Abstract
Background
This is the first update of a review published in 2009. Sustained moderate to severe elevations in resting blood pressure leads to a critically important clinical question: What class of drug to use first‐line? This review attempted to answer that question.
Objectives
To quantify the mortality and morbidity effects from different first‐line antihypertensive drug classes: thiazides (low‐dose and high‐dose), beta‐blockers, calcium channel blockers, ACE inhibitors, angiotensin II receptor blockers (ARB), and alpha‐blockers, compared to placebo or no treatment.
Secondary objectives: when different antihypertensive drug classes are used as the first‐line drug, to quantify the blood pressure lowering effect and the rate of withdrawal due to adverse drug effects, compared to placebo or no treatment.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials up to November 2017: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We contacted authors of relevant papers regarding further published and unpublished work.
Selection criteria
Randomized trials (RCT) of at least one year duration, comparing one of six major drug classes with a placebo or no treatment, in adult patients with blood pressure over 140/90 mmHg at baseline. The majority (over 70%) of the patients in the treatment group were taking the drug class of interest after one year. We included trials with both hypertensive and normotensive patients in this review if the majority (over 70%) of patients had elevated blood pressure, or the trial separately reported outcome data on patients with elevated blood pressure.
Data collection and analysis
The outcomes assessed were mortality, stroke, coronary heart disease (CHD), total cardiovascular events (CVS), decrease in systolic and diastolic blood pressure, and withdrawals due to adverse drug effects. We used a fixed‐effect model to to combine dichotomous outcomes across trials and calculate risk ratio (RR) with 95% confidence interval (CI). We presented blood pressure data as mean difference (MD) with 99% CI.
Main results
The 2017 updated search failed to identify any new trials. The original review identified 24 trials with 28 active treatment arms, including 58,040 patients. We found no RCTs for ARBs or alpha‐blockers. These results are mostly applicable to adult patients with moderate to severe primary hypertension. The mean age of participants was 56 years, and mean duration of follow‐up was three to five years.
High‐quality evidence showed that first‐line low‐dose thiazides reduced mortality (11.0% with control versus 9.8% with treatment; RR 0.89, 95% CI 0.82 to 0.97); total CVS (12.9% with control versus 9.0% with treatment; RR 0.70, 95% CI 0.64 to 0.76), stroke (6.2% with control versus 4.2% with treatment; RR 0.68, 95% CI 0.60 to 0.77), and coronary heart disease (3.9% with control versus 2.8% with treatment; RR 0.72, 95% CI 0.61 to 0.84).
Low‐ to moderate‐quality evidence showed that first‐line high‐dose thiazides reduced stroke (1.9% with control versus 0.9% with treatment; RR 0.47, 95% CI 0.37 to 0.61) and total CVS (5.1% with control versus 3.7% with treatment; RR 0.72, 95% CI 0.63 to 0.82), but did not reduce mortality (3.1% with control versus 2.8% with treatment; RR 0.90, 95% CI 0.76 to 1.05), or coronary heart disease (2.7% with control versus 2.7% with treatment; RR 1.01, 95% CI 0.85 to 1.20).
Low‐ to moderate‐quality evidence showed that first‐line beta‐blockers did not reduce mortality (6.2% with control versus 6.0% with treatment; RR 0.96, 95% CI 0.86 to 1.07) or coronary heart disease (4.4% with control versus 3.9% with treatment; RR 0.90, 95% CI 0.78 to 1.03), but reduced stroke (3.4% with control versus 2.8% with treatment; RR 0.83, 95% CI 0.72 to 0.97) and total CVS (7.6% with control versus 6.8% with treatment; RR 0.89, 95% CI 0.81 to 0.98).
Low‐ to moderate‐quality evidence showed that first‐line ACE inhibitors reduced mortality (13.6% with control versus 11.3% with treatment; RR 0.83, 95% CI 0.72 to 0.95), stroke (6.0% with control versus 3.9% with treatment; RR 0.65, 95% CI 0.52 to 0.82), coronary heart disease (13.5% with control versus 11.0% with treatment; RR 0.81, 95% CI 0.70 to 0.94), and total CVS (20.1% with control versus 15.3% with treatment; RR 0.76, 95% CI 0.67 to 0.85).
Low‐quality evidence showed that first‐line calcium channel blockers reduced stroke (3.4% with control versus 1.9% with treatment; RR 0.58, 95% CI 0.41 to 0.84) and total CVS (8.0% with control versus 5.7% with treatment; RR 0.71, 95% CI 0.57 to 0.87), but not coronary heart disease (3.1% with control versus 2.4% with treatment; RR 0.77, 95% CI 0.55 to 1.09), or mortality (6.0% with control versus 5.1% with treatment; RR 0.86, 95% CI 0.68 to 1.09).
There was low‐quality evidence that withdrawals due to adverse effects were increased with first‐line low‐dose thiazides (5.0% with control versus 11.3% with treatment; RR 2.38, 95% CI 2.06 to 2.75), high‐dose thiazides (2.2% with control versus 9.8% with treatment; RR 4.48, 95% CI 3.83 to 5.24), and beta‐blockers (3.1% with control versus 14.4% with treatment; RR 4.59, 95% CI 4.11 to 5.13). No data for these outcomes were available for first‐line ACE inhibitors or calcium channel blockers. The blood pressure data were not used to assess the effect of the different classes of drugs as the data were heterogeneous, and the number of drugs used in the trials differed.
Authors' conclusions
First‐line low‐dose thiazides reduced all morbidity and mortality outcomes in adult patients with moderate to severe primary hypertension. First‐line ACE inhibitors and calcium channel blockers may be similarly effective, but the evidence was of lower quality. First‐line high‐dose thiazides and first‐line beta‐blockers were inferior to first‐line low‐dose thiazides.
Plain language summary
Thiazides best first choice for hypertension
Review Question(s)
In this first update of a review published in 2009, we wanted to determine which drug class was the best first‐line choice in treating adult patients with raised blood pressure.
We searched the available medical literature to find all the trials that compared the drugs to placebo or no treatment to assess this question. The data included in this review are up to date as of November 2017.
Background
High blood pressure or hypertension can increase the risk of heart attacks and stroke. One of the most important decisions in treating people with elevated blood pressure is what drug class to use first. This decision has important consequences in terms of health outcomes and cost.
Study characteristics
We found no new trials in this updated search. In the original review, we found 24 studies that randomly assigned 58,040 adult people (mean age 62 years) with high blood pressure, to four different drug classes or placebo. Duration of these studies ranged from three to five years. Drug classes studied included thiazide diuretics, beta‐blockers, ACE inhibitors, and calcium channel blockers.
Key Results
We concluded that most of the evidence demonstrated that first‐line low‐dose thiazides reduced mortality, stroke, and heart attack. No other drug class improved health outcomes better than low‐dose thiazides. Beta‐blockers and high‐dose thiazides were inferior.
Conclusions
High‐quality evidence supported that low‐dose thiazides should be used first for most patients with elevated blood pressure. Fortunately, thiazides are also very inexpensive.
Quality of evidence
The evidence for first‐line low dose thiazides was high quality. For the other classes, we judged the evidence to be moderate or low quality.
Summary of findings
Background
Elevated blood pressure (hypertension) is a chronic condition in which the blood pressure in the arteries is persistently elevated. It has been divided into three categories, based on resting blood pressures, measured in a standard way: mild hypertension (140 to 159/90‐99 mmHg), moderate hypertension (160 to 179/100 to 109 mmHg), and severe hypertension (180/110 mmHg or higher). Most people with high blood pressure have no signs or symptoms, even if blood pressure readings are very high. For most adults with primary or essential hypertension, there is no identifiable cause for the high blood pressure. Some people have high blood pressure, called secondary hypertension, caused by underlying conditions such as adrenal gland tumours, kidney problems, thyroid problems, excessive alcohol intake, or use of certain medications, such as birth control pills. Isolated systolic hypertension is a condition in which the diastolic pressure is normal (less than 90 mmHg), but systolic pressure is high (160 mmHg or greater). This is a common type of high blood pressure among older people.
Blood pressure tends to increase with age. High blood pressure is more common in men in early middle age, more common in women after age 65, and more common in Blacks compared to Caucasians. The risk of high blood pressure is increased when there is a family history of high blood pressure, in the presence of obesity, or when physically inactive. High blood pressure is associated with smoking, too much salt in the diet, drinking excessive amounts of alcohol, high levels of stress, and chronic conditions such as diabetes, kidney disease, and sleep apnea.
Uncontrolled persistent resting high blood pressure increases the risk of stroke, heart attack, heart failure, kidney damage, and vision loss.
Description of the condition
When drug treatment is indicated in the management of patients with elevated blood pressure, an important decision is which drug to choose first. The decision should be informed by the best available evidence on reduction of the outcomes that are important to the patient, i.e. the ability of the drug to reduce the adverse health outcomes associated with elevated blood pressure (disabling stroke, myocardial infarction, heart failure, and mortality).
Description of the intervention
High blood pressure should initially be managed with changing life style — eating a healthy diet with less salt, exercising regularly, quitting smoking, and maintaining a healthy weight. When these life‐style changes are not enough, treatment with antihypertensive drugs is recommended. Several different classes of medications are available to reduce blood pressure. The six main drug classes, included in this review, are thiazide diuretics, beta‐blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, calcium channel blockers, and alpha blockers.
How the intervention might work
Different classes of antihypertensive drugs have different mechanisms of action.
Thiazide and thiazide‐like diuretics: The mechanism of action by which thiazide diuretics lower blood pressure in the long term is not fully understood (Zhu 2005). After chronic use, thiazides lower peripheral resistance. The mechanism of these effects is uncertain, as it may involve effects on the whole body, renal autoregulation, or direct vasodilator actions (Hughes 2004). Thiazides act on the kidney to inhibit reabsorption of sodium (Na+) and chloride (Cl‐) ions from the distal convoluted tubules in the kidneys, by blocking the thiazide‐sensitive sodium‐chloride symporter (Duarte 2010). They also increase calcium reabsorption at the distal tubule, and increase the reabsorption of calcium ions (Ca2+), by a mechanism involving the reabsorption of sodium and calcium in the proximal tubule in response to sodium depletion.
Beta‐blockers: Beta‐blockers are competitive antagonists that block the receptor sites for epinephrine (adrenaline) and norepinephrine on adrenergic beta‐receptors. Some block activation of all types of beta‐adrenergic receptors (β1, β2, and β3), and others are selective for one of the three types of beta receptors (Frishman 2005).
Angiotensin converting enzyme (ACE) inhibitors: ACE inhibitors block the conversion of angiotensin I (AI) to angiotensin II (AII), and thus decrease the actions of angiotensin II. The end result is to lower arteriolar resistance and increase venous capacity; decrease cardiac output, cardiac index, stroke work, and volume; lower resistance in blood vessels in the kidneys; and increase excretion of sodium in the urine. Renin and AI increases in concentration in the blood as a result of negative feedback of the conversion of AI to AII. AII and aldosterone levels decrease. Bradykinin increases, because ACE is also responsible for inactivation of bradykinin.
Angiotensin receptor blockers (ARBs): ARBs block the activation of angiotensin II AT1 receptors. Blockage of AT1 receptors directly causes vasodilation, reduces secretion of vasopressin, and reduces the production and secretion of aldosterone.
Calcium channel blockers (CCBs): CCBs block calcium channel and inhibit calcium ion influx into vascular smooth muscle and myocardial cells. They reduce blood pressure through various mechanisms: by vasodilation, by reducing the force of contraction of the heart, by slowing the heart rate, and by directly reducing aldosterone production.
Alpha blockers: α1 adrenergic receptor blockers inhibit the binding of norepinephrine (noradrenaline) to the α1 receptors on vascular smooth muscle cells. The primary effect of this inhibition is vasodilation, which decreases peripheral vascular resistance, leading to decreased blood pressure.
Why it is important to do this review
There have been a number of reviews of the effectiveness of antihypertensive therapy, but most have emphasized effectiveness of all drug classes (Collins 1990; Gueyffier 1996), or effectiveness of all drug classes in special populations, such the elderly (Insua 1994; MacMahon 1993; Mulrow 1994; Mulrow 1998; Thijs 1992). When all drug therapies are included in one review, there is an underlying assumption that the benefits of lowering blood pressure are independent of the mechanism by which it is achieved. This assumption has not been proven, and it is likely that different classes of drugs will have different blood pressure lowering effects, and will have effects that are independent of the blood pressure lowering effect. A drug that lowers blood pressure could have pharmacological and physiological actions independent of blood pressure lowering, and these other actions (both known and unknown) could enhance or negate the effects on health outcomes associated with the decrease in blood pressure. This possibility is supported by a recent analysis that suggested that blood pressure lowering only explains about 50% of the treatment effect in antihypertensive trials (Boissel 2005).
This review update aims to 1) document the best available evidence of effectiveness for different classes of drugs and doses used as first‐line therapy, compared to placebo or no treatment, and 2) present the outcome data in a way that best assists clinicians in the choice of a first‐line drug.
Objectives
Primary objective
To quantify the mortality and morbidity effects from different first‐line anti‐hypertensive drug classes: thiazides (low dose and high dose), beta‐blockers, calcium channel blockers, ACE inhibitors, angiotensin II receptor blockers, and alpha‐blockers, compared to placebo or no treatment.
Secondary objectives
To quantify the blood pressure lowering effect of antihypertensive treatment when different drug classes are used as the first‐line drug.
To quantify the rate of withdrawal due to adverse drug effects of different first‐line antihypertensive class drugs, compared to placebo or no treatme
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCT) of at least one year duration. The comparative group was a placebo, or an untreated control. We required the following data from the trial: baseline patient characteristics, clearly defined morbidity and mortality endpoints, and outcome data presented using the intention‐to‐treat principle.
We excluded trials using other than randomized allocation methods, such as alternate allocation, week of presentation, or retrospective controls. We also excluded trials that compared two specific antihypertensive first‐line therapies without a placebo or untreated control.
Types of participants
Blood pressure was measured using proper technique at least two times, with the patient resting for at least five minutes. All patients must have had a baseline resting blood pressure of at least 140 mmHg systolic or a diastolic blood pressure of at least 90 mmHg. Trials that included both hypertensive and normotensive patients were acceptable if the majority (> 70%) of patients had elevated blood pressure, or the trial separately reported outcome data on patients with elevated blood pressure. Trials were not limited by any other factor or baseline risk. It was assumed that age and comorbidities did not affect the risk ratio of outcomes associated with drug treatment.
Types of interventions
Treatment was to be clearly defined as a specific class of first‐line antihypertensive therapy in one of the following classes: thiazide diuretics, beta blockers, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, or alpha adrenergic blockers. The majority (> 70%) of the patients in the treatment group were to be taking the drug class of interest after one year. We allowed initial combined therapies with drug classes not in the defined categories. We also allowed supplemental drugs from other drug classes of interest as stepped therapy, but only as long as they were not taken by over 50% of the patients. We assumed that these supplemental drugs did not systematically interact to affect the occurrence of the outcomes studied. We also stratified the analysis by the thiazide dose. We classified thiazide doses as high‐dose and low‐dose by selecting hydrochlorothiazide as the standard, and translating the doses of other drugs in the class into hydrochlorothiazide equivalents. We assumed that each thiazide had a similar dose‐response curve, and the usual prescription dose range represented a similar range on the dose‐response curve.
We classified groups according to the starting dose in the trial:
High‐dose thiazide group: starting dose
hydrochlorothiazide ≥ 50 mg per day
chlorothiazide ≥ 500 mg per day
chlorthalidone ≥ 50 mg per day
bendrofluazide ≥ 5 mg per day
methylclothiazide ≥ 5 mg per day
trichlormethiazide ≥ 2 mg per day
indapamide ≥ 5 mg per day
Low‐dose thiazide group: starting dose
hydrochlorothiazide < 50 mg per day
chlorthiazide < 500 mg per day
chlorthalidone < 50 mg per day
bendrofluazide < 5 mg per day
methylclothiazide < 5 mg per day
trichlormethiazide < 2 mg per day
indapamide < 5 mg per day
We calculated the average dose in the high‐dose and low‐dose group as a weighted average from the trials in which the average dose was reported, or could be estimated.
Types of outcome measures
Primary outcomes
Total mortality (death from all causes)
Total stroke (fatal and non‐fatal strokes)
Total coronary heart disease (CHD; fatal and non‐fatal myocardial infarction, and sudden or rapid cardiac death).
Total cardiovascular events (total stroke, total CHD, hospitalization or death from congestive heart failure and other significant vascular deaths, such as ruptured aneurysms. It does not include angina, transient ischemic attacks, surgical or other procedures, or accelerated hypertension).
When the primary trials did not report outcomes that fit the above definitions, we based our decisions on maximizing the inclusion of the data and maintaining concordance, with how the data were classified in previous reviews. We assumed that the effect of antihypertensive treatment on outcomes was independent of whether elevated blood pressure was defined in terms of systolic or diastolic pressure.
Secondary outcomes
Reduction in systolic and diastolic blood pressure during the first year
Patient withdrawal due to adverse drug effects
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist (DS) conducted systematic searches in the following databases for RCTs without language, publication year, or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web; searched 24 November 2017);
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web; searched 24 November 2017);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 24 November 2017);
Embase Ovid (searched 24 November 2017);
ClinicalTrials.gov (www.clinicaltrials.gov; searched 24 November 2017);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch; searched 24 November 2017).
The Cochrane Hypertension Information Specialist (DS) modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions (Box 6.4.b; Higgins 2011a). The MEDLINE search strategy was translated into the other databases using the appropriate controlled vocabulary, as applicable (Appendix 1).
Searching other resources
We used previously published meta‐analyses on the treatment of hypertension to help identify references to trials (BBLTTC 2005; BPLTTC 2000; Collins 1990; Goeres 2014; Gueyffier 1996; Gueyffier 1999; Insua 1994; Kang 2004; Kızılırmak 2017; Law 2009; MacMahon 1993; Mulrow 1994; Mulrow 1998; Musini 2009; Nikolaus 2000; Parsons 2016; Pearce 1995; Psaty 1997; Psaty 2003; Quan 1999; Sundstro¨m 2015; Tan 2016; Thijs 1992; Thomopoulos 2014; Thomopoulos 2016; Turnbull 2003; Wiysonge 2017; Zanchetti 2015). We carefully screened the bibliographies from these systematic reviews to make sure that any study that met the inclusion criteria was not missed.
We contacted experts in the field to identify any other trials we may have missed in our search. We checked reference lists of included studies, and contacted relevant individuals for information about unpublished or ongoing studies.
Data collection and analysis
Selection of studies
We rejected articles on the initial screening if we could determine from the title or the abstract that the article was not a report of a randomized controlled trial, or that there was no possibility that the trial would fit the requirements of this review. Of the articles selected for further review, two reviewers (JMW and VM) independently assessed whether they would be included or excluded.
Data extraction and management
The data abstraction form included details of study design, randomization, blinding, duration of treatment, baseline characteristics, number of patients lost to follow‐up, outcomes, intervention, statistical analysis, and reporting. Two reviewers (JMW and VM) independently extracted the data, cross‐checked, and compared, whenever possible, to data from previously published meta‐analyses. We detailed trial characteristics in the 'Characteristics of included studies' table. We detailed trials that were excluded in the 'Characteristics of excluded studies' table.
Assessment of risk of bias in included studies
Two review authors (VM and RG) independently assessed risk of bias of each included trial; a third review author (JMW) sorted any disagreements. We assessed risk of bias according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed seven domains: randomization and allocation concealment to assess selection bias; blinding of the participants and physician to assess performance bias; blinding of the outcome assessor to assess detection bias; incomplete outcome reporting to assess attrition bias; selective reporting of outcomes to assess selective reporting bias; and we added an additional category ‐ other bias, to assess whether the study was funded by the manufacturer and conflict of interest was present, which we assessed as high risk of bias, since it has been shown to overestimate treatment effect.
'Summary of findings' table
We used GRADEpro GDT software to present the 'Summary of findings' table (GRADEpro GDT). We decided to include all clinically relevant primary and secondary outcomes: total mortality, total stroke, total coronary heart disease, total cardiovascular events, and withdrawal due to adverse events. We did not include the magnitude of systolic and diastolic blood pressure reduction.
We considered five factors in grading the overall quality of evidence: limitations in study design and implementation, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision in results, and high probability of publication bias. This approach specifies four levels of quality: high‐, moderate‐, low‐, and very low‐quality evidence. The highest quality rating is for randomized trial evidence. Quality rating is downgraded by one level for each factor, up to a maximum of three levels for all factors. If there are severe problems for any one factor (when assessing limitations in study design and implementation, in concealment of allocation, loss of blinding, or attrition over 50% of participants during follow‐up), randomized trial evidence may fall by two levels due to that factor alone.
Measures of treatment effect
We used Review Manager 5 for data synthesis and analyses (RevMan 2014). We based quantitative analyses of outcomes on intention‐to‐treat results. We used risk ratios (RR) with 95% confidence intervals (CI) to combine outcomes across trials. If there was a significant difference in any outcome measure, we presented an absolute risk reduction (ARR), and number needed to treat for an additional beneficial (NNTB) or harmful (NNTH) outcome in the 'Summary of findings' table. This estimate, with 95% confidence intervals (CI), is considered the best estimate of the average benefit and the range of that benefit in populations with different baseline risks.
For continuous outcomes (systolic and diastolic blood pressure), we calculated the mean difference (MD) with 99% CI to combine outcomes across studies. Systolic and diastolic blood pressure readings were taken at one year, or the earliest time after one year, to include the data from the maximum number of randomized patients. Mean blood pressure values only reflected data for patients in whom blood pressure was measured. We used standard deviation of the change (SD) at one year if available.
Unit of analysis issues
For all outcomes measures reported, we used data from each trial at the end of the follow‐up period mentioned in each trial, which varied from 1.1 to 10 years.
Dealing with missing data
When participants were lost to follow‐up, we used data as reported for participants who were followed until the end of the study, in the analyses. We outlined how data were accounted for and included in each study under assessment of attrition bias in the 'Risk of bias in included studies' table.
For example, in the MRC‐TMH 1985 study, for events such as non‐fatal stroke or myocardial infarction, which terminated participation in the study, the investigators did not follow‐up these participants to the end of the study. In such instances, investigators included data available up to the time point during which participants were followed in the analyses.
If the SD value for reduction in systolic and diastolic blood pressure was not reported at one year, we imputed the SD of the change at other time points during treatment. If the SD of the change was not available at all, we imputed the SD of the endpoint systolic or diastolic blood pressure. In cases where these values were also missing, we imputed the mean weighted SD of the change from other trials. This imputation is a limitation, and to reduce the impact of this limitation, we used a 99% level of significance for the blood pressure measurements, instead of the standard 95%.
Assessment of heterogeneity
We tested heterogeneity of treatment effect between the trials using a standard Chi² statistic for heterogeneity. We used the fixed‐effect model to obtain summary statistics of pooled trials, unless there was significant between‐study heterogeneity, in which case we used the random‐effects model to test statistical significance.
Assessment of reporting biases
For each study, we evaluated whether selective reporting of outcomes was suspected. In the case of suspected reporting bias, we contacted study authors for clarification.
We had planned to use a funnel plot to assess the possibility of publication bias for outcomes that were reported in 10 or more studies. A test for funnel plot asymmetry (small study effects) formally examines whether the association between estimated intervention effects and a measure of study size is greater than might be expected to occur by chance.
Data synthesis
We used Review Manager 5 to perform data synthesis and analyses (RevMan 2014). We presented dichotomous outcomes as RR with 95% CI using a fixed‐effect model, and continuous outcomes (systolic and diastolic blood pressure) as MD with 99% CI.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses: different race: Black versus Caucasian versus Asian; and baseline severity of hypertension: mild, moderate, severe. Unfortunately, it was not possible to do any subgroup analyses, because there were a lack of data specific to race, and most trial participants had moderate to severe hypertension, but data were not available separately.
When heterogeneity was significant (I² greater than 50%), we attempted to identify trials that would contribute to heterogeneity, and explore their population characteristics, baseline blood pressure, blinded or open‐label study design, use of antihypertensive drugs as fixed dose or stepped‐up therapy, or response to placebo that would possibly explain the reason for heterogeneity. As the decrease in systolic and diastolic blood pressure showed significant heterogeneity, we present results as MD with 99% CI using both a fixed‐effect as well as random effect model.
Sensitivity analysis
To test for robustness of results, we conducted several sensitivity analyses. This was done by deselecting trials in the following categories: trials that were not placebo‐controlled and blinded, trials restricted to patients with isolated systolic hypertension, trials that enrolled more than 80% of patients with previous stroke, and myocardial infarction of peripheral vascular disease (secondary prevention), trials using combined starting drugs, and trials using supplemental drugs from other defined classes.
Results
Description of studies
Results of the search
The initial search up to 2009 resulted in 6232 citations, 5985 of which we excluded on reading titles and abstracts. We retrieved 247 citations for more detailed evaluation, 13 of which were review articles. We further evaluated 234 reports; we included 127 reports of 24 trials, and excluded 107 reports.
The updated search in November 2017 resulted in 11,855 citations. We screened the titles and abstracts of these citations, and found 11,500 to be irrelevant. We requested the full text of 355 citations, but none of them met the minimum inclusion criteria. We found 6 additional reports of previously included studies.
For this update of a total of 87 potential trials identified, we included 133 reports of 24 trials, (58,040 patients), and excluded 63 trials. Refer to Figure 1 for study flow diagram.
1.
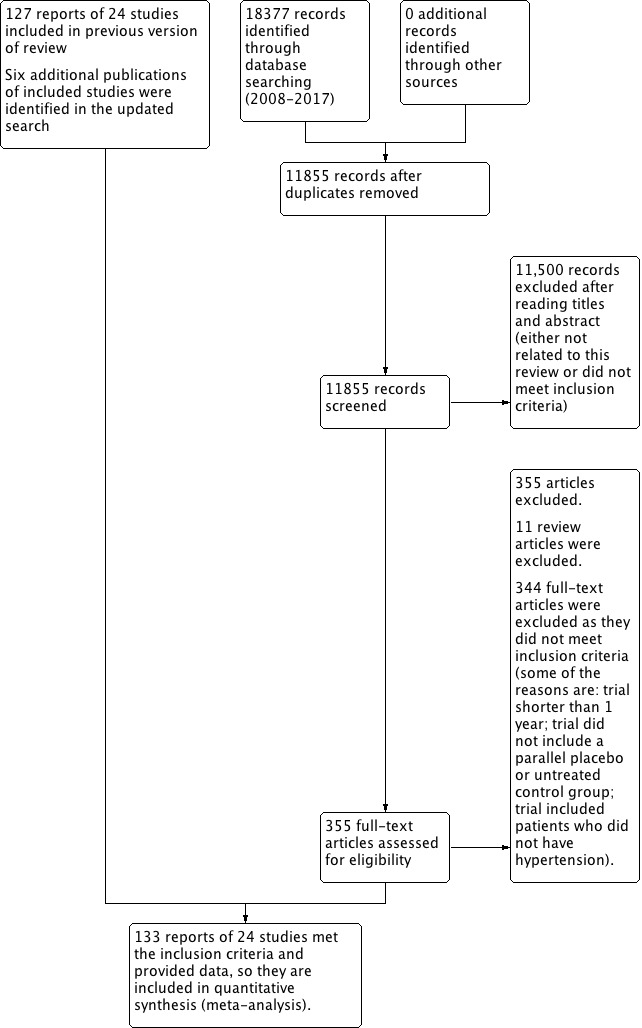
Study flow diagram
Included studies
We found 24 trials, with 28 active treatment arms, studying 58,040 patients, which met the inclusion criteria. Trials were only available to evaluate the effectiveness of four drug classes as first‐line drugs thiazides (19 trials in 39,713 patients), beta‐blockers (five trials in 19,313 patients), ACE inhibitors (three trials in 6002 patients), and calcium channel blockers (one trial in 4695 patients). Two trials evaluated thiazides as well as beta‐blockers versus placebo (MRC‐O 1992; MRC‐TMH 1985;); UKPDS 39 1998 evaluated beta‐blockers as well as ACE inhibitors versus placebo, and HYVET pilot 2003 evaluated thiazides as well as ACE inhibitors versus placebo, making 28 total comparisons from 24 trials. We did not identify any randomized controlled trials that compared first‐line alpha‐adrenergic blockers or angiotensin receptor blockers to placebo or untreated control group.
The average age of participants across all included trials was 62 years. Six trials were limited to patients over 60 years of age (EWPHE 1985; HYVET 2008; HYVET pilot 2003; Kuramoto 1981; MRC‐O 1992; SHEP 1991; SHEP‐P 1989; SYST‐EUR 1997). The age range in other trials was between 21 and 80 years. The mean age of patients from the four classes was: thiazide ‒ 61 years, beta‐blockers ‒ 56 years, ACE inhibitors ‒ 67 years, and calcium channel blockers ‒ 70 years.
Most participants were recruited from Western industrialized countries. Two trials did not report percentage of participants from different countries (HOPE HYP 2000; HYVET 2008). In 22 trials reporting recruitment of participants, 7750 (15.5%) were from USA, 32,907 (66%) from Europe; 3427 (6.9%) from Australia, and 91 (0.2%) from Japan. Females represented 45% of the population studied. Four trials included only men (OSLO 1986; VA‐I 1967; VA‐II 1970; VA‐NHLBI 1978) .
Fourteen trials did not report ethnicity (Barraclough 1973; Carter 1970; Dutch TIA 1993; EWPHE 1985; HOPE HYP 2000; HYVET 2008; HYVET pilot 2003; Kuramoto 1981; MRC‐TMH 1985; MRC‐O 1992; OSLO 1986; PATS 1996; SYST‐EUR 1997; TEST 1995). Ten trials reported ethnicity. African‐Americans comprised the following percentages in these trials: ATTMH 1980 (0%), HSCSG 1974 (80%), SHEP 1991 (13.8%), SHEP‐P 1989 (18%), UKPDS 39 1998 (7.6%), USPHSHCSG 1977 (28%), VA‐I 1967 ( 53.8%), VA‐II 1970 (42%), VA‐NHLBI 1978 (25%), and Wolff 1966 (89.6%).
The study population consisted of predominantly ambulatory patients recruited from the community, primary care centres, or hospital‐based clinics in 22 trials (57,982 patients, 99.7% of all patients included in this review). In the Kuramoto 1981 trial, 91 (0.2% of total) subjects were recruited from a home for the aged. Carter 1970 recruited 97 (0.3% of total) participants admitted to the hospital, who had survived an ischemic‐type major stroke.
In most trials, it was possible to determine whether the participants in the trials represented primary or secondary prevention. All trials excluded patients with angina and congestive heart failure, as these conditions would require use of antihypertensive drugs for reasons independent of their antihypertensive action. Some trials allowed patients with prior myocardial infarction or stroke, as long as they were not recent (e.g. within the previous three months). Thus, by determining the baseline prevalence of stroke and myocardial infarction, it was possible to calculate the percentage that represented secondary prevention. Three trials did not report prevalence of stroke or myocardial infarction, but it was likely low in these trials (Barraclough 1973; Kuramoto 1981; VA‐II 1970; 587 participants, (1.0% of total randomized participants). Six trials (11,157 patients) were primary prevention with less than 1% secondary prevention patients (ATTMH 1980 (0.4%); MRC‐O 1992 (0%); OSLO 1986 (0%); UKPDS 39 1998 (0%); USPHSHCSG 1977 (0%); and VA‐NHLBI 1978 (0%). Six trials (12,042 patients) were secondary prevention (Dutch TIA 1993 (100%); HOPE HYP 2000 (88%); HSCSG 1974 (96%); OSLO 1986 (0%); PATS 1996 (100%); and TEST 1995 (100%). Nine trials (34,041 patients) were mostly primary prevention patients (EWPHE 1985 (it was reported that the baseline prevalence of cardiovascular complications was 36% and these included conditions other than proven myocardial infarction and stroke); HYVET 2008 (12%); HYVET pilot 2003 (7%); MRC‐TMH 1985 (2.2%); SHEP 1991 (6.4%); SHEP‐P 1989 (5.5%); SYST‐EUR 1997 (reported as 30% patients with cardiovascular complications); VA‐I 1967 (7%);and Wolff 1966 (29%). The percentage of secondary patients in the 10 mostly primary prevention trials was 3212 (5.6%) of total randomized patients.
Thus, since 42,196 (72.7%) of total randomized people were primary prevention, the conclusions from this review are primarily relevant to the primary prevention setting.
Baseline prevalence of diabetes was reported in 8 trials as follows: HOPE HYP 2000 (38%); HYVET 2008 (7%); MRC‐O 1992 (0%); SHEP 1991 (10.1%); UKPDS 39 1998 (100%); USPHSHCSG 1977 (0%); VA‐I 1967 (9.1%); and Wolff 1966 (16.0%). Baseline prevalence of smoking was as follows: ATTMH 1980 (25.0%); EWPHE 1985 (16.0%); HYVET 2008 (6.5%); OSLO 1986 (41.7%); MRC‐TMH 1985 (28.5%); MRC‐O 1992 (36.0%); SHEP 1991 (13.0%); SHEP‐P 1989 (11.0%); SYST‐EUR 1997 (7.0%); UKPDS 39 1998 (22.3%); and USPHSHCSG 1977 (46.7%).
Recent trials defined stroke as the presence of neurological deficit lasting for more than 24 hours. It includes some patients with no disability. Older trials, like the HSCSG 1974, defined stroke as a neurological deficit lasting more than 24 hours, or a marked increase in transient ischemic attacks (twice the weekly pre‐randomization level of occurrence, more than four per week, or deterioration of more than eight points in neurological score). VA‐NHLBI 1978 defined stroke as typical weakness or paralysis. In some trials, stroke was not defined. In our opinion, lumping all strokes, including reversible ischemic neurological deficit (RIND), into one outcome is not optimal. More clinically relevant interpretations could be made if strokes were subdivided into three groups: strokes with no disability, strokes with mild disability, and strokes with severe disability. Myocardial infarction and sudden death were defined consistently across most trials. Myocardial infarction was defined as typical chest pain with ECG changes or increased cardiac enzymes; sudden death was defined as death within 24 hours of first evidence of acute cardiovascular disease, or unrelated to other known pre‐existing diseases.
Five trials restricted recruitment to persons with systolic hypertension; defined as systolic pressure 160 to 219 mmHg, and diastolic pressure less than 90 mmHg (SHEP 1991; SHEP‐P 1989), diastolic pressure less than 95 mmHg (SYST‐EUR 1997), or systolic pressure higher than 140 mmHg (TEST 1995), or higher than 160 mmHg (HYVET 2008). Six trials based entry on diastolic hypertension (Barraclough 1973; USPHSHCSG 1977; VA‐I 1967; VA‐II 1970; VA‐NHLBI 1978; Wolff 1966); and 10 trials based entry on either systolic or diastolic hypertension (ATTMH 1980; Carter 1970; EWPHE 1985; HSCSG 1974; HYVET pilot 2003; Kuramoto 1981; MRC‐TMH 1985; MRC‐O 1992; OSLO 1986; UKPDS 39 1998). HOPE HYP 2000 represented the subgroup of the HOPE trial that had a baseline blood pressure higher than 140 mmHg systolic, or higher than 90 mmHg diastolic. Two trials were included because more than 70% of patients at entry had a systolic BP higher than 140 mmHg (Dutch TIA 1993; PATS 1996).
