Abstract
Background
About one in five strokes occur during sleep (wake‐up stroke). People with wake‐up strokes have traditionally been considered ineligible for thrombolytic treatment because the time of stroke onset is unknown. However, some studies suggest that these people may benefit from recanalisation therapies.
Objectives
To assess the effects of intravenous thrombolysis and other recanalisation therapies versus control in people with acute ischaemic stroke presenting on awakening.
Search methods
We searched the Cochrane Stroke Group Trials Register (last search: 9 January 2018). In addition, we searched the following electronic databases in December 2017: Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11) in the Cochrane Library, MEDLINE, Embase, US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), the ISRCTN registry, and Stroke Trials Registry. We also screened references lists of relevant trials, contacted trialists, undertook forward tracking of relevant references, and contacted manufacturers of relevant devices and equipment.
Selection criteria
Randomised controlled trials of intravenous thrombolytic drugs or intra‐arterial therapies in people with acute ischaemic stroke presenting upon awakening.
Data collection and analysis
Two review authors applied the inclusion criteria, extracted data, and assessed trial quality and risk of bias using the GRADE approach. We obtained both published and unpublished data.
Main results
We included one pilot trial with nine participants. The trial was a feasibility trial that included participants with an unknown onset of stroke and signs on perfusion computed tomography of ischaemic tissue at risk of infarction, who were randomised to alteplase (0.9 mg/kg) or placebo. One trial was prematurely terminated due to signs of efficacy of the intervention arm; we did not include this trial because we were not able to obtain data for the portion of the participants with wake‐up stroke after requesting this information from the trial authors. We identified six ongoing trials.
Authors' conclusions
There is insufficient evidence from randomised controlled trials for recommendations concerning recanalisation therapies for wake‐up stroke. Results from ongoing trials will hopefully establish the efficacy and safety of such therapies.
Plain language summary
Recanalisation therapies for wake‐up stroke
Review question: Do people who wake up with stroke benefit from treatments to reopen blood vessels (recanalisation therapies)?
Background: Most strokes are caused by a blockage of a blood vessel in the brain by a blood clot (ischaemic stroke), which are a leading cause of death and disability. Treatments to reopen blood vessels (such as clot‐dissolving drugs or devices to remove blood clots) may improve recovery after ischaemic stroke if blood flow is rapidly restored.
About one in five strokes occur during sleep (wake‐up stroke). People with wake‐up stroke have traditionally been considered ineligible for recanalisation therapies because the time of stroke onset is unknown. However, some studies suggest that these people may benefit from recanalisation therapies.
Search date: We searched for randomised controlled trials (a type of experiment in which people are randomly allocated to one or more treatment groups) up until 9 January 2018.
Study characteristics: We included one trial with nine participants randomised to a recanalisation therapy or to placebo (dummy treatment). The trial was a feasibility study for perfusion computed tomography‐guided thrombolysis in people with unknown onset of stroke.
Key results: There is insufficient evidence to determine if recanalisation therapies improve outcome in people with wake‐up stroke. There are six ongoing trials that may contribute to our review when completed.
Quality of evidence: Low. There were insufficient data to assess the effect of treatment.
Summary of findings
Summary of findings for the main comparison. Recanalisation therapies compared to no recanalisation therapies for wake‐up stroke.
| Recanalisation therapies compared to no recanalisation therapies for wake‐up stroke | ||||||
| Patient or population: wake‐up stroke Setting: in‐hospital Intervention: recanalisation therapies Comparison: no recanalisation therapies | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no recanalisation therapies | Risk with recanalisation therapies | |||||
| Independent functional outcome (mRS score 0 to 2) at end of follow‐up ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Too few participants for analysis |
| Symptomatic intracranial haemorrhage at 14 days follow‐up ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Too few participants for analysis |
| Dead at end of follow‐up ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Too few participants for analysis |
| Quality of life at end of follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | Too few participants for analysis |
| Neurological status at 7 to 14 days and at end of follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | Too few participants for analysis |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; mRS: modified Rankin Scale | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Background
Acute ischaemic stroke is a major cause of death and disability worldwide (Lozano 2012). Intravenous thrombolysis and other recanalisation therapies may restore perfusion and improve clinical outcomes if given within a few hours after stroke onset (Wardlaw 2012).
Approximately one in five strokes occur during sleep (Bassetti 1999). Individuals with stroke symptoms presenting on awakening have traditionally been considered ineligible for thrombolytic treatment because the time of stroke onset is unknown. However, these people may benefit from thrombolytic treatment if the onset of stroke was shortly before awakening. Several studies suggest that the onset of stroke during sleep is close to awakening, and that people with wake‐up stroke and people with stroke onset within 4.5 hours of waking share many clinical findings on brain imaging (Roveri 2011; Silva 2010). Registry studies suggest that intravenous thrombolysis is safe for people with wake‐up stroke (Barreto 2009; Manawadu 2013; Meretoja 2010), but the efficacy and safety of intravenous thrombolysis and other recanalisation therapies in people with acute ischaemic stroke on awakening have not been established.
Other reviews have assessed the benefits of intravenous thrombolytic therapy and intra‐arterial stroke therapy (O'Rourke 2010; Wardlaw 2012). However, the effects of recanalisation therapies in people with wake‐up stroke may differ from those in people with stroke whilst awake because the onset of stroke in wake‐up stroke is unknown and because changes in cerebral blood flow and metabolism occur during sleep (Madsen 1991).
We aimed to perform a systematic review of all randomised controlled trials of intravenous thrombolytic drugs and other recanalisation therapies versus control in people with acute ischaemic stroke presenting on awakening.
Description of the condition
Stroke is globally the second leading cause of death and the third leading cause of loss of disability‐adjusted life‐years (Lozano 2012; Murray 2012). Most strokes are caused by the blockage of an intracranial artery by a clot (ischaemic stroke).
