Abstract
Background
Pain during dental treatment, which is a common fear of patients, can be controlled successfully by local anaesthetic. Several different local anaesthetic formulations and techniques are available to dentists.
Objectives
Our primary objectives were to compare the success of anaesthesia, the speed of onset and duration of anaesthesia, and systemic and local adverse effects amongst different local anaesthetic formulations for dental anaesthesia. We define success of anaesthesia as absence of pain during a dental procedure, or a negative response to electric pulp testing or other simulated scenario tests. We define dental anaesthesia as anaesthesia given at the time of any dental intervention.
Our secondary objective was to report on patients' experience of the procedures carried out.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2018, Issue 1), MEDLINE (OVID SP), Embase, CINAHL PLUS, WEB OF SCIENCE, and other resources up to 31 January 2018. Other resources included trial registries, handsearched journals, conference proceedings, bibliographies/reference lists, and unpublished research.
Selection criteria
We included randomized controlled trials (RCTs) testing different formulations of local anaesthetic used for clinical procedures or simulated scenarios. Studies could apply a parallel or cross‐over design.
Data collection and analysis
We used standard Cochrane methodological approaches for data collection and analysis.
Main results
We included 123 studies (19,223 participants) in the review. We pooled data from 68 studies (6615 participants) for meta‐analysis, yielding 23 comparisons of local anaesthetic and 57 outcomes with 14 different formulations. Only 10 outcomes from eight comparisons involved clinical testing.
We assessed the included studies as having low risk of bias in most domains. Seventy‐three studies had at least one domain with unclear risk of bias. Fifteen studies had at least one domain with high risk of bias due to inadequate sequence generation, allocation concealment, masking of local anaesthetic cartridges for administrators or outcome assessors, or participant dropout or exclusion.
We reported results for the eight most important comparisons.
Success of anaesthesia
When the success of anaesthesia in posterior teeth with irreversible pulpitis requiring root canal treatment is tested, 4% articaine, 1:100,000 epinephrine, may be superior to 2% lidocaine, 1:100,000 epinephrine (31% with 2% lidocaine vs 49% with 4% articaine; risk ratio (RR) 1.60, 95% confidence interval (CI) 1.10 to 2.32; 4 parallel studies; 203 participants; low‐quality evidence).
When the success of anaesthesia for teeth/dental tissues requiring surgical procedures and surgical procedures/periodontal treatment, respectively, was tested, 3% prilocaine, 0.03 IU felypressin (66% with 3% prilocaine vs 76% with 2% lidocaine; RR 0.86, 95% CI 0.79 to 0.95; 2 parallel studies; 907 participants; moderate‐quality evidence), and 4% prilocaine plain (71% with 4% prilocaine vs 83% with 2% lidocaine; RR 0.86, 95% CI 0.75 to 0.99; 2 parallel studies; 228 participants; low‐quality evidence) were inferior to 2% lidocaine, 1:100,000 epinephrine.
Comparative effects of 4% articaine, 1:100,000 epinephrine and 4% articaine, 1:200,000 epinephrine on success of anaesthesia for teeth/dental tissues requiring surgical procedures are uncertain (RR 0.85, 95% CI 0.71 to 1.02; 3 parallel studies; 930 participants; very low‐quality evidence).
Comparative effects of 0.5% bupivacaine, 1:200,000 epinephrine and both 4% articaine, 1:200,000 epinephrine (odds ratio (OR) 0.87, 95% CI 0.27 to 2.83; 2 cross‐over studies; 37 participants; low‐quality evidence) and 2% lidocaine, 1:100,000 epinephrine (OR 0.58, 95% CI 0.07 to 5.12; 2 cross‐over studies; 31 participants; low‐quality evidence) on success of anaesthesia for teeth requiring extraction are uncertain.
Comparative effects of 2% mepivacaine, 1:100,000 epinephrine and both 4% articaine, 1:100,000 epinephrine (OR 3.82, 95% CI 0.61 to 23.82; 1 parallel and 1 cross‐over study; 110 participants; low‐quality evidence) and 2% lidocaine, 1:100,000 epinephrine (RR 1.16, 95% CI 0.25 to 5.45; 2 parallel studies; 68 participants; low‐quality evidence) on success of anaesthesia for teeth requiring extraction and teeth with irreversible pulpitis requiring endodontic access and instrumentation, respectively, are uncertain.
For remaining outcomes, assessing success of dental local anaesthesia via meta‐analyses was not possible.
Onset and duration of anaesthesia
For comparisons assessing onset and duration, no clinical studies met our outcome definitions.
Adverse effects (continuous pain measured on 170‐mm Heft‐Parker visual analogue scale (VAS))
Differences in post‐injection pain between 4% articaine, 1:100,000 epinephrine and 2% lidocaine, 1:100,000 epinephrine are small, as measured on a VAS (mean difference (MD) 4.74 mm, 95% CI ‐1.98 to 11.46 mm; 3 cross‐over studies; 314 interventions; moderate‐quality evidence). Lidocaine probably resulted in slightly less post‐injection pain than articaine (MD 6.41 mm, 95% CI 1.01 to 11.80 mm; 3 cross‐over studies; 309 interventions; moderate‐quality evidence) on the same VAS.
For remaining comparisons assessing local and systemic adverse effects, meta‐analyses were not possible. Other adverse effects were rare and minor.
Patients' experience
Patients' experience of procedures was not assessed owing to lack of data.
Authors' conclusions
For success (absence of pain), low‐quality evidence suggests that 4% articaine, 1:100,000 epinephrine was superior to 2% lidocaine, 1:100,000 epinephrine for root treating of posterior teeth with irreversible pulpitis, and 2% lidocaine, 1:100,000 epinephrine was superior to 4% prilocaine plain when surgical procedures/periodontal treatment was provided. Moderate‐quality evidence shows that 2% lidocaine, 1:100,000 epinephrine was superior to 3% prilocaine, 0.03 IU felypressin when surgical procedures were performed.
