Abstract
Background
There are controversies about the amount of calories and the type of nutritional support that should be given to critically‐ill people. Several authors advocate the potential benefits of hypocaloric nutrition support, but the evidence is inconclusive.
Objectives
To assess the effects of prescribed hypocaloric nutrition support in comparison with standard nutrition support for critically‐ill adults
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library), MEDLINE, Embase and LILACS (from inception to 20 June 2017) with a specific strategy for each database. We also assessed three websites, conference proceedings and reference lists, and contacted leaders in the field and the pharmaceutical industry for undetected/unpublished studies. There was no restriction by date, language or publication status.
Selection criteria
We included randomized and quasi‐randomized controlled trials comparing hypocaloric nutrition support to normo‐ or hypercaloric nutrition support or no nutrition support (e.g. fasting) in adults hospitalized in intensive care units (ICUs).
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We meta‐analysed data for comparisons in which clinical heterogeneity was low. We conducted prespecified subgroup and sensitivity analyses, and post hoc analyses, including meta‐regression. Our primary outcomes were: mortality (death occurred during the ICU and hospital stay, or 28‐ to 30‐day all‐cause mortality); length of stay (days stayed in the ICU and in the hospital); and Infectious complications. Secondary outcomes included: length of mechanical ventilation. We assessed the quality of evidence with GRADE.
Main results
We identified 15 trials, with a total of 3129 ICU participants from university‐associated hospitals in the USA, Colombia, Saudi Arabia, Canada, Greece, Germany and Iran. There are two ongoing studies. Participants suffered from medical and surgical conditions, with a variety of inclusion criteria. Four studies used parenteral nutrition and nine studies used only enteral nutrition; it was unclear whether the remaining two used parenteral nutrition. Most of them could not achieve the proposed caloric targets, resulting in small differences in the administered calories between intervention and control groups. Most studies were funded by the US government or non‐governmental associations, but three studies received funding from industry. Five studies did not specify their funding sources.
The included studies suffered from important clinical and statistical heterogeneity. This heterogeneity did not allow us to report pooled estimates of the primary and secondary outcomes, so we have described them narratively.
When comparing hypocaloric nutrition support with a control nutrition support, for hospital mortality (9 studies, 1775 participants), the risk ratios ranged from 0.23 to 5.54; for ICU mortality (4 studies, 1291 participants) the risk ratios ranged from 0.81 to 5.54, and for mortality at 30 days (7 studies, 2611 participants) the risk ratios ranged from 0.79 to 3.00. Most of these estimates included the null value. The quality of the evidence was very low due to unclear or high risk of bias, inconsistency and imprecision.
Participants who received hypocaloric nutrition support compared to control nutrition support had a range of mean hospital lengths of stay of 15.70 days lower to 10.70 days higher (10 studies, 1677 participants), a range of mean ICU lengths of stay 11.00 days lower to 5.40 days higher (11 studies, 2942 participants) and a range of mean lengths of mechanical ventilation of 13.20 days lower to 8.36 days higher (12 studies, 3000 participants). The quality of the evidence for this outcome was very low due to unclear or high risk of bias in most studies, inconsistency and imprecision.
The risk ratios for infectious complications (10 studies, 2804 participants) of each individual study ranged from 0.54 to 2.54. The quality of the evidence for this outcome was very low due to unclear or high risk of bias, inconsistency and imprecision
We were not able to explain the causes of the observed heterogeneity using subgroup and sensitivity analyses or meta‐regression.
Authors' conclusions
The included studies had substantial clinical heterogeneity. We found very low‐quality evidence about the effects of prescribed hypocaloric nutrition support on mortality in hospital, in the ICU and at 30 days, as well as in length of hospital and ICU stay, infectious complications and the length of mechanical ventilation. For these outcomes there is uncertainty about the effects of prescribed hypocaloric nutrition, since the range of estimates includes both appreciable benefits and harms.
Given these limitations, results must be interpreted with caution in the clinical field, considering the unclear balance of the risks and harms of this intervention. Future research addressing the clinical heterogeneity of participants and interventions, study limitations and sample size could clarify the effects of this intervention.
Plain language summary
Does the prescription of low‐calorie (hypocaloric) nutrition support improve the recovery of critically‐ill adult patients?
Review question
Does low‐calorie nutrition delivered into the stomach or small intestine (enteral), or into a vein (parenteral) improve clinical outcomes in critically‐ill adults admitted to an intensive care unit (ICU), when compared with standard calorie nutrition support?
The main outcomes were death (in the hospital, in the ICU and at 30 days); length of ICU and hospital stay; infectious complications and length of time the person was mechanically ventilated (a machine used in ICU to help a person breath) .
Background
Critically‐ill people experience major metabolic changes (one chemical is transformed through a series of steps into another chemical) during injury or sepsis (a life‐threatening condition in which the body's response to infection causes injury to its own organs). They receive nutritional support to prevent or minimize some adverse effects. Nevertheless, both overfeeding and starvation can be harmful.
There is currently no agreement about the amount of calories we should give to these critically‐ill people. Normal caloric feeding provides the estimated caloric needs. Hypocaloric feeding provides an intentionally lower amount of calories.
Study characteristics
We included 15 trials with 3129 ICU surgical or medical participants from academic hospitals. Four studies used parenteral nutrition and nine studies used only enteral nutrition. The route was unclear in the remaining two studies. While the studies planned to give different amounts of calories in the experimental and control groups, the actual difference in calories was small. Most studies were funded by the US government or non‐governmental associations, but three studies received funding from the industry. Five studies did not state how they were funded.
Key results
The differences in the type of nutrition and type of participants across studies did not allow us to combine study results, so we describe the range of results across the individual studies.
The number of deaths at the hospital, in the ICU and at 30 days in those who received low‐calorie nutrition was similar to those in the control group. The length of hospital and ICU stay and the length of mechanical ventilation varied across studies, sometimes shorter and sometimes longer when compared to the control group. The number of infections also varied across studies. We tried to analyse subgroups of participants in order to clarify this variation, but the results were not consistent.
Quality of evidence
The overall quality of evidence for each outcome according to GRADE classification varied from very low to low. This was due to problems in the design and conduct of the studies, the variation in the study results (inconsistency between studies) and the wide range of possible results (imprecision).
Summary of findings
Summary of findings for the main comparison. Hypocaloric nutrition compared to control for critically‐ill adults.
| Hypocaloric nutrition compared to control for critically‐ill adults | |||
|
Patient or population: critically‐ill adults
Settings: Hospitals (intensive care units), eight in USA, two in Colombia, one in Saudi Arabia and Canada, and one each in Saudi Arabia, Germany, Greece and Iran
Intervention: hypocaloric nutrition Comparison: control nutritional support with a higher caloric intake than the 'intervention' group | |||
| Outcomes | Effect estimate (range of results of individual studies) | N of Participants (studies) | Quality of the evidence (GRADE) |
| Mortality in hospital: death occurring during the hospital stay | Range of risk ratios from 0.23 to 5.54a | 1775 (9 studies) |
⊕⊕⊝⊝ very lowb,c,d |
| Mortality in ICU: death occurred during the ICU stay | Range of risk ratios from 0.81 to 5.54a | 1291 (4 studies) | ⊕⊕⊝⊝ very lowb,c,d |
| Mortality at 30 days: 28 to 30 days all‐cause mortality | Range of risk ratios from 0.79 to 3.00a | 2611 (7 studies) | ⊕⊕⊝⊝ very lowb,c,d |
| Length of hospital stay: days stayed in the hospital | Range of length of hospital stay from 15.70 days lower to 10.70 days highera | 1677 (10 studies) | ⊕⊝⊝⊝ very lowb,c,e |
| Length of ICU stay: days stayed in the ICU | Range of length of ICU stay from 11.00 days lower to 5.40 days highera | 2942 (11 studies) | ⊕⊝⊝⊝ very lowb,c,e |
| Infectious complications: events of any type of infectious complications occurred during the hospital stay, registered by the study authors according to their diagnostic criteria of infections. | Range of risk ratios from 0.54 to 2.54a | 2804 (10 studies) | ⊕⊝⊝⊝ very lowb,c,e |
| Length of mechanical ventilation: days on mechanical ventilation during ICU stay | Range of mean differences: 13.20 days lower to 8.36 days highera | 3000 (12 studies) |
⊕⊝⊝⊝ very lowb,c,e |
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||
aResults were not combined due to clinical heterogeneity. bDowngraded one level due to risk of bias: most studies had unclear or high risk of bias. cDowngraded one level due to imprecision issues: very wide confidence intervals. dDowngraded one level due to inconsistency: wide variance of point estimates across studies. eDowngraded one level due to inconsistency: high statistical heterogeneity I2 > 50%.
Background
Description of the condition
Most critically‐ill people treated for injury or sepsis have some degree of hypermetabolism and hypercatabolism and are also unable to feed themselves. For these reasons, it was recommended to provide them with nutrition support by enteral or parenteral routes, in order to prevent or minimize depletion of protein and caloric stores; to enhance protein synthesis; and to avoid deficiencies in essential and semi‐essential nutrients (Cerra 1997). However, there are several aspects of nutrition support for the critically‐ill that are still under debate, such as: the time at which to initiate nutrition support; the route (enteral, parenteral or combined); the caloric and protein requirements; the amount and type of protein to give; the composition of lipids; the supplementation of some amino acids and micronutrients; and the occurrence and type of some complications. Several of these topics were recently discussed (Berger 2012; Biolo 2002; Bost 2014; Heyland 2003; Kreymann 2006;Preiser 2015; Wischmeyer 2012; Wischmeyer 2013), and some of these aspects are included in Cochrane Reviews on adults (Alkhawaja 2015; Allingstrup 2015; Fuentes Padilla 2016; Lewis 2016; Tao 2014), and children (Joffe 2016), as well as in a Cochrane protocol (Dushianthan 2016). This current review focuses on the prescription of hypocaloric versus normocaloric feeding debate in nutrition support for critically‐ill adults.
During the 1970s, the proposed goal of nutrition support was to provide sufficient calories to match the measured increased resting energy expenditure (hypermetabolism) in order to prevent protein depletion. As indirect calorimetry (the gold standard) is not available in most intensive care units (ICUs) or it is not possible to perform it in certain patients, it is usual to estimate the daily caloric requirements using formulae. For years the most frequently used one was the Long equation (resting metabolic expenditure calculated by the Harris‐Benedict equation with the addition of an injury factor and an activity factor; Long 1979). This approach often led to overestimation of caloric requirements (compared with the values obtained by indirect calorimetry), mainly in ventilated and sedated patients (McClave 1992). It also induced some degree of overfeeding with nutrition support, which was associated with several metabolic complications (Klein 1998), such as hypertriglyceridaemia, increased production of CO2, hepatic steatosis and hyperglycaemia, which also behaves as an independent factor for increased mortality in critically‐ill patients (Badawi 2012; Krinsley 2003).
It is currently known that the caloric requirements for nutrition support of a critically‐ill person could differ from the estimated resting or total energy expenditure (Reid 2004). We must take into account variability due to several factors: the presence of injury or sepsis (type, severity and metabolic response of the host) (Hoffer 2003); the time course of the disease or the elapsed time in the ICU (Monk 1996; Uehara 1999); current ICU care and treatments (Boulanger 1994); the nutrition state or the fat‐free mass (Zauner 2006); the complications and some factors associated with the disease states (Magnuson 2011; Stahel 2010), and comorbidities. This variability contributes to the difficulty in estimating energy needs for the nutrition support of these patients (Frankenfield 2011). The use of predictive equations (Cooney 2012) could be one of the causes of underfeeding or overfeeding in some critically‐ill people (Reid 2006).
There is consensus about some aspects of caloric and protein requirements for nutrition support of the critically‐ill ventilated person: a) the degree of hypermetabolism due to injury or sepsis is lower than that reported at the beginning of the 1970s (Liggett 1990), particularly during the first days in the ICU (Biolo 2002; Heyland 2003; Kreymann 2006); b) positive or neutral energy balance failed to decrease the protein catabolic rate or nitrogen loss and did not prevent negative nitrogen balance and protein depletion (Frankenfield 1997; Plank 2003); c) positive energy balance is associated with increased fat mass, without changes in lean body mass (Hart 2002; Streat 1987); d) the main determinant of a positive, or less negative, nitrogen balance during nutrition support seems to be the nitrogen intake (Iapichino 1984; Weijs 2013); e) nutrition support did not modify the rate of protein catabolism, but was able to preserve some nitrogen loss (less negative nitrogen balance) by promoting whole‐body protein synthesis, with protein intake of up to 1.5 g/kg/day (Shaw 1987).
The well‐known clinical guidelines for the nutrition support of critically‐ill people (ASPEN / SCCM guidelines 2016; ASPEN / SCCM guidelines 2009; ESPEN guidelines 2009) sometimes disagreed with each other, and in the literature there are some open debates. For example, when and how to initiate the nutrition support; when to begin lipid administration by the parenteral route and the type of lipids to be used; the role and timing for supplemental parenteral nutrition; the amount of protein or the non‐protein calories/nitrogen ratio to prescribe; the dose and type of supplemental trace elements and antioxidant vitamins; the best way to estimate the caloric requirements; if caloric provision should be optimized to prevent a caloric deficit during the first days of ICU in order to minimize the initial or delayed complications associated with undernutrition (Dvir 2006; Heidegger 2013; Rubinson 2004; Wischmeyer 2013), or if it is better to give hypocaloric nutrition during the first days of intense inflammatory response (and metabolic changes) induced by injury or sepsis (Berger 2007; Berger 2012; Casaer 2014; Cooney 2012; Dickerson 2011; Kreymann 2012; Singer 2010; Weijs 2013; Wischmeyer 2012). This review focuses only on the clinical results of providing hypocaloric nutrition support compared to normocaloric nutrition to critically‐ill adults.
Description of the intervention
More than 20 years ago, Zaloga 1994 proposed a short period of dietary restriction during the first few days of acute injury or sepsis, originally designated "permissive underfeeding" and later "hypocaloric nutrition support" (Patiño 1999). The provision of hypocaloric nutrition support with high‐protein content was successfully used in a group of obese stressed patients (Dickerson 1986). This approach was first reviewed (Kushner 2011), suggested by a group of experts for critically‐ill people (McClave 2011), and recommended in some clinical guidelines (ASPEN guidelines 2013; ASPEN / SCCM guidelines 2016). The use of hypocaloric nutrition support in critically‐ill people, mainly during the first days of ICU stay, has been frequently mentioned in the literature; some evidence and opinions were reported in several narrative reviews ( Boitano 2006; Berger 2007; Jeejeebhoy 2004; Malone 2007; Stapleton 2007).
How the intervention might work
Severely critically‐ill people experiencing major metabolic changes during the acute phase of systemic inflammatory response induced by injury or sepsis could benefit from this approach. This may be explained by: avoidance of the well‐known deleterious effects of overfeeding or the consequences of starvation; diminishing metabolic disturbances, especially hyperglycaemia, and the level of inflammatory cytokines. In certain animal models, hypocaloric nutrition during acute stress seemed to lower morbidity and mortality. This could also be possible in critically‐ill people, but the available data are not conclusive about the potential benefits of hypocaloric feeding. On the contrary, there is some evidence that underfeeding could be associated with complications and worse outcomes for critically‐ill people (Dvir 2006; Villet 2005), and that the effect of hypocaloric nutrition support could be different in malnourished and well‐nourished people (Braunschweig 2001). The possible role of starvation‐induced autophagy is currently under consideration (Marik 2016a).
Why it is important to do this review
We do not so far have conclusive evidence for how many calories we should give to critically‐ill people in order to improve outcomes and diminish complications. However, today we certainly know that caloric requirements are rather less than that proposed in the 1970s or 1980s (Krishnan 2003; Rubinson 2004). Currently, in several countries there are intensive care or nutrition support specialists providing hypocaloric nutrition support to most of their critically‐ill patients during the first few days of illness, or tolerating the administration of less than prescribed enteral nutrition (fewer calories than the estimated ones) for their patients. This is based more on observational evidence or expert opinions than on scientific data.
Several authors consider it important to optimize the energy provision, targeting measured or estimated requirements, in order to avoid caloric deficits ("caloric debt") during the first days of ICU stay (Faysy 2008; Singer 2010; Singer 2011; Wischmeyer 2013; Wischmeyer 2015), or, even more importantly, to also target the protein supply (Weijs 2012; Weijs 2014; Hoffer 2012; Nicolo 2016), or give some supplementary protein (Alberda 2009).
Due to these unanswered questions, the controversial data and the different interpretations of it, it is necessary to perform systematic reviews of each contentious topic and to analyse the clinical significance of each nutritional approach. We have therefore conducted this systematic review to explore the effects of prescribed hypocaloric enteral or parenteral nutrition on clinical and metabolic outcomes in critically‐ill adults.
Objectives
To assess the effects of prescribed hypocaloric nutrition support in comparison with standard nutrition support for critically‐ill adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized controlled trials. We considered the inclusion of quasi‐randomized controlled trials in order to enlarge the evidence about the efficacy and safety of hypocaloric nutrition support (Schneider 2007; Shadish 2002).
Types of participants
We included all adult participants (aged 18 years or more) hospitalized for different diseases and severity at medical, surgical or disease‐specific (burns, trauma, neurological, etc.) intensive care units (ICUs) and requiring any type of nutrition support.
Exclusion criteria: none.
Types of interventions
The experimental intervention evaluated was: hypocaloric nutrition support with fewer total calories than measured resting energy expenditure (REE) by indirect calorimetry or, if not measured, less than 25 kcal/kg/day. This could be done through restricted doses of carbohydrates or lipids, or both, with either normal or increased protein dose. The control intervention was:
Normo‐ or hypercaloric nutrition support: equal to or more than the measured REE or than 25 kcal/kg/day (with the same characteristics as above); or
No nutrition support at all: fasting or dextrose solutions.
We evaluated the results of trials designed to compare prescribed hypocaloric enteral or parenteral nutrition support (or permissive underfeeding) with standard nutrition support, or with no nutrition, even if those trials did not reach their caloric goals in the intervention or control groups (intention‐to‐treat analysis). Furthermore, we did not include trials that planned to provide full nutrition support but resulted in unintended hypocaloric provision (for any reason).
Types of outcome measures
Primary outcomes
The primary outcomes were the following clinical outcomes:
Mortality. Death occurring during the ICU and hospital stay, or 28‐ to 30‐day all‐cause mortality.
Length of stay. Days stayed in the ICU and in the hospital.
Infectious complications. Events of any type of infectious complications occurring during the hospital stay, registered by the study authors according to their diagnostic criteria of infections.
Secondary outcomes
The secondary outcomes were one or more of the following outcomes:
Length of mechanical ventilation. Days on mechanical ventilation during ICU stay.
Non‐infectious complications. Events of any non‐infectious complication during the hospital stay, potentially associated with the nutrition status or the nutrition support, according the criteria of the study authors (for example: wound dehiscence, decubitus ulcers, etc.)
Carbohydrate metabolic outcomes. Events of hyperglycaemia (glycaemia higher than 150 mg/dl) during ICU stay. Events of hypoglycaemia (glycaemia lower than 70 mg/dl) during ICU stay.
Lipid metabolic outcomes. Events of hypertriglyceridaemia (higher than 200 mg/dl) or any lipid metabolic complication associated with the nutrition support according to the criteria of the study authors.
Protein metabolic outcomes. Nitrogen balance (positive or negative in grams/day) or any protein metabolic complication associated with the nutrition support according to the criteria of the study authors.
Nutrition status or clinical condition at ICU discharge. Nutrition or functional evaluation, made at the time of ICU discharge with any method of assessment used by the study authors.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library Issue 5, 2017); MEDLINE/Ovid (1946 to 20 June 2017); Embase (1980 to 20 June 2017), and LILACS (1992 to 20 June 2017). We developed a specific strategy for each database (see Appendix 1 for CENTRAL, Appendix 2 for MEDLINE, Appendix 3 for Embase and Appendix 4 for LILACS).
We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy phases one and two, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The filter used to identify randomized controlled trials (RCTs) in the search strategy for MEDLINE was from Glanville 2006. For Embase we applied the trial filter for therapy maximizing sensitivity developed by Health Information Research Unit (HIRU) at McMaster University: hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx).
We did not apply restrictions by language or by publication status.
We also searched (up to 20 June 2017) for relevant ongoing trials in specific trial registries:
ClinicalTrials.Gov: clinicaltrials.gov/
International Clinical Trials Registry Platform: apps.who.int/trialsearch/
ISRCTN Registry: www.isrctn.com/
Searching other resources
We searched the Conference Proceedings of the annual congresses of the following four societies, as published in their respective journals, in order to find papers presented at different meetings:
American Society for Parenteral and Enteral Nutrition (ASPEN), through the Journal of Parenteral and Enteral Nutrition (1990 to 30 June 2017).
European Society for Clinical Nutrition and Metabolism (ESPEN), through the journal Clinical Nutrition (1990 to 30 June 2017).
Society of Critical Care Medicine (SCCM), through the journal Critical Care Medicine (1990 to 30 June 2017).
European Society of Intensive Care Medicine (ESICM), through the journal Intensive Care Medicine (1997 to 30 June 2017).
We also handsearched the original papers published in the following journals:
Journal of Parenteral and Enteral Nutrition (1990 to 30 June, 2017).
Clinical Nutrition (1990 to 30 June 2017).
Nutrition (1990 to 30 June 2017).
Nutrition Clinique et Métabolisme (1994 to 30 June 2017).
We also checked the reference list and citations of the relevant articles and reviews related to hypocaloric feeding and to caloric and protein requirements of critically‐ill people (1970 to 30 June 2017).
Correspondence
We contacted main authors of relevant trials and reviews to identify any additional studies, and relevant pharmaceutical companies for published and unpublished reports.
Data collection and analysis
Selection of studies
Three review authors (MP, ACr and CL) independently scanned the titles and abstracts of reports identified by electronic searching, manual searching, snowballing and by contacts with clinical experts and the pharmaceutical industry. We retrieved and evaluated potentially relevant studies, chosen by at least one review author, in full‐text versions. These review authors independently selected trials that met the inclusion criteria using a checklist designed in advance for that purpose. We resolved any disagreement through consultation with a fourth review author (GP). We rejected articles at the initial screening only if we could determine from the title and abstract that the study was not a report of a randomized or quasi‐randomized controlled trial; or that it did not address enteral and/or parenteral nutrition in critically‐ill adults. When we could not reject a study with certainty, we obtained the full text of the article for further evaluation.
Data extraction and management
Two review authors (ACr and CL) independently extracted data using a standardized checklist. We registered it in the data extraction form. We resolved any disagreement through consultation with a third review author (MP).
Assessment of risk of bias in included studies
Two review authors (GP and CL) independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement through discussion and consultation with a third assessor (ACi).
(1) Sequence generation (checking for possible selection bias)
We looked for the description of methods used in each included study to generate the allocation sequence, and assessed if they were adequate to produce comparable groups (unbiased selection). We classified methods as being at low, high or unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We looked for the description of methods used in each included study to conceal the allocation sequence and assessed if they were adequate to avoid the intervention allocation being foreseen or changed. We classified methods as being at low, high or unclear risk of bias.
(3) Blinding (checking for possible performance bias)
We looked for the description of methods used, if any, in each included study to blind study participants and personnel from knowledge of which intervention a participant received. We also considered partial blinding (e.g. where it had not been feasible to blind participants but outcome assessment was carried out without knowledge of group assignment). Where blinding was not possible we assessed whether the lack of blinding was likely to have introduced bias. We classified methods as being at low, high or unclear risk of bias.
We also assessed any information about whether the intended blinding was effective.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We looked for the completeness of outcome data in each included study, for each main outcome, including attrition and exclusions from the analysis. We assessed whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition/exclusion, and any re‐inclusions in analyses. We classified methods as being at low, high or unclear risk of bias.
(5) Selective reporting bias
We assessed this by comparing the study protocol, when available, and all of the study's pre‐specified outcomes that are of interest in the review. We classified methods as being at low, high or unclear risk of bias.
(6) Other sources of bias
We looked for any important concerns about other possible sources of bias in each included study. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent? Has the researcher gained sponsorship from agencies with a vested interest in the findings? We assessed whether each study was free of other problems that could put it at risk of bias. We classified methods as being at low, high or unclear risk of bias.
(7) Overall risk of bias
We made an explicit judgement about whether studies were at an overall high, low or unclear risk of bias, according to the following criteria: low risk if all six 'Risk of bias' domains were rated low for that study; unclear risk if at least one domain was rated at unclear risk; high risk if at least one domain was rated at high risk of bias.
We assessed the likely magnitude and direction of identified risks of bias, and whether we considered this could have a significant effect on the findings. We explored the impact of the level of bias through sensitivity analyses.
Measures of treatment effect
For dichotomous outcomes we calculated risk ratios (RRs) and 95% confidence intervals (95% CIs). We calculated the mean difference (MD) for continuous outcomes measured using the same scales, or the standardized mean difference (SMD) if they used different scales.
Unit of analysis issues
The unit of analysis was the participant in each trial arm. All included studies had a parallel‐group design, so there was no need for adjustment for a cluster or cross‐over design.
Dealing with missing data
We obtained missing data from study authors, if feasible, and performed intention‐to‐treat analyses if data were available; otherwise, we performed available‐case analyses. We investigated attrition rates, such as dropouts, losses to follow‐up and withdrawals, and we critically appraised issues of missing data. We did not impute missing data.
We contacted by email the first authors of the following included and ongoing trials:
Ahrens 2005. The first author sent the estimates of continuous outcomes as means and standard deviations for length of hospital and ICU stay and for length of mechanical ventilation.
Arabi 2015 The first author sent us the length of hospital and ICU stay and of mechanical ventilation in means and standard deviation.
Charles 2014 The first author sent us mean and standard deviation of days on mechanical ventilation, and additional information to complete the 'Risk of bias' table.
NHLBI 2012 and Rice 2011 The corresponding author sent us all the information required to render their data compatible, and also some additional unpublished results: length of hospital stay, ICU and mechanical ventilation in means and standard deviations, number of participants with infections and hyperglycaemic episodes, and amount of calories received by participants in both groups.
Ochoa 2017 We contacted the lead author. He replied that he would try to recover and send the requested study results, but we have not received them yet.
Petros 2016. The study was initially published only in abstract form. The first author sent us all the information we required from its finished but unpublished pilot study. The full paper of the pilot trial was recently published (Petros 2016).
Rugeles 2013 We initially identified the study before publication. The first author sent us the full paper ready to be published in advance of publication, and some additional considerations to better assess the risk of bias and the number of participants with hyperglycaemia.
Rugeles 2016 The first author sent us the full paper of this clinical trial before it was indexed in MEDLINE (It was registered in clinicaltrials.gov as NCT02577211). The second author gave us the means and standard deviations for length of ICU stay and of mechanical ventilation, and also some additional information to complete the 'Risk of bias' table.
