.19 to 0.01) | 48% | x |
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Usual care | Modified Control Preference Scale | 261 | ‐0.01 (‐0.14 to 0.13) | |||
| Landrey 2012 | Patient‐mediated intervention (mailed flyer) | Usual care | Modified Control Preference Scale | 152 | ‐0.03 (‐0.19 to 0.12) | x | ||
| Murray 2001 | Patient‐mediated intervention (decision aid) | Usual care | Modified Control Preference Scale | 105 | ‐0.28 (‐0.44 to ‐0.12) | x | ||
| Sheridan 2014 | Patient‐mediated intervention (decision aid) | Usual care | Shared decision | 114 | ‐0.17 (‐0.35 to 0.002) | x | ||
| Vestala 2013 | Patient‐mediated intervention (patient participation in nursing documentation) | Usual care | Modified Control Preference Scale | 39 | 0.00 (‐0.30 to 0.30) | x | ||
| Vodermaier 2009 | Patient‐mediated intervention (decision aid) | Usual care | Modified Control Preference Scale | 107 | ‐0.01 (‐0.19 to 0.17) | x | ||
| Patient‐reported outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | Direct quote | ||||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Who made the decision in today's consultation | Intervention: Median (3) ‐ Range (1‐5); Control: Median (3) ‐ Range (1‐5) ‐ No difference | na | |||
| van der Krieke 2013 | Patient‐mediated intervention (web‐based information and decision tool) | Usual care | COMRADE (confidence) | COMRADE confidence in decision: F(1.67) = 0.086. P=0.77. see also Table 3 | na | |||
| Vodermaier 2009 | Patient‐mediated intervention (decision aid) | Usual care | Man‐Son‐Hing Instrument | No data | na | |||
| van Tol‐Geerdink 2016 | Patient‐mediated intervention (decision aid) | Usual care | Patient participation | "At t2, 95% of the patients in the decision aid group indicated that they actually had been involved in the decision, compared to 83% in the usual care group (P = 0.002). As such, the decision aid had the expected effect on patient participation."Page 466 | na | |||
| Wolderslund 2017 | Patient‐mediated intervention (question prompt list) + other (digital audio recording) | Usual care | Involvement in decision making | "Intention‐to‐treat analyses of the participants’ perception of the consultation showed that on average, 4.5% more patients (range: 4.0‐5.5), regardless of intervention group, rated their satisfaction with the treatment, confidence in and relationship with the health professional, and involvement in decision making in the highest reply category compared with patients in the control group. This increase was significant (p = 0.001) except for decision making (p = 0.044)." Page 247 | na | |||
| Wolderslund 2017 | Other (digital audio recording) | Usual care | Involvement in decision making | "Intention‐to‐treat analyses of the participants’ perception of the consultation showed that on average, 4.5% more patients (range: 4.0‐5.5), regardless of intervention group, rated their satisfaction with the treatment, confidence in and relationship with the health professional, and involvement in decision making in the highest reply category compared with patients in the control group. This increase was significant (p = 0.001) except for decision making (p = 0.044)." Page 247 | na | |||
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | Involvement in decision making | There was no difference between intervention and control group regarding those who "totally agree" that the doctor has involved them in the decision (37.3% vs 39.1%; fig 2) | na | |||
ASK: Ask Share Know; CI: confidence interval; COMRADE: Combined Outcome Measure for Risk communication And treatment Decision making Effectiveness; GI PROMIS: gastrointestinal Patient Reported Outcomes Measurement Information System; N: sample size; na: not applicable; OPTION: observing patient involvement; QPL: question prompt list; RD: risk difference; SDM: shared decision making; SMD: standardized mean difference.
2. Effect of interventions on primary outcome: interventions targeting healthcare professionals compared to usual care.
| Observer‐based outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Fossli 2011 | Educational meeting + audit and feedback + distribution of educational material | Usual care | Fours Habits Coding Scheme (4HCS) | 51 | 0.38 (‐0.17 to 0.94) | 0.60 (0.15 to 1.06) | 80% | x |
| Shepherd 2011 | Educational outreach visit | Usual care | Assessing Communication about Evidence and Patient Preferences (ACEPP) | 36 | 1.25 (0.53 to 1.97) | |||
| Shepherd 2011 | Educational outreach visit | Usual care | OPTION | 36 | 0.90 (0.21 to 1.58) | x | ||
| Stacey 2006 | Distribution of educational materials + educational meeting + audit and feedback and barriers assessment | Usual care | Decision Support Analysis Tool (DSAT) | 38 | 2.07 (1.26 to 2.87) | x | ||
| Sanders 2017 | Educational meeting + Audit feed‐back | Usual care | OPTION | 175 | 0.93 (0.62 to 1.25) | |||
| Sanders 2017 | Educational meeting + Audit feed‐back | Usual care | Level of autonomy (CPS) (2=SDM) | 175 | 0.85 (0.54 to 1.16) | x | ||
| Ampe 2017 | Educational meetings (Training) | usual care | OPTION (/100) | 21 | ‐0.10 (‐0.96 to 0.76) | x | ||
| Cox 2017 | Educational meeting + Distribution of educational material | Usual care | Family engagement | Pre: 144 post: 154 | 0.11 (‐0.21 to 0.42) | x | ||
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | usual care | OPTION (/100) | 25 | 0.08 (‐0.84 to 0.99) | x | ||
| Observer‐based outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Observer‐based outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Bernhard 2011 | Educational meeting + audit and feedback | Usual care | SDM framework (DAS‐O subscale) (SGA) | There was no effect for this variable for SGA doctors (estimated population mean difference = 0.52, SE = 1.39, ES = 0.04, P = 0.71) | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback | Usual care | SDM framework (DAS‐O subscale) (ANZ) | "In the ANZ cohort, the estimated population mean of the difference for establishing the SDM framework (subscale 1 of the DAS‐O) was statistically significant, indicating that after the training workshop, doctors in the experimental group within the ANZ cohort displayed more behaviours designed to establish the SDM framework than doctors in the control group (estimated population mean difference = 3.42, SE = 1.50, ES = 0.30, P = 0.03). However, the ES was small." Page 2578 | na | |||
| Murray 2010 | Educational meeting + audit and feedback + distribution of educational materials + educational outreach + barriers assessment | Usual care | Decision Support Analysis Tool (DSAT) | "The mean score change from baseline in the intervention group 3.75 (95% CI 2.46 to 5.03) was significantly greater than the mean score change in the control group ‐0.667 (95% CI ‐1.57 to 0.24) using the two sided t‐test (P < 0.0001)" Page 116 | na | |||
| Patient‐reported outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% IC) | I2 | |
| Cooper 2011 | Educational meeting | Usual care | Participatory Decision making (PDM) | 94 | 0.11 (‐0.30 to 0.51) | 0.03 (‐0.15 to 0.20) | 83% | x |
| Kennedy 2013 | Educational meeting | Usual care | Shared decision making (short‐form healthcare climate questionnaire) ‐ 12 month vs baseline | 4005 | ‐0.05 (‐0.12 to 0.01) | x | ||
| Koerner 2014 | Educational meeting | Usual care | SDM‐Q‐9 (post‐intervention) | Pre: 402; Post: 463 | ‐0.08 (‐0.26 to 0.11) | x | ||
| Koerner 2014 | Educational meeting | Usual care | SDM‐Q‐9 (Six‐months after) | Pre: 402; Post: 461 | ‐0.03 (‐0.22 to 0.16) | |||
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | SDM‐Q‐9 (6 months) | Pre: 940; Post: 731 | 0.32 (0.17 to 0.46) | x | ||
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | SDM‐Q‐9 (12 months) | Pre: 940; Post: 628 | 0.16 (0.00 to 0.32) | |||
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | SDM‐Q‐9 (18 months) | Pre: 940; Post: 570 | 0.25 (0.08 to 0.41) | |||
| Wilkes 2013 | Distribution of educational material | Usual care | Overall PSA SDM perception | 479 | ‐0.13 (‐0.32 to 0.05) | x | ||
| Patient‐reported outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| Légaré 2012 | Educational meeting and distribution of educational material | Usual care | Modified Control Preference Scale | Pre: 353; Post: 353 | 0.09 (‐0.01 to 0.19) | 0.01 (‐0.03 to 0.06) | 74% | x |
| O'Cathain 2002 | Educational meeting and distribution of educational material | Usual care | Modified Control Preference Scale (antenatal sample) | Pre: 2745; Post: 2737 | ‐0.02 (‐0.05 to 0.01) | x | ||
| O'Cathain 2002 | Educational meeting and distribution of educational material | Usual care | Modified Control Preference Scale (postnatal sample) | Pre: 3156; Post 3213 | 0.02 (‐0.01 to 0.05) | x | ||
| Patient‐reported outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of educational material | Usual care | Patient involvement preference and actual involvement | "There was considerable variation in patient outcomes between the SGA and ANZ cohorts and no substantial training effect." Page 6 | na | |||
| Légaré 2012 | Educational meeting and distribution of educational material | Usual care | D‐OPTION | 359 | Pre: ‐0.48 (‐0.69 to ‐0.27) | na | ||
ANZ: Australia, New Zealand; CI: confidence interval; CPS: control preference scale; DAS‐O: Decision Analysis System for Oncology; N: sample size; na: not applicable; OPTION: observing patient involvement; PSA: prostatic specific antigen; RD: risk difference; SE: standard error; SGA: Switzerland, Germany and Austria; SDM: shared decision making; SMD: standardized mean difference.
3. Effect of interventions on primary outcome: interventions targeting both patients and healthcare professionals compared to usual care.
| Observer‐based outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Branda 2013 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Level of patient engagement (OPTION) | 39 | 0.85 (0.19 to 1.52) | 1.10 (0.42 to 1.79) | 96% | x |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | OPTION | 421 | 0.54 (0.35 to 0.74) | x | ||
| Hess 2012 | Patient‐mediated intervention + educational meeting | Usual care | OPTION | 200 | 2.83 (2.43 to 3.21) | x | ||
| Coylewright 2016 | Patient‐mediated intervention + Educational meeting | Usual care | OPTION (/100) | 54 | 0.51 (‐0.05 to 1.07) | x | ||
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | OPTION (/100) | 536 | 1.36 (1.17 to 1.55) | X | ||
| Tai‐Seale 2016 | Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers) | Usual care | OPTION5 (/100) | 20 | 0.35 (‐0.53 to 1.24) | x | ||
| Tai‐Seale 2016 | [Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers)] + Patient mediated intervention (one‐page ASK handout) | Usual care | OPTION5 (/100) | 20 | 0.04 (‐0.83 to 0.92) | |||
| Observer‐based outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Observer‐based outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Haskard 2008 | Patient‐mediated intervention + Distribution of educational material + education meeting | Usual care | Physician informative and participatory | Unit of error analysis | na | |||
| Haskard 2008 | Patient‐mediated intervention + Distribution of educational material + education meeting | Usual care | Patient active | Unit of error analysis | na | |||
| Haskard 2008 | Patient‐mediated intervention + Distribution of educational material + education meeting | Usual care | Physician‐patient interaction | Unit of error analysis | na | |||
| Patient‐reported outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Cooper 2011 | Patient‐mediated intervention + Educational meeting | Usual care | Participatory Decision making (PDM) | 101 | 0.16 (‐0.23 to 0.56) | 0.13 (‐0.02 to 0.28) | 44% | x |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | SDM‐Q‐9 | 413 | ‐0.07 (‐0.26 to 0.12) | x | ||
| Hamann 2007 | Patient‐mediated intervention (decision aid) + Educational meeting | Usual care | COMRADE | 82 | 0.16 (‐0.28 to 0.61) | x | ||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Treatment Alliance Scale (TAS) | 75 | 0.07 (‐0.38 to 0.52) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Patient Activation Measure (PAM) | 75 | 0.13 (‐0.32 to 0.58) | x | ||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Patient participation (PP) | 75 | ‐0.09 (‐0.55 to 0.36) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Treatment Alliance Scale (TAS) ‐ 6 months | 64 | 0.00 (‐0.49 to 0.49) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Patient Activation Measure (PAM) ‐ 6 months | 64 | 0.12 (‐0.37 to 0.61) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Patient participation (PP) ‐ 6 months | 64 | 0.09 (‐0.40 to 0.58) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Treatment Alliance Scale (TAS) ‐ 12 months | 63 | 0.16 (‐0.34 to 0.65) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Patient Activation Measure (PAM) ‐ 12 months | 63 | 0.21 (‐0.28 to 0.71) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + Educational meeting | Usual care | Patient participation (PP) ‐ 12 months | 63 | 0.28 (‐0.22 to 0.78) | |||
| Wilkes 2013 | Patient mediated intervention (web‐based educational program for patients) + Distribution of educational material | Usual care | Overall PSA SDM perception | 393 | 0.20 (‐0.03 to 0.43) | x | ||
| Tai‐Seale 2016 | Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers) | Usual care | CollaboRATE (/100) | 150 | 0.51 (0.19 to 0.84) | x | ||
| Tai‐Seale 2016 | [Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers)] + Patient mediated intervention (one‐page ASK handout) | Usual care | CollaboRATE (/100) | 150 | 0.53 (0.21 to 0.86) | |||
| Epstein 2017 | Patient‐mediated intervention (patients & caregivers coaching session + question prompt list) + Educational meeting | Usual care | Health Care Climate Questionnaire (HCCQ) | 265 | 0.00 (‐0.24 to 0.24) | x | ||
| Patient‐reported outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| Mathers 2012 | Patient‐mediated intervention (decision aid) + Educational meeting | Usual care | Modified Control Preference Scale | 169 | ‐0.09 (‐0.23; 0.05) | ‐0.01 (‐0.20 to 0.19) | 60% | x |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Patient perception scale (PPS) | 97 | 0.11 (‐0.10 to 0.31) | x | ||
| Patient‐reported outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Leighl 2011 | Patient‐mediated intervention + educational meeting | Usual care | Modified Control Preference Scale | There was no difference after the intervention: the mean score of the item on the CPS scale in the intervention group was: 2.86 (0.92), it was 2.87 (1.04) in the control group. See Figure 4, page 2082. Data are from the authors. | na | |||
| Loh 2007 | Patient‐mediated intervention + educational meeting | Usual care | PPS (Man‐Son‐Hing) | "In the intervention group, significantly higher patient participation from pre‐ to post‐intervention was found for … the Man‐Son‐Hing patient participation scale." P = 0.10. Page 329 | na | |||
| Wetzels 2005 | Patient‐mediated Intervention (leaflet) + educational outreach visit | Usual care | COMRADE ‐ 4 items | Unable to calculate. No differences between groups were detected. | na | |||
CI: confidence interval; COMRADE: Combined Outcome Measure for Risk communication And treatment Decision making Effectiveness; N: sample size; na: not applicable; OPTION: observing patient involvement; PCOMS: Partners for Change Outcome Management System; PSA: prostatic specific antigen; RD: risk difference; SDM: shared decision making; SMD: standardized mean difference.
4. Effect of interventions on primary outcome: interventions targeting patients compared to other interventions targeting patients.
| Observer‐based outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (usual care and booklet) | OPTION | 100 | 1.21 (0.78 to 1.64) | 0.88 (0.39. to1.37) | 74% | x |
| Nannenga 2009 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (pamphlet) | OPTION | 91 | 1.04 (0.60 to 1.48) | x | ||
| Jouni 2017 | Patient‐mediated intervention (conventional risk and genetic risk information + decision aid) | Patient‐mediated intervention (conventional risk information + decision aid) | OPTION5 (/100) | 80 | 0.38 (‐0.06 to 0.82) | x | ||
| Observer‐based outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Observer‐based outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| No study | ||||||||
| Patient reported outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Causarano 2014 | Patient‐mediated intervention (Routine education + educational meeting to patient) | Patient‐mediated intervention (Routine education) | Decision making subscale (M‐PICS) | 39 | ‐0.44 (‐1.08 to 0.19) | 0.03 (‐0.16 to 0.23) | 76% | x |
| Deen 2012 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Patient Activation) | Patient Activation Measure (PAM) | 142 | ‐0.15 (‐0.48 to 0.18) | |||
| Deen 2012 | Patient‐mediated intervention (decision aid + Patient Activation) | Patient‐mediated intervention (Patient Activation) | Patient Activation Measure (PAM) | 141 | ‐0.05 (‐0.38 to 0.28) | x | ||
| Deen 2012 | Patient‐mediated intervention (decision aid + Patient Activation) | Patient‐mediated intervention (decision aid) | Patient Activation Measure (PAM) | 137 | 0.11 (‐0.22 to 0.45) | |||
| Hamann 2011 | Patient‐mediated intervention (SDM training) | Patient‐mediated intervention (cognitive training) | Who makes important decisions about your medical treatment? (postintervention) | 61 | ‐0.18 (‐0.68 to 0.32) | x | ||
| Hamann 2011 | Patient‐mediated intervention (SDM training) | Patient‐mediated intervention (cognitive training) | Who makes important decisions about your medical treatment? (at 6 months) | 48 | ‐1.09 (‐1.70 to ‐0.48) | |||
| Schroy 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Educational material) | Satisfaction with the decision making process | 422 | 0.66 (0.46 to 0.85) | |||
| Schroy 2011 | Patient‐mediated intervention (decision aid + YDR) | Patient‐mediated intervention (Educational material) | Satisfaction with the decision making process | 431 | 0.63 (0.44 to 0.83) | x | ||
| Schroy 2011 | Patient‐mediated intervention (decision aid + YDR) | Patient‐mediated intervention (decision aid) | Satisfaction with the decision making process | 419 | ‐0.03 (‐0.22 to 0.16) | |||
| van Roosmalen 2004 | Patient‐mediated intervention (Shared decision making intervention +decision aid) | Patient‐mediated intervention (decision aid) | Perceived participation in DM (T4) | 78 | 0.30 (‐0.14 to 0.75) | x | ||
| van Roosmalen 2004 | Patient‐mediated intervention (Shared decision making intervention +decision aid) | Patient‐mediated intervention (decision aid) | Perceived participation in DM (T5) | 71 | 0.15 (‐0.31 to 0.62) | |||
| Schroy 2016 | Patient‐mediated intervention (decision aid + risk assessment tool) | Patient‐mediated intervention (decision aid) | Satisfaction with Decision making process (SDMP) | 324 | 0.00 (‐0.22 to 0.22) | x | ||
| Adarkwah 2016 | Patient‐mediated intervention (Computerised decision aid (Time‐to‐event)) | Patient‐mediated intervention (Computerised decision aid (emoticon) | PEF‐FB‐9 (SDM‐Q9) | 304 | ‐0.09 (‐0.31 to 0.14) | x | ||
| Barton 2016 | Patient‐mediated intervention (adapted guide) | Patient‐mediated intervention (Existing medication guide) | The Interpersonal Processes of Care (IPC) measure (which includes a 2‐item decision‐making subscale) (in %) | 97 | ‐0.21 (‐0.61 to 0.19) | |||
| Barton 2016 | Patient‐mediated intervention (adapted guide + decision aid) | Patient‐mediated intervention (Existing medication guide) | The Interpersonal Processes of Care (IPC) measure (which includes a 2‐item decision‐making subscale) (in %) | 110 | ‐0.19 (‐0.56 to 0.19) | |||
| Barton 2016 | Patient‐mediated intervention (adapted guide + decision aid) | Patient‐mediated intervention (adapted guide) | The Interpersonal Processes of Care (IPC) measure (which includes a 2‐item decision‐making subscale) (in %) | 99 | 0.03 (‐0.37 to 0.43) | x | ||
| Eggly 2017 | Patient mediated intervention (QPL‐plus‐Coach) | Patient‐mediated intervention (QPL‐only) | Patient role in treatment decision | 56 | ‐0.18 (‐0.70 to 0.35) | x | ||
| Hamann 2017 | Patient‐mediated intervention (SDM Training for patients) | Patient‐mediated intervention (Cognitive training for patient ‐ control group) | Who makes important decision about your medical treatment ? | 215 | ‐0.26 (‐0.53 to 0.01) | x | ||
| Jouni 2017 | Patient‐mediated intervention (conventional risk and genetic risk information + decision aid) | Patient‐mediated intervention (conventional risk information + decision aid) | SDM‐Q (0‐11) | 207 | 0.13 (‐0.15 to 0.40) | x | ||
| Smallwood 2017 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (web‐based information) | Shared decision Making | 50 | 0.22 (‐0.34 to 0.79) | x | ||
| Patient‐reported outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| Butow 2004 | Patient‐mediated intervention (booklet + brochure + question prompt sheet) | Patient‐mediated intervention (booklet) | Modified Control Preference Scale | 131 | 0.04 (‐0.11 to 0.20) | 0.03 (‐0.02 to 0.08) | 32% | x |
| Davison 1997 | Patient‐mediated intervention (individual empowerment sessions) | Patient‐mediated intervention (information package) | Modified Control Preference Scale | 60 | ‐0.17 (‐0.41 to 0.08) | x | ||
| Deschamps 2004 | Patient‐mediated intervention (pharmacist consultation, patient‐specific information and a 40‐minute consultation with pharmacist) | Patient‐mediated intervention (decision aid) | Modified Control Preference Scale | 90 | 0.11 (‐0.09 to 0.32) | x | ||
| Dolan 2002 | Patient‐mediated intervention (preliminary phase + decision aid) | Patient‐mediated intervention (preliminary phase + educational phase) | Modified Control Preference Scale | 86 | 0.12 (‐0.09 to 0.32) | x | ||
| Kasper 2008 | Patient‐mediated intervention | Patient‐mediated intervention | Modified Control Preference Scale | 278 | 0.03 (‐0.08 to 0.15) | x | ||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Patient‐mediated intervention (decision aid brochure) | Modified Control Preference Scale | 372 | 0.00 (‐0.10 to 0.09) | x | ||
| Raynes‐Greenow 2010 | Patient‐mediated intervention (decision aid (Audio)) | Patient‐mediated intervention (Pamphlet) | Modified Control Preference Scale ‐ First Follow‐up | 351 | 0.04 (‐0.04 to 0.13) | x | ||
| Raynes‐Greenow 2010 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Pamphlet) | Modified Control Preference Scale ‐ First Follow‐up | 343 | 0.04 (‐0.04 to 0.13) | |||
| Raynes‐Greenow 2010 | Patient‐mediated intervention (decision aid (Audio)) | Patient‐mediated intervention (Pamphlet) | Modified Control Preference Scale ‐ Second Follow‐up | 277 | 0.04 (‐0.04 to 0.13) | |||
| Raynes‐Greenow 2010 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Pamphlet) | Modified Control Preference Scale ‐ Second Follow‐up | 286 | 0.07 (‐0.02 to 0.13) | |||
| Stiggelbout 2008 | Patient‐mediated intervention (individualized brochure) | Patient‐mediated intervention (general brochure) | Modified Control Preference Scale | 64 | ‐0.21 (‐0.44 to 0.02) | x | ||
| Thomson 2007 | Patient‐mediated intervention (Computerised decision aid) | Patient‐mediated intervention (Guidelines) | DM role experienced (patient more important in DM) | 106 | 0.23 (0.05; 0.41) | x | ||
| Davison 2002 | Patient‐mediated intervention (computer) | Patient‐mediated intervention (discussion with research nurse) | Modified Control Preference Scale | 734 | 0.02 (‐0.06 to 0.09) | x | ||
| Patient reported outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education program) | Patient‐mediated intervention (4h MS‐specific stress management program.) | Decision autonomy (at 12 months) | "Overall, 70 (IG) and 72 (CG) decisions on DMDs were reported during the 12 months of follow‐up. In both groups, most decisions were reported to be solely of mostly driven by the patient or were shared between patients and physicians with no differences between groups." Page 414 | na | |||
| Lalonde 2006 | Patient‐mediated intervention (decision aid + personal risk profile) | Patient‐mediated intervention (decision aid + personal risk profile + personal risk assessment) | Decision satisfaction inventory | No statistically significant differences in patient satisfaction with the decision‐making process were detected between the study groups. Page 55 | na | |||
| Street 1995 | Patient‐mediated intervention (Interactive multimedia program (decision aid)) | Patient‐mediated intervention (brochure (decision aid) | Perceived Decision Control Instrument | "The experimental manipulation (computer program versus brochure) had very little effect on the dependent variables." Page 2280 | na | |||
| Butow 2004 | Patient‐mediated intervention (booklet + brochure + question prompt sheet) | Patient‐mediated intervention (booklet) | Physician behaviours facilitating patient involvement | "On average, oncologists demonstrated about 7.5 of the 12 behaviours, with no significant differences between the groups (cancer consultation preparation package (CCPP) versus control booklet)." Page 4406 | na | |||
| Wolderslund 2017 | Patient‐mediated intervention (question prompt list) + other (digital audio recording) | Other (digital audio recording) | Involvement in decision making | "Intention‐to‐treat analyses of the participants’ perception of the consultation showed that on average, 4.5% more patients (range: 4.0‐5.5), regardless of intervention group, rated their satisfaction with the treatment, confidence in and relationship with the health professional, and involvement in decision making in the highest reply category compared with patients in the control group. This increase was significant (p = 0.001) except for decision making (p = 0.044)." Page 247 | na | |||
CG: control group; CI: confidence interval; DM: decision making; IG: intervention group; M‐PICS: modified perceived involvement in care scale; N: sample size; na: not applicable; OPTION: observing patient involvement; QPL: question prompt list; RD: risk difference; SDM: shared decision making; SMD: standardized mean difference; YDR: Web‐based ‘‘Your Disease Risk’’ colorectal cancer risk assessment tool.
5. Effect of interventions on primary outcome: interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals.
| Observer‐based outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Elwyn 2004 | Educational Meeting + audit and feedback | Educational Meeting + audit and feedback | OPTION | 20 | ‐0.30 (‐1.19 to 0.59) | ‐0.30 (‐1.19 to 0.59) | na | |
| Observer‐based outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Observer‐based outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Feng 2013 | Distribution of educational material (Intervention A) | Distribution of educational material (Brochure + control) | Prostate Cancer Screening Abstraction Tool | P‐value < 0.05 | na | |||
| Patient‐reported outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Elwyn 2004 | Educational meeting + audit and feedback | Educational Meeting + audit and feedback | COMRADE (communication) ‐ Time 1 | Pre: 187; Post: 327 | ‐0.07 (‐0.29 to 0.15) | 0.24 (‐0.10 to 0.58) | 87% | |
| Elwyn 2004 | Educational meeting + audit and feedback | Educational Meeting + audit and feedback | COMRADE (communication) ‐ Time 2 | Pre: 163; Post: 290 | ‐0.11 (‐0.34 to 0.13) | |||
| Elwyn 2004 | Educational meeting + audit and feedback | Educational Meeting + audit and feedback | COMRADE (confidence) ‐ Time 1 | Pre: 187; Post: 327 | 0.05 (‐0.17 to 0.27) | x | ||
| Elwyn 2004 | Educational meeting + audit and feedback | Educational Meeting + audit and feedback | COMRADE (confidence) ‐ Time 2 | Pre: 163; Post: 290 | ‐0.18 (‐0.42 to 0.05) | |||
| Krones 2008 (ARRIBA‐Herz) | Educational meeting + audit and feedback + educational material + educational outreach visit | Educational Meeting | PPS (Man Son‐Hing): I made the decision jointly (Score inversé pour respecter le sens de l'échelle) | 1132 | 0.48 (0.36 to 0.60) | |||
| Krones 2008 (ARRIBA‐Herz) | Educational meeting + audit and feedback + educational material + educational outreach visit | Educational Meeting | SDM‐Q | 1132 | 0.40 (0.28 to 0.52) | x | ||
| Krones 2008 (ARRIBA‐Herz) | Educational meeting + audit and feedback + educational material + educational outreach visit | Educational Meeting | PPS (Man‐Son‐Hing) | 1052 | 6.11 (5.82 to 6.40) | |||
| Patient‐reported outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Patient‐reported outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| No study | ||||||||
CI: confidence interval; COMRADE: Combined Outcome Measure for Risk communication And treatment Decision making Effectiveness; N: sample size; na: not applicable; OPTION: observing patient involvement; PPS: patient participation scale; RD: risk difference; SDM: shared decision making; SMD: standardized mean difference.
6. Effect of interventions on primary outcome: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals.
| Observer‐based outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Tai‐Seale 2016 | [Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers)] + Patient mediated intervention (one‐page ASK handout) | Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers) | OPTION5 (/100) | 20 | ‐0.29 (‐1.17 to 0.60) | ‐0.29 (‐1.17 to 0.60) | na | |
| Observer‐based outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| Myers 2011 | Patient‐mediated intervention (pamphlet and counseling) + reminders | Patient‐mediated intervention (pamphlet) + reminders | Informed decision making scale (IDM) | 134 | ‐0.04 (‐0.13 to 0.04) | ‐0.04 (‐0.13 to 0.04) | na | |
| Observer‐based outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| No study | ||||||||
| Patient‐reported outcome measure ‐ Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Tai‐Seale 2016 | [Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers)] + Patient mediated intervention (one‐page ASK handout) | Educational material (video) + patient mediated intervention (booklet) + educational meeting (coaching session for providers) | CollaboRATE (/100) | 150 | 0.00 (‐0.32 to 0.32) | 0.00 (‐0.32 to 0.32) | na | |
| Patient‐reported outcome measure ‐ Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Patient‐reported outcome measure ‐ Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Cooper 2013 | Patient‐mediated intervention + educational outreach visit + distribution of educational material + audit and feedback (patient‐centered group) | Patient‐mediated intervention + educational outreach visit + distribution of educational material (Standard group) | Patient Rating of their clinicians participatory decision‐making skills (from baseline to 12 months) | OR = 0.7 (IC 95% 0.3‐1.9) ‐ No significant | na | |||
| Cooper 2013 | Patient‐mediated intervention + educational outreach visit + distribution of educational material + audit and feedback (Patient‐centered group) | Patient‐mediated intervention + educational outreach visit + distribution of educational material (Standard group) | Patient Rating of their clinicians participatory decision‐making skills (from baseline to 18 months) | OR = 0.6 (IC 95% 0.2‐1.8) ‐ No significant | na | |||
ASK: Ask Share Know; CI: confidence interval; N: sample size; na: not applicable; OPTION: observing patient involvement; OR: odds ratio; RD: risk difference; SMD: standardized mean difference.
7. Effect of interventions on secondary outcomes: interventions targeting patients compared with usual care.
| Continous Data | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Landrey 2012 | Patient‐mediated intervention (mailed flyer) | Usual care | Knowledge/knowledge not addressed in decision aid | 148 | 0.14 (‐0.19 to 0.46) | 0.38 (0.16 to 0.61) | 44% | x |
| van Peperstraten 2010 | Patient‐mediated intervention (decision aid + support call) | Usual care | Pre: 304 Post: 262 | 0.52 (0.27 to 0.77) | x | |||
| van Peperstraten 2010 | Patient‐mediated intervention (decision aid + support call) | Usual care | 262 | 0.81 (0.55 to 1.06) | ||||
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Pre: 167; Post: 155 | 0.45 (0.13 to 0.77) | x | |||
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Knowledge (items addressed in decision aid) | Pre: 164; Post: 152 | 0.77 (0.44 to 1.10) | 0.77 (0.44 to 1.10) | na | na |
| Vodermaier 2009 | Patient mediated intervention (decision aid) | Usual care | Satisfaction with decision and treatment | 107 | 0.14 (‐0.24 to 0.52) | 0.14 (‐0.24 to 0.52) | na | na |
| Murray 2001 | Patient‐mediated intervention (decision aid) | Usual care | Decisional conflict | 105 | ‐0.66 (‐1.06 to ‐0.27] | ‐0.30 (‐0.68 to 0.09) | 71% | x |
| Vodermaier 2009 | Patient‐mediated intervention (decision aid) | Usual care | 107 | ‐0.28 (‐0.66 to 0.10) | x | |||
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | 155 | 0.01 (‐0.31 to 0.32) | x | |||
| van Tol‐Geerdink 2016 | Patient‐mediated intervention (decision aid) | Usual care | Decision regret (6 months) | 212 | ‐0.10 (‐0.39 to 0.19) | ‐0.10 (‐0.39 to 0.19) | na | na |
| van Tol‐Geerdink 2016 | Patient‐mediated intervention (decision aid) | Usual care | Decision regret (12 months) | 201 | ‐0.20 (‐0.50 to 0.10) | ‐0.20 (‐0.50 to 0.10) | ||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Patient‐physician communication (number of topics raised by the patient) | 100 | 0.26 (‐0.13 to 0.65) | 0.26 (‐0.13 to 0.65) | na | x |
| Deen 2012 | Patient‐mediated intervention (decision aid) | Usual care | Decision self‐efficacy | 137 | 0.07 (‐0.26 to 0.41) | 0.16 (‐0.08 to 0.40) | 0% | |
| Deen 2012 | Patient‐mediated intervention (Patient Activation) | Usual care | 142 | 0.18 (‐0.15 to 0.51) | x | |||
| Deen 2012 | Patient‐mediated intervention (decision aid + Patient Activation) | Usual care | 135 | 0.08 (‐0.25 to 0.42) | ||||
| Maranda 2014 | Patient mediated intervention | Usual care | 132 | 0.14 (‐0.20 to 0.48) | x | |||
| Pickett 2012 | Patient‐mediated intervention (training for patients) | Usual care | Empowerment (time 2) | Pre: 428; Post: 342 | 0.26 (0.05 to 0.48) | 0.26 (0.05 to 0.48) | na | na |
| Pickett 2012 | Patient‐mediated intervention (training for patients) | Usual care | Empowerment (time 3) | Pre: 428; Post: 318 | 0.17 (‐0.05 to 0.39) | 0.17 (‐0.05 to 0.39) | ||
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | Health‐related QoL (physical) | Pre: 133; Post: 116 | 0.00 (‐0.36 to 0.36) | 0.00 (‐0.36 to 0.36) | na | x |
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | Health‐related QoL (mental) | Pre: 133; Post: 116 | 0.10 (‐0.26 to 0.46) | 0.10 (‐0.26 to 0.46) | na | x |
| van Peperstraten 2010 | Patient‐mediated intervention (decision aid + support call) | Usual care | Anxiety (20‐80) | Pre: 304 Post: 262 | ‐0.17 (‐0.49 to 0.14) | ‐0.17 (‐0.49 to 0.14) | na | na |
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Anxiety (state) | Pre: 166; Post: 157 | 0.18 (‐0.06 to 0.43) | 0.18 (‐0.06 to 0.43) | na | na |
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Consultation length (min: sec) | 100 | 0.35 (‐0.04 to 0.75) | 0.10 (‐0.39 to 0.58) | 70% | x |
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Consultation length | 124 | ‐0.15 (‐0.50 to 0.21) | x | ||
| Murray 2001 | Patient mediated intervention (decision aid) | Usual care | Cost (excluding intervention) | 105 | 0.25 (‐0.14 to 0.63) | 0.25 (‐0.14 to 0.63) | na | na |
| Murray 2001 | Patient mediated intervention (decision aid) | Usual care | Cost (including intervention) | 105 | 0.82 (0.42 to 1.22) | 0.82 (0.42 to 1.22) | ||
| Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | N | RD | RD (95% CI) | I2 | |
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | Overall knowledge/Knowledge of risk without medication | 41 | 0.27 (‐0.05 to 0.60) | 0.17 (0.05 to 0.29) | 0% | x |
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | 41 | 0.38 (0.06 to 0.69) | ||||
| Krist 2007 | Patient‐mediated intervention (decision aid brochure) | Usual care | 271 | 0.16 (0.03 to 0.29) | x | |||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Usual care | 301 | 0.16 (0.03 to 0.28) | ||||
| Sheridan 2014 | Patient‐mediated intervention (decision aid) | Usual care | Patient‐physician communication (patient raised discussion) | 157 | 0.29 (0.14 to 0.44) | 0.29 (0.14 to 0.44) | na | x |
| Sheridan 2014 | Patient mediated intervention (decision aid) | Usual care | Patient‐physician communication (patient participation in discussion) | 157 | 0.27 (0.13 to 0.42) | 0.27 (0.13 to 0.42) | na | x |
| van Peperstraten 2010 | Patient‐mediated intervention (decision aid + support call) | Usual care | Empowerment (number of fully empowered couples) | Pre: 304 Post: 262 | 0.18 (0.09 to 0.27) | 0.18 (0.09 to 0.27) | na | na |
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Adherence (% of patients who filled their prescription within 30 days) | 197 | ‐0.07 (‐0.15 to 0.01) | ‐0.07 (‐0.15 to 0.01) | na | na |
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Adherence (% of patients with a PDC > 80%) | 206 | ‐0.03 (‐0.08 to 0.02) | ‐0.03 (‐0.08 to 0.02) | na | na |
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Adherence to medication (sometimes forget take cholesterol medicine) | 98 | ‐0.04 (‐0.23 to 0.15) | ‐0.04 (‐0.23 to 0.15) | na | na |
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Adherence to medication (did not miss a dose last week) | 97 | 0.07 (‐0.06 to 0.20) | 0.07 (‐0.06 to 0.20) | na | na |
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | Anxiety (mild, moderate and severe) | Pre: 138; Post: 127 | 0.04 (‐0.07 to 0.15) | 0.04 (‐0.07 to 0.15) | na | na |
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | Depression (mild, moderate and severe) | Pre: 138; Post: 127 | 0.16 (0.05 to 0.28) | 0.16 (0.05 to 0.28) | na | na |
| Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Control | Outcome | Direct quotes | ||||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | Knowledge (overall: generic and tailored) | No significant differences between groups (table 2, page 9 of the publication and table 2 of the supplementary material) | na | |||
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Significant difference in favor of the intervention group (p=0.03) (table 2; page 1767) | na | ||||
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | "Intervention patients ... had a better knowledge of prosthetic valves (85% versus 68%; P=0.004)" Page 1 | na | ||||
| Sheridan 2014 | Patient‐mediated intervention (decision aid) | Usual care | Results reported only for the intervention group | na | ||||
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Knowledge : Generic | No differences between groups (p=0.65) (table 2; page 1767) | na | |||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | No significant differences betwwen groups (table 2, page 9 of the publication and table 2 of the supplementary material) | na | ||||
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Knowledge : Tailored to information in the decision aid | Significant difference in favour of the intervention group (p<0.001) (table 2; page 1767) | na | |||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | Significant differences between groups (table 2, page 9 of the publication and table 2 of the supplementary material) | na | ||||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Satisfaction with the consultation | Intervention group (median; range) 5.0 (4–5) vs control group 5.0 (3–5) (p‐value=0.27). Page 231 (table 2) | na | |||
| Almario 2016 | Patient‐mediated intervention (GI PROMIS) | Usual care | "Table 3 presents the CG‐CAHPS provider rating scores for the GI PROMIS and control arms in the intention‐to‐treat analysis. After adjusting for confounders, we found no difference in provider rating between groups." Page 7 | na | ||||
| Haskard 2008 | Patient‐mediated intervention | Usual care | Satisfaction with care | "There were no significant main effects of patient training on patient satisfaction questionnaire items." Page 518 | na | |||
| van der Krieke 2013 | Patient‐mediated intervention (web‐based information and decision tool) | Usual care | "Patients also did not differ in self‐reported satisfaction with care (CSQ) (F1,70=0.014, P=.91)." (no page number, in the resuts section) | na | ||||
| Landrey 2012 | Patient‐mediated intervention (mailed flyer) | Usual care | Satisfaction with the intervention | “Among patients who reported receiving the flyer, 86.4% felt the content was clearly presented, 86.4% felt it contained about the right amount of information, 45.5% felt the information was completely balanced, and 43.2% viewed it as biased against PSA testing; 88.6% would recommend it to others.” Page 71 | na | |||
| Murray 2001 | Patient‐mediated intervention (decision aid) | Usual care | “Patients reacted positively to the decision aid (table 3)” Page 5 | na | ||||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | "Fifteen patients rated the QPS as not helpful, while 16 rated it as somewhat helpful and 20 patients as helpful." Page 230 | na | ||||
| LeBlanc 2015a | Patient mediated intervention (decision aid) | Usual care | "After the encounters with their clinicians, patients in the decision aid arm ... were more … satisfied (ranging from risk ratio [RR], 1.25 [P = .81] to RR, 2.40 [P = .002]) compared with patients in the control arm (Table 2)." Page 1765 | na | ||||
| van der Krieke 2013 | Patient‐mediated intervention (web‐based information and decision tool) | Usual care | "They agreed or completely agreed with the following statements: “I have been well informed about the treatment options offered by Friesland Mental Health Care Service by the decision aid” (22/29, 76%), “The advice presented by the decision aid has helped me to reflect on what I want” (22/29, 76%), “The decision aid was easy to use” (20/28, 71%), “I would recommend the decision aid to others” (20/27, 74%) and “The decision aid helped me to get a clearer view on what my problem areas or points of interest are” (17/28, 61%). Patients were divided on whether the decision aid helped them to better prepare the evaluation meeting with their clinicians, 44% (12/27) said it did help; 56% (15/27) were neutral or said it did not help. " (no page number, in the result section) | na | ||||
| Eggly 2017 | Patient‐mediated intervention (question prompt list) | Usual care | "The mean patient response across the eight questions about the QPL booklet was 2.80 (SD = 0.23). T‐tests showed no significant differences between the two intervention arms on any of these questions (p’s > 0.05). The mean response across the five questions about coaching was 2.83 (SD = 0.29) (Table 3)." Page 823 | na | ||||
| Eggly 2017 | Patient‐mediated intervention (question prompt list + communication coach) | Usual care | "The mean patient response across the eight questions about the QPL booklet was 2.80 (SD = 0.23). T‐tests showed no significant differences between the two intervention arms on any of these questions (p’s > 0.05). The mean response across the five questions about coaching was 2.83 (SD = 0.29) (Table 3)." Page 823 | na | ||||
| Krist 2007 | Patient‐mediated intervention (decision aid brochure) | Usual care | Decisional conflict | "DCS scores among all 3 groups were equally low and did not differ signifi cantly (control, 1.58; brochure, 1.54; and Web site, 1.55)." Page 115 | na | |||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Usual care | "DCS scores among all 3 groups were equally low and did not differ significantly (control, 1.58; brochure, 1.54; and website, 1.55)." Page 115 | na | ||||
| Sheridan 2014 | Patient‐mediated intervention (decision aid) | Usual care | Results reported only for the intervention group | na | ||||
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | No differences between groups (p>0.05). Page 5; table 2 | na | ||||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | "Decision conflict was low for both groups, and was lower in the Decision Aid arm, but no significant difference was found in the overall scale or in its subscales across arms (Table 2)." Page 8 | na | ||||
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Decisional conflict (0 = conflict, 100 = comfort) | "After the encounters with their clinicians, patients in the decision aid arm reported significantly higher comfort with the decision (mean difference [MD], 5.3 out of 100; 95% CI, 1.1‐9.5; P = .01)" Page 1765 | na | |||
| Korteland 2017 | Patient‐mediated intervention (decision aid) | Usual care | Decision regret (3 months post‐operative) | "Three months postoperative regret with regard to prosthetic valve choice ranged from 0 to 55, with no statistical difference between the intervention and control groups. The majority of patients in the intervention and control groups did not experience any regret (70% versus 64%, respectively; P=0.513)." Page 4 | na | |||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Patient‐physician communication (Dominant behaviour of physician) | There was more dominant behaviour in the control group compared to the intervention group (Median, range: intervention group 2.0 (0–4) control group 3.0 (0–4); p= 0.03) (table 2) | na | |||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Patient‐physician communication (Dominant behaviour of patient) | There no difference between groups (Median, range: intervention group 1.0 (0–4) control group 1.0 (0–4); p= 0.46) (table 2) | na | |||
| Hamann 2014 | Patient‐mediated intervention (question prompt sheet) | Usual care | Patient‐physician communication (Patient shows interest, raises questions) | There no difference between groups (Median, range: intervention group 2.0 (0–4) control group 1.0 (0–3); p= 0.31) (table 2) | na | |||
| Sheridan 2014 | Patient‐mediated intervention (decision aid) | Usual care | Patient‐physician communication (Patients’ perceptions of discussions and the health care visit) | "Intervention participants also tended to report better interactions with their provider, with improvements for the following 3 of 6 items from the Healthcare Climate questionnaire: “My provider provided me with choices and options about lowering my chances of heart disease” (+15 percentage points; adjusted p = .02); “My provider listened to how I would like to do things” (+21 percentage points; adjusted p < .01); and “My provider tried to understand how I see things before suggesting new ways to lower my chances of heart disease” (+15 percentage points; adjusted p = .05)." Page 5 | na | |||
| Vestala 2013 | Patient‐mediated intervention (patient participation in nursing documentation) | Usual care | Empowerment | "No statistical difference was identified between the intervention and control group with regards to empowerment." page 70 | na | |||
| Krist 2007 | Patient‐mediated intervention (decision aid brochure) | Usual care | Match between preferred and actual level of participation in decision making | "Concordance did not differ between the 3 study groups (P1 = .41)." Page 117 | na | |||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Usual care | "Concordance did not differ between the 3 study groups (P1 = .41)." Page 117 | na | ||||
| Cooper 2011 | Patient‐mediated intervention | Usual care | Adherence to medication | “Changes in patient‐reported adherence to medications at 12 months did not differ for any of the intervention groups compared to the patient+physician minimal intervention group.” Page 1300 | na | |||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Differents forms of usual care [Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) OR usual care] | "No difference was found in adherence to the medication that was prescribed for both, those who filled their initial prescription [PDC Median 46.7%, IQR (30, 62) for Decision Aid arm vs. 85%, IQR (55.3, 92.6) for FRAX/Usual Care arm, Table 3] or for all that were prescribed bisphosphonates [PDC Median (IQR) 46.7%, (7.8, 46.7) for Decision Aid arm vs. 0%, (0, 72.5) for FRAX/Usual Care arm]. Only one patient in the Decision Aid arm and 3 in the FRAX/Usual Care arm had PDC >80%." page 8 | na | ||||
| Murray 2001 | Patient‐mediated intervention (decision aid) | Usual care | Health status and physical function | "We found no difference between the two groups in the trends over time in the EQ5D responses nor in the SF36 scores." Page 5 | na | |||
| Murray 2001 | Patient‐mediated intervention (decision aid) | Usual care | Health states and valuation of health states | "We found no difference between the two groups in the trends over time in the EQ5D responses nor in the SF36 scores." Page 5 | na | |||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | Usual care | Health‐related QoL | "We found no difference between the FRAX and usual care arms, nor were overall results significantly impacted by analyses comparing the three arms versus only two arms (Decision aid arm vs. FRAX/usual care arms together, see Tables A, B, C, D, and E in S1 File. Therefore, the results comparing the FRAX arm and the Usual Care arm were combined and all subsequent results are presented as Decision Aid vs. FRAX/Usual Care arm (i.e. different forms of usual care)." page 7 There were no difference between group regarding quality of life [Median (IQR); Decision aid: 85 (80 to 95) FRAX/UC: 85 (73 to 90); p=0.19 (table 4)] | na | |||
| Murray 2001 | Patient‐mediated intervention (decision aid) | Usual care | Anxiety | "The Spielberger scores were similar at the final assessment in the two groups (MannWhitney U test). " page 5 | na | |||
| van Peperstraten 2010 | Patient‐mediated intervention (decision aid + support call) | Usual care | Depression (number with subclinical depression) | "At uptake of in vitro fertilisation the frequency of subclinical depression did not differ between the intervention and control group: 11% (16/147) v 9% (113/151). After patients received the empowerment strategy, however, this frequency was higher in the intervention group (13% (16/126) v 4% (5/136); P=0.01); this difference diminished after embryo transfer, however (14% (17/123) v 14% (17/120); P=0.94)." Page 5 | na | |||
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Depression (symptoms) ‐ 3 months | "There was no observed difference across arms in control of depression symptoms (mean PHQ‐9 score), remission rate (PHQ‐9 score <5), or responsiveness (>50% PHQ‐9 improvement) at 3 and 6 months" Pages 1765‐1766 | na | |||
| LeBlanc 2015a | Patient‐mediated intervention (decision aid) | Usual care | Depression (symptoms) ‐ 6 months | "There was no observed difference across arms in control of depression symptoms (mean PHQ‐9 score), remission rate (PHQ‐9 score <5), or responsiveness (>50% PHQ‐9 improvement) at 3 and 6 months" Pages 1765‐1766 | na | |||
| Vestala 2013 | Patient‐mediated intervention (patient participation in nursing documentation) | Usual care | Depression | Not reported | na | |||
| Perestelo‐Perez 2016 | Patient‐mediated intervention (decision aid) | Usual care | Stress (diabete related) | No difference between groups (page 298; table 2) | na | |||
| Krist 2007 | Patient‐mediated intervention (decision aid brochure) | Usual care | Consultation length | “These [discussion times] patient‐physician differences did not differ significantly across the control, brochure, and Web groups.” Page 116 | na | |||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Usual care | “These [discussion times] patient‐physician differences did not differ significantly across the control, brochure, and Web groups.” Page 116 | na | ||||
| Vodermaier 2009 | Patient‐mediated intervention (decision aid) | Usual care | “No time differences emerged in the length of the treatment decision consultation with the physicians on patient self‐reports. The mean time for the treatment decision making appointment was about 15 minutes” Page 593 | na | ||||
| Eggly 2017 | Patient‐mediated intervention (question prompt list) | Usual care | "There were no significant differences in interaction length between either of the two intervention arms and the usual care arm (Arm 2 vs. Arm 1, p = 0.21); Arm 3 vs. Arm 1, p = 0.11)." Page 823 | na | ||||
| Eggly 2017 | Patient‐mediated intervention (question prompt list + communication coach) | Usual care | "There were no significant differences in interaction length between either of the two intervention arms and the usual care arm (Arm 2 vs. Arm 1, p = 0.21); Arm 3 vs. Arm 1, p = 0.11)." Page 823 | na | ||||
| LeBlanc 2015b | Patient‐mediated intervention (decision aid) | usual care] | "Encounter duration in the FRAX/Usual Care arm had a median of 10.7 minutes and a range of 2.5 to 54.9 minutes, where encounters in the Decision Aid arm had a median duration of 11.5 with a range of 5.4 to 21.4 minutes (median difference 0.8 minutes, range ‐33.6 to 3.0)." Page 10 | na | ||||
| Maclachlan 2016 | Patient‐mediated intervention (educational meeting for patient) | Usual care | "The overall average length of consultations was 5.58 minutes, 5.78 minutes in the trained group and 5.37 minutes in the untrained group." Page 5 | na | ||||
| van Peperstraten 2010 | Patient‐mediated intervention (decision aid + support call) | Usual care | Cost | “The mean total savings in the intervention group were calculated to be EURO169.75 per couple included from the waiting list for in vitro fertilisation” Page 5 | na | |||
CI: confidence interval; CSQ: Client Satisfaction Questionnaire; DA: decision aid; DCS: Decisional Conflict Scale; GI PROMIS: gastrointestinal Patient Reported Outcomes Measurement Information System; N: sample size; na: not applicable; PDC: percentage of days covered; PHQ‐9: Patient Health questionnaire for depression; PSA: Prostatic Specific Antigen; QPL: question prompt list; QPS: question prompt sheet; QoL: quality of life; RD: risk difference; RR: relative risk; SD: standard deviation; SMD: standardized mean difference.
8. Effect of interventions on secondary outcomes: interventions targeting healthcare professionals compared to usual care.
| Continous Data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Murray 2010 | Educational meeting + audit and feedback + distribution of educational material + educational outreach visit + barriers assessement | Usual care | Knowledge | 70 | 0.55 (0.07 to 1.03) | 0.26 (‐0.16 to 0.69) | 68% | x |
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | Knowledge (time 1) | Pre: 1083; Post: 899 | 0.10 (‐0.03 to 0.23) | x | ||
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | Knowledge (time 2) | Pre: 1083; Post: 794 | 0.09 (‐0.05 to 0.23) | |||
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | Knowledge (time 3) | Pre: 1083; Post: 714 | 0.17 (0.02 to 0.31) | |||
| Wilkes 2013 | Distribution of educational material | Usual care | Satisfaction with consultation | 479 | 0.00 (‐0.18 to 0.18) | 0.00 (‐0.18 to 0.18) | na | na |
| Légaré 2012 | Educational meeting and distribution of Educational material | Usual care | Decision regret (2 weeks) | Pre: 329 Post: 326 | 0.29 (0.07 to 0.51) | 0.29 (0.07 to 0.51) | na | na |
| Kennedy 2013 | Educational meeting | Usual care | Self‐efficacy (6 months) | Pre: 5599; Post: 4475 | ‐0.03 (‐0.09 to 0.03) | ‐0.03 (‐0.09 to 0.03) | na | na |
| Kennedy 2013 | Educational meeting | Usual care | Self‐efficacy (12 months) | Pre: 5599; Post: 4005 | ‐0.04 (‐0.10 to 0.03) | ‐0.04 (‐0.10 to 0.03) | na | na |
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | Adherence to medication (time 1) | Pre: 1070; Post: 827 | ‐0.08 (‐0.21 to 0.06) | ‐0.08 (‐0.21 to 0.06) | na | na |
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | Adherence to medication (time 2) | Pre: 1070; Post: 744 | ‐0.01 (‐0.16 to 0.13) | ‐0.01 (‐0.16 to 0.13) | na | na |
| Tinsel 2013 | Distribution of educational material + educational meeting | Usual care | Adherence to medication (time 3) | Pre: 1070; Post: 675 | 0.10 (‐0.05 to 0.25) | 0.10 (‐0.05 to 0.25) | na | na |
| Kennedy 2013 | Educational meeting | Usual care | General health | Pre: 5599; Post: 4056 | 0.02 (‐0.04 to 0.08) | 0.02 (‐0.04 to 0.08) | na | na |
| Kennedy 2013 | Educational meeting | Usual care | Psychological well‐being | 4052 | 0.00 (‐0.06 to 0.06) | 0.00 (‐0.06 to 0.06) | na | na |
| Légaré 2012 | educational meeting and distribution of educational material | Usual care | Health‐related QoL (physical) | 359 | 0.16 (‐0.05 to 0.36) | 0.16 (‐0.05 to 0.36) | na | na |
| Légaré 2012 | Educational meeting and distribution of Educational material | Usual care | Health‐related QoL (mental) | 359 | 0.28 (0.07 to 0.49) | 0.28 (0.07 to 0.49) | na | na |
| Kennedy 2013 | Educational meeting | Usual care | Health‐related QoL (6 months) | Pre: 5598; Post: 4449 | 0.00 (‐0.06 to 0.06) | 0.00 (‐0.06 to 0.06) | na | na |
| Kennedy 2013 | Educational meeting | Usual care | Health‐related QoL (12 months) | Pre: 5598; Post: 3991 | 0.00 (‐0.06 to 0.06) | 0.00 (‐0.06 to 0.06) | ||
| Sanders 2017 | Educational meeting + audit and feed‐back | Usual care | Consultation length (minutes) | 175 | 0.51 (0.21 to 0.81) | 0.51 (0.21 to 0.81) | na | na |
| Cox 2017 | Distribution of educatuonal material (FCR checklist) + Educational meeting | Usual care | Safety (parent perception of hospital safety) | Pre: 144; Post: 154 | 0.00 (‐0.32 to 0.32) | 0.00 (‐0.32 to 0.32) | na | na |
| Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | Knowledge of risk without medication | 40 | ‐0.10 (‐0.42 to) 0.23] | ‐0.10 (‐0.42 to 0.23) | na | na |
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | Knowledge of risk risk post‐treatment | 40 | ‐0.17 (‐0.49 to 0.16) | ‐0.17 (‐0.49 to 0.16) | na | na |
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Satisfaction with amount of information (postnatal period) | Pre: 1671 Post: 1492 | 0.02 (‐0.02 to 0.07) | 0.02 (‐0.02 to 0.07) | na | na |
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Satisfaction with the decision making process (how choice had been made) (postnatal period) | Pre: 1666 Post: 1488 | ‐0.03 (‐0.07 to 0.02) | ‐0.03 (‐0.07 to 0.02) | na | na |
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Satisfaction with discussion with HCP (View of whether they had had sufficient discussion with HCP) (postnatal period) | Pre: 1657 Post: 1483 | ‐0.00 (‐0.05 to 0.05) | ‐0.00 (‐0.05 to 0.05) | na | na |
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Anxiety (more anxious) ‐ antenatal period | Pre: 1195 Post: 1527 | ‐0.00 (‐0.03 to 0.03) | ‐0.00 (‐0.02 to 0.02) | 0% | x |
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Anxiety (more anxious) ‐ postnatal period | Pre: 1651 Post: 1476 | 0.00 (‐0.03 to 0.03) | x | ||
| Wilkes 2013 | Distribution of Educational material | Usual care | Consultation length (10‐20 minutes) | 479 | ‐0.04 (‐0.13 to 0.05) | ‐0.04 (‐0.13 to 0.05) | na | na |
| Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Knowldege (antenatal period) | Adjusted mean difference 95%CI : 0.20 (‐0.09 to 0.49) (Page 3, table 3) | na | |||
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Knowldege (postnatal period) | Adjusted mean difference 95%CI : 0.20 (‐0.05 to 0.44) (Page 3, table 3) | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Knowledge | "Although there were some tendencies in the expected direction, there was no overall effect by the training in the secondary patient outcomes in either language cohort." Pages 1269‐1270 | na | |||
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | Knowledge (overall) | No differences between group (table 2; supplementary material) | na | |||
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | Knowledge (Items not addressed in DA ) (4 items) | No differences between group (table 2; supplementary material) | na | |||
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | Knowledge (Items addressed in DA ) (9 items) | Significant differences between group (table 2; supplementary material) | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Satisfaction with consultation | "There was higher satisfaction with doctor’s consultation skills for patients in ANZ than for patients in SGA, and all cohorts showed improved satisfaction with doctor’s consultation skills except for the ANZ control group (Figure 3c), although not statistically significant (p = 0.08 for SGA and p = 0.26 for ANZ)." Page 1270 | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Satisfaction with decision (2 weeks) | "Overall, patients were satisfied with their treatment decision (Figure 3a). Although there were some tendencies in the expected direction, there was no overall effect by the training in the secondary patient outcomes in either language cohort." Pages 1269‐1270 | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Satisfaction with decision (4 months) | "Although there were some tendencies in the expected direction, there was no overall effect by the training in the secondary patient outcomes in either language cohort." Pages 1269‐1270 | na | |||
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Satisfaction with the decision making process (how choice had been made) (antenatal period) | No difference between groups (Page 3; table 2) | na | |||
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Satisfaction with discussion with HCP (View of whether they had had sufficient discussion with HCP) (antenatal period) | No difference between groups (Page 3; table 2) | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Satisfaction with doctor communication | "Although there were some tendencies in the expected direction, there was no overall effect by the training in the secondary patient outcomes in either language cohort." Pages 1269‐1270 | na | |||
| Fossli 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Satisfaction (Patient global satisfaction) | "The duration of encounters (min:sec) did not change significantly (1:03 (p = 0.69, 95% CI 6:13; 4:07)) from pre to post, and neither did patient global satisfaction (0.3 (p = 0.38, 95% CI 0.3; 0.8))." Page 166 | na | |||
| Murray 2010 | Educational meeting + audit and feedback + distribution of Educational material + Educational outreach visit + barriers assessement | Usual care | Satisfaction with the intervention (acceptability and utility of intervention components) | All the intervention group members who logged to the autotutorial and completed the satisfaction survey (n=8), rated the tutorial as excellent to very good, seven indicated that the tutorial was helpful, and six reported that it was very easy to complete. "In all, 37 members of the intervention group (97%) commented on the acceptability of the skills building workshop. Most (n=35 (94%)) said they would recommend the workshop to others, and 26 (68%) gave it an overall rating of excellent. Workshop participants rated the workshop very good at helping them to understand and use decision support…" Overall, the POC PtDA was rated as acceptable and clinically usefull by the 38 participants who provided data...31(81%) agreed that the PtDA would be acceptable to patients, while 24 (63%) agreed that it would be acceptable to practitioners.” Page 117 “All 36 who participated in the Educational outreach call indicated an interested in using the POC PtDa and express frustration that it was not available for use in their clinical practice setting.” Page 118 | na | |||
| O'Cathain 2002 | Educational meeting + distribution of Educational material | Usual care | Satisfaction with amount of information (antenatal period) | Difference between groups: adjusted OR 1.40 (1.05 to 1.88) (Page 3; table 2) | na | |||
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | Decisional conflict | "Decision conflict was low for both groups, and was lower in the Decision Aid arm, but no significant difference was found in the overall scale or in its subscales across arms (Table 2)." page 8 | na | |||
| Légaré 2012 | Educational meeting + distribution of Educational material | Usual care | "The training had no statistically significant effect on decisional conflict,…" Page E731 | na | ||||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | "Overall, patients in the SGA cohort reported higher conflict scores than those in the ANZ cohort… Post‐randomisation, although there was no change in conflict in the SGA cohort, in the ANZ cohort there was an increased level of conflict in the control group (estimate = 0.28; ES = 0.17: p = 0.003). This change exceeded the improvement in the training group (estimate = 0.14; ES = 0.09; p = 0.13). In summary, the training appeared to have no overall effect on decisional conflict in either language cohort." Pages 1268‐1269 | na | ||||
| Légaré 2012 | Educational meeting + distribution of Educational material | Usual care | Adherence to decision | No differences between group (page E733; table 4) | na | |||
| Cooper 2011 | Educational meeting | Usual care | Adherence to medication | Changes in patient‐reported adherence to medications at 12 months did not differ for any of the intervention groups compared to the patient+physician minimal intervention group.”Page 1300 | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Health‐related QoL | "The quality of life indicators showed similar findings (data not shown)." Page 1270 | na | |||
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | "We found no difference between the FRAX and usual care arms, nor were overall results significantly impacted by analyses comparing the three arms versus only two arms (Decision aid arm vs. FRAX/usual care arms together, see Tables A, B, C, D, and E in S1 File. Therefore, the results comparing the FRAX arm and the Usual Care arm were combined and all subsequent results are presented as Decision Aid vs. FRAX/Usual Care arm (i.e. different forms of usual care)." Page 7 There were no difference between group regarding quality of life [Median (IQR); Decision aid: 85 (80, 95) FRAX/UC: 85 (73, 90); p=0.19 (table 4)] | na | ||||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Anxiety (state) ‐ 2 weeks | "Anxiety slightly decreased over time for all cohorts. Patients in the SGA (Figure 4a) and ANZ (Figure 4b) cohorts reported comparable anxiety levels at each time point." Page 1270 | na | |||
| Bernhard 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Anxiety (state) ‐ 4 months | "Anxiety slightly decreased over time for all cohorts. Patients in the SGA (Figure 4a) and ANZ (Figure 4b) cohorts reported comparable anxiety levels at each time point." Page 1270 | na | |||
| Fossli 2011 | Educational meeting + audit and feedback + distribution of Educational material | Usual care | Consultation length | "The duration of encounters (min:sec) did not change significantly ( 1:03 (p = 0.69, 95% CI 6:13; 4:07)) from pre to post, and neither did patient global satisfaction (0.3 (p = 0.38, 95% CI 0.3; 0.8))." Page 166 | na | |||
| Murray 2010 | Educational meeting + audit and feedback + distribution of Educational material + Educational outreach visit + barriers assessement | Usual care | “At baseline there was no significant difference. However, in the post‐calls, the mean call duration was longer in the intervention group at 13,47 minutes (95% confidence interval 11.8;14.21), than in the control group at 10.29 minutes (95% CI 8.79 to 11.79 P = 0.004)” Page 117 | na | ||||
| Shepherd 2011 | Educational outreach visit | Usual care | “These effects occurred without any significant difference in consultation length, mean consultation lengths were 26 minutes for control and intervention visits.” Page 381 | na | ||||
| LeBlanc 2015b | Reminder (copy of patient's estimated risk of fracture as computed by the FRAX) | Usual care | "We found no difference between the FRAX and usual care arms, nor were overall results significantly impacted by analyses comparing the three arms versus only two arms (Decision aid arm vs. FRAX/usual care arms together, see Tables A, B, C, D, and E in S1 File..." Page 7 | na | ||||
ANZ: Australia, New Zealand; CI: confidence interval; DA: decision aid; DCS: Decisional Conflict Scale; ES: effect size; HCP: healthcare professional; IQR: interquartile range; N: sample size; na: not applicable; POC: place‐of‐care; PtDA: patient decision aid; QoL: quality of life; OR: odds ratio; RD: risk difference; SGA: Switzerland, Germany and Austria; SMD: standardized mean difference.
