Abstract
Background
Cystic fibrosis is an inherited recessive disorder of chloride transport that is characterised by recurrent and persistent pulmonary infections from resistant organisms that result in lung function deterioration and early mortality in sufferers.
Meticillin‐resistant Staphylococcus aureus (MRSA) has emerged as, not only an important infection in people who are hospitalised, but also as a potentially harmful pathogen in cystic fibrosis. Chronic pulmonary infection with MRSA is thought to confer people with cystic fibrosis with a worse clinical outcome and result in an increased rate of lung function decline. Clear guidance for MRSA eradication in cystic fibrosis, supported by robust evidence, is urgently needed. This is an update of a previous review.
Objectives
To evaluate the effectiveness of treatment regimens designed to eradicate MRSA and to determine whether the eradication of MRSA confers better clinical and microbiological outcomes for people with cystic fibrosis. To ascertain whether attempts at eradicating MRSA can lead to increased acquisition of other resistant organisms (including P aeruginosa) or increased adverse effects from drugs, or both.
Search methods
Randomised and quasi‐randomised controlled trials were identified by searching the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register, PubMed, MEDLINE, clinical trial registries (Clinicaltrials.gov, WHO ICTRP, ISRCTN Registry), handsearching article reference lists and through contact with experts in the field.
Date of the last search of the Group's Cystic Fibrosis Trials Register: 27 July 2017.
Ongoing trials registries were last searched: 07 August 2017.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing any combinations of topical, inhaled, oral or intravenous antimicrobials with the primary aim of eradicating MRSA compared with placebo, standard treatment or no treatment.
Data collection and analysis
The authors independently assessed all search results for eligibility. They used the GRADE methodology to assess the quality of the evidence.
Main results
The review includes two trials with a total of 106 participants with MRSA infection. In both trials the active treatment was oral trimethoprim and sulfamethoxazole combined with rifampicin; however, one trial administered this combination for two weeks alongside nasal, skin and oral decontamination and a three‐week environmental decontamination, while the second trial administered this drug combination for 21 days with five days intranasal mupirocin. In both trials the control arm was observation only.
Both trials reported successful eradication of MRSA in people with CF as an outcome; however, the definition used for MRSA eradication differed. The first trial (n = 45) defined MRSA eradication as negative MRSA respiratory cultures at day 28, and reported that, when compared to control, oral trimethoprim and sulfamethoxazole combined with rifampicin may lead to a higher proportion of negative cultures, odds ratio (OR) 12.6 (95% confidence interval (CI) 2.84 to 55.84; low‐certainty evidence); however, by day 168 of follow‐up there was no difference in the proportion of participants who remained MRSA‐negative in either treatment arm, OR 1.17 (95% CI 0.31 to 4.42) (low‐quality evidence). In the second trial, successful eradication was defined as the absence of MRSA following treatment (oral co‐trimoxazole and rifampicin with intranasal mupirocin or observation) in at least three cultures over a period of six months. At the time of reporting, 40 out of 61 participants had completed follow‐up, but results showed no difference between groups. Eradication was achieved in 12 out 29 participants (41%) receiving active treatment, and in 9 out of 32 participants (28%) on the observation arm, OR 1.80 (95% CI 0.62 to 5.25) (very low‐quality evidence).
With regards to this review's secondary outcomes, these were reported in the first trial only. The trial reports that no differences were observed between the two arms in terms of pulmonary exacerbations (from screening to day 28), nasal colonisation, lung function, weight or participant‐reported outcomes. While not a specific outcome of this review, investigators reported that the rate of hospitalisation from screening through day 168 was lower with oral trimethoprim and sulfamethoxazole combined with rifampicin compared to control, rate ratio 0.22 (95% CI 0.05 to 0.72) (P = 0.0102).
Authors' conclusions
Early eradication of MRSA is possible in people with cystic fibrosis, with one trial demonstrating superiority of active MRSA treatment compared with observation only in terms of the proportion of MRSA‐negative respiratory cultures at day 28. However, by six months, the proportion of participants who remained MRSA‐negative did not differ between treatment arms in either trial. Moreover, the longer‐term clinical consequences in terms of lung function, mortality and cost of care, remain unclear.
Using GRADE methodology, we judged the quality of the evidence provided by this review to be very low to low, due to potential biases from the open‐label design and unclear detail reported in one trial. Based on the available evidence, it is the opinion of the authors that whilst early eradication of respiratory MRSA in people with cystic fibrosis is possible, there is not currently enough evidence regarding the clinical outcomes of eradication to support the use of the interventions studied.
Plain language summary
Interventions (treatments) to clear meticillin‐resistant Staphylococcus aureus (MRSA) from the lungs of people with cystic fibrosis
Review question
We looked for evidence for the effects of different ways of clearing meticillin‐resistant Staphylococcus aureus (MRSA) from the lungs of people with cystic fibrosis.
Background
MRSA is the name given to particular bacteria which are resistant to some types of antibiotics. This is particularly worrying for people with cystic fibrosis, which is an inherited condition which amongst other things causes thick mucus to build up in the lungs. It is very difficult for people with cystic fibrosis to cough up this thick mucus, making it an ideal breeding ground for bacteria, including MRSA, and making these people more prone to chest infections. It is thought that MRSA can cause more damage than other bacteria, which are not resistant to antibiotics. We wanted to identify research evidence to support the best way for treating MRSA infections and also to see if this would improve the lives of people with cystic fibrosis. This is an update of a previously published review.
Search date
The evidence is current to: 27 July 2017.
Key results
We found two trials which included people with cystic fibrosis and a diagnosed MRSA infection. In one trial the active treatment was oral trimethoprim and sulfamethoxazole combined with rifampicin and some additional decontamination treatment and in the second trial it was oral co‐trimoxazole and rifampicin with intranasal mupirocin; in both trials the comparison treatment was just observation and no active treatment. The results of these trials showed that clearing MRSA from the airways of people with CF is possible. Although a larger proportion of those who were treated became clear of MRSA in both trials, some of the individuals who were untreated also cleared MRSA spontaneously. Also, six months after treatment, the number of individuals who still had MRSA was not different between those who received treatment and those who did not.
In one of the trials, fewer people who were treated with antibiotics were admitted to hospital in the first 168 days. There were no other differences seen between the two groups (treated or untreated) in terms of their lung function, weight or chest exacerbations at six months.
Treating MRSA early in people with CF has been shown to be possible, but it is not clear what longer‐term implications this will have.
Quality of the evidence
Using GRADE methodology, we judged the quality of the evidence we found to be very low to low for the different outcomes. This was due to potential issues from the trial design where people knew which treatment each of the participants were receiving (groups were either given medication or just observed) and because there were small numbers of people included in each trial. Also, one of the trials did not report all details clearly.
Summary of findings
for the main comparison.
| Active treatment compared with observation only for eradicating MRSA in people with cystic fibrosis | ||||||
|
Patient or population: adults or children with positive microbiological isolate of MRSA from a respiratory tract specimen Settings: outpatient and inpatient Intervention: any combinations of topical, inhaled, oral or intravenous antimicrobials with the primary aim of eradicating MRSA Comparison: placebo, standard treatment or no treatment. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation only | Active treatment | |||||
|
Eradication of MRSA (defined by negative respiratory culture at day 28 for MRSA) |
263 per 1000. | 818 per 1000 (from 504 to 952). | OR 12.60 (95% CI 2.84 to 55.84) | 41 (1 study) | Low1, 2 | Trial stopped early based on DMC recommendations due to treatment efficacy at day 28, so did not reach planned recruitment target. However, by day 168 of follow‐up the proportion of participants who remained MRSA negative in either treatment arm was no longer significantly different. |
|
Eradication of MRSA (defined by ≥3 negative respiratory cultures for MRSA over 6 months) |
281 per 1000. | 413 per 1000 (from 195 to 673). | OR 1.80 (95% CI 0.62 to 5.25) | 61 (1 study) | Very low1, 2, 3 | |
|
Time until next positive MRSA isolate (from clinically relevant respiratory culture) |
Outcome not reported. | NA | NA | NA | ||
| Lung function | There were no differences between treatment arms in terms of FEV1 (absolute and relative change) or FEV1% predicted (absolute change) at day 28 or at day 168 of follow up. | 35 (1 study) | Low1, 2 | |||
| Frequency of exacerbations | 333 per 1000 | 120 per 1000 (from 27 to 430). | OR 0.29 (95% CI 0.06 to 1.30) | 45 (1 study) | Low1, 2 | |
| Growth and nutritional status (weight at day 168) | The mean change in weight from baseline was 1.97kg in the observation group. | The mean difference in weight (kg) was 0.19 lower (1.70 kg lower to 1.32 kg higher) in the active treatment group. | NA | 45 (1 study) | Low1, 2 | |
| Adverse effects of treatment | There were 3 instances of oral antibiotic discontinuation due to adverse events 'probably related' to the trial drug: 2 were temporary discontinuation of rifampin due to gastrointestinal complaints, whereas 1 participant had to discontinue all antibiotics due to urticaria. None of the adverse events was considered serious or required hospitalisation. |
NA | 45 (1 study) | Low1, 2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMC: data monitoring committee; MRSA: meticillin‐resistant Staphylococcus aureus; NA: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Downgraded by one level due to risk of performance bias; trial was open label and due to lack of blinding is at risk of performance and detection bias.
2. Downgraded by one level due to serious imprecision (small sample size and wide CIs).
3. Downgraded by one level due to unclear risk of selection, attrition, and reporting bias; data presented in abstract form only, so methodology was unclear.
Background
Description of the condition
Cystic fibrosis (CF) is the most common autosomal inherited condition in white populations, with a gene carrier rate of 1 in 25 and affecting around 1 in 2500 newborns in the UK (CF Trust UK 2016). It is a multisystem disorder resulting from a disruption in chloride transport at the cellular level leading to abnormal, dehydrated secretions within the lungs. This results in impaired mucociliary clearance leading to recurrent pulmonary infections, bronchiectasis and progressively deteriorating lung function, which is the main cause of the morbidity and mortality seen in CF.
Organism
The abbreviation MRSA stands for meticillin‐resistant Staphylococcus aureus (S aureus). Meticillin is an antibiotic that is no longer in clinical use, but MRSA is resistant to antibiotics within the same class. This includes flucloxacillin, which is prescribed both for prophylaxis and treatment of infection with S aureus in people with CF in the UK. Furthermore, MRSA is also resistant to other antibiotics in the beta lactam family such as cephalosporins (e.g. ceftazidime) and carbapenems (e.g. meropenem). Resistance is not due to production of beta lactamase enzymes, but rather to the production of altered penicillin‐binding proteins coded on the mecA gene.
Most MRSA infections in both the non‐CF and CF populations have been so‐called 'healthcare associated' (HA‐MRSA), which occur in those who have been hospitalised, had surgery, are on dialysis, or who have had invasive procedures. However, in recent years outbreaks of 'community‐acquired' MRSA (CA‐MRSA) have occurred in otherwise healthy people with no link to a healthcare facility (Chambers 2009). This distinction by patient location at time of infection is becoming increasingly difficult, given outbreaks of strains of CA‐MRSA in hospitals, and the spread of HA‐MRSA strains in the community through people with chronic illnesses.
It is possible to further classify MRSA according to the staphylococcal chromosome cassette mec (SCCmec) type, on which the mecA gene is located. Several distinct types have been described to date, of which HA‐MRSA is associated with types I to III. These SCCmec types also encode for resistance to other classes of antibiotics, thus making HA‐MRSA overall more resistant. So‐called CA‐MRSA carries SCCmec types IV and V. Although CA‐MRSA usually has the smaller type IV SCCmec type, which lacks some of the antibiotic resistance determinants possessed by types I to III, it is also more frequently associated with the production of the virulence factor Panton‐Valentine leucocidin (PVL), a cytotoxin which causes leucocyte destruction and tissue necrosis.
Although people with MRSA have been found to require a higher intensity of treatment when compared with their meticillin‐sensitive S aureus (MSSA) counterparts, this is further complicated by differences observed between different MRSA types (Muhlebach 2011). For instance, the emergence of PVL‐positive CA‐MRSA within the CF population has been described and one report suggests this to be associated with a more severe clinical course acutely compared with PVL‐negative CA‐ or HA‐MRSA strains (Elizur 2007). This has not been replicated in other reports.
Prevalence
The prevalence of MRSA varies throughout Europe. Though the occurrence of MRSA is stabilising, or even decreasing, in several European countries; the percentage of MRSA among all S aureus isolates remains above 25% in seven of the 29 reporting countries from the European Union or European Economic Area. In the UK 10% to 25% of isolates of S aureus are found to be MRSA compared to less than 1% in Sweden (ECDC 2016). In the USA, the proportion of healthcare‐associated S aureus infections found in intensive care units that are attributable to MRSA had increased from 2% in 1974 to 64% in 2004 (Klevens 2006). Whilst a 2016 progress document by the Centers for Disease Control and Prevention (CDC) reported a 13% decrease in MRSA bacteremia between 2011 and 2014 (CDC 2016); a separate review of the National Inpatient Sample showed that invasive MRSA‐related hospitalisations had remained largely unchanged between 2010 to 2014 (Klein 2014).
Amongst people with CF, the prevalence of chronic MSSA (defined as three or more recorded isolates) in the UK has increased from 7.3% in 2001 to 15.2% in 2009, with the latest data reporting a prevalence in adults of 20.1% (CF Trust 2016). The prevalence of MRSA (defined as any single isolate) remains largely unchanged at 2.5% in 2009 (CF Trust 2009) and 2.7% (in adults) in 2015 (CF Trust 2016).
The USA CF registry data from 2009 recorded any isolate of MSSA at 51.3% and any isolate of MRSA at 23.7%, with 65.8% of their CF population having positive cultures for either MSSA or MRSA (CF Foundation 2009). 2010 data showed a further increase in prevalence of MSSA at 67% and MRSA at 25.7% (CF Foundation 2010). The most recent report from 2015 has shown a decrease in MSSA and stabilisation in number of MRSA cases with prevalence of MSSA at 54% and MRSA at 26% (CF Foundation 2016).
In Australia, the 2009 CF registry reported a MSSA prevalence of 43% and MRSA prevalence of 4.2% as a proportion of tested patients via any culture method and including any single positive isolate (Cystic Fibrosis Australia 2011). The latest data from 2016 also revealed a decrease in prevalence of both MSSA (42%) and MRSA (2.6%) (Cystic Fibrosis Australia 2014).
Condition
As described above, one of the early key pathogens in CF‐lung disease is MSSA, but increasingly MRSA has been cultured from the lower respiratory tracts of people with CF. The role of MRSA in CF‐lung disease remains debated.