Adverse events were rare. Moderate‐quality evidence shows no difference in pain on injection when 4% articaine, 1:100,000 epinephrine and 2% lidocaine, 1:100,000 epinephrine were compared, although lidocaine resulted in slightly less pain following injection.
Many outcomes tested our primary objectives in simulated scenarios, although clinical alternatives may not be possible.
Further studies are needed to increase the strength of the evidence. These studies should be clearly reported, have low risk of bias with adequate sample size, and provide data in a format that will allow meta‐analysis. Once assessed, results of the 34 ‘Studies awaiting classification (full text unavailable)’ may alter the conclusions of the review.
Plain language summary
Injectable local anaesthetic agents for preventing pain in participants requiring dental treatment
Review question
This review assessed the evidence for providing successful local anaesthesia that prevents pain during a dental procedure. Included studies compared injections of local anaesthetic to help people requiring dental treatment and to prevent painful sensations tested in an experimental way (such as using cold, a sharp probe, or an electric stimulus).
Background
An injection of local anaesthetic prevents a person from feeling pain. It is given in one specific area rather than in the whole body. Although pain during dental treatment can be successfully managed, it is a common fear of patients.
Several different local anaesthetics are available to dentists, as well as a variety of ways to deliver them, to prevent pain. Factors that appear to influence success include increased difficulty in anaesthetizing teeth in the presence of inflammation, variable susceptibility of different teeth to local anaesthesia, different operative procedures performed on the tooth (for example, it appears easier to achieve successful anaesthesia for dental extractions than for root canal treatment), and various techniques and solutions used to give the local anaesthetic.
We investigated whether injection of one local anaesthetic solution was more effective than another for preventing pain during dental treatment or during an experimental study, and whether this effect occurred quickly or lasted a sufficient length of time, if any unwanted effects occurred, and people’s experience of the dental procedures. Local adverse events might include pain during or after injection, or long‐lasting anaesthesia. Systemic effects due to the local anaesthetic solution can include allergic reactions and changes in heart rate and blood pressure.
Study characteristics
Two reviewers searched the literature to identify studies that compared different local anaesthetic solutions injected into people undergoing dental treatment or volunteers who had the same outcomes measured in experimental ways. Within every trial, each person was randomly assigned to receive one of the local anaesthetics under study. The search was up‐to‐date as of 31 January 2018.
We found 123 trials with 19,223 male and female participants. These trials investigated pain experienced during dental treatment including surgery, extraction, periodontal (gum) treatment, tooth preparation, root canal treatment, anaesthesia of nerves within teeth (pulps) tested using an electric pulp tester or cold stimulant, and anaesthesia of soft tissues measured following pricking of gums or self‐reported by the participant. We pooled data from 68 studies (6615 participants). This resulted in eight outcomes when seven different local anaesthetic solutions were tested during dental treatment, two outcomes assessing pain during and after injection of local anaesthetic, and 47 outcomes tested with a pulp tester or by pricking of gums or self‐reported by participants.
Key results
The review suggests that of the 14 types of local anaesthetic tested, evidence to support the use of one over another is limited to the outcome of success (absence of pain), from three comparisons of local anaesthetic. Findings show that 4% articaine, 1:100,000 epinephrine was superior to 2% lidocaine, 1:100,000 epinephrine in posterior teeth with inflamed pulps requiring root canal treatment. No difference between these solutions was seen when pain on injection was assessed, and although lidocaine resulted in less post‐injection pain, the difference was minimal. Researchers found that 2% lidocaine, 1:100,000 epinephrine was superior to 3% prilocaine, 0.03 IU felypressin and 4% prilocaine plain for surgical procedures and surgical procedures/periodontal treatment, respectively. Speeds of onset were within clinically acceptable times, and durations were variable, making them suitable for different applications. Both of these latter outcomes were tested in experimental ways that may not reflect clinical findings. Unwanted effects were rare. Patients' experience of the procedures was not assessed owing to lack of data.
Quality of the evidence
From comparisons of local anaesthetics in this review, all appeared effective and safe with little difference between them. Available evidence ranged from moderate to very low in quality. Some studies fell short, in terms of quality, owing to small numbers of participants, unclear reporting of study methods, and reporting of data in a format that was not easy to combine with other data. Further research is required to clarify the effectiveness and safety of one local anaesthetic over another.
Summary of findings
Background
Description of the condition
Local anaesthesia is the most common form of pain control in dentistry. Several different formulations and various techniques are used to attain local anaesthesia in the mouth. Some of these methods, such as periodontal ligament and intrapulpal anaesthesia, are unique to dentistry. Pain can occur during a variety of dental interventions, which commonly involve some form of surgery or stimulation of the dental pulp by cutting dentine. Common dental treatments causing pain, which can be prevented by using local anaesthetic, include the placement of restorations, endodontic treatment in teeth with irreversible pulpitis, and extraction of teeth. During these treatments, pain is always felt, and completion may be impossible without local anaesthetic. Even with local anaesthetic delivered by infiltration or block anaesthesia, certain treatments such as endodontic treatment in teeth with irreversible pulpitis may still be painful, with the success rate of local anaesthesia as low as 23% (Claffey 2004).
As well as producing the desired local effect of pain control, dental local anaesthetic solutions may produce unwanted localized and systemic effects.
Description of the intervention
Although local anaesthesia is perceived to be a technique associated with a high success rate, failure of local anaesthetic injections is a feature of dental practice (Kaufman 1984). A search of the literature reveals that the efficacy of dental local anaesthesia varies. For example, the success rate reported for anaesthesia of mandibular permanent central incisor teeth ranges from 0% ‐ in Meechan 2002 ‐ to 100% ‐ in Rood 1977.