Theodorakopoulou 2016 We did not received an answer to several questions about the abstract of the trial.
Assessment of heterogeneity
In cases of statistical heterogeneity, i.e. a Chi2 test with a P value less than 0.10 or an I2 greater than 30% (Higgins 2002), we examined the potential causes of the heterogeneity by prespecified subgroup and sensitivity analyses. We followed the suggestions in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions and interpreted and rated heterogeneity according to the I2 value as follows: 'not important' if 40% or less, 'moderate' with I2 between 30% and 60%, 'substantial' with I2 between 50% and 90%, and 'considerable' if I2 is higher than 75% (see Data synthesis section for levels of I2 that allowed us to report numerical results or not) (Higgins 2002).
We assessed clinical heterogeneity by considering different parameters of clinical practice. We considered the objectives and methodology of the trials, the type/severity of the participants (surgical, medical and others), and several aspects of the nutrition support, such as time to initiation, route, duration and amount of calories and protein received by the intervention and the control groups. The most important parameters of this pragmatic and subjective assessment were the amount of calories received by each group of participants, and the difference in calories received by the intervention and control groups. We defined clinical heterogeneity as 'low', 'moderate' or 'important', according to a clinical judgement about the possibility of comparing trials with small, moderate or important differences according to the above parameters.
Some of the parameters we used to define clinical heterogeneity were also used for subgroup and sensitivity analyses, to investigate the heterogeneity (Subgroup analysis and investigation of heterogeneity). In addition, where we identified important statistical or clinical heterogeneity we performed meta‐regression in order to explore the possible causes.
Assessment of reporting biases
The search strategy included consultation with leaders in the field, the pharmaceutical industry, conference and congress proceedings and snowballing techniques to maximize the possibility of finding unpublished studies. We performed funnel plot analyses when eight or more studies were included in each outcome analysis.
Data synthesis
We first reviewed the data from included studies qualitatively. Then, if possible, we combined them quantitatively by population, intervention and outcome, using Cochrane statistical software (Review Manager 2014). We based the quantitative analyses of outcomes on intention‐to‐treat (ITT) results.
In case of unimportant statistical heterogeneity between studies (I2 of 30% or less), we performed meta‐analyses using the fixed‐effect model. In case of I2 between 30% and 50%, we used a random‐effects model to produce more conservative confidence intervals. If the I2 was above 50%, we did not report pooled estimates of the meta‐analysis. In cases of important clinical heterogeneity we did not report pooled estimates of the meta‐analyses, even in the absence of statistical heterogeneity.
In the subgroup analyses we reported results using a random‐effects model if one or more of the subgroups had an I2 between 30% and 50%, for a more conservative analysis. If the total statistical heterogeneity test showed I2 above 50% or if the clinical heterogeneity was important, we did not report summary estimates of the meta‐analysis.
In all cases where it was not possible to perform or report total or subtotal analyses, we produced a short descriptive comment about the results of the studies for each outcome.
Subgroup analysis and investigation of heterogeneity
The prespecified possible causes of heterogeneity were the following:
Age: 18 to 65 years old, 66 to 75 years old, and more than 75 years old.
Primary disease of the participants: major surgery, trauma, sepsis, medical diseases.
Disease severity with or without organ failure: acute physiology and chronic health evaluation II (APACHE II); simplified acute physiology score II (SAPS II); sequential organ failure assessment (SOFA); multiple organ dysfunction score (MODS); logistic organ dysfunction system (LODS), other scores.
With or without comorbidities: assessed by the Charlson score or similar.
Nutrition status: obese, malnourished or well‐nourished.
Level of inflammation (by determination of plasma level of C reactive protein or other acute phase reactants) or hypermetabolism (by indirect calorimetry) or hypercatabolism (by measured or estimated total urinary nitrogen).
Amount of calories in the intervention group: low versus very low amount of calories.
After retrieval of studies, we acknowledged that there were important differences among them that we should consider in the assessment of clinical heterogeneity. We therefore added other non‐prespecified explorations of heterogeneity:
Subgroup analysis by route of nutrition support: enteral or parenteral nutrition.
Meta‐regressions (using STATA 14.1; Stata), to explore the effect of the following variables on the main outcomes: type of participants, the calories received, and the difference in calories received by the intervention and control groups.
To investigate differences between two or more subgroups we used the test for heterogeneity across subgroup results rather than across individual study results. We also calculated an I2 statistic for subgroup differences (Higgins 2011). We considered a P value less than 0.05 as statistically significant.
Sensitivity analysis
Trial design: we performed three prespecified sensitivity analyses: 1) excluding the quasi‐randomized trials: 2) excluding those studies with at least one high 'Risk of bias' criterion; and 3) in all the outcomes performed with the fixed‐effect model, we also conducted the analysis with the random‐effects model.
We undertook two more non‐prespecified sensitivity analyses, excluding trials with a primary goal different from prescribed hypocaloric enteral or parenteral nutrition.
'Summary of findings' table and GRADE
We present the overall quality of the evidence for selected outcomes using the GRADE approach (Schünemann 2011). This approach takes into account five criteria:
Risk of bias
Inconsistency
Imprecision
Directness
Publication bias
For each comparison, two review authors (JVAF, ACi) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low', using GRADEpro GDT software. We resolved any discrepancies by consensus, or, if needed, by arbitration by a third review author (MP).
We present the results for the comparison of hypocaloric nutrition versus control for the following outcomes:
Mortality in hospital
Mortality in ICU
Mortality at 30 days
Length of hospital stay (days)
Length of ICU stay (days)
Infectious complications
Length of mechanical ventilation (days)
Since meta‐analysis was not possible in most cases due to both statistical and clinical heterogeneity, we present the range of effect estimates of the individual studies along with the number of participants, number of included studies and confidence in the effect estimates (Guyatt 2011; Schünemann 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search strategy from electronic databases, updated to 20 June 2017, retrieved 5055 records. We found four more studies by handsearching. One full paper was sent by the first author before it was indexed in MEDLINE (original reference in clinicaltrials.gov, with the identifier NCT02577211). After removing duplicates we screened the remaining 4880 records. After title and abstract evaluation, we eliminated 4840 records as irrelevant. We found two ongoing trials. We assessed 47 full‐text reports for eligibility and excluded 20 of them for different reasons (see Characteristics of excluded studies). We therefore included the remaining 15 studies (18 reports, Characteristics of included studies). See the updated flow diagram of the studies in Figure 1.
1.
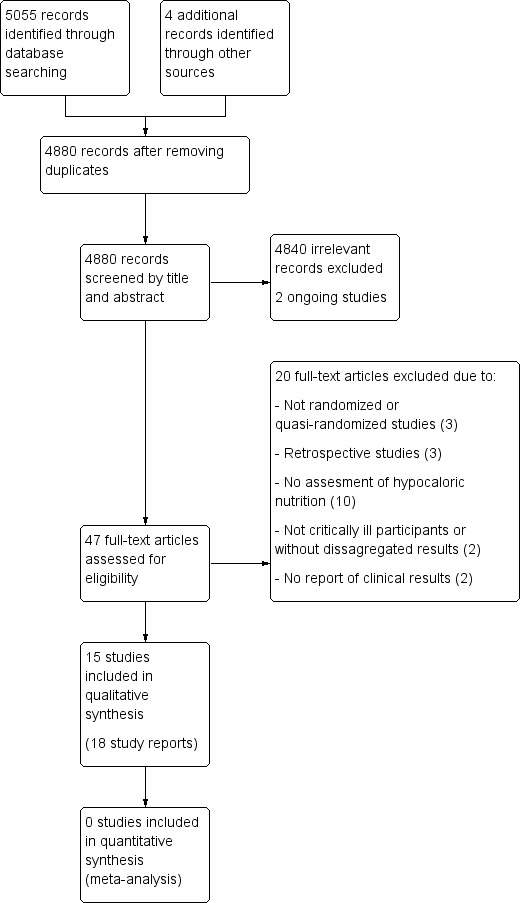
Updated study flow diagram, 20 June 2017
Included studies
Fifteen studies fulfilled the inclusion criteria (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Choban 1997; Ibrahim 2002; McCowen 2000; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016; Theodorakopoulou 2016). Two studies (Norouzy 2013; Theodorakopoulou 2016), were available as abstract only, so some of the study characteristics are missing.
Sample sizes
The total number of ICU participants included was 3129. The range of number of ICU participants included in the trials varied from 13 to 1000.
Setting
Eight included studies were performed in the USA, two in Colombia, one in Saudi Arabia and Canada, and one in each of the following countries: Saudi Arabia, Greece, Germany and Iran. Fourteen of the included studies were RCTs and one was a quasi‐randomized trial (Ibrahim 2002). The setting was mostly university‐associated hospitals.
Participants
Two studies (Ahrens 2005; Choban 1997), reported data of participants in the ICU and on a regular patient care floor. In those studies we only included the data of the ICU participants. The rest of the trials only included ICU participants. The type of ICU was reported in the studies as medical, medical‐surgical or trauma ICU, but after evaluating the reported diagnoses of the included participants, we considered only two categories: surgical participants in five trials (Ahrens 2005; Battistella 1997; Charles 2014; Choban 1997; McCowen 2000;), and medical participants in 10 trials (Arabi 2011; Arabi 2015, Ibrahim 2002; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016; Theodorakopoulou 2016). Some inclusion criteria considered participants with specific conditions, such as hyperglycaemia (Arabi 20111), obesity (Choban 1997), sepsis (Theodorakopoulou 2016), or mechanical ventilation for at least 24 hours (Ibrahim 2002; Rice 2011). In four studies the participants received parenteral nutrition (Ahrens 2005; Battistella 1997; Choban 1997; McCowen 2000). Nine studies used only enteral nutrition (Arabi 2011; Arabi 2015; Ibrahim 2002; NHLBI 2012; Norouzy 2013; Rice 2011; Rugeles 2013; Rugeles 2016; Theodorakopoulou 2016). In two studies the indication was enteral nutrition, but if this was not possible they used parenteral nutrition (Petros 2016; Charles 2014), (see Table 2).
1. Differences in participants, interventions and outcomes across included studies.
| Study ID |
Type of participants Primary outcomes |
Arm | Number of ICU participants | APACHE II score mean ± SD | Route (enteral or parenteral) | Duration of PN or EN (days) |
Mechanical ventilation (% of participants) |
ICU mortality % | Hospital mortality % |
| Ahrens 2005 | Surgical participants with PN requirement Incidence/severity hyperglycaemia and insulin received by the participants |
Hypoc. | 8 (other 12 non‐ICU) | 20 ± 9 | Parenteral | 6 (4 to 10) | 100 | Not reported | Not reported |
| Control | 10 (other 10 non‐ICU) | 19 ± 11 | 7 (5 to 10) | 80 | |||||
| Arabi 2011 | Medical (mainly) and surgical participants with EN. 2 x 2 factorial trial with Intensive Insuline therapy 28 days all‐cause mortality |
Hypoc. | 120 | 25 ± 8 | Enteral | Not reported | 99 | 18 | 30 |
| Control | 120 | 25 ± 8 | 99 | 22 | 43 | ||||
| Arabi 2015 | Critically‐ill participants (75% medical) 90‐day all‐cause mortality |
Hypoc. | 448 | 21 ± 7.9 | Enteral | 9.1 ± 4.6 | 97.3 | 16.1 | 24.2 |
| Control | 446 | 21 ± 8.2 | 9.4 ± 4.4 | 96.2 | 19.1 | 27.6 | |||
| Battistella 1997 | Trauma participants with PN requirement Length of hospital stay, length of stay in the ICU, number of days on mechanical ventilation and infectious complications. |
Hypoc. | 27 | 22 ± 5 | Parenteral | 10 | Not reported | 7.4 | Not reported |
| Control | 30 | 23 ± 6 | 10 | 0 | |||||
| Charles 2014 | Critically‐ill surgical participants Hospital‐acquired infection |
Hypoc. | 41 | 16.6 ± 0.9 | Enteral & parenteral | 12.6 ± 2.8 | 68 | N/A | 7.3 |
| Control | 42 | 17.3 ± 0.8 | 10.4 ± 1.1 | 57 | N/A | 9.5 | |||
| Choban 1997 | Obese participants with PN requirement. Predominantly surgical diseases Achievement of nitrogen balance |
Hypoc. | 6 (other 10 non‐ICU) | 13 ± 5 | Parenteral | 10 ± 3 | Not reported | Not reported | 0 |
| Control | 7 (other 7 non‐ICU) | 15 ± 5 | 11 ± 2 | 28.6 | |||||
| Ibrahim 2002 | Medical ICU participants with EN Incidence of ventilator‐associated pneumonia |
Hypoc. | 75 | 26 ± 8 | Enteral | 5 ± 6 | 100 | Not reported | 27 |
| Control | 75 | 25 ± 8 | 10 ± 12 | 100 | 20 | ||||
| McCowen 2000 | Participants with predominantly surgical diseases requiring PN Glycaemic control and Infections |
Hypoc. | 21 | not reported | Parenteral | ≥ 5 | 50 | 10 | Not reported |
| Control | 19 | not reported | ≥ 5 | 33 | 16 | ||||
| NHLBI 2012 | Acute lung injury predominantly due to medical diseases (61% and 63% of participants) with EN Ventilator‐free days at study day 28 |
Hypoc. | 508 | APACHE III 92 ± 28 | Enteral | 6 | 100 | Not reported | 22.4 |
| Control | 492 | APACHE III 90 ± 27 | Enteral | 6 | 100 | 19.6 | |||
| Norouzy 2013 | Critically‐ill head trauma participants 28 days of all‐cause mortality |
Hypoc. | 30 | Not reported | Enteral | 7 | Not reported | Not reported | 10.7a |
| Control | 30 | 7 | 3.8a | ||||||
| Petros 2016 | Medical ICU with EN and/or PN requirement Glycaemic control and mortality |
Hypoc. | 46 | 31 ± 9 | Enteral & parenteral | 7 | not reported | 22 | 37 |
| Control | 54 | 28 ± 8 | 7 | 22 | 31 | ||||
| Rice 2011 | Acute lung injury, predominantly due to medical diseases with EN Ventilator‐free days at study day 28 |
Hypoc. | 98 | 27 ± 8 | Enteral | 6 ± 4 | 100 | Not reported | 22 |
| Control | 102 | 27 ± 7 | 5 ± 3 | 100 | 20 | ||||
| Rugeles 2013 | Medical ICU participants with EN requirement Change in SOFA score at 48 hours |
Hypoc. | 40 | 14 ± 5 | Enteral | 7 | Not reported | Not reported | Not reported |
| Control | 40 | 15 ± 6 | |||||||
| Rugeles 2016 | Medical ICU participants with EN requirement Change in SOFA score at 48 hours |
Hypoc. | 60 | 13.5 ± 6.4 | Enteral | 7 | Not reported | Not reported | 30a |
| Control | 60 | 13.7 ± 6.8 | 27a | ||||||
| Theodorakopoulou 2016 | Septic, mechanically ventilated critically‐ill participants 28‐day mortality |
Hypocal. | Total sample of 74 participants | Total sample 22 ± 4 |
Enteral | Not reported | Not reported | Not reported | Not reported |
| Control |
a28‐day mortality.
EN = Enteral nutrition; ICU = Intensive Care Unit; N/A: not available; PN = Parenteral nutrition; SOFA = Sequential Organ Failure Assessment
Interventions and study design
All studies had a parallel‐group design, except for two (Arabi 2011; NHLBI 2012) which had a factorial design. These also evaluated, respectively, intensive insulin treatment versus standard insulin treatment, and a nutritional supplement containing omega‐3 fatty acids and antioxidants versus an isocaloric formula. The 15 included studies had a control group with prescribed normocaloric nutrition support. None of the included studies had fasting or only hydration as a comparator. See Table 2; Table 3. Most of the included studies did not achieve the proposed caloric target, with a difference in calories between the intervention and control groups in the range of 2 to 14 kcal/kg/day.
2. Calories and protein received in both study groups.
| Studies | How data was reported |
Hypocaloric (intervention) group |
Control group |
Calories received by the "hypocaloric" intervention group (kcal/kg/day) |
Calories received by the "normocaloric" control group (kcal/kg/day) |
Categories denominated by the calories really received in the intervention and the control groups a |
| Ahrens 2005 | Total calories/kg/day (median (IQ))b | 26.6 (26.2 to 27.5) | 37 (36.0 to 38.4) | 26.60 (median) | 37.00 (median) | Normocaloric vs hypercaloric |
| Protein g/kg/day (mean± SD) | 1.61 ± 0.13 | 1.53 ± 0.26 | ||||
| Arabi 2011 | Calories/day (mean ± SD) | 1066.6 ± 306.1 | 1251.7 ± 432.5 | 13.85 | 16.40 | Hypocaloric vs hypocaloric |
| Protein g/day (mean ± SD) | 47.5 ± 21.2 | 43.6 ± 18.9 | ||||
| Arabi 2015 | Calories/day (mean ± SD) | 835 ± 297 | 1299 ± 2470 | 10.56 | 16.04 | Hypocaloric vs hypocaloric |
| Protein g/day (mean ± SD) | 57 ± 24 | 59 ± 25 | ||||
| Battistella 1997 | Calories/kg ideal body weight/day (mean ± SD) | 27.4 ± 2 | 34.4 ± 2 | 27.4 (of ideal body weight) | 34.4 (of ideal body weight) | Normocaloric vs. normocaloric |
| Protein g/kg ideal body weight/day (mean± SD) | 1.6 ± 0.1 | 1.6 ± 0.2 | ||||
| Charles 2014 | Calories/kg/day (mean ± SD) | 12.3 ± 0.7 | 17.1 ± 1.1 | 12 | 17 | Hypocaloric vs hypocaloric |
| Protein g/kg/day (mean ± SD) | 1.1 ± 0.1 | 1.1 ± 0.1 | ||||
| Choban 1997 | Kcal/kg actual body weight/day (mean ± SD) Kcal/kg ideal body weight/day (mean ± SD) |
8.6 ± 2.39 13.88 ± 2.87 |
17.45 ± 4.06 27.99 ± 3.83 |
14.00 (of ideal body weight) | 28.00 (of ideal body weight) | Hypocaloric vs normocaloric |
| Protein g/kg actual body weight/day (mean ± SD) Protein g/kg ideal body weight/day (mean ± SD) |
1.2 ± 0.2 2.0 ± 0.1 |
1.2 ± 1.2 2.0 ± 0.1 |
||||
| Ibrahim 2002 | Calories/day (mean ± SD) | 126 ± 115 | 474 ± 400 | 1.53 | 5.81 | Very hypocaloric vs very hypocaloric |
| Proteins g/day (mean) (mean ± SD) | 5.3 ± 5.3 | 18.7 ± 15.4 | ||||
| McCowen 2000 | Calories/kg/day (mean ± SD) | 14 ± 3 | 18 ± 4 | 14.30 | 18.40 | Hypocaloric vs hypocaloric |
| Proteins g/kg/day (mean ± SD) | 1.1 ± 0.2 | 1.3 ± 0.2 | ||||
| NHLBI 2012 | Calories/day (mean ± SD) | 399 ± 225 | 1365 ± 596 | 4.64 (estimated by kcal/day divided by weight from the baseline table) |
15.69 (estimated by kcal/day divided by weight from the baseline table) |
Very hypocaloric vs hypocaloric |
| Proteins: information not collected | ‐ | ‐ | ||||
| Norouzy 2013 | Calories/kg/day (mean ± SD) | Not reported | Not reported | N/A | N/A | N/A |
| Protein g/kg/day (mean ± SD) | Not reported | Not reported | ||||
| Petros 2016 | Calories/kg/day (mean ± SD) | 11.3 ± 3.1 | 19.7 ± 5.7 | 11.30 | 19.70 | Hypocaloric vs hypocaloric |
| Protein | Data not reported | Data not reported | ||||
| Rice 2011 | Calories/day (mean ± SD of study days 1 to 5) | 300 ± 149 | 1418 ± 686 | 3.60 | 17.31 | Very hypocaloric vs hypocaloric |
| Proteins g/day (mean ± SD of study days 1 to 5) | 10.9 ± 6.8 | 54.4 ± 33.2 | ||||
| Rugeles 2013 | Calories/kg/day (mean ± SD) | 12 ± 3.9 | 14 ± 6.2 | 12.00 | 14.00 | Hypocaloric vs hypocaloric |
| Protein g/kg/day (mean ± SD) | 1.4 ± 0.44 | 0.76 ± 0.32 | ||||
| Rugeles 2016 | Total calories/kg ideal body weight/day (mean ± SD) | 12.6 ± 3.4 | 20.5 ± 5.1 | 13 | 21 | Hypocaloric vs hypocaloric |
| Protein g/kgIBW/day (mean ± SD) | 1.4 ± 0.4 | 1.4 ± 0.3 | ||||
| Theodorakopoulou 2016 | Calories/day (mean ± SD) | 962 ± 314 | 1308 ± 513 | Not reported Estimatedc 16.63 kcal/kg/day |
Not reported Estimatedc 22.62 kcal/kg/day |
Estimatedc Hypocaloric vs normocaloric |
| Protein g/day (mean ± SD) |
57 ± 24 | 59 ± 25 | Not reported Estimatedc 0.99 g/kg/day |
Not reported Estimatedc 1.02 g/kg/day |
aCategories denominated by the amount of calories really received by both study groups, according to the following: very hypocaloric = < 10 kcal/kg/day; hypocaloric = ≥ 10 to < 25 kcal/kg/day; normocaloric = ≥ 25 to < 35 kcal/kg/day; hypercaloric = ≥ 35 kcal/kg/day. bIQ: interquartile range ‐ Median total calories received by all 20 participants (ICU and non‐ICU participants) in each group (the total calories received by the 8 and 10 ICU participants in each group were not reported). cNot reported in the abstract. The numbers are a crude estimation of kcal and grams of protein/kg/day from the whole sample data of height and BMI.
BMI = Body Mass Index; g = gram; ICU = Intensive Care Unit; kcal = kilocalories; N/A: not available; SD = standard deviation; vs = versus
Outcomes
For full details of the reported outcomes see Table 4 and Characteristics of included studies.
3. Main outcomes in individual studies ordered by the magnitude of the differences in calories received between the control and hypocaloric groups.
| Study |
Difference in calories between groups (kcal/kg/day) |
Hospital mortality (%) IG vs CG |
ICU mortality (%) IG vs CG |
Mortality at 30 days (%) IG vs CG |
Infectious complications (%) IG vs CG |
Length of hospital stay (days)a IG vs CG |
ICU length of stay (days)a IG vs CG |
Length of mechanical ventilation (days)a IG vs CG |
Categories denominated by the calories really received in the intervention and the control groupsb |
| Rugeles 2013 | 2.00 | N/A | N/A | N/A | N/A | N/A | 9.5 vs 10.4 | 8.5 vs 9.7 | Hypocaloric vs hypocaloric |
| Arabi 2011 | 2.55 | 30% vs 42.5% | 17.5% vs 21.7% | 18.3% vs 23.3% | 44.2% vs 46.7% | 70.2 vs 67.2 | 11.7 vs 14.5 | 10.6 vs 13.2 | Hypocaloric vs hypocaloric |
| McCowen 2000 | 4.10 | 9.5% vs 15.8% | N/A | N/A | 28.6% vs 52.6% | 19 vs 17 | N/A | N/A | Hypocaloric vs hypocaloric |
| Ibrahim 2002 | 4.28 | 26.7% vs 20% | N/A | N/A | 30.7% vs 49.3% | 16.7 vs 22.9 | 9.8 vs 13.6 | 8.1 vs 12.9 | Very hypocaloric vs very hypocaloric |
| Charles 2014 | 5.00 | 7.3% vs 9.5% | N/A | N/A | 56.1% vs 57.1% | 35.2 vs 31 | 16.7 vs 13.6 | 10.8 vs 8.3 | Hypocaloric vs hypocaloric |
| Arabi 2015 | 5.48 | 24.2% vs 27.6% | 16.1% vs 19.1% | 20.8% vs 21.8% | 35.9% vs 37.9% | 48.3 vs 54.4 | 15.8 vs 16.4 | 11.3 vs 13.5 | Hypocaloric vs hypocaloric |
| Battistella 1997 | 7.00 | 7.4% vs 0% | 7.4% vs 0% | N/A | 48.2% vs 73.3% | 27 vs 39 | 18 vs 29 | 15 vs 27 | Normocaloric vs normocaloric |
| Rugeles 2016 | 7.90 | N/A | N/A | 30% vs 26.7% | N/A | N/A | 13.2 vs 13.5 | 10.8 vs 10.8 | Hypocaloric vs hypocaloric |
| Petros 2016 | 8.40 | 37% vs 31.5% | 21.7% vs 22.2% | 39.1% vs 33.3% | 28.3% vs 11.1% | 38.1 vs 27.4 | 22.4 vs 17 | 20.7 vs 12.4 | Hypocaloric vs hypocaloric |
| Ahrens 2005 | 10.40 | N/A | N/A | N/A | 25% vs 10% | 23.4 vs 27.8 | 16.8 vs 23 | 11.1 vs 20.3 | Normocaloric vs hypercaloric |
| NHLBI 2012 | 11.05 | N/A | N/A | 19.5% vs 19.3% | 18.9% vs 16.1% | N/A | 11.5 vs 11 | 10.5 vs 10.2 | Very hypocaloric vs hypocaloric |
| Rice 2011 | 13.71 | 22.4% vs 19.6% | N/A | 22.4% vs 19.6% | 30.6% vs 32.4% | N/A | 8.1 vs 7.6 | 5.7 vs 6.2 | Very hypocaloric vs hypocaloric |
| Choban 1997 | 14.00 | 0% vs 29% | N/A | N/A | N/A | 48 vs 45 | N/A | N/A | Hypocaloric vs normocaloric |
| Norouzy 2013 | N/A | N/A | N/A | 10% vs 3.3% | N/A | 19.9 vs 35.6 | N/A | 4.7 vs 17.9 | N/A |
| Theodorakopoulou 2016 | N/A | N/A | N/A | 18.4% vs 28.9% |
N/A | N/A | N/A | N/A | Hypocaloric vs normocaloric |
aLengths of hospital, ICU stays and of mechanical ventilation presented in mean days. bCategories denominated by the amount of calories really received by both study groups, according to the following: very hypocaloric = < 10 kcal/kg/day; hypocaloric = ≥ 10 to < 25 kcal/kg/day; normocaloric = ≥ 25 to < 35 kcal/kg/day; hypercaloric = ≥ 35 kcal/kg/day.