A large observational study looking at 1834 participants who had positive respiratory cultures for S aureus (MRSA or MSSA) found that presence of MRSA in respiratory cultures was associated with poorer lung function, more courses of antibiotics and longer hospital stays when compared with those colonised with MSSA (Ren 2007). However, the authors were unable to conclude whether their findings were due to cause or effect.
Two studies were published in 2008 addressing this point, but came to differing conclusions (Dasenbrook 2008; Sawicki 2008). Dasenbrook suggested that chronic, though not intermittent, detection of MRSA in respiratory tract cultures of people with CF (as defined by reports from the CF Foundation Registry) is associated with poorer survival and reduced lung function (Dasenbrook 2008; Dasenbrook 2010). By contrast, Sawicki concluded that although MRSA was a marker for more aggressive therapy and may reflect increased disease severity, MRSA detection was not associated with a significant decline in lung function (Sawicki 2008).
Although both were longitudinal studies, Sawicki analysed data from an observational study of people with CF in North America (Epidemiologic Study of Cystic Fibrosis (ESCF) (Morgan 1999)) using multivariate linear regression analysis to study the impact of MRSA on lung function (forced expiratory volume in one second (FEV1) per cent (%) predicted); whilst Dasenbrook used data from the CF Foundation Registry. One of the fundamental differences between the two studies is the inclusion criteria. Sawicki included patients for analysis who had only one positive culture for MRSA (23% of cohort) whilst Dasenbrook studied patients with three or more positive cultures, those with one or two MRSA cultures were excluded.
Despite these differences, both studies reported an increased rate of decline in FEV1 % predicted of around 0.5% in their 'before' and 'after' MRSA groups. It is possible that this did not reach statistical significance in the Sawicki paper secondary to the smaller cohort size (593 versus 1732). An increased rate of decline of 0.8% has more recently been reported by a group in Belgium who conducted a retrospective case‐control study based at a single centre (Vanderhelst 2012).
In terms of survival, Dasenbrook found that the detection of MRSA from the respiratory tract of people with CF was associated with an increased risk of death when compared with individuals in whom MRSA had never been detected, hazard ratio 1.27 (95% confidence interval (CI) 1.11 to 1.45) (Dasenbrook 2010). Perhaps of more clinical importance however, is that they also found that those who clear MRSA within one year have the same risk of death as those who never have a positive culture for MRSA. This emphasizes the importance and need for clear guidance on how we manage MRSA infection in CF.
Description of the intervention
Currently in the UK, children are prescribed prophylactic anti‐staphylococcal antibiotics (flucloxacillin) from diagnosis until three years of age with resultant fewer isolates of S aureus, though the clinical significance of this finding remains uncertain (Smyth 2017). However, the US Cystic Fibrosis Foundation recommend against the use of prophylaxis in anticipation that this may lead to an increase in colonisation of Pseudomonas aeruginosa (P aeruginosa) (Flume 2007).
Some authors suggest a pragmatic approach would be to treat every isolate of MRSA or MSSA with eradication therapy (Solis 2003). However, this approach, with its frequent use of antibiotics, would run the risk of increasing the incidence of multi‐resistant organisms that are less susceptible to treatment, whilst potentially adding to the already substantial treatment burden that people with CF face.
Certainly in the case of HA‐MRSA infections, there has been encouraging progress since the introduction of stringent MRSA screening and eradication measures in hospitals. The 2010 report by the CDC showed a 28% decline in invasive MRSA infections originating in hospitals between 2005 and 2008 in the USA (Kallen 2010). Whilst in the UK, the Department of Health target to reduce MRSA bloodstream infections by 50% from its peak levels in 2003 to 2004 was achieved by 2008 (Liebowitz 2009; Pearson 2009).
How the intervention might work
Treatment strategies designed to target MRSA when it is first isolated from the respiratory samples of people with CF may be successful at eradicating MRSA from subsequent respiratory cultures, and result in improved clinical outcomes in the long term.
Why it is important to do this review
Despite the increasing prevalence of MRSA, its clinical significance remains unclear and there remains no international consensus for its management. With the increasing prevalence of resistant strains of S aureus, it becomes more important for any therapeutic approaches with antibiotics to be justified with the most up‐to‐date evidence, especially in those with chronic medical conditions.
A previous Cochrane Review could not find sufficient evidence to support the use of any single or combination of therapies for eradicating nasal or extra‐nasal colonisation of MRSA over another in the general population (Loeb 2003). Most studies addressing MRSA colonisation have been done in either healthy carriers or people in chronic care facilities, but not in those with chronic lung disease as seen in CF. Such reports include a variety of interventions, often focusing on nasal and skin colonisation, thus such findings may not be directly applicable to CF. However, a retrospective review of MRSA eradication practice in a single large UK adult CF centre showed some promise (Doe 2010). They used varying eradication regimens based on sensitivity patterns and individual tolerability, including stringent patient segregation and topical decolonisation, to attempt MRSA eradication from sputum and skin of people with CF. Over a 10‐year period they reported an eradication rate of 81% (defined as three consecutive negative sputum and peripheral cultures over six months), though the clinical impact of what successful MRSA eradication meant for patients was not reported.
The 2008 UK CF Trust consensus statement document stated that in the absence of prospective randomised clinical trials looking at the effect on lung function which chronic carriage with MRSA confers, MRSA infection will lead to a reduction in antibiotic treatment options and a likelihood of a deterioration in lung function. It is therefore their recommendation that the eradication of MRSA should be attempted for positive cases (CF Trust 2008).
The rationale for this review is to determine the success of MRSA eradication for people with CF, and to question whether eradication confers improved clinical outcomes. This version of the review is an update of a previously published review (Lo 2013; Lo 2015).
Objectives
To evaluate the effectiveness of treatment regimens designed to eradicate MRSA and to determine whether the eradication of MRSA confers better clinical and microbiological outcomes for people with CF.
To ascertain whether attempts at eradicating MRSA can lead to increased acquisition of other resistant organisms (including P aeruginosa) or increased adverse effects from drugs, or both.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Children and adults diagnosed with CF clinically and by sweat or genetic testing with a confirmed positive microbiological isolate of MRSA on clinically relevant CF respiratory cultures (bronchoalveolar lavage (BAL), cough or oropharyngeal swab, spontaneous or induced sputum culture) specimen prior to enrolment into the trial.
We included all disease severities. We did not include participants with nasal carriage of MRSA alone in this review.
Types of interventions
Any combinations of topical, inhaled, oral or intravenous antimicrobials with the primary aim of eradicating MRSA once detected on clinically relevant CF respiratory cultures compared with placebo, standard treatment or no treatment.
Types of outcome measures
Primary outcomes
Eradication of MRSA (as defined by negative respiratory culture after completion of the eradication protocol)
Time until next positive MRSA isolate from clinically relevant respiratory culture
Secondary outcomes
-
Lung function
forced expiratory volume at one second (FEV1) % predicted
forced vital capacity (FVC) % predicted
other validated measures of lung function
Overall antibiotic use
Mortality
-
Quality of life measured using a validated tool
CF Questionnaire‐Revised version (CFQ‐R) (Quittner 2009)
CF Quality of Life Questionnaire (CFQoL) (Gee 2000)
-
Isolation of MRSA or other organisms with new antibiotic resistant phenotypes
P aeruginosa
other previously uncultured organism
small colony variants of S aureus
-
Growth and nutritional status
weight (kg)
height (cm)
body mass index (BMI) (kg/m²)
lean body mass (%)
fat body mass (%)
-
Adverse effects to treatment
mild (not requiring treatment)
moderate (requiring treatment or admission or cessation of treatment, or a combination of any of these)
severe (life‐threatening)
Elimination of carrier status (nasal or skin)
Frequency of exacerbations
Cost of care
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
We identified relevant studies from the Group's Cystic Fibrosis Trials Register using the terms: (staphylococcus aureus or mixed infections) AND (eradication OR unknown). The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group website.
Date of the latest search: 27 July 2017.
We also searched the following databases and trial registries:
MEDLINE Ovid (1946 to 07 August 2017);
PubMed (www.ncbi.nlm.nih.gov/pubmed/; 1946 to 07 August 2017);
US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov (www.clinicaltrials.gov; searched 07 August 2017);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch; searched 07 August 2017);
ISRCTN registry (www.isrctn.com/; searched 07 August 2017).
For details of our search strategies, please see Appendix 1.
Searching other resources
We also contacted primary authors and research institutions of ongoing identified trials for unpublished data.
Data collection and analysis
Selection of studies
Two authors (DL and AS) independently screened trials for inclusion in this review in accordance with methods described by Higgins in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Both authors independently examined the title and abstracts to exclude duplicate publications, case reports, review articles and unrelated articles. The two authors (DL and AS) independently examined the full‐text publications of the remaining trials to determine if they met the review's eligibility criteria. The authors planned to resolve any queries on the eligibility of trials by consulting with the third author (MM) for advice and reaching a consensus through discussion between all authors.
Data extraction and management
Two authors (DL and AS) extracted data using standardised data acquisition forms, upon which all authors had agreed. They resolved disagreements through discussion between all three authors. Where information was incomplete or unclear, the authors contacted the lead author of the paper where possible.
We planned to group outcome data into those measured at up to 14 days, up to one month, up to three months, up to six months and up to 12 months after MRSA therapy. For this update, in a post hoc change, as grouping of data was not appropriate, we have reported data at day 28, day 168 and six months as per the original included studies. In future searches, if trials report data at other time intervals, the authors plan to consider these for inclusion and highlight this in the review.
Assessment of risk of bias in included studies
The authors (DL and AS) assessed the risk of bias using methods described in theCochrane Handbook for Systematic Reviews for Interventions (Higgins 2011b). In particular each author examined the methods to determine the adequacy of randomisation and blinding, and also whether any participants lost to follow‐up were accounted for and justified. They sought to identify any selective reporting by comparing the full report to the protocol.
In addition, each author independently used the 'risk of bias' assessment tool available in section 8.5 of the Cochrane Handbook for Systematic Reviews for Interventions in order to judge each of the described seven domains as having low, high or unclear risk of bias (Higgins 2011b).
Measures of treatment effect
For dichotomous data (e.g. eradication achieved or not), the authors analysed the data on an intention‐to‐treat basis, irrespective of compliance or dropout secondary to adverse effects. They sought data based on each possible outcome event for each treatment arm and calculate the odds ratio (OR) and its 95% CI.
For continuous data, the authors reported the mean difference (MD) of effect of each variable along with its 95% CI. If two or more trials had reported the same outcome but using different scales, they planned to calculate the standardised mean difference (SMD) with its 95% CI.
If the data had allowed, the authors planned to extract ordinal and count data in all forms in which they are reported and planned to analyse these as per continuous data for common outcomes; for rare outcomes they would follow the advice in section 9.2.5 of theCochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If it had been reported, for time‐to‐event data (e.g. time to next exacerbation), they planned to calculate the hazard ratio (HR) at individual time points (at 14 days, then 1, 3, 6 and 12 months) along with its 95% CI.
Unit of analysis issues
Cross‐over trials were not eligible for inclusion within this review since the authors were reviewing how efficacious the initial attempt at eradication of MRSA was when compared with placebo, usual treatment or no treatment. Subsequently, they aimed to evaluate the time until the next positive MRSA culture and number of further courses of antibiotics required following each arm of therapy.
The authors did not plan to include cluster‐RCTs. When randomisation is performed according to participant groups, certain strains of MRSA (which may differ between communities) could potentially be over‐represented in either the treatment or placebo arm and hence bias the results.
Dealing with missing data
In cases where data relating to either the review's primary or secondary outcomes were missing, the authors contacted the primary investigator(s) for clarification.
Assessment of heterogeneity
In order to assess heterogeneity between outcomes the authors used the I² statistic and the Chi² test. As stated in theCochrane Handbook for Systematic Reviews of Interventions, the importance of the observed value of I² depends on (i) the magnitude and direction of effects and (ii) the strength of evidence for heterogeneity (e.g. P value for Chi²) (Deeks 2011). The authors planned to consider values of 0% to 40% to represent little to no heterogeneity, 30% to 60% moderate, 60% to 90% substantial and values of more than 90% as demonstrating considerable heterogeneity.
Assessment of reporting biases
The authors assessed for selective reporting of results by comparing (where available) the outcomes listed in the original protocol to those reported in the final paper. They also searched clinical trials registers for the included studies. They contacted the primary investigator(s) of included trials to determine whether they were aware of any relevant unpublished data. The authors aimed to identify publication bias with the construction of funnel plots; however insufficient trials were eligible for inclusion in this version of the review. The authors plan to undertake this analysis in future if they are able to include more trials.
Data synthesis
The authors planned to analyse extracted data using a fixed‐effect meta‐analysis, however as they found the heterogeneity between the two trials to be substantial (more than 60%), they performed a random‐effects meta‐analysis.
Subgroup analysis and investigation of heterogeneity
If the authors had identified a sufficient number of trials (more than 10) and also found substantial heterogeneity between trials, they would have investigated this with subgroup analysis of the following:
eradication therapy commenced at initial acquisition versus following chronic colonisation (three or more positive cultures over a 12‐month period);
duration of eradication therapy (up to and including 6 weeks, 7 to 12 weeks, over 12 weeks);
intravenous versus aerosolised versus oral administration of antibiotics;
efficacy of regimens which include methods for skin or nasal eradication, or both, versus those that do not.
Sensitivity analysis
Where outcome measures had been chosen which use arbitrary numerical endpoints (i.e. number of days, or percentage change), the authors planned to re‐evaluate the effect that alternative endpoints have on their findings where available data allows.
If the authors had included smaller studies (20 participants in each group or less) in the initial meta‐analyses, they would have aimed to repeat the analyses without these smaller studies to determine their effect.
Summary of findings table
In a post hoc change in line with current Cochrane guidance, in the 2018 update we added a summary of findings table for the comparison of active treatment versus observation only (control) for respiratory MRSA in people with CF (Table 1). We selected the following seven outcomes to report:
eradication of MRSA from respiratory culture (as defined by negative culture at day 28);
eradication of MRSA from respiratory culture (as defined by three negative cultures over six months);
time until next positive MRSA isolate;
lung function;
frequency of exacerbations;
growth and nutritional status;
adverse effects of treatment.
The authors determined the trial quality using the GRADE approach, and rated quality with regard to the risk of bias or trial limitations, directness, consistency of results, precision, publication bias and effect size (Schunemann 2011). The quality of evidence was downgraded by one level for trial limitations related to bias.
Results
Description of studies
Results of the search
A total of 52 trials were identified from the CFGD Group's CF Trials Register and 11 additional trials were identified from separate additional searches. Two ongoing studies were identified from the ongoing trials registers (www.clinicaltrials.gov; www.isrctn.org; www.who.int/ictrp/search/en/).