How the intervention might work
Although no systematic review has examined the topic of failure of all dental local anaesthetic solutions, a number of factors appear to influence success. Teeth are more difficult to anaesthetize in the presence of inflammation. It has been reported that patients with irreversible pulpitis are eight times more likely than controls to suffer failure of local dental anaesthesia (Hargreaves 2001). Different teeth vary in their susceptibility to local anaesthesia. Mandibular incisor teeth are more difficult to anaesthetize than posterior teeth after inferior alveolar nerve block injection (IANB) (Clark 1999). The success of intraligamentary injections has been reported to be poorer with mandibular incisors than with maxillary teeth (White 1998). The success of dental anaesthesia varies with the operative procedure performed on the tooth, for example, it appears easier to achieve successful anaesthesia for dental extractions than for endodontic therapy (Malamed 1982). The method of dental local anaesthesia used affects success. It has been reported that mandibular central incisor teeth are more likely to be anaesthetized by an infiltration injection than by a periodontal ligament anaesthesia (Meechan 2002). The local anaesthetic solution chosen has been shown to influence efficacy. The effectiveness of periodontal ligament anaesthesia has been reported to be much greater when a vasoconstrictor is included in the formulation (Gray 1987). The concentration and choice of local anaesthetic agent also appear to be important (Rood 1976). The efficacy of infiltration techniques in the mandible seems to be influenced by the choice of solution (Meechan 2010).
Unwanted effects of dental local anaesthesia may be localized or systemic. Local adverse events include trismus; long‐lasting anaesthesia or paraesthesia (Garisto 2010; Haas 1995; Hillerup 2006); paralysis of motor nerves; and interference with special senses such as vision (Rood 1972). Systemic effects may be due to the local anaesthetic or an added vasoconstrictor. Allergy is rare. Systemic effects that may occur include toxicity from the local anaesthetic that may manifest as altered cardiovascular or central nervous system effects. Systemic effects of the vasoconstrictor principally affect the cardiovascular system and are seen as changes in heart rate and blood pressure (Meechan 2001). Drug interactions with concurrent medication may also occur (Meechan 1997).
Why it is important to do this review
We are conducting this systematic review to determine which local anaesthetic solution is most successful for dental interventions owing to the current popularity of some formulations, such as those of articaine, for which growing evidence suggests that they provide more successful anaesthesia than other formulations. A rigorous systematic review of the success rate of local anaesthesia is needed to inform evidence‐based practice. This review will consider only injectable agents used for dental blocks or infiltration, while excluding supplemental injections.
Objectives
Our primary objectives were to compare the success of anaesthesia, the speed of onset and duration of anaesthesia, and systemic and local adverse effects amongst different local anaesthetic formulations for dental anaesthesia. We define success of anaesthesia as absence of pain during a dental procedure, or a negative response to electric pulp testing or other simulated scenario tests. We define dental anaesthesia as anaesthesia given at the time of any dental intervention.
Our secondary objective was to report on patients' experience of the procedures carried out.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that tested different formulations of local anaesthetic. These RCTs looked at either clinical procedures carried out under local anaesthesia or simulated scenario studies that made objective measurements of the success of local anaesthetic.
Clinical and simulated scenario studies were of a parallel or cross‐over design to compare solutions. When suitable data were available from cross‐over trials and it was appropriate to include them in a meta‐analysis, we adopted the approach recommended by Elbourne 2002. When possible, we included the data showing results from paired analyses (i.e. when estimates of within‐patient treatment effects and standard errors were available, or could be obtained from authors, or could be computed). If this was not possible, we combined data from the first period only as if they were derived through a parallel study design. We also used this approach if the study used a cross‐over design but the cross‐over design was in fact inappropriate (e.g. when the duration of carry‐over effect exceeded the wash‐out period). When paired data, or data from the first period, were not available, we treated the data from cross‐over studies as if derived from a parallel study, then performed sensitivity analysis with cross‐over data removed.
We also used RCTs to assess participants' experience and to look at local and systemic adverse effects.
Types of participants
We included participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested.
We define adults as over 16 years of age.
We excluded any participants taking regular medications that may alter their pain perception.
Types of interventions
Interventions in participants undergoing clinical procedures or participating in simulated scenario trials included:
all commercial preparations of dental local anaesthetic versus all other commercial preparations of dental local anaesthetic;
one dosage of local anaesthetic versus a different dose of local anaesthetic administered by the same injection technique (the higher dosage may be delivered in one injection or more); and
one concentration of local anaesthetic versus a similar volume but higher concentration of local anaesthetic given by the same injection technique.
Examples of commercial local anaesthetic solutions considered for inclusion in the review include:
2% lidocaine (with no epinephrine, 1:50,000 epinephrine, 1:80,000 epinephrine, 1:100,000 epinephrine, or 1:200,000 epinephrine);
4% articaine hydrochloride (HCl) (with no epinephrine, 1:100,000 epinephrine, 1:100,000 epinephrine, or 1:400,000 epinephrine);
3% prilocaine HCl (with 0.03 international units/mL (IU/mL) octapressin);
4% prilocaine HCl (with no epinephrine, or 1:200,000 epinephrine);
2% mepivacaine (with 1:20,000 levonordefrin or 1:100,000 epinephrine);
3% mepivacaine (with no epinephrine); and
0.5% bupivacaine (with 1:200,000 epinephrine).
We considered only primary infiltration and block anaesthesia and did not consider supplemental anaesthesia.
Types of outcome measures
Primary outcomes
Our primary outcome measure was the degree of anaesthesia.
Success of local anaesthesia, measured by the absence of pain during a procedure via a visual analogue scale (VAS) or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia by an electric pulp tester or cold stimulus.
Speed of onset (from time of injection to complete anaesthesia) and duration (time from onset until anaesthesia disappeared) of anaesthesia, measured by the absence of pain during a procedure seen on a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia by an electric pulp tester or cold stimulus.
-
Adverse effects: local and systemic, when the cause of the harmful effect is attributed to the local anaesthetic formulation, including:
pain on injection (solution deposition), measured on a VAS;
pain following injection, measured by VAS;
paraesthesia following injection; and
allergy to local anaesthetic.
Outcomes were classified separately by the oral tissues tested or the testing method used, which included the following.
-
Clinical testing of:
healthy pulps ‐ hard and soft tissues;
healthy pulps;
diseased pulps with irreversible pulpitis;
different tissues, pooled; and
tissues, when tissues tested were unclear.