IG = Intervention Group; CG = Control Group; N/A = Not available; vs = versus
Funding
Studies were funded by non‐governmental associations or foundations (Arabi 2015; Choban 1997; Ibrahim 2002), or by the US government (Battistella 1997; Charles 2014; NHLBI 2012; Rice 2011). Three studies received funding from the industry (Arabi 2011; Rugeles 2013; Rugeles 2016), and five studies did not specified their sources of funding (Ahrens 2005; McCowen 2000; Norouzy 2013; Petros 2016; Theodorakopoulou 2016).
Excluded studies
Out of the 47 full papers we initially assessed for eligibility, we finally excluded 20 for the following reasons:
Three were not randomized or quasi‐randomized controlled trials (Alberda 2009; Arabi 2010; Müller 1995).
Three were retrospective studies (Casadei 2006; Dickerson 2002; Lau 2010).
Ten studies did not assess hypocaloric nutrition (Desachy 2008; Dissanaike 2007; Doig 2013; Fiaccadori 2005; Garrel 1995; Mackenzie 2005; Moses 2009; Rodríguez 2005; Esterle 2010; Wewalka 2010).
Two studies did not include critically‐ill participants or only some of them without disaggregated results (Owais 2014; Schricker 2005).
Two studies did not report clinical results (Berg 2013; Iapichino 1990).
Refer to the Characteristics of excluded studies for further details.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
There are two ongoing studies.
We identified one study (NCT01665664) through clinical trial registries. It is set in Israel, and plans to include adult participants with mechanical ventilation and to compare hypocaloric nutrition to normocaloric nutrition. The study outcomes include all‐cause mortality, ICU mortality, hospital mortality, length of stay (hospital and ICU), length of mechanical ventilation, rate of infections, ventilator‐free days and rate of ventilator‐associated pneumonia. This study was last verified in 2012 in ClinicalTrials.gov and was "not recruiting". We were unsuccessful in contacting the study author.
We identified the second ongoing study in a conference proceeding (Ochoa 2017). This multicentre RCT includes adult, obese, critically‐ill and mechanically ventilated participants requiring enteral nutrition, and compares hypocaloric versus normocaloric enteral nutrition support. The study outcomes include events of hyperglycaemia and hypoglycaemia. Since the abstract included limited information about a preliminary interim analysis we contacted the study author for further information. This study is funded by Nestlé Health Science.
Refer to the Characteristics of ongoing studies
Risk of bias in included studies
We assessed seven domains of possible biases, according to prespecified criteria. Details for each included study are provided in their corresponding 'Risk of bias' table in the Characteristics of included studies. A graphical summary can be seen in Figure 2 and Figure 3 (showing overall percentages of risk level for each domain, and levels of risk of bias for each study, respectively).
2.
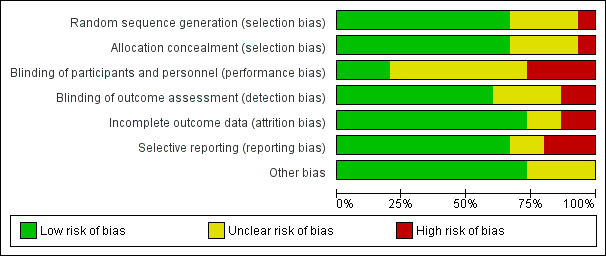
Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies.
3.
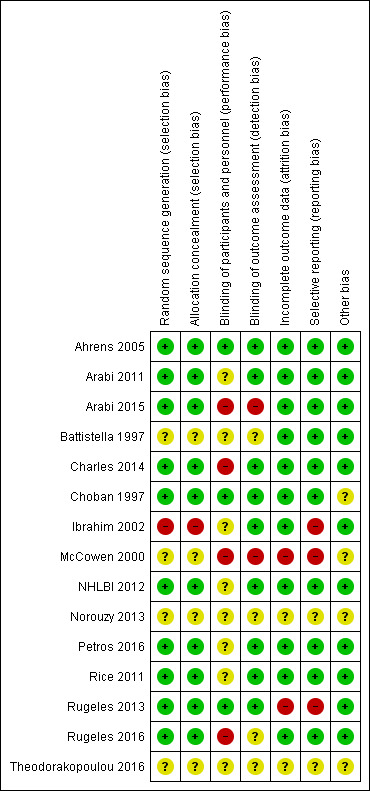
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study. Red colour represents high risk of bias; green, low risk of bias; and yellow, unclear risk of bias.
Overall, only one study had low risk of bias in all the evaluated domains (Ahrens 2005). Six studies had at least one high 'Risk of bias' criterion (Arabi 2015; Charles 2014; Ibrahim 2002; McCowen 2000; Rugeles 2013; Rugeles 2016). The eight remaining studies had at least one unclear 'Risk of bias' criterion. In six of them (Arabi 2011; Battistella 1997, NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011), this was attributable to an unblinded study design. In these cases, although most outcomes were objective or well‐defined with low risk of detection bias, the descriptions of the processes of care by clinical personnel did not have enough detail to assess whether this could have led to a performance bias.
For publication bias, the funnel plots for the outcomes with at least eight trials did not show significant asymmetry.
Allocation
The random sequence generation and the allocation concealment were appropriately performed in 10 studies (Ahrens 2005; Arabi 2011; Arabi 2015; Charles 2014; Choban 1997; NHLBI 2012; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016). One study was quasi‐randomized (Ibrahim 2002), and therefore had a high risk of bias. Four studies (Battistella 1997; McCowen 2000; Norouzy 2013; Theodorakopoulou 2016) did not clearly describe these processes, and we classified them as being at unclear risk of bias.
Blinding
Lack of blinding (open‐label or blinding only participants) was the main driver of the high or unclear risks of bias in most studies (Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; McCowen 2000; NHLBI 2012; Petros 2016; Rice 2011; Norouzy 2013; Rugeles 2016; Theodorakopoulou 2016). The inherent difficulty of blinding a nutrition support strategy in critically‐ill people explains the fact that 80% of the studies could not blind the healthcare personnel. Nevertheless, three studies managed to do it (Ahrens 2005; Choban 1997; Rugeles 2013).
Incomplete outcome data
Only two studies had a high risk of attrition bias (McCowen 2000; Rugeles 2013). They excluded participants because they did not fulfil the prespecified follow‐up criteria. Nevertheless, they should have reported all included participants in an intention‐to‐treat analysis. We classified two studies as being at unclear risk, due to a lack of information in these trials which were only published as conference abstracts (Norouzy 2013; Theodorakopoulou 2016). The other 11 studies reported outcomes for all included participants .
Selective reporting
Three studies had a high risk of reporting bias (Ibrahim 2002; McCowen 2000; Rugeles 2013). For Ibrahim 2002, some prespecified secondary outcomes (duration of mechanical ventilation, need for gastrostomy tube) were not reported. For McCowen 2000, "nitrogen balance was only measured in 12 participants (57%) in the hypocaloric and 10 (53%) of the control group, usually because of an error during collection". Rugeles 2013 did not report mortality. The authors justified this by explaining that they excluded participants who did not fulfil the 96 hours of enteral nutrition requirement. They therefore did not report mortality because this result would have been biased (they only measured mortality in participants who completed the 96 hours). A better approach would have been to perform an intention‐to‐treat analysis and also to report premature deaths. In Norouzy 2013 and Theodorakopoulou 2016, the information was not provided, so we classified them as being at unclear risk. We rated all the other studies at low risk of reporting bias.
Other potential sources of bias
Choban 1997 was partially funded by a corporation. Since we could not guarantee that this sponsorship had no material interest in the findings of the study, we classified it as being at unclear risk of bias.
The lack of detail in the description of the methods section of McCowen 2000 could not warrant a 'low risk' rating for Other sources of bias. We therefore classified it as being at unclear risk of bias. Due to the lack of information in the abstracts of Norouzy 2013 and Theodorakopoulou 2016 we also classified them as being at unclear risk of Other potential sources of bias.
Effects of interventions
See: Table 1
The 15 included studies showed significant clinical heterogeneity between them, mainly related to the amount of calories provided to the intervention and control groups (Table 3), and also to some differences in trials methodology, the target participants and the feeding strategies. As stated in Assessment of heterogeneity and in Data synthesis, the degree of clinical or statistical heterogeneity precluded us from reporting the numerical summary results of the meta‐analysis for all the primary and secondary outcomes (Analysis 1.1 to Analysis 1.11). We used similar criteria to report the sensitivity or subgroup analyses.
1.1. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 1 Mortality in hospital.
1.11. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 11 Nitrogen balance (g/day).
When we could not report results due to clinical or statistical heterogeneity or both, we did a qualitative synthesis of the trial results. We also reported trial results of the included studies in tabular form: percentages and means of the hypocaloric and the control group of the seven main outcomes (Table 4).
Primary outcomes
1.1 Mortality in hospital
For this outcome we found nine relevant trials (1775 participants) (Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Choban 1997; Ibrahim 2002; McCowen 2000; Petros 2016; Rice 2011). We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to the differences in the underlying diagnoses of the medical or surgical ICU participants and the route/characteristics of administration of enteral or parenteral nutrition or both, but mainly to the wide differences in calories and protein received by the participants in the included trials (Table 1; Table 2). We therefore did not pool the point estimates in meta‐analysis (Analysis 1.1). There were 210 deaths in the 881 participants who received hypocaloric nutrition, and 235 deaths in the 894 participants who received the control intervention. All studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.1). The central estimates of risk ratios for hospital mortality of each individual studies ranged from 0.23 to 5.54. When we excluded Battistella 1997, the range of risk ratio estimates was narrower, since this study has a more extreme estimate due to small sample size and zero events in the control group. The quality of the evidence for this outcome was very low, due to high risk of attrition bias, imprecision and inconsistency (wide variance of point estimates across studies) (Summary of findings table 1).
1.2 Mortality in the intensive care unit (ICU)
We found four relevant trials for this outcome (1291 participants) (Arabi 2011; Arabi 2015; Battistella 1997; Petros 2016). We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to the type of participants, the nutrition methodology and the amount of calories received by the participants (Table 2; Table 3). We therefore have not pooled the point estimates (Analysis 1.2). There were 105 deaths in the 641 participants who received hypocaloric nutrition, and 123 deaths in the 650 participants who received the control intervention. All studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.2). The central estimates of risk ratios for ICU mortality of each individual studies ranged from 0.81 to 5.54. When we excluded Battistella 1997, the range of risk ratio estimates was narrower, since this study has a more extreme estimate due to small sample size and zero events in the control group. The quality of the evidence for this outcome was very low, due to a high risk of attrition bias, imprecision and inconsistency (wide variance of point estimates across studies) (Summary of findings table 1).
1.2. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 2 Mortality in ICU.
1.3 Mortality at 30 days
For this outcome we found seven relevant trials (2611 participants) (Arabi 2011; Arabi 2015; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2016). We found the abstract of an additional trial (Theodorakopoulou 2016), with mortality reported narratively for 38 participants. We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to participants' diagnoses, type and characteristics of the nutrition support, the amount of calories and the differences in calories received by the participants of both groups in the analysed trials (Table 2; Table 3). We therefore did not pool the point estimates (Analysis 1.3). There were 275 deaths in the 1309 participants who received hypocaloric nutrition, and 275 deaths in the 1302 participants who received the control intervention. All studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.3). The central estimates of risk ratios for mortality at 30 days of the individual studies ranged from 0.79 to 3.00. The quality of the evidence for this outcome was very low, due to a high risk of attrition bias, imprecision and inconsistency (wide variance of point estimates across studies) (Summary of findings table 1).
1.3. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 3 Mortality at 30 days.
2. 1 Length of hospital stay (days)
We found 10 relevant trials for this outcome (1677 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Choban 1997; Ibrahim 2002; McCowen 2000; Norouzy 2013; Petros 2016). We found considerable statistical heterogeneity (I2 = 78%) and important clinical heterogeneity due to differences in participants, nutrition methodology, and calories received by the participants of the intervention and control groups (Table 2; Table 3). We therefore did not pool the estimates (Analysis 1.4). Participants who received hypocaloric nutrition support had a mean length of stay of 15.70 days lower to 10.70 days higher compared to those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Summary of findings table 1).
1.4. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 4 Length of Hospital stay (days).
2. 2 Length of ICU stay (days)
For this outcome we found 11 relevant trials (2942 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; NHLBI 2012; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016). We found considerable statistical heterogeneity (I2 = 81%) and important clinical heterogeneity due to differences in the type of participants, nutrition methodology and the differences in total amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial ( Table 2; Table 3). We therefore have not pooled the effect estimates (Analysis 1.5). Participants who received hypocaloric nutrition support had a mean length of stay 11.00 days lower to 5.40 days higher compared to those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Summary of findings table 1).
1.5. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 5 Length of ICU stay (days).
3. Infectious complications. Events of any type of infectious complications occurring during the hospital stay, registered by the study authors according to their diagnostic criteria of infections
Ten studies reported this outcome (2804 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; McCowen 2000; NHLBI 2012; Petros 2016; Rice 2011). We found moderate statistical heterogeneity (I2 = 49%) and important clinical heterogeneity due to the type of participants, study methodology and amount of calories and protein received by the participants (Table 2; Table 3). We therefore have not pooled the estimates. There were 423 participants with infections in the 1404 participants who received hypocaloric nutrition, and 438 infections in the 1400 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.6). The range of the central estimate of risk ratios for infectious complications of the individual studies ranged from 0.54 to 2.54. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias, inconsistency and imprecision (Summary of findings table 1).
1.6. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 6 Infectious complications.
Secondary outcomes
1. Length of mechanical ventilation. Days on mechanical ventilation during ICU stay
For this outcome we found 12 relevant trials (3000 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016). We found substantial statistical heterogeneity (I2 = 69%) and important clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 2; Table 3). We therefore did not pool the effect estimates. Participants who received hypocaloric nutrition support had a mean length of mechanical ventilation of 13.20 days lower to 8.36 days higher compared with those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Analysis 1.7; Table 1).
1.7. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 7 Length of mechanical ventilation (days).
2. Non‐infectious complications. Events of any non‐infectious complication during the hospital stay, potentially associated with the nutrition status or the nutrition support, according to the criteria of the study authors (diarrhoea)
Three studies reported this outcome (1994 participants) (Arabi 2015; NHLBI 2012; Petros 2016). We found considerable statistical heterogeneity (I2 = 76%) and important clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 2, Table 3). We therefore did not pool the effect estimates. There were 187 participants with non‐infectious complications (diarrhoea) in the 1002 participants who received hypocaloric nutrition, and 242 participants with non‐infectious complications in the 992 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value. The range of the central estimate of risk ratios for non‐infectious complications of the individual studies ranged from 0.32 to 0.85. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias, inconsistency and imprecision (Analysis 1.8).
1.8. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 8 Non‐infectious complications (diarrhoea).
3.1 Carbohydrate metabolic outcomes: hyperglycaemia (glycaemia higher than 150 mg/dl) during ICU stay
For this outcome we found six relevant trials (1380 participants) (Ahrens 2005; McCowen 2000; NHLBI 2012; Petros 2016; Rugeles 2013; Rugeles 2016). We found substantial statistical heterogeneity (I2 = 62%) with moderate clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 2; Table 3). We therefore did not pool the effect estimates. There were 205 participants who suffered hyperglycaemia in the 695 participants who received hypocaloric nutrition, and 279 participants who suffered hyperglycaemia in the 685 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value. The central estimate of risk ratios for hyperglycaemia of the individual studies ranged from 0.36 to 0.93. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias, inconsistency and imprecision (Analysis 1.9).
1.9. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 9 Hyperglycaemia.
3.2 Carbohydrate metabolic outcomes: events of hypoglycaemia (glycaemia lower than 70 mg/dl) during ICU stay
We found five relevant trials for this outcome (1394 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Petros 2016; Rugeles 2016). We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants , as well as the caloric difference between the groups in each trial (Table 2; Table 3). We therefore did not pool the effect estimates. There were 46 participants who suffered hypoglycaemia in the 694 participants who received hypocaloric nutrition, and 38 participants who suffered hypoglycaemia in the 700 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value. The central estimate of risk ratios for hypoglycaemia of the individual studies ranged from 0.85 to 1.76. In Rugeles 2016, a risk ratio was not estimable due to no events in either group. The quality of the evidence for this outcome was low, due to unclear or high risk of bias and imprecision (Analysis 1.10).
1.10. Analysis.
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 10 Hypoglicaemia.
4. Lipid metabolic outcomes. Events of hypertriglyceridaemia (higher than 200 mg/dl) or any lipid metabolic complication associated with the nutrition support according to the criteria of the study authors
None of the included trials reported this outcome
5. Protein metabolic outcomes: nitrogen balance
For this outcome we found three relevant trials (92 participants) (Battistella 1997; Choban 1997; McCowen 2000). We found substantial statistical heterogeneity (I2 = 72%) with moderate clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 2; Table 3). We therefore did not pool the effect estimates (Analysis 1.11). Participants who received hypocaloric nutrition support had a mean nitrogen balance of −7.70 g/day to +2.00 g/day compared to those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Analysis 1.11).
6. Nutrition status or clinical condition at ICU discharge. Nutrition or functional evaluation, made at the time of ICU discharge with any method of assessment used by the study authors.
None of the included trials reported this outcome
Subgroup analyses
We focused our subgroup analyses on the seven outcomes reported in Table 1. Out of these seven outcomes only four had considerable statistical heterogeneity: length of hospital and ICU stay, infectious complications and length of mechanical ventilation. We explored sources of statistical heterogeneity and assessed whether meta‐analysis was possible, considering clinical heterogeneity in the predefined subgroups.
Due to insufficient information available, we were unable to perform subgroup analysis by: age, disease severity, presence of comorbidities, nutrition status (malnourished or well‐nourished), level of inflammation, hypermetabolism or hypercatabolism. It was only possible to perform prespecified subgroup analyses by: obesity status (as a condition of nutrition status), type of underlying medical condition (surgical or medical), amount of calories actually received by participants in the intervention and control groups. During the process of data extraction we realized that the included trials had several methodological differences between them. At this time (before any analysis of results), we decided to perform two additional analyses not prespecified in the protocol (Subgroup analysis and investigation of heterogeneity): subgroup analysis by route of nutrition support (enteral or parenteral) and meta‐regression (see below). The I2 values for these subgroup analyses are shown in Table 5. Most of the subgroup analyses were unable to explain the statistical heterogeneity of the results across studies:
4. Subgroup analyses.
| Subgroup | N participants (n studies) | Subgroup testing |
| 1. Nutrition status | ||
| 1.1. Length of hospital stay | ||
| Obese | 13 (1 RCT) | I2 = 0%, P = 0.76 |
| General | 1664 (9 RCTs) | |
| 2. Route of nutrition support | ||
| 2.1. Length of hospital stay | ||
| Parenteral | 150 (4 RCTs) | I2 = 0%, P = 0.72 |
| Enteral | 1725 (6 RCTs) | |
| 2.2. Length of ICU stay | ||
| Parenteral | 75 (2 RCTs) | I2 = 83.3%, P < 0.01 |
| Enteral | 2867 (9 RCTs) | |
| 2.3. Infectious complications | ||
| Parenteral | 137 (3 RCTs) | I2 = 0%, P = 0.35 |
| Enteral | 2667 (7 RCTs) | |
| 2.4. Length of mechanical ventilation | ||
| Parenteral | 73 (2 RCTs) | I2 = 85.4%, P < 0.01 |
| Enteral | 2927 (10 RCTs) | |
| 3. Type of participant | ||
| 3.1. Length of hospital stay | ||
| Surgical participants | 223 (5 RCTs) | I2 = 0%, P = 0.55 |
| Medical participants | 1354 (5 RCTs) | |
| 3.2. Length of ICU stay | ||
| Surgical participants | 158 (3 RCTs) | I2 = 0%, P = 0.52 |
| Medical participants | 2784 (8 RCTs) | |
| 3.3. Infectious complications | ||
| Surgical participants | 220 (4 RCTs) | I2 = 0%, P = 0.45 |
| Medical participants | 2584 (6 RCTs) | |
| 3.4. Length of mechanical ventilation | ||
| Surgical participants | 156 (3 RCTs) | I2 = 0%, P = 0.45 |
| Medical participants | 2854 (9 RCTs) | |
| 4. Amount of calories received by each study group | ||
| 4.1. Length of hospital stay | ||
| Normo‐hypercaloric | 97 (2 RCTs) | I2 = 84.1%, P < 0.01 |
| Hypocaloric | 1370 (6 RCT) | |
| Very hypocaloric | 150 ( RCT) | |
| 4.2. Length of ICU stay | ||
| Normo‐hypercaloric | 75 (2 RCTs) | I2 = 0%, P = 0.42 |
| Hypocaloric | 1517 (6 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
| 4.3. Infectious complications | ||
| Normo‐hypercaloric | 97 (2 RCTs) | I2 = 0%, P = 0.94 |
| Hypocaloric | 1357 (5 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
| 4.4. Length of mechanical ventilation | ||
| Normo‐hypercaloric | 73 (2 RCTs) | I2 = 73.1%, P = 0.02 |
| Hypocaloric | 1517 (6 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
RCT = randomized controlled trial; ICU = Intensive care unit
In the subgroup analysis by nutrition status, limited to obesity, we did not observe subgroup differences in length of hospital stay.
In the subgroup analysis by route of nutrition support we found considerable subgroup differences in length of stay in ICU and in duration of mechanical ventilation.
In the subgroup analysis by the type of participant we did not find subgroup differences between the surgical or medical participants in any of the outcomes analysed
In the subgroup analysis by the amount of calories received by each study group we found considerable subgroup differences in length of hospital stay and in duration of mechanical ventilation.
Sensitivity analyses
Excluding quasi‐randomized trials. The sensitivity analysis after excluding the only quasi‐randomized trial (Ibrahim 2002) did not show major changes in the overall results for the outcomes of mortality in hospital, length of hospital stay, length of ICU stay and infectious complications. We only observed a change in the outcome of duration of mechanical ventilation. After excluding this trial, the statistically significant difference in favour of the hypocaloric group disappeared. However, this result should be interpreted with caution due to the substantial statistical heterogeneity (I2 = 69%) and important clinical heterogeneity.
Excluding trials with at least one high 'Risk of bias' criterion. The sensitivity analysis after excluding the six trials with at least one high 'Risk of bias' criterion ( Arabi 2015; Charles 2014; Ibrahim 2002; McCowen 2000; Rugeles 2013; Rugeles 2016) did not show major changes in the results of the primary and secondary outcomes analysed, nor in the subgroup analyses. We only observed some minor changes in the statistical heterogeneity of several subgroups analysed.
By fixed‐effect or random‐effects models. We analysed the primary and secondary outcomes by fixed‐effect or random‐effects models according to the value of I2, as stated in Data synthesis. In all the analyses where the pooled estimates were done with the fixed‐effect model, we conducted a sensitivity analysis with the random‐effects model to explore the robustness of results. No primary or secondary outcomes or subgroup analyses showed a major change in their statistical significance or heterogeneity.
By different primary goal of enteral nutrition trials. We performed a post hoc sensitivity analysis (see Sensitivity analysis), excluding the three studies (Ibrahim 2002; NHLBI 2012; Rice 2011) with a primary goal to assess the effects of early initiation of trophic (hypocaloric) enteral nutrition or standard (normocaloric) enteral feeding from the beginning. We did not find major differences in the primary or secondary outcomes, except for minor changes in two unreported outcomes due to the heterogeneity: length of mechanical ventilation and hyperglycaemia.
By different primary goal of parenteral nutrition trial. Another post hoc sensitivity analysis was the exclusion of the Battistella 1997 trial, because its primary goal was the evaluation of parenteral nutrition with or without lipids (equivalent to normocaloric or hypocaloric nutrition, respectively). After excluding this trial, we did not see major changes in the primary and secondary outcomes evaluated, with the exception of the length of mechanical ventilation: loss of statistical significance in favour of the hypocaloric group (result not reported due to the substantial statistical heterogeneity).
Meta‐regression
Considering that we found high levels of clinical and statistical heterogeneity, we performed non‐prespecified meta‐regressions using STATA 14.1 to explore the effect of covariates for which we had data (Table 3; Table 4):
Type of participants (medical or surgical participants)
The calories received in the hypocaloric group, based on the three aforementioned categories (see Subgroup analysis by the amount of calories received by each study group): normo‐hypercaloric, hypocaloric or very low hypocaloric.
The difference in calories received between study groups (control minus intervention groups).
We performed meta‐regression on the primary outcomes with results of nine or more trials: hospital mortality, infectious complications, hospital length of stay and ICU length of stay. We did not find significant results explaining sources of heterogeneity using this analysis. None of the analysed explanatory variables influenced the size of the intervention effect of the outcome variables. The details on the definition of variables, dataset and outcome measures are available in Appendix 5 and Appendix 6.
Table 4: In order to show some aspects of the heterogeneous results, in Table 4 we present crude results of the primary outcomes and of length of mechanical ventilation for the 15 included studies. The files of the table were ordered from top to bottom by the difference in the amount of calories receive by the control groups minus those received by the hypocaloric groups (second column from the left).
Assessment of reporting bias
We performed a funnel plot for the outcomes with more than eight included studies (Analysis 1.1; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7). We did not see important asymmetries in the funnel plots, suggesting publication bias (we give one example in Figure 4).
4.
Funnel plot of comparison: 1 Hypocaloric nutrition (intervention) vs. Control, outcome: 1.1 Mortality in hospital.
Discussion
Summary of main results
We identified 15 trials including a total of 3129 ICU participants. The included trials had different objectives, participant characteristics and methodology for the administration of the nutrition support. The consequence of this was important methodological diversity between the included trials (Table 2). Due to the high clinical and statistical heterogeneity, we did not report summary estimates for the primary and secondary outcome analyses. Of all the causes of clinical heterogeneity, the most relevant ones precluding the report of summary estimates were the disparity in the amount of calories/protein received by the intervention and control groups, and the disparity in the differences in the calories received between the study groups in the included trials (Table 3; Table 4). For the same reason, we did not report total estimates of subgroup analyses (See Table 5).
In a descriptive analysis of the results of the included trials for the main outcomes (See Table 1), we can summarize the following:
We found very low‐quality evidence for the outcomes related to mortality (in hospital, in ICU and at 30 days), with no statistical but important clinical heterogeneity. Most studies did not find differences in the incidence of mortality between hypocaloric and control groups. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
We found very low‐quality evidence for the outcome length of hospital and ICU stay, with both clinical and statistical heterogeneity. In smaller studies, there was a tendency towards a shorter length of stay in participants in the hypocaloric group, but the results across studies were inconsistent, some favouring hypocaloric nutrition support and some control. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
We found very low‐quality evidence for the outcome infectious complications, with moderate statistical and important clinical heterogeneity. The results across studies were inconsistent, some favouring hypocaloric nutrition support and some control. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
We found very low‐quality evidence for the outcome length of mechanical ventilation, with both clinical and statistical heterogeneity. The results across studies were inconsistent, some favouring hypocaloric nutrition support and some control. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
Other outcomes
For diarrhoea (non‐infectious complications) the statistical heterogeneity was considerable and the clinical heterogeneity important. The central estimates of the individual studies favoured hypocaloric nutrition support, but the quality of this evidence was very low, due to unclear or high risk of bias in the included studies, inconsistency and imprecision.