Weighted mean baseline blood pressure for all the trials was 168/94 mmHg. When this was broken down into those that used systolic blood pressure as entry criteria, it was 173/84 mmHg; for those using diastolic pressure as entry criteria, it was 162/106 mmHg; and for those using both systolic and diastolic pressure as entry criteria, it was 167/97 mmHg. Two trials did not report baseline systolic pressure levels (Barraclough 1973;VA‐NHLBI 1978), and one trial did not report baseline diastolic pressure levels (VA‐NHLBI 1978).
For complete description of the blood pressure inclusion criteria for each study, see 'Participants' in the 'Characteristics of included studies' table.
A stepped approach to antihypertensive drug administration was used in 18 of the 24 trials. The exceptions were Dutch TIA 1993; HOPE HYP 2000; HSCSG 1974; PATS 1996; TEST 1995; and USPHSHCSG 1977, which used a standard dose of drug in the intervention arm. In 19 of the trials, a thiazide was the first‐line therapy in one of the arms of the trial. Because of a relatively large amount of data for thiazides, we were able to divide these 19 trials into those in which the thiazide starting dose was defined as low (8/19 trials, 874 patients) or high (11/19 trials, 19,839 patients), as explained in the methods. Three of the trials did not specify the thiazide dose, but were included in the high‐dose group because prescribing high doses of thiazides was common when those trials were conducted (Barraclough 1973; Carter 1970; OSLO 1986). The weighted mean dose of thiazide, in hydrochlorothiazide equivalents, was 90 mg for the high‐dose trials and 24 mg for the low‐dose trials. In five trials, a beta‐blocker was used as first‐line therapy in one of the arms of the trial (Dutch TIA 1993; MRC‐O 1992; MRC‐TMH 1985; TEST 1995; UKPDS 39 1998). Three trials used an angiotensin converting enzyme inhibitor, (HOPE HYP 2000; HYVET pilot 2003; UKPDS 39 1998). One trial used the calcium channel blocker nitrendipine (SYST‐EUR 1997). Second‐ and third‐line drugs included beta‐blockers, centrally‐acting drugs, peripherally‐acting anti‐adrenergic agents, vasodilators, thiazides, ACE inhibitors, alpha blockers, calcium channel blockers, and loop diuretics. See 'Interventions' in the 'Characteristics of included studies' table for a complete description of each study's drug treatment protocol.
Mean duration of follow‐up ranged from 1.1 years for the HYVET pilot 2003 trial to 10 years for the USPHSHCSG 1977 trial. The weighted average follow‐up was 4.1 years for the thiazide trials, 5.3 years for the beta‐blocker trials, 4.9 years for the ACE Inhibitor trials, and 2.5 years for the one calcium‐channel blocker trial.
Excluded studies
We detailed the reasons for excluding 63 trials in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We assessed risk of bias for each included study using the Cochrane 'Risk of bias' tool for RCTs, described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Potential parameters of methodological quality listed in the 'Risk of bias' table include: method used to randomize participants, whether randomization was completed in an appropriate and blinded manner; whether participants, providers, outcome assessors, or a combination of these, were blinded to assigned therapy; whether the control group received a placebo or no treatment; percent of participants who did not complete follow‐up (dropouts); percent of participants not on assigned active or placebo therapy at study completion; selective reporting of outcomes; and other bias, in terms of funding of the trial by the manufacturer.
Refer to Figure 2 for the 'Risk of bias' graph, which provides review authors' judgements about each risk of bias item, presented as percentages across all included studies. Refer to Figure 3 for the 'Risk of bias' summary, which provides review authors' judgements about each risk of bias item for each included study.
2.
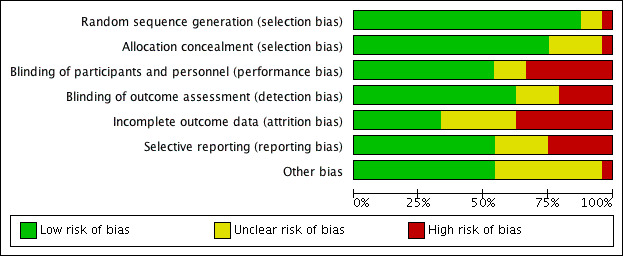
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
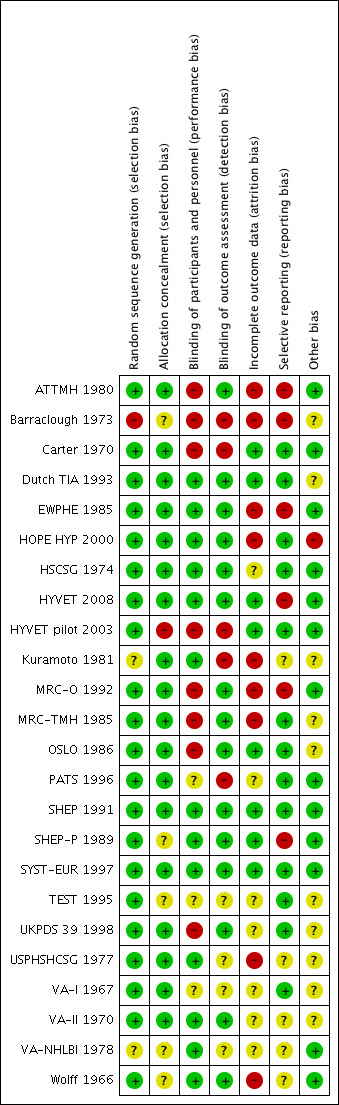
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomization was at low risk of bias in 22 trials (ATTMH 1980; Carter 1970; Dutch TIA 1993; EWPHE 1985; HOPE HYP 2000; HSCSG 1974; HYVET 2008; HYVET pilot 2003; Kuramoto 1981; MRC‐O 1992; MRC‐TMH 1985; OSLO 1986; PATS 1996; SHEP 1991; SHEP‐P 1989; SYST‐EUR 1997; TEST 1995; UKPDS 39 1998; USPHSHCSG 1977; VA‐I 1967; VA‐II 1970; Wolff 1966). It was judged as unclear risk of bias in one trial (VA‐NHLBI 1978), and as high risk of bias in one trial (Barraclough 1973).
Allocation concealment was at low risk of bias in 18 trials ((ATTMH 1980; Carter 1970; Dutch TIA 1993; EWPHE 1985; HOPE HYP 2000; HSCSG 1974; HYVET 2008; Kuramoto 1981; MRC‐O 1992; MRC‐TMH 1985; OSLO 1986; PATS 1996; SHEP 1991; SYST‐EUR 1997; UKPDS 39 1998; USPHSHCSG 1977; VA‐I 1967; VA‐II 1970), unclear risk of bias in five trials (Barraclough 1973; SHEP‐P 1989; TEST 1995; VA‐NHLBI 1978; Wolff 1966), and high risk of bias in one trial (HYVET pilot 2003).
Blinding
Blinding of participant and personnel was at low risk of bias in 13 trials (Dutch TIA 1993; EWPHE 1985; HOPE HYP 2000; HSCSG 1974; HYVET 2008; Kuramoto 1981; SHEP 1991; SHEP‐P 1989; SYST‐EUR 1997; USPHSHCSG 1977; VA‐II 1970; VA‐NHLBI 1978; Wolff 1966), as unclear risk of bias in three trials ( PATS 1996; TEST 1995; VA‐I 1967), and high risk of bias in eight trials (ATTMH 1980; Barraclough 1973; Carter 1970; HYVET pilot 2003; MRC‐O 1992; MRC‐TMH 1985; OSLO 1986; UKPDS 39 1998).
Blinding of outcome assessor was at low risk of bias in 15 trials (ATTMH 1980; Dutch TIA 1993; EWPHE 1985; HOPE HYP 2000; HSCSG 1974; HYVET 2008; MRC‐O 1992; MRC‐TMH 1985; OSLO 1986; SHEP 1991; SHEP‐P 1989; SYST‐EUR 1997; UKPDS 39 1998; VA‐II 1970; Wolff 1966), unclear risk of bias in four trials (TEST 1995; USPHSHCSG 1977; VA‐I 1967; VA‐NHLBI 1978), and high risk of bias in five trials (Barraclough 1973; Carter 1970; HYVET pilot 2003; Kuramoto 1981; PATS 1996)
Incomplete outcome data
Incomplete outcome data was at low risk of bias in eight trials (Carter 1970; Dutch TIA 1993; HYVET 2008; HYVET pilot 2003; OSLO 1986; SHEP 1991; SHEP‐P 1989; SYST‐EUR 1997), unclear risk of bias in seven trials (HSCSG 1974; PATS 1996; TEST 1995; UKPDS 39 1998; VA‐I 1967; VA‐II 1970; VA‐NHLBI 1978), and high risk of bias in nine trials (ATTMH 1980; Barraclough 1973; EWPHE 1985; HOPE HYP 2000; Kuramoto 1981; MRC‐O 1992; MRC‐TMH 1985; USPHSHCSG 1977; Wolff 1966).
Selective reporting
Selective reporting was at low risk of bias in 13 trials (Carter 1970; Dutch TIA 1993; HOPE HYP 2000; HSCSG 1974; HYVET pilot 2003; OSLO 1986; MRC‐TMH 1985; PATS 1996; SHEP 1991; SYST‐EUR 1997; TEST 1995; UKPDS 39 1998;VA‐I 1967 ), unclear risk of bias in five trials (Kuramoto 1981; USPHSHCSG 1977; VA‐II 1970; VA‐NHLBI 1978; Wolff 1966), and high risk of bias in six trials (ATTMH 1980; Barraclough 1973; EWPHE 1985; HYVET 2008; MRC‐O 1992; SHEP‐P 1989).
Other potential sources of bias
Other potential bias was judged low risk in 13 trials (ATTMH 1980; Carter 1970; EWPHE 1985; HSCSG 1974; HYVET 2008; HYVET pilot 2003; MRC‐O 1992; PATS 1996; SHEP 1991; SHEP‐P 1989; SYST‐EUR 1997; VA‐NHLBI 1978; Wolff 1966), unclear risk in 10 trials (Barraclough 1973; Dutch TIA 1993; Kuramoto 1981; MRC‐TMH 1985; OSLO 1986; TEST 1995; UKPDS 39 1998; USPHSHCSG 1977; VA‐I 1967; VA‐II 1970), and high risk in one trial due to run‐in period leading to patient selection bias, plus funding bias (HOPE HYP 2000).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. First‐line low‐dose thiazide compared to placebo for hypertension.
| First‐line low‐dose thiazide compared to placebo for hypertension | ||||||
| Patient or population: adult patients with primary hypertension Setting: outpatients Intervention: First‐line low‐dose thiazide (mean duration 4.1 years) Comparison: placebo or untreated | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
№ of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Risk with Placebo |
Risk with Low dose thiazide |
|||||
| Total mortality | 110 per 1000 | 98 per 1000 (90 to 107) | RR 0.89 (0.82 to 0.97) | 19,874 (8 RCTs) | ⊕⊕⊕⊕ HIGH | ARR = 1.2%; NNTB = 83 |
| Total stroke | 62 per 1000 | 42 per 1000 (37 to 48) | RR 0.68 (0.60 to 0.77) | 19,874 (8 RCTs) | ⊕⊕⊕⊕ HIGH | ARR = 2%; NNTB = 50 |
| Total coronary heart disease | 39 per 1000 | 28 per 1000 (24 to 33) | RR 0.72 (0.61 to 0.84) | 19,022 (7 RCTs) | ⊕⊕⊕⊕ HIGH | ARR = 1.1%; NNTB = 91 |
| Total cardiovascular events | 129 per 1000 | 90 per 1000 (83 to 98) | RR 0.70 (0.64 to 0.76) | 19,022 (7 RCTs) | ⊕⊕⊕⊕ HIGH | ARR = 3.9%; NNTB = 26 |
| Withdrawal due to adverse effects | 50 per 1000 | 118 per 1000 (102 to 136) | RR 2.38 (2.06 to 2.75) | 8870 (3 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ARI = 6.8%; NNTH = 15 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomized controlled trial; ARR: Absolute risk reduction; ARI: Absolute risk increase; NNTB: Number needed to treat for an additional beneficial outcome; NNTH: Number needed to treat for an additional harmful outcome. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded due to risk of selective reporting bias; only 3 of the 8 studies reported withdrawal due to adverse effects
2. Downgraded due to inconsistency; I² = 96%
Summary of findings 2. First‐line high‐dose thiazide compared to placebo for hypertension.
| First‐line high‐dose thiazide compared to placebo for hypertension | ||||||
| Patient or population: adult patients with primary hypertension Setting: outpatients Intervention: First‐line high‐dose thiazide (mean duration 4.1 years) Comparison: placebo or untreated | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
№ of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Risk with Placebo |
Risk with High dose thiazide |
|||||
| Total mortality | 31 per 1000 | 28 per 1000 (24 to 33) | RR 0.90 (0.76 to 1.05) | 19,839 (11 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Not significant |
| Total stroke | 19 per 1000 | 9 per 1000 (7 to 12) | RR 0.47 (0.37 to 0.61) | 19,839 (11 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | ARR = 1%; NNTB = 100 |
| Total coronary heart disease | 27 per 1000 | 27 per 1000 (23 to 33) | RR 1.01 (0.85 to 1.20) | 19,839 (11 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | Not significant |
| Total cardiovascular events | 51 per 1000 | 37 per 1000 (32 to 42) | RR 0.72 (0.63 to 0.82) | 19,839 (11 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | ARR = 1.4%; NNTB = 71 |
|
Withdrawal due to adverse effects |
22 per 1000 | 98 per 1000 (84 to 115) | RR 4.48 (3.83 to 5.24) |
15,170 ( 7 RCTs) | ⊕⊕⊝⊝ LOW 2,3 | ARI = 7.6%; NNTH = 13 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomized controlled trial; ARR: Absolute risk reduction; ARI: Absolute risk increase; NNTB: Number needed to treat for an additional beneficial outcome; NNTH: Number needed to treat for an additional harmful outcome. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded due to imprecision; wide confidence limits.
2 Downgraded due to bias secondary to lack of blinding of investigators.
3 Downgraded due to high risk of selective reporting bias as only 7 of the 11 trials report this outcome.
Summary of findings 3. First‐line beta‐blocker compared to placebo for hypertension.
| First‐line beta‐blocker compared to placebo for hypertension | ||||||
| Patient or population: adult patients with primary hypertension Setting: outpatients Intervention: First‐line beta‐blocker (mean duration 5.3 years) Comparison: placebo or untreated | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Placebo |
Risk with Beta‐ blocker |
|||||
| Total mortality | 62 per 1000 | 60 per 1000 (54 to 67) | RR 0.96 (0.86 to 1.07) | 19,313 (5 RCTs) | ⊕⊕⊕⊝ MODERATE1 | Not significant |
| Total stroke | 34 per 1000 | 28 per 1000 (24 to 33) | RR 0.83 (0.72 to 0.97) | 19,313 (5 RCTs) | ⊕⊕⊕⊝ LOW 1,2 | ARR = 0.6% NNTB = 167 |
| Total coronary heart disease | 44 per 1000 | 39 per 1000 (34 to 45) | RR 0.90 (0.78 to 1.03) | 19,313 (5 RCTs) | ⊕⊕⊕⊝ LOW 1,2 | Not significant |
| Total cardiovascular events | 76 per 1000 | 68 per 1000 (62 to 75) | RR 0.89 (0.81 to 0.98) | 19,313 (5 RCTs) | ⊕⊕⊝⊝ LOW 2,3 | ARR = 0.8% NNTB = 125 |
|
Withdrawal due to adverse effects |
31 per 1000 | 144 per 1000 (129 to 161) | RR 4.59 (4.11 to 5.13) | 18,565 (4 RCTs) | ⊕⊕⊕⊕ LOW 2,3 | ARI = 11.3% NNTH = 9 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomized controlled trial; ARR: Absolute risk reduction; ARI: Absolute risk increase; NNTB: Number needed to treat for an additional beneficial outcome; NNTH: Number needed to treat for an additional harmful outcome. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded due to imprecision; wide confidence limits
2. Downgraded due to high risk of performance and detection bias.
3. Downgraded due to inconsistency; I² > 50%
Summary of findings 4. First‐line angiotensin converting enzyme inhibitor compared to placebo for hypertension.
| First‐line angiotensin converting enzyme (ACE) inhibitor compared to placebo for hypertension | ||||||
| Patient or population: adult patients with primary hypertension Setting: outpatient Intervention: First‐line ACE inhibitor (mean duration 4.9 years) Comparison: placebo or untreated | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Placebo |
Risk with ACE inhibitors |
|||||
| Total mortality | 136 per 1000 | 113 per 1000 (98 to 129) | RR 0.83 (0.72 to 0.95) | 6002 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ARR = 2.3%; NNTB = 43 Mostly secondary prevention population |
| Total stroke | 60 per 1000 | 39 per 1000 (31 to 49) | RR 0.65 (0.52 to 0.82) | 6002 (3 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ARR = 2.1%; NNTB = 48 Mostly secondary prevention population |
| Total coronary heart disease | 135 per 1000 | 110 per 1000 (95 to 127) | RR 0.81 (0.70 to 0.94) | 5145 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ARR = 2.5%; NNTB = 40 Mostly secondary prevention population |
| Total cardiovascular events | 201 per 1000 | 153 per 1000 (135 to 171) | RR 0.76 (0.67 to 0.85) | 5145 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ARR = 4.8%; NNTB = 21 Mostly secondary prevention population |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomized controlled trial; ARR: Absolute risk reduction; ARI: Absolute risk increase; NNTB: Number needed to treat for an additional beneficial outcome. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded due to high risk of attrition and other bias.
2. Downgraded due to imprecision (wide confidence interval).
Summary of findings 5. First‐line calcium channel blocker compared to placebo for hypertension.
| First‐line calcium channel blocker (CCB) compared to placebo for hypertension | ||||||
| Patient or population: adult patients with primary hypertension Setting: outpatient Intervention: First‐line CCB (mean duration 2.5 years) Comparison: placebo or untreated | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with CCB | |||||
| Total mortality | 60 per 1000 | 51 per 1000 (41 to 65) | RR 0.86 (0.68 to 1.09) | 4695 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | Not significant |
| Total stroke | 34 per 1000 | 19 per 1000 (14 to 28) | RR 0.58 (0.41 to 0.84) | 4695 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | ARR = 1.5% NNTB = 67 |
| Total coronary heart disease (CHD) | 31 per 1000 | 24 per 1000 (17 to 34) | RR 0.77 (0.55 to 1.09) | 4695 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | Not significant |
| Total cardiovascular events | 81 per 1000 | 57 per 1000 (46 to 70) | RR 0.71 (0.57 to 0.87) | 4695 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | ARR = 2.4% NNTB = 42 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomized controlled trial; ARR: Absolute risk reduction; ARI: Absolute risk increase; NNTB: Number needed to treat for an additional beneficial outcome. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded due to imprecision (wide confidence intervals).
2. Downgraded by 1 more level as there was only 1 trial
Refer to five Summary of findings tables: Table 1; Table 2; Table 3; Table 4; Table 5.
Primary outcomes by drug class
Thiazides
Because of the large number of trials, and the high heterogeneity in the effect between low dose and high dose thiazides on coronary heart disease events, we presented data for the two subgroups only, and not overall results for all thiazides.
Low‐dose thiazides
First‐line low‐dose thiazide significantly reduced all the primary outcomes: mortality (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.82 to 0.97; N = 19874; RCTs = 8; I² = 0%; Analysis 1.1), stroke (RR 0.68, 95% CI 0.60 to 0.77; N = 19874; RCTs = 8; I² = 0%; Analysis 1.2), coronary heart disease (RR 0.72, 95% CI 0.61 to 0.84; N = 19,022; RCTs = 7; I² = 0%; Analysis 1.3), and total cardiovascular events (RR 0.70, 95% CI 0.64 to 0.76; N = 19,022; RCTs = 7; I² = 0%; Analysis 1.4). The SHEP 1991 trial was the only one that reported total hospitalizations, an acceptable measure of total serious adverse events. It showed a numerical reduction with treatment (RR 0.95, 95% CI 0.89 to 1.01, N = 4736; RCT = 1; Analysis 1.5).
1.1. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 1 Total mortality.
1.2. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 2 Total stroke.
1.3. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 3 Total coronary heart disease.
1.4. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 4 Total cardiovascular events.
1.5. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 5 Total hospitalizations.
High‐dose thiazides
First‐line high‐dose thiazide, in contrast, significantly reduced stroke (RR 0.47, 95% CI 0.37 to 0.61; N = 19,839; RCTs = 11; I² = 46%; Analysis 1.2), and total cardiovascular events (RR 0.72, 95% CI 0.63 to 0.82; N = 19,839; RCTs = 11; I² = 35%; Analysis 1.4), but not mortality (RR 0.90, 95% CI 0.76 to 1.05; N = 19,839; RCTs = 11; I² = 21%; Analysis 1.1), or coronary heart disease (RR 1.01, 95% CI 0.85 to 1.20; N = 19,839; RCTs = 11; I² = 0%; Analysis 1.3).
Beta‐blockers
First‐line beta‐blockers reduced stroke (RR 0.83, 95% CI 0.72 to 0.97; N = 19313; RCTs = 5; I² = 7%; Analysis 2.2), and total cardiovascular events (RR 0.89, 95% CI 0.81 to 0.98; N = 19313; RCTs = 5; I² = 54%; Analysis 2.4), but not mortality (RR 0.96, 95% CI 0.86 to 1.07; N = 19313; RCTs = 5; I² = 25%; Analysis 2.1), or coronary heart disease (RR 0.90, 95% CI 0.78 to 1.03; N = 19313; RCTs = 5; I² = 4%; Analysis 2.3).
2.2. Analysis.
Comparison 2 First‐line beta‐blocker vs placebo, Outcome 2 Total stroke.
2.4. Analysis.
Comparison 2 First‐line beta‐blocker vs placebo, Outcome 4 Total cardiovascular events.
2.1. Analysis.
Comparison 2 First‐line beta‐blocker vs placebo, Outcome 1 Total mortality.
2.3. Analysis.
Comparison 2 First‐line beta‐blocker vs placebo, Outcome 3 Total coronary heart disease.
Angiotensin converting enzyme (ACE) inhibitors
First‐line ACE inhibitors reduced mortality (RR 0.83, 95% CI 0.72 to 0.95; N = 6002; RCTs = 3; I² = 0%; Analysis 3.1), stroke (RR 0.65, 95% CI 0.52 to 0.82; N = 6002; RCTs = 3; I² = 0%; Analysis 3.2), coronary heart disease (RR 0.81, 95% CI 0.70 to 0.94; N = 5145; RCTs = 2; I² = 0%; Analysis 3.3), and total cardiovascular events (RR 0.76, 95% CI 0.67 to 0.85; N = 5145; RCTs = 2; I² = 0%; Analysis 3.4).
3.1. Analysis.
Comparison 3 First‐line ACE inhibitor vs Placebo, Outcome 1 Total mortality.
3.2. Analysis.
Comparison 3 First‐line ACE inhibitor vs Placebo, Outcome 2 Total stroke.
3.3. Analysis.
Comparison 3 First‐line ACE inhibitor vs Placebo, Outcome 3 Total coronary heart disease.
3.4. Analysis.
Comparison 3 First‐line ACE inhibitor vs Placebo, Outcome 4 Total cardiovascular events.
Calcium channel blockers
First‐line calcium channel blockers reduced stroke (RR 0.58, 95% CI 0.41 to 0.84; N = 4695; RCT = 1; Analysis 4.2), and total cardiovascular events (RR 0.71, 95% CI 0.57 to 0.87; N = 4695; RCT = 1; Analysis 4.4), but not mortality (RR 0.86, 95% CI 0.68 to 1.09; N = 4695; RCT = 1; Analysis 4.1), or coronary heart disease (RR 0.77, 95% CI 0.55 to 1.09; N = 4695; RCT = 1; Analysis 4.3).
4.2. Analysis.
Comparison 4 First‐line calcium channel blocker vs Placebo, Outcome 2 Total stroke.
4.4. Analysis.
Comparison 4 First‐line calcium channel blocker vs Placebo, Outcome 4 Total cardiovascular event.
4.1. Analysis.
Comparison 4 First‐line calcium channel blocker vs Placebo, Outcome 1 Total mortality.
4.3. Analysis.
Comparison 4 First‐line calcium channel blocker vs Placebo, Outcome 3 Total coronary heart disease.
Secondary outcomes
Reduction in systolic and diastolic blood pressure
Antihypertensive drug therapy significantly lowered both systolic and diastolic blood pressure, compared to the control group. Please refer to Table 6 for details.
1. Blood pressure lowering efficacy with different drug classes.
| Fixed Effects | Random Effects | |||
| First‐line drug class |
SBP mmHg MD (99% CI) |
DBP mmHg MD (99% CI) |
SBP mmHg MD (99% CI) |
DBP mmHg MD (99% CI) |
| Low‐dose thiazide | ‐12.56 (‐13.22 to ‐11.91) | ‐4.73 (‐5.12 to ‐4.34) | ‐14.10 (‐19.57 to ‐8.63) | ‐5.59 (‐8.41 to ‐2.76) |
| High‐dose thiazide | ‐13.66 (‐14.40 to ‐12.91) | ‐6.82 (‐7.24 to ‐6.41) | ‐23.29 (‐32.62 to ‐13.97) | ‐12.78 (‐16.20 to ‐9.36) |
| Beta‐blocker | ‐9.51 (‐10.16 to ‐8.85) | ‐5.64 (‐6.06 to ‐5.22) | ‐8.20 (‐11.21 to ‐5.20) | ‐4.85 (‐6.52 to ‐3.19) |
| ACE inhibitor | ‐21.14 (‐23.13 to ‐19.15) | ‐9.64 (‐10.70 to ‐8.58) | ‐16.53 (‐35.72 to 2.66) | ‐7.59 (‐16.74 to 1.55) |
| Calcium channel blocker | ‐8.90 (‐10.14 to ‐7.66) | ‐4.50 (‐5.10 to ‐3.90) | ‐8.90 (‐10.14 to ‐7.66) | ‐4.50 (‐5.10 to ‐3.90) |
SBP: systolic blood pressure; DBP: diastolic blood pressure; MD: mean difference; CI: confidence interval
First‐line low‐dose thiazides, compared to placebo or no treatment, decreased systolic blood pressure (mean difference (MD) ‐12.56, 99% CI ‐13.22 to ‐11.91; N = 18,685; RCTs = 8; I² = 98%; Analysis 1.7), and diastolic blood pressure (MD ‐4.73, 99% CI ‐5.12 to ‐4.34; N = 18,685; RCTs = 8; I² = 98%; Analysis 1.8).
1.7. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 7 Systolic blood pressure.
1.8. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 8 Diastolic blood pressure.
First‐line high‐dose thiazides, compared to placebo or no treatment, decreased systolic blood pressure (MD ‐13.66, 99% CI ‐14.40 to ‐12.91; N = 14,906; RCTs = 6; I² = 98%; Analysis 1.7), and diastolic blood pressure (MD ‐6.82, 99% CI ‐7.24 to ‐6.41; N = 19,347; RCTs = 10; I² = 97%; Analysis 1.8).
First‐line beta‐blockers, compared to placebo or no treatment, reduced systolic blood pressure (MD ‐9.51, 99% CI ‐10.16 to ‐8.85; N = 18,833; RCTs = 5; I² = 92%; Analysis 2.6), and diastolic blood pressure (MD ‐5.64, 99% CI ‐6.06 to ‐5.22; N = 18,833; RCTs = 5; I² = 89%; Analysis 2.7).
2.6. Analysis.
Comparison 2 First‐line beta‐blocker vs placebo, Outcome 6 Systolic blood pressure.
2.7. Analysis.
Comparison 2 First‐line beta‐blocker vs placebo, Outcome 7 Diastolic blood pressure.
First‐line ACE Inhibitors, compared to placebo or no treatment, decreased systolic blood pressure (MD ‐21.14, 99% CI ‐23.13 to ‐19.15; N = 1071; RCTs = 2; I² = 98%; Analysis 3.5), and diastolic blood pressure (MD ‐9.64, 99% CI ‐10.70 to ‐8.58; N = 1071; RCTs = 2; I² = 98%; Analysis 3.6).
3.5. Analysis.
Comparison 3 First‐line ACE inhibitor vs Placebo, Outcome 5 Systolic blood pressure.
3.6. Analysis.
Comparison 3 First‐line ACE inhibitor vs Placebo, Outcome 6 Diastolic blood pressure.
First‐line calcium‐channel blockers, compared to placebo or no treatment, reduced systolic blood pressure (MD ‐8.90, 99% CI ‐10.14 to ‐7.66; N = 4695; RCT = 1; Analysis 4.6), and diastolic blood pressure (MD ‐4.50, 99% CI ‐5.10 to ‐3.90; N = 4695; RCT = 1; Analysis 4.7).
4.6. Analysis.
Comparison 4 First‐line calcium channel blocker vs Placebo, Outcome 6 Systolic blood pressure.
4.7. Analysis.
Comparison 4 First‐line calcium channel blocker vs Placebo, Outcome 7 Diastolic blood pressure.
For each class of drugs, the blood pressure data were heterogeneous, however the effects remained highly significant, using the random‐effects model. Because of the high heterogeneity, and the fact that in many trials, other drugs were allowed, we did not think this was an accurate reflection of the blood pressure lowering effect of the first‐line drug. For the same reason, we did not present these data in the 'Summary of findings' tables, and no attempt was made to indirectly compare the different drugs for blood pressure.
Withdrawal due to adverse effects
This outcome was not reported in most of the trials. Where it was reported, drug therapy increased withdrawals due to adverse effects, compared to placebo or no treatment: low‐dose thiazides (RR 2.38, 95% CI 2.06 to 2.75; N = 8870; RCTs = 3; I² = 96%; Analysis 1.6), high‐dose thiazides (RR 4.48, 95% CI 3.83 to 5.24; N = 15,170; RCTs = 7; I² = 31%; Analysis 1.6), and beta‐blockers (RR 4.59, 95% CI 4.11 to 5.13; N = 18,565; RCTs = 4; I² = 96%; Analysis 2.5).
1.6. Analysis.
Comparison 1 First‐line thiazide vs placebo, Outcome 6 Withdrawal due to adverse effects.
2.5. Analysis.
Comparison 2 First‐line beta‐blocker vs placebo, Outcome 5 Withdrawal due to adverse effects.
Because many of the trials did not report this outcome, and there was high heterogeneity between trials that did, we judged these data to have a high risk of bias. We could not calculate this information for the calcium channel blocker therapy, because withdrawals due to adverse drug effects were not reported in the SYST‐EUR 1997 trial, and authors declined to provide the information when requested. We could not use the data from the only ACE inhibitor trial that reported withdrawals due to adverse effects, as the untreated control group was not blinded (UKPDS 39 1998).
Discussion
Summary of main results
This review, with a large amount of thiazide trial data (19 randomized controlled trials (RCTs), 39,713 participants), demonstrates the benefits of starting with a low‐dose thiazide as first‐line therapy for elevated blood pressure. The pooled data showed a reduction in total mortality when using a thiazide as the first‐line choice, and suggested that as first‐line therapy, low‐dose thiazide, reduced coronary heart disease events, whereas high‐dose thiazide did not.
Five RCTs used a first‐line beta‐blocker (19,313 participants), and provided enough data to compare with first‐line thiazides. Analyses suggested that beta‐blockers reduced total stroke and total cardiovascular events less than all thiazides, and they reduced coronary heart disease less than a first‐line low‐dose thiazide. It is important to note that in four of the five beta‐blocker trials, atenolol was the beta‐blocker used. It is possible that the reduced effectiveness of first‐line beta blockers was limited to atenolol.
First‐line angiotensin converting enzyme (ACE) inhibitors, in three RCTs and a smaller population (6002 participants), were associated with similar benefits, but wider confidence intervals than first‐line low‐dose thiazides for all outcomes.
The amount of data for first‐line calcium channel blockers in one trial (4695 participants) was insufficient to make any meaningful comparisons; this can be appreciated by noting the wide confidence intervals associated with the treatment effects for this drug class (SYST‐EUR 1997).
Relative risk (risk ratio) is the best way to indirectly compare the effectiveness between different drug classes. When we compared risk ratios (RR), beta blockers (RR 0.89, 95% confidence interval (CI) 0.81 to 0.98) appeared to be less effective than low‐dose thiazides (RR 0.70, 95% CI 0.64 to 0.76) in reducing total cardiovascular events. First‐line ACE inhibitors (RR 0.76, 95% CI 0.67 to 0.85), and calcium channel blockers (RR 0.71, 95% CI 0.57 to 0.87), had less data, but could not be distinguished from low‐dose thiazides for the effect on total cardiovascular events, or other outcomes.
However, for the patient, it is more meaningful to have a measure of the absolute risk reduction (ARR) over a specified period of time. We calculated this summary measure for total cardiovascular events for the four drug classes in three clinical settings, where it was possible: secondary prevention, primary prevention (moderate to severe hypertension), and primary prevention (mild to moderate hypertension).
Secondary prevention: For the three secondary prevention trials using thiazides, and the one secondary trial using an ACE inhibitor, the average baseline blood pressure was approximately 155/94 mmHg. The average total cardiovascular event rate was 23.1% over five years in the control group, and the RR with treatment was 0.76. Therefore, the ARR over five years was 5.5% (23.1 X 0.24). For the two secondary prevention beta‐blocker trials, there was no clear reduction in total cardiovascular events (RR 1.01, 95% CI 0.84 to 1.21). There were no secondary prevention trials that used calcium channel blockers.
Primary prevention (moderate to severe hypertension): For the seven low‐dose thiazide trials in this category, the baseline systolic blood pressure was 175 mmHg, and the average total cardiovascular event rate over five years in the control groups was 16%. The RR with treatment was 0.68, for an ARR over five years of 5.1% (16 X 0.32). This is similar to the 5.5% calculated for secondary prevention. For the two beta‐blocker trials in this category, there was no significant reduction in total cardiovascular events (RR 0.88, 95% CI 0.71 to 1.02). In the one calcium channel blocker trial, the ARR over five years was 4.6% (16 X 0.29), and in the one ACE inhibitor trial, the estimated ARR over five years was 3.7% (16 X 0.23; UKPDS 39 1998).
Primary prevention (mild to moderate hypertension): There were five first‐line high‐dose thiazide trials in this category, with an average baseline systolic blood pressure of 160 mmHg. In these five trials, the average cardiovascular event rate in the control group over five years was 4.1%. The RR with first‐line high‐dose thiazide treatment was 0.80 (95% CI 0.69 to 0.94), and the ARR over five years was 0.82% (4.1 X 0.2). For the one beta‐blocker trial in this category, the RR with treatment was 0.82, and the ARR over five years was 0.74% (4.1 X 0.18).
This demonstrates that the absolute benefits are at least as good for first‐line low dose thiazides as the other classes of antihypertensives. The number needed to treat for an additional beneficial outcome (NNTB) for a low‐dose thiazide in moderate to severe hypertension (average systolic 175 mmHg) is about 20 over a five‐year duration. The NNTB was only a little lower in a secondary prevention setting, though the baseline blood pressures (average systolic 155 mmHg) were also lower.