-
Simulated scenario testing of:
healthy pulps;
diseased pulps with irreversible pulpitis; and
soft tissues.
Secondary outcomes
Our secondary outcome measure was the experience of participants:
including but not limited to preference and overall experience.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2018, Issue 1), which contains the Cochrane Oral Health and Anaesthesia, Critical and Emergency Care Groups' Trials Registers (see Appendix 1 for the detailed search strategy); MEDLINE (Ovid SP, 1946 to January 2018; see Appendix 2); Embase (Ovid SP, 1980 to January 2018; see Appendix 3); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) PLUS (EBSCOhost, 1937 to January 2018; see Appendix 4); and the Institute for Scientific Information (ISI) Web of Science (1956 to January 2018; Appendix 5). We ran all searches on 31 January 2018.
Our search strategy combined the subject search with the Cochrane Highly Sensitive Search Strategy for identifying Randomized Controlled Trials (RCTs) (as published in the Cochrane Handbook for Systematic Reviews of Interventions;Higgins 2011a). The subject search used a combination of controlled and free‐text terms.
Other electronic sources
We searched other available databases including the following.
IndMED (1985 to January 2018).
KoreaMED (1958 to January 2018).
Panteleimon (1998 to January 2018).
Australian New Zealand Clinical Trials Registry (ANZCTR) (2005 to January 2018).
Ingenta Connect (1973 to January 2018).
We ran all searches on 31 January 2018.
We also searched bibliographies, reference lists, and web sites related to local anaesthetic use.
We did not impose a language restriction. We included publications published in all languages following translation.
Searching other resources
Handsearching
We handsearched the following journals when they had not already been searched as part of the Cochrane handsearching programme.
Anesthesia Progress (March 1966 to January 2018).
Journal of Endodontics (January 1975 to January 2018).
International Endodontic Journal (April 1967 to January 2018).
International Journal of Oral Surgery (1972 to December 1985), continued as International Journal of Oral and Maxillofacial Surgery (February 1986 to January 2018).
Oral Surgery, Oral Medicine, Oral Pathology (January 1948 to December 1994), continued asOral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics (January 1995 to December 2011), then asOral Surgery, Oral Medicine, Oral Pathology and Oral Radiology (January 2012 to January 2018).
Journal of the American Dental Association (January 1948 to January 2018).
Pediatric Dentistry (March 1979 to January 2018).
British Dental Journal (January 1948 to January 2018).
Journal of Dental Research (February 1948 to January 2018).
General Dentistry (January 1976 to January 2018).
Journal of the Canadian Dental Association (February 1948 to January 2018).
We carried out all searches on 31 January 2018.
We checked the bibliographies of papers and review articles to find any studies not revealed by other search methods.
Unpublished trials
We searched OpenSIGLE (System for Information on Grey Literature in Europe) (1996 to 31 January 2018) for any relevant unpublished dissertations. We searched for additional relevant trials in:
National Research Register Archive (2000 to 2007) (database has now been archived);
UK Clinical Research Network (UKCRN) Study Portfolio (January 2008 to 31 January 2018); and
metaRegister of Controlled Trials (2000 to 31 January 2018).
We attempted to identify unpublished studies and ongoing trials by contacting:
editors of relevant journals;
authors of RCTs already identified;
local anaesthetic manufacturers; and
researchers known to the review authors.
Evidence on adverse effects
We gathered information on adverse effects from RCTs and from national adverse drug effect databases (searched up to 31 January 2018).
Medicines and Healthcare Products Regulatory Agency.
European Database of Suspected Adverse Drug Reaction Reports (European Medicines Agency).
Conference proceedings
We considered conference proceedings if, during our search, full‐text articles had been published or data from trial authors were made available. These included conference proceedings from:
Annual Session of the American Association of Endodontists (1985 to 31 January 2018).
Data collection and analysis
Selection of studies
Two review authors (GST and AM) independently read all titles and abstracts of publications retrieved through our search. We obtained any papers considered suitable for the review (which met our inclusion criteria) in their full version, including those for which a decision could not be made from just the title and abstract. When we were initially unable to make a decision, we (GST and AM) independently assessed the papers to see whether inclusion criteria for the review were met. We resolved disagreements initially by mutual discussion; when we could not resolve a difference of opinion, we involved a third review author ‐ John Meechan (JM). We assessed the degree of agreement by using the kappa statistic.
Our inclusion and exclusion criteria for the main study of effects were as follows.
Inclusion criteria
Randomized controlled trials (RCTs) evaluating the efficacy of a commercially available dental local anaesthetic agent
Exclusion criteria
Trials investigating postoperative pain control
Data extraction and management
Two review authors carried out the data abstraction independently (GST and AM).
Two review authors (GST and AM) used a data extraction form to record data from individual studies. We used five studies previously chosen as fulfilling the review selection criteria to pilot the form to ensure that data obtained were adequate for the review's purposes. We obtained or clarified missing or unclear data by contacting study authors.
We obtained data as follows.
Study characteristics
Study authors
Year of trial
Country where study was performed
Source of funding
Study design
Method of randomization
Method of allocation
Study population inclusion and exclusion criteria
Age
Blinding of participants, operator, and assessor
Intervention description
Number of participants recruited and number completing the trial
Reasons for withdrawal
Overall sample size
Methods used to estimate sample size (statistical power)
Statistical methods used
Unit of analysis
Use of intention‐to‐treat (ITT) analysis
Outcomes and/or confounders
Presence or absence of pain during a procedure measured by VAS or other appropriate method
Measurement of pulpal anaesthesia by an electric pulp tester
Speed of onset of anaesthesia
Duration of anaesthesia
Measurement of area of soft tissue anaesthesia
Patient experiences ‐ these include but are not limited to preferences and overall experience
Adverse events
After extracting data, we performed double data entry and screened the database for inconsistencies as a quality assurance measure.