Two trials were deemed eligible for inclusion in this review (Muhlebach 2017; Neri 2016). One of these trials was identified as ongoing in a previous version of this review (Muhlebach 2017). A total of 61 trials were excluded.
One previously identified ongoing trial has since reported findings in abstract form, but has not currently reported data on MRSA eradication (Dasenbrook 2015). For this reason it is now listed as awaiting classification (Characteristics of studies awaiting classification). A further trial is ongoing, but no longer recruiting and currently performing data analysis (Dasenbrook 2012).
Please also see the PRISMA diagram (Figure 1).
1.
Study flow diagram.
Included studies
We identified two trials which were eligible for inclusion (Muhlebach 2017; Neri 2016). One trial has been fully completed and published (Muhlebach 2017); the second trial has been recently completed, but follow‐up data and full data analysis are awaited (Neri 2016). Prelimary data for this second trial have been published in abstract form and presented in this review.
Trial design
Both trials were multicentre, non‐blinded, open‐label RCTs. One trial involved six centres in Italy where participants were followed up for six months (Neri 2016). The second trial involved 14 centres in the USA where participants were followed up to 168 days (Muhlebach 2017).
Participant
Both trials included people with CF over four years of age with newly‐acquired MRSA from respiratory culture. One trial recruited 45 participants (44% female, mean age 11.5 years) (Muhlebach 2017). The second trial recruited 61 participants (57% female, mean age 19.1 years) (Neri 2016).
Interventions
Both trials compared active interventions to observation only. In both trials the active treatment comprised oral trimethoprim and sulfamethoxazole combined with rifampicin; however, one trial administered this combination for two weeks combined with nasal, skin and oral decontamination and a three‐week environmental decontamination (Muhlebach 2017), while the second trial administered this drug combination for 21 days, with five days intranasal mupirocin (Neri 2016). In both trials the control arm was observation only (Muhlebach 2017; Neri 2016).
Outcome measures
The primary outcome for both trials was MRSA eradication from the respiratory cultures of participants; however, the two trials used different definitions. One trial defined eradication as a MRSA‐negative respiratory culture at day 28 (Muhlebach 2017). Whilst the second trial defined successful eradication as the absence of MRSA in at least three respiratory cultures over a period of six months (Neri 2016).
One trial also evaluated the safety and tolerability of the treatment regimen, protocol adherence, duration of microbiological effect, number of pulmonary exacerbations, use of antibiotics, change in spirometry (measured by FEV1), respiratory symptoms as measured by the CF‐specific patient outcomes: Cystic Fibrosis Questionnaire Revised respiratory domain scores and Cystic Fibrosis Respiratory Symptom Diary Chronic Respiratory Infection Symptom Scale, and weight change over the six‐month study period (Muhlebach 2017).
The only other stated outcome measure from the abstract of the second trial is antibiotic susceptibility of MRSA strains at first isolation (Neri 2016).
Excluded studies
A total of 61 trials were excluded (Characteristics of excluded studies).
One trial was a tolerability study (Adeboyeku 2001). A total of 13 were pharmacokinetic trials (Coates 2011; Davis 1987; Geller 2004; Goldfarb 1986; Griffith 2008; Huls 2000; Keel 2011; Pai 2006; Rosenfeld 2006; Vitti 1975; Roberts 1993; Smith 1997; Stutman 1987). In 19 trials the interventions were not relevant to our review (Amelina 2000; Chua 1990; Degg 1996; Dodd 1997; Dodd 1998; Flume 2015; Frederiksen 2006; Gulliver 2003; Hodges 2014; Khorasani 2009; Labiris 2004; Loening ‐Bauke 1979; NCT03181932; Nolan 1982; Postnikov 2001a; Postnikov 2001b; Ramstrom 2000; Sharma 2016; Wood 1996), and 17 trials were excluded because the participants were not relevant to our review (Carswell 1987; Conway 1996; Cooper 1985; Flume 2016; Heininger 1993; Hjelte 1988; Huang 1979; Junge 2001; Kapranov 1995; Knight 1979; Nathanson 1985; Postnikov 2000; Romano 1991; Sahl 1992; Shapera 1981; Singh 2013; Van Devanter 2014). A further 11 trials had relevant participants, interventions and outcomes but were not randomised or controlled trials. Of these 11 trials, two were case reports, one of a 10‐year old boy (Maiz 1998) and one of a 28‐year old man (Serisier 2004), seven were observational studies (Dalbøge 2013; Garske 2004; Hall 2015; Kappler 2016; Macfarlane 2007; Vallieres 2016; Vanderhelst 2013) and two were retrospective studies (Bittencourt 2016; Solis 2003).
Studies awaiting classification
One trial has now completed recruitment and we are awaiting data analysis and publication of results (Dasenbrook 2015). Although the primary outcome is not MRSA eradication, the lead investigator has informed us that MRSA eradication will be reported as an outcome, and so this trial may be eligible for inclusion in future updates of this review. See Characteristics of studies awaiting classification.
Ongoing studies
The authors identified one ongoing trial, NCT01594827, from ClinicalTrials.gov (Dasenbrook 2012). The trial is currently listed as "ongoing, but not recruiting participants" and details were last updated in January 2018 (Dasenbrook 2012). The primary investigators confirmed to us that participant enrolment has been completed and results are being analysed. This trial may be eligible for inclusion into future versions of this review and further details of this trial can be found in the tables (Characteristics of ongoing studies).
The RCT is double‐blinded and based in the USA. Participants are people with CF and confirmed respiratory MRSA on culture who are aged 12 years and over. Active treatment consists of 28 days of inhaled vancomycin (250 mg twice daily) plus 28 days of oral rifampin and a second oral antibiotic (trimethoprim/sulfamethoxazole or doxycycline), mupirocin intranasal cream and chlorhexidine body washes versus comparator treatment of 28 days of inhaled sterile placebo (saline) plus the same oral and skin antibiotics as the active arm. The primary outcome measure of MRSA eradication and participants will be followed up for three months after completion of the treatment protocol.
Risk of bias in included studies
The design of the included trials are summarised in the tables (Characteristics of included studies) and a summary of risk of bias judgements of the included trials is illustrated in Figure 2.
2.
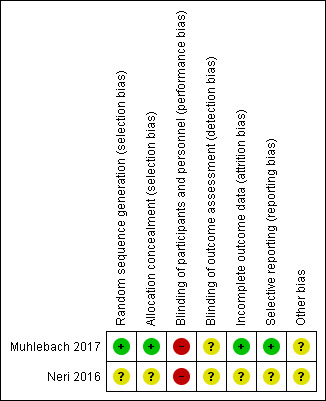
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Using GRADE and incorporating the risk of bias judgements, the quality of evidence for outcomes reported ranged from very low to low (Table 1).
Allocation
Randomisation
Both trials were described as randomised (Muhlebach 2017; Neri 2016). In the Muhlebach trial, assignments were generated via a centralised, secure web‐based system for each enrolled participant, so we judged there to be a low risk of bias (Muhlebach 2017). Since the Neri trial has only been presented as an abstract, there are no details regarding the method of randomisation available, we therefore judge the risk of bias to be unclear (Neri 2016).
Allocation concealment
One trial utilised a centralised, randomisation system for each enrolled participant, so it was not possible for the investigators to know the allocation sequence in advance (Muhlebach 2017). We judged the risk of bias for this trial to be low. There were no details on allocation concealment available for the second trial, so we judge the risk of bias to be unclear (Neri 2016).
Blinding
Neither the participants or trial personnel were blinded to the treatment regimen in either trial. In one trial this was due to part of the regimen involving enhanced house cleaning and so it would not have been possible to blind participants (Muhlebach 2017). Furthermore, blinding would have been difficult in both trials because rifampicin discolours urine and secretions that would be difficult to mimic with placebo. We therefore judged the risk of bias from blinding to be high in both trials (Muhlebach 2017; Neri 2016).
Incomplete outcome data
We judged one trial to have a low risk of bias due to incomplete outcome data where 41 of the 45 randomised participants were included in the intention‐to‐treat analysis. The remaining four randomised participants had missing MRSA culture results at day 28 and were all accounted for (two from the observation only group and two from the active treatment group) (Muhlebach 2017).
It is unclear from the second trial's abstract whether any participants withdrew once randomised, and so the risk of bias is judged to be unclear (Neri 2016).
Selective reporting
There was no evidence of selective reporting in the trial which is reported in a full publication, where both primary and secondary outcome measures were reported as described on the trials database (ClinicalTrials.gov); we therefore judge this trial to have a low risk of bias (Muhlebach 2017). Since the Neri trial has only been presented as an abstract there are no details regarding the planned outcomes available, we therefore judge the risk of bias to be unclear (Neri 2016).
Other potential sources of bias
We judged there to be an unclear risk for both trials. In one trial the power calculation required the randomisation of 90 participants; however, the data monitoring committee recommended stopping the trial, after 45 participants had been enrolled, on the grounds of clinical efficacy (Muhlebach 2017). The second trial is currently only reported in abstract form which does not provide sufficient information for us to make a clear assessment (Neri 2016).
Effects of interventions
See: Table 1
The quality of the evidence has been graded for those outcomes included in the summary of findings table. For the definitions of these gradings, please refer to the summary of findings tables (Table 1).
Primary outcomes
1. Eradication of MRSA
Both trials reported this outcome (n = 77) but used different definitions of eradication (Muhlebach 2017; Neri 2016).
One trial reported the number of participants who were MRSA negative at day 28, and the number who remained MRSA‐negative by day 168 (Muhlebach 2017). At day 28, 18 out of 22 (82%) participants on active treatment were MRSA‐negative compared to 5 out of 19 (26%) participants in the control group (P < 0.001), OR 12.60 (95% CI 2.84 to 55.84) (low‐quality evidence). However, by day 168, 12 out of 21 participants (57%) in the active treatment arm compared to 8 out of 15 (53%) participants in the control group remained MRSA‐negative, OR 1.17 (95% CI 0.31 to 4.42) (Analysis 1.1) (low‐quality evidence).
1.1. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 1 Eradication of MRSA (negative MRSA respiratory culture following treatment).
Neri reported on successful eradication, defined as three negative MRSA respiratory cultures over six months (Neri 2016). In the active treatment arm 12 out of 29 participants (41%) fulfilled this definition compared to 9 out of 32 (28%) participants in the control group, OR 1.80 (95% CI 0.62 to 5.25) (Analysis 1.2) (very low‐quality evidence). Thus, by six months the second trial had a higher proportion of participants with negative MRSA respiratory cultures in the active treatment arm, but this did not reach statistical significance (Neri 2016).
1.2. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 2 Eradication of MRSA (3 negative MRSA respiratory cultures over 6 months).
2. Time until next positive MRSA isolate from clinically relevant respiratory culture
This outcome was not reported by either trial (Muhlebach 2017; Neri 2016).
Secondary Outcomes
1. Lung function
Only one trial (n = 45) reported this outcome as FEV1 measured in L (both absolute and relative change from baseline) and % predicted (absolute change from baseline only) (Muhlebach 2017). No results were statistically significant, although mean values were consistently greater in the active treatment group compared to the observation group at both day 28 and day 168 (low‐quality evidence).
The absolute change from baseline in FEV1 (L) was greater at day 28, MD 0.11 L (95% CI ‐0.01 to 0.23), than at day 168, MD 0.06 L (95% CI ‐0.06 to 0.18) (Analysis 1.3). This was replicated in the data for the relative change from baseline in FEV1 (L) at day 28, MD 4.89% (95% CI ‐0.61 to 10.39) and at day 168, MD 3.08% (95% CI ‐2.21 to 8.37) (Analysis 1.4). Likewise, the absolute change from baseline in FEV1 % predicted was greater at day 28, MD 4.79% (95% CI ‐0.89 to 10.47), than at day 168, MD 4.68% (95% CI ‐0.29 to 9.65) (Analysis 1.5).
1.3. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 3 FEV1 (L) ‐ absolute change from baseline.
1.4. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 4 FEV1 (L) ‐ relative change from baseline.
1.5. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 5 FEV1 (% predicted) ‐ absolute change from baseline.
2. Overall antibiotic use
Only one trial (n = 45) reported this outcome (Muhlebach 2017). There was no significant difference between the rate of anti‐MRSA antibiotic usage between the two treatment arms; between day 28 to 168, nine (38%) participants in the treatment and nine (43%) in the control arm were treated with anti‐MRSA antibiotics, OR 0.80 (95% CI 0.24 to 2.64) (Analysis 1.6).
1.6. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 6 Overall anti‐MRSA antibiotic use.
The paper also reported that the use of non‐MRSA antibiotics (oral, inhaled or intravenous) was similar across groups throughout the trial (Muhlebach 2017).
3. Mortality
No deaths were reported during either trial (Muhlebach 2017; Neri 2016).
4. Quality of life
Only one trial (n = 45) reported this outcome (Muhlebach 2017). No significant differences in patient‐reported outcomes were found between treatment arms based on responses to the Cystic Fibrosis Respiratory Symptom Diary Chronic Respiratory Infection Symptom Scale (CFRSD‐CRISS) either at day 28, MD ‐6.72 (95% CI ‐14.36 to 0.92) or at day 168, MD 5.14 (95% CI ‐5.06 to 15.34) (Analysis 1.7). Similarly, there were no differences between groups for the Cystic Fibrosis Questionnaire Revised respiratory domain scores (CFQ‐R RSS) at day 28, MD ‐0.26 (95% CI ‐11.32 to 10.80) or at day 168, MD ‐3.94 (95% CI ‐13.96 to 6.08) (Analysis 1.8).
1.7. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 7 Patient outcomes (absolute change from baseline) measured using CFRSD‐CRISS.
1.8. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 8 Patient outcome (absolute change from baseline) measured using CFQ‐R RSS.
5. Isolation of MRSA or other organisms with new antibiotic resistant phenotypes
Only one trial (n = 45) reported this outcome (Muhlebach 2017). No emergent MRSA resistances to the antibiotics used or the appearance of small colony variants were identified in either treatment arm. In particular, investigators found no difference between treatment arms in the proportion of participants testing positive for P aeruginosa from screening through to day 168, OR 0.67 (95% CI 0.11 to 3.87) (Analysis 1.9).
1.9. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 9 Participants positive for Pseudomonas aeruginosa.
6. Growth and nutritional status
Only one trial (n = 45) reported weight for this outcome (Muhlebach 2017).
a. weight (kg)
At day 28, the difference in the change in weight from baseline between treatment arms was MD 0.07 kg (95% CI ‐0.77 to 0.91) and at day 168 it was MD ‐0.19 kg (95% CI ‐1.70 to 1.32) (Muhlebach 2017). Neither of these were statistically significant (Analysis 1.10) (low‐quality evidence).
1.10. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 10 Weight (kg) (absolute change from baseline).