For hyperglycaemia the statistical heterogeneity was substantial and the clinical heterogeneity moderate. The central estimates of the individual studies favoured hypocaloric nutrition support, but the quality of this evidence was very low, due to unclear or high risk of bias in the included studies, inconsistency and imprecision.
For hypoglycaemia, the clinical heterogeneity was important, but with no statistical heterogeneity. The individual studies did not find differences in the incidence of hypoglycaemia between hypocaloric and control groups, but the quality of this evidence was low, due to unclear or high risk of bias in the included studies and imprecision.
For nitrogen balance, the statistical heterogeneity was substantial and the clinical heterogeneity moderate. The results were inconsistent, some favouring hypocaloric nutrition support and some control; the quality of evidence was very low, due to unclear or high risk of bias in the included studies, inconsistency and imprecision.
We did not find data to perform several of the subgroup analyses proposed in the review protocol. We performed subgroup analyses for the main outcomes, but these could not comprehensively explain the statistical heterogeneity (See Table 5).
In the three prespecified sensitivity analyses (excluding the quasi‐randomized trial; the three trials with at least one high 'Risk of bias' domain; or the change of results from fixed‐effect to random‐effects model) we did not see major changes in the results, nor in the post hoc sensitivity analysis excluding three studies with a primary goal to assess hypocaloric trophic enteral nutrition versus standard enteral feeding. In the other post hoc sensitivity analysis excluding Battistella 1997, in the primary and secondary outcomes we only observed the loss of an unreported significant difference in length of mechanical ventilation.
As we established in the subgroup and sensitivity analyses, Ahrens 2005 and Battistella 1997 had very dissimilar results compared to the other included studies. In both trials, the control groups received a high caloric dose, a median 37 total kcal/kg/day in Ahrens 2005, and 34.4 total kcal/kg ideal body weight/day in Battistella 1997. Moreover, in Ahrens 2005, the control participants not only received hypercaloric parenteral nutrition but also more dextrose than currently recommended for critically‐ill participants (ASPEN / SCCM guidelines 2009; ESPEN guidelines 2009), with a median (interquartile (IQ) range) of 4.9 (4.79 to 5.07) mg dextrose/kg/min. (The authors also reported that the administration of more dextrose than 4 mg/kg/min behaved as a predictor of hyperglycaemia). Finally, It is also important to remember that Battistella 1997 compared parenteral nutrition with and without lipid emulsions. It is therefore difficult to discriminate whether the observed results were due to the amount of calories administered, to the withholding of soy‐derived lipid emulsions in the parenteral nutrition, or both.
In order to evaluate causes of heterogeneity or to formulate hypotheses about them, we performed a non‐prespecified meta‐regression with the available covariates for the primary outcomes with nine or more trials. We did not find significant results explaining sources of heterogeneity (Appendix 5; Appendix 6). However, the results of the meta‐regression should be considered cautiously, due to the fact of post hoc analysis, and to the limited number of studies for the number of covariates in the model.
Overall completeness and applicability of evidence
The research strategy was comprehensive and inclusive. Given the scarcity of evidence, it sought to include all possible trials with a design and goal to evaluate hypocaloric versus normocaloric nutrition support in critically‐ill people. This is why we also included quasi‐randomized controlled trials, different types of ICU settings (medical, surgical, mixed), types of participants (age, medical condition, etc.), administration routes (enteral, parenteral or both), and also considered trials with a different primary goal or methodology to achieve prescribed hypocaloric feeding. We therefore believe that the included studies represent a complete set of up‐to‐date evidence on hypocaloric nutrition support in critically‐ill adults.
Nevertheless, breadth of scope was at the expense of clinical and statistical heterogeneity. Some of these differences in participants, interventions and outcomes of the included trials can be seen in Table 2 and Table 3. In addition, all studies were performed at university‐associated or teaching hospitals, which are probably different from other clinical settings. It is therefore arguable whether our results could be generalized. The clinical and statistical heterogeneity precluded a quantitative synthesis of all the outcomes and most of the subgroup analyses. In the clinical field, the results should be interpreted with caution, considering all these issues.
When we analyse the amount of calories actually received by the groups in each trial (many of them different from those prespecified in their study protocols), we find that most of the included studies did not really evaluate the administration of hypocaloric versus normocaloric nutrition support, but a wide range of calories administered (Table 3). Moreover, the difference in calories received by the control minus the hypocaloric group was quite small in some of the included trials (Table 4). Both factors not only contributed to the clinical heterogeneity, but could also have been associated with the lack of statistically significant differences (if any) between the study groups.
Most of the included trials did not analyse the role of protein administration in the outcomes evaluation. The amount of protein administered to the intervention and control groups was reported in 12 trials: quite diverse in three of them, rather similar in four, and more or less the same in the other five (Table 3). There is wide consensus that obese critically‐ill people should receive hyperproteic hypocaloric feeding (ASPEN / SCCM guidelines 2016; Choban 2013; Dickerson 1986), but there is a current debate about the best protein dose for the non‐obese people: higher doses of proteins seemed to be associated with better outcomes in the critically‐ill people (Dickerson 2012; Weijs 2012; Weijs 2013; Van Zanten 2016). The different daily protein administered to the study groups in the included trials should be considered as another component of the clinical heterogeneity.
Even though our results did not find conclusive significant evidence in favour of the hypocaloric nutrition support, it is also interesting to note that we did not find high‐quality evidence for harms. This is in contrast to two observational studies that reported some poorer clinical outcomes or complications when certain levels of calories were not achieved (Rubinson 2004; Villet 2005).
Quality of the evidence
According to GRADE, the quality of evidence for the primary outcomes was very low (see Table 1).
Six out of 15 included studies presented one or more high 'Risk of bias' criteria and eight studies had one or more unclear 'Risk of bias' criteria. Given the complexity of nutrition support in critically‐ill people, blinding the personnel (the major driver for high risk of bias in this systematic review) is challenging, although some studies were able to do it. Only one included trial (Ahrens 2005) had low risk of bias in all predefined criteria (Figure 3). The quality of evidence according to GRADE was low to very low for all the primary outcomes.
Another reason for downgrading the quality of evidence was inconsistency. We explored the qualitative characteristics that could explain inconsistency, but we were unable to identify them in subgroup analyses (Table 5), sensitivity analyses and meta‐regression. Inconsistency was also evident in the wide variance of point estimates for mortality.
Imprecision affected the quality of all main outcomes, especially mortality, due to the low number of events. The confidence intervals were wide and we could not improve precision by pooling results in most cases, due to the clinical heterogeneity.
For publication bias, the funnel plots for the outcomes with at least eight trials did not show significant asymmetry.
Potential biases in the review process
We followed the procedures of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), in order to minimize biases in the review process. The search strategy was defined by a senior librarian and evaluated by another independent expert. Our search strategy was comprehensive, including consultation with opinion leaders, the pharmaceutical industry, conference and congress proceedings and snowballing techniques to maximize the chances of retrieving all existing studies, published or unpublished. Three review authors independently screened the trials, and data extraction and assessment of risks of bias were also done by two independent review authors. We resolved any disagreement through consultation with a third review author.
We strictly followed the inclusion‐exclusion criteria of our protocol (Perman 2009). Our included trials are non‐homogeneous, with different objectives and methodologies. The main differences related to the type and conditions of the participants and the methodology for the administration of the nutrition support (goals, time of initiation, route, strategy of delivery and calories administered, among others) (Table 2). This clinical and methodological heterogeneity added complexity to the analysis of data and the interpretation of results. It was not possible to report summary estimates due to the clinical or statistical heterogeneity or both.
We acknowledge that the multiplicity of subgroup analyses and post hoc analyses could have yielded false positive results. We added these post hoc analyses in order to explore the clinical heterogeneity found in the included studies. We tried to reduce the risk of false‐positive results by restricting the exploration of subgroups to those outcomes in which we found statistical heterogeneity, as we had defined in our protocol.
Agreements and disagreements with other studies or reviews
There are eight previous systematic reviews and meta‐analyses directly or indirectly related to the topic of this review. They have similar purposes, but different review questions and inclusion criteria.
The first review (Jiang 2011), evaluated randomized controlled trials comparing hypocaloric parenteral nutrition (≤ 20 non‐protein kcal/kg/day) versus standard or high‐energy parenteral nutrition (≥ 25 or > 30 non‐protein kcal/kg/day, respectively) in surgical or trauma participants. According to their inclusion‐exclusion criteria, they included five trials, two of which (Ahrens 2005; Battistella 1997) we also include. The other three studies (in the Chinese language) were trials of postoperative, not critically‐ill participants (according to the titles and one abstract of the studies) (Jiang 2003; Mao 2015; Zhan 2007). They reported a statistically significant reduction in infectious complications and length of hospital stay, with moderate heterogeneity, in the surgical participants receiving hypocaloric parenteral nutrition. Those results were more consistent and with less heterogeneity when they excluded the small‐sample size trials. The calories administered to the participants seemed to be more homogeneous, but the authors did the analysis with administered non‐protein calories. If we add the caloric content of the administered protein, the intervention group received an average of 24.0 (range 20.5 to 27.0) total kcal/kg/day and the control group 34.5 (range 32.5 to 36.0) total kcal/kg/day. This means that the study compared almost normocaloric versus normo‐ to hypercaloric parenteral nutrition. The favourable effects of the lower‐caloric parenteral nutrition on infectious complications and length of hospital stay reported in this meta‐analysis should therefore be limited to surgical participants receiving parenteral nutrition with higher than recommended caloric dose. In our subgroup analyses we observed similar results with the analyses of Ahrens 2005 and Battistella 1997.
In 2015 the Canadian Critical Care Nutrition Clinical Practice Guidelines Committee updated the Canadian Clinical Practice Guidelines for nutrition support for critically‐ill adults. They produced three different but related systematic reviews and meta‐analyses, with the following titles: Intentional Underfeeding: Trophic Feeds vs. Full Feeds (Canadian Guideline 3.3a 2015); Intentional Underfeeding: Hypocaloric Enteral Nutrition (Canadian Guideline 3.3b 2015); and Strategies to Optimize Parenteral Nutrition and Minimize Risks: Dose of PN (Canadian Guideline 10.1 2015). This approach was guidelines‐oriented, but also served to diminish the clinical heterogeneity of the included trials and the statistical heterogeneity in some analyses.
For the evaluation of trophic (hypocaloric) versus full (normocaloric) feeding (Canadian Guideline 3.3a 2015), the Canadian group evaluated two studies, also included in our review (NHLBI 2012; Rice 2011), but they did not include a quasi‐randomized trial with a similar goal and methodology (Ibrahim 2002). The meta‐analysis did not show statistical differences in mortality or ventilator‐associated pneumonia between the study groups. They did not report results of length of hospital stay, of ICU stay or of mechanical ventilation, due to the way the data were reported in the trials. However, we could analyse these results after receiving the information from the first author of each trial. We did not find statistically significant differences between the study groups.
To update the 2015 guideline Intentional Underfeeding: Hypocaloric Enteral Nutrition (Canadian Guideline 3.3b 2015), the Canadian Committee included four trials in the meta‐analysis. We included all four of them in our review (Arabi 2011; Arabi 2015; Charles 2014; Petros 2016). They found that hypocaloric enteral nutrition was associated with a trend towards lower hospital and ICU mortality, and a statistically significant reduction in the length of mechanical ventilation. They did not find significant differences for infectious complications or length of hospital and ICU stay. In the enteral nutrition subgroup analysis we were prevented from reporting summary estimates of the six outcomes evaluated, due to the high clinical or statistical heterogeneity. When we did the same analysis as they did, the results were almost the same (some minor numerical differences).
The Canadian group included four trials in their meta‐analysis of parenteral nutrition (Canadian Guideline 10.1 2015), which we also included in our review (Ahrens 2005; Battistella 1997; Choban 1997; McCowen 2000). (They included results from an "unpublished Ahrens 2003" trial, which were the same as our included Ahrens 2005 trial). They did not find statistically significant differences between the intervention and control groups for hospital mortality or infectious complications. In our subgroup analysis of parenteral nutrition, we found some minor numerical differences from the Canadian Guideline in the same two outcomes, but the results were essentially the same. They also reported some additional results (sensitivity analysis and results of individual studies), but not significant ones.
Another systematic review and meta‐analysis (Choi 2015), compared the effect of initial enteral nutrition with an underfeeding dose versus initial full‐feeding dose of enteral nutrition in critically‐ill adults. They included four trials, three of which we also included in our review (Arabi 2011; NHLBI 2012; Rice 2011), and one which we excluded due to a different primary objective (Desachy 2008). They did not find significant differences in overall mortality and other clinical outcomes between the underfeeding and the full‐feeding groups. In the subgroup analysis, the underfeeding subgroup that received 33.3% or more of the standard caloric requirement showed a significantly lower overall mortality, compared with the full‐feeding group. This was not seen in the underfeeding subgroup that received less than a 33.3% dose of enteral nutrition. This suggests the possibility that a moderate underfeeding enteral nutrition, but not a minimal intake, could be associated with a better prognosis. Nevertheless, the included trials showed clinical heterogeneity, as well as our subgroup analysis of enteral nutrition, where we did not see differences in hospital mortality.
The Tian 2015 meta‐analysis included eight randomized trials showing significantly different calories administered by the enteral route. We included four of these trials in our review (Arabi 2011; Charles 2014; NHLBI 2012; Rice 2011). They did not find significant differences between the low‐ and high‐energy groups for mortality; infectious complications; pneumonia; gastrointestinal intolerance and the length of hospital stay, of ICU stay and of mechanical ventilation. In the subgroup analysis, the low‐energy groups who received between 33.3% and 66.6% of the caloric goal had a significantly lower mortality compared with the high‐energy group. In the subgroup analysis with different amount of protein administration, they found that high protein administration (more than 0.85 g/kg/day) plus high energy could decrease the rate of infectious complication. In our subgroup analysis with three categories of calories administered, we did not find any significant result, but it is necessary to keep in mind the results of the Choi 2015 and Tian 2015 meta‐analyses for the dose of calories with enteral nutrition, as well as the dose of protein (Tian 2015).
The Marik 2016b systematic review and meta‐analysis compared normocaloric (80% to 100% of daily energy expenditure) with intentional hypocaloric enteral nutrition, dividing it into two different strategies: 'permissive underfeeding' (less than 70% of daily energy expenditure) and 'trophic' (20% of the dose during the first week). They included six trials, which we also include in our review, but analysed them separately in the subgroup 'trophic' (NHLBI 2012; Rice 2011), and 'permissive underfeeding' (Arabi 2011; Arabi 2015; Charles 2014; Petros 2016). In the meta‐analysis the statistical heterogeneity was low and they did not find significant differences between the study groups for infectious complications, length of ICU stay and hospital mortality (only a trend towards a lower mortality in the permissive underfeeding subgroup) and ventilator‐free days. In line with our protocol, we performed different subgroup and sensitivity analyses, with conceptually similar results. However, their subgroups approach should be considered in future systematic reviews.
In a second systematic review and meta‐analysis (Tian 2017), the authors included 11 studies comparing low‐ and high‐energy enteral nutrition (in two studies also enteral plus supplemental parenteral nutrition), administered to adults who were critically‐ill but not malnourished. We also included five of these studies (Arabi 2011; Arabi 2015; Charles 2014; NHLBI 2012; Rice 2011). In the meta‐analysis, they did not find statistically significant differences between low‐ and high‐energy groups for mortality, infectious complications, pneumonia, length of hospital and ICU stay, and length of mechanical ventilation. They found significantly less gastrointestinal intolerance in the low‐energy group. In the subgroup analysis for mortality, they observed significantly less mortality in the low‐energy group but only within the range of 33.3% to 66.6% of the goal calories. In another subgroup analysis, the incidence of infectious complications was significantly lower in the high‐energy group, but only when the enteral nutrition also provided higher amounts of protein. Even though one might question their decision to perform meta‐analysis with such high clinical heterogeneity, we should consider in future studies the role of the enteral nutrition dose between 33.3% and 66.6% of the caloric goal and the amount of protein administered.
It is important to emphasize that, in line with our protocol (Perman 2009), we only included trials comparing any type of 'prescribed' hypocaloric nutrition support with different control groups in critically‐ill adults. Although there are several reports in the literature of critically‐ill people receiving hypocaloric enteral nutrition due to difficulties, intolerance or complications during the administration (occurring in 59% of the cases, as reported in the international survey done in 158 ICUs by Cahill 2010), we did not include any study assessing this 'non‐ prescribed', unintentional hypocaloric nutrition support. Nevertheless, we point out that many trials included in our study could not achieve their prespecified caloric goals.
We included three studies that indirectly assessed our review question by the intentional administration of trophic (hypocaloric) enteral feeding during the first five or six days in ICU versus full enteral (normocaloric) feeding from the beginning of the ICU stay (Ibrahim 2002; NHLBI 2012; Rice 2011). In order to be as inclusive as possible, we also included Battistella 1997 (primary objective to evaluate parenteral nutrition with or without soy‐lipid emulsions). It is important to highlight that the results of the latter trial could be associated with the less‐caloric parenteral nutrition, with the lack of lipids, or a combination of both factors (ASPEN 2012; Ren 2013). When we did a sensitivity analysis excluding those trials, we found only minor differences in the results.
We did not include studies evaluating enteral nutrition optimized with supplemental parenteral nutrition to reach the 'target energy' (measured by indirect calorimetry or estimated by formulae) to avoid “caloric debts” (Heidegger 2013), or to assess when to initiate supplemental parenteral nutrition (Doig 2013). Both topics are the subject of currently debate.
Authors' conclusions
Implications for practice.
The inclusion criteria and the data analyses by intention‐to‐treat defined in the protocol resulted in important clinical and statistical heterogeneity of the included trials. This heterogeneity did not allow us to report pooled estimates of the primary and secondary outcomes, so we have described them in a narrative fashion. We found very low‐quality evidence for the effects of prescribed hypocaloric nutrition support on mortality in hospital, in the ICU and at 30 days, as well as in length of hospital and ICU stay, infectious complications and the length of mechanical ventilation. The reasons for downgrading this evidence were unclear or high risk of bias in the included studies, imprecision and inconsistency. For these outcomes there is uncertainty about the effects of prescribed hypocaloric nutrition, since the range of estimates includes both appreciable benefits and harms. Using subgroup and sensitivity analyses, as well as meta‐regression, we were not able to explain the causes of the observed heterogeneity.
Implications for research.
The evidence available is sparse, heterogeneous, and with limitations in its quality. It is important to have more well‐designed, well‐powered and well‐conducted randomized controlled trials to assess the effects of hypocaloric nutrition support in critical outcomes such as mortality, infectious complications, length of stay and length of mechanical ventilation.
To minimize heterogeneity and to improve external validity, it is important for future studies to better categorize the participants and their nutritional treatments. The adequate report of these categorizations could help us understand the inconsistencies in the findings. Considering that nutrition support is a complex intervention, study authors should consider the following factors: a) the clinical characteristic of included participants (diagnostic category, severity of disease, metabolic changes, acute or prolonged critical state, nutritional status, comorbidities, and other factors according to the goals of the trials); b) the methods of nutrition support (early or late initiation, duration, amount of prescribed and administered calories to the intervention and control groups, reported in kcal/kg/day); c) the detailed amount of prescribed proteins and the amounts effectively administered to participants (reported in grams/kg/day).
Individual‐patient data (IPD) meta‐analysis could be applied to model the effect of the interventions considering these covariables.
Furthermore, it is important to properly report all research methods (avoiding 'unclear' domains in 'Risk of bias' assessments) and ideally to conduct masked studies, taking into account the difficulties in effectively implementing a prescribed hypocaloric nutrition (performance bias) and in assessing outcomes subject to bias, such as lengths of stay and length of mechanical ventilation (detection bias).
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 6, 2018
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Co‐ordinating Editor |
Acknowledgements
During the prepublication editorial process, the protocol has been commented on by a content editor and two peer reviewers (who are external to the editorial team), members of the Cochrane Consumer network’s international panel of consumers and the Anaesthesia Group’s Information Specialist. For that, we thank Martha Delgado, Khursheed Jeejeebhoy, Alison Avenell, Anne E. Fonfa and Janet Wale. We are especially grateful to Jane Cracknell for her help and patience during the protocol development (Perman 2009), and publication process, to Karen Hovhannisyan and Daniel Comandé for their help with the search strategies and obtaining copies of trials, and to Marit Johansen for the SciSearch information.
We also thank Kursheed Jeejeebhoy, Mette Berger, Roland Dickerson, José F. Patiño, Ewald Schlotzer (Fresenius‐Kabi AG, Germany); Rolf Franke and Ute Brauer (B Braun Melsungen AG, Germany) for their help in suggesting potential studies to be considered, and to the first authors of several trials for giving all the information requested to complete the data for this review: Christine Ahrens sent information on length of ICU and hospital stay and on mechanical ventilation expressed as mean and standard deviation (Ahrens 2005); Sirak Petros completed the data of their initially unpublished but finished pilot study (Petros 2016); Todd Rice, for his own study (Rice 2011) and on behalf of the ARDS Network (NHLBI 2012) gave us important additional information and interpretation of results of both trials; Saúl Rugeles (Rugeles 2013), sent the final version of the trial before it was published and also additional information about participants with hyperglycaemia and to estimate the risks of bias. He also sent us in advance the full paper of his second trial (Rugeles 2016), with some additional information; Yaseen Arabi (Arabi 2015) sent us means and standard deviations of several outcomes; Eric Charles (Charles 2014) sent us the mean and standard deviation value of length of mechanical ventilation and additional information to complete the 'Risk of bias' table.
During the preparation of the final manuscript, we received several comments and advice. We would like to thank Rodrigo Cavallazzi (content editor) and Harald Herkner (content and co‐ordinating editor), Nathan Pace (statistical editor), Bill Simpson, Khursheed Jeejeebhoy, Thomas Bongers (peer reviewers), Janet Wale (consumer editor) and Daren Heyland for their help and editorial advice during the preparation of this systematic review. Once more, we also want to express our special gratitude to Jane Cracknell (Managing Editor) for her help during the editorial process and to Nathan Pace (statistical editor) for his advice about how to perform and report several statistical results.
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor: [Critical Illness] explode all trees #2 stressed:ti,ab,kw #3 critical* next ill*:ti,ab,kw #4 MeSH descriptor: [Critical Care] explode all trees #5 MeSH descriptor: [Intensive Care Units] explode all trees #6 #1 or #2 or #3 or #4 or #5 #7 (feeding or food or nutrition* or diet* or intake*) .ti #8 (eucalor* or hypoenerg* or underfeed* or (low calor*) or hypocalor*):ti,ab,kw #9 MeSH descriptor: [Diet] explode all trees #10 MeSH descriptor: [Parenteral Nutrition] explode all trees #11 MeSH descriptor: [Enteral Nutrition] explode all trees #12 #7 or #8 or #9 or #10 or #11
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1 exp Critical Illness/ 2 stressed.ti,ab. 3 (critical adj3 ill*).mp. 4 Critical Care/ 5 Intensive Care/ 6 Intensive Care Units/ 7 1 or 2 or 3 or 4 or 5 or 6 8 (feeding or food or nutrition* or diet* or intake*).ti. 9 (eucalor* or hypoenerg* or underfeed* or (low adj3 calor*) or h?pocalor*).mp. 10 Diet/ or Parenteral‐Nutrition/ or Enteral‐Nutrition/ 11 8 or 9 or 10 12 7 and 11 13 "Randomized Controlled Trial".pt. 14 "Controlled Clinical Trial".pt. 15 randomi?ed.ti,ab. 16 placebo*.ti,ab. 17 "drug therapy".sh. 18 randomly.ti,ab. 19 trial.ti,ab. 20 groups.ti,ab. 21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 22 Animals/ not (Humans/ and Animals/) 23 21 not 22 24 12 and 23
Appendix 3. Search strategy for Embase (Ovid SP)
1 eucalor*.ti,ab. 2 hypoenerg*.ti,ab. 3 underfeed*.ti,ab. 4 (low adj3 calor*).ti,ab. 5 h?pocalor*.ti,ab. 6 1 or 2 or 3 or 4 or 5 7 (feeding or food or nutrition* or diet* or intake*).ti. 8 *diet/ 9 *parenteral nutrition/ 10 6 or 7 or 8 or 9 11 critical*.ti,ab. 12 stressed.ti,ab. 13 *intensive care unit/ 14 *intensive care/ 15 *critical illness/ 16 11 or 12 or 13 or 14 or 15 17 10 and 16 18 random.tw. or placebo.mp. or double‐blind.tw. 19 17 and 18
Appendix 4. Search strategy for LILACS, (BIREME)
(tw:(("ENFERMEDAD CRITICA" OR "UNIDADES DE TERAPIA intensiva" OR "CUIDADOS INTENSIVOS" OR trauma* OR "TRAUMA multiple" OR "SEPSIS" OR septicemia* OR "ENFERMEDAD AGUDA" OR "cuidados criticos" OR "cuidado critico" OR "cuidado intensivo" OR "cuidados intensivos" OR icu* OR uti*))) AND
(tw:((desnutricion* OR hypocalor* OR hipocalor* OR hypoenerg* OR hipoenerg* OR underfeed* OR subaliment* OR "bajas calorias" OR "bajo valor" OR hiponutr* OR malnutr* OR calorimetr*)))
AND (instance:"regional") AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
Appendix 5. Meta‐regression
STATA 14.1 outputs exploring the effect of several explanatory variables on the primary outcomes with the highest number of included studies: mortality in hospital, infectious complications, length of hospital stay and length of ICU stay. The covariates included in the models were: type of participants [typepatient]; calories received by the intervention group in three categories categorized [catcal]; difference in the amount of calories received by the control groups minus the intervention group [difcal].
The explanatory variables were defined as follow:
typepatient: surgical participant vs medical participant (all surgical participants received parenteral nutrition and medical received enteral nutrition) (See Table 2).
catcal: categories denominated by the amount of calories really received by the intervention groups, according to the following: very hypocaloric ≤ 10 kcal/kg/day (icatcal 2); hypocaloric ≥ 10 to < 25 kcal/kg/day (icatcal 1); normocalcaloric or hypercaloric ≥ 25 kcal/kg/day (icatcal 0) (see Table 3).
difcal: absolute difference in kcal/kg/day received by the control minus the intervention groups (see Table 4).