Assessment of risk of bias in included studies
Two review authors (GST and AM) independently assessed the quality of the chosen RCTs. We assessed those trials selected in four areas that have been shown to affect the size of treatment effect, including:
method of randomization;
concealed allocation of treatment;
blinding of participants, therapists, and outcome assessors; and
information on reasons for withdrawal by trial group (ITT analysis).
We resolved disagreements by discussion between authors.
We based the quality components on those derived from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), defined as follows.
Randomization
We graded this as:
adequate if the randomization sequence was generated by a random number table (computer‐generated or not), a tossed coin, shuffled cards, or picking randomly mixed, masked cartridges of local anaesthetic from a container;
unclear if the randomization method used was not explained well or no method was reported; or
inadequate if randomization methods included alternate assignment, hospital number, and odd/even birth date.
Concealment of allocation
Adequate allocation concealment methods included:
central concealment of allocation such as by telephone to pharmacy or trial office;
pharmacy use of sequentially numbered or coded containers; or
use of sequentially numbered, opaque envelopes.
Allocation concealment was unclear if the study referred to allocation concealment but did not adequately explain the method, or if the study reported no method of allocation concealment.
Concealment was inadequate in studies for which randomization methods could not be concealed, such as alternate assignment, hospital number, and odd or even birth date.
Blinding
An assessment was made of the adequacy of blinding of participants, caregivers, and examiners. Blinding was assessed as:
adequate;
inadequate; or
unclear.
Participants entering studies were assessed to ensure that any who failed to complete their trials were accounted for. Studies utilizing an intention‐to‐treat (ITT) analysis were included.
When data were unclear or missing, we contacted the authors of studies to clarify the data. In circumstances for which clarification was not possible, we assessed the effect of inclusion of studies by performing sensitivity analysis.
Measures of treatment effect
For binary data, we expressed pooled outcomes as pooled odds ratios (ORs), risk ratios (RRs), and associated 95% confidence intervals (CIs). For continuous data, we expressed pooled outcomes as pooled mean differences (MDs) and associated 95% CIs.
When a data and analysis had only one included study (orphan study), it was not entered into a data and analysis table. Instead, the outcome was placed in the appropriate additional table (Table 9; Table 10; Table 11; Table 12; Table 13). When an orphan study was the sole study entered into a subgroup, its data were still analysed if data were available from other studies included in other subgroups in the data and analysis table.
1. Pulp anaesthesia onset (time in minutes).
| Study | Local anaesthetic solution | Jaw/Tooth | Onset | Standard deviation |
| Abdulwahab 2009 | BI (0.9 mL) of:
|
Mandibular first molars | 8 10 14 12 11 9 |
* |
| Batista da Silva 2010 | Mental/incisive nerve block (0.6 mL) of:
|
Mandibular canines Mandibular first premolars Mandibular second premolars |
8** 5** 4** 4** 3** 2** |
5‐9*** 4‐6*** 2‐6*** 2‐4*** 2‐4.5*** 2‐4*** |
| Burns 2004 | Palatal‐anterior superior alveolar injection (1.4 mL) of:
|
Maxillary central incisors, lateral incisors, and canines | Insufficient numbers for matched pair comparison. Onset for central incisors was within 4‐8 minutes for both anaesthetic solutions | * |
| Caldas 2015 | Maxillary BI (1.8 mL) of:
|
Right maxillary canines | 1.29 1.10 |
± 1.90## ± 1.47## |
| Costa 2005 | Maxillary BI (1.8 mL) of:
|
Maxillary posterior teeth | 2.8 1.4 1.6 |
* |
| Donaldson 1987 | Standard IANB (1.8 mL) or maxillary BI (0.6 mL) of:
|
Not stated | Inf' = 1.49 IANB = 1.37 Inf' = 1.35 IANB = 1.66 |
± 0.83 ± 0.80 ± 0.82 ± 1.13 |
| Forloine 2010 | High‐tuberosity maxillary second division nerve blocks (4.0 mL) of:
|
Mandibular first molars | 2.5 2.3 |
* |
