Abstract
Background
Despite major advances in medical technology, the incidence of preterm birth remains high. The use of antenatal corticosteroid administered transplacentally, by intramuscular injection to women at risk of preterm birth, has reduced the incidence of respiratory distress syndrome and increased the survival rates of preterm infants. However, this intervention also comes with its own risks and side effects. Animal studies and early studies in pregnant women at risk of preterm birth have reported the use of an alternative route of administration, by direct intramuscular injection of corticosteroid into the fetus under ultrasound guidance, in an attempt to minimise the side‐effect profile. Direct fetal corticosteroid administration may have benefits over maternal administration in terms of safety and efficacy.
Objectives
To assess if different routes of corticosteroid administration (maternal versus direct fetal) have effects on health outcomes for women and their babies.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (25 October 2017), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (25 October 2017) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials comparing maternal with direct fetal routes of antenatal corticosteroid administration in women at risk of preterm birth.
Data collection and analysis
The two review authors independently assessed study eligibility. In future updates of this review, at least two review authors will extract data and assess the risks of bias in included studies. We will also assess the quality of the evidence using the GRADE approach.
Main results
We did not identify any eligible randomised controlled trials to include in this review.
Authors' conclusions
The available clinical studies carried out so far on animals and human have shown that direct intramuscular injection of corticosteroid into the fetus under ultrasound guidance is feasible, but data on health outcomes are lacking. Uncertainty therefore persists as to which method could provide better efficacy and safety. Randomised controlled trials are required focusing on the benefits and harms of transplacental versus direct fetal corticosteroid treatment. Until the uncertainties have been addressed, it is advisable to stay with the current standard of antenatal transplacental maternally‐administered corticosteroid treatment.
Plain language summary
Direct injection of corticosteroids into the fetus compared with injection into the mother for improving fetal outcomes when the mother is at risk of preterm birth
What is the issue?
Babies born preterm (before 37 weeks of pregnancy) are at risk of dying, having bleeding into their brain and problems with their breathing because their lungs are not fully developed. Corticosteroid treatment given to the mother before early birth has been shown to be effective in preventing these problems and has become standard care in many countries. The common method of giving corticosteroid is by injecting into the mother's muscles. The corticosteroid treatment then transfers across the placenta (known as transplacental transfer) to the fetus. This treatment has its own risks, such as reducing fetal growth and brain development as well as increasing the baby's risks of diseases such as diabetes and high blood pressure. Injecting corticosteroid directly into the fetus is feasible with ultrasound guidance.
Why is this important?
Injecting corticosteroid directly into the fetus, instead of injecting into the mother's muscles, may prevent the risk of increased blood pressure, increased blood glucose levels, and susceptibility to sepsis in the mother. It may also reduce the amount of corticosteroid needed. However, it carries a risk of infection of the uterus and fetal injury, and may cause preterm labour and birth. We found that there have been no studies assessing the benefits and harms of direct injection into the fetus compared with injection into the mother.
What evidence did we find?
We searched for evidence on 25 October 2017 and we did not find any completed randomised controlled trials that assess the benefits and harms of direct injection of corticosteroid into the fetus compared with injection into the mother, for women who are at risk of preterm birth. We found two studies, but one was not a randomised controlled trial, and in the other study the methods were unclear, so we have contacted the study authors for further information.
What does this mean?
We need further studies to assess the effects of injecting corticosteroid directly to the fetus compared with injecting into the mother's muscles. The babies in these trials need to be followed up over a long period so that we can monitor the effects of corticosteroids on childhood development, including impairments or disabilities such as cerebral palsy. We need good‐quality randomised trials, to establish if one method is better than the other.