Description of the intervention
Recanalisation therapies for acute ischaemic stroke include intravenous administration of thrombolytic drugs and intra‐arterial therapies.
Thrombolytic drugs given intravenously are used most commonly and work by dissolving blood clots. These drugs include urokinase, recombinant pro‐urokinase (rpro‐UK), streptokinase (SK), and recombinant tissue plasminogen activator (rt‐PA) including alteplase, duteplase, lumbrokinase, tenecteplase, reteplase, and desmoteplase. Alteplase is the only thrombolytic drug licenced to treat acute ischaemic stroke up to 4.5 hours after symptom onset. The recommended dose of alteplase is 0.9 mg per kilogram of body weight (maximum 90 mg), with 10% as a bolus and the rest infused intravenously over 60 minutes.
Intra‐arterial therapies include administration of thrombolytic drugs through an intra‐arterial catheter, mechanical thrombus disruption using a microcatheter or guidewire, angioplasty, and the use of endovascular devices. The benefit of mechanical thrombus disruption and endovascular devices is covered in another Cochrane Review (O'Rourke 2010). Our review differs from O'Rourke 2010 in that we also include intravenous thrombolysis and only people with wake‐up stroke.
How the intervention might work
Interventions may restore perfusion to the ischaemic brain parenchyma, which may reduce damage to the brain parenchyma and improve clinical outcome.
Why it is important to do this review
Intravenous thrombolysis with alteplase is the only approved reperfusion drug therapy for acute ischaemic stroke. Currently, only a minority of people with stroke are treated because of strict inclusion criteria. Approximately one in five strokes occur during sleep; these people are ineligible for thrombolytic drug therapy because the time of stroke onset is unknown. However, they may benefit from reperfusion therapies if stroke onset was shortly before awakening.
The efficacy and safety of intravenous thrombolytic drugs and intra‐arterial treatments in people with acute ischaemic stroke upon awakening have not been established. If recanalisation therapies are shown to provide any benefit for such individuals, the proportion of people with stroke who might benefit from such treatments may be increased.
Objectives
To assess the effects of intravenous thrombolysis and other recanalisation therapies versus control in people with acute ischaemic stroke presenting on awakening.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of intravenous thrombolytic drugs or intra‐arterial therapies versus control in people with acute ischaemic stroke presenting upon awakening.
Types of participants
People with acute ischaemic stroke presenting upon awakening (with neuroimaging excluding intracranial haemorrhage before randomisation). If a trial recruited both people with wake‐up strokes and those whose strokes occurred while awake, we contacted the trial authors to request data for only those participants with wake‐up strokes.
Types of interventions
We included all types of thrombolytic drugs, given in any dose by intravenous route: urokinase, recombinant pro‐urokinase, streptokinase, and tissue plasminogen activator including alteplase, duteplase, lumbrokinase, tenecteplase, and desmoteplase.
We included all types of intra‐arterial treatments: administration of thrombolytic drugs through intra‐arterial catheters, mechanical thrombus disruption using a microcatheter or guidewires or both, angioplasty, and the use of endovascular devices.
The comparison therapy was standard medical care or placebo.
Types of outcome measures
Primary outcomes
Functional outcome at the end of the follow‐up period. We defined favourable functional outcome as a modified Rankin scale (mRS) score of 0 to 2. If the mRS score was not reported, we used the trial's definition of functional outcome.
Secondary outcomes
Death from all causes within seven to 14 days and at the end of follow‐up
Symptomatic intracranial haemorrhage within seven to 14 days
Quality of life at the end of follow‐up
Neurological status at seven to 14 days and at the end of follow‐up
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged for the translation of relevant articles when necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched on 9 January 2018) and the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11) in the Cochrane Library (4 December 2017) (Appendix 1)
MEDLINE Ovid (from 1948 to 7 December 2017) (Appendix 2)
Embase Ovid (from 1980 to 7 December 2017) (Appendix 3)
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist.
We searched the following trial registries for ongoing studies.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 7 December 2017) (Appendix 4)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch; searched 7 December 2017) (Appendix 5)
ISRCTN registry (www.isrctn.com; searched 7 December 2017) (Appendix 6)
Stroke Trials Registry, the Internet Stroke Centre; (www.strokecenter.org/trials/; searched 7 December 2017) (Appendix 7)
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials, we:
screened reference lists of relevant trials;
contacted principal investigators of identified trials (we received replies from Masatoshi Koga and Christian Gerloff);
used the Science Citation Index Cited Reference search for forward tracking of relevant references;
contacted manufacturers of relevant devices and equipment (we received a reply from Penumbra Inc.).
Data collection and analysis
Selection of studies
Two review authors (MBR and HL) independently screened titles and abstracts of references obtained as a result of the searches and excluded obviously irrelevant reports. We retrieved the full‐text articles for the remaining references, and two review authors (HL and EBM) independently screened the full‐text articles and identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (EB) when necessary. We collated multiple reports of the same study so that each study, rather than each reference, was the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram.
Data extraction and management
Two review authors (MBR and HL) independently extracted data from the report of each eligible trial onto a specially designed data extraction form. The review authors were not blinded to journal or institution.
We extracted the following data from each report.
Method of randomisation
Allocation concealment
Blinding of participants, personnel, and outcome assessment
Whether data were reported completely
Whether data were reported selectively
Other bias
We extracted the numbers of participants in the intervention and control groups who:
were independent (mRS score 0 to 2) at end of follow‐up: if possible, we also extracted the number of participants in each mRS category;
died within the first seven to 14 days;
died at the end of follow‐up;
developed symptomatic intracranial haemorrhage within the first seven to 14 days after stroke.