| Gazal 2015 | IANB (1.8 mL) of 2% mepivacaine, 1:100,000 epinephrine, followed by BI (1.8 mL) of 1 of the following solutions:
|
Mandibular first molars | 4.26 2.78 |
± 1.94 ± 1.00 |
| Gazal 2017 | Maxillary BI (1.4 mL) and PI (0.4 mL) using the following:
|
Various maxillary teeth | 3.37 1.96 |
± 3.05 ± 1.93 |
| Hinkley 1991 | IANB (1.8 mL) of:
|
Lateral incisors First premolars Mandibular first molars |
16.3 11.0 12.3 10.1 11.7 10.6 10 9.6 8.8 |
± 3.2† ± 2.0† ± 1.9† ± 1.7† ± 2.3† ± 1.6† ± 2.2† ± 1.9† ± 1.8† |
| Jaber 2010 | BI (0.9 mL) of:
|
Mandibular central incisors | 3.3 3.4 |
2‐14††† 2‐6††† |
| Kammerer 2014 | BI (1.7 mL) of:
|
Maxillary central incisors | 6.5 5.0 4.7 5.3 |
± 1.5 ± 3.2 ± 2.6 ± 2.3 |
| Kanaa 2012; | BI (2.0 mL) of:
|
Maxillary teeth | 4.9 5.1 |
± 2.7 ± 2.4 |
| Katz 2010; | BI (1.8 mL) of:
|
Maxillary lateral incisors Maxillary first molars |
2.3 1.8 3.5 3.9 |
± 2.9 ± 1.5 ± 2.2 ± 2.3 |
| Knoll‐Kohler 1992a; | BI (0.5 mL) of:
|
Right maxillary incisors | 3.5 3.9 5.1 |
± 2.37† ± 2.23† ± 1.95† |
| Kramer 1958 | Maxillary and mandibular injections of 1 or more cartridges of:
|
Not stated | Mand' < 5 minutes = 57.3% > 5 minutes = 42.7% Max' < 5 minutes = 60% > 5 minutes = 40% Mand' < 5 minutes = 36% > 5 minutes = 64% Max' < 5 minutes = 49.2% > 5 minutes = 50.8% |
* |
| Maruthingal 2015 | Mandibular BI (1.7 mL) of 1 of the following:
|
Mandibular first molars | 10.352 6.928 |
± 4.54 ± 3.463 |
| McEntire 2011 | Mandibular BI (1.8 mL) of:
|
Mandibular first molars | 4.7 4.6 |
± 3.3# ± 3.3# |
| McLean 1993 | IANB (1.8 mL) of:
|
Mandibular lateral incisors Mandibular first premolars Mandibular first molars |
12.3 14.6 13.7 10.0 11.0 8.2 |
± 2.4† ± 3.3† ± 2.2† ± 1.7† ± 2.2† ± 2.0† |
| Mumford 1961 | Infiltration (1.0 mL) and regional injection (1.5 mL) of:
|
Various teeth | Inf’ 2.75†† Regional 3.5†† Inf’ 3.00†† Regional 3.25†† Inf’ 2.75†† Regional 4.25†† |
* |
| Nordenram 1990; | BI (0.6 mL) of:
|
Maxillary anterior teeth | (Young and elderly combined) < 2 minutes = 23/38 > 2 minutes = 15/38 < 2 minutes = 21/34 > 2 minutes = 13/34 < 2 minutes = 25/34 >2 minutes = 9/34 |
* |
| Oliveira 2004; | Maxillary infiltration, buccally (1.8 mL) and palatally (0.35 mL) of:
|
Right maxillary canines | 1.0** 3.0** |
1.0–13.0††† 1.0–7.0††† |
| Vahatalo 1993; | BI (0.6 mL) of:
|
Maxillary lateral incisors | 3.35 3.12 |
± 1.47 ± 1.1 |
| Vreeland 1989; | IANBs of:
|
Mandibular lateral incisors Mandibular canines Mandibular first molars |
13.20 8.63 13.60 7.43 8.44 7.12 |
± 2.35# ± 2.25# ± 2.79# ± 1.05# ± 1.85# ± 1.87# |
* Not available; ** median; *** lower‐upper quartiles; † standard error; †† clinical anaesthesia (no pain at start of procedure (onset) or throughout the procedure); ††† range; # author unsure whether measurement is standard error or standard deviation; ## unsure whether measurement is standard error or standard deviation.
BI = buccal infiltration; IANB = Inferior alveolar nerve block; Inf' = infiltration injection; Mand' = mandibular; Max' = maxillary; PI = palatal infiltration.
2. Soft tissue anaesthesia onset (time in minutes).
| Study | Local anaesthetic solution | Soft tissues tested | Onset (mean) | Standard deviation |
| Abdulwahab 2009 | BI (0.9 mL) of:
|
Soft tissues adjacent to mandibular first molars | Occurred between 7 and 15 minutes after injection for the 6 formulations (individual data not available) |
* |
| Albertson 1963 | Injections (type and volume not specified) of:
|
Method not stated | 1.25 0.97 |
2.48 1.58 |
| Batista da Silva 2010 | Mental/incisive nerve blocks (0.6 mL) of:
|
Lower lip | 2** 2** |
* |
| Bradley 1969 | Infiltration and “mandibular” injection (0.8‐3.6 mL) of:
|
Tissues of upper and lower jaws (exact tissues and method of measurement not stated) | Inf' = 0.83** Mand' = 0.67** Inf' = 1.08** Mand' = 0.75** |
0.17‐3.83 †††† 0.17‐3.00†††† 0.25‐4 †††† 0.083‐4.17 †††† |
| Chapman 1988; | IANB (2.0 mL) and BI (1.0 mL) of:
|
Lower lip | 2 2 |
* |
| Chilton 1971; | IANB (1.8 mL) and infiltration (1.5 mL) of:
|
Maxillary and mandibular soft tissues | Inf' = 0.9 IANB = 1.4 Inf' = 0.9 IANB = 1.8 |
± 0.6 ± 0.9 ± 0.5 ± 1.8 |
| Colombini 2006 | IANB (1.8 mL) and BI (0.9 mL) of:
|
Lower lip, tongue, and mucosa | 2.50 2.50 |
± 0.13† ± 0.24† |
| Gazal 2017 | Maxillary BI (1.4 mL) and PI (0.4 mL) using the following:
|