7. Adverse effects to treatment
Only one trial (n = 45) reported adverse effects (Muhlebach 2017). The most frequently occurring adverse events in both treatment arms were gastrointestinal disorders; affecting 46% of participants randomised to active treatment and 24% to observation only, OR 1.93 (95% CI 0.80 to 4.64). None of the adverse events were considered serious or required hospitalisation. There were no statistical differences found between treatment arms for any of the reported adverse event types (all reported adverse events are presented in Analysis 1.11) (low‐quality evidence).
1.11. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 11 Adverse effects to treatment.
Two gastrointestinal complaints led to a temporary discontinuation of rifampin, whereas one participant had to discontinue all antibiotics due to urticaria. Two serious adverse events occurred during the first 28 days of the trial, one in the treatment arm (increased cough) and one in the control arm (cellulitis of the eyelid). Three instances of antibiotic discontinuation due to adverse events "probably" related to the trial drug were reported in this trial (Muhlebach 2017).
8. Elimination of carrier status (nasal)
Only one trial (n = 45) reported this outcome (Muhlebach 2017). At screening, 14 of 45 participants had nasal MRSA colonisation with similar distribution across groups: 6 out of 24 (25%) in the active treatment and 8 out of 21 (38%) in the control (P = 0.52) arms. No treatment‐related differences emerged during the course of the trial. No other data were available for analysis.
9. Frequency of exacerbations
Only one trial (n = 45) reported this outcome (Muhlebach 2017). Between screening and day 28, 13% of participants in the treatment arm experienced at least one pulmonary exacerbation compared to 33% in the observation arm (calculated as the proportion of participants experiencing an event per 28 days of follow‐up). This was not statistically significant, OR 0.29 (95% CI 0.06 to 1.30) (Analysis 1.12) (low‐quality evidence).
1.12. Analysis.
Comparison 1 Active treatment versus observation only, Outcome 12 Number of participants with pulmonary exacerbations.
Though not a stated outcome for this review, we feel it is important to present the data that Muhlebach reported for the rate of hospitalisation of participants from screening through day 168; this was significantly lower in the treatment arm compared to the observation arm, rate ratio 0.22 (95% CI 0.05 to 0.72) (P = 0.01) (Muhlebach 2017).
10. Cost of care
Although no health economic analysis was performed in either trial, it could be speculated that the lower rate of hospitalisations in the treatment arm of the Muhlebach trial would equate to a lower cost of care, however based on the evidence provided we are unable to comment further (Muhlebach 2017).
Discussion
Summary of main results
Although MRSA is an important emerging pathogen in CF respiratory illness, there is no widely accepted consensus for its optimal management. The broad search terms used in this review identified a large number of studies, unfortunately only two were eligible for inclusion at the time of this update (Muhlebach 2017; Neri 2016). Most of the other studies identified dealt either with reduction of MRSA bacterial density or were retrospective reports of MRSA treatment.
The results from the included trials demonstrated success in achieving MRSA eradication in people with CF with newly‐acquired MRSA on respiratory cultures; with one trial showing superiority of active treatment over control at day 28 (Muhlebach 2017). However, by six months, neither trial demonstrated a statistically significant difference in MRSA status between participants on either the active treatment or control arms of the trials in terms of MRSA respiratory status.
There were no differences observed between treatment arms in terms of lung function, exacerbation rates, participant‐reported outcomes, or weight. Although one trial reported fewer hospitalisations of participants who received active treatment when compared with controls over the trial period (Muhlebach 2017).
Overall completeness and applicability of evidence
Both trials included relevant participants with positive MRSA cultures obtained from clinically relevant samples; however, both excluded children younger than four years of age (Muhlebach 2017; Neri 2016); and one trial excluded adults over 45 years of age (Muhlebach 2017). Therefore, the generalisability of results to people outside of this age range cannot be assumed.
The aim of treating early MRSA infection in CF is to achieve both eradication and improvement in an individual's clinical outcomes. At present, only one trial has reported data to inform the review's secondary outcomes, which do not show superiority of active treatment compared with control (Muhlebach 2017). Of note, the Neri trial has only currently reported data in abstract form, and the full data analysis is still awaited to be included into the next version of this review (Neri 2016).
Quality of the evidence
One trial was judged to have an overall low risk of bias and the methodology of the trial is robust (Muhlebach 2017). The early termination of the trial, as recommended by the data monitoring committee, has meant that it did not achieve calculated sample size. The design and objectives were set out clearly with no evidence of selective reporting of results.
As data from the second included trial is currently only published in abstract form, it was not possible to make accurate comments regarding its methodology (Neri 2016). Having contacted one of the authors, we understand that they are currently analysing data and will publish the full report soon.
Based on GRADE criteria, the quality of evidence from both studies were downgraded by one point based on both trials being open‐label and so introducing performance bias, and a further point due to imprecision (small sample sizes and wide CIs) (Table 1). Results from one trial were further downgraded by one point due to unclear methodology resulting in unclear risk of selection, attrition, and reporting bias (Neri 2016).
Potential biases in the review process
One of the co‐authors of this review (MM) is the lead investigator in the included trial (Muhlebach 2017), however MM was not involved in data extraction or risk of bias assessment for her own trial. No other potential biases in the review process were identified.
Agreements and disagreements with other studies or reviews
Various strategies have been proposed for the eradication of MRSA when isolated from CF respiratory samples. It has become apparent from this review that these are based on anecdotal evidence or, at best, a small number of observational studies involving small numbers of participants as detailed below.
The authors identified 11 non‐randomised and non‐controlled studies. Four of these were in paediatric participants (age range 1 to 16 years), four in adults and three in mixed paediatric and adult groups. With the exception of a case report on one 10‐year old boy (Maiz 1998) and a cohort study which reports on efficacy of S aureus eradication, where only 0.3% of participants were MRSA‐positive (Dalbøge 2013), the remaining nine studies reported successful eradication of MRSA in at least a proportion of their participants (Bittencourt 2016; Garske 2004; Hall 2015; Kappler 2016; Macfarlane 2007; Serisier 2004; Solis 2003; Vallieres 2016; Vanderhelst 2013).
Whilst in the case report MRSA was not eradicated after the 17‐month treatment with daily continuous inhaled vancomycin, the authors did report improvements in lung function and symptom score in the child (Maiz 1998). One study (n = 11) reported that after successful eradication of MRSA, there was a non‐statistically significant trend in improvement of forced expiratory volume at one second (FEV1) % predicted (Vanderhelst 2013). The largest cohort study (n = 65) successfully eradicated S aureus from the sputum samples of participants and reported a statistically significant median (range) improvement in FEV1 % predicted of 3.3% (−25% to 36%; P < 0.0001); however, they did not differentiate between those individuals who grew MSSA or those who grew MRSA from their sputum (Dalbøge 2013). This finding is contradictory to three other studies, which reported no significant differences in lung function between participants where MRSA was successfully eradicated when compared to those in whom it failed (Garske 2004; Hall 2015; Solis 2003). However, this may be because the numbers were too small to detect a difference.
With regards to long‐term microbiological outcome, one study followed their cohort up for three years following initial eradication (dual intravenous antibiotic treatment over three weeks, accompanied by hygenic directives and topical therapy for five days followed by a six‐week period with dual oral antibiotic therapy and inhalation with vancomycin) (Kappler 2016). Long‐term success of eradication following a therapy per protocol was 84% (n = 31) but MRSA was still detectable in the third year of observation in six participants (16%).
The final four studies reported successful eradication of MRSA in 94% of participants (Macfarlane 2007), in 80% of participants (Bittencourt 2016), in 79% of participants (Vallieres 2016) and in one 28‐year old (Serisier 2004), but did not report on lung function or clinical status during or following eradication.
Authors' conclusions
Implications for practice.
We have included the only reported randomised control trials to date in this review (Muhlebach 2017; Neri 2016). One trial reports meticillin‐resistant Staphylococcus aureus (MRSA) eradication favouring the treatment arm compared to controls (observation only) at day 28 (Muhlebach 2017), but results from both trials show no significant difference between treatment arms by six months (Muhlebach 2017; Neri 2016). Fewer hospital admissions during follow‐up were seen in participants in the active treatment arm of one trial (Muhlebach 2017). The trial was unable to demonstrate significant differences in other clinically relevant outcomes, however it was not powered to do so. The currently available evidence does not demonstrate that routine treatment of respiratory MRSA in people with cystic fibrosis (CF) is effective.
Implications for research.
This review has highlighted the lack of evidence supporting the present management of MRSA respiratory infections in CF and emphasizes the need for well‐designed, adequately‐powered trials with long‐term follow‐up in order to address this issue.
Such trials will need to address these questions.
Does eradication of MRSA confer a favourable long‐term prognosis (see Types of outcome measures) for people with CF?
What is the optimal duration of treatment?
Should there be recurrent treatment cycles to avoid recurrence?
What is the most effective method of providing treatment (oral or intravenous or inhaled)?
Are there any pitfalls to treating MRSA aggressively (i.e. selection for other resistant pathogens, reduced tolerability, increased adverse effects)?
When should treatment be initiated?
The published reports of the two ongoing trials identified are keenly awaited and the authors look forward to assessing the published data of these for inclusion into a future update of this review.
What's new
| Date | Event | Description |
|---|---|---|
| 28 June 2018 | New citation required and conclusions have changed | The inclusion of two trials in a previously empty review has enabled us to revise our earlier conclusions. |
| 28 June 2018 | New search has been performed | A search of the Group's Cystic Fibrosis Register identified nine new references (Frederiksen 2006, Singh 2013, Sharma 2016, Khorasani 2009, Flume 2015, Hodges 2014; Muhlebach 2017; Neri 2016). Two trials were eligible for inclusion (Neri 2016; Muhlebach 2017). One article reports the data from a recently completed study identified in a previous version of this review (Muhlebach 2017). A search of PubMed and MEDLINE identified a further four trials, none of which were not eligible and are listed under 'Excluded studies' (Bittencourt 2016; Hall 2015; Kappler 2016; Vallieres 2016). A search of two ongoing trials registers (www.clinicaltrials.gov; www.isrctn.org) identified one new trial, which is not eligible for inclusion in the review (NCT03181932). Two of the ongoing trials identified in the previous version of this review are now listed as completed ‐ NCT01349192 (Muhlebach 2017) and NCT01746095 (Dasenbrook 2015). Data from one of these are included in this review (Muhlebach 2017), but currently the only published data identified from the Dasenbrook trial has been in abstract form only and does not provide sufficient information to assess eligibility for inclusion (Dasenbrook 2015). Therefore this trial is currently listed under "Studies awaiting classification". The third previously identified ongoing trial is still ongoing but no longer recruiting participants (Dasenbrook 2012). At this update a summary of findings table has been added to the review. |
History
Protocol first published: Issue 2, 2012 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 18 February 2015 | New citation required but conclusions have not changed | Given that no new data have been added to this review, our conclusions remain the same. |
| 18 February 2015 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register identified no new studies to be included in this review. A search of PUBMED, Embase and MEDLINE identified a further three studies, none of which were eligible for inclusion in the analysis (Dalbøge 2013; Serisier 2004; Vanderhelst 2013). A search of the ongoing trials registers (www.clinicaltrials.gov; www.isrctn.org) identified one further ongoing study, which has been listed in the review (Dasenbrook 2015a). |
Acknowledgements
The authors would like to thank Nikki Jahnke and Tracey Remmington for all their support with the development of this review. We would also like to thank Natalie Hall for her help with the literature searches.
Appendices
Appendix 1. Electronic searches
| Database/Resource | Strategy |
| Medline Ovid (1946 onwards) | 1 cystic fibrosis or CF 2 methicillin resistant staphylococcus aureus OR meticillin resistant staphylococcus aureus OR MRSA 3 1 OR 2 |
| PubMed (1946 onwards) |
[PubMed Advanced Search Builder] #1 (cystic fibrosis[Title]) OR CF[Title] #2 ((methicillin resistant staphylococcus aureus[Title]) OR meticillin resistant staphylococcus aureus[Title]) OR MRSA[Title] #3 1 AND 2 |
| Clinicaltrials.gov | Condition/Disease: cystic fibrosis Other terms: mrsa |
| WHO ICTRP | cystic fibrosis AND mrsa |
| ISRCTN registry | cystic fibrosis AND mrsa |
Data and analyses
Comparison 1. Active treatment versus observation only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication of MRSA (negative MRSA respiratory culture following treatment) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At day 28 | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.60 [2.84, 55.84] |
| 1.2 At day 168 | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.31, 4.42] |
| 2 Eradication of MRSA (3 negative MRSA respiratory cultures over 6 months) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 6 months | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.62, 5.25] |
| 3 FEV1 (L) ‐ absolute change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At day 28 | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.01, 0.23] |
| 3.2 At day 168 | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.06, 0.18] |
| 4 FEV1 (L) ‐ relative change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At day 28 | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 4.89 [‐0.61, 10.39] |
| 4.2 At day 168 | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 3.08 [‐2.21, 8.37] |
| 5 FEV1 (% predicted) ‐ absolute change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At day 28 | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 4.79 [‐0.89, 10.47] |
| 5.2 At day 168 | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 4.68 [‐0.29, 9.65] |
| 6 Overall anti‐MRSA antibiotic use | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 At day 28 | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.24, 2.64] |
| 7 Patient outcomes (absolute change from baseline) measured using CFRSD‐CRISS | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At day 28 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐6.72 [‐14.36, 0.92] |
| 7.2 At day 168 | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [‐5.06, 15.34] |
| 8 Patient outcome (absolute change from baseline) measured using CFQ‐R RSS | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 At day 28 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐11.32, 10.80] |
| 8.2 At day 168 | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐3.94 [‐13.96, 6.08] |
| 9 Participants positive for Pseudomonas aeruginosa | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 At screening | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.18, 3.92] |
| 9.2 At day 28 | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.11, 3.06] |
| 9.3 At day 84 | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.11, 3.02] |
| 9.4 At day 168 | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.87] |
| 10 Weight (kg) (absolute change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 At day 28 | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.77, 0.91] |
| 10.2 At day 168 | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.70, 1.32] |
| 11 Adverse effects to treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Gastrointestinal disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.79] |
| 11.2 Skin and subcutaneous tissue disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.26 [0.56, 49.29] |
| 11.3 Injury, poisoning and procedural complications | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.34, 143.85] |
| 11.4 Nervous system disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.78 [0.22, 105.36] |
| 11.5 General disorders and administration site conditions | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.27, 29.80] |
| 11.6 Renal and urinary disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.11, 71.04] |
| 11.7 Musculoskeletal and connective tissue disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.11, 71.04] |
| 11.8 Immune system disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.11, 71.04] |
| 11.9 Eye disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.15, 21.62] |
| 11.10 Ear and labyrinth disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.15, 21.62] |
| 11.11 Infections and infestations | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.20, 9.02] |
| 11.12 Psychiatric disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.05, 14.82] |
| 11.13 Blood and lymphatic system disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.05, 14.82] |
| 11.14 Metabolism and nutrition disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.11, 6.73] |
| 11.15 Investigations | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.15, 4.79] |
| 11.16 Congenital, familial and genetic disorders | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.22] |
| 12 Number of participants with pulmonary exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 At day 28 | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Muhlebach 2017.