We analysed several different models for each outcome. We only presented the model with the three covariates of each outcome, including the full output of the STATA 14.1 statistics.
In each model the covariates were typed in bold (see above definitions). The other codes in tables were:
logrr: Relative risk of dichotomic outcomes.
ES: Mean difference of continuous outcomes.
Coef.: Value of the relative risk or the mean difference in their units
P > t: Probability that the Logrr difference adjusted by other covariates could be related to chance if P is higher than 0.05
Std. Err: Standard error of the coefficient.
t: test.
P > t: Probability that the Logrr difference adjusted by other covariates could be related to chance if P is higher than 0.05 (not significant).
95% conf. interval: 95% confidence interval of the Logrr or ES values.
It is important to state the limitations of this meta‐regression because of the limited number of studies for the number of covariates in the model. Meta‐regression should generally not be considered when there are fewer than 10 studies in a meta‐analysis.
1. Mortality in hospital
xi: metareg logrrdifcal i.catcal typepatient, wsse(selogrr) bsest(reml)
i.catcal _Icatcal_0‐2 (naturally coded; _Icatcal_0 omitted)
note: _Icatcal_1 dropped because of collinearity
numerical derivatives are approximate
nearby values are missing
Meta‐regression Number of observations = 7
REML estimate of between‐study variance tau2 = 0
% residual variation due to heterogeneity I‐squared_res = 0.00%
Proportion of between‐study variance explained Adj R‐squared = 100.00%
Joint test for all covariates Model F(3,3) = 1.16
With Knapp‐Hartung modification Prob > F = 0.4542
| logrra | Coef. | Std. Err. | t | P > t | (95% Conf. | Interval) |
| difcal | .0237277 | .0333315 | 0.71 | 0.528 | −.0823481 | .1298035 |
| _Icatcal_2 | .2621164 | .2656104 | 0.99 | 0.396 | −.5831745 | 1.107407 |
| typepatient | −.3222415 | .8614032 | −0.37 | 0.733 | −3.063611 | 2.419128 |
| _cons | −.2805905 | .1894936 | −1.48 | 0.235 | −.8836437 | .3224628 |
aRelative Risk
Interpretationof hospital mortality. None of the covariates had a statistically significant influence on the size of the intervention effect on hospital mortality (P > 0.05).
2. Infectious complications
xi: metareg logrr difcal i.catcal typepatient, wsse(selogrr) bsest(reml)
i.catcal _Icatcal_0‐2 (naturally coded; _Icatcal_0 omitted)
Meta‐regression Number of obs = 10
REML estimate of between‐study variance tau2 = .0115
% residual variation due to heterogeneity I‐squared_res = 40.22%
Proportion of between‐study variance explained Adj R‐squared = 24.55%
Joint test for all covariates Model F(4,5) = 1.48
With Knapp‐Hartung modification Prob > F = 0.3346
| logrra | Coef. | Std. Err. | t | P > t | (95% Conf. Interval) | |
| difcal | .0660771 | .0343893 | 1.92 | 0.113 | −.0223233 | .1544776 |
| _Icatcal_1 | −.1032021 | .5736509 | −0.18 | 0.864 | −1.577819 | 1.371415 |
| _Icatcal_2 | −.511068 | .5948386 | −0.86 | 0.430 | −2.040149 | 1.018013 |
| typepatient | −.5686713 | .502095 | −1.13 | 0.309 | −1.859348 | .7220049 |
| _cons | −.209952 | .6345331 | −0.33 | 0.754 | −1.841071 | 1.421167 |
aRelative Risk
Interpretation of infectious complications. None of the covariates had a statistically significant influence on the size of the intervention effect on infectious complications (P > 0.05).
3. Hospital length of stay
xi: metareg _ES difcal i.catcal typepat, wsse(_seES) bsest(reml)
i.catcal _Icatcal_0‐2 (naturally coded; _Icatcal_0 omitted)
Meta‐regression Number of obs = 9
REML estimate of between‐study variance tau2 = .1866
% residual variation due to heterogeneity I‐squared_res = 84.83%
Proportion of between‐study variance explained Adj R‐squared = ‐0.95%
Joint test for all covariates Model F(4,4) = 0.95
With Knapp‐Hartung modification Prob > F = 0.5178
| _ESa | Coef. | Std. Err. | t | P > t | (95% Conf. | Interval) |
| difcal | .0227448 | .0664972 | 0.34 | 0.750 | −.1618811 | .2073708 |
| _Icatcal_1 | .5450151 | .5673605 | 0.96 | 0.391 | −1.03023 | 2.12026 |
| _Icatcal_2 | −.1219102 | .7756573 | −0.16 | 0.883 | −2.27548 | 2.03166 |
| typepat | −.2502513 | .5116285 | −0.49 | 0.650 | −1.67076 | 1.170257 |
| _cons | −.3512504 | .7353122 | −0.48 | 0.658 | −2.392804 | 1.690304 |
aMean difference
Interpretation of length of hospital stay. None of the covariates had a statistically significant influence on the size of the intervention effect on hospital length of stay (P > 0.05).
4. ICU length of stay
xi: metareg _ES difcal i.catcal typepat, wsse(_seES) bsest(reml)
i.catcal _Icatcal_0‐2 (naturally coded; _Icatcal_0 omitted)
note: typepat dropped because of collinearity
Meta‐regression Number of obs = 11
REML estimate of between‐study variance tau2 = .2453
% residual variation due to heterogeneity I‐squared_res = 83.29%
Proportion of between‐study variance explained Adj R‐squared = ‐3.80%
Joint test for all covariates Model F(3,7) = 0.99
With Knapp‐Hartung modification Prob > F = 0.4503
| _ESa | Coef. | Std. Err. | t | P > t | (95% Conf. | Interval) |
| difcal | .0459442 | .0578779 | 0.79 | 0.453 | −.0909154 | .1828038 |
| _Icatcal_1 | .8943999 | .5321506 | 1.68 | 0.137 | −.3639364 | 2.152736 |
| _Icatcal_2 | .4275402 | .5473569 | 0.78 | 0.460 | −.8667533 | 1.721834 |
| _cons | −.9390958 | .6599035 | −1.42 | 0.198 | −2.49952 | .621328 |
aMean difference
Interpretation of length of ICU stay. None of the covariates had a statistically significant influence on the size of the intervention effect on length of ICU stay (P > 0.05).
Appendix 6. Database for meta‐regression
We conducted the meta‐regressions of each of the outcomes according to the following databases. The codes used to identify each column of the databases were:
trialnam: study ID.
cases1: number of events in the intervention group.
cases0: number of events in the control group.
tot1: number of participants in the intervention group.
tot0: number of participants in the control group.
mean 1: mean value in the intervention group.
SD 1: standard deviation in the intervention group.
total 1: total number of participants in the intervention group.
mean 2: mean value in the control group.
SD 2: standard deviation in the control group.
total 2: total number of participants in the control group.
difcal: absolute difference in kcal/kg/day between the control minus the study group.
catcal: categories according the amount of calories received by the intervention groups. 0 ≥ 25 kcal/kg/day; 1 ≥ 10 to < 25 kcal/kg/day; 2 < 10 kcal/kg/day.
typepatient: medical participants 0; surgical participants 1 (also equivalent to enteral nutrition and parenteral nutrition respectively)
n/a: not available.
Database of mortality in hospital
| Mortality in hospital | |||||||
| trial name | cases1 | tot1 | cases0 | tot0 | difcal | catcal | typepatient |
| Arabi 2011 | 36 | 120 | 51 | 120 | 2.55 | 1 | 0 |
| Arabi 2015 | 108 | 447 | 123 | 445 | 5.48 | 1 | 0 |
| Battistella 1997 | 2 | 27 | 0 | 30 | 7 | 0 | 1 |
| Charles 2014 | 3 | 41 | 4 | 42 | 5 | 1 | 0 |
| Choban 1997 | 0 | 6 | 2 | 7 | 14 | 1 | 1 |
| Ibrahim 2002 | 20 | 75 | 15 | 75 | 4.28 | 2 | 0 |
| McCowen 2000 | 2 | 21 | 3 | 19 | 4.1 | 1 | 1 |
| Petros 2016 | 17 | 46 | 17 | 54 | 8.4 | 1 | 0 |
| Rice 2011 | 22 | 98 | 20 | 102 | 13.71 | 2 | 0 |
Database of infectious complications
| Infectious complications | |||||||
| trial name | cases1 | tot1 | cases0 | tot0 | difcal | catcal | typepatient |
| Ahrens 2005 | 5 | 20 | 2 | 20 | 10.4 | 0 | 1 |
| Arabi 2011 | 53 | 120 | 56 | 120 | 2.55 | 1 | 0 |
| Arabi 2015 | 161 | 448 | 169 | 446 | 5.48 | 1 | 0 |
| Battistella 1997 | 13 | 27 | 22 | 30 | 7 | 0 | 1 |
| Charles 2014 | 23 | 41 | 24 | 42 | 5 | 1 | 0 |
| Ibrahim 2002 | 23 | 75 | 37 | 75 | 4.28 | 2 | 0 |
| McCowen 2000 | 6 | 21 | 10 | 19 | 4,1 | 1 | 1 |
| NHLBI 2012 | 96 | 508 | 79 | 492 | 11.05 | 2 | 0 |
| Petros 2016 | 13 | 46 | 6 | 54 | 8.4 | 1 | 0 |
| Rice 2011 | 30 | 98 | 33 | 102 | 13.71 | 2 | 0 |
Database of length of hospital stay
| Length of hospital stay | |||||||||
| trial name | Mean 1 | SD 1 | total 1 | mean 2 | SD 2 | total 2 | difcal | catcal | typepatient |
| Ahrens 2005 | 23.4 | 23.92 | 20 | 27.8 | 17.4 | 20 | 10.4 | 0 | 1 |
| Arabi 2011 | 70.2 | 106.9 | 120 | 67.2 | 93.6 | 120 | 2.55 | 1 | 0 |
| Arabi 2015 | 48.3 | 67.7 | 448 | 54.4 | 73.9 | 446 | 5.48 | 1 | 0 |
| Battistella 1997 | 27 | 16 | 27 | 39 | 24 | 30 | 7 | 0 | 1 |
| Charles 2014 | 35.2 | 4.9 | 41 | 31 | 2.5 | 42 | 5 | 1 | 0 |
| Choban 1997 | 48 | 30 | 6 | 45 | 38 | 7 | 14 | 1 | 1 |
| Ibrahim 2002 | 16.7 | 12.5 | 75 | 22.9 | 19.7 | 75 | 4.28 | 2 | 0 |
| McCowen 2000 | 19 | 14 | 21 | 17 | 15 | 19 | 4.1 | 1 | 1 |
| Norouzy 2013 | 19.9 | 11 | 30 | 35.6 | 25 | 30 | n/a | n/a | 0 |
| Petros 2016 | 38.1 | 33.4 | 46 | 27.4 | 21.9 | 54 | 8.4 | 1 | 0 |
Database of length of ICU stay
| Length of ICU stay | |||||||||
| trial name | mean 1 | SD 1 | total 1 | mean 2 | SD 2 | total 2 | difcal | catcal | typepatient |
| Ahrens 2005 | 16.75 | 10.35 | 8 | 23 | 15.2 | 10 | 10.4 | 0 | 1 |
| Arabi 2011 | 11.7 | 8.1 | 120 | 14.5 | 15.5 | 120 | 2.55 | 1 | 0 |
| Arabi 2015 | 15.8 | 11.6 | 448 | 16.4 | 12.1 | 446 | 5.48 | 1 | 0 |
| Battistella 1997 | 18 | 12 | 27 | 29 | 22 | 30 | 7 | 0 | 1 |
| Charles 2014 | 16.7 | 2.7 | 41 | 13.5 | 1.1 | 42 | 5 | 1 | 0 |
| Ibrahim 2002 | 9.8 | 7.4 | 75 | 13.6 | 14.2 | 75 | 4.28 | 2 | 0 |
| NHLBI 2012 | 11.5 | 11 | 508 | 11 | 9.8 | 492 | 11.05 | 2 | 0 |
| Petros 2016 | 22.4 | 25.5 | 46 | 17 | 16.1 | 54 | 8.4 | 1 | 0 |
| Rice 2011 | 8.1 | 6.1 | 98 | 7,6 | 5.9 | 102 | 13.71 | 2 | 0 |
| Rugeles 2013 | 9.5 | 5.5 | 40 | 10.4 | 5 | 40 | 2 | 1 | 0 |
| Rugeles 2016 | 13.23 | 6.03 | 60 | 13.45 | 8.33 | 60 | 7.9 | 1 | 0 |
Data and analyses
Comparison 1. Hypocaloric nutrition (intervention) vs. Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality in hospital | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mortality in ICU | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality at 30 days | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Length of Hospital stay (days) | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Length of ICU stay (days) | 11 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Infectious complications | 10 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Length of mechanical ventilation (days) | 12 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Non‐infectious complications (diarrhoea) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Hyperglycaemia | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Hypoglicaemia | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Nitrogen balance (g/day) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahrens 2005.
| Methods |
Study design: prospective, randomized controlled trial. Study dates: “study dates not available" Setting: level‐1 trauma centre. Department of Surgery, Detroit Receiving Hospital, Wayne State University, Detroit, Michigan Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size: calculated sample size of 26 participants to detect an absolute difference in glucose area under the curve of 50 mg hr/dl with 80% power (P = 0.05). 40 participants were randomized: 20 to each group. Only 18 were ICU participants (8 of the low caloric and 10 of the standard group). At baseline both groups were well matched, with exception of lower creatinine clearance in the standard group. Age (years mean ± SD) group 1: 45.3 ± 17.2; group 2: 53.1 ± 17.9 Sex (male, %) group 1: 75; group 2: 80 Most frequent admitting diagnosis (groups 1 and 2 respectively): pancreatitis 6 & 6, trauma 7 & 3, bowel obstruction 4 & 5. ICU participants (n). group 1: 8; S group 2: 10 APACHE II score (mean ± SD of participants in ICU). Group 1: 20.1 ± 9.1; Group 2: 18.6 ± 11.1 Mechanical ventilation (n). 8 participants in each group Baseline nutrition status No major differences between ideal and actual body weight in both groups Duration of parenteral nutrition (days; median (interquartile range)). group 1 6 (4 to 10); group 2 7 (5 to 10) |
|
| Interventions |
Group 1, low caloric parenteral nutrition (n = 20)
Group 2, standard parenteral nutrition (n = 20)
In both groups, parenteral nutrition was administered by a multiple‐bottle system. Lipids administration was standardized to 1000 kcal 3 times weekly. Proteins administered according the levels of estimated metabolic stress of the disease (mild 1.2 ‐ 1.4; moderate 1.5 ‐ 1.7; or severe 1.8 ‐ 2.2 gr/kg/day) |
|
| Outcomes |
Primary outcomes
Incidence of hyperglycaemia was calculated as the number of assessments of glycaemia ≥ 200 mg/dl divided by the total number of assessments Severity of hyperglycaemia was assessed by measuring the area under the curve Secondary outcomes
|
|
| Funding sources | Not available | |
| Declarations of interest | The authors have no financial interests to disclose | |
| Notes | Total calories administered/kg (median (interquartile range)) were: 26.6 (26.2 to 27.5) and 37.0 (36.6 to 38.4); the amount of protein administered and the duration of PN therapy were similar. The first author sent the data of continuous outcomes expressed as mean and standard deviation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by means of a computer‐generated random‐numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacist) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians were blinded to which caloric group participants were randomized to, with the exception of the critical care pharmacist who calculated the formula. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians were blinded to which caloric group participants were randomized to, with the exception of the critical care pharmacist who calculated the formula. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data were available for all participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Arabi 2011.
| Methods |
Study design: prospective, randomized controlled trial Study dates: April 2006 to January 2008 Setting: 1 tertiary care academic hospital Country: Saudi Arabia |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size: authors estimated a relative difference of 50% in ICU mortality between participants receiving .90% of caloric requirements and those receiving 60% to70% of caloric requirements (28% compared with 14%). Quote: “on the basis of an estimated 28‐d mortality rate of 25%, a power of 0.8, and an α of 0.05, the number of subjects needed to show a reduction in mortality was 120 in each group.” Age (years): intervention group: 50.3 ± 21.3; Control group: 51.9 ± 22.1 Sex (male, %): intervention group: 71.1; Control group: 65 Primary disease of the participants Intervention; Control group Admission category (n (%)) Nonoperative 95 (79.2); 103 (85.8) Postoperative 25 (20.8); 17 (14.2) Traumatic brain injury 35 (29.2); 31 (25.8) Disease severity score: APACHE II Intervention group: 25.2 ± 7.5; Control group: 25.3 ± 8.2 Mechanical ventilation n (%) Intervention group: 119 (99.2); Control group: 119 (99.2) Comorbidities: not available Nutrition status: intervention group; Control group: Not available Level of inflammation: not available |
|
| Interventions |
Intervention Group 1 (n = 120)
Control Group 2 (n = 120)
Quote: “for both groups, caloric requirement was estimated by the dietitian using the Harris‐Benedict equations and adjusting for stress factors. The selection of formula was left to discretion of the attending physician as long as it satisfied the total caloric intake criteria and was not enriched with immunonutrients. Calculation of caloric intake took into account intravenous dextrose and propofol infusions.” Quote: “the patients were followed until discharge from the ICU, except if the patient tolerated oral feeding, had a do‐not‐resuscitate order written (after enrolment), or became brain dead (after enrolment). In the latter situations, the intervention was stopped but the outcome data were collected.” Co‐interventions Quote:“The protein requirement was calculated as 0.8–1.5 g/kg on the basis of patient condition and underlying diseases. To avoid protein malnutrition in the permissive underfeeding group, additional protein (Resource Beneprotein; Nestle Healthcare Nutrition Inc, Minneapolis, MN) was added to maintain the full protein requirement without affecting the assigned caloric intake.” |
|
| Outcomes |
Primary outcome
Secondary outcomes
How measured or definition and time point measured
Subgroups
|
|
| Funding sources | Funded by King Abdulaziz City for Science and Technology (LG 10‐30). | |
| Declarations of interest | No potential conflict of interest relevant to this article was reported. | |
| Notes | As it was a 2 x 2 factorial trial, the enrolled participants were randomly assigned by using concealed envelops to 1 of the 4 study groups: 1‐permissive underfeeding with intensive insulin therapy (IIT), 2‐permissive underfeeding with conventional insulin therapy (CIT), 3‐target feeding with IIT, and 4‐target feeding with CIT. We grouped 1 and 2; 3 and 4. Blood glucose concentration target was 4.4 – 6.1 mmol/L (80 – 110 mg/dL) in the IIT group and 10 – 11.1 mmol/L (180 – 200 mg/dL) in the CIT group. The frequency of blood glucose monitoring increased to every 20 mins when blood glucose concentrations decreased to > 3.2 mmol/L (58 mg/dL) and reduced to every 2 – 4 hrs when measurements were stable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On the basis of (quote:) "computer‐generated random permuted blocks" |
| Allocation concealment (selection bias) | Low risk | The enrolled participants were randomly assigned by using concealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded study. Details on healthcare processes to be followed by personnel (e.g. co‐interventions) were not described in order to make an appropriate judgement on possible performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but main and secondary outcomes well‐defined. We judge that the outcome measurement was probably not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data were available for all participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Arabi 2015.
| Methods |
Study design: prospective, randomized controlled trial Study dates: November 2009 to September 2014 Setting: 7 tertiary care centres Country: Saudi Arabia and Canada |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size With 432 participants in each group; with an estimated 3% loss to follow‐up, the final calculated sample size was 892 participants. Permissive underfeeding would be associated with an absolute risk reduction in mortality of 8 percentage points. Assuming an estimated 90‐day mortality of 25% with standard feeding, they estimated that enrolment of 432 participants in each group would give the study 80% power. Age (years): intervention group: 50.2 ± 19.5; Control group: 50.9 ± 19.4 Sex (male, %): intervention group: 65.2; Control group: 63.2 Primary disease of the participants Intervention; Control group Medical no. (%) 336 (75.0); 335 (75.1) Surgical no. (%) 19 (4.2); 12 (2.7) Nonoperative trauma no. (%) 93 (20.8); 99 (22.2) Severe sepsis at admission no. (%) 159 (35.5); 133 (29.8) Traumatic brain injury no. (%) 55 (12.3); 63 (14.1) Disease severity score: APACHE II Intervention group: 21.0 ± 7.9; Control group: 21.0 ± 8.2 Mechanical ventilation no. (%) Intervention group: 436 (97.3); Control group: 429 (96.2) Comorbidities: not available Nutrition status: intervention group; Control group Albumin g/litre 28 ± 7; 28 ± 6 Prealbumin g/litre 0.15 ± 0.13; 0.14 ± 0.12 Transferrin g/litre 1.36 ± 0.49; 1.38 ± 0.50 24‐hour urinary nitrogen excretion mmol 284 ± 176; 303 ± 219 Level of inflammation : not available |
|
| Interventions |
Intervention Group (n = 448)
Control group (n = 446)
For both groups, the calculation of caloric requirements was using the Penn State equation for mechanically‐ventilated participants who had a BMI < 30 and using the 1992 Ireton‐Jones equation for mechanically‐ventilated participants who had a BMI of 30 or higher and for spontaneously‐breathing participants. Protein requirements were calculated at 1.2 to1.5 g per kilogram of body weight a day, in accordance with clinical practice guidelines. Co‐interventions Quote. "to ensure that enteral protein and volume delivery in the permissive‐underfeeding group would be similar to those in the standard‐feeding group, the permissive‐underfeeding group received additional protein (Beneprotein, Nestlé Nutrition) and normal saline or water at a dose of 2 ml per kilogram every 4 hours unless otherwise specified by the clinical team. The assigned intervention was continued for up to 14 days or until ICU discharge, initiation of oral feeding, death, or withholding of nutrition as part of palliation." The study protocol provided suggestions on the selection of enteral formulas on the basis of published guidelines; however, the decision was left to the clinical team. Study centres used their own insulin protocols, with a target blood glucose level of 4.4 to 10 mmol. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Tertiary outcomes
How measured or definition and time point measured
Subgroups
|
|
| Funding sources | Funded by the King Abdullah International Medical Research Center | |
| Declarations of interest | No potential conflict of interest relevant to this article was reported. | |
| Notes | The total caloric intake included calories from propofol, intravenous dextrose and parenteral nutrition. The author provided additional information about mean and standard deviation values of length of hospital and ICU stay and of mechanical ventilation. In 2017, the researchers published a subgroup analysis using a Nutrition Risk In Critically ill (NUTRIC) score. However these subgroup analyses did not contribute to our review objectives. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomization list was computer‐generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "enrolled patients were randomly assigned to the permissive‐underfeeding group or the standard‐feeding group with the use of opaque, sealed, sequentially numbered envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | PermiT was a multicentre, pragmatic, open‐label international randomized clinical trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data were available for 445/448 and 440/446 participants in the intervention and control group respectively. |
| Selective reporting (reporting bias) | Low risk | Authors reported all protocol outcomes. |
| Other bias | Low risk | No evidence of other bias |
Battistella 1997.
| Methods |
Study design: prospective, randomized controlled trial Study dates: September 1992 to July 1994 Setting: Trauma surgery service. University of California, Davis, Medical Center Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size: 60 participants randomized, data analysed of 57 participants Age (years; mean ± SD). Group 1: 32 ± 9; Group 2: 33 ± 10 Sex (male, %). Group 1: 85%; Group 2: 80% Type of injury (blunt trauma %): Group 1: 85%; Group 2: 80% APACHE II score (mean ± SD). Group 1: 22 ± 5; Group 2: 23 ± 6 Injury severity score (mean ± SD). Group 1: 30 ± 9; Group 2: 27 ± 8 Nutrition status. On admission no participants weighted less than ideal body weight |
|
| Interventions | Participants randomized at the 5th post‐injury day. 10 days study period with parenteral nutrition. No lipid group (Group 1) (n = 27)
Lipid group (Group 2) (n = 30)
|
|
| Outcomes |
Outcomes evaluated after 10 days of parenteral nutrition Other outcomes
|
|
| Funding sources | Study supported in part by National Institutes of Health Grant P30 DK‐35747 | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasonable explanation: quote: "Of the 60 patients enrolled, only 57 had data that could be analysed. One patient was ineligible for the study because he had been admitted for management of an entero‐cutaneous fistula that had resulted as a complication of a remote trauma and two patients died before being randomized (before the fifth post injury day)". |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias. |
Charles 2014.
| Methods |
Study design: prospective, randomized controlled trial Study dates: March 2008 to November 2011 Setting: Surgical/trauma ICU at a tertiary‐care hospital. Department of Surgery, University of Virginia Health System. Charlottesville, Virginia Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size: From 2892 admissions to the ICU 83 participants were enrolled and randomized: 41 to the hypocaloric group and 42 to the eucaloric group (detailed flow diagram given of the randomization, exclusion and study end) Age (years; mean ± SD). Hypocaloric group 50.4 ± 2.8; Eucaloric group 53.4 ± 2.7 Sex (male, %). Group 1: 58.3; Group 2: 73.8 Primary disease. Trauma admission (%). Group 1: 68.3; Group 2: 59.5. The other participants in the surgical ICU were abdominal, vascular, orthopaedic and liver transplant surgery. Disease severity, APACHE II score (mean ± SD) Group 1: 16.6 ± 0.9; Group 2: 17.3 ± 0.8 Mechanical‐ventilation dependence (%). Group 1: 68.3; Group 2: 57.1 Comorbidities. Diabetes mellitus and coronary artery disease (%). Group 1. 19.5 and 17.1 respectively; Group 2: 14.3 and 11.9 respectively Nutrition status BMI (kg/m2, mean ± SD). Group 1: 32.9 ± 2.0; Group 2: 28.1 ± 0.9 Risk of refeeding syndrome at admission (due to weigh loss, poor caloric intake or alcohol abuse) (%). Group 1: 31.7; Group 2: 54.8 Level of inflammation: not available |
|
| Interventions |
Group 1 hypocaloric (n = 41)
Group 2 eucaloric (n = 42)
Co‐interventions: the protein goal of the 2 groups was 1.5 grams protein/kg/day. If the participant’s actual weight was > 130% of ideal weight, adjusted weight was used. Participants with severe malnutrition not able to receive enteral nutrition were considered for parenteral nutrition, all others received enteral nutrition. In cases of enteral feeding intolerance, parenteral nutrition was started after 5 to 7 days. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Subgroups
|
|
| Funding sources | Supported by grant 5‐T32‐AI‐078875‐03 from the National Institute of Health, USA | |
| Declarations of interest | The authors stated that “No conflicts of interest were reported” | |
| Notes | Due to slow enrolment, the study was closed before the planned enrolment of 116 participants. Enteral nutrition was given initially. Participants were considered for parenteral nutrition if they were severely malnourished and could not receive enteral feeding, or in case of continuous intolerance of enteral nutrition lasting more than 5 to 7 days. The author provided additional information: mean and standard deviation of the length of mechanical ventilation and to complete the 'Risk of bias' table. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated 1:1 by using a computer‐based random number generator |
| Allocation concealment (selection bias) | Low risk | Quote: "investigators were blinded to the preparation of the randomization envelopes, and the randomization assignment was determined by opening sequential opaque security envelopes containing the randomization assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded (written information provided by the author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data were available for all participants. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No evidence of other bias |
Choban 1997.