One review author (HL) entered the data into Review Manager 5 (RevMan 2014). Another review author (MBR) checked these data against the hard‐copy data extraction forms to correct any clerical data entry errors. If any relevant data were missing from the available publications, we made direct contact with the relevant principal investigators.
Assessment of risk of bias in included studies
Two review authors (MBR and HL) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving another review author (EB). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We graded the risk of bias for each domain as high, low, or unclear and provided information from the study report together with a justification for our judgement in the 'Risk of bias' tables.
Measures of treatment effect
For dichotomous outcomes, we intended to calculate a weighted estimate of treatment effects across trials and to report odds ratios (ORs) with 95% confidence intervals (CIs). When continuous scales of measurement were used to assess the effects of treatment, we intended to use the mean difference (MD). For studies that used different scales for assessment of similar outcomes, we intended to report standardised mean differences (SMDs).
Unit of analysis issues
For each study, we considered whether groups of individuals were randomised together to the same intervention (cluster‐randomised trial), individuals underwent more than one intervention (cross‐over trial), or there were multiple observations for the same outcome.
Dealing with missing data
If the published information did not allow intention‐to‐treat analysis, we contacted the study authors to ask for follow‐up data that were as complete as possible on all randomly assigned participants for the originally proposed period of follow‐up. In this sensitivity analysis, we assumed that participants who were lost to follow‐up in the treatment group had the worst outcomes and participants who were lost to follow‐up in the control group had the best outcomes.
Assessment of heterogeneity
We intended to use the I2 statistic to measure heterogeneity among the trials in each analysis. We intended to assess heterogeneity according to Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, studies were insufficient to allow this.
Assessment of reporting biases
We intended to use funnel plots to assess reporting bias.
Data synthesis
We intended to calculate a weighted estimate of the typical treatment effect across trials by means of a random‐effects model.
When more studies are available for inclusion in the review, we will use the GRADE approach to assess the quality of the body of evidence as described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will use GRADEpro GDT to complete the Table 1 (GRADEpro GDT 2015).
Subgroup analysis and investigation of heterogeneity
We intended to perform separate analyses in the following subgroups.
Participants characterised by specific imaging criteria (e.g. findings of ischaemic penumbra)
Participants treated at different time intervals (e.g. within three hours after awakening or longer than three hours after awakening)
Sensitivity analysis
We intended to perform a sensitivity analysis to test whether results differed when we excluded trials with high risk of bias.
Results
Description of studies
Results of the search
The searches yielded 1481 references. We excluded 248 duplicates that were not relevant to the review objective. We assessed a total of 1233 records and excluded 1231 records because the abstract showed that they were not randomised trials of wake‐up stroke.
We assessed two records in full. We included one study (one record). We excluded one study (one record) because it included both non‐wake‐up strokes and wake‐up strokes, and we were unable to obtain the data from the trial authors for the portion of participants with wake‐up stroke in the trial. The PRISMA flow diagram is given in Figure 1.
1.
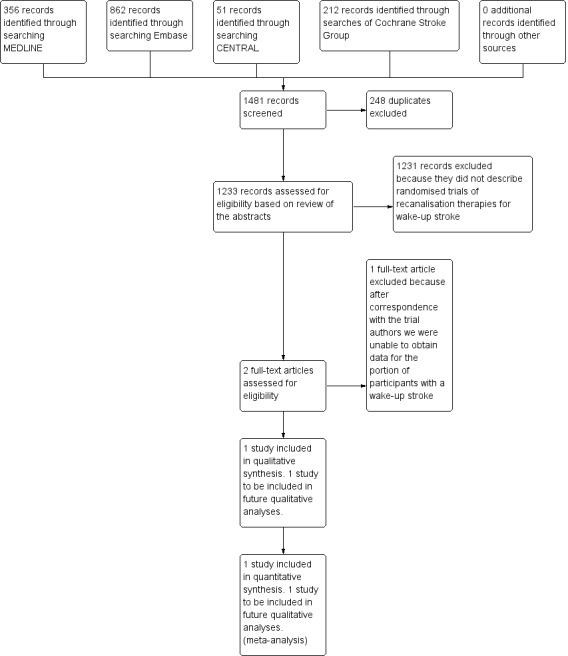
PRISMA flow diagram.
Included studies
We included one study that recruited people with wake‐up strokes and people with strokes that occurred whilst the individual was awake (Michel 2012). This pilot trial randomised people with an unknown onset of stroke and signs on perfusion computed tomography (CT) of ischaemic tissue at risk of infarction. The trial was managed from Switzerland. The principal investigator provided data on the nine participants included in the trial. Four participants were randomised to intravenous thrombolytic therapy with alteplase (0.9 mg/kg), and five participants were randomised to placebo.
We identified six ongoing trials (NCT01455935; NCT01525290; NCT01580839; NCT01852201; NCT02002325; NCT03181360).
Excluded studies
We excluded one study that recruited both people with wake‐up strokes and people with strokes that occurred whilst the individual was awake (NCT02142283). Participants were randomised to intra‐arterial treatment or control. The trial was ended after interim analyses showed effect of intra‐arterial treatment. We contacted the trial authors, but they did not wish to share data until their secondary papers are published.
Risk of bias in included studies
See Figure 2. The quality of randomisation and blinding in the included study was adequate (Michel 2012). No participants were lost to follow‐up. The study reported intention‐to‐treat analyses. This was a pilot trial and had limited statistical power.
2.
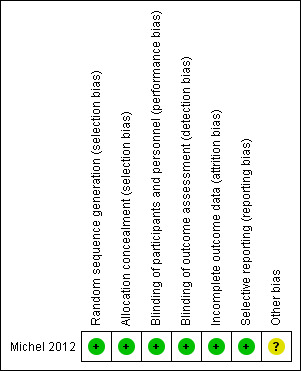
Risk of bias summary: judgements about each risk of bias item for the included study.