Soft tissues adjacent to various maxillary teeth |
Buccal 1.74 1.05 Palatal 0.90 minutes 0.52 minutes |
± 2.14 ± 1.68 ± 0.96 ± 0.20 |
| Gangarosa 1967 | Mandibular block and infiltration (volume not stated) of:
|
Not stated | Within 2 minutes = 38/100†† 5 or more minutes = 62/100†† Within 2 minutes = 50/100†† 5 or more minutes = 50/100†† |
* |
| Hersh 1995 | IANB (1.8 mL) of:
|
Lower lip and tongue | Within 5 minutes Within 5 minutes Within 5 minutes |
* |
| Hinkley 1991 | IANB (1.8 mL) of:
|
Lower lip, tongue, and mucosa Mucosal probing |
6.3 5.3 6.1 10.8 9.1 10.6 |
± 1.1† ± 0.8† ± 0.8† ± 1.8† ± 1.6† ± 1.9† |
| Jain 2016 | IANB and BI (1.7 mL in total) of:
|
Inferior lip, corresponding half of the tongue, and buccal mucosa Measured subjectively and objectively (methods not detailed) but only 1 outcome presented in the journal article |
1.47 0.94 |
± 0.22 ± 0.16 |
| Kammerer 2012 | IANB and BI (up to 2.2 mL) of:
|
Vestibular mucosa and oral gingivae | 7.2 9.2 |
± 2.97 ± 2.7 |
| Karm 2017 | IANB and BI (1.8 mL in total) of:
|
Lower lip, corresponding half of tongue and mucosa | 4.9 5.2 |
± 4.1 ± 4.1 |
| Lasemi 2015 | IANB (volume not stated) of:
|
Lower lip | 1.4 2.0 |
± 0.42## ± 0.45## |
| Maruthingal 2015 | Mandibular BI (1.7 mL) of 1 of the following:
|
Lip and lingual mucosa | 4.937 3.562 |
± 1.366 ± 1.664 |
| McLean 1993 | IANB (1.8 mL) of:
|
Lower lip, tongue, and adjacent soft tissues Mucosal sticks |
5.0 5.0 4.5 7.8 10.7 8.4 |
± 0.65† ± 0.55† ± 0.61† ± 1.49† ± 1.52† ± 1.92† |
| Nespeca 1976 | Various types of injections (1.5‐2.0 mL) of:
|
Various soft tissues | 2.40†† 4.48†† |
± 0.16† ± 0.28† |
| Odabas 2012 | Maxillary BI (1.8 mL) of:
|
Upper lip | 1 1 |
± 0.00 ± 0.15 |
| Pellicer‐Chover 2013 | IANB (1.8 mL) and BI (1.8 mL) of:
|
Lower lip and tongue | 3.1 2 |
± 1.5 ± 1.4 |
| Ram 2006 | IANB and maxillary infiltration (up to 1 cartridge) of:
|
Maxillary and mandibular soft tissues | Immediate (< 2 minutes) in > 80% of cases with either solution | * |
| Sadove 1962 | Various types of dental blocks and infiltrations (volume not stated) of:
|
Various soft tissues | 2.03 1.79 |
0.13† 0.09† |
| Sancho‐Puchades 2012 | IANB (1.8 mL) of:
|
Lower lip and tongue | 1.9 1.8 |
±1.2 ±1.2 |
| Santos 2007 | IANB (1.8 mL) and BI (0.9 mL) of:
|
Lower lip, tongue, and mucosa | 1.64 1.58 |
± 0.08† ± 0.08† |
| Sherman 1954 | Mandibular and maxillary injections (1.1‐2.2 mL) of:
|
Maxillary and mandibular soft tissues | Inf' = 1** Block = 2** Inf' = 1** Block = 2** |
* |
| Sierra Rebolledo 2007 | IANB (1.8 mL) and BI (1.8 mL) of:
|
Lower lip | 1.25 0.93 |
± 0.23 ± 0.16 |
| Stibbs 1964 | "Mandibular" injection and "infiltration" (varying volumes) of:
|
Maxillary and mandibular soft tissues | Mand' 1.74 Inf' 1.23 Other 1.51 Mand' 1.86 Inf' 1.25 Other 1.48 |
± 0.15† ± 0.13† ± 0.17† ± 0.15† ± 0.16† ± 0.24† |
| Thakare 2014 | BI (1.4 mL) of:
|
Maxillary soft tissues? | 0.71 1.0 |
± 0.28 ± 0.44 |
| Trieger 1979 | IANB and BI (varying volumes) of:
|
Tissues adjacent to extraction site | 8.1 6.5 |
< 5‐15††† < 5‐10††† |
| Vilchez‐Perez 2012 | BI (0.9 mL) of:
|
Upper lip | 85% of participants felt anaesthesia before withdrawal of the needle 10% < 30 seconds 5% > 30 seconds 80% of participants felt anaesthesia before withdrawal of the needle 10% < 30 seconds 10% > 30 seconds |
* |
| Vreeland 1989 | IANB of:
|
Lower lip and tongue (subjective) Labial and lingual to the test canine and buccal to the test molar (alveolar mucosal sticks) |
8.80 6.70 6.23 4.47 |
± 1.290# ± 0.757# ± 0.748# ± 0.722# |
| Wali 2010 | IANB (1.8 mL) of:
|
Lower lip | 4.4 5.9 |
± 0.4† ± 0.5† |
| Weil 1961 | Infiltration and "mandibular" injection (1 or more cartridges) of:
|
Maxillary and mandibular soft tissues | Inf' 0.83 Mand' 1.4 Inf' 0.7 Mand' 1.07 |
± 0.06 ± 0.12 ± 0.09 ± 0.15 |
* Not available; ** median; † standard error; †† clinical anaesthesia (no pain at start of procedure (onset) or throughout the procedure); ††† range; †††† 90% range; # author unsure whether measurement is standard error or standard deviation; ## unsure whether measurement is standard error or standard deviation.
BI = buccal infiltration; IANB = inferior alveolar nerve block; Inf' = infiltration injection; Mand' = mandibular injection; PI = palatal infiltration.