9. Effect of interventions on secondary outcomes: interventions targeting both patients and healthcare professionals compared to usual care.
| Continous Data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Coylewright 2016 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Knowledge | 107 | 0.98 (0.57 to 1.38) | 0.41 (0.28 to 0.53) | 0% | |
| Coylewright 2016 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | 106 | 0.48 (0.09 to 0.87) | x | |||
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | 898 | 0.40 (0.27 to 0.53) | x | |||
| Loh 2007 | Patient‐mediated intervention + educational meeting | Usual care | Satisfaction with care (overall) | 287 | 0.92 (0.67 to 1.18) | 0.51 (‐0.34 to 1.36) | 91% | x |
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + educational meeting | Usual care | Satisfaction with care (6 weeks) | 75 | 0.05 (‐0.40, 0.51) | x | ||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + educational meeting | Usual care | Satisfaction with care (6 months) | 64 | 0.00 (‐0.49 to 0.49) | |||
| Rise 2012 | Patient‐mediated intervention (use of PCOMS) + educational meeting | Usual care | Satisfaction with care (12 months) | 63 | 0.21 (‐0.28 to 0.71) | |||
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Satisfaction with decision (post‐consultation) | 424 | 0.24 (0.05 to 0.43) | 0.24 (0.05 to 0.43) | na | na |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Satisfaction with decision (3 months) | 366 | ‐0.47 (‐0.67 to ‐0.26) | ‐0.47 (‐0.67 to ‐0.26) | na | na |
| Wilkes 2013 | Patient‐mediated intervention (web‐based educational program for patients) + distribution of educational material | Usual care | Satisfaction with consultation | 393 | 0.00 (‐0.23 to 0.23) | 0.00 (‐0.23 to 0.23) | na | na |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Decisional conflict (confidence in decision) (post‐consultation) | 414 | 0.03 (‐0.17 to 0.22) | 0.03 (‐0.17 to 0.22) | na | na |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Decisional conflict (confidence in decision) (3 months) | 371 | ‐0.32 (‐0.53 to ‐0.12) | ‐0.32 (‐0.53 to ‐0.12) | na | na |
| Mathers 2012 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Decisional conflict | 167 | ‐0.19 (‐0.32 to ‐0.06) | ‐0.35 (‐0.71 to 0.01) | 79% | x |
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | 898 | ‐0.57 (‐0.88 to ‐0.26) | x | |||
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Decision regret (3 months) | 369 | 0.13 (‐0.08 to 0.33) | 0.13 (‐0.08 to 0.33) | na | na |
| Epstein 2017 | Patient‐mediated intervention (patients & caregivers coaching session + question prompt list) + educational meeting | Usual care | Patient‐physician communication (patient‐centred communication) | Pre: 118; Post: 265 | 0.23 (‐0.01 to 0.47) | 0.23 (‐0.01 to 0.47) | na | na |
| Loh 2007 | Patient‐mediated intervention + educational meeting | Usual care | adherence to medcation (patient's assessment) | 287 | 0.44 (‐0.17 to 1.05) | 0.44 (‐0.17 to 1.05) | na | na |
| Loh 2007 | Patient‐mediated intervention + educational meeting | Usual care | adherence to medication (physician's assessment) | 287 | 0.62 (0.37 to 0.87) | 0.62 (0.37 to 0.87) | na | na |
| Epstein 2017 | Patient‐mediated intervention (patients & caregivers coaching session + question prompt list) + educational meeting | Usual care | Health‐related QoL | 265 | 0.08 (‐0.16 to 0.33) | 0.08 (‐0.16 to 0.33) | na | na |
| Rise 2012 | Patient‐mediated intervention + educational meeting | Usual care | Health‐related QoL (physical)‐ 6 weeks | Pre: 75; Post: 75 | 0.08 (‐0.37 to 0.54) | 0.08 (‐0.37 to 0.54) | na | na |
| Rise 2012 | Patient‐mediated intervention + educational meeting | Usual care | Health‐related QoL (physical)‐ 6 months | Pre:75; Post: 64 | ‐0.09 (‐0.58 to 0.40) | ‐0.09 (‐0.58 to 0.40) | na | na |
| Rise 2012 | Patient‐ mediated intervention + educational meeting | Usual care | Health‐related QoL (physical)‐ 12 months | Pre: 75; Post: 63 | 0.11 (‐0.39 to 0.60) | 0.11 (‐0.39 to 0.60) | na | na |
| Rise 2012 | Patient‐mediated intervention + educational meeting | Usual care | Health‐related QoL (mental)‐ 6 weeks | Pre: 75; Post: 75 | 0.01 (‐0.44 to 0.46) | 0.01 (‐0.44 to 0.46) | na | na |
| Rise 2012 | Patient‐mediated intervention + educational meeting | Usual care | Health‐related QoL (mental)‐ 6 months | Pre:75; Post: 64 | 0.24 (‐0.25 to 0.74) | 0.24 (‐0.25 to 0.74) | na | na |
| Rise 2012 | Patient‐mediated intervention + educational meeting | Usual care | Health‐related QoL (mental)‐ 12 months | Pre: 75; Post: 63 | 0.15 (‐0.34 to 0.65) | 0.15 (‐0.34 to 0.65) | na | na |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Anxiety ‐ post‐consultation | 419 | ‐0.12 (‐0.31 to 0.08) | ‐0.12 (‐0.31 to 0.08) | na | na |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Anxiety ‐ 3 months | 367 | ‐0.85 (‐1.06 to ‐0.63) | ‐0.85 (‐1.06 to ‐0.63) | na | na |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Depression ‐ post‐consultation | 418 | ‐0.14 (‐0.33 to 0.05) | ‐0.14 (‐0.33 to 0.05) | na | na |
| Härter 2015 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Depression ‐ 3 months | 364 | ‐0.59 (‐0.80 to ‐0.38) | ‐0.59 (‐0.80 to ‐0.38) | na | na |
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | Consultation length (minutes) | 536 | 3.72 (3.44 to 4.01) | 3.72 (3.44 to 4.01) | na | na |
| Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| Sheridan 2012 | Patient‐mediated intervention (video + coahcing session) + educational meeting | Usual care | Knowledge | 128 | 0.34 (0.19 to 0.49) | 0.28 (0.05 to 0.51) | 92% | x |
| Branda 2013 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | 59 | 0.08 (‐0.18 to 0.34) | ||||
| Branda 2013 | Patient mediated intervention (decision aid) + educational meeting | Usual care | 59 | 0.28 (0.02 to 0.53) | x | |||
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | 898 | 0.02 (0.00 to 0.03) | ||||
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | 898 | 0.47 (0.41 to 0.53) | x | |||
| Mathers 2012 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | 175 | 0.23 (0.09 to 0.37) | ||||
| Mathers 2012 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | 175 | 0.02 (‐0.12 to 0.15) | x | |||
| Härter 2015 | Patient‐mediated intervention + educational meeting | Usual care | Match between preferred and actual level of participation in decision making | 96 | ‐0.01 (‐0.19 to 0.18) | ‐0.03 (‐0.16 to 0.10) | 0% | x |
| Sheridan 2012 | Patient‐mediated intervention (video + coahcing session) + educational meeting | Usual care | 89 | ‐0.05 (‐0.24 to 0.13) | x | |||
| Hamann 2007 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Adherence (good) (6 months) | 86 | ‐0.14 (‐0.35 to 0.07 | 0.00 (‐0.15 to 0.15) | 0% | x |
| Hamann 2007 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Adherence (good) (12 months) | 68 | 0.02 (‐0.21 to 0.26) | |||
| Branda 2013 | Patient‐mediated intervention decision aid) + educational meeting | Usual care | Adherence to medication (% of patients with ≥ 80% of days covered) | 77 | ‐0.01 (‐0.20 to 0.18) | x | ||
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | Safety (whether a patient experienced a major adverse cardiac event): MACE within 30 days | 898 | 0.00 (‐0.00 to 0.01) | 0.00 (‐0.00 to 0.01) | na | na |
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | Safety (whether a patient experienced a major adverse cardiac event): Acute myocardial infarction | 898 | 0.01 (‐0.00 to 0.02) | 0.01 (‐0.00 to 0.02) | na | na |
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | Safety (whether a patient experienced a major adverse cardiac event): Death of cardiac or unknown cause | 898 | 0.00 (‐0.00 to 0.00) | 0.00 (‐0.00 to 0.00) | na | na |
| Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Hamann 2007 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Knowledge | "Patients in the intervention group knew significantly more about their disease and treatment at the time of discharge." Page 270 (Hamann 2006) | na | |||
| Hess 2012 | Patient‐mediated intervention + educational meeting | Usual care | Knowledge | "Patients randomized to the shared decision‐making arm answered a greater number of questions in the knowledge questionnaire correctly (Table 2)." page 255 "They also had greater knowledge regarding their exact pretest probability of ACS within 45 days (25% versus 1%; mean difference, 24%; 95% CI, 22%–26%)." Page 255 | na | |||
| Branda 2013 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Knowledge (baseline) | Decision aid use significantly increased knowledge transfer at baseline (Table 3). | na | |||
| Branda 2013 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Knowledge (3 months) | No difference between group (page 6; table 3) | na | |||
| Branda 2013 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Knowledge (6 months) | No difference between group (page 6; table 3) | na | |||
| Leighl 2011 | Patient‐mediated intervention + educational meeting | Usual care | Satisfaction with decision (4 weeks) | "Decision satisfaction and decisional conflict scores were similar in both arms. There were also no differences in decisional conflict subscale scores between arms. Other outcomes were similar between groups (Table 3)." Page 2080 | na | |||
| Hess 2012 | Patient‐mediated intervention + educational meeting | Usual care | Satisfaction with the decision making process | "Patients who used the decision aid reported greater satisfaction with the decision making process (strongly agree, 61% versus 40%; absolute difference, 21%; 95% CI, 7%–33%)." Page 255 | na | |||
| Leighl 2011 | Patient‐mediated intervention + educational meeting | Usual care | Satisfaction with consultation (post visit) | "Decision satisfaction and decisional conflict scores were similar in both arms. There were also no differences in decisional conflict subscale scores between arms. Other outcomes were similar between groups (Table 3)." Page 2080 | na | |||
| Wetzels 2005 | Patient‐mediated Intervention (leaflet) + educational outreach visit | Usual care | Satisfaction with care | "Finally, patients were very satisfied with the way their GP behaved during the consultation. No differences between intervention and control group were detected." Page 290 | na | |||
| Hamann 2007 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | "Patients in the intervention group did not differ from the patients in the control group in regard to overall satisfaction with treatment (ZUF 8)…" Page 270 (Hamann 2006) | na | ||||
| Haskard 2008 | Patient‐mediated intervention + distribution of educational material + educational meeting | Usual care | "The following significant ( p < .05) effects emerged: physician training improved patients’ satisfaction with information and overall care" (page 513). "Physician training improved patient satisfaction with “overall care” (the linear time physician training interaction)" Page 518 | na | ||||
| Hess 2012 | Patient‐mediated intervention + educational meeting | Usual care | Satisfaction with the intervention | "The decision aid was acceptable, clear, and helpful to patients and clinicians (Table 2)." Page 255 | na | |||
| Leighl 2011 | Patient‐mediated intervention + educational meeting | Usual care | "Ninety percent of those who received the DA reported that it was helpful or very helpful in making their treatment decision." Page 2081 | na | ||||
| Branda 2013 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | "Patients were similarly satisfied with usual care and the decision aid; 71% of decision aid patients found the information provided helpful compared to 53% of patients in the usual care arm (p=.17) (Table 4)." Page 4 | na | ||||
| Mathers 2012 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | "Most of the PDA users found the PDA useful. When asked about their opinion of the PDA, 83.2% (n=88), 86.3% (n=89), 86.3% (n=89) and 88.4% (n=90) thought that the PDA had helped them to recognise that a decision needs to be made; know that the decision depends on what matters most to them; think about how involved they wanted to be in the decision; and prepare to talk to the nurse or doctor about what mattered most to them’,respectively." Pages 7‐8 | na | ||||
| Hess 2016 | Patient‐mediated intervention (decision aid) + reminder (quantitative pretest probability web tool) | Usual care | "Patients randomized to the decision aid found the information discussed to be of greater clarity, and a greater proportion (decision aid, 88.0% v usual care, 79.9%; absolute difference 8.1%, P=0.004) would recommend the way they discussed management options with their clinician to others." page 6 | na | ||||
| Leighl 2011 | Patient‐mediated intervention + educational meeting | Usual care | Decisional conflict | "There were also no differences in decisional conflict subscale scores between arms. (Table 3)." Page 2080 | na | |||
| Branda 2013 | Patient‐mediated intervention (decision aid + educational meeting | Usual care | "Patient decisional comfort was similarly high in both trial arms." Page 5 | na | ||||
| Coylewright 2016 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | "The decision aid significantly improved the degree to which patients felt informed regarding their choices (decisional conflict score in decision aid arm: 15.4 versus UC: 21.9; P=0.043); overall decisional conflict was not different between the 2 arms." Page 773 | na | ||||
| Hess 2012 | Patient‐mediated intervention + educational meeting | Usual care | "Patients who used the decision aid also experienced less decisional conflict when engaging in management decisions regarding their care." Page 255 | na | ||||
| Mathers 2012 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Decision regret (6 months) | "Table 9 shows that there was no difference at 6 months in the Regret Scale" Page 7 | na | |||
| Epstein 2017 | Patient‐mediated intervention (patients & caregivers coaching session + question prompt list) + educational meeting | Usual care | Self‐efficacy | "There were no statistically significant effects of the intervention on the PEPPI..." Page 96 | na | |||
| Leighl 2011 | Patient‐mediated intervention + educational meeting | Usual care | Match between preferred and actual level of participation in decision making | "After arriving at a treatment decision, 32% of those who received the DA and 35% in the standard arm reported perceiving a role in decision making that matched their preference. Most perceived playing a greater role than initially preferred (DA arm, 38%; standard arm, 41%). There were no significant differences between study arms." Page 2081 | na | |||
| Cooper 2011 | Patient‐mediated intervention + educational meeting | Usual care | Adherence to medication | “Changes in patient‐reported adherence to medications at 12 months did not differ for any of the intervention groups compared to the patient+physician minimal intervention group.” Page 1300 | na | |||
| Mathers 2012 | Patient‐mediated intervention (decision aid) + educational meeting | Usual care | Persistence with the chosen option | "Patients in the intervention group were rather more likely to persist with their chosen option." Page 7 [68.1% vs 56.3%; adjusted OR (95% CI): 1.17 (1.00 to 1.36); p= 0.041] | na | |||
| Leighl 2011 | Patient‐mediated intervention + educational meeting | Usual care | Anxiety | "Patient anxiety was low to moderate at all time points, with a minor decrease over time. There was no difference between study arms, demonstrating no adverse impact of the DA (Fig 3)." Page 2080 | na | |||
| Loh 2007 | Patient‐mediated intervention + educational meeting | Usual care | Depression | No difference between group (page 329; table 2) | na | |||
| Loh 2007 | Patient‐mediated intervention + educational meeting | Usual care | Consultation length (minutes) | "No differences were found between study groups for length of consultation." Page 329 | na | |||
| Wetzels 2005 | Patient‐mediated intervention (leaflet) + educational outreach visit | Usual care | There were no differences between intervention and control group regarding consultation length (page 292, table 3) | na | ||||
ACS: acute coronary syndrom; CI: confidence interval; DA: decision aid; MACE: major averse cardiac events; N: sample size; na: not applicable; PCOMS: Partners for Change Outcome Management System; PEPPI: Perceived Efficacy in Patient‐Physician Interactions; PDA: patient decision aid; QoL: quality of life; OR: odds ratio; RD: risk difference; SMD: standardized mean difference.
10. Effect of interventions on secondary outcomes: interventions targeting patients compared to other interventions targeting patients.
| Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Raynes‐Greenow 2010 | Patient‐mediated intervention (audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | Knowledge | 596 | 0.30 (0.13 to 0.47) | 0.30 (0.13 to 0.47) | na | na |
| Raynes‐Greenow 2010 | Patient‐mediated intervention (audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | Satisfaction with decision (first follow‐up) | 596 | 0.07 (‐0.10 to 0.24) | 0.07 (‐0.10 to 0.24) | na | na |
| Raynes‐Greenow 2010 | Patient‐mediated intervention (audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | Satisfaction with decision (second follow‐up) | 596 | 0.11 (‐0.06 to 0.28) | 0.11 (‐0.06 to 0.28) | na | na |
| Hamann 2011 | Patient‐mediated intervention (SDM training) | Patient‐mediated intervention (cognitive training) | Satisfaction with treatment | 61 | ‐0.32 (‐0.83 to 0.19) | ‐0.09 (‐0.34 to 0.16) | 4% | x |
| Hamann 2017 | Patient‐mediated intervention (training for patient‐ SDM training) | Patient‐mediated intervention (training for patient‐ cognitive training) | 206 | ‐0.02 (‐0.29 to 0.25) | x | |||
| Jouni 2017 | Patient‐mediated intervention (conventional risk and genetic risk information + decision aid) | Patient‐mediated intervention (conventional risk information + decision aid) | Satisfaction with consultation | 207 | ‐0.14 (‐0.42 to 0.13) | ‐0.14 (‐0.42 to 0.13) | na | na |
| Causarano 2014 | Patient‐mediated intervention (Routine education + educational meeting to patient) | Patient‐mediated intervention (Routine education) | Satisfaction with the information provided | 39 | 0.11 (‐0.52 to 0.73) | 0.11 (‐0.52 to 0.73) | na | na |
| Dolan 2002 | Patient‐mediated intervention (preliminary phase + decision aid) | Patient‐mediated intervention (preliminary phase + educational phase) | Decisional conflict | 88 | ‐0.29 (‐0.71 to 0.13) | ‐0.20 (‐0.48 to 0.08) | 72% | x |
| Lalonde 2006 | Patient‐mediated intervention (decision aid + four‐step decision making strategy) | Patient‐mediated intervention (decision aid) | Decisional conflict | 50 | 0.10 (‐0.46 to 0.65) | x | ||
| Raynes‐Greenow 2010 | Patient‐mediated intervention (audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | Decisional conflict (primary follow‐up) | 596 | ‐0.09 (‐0.26 to 0.08) | x | ||
| Raynes‐Greenow 2010 | Patient‐mediated intervention (audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | Decisional conflict (second follow‐up) | 596 | ‐0.02 (‐0.19 to 0.15) | |||
| Adarkwah 2016 | Patient‐mediated intervention (decision aid‐Emoticon group) | Patient‐mediated intervention (decision aid‐TTE group) | Decisional conflict | 304 | 0.05 (‐0.18 to 0.27) | x | ||
| Smallwood 2017 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (web‐based information) | Decisional conflict (post‐intervention) | 50 | ‐1.15 (‐1.76 to ‐0.54) | x | ||
| Smallwood 2017 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (web‐based information) | Decisional conflict (3 months) | 50 | ‐0.51 (‐1.08 to 0.06) | |||
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | Decision uncertainty ‐ time 4 | 80 | ‐0.21 (‐0.65 to 0.23) | ‐0.21 (‐0.65 to 0.23) | na | na |
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | Decision uncertainty ‐ time 5 | 71 | ‐0.42 (‐0.89 to 0.05) | ‐0.42 (‐0.89 to 0.05) | na | na |
| Causarano 2014 | Patient‐mediated intervention (routine education + educational meeting to patient) | Patient‐mediated intervention (Routine education) | Decision self‐efficacy | 39 | ‐0.12 (‐0.75 to 0.51) | ‐0.02 (‐0.41 to 0.37) | 0% | x |
| Hamann 2011 | Patient‐mediated intervention (SDM training) | Patient‐mediated intervention (cognitive training) | 61 | 0.04 (‐0.46 to 0.55) | x | |||
| Hamann 2017 | Patient‐mediated intervention training for patient‐ SDM training) | Patient‐mediated intervention (training for patient‐ cognitive training) | Adherence to medication ‐ 6 months after discharge | 100 | 0.05 (‐0.35 to 0.44) | 0.05 (‐0.35 to 0.44) | na | na |
| Hamann 2017 | Patient‐mediated intervention training for patient‐ SDM training) | Patient‐mediated intervention (training for patient‐ cognitive training) | Adherence to medication ‐ 12 months after discharge | 85 | ‐0.18 (‐0.61 to 0.25) | ‐0.18 (‐0.61 to 0.25) | na | na |
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | General health (t4) | 88 | ‐0.19 (‐0.61 to 0.23) | ‐0.19 (‐0.61 to 0.23) | na | na |
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | General health (t5) | 82 | 0.53 (0.09 to 0.97) | 0.53 (0.09 to 0.97) | na | na |
| Raynes‐Greenow 2010 | Patient‐mediated intervention (Audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | Anxiety (State) ‐ 1st follow‐up | 596 | ‐0.10 (‐0.27 to 0.07) | ‐0.11 (‐0.27 to 0.05) | 0% | x |
| Raynes‐Greenow 2010 | Patient‐mediated intervention (Audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | Anxiety (State) ‐ 2nd follow‐up | 596 | 0.05 (‐0.12 to 0.21) | |||
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | Anxiety (State) ‐ time 4 | 86 | ‐0.18 (‐0.60 to 0.25) | x | ||
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | Anxiety (State) ‐ time 5 | 87 | ‐0.36 (‐0.78, 0.07) | |||
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | Depression ‐ time 4 | 86 | ‐0.27 (‐0.69 to 0.16) | ‐0.27 (‐0.69 to 0.16) | na | na |
| van Roosmalen 2004 | Patient‐mediated intervention (shared decision making intervention+decision aid) | Patient‐mediated intervention (decision aid) | Depression ‐ time 5 | 87 | ‐0.39 (‐0.82 to 0.03) | ‐0.39 (‐0.82 to 0.03) | na | na |
| Causarano 2014 | Patient‐mediated intervention (Routine education + educational meeting to patient) | Patient‐mediated intervention (Routine education) | Consultation length (minutes) | 39 | ‐0.65 (‐1.29 to ‐0.00) | ‐0.65 (‐1.29 to ‐0.00) | na | na |
| Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| Barton 2016 | Patient‐mediated intervention (Low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | Knowledge | 106 | 0.09 (‐0.10 to 0.28) | 0.16 (‐0.10 to 0.42) | 91% | x |
| Barton 2016 | Patient‐mediated intervention (decision aid + low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | 118 | 0.25 (0.08 to 0.41) | ||||
| Barton 2016 | Patient‐mediated intervention (decision aid + low literacy medication guide) | Patient‐mediated intervention (Low literacy medication guide) | 108 | 0.16 (‐0.01 to 0.33) | ||||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education programme) | Patient‐mediated intervention (4h MS‐specific stress management programme) | Pre: 192; Post: 178 | 0.39 (0.26 to 0.53) | x | |||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Patient‐mediated intervention (decision aid brochure) | 422 | 0.00 (‐0.09 to 0.09) | x | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Concordance between preferred and assumed role in decision making | 131 | ‐0.13 (‐0.32 to 0.06) | ‐0.10 (‐0.16 to ‐0.05) | 20% | x |
| Dolan 2002 | Patient‐mediated intervention (preliminary phase + decision aid) | Patient‐mediated intervention (preliminary phase + educational phase) | 86 | 0.05 (‐0.14 to 0.24) | x | |||
| Kasper 2008 | Patient‐mediated intervention (decision aid + information booklet about immunotherapy) | Patient‐mediated intervention (decision aid + standard information package) | 278 | ‐0.09 (‐0.17 to 0.00) | x | |||
| Davison 2002 | Patient‐mediated intervention (computer generated information and decision preference profiles + computer generated prompt sheet) | Patient‐mediated intervention (discussion with research nurse) | 734 | ‐0.14 (‐0.20 to ‐0.08) | x | |||
| Schroy 2016 | Patient‐mediated intervention (decision aid + electronic risk assessment tool) | Patient‐mediated intervention (decision aid) | Concordance between preferred and chosen option | 341 | ‐0.03 (‐0.10 to 0.04) | ‐0.20 (‐0.60 to 0.20) | 81% | x |
| Causarano 2014 | Patient‐mediated intervention (Routine education + educational meeting to patient) | Patient‐mediated intervention (Routine education) | 22 | ‐0.45 (‐0.79 to ‐0.10) | x | |||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | Adherence to medication (self‐report): did not miss a dose in last week | 36 | 0.02 (‐0.30 to 0.33) | 0.01 (‐0.10 to 0.12) | 0% | x |
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | Adherence to medication (pharmacy record): > 80% d covered | 42 | 0.26 (0.06 to 0.47) | |||
| Barton 2016 | Patient‐mediated intervention (Low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | Adherence to medication: did not miss dose in past week | 81 | ‐0.23 (‐0.44 to ‐0.03) | |||
| Barton 2016 | Patient‐mediated intervention (decision aid + low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | Adherence to medication: did not miss dose in past week | 93 | ‐0.14 (‐0.33 to 0.05) | |||
| Barton 2016 | Patient‐mediated intervention (decision aid + low literacy medication guide) | Patient‐mediated intervention (Low literacy medication guide) | Adherence to medication: did not miss dose in past week | 92 | 0.10 (‐0.11 to 0.30) | x | ||
| Hamann 2017 | Patient‐mediated intervention training for patient‐ SDM training) | Patient‐mediated intervention (training for patient‐ cognitive training) | Adherence to medication and outpatient visits (good adherence) | 173 | ‐0.04 (‐0.18 to 0.11) | x | ||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education programme) | Patient‐mediated intervention (4h MS‐specific stress management programme) | Adherence to medication (DMD discontinuation) | 86 | ‐0.14 (‐0.31 to 0.02) | ‐0.14 (‐0.31 to 0.02) | na | na |
| Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | Knowledge (DA specific) | Knowledge DA specific (P = 0.001) Table 2, page 553 | na | |||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | Knowledge (Not in the DA) | Knowledge not in the DA (P = 0.35) Table 2, page 553 | na | |||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education programme) | Patient‐mediated intervention (4h MS‐specific stress management programme) | Knowledge | Significant difference between groups (p < 0.001) (Page 415; table 3) | na | |||
| Schroy 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (educational material) | "Mean [standard deviation] cumulative pretest knowledge scores were comparable (P = 0.91) for the 3 groups (decision aid plus YDR, 7.6 [2.8]; decision aid alone, 7.7 [2.9]; control, 7.5 [2.7]). Cumulative posttest scores, however, were significantly higher (P < 0.001) for the 2 intervention groups (decision aid plus YDR, 10.7 [1.8]; decision aid alone, 10.9 [1.6]) compared with the control group (8.6 [2.7]), with differences corresponding to large effect sizes of d = 1.15 and d = 1.27 for the decision aid plus YDR group and decision aid alone group versus control, respectively." Pages 7 and 8 | na | ||||
| Schroy 2011 | Patient‐mediated intervention (decision aid + YDR) | Patient‐mediated intervention (educational material) | "Mean [standard deviation] cumulative pretest knowledge scores were comparable (P = 0.91) for the 3 groups (decision aid plus YDR, 7.6 [2.8]; decision aid alone, 7.7 [2.9]; control, 7.5 [2.7]). Cumulative posttest scores, however, were significantly higher (P < 0.001) for the 2 intervention groups (decision aid plus YDR, 10.7 [1.8]; decision aid alone, 10.9 [1.6]) compared with the control group (8.6 [2.7]), with differences corresponding to large effect sizes of d = 1.15 and d = 1.27 for the decision aid plus YDR group and decision aid alone group versus control, respectively." Pages 7 and 8 | na | ||||
| Lalonde 2006 | Patient‐mediated intervention (decision aid + four‐step decision making strategy) | Patient‐mediated intervention (decision aid) | "Since similar CVD knowledge and risk perception before and after the intervention were observed in DA and PRP groups, the participants in both groups were combined." page 54 ...” However, knowledge of the estimated benefits of treatment tended to improve after the intervention (29% versus 58%; P = 0.06)“ Page 55 | na | ||||
| Nannenga 2009 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (pamphlet) | "Use of the decision aid resulted in higher knowledge scores (mean difference 1.6, 95% CI: 0.7, 2.5)..." Page 42 | na | ||||
| Street 1995 | Patient‐mediated intervention (Interactive multimedia program decision aid) | Patient‐mediated intervention (brochure decision aid) | “The effect for method of communication approached significance (F = 3.30, P = 0.07) as patients in the computer group tended to learn more (mean, 75.5%; SD13.64%) than did patients in the brochure group (mean, 71.4%; SD, 15.7%)” Page 2279 | na | ||||
| Street 1995 | Patient‐mediated intervention (Interactive multimedia program decision aid) | Patient‐mediated intervention (brochure decision aid) | “The effect for method of communication approached significance (F = 3.30, P = 0.07) as patients in the computer group tended to learn more (mean, 75.5%; SD13.64%) than did patients in the brochure group (mean, 71.4%; SD, 15.7%)” Page 2279 | na | ||||
| Thomson 2007 | Patient‐mediated intervention (Computerised decision aid) | Patient‐mediated intervention (Guidelines) | "Although the overall knowledge scores improved slightly post‐clinic, by three months they were back to pre‐clinic levels; there was no difference between decision aid and guidelines groups at any point." Page 220 | na | ||||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Satisfaction with consultation | “No significant differences were found between the groups in satisfaction with either the consultation or treatment decision” Page 4407 | na | |||
| Davison 2002 | Patient‐mediated intervention (computer generated information and decision preference profiles + computer generated prompt sheet) | Patient‐mediated intervention (discussion with research nurse) | "The 14‐item satisfaction questionnaire was found to be reliable (Cronbach’s Alpha = .885) (see Figure 3 for items included in the questionnaire). Women did not significantly differ on any of the items, and both groups were satisfied with their clinic visits. The overall mean score for this scale was 1.64 (SD = .54)." (no page number, in the results section) | na | ||||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Satisfaction with decision (treatment) | “No significant differences were found between the groups in satisfaction with either the consultation or treatment decision” Page 4407 | na | |||
| Deschamps 2004 | Patient‐mediated intervention (pharmacist consultation + patient specific information + letter) | Patient‐mediated intervention (decision aid) | “Women in the pharmacist and decision‐aid groups had mean SWD scores of 4.3 and 4.4 respectively (scale range: 1 to 5) with no significant differences being reported between groups. Page 26 | na | ||||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education programme) | Patient‐mediated intervention (4h MS‐specific stress management programme) | "In both groups, almost all decisions were reported as satisfactorily (data not shown)." Page 414 | na | ||||
| Kasper 2008 | Patient‐mediated intervention (decision aid + information booklet about immunotherapy) | Patient‐mediated intervention (decision aid + standard information package) | Satisfaction with the intervention | "Patients in the IG rated the value of the received information for the decision‐making process significantly higher than did those in the CG (P < 0.001). This result refers to the IG patients feeling of being better informed (P < 0.001), getting important questions more adequately answered (P < 0.01), and being better supported in finding their preferred role (P < 0.05), compared to patients in the CG." Pages 1349‐1350 | na | |||
| Lalonde 2006 | Patient‐mediated intervention (decision aid + four‐step decision making strategy) | Patient‐mediated intervention (decision aid) | "As reported in table 2, comparison of the acceptability of each educational tool revealed no satistically significant differences." Page 54 | na | ||||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | "Patients receiving the decision aid were satisfied with this mode of information transfer to the same extent as patients in usual care encounters (Table 2)." Page 553 | na | ||||
| Raynes‐Greenow 2010 | Patient‐mediated intervention (Audio + non‐audio decision aid ) | Patient‐mediated intervention (Pamphlet) | "Equally both groups would recommend the intervention they received to a pregnant friend (decision aid group 94% compared to pamphlet group 93%, chi‐square, df=1, P = 0.57)” Page 7 | na | ||||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | "No significant differences were found between groups in terms of reported anxiety provoked, perceived utility, or ease of understanding of the materials." Page 4405 | na | ||||
| Barton 2016 | Patient‐mediated intervention (low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | "The acceptability scale completed by patients immediately postvisit did not differ significantly across the 3 trial arms (P50.24)." Page 894 | na | ||||
| Barton 2016 | Patient‐mediated intervention (decision aid + low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | "The acceptability scale completed by patients immediately postvisit did not differ significantly across the 3 trial arms (P50.24)." Page 895 | na | ||||
| Barton 2016 | Patient‐mediated intervention (decision aid + low literacy medication guide) | Patient‐mediated intervention (Low literacy medication guide) | "The acceptability scale completed by patients immediately postvisit did not differ significantly across the 3 trial arms (P50.24)." Page 896 | na | ||||
| Hamann 2017 | Patient‐mediated intervention training for patient‐ SDM training) | Patient‐mediated intervention (training for patient‐ cognitive training) | "Overall patients enjoyed visiting both, the intervention and the control group. However, their ratings were more positive regarding the specific content of the intervention group and more patients in the intervention group planned to play a more active role in future consultations compared with the control group (Table 2)." Page 178 | na | ||||
| Warner 2015 | Patient‐mediated intervention (decision aid + patient education brochure) + education meeting | Patient‐mediated intervention (patient education brochure) | Satisfaction with discussion | "The measure of satisfaction with the smoking discussion assessed on the morning of surgery was significantly higher in patients receiving the decision aid (81 [24] and 90 [19] for the usual care and decision aid groups, respectively; P = 0.02). However, these differences did not persist; by 30 days after surgery, satisfaction was not different between groups (84 [21] and 84 [24] for the usual care and the decision aid groups, respectively, at day 30; P = 0.90)." Page 23 | na | |||
| Warner 2015 | Patient‐mediated intervention (decision aid + patient education brochure) + education meeting | Patient‐mediated intervention (patient education brochure) | Satisfaction with information provided | "However, clarity of information and helpfulness were rated significantly higher by patients receiving the decision aid, whereas willingness to recommend to others and assessment of the amount of information presented did not differ significantly (table 3)" Page 23 | na | |||
| Barton 2016 | Patient‐mediated intervention (Low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | Decisional conflict | No significant differences between groups (page 895; table 2) | na | |||
| Barton 2016 | Patient‐mediated intervention (decision aid + low literacy medication guide) | Patient‐mediated intervention (Existing medication guide) | Significant difference between groups (page 895; table 2) | na | ||||
| Causarano 2014 | Patient‐mediated intervention (Routine education + educational meeting to patient) | Patient‐mediated intervention (Routine education) | "The decrease in decisional conflict was greater in the intervention group (−37.7, SD=22.5) compared to routine education (−24.3, SD=16.0). The Cohen’s d effect size of 0.69 (95 % CI= 0.02–1.42) suggested a moderate to large effect size." (page 1371) | na | ||||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Patient‐mediated intervention (decision aid brochure) | "DCS scores among all 3 groups were equally low and did not differ signifi cantly (control, 1.58; brochure, 1.54; and Web site, 1.55)." Page 115 | na | ||||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | No difference between groups (p=0.725) (table 2) | na | ||||
| Deschamps 2004 | Patient‐mediated intervention (pharmacist consultation + patient specific information + letter) | Patient‐mediated intervention (decision aid) | The differences between groups were non‐significant (Table 2), Page 25 | na | ||||
| Nannenga 2009 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (pamphlet) | "use of the decision aid resulted in higher knowledge scores (mean difference 1.6, 95% CI: 0.7, 2.5) and less decisional conflict (mean difference ‐9.8, 95% CI: ‐14.2, ‐5.4)." Page 42 | na | ||||
| Thomson 2007 | Patient‐mediated intervention (Computerised decision aid) | Patient‐mediated intervention (Guidelines) | "For the decision conflict scale (the primary outcome measure), the difference in total scores between groups (maximum score 5 for high decision conflict) was estimated on each occasion. The mean (95% CI) differences for decision aid group versus the guideline group were 0.02 (20.22 to 0.26), 20.18 (20.34 to 20.01) and 20.15 (20.37 to 0.06) at pre‐clinic, post‐clinic and three month follow‐up respectively with a negative difference representing a lower decision conflict. While decision conflict fell in both groups post‐clinic compared to pre‐clinic, the difference between groups post‐clinic was significant at the 5% level (t=2.12, df=107, p=0.036)." Page 219 | na | ||||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education programme) | Patient‐mediated intervention (4h MS‐specific stress management programme) | "Decisional conflict scores were low for all subgroup categories with no differences between groups neither before the intervention nor after 12 months (data not shown). Overall, values further decreased during the study for both groups." Page 415 | na | ||||
| Stiggelbout 2008 | Patient‐mediated intervention (individualized brochure) | Patient‐mediated intervention (general brochure) | Patient‐clinician communication (understanding) | "The only difference that was seen for the items related to understanding was a difference in favor of the IB group in the stated understanding of the issues that were important in the treatment decision: 84% (n=32) of the IB group felt that due to the brochure they had better understanding, v. 62% (n=21) of the GB group (chi‐square test P =0:04)." Page 756 | na | |||
| Stiggelbout 2008 | Patient‐mediated intervention (individualized brochure) | Patient‐mediated intervention (general brochure) | Patient‐clinician communication (consultation with surgeon) | "A main difference between the 2 groups was seen in satisfaction with the duration of the consultation. Whereas 89% of the IB group was (rather) satisfied, all patients (100%) in the GB group were satisfied with the duration of the consultation (chi‐square test P =0:04). For patients’ impression whether the surgeon perceived them more as a medical problem than as a person with a problem, an interaction effect was observed, F(1, 68)=4.31, P =0:04. Further analysis showed that in the IB group from 1st to 2nd consultation, the feeling increased that the surgeon perceived them more as a medical problem than as a person with a problem (mean increased from 1.9, s 1.3, to 2.3, s 1.4), whereas for the GB group this feeling decreased (from 2.0, s 1.3, to 1.7, s 1.2)." Page 757 | na | |||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Patient‐mediated intervention (decision aid brochure) | Concordance between preferred and assumed role in decision making | "Concordance did not differ between the 3 study groups (P1 = .41)." (figure 2, page 117) | na | |||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | Adherence to medication (pharmacy record): % of days covered | No difference between groups (p=0.09) (table 5) | na | |||
| Deschamps 2004 | Patient‐mediated intervention (pharmacist consultation + patient specific information + letter) | Patient‐mediated intervention (decision aid) | Adherence to medication (HRT) | ”There was no statistically significant difference in adherence between the study groups“ Page 26 | na | |||
| Thomson 2007 | Patient‐mediated intervention (Computerised decision aid) | Patient‐mediated intervention (Guidelines) | Adherence to initial decision | "Fitting first a difference between groups, participants in the decision aid group were less likely to make a definite decision to start or continue warfarin than participants in the guidelines arm (OR=0.33, 95% CI 0.12 to 0.95)." Page 220 | na | |||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | Persistence (pharmacy record): Number of days covered | No difference between group (p=0.38) (table 5) | na | |||
| Stiggelbout 2008 | Patient‐mediated intervention (individualized brochure) | Patient‐mediated intervention (general brochure) | Health‐related QoL | “Patients’ quality of life was stable over time, in both groups. No effects were observed in the repeated measures for the anxiety and depression scales of the HADS, nor on the quality of life scales” Page 757 | na | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Anxiety (State) ‐ before consultation | "Before the consultation, patients who had received the CCPP were significantly more anxious than those who received the control booklet (mean, 42 v 38, respectively; t 2.0; P .04)." Page 4406 | na | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Anxiety (State) ‐ after consultation | "In both groups, anxiety decreased by 3 points after the consultation, and there was no significant difference between the groups immediately after the consultation and 1 month later." Page 4407 | na | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Anxiety (State) ‐ 1 month | "In both groups, anxiety decreased by 3 points after the consultation, and there was no significant difference between the groups immediately after the consultation and 1 month later." Page 4407 | na | |||
| Stiggelbout 2008 | Patient‐mediated intervention (individualized brochure) | Patient‐mediated intervention (general brochure) | Anxiety | “Patients’ quality of life was stable over time, in both groups. No effects were observed in the repeated measures for the anxiety and depression scales of the HADS, nor on the quality of life scales” Page 757 | na | |||
| Thomson 2007 | Patient‐mediated intervention (Computerised decision aid) | Patient‐mediated intervention (Guidelines) | "There was a significant fall in anxiety immediately after the clinic (mean change pre‐clinic to post‐clinic of 24.57 (95% CI 26.30 to 22.84)) but no evidence that this reduction varied between the two groups (F1,95=0.001; p=0.98)." Page 220 | na | ||||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education programme) | Patient‐mediated intervention (4h MS‐specific stress management programme) | "Mean scores of anxiety and depression were low and no significant differences between groups and between measurement points were found for Hospital Anxiety and Depression Scale assessments. At baseline, results were 7±3.6 (IG) and 7±3.7 (CG) for anxiety and 4.1±3.8 (IG) and 4.8±4.1 (CG) for depression. After 12 months, results were 6.6±3.6 (IG) and 6.8±4.1 (CG) for anxiety and 3.8±3.4 (IG) and 4.5±4.1 (CG) for depression, indicating that neither intervention had an influence on participants’ anxiety and depression levels." Page 415 | na | ||||
| Davison 1997 | Patient‐mediated intervention (individual empowerment sessions) | Patient‐mediated intervention (information package) | Anxiety (trait) | “There was no evidence trait scores were different among groups, among measurement times, or between groups and measurement times” Page 195 | na | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Depression ‐ before consultation | "The groups’ depression levels were similar at baseline; both were in the low range (mean, 16.25)." Page 4406 | na | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Depression ‐ after consultation | "No significant differences between groups were observed in raw or change scores on depression immediately after the consultation or 1 month later." Page 4407 | na | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Depression ‐ 1 month | "No significant differences between groups were observed in raw or change scores on depression immediately after the consultation or 1 month later." Page 4407 | na | |||
| Davison 1997 | Patient‐mediated intervention (individual empowerment sessions) | Patient‐mediated intervention (information package) | Depression | “No significant differences in mean depression scores were found among the groups, among measurement times, or between groups and measurement times” Page 196 | na | |||
| Stiggelbout 2008 | Patient‐mediated intervention (individualized brochure) | Patient‐mediated intervention (general brochure) | Depression | “Patients’ quality of life was stable over time, in both groups. No effects were observed in the repeated measures for the anxiety and depression scales of the HADS, nor on the quality of life scales” Page 757 | na | |||
| Köpke 2014 | Patient‐mediated intervention (interactive‐4h education programme) | Patient‐mediated intervention (4h MS‐specific stress management programme) | Depression | "Mean scores of anxiety and depression were low and no significant differences between groups and between measurement points were found for Hospital Anxiety and Depression Scale assessments. At baseline, results were 7±3.6 (IG) and 7±3.7 (CG) for anxiety and 4.1±3.8 (IG) and 4.8±4.1 (CG) for depression. After 12 months, results were 6.6±3.6 (IG) and 6.8±4.1 (CG) for anxiety and 3.8±3.4 (IG) and 4.5±4.1 (CG) for depression, indicating that neither intervention had an influence on participants’ anxiety and depression levels." Page 415 | na | |||
| Butow 2004 | Patient‐mediated intervention (consultation preparation package) | Patient‐mediated intervention (booklet) | Consultation length (minutes) | "Consultation length was similar between groups—on average, 36 minutes per consultation." Pages 4406‐4407 | na | |||
| Krist 2007 | Patient‐mediated intervention (decision aid web) | Patient‐mediated intervention (decision aid brochure) | Consultation length | “These [discussion times] patient‐physician differences did not differ significantly across the control, brochure, and Web groups.” Page 116 | na | |||
| Montori 2011 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (Usual care and booklet) | Consultation length (minutes) | “The median (range)duration of osteoporosis discussions was 12.4 minutes (2.3‐27.4) in the decision aid arm compared with 9.4 minutes (2.1‐58) in the usual care arm (P .045)” Pages 552‐553 | na | |||
| Nannenga 2009 | Patient‐mediated intervention (decision aid) | Patient‐mediated intervention (pamphlet) | Consultation length (minutes) | “We found no significant difference in face‐to‐face consultation duration with the staff endocrinologist (mean difference 3.8 min longer with the decision aid, 95% CI ‐ 2.9 to 10.5).” Page 42 | na | |||
CCPP: cancer consultation preparation package; CG: control group; CI: confidence interval; CVD: cardiovascular diseases; DA; decision aid; DCS: Decisional Conflict Scale; df: degrees of freedom; DMD: disease modifying drug; GB: general brochure; HADS: Hospital Anxiety and Depression Scale; HRT: Hormone Replacement Therapy; IB: individualized brochure; IG: intervention group; MS: Multiple Sclerosis; N: sample size; na: not applicable; OR: odds ratio; QoL: quality of life; RD: risk difference; SD: standard deviation; SDM: shared decision making; SMD: standardized mean difference; SWD: Satisfaction With Decision; TTE: time‐to‐event; YDR: Web‐based ‘‘Your Disease Risk’’ colorectal cancer risk assessment tool.
11. Effect of interventions on secondary outcomes: interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals.
| Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Elwyn 2004 | Educational meeting + audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Health status (mental) time 1 | Pre: 169 Post: 295 | 0.24 (0.01 to 0.47) | 0.24 (0.01 to 0.47) | na | na |
| Elwyn 2004 | Educational meeting + audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Health status (mental) time 2 | Pre: 147 Post: 257 | 0.17 (‐0.07 to 0.42) | 0.17 (‐0.07 to 0.42) | na | na |
| Elwyn 2004 | Educational meeting + audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Health status (physical) time 1 | Pre: 169 Post: 295 | 0.05 (‐0.19 to 0.28) | 0.05 (‐0.19 to 0.28) | na | na |
| Elwyn 2004 | Educational meeting + audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Health status (physical) time 2 | Pre: 147 Post: 257 | ‐0.01 (‐0.26 to 0.24) | ‐0.01 (‐0.26 to 0.24) | na | na |
| Elwyn 2004 | Educational meeting + audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Anxiety ‐ time 1 | Pre: 186 Post: 325 | 0.04 (‐0.18 to 0.26) | 0.04 (‐0.18 to 0.26) | na | na |
| Elwyn 2004 | Educational meeting + audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Anxiety ‐ time 2 | Pre: 165 Post: 281 | 0.25 (0.02 to 0.49) | 0.25 (0.02 to 0.49) | na | na |
| Elwyn 2004 | Educational meeting + audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Anxiety ‐ time 3 | Pre: 136 Post: 237 | 0.15 (‐0.11 to 0.40) | 0.15 (‐0.11 to 0.40) | na | na |
| Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Krones 2008 (ARRIBA‐Herz) | Educational meeting + audit and feedback + educational material and Educational outreach visit | Educational meeting | Knowledge | "Knowledge did not improve through intervention." Page 223. Difference intervention ‐ control (95% CI): 0.11 (‐0.01 to 0.24); p=0.07 (table 4) | na | |||
| Elwyn 2004 | Educational meeting and audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Satisfaction with information provided | ”No significant effects of the risk communication or SDM intervention were seen on the whole range of patient‐based outcomes“ Page 351 | na | |||
| Elwyn 2004 | Educational meeting and audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Satisfaction with decision | ”No significant effects of the risk communication or SDM intervention were seen on the whole range of patient‐based outcomes“ Page 351 | na | |||
| Krones 2008 (ARRIBA‐Herz) | Educational meeting + audit and feedback + Educational material and Educational outreach visit | Educational meeting | Decision regret (6 months) | "After 6 months, patients who could remember the decision and had completed the decisional regret scale (385 interventions, 377 controls), reported less decisional regret in the intervention arm. " Page 223. Difference intervention ‐ control (95% CI): ‐3.39 (‐6.26 to ‐0.53); p=0.02 (table 4) | na | |||
| Elwyn 2004 | Educational meeting and audit and feedback (SDM skills) | Educational meeting + audit and feedback (risk communication skills) | Consultation length (minutes) | “There was no difference in the mean consultation lengths at baseline, phase 1 and phase 2 (overall consultation mean duration was 12.5 minutes)” Page 342 | na | |||
CI: confidence interval; N: sample size; na: not applicable; RD: risk difference; SDM: shared decision making; SMD: standardized mean difference.
12. Effect of interventions on secondary outcomes: interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals.
| Continous data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | SMD | SMD (95% CI) | I2 | |
| Myers 2011 | Patient‐mediated intervention (pamphlet+counselling) + reminders (prompting) | Patient‐mediated intervention + reminders (prompting) | Decisional conflict | 286 | ‐0.07 (‐0.30 to 0.16) | ‐0.07 (‐0.30 to 0.16) | na | na |
| Categorical data | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | N | RD | RD (95% CI) | I2 | |
| No study | ||||||||
| Qualitative statement | Meta‐analysis | |||||||
| Study | Intervention | Intervention | Outcome | Direct quote | ||||
| Cooper 2013 | Patient‐mediated intervention, educational outreach visit + distribution of educational material + audit and feedback (Patient‐centred group) | Patient‐mediated intervention + educational outreach visit + distribution of educational material (Standard group) | Satisfaction with the intervention (helpfulness of the Depression Case Manager) | "At 12 months, compared with patients in the standard group, patients in the patient‐centred group had statistically significantly higher odds of rating their DCM as extremely helpful at identifying concerns (OR, 3.00; 95 percent CI, 1.23, 7.30) and improving adherence to treatment (OR, 2.60; 95 percent CI, 1.11, 6.08). Similar patterns were present, but not statistically significant, for other ratings of the DCMs (data not shown)." Page 166 | na | |||
| Cooper 2013 | Patient‐mediated intervention, educational outreach visit + distribution of educational material + audit and feedback (Patient‐centred group) | Patient‐mediated intervention + educational outreach visit + distribution of educational material (Standard group) | Depression (symptom reduction) | "Both groups experienced statistically highly significant reductions in mean depression severity score over time that are clinically meaningful. However, none of the adjusted between‐group differences in CES‐D over the follow‐up period were statistically significant (at 6 months, 1.8 points, 95 percent CI ‐3.4, 6.9; at 12 months, 2.4 points, 95 percent CI ‐7.7, 2.9; and at 18 months, 2.9 points, 95 percent CI, ‐8.2, 2.4)." Page 164 | na | |||
| Cooper 2013 | Patient‐mediated intervention, educational outreach visit + distribution of educational material + audit and feedback (Patient‐centred group) | Patient‐mediated intervention + educational outreach visit + distribution of educational material (Standard group) | Depression (remission) | "At 12 months, 33 percent of the patient‐centred group and 42 percent of the standard group achieved remission from depression (as measured by the CIDI); this difference was not statistically significant (adjusted OR 0.97; 95 percent CI, 0.34, 2.80)." Page 165 | na | |||
CES‐D: centre for Epidemiological Studies Depression Scale; CI: confidence interval; CIDI: Composite International Diagnostic Interview; DCM: depression case manager; N: sample size; na: not applicable; OR: odds ratio; RD: risk difference; SDM: shared decision making; SMD: standardized mean difference.
4.
Funnel plot of comparison: 1 Group 1: Interventions targeting patients compared to usual care, outcome: 1.2 Shared decision making (PROM, continuous).
5.
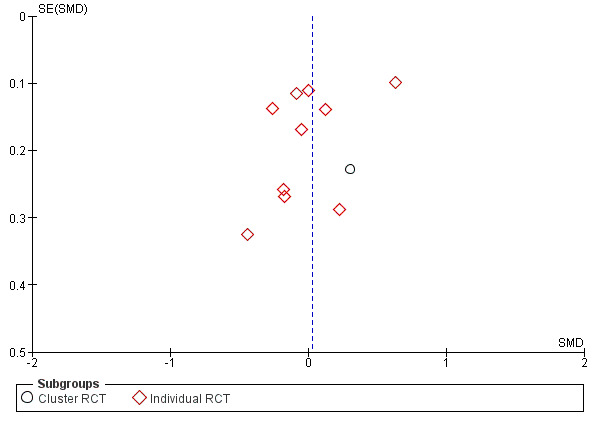
Funnel plot of comparison: 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, outcome: 4.2 Shared decision making (PROM, continuous).
6.
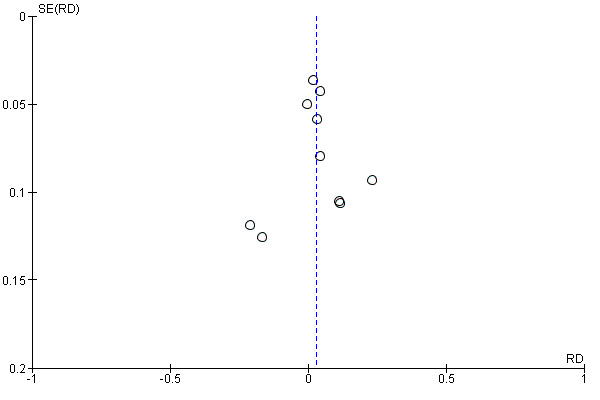
Funnel plot of comparison: 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, outcome: 4.6 Shared decision making (PROM, categorical).
Primary outcome ‐ Shared decision making
Observer‐based outcome measures (OBOMs) studies: comparisons with usual care
We are uncertain whether interventions increase the use of shared decision making (SDM) among healthcare professionals compared with usual care as measured by continuous OBOMs, as the certainty of the evidence was very low for all the six comparisons included in the review.
Interventions targeting patients versus usual care (six studies)
Six studies reported on seven continuous OBOMs of shared decision‐making (Hamann 2014; Haskard 2008; LeBlanc 2015a; LeBlanc 2015b; Maclachlan 2016; Tai‐Seale 2016). (Analysis 1.1; four studies, 424 observations, very low‐certainty evidence). The estimate of the standardized mean difference (SMD) was 0.54 (95% confidence interval (CI): ‐0.13 to 1.22). A unit of analysis error was observed in one study, and so we could not estimate the effect size (Haskard 2008).
1.1. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 1 Shared decision making (OBOM, continuous).
Interventions targeting healthcare professionals versus usual care (nine studies)
Eight randomized studies reported on seven continuous OBOMs of shared decision‐making (Bernhard 2011; Cox 2017; Fossli 2011; LeBlanc 2015b; Murray 2010; Shepherd 2011; Sanders 2017; Stacey 2006). (Analysis 2.1; six studies, 479 observations, very low‐certainty evidence). The pooled estimate of the SMD was 0.70 (95% CI: 0.21 to 1.19) in observer‐reported SDM in favor of the group that received the intervention. We are uncertain whether the intervention increases SDM as measured by continuous OBOMs as the certainty of the evidence was very low. The two randomized studies not included in the meta‐analysis reported that outcomes improved after exposure of study participants to the intervention (Bernhard 2011; Murray 2010).
2.1. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 1 Shared decision making (OBOM, continuous).
One controlled before and after study (Ampe 2017) showed an effect size (SMD) of ‐0.10 (95% CI: ‐0.96 to 0.76) (Analysis 2.2; one study, 21 observations, very low‐certainty evidence).
2.2. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 2 Shared decision making (OBOM, continuous) ‐ CBAs.
Interventions targeting both patients and healthcare professionals versus usual care (seven studies)
Seven studies reported on five continuous OBOMs of shared decision‐making (Branda 2013; Coylewright 2016; Härter 2015; Haskard 2008; Hess 2012; Hess 2016; Tai‐Seale 2016). Data from six studies were available for meta‐analysis (Analysis 3.1; six studies, 1270 observations, very low‐certainty evidence). The pooled estimate of the SMD was 1.10 (95% CI: 0.42 to 1.79) in observer‐reported SDM in favor of the group that received the intervention, although we cannot be certain of the effect estimate due to the very low certainty of the evidence. A unit of analysis error was observed in one study and so we could not estimate the effect size (Haskard 2008).
3.1. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 1 Shared decision making (OBOM, continuous).
Patient‐reported outcome measures (PROMs) studies: comparisons with usual care
We are uncertain whether interventions increase the use of SDM among healthcare professionals compared with usual care as measured by PROMs, as the certainty of the evidence was very low for the six comparisons included in the review.
Interventions targeting patients versus usual care (19 studies)
Eleven randomized studies reported on 10 continuous PROMs ( Cooper 2011; Deen 2012; Eggly 2017; Hamann 2014; Maranda 2014; Perestelo‐Perez 2016; Pickett 2012; van der Krieke 2013; van Peperstraten 2010; Tai‐Seale 2016; Vodermaier 2009). Data from nine studies were available for meta‐analysis (Analysis 1.2; nine studies, 1386 patients, very low‐certainty evidence). The pooled estimate of the SMD was 0.32 (95% CI: 0.16 to 0.48) in patient‐perceived SDM in favor of the group that received the intervention, although we have very little confidence in the effect estimate due to the very low‐certainty of the evidence. One of the two randomized studies not included in the meta‐analysis did not report that outcomes improved after exposure of study participants to the intervention (Hamann 2014); the other study did not provide data (Vodermaier 2009).
1.2. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 2 Shared decision making (PROM, continuous).
One non‐randomized study reported on one continuous PROM (Almario 2016) and showed an effect size (SMD) of 0.02 (95% CI: ‐0.21 to 0.25) (Analysis 1.3; one study, 303 patients, very low‐certainty evidence).
1.3. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 3 Shared decision making (PROM, continuous) ‐ NRCT.
Nine studies reported on two categorical PROMs (Korteland 2017; Krist 2007; Landrey 2012; Murray 2001; Sheridan 2014; van Tol‐Geerdink 2016; Vestala 2013; Vodermaier 2009; Wolderslund 2017). Data from six studies were available for meta‐analysis (Analysis 1.4; six studies, 754 patients, very low‐certainty evidence). We calculated a 0.09 reduction in the pooled estimate of the risk difference (RD) for these outcomes (95% CI: ‐0.19 to 0.01). The certainty of the evidence was very low. Among the three studies not included in the meta‐analysis, two studies reported that outcomes improved after exposure of study participants to the intervention (van Tol‐Geerdink 2016; Wolderslund 2017), and one study found little or no improvement (Korteland 2017).
1.4. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 4 Shared decision making (PROM, categorical).
Interventions targeting healthcare professionals versus usual care (eight studies)
Six studies reported on five continuous PROMs (Cooper 2011; Kennedy 2013; Koerner 2014; Légaré 2012; Tinsel 2013; Wilkes 2013). Data from five studies were used for meta‐analysis (Analysis 2.3; five studies, 5772 patients, very low‐certainty evidence). The pooled estimate of the SMD was 0.03 (95% CI: ‐0.15 to 0.20). The one study not included in the meta‐analysis reported that outcomes improved after exposure of study participants to the intervention (Légaré 2012).
2.3. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 3 Shared decision making (PROM, continuous).
Three studies reported on one categorical PROM (Bernhard 2011; Légaré 2012; O'Cathain 2002). Data from two studies were available for meta‐analysis (Analysis 2.4; two studies, 6303 patients, very low‐certainty evidence). The pooled estimate of the RD was 0.01 (95%CI: ‐0.03 to 0.06). The one study not included in the meta‐analysis did not report improvement after exposure of study participants to an intervention (Bernhard 2011).
2.4. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 4 Shared decision making (PROM, categorical).
Interventions targeting both patients and healthcare professionals versus usual care (11 studies)
Ten studies reported on 11 continuous PROMs (Cooper 2011; Epstein 2017; Härter 2015; Hamann 2007; Leighl 2011; Loh 2007; Rise 2012; Tai‐Seale 2016; Wetzels 2005; Wilkes 2013). Data from seven studies were available for meta‐analysis (Analysis 3.2; seven studies, 1479 patients, very low‐certainty evidence). The pooled estimate of the SMD was 0.13 (95% CI: ‐0.02 to 0.28). One of the studies not included in the meta‐analysis reported that outcomes improved after exposure of study participants to the intervention (Loh 2007).
3.2. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 2 Shared decision making (PROM, continuous).
Two studies reported on one categorical PROM (Härter 2015; Mathers 2012) and were both included in the meta‐analysis (Analysis 3.3; two studies, 266 patients, very low‐certainty evidence). The estimate of the RD was ‐0.01 (95% CI: ‐0.20 to 0.19).
3.3. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 3 Shared decision making (PROM, categorical).
Observer‐based outcome measures (OBOMs) studies: comparisons of interventions of the same type
We are uncertain whether interventions increase the use of SDM among healthcare professionals compared with other interventions of the same type, as measured by continuous OBOMs, as the certainty of the evidence was very low for all six comparisons.
Interventions targeting patients versus other interventions targeting patients (three studies)
The pooled estimate of the SMD was 0.88 (95% CI: 0.39 to 1.37) in observer‐reported SDM in favor of the group that received a patient decision aid compared to the group that received a booklet (SMD of 1.21; 95% CI: 0.78 to 1.64) or a pamphlet (SMD of 1.04; 95% CI: 0.60 to 1.48) (Jouni 2017; Montori 2011; Nannenga 2009). (Analysis 4.1; three studies, 271 observations,very low‐certainty evidence).
4.1. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 1 Shared decision making (OBOM, continuous).
Interventions targeting healthcare professionals versus other interventions targeting healthcare professionals (two studies)
Two studies reported on two continuous OBOMs (Elwyn 2004; Feng 2013). Data from one study were available for the analysis (Analysis 5.1; one study, 20 observations, very low‐certainty evidence). The SMD for this study was ‐0.30 (95% CI: ‐1.19 to 0.59). The study not included in the meta‐analysis reported a significant improvement for the group that received an interactive web‐based curriculum compared to the group that received a brochure (Feng 2013).
5.1. Analysis.
Comparison 5 Group 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals, Outcome 1 Shared decision making (OBOM, continuous).
Interventions targeting both patients and healthcare professionals versus other interventions targeting both patients and healthcare professionals (two studies)
One study reported on one continuous OBOM (Tai‐Seale 2016). The SMD for this study was ‐0.29 (95%CI: ‐1.17 to 0.60) (Analysis 6.1; one study, 20 observations, very low‐certainty evidence).
6.1. Analysis.
Comparison 6 Group 6: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals, Outcome 1 Shared decision making (OBOM, continuous).
One study reported on one categorical OBOM (Myers 2011). The RD for this study was ‐0.04 (95%CI: ‐0.13 to 0.04) (Analysis 6.2; one study, 134 observations, very low‐certainty evidence).
6.2. Analysis.
Comparison 6 Group 6: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals, Outcome 2 Shared decision making (OBOM, categorical).
Patient‐reported outcome measures( PROMs) studies: comparisons of interventions of the same type
We are uncertain whether interventions increase the use of SDM among healthcare professionals compared with usual care, as measured by PROMs, as the certainty of the evidence was very low for all six comparisons included in the review.
Interventions targeting patients versus other interventions targeting patients (26 studies)
Thirteen randomized studies reported 11 continuous PROMs (Adarkwah 2016; Causarano 2014; Deen 2012; Eggly 2017; Hamann 2011; Hamann 2017; Jouni 2017; Lalonde 2006; Schroy 2011; Schroy 2016; Smallwood 2017; Street 1995; van Roosmalen 2004), 11 of which were available for meta‐analysis (Analysis 4.2; 11 studies, 1906 patients, very low‐certainty evidence). The pooled estimate of the SMD was 0.03 (95% CI: ‐0.18 to 0.24). The two randomized studies not included in the meta‐analysis reported little or no difference between groups (Lalonde 2006; Street 1995).
4.2. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 2 Shared decision making (PROM, continuous).
One non‐randomized study (Barton 2016) reported an effect size for PROM of an SMD of ‐0.21 (95% CI: ‐0.61 to 0.19) (Analysis 4.3; one study, 97 patients, very low‐certainty evidence) when comparing an adapted guide with an existing medication guide. This study showed an effect size (SMD) of ‐0.19 (95% CI: ‐0.56 to 0.19) (Analysis 4.4; one study, 110 patients, very low‐certainty evidence) when comparing an adapted guide combined with a decision aid with an existing medication guide. Finally, this study showed an effect size (SMD) of 0.03 (95% CI: ‐0.37 to 0.43) (Analysis 4.5; one study, 99 patients, very low‐certainty evidence) when comparing an adapted guide combined with a decision aid with an adapted guide alone.
4.3. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 3 Shared decision making (PROM, continuous) comp1 ‐ NRCT.
4.4. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 4 Shared decision making (PROM, continuous) comp2 ‐ NRCT.
4.5. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 5 Shared decision making (PROM, continuous) comp3 ‐ NRCT.
Twelve studies reported on four categorical PROMs (Butow 2004; Davison 1997; Davison 2002; Deschamps 2004; Dolan 2002; Kasper 2008; Köpke 2014; Krist 2007; Raynes‐Greenow 2010; Stiggelbout 2008; Thomson 2007; Wolderslund 2017). Data from 10 studies were available for the meta‐analysis (Analysis 4.6; 10 studies, 2272 patients, very low‐certainty evidence). The pooled estimate of the RD was 0.03 (95% CI: ‐0.02 to 0.08).
4.6. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 6 Shared decision making (PROM, categorical).
Interventions targeting healthcare professionals versus other interventions targeting healthcare professionals (two studies)
The pooled estimate of the SMD was 0.24 (95%CI: ‐0.10 to 0.58) (Analysis 5.2; two studies, 1459 patients, very low‐certainty evidence) (Elwyn 2004; Krones 2008 (ARRIBA‐Herz)).
5.2. Analysis.
Comparison 5 Group 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals, Outcome 2 Shared decision making (PROM, continuous).
Interventions targeting both patients and healthcare professionals versus other interventions targeting both patients and healthcare professionals (two studies)
One study reported on one continuous PROM (Tai‐Seale 2016) an SMD of 0.00 (95%CI: ‐0.32 to 0.32) (Analysis 6.3; 150 patients, very low‐certainty evidence).
6.3. Analysis.
Comparison 6 Group 6: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals, Outcome 3 Shared decision making (PROM, continuous).
One study reported on one categorical PROM but data were not available to compute a risk difference (Cooper 2013). Authors of this study reported little or no difference between the two interventions.
Secondary outcomes
Interventions targeting patients versus usual care
Patient outcomes
Affective‐cognitive outcomes
Knowledge
Eight studies reported on knowledge (Korteland 2017; Krist 2007; Landrey 2012; LeBlanc 2015a; LeBlanc 2015b; Perestelo‐Perez 2016; Sheridan 2014; van Peperstraten 2010). Data from five studies were available for statistical analysis (Analysis 1.5; Analysis 1.6). On a continuous scale, the SMD was 0.38 (95% CI: 0.16 to 0.61; three studies, 565 participants; Analysis 1.5) for overall knowledge (knowledge not addressed in a decision aid) and 0.77 (95% CI: 0.44 to 1.10;) for knowledge addressed in a decision aid. This indicates an increase in knowledge for the group that received the intervention targeting patients (Analysis 1.5).
1.5. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 5 Knowledge.
1.6. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 6 Knowledge (categorical).
The RD was 0.17 (95% CI: 0.05 to 0.29; two studies, 312 participants) for knowledge of risk without medication/overall knowledge, indicating a small improvement for the group that received an intervention targeting patients (Analysis 1.6). Among studies that did not report enough data to perform a meta‐analysis, two studies reported improvement in overall knowledge in favor of the group that received the intervention (Korteland 2017; LeBlanc 2015a) and two studies reported an improvement in knowledge addressed in decision aid in favor of the group that received the intervention (LeBlanc 2015a; LeBlanc 2015b).
Satisfaction
Nine studies reported on satisfaction (Almario 2016; Eggly 2017; Hamann 2014; Haskard 2008; Landrey 2012; LeBlanc 2015a; Murray 2001; van der Krieke 2013; Vodermaier 2009). Data from one study were available for statistical analysis (Analysis 1.7); the SMD was 0.14 (95% CI: ‐0.24 to 0.52; 107 participants) indicating little or no difference in satisfaction with the decision and treatment for the group that received the intervention. Among the studies not used for statistical analysis, there was little or no difference between groups in studies that reported on satisfaction with the consultation (Almario 2016; Hamann 2014); nor in the studies that reported on satisfaction with care (Haskard 2008; van der Krieke 2013). Among the studies that reported on satisfaction with the intervention (Eggly 2017; Hamann 2014; Landrey 2012; LeBlanc 2015a; Murray 2001; van der Krieke 2013), most studies reported little or no difference in satisfaction with the intervention.
1.7. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 7 Satisfaction.
Decisional conflict
Eight studies reported on decisional conflict (Korteland 2017; Krist 2007; Murray 2001; LeBlanc 2015a; LeBlanc 2015b; Perestelo‐Perez 2016; Sheridan 2014; Vodermaier 2009). Data from three studies (367 participants) were available for statistical analysis (Analysis 1.8), the SMD was ‐0.30 (95% CI: ‐0.68 to 0.09) indicating little or no difference between groups. Among the five studies not included in the meta‐analysis (Korteland 2017; Krist 2007; LeBlanc 2015a; LeBlanc 2015b; Sheridan 2014), most reported little or no difference between groups for decisional conflict.
1.8. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 8 Decisional conflict.
Decision regret
Two studies reported on decision regret (Korteland 2017; van Tol‐Geerdink 2016). Data from one study were available for statistical analysis (Analysis 1.9), the estimate of the SMD was ‐0.10 (95% CI: ‐0.39 to 0.19) and ‐0.20 (95% CI: ‐0.50 to 0.10) at six months and 12 months respectively, indicating little or no difference between groups regarding regret after the decision. We are uncertain whether the intervention has an effect on decision regret as the certainty of the evidence was very low (one study, 212 participants). The study not included in the analysis reported little or no difference between groups regarding decision regret at three months (Korteland 2017).
1.9. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 9 Decision regret.
Patient‐clinician communication
Two studies reported on patient‐clinician communication during the encounter (Hamann 2014; Sheridan 2014). One study (157 participants) reported an improvement in discussion raising by patient (RD 0.29; 95% CI: 0.14 to 0.44; Analysis 1.11), patient participation in discussion (RD 0.27; 95% CI: 0.13 to 0.42;157 participants; Analysis 1.12), and interaction with provider (Sheridan 2014) in the group that received the intervention. The other study reported more dominant behavior by the physician in the usual care group than in the group that received the intervention (Hamann 2014; Analysis 1.10). However, little or no difference was found regarding the number of topics raised by patients and dominant behavior by the patient (Hamann 2014).
1.11. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 11 Patient‐physician communication (patient raised discussion).
1.12. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 12 Patient‐physician communication (patient participation in discussion).
1.10. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 10 Patient‐physician communication (number of topics raised by patients).
Self‐efficacy
Two studies (274 participants) reported on decision self‐efficacy (Deen 2012; Maranda 2014) and were included in the meta‐analysis (Analysis 1.13). The pooled estimate of the SMD was 0.16 (95% CI: ‐0.08 to 0.40) indicating little or no increase in decision self‐efficacy for the group that received the intervention.
1.13. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 13 Decision self‐efficacy.
Empowerment
Three studies reported on empowerment (Pickett 2012; van Peperstraten 2010; Vestala 2013). Two were used for statistical analysis. On a continuous scale, the SMD was 0.26 (95% CI: 0.05 to 0.48; one study, 342 participants), indicating an increase in empowerment just after the intervention for the group that received the intervention (Analysis 1.14). On a categorical scale, the RD was 0.18 (95% CI: 0.09 to 0.27; one study, 262 participants) for empowerment indicating a small improvement for the group that received the intervention (Analysis 1.15). The study not included in statistical analysis reported little or no difference between groups (Vestala 2013).
1.14. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 14 Empowerment.
1.15. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 15 Empowerment (categorical).
Behavioral outcomes
Match between preferred and actual level of participation in decision making
One study reported on match between preferred and actual level of participation in decision making (Krist 2007) and found little or no difference between groups .
Match between option preferred and decision made
No studies targeting patients compared with usual care reported on match between option preferred and decision made.