Allocation
Enrolment of participants and allocation was performed by a blinded physician. We assessed the risk of bias to be low.
Blinding
The trial was placebo controlled and employed blinded outcome assessment. We assessed the risk of bias to be low.
Incomplete outcome data
There was no loss to follow‐up. We assessed the risk of bias to be low.
Selective reporting
There was no selective reporting. We assessed the risk of bias to be low.
Other potential sources of bias
We identified no other potential sources of bias. We assessed the risk of bias to be unclear.
Effects of interventions
See: Table 1
Good functional outcome (mRS 0 to 2) at the end of follow‐up was observed in four of four participants in the intervention group and two of five participants in the control group. No participants were dead at the end of follow‐up. No participants had symptomatic intracranial haemorrhage within the first two weeks. There were no results for quality of life at the end of follow‐up or for neurological status at the end of follow‐up.
We considered the sample size to be inadequate for statistical analyses.
Discussion
There are six ongoing randomised controlled trials of wake‐up stroke (NCT01455935; NCT01525290; NCT01580839; NCT01852201; NCT02002325; NCT03181360). One trial was recently stopped after interim analyses showed effect of intra‐arterial treatment (NCT02142283). This study supports the role of intra‐arterial treatment in carefully selected people with wake‐up stroke. However, the findings should be interpreted with caution with respect to people that do not fulfil the inclusion criteria of that study. NCT02142283 included participants with evidence of occlusion of the intracranial internal carotid artery or the proximal middle cerebral artery that had mismatch between the severity of the clinical deficit and infarct volume on diffusion‐weighted magnetic resonance imaging (MRI). Furthermore, a large proportion of the participants (> 40%) in NCT02142283 had late presenting stroke and not wake‐up stroke. We excluded this trial from the review because we could not obtain extracted data on the portion of participants who had wake‐up stroke.
Four trials are testing alteplase versus placebo or control (NCT01455935; NCT01525290; NCT01580839; NCT02002325). One trial is testing tenecteplase (NCT03181360), and two trials are testing the effect of intra‐arterial interventions for wake‐up strokes (NCT01852201; NCT02142283).
The time window for recanalisation therapies in people with wake‐up stroke is unknown. NCT01455935, NCT01525290, NCT02002325, and NCT03181360 allow thrombolytic treatment to be started within 4.5 hours of symptom recognition (e.g. awaking). NCT01580839 allows thrombolytic treatment to be started nine hours from the midpoint between sleep onset or last known to be normal and time of waking. NCT02142283 includes participants between six and 24 hours after last seen well. NCT01852201 includes participants between six and 12 hours after last seen well.
The benefit of advanced imaging modalities in selecting people to treatment is unknown, as demonstrated in the MR RESCUE trial, which did not find a benefit of penumbra imaging in selecting people with stroke to intra‐arterial interventions (Kidwell 2013). Six of the seven ongoing randomised controlled trials on wake‐up stroke use advanced imaging modalities such as magnetic resonance imaging diffusion weighted imaging/fluid attenuated inversion recovery (MRI DWI/FLAIR) mismatch or CT/MRI penumbral mismatch for selection of participants. NCT01525290, NCT01580839, and NCT02002325 use MRI DWI/FLAIR indicating that the patient has an ischaemic lesion within the time window of thrombolytic treatment, whilst NCT01455935 and NCT01580839 use penumbra‐based imaging visualising a salvageable penumbral area around the infarct core. However, the interobserver agreement for MRI DWI/FLAIR mismatch is moderate, and the sensitivity and negative predictive value is low to moderate (Thomalla 2011). Even if it is possible to include patients based on MRI DWI/FLAIR mismatch, a substantial proportion of patients that may benefit from recanalisation therapies will be excluded based on mismatch imaging.
Summary of main results
Recanalisation therapies show promise in the treatment of wake‐up stroke.
The American Stroke Association recommends that thrombolytic treatment for wake‐up stroke with time last known to be well at more than 4.5 hours shall not be given outside clinical trials (Demaerschalk 2016), as more evidence is needed from randomised controlled trials on the risk and benefit of recanalisation therapies for wake‐up stroke.
Overall completeness and applicability of evidence
Evidence was limited to one small‐sampled trial. Based on the current evidence, it is not possible to conclude whether recanalisation therapies can be recommended for wake‐up stroke.
Quality of the evidence
Quality of evidence was not applicable, as the data were insufficient for statistical analysis.
Potential biases in the review process
We have not included trials that were not published on the date we carried out our searches.
Agreements and disagreements with other studies or reviews
A brief literature search did not identify other reviews on this topic. Evidence of the benefit of recanalisation therapies for wake‐up stroke is limited to comparison with historical controls. These studies suggest that intravenous thrombolytic drugs and intra‐arterial treatments are safe to use and may improve outcome in people with wake‐up stroke (Barreto 2009; Manawadu 2013; Meretoja 2010).
Authors' conclusions
Implications for practice.
There is too little evidence from randomised controlled trials to support the routine use of recanalisation therapies in people with wake‐up stroke outside of clinical trials.
Implications for research.
Evidence from randomised controlled trials is highly warranted in order to evaluate the benefit of recanalisation therapies in wake‐up stroke. Six trials are ongoing and may contribute to this review when completed.
Acknowledgements
We thank Brenda Thomas from the Cochrane Stroke Group for developing the search strategies used in the review and for performing the searches. We also thank Dr Patrik Michel, University of Lausanne, for contributing unpublished data from Michel 2012 to this review.