Background
Description of the condition
The World Health Organization (WHO) defines preterm birth as birth before 37 weeks of gestation (WHO 2015) and this condition is associated with high neonatal morbidity and mortality. Prematurity is the main cause of perinatal mortality and morbidity in high‐income countries (Evans 1993; Goldenberg 2007). In 2013, preterm birth accounted for 16% of all perinatal mortality in Australia (AIHW 2015). Despite advances in medical technology, the primary cause of early neonatal death in preterm infants remains respiratory distress syndrome (RDS) as a consequence of immature lung development and surfactant insufficiency. With increasing gestational age, organ systems are more mature and this increases the survival rate (Doyle 2001; Saigal 2007). Preterm infants who survive the neonatal period are at increased risk of chronic disability, including but not limited to neurological disability and chronic pulmonary disease (Doyle 2001; Evans 1993). As preterm birth is associated with high mortality and long‐term adverse health impact compared with birth at term, this clearly signifies a prominent clinical as well as economic burden on healthcare resources (Goldenberg 2007; WHO 2015).
Description of the intervention
Single (Roberts 2017) or multiple (Crowther 2015) courses of antenatal corticosteroid in women at risk of preterm birth have been shown to reduce the incidence of RDS, as first described by Liggins 1972. Corticosteroid is a steroid hormone that acts by increasing protein and phospholipid synthesis, increasing levels of surfactant in the fetal lung and accelerating maturation of the fetal lung (Ballard 1995; Evans 1993). In addition to reducing the incidence of RDS, antenatal corticosteroid treatment prior to preterm birth has been shown to reduce the risk of perinatal death, neonatal death, intraventricular haemorrhage, infections, intensive care unit admission and developmental delay in childhood (Roberts 2017). These outcomes also depend on the gestational age of the pregnancy. Antenatal corticosteroids have become the standard of care for women at risk of preterm birth in many countries (Antenatal Corticosteroid Guidelines Panel 2015; Haram 2003; Jobe 2004; NIH 1995; WHO 2015).
However, the short‐ and long‐term safety profile of antenatal corticosteroid treatment is still debatable, especially in multiple corticosteroid administrations. Corticosteroids are known to inhibit cell growth and DNA replication. Animal studies have demonstrated that maternal corticosteroid administration at the minimal effective dose inhibits fetal growth, increases fetal blood pressure and perhaps modifies neurodevelopment (Fowden 1996; Jobe 1998; Moss 2003). The severity increases as the dose and number of administrations increase (Moss 2003). The known short‐term effects of antenatal corticosteroids in the fetus are decreased fetal breathing and movements, and a reduction in the amniotic fluid volume (Babovic 2009; Jackson 2003). Exposure to high levels of cortisol in a normally low fetal cortisol environment, in addition to other stress hormones produced with growth restriction, may have lifelong effects, leading to fetal programming for adult diseases, such as hypertension, insulin resistance, diabetes mellitus and metabolic syndrome (Benediktsson 1993; Dalziel 2005; Newnham 1999). Maternal administration of corticosteroid (orally, intramuscularly or intravascularly) not only affects the fetus but has the potential for negative maternal side effects, such as elevation of maternal blood pressure (Babovic 2009) and blood glucose concentrations, increasing susceptibility to sepsis (Evans 1993).
There remains variation in clinical practice on issues regarding the use of antenatal corticosteroids, including the type of corticosteroid to use, the dose and the optimal route of administration (Brownfoot 2013; Jobe 2004). The common route of administration is intramuscularly to the mother, with transplacental transfer to the fetus (Roberts 2017). Animal studies and early studies in pregnant women at risk of preterm birth have reported the use of an alternative route of administration, by direct intramuscular injection of corticosteroid into the fetus under ultrasound guidance, in an attempt to minimise the side‐effect profile. These early studies indicate that direct fetal administration may be feasible in humans but further research still needs to be conducted (Moss 2003).
How the intervention might work
Direct fetal ultrasound‐guided injection of corticosteroid is believed to induce lung maturation of the preterm fetus, with different side‐effect profiles compared to indirect administration. The most important advantages of direct administration could include the avoidance of maternal toxicity and the metabolic side effects, as well as increasing the efficacy of bypassing the placenta and therefore inducing a more rapid fetal lung maturation (Evans 1993; Jobe 1993).