Adherence to decision made
Four studies reported on adherence (Cooper 2011; LeBlanc 2015a; LeBlanc 2015b; Perestelo‐Perez 2016) and two were used for statistical analysis (Analysis 1.16). For the four measures of adherence used (proportion of patients who filled their prescription within 30 days, proportion of patients with > 80% of days covered, proportion of patients who sometimes forgot to take their cholesterol medicine, proportion of patients who did not miss a dose the previous week) little or no difference between groups were found. The two studies not included in statistical analysis reported little or no difference between groups (Cooper 2011; LeBlanc 2015b).
1.16. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 16 Adherence.
Health outcomes
Health status
One study reported on health status (Murray 2001) and found little or no difference between groups.
Health‐related quality of life
Two studies reported on health‐related quality of life (Korteland 2017; LeBlanc 2015b). Data from one study were available for analysis, the estimate of the SMD was 0.00 (95% CI: ‐0.36 to 0.36) for physical components of quality of life (Analysis 1.17) and 0.10 (95% CI: ‐0.26 to 0.46) for mental components of quality of life (Analysis 1.18). We are uncertain whether the intervention improves health‐related quality of life, as the certainty of the evidence was very low (one study, 116 participants). The study not included in the analysis reported little or no difference between groups (LeBlanc 2015b).
1.17. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 17 Health‐related quality of life (physical).
1.18. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 18 Health‐related quality of life (mental).
Anxiety
Four studies reported on anxiety (Korteland 2017; Murray 2001; Perestelo‐Perez 2016; van Peperstraten 2010), three of which were included in the statistical analysis. On a continuous scale, the SMD was ‐0.17 (95% CI: ‐0.49 to 0.14) and 0.18 (95% CI: ‐0.06 to 0.43; two studies, 419 participants) for anxiety and state of anxiety, respectively (Analysis 1.19). This indicated little or no difference between groups (Perestelo‐Perez 2016; van Peperstraten 2010). On a categorical scale, the RD was 0.04 (95% CI: ‐0.07 to 0.15; one study 127 participants) for anxiety (Analysis 1.20), indicating little or no improvement for the group that received the intervention (Korteland 2017). The study not included in statistical analysis reported little or no difference between groups (Murray 2001).
1.19. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 19 Anxiety.
1.20. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 20 Anxiety (categorical).
Depression
Four studies reported on depression (Korteland 2017; LeBlanc 2015a; van Peperstraten 2010; Vestala 2013). For the one study included in the analysis (127 participants), the RD was 0.16 (95% CI: 0.05 to 0.28) indicating a small increase in depression for the group that received the intervention (Analysis 1.21). Among the three studies not used in statistical analysis, two reported little or no difference between groups and one reported a transient increase in frequency of subclinical depression (van Peperstraten 2010).
1.21. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 21 Depression.
Stress
One study reported on diabetes‐related stress (Perestelo‐Perez 2016) and found little or no difference between groups.
Distress
No studies targeting patients compared with usual care reported on distress.
Process outcomes
Consultation length
Seven studies reported on consultation length (Eggly 2017; Hamann 2014; Krist 2007; LeBlanc 2015b; Maclachlan 2016; Perestelo‐Perez 2016; Vodermaier 2009). Data from two studies were available for meta‐analysis and the estimate of the SMD was 0.10 (95% CI: ‐0.39 to 0.58) (Analysis 1.22). We are uncertain whether the intervention increases consultation length (two studies, 224 participants, very low‐certainty evidence). The five studies not included in the meta‐analysis reported little or no difference between groups (Eggly 2017; Krist 2007; LeBlanc 2015b; Maclachlan 2016; Vodermaier 2009).
1.22. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 22 Consultation length.
Cost
Two studies reported on cost (Murray 2001; van Peperstraten 2010). Data from one study were available for statistical analysis, with an estimate of SMD of 0.82 (95% CI: 0.42 to 1.22) (Analysis 1.23). As the certainty of the evidence was very low, we are uncertain about the effect of the intervention on cost (one study, 105 participants). The study not included in the meta‐analysis reported a decrease in cost for the group that received the intervention (van Peperstraten 2010).
1.23. Analysis.
Comparison 1 Group 1: Interventions targeting patients compared to usual care, Outcome 23 Cost.
Equity
No studies targeting patients compared with usual care reported on equity.
Adverse effects
No studies targeting patients compared with usual care reported on adverse effects.
Interventions targeting healthcare professionals versus usual care
Patient outcomes
Affective‐cognitive outcomes
Knowledge
Five studies reported on knowledge (Bernhard 2011; LeBlanc 2015b; Murray 2010; O'Cathain 2002; Tinsel 2013). Data from three studies were available for statistical analysis (Analysis 2.5; Analysis 2.6). The SMD was 0.26 (95% CI: ‐0.16 to 0.69; two studies, 969 participants), indicating little or no improvement in knowledge for the group that received the intervention (Analysis 2.5). Among studies that did not report enough data to perform meta‐analysis, most reported little or no difference in knowledge addressed in the decision aid and one study reported an increase in knowledge in favor of the group that received the intervention (LeBlanc 2015b).
2.5. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 5 Knowledge.
2.6. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 6 Knowledge (categorical).
Satisfaction
Five studies reported on satisfaction (Bernhard 2011; Fossli 2011; Murray 2010; O'Cathain 2002; Wilkes 2013). Data from two studies were available for statistical analysis. On a continuous scale, the SMD was 0.00 (95% CI: ‐0.18 to 0.18; one study. 479 participants) indicating little or no increase in satisfaction with the decision and treatment in the group that received the intervention (Analysis 2.7). On a categorical scale (RD), there was little or no difference between groups regarding satisfaction with the amount of information (one study, 1492 participants; Analysis 2.8), satisfaction with the decision‐making process (one study, 1488 participants; Analysis 2.9), and satisfaction with the discussion with healthcare professional (Analysis 2.10). Among the studies not used for statistical analysis, there was little or no difference between the groups in studies that reported on satisfaction with the consultation, satisfaction with the decision, satisfaction with the decision‐making process, satisfaction with the discussion with the healthcare professional, satisfaction with the doctor’s communication, overall patient satisfaction and satisfaction with the intervention (Bernhard 2011; Fossli 2011; Murray 2010; O'Cathain 2002). One study reported an increase in satisfaction with the amount of information received in the prenatal period in favor of the group that received the intervention (O'Cathain 2002).
2.7. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 7 Satisfaction with consultation.
2.8. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 8 Satisfaction with information.
2.9. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 9 Satisfaction with decision making process.
2.10. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 10 Satisfaction with discussion.
Decisional conflict
Three studies reported on decisional conflict (Bernhard 2011; LeBlanc 2015b; Légaré 2012), finding little or no difference between groups.
Decision regret
One study reported an increase in decision regret in the group that received the intervention (SMD 0.29; 95% CI: 0.07 to 0.51) (Analysis 2.11). As the certainty of the evidence was very low, we cannot be certain whether the intervention has an effect on decision regret (one study, 326 participants).
2.11. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 11 Decision regret.
Self‐efficacy
Kennedy 2013 (4475 participants) reported on decision self‐efficacy and found little or no difference between groups either at six months (SMD ‐0.03; 95% CI: ‐0.09 to 0.03), or at 12 months (SMD ‐0.04; 95% CI: ‐0.10 to 0.03) (Analysis 2.12).
2.12. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 12 Self‐efficacy.
Other affective‐cognitive outcomes
No studies targeting healthcare professionals compared with usual care reported on patient‐clinician communication or empowerment.
Behavioral outcomes
Adherence to decision made
Three studies reported on adherence (Cooper 2011; Légaré 2012; Tinsel 2013) and one was included in statistical analysis (Analysis 2.13). SMDs were: (‐0.08; 95% CI: ‐0.21 to 0.06), (‐0.01; 95% CI: ‐0.16 to 0.13), and (0.10; 95% CI: ‐0.05 to 0.25) at six, 12 and 18 months, respectively. This indicates little or no increase in adherence to medication for the group that received the intervention. The two studies not included in statistical analysis reported little or no difference between groups (Cooper 2011; Légaré 2012).
2.13. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 13 Adherence.
Other behavioral outcomes
No studies targeting healthcare professionals compared with usual care reported on match between preferred and actual level of participation in decision making or match between option preferred and decision made.
Health outcomes
Health status
Kennedy 2013 reported on health status and found little or no difference between groups either regarding general health (SMD 0.02; 95% CI: ‐0.04 to 0.08); Analysis 2.14); or psychological well‐being (SMD 0.00; 95% CI: ‐0.06 to 0.06); Analysis 2.15).
2.14. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 14 General health.
2.15. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 15 Psychological well‐being.
Health‐related quality of life
Four studies reported on health‐related quality of life (Bernhard 2011; Kennedy 2013; LeBlanc 2015b; Légaré 2012), two of which were included in the pooled analyses; (SMD 0.16; 95% CI: ‐0.05 to 0.36) for the physical component (Analysis 2.16; Légaré 2012), (SMD 0.28; 95% CI: 0.07 to 0.49) for the mental component (Analysis 2.17; Légaré 2012), and (SMD 0.00; 95% CI: ‐0.06 to 0.06; 4449 participants) for quality of life in general (Analysis 2.18; Kennedy 2013). The intervention might slightly improve mental health‐related quality of life (one study, 359 participants, low‐certainty evidence) and for physical health‐related quality of life the intervention might make little or no difference (one study, 359 participants, low‐certainty evidence). The two studies not included in the analysis reported little or no difference between groups (Bernhard 2011; LeBlanc 2015b).
2.16. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 16 Health‐related quality of life (physical).
2.17. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 17 Health‐related quality of life (mental).
2.18. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 18 Health‐related quality of life.
Anxiety
Two studies reported on anxiety (Bernhard 2011; O'Cathain 2002) and the data from one study (3003 participants) were used for statistical analysis (Analysis 2.19). The RD was ‐0.00 (95% CI: ‐0.02 to 0.02) indicating little or no increase in anxiety for the group that received the intervention targeting healthcare professionals. The study not included in the analysis reported little or no difference between groups (Bernhard 2011).
2.19. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 19 Anxiety.
Other health outcomes
No studies targeting healthcare professionals compared with usual care reported on depression, stress and distress.
Process outcomes
Consultation length
Six studies reported on consultation length (Fossli 2011; LeBlanc 2015b; Murray 2010; Shepherd 2011; Sanders 2017; Wilkes 2013), two of which were included in the analysis. In one study, the consultation length increased in the group that received the intervention (SMD 0.51; 95% CI: 0.21 to 0.81) (Analysis 2.20). We are uncertain about the effects of the intervention on consultation length as the certainty of the evidence was very low (one study, 175 participants). Another study (479 participants) reported little or no difference between groups for a consultation length of between 10 to 20 minutes (RD ‐0.04; 95% CI: ‐0.13 to 0.05) (Analysis 2.21), indicating that the intervention might make little or no difference for consultation length as measured by 10‐minute blocks. Among the four studies not included in the statistical analysis, most reported little or no difference between groups and one reported an increase in consultation length in the group that received the intervention (Murray 2010).
2.20. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 20 Consultation length.
2.21. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 21 Consultation length (10‐20 min).
Other process outcomes
No studies targeting healthcare professionals compared with usual care reported on costs or equity.
Adverse effects
One study (154 participants) reported on parent perception of hospital safety (Cox 2017) and found little or no difference between groups (SMD 0.00; 95% CI: ‐0.32 to 0.32) (Analysis 2.22).
2.22. Analysis.
Comparison 2 Group 2: Interventions targeting healthcare professionals compared to usual care, Outcome 22 Safety.
Interventions targeting both patients and healthcare professionals versus usual care
Patient outcomes
Affective‐cognitive outcomes
Knowledge
Seven studies reported on knowledge (Branda 2013; Coylewright 2016; Hamann 2007; Hess 2012; Hess 2016; Mathers 2012; Sheridan 2012). Data from five studies were available for statistical analysis. On a continuous scale, the SMD was 0.41 (95% CI: 0.28 to 0.53; two studies, 1004 participants), indicating an increase in knowledge for the group that received the intervention (Analysis 3.4). On a categorical scale, the RD was 0.28 (95% CI: 0.05 to 0.51; four studies, 1260 participants), indicating an increase in knowledge for the group that received the intervention (Analysis 3.5). The three studies not included in the pooled analyses reported an increase in knowledge in favor of the group that received the intervention (Branda 2013; Hamann 2007; Hess 2012).
3.4. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 4 Knowledge.
3.5. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 5 Knowledge (categorical).
Satisfaction
Twelve studies reported on satisfaction (Branda 2013; Hamann 2007; Härter 2015; Haskard 2008; Hess 2012; Hess 2016; Leighl 2011; Loh 2007; Mathers 2012; Rise 2012; Wetzels 2005; Wilkes 2013), four of which were included in the statistical analysis. The SMD was 0.51 (95% CI: ‐0.34 to 1.36; two studies, 362 participants), indicating little or no increase in satisfaction with care for the group that received the intervention (Analysis 3.6). For satisfaction with the decision after consultation, the SMD was 0.24 (95% CI: 0.05 to 0.43; one study, 424 participants), indicating a small increase for the group that received the intervention (Analysis 3.7). Little or no difference between groups was found for satisfaction with the consultation (SMD 0.00; 95% CI: ‐0.23 to 0.23; one study, 383 participants) (Analysis 3.8).
3.6. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 6 Satisfaction with care.
3.7. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 7 Satisfaction with decision.
3.8. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 8 Satisfaction with consultation.
Among the studies that did not provide enough information for statistical analysis, there was little or no difference between groups in studies that reported on satisfaction with the decision and satisfaction with the intervention. One study each reported an increase in satisfaction with the decision‐making process (Hess 2012) and satisfaction with overall care (Haskard 2008), both in favor of the group that received intervention.
Decisional conflict
Seven studies reported on decisional conflict (Branda 2013; Coylewright 2016; Härter 2015; Hess 2016; Hess 2012; Leighl 2011; Mathers 2012). Data from two studies (1065 participants) were available for statistical analysis (Analysis 3.9). Results indicated little or no difference between groups (SMD ‐0.35, 95% CI: ‐0.71 to 0.01). Regarding confidence in the decision, the SMD was 0.03 (95% CI: ‐0.17 to 0.22; one study, 414 participants), indicating no increase in confidence post‐consultation for the group that received the intervention (Analysis 3.10). Among the studies that were not pooled, most reported little or no difference between groups and one study reported lower decisional conflict in the intervention group (Hess 2012).
3.9. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 9 Decisional conflict.
3.10. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 10 Confidence in decision.
Decision regret
Two studies reported on decision regret (Härter 2015; Mathers 2012), of which one reported data for analysis. The estimate of the SMD was 0.13 (95% CI: ‐0.08 to 0.33) at two months indicating little or no difference between groups (Analysis 3.11). The intervention might make little or no difference for decision regret (one study, 369 participants, low‐certainty evidence). The study not included in the analysis reported little or no difference between groups regarding decision regret at six months (Mathers 2012).
3.11. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 11 Decision regret.
Patient‐clinician communication
One study (265 participants) reported on patient‐clinician communication and found little or no difference between groups regarding patient‐centered communication (SMD 0.23; 95% CI: ‐0.01 to 0.47) (Analysis 3.12).
3.12. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 12 Patient‐physician communication (patient‐centered communication).
Self‐efficacy
Epstein 2017 reported on decision self‐efficacy and found little or no difference between groups.
Empowerment
No studies targeting both patients and healthcare professionals compared with usual care reported on empowerment.
Behavioral outcomes
Match between preferred and actual level of participation in decision making
Three studies reported on match between preferred and actual level of participation in decision making (Härter 2015; Sheridan 2012; Leighl 2011) and two (185 participants) were used for statistical analysis (Analysis 3.13). The estimate of the RD was ‐0.03 (95% CI: ‐0.16 to 0.10) indicating that the intervention may make little or no difference to increase in match between preferred and actual level of participation. The study not included in the analysis reported little or no difference between groups regarding the match between preferred and actual level of participation in decision making (Leighl 2011).
3.13. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 13 Match between preferred and actual level of participation in decision making.
Adherence to decision made
Four studies reported on adherence (Cooper 2011; Branda 2013; Hamann 2007; Loh 2007) and three were used for statistical analysis. On a continuous scale, the SMD was 0.44 (95% CI: ‐0.17 to 1.05) for patient’s self‐assessment of adherence and 0.62 (95% CI: 0.37 to 0.87) for physician’s assessment of adherence, indicating an improvement in adherence to medication for the group that received the intervention (Analysis 3.14). On a categorical scale, the RD was 0.00 (95% CI: ‐0.15 to 0.15; two studies, 145 participants), indicating little or no difference between groups in adherence to medication as reported by patients (Analysis 3.15). The one study not included in statistical analysis reported little or no difference between groups for adherence to medication as reported by patients (Cooper 2011).
3.14. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 14 Adherence.
3.15. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 15 Adherence (categorical).
One study reported on persistence with the chosen option (Mathers 2012) and found that patients in the intervention group were more likely to persist with their chosen option.
Other behavioral outcomes
No studies targeting both patients and healthcare professionals compared with usual care reported on match between option preferred and decision made.
Health outcomes
Health‐related quality of life
Two studies reported on health‐related quality of life (Epstein 2017; Rise 2012). The SMDs were 0.08 (95% CI: ‐0.37 to 0.54) for the physical component (Analysis 3.17), 0.01 (95% CI: ‐0.44 to 0.46) for the mental component (Analysis 3.18), and SMD 0.08 (95% CI: ‐0.16 to 0.33; one study, 265 participants) for quality of life in general (Analysis 3.16). We are uncertain whether the intervention improves physical health‐related quality of life or mental health‐related quality of life as the certainty of the evidence has been assessed as very low (one study; 75 participants).
3.17. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 17 Health‐related quality of life (physical).
3.18. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 18 Health‐related quality of life (mental).
3.16. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 16 Health‐related quality of life.
Anxiety
Two studies reported on anxiety (Härter 2015;Leighl 2011) and one was included in statistical analysis (Analysis 3.19). The SMD was ‐0.12 (95% CI: ‐0.31 to 0.08; one study, 419 participants) post‐consultation and ‐0.85 (95% CI: ‐1.06 to ‐0.63) at three months, indicating an increase in anxiety for the group that received usual care. The study not included in statistical analysis reported little or no difference between groups (Leighl 2011).
3.19. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 19 Anxiety.
Depression
Two studies reported on depression (Härter 2015;Loh 2007) and one was included in statistical analysis (Analysis 3.20). The SMD was ‐0.14 (95% CI: ‐0.33 to 0.05) post‐consultation and ‐0.59 (95% CI: ‐0.80 to ‐0.38) at three months, indicating an increase in depression for the group that received usual care. The study not included in statistical analysis reported little or no difference between groups (Loh 2007).
3.20. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 20 Depression.
Other health outcomes
No studies targeting both patients and healthcare professionals compared with usual care reported on health status, stress or distress.
Process outcomes
Consultation length
Three studies reported on consultation length (Hess 2016; Loh 2007; Wetzels 2005) and one was used for the analysis (Analysis 3.21). The SMD was 3.72 (95% CI: 3.44 to 4.01), indicating an increase in consultation length for the group that received the intervention, although the very low‐certainty evidence means we have very little confidence in the effect estimate (one study, 536 participants). The two other studies reported little or no difference between groups (Loh 2007; Wetzels 2005).
3.21. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 21 Consultation length.
Other process outcomes
No studies targeting both patients and healthcare professionals compared with usual care reported on costs or equity.
Adverse effects
One study reported on safety (Hess 2016) and found little or no difference between groups regarding occurrence of major adverse cardiac events (Analysis 3.22).
3.22. Analysis.
Comparison 3 Group 3: Interventions targeting both patients and healthcare professionals compared to usual care, Outcome 22 Safety.
Comparisons of interventions of the same type
Interventions targeting patients versus other interventions targeting patients
Patient outcomes
Affective‐cognitive outcomes
Knowledge
Ten studies reported on knowledge (Barton 2016; Köpke 2014; Krist 2007; Lalonde 2006; Montori 2011; Nannenga 2009; Raynes‐Greenow 2010; Schroy 2011; Street 1995; Thomson 2007), and four were included in the statistical analysis. On a continuous scale, the SMD was 0.30 (95% CI: 0.13 to 0.47; one study, 596 participants), indicating an improvement in knowledge for the group that received an audio and non‐audio decision aid compared to the group that received a pamphlet (Analysis 4.7). On a categorical scale, the RD was 0.16 (95% CI: ‐0.10 to 0.42; three studies, 706 participants), indicating little or no difference between groups (Analysis 4.8). Among the seven studies not pooled, most found little or no difference between groups. Two studies reported an increase in knowledge in the group that received a decision aid compared to the group that received patient educational material (pamphlet) (Nannenga 2009; Schroy 2011), and one study reported an increase in knowledge in favor of the group that received an interactive four‐hour education program compared to the group that received a four‐hour Multiple Sclerosis‐specific stress management program (Köpke 2014).
4.7. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 7 Knowledge.
4.8. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 8 Knowledge (categorical).
Satisfaction
Fourteen studies reported on satisfaction (Barton 2016; Butow 2004; Causarano 2014; Davison 2002; Deschamps 2004; Hamann 2011; Hamann 2017; Jouni 2017; Kasper 2008; Köpke 2014; Lalonde 2006; Montori 2011; Raynes‐Greenow 2010; Warner 2015). Data from five studies were available for statistical analysis. Little or no difference between groups were found regarding satisfaction with the decision (SMD 0.07; 95% CI: ‐0.10 to 0.24; one study, 596 participants; Analysis 4.9), satisfaction with treatment (SMD ‐0.09; 95% CI: ‐0.34 to 0.16; two studies, 267 participants; Analysis 4.10), satisfaction with consultation (SMD ‐0.14; 95% CI: ‐0.42 to 0.13; ;one study, 207 participants; Analysis 4.11), and satisfaction with information provided (SMD 0.11; 95% CI: ‐0.52 to 0.73; one study, 39 participants; Analysis 4.12). Among the studies that did not provide enough information for statistical analysis, most studies reported little or no difference between groups. One study reported an increase in satisfaction with the intervention in favor of the group that received a decision aid and an information booklet about immunotherapy compared to the group that received a decision aid and a standard information package (Kasper 2008).
4.9. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 9 Satisfaction with decision.
4.10. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 10 Satisfaction with treatment.
4.11. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 11 Satisfaction with consultation.
4.12. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 12 Satisfaction with information provided.
Decisional conflict
Fourteen studies reported on decisional conflict (Adarkwah 2016; Barton 2016; Causarano 2014; Deschamps 2004; Dolan 2002; Köpke 2014; Krist 2007; Lalonde 2006; Montori 2011; Nannenga 2009; Raynes‐Greenow 2010; Smallwood 2017; Thomson 2007; van Roosmalen 2004), six of which were included in a pooled analysis. Regarding decisional conflict, the SMD was ‐0.20 (95% CI: ‐0.48 to 0.08, five studies, 1088 participants), indicating little or no difference between groups (Analysis 4.13). Little or no difference was found for decision uncertainty (1, 80 participants; Analysis 4.14). Among the eight studies that did not provide enough information for statistical analysis, most reported little or no difference between groups. One study reported lower decisional conflict in the intervention group with routine education and educational meeting for patients compared to the group with routine education alone (Causarano 2014); another study reported a decrease in decisional conflict in the group with a computerized decision aid compared to the group with guidelines (Thomson 2007), and finally, a third study reported a decrease in decisional conflict in the group with a decision aid and low‐literacy medication guide compared to the group with the existing medication guide (Barton 2016).
4.13. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 13 Decisional conflict.
4.14. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 14 Decision uncertainty.
Patient‐clinician communication
One study reported on patient‐clinician communication (Stiggelbout 2008) and found that the group who received an individualized brochure had better understanding (84%) than the group who received a general brochure (62%).
Self‐efficacy
Two studies (100 participants) reported on decision self‐efficacy (Analysis 4.15). The pooled estimate of the SMD was ‐0.02 (95% CI: ‐0.41 to 0.37), indicating little or no difference between groups.
4.15. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 15 Decision self‐efficacy.
Other affective‐cognitive outcomes
No studies targeting patients compared with other interventions targeting patients reported on decision regret or empowerment.
Behavioral outcomes
Match between preferred and actual level of participation in decision making
Five studies reported on the match between preferred and actual level of participation in decision making (Butow 2004; Davison 2002; Dolan 2002; Kasper 2008; Krist 2007). Data from four studies (1206 participants) were available for meta‐analysis (Analysis 4.16). The estimate of the RD was ‐0.10 (95% CI: ‐0.16 to ‐0.05), indicating a very small increase in match between preferred and actual level of participation in decision making for the group that received a discussion with a research nurse compared to the group that received computer‐generated information and decision‐preference profiles and a computer‐generated prompt sheet; and a better match for the group that received a decision aid and a standard information package than for the group that received a decision aid and an information booklet about immunotherapy. The study not included in the meta‐analysis reported little or no difference between groups (Krist 2007).
4.16. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 16 Match between preferred and actual level of participation in decision making.
Match between option preferred and decision made
Two studies (363 participants) reported on match between option preferred and decision made (Causarano 2014; Schroy 2016). Data from both studies were available (Analysis 4.17). The estimate of the RD was ‐0.20 (95% CI: ‐0.60 to 0.20) indicating little or no difference between groups.
4.17. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 17 Match between preferred option and decision made.
Adherence to decision made
Six studies reported on adherence (Barton 2016; Deschamps 2004; Hamann 2017; Köpke 2014; Montori 2011; Thomson 2007) and four were used for statistical analysis. On a continuous scale, the SMD was 0.05 (95% CI: ‐0.35 to 0.44) at six months, one study 100 participants, indicating little or no difference between groups (Analysis 4.18). On a categorical scale, the RD was 0.01 (95% CI: ‐0.10 to 0.12), three studies, 301 participants, indicating little or no difference between groups in adherence to medication (Analysis 4.19). One study (Köpke 2014) reported little or no difference between groups regarding medication discontinuation (RD: ‐0.14; 95% CI: ‐0.31 to 0.02). Among the studies not included in statistical analysis, one study reported that participants in the group using a decision aid were less likely to make a definite decision to start or continue medication than participants in the group using guidelines (Thomson 2007). One study reported on persistence with the chosen option (Montori 2011), finding little or no difference between groups.
4.18. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 18 Adherence.
4.19. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 19 Adherence (categorical).
Health outcomes
Health status
One study (88 participants) reported on general health (Analysis 4.20). Little or no difference between groups was reported at three months (SMD ‐0.19 ; 95% CI: ‐0.61 to 0.23). At nine months, the SMD was 0.53 (95% CI: 0.09 to 0.97), indicating an improvement in general health in the intervention group receiving a SDM intervention and decision aid compared to the group receiving a decision aid alone.
4.20. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 20 General health.
Health‐related quality of life
One study reported on health‐related quality of life (Stiggelbout 2008) and found little or no difference between groups.
Anxiety
Seven studies reported on anxiety (Butow 2004; Davison 1997; Köpke 2014; Raynes‐Greenow 2010; Stiggelbout 2008; Thomson 2007; van Roosmalen 2004) and two (682 participants) were used for statistical analysis (Analysis 4.21). The SMD was ‐0.11 (95% CI: ‐0.27 to 0.05) indicating little or no difference between groups. Studies not included in statistical analysis reported little or no difference between groups.
4.21. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 21 Anxiety.
Depression
Five studies reported on depression (Butow 2004; Davison 1997; Köpke 2014; Stiggelbout 2008; van Roosmalen 2004) and one study (86 participants) was included in statistical analysis (Analysis 4.22)). Little or no difference between groups was reported at three (SMD ‐0.27 ; 95% CI: ‐0.69 to 0.16) or nine months (SMD ‐0.39 ; 95% CI: ‐0.82 to 0.03). The studies not included in statistical analysis reported little or no difference between groups.
4.22. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 22 Depression.
Other health outcomes
No studies targeting patients compared with other interventions targeting patients reported on stress or distress.
Process outcomes
Consultation length
Five studies reported on consultation length (Butow 2004; Causarano 2014; Krist 2007; Montori 2011; Nannenga 2009), one of which was included in the analysis (Analysis 4.23). The SMD was ‐0.65 (95% CI: ‐1.29 to ‐0.00) suggesting an increase in consultation length for the group that received routine education compared to the group that received routine education and a patient educational meeting. The certainty of evidence was very low (one study, 39 participants). Among the four studies not included in the analysis, most reported little or no difference between groups and one reported an increase in consultation length for the group that received decision aid compared to the group that received usual care and booklet (Montori 2011).
4.23. Analysis.
Comparison 4 Group 4: Inteventions targeting patients compared to other interventions targeting patients, Outcome 23 Consultation length.
Other process outcomes
No studies targeting patients compared with other interventions targeting patients reported on costs or equity.
Adverse effects
No studies targeting patients compared with other interventions targeting patients reported on adverse effects.
Interventions targeting healthcare professionals versus other interventions targeting healthcare professionals
Patient outcomes
Affective‐cognitive outcomes
Knowledge
Krones 2008 (ARRIBA‐Herz) reported on knowledge, finding little or no difference between groups.
Satisfaction
Elwyn 2004 reported on satisfaction finding little or no difference between groups either in satisfaction with the information provided or in satisfaction with the decision.
Decision regret
One study reported on decision regret at six months (Krones 2008 (ARRIBA‐Herz)), and found less decision regret in the group that received an educational meeting, audit and feedback, educational material and an educational outreach visit than in the group that received an educational meeting alone.
Other affective‐cognitive outcomes
No studies targeting healthcare professionals compared with other interventions targeting healthcare professionals reported on decisional conflict, patient‐clinician communication, self‐efficacy or empowerment.
Behavioral outcomes
No studies targeting healthcare professionals compared with other interventions targeting healthcare professionals reported on match between preferred and actual level of participation in decision making, match between option preferred and decision made or adherence to decision made.
Health outcomes
Health status
One cross‐over study (295 participants) reported on mental and physical health status at two points in time (Elwyn 2004). The SMD was 0.24 (95% CI: 0.01 to 0.47) for mental health status at time point 1 (Analysis 5.3), indicating a small improvement in the group that received training in SDM compared to the group that received training in risk communication. Little or no difference between groups was observed for physical health status (Analysis 5.4).
5.3. Analysis.
Comparison 5 Group 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals, Outcome 3 Health status (mental).
5.4. Analysis.
Comparison 5 Group 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals, Outcome 4 Health status (physical).
Anxiety
Elwyn 2004 reported on anxiety at three points in time. The SMD was 0.25 (95% CI: 0.02 to 0.49) for anxiety at time point 2, indicating a small increase in the group that received training in shared decision making compared to the group that received training in risk communication (Analysis 5.5).
5.5. Analysis.
Comparison 5 Group 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals, Outcome 5 Anxiety.
Other health outcomes
No studies targeting healthcare professionals compared with other interventions targeting healthcare professionals reported on health‐related quality of life, depression, stress or distress.
Process outcomes
Consultation length
One study reported on consultation length (Elwyn 2004) and found little or no difference between groups.
Other process outcomes
No studies targeting healthcare professionals compared with other interventions targeting healthcare professionals reported on costs or equity.
Adverse events
No studies targeting healthcare professionals compared with other interventions targeting healthcare professionals reported on adverse events.
Interventions targeting both patients and healthcare professionals versus other interventions targeting both patients and healthcare professionals
Patient outcomes
Affective‐cognitive outcomes
Satisfaction
Cooper 2013 reported on satisfaction with the intervention and found that, at 12 months, compared with patients in the standard group, patients in the patient‐centered group had higher odds of rating their depression case manager as extremely helpful at identifying concerns (odds ratio (OR), 3.00; 95% CI, 1.23 to 7.30) and improving adherence to treatment (OR, 2.60; 95% CI, 1.11 to 6.08).
Decisional conflict
One study (286 participants) reported on decisional conflict (Myers 2011) and found little or no difference between groups (SMD ‐0.07; 95% CI ‐0.30 to 0.16; Analysis 6.4).
6.4. Analysis.
Comparison 6 Group 6: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals, Outcome 4 Decisional conflict.
Other affective‐cognitive outcomes
No studies targeting both patients and healthcare professionals compared with other interventions targeting both patients and other healthcare professionals reported on knowledge, decision regret, patient‐clinician communication, self‐efficacy or empowerment.
Behavioral outcomes
No studies targeting both patients and healthcare professionals compared with other interventions targeting both patients and other healthcare professionals reported on match between preferred and actual level of participation in decision making, match between option preferred and decision made or adherence to decision made.
Health outcomes
Depression
Cooper 2013 reported on depression and found little or no difference between study groups.
Other health outcomes
No studies targeting both patients and healthcare professionals compared with other interventions targeting both patients and other healthcare professionals reported on health status, health‐related quality of life, anxiety, stress or distress.
Process outcomes
No studies targeting both patients and healthcare professionals compared with other interventions targeting both patients and other healthcare professionals reported on consultation length, costs or equity.
Adverse events
No studies targeting both patients and healthcare professionals compared with other interventions targeting both patients and other healthcare professionals reported on adverse events.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis by study design. We observed a significant difference between the subgroup of individual randomized trials and that of cluster‐randomized trials when interventions targeting healthcare professionals were compared with other interventions targeting healthcare professionals and assessed using OBOMs on a continuous scale. The study within the subgroup of individual trials showed an effect size (SMD) of 0.40 (95% CI: 0.28 to 0.52) (Analysis 5.2; 1132 observations) in favor of the group that received educational meeting and audit and feedback and educational material and educational outreach visit when compared with the group that received educational meeting alone (Krones 2008 (ARRIBA‐Herz). The study within the subgroup of cluster trials did not show any effect (Elwyn 2004). No differences between subgroups were observed for the other comparisons categories. For more details see Analysis 1.1, Analysis 1.2, Analysis 2.1, Analysis 2.3, Analysis 3.1, Analysis 3.2, Analysis 3.3, Analysis 4.2.
Statistical heterogeneity among studies measuring the use of SDM by healthcare professionals was partly explained by methodological heterogeneity namely, the study design. Looking only at cluster‐randomized trials, when interventions targeting patients were compared to usual care and assessed using OBOMs on a continuous scale, the I2 statistic moved from 84% (P = 0.0002) to 31% (P = 0.23) (Analysis 1.1). When the same comparison was assessed using PROMs on a continuous scale, the I2 moved from 50% (P = 0.04) to 0% (P = 0.40) ( Analysis 1.2). When interventions targeting both patients and healthcare professionals were compared to usual care and assessed using OBOMs on continuous scale, the I2 moved from 96% (P < 0.00001) to 0% (P = 0.37) (Analysis 3.1). Other potential sources of variation could be other methodological heterogeneity (the studies differed in their risk of bias from one domain to another) and extensive clinical heterogeneity (the studies differed considerably in types of interventions studied and in clinical contexts).
Discussion
Summary of main results
This updated search added 48 new studies to the 39 studies from the first update of the original Cochrane review, for a total of 87 studies that recruited a total of 48,754 participants: 3113 healthcare professionals, with a minimum enrolment of one and a maximum of 363; and 45,641 patients, with a minimum of 26 patients and a maximum of 10,070. The number of included studies more than doubled in five years. This is not surprising considering that this field is rapidly expanding. Evidence shows that shared decision making (SDM) publications increased exponentially in major medical journals from 1996 to 2011. The absolute number of publications per journal ranged from two to 273 over 16 years (Blanc 2014). This growth reflects increased dissemination of the SDM concept to the medical community and increasing inclusion of SDM in health policy in many countries (Harter 2017). Most countries represented were in Europe or the USA. Consistent with a recent update on international accomplishments in SDM policy, research and implementation (Harter 2017), we observed few studies by international collaborations and only one conducted in a low‐income country. Primary care was the setting of most of the studies. In addition, most studies focused on licensed healthcare professionals, demonstrating the need for further studies involving healthcare professionals in training as well. The most common clinical conditions targeted were cancer, psychiatric and cardiovascular diseases. Implementation studies in SDM are thus addressing the diseases that have been identified as among the most important causes of the global burden of disease (Institute for Health Metrics and Evaluation 2013).
To assess the effect of interventions for increasing the use of SDM by healthcare professionals, we divided studies based on the population targeted (patients, healthcare professionals, or both), and what the intervention was being compared with (usual care or other interventions of the same type), resulting in six comparisons. We also considered whether the primary outcome of interest was measured with observer‐based outcome measures (OBOMs), used by a third‐party observer during an encounter between a healthcare professional and a patient, or patient‐reported outcome measures (PROMs), which collect information directly from patients. We graded the certainty of the evidence for the primary outcome of interest as very low for all the six comparisons and, for secondary outcomes (decision regret, physical and mental health‐related quality of life, consultation length and cost), as low to very low. Studies did not report any adverse effects associated with the interventions.
Overall completeness and applicability of evidence
Overall, when reviewing studies assessing the impact of any interventions for increasing the use of SDM by healthcare professionals, we observed that the evidence was of low or very low certainty. There is still no consensus on which type of measure (OBOMs or PROMs) is most accurate for SDM. Therefore, we decided to include studies that had either used OBOMs, PROMs or both to ensure completeness of this review. Had we favored one type of measure over the other, this review would not have reflected the state of the science regarding the impact of any interventions for increasing the use of SDM by healthcare professionals. In OBOM studies, the most commonly used instrument was OPTION (Elwyn 2003), and in PROM studies the “perceived level of control in decision making” scale (adapted from the Control Preference Scale) (Degner 1992) was most common. As for studies not using either of these two scales, there were as many instruments as there were studies. These findings confirm that there is still no standardized instrument for assessing the use of SDM by healthcare professionals. They also confirm that measurement of SDM clearly needs improvement.
It is important to note that in line with the EPOC taxonomy of interventions, in our 'Summary of findings' tables, we refer to patient‐mediated interventions (i.e. interventions targeting patients) as single entities. Unlike in the last update, this time we used head‐to‐head comparisons to disentangle the separate components of multifaceted patient‐mediated interventions. This allowed us to investigate which of these separate interventions were more effective than others.
We have not reported on comparisons between different target categories (e.g. interventions targeting patients compared with interventions targeting healthcare professionals) because not enough additional studies were found for this update and therefore no further conclusions were possible.
Certainty of the evidence
Surprisingly, the large number of eligible studies did not translate into more certainty of the evidence for the primary outcome of interest, namely the use of SDM by healthcare professionals. Overall, the certainty of the evidence for the main outcome of this review, i.e. use of SDM by healthcare professionals, was graded as very low, which means we have very little confidence in the effect estimate. The 87 studies reviewed in this study (including the 48 added for this update) either did not provide reliable indication of the likely effect, or else there was high likelihood of the effect being substantially different. Evidence on secondary outcomes, i.e. decision regret, physical and mental health‐related quality of life, consultation length and cost, was also uncertain.
A number of factors may explain this. First, only 22 of the 87 included studies had the same primary outcome as the primary outcome of interest of this review, i.e. the use of SDM by healthcare professionals, and therefore they may not have been sufficiently powered to accurately assess it. Second, in our assessment of risk of bias within studies, we scored several studies as “high risk” regarding protection against contamination. This was mostly because randomization was at the patient level instead of at the cluster level (e.g. clinics). This issue was reflected in the high methodological heterogeneity that occurred when cluster‐randomized studies were mixed with individual‐randomized studies. Further studies should address risk of contamination by adopting cluster‐level randomization. When this is not possible, study authors should report in detail what steps they took to mitigate the risk of contamination. Third, we observed the use of a large number of diverse SDM measures. If researchers agreed on a common set of validated measures for evaluating the impact of interventions to increase the use of SDM, the certainty of the evidence would also improve. Fourth, except for decision aids, very few studies assessed the impact of the same intervention. Lastly, it should also be noted that when assessing risk bias, for many studies we scored key domains “unclear risk” mainly because we did not have enough information to make a judgement. Authors should report the methods and results of their trials in more detail by following reporting guidelines more strictly (Campbell 2012; Schulz 2010).
The effects of interventions as measured with PROMs were not consistent with those as measured with OBOMs. Although we scored the evidence as of very low certainty, in three of the six comparisons OBOM studies indicated the intervention had an effect, while PROM studies did not; in one comparison, it was the reverse; and in the remaining two comparisons, OBOM and PROM studies were in agreement that the intervention had no effect. Different understandings of what constitutes participation in decisions between patients and healthcare professionals may partly explain these findings. For example, discrepancies between patients’ interpretation of the Control Preferences Scale and its intended meaning have been reported (Davey 2004; Entwistle 2001). To ensure validity and reliability of PROMs, further investigation of how patients interpret and understand instructions, items and response options on which these measures rely is essential (Barr 2016).
In conclusion, studies in this field of research are no different from those in other fields in that their methods may be inadequate; they may be too small; many fail to deal adequately with bias; and most are not replicated (Chalmers 2009). Therefore, more and better research is required to strengthen the certainty of the evidence.
Potential biases in the review process
Assessment of publication bias within comparison categories did not show any clear evidence of reporting bias. However, since funnel plots were used to perform this assessment, the number of studies in some comparison categories may not have been sufficient.
Overall, we were unable to extract much information regarding the general context of the included studies. We relied on published and publicly available material and contacted authors of included studies to obtain more information when needed. However, we were not able to always get an answer from them.
Agreements and disagreements with other studies or reviews
We found no other review assessing the impact of interventions for increasing the use of SDM by healthcare professionals with the same primary outcome as our review, namely the use of SDM by healthcare professionals. However, we found reviews that had assessed the impact of interventions for increasing the use of SDM by healthcare professionals on other outcomes, such as levels of patient satisfaction or knowledge, namely the secondary outcomes of this review. In a Cochrane systematic review of interventions to facilitate SDM to address antibiotic use for acute respiratory infections in primary care, Coxeter and colleagues found that interventions to promote SDM reduce antibiotic use for acute respiratory infections in the short term (immediately after or within six weeks of the consultation), compared with usual care, without decreasing satisfaction with the consultation (Coxeter 2015). With pediatric patients, Wyatt and colleagues found that interventions to engage pediatric patients, parents, or both in medical decisions significantly improved knowledge and reduced decisional conflict (Wyatt 2015). However, none of these reviews could inform us about the effect of these interventions on the use of SDM by healthcare professionals.
Shay and Lafata systematically reviewed the empirical evidence linking patient outcomes with SDM, and identified which measurement perspectives (OBOM or PROM) are associated with which types of patient outcomes (affective‐cognitive, behavioral, and health) (Shay 2015). As in our review, in a high number of their included studies (33 out of 39), the measures of SDM were patient‐reported. Of 97 unique patient outcomes assessed, 42 studies (43%) found a significant and positive relationship between SDM and the patient outcome. This proportion varied according to the measurer (patient or observer) and the outcome category. The authors reported that more than half of outcomes assessed with patient‐reported SDM were significant and positive, compared with 21% of those that were observer‐rated. These results, along with ours, confirm two important facts: 1) patient‐reported measures are those most commonly used in the assessment of patient involvement in decision making; 2) results vary according to the perspective (observer‐based or patient‐reported). This investment in patient‐reported measures may be related to increasing interest among researchers in assessing whether health systems deliver what matters most to patients. Reliance on clinical indicators gives only a partial view of what patients and their caregivers prefer or value. What people care about is the impact of health services on their well‐being and their ability to play an active role in decision making. The only way to evaluate this is to ask patients themselves. Therefore it is not surprising that Patient‐Reported Experience Measures (PREMs) and Patient‐Reported Outcome Measures (PROMs) seem poised to become the methods of choice for comparative performance assessment in research involving patients and their caregivers (Coulter 2017).
Authors' conclusions
Implications for practice.
The results of this Cochrane review update allow us to confirm the conclusions of the previous update regarding the types of intervention that are the most effective for increasing the use of shared decision making (SDM).
It is uncertain whether any interventions for increasing the use of SDM by healthcare professionals (i.e. those targeting solely patients or healthcare professionals or both) as measured by observers or reported by patients are effective because the certainty of the evidence is very low.
Implications for research.
There are several gaps in knowledge about the effectiveness of interventions focused on increasing shared decision making (SDM) among healthcare professionals.
Future studies should be designed to minimize bias regarding risk of contamination and should have enough power to estimate the effects of active interventions on increased use of SDM among healthcare professionals (primary outcome).
Future studies should report their methods and results in enough detail and according to the recommended reporting guidelines to allow extensive assessment of risk of bias.
Further research is needed to develop better patient‐derived measures of SDM. Improved methods for measurements might produce consistency between ratings of SDM by external observers and ratings by patients.
Further research is required to assess the same intervention across multiple clinical contexts, health professionals and also across diverse jurisdictions (i.e. international collaborations).
Further research is required to more clearly determine the cost of interventions to increase the use of SDM and the impact of different clinical care payment mechanisms on the use of SDM.
What's new
| Date | Event | Description |
|---|---|---|
| 15 June 2017 | New search has been performed | This is the second update of this Cochrane review first published in 2010. A new search was conducted and other content updated. Forty‐eight new studies were added to the review. |
| 15 June 2017 | New citation required but conclusions have not changed | Forty‐eight new studies were added to the review. The review includes 87 studies. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 5, 2010
| Date | Event | Description |
|---|---|---|
| 15 September 2014 | New search has been performed | Included observer‐reported and patient‐reported outcomes to 2012 |
| 30 November 2011 | Amended | |
| 29 September 2011 | New search has been performed | Updated observer‐reported outcomes to 2010 |
Acknowledgements
We thank Stéphane Ratté who developed the search strategy of this review, and Marie‐Joëlle Cossi and Karine Gravel for their work on previous updates.
We thank Paul Miller for helping us to identify records in electronic databases.
We thank Daniela Gonçalves Bradley, Sasha Shepperd and Julia Worswick who provided us with editorial guidance and critical assessment of the intellectual content of the review. We also thank the referees for their constructive comments: Linda Lehman, Nicole Seefeldt, Jonathan Fuchs, Terrie Cowley, Mary Ann O’Brien, Dell Horey, Hilary Thompson, Paul Miller, and Keith R Abrams.
We thank Ndeye Thiab Diouf (NTD), Jessica Hébert (JH), Sara Chenacher (SC), Ali Ben Chérif (ABC), Hubert Robitaille (HR), Patrice Alain Ngange (PAN), Alexandrie Boucher (AB), Élodie Chenard (EC), Lionel Adisso (LA), Hélène Élidor (HE) and Tatiana Agbadjè (TA) for their valuable contributions in identification of eligible studies and data extraction.
We thank Geneviève Painchaud‐Guérard who helped complete tables of characteristics of included studies, ongoing studies and studies awaiting classification.
We thank Louisa Blair and Heather Maxwell for their editorial assistance.
This review update was supported by Oxford editorial base. National Institute for Health Research, via Cochrane Infrastructure funding to the Effective Practice and Organisation of Care Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
PubMed
Search date: 15 June 2017
| No. | Search terms | Results |
| #1 | (shared decision*[tiab] or sharing decision*[tiab] or informed decision*[tiab] or informed choice*[tiab] or decision aid*[tiab] or ((share*[ti] or sharing*[ti] or informed*[ti]) and (decision*[ti] or deciding*[ti] or choice*[ti]))) | 14116 |
| #2 | (decision making[mh:noexp] or decision support techniques[mh:noexp] or decision support systems, clinical[mh] or choice behaviour[mh:noexp] or decision making*[tiab] or decision support*[tiab] or choice behaviour*[tiab] or ((decision*[ti] or choice*[ti]) and (making*[ti] or support*[ti] or behaviour*[ti]))) | 195864 |
| #3 | (patient participation[mh] or patient participation*[tiab] or consumer participation*[tiab] or patient involvement*[tiab] or consumer involvement*[tiab] or ((patient*[ti] or consumer*[ti]) and (involvement*[ti] or involving*[ti] or participation*[ti] or participating*[ti]))) | 29921 |
| #4 | (professional‐patient relations[mh] or ((nurses[mh] or physicians[mh] or nurse*[ti] or physician*[ti] or clinician*[ti] or doctor*[ti] or general practitioner*[ti] or gps[ti] or health care professional*[ti] or healthcare professional*[ti] or health care provider*[ti] or healthcare provider*[ti] or resident*[ti]) and (patients[mh] or patient*[ti] or consumer*[ti] or people*[ti]))) | 160812 |
| #5 | (clinical trial[pt:noexp] or randomized controlled trial[pt] or controlled clinical trial[pt] or evaluation studies[pt] or comparative study[pt] or intervention studies[mh] or Evaluation Studies as Topic[mh:noexp] or program evaluation[mh:noexp] or random allocation[mh] or random*[tiab] or double blind*[tiab] or controlled trial*[tiab] or clinical trial*[tiab] or pretest*[tiab] or pre test*[tiab] or posttest*[tiab] or post test*[tiab] or prepost*[tiab] or pre post*[tiab] or controlled before*[tiab] or "before and after"[tiab] or interrupted time*[tiab] or time serie*[tiab] or intervention*[tiab]) | 4001044 |
| #6 | ((#1 OR (#2 AND #3) OR (#2 AND #4) OR (#3 AND #4)) AND #5) | 8177 |
| #7 | ((#1 OR (#2 AND #3) OR (#2 AND #4) OR (#3 AND #4)) AND #5) Filters: Publication date from 2015/01/01 to 2017/12/31 | 1760 |
Embase (OVID)
Embase database used: Embase <1974 to 2017 June 14>
Search date: 15 June 2017
| No. | Search terms | Results |
| 1 | (shared decision or sharing decision or informed decision or informed choice or decision aid).ti,ab. or ((share* or sharing* or informed*) and (decision* or deciding* or choice*)).ti. | 12773 |
| 2 | exp clinical decision making/ or exp decision making/ or exp decision support system/ or exp ethical decision making/ or exp family decision making/ or exp medical decision making/ or exp patient decision making/ or (decision making or decision support or choice behaviour).ti,ab. or ((decision* or choice*) and (making* or support* or behaviour*)).ti. | 355815 |
| 3 | exp patient participation/ or (patient participation or consumer participation or patient involvement or consumer involvement).ti,ab. or ((patient* or consumer*) and (involvement* or involving* or participation* or participating*)).ti. | 33527 |
| 4 | exp doctor patient relation/ or exp nurse patient relationship/ or ((exp nurse/ or exp physician/ or (nurse* or physician* or clinician* or doctor* or general practitioners or gps or health care professionals or healthcare professionals or health care providers or healthcare providers or resident*)).ti. and (exp patient/ or (patient* or consumer* or people*).ti.)) | 412940 |
| 5 | 1 or (2 and 3) or (2 and 4) or (3 and 4) | 46996 |
| 6 | exp clinical trial/ or exp randomized controlled trial/ or exp controlled clinical trial/ or exp controlled trial/ or exp pretest posttest control group design/ or exp comparative study/ or exp evaluation research/ or exp intervention study/ or exp randomization/ or (random* or double blind or controlled trial or clinical trial or pretest* or pre test or pre tests or posttest* or post test or post tests or prepost* or pre post or controlled before or "before and after" or interruped time or time serie? or intervention*).ti,ab. | 8427337 |
| 7 | (2015* or 2016* or 2017*).yr. | 3508644 |
| 8 | 5 and 6 and 7 | 4460 |
| 9 | limit 8 to embase | 2206 |
The Cochrane Library (Wiley) Search date: 15 June 2017
| No. | Search terms | Results |
| #1 | ((shar* or inform*) near/3 (decision* or aid* or deciding* or choice*)):ti,ab,kw | 2463 |
| #2 | ((decision* or choice*) near/3 (making* or support* or behaviour*)):ti,ab,kw | 11115 |
| #3 | ((patient* or consumer*) near/3 (involvement* or involving* or participation* or participating*)):ti,ab,kw | 8416 |
| #4 | ((nurse* or physician* or clinician* or doctor* or general practitioner* or gps or health care professional* or healthcare professional* or health care provider* or healthcare provider* or resident*) near/3 (patient* or consumer* or people*)):ti,ab,kw | 15782 |
| #5 | #1 or (#2 and #3) or (#2 and #4) or (#3 and #4) | 4248 |
| #6 | #1 or (#2 and #3) or (#2 and #4) or (#3 and #4) Publication Year from 2015 to 2017 | 1360 |
Cinahl (EBSCO) Search date: 15 June 2017
| No. | Search terms | Results |
| S1 | AB Shared Decision* OR TI Shared Decision* OR AB Sharing Decision* OR TI Sharing Decision* OR AB Informed Decision* OR TI Informed Decision* OR AB Informed Choice* OR TI Informed Choice* OR AB Decision Aid* OR TI Decision Aid* OR ((TI Share* OR TI Sharing OR TI Informed*) AND (TI Decision* OR TI Deciding* OR TI Choice*)) | 7,369 |
| S2 | MH "Decision Making+" OR MW Decision Support OR AB Decision Making* OR TI Decision Making* OR AB Decision Support* OR TI Decision Support* OR AB Choice Behaviour* OR TI Choice Behaviour* OR ((TI Decision* OR TI Choice*) AND (TI Making* OR TI Support* OR TI Behaviour*)) | 85,579 |
| S3 | MH Consumer Participation OR AB Patient Participation* OR TI Patient Participation* OR AB Consumer Participation* OR TI Consumer Participation* OR AB Patient Involvement* OR TI Patient Involvement* OR AB Consumer Involvement* OR TI Consumer Involvement* OR ((TI Patient* OR TI Consumer*) AND (TI Participating* OR TI Participation* OR TI Involving* OR TI Involvement*)) | 17,733 |
| S4 | MH Professional Patient Relations OR MH Nurse Patient Relations OR MH Physician Patient Relations OR ((MH Nurses+ OR MH Physicians+ OR TI Nurse* OR TI Physician* OR TI Clinician* OR TI Doctor* OR TI General Practitioner* OR TI GPs OR TI Health Care Professional* OR TI Healthcare Professional* OR TI Health Care Provider* OR TI Healthcare Provider* OR TI Resident*) AND (MH Patients+ OR TI Patient* OR TI Consumer* OR TI People*)) | 47,297 |
| S5 | MH Experimental Studies+ OR MH Quasi‐Experimental Studies OR MH Comparative Studies OR MH Evaluation Research OR AB Random* OR TI Random* OR AB Double Blind* OR TI Double Blind* OR AB Controlled Trial* OR TI Controlled Trial* OR AB Clinical Trial* OR TI Clinical Trial* OR AB Pretest* OR TI Prestest* OR AB Pre Test* OR TI Pre Test* OR AB Posttest* OR TI Posttest* OR AB Post Test* OR TI Post Test* OR AB Prepost* OR TI Prepost* OR AB Pre Post* OR TI Pre Post* OR AB Controlled Before* OR TI Controlled Before* OR AB "Before and After*" OR TI "Before and After*" OR AB Interruped Time* OR TI Interrupted Time* OR AB Time Serie* OR TI Time Serie* OR AB Intervention* OR TI Intervention* | 521,147 |
| S6 | (S1 OR (S2 AND S3) OR (S2 AND S4) OR (S3 AND S4)) AND S5 | 3,439 |
| S7 | S6 Limiters ‐ Published Date: 20150101‐20171231; Exclude MEDLINE records | 341 |
PsycINFO (OVID) PsycINFO database used:PsycINFO <1967 to June Week 1 2017>
Search date: 15 June 2017
| No. | Search terms | Results |
| 1 | (shared decision or sharing decision or informed decision or informed choice or decision aid).ab. or ((share* or sharing* or informed*) and (decision* or deciding* or choice*)).ti. | 3799 |
| 2 | (decision making or decision support or choice behaviour).ab. or ((decision* or choice*) and (making* or support* or behaviour)).hw. | 98574 |
| 3 | client participation.hw. or (consumer participation or consumer involvement or patient participation or patient involvement).ab. or ((patient* or consumer*) and (participating* or participation* or involving* or involvement*)).hw. | 3741 |
| 4 | therapeutic processes.hw. or (((nurses or physicians or general practitioners or gynecologists or internists or neurologists or obstetricians or pathologists or pediatricians or psychiatrists or surgeons).hw. or (nurse* or physician* or clinician* or doctor* or general practitioner or gps or health care professional or healthcare professional or health care provider* or healthcare provider).ti.) and ((patients or outpatients).hw. or (patient* or consumer* or people* or outpatient*).ti.)) | 33440 |
| 5 | 1 or (2 and 3) or (2 and 4) or (3 and 4) | 6170 |
| 6 | (2015* or 2016* or 2017*).yr. | 441961 |
| 7 | 5 and 6 | 1124 |
| 8 | (clinical trial or empirical study or experimental replication or followup study or longitudinal study or prospective study or quantitative study or treatment outcome).md. | 2121872 |
| 9 | experimental design/ | 10194 |
| 10 | between groups design/ | 107 |
| 11 | quantitative methods/ | 2763 |
| 12 | quasi experimental methods/ | 141 |
| 13 | (randomised or randomized or randomly or controlled or control group? or evaluat* or time series or time point or time points or quasi experiment* or quasiexperiment* or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or multicenter study or multicentre study or multi center study or multi centre study or repeated measur*).ti,ab. | 680691 |
| 14 | (trial or effect? or impact? or intervention?).ti. | 390327 |
| 15 | exp clinical trial/ | 10376 |
| 16 | ((clinical or control*) adj3 trial*).ti,ab. | 58915 |
| 17 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. | 23288 |
| 18 | (volunteer* or control group or controls).ti,ab. | 208831 |
| 19 | placebo/ or placebo*.ti,ab. | 35733 |
| 20 | pretesting/ | 229 |
| 21 | posttesting/ | 135 |
| 22 | repeated measures/ | 625 |
| 23 | time series/ | 1783 |
| 24 | or/8‐23 | 2459274 |
| 25 | 7 and 24 | 828 |
ClinicalTrials.gov
Search date: 4 August 2017
| Search terms | Results |
| "informed choice" | 101 |
| "decision making" | 435 |
| "decision support" | 295 |
| "informed decision" | 85 |
| "decision aid" | 297 |
| "sharing decision" | 141 |
| "shared decision" | 122 |
| 1,476 |
WHO International Clinical Trials Registry Platform
Search date: 4 August 2017
| "informed choice" | 16 |
| "decision making" | 672 |
| "decision support" | 317 |
| "informed decision" | 33 |
| "decision aid" | 269 |
| "sharing decision" | 0 |
| "shared decision" | 153 |
| 1,460 |
Appendix 2. International Shared Decision Making Conference 2013, 2015, 2017 (ISDM)
| [Search in ISDM proceedings] | [Results] |
| References | 700 |
Appendix 3. Society for Medical Decision Making 2012, 2013, 2014, 2015, 2016 (SMDM)
| [Search in SMDM proceedings] | [Results] |
| References | 1607 |
Appendix 4. Reference lists of systematic reviews
| [Reference lists of systematic reviews] | [Results] |
| Reference | 587 |
Appendix 5. Reference lists of primary studies included
| [Reference list of primary studies] | [Results] |
| Reference | 976 |
Appendix 6. Impact of studies not included in the quantitative synthesis on the pooled results (for the randomized trials)
| Comparison 1: Interventions targeting patients compared with usual care | ||
| Observer‐based outcome measure ‐ continuous data | The study not included in the quantitative synthesis supported the pooled result | |
| Studies included in the quantitative synthesis (n = 424) | SMD (95% IC) | |
| LeBlanc 2015a | 0.54 (‐0.13 to 1.22) | |
| Maclachlan 2016 | ||
| LeBlanc 2015b | ||
| Tai‐Seale 2016 | ||
| Observer‐based outcome measure ‐ continuous data | ||
| Studies not included in the quantitative synthesis | Results | |
| Hamann 2014 | n= 100, no difference between the study groups, unclear risk of bias | |
| Haskard 2008 | Unit of error analysis | |
| Patient reported outcome measure ‐ continuous data | It is unlikely that the study not included in the quantitative synthesis would change the direction of the effect size estimate given that its sample size is not very large | |
| Studies included in the quantitative synthesis (n = 1386) | SMD (95% IC) | |
| Deen 2012 | 0.32 (0.16 to 0.48) | |
| Maranda 2014 | ||
| Pickett 2012 | ||
| van der Krieke 2013 | ||
| van Peperstraten 2010 | ||
| Cooper 2011 | ||
| Perestelo‐Perez 2016 | ||
| Tai‐Seale 2016 | ||
| Eggly 2017 | ||
| Patient‐reported outcome measure ‐ continuous data | ||
| Studies not included in the quantitative synthesis | Results | |
| Hamann 2014 | n = 100, no difference between the study groups, unclear risk of bias | |
| Patient‐reported outcome measure ‐ categorical data | Van Tol‐Geerdink 2016 does not support the pooled result but given that the pooled estimate of the effect size is in favor of the control group, it is likely that adding this study would move the pooled estimate of the effect size towards a null effect. Wolderslund 2017 does not support the pooled result but given its very large sample size, it is likely that adding this study would move the pooled estimate of the effect size towards a positive effect. Korteland 2017 supports the pooled result (null effect). |
|
| Studies included in the quantitative synthesis (n = 754) | RD (95% IC) | |
| Krist 2007 | ‐0,09 (‐0.19 to 0.01) | |
| Landrey 2012 | ||
| Murray 2001 | ||
| Sheridan 2014 | ||
| Vestala 2013 | ||
| Vodermaier 2009 | ||
| Patient‐reported outcome measure ‐ categorical data | ||
| Studies not included in the quantitative synthesis | Results | |
| van Tol‐Geerdink 2016 | n = 240, significant difference in favor of the intervention group, high risk of bias | |
| Wolderslund 2017 | n = 4349, significant difference in favor of the intervention group, high risk of bias | |
| Korteland 2017 | n = 155, no difference between the study groups, high risk of bias | |
| Comparison 2. Effect of intervention: Interventions targeting healthcare professionals compared to usual care | ||
| Observer‐based outcome measure ‐ continuous data | Murray 2010 and Bernhard 2011 ‐ ANZ (Australia and New Zealand sub‐sample) support the pooled result. Bernhard 2011 ‐ SGA (Switzerland, Germany and Austria sub‐sample) does not support the pooled result but it is unlikely that adding these data to the quantitative synthesis would move the pooled estimate of the effect size toward a null effect or in the opposite direction, given its very small sample size. Moreover, the different results observed in the two sub‐samples is a possible indication of an effect modification |
|
| Studies included in the quantitative synthesis (n = 479) | SMD (95% IC) | |
| Fossli 2011 | 0.70 (0.21 to 1.19) | |
| Shepherd 2011 | ||
| Stacey 2006 | ||
| Sanders 2017 | ||
| Cox 2017 | ||
| LeBlanc 2015b | ||
| Observer‐based outcome measure ‐ continuous data | ||
| Studies not included in the quantitative synthesis | Results | |
| Bernhard 2011‐ SGA | n = 32, no difference between the study groups, high risk of bias | |
| Bernhard 2011‐ ANZ | n = 21, significant difference in favor of the intervention group, high risk of bias | |
| Murray 2010 | n = 88, significant difference in favor of the intervention group, low risk of bias | |
| Patient‐reported outcome measure ‐ continuous data | Légaré 2012 does not support the pooled result. However, it is unlikely that adding these data to the quantitative synthesis would move the pooled estimate of the effect size from the null effect to a significant positive effect. | |
| Studies included in the quantitative synthesis (n = 5772) | SMD (95% IC) | |
| Cooper 2011 | 0.03 (‐0.15 to 0.20) | |
| Kennedy 2013 | ||
| Koerner 2014 | ||
| Tinsel 2013 | ||
| Wilkes 2013 | ||
| Patient reported outcome measure ‐ continuous | ||
| Studies not included in the quantitative synthesis | Results | |
| Légaré 2012 | n = 359, significant difference in favor of the intervention group, unclear risk of bias | |
| Patient ‐reported outcome measure ‐ categorical data | Bernhard 2011 supports the pooled result. | |
| Studies included in the quantitative synthesis (n = 6303) | RD (95% IC) | |
| Légaré 2012 | 0,01 (‐0.03 to 0.06) | |
| O'Cathain 2002 | ||
| Patient‐reported outcome measure ‐ categorical | ||
| Studies not included in the quantitative synthesis | Results | |
| Bernhard 2011 | n = 694, no difference between the study groups, high risk of bias | |
| Comparison 3: Interventions targeting both patients and healthcare professionals compared to usual care | ||
| Patient‐reported outcome measure ‐ continous data | Although the confidence interval of the pooled estimate of the effect size contains the null effect, the estimate is in favor of the intervention group. Adding data from Loh 2007 would move the pooled estimate towards a positive effect. Adding Wetzels 2005 would move the result towards a null effect. |
|
| Studies included in the quantitative synthesis (n = 1479) | SMD (95% IC) | |
| Cooper 2011 | 0.13 (‐0.02 to 0.28) | |
| Härter 2015 | ||
| Hamann 2007 | ||
| Rise 2012 | ||
| Wilkes 2013 | ||
| Tai‐Seale 2016 | ||
| Epstein 2017 | ||
| Patient reported outcome measure ‐ continuous data | ||
| Studies not included in the quantitative synthesis | Results | |
| Loh 2007 | n = 405, significant difference in favor of the intervention group, unclear risk of bias | |
| Wetzels 2005 | n = 263, no difference between the study groups, high risk of bias | |
| Patient‐reported outcome measure ‐ categorical data | Leighl 2011 supports the pooled result | |
| Studies included in the quantitative synthesis (n = 266) | RD (95% IC) | |
| Mathers 2012 | ‐0.01 (‐0.20 to 0.19) | |
| Härter 2015 | ||
| Patient‐reported outcome measure ‐ categorical data | ||
| Studies not included in the quantitative synthesis | Results | |
| Leighl 2011 | n = 207, no difference between the study groups, unclear risk of bias | |
| Comparison 4: Interventions targeting patients compared to other interventions targeting patients | ||
| Patient‐reported outcome measure ‐ continuous data | Lalonde 2006 and Street 1995 support the pooled results | |
| Studies included in the quantitative synthesis (n = 1906) | SMD (95% IC) | |
| Causarano 2014 | 0.03 (‐0.18 to 0.24) | |
| Deen 2012 | ||
| Hamann 2011 | ||
| Schroy 2011 | ||
| van Roosmalen 2004 | ||
| Schroy 2016 | ||
| Adarkwah 2016 | ||
| Eggly 2017 | ||
| Hamann 2017 | ||
| Jouni 2017 | ||
| Smallwood 2017 | ||
| Patient‐reported outcome measure ‐ continuous data | ||
| Studies not included in the quantitative synthesis | Results | |
| Lalonde 2006 | n = 26, no difference between study groups, high risk of bias | |
| Street 1995 | n = 60, no difference between study groups, high risk of bias | |
| Patient‐reported outcome measure ‐ categorical data | Kopke 2014 and Butow 2004 support the pooled results. Wolderslund 2017 does not support the pooled result but given its very large sample size, it is likely that adding this study would move the pooled estimate of the effect size toward a positive effect. | |
| Studies included in the quantitative synthesis (n = 2272) | RD (95% IC) | |
| Butow 2004 | 0,03 (‐0.02 to 0.08) | |
| Davison 1997 | ||
| Deschamps 2004 | ||
| Dolan 2002 | ||
| Kasper 2008 | ||
| Krist 2007 | ||
| Raynes‐Greenow 2010 | ||
| Stiggelbout 2008 | ||
| Thomson 2007 | ||
| Davison 2002 | ||
| Patient‐reported outcome measure ‐ categorical data | ||
| Studies not included in the quantitative synthesis | Results | |
| Köpke 2014 | n = 192, no difference between the study groups, high risk of bias | |
| Butow 2004 | n =164, no difference between the study groups, high risk of bias | |
| Wolderslund 2017 | n = 4349, significant difference in favor of the intervention group, high risk of bias | |
| Comparison 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals | ||
| Observer‐based outcome measure ‐ continuous data | Contrary to Elwyn 2004, which reported null results, Feng 2013 reported significant positive results. However it is unlikely that combining these two studies would move the estimate of the effect towards a significant positive result. More studies are needed to draw robust conclusions. | |
| Studies included in the quantitative synthesis (n = 20) | SMD (95% IC) | |
| Elwyn 2004 | ‐0,30 (‐1.19 to 0.59) | |
| Observer‐based outcome measure ‐ continuous data | ||
| Studies not included in the quantitative synthesis | Results | |
| Feng 2013 | n =118, significant difference in favor of the intervention group, high risk of bias | |
Appendix 7. Previous review on interventions promoting the adoption of SDM
| [Previous review (Légaré 2014] | [Results] |
| References | 20,792 |
Appendix 8. Certainty assessment of evidence for primary outcome
| Name of outcome | 1. Certainty of evidence | 2. Starting score for certainty of evidence according to study design | 3. Criteria to downgrade score | ||||
| 3a. Study limitation | 3b. Indirectness of evidence | 3c. Inconsistency | 3d. Imprecision | 3e. Publication bias | |||
| Comparison 1: Interventions targeting patients compared with usual care | |||||||
| OBOM ‐ Continuous | 1 | 4 | ‐2 | ‐1 | ‐2 | ‐2 | N/A |
| PROM ‐ Continuous | 1 | 4 | ‐2 | ‐1 | ‐2 | 0 | 0 |
| PROM ‐ Continuous ‐ (Non‐randomized control trial) | 1 | 2 | ‐2 | ‐1 | N/A | ‐1 | N/A |
| PROM ‐ Categorical | 1 | 4 | ‐2 | ‐1 | ‐1 | 0 | 0 |
| Comparison 2: Interventions targeting healthcare professionals compared to usual care | |||||||
| OBOM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | ‐2 | ‐1 | 0 |
| OBOM ‐ Continuous ‐ (Controlled before‐after study) | 1 | 2 | ‐2 | ‐1 | N/A | ‐1 | N/A |
| PROM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | ‐2 | ‐1 | 0 |
| PROM ‐ Categorical | 1 | 4 | ‐1 | ‐1 | ‐2 | 0 | N/A |
| Comparison 3: Interventions targeting both patients and healthcare professionals compared to usual care | |||||||
| OBOM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | ‐2 | ‐1 | ‐1 |
| PROM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | ‐1 | 0 | 0 |
| PROM ‐ Categorical | 1 | 4 | ‐1 | ‐1 | ‐1 | ‐1 | N/A |
| Comparison 4: Interventions targeting patients compared to other interventions targeting patients | |||||||
| OBOM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | ‐2 | ‐1 | N/A |
| PROM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | ‐2 | ‐1 | 0 |
| PROM ‐ Continuous ‐ (Non‐randomized control trial) | 1 | 2 | ‐2 | ‐1 | N/A | ‐1 | N/A |
| PROM ‐ Categorical | 1 | 4 | ‐2 | ‐1 | ‐1 | 0 | 0 |
| Comparison 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals | |||||||
| OBOM ‐ Continuous | 1 | 4 | 0 | ‐1 | N/A | ‐2 | N/A |
| PROM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | ‐2 | ‐1 | N/A |
| Comparison 6: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals | |||||||
| OBOM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| OBOM ‐ Categorial | 1 | 4 | ‐2 | ‐1 | N/A | ‐1 | N/A |
| PROM ‐ Continuous | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
Appendix 9. Certainty assessment of evidence for secondary outcomes
| Name of outcome | 1. Certainty of evidence | 2. Starting score for certainty of evidence according to study design | 3. Criteria to downgrade score | ||||
| 3a. Study limitation | 3b. Indirectness of evidence | 3c. Inconsistency | 3d. Imprecision | 3e. Publication bias | |||
| Comparison 1: Interventions targeting patients compared with usual care | |||||||
| Continous | |||||||
| Decision regret | 1 | 4 | ‐2 | ‐1 | N/A | ‐1 | N/A |
| Health‐related QoL (physical) | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Health‐related QoL (mental) | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Consultation length | 1 | 4 | ‐1 | ‐1 | ‐1 | ‐1 | N/A |
| Cost | 1 | 4 | ‐2 | ‐1 | N/A | ‐1 | N/A |
| Categorical | |||||||
| No study | |||||||
| Comparison 2. Effect of intervention: Interventions targeting healthcare professionals compared to usual care | |||||||
| Continous | |||||||
| Decision regret | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Health‐related QoL (physical) | 2 | 4 | ‐1 | ‐1 | N/A | 0 | N/A |
| Health‐related QoL (mental) | 2 | 4 | ‐1 | ‐1 | N/A | 0 | N/A |
| Consultation length | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Categorical | |||||||
| No study | |||||||
| Comparison 3: Interventions targeting both patients and healthcare professionals compared to usual care | |||||||
| Continous | |||||||
| Decision regret | 2 | 4 | ‐1 | ‐1 | N/A | 0 | N/A |
| Health‐related QoL (physical) | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Health‐related QoL (mental) | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Consultation length | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Categorical | |||||||
| No study | |||||||
| Comparison 4: Interventions targeting patients compared to other interventions targeting patients | |||||||
| Continous | |||||||
| Consultation length | 1 | 4 | ‐1 | ‐1 | N/A | ‐1 | N/A |
| Categorical | |||||||
| No study | |||||||
| Comparison 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals | |||||||
| Continous | |||||||
| No study | |||||||
| Categorical | |||||||
| No study | |||||||
| Comparison 6: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals | |||||||
| Continous | |||||||
| No study | |||||||
| Categorical | |||||||
| No study | |||||||
Data and analyses
Comparison 1. Group 1: Interventions targeting patients compared to usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shared decision making (OBOM, continuous) | 4 | 424 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.13, 1.22] |
| 1.1 Cluster RCT | 2 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.22, 1.29] |
| 1.2 Individual RCT | 2 | 308 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.76, 1.72] |
| 2 Shared decision making (PROM, continuous) | 9 | 1386 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.16, 0.48] |
| 2.1 Cluster RCT | 2 | 303 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.18, 0.63] |
| 2.2 Individual RCT | 7 | 1083 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.10, 0.49] |
| 3 Shared decision making (PROM, continuous) ‐ NRCT | 1 | 303 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.21, 0.25] |
| 4 Shared decision making (PROM, categorical) | 6 | 754 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.19, 0.01] |
| 5 Knowledge | 3 | 565 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.16, 0.61] |
| 6 Knowledge (categorical) | 2 | 312 | Risk Difference (M‐H, Random, 95% CI) | 0.17 [0.05, 0.29] |
| 7 Satisfaction | 1 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.24, 0.52] |
| 8 Decisional conflict | 3 | 367 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.68, 0.09] |
| 9 Decision regret | 1 | 212 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.39, 0.19] |
| 10 Patient‐physician communication (number of topics raised by patients) | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.13, 0.65] |
| 11 Patient‐physician communication (patient raised discussion) | 1 | 157 | Risk Difference (M‐H, Random, 95% CI) | 0.29 [0.14, 0.44] |
| 12 Patient‐physician communication (patient participation in discussion) | 1 | 157 | Risk Difference (M‐H, Random, 95% CI) | 0.27 [0.13, 0.42] |
| 13 Decision self‐efficacy | 2 | 274 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.08, 0.40] |
| 14 Empowerment | 1 | 342 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.05, 0.48] |
| 15 Empowerment (categorical) | 1 | 262 | Risk Difference (M‐H, Random, 95% CI) | 0.18 [0.09, 0.27] |
| 16 Adherence | 2 | 598 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 17 Health‐related quality of life (physical) | 1 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 18 Health‐related quality of life (mental) | 1 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.26, 0.46] |
| 19 Anxiety | 2 | 419 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.33, 0.37] |
| 20 Anxiety (categorical) | 1 | 127 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.07, 0.15] |
| 21 Depression | 1 | 127 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [0.05, 0.28] |
| 22 Consultation length | 2 | 224 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.39, 0.58] |
| 23 Cost | 1 | 105 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.42, 1.22] |
Comparison 2. Group 2: Interventions targeting healthcare professionals compared to usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shared decision making (OBOM, continuous) | 6 | 479 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.21, 1.19] |
| 1.1 Cluster RCT | 2 | 329 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.25, 1.21] |
| 1.2 Individual RCT | 4 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.85 [0.06, 1.64] |
| 2 Shared decision making (OBOM, continuous) ‐ CBAs | 1 | 21 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.96, 0.76] |
| 3 Shared decision making (PROM, continuous) | 5 | 5772 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.15, 0.20] |
| 3.1 Cluster RCT | 4 | 5678 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.17, 0.20] |
| 3.2 Individual RCT | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.30, 0.51] |
| 4 Shared decision making (PROM, categorical) | 2 | 6303 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.06] |
| 5 Knowledge | 2 | 969 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.16, 0.69] |
| 6 Knowledge (categorical) | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | ‐0.13 [‐0.36, 0.10] |
| 7 Satisfaction with consultation | 1 | 479 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.18, 0.18] |
| 8 Satisfaction with information | 1 | 1492 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.02, 0.07] |
| 9 Satisfaction with decision making process | 1 | 1488 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 10 Satisfaction with discussion | 1 | 1483 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 11 Decision regret | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.07, 0.51] |
| 12 Self‐efficacy | 1 | 4475 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 13 Adherence | 1 | 827 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.21, 0.06] |
| 14 General health | 1 | 4056 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 15 Psychological well‐being | 1 | 4052 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 16 Health‐related quality of life (physical) | 1 | 359 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.05, 0.36] |
| 17 Health‐related quality of life (mental) | 1 | 359 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.07, 0.49] |
| 18 Health‐related quality of life | 1 | 4449 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 19 Anxiety | 1 | 3003 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 20 Consultation length | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.21, 0.81] |
| 21 Consultation length (10‐20 min) | 1 | 479 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.05] |
| 22 Safety | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.32, 0.32] |
Comparison 3. Group 3: Interventions targeting both patients and healthcare professionals compared to usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shared decision making (OBOM, continuous) | 6 | 1270 | Std. Mean Difference (IV, Random, 95% CI) | 1.10 [0.42, 1.79] |
| 1.1 Cluster RCT | 2 | 59 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.14, 1.20] |
| 1.2 Individual RCT | 4 | 1211 | Std. Mean Difference (IV, Random, 95% CI) | 1.31 [0.45, 2.17] |
| 2 Shared decision making (PROM, continuous) | 7 | 1479 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.02, 0.28] |
| 2.1 Cluster RCT | 4 | 890 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.01, 0.41] |
| 2.2 Individual RCT | 3 | 589 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.17, 0.16] |
| 3 Shared decision making (PROM, categorical) | 2 | 266 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.20, 0.19] |
| 3.1 Cluster RCT | 1 | 169 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 3.2 Individual RCT | 1 | 97 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [‐0.10, 0.31] |
| 4 Knowledge | 2 | 1004 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.28, 0.53] |
| 5 Knowledge (categorical) | 4 | 1260 | Risk Difference (M‐H, Random, 95% CI) | 0.28 [0.05, 0.51] |
| 6 Satisfaction with care | 2 | 362 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.34, 1.36] |
| 7 Satisfaction with decision | 1 | 424 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.05, 0.43] |
| 8 Satisfaction with consultation | 1 | 393 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 9 Decisional conflict | 2 | 1065 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.71, 0.01] |
| 10 Confidence in decision | 1 | 414 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.17, 0.22] |
| 11 Decision regret | 1 | 369 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.08, 0.33] |
| 12 Patient‐physician communication (patient‐centered communication) | 1 | 265 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.01, 0.47] |
| 13 Match between preferred and actual level of participation in decision making | 2 | 185 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.16, 0.10] |
| 14 Adherence | 1 | 489 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.36, 0.83] |
| 15 Adherence (categorical) | 2 | 145 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.15, 0.15] |
| 16 Health‐related quality of life | 1 | 265 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.16, 0.33] |
| 17 Health‐related quality of life (physical) | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.37, 0.54] |
| 18 Health‐related quality of life (mental) | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.44, 0.46] |
| 19 Anxiety | 1 | 419 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.31, 0.08] |
| 20 Depression | 1 | 418 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.33, 0.05] |
| 21 Consultation length | 1 | 536 | Std. Mean Difference (IV, Random, 95% CI) | 3.72 [3.44, 4.01] |
| 22 Safety | 1 | 898 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
Comparison 4. Group 4: Inteventions targeting patients compared to other interventions targeting patients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shared decision making (OBOM, continuous) | 3 | 271 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.39, 1.37] |
| 2 Shared decision making (PROM, continuous) | 11 | 1906 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.18, 0.24] |
| 2.1 Cluster RCT | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.14, 0.75] |
| 2.2 Individual RCT | 10 | 1828 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.22, 0.23] |
| 3 Shared decision making (PROM, continuous) comp1 ‐ NRCT | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.61, 0.19] |
| 4 Shared decision making (PROM, continuous) comp2 ‐ NRCT | 1 | 110 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.56, 0.19] |
| 5 Shared decision making (PROM, continuous) comp3 ‐ NRCT | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.37, 0.43] |
| 6 Shared decision making (PROM, categorical) | 10 | 2272 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 7 Knowledge | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.13, 0.47] |
| 8 Knowledge (categorical) | 3 | 706 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [‐0.10, 0.42] |
| 9 Satisfaction with decision | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.10, 0.24] |
| 10 Satisfaction with treatment | 2 | 267 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.34, 0.16] |
| 11 Satisfaction with consultation | 1 | 207 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.42, 0.13] |
| 12 Satisfaction with information provided | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.52, 0.73] |
| 13 Decisional conflict | 5 | 1088 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.48, 0.08] |
| 14 Decision uncertainty | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.65, 0.23] |
| 15 Decision self‐efficacy | 2 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.41, 0.37] |
| 16 Match between preferred and actual level of participation in decision making | 4 | 1206 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.05] |
| 17 Match between preferred option and decision made | 2 | 363 | Risk Difference (M‐H, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 18 Adherence | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.35, 0.44] |
| 19 Adherence (categorical) | 3 | 301 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
| 20 General health | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.61, 0.23] |
| 21 Anxiety | 2 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.27, 0.05] |
| 22 Depression | 1 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.69, 0.16] |
| 23 Consultation length | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.29, ‐0.00] |
Comparison 5. Group 5: Interventions targeting healthcare professionals compared to other interventions targeting healthcare professionals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shared decision making (OBOM, continuous) | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.19, 0.59] |
| 2 Shared decision making (PROM, continuous) | 2 | 1459 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.10, 0.58] |
| 2.1 Cluster RCT | 1 | 327 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.17, 0.27] |
| 2.2 Individual RCT | 1 | 1132 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.28, 0.52] |
| 3 Health status (mental) | 1 | 295 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.01, 0.47] |
| 4 Health status (physical) | 1 | 295 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.19, 0.28] |
| 5 Anxiety | 1 | 843 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [0.00, 0.28] |
Comparison 6. Group 6: Interventions targeting both patients and healthcare professionals compared to other interventions targeting both patients and healthcare professionals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shared decision making (OBOM, continuous) | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐1.17, 0.60] |
| 2 Shared decision making (OBOM, categorical) | 1 | 134 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.04] |
| 3 Shared decision making (PROM, continuous) | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.32, 0.32] |
| 4 Decisional conflict | 1 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.30, 0.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adarkwah 2016.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: primary care, Germany Health professionals: 32; general practitioners; fully trained Patients: 304; cardiovascular risk prevention; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (Computerised decision aid (TTE)) Quote: "Immediately after giving their informed consent, patients were randomized to consultation with the emoticons (Fig. 2) or the TTE illustration (Fig. 3). GPs entered a study ID into the decision support software, which automatically allocated each patient into one of the two conditions according to an a priori randomized sequence. GPs learned about each patient’s allocation by the illustration displayed by the software. They then started a discussion with their patients on the basis of the allocated display, i.e. either emoticons, or TTE, respectively." Page 3, figure 3 Single intervention: patient‐mediated intervention (Computerised decision aid (emoticon)). Figure 2 |
|
| Outcomes | PEF‐FB‐9 (SDM‐Q9) (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "GPs entered a study ID into the decision support software, which automatically allocated each patient into one of the two conditions according to an a priori randomized sequence." page 3 |
| Allocation concealment (selection bias) | Low risk | Quote: "GPs entered a study ID into the decision support software, which automatically allocated each patient into one of the two conditions according to an a priori randomized sequence." page 3 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Performance bias. Quote: "GPs recorded the decision made, such as specific medications, dose adjustments, behavioral measures or no change at all." page 3 Quote: "GPs learned about each patient’s allocation by the illustration displayed by the software." page 3 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: we cannot assume that all patients replied to all questions related to outcomes. Missing outcome data were not specified. |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes pre‐specified in the study protocol were reported in the results (Clinical Trials Register Platform (ICTRP, ID DRKS00004933)). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of primary outcome. |
| Protection against contamination? | High risk | Comment: patients were randomized. |
| Baseline characteristics patients | Low risk | Quote: "Both study arms were well‐balanced regarding socio demographic and clinical variables." page 6. See Table 1 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Almario 2016.