| Methods |
Study design: prospective, randomized controlled trial Study dates: Not stated Setting: participants referred to the Nutrition Support Service of the Ohio State University Hospital. Departments of Surgery and Medical Dietetics. College of Medicine. Ohio State University. Columbus, Ohio Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size: 30 participants, stratified according their hospitalization in ICU (n = 13) or regular floor (n = 17) (randomized with separate randomization tables) Age (years; mean ± SD; whole sample): Group 1: 52 ± 19; Group 2: 52 ± 15 Sex (male, %: whole sample): Group 1: 31.25; Group 2: 14.29 Primary disease of the participants, surgical diseases. 70% of the whole‐sample diagnosis were cancer with or without enterocutaneous fistulae and pancreatic disease. Nutrition status. Body weight/BMI (kg and kg/m2 respectively; mean ± SD; whole sample). Group 1: 97 ± 19 and 36 ± 5. Group 2: 90 ± 17 and 34 ± 6 Comorbidities. Diabetes type 1 and 2 (n of ICU participants) Group 1: 2 and 1; Group 2: 2 and 2 Disease severity score. APACHE II score at the time of enrolment (mean ± SD of the ICU participants). Group 1: 13 ± 5; Group 2: 15 ± 5 Level of inflammation Initial urinary urea nitrogen (grams/24 hours; mean ± SD of the ICU participants). Group 1: 10.1 ± 9.0; Group 2: 10.0 ± 4.2 Duration of PN (days; mean ± SD). Group 1: 10 ± 3; Group 2: 12 ± 2 |
|
| Interventions |
Group 1 hypocaloric PN (whole sample n = 16; n of ICU participants = 6) has 50% of the carbohydrate and lipid compared with the standard PN. Group 2 standard PN (whole sample n = 14; n of ICU participants = 7) Co‐interventions: both PN solutions were isonitrogenous, providing 2 grams of protein/kg ideal body weight/day, added with electrolytes, vitamins and trace elements, administered during ≤ 14 days or until they could receive enteral or oral feeding. |
|
| Outcomes |
Time points reported
Subgroups
|
|
| Funding sources | Supported by funds from the Bremer Foundation, Department of Surgery Medical Research Development Fund, and Surgical Research, Inc. | |
| Declarations of interest | Not available | |
| Notes | Both groups of participants in ICU had moderate severity of diseases by APACHE II scores, the initial urinary urea nitrogen and the mortality rate (15%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were then randomly assigned to receive either the control parenteral nutrition (PN) formula or the hypoenergetic PN formula by using separate randomization tables by the investigational pharmacist in the research pharmacy of the hospital. |
| Allocation concealment (selection bias) | Low risk | Participants randomly assigned to receive either the control PN formula or the hypoenergetic PN formula by using separate randomization tables (ICU or regular floor) by the research pharmacist of the hospital (Central allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All care providers as well as participants were blinded to the nutrient composition of the parenteral formulas. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded. All care providers as well as participants were blinded to the nutrient composition of the parenteral formulas. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data were available for all participants. |
| Selective reporting (reporting bias) | Low risk | Not clearly described research outcomes, although: (quote:) "this study was designed to determine whether a restricted energy parenteral formulation providing 2 gr protein/kg ideal body weight could be administered to acutely ill obese participants with the same degree of efficacy as a standard parenteral nutrition solution provided to a comparable group of patients". Participants located in the intensive care unit and those with diabetes mellitus were included in the study population to determine the efficacy of this treatment in critically‐ill participants and to assess the effect on glycaemic control in obese diabetic participants. Results were reported regarding this description and more detailed measurement methods described in the appropriate section. |
| Other bias | Unclear risk | Not clear if any bias could have been introduced by some of the funders |
Ibrahim 2002.
| Methods |
Study design: prospective, randomized controlled trial Study dates: May 1999 to December 2000 Setting: Medical ICU, Barnes‐Jewish Hospital, affiliated to Washington University School of Medicine. St. Louis, Missouri Country: USA. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size 189 consecutive participants were evaluated for enrolment, with 39 not included for different reasons, and 150 finally included and analysed. 75 participants were randomized to each study group. The estimated sample size for a significant reduction of the incidence of pneumonia (primary outcome) was 82 participants in each study group. Age (years, mean ± SD). Group 1: 59.1 ± 19.0; Group 2: 56.5 ± 15.6 Sex (% of male). Group 1: 46.7; Group 2: 37.3 Primary reason for ICU admission. Respiratory diseases (%). Group 1: 58.7; Group 2: 64.0 Disease severity: APACHE II score. Group 1: 25.6 ± 8.3; Group 2: 24.7 ± 8.4 PaO2/FiO2 (mean ± SD). Group 1: 204 ± 108; Group 2: 207 ± 126 Predicted mortality based on APACHE II score (%, mean ± SD). Group 1: 48.7 ± 24.9; Group 2: 49.6 ± 23.9 Process of care variables: with 2 exceptions, all of them had statistically non‐significant differences between the study groups: Duration of enteral nutrition and of mechanical ventilation (days, mean ± SD respectively). Group 1: 5.2 ± 5.9 and 8.1 ± 7.4; Group 2: 9.9 ± 12.3 and 12.9 ± 15.7 respectively. Comorbidities, nutrition status and level of inflammation: not reported |
|
| Interventions |
Group 1 late feeding‐hypocaloric (n = 75)
Group 2 early feeding‐normocaloric (n = 75)
Co‐interventions The goal for enteral nutrition daily requirements were defined as 25 kcal/kg ideal body weight/day and 1 to 1.3 grams of protein/kg ideal body weight/day. The enteral nutrition, with a polymeric iso‐osmolar formula, was administered in the stomach by bolus feeding, through an orogastric tube inserted on day 1 of mechanical ventilation. In case of 3 consecutive gastric residual volumes > 150 ml, a post‐pyloric enteral tube was inserted for continuous drop enteral nutrition. |
|
| Outcomes |
Primary outcome
Secondary outcomes
How measured or defined
Time of measurements
Subgroups
|
|
| Funding sources | Supported in part by a grant from the Barnes‐Jewish‐Christian Health Care Innovations Program | |
| Declarations of interest | Information not available | |
| Notes | The total calories and protein received by the participants showed a statistically significant difference between the study groups, but participants in each group only received a percentage of the defined goals during the first 5 days of mechanical ventilation: in the hypocaloric group the participants received 7% of their estimated caloric requirements and 7.7% of the estimated protein requirements, and in the control group they received 27.9% and 26.9% respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study allocated participants to treatment groups based on the date of their ICU admission using a quasi‐randomized design (odd/even‐numbered days). |
| Allocation concealment (selection bias) | High risk | The study allocated participants to treatment groups based on the date of their ICU admission using a quasi‐randomized design. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded study. Details on healthcare processes to be followed by personnel (e.g. co‐interventions) were not described in order to make an appropriate judgement on possible performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but main and secondary outcomes well‐defined. We judge that the outcome measurement was probably not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data were available for all participants. |
| Selective reporting (reporting bias) | High risk | Some prespecified secondary outcomes (duration of mechanical ventilation, need for gastrostomy tube) not reported |
| Other bias | Low risk | No evidence of other bias |
McCowen 2000.
| Methods |
Study design: prospective, randomized, controlled non‐blinded trial Study dates: Not stated Setting: single‐centre, university‐affiliated teaching hospital with a dedicated total parenteral nutrition (TPN) service. Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size 48 participants were initially included, but 4 in each group were excluded from the analysis because of PN duration ≤ 4 days, leaving 21 participants in the hypocaloric group and 19 in the control group Age (years; mean ± SD). Group 1 hypocaloric: 57.5 ± 14.9; Group 2 control: 56.6 ± 20.4 Sex (% male): Group 1: 57; Group: 53 Primary disease of the participants. Mainly surgical participants with different types of complications. Major differences between groups: Group 1 acute pancreatitis and bowel surgery/postoperative ileus: n = 6 and 3 participants respectively; Group 2 n = 1 and 6 respectively. Mechanically‐ventilated participants (n). Hypocaloric group: 11; Control group: 6 Comorbidities. Diabetes (n). Group 1: 5 participants; Group 2: 2 participants. Obesity: 4 participants in each group Nutrition status. BMI (mean ± SD). Group 1: 27.6 ± 8.1; Group 2: 25.7 ± 6.2 |
|
| Interventions |
Group 1 hypocaloric (n = 21)
Group 2 control (n = 19)
Co‐interventions
|
|
| Outcomes |
Time points of measurements
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Due to a protocol violation, fat was given to 1 participant in the hypocaloric group. Some results associated with hospital rules to avoid iatrogenic hyperglycaemia by gradual increase of nutrients to avoid complications.The hypocaloric group also received less protein than the control group. More participants in the hypocaloric group had acute pancreatitis and mechanical ventilatory support than in the control group. The hypocaloric group received 14 ± 3 kcal/kg/day and the control group 18 ± 4 kcal/kg/day (also hypocaloric). The hypocaloric group not only received significantly fewer calories than the control group (due to fewer dextrose and fat calories), but also less protein (1.1 ± 0.2 versus 1.3 ± 0.2 in the control group). If the infection rate trend observed were to persist, they calculated the study would have required ˜174 participants to see a statistical difference between the 2 groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding: the standard group received parenteral nutrition as 3‐in‐1 bags, and the hypocaloric group received 1 litre of fat‐free parenteral nutrition. Outcomes could have been influenced by different performance of clinical personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and not clearly‐defined and objective outcomes that would warrant a low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 participants in each group were excluded from the data analysis because of a TPN duration of ≤ 4 days (not prespecified exclusion criteria). |
| Selective reporting (reporting bias) | High risk | Nitrogen balance was only measured in 12 participants (57%) in the hypocaloric and 10 (53%) of the control group, usually because of an error during collection. |
| Other bias | Unclear risk | The lack of detail in the description of the Methods section could not warrant a low risk of other sources of bias. |
NHLBI 2012.
| Methods |
Study design: prospective, randomized controlled trial Study dates: January 2008 to April 2011 Setting: 44 ICUs of the National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size 500 participants for each arm, to detect a 2¼‐day difference in ventilator‐free days (VFDs), assuming a mean of 14 ± 10.5 VFDs. power: 91% α: 0.05 Age (years): intervention group: 52 ± 17; Control group: 52 ± 16 Sex (male, %): intervention group: 53; Control group: 49 Primary disease of the participants Diagnosis:% intervention group/% control group Medical ICU: 61; 63 Primary lung injury category % intervention group/control group Pneumonia 67; 63 Sepsis 16; 13 Aspiration 8; 11 Trauma 3; 4 Transfusion 1; 2 Disease severity score: APACHE III Intervention group: 92 ± 28; Control group: 90 ± 27 Mechanical ventilation 100% in each group (inclusion criterion) Comorbidities: % intervention group; % control group Diabetes: 27; 29 No other data available Nutrition status: not available Level of inflammation: not available |
|
| Interventions |
Intervention (trophic) Group 1 (n = 508)
Control Group 2 (n = 492)
Co‐interventions
|
|
| Outcomes |
Primary outcome
Secondary outcomes
How measured or definition
Subgroups
|
|
| Funding sources | Supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268200536165C and HHSN268200536179C | |
| Declarations of interest | Authors have not disclosed any potential conflicts of interest. | |
| Notes | The initial 272 participants were also simultaneously randomized to a separate trial (the OMEGA study) comparing a nutritional supplement containing omega‐3 fatty acids and antioxidants with an isocaloric, isovolemic control in a 2 x 2 factorial design. After the Data and Safety Monitoring Board stopped the OMEGA portion of the factorial design, participants randomized to the initial trophic‐feeding group received additional calories to compensate for the calories that had been received in the OMEGA study (240 ml volume a day). We asked the first author for some data not reported in the manuscript or reported differently. He gave us the data we used in the meta‐analysis for the following outcomes: 28‐day mortality, length of ICU stay (days from randomization to first ICU discharge); length of mechanical ventilation (ventilator days up to day 28); hyperglycaemia (participants with any on‐study glucose > 200 mg/dl); incidence of total infectious complications and of diarrhoea, and the amount of calories received by both groups of participants. The author also informed they did not have duplicate participants with the Rice 2011 study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized by a web‐based randomization system, stratified by site and presence of shock at enrolment, to receive either trophic or full enteral feeding for the first 6 days of mechanical ventilation. |
| Allocation concealment (selection bias) | Low risk | Participants were randomized by a web‐based randomization system, stratified by site and presence of shock at enrolment, to receive either trophic or full enteral feeding for the first 6 days of mechanical ventilation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded study. Details on healthcare processes to be followed by personnel (e.g. co‐interventions) were not described in order to make an appropriate judgement on possible performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was not blinded but most outcomes were objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant lost, from the control group. All analyses were by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported. All analyses were by intention‐to‐treat. |
| Other bias | Low risk | No evidence of other bias |
Norouzy 2013.
| Methods |
Study design: single‐centre double‐blind, randomized controlled trial Study dates: Not stated Setting: Nutrition and neurosurgery departments. Mashad University of Medical Sciences. Mashad Country: Islamic Republic of Iran |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size 60 participants randomized Age: not reported Sex: not reported Primary disease: head trauma Disease severity: not reported Mechanical ventilation: number of participants not reported Comorbidities: not reported Nutrition status: not reported Level of inflammation: not reported |
|
| Interventions |
Group 1 permissive underfeeding (n = not reported )
Group 2 standard full calorie (n = not reported)
All participants received enteral nutrition |
|
| Outcomes |
Primary outcome
Secondary outcomes
No information about measures or definition of the outcomes The participants in the permissive‐underfeeding group received full enteral feeding after the 7th day of the study. No subgroups reported |
|
| Funding sources | Not available | |
| Declarations of interest | None declared | |
| Notes | Available only in abstract form. Poster presentation in the 35th ESPEN Congress (Leipzig, Germany, August 2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned in the abstract (quote:) "head trauma randomly assigned to a double‐blind randomized controlled clinical trial" |
| Allocation concealment (selection bias) | Unclear risk | Same as above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Mentioned that was double‐blind, but did not report the methodology |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Mentioned that was double‐blind, but did not report the methodology |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned in the abstract |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned in the abstract |
| Other bias | Unclear risk | Insuficient information to make judgement (abstract only) |
Petros 2016.
| Methods |
Study design: prospective, randomized controlled trial Study dates: July 2008 to December 2010 Setting: 1 tertiary medical ICU Country: Germany |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size: not available Age (years): intervention group: 67.6 ± 11.5; Control group: 64.3 ± 11.5 Sex (male, %): intervention group: 70; Control group: 63 Primary disease of the participants Diagnosis: % intervention group; % control group Sepsis: 25; 28 Acute cardiovascular dysfunction: 30; 46 Acute respiratory insufficiency: 22; 33 Other: 9; 11 Disease severity score: APACHE II Intervention group: 28.6 ± 6.5; Control group: 27.7 ± 8.4 Mechanical ventilation: not available Comorbidities: % intervention group; % control group Underlying chronic disease: None: 26; 43 Diabetes mellitus: 33; 20 Respiratory: 31; 22 Cardiovascular: 19; 20 Neuropsychiatric: 0; 20 Other: 9; 13 Nutrition status: not available Level of inflammation: not available |
|
| Interventions |
Intervention group 1 (n = 54)
Control group 2 (n = 46)
For both groups, energy expenditure was measured with an indirect calorimeter (Deltatrac II, Datex Ohmeda, Helsinki, Finland). If this was not possible, the Ireton‐Jones prediction equation was used. Co‐interventions Quote: “artificial nutrition support was started within 24 hours of ICU admission. Enteral feeding was favoured in every case if there was no sign of gastrointestinal intolerance (defined as gastric aspirate > 300 mL/d) and/or diarrhoea. Diarrhoea was defined as at least 3 watery bowel movements per day or continuous watery stool. In case of enteral feeding, the target energy supply was to be achieved on day 3 at the latest. A commercially available standard solution with a caloric concentration of 1 kcal/mL was used in every case. If at least 70% of the target caloric supply was considered not to be achieved on day 3 via the enteral route based on gastrointestinal tolerance and the consensus of the managing physicians together with members of the trial group, participants received supplementary parenteral nutrition. The expected deficit was calculated everyday during the morning hours by one of the study authors and supplementary PN prescribed as required. If enteral nutrition (EN) was to be interrupted for unforeseen reasons during the course of the day (diagnostic or therapeutic procedures), adjustment of the supply rate was carried out depending on clinical judgment as to whether an increased rate would be tolerated by the participant. In such cases, possible caloric deficits were not compensated with PN. Causes of the feeding interruptions were recorded if the interruption lasted at least an hour. The blood glucose level was monitored every 3 hours. The insulin dose was adjusted to a target blood glucose level of 6–8 mmol/L.” |
|
| Outcomes |
Primary end point
Secondary end points
|
|
| Funding sources | None declared | |
| Declarations of interest | None declared | |
| Notes | Study originally published as a congress abstract with few results. The first author answered several questions, so some of the results originally included in the review came from the information provided by him. During the editorial process the study was published (Petros 2016). All the published data were the same as the first author had originally reported to us. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic randomization list |
| Allocation concealment (selection bias) | Low risk | Quote: "yes, the allocation was concealed. The electronic randomization was managed by coauthors not directly involved in the management of the patients” (written information provided by the author) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was single‐blinded (participants were blinded, the ICU personnel were not). Details on healthcare processes to be followed by personnel (e.g. co‐interventions) were not described in order to make an appropriate judgement on possible performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was not blinded but outcomes were objective (written information provided by the author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data were available for all participants (written information provided by the author) |
| Selective reporting (reporting bias) | Low risk | All outcome assessed were reported (written information provided by the author). |
| Other bias | Low risk | No evidence of other bias |
Rice 2011.
| Methods |
Study design: prospective, randomized controlled trial Study dates: August 2003 to July 2009 Setting: 2 ICUs at a single academic centre Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size 94 participants were randomized in each arm. An independent sample t test, designed to demonstrate a 15% relative increase of 3.0 VFDs with 80% power and a 2‐sided P value of 0.05. The study enrolled 200 to allow for a 5% withdrawal rate and compensate for the single interim analysis. Age (years): intervention group: 53 ± 19; Control group: 53 ± 19 Sex (male, %): intervention group: 39.8; Control group: 46.1 Primary disease of the participants: 100% medical diagnosis Acute lung injury: 21; 20 Pneumonia: 15; 19 Altered mental status/neurologic: 14; 15 Sepsis: 10; 12 Overdose: 10; 7 Disease severity score: APACHE II Intervention group: 26.9 ± 8.1; Control group: 26.9 ± 6.6 Mechanical ventilation 100% in each group (inclusion criteria) Comorbidities:% intervention group/ % control group Hypertension 42; 37 Cardiac disease 24; 23 Diabetes 22; 23 Chronic renal insufficiency 18; 12 Chronic obstructive pulmonary disease 16; 18 Immunosuppression 14; 16 Peptic ulcer disease 4; 4 Gastroesophageal reflux 4; 4 Nutrition status: not available Albumin concentration (g/dL) 2.8± 0.6; 2.8± 0.7 Level of inflammation: not available |
|
| Interventions |
Group 1 (n = 98): trophic group
Group 2 (n = 102): control group
Co‐interventions For both groups, in participants who were extubated and then required re‐intubation, enteral nutrition was started and managed according to the study protocol through study day 28. Elevated gastric residual volumes (GRV) were defined as > 300 cc of gastric contents withdrawn from the gastric tube at one time. GRVs were checked every 6 hours while feeding rates were being increased to full‐energy rates and every 12 hours if the participant was receiving trophic rates or once full‐energy rate was achieved. Gastric residuals were only measured in participants with post‐pyloric feeding tubes if a separate gastric port on the feeding tube or separate gastric tube was in place. Since a single, isolated elevated GRV has been shown to be a poor predictor of enteral nutrition intolerance, feeding rates were not adjusted after a single elevated GRV. After the first episode of elevated GRV, 300 cc was replaced and the feeding rate was maintained. GRV was rechecked in 2 hours. If this recheck was also above 300 cc, feeds were held until GRV decreased below 300 cc and restarted at a rate of 25 cc/hr < the previous rate in the full‐energy group and at 10 cc/hr in the trophic group. |
|
| Outcomes |
How measured or definition
Time points measured and time points reported
Subgroups
|
|
| Funding sources | Supported, in part, by grants K23HL81431(TWR), P30DK058404 (TWR), and 1 UL1 RR024975 (TWR, GRB) from the National Institutes of Health (Bethesda, MD) | |
| Declarations of interest | Dr Rice, Dr Bernard, and Dr Wheeler received funding from the National Institutes of Health. The remaining authors have not disclosed any potential conflicts of interest. | |
| Notes | Variables were assessed by intention‐to‐treat analyses. Upon our request, the data for the following outcomes was provided by the first author: hospital and 28‐day mortality, length of mechanical ventilation, length of ICU stay and incidence of infectious complications. None of the participants included in this study was included in NHLBI 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block scheme with a random block size of 2, 4 or 6 participants. |
| Allocation concealment (selection bias) | Low risk | Assignments were placed in consecutively‐numbered, opaque envelopes that were sealed before the start of the study by personnel not associated with the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded study. Details on healthcare processes to be followed by personnel (e.g. co‐interventions) were not described in order to make an appropriate judgement on possible performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open‐label study but most outcomes were objective. The number of ventilator‐free days to study day 28 was the primary efficacy measure. Secondary end points included 28‐day and hospital all‐cause mortality, organ‐failure‐free days, ICU‐free days, and hospital‐free days to study day 28. Only gastrointestinal intolerance and infections are more subjective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants had complete follow‐up to death or hospital discharge. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Rugeles 2013.
| Methods |
Study design: prospective, randomized controlled trial Study dates: August 2011 to July 2012 Setting: 30‐bed ICU of a tertiary‐level university hospital Country: Colombia |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size 80 participants: 40 participants in each group to detect an absolute difference in the SOFA score between the 2 measurements of 15% (8.0 expected total score and 1.2 for expected delta SOFA) and a SD between the difference of the means of 3.0. 80% power α error of 0.05 Age (years): intervention group: 53.3 (19.5); Control group: 55.7 (19.5) Sex (male, %): intervention group: 55; Control group: 60 Primary disease of the participants Reasons for admission‐ Intervention/Control group n (%) Respiratory disease 16 (40); 14 (35) CNS disorder 13 (33); 12 (30) Cardiac disease 2 (5); 4 (10) Gastrointestinal disease 0 (0); 3 (8) Other 9 (23); 7 (18) Disease severity score: APACHE II Intervention group: 13.9 ± 4.8; Control group: 15.1 ± 6.2 Mechanical ventilation no. (%) Not available Comorbidities: not available Nutrition status: not available Level of inflammation: not available |
|
| Interventions |
Intervention Group 1 (n = 40)
Control Group 2 (n = 40)
Co‐interventions "for both groups, it was used an enteral formula in continuous feeding. To reach the protein goal, the study group regimen was enriched with additional protein modules, based on soy protein diluted in water and administered in two daily boluses. Participants in the study group received hyperproteic regimen until day 7, if they needed any further enteral nutrition they were switched to standard nutritional regimen with a goal of 25 kcal/kg/day without protein boluses." |
|
| Outcomes |
Primary outcome
Secondary outcomes
Subgroups Not available |
|
| Funding sources | This research was supported by an unrestricted grant from Lafrancol Colombia. | |
| Declarations of interest | No potential conflict of interest relevant to this article was reported. | |
| Notes | The first author sent us the final manuscript of the study before publication, and answered our questions about the average time of the participants on enteral nutrition, the standard deviation of the calories and proteins received by both groups, why they did not report mortality and the way they gave the protein supplements to achieve the double blinding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using dark sealed envelopes with computer‐generated random allocations. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed using dark sealed envelopes with computer‐generated random allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind clinical trial. Although one of the investigators was not blind: (quote:) "only one of the members of the team (JDR) knew patient allocation, prescribed the formulations, and supervised the administration of the regimens; but ICU staff, who decided on daily care patient, was blind to patient allocation". The authors, upon request, gave further explanations about how there was low risk of blinding being broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind clinical trial. Although one of the investigators was not blind (quote:) "only one of the members of the team (JDR) knew patient allocation, prescribed the formulations, and supervised the administration of the regimens; but ICU staff, who decided on daily care patient, was blind to patient allocation". The authors, upon request, gave further explanations about how there was low risk of blinding being broken. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "only patients who completed 96 hours of follow‐up were considered for the analysis; patients who did not fulfil the follow‐up period were excluded, and the envelope was returned to the sequence for patient replacement, until completion of the sample size (40 in each group)". Although the inclusion criteria stated that "Study population consisted of adult patients (18 years or older) admitted in the ICU, who were expected to require enteral nutrition through nasoenteric tube for at least 96 hours.", having participants randomized, intervened, and then excluded if they did not have 96 hours of enteral feeding could lead to a high risk of selection bias. Especially if the primary endpoint was "change in SOFA score at 48 hours". The number of excluded participants was significant: "In total, 115 potential patients met the initial inclusion criteria for enrolment, but only 80 completed the follow‐up and were included in the per protocol analysis". |
| Selective reporting (reporting bias) | High risk | Mortality, a secondary outcome, was not reported. Nevertheless, upon request, the authors responded that given that they excluded participants that did not fulfil the 96 hours of enteral nutrition requirement, they did not report mortality because this result would have been biased (they only measured mortality in participants who completed the 96 hours). This is why they did not report it. This is correct, although the best thing would have been to perform an intention‐to‐treat analysis and also report premature deaths. |
| Other bias | Low risk | No evidence of other bias |
Rugeles 2016.