Appendices
Appendix 1. CENTRAL search strategy
#1[mh ^"cerebrovascular disorders"] or [mh ^"basal ganglia cerebrovascular disease"] or [mh ^"brain ischemia"] or [mh "brain infarction"] or [mh ^"hypoxia‐ischemia, brain"] or [mh ^"carotid artery diseases"] or [mh ^"carotid artery thrombosis"] or [mh ^"carotid artery, internal, dissection"] or [mh ^"intracranial arterial diseases"] or [mh "cerebral arterial diseases"] or [mh ^"infarction, anterior cerebral artery"] or [mh ^"infarction, middle cerebral artery"] or [mh ^"infarction, posterior cerebral artery"] or [mh "intracranial embolism and thrombosis"] or [mh stroke] or [mh ^"vertebral artery dissection"] #2isch*emi* near/6 (stroke* or apoplex* or cerebral next vasc* or cerebrovasc* or cva or attack*):ti,ab #3(brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle next cerebr* or mca* or "anterior circulation") near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*):ti,ab #4#1 or #2 or #3 #5[mh ^wakefulness] or [mh ^sleep] #6("wake up" or "wake‐up" or "wakes up" or "wakes‐up"):ti,ab #7(waking* or awake* or awoke):ti,ab #8(during near/5 sleep*):ti,ab #9(whil* near/5 (sleep* or asleep)):ti,ab #10((unknown or unclear or uncertain or indefinite or "not known") near/10 onset):ti,ab #11#5 or #6 or #7 or #8 or #9 or #10 #12[mh ^"thrombolytic therapy"] #13[mh ^"fibrinolytic agents"] or [mh ^plasmin] or [mh ^plasminogen] or [mh ^"tissue plasminogen activator"] or [mh "plasminogen activators"] or [mh ^"urokinase‐type plasminogen activator"] or [mh streptokinase] #14[mh ^fibrinolysis] #15(thromboly* or fibrinoly* or recanalis* or recanaliz*):ti,ab #16((clot* or thrombus) near/5 (lyse or lysis or dissolve* or dissolution or bust*)):ti,ab #17(tPA or t‐PA or rtPA or rt‐PA or plasminogen or plasmin or alteplase or actilyse):ti,ab #18(anistreplase or streptodornase or streptokinase or urokinase or pro*urokinase or rpro*uk or lumbrokinase or duteplase or lanoteplase or pamiteplase or reteplase or saruplase or staphylokinase or streptase or tenecteplase or desmoteplase or amediplase or monteplase or nasaruplase or silteplase):ti,ab #19[mh ^"radiography, interventional"] or [mh ^"radiology, interventional"] #20[mh ^catheterization] or [mh ^angioplasty] or [mh ^"angioplasty, balloon"] or [mh ^"angioplasty, balloon, laser‐assisted"] or [mh ^"angioplasty, laser"] or [mh ^atherectomy] or [mh ^"balloon dilatation"] or [mh ^"catheter ablation"] #21[mh ^Stents] #22[mh ^"mechanical thrombolysis"] or [mh ^thrombectomy] or [mh ^embolectomy] #23[mh ^"blood vessel prosthesis"] or [mh ^"blood vessel prosthesis implantation"] #24[mh ^"Cerebral Revascularization"] or [mh ^reperfusion] or [mh ^dilatation] #25(interventional near/3 (radiolog* or radiograph* or neuroradiolog*)):ti,ab #26(angioplast* or stent*):ti,ab #27(thrombectomy or embolectomy or atherect*):ti,ab #28(thromboaspiration or arterial next recanali*ation):ti,ab #29((mechanical or radiolog* or pharmacomechanical or laser or endovascular or neurovascular) near/5 (thrombolys* or reperfusion or fragment* or aspiration or recanali*ation or clot next lys*)):ti,ab #30((clot or thrombus or thrombi or embol*) near/5 (aspirat* or remov* or retriev* or fragment* or retract* or extract* or obliterat* or dispers* or disrupt* or disintegrate*)):ti,ab #31((retrieval or extraction) near/5 device*):ti,ab #32(endoluminal next repair*):ti,ab #33(blood vessel near/5 (prosthesis or implantat*)):ti,ab #34((merci or concentric) next retriever):ti,ab #35(endovascular next snare* or neuronet or microsnare or "X‐ciser" or angiojet):ti,ab #36[mh ^ultrasonics] or [mh ^"ultrasonic therapy"] or [mh ^ultrasonography] or [mh "ultrasonography, Doppler"] or [mh ^"ultrasonography, interventional"] #37(ultrasound* or ultrasonic* or ultrasonogra* or sonograph* or insonation):ti,ab #38((transcranial near/5 doppler) or TCD or TCCD):ti,ab #39[mh /US] #40(sonothrombolysis or sonothromboly* or sonolys* or sonothrombotripsy or thrombotripsy):ti,ab #41{or #12‐#40} #42#4 and #11 and #41
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or hypoxia‐ischemia, brain/ or carotid artery diseases/ or carotid artery thrombosis/ or carotid artery, internal, dissection/ or intracranial arterial diseases/ or cerebral arterial diseases/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or exp "intracranial embolism and thrombosis"/ or exp stroke/ or vertebral artery dissection/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. wakefulness/ or sleep/ 6. (wake up or wake‐up or wakes up or wakes‐up).tw. 7. (waking$ or awake$ or awoke).tw. 8. (during adj5 sleep$).tw. 9. (whil$ adj5 (sleep$ or asleep)).tw. 10. ((unknown or unclear or uncertain or indefinite or "not known") adj10 onset).tw. 11. 5 or 6 or 7 or 8 or 9 or 10 12. thrombolytic therapy/ 13. fibrinolytic agents/ or fibrinolysin/ or plasminogen/ or tissue plasminogen activator/ or exp plasminogen activators/ or urokinase‐type plasminogen activator/ or exp streptokinase/ 14. fibrinolysis/ 15. (thromboly$ or fibrinoly$ or recanalis$ or recanaliz$).tw. 16. ((clot$ or thrombus) adj5 (lyse or lysis or dissolve$ or dissolution or bust$)).tw. 17. (tPA or t‐PA or rtPA or rt‐PA or plasminogen or plasmin or alteplase or actilyse).tw. 18. (tPA or t‐PA or rtPA or rt‐PA or plasminogen or plasmin or alteplase or actilyse).nm. 19. (anistreplase or streptodornase or streptokinase or urokinase or pro?urokinase or rpro?uk or lumbrokinase or duteplase or lanoteplase or pamiteplase or reteplase or saruplase or staphylokinase or streptase or tenecteplase or desmoteplase or amediplase or monteplase or nasaruplase or silteplase).tw. 20. (anistreplase or streptodornase