| Methods | RCT (open‐label). Design: parallel. Location: multicentre (14 centres) in USA. Duration: 14 days treatment, follow up to 6 months. |
|
| Participants | People with first or early (≤ 2 positive cultures within 3 years) MRSA‐positive culture without MRSA active antibiotics within 4 weeks. Between 1 April 2011 to September 2014, 45 participants were randomised 1:1 to treatment or control (24 in the treatment group, 21 in the control group). Age (mean): 11.5 years (6.1) (ages 4 – 45 years were eligible for inclusion). Gender: 44% female. No significant differences in lung function, weight or Pseudomonas aeruginosa status between treatment arms |
|
| Interventions |
Eradication protocol: 14‐day oral rifampicin plus TMP‐SMX or minocycline in people with contraindications to TMP‐SMX; chlorhexidine mouthwash for 2 weeks; nasal mupirocin and chlorhexidine body wipes for 5 days and, in addition, environmental decontamination (wipe down high‐touch surfaces and medical equipment with surface disinfecting wipes daily for the first 21 days. Wash all linens and towels in hot water 1x weekly for 3 weeks). Drug: rifampin (adult dose: 300 mg 2x daily for 14 days; paediatric dose: <40 kg: 15 mg/kg daily for 14 days divided every 12 hours). Drug: TMP‐SMX (adult dose: 320/1600 orally 2x daily for 14 days; paediatric dose: < 40 kg: 8 mg/kg trimethoprim, > 40 mg/kg sulfamethoxazole twice daily for 14 days). Drug: minocycline (only for participants ≥ 8 years of age, who can not tolerate TMP‐SMX or whose screening MRSA is resistant to TMP/SMX. Adult dose: 100 mg orally 2x daily for 14 days. Paediatric dose: < 50 kg: 2 mg/kg orally twice daily for 14 days not to exceed 200 mg/day). Drug: mupirocin (1 g 2% nasal ointment generously applied to each nostril using a cotton swab twice daily for 14 days). Drug: chlorhexidine gluconate oral rinse (0.12% chlorhexidine gluconate oral rinse twice daily for 14 days). Drug: 2% chlorhexidine solution wipes (whole body wash solution wipes once daily for the first 5 days). Control group: observation with current standard of care, i.e. treatment for MRSA only with pulmonary exacerbations. |
|
| Outcomes |
Primary outcome measure 1. Proportion of participants in each arm with MRSA‐negative respiratory cultures at day 28. Secondary outcome measures 1. Safety and tolerability of treatment regime 2. Protocol adherence 3. Duration of microbiological effect 4. Number of pulmonary exacerbations 5. Use of antibiotics 6. Change in spirometry (FEV1) 7. Respiratory symptoms as measured by the CF‐specific patient outcomes: Cystic Fibrosis Questionnaire Revised respiratory domain scores and Cystic Fibrosis Respiratory Symptom Diary Chronic Respiratory Infection Symptom Scale 8. Weight |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised (1:1) to an MRSA eradication protocol...or to no treatment" using a secure web‐based randomisation system. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation assignments were generated via a centralised, secure web based randomisation system for each enrolled subject" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Study personnel and participants were not blinded to the treatment regimen". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinical evaluations, physical examination and spirometry were performed on day 1 (randomisation), day 15, day 28, day 84 and day 168; but not details of blinding of outcome assessors given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47 participants were randomised. 2 withdrew immediately post randomisation. Of the remaining 45 participants, 4 had "missing" MRSA culture results at day 28 (2 from each arm) and so not included in ITT‐E analysis. All missing participants accounted for. |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified. Reported outcomes matched stated outcomes on clinical trials registry. |
| Other bias | Unclear risk | Trial stopped early based on recommendations of data monitoring committee due to treatment efficacy, so did not reach planned recruitment target. |
Neri 2016.
| Methods | RCT (open‐label). Design: parallel. Location: multicentre (6 centres) in Italy. Duration: 21 days treatment with follow up to 6 months. |
|
| Participants | People with CF over 4 years of age with first or new MRSA infection, not infected by B cepacia complex and with no clinical signs of respiratory exacerbation and who could demonstrate regular visits to the centre. From 2013, 61 participants randomised: 29 to treatment arm and 32 to control arm. Mean age 19.1 (12.9) years. |
|
| Interventions |
Eradication protocol: an association of antibiotics according to susceptibility pattern (oral co‐trimoxazole and rifampicin for 21 days associated with intranasal mupirocin for 5 days). Control group: observation only. |
|
| Outcomes | Successful eradication or failure, where successful eradication is defined as the absence of MRSA following antibiotic treatment in at least 3 antibiograms over a period of 6 months. | |
| Notes | Awaiting data analysis and full publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly" assigned to active treatment arm or observation only arm. However, the procedure for randomisation is unclear from the abstract. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed in abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear from abstract data. |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not published. |
| Other bias | Unclear risk | Unclear from abstract data |
B cepacia: Burkholderia cepacia CF: cystic fibrosis IV: intravenous MRSA: meticillin‐resistant Staphyloccocus aureus RCT: randomised controlled trial TMP‐SMX: trimethoprim‐sulfamethoxazole
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adeboyeku 2001 | Not a relevant intervention ‐ tolerability study of differing dosages of nebulised colistin. |
| Amelina 2000 | Not a relevant intervention ‐ difference in quality of life between home versus hospital IV treatment. |
| Bittencourt 2016 | Not randomised. Retrospective cohort study. |
| Carswell 1987 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Chua 1990 | Not a relevant intervention ‐ used differing tonicities of inhaled antibiotics to assess airway responsiveness. |
| Coates 2011 | Pharmocokinetic study. |
| Conway 1996 | Not relevant participants ‐ did not differentiate between organisms causing exacerbation leading to inclusion into the trial. |
| Cooper 1985 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Dalbøge 2013 | An observational study. Not randomised. |
| Davis 1987 | Pharmocokinetic study. |
| Degg 1996 | Not a relevant intervention ‐ study on long‐term effects of gentamicin on hearing. Participants not selected on basis of microbial colonisation. |
| Dodd 1997 | Not a relevant intervention ‐ testing differences in lung function relating to tonicity of nebulised colistin. |
| Dodd 1998 | Not a relevant intervention ‐ a compliance study. No suitable control. |
| Flume 2015 | Not a relevant intervention ‐ safety evaluation of levofloxacin inhalation solution |
| Flume 2016 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Frederiksen 2006 | Not a relevant intervention ‐ not an eradication study |
| Garske 2004 | An observational study. |
| Geller 2004 | Pharmocokinetic study. |
| Goldfarb 1986 | Pharmocokinetic study. |
| Griffith 2008 | Pharmocokinetic/tolerability study. |
| Gulliver 2003 | Not a relevant intervention ‐ testing whether nebulised IV tobramycin solution induced cough or bronchoconstriction or both. |
| Hall 2015 | Observational study. No randomisation |
| Heininger 1993 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Hjelte 1988 | Not relevant participants ‐ investigated affect of home IV antibiotics for P. aeruginosa on quality of life. |
| Hodges 2014 | Not a relevant intervention |
| Huang 1979 | Not relevant participants ‐ did not differentiate between organisms causing exacerbation leading to inclusion into trial. |
| Huls 2000 | Pharmocokinetic study. |
| Junge 2001 | Not relevant participants ‐ investigating risk of ototoxicity or cochlea damage in once daily versus 3‐times daily IV tobramycin. |
| Kappler 2016 | An observational study. Not randomised. |
| Kapranov 1995 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Keel 2011 | Pharmocokinetic study. |
| Khorasani 2009 | Not a relevant intervention ‐ primary objective was not to eradicate MRSA |
| Knight 1979 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Labiris 2004 | Not a relevant intervention ‐ objective was to determine whether preservative containing inhaled tobramycin causes airway inflammation. |
| Loening ‐Bauke 1979 | Not a relevant intervention ‐ used cephalexin as prophylaxis. |
| Macfarlane 2007 | An observational study. |
| Maiz 1998 | A case report of one 10‐year old boy. |
| Nathanson 1985 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| NCT03181932 | Not relevant intervention ‐ primary outcome is change in lung function |
| Nolan 1982 | Not a relevant intervention ‐ prophylaxis rather than eradication. |
| Pai 2006 | Pharmocokinetic study. |
| Postnikov 2000 | Not relevant participants ‐ compared children with CF and aplastic anaemia |
| Postnikov 2001a | Not a relevant intervention ‐ describes risk of quinolone arthropathy in children. |
| Postnikov 2001b | Not a relevant intervention ‐ investigated the effect on growth with the addition of ciprofloxacin to the treatment of children with CF. |
| Ramstrom 2000 | Not a relevant intervention ‐ compared quality of life scores in patients who received pre‐made infusion devices compared to those who reconstituted drugs themselves. |
| Roberts 1993 | Pharmocokinetic study. |
| Romano 1991 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Rosenfeld 2006 | Pharmocokinetic study. |
| Sahl 1992 | Not relevant participants ‐ MRSA not required for entry into study. |
| Serisier 2004 | A case report of one 28‐year old man. |
| Shapera 1981 | Not relevant participants ‐ did not differentiate between MRSA and MSSA in inclusion criteria. Unclear how randomisation was achieved. |
| Sharma 2016 | Not a relevant intervention |
| Singh 2013 | Not relevant participants ‐ study of efficacy of interventions for pre‐pseudomonal pathogens |
| Smith 1997 | Pharmocokinetic study. |
| Solis 2003 | Retrospective study. |
| Stutman 1987 | Pharmacokinetic study of P. aeruginosa treatment. |
| Vallieres 2016 | An observational study. Not randomised. |
| Van Devanter 2014 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Vanderhelst 2013 | An observational study. Not randomised. |
| Vitti 1975 | Pharmocokinetic study. |
| Wood 1996 | Not a relevant intervention ‐ compared aminoglycoside toxicity in twice and 3‐times daily dosing regimens. |
CF: cystic fibrosis IV: intravenous MRSA: meticillin‐resistant Staphylococcus aureus MSSA: meticillin‐sensitive Staphylococcus aureus P. aeruginosa: Pseudomonas aeruginosa
Characteristics of studies awaiting assessment [ordered by study ID]
Dasenbrook 2015.
| Methods | RCT (Phase‐2, double‐blind). Design: parallel. Location: USA. Duration: treatment 28 days, follow up 56 days. |
| Participants | 40 adults and children (≥12 years of age) with CF and sputum culture positive for MRSA at screening, with at least 10,000 CFUs/mL of MRSA. |
| Interventions |
Cohort 1 treatment group: vancomycin inhalation powder (AeroVanc™) 32 mg 2x daily. Cohort 1 control group: placebo inhalation powder 2x daily. Cohort 2: prior to starting enrolment in Cohort 2, a safety evaluation will be carried out by the DMC based on treatment data from the first 20 participants in Cohort 1. Subject to the sponsor's written communication of the DMC's opinion of acceptable safety, the dose for the active arm in Cohort 2 will be escalated to 64 mg 2x daily. Optionally, the active arm for Cohort 2 may also be kept the same (32 mg 2x daily), or reduced to 16 mg 2x daily, depending on the outcome of the DMC's safety evaluation. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Completed. Full data not yet published. |
CF: cystic fibrosis CF‐RSD: cystic fibrosis respiratory symptom diary CFU: colony forming units CRP: C‐reactive protein DMC: data monitoring committee IV: intravenous MRSA: meticillin‐resistant Staphyloccocus aureus RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Dasenbrook 2012.
| Trial name or title | Persistent methicillin‐resistant Staphylococcus aureus eradication protocol (PMEP). |
| Methods | RCT ‐ participants will be assigned in a 1:1 ratio to either treatment or control group. Design: parallel. Location: dual centre in the USA. Duration: 28 days with additional 3‐month follow‐up. |
| Participants | 40 participants with persistent respiratory tract MRSA infection will be enrolled in this trial. Inclusion criteria:
|
| Interventions |
Treatment group: 28‐day course of vancomycin for inhalation (250 mg 2x daily) plus oral rifampicin and oral TMP‐SMX. Control group: taste‐matched inhaled placebo (sterile water) plus oral rifampicin and oral TMP‐SMX. In addition, both groups will receive oral rifampin, a second oral antibiotic (TMP‐SMX or doxycycline, protocol determined), mupirocin intranasal cream and chlorhexidine body washes |
| Outcomes |
Primary outcomes
Secondary objectives
|
| Starting date | Oct 2012. |
| Contact information | Michael Boyle, Associate Professor of Medicine, Johns Hopkins University. |
| Notes | Currently actively recruiting. Estimated completion date: March 2015. |
CF: cystic fibrosis CFQ‐R: Cystic Fibrosis Questionnaire‐Revised CFTR: cystic fibrosis transmembrane conductance regulator CFU: colony forming unit FEV1: forced expiratory volume at one second MRSA: meticillin‐resistant Staphylococcus aureus NPD: nasal potential difference P aeruginosa : Pseudomonas aeruginosa RCT: randomised controlled trial TMP‐SMX: trimethoprim‐sulfamethoxazole
Differences between protocol and review
In the 2015 update we have changed the spelling of 'methicillin' to 'meticillin' in line with the change in the international non‐proprietary name (although we are aware that in some parts of the world the drug is still known as methicillin).
In the 2018 update, we reported the rate of hospitalisations of participants (from screening through to end of trial) under the existing outcome of 'Frequency of exacerbations'. Even though the rate of hospitalisation was not a stated outcome within the original protocol, we felt that hospital admissions represent a significant morbidity and important health outcome for people with CF.
In the 2018 update, as grouping of MRSA eradication data was not appropriate, we have reported data at day 28, day 168 and six months as per the original included studies.
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO WILL UNDERTAKE THE TASK? |
| Protocol stage: draft the protocol | David Lo |
| Review stage: select which trials to include (2 + 1 arbiter) | David Lo, Marianne Muhlebach, Alan Smyth |
| Review stage: extract data from trials (2 people) | David Lo, Alan Smyth |
| Review stage: enter data into RevMan | David Lo |
| Review stage: carry out the analysis | David Lo |
| Review stage: interpret the analysis | David Lo, Marianne Muhlebach, Alan Smyth |
| Review stage: draft the final review | David Lo |
| Update stage: update the review | David Lo |
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
David Lo: none known.