One of the first few animal studies of direct fetal corticosteroid treatment used ultrasound to deliver direct fetal injection of corticosteroid or saline control to 36 pregnant ewes. The preterm lambs were delivered at 128 days gestational age, to assess the postnatal lung function (Jobe 1993). The authors found that relative to the saline control group, corticosteroid given as a single injection 48 hours before birth resulted in a significant improvement in postnatal lung function (Jobe 1993).
Another animal study comparing direct fetal against maternal administration of corticosteroid showed that administration of repeated doses of corticosteroid directly to the fetus did not produce the growth restriction induced by maternal administration (Newnham 1999). There was no reduction in the fetal birthweight or weights of major organs, with the exception of the liver (Newnham 1999).
The first human study (a single‐arm trial) administered direct intramuscular fetal therapy to six women at risk of preterm birth (Ljubic 1999). In five cases, there was an uneventful outcome of fetuses, indicating that direct fetal corticosteroid treatment improved postnatal lung function in preterm fetuses. Another single‐arm trial involving 41 women at risk of preterm birth found that direct intramuscular fetal corticosteroid administration led to an increase in fetal breathing but no change in fetal movement and growth parameters (Babovic 2009).
In an animal study involving direct corticosteroid administration, higher corticosteroid peak concentrations were found in the fetal circulation compared with maternal intramuscular administration of the same dose (Moss 2003). However, the duration of maternal and fetal exposure to corticosteroid has been shown to be shorter after direct fetal injection (Babovic 2009). These differences in corticosteroid concentration and length of exposure are thought to lead to a reduced risk of growth retardation and other maternal and fetal complications (Moss 2003). Neurodevelopmental outcomes and long‐term effects on the risk of hypertension, diabetes and metabolic syndrome in relation to direct corticosteroid treatment are as yet unknown.
As an invasive procedure, direct fetal administration of corticosteroids carries additional risks which are not present with maternal administration. These risks are likely to be similar to those of amniocentesis, which carries a 1:1000 risk of intrauterine infection and 1:100 risk of pregnancy loss (RCOG 2010). In addition, there are potential risks of fetal injury, initiation of preterm labour (Newnham 1999), preterm prelabour rupture of membranes, placental abruption and maternal‐fetal haemorrhage (Gordon 2002).
Why it is important to do this review
Despite the major advances in medical technology, the incidence of preterm birth remains high. This could be due multiple factors such as increasing rates of multiple pregnancies, greater use of reproductive technologies, increases in maternal age and changes in clinical practice (Beck 2010). Preterm birth has significant impacts on maternal health, neonatal and childhood health, families and the economy (Goldenberg 2007; Saigal 2007). The introduction of the use of antenatal corticosteroid administered by intramuscular injection to women at risk of preterm birth has reduced the incidence of RDS and increased the survival rates of preterm infants. However, this intervention also comes with its own risks and side effects. There remains uncertainty about the type of corticosteroid to use and the optimal method of administration (Brownfoot 2013). Direct fetal corticosteroid administration may have benefits over maternal administration in terms of safety and efficacy. This review assesses the effects of direct corticosteroid administration to the preterm fetus, compared with maternal administration, using the best available evidence.
Objectives
To assess if different routes of corticosteroid administration (maternal versus direct fetal) have effects on health outcomes for women and their babies.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials comparing maternal with direct fetal routes of antenatal corticosteroid administration in women at risk of preterm birth. We planned not to include quasi‐randomised trials, cluster‐randomised trials or cross‐over trials. We intended to consider studies presented only as abstracts.
Types of participants
All women at risk of preterm birth (less than 37 weeks) receiving any antenatal corticosteroid treatment prior to birth, by any maternal transplacental or direct fetal route of administration.