Notably, the NNTB was much higher, about 120 over five years, for primary prevention patients with mild to moderate hypertension. The low absolute benefit in individuals with lower blood pressure at baseline reflected two differences: the lesser relative benefit of a RR of 0.8 versus a RR of 0.7, and the lower five‐year event rate in the control groups of 4%. One of the limitations of this analysis was that the first‐line drug used in this population was a high‐dose thiazide, which the evidence suggests is not as effective. It is possible that using a low‐dose thiazide in these trials would have improved the benefit to a RR of 0.7. However, even if that was true, the absolute benefit would still be small (ARR = 1.2%; NNTB 83 over five years). The low absolute benefit of antihypertensive therapy for mild to moderate elevations in blood pressure in primary prevention needs to be reflected by authors of hypertension guidelines.
Many of the patients in these trials also had co‐morbidities, such as diabetes mellitus. In this review, it was not possible to assess these patients separately, but SHEP 1991 analyzed their diabetes subgroup, and found the relative benefit was the same, and the absolute benefit was greater in diabetic patients than in non‐diabetic patients.
Overall completeness and applicability of evidence
Since 72.7% of participants in this review were primary prevention, the data are primarily relevant to a primary prevention population. Three of the included first‐line thiazide trials included participants with a prior stroke or transient ischemic attack (TIA; (Carter 1970; HSCSG 1974; PATS 1996)). When we deselected these trials from the thiazide analyses, the treatment effect estimates did not change. When we deselected all trials in which the baseline prevalence of myocardial infarction was either not reported (Barraclough 1973; Kuramoto 1981; MRC‐O 1992; VA‐II 1970), or was greater than 10% (Carter 1970; EWPHE 1985; HSCSG 1974; Wolff 1966), the treatment effect for total cardiovascular events was not different. For first‐line ACE inhibitors, HOPE HYP 2000 was predominantly secondary prevention, and the relative benefit with treatment was similar to the other two primary prevention trials (HYVET pilot 2003; UKPDS 39 1998).
For sensitivity analyses, we deselected trials that were not placebo controlled and blinded, trials that were restricted to patients with isolated systolic hypertension, small trials, and trials using supplemental drugs from other defined classes. In all of these instances, there was no clinically important change in the treatment effect estimate.
The data on withdrawals due to adverse effects were incomplete and heterogeneous. Therefore, it was difficult to compare the different drug classes for this outcome. However, there was nothing to suggest that other classes of drugs were better tolerated than first‐line low‐dose thiazides.
The blood pressure data in this systematic review were heterogeneous. This was because the number of drugs used in the trials differed, and only 16 of the 24 trials were double‐blinded. Blood pressure measurements are subject to bias if the observer and the patient know what is being administered. Because of these factors, it was not possible to use the blood pressure data to assess the blood pressure lowering efficacy of the different classes of drugs, or to compare low‐dose and high‐dose first‐line thiazides.
There is growing evidence that the surrogate marker ‐ the lowering of blood pressure ‐ is inadequate to predict health outcomes with antihypertensive therapy. Despite similar reductions in blood pressure, we found that first‐line low‐dose and high‐dose thiazide therapy had different impacts on the incidence of coronary heart disease.
In the 15 of the 24 trials where it could be assessed, despite dose titration and the addition of supplemental drugs, about 40% of the patients did not achieve the target blood pressure. Four trials reported that 34% of the patients did not achieve the target systolic pressure of less than 160 mm Hg (Kuramoto 1981; MRC‐O 1992; SHEP 1991; SHEP‐P 1989). In two trials, over 50% did not achieve the target systolic pressure of less than 150 mmHg (HYVET 2008; HYVET pilot 2003). The other nine trials reported that 40% of the patients did not achieve the target diastolic pressure of less than 90 mmHg (ATTMH 1980; EWPHE 1985; HSCSG 1974; MRC‐TMH 1985; OSLO 1986; USPHSHCSG 1977; VA‐I 1967; VA‐II 1970; Wolff 1966). This observation is extremely important clinically, as it means that physicians can only expect to achieve blood pressure targets in about 60% of the patients they treat. However, this does not mean that the patients who do not achieve the target will benefit less than those who do, as blood pressure reduction is only partly responsible for the risk reduction from antihypertensive treatment (Boissel 2005).
The evidence showed that starting with a low dose of thiazide, and only titrating up if necessary, significantly reduced the risk of coronary heart disease. In contrast, starting with a high dose (50 mg of hydrochlorothiazide or the equivalent) did not. Since the high‐dose therapy has no proven advantages, there is no justification for using high doses, or for comparing low‐dose and high‐dose thiazide therapies in a future trial. Therefore, the current recommendation for the use of thiazides in the management of hypertension is justifiable, based on the evidence presented here: start with a low dose (12.5 mg hydrochlorothiazide, or the equivalent), increase the dose if necessary, and do not exceed a dose of 50 mg of hydrochlorothiazide, or the equivalent.
The fact that thiazides are similarly, or more effective than other drug classes in reducing mortality and morbidity, and that the evidence is most robust for thiazides, is reason by itself to prescribe them first for most patients with hypertension. The value of this approach is further supported by the fact that of the drug classes studied, the thiazides are the least expensive drug class in most countries. If more than one thiazide or thiazide‐like drug is available, then the least expensive one is the most rational choice.
Additional information supporting the use of thiazides was recently published by Puttnam 2017. By using data from a national database, a post‐trial cohort surveillance study of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack trial (ALLHAT) examined whether the use of thiazide diuretics for the treatment of hypertension was associated with reduced risk of fracture, compared with non‐use. ALLHAT was a large randomized clinical trial that compared the effect of first‐step therapy with different classes of antihypertensive drug therapy (chlorthalidone, amlodipine, and lisinopril) in preventing fatal coronary heart disease (CHD), nonfatal myocardial infarction (primary outcome), or other cardiovascular disease (CVD) events. Hospitalized hip and pelvic fractures were chosen as endpoints, because they are almost always associated with hospitalization. A total of 22,180 participants, with a mean age of 70.4 years, were followed for up to eight years (mean 4.9 years) during masked therapy. Participants randomized to receive chlorthalidone versus amlodipine or lisinopril had a lower risk of fracture on adjusted analyses (hazards ratio (HR) 0.79, 95% CI 0.63 to 0.98; P = 0.04). The authors concluded that "findings from a large randomized clinical trial provide evidence of a beneficial effect of thiazide‐type diuretic therapy in reducing hip and pelvic fracture compared with treatment with other antihypertensive medications".
Quality of the evidence
We graded the overall quality of the evidence and developed 'Summary of findings' tables, using GRADEpro GDTsoftware (GRADEpro GDT).
We created five 'Summary of findings' tables to display the quality of evidence and summary of the effects on clinically important outcomes, for first‐line low‐dose thiazides (Table 1), high‐dose thiazides (Table 2), beta‐blockers (Table 3), ACE inhibitors (Table 4), and calcium channel blockers (Table 5).
We found high‐quality evidence that first‐line low‐dose thiazides reduced mortality, stroke, CHD, and total cardiovascular events more than placebo. We found moderate‐quality evidence that high‐dose thiazides reduced stroke, and CVS events more than placebo, and low‐quality evidence that first‐line high‐dose thiazides did not reduce CHD more than placebo.
We found low‐quality evidence that beta‐blockers reduced stroke, and total cardiovascular events more than placebo.
We found moderate‐quality evidence that ACE inhibitors reduced mortality and total CVS events, and low‐quality evidence that they reduced stroke and CHD more than placebo.
We found low‐quality evidence that calcium channel blockers reduced mortality, stroke, CHD, and CVS events more than placebo.
We found low‐quality evidence that thiazides and beta‐blockers caused more participants to withdraw from the trials due to adverse effects than those who took placebo.
We found low‐ to very low‐quality evidence that all four drug classes reduced systolic and diastolic blood pressure.
Potential biases in the review process
A potential limitation of attributing the benefit predominantly to the first‐line thiazide is the fact that in some of the thiazide trials, the first‐line therapy included the thiazide combined with another drug. In two trials, the first‐line therapy was a combination of a thiazide plus a potassium sparing diuretic (EWPHE 1985; MRC‐O 1992). Deselecting these two trials had no impact on the treatment effect. In five trials, a reserpine derivative was combined with the thiazide as first‐line therapy (HSCSG 1974; USPHSHCSG 1977; VA‐I 1967; VA‐II 1970; Wolff 1966. When these five trials were deselected, the treatment effect did not change either. Therefore, the data for first‐line thiazides appear robust, and the benefits achieved are most likely attributable to the first‐line thiazide therapy.
Agreements and disagreements with other studies or reviews
It is important in this type of analysis not to include trials in which there is significant contamination by drugs from other drug classes of interest. In our opinion, Psaty 1997 incorrectly included the trial by Coope 1986 and the STOP 1991 trial in their beta‐blocker group. In the trial by Coope 1986, 67% of patients in the active treatment group received bendrofluazide, and 70% received atenolol; in the STOP 1991 trial, more than 70% in the active treatment group received thiazides and more than 70% received beta‐blockers. Therefore, in those two trials, the treatment effect could not be attributed to first‐line thiazides or first‐line beta‐blockers. Other minor differences in the trials in this review and that by Psaty 1997 are as follows. We classified Kuramoto 1981 as a low‐dose trial, and they classified it as high‐dose trial. It fit into our low‐dose category, based on the starting dose of 1 mg of trichlormethiazide. Moving the trial from the low‐dose to the high‐dose group did not change our findings. Unlike Psaty 1997, we excluded the Hypertension Detection and Follow‐up Program trial because it did not have an untreated control group, and the intervention group was treated with life‐style therapies in addition to drug therapy (HDFP 1984). We included one small trial by Wolff 1966, not referenced by Psaty 1997. This trial was also included in the meta‐analysis in Collins 1990 and in Gueyffier 1996, The meta‐analysis by the blood pressure trialists group was incomplete and misleading because of their decision to exclude all trials prior to 1995 (BBLTTC 2005).
The network meta‐analysis included most of the trials comparing treatment versus placebo or no treatment that were included in this review (Psaty 2003). However, they again incorrectly included two trials in their beta‐blocker group (Coope 1986; STOP 1991), and in our opinion, inappropriately included PROGRESS 2001, IDM 2001, and Lewis 2001 as evidence for first‐line treatment in hypertension, when most patients did not have hypertension. Most importantly, this review includes four trials not included in the Psaty 2003 review (HOPE HYP 2000; HYVET pilot 2003; UKPDS 39 1998).
Our reasons for classifying UKPDS 39 1998 in this review deserve mention. First, in UKPDS 39 1998, the treatment target for the control group was over 200/105 mmHg for the first five years, after which it was lowered to over 180/105 mmHg. This was similar to the escape therapy in other trials that classified the control group as no treatment. For example, in the OSLO 1986 trial, drug treatment in the control group was started if blood pressure exceeded 179/109 mmHg and was sustained. Second, the UKPDS 39 1998 target in the intervention group (less than 150/85 mmHg) was very similar to the targets in the intervention groups of the included studies in this review (less than 140 to 160/less than 80 to 90 mmHg). Third, the difference in blood pressure achieved between the treatment and control group over the nine years of the trial (10 to 11/4 to 6 mmHg) is similar to the difference in BP for other included trials in this review (9 to 12/3 to 6 mmHg; (ATTMH 1980; Kuramoto 1981; MRC‐TMH 1985; SYST‐EUR 1997)).
Wiysonge 2017 also included UKPDS 39 1998 in their beta‐blocker review, published in 2007. In contrast to Wiysonge 2017, we did not include IPPPSH 1985 as representing first‐line beta blockers, as more than 65% of the patients also received a thiazide. We did include Dutch TIA 1993 and TEST 1995, which Wiysonge 2017 did not. Other reviews did not include HOPE HYP 2000, as the data separated for the hypertensive group had not been published. We are thankful to the authors of HOPE for providing these data. The HYVET pilot 2003 trial and the HYVET 2008 trial are more recent trials included in our review. Despite these differences, the conclusions and interpretation of the findings in this review are concordant with Psaty 2003, who concluded that thiazides were the first‐line drug of choice, and Wiysonge 2017, who concluded that beta‐blockers were not an appropriate first‐line drug choice. Refer to Table 7 for further details.
2. First‐line beta‐blocker compared to placebo. Comparison of this review with Wysonge 2017.
| Present review | Wiysonge 2017 | |
| No of included studies* (participants) | 5 (19,313) | 4 (23,613) |
|
Mortality RR (95% CI) Quality of evidence |
0.96 (0.86 to 1.07) Moderate |
0.99 (0.88 to 1.11) Moderate |
|
Total cardiovascular events RR (95% CI) Quality of evidence |
0.89 (0.81 to 0.98) Low |
0.88 (0.79 to 0.97) Low |
|
Total stroke RR (95% CI) Quality of evidence |
0.83 (0.72 to 0.97) Low |
0.80 (0.66 to 0.96) Low |
|
Total coronary heart disease (CHD) RR (95% CI) Quality of evidence |
0.90 (0.78 to 1.03) Low |
0.93 (0.81 to 1.07) Moderate |
|
Withdrawal due to adverse effects RR (95% CI) Quality of evidence |
4.59 (4.11 to 5.13) Low |
3.38 (0.82 to 13.95) Low |
The 2 systematic reviews differed in study inclusion criteria; total number of studies included and quality of evidence for CHD.
RR: relative risk; CI: confidence interval
The recent Taverny 2016 review assessed the effects of antihypertensive pharmacotherapy on three separate cardiac outcomes ‐ sudden cardiac death, fatal myocardial infarction, and nonfatal myocardial infarction in hypertensive individuals. In our review, these three outcomes were combined as total CHD events. In Taverny 2016, based on 15 randomized placebo‐controlled trials, in 39,908 patients, for a mean duration of 4.2 years, moderate‐quality evidence showed that antihypertensive therapy did not reduce sudden cardiac death (RR 0.96, 95% CI 0.81 to 1.15), but did reduce both nonfatal myocardial infarction (RR 0.85, 95% CI 0.74 to 0.98), and fatal myocardial infarction (RR 0.75, 95% CI 0.62 to 0.90). However, they did not report results separately for specific first‐line antihypertensive drug classes. Withdrawals due to adverse effects were increased in the drug treatment group to 12.8%, compared with 6.2% in the no treatment group. A Cochrane review, Pharmacotherapy for mild hypertension, has been published and is being updated (Diao 2012). A systematic review, Pharmacotherapy for hypertension in adults (18 to 59 years), has recently been published (Musini 2017). The Cochrane review, Pharmacotherapy for hypertension in the elderly (60 years or older), is being updated (Musini 2009).
Since more trials comparing drug therapy with a placebo or no treatment are unlikely to be forthcoming, future evidence of effectiveness of first‐line therapy will need to come from head‐to‐head comparisons. Two Cochrane reviews have compared first‐line calcium channel blockers (Chen 2010) and first‐line drugs inhibiting the renin angiotensin system (Xue 2015) with first‐line thiazides. These reviews confirm the likely morbidity benefits of first‐line thiazides. We are also currently working on a meta‐analysis of head‐to‐head trials in a separate systematic review. That review will focus on head‐to‐head trials comparing first‐line thiazides with other classes of drugs (Reinhart 2011).
Authors' conclusions
Implications for practice.
Choice of first‐line treatment versus placebo or no treatment: the evidence for morbidity and mortality.
Most of the available evidence justifying treatment of patients with elevated blood pressure used a thiazide as the first‐line drug.
First‐line low‐dose thiazides were more effective than first‐line high‐dose thiazides and first‐line beta‐blockers.
The treatment effect for first‐line ACE inhibitors was similar to low‐dose thiazides but less robust, and ACE inhibitors are more expensive than thiazides.
Evidence for effectiveness of first‐line calcium channel blockers was insufficient.
There were no RCTs comparing first‐line use of angiotensin receptor blockers or alpha blockers.
Morbidity and mortality benefit with antihypertensive treatment depended on the drug class received, not the blood pressure achieved.
Blood pressure measurement.
Blood pressure must be measured (average of multiple readings) using proper technique, with the patient non‐stimulated and resting for at least five minutes.
Population.
Secondary prevention, survivors of transient ischemic attacks, stroke, or myocardial infarction, aged 55 to 80 years, with an average blood pressure of 155/94 mmHg (five‐year ARR 5.5%) (Moderate quality evidence).
Primary prevention in adult patients with moderate to severe hypertension, with an average systolic blood pressure of 175 mmHg (five‐year ARR 5.1%) (High quality evidence).
Primary prevention in adult patients with mild to moderate hypertension, with an average systolic blood pressure of 160 mmHg (five‐year ARR 0.8% to 1.2%) (Low quality evidence).
Blood pressure targets are only achieved in about 60% of patients with mild, moderate, and severe hypertension treated with stepped‐care antihypertensive drugs.
Implications for research.
At the present time, RCT data for antihypertensives as first‐line treatment versus placebo or no treatment are lacking for all classes other than thiazides. Since we have clear evidence of effectiveness of first‐line low‐dose thiazides in primary prevention, patients with blood pressure of 160/100 mmHg or higher, and in secondary prevention, in patients with lower blood pressures, it would be unethical to do further trials for this population compared to a placebo or untreated control group. Future RCTs in these populations should be done with low‐dose first‐line thiazides as the comparison group. The benefits and harms of primary prevention treatment of patients with lower blood pressures remains uncertain at the present time. Large trials recruiting primary prevention patients in lower blood pressure categories and using first‐line low‐dose thiazides compared to placebo are needed.
Feedback
Was Kuramoto study randomized?, 17 April 2014
Summary
In the table of included studies, the Cochrane review authors state that the included study by Kuramoto, a Japanese study is a randomised, double‐blind placebo‐controlled trial conducted in Japan. Internal validity, risk of bias for allocation is low risk.
It appears to me that the Kuramoto study may not have been randomized, but is a matched case control study.
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Thank you for your comment. Kuramoto 1981 study is a placebo‐controlled single site study conducted in ambulatory patients in home for the aged in Tokyo, Japan. This study was included as a randomised study in the "Pharmacotherapy for hypertension in the elderly" original review by Mulrow 1998 based on unpublished information as per personal conversation with study author. Mulrow 1998 review stated that "Allocation of individuals within matched pairs to treatment and control groups was made by a blinded statistical coordinator, thought to be randomised but not entirely clear. Both patients and providers were blinded." Based on this information from Mulrow 1998 review we have decided to include this study and reassessed the risk of bias for the two domains ‐ allocation concealment and blinding of patient and physician as "Low risk" of bias.
Contributors
Kirsty Loudon, PhD Student
Affiliation: University of Dundee k.loudon@dundee.ac.uk
What's new
| Date | Event | Description |
|---|---|---|
| 9 February 2018 | New citation required but conclusions have not changed | Risk of bias of 24 included studies was assessed and included in this first update. Quality of evidence is graded overall and reported in five 'Summary of findings' tables. |
| 24 November 2017 | New search has been performed | Search findings were updated until 24 November 2017. The updated search failed to identify any new trials. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 19 January 2017 | Feedback has been incorporated | New feedback: Was Kuramoto study randomized? |
| 25 October 2008 | Amended | Converted to new review format. |
| 6 June 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Stephen Adams for finding references and Ciprian Jauca for assistance with formatting and copy‐editing.
Appendices
Appendix 1. Search strategies
Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web) Search Date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (loop OR ceiling) NEXT (diuretic OR diuretics) AND INSEGMENT #2 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide or thiazides) AND INSEGMENT #3 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide) AND INSEGMENT #4 #1 OR #2 OR #3 AND INSEGMENT #5 "angiotensin converting enzyme" NEXT inhibit* AND INSEGMENT #6 ace NEAR3 inhibit* AND INSEGMENT #7 acei AND INSEGMENT #8 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril or pivopril or quinapril or ramipril or ramiprilat or rentiapril or saralasin or s nitrosocaptopril or spirapril or temocapril or teprotide or trandolapril or utibapril or zabicipril or zofenopril) AND INSEGMENT #9 #5 OR #6 OR #7 OR #8 AND INSEGMENT #10 angiotensin NEAR3 (receptor antagonist* OR receptor block*) AND INSEGMENT #11 (arb OR arbs) AND INSEGMENT #12 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan) AND INSEGMENT #13 #10 OR #11 OR #12 AND INSEGMENT #14 (amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil) AND INSEGMENT #15 calcium NEAR2 (antagonist* OR block* OR inhibit*) AND INSEGMENT #16 #14 OR #15 AND CENTRAL:TARGET #17 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa):ti,ab,kw AND CENTRAL:TARGET #18 (reserpine or serpentina or rauwolfia or serpasil):ti,ab,kw AND CENTRAL:TARGET #19 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets) AND INSEGMENT #20 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat) AND INSEGMENT #21 #17 OR #18 OR #19 OR #20 AND INSEGMENT #22 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) AND INSEGMENT #23 beta NEAR2 (adrenergic or antagonist or antagonists or blocker or blockers or blocking or receptor or receptors) AND INSEGMENT #24 #22 OR #23 AND INSEGMENT #25 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin) AND INSEGMENT #26 adrenergic NEAR2 (alpha OR antagonist OR antagonists) AND INSEGMENT #27 (adrenergic or alpha or receptor or receptors) NEAR2 (blocker or blockers or blocking) AND INSEGMENT #28 #25 OR #26 OR #27 AND INSEGMENT #29 #4 OR #9 OR #13 OR #16 OR #21 OR #24 OR #28 AND INSEGMENT #30 hypertens* AND INSEGMENT #31 (elevate* OR high* OR rais*) NEAR2 blood pressure AND INSEGMENT #32 #30 OR #31 AND INSEGMENT #33 RCT:DE AND INSEGMENT #34 Review:MISC2 AND INSEGMENT #35 #33 OR #34 AND INSEGMENT #36 #29 AND #32 AND #35 AND INSEGMENT #37 #36 AND (23/01/2017_TO_24/11/2017:CRSCREATED) AND INSEGMENT *************************** Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web) Search Date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Thiazides EXPLODE ALL AND CENTRAL:TARGET #2 MeSH DESCRIPTOR Sodium Chloride Symporter Inhibitors EXPLODE ALL AND CENTRAL:TARGET #3 MeSH DESCRIPTOR Sodium Potassium Chloride Symporter Inhibitors EXPLODE ALL AND CENTRAL:TARGET #4 ((loop or ceiling) next (diuretic or diuretics)):ti,ab AND CENTRAL:TARGET #5 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide or thiazides):ti,ab AND CENTRAL:TARGET #6 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide):ti,ab AND CENTRAL:TARGET #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 AND CENTRAL:TARGET #8 MeSH DESCRIPTOR Angiotensin‐Converting Enzyme Inhibitors EXPLODE ALL AND CENTRAL:TARGET #9 "angiotensin converting enzyme" next inhibit*:ti,ab AND CENTRAL:TARGET #10 ace near3 inhibit*:ti,ab AND CENTRAL:TARGET #11 acei:ti,ab AND CENTRAL:TARGET #12 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril or pivopril or quinapril or ramipril or ramiprilat or rentiapril or saralasin or s nitrosocaptopril or spirapril or temocapril or teprotide or trandolapril or utibapril or zabicipril or zofenopril):ti,ab AND CENTRAL:TARGET #13 #8 OR #9 OR #10 OR #11 OR #12 AND CENTRAL:TARGET #14 MeSH DESCRIPTOR Angiotensin Receptor Antagonists EXPLODE ALL AND CENTRAL:TARGET #15 angiotensin near3 (receptor antagonist* or receptor block*):ti,ab AND CENTRAL:TARGET #16 (arb OR arbs):ti,ab AND CENTRAL:TARGET #17 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan):ti,ab AND CENTRAL:TARGET #18 #14 OR #15 OR #16 OR #17 AND CENTRAL:TARGET #19 MeSH DESCRIPTOR Calcium Channel Blockers EXPLODE ALL AND CENTRAL:TARGET #20 (amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil):ti,ab AND CENTRAL:TARGET #21 calcium near2 (antagonist* or block* or inhibit*):ti,ab AND CENTRAL:TARGET #22 #19 OR #20 OR #21 AND CENTRAL:TARGET #23 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa):ti,ab,kw AND CENTRAL:TARGET #24 (reserpine or serpentina or rauwolfia or serpasil):ti,ab,kw AND CENTRAL:TARGET #25 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets):ti,ab,kw AND CENTRAL:TARGET #26 MeSH DESCRIPTOR Hydralazine EXPLODE ALL AND CENTRAL:TARGET #27 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat):ti,ab,kw AND CENTRAL:TARGET #28 #23 OR #24 OR #25 OR #26 OR #27 AND CENTRAL:TARGET #29 MeSH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #30 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol):ti,ab AND CENTRAL:TARGET #31 beta near2 (adrenergic or antagonist or antagonists or blocker or blockers or blocking or receptor or receptors):ti,ab AND CENTRAL:TARGET #32 #29 OR #30 OR #31 AND CENTRAL:TARGET #33 MeSH DESCRIPTOR Adrenergic alpha‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #34 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin):ti,ab AND CENTRAL:TARGET #35 adrenergic near2 (alpha or antagonist or antagonists):ti,ab AND CENTRAL:TARGET #36 (adrenergic or alpha or receptor or receptors) near2 (blocker or blockers or blocking):ti,ab AND CENTRAL:TARGET #37 #33 OR #34 OR #35 OR #36 AND CENTRAL:TARGET #38 MeSH DESCRIPTOR Hypertension AND CENTRAL:TARGET #39 hypertens*:ti,ab AND CENTRAL:TARGET #40 (elevate* OR high* OR raise*) NEAR2 blood pressure:ti,ab AND CENTRAL:TARGET #41 #38 OR #39 OR #40 AND CENTRAL:TARGET #42 #7 OR #13 OR #18 OR #22 OR #28 OR #32 OR #37 AND CENTRAL:TARGET #43 #41 AND #42 AND CENTRAL:TARGET #44 #43 AND (23/01/2017_TO_24/11/2017:CRSCREATED) AND CENTRAL:TARGET
*****************************************************
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazides/ 2 exp sodium chloride symporter inhibitors/ 3 exp sodium potassium chloride symporter inhibitors/ 4 ((ceiling or loop) adj diuretic?).tw. 5 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. 6 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. 7 or/1‐6 8 exp angiotensin‐converting enzyme inhibitors/ 9 angiotensin converting enzyme inhibit$.tw. 10 (ace adj2 inhibit$).tw. 11 acei.tw. 12 (alacepril or altiopril or ancovenin or benazepril$ or captopril or ceranapril or ceronapril or cilazapril$ or deacetylalacepril or delapril or derapril or enalapril$ or epicaptopril or fasidotril$ or foroxymithine or fosinopril$ or gemopatrilat or idapril or imidapril$ or indolapril or libenzapril or lisinopril or moexipril$ or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. 13 or/8‐12 14 exp Angiotensin Receptor Antagonists/ 15 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. 16 arb?.tw. 17 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw. 18 or/14‐17 19 exp calcium channel blockers/ 20 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. 21 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. 22 or/19‐21 23 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 24 (reserpine or serpentina or rauwolfia or serpasil).mp. 25 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 26 exp hydralazine/ 27 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw. 28 or/23‐27 29 exp adrenergic beta‐antagonists/ 30 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. 31 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. 32 or/29‐31 33 exp adrenergic alpha antagonists/ 34 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. 35 (adrenergic adj2 (alpha or antagonist?)).tw. 36 ((adrenergic or alpha or receptor?) adj2 block$).tw. 37 or/33‐36 38 hypertension/ 39 hypertens$.tw. 40 ((high or elevat$ or rais$) adj2 blood pressure).tw. 41 or/38‐40 42 randomized controlled trial.pt. 43 controlled clinical trial.pt. 44 randomized.ab. 45 placebo.ab. 46 clinical trials as topic/ 47 randomly.ab. ) 48 trial.ti. 49 or/42‐48 50 animals/ not (humans/ and animals/) 51 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 52 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. 53 49 not (50 or 51 or 52) 54 (7 or 13 or 18 or 22 or 28 or 32 or 37) and 41 and 53 55 54 and (2016$ or 2017$).ed. 56 remove duplicates from 55 *************************** Database: Embase <1974 to 2017 November 22> Search Date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazide diuretic agent/ 2 exp loop diuretic agent/ ) 3 ((loop or ceiling) adj diuretic?).tw. 4 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. 5 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. 6 or/1‐5 7 exp dipeptidyl carboxypeptidase inhibitor/ 8 angiotensin converting enzyme inhibit$.tw. 9 (ace adj2 inhibit$).tw. 10 acei.tw. 11 (alacepril or altiopril or ancovenin or benazepril$ or captopril or ceranapril or ceronapril or cilazapril$ or deacetylalacepril or delapril or derapril or enalapril$ or epicaptopril or fasidotril$ or foroxymithine or fosinopril$ or gemopatrilat or idapril or imidapril$ or indolapril or libenzapril or lisinopril or moexipril$ or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. 12 or/7‐11 13 exp angiotensin receptor antagonist/ 14 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. 15 arb?.tw. 16 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw. 17 or/13‐16 18 calcium channel blocking agent/ 19 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. 20 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. 21 or/18‐20 22 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 23 (reserpine or serpentina or rauwolfia or serpasil).mp. 24 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 25 hydralazine/ 26 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw. 27 or/22‐26 28 exp beta adrenergic receptor blocking agent/ 29 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. 30 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. 31 or/28‐30 32 exp alpha adrenergic receptor blocking agent/ 33 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. 34 (adrenergic adj2 (alpha or antagonist?)).tw. 35 ((adrenergic or alpha or receptor?) adj2 block$).tw. 36 or/32‐35 37 exp hypertension/ 38 (hypertens$ or antihypertens$).tw. 39 ((high or elevat$ or rais$) adj2 blood pressure).tw. 40 or/37‐39 41 double blind$.mp. 42 placebo$.tw. 43 blind$.tw. 44 or/41‐43 45 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 46 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 47 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. 48 44 not (45 or 46 or 47) 49 (6 or 12 or 17 or 21 or 27 or 31 or 36) and 40 and 48 50 49 and (2016$ or 2017$).dc. 51 remove duplicates from 50 *************************** Database: ClinicalTrials.gov Search Date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Other Terms: randomized Study Type: Interventional Studies Condition / Disease: hypertension Intervention / Treatment: Antihypertensive Agents First Received: From 01/23/2017 to 11/24/2017 *************************** Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ antihypertens* AND hypertens* AND randomized antihypertens* AND high blood pressure AND randomized
Data and analyses
Comparison 1. First‐line thiazide vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low‐dose thiazide | 8 | 19874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.97] |
| 1.2 High‐dose thiazide | 11 | 19839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.05] |
| 2 Total stroke | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low‐dose thiazide | 8 | 19874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.60, 0.77] |
| 2.2 High‐dose thiazide | 11 | 19839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.37, 0.61] |
| 3 Total coronary heart disease | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Low‐dose thiazide | 7 | 19022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.84] |
| 3.2 High‐dose thiazide | 11 | 19839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| 4 Total cardiovascular events | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low‐dose thiazide | 7 | 19022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.64, 0.76] |
| 4.2 High‐dose thiazide | 11 | 19839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.63, 0.82] |
| 5 Total hospitalizations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Low‐dose thiazide | 1 | 4736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.89, 1.01] |
| 6 Withdrawal due to adverse effects | 10 | 24040 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [2.90, 3.57] |
| 6.1 Low‐dose thiazide | 3 | 8870 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [2.06, 2.75] |
| 6.2 High‐dose thiazide | 7 | 15170 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.48 [3.83, 5.24] |
| 7 Systolic blood pressure | 14 | 33591 | Mean Difference (IV, Fixed, 99% CI) | ‐13.04 [‐13.53, ‐12.55] |
| 7.1 Low‐dose thiazide | 8 | 18685 | Mean Difference (IV, Fixed, 99% CI) | ‐12.56 [‐13.22, ‐11.91] |
| 7.2 High‐dose thiazide | 6 | 14906 | Mean Difference (IV, Fixed, 99% CI) | ‐13.66 [‐14.40, ‐12.91] |
| 8 Diastolic blood pressure | 18 | 38032 | Mean Difference (IV, Fixed, 99% CI) | ‐5.71 [‐5.99, ‐5.42] |
| 8.1 Low‐dose thiazide | 8 | 18685 | Mean Difference (IV, Fixed, 99% CI) | ‐4.73 [‐5.12, ‐4.34] |
| 8.2 High‐dose thiazide | 10 | 19347 | Mean Difference (IV, Fixed, 99% CI) | ‐6.82 [‐7.24, ‐6.41] |
Comparison 2. First‐line beta‐blocker vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 5 | 19313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 2 Total stroke | 5 | 19313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.97] |
| 3 Total coronary heart disease | 5 | 19313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.03] |
| 4 Total cardiovascular events | 5 | 19313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.98] |
| 5 Withdrawal due to adverse effects | 4 | 18565 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.59 [4.11, 5.13] |
| 6 Systolic blood pressure | 5 | 18833 | Mean Difference (IV, Fixed, 99% CI) | ‐9.51 [‐10.16, ‐8.85] |
| 7 Diastolic blood pressure | 5 | 18833 | Mean Difference (IV, Fixed, 99% CI) | ‐5.64 [‐6.06, ‐5.22] |
Comparison 3. First‐line ACE inhibitor vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 3 | 6002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.95] |
| 2 Total stroke | 3 | 6002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.52, 0.82] |
| 3 Total coronary heart disease | 2 | 5145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.94] |
| 4 Total cardiovascular events | 2 | 5145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.85] |
| 5 Systolic blood pressure | 2 | 1071 | Mean Difference (IV, Fixed, 99% CI) | ‐21.14 [‐23.13, ‐19.15] |
| 6 Diastolic blood pressure | 2 | 1071 | Mean Difference (IV, Fixed, 99% CI) | ‐9.64 [‐10.70, ‐8.58] |
Comparison 4. First‐line calcium channel blocker vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 1 | 4695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
| 2 Total stroke | 1 | 4695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.41, 0.84] |
| 3 Total coronary heart disease | 1 | 4695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.55, 1.09] |
| 4 Total cardiovascular event | 1 | 4695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.57, 0.87] |
| 5 Heart Failure | 1 | 4695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.12] |
| 6 Systolic blood pressure | 1 | 4695 | Mean Difference (IV, Fixed, 99% CI) | ‐8.9 [‐10.14, ‐7.66] |
| 7 Diastolic blood pressure | 1 | 4695 | Mean Difference (IV, Fixed, 99% CI) | ‐4.5 [‐5.10, ‐3.90] |
4.5. Analysis.
Comparison 4 First‐line calcium channel blocker vs Placebo, Outcome 5 Heart Failure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ATTMH 1980.