Marianne Muhlebach is one of the principle investigators for a randomised controlled trial evaluating early treatment of MRSA (Muhlebach 2017).
Alan Smyth is the Co‐ordinating Editor of the Cochrane Cystic Fibrosis and Genetic Disorders Group and declares relevant activities of: membership of a REMPEX steering committee; consultancies for Novartis, Biocontrol and Rempex Pharma (both make aerosolised antibiotics which are active against some strains of Staphylococcus aureus); and also a lecture paid for by Chiesi Pharma.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Muhlebach 2017 {published data only}
- Goss CH, Thompson V, Popowitch E, Howe DL, Baines A, Mayer‐Hamblett N, et al. Efficacy of a protocol for eradication of newly acquired MRSA: results of the STAR‐too trial. Journal of Cystic Fibrosis 2015;14 Suppl 1:S3. [Abstract no: WS02.1; CFGD Register: PI286a] [Google Scholar]
- Muhlebach MS, Beckett V, Popowitch E, Miller MB, Baines A, Mayer‐Hamblett N, et al. Microbiological efficacy of early MRSA treatment in cystic fibrosis in a randomised controlled trial. Thorax 2017;72(4):318‐26. [CENTRAL: 1292548; CFGD Register: PI286b; CRS: 5500135000001800; EMBASE: 613492003; PUBMED: 27852955] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlebach MS, Beckett V, Popowitch E, Miller MB, Baines A, Mayer‐Hamblett N, et al. Microbiological efficacy of early MRSA treatment in cystic fibrosis in a randomised controlled trial. Thorax 2017;72(4):318‐26. Online supplementary data. [CENTRAL: 1383326; CFGD Register: PI286c; CRS: 5500135000001803] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlebach MS, Thompson V, Popowitch E, Miller MB, Howe D, Baines A, et al. MRSA types in new onset infection‐results from Star‐Too trial. Pediatric Pulmonology 2015;50 Suppl 41:327. [Abstract no: 360; CENTRAL: 1198456; CFGD Register: PI286d ; CRS: 5500135000002069; EMBASE: 72081639] [Google Scholar]
- NCT01349192. Early methicillin‐resistant Staphylococcus aureus (MRSA) therapy in cystic fibrosis (CF) (STAR‐Too) [Early MRSA therapy in CF ‐ culture based vs. observant therapy (treat or observe) (Star‐TOO ‐ STaph Aureus Resistance ‐ Treat or Observe)]. http://clinicaltrials.gov/ct2/show/NCT01349192 06 May 2011.
Neri 2016 {published data only}
- Neri S, Campana S, Dolce D, Ravenni N, Braggion C, Galici V, et al. Early antibiotic treatment for MRSA eradication in cystic fibrosis patients: a multicenter RCT. Pediatric Pulmonology 2016;51 Suppl 45:309. [Abstract no: 309; CENTRAL: 1212553; CFGD Register: PI291; CRS: 5500135000002066; EMBASE: 612359126] [Google Scholar]
References to studies excluded from this review
Adeboyeku 2001 {published data only}
- Adeboyeku DU, Agent P, Jackson V, Hodson M. A double blind randomised study to compare the safety and tolerance of differing concentrations of nebulised colistin administered using HaloLite in cystic fibrosis (CF) patients. Pediatric Pulmonology 2001;32(S22):288. [Google Scholar]
Amelina 2000 {published data only}
- Amelina E, Senkevich N, Cherniak A, Cherniaev A, Chuchalin A. Home intravenous therapy in adult cystic fibrosis patients. The impact on lung function and quality of life. European Respiratory Journal 2000;16 Suppl 31:123s. [Google Scholar]
Bittencourt 2016 {published data only}
- Bittencourt PH, Pimentel CSS, Bonfim BS, Godoy Almeida C, Marostica PJC, Souza ELS. Incidence and treatment of methicillin‐resistant Staphylococcus aureus infection in cystic fibrosis patients: a cohort study. Pediatric Pulmonology 2016;51 Suppl 42:S40. [Abstract no: 115] [Google Scholar]
Carswell 1987 {published data only}
- Carswell F, Ward C, Cook DA, Speller DC. A controlled trial of nebulized aminoglycoside and oral flucloxacillin versus placebo in the outpatient management of children with cystic fibrosis. British Journal of Diseases of the Chest 1987;81(4):356‐60. [DOI] [PubMed] [Google Scholar]
Chua 1990 {published data only}
- Chua H, Collis G, Souef P. Bronchial response of children with cystic fibrosis to nebulised antibiotics. Australian and New Zealand Journal of Medicine 1990;20:537. [CFGD Register: PI66b] [Google Scholar]
- Chua HL, Collis GG, Souef PN. Bronchial response to nebulized antibiotics in children with cystic fibrosis. European Respiratory Journal 1990;3(10):1114‐6. [CFGD Register: PI66a] [PubMed] [Google Scholar]
Coates 2011 {published data only}
- Coates AL, Denk O, Leung K, Ribeiro N, Chan J, Green M, et al. Higher tobramycin concentration and vibrating mesh technology can shorten antibiotic treatment time in cystic fibrosis. Pediatric Pulmonology 2011;46(4):401‐8. [CFGD CF Register: PI241b] [DOI] [PubMed] [Google Scholar]
- Denk O, Coates AL, Keller M, Leung K, Green M, Chan J, et al. Lung delivery of a new tobramycin nebuliser solution (150mg/1.5ml) by an investigational eFlow® nebuliser is equivalent to TOBI® but in a fraction of time. Journal of Cystic Fibrosis 2009;8 Suppl 2:S66. [Abstract no: 264; CFGD Register: PI241c] [Google Scholar]
- Keller M, Coates AL, Griese M, Denk O, Schierholz J, Knoch M. In‐vivo data support equivalent therapeutic efficacy of a new tobramycin inhalation solution (150mg/1.5ml) administered by the eFlow® electronic nebuliser compared to TOBI® in the PARI LC PLUS®. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [CFGD Register: PI241a] [Google Scholar]
Conway 1996 {published data only}
- Conway SP. Ceftazidime 3G BD is as effective as ceftazidime 2G TDS in the treatment of respiratory exacerbations in cystic fibrosis. Israel Journal of Medical Sciences 1996;32 Suppl:S256. [CFGD Register: PI78] [Google Scholar]
Cooper 1985 {published data only}
- Cooper DM, Harris M, Mitchell I. Comparison of intravenous and inhalation antibiotic therapy in acute pulmonary deterioration in cystic fibrosis. American Review of Respiratory Disease 1985;131:A242. [CFGD Register: PI129] [Google Scholar]
Dalbøge 2013 {published data only}
- Dalbøge C, Pressler T, Høiby N, Nielsen K, Johansen H. A cohort study of the Copenhagen CF Centre eradication strategy against Staphylococcus aureus in patients with CF. Journal of Cystic Fibrosis 2013;12(1):42‐8. [DOI] [PubMed] [Google Scholar]
Davis 1987 {published data only}
- Davis RL, Koup JR, Williams‐Warren J, Weber A, Heggen J, Stempel D, et al. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrobial Agents and Chemotherapy 1987;31(6):915‐9. [CFGD Register: PI50] [DOI] [PMC free article] [PubMed] [Google Scholar]
Degg 1996 {published data only}
- Degg C, Mulheran M. The effect of frequent exposure to gentamicin on distortion product OAEs in patients with cystic fibrosis. British Journal of Audiology 1996;30(2):99‐100. [CFGD Register: PI167] [Google Scholar]
Dodd 1997 {published data only}
- Dodd M, Maddison J, Abbott J, Webb AK. The effect of the tonicity of nebulised colistin on lung function in adults with cystic fibrosis. 18th European Cystic Fibrosis Conference; 1993 May 21‐26; Madrid, Spain. 1993:121. [CFGD Register: PI100a]
- Dodd ME, Maddison J, Abbot J, Moorcroft AJ, Webb AK. Effect of tonicity of nebulised colistin on chest tightness and pulmonary function in adults with cystic fibrosis. Thorax 1997;52(7):656‐8. [CFGD Register: PI100b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd ME, Maddison J, Abbot J, Webb AR. The effect of the tonicity of nebulised colistin on chest tightness and lung function in adults with cystic fibrosis. European Respiratory Journal 1993;6 Suppl 17:515s. [CFGD Register: PI100c] [Google Scholar]
Dodd 1998 {published data only}
- Dodd ME, Haworth CS, Moorcroft AJ, Miles J, Webb AK. Is medicine evidence‐based when there is discrepancy between patient reported and objective measures of compliance in clinical trials?. Pediatric Pulmonology 1998;26 Suppl 17:389‐90. [CFGD Register: PI237] [Google Scholar]
Flume 2015 {published data only}
- Conrad D, Flume P, Sindel L, Andrews S, Morgan L, Loutit J, et al. Phase 2b study of inhaled MP‐376 (Aeroquin, levofloxacin inhalation solution) in stable cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa (PA) lung infection. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A2239. [CENTRAL: CN‐01031676; CFGD Register: PI240g; CRS: 1762281; EMBASE: 70839804] [Google Scholar]
- Flume P, Geller DE, Sindel L, Staab D, Fischer R, Riethmuller J, et al. Effects of inhaled MP‐376 (Aeroquin, levofloxacin inhalation solution) on lung function in stable cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa (PA) lung infection. Journal of Cystic Fibrosis 2010;9 Suppl 1:S23. [Abstract no.: 86; CENTRAL: CN‐00774683; CFGD Register: PI240a; CRS: 1572247] [Google Scholar]
- Flume P, VanDevanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis 2015; Vol. 14 Suppl 1:S87. [Abstract no: 117; CENTRAL: CN‐01077213; CFGD Register: PI240f // PI283c // PI284c; CRS: 1807783]
- Flume PA, Geller DE, Loutit JS, Dudly MN, Conrad D, Mpex 204 Study Group. Effects of inhaled MP‐376 (Aeroquin™ levofloxacin inhalation solution) on cystic fibrosis patients with both Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) lung infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S22. [Abstract no: 87; CENTRAL: CN‐00849020; CFGD Register: PI240b; CRS: 1625093] [Google Scholar]
- Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ. Levofloxacin inhalation solution (MP‐376) in patients with cystic fibrosis with Pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2011;183(11):1510‐6. Online supplemental methods. [CENTRAL: CN‐01073292; CFGD Register: PI240e; CRS: 1803862] [DOI] [PubMed] [Google Scholar]
- Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ, et al. Levofloxacin inhalation solution (MP‐376) in patients with cystic fibrosis with Pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2011;183(11):1510‐6. [CENTRAL: CN‐00800852; CFGD Register: PI240d; CRS: 1597245; PUBMED: 21471106] [DOI] [PubMed] [Google Scholar]
- Geller DFPA, Sindel L, Staab D, Fischer R, Loutit J, Conrad D. Effects of inhaled MP‐376 (aeroquin, levofloxacin inhalation solution) on the need for other anti‐pseudomonal antimicrobials in stable CF patients with chronic pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2010;45 Suppl 33:301. [Abstract no: 232; CENTRAL: CN‐00848914; CFGD Register: PI240c; CRS: 1624989] [Google Scholar]
Flume 2016 {published data only}
- Flume P, VanDevanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: CN‐01077213; CFGD Register: PI240f // PI283c // PI284c; CRS: 1807783] [Google Scholar]
- Flume PA, VanDevanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis 2016;15(4):495‐502. [CENTRAL: CN‐01178921; CFGD Register: PI284d; CRS: 4429577; EMBASE: 20160108146; PUBMED: 26852040] [DOI] [PubMed] [Google Scholar]
- Flume PA, VanDevanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2016;15(4):495‐502. Online supplement. [CFGD Register: PI284e; CRS: 5462943] [DOI] [PubMed] [Google Scholar]
- NCT01180634. MP‐376 (Aeroquin™, Levofloxacin for Inhalation) in patients with cystic fibrosis [A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of MP‐376 (Levofloxacin Inhalation Solution; Aeroquin™) in stable cystic fibrosis patients]. clinicaltrials.gov/show/nct01180634 12 August 2010. [CENTRAL: CN‐01012532; CFGD Register: PI284a; CRS: 1743149]
- Devanter D, Flume PA, Fleming R, Elborn J. How often is pulmonary exacerbation defined by ≥4 Fuchs criteria associated with antibiotic treatment?. Pediatric Pulmonology 2014;49 Suppl 38:356. [Abstract no: 388; CENTRAL: CN‐01012531; CFGD Register: PI283b // PI284b; CRS: 1743148] [Google Scholar]
Frederiksen 2006 {published data only}
- Frederiksen B, Koch C, Hoiby N, Pressler T, Hansen A. Effect of aerosolised rhDnase (Pulmozyme®) on pulmonary infections in CF: an open randomised study. Pediatric Pulmonology 2000;Suppl 20:246. [CENTRAL: 792917; CFGD Register: BD97a; CRS: 5500100000003555] [Google Scholar]
- Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatrica 2006;95(9):1070‐4. [CENTRAL: 571885; CFGD Register: BD97b; CRS: 5500100000002869; EMBASE: 2006449673; PUBMED: 16938752] [DOI] [PubMed] [Google Scholar]
Garske 2004 {published data only}
- Garske LA, Kidd TJ, Gan R, Bunting JP, Franks CA, Coulter C, et al. Rifampicin and sodium fusidate reduces the frequency of methicillin‐resistant Staphylococcus aureus (MRSA) isolation in adults with cystic fibrosis and chronic MRSA infection. Journal of Hospital Infection 2004;56(3):208‐14. [DOI] [PubMed] [Google Scholar]
Geller 2004 {published data only}
- Geller DE, Rodriguez CA, Howenstine M, Murphy T, Voter K, Nickerson B, et al. The effects of doubling concentration of tobramycin solution for inhalation on pharmacokinetics (PK), safety and delivery time in patients with cystic fibrosis (CF). American Journal of Respiratory and Critical Care Medicine 2004;169(7):A391. [CFGD Register: PI183a] [Google Scholar]
- Rosenfeld M, Geller DE, Rodriguez CA, Howenstine M, Konstan M, Ordonez C, et al. Serum pharmacokinetics of two preparations of tobramycin solution for inhalation in young cystic fibrosis patients. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A386. [CFGD Register: PI183b] [Google Scholar]
Goldfarb 1986 {published data only}
- Goldfarb J, Wormser GP, Inchiosa MA Jr, Guideri G, Diaz M, Gandhi R, et al. Single‐dose pharmacokinetics of oral ciprofloxacin in patients with cystic fibrosis. Journal of Clinical Pharmacology 1986;26(3):222‐6. [CFGD Register: PI44] [DOI] [PubMed] [Google Scholar]