Types of interventions
Transplacental (oral, intramuscular or intravascular) versus direct fetal corticosteroid treatment given to women at risk of preterm birth (less than 37 weeks). We planned to consider any type, dose and regimen of corticosteroid treatment.
Types of outcome measures
Primary outcomes
Maternal
Maternal sepsis (as defined by authors)
Infant
Death (stillborn or death of a live‐born infant prior to primary hospital discharge)
Respiratory distress syndrome
Child
Survival free of any disability (as defined by authors)
Neurodevelopmental impairment (as defined by authors)
Child as an adult
Survival free of cardiometabolic disease
Neurodevelopmental impairment (as defined by authors)
Secondary outcomes
Maternal
Chorioamnionitis
Pyrexia after trial entry requiring the use of antibiotics
Intrapartum pyrexia
Postnatal pyrexia
Intensive care unit admission
Glucose tolerance (as defined by authors)
Hypertension (as defined by authors)
Breastfeeding
Infant
Gestational age at birth
Low Apgar score (less than seven at five minutes)
Intraventricular haemorrhage
Periventricular leukomalacia
Body size (birthweight, head circumference, length and skinfold thickness)
Placental weight
Neonatal blood pressure
Bronchopulmonary dysplasia (chronic lung disease) (as defined by authors)
Necrotising enterocolitis
Admission to neonatal intensive care
Composite of serious infant outcomes (as defined by authors)
Systemic infection in the first 48 hours of life
Hypothalamo‐pituitary‐adrenal axis function (as defined by authors)
Child
Total deaths
Body size measurements (including z scores for weight, height, head circumference and body mass index (BMI))
Asthma/wheeze
Risk factors for cardiovascular disease
Emotional and behavioural problems
Child as an adult
Growth measurements (including weight, head circumference, height, skin fold thickness and BMI)
Age at puberty
Abnormal lung function (including z scores for forced expiratory volume in one second, forced vital capacity and forced expiratory flow at 25% to 75% of forced vital capacity)
Health‐related quality of life
Employment status
Health services
Length of antenatal hospitalisation for women
Length of postnatal hospitalisation for women
Length of neonatal hospitalisation
Cost of maternal care
Cost of neonatal care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (25 October 2017)
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed by the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Excluded studies; Studies awaiting classification).
We also searched the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (25 October 2017). See Appendix 1 for search terms used.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
There are no included studies in this review. SeeAppendix 2 for full methods of data collection and analysis to be used in future updates of this review, as more data become available.
Selection of studies
Two review authors independently assessed for inclusion the potential studies identified by the search strategy. We resolved any disagreement through discussion, or consulted a third person.
Results
Description of studies
Results of the search
In the previous version of this review, we identified only one potential abstract (Ljubic 2000). The Ljubic 2000 study compared the efficacy and safety of transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation. The authors examined effects of single‐dose, ultrasound‐guided fetal administration of 4 mg dexamethasone compared with standard transplacental maternal administration of 24 mg dexamethasone. We contacted the author about the study design and await further information. The study is currently listed under Studies awaiting classification.
The updated search in 2017 identified one potential study (Babovic 2015) which we have excluded from the review as it was a non‐randomised trial (see: Excluded studies).
Our search of the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov in October 2017 did not identify any further trials.
See: Figure 1.
1.
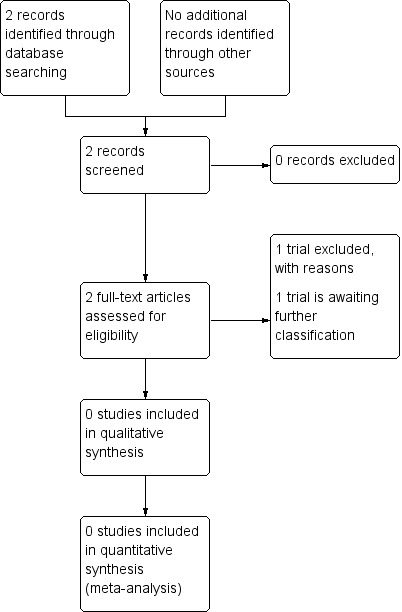
Study flow diagram.