| Methods | Randomized, placebo‐controlled, single‐blind trial conducted in 4 centres in Australia. | |
| Participants | Ambulatory Caucasian patients, mean age 50.5 years, range (30 to 69 years). Male (37%). Baseline SBP/DBP was 157/100.5 mmHg and pulse pressure was 57 mmHg. Inclusion criteria: SBP of < 200 mmHg and DBP 95‐110 mmHg Follow‐up: 4 years Target BP: less than 90 mmHg, which was lowered to < 80 mmHg after 2 years |
|
| Interventions | Treatment: First‐line ‐ chlorothiazide 500 mg Second‐line ‐ dose increased to 1000 mg, or addition of Methyldopa, propranolol, or pindolol Third‐line drugs added were hydralazine or clonidine Control: placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF (patients were censored after the first outcome so data are limited to first outcome in each category) | |
| Notes | "Of the 104,171 subjects screened, 3931 were randomised. Number eligible to start tablets previously defined as trial population was 3427 (3.3%) of originally screened population". "Thus, 504 subjects originally randomised, who at no time throughout the trial became eligible for tablets, were eliminated." "62 subjects in active group and 46 in the placebo group who by mistake, did not start tablets within 4 months of becoming eligible. As required by the study design they were included in the trial population but withdrawn from the regimen after the 4‐month period of grace". "About one third of the trial population prematurely stopped the regimen to which they had been randomised. Those who stopped had a higher proportion of smokers (29% vs 23%) and higher proportion of women (42% vs 34%)". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Eligible subjects who agreed to enter the study were randomly allocated, with stratification by age and sex, to one of the two trial regimens, to take either pharmacologically active tablets, the "active group", or placebo tablets, the "placebo group". Comment: method of randomization was not reported. Baseline characteristics were well matched at entry. |
| Allocation concealment (selection bias) | Low risk | Method of allocation was not reported, however baseline characteristics were well matched at entry. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study in which patients were not aware of which treatment they received. "Placebo tablets were identical in appearance to the active tablets." "The study centre staff knew the trial regimen of each subject, and this information was available, on request, to a subject’s local doctor. An ethics committee was kept aware of all aspects of the trial including the progressive distribution of trial endpoints between the groups." Comment: single‐blind study in which treating physicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "During the trial the members were not aware of the distribution of trial endpoints between active and placebo groups until the day the decision was taken to stop, except that one member was on the ethics committee and three members prepared the data on which the decision to stop was based. A trial endpoint committee, unaware of the subject’s treatment group and blood‐pressure, made the final decision on acceptance of a trial endpoint. An ECG committee, similarly "blind", reported on all electrocardiographic tracings." Comment: outcome assessors were blinded to treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The occurrence of any trial endpoint (table 11) terminated the subject’s participation in the study." "There were more withdrawals initiated by subjects’ doctors in the placebo than in the active group. Of the 88 subjects lost to follow‐up, 42 were in the active and 46 in the placebo group." Comment: outcome data after termination of subject's participation due to occurrence of trial endpoint were not reported. |
| Selective reporting (reporting bias) | High risk | All stated outcomes were reported. However, occurrence of any trial endpoint terminated patient's participation in the study, so follow‐up of these patients was not done, and outcome data were missing for the entire duration of the trial. |
| Other bias | Low risk | Study was initiated and administered by National Health Foundation of Australia. It was jointly sponsored by the National Health and Medical Research Council of Australia, the Life Insurance Medical research Fund of Australia and New Zealand, the Raine Medical Research Foundation of western Australia, the Ramaciotti Foundation and the Victorian government. |
Barraclough 1973.
| Methods | Single‐blind, placebo‐controlled trial conducted in Cardiff and London UK. | |
| Participants | 116 ambulatory patients, ethnicity was not reported, mean age 55 years, range (45 to 69 years). Male (50%). Baseline mean DBP was 110 mmHg; SBP and pulse pressure were not reported. Inclusion criteria: Men and women between 45 and 69 years with two casual, sitting diastolic blood pressures of between 100 and 120 mmHg on each of two occasions, separated by an interval of at least two weeks were included. Exclusion criteria: Patients were excluded if: (a) there was evidence of renal or cardiac failure or papilloedema; (b) there was a history of cerebrovascular accident or myocardial infarct within the preceding three months; (c) any serious or potentially fatal disease or disability was present that would prevent regular attendances or which contraindicated hypotensive therapy; (d) they were currently receiving antihypertensive therapy; or (e) there was evidence that hypertension was secondary to a surgically remediable condition. Follow‐up: 1.5 years |
|
| Interventions | Treatment: bendrofluazide (93%), methlydopa, and debrisoquine Control: placebo (received calcium lactate tablets) |
|
| Outcomes | Mortality, CHD, stroke, CHF, and diastolic BP | |
| Notes | Doses were not specified, assumed to be high dose bendrofluazide. K supplement was given automatically. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Random samples of the general population and hospital patients." "The patients in the control and treatment groups were compared for age, weight, the levels of blood glucose, and blood urea at the time of entry to the trial (table I)." Comment: simple random sampling was done. Two groups were comparable at the outset, but within 18 months this comparability had disappeared. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were allocated at random to either the control or treatment group. The series was balanced for age and sex after every 10 allocations." Comment: method of allocation concealment was not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Those in the treatment group were treated with any combination of bendrofluazide with potassium supplement, methyldopa, or debrisoquine, the choice of treatment being at the discretion of the physician. The physicians knew which treatment was given." "Progression from one regimen to the next depended on the blood pressure response and incidence of side effects. If the diastolic blood pressure rose to 130 mmHg or over in a patient in the control group the patient was immediately withdrawn from the trial and given hypotensive treatment." Comment: although stated as single‐blind, neither the physicians nor the participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Information on the outcome assessors was not reported in the study. Probably, blinding was not carried out. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Total of 42 of the 58 (72.4%) randomised patients left the trial. Six patients in control group were withdrawn from the trial as DBP > 130 mmHg. Seventeen patients left for medical reasons 14 in control group and 3 in treatment group. Nineteen left for non‐medical reasons." "Medical indications in control group for leaving trial were diastolic pressure greater than 130 mmHg, myocardial infarction, pulmonary embolus, cardiac septum infarct, and cardiac failure; while indications for treatment group were senility, fractured skull, and myocardial infarction." Comment: attrition rate was very high and unequal across groups. How data were collected or analyzed in patients who withdrew is not explained or reported. By the end of one year, the two groups lost comparability. Statistical tests used for analysis were not mentioned. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and morbidity data was not clearly stated and list of side effects were not reported. It is not clear whether data from patients who withdrew were collected or reported until end of study. |
| Other bias | Unclear risk | Conflict of interest of authors was not reported. |
Carter 1970.
| Methods | Randomized single‐site study conducted in UK. Patients were stroke survivors admitted to the hospital and followed in clinics. | |
| Participants | 99 participants,71 of whom were aged 18 to 59 years; 54% men; age range: 40 to 79; mean: 69 years; race/ethnicity: not reported
Mean BP at entry: not reported Pre‐existing factors: stroke: 100%; BP entry criteria: SBP > 160 mmHg and DBP < 110 mmHg, or DBP ≥ 110 mmHg irrespective of SBP Exclusion criteria: cerebral haemorrhage; embolism; tumour; accelerated hypertension; "those with an obvious need for hypotensive therapy;" left ventricular failure; congestive cardiac failure; gross radiological cardiac enlargement; various cardiac arrhythmias, or evidence of renal failure." Mean follow‐up: 4.0 years |
|
| Interventions | Treatment: first choice: thiazide diuretic (dose or type of thiazide not specified; assumed to be high‐dose thiazide); second choice: methyldopa; third choice: bethanidine, debrisoquine or guanethidine Control: observation without placebo |
|
| Outcomes | Stroke, mortality, CHD, CHF Dropouts due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | Percentage not on assigned therapy at study end: not reported. Difference in blood pressure at study end: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Placed at random into treated (50) or control (49) groups. The two groups matched reasonably closely with regard to numbers, age, sex, and severity of hypertension." Comment: Method of randomization was not described. Probably randomization achieved as groups matched at baseline. |
| Allocation concealment (selection bias) | Low risk | Method for allocation concealment was not mentioned. Probably OK as groups were matched well at baseline. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study does not state blinding of participants or personnel. The treating physicians were aware of the treatment being prescribed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study does not mention blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 out of 99 patients (0.02%) have been lost to follow‐up, a treated man aged 65 and untreated women of 70 ‐ so results are available for 49 treated and 48 untreated patients." Comment: The attrition rate was extremely low and although reason for loss to follow‐up was not mentioned, it could not have affected the outcome analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available to confirm reporting bias. Mortality rate and recurrence rate of strokes mentioned as study objectives were reported in the results section. "Figures for minor strokes or transient cerebral ischaemic attacks are not available". |
| Other bias | Low risk | "Part of the expenses of this research project was covered by a grant from the clinical research subcommittee of the North West Metropolitan Regional Hospital Board." |
Dutch TIA 1993.
| Methods | Randomized double‐blind, placebo‐controlled trial conducted in hospital in Netherland. No run‐in period in the study design | |
| Participants | 1473 patients age > 65 years (range not reported); 64% male with TIA or nondisabling stroke TIAs should not include loss of consciousness, convulsions, incontinence, or prominent headache. Time course: the symptoms should develop within a few seconds, should not progress from one part of the body to another in an orderly march, and should last between 1 minute and 24 hours. Mean baseline BP 158/91 mmHg. Race not stated Exclusions: cerebral ischemia from identifiable causes other than arterial thrombosis or arterial embolism, patients with a contraindication against or a strict indication for a beta‐blocker, last TIA > 3 months before, disorders that may mimic cerebral ischemia, factors likely to confound interpretation of the results. Follow‐up: 2.6 years |
|
| Interventions | Treatment: atenolol 50 mg daily Control: Identical placebo tablet | |
| Outcomes | Mortality, CHD, stroke, total CV events | |
| Notes | Percentage on assigned treatment at end of study: Beta‐blocker arm: 71% at 2 years (64% at 3 years); Placebo: 75% at 2 years (68% at 3 years) Inappropriate sample size calculation ‐ "...as the size of the Dutch TIA trial was determined primarily by the number of patients required for the aspirin study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Double‐blind and placebo‐controlled randomized clinical trial." "Balance between treatment allocations within hospitals was achieved by the use of random permuted blocks; blinded randomization codes were distributed by telephone." Comment: baseline characteristics were well matched. |
| Allocation concealment (selection bias) | Low risk | Quote: "Balance between treatment allocations within hospitals was achieved by the use of random permuted blocks; blinded randomization codes were distributed by telephone." Comment: allocation concealment was adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind and placebo‐controlled randomized clinical trial. Atenolol was supplied as 50 mg tablets to be taken once a day; placebo tablets had identical appearance and taste." Comment: participants and the treating physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All outcome events were independently classified by at least three members of the Auditing Committee for Outcome Events, without knowledge of treatment allocation. All possible adverse effects as reported by the patients were recorded; the physicians inquired about such effects in a general fashion." Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients had their last follow‐up visit between March 1, 1990, and June 30, 1990; the mean duration of follow‐up was 32 months, with a minimum of 12 and a maximum of 52 months. No patient was lost to follow‐up." "The primary data analysis was based on the intention‐to‐treat principle; whether or not medication was taken, patients were analyzed in their originally allocated treatment group until the last follow‐up visit." Comment: No attrition bias as all patients were included in an ITT analysis. |
| Selective reporting (reporting bias) | Low risk | All the primary outcomes (occurrence of death from the vascular causes, nonfatal stroke, or nonfatal myocardial infarction, whichever occurred first), secondary outcomes (death from all causes, death from vascular causes, plus nonfatal stroke), tertiary outcomes (fatal stroke, the combination of fatal and nonfatal stroke, cardiac death, and the combination of cardiac death and nonfatal myocardial infarction) analyses, along with the adverse effects were reported. Comment: Although protocol was not available, probably all outcomes were reported. |
| Other bias | Unclear risk | Conflict of interest was not reported. |
EWPHE 1985.
| Methods | Multisite randomized, placebo‐controlled, double‐blind trial conducted in Europe, stratified by age, sex, presence or absence of cardiovascular complications, and site. | |
| Participants | 840 ambulatory elderly patients; 69.8% female; age range: 60 to 97; mean: 72.0 years; Ethnicity not reported. Baseline SBP/DBP was 183/101 mmHg and pulse pressure was 82 mmHg. Geographic region: Europe (Belgium (25%), United Kingdom (19%), Finland (17%), France (14%), Italy (7%), The Netherlands (7%), Ireland (6%), Portugal (3%), Norway (2%), West‐Germany (1%). Study setting: hospitals (geriatric); physician offices; nursing home. Inclusion criteria: SBP 160 to 239 mmHg and DBP 90 to 119 mmHg Exclusion criteria: curable causes of high blood pressure; certain complications of hypertension (i.e. retinopathy grade III or IV, congestive heart failure, history of cerebral or subarachnoid haemorrhage); concurrent diseases, such as hepatitis or cirrhosis, gout, malignancy, and diabetes mellitus requiring insulin treatment Follow‐up: 7 years. Average follow‐up: placebo 4.63 years; treatment 4.69 years |
|
| Interventions | Treatment: Step 1 ‐ hydrochlorothiazide 25 mg to 50 mg + triamterene 50 mg to 100 mg daily; Step 2 ‐ methyldopa 250 mg to 2000 mg daily Control: matching placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF, systolic BP and diastolic BP Dropouts due to side effects: not stated Quality of life or functional outcomes: not stated |
|
| Notes | Only ITT data included. Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐22/‐10 mmHg Percentage of participants not on assigned therapy at study end: placebo group: > 35%; treatment group: > 35% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 840 patients were randomised to placebo (N = 424) or active treatment (N = 416). The placebo and active treatment groups were similar in sex ratio, age, sitting blood pressure at randomisation, weight, and percentage with cardiovascular complications on admission to the trial." Comment: stratified randomization was utilized but method of random allocation was not stated. Baseline characteristics were matched. |
| Allocation concealment (selection bias) | Low risk | Stratified randomization was utilized but method of random allocation was not stated. Baseline characteristics were matched. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A double‐blind randomised placebo‐controlled trial of antihypertensive treatment was conducted in patients over the age of 60." "Tablets and matching placebos are identical in shape, taste and colour." Comment: both patients and physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data were sent to the co‐ordinating office every three months on specially designed forms, and deaths and other terminating events were classified independently by two investigators into previously agreed categories. These investigators were not aware of the treatment group to which the patients had been assigned. After leaving the double‐blind part of the trial, the surviving patients were followed up until July 1984, but only date and cause of death were recorded." Comments: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The intention‐to‐treat analysis was restricted to the cause and date of death because data on non‐fatal events in patients who dropped out from randomised treatment were not available." "During randomised treatment 128 patients defaulted from follow‐up and 52 refused to continue their randomised treatment for various reasons, but continued to attend. 38 patients were withdrawn from randomised treatment because of serious inter current illnesses (mainly neoplasms). Withdrawal was less frequent in the actively treated group (P = 0.022)." "One centre with 21 patients withdrew from the trial before its end. In another centre, the double‐blind phase was terminated in 29 patients, each followed for 5 years, because this was the duration to which the patients had agreed. 11 patients were withdrawn from randomised treatment by the local investigators owing to a moderate increase in blood pressure that did not, however, reach the previously established study‐terminating criteria. Similarly, 17 patients were withdrawn by the local investigators on discovery that the patients were no longer hypertensive during a brief period without treatment. In 6 patients, the treatment code was broken, e.g. at the request of an anaesthetist. 2 patients had treatment stopped in error, and 2 others were withdrawn because the double‐blind drug supply was not available. There were 291 patients still in the double‐blind part of the trial when it was stopped in the summer of 1984." "Both analyses on randomised treatment in the double‐blind part of the trial (on‐randomised treatment or per‐protocol analysis) and an overall intention‐to‐treat analysis was performed. The latter was confined to mortality owing to the difficulty in determining morbidity outside the period of double‐blind follow‐up." Comment: 16.3% patients in the placebo group and 14.2% in treatment group were lost to follow‐up and data on nonfatal events in patients who dropped out of the trial were not available. |
| Selective reporting (reporting bias) | High risk | Patients were censored if they had "one of the specific study terminating events, including death, nonfatal cerebral or subarachnoid haemorrhage, development of hypertensive retinopathy grade III or IV, dissecting aneurysm, congestive heart failure not controllable without diuretics or antihypertensive drugs, hypertensive encephalopathy, severe increase in left ventricular hypertrophy, and a rise in blood pressure exceeding the defined limits." Comment: although all the terminating fatal events (cardiovascular, non‐cardiovascular non‐renal, renal, and other causes), as well as nonfatal, morbid cardiovascular terminating events and nonfatal, non‐morbid cardiovascular terminating events were reported in the results section, censoring of patients lead to high risk of bias. |
| Other bias | Low risk | Quote: "This study is supported by the Belgian Hypertension Committee and the World Health Organization. Tablets of alpha methyldopa and placebo were supplied by Merck, Sharp and Dohme; capsules of hydrochlorothiazide and triamterene by Smith, Kline and French." Comment: Conflict of interest was not reported. However, it was not funded by the manufacturer. |
HOPE HYP 2000.
| Methods | Double‐blind randomized trial with a two by two factorial design. | |
| Participants | Patients 55 years or older with previous coronary artery disease, cerebrovascular disease, or peripheral vascular disease, or diabetes plus one additional risk factor (high blood pressure > 160 or > 90 mmHg, total cholesterol > 5.2 mmol/L, HDL cholesterol < 0.9 mmol/L, current cigarette smoking, or known microalbuminuria). This analysis was limited to patients with blood pressure of 140/ 90 mmHg or higher at baseline. Exclusion criteria: Patients were excluded if they had heart failure, were known to have a low ejection fraction (< 0.40), were taking an angiotensin‐converting enzyme inhibitor or vitamin E, had uncontrolled hypertension or overt nephropathy, or had had a myocardial infarction or stroke within four weeks before the study began. Follow‐up: 4.5 years |
|
| Interventions | Treatment: ramipril 2.5 mg, titrating up to 10 mg Control: placebo Vitamin E 400 IU/day in both groups |
|
| Outcomes | Primary: composite of myocardial infarction, stroke, or cardiovascular death (total CV events),
total mortality, total stroke, total CHD Secondary outcomes included the need for revascularization, hospitalization for unstable angina or heart failure, and complications related to diabetes (whether or not hospitalization was required). Other outcomes were worsening angina, cardiac arrest, heart failure (whether or not hospitalization was required), unstable angina with electrocardiographic changes, and the development of diabetes. |
|
| Notes | All patients entered a run‐in phase in which they received 2.5 mg of ramipril daily for 7 to 10 days with measurement of creatinine and potassium. 1035 were not randomized after this run‐in period. "All 10,576 eligible patients participated in a run‐in phase in which they received 2.5 mg of ramipril orally once daily for 7 to 10 days followed by matching placebo for 10 to 14 days. A total of 1035 patients were subsequently excluded from randomization because of noncompliance (< 80% of pills taken), side effects, abnormal serum creatinine or potassium levels, or withdrawal of consent." "Four formal interim analyses were planned." “Co‐administration of vitamin E and interim analysis in the favour of the ramipril group could have affected the outcome measures." "Among the patients who were randomly assigned to receive placebo, 3.4% were receiving an ACE inhibitor at one year, 6.0% were doing so at two years, 8.1% were doing so at three years, 10.8% were doing so at four years, and 12.3% were doing so at five years. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central telephone randomization: randomization is done internationally by a telephone call to a central office. After receipt of appropriate baseline data over the telephone, the patient is randomized to ramipril (2.5 mg for one week, then 5 mg every day for three weeks) or matching placebo and vitamin E (400 IU) or matching placebo by a 2 x 2 factorial design (Table 3)." Comment: selection of participants done in a random fashion. |
| Allocation concealment (selection bias) | Low risk | Quote: "At randomization, patients were assigned to receive ramipril (or matching placebo) at a dose of 2.5 mg once a day for one week, 5 mg for the next three weeks, and then 10 mg. In addition, all patients were randomly assigned to receive 400 IU of vitamin E per day or matching placebo. Comment: method employed for the random allocation was clearly stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The double‐blind, two‐by‐two factorial, randomized Heart Outcomes Prevention Evaluation study." (Page 146) "Emergency unblinding is available centrally and locally but will only be done when absolutely necessary and after a check‐list is completed by telephone call to the project office.” Comment: patients and the treating physicians were both blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Central adjudication of all events: source documentation for every event is sent to the Canadian project office. A member of the Event Adjudication Sub‐committee then reviews the event based on study definitions of primary and secondary endpoints. All suspected cardiovascular deaths, MI, stroke or secondary endpoints for which there is any discrepancy between the event forms and the supporting documentation will then be reviewed by another member of the Events Adjudication Committee. Events are reviewed on a quarterly basis." (Page 133, HOPE study investigators 1996). Comment: Independent outcome assessment was done properly. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: Attrition rate ‐ permanent discontinuation of study medication was high (28.9% in ramipril group and 27.3% in the placebo group). Reasons for discontinuation due to cough was higher in ramipril group (7.3%) vs placebo group (1.8%). Higher percentage of patients discontinued from placebo group compared to ramipril group due to clinical events 9% vs 6.7% and uncontrolled hypertension 3.9% vs 2.3%, respectively. Comment: attrition rates high and how data were collected and analyzed in these patients was not reported. |
| Selective reporting (reporting bias) | Low risk | All analyses for the primary, secondary and other outcomes mentioned in the method section were reported in the results. |
| Other bias | High risk | Funded by the Medical Research Council of Canada, Hoechst–Marion Roussel, AstraZeneca, King Pharmaceuticals, Natural Source Vitamin E Association and Negma, and the Heart and Stroke Foundation of Ontario. Dr. Yusuf was supported by a Senior Scientist Award of the Medical Research Council of Canada and is the Heart and Stroke Foundation of Ontario Research Chair. Comment: The prior administration of ramipril in all the patients without wash‐out period before randomization; excluding patients who were non‐compliant and those with adverse effects led to highly selective patient population being included in the study. Partially funded by the manufacturer |
HSCSG 1974.
| Methods | Randomized, double‐blind, placebo‐controlled trial conducted in USA with a six week drug run‐in phase. | |
| Participants | 452 ambulatory stroke survivors with mild to moderate hypertension, 80% African‐Americans, mean age 59 years, range < 75 years, 60% men. Baseline SBP/DBP 167/100 mmHg, pulse pressure 67 mmHg. 80% of participants had completed stroke in year before randomization. 16% had mixtures of completed stroke and TIA, and 4% had only TIAs. Inclusion criteria: SBP ≥ 140 to 220 mmHg and DBP 90 to 115 mmHg and stroke or TIA, or both, in previous year. Ambulatory, capable of long‐term attendance at treatment clinic, < 75 years of age and no concomitant disease that might be influenced adversely by prolonged treatment with drug or placebo. Follow‐up: 3 years |
|
| Interventions | Treatment: deserpidine 1 mg + methyclothiazide 10 mg Control: no treatment |
|
| Outcomes | Mortality, stroke, CHD, CHF, systolic BP, and diastolic BP | |
| Notes | Definition of stroke used in the trial – “A marked increase in frequency of TIAs (twice the weekly pre‐randomization level of occurrence, and more than four per week), or a deterioration of more than eight points in the neurological score, also qualified as a stroke endpoint.” A stroke endpoint was defined by the same criteria used for entry into the study. It also was confirmed by a majority of a committee consisting of two members outside of the study and the Central Registry neurologist. The scoring system of residual deficits by the neurologist was based on a total of 100 points, allowed a maximum of 35 points for level of consciousness and mentation, 9 points for cranial nerve function, 30 points for motor system, 3 points for reflexes, 3 points for sensory function, and 20 points for 'health and performance' function. "The study was terminated earlier than planned when it became evident that further follow‐up would not significantly affect the results. All patients without endpoints were under observation for at least one year; the mean follow‐up period for all individuals including those with endpoints was 27.4 months, and for those not having endpoints, it was 38.6 months". " Forty‐nine who entered the drug trial were not subsequently randomized." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A prospective double‐blind co‐operative study was undertaken to determine the influence of treatment on the prognosis in stroke survivors with mild to moderate hypertension." If no intolerable side effects occurred, the patient was placed on a regimen of two tablets daily of randomized medication.” "To ensure that drug and placebo were balanced among groups with characteristics of possible prognostic importance, patients were divided into cells based on these characteristics, and drug or placebo was prescribed to maintain a balance within these cells. The characteristics for which this randomization was conducted were sex, race, diastolic blood pressure above or below 100 mm Hg, and the four stroke categories." "Although no effort was made to assure an equal distribution of drug‐treated and placebo‐treated patients within each clinic, the drug‐placebo ratio differed appreciably in only two of the ten clinics." "No statistically significant differences were noted in the frequency of abnormalities in the laboratory findings, ECGs, and chest X ray films between the drug and placebo groups." Comment: randomization was probably done. However, the method for random sequence generation was not mentioned. Baseline characteristics were well matched. |
| Allocation concealment (selection bias) | Low risk | Quote: "The biostatistical section was responsible for assignment of patients to drug or placebo regimens, distribution of medication by mail to the individual clinics, data preparation, coding, and analysis." “For use in an emergency, a sealed envelope held by a disinterested person at the local clinic identified the type of medication the patient was receiving." Comment: method of allocation concealment was not reported. Baseline characteristics were well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A prospective double‐blind cooperative study." "Neither the doctor nor the patient was aware of whether placebo or drug had been supplied. For use in an emergency, a sealed envelope held by a disinterested person at the local clinic identified the type of medication the patient was receiving." Comment: participants and treating physicians were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the report of the stroke event and the neurological findings were submitted to Central Registry for confirmation. A stroke endpoint was defined by the same criteria for entry into the study. It also was confirmed by a majority of a committee consisting of two members outside of the study and the Central Registry neurologist." "Similarly, any medical event justifying removal of the patient from the study was carefully reviewed and classified into cardiovascular and non‐cardiovascular categories. The events of a cardiovascular nature were confirmed by an outside cardiologist." Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Five‐hundred and one patients were exposed to a pre‐randomization drug trial. Forty‐nine who entered the drug trial were not subsequently randomized." Of the 452 patients randomized, total withdrawals are not reported. "The study was terminated earlier than planned (3 years follow‐up) when it became evident that further follow‐up would not significantly affect the results. All patients without endpoints were under observation for at least one year; the mean follow‐up period for all individuals including those with endpoints was 27.4 months, and for those not having endpoints, it was 38.6 months." Comment: attrition rate was not mentioned and how data were analyzed was not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol was not available. Cerebrovascular and cardiovascular outcomes, blood pressure measurements, drug intolerance, laboratory measurements were reported. |
| Other bias | Low risk | This investigation was supported by grants from the National Institute of Neurological Diseases and Stroke. |
HYVET 2008.
| Methods | Randomized double‐blind placebo‐controlled multisite outpatient study conducted in Western Europe (86 patients), Eastern Europe (2144), China (1526), Australasia (19), and Tunisia (70). | |
| Participants | 3845 participants (61% women); age range: 80 to 105, mean age = 84 years Pre‐existing factors: cardiovascular disease = 12.0%; hypertension = 89.9%; antihypertensive treatment = 64%; stroke = 6.8%; myocardial infarction = 3.1%; diabetes = 6.8%; heart failure = 2.9%; smoking = 6.5% Blood pressure (BP) entry criteria: mean of the four systolic blood pressure measurements taken at the second and third visits (two at each visit) was between 160 and 199 mmHg. Baseline BP 173.0/90.8 mmHg. Pulse pressure 82.2 mmHg. Target BP was < 150/80 mmHg. Exclusion criteria: accelerated hypertension (retinal haemorrhage, exudates, or papilledema), overt clinical congestive heart failure requiring treatment with diuretic, vasodilator or ACE inhibitor, renal failure, documented cerebral or subarachnoid haemorrhage, condition expected to severely limit survival, e.g. terminal illness, unable to stand up, require BP lowering treatment for reasons other than hypertension e.g. angina, peripheral ischemia, gout, renal artery stenosis, those with dementia (Mental Test score < 7/10) Follow‐up: 2.1 years (median 1.8 years) |
|
| Interventions | Treatment: Step 1 ‐ indapamide 1.5 mg daily. Step 2 ‐ perindopril 2 mg daily. Step 3 ‐ perindopril 4 mg daily Control: placebos for each step | |
| Outcomes | Total stroke, total coronary artery disease, total mortality, total cardiovascular events (including CHF) Dropouts due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: sitting ‐15.0/‐6.1 mmHg, standing ‐14.7/‐5.4 mmHg. Percentage of patients not on assigned therapy at study end: active treatment 0.8%, placebo 0.6%. Corresponded with the author for missing information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization: Sequence generation was not reported. Randomization was stratified according to age (80 to 89 years and 90 years or older) and sex; permuted blocks of 4 and 6 of any 10 patients were used to ensure roughly equal assignment to each of the two groups within large centres. Comment: method used for randomization was not mentioned. Baseline characteristics were similar in the two groups |
| Allocation concealment (selection bias) | Low risk | An interactive voice response system (IVRS) was employed to tell the investigator which 6‐month drug pack to prescribe. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The main trial was a randomized, double‐blind, placebo‐controlled trial. Patients and providers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The Endpoint Committee will provide an objective blinded evaluation of previously defined endpoints." "All events that were possible endpoints were reviewed by an independent committee, unaware of the group assignment, using predefined definitions from the protocol." Comment: outcome assessment done in an independent manner and outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage lost to follow‐up: active treatment 0.3%, placebo 0.6%. Reported on the number of patients lost to follow‐up (16 patients) "...vital status was unknown in 17 patients..." "The primary analysis was performed according to the intention‐to‐treat principle." Comment: small loss to follow‐up and ITT analysis used |
| Selective reporting (reporting bias) | High risk | All the primary and secondary outcomes mentioned in the objectives were reported in the results. Could not extract the number of patients in each group that had nonfatal myocardial infarctions. Correspondence with the author Question: "The serious adverse events noted in the publication...are the numbers the total serious adverse events OR was the first event counted and analyzed? Answer: It is the total number of SAEs. Patients could contribute more than one SAE." Question: "If a patient had an event after being censored, were those events counted? If not, is it possible to see those data? Answer: It would depend on the event. If it was a recurrent endpoint then it was not counted (e.g. a further non‐fatal stoke). If the event was a new endpoint (e.g. a fatal MI in someone who had previously had a nonfatal stroke), then it was. |
| Other bias | Low risk | Quote: "Supported by grants from the British Heart Foundation and the Institut de Recherches Internationales Servier. Drs. Beckett and Peters and Mr. Banya report receiving grant support from the Institut de Recherches Internationales Servier; Dr. Staessen, consulting fees from Pfizer, Tanabe, Daiichi‐Sankyo, and Sigma‐Tau and speakers’ fees from Pfizer, Tanabe, and Bayer; Dr. Anderson, consulting fees from Boehringer Ingelheim and Servier and speakers’ fees from Boehringer Ingelheim, Servier, AstraZeneca, and Sanofi‐Aventis; Dr. Forette, consulting fees from Wyeth Elan, Sanofi‐Aventis and Bristol‐Myers Squibb and speakers’ fees from Servier, AstraZeneca, and Sanofi‐Aventis; Dr. Rajkumar, speakers’ fees from Schering‐Plough, Merck Sharp & Dohme, and Menarini; and Dr. Bulpitt, consulting fees from Imperial College Consulting, a consultancy funded by a grant from the Institut de Recherches Internationales Servier. No other potential conflict of interest relevant to this article was reported." Comment: some doctors received consulting fee and speakers' fees from the pharmaceutical companies, although the research received grants from the British Heart Foundation and the Institut de Recherches Internationales Servier. |
HYVET pilot 2003.
| Methods | Randomized, open, multisite trial conducted in Europe. Most patients enrolled were from Bulgaria 1130 (88%), 39 (3%) in Spain, 39 (3%) in Romania, 32 (2.5%) in the UK, 20 (1.5%) in Poland, and smaller numbers in Finland, Lithuania, Ireland, Greece, and Serbia. | |
| Participants | Study setting: both primary and secondary care
1283 participants (63% women); age range: 79.5 to 96.1, mean age = 84 years; race: not stated Blood pressure (BP) entry criteria: systolic blood pressure (average of four readings) 160 to 219 mmHg, diastolic blood pressure 95 to 109 mmHg (later changed to 90 to 109 mmHg), standing systolic blood pressure > 140 mmHg (average of two readings). Mean blood pressure at entry: systolic blood pressure averaged 181.5 + 11.3 mmHg (range 160–217 mmHg) and entry diastolic pressure averaged 99.6 + 3.4 mmHg (range 90–114 mmHg). Pulse pressure was 82 mmHg. Target blood pressure was < 150/ 80 mmHg Pre‐existing factors: patients were not obese, with an average body mass index of 25 kg/m2; 48% had been previously treated, 3.0% had had a previous myocardial infarction, 4.5% a previous stroke, and 20.7% drank more than 1 unit of alcohol per day. Smoking: 4.2% The target blood pressures were a sitting systolic pressure less than 150 mmHg plus a sitting diastolic pressure less than 80 mmHg Exclusion criteria: serum creatinine > 150 mol/l, accelerated hypertension, congestive heart failure requiring treatment, inability to stand, cerebral or subarachnoid haemorrhage in past 6 months, need for blood pressure‐decreasing treatment because of angina, etc., the presence of gout, renal artery stenosis, dementia (abbreviated mental test score, 7/10), and a condition expected to limit survival severely Follow‐up: 13 months |
|
| Interventions | Treatment: Step1: diuretic (usually bendrofluazide 2.5 mg), an ACE inhibitor (usually lisinopril 2.5 mg), or no treatment Step 2 involved doubling the dose of the first drug Step 3 involved adding diltiazem slow‐release 120 mg daily Step 4 involved adding diltiazem slow‐release 240 mg daily Control: no treatment |
|
| Outcomes | Total stroke, total mortality, cardiovascular mortality, cardiac mortality, sitting systolic BP and diastolic BP Dropouts due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | "As the trial was a pilot trial with limited numbers and a short period of follow‐up, interim analyses were not performed. Similarly, although power calculations are published, they are not relevant to the pilot trial. All analyses are presented on an intention‐to‐treat basis." "The main weaknesses of the pilot trial were that it was an open study and also was not conducted to the standards of Good Clinical Practice. The problem with the use of an open design is that both patient and investigator know the treatment given. This can lead to bias in several different ways. Investigator bias may affect what is written on a death certificate: for example, if the patient has both a myocardial infarction and a stroke before death, the investigator may tend to record a stroke as the underlying cause of death if the patient is receiving no treatment and blood pressure is high." Percentage of patients not on assigned therapy at study end: diuretic 97%, ACEI 96%, no treatment 99.2%. Difference in blood pressure at study end (Treatment ‐ Control): sitting BP difference between diuretic/ACEI and no treatment ‐23/‐11 mmHg; standing BP difference between diuretic and no treatment ‐23/‐11 mmHg, and difference between ACEI and no treatment ‐24/‐12 mmHg. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In the pilot trial, patients older than 80 years and with hypertension were allocated randomly but equally to groups to receive a diuretic‐based regimen, an angiotensin‐converting enzyme (ACE)‐based regimen or to no treatment." "The unit of randomisation was the individual and the SAS Random Allocation of Treatments Balanced in Blocks Program was used to generate the schedule." Restricted random allocation to groups was used to ensure equal allocation per group within each centre and allocation to groups was performed centrally. Stratified into four groups on the basis of sex and age (80 to 89 years, and > 90 years). Baseline characteristics were similar in all treatment groups". |
| Allocation concealment (selection bias) | High risk | Quote: "Restricted random allocation to groups was used to ensure equal allocation per group within each centre and allocation to groups was performed centrally." "The pilot HYVET trial was an open design that worked well, but concerns were expressed that only the results of a double‐blind trial conducted to Good Clinical Practice guidelines would be acceptable in the 21st century." Comment: method used for allocation concealment was not specified and probably not done as it was an open‐label pilot study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The trial recruited individuals from both primary and secondary care and was of an open design." Comment: patients and providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 1283 patients who were assigned to groups, only 27 (2.1%) were lost to follow‐up (had no end‐of‐trial information)." (Diuretic 2%, ACEI 2%, no treatment 2%) "Of the 426 patients allocated randomly to a diuretic‐based treatment, 385 (88.5%) were alive and provided information at the end of the trial. The corresponding numbers were 397 (89.8%) for ACE‐based treatment, and 394 (90.1%) for no treatment." "Both the investigators’ and the patients’ knowledge of treatment may affect the withdrawal rates, for example, favouring the removal from the trial of a patient who is receiving no treatment but has high blood pressure that approaches but does not exceed a terminating outcome." Comment: number of patients lost to follow‐up low and reasons for attrition were not mentioned, although the small attrition could not have affected the outcome. |
| Selective reporting (reporting bias) | Low risk | Quote: "The main endpoints of the trial were stroke events, total mortality and cardiovascular, cardiac and stroke mortality." "As this was an open study, the randomised treatment could be continued after a nonfatal event." Comment: all endpoints were reported in the results section. |
| Other bias | Low risk | Quote: "The pilot trial was supported by the British Heart Foundation." |
Kuramoto 1981.