Griffith 2008 {published data only}
- Geller DE, Flume P, Schwab R, Fornos P, Conrad DJ, Morgan E, et al. A phase 1 safety, tolerability and pharmacokinetic (PK) study of MP‐376 (levofloxacin solution for inhalation) in stable cystic fibrosis (CF) patients. Pediatric Pulmonology 2008;43 Suppl 31:315. [CFGD Register: PI210b] [Google Scholar]
- Griffith DC, Hansen C, Pressler T, Balchen T, Jensen TJ, Geller DE, et al. Single‐dose pharmacokinetics of aerosol MP‐376 (levofloxacin solution for inhalation) in cystic fibrosis patients: PK‐PD implications. Journal of Cystic Fibrosis 2008;7 Suppl 2:S26. [CFGD Register: PI210a] [Google Scholar]
- Kearns GL, Rubino CM, Griffith DC, Geller DE, Forrest A, Bhavnani SM, et al. Levofloxacin pharmacokinetics (PK) after administration of MP‐376 (Levofloxacin inhalation solution; Aeroquin) in children with cystic fibrosis. Journal of Cystic Fibrosis 2011;10 Suppl 1:S23. [Abstract no: 88; CFGD Register: PI210d] [Google Scholar]
- Stockmann C, Hillyard B, Ampofo K, Spigarelli MG, Sherwin CM. Levofloxacin inhalation solution for the treatment of chronic Pseudomonas aeruginosa infection among patients with cystic fibrosis. Expert Review of Respiratory Medicine 2015;9(1):13‐22. [CFGD Register: PI210c] [DOI] [PubMed] [Google Scholar]
Gulliver 2003 {published data only}
- Gulliver T, Wilson S, Williams G, Harris M, Cooper D. Nebulized tobramycin (intravenous solution) is tolerated without inducing cough and wheeze in cystic fibrosis patients. Thoracic Society of Australia & New Zealand Annual Scientific Meeting; 2003 April 4‐9; Adelaide, Australia. 2003:A139. [CFGD Register: PI184]
Hall 2015 {published data only}
- Hall H, Gadhok R, Alshafi K, Bilton D, Simmonds NJ. Eradication of respiratory tract MRSA at a large adult cystic fibrosis centre. Respiratory Medicine 2015;109(3):357‐63. [DOI: 10.1016/j.rmed.2015.01.013] [DOI] [PubMed] [Google Scholar]
Heininger 1993 {published data only}
- Heininger U, Bowing B, Stehr K, Solbach W. Aminoglycosides in patients with mucoviscidosis and pulmonary exacerbation. Comparison of once or three times daily administration [Aminoglykoside bei Patienten mit Mukoviszidose und pulmonaler Exazerbation: vergleich von Einmal‐ und Dreimalgabe]. Klinische Padiatrie 1993;205(1):18‐22. [CFGD Register: PI74] [DOI] [PubMed] [Google Scholar]
Hjelte 1988 {published data only}
- Hjelte L, Widen B, Malmborg AS, Freyschuss U, Strandvik B. Intravenous administration of antibiotics at home in patients with cystic fibrosis improves quality of life [Intravenos antibiotikabehandling i hemmet vid cystisk fibros ger okad livskvalitet]. Lakartidningen 1988;85(18):1614‐7. [CFGD Register: PI206] [PubMed] [Google Scholar]
Hodges 2014 {published data only}
- Hodges L, MacGregor G, Stevens H, Dessen A, Myrset A. An open label, randomised, two‐way crossover scintigraphic study to investigate lung deposition of radiolabelled alginate oligosaccharide delivered as a dry powder and as a nebulised solution in cystic fibrosis patients. Pediatric Pulmonology 2014;49 Suppl 38:305. [Abstract no: 251; CENTRAL: 1012384; CFGD Register: BD215; CRS: 5500131000000154] [Google Scholar]
Huang 1979 {published data only}
- Huang N, Palmer J, Schidlow D, Hsuan F, Hsu C, Goldberg M, et al. Evaluation of antibiotic therapy in patients with cystic fibrosis. Chest 1979;76(3):354‐5. [CFGD Register: PI113a] [Google Scholar]
- Huang NN, Palmer J, Braverman S, Keith HH, Schidlow D. Therapeutic efficacy of ticarcillin and carbenicillin in patients with cystic fibrosis: a double blind study. 23rd Annual Meeting Cystic Fibrosis Club Abstracts; 1982 May 14; Washington D.C. 1982:124. [CFGD Register: PI113b]
Huls 2000 {published data only}
- App EM, Huls G, Bittner‐Dersch P, Stolz S, Lindemann H, Matthys H. Impaired lung function influences the serum concentration of inhaled drugs in cystic fibrosis. Pediatric Pulmonology 2000;30 Suppl 20:279‐80. [CFGD Register: PI156b] [Google Scholar]
- Huls G, App EM, Bittner‐Dersch P, Stolz S, Lindemann H. Impaired lung function influences the serum concentration of inhaled drugs in cystic fibrosis. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:177. [CFGD Register: PI156a]
Junge 2001 {published data only}
- Junge S, Kruger K, Schonweiler R, Ptok M, Ballmann M. Once daily dosage of intravenous tobramycin ‐ increased risk for cochlea damage in children with cystic fibrosis. Pediatric Pulmonology 2001;32 Suppl 22:291. [CFGD Register: PI160b] [Google Scholar]
- Kruger K, Junge S, Schonweiler R, Ptok M, Ballmann M. Once daily dosage of intravenous tobramycin in patients with cystic fibrosis ‐ increased risk for cochlea damage?. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P191. [CFGD Register: PI160a]
Kappler 2016 {published data only}
- Kappler M, Nagel F, Feilcke M, Kröner C, Pawlita I, Naehrig S, et al. Eradication of methicillin resistant Staphylococcus aureus detected for the first time in cystic fibrosis: a single center observational study. Pediatric Pulmonology 2016;51(10):1010‐9. [DOI: 10.1002/ppul.23519] [DOI] [PubMed] [Google Scholar]
Kapranov 1995 {published data only}
- Kapranov NI, Belousov YB, Kashyrskaya NY, Smirnova EY. Quinoline therapy in children with cystic fibrosis. 20th European Cystic Fibrosis Conference; 1995 June 18‐21; Brussels, Belgium. 1995:P19. [CFGD Register: PI104]
Keel 2011 {published data only}
- Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2011;55(7):3393‐8. [CFGD Register: PI251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khorasani 2009 {published data only}
- Khorasani EN, Mansouri F. Effect of zinc supplementation on respiratory infections in children with cystic fibrosis. European Respiratory Society Annual Congress; 2009; Sept 12‐16; Vienna, Austria. 2009:722s. [Abstract no: P4032; CENTRAL: 793188; CFGD Register: GN255; CRS: 5500050000000367]
Knight 1979 {published data only}
- Knight RK, Batten JC, Mearns M. A double blind trial of cephalexin in cystic fibrosis patients with pseudomonas in the sputum. 9th Meeting European Working Group for Cystic Fibrosis; 1979 June 12‐13; Noordwijkerhout, The Netherlands. 1979:52. [CFGD Register: PI124]
Labiris 2004 {published data only}
- Labiris R, Freitag A, Pratt B, Efthimiadis A, Hargreave F, Dolovich M. Does inhalation of preservatives from IV tobramycin preparations (TOB) cause airway inflammation?. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A307. [CFGD Register: PI182] [Google Scholar]
Loening ‐Bauke 1979 {published data only}
- Loening Baucke VA, Mischler E, Myers MG. A placebo‐controlled trial of cephalexin therapy in the ambulatory management of patients with cystic fibrosis. Journal of Pediatrics 1979;95(4):630‐7. [CFGD Register: PI19b] [DOI] [PubMed] [Google Scholar]
- Loening‐Baucke VA, Mischler EH, Myers MG. Cephalexin in cystic fibrosis: a placebo‐controlled study. Pediatric Research 1978;12(4 Pt 2):495. [CFGD Register: PI19c] [Google Scholar]
- Loening‐Bauke, Mischler EH, Myers MG. Cephalexin compared to placebo in the management of patients with cystic fibrosis. 19th Cystic Fibrosis Club Abstracts; 1978. 1978:69. [CFGD Register: PI19a]
Macfarlane 2007 {published data only}
- Macfarlane M, Leavy A, McCaughan J, Fair R, Reid AJM. Successful decolonization of methicillin‐resistant Staphylococcus aureus in paediatric patients with cystic fibrosis (CF) using a three‐step protocol. Journal of Hospital Infection 2007;65(3):231‐6. [DOI] [PubMed] [Google Scholar]
Maiz 1998 {published data only}
- Maiz L, Canton R, Mir N, Baquero F, Escobar H. Aerosolized vancomycin for the treatment of methicillin‐resistant Staphylococcus aureus infection in cystic fibrosis. Pediatric Pulmonology 1998;26(4):287‐9. [DOI] [PubMed] [Google Scholar]
Nathanson 1985 {published data only}
- Nathanson I, Cropp GJA, Li P, Neter E. Effectiveness of aerosolized gentamicin in cystic fibrosis (CF). Cystic Fibrosis Club Abstracts; 1985. 1985:145. [CFGD Register: PI130]
NCT03181932 {published data only}
- NCT03181932. A study of AeroVanc for the treatment of MRSA infection in CF patients [A phase III, randomized, double‐blind, placebo‐controlled study of AeroVanc for the treatment of persistent methicillin‐resistant Staphylococcus aureus lung Infection in cystic fibrosis patients]. clinicaltrials.gov/ct2/show/NCT03181932 09 June 2017.
Nolan 1982 {published data only}
- Nolan G, McIvor P, Levison H, Fleming PC, Corey M, Gold R. Antibiotic prophylaxis in cystic fibrosis: inhaled cephaloridine as an adjunct to oral cloxacillin. Journal of Pediatrics 1982;101(4):626‐30. [DOI] [PubMed] [Google Scholar]
Pai 2006 {published data only}
- Pai MP, Allen SE, Amsden GW. Altered steady state pharmacokinetics of levofloxacin in adult cystic fibrosis patients receiving calcium carbonate. Journal of Cystic Fibrosis 2006;5(3):153‐7. [CFGD Register: PI199] [DOI] [PubMed] [Google Scholar]
Postnikov 2000 {published data only}
- Postnikov SS, Semykin SI, Kapranov NI, Perederko LV, Polikarpova SV, Khamidullina KF. Evaluation of tolerance and efficacy of pefloxacin in the treatment and prevention of severe infections in children with mucoviscidosis and aplastic anemia. Antibiotiki i Khimioterapiia 2000;45(8):25‐30. [CFGD Register: PI171] [PubMed] [Google Scholar]
Postnikov 2001a {published data only}
- Postnikov SS, Semykin SJ, Najimov VP. Safety of fluoroquinolones in children. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P213. [CFGD Register: PI162]
Postnikov 2001b {published data only}
- Postnikov SS, Semiakin SI, Nazhimov VP, Kapranov NI. Comparative yearly growth rate of children with mucoviscidosis treated and not treated with ciprofloxacin: clinicomorphological comparisons. Antibiotiki I Khimioterapiia 2001;46(10):11‐3. [CFGD Register: PI169] [PubMed] [Google Scholar]
Ramstrom 2000 {published data only}
- Ramstrom H, Erwander I, Mared L, Kornfalt R, Seiving B. Pharmaceutical intervention in the care of cystic fibrosis patients. Journal of Clinical Pharmacy and Therapeutics 2000;25(6):427‐34. [CFGD Register: PI159] [DOI] [PubMed] [Google Scholar]
Roberts 1993 {published data only}
- Roberts GW, Nation RL, Jarvinen AO. Measurement of serum tobramycin in the presence of ticarcillin or piperacillin. Australian Journal of Hospital Pharmacy 1992;22(2):152‐4. [CFGD Register: PI132b] [Google Scholar]
- Roberts GW, Nation RL, Jarvinen AO, Martin AJ. An in vivo assessment of the tobramycin/ticarcillin interaction in cystic fibrosis patients. British Journal of Clinical Pharmacology 1993;36:372‐5. [CFGD Register: PI132a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 1991 {published data only}
- Romano L, Girosi D, Spallone E, Parisi F, Minicucci L, Romano C. The use of ofloxacin in cystic fibrosis patients. Minerva Pediatrica 1992;44(3):79‐86. [CFGD Register: PI68b] [PubMed] [Google Scholar]
- Romano L, Minicucci L, Spallone E, Girosi D, Campelli A, Fabbri A, et al. Role of home therapy with ofloxacin in patients with cystic fibrosis (CF). Giornale Italiano de Chemioterapia 1991;38(1‐3):181‐3. [CFGD Register: PI68a] [PubMed] [Google Scholar]
Rosenfeld 2006 {published data only}
- Rosenfeld M, Emerson J, Uh D, Anderson G, Genatossio A, McNamara S, et al. Does tobramycin accumulate in respiratory secretions with repeated aerosol administration: a pilot study. Pediatric Pulmonology 2006;41 Suppl 29:327. [CFGD Register: PI203] [Google Scholar]
Sahl 1992 {published data only}
- Salh B, Bilton D, Dodd M, Abbot J, Webb K. A comparison of aztreonam and ceftazidime in the treatment of respiratory infections in adults with cystic fibrosis. Scandinavian Journal of Infectious Diseases 1992;24(2):215‐8. [CFGD Register: PI72] [DOI] [PubMed] [Google Scholar]
Serisier 2004 {published data only}
- Serisier D, Jones G, Carroll M. Eradication of pulmonary methicillin‐resistant Staphylococcus aureus (MRSA) in cystic fibrosis with linezolid. Journal of Cystic Fibrosis 2004;3(1):61. [DOI] [PubMed] [Google Scholar]
Shapera 1981 {published data only}
- Shapera RM, Warwick WJ, Matsen JM. Clindamycin therapy of staphylococcal pulmonary infections in patients with cystic fibrosis. Journal of Pediatrics 1981;99(4):647‐50. [CFGD Register: P111] [DOI] [PubMed] [Google Scholar]
Sharma 2016 {published data only}
- Sharma G, Lodha R, Shastri S, Saini S, Kapil A, Singla M, et al. Zinc supplementation for one year among children with cystic fibrosis does not decrease pulmonary infection. Respiratory Care 2016;61(1):78‐84. [CFGD Register: GN256; CRS: 5500135000001517; PUBMED: 26443019] [DOI] [PubMed] [Google Scholar]
Singh 2013 {published data only}
- Singh SB, Shelton AU, Kotek K, Starner TD. A clinically‐embedded trial to evaluate the efficacy of interventions for pre‐pseudomonal pathogens. Pediatric Pulmonology 2013;48 Suppl 36:335. [Abstract no: 358; CENTRAL: 999884; CFGD Register: PI274; CRS: 5500127000000006] [Google Scholar]
Smith 1997 {published data only}
- Smith A, Weber A, Pandher R, Williams‐Warren J, Cohen MJ, Ramsey B. Utilization of salivary concentrations of ciprofloxacin in subjects with cystic fibrosis. Infection 1997;25(2):106‐8. [CFGD Register: PI145] [DOI] [PubMed] [Google Scholar]
Solis 2003 {published data only}