Excluded studies
The Babovic 2015 study assessed the short‐term effects (0 to 4 hours) of transplacental versus direct fetal corticosteroid treatment on the fetal biophysical profile, baseline fetal heart rate, non‐stress test and perinatal outcomes. The authors assessed the effects of a single‐dose, ultrasound‐guided fetal administration of 4 mg dexamethasone compared with standard transplacental maternal administration of 24 mg dexamethasone (Babovic 2015). We contacted the trial author, who responded and provided clarity about the study's methodology.
Risk of bias in included studies
There are no included studies in this review.
Effects of interventions
There are no included studies in this review.
Discussion
We did not identify any randomised controlled trials for inclusion in this review comparing the benefits and harms of transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation where there is a risk of preterm birth.
The available studies carried out so far on animals and humans have shown that direct intramuscular injection of corticosteroid into the fetus under ultrasound guidance is feasible, but good‐quality data on health outcomes are lacking.
In the absence of sufficient data on which to base a clinical decision, uncertainty persists as to which method could provide better efficacy and safety. Randomised controlled trials are needed, focusing on the benefits and harms of maternal administration of transplacental corticosteroid treatment versus direct fetal corticosteroid treatment. Until the uncertainties have been addressed, it is advisable to stay with the current standard of transplacental maternally administered corticosteroid treatment (Roberts 2017).
Authors' conclusions
Implications for practice.
As there has been a lack of research completed in this area and as there is an absence of randomised controlled trial data, it is advisable to stay with the current standard of transplacental maternally administered corticosteroid treatment.
Implications for research.
The available animal studies of direct fetal corticosteroid treatment provide some insight into the potential benefits of this intervention. Randomised controlled trials are required, however, to provide the most reliable evidence about any potential benefits and harms of giving pregnant women at risk of preterm birth direct fetal corticosteroid treatment. Such trials must be of high quality, and of a sufficient sample size to assess the comparative effects on fetal, infant and child mortality and morbidity, maternal outcomes, and the use of health services.
What's new
| Date | Event | Description |
|---|---|---|
| 25 October 2017 | New citation required but conclusions have not changed | We found no new trials. The review remains empty. |
| 25 October 2017 | New search has been performed | Search updated and planned methods updated. |
History
Protocol first published: Issue 2, 2011 Review first published: Issue 9, 2011
| Date | Event | Description |
|---|---|---|
| 10 February 2012 | Amended | Contact details updated. |
Acknowledgements
In this update, we thank Emily Shepherd who assisted in updating the planned methods of the review.
As part of the pre‐publication editorial process, the previous version of this review was commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms used for ICTRP and ClinicalTrials.gov
International Clinical Trials Registry Platform (ICTRP) (25 October 2017)
We ran each line separately:
direct AND steroid AND antenatal
direct AND steroid AND prenatal
direct AND corticosteroid AND antenatal
direct AND corticosteroid AND prenatal
direct AND corticosteroid AND fetal
ClinicalTrials.gov (25 October 2017)
Advanced search
Interventiional studies
Intervention/Treatment: corticosteroid
Other terms: direct AND antenatal; direct AND prenatal; direct AND fetal
Appendix 2. Methods of data collection and analysis to be used in future updates of this review.
Data collection and analysis
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third person.