| Methods |
Study design: non‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: ambulatory care, specialized care, USA Health professionals: various types; fully trained and in training Patients: 371; gastrointestinal disorders; male and female |
|
| Interventions |
Single intervention ‐ patient‐mediated (GI PROMIS)
Quote: "Intervention patients completed GI PROMIS symptom questionnaires on an e‐portal one week before their visit." page 1
Quote: "Using modern psychometric techniques, such as item response theory and computerized adaptive testing,(14, 15) PROMIS offers state‐of‐the‐art psychometrics, establishes common‐language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires are administered electronically and efficiently, allowing implementation in busy clinical settings. Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a gastrointestinal (GI) item bank, which our group developed." page 3 Usual care Quote: "Usual care patients were managed according to customary practices." page 2 |
|
| Outcomes | SDM‐Q‐9 (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: no randomization. |
| Allocation concealment (selection bias) | High risk | Quote: "We used a pragmatic, off‐on study design alternating weekly between the PROMIS intervention and control arms." page 4 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Quote: "Specifically, the missing outcome data for this group was imputed to the corresponding mean value calculated from controls for each item. Because this assumption biases towards the null, we also performed a sensitivity analysis using a per‐protocol approach where we excluded patients without follow‐up data." page 6 |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. |
| Other bias | Unclear risk | Quote: "We only evaluated patients with GI symptoms, so we cannot know whether using other PROMIS questionnaires, such as those for fatigue, physical function, or pain, among many others, would also fail to show a difference vs. usual care." page 8 |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: insufficient information to make a judgement. |
| Protection against contamination? | Unclear risk | Comment: insufficient information to make a judgement. |
| Baseline characteristics patients | Low risk | Quote: "No differences were seen in gender and race/ethnicity between groups (Appendix Table 2)." page 7 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Ampe 2017.
| Methods |
Study design: non‐randomized trial Unit of allocation: practice Unit of analysis: provider Power calculation: unclear |
|
| Participants |
Care setting: non‐ambulatory care, primary care, Belgium Health professionals: 90; various types; fully trained and in training; male and female Patients: advance care planning, dementia care units |
|
| Interventions |
Single intervention ‐ educational meeting (We DECide)
"We DECide’ was a communication intervention for nursing home staff working in dementia care units, in which competences were trained for realizing SDM in ACP conversations with residents with dementia and their families. It was developed for this study and aimed at practising how to conduct ACP conversations with residents with dementia and their family caregivers, by applying the three‐step model for SDM by Elwyn and colleagues. This model describes the three steps that are necessary for realizing SDM in a clinician‐patient encounter: the ‘Choice talk’, talking about the fact that different choices exist; the ‘Option talk’, talking about the different options and choices; and the 'Decision talk', talking about a final decision. ‘We DECide’ consisted of three modules (two 4‐hour workshops and a homework assignment) that were based on the three steps of the model for SDM. Each module was designed to train the specific competences that are necessary to complete the corresponding step. Three types of conversations that are crucial for talking about ACP in the nursing home were used for practising SDM. Conversations at the time of admission were used as a prototype for the ‘Choice talk’ in the first workshop, since these conversations are crucial for indicating that certain choices for care exist. As a homework assignment participants were to practise the ‘Option talk’ by engaging in conversations with residents about preferences in routine care situations, and thus to talk about the different care options. Conversations in crisis situations were used as a prototype for the ‘Decision talk’ in the second workshop (which took place after the homework assignment), since the urgency of crisis situations require that certain decisions have to be made. The overview of the ‘we DECide'‐ modules are represented in Fig. 1. ‘We DECide’ was taught in small groups (approximately 10 participants per session) by an experienced communication trainer, in order to ensure active participation of each participant. The intervention took place in a time span of maximum 4 weeks." page 140 Usual care (control group) |
|
| Outcomes | OPTION (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: no randomization. |
| Allocation concealment (selection bias) | High risk | Quote: "Therefore, because ‘we DECide’ was designed for participants with sufficient learning opportunities, the nine care units with the lowest scores were included in the intervention group." pages 140‐141 Quote: "We created participant groups of comparable size and composition. In order to make groups with staff from two nursing home units, we chose to include a unit from the control group." page 141 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | COmment: insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | High risk | Quote: "Thirteen units recorded one or more conversations. Five units (two from the intervention group) did not record any conversations, due to one of the following reasons: no admission of new residents with dementia due to relocation of the care unit to a new building, or absence of staff members due to illness... A total of 21 conversations were analysed." page 142 "Only a small number of conversation recordings was provided. Maybe a longer time period would have allowed dementia care units to conduct more conversations and to provide a more complete picture of resident involvement in ACP in the dementia care unit." page 145 |
| Selective reporting (reporting bias) | High risk | Comment: Some relevant outcomes prespecified in the study protocol were not reported in the results (see IFC questionnaire). |
| Other bias | Low risk | Comment: No evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Low risk | Quote: "When OPTION scores at pre‐test and post‐test were compared, no statistically significant differences were found for the intervention group (average pre‐test score: 41.32/100, SD 10.84), nor the control group (average pre‐test score: 47.61/100, SD 20.54 (see Table 6)." page 143 |
| Protection against contamination? | Unclear risk | Insufficient information to make a judgement. Quote: "To increase standardisation of the intervention, we created participant groups of comparable size and composition. In order to make groups with staff from two nursing home units, we chose to include a unit from the control group. In this way, the training could be offered to five small groups separately, each of which contained staff from two different nursing home units." page 141 |
| Baseline characteristics patients | Unclear risk | No report of characteristics |
| Baseline characteristics healthcare professionals | Unclear risk | Characteristics are mentioned in text but no data were presented. Quote: "We created participant groups of comparable size and composition." page 141 |
Barton 2016.
| Methods |
Study design: non‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory care, specialized care, USA Health professionals: unclear type; fully trained Patients: 166; rheumatoid arthritis; male and female |
|
| Interventions |
Single intervention ‐ patient‐mediated intervention: (adapted guide prior to visit)
Patient‐mediated intervention
Quote: "Briefly, the existing AHRQ guide was discussed in patient and clinician focus groups; transcripts and field notes were analyzed and informed the content of the adapted guide, which included chapters on “What is RA?” and “What can RA medicines do for you?” The design and development process for RA Choice was based on a tool created for diabetes mellitus medications for use in the clinic to facilitate a conversation between clinician and patient. Tool development involved field‐testing low‐fidelity prototypes (drafts or incomplete versions) in real clinical encounters followed by modifications and iterative field testing." page 891. "All participating clinicians received brief (5 minutes), in‐person training about RA Choice." page 891 Single intervention ‐ patient‐mediated intervention: (adapted guide + decision aid during visit ) Literacy‐appropriate medication guide and decision aid (RA Choice) Single intervention ‐ patient‐mediated intervention: Control Group Quote: "Patients received existing medication guide prior to clinic visit." page 889 |
|
| Outcomes | The Interpersonal Processes of Care (IPC) (Continous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "We used this method rather than randomizing by physician to allow for the broadest possible group of physicians to participate in arm 3, to allow for additional time to complete the design and testing of materials, and to avoid contamination of the groups." page 890 |
| Allocation concealment (selection bias) | High risk | Quote: "After completing enrollment for the control group, patients were enrolled into arm 2, in which patients received the adapted guide prior to the clinic visit, and then into arm 3, where patients received the adapted guide prior to the visit and the decision aid (used in the clinical encounter). We used this method rather than randomizing by physician to allow for the broadest possible group of physicians to participate in arm 3, to allow for additional time to complete the design and testing of materials, and to avoid contamination of the groups." page 890 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to make a judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | Low risk | Quote: "We used this method rather than randomizing by physician to allow for the broadest possible group of physicians to participate in arm 3, to allow for additional time to complete the design and testing of materials, and to avoid contamination of the groups" page 890 |
| Baseline characteristics patients | Low risk | Quote: "There were no significant differences in characteristics across the 3 study arms with the exception of sex (more women in arms 1 and 3; P 5 0.02) and clinic site (78% in arm 1 were from the county hospital compared to 60% each in arms 2 and 3) the possibility that patients enrolled in arm 1 may have differed in characteristics from those in arm 2 or 3; however, the only significant differences were in sex and clinic site 2‐ Adjusted models control for clinic site and sex." page 893 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Bernhard 2011.
| Methods |
Study design: clinician‐randomized trial Unit of allocation: clinician Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: specialized care; ambulatory care; Australia, New Zealand, Switzerland, Germany, and Austria Health professionals: 62; various type of physician (medical, surgical, radiation and gynacological oncologists); fully trained Patients: 694; breast cancer; female |
|
| Interventions |
Multifaceted intervention: educational meeting, audit and feedback, distribution of educational materials (interactive face‐to face workshop and two follow‐up telephone calls) Quote: "The training consisted of a 7 hours interactive face to‐face workshop with one to two follow‐up telephone calls over 2 months. The elements of this training were evidence‐based .... The training focused on four key concepts: ... The workshops were held at the participating centres and conducted in the local language by one to two clinical psychologists ... The teaching materials were in English .... Before the workshop, participants were expected to have read the strategies document." Page 1267 Usual care (control): No training workshop Quote: "Following baseline assessment and before the scheduled training workshop, they were randomly assigned to ... or control (no training workshop) group." Page 1267 |
|
| Outcomes | Patient involvement preference and actual involvement; SDM framework (DAS‐O subscale) (qualitative) | |
| Notes |
Additional information Number of approached patients (eligible): SGA (Swiss/German/Austrian): 429; ANZ (Australian/New Zealand): 340 Number of patients per physician: SGA (Swiss/German/Austrian): 41; ANZ (Australian/New Zealand): 21 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly assigned to the experimental (training workshop) or control (no training workshop) group." page 1266 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Quote: "Two raters applied the DAS‐O coding system to the consultations." page 7 (Butow 2014). Comment: in the paper, it is not specified if the raters were unaware of the allocation of the audiotaped record. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: Not specified in the paper. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | High risk | Quote: "53 (85,5%) doctors… were assessable with regard to the primary endpoint for this analysis: doctor behavior…" page 3 (Butow 2014). In the ANZ group, 75% of the consultation requested were audiotaped. In the SGA group, 9 doctors did not provide the an audiotape (7 were gynecologists). 74 % of the requested consultation among the 32 who provided audiotape were audiotaped. Quote: "Characteristics of doctors with... and without... audio tapes were compared... results suggest a difference in doctor speciality... Physicians without tapes were also younger" page 4‐5 (Butow 2004). Comment: it is likely that the missing primary outcome data (non audiotaped) were related to doctors behaviors. If the missing outcomes were related to a characteristic that is related to the intervention, it may be a selection bias. However, age and specialty are similar at baseline among doctors. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: for our primary outcome, but low risk for the primary outcome of the study (decisional conflict). |
| Selective reporting (reporting bias) | High risk | Comment: outcomes of interest are reported incompletely so they cannot be entered in a meta‐analysis. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment; no baseline measure of our primary outcome. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "Within two weeks of their initial consultation discussing treatment options, patients gave informed consent and completed a baseline questionnaire gathering demographics; preferences for information (degree of detail required on a Likert scale from ‘prefer few details’ to ‘prefer as many details ... " page 1266 |
| Protection against contamination? | Unclear risk | Comment: physicians within centre were allocated to intervention or control. |
| Baseline characteristics patients | Low risk | Quote: "Baseline characteristics of eligible patients are summarised for the pre‐randomisation and post‐randomisation cohorts in Table 2." page 1268 |
| Baseline characteristics healthcare professionals | Low risk | Quote: "Baseline characteristics of eligible doctors are shown in Table 1." page 1268 |
Bieber 2006.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: specialized care and ambulatory care (Rheumatologic Outpatient Clinic of the University of Heidelberg); Germany
Health professionals: 10; internal medicine; fully trained Patients: 149; fibromyalgia syndrome; male and female |
|
| Interventions |
Multifaceted intervention: educational meeting with physician (18 hours); patient‐mediated intervention (computer‐based visualized information tool). The computer‐based tool provided information on fibromyalgia syndrome, combining textual information with diagrams and short video sequences. The educational meeting involved training physicians to improve patient‐centered communication and interaction skills. Single intervention (control): patient‐mediated intervention (computer‐based visualized information tool) The tool was the same as the multifaceted intervention. |
|
| Outcomes | Doctor‐patient interaction, from the patient perspective, using the QQPPI (Questionnaire on the Quality of Physician‐Patient Interaction) (continuous); joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: patients were randomized but the method was unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Patients were informed on the intervention but they were blinded to the fact in which group they were being treated." Page 359 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: 64/149 (43%) patients were excluded after randomization (did not meet inclusion criteria, refused to complete questionnaire); Information about missing data in our primary outcome were lacking. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: baseline measurements for the FAPI are not reported, nor were they measured. |
| Protection against contamination? | High risk | Comment: it was the patients who were randomized in an university outpatient setting. |
| Baseline characteristics patients | Low risk | Quote: "All three patient group were comparable as to socio‐economic (see Table 1) and health related variables (see Table 3)." page 359 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Branda 2013.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: practice Unit of analysis: provider and patient Power calculation: not done |
|
| Participants |
Care setting: primary care; ambulatory care; USA Health professionals: 41; various type (physician, nurse practitioner, physician assistant, resident/fellow); fully trained and in training Patients: 110; Type 2 diabetes; male and female |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention, educational meeting. Quote: "The intervention will consist of the use of a decision aid (Statin Choice and Aspirin Choice, or Diabetes Medication Choice) by patients and their primary care clinician during the clinical encounter ." page 3 of the study protocol. Quote: "A study team member will conduct a demonstration showing how to use the decision aid at the time of the initial in‐person discussion with clinics. The focal points of the demonstration will be that decision aids serve as guides for conversation rather than scripted discussions... Brief video clips and storyboards that demonstrate the basic use of decision aids are publicly available at http://kercards.e‐bm.info for clinicians to review at their convenience. A study team member will remain available to do one‐on‐one demonstrations after the initial group demonstration if needed." page 4 of the study protocol Usual care (control): Quote: "For patients in the usual care arm, clinicians will manage the discussion about medication regimen as usual, without using decision aids. " page 4 of the study protocol. |
|
| Outcomes | Level of patient engagement (OPTION) (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described in the paper. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A study statistician will perform the randomization centrally after the practice has been enrolled." (Study protocol page 3, column 2). However, intervention and usual care patients were recruited within each practice (diabetes DA and UC, statin DA and UC). Investigators, clinicians were not blinded to the practice status. If the persons who enrolled the patients were not blinded too, it may have biased the selection. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: data collectors and analysts were blinded to allocation (p7 para 1) but it is not specified whether investigators who assessed videos recorded were blinded. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Quote: "We were able to obtain video recordings from 38% of encounters. This limits our ability to use our checklist and to obtain an OPTION score in all encounters thus reducing our confidence in the inferences related to fidelity and clinicians’ efforts to engage patients in decision making, respectively." page 6 para 3 DA: 41,5% UC: 34% |
| Selective reporting (reporting bias) | High risk | Comment: see study protocol: not all the study's prespecified relevant outcomes were reported (quality of life, costs and resources utilization). Morevover some relevant outcomes are specified differently in the results: e.g. decision comfort instead of decisional conflict, patient satisfaction with knowledge transfer instead of satisfaction with decision making. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | Low risk | Comment: intervention and usual care patients were recruited within each practice (diabetes DA and UC, statin DA and UC). |
| Baseline characteristics patients | High risk | Quote: "All patient factors were well balanced across both arms with a difference found in the type of discussion that patients had (statins vs. diabetes medication; Table 1); subsequent results adjust for this difference." page 4 column 2 Comment: there is imbalance in race and HbA1c too. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: there is mention of participant characteristics in table 2 but comparisons between intervention and control arms were not presented. |
Butow 2004.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Setting of care: specialized care; ambulatory care (University of Sydney teaching hospital); Australia Healthcare professionals: 4; medical oncologists (2) and radiation oncologists (2); fully trained Patients: 164; cancer; male or female |
|
| Interventions |
Single intervention: patient‐mediated intervention (consultation preparation package: booklet "How treatment decisions are made" + brochure "Your right and responsibilities" + question prompt sheet) Patients received an information package at least 48 hours before their first oncology appointment. The information package included a question prompt sheet, booklets on clinical decision making and patient rights, and an introduction to the clinic. Single intervention (control): patient‐mediated intervention (booklet NSW Cancer council booklet on living with cancer). Patients received the control booklet at least 48 hours before their first oncology appointment. This booklet contained only the introduction to the clinic. |
|
| Outcomes | Quote: "Physician encouragement of patient participation in the consultation and decision making process" (page 4404) subscale of the behaviours coding system (categorical); SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process Perceived level of control in the decision making process; SDM is assessed as the joint process between healthcare professionals and patients to make decisions. |
|
| Notes |
Additional information Number of approached patients (eligible): 246 Number of patients per physician: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Research nurse assigned an identification to consenting patients, determined random assignment, and sent the appropriate package with a consent form…" page 4403 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Research nurse assigned an identification to consenting patients, determined random assignment, and sent the appropriate package with a consent form…" page 4403 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in paper. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: for our primary outcome, 160/164. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: for our primary outcome, similar proportion in the two groups (Cancer Consultation Package Group (CCPP) group: 62/80; Booklet group: 69/84). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: No evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: baseline measurements are not reported for Quote: "Physician behaviours facilitating patient involvement". |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "There were no significant differences between the groups in information and involvement preferences measured before the consultation." age 4406 |
| Protection against contamination? | High risk | Comment: one of the outcomes is patient reported and the intervention is patient allocated; consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Quote: "Given that no significant differences were found on demographic or disease variables between control and intervention arms... " Page 4404 |
| Baseline characteristics healthcare professionals | High risk | Comment: not specified in the paper. |
Causarano 2014.
| Methods |
Study design: patient‐randomized trial (pilot) Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Setting of care: specialized care; Canada Healthcare professionals: various types (plastic surgeon, breast reconstruction clinical nurse specialist, social worker); fully trained Patients: 41; post‐mastectomy breast reconstruction; female |
|
| Interventions |
Single intervention: patient‐mediated intervention (routine education + educational meeting to patient) Quote: "Patients randomized to the intervention group participated in a pre‐consultation educational group intervention in addition to receiving routine education." page 1367 Single intervention (control): patient‐mediated intervention (routine education) |
|
| Outcomes | Decision making subscale (M‐PICS) (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): 57 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated random allocation sequence was created with a 1:1 allocation to the intervention or routine education (control) in blocks of 10. To achieve allocation concealment, the randomization allocation list was developed by a statistician independent from the coordinator using PROC PLAN in SAS." Page 1366 |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer generated random allocation sequence was created with a 1:1 allocation to the intervention or routine education (control) in blocks of 10. To achieve allocation concealment, the randomization allocation list was developed by a statistician independent from the coordinator using PROC PLAN in SAS." Page 1366 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "patients were not blinded to their treatment arm; the surgeon leading the intervention could not be blinded as she/he also conducted the consultation." page 1366 last paragraph. Comment: it is not mentioned if patients were aware of the objective of the study but outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: although reasons of non completion of the entire outcome questionnaires by 2 patients are not specified, missing outcome data are balanced in numbers across groups. Retention rate was 95% (39/41). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | Low risk | Quote: "Consultations were scheduled in batches to prevent contamination, such that the two groups were not in the clinic waiting room at the same time." page 1367 |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 2). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Cooper 2011.
| Methods |
Study design: randomized trial (factorial design) Unit of allocation: physician and patient Unit of analysis: physician and patient Power calculation: unclear |
|
| Participants |
Care setting: primary care, ambulatory care (especially low SES service), USA Health professionals: 41, physicians fully trained Patients: 279, hypertensive; 184 female |
|
| Interventions |
Four arms:
Quote: "The physician communication skills program was designed to provide physicians with personalized feedback based on their videotaped performance with a simulated patient scheduled for an office appointment. ... Intervention group physicians reviewed the videotape of their personal interviews with the simulated patient and completed exercises on the CD‐ROM or in the workbook." page 1298 Quote: "Control group physicians participated in the simulated visit but did not receive any feedback until the end of the study" page 1298 |
|
| Outcomes | Participatory Decision making (PDM); Patient involvement in care | |
| Notes |
Additional information Number of approached patients (eligible): 980 Number of patients per physician: 50 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random blocks of size two and four were used, and a list of random numbers between zero and one was generated in Stata version 7.0." protocol |
| Allocation concealment (selection bias) | Low risk | Quote: "The study statistician generated the allocation sequence for both physicians and patients and placed the intervention assignment for each subject in opaque envelopes to be opened by research assistants." Protocol |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "Due to the nature of the interventions, complete masking of participants, investigators and CHWs was not possible." page 1299 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: we do not know if missing outcomes were balanced across intervention groups. Imputation was used by assuming that data are MAR but the mechanism and the reasons of missing data were not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes were described in the protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: process measures at baseline and change at 12‐month follow‐up by intervention group. |
| Protection against contamination? | Unclear risk | Comment: professionals were allocated within a clinic. |
| Baseline characteristics patients | Low risk | Comment: see table 2. |
| Baseline characteristics healthcare professionals | Low risk | Comment: see table 1. |
Cooper 2013.
| Methods |
Study design: provider‐randomized trial Unit of allocation: provider Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: primary care; USA Health professionals: 36 (but 27 contributed patients); various type (general internists, family physicians, nurse practitioners); fully trained Patients: 132; major depressive disorders; male and female |
|
| Interventions |
Multifaceted intervention (Patient‐centered group): patient‐mediated intervention, educational outreach visit, distribution of educational material, audit and feedback Quote: "Table 1 provides the rationale and expected outcomes for each intervention component and a comparison of the two intervention approaches." page 154 Multifaceted intervention (Standard group): patient‐mediated intervention, educational outreach visit, distribution of educational material Quote: "Table 1 provides the rationale and expected outcomes for each intervention component and a comparison of the two intervention approaches." page 154 |
|
| Outcomes | Patient rating of their clinicians participatory decision‐making skills (PDM) (categorical) | |
| Notes |
Additional information Number of approached patients (eligible): 1486 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: within each study site (stratum), a randomization schedule was generated through computer by the study statistician using the Moses and Oakford algorithm page 5, randomization. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was an allocation concealment but the method of concealment was not described in sufficient details to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Interviewers who collected data at 6 and 12 months were masked to clinician and patient intervention assignment. Outcome assessors at 18 months were not blinded to intervention assignment. The 12‐month assessments remained the primary outcome." page 158 paragraph 2 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: see patient flow chart of the study protocol Quote: "Overall, 89 percent (N = 117) of the sample completed the 6‐month interview, 85 percent (N = 113) completed the 12‐month interview, and 55 percent (N = 73) completed the 18‐month interview. Follow‐up rates for standard and patient‐centered groups were as follows: 88 percent versus 90 percent at 6 months and 83 percent versus 88 percent at 12 months. There were no significant differences in characteristics between participants who completed the trials and those who were lost to follow‐up." page 162‐163 In addition: reasons for missing data were similar across groups. |
| Selective reporting (reporting bias) | High risk | Comment: see table 3 and 4 of the study protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: see Table 3. |
| Protection against contamination? | Unclear risk | Comment: allocation was by clinician within a clinic. |
| Baseline characteristics patients | Low risk | Quote: "patients in then standard group had higher mean scores on readiness for treatment (7.2 vs. 6.6, p = .02). A higher proportion of patients in the patient‐centered group received care from an African American clinician (race‐concordant relationship) (61.2 percent vs. 6.2 percent, p < .001)." page 162 Quote: "However, because race concordance between clinicians and patients is an important predictor of patient‐reported outcomes (Cooper et al. 2003a), it was included as a covariate. Clinician age and patient attitudes were also included as covariates in separate mean models to examine the robustness of main inferences." page 161 |
| Baseline characteristics healthcare professionals | Low risk | Comment: see table 2 Clinician race was taken into account in the analysis through the race concordance. |
Cox 2017.
| Methods |
Study design: randomized trial Unit of allocation: other Unit of analysis: other Power calculation: not done |
|
| Participants |
Care setting: non‐ambulatory care, specialized care, USA Health professionals: various types; fully trained and in training Patients: 298; hospitalized children (general pediatric hospital services, the pulmonary service, and the hematology/oncology service) for breathing problems, gastrointestinal problems, and fever; male and female |
|
| Interventions |
Multifaced intervention : Distribution of educational material and Educational meeting (FRC Cheklist intervention), page 2
Quote: "To optimize implementation, the checklist was bundled with a 1‐hour interactive training, a brief refresher training, tools to monitor implementation, and laminated checklists for use as prompts, constituting the FCR checklist intervention (toolkit available at www. hipxchange. org/ familyrounds)." page 2 Usual care: page 2 Usual care services (the other hospitalist service and the pulmonary service) |
|
| Outcomes | Family engagement communication (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One research team member (R.L.B.) used a computer algorithm to randomly designate intervention (1 hospitalist service and the hematology/oncology service) and usual care services (the other hospitalist service and the pulmonary service)." page 2 |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment method is not clearly described. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "Coders were blinded to intervention or usual care status. Page 8 Coders were blinded, but they may have been able to distinguish between arms after coding multiple videos." page 3 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Comment: insufficient information to make a judgement. |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes pre‐specified in the study protocol were reported in the results (NCT02625142). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Low risk | Seem similar according to Table 1 Descriptive Characteristics of the Participants and Study Outcomes for Usual Care and Intervention Services, Pre‐ and Postintervention. |
| Protection against contamination? | Low risk | Practices (service) were randomized. |
| Baseline characteristics patients | Low risk | Usual care and intervention arms were comparable across numerous patient and parent characteristics. The only significantly different characteristic was length of stay (χ2, P = .04) (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | No report of characteristics. |
Coylewright 2016.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: ambulatory care, specialized care, USA Health professionals: 36; various types; fully trained; male and female Patients: 132 (124 included in analysis); stable coronary artery disease; male and female |
|
| Interventions |
Single intervention ‐ Patient mediated intervention and educational meeting
Quote: "The decision aid arm included use of a paper‐based decision aid that was stratified by angina type (CCS class I–II angina versus class III angina; Figure 2A and 2B). The decision aid was designed with a user‐centered approach for use during the clinical encounter (in visit), and its development is described elsewhere." page 769
Educational meeting: Quote: "Training sessions were given on the decision aid to all participating clinicians in the form of several grand round presentations and at the time of initial consent into the study. In addition, the study coordinator and principal investigator offered just‐in time training before each visit to review decision aid content and recommendations for its use; this took 1 to 3 minutes. A video was created demonstrating use of the decision aid and was available for viewing." page 769 Usual care (control group) |
|
| Outcomes | OPTION (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study coordinator then randomized the patient to UC versus decision aid (Figure 1), with a dynamic allocation balanced across sex and presence of type 2 diabetes mellitus. The randomization took place on a secure study website using a computer generated allocation sequence, which randomized patients in a concealed fashion to decision aid versus UC." page 768 |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization took place on a secure study website using a computer‐ generated allocation sequence, which randomized patients in a concealed fashion to decision aid versus UC." page 768 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Quote: "Blinding was not possible for patients and involved clinicians, given physical presence of the decision aid." page 768 Comment: we did not know if coders were blinded |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Quote: "Because of the fact that less than half of clinic visits were recorded (decision aid: 34/65, 52%, and UC: 20/59, 37%, total 45%), these results are deemed hypothesis‐generating only. Reasons for a lack of recording were not formally documented and ranged from clinician, patient, or family preference or lack of availability of recording equipment because of simultaneous patient enrollment." page 773 Comment: missing outcome unbalanced between groups and we don't know if reasons of not recording are well balanced between groups. |
| Selective reporting (reporting bias) | High risk | Comment: primary outcome measures: Efficacy of the PCI choice decision aid vs. usual care assessed by patient and provider surveys, and encounter Video/Audio recordings (time frame: baseline to three months) Efficacy in improving measures of patient knowledge and involvement, decision making quality, treatment choice and clinician satisfaction of the decision aid. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of primary outcome. |
| Protection against contamination? | Low risk | Patients were randomized but, Quote: "Detailed analysis of the recorded visits suggested high fidelity of decision aid delivery and did not demonstrate evidence of contamination." page 774 |
| Baseline characteristics patients | Low risk | Quote: "There were no significant differences in any of the baseline characteristics." page 770 |
| Baseline characteristics healthcare professionals | Low risk | Comment: clinical visits per clinician for usual care and DA are similar (Table 2). |
Davison 1997.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: specialized care and ambulatory care (Winnipeg Community Clinic); Canada
Health professionals: 2; urologist; fully trained Patients: 60; prostate cancer; men |
|
| Interventions |
Single intervention; patient‐mediated intervention (individual empowerment sessions) This session helped them to think on how to discuss with the doctor what treatment is best for them and what questions to ask the physician. Single intervention (control); patient‐mediated intervention (information package) A list of questions, also found in the empowerment session. |
|
| Outcomes | Perceived level of control in the decision‐making process (categorical); joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 60 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The group to which subjects were assigned was predetermined by a block randomization procedure" n.p. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: for our primary outcome (n = 60). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "At the pre‐test, no significant differences were found between the role preference of the groups (Chi2 = 4.365, P = 0.113)." page 194 |
| Protection against contamination? | High risk | Comment: it was the patients who were randomized in one community clinic. |
| Baseline characteristics patients | Low risk | Quote: "The two groups were not significantly different from one another with reference to: age category, years of education, education category, marital status, residence, days from first interview, and intended/received treatment" n.p. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Davison 2002.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: ambulatory care, specialized care, Canada Health professionals: various types; fully trained Patients: 749 (736 for assumed role 734 for type of assumed role); oncology, breast cancer, female |
|
| Interventions |
Single intervention : patient MI ( computer)
Section Procedure: Quote: "The first part of the computer program used the Control Preferences Scale developed by Degner 35 to elicit patients’ preferences for control over treatment decision making. The tool consists of 5 statements about different roles individuals can assume in treatment decision making. After an introductory screen provides the patient with instructions, two statements appear on each screen in a fixed order. When the patient makes a choice between the two statements, the next two statements appear on the screen... The second part of the computer program is based on a paper‐and‐pencil survey questionnaire previously developed and validated with a group of women with breast cancer. The 9 information categories include chances of cure, spread of disease, side effects, treatment options, social activities, effect on family; family risk, home self‐care, and sexuality." page 3 Single intervention ‐ control group : patient‐mediated intervention (discussion with research nurse) Quote: "Women in the control group did not use the computer program. They were asked to select the 1 statement from the 5 statements of the CPS that best described their preferred roles in decision making with their physicians that day. The RN talked to this group of women about general issues for the same length of time it took to generate the computer printouts (approximately 15 minutes)." page 3 |
|
| Outcomes | Assumed role in decision (CPS) (categorical) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subject were assigned in the order of accrual." page 4 |
| Allocation concealment (selection bias) | Low risk | Quote: "Separate consents were used to conceal group assignment because only women in the experimental group used the computer program." page 4 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: not enough information about missing data and how they were treated. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Other bias | Unclear risk | Comment: selection bias: perhaps only women who were contemplating treatment‐related discussions with their physician should have been recruited into this study. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: prior to the intervention, all women completed short demographic questionnaires. No other measures before the computer program. |
| Protection against contamination? | High risk | Comment: Patients were randomized |
| Baseline characteristics patients | Unclear risk | Quote p.7 : « Women in the control group were more likely to have less than a high school education». The autor also said : « The two groups were remarkably similar» |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Deen 2012.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary care; specialized care and ambulatory care (health center); USA Health professionals: not mentioned in paper Patients: 279; no one particular type of clinical condition; 103 males and 176 females |
|
| Interventions |
Four arms:
Quote: "Individuals agreeing to participate provided informed consent and were then randomly assigned to one of 4 groups: no intervention (control = data collection and doctor visit), pre‐visit exposure to a PAI, pre‐visit exposure to the DA, and pre‐visit exposure to both DA and the intervention (DA + PAI). The DA selected for this project, ..., to impart general information to patients about their role in gaining information and care within a medical setting." page 179 |
|
| Outcomes | Patient Activation Measure (PAM); the fostering by healthcare professional of active participating of patients in the decision‐making process. | |
| Notes |
Additional information Number of approached patients (eligible): 945 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Individuals agreeing to participate provided informed consent and were then randomly assigned to one of 4 groups." page 179 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: no information about the number of participants excluded in the analysis in the study arms. Exclusion of participants after the randomization may not preserve the benefit of randomization. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "Pre and post‐visit data were collected in the CHC waiting room prior to and following a physician visit" |
| Protection against contamination? | High risk | Comment: It was the patients who were randomised |
| Baseline characteristics patients | High risk | Comment: gender and race/ethinicty were not evenly distributed across the study arms (page 182). Moreover, PAM scores were associated with ethnicity (table 1). Analysis did not adjust for these variables. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Deinzer 2009.
| Methods |
Study design: non‐randomized trial Unit of allocation:patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: specialized palliative care, non‐ambulatory care, Germany Healthcare professionals: >15 (total only reported in intervention group); physicians: fully trained Patients: 86, hypertensive, male and female |
|
| Interventions |
Multifaceted intervention: educational meetings (training for physicians), patient‐mediated intervention (patient education program); training for physicians with 4 special consultations Quote: "The SDM interventions were performed ... by physicians who had undergone special communication Training ... " page 267 "Subjects in both the SDM and control groups took part in the patient education program which consisted of modules on the main topics of hypertension ..." page 267 Single intervention: patient‐mediated intervention (patient education program) Quote: "Subjects in both the SDM and control groups took part in the patient education program which consisted of modules on the main topics of hypertension ..." page 267 Quote: "Physicians of control patients were just informed about patient empowerment." page 267 |
|
| Outcomes | COMRADE (continuous, score); SDM is assessed as the joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: non‐randomised trial. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: n for outcomes were not specified in the paper. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "The degree of SDM was significantly higher in the SDM group at baseline and after 1 year visits." page 268 |
| Protection against contamination? | Low risk | Quote: "Physicians of control patients did not take part in such a special communication program thereby avoiding any contamination with the SDM group." page 267 |
| Baseline characteristics patients | Low risk | Comment: see table 2. Differences in duration of HTA may be due to chance alone. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Deschamps 2004.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary and ambulatory care (a family medicine clinic); Canada
Health professionals: unknown number; general practitioners; unclear level of training Patients: 128; hormone replacement therapy; female |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (pharmacist consultation, patient‐specific information and a 40‐minute consultation with pharmacist) and other (a letter to the patient's physicians). The letter to the physician highlights the decision made during the pharmacist consultation. Single intervention (control): patient‐mediated intervention (decision aid: "Making choices: hormones after menopause") The decision aid package was created by the Ottawa Health Decision Centre; it describes both the risks and the benefit of the therapy or therapies. |
|
| Outcomes | Perceived level of control in the decision making process (categorical); joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Volunteers were randomly assigned to one of the two study arms." page 22 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: patient‐mediated intervention and patient reported outcome, so the patient was not really blinded. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: 24/67 missing (35,8%, pharmacist consultation) vs 13/61 missing (21,3%, decision aid). |
| Selective reporting (reporting bias) | Unclear risk | Comment: No evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: No evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Comment: see Table 1. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Dolan 2002.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: primary and ambulatory care (two practices in Rochester New York); USA
Health professionals: 6, general internist; 5 fully trained and 1 in training Patients: 96; colorectal cancer screening patients; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (preliminary phase + detailed analysis of the decision using the analytic hierarchy process (decision aid) Quote: "The preliminary phase describes colorectal cancer, the study, administers a demographic survey, ask about family and personal history, established past screening and patients' preference and a knowledge test." pages 126 ‐ 127) Single intervention (control): patient‐mediated intervention (preliminary phase and educational phase) Quote: "The educational phase consisted of a short description of colorectal cancer and the 5 screening programs for average risk patients." page 127 |
|
| Outcomes | Perceived level of control in the decision making process (categorical); Joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 178 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All randomisation schedules were created using a computer random number generator before the onset of patient enrolment." page 126 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: patient‐mediated intervention and patient reported outcome, so the patient was not really blinded. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: 43/50 missing (86%, experimental) vs 43/47 missing (91%, control). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "There were no significant differences between study groups in pre‐intervention views about how screening decisions should be made (chi‐square = 4.54 df=2 P = 0.10) or in patients' perception about how decisions should be made (Chi2 = 2.1 df = 2 P = 0.34)." page 132 |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Comment: see Table 1. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Eggly 2017.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: ambulatory care, specialized care, USA Health professionals: 18; specialists; fully trained; male and female Patients: 114; breast, colon, or lung cancer; male and female |
|
| Interventions |
Single intervention (QPL‐only) ‐ patient MI
Quote: "QPL booklet : The QPL was a booklet designed to be accessible to patients with low levels of education and health literacy. The booklet included 43 questions related to diagnosis, treatment, chemotherapy, side effects, daily life during treatment, treatment plan and schedule, help with costs, and help with coping." page 820 Single intervention (QPL + Coach) ‐ patient MI Quote: "Discussion with a communication coach. Coaches were three Black female research staff trained to use a strategy developed by the investigators called'GPS: Generate, Prioritize, Summarize.' Specifically, they read each question aloud (“generate”), and then asked patients whether they wanted to ask the oncologist this question, and why or why not (“prioritize”). Coaches reviewed questions patients indicated wanting to ask (“summarize”), asked if there were other questions they wanted to ask, and offered the opportunity to practice asking the questions." page 821 Usual Care (control group) Quote: "Patients assigned to the usual care arm (Arm 1) did not receive the QPL booklet or any other intervention." page 821 |
|
| Outcomes | Patient role in treatment decision (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following completion of baseline measures, the software randomized patients (1:1:1) to either the usual care arm or one of two intervention arms (QPL‐Only or QPL‐plus‐Coach)." page 820 |
| Allocation concealment (selection bias) | Low risk | Quote : "Interested patients met with research staff to provide consent and complete baseline measures, using a tablet device with survey software (Qualtrics©). Following completion of baseline measures, the software randomized patients (1:1:1) to either the usual care arm or one of two intervention arms (QPL‐Only or QPL‐plus‐Coach)." page 820 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: no report about if patients were aware or not of their arm. Insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: for the outcome Patient Role in Treatment Decision the sample size was n = 85, and the total sample size was n = 114. Eight patients were excluded after randomization because they provided incomplete responses to baseline or outcome measures. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient information to make a judgement |
| Other bias | Unclear risk | The possibility of a selection bias exists. Quote : "Also, many eligible patients could not be reached, and among those who were contacted, only half agreed to participate. Thus, the possibility of a selection bias exists; however, an analysis of zip codes of participants and nonparticipants suggested they came from areas with similar socio‐demographic characteristics." page 825 |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment : no baseline mesures for Patient Role in Treatment Decision |
| Protection against contamination? | High risk | Comment : Patients were randomized Quote: "Patients assigned to the QPL‐Only arm (Arm 2) received the QPL booklet, along with a brief explanation and encouragement to read it, show it to friends and family, and bring it to the visit because “asking questions during medical visits is important." page 821 |
| Baseline characteristics patients | Low risk | Comment : Patient characteristics across study arms are similar (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: Insufficient information to make a judgement. |
Elwyn 2004.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: provider (one per practice) Unit of analysis: provider Power calculation: done |
|
| Participants |
Care setting: primary care; ambulatory care (usual practice and protected research clinics; urban and rural in Gwent, South Wales); UK Healthcare professionals: 21; general practitioners; fully trained Patients: 747 included in COMRADE, 352 in OPTION; non‐valvular atrial fibrillation or prostatism or menorrhagia or menopausal symptoms; male or female |
|
| Interventions |
Multifaceted intervention: educational meeting (SDM skills) and audit and feedback; 5 hours Practitioners attended two workshops. During the first workshop, the background literature on SDM was outlined and participants were asked to debate its relevance to clinical practice. The skills of SDM were described and demonstrated using simulated consultations. This provided opportunities for all the participants to comment on the method, using an observational competence checklist. Simulated patients were also encouraged to comment. Participants were asked to consult with the simulated patients using pre‐prepared scenarios involving the study conditions. At the second workshop, participants were asked to consider the competences in more depth. By the end of the workshop, all participants had conducted and received feedback from at least one consultation with a simulated patient. Multifaceted intervention (control): educational meeting (risk communication skills) with audit and feedback; 5 hours A risk communication aid was presented for the four study conditions. The risk data were based on systematic reviews and presented as the best evidence available at the time of the trial. The participants were provided with treatment outcome information for the study conditions. Participants were asked to use them in simulated patient consultations. The consultations were conducted in pairs, where colleagues alternated between clinician and observer roles. This was repeated until each participant had received feedback after conducting two or three consultations using the risk communication aids across a range of conditions. A plenary group discussion, which included the patient simulators, allowed the group to share learning points and consider the application of the materials in clinical practice. |
|
| Outcomes | OPTION (continuous); SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process COMRADE (continuous); joint process between healthcare professionals and patients to make decisions |
|
| Notes |
Additional information Number of approached patients (eligible): 2585 Number of patients per physician:12 or 24 patients per physician according the phase (baseline, first and second intervention) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All randomizations were undertaken by random number generation, and allocations by the trial statistician (KH) were concealed from those implementing the interventions or assessments." page 339 |
| Allocation concealment (selection bias) | Low risk | Comment: unit of allocation was by provider or practice. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "All consultation recordings were intended to be rated by two raters and rating were undertaken blind to study group allocation of clinicians or patients." page 340 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Both clinicians and patients were informed that the trial was investigating "communication skills" but were otherwise blinded to the decision‐making or risk communication focus of the intervention." page 339 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: one summary measure for all physician. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: follow‐up was not clear in the 2 articles. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Low risk | Quote: "Unit of allocation is the provider. Only one practitioner per practice would be recruited." page 338 |
| Baseline characteristics patients | Low risk | Comment: see Table 1 in Edwards et al. page 351 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no mention of provider characteristics in the two papers. |
Epstein 2017.
| Methods |
Study design: randomized trial Unit of allocation: provider Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory care, specialized care, USA Health professionals: 38; specialists; fully trained; male and female Patients: 265; non hematologic cancer; male and female |
|
| Interventions |
Multifaced intervention Patient MI + educational meeting
Quote: "(1) a 2‐session in‐office physician training (1.75 hours) using a brief video, feedback from standardized patients portraying roles of patients with advanced cancer, audio recorded study patient visits, and (2) a single 1‐hour patient and caregiver coaching session incorporating a question prompt list to help patients bring their most important concerns to their oncologist’s attention at an upcoming office visit, plus up to 3 follow‐up phone calls (Table 1; eTable 2 in Supplement 3). Trainers and coaches underwent 3‐day on‐site training. To promote patient centered communication about disease course, prognosis, treatment decisions and end‐of‐life care, physician and patient interventions focused on the same 4 key domains of patient centered communication." page 94 Usual care Quote from the abstract : "Control participants received no training." |
|
| Outcomes | Health Care Climate Questionnaire (HCCQ) (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the study statisticians were aware of the random number sequences and treatment assignment, preserving blinding among transcriptionists, coders, and abstractors." Page 95 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: missing outcome data were not specified. |
| Selective reporting (reporting bias) | High risk | Comment: some relevant outcomes prespecified in the study protocol were not reported in the results: preferred role and actual role in decision making, PEACE (NCT01485627). |
| Other bias | Low risk | |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: insufficient information to permit judgement. |
| Protection against contamination? | Unclear risk | Comment: professionals were allocated within a clinic or practice and it is possible that communication between intervention and control professionals could have occurred. |
| Baseline characteristics patients | Low risk | Comment: see Table 1: no statistically significant differences between intervention and control for pre‐randomization or cluster‐RCT |
| Baseline characteristics healthcare professionals | Low risk | COmment: eTable 1: no statistically significant differences between intervention and control for pre‐randomization or cluster‐RCT |
Feng 2013.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: waiting area Unit of analysis: provider Power calculation: unclear |
|
| Participants |
Care setting: primary care; ambulatory care; USA Health professionals: 118; general practitioners;fully trained Patients: prostate cancer screening; male |
|
| Interventions |
Three arms: Distribution of educational material (intervention A): Quote: "...intervention physicians were exposed to an interactive, 30‐minute, Web‐based curriculum that included interactive roulette wheels, illustrative video vignettes, and other content to illustrate the potential harms, benefits, and downstream consequences of receiving prostate cancer screening, as well as methods of enhancing shared decision making." page 316 "Intervention physicians were further divided into those who participated in the intervention (intervention A), and those who participated in the intervention and had up to 3 of their regular clinic patients activated to discuss prostate cancer screening by participating in a similar patient‐focused, Web‐based tool immediately before a scheduled clinic visit (intervention B)." page 316 Patient mediated intervention, distribution of educational material (intervention B): Quote: "Intervention physicians were further divided into those who participated in the intervention (intervention A), and those who participated in the intervention and had up to 3 of their regular clinic patients activated to discuss prostate cancer screening by participating in a similar patient‐focused, Web‐based tool immediately before a scheduled clinic visit (intervention B).The patient intervention included video vignettes that depict the potential harms and benefits of undergoing prostate cancer screening." page 316 Distribution of educational material (brochure, control): "Control physicians received a brochure on prostate cancer screening that was distributed by the Centers for Disease Control and Prevention..." page 316 |
|
| Outcomes | Prostate Cancer Screening Abstraction Tool (continuous) | |
| Notes |
Additional information Number of approached physicians (eligible): 130 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment:iInsufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | High risk | Comment: as treated analysis with 19,5% of physicians allocated to intervention A who moved to control group and 22,2% of physicians allocated to intervention B who moved to control group. |
| Selective reporting (reporting bias) | Low risk | Comment: the study is registered (under the number NCT 00207649) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | Unclear risk | Comment: insufficient information to make a judgement. |
| Baseline characteristics patients | Unclear risk | Comment: not reported. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: not clear in the paper. |
Fiks 2015.
| Methods |
Study design: randomized trial Unit of allocation: other Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: ambulatory care, primary care, USA Health professionals: various types; unclear level of training and gender Patients: 60; pediatric asthma; unclear gender |
|
| Interventions |
Multifaced intervention : patient‐mediated intervention + reminder (EHR‐linked SDM portal (MyAsthma) Quote: "MyAsthma was developed with input from families and clinicians with the goal of fostering ongoing SDM. MyAsthma provided decision support to both clinicians and parents. The clinician interface appeared seamlessly in the EHR, and the parent interface appeared seamlessly within MyChart, the EHR vendor’s patient portal. The features of MyAsthma (Supplemental Appendix 1) include identification of parents’ concerns and goals for asthma treatment; monthly tracking of symptoms, medication side effects, and progress toward goals; asthma educational content including videos; and access to the child’s asthma care plan. Parents were encouraged with E‐mail reminders to complete monthly portal surveys with input from their affected child (Supplemental Appendix 2). In response to these surveys, families and clinicians received guideline‐based decision support that directed them to speak to one another if asthma was not well‐controlled or if there were side effects, or to continue current therapy." page e966 Single intervention ‐ reminder Quote: "Families in the control group did not have access to the portal; however, clinicians caring for control group children had access to a clinician focused decision support system proven effective in fostering guideline‐based care." page e967 |
|
| Outcomes | Parent Patient Activation Measure (PPAM‐13) (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make judgement. Quote: "A randomization sequence was generated by the study coordinator (SLM)." page e966 |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes were used to ensure blinding of study staff to treatment condition before enrollment and randomization." page e966 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Patients were not blinded. Quote: "Sealed envelopes were used to ensure blinding of study staff to treatment condition before enrollment and randomization." page e966 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Quote (discussion): "We found that more than half of intervention families completed portal surveys for at least 5 of the 6 study months and 77% completed the survey more than once." page e970 Quote (bottom of table 4 ): "At baseline, n = 30 for each group. At follow‐up, n = 26 for intervention, n = 27 for control." page e970 Comment: missing data similar in the 2 groups. The remaining 7 families were unable to be reached by phone or E‐mail. |
| Selective reporting (reporting bias) | High risk | Comment: some relevant outcomes prespecified in the study protocol were not reported in the results: preferred role and actual role in decision making, PEACE (NCT01715389): Observing Parent Involvement (OPTION) scale. |
| Other bias | High risk | Selection bias: study participants were a convenience sample; some were recommended by their primary care providers and others were enrolled based on EHR rosters.