| Methods | Placebo‐controlled single site study conducted in ambulatory patients in home for the aged in Tokyo, Japan. Allocation of individuals within matched pairs to treatment and control groups was made by a blinded statistical coordinator, thought to be randomised but not entirely clear. (Unpublished information as per personal conversation with author as stated in Mulrow 1998 review. |
|
| Participants | 91 participants (45% female); age range: > 60, mean: 76.1 years, race: not stated The inclusion criteria were SBP/DBP 160/90 mmHg to < 200/110 mmHg Pre‐existing factors: not reported. Blood pressure (BP) entry criteria: not clearly stated Mean blood pressure at entry: 169/86 mmHg (isolated systolic hypertension in 44% of subjects) Pulse pressure was 83 mmHg. Exclusion criteria were not mentioned. Patients were excluded from the trial "when the blood pressure exceeded 200/110, and appearance of cerebrovascular or cardiac complications, other diseases which needed hospital admission, death or moving out from the home were considered to be dropouts." Follow‐up: 2.7 years |
|
| Interventions | Treatment: trichlormethiazide 1 mg to 4 mg, 80% monotherapy. Reserpine (0.3 mg), methyldopa (125 mg to 500 mg) and hydralazine (50 mg to 100 mg) added as stepped care approach when needed. Control: placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF, systolic BP and diastolic BP Dropout due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | Difference in blood pressure at study end (based on only 29 patients; Treatment ‐ Control) systolic/diastolic: 0.8/1.3 mmHg Reporting of study methods was inadequate in the publication. We have used unpublished information as per conversation with the study author as stated in the Mulrow 1998 review to assess the risk of bias of this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The matched pair group was selected by the age, sex, and blood pressure levels during the drug‐off control period of about 1 year." Comment: method of randomization not described. However, the study reports that "Forty four drug treated cases and 47 placebo treated cases were comparable in blood pressure as well as in laboratory data". |
| Allocation concealment (selection bias) | Low risk | Allocation of individuals within matched pairs to treatment and control groups was made by a blinded statistical co‐ordinator; thought to be randomized, but not entirely clear (unpublished information as per personal conversation with author by Mulrow 1998). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study does not state whether patients and physicians were blinded. Comment: Patient and providers were blinded (unpublished information as per personal conversation with author by Mulrow 1998). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Patients were excluded from the trial when the blood pressure exceeded 200/110 and appearance of cerebrovascular or cardiac complications, other diseases which needed hospital admission, death, or moving out from home were considered to be drop out." "As a whole, 9 out of 41 cases or 22.0% in the placebo group, and 4 out of 38 cases or 10.5% in the drug group dropped out by cerebrovascular or cardiac complications. In addition to the cerebrovascular and cardiac complications, dropouts due to blood pressure elevation were observed in 8 cases in the placebo group, and total dropouts in the placebo group were 17 cases or 41.5%. This incidence was significantly higher than that in the drug treated group (Table IV)." "Six cases of dropout due to moving out from the home were observed in both groups, and follow‐up cases were 38 in the drug group and 41 in the placebo group." For blood pressure measurements, the number of he follow‐up cases in the placebo group decreased markedly from 47 to 32, 24, 13, and 7 at the end of each year. The number of the follow‐up cases at the end of each year in the drug group declined from 44 to 32, 26, 25, and 22 due to dropouts. Comment: follow‐up of patients was incomplete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Insufficient information to judge selective reporting bias. |
| Other bias | Unclear risk | Comment: no mention about statistical methods, source of funding, members/team involved in the conduct of trial and conflict of interest. |
MRC‐O 1992.
| Methods | Randomized, single‐blind, placebo‐controlled multisite study conducted in general practice setting in England, Scotland, and Wales. | |
| Participants | 4396 participants (58% female); ambulatory patients; age range: 60 to 74, mean: 70.3 years; male (42%); race: not reported
Blood pressure (BP) entry criteria: systolic BP 160 to 209 mm Hg and diastolic BP < 115 mm Hg; Mean blood pressure at entry: 184/91 mmHg and pulse pressure was 94 mmHg Exclusions: known or suspected secondary hypertension; taking antihypertensive drugs; cardiac failure or any other accepted indication for antihypertensive treatment; receiving treatment for angina pectoris; history of myocardial infarction or stroke within preceding three months; impaired renal function; diabetic; asthma; serious inter current disease, including malignancy, known to be present at time of examination; serum potassium concentration ≤ 3.4 mmol/L or > 5.0 mmol/L Pre‐existing risk factors: myocardial infarction: excluded if within last 3 months; stroke: excluded if within last 3 months; diabetes: excluded; smoking: 17.5% Follow‐up: 5.8 years |
|
| Interventions | Treatment: Diuretic Arm: Step 1 ‐ hydrochlorothiazide 25 mg or 50 mg + amiloride 2.5 mg or 5 mg daily; Step 2 ‐ atenolol 50 mg daily; Step 3 ‐ nifedipine up to 20 mg daily; Step 4 ‐ other drugs Beta‐blocker Arm: Step 1 ‐ atenolol 50 mg daily; Step 2 ‐ hydrochlorothiazide 25 mg or 50 mg + amiloride 2.5 mg or 5 mg daily; Step 3 ‐ nifedipine up to 20 mg daily; Step 4 ‐ other drugs Control: placebo |
|
| Outcomes | Mortality, stroke, CHD, systolic BP and diastolic BP Dropouts due to side effects Quality of life or functional outcomes |
|
| Notes | Percentage not on assigned therapy at study end (including withdrawals and lost to follow‐up): placebo group: 53%; diuretic arm: 48%; beta‐blocker arm: 63% Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐6.3/‐5.9 mmHg Dropouts due to side effects: control group: 82 (3.7%); diuretic arm: 160 (14.8%); beta‐blocker arm: 333 (30.2%) Quality of life or functional outcomes: no perceptible negative effect of treatment compared to control on measures of cognitive function. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All trial entrants were randomly allocated in equal proportions to one of the four treatment categories... Randomization was stratified by gender and site; at each site, subjects were assigned to therapy based on computer generated lists." Comment: baseline characteristics were similar. |
| Allocation concealment (selection bias) | Low risk | Comment: method of allocation concealment was not described. Baseline characteristics were similar. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This trial was single‐blind; patients did not know which treatment group they were in; but the doctors and nurses..." Comment: patients were blinded; providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The records of all patients were "flagged" at Southport NHS center register to ensure notification of death. The diagnostic evidence for each terminating event was assessed by the arbitrator, blind to the treatment regimen. World Health Organization criteria for classification of strokes and coronary events were used. All available documentation was reviewed, including copies of general practitioners' notes, hospital inpatient and outpatient notes, electrocardiographic recordings, necropsy findings, and death certificates." "Data on terminating events were analysed after every 5000 patient years and were reviewed by an independent monitoring and ethics committee." Comment: outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Over five and a half years about 25% of people were lost to follow‐up. The cumulative percentage of people who stopped taking their randomised treatment, including both those withdrawn but continuing on follow‐up and those lost to follow‐up, 48% of the diuretic group, 63% of the beta‐blocker group, and 53% of the placebo group." "Overall, the beta‐blocker group had significantly more withdrawals than diuretic group." Comment: loss to follow‐up was high, only selected reasons for the beta‐blocker group was provided. The reasons pertinent to respective groups were not mentioned. Insufficient detail to determine if ITT was carried out correctly. |
| Selective reporting (reporting bias) | High risk | "A patient's participation in a trial ended with a stroke, whether nonfatal or fatal; coronary events; other cardiovascular events, and death from any cause." "If a patient had a nonfatal event followed by a fatal event in the same category, only the fatal event was included in the analyses. If a patient had two events in different categories, for example, a nonfatal stroke, then a coronary event (fatal or nonfatal), then both were included." Morbidity and mortality data were reported as stated in the objectives. |
| Other bias | Low risk | Quote: "The trial was supervised by an MRC working party and coordinated by the MRC Epidemiology and Medical Care Unit at Northwick Park Hospital, Harrow." Comment: conflict of interest was not reported. The source of funding for carrying out the trial was not mentioned, nor was the relation of investigators or any member of the MRC working party to the manufacturers/suppliers of medications for the trial. |
MRC‐TMH 1985.
| Methods | Randomized single‐blind study comparing 2 treatments and placebo in ambulatory young patients in England, Scotland & Wales. | |
| Participants | 17,354 participants (8306 male and 9048 female); mean age 52 years, range 35 to 64 years; ethnicity not reported; male (49%); baseline mean SBP/DBP was 161.4/98.2 mmHg; and pulse pressure was 63 mmHg. The inclusion criteria was SBP < 200 mmHg and DBP 90 to 109 mmHg. Exclusion criteria: secondary hypertension, taking antihypertensive treatment, normally accepted indications for antihypertensive treatment (such as congestive cardiac failure) present, myocardial infarction or stroke within the previous three months, presence of angina, intermittent claudication, diabetes, gout, bronchial asthma, serious inter current disease or pregnancy. Follow‐up: 5 years |
|
| Interventions | Treatment: bendrofluazide 10 mg daily, propranolol 80 mg to 240 mg daily + methyldopa added if required Control: placebo Note: 288 patients were randomly assigned to observation only, taking no tablets, and were merged with placebo. |
|
| Outcomes | Mortality, stroke, CHD, systolic BP and diastolic BP No CHF data |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated at entry... Randomisation was in stratified blocks of eight within each sex, 10 year age group, and clinic." Comment: no information provided for sequence generation. Random sequence generation achieved properly and the baseline characteristics were well matched. |
| Allocation concealment (selection bias) | Low risk | No description of method for allocation concealment provided. Baseline characteristics were well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "four treatments: the thiazide diuretic bendrofluazide, placebo tablets that looked like bendrofluazide, the beta‐blocker propranolol, and placebo tablets that looked like propranolol. The two placebo groups were treated as one in all analyses." Quote: "When the protocol was written, it was judged unreasonable to ask general practitioners to undertake such adjustments in a double‐blind study, and the trial was therefore single‐blind only." Comment ‐ participants were blinded but not the physician. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evidence on which the diagnosis of each terminating event was based was assessed by an arbitrator ignorant of the treatment regimen... The arbitrator used WHO criteria for classification." "All events were assessed by an independent arbiter who was blind to the treatment regimen." "Each electrocardiogram tracing was read by two observers who were blind to the treatment regimen; the second reader was also blind to the first reader's coding. If these two readers disagreed, a third reader was used." Comment ‐ adjudication was independent and blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All analyses presented here are based on randomised treatment (intention‐to‐treat) categories. Thus data for all participants are presented as if the individual was still in the treatment group to which he was originally randomised, although substantial percentages of patients (see below) were in fact withdrawn from their randomly allocated regimen during follow‐up." Quote: "The total five and a half year cumulative percentages of men who stopped taking their randomised treatment, including both those withdrawn from their randomly allocated regimen but continuing on follow‐up and those lapsing from the trial, were 43% of the bendrofluazide group, 42% of the propranolol group, and 47% of the placebo group. For women, the figures were 33%, 40%, and 40% respectively. The cumulative percentages of people not taking either primary active drug by five and a half years were smaller: 33% of men originally randomised to bendrofluazide, and 34% of men randomised to propranolol, and 28% and 31% respectively of women." Quote: "Events terminating a patient’s participation were: stroke, whether fatal or nonfatal; coronary events, including sudden death thought to be due to a coronary cause, death known to be due to myocardial infarction, and nonfatal myocardial infarction; other cardiovascular events, including deaths due to hypertension (ICD 400 to 404), and to rupture or dissection of an aortic aneurysm, and death from any other cause. Clinic staff reported these events to the co‐ordinating centre. The records of all patients who suffered nonfatal terminating events and of any others, who lapsed from the trial, whatever the reason, were 'flagged' at the Southport NHS central register to ensure notification of death." Comment: myocardial infarction and stroke were reasons for terminating the study follow‐up, except for death flagging. This induced a censoring attrition bias, limited to the occurrence of nonfatal events of myocardial infarction or stroke. |
| Selective reporting (reporting bias) | Low risk | No information about pre‐specified outcomes was available on which to make this assessment. However, aim of the study was to study mortality and morbidity, which have been reported. |
| Other bias | Unclear risk | Conflict of interest was not reported. "The working party thanks the general practitioners and nurses collaborating in the trial; the staff at the coordinating centre; the staff of the Wolfson Research Laboratories, Queen Elizabeth Medical Centre, Birmingham, for carrying out the biochemical analyses; Duncan, Flockhart and Co Ltd for tablets of bendrofluazide and placebo; Imperial Chemical Industries Ltd for financial support and for tablets of propranolol and placebo; Ciba Laboratories for supplies of guanethidine; and Merck Sharp and Dohme Ltd for a mobile screening unit, funds for its staffing, and supplies of methyldopa." |
OSLO 1986.
| Methods | Open randomized trial conducted in ambulatory young male patients randomized to treatment or not in Norway. | |
| Participants | 785 patients with mean age 45.3 years, range 40 to 49 years. Ethnicity not reported. Baseline mean SBP/DBP was 156.2/97 mmHg and pulse pressure was 59 mmHg. Inclusion criteria: SBP 150 to 179 mmHg and DBP < 110 mmHg. Target < 140/90 mmHg. Exclusion criteria: New or previous coronary heart disease, cardiovascular disease, intermittent claudication, congestive heart failure or valvular heart disease, drug‐treated hypertension during the last year, diabetes mellitus (fasting blood sugar > 8.3 mmol/L), retinopathia (Keith‐Wagener grade 3 and 4), renal disease (proteinuria, hematuria, creatininc > 123.8 mmol/L, chronic nephritis), hepatic disease, psychosis, severe neurosis, persons regularly treated with psychopharmacologic drugs, malignant disease, and such chronic disease as rheumatoid arthritis, endocrine disorders, obvious alcohol abuse and social maladjustment, secondary hypertension, electrocardiographic changes at rest, left bundle branch block, atrial fibrillation, S‐T segment depressions Sl mm, marked left ventricular hypertrophy: R max + S max in precordial leads > 45 mm and simultaneous ST‐T changes (Minnesota code 4‐l, S‐T segment depression, and T‐wave flattening or inversion). Mean follow‐up: 5 years |
|
| Interventions | Treatment: hydrochlorothiazide (95%), methyldopa, and propranolol (26%). At 5‐year follow‐up, 36.7% were on HCTZ alone, 26% were on HCTZ + propanolol, 20% were on HCTZ + methyldopa, and 18% patients were on other drugs. Control group: no treatment |
|
| Outcomes | Stroke, CHD, mortality, CHF, systolic BP and diastolic BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "During 1973, 785 men, aged 40 to 49, with mild, symptom‐free hypertension were randomly assigned for a five‐year controlled drug treatment study, 406 men in the treatment group and 379 in the control group." "The randomization was performed by a random number table.” Comment: participants randomly allocated using random number tables and baseline characteristics were similar. |
| Allocation concealment (selection bias) | Low risk | The method used for allocation concealment was not mentioned. Participants and physician were aware of treatment given. Baseline characteristics were similar. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Drug treatment was started with hydrochlorothiazide. If SBP remained above 140 mmHg, DBP above 90 mmHg, or both, alpha methyldopa was added. If there were side effects, methyldopa was replaced with propranolol. The control group was not given a placebo." Comment: no mention of blinding, both the participants and physicians were aware of the treatment provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Possible and definite coronary events and other cardiac complications were also evaluated by a “blind” diagnostic board of two independent cardiologists." Comment: blinding of the outcome assessor was probably done, though it was not clearly stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In this study the patients have seen the same physicians and the same paramedical staff during 4 years and the drop‐out rate has been small, 0.6 per cent and the same in both groups." "Three men refused the drugs, and 13 (1.7%) dropped out of the study, three in the treatment group and 10 in the control group." "The mean observation time was 66 months (range: 60 to 76). Only 13 (1.7%) men failed to report for regular examinations. However, these men were followed for possible cardiovascular events at the end of the study." Comment: number of drop‐outs was low and patients were followed until end of study to account for all outcomes. |
| Selective reporting (reporting bias) | Low risk | Quote: “Each patient with cardiovascular events has been counted once, i.e., the number of events is identical with the number of patients with events. If a patient had more than one event, the most serious was counted. Nobody had both coronary heart disease and a cerebrovascular event”. Comment: all outcomes (coronary, cerebrovascular and other events) were properly reported and accounted for in the results section. |
| Other bias | Unclear risk | Conflict of interest was not reported. Source of funding was not stated. |
PATS 1996.
| Methods | Randomized, double‐blind placebo controlled trial conducted in China from 44 clinical centres. | |
| Participants | 5665 Chinese men (72%) and women (28%) with a history of transient ischemic attack, minor stroke or major stroke without severe disability. Mean age + SD was 60 + 8 years. Baseline BP 154/93 mmHg. 16 % of patients were not hypertensive BP <140/90 mm Hg. Exclusion criteria: malignant neoplasm, rheumatic valvular disease, congestive cardiomyopathy, permanent atrial fibrillation, secondary hypertension, hyperthyroidism, severe hepatic or renal disease, haemorrhagic disease, insulin dependent diabetes mellitus etc. Follow‐up: 2 years |
|
| Interventions | Treatment: Indapamide 2.5 mg daily Control: placebo | |
| Outcomes | Mortality, stroke, coronary heart disease, blood pressure | |
| Notes | Secondary prevention trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Conducted as a double‐blind placebo‐controlled multicentre trial in China." "After a single‐blind, run‐in phase on placebo, eligible patients were randomized to indapamide treatment or to placebo." Comment: method used for the random selection of participants was not mentioned. Baseline characteristics were balanced at study entry. |
| Allocation concealment (selection bias) | Low risk | Quote: "The sealed envelope system was used to randomize the participants. Investigators in every clinical centre assigned all the eligible patients to either the indapamide treatment group or the placebo group according to the order of the sealed envelopes supplied by the Coordinating Office. Patients would enter into the double‐blind period on the date of randomization." "The hypotensive treatment protocol was fixed, i.e. a tablet of indapamide (2.5 mg) per day in treatment group and a pill of matching placebo per day in the placebo group." Comment: sealed envelopes whether transparent or opaque used for the allocation was not mentioned. Baseline characteristics were balanced at study entry. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Conducted as a double‐blind placebo‐controlled multicentre trial in China." "A physician might withdraw a patient from the double‐blind treatment when he thought the elevated or lowered blood pressure was harmful to the patient. During the double‐blind phase, if the double‐blind treatment was harmful to the participants because of the elevation or lowering of blood pressure to an intolerable level, treatment can be modified." Comment: participants of the study were blinded but it is unclear from the statement that physicians could withdrew the patients from the treatment or placebo group depending on the patient's medical condition. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 162 (5.7%) in treatment group and 150 (5.3%) in placebo group defected from the trial for non‐medical reasons. All patients who left DB period alive were followed to allow ITT analysis of mortality and morbidity. These patients were examined once a year. The loss to follow‐up was not mentioned. The drug withdrawal was more in the placebo group. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes (primary and secondary as well as fatal and non‐fatal events) as mentioned in the objectives analyzed and reported in the results section. |
| Other bias | Low risk | This study was supported financially by the World bank Office, Ministry of Public Health, China and Clinical Trial service Unit of Oxford University, U.K. |
SHEP 1991.
| Methods | Randomized, double‐blind, placebo controlled multisite study in community ambulatory patients conducted in USA. | |
| Participants | 4736 participants (55.8% female); age range 60 to > 80 mean: 72 years; male 43%; race: white non‐Hispanic (79.2%), Black (13.8%), Hispanic (1.8%), Asian (4.3%), other (0.9%); mean blood pressure at entry: 170/77 mmHg Pre‐existing risk factors: myocardial infarction: 4.9%; stroke: 1.4%; diabetes: 10.1%; smoking: 12.7%; Blood pressure (BP) entry criteria: systolic BP 160 ‐ 219 mm Hg and diastolic BP < 90 mm Hg. Baseline mean SBP/DBP was 170/77 mmHg and pulse pressure was 93 mmHg. Exclusion criteria: history, signs, or both, of major cardiovascular diseases likely to require pharmacologic and other treatment (e.g. previous myocardial infarction, coronary artery surgery, major arrhythmias, conduction defect, recent stroke, carotid artery disease, history of transient ischemic attack (TIA) with bruit matched with TIA localization, two or more TIAs and signs or symptoms in a single neurological distribution); other major diseases (e.g. cancer, alcoholic liver disease, established renal dysfunction) with competing risk factors for the primary endpoint ‐ stroke; presence of medical management problems (e.g. insulin dependent diabetes, history of dementia, evidence of alcohol abuse); bradycardia; people maintained on beta‐blockers, diuretics, other antihypertensive drugs, anticoagulants, or experimental drugs on recommendation of their physicians. Follow‐up: 4.5 years |
|
| Interventions | Treatment: Step 1 ‐ chlorthalidone 12.5 mg or 25 mg daily Step 2 ‐ atenolol 25 mg or 50 mg or reserpine 0.05 mg or 0.10 mg daily Control: placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF, systolic BP and diastolic BP Dropouts due to side effects Quality of life or functional outcomes |
|
| Notes | Percentage not on assigned therapy at study end: placebo group: 44%, and treatment group: 10%. Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐11.1/‐3.4 mmHg. Dropouts due to side effects: Control group: 7%; Treatment group: 13%. Quality of life or functional outcomes: no perceptible negative effect of treatment compared to control on measures of cognitive, physical, and emotional function. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Stratified randomization by antihypertensive drug treatment status at initial contact and by center produced two SHEP groups—assigned to active treatment and placebo—comparable at baseline.” "Each randomisation was carried out by telephone." “Both treatment groups were generally comparable to the several traits assessed.” Comment: randomization was adequately done and baseline characteristics of two groups were well matched. |
| Allocation concealment (selection bias) | Low risk | Quote: "The random assignment to one of the two study groups was to be made by the Coordinating Center and transmitted to the clinical center by telephone after verification of eligibility (inclusion and exclusion criteria). Each participant was to be assigned a drug bottle number for the first step and dosage of the treatment program. A randomization report was then to be mailed to each clinical center." Comment: patients were randomly allocated by co‐ordinating centre and allocation concealment seems to be performed adequately. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "SHEP was a long term, multicenter, randomized, double‐blind, placebo‐controlled trial sponsored by the National Heart, Lung and Blood Institute and National Institute of Ageing." "Participants were to be randomized at each center to either chlorthalidone or matching placebo in a double‐blind manner." “Drug dosage was doubled (including matching placebo) for participants failing to achieve the SBP goal at follow‐up visits.” Comment: neither participants nor treating physicians were aware of the treatment given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Occurrence of study events listed above was confirmed by a coding panel of three physicians blind to randomization allocation.” "The SHEP endpoint committee, which was masked to results by treatment group and individual participant treatment assignment, coded strokes, causes of death, and selected nonfatal outcomes. Documented criteria (1, 2a, 2b) were used in assessing outcomes. At each of its meetings, the DSMB was satisfied that the ascertainment of outcomes was not biased." "The progress of the study and the safety of the participants were reviewed on a regular basis by an independent data and safety monitoring board." Comment: morbidity and mortality outcome assessment was carried out independently |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "By July 1990, there were 661 initial reports of strokes and deaths. Of these, 90.3%, or 587, had complete information of which 579 had been coded by the endpoint committee. By December 1990, there were 721 reports of strokes and deaths, and 94.9%, or 684, had complete information and 666 had been coded. Primary outcome determination was complete for 99.8% of the participants." “All analyses are to be based on participants’ original treatment group assignment (i.e. the intention‐to‐treat principle)." Comment: complete follow‐up of 99.8% patients therefore it is assessed as low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: The primary endpoints, such as nonfatal and fatal stroke over a 5‐year period; secondary endpoints such as nonfatal myocardial infarction and fatal coronary heart disease, and major CVD morbidity and mortality were reported. |
| Other bias | Low risk | Quote: "The SHEP trial was supported by contracts with the National Heart, Lung and Blood Institute and the National Institute on Aging. Drugs were supplied by the Lemmon Co., Sellersville, Pa; Wyeth laboratories/Ayerst laboratories and AH Robims Co.; Richmond Va; Stuart Pharmaceuticals, Welmington, Del. It is pleasure to acknowledge the contribution of the investigators and the staff at the 16 clinical centers and coordination and service centers of the SHEP Cooperative Research Group." Comment: study sponsored by the NHLBI; no conflict of interest was declared. |
SHEP‐P 1989.
| Methods | Randomized, double‐blind, placebo‐controlled multi site study in community ambulatory patients in United States of America. | |
| Participants | 551 participants (63% female); age range: > 60 (15% > 80) mean: 72 years; race: white (82%); non‐white (18%); male (37%) Inclusion criteria: SBP 160 to 219 mmHg and DBP < 90 mmHg. Mean blood pressure at entry: 172/75 mmHg and pulse pressure was 93 mmHg. Pre‐existing risk factors: myocardial infarction (4%), stroke (1%), smoking (11%) Exclusion criteria: coronary bypass surgery within 2 years, heart attack within 6 months, stroke with residual, current treatment with antihypertensive drugs, insulin or anticoagulants, allergy to study medications, specified arrhythmias or a pacemaker, uncontrolled congestive heart failure, serum creatinine level of 2.0 mg/dL or more, alcohol abuse, cancer or other life‐threatening disease, chronic obstructive pulmonary disease, peripheral vascular disease with tissue injury, senile dementia, residence in a nursing home, carotid bruit with history of transient ischemic attacks, history of malignant hypertension Follow‐up: 3 years |
|
| Interventions | Treatment: Step 1 ‐ chlorthalidone 25 mg to 50 mg daily (87%) Step 2 ‐ randomized to hydralazine 25 mg twice daily, reserpine 0.05 mg twice daily or metoprolol 50 mg twice daily (13%) Control: placebo |
|
| Outcomes | Mortality, CHD, stroke, CHF, systolic BP and diastolic BP Dropouts due to side effects reported at 12 months Quality of life or functional outcomes not reported |
|
| Notes | Percentage not on assigned therapy at study end: placebo group 40%, and treatment group 30%. Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic ‐17/‐5 mmHg. Dropouts due to side effects (at 12 months; data not reported for end of study): control group 2 (1.8%), treatment group 7 (1.6%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The pilot study of the Systolic Hypertension in the Elderly Program was a randomized, double‐blind, placebo‐controlled trial of drug therapy for isolated systolic hypertension." “Each randomization was carried out by telephone between the clinic staff and the coordinating center data manager, who checked that eligibility criteria were met before assigning the participant to chlorthalidone or placebo. We used an adaptive randomization procedure that varied treatment assignment probabilities by 10% in one or the other direction in order to balance the step I study groups within race, sex, age and baseline systolic BP strata.” Comment: randomization was carried out in a proper manner and baseline characteristics were matching, minor differences seen in the medical history and physical examination were relatively small and could not affect the outcome. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The pilot study of systolic hypertension in the Elderly Program (SHEP‐PS) was a randomized, double‐blind, placebo‐controlled trial, following participants for an average of 34 months." "Upon randomization into the study, participants entered the step‐up protocol and received 25 mg/day of chlorthalidone or placebo (supplied as identical capsules by USV Pharmaceutical Corp)." “Participants receiving step I placebo who had not reached goal underwent a dummy randomization, and all received step II placebo twice daily. Twelve weeks later, the dosage for participants who still had not reached goal was doubled.” Comment: participants and treating physicians were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "When the necessary documentation for a morbid event was assembled at the Coordinating Center, it was copied and mailed to the three members of the Morbidity and Mortality Committee (a neurologist and two internists). Working independently and without knowledge of the participant's treatment group assignment, each member made a diagnosis based on the criteria of Table 1. The diagnosis of 'no event' was also acceptable and was the final diagnosis for five suspected morbid events. A diagnosis was accepted when the three members agreed unanimously." Comment: outcome assessment was done in an independent manner; outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “At the end of SHEP‐PS, the vital status of all participants was known; 512 were alive.” “Analysis was by intention‐to‐treat according to randomization to Step I medication (chlorthalidone or placebo), regardless of whether a Step II medication was added subsequently.” "We specified an intention‐to‐treat rule (with study groups divided by the randomized assignment regardless of subsequent crossovers) and a plan for replacing any missing annual visit BP with the last available value." Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | All cardiovascular events, such as stroke, left ventricular failure, transient ischaemic attack, myocardial infarction, sudden death, angina pectoris, coronary artery surgery, peripheral vascular disease were reported in the results section. "For any participant who had two or more events, one was designated the study event based on a hierarchal classification headed by death, followed by four categories of nonfatal events in rank order of stroke, other hypertensive events, atherosclerotic events, and non‐cardiovascular events. When there were two events in one category, the event that occurred first was used." Comment: not all events were reported if they occurred in the same category. |
| Other bias | Low risk | Quote: "Sponsorship: This study was supported by the National Heart, Lung and Blood Institute: The National Institute of Ageing; in part by the National Institute of Mental Health." Comment: conflict of interest declared and source of funding was provided. Since it was not industry sponsored, we assessed it as low risk of bias. |
SYST‐EUR 1997.
| Methods | Randomized double‐blind placebo controlled multi site study conducted in ambulatory community based patients from referral clinic in Europe (23 countries across western and eastern Europe, mainly from Finland, Bulgaria, the Russian Federation, Belgium, Italy, Israel, UK, France, Estonia, Lithuania, Spain, Poland and Romania). | |
| Participants | 4695 participants (66.8% female); age range 60+, mean 70.3 years, race not reported, male (31%) Inclusion criteria: SBP 160 to 219 mmHg and DBP < 95 mmHg. Mean blood pressure at entry 174/86 mmHg. Pre‐existing risk factors: myocardial infarction (1.2%), stroke (3.5%), smoking (7.3%), BP target: reduce systolic by > 20 mmHg or systolic < 150 mmHg Exclusion criteria: hypertension secondary to a disorder that needed specific medical or surgical treatment, retinal haemorrhage or papilledema, congestive heart failure, dissecting aortic aneurysm, serum creatinine concentration at presentation of 180 micromols/L or more, history of severe nose bleeds, stroke, or myocardial infarction in the year before the study, dementia, substance abuse, any disorder prohibiting a sitting or standing position, any severe concomitant cardiovascular or non‐cardiovascular disease Follow‐up: 2.5 years |
|
| Interventions | Treatment: Step 1 ‐ nitrendipine 10 mg daily, 10 mg twice a day, 20 mg twice a day Step 2 ‐ enalapril 5 mg, 10 mg, 20 mg daily in evening, hydrochlorothiazide 12.5 mg to 25 mg/day in morning, or both Control: placebos |
|
| Outcomes | Mortality, stroke, CHD, CHF, systolic BP and diastolic BP | |
| Notes | Percentage not on assigned therapy at study end (2 years) including open follow‐up and loss to follow‐up: placebo group 27% and treatment group 18%. Percentage receiving nitrendipine fell from 80% in year 1 to 50% in year 4. Difference in blood pressure at end of study (Treatment ‐ Control) systolic/diastolic ‐10.1/‐4.5 mmHg at 2 years. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomized to double‐blind treatment with active medication or placebo by means of a computerized random function.” "Randomization was stratified by centre, sex and previous cardiovascular complications. Group allocation determined by computerized random function." Comment: randomization was properly done. |
| Allocation concealment (selection bias) | Low risk | Quote: "All bottles with study medication are identified by a unique number, allowing persons with access to the code to distinguish between placebo and active medication ... The responsible officer at the RDDC is instructed by the Coordinating Office whether the patient should receive placebo or active medication. The officer then writes the patient identification number on the labels of the bottles with the study medications and ships a one‐year supply to the local investigator. Under no circumstances is the officer at the RDDC allowed to disclose a patient's code. The physician, who proposed the patient for entry into the trial, receives the patient's identification number and a sealed envelope with patient's code from the Coordinating Office. This envelop will be collected at the end of study, and can only be opened in a medical emergency that cannot be dealt otherwise. The investigator verifies whether the patient identification number on the label of each medicine bottle corresponds with the number given by the Coordinating Office." Comment: allocation of treatment was concealed via proper methodology. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Sys‐Eur is conducted as a double‐blind placebo‐controlled multicentre trial." "In the active treatment, tablets with 20 mg nitrendipine, 10 mg enalapril, and 25 mg hydrochlorothiazide were used. The matching placebos in the control patients do not contain any active substance." "Placebo tablets were identical to the study drugs, with a similar schedule." Comment: neither patients nor physicians were aware of treatment provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The endpoint committee, which was unaware of the patients’ treatment status, identified all major endpoints by reviewing the patients’ files and other source documents, or by requesting detailed written information from the investigators, or by both approaches." "All other events were checked at the coordinating office by doctors who were unaware of the treatment‐group status." Comment: outcome assessment carried out in an independent manner; outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In patients who do not continue to attend clinics (non‐supervised open follow‐up), the following information is obtained by writing, telephone or personal contact either from the patients themselves or where appropriate, their general practitioner, family members, or via office of vital statistics: vital status, if deceased, cause of death, information on current medical treatment, and the incidence of nonmorbid fatal events." "Patients without any report within the year before the trial stopped were counted as lost to follow‐up." Comment: follow‐up was as complete as possible, lost to follow‐up: 2% at 2 years. |
| Selective reporting (reporting bias) | Low risk | Comment: all fatal and nonfatal cerebrovascular and cardiovascular outcomes were reported. |
| Other bias | Low risk | Quote: "The trial was sponsored by Bayer AG, Wuppertal, Germany. The National Fund for Scientific Research, Brussels, Belgium, provided additional support. The study medication was donated by Bayer AG and Merck Sharpe & Dohme Inc, West Point, Pa. The Syst‐Eur trial, initiated by Antoon Amery, MD, who died on November 2, 1994, was a concerted action of the BIOMED Research Program sponsored by the European Union. The trial was carried out in consultation with the World Health Organization, International Society of Hypertension, European Society of Hypertension, and World Hypertension League." Comment: conflict of interest not declared |
TEST 1995.