| Methods |
Study design: prospective, randomized controlled trial Study dates: December 2013 to July 2015 Setting: 30‐bed ICU of a tertiary‐level university hospital Country: Colombia |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size 60 participants in each group to detect a 15% (1.7 points) difference in SOFA at 48 hours between the 2 groups with an SD of 1.9 with a 2‐tailed t test. 80% power α error of 0.05 Age (years): intervention group: 53.8 ± 19.0; Control group: 51.8 ± 20.3 Sex (male, %): intervention group: 45; Control group: 55 Primary disease of the participants Reasons for admission‐ Intervention/Control group n (%) Cardiovascular 7 (12%); 7 (12%) Gastrointestinal 4 (7%); 6 (10%) Hematology 4 (7%); 1 (2%) Orthopaedics 0 (0%); 1 (2%) Respiratory 31 (52%); 22 (37%) Central nervous system 8 (13%); 18 (30%) Trauma 1 (2%); 1 (2%) Urology 1 (2%); 0 (0%) Other 4 (7%); 4 (7%) Disease severity score: APACHE II Intervention group: 13.5 ± 6.4; Control group: 13.7 ± 6.8 Mechanical ventilation no. (%) Not available Comorbidities: not available Nutrition status: intervention group; Control group Subjective global assessment nutritional status, n (%) b A 4 (7%); 4 (7%) B 36 (60%); 43 (72%) C 20 (33%); 13 (22%) Level of inflammation: not available |
|
| Interventions |
Intervention Group 1 (n = 60)
For both groups, ideal body weight was used to calculate caloric and protein requirements. A commercial enteral formula was adjusted to achieve caloric goals and was enriched with additional modules of whey and soy protein diluted in water, given in 3 or 4 daily boluses. All participants received allocated nutritional regimen until day 7. If further EN was necessary, all participants received normocaloric nutrition. Co‐interventions
|
|
| Outcomes |
Primary outcome
Secondary outcomes
Subgroups
|
|
| Funding sources | This research was supported by an unrestricted grant from Lafrancol Colombia and Hospital Universitario San Ignacio. | |
| Declarations of interest | No potential conflict of interest relevant to this article was reported. | |
| Notes | The study sponsor (Lafrancol S.A) provided an unrestricted grant and was not involved in any of the stages of the study. The authors sent us the full paper of this clinical trial before it was indexed in MEDLINE (registered in clinicaltrials.gov with the Identifier: NCT02577211). They gave us the mean and SD values for length of ICU stay and of mechanical ventilation, and also some additional information to complete the 'Risk of bias' table. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using dark sealed envelopes with computer‐generated random allocations. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed using dark sealed envelopes with computer‐generated random allocations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors considered 1 limitation of the study could be lack of proper blinding of ICU staff. One investigator knew participant allocation and prescribed and supervised the administration of nutritional regimens after randomization. Participants and ICU staff deciding on the rest of medical care were blinded to participant allocation. Nutritional information and regimen formulation were not registered in clinical records, except for general information such as total liquids administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if outcome assessors were blinded to participant allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data were reported for non‐excluded participants. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were registered and reported (written information provided by the author) |
| Other bias | Low risk | No other bias (written information provided by the author) |
Theodorakopoulou 2016.
| Methods |
Study design: prospective, randomized controlled trial Study dates: period of one year, but study dates not available Setting: single centre. ICU at Attikon University Hospital. Athens. Greece |
|
| Participants |
Inclusion criteria
Exclusion criteria
Sample size Total number of participants enrolled: 74 Age (years): whole group age of 68.4 ± 18.4 years Sex (male, %): 38 men included (100%) Primary disease of the participants: all participants met the consensus criteria for sepsis. Disease severity score: at entry overall APACHE II score 22 ± 4. etc. and SOFA score 8 ± 4 Mechanical ventilation: 100% of the participants were mechanically ventilated Comorbidities: not reported Nutrition status: non‐obese participants. Overall BMI ≈ 21.5 ± 3.4 kg/m2 |
|
| Interventions |
Permisive underfeeding group (n = not available )
Standar protocol feeding group (n = not available)
Same protein intake for both groups: 1.5 gr protein/kg/day Each participant monitored for 14 days |
|
| Outcomes |
Primary outcome
|
|
| Funding sources | Not available. | |
| Declarations of interest | Not available | |
| Notes | This information was extracted from an abstract. We contacted Dr. Maria Theodorakoupoulou to request the missing data (including outcome data). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information for judgement (abstract only) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information for judgement (abstract only) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement (abstract only) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information for judgement (abstract only) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information for judgement (abstract only) |
| Other bias | Unclear risk | Insufficient information for judgement (abstract only) |
Abbreviations:
APACHE = acute physiology and chronic health evaluation; BMI = Body Mass Index ; CD = cluster of differentiation; CIT = conventional insulin therapy; dl = decilitre; EN = enteral nutrition; gr = gram; GRV = gastric residual volumes; hr = hour; ICU = intensive care unit; IIT = intensive insulin therapy; IVFE = Intravenous fat emulsion; kcal = kilocalories; kg = kilograms; mg = milligrams; NHLBI = National Heart, Lung, and Blood Institute; NNIS = National Nosocomial Infection Surveillance; OMEGA = OMEGA study (Rauch 2010); PN = parenteral nutrition; SD = standard deviation; SOFA = sequential organ failure assessment; TPN = total parenteral nutrition; UAB = unassisted breathing; VFD = ventilator‐free days
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alberda 2009 | Non‐randomized or quasi‐randomized controlled trial. Observational cohort study to examine the relationship between the amount of energy and protein administered and clinical outcomes. |
| Arabi 2010 | Non‐randomized or quasi‐randomized controlled trial assessing hypocaloric nutrition versus control. It is a nested cohort study of participants enrolled in a randomized controlled clinical trial that compared intensive to conventional insulin therapy. The clinical outcomes were analysed according to tertiles of caloric administration. |
| Berg 2013 | Study of whole‐body protein turnover with d5‐phenylalanine and 13C.leucine tracers. The only clinical parameter evaluated was nitrogen balance. |
| Casadei 2006 | Non‐randomized nor quasi‐randomized controlled trial. Retrospective study |
| Desachy 2008 | Not primarily hypocaloric nutrition support study; the goal was to evaluate caloric intake and tolerability of 2 early enteral nutrition protocols in which the optimal flow rate was introduced either immediately or gradually. |
| Dickerson 2002 | Non‐randomized or quasi‐randomized controlled trial. Retrospective study |
| Dissanaike 2007 | Not hypocaloric nutrition support study. Not randomized clinical trial (cohort study) |
| Doig 2013 | Multicentre, randomized, single‐blind clinical trial in critically‐ill adults with relative contraindications to early enteral nutrition. Random allocation to pragmatic standard care or early parenteral nutrition. The objective was different from prescribed hypocaloric nutrition (determine if early parenteral nutrition alters outcomes). No numerical data of calories administered to the groups (only in 1 figure). |
| Esterle 2010 | Hypocaloric nutrition support was not evaluated. Their goal was to evaluate if volume‐based enteral nutrition causes less caloric deficit than rate‐base feeding in critically‐ill ventilated participants. |
| Fiaccadori 2005 | Not hypocaloric nutrition support trial. Open‐label, cross‐over trial in critically‐ill people with acute renal failure and renal replacement therapy, comparing iso‐nitrogenous parenteral nutrition providing 30 and 40 kcal/kg/day (normocaloric versus hypercaloric parenteral nutrition) |
| Garrel 1995 | Not hypocaloric nutrition support trial. They compared isocaloric enteral nutrition with less fat (but more carbohydrates) in people with burns. |
| Iapichino 1990 | Non‐randomized or quasi‐randomized controlled trial assessing hypocaloric nutrition versus control. During 3 days, the participants received randomly 4 different types of parenteral nutrition (2 types of amino acids and 2 different doses of glucose). The authors only assessed metabolic outcomes (no clinical outcomes). |
| Lau 2010 | Retrospective study to evaluate 3 different caloric regimes on the incidence of hyperglycaemia and hypoglycaemia in critically‐ill participants on intensive insulin treatment |
| Mackenzie 2005 | Not a prospective controlled trial of hypocaloric nutrition support. Prospective study to evaluate the proportion of participants meeting their caloric goals with the implementation of an evidence‐based enteral nutrition protocol. |
| Moses 2009 | Hypocaloric nutrition support was not evaluated against normo‐ or hypercaloric feeding. Prospective controlled randomized trial realized exclusively in ventilated participants with acute organophosphate poisoning, to evaluate if enteral nutrition could be possible (due to the treatment with high dose of atropine) and had different clinical outcomes than the participants on intravenous fluids |
| Müller 1995 | Not randomized trial to study the metabolic effects of different caloric regimens in medical participants with multiple organ failures. The participants received 7 parenteral nutrition regimens with different amounts of calories, carbohydrates, amino‐acids and lipids, for 12 hours each regimen. |
| Owais 2014 | Single‐blinded randomized clinical trial of 50 consecutive participants requiring parenteral nutritional support; permissive underfeeding in participants requiring parenteral nutrition. Participants were randomized to receive either normocaloric or hypocaloric feeding (respectively 100% vs 60% of estimated requirements). The primary end point was septic complication and the secondary end points included the metabolic, physiological and clinical outcomes to the 2 feeding protocols. Only 26% (12 out of 46) of included participants were ICU participants and the results did not distinguish between ICU and non‐ICU participants. |
| Rodríguez 2005 | Hypocaloric nutrition support was not evaluated. They assess clinical results with 2 different calories/protein relationships. |
| Schricker 2005 | Not critically‐ill participants . Surgical participants (hemicolectomy, sigmoid colectomy) to assess if hypocaloric nutrition could induce anabolism in participants with perioperative epidural analgesia. |
| Wewalka 2010 | Hypocaloric nutrition support was not evaluated. The aim of the study was the evaluation of 2 nutrition support programmes: isocalorically right from the beginning compared with a hypocaloric beginning (50% of the dose in the first day, 75% the second day and 100% from the third day): abstract with no results of the clinical outcomes. |
Abbreviations:
kcal = kilocalories; kg = kilograms
Characteristics of ongoing studies [ordered by study ID]
NCT01665664.
| Trial name or title | Hypocaloric vs full‐energy enteral feeding in critically ill patients guided by indirect calorimetry, a prospective, blinded, randomized controlled trial |
| Methods | Study design: randomized controlled double‐blind trial with measurement of REE by indirect calorimetry to establish the exact amount of calories to be delivered to the intervention and control groups |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Group 1 hypocaloric feeding group
Full energy feeding group
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | September 2012 |
| Contact information | Arie Soroksky: soroksky@gmail.com (Israel) |
| Notes | Unknown state of the trial up to the end of June 2016. The principal investigator did not answer a question about the state of the trial. Clinical trial record states: (quote:) "the recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years." "Verified August 2012 by Soroksky Arie, Wolfson Medical Center. Recruitment status was: not yet recruiting" |
Ochoa 2017.
| Trial name or title | Hypocaloric high‐protein enteral nutrition improves glucose management in critically ill patients |
| Methods |
Study design: prospective, randomized, multicenter clinical trial Settings and countries: ICU of 7 academic centres at USA and Canada. In USA: Wake Forest University, Winston‐Salem, North Carolina; University of Kentucky, Lexington, Kentucky; Emory University, Atlanta, Georgia; Medicine, University of Chicago, Hinsdale, Illinois; Pulmonary Medicine, Regions Hospital, St Paul, Minnesota; Vanderbilt University, Nashville, Tennessee. In Canada: Kingston Hospital, Kingston, Ontario. Funding: Nestlé Health Science |
| Participants |
Inclusion criteria
Exclusion criteria
Sample size: calculated sample size of 100 participants per group, based in a reduction of “out‐of‐range” glycaemic events and their standard deviation (glucose variability). Sample size of each arm of the study not reported. “Ninety‐eight subjects were randomized into the study at the time of interim analysis. Of these subjects, 40 had at least 5 days of data collected. The remaining subjects withdrew primarily due to removal of the feeding tube” Age (years, mean ± SD): Group 1: hypocaloric: 60.7 ± 15.07; Group 2: 62.6 ± 12.09 Sex (% of women): Group 1: 42.9; Group 2: 55.1 Primary disease of the participants. Not reported Disease severity: APACHE II score (mean ± SD). Group 1: 25.1 ± 9.0; Group 2: 26.3 ± 9.24 Nutrition status: BMI (kg/m2; mean ± SD). Group 1: 33.7 ± 4.57; Group 2: 32.5 ± 5.65 Mechanical ventilation: not available Comorbidities: not available Level of inflammation: not available |
| Interventions |
Group 1 hypocaloric (n = not available)
Group 2 (n = not available)
Co‐interventions In both study groups the quantity of the assigned formula was enough to provide 1.5 grams of protein/kg ideal body weight/day |
| Outcomes |
Primary endpoint
Other endpoints
Outcomes and time points: not clearly defined Subgroups: not available |
| Starting date | Not available |
| Contact information | Juan.Ochoa@US.nestle.com. We contacted the study author and he replied that he would send us the study results. |
| Notes | An interim analysis was scheduled when 40 participants completed at least 5 days of data collection. All the current information comes from the abstract of a congress presentation (ASPEN, CNW, Orlando, Florida, 18 to 21 February, 2017) regarding the preliminary analysis of the intention‐to‐treat data. |
Abbreviations:
APACHE = Acute Physiology And Chronic Health Evaluation II; BMI = Body Mass Index; ICU = Intensive Care Unit; REE = resting energy expenditure; SD = standard deviation; μg/kg/min = micrograms/kilograms/minute
Differences between protocol and review
Background section
The original Background section contained a single description without subheadings. We updated the references and divided them into level two subheadings according to the Cochrane Handbook for Systematic Reviews of Interventions recommendations (Higgins 2011).
Objectives and outcomes
Modifications in order to comply with the latest MECIR standards (Higgins 2016): we modified the wording of the objectives in order to comply with Standard R5 and R22; we provided additional detail for the definition of outcomes in order to comply with Standard R32; we provided detail on the GRADE methods in order to comply with Standard C23 and R98; we provided detail on subgroup analysis (Standard R52)
In order to have only three primary outcomes (according to Higgins 2011), we changed the order of the primary and secondary outcomes stated in the protocol (Perman 2009), while maintaining all the predefined ones. The primary outcomes for this review were: mortality (in hospital, in lCU and at 30 days); length of stay (in hospital and in ICU) and infectious complications. The secondary outcomes we were able to evaluate were: length of mechanical ventilation, non‐infectious complications, carbohydrate metabolic outcomes (hyperglycaemia, hypoglycaemia), protein metabolic outcomes (nitrogen balance). However, we include seven outcomes in the 'Summary of findings' table. These main outcomes were considered for the subgroup analysis.
Criteria for inclusion of studies
We provided further detail on the inclusion criteria for the Types of interventions that initially was broadly defined as " 1) normo‐ or hypercaloric NS: equal or more than the measured REE or 25 kcal/kg/day (with the same characteristics as above); or 2) no nutrition support at all: fasting or dextrose solutions". We added "We evaluated results of trials designed to compare prescribed hypocaloric enteral or parenteral nutrition support (or permissive underfeeding) with standard nutrition support, or with no nutrition, even if those trials did not reach their caloric goals in the intervention or control groups (intention‐to treat analysis). We did not include trials that planned to provide full nutrition support but resulted in unintended hypocaloric provision (for any reason)."
Search methods
In Electronic searches we made some changes: we applied the trial filter for therapy, maximizing sensitivity developed by HIRU (Health Information Research Unit at McMaster University: hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx. We consulted the following trial registries: ClinicalTrials.Gov: clinicaltrials.gov/ct2/home; International Clinical Trials Registry Platform (WHO): apps.who.int/trialsearch/); and ISRCTN Registry: www.isrctn.com/. The LILACS strategy was improved (Appendix 4). We did not perform ISI SciSearch due to lack of access to the database. We did not contact relevant societies to identify abstracts, since we checked the conference proceedings of those societies directly.
Data collection and analysis
We updated the sections Selection of studies, Data extraction and management, Assessment of risk of bias in included studies according to the latest MECIR standards (2016) and Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They were initially in different subheadings and now use the current recommended subheadings.
We added the sections Measures of treatment effect, Unit of analysis issues, Dealing with missing data and Assessment of reporting biases that were not present in the original protocol.
We constructed the section Assessment of heterogeneity and Data synthesis with the information present in "Analysis" in the original protocol.
We modified the sections Assessment of heterogeneity and Data synthesis, to adjust the cut‐off points to classify and report heterogeneity according to Higgins 2011 (Section 9.5.2).
We added a section for the methods used to develop the 'Summary of findings' table using the GRADE approach (see Sensitivity analysis).
Methods not implemented
Several outcomes stated in the protocol were not reported in the trials; for this reason we were not able to conduct some predefined subgroup analyses. We did a subgroup analysis not prespecified in the protocol to assess the effect of the route of nutrition support (enteral or parenteral). We considered this to be relevant after the search strategy was performed, but before we had conducted any analysis.
Post hoc analysis
After collecting the data about the calories received by both groups of participants in the included studies, and before the analysis of results, we decided to perform the subgroup analysis of the amount of calories received according to the following categories: very hypocaloric, hypocaloric, normocaloric and hypercaloric.
We performed two sensitivity analyses not previously stated in the protocol. In one of them we excluded three studies (Ibrahim 2002; NHLBI 2012; Rice 2011) with a different primary goal: they evaluated early initiation of low‐dose enteral nutrition (hypocaloric trophic feeding) against full enteral dose from the beginning (normocaloric standard feeding). In the other sensitivity analysis, we excluded a study (Battistella 1997), primarily designed to compare parenteral nutrition without the administration of intravenous lipid emulsion (hypocaloric) and with lipids (normocaloric).
When we found high levels of clinical and statistical heterogeneity, we performed a non‐prespecified meta‐regression using STATA 13.1 to explore the effect of several covariates on the main outcomes (Appendix 5).
Change in authorship
Juan VA Franco has joined the review team.
Contributions of authors
Conception, design and coordination of the review: Mario I Perman (MP) Design of search strategies: Virginia Garrote (VG), MP, Agustín Ciapponi (ACi) Searching: VG, MP Manual searches: Cecilia Loudet (CL), Adriana Crivelli (ACr), VG and MP. Screening search results: MP, CL, ACr. Organizing retrieval of papers: VG, MP. Obtaining copies of trials: MP, ACr, CL, GP, VG Screening retrieved paper against inclusion criteria: MP, CL, ACr, GP. Selection of trials to include: MP, CL, ACr, GP. Appraising quality of papers: GP, CL, AC, Juan Franco (JF). Extracting data from trials: MP, CL, ACr.
Meta‐regression in STATA 14.1 AC Writing to authors of papers for additional information: MP.
Data management for the review
Entering data into RevMan: MP, GP, ACi. Analysis of data: MP, ACi, GP, CL, ACr, JF. Interpretation of data: MP, GP, ACi, CL, JF. Providing a methodological perspective: ACi, JF. Providing a clinical perspective: MP, GP, ACi, CL. Writing the review: MP, ACi, GP, CL, JF. Update of the review: MP.
Sources of support
Internal sources
None, Argentina.
External sources
None, Argentina.
Declarations of interest
Mario I Perman received honoraria from laboratory Fresenius‐Kabi S.A (Buenos Aires‐Argentina) for doing educational activities in the field of parenteral nutrition in different clinical settings. He has not received any type of financial support from Fresenius‐Kabi S.A. for doing or writing this Cochrane review.
Agustín Ciapponi: none declared.
Cecilia Loudet: none declared.
Adriana Crivelli: none declared.
Virginia Garrote: none declared.
Gastón Perman: none declared.
Juan VA Franco: none declared.
Edited (no change to conclusions)
References
References to studies included in this review
Ahrens 2005 {published data only}
- Ahrens CL, Barletta JF, Kanji S, Tyburski JG, Wilson RF, Janisse JJ, et al. Effect of low‐calorie parenteral nutrition on the incidence and severity of hyperglycemia in surgical patients: a randomized, controlled trial. Critical Care Medicine 2005;33(11):2507‐12. [PUBMED: 16276174] [DOI] [PubMed] [Google Scholar]
Arabi 2011 {published data only}
- Arabi M, Tamim HM, Dhar GS, Al‐Dawood A, Al‐Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: A randomized controlled trial. American Journal of Clinical Nutrition 2011;93(3):569‐77. [PUBMED: 21270385 ] [DOI] [PubMed] [Google Scholar]
- Arabi Y, Tamim H, Shifaat G, Sakkijha M, Al‐Dawood A, Al‐Sultan M. Permissive underfeeding versus target feeding in critically ill patients: randomized controlled trial. American Journal Respiratory Critical Care Medicine 2009;179:A2167. [Google Scholar]
Arabi 2015 {published data only}
- Arabi YM, Aldawood AS, Al‐Dorzi HM, Tamim HM, Haddad SH, Jones G, et al. Permissive underfeeding or standard enteral feeding in high‐ and low‐nutritional‐risk critically ill adults. Post hoc analysis of the PermiT trial. American Journal of Respiratory and Critical Care Medicine 2017;195(5):652‐62. [PUBMED: 27589411] [DOI] [PubMed] [Google Scholar]
- Arabi YM, Aldawood AS, Haddad SH, Al‐Dorzi HM, Tamim HM, Jones G, et al. PermiT Trial Group. Permissive underfeeding or standard enteral feeding in critically ill adults. New England Journal of Medicine 2015;372(25):2398‐408. [PUBMED: 25992505 ] [DOI] [PubMed] [Google Scholar]
Battistella 1997 {published data only}
- Battistella FD, Widergren JT, Anderson JT, Siepler JK, Weber JC, MacColl K. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. Journal of Trauma 1997;43(1):52‐8; discussion 58‐60. [PUBMED: 9253908] [DOI] [PubMed] [Google Scholar]
Charles 2014 {published data only}
- Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, et al. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: a randomized controlled trial. American Journal of Clinical Nutrition 2014;100(5):1337‐43. [PUBMED: 25332331 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choban 1997 {published data only}
- Choban PS, Burge JC, Scales D, Flancbaum L. Hypoenergetic nutrition support in hospitalized obese patients: a simplified method for clinical application. American Journal of Clinical Nutrition 1997;66(3):546‐50. [PUBMED: 9280171] [DOI] [PubMed] [Google Scholar]
Ibrahim 2002 {published data only}
- Ibrahim EH, Mehringer L, Prentice D, Sherman G, Schaiff R, Fraser V, et al. Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. Journal of Parenteral and Enteral Nutrition 2002;26(3):174‐81. [PUBMED: 12005458] [DOI] [PubMed] [Google Scholar]
McCowen 2000 {published data only}
- McCowen KC, Friel C, Sternberg J, Chan S, Forse RA, Burke PA, et al. Hypocaloric total parenteral nutrition: effectiveness in prevention of hyperglycemia and infectious complications‐‐a randomized clinical trial. Critical Care Medicine 2000;28(11):3606‐11. [PUBMED: 11098961] [DOI] [PubMed] [Google Scholar]
NHLBI 2012 {published data only}
- Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, et al. National Heart Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury. The EDEN randomized trial. JAMA 2012;307(8):795‐803. [PUBMED: 22307571] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norouzy 2013 {published data only}
- Norouzy A, Kazemi M, Samini F, Nematy M. Early permissive enteral underfeeding in critically ill head trauma patients: a double blind randomized controlled trial. Clinical Nutrition 2013;32(Supplement):S27. [Google Scholar]
Petros 2016 {published data only}
- Petros S, Horbach M, Seidel F, Weidhase L. Hypocaloric vs normocaloric nutrition in critically ill patients: a prospective randomized pilot trial. Journal of Enteral and Parenteral Nutrition 2016;40(2):242‐9. [PUBMED: 24699555] [DOI] [PubMed] [Google Scholar]
Rice 2011 {published data only}
- Rice T, Mogan S, Hays M, Bernard G, Wheeler A, Jensen G. Initial low volume trophic vs. full‐calorie enteral feeds in acute respiratory failure. Critical Care Medicine 2009;37(12 (Suppl)):A442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full‐energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Critical Care Medicine 2011;39(5):967‐74. [PUBMED: 21242788] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rugeles 2013 {published data only}
- Rugeles SJ, Rueda JD, Díaz CE, Rosselli D. Hyperproteic hypocaloric enteral nutrition in the critically ill patient: a randomized controlled clinical trial. Indian Journal Critical Care Medicine 2013; Vol. 17, issue 6:343‐9. [PUBMED: 24501485] [DOI] [PMC free article] [PubMed]
Rugeles 2016 {published data only}
- Rugeles S, Villarraga‐Angulo LG, Ariza‐Gutiérrez A, Chaverra‐Kornerup S, Lasalvia P, Rosselli D. High‐protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: a randomized clinical trial. Journal of Critical Care 2016;35:110‐4. [PUBMED: 27481744] [DOI] [PubMed] [Google Scholar]
Theodorakopoulou 2016 {published data only}
- Theodorakopoulou M, Diamantakis A, Kontogiorgi M, Chrysanthopoulou E, Christodoulopoulou T, Frantzeskaki F, et al. Permissive underfeeding of mechanically ventilated septic ICU patients. Intensive Care Medicine Experimental 2016;4(Suppl 1):A248. [Google Scholar]
References to studies excluded from this review
Alberda 2009 {published data only}
- Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Medicine 2009;35(10):1728‐37. [PUBMED: 19572118] [DOI] [PubMed] [Google Scholar]
Arabi 2010 {published data only}
- Arabi YM, Haddad SH, Tamim HM, Rishu AH, Sakkijha MH, Kahoul SH, et al. Near‐target caloric intake in critically ill medical‐surgical patients is associated with adverse outcomes. Journal of Parenteral and Enteral Nutrition 2010;34(3):280‐8. [PUBMED: 20467009] [DOI] [PubMed] [Google Scholar]
Berg 2013 {published data only}
- Berg A, Rooyackers O, Bellanders BM, Wernerman J. Whole body protein kinetics during hypocaloric and normocaloric feeding in critically ill patients. Critical Care 2013;17:R158. [PUBMED: 23883571 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Casadei 2006 {published data only}
- Casadei E, Scolletta S, Franchi F, Mongelli P, Giomarelli P. Effects of hypocaloric feeding on clinical outcome in ICU patients. Critical Care 2006;10(Suppl 1):P217. [Google Scholar]
Desachy 2008 {published data only}
- Desachy A, Clavel M, Vuagnat A, Normand S, Gissot V, François B. Initial efficacy and tolerability of early enteral nutrition with immediate or gradual introduction in intubated patients. Intensive Care Medicine 2008;34(6):1054‐9. [PUBMED: 18210092] [DOI] [PubMed] [Google Scholar]
Dickerson 2002 {published data only}
- Dickerson RN, Boschert KJ, Kudsk KA, Brown RO. Hypocaloric enteral tube feeding in critically III obese patients. Nutrition 2002;18(3):241‐6. [PUBMED: 11882397] [DOI] [PubMed] [Google Scholar]
Dissanaike 2007 {published data only}
- Dissanaike S, Shelton M, Warner K, O'Keefe GE. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Critical Care 2007;11(5):R114. [PUBMED: 17958913] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doig 2013 {published data only}
- Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early PN Investigators of the ANZICS Clinical Trials Group. Early parenteral nutrition in critically ill patients with short‐term relative contraindications to early enteral nutrition. JAMA 2013;309(20):2130‐8. [PUBMED: 23689848] [DOI] [PubMed] [Google Scholar]
Esterle 2010 {published data only}
- Esterle ME, Kellie S, Mohammad S, McClave S. Volume‐based feeding in the critically ill admitted to the medical intensive care unit: a proof of concept. CHEST Journal. 138 (4_MeetingAbstracts) 2010;138(4 (Meeting Abstracts)):908A. [Google Scholar]
Fiaccadori 2005 {published data only}
- Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Picetti E, Parenti E, et al. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrology Dialysis Transplantation 2005;20(9):1976‐80. [PUBMED: 15998652] [DOI] [PubMed] [Google Scholar]
Garrel 1995 {published data only}
- Garrel DR, Razi M, Larivière F, Jobin N, Naman N, Emptoz‐Bonneton A, et al. Improved clinical status and length of care with low‐fat nutrition support in burn patients. Journal Parenteral Enteral Nutrition 1995;19(6):482‐91. [PUBMED: 8748363] [DOI] [PubMed] [Google Scholar]
Iapichino 1990 {published data only}
- Iapichino G, Radrizzani D, Bonetti G, Colombo A, Leoni L, Ronzoni G, et al. Influence of parenteral nutrition on leg nitrogen exchange in injured patients. Critical Care Medicine 1990;18(12):1367‐73. [PUBMED: 2123142] [DOI] [PubMed] [Google Scholar]
Lau 2010 {published data only}
- Lau YT, Oyen LJ, Malinchoc M, Arendt CJ, Barth MM. A retrospective analysis on the impact of caloric intake on glycemic control in critically ill patients. Intensive Care Med 2010;36(4):725‐6. [PUBMED: 19894034] [DOI] [PubMed] [Google Scholar]
Mackenzie 2005 {published data only}
- Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SM. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. Journal Parenteral Enteral Nutrition 2005;29(2):74‐80. [PUBMED: 15772383] [DOI] [PubMed] [Google Scholar]
Moses 2009 {published data only}
- Moses V, Mahendri NV, John G, Peter JV, Ganesh A. Early hypocaloric enteral nutritional supplementation in acute organophosphate poisoning‐‐a prospective randomized trial. Clinical Toxicology (Phila) 2009;47(5):419‐24. [PUBMED: 19492933] [DOI] [PubMed] [Google Scholar]
Müller 1995 {published data only}
- Müller TF, Müller A, Bachem MG, Lange H. Immediate metabolic effects of different nutritional regimens in critically ill medical patients. Intensive Care Medicine 1995;21(7):561‐66. [PUBMED: 7593897] [DOI] [PubMed] [Google Scholar]
Owais 2014 {published data only}
- Owais AE, Kabir SI, Macnaught C, Gatt M, MacFie J. A single‐blinded randomised clinical trial of permissive underfeeding in patients requiring parenteral nutrition. Clinical Nutrition 2014;33(6):997‐1001. [PUBMED: 24467878] [DOI] [PubMed] [Google Scholar]
Rodríguez 2005 {published data only}
- Rodríguez Perón J, Hernández Pedroso W, Pérez Salido J. Comparison of two nutritional regimes in a group of critically ill patients [Comparación de dos regímenes nutricionales en un grupo de pacientes graves]. Revista Cubana Medicina Militar 2005;34(2):0‐0. [Google Scholar]
Schricker 2005 {published data only}
- Schricker T, Wykes L, Eberhart L, Carli F, Meterissian S. Randomized clinical trial of the anabolic effect of hypocaloric parenteral nutrition after abdominal surgery. British Journal of Surgery 2005;92(8):947‐53. [PUBMED: 16034820] [DOI] [PubMed] [Google Scholar]
Wewalka 2010 {published data only}
- Wewalka M, Kitzberger R, Fuhrmann V, Schneeweiss B, Zauner C. Isocaloric artificial nutrition right from the beginning causes no increase of nutritional related side effects in critically ill medical patients. Intensive Care Medicine 2010;2010:S371. [Google Scholar]
References to ongoing studies
NCT01665664 {published data only}
- NCT01665664. Hypocaloric vs full energy enteral feeding in critically ill patients [Hypocaloric vs full energy enteral feeding in critically ill patients guided by indirect calorimetry, a prospective, blinded, randomized controlled trial.]. clinicaltrials.gov/ct2/show/NCT01665664 (first received 15 August 2012).