We will create a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third person. We will enter data into Review Manager 5 software (RevMan 2014) and check for accuracy. When information about any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We will assess the quality of the evidence using the GRADE approach as outlined in the GRADE handbook, in order to evaluate the body of evidence relating to the following outcomes for the main comparison:
Maternal
Maternal sepsis (as defined by authors)
Infant
Death (stillborn or death of a live‐born infant prior to primary hospital discharge)
Respiratory distress syndrome
Child
Survival free of any disability (as defined by authors)
Neurodevelopmental impairment (as defined by authors)
Child as an adult
Survival free of cardio‐metabolic disease
Neurodevelopmental impairment (as defined by authors)
We will use GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create ’Summary of findings’ tables. We will produce a summary of the intervention effect and a measure of quality for each of the above outcomes, using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as a summary risk ratio with a 95% confidence interval.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to exclude cluster‐randomised trials.
Cross‐over trials
We planned to exclude cross‐over trials.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and will analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We will regard heterogeneity as substantial if I2 is greater than 30% and either Tau2 is greater than zero, or there is a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using Review Manager 5 software (RevMan 2014). We will use a fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and we judge the trials’ populations and methods to be sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if we find substantial statistical heterogeneity, we will use a random‐effects meta‐analysis to produce an overall summary if we consider that an average treatment effect across trials is clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, will use a random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
Type of corticosteroid use (betamethasone versus dexamethasone).
Dose of corticosteroid use.
Gestational age at trial entry (less than 28 weeks versus equal to or above 28 weeks).
Singleton versus multiple pregnancy.
We will use the primary outcomes in subgroup analyses.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value.
Sensitivity analysis
We will carry out sensitivity analyses to explore the effects of trial quality assessed by sequence generation and allocation concealment, by omitting studies rated at 'high risk of bias' or 'unclear risk of bias' for these components. We will restrict this to the primary outcomes.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babovic 2015 | This was not a randomised controlled trial; quote: "They were divided into two groups". Dr Babovic was contacted by email, and provided additional detail about the study methodology. |
Characteristics of studies awaiting assessment [ordered by study ID]
Ljubic 2000.
| Methods | Unknown |
| Participants | 65 women at risk of preterm birth |
| Interventions | 35 women (group A) received single 4 mg dose of dexamethasone delivered intramuscularly to the fetus under ultrasound guidance, and 30 women (group B) received single 24 mg dose of dexamethasone intramuscularly. |
| Outcomes | Apgar score, perinatal mortality, RDS, intracranial haemorrhage, maternal morbidity (aggravation of hypertension and diabetes mellitus). |
| Notes | We have contacted the author about the study's design and particularly the method of treatment allocation. We are awaiting a reply. |
RDS: respiratory distress syndrome
Differences between protocol and review
In this update of the review:
we have updated our primary and secondary review outcomes to be in line with those prespecified in the antenatal corticosteroid Cochrane overview protocol (McGoldrick 2016);
The following are new primary and secondary outcomes added for this update:
Maternal sepsis (as defined by authors)
Death (stillborn or death of a live‐born infant prior to primary hospital discharge)
Survival free of any disability (as defined by authors)
Neurodevelopmental impairment (as defined by authors)
Survival free of cardiometabolic disease (child as an adult)
Neurodevelopmental impairment (child as an adult)
Bronchopulmonary dysplasia (chronic lung disease) (as defined by authors)
Composite of serious infant outcomes (as defined by authors)
Asthma/wheeze (child)
Risk factors for cardiovascular disease (child)
Emotional and behavioural problems (child)
Child as an adult: growth measurements (including weight, head circumference, height, skin fold thickness and BMI); age at puberty; abnormal lung function (including z scores for forced expiratory volume in one second, forced vital capacity and forced expiratory flow at 25% to 75% of forced vital capacity); health‐related quality of life; employment status
Cost of maternal care
Cost of neonatal care
The following secondary infant and child outcomes are no longer included in this update.
Blood pressure; glucose intolerance (however defined by authors); dyslipidaemia (however defined by authors); visual impairment (however defined by authors); hearing impairment (however defined by authors); intellectual impairment (intelligence quotient less than two standard deviation below population mean); developmental delay (developmental quotient less than two standard deviation below population mean); behavioural problems; cerebral palsy (however defined by authors)
we have updated the planned methods to be in line with those in the standard template used by Cochrane Pregnancy and Childbirth;
we have updated the planned methods to include the GRADE approach to assess the quality of the body of evidence and we have included ’Summary of findings’ tables.