| Methods | Randomized double‐blind placebo controlled trial conducted in 21 centers in Sweden. Study setting: Not stated. | |
| Participants | 720 Swedish patients >40 years old, within 3 weeks of a stroke or transient ischemic attack. 60% men. Race: Not stated, mean age 70.1 years. Patients had to have a systolic BP > 140 mmHg. Mean baseline BP 161/89 mmHg. Co‐morbid conditions: smoking (23%), previous myocardial infarction (10%), diabetes (12.5%), congestive heart failure (4%), angina pectoris (15%). Exclusion criteria: SBP ≤ 140 mmHg, DBP ≤ 80 mmHg, bradycardia ≤ 50 beats/min, manifest heart failure, atrioventricular block I to III, previous side effects of beta‐blockers, patients in poor general condition, patients completely dependent on help for ADL, patients with specific indications for beta‐blockade. Follow‐up: 2.5 years |
|
| Interventions | Treatment: atenolol 50 mg daily Control: placebo | |
| Outcomes | Mortality, stroke, CHD, hospitalizations, BP | |
| Notes | "The study was initially designed to include 1900 patients to be followed for 2 years. However, only 720 patients could be recruited." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This trial was designed as multicentre study including 21 centres in Sweden. In all, 720 patients were included, 372 randomised to group treated with atenolol and 348 to the placebo group. A computer‐generated random scheme using a random permuted block design with a block size of four was used for randomisation, which was stratified for centre, age and the Scandinavian treatment score." Comment: random sequence was properly generated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method used for allocation concealment of participants was not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: mentioned as double‐blind study, details were not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor was not mentioned. An independent committee reviewed fatal endpoints by examination of case notes, death certification and post mortem reports as appropriate. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The loss to follow‐up was not mentioned. The mean follow‐up time was 30.7 months in placebo group and 30.6 months in the atenolol group (minimum 13 and 12 respectively, and maximum 47 months in both groups). The different rates of treatment discontinuation as 10% in the placebo group and 17% in the treatment group might have affected the outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the objectives reported. It included total deaths, cardiovascular deaths, cerebrovascular deaths, cardiac death, nonfatal myocardial infarction, nonfatal recurrent stroke, critical events I and critical events II. |
| Other bias | Unclear risk | Conflict of interest was not reported. |
UKPDS 39 1998.
| Methods | Randomized controlled open‐label trial conducted in England, Scotland, Northern Ireland. | |
| Participants | Newly diagnosed patients with type 2 diabetes mellitus and hypertension (SBP 160 mmHg or higher, DBP 90 mmHg or higher, or both in patients not on antihypertensive therapy and SBP 150 mmHg or higher, DBP 85 mmHg or higher, or both in patients on antihypertensive therapy), mean age 56 years (range 25 to 65 years), male (55%), white (86%), baseline SBP/DBP was 160/94 mmHg, and pulse pressure was 66 mmHg. Exclusion criteria were ketonuria > 3 mmol/L, a history of myocardial infarction in the previous year, current angina or heart failure, more than one major vascular episode, serum creatinine concentration > 175 mmol/L, retinopathy requiring laser treatment, malignant hypertension, an uncorrected endocrine abnormality, an occupation which would preclude insulin treatment (such as heavy goods vehicle driver), a severe concurrent illness likely to limit life or require extensive systemic treatment, or inadequate understanding or unwillingness to enter the study. Follow‐up: 8.4 years |
|
| Interventions | Treatment: tight BP control group (Captopril 25 mg to 50 mg twice a day or atenolol 50 mg to 100 mg/day. Supplemental drugs added frusemide 20 mg to 40 mg twice a day, slow release nifedipine 10 mg to 40 mg twice a day, methyldopa 250 mg to 500 mg twice a day, prazosin 1 mg to 5 mg three times a day given sequentially to achieve target BP) . Control: no treatment. Participants in this group were given treatment if SBP 200 mmHg or higher, DBP 105 mmHg or higher, or both (frusemide, long acting nifedipine, methyldopa, prazosin given sequentially to control BP. If possible, ACE inhibitors and beta‐blockers were avoided). |
|
| Outcomes | Mortality, stroke, CHD and CHF, systolic BP and diastolic BP | |
| Notes | The less tight control group did not receive any treatment unless their BP rose 200/105 mmHg or higher before 1992, or 180/105 mmHg or higher after 1992. In the control group at 1 year, 50% of the patients were treated for SBP 180 mmHg or higher, DBP 105 mmHg or higher, or both, with specified drug therapy (14% were on ACE inhibitors or beta‐blockers). Patients remaining on assigned therapy at study end: beta‐blocker arm 65%, ACE inhibitor arm 78% "During the study, patients assigned captopril and atenolol took their treatment for 80% and 74%, respectively, of the total person years of follow‐up." "Increasing number of agents were required to obtain the tight blood pressure control target of < 150/85 mmHg. A similar proportion of patients were taking three or more agents in the two groups." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Full details of this study, including the recruitment and randomisation procedure, are reported in the accompanying paper." "Randomisation stratified for those with or without previous treatment for hypertension was performed by the coordinating centre." "Randomisation produced balanced numbers of patients allocated to the various glucose and blood pressure treatment combinations for the UK prospective diabetes study and hypertension in diabetes group." Comment: stratified randomization was used for random sequence generation. The risk was judged to be low as the accompanying paper mentioned the randomization method used. |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes were used and checked as described for the UK prospective diabetes study." "Figure 1 in the accompanying paper shows that two thirds of the patients (758) were randomly allocated tight control of blood pressure aiming for a blood pressure of < 150/85 mmHg by the co‐ordinating centre; 400 patients were randomly allocated to captopril and 358 to atenolol. The small imbalance in the numbers of patients allocated to these two treatments occurred by chance as the randomisation was not blocked. The other 390 patients were randomly allocated less tight control of blood pressure, aiming at a blood pressure of < 180/105 mmHg but avoiding treatment with angiotensin converting enzyme inhibitors or beta blockers." Comment: allocation concealment properly carried out and the two groups matched in terms of biometric and biochemical characteristics. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label study" "Captopril was usually started at a dose of 25 mg twice daily, increasing to 50 mg twice daily, and atenolol at a daily dose of 50 mg, increasing to 100 mg if required. If the blood pressure control criteria were not met in the tight control group despite maximum allocated treatment, other agents were added, the suggested sequence being frusemide 20 mg daily (maximum 40 mg twice daily), slow release nifedipine 10 mg (maximum 40 mg) twice daily, methyldopa 250 mg (maximum 500 mg) twice daily, and prazosin 1 mg (maximum 5 mg) thrice daily." Comment: participants and treating physicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All available clinical information was gathered for possible endpoints, for example copies of admission notes, operation records, death certificates and necropsy reports. Copies of these without reference to the patient's allocated or actual treatment, were formally presented to two independent physicians who allocated an appropriate code from the ninth revision of the international classification of diseases (ICD‐9) if the criteria for any particular clinical endpoint had been met. Any disagreement between the two assessors was discussed and the evidence reviewed. If agreement was not possible, the information was submitted to a panel of two further independent assessors for final arbitration." Comment: blinding was not mentioned in this paper, but the quote was taken from the accompanying article UKPDS 38. BMJ 1998; 317:70313. The risk was judged to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no information for the patients lost to follow‐up, though the reasons for non‐compliance were mentioned for the two groups, which were varying. The treatment discontinuation was nearly 20% to 25%, as well as reasons varying between the two groups, and statistical analysis was carried out using intention‐to‐treat. The trial did not assess the sole action of intervention as other drugs were also allowed. Other confounding factors, such as diabetes, were not excluded and could affect the outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: all primary and secondary outcome measures, along with microvascular and macrovascular diseases' surrogate endpoints as described in the objective were reported in the results section. |
| Other bias | Unclear risk | Funding: The main grants for this study were from the Medical Research Council, British Diabetic Association, the Department of Health, the National Eye Institute and the National Institute of Digestive, Diabetes and Kidney Disease in the National Institutes of Health in the United States, the British Heart Foundation, NovoNordisk, Bayer, Bristol Myers Squibb, Hoechst, Lilly, Lipha, and Farmitalia Carlo Erba. Other funding companies and agencies are listed in the accompanying paper. Authors stated conflict of interest: none |
USPHSHCSG 1977.
| Methods | Randomized, double‐blind, placebo controlled trial conducted in ambulatory young patients in USA | |
| Participants | 389 participants, mean age 44.3 years, range 21 to 55 years, 28% were African‐Americans, male (80%), baseline mean SBP/DBP was 146.9/99 mmHg and pulse pressure was 48 mmHg. Inclusion criteria: DBP 90 to 115 mmHg. Target: None (medication was not titrated) Exclusion criteria: diabetes mellitus, renal insufficiency, or hypercholesterolemia, abnormal ECG including single or double Master test, radiographic cardiomegaly, Grade III or IV retinopathy, clinical history or findings of (a) previous arterial thrombosis or vascular insufficiency, whether coronary, cerebral or peripheral, (b) congestive heart failure, (c) angina pectoris, (d) valvular heart disease, or (e) secondary or correctable hypertension, and known sensitivities to the intervention agents Follow‐up: 10 years |
|
| Interventions | Treatment: chlorothiazide 500 mg twice a day plus rauwolfia serpentina 100 mg twice a day Control: placebo |
|
| Outcomes | Mortality, CHD, stroke, CHF, systolic BP and diastolic BP | |
| Notes | The study was carried out in a middle‐aged population with mild hypertension, which are low‐risk factors for studying mortality and morbidity data. The study was terminated once patient had a stroke. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned to treatment or placebo and then matched by race and sex for two broad age groups (under 46 and 46 to 55). The randomization was carried out within each of the six participating clinics." "The distribution of all pre‐treatment characteristics into the active drug and placebo groups was uniform." Comment: randomization was carried out, but method used for the generation of random numbers was not specified. |
| Allocation concealment (selection bias) | Low risk | Quote: "At the conclusion of the trial period, subjects were randomly assigned either active or placebo treatment, and this medication was substituted for the identical placebo of the trial period and administered in double‐blind fashion. Active therapy consisted of chlorothiazide, 500 mg, plus rauwolfia serpentina, 100 mg, in one tablet taken twice daily. There was no intervention on diet or smoking or other behavioral factors." Comment: allocation carried out randomly but methodology used to conceal allocation was not mentioned; however, baseline characteristics were well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "At the conclusion of the trial period, subjects were randomly assigned either active or placebo treatment, and this medication was substituted for the identical placebo of the trial period and administered in double‐blind fashion." "The complications were also classified in terms of those events considered likely to be the consequence of elevated pressure per se and those which are predominantly associated with vascular sclerosis (Table 2). All such events were reviewed by two consultants otherwise unassociated with the trial, who were provided with all pertinent information except knowledge of the treatment regimen." Comment: participants and the treating physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "... administered in double‐blind fashion." "Follow‐up continued for another 4 months, the last 2 weeks of which included home blood pressures again. At that point, the annual examination procedures were repeated. Thereafter, the regimens were unblinded and investigators were at liberty to treat as clinically indicated." "Of the complications observed, only stroke required termination from the regimen to which they were randomized. For myocardial infarction, it was elective, depending on the clinical circumstances. Thus, by design, most subjects continued on the same double‐blind follow‐up after their first morbid event and were at risk for additional subsequent events. Others were followed on known medication." Comment: blinding of outcome assessor was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of importance in considering the morbidity and side effects data in this report is the fact that there was no differential dropout rate between the treatment and control groups (33.2% vs 34.7%). This applies to those who simply failed to return (lost to follow‐up) as well as those who voluntarily 'withdrew' from assigned therapy but remained under follow‐up. The number for whom vital status is unknown is also similar in the two groups (14 vs12)." "During that time, 206 (52.9%) were terminated from their assigned regimen (Table 5). Dropouts accounted for 132 (33.9%), of whom 75 have been lost to regular follow‐up. The vital status of 26 of the dropouts is unknown. Drug intolerance necessitated terminations in 23 cases and major morbid events in 27, four of which were deaths. The remainder of those terminated have continued under regular follow‐up on known medications, including 24 who were terminated as treatment failures on the basis of progressive elevation of blood pressure to above a predetermined level." "The dropout rate of 33.9% overall is within that allowed for in the calculation of sample size (5% per year of follow‐up). At the beginning of closeout, one‐half remained on their assigned coded medication." Comment: attrition rate was high. Attrition rates per year not mentioned as the cut off was kept, 5% lost to follow‐up, if > 5% the outcome measures would be affected. The reasons for withdrawal and loss to follow‐up were not stated. |
| Selective reporting (reporting bias) | Unclear risk | Comment: secondary and tertiary outcomes as mentioned in the objectives were not mentioned in the results section |
| Other bias | Unclear risk | Comment: conflict of interest was not reported. Source of funding was not stated. |
VA‐I 1967.
| Methods | Randomized, double‐blind, placebo‐controlled trial conducted in ambulatory patients in USA | |
| Participants | N = 143, mean age 51 years, range not reported. 53.8% patients were African‐Americans. Male (100%). Baseline mean SBP/DBP was 186/121 mmHg and pulse pressure was 65 mmHg. The inclusion criterion was DBP < 115 to 129 mmHg. Patients were followed for 1.5 years. Exclusion criteria: surgically curable hypertension, uremia, and concomitant fatal diseases such as carcinoma. Patients with hemorrhages, exudates, or papilledema in the optic fundi, history of cerebral or subarachnoid hemorrhage, dissecting aneurysm, or congestive heart failure resistant to digitalis and mercurial diuretics were excluded. Additional exclusions included patients who wished to return to the care of their private physicians, those who would be unable to attend clinic regularly, for geographical or other reasons, and patients of dubious reliability such as alcoholics, vagrants, and poorly motivated patients. |
|
| Interventions | Step 1. HCTZ 100 mg plus reserpine 0.2 mg plus hydralazine 75 mg Step 2. hydralazine 150 mg | |
| Outcomes | Mortality, stroke, CHD, CHF, and diastolic BP | |
| Notes | Study design published: Freis ED. In: Gross F, editor(s). Antihypertensive Therapy; Principles and Practice, an International Symposium. New York: Springer‐Verlag, 1966:345‐54. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A group of 143 male hypertensive patients with diastolic blood pressures (at the clinic) averaging between 115 mmHg and 129 mmHg were randomly assigned to either active (hydrochlorothiazide plus reserpine plus hydralazine hydrochloride) or placebo treatment." "A table of random numbers was utilized by the statistician in determining the assignments." "Patients classified by severity scores as having mild hypertension were randomized in a separate stratification from those with moderate hypertension." "There were no significant differences with regard to age, weight, duration of known hypertension, or family history of hypertension, between the placebo and active treatment groups (Tables 1 and 2). There were more Negro and diabetic patients in the actively treated than in the placebo group, but the differences were not significant. The various indices of severity, such as hospital and clinic blood pressure, funduscopic, cardiac, central nervous system, and renal abnormalities were essentially similar in the two groups." Comment: method of randomization was adequate. |
| Allocation concealment (selection bias) | Low risk | "At the time of randomization, a sealed envelope was opened, which assigned the patient to one of two possible regimens—active antihypertensive medications or their placebos." Fries 1966 stated allocation was to be accomplished by opening a numbered sealed envelope containing a card assigning patient to a code number regimen. The cards were made by statistician from table of random numbers. Comment: Allocation concealment was probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The tablets of hydralazine and the Veratrum compound were made up to appear and taste the same. Chlorothiazide, reserpine, and hydralazine also were prepared to resemble their respective placebos. As in the previous study, three different code numbers were assigned to each preparation, including the placebos." "A similar appearing placebo also was manufactured. These tablets were given the same code number identifications as used in the prior study except that the letter 'C' preceded the series of digits. By substituting the 'C' series medication for the prior 'A' series, those patients taking either reserpine plus hydralazine or reserpine plus placebo of hydralazine had chlorothiazide 500 mg twice daily added to their regimens, while those patients who were not treated with active preparations had only placebo of chlorothiazide added." "The double‐blind technique was employed by utilizing a series of complex code numbers to disguise the identity of the randomized treatments and by making active drugs and placebos identical in appearance. It is realized, however, that blood pressure levels and side effects made the maintenance of such a double‐blind study difficult and imperfect." Comment: although blinding was attempted, it was probably not successful. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of the outcome assessor was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The total number of dropouts was 12, or 8.4%. Nine occurred during the first two months following randomization. Seven had been randomized to placebos and five to active drugs. Thus, the dropout rate was small and was approximately equally divided between the active‐ and placebo‐treated patients. There is considerable variation in duration of observation as patients recruited from April 1964 to December 1966, and study ended in May 1967. 38% of patients followed for two years or more. Duration of study averaged 15.7 months for placebo‐treated patients and 20.7 months for active‐treated group. |
| Selective reporting (reporting bias) | Low risk | The study reports blood pressure data and morbid events, such as death, dissecting aortic aneurysm, ruptured aortic aneurysm, cerebral hemorrhage, fundi striate hemorrhage, high BP leading to re hospitalization, cerebrovascular accident, increased creatinine and BUN, hyperglycemia and depression as terminating events Non‐terminating events reported are MI, CHF, cerebrovascular thrombosis, and TIA. Treatment failures were also reported. |
| Other bias | Unclear risk | COI was not reported |
VA‐II 1970.
| Methods | Randomized, double‐blind, placebo‐controlled study conducted in ambulatory patients in USA | |
| Participants | 380 men, mean age 52 years, range not reported. 42% patients were African‐Americans. Baseline mean SBP/DBP was 162/104 mmHg and pulse pressure was 58 mmHg. Inclusion criterion: DBP > 90 to 114 mmHg Exclusion criteria: history of a severe hypertensive complication such as a cerebral or subarachnoid hemorrhage, hypertensive neuro retinopathy, dissecting aneurysm, or renal failure, but did not include atherosclerotic complications such as coronary artery disease or cerebrovascular thrombosis. Also excluded were (1) patients with surgically curable hypertension, (2) with unrelated fatal diseases such as malignant tumors, (3) those unwilling or unable to return to clinic, and (4) poorly motivated or otherwise uncooperative or unreliable patients. Follow‐up: 3.7 years |
|
| Interventions | Treatment: Step 1. HCTZ 100 mg plus reserpine 0.2 mg Step 2. hydralazine 75 mg to 150 mg Control: placebo |
|
| Outcomes | Mortality, CHD, stroke, CHF, systolic BP and diastolic BP | |
| Notes | "The study was terminated in the subgroup of 143 patients whose diastolic blood pressures averaged 115 through 129 mmHg prior to randomization." "Many uncooperative and unreliable patients were identified and eliminated from the trial on the basis of pill counts, urine fluorescence test results, and irregularity of clinic attendance during a pre‐randomization observation period. Treatment obviously would not have been as effective in a group of patients less carefully selected with regard to their desire to cooperate. The population was further limited in that it excluded female patients and patients with labile hypertension, whose diastolic blood pressures averaged lower than 90 mmHg during the fourth through the sixth day of hospitalization." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Three hundred and eighty male hypertensive patients with diastolic blood pressures averaging 90 to 114 mmHg were randomly assigned to either active antihypertensive agents or placebos." Comment: although method used for random sequence generation was not stated, it was probably done. Tables 1 and 2 indicate that the two groups were comparable according to the indicated variables. |
| Allocation concealment (selection bias) | Low risk | Quote: "Accepted patients were then randomly assigned double‐blind to either active drugs or placebos." Comment: method used for allocation concealment was not reported, however baseline characteristics were well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Accepted patients were then randomly assigned double‐blind to either active drugs or placebos." "Active drugs consisted of two types of tablets, one being a combination tablet containing 50 mg hydrochlorothiazide and 0.1 mg reserpine which was given twice daily. The other was 25 mg of hydralazine hydrochloride given three times daily. The latter medication was raised to 50 mg three times daily if the diastolic blood pressure remained at 90 mm Hg or higher. Obviously, practically all of the patients in the placebo group had their doses raised to this level." "Patients in the control group received placebos identical in taste and appearance to the active drugs." "In order to avoid losses to protocol because of side effects presumably caused by one or the other of the two agents, provision was made to permit substitution of a tablet which contained either reserpine or hydrochlorothiazide alone, and omitted the offending medication. These special tablets were made available on request of a participating physician. Similar appearing placebo tablets were made available for the control patients and the physician did not know whether the substitution represented active drugs or placebos." Comment: trial was a double‐blinded where participants and physicians were not aware of the treatment allocated to either group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The records of the patients reported as having assessable morbid events were reviewed by two consulting physicians who had not participated in the trial." "All available data pertaining to each organic complication, except the type of protocol treatment and the level of blood pressure, were presented to the reviewers and their decisions regarding the occurrence and classification of an event according to the definitions given in the protocol (see list of assessable events at the end of the communication) were accepted as final." Comment: outcome assessors probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Fifty‐six, or 15% of the 380 randomized patients were classified as dropouts during the course of the trial. Of this number, 27 had been randomized to receive placebos and 29 to receive active drugs. The average period of follow‐up prior to dropping out was 17.6 months, with a range from less than 1 month to 49 months." "Thus, the earliest entrants were observed for 5.5 years and the latest entrants for a minimum of 1 year. The average potential duration of observation, disregarding losses and terminations, was 3.9 years for the control group and 3.7 years for the treated patients. However, because of the losses and terminations due to elevated diastolic blood pressure described below, the actual duration of post randomization observation was 3.3 years for the control group and 3.2 years for the treated patients." Comment: reasons for dropouts were mentioned, though the reasons were not given separately for the two groups. How data were analyzed in these patients was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol was not available The mortality (various causes of death) and morbidity (various terminating morbid events other than death) data were reported. |
| Other bias | Unclear risk | Conflict of interest was not reported. |
VA‐NHLBI 1978.
| Methods | Randomized, double‐blind, placebo‐controlled trial conducted in ambulatory patients in USA | |
| Participants | Mean age 37.5 years, range 21 to 50 years. 25% patients were African‐Americans. Male (81%). Baseline mean DBP was 93.3 mmHg. Inclusion criterion: DBP 85 to 105 mmHg. Target < 85 mmHg Exclusion criteria: significant cardiovascular renal abnormalities, insulin‐requiring diabetes, treatment with vasoactive drugs, a concomitant 'fatal' disease, a history of depression or of recent (within the last 2 years) gout or peptic ulcer, and finally any conditions felt to make noncompliance likely Follow‐up: 2 years |
|
| Interventions | Treatment: Step 1. chlorthalidone 50 mg Step 2. 100 mg (53% chlorthalidone alone) Step 3. chlorthalidone 100 mg + reserpine 0.25 mg Control: placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF, and diastolic BP | |
| Notes | No intervention on diet, smoking, or other behavioral factors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the conclusion of the trial period, subjects were randomly assigned either active or placebo treatment, and this medication was substituted for the identical placebo of the trial period and administered in double‐blind fashion." Comment: method for random sequence generation was not specified; table for baseline characteristics of the 2 groups were not provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment was not mentioned; could not assess baseline characteristics of the 2 groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The Cooperative Studies Program Central Research Pharmacy was responsible for distribution of coded double‐blind study drug to each clinical center and for assuring proper handling of these drugs when they were distributed to the individual subjects." "The blinded active and placebo drugs were both designated by small letters in parentheses, whereas the known drugs were designated by underlined capital letters: C, 2C, 1/2C and R. The protocol defined three standard successive therapeutic steps: (c), (2c), and (2c)+(r). Each subject began (c) when he was randomized." "The study biostatistician supervised data management and reporting. The data center supplied each clinical center with instruction manuals, data forms, randomization numbers, and individual subject identification labels for all forms, drug bottles, sample containers, electrocardiograms, and x‐rays." Comment: trial stated as double‐blind and probably patient and physician were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "By preliminary count there were 98 losses to study in the active group and 104 in the placebo group, making the total cumulative dropout rate equal to 20% with a dropout being defined as a subject with an appointment overdue for more than 60 days." Comment: dropout rates 19.3% (98/508) in treatment group and 20.6% (104/504) in the placebo group. Reasons for the loss to follow‐up was not stated separately for each group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Comment: major and minor morbid events were reported along with adverse events for each group. |
| Other bias | Low risk | Quote: "This project was jointly supported by the Cooperative Studies Program of the Medical Research Service of the Veterans Administration and by an Interagency Agreement (2 Y01‐HV‐40012‐04) awarded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health, Education, and Welfare." "A feasibility trial to investigate the practicality of determining the advantages and disadvantages of prompt pharmacologic treatment for mild hypertension was jointly funded by the Veterans Administration and the National Heart, Lung and Blood Institute." |
Wolff 1966.
| Methods | Double‐blind placebo controlled trial conducted in ambulatory patients in USA | |
| Participants | 87 participants, mean age 50 years, range not reported. 89.6% patients were African‐American. Male 32%. Baseline mean SBP/DBP was 178/109 mmHg and pulse pressure was 69 mmHg. Inclusion criteria: male and female patients with a diastolic pressure of 100 mmHg or more (taken on three separate occasions at least one week apart) at some time during the recent course of their hypertension; patients with coronary artery disease and cerebrovascular disease Exclusion criteria: patients with a history of, or presently manifesting signs of malignant or accelerated severe hypertension, that is, patients showing very high blood pressures (diastolic pressures > 130 mmHg), retinal deterioration with hemorrhages, exudates and papilledema and evidence of impaired renal function (serum urea nitrogen levels > 60 mg per 100 mL), patients with chronic renal disease with serum urea nitrogen levels above 60 mg per 100 mL, patients with surgically correctable lesions of the adrenal (primary hyper‐aldosteronism, pheochromocytoma, Cushing’s disease) or kidney (renal arterial Iesions) Patients were followed for 2 years |
|
| Interventions | Treatment: reserpine 0.25 mg three times daily, chlorthiazide 0.5 g twice daily, or HCTZ 25 mg four times a day plus guanethidine if needed Control: placebo |
|
| Outcomes | Mortality, stroke, MI, CHF, systolic BP and diastolic BP Other outcomes: the appearance of the optic fundi, and biochemical tests of renal function, carbohydrate metabolism, serum uric acid and electrolytes |
|
| Notes | Treatment failure was decided by 2 physicians:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were placed randomly on either hypotensive drug therapy (reserpine, thiazide, guanethedine) or matched placebos." "A table of random numbers was utilized to divide the patients accepted into the study into two groups (treated and placebo)." Comment: baseline characteristics similar in both groups (age, sex, weight, SBP and DBP).The incidence of past history of hypertension or diabetes, the duration of known hypertension, and the incidence of left ventricular hypertrophy similar in the two groups. The placebo group had more patients with evidence of coronary artery disease at the inception of the study, while more patients in the treatment group had a positive history of cerebrovascular disease and headache. |
| Allocation concealment (selection bias) | Unclear risk | Method used for allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The ‘prescribers’ took the blood pressures on each visit, were aware of the original random allocation of treatment or placebo, and were responsible for the titration of these patients with their medications. The second group of physicians (the ‘examiners’), unaware of the patients’ blood pressure and treatment, examined the patients at intervals ranging from 1 week to 5 months (routinely at 2‐month intervals)." "Placebo group. These patients received matched placebos which were the same shape and size and were administered at the same intervals as those medications received by their matched cases in the treatment group." Comment: participants and physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Cardiac, cerebral, retinal, renal, and general medical status were followed by observers unaware of the blood pressure readings or drug schedule." Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The cause of the absenteeism of ten patients in the treated group as opposed to one in the placebo group remains obscure." "A follow‐up of six of the treated absentees in their homes by visiting nurses indicated that all were delinquent for social rather than medical reasons. None admitted any increase in symptoms suggestive of treatment failure." "The incidence of complications sufficient to terminate the patients’ participation in the study was significantly higher in the placebo group than in the treated group." Comment: more participants were absent in the treatment group (10/45 = 22.2%) versus placebo group (1/42 = 2.4%). |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available to confirm what specific outcome measures were to be reported as primary or secondary outcomes. Comment: morbidity data and target organ function were provided in the results section along with the various reasons for treatment failure. |
| Other bias | Low risk | Quote: "This study was supported by NH1 Grant HE‐04788‐04.