or streptokinase or urokinase or pro?urokinase or rpro?uk or lumbrokinase or duteplase or lanoteplase or pamiteplase or reteplase or saruplase or staphylokinase or streptase or tenecteplase or desmoteplase or amediplase or monteplase or nasaruplase or silteplase).nm. 21. radiography, interventional/ or radiology, interventional/ 22. catheterization/ or angioplasty/ or angioplasty, balloon/ or angioplasty, balloon, laser‐assisted/ or angioplasty, laser/ or atherectomy/ or balloon dilatation/ or catheter ablation/ 23. Stents/ 24. mechanical thrombolysis/ or thrombectomy/ or embolectomy/ 25. blood vessel prosthesis/ or blood vessel prosthesis implantation/ 26. Cerebral Revascularization/ or reperfusion/ or dilatation/ 27. (interventional adj3 (radiolog$ or radiograph$ or neuroradiolog$)).tw. 28. (angioplast$ or stent$).tw. 29. (thrombectomy or embolectomy or atherect$).tw. 30. (thromboaspiration or arterial recanali?ation).tw. 31. ((mechanical or radiolog$ or pharmacomechanical or laser or endovascular or neurovascular) adj5 (thrombolys$ or reperfusion or fragmentation or aspiration or recanali?ation or clot lys$)).tw. 32. ((clot or thrombus or thrombi or embol$) adj5 (aspirat$ or remov$ or retriev$ or fragment$ or retract$ or extract$ or obliterat$ or dispers$ or disrupt$ or disintegrate$)).tw. 33. ((retrieval or extraction) adj5 device$).tw. 34. endoluminal repair$.tw. 35. (blood vessel adj5 (prosthesis or implantat$)).tw. 36. ((merci or concentric) adj retriever).tw. 37. (endovascular snare$ or neuronet or microsnare or X‐ciser or angiojet).tw. 38. ultrasonics/ or ultrasonic therapy/ or ultrasonography/ or exp ultrasonography, doppler/ or ultrasonography, interventional/ 39. (ultrasound$ or ultrasonic$ or ultrasonogra$ or sonograph$ or insonation).tw. 40. ((transcranial adj5 doppler) or TCD or TCCD).tw. 41. ultrasonography.fs. 42. (sonothrombolysis or sonothromboly$ or sonolys$ or sonothrombotripsy or thrombotripsy).tw. 43. or/12‐42 44. 4 and 11 and 43 45. exp animals/ not humans.sh. 46. 44 not 45
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or lacunar stroke/ or cardioembolic stroke/ or carotid artery disease/ or exp carotid artery obstruction/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ or stroke patient/ or stroke unit/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. wakefulness/ or sleep/ 6. (wake up or wake‐up or wakes up or wakes‐up).tw. 7. (waking$ or awake$ or awoke).tw. 8. (during adj5 sleep$).tw. 9. (whil$ adj5 (sleep$ or asleep)).tw. 10. ((unknown or unclear or uncertain or indefinite or "not known") adj10 onset).tw. 11. 5 or 6 or 7 or 8 or 9 or 10 12. fibrinolytic therapy/ 13. fibrinolytic agent/ or plasmin/ or plasminogen/ or exp plasminogen activator/ 14. blood clot lysis/ 15. fibrinolysis/ 16. (thromboly$ or fibrinoly$ or recanalis$ or recanaliz$).tw. 17. ((clot$ or thrombus) adj5 (lyse or lysis or dissolve$ or dissolution or bust$)).tw. 18. (tPA or t‐PA or rtPA or rt‐PA or plasminogen or plasmin or alteplase or actilyse).tw. 19. (anistreplase or streptodornase or streptokinase or urokinase or pro?urokinase or rpro?uk or lumbrokinase or duteplase or lanoteplase or pamiteplase or reteplase or saruplase or staphylokinase or streptase or tenecteplase or desmoteplase or amediplase or monteplase or nasaruplase or silteplase).tw. 20. interventional radiology/ or endovascular surgery/ 21. percutaneous transluminal angioplasty/ or angioplasty/ or laser angioplasty/ or catheterization/ or catheter ablation/ or balloon dilatation/ or exp atherectomy/ 22. stent/ 23. thrombectomy/ or exp percutaneous thrombectomy/ or embolectomy/ 24. artery prosthesis/ 25. cerebral revascularization/ or reperfusion/ or artery dilatation/ or recanalization/ 26. (interventional adj3 (radiolog$ or radiograph$ or neuroradiolog$)).tw. 27. (angioplast$ or stent$).tw. 28. (thrombectomy or embolectomy or atherect$).tw. 29. (thromboaspiration or arterial recanali?ation).tw. 30. ((mechanical or radiolog$ or pharmacomechanical or laser or endovascular or neurovascular) adj5 (thrombolys$ or reperfusion or fragment$ or aspiration or recanali?ation or clot lys$)).tw. 31. ((clot or thrombus or thrombi or embol$) adj5 (aspirat$ or remov$ or retriev$ or fragment$ or retract$ or extract$ or obliterat$ or dispers$ or disrupt or disintegrate$)).tw. 32. ((retrieval or extraction) adj5 device$).tw. 33. endoluminal repair$.tw. 34. ((blood vessel or artery) adj5 (prosthesis or implantat$)).tw. 35. ((merci or concentric) adj retriever).tw. 36. (endovascular snare$ or neuronet or microsnare or X‐ciser or angiojet).tw. 37. ultrasound/ or exp ultrasound therapy/ or echography/ or doppler echography/ or intravascular ultrasound/ 38. (ultrasound$ or ultrasonic$ or ultrasonogra$ or sonograph$ or insonation).tw. 39. ((transcranial adj5 doppler) or TCD or TCCD).tw. 40. (sonothrombolysis or sonothromboly$ or sonolys$ or sonothrombotripsy or thrombotripsy).tw. 41. or/12‐40 42. 4 and 11 and 41 43. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/) 44. 42 not 43
Appendix 4. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov search strategy
Advanced search: Recruitment status: All studies Condition: Stroke Other terms: awakening OR wake‐up
Appendix 5. WHO International Clinical Trials Registry Platform search strategy
Advanced search: Recruitment Status: ALL Condition: Stroke Other terms: awakening OR wake‐up
Appendix 6. ISRCTN Registry search strategy
Advanced search: Text search: (awakening OR "wake‐up") AND stroke
Appendix 7. Stroke Trials Registry search strategy
Keywords: wake
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Michel 2012.