In 2011 we added in a search of the International Clinical Trials Registry Platform (ICTRP) portal which was not prespecified in the original protocol, and in 2017 we also searched ClinicalTrials.gov.
Contributions of authors
For this update, and previous versions of the review, Debby Utama developed the initial draft. Caroline Crowther and Debby Utama edited all the subsequent drafts and finalised the review.
Sources of support
Internal sources
ARCH: Australian Research Centre for Health of Women and Babies, Robinson Research Institute,The University of Adelaide, Adelaide, Australia.
External sources
Australia Department of Health and Ageing, Australia.
National Health and Medical Research Council, Australia.
Declarations of interest
Debby P Utama: none known.
Caroline A Crowther: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Babovic 2015 {published data only}
- Babovic I, Radojicic Z, Plesinac S, Kastratovic Kotlica B, Sparic R. Direct intramuscular fetal or maternal antenatal corticosteroid therapy: short‐time effects on fetal behavior and oxygenation: a comparative study. Journal of Maternal‐fetal & Neonatal Medicine 2016; Vol. 29, issue 19:3213‐7. [DOI] [PubMed]
References to studies awaiting assessment
Ljubic 2000 {published data only}
- Ljubic A, Cvetkovic M, Sulovic V, Dukanac J, Antonovic O, Petkovic S. RDS prevention: direct fetal versus transplacental corticosteroid therapy [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 2); 2000 Sept 3‐8; Washington DC, USA. 2000:156.
Additional references
AIHW 2015
- Australian Institute of Health and Welfare (AIHW). Australia's Mothers and Babies 2013—in brief. Perinatal statistics series no. 31. Cat. no. PER 72. Canberra: AIHW, 2015. [Google Scholar]
Antenatal Corticosteroid Guidelines Panel 2015
- Antenatal Corticosteroid Clinical Practice Guidelines Panel. Antenatal Corticosteroids Given to Women Prior to Birth to Improve Fetal, Infant, Child and Adult Health: Clinical Practice Guidelines 2015. The University of Auckland, Auckland. New Zealand.: Liggins Institute, 2015. [Google Scholar]
Babovic 2009
- Babovic I, Plesinak S, Opalik J, Devrnjaa V, Pavlovic I, Radojicic Z, et al. Intramuscular fetal corticosteroid therapy: short term effects on the fetus. Fetal Diagnosis and Therapy 2009;25(1):98‐101. [DOI] [PubMed] [Google Scholar]
Ballard 1995
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. American Journal of Obstetrics and Gynecology 1995;173(1):254‐62. [DOI] [PubMed] [Google Scholar]
Beck 2010
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010;88(1):31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benediktsson 1993
- Benediktsson R, Lindsay RS, Noble J, Secki JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 1993;341(8841):339‐41. [DOI] [PubMed] [Google Scholar]
Brownfoot 2013
- Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006764.pub3] [DOI] [PubMed] [Google Scholar]
Crowther 2015
- Crowther CA, McKinlay CJD, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD003935.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalziel 2005
- Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trials. BMJ 2005;331(7518):665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2001
- Doyle LW, Victorian Infant Collaborative Study Group. Outcome at 5 years of age of children 23 to 27 weeks gestation: refining the prognosis. Pediatrics 2001;108(1):134‐41. [DOI] [PubMed] [Google Scholar]
Evans 1993
- Evans MI, Pryde PG, Reichler A, Bardicef M, Johnson MP. Fetal drug therapy. Western Journal of Medicine 1993;159(3):325‐32. [PMC free article] [PubMed] [Google Scholar]
Fowden 1996
- Fowden AL, Szemere J, Hughes RS, Forhead AJ. The effects of cortisol on the growth rate of the sheep fetus during late gestation. Journal of Endocrinology 1996;151(1):97‐105. [DOI] [PubMed] [Google Scholar]