Recipient of the Career Scientist Award of the Health Research Council of the City of New York, under contract i‐342." "The population utilized in this study was primarily a lower‐income Negro group. Negro population may be significantly different from that observed in the white population." |
BP: blood pressure; CHD: coronary heart disease; CHF: congestive heart failure; DBP: diastolic blood pressure; ECG: electrocardiogram; SBP: systolic blood pressure; TIA: transient ischemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTIVE I 2011 | RCT comparing irbesartan 300 mg/day or double‐blind placebo for a mean follow‐up of 4.1 years in patients with a history of risk factors for stroke and a systolic blood pressure of at least 110 mmHg. Patients at study entry were required to have one of the following risk factors: an age of 75 years or older; treatment for hypertension; a history of stroke, transient ischemic attack, or non‐central nervous system systemic embolism; a left ventricular ejection fraction of less than 45%; peripheral vascular disease; or an age of 55 to 74 years, plus either diabetes mellitus or coronary artery disease. Not all patients had hypertension. 52% patients had hypertension at baseline. |
| ADVANCE 2007 | The study is a 2 x 2 factorial randomized controlled trial that includes 11,140 adults with type 2 diabetes at elevated risk of vascular disease. Following 6 weeks on open label perindopril‐indapamide combination, eligible individuals were randomized to continued perindopril‐indapamide or matching placebo, and to an intensive gliclazide MR‐based glucose control regimen (aiming for HbA1c of 6.5% or lower) or usual guidelines‐based therapy for a mean of 4·3 years of follow‐up. More than 75% patients had hypertension at baseline. Control group included non‐specific antihypertensive therapy. |
| ALLHAT 2000 | Drug‐drug comparison of different drug classes with no placebo or untreated control group. |
| BENEDICT 2004 | This is a multicenter DBRCT in 1204 subjects, 40 years of age or older, who had hypertension and a known history of type 2 diabetes mellitus not exceeding 25 years, a urinary albumin excretion rate of less than 20 µg per minute in at least two of three consecutive, sterile, overnight samples, and a serum creatinine concentration of no more than 1.5 mg/dL (133 µmol/L). After a six‐week washout period during which any previous therapy with agents that inhibit the renin–angiotensin system was discontinued, and a three‐week washout period during which any previous therapy with non‐dihydropyridine calcium channel blockers was discontinued, eligible subjects were randomly assigned to receive one of the study treatments: the non‐dihydropyridine calcium channel blocker verapamil (in a sustained‐release formulation, at a dose of 240 mg per day), the ACE inhibitor trandolapril (2 mg per day), the combination of verapamil (in a sustained‐release formulation, 180 mg per day) plus trandolapril (2 mg per day), or placebo for a median follow‐up of 3.6 years. Additional antihypertensive drugs were allowed to achieve the target blood pressure of 120/80 mmHg. At baseline 56% patients received antihypertensive medications in placebo group, which was increased to 67% patients at the end of follow‐up. There was no true placebo group. |
| BENEDICT A 2006 | This is a randomized, double‐blind placebo‐controlled study in 590 hypertensive patients (age 30 to 70 years) with type 2 diabetes and microalbuminuria. The patients were randomly assigned to receive irbesartan in a dose of 150 mg once daily, irbesartan in a dose of 300 mg once daily, or matching placebo once daily. There was no true placebo group as 56 percent of the patients in the placebo group were receiving blood pressure–lowering therapy at the end of the two years of follow‐up. |
| Berglund 1981 | Drug‐drug comparison of bendrofluazide 2.5 mg versus propanolol 160 mg, with no placebo or untreated control group. |
| CASTEL 1994 | Drug‐drug comparison with no placebo or untreated control group. Control group included non‐specific antihypertensive therapy. |
| Coope 1986 | Randomized trial in 884 patients aged 60 to 79 years with hypertension. It could not be used to evaluate thiazides or beta‐blockers as first‐line therapy because 67% of patients received bendrofluazide and 70% received atenolol. Five percent were not on any treatment throughout the study. In the control group 2% were put on antihypertensive therapy because of rise in BP above 280/120 mmHg; seven percent were put on diuretics due to ventricular failure. |
| DIABHYCAR 2004 | This was a randomized, double‐blind, parallel group trial comparing ramipril (1.25 mg/day) with placebo (on top of usual treatment) for cardiovascular and renal outcomes for at least three years in 4937 patients with type 2 diabetes and high urinary albumin excretion. 56% patients had hypertension at baseline. There was no true placebo control group. |
| DREAM 2006 | This was a double‐blind, randomized clinical trial in 5269 participants without cardiovascular disease but with impaired fasting glucose levels (after an 8‐hour fast) or impaired glucose tolerance. Patients were randomized to ramipril (up to 15 mg per day) or placebo (and rosiglitazone or placebo) and followed for a median of 3 years. 43.7% patients at baseline had a history of hypertension. Not all patients had hypertension at baseline. |
| EUROPA 2003 | This was a randomized double‐blind trial conducted in 13,655 patients with previous myocardial infarction (64%), angiographic evidence of coronary artery disease (61%), coronary revascularisation (55%), or a positive stress test only (5%). After a run‐in period of 4 weeks, in which all patients received perindopril, 12,218 patients were randomly assigned perindopril 8 mg once daily (N = 6110), or matching placebo (N = 6108). The mean follow‐up was 4.2 years. There was no true placebo group. |
| Fuchs 2011 | Randomized, double‐blind, clinical trial, controlled by placebo in patients 30 to 70 years of age with pre‐hypertension |
| GENERIC 2010 | A single‐centre randomized double‐blind placebo‐controlled parallel trial comparing the effects of moexipril and placebo on insulin sensitivity and 24‐hour blood pressure control in post‐menopausal women with essential hypertension. Excluded because it was only 8 weeks in duration. |
| GENRES 2007 | GENRES was a prospective randomized double‐blind placebo‐controlled cross‐over study in 208 moderately hypertensive Finnish men (aged 35 to 60 years) treated with 4 weeks of antihypertensive drugs with 4 weeks placebo in between treatment periods. It was not 52 weeks duration of drug therapy. |
| GLANT 1995 | Employed alternate allocation (i.e. not random allocation). Drug‐drug comparison of delapril 30 to 120 mg versus several dihydropyridine calcium channel blockers with no placebo or untreated control group. |
| HAPPHY 1987 | Drug‐drug comparison of bendrofluazide 5 mg or HCTZ 50 mg versus atenolol 100 mg or metroprolol 200 mg with no placebo or untreated control group. |
| HDFP 1984 | Treated group included various lifestyle measures in addition to antihypertensive drug therapy. Control group was usual care and not necessarily untreated controls. |
| Hood 2007 | A placebo‐controlled, double‐blind, randomized cross‐over trial. Patients then received 10 cycles of double‐blind treatment comprising spironolactone 50 mg to 100 mg, amiloride 20 mg to 40 mg, bendroflumethiazide 2.5 mg to 5 mg at the 2 doses shown, losartan 100 mg, and placebo. Order of drugs and doses were randomized, except that the higher doses of diuretic and the placebo were administered in alternate cycles, and the 2 doses of each diuretic were separated by at least 3 intervening cycles. Each cycle of treatment lasted 5 weeks. There were no washout periods, and the entire study lasted 44 weeks for each patient. Study treatment was not given for minimum duration of 1 year. |
| HOPE 3 2016 | A total of 12,705 women 65 years or older and men 55 years or older with at least 1 CV risk factor, no known CV disease, and without any clear indication or contraindication to the study drugs were randomized to double‐blind treatment with rosuvastatin 10 mg/d or placebo and to candesartan/hydrochlorothiazide 16/12.5 mg/d or placebo (22 factorial design) and were followed for a mean of 5.8 years. Participants were not selected on the basis of history of either hypertension or hyperlipidemia, and the trial did not mandate strict blood pressure or lipid levels for entry. Persons with a history of hypertension could be enrolled if the blood pressure was adequately controlled (in the assessment of the recruiting physician) with lifestyle or drugs other than an angiotensin receptor blocker, ACE inhibitor, or thiazides. Only 38% patients had hypertension at baseline and 29% were taking antihypertensive agents (other than ARBs, ACE inhibitors, or thiazides). Participants were allowed open label use of ARBs, ACE inhibitors, thiazides, and other blood pressure‐lowering drugs |
| HOT 1995 | Evaluated the effects of achieving pre‐specified levels of diastolic blood pressure control with all patients receiving antihypertensive treatment. |
| IDM 2001 | Other antihypertensive drugs were prescribed to 56% of the placebo group |
| IDNT 2003 | This was a randomized double‐blind, placebo‐controlled trial with a median follow‐up of 2.6 years in 1715 adults with type 2 diabetic nephropathy and hypertension treated with irbesartan, amlodipine, or placebo. The distribution of non‐study drugs used to achieve the target blood pressure was similar in the three groups (Table 2). The placebo group received an average of 3.3 non‐study drugs, and the other two groups received an average of 3.0 drugs. There was no true placebo control group. |
| IMAGINE 2008 | This was a double‐blind, placebo‐controlled study of 2553 patients after CABG who were randomly assigned to quinapril, target dose 40 mg/d, or placebo, who were followed for a maximum of 43 months. Blood pressure entry criteria were not required. 47% had hypertension at baseline and baseline SBP/DBP was 122/70 mmHg. Before CABG, 91% of subjects were taking aspirin, 65% were taking a statin, 79% were taking a beta‐blocker, 23% were taking an ACE inhibitor or angiotensin receptor blocker, and 37% were taking a calcium channel blocker. After randomization, over the entire study period, the use of antiplatelet agents averaged 95%, whereas lipid‐lowering drug use averaged 85% (statins 83%), and beta‐blocker use averaged 63%. Among patients randomized to placebo, 5% were taking an ACE inhibitor at 1 year, 8% at 2 years, and 11% at 3 years. |
| Imai 2011 | A RCT in 577 patients treated with antihypertensive therapy (73.5% (N = 424) received concomitant ACE inhibitors), were given either once‐daily olmesartan (10 mg to 40 mg; N = 288) or placebo (N = 289) over 3.2 ± 0.6 years (mean±SD). 282 received olmesartan and 284 received placebo in addition to conventional antihypertensive therapy. There was no true placebo control group. |
| INSIGHT 1996 | No placebo or untreated control group |
| IPPPSH 1985 | Thiazide was given to over 67% of patients in both the treated and control group. |
| Kondo 2003 | In this study patients with a history of coronary intervention and no significant coronary stenosis on follow‐up angiography 6 months after intervention were randomly assigned into a candesartan group (N = 203; baseline treatment plus candesartan 4 mg/d) or a control group (N = 203; baseline treatment alone). No placebo tablets were administered in the control group. |
| Kuramoto‐2 1994 | Head‐to‐head comparison of different drug therapies (nicardipine vs trichlormethiazide) without a placebo or untreated control group. |
| Lewis 1993 | This was a randomized controlled trial in 207, comparing captopril with placebo in patients with insulin dependent diabetes mellitus in whom urinary protein excretion was > 500 mg/day and serum creatinine concentration was < 2.5 mg/dL regardless of previous blood pressure status or a previous need for antihypertensive medication. 75.5% patients were hypertensive at baseline. Median follow‐up of 1.7 years. Patients receiving calcium channel blockers or ACE inhibitors were eligible provided their blood pressure could be maintained with BP goals required by the trial. There was no true placebo group and not all patients had hypertension at baseline. |
| Lewis 2001 | The placebo group received an average of 3.3 antihypertensive drugs per patient during the study. |
| MacMahon 2000 | This was a double‐blind placebo‐controlled randomized trial in patients aged 75 years or younger, if they had a hospital diagnosis (within five years of enrolment) of any of the following: acute myocardial infarction (MI), angina with coronary disease confirmed by angiography or exercise electrocardiogram, transient ischemic attack (TIA) or intermittent claudication. Patients were excluded for several reasons, one of them was a diastolic blood pressure (BP) > 100 mmHg, a systolic BP > 160 mmHg, or SBP < 100 mmHg during the pre‐randomization run‐in period. Patients (N = 617) were randomized to ramipril 5 mg or 10 mg daily or placebo for a duration of 4 years. The primary outcomes were carotid atherosclerosis, assessed by B‐mode (brightness mode) ultrasound, and left ventricular mass, assessed by M‐mode (Motion mode) echocardiography. At baseline 42% patients were on beta‐blockers and 25% were on calcium antagonists. The percentage of patients at baseline with hypertension was not reported. The average BP at entry was 133/79 mm Hg. |
| MAPHY 1988 | Represents a subgroup of the patients included in the HAPPHY trial. Excluded as drug‐drug comparison of bendrofluazide 5 mg or HCTZ 50 mg versus atenolol 100 mg or metroprolol 200 mg with no placebo or untreated control group. |
| Materson 1997 | Randomized double‐blind placebo‐controlled study of 1292 male veterans with DBP of 95 mmHg to 109 mmHg. After placebo washout, patients randomized to placebo or one of the six drugs ‐ HCTZ 12.5 mg to 50 mg/day; atenolol 25 mg to 100 mg/day; captopril 25 mg to 100 mg/day; clonidine 0.2 mg to 0.6 mg/day; a sustained preparation of diltiazem 120 mg to 360 mg/day or prazosin 4 mg to 20 mg/day for a period of 1 year. Morbidity and mortality outcomes were not reported for different drug classes. Blood pressure control and incidence of termination of treatment were the main outcomes. |
| MIDAS 1996 | Drug‐drug comparison of HCTZ 25 mg versus isradipine 5 mg with no placebo or untreated control group. |
| Morgan 1980 | Allocation to groups was not random, it was based on week of presentation at the clinic. |
| NAVIGATOR 2010 | Randomized double‐blind placebo‐controlled study in 9306 patients with impaired glucose tolerance and established cardiovascular disease or cardiovascular risk factors to receive valsartan (up to 160 mg daily) or placebo (and nateglinide or placebo) in addition to lifestyle modification, to achieve and maintain a 5% weight loss, reduce intake of saturated and total dietary fat, and increase physical activity to 150 minutes weekly. 77.5% patients were hypertensive at baseline. Patients with cardiovascular disease were treated more intensively at baseline than were those who had risk factors only: 21.5% received an ACE inhibitor, compared with 2.7% of those with risk factors only; 75.8% received antiplatelet treatment, compared with 24.2% of those with risk factors only; 61.7% received a beta‐blocker, compared with 32.2% of those with risk factors only; and 64.1% received lipid‐modifying therapy, compared with 30.2% of those with risk factors only. The use of diuretics and calcium‐channel blockers was similar in the two groups. At the last study visit, 20.4% of patients in the valsartan group and 24.0% of those in the placebo group were receiving an open‐label renin–angiotensin inhibitor. There was no true placebo group. |
| NICOLE 2003 | The NICOLE study (NIsoldipine in COronary artery disease in LEuven) was a single centre, randomized, double‐blind, placebo‐controlled trial with coronary angiography at baseline, six months, and three years of follow‐up. 826 patients who had undergone successful coronary angioplasty were randomized to nisoldipine 40 mg once daily or placebo. Hypertension at baseline reported in 41.7% patients in Nisoldipine group and in 39.4% patients in placebo group. Data were not reported separately in patients with hypertension. |
| NORDIL 2000 | No placebo or untreated control group |
| PEACE 2004 | This was a randomized, double‐blind, placebo‐controlled trial in which 8290 patients with stable coronary artery disease and normal or slightly reduced left ventricular function, were randomly assigned to receive either trandolapril at a target dose of 4 mg per day (4158 patients) or matching placebo (4132 patients) for a median follow‐up of 4.8 years. 45.5% patients were hypertensive at baseline. 68.6% of the treated group and 77.7% of the placebo group were taking the target dose of 4 mg of trandolapril or placebo, respectively, per day. |
| Pool 2007 | This study was a 8‐week, multicentre, randomized, double‐blind, placebo‐controlled, parallel‐group trial that compared the efficacy and tolerability of the combination of valsartan/HCTZ at doses up to 320 mg/25 mg with monotherapy of both drugs. Did not meet the minimum inclusion criteria of 52‐week duration. |
| PRoFESS 2008 | This was a multicenter trial in 20,332 patients who recently had an ischemic stroke and were randomly assigned to receive telmisartan (80 mg daily) and placebo for a mean follow‐up of 2.5 years. 74% patients at baseline had a history of hypertension. By the end of the study, the use of diuretics, ACE inhibitors, calcium channel blockers, and beta‐blockers was more frequent in the placebo group than in the telmisartan group. There was no true placebo control group in this study. |
| PROGRESS 2001 | Less than 50% of patients had elevated blood pressure and about 50% of patients were receiving other antihypertensive therapy at baseline and throughout the trial. |
| QUIET 2001 | Most patients did not have elevated blood pressure. 25% of patients were receiving a beta‐blocker. |
| REIN 1997 | This was a prospective double‐blind trial in 352 patients classified according to baseline proteinuria (stratum 1: 1 to 3 g/24 h; stratum 2: ≥ 3 g/24 h), and randomly assigned to ramipril or placebo plus conventional antihypertensive therapy targeted at achieving diastolic blood pressure under 90 mmHg. There was no true placebo control group. |
| RENAAL 2001 | This was a double‐blind randomized controlled trial (N = 1513) comparing losartan (50 mg to 100 mg once daily) with placebo, both taken in addition to conventional antihypertensive treatment (calcium channel antagonists, diuretics, alpha‐blockers, beta‐blockers, and centrally acting agents), for a mean of 3.4 years. 93% patients had hypertension at baseline.There was no true placebo control group. |
| ROAD 2007 | This was a prospective, randomized, open, blinded endpoint (PROBE) study with median follow‐up of 3.7 years in patients with chronic renal insufficiency. A total of 360 patients were randomly assigned to four groups. Patients received open‐label treatment with a conventional dosage of benazepril (10 mg/d), individual up titration of benazepril (median 20 mg/d; range 10 mg/d to 40 mg/d), a conventional dosage of losartan (50 mg/d), or individual up titration of losartan (median 100 mg/d; range 50 mg/d to 200 mg/d). Up titration was performed to optimal antiproteinuric and tolerated dosages, and then these dosages were maintained. After 4 weeks of therapy with the study drugs, patients who continued to show inadequate BP control (i.e. SBP of 130 mmHg, DBP of 80 mmHg, or both) had an additional antihypertensive agent (diuretic, calcium channel blocker, centrally acting agent, or combination of these medications, excluding ACE inhibitors and ARB) added to their treatment regimen. There was no true placebo or no treatment control group. |
| ROADMAP 2011 | This was a double‐blind placebo‐controlled randomized trial in 4447 patients with type 2 diabetes comparing olmesartan 40 mg once daily or placebo for a median duration of 3.2 years. The study enrolled patients with type 2 diabetes, among whom there was a wide range of blood pressure values, including some that were in the normal range. 82% patients had hypertension at baseline. Additional antihypertensive drugs (except angiotensin converting–enzyme inhibitors or ARBs) were used as needed to lower blood pressure to less than 130/80 mm Hg. There was no true placebo or no treatment control group. |
| SCAST 2015 | SCAST was a randomized placebo‐controlled, double‐masked trial of the angiotensin receptor blocker candesartan in 2029 patients presenting within 30 hours of acute ischemic or hemorrhagic stroke and with systolic blood pressure ≥140 mmHg. Patients were treated with candesartan or placebo for seven days, with doses increasing from 4 mg to 16 mg once daily during the first three days, and were followed for six months. Minimum duration of 1 year criteria was not met. |
| SCAT 2000 | This was a double‐blinded randomized controlled, 2 x 2 factorial, angiographic trial evaluating the effects of cholesterol lowering and angiotensin‐converting enzyme inhibition on coronary atherosclerosis in normo‐cholesterolemic patients. There were a total of 460 patients: 230 received simvastatin, and 230, a simvastatin placebo, and 229 received enalapril and 231, an enalapril placebo (some subjects received both drugs and some received a double placebo). Over 60% did not have hypertension and about half were taking beta‐blockers at baseline and throughout. |
| Schmieder 2012 | This was a double‐blind randomized placebo‐controlled study. After a 2‐ to 4‐week placebo run‐in, 1124 patients were randomized to aliskiren 150 mg, hydrochlorothiazide 12.5 mg, or placebo once daily. Forced titration (to aliskiren 300 mg or hydrochlorothiazide 25 mg) occurred at week 3; at week 6, patients receiving placebo were reassigned (1:1 ratio) to aliskiren 300 mg or hydrochlorothiazide 25 mg. From week 12, amlodipine 5 mg was added and titrated to 10 mg from week 18, for patients whose BP remained uncontrolled. This study was not a placebo or no treatment controlled study of 52 weeks duration. |
| SCOPE 2003 | SCOPE was a study of 4964 patients aged 70 to 89 years, with systolic blood pressure 160 to 179 mmHg, diastolic blood pressure 90 to 99 mmHg, or both, and a Mini Mental State Examination (MMSE) test score > 24. Patients were assigned randomly to receive the angiotensin receptor blocker candesartan or placebo, with open‐label active antihypertensive therapy added as needed. As a consequence, active antihypertensive therapy was extensively used in the control group (84% of patients). Mean follow‐up was 3.7 years. There was no true placebo or no treatment control group. |
| SHELL 1995 | No placebo or untreated control group |
| Sprackling 1981 | 123 elderly subjects were randomly allocated to simple observation or to treatment with methyldopa. Methyldopa was used at an initial dose of 250 mg twice daily, which was subsequently adjusted as necessary to bring the standing diastolic pressure towards the target of 90 mmHg. Excluded because alpha‐methyldopa was not one of the first‐line drug classes specified for this review. |
| STONE 1996 | A single‐blind trial in 1632 subjects, aged 60 to 79 years, alternatively allocated by entry order numbers to either nifedipine or placebo, with a mean follow‐up of 30 months. No randomized allocation. |
| STOP 1991 | STOP was a prospective, double‐blind randomized controlled trial, set up to compare the effects of active antihypertensive therapy (three beta‐blockers and one diuretic) and placebo on the frequency of fatal and nonfatal stroke and myocardial infarction, and other cardiovascular death in hypertensive Swedish men and women aged 70 to 84 years. Could not be used to represent first‐line thiazide or first‐line beta blocker as 67% received a beta blocker and > 70 % received a thiazide. |
| STOP‐2 1993 | Head‐to‐head comparison of different drug therapies without a placebo or untreated control group. |
| Strandberg 1991 | Treatment group had multiple interventions. Control group was usual treatment, not untreated control. |
| SYST‐CHINA 1993 | Allocation to treatment and control groups not randomized (alternate allocation was employed). |
| TOMHS 1993 | TOMHS was a 4 year double‐blind randomized controlled trial comparing six treatments for long‐term care of people with mild hypertension aged 45 to 69 years. All randomized participants received intensive nutritional‐hygienic intervention aimed at weight loss with a fat modified diet, lowering dietary sodium and alcohol intake, and increasing leisure‐time physical activity. 902 men and women with mild hypertension (average blood pressure 140/91 mmHg) were randomized to receive nutritional‐hygienic intervention plus one of six treatments: (1) placebo; (2) diuretic (chlorthalidone); (3) beta‐blocker (acebutolol); (4) alpha 1 antagonist (doxazosin mesylate); (5) calcium antagonist (amlodipine maleate); or (6) angiotensin‐converting enzyme inhibitor (enalapril maleate). The primary outcomes of TOMHS were changes in BP. Morbidity and mortality events were not reported separately for the different drug treatments. Corresponding author was contacted and refused to provide the data. |
| TRANSCEND 2008 | 5926 patients intolerant to ACE inhibitors with cardiovascular disease or diabetes with end‐organ damage were randomized to receive telmisartan 80 mg/day (N = 2954) or placebo (N = 2972). Many of these patients were receiving concomitant proven therapies. 76.4% patients had hypertension at baseline. Other non‐study blood pressure‐lowering agents were used more frequently in the placebo group than in the telmisartan group by the end of the study (telmisartan vs placebo—diuretics: 888 (33.7%) vs 1059 (40.0%), P < 0.0001; calcium channel blockers: 1003 [38.0%) vs 1215 (45.9%), P < 0.0001; beta‐blockers: 1492 (56.6%) vs 1561 (59.0%), P = 0.081; alpha‐blockers: 140 (5.3%) vs 197 (7.5%), P = 0.002). There was no true placebo or no treatment control group. |
| VACS 1982 | Drug‐drug comparison of HCTZ 50 mg versus propanolol 80 mg with no placebo or untreated control group. |
| VHAS 1997 | Drug‐drug comparison of chlorthalidone 25 mg versus verapamil 240 mg with no placebo or untreated control group. |
| White 1995 | No placebo group and patients were not randomly allocated to moexipril or moexipril plus hydrochlorothiazide. |
HCTZ = hydrochlorothiazide; DBP = diastolic blood pressure; SBP = systolic blood pressure; M‐mode echocardiography =Motion mode; CABG = Coronary Artery Bypass Grafting; ACE inhibitor = Angiotensin Converting enzyme inhibitor; ARB = Angiotensin Receptor Blocker
Differences between protocol and review
As the original version of this review did not include 'Risk of bias' assessment of all included studies, according to chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions, the protocol was updated to include it, using standard Cochrane methodology. Also, this update meets MECIR standards, including the addition of five 'Summary of findings' tables.
A third author, Rupam Gill has been added to the updated review. RG independently assessed risk of bias of the 24 included studies as a second reviewer.
Although the protocol did not specify primary and secondary outcome measures separately, we have categorized total mortality, total cardiovascular events, total stroke, and total CHD as the primary outcome measures. Reduction in systolic and diastolic blood pressure and withdrawal due to adverse effects are secondary outcome measures.
Contributions of authors
Jim Wright (JMW) was responsible for the design of the protocol, assessed all trials for inclusion or exclusion, checked the data entry, and contributed to the data analysis and to the interpretation and final draft of the review.
Vijaya Musini (VM) assessed all trials for inclusion or exclusion, extracted data, checked data entry, assessed risk of bias of all included studies, contributed to data analysis, interpretation and final draft of the review. Summary of Findings table was also prepared by VM.
Rupam Gill (RG) was the second reviewer who assessed risk of bias of all included studies.
Sources of support
Internal sources
-
Faculty of Medicine, University of British Columbia, Canada.
Salary, space and infrastructure
External sources
-
CIHR grant to the Hypertension Review Group, Canada.
Infrastructure support to the Cochrane Hypertension Group
Declarations of interest
JMW: none.
VM: none.
RG: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ATTMH 1980 {published data only}
- Abernethy JD. The Australian therapeutic trial in mild hypertension. Hypertension 1984;6(5):774‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Abernethy JD. The need to treat mild hypertension. Misinterpretation of results from the Australian trial. JAMA 1986;256(22):3134‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Doyle AE. Cardiovascular morbidity and mortality in mild hypertension: the Australian trial. Journal of Cardiovascular Pharmacology 1985;7(Suppl 2):S10‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Doyle AE. The Australian therapeutic trial in mild hypertension. Nephron 1987;47(Suppl 1):115‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Management Committee. Initial results of the Australian therapeutic trial in mild hypertension. Report by the Management Committee. Clinical Science 1979;57:449s‐52s. [MEDLINE: ] [PubMed] [Google Scholar]
- Management Committee. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet 1980;1:1261‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Management Committee. Untreated mild hypertension. A report by the Management Committee of the Australian Therapeutic Trial in Mild Hypertension. Lancet 1982;1(8265):185‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- National Heart Foundation of Australia. Treatment of mild hypertension in the elderly. A study initiated and administered by the National Heart Foundation of Australia. Medical Journal of Australia 1981;2:398‐402. [MEDLINE: ] [PubMed] [Google Scholar]
- Parry CA. Australian therapeutic trial in mild hypertension. Lancet 1980;2(8191):425. [MEDLINE: ; Letter] [DOI] [PubMed] [Google Scholar]
- Reader R. Australian therapeutic trial in mild hypertension. Medical Journal of Australia 1984;140(13):752‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barraclough 1973 {published data only}
- Barraclough M, Joy MD, MacGregor GA, Foley TH, Lee MR, Rosendorff C, et al. Control of moderately raised blood pressure. Report of a co‐operative randomized controlled trial. British Medical Journal 1973;III:434‐6. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Carter 1970 {published data only}
- Carter A. Hypotensive therapy in stroke survivors. Lancet 1970;I:485‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dutch TIA 1993 {published data only}
- Dutch TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or non‐disabling ischemic stroke. Stroke 1993;24:543‐8. [DOI] [PubMed] [Google Scholar]
EWPHE 1985 {published data only}
- Amery A, Berthaux P, Birkenhäger W, Boel A, Brixko P, Bulpitt C, et al. Antihypertensive therapy in patients above age 60 years. Fourth interim report of the European Working Party on High Blood Pressure in the Elderly. Clinical Science and Molecular Medicine 1978;55:263s‐70s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Berthaux P, Birkenhäger W, Boel A, Brixko P, Bulpitt C, et al. Antihypertensive therapy in patients above age 60. Third interim report of the European Working Party on High Blood Pressure in the Elderly. Acta Cardiologica 1978;33(2):113‐34. [MEDLINE: ] [PubMed] [Google Scholar]
- Amery A, Berthaux P, Birkenhäger W, Bulpitt C, Clement D, Schaepdryver A, et al. Antihypertensive therapy in elderly patients. Pilot trial of the European Working Party on High Blood Pressure in the Elderly. Gerontology 1977;23(6):426‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Birkenhäger W, Brixko P, Bulpitt C, Clement D, Deruyttere M, et al. Glucose intolerance during diuretic therapy in elderly hypertensive patients. A second report from the European Working Party on High Blood Pressure in the Elderly. Postgraduate Medical Journal 1986;62(732):919‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amery A, Birkenhäger W, Brixko P, Bulpitt C, Clement D, Deruyttere M, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985;1(8442):1349‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Birkenhäger W, Brixko P, Bulpitt C, Clement D, Leeuw P, et al. Efficacy of antihypertensive drug treatment according to age, sex, blood pressure and previous cardiovascular disease in patients over age of 60. Lancet 1986;2(8507):589‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Birkenhäger W, Brixko P, Bulpitt C, Clement D, Leeuw P, et al. Influence of antihypertensive drug treatment on morbidity and mortality in patients over the age of 60 years. European Working Party on High blood pressure in the Elderly (EWPHE) results: sub‐group analysis on entry stratification. Journal of Hypertension 1986;4(6):S642‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Amery A, Birkenhäger W, Brixko P, Bulpitt C, Clement D, Leeuw P, et al. Influence of hypotensive drug treatment in elderly hypertensives: study terminating events in the trial of the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension 1985;3(Suppl 3):S501‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Amery A, Schaepdrijver A. European Working Party on High Blood Pressure in the Elderly (EWPHE): organization of a double‐blind multicenter trial on antihypertensive therapy in elderly patients. Clinical Science and Molecular Medicine 1973;45(Suppl):71s‐3s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Schaepdryver A. The European Working Party on High Blood Pressure in the Elderly. The American Journal of Medicine 1991;90(Suppl 3A):1S‐4S. [MEDLINE: ] [PubMed] [Google Scholar]
- Leeuw PW. Renal function in the elderly: results from the European Working Party on High Blood Pressure in the Elderly trial. The American Journal of Medicine 1991;90(Suppl 3A):45S‐9S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- European Working Party on High Blood Pressure in the Elderly (EWPHE). An international trial of antihypertensive therapy in elderly patients. Objectives, protocol and organization. Archives internationales de pharmacodynamie et de thérapie 1985;275:300‐34. [MEDLINE: ] [PubMed] [Google Scholar]
- European Working Party on High Blood Pressure in the Elderly (EWPHE). Antihypertensive therapy in the elderly. Ninth interim report. Netherlands Journal of Medicine 1984;27:165‐70. [MEDLINE: ] [PubMed] [Google Scholar]
- European Working Party on high blood pressure in the elderly trial. Discussion after the presentation of the results of the European Working Party on High Blood Pressure in the Elderly trial (EWPHE). Journal of Hypertension 1985;3(Suppl 3):S509‐11. [MEDLINE: ] [Google Scholar]
- Fagard R. Serum cholesterol levels and survival in elderly hypertensive patients: analysis of data from the European Working Party on High Blood Pressure in the Elderly. The American Journal of Medicine 1991;90(Suppl 3A):62S‐3S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fagard R, Staessen J, Amery A. Experience with diuretics in the study conducted by the European Working Party on High Blood Pressure in the Elderly (EWPHE). Progress in Pharmacology and Clinical Pharmacology 1992;9:463‐72. [Accession number 1992230803] [Google Scholar]
- Fletcher A, Amery A, Birkenhäger W, Bulpitt C, Clement D, Leeuw P, et al. Risks and benefits in the trial of the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension 1991;9:225‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fletcher AE. Adverse treatment effects in the trial of the European Working Party on High Blood Pressure in the Elderly. The American Journal of Medicine 1991;90(Suppl 3A):42S‐4S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Malley K, Cox JP, O'Brien E. Further learnings from the European Working Party on High Blood Pressure in the Elderly (EWPHE) study: focus on systolic hypertension. Cardiovascular Drugs and Therapy. 1990;4:1249‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Malley KO, McCormack P, O'Brien ET. Isolated systolic hypertension: data from the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension 1988;6(suppl 1):S105‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Prisant LM, Carr AA. Overview of the findings of the European Party on High Blood Pressure in the Elderly. Geriatric Medicine Today 1990;9(5):35‐8. [Accession number 1990224411] [Google Scholar]
- Staeesen J. The determinants and prognostic significance of serum uric acid in elderly patients of the European Working Party on High Blood Pressure in the Elderly trial. The American Journal of Medicine 1991;90(Suppl 3A):50S‐4S. [MEDLINE: ] [PubMed] [Google Scholar]
- Staessen J. Mortality and treatment of blood pressure in patients of the European Working Party on High Blood Pressure in the Elderly. The American Journal of Medicine 1991;90(Suppl 3A):60S‐1S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staessen J, Bulpitt C, Clement D, Leeuw P, Fagard R, Fletcher A, et al. Relation between mortality and treated blood pressure in elderly patients with hypertension: report of the European Working Party on High Blood Pressure in the Elderly. BMJ 1989;298:1552‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs L. Age‐related hypotensive effect of placebo and active treatment in patients older than 60 years. The American Journal of Medicine 1991;90(Suppl 3A):24S‐6S. [MEDLINE: ] [PubMed] [Google Scholar]
- Tuomilehto J. Body mass index and prognosis in elderly hypertensive patients: a report from the European Working Party on High Blood Pressure in the Elderly. The American Journal of Medicine 1991;90(Suppl 3A):34S‐41S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoof R. Left ventricular hypertrophy in elderly hypertensive patients: a report from the European Working Party on High Blood Pressure in the Elderly trial. The American Journal of Medicine 1991;90(Suppl 3A):55s‐59s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoof R, Staessen J, Fagard R, Thijs L, Amery A. The effect of antihypertensive treatment on electrocardiogram voltages in the European Working Party on High Blood Pressure in the Elderly trial. Journal of Cardiovascular Pharmacology 1991;17(Suppl 2):S101‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOPE HYP 2000 {published data only}
- The HOPE Study Investigators. The HOPE (Heart Outcomes Prevention Evaluation) Study: the design of a large, simple randomized trial of angiotensin‐converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. Canadian Journal of Cardiology 1996;12:127‐37. [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Outcomes Study Investigators. New England Journal of Medicine 2000;342(3):145‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HSCSG 1974 {published data only}
- Hypertension‐Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA 1974;229:409‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HYVET 2008 {published data only}
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. New Egyptian Journal of Medicine 2008;358(18):1887‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ, Fletcher AE, Amery A, Coope J, Evans JG, Lightowlers S, et al. The Hypertension in the Very Elderly Trial (HYVET). Journal of Human Hypertension 1994;8(8):631‐2. [PubMed] [Google Scholar]
- Bulpitt CJ, Fletcher AE, Amery A, Coope J, Evans JG, Lightowlers S, et al. The Hypertension in the Very Elderly Trial (HYVET): rationale, methodology and comparison with previous trials. Drugs & Aging 1994;5(3):171‐83. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. HYVET investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET‐COG): a double‐blind, placebo controlled trial. Lancet 2008;7(8):683‐9. [DOI] [PubMed] [Google Scholar]
HYVET pilot 2003 {published data only}
- Beckett NS, Connor M, Sadler JD, Fletcher AE, Bulpitt CJ. Orthostatic fall in blood pressure in the very elderly: results from the Hypertension in the Very Elderly trial (HYVET) ‐ pilot. Journal of Human Hypertension 1999;13(12):839‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ, Beckett NS, Cooke J, Dumitrascu DL, Gil‐Extremera B, Nachev C, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. Journal of Hypertension 2003;21:2409‐17. [DOI] [PubMed] [Google Scholar]
Kuramoto 1981 {published data only}
- Kuramoto K, Matsushita S, Kuwajima I, Murakami M. Prospective study on the treatment of mild hypertension in the aged. Japanese Heart Journal 1981;22:75‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MRC‐O 1992 {published data only}
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. British Medical Journal 1992;304:405‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MRC‐TMH 1985 {published data only}
- Medical Research Council Working Party. Comparison of the antihypertensive efficacy and adverse reactions to two doses of bendrofluazide and hydrochlorothiazide and the effect of potassium supplementation on the hypotensive action of bendrofluazide: sub studies of the Medical Research Council's trials of treatment of hypertension: Medical Research Council Working Party. Journal of Clinical Pharmacology 1987;27(4):271‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Medical Research Council Working Party. Randomised controlled trial of treatment of mild hypertension: design and pilot trial. Report of the Medical Research Council Working party on Mild to Moderate Hypertension. British Medical Journal 1977;1(6074):1437‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Adverse reactions to bendrofluazide and propanolol for the treatment of mild hypertension. Report of Medical Research Council Working Party on Mild to Moderate Hypertension. Lancet 1981;2(8246):539‐43. [MEDLINE: ] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Comparison of the antihypertensive efficacy and adverse reactions of two doses of bendrofluazide and hydrochlorothiazide and the effect of potassium supplementation on the hypotensive action of bendrofluazide: sub studies of the Medical Research Council's trials of treatment of mild hypertension. Journal of Clinical Pharmacology 1987;27(4):271‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Coronary heart disease in Medical Research Council trial of mild hypertension. British Heart Journal 1988;59(3):364‐78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Course of blood pressure in mild hypertension after withdrawal of long term antihypertensive treatment. British Medical Journal (Clinical Research Ed.) 1986;293(6553):988‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. MRC trial of treatment of mild hypertension: principal results. British Medical Journal 1985;291(6488):97‐104. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Stroke and coronary heart disease in mild hypertension: risk factors and the value of treatment. BMJ 1988;296(6636):1565‐70. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Miall WE, Brennan PJ, Mann AH. Medical Research Council's Treatment Trial for mild hypertension: an interim report. Clinical Science and Molecular Medicine 1976;51:563s‐5s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Peart S. Results of the MRC (UK) trial of drug therapy for mild hypertension. Clinical and Investigative Medicine 1987;10(6):616‐20. [MEDLINE: ] [PubMed] [Google Scholar]