| Methods | Randomised, double‐blinded, placebo‐controlled pilot trial | |
| Participants | 12 participants with a supratentorial stroke of unknown onset in the middle cerebral artery territory and significant volume of at‐risk tissue on perfusion computed tomography. 9 of the participants had wake‐up stroke, and 3 had a non‐wake‐up stroke of unknown onset. The study authors contributed unpublished data on the 9 participants with wake‐up stroke. |
|
| Interventions |
|
|
| Outcomes | Primary outcome: feasibility of study Secondary outcome: mRS 0 to 2 at 90 days' follow‐up |
|
| Notes | Principal Investigator: Patrik Michel, University of Lausanne, Lausanne, Switzerland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table generated by independent pharmacist |
| Allocation concealment (selection bias) | Low risk | Enrolment of participants and allocation performed by blinded physician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Unclear risk | None found. |
mRS: modified Rankin Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT02142283 | We were unable to obtain the portion of data for the wake‐up stroke participants (approximately 60%). We contacted the trial authors, but they did not wish to share data until their secondary papers are published. |
Characteristics of ongoing studies [ordered by study ID]
NCT01455935.
| Trial name or title | WAke up Symptomatic Stroke in Acute Brain Ischemia (WASSABI) trial |
| Methods | Randomised, single‐blinded, controlled trial |
| Participants | 90 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | mRS at 90 days' follow‐up |
| Starting date | November 2011 |
| Contact information | Principal Investigator: Tareq Kass‐Hout, Jacobs Neurological Institute, University at Buffalo Neurosurgery, USA E‐mail: kasshouttareq@gmail.com |
| Notes | ClinicalTrials.gov identifier: NCT01455935 |
NCT01525290.
| Trial name or title | Efficacy and safety of magnetic resonance imaging‐based thrombolysis in wake‐up stroke (WAKE‐UP) |
| Methods | Randomised, double‐blinded, placebo‐controlled trial |
| Participants | 800 participants Clinical inclusion criteria
Imaging inclusion criteria
Clinical exclusion criteria
Imaging exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2012 |
| Contact information | Principal Investigator: Goetz Thomalla, Universitätsklinikum Hamburg‐Eppendorf, Germany E‐mail: thomalla@uke.uni‐hamburg.de |
| Notes | ClinicalTrials.gov identifier: NCT01525290 |
NCT01580839.
| Trial name or title | EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 400 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | mRS 0 to 1 at 90 days' follow‐up |
| Starting date | June 2010 |
| Contact information | Principal Investigator: Geoffrey Donnan, National Stroke Research Institute, Australia
Principal Investigator: Stephen Davis, University of Melbourne, Australia Contact: Sue Bates, e‐mail: sbates@neurotrialsaustralia.com |
| Notes | ClinicalTrials.gov identifier: NCT01580839 (Australian part) and NCT00887328 (international part) |
NCT01852201.
| Trial name or title | PerfusiOn imaging Selection of Ischemic sTroke patIents for endoVascular thErapy (POSITIVE) |
| Methods | Randomised, single‐blinded trial |
| Participants | 750 participants Inclusion criteria
Exclusion criteria
Head CT or MRI scan exclusion criteria
|
| Interventions | Endovascular treatment plus best medical treatment or best medical treatment alone |
| Outcomes | mRS score at 90 days' follow‐up |
| Starting date | September 2013 |
| Contact information | Adrian Parker 843‐792‐3164 parkerad@musc.edu |
| Notes | ClinicalTrials.gov identifier: NCT01852201 Not a trial of wake‐up stroke per se, but the trial will include a substantial proportion of participants with wake‐up stroke. |
NCT02002325.
| Trial name or title | THrombolysis for Acute Wake‐up and unclear‐onset Strokes with alteplase at 0.6 mg/kg Trial (THAWS) |
| Methods | Randomised, single‐blinded, controlled trial |
| Participants | 300 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Favourable outcome (mRS score 0 to 1) at 90 days' follow‐up |
| Starting date | April 2014 |
| Contact information | Principal Investigator: Kazunori Toyoda, National Cerebral and Cardiovascular Center, Japan Contact person: Masatoshi Koga, koga@ncvc.go.jp |
| Notes | ClinicalTrials.gov identifier: NCT02002325 |
NCT03181360.
| Trial name or title | Tenecteplase in Wake‐up Ischaemic Stroke Trial (TWIST) |
| Methods | PROBE; prospective, randomised, open, blinded‐endpoint |
| Participants | 500 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2017 |
| Contact information | Trial Manager: Melinda B Roaldsen; e‐mail: melinda.b.roaldsen@uit.no or twist@uit.no |
| Notes | ClinicalTrials.gov identifier NCT03181360 |
aPTT: activated partial thromboplastin time ASPECTS: Alberta Stroke Program Early CT score CT: computed tomography DWI: diffusion‐weighted imaging FLAIR: fluid attenuated inversion recovery INR: international normalised ratio IV: intravenous MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale PWI: perfusion‐weighted imaging
Differences between protocol and review
There are no differences between protocol and review.