Ochoa 2017 {published data only}
- Ochoa J, Huhmann M, Files DC. Hypocaloric high‐protein enteral nutrition improves glucose management in critically ill patients (Clinical nutrition week 2017: Orlando, Florida, 18–21 February 2017). Journal of Parenteral and Enteral Nutrition 2017;41(2):266–300. [DOI] [PubMed] [Google Scholar]
Additional references
Alkhawaja 2015
- Alkhawaja S, Martin C, Butler RJ, Gwadry‐Sridhar F. Post‐pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD008875.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allingstrup 2015
- Allingstrup M, Afshari A. Selenium supplementation for critically ill adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD003703.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASPEN / SCCM guidelines 2009
- McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. Journal of Parenteral and Enteral Nutrition 2009;33(3):277‐316. [PUBMED: 19398613] [DOI] [PubMed] [Google Scholar]
ASPEN / SCCM guidelines 2016
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Journal of Parenteral and Enteral Nutrition 2016;40(2):159‐211. [PUBMED: 26773077 ] [DOI] [PubMed] [Google Scholar]
ASPEN 2012
- Vanek VW, Seidner DL, Bistrian B, Gura K, Valentine CJ, Novel Nutrient Task Force, Intravenous Fat Emulsions Workgroup, American Society for Parenteral and Enteral Nutrition Board of Directors. A.S.P.E.N. position paper: Clinical role for alternative intravenous fat emulsions. Nutrition in Clinical Practice 2012;27(2):150‐2. [PUBMED: 22378798] [DOI] [PubMed] [Google Scholar]
ASPEN guidelines 2013
- Choban P, Dickerson R, Malone A, Worthington P, Compher CH, American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. Clinical guidelines: nutrition support of hospitalized adult patients with obesity. Journal of Parenteral and Enteral Nutrition 2013;37(6):714‐44. [PUBMED: 23976769] [DOI] [PubMed] [Google Scholar]
Badawi 2012
- Badawi O, Waite MD, Fuhrman SA, Zuckerman IH. Association between intensive care unit‐acquired dysglycemia and in‐hospital mortality. Critical Care Medicine 2012;40(12):3180‐8. [PUBMED: 22971590] [DOI] [PubMed] [Google Scholar]
Berger 2007
- Berger MM, Chioléro RL. Hypocaloric feeding: pros and cons. Current Opinion in Critical Care 2007;13(2):180‐6. [PUBMED: 17327740] [DOI] [PubMed] [Google Scholar]
Berger 2012
- Berger MM, Pichard C. Best timing for energy provision during critical illness. Critical Care 2012;16(2):215. [PUBMED: 22429787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biolo 2002
- Biolo G, Grimble G, Preiser JC, Leverve X, Jolliet P, Planas M, et al. Position paper of the ESICM Working Group on Nutrition and Metabolism. Intensive Care Medicine 2002;28(11):1512‐20. [PUBMED: 12415440 ] [DOI] [PubMed] [Google Scholar]
Bost 2014
- Bost RBC, Tjan DHT, Zanten AR. Timing of (supplemental) parenteral nutrition in critically ill patients: a systematic review. Annals of Intensive Care 2014;4:31. [PUBMED: 25593747] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulanger 1994
- Boulanger BR, Naman R, McLean RF, Phillips E, Rizoli SB. What are the clinical determinants of early energy expenditure in critically injured adults?. Journal of Trauma 1994;37(6):969‐74. [PUBMED: 7996613] [DOI] [PubMed] [Google Scholar]
Braunschweig 2001
- Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta‐analysis. American Journal of Clinical Nutrition 2001;74(4):534‐42. [PUBMED: 11566654] [DOI] [PubMed] [Google Scholar]
Cahill 2010
- Cahill N, Dhaliwal R, Dau AG, Jiang X, Heyland DK. Nutrition therapy in the critical care setting: What is “best achievable” practice? An international multicenter observational study. Critical Care Medicine 2010;38(2):395‐40. [PUBMED: 19851094] [DOI] [PubMed] [Google Scholar]
Canadian Guideline 10.1 2015
- Critical Care Nutrition Committee. Canadian Clinical Practice Guidelines. 10.1. Strategies to optimize parenteral nutrition and minimize risks: dose of PN. Critical Care Nutrition 2015 March (updated 2015, cited 1 November 2013) (accessed 28 May 2018); Vol. Available from: www.criticalcarenutrition.com/docs/CPGs%202015/10.1%202015.pdf.
Canadian Guideline 3.3a 2015
- Critical Care Nutrition Committee. Canadian Clinical Practice Guidelines 3.3a Intentional underfeeding: hypocaloric enteral nutrition. Critical Care Nutrition 2015 May (updated 2015, cited October 2015) (accessed 28 May 2018); Vol. Available from: www.criticalcarenutrition.com/docs/CPGs%202015/3.3a%202015.pdf.
Canadian Guideline 3.3b 2015
- Critical Care Nutrition Committee. Canadian Clinical Practice Guidelines. 3.3b. Intentional underfeeding. hypocaloric enteral nutrition. Critical Care Nutrition 2015 May (updated 2015, cited October 2015) (accessed 28 May 2018); Vol. Available from: www.criticalcarenutrition.com/docs/CPGs%202015/3.3b%202015.pdf.
Casaer 2014
- Casaer MP, Berghe G. Nutrition in the acute phase of critical illness. New England Journal Medicine 2014;370(13):1227‐36. [PUBMED: 24670169] [DOI] [PubMed] [Google Scholar]
Cerra 1997
- Cerra FB, Rios Benitez M, Blackburn GL, Irwin RS, Jeejeebhoy K, Katz DP, et al. Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest 1997;111(3):769‐78. [PUBMED: 9118718] [DOI] [PubMed] [Google Scholar]
Choban 2013
- Choban P, Dickerson R, Malone A, Worthington P, Compher C, American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. Clinical Guidelines: nutrition support of hospitalized adult patients with obesity. Journal of Parenteral & Enteral Nutrition 2013;37(6):714‐44. [PUBMED: 23976769] [DOI] [PubMed] [Google Scholar]
Choi 2015
- Choi EY, Park DA, Park J. Calorie intake of enteral nutrition and clinical outcomes in acutely critically ill patients: a meta‐analysis of randomized controlled trials. Journal of Parenteral and Enteral Nutrition 2015;39(3):291‐300. [PUBMED: 25078609 ] [DOI] [PubMed] [Google Scholar]
Cooney 2012
- Cooney RN Frankenfield DC. Determining energy needs in critically ill patients: equations or indirect calorimeters. Current Opinion Critical Care 2012;18(2):174‐7. [PUBMED: 22322264 ] [DOI] [PubMed] [Google Scholar]
Dickerson 1986
- Dickerson RN, Rosato EF, Mullen JL. Net protein anabolism with hypocaloric parenteral nutrition in obese stressed patients. American Journal of Clinical Nutrition 1986;44(6):747‐55. [DOI] [PubMed] [Google Scholar]
Dickerson 2011
- Dickerson RN. Optimal caloric intake for critically ill patients: first, do not harm. Nutrition in Clinical Practice 2011;26(1):48‐54. [DOI] [PubMed] [Google Scholar]
Dickerson 2012
- Dickerson RN, Pitts SL, Maish GO 3rd, Schroeppel TJ, Magnotti LJ, Croce MA, et al. A reappraisal of nitrogen requirements for patients with critical illness and trauma. Journal Trauma Acute Care Surgery 2012;73(3):549‐57. [DOI] [PubMed] [Google Scholar]
Dushianthan 2016
- Dushianthan A, Cusack R, Burgess VA, Grocott MP, Calder PC. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dvir 2006
- Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: An observational study. Clinical Nutrition 2006;25(1):37‐44. [DOI] [PubMed] [Google Scholar]
Emori 1991
- Emori TG, Culver DH, Horan TC, Jarvis WR, White JW, Olson DR, et al. National nosocomial infections surveillance system (NNIS): description of surveillance methods. American Journal of Infection Control 1991;19(1):19‐35. [PUBMED: 1850582] [DOI] [PubMed] [Google Scholar]
ESPEN guidelines 2009
- Singer P, Berger MM, Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN. ESPEN guidelines on parenteral nutrition: intensive care. Clinical Nutrition 2009;28(4):387‐400. [DOI] [PubMed] [Google Scholar]
Faysy 2008
- Faisy C, Lerolle N, Dachraoui F, Savard JF, Abboud I, Tadie JM, et al. Impact of energy deficit calculated by a predictive method on outcome in medical patients requiring prolonged acute mechanical ventilation. British Journal of Nutrition 2008;101(7):1079‐87. [DOI] [PubMed] [Google Scholar]
Frankenfield 1997
- Frankenfield DC, Smith JA, Cooney RN. Accelerated nitrogen loss after traumatic injury is not attenuated by achievement of energy balance. Journal of Parenteral and Enteral Nutrition 1997;21(6):324‐9. [DOI] [PubMed] [Google Scholar]
Frankenfield 2011
- Frankenfield DC, Ashcraft CM. Estimating energy needs in nutrition support patients. Journal of Parenteral and Enteral Nutrition 2011;35(5):563‐70. [DOI] [PubMed] [Google Scholar]
Fuentes Padilla 2016
- Fuentes Padilla P, Martínez G, Vernooij RW, Urrútia G, Roqué i Figuls M, Bonfill Cosp X. Early versus delayed enteral nutrition support for critically ill adults. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Hart 2002
- Hart DL, Wolf SE, Herndon DN, Chinkes DL, Lal SO, Obeng MK, et al. Energy expenditure and caloric balance after burn. Increased feeding leads to fat rather than lean mass accretion. Annals of Surgery 2002;235(1):152‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heidegger 2013
- Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 2013;381(9864):385‐93. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2016
- Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological expectations of Cochrane intervention reviews (MECIR). Available at community.cochrane.org/mecir‐manual 2016.
Hoffer 2003
- Hoffer LJ. Protein and energy provision in critical illness. American Journal of Clinical Nutrition 2003;78(5):906‐11. [DOI] [PubMed] [Google Scholar]
Hoffer 2012
- Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. American Journal of Clinical Nutrition 2012;96(3):591‐600. [DOI] [PubMed] [Google Scholar]
Iapichino 1984
- Iapichino G, Radrizzani D, Solca M, Pesenti A, Gattinoni L, Ferro A, et al. The main determinants of nitrogen balance during total parenteral nutrition in critically ill injured patients. Intensive Care Medicine 1984;10(5):251‐4. [DOI] [PubMed] [Google Scholar]
Jeejeebhoy 2004
- Jeejeebhoy K. Permissive underfeeding of the critically ill patient. Nutrition in Clinical Practice 2004;19(5):477‐80. [DOI] [PubMed] [Google Scholar]
Jiang 2003
- Jiang ZM, Wang XR, Wei JM, Jia ZG, Tang Y, Zhang ZT. The impact of hypocaloric and low nitrogen parenteral nutrition on blood glucose level, infection related complication, hospital stay and cost in post‐operative patients (multi‐center, randomized, controlled clinical trial for 100 cases). Chinese Journal of Clinical Nutrition 2003;11:179‐83. [Google Scholar]
Jiang 2011
- Jian H, Sun MW, Hefright B, Chen W, Lu CD, Zeng J. Efficacy of hypocaloric parenteral nutrition for surgical patients: A systematic review and meta‐analysis. Clinical Nutrition 2011;30(6):730‐7. [DOI] [PubMed] [Google Scholar]
Joffe 2016
- Joffe A, Anton N, Lequier L, Vandermeer B, Tjosvold L, Larsen B, et al. Nutritional support for critically ill children. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD005144.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 1998
- Klein C, Stanek GS, Wiles CE. Overfeeding macronutrients to critically ill adults: metabolic complications. Journal of the American Dietetic Association 1998;98(7):795‐806. [DOI] [PubMed] [Google Scholar]
Kreymann 2006
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clinical Nutrition 2006;25(2):210‐23. [DOI] [PubMed] [Google Scholar]
Kreymann 2012
- Kreymann G, DeLegge MH, Luft G, Hise ME, Zaloga GP. The ratio of energy expenditure to nitrogen loss in diverse patient groups ‐ a systematic review. Clinical Nutrition 2012;31(2):168‐75. [DOI] [PubMed] [Google Scholar]
Krinsley 2003
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic Proceedings 2003;78:1471‐78. [DOI] [PubMed] [Google Scholar]
Krishnan 2003
- Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients. Consistency of care with guidelines and relationship to clinical outcomes. Chest 2003;124(1):297‐305. [DOI] [PubMed] [Google Scholar]
Kushner 2011
- Kushner RF, Drover JW. Current strategies of critical care assessment and therapy of the obese patient (hypocaloric feeding): what are we doing and what do we need to do. Journal of Parenteral and Enteral Nutrition 2011;35(Suppl 1):36S‐43S. [DOI] [PubMed] [Google Scholar]
Lewis 2016
- Lewis SR, Schofield‐Robinson OJ, Alderson P, Smith AF. Enteral versus parenteral nutrition for adults in the intensive care unit. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liggett 1990
- Liggett SB, Renfro AD. Energy expenditures of mechanically ventilated non surgical patients. Chest 1990;98(3):682‐6. [DOI] [PubMed] [Google Scholar]
Long 1979
- Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. Journal of Parenteral and Enteral Nutrition 1979;3(6):452‐6. [DOI] [PubMed] [Google Scholar]
Magnuson 2011
- Magnuson B, Peppard A, Auer Flomenhoft D. Hypocaloric considerations in patients with potentially hypometabolic disease states. Nutrition in Clinical Practice 2011;26(3):253‐60. [DOI] [PubMed] [Google Scholar]
Malone 2007
- Malone AM. Permissive underfeeding: Its appropriateness in patients with obesity, patients on parenteral nutrition, and non‐obese patients receiving enteral nutrition. Current Gastroenterology Reports 2007;9(4):317‐22. [DOI] [PubMed] [Google Scholar]
Mao 2015
- Mao YL, Lu X, Sang XT, Wang XR, Zhong SX, Huang JF. Permissive underfeeding in post‐operative patients: results of a prospective, randomized, controlled clinical trial. Chinese Journal of General Surgery 2005;20:612‐5. [Google Scholar]
Marik 2016a
- Marik PE. Is early starvation beneficial for the critically ill patient?. Current Opinion Clinical Nutrition & Metabolic Care 2016;19(2):155‐60. [DOI] [PubMed] [Google Scholar]
Marik 2016b
- Marik PE, Hooper MH. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta‐analysis. Intensive Care Medicine 2016;42(3):316‐23. [DOI] [PubMed] [Google Scholar]
McClave 1992
- McClave SA, Snider HL. Use of indirect calorimetry in clinical nutrition. Nutrition in Clinical Practice 1992;7(5):207‐21. [DOI] [PubMed] [Google Scholar]
McClave 2011
- McClave SA, Kushner R, Way CW, Cave M, DeLegge M, Dibaise J, et al. Nutrition therapy of the severely obese, critically ill patient: Summation of conclusions and recommendations. Journal of Parenteral and Enteral Nutrition 2011;35(Suppl 1):88S‐96S. [DOI] [PubMed] [Google Scholar]
Monk 1996
- Monk DN, Plank LD, Franc‐Arcas G, Finn PJ, Streat SL, Hill GL. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Annals of Surgery 1996;233(4):395‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicolo 2016
- Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C. Clinical outcomes related to protein delivery in a critically ill population: a multicenter, multinational observation study. Journal Parenteral Enteral Nutrition 2016;40(1):45‐5. [DOI] [PubMed] [Google Scholar]
Patiño 1999
- Patiño JF, Pimiento SE, Vergara A, Savino P, Rodríguez M, Escallón J. Hypocaloric support in the critically ill. World Journal of Surgery 1999;23(6):553‐9. [DOI] [PubMed] [Google Scholar]
Plank 2003
- Plank LD, Hill GL. Energy balance in critical illness. Proceedings of the Nutrition Society 2003;62(2):545‐52. [DOI] [PubMed] [Google Scholar]
Preiser 2015
- Preiser JC, Zanten AR, Berger MM, Biolo G, Casaer MP, Doig GS, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Critical Care 2015;19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rauch 2010
- Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation 2010;122(21):2152‐9. [PUBMED: 21060071] [DOI] [PubMed] [Google Scholar]
Reid 2004
- Reid CL. Nutritional requirements of surgical and critically‐ill patients: do we really know what they need?. Proceedings of the Nutrition Society 2004;63(3):467‐72. [DOI] [PubMed] [Google Scholar]
Reid 2006
- Reid C. Frequency of under‐ and overfeeding in mechanically ventilated ICU patients: causes and possible consequences. Journal of Human Nutrition and Dietetics 2006;19(1):13‐22. [DOI] [PubMed] [Google Scholar]
Ren 2013
- Ren T, Cong L, Wang Y, Tang Y, Tian B, Sin X, et al. Lipid emulsions in parenteral nutrition: current applications and future developments. Expert Opinion Drug Delivery 2013;10(1):1533‐49. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubinson 2004
- Rubinson L, Diette GB, Song X, Brower R, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Critical Care Medicine 2004;32(2):350‐7. [DOI] [PubMed] [Google Scholar]
Schneider 2007
- Schneider B, Carnoy M, Kilpatrick J, Schmidt WH, Shavelson RJ. Estimating causal effects using experimental and observational designs (report from the Governing Board of the American Educational Research Association Grants Program). Available from www.aera.net/Publications/Books/Estimating‐Causal‐Effects‐Using‐Experimental‐and‐Observational‐Designs 2007 (accessed 28 May 2018).
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shadish 2002
- Shadish WR, Cook TD, Campell DT. Experimental and quasi‐experimental designs for generalized causal inference. Available from pdfs.semanticscholar.org/9453/f229a8f51f6a95232e42acfae9b3ae5345df.pdf 2002 (accessed 28 May 2018).
Shaw 1987
- Shaw JH, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. Annals of Surgery 1987;205(3):288‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singer 2010
- Singer P, Pichard C, Heidegger P, Wernerman J. Considering energy deficit in the intensive care unit. Current Opinion Clinical Nutrition Metabolic Care 2010;13(2):170‐6. [DOI] [PubMed] [Google Scholar]
Singer 2011
- Singer P, Anbar R, Cohen J, Shapiro H, Shalita‐Chesner M, Lev S, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Medicine 2011;37(4):601‐9. [DOI] [PubMed] [Google Scholar]
Stahel 2010
- Stahel PF, Flierl MA, Moore EE. "Metabolic staging" after major trauma ‐ a guide for clinical decision making?. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2010;18:34‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata [Computer program]
- StataCorp. Stata. College Station, TX, USA: StataCorp, 2015.
Streat 1987
- Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. Journal of Trauma 1987;27(3):262‐6. [DOI] [PubMed] [Google Scholar]
Tao 2014
- Tao K‐M, Li X‐Q, Yang L‐Q, Yu W‐F, Lu Z‐J, Sun Y‐M, et al. Glutamine supplementation for critically ill adults. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD010050.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tian 2015
- Tian F, Wang X, Gao X, Wan X, Wu C, Zhang L, et al. Effect of initial calorie intake via enteral nutrition in critical illness: a meta‐analysis of randomised controlled trials. Critical Care 2015;19:180‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tian 2017
- Tian F, Gao X, Wu C, Zhang L, Xia X, Wang X. Initial energy supplementation in critically ill patients receiving enteral nutrition: a systematic review and meta‐analysis of randomized controlled trials. Asia Pacific Journal of Clinical Nutrition 2017;26(1):11‐9. [DOI] [PubMed] [Google Scholar]
Uehara 1999
- Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Critical Care Medicine 1999;27(7):1295‐302. [DOI] [PubMed] [Google Scholar]
Van Zanten 2016
- Zanten AR. Should we increase protein delivery during critical illness?. Journal of Parenteral and Enteral Nutrition 2016;40(6):756‐62. [DOI] [PubMed] [Google Scholar]
Villet 2005
- Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux MC, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clinical Nutrition 2005;24(4):502‐9. [DOI] [PubMed] [Google Scholar]
Weijs 2012
- Weijs PJ, Stapel SN, Groot SD, Driessen RH, Jong E, Girbes AR, et al. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational cohort study. Journal of Parenteral and Enteral Nutrition 2012;36(1):60‐8. [DOI] [PubMed] [Google Scholar]
Weijs 2013
- Weijs PJ, Wischmeyer PE. Optimizing energy and protein balance in the ICU. Current Opinion in Clinical Nutrition and Metabolic Care 2013;16(2):194‐201. [DOI] [PubMed] [Google Scholar]
Weijs 2014
- Weijs PJ, Cynober L, DeLegge M, Kreymann G, Wernerman J, Wolfe RR. Proteins and amino acids are fundamental to optimal nutrition support in critically ill patients. Critical Care 2014;18(6):591‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wischmeyer 2012
- Wischmeyer P. Parenteral nutrition and calorie delivery in the ICU: controversy, clarity, or call to action?. Current Opinion Critical Care 2012;18(2):164‐3. [DOI] [PubMed] [Google Scholar]
Wischmeyer 2013
- Wischmeyer PE. The evolution of nutrition in critical care: how much, how soon?. Critical Care 2013;17(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wischmeyer 2015
- Wischmeyer PE. Ensuring optimal survival and post‐ICU quality of life in high‐risk ICU patients: permissive underfeeding is not safe!. Critical Care Medicine 2015;43(8):1769‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaloga 1994
- Zaloga GP, Roberts P. Permissive underfeeding. New Horizons 1994;2(2):257‐63. [PubMed] [Google Scholar]
Zauner 2006
- Zauner A, Schneeweiss B, Kneidinger N, Lindner G, Zauner C. Weight‐adjusted resting energy expenditure is not constant in critically ill patients. Intensive Care Medicine 2006;32(3):428‐34. [DOI] [PubMed] [Google Scholar]
Zhan 2007
- Zhan WH, Jiang ZM, Tang Y, Tang Y, Wu YP, Liu JW, et al. Impact of hypocaloric and hyponitrogenic parenteral nutrition on clinical outcome in postoperative patients: a multi‐center randomized controlled trial of 120 cases. National Medical Journal of China 2007;87(25):1729‐33. [PubMed] [Google Scholar]
References to other published versions of this review
Perman 2009
- Perman MI, Ciapponi A, Crivelli A, Garrote V, Loudet C, Perman G. Prescribed hypocaloric nutrition support for critically ill adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007867] [DOI] [PMC free article] [PubMed] [Google Scholar]