Quote: "...because this study was confined to practices within 1 health system with an interest in improving asthma care, this sample may not be representative of the larger population of children with asthma." page e971 Because of the small number of subjects in this study, randomization did not fully balance asthma severity between intervention and control subjects." page e971 |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: there were no significant differences in baseline control, quality of life, or parent activation between the 2 study arms (page 2 for all comparisons). |
| Protection against contamination? | High risk | Comment: Family were randomized within practices |
| Baseline characteristics patients | Low risk | Comment : See table 1 Quote: "No significant differences between intervention and control groups" (bottom of table 1) ; Quote: "We did not observe any significant difference between frequent users and other intervention families in demographic characteristics;" page e968 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Fossli 2011.
| Methods |
Study design: clinician‐randomized, cross‐over trial Unit of allocation: clinician Unit of analysis: clinician Power calculation: done |
|
| Participants |
Setting of care: primary care, ambulatory care; Norway Healthcare professionals: 72; various types of physician (residents, consultants, medical surgeons, neurologist, podiatrists, gynecologist), fully trained and residents Patients: not reported |
|
| Interventions |
Multifaceted intervention: educational meeting, distribution of educational materials, Audit and feedback after role‐play Quote: "Doctors participated in the 20 hours (a 45 min) course over two consecutive days. …The course consisted of a 50/50 mix of theory and 45 min group sessions (3–7 participants and two teachers per group) including role‐plays, with plenary debriefs after each group." page 2 "Our course was based on the same content as the 5‐day course Communication Skills Intensive offered by Kaiser Permanente." page 2 "At the conclusion of the course, all participants received a one‐sheet overview of the Four Habits to carry in their pockets as reminder in everyday work." page 3 Usual care (control) |
|
| Outcomes | Four Habits Coding Scheme (continuous, score); SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported, planned for eight video consultations per physicians |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The doctors were randomized to receive the intervention in the summer of 2007 or the winter of 2008." page 3 |
| Allocation concealment (selection bias) | Unclear risk | Comment: unit of allocation is the doctor within a clinic. No details on allocation procedure. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "Raters were blinded to all information about the doctors and the encounters, including whether the video was made before or after the intervention." page 3 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: reasons for missing outcome data unlikely to be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases |
| Baseline measurement? Observer‐based outcome | Low risk | Quote: "All included doctors had two encounters videotaped before the first course (period A ‐ baseline)." page 2 |
| Protection against contamination? | Unclear risk | Comment: doctor were allocated within a clinic. |
| Baseline characteristics patients | Unclear risk | Comment: not reported |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No comparison between intervention and control group |
Hamann 2007.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: group of providers for wards Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: specialized and non‐ambulatory care (12 acute psychiatric wards of two state hospitals); Germany
Health professionals: unknown number; specialists (psychiatrists) Patients: 107; schizophrenic; male and female |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (decision aid) + educational meeting with nurses, aided by various charts, lasting 30‐60 minutes. A nurse assisted the patient work through the decision aid. Patients met with their physician 24 hours after having consulted the decision aid. Usual care (Control) |
|
| Outcomes | COMRADE (continuous); Joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization of the wards", page 993 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization of the wards", page 993 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: missing data on outcomes (see table 4 of Hamann 2006) but insufficient reporting to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper |
| Protection against contamination? | Low risk | Comment: wards were randomized, patients remained in their respective wards. |
| Baseline characteristics patients | Low risk | Comment: see table 1 of reference 11 (Hamann 2006). Covariates that were unbalanced were adjusted in the analysis. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Hamann 2011.
| Methods |
Study design: patient‐randomized trial (pilot) Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: specialized and non‐ambulatory care; Germany
Health professionals: unknown number; specialists (psychiatrists) Patients: 61; schizophrenia and schizoaffective disorder; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (SDM training) Quote: "The training consisted of five one hour sessions for a group of five to eight patients. The content of the training was derived from theoretical considerations about patients’ contributions to the shared decision‐making process, from an adaptation of related approaches from somatic medicine, and from pilot testing the training. The training sessions included motivational aspects (such as prospects of participation) and behavioral aspects (including role‐play exercises). The training emphasized interaction between moderators and patients as well as mutual support. All sessions were led by a psychiatrist and a psychologist, neither of whom was in charge of the specific care of these patients." page 1218 Single intervention (Control): patient‐mediated intervention (cognitive training) Quote: "Patients in the control condition participated in a five‐session cognitive training group." page 1218 |
|
| Outcomes | Patients were asked who was making important medical decisions concerning their health (continuous); joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information about the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Comment: numbered closed‐allocation concealment envelopes. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Comment: see protocol in clinical trial register (masking: open‐label). |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: for our primary outcome. 25% in intervention group and 12% in control group. No reasons for missing data to permit judgement. |
| Selective reporting (reporting bias) | Low risk | Comment:the study is registered at clinicaltrial.gov (under the number NCT01313013) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | High risk | Comment: randomization was made by patient within one single practice. |
| Baseline characteristics patients | Unclear risk | Comment: characteristics are mentioned in text but no data were reported. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Hamann 2014.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: specialized and ambulatory care; Germany
Health professionals: 1, specialist, fully trained Patients: 100; affective disorders; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention Quote: "The QPS for outpatients with an affective disorder was developed by four experienced clinicians (two psychiatrists, two psychologists)...The final version of the QPS was a one‐page leaflet in which patients were encouraged to behave actively in the consultation (‘Make the best out of the consultation’), to write down notes about their wishes for today’s consultation and to tick up to 15 standard questions that were provided on the QPS (e.g. ‘What is my diagnosis?’, ‘What treatment options are still available for my complaints?’ etc.). Finally, the QPS stated that patients could refer to the leaflet during the consultation..." page 228 Usual care (Control): patient‐mediated intervention (cognitive training) Quote: "Patients in the control condition went to the consultation without receiving the QPS." page 228 |
|
| Outcomes | Patients self‐report of who made the decision during the day's consultation (continuous); Third‐party assessment of who made the decision during the day's consultation (continuous). | |
| Notes |
Additional information Number of approached patients (eligible): 152 Number of patients per physician: 100 (only one physician) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to make a judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Every patient was given a numbered, sealed, allocation concealment envelope that contained allocation to their group and all study materials." page 228 column 2 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Quote: "All consultations were audio‐taped and subsequently analysed." page 229 column 1 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: I cannot assume that all patient replied to all questions related to outcomes. Missing outcome data were not specified. |
| Selective reporting (reporting bias) | High risk | Comment: participant flow was not fully drawn and missing outcomes data were not specified. One outcome was not specified but reported in table 2: Quote: "what influence did you have on what had been decided during the consultation?" Oucomes like: number of questions ticked among the 15, number of patients who ticked at least 1 question in the QPS, topics raised in the QPS and factors associated with the number of questions ticked were not pre‐specified in the paper. As median and range are mostly reported, it will be difficult to compute an effect size for a meta‐analysis. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | Low risk | Quote: "All patients in the intervention group were provided with the QPS prior to the consultation… and were asked to work through it in a separate room." page 228 column 2 |
| Baseline characteristics patients | Low risk | Comment: reported and similar (table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Hamann 2017.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: non‐ambulatory care, specialized care, Germany Health professionals: specialists; fully trained Patients: 264 (215 included in analysis); psychiatric hospital ‐ schizophrenia; male and female |
|
| Interventions |
Single intervention : patient mediated intervention (SDM Training for patients)
Quote: "5‐session training (60 min/session) addressing patient competencies for SDM. "The group was led by a psychiatrist (J.H. or A.P.), who was not involved in the patients’ treatment, and another mental health professional (e.g. nurse, psychologist) and comprised 5–8 patients. The content of the group builds upon conceptual and empirical research on patient competences in the medical encounter (e.g. [15, 16]) and had been subject to extensive pilot testing. The intervention follows a structured manual which is available on request from the authors. Group sessions took place twice a week and addressed the following topics:
• Patient rights
• Prospects of SDM (better health)
• Communication skills (asking questions, information
provision, being assertive)
• Preparing for ward rounds and consultations
The skills were introduced and rehearsed using role plays and homework (e.g. pose a question to the doctor in charge, prepare oneself for the next ward round). Patients in the control condition received a 5‐session cognitive training (finding differences, completing lists etc) including also elements of euthymic therapy (e.g. 'using all five senses')but with no reference to doctor‐patient communication." page 176 Single intervention : patient mediated intervention (Training for patients) 5‐sessions of cognitive training with no reference to doctor‐patient communication |
|
| Outcomes | Who makes important decision about your medical treatment? (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information to make judgement about sequence generation. Quote: "Separate randomization lists for every study center (block size = 4) and numbered closed allocation concealment envelopes were generated prior to the study by our statistical department. Patients were recruited until group size was reached, then randomized to the intervention/control condition." page 177 |
| Allocation concealment (selection bias) | Low risk | Quote: "numbered closed allocation concealment envelopes were generated prior to the study by our statistical department." page 177 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "As to the nature of our intervention patients were not blinded. Psychiatrists in charge who also did the ratings were neither informed about allocation of their patients nor intentionally blinded." page 177 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: did not mention how missing data was treated (105 between T1 and T3). Responsibility for decision‐making, N = 192 ; Responsibility for decision‐making, N = 118 ; Responsibility for decision‐making, N = 87 Quote: "49 patients dropped out of the trial during the inpatient and intervention phase, most of them because they were suddenly discharged or left the hospital against their doctor’s advice, and were therefore excluded from the analysis." page 177 |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to make a judgement. Protocol not available |
| Other bias | High risk | Quote: "Selection bias = As only patients who were judged to tolerate a 60 min intervention were recruited, we surely had a recruitment bias towards less ill patients which results in a possible lack of generalizability of our data." page 179 |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measures. |
| Protection against contamination? | High risk | Comment: patients were randomized. |
| Baseline characteristics patients | Low risk | Quote: "Apart from duration of illness there were no significant differences between the intervention and the control group with regard to socio‐demographic or clinical variables at baseline." page 177 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no characteristics reported. |
Haskard 2008.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: provider Unit of analysis: provider Power calculation: not done |
|
| Participants |
Care setting: primary care; ambulatory care (a west coast university medical centre, a Department of Veterans Affairs clinic and a staff model HMO); USA Healthcare professionals: 156; from three primary care specialties, Various type of physician (obstetrics/gynecology, family medicine, internal medicine); fully trained (87) and in training (69) Patients: 2196; various clinical conditions; male or female |
|
| Interventions |
Multifaceted intervention (physician and patient trained arm): educational meeting + distribution of educational materials + patient‐mediated intervention; 20 hours and 20 minutes. Physician received a 3X6 hours interactive workshop over a period of 3 months. The first workshop focused on core communication skills in healthcare (engaging; empathising; educating patients of diagnosis, prognosis, and treatment; and enlisting patients in mutually agreed upon treatment plans). The second workshop focused on patient adherence, enhancing patients’ health lifestyles, reducing health risk behaviours, and building confidence and conviction in patients to make healthy behaviour changes. The third workshop focused on sources and nature of interpersonal difficulties between clinicians and patients, recognizing and assessing tension in relationships, acknowledging problems, discovering meaning, showing compassion, setting boundaries, and helping patients find additional support. Each workshop was followed by the utilization and distribution of educational materials about the main topic covered during the workshop. Patient received a 20‐minute waiting room pre‐visit intervention. This intervention involved listening to audio CD with accompanying patient guide book focusing on planning and organizing concerns and questions for physician and encouragement to discuss treatment choices, negotiate best plan, repeat their understanding of the plan, follow up of care with their physician, asking questions about medications, tests, procedures, and referrals. Multifaceted intervention (physician only trained arm): educational meeting + distribution of educational materials; 20 hours See the above description for the physician intervention Single intervention (patient only trained arm): patient‐mediated intervention; 20 minutes See the above description for the patient intervention No intervention (control) |
|
| Outcomes | Physician‐patient global rating (continuous). SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision making process | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: up to 24 patients per physician |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… physicians were randomised to one of four conditions using a computer‐generated random order." page 515 |
| Allocation concealment (selection bias) | Unclear risk | Comment: unit of allocation is the provider and not separated by practice. No details on allocation procedure. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: some physician dropped out before training was completed and post training assessments. Reasons were balanced across groups. Although reasons for loss to follow up were not reported, loss to follow up were quite balanced in numbers across groups. Proportion of missing data was 18.6%. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Unclear risk | Comment: unit of allocation is the provider and not separated by practice. |
| Baseline characteristics patients | Unclear risk | Comment: not reported |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: there is mention of participant characteristics on page 514 but comparison between intervention and control arms were not presented. |
Hess 2012.
| Methods |
Study design: patient randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: tertiary care; ambulatory care, USA Health professionals: 102; physicians, residents; fully trained and in training Patients: 204; chest pain; male and female: 120 females, 84 males |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (one brief demonstration of the use of the decision aid) and educational meeting (one hour training session) Quote: "Participating clinicians were oriented during a 1‐hour training session given by the lead investigator (E.P.H.) as well as a brief (3 min) demonstration from the study coordinator on how to use the decision aid before meeting the first enrolled patient and as needed." page 252 No intervention, standard care (control) |
|
| Outcomes | Observing Patient Involvement (OPTION) scores; The fostering by healthcare professionals of active participation of patients in the decision‐making process | |
| Notes |
Additional information Number of approached patients (eligible): 310 Number of patients per physician: 208 patients for 51 clinicians |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised to either usual care or shared decision making through a Web‐based, computer‐generated allocation sequence in a 1:1 concealed fashion …" page 253 |
| Allocation concealment (selection bias) | Unclear risk | Comment: the centralized randomization scheme was unclear |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "Third investigator (H.H.T.), who was also blinded to allocation, reviewed all potentially positive outcomes." page 254 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: 3 missing outcomes in DA and 5 in control group. Reasons unlikely to be related to our outcome of interest. |
| Selective reporting (reporting bias) | Low risk | Comment: pre‐specified relevant outcomes were reported in the results (see clinical tria.gov NCT 01077037). |
| Other bias | Low risk | Comment: no other evidence of risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Low risk | Comment: it was the patients who were randomized in an ED Hospital. |
| Baseline characteristics patients | Low risk | Comment: see table 1 for patient baseline characteristics, page 255 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Hess 2016.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory care, primary care, USA Health professionals: 361; various types; fully trained Patients: 913 (898 included in analysis); emergency: low risk chest pain; male and female |
|
| Interventions |
Multifaceted intervention ‐ Patient mediated intervention (Decision aid) + reminder (quantitative pretest probability web‐based tool)
Quote: "For patients randomized to the decision aid, a study
coordinator collected each of the variables needed to populate the quantitative probability web tool, 14 asked the treating clinician to sign off on their accuracy, and calculated the patient’s pretest probability of acute coronary syndrome, incorporating the result of the first troponin test but prior to subsequent biomarker testing (fig 2). After selecting the decision aid corresponding to the appropriate level of risk, the study coordinator offered to provide the clinician with a concise refresher of the content. The treating clinician, after evaluating the patient and the results of the initial ECG and cardiac troponin tests, then used the decision aid to educate the patient about the results of the two tests, the potential need for observation and further cardiac testing, subsequent cardiac troponin testing to definitively rule out acute myocardial infarction, if required, and their personalized 45 day risk for acute coronary syndrome. The clinician then engaged the patient in selecting the management option most closely aligned to his or her values and preferences." page 2 Control group ‐ usual care Quote: "For patients randomized to usual care, a study coordinator instructed the clinician to discuss the results of diagnostic investigations and management options according to the clinician’s usual manner. Clinicians treating patients in the usual care arm did not have access to the quantitative probability web tool or to the decision aid. As the trial was intentionally pragmatic in design, usual care was not standardized." page 4 |
|
| Outcomes | OPTION (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: online randomization algorithm |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed by an online password protected randomization algorithm (Medidata Balance; Medidata Solutions, New York City, NY)." page 2 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "Patients, study coordinators, and treating clinicians were not masked to allocation. All other investigators were blinded to allocation." Coders were blinded." page 2 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Quote: "The main reasons recordings were not obtained were clinician and patient refusal and technical difficulties with recording equipment."
Quote: "We were unable to obtain video recordings in 40% of the encounters." page 9 Comment: we do not know how missing outcome data are balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Quote: "There is only one primary outcome for the study: patient knowledge. The sentence “Test if Chest Pain Choice safely improves validated patient‐centered outcome measures” refers to the five additional outcome measures listed as secondary outcomes at clinicaltrials.gov (a through e) and is redundant. This is documented in the study protocol, which was published prior to completion of enrollment for the trial." (ClinicalTrials.gov NCT01969240), page 5 |
| Other bias | Low risk | Comment: no evidence of other risk of biases |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of primary outcome. |
| Protection against contamination? | Low risk | Randomisation was done by patients. Quote: "To limit the risk of contamination, the quantitative pretest probability web tool was password protected, and coordinators did not provide clinicians access to the decision aid. However, even if contamination were to occur, this would bias the results of the trial toward the null, and we observed a positive effect of the intervention despite the potential for contamination." page 9 |
| Baseline characteristics patients | Low risk | Quote: "There were no significant differences in baseline characteristics between the study arms." page 6 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no characteristics reported. |
Härter 2015.
| Methods |
Study design: provider randomized trial Unit of allocation: provider Unit of analysis: provider and patient Power calculation: unclear |
|
| Participants |
Care setting: ambulatory and non‐ambulatory care, Germany Health professionals: 86; type: specialists; level of training: unclear Patients: 160; breast and colon cancer; male and female |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (decision aid) and educational meeting (training) Quote: "Physicians in the intervention group participated in shared decision making training consisting of 12 training units, including a unit on the use of patient decision aids." page 673 Usual care (control) Quote "Physicians in the control group provided treatment as usual." page 673 |
|
| Outcomes | Observing Patient Involvement (OPTION) scores (continuous); SDM‐Q‐9 (continuous); Patient perception scale (PPS) (categorical). | |
| Notes |
Additional information Number of approached physicians (eligible): 900 Number of patients per physician (mean): at T1 4 in the intervention group and 6 in the control group; at T2 3 in the intervention group and 5 in the control group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Physicians were randomized to the intervention group or control group at a ratio of 1:1 by an independent statistician, using a computer‐based procedure." page 674 |
| Allocation concealment (selection bias) | Low risk | Quote: "Physicians were randomized to the intervention group or control group at a ratio of 1:1 by an independent statistician, using a computer‐based procedure." page 674 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Patient were blinded to the group to which they had been randomized." page 674 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: although there are more than 20% of lost to follow‐up at T1 among physicians, lost to follow‐up are balanced in number and reasons across groups (24 vs 29) (OPTION scale was assessed at T1). |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: insufficient reporting of attrition/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Low risk | Comment: the study is registered in the German Clinical Trials Register (under the number DRKS00000539) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of outcome. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of outcome. |
| Protection against contamination? | Unclear risk | Comment: insufficient information to make a judgement. |
| Baseline characteristics patients | Low risk | Comment: reported and similar (table 1) |
| Baseline characteristics healthcare professionals | Low risk | Comment: reported and similar (table 1) |
Jouni 2017.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: ambulatory care, USA Health professionals: 6; various types; fully trained; unclear gender Patients: 207; coronary heart disease; male and female |
|
| Interventions |
Single intervention : patient‐mediated intervention
(CRS: 10‐year risk of CHO based on conventional risk factors alone)
Quote: "The Statin Choice decision aid was originally developed to disclose CHD risk and help patients as well as clinicians review the benefits and downsides of taking a statin medi cation to reduce CHD risk. The tool displays the 10‐year probability of CHD based on CRS in addition to the absolute risk reduction with the use of statin drugs, and the associated costs/side effects. lt can be freely accessed online at http://statindecisionaid.mayoclinic.org.The modified tool can be accessed online at http://migenesstudy.mayoclinic.org ( password: migenes‐use of this decision aid should be limited to research purposes only). Afterwards, the provider can discuss the benefits of starting standard versus high dose statins as well as potential sicle effects ( figure 3). CHD risk was disclosed using scripted language as follows: 'Out of 100 people like you ...' The benefit of statins was conveyed in a similar manner stressing the absolute risk reduction while minimizing framing by presenting the groups helped and not helped by using statins." page 683 Single intervention : patient‐mediated intervention (CRS + GRS: conventional risk factors alone with a genetic risk score) Quote: "The tool was also equipped with a report generating function and a frequently asked questions page that includes additional information about GRS." page 683 |
|
| Outcomes | SDM‐Q and OPTION5 (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomization was performed by means of a computer‐generated random sequence with stratification for age, gender, and positive family history for CGD using the Pocock and Simon method." page 682 |
| Allocation concealment (selection bias) | Low risk | Quote: "One of the investigators (HJ) generated the random allocation sequence and study arm assignment using the computer software described earlier." page 682 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Quote: A random sample of 40 CRS encounters and 40 age‐ and sex‐matched CRS*GRS encounters was obtained and video recordings were analyzed by one of the authors (TSM). page 683 Comment : it is not said if the author was blinded. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "The study sample size was relatively small and the intervention was not blinded." page 1186 (primary article) |
| Incomplete outcome data (attrition bias) Observer‐based outcome | High risk | All encounters were not recorded. Quote: "Encounters with the genetic counselor and physician were video‐recorded in 187 patients who consented to the recording." page 682 Quote: "A random sample of 40 CRS encounters and 40 age‐ and sex‐matched CRS*GRS encounters was obtained and video recordings were analyzed by one of the authors (TSM)." page 683 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: The SDM survey was completed by 206 study participants, and the physician visit satisfaction survey was completed by al study participants (one missing data for SDM). |
| Selective reporting (reporting bias) | High risk | Comment: some relevant outcomes pre‐specified in the study protocol were not reported in the results (Trial registration number NCT01936675). |
| Other bias | Unclear risk | Quote: "Our study participation had higher than average educational and socioeconomic background and may have been more adept in understanding genetic results." Comment: Selection bias ; Study participants were recruited from the Maya Clinic BioBank and may not be fully representative of the general population." page 687 |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of primary outcome. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of primary outcome. |
| Protection against contamination? | High risk | Comment: patients were randomized. |
| Baseline characteristics patients | Low risk | Quote: "Baseline characteristics including age, sex, smoking status, and other CHD conventional risk factor were similar between the two groups." page 684 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no characteristics reported. |
Kasper 2008.
| Methods |
Study design: patient randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: specialized care and ambulatory care (Hamburg University Hospital); Germany
Health professionals: unknown number; physicians; unclear level of training Patients: 297; multiple sclerosis; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (decision aid including a patient information booklet about immunotherapy options and an interactive workshop) The decision aid was formulated after assessing patients' needs and determining its feasibility. Single intervention (control); patient‐mediated intervention (decision aid consisting of a standard information package) This information can be found on the Internet. |
|
| Outcomes | Perceived level of control in the decision‐making process (categorical); joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 304 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by concealed allocation using computer generated random numbers." page 1346 |
| Allocation concealment (selection bias) | Low risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "To preserve blinding assessors explicitly asked patients not to refer to details of the information materials … However, [the treating physicians] were not informed about their patient's allocation and did not receive the patient information." Page 1347 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: missing outcomes are not well‐balanced in number and reasons but the proportion of missing outcome is 6,4% and the ratio of participants with missing data to participants with events (SDM) is 0,17. |
| Selective reporting (reporting bias) | Low risk | Comment: see protocol ISRCTN25267500. Relevant pre‐specified outcomes were included in the analysis. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "In the intervention, 18 preferred shared and 122 prefer another style, in the control group 34 prefer shared, 109 prefer another style. This yields a Chi2 value of 5.96, p<0.05." page 1349 |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Comment: see table 2 and 3 and the support for judgement of the criteria baseline outcome. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Kennedy 2013.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: practice Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary care; ambulatory care; UK Health professionals: various type (doctors, nurses, technicians, administration staff); fully trained Patients: 5599; diabetes, COPD, irritable bowel syndrome; male and female |
|
| Interventions |
Single intervention: educational meeting Quote: "Training (developed and piloted with two non‐trial practices) was delivered in each practice over two sessions, which we estimated through informed feedback was the maximum feasible in UK primary care using current educational structures." page 2 Usual care (control): Quote: "We used a wait list comparator group." page 3 |
|
| Outcomes | Shared decision making (short‐form healthcare climate questionnaire) ‐ 12 month vs baseline (continuous). | |
| Notes |
Additional information Number of approached practices (eligible): 51 Number of patients per cluster (mean): 121 in the intervention group, 151 in the control group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a minimisation procedure based on practice size…" page 3 |
| Allocation concealment (selection bias) | Low risk | Comment: practices were randomized |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "There is no blinding of patients or outcome assessors, although all outcomes are self‐report." study protocol page 6 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: missing outcome data balanced in proportion across intervention groups (71,9% vs 73,5%) but not in number (646 vs 877). No reasons for missing data were provided. Missing outcomes were not imputed but I do not know if to address it is widely acknowledged (and what the assumption underlying the use). However, a sensitivity analysis has been performed.CM6 |
| Selective reporting (reporting bias) | High risk | Comment: results of cost‐effectiveness analysis that was prespecified in the study protocol were not reported. Techniques for treatment of missing data was not prespecified in the protocol; moreover, assumptions underlying the techniques used were not reported in the paper. One prespecified secondary outcome: management options was not reported. The authors treated the 6‐month score of the 3 main outcome measures and self care activity as additional secondary outcomes but it was not prespecified in the study protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: no difference across groups for our primary outcome (Table 1). |
| Protection against contamination? | Low risk | Comment: practices were randomized. |
| Baseline characteristics patients | Low risk | Comment: Reported and similar (Table 1) |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Koerner 2014.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: practice Unit of analysis: provider and patient Power calculation: unclear |
|
| Participants |
Care setting: specialized care; non‐ambulatory care; Germany Health professionals: 363; various type (physicians, nurses, psychosocial therapists, physical therapists); fully trained Patients: 1326; chronic diseases; male and female |
|
| Interventions |
Single intervention: educational meeting Quote: "The train‐the‐trainer programme ‘Fit for Shared Decision‐Making’ (see Appendix 1, available online) was implemented in the intervention clinics after the first data collection period (preintervention)..." page 22 Usual care (control): Quote: "...whereas the control clinics were offered training after the data collection had been completed in all clinics (waiting control group)." page 22 |
|
| Outcomes | SDM‐Q‐9 score (continuous) | |
| Notes |
Additional information Number of approached practices (eligible):92 Number of patients per practice: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We put the name of each clinic of a pair on a piece of paper, placed them in a bag so that the name could not be seen and drew out one name which was allocated to the intervention group. The other was placed into the control group. One centre was not matched,and was allocated to the intervention group, as we expected more cancellations in the intervention." page 22 |
| Allocation concealment (selection bias) | Low risk | Comment: practices were randomized. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "The patients were not aware of which group they were in… but the study coordinators and those analysing the data… were." page 22 column 2 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: see: flow chart and comments in the text, data analysis (missing values) and limitation of the study in the discussion. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: see Table 5. |
| Protection against contamination? | Low risk | Comment: practices were randomized. |
| Baseline characteristics patients | Low risk | Comment: reported and similar (table 2). |
| Baseline characteristics healthcare professionals | Low risk | Comment: reported and similar (table 3). |
Korteland 2017.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: ambulatory care, specialized care, the Netherlands Health professionals: specialists; fully trained Patients: 155; prosthetic heart valve selection; male and female |
|
| Interventions |
Single intervention : patient MI (PDA decision tool)
Quote: "The final PDA is an online tool (www.hartklepkeuze.nl) and contains 2 sections: an information section on heart function, heart valve disease, available heart valve prostheses, the operation, living with a heart valve prosthesis, and hyperlinks for further information; and the actual PDA, which is made up of 7 parts: (1) introduction and personal information (patients may optionally enter age and sex), (2) information on the 2 options (mechanical or biological valve), (3) a comparison of the options (if patient has entered age and sex, then age‐ and sex‐specific estimates of the lifetime risk of bleeding with a mechanical prosthesis and reoperation with a biological valve are displayed), (4) exploration of personal feelings about the 2 options, (5) a knowledge quiz, (6) exploration of patient preference, and (7) a summary of the results of the PDA that can be printed or e‐mailed for use in the doctor’s office." page 2 Usual care : standard preoperative care (control group) |
|
| Outcomes | Involvement in decision making (qualitative) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned (1:1) to standard preoperative care or standard preoperative care plus additional use of the PDA with permuted block sizes of 10, stratified by center. The randomization sequence was generated by an independent statistician using a random number generator." page 4 |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocations were placed in serially numbered, opaque, sealed envelopes by 2 independent research assistants." page 4 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "Because of the nature of the intervention, it was not possible to blind investigators and patients to the allocation." page 4 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: missing outcome data were not specified for our primary outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes prespecified in the study protocol are reported in the results (NTR4350). |
| Other bias | Unclear risk | Comment 1: not all randomized patients completed the preoperative questionnaire, which may have resulted in selection bias. Comment 2: selection bias page 9 Quote: "Mainreasons were the absence of a computer at home, a language barrier (the Netherlands has an increasingly diverse population with many nationalities and cultural backgrounds)" Comment: Desirability ?? Quote: "Questionnaires were completed at home, and patients may have been influenced by family members or friends." page 8 |
| Baseline measurement? Participant‐reported outcome | Unclear risk | COmment: no baseline measure of primary outcome. |
| Protection against contamination? | High risk | Comment: patients were randomized. |
| Baseline characteristics patients | Low risk | Quote: "As there seemed to be potential imbalances in the baseline characteristics preoperative consultation and involved in prosthetic valve choice, we first performed an ordinal regression analysis to assess the effect of the use of the DA on the primary outcome DCS without correction for these potential imbalances, and next, a multivariable ordinal regression analysis with the 2 baseline characteristics included." page 4 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no characteristics reported. |
Krist 2007.
| Methods |
Study design: patient randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: primary care and ambulatory care (1 large family practice centre in suburban northern Virginia); USA
Health professionals: 29; family physicians; 13 fully trained and 16 in training Patients: 497; prostate cancer screening; male |
|
| Interventions |
Single intervention: patient‐mediated intervention (mailed paper version of the decision aid) The brochure duplicated the content of the website. Single intervention (control): patient‐mediated intervention (Internet‐based decision aid) The web‐based decision aid was created by the author and reviewed by experts, presents evidence of prostate cancer. No intervention (control) |
|
| Outcomes | Perceived level of control in the decision‐making process (categorical). Joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 1073 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the time of enrolment, the allocation was concealed from the coordinator … the coordinator referred to pre‐generated randomisation tables to inform the participant to which arm he was randomised." page 113‐114 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation was concealed from the coordinator…" page 113 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: patient‐mediated intervention and patient‐reported outcome, so the patient was not really blinded. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Quote: "Questionnaires were completed by 87% of patients and 91% of physicians overall." page 114 |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Comment: baseline demographics for the control, brochure, and Web site groups were similar (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Krones 2008 (ARRIBA‐Herz).
| Methods |
Study design: clinician‐randomized trial Unit of allocation: clinician Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary care; ambulatory care (CME groups in Hessen); Germany Health professionals: 91; family doctors; fully trained Patients: 1132; cardiovascular; male and female (Krones 2008) |
|
| Interventions |
Multifaceted intervention: educational meeting, audit and feedback, distribution of educational materials, educational outreach visit Educational meeting two 2 hr sessions (risk of CVD, ethics of SDM, practical communication strategies), audit and feedback (after role‐play feedback was given by their peers), distribution of educational materials (ARRIBA‐Heart counseling sheet), educational outreach (CME members were invited to moderate the sessions). Quote: "In the sessions they discussed epidemiological background of global cardiovascular disease risk calculation and ethics of SDM. ... emphasis on practical communication strategies ... Use of script‐like decision aid was practiced through role play, participants received feedback from their peers ...." page 324 The participating family doctors were taught how to moderate a session Single intervention (control):placebo educational meeting Quote: "Family doctors in the control arm were offered seminars on defined alternative topics that would not interfere with CVD prevention." page 324 |
|
| Outcomes | Patient Participation scale, SDM‐Q; Joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): NA Number of patients per physician: at least one patient per physician (Hirsch 2010) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization to intervention or control group was stratified by the rural or urban location of member practices…" page 219 |
| Allocation concealment (selection bias) | Low risk | Comment: CME groups (group of providers) were randomized |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Quote: "Patients were informed that different kinds of risk communication and decision support would be assessed; they were unaware of their physicians' group allocation.." page 219 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: 19% of patients were lost to follow up; 16.3% in one group and 19.9% in the other. 37.8% vs 41 % of the lost to follow‐up patient were lost because their physicians no longer participated in the study. Reasons for the rest of the lost to follow‐up patients were not documented. In addition, reasons for study discontinuation by physician were not reported. 50% vs 50% of physicians/practices were analyzed (balanced) although reasons of discontinuation may be related to the outcomes... The proportion of missing outcome among the physicians is very high and may have a potential impact on the results. Missing data for our primary outcome are not specified. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | High risk | Quote: "Patients' participation preference in decision making also differed significantly in the 2 study arms, which might represent a selection bias in the intervention effect." page 222 |
| Protection against contamination? | Low risk | Comment: the intervention was stratified in accordance to CME groups. |
| Baseline characteristics patients | Low risk | Comment: see table 3. Quote: "…we included diabetes… in addition to CVD… as covariate in our analysis… page 222 |
| Baseline characteristics healthcare professionals | Low risk | Comment: see table 2. Quote: "Because there were slight imbalances with regard to family doctors' age and practice size, we included these characteristics in all multivariate analyses…" page 222 |
Köpke 2014.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory care; Germany Health professionals: type: unclear; level of training: unclear Patients: 192; clinical isolated syndrome or definite relapsing‐remitting multiple sclerosis; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (interactive‐4‐hour education program) Quote: "The intervention group (IG) received an interactive 4‐h education programme, presenting the best available evidence regarding diagnostic testing in MS, prognosis of MS and early MS DMD therapy (table 1)." Page 412 Single intervention: patient mediated‐intervention (4‐hour MS‐specific stress management program) Quote: "To control for unspecific attention effects and enable patient blinding, control group (CG) participants took part in a 4‐h MS‐specific stress management programme led by a specially trained psychologist." page 412 |
|
| Outcomes | Decision autonomy (qualitative) | |
| Notes |
Additional information Number of approached patients (eligible): 252 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We performed a double‐blind randomised controlled trial with a follow‐up of 12 months using computer‐generated randomisation lists for concealed allocation of participants by external central telephone." page 411 |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment method is not clearly described. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Participants were blinded to study groups as they were not informed about the ‘active’ intervention. Outcomes were assessed via blinded telephone calls and mailed questionnaires." page 412 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: For our primary outcome (decision autonomy) proportion of missing values are balanced in number and proportion across groups: 23 (24,7%) vs 27 (27,3%). But reasons for missing were not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: there is no evidence that outcomes were selectively reported (all relevant outcomes in the trial registry are reported in the results section). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: incomplete information to permit judgement. |
| Protection against contamination? | High risk | Comment: patients were randomized. |
| Baseline characteristics patients | Low risk | Quote: "Baseline demographics were similar between groups (table 2). Results from the cognitive items of the quality of life and the disability assessment indicate few participants with important cognitive impairment with no differences between groups (data not shown)." page 413 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Lalonde 2006.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: primary care and ambulatory care (10 community pharmacies in Montréal); Canada
Health professionals: unknown number; pharmacist; unclear level of training Patients: 26; cardiovascular problems; male and female |
|
| Interventions |
Multifaceted intervention: distribution of educational materials (decision aid + personal risk profile) + patient‐mediated intervention (decision aid) The decision aid is made of a booklet providing general information on the illness, the risk factors and lifestyle change and treatment option.Quote: "A four‐step decision making strategy is suggested ( Page 52)". It also included a personal worksheet which summarizes their risk and allows them to create an action plan. Multifacted intervention (control); distribution of educational materials (decision aid + personal risk assessment) + patient‐mediated intervention (personal risk profile) The risk profile identifies the patient risk factors and estimates a 10‐year CVD risk, changing as the patient changes their risk factors. It also includes a four‐page information handout. |
|
| Outcomes | Decision satisfaction inventory (continuous). Joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 42 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was stratified by community pharmacy." page 52 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: In all, 88% of the patients were included in the follow‐up. Missing data were balanced in number across groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | High risk | Comment: imbalances between patient characteristics (table 1 and p54). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Landrey 2012.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not clear |
|
| Participants |
Care setting: primary care and ambulatory care, USA
Health professionals: 44, physicians; fully trained Patients: 303; prostate cancer screening; male Males with no history of prostate cancer |
|
| Interventions |
Single intervention (mailed flyer), patient‐mediated intervention Quote: "One week prior to their upcoming annual health maintenance visits, eligible patients were randomised to receive a mailed flyer (intervention group) or no flyer (usual care group)." page 2 No intervention (control) |
|
| Outcomes | Control Preference Scale (CPS). Joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): 752 Number of patients per physician: 303 patients for 44 providers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomized to receive a mailed flyer (intervention group) or no flyer (usual care group)." page 2 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Two research assistants blinded to group assignment collected chart outcome information by reviewing clinic notes following patient appointment." page 2 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: missing outcomes were balanced in numbers across groups. At the follow‐up survey 50% of outcome were missing and reasons for lost to follow‐up were not documented. |
| Selective reporting (reporting bias) | Low risk | Comment: prespecified outcomes in the protocol were reported in the results. NCT01516801 |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | High risk | Comment: there was no baseline, a follow‐up telephone survey consisting of 13 items was conducted within 2 weeks of the clinic visit. |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Quote: "There were no significant study group differences in baseline characteristics." page 69, table 1 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
LeBlanc 2015a.
| Methods | Study design: cluster‐randomized trial Unit of allocation: practice Unit of analysis: provider and patient Power calculation: done | |
| Participants | Care setting: primary care and ambulatory care, USA Health professionals: 117, various types (physician, nurse practitioner, physician assistant (see study protocol page 3)); fully trained and in training Patients: 301; depression; male and female | |
| Interventions | Single intervention: patient‐mediated intervention Quote: "Clinicians in the intervention group were to use the decision aid during the consultation with their patients..." page 1764 Usual care (control): Quote: "...whereas clinicians in the control arm did not have access to the decision aid (usual care)." page 1764 | |
| Outcomes | OPTION (continuous) | |
| Notes | Additional information Number of approached practices (eligible): not reported Number of patients per physician: 2, number of patients per practice: 34; number of clinician per practice: 7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Low risk | Quote: "The lead study statistician therefore stratified practices by their history of accrual and the presence of the DIAMOND [Depression Improvement Across Minnesota ‐ Offering New Direction] program (a practice redesign initiative to improve depression care present in numerous Minnesota practices at the time of the study), and centrally randomized practices within these strata to either care with or without Depression Medication Choice." page 1763 |
| Blinding (performance bias and detection bias) Observer‐based outcome | High risk | Quote: "Study team members, practices, and clinicians were aware of the assigned arms." page 8 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: "There was no difference in the attrition of participants or completeness of the data across arms (Figure 2)." page 11 |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Comment: No evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment:No baseline measure of our primary outcome. |
| Protection against contamination? | Low risk | Comment: practices were randomized. |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 1). |
| Baseline characteristics healthcare professionals | Low risk | Comment: reported and similar (See table 1). |
LeBlanc 2015b.
| Methods | Study design: cluster‐randomized trial Unit of allocation: patient Unit of analysis: provider and patient Power calculation: done | |
| Participants |
Care setting: ambulatory care, primary care, USA Health professionals: 41; general practitioners; male and female; fully trained Patients: 79; osteoporosis; female |
|
| Interventions |
Single intervention: patient‐mediated intervention (Decision aid) Quote: ''The intervention in the first arm (Decision Aid) consisted of the use of the Osteoporosis Choice decision aid by the clinician and patient during the clinical encounter (Fig 1). The decision aid included (a) the individualized 10‐year risk of having a bone fracture (estimated using the FRAX calculator) with and without use of bisphosphonates (i.e., showing the absolute reduction with bisphosphonates) represented using an evidence‐based pictograph and assuming a treatment‐related reduction in overall fractures of 40%[10]; and (b) potential harms and other downsides of using bisphosphonates.'' page 3 Single intervention : reminder Quote: ''The intervention in the second arm (FRAX) consisted of giving clinicians a copy of the patient’s individualized 10‐year risk of having a bone fracture estimated using the FRAX calculator before the visit for use during the clinical encounter.'' page 4 Usual care (control) Quote: ''In the third arm (Usual Care), clinicians discussed risk of fractures and treatment as usual without any research‐related intervention. No specific guidance was provided to support decisions about non‐pharmacological interventions to reduce falls and fractures in any of the three arms.'' page 4 |
|
| Outcomes | OPTION (qualitative) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were the unit of randomization and were allocated using a computer‐generated sequence that randomized them 1:1:1 in a concealed fashion." page 5 |
| Allocation concealment (selection bias) | Low risk | Quote: "... using a computer‐generated sequence that randomized them 1:1:1 in a concealed fashion" page 5. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Quote: "Patients and clinicians were aware of the overall objective, presented as improvement in communication between patients and clinicians during the clinical encounter, but remained blinded to the specific aims." page 5 "After randomization, only data analysts remained blind to allocation." page 5 Comment: however authors did not talk about blinding of those who coded OPTION. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Comment: insufficient information about our primary outcome to make a judgement. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol was registered (Identifier: NCT00949611). The main outcomes pre‐specified in the protocol were reported in the results. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | No baseline measure of the primary outcome. |
| Protection against contamination? | Low risk | Quote: "there was no evidence of contamination as clinicians in the FRAX/Usual Care arm covered significantly fewer items [17%, 95%CI (12, 23), t‐test p< 0.0001]." page 9 |
| Baseline characteristics patients | Low risk | Caracteristics between groups were similar at baseline (table 1). |
| Baseline characteristics healthcare professionals | Low risk | Similar repartition between groups (Table 1). |
Leighl 2011.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: specialized care,aAmbulatory care; Australia, Canada Health professionals: 13 oncologists; fully trained Patients: 207, advanced colorectal cancer; male and female: 120 males, 87 females |
|
| Interventions |
Multifaceted intervention: Patient‐mediated intervention (decision aid), physician training (educational meeting) Decision aid: booklet with accompanying narration on an audiotape or CD Quote: "The DA used in this study was developed as a booklet with accompanying narration on an audiotape or compact disc for patients to take home ... Oncologists were trained to use the DA during the consultation and instructed to have patients return after the initial consultation for a final treatment decision as part of the study." page 2079 No intervention, (control): standard consultation |
|
| Outcomes | Modified Control Preferences Scale. Joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 229 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization lists, stratified by the consulting oncologists, were computer‐generated…" page 2078 |
| Allocation concealment (selection bias) | Low risk | Quote: "…and the code was concealed in a sealed envelope until the time of random assignment." page 2078 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: losses to follow‐up were balanced in number and percentage across group (overall) but reasons for lost to follow‐up were not reported. Missing data related to our primary outcome are not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: see table 1 for measurement prior to consultation, page 2078 |
| Protection against contamination? | Low risk | Quote: "Those receiving the DA were counselled not to share it with others in the waiting room to avoid contamination of the standard arm. To further minimize contamination between the arms, five consultations were audiotaped before study commencement as a baseline for comparison with consultations in the standard arm." page 2078 |
| Baseline characteristics patients | Unclear risk | Comment: "Patient characteristics were well balanced between the groups (Table 2), although more patients randomly assigned to receive the DA reported English as their first language." page 2080. Comment: language may be related to some outcomes namely patient understanding and decision involvement but the authors did not specified if they adjusted that variable in the multivariate analysis. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Loh 2007.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: provider Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: primary care and ambulatory care (Department of Primary Care at University Hospital of Freiburg); Germany
Health professionals: 30; primary care physicians; fully trained Patients: 405; depressive disorders; male and female |
|
| Interventions |
Multifaceted intervention: educational meeting with physicians and patient‐mediated intervention (decision aid as well as a patient information leaflet); 20 hours(educational meeting) Quote: "Physician followed modules (lectures, round discussions, facilitation practice, role‐play, videos, standardized case vignettes and case studies) for guidelines concerning depression care, including how to how to include patients in the decision. The SDM portion was based on the works of Towle and Godlphin, as well as those of Elwyn and colleagues." page 326 The physicians were given the decision aid and patient information leaflet to be used during the consultation. The patient's leaflet was based on the Clinical Practice Guideline on Depression in Primary Care of the Agency for Health Care and Policy. No intervention (control) |
|
| Outcomes | Man‐Son‐HIng Instrument (continuous). joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… were randomly assigned by drawing blinded lots under supervisions of the principal investigators…" page 326 |
| Allocation concealment (selection bias) | Unclear risk | Comment: unit of allocation is the provider and not separated by practice. No details on allocation procedure. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: 29,1% of patient were lost to follow‐up (27.4% in intervention group vs 32,4% in control group) but reasons were not documented for pre and post‐intervention phases. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: baseline measurements for our primary outcome were reported. |
| Protection against contamination? | Unclear risk | Comment: unit of allocation is the provider and not separated by practice. |
| Baseline characteristics patients | Low risk | Quote: "Statistically significant differences were found between groups and measurement points for age, family status and educational level; therefore all outcome analyses were controlled for these variables." page 329, table1 |
| Baseline characteristics healthcare professionals | Low risk | Quote: "Gender, age, and professional experience did not differ significantly between the study groups (p > .10)." page 328 |
Légaré 2012.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: family practice teaching units Unit of analysis: family physicians and patients Power calculation: done |
|
| Participants |
Care setting: primary care (family practice), ambulatory care, Canada Health professionals: 270 family physician; teachers and residents; fully trained and in training Patients: 712; acute respiratory infections; male and female |
|
| Interventions |
Multifaceted intervention: educational meeting, distribution of educational materials (online tutorial and workshop) Quote: "DECISION+2 consisted of a 2‐hour online tutorial followed by a 2‐hour on‐site interactive workshop" Usual care (control): Quote: "Physicians in the control group were asked to provide usual care" Page E728 |
|
| Outcomes | Control Preference Scale (CPS). Joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A biostatistician used Internet‐based software to simultaneously randomise all 12 family practice teaching units to either the intervention group (DECISION+2) or control group." page E728 |
| Allocation concealment (selection bias) | Low risk | Comment: practice were randomized. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: not enough information to make a judgement regarding our primary outcome (M‐CPS). |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes prespecified in the trial register (NCT01116076) were reported in the result paper. |
| Other bias | Low risk | Comment: no evidence of other risk of biases |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: Family physicians' intentions to engage in shared decision‐making … were recorded at baseline and again at the end of the study." page E729 |
| Protection against contamination? | Low risk | Quote: To avoid contamination bias, access to the online tutorial was denied to participants in the control group during the trial." page E728 |
| Baseline characteristics patients | Low risk | Comment: in general, key characteristics of the patients (Table 1) and family practice teaching units and physicians (Table 2) in the DECISION+ 2 group were similar to those in the control group. E730 |
| Baseline characteristics healthcare professionals | Low risk | Comment: in general, key characteristics of the patients (Table 1) and family practice teaching units and physicians (Table 2) in the DECISION+ 2 group were similar to those in the control group. E730 |
Maclachlan 2016.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory care, primary care, Namibia Health professionals: general practitioners Patients: 592; HIV, male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (Trainings for patients) Quote: "The intervention consisted in three, two‐hour trainings in active participation, patient empowerment, and communication." page 621. Usual care (control, wait list) Quote: "Six months after their enrollment date, participants in the control group (Group 2) were also offered training sessions as an ethically important intervention benefit." page 621‐622 |
|
| Outcomes | RIAS for patients (Patient activation and engagement) (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Commnent: not enough information to make a judgement. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Quote: "The same clinicians at each site saw both groups of patients but were blinded to the extent possible as to participant group assignment." page 622. Comment: however authors did not talk about blinding of those who coded RIAS. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | High risk | Comment: level of losses of follow‐up is high: 14% (intervention group) and 9% (control group) of losses to follow‐up. Some of the reasons of follow‐up could be linked to the intervention such as, "withdrew" or "did not return" (these reasons are not well‐balanced between the groups). |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available to permit judgement. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measure of our primary outcome |
| Protection against contamination? | Low risk | Comment: patients were randomized and intervention and control groups were in the same hospital. |
| Baseline characteristics patients | Low risk | Comment: characteristics between groups were similar at baseline (table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about clinicians at baseline. |
Maindal 2014.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patients Unit of analysis: patients Power calculation: done |
|
| Participants |
Care setting: primary care, ambulatory care, Denmark Health professionals: number: not reported; various types; level of training: unclear Patients: 509; type 2 diabetes, impaired fasting glucose/impaired glucose tolerance; male and female |
|
| Interventions |
Multifaceted intervention: patient mediated‐intervention, educational meeting Quote: "GPs of participants in the ADDITION‐Denmark treatment arm were trained to provide target‐driven intensive behavioural and pharmacological treatment for people with Type 2 diabetes." page 978 Quote: "The intervention group received intensive treatment of diabetes and routine care of impaired fasting glucose/ impaired glucose tolerance and an invitation to take part in the Ready to Act health education programme. The programme aimed to promote health‐related action competence including motivation, informed decision‐making, action experience and social involvement. Before the programme, nurses, dieticians, physiotherapists and GPs received formal training in autonomy support, participant‐centred communication and action plan support." pages 978‐979 Single intervention (control): educational meeting Quote: "All general practioners (GPs) in the ADDITION study were trained to motivate and to provide target driven intensive nonpharmacological and pharmacological treatment to people with screen‐detected T2D." page 263 of reference 14 |
|
| Outcomes | PAM‐13 (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): 521 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Comment: the pre‐randomization scheme may include confounding because the participants who did not signed up for the intervention program may imbalance the characteristics between the group and hinder the benefice of the randomization. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Measurements, data entry and laboratory analysis were conducted by people blinded to the participants’ study group allocation." page 979 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: more than 20% of missing data for primary outcome and no reasons for missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes in the trial registry are reported in the results. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | High risk | Comment: patients were randomized. |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Maranda 2014.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: primary care, ambulatory care, USA Health professionals: number: not reported; types: unclear; level of training: unclear Patients: 132; context: not specified; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention Quote: "The patient activation intervention (PAI) objective is to help patients identify medical decisions and the questions that inform those decisions, and then to use that information to prepare questions for their impending doctor visit. The PAI was developed and implemented previously at community health centers in NYC24 and is based on principles that empower patients who are not effective advocates for themselves." page 593 Usual care (control): no exposure to the PAI |
|
| Outcomes | PAM (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): 945 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information about the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment method is not clearly described. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Quote: "Data collection was incomplete for 43 individuals who were excluded from the analysis. Reasons for incomplete data collection included: participant was called in to see the physician before finishing the pre‐ visit questionnaire; participant failing to complete the PAM; or participant left without completing the post‐ visit interview." page 596 Comment: missing outcome data are likely to bias the results because: more participants were lost from the control than from the intervention group (26 vs 16); so the exposure may be associated to the selection ‐ because some excluded participants were called before finishing the pre‐visit questionnaire and some participants failed to complete the PAM, the outcome (PAM) maybe associated to the selection (those patient may have low level of PAM). |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: see Table 1. |
| Protection against contamination? | High risk | Comment: patients rather than professionals were randomized at 1 CHC. |
| Baseline characteristics patients | Low risk | Table 1, Quote: "The control group had a smaller proportion of participants with less than a high school education (45.9%) than the intervention group (58.6%); however, this difference was not statistically significant." page 596 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Mathers 2012.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: practice Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: primary care, ambulatory care, UK Health professionals: number: not reported; various type (doctors and nurses); level of training: unclear Patients: 175; type 2 diabetes mellitus; male and female |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention, educational meeting Quote: "This was a complex intervention comprising three components: PDA; healthcare professional training workshop and use of the PDA in a consultation." page 3 Usual care (control): Quote: "In the control group, the GP and the practice nurse did not receive any training and the PANDAs decision aid was not used. The GPs or the nurses conducted a normal consultation with the patient." page 3 |
|
| Outcomes | Modified control preference scale (categorical) | |
| Notes |
Additional information Number of approached patients (eligible): 182 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was a pragmatic trial and all eligible and willing practices were randomly allocated by a computer to two groups: the intervention group used the PANDAs decision aid when making the specified treatment choices and the control group delivered usual care." page 3 |
| Allocation concealment (selection bias) | Low risk | Quote: "A statistician generated the random allocation sequence while a secretary who was not involved in the research study assigned participants to either the intervention or control groups." page 3, column 1 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "Blinding of the intervention and assessment of the process measures were not feasible in view of the nature of the intervention studied." page 3, column 1 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: missing outcome not enough to have clinically relevant impact on the intervention effect. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available. All primary outcomes were reported. However duration of consultation was not reported though prespecified. In addition, some information is missing in table 9 (SD of the means, denominators of the percentage). |
| Other bias | Low risk | |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: No baseline measure of our primary outcome |
| Protection against contamination? | Low risk | Comment: Practices were randomised |
| Baseline characteristics patients | High risk | Comment: Imbalance with number with diabetic complications (table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Montori 2011.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: physicians and patients Power calculation: done |
|
| Participants |
Care setting: primary care, ambulatory care, USA Health professionals: 60; primary care physicians;fully trained Patients: 100 osteopenia/osteoporosis; 100% of female |
|
| Interventions |
Single intervention: patient‐mediated intervention; decision aid,Osteoporosis Choice decision aid Quote: "The Osteoporosis Choice decision aid provides the patient’s individualized 10‐year risk estimate risk of having a major osteoporotic fracture ... .The decision aid also showed the absolute risk reduction in fracture risk with alendronate, ... In addition, the decision aid described the potential downsides of taking bisphosphonates. The decision aid also prompted further discussion with the question What would you like to do?" page 550 Other single intervention (control): usual care and booklet Quote: "In addition to usual care ... , patients randomised to the control group received the National Osteoporosis Foundation booklet, “Boning Up On Osteoporosis: A Guide To Prevention and Treatment." page 550 |
|
| Outcomes | OPTION to quantify the extent to which clinicians are able to involve patients in the decision‐making process. | |
| Notes |
Additional information Number of approached patients (eligible): 14,060 Number of patients per physician: 13 clinicians enrolled more than one patient; five clinicians enrolled more than two |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated allocation sequence randomised patients 1:1 in a concealed fashion (using a secure study website) to control (usual care booklet) or intervention (Osteoporosis Choixe decision aid)." page 551 |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated allocation sequence randomised patients 1:1 in a concealed fashion (using a secure study website) to control (usual care booklet) or intervention (Osteoporosis Choixe decision aid)." page 551 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "After randomisation, data collectors and data analysts were blind to allocation." page 551 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: 38 (73%) decision aid visits and 32 (66%) usual care visits were video recorded. Reasons for non recording were non agreement. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes were described in the protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | High risk | Comment: not specified in the paper. |
| Protection against contamination? | Unclear risk | Comment: despite of the exploration of possible clinician contamination descriptively, the unit of allocation is the patient and not separated by the 10 general medicine. |
| Baseline characteristics patients | Low risk | Comment: see table1, balanced risk factors. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Mullan 2009.
| Methods |
Study design: clinician‐randomized trial Unit of allocation: clinicians Unit of analysis: patient Power calculation: done |
|
| Participants |
Setting of care: primary care, ambulatory care, USA Healthcare professionals: 40; various healthcare professional and inter‐professional (physicians, physicians assistant, nurse practitioners managing diabetes); Fully trained and residents Patients: 85; diabetes type 2; males and females |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (decision aid used during the clinical encounter); and educational training (how to use decision aid) Quote: "[The Diabetes Medication choice decision aid tool] is designed to enable clinicians to discuss with patients the potential advantages and disadvantages of adding an [antihyperglycemics pharmaceutical] agent." page 1562 « Ideally, the clinician presents all 6 cards [describing the possible side effect of the medication] to the patient and asks which of the cards the patient would like to discuss first. After reviewing and discussing the cards that the patient and the clinician choose [what] to discuss." page 1562 Quote; "The patient receives a copy of the cards in the form of a take‐home pamphlet." page 1562 "Clinicians randomised to the intervention arm received a brief demonstration from the study coordinator on how to use the decision aid prior to meeting the first enrolled patient." page 1562 Single intervention (control): patient‐mediated intervention (decision aid). Quote: "... 12‐page general pamphlet on oral antihyperglycemics medication to take home." page 1562 |
|
| Outcomes | OPTION (continuous, score) and validated pictorial instrument ; SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process. | |
| Notes |
Additional information Number of approached patients (eligible): 1341 Number of patients per physician: at least one, page 1563 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated allocation sequence, unavailable to personnel enrolling patients or clinicians, randomized clinicians to intervention (decision aid) or usual care and was accessed by the study coordinators via telephone." page 1562 |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated allocation sequence, unavailable to personnel enrolling patients or clinicians, randomized clinicians to intervention (decision aid) or usual care and was accessed by the study coordinators via telephone." page 1562 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: 18 vs 16 visits were recorded. In both groups reasons were either because patient/clinician did not wish to be recorded or technical difficulties. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: lost to follow‐up patients were balanced in number and reasons across groups (fig 2). |
| Selective reporting (reporting bias) | High risk | Comment: the PROM was not reported (validated pictorial instrument). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Unclear risk | Comment: unit of allocation is the clinician and not separated by primary care and family medicine sites. |
| Baseline characteristics patients | Low risk | Comment: see table 1. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: insufficient data about provider in table 1. There are more physicians and nurses in the DA group. |
Murray 2001.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: primary care and ambulatory care (33 practices in two urban areas (Oxford and London), one suburban area (Harrow),and one in a semi‐rural area (Thames and the Chilterns); UK
Health professionals: unknown number; general practitioners; level of training unclear Patients: 112; benign prostatic hypertrophy; male |
|
| Interventions |
Single‐intervention: patient‐mediated intervention (decision aid); 60 minutes Information of the decision aid HealthDialog interactive videodisc on options, outcomes, clinical problem, outcome probability, and other's opinion. Usual care (control) |
|
| Outcomes | Percived level of control in decision making process (categorical); joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 159 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule, stratified according to recruitment centre, was generated by computer." page 3 |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocations were sealed in opaque numbered envelopes, opened by the study nurse after collection of the baseline data." page 3 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: "In all, 91% patients were included in the follow up. However, imbalance in reasons, number and proportion of lost to follow up patients (5,2% vs 23,6%). Reasons for lost to follow in the control group at 3 months were not specified." page 4 |
| Selective reporting (reporting bias) | High risk | Comment: Time spent during the consultation, patients' choice and satisfaction with the choice, prostatectomy rate were relevant outcome prespecified but not reported in the analysis. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | High risk | Comment: outcome is patient reported and the intervention is patient allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Comment: see table 1. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Murray 2010.
| Methods |
Study design: clinician‐randomized trial Unit of allocation: clinician Unit of analysis: clinician Power calculation: done |
|
| Participants |
Setting of care: specialized palliative care, non‐ambulatory care, Canada Healthcare professionals: 88; various healthcare professional (nurses, pharmacists, non‐nurse case managers, social works); fully trained Patients: 5; simulated patients |
|
| Interventions |
Multifaceted intervention: including educational meetings, audit and feedback, distribution of education materials; educational outreach; barriers assessment. Interventions were chosen to target identified barriers to providing decision support for place of end‐of‐life care and were based on their proven effectiveness in improving practitioners' decision support knowledge and skills Quote: "Three components were delivered over six weeks. The first was an online, self‐directed, module‐based tutorial. ... The second component was a three‐hour skills building workshop … Participants were given feedback on their decision support skills during their baseline standardized calls. Next, participants viewed and rated the quality of decision support ... then they practised providing decision support using the [Place‐of‐care patient decision aid] during role‐playing sessions. ... Based on evidence from social marketing, education outreach was chosen as the third component." page 114 Usual care (control) |
|
| Outcomes | DSAT10 (continuous, score); SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process. | |
| Notes |
Additional information Number of approached patients (eligible): not applicable, the patients are simulated Number of patients per physician: 1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation was conducted through a computer‐generated random numbers table provided centrally by a statistician external to the study." page 114 |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was conducted through a computer‐generated random numbers table provided centrally by a statistician external to the study." page 114 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "DSAT10 scoring was done by one of two raters who were blinded to group assignment." page 115 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: in total 88 consented, 78 were included in the analysis (N = 36 intervention; n = 42 control), yielding a 88% follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes pre‐specified in the trial protocol are reported in the results (trial registry number NCT00614003). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Low risk | Quote: "Baseline scores for non‐retained calls were non significantly different from baseline scores for complete cases (P = 0.866). The baseline score change from baseline …" page 116 |
| Protection against contamination? | Low risk | Comment: separated geographically. |
| Baseline characteristics patients | Unclear risk | Comment: simulated patients. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: characteristics are mentioned in the text but no data were presented. |
Myers 2011.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Setting of care: primary care, ambulatory care, USA Healthcare professionals: 22 physicians; fully trained (board certified practitioners) Patients: 313; eligible for prostate cancer screening; males |
|
| Interventions |
Multifaceted intervention: Including patient‐mediated interventions (pamphlet and counseling) and reminders (prompting) Quote: "... mailed a12‐page information brochure on prostate cancer and screening to all participants." page 241 "The nurse educators met EI Group men at the office visit, reviewed the content of the mailed booklet, and conducted a structured decision counselling session about prostate cancer. [The nurses] elicited factors that were likely to influence the participant's screening decision, align with their relative influence and strength. Then nurse educator then used a hand‐held computer with a pre‐programmed algorithm to compute each participants's decision preference score ..." page 241 "... the nurse educator also placed a generic note on each EI group participant's medical chart to prompt the physician to discuss prostate cancer screening." page 241 Multifaceted intervention: Including patient‐mediated interventions and reminders (prompting) (control). The brochure and the prompt were the same as those in the intervention group. |
|
| Outcomes | Informed decision‐making scale; SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process. | |
| Notes |
Additional information Number of approached patients (eligible): 1245 Number of patients per physician: median number of patients per physician is 8. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using a system of sealed envelopes, the nurse educator then determined the participants's study group assignment to either …" page 241 |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a system of sealed envelopes, the nurse educator then determined the participants's study group assignment to either …" page 241 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Comment: for the entire study, there was an over 90% follow‐up, however, only 50% audio‐recorded encounters (46% in SI group, 55% in EI group); 84% of the audio. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | High risk | Quote: "Certain patients in either the groups received their unassigned interventions." page 242 |
| Baseline characteristics patients | Low risk | Comment: "The data show that the two groups were well balanced on all the measured variables." page 242 (table 2) |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Nannenga 2009.
| Methods |
Study design: provider‐randomized trial (factorial 2 x 2 randomized trial) Unit of allocation: provider and patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Setting of care: specialized care; ambulatory care (clinic for diabetes at Mayo Clinic in Rochester, MN); USA Healthcare professionals: 16; endocrinologists; fully trained Patients: 98; Type 2 diabetes; male or female |
|
| Interventions |
Single intervention: decision aid administered by provider during visit Statin Choice decision aid is a one‐page document tailored to the individual patient including the patients name, cardiovascular risk factors and estimated cardiovascular risk. Benefits and downsides were presented Single intervention: patient‐mediated intervention (decision aid administered by researcher prior to visit). See the above description of the decision aid. Single intervention (control): pamphlet administered by provider during visit. The standard Mayo patient education pamphlet outlined guidelines for reducing hyperlipidemia, cholesterol, and triglycerides without consideration of patient‐specific cardiovascular risk. It defined lipid disorders and provided primarily dietary guidelines for control of cholesterol along with general statements encouraging exercise and smoking cessation. Single intervention (control): patient‐mediated intervention (pamphlet administered by researcher prior to visit). See the above description of the pamphlet. |
|
| Outcomes | OPTION (continuous); SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process. | |
| Notes |
Additional information: Number of approached patients (eligible): 260 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated allocation sequence, unavailable to personnel enrolling patients, randomized providers to intervention (decision aid) or control groups …." (Page 1077, Weymiller) |
| Allocation concealment (selection bias) | Low risk | Quote: "randomized by concealed central allocation to a 2 x 2 cluster factorial design…." page 40 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "Using the videotaped encounters, reviewers blinded to questionnaire result quantified encounter duration and used the OPTION scale to quantify the extent to which clinicians invited patient participation in decision making." page 41 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: 48/52 (intervention); 43/46 (control). |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes pre‐specified in the trial protocol are reported in the results (trial registry number NCT00217061). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper |
| Protection against contamination? | High risk | Comment: professionals and patients were randomized within a single‐centered study. |
| Baseline characteristics patients | High risk | Quote: "Baseline cardiovascular risk factors were generally well‐balanced (Table 1), although the decision aid group had significantly fewer women, greater high school completion, and a higher baseline HbA1C. Distance from place of residence to Mayo Clinic was similar for intervention and control groups." Page 41 |
| Baseline characteristics healthcare professionals | High risk | Comment: Insufficient data about diabetologists |
O'Cathain 2002.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: group of providers Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary care and ambulatory care (maternity units); UK
Health professionals: unknown number; physicians in maternity care and midwives; unclear level of training Patients: 10,070; maternity care; female |
|
| Interventions |
Multifaceted‐intervention: education meeting with staff + distribution of educational materials ; 2 hours (educational meeting) The educational materials consisted of pairs of "Informed Choice" leaflets (given at different periods during gestation) which provided information concerning the benefits and risks of available options concerning labour, and a detailed professional leaflet. The staff in the units receiving the units were trained Usual care (control) |
|
| Outcomes | Perceived level of control in decision‐making process (categorical); joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): 10,070 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Members of pairs were randomly assigned by tossing a coin to receive the set of leaflets (five intervention units) or to the continue with usual care (five control units)" Page 1 |
| Allocation concealment (selection bias) | Low risk | Quote: "We randomised maternity units rather the individual women because of the risk of women sharing the leaflets in an individual level trial" Page 1 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: the patients before the intervention are not the same as the patients after intervention. However responses rates were similar across group in each samples. Reasons for non responses were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: baseline measurements for our primary outcome were reported. |
| Protection against contamination? | Low risk | Quote: "We randomised maternity units rather the individual women because of the risk of women sharing the leaflets in an individual level trial." page 1 |
| Baseline characteristics patients | Low risk | Comment: see table 1 before the intervention. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about professionals. |
Perestelo‐Perez 2016.
| Methods |
Study design: randomized trial Unit of allocation: provider Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: ambulatory care, primary care, Spain Health professionals: general practitioners; fully trained Patients: 168; diabetes; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (decision aid) Quote: "Physicians in the intervention group were trained to apply the DA by a member of the research team, in group sessions of one hour." page 296 Usual care (control) |
|
| Outcomes | Satisfaction with decision making process (SDMP) (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: Not enough information to make a judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: too many losses in the follow up, however proportion of lost to follow‐up were well balanced between groups and reasons of losses to follow‐up were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available to permit judgement. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no outcome measure at baseline. |
| Protection against contamination? | Unclear risk | Comment: physicians were allocated within a primary care center and it is possible that communication between intervention and control professionals could have occurred (e.g. physicians within practices were allocated to intervention or control). |
| Baseline characteristics patients | Low risk | Comment: the characteristics at baseline were different. But the authors tried to adjust for those variables to minimize confounding. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about clinicians at baseline. |
Pickett 2012.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: level of care: unclear; ambulatory care; USA
Health professionals: unknown number; type: unclear; unclear level of training Patients: 428; mental illness; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention Quote: "...BRIDGES [Building Recovery of Individual Dreams and Goals] is an 8‐week, manualized peer‐led education course designed to empower adults with psychiatric disabilities and enhance their recovery...Topics covered in the BRIDGES curriculum include: self‐advocacy; communication and problem‐solving skills; philosophy of recovery; social support; psychiatric diagnoses, medications and mental health treatment; and crisis planning." page 424 Usual care (control): Quote: "As described above, a total of 216 participants were randomly assigned to a BRIDGES course waiting list (control condition) and were guaranteed an opportunity to receive BRIDGES from the study after completing their third and final interview. During their participation in the project, control group participants received services as usual..." page 425 |
|
| Outcomes | Patient self‐advocacy (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by UIC SRL [University of Illinois Survey Research Laboratory] research staff at the end of each T1 interview. A random allocation sequence programmed into the CAPI administration software blinded both interviewers and participants to subjects’ study assignment (Gluud 2006)." page 422 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by UIC SRL research staff at the end of each T1 interview. A random allocation sequence programmed into the CAPI administration software blinded both interviewers and participants to subjects’ study assignment (Gluud 2006)." page 422 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "A random allocation sequence programmed into the CAPI administration software blinded both interviewers and participants to subjects’ study assignment (Gluud 2006). To monitor the integrity of the blind, interviewers were asked at the end of each follow‐up interview whether subjects had directly or indirectly shared their study status; this occurred in only 7.2 % of all T2 and T3 interviews." page 422 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Quote: "A total of 343 subjects (80.1 %) completed T2 interviews, and 320 subjects (74.8 %) completed T3 interviews, for an attrition rate of 25.2 %. There were no statistically significant differences in follow‐up rates between intervention and control conditions. At T2, interviews were completed by 171 (80.7 %) intervention group participants and 172 (79.6 %) control group participants (X1 2 = 0.071, p = .810). At T3, assessments were completed by 157 (74.1 %) intervention group participants 163 (75.5 %) of the control group (X1 2 = 0.112, p = .740)." page 427 |
| Selective reporting (reporting bias) | Low risk | Comment: there is selective outcome reporting because of the objective of the paper. The other outcomes were reported in another paper. ClinicalTrials.gov: NCT01297985 |
| Other bias | High risk | Quote: "although we assessed changes in participants’ self‐reported empowerment and self‐advocacy, we did not observe their interactions with their treatment providers. Thus, we do not know if BRIDGES participants actually asked questions and/or asserted themselves in treatment discussions with providers." page 428 |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: see table 2. |
| Protection against contamination? | Low risk | Quote: "No BRIDGES classes were offered outside of the study in any of the sites during the intervention or 6‐months follow‐up period; thus, the intervention was not available to any control group participants." page 425 |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Raynes‐Greenow 2010.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: Specialized care (2 obstetric hospital, Sydney); ambulatory care; Australia Health professionals: unknown; unclear level of training Patients: 596; primiparous women in their final trimester planning a vaginal birth of a single infant; female |
|
| Interventions |
Single intervention: patient‐mediated intervention (decision aid: booklet and audio guide) Single intervention : patient‐mediated intervention (decision aid: booklet) The booklet was 55 pages and the audio guide 40 minutes. Quote: "Information was presented in a style that was sparse." page 2 The content included both pharmacological and non‐pharmacological analgesics Single intervention (comparison group): patient‐mediated (pamphlet) Same booklet as intervention group, page 2 |
|
| Outcomes | Perceived level of control in decision‐making process (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): 1065 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was randomly generated computer using random variable block sizes." page 3 |
| Allocation concealment (selection bias) | High risk | Quote: "It was not possible to conceal allocation once randomised" Page 3 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: patient‐mediated intervention and patient‐reported outcome, so the patient was not really blinded. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: in all, 76%patients were present at follow‐up. Moreover, incomplete data forms/lost to follow‐up in DA group were double of those in the PA. Voir page 6 |
| Selective reporting (reporting bias) | Low risk | Comment: relevant outcomes pre‐specified in the trial protocol are reported in the results (trial registry number ISRCTN52287533). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Low risk | Quote: "It was not possible to conceal allocation once randomized; however, to minimize contamination a research assistant worked at each centre and the antenatal staff were kept blinded to the treatment allocation and the actual content of the decision aid." page 4 (Raynes‐Greenow 2009) |
| Baseline characteristics patients | Low risk | Quote: "As seen in Table 2, maternal demographic characteristics and baseline measures of cognitive and affective outcomes were comparable between these two groups." page 6 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Rise 2012.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: specialized care; ambulatory care; Norway Health professionals: 25; various type; fully trained Patients: 75; mental health; male and female |
|
| Interventions |
Multifaceted intervention: Patient mediated intervention (PCOMS), Educational meeting (training of therapists) Quote: "The intervention therapists were trained to administer the feedback system Partners for Change Outcome Management System (PCOMS) [30] during the treatment they usually provide. PCOMS therapists received 12 h of training during two days, with four weeks apart, with respectively eight and four hours of training...The use of the PCOMS consisted of administering two feedback scales in every treatment session, one at the beginning of the session (the Outcome Rating Scale, or ORS), and one at the end (the Session Rating Scale, or SRS)... In the ORS the patients rate their own functioning during the last week, or since the last treatment session, individually, interpersonally, socially, and generally. On the SRS, the patients rate the current session on relations with the therapist and the degree of agreement on goals, methods, and treatment approach...The intervention thus consisted of systematically using the ORS and SRS scales to assess feedback from the patient on treatment outcome and the quality of the session." page 4 Usual care (control): Quote: "The controls received treatment as usual." page 4 |
|
| Outcomes | Treatment Alliance Scale (continuous), Patient Activation Measure (continuous), Patient Participation (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): 395 Number of patients per therapist (median): 5 in the intervention group, 1.5 in the control group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "University internet based computerised randomisation service" page 6 |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "This was an open study and no blinding was performed." page 6 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: there are missing data, but sensitivity analysis was performed comparing intention‐to‐treat analyses to per‐protocol analyses and the results of the outcomes were similar. Number lost to follow‐up and reasons for loss to follow‐up were well‐balanced between groups. |
| Selective reporting (reporting bias) | Low risk | The protocol was registered (Identifier: NCT01083225) The main outcomes pre‐specified in the protocol were reported in the results. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no applicable for Treatment Alliance Scale, done for Patient Activation Measure, but not done for Patient Participation |
| Protection against contamination? | Unclear risk | Quote: "To fortify fidelity of the treatment as usual group the therapists were repeatedly instructed to avoid using any feed‐back scales during treatment" Page 4 |
| Baseline characteristics patients | Unclear risk | Comment: Table 1: imbalance between groups for some factors (gender (female), living alone, can confide in 2 or more persons, level of education, working). But it is not specified if some of those factors are known risk factors of the issues. Moreover, The sample size is not that large. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: No report of characteristics |
Roter 2012.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: physicians and patients Power calculation: unclear |
|
| Participants |
Care setting: primary care, ambulatory care; USA Health professionals: 29 family physicians fully‐trained and in training Patients: 197; type of clinical condition not mentioned; 50 females and 80 males |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (decision aid); distribution of educational materials Separate interactive video glossaries demonstrating communication skills organized by the LEAPS (Listen, Educate, Assess, Partner and Support) heuristic. Quote: "The interventions were comprised of separate interactive video glossaries demonstrating communication skills organized by the LEAPS heuristic. The patient glossary included the performance of 228 10‐s video clips demonstrating the 18 targeted patient communication skills in various ways ... " page 407 Single intervention (control): distribution of educational materials. Quote: "Since control group patients would have benefited from seeing web exposed physicians as well as intervention group patients." page 412 |
|
| Outcomes | Separate interactive video glossaries demonstrating communication skills to patients and to clinicians | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Some practices assigned patients to study groups on alternating days and others used a random numbering system." page 407 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "Communication behaviours were assessed at baseline and after a follow‐up visit through an 18‐item self‐report questionnaire." page 408 |
| Protection against contamination? | High risk | Comment: Outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Comment: study groups did not differ on any of these characteristics. Table 3 page 410 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Sanders 2017.