Contributions of authors
MBR: data collection, drafting of the protocol. HL: conception and design of the review, data collection, drafting of the protocol. EBM and EB: conception and design of the review, commenting on protocol drafts.
Sources of support
Internal sources
University of Tromsø, Norway.
External sources
No sources of support supplied
Declarations of interest
MBR: none known HL: none known EBM: none known EB: none known
New
References
References to studies included in this review
Michel 2012 {published and unpublished data}
- Michel P, Ntaios G, Reichhart M, Schindler C, Bogousslavsky J, Maeder P, et al. Perfusion‐CT guided intravenous thrombolysis in patients with unknown‐onset stroke: a randomized, double‐blind, placebo‐controlled, pilot feasibility trial. Neuroradiology 2012;54:579‐88. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
NCT02142283 {published data only}
- NCT02142283. Clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN). clinicaltrials.gov/ct2/show/NCT02142283 (first received 20 May 2014).
References to ongoing studies
NCT01455935 {published data only}
- NCT01455935. Wake up symptomatic stroke ‐ benefit of intravenous clot busters or endovascular intervention (WASSABI). clinicaltrials.gov/ct2/show/NCT01455935 (first received 20 October 2011).
NCT01525290 {published data only}
- NCT01525290. Efficacy and safety of MRI‐based thrombolysis in wake‐up stroke (WAKE‐UP). clinicaltrials.gov/ct2/show/NCT01525290 (first received 2 February 2012).
NCT01580839 {published data only}
- NCT01580839. EXTEND (International): Extending the Time for Thrombolysis in Emergency Neurological Deficits (International) (EXTEND). clinicaltrials.gov/ct2/show/NCT01580839 (first received 19 April 2012).
NCT01852201 {published data only}
- NCT01852201. POSITIVE Stroke Clinical Trial. clinicaltrials.gov/ct2/show/NCT01852201 (first received 13 May 2013).
NCT02002325 {published data only}
- NCT02002325. THrombolysis for Acute Wake‐up and unclear‐onset Strokes with alteplase at 0.6 mg/kg trial (THAWS). clinicaltrials.gov/ct2/show/NCT02002325 (first received 5 December 2013).
NCT03181360 {published and unpublished data}
- NCT03181360. Tenecteplase in Wake‐up Ischaemic Stroke Trial (TWIST). clinicaltrials.gov/ct2/show/NCT03181360 (first received 8 June 2017).
Additional references
Barreto 2009
- Barreto AD, Martin‐Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, et al. Thrombolytic therapy for patients who wake‐up with stroke. Stroke 2009;40:827‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassetti 1999
- Bassetti C, Aldrich M. Night time versus daytime transient ischaemic attack and ischaemic stroke. Journal of Neurology, Neurosurgery and Psychiatry 1999;67:463‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demaerschalk 2016
- Demaerschalk B, Kleindorfer DO, Adeoye MO, Demchuk AM, Fugate JE, Grotta JC, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke. Stroke 2016;47:581‐641. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kidwell 2013
- Kidwell CS, Jahan R, Gombein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. New England Journal of Medicine 2013;368:914‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2095‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madsen 1991
- Madsen PL, Schmidt JF, Wildschiodtz G, Friberg L Holm S, Vorstrup, et al. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid‐eye‐movement sleep. Journal of Applied Physiology 1991;70:2597‐601. [DOI] [PubMed] [Google Scholar]
Manawadu 2013
- Manawadu D, Bodla S, Keep J, Jarosz J, Kalra L. An observational study of thrombolysis outcomes in wake‐up ischemic stroke patients. Stroke 2013;44:427‐31. [DOI] [PubMed] [Google Scholar]
Meretoja 2010
- Meretoja A, Putaala J, Tatlisumak T, Atula S, Artto V, Curtze S, et al. Off‐label thrombolysis is not associated with poor outcome in patients with stroke. Stroke 2010;41:1450‐8. [DOI] [PubMed] [Google Scholar]
Murray 2012
- Murray CJL, Vos T, Lozanno R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197‐223. [DOI] [PubMed] [Google Scholar]
O'Rourke 2010
- O'Rourke K, Berge E, Walsh CD, Kelly PJ. Percutaneous vascular interventions for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007574.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roveri 2011
- Roveri L, Gioia S, Ghidinelli C, Anzalone N, Filippis C, Comi G. Wake‐up stroke within 3 hours of symptom awareness: imaging and clinical features compared to standard recombinant tissue plasminogen activator treated stroke. Journal of Stroke and Cerebrovascular Diseases 2011;22:703‐8. [DOI] [PubMed] [Google Scholar]
Silva 2010
- Silva GS, Lima FO, Camargo EC, Smith WS, Singhal AB, Greer DM, et al. Wake‐up stroke: clinical and neuroimaging characteristics. Cerebrovascular Diseases 2010;29:336‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomalla 2011
- Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI‐FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE‐FLAIR): a multicentre observational study. Lancet Neurology 2011;10:978‐86. [DOI] [PubMed] [Google Scholar]
Wardlaw 2012
- Wardlaw JM, Murray M, Berge E, Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta‐analysis. Lancet 2012;379:2364‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lindekleiv 2014
- Lindekleiv H, Mathiesen EB, Berge E. Recanalisation therapies for wake‐up stroke. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010995] [DOI] [PMC free article] [PubMed] [Google Scholar]