- Solís A, Brown D, Hughes J, Saene HK, Heaf DP. Methicillin‐resistant Staphylococcus aureus in children with cystic fibrosis: an eradication protocol. Pediatric Pulmonology 2003;36(3):189‐95. [DOI] [PubMed] [Google Scholar]
Stutman 1987 {published data only}
- Shalit I, Stutman HR, Marks MI, Chartrand SA, Hilman BC. Randomized study of two dosage regimens of ciprofloxacin for treating chronic bronchopulmonary infection in patients with cystic fibrosis. American Journal of Medicine 1987;82 Suppl 4A:189‐95. [CFGD Register: PI48b] [PubMed] [Google Scholar]
- Stutman HR, Shalit I, Marks MI, Greenwood R, Chartrand SA, Hillman BC. Pharmacokinetics of two dosage regimens of ciprofloxacin during a two‐week therapeutic trial in patients with cystic fibrosis. American Journal of Medicine 1987;82 Suppl 4A:142‐5. [CFGD Register: PI48a] [PubMed] [Google Scholar]
Vallieres 2016 {published data only}
- Vallières E, Rendall JC, Moore JE, McCaughan J, Hoeritzauer AI, Tunney MM, et al. MRSA eradication of newly acquired lower respiratory tract infection in cystic fibrosis. ERJ Open Research 2016;2(1):pii: 00064‐2015. [DOI: 10.1183/23120541.00064-2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderhelst 2013 {published data only}
- Vanderhelst E, Wachter E, Willekens J, Piérard D, Vincken W, Malfroot A. Eradication of chronic methicillin‐resistant Staphylococcus aureus infection in cystic fibrosis patients. An observational prospective cohort study of 11 patients. Journal of Cystic Fibrosis 2013;12(6):662‐6. [DOI] [PubMed] [Google Scholar]
Van Devanter 2014 {published data only}
- Fischer R, Flume PA, Devanter DR, Polu K, Pecoraro M, Bhatt N, et al. Pulmonary exacerbations and changes in lung function in CF adults with P aeruginosa treated with inhaled levofloxacin (Quinsair®) or tobramycin. Journal of Cystic Fibrosis 2016;51 Suppl 45:359. [Abstract no: 436; CENTRAL: CN‐01262259; CFGD Register: PI283d; CRS: 5170002] [Google Scholar]
- Flume P, Elborn JS, Polu K, Llorens L, Pecoraro ML, Bhatt N, et al. History of pulmonary exacerbations (pex) AS a predictor of response to nebulized levofloxacin compared with nebulized tobramycin. Journal of Cystic Fibrosis 2016;2015 Suppl 1:S61. [Abstract no: 38; CENTRAL: CN‐01171477; CFGD Register: PI283e; CRS: 4378591] [Google Scholar]
- Flume P, Devanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: CN‐01077213; CFGD Register: PI240f // PI283c // PI284c; CRS: 1807783] [Google Scholar]
- NCT01270347. Trial of aeroquin versus tobramycin inhalation solution (TIS) in cystic fibrosis (CF) patients [Phase 3, open‐label, randomized trial to evaluate the safety and efficacy of MP‐376 inhalation solution (Aeroquin) vs. tobramycin inhalation solution (TIS) in stable CF patients]. clinicaltrials.gov/ct2/show/NCT01270347 05 January 2011. [CENTRAL: CN‐01012530; CFGD Register: PI283a; clinicaltrials.gov: NCT01270347; CRS: 1743147]
- Devanter D, Flume PA, Fleming R, Elborn J. How often is pulmonary exacerbation defined by ≥4 Fuchs criteria associated with antibiotic treatment?. Pediatric Pulmonology 2014;49 Suppl 38:356. [Abstract no: 388; CENTRAL: CN‐01012531; CFGD Register: PI283b // PI284b; CRS: 1743148] [Google Scholar]
Vitti 1975 {published data only}
- Vitti TG, Berg TJ, Pagtakhan RD. The effect of pancreatic enzyme supplement on the intestinal absorption of ampicillin and cloxacillin in children with cystic fibrosis. 16th Cystic Fibrosis Club Abstracts. 1975:56. [CFGD Register: PI81]
Wood 1996 {published data only}
- Wood PJ, Ioannides‐Demos LL, Li SC, Williams TJ, Hickey B, et al. Minimisation of aminoglycoside toxicity in patients with cystic fibrosis. Thorax 1996;51(4):369‐73. [CFGD Register: PI109] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dasenbrook 2015 {published data only}
- Dasenbrook EC. Emerging therapies in cystic fibrosis: aerovanc for the treatment of chronic MRSA. Pediatric Pulmonology 2015;50 Suppl 41:149. [Abstract no: S11.4; CENTRAL: 1092196; CFGD Register: PI289a; CRS: 5500135000001385] [Google Scholar]
- Marich C, Lord J, Dasenbrook EC, Flume PA, Jouhikainen T. Pharmacokinetics of vancomycin in plasma and sputum following pulmonary administration in cystic fibrosis patients with persistent methicillin‐resistant Staphylococcus aureus infection. Pediatric Pulmonology 2016;51 Suppl 45:298‐99. [Abstract no: 282; CENTRAL: 1212605; CFGD Register: PI289b; CRS: 5500135000002071; EMBASE: 612358635] [Google Scholar]
- NCT01746095. Efficacy and safety study of AeroVanc for the treatment of persistent MRSA lung infection in cystic fibrosis patients [A phase 2, randomized, double blind, placebo‐controlled study of AeroVanc for the treatment of persistent methicillin‐resistant staphylococcus aureus lung Infection in cystic fibrosis patients]. clinicaltrials.gov/ct2/show/NCT01746095 10 December 2012.
References to ongoing studies
Dasenbrook 2012 {published data only}
- Dasenbrook EC. Design of a clinical trial to eradicate persistent MRSA: The path from hypothesis to implementation. Pediatric Pulmonology 2012;47 Suppl:123‐4. [CENTRAL: 1004547; CFGD Register: PI285c; CRS: 5500135000001960; EMBASE: 70891676] [Google Scholar]
- Dezube R, Jennings M, Rykiel M, Weaver D, Boyle MP, Dasenbrook E. Update on the persistent methicillin‐resistant Staphylococcus aureus eradication protocol (PMEP) trial. Pediatric Pulmonology 2015;50 Suppl 41:314. [Abstract no: 327; CENTRAL: 1092176; CFGD Register: PI285b; CRS: 5500135000001372] [Google Scholar]
- Jennings MT, Boyle MP, Weaver D, Callahan KA, Dasenbrook EC. Eradication strategy for persistent methicillin‐resistant Staphylococcus aureus infection in individuals with cystic fibrosis‐the PMEP trial: study protocol for a randomized controlled trial. Trials 2014;15(1):223. [CENTRAL: 995772; CFGD Register: PI285a; CRS: 5500050000000166; DOI: 10.1186/1745-6215-15-223; EMBASE: 2014424291] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01594827. Persistent methicillin resistant Staphylococcus aureus eradication protocol (PMEP) [Persistent MRSA eradication protocol (PMEP)]. clinicaltrials.gov/ct2/show/NCT01594827 09 May 2012.
Additional references
CDC 2016
- Centers for Disease Control and Prevention. 2014 national and state healthcare‐associated infections progress report; 2016. www.cdc.gov/hai/progress‐report/index.html (accessed 03 Aug 2017).
CF Foundation 2009
- Cystic Fibrosis Foundation. Patient Registry. 2009 Annual Data Report; Bethesda, Maryland 2009.
CF Foundation 2010
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry annual data report 2010. www.cysticfibrosisdata.org/ReportsUS.html. Bethesda, Maryland: Cystic Fibrosis Foundation, (accessed prior to 08 November 2017).
CF Foundation 2016
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry annual data report 2015. www.cff.org/Our‐Research/CF‐Patient‐Registry/2015‐Patient‐Registry‐Annual‐Data‐Report.pdf (accessed 03 Aug 2017).
CF Trust 2008
- UK Cystic Fibrosis Trust. Methicillin‐resistant Staphylococcus aureus (MRSA). A Report of the UK Cystic Fibrosis Trust Infection Control Working Group 2008.
CF Trust 2009
- Cystic Fibrosis Trust. UK CF Registry annual data report 2009; 2011 March. https://www.cysticfibrosis.org.uk/registryreports (accessed prior to 08 November 2011).
CF Trust 2016
- Cystic Fibrosis Trust. UK Cystic Fibrosis Registry 2015 annual data report; 2016 August. www.cysticfibrosis.org.uk/registryreports (accessed 03 Aug 2017).
CF Trust UK 2016
- CF Trust. What is cystic fibrosis?. www.cysticfibrosis.org.uk/what‐is‐cystic‐fibrosis/faqs#How common is cystic fibrosis? (accessed 23 September 2016).
Chambers 2009
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Reviews Microbiology 2009;7(9):629‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cystic Fibrosis Australia 2011
- Cystic Fibrosis Australia. Cystic fibrosis in Australia 2009. 12th annual report from the Australian Cystic Fibrosis Data Registry; 2011. www.cysticfibrosis.org.au/media/wysiwyg/CF‐Australia/PDF_files/Cystic_Fibrosis_in_Australia_2009.pdf (accessed prior to 08 November 2017).
Cystic Fibrosis Australia 2014
- Cystic Fibrosis Australia. Cystic fibrosis in Australia 2014. 17th annual report from the Australian Cystic Fibrosis Data Registry; 2016. www.cysticfibrosis.org.au/media/wysiwyg/CF‐Australia/medical‐documents/CFA_DataRegistryReport_2014_Final.pdf (accessed 03 Aug 2017).
Dasenbrook 2008
- Dasenbrook EC, Merlo CA, Diener‐West M, Lechtzin N, Boyle MP. Persistent Methicillin‐resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2008;178(8):814‐21. [DOI] [PubMed] [Google Scholar]
Dasenbrook 2010
- Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin‐resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010;303(23):2386‐92. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG on behalf of the CSMG, editor(s). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Doe 2010
- Doe SJ, McSorley A, Isalska B, Kearns AM, Bright‐Thomas R, Brennan AL, et al. Patient segregation and aggressive antibiotic eradication therapy can control methicillin‐resistant Staphylococcus aureus at large cystic fibrosis centres. Journal of Cystic Fibrosis 2010;9(2):104‐9. [DOI] [PubMed] [Google Scholar]
ECDC 2016
- European Centre for Disease Prevention and Control. Proportion of methicillin resistant Staphylococcus aureus (MRSA) isolates in participating countries in 2014. ecdc.europa.eu/en/healthtopics/antimicrobial‐resistance‐and‐consumption/antimicrobial_resistance/database/Pages/map_reports.aspx (accessed 23 September 2016).
Elizur 2007
- Elizur A, Orscheln RC, Ferkol TW, Atkinson JJ, Dunne WM, Buller RS, et al. Panton‐Valentine leukocidin‐positive methicillin‐resistant Staphylococcus aureus lung infection in patients with cystic fibrosis. Chest 2007;131(6):1718‐25. [DOI] [PubMed] [Google Scholar]
Flume 2007
- Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr, Willey‐Courand DB, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. American Journal of Respiratory and Critical Care Medicine 2007;176(10):957‐69. [DOI] [PubMed] [Google Scholar]
Gee 2000
- Gee L, Abbott J, Conway S, Etherington C, Webb A. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s) on behalf of the CSMG and the CBMG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kallen 2010
- Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, et al. Health care‐associated invasive MRSA infections, 2005‐2008. JAMA 2010;304(6):641‐9. [DOI] [PubMed] [Google Scholar]
Klein 2014
Klevens 2006
- Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R, et al. Changes in the epidemiology of methicillin‐resistant Staphylococcus aureus in intensive care units in US hospitals, 1992‐2003. Clinical Infectious Diseases 2006;42(3):389‐91. [DOI] [PubMed] [Google Scholar]
Liebowitz 2009
- Liebowitz LD. MRSA burden and interventions. International Journal of Antimicrobial Agents 2009;34 Suppl 3:S11‐3. [DOI] [PubMed] [Google Scholar]
Loeb 2003
- Loeb MB, Main C, Eady A. Antimicrobial drugs for treating methicillin‐resistant Staphylococcus aureus colonization. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003340] [DOI] [PubMed] [Google Scholar]
Morgan 1999
- Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, et al. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the US and Canada. Pediatric Pulmonology 1999;28(4):231‐41. [DOI] [PubMed] [Google Scholar]
Muhlebach 2011
- Muhlebach MS, Miller M, LaVange LM, Mayhew G, Goodrich JS, Miller MB. Treatment intensity and characteristics of MRSA infection in CF. Journal of Cystic Fibrosis 2011;10(3):201‐6. [DOI] [PubMed] [Google Scholar]
Pearson 2009
- Pearson A, Chronias A, Murray M. Voluntary and mandatory surveillance for methicillin‐resistant Stapylococcus aureus (MRSA) and methicillin‐susceptible S. aureus (MSSA) bacteraemia in England. Journal of Antimicrobial Chemotherapy 2009;64 Suppl 1:i11‐7. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ren 2007
- Ren CL, Morgan WJ, Konstan MW, Schechter MS, Wagener JS, Fisher KA, et al. Presence of methicillin‐resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatric Pulmonology 2007;42(6):513‐8. [DOI] [PubMed] [Google Scholar]
Sawicki 2008
- Sawicki GS, Rasouliyan L, Pasta DJ, Regelmann WE, Wagener JS, Waltz DA, et al. The impact of incident methicillin‐resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatric Pulmonology 2008;43(11):1117‐23. [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Smyth 2017
- Smyth AR, Rosenfeld M. Prophylactic anti‐staphylococcal antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD001912.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderhelst 2012
- Vanderhelst E, Meirleir L, Verbanck S, Pierard D, Vincken W, Malfroot A. Prevalence and impact on FEV1 decline of chronic methicillin‐resistant Staphylococcus aureus (MRSA) colonization in patients with cystic fibrosis. A single‐center, case control study of 165 patients. Journal of Cystic Fibrosis 2012; Vol. 11, issue 1:2‐7. [DOI] [PubMed]
References to other published versions of this review
Lo 2013
- Lo DKH, Hurley MN, Muhlebach MS, Smyth AR. Interventions for the eradication of methicillin‐resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009650.pub2] [DOI] [PubMed] [Google Scholar]
Lo 2015
- Lo DKH, Hurley MN, Muhlebach MS, Smyth AR. Interventions for the eradication of methicillin‐resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD009650.pub3] [DOI] [PubMed] [Google Scholar]