| Methods |
Study design: randomized trial Unit of allocation: provider Unit of analysis: provider and patient Power calculation: not done |
|
| Participants |
Care setting: Ambulatory care, primary care, Netherlands Health professionals: 42; general practitioners; male and female; fully trained Patients: 175; back pain; male and female |
|
| Interventions |
Multifaceted intervention: educational meeting + audit and feed back Quote: "GPs in the intervention group received two training sessions that were each two and a half hours in duration and were held in small groups of approximately three to five participants. The training focused on the SDM process and evidence‐based treatment of low back pain according to professional guidelines" Page 564 "In addition to receiving training sessions, the GPs in the intervention group received personalised feedback on each videotaped consultation for a maximum of two consultations between the training sessions and for all consultations with recruited patients." page 565 Usual care (Control): Quote: GPs in the control group were not trained and provided usual care." page 565 |
|
| Outcomes | OPTION (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Quote: "Control GPs were not blind to the allocation or scope of the intervention, but they were not familiar with the content of the intervention." Page 569. No test was done to know if GPs were familiar or not or the level of familiarity with the content of the intervention "Two blind observers (AL and IvdE) scored the videotapes using Observer (Noldus, 7th edition), a program designed to aid in the observation of videotapes." page 566 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Quote: "Not all trained GPs videotaped consultations. However, we do not believe that this limitation induced selection bias because we did not find differences in the baseline variables between recruited and non‐recruited patients." page 569 |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available to permit judgement. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no measure of the outcome at baseline. |
| Protection against contamination? | Low risk | Comment: possible contamination, because it is in the same hospital.However the GPs from different groups were not present at the same working time. |
| Baseline characteristics patients | Low risk | Comment: characteristics between groups were similar at baseline (table 1) |
| Baseline characteristics healthcare professionals | Low risk | Comment: characteristics at baseline for HCP were similar. |
Schroy 2011.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary care (Boston Medical Care centre, South Boston Community Health Centre); ambulatory care; USA Health professionals: 50; Various healthcare professional with inter‐professional (board‐certified general internist, nurse practitioners); fully trained Patients: 666; colorectal cancer screening; female and male |
|
| Interventions |
Single (first intervention group): patient‐mediated intervention (DVD audio‐visual touch screen decision aid explaining screening importance, epidemiology of disease, recommended methods and their comparison, and decision guidance: Your Disease risk assessment tool with feedback). Single intervention (second intervention group): patient‐mediated intervention (DVD audio‐visual touch screen decision aid explaining screening importance, epidemiology of disease, recommended methods and their comparison, and decision guidance). Single intervention (control): educational materials (a modified Quote: “9 ways to stay healthy and prevent disease"). |
|
| Outcomes | 12‐item satisfaction with the decision‐making process scale (categorical) | |
| Notes |
Additional information Number of approached patients (eligible): 9869 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: patients were randomized but the method was unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: in all, 100% of the patiens were included at follow‐up. Page 5. figure 2 |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | High risk | Quote: "Patient satisfaction with the decision‐making process was assessed on the posttest using the validated 12‐item Satisfaction with the Decision‐Making Process Scale (Appendix 2)." page 6 |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves |
| Baseline characteristics patients | Low risk | Comment: as shown in Table 1, the 3 study arms were well‐balanced with respect to patient age, sex, race, ethnicity, education, prior FOBT, insurance status. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Schroy 2016.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory care, primary care, USA Health professionals: various types; fully trained Patients: 341; colorectal cancer; male and female |
|
| Interventions |
Multifaceted intervention : patient‐mediated intervention (decision aid + risk assessment tool): Quote: "An updated web‐based version (http://www.colorectalcancerscreening4u.com) of our validated DVD‐formatted decision aid 9 was employed in this study." Page 528 "The risk index employed is this study was developed from a cross sectional study of 3457 average‐risk patients undergoing screening colonoscopy at BMC." page 528 "After completing the electronic risk assessment tool, patients received a printed form describing their risk category (low versus intermediate/high) and pictographs with absolute risk estimates." page 528 Single intervention: patient‐mediated intervention (decision aid): Quote: "An updated web‐based version (http://www.colorectalcancerscreening4u.com) of our validated DVD‐formatted decision aid was employed in this study." page 528. |
|
| Outcomes | OPTION (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomized 1:1 via a preset block randomization table within the study website." page 527 |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were then randomized 1:1 via a preset block randomization table within the study website that was inaccessible to study coordinators to a control arm (decision aid only) or experimental arm (decision aid plus risk assessment), within strata by provider." page 527 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "A prospective, unblinded, parallel‐group randomized controlled trial was conducted between September 2012 and September 2014 at Boston Medical Center (BMC) to evaluate the impact of risk stratification for ACN on shared decision‐making for CRC screening." page 527 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: very low level of lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available to permit judgement. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no outcome measure at baseline. |
| Protection against contamination? | High risk | Comment: randomized patients were in the same medical center. |
| Baseline characteristics patients | Low risk | Comment: characteristics between groups were similar at baseline (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about clinicians at baseline. |
Shepherd 2011.
| Methods |
Study design: randomized trial (cross‐over trial) Unit of allocation: the order of the standardized patients visits Unit of analysis: physicians and patients Power calculation: done |
|
| Participants |
Care setting: Primary care, ambulatory care, Australia Health professionals: 36; family physicians; Fully trained Patients: 2, depression ; patients are simulated, male or female not reported |
|
| Interventions |
Single intervention: educational outreach visit. Healthcare professional visited by an unannounced and standardized patient who asked three questions. Usual care (control): no intervention (the control standardized patient did not ask the three questions). |
|
| Outcomes | Assessing Communication about Evidence and Patient Preferences (ACEPP); Observing Patient Involvement (OPTION) scores; The fostering by healthcare professionals of active participation of patients in the decision‐making process. | |
| Notes |
Additional information number of approached patients (eligible): NA, simulated patients were used in the study number of patients per physician: NA, simulated patients were used in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The order of the standardized patient visits (intervention vs control) was allocated randomly." page 380 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "The transcribed consultations were analysed using ACEPP and OPTION by two trained coders who were not investigators on the study and blinded to the study purpose …." page 381 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Selective reporting (reporting bias) | High risk | Comment: some outcomes prespecified in the trial registry are not reported in the results paper: assessment of nature and variability of patient‐doctor communication in both general and specialist practice in Australia; differences in management recommended in consultations (see Australian New Zealand Clinical Trials Registry no. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Unclear risk | Comment: not specified in the paper. |
| Baseline characteristics patients | Unclear risk | Comment: simulated patients. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: insufficient information about professionals. |
Sheridan 2012.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: level of care not clear, ambulatory care, USA Health professionals: 28; type: unclear; level of training: unclear Patients: 128, prostate cancer screening, male |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (video + coaching session), Educational meeting Quote: "Our intervention consisted of 2 components designed by investigators (see Table 1): 1) a video‐based decision aid for patients and 2) a coaching session for patients." page 3 Single intervention (control): Quote: "Highway safety control video." page 5 |
|
| Outcomes | Shared decision post‐visit (qualitative) | |
| Notes |
Additional information Number of approached patients (eligible): 474 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization used computer‐generated random numbers that were sealed in opaque envelopes." page 5 column 1 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization used computer‐generated random numbers that were sealed in opaque envelopes." page 5 column 1 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Comment: no blinding. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: 2 false inclusions. No missing at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not seen, but the preference in participation in DM was not reported. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome (it is preference in participation not actual participation that was measured at baseline). |
| Protection against contamination? | High risk | Allocation by patients within a practice : Quote: "Third, because we randomized at the patient level, physicians saw patients in both the intervention and control groups, creating the possibility for contamination." page 9 column 2 |
| Baseline characteristics patients | Unclear risk | Baseline imbalance. Quote: "Second, despite randomization, the small size of our study resulted in differential distribution of confounders among study groups. We controlled for this in multivariate analysis, but recognize the potential that unmeasured confounders may have affected our results." page 9 column 2 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Sheridan 2014.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: specialized care, ambulatory care, USA Health professionals: 24; specialists; fully trained and in training Patients: 160, coronary heart disease risk reduction, male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention (decision aid) Quote: "Our intervention consisted of two parts: a decision aid delivered prior to a provider visit (at the primary study visit) and a series of three tailored adherence messages delivered between the primary and follow‐up study visits. In this paper, we focus on the independent effects of the decision aid, which includes three components: individualized risk assessment and education; values clarification; and coaching..." page 3 Usual care (control): Quote: "Patients randomized to the control group did not present early to their previously scheduled clinic visit and received usual care from their provider." page 3 |
|
| Outcomes | Shared decision (categorical) | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: 24 eligible providers enrolled in the study 160 eligible patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "Staff told patients only that they were participating in a study about “prevention of heart disease.” Physicians were not blinded and saw patients in both the intervention and control group." page 2 column 2 (Sheridan 2011) |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: similar reasons for missing data across groups (visit no shows, declined further participation). The proportion of missing outcomes compared with observed event not enough to have relevant impact on the intervention effect estimate (4/81 vs 2/11) (see flow chart Sheridan 2011). |
| Selective reporting (reporting bias) | High risk | Comment: authors did not compute between group comparisons of the effect of the decision aid on knowledge, accuracy of risk perception and values clarity (in post visit) NCT00494052 |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome (it is preference in participation not actual participation that was measured at baseline). |
| Protection against contamination? | High risk | Comment: allocation by patients within a practice. |
| Baseline characteristics patients | Low risk | Comment: imbalance for education but this variable was adjusted for in the analysis. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Smallwood 2017.
| Methods |
Study design: pilot‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: ambulatory care, primary care, USA Health professionals: various types; fully trained Patients: 50; female; osteoporosis |
|
| Interventions |
Single Intervention : patient‐mediated intervention (decision aid): Quote: "The final decision aid included information about osteoporosis including causes, risk factors, how to determine if you have osteoporosis personalized fracture risk based on FRAX, details about medication and nonprescription treatment, and a values elicitation exercise related to the treatment decision." page 568. Single intervention: patient‐mediated intervention (web‐based information): Quote: "Participants in the experimental group received the decision aid, while those in the control group were directed to the National Institute on Aging homepage (www.nia.nih.gov) rather than the decision aid. This control site provided web‐based information relevant to aging but not specific to osteoporosis." page 568. |
|
| Outcomes | Shared Decision Making (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two predetermined block randomization schedules for osteoporosis and osteopenia were created using a computer random number generator and maintained electronically.'' page 568. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Quote: "Neither patients nor physicians could be adequately blinded to their treatment arm'' page 575 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: no loss to follow‐up and no missing data for primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available to permit judgement. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no outcome measure at baseline. |
| Protection against contamination? | Low risk | Comment: participants in the experimental group and the control group were on different platform. |
| Baseline characteristics patients | Low risk | Comment: characteristics between groups were similar at baseline (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about clinicians at baseline. |
Stacey 2006.
| Methods |
Study design: provider‐randomized trial Unit of allocation: provider Unit of analysis: provider Power calculation: done |
|
| Participants |
Setting of care: primary care; ambulatory care (province‐wide health call centre in British Columbia); Canada Healthcare professionals: 41; nurse; fully trained Patients: simulated patients; decisions about amniocentesis, treatment for attention deficit disorder and herniated disk, decisions about allergy injections, and treatment for gall bladder attacks and borderline hypercholesterolemia. |
|
| Interventions |
Multifaceted intervention: distribution of educational materials, educational meeting, as well as audit and feedback; barriers assessment; 6 hours. The intervention involved a structured coaching protocol, a 3‐hour online tutorial and a 3‐hour skill‐building workshop that included performance feedback from baseline calls with simulated patients. The coaching protocol was introduced in the tutorial, used in the workshop and available exclusively to trained nurses for use with routine calls. Usual care (control) |
|
| Outcomes | Decision Support Analysis Tool (continuous); SDM is assessed as the fostering by healthcare professionals of active participation of patients in the decision‐making process. | |
| Notes |
Additional information Number of approached patients (eligible): not reported (simulated patients) Number of patients per physician: not reported (simulated patients). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation schedule was computer‐generated centrally by a statistician." page 411 |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation schedule was computer‐generated centrally by a statistician. Allocation was concealed until after the nurses completed their baseline simulated call." page 411 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Quote: "In the present study, two of five raters trained in the use of the DSAT and blinded to group assignment, assessed the recorded calls independently." page 412 |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: Of 41 randomized nurses, 2 dropped out and 1 baseline call was not recorded due to technical errors. There was a 93% follow up rate. Page 411 |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Unclear risk | Comment: Unit of allocation is the provider within a province wide call centre |
| Baseline characteristics patients | Unclear risk | Comment: simulated patients |
| Baseline characteristics healthcare professionals | Low risk | Comment: The intervention and control groups were similar in the demographic characteristics of participants and in the quality and length of their baseline calls with simulated patients (Table 2). |
Stiggelbout 2008.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: specialized care and ambulatory care (outpatient clinic of 2 teaching hospitals in the west of the country); the Netherlands Health professionals: 15; vascular surgeon; fully trained and in training Patients: 113; abdominal aortic aneurysm; male and female |
|
| Interventions |
Single‐intervention: patient‐mediated intervention (individualized brochure) This brochure contained an output providing information on three strategies concerning the management of the patient, ranked in accordance to the patients' risk. Single‐intervention (control): patient‐mediated intervention (general brochure) |
|
| Outcomes | Patients' decisional role subscale (continuous); joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): 136 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to receive an individualized brochure (IB) or a general brochure (GB) about surgery for abdominal aneurysm. Randomization was stratified by the surgeon." page 752 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: in all, 88% of the patients are present in the follow‐up. Lost to follow‐up were balanced in numbers across groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Quote: "…whereas the IB group had preferred a (non significant) more active decision‐making role before hand (mean 2,9, SD 1,3 versus mean 2,5, SD 0,9, P = 0,15)." page 757 |
| Protection against contamination? | Low risk | Quote: "The fact that we did not randomize surgeons may also have led to some of the similarities between the arms of the trial, because one runs a risk of contamination if a surgeon sees both intervention and control patients. But it is highly unlikely that surgeons could have reproduced the individualized information without access to the model." page 758 |
| Baseline characteristics patients | Low risk | Comment: patients in the index and control arm were similar with respect to sociodemographic characteristics and major medical characteristics (see Table 2). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Street 1995.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: specialized care and ambulatory care (Scott and White clinic and Hospital (Texas)); USA Health professionals: 10; various type of physician (4 medical oncologists, 2 radiation oncologists, 4 surgeons); fully trained Patients; 60; breast cancer; female |
|
| Interventions |
Single‐intervention: patient‐mediated intervention (Interactive multimedia program (decision aid));15‐20 minutes. Quote: "The program "Options for treating breast cancer" is an interactive program using a touch‐screen monitor containing audio‐visual elements. It provides an introductions, elaborate the problem, treatment options and provides testimonies of other women's experiences." page 2277 Single‐intervention (control): patient‐mediated intervention (brochure (decision aid)) Quote: "This is an eight page brochure entitled "Care of patients with early breast cancer". It contains comments by other women, elaborates the problem and presents treatment options. The medical information is the same in both the multimedia format and the brochure format." page 2278 |
|
| Outcomes | Perceived decision control (continuous); joint process between healthcare professionals and patients to make decisions. | |
| Notes |
Additional information Number of approached patients (eligible): not reported Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: patients were randomized but the method was unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Selective reporting (reporting bias) | High risk | Comment: patient participation in the consultations was not reported in each study group but according to age and education (contrary to the hypotheses 2). Thus, we are not able to see if patient using the computer program will be more involved in the DM than will patients reading the brochure. |
| Other bias | Low risk | Comment: no evidence of other risk of biases |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | High risk | Comment: outcome is patient reported and the intervention is patient allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Quote: "… there were no significant differences between the multimedia or brochure group with respect to the patient's age, education, disease stage, or ethnicity." page 2277 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Tai‐Seale 2016.
| Methods |
Study design: pilot‐randomized trial Unit of allocation: practice Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: primary care and ambulatory care, USA Health professionals: 26; various type; unclear level of training; male and female Patients: 300; clinical context: biomedical, health behavior, mental health and psychosocial; male and female. |
|
| Interventions |
Multifaceted intervention: educational material (video) + patient‐mediated intervention (booklet) + educational meeting (coaching session for providers) Multifaceted intervention: OpenCom + patient‐mediated intervention (one‐page ASK Handout) Single intervention: patient‐mediated intervention (one‐page ASK Handout) Usual care (Control) Quote: ''A multidimensional intervention, called Open Communication (OpenComm), emerged from our work. The first element of this intervention was a two‐minute animated video, developed to illustrate open communication behaviors for patients and primary care providers. The video normalized setting a joint agenda, asking questions, and requesting information on other options. The second component was a Visit Companion booklet for patients that enabled them to delineate issues that matter the most to them before their visit and to review and record their next steps during the visit... Lastly, in an initiative modeled after the VOICE study, a standardized patient instructor provided communication coaching for primary care providers, consisting of two thirty‐minute, individually tailored sessions that occurred during usual clinic time at the providers’ practices. These sessions occurred approximately one month apart.'' page 606 ''As we stated earlier, our pilot examined the efficacy of our novel intervention by comparing it to an existing intervention, ASK, which poses three questions: “What are my options? What are the possible benefits and risks of each option? How likely are the benefits and risks of each option to occur? ASK has been used to improve patients’ involvement in health care consultations. To undertake this comparison, we handed patients a one‐page ASK handout before their visits.'' page 607 "We compared OpenComm (which we designed), ASK, OpenComm plus ASK, and usual care, in a fully crossed 2x2 factorial design.'' page 607. |
|
| Outcomes | Collaborate (continuous); OPTION5 (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Observer‐based outcome | Low risk | Comment: rating was done by two members of the research team, ED and CS, who were blinded to each visit’s intervention arm. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not enough information to make a judgement. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Unclear risk | Comment: two PCPs chose not to participate in audio‐recording. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: no follow‐up or small rate of loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Comment: one outcome that was pre‐specified in the protocol was not reported in the results (NCT02522286). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no baseline measurement. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measurement. |
| Protection against contamination? | Low risk | Quote: "Four primary care clinics were randomized, one to each arm.'' page 607 |
| Baseline characteristics patients | Low risk | Comment: characteristics between groups were similar at baseline (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: not enough information to make a judgement. |
Thomson 2007.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: setting: unclear; primary care; UK Health professionals: number unknown; type: unclear; level of training: unclear Patients; 145; atrial fibrillation; male and female |
|
| Interventions |
Single‐intervention: patient‐mediated intervention (computerized‐decision aid) Quote: "All participants were seen in one of two research clinics each conducted by a single doctor, trained in delivering either the decision aid or guidelines but blinded to the alternative method." table 1,page 217 Single‐intervention (control): patient‐mediated intervention (guidelines) Table 1, page 217 |
|
| Outcomes | Decision Making role experienced (categorical) | |
| Notes |
Additional information Number of approached patients (assessed for eligibility): 1360 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised… using electronically‐generated random permuted blocks via web‐based randomisation service provided by the Centre for health Services Research." page 217 |
| Allocation concealment (selection bias) | Low risk | Comment: centralized randomization scheme. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: see the flow chart page 218 |
| Selective reporting (reporting bias) | High risk | Comment: decision‐making preference. Some outcomes were not reported in enough details. |
| Other bias | Low risk | Comment: no evidence of other risk of bias. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: insufficient information to permit judgement. |
| Protection against contamination? | Unclear risk | Quote: "Randomization was done by patients but "all participants were seen in one of the two research clinics each conducted by a single doctor, trained in delivering either the decision aid or guidelines but blinded to the alternative method." page 217 (probably to avoid contamination but patient may still communicate each other in the practices where they were recruited). |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 2). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Tinsel 2013.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: practice Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary care and ambulatory care; Germany
Health professionals: number unknown; general practitioners; fully trained Patients: 1120; hypertension; male and female |
|
| Interventions |
Multifaceted intervention: distribution of educational material, educational meeting Quote: "Those GPs who had been allocated to the intervention group took part in an SDM training programme... which had been evaluated in various studies...The training included the following elements: (1) information on arterial hypertension, (2) physician‐patient communication and risk communication, (3) the process steps of SDM, (4) motivational interviewing [40,41], (5) introduction of a decision table listing options to lower CVR, and (6) use of case vignettes for role plays simulating physician‐patient consultations. Additionally, we recommended implementing a cardiovascular risk calculator for GPs which included elements of SDM... Furthermore we delivered patient information flyers...to the GPs of the intervention group." page 3 Usual care (control) Quote: "GPs of the control group treated their patients as usual." page 3 |
|
| Outcomes | SDM‐Q‐9 (continuous) | |
| Notes |
Additional information Number of approached practices (eligible): 115 Number of patients per practices: 32.5 in intervention group, 29.9 in control group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not reported in the paper. |
| Allocation concealment (selection bias) | Low risk | Comment: randomization at the GP practice level. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "The patients were blinded to the allocation of the intervention (single‐blinded study)." page 3 column 2 paragraph 1 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: lost to follow‐up at T3 and non response to outcomes is higher in higher in control group than in intervention group. Was the follow up in the 2 groups comparable? Are the principal reason for missing data related to outcomes (patient desire)? What is the pattern of reasons for discontinuation across groups? There is no clear pattern of missing data. |
| Selective reporting (reporting bias) | Low risk | Commment: tables 1,2,3 and study protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Low risk | Comment: see table 2. |
| Protection against contamination? | Low risk | Comment: randomization at the GP practice level. |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 2). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about professionals. |
van der Krieke 2013.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: unclear |
|
| Participants |
Care setting: specialized and ambulatory care; the Netherlands
Health professionals: number unknown; various type (psychiatrists, community psychiatric nurses, psychologists); fully trained Patients: 250; Psychotic disorders; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention Quote: "Patients in the intervention condition received care as described in the local disease management program for the treatment of people with psychosis plus they were offered the opportunity to make use of the Web‐based information and decision tool..." page 3 Usual care (control) Quote: "Patients in the control condition received care as usual, as described in the local disease management program for the treatment of people with psychosis." page 3 |
|
| Outcomes | COMRADE (continuous) | |
| Notes |
Additional information Number of approached patient (eligible): not reported Number of patients per provider: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of patients was conducted by using the online Research Randomizer." page 4 |
| Allocation concealment (selection bias) | Low risk | Quote: "Another research assistant located at our research center added the randomization conditions to the spreadsheet, assigning participants to the interventions." page 4 Comment: centralized randomization scheme. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: missing outcome data balanced in numbers across groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | High risk | Comment: patients in the same institution were randomized. |
| Baseline characteristics patients | Low risk | Comment: reported and similar (see table 2). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
van Peperstraten 2010.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient (client couple) Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: specialized care (fertilization clinics); ambulatory care; the Netherlands Health professionals: NA; nurses and staff at the fertilization clinics; fully trained Patients: 308, need in vitro fertilization; females and males (client couple) |
|
| Interventions |
Single intervention: patient‐mediated intervention (decision aid, support call), reimbursement of fees; barriers assessment. Decision Aid and reimbursement; discussion; telephone call discussion Quote: "The multifaceted strategy aimed to empower couples ... The strategy consisted of a decision aid, support of a nurse specialising in vitro fertilisation, and the offer of reimbursement by way of an extra treatment cycle." page 1 No intervention, usual care (control) No intervention (usual discussion) Quote: "The control group received standard care for in vitro fertilisation." page 1 |
|
| Outcomes | Decision Evaluation Scale (informed choice). Joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 344 Number of patients per physician: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation took place centrally using a computer generated randomisation list. Participants were randomised in blocks of four couples. A secretary outside our department was the only person with access to the randomisation list. She randomised the couples on the day consent was received and informed the couple that same day." page 2 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation took place centrally using a computer generated randomisation list. Participants were randomised in blocks of four couples. A secretary outside our department was the only person with access to the randomisation list. She randomised the couples on the day consent was received and informed the couple that same day." page 2 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "Because of the nature of the intervention it was not possible to blind the participants or in vitro fertilisation doctors to the allocation." page 2 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Unclear risk | Comment: 124 vs 128 reported about our primary outcome (informed choice subscale of the DES). Data were missing for 28 couples in each groups (18,4% vs 17,9%). However, the reasons for missing data were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measurement for our primary outcome (see table 3, page 5). |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Quote: "Table 1 shows the characteristics of the couples in the two groups. No relevant differences were observed between the groups. " page 4. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
van Roosmalen 2004.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: family Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: specialized care; setting: unclear; the Netherlands Health professionals: number unknown; type: unclear; level of training: unknown Patients: 88, deleterious BRCA1/2 mutation; female |
|
| Interventions |
Multifaceted intervention: patient‐mediated intervention (SDMI+DA) Quote: "The SDMI was provided by a trained research assistant and consisted of three sessions with an interval of 1 to 2 weeks. In the first session, individual values for the treatment options (screening and prophylactic surgery) were assessed in a face‐to‐face interview by use of the TTO method.In the second session, the TTO interview was repeated by telephone...In the first part of the study (T1 to T3; Fig 1), not reported here, women were randomly assigned to the DA group (the DA was provided 2 weeks after blood sampling) or to the control group (receiving usual care)." see figure 1 pages 3294‐3295 Single intervention (control): patient‐mediated intervention (DA) Quote: "The DA was added to usual care and was to be viewed at home. It consisted of a brochure and video providing information on screening and prophylactic surgery, and the physical, emotional, and social consequences." page 3295 |
|
| Outcomes | Perceived participation in decision making (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): 453 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer generated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "Neither study participants nor members of the study staff were blinded to intervention assignment." page 3296 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: the follow‐up at T4 was 100%. At T5, one woman from the control group was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | Low risk | Quote: "Randomization took place by family to avoid contamination." page 3296 |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
van Tol‐Geerdink 2016.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: ambulatory care, specialized care, the Netherlands Health professionals: specialists; fully trained Patients: 240; prostate cancer; male |
|
| Interventions |
Single intervention: patient‐mediated intervention (decision aid) Quote: ''The decision aid explained that there are different treatment options with different pros and cons. Radial protatectomy and external beam radiotherapy were presented to all patients. A third option, bradytherapy was presented only to eligible patients.'' page 463 Usual care (control) |
|
| Outcomes | Patient participation (qualitative) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information about sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was centralized to avoid allocation bias and was blocked in groups of 3 per hospital, thus stratifying for hospital site." page 461 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "Patients and caregivers could not be blinded to the intervention." page 460 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: there are losses to follow‐up after randomization and missing data and reasons were not well‐balanced (flow chart of the study). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information about registration of the protocol. |
| Other bias | Low risk | |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no outcome measure at baseline. |
| Protection against contamination? | High risk | Comment: randomization was done at the patient level. |
| Baseline characteristics patients | Low risk | Quote: "Patient characteristics in the decision aid group and the usual care group were comparable for education, age, baseline physical functioning and tumour characteristics." page 465 |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about clinicians at baseline. |
Vestala 2013.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: level of care: unclear; non‐ambulatory care; Sweden Health professionals: number unknown; nurses; fully trained Patients: 39, chronic diseases: diabetes, inflammatory bowel diseases, liver disease, coronary artery disease, COPD; male and female |
|
| Interventions |
Single intervention: patient‐mediated intervention Quote: "The study intervention meant that during their stay in the ward, the patient participated in the nursing documentation together with their nurse. General health status, care goals, and care plans were documented by the nurse and the patient together. The documentation was completed daily. The patients received a printed copy of his/her nursing record and documentation was changed according to the patients’ comments utilizing documentation standards. The nurses used a laptop computer to complete all nursing documentation, to facilitate patient presence and direct documentation in the patient record." page 67 Usual care (control) |
|
| Outcomes | Control Preference Scale (Categorical) | |
| Notes |
Additional information Number of approached patients (eligible): 70 Number of patients per provider: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Patients were not blinded: Quote: "The patients who chose to participate were randomised either to a group participating in nursing documentation or to a control group, depending on the content of the patient information letter. " page 67, column 2 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: for perceived role in decision making: missing data balanced in number across groups (2 in intervention group, 2 in control group). |
| Selective reporting (reporting bias) | High risk | Comment: relevant outcomes like mastery, self‐esteem, empowerment, depression were reported incompletely. Many sub‐group analysis and correlations were computed but not prespecified in the methods. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | High risk | Comment: patients of a medical ward were randomized. |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Vodermaier 2009.
| Methods |
Study design: patient‐randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: not done |
|
| Participants |
Care setting: specialized care and non‐ambulatory care (gynecological department of the University of Munich‐Grosshadern; Germany Health professionals: Unknown number; physicians; unclear level of training Patients: 152; breast cancer; female |
|
| Interventions |
Single‐intervention: patient‐mediated intervention (decision aid) The decision aid took the form of three decision boards (corresponding to tumour size) relating to chemotherapy information with hormone‐responsive breast cancer, for preoperative chemotherapy. They are presented in 20 minute sessions going over the options so that the patient understands and can discuss them; they also present how the patient can participate in the decision making. They receive a brochure summarizing the boards content. Usual care (control) |
|
| Outcomes |
|
|
| Notes |
Additional information Number of approached patients (eligible): 246 Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random assignment was performed by means of numbered cards in envelopes for the intervention and the control group..." page 591 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified in the paper. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Quote: "Blinding was not possible within the hospital procedures." page 591 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: 27% of missing outcome. However lost to follow‐up were equal in numbers across groups (and unlikely to bias the results) and; exclusions from data analysis were similar in number and reasons across groups. Morevover the ratio of participants with missing data to participants with events was 12/71 = 0,17. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | High risk | Comment: outcome is patient‐reported and the intervention is patient‐allocated. Consequently patients could discuss the intervention among themselves. |
| Baseline characteristics patients | Low risk | Quote: "No group differences in terms of demographic and tumour‐related variables were found." (Table 2). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no report of characteristics. |
Warner 2015.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory and non‐ambulatory care, specialized care, USA Health professionals: 24; fully trained and in training Patients: 130; preoperative context; male and female |
|
| Interventions |
Multifaceted intervention: Patient‐mediated intervention (decision aid + patient education brochure) + education meeting: Quote: "Patients receiving the decision aid received the decision aid packet from the personnel who brought them into the examination room and who read them the instructions printed on the packet sleeve (“You need to make a decision about how to handle smoking around the time of your surgery. Here is information to help you make that decision. Read both sides of these cards, Consider which is right for you, Choose one, and Give that card to your doctor”). A supply of the same standard patient education brochure distributed to the usual care group was made available in the rooms for use by the clinician if the patient wanted more information regarding available resources to support quitting." page 21 "Those clinicians delivering the decision aid watched an 8‐min video demonstrating the use of the decision aid and had an opportunity to ask questions. The total length of the briefing did not exceed 30 min for any clinician." page 21 Single intervention: patient‐mediated intervention (patient education brochure) Quote: "Patients receiving usual care received from the personnel who brought them into the examination room a standard patient education brochure in clinical use outlining the risk of smoking in the perioperative period, the benefits of quitting, and resources available to support quitting. Clinicians caring for these patients were not instructed regarding how to discuss smoking, but all incorporated advice to quit smoking as a part of their discussion per usual clinical practice in the POE." page 21 |
|
| Outcomes | OPTION and COMRADE (continuous) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For each stratum, a randomization schedule was generated by the Mayo Clinic Division of Biostatistics." page 21 |
| Allocation concealment (selection bias) | Low risk | Quote: "At the time of enrollment, group assignment was determined according to the appropriate stratum using sealed envelopes." page 21 |
| Blinding (performance bias and detection bias) Observer‐based outcome | Unclear risk | Comment: not enough information to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Unclear risk | Comment: not enough information to make a judgement. |
| Incomplete outcome data (attrition bias) Observer‐based outcome | Low risk | Comment: small proportion of losses of follow up (flow chart). |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | Low risk | Comment: small proportion of losses of follow up (flow chart). |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol was registered (Identifier: NCT01575119). |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Observer‐based outcome | Unclear risk | Comment: no outcome measure at baseline. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no outcome measure at baseline. |
| Protection against contamination? | High risk | Comment: randomization was done at the patient level. |
| Baseline characteristics patients | Low risk | Comment: characteristics between groups were similar at baseline (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about clinicians at baseline. |
Wetzels 2005.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: group of providers (a practice) Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: primary care and ambulatory care (20 practices in south‐eastern Netherlands) Health professionals: 25; general practitioners, unclear level of training Patients: 1246; various clinical conditions; male and female |
|
| Interventions |
Multifaceted intervention: educational outreach visit , patient‐mediated intervention; 30 minutes (educational outreach visit). All patients received a consultation leaflets by mail. The leaflet provided a motivational text, including a series of questions, encouraging patient involvement. The general practitioners received a 30‐minute visit, in which they were motivated to involve the patient and to use the brochure. No intervention (control) |
|
| Outcomes | COMRADE (4 items, continuous); joint process between healthcare professionals and patients to make decisions | |
| Notes |
Additional information Number of approached patients (eligible): 1246 Number of patients per physician: approximately 30 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "To secure blinding of allocation, practices were numbered in the order of their arrival in our mail. All participating GPs in a particular practice were randomised to the same intervention." page 287 |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent person, who was blinded for the practices as these were numbered, performed the allocation." page 287 |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: All GPs in one practice were assigned to an intervention by a person blinded to the study." page 287 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: response rates were balanced in proportion across group in pre intervention (52,6% vs 52,7%) and postintervention. However, reasons for non response were not reported Quote: "Secondly, there were many missing values, suggesting that the questionnaire might have been too difficult for our study subjects." page 293 |
| Selective reporting (reporting bias) | Unclear risk | Comment: no evidence that outcomes were selectively reported, but no protocol. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: not specified in the paper. |
| Protection against contamination? | Low risk | Comment: practices were randomized, GPs remained in their respective practices. |
| Baseline characteristics patients | Low risk | Comment: see table 1 and 2. |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about professionals. |
Wilkes 2013.
| Methods |
Study design: cluster‐randomized trial Unit of allocation: waiting areas Unit of analysis: provider and patient Power calculation: done |
|
| Participants |
Care setting: primary care and ambulatory care; USA Health professionals: 120; physicians in internal and family medicine; fully trained Patients: 712 patients + unknown number of simulated patients; prostate cancer screening; male |
|
| Interventions |
Three arms Multifaceted intervention: patient‐mediated intervention, distribution of educational material Quote: "The study had 3 arms: usual practice (control) and 2 intervention arms (Figure1). Physicians in both intervention arms participated in an interactive Web‐based educational program. In one intervention arm physicians saw only the educational program (MD‐Ed). The other intervention also including activated patients (MD‐Ed+A), who viewed a different, but related, program that both provided information and encouraged them to participate actively in the decision to pursue prostate cancer screening...Brochures on prostate cancer screening from the Centers for Disease Control and Prevention (the only materials provided for control patients) were available in the waiting areas of all enrolled practices. We developed 2, 30‐minute interactive educational Web‐based programs on prostate cancer screening, one for physicians and another for patients...We also sent laminated screen shots of essential diagrams to physicians in both intervention arms for use while counseling patients about likelihood of harm and benefit around prostate cancer screening...The patient program includes video vignettes to depict the potential harms for 2 scenarios: (1) not having prostate cancer screening (a regretful patient dying of advanced prostate cancer), and (2) having prostate cancer screening with a false‐positive result (a regretful patient with impotence from an ostensibly nontherapeutic prostatectomy)." pages 325‐326 Single intervention: distribution of educational material Usual care (control) |
|
| Outcomes | Overall PSA (prostate‐specific andogen) Shared Decision Making perception (continuous) | |
| Notes |
Additional information Number of approached patients (eligible): 2913 in the MD‐Ed+A Intervention arm, 2952 in the MD‐Ed Intervention arm, 3517 in the control arm were solicited by mail; 134 physicians were assessed for eligibility Number of patients per physician: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not reported in the paper. |
| Allocation concealment (selection bias) | Low risk | Comment: waiting areas were randomized. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | Low risk | Quote: "With regard to blinding, patients and standardized patients were not aware of the multiple study arms or the arm to which their physician was assigned. The standardized patients were told that they were assessing standard differences in physician communication styles." page 325 column 2 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: imbalance of missing outcomes data across groups: control 13,6%, MD‐Ed 19,5%, MD‐Ed+A 4,4%. It is possible that a particular attention has been done in the follow‐up of the MD‐Ed+A group and this may be related to the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no baseline measure of our primary outcome. |
| Protection against contamination? | Low risk | Quote: "We chose a cluster randomized design because we assumed that physicians who share a common waiting area (3 to 8 physicians) would interact with each other, as might their patients, creating potential contamination." page 325, column 2 |
| Baseline characteristics patients | Low risk | Comment: reported and similar (See table 1). |
| Baseline characteristics healthcare professionals | Low risk | Comment: reported and similar (See table 2). |
Wolderslund 2017.
| Methods |
Study design: randomized trial Unit of allocation: patient Unit of analysis: patient Power calculation: done |
|
| Participants |
Care setting: ambulatory care, specialized care, Denmark Health professionals: 49; various types; fully trained Patients: 4349; pediatrics |
|
| Interventions |
Multifaceted intervention: Patient‐mediated intervention (question prompt list) + other (digital audio recording) Single intervention: other (digital audio recording) Usual care: Quote: "The study was designed as a three‐armed randomised controlled trial. One group of patients received standard care (Control), while the other two groups received either QPL in combination with a recording of their consultation (QPL‐DAR) or only the recording (DAR)." page 244 |
|
| Outcomes | Involvement in decision making (qualitative) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned on a weekly basis to one of the three groups using computer‐generated random numbers." page 244 |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information to make a judgement. |
| Blinding (performance bias and detection bias) Participant‐reported outcome | High risk | Comment: Quote: "Neither the patients nor the health professionals were blinded to the randomisation group. Blinding would have required recordings of consultations in the control group without the patients having the possibility to replay the consultation, which we found unethical." page 248 |
| Incomplete outcome data (attrition bias) Participant‐reported outcome | High risk | Comment: very high rate of loss to follow‐up and proportion of losses to follow‐up were not well‐balanced between groups (flow chart). |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available to permit judgement. |
| Other bias | Low risk | Comment: no evidence of other risk of biases. |
| Baseline measurement? Participant‐reported outcome | Unclear risk | Comment: no information. |
| Protection against contamination? | Unclear risk | Comment: not enough information to make a decision. |
| Baseline characteristics patients | Low risk | Comment: characteristics between groups were similar at baseline (Table 1). |
| Baseline characteristics healthcare professionals | Unclear risk | Comment: no information about clinicians at baseline. |
ACEPP: Assessing Communication about Evidence and Patient Preferences; ACP: Advanced care planning; ADDITION: Anglo‐Danish‐Dutch Study of Intensive Treatment;AHRQ: Agency for Healthcare Research and Quality; ASK: Ask Share Know; BRCA: Breast cancer susceptibility gene; CAPI: Computer Assisted Personal Interviewing; CCS: Canadian Cardiovascular Society; CGD: Chronic granulomatous disorder; CHC: Community health centre; CHD: Coronary heart disease; CHW: Community health worker; CME: Continuing medical education; COMRADE: Combined Outcome Measure for Risk Communication and Treatment Decision‐making Effectiveness; COPD: Chronic obstructive pulmonary disease; CPS: Control Preferences Scale; CRC: Colorectal cancer; CRS: Conventional risk score; CVD: Cardiovascular disease; DA: Decision aid; DAS‐O: Decision Analysis System for Oncology; DCS: Decision Control Scale; EHR: Electronic health record; EI: Enhanced intervention; FAPI: Fragebogen zur Arzt‐Patient‐Interaktion (quality of physician‐patient interaction scale); FCR: Family‐centered rounds; FRAX: WHO online calculator for discussing treatment options; GI: gastrointestinal; GP: General practitioner; GRS: Genetic risk score; HCCQ: Health Care Communication Questionnaire; HCP: Healthcare professional; HMO: Health maintenance organization; ICTRP: International Clinical Trials Registry Platform (WHO); ID: Identification; IPC: Interpersonal Processes of Care; MI: Mediated intervention; MS: Multiple sclerosis; NA: Not applicable; OPTION: Observing patient involvement; PAM: Patient Activation Measure; PANDA: Patient decision aid; PCI: Percutaneous coronary intervention; PCP: Primary care physician; PDA: Patient decision aid; PDM: Participatory Decision Making; PEF‐FB‐9: Partizipative Entscheidungsfindung‐Fragebogen‐9; PP: Patient participation; PROM: Patient‐reported outcome measure; PROMIS: Patient‐Reported Outcomes Measurement Information System; QPL: Question prompt list; QPS: Question prompt sheets;QQPPI: Questionnaire on the Quality of Physician‐Patient Interaction; RA: Rheumatoid arthritis; SAS: Statistical Analysis System; SDM‐Q9: Shared Decision Making Questionnaire (9‐item); SDM: Shared decision making; SDMI: Shared decision making intervention; SES: Socioeconomic status; TAS: Treatment Alliance Scale; TTE: Time to event; TTO: Time trade‐off; UC: Usual care; VOICE: Valuing Opinions, Individual Communication and Experience.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 2006 | The design of the study was not appropriate |
| Aljumah 2015 | Data regarding the outcome were not available |
| Allen 2009 | The study type was not appropriate. This is a one group pre/post‐test quasi‐experimental design |
| Boehmer 2014 | The outcome was not appropriate |
| Boyd 2010 | The outcome was not appropriate |
| Brinkman 2013 | The study design was not appropriate |
| Brown 2004 | The outcome was inappropriate, only preference was stated |
| Davison 2007 | The intervention was after the consultation |
| Golnik 2012 | The design of the study was not appropriate. Inappropriate number of control sites, less than four |
| Green 2011 | The outcome was not appropriate |
| Hack 2007 | The intervention was after the consultation |
| Hanson 2011 | The outcomes were not appropriate |
| Harmsen 2014 | The intervention was not appropriate |
| Hermansen Kobulnicky 2002 | Relevant data were not presented and are clearly unobtainable |
| Hoffman 2014 | Hypothetical scenario |
| Jangland 2012 | The study design was not appropriate |
| Koekkoek 2012 | The study design was not appropriate |
| Kopke 2009 | The outcomes were not appropriate, only the active patient was reported and not the shared decision |
| Kupke 2013 | The outcome was not appropriate |
| Langewitz 1998 | The outcome related to SDM is limited to a single item from an observer‐based multiple instrument |
| Leader 2012 | The outcomes of the study were not appropriate |
| LeBlanc 2017 | The outcome was not appropriate |
| Man‐Son‐Hing 1999 | The outcomes of the study were not appropriate |
| Maslin 1998 | Relevant data were not presented and are clearly unobtainable |
| McCormack 2011 | The design of the study was not appropriate. Inappropriate number of control sites, less than four |
| NCT01550731 | The outcome was not appropriate |
| NCT02033499 | The outcome was not appropriate |
| NCT02319525 | The outcome was not appropriate |
| Ockhuysen‐Vermey 2008 | The outcomes of the study were not appropriate |
| Price‐Haywood 2014 | The outcome was reported by a standardized patient |
| Riippa 2014 | The study design was not appropriate |
| Roelands 2004 | The outcomes of the study were not appropriate |
| Schwalm 2012 | The outcomes of the study were not appropriate |
| Simon 2012 | The participants in the study were not appropriate. The healthcare professional was virtual, so it was difficult to measure SDM |
| Smith 2010a | The outcomes of the study were not appropriate; we could not be sure if the preference for involvement in the screening decision was the actual or preferred involvement. |
| Spertus 2011 | The design of the study was not appropriate. This is a pre‐post‐ cross‐sectional study |
| van Tol‐Geerdink 2008 | The design of the study was not appropriate |
| Wagner 2012 | The outcome was not appropriate |
| Whelan 2003 | The outcomes of the study were not appropriate, only the active patient was reported and not the shared decision |
SDM: shared decision making.
Characteristics of studies awaiting assessment [ordered by study ID]
Simmons 2017.
| Methods | Study design not clear (a variety of qualitative and quantitative methods were used) |
| Participants | Young people with emerging or established mental disorders |
| Interventions | Decision aids |
| Outcomes | Decisional conflict, perceived involvement, choice of guideline concordant treatment option, depression scores (the list is not exhaustive) |
| Notes |
van Veenendaal 2017.
| Methods | Study design not clear (we are not sure if there is a control group) |
| Participants | Breast cancer patients |
| Interventions | The introduction of time‐out periods and an participation in implementation program for SDM
The hospitals participated in a tailor‐made implementation program consisting of:
|
| Outcomes | Involvement of breast cancer patients in decision‐making (OPTION5, SDM‐Q9) |
| Notes |
OPTION: Observing patient involvement; SDM: Shared decision making; SDM‐Q9: Shared Decision Making Questionnaire (9‐item)
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000593639.
| Trial name or title | The impact of guiding patients suffering from wisdom tooth problems through Dental Open Educational Resources (DOER) on enhancing shared clinical decision‐making and improving health care outcomes: a randomized controlled trial. |
| Methods | RCT |
| Participants | Inclusion criteria: participants who have been diagnosed with wisdom teeth problems otherwise fit and healthy, aged 18‐39 years, internet users, signed the informed consent Exclusion criteria: non internet user, have serious health problems, non English speakers. |
| Interventions |
Intervention group (study group) Participants of this group will receive a list of recommended Dental Open Educational Resources as an additional resources for patient educational materials one month before consultation. Participants can access these information at any time as they are freely accessible online. Knowledge and anxiety will be assessed in pre‐consultation survey. Patient participation in SDM, satisfaction and QoL will be evaluated post intervention pre‐consultation and at one month post consultation/surgery via a survey that will be posted to them. In the pre‐consultation survey, participants will be asked if they reviewed the provided resources. A sample of 25 participants will be invited for structured interview that will take place face to face, online, over the phone depending on patient preference. Control group Participants will receive standard care patient education (verbal communication and information leaflets) at consultation. At consultation participants will be requested to fill in a pre‐consultation survey to assess their knowledge and anxiety. Patient participation in SDM, satisfaction and quality of life will be evaluated through a one‐month post consultation/surgery survey that will be conducted either online or by post depending on patient preference. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | September 2014 |
| Contact information | Dr Kamal Hanna, +61, 08, 83135626, kamal.hanna@adelaide.edu.au |
| Notes |
ACTRN12616000213448.
| Trial name or title | Development and evaluation of an Australian adult health literacy program for socially disadvantaged adults attending TAFE (Technical And Further Education). |
| Methods | Randomized controlled trial |
| Participants | People with low literacy and low health literacy |
| Interventions | Classes (clusters) are randomly allocated to receive either the health literacy intervention (an 18‐week program with health knowledge and skills embedded in language, literacy, and numeracy training (LLN)), or the standard Language Literacy and Numeracy (LLN) program (usual LLN classes, specifically excluding health content). |
| Outcomes | Primary outcome: functional health literacy skills – knowing how to use a thermometer, and read and interpret food and medicine labels. Secondary outcomes: self‐reported confidence, health literacy; shared decision making skills, patient activation, health knowledge and self‐reported health behavior. |
| Starting date | February 2014 |
| Contact information | Prof Kirsten McCaffery; +61 2 9351 7220; kirsten.mccaffery@sydney.edu.au |
| Notes | Recruitment is completed |
ACTRN12616000644460.
| Trial name or title | Effect of decision aids for acute respiratory infections on the use of antibiotics in general practice: a cluster‐randomised controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria
General practice in the recruitment region, minimum age 18 years Exclusion criteria General practice is currently, or within the last 2 years, participated in a research study aimed at reducing antibiotic prescribing |
| Interventions | Brief name: Decision aids for ARIs GPs will be given copies of three patient decision aids (one each for acute otitis media, sore throat, and acute bronchitis) and a brief training package (to be completed at their convenience). Each decision aid is a two‐page (double‐sided) document; the training package is a short video (˜15 minutes) explaining about SDM and use of decision aids; a list of frequently asked questions (by GPs) about the decision aids will also be provided. Usual care: GPs at the control practices will not receive access to the training package or the decision aids. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | June 2016 |
| Contact information | Prof Tammy Hoffmann, +61 7 5595 5522, thoffmann@bond.edu.au |
| Notes |
ACTRN12617000614392.
| Trial name or title | Whakapai e Te Ara Ha: Asthma Self‐Management Programme. A single‐blinded, parallel group, randomised controlled trial of the impact of a culturally‐relevant peer‐support and self‐management programme, on activation and quality of life among the parents/caregivers of Maori children aged 4‐13 years old with asthma in New Zealand, and asthma control, quality of life and health‐care utilization among their children. |
| Methods | RCT |
| Participants |
Inclusion criteria Parents/caregivers will be eligible to participate if their child is: a) between 4‐13 years, b) identifies as NZ Maori ethnicity (prioritized, self‐/parental‐reported), c) has a doctor's diagnosis of asthma, d) has a previous hospitalization or ED presentation for asthma or wheeze (ICD‐10‐AM code: J45,J46 or R06.2), and e) usually resides within the geographical catchment area of the participating DHBs (District Health Boards). Exclusion criteria Parents/caregivers will be excluded from participating if their child: a) has previously been enrolled in the study, b) has a sibling on the study, c) does not reasonably expect to remain in the region for the duration of the intervention and follow‐up, or d) have other chronic respiratory comorbidities that may be deemed to interfere with the study (e.g. bronchiectasis). |
| Interventions | The intervention group will receive a six‐month holistic, culturally‐based peer‐support program. The intervention will comprise two phases: an initial 6‐week intensive 'whakawhanaungatanga’ (relationship building) period and an ‘awhi’ (support) maintenance period over the remainder of the 6 months. Usual Care: participants in the control group will receive appropriate educational resources (Children and Asthma Booklet) from the Asthma Foundation of NZ and be followed‐up by their usual GP without limitation. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | May 2017 |
| Contact information | Dr Tristram Ingham, +6449186842, tristram.ingham@otago.ac.nz |
| Notes |
ACTRN12617000840381.
| Trial name or title | Phase II randomised controlled trial evaluation of treatment decision‐aid for patients with bipolar II disorder and their family considering treatment options for relapse prevention |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment decision‐aid intervention: This decision‐aid website for bipolar II disorder, developed by PI Juraskova, CIs and professional web designers/developers. The decision‐aid explains main available medication and psychological treatment options for relapse prevention in bipolar II disorder, based on available guidelines; with specific sections for patients’ family.The decision‐aid website can be accessed by the patient participant and/or family member at any frequency and duration in the period following diagnosis and leading up to follow‐up consultation/s with their managing psychiatrist, GP and/or clinical psychologist. Usual care/attention control will comprise: any information materials (e.g. fact sheets) that patients are already routinely provided with, or advised to consult during their appointment at the Black Dog Institute (recruitment site); and the existing Black Dog Institute web pages on treatments for bipolar disorder. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2017 |
| Contact information | A/Prof Ilona Juraskova, +61 2 9351 6811, ilona.juraskova@sydney.edu.au |
| Notes |
Adekpedjou ongoing.
| Trial name or title | Improving the decision‐making process with caregivers of elderly people about housing options: a cluster randomised trial (NCT02244359) |
| Methods | Cluster‐randomised trial |
| Participants | Inter‐professional teams involved with eligible caregivers in decision‐making about planning care for their loved one, caregivers of cognitively impaired elderly people |
| Interventions | Training in SDM and use of a decision guide |
| Outcomes | Primary outcome: role assumed in the decision‐making process as assessed by caregivers using a modified version of the Control Preferences Scale. Secondary outcomes: preferred option and decision made, match between role preferred and assumed in decision‐making, decisional conflict, decision regret, and burden of care of caregivers. |
| Starting date | September 2014 |
| Contact information | France Légaré; (418) 663‐5919; france.legare@mfa.ulaval.ca |
| Notes | The study is completed but results of the trial are not published yet |
Altshuler 2016.
| Trial name or title | Transforming the patient role to achieve better outcomes through a patient empowerment program: a randomized wait‐list control trial protocol |
| Methods | Randomized controlled trial |
| Participants | English‐speaking adult patients with type 2 diabetes mellitus from three urban clinical sites in New York City |
| Interventions | The PEP (Patient Empowerment Program) intervention consists of two facilitated small group sessions. Session 1 focuses on defining HCP and patient roles in the medical encounter by introducing ideal communication behaviors in each role and by providing both positive and negative examples of patient‐HCP encounters. Session 2 focuses on practicing communication skills by role‐playing with actors who serve as standardized healthcare providers. After the role play, participants set goals for their own health care and for future interactions with their HCPs. |
| Outcomes | Outcome measures include the Patient Activation Measure; Ask, Understand, Remember Assessment; Krantz Health Opinion Survey; SF‐12v2 Health Survey; Diabetes Self‐Management Questionnaire; and HbA1c. |
| Starting date | |
| Contact information | Lisa Altshuler, PhD; 1 (646) 501 4136; Lisa.Altshuler [at] nyumc.org |
| Notes |
DRKS00000191.
| Trial name or title | Advance directives as an example for shared decision making in the General Practitioner practice |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Arm 1: consultation for completing an Advance Directive after training of General Practitioners in Shared Decision Making Arm 2: control group, treatment as usual |
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | November 2009 |
| Contact information | Lehrbereich Allgemeinmedizin Universitätsklinik Freiburg Elsässerstr. 2 79110 Freiburg i. Br. Germany |
| Notes |
DRKS00010880.
| Trial name or title | Shared Decision Making PLUS – a cluster‐randomized trial with inpatients suffering from schizophrenia |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Arm 1: SDM PLUS intervention: training for treatment teams how to implement SDM (2x4h workshops on SDM and other communicative techniques) + training for patients how to facilitate SDM (5 x 60 min. interactive group training) Arm 2: treatment as usual |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | October 2016 |
| Contact information | Mr. Prof. Dr. Johannes Hamann Ismaninger Straße 22 81675 München Germany Telephone: 089/41404282 Fax: 089/41406688 E‐mail: j.hamann at tum.de |
| Notes |
Dwinger ongoing.
| Trial name or title | Development and pilot testing of a face‐to‐face SDM coaching intervention for oncologists |
| Methods | Pilot study for a RCT |
| Participants | Patients and physicians |
| Interventions | The content of the training is based on a SDM manual evaluated in previous studies. The training is structured in two parts. First the SDM approach, including information models, definition of SDM, and the applicability of SDM are explained in a dialogue. Second the steps of SDM (team talk, option talk, decision talk) are explained and discussed using the videotape of the first consultation with the simulation patient. |
| Outcomes | OPTION12 and MAPPIN’ SDM from three perspectives: physician, patient and observer |
| Starting date | Unknown |
| Contact information | Sarah Dwinger, University Medical Center Hamburg‐Eppendorf, Department of Medical Psychology, Germany |
| Notes | Information retrieved from the 9th International Shared Decision Making Conference 2017 Book of Abstract |
Fagerlin ongoing.
| Trial name or title | The impact of shared decision making on patient involvement in two prostate cancer decision aid trials |
| Methods | Randomized controlled trial |
| Participants | Men with Gleason ≤ 7 localized disease at 1 University medical center |
| Interventions | Decision aid |
| Outcomes | Self‐efficacy to participate in shared decision‐making skills |
| Starting date | |
| Contact information | Angela Fagerlin, PhD; 801‐587‐2100; Department of Population Health Sciences 295 Chipeta Way, Room: Room 1S105 Salt Lake City, UT 84108 |
| Notes |
Finderup 2017.
| Trial name or title | Developing and pilot testing a shared decision‐making intervention for dialysis choice |
| Methods | Study design not clear |
| Participants | Patient facing the decision to choose the dialysis modality |
| Interventions | Intervention for SDM targeting the choice of dialysis modality (a manual for SDM, including a variety of decision aids) |
| Outcomes | Dialysis choice, shared decision making (SDM‐Q9) |
| Starting date | |
| Contact information | |
| Notes |
Henselmans ongoing.
| Trial name or title | Effect of a skills training for oncologists on shared decision making about palliative chemotherapy in simulated encounters |
| Methods | Randomized controlled trial |
| Participants | Oncologists and oncology residents |
| Interventions | SDM training The training was based on a 4‐step model of SDM, including (1) setting the SDM agenda, (2) informing about options, (3) exploring patient values, and (4) making a decision. The training focused on SDM about palliative systemic treatment and consisted of a reader, two 3.5‐hour group sessions using modeling videos and role play, a booster session including individual feedback on a audio‐recorded consultation in clinical practice, and a consultation card with the SDM steps and example phrases. |
| Outcomes |
Primary outcome: observed SDM as assessed with the OPTION12 (Observing Patient Involvement). Secondary outcomes: observed SDM per step (self‐developed instrument), general communication skills ratings (providing information and anticipating/responding to patient emotions) and oncologists’ satisfaction with communication (PSQ). |
| Starting date | |
| Contact information | Inge Henselmans, PhD |
| Notes |
ISRCTN37929939.
| Trial name or title | Nurse‐led immunotherapy decision coaching in persons with relapsing‐remitting multiple sclerosis (DECIMS) |
| Methods | Evaluator‐blinded cluster‐randomized controlled trial |
| Participants | Patients with suspected or the relapsing form of MS (‘relapsing‐remitting MS’) who are facing a decision on starting, stopping, or changing MS immunotherapy |
| Interventions |
Experimental intervention One to three counseling (decision coaching) sessions with specially trained nurses, supported by an evidence‐based online patient information tool prior to a decisional encounter with a physician. Control intervention Counselling as usual and access to an evidence‐based online information tool. |
| Outcomes |
Primary outcomes: informed choice (Multi‐dimensional Measure of Informed Choice (MMIC)) including the sub‐dimensions risk knowledge, attitude and uptake; attitude towards immunotherapy; uptake of immunotherapy; risk knowledge Secondary outcome: Decisional Conflict Scale (DCS); control Preference Scale (CPS); planned Behaviour in MS (PBMS); the Coping‐Self‐Efficacy‐Scale (CSES); duration of physician encounters; decision adherence |
| Starting date | June 2014 |
| Contact information | Prof. Christoph Heesen; heesen@uke.de |
| Notes | The study is completed but results of the trial are not published yet |
ISRCTN46305518.
| Trial name or title | Informed shared decision making supported by decision coaches for women with ductal carcinoma in situ |
| Methods | RCT |
| Participants | Women are eligible if they are at least 18 years old, have no known BRCA1/2‐mutation, are not pregnant and have a primary DCIS. All participants need sufficient German language skills. |
| Interventions | The intervention includes a four‐day training program in SDM for specialized nurses, a two hour‐lasting workshop in SDM for physicians and an evidence‐based patient decision aid and decision coaching for women. Women in the control group receive standard care. |
| Outcomes | Involvement in treatment decision making and decisional conflict |
| Starting date | October 2014 |
| Contact information | Martin‐Luther‐King‐Platz 6 Hamburg 20146 Germany +49 40 42838 7152 Birte.Berger‐Hoeger@uni‐hamburg.de |
| Notes |
ISRCTN63110516.
| Trial name or title | ACTION: cancer patient involvement in medical decision making |
| Methods | Cluster‐randomized trial |
| Participants | Adult patients with advanced stages of lung or colorectal cancer |
| Interventions | The 'Respecting Choices Program' is a formalized model of advance care planning developed and currently being used in the USA and Australia. In this program, a trained 'Respecting Choices Facilitator' invites patients to reflect on their personal goals, values and beliefs, to discuss and document their choices regarding their future treatment and care and to nominate someone who they may wish to be consulted about their treatment or care if they are not able to make decisions for themselves. |
| Outcomes |
Primary outcome: quality of life and symptoms Secondary outcomes: coping with their illness, decisional Quality and Patient Activation, satisfaction with care, satisfaction with the intervention |
| Starting date | 01/11/2014 |
| Contact information | Miss Lesley Dunleavy; l.dunleavy@lancaster.ac.uk |
| Notes | The study is completed but results of the trial are not published yet |
Lifford ongoing.
| Trial name or title | Bridging the Age Gap trial of decision support interventions for older women with breast cancer: Preliminary process evaluation |
| Methods | RCT |
| Participants | 40‐60 patients from a subsample of trial sites and 12‐20 clinicians (surgeons, oncologists and specialist nurses) |
| Interventions | The decision support interventions (DESIs) consist of an online algorithm (primarily for clinicians, with a patient print‐out available) which predicts personalized survival rates with each treatment, a short tool (for use within consultations) and a booklet of information with a values‐clarification section (for use outside consultations). |
| Outcomes | Usage of the decision support interventions and levels of SDM |
| Starting date | Unknown |
| Contact information | Kate Lifford, Cardiff University, Cardiff, UK |
| Notes | Information retrieved from the 9th International Shared Decision Making Conference 2017 Book of Abstract |
Ludden ongoing.
| Trial name or title | Who Made the Decision Today?” Surveying asthma patients level of shared decision making in an RCT |
| Methods | Randomized controlled trial |
| Participants | Asthma patients |
| Interventions | The FLOW intervention involved customized training sessions with clinics to incorporate the SDM toolkit into workflows unique to each practice. |
| Outcomes | Shared decision making [Who made the decision in your meeting with the care team (health coach and provider) about what your asthma treatment would be?] |
| Starting date | |
| Contact information | Dr Thomas Ludden |
| Notes |
NCT01485627.
| Trial name or title | VOICE: Values and Options in Cancer Care (VOICE) |
| Methods | RCT |
| Participants | Age : 21 Years and older Physicians
Patients
Caregivers
|
| Interventions | Oncologists will receive communication training. Patients will be coached to make the most of the oncologist visit. |
| Outcomes |
Primary outcome: improved patient‐physician‐caregiver communication; prolonged grief symptoms (Caregiver Bereavement). Secondary outcomes: improved patient‐perceived communication; patient and caregiver well‐being; caregiver physical health outcomes; health care utilization. Other outcomes; examine whether caregiver outcomes are mediated by patient‐reported quality of life and patient healthcare utilization (quantitative). Explore caregiver perspectives on decision‐making and communication processes to link bereavement outcomes with VOICE study communication outcomes (qualitative). |
| Starting date | April 2011 |
| Contact information | Contact information is only displayed when the study is recruiting participants |
| Notes |
NCT01519999.
| Trial name or title | Colorectal cancer screening with improved shared decision making (CRCS‐WISDM) |
| Methods | Non‐randomized controlled trial |
| Participants | Patients 50‐75 years seen in the participating primary care clinics during the study period who are non‐adherent to CRCS recommendation |
| Interventions |
Behavioral: SDM for colorectal cancer screening Quote: "Age‐eligible adults in the intervention communities will be exposed to the SDM intervention when they are seen in the primary care clinics (N = all patients 50‐75 years seen during the study period who are non‐adherent to CRCS recommendation). Additionally, among patients with primary care visits scheduled one week or more before the visit, they will be randomized to receive either a mailed decision aid booklet or an informational flyer on shared decision making and CRCS prior to the visit. Patients with primary care visits scheduled less than one week prior to the visit will not be mailed materials in advance. The effect of the pre‐visit materials on referral to SDM session and CRCS adherence between these groups will be compared. They will also be exposed to SDM tools and resources available through the community‐wide intervention activities." |
| Outcomes | Colorectal cancer screening adherence. |
| Starting date | May 2012 |
| Contact information | Principal Investigator: Resa M Jones, MPH, PhD from Virginia Commonwealth University. |
| Notes |
NCT01828567.
| Trial name or title | Will veterans engage in prevention after HRA‐guided shared decision making? (ACTIVATE) |
| Methods | RCT |
| Participants | Veterans enrolled in primary care at the Durham or Ann Arbor Health Care Systems and who have one modifiable risk factor identified by a healthy living assessment (physical inactivity, overweight or obese by BMI, or tobacco user) |
| Interventions | Behavioral: SDM with a prevention coach. A series of two phone sessions with a prevention coach. The first to engage the veteran to choose a preferred prevention program and link them to PACT, and a follow‐up call one month later to assess the progress of the prevention plan. |
| Outcomes |
Primary outcome: enrollment in prevention services. Secondary outcomes: patient activation measures, Framingham Risk Score |
| Starting date | October 2014 |
| Contact information | Principal Investigator: Eugene Z Oddone, MD MHSc; Durham VA Medical Center, Durham, NC, United States, 27705 Principal Investigator: Laura J. Damschroder, MPH; VA Ann Arbor Healthcare System, Ann Arbor, MI, USA, 48105 |
| Notes |
NCT01837953.