Goldenberg 2007
- Goldenberg R, Culhane JF, Iams J, Romero R. Epidemiology and causes of preterm birth. Lancet 2007;371(9606):73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2002
- Gordon MC, Narula K, O'Shaughnessy R, Barth WH Jr. Complications of third trimester amniocentesis using continuous ultrasound guidance. Obstetrics & Gynecology 2002;99(2):255‐9. [DOI] [PubMed] [Google Scholar]
Haram 2003
- Haram K, Mortensen JH, Wollen AL. Preterm delivery: an overview. Acta Obstetricia et Gynecologica Scandinavica 2003;82(8):687‐704. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jackson 2003
- Jackson JR, Kleeman S, Doerzbacher M, Lambers DS. The effect of glucocorticosteroid administration on fetal movements and biophysical profile scores in normal pregnancies. Journal of Maternal‐fetal & Neonatal Medicine 2003;13(1):50‐3. [DOI] [PubMed] [Google Scholar]
Jobe 1993
- Jobe AH, Polk D, Ikegami M, Newnham J, Sly P, Kohen R, et al. Lung responses to ultrasound‐guided fetal treatments with corticosteroids in preterm lambs. Journal of Applied Physiology 1993;75(5):2099‐105. [DOI] [PubMed] [Google Scholar]
Jobe 1998
- Jobe AH, Newnham J, Willet K, Sly P, Ikegami M. Fetal versus maternal and gestational age effects of repetitive antenatal glucocorticoids. Pediatrics 1998;102(5):1116‐25. [DOI] [PubMed] [Google Scholar]
Jobe 2004
- Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. American Journal of Obstetrics and Gynecology 2004;190(4):878‐81. [DOI] [PubMed] [Google Scholar]
Liggins 1972
- Liggins GC, Howie RNA. A control trial of antepartum glucocorticoid treatment in prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50(4):515‐25. [PubMed] [Google Scholar]
Ljubic 1999
- Ljubic A, Cvetkovic M, Sulovic V, Radunovic N, Antonovic O, Vukolic D, et al. New technique for artificial lung maturation. Direct intramuscular fetal corticosteroid therapy. Clinical and Experimental Obstetrics and Gynecology 1999;26(1):16‐9. [PubMed] [Google Scholar]
McGoldrick 2016
- McGoldrick E, Brown J, Middleton P, McKinlay CJD, Haas DM, Crowther CA. Antenatal corticosteroids for fetal lung maturation: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012156] [DOI] [Google Scholar]
Moss 2003
- Moss TJ, Doherty DA, Nitsos I, Harding R, Newnham JP. Pharmacokinetics of betamethasone after maternal or fetal intramuscular administration. American Journal of Obstetrics and Gynecology 2003;189(6):1751‐7. [DOI] [PubMed] [Google Scholar]
Newnham 1999
- Newnham JP, Evans SF, Godfrey ME, Huang WL, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. Journal of Maternal‐Fetal Medicine 1999;8(3):81‐7. [DOI] [PubMed] [Google Scholar]
NIH 1995
- Anonymous. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 1995;273(5):413‐8. [DOI] [PubMed] [Google Scholar]
RCOG 2010
- Royal College of Obstetricians and Gynaecologists. Amniocentesis and Chorionic Villus Sampling Green‐top Guideline 8. London: RCOG, June 2010. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2017
- Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD004454.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saigal 2007
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2007;371(9608):261‐9. [DOI] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
References to other published versions of this review
Utama 2011a
- Utama DP, Crowther CA. Transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation where there is a risk of preterm birth. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008981] [DOI] [PubMed] [Google Scholar]
Utama 2011b
- Utama DP, Crowther CA. Transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation where there is a risk of preterm birth. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008981.pub2] [DOI] [PubMed] [Google Scholar]