OSLO 1986 {published data only}
- Helgeland A. Treatment of mild hypertension: a five year controlled drug trial. The Oslo study. American Journal of Medicine 1980;69:725‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Helgeland A, Baksaas IA, Leren P. Mild hypertension ‐ early drug treatment or follow‐up only? the Oslo study. Acta Medica Scandinavica. Supplementum 1979;626:34‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Helgeland A, Hjermann I, Holme I, Liren P. Serum triglycerides and serum uric acid in untreated and thiazide‐treated patients with mild hypertension. American Journal of Medicine 1978;64:34‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Helgeland A, Leren P. Oslo study: treatment of mild hypertension. A five‐year controlled drug study. Nephron 1987;47(Suppl 1):108‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Helgeland A, Leren P, Foss OP, Hjermann I, Holme I, Lund‐Larsen PG. Serum glucose levels during long‐term observation of treated and untreated men with mild hypertension. American Journal of Medicine 1984;76:802‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holme I. Coronary risk factors and their possible causal role in the development of coronary heart disease: the Oslo study. Journal of the Oslo City Hospitals 1982;32(7‐8):80‐105. [MEDLINE: ] [PubMed] [Google Scholar]
- Holme I1, Helgeland A, Hjermann I, Leren P, Mogensen SB. Correlates of blood pressure change in middle‐aged male mild hypertensives: results from the untreated control group in the Oslo hypertension trial. The Oslo Study. American Journal of Epidemiology 1988;127(4):742‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leren P, Eide I, Foss OP, Helgeland A, Hjermann I, Holme I, et al. Blood lipids and antihypertensive drugs. The Oslo study. Journal de pharmacologie (Paris) 1983;14(Suppl II):217‐20. [MEDLINE: ] [PubMed] [Google Scholar]
- Leren P, Hegeland A, Hjermann I, Holme I. The Oslo study: CHD risk factors, socioeconomic influences, and interventions. American Heart Journal 1983;106:1200‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leren P, Helgeland A. Coronary heart disease and treatment of hypertension. Some Oslo Study data. American Journal of Medicine 1986;80(Suppl 2A):3‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leren P, Helgeland A. Oslo hypertension study. Drugs 1986;31(Suppl 1):41‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PATS 1996 {published data only}
- PATS collaborating group. Post‐stroke antihypertensive treatment study. A preliminary result. Chinese Medical Journal 1995;108(9):710‐7. [MEDLINE: ] [PubMed] [Google Scholar]
SHEP 1991 {published data only}
- Applegate WB, Davis BR, Black HR, et al. Prevalence of postural hypotension at baseline in the systolic hypertension in the elderly program (SHEP) cohort. Journal of American Geriatric Society 1991;39:1057‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bearden D. Allman R. McDonald R, et al. Age, race, and gender variation in the utilization of coronary artery bypass surgery and angioplasty in SHEP. Journal of the American Geriatric Society 1994;42:1143‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Black HR. Unger D. Burlando A, et al. Part 6: Baseline physical examination findings. Hypertension 1991;17(3 (Suppl II)):II‐77‐II‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Borhani NO. Applegate WB. Cutler JA, et al. Part 1: rationale and design. Hypertension 1991;17(3 (Suppl II)):II‐2‐II‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brittain E. Palensky J. Blood J and Wittes J. Blinded subjective rankings as a method of assessing treatment effect: a large sample example from the systolic hypertension in the elderly program (SHEP). Statistics in Medicine 1997;16:681‐693. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curb JD. Lee M. Jensen J, Applegate W. Part 4: baseline medical history findings. Hypertension 1991;17(3 (Suppl II)):II‐35‐II‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curb JD. Pressel SL. Cutler JA, et al. Effect of diuretic based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA 1996;276:1886‐92. [MEDLINE: ] [PubMed] [Google Scholar]
- Davis BR. Wittes J. Pressel S, et al. Statistical considerations in monitoring the systolic hypertension in the elderly program (SHEP). Controlled Clinical Trials 1993;14:350‐361. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Franse LV. Pahor M. Di Bari M, et al. serum uric acid, diuretic treatment and risk of cardiovascular events in the systolic hypertension in the elderly program. Journal of Hypertension 2000;18:1149‐54. [Accession number Current contents 343FE‐0021] [DOI] [PubMed] [Google Scholar]
- Frost PH. Davis BR. Burlando AJ, et al. Serum lipids and incidence of coronary heart disease. Findings from the systolic hypertension in the elderly program. Circulation 1996;94:2381‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hall WD. Davis BR. Frost P, et al. Part 7: Baseline laboratory characteristics. Hypertension 1991;17(3 (Suppl II)):II‐102‐II‐122. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hawkins CM. Isolated systolic hypertension, morbidity and mortality: The SHEP experience. American Journal of Geriatric Cardiology 1993;September/October:25‐27. [Accession number 1993303455] [PubMed] [Google Scholar]
- Kostis JB. Allen R. Berkson DM, et al. Correlates of ventricular ectopic activity in isolated systolic hypertension. American Heart Journal 1994;127:112‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kostis JB. Davis BR. Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997;278(3):212‐216. [MEDLINE: ] [PubMed] [Google Scholar]
- Kostis JB. Lacy CR. Hall D, et al. The effect of chlorthalidone on ventricular ectopic activity in patients with isolated systolic hypertension. American Journal of Cardiology 1994;74:464‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kostis JB. Prineas R. Curb JD, et al. Part 8: Electrocardiographic characteristics. Hypertension 1991;17(3 (Suppl II)):II‐123‐II‐151. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menard J. Day M. Chatellier G, Laragh JH. Some lessons from systolic hypertension in the elderly program (SHEP). American Journal of Hypertension 1992;5:325‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Newman AB. Tyrrell KS, Kuller LH. Mortality over four years in SHEP participants with a low ankle‐arm index. Journal of American Geriatric Society 1997;45:1472‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perry HM. Davis BR. Price TR, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke. JAMA 2000;284(4):465‐471. [Accession number Current contents 335QB‐0033] [DOI] [PubMed] [Google Scholar]
- Petrovich H. Byington R. Bailey G, et al. Part 2: screening and recruitment. Hypertension 1991;17 (Suppl II)(3):II‐17‐II‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Probstfield JL. Applegate WB. Borhani NO, et al. The systolic hypertension in the elderly program (SHEP): an intervention trial on isolated systolic hypertension. Clinc. and Exper. Hyper.‐Theory and Practice 1989;A11(5 & 6):973‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- SHEP cooperative Research Group. Rationale and design of a randomized clinical trial on prevention of stroke in isolated systolic hypertension. Journal of Clinical Epidemiology 1988;41(12):1197‐1208. [Accession number 1989036523] [DOI] [PubMed] [Google Scholar]
- SHEP cooperative research group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the SHEP. ACP Journal Club 1991;115(3):65. [Accession number 1992021057] [PubMed] [Google Scholar]
- SHEP cooperative research group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program. JAMA 1991;265:3255‐3264. [MEDLINE: ] [PubMed] [Google Scholar]
- Savage PJ. Pressel SL. Curb D, et al. Influence of long‐term, low‐dose, diuretic‐based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension. Archives of Internal Medicine 1998;158:741‐751. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vogt TM. Schron E. Pressel S, et al. Part 3: Sociodemographic characteristics. Hypertension 1991;17(3 (Suppl II)):II‐24‐II‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wassertheil S. Applegate WB. Berge K, et al. Change in depression as a precursor of cardiovascular events. Archives of Internal Medicine 1996;156:553‐561. [MEDLINE: ] [PubMed] [Google Scholar]
- Wasserthell‐Smoller S. FannC. Allman RM, et al. Relation of low body mass to death and stroke in the systolic hypertension in the elderly program. Archives of Internal Medicine 2000;160:494‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weiler PG. Camel GH. Chiappini M, et al. Part 9: Behavorial characteristics. Hypertension 1991;17(3 (Suppl II)):II‐152‐II‐161. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wittes J. Davis B. Berge K, et al. Part 10: Analysis. Hypertension 1991;17(3 (Suppl II)):II‐162‐II‐167. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SHEP‐P 1989 {published data only}
- Black DM. Brand RJ. Greenlick M, et al. Compliance to treatment for hypertension in elderly patients: The SHEP pilot study. Journal of Gerontology 1987;42(5):552‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furberg CD. Black DM. for the SHEP research group. The systolic hypertension in the elderly program: Methodological issues. European Heart Journal 1988;9:223‐227. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hulley SB. Feigal D. Ireland C, et al. Systolic hypertension in the elderly program (SHEP). Journal of the American Geriatric Society 1986;34(2):101‐105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hulley SB. Furberg CD. Gurland B. McDonald R, et al. Systolic Hypertension in the Elderly (SHEP): antihypertensive efficacy of chlorthalidone. The American Journal of cardiology 1985;56(15):913‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perry HM, et al. Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) Pilot study. Stroke 1989;20:4‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perry Jr HM. McDonald RH. Hulley SB, et al. Systolic hypertension in the elderly program, pilot study (SHEP‐PS): morbidity and mortality experience. Journal of Hypertension 1986;4(Suppl 6):S21‐S23. [MEDLINE: ] [PubMed] [Google Scholar]
- Vogt TM. Ireland CC. Black D, et al. Recruitment of elderly volunteers for a multicenter clinical trial: The SHEP pilot study. Controlled Clinical Trials 1986;7:118‐133. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vogt TM. Ireland CC. Greenlick MR, Hughes GH. Relation of life events to blood pressure control in the SHEP trial. American Journal of Preventive Medicine 1988;4:1‐4. [MEDLINE: ] [PubMed] [Google Scholar]
SYST‐EUR 1997 {published data only}
- Amery A. Birkenhager W. Bulpitt CJ, et al. Syst‐Eur. A multicenter trial on the treatment of isolated systolic hypertension in the elderly: objectives, protocol and organization. Aging 1991;3(3):287‐302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Birkenhager WH. Staessen J. Gasowski J, Leeuw PW. Effects of antihypertensive treatment on endpoints in the diabetic patients randomized in the systolic hypertension in Europe (Syst‐Eur) trial. Journal of Nephrology 2000;13:232‐237. [Accession number 331TE‐ 0011] [PubMed] [Google Scholar]
- Gasowski J. Birkenhager WH. Staessen J, Leeuw PW. Benefit of antihypertensive treatment in diabetic patients enrolled in the systolic hypertension in Europe (Sys‐Eur) trial. Cardiovascular Drugs and Therapy 2000;14:49‐53. [Accession number 281MJ‐ 0006] [DOI] [PubMed] [Google Scholar]
- Girerd X, Amery A, Birkenhager W, Bulpitt C, Cox J, Leeuw P. SYST‐EUR: a multicenter trial of treatment of systolic hypertension in aged subjects. An initial report (French). Archives des Maladies du Coeur et des Vaisseaux 1992;85(8):1243‐7. [PubMed] [Google Scholar]
- Slovick D, Staessen J, Bert P, Bulpitt C, Cort P, Fagard R, et al. Nitrendipine in older patients with isolated systolic hypertension: second progress report on the Syst‐Eur trial. J Hum Hypertens 1993;7(4):411‐2. [PubMed] [Google Scholar]
- Slovick DI, Amery A, Birkenhager W, Bulpitt CJ, Cox J, Leeuw P, et al. Syst‐Eur multicenter trial on the treatment of isolated hypertension in the elderly: first interim report. J Hum Hypertens 1993;7(2):201‐3. [PubMed] [Google Scholar]
- Staessen J. Dekempeneer L. Fagard R, et al. Treatment of isolated systolic hypertension. Journal of Cardiovascular Pharmacology 1991;18(Suppl 1):S34‐S40. [MEDLINE: ] [PubMed] [Google Scholar]
- Staessen JA. Amery A. Birkenhager W, et al. Syst‐Eur‐ a multicenter trial on treatment of isolated systolic hypertension in the elderly: first interim report. Journal of Cardiovascular Pharmacology 1992;19:120‐125. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staessen JA. Fagard R. Thijs L. Celis H. Arabidze GG, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757‐764. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staessen JA. Fagard R. Thijs L, et al. Subgroup and per protocol analysis of the randomized European trial on isolated systolic hypertension in the elderly. Archives of Internal medicine 1998;158:1681‐1691. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TEST 1995 {published data only}
- Eriksson S, Olofsson BO, Wesley PO, for the TEST study group. Atenolol for secondary prevention after stroke. Cerebrovasc Dis 1995;5:21‐25. [Google Scholar]
UKPDS 39 1998 {published data only}
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. Brit Med J 1998;317:713‐720. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Brit Med J 1998;317:703‐713. [PMC free article] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study Group. UK prospective diabetes study VIII: study design, progress and performance. Diabetologia 1991;34:877‐90. [PubMed] [Google Scholar]
USPHSHCSG 1977 {published data only}
- Smith WM, Bouchard RJ, Bromer L, et al. Morbidity and mortality in mild essential hypertension. Circ Res 1972;30/31(Suppl II):110‐20. [PubMed] [Google Scholar]
- Smith WM, Johnson WP, Bromer L. Intervention trial in mild hypertension,U.S. Public Health Service Hospitals Cooperative Study Group. Intervention trial in mild hypertension, U.S. Public Health Service Hospitals Cooperative Study Group. Miami, Symposia Specialists: In Epidemiology and Control of Hypertension, edited by OPaul, 1975:461‐83. [Google Scholar]
- US. public health service hospital cooperative study group (USPHSHCSG). Treatment of mild hypertension. Results of a ten‐year intervention trial. Circ Res 1977;40(Suppl 1):98‐105. [MEDLINE: ] [PubMed] [Google Scholar]
VA‐I 1967 {published data only}
- Freis ED. Antihypertensive Therapy; Principles and Practice, an International Symposium. In: Gross F editor(s). Antihypertensive Therapy; Principles and Practice, an International Symposium. New York: Springer‐Verlag, 1966:345‐54. [Google Scholar]
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. A double‐blind control study of antihypertensive agents. III. Chlorothiazide alone and in combination with other agents: preliminary results. Arch Intern Med 1962;110:230‐5. [No unique identifier] [Google Scholar]
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967;202:1028‐1034. [MEDLINE: ] [PubMed] [Google Scholar]
VA‐II 1970 {published data only}
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA 1970;213:1143‐1152. [MEDLINE: ] [PubMed] [Google Scholar]
VA‐NHLBI 1978 {published data only}
- Perry Jr HM, et al. Evaluation of drug treatment in mild hypertension: VA‐NHLBI feasibility trial. Ann N Y Acad Sci 1978;304:267‐288. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perry Jr HM, et al. Treatment of mild hypertension. Preliminary results of a two year feasibility trial. Circ Res 1977;40 suppl:1180‐187. [MEDLINE: ] [PubMed] [Google Scholar]
Wolff 1966 {published data only}
- Wolff FW, Lindeman RD. Effects of treatment in hypertension. Results of a controlled study. J Chron Dis 1966;19:227‐240. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTIVE I 2011 {published data only}
- The Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE I)Investigators. Irbesartan in patients ACTIVE 1 with atrial fibrillation. N Engl J Med 2011;364:928‐33. [Google Scholar]
ADVANCE 2007 {published data only}
- Patel A, ADVANCE Collaborative Group, MacMahon S, Chalmers J, Neal B, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829‐40. [DOI] [PubMed] [Google Scholar]
ALLHAT 2000 {published data only}
- ALLHAT Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2000;283:1967‐75. [MEDLINE: ] [PubMed] [Google Scholar]
- Davis BR. Cutler JA. Gordon DJ. et al for the ALLHAT Research Group. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to prevent Heart Attack Trial (ALLHAT). Am J Hypertens 1996;9:342‐360. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
BENEDICT 2004 {published data only}
- Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene‐Iordache B, Gaspari F, Perna A, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004;351:1941‐51. [DOI] [PubMed] [Google Scholar]
BENEDICT A 2006 {published data only}
- Parving H, Lehnert H, Brochner ‐ Mortensen J, Gomis R, et al. The effect of irbesartan on teh development of diabetic nephropathy in patients with type 2 diabetes. NEJM 2001;345:870‐8. [DOI] [PubMed] [Google Scholar]
Berglund 1981 {published data only}
- Berglund G. Andersson O. Beta‐blockers of diuretics in hypertension? A six year follow‐up of blood pressure and metabolic side effects. Lancet 1981;I:744‐747. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CASTEL 1994 {published data only}
- Casigila E. Spolaore P. Mazza A. Ginocchio G, et al. Effect of two different therapeutic approaches on total and cardiovascular mortality in a Cardiovascular Study in the Elderly (CASTEL). Jpn Heart J 1994;35(5):589‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coope 1986 {published data only}
- Coope J. Randomised trial of treatment of hypertension in elderly patients in primary care. Brit Med J 1986;293:1145‐1151. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DIABHYCAR 2004 {published data only}
- Marre M, Lievre M, Chatellier G, Mann JF, Passa P, Ménard J, DIABHYCAR Study Investigators. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ 2004;328:495‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
DREAM 2006 {published data only}
- DREAM trial investigators. Effect of ramipril on the incidence of diabetes. The DREAM trial investigators. N Engl J Med 2006;355:1551‐62. [DOI] [PubMed] [Google Scholar]
EUROPA 2003 {published data only}
- Fox KM, for the EURopean trial On reduction of‐cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of‐ cardiovascular events among patients with stable coronary artery disease: Randomised, double‐blind, placebo‐controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782‐8. [DOI] [PubMed] [Google Scholar]
Fuchs 2011 {published data only}
- Fuchs FD, Fuchs SC, Moreira LB, Gus M, Nobrega AC, et al. Prevention of hypertension in patients with pre‐hypertension: protocol for the PREVER‐prevention trial. Trials 2011;12(65):1‐7. [DOI: 10.1186/1745‐6215‐12‐65] [DOI] [PMC free article] [PubMed] [Google Scholar]
GENERIC 2010 {published data only}
- Punzi HA, Lewin AJ, Lukic T, Goodin T, Chen W. Efficacy and safety of nebivolol monotherapy in Hispanics with Stage I‐II Hypertension (Abstract). Journal of clinical hypertension 2010;12:536. [DOI] [PubMed] [Google Scholar]
GENRES 2007 {published data only}
- Hitunen TP, Suonsyrja T, Hannila‐Handelberg T, Paavonen KJ, et al. Predictors of antihypertensive drug responses: initial data from a placebo‐controlled , randomized, cross over study with four antihypertensive drugs. Journal of Hypertension 2009;27:2001‐9. [DOI] [PubMed] [Google Scholar]
GLANT 1995 {published data only}
- Study Group on Long‐term Antihypertensive Therapy. A 12‐month comparison of ACE inhibitor and CA antagonist therapy in mild to moderate essential hypertension‐ the GLANT study. Hypertens Res 1995;18:235‐244. [MEDLINE: ] [PubMed] [Google Scholar]
HAPPHY 1987 {published data only}
- Wilhelmsen L. Berglund B. Elmfeldt D. Fitzsimons T, et al. Beta‐blockers versus diuretics in hypertensive men: Main results from the HAPPHY trial. J Hypertens 1987;5:561‐572. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HDFP 1984 {published data only}
- HDFP. Effect of stepped care treatment on the incidence of myocardial infarction and angina pectoris. 5‐year findings of the hypertension detection and follow‐up program. Hypertension 1984;6(Suppl 1):198‐206. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hood 2007 {published data only}
- Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The Spironolactone, Amiloride, Losartan, and Thiazide (SALT) Double‐Blind Crossover Trial in Patients With Low‐Renin Hypertension and Elevated Aldosterone‐Renin Ratio. Circulation 2007;116:268‐75. [DOI] [PubMed] [Google Scholar]
HOPE 3 2016 {published data only}
- Lonn EM, Bosch J, Lopez‐ Jaramillo P, Zhu J, et al. Blood‐Pressure Lowering in Intermediate‐Risk Persons without Cardiovascular Disease. N Engl J Med 2016;374:2009‐20. [DOI] [PubMed] [Google Scholar]
HOT 1995 {published data only}
- Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study: 12‐month Data on Blood Pressure and Tolerability. With Special Reference to Age and Gender. Blood Pressure 1995;4(5):313‐19. [DOI] [PubMed] [Google Scholar]
IDM 2001 {published data only}
- Parving HH, Lehnert H, Brochner‐Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. NEJM 2001;345:870‐878. [DOI] [PubMed] [Google Scholar]
IDNT 2003 {published data only}
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Parikh CR, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ, Irbesartan Diabetic Nephropathy Trial. Collaborative Study Group. Cardiovascular outcomes in the Irbesartan diabetic nephropathy trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med 2003;138:542‐9. [DOI] [PubMed] [Google Scholar]
IMAGINE 2008 {published data only}
- Rouleau JL, Warnica WJ, Baillot R, Block PJ, Chocron S, Johnstone D, Myers MG, Calciu CD, Dalle‐Ave S, Martineau P, Mormont C, Gilst WH, IMAGINE (Ischemia Management with Accupril post‐bypass Graft via Inhibition of the coNverting Enzyme) Investigators. Effects of angiotensin converting enzyme inhibition in low‐risk patients early after coronary artery bypass surgery. Circulation 2008;117:24‐31. [DOI] [PubMed] [Google Scholar]
Imai 2011 {published data only}
- Imai E, Chan J, Ito S, Yamasaki T, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo‐controlled study. Diabetologia 2011;54:2978‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
INSIGHT 1996 {published data only}
- Brown MJ. Castaigne A. De Leeuw PW. Mancia G, et al. INSIGHT: International Nifedipine GITS study: Intervention as a goal in hypertension treatment. J Hum Hypertens 1996;10((Suppl3)):S157‐160. [MEDLINE: ] [PubMed] [Google Scholar]
IPPPSH 1985 {published data only}
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta‐blocker, oxprenolol: The international prospective primary prevention study in hypertension (IPPPSH). J Hypertens 1985;3:379‐392. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kondo 2003 {published data only}
- Kondo J, Sone T, Tsuboi H, Mukawa H, Morishima I, Uesugi M, Kono T, Kosaka T, Yoshida T, Numaguchi Y, Matsui H, Murohara T, Okumura K. Effects of low‐dose angiotensin II receptor blocker candesartan on cardiovascular events in patients with coronary artery disease. Am Heart J 2003;146:E20‐25. [DOI] [PubMed] [Google Scholar]
Kuramoto‐2 1994 {published data only}
- Kuramoto K. Treatment of the elderly hypertensives in Japan: National Intervention Cooperative Study in Elderly Hypertensives. The National Intervention Cooperative study Group. J Hypertens 1994;12(6):S35‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Lewis 1993 {published data only}
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456‐62. [DOI] [PubMed] [Google Scholar]
Lewis 2001 {published data only}
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to Type 2 Diabetes. NEJM 2001;345:851‐860. [DOI] [PubMed] [Google Scholar]
MacMahon 2000 {published data only}
- MacMahon S, Sharpe N, Gamble G, Clague A, Mhurchu CN, Clark T, et al. PART‐2 Collaborative Research Group. Randomized, placebo‐controlled trial of the angiotensin‐converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. J Am Coll Cardiol 2000;36:438‐43. [DOI] [PubMed] [Google Scholar]
MAPHY 1988 {published data only}
- Wikstrand J. Warnold I. Olsson G, et al. Primary prevention with metoprolol in patients with hypertension: mortality results from the MAPHY study. JAMA 1988;259:1976‐1982. [MEDLINE: ] [PubMed] [Google Scholar]
- Wikstrand J, et al. Metoprolol versus thiazide diuretics in hypertension. Morbidity results from the MAPHYstudy Hypertension 1991;17:579‐588. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Materson 1997 {published data only}
- Materson BJ. Reda DJ. Cushman WC. Massie BM, et al. Single‐drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo. NEJM 1997;328:914‐921. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MIDAS 1996 {published data only}
- Borhani NO. Mercuri M. Borhani PA. Buckalew VM. Canossa‐Terris M, et al. Final outcome results of the multicenter isradipine diuretic atherosclerosis study (MIDAS) A randomised controlled trial. JAMA 1996;276:785‐791. [MEDLINE: ] [PubMed] [Google Scholar]
Morgan 1980 {published data only}
- Morgan TO. Adams WR. Hodgson M, Gibberd RW. Failure of therapy to improve prognosis in elderly males with hypertension. Med J Aust 1980;2(1):27‐31. [MEDLINE: ] [PubMed] [Google Scholar]
NAVIGATOR 2010 {published data only}
- McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477‐90. [DOI] [PubMed] [Google Scholar]
NICOLE 2003 {published data only}
- Dens JA, Desmet WJ, Coussement P, Scheerder KD, et al. Long term effects of nisoldipine on the progression of coronary atherosclerosis and the occurrence of clinical events: the NICOLE study. Heart 2003;89:887‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
NORDIL 2000 {published data only}
- Hansson L. Hedner T. Lund‐Johnson P. Kjeldsen SE, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta‐blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Dilitazem (NORDIL) study. Lancet 2000;356(9227):359‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PEACE 2004 {published data only}
- Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, Pfeffer MA, Rice MM, Rosenberg YD, Rouleau JL, PEACE Trial Investigators. Angiotensin converting‐ enzyme inhibition in stable coronary artery disease. N Engl J Med 2004;351:2058‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pool 2007 {published data only}
- Pool JL, Glazer R, Weinberger M, et al. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25mg versus monotherapy: a double‐blind, placebo‐controlled study followed by long‐term combination therapy in hypertensive adults. Clin Ther 2007;29(1):61‐73. [DOI] [PubMed] [Google Scholar]
PRoFESS 2008 {published data only}
- Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, etal. PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008;359:1225‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
PROGRESS 2001 {published data only}
- PROGRESS Collaborative Group. Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke or transient ischemic attack.. Lancet 2001;358:1033‐41. [DOI] [PubMed] [Google Scholar]
QUIET 2001 {published data only}
- Pitt B, O'Neill B, Feldman R, Ferrari R, Schwartz L, Judra H, Bass T, et al. The QUinapril Ischemic Event Trial (QUIET): Evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol 2001;87:1058‐1063. [DOI] [PubMed] [Google Scholar]
REIN 1997 {published data only}
- GISEN Group. Randomised placebo‐controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non‐diabetic nephropathy [Gruppo Italiano di Studi Epidemiologici in Nefrologia]. Lancet 1997;349:1857‐63. [PubMed] [Google Scholar]
RENAAL 2001 {published data only}
- Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861‐9. [DOI] [PubMed] [Google Scholar]
ROAD 2007 {published data only}
- Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, Guo ZJ, Jiang JP. Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 2007;18:1889‐98. [DOI] [PubMed] [Google Scholar]
ROADMAP 2011 {published data only}
- Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, et al. ROADMAP Trial Investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907‐17. [DOI] [PubMed] [Google Scholar]
SCAST 2015 {published data only}
- Jusufovic M, Sandset EC, Bath PM, Karlson BW, Berge E. Scandinavian Candesartan Acute Stroke Trial Study Group. Effects of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis. International Journal of Stroke 2015;10:354‐9. [DOI] [PubMed] [Google Scholar]
SCAT 2000 {published data only}
- Teo KK, Burton JR, Buller CE, Plante S, Catellier D, Tymchak W, Dzavik V, Taylor D, Yokoyama S, Montague TJ. Long‐term effects of cholesterol lowering and angiotensin converting enzyme inhibition on coronary atherosclerosis.. Circulation 2000;102:1748‐54. [DOI] [PubMed] [Google Scholar]
Schmieder 2012 {published data only}
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, et al. Long‐term antihypertensive efficacy and safety of the oral direct renin Inhibitor aliskiren. A 12‐month randomized, double‐blind comparator trial with hydrochlorothiazide. Circulation 2009;119:417‐25. [DOI] [PubMed] [Google Scholar]
SCOPE 2003 {published data only}
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double‐blind intervention trial. J Hypertension 2003;21:875‐886. [DOI] [PubMed] [Google Scholar]
SHELL 1995 {published data only}
- Zanchetti A. Evaluating the benefits of an antihypertensive agent using trials based on event and organ damage: the systolic Hypertension in the Elderly Long‐term Lacidipine (SHELL) trial and European Lacidipine Study on Atherosclerosis (ELSA). J of Hypertension‐ supplement 1995;13(4):S35‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sprackling 1981 {published data only}
- Sprackling ME, Mitchell JRA, Short AH, Watt G. Blood pressure reduction in the elderly: a randomised controlled trial of methyldopa. BMJ 1981;283:1151‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
STONE 1996 {published data only}
- Gong L. Zwang W. Zhu Y, et al. Shanghai Trial of Nifedipine in the Elderly (STONE) Study. J Hypertens 1996;14:1237‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STOP 1991 {published data only}
- Dahlof B, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOPHypertension). Lancet 1991;338:1281‐1285. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STOP‐2 1993 {published data only}
- Dahlof B. Hansson L. Lindholm LH, et al. STOP ‐ Hypertension 2: A prospective intervention trial of "newer" vs "older" treatment alternatives in old patients with hypertension. Blood Pressure 1993;2:136‐141. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strandberg 1991 {published data only}
- Strandberg E, et al. Long‐term mortality after 5‐year multifactorial primary prevention of cardiovascular diseases in middle‐aged men. JAMA 1991;266:1255‐1259. [MEDLINE: ] [PubMed] [Google Scholar]
SYST‐CHINA 1993 {published data only}
- Anonymous. [Systolic hypertension in the elderly: Chinese trial (Syst‐China)‐‐second interim report]. [Chinese] Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 1993;21(3):135‐7,185. [Accession number 1993350344] [PubMed] [Google Scholar]
TOMHS 1993 {published data only}
- Neaton JD. Brimm RH. Prineas RJ, et al. Treatment of mild hypertension study. Final results. JAMA 1993;270:713‐724. [MEDLINE: ] [PubMed] [Google Scholar]
TRANSCEND 2008 {published data only}
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators, Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P. Effects of the angiotensin‐receptor blocker telmisartan on cardiovascular events in high‐risk patients intolerant to angiotensin converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372:1174‐83. [DOI] [PubMed] [Google Scholar]
VACS 1982 {published data only}
- Veterans Administrative Cooperative Study Group on Antihypertensive Agents. Comparison of propanolol and hydrochlorothiazide for the initial treatment of hypertension. II Results of long term therapy. JAMA 1982;248:2004‐2011. [MEDLINE: ] [PubMed] [Google Scholar]
VHAS 1997 {published data only}
- Agabiti RE, Dal Palu D, Leonetti G, Magnani G, Pessina A, Zanchetti A. Clinical results of the verapamil in hypertension and atherosclerosis study.. J Hypertens 1997;15:1337‐1344. [DOI] [PubMed] [Google Scholar]
White 1995 {published data only}
- White WB, Stimpel M. Long‐term safety and efficacy of moexipril alone and in combination with hydrochlorothiazide in elderly patients with hypertension. J Hum Hypertension 1995;9(11):879‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
BBLTTC 2005
- Turnbull F, Neal B, Albert C, Chalmers J, Chapman N, Cutler J, Woodward M, MacMahon S: Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials.. Arch Intern Med 2005;165(12):1410‐1419. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boissel 2005
- Boissel JP, Gueyffier F, Boutitie F, Pocock S, Fagard R. Apparent effect on blood pressure is only partly responsible for the risk reduction due to antihypertensive treatments.. Fund Clin Pharmacol 2005;19:579‐584. [DOI] [PubMed] [Google Scholar]
BPLTTC 2000
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood pressure‐lowering drugs: results of prospectively designed overviews of randomised trials. Lancet 2000;355:1955‐64. [DOI] [PubMed] [Google Scholar]
Chen 2010
- Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, Wang Y, Yang X, He L. Calcium channel blocker versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD003654.pub4] [DOI] [PubMed] [Google Scholar]
Collins 1990
- Collins, R, Peto, R, Macmahon, S, Hebert, P, Fiebach, N.H, Eberlein, k.A, Godwin, J, Qizilbash, N, Taylor, J.O, Hennekens, C.H. Blood pressure, stroke, and coronary heart disease‐ Part 2. Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diao 2012
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD006742.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duarte 2010
- Duarte JD, Cooper‐DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide‐like diuretics. Expert Rev Cardiovasc Ther. 2010;8(6):793‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frishman 2005
- Frishman W.H, Cheng‐Lai A, Nawarskas J. Current Cardiovascular Drugs. Fourth. Current Science Group, 2005:153. [Google Scholar]
Goeres 2014
- Goeres LM, Eckstrom E, Lee DS. Pharmacotherapy for Hypertension in Older Adults: A Systematic Review. Drugs Aging 2014;31:897‐910. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gueyffier 1996
- Gueyffier F. Froment A, Gouton M. New meta‐analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. J Human Hyperten 1996;10:1‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Gueyffier 1999
- Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, et al. for the INDANA Group. Antihypertensive drugs in very old people: a subgroup meta‐analysis of randomised controlled trials. Lancet 1999;353:793‐96. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org, 2011. [Google Scholar]
Hughes 2004
- Hughes AD. "How do thiazide and thiazide‐like diuretics lower blood pressure?". J Renin Angiotensin Aldosterone Syst 2004;5(4):155‐60. [DOI] [PubMed] [Google Scholar]
Insua 1994
- Insua JT. Sacks HS. Lau TS. Lau J, et al. Drug treatment of hypertension in the elderly: A meta‐analysis. Ann Intern Med 1994;121:355‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kang 2004
- Kang S, Wu FY, An N, Ren M. A Systematic Review and Meta‐Analysis of the Efficacy and Safety of a Fixed,Low‐Dose Perindopril‐Indapamide Combination as First‐Line Treatment of Hypertension. Clinical Therapeutics 2004;26(2):257‐70. [DOI] [PubMed] [Google Scholar]
Kızılırmak 2017
- Kızılırmak P, Uresin Y, Ozdemir O, Kilickiran B, et al. Renin‐angiotensin‐aldosterone system blockers and cardiovascular outcomes: a meta‐analysis of randomized clinical trials [Renin‐anjiyotensin‐aldosteron sistemi blokerleri ve kardiyovasküler sonuçları: Randomize klinik çalışmaların meta‐analizi]. Turk Kardiyol Dern Ars 2017;45(1):49‐66. [DOI: 10.5543/tkda.2016.78006] [DOI] [PubMed] [Google Scholar]
Law 2009
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacMahon 1993
- MacMahon S, Rogers A. The effects of blood pressure reduction in older patients: an overview of five randomized controlled trials in elderly hypertensives. Clin Exper Hypertension 1993;15(6):967‐78. [Accession number 1993344587] [DOI] [PubMed] [Google Scholar]
Mulrow 1994
- Mulrow CD, Cornell JA, Herrera CR, Kadri A, et al. Hypertension in the elderly: Implications and generalizability of randomized trials. JAMA 1994;272:1932‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Mulrow 1998
- Mulrow C, Lau J, Cornell J, Brand M. Pharmacotherapy for hypertension in the elderly. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000028] [DOI] [PubMed] [Google Scholar]
Musini 2009
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy of hypertension in the elderly. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000028.pub2] [DOI] [PubMed] [Google Scholar]
Musini 2017
- Musini VM, Gueyffier F, Puil L, Salzwedel DM, Wright JM. Pharmacotherapy for hypertension in adults aged 18 to 59 years. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD008276.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikolaus 2000
- Nikolaus T, Sommer N, Becker C. Treatment of arterial hypertension with diuretics, beta‐ and calcium channel blockers in old patients. Z Gerontol Geriat 2000;33:427‐32. [DOI] [PubMed] [Google Scholar]
Parsons 2016
- Parsons C, Murad MH, Andersen S, Mookadam F, Labonte H. The effect of antihypertensive treatment on teh incidence of stroke and cognitive decline in the elderly: a meta‐analysis. Future Cardiology 2016;12(2):237‐48. [DOI] [PubMed] [Google Scholar]
Pearce 1995
- Pearce KA, Furberg CD, Rushing J. Does Antihypertensive Treatment of the Elderly Prevent Cardiovascular Events or Prolong Life? A Meta‐analysis ofHypertension Treatment Trials. Archives of Family Medicine 1995;4:943‐50. [DOI] [PubMed] [Google Scholar]
Psaty 1997
- Psaty BM, Smith NL, Siscovick DS, Koepsell TD, et al. Health outcomes associated with antihypertensive therapies used as first‐line agents. A systematic review and meta‐analysis.. JAMA 1997;277:739‐745. [MEDLINE: ] [PubMed] [Google Scholar]
Psaty 2003
- Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first‐line agents: A network meta‐analysis. JAMA 2003;289:2534‐2544. [DOI] [PubMed] [Google Scholar]
Puttnam 2017
- Puttnam J, Davis BR, Pressel SL, Whelton PK, Cushman WC, Louis GT, Margolis KL, Oparil S, Williamson J, Ghosh A, Einhorn PT and, Barzilay JI. Association of 3 different antihypertensive medications with hip and pelvic fracture risk in older adults. Secondary analysis of a randomized clinical trial. JAMA Internal Medicine 2017;177(1):67‐76. [DOI] [PubMed] [Google Scholar]
Quan 1999
- Quan A, Kerlikowske K, Gueyffier F, Boissel JP and INDANA investigators. Efficacy of Treating Hypertension in Women. J General Internal Medicine 1999;14:718‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinhart 2011
- Reinhart M, Musini VM, Salzwedel DM, Dormuth C, Wright JM. First‐line diuretics versus other classes of antihypertensive drugs for hypertension. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008161.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sundstro¨m 2015
- Sundstro¨m J, Arima H, Jackson R, Turnbull F, et al. Effects of Blood Pressure Reduction in Mild Hypertension. A Systematic Review and Meta‐analysis. Annals of Internal Medicine 2015;162:184‐191. [DOI] [PubMed] [Google Scholar]
Tan 2016
- Tan Xue‐ Ying, Hu Jing‐Bo. ACEIs/ARBs for the prevention of type 2 diabetes in patients with cardiovascular diseases: a systematic review and meta‐analysis. Int J Clin Exp Med 2016;9(5):7624‐37. [Google Scholar]
Taverny 2016
- Taverny G, Mimouni Y, LeDigarcher A, Chevalier P, Thijs L, Wright JM, Gueyffier F. Antihypertensive pharmacotherapy for prevention of sudden cardiac death in hypertensive individuals. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD011745.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thijs 1992
- Thijs L. Fagard R. Lijnen P. Staessen J, et al. A meta‐analysis of outcome trials in elderly hypertensives. Journal of Hypertension 1992;10:1103‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomopoulos 2014
- Thomopoulos C, Parati G, Zanchetti. A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. Journal of Hypertension 2014;32(12):2285‐95. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2016
- Thomopoulos, C, Parati, G, Zanchetti, A. Effects of blood pressure‐lowering treatment. 6. Prevention of heart failure and new‐onset heart failure ‐ Meta‐analyses of randomized trials. Journal of Hypertension March 2016;34(3):373‐84. [DOI] [PubMed] [Google Scholar]
Turnbull 2003
- Turnbull F. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003;362:1527‐35. [DOI] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge, Charles S. Bradley, Hazel A. Volmink, Jimmy. Mayosi, Bongani M. Opie, Lionel H. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002003.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xue 2015
- Xue H, Lu Z, Tang WL, Pang LW, Wang GM, Wong GWK, Wright JM. First‐line drugs inhibiting the renin angiotensin system versus other first‐line antihypertensive drug classes for hypertension. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008170.pub2] [DOI] [PubMed] [Google Scholar]
Zanchetti 2015
- Zanchetti, A, Thomopoulos, C, Parati, G. Randomized Controlled Trials of Blood Pressure Lowering in Hypertension: A Critical Reappraisal. Circulation Research 2015;116(6):1058‐73. [DOI] [PubMed] [Google Scholar]
Zhu 2005
- Zhu Z, Zhu S, Liu D, Cao T, Wang L, Tepel M. Thiazide‐like diuretics attenuate agonist‐induced vasoconstriction by calcium desensitisation linked to Rho kinase". Hypertension 2005;45(2):233‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wright 1999
- Wright JM. Cheng‐Han Lee, Chambers KC. Systematic review of antihypertensive therapies: does the evidence assist in choosing a first‐line drug?. CMAJ 1999;161(1):25‐32. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]