| Trial name or title | Stepped care for binge eating disorder: predicting response to minimal intervention in a randomized controlled trial |
| Methods | RCT |
| Participants | Adults aged 18 years and older with Binge Eating Disorder (BED) |
| Interventions | Two interventions. Behavioral: Group Psychodynamic Interpersonal Psychotherapy Quote: "For those participants randomized to the USH (Unguided Self‐Help) + Group Psychodynamic Interpersonal Psychotherapy (GPIP) condition, this intervention will consist of 16 weekly 90 minute sessions of GPIP. GPIP was developed and empirically tested in a randomized controlled trial (RCT) at our Centre. GPIP will be preceded by an individual pre‐group preparation session conducted by a psychologist trained in GPIP to orient the patient to the therapy. Patients are given a rationale for the treatment. Examples of the patient's cyclical relational patterns (CRPs) that may underlie their symptoms are discussed and the patient will be encouraged to work on these in the groups. Therapists will be given a written summary of each patient's CRP." Behavioral: USH Quote: "All participants will first receive 10 weeks of USH. The USH will be based on Dr. Christopher Fairburn's CBT‐oriented and evidence based self‐help treatment plan for binge eating explained in his book, Overcoming Binge Eating. The USH program follows six steps: (1) Getting Started: Self‐monitoring, weekly weighing; (2) Regular Eating: Establishing a pattern of regular eating; (3) Alternatives to Binge Eating: Substituting alternative activities; (4) Problem Solving and Taking Stock: Practicing problem solving and reviewing progress; (5) Dieting and Related Forms of Avoidance: Tackling the three forms of dieting and other forms of avoidance eating; and (6) What Next? Preventing relapse and dealing with other problems." |
| Outcomes |
Primary outcome: Binge Eating Episodes in the Past 28 Days. Secondary outcomes: Body Mass Index (BMI); Center for Epidemiologic Studies Depression Scale (CES‐D); Experiences in Close Relationships Scale (ECR); Inventory of Interpersonal Problems (IIP‐64); Patient Health Questionnaire 9 (PHQ‐9); Rapid Response to Treatment: Self‐Monitoring; Rosenberg Self Esteem Scale (RSES); Eating Disorder Diagnostic Scale (EDDS); Experiences in Close Relationships Scale Short Form (ECR‐S); Therapeutic Factors Inventory (TFI); Outcome Rating Scale (ORS); Working Alliance Inventory Short (WAI‐S); Eating Disorder Examination ‐ Questionnaire (EDE‐Q); Depression Anxiety and Stress Scales 21(DASS‐21) |
| Starting date | November 2012 |
| Contact information | Contact information is only displayed when the study is recruiting participants |
| Notes |
NCT01838226.
| Trial name or title | Randomized controlled trial of group prevention coaching |
| Methods | RCT |
| Participants | Adults aged 21 years and older with:
|
| Interventions |
Behavioral: Problem Solving A group problem‐solving intervention, with interval phone calls delivered to check in on goal progress and reinforce group learning. Groups will meet monthly for 6 months, and each patient will be called once between each group session. Each group will consist of 10 patients. Problem‐solving teaches patients to overcome internal barriers to healthful behaviors. Problem solving will be combined, at all group sessions, with self‐efficacy training, so that patients will be taught simultaneously to overcome both internal and external barriers. Participants will be asked to develop personal goals related to CVD‐related behaviors (e.g., smoking and weight reduction). |
| Outcomes |
Primary outcome: risk of fatal coronary event or non‐fatal MI Secondary outcomes: International Physical Activity Questionnaire; Block Brief 2000 Food Frequency Questionnaire; Patient Activation Measure. |
| Starting date | August 2014 |
| Contact information | Michael Owings, BS 716‐862‐8590 Michael.Owings2@va.gov David Edelman, MD MHS (919) 286‐6936 david.edelman@va.gov |
| Notes |
NCT01866228.
| Trial name or title | Clinical trial of the impact of treatment consultation recordings on cancer patient outcomes |
| Methods | RCT |
| Participants | Adults aged 18 years and older presenting with a primary diagnosis of non‐recurrent or metastatic brain, or neuroendocrine cancer |
| Interventions | Consultation Recording. The main goal of this study is to demonstrate the benefits of giving cancer patients an audio‐recording of their first consultation with their cancer doctor. Patients will receive their recording immediately after their consultation, and will be able to listen to the recording at any time either alone, or with family and friends. |
| Outcomes | Primary outcomes: Control Preferences Scale; Patient Satisfaction with Cancer Care Scale; PrestMan Satisfaction with Doctor Scale; Hospital Anxiety and Depression Scale; Perception of Being Informed Scale. |
| Starting date | June 2013 |
| Contact information | Contact information is only displayed when the study is recruiting participants. |
| Notes |
NCT01992926.
| Trial name or title | Facilitating anemia treatment risk communication for patients with kidney disease: decision aid trial |
| Methods | Randomized controlled trial |
| Participants | Patients (over 18, under 80 years of age) with chronic kidney disease or end stage renal disease |
| Interventions | Interactive educational intervention:use of a concise, literacy‐sensitive, physician‐led, educational interaction with the patient. |
| Outcomes | Primary outcome measures: change in patient understanding of anemia and treatment options |
| Starting date | November 2013 |
| Contact information | Contact information is only displayed when the study is recruiting participants |
| Notes | The study is completed but results of the trial are not published yet |
NCT02047929.
| Trial name or title | Comparing types of implementation of a shared decision making Intervention (ADAPT‐NC) |
| Methods | RCT |
| Participants | 1 Year and older Medicaid patients with the diagnosis of asthma |
| Interventions | Asthma Shared Decision Making (SDM) Toolkit Quote: "A potential solution to improving asthma outcomes is the use of patient‐centered approaches like Shared Decision Making (SDM), identified by both the Institute of Medicine and the Patient‐Centered Outcomes Research Institute as an important new means of improving patient outcomes. In the SDM process, patients and their health care providers are engaged jointly in making decisions about medical tests and treatments. The research team for this proposal was funded by the Agency for Health Care Research and Quality to build, disseminate and evaluate a novel Asthma SDM Toolkit ‐ The Asthma Comparative Effectiveness Study. The Toolkit development was completed in 2010 and has been in evaluation for 2 years. Initial results show marked improvement in patient adherence to medications, decreases in utilization of the ED and hospital for asthma care. This study will continue to evaluate the Toolkit in a wide array of practices across North Carolina while testing a new method of dissemination." |
| Outcomes |
Primary outcome
Patient perception of shared decision making. Secondary outcomes: Asthma exacerbations, medication adherence. |
| Starting date | August 2013 |
| Contact information | Contact information is only displayed when the study is recruiting participants |
| Notes |
NCT02063087.
| Trial name or title | Sharedd decision making in parents of children with head trauma: Head CT Choice (Head CT Choice) |
| Methods | RCT |
| Participants | Parents and their child, seeking care for a child who:
|
| Interventions |
Head CT Decision Aid Quote: "The decision aid, Head CT Choice, educates parents regarding how the clinician determined the severity of their child's head trauma, their child's quantitative risk for a clinically‐important TBI, the pros and cons of cranial CT compared to active observation, and what signs and symptoms parents should watch for in the next 24 hours that should prompt a return visit to the ED." |
| Outcomes |
Primary outcome: assess parents' knowledge regarding their child's risk for a significant brain injury. Secondary outcomes: patient engagement in the decision‐making process (OPTION); decisional conflict; trust in the physician; parental satisfaction; proportion of children who undergo head CT; healthcare utilization; rate of clinically important traumatic brain injury (ciTBI); fidelity. |
| Starting date | April 2014 |
| Contact information | Erik Hess, MD, MSc; (507)284‐7221; hess.erik@mayo.edu. Melissa Kuntz, BA; (507) 293‐1239; kuntz.melissa@mayo.edu. |
| Notes |
NCT02136732.
| Trial name or title | Chronic care management for adults at federally qualified health centers |
| Methods | RCT |
| Participants | 45 years of age or older, 2 or more chronic conditions, 2 or more emergency department visits or hospital admissions in previous 12 months. |
| Interventions |
Experimental: active self‐management intervention. Participants will receive home visits and phone calls from a registered nurse and social worker. The registered nurse and social worker will provide participants one on one coaching, education, support and referrals to community resources to help them manage their chronic conditions. Active Comparator: attention control phone calls. Participants will receive an initial visit and then a phone call every other month from a social services aide who can provide information about community resources that might be helpful. |
| Outcomes |
Primary outcome: patient activation. Secondary outcome: acute care utilization. Other outcome: participant's health‐related quality of life. |
| Starting date | October 2013 |
| Contact information | Mike Wisor 509‐444‐8888; mwisor@chas.org. Kaleena Reynolds 509‐444‐888; kreynolds@chas.org |
| Notes |
NCT02138448.
| Trial name or title | Implementing personal health records to promote evidence‐based cancer screening |
| Methods | RCT |
| Participants |
|
| Interventions | Intervention practices will implement an interactive preventive health record in addition to their standard personal health record functionality. |
| Outcomes |
Primary outcome: percentage of patients who are up‐to‐date with recommended cancer screening tests in intervention versus control practices. Secondary outcomes: SDM outcomes (knowledge, communication, decisional conflict, and decision control) reported by patients in intervention versus control practices. To assess whether cancer screening rates differ for disadvantaged patients, defined as minorities and Medicaid beneficiaries. To assess whether SDM differ for disadvantaged patients, defined as minorities and Medicaid beneficiaries. To assess whether perceptions of the technology differ for disadvantaged patients, defined as minorities and Medicaid beneficiaries. |
| Starting date | February 2014 |
| Contact information | Alexander H Krist, MD, MPH; 804‐827‐6750; ahkrist@vcu.edu. Rebecca A Aycock, PhD; 8048274121; raycock@vcu.edu. |
| Notes |
NCT02146573.
| Trial name or title | Pediatric Continuity Care Intensivist (CCI) |
| Methods | RCT |
| Participants |
CCI Provider
Usual Care (UC) Provider
Parent‐Patient Dyads
|
| Interventions |
CCI Provider for Parent‐patient dyad Parents and patients are randomly assigned to a Continuity Care Intensivist (CCI) Provider who has received specialized communication training. The parent‐patient dyad will receive standardized care from the CCI throughout their time in the PICU in addition to being assigned a rotating physician of record. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | May 2014 |
| Contact information | Contact information is only displayed when the study is recruiting subjects. |
| Notes |
NCT02165735.
| Trial name or title | Get Ready And Empowered About Treatment (GREAT) |
| Methods | RCT |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental: patient empowerment Participants will take part in six 90‐minute sessions focused on development of basic information technology competency within a context that supports patient autonomy, competence and human relationships. No Intervention: standard care Participants will be followed through usual source of care, without receiving the empowerment training. |
| Outcomes | Primary outcome : patient empowerment based on changes in the Patient Activation Measure (PAM) |
| Starting date | June 2014 |
| Contact information | Kevin A Fiscella, MD MPH, 585‐271‐1206 and Jonathan Tobin, PhD, 212‐382‐0699 ext 234, JNTobin@CDNetwork.org |
| Notes |
NCT02198690.
| Trial name or title | Trial of a mammography decision aid for women aged 75 and older |
| Methods | RCT |
| Participants |
|
| Interventions |
Experimental: Mammography Decision Aid Quote: "Development and pilot testing of the decision aid (DA) has been described previously. In brief, the DA is written at a 6th grade reading level and includes information on 1) breast cancer risk factors for women >75 years; 2) health/life expectancy; 3) likely outcomes if screened and not screened with mammography; 4) competing mortality risks; 5) breast cancer treatments; and 6) a values clarification exercise. The last page asks users their intentions of being screened on a 15‐point validated scale and invites users to share this information with their clinician. PCPs whose patients are randomized to receive the DA will be sent a copy of the DA via email and a link to an optional training on using the DA (5 informational slides and a 3‐minute video)." Placebo comparator: Home safety pamphlet Quote:"To reduce response bias and to compensate for the time and attention required by the intervention group to read the DA, patients in the control arm will be provided a two page pamphlet on home safety for older adults developed by the American Geriatrics Society (AGS) Foundation for Health in Aging. PCPs whose patients are randomized to the receive the home safety pamphlet, will be sent an email informing them that their patient will be coming in early to read health educational materials for older adults as part of a study. We otherwise do not plan any intervention for control group PCPs because we do not want to change their usual behavior. However, if PCPs in the control arm request a copy of the educational materials then we will email them a copy of the home safety pamphlet." |
| Outcomes |
Primary outcome: receipt of mammography screening. Secondary outcomes: screening intentions; knowledge of the pros and cons of mammography screening; Decisional Conflict Scale (DCS); Decision‐making role: preparation for decision‐making; acceptability; anxiety; home safety; screening discussions; home safety discussions. |
| Starting date | September 2014 |
| Contact information | Mara A Schonberg, MD, MPH; 617‐754‐1414; mschonbe@bidmc.harvard.edu. Gianna Aliberti; 617‐754‐1435; galibert@bidmc.harvard.edu. |
| Notes |
NCT02278900.
| Trial name or title | Supporting doctor‐patient communication in oncology |
| Methods | RCT |
| Participants |
|
| Interventions |
Communication aids Quote: "Patients in the intervention group will receive the QPL at home in advance of the consultation along with information about the clinic.The consultations will be recorded in both the control and intervention group. The recording will be done on the computer, and the patients in the intervention group will be given the recording immediately after the consultation on a memory stick." |
| Outcomes |
Primary outcome: difference in number of questions asked, and especially concerning prognosis. Secondary outcome: difference in SDM. Other outcomes: hospital anxiety and depression scale (HADS) score; difference in satisfaction with the consultation and information retrieved; difference in health‐related quality of life |
| Starting date | April 2014 |
| Contact information | Contact information is only displayed when the study is recruiting participants. |
| Notes |
NCT02282722.
| Trial name or title | Improving informed consent for palliative chemotherapy |
| Methods | RCT |
| Participants |
|
| Interventions |
Experimental: investigational informed consent Study participant will receive investigational informed consent for chemotherapy materials that were developed by the study team. Active Comparator: usual informed consent Study participant will receive usual, standard‐of‐care informed consent for chemotherapy materials. |
| Outcomes |
Primary outcome Proportion of participants at 4 months who understand the benefits of palliative chemotherapy Secondary outcomes
|
| Starting date | December 2013 |
| Contact information | Deborah Schrag, MD MPH; 617‐582‐8301; deb_schrag@dfci.harvard.edu. Andrea Enzinger, MD; 617‐582‐7335; andrea_enzinger@dfci.harvard.edu. |
| Notes |
NCT02285881.
| Trial name or title | Shared decision making between patients and GPs in the treatment of Type 2 diabetes in primary care |
| Methods | Cluster‐randomized trial |
| Participants | Patients with type 2 diabetes mellitus aged 60 years to 80 years |
| Interventions |
Experimental: shared decision making Quote: "In the intervention practices the SDM process is used. In the SDM process the patient and GP use a decision aid to discuss the pros and cons of two evidence based treatment possibilities, according to the Dutch College of General Practitioners (NHG) versus the ADDITION guideline, and the patients' preferences for either of these treatments. Together they choose one of these treatments, and set the five treatment targets (blood pressure, cholesterol, HbA1c, smoking status and weight) in order of priority. Subsequent treatment will take place according to the priorities of these OPTIMAL treatment targets. The priorities will be evaluated every 12 months." No Intervention: control group Quote: "Patients in the control practices will receive treatment‐as‐before, which means that the patients will not be offered the structured SDM process. So the GP will treat the former ADDITION patients as they were used during the period that followed after the ADDITION study (2009), either according to the national guidelines or to the ADDITION intensive treatment algorithm." |
| Outcomes |
Primary outcome The between groups difference in the proportion of patients which achieve the treatment goals for HbA1c, blood pressure, and total cholesterol. Secondary outcomes
Other Outcome Measures
|
| Starting date | March 2012 |
| Contact information | Contact information is only displayed when the study is recruiting participants |
| Notes | The study is completed but not published yet |
NCT02328326.
| Trial name or title | Caring Others Increasing EngageMent in PACT (CO‐IMPACT) |
| Methods | RCT |
| Participants |
Patient inclusion criteria
Care partner inclusion criteria
|
| Interventions |
Experimental: CO‐IMPACT Quote: "Patient and supporter (dyad) receive one coaching session on action planning, communicating with providers, navigation skills and support skills; preparation by phone before patients? primary care visits; after‐visit summaries by mail; and biweekly automated phone calls to prompt action on new patient health concerns." Active comparator: PACT Quote: "Patient and their health supporter (dyad) will receive PACT care for high‐risk diabetes, which includes (at primary care team discretion): nurse care manager visits, diabetes education classes, chronic disease self‐management groups, telehealth, clinical pharmacist visits." |
| Outcomes |
Primary outcomes:
|
| Starting date | November 2016 |
| Contact information | Shelley C Stoll, MPH; (734) 845‐5085; Shelley.Stoll@va.gov. Ann‐Marie Rosland, MD MS; (734) 222‐7621; Ann‐Marie.Rosland@va.gov. |
| Notes |
NCT02344576.
| Trial name or title | PCORI‐1310‐06998 Trial of a decision support intervention for Patients and Caregivers Offered Destination Therapy Heart Assist Device (DECIDE‐LVAD) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
No intervention: control: usual care Quote: "Patients and caregivers will receive the current usual education and consent process for DT LVAD at each hospital. This often means viewing consent forms and industry materials." Experimental: DT LVAD Decision Support Intervention Quote: "In the intervention phase of the study, patients and caregivers will receive the new decision support intervention, which consists primarily of decision aid materials about DT LVAD. The standard consent process will also still take place, but will be supplemented with additional decision support." |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | May 2015 |
| Contact information | Principal investigator: Larry Allen, MD, MHS, University of Colorado School of Medicine |
| Notes | No contact details provided |
NCT02379078.
| Trial name or title | Impact of an interprofessional shared decision‐making and goal‐setting decision aid for patients with diabetes |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Shared decision‐making aid Quote:"At study start (step 1: provider‐directed intervention phase): Online shared decision‐making aid, 1‐page provider enabler, provider training video made available to health care providers. At 6 months (step 2: provider‐ and patient‐directed phase): Online shared decision‐making aid, 1‐page patient enabler, patient training video also made available to patients (in addition to health care providers). Placebo Comparator: Generic hard‐copy diabetes resources Quote: "At study start (step 1: Provider‐directed intervention phase): A hard copy of the executive summary of the CDA CPG and postcard outlining online resources made available to health care providers. At 6 months (step 2: provider‐ and patient‐directed phase): A CDA patient education pamphlet regarding diabetes self‐management also made available to patients. In addition, provider‐ and patient‐directed guideline dissemination tools (not incorporating SDM) will also be publicly accessible from the CDA website." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | March 2016 |
| Contact information | Principal investigator: Catherine H Yu, MD FRCPC, St. Michael's Hospital, Toronto |
| Notes | No contact details provided |
NCT02429115.
| Trial name or title | Peer‐mentoring, quality of life and caregiver burden in patients with chronic kidney disease and their caregivers |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: face‐to‐face peer mentoring Will receive 6 months of face‐to‐face peer mentoring by a trained peer mentor. Experimental: online peer mentoring Will receive 6 months of face‐to‐face peer mentoring by a trained peer mentor. No Intervention: control Will not receive peer mentoring. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | February 2015 |
| Contact information | Tabitha Rothenberger, 717‐652‐8123 ext 102, Tabitha@kfcp.org and Tara Liaghat, taraliaghat@yahoo.com |
| Notes |
NCT02507349.
| Trial name or title | Person‐centered versus measurement‐based care in mental health (PCORI‐SDM) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Active Comparator: Person‐Centered Care Quote: "Decision support center staffed by peers. Patient uses the CommonGround program prior to medication visit to prepare a personal report, with support from peer(s). The CommonGround report expresses goals for medication, how other strategies help with functioning, current problems, and medication side effects. Patient brings report into the medication visit. Prescriber and patient discuss medication options, and prescriber enters the shared decision into CommonGround during the visit." Active comparator: Measurement‐Based Care Quote: "Clinic staff asks each patient to use a tablet computer to complete a brief assessment of symptoms and problems prior to medication visit. Prescriber views assessment results on office computer and discusses next steps in medication management with the patient." |
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | August 2014 |
| Contact information | Principal investigators: Gregory J McHugo, PhD, Dartmouth Psychiatric Research Center, The Geisel School of Medicine at Dartmouth, Kim MacDonald‐Wilson, ScD, CRC, CPRP, UPMC Center for High‐Value Health Care, Patricia E Deegan, PhD, Pat Deegan, PhD & Associates, LLC |
| Notes | No contact details provided |
NCT02592525.
| Trial name or title | Implementing shared decision making in interprofessional home care teams (IPSDM‐SW) |
| Methods | RCT |
| Participants |
Inclusion criteria Clients or caregivers of clients
In the case clients are not able to provide informed consent, their caregiver will be eligible. Exclusion criteria
|
| Interventions |
Behavioral: IP‐SDM training for health professionals Quote: "Multifaceted SDM training program for providers: i) 1.5‐hour online tutorial, ii) 3.5‐hour skills building workshop; iii) video‐clip demonstrating SDM in the context of an IP home care team with an aging adult making a decision about location of care (to be used with clients and providers as well); and iv) performance feedback to providers (role play during the workshop)." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | November 2015 |
| Contact information | Geneviève Painchaud Guérard, MSc, 418‐525‐4444 ext 52581, Genevieve.Painchaud‐Guerard@crchudequebec.ulaval.ca and Hubert Robitaille, PhD, 418‐525‐4444 ext 52341, hubert.robitaille@crchudequebec.ulaval.ca |
| Notes |
NCT02611050.
| Trial name or title | Treatment decisions for multi‐vessel CAD |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Option Grid Quote: "Patients randomized to the Option Grid arm will receive the Multi‐vessel Coronary Artery Disease Option Grid at the time of enrollment. The treating physician will then discuss the patient diagnosis and treatment choice reviewing the Option Grid within the conversation to facilitate patient understanding and shared decision making" Usual Care: Quote: "Patients randomized to usual care will discuss the patient diagnosis and treatment options typical to the physician's routine care.2 |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | December 2015 |
| Contact information | Principal investigator: Elizabeth L Nichols, MS, The Dartmouth Institute |
| Notes | No contact details provided |
NCT02623335.
| Trial name or title | PCORI‐1502‐27462 Navigating high risk surgery: empowering older adults to ask questions that iInform decisions about surgical treatment |
| Methods | RCT |
| Participants |
Surgeons Inclusion criteria
Exclusion criteria
Patients Inclusion criteria
Exclusion criteria
Family members Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: QPL (question prompt list) brochure Patients will be mailed the QPL (question prompt list) prior to their appointment with an enrolled surgeon. No intervention: usual care The investigators have observed that usual care includes informed consent and a surgeon‐directed deliberative phase in which surgeons present their own evaluation of the trade‐offs and goals of the proposed intervention. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | February 2016 |
| Contact information | Principal investigators: Gretchen Schwarze, University of Wisconsin, Madison, Emily Finlayson, University of California, San Francisco, Zara Cooper, Brigham and Women's Hospital, Anne Mosenthal, Rutgers New Jersey Medical School, Ana Berlin, Rutgers New Jersey Medical School, Karen Brasel, Oregon Health and Science University |
| Notes | No contact details provided |
NCT02631200.
| Trial name or title | Advance Care Planning with older patients who have End‐stage Kidney Disease (ACREDiT) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Advance care plan Participants will be offered the opportunity to complete an advance care plan. No Intervention: usual care Participants will be offered usual care for 12 weeks (and only then be offered the opportunity to complete an advance care plan). |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | December 2016 |
| Contact information | Peter D O'Halloran, PhD, +44 (0) 289097 2490, p.ohalloran@qub.ac.uk and Helen Noble, PhD, +44 (0) 289097 2472, helen.noble@qub.ac.uk |
| Notes |
NCT02646423.
| Trial name or title | Effect of a patient‐centered decision app on TOLAC (PROCEED) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Prior CD Decision App (PCDDA) Quote: "Women who are randomized to PCDDA will be provided access to a tablet which they can use to view the Prior CD Decision App at their own pace. The research assistant will print a summary of the participant's predicted likelihood of a VBAC if she undergoes a trial of labor after Caesarean (TOLAC), as well as her answers to the values clarification exercises, that she can review and share with whomever she chooses, including her provider." No intervention: Usual care ‐ No App Quote "Women randomized to the Usual Care ‐ No App group will simply continue with usual care." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | January 2016 |
| Contact information | Miriam Kuppermann, PhD, MPH, (415) 502‐4089, miriam.kuppermann@ucsf.edu |
| Notes |
NCT02653170.
| Trial name or title | Michigan Stroke Transitions Trial (MISTT) |
| Methods | RCT |
| Participants |
Patient inclusion criteria
Patient exclusion criteria
Caregiver inclusion criteria
|
| Interventions |
No intervention: usual care Patients in this group will receive the hospitals' usual transitional care approach. Experimental: SCM One intervention is provided:
Experimental: SCM and VSSP Two interventions are provided:
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | January 2016 |
| Contact information | Michele C Fritz, BSc, LVT, 517‐353‐8623 ext 209, mfritz@epi.msu.edu and Mathew J Reeves, BVSc, PhD, 517‐353‐8623 ext 130, reevesm@msu.edu |
| Notes |
NCT02663245.
| Trial name or title | INTEGRA Study: Primary care Intervention in Type 2 diabetes patients with poor glycaemic control |
| Methods | NRCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: intervention 1 Diabetes specific consultation + multicomponent intervention aimed at professionals and patients Experimental: intervention 2 Multicomponent intervention aimed at professionals and patients minus the diabetes specific consultation. No Intervention: control group No intervention. Data of the control groups will be retrieved from the SIDIAP. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | December 2015 |
| Contact information | Esther Rubinat, PhD, RN, 646186720, rubinatesther@gmail.com |
| Notes |
NCT02668900.
| Trial name or title | Decision support for adults facing implantable cardioverter‐defibrillator pulse generator replacement |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: decision support Quote: "The decision support intervention includes a patient decision and a decision coaching session. The patient decision aid includes a summary about the ICD's function, and the risks and benefits (including probabilities) associated with the option of replacing or not replacing the ICD. The decision coaching session will be led by a trained, non‐directive decision coach who will provide support that aims to develop patients' skills in thinking about the options, assess their values associated with each option, and prepare them to discuss the decision in a consultation with their physician. The final decision, whether to replace or not replace the ICD, will be made with their treating physician (e.g., cardiologist, electrophysiologist)." No intervention: usual care Quote: "The control group will not receive the decision support intervention prior to consultation with the physician." |
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | April 2016 |
| Contact information | Krystina B Lewis, RN, MN, kblewis@ottawaheart.ca and David Birnie, MD, 613 696 7269, dbirnie@ottawaheart.ca |
| Notes |
NCT02674360.
| Trial name or title | RCT regarding SDM online training andfFace‐to‐face SDM training |
| Methods | RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
Active comparator: SDM Online Training Quote: "The intervention consists of a SDM training for oncologists, which is conducted in the form of a web‐based SDM online Training (intervention group I). During training, the oncologists are guided to use decision aids for breast and colon cancer patients in their consultations, which were developed and evaluated in a previous project. The SDM training has the same duration (one session à 120 minutes) in both intervention groups. Doctors in the intervention group receive decision aids for breast cancer and colorectal cancer patients during training. The training contents are based on an already developed, evaluated and published SDM manual. The SDM online training works on the modeling principle." Active comparator: Face‐to‐Face SDM Training Quote: "The intervention consists of a SDM training for oncologists, which is conducted in the form of an individualized, context‐based SDM individual face‐to‐face training at the workplace of the participants (intervention group II). During training, the oncologists are guided to use decision aids for breast and colon cancer patients in their consultations, which were developed and evaluated in a previous project. The SDM training has the same duration (one session à 120 minutes) in both intervention groups. Doctors in the intervention group receive decision aids for breast cancer and colorectal cancer patients during training. The training contents are based on an already developed, evaluated and published SDM manual. The individual training works on the coaching principle." No intervention: Control Group Quote: "The Control Group receives no SDM Training. All participants of the Control Group will be offered to participate in the SDM Online Training after T2." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | May 2016 |
| Contact information | Kathrin M Gschwendtner, Dr, +49 6221/56‐34587, kathrin.gschwendtner@med.uni‐heidelberg.de |
| Notes |
NCT02686775.
| Trial name or title | The PACO Project: A Clinical Study of a PAtient COach Program in vulnerable lung cancer patients (PACO) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: patient coach Standard care and patient coach. 5 face‐to‐face sessions of approximately 1‐2 hours duration and 3 phone calls from inclusion to one month after end of first line treatment. Deviations from this schedule might depend on the treatment modules and on the wishes and needs of the patient. Several patients will continue directly into palliative care and the coach will thus support this transition. Active Comparator: standard treatment Standard care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | January 2016 |
| Contact information | Trille Kjaer, Postdoc, +4535257608, trille@cancer.dk and Susanne O Dalton, Senior researcher, sanne@cancer.dk |
| Notes |
NCT02721810.
| Trial name or title | Patient Engagement Initiative (PEI) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
No intervention: control Prompts standard to rounds or electronic medical records Experimental: prompting Intervention Prompting consideration of 3‐month functional outcome |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | October 2016 |
| Contact information | Alison E Turnbull, DVM,MPH,PhD, (410)‐955‐2190, turnbull@jhmi.edu |
| Notes |
NCT02759939.
| Trial name or title | Right For Me: Birth control decisions made easier |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Arm 1 Video + prompt card Experimental: Arm 2 Decision aids + training Experimental: Arm 3 Video + prompt card and decision aids + training |
| Outcomes |
Primary outcome measure
Secondary outcome measures
Other outcomes
|
| Starting date | July 2016 |
| Contact information | Principal investigator: Rachel Thompson, PhD, Dartmouth College |
| Notes | No contact details provided |
NCT02823262.
| Trial name or title | A breast cancer treatment decision aid for women aged 70 and older |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: decision aid Post Initial Surgical Consultation
Active Comparator: No decision aid Post Initial Surgical Consultation
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | July 2016 |
| Contact information | Mara Schonberg, MD MPH, 617‐754‐1414 |
| Notes |
NCT02842047.
| Trial name or title | The mediating effects of decentering on self‐management of stress and end of life planning |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: End of Life Care with Meditation Quote: "The intervention has two content components: end of life planning education (using end of life planning videos) and strategies and kindness based meditation (using the Stop, Breathe & Think™ app). The activities comprising these components work together to improve both analytic neural processing (e.g. improving knowledge about goal setting and EOL (end‐of‐life) planning, learning self‐monitoring of EOL values and goals of care, and self‐regulation skills of monitoring symptoms of distress and anxiety) and emotional neural processing (e.g. teaching participants to experience the moment non‐judgmentally and directing thoughts to think positive thoughts and feel positive feelings like kindness and compassion." Active Comparator: Meditation Only Quote: "This arm has the single content component of kindness based meditation delivered by using the Stop, Breathe & Think™ application. This group will also be instructed to view 3 caregiver wellness videos." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | July 2016 |
| Contact information | Principal investigator: Sara Douglas, PhD, RN, Case Comprehensive Cancer Center |
| Notes | No contact details provided |
NCT02866799.
| Trial name or title | Multi‐PAP RCT: Improving prescription in primary care patients with multimorbidity and polypharmacy (Multi‐PAP) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: multi‐PAP intervention Complex intervention with general practitioners and patients Active comparator: usual care Patients will receive the usual clinical care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | November 2016 |
| Contact information | Principal investigators: Alexandra Prados‐Torres, MD, PhD, Instituto Aragonés de Ciencias de la Salud (IACS), Daniel Prados‐Torres, MD, PhD, Servicio Andaluz de Salud (Andaluz Health Service), Isabel Del Cura‐González, MD, PhD, Gerencia de Atención Primaria, Madrid |
| Notes | No contact details provided |
NCT02868983.
| Trial name or title | Integrating Behavioral Health and Primary Care for comorbid behavioral and medical problems (IBHPC) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria No exclusions apply. |
| Interventions |
Experimental: Integration The intervention consists of training for practice leaders, BHCs, PCPs, and office staff, a Protocolized Redesign Process support for practice redesign, and a toolkit of suggested tactics for implementing Tasks A through D: A. Identification B. Assessment C. Treatment D. Surveillance No Intervention: Co‐Location A Behavioral Health Clinician (BHC) such as a psychologist or counselor is housed in or near the primary care practice. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | April 2016 |
| Contact information | Principal investigator: Benjamin Littenberg, MD, University of Vermont |
| Notes | No contact details provided |
NCT02890615.
| Trial name or title | CanDirect: Effectiveness of a telephone‐supported Depression Self‐care Intervention for Cancer Survivors (CanDirect) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Depression Self‐care Intervention (SCI) Quote: "Intervention group participants will receive the Depression Self‐Care Toolkit for Cancer Survivors and will be supported by telephone by a coach who will help to activate them, guide them through the materials, help in selecting appropriate tools, and provide positive reinforcement. Coach contacts will be made every week for 3 months followed by 3 monthly contacts, up to a maximum of 15 contacts, lasting 10‐20 minutes each. The coach uses a stepped approach (i.e. educate about depression, initiate mood monitoring, determine participant's goals with respect to reducing depressive symptoms, and help with the use of specific tools). A suggested script is provided for the coach as a framework for each call. Tailoring of the SCI to different participants will be based on problems, depressive symptoms (from the PHQ‐9), or concerns a participant may raise during the call." No Intervention: Control group Quote: "Members of both groups will continue to receive "usual care" for their depression. We will not interfere with usual care beyond recommending that participants discuss their depressive symptoms with their doctor. If participants consent, a short progress report will be send to their treating physician at the end of the study. At each follow‐up, we will ask participants about specific treatment they have received for depression since entering the study (antidepressant medication initiation, discontinuation, change of dose, or psychotherapy) and use of community resources. The Intervention group will receive the Depression SCI. The Control group will receive only usual care for 6 months after randomization; they will be given the Toolkit with a single coaching call upon completion of the final interview, to ensure their access to depression treatment." |
| Outcomes |
Primary outcome
Secondary outcome measures
|
| Starting date | July 2016 |
| Contact information | Manon de Raad, 514‐345‐3511 ext 3074, manon.deraad@ssss.gouv.qc.ca |
| Notes |
NCT02905032.
| Trial name or title | SDM for stroke prevention in atrial fibrillation (SDM4Afib) |
| Methods | RCT |
| Participants |
Clinician inclusion criteria
Patient inclusion criteria
Patient exclusion criteria
|
| Interventions |
No intervention: standard care Observations in clinical encounter via video, audio or observational notes. Active comparator: standard care + decision aid Observation of clinical encounter using the decision aid via video, audio, or observational notes. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | September 2016 |
| Contact information | Principal investigator: Peter A Noseworthy, Mayo Clinic |
| Notes | No contact details provided |
NCT02917603.
| Trial name or title | Shared decision making to improve palliative care in the nursing home |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: intervention These family members and residents will use web conferencing technology to attend their quarterly care conferences No Intervention: control These families and residents will receive usual care |
| Outcomes |
Primary outcome measure
Secondary outcomes
|
| Starting date | September 2016 |
| Contact information | Debra Oliver, PhD, 573‐884‐5301, oliverdr@health.missouri.edu and Karla Washington, PhD, (573) 884‐2119, washingtonkar@health.missouri.edu |
| Notes |
NCT02920086.
| Trial name or title | Improving partnerships with family members of ICU patients (IMPACT) |
| Methods | RCT |
| Participants |
Inclusion criteria for patients
Inclusion criteria for family member
Exclusion criteria
|
| Interventions |
Experimental: Nutrition Education Program Nutrition education for family members of an elderly critically ill patient Experimental: Decision Support Program Decision support education for family members of an elderly critically ill patient No Intervention: usual care No intervention |
| Outcomes |
Primary outcomes
|
| Starting date | January 2017 |
| Contact information | Daren Heyland, MD, 613‐549‐6666 ext 3339, dkh2@queensu.ca |
| Notes |
NCT02935920.
| Trial name or title | A study on optimizing follow‐up for postmenopausal women with breast cancer treated with adjuvant endocrine therapy |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: individual, tailored follow‐up Patient symptoms are evaluated by the use of PRO‐data to uncover the needs of a consultation. The outcome of the questionnaire is used to customize the follow‐up program to the individual patient. No intervention: standard follow‐up Scheduled clinical examination every six months throughout the course of adjuvant treatment, performed by a doctor or nurse. |
| Outcomes |
Primary outcome
|
| Starting date | April 2016 |
| Contact information | Cathrine L. Riis, MD, cathrine.lundgaard.riis@rsyd.dk and Karina D. Steffensen, MD, PhD, karina.dahl.steffensen@rsyd.dk |
| Notes |
NCT02971163.
| Trial name or title | Syncope Decision Aid for emergency care (SynDA) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: SynDA The research coordinator will print the appropriate version of the SynDA based on the patient's individualized risk score and the corresponding estimated probability of a serious medical event within 30 days. No Intervention: Control Patients in the control arm will receive usual emergency care pertaining to syncope. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | January 2017 |
| Contact information | Marc Probst, MD, MS, 212‐824‐8094, marc.probst@mssm.edu |
| Notes |
NCT02987608.
| Trial name or title | A feasibility trial of power up |
| Methods | NRCT |
| Participants |
Inclusion criteria
|
| Interventions |
No Intervention: control phase (No Power Up) 60 young people will receive CAMHS treatment as usual. Measures of empowerment, activation, and symptoms will be completed by participants soon after their referral to the service. The same measures plus shared decision making questionnaires will be administered three months later. Experimental: intervention phase (Power Up) 60 young people use Power Up alongside CAMHS treatment as usual. Measures of empowerment, activation, and symptoms will be completed by participants soon after their referral to the service. The same measures plus shared decision making questionnaires and a Power Up feedback form will be administered three months later. |
| Outcomes |
Primary outcomes
Secondary Outcomes
Other Outcomes
|
| Starting date | September 2016 |
| Contact information | Louise N Chapman, 020 7443 2205, louise.chapman@annafreud.org and Julian Edbrooke ‐ Childs, 020 7443 2275, julian.edbrooke‐childs@annafreud.org |
| Notes |
NCT02988661.
| Trial name or title | Women Empowered to Live With Lupus Study (WELL) |
| Methods | NRCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Active comparator: Chronic Disease Self‐management Program (CDSMP) A random sample of African American women with SLE selected from the Georgians Organized Against Lupus (GOAL) parent cohort will be used to recruit participants into the CDSMP. This group will be identified as the WELL Cohort. No intervention: usual care African American women consented into the parent Georgians Organized Against Lupus (GOAL) cohort who have not been selected to be enrolled in the intervention will comprise the usual care group. This group will continue their longitudinal assessments as part or the GOAL cohort data collection efforts. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | January 2017 |
| Contact information | Charmayne M Dunlop‐Thomas, MS, MPH, 404‐251‐8898, cmdunlo@emory.edu |
| Notes |
NCT03012087.
| Trial name or title | Using m‐health tools to reduce the misuse of opioid pain relievers |
| Methods | Randomized controlled trial |
| Participants | Patients (18 years and older) visiting emergency department for an injury‐ or pain‐related chief complaint |
| Interventions |
Intervention group: MyHealthyChoices. Quote:"My Healthy Choices explains what opioid pain medications are, assesses and explains the patient's risk factors related to taking opioids, assesses patient preferences about pain medications, and produces a tailored patient report based on the answers. The patient is encouraged to show the report to the treating ED clinician so they can discuss medication options for treating the patient's pain. Following discharge from the ED, intervention group participants discharged with a prescription pain reliever receive messages about safe medication use, storage, and disposal and access to an educational web portal that contains more information on prescription pain medications and safety." Control group: Health Risk Assessment. Quote: "The WellSource health risk assessment content focuses on general health promotion, and the participant's overall health and wellness. A summary report based on the participants' answers is sent to their email address." |
| Outcomes |
Primary outcome:
Change in self‐reported preference for opioid pain reliever
Secondary outcomes: Change in knowledge about prescription pain medication side effects and safe practices for taking, storing and disposing prescription pain medications. |
| Starting date | September 2016 |
| Contact information | Contact information is only displayed when the study is recruiting participants |
| Notes | The study is completed but results of the trial are not published yet |
NCT03037112.
| Trial name or title | Resetting the default: improving provider‐patient communication to reduce antibiotic misuse |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Active comparator: education Quote: "All providers will receive identical training on the appropriate prescribing of antibiotics for ARTIs in a 20 minute presentation. Follow up refresher video clips will also be available for all providers to view at their convenience throughout the study. Parents in both arms will receive identical high quality education on the pros and cons of antibiotics and tips for communicating with their provider." Active comparator: Communication skills Quote: "Providers randomized to the communication intervention will receive additional training on communication skills in a 40 minute communication skills training session. This training session will include good and bad communication examples, training on positive and negative behavioral framing, and education regarding key drivers of patient satisfaction." |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | March 2017 |
| Contact information | Emily Hurley, PhD, 816‐302‐0251, eahurley@cmh.edu and Areli Ramphal, MSW, 314‐747‐5128, aramphal@cmh.edu |
| Notes |
NCT03084159.
| Trial name or title | Multi‐disciplinary participatory design of a process to deliver a CKD diagnosis in primary care |
| Methods | NRCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: participatory design and intervention Quote: "Patients in this arm will receive the intervention of using an education worksheet during their appointment with their provider. They will be asked to complete post intervention surveys. Providers and staff at this site have been involved in the design of the intervention process, to make it streamlined and efficient for application in practice." Experimental: intervention only Quote: "A future arm will include patients at another site that will also receive the intervention of using an education worksheet during their appointment and fill out post intervention surveys. Providers/staff have not been involved in the initial design of the intervention process but will use it as part of the intervention delivery." No Intervention: usual care Quote: "A third site will include usual care, which does not include the intervention. Participants will be given post visit surveys similar to those in the two other study / intervention arms. This site will serve as a usual care comparison." |
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcome
|
| Starting date | June 2017 |
| Contact information | Emily Chen, MA, 734‐232‐4508, emilypc@med.umich.edu |
| Notes |
NCT03109145.
| Trial name or title | MyHealtheVet to enable shared decision making regarding menopausal in postmenopausal women veterans (MEANS) |
| Methods | NRCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: menopause educational secure messaging Secure messages that provide information about menopause and treatment option for menopause symptoms. No intervention: usual care: control Control group of eligible women patients between 45‐60 years who do not receive the intervention at the West Palm Beach and Orlando Veterans Healthcare System. Usual care participants did not receive the educational secure messages. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | October 2014 |
| Contact information | Stuti Dang, MD, MPH, 305‐575‐7000 ext 3388, stuti.dang@va.gov |
| Notes |
NCT03134092.
| Trial name or title | The Life STORRIED Study |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
No Intervention: Generalized Risk Communication (GRC) Active Comparator: Probabilistic Risk Communication (PRT) Experimental: Narrative Enhanced Risk Tool (NERT) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | June 2017 |
| Contact information | Erica B Goldberg, MSW, 215‐573‐2944, erica.goldberg@uphs.upenn.edu and Camille Lin, BA, 215‐746‐5608, camille.lin@uphs.upenn.edu |
| Notes |
NCT03136367.
| Trial name or title | What matters most: choosing the right breast cancer surgery for you |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Arm 1: Option Grid Quote: "Patients in this arm will receive the Option Grid for breast cancer surgery, an encounter decision aid, when they first meet with the breast surgeon to discuss their surgical options for breast cancer treatment." Experimental: Arm 2: Picture Option Grid Quote: "Patients in this arm will receive the Picture Option Grid for breast cancer surgery, an encounter decision aid, when they first meet with the breast surgeon to discuss their surgical options for breast cancer treatment." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | September 2017 |
| Contact information | Marie‐Anne Durand, MSc, PhD, 603‐653‐0851, marie‐anne.durand@dartmouth.edu and Renata Yen, MPH, 603‐650‐1494, renata.west.yen@dartmouth.edu |
| Notes |
NCT03216109.
| Trial name or title | Improving supportive care for patients with thoracic malignancies |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Weekly telephone symptom assessment Quote: "Each patient who is enrolled in the intervention will receive a weekly phone call from the Research Assistant for a total of 9 months to assess symptoms using the Edmonton Symptom Assessment Scale. Results of the symptom assessments will be provided to the clinic staff (RN and MD) for review each week. Symptom assessments will be documented into an encrypted, HIPAA compliant digital platform which provides longitudinal symptom data management and also provides symptom assessment tools for the clinical team in their intervention strategies. In addition, patients will complete symptom and quality of life surveys at 0, 3, 6 and 9 months." No intervention: control arm Quote: "Patients randomized to usual clinical care will receive standard of care for thoracic malignancies as provided by the VA Palo Alto Health Care System. Patients will complete outcome surveys at 0, 3, 6, and 9 months." |
| Outcomes |
Primary outcome:
Secondary outcomes
|
| Starting date | May 2017 |
| Contact information | Manali Patel, MD, MPH, 650‐498‐6000, manalip@stanford.edu and Evan Hall, MD, MPhil, 650‐498‐6000, ethall@stanford.edu |
| Notes |
NCT03221556.
| Trial name or title | Improving outcomes for low‐income mothers With depression |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Active comparator: Engagement‐Focused Care Coordination Quote: "The brief intervention in Engagement‐Focused Care Coordination is the Engagement Interview. In this model, providers meet one to two times with mothers who screen positive for depression, and use techniques of shared decision‐making to help mothers process the results of the screen; explore treatment options; and connect with formal mental health services. Engagement‐Focused Care Coordination emphasizes referral to formal mental health services." Active comparator: Problem Solving Education (PSE) Quote: "The brief Problem Solving Education (PSE) is a six‐session cognitive‐behavioral program. PSE offers immediate intervention in the PCMH, followed by referral to further treatment if symptoms persist." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | September 2017 |
| Contact information | Michael Silverstein, MD MPH, (617) 414‐7903, misilve@bu.edu and Winta Z Haile, 617‐414‐3638, whaile@bu.edu |
| Notes |
NCT03228615.
| Trial name or title | IBD shared decision making intervention |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Experimental: Shared Decision Making Intervention No Intervention: usual care group |
| Outcomes |
Primary outcomes
|
| Starting date | July 2017 |
| Contact information | Ellen A Lipstein, MD, MPH, 513‐803‐1626, ellen.lipstein@cchmc.org, Cassandra M Dodds, MA, CCRP, 513‐803‐3144, cassandra.dodds@cchmc.org |
| Notes |
NCT03234322.
| Trial name or title | The impact of a diabetes risk prediction model in primary care |
| Methods | RCT |
| Participants |
Inclusion criteria for participation of medical practitioners
Exclusion criteria for participation of medical practitioners
Inclusion criteria for participation of participants
Exclusion criteria for participation of participants
|
| Interventions |
Experimental: intervention group In the intervention group the routine health check is expanded by usage of a non‐invasive diabetes risk score. No intervention: control group In the control group the routine health check is conducted. |
| Outcomes |
Primary outcome
Secondary outcomes
Other outcome
|
| Starting date | September 2017 |
| Contact information | Principal investigator: Wolfgang Rathmann, Dr, German Diabetes Center |
| Notes | No contact details provided |
NTR4554.
| Trial name or title | Prostate Cancer Patient‐centered Care (PCPCC): impact of a treatment decision aid in a pragmatic, cluster randomized controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | The control group will receive usual care; information provision and provided decisional support will be given according to the hospital standards. In addition to usual care, the intervention group will be granted access to an online decision aid, providing patients with structured information on the risks and benefits of the different treatment options and offer value‐clarification tasks. A summary is obtained to discuss with their physician during the following consultation. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | May 2014 |
| Contact information | M. Cuypers, +31 13 466 28 55, M.Cuypers@uvt.nl |
| Notes |
NTR4879.
| Trial name or title | Implementation of shared decision making in a clinical setting; how to make it fit in the daily workflow? |
| Methods | Non‐randomised, single arm intervention |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Implementing shared decision making (SDM) using a patient decision aid |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | October 2014 |
| Contact information | W. Savelberg, 043‐3882336, wilma.savelberg@maastrichtuniversity.nl |
| Notes |
NTR5177.
| Trial name or title | (Cost‐)effectiveness and implementation of a decision aid for patients with localized prostate cancer and their partners: study protocol of a stepped wedge cluster randomized controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Control group: patients with prostate cancer (and their partners), who have a choice for a curative treatment option and who receive care as usual by health care providers in participating centers. Intervention group: patients with prostate cancer (and their partners), who have a choice for a curative treatment option and who additionally to care as usual by health care providers in participating centers, will receive the PDA. |
| Outcomes |
Primary outcomes
Secondary outcomes Patients
Cost‐evaluation
Satisfaction with intervention
Partners
Satisfaction with intervention
Moderating factors patients and partners
Implementation
|
| Starting date | February 2014 |
| Contact information | Dr. André Vis, +31 20 444 0261, a.vis@vumc.nl |
| Notes |
NTR5262.
| Trial name or title | Shared decision making in mental health with Routine Outcome Monitoring (ROM) as an information source |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Appliance of Routine Outcome monitoring in Shared Decision Making about treatment options between client and practitioner. Breakthrough, intervention teams, receive training in SDM & ROM model, support and coaching in the implementation. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2015 |
| Contact information | Drs. Metz Margot, 06 51 43 72 69, mmetz@trimbos.nl |
| Notes |
NTR5489.
| Trial name or title | CHOICE: CHOosing treatment together In Cancer at the End of life |
| Methods | RCT |
| Participants |
Inclusion criteria Patients
Oncologists
Exclusion criteria Patients
Oncologist
|
| Interventions | Quote: "The oncologist skills training is based on a four‐step model of SDM and on techniques known from behavior change theories. The training is provided in small groups (n=3‐5) by a professional trainer and actor. It consists of a reader, two half days of training making use of modelling videos and role play, a booster session and a consultation room tool. The ‘Gesprekswijzer’ consists of a Question Prompt List and Value Clarification Exercises, i.e., two known methods to empower patients in communication and decision making. The booklet comprises (1) an explanation that, when cure is no longer an option, treatment decisions are highly dependent on individual preferences, (2) example question patients may wish to pose in the upcoming consultation with the oncologist and (3) questions to help patients think about their values." |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | December 2015 |
| Contact information | Dr. Inge Henselmans, 020‐5668735, I.Henselmans@amc.uva.nl |
| Notes |
NTR5677.
| Trial name or title | Empowerment in mental health care using e‐health in a redesigned intake process: a cluster randomised controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | The intake‐teams randomized to the intervention group implement e‐health interventions in a redesigned intake process. To implement this new way of working, the clinicians of the intervention teams follow a training aiming to gain insight, knowledge and skills in the application of recovery supported care, shared decision making and e‐health with the purpose to motivate and empower patients in gaining an active role in their recovery and stimulating an equivalent interplay between patients and clinicians. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | September 2016 |
| Contact information | Margot Metz, 06‐51437269, m.metz@ggzbreburg.nl |
| Notes |
NTR6106.
| Trial name or title | A decision aid for the treatment of Benign Prostatic Hyperplasia: a prospective cohort study to investigate the effect |
| Methods | Prospective cohort study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Using a BPH decision aid |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | September 2016 |
| Contact information | Fieke van der Wijden, f.vanderwijden@etz.nl |
| Notes |
NTR6227.
| Trial name or title | Secondary manifestations of arterial disease ‐ influence of cardiovascular prognosis and treatment effect predictions on patient and physician decision‐making: a three‐armed, blinded, randomized controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | The three‐arms of this trial are: Standard‐communication practices only (Control Group) Standard‐ communication practices plus personalized information on:
Standard‐communication practices plus personalized information on:
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | March 2017 |
| Contact information | Nicole N. M. Jaspers, +31 88 75 556 50, N.E.M.Jaspers@umcutrecht.nl |
| Notes |
NTR6379.
| Trial name or title | Shared decision making in patients with Castration‐Resistant Prostate Cancer ‐ the impact of implementation of a treatment decision aid in CRPC patients |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Decision aid with value clarification exercise |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | September 2016 |
| Contact information | Prof. Dr. J. J. M. Takkenberg, +31 (0)10 7035413, j.j.m.takkenberg@erasmusmc.nl |
| Notes |
NTR6487.
| Trial name or title | A multicentre stepped‐wedge cluster‐randomised trial studying the level of shared decision‐making during vascular surgical consultation before and after the introduction of decision support tools |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Decision support tools: decision aids, decision tables, decision cards, training |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2017 |
| Contact information | Sylvana de Mik, +31 (0)20 566 2971, s.m.demik@amc.nl |
| Notes |
Simmons ongoing.
| Trial name or title | Skills training for shared decision making: a randomized pilot study |
| Methods | Randomized pilot study |
| Participants | Physicians from Partners HealthCare System (PHS) in Boston, MA |
| Interventions | The study randomly assigned participants to watch a webinar (developed by investigators and featuring demonstrations of SDM skills) or review video decision aids (DAs), and to receive individual feedback on their SPI or not. |
| Outcomes | Presence/absence of 9 key elements of SDM using the Braddock’s Informed Decision Making (IDM) framework, confidence in SDM skills, satisfaction with the skills training and completion of the assigned training arm |
| Starting date | |
| Contact information | Leigh Simmons, MD; 617‐726‐2368 |
| Notes |
Steele Gray 2016.
| Trial name or title | Supporting goal‐oriented primary health care for seniors with complex care needs Using mobile technology: evaluation and implementation of the Health System Performance Research Network, Bridgepoint Electronic Patient Reported Outcome Tool |
| Methods | RCT |
| Participants |
Inclusion criteria
|
| Interventions | Use of the electronic patient reported outcomes (ePRO) tool. The ePRO mobile app and portal offers an innovative approach to creating and monitoring goal‐oriented patient‐care plans to improve patient self‐management and shared decision‐making between patients and healthcare providers. The ePRO tool also supports proactive patient monitoring by the patient, caregiver(s), and healthcare provider. |
| Outcomes |
Primary outcome
Secondary outcomes Patient self‐management using the 13‐item patient activation measure (PAM) Efficiency using a cost‐effectiveness analysis |
| Starting date | Unknown |
| Contact information | Carolyn Steele Gray, Bridgepoint Collaboratory , Lunenfeld‐Tanenbaum Research Institute, Sinai Health System, 1 Bridgepoint Drive, AM.37, Toronto, ON M4M 2B5, Canada, Phone: 1 416 461 8252 ext 2908, Fax: 1 416 461 0656, Email: ac.metsyshtlaehianis@yargeleets.nylorac |
| Notes | The expected completion date of the study is November 2019 |
Thompson 2017.
| Trial name or title | Should I continue taking my acid reflux medication? Design of a pilot before/after study evaluating a patient decision aid |
| Methods | Before/after study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Patients will have an appointment with a pharmacist to go through a patient decision aid to discuss the decision (probabilities of benefit and harm, individual values and preferences). Following the appointment, patients can follow up with their family physician should they wish to pursue de‐prescribing or can receive instructions from the pharmacist. |
| Outcomes |
|
| Starting date | Unknown |
| Contact information | wthomp01@gmail.com |
| Notes |
UMIN000009239.
| Trial name or title | Comparison of Shared Decision Making (SDM) and routine care for University students in psychiatric outpatient clinic. A Randomized controlled trial |
| Methods | Randomized controlled trial |
| Participants | Patient (20 years and older) at first visit to psychiatric clinic at Waseda University Health Support Center |
| Interventions | Shared decision making intervention |
| Outcomes |
|
| Starting date | November 2012 |
| Contact information | |
| Notes | Recrutment completed but results not published |
UMIN000022832.
| Trial name or title | A shared decision making communication training program for clinicians treating: a pilot clustered randomised controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria Eligible therapists:
Eligible patients:
Exclusion criteria Eligible therapists:
Eligible patients:
|
| Interventions | Education program (communication) Education program (stroke) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | July 2016 |
| Contact information | Noriko Kon, 03‐5307‐5151, nobinobi.00.nontan.sc@gmail.com |
| Notes |
ARI: Acute respiratory infection; ARTI: Acute respiratory tract infection; BHC: Behavioural health clinician; CAHPS: Consumer Assessment of Healthcare Providers and Systems; CANSAS‐P: Camberwell Assessment of Need, Short Appraisal Schedule ‐ Patient; CHA2D2‐VASc– Congestive heart failure/Hypertension/Age ‐ Diabetes mellitis ‐ Vascular disease/Age/Sex category [clinical prediction rule]; CI: Co‐investigators; CRCS: Colorectal cancer screening; CSI: Caregiver Strain Index; CVR: Cardiovascular risk; DECIMS: Decision coaching in multiple sclerosis; DST: Decision support tools; ED: Emergency department; EORTC: European Organisation for Research and Treatment of Cancer; EPDS: Edinburgh Postnatal Depression Scale; EQ5D: EuroQol 5 Dimensions (self‐rated health); EUROHIS: European Health Inverview Surveys; HAS‐P: Helping Alliance Scale ‐ Patient;HIPAA: Health Insurance Portability and Accountability Act; HRA: Health Risk Assessment; IBD: Inflammatory bowel disease;IDAF: Index of Dental Anxiety and Fear; MAPPIN’SDM: Multifocal approach to sharing in shared decision making; MARS: Medication Adherence Rating Scale; NYHA: New York Heart Association; OHIP: Oral Health Impact Profile; PACT: Patient‐Aligned Care Team; PCMH: Patient‐centered medical home; PCORI: Patient‐Centered Outcomes Research Institute; PEP: Patient empowerment program; PEPPI: Perceived Efficacy in Patient‐Physician Interactions; PHQ: Patient Health Questionnaire; PREP‐DM: Preparation for Decision Making scale; PRODISQ: Productivity and Disease Questionnaire; PROMIS: Patient‐Reported Outcomes Measurement Information System; PSA: Prostate‐specific antigen; PtDA: Patient decision aid; QoL: Quality of life/QOL; QPL: Question prompt list; QPS: Question prompt sheets; QQPPI: Questionnaire on the Quality of Physician‐Patient Interaction; RA: Rheumatoid arthritis; SAS: Statistical Analysis System; SCIP: Satisfaction with Cancer Information Profile;SCNS: Supportive Care Needs Survey; SDM‐Q9: Shared Decision Making Questionnaire (9‐item); SDM: Shared decision making; SDMI: Shared decision making intervention; SES: Socioeconomic status; SETS: Stanford Expectations of Treatment Scale; SF: Short‐form; SURE [screening test]: Sure of myself; Understand information; Risk‐benefit ratio; Encouragement; TAS: Treatment Alliance Scale;TiC‐P: Trimbos/iMTA questionnaire for costs associated with psychiatric illness; TTE: Time to event; TTO: Time trade‐off; UC: Usual care; USH: Unguided self‐help; VA: Veteran Affairs; VBAC: Vaginal birth after caesarean;VOICE: Values and Options in Cancer Care (VOICE); VOICE: Valuing Opinions, Individual Communication and Experience; ZUF: Fragebogen zur Patientenzufriedenheit [questionnaire on patient satisfaction].
Differences between protocol and review
Since publishing the protocol and the 2010 version of this review (Légaré 2010), we organized the types of intervention defined by the Effective Practice and Organisation of Care (EPOC) taxonomy into three target categories: interventions targeting patients (i.e. patient‐mediated interventions), interventions targeting healthcare professionals (e.g. distribution of printed educational material, educational meetings, audit and feedback, reminders and educational outreach visits), and interventions targeting both patients and healthcare professionals (i.e. patient‐mediated interventions combined with interventions targeting the healthcare professional). These three categories correspond to the specific objectives of the review. We also split the outcomes into observer‐based outcomes and patient‐reported outcomes because measures for observer‐based outcomes are more objective than patient‐reported outcomes. We used GRADE tools to summarize our findings (see 'Summary of findings' tables). Since publishing the protocol, three review authors have been removed (S Ratté, K Gravel and M‐J Cossi) and six new review authors added (RA, AL, MCP, RT, GE and NDB).
In this update, instead of considering nine comparisons between intervention categories, we considered six: comparisons between each target category and usual care (three) and comparisons between each target category and other interventions with the same targets, or head‐to‐head comparisons (three). More specifically, our six categories were as follows:
comparisons between interventions targeting patients and usual care;
comparisons between interventions targeting healthcare professionals and usual care;
comparisons between interventions targeting both healthcare professionals and patients and usual care;
comparisons between interventions targeting patients and other interventions targeting patients;
comparisons between interventions targeting healthcare professionals and other interventions targeting healthcare professionals;
comparisons between interventions targeting both healthcare professionals and patients and other interventions targeting both healthcare professionals and patients.
Comparisons between different target categories were not considered in this review as they would have added no additional information to the former one, and there were few studies in these comparisons.
To address some theoretical confusion related to the previous title, a slight change has been made from “Interventions for improving the adoption of shared decision making by healthcare professionals” to “Interventions for increasing the use of shared decision making by healthcare professionals”.
Contributions of authors
FL, RA, JH, ST, PAN, AB, EC, LA and HR identified eligible studies for the update of this review.
RA, NTD, ST, SC, ABC, JH, AB, LA and HE helped with data extraction including the 'Risk of bias' assessment.
RA and ST assisted with data analysis.
FL, RA and AB assisted in evaluation of the certainty of the evidence (GRADE).
RA and ST drafted the review.
FL, RA, AL, DS, ST, JK, IDG, MCP, RT, GE and NDB reviewed and participated in the writing of the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
Tier 2 Canada Research Chair in Implementation of Shared Decision Making in Primary Care, Université Laval, Québec, Canada.
Consortium de recherche sur les services de génétique de laboratoire (CanGènetest), Québec, Canada.
Centre de recherche du Centre Hospitalier Universitaire de Québec, Québec, Canada.
Tier 1 Canada Research Chair in Shared Decision Making and Knowledge Translation, Canada.
Declarations of interest
FL: none known. DS: none known. ST: none known. JK: none known. IDG: none known. AL: none known. MCP: is on the Medication Adherence Advisory Board for Merck. RT: none known. GE: none known. NDB: none known. RA: none known.
This review includes studies that were published by some of its authors (DS, FL, GE, IDG, NDB). FL was involved in the evaluation of the certainty of the evidence but was blinded to the identification of the included studies to avoid conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adarkwah 2016 {published data only}
- Adarkwah CC, Jegan N, Heinzel‐Gutenbrunner M, Kuhne F, Siebert U, Popert U, et al. Time‐to‐event versus ten‐year‐absolute‐risk in cardiovascular risk prevention ‐ does it make a difference? Results from the Optimizing‐Risk‐Communication (OptRisk) randomized‐controlled trial. BMC Medical Informatics and Decision Making 2016;16(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Almario 2016 {published data only}
- Almario CV, Chey WD, Khanna D, Mosadeghi S, Ahmed S, Afghani E, et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. American Journal of Gastroenterology 2016;111(11):1546‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ampe 2017 {published data only}
- Ampe S, Sevenants A, Smets T, Declercq A, Audenhove C. Advance care planning for nursing home residents with dementia: Influence of 'we DECide' on policy and practice. Patient Education & Counselling 2017;100(1):139‐46. [DOI] [PubMed] [Google Scholar]
Barton 2016 {published data only}
- Barton JL, Trupin L, Schillinger D, Evans‐Young G, Imboden J, Montori V M, et al. Use of low‐literacy decision aid to enhance knowledge and reduce decisional conflict among a diverse population of adults with rheumatoid arthritis: results of a pilot study. Arthritis Care Research 2016;68(7):889‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernhard 2011 {published data only}
- Bernhard J, Butow P, Aldridge J, Juraskova I, Ribi K, Brown R. Communication about standard treatment options and clinical trials: can we teach doctors new skills to improve patient outcomes?. Psycho‐Oncology 2011;21(12):1265–74. [DOI: 10.1002/pon.2044] [DOI] [PubMed] [Google Scholar]
- Butow P, Brown R, Aldridge J, Juraskova I, Zoller P, Boyle F, et al. Can consultation skills training change doctors' behaviour to increase involvement of patients in making decisions about standard treatment and clinical trials: a randomized controlled trial. Health Expectations 2015;18(6):2570‐83. [PUBMED: 24975503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bieber 2006 {published data only}
- Bieber C, Muller KG, Blumenstiel K, Schneider A, Richter A, Wilke S, et al. Long‐term effects of a shared decision‐making intervention on physician‐patient interaction and outcome in fibromyalgia. A qualitative and quantitative 1 year follow‐up of a randomized controlled trial. Patient Education and Counseling 2006;63(3):357‐66. [CENTRAL: 00571329] [DOI] [PubMed] [Google Scholar]
Branda 2013 {published data only}
- Branda ME, LeBlanc A, Shah ND, Tiedje K, Ruud K, Houten H, et al. Shared decision making for patients with type 2 diabetes: A randomized trial in primary care. BMC Health Services Research 2013;13:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butow 2004 {published and unpublished data}
- Butow P, Devine R, Boyer M, Pendlebury S, Jackson M, Tattersall MH. Cancer consultation preparation package: changing patients but not physicians is not enough. Journal of Clinical Oncology 2004;22(21):4401‐9. [CENTRAL: 00492482] [DOI] [PubMed] [Google Scholar]
Causarano 2014 {published data only}
- Causarano N, Platt J, Baxter NN, Bagher S, Jones JM, Metcalfe KA, et al. Pre‐consultation educational group intervention to improve shared decision‐making for postmastectomy breast reconstruction: a pilot randomized controlled trial. Support Care Cancer 2015;23(5):1365–75. [DOI: 10.1007/s00520-014-2479-6] [DOI] [PubMed] [Google Scholar]
Cooper 2011 {published and unpublished data}
- Cooper LA, Roter DL, Carson KA, Bone LR, Larson SM, Miller ER 3rd, et al. A randomized trial to improve patient‐centered care and hypertension control in underserved primary care patients. Journal of General Internal Medicine 2011;26(11):1297‐304. [DOI: 10.1007/s11606-011-1794-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2013 {published data only}
- Cooper LA, Ghods Dinoso BK, Ford DE, Roter DL, Primm AB, Larson SM, et al. Comparative effectiveness of standard versus patient‐centered collaborative care interventions for depression among African Americans in primary care settings: the BRIDGE Study. Health Research and Educational Trust 2013;48(1):150‐74. [DOI: 10.1111/j.1475-6773.2012.01435.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2017 {published data only}
- Cox ED, Jacobsohn GC, Rajamanickam VP, Carayon P, Kelly MM, Wetterneck TB, et al. A family‐centered rounds checklist, family engagement, and patient safety: a randomized trial. Pediatrics 2017;139(5):e20161688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coylewright 2016 {published and unpublished data}
- Coylewright M, Dick S, Zmolek B, Askelin J, Hawkins E, Branda M, et al. PCI Choice Decision Aid for stable coronary artery disease: a randomized trial. Circulation: Cardiovascular Quality and Outcomes 2016;9(6):767‐76. [DOI] [PubMed] [Google Scholar]
Davison 1997 {published data only}
- Davison BJ, Degner LF. Empowerment of men newly diagnosed with prostate cancer. Cancer Nursing 1997;20(3):187‐96. [CENTRAL: 00140696] [DOI] [PubMed] [Google Scholar]
Davison 2002 {published data only}
- Davison BJ, Degner LF. Feasibility of using a computer‐assisted intervention to enhance the way women with breast cancer communicate with their physicians. Cancer Nursing 2002;25(6):417‐24. [DOI] [PubMed] [Google Scholar]
Deen 2012 {published data only}
- Deen D, Lu WH, Weintraub MR, Maranda MJ, Elshafey S, Gold MR. The impact of different modalities for activating patients in a community health center setting. Patient Education and Counseling 2012;89(1):178‐83. [DOI: 10.1016/j.pec.2012.04.012] [DOI] [PubMed] [Google Scholar]
Deinzer 2009 {published data only}
- Deinzer A, Veelken R, Kohnen R, Schmieder RE. Is a shared decision‐making approach effective in improving hypertension management?. Journal of Clinical Hypertension 2009;11(5):266‐70. [PUBMED: 19534034] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deschamps 2004 {published and unpublished data}
- Deschamps MA, Taylor JG, Neubauer SL, Whiting S, Green K. Impact of pharmacist consultation versus a decision aid on decision making regarding hormone replacement therapy. International Journal of Pharmacy Practice 2004;12(1):21‐8. [EMBASE: 2004506946] [Google Scholar]
Dolan 2002 {published data only}
- Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Medical Decision Making 2002;22(2):125‐39. [CENTRAL: 00408555] [DOI] [PubMed] [Google Scholar]
Eggly 2017 {published and unpublished data}
- Eggly S, Hamel LM, Foster TS, Albrecht TL, Chapman R, Harper FWK, et al. Randomized trial of a question prompt list to increase patient active participation during interactions with black patients and their oncologists. Patient Education and Counseling 2017;100(5):818‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2004 {published and unpublished data}
- Edwards A, Elwyn G, Hood K, Atwell C, Robling M, Houston H, et al. Patient‐based outcome results from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Family Practice 2004;21(4):347‐54. [DOI: 10.1093/fampra/cmh402] [DOI] [PubMed] [Google Scholar]
- Elwyn G, Edwards A, Hood K, Robling M, Atwell C, Russell I, et al. Study Steering Group. Achieving involvement: Process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Family Practice 2004;21(4):337‐46. [CENTRAL: 00490204] [DOI] [PubMed] [Google Scholar]
- Longo MF, Cohen DR, Hood K, Edwards A, Robling M, Elwyn G, et al. Involving patients in primary care consultations: assessing preferences using discrete choice experiments. British Journal of General Practice 2006;56(522):35‐42. [PUBMED: 16438813] [PMC free article] [PubMed] [Google Scholar]
Epstein 2017 {published and unpublished data}
- Epstein RM, Duberstein PR, Fenton JJ, Fiscella K, Hoerger M, Tancredi DJ, et al. Effect of a patient‐centered communication intervention on oncologist‐patient communication, quality of life, and health care utilization in advanced cancer: The VOICE randomized clinical trial. JAMA Oncology 2017;3(1):92‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2013 {published data only}
- Feng B, Srinivasan M, Hoffman JR, Rainwater JA, Griffin E, Dragojevic M, et al. Physician communication regarding proatate cancer screening: analysis of unannounced standardized patient visits. Annals of Family Medicine 2013;11(4):315‐23. [DOI: 10.1370/afm.1509] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiks 2015 {published data only}
- Fiks AG, Mayne SL, Karavite DJ, Suh A, O'Hara R, Localio AR, et al. Parent‐reported outcomes of a shared decision‐making portal in asthma: a practice‐based RCT. Pediatrics 2015;135(4):e965‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fossli 2011 {published and unpublished data}
- Fossli JB, Gulbrandsen P, Dahl FA, Krupat E, Frankel RM, Finset A. Effectiveness of a short course in clinical communication skills for hospital doctors: results of a crossover randomized controlled trial (ISRCTN22153332). Patient Education and Counseling 2011;84(2):163‐9. [PUBMED: 21050695] [DOI] [PubMed] [Google Scholar]
Hamann 2007 {published data only}
- Hamann J, Cohen R, Leucht S, Busch R, Kissling W. Shared decision making and long‐term outcome in schizophrenia treatment. Journal of Clinical Psychiatry 2007;68(7):992‐7. [PUBMED: 17685733] [DOI] [PubMed] [Google Scholar]
Hamann 2011 {published data only}
- Hamann J, Mendel R, Meier A, Asani F, Pausch E, Leucht S, et al. “How to Speak to Your Psychiatrist”: Shared Decision‐Making Training for Inpatients with Schizophrenia. Psychiatric Services 2011;62(10):1218‐21. [DOI: 10.1176/appi.ps.62.10.1218] [DOI] [PubMed] [Google Scholar]
Hamann 2014 {published data only}
- Hamann J, Maris N, Iosifidou P, Mendel R, Cohen R, Wolf P, et al. Effects of a question prompt sheet on active patient behaviour: a randomized controlled trial with depressed outpatients. International Journal of Social Psychiatry 2014;60(3):227–35. [DOI: 10.1177/0020764013482311] [DOI] [PubMed] [Google Scholar]
Hamann 2017 {published and unpublished data}
- Hamann J, Parchmann A, Sassenberg N, Bronner K, Albus M, Richter A, et al. Training patients with schizophrenia to share decisions with their psychiatrists: a randomized‐controlled trial. Social Psychiatry and Psychiatric Epidemiology 2017;52(2):175‐82. [DOI] [PubMed] [Google Scholar]
Härter 2015 {published data only}
- Bieber C, Nicolai J, Gschwendtner K, Muller N, Reuter K, Buchholz A, et al. How does a Shared Decision‐Making (SDM) intervention for oncologists affect participation style and preference matching in patients with breast and colon cancer?. Journal of Cancer Education 2016;[Epub ahead of print]:1‐8. [PUBMED: 27966192] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härter M, Buchholz A, Nicolai J, Reuter K, Komarahadi F, Kriston L, Kallinowski B, Eich W, Bieber C. Shared decision making and the use of decision aids. Deutsches Ärzteblatt International 2015 Oct;112(40):672‐9. [DOI: 10.3238/arztebl.2015.0672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haskard 2008 {published and unpublished data}
- Haskard KB, Williams SL, DiMatteo MR, Rosenthal R, White MK, Goldstein MG. Physician and patient communication training in primary care: effects on participation and satisfaction. Health Psychology 2008;27(5):513‐22. [CENTRAL: 00670391] [DOI] [PubMed] [Google Scholar]
Hess 2012 {published data only}
- Hess EP, Knoedler MA, Shah ND, Kline JA, Breslin M, Branda ME, et al. The chest pain choice decision aid: a randomized trial. Circulation: Cardiovascular Quality and Outcomes 2012;5(3):251‐9. [DOI: 10.1161/CIRCOUTCOMES.111.964791] [DOI] [PubMed] [Google Scholar]
Hess 2016 {published data only}
- Hess EP, Hollander JE, Schaffer JT, Kline JA, Torres CA, Diercks DB, et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ 2016;355:i6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jouni 2017 {published data only}
- Jouni H, Haddad RA, Marroush TS, Brown SA, Kruisselbrink TM, Austin EE, et al. Shared decision‐making following disclosure of coronary heart disease genetic risk: results from a randomized clinical trial. Journal of Investigative Medicine 2017;65(3):681‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasper 2008 {published data only}
- Kasper J, Köpke S, Mühlhauser I, Nübling M, Heesen C. Informed shared decision making about immunotherapy for patients with multiple sclerosis (ISDIMS): a randomized controlled trial. European Journal of Neurology 2008;15(12):1345‐52. [PUBMED: 17503119] [DOI] [PubMed] [Google Scholar]
Kennedy 2013 {published data only}
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew‐Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013 May 13;346:f2882. [DOI: 10.1136/bmj.f2882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koerner 2014 {published data only}
- Koerner M, Wirtz M, Michaelis M, Ehrhardt H, Steger AK, Zerpies E, et al. A multicentre cluster randomized controlled study to evaluate a train‐the‐trainer programme for implementing internal and external participation in medical rehabilitation. Clinical Rehabilitation 2014;28(1):20–35. [DOI: 10.1177/0269215513494874] [DOI] [PubMed] [Google Scholar]
Köpke 2014 {published data only}
- Köpke S, Kern S, Ziemssen T, Berghoff M, Kleiter I, Marziniak M, et al. Evidence‐based patient information programme in early multiple sclerosis: a randomised controlled trial. Journal of Neurology, Neurosurgery and Psychiatry 2014 Apr;85(4):411‐8. [DOI: 10.1136/jnnp-2013-306441] [DOI] [PubMed] [Google Scholar]
Korteland 2017 {published and unpublished data}
- Korteland NM, Ahmed Y, Koolbergen DR, Brouwer M, Heer F, Kluin J, et al. Does the use of a decision aid improve decision making in prosthetic heart valve selection? A multicenter randomized trial. Circulation: Cardiovascular Quality and Outcomes 2017;10(2):e003178. [DOI] [PubMed] [Google Scholar]
Krist 2007 {published data only}
- Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Annals of Family Medicine 2007;5(2):112‐9. [CENTRAL: 00579543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krones 2008 (ARRIBA‐Herz) {published and unpublished data}
- Hirsch O, Keller H, Albohn‐Kuhne C, Krones T, Donner‐Banzhoff N. Satisfaction of patients and primary care physicians with shared decision making. Evaluation and the health professions 2010;33(3):321‐42. [DOI: 10.1177/0163278710376662] [DOI] [PubMed] [Google Scholar]
- Krones T, Keller H, Sonnichsen A, Sadowski EM, Baum E, Wegscheider K, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Annals of Family Medicine 2008;8(23):218‐27. [DOI: 10.1370/afm.854] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalonde 2006 {published and unpublished data}
- Lalonde L, O'Connor AM, Duguay P, Brassard J, Drake E, Grover SA. Evaluation of a decision aid and a personal risk profile in community pharmacy for patients considering options to improve cardiovascular health: the OPTIONS pilot study. International Journal of Pharmacy Practice 2006;14(1):51‐62. [CENTRAL: 00622580] [Google Scholar]
Landrey 2012 {published and unpublished data}
- Landrey A, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate‐specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. Journal of Primary Care and Community Health 2012;4(1):67‐74. [DOI: 10.1177/2150131912447074] [DOI] [PubMed] [Google Scholar]
LeBlanc 2015a {published data only}
- LeBlanc A, Herrin J, Williams MD, Inselman JW, Branda ME, Shah ND, et al. Shared decision making for antidepressants in primary care: a cluster randomized trial. JAMA Internal Medicine 2015;175(11):1761‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
LeBlanc 2015b {published and unpublished data}
- LeBlanc A, Wang AT, Wyatt K, Branda ME, Shah ND, Houten H, et al. Encounter decision aid vs. clinical decision support or usual care to support patient‐centered treatment decisions in osteoporosis: the Osteoporosis Choice Randomized Trial II. PLOS One 2015;10(5):e0128063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Légaré 2012 {published and unpublished data}
- Légaré F, Guerrier M, Nadeau C, Rhéaume C, Turcotte S, Labrecque M. Impact of DECISION + 2 on patient and physician assessment of shared decision making implementation in the context of antibiotics usefor acute respiratory infections. Implementation Science 2013 Dec 26;8:144. [DOI: 10.1186/1748-5908-8-144] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision‐making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. Canadian Medical Association Journal 2012;184(13):E726‐34. [DOI: 10.1503/cmaj.120568] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leighl 2011 {published data only}
- Leighl NB, Shepherd HL, Butow PN, Clarke SJ, McJannett M, Beale PJ, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. Journal of Clinical Oncology 2011;29(15):2077‐84. [DOI] [PubMed] [Google Scholar]
Loh 2007 {published data only}
- Loh A, Simon D, Wills CE, Kriston L, Niebling W, Harter M. The effects of a shared decision‐making intervention in primary care of depression: a cluster‐randomized controlled trial. Patient Education and Counseling 2007;67(3):324‐32. [CENTRAL: 00609803] [DOI] [PubMed] [Google Scholar]
Maclachlan 2016 {published data only}
- Maclachlan EW, Shepard‐Perry MG, Ingo P, Uusiku J, Mushimba R, Simwanza R, et al. Evaluating the effectiveness of patient education and empowerment to improve patient‐provider interactions in antiretroviral therapy clinics in Namibia. AIDS Care 2016;28(5):620‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maindal 2014 {published data only}
- Maindal HT, Carlsen AH, Lauritzen T, Sandbaek A, Simmons RK. Effect of a participant‐driven health education programme in primary care for people with hyperglycaemia detected by screening: 3‐year results from the Ready to Act randomized controlled trial (nested within the ADDITION‐Denmark study). Diabetic Medicine 2014 Aug;31(8):976‐86. [DOI: 10.1111/dme.12440] [DOI] [PubMed] [Google Scholar]
- Maindal HT, Sandbæk A, Kirkevold M, Lauritzen T. Effect on motivation, perceived competence, and activation after participation in the ''Ready to Act'' programme for people with screen‐detected dysglycaemia: a 1‐year randomised controlled trial, Addition‐DK. Scandinavian Journal of Public Health 2011 May;39(3):262‐71. [DOI: 10.1177/1403494811402721] [DOI] [PubMed] [Google Scholar]
Maranda 2014 {published data only}
- Maranda MJ, Deen D, Elshafey S, Herrera M, Gold MR. Response to a patient activation intervention among Spanish‐speaking patients at a community health center in New York City. Journal of Health Care for the Poor and Underserved 2014 May;25(2):591‐604. [DOI: 10.1353/hpu.2014.0110] [DOI] [PubMed] [Google Scholar]
Mathers 2012 {published data only}
- Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open 2012 Nov 5;2(6):pii: e001469. [DOI: 10.1136/bmjopen-2012-001469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montori 2011 {published and unpublished data}
- Montori VM, Shah ND, Pencille LJ, Branda ME, Van Houten HK, Swiglo BA, et al. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. American Journal of Medicine 2011;124(6):549‐56. [DOI] [PubMed] [Google Scholar]
Mullan 2009 {published and unpublished data}
- Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Archives of Internal Medicine 2009;169(17):1560‐8. [DOI] [PubMed] [Google Scholar]
Murray 2001 {published data only}
- Murray E, Davis H, Tai SS, Coulter A, Gray A, Haines A. Randomised controlled trial of an interactive multimedia decision aid on benign prostatic hypertrophy in primary care. BMJ 2001;323(7311):493‐6. [DOI: 10.1136/bmj.323.7311.493] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2010 {published data only}
- Murray MA, Stacey D, Wilson KG, O'Connor AM. Skills training to support patients considering place of end‐of‐life care: a randomized control trial. Journal of Palliative Care 2010;26(2):112‐21. [PUBMED: 20718396] [PubMed] [Google Scholar]
Myers 2011 {published data only}
- Myers RE, Daskalakis C, Kunkel EJ, Cocroft JR, Riggio JM, Capkin M, et al. Mediated decision support in prostate cancer screening: a randomized controlled trial of decision counselling. Patient Education and Counseling 2011;83(2):240‐6. [PUBMED: 20619576] [DOI] [PubMed] [Google Scholar]
Nannenga 2009 {published and unpublished data}
- Jones LA, Weymiller AJ, Shah N, Bryant SC, Christianson TJH, Guyatt GH, et al. Should clinicians deliver decision aids? Further exploration of the statin choice randomized trial results. Medical Decision Making 2009;29(4):468‐74. [DOI: 10.1177/0272989X09333120] [DOI] [PubMed] [Google Scholar]
- Nannenga MR, Montori VM, Weymiller AJ, Smith SA, Christianson TJ, Bryant SC, et al. A treatment decision aid may increase patient trust in the diabetes specialist. The Statin Choice randomized trial. Health Expectations 2009;12(1):38‐44. [PUBMED: 19250151] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymiller AJ, Montori VM, Jones LA, Gafni A, Guyatt GH, Bryant SC, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Archives of Internal Medicine 2007;167(10):1076‐82. [DOI] [PubMed] [Google Scholar]
O'Cathain 2002 {published data only}
- O'Cathain A, Walters SJ, Nicholl JP, Thomas KJ, Kirkham M. Use of evidence based leaflets to promote informed choice in maternity care: randomised controlled trial in everyday practice. BMJ 2002;324(7338):643. [DOI: 10.1136/bmj.324.7338.643] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perestelo‐Perez 2016 {published data only}
- Perestelo‐Perez L, Rivero‐Santana A, Boronat M, Sanchez‐Afonso JA, Perez‐Ramos J, Montori VM, et al. Effect of the statin choice encounter decision aid in Spanish patients with type 2 diabetes: a randomized trial. Patient Education and Counselling 2016;99(2):295‐9. [DOI] [PubMed] [Google Scholar]
Pickett 2012 {published data only}
- Pickett SA, Diehl SM, Steigman PJ, Prater JD, Fox A, Shipley P, et al. Consumer empowerment and self‐advocacy outcomes in a randomized study of peer‐led education. Community Mental Health Journal 2012;48(4):420‐30. [DOI: 10.1007/s10597-012-9507-0] [DOI] [PubMed] [Google Scholar]
Raynes‐Greenow 2010 {published data only}
- Raynes‐Greenow CH, Nassar N, Torvaldsen S, Trevena L, Roberts CL. Assisting informed decision making for labour analgesia: a randomised controlled trial of a decision aid for labour analgesia versus a pamphlet. BMC Pregnancy and Childbirth 2010;10:15. [PUBMED: 20377844] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rise 2012 {published data only}
- Rise MB, Eriksen L, Grimstad H, Steinsbekk A. The long‐term effect on mental health symptoms and patient activation of using patient feedback scales in mental health out‐patient treatment. A randomised controlled trial. Patient Education and Counselling 2016;99(1):164‐8. [DOI] [PubMed] [Google Scholar]
- Rise MB, Eriksen L, Grimstad H, Steinsbekk A. The short‐term effect on alliance and satisfaction of using patient feedback scales in mental health out‐patient treatment. A randomised controlled trial. BMC Health Services Research 2012 Oct 3;12:348. [DOI: 10.1186/1472-6963-12-348] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roter 2012 {published data only}
- Roter DL, Wexler R, Naragon P, Forrest B, Dees J, Almodovar A, et al. The impact of patient and physician computer mediated communication skill training on reported communication and patient satisfaction. Patient Education and Counseling 2012;88(3):406‐13. [DOI: 10.1016/j.pec.2012.06.020] [DOI] [PubMed] [Google Scholar]
Sanders 2017 {published data only}
- Sanders AR, Bensing JM, Essed MA, Magnee T, Wit NJ, Verhaak PF. Does training general practitioners result in more shared decision making during consultations?. Patient Education and Counselling 2017;100(3):563‐74. [DOI] [PubMed] [Google Scholar]
Schroy 2011 {published data only}
- Schroy PC 3rd, Emmons K, Peters E, Glick JT, Robinson PA, Lydotes MA, et al. The impact of a novel computer‐based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Medical Decision Making 2011;31(1):93‐107. [DOI: 10.1177/0272989X10369007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroy 2016 {published and unpublished data}
- Schroy PC 3rd, Duhovic E, Chen CA, Heeren TC, Lopez W, Apodaca DL, et al. Risk stratification and shared decision making for colorectal cancer screening: a randomized controlled trial. Medical Decision Making 2016;36(4):526‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 2011 {published and unpublished data}
- Shepherd HL, Barratt A, Trevena LJ, McGeechan K, Carey K, Epstein RM, et al. Three questions that patients can ask to improve the quality of information physicians give about treatment options: a cross‐over trial. Patient Education and Counseling 2011;84(3):379‐85. [DOI: 10.1016/j.pec.2011.07.022] [DOI] [PubMed] [Google Scholar]
Sheridan 2012 {published data only}
- Sheridan SL, Golin C, Bunton A, Lykes JB, Schwartz B, McCormack L, et al. Shared decision making for prostate cancer screening: the results of a combined analysis of two practice‐based randomized controlled trials. BMC Medical Informatics and Decision Making 2012 Nov 13;12:130. [DOI: 10.1186/1472-6947-12-130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2014 {published data only}
- Sheridan SL, Draeger LB, Pignone MP, Rimer B, Bangdiwala SI, Cai J, et al. The effect of a decision aid intervention on decision making about coronary heart disease risk reduction: secondary analyses of a randomized trial. BMC Medical Informatics and Decision Making 2014 Feb 28;14:14. [DOI: 10.1186/1472-6947-14-14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smallwood 2017 {published and unpublished data}
- Smallwood AJ, Schapira MM, Fedders M, Neuner JM. A pilot randomized controlled trial of a decision aid with tailored fracture risk tool delivered via a patient portal. Osteoporosis International 2017;28(2):567‐76. [DOI] [PubMed] [Google Scholar]
Stacey 2006 {published data only}
- Stacey D, O'Connor AM, Graham ID, Pomey MP. Randomized controlled trial of the effectiveness of an intervention to implement evidence‐based patient decision support in a nursing call centre. Journal of Telemedicine and Telecare 2006;12(8):410‐5. [DOI: 10.1258/135763306779378663] [DOI] [PubMed] [Google Scholar]
- Stacey D, Taljaard M, Drake ER, O'Connor AM. Audit and feedback using the brief Decision Support Analysis Tool (DSAT‐10) to evaluate nurse–standardized patient encounters. Patient Education and Counseling 2008;73(3):519‐25. [DOI: 10.1016/j.pec.2008.07.016] [DOI] [PubMed] [Google Scholar]
Stiggelbout 2008 {published data only}
- Stiggelbout AM, Molewijk AC, Otten W, Bockel JH, Bruijninckx CM, Salm I, et al. The impact of individualized evidence‐based decision support on aneurysm patients' decision making, ideals of autonomy, and quality of life. Medical Decision Making 2008;28(5):751‐62. [PUBMED: 18626126] [DOI] [PubMed] [Google Scholar]
Street 1995 {published data only}
- Street RL Jr, Voigt B, Geyer C Jr, Manning T, Swanson GP. Increasing patient involvement in choosing treatment for early breast cancer. Cancer 1995;76(11):2275‐85. [CENTRAL: 00124857] [DOI] [PubMed] [Google Scholar]
Tai‐Seale 2016 {published and unpublished data}
- Dillon EC, Stults CD, Wilson C, Chuang J, Meehan A, Li M, et al. An evaluation of two interventions to enhance patient‐physician communication using the observer OPTION5 measure of shared decision making. Patient Education and Counseling 2017;100(10):1910‐7. [PUBMED: 28532861] [DOI] [PubMed] [Google Scholar]
- Tai‐Seale M, Elwyn G, Wilson C J, Stults C, Dillon E C, Li M, et al. Enhancing shared decision making through carefully designed interventions that target patient and provider behavior. Health Affairs 2016;35(4):605‐12. [DOI] [PubMed] [Google Scholar]
Thomson 2007 {published data only}
- Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, May CR. A patient decision aid to support shared decision‐making on anti‐thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Quality & Safety in Health Care 2007 Jun;16(3):216‐23. [DOI: 10.1136/qshc.2006.018481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinsel 2013 {published data only}
- Tinsel I, Buchholz A, Vach W, Siegel A, Dürk T, Buchholz A, et al. Shared decision‐making in antihypertensive therapy: a cluster randomised controlled trial. BMC Family Practice 2013 Sep 11;14:135. [doi: 10.1186/1471‐2296‐14‐135] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Krieke 2013 {published data only}
- Krieke L, Emerencia AC, Boonstra N, Wunderink L, Jonge P, Sytema S. A web‐based tool to support shared decision making for people with a psychotic disorder: randomized controlled trial and process evaluation. Journal of Medical Internet Research 2013 Oct 7;15(10):e216. [10.2196/jmir.2851] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Peperstraten 2010 {published data only}
- Peperstraten A, Nelen W, Grol R, Zielhuis G, Adang E, Stalmeier P, et al. The effect of a multifaceted empowerment strategy on decision making about the number of embryos transferred in in vitro fertilisation: randomised controlled trial. BMJ 2010:341:c2501. [DOI: 10.1136/bmj.c2501] [DOI] [PMC free article] [PubMed]
- Peperstraten AM, Hermens RP, Nelen WL, Stalmeier PF, Wetzels AM, Maas PH, et al. Deciding how many embryos to transfer after in vitro fertilisation: development and pilot test of a decision aid. Patient Education and Counselling 2010;78(1):124‐9. [DOI: 10.1016/j.pec.2009.04.007] [DOI] [PubMed] [Google Scholar]
van Roosmalen 2004 {published data only}
- Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra‐Weebers JE, Oosterwijk JC, Hoogerbrugge N, et al. Randomized trial of a shared decision‐making intervention consisting of trade‐offs and individualized treatment information for BRCA1/2 mutation carriers. Journal of Clinical Oncology 2004 Aug 15;22(16):3293‐301. [DOI: 10.1200/JCO.2004.05.066] [DOI] [PubMed] [Google Scholar]
van Tol‐Geerdink 2016 {published and unpublished data}
- Tol‐Geerdink JJ, Leer JW, Wijburg CJ, Oort IM, Vergunst H, Lin EJ, et al. Does a decision aid for prostate cancer affect different aspects of decisional regret, assessed with new regret scales? A randomized, controlled trial. Health Expectations 2016;19(2):459‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vestala 2013 {published data only}
- Vestala H, Frisman GH. Can participation in documentation influence experiences of involvement in care decision‐making?. Open Nursing Journal 2013 May 16;7:66‐72. [DOI: 10.2174/1874434620130516002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vodermaier 2009 {published data only}
- Vodermaier A, Caspari C, Koehm J, Kahlert S, Ditsch N, Untch M. Contextual factors in shared decision making: a randomised controlled trial in women with a strong suspicion of breast cancer. British Journal of Cancer 2009;100(4):590‐7. [DOI: 10.1038/sj.bjc.6604916] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warner 2015 {published data only}
- Warner DO, LeBlanc A, Kadimpati S, Vickers KS, Shi Y, Montori VM. Decision aid for cigarette smokers scheduled for elective surgery. Anesthesiology 2015;123(1):18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetzels 2005 {published data only}
- Wetzels R, Wensing M, Weel C, Grol R. A consultation leaflet to improve an older patient's involvement in general practice care: a randomized trial. Health Expectations 2005;8(4):286‐94. [CENTRAL: 00531671] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkes 2013 {published data only}
- Wilkes MS, Day FC, Srinivasan M, Griffin E, Tancredi DJ, Rainwater JA, et al. Pairing physician education with patient activation to improve shared decisions in prostate cancer screening: a cluster randomized controlled trial. Annals of Family Medicine 2013;11(4):324‐34. [DOI: 10.1370/afm.1550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolderslund 2017 {published data only}
- Wolderslund M, Kofoed PE, Holst R, Axboe M, Ammentorp J. Digital audio recordings improve the outcomes of patient consultations: a randomised cluster trial. Patient Education and Counselling 2017;100(2):242‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 2006 {published data only}
- Alexander SC, Keitz SA, Sloane R, Tulsky JA. A controlled trial of a short course to improve residents' communication with patients at the end of life. Academic Medicine 2006;81(11):1008‐12. [DOI: 10.1097/01.ACM.0000242580.83851.ad] [DOI] [PubMed] [Google Scholar]
Aljumah 2015 {published data only}
- Aljumah K, Hassali MA. Impact of pharmacist intervention on adherence and measurable patient outcomes among depressed patients: a randomised controlled study. BMC Psychiatry 2015;15:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2009 {published data only}
- Allen JD, Mohllajee AP, Shelton RC, Drake BF, Mars DR. A computer‐tailored intervention to promote informed decision making for prostate cancer screening among African American men. American Journal of Men's Health 2009; Vol. 3, issue 4:340‐51. [DOI: 10.1177/1557988308325460] [DOI] [PMC free article] [PubMed]
Boehmer 2014 {published data only}
- Boehmer KR, Egginton JS, Branda ME, Kryworuchko J, Bodde A, Montori VM, et al. Missed opportunity? Caregiver participation in the clinical encounter. A videographic analysis. Patient Education and Counseling 2014;96(3):302‐7. [DOI: 10.1016/j.pec.2014.05.016] [DOI] [PubMed] [Google Scholar]
Boyd 2010 {published data only}
- Boyd CM, Reider L, Frey K, Scharfstein D, Leff B, Wolff J, et al. The effects of guided care on the perceived quality of health care for multi‐morbid older persons: 18‐month outcomesfrom a cluster‐randomized controlled trial. Journal of General Internal Medicine 2010;25(3):235‐42. [DOI: 10.1007/s11606-009-1192-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brinkman 2013 {published data only}
- Brinkman WB, Hartl Majcher J, Poling LM, Shi G, Zender M, Sucharew H, et al. Shared decision‐making to improve attention‐deficit hyperactivity disorder care. Patient Education and Counseling 2013;93(1):95‐101. [DOI: 10.1016/j.pec.2013.04.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2004 {published data only}
- Brown RF, Butow PN, Sharrock MA, Henman M, Boyle F, Goldstein D, et al. Education and role modelling for clinical decisions with female cancer patients. Health Expectations 2004;7(4):303‐16. [CENTRAL: 00504159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davison 2007 {published data only}
- Davison BJ, Goldenberg SL, Wiens KP, Gleave ME. Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer. Cancer Nursing 2007;30(5):E7‐15. [DOI: 10.1097/01.NCC.0000290819.22195.d6] [DOI] [PubMed] [Google Scholar]
Golnik 2012 {published data only}
Green 2011 {published data only}
- Green MJ, Levi BH. Teaching advance care planning to medical students with a computer‐based decision aid. Journal of Cancer Education 2011;26(1):82‐91. [PUBMED: 20632222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hack 2007 {published data only}
- Hack TF, Pickles T, Bultz BD, Ruether JD, Degner LF. Impact of providing audiotapes of primary treatment consultations to men with prostate cancer: a multi‐site, randomized, controlled trial. Psycho‐Oncology 2007;16(6):543‐52. [PUBMED: 16991107] [DOI] [PubMed] [Google Scholar]
Hanson 2011 {published data only}
- Hanson LC, Carey TS, Caprio AJ, Lee TJ, Ersek M, Garrett J, et al. Improving decision‐making for feeding options in advanced dementia: A randomized, controlled trial. Journal of the American Geriatrics Society 2011;59(11): 2009‐16. [DOI: 10.1111/j.1532-5415.2011.03629.x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmsen 2014 {published data only}
- Harmsen CG, Kristiansen IS, Larsen PV, Nexøe J, Støvring H, Gyrd‐Hansen D, et al. Communicating risk using absolute risk reduction or prolongation of life formats: cluster‐randomised trial in general practice. British Journal of General Practice 2014;64(621):e199‐207. [DOI: 10.3399/bjgp14X677824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermansen Kobulnicky 2002 {published data only}
- Hermansen Kobulnicky CJ. The behavioral impact of teaching patients to monitor side effects of chemotherapy: an intervention study. Madison: MI: University of Michigan, 2002. [Google Scholar]
Hoffman 2014 {published data only}
- Hoffmann TC, Bennett S, Tomsett C, Mar C. Brief training of student clinicians in shared decision making: a single‐blind randomized controlled trial. Journal of General Internal Medicine 2014;29(6):844‐9. [DOI: 10.1007/s11606-014-2765-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jangland 2012 {published data only}
- Jangland E, Carlsson M, Lundgren E, Gunningberg L. The impact of an intervention to improve patient participation in a surgical care unit: a quasi‐experimental study. International Journal of Nursing Studies 2012 May;49(5):528‐38. [DOI: 10.1016/j.ijnurstu.2011.10.024] [DOI] [PubMed] [Google Scholar]
Koekkoek 2012 {published data only}
- Koekkoek B, Meijel B, Schene A, Smit A, Kaasenbrood A, Hutschemaekers G. Interpersonal Community Psychiatric Treatment for non‐psychoticchronic patients and nurses in outpatient mental health care: a controlled pilot study on feasibility and effects. International Journal of Nursing Studies 2012;49(5):549‐59. [DOI: 10.1016/j.ijnurstu.2011.11.003] [DOI] [PubMed] [Google Scholar]
Kopke 2009 {published data only}
- Köpke S, Kasper J, Mühlhauser I, Nübling M, Heesen C. Patient education program to enhance decision autonomy in multiple sclerosis relapse management: a randomized‐controlled trial. Multiple Sclerosis 2009;15(1):96‐104. [DOI: 10.1177/1352458508095921] [DOI] [PubMed] [Google Scholar]
Kupke 2013 {published data only}
- Kupke J, Wicht MJ, Stützer H, Derman SH, Lichtenstein NV, Noack MJ. Does the use of a visualised decision board by undergraduate students during shared decision‐makingenhance patients' knowledge and satisfaction? A randomised controlled trial. European Journal of Dental Education 2013;17(1):19‐25. [DOI: 10.1111/eje.12002] [DOI] [PubMed] [Google Scholar]
Langewitz 1998 {published data only}
- Langewitz WA, Eich P, Kiss A, Wössmer B. Improving communication skills a randomized controlled behaviorally oriented intervention study for residents in internal medicine. Psychosomatic Medicine 1998;60(3):268‐76. [PUBMED: 9625213] [DOI] [PubMed] [Google Scholar]
Leader 2012 {published data only}
LeBlanc 2017 {published data only}
- LeBlanc A, Egginton J, Inselman J, Dick S, Schuerman J, Branda M, et al. Dissemination and Implementation of Evidence‐Based Medicine in Primary Care Through The Use of Encounter Decision Aids: The ShareEBM Pragmatic Trial. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Oral communications. 2017.
Man‐Son‐Hing 1999 {published data only}
- Man‐Son‐Hing M, Laupacis A, O'Connor AM, Biggs J, Drake E, Yetisir E, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA 1999;282(8):737‐43. [DOI: 10.1001/jama.282.8.737] [DOI] [PubMed] [Google Scholar]
Maslin 1998 {published data only}
- Maslin AM, Baum M, Walker JS, A'Hern R, Prouse A. Using an interactive video disk in breast cancer patient support. Nursing Times 1998;94(44):52‐5. [PUBMED: 9919256] [PubMed] [Google Scholar]
McCormack 2011 {published data only}
- McCormack L, Treiman K, Bann C, Williams‐Piehota P, Driscoll D, Poehlman J, et al. Translating medical evidence to promote informed health care decisions. Health Services Research 2011; Vol. 46, issue 4:1200‐23. [DOI: 10.1111/j.1475-6773.2011.01248] [DOI] [PMC free article] [PubMed]
NCT01550731 {published data only}
- NCT01550731. Preparing older veterans with serious and cronic illness for decision making (PREPARE). https://clinicaltrials.gov/ct2/show/NCT01550731 (accessed 25 September 2017).
NCT02033499 {published data only}
- NCT02033499. Three approaches to glucose monitoring in non‐insulin treated diabetes. https://clinicaltrials.gov/show/NCT02033499 (accessed December 2016).
NCT02319525 {published data only}
- NCT02319525. Individualized patient decision making for treatment choices among minorities with Lupus. https://clinicaltrials.gov/ct2/show/NCT02319525 (accessed 25 September 2017).
Ockhuysen‐Vermey 2008 {published data only}
- Ockhuysen‐Vermey CF, Henneman L, Asperen CJ, Oosterwijk JC, Menko FH, Timmermans D. Design of the BRISC study: a multicentre controlled clinical trial to optimize the communication of breast cancer risks in genetic counselling. BMC Cancer 2008;8:283. [DOI: 10.1186/1471-2407-8-283] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price‐Haywood 2014 {published data only}
- Price‐Haywood EG, Harden‐Barrios J, Cooper LA. Comparative effectiveness of audit‐feedback versus additional physician communication training to improve cancer screening for patients with limited health literacy. Journal of General Internal Medicine 2014;29(8):1113‐21. [DOI: 10.1007/s11606-014-2782-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riippa 2014 {published data only}
- Riippa I, Linna M, Rönkkö I. The effect of a patient portal with electronic messaging on patient activation among chronically ill patients: controlled before‐and‐after study. Journal of Medical Internet Research 2014 Nov 19;16(11):e257. [DOI: 10.2196/jmir.3462.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roelands 2004 {published data only}
- Roelands M, Van Oost P, Stevens V, Depoorter A, Buysse A. Clinical practice guidelines to improve shared decision‐making about assistive device use in home care: a pilot intervention study. Patient Education and Counseling 2004;55(2):252‐64. [DOI: ] [DOI] [PubMed] [Google Scholar]
Schwalm 2012 {published data only}
- Schwalm JD, Stacey D, Pericak D, Natarajan MK. Radial artery versus femoral artery access options in coronary angiogram procedures: randomized controlled trial of a patient‐decision aid. Circulation: Cardiovascular Quality and Outcomes 2012; Vol. 5, issue 3:260‐6. [DOI: 10.1161/CIRCOUTCOMES] [DOI] [PubMed]
Simon 2012 {published data only}
- Simon D, Kriston L, von Wolff A, Buchholz A, Vietor C, Hecke T, et al. Effectiveness of a web‐based, individually tailored decision aid for depression or acute low back pain: a randomized controlled trial. Patient Education and Counseling 2012;87(3):360‐8. [DOI: 10.1016/j.pec.2011.10.009] [DOI] [PubMed] [Google Scholar]
Smith 2010a {published data only}
- Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. BMJ 2010; Vol. 341:c5370. [DOI: 10.1136/bmj.c5370] [DOI] [PMC free article] [PubMed]
Spertus 2011 {unpublished data only}
- Spertus J. Testing evidence‐based, individualized informed consent forms to improve patients' experiences with percutaneous coronary intervention. Late‐Breaking Clinical Trial and Clinical Science: Special Reports Abstracts from the American Heart Association’s Scientific Sessions 2011.
van Tol‐Geerdink 2008 {published data only}
- Tol‐Geerdink JJ, Leer JW, van Lin EN, Schimmel EC, Huizenga H, van Daal WA, et al. Offering a treatment choice in the irradiation of prostate cancer leads to better informed and more active patients, without harm to well‐being. International Journal of Radiation Oncology, Biology, Physics 2008;70(2):442‐8. [PUBMED: 17765404] [DOI] [PubMed] [Google Scholar]
Wagner 2012 {published data only}
- Wagner PJ, Dias J, Howard S, Kintziger KW, Hudson MF, Seol YH, et al. Personal health records and hypertension control:a randomized trial. Journal of the American Medical Informatics Association 2012;19(4):626‐34. [DOI: 10.1136/amiajnl-2011-000349] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whelan 2003 {published data only}
- Whelan T, Sawka C, Levine M, Gafni A, Reyno L, Willan A, et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node‐negative breast cancer. Journal of the National Cancer Institute 2003;95(8):581‐7. [DOI: 10.1093/jnci/95.8.581] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Simmons 2017 {published data only}
- Simmons M. ‘If I could see on a piece of paper options for treatment that would just be insane’: Shared decision making in mental health. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Posters Sessions. 2017.
van Veenendaal 2017 {published data only}
- Veenendaal H, Voogdt‐Pruis H, Schuurman M, Visserman E, Oskam J, Ubbink D, et al. Implementing shared decision‐making (SDM) and time‐out for breast cancer patients in six Dutch Hospitals. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Posters Sessions. 2017.
References to ongoing studies
ACTRN12614000593639 {published data only}
- ACTRN12614000593639. Improving the engagement of patients suffering from wisdom tooth problems in shared clinical decision making using different formats of health information toward achieving better healthcare outcomes. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000593639 (accessed 25 September 2017).
ACTRN12616000213448 {published data only}
- ACTRN12616000213448. Development and evaluation of an Australian adult health literacy program for socially disadvantaged adults attending TAFE. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000213448 (accessed 25 September 2017).
ACTRN12616000644460 {published data only}
- ACTRN12616000644460. Evaluating decision aids for acute respiratory infections on the use of antibiotics in general practice. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000644460 (accessed 25 September 2017).
ACTRN12617000614392 {published data only}
- ACTRN12617000614392. Whakapai e Te Ara Ha: Asthma self‐management programme for the whanau of tamariki Maori with asthma. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000614392 (accessed 25 September 2017).
ACTRN12617000840381 {published data only}
- ACTRN12617000840381. Enhancing decision‐making about treatment in bipolar II disorder: evaluation of a treatment decision‐aid for patients and their family. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000840381 (accessed 25 September 2017).
Adekpedjou ongoing {published data only}
- Adekpedjou R, Stacey D, Brière N, Freitas A, Garvelink M, Turcotte S, et al. Improving the decision‐making process with caregivers of elderly people about housing options: a cluster randomised trial. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Oral communications. 2017.
Altshuler 2016 {published data only}
- Altshuler L, Plaksin J, Zabar S, Wallach A, Sawicki C, Kundrod S, et al. Transforming the patient role to achieve better outcomes through a patient empowerment program: a randomized wait‐list control trial protocol. JMIR Research Protocols 2016;5(2):e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00000191 {published data only}
- DRKS00000191. Umsetzung des Modells der Partizipativen Entscheidungsfindung in der hausärztlichen Praxis am Beispiel der Patientenverfügung‐Patientenverfügung im Partizipativen Prozess (PPP). http://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00000191 (accessed 25 September 2017).
DRKS00010880 {published data only}
- DRKS00010880. Shared Decision Making PLUS – eine Cluster‐randomisierte Studie mit stationär behandelten Patienten mit Schizophrenie. http://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00010880 (accessed 25 September 2017).
Dwinger ongoing {published data only}
- Dwinger S, Gschwendtner K, Müller N, Bieber C, Härter M, Bergelt C. Development and pilot testing of a face‐to‐face SDM coaching intervention for oncologists. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Oral communications. 2017.
Fagerlin ongoing {published data only}
- Fagerlin A, Holmes‐Rovner M, Scherer LD, Kahn VC, Ubel PA. The impact of shared decision making on patient involvement in two prostate cancer decision aid trials. ISDM2015. Sydney, Australia: Joint International Shared Decision‐Making and International Society for Evidence‐Based Health Care Conference. 2015.
Finderup 2017 {published data only}
- Finderup J, Dam Jensen JK, Lomborg K. Developing and pilot testing a shared decision‐making intervention for dialysis choice. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Oral communications. 2017.
Henselmans ongoing {published data only}
- Henselmans H, Laarhoven HWM, Haes HCJM, Tokat M, Engelhardt EG, Maarschalkerweerd P, et al. Effect of a Skills Training for oncologists on shared decision making about palliative chemotherapy in simulated encounters. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Posters Sessions. 2017.
ISRCTN37929939 {published data only}
- ISRCTN37929939. Nurse‐led immunotherapy decision coaching in persons with relapsing‐remitting multiple sclerosis (DECIMS). http://www.isrctn.com/ISRCTN37929939 (accessed 25 September 2017). [DOI] [PMC free article] [PubMed]
ISRCTN46305518 {published data only}
- ISRCTN46305518. Informed shared decision making supported by decision coaches for women with ductal carcinoma in situ. http://www.isrctn.com/ISRCTN46305518 (accessed 25 September 2017). [DOI] [PMC free article] [PubMed]
ISRCTN63110516 {published data only}
- ISRCTN63110516. ACTION: cancer patient involvement in medical decision making. http://www.isrctn.com/ISRCTN63110516 (accessed 25 September 2017).
Lifford ongoing {published data only}
- Lifford K, Burton M, Armitage F, Collins K, Reed M, Wyld L, et al. Bridging the Age Gap trial of decision support interventions for older women with breast cancer: Preliminary process evaluation. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Posters Sessions. 2017.
Ludden ongoing {published data only}
- Ludden T, Taylor Y, Kuhn L, McWilliams A, Dulin M, Tapp H. “Who Made the Decision Today?” Surveying asthma patients level of shared decision making in an RCT. ISDM2015. Sydney, Australia: Joint International Shared Decision‐Making and International Society for Evidence‐Based Health Care Conference. 2015.
NCT01485627 {published data only}
- NCT01485627. VOICE: Values and Options in Cancer Care (VOICE). https://clinicaltrials.gov/show/NCT01485627 (accessed 16 December 2016).
NCT01519999 {published data only}
- NCT01519999. Colorectal cancer screening with iImproved shared decision making. https://clinicaltrialbase.com/study/NCT01519999 (accessed 19 December 2016).
NCT01828567 {published data only}
- NCT01828567. Will veterans engage in prevention after HRA‐guided shared decision making? (ACTIVATE). https://clinicaltrials.gov/show/NCT01828567 (accessed 16 December 2016).
NCT01837953 {published data only}
- NCT01837953. Stepped care for binge eating disorder: predicting response to minimal intervention in a randomized controlled trial. https://clinicaltrials.gov/show/NCT01837953 (accessed 16 December 2016).
NCT01838226 {published data only}
- NCT01838226. Randomized controlled trial of group prevention coaching. https://clinicaltrials.gov/show/NCT01838226 (accessed 16 December 2016).
NCT01866228 {published data only}
- NCT01866228. Clinical trial of the impact of treatment consultation recordings on cancer patient outcomes. https://ClinicalTrials.gov/show/NCT01866228 (accessed 16 December 2016).
NCT01992926 {published data only}
- NCT01992926. Facilitating anemia treatment risk communication for patients with kidney disease: decision aid trial. https://clinicaltrials.gov/ct2/show/NCT01992926 (accessed 25 September 2017).
NCT02047929 {published data only}
- NCT02047929. Comparing types of implementation of a shared decision making intervention (ADAPT‐NC). https://clinicaltrials.gov/show/NCT02047929 (accessed 16 December 2016).
NCT02063087 {published data only}
- NCT02063087. Shared decision making in parents of children with head trauma: Head CT Choice (Head CT Choice). https://clinicaltrials.gov/show/NCT02063087 (accessed 16 December 2016).
NCT02136732 {published data only}
- NCT02136732. Chronic care management for adults at FQHCs. https://clinicaltrials.gov/show/NCT02136732 (accessed 16 December 2016).
NCT02138448 {published data only}
- NCT02138448. Implementing personal health records to promote evidence‐based cancer screening. https://clinicaltrials.gov/show/NCT02138448 (accessed 16 December 2016).
NCT02146573 {published data only}
- NCT02146573. Pediatric Continuity Care Intensivist (CCI). https://clinicaltrials.gov/show/NCT02146573 (accessed 16 December 2016).
NCT02165735 {published data only}
- NCT02165735. Get Ready And Empowered About Treatment (GREAT). https://clinicaltrials.gov/ct2/show/NCT02165735 (accessed 25 September 2017).
NCT02198690 {published data only}
- NCT02198690. Trial of a mammography decision aid for women aged 75 and older. https://clinicaltrials.gov/show/NCT02198690 (accessed 16 December 2016).
NCT02278900 {published data only}
- NCT02278900. Supporting doctor‐patient communication in oncology. https://clinicaltrials.gov/show/NCT02278900 (accessed 16 December 2016).
NCT02282722 {published data only}
- NCT02282722. Improving informed consent for palliative chemotherapy. https://clinicaltrials.gov/show/NCT02282722 (accessed 16 December 2016).
NCT02285881 {published data only}
- NCT02285881. Shared decision making between patients and GPs in the treatment of Type 2 diabetes in primary care. https://clinicaltrials.gov/ct2/show/NCT02285881 (accessed 25 September 2017).
NCT02328326 {published data only}
- NCT02328326. Caring Others Increasing EngageMent in PACT (CO‐IMPACT). https://clinicaltrials.gov/show/NCT02328326 (accessed 16 December 2016).
NCT02344576 {published data only}
- NCT02344576.. PCORI‐1310‐06998 Trial of a decision support intervention for Patients and Caregivers Offered Destination Therapy Heart Assist Device (DECIDE‐LVAD) (accessed 25 September 2017).
NCT02379078 {published data only}
- NCT02379078. Impact of an interprofessional shared decision‐making and goal‐setting decision aid for patients with diabetes (accessed 25 September 2017).
NCT02429115 {published data only}
- NCT02429115. Peer‐mentoring, quality of life and caregiver burden in patients with chronic kidney disease and their caregivers. https://clinicaltrials.gov/show/NCT02429115 (accessed 25 September 2017).
NCT02507349 {published data only}
- NCT02507349. Person‐centered versus measurement‐based care in mental health (PCORI‐SDM). https://clinicaltrials.gov/show/NCT02507349 (accessed 25 September 2017).
NCT02592525 {published data only}
- NCT02592525. Implementing shared decision making in interprofessional home care teams (IPSDM‐SW). https://clinicaltrials.gov/show/NCT02592525 (accessed 25 September 2017).
NCT02611050 {published data only}
- NCT02611050. Treatment decisions for multi‐vessel CAD. https://clinicaltrials.gov/show/NCT02611050 (accessed 25 September 2017).
NCT02623335 {published data only}
- NCT02623335. PCORI‐1502‐27462 Navigating high risk surgery: empowering older adults to ask questions that inform decisions about surgical treatment. https://clinicaltrials.gov/show/NCT02623335 (accessed 25 September 2017).
NCT02631200 {published data only}
- NCT02631200. Advance Care Planning with older patients who have End‐stage Kidney Disease (ACREDiT). https://clinicaltrials.gov/show/NCT02631200 (accessed 25 September 2017).
NCT02646423 {published data only}
- NCT02646423. Effect of a Patient‐Centered Decision App on TOLAC (PROCEED). https://clinicaltrials.gov/show/NCT02646423 (accessed 25 September 2017).
NCT02653170 {published data only}
- NCT02653170. Michigan Stroke Transitions Trial (MISTT). https://clinicaltrials.gov/show/NCT02653170 (accessed 25 September 2017).
NCT02663245 {published data only}
- NCT02663245. INTEGRA Study: Primary care intervention in Type 2 diabetes patients with poor glycaemic control. https://clinicaltrials.gov/show/NCT02663245 (accessed 25 September 2017). [DOI] [PMC free article] [PubMed]
NCT02668900 {published data only}
- NCT02668900. Decision support for adults facing implantable cardioverter‐defibrillator pulse generator replacement. https://clinicaltrials.gov/show/NCT02668900 (accessed 25 September 2017).
NCT02674360 {published data only}
- NCT02674360. RCT regarding SDM online training and face‐to‐face SDM training. https://clinicaltrials.gov/show/NCT02674360 (accessed 25 September 2017).
NCT02686775 {published data only}
- NCT02686775. The PACO Project: a clinical study of a PAtient COach program in vulnerable lung cancer patients (PACO). https://clinicaltrials.gov/show/NCT02686775 (accessed 25 September 2017).
NCT02721810 {published data only}
- NCT02721810. Patient Engagement Initiative (PEI). https://clinicaltrials.gov/show/NCT02721810 (accessed 25 September 2017).
NCT02759939 {published data only}
- NCT02759939. Right For Me: birth control decisions made easier. https://clinicaltrials.gov/show/NCT02759939 (accessed 25 September 2017).
NCT02823262 {published data only}
- NCT02823262. A breast cancer treatment decision aid for women aged 70 and older. https://ClinicalTrials.gov/show/NCT02823262 (accessed 25 September 2017).
NCT02842047 {published data only}
- NCT02842047. The mediating effects of decentering on self‐management of stress and end of life planning. https://clinicaltrials.gov/show/NCT02842047 (accessed 25 September 2017).
NCT02866799 {published data only}
- NCT02866799. Multi‐PAP RCT: improving prescription in primary care patients with Multimorbidity and Polypharmacy (Multi‐PAP). https://clinicaltrials.gov/show/NCT02866799 (accessed 25 September 2017).
NCT02868983 {published data only}
- NCT02868983. Integrating Behavioral Health and Primary Care for comorbid behavioral and medical problems (IBHPC). https://clinicaltrials.gov/show/NCT02868983 (accessed 25 September 2017).
NCT02890615 {published data only}
- NCT02890615. CanDirect: effectiveness of a telephone‐supported Depression Self‐care Intervention for Cancer Survivors (CanDirect). https://clinicaltrials.gov/show/NCT02890615 (accessed 25 September 2017). [DOI] [PubMed]
NCT02905032 {published data only}
- NCT02905032. SDM for Stroke Prevention in Atrial Fibrillation (SDM4Afib). https://clinicaltrials.gov/show/NCT02905032 (accessed 25 September 2017).
NCT02917603 {published data only}
- NCT02917603. Shared decision making to improve palliative care in the nursing home. https://clinicaltrials.gov/show/NCT02917603 (accessed 25 September 2017).
NCT02920086 {published data only}
- NCT02920086. Improving Partnerships with family members of ICU patients (IMPACT). https://clinicaltrials.gov/show/NCT02920086 (accessed 25 September 2017).
NCT02935920 {published data only}
- NCT02935920. A study on optimizing follow‐up for postmenopausal women with breast cancer treated with adjuvant endocrine therapy. https://clinicaltrials.gov/show/NCT02935920 (accessed 25 September 2017).
NCT02971163 {published data only}
- NCT02971163. Syncope Decision Aid for emergency care (SynDA). https://clinicaltrials.gov/show/NCT02971163 (accessed 25 September 2017).
NCT02987608 {published data only}
- NCT02987608. A feasibility trial of power up. https://clinicaltrials.gov/show/NCT02987608 (accessed 25 September 2017).
NCT02988661 {published data only}
- NCT02988661. Women Empowered to Live with Lupus study (WELL). https://clinicaltrials.gov/show/NCT02988661 (accessed 25 September 2017).
NCT03012087 {published data only}
- NCT03012087. Using m‐Health tools to reduce the misuse of opioid pain relievers. https://clinicaltrials.gov/ct2/show/NCT03012087 (accessed 25 September 2017). [DOI] [PubMed]
NCT03037112 {published data only}
- NCT03037112. Resetting the default: improving provider‐patient communication to reduce antibiotic misuse. https://clinicaltrials.gov/show/NCT03037112 (accessed 25 September 2017).
NCT03084159 {published data only}
- NCT03084159. Multi‐disciplinary participatory design of a process to deliver a CKD diagnosis in primary care. https://clinicaltrials.gov/show/NCT03084159 (accessed 25 September 2017).
NCT03109145 {published data only}
- NCT03109145. MyHealtheVet to enable shared decision making regarding menopausal in postmenopausal women veterans (MEANS). https://clinicaltrials.gov/show/NCT03109145 (accessed 25 September 2017).
NCT03134092 {published data only}
- NCT03134092. The Life STORRIED Study. https://clinicaltrials.gov/show/NCT03134092 (accessed 25 September 2017).
NCT03136367 {published data only}
- NCT03136367. What matters most: choosing the right breast cancer surgery for you. https://clinicaltrials.gov/show/NCT03136367.
NCT03216109 {published data only}
- NCT03216109. Improving supportive care for patients with thoracic malignancies. https://clinicaltrials.gov/show/NCT03216109 (accessed 25 September 2017).
NCT03221556 {published data only}
- NCT03221556. Improving outcomes for low‐income mothers with depression. https://clinicaltrials.gov/show/NCT03221556 (accessed 25 September 2017).
NCT03228615 {published data only}
- NCT03228615. IBD shared decision making intervention. https://clinicaltrials.gov/show/NCT03228615 (accessed 25 September 2017).
NCT03234322 {published data only}
- NCT03234322. The impact of a diabetes risk prediction model in primary care. https://clinicaltrials.gov/show/NCT03234322 (accessed 25 September 2017).
NTR4554 {published data only}
- NTR4554. Prostate Cancer Patient‐centered Care (PCPCC): impact of a treatment decision aid in a pragmatic, cluster randomized controlled trial. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4554 (accessed 26 September 2017).
NTR4879 {published data only}
- NTR4879. Implementation of shared decision making in a clinical setting; how to make it fit in the daily workflow?. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4879 (accessed 26 September 2017).
NTR5177 {published data only}
- NTR5177. (Cost‐)effectiveness and implementation of a decision aid for patients with localized prostate cancer and their partners: study protocol of a stepped wedge cluster randomized controlled trial. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5177 (accessed 26 September 2017). [DOI] [PMC free article] [PubMed]
NTR5262 {published data only}
- NTR5262. Shared decision making in mental health with Routine Outcome Monitoring (ROM) as an information source. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5262 (accessed 26 September 2017).
NTR5489 {published data only}
- NTR5489. CHOICE: CHOosing treatment together In Cancer at the End of life. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5489.
NTR5677 {published data only}
- NTR5677. Empowerment in mental health care using e‐health in a redesigned intake process: a cluster randomised controlled trial. Study protocol. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5677 (accessed 26 September 2017).
NTR6106 {published data only}
- NTR6106. A decision aid for the treatment of Benign Prostatic Hyperplasia: a prospective cohort study to investigate the effect. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6106 (accessed 26 September 2017).
NTR6227 {published data only}
- NTR6227. Secondary manifestations of arterial disease ‐ influence of cardiovascular prognosis and treatment effect predictions on patient and physician decision‐making: a three‐armed, blinded, randomized controlled trial. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6227 (accessed 26 September 2017).
NTR6379 {published data only}
- NTR6379. Shared decision making in patients with Castration‐Resistant Prostate Cancer ‐ The impact of implementation of a treatment decision aid in CRPC patients. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6379 (accessed 26 September 2017).
NTR6487 {published data only}
- NTR6487. A multicentre stepped‐wedge cluster‐randomised trial studying the level of shared decision‐making during vascular surgical consultation before and after the introduction of decision support tools. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6487 (accessed 26 September 2017).
Simmons ongoing {published data only}
- Simmons L, Meyer C, Marques F, Leavitt L, Sepucha K. Skills training for shared decision making: a randomized pilot study. ISDM2017. Lyon, France: 9th International Shared Decision Making Conference. Book of Abstract, Oral communications. 2017.
Steele Gray 2016 {published data only}
- Steele Gray C, Wodchis W P, Upshur R, Cott C, McKinstry B, Mercer S, et al. Supporting goal‐oriented primary health care for seniors with complex care needs using mobile technology: evaluation and implementation of the health system performance research network, bridgepoint electronic patient reported outcome tool. JMIR Research Protocols 2016;5(2):e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2017 {published data only}
- Thompson W, Farrell B, Welch V, Tugwell P, Bjerre L M. Should I continue taking my acid reflux medication? Design of a pilot before/after study evaluating a patient decision aid. Canadian Pharmacists Journal (Ott) 2017;150(1):19‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000009239 {published data only}
- UMIN000009239. Comparison of Shared Decision Making (SDM) and routine care for University students in psychiatric outpatient clinic. A randomized controlled trial. https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000010849 (accessed 25 September 2017).
UMIN000022832 {published data only}
- UMIN000022832. A shared decision making communication training program for clinicians treating: a pilot clustered randomised controlled trial. https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000025946 (accessed 25 September 2017).
Additional references
Alston 2012
- Alston C, Paget L, Halvorson G, Novelli B, Guest J, McCabe P, et al. Communicating with patients on health care evidence. Washington, DC: Institute of Medicine of the National Academes. 2012.
Andrews 2013
- Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation‐determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726‐35. [PUBMED: 23570745] [DOI] [PubMed] [Google Scholar]
Barr 2015
- Barr PJ, O'Malley AJ, Tsulukidze M, Gionfriddo MR, Montori V, Elwyn G. The psychometric properties of Observer OPTION(5), an observer measure of shared decision making. Patient Education and Counseling 2015;98(8):970‐6. [PUBMED: 25956069] [DOI] [PubMed] [Google Scholar]
Barr 2016
- Barr PJ, Elwyn G. Measurement challenges in shared decision making: Putting the 'patient' in patient‐reported measures. Health Expectations 2016;19(5):993‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanc 2014
- Blanc X, Collet TH, Auer R, Fischer R, Locatelli I, Iriarte P, et al. Publication trends of shared decision making in 15 high impact medical journals: a full‐text review with bibliometric analysis. BMC Medical Informatics and Decision Making 2014;14:71. [PUBMED: 25106844] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouwers 2010
- Brouwers M, Stacey D, O'Connor A. Knowledge creation: synthesis, tools and products. Canadian Medical Association Journal 2010;182(2):E68‐72. [DOI: 10.1503/cmaj.081230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2012
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ (Clinical research ed.) 2012;345:e5661. [PUBMED: 22951546] [DOI] [PubMed] [Google Scholar]
Chalmers 2009
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet (London, England) 2009;374(9683):86‐9. [PUBMED: 19525005] [DOI] [PubMed] [Google Scholar]
Charles 1997
- Charles C, Gafni A. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science & Medicine 1997;44(5):681‐92. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: L Erlbaum Associates, 1988. [Google Scholar]
Coulter 2006
- Coulter A. Engaging patients in their healthcare. How is the UK doing relative to other countries?. Oxford: Picker Institute Europe 2006.
Coulter 2017
- Coulter A. Measuring what matters to patients. BMJ 2017;356:j816. [DOI: 10.1136/bmj.j816] [DOI] [PubMed] [Google Scholar]
Couët 2013
- Couët N, Desroches S, Robitaille H, Vaillancourt H, Leblanc A, Turcotte S, et al. Assessments of the extent to which health‐care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expectations 2013;Epub ahead of print:1‐20. [DOI: 10.1111/hex.12054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coxeter 2015
- Coxeter P, Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD010907.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davey 2004
- Davey HM, Lim J, Butow PN, Barratt AL, Redman S. Women's preferences for and views on decision‐making for diagnostic tests. Social Science & Medicine 2004;58(9):1699‐707. [DOI: 10.1016/S0277-9536(03)00339-3] [DOI] [PubMed] [Google Scholar]
Degner 1992
- Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play?. Journal of Clinical Epidemiology 1992;45(9):941‐50. [PUBMED: 1432023] [DOI] [PubMed] [Google Scholar]
Diouf 2016
- Diouf NT, Menear M, Robitaille H, Painchaud Guérard G, Légaré F. Training health professionals in shared decision making: Update of an international environmental scan. Patient Education and Counselling 2016;99(11):1753‐8. [DOI: 10.1016/j.pec.2016.06.008] [DOI] [PubMed] [Google Scholar]
Elwyn 1999
- Elwyn G, Edwards A, Kinnersley P. Shared decision‐making in primary care: the neglected second half of the consultation. British Journal of General Practice 1999;49(443):477‐82. [PUBMED: 10562751] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2003
- Elwyn G, Edwards A, Wensing M, Hood K, Atwell C, Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Quality & safety in health care 2003;12(2):93‐9. [PUBMED: 12679504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Entwistle 2001
- Entwistle VA, Skea ZC, O'Donnell MT. Decisions about treatment: interpretations of two measures ofcontrol by women having a hysterectomy. Social Science & Medicine 2001 Sep;53(6):721‐32. [DOI] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). EPOC Taxonomy; 2015. Available at: https://epoc.cochrane.org/epoc‐taxonomy.
EPOC 2017
- Cochrane Effective Practice, Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC resources for review authors, 2017. Available at: http://epoc.cochrane.org/resources/epoc‐resources‐review‐authors.
EPOC 2017b
- Cochrane Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors, 2017. Available at: http://epoc.cochrane.org/resources/epoc‐resources‐review‐authors.
EPOC 2017c
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors, 2017. http://epoc.cochrane.org/resources/epoc‐resources‐review‐authors.
EPOC 2017d
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors, 2017. Available at: http://epoc.cochrane.org/resources/epoc‐resources‐review‐authors.
Frosch 2009
- Frosch DL, Légaré F, Fishbein M, Elwyn G. Adjuncts or adversaries to shared decision‐making? Applying the Integrative Model of behavior to the role and design of decision support interventions in healthcare interactions. Implementation Science 2009;4:73. [DOI: 10.1186/1748-5908-4-73] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giguère 2012
- Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD004398.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Godin 2008
- Godin G, Belanger‐Gravel A, Eccles M, Grimshaw J. Healthcare professionals' intentions and behaviours: a systematic review of studies based on social cognitive theories. Implementation Science 2008;3:36. [DOI: 10.1186/1748-5908-3-36] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimshaw 2004
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment 2004;8(6):1‐72. [PUBMED: 14960256] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011 Apr;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Harter 2017
Hibbard 2004
- Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Services Research 2004;39(4 Pt 1):1005‐26. [PUBMED: 15230939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hibbard 2005
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Services Research 2005;40(6 Pt 1):1918‐30. [PUBMED: 16336556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org 2011.
Institute for Health Metrics and Evaluation 2013
- Institute for Health Metrics and Evaluation. The Global Burden of Disease: Generating Evidence, Guiding Policy. Seattle: Institute for Health Metrics and Evaluation (IHME), 2013. [Google Scholar]
Kiesler 2006
- Kiesler DJ, Auerbach SM. Optimal matches of patient preferences for information, decision‐making and interpersonal behavior: evidence, models and interventions. Patient Education and Counseling 2006 Jun;61(3):319‐41. [DOI: 10.1016/j.pec.2005.08.002] [DOI] [PubMed] [Google Scholar]
Kriston 2010
- Kriston L, Scholl I, Holzel L, Simon D, Loh A, Harter M. The 9‐item Shared Decision Making Questionnaire (SDM‐Q‐9). Development and psychometric properties in a primary care sample. Patient Education and Counseling 2010;80(1):94‐9. [PUBMED: 19879711] [DOI] [PubMed] [Google Scholar]
LeBlanc 2009
- LeBlanc A, Kenny DA, O'Connor AM, Legare F. Decisional conflict in patients and their physicians: a dyadic approach to shared decision making. Medical Decision Making 2009;29(1):61‐8. [DOI: 10.1177/0272989X08327067] [DOI] [PubMed] [Google Scholar]
Légaré 2007a
- Légaré F, Graham ID, O'Connor AC, Aubin M, Baillargeon L, Leduc Y, et al. Prediction of health professionals' intention to screen for decisional conflict in clinical practice. Health Expectations 2007;10(4):364‐79. [DOI: 10.1111/j.1369-7625.2007.00465.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Légaré 2011
- Légaré F, Stacey D, Pouliot S, Gauvin FP, Desroches S, Kryworuchko, J, et al. Interprofessionalism and shared decision‐making in primary care: a stepwise approach towards a new model. Journal of Interprofessional Care 2011;25(1):18‐25. [DOI: 10.3109/13561820.2010.490502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Légaré 2013
- Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Affairs (Millwood) 2013 Feb;32(2):276‐84. [DOI: 10.1377/hlthaff.2012.1078] [DOI] [PubMed] [Google Scholar]
Makoul 2006
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Education and Counseling 2006;60(3):301‐12. [DOI: 10.1016/j.pec.2005.06.010] [DOI] [PubMed] [Google Scholar]
Michie 2009
- Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implementation Science 2009;4:40. [DOI: 10.1186/1748-5908-4-40] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulley 2012
- Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients' preferences matter. BMJ 2012;345:e6572. [DOI: 10.1136/bmj.e6572] [DOI] [PubMed] [Google Scholar]
Salzburg Global Seminar 2011
- Salzburg Global Seminar. Salzburg Global Seminar 2011.
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):726‐32. [PUBMED: 20335313] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results, drawing conclusions. In: Higgins JPT. Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from http://training.cochrane.org/handbook.
Shafir 2012
- Shafir A, Rosenthal J. Shared Decision Making: Advancing Patient‐Centered Care through State and Federal Implementation. National Academy for State Health Policy, 2012:1‐38. [Google Scholar]
Shay 2015
- Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Medical Decision Making 2015 Jan;35(1):114‐31. [DOI: 10.1177/0272989X14551638] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2017
- Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD001431.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straub 2008
- Straub C, Nebling T, Muller H. Translating research into practice: a German sickness fund supporting patient participation. Patient Education and Counseling 2008;73(3):544‐50. [DOI: 10.1016/j.pec.2008.07.019] [DOI] [PubMed] [Google Scholar]
Street 2009
- Street RL Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician‐patient communication to health outcomes. Patient Education and Counseling 2009;74(3):295‐301. [PUBMED: 19150199] [DOI] [PubMed] [Google Scholar]
Towle 1999
- Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ (Clinical research ed.) 1999;319(7212):766‐71. [DOI: 10.1136/bmj.319.7212.766] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PUBMED: 10982317] [PubMed] [Google Scholar]
Velentgas 2013
- Velentgas P, Dreyer NA, Wu AW. Outcome definition and measurement. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM editor(s). Developing a Protocol for Observational Comparative Effectiveness Research: A User's Guide. Rockville (MD): Agency for Healthcare Research and Quality (US), 2013 Jan. [PubMed] [Google Scholar]
Wennberg 2004
- Wennberg JE. Practice variation: implications for our health care system. Managed Care 2004;13(9 Suppl):3‐7. [PUBMED: 15493217] [PubMed] [Google Scholar]
Weston 2001
- Weston WW. Informed and shared decision‐making: the crux of patient‐centered care. Canadian Medical Association Journal 2001;165(4):438‐9. [PUBMED: 11531054] [PMC free article] [PubMed] [Google Scholar]
Wyatt 2015
- Wyatt KD, List B, Brinkman WB, Prutsky Lopez G, Asi N, Erwin P, et al. Shared decision making in pediatrics: a systematic review and meta‐analysis. Academic Pediatrics 2015 Nov‐Dec;15(6):573‐83. [DOI: 10.1016/j.acap.2015.03.011] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Légaré 2007
- Légaré F, Ratté S, Stacey D, Kryworuchko J, Gravel K, Turcot L, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2007, Issue Issue 3. [DOI: 10.1002/14651858.CD006732] [DOI] [PubMed] [Google Scholar]
Légaré 2010
- Légaré F, Ratté S, Stacey D, Kryworuchko J, Gravel K, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD006732.pub2] [DOI] [PubMed] [Google Scholar]
Légaré 2014
- Légaré F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006732.pub3] [DOI] [PubMed] [Google Scholar]