3. Pulp anaesthesia duration (time in minutes).
| Study | Local anaesthetic solution | Jaw/Tooth | Duration | Standard deviation |
| Batista da Silva 2010 | Incisive/mental nerve block (0.6 mL) of:
|
Mandibular canines Mandibular first premolars Mandibular second premolars |
10** 10** 10** 20** 10** 20** |
10 ‐ 20*** 10 ‐ 20*** 10 ‐ 20*** 10 ‐ 30*** 10 ‐ 20*** 10 ‐ 32.5*** |
| Caldas 2015 | Maxillary BI (1.8 mL) of:
|
Right maxillary canines | 41.61 41.03 |
± 14.16## ± 17.79## |
| Costa 2005 | Maxillary BI (1.8 mL) of:
|
Maxillary posterior teeth | 39.2 66.3 56.7 |
* |
| Donaldson 1987 | IANB (1.8 mL) or maxillary BI (0.6 mL) of:
|
Not stated | Data presented in life tables; therefore cannot be used | * |
| Fernandez 2005 | IANB (1.8 mL) of:
|
Lateral incisors First premolars Second premolars First molars Second molars |
127 244 154 256 152 258 138 232 148 232 |
± 8.1† ± 18† ± 5.9† ± 15.8† ± 6.0† ± 15.5† ± 8.1† ± 16.6† ± 6.4† ± 16.3† |
| Gazal 2015 | IANB (1.8 mL) of 2% mepivacaine, 1:100,000 epinephrine, followed by BI (1.8 mL) of 1 of the following solutions:
|
Mandibular first molars | 40.74 42.22 |
± 1.94 ± 1.00 |
| Kammerer 2014 | BI (1.7 mL) of:
|
Maxillary central incisors | 14.75 77.6 54.8 35.9 |
± 5.8 ± 30.1 ± 17.5 ± 15.1 |
| Knoll‐Kohler 1992a; | BI (0.5 mL) of:
|
Right maxillary incisors | 78.6 61.7 26.5 |
± 24.95† ± 15.72† ± 18.31† |
| Mumford 1961; | Infiltration (1.0 mL) and regional injection (1.5 mL) of:
|
Various teeth | Inf’ 31†† Regional 34†† Inf’ 20†† Regional 33†† Inf’ 32†† Regional 40†† |
* |
| Nordenram 1990; | BI (0.6 mL) of:
|
Maxillary anterior teeth | Elderly = 59.3 Young = 44.8 Elderly = 26.6 Young = 17.5 Elderly = 43.2 Young = 24.8 |
± 34.3 ± 18.7 ± 13.3 ± 6.1 ± 29.2 ± 11.8 |
| Oliveira 2004; | BI (1.8 mL) and PI (0.35) of:
|
Right maxillary canines | 67.0** 46.5** |
27.0–117.0††† 25.0–107.0††† |
| Vahatalo 1993; | BI (0.6 mL) of:
|
Maxillary lateral incisors | 23.8 24.5 |
± 8.6 ± 10.0 |
| Weil 1961 | Infiltration and mandibular injection (1 or more cartridges) of:
|
Various teeth | Inf ' 41.71†† Mand' 40.00†† Inf' 76.33†† Mand' 45.00†† |
± 4.11 ± 7.45 ± 6.77 ± 12.22 |
* Not available; ** median; *** lower‐upper quartiles; † standard error; †† clinical anaesthesia (no pain at start of procedure (onset) or throughout procedure); ††† range.
BI = buccal infiltration; IANB = inferior alveolar nerve block; Inf' = infiltration injection; Mand' = mandibular; PI = palatal infiltration.
4. Soft tissue anaesthesia duration (time in minutes).
| Study | Local anaesthetic solution | Jaw/Tooth | Duration | Standard deviation |
| Batista da Silva 2010 | Mental/Incisive nerve block (0.6 mL) of:
|
Lower lip | 156** 165** |
135.5‐184.25*** 145.75‐198.5*** |
| Bortoluzzi 2009 | BI (0.18 mL) of:
|
Lower lip | 111.3 104.5 |
± 26 ± 26.7 |
| Bradley 1969 | Infiltration and “mandibular” injection (0.8‐3.6 mL) of:
|
Upper and lower jaws (1.8 mL) | Inf' = 139** IANB = 178** Inf' = 96** IANB = 182** |
37‐254††† 64‐294††† 23‐238††† 127‐277††† |
| Caldas 2015 | Maxillary BI (1.8 mL) of:
|
Vestibular mucosa | 148.06 137.93 |
± 58.10# ± 70.67# |
| Chapman 1988; | IANB (2.0 mL) and BI (1.0 mL) of:
|
Mental region | 216 510 |
± 36 ± 150 |
| Elbay 2016 | IANB (0.9 mL) of 1 of the following:
|
Lower lip and adjacent soft tissues | 149.10 139.68 |
49.08 45.76 |
| Fertig 1968 | IANB (1.8 mL) of:
|
Lower lip | 191.5 189.38 206.25 |
* |
| Gangarosa 1967 | IANB and infiltration (volume not stated) of:
|
Maxillary and mandibular soft tissues | 169 144 |
* |
| Hellden 1974 | IANB (1.8 mL) and local infiltration (1.8 mL) of:
|
Lower lip and adjacent soft tissues | 185 152 |
± 3.5† ± 5.3† |
| Hersh 1995 | IANB (1.8 mL) of:
|
Lower lip and tongue | Exact figures not given | * |
| Jain 2016 | IANB and BI (1.7 mL) of:
|
Inferior lip, corresponding half of the tongue, and buccal mucosa Only postoperative duration measured |
174.80 231 |
± 37.62 ± 57.15 |
| Kalia 2011 | IANB, IANB and BI, IONB and greater palatine nerve block (volume not stated) of:
|
Lip, buccal mucosa, tongue, and palate Only postoperative duration measured |
161.13 232.99 |
± 27.03 ± 32.44 |
| Kambalimath 2013 | IANB and BI (volume not stated) of:
|
Lower lip and adjacent soft tissues Duration measured only up to when local anaesthetic effect began to fade |
175.9 196.8 |
± 51.7 ± 57.3 |
| Kammerer 2012 | IANB and mandibular BI (up to 2.2 mL) of:
|
Lower lip, tongue, and mucosa Figures for duration of soft tissue anaesthesia in the journal article are for all participants who may have had 1 or 2 sets of injections. Following communication, study author provided data for participants (70) who had only 1 injection (original data for 1 and 2 injections are given in brackets) |
216 (228) 138 (150) |
24 (34.2) 44.4 (58.2) |
| Kammerer 2014 | BI (1.7 mL) of:
|
Adjacent soft tissues | 60.3 151.7 129.3 104.0 |
± 24.2 ± 27.6 ± 19.2 ± 22.5 |
| Karm 2017 | IANB and BI (1.8 mL) of:
|
Lower lip, corresponding half of tongue, and mucosa | 183.5 182.2 |
± 5.0 ± 5.4 |
| Kramer 1958 | Mandibular and maxillary injections (1 or more cartridges) of:
|
Mandibular and maxillary soft tissues | Mand' = 178** Max' = 157** Mand' = 185** Max' = 153** |
* |
| Lasemi 2015 | IANB (volume not stated) of:
|
Lower lip | 235.5 230 |
± 13.32# ± 14.10# |
| Maniglia‐Ferreira 2009 | IANB (1 cartridge) of:
|
Tissues not stated (possibly lower lip) | > 90 > 90 |
* |
| Mumford 1961 | "Regional" (1.5 mL) and infiltration (1.0 mL) of:
|
Maxillary and mandibular soft tissues | Inf’ 172.2 Regional 188.4 Inf’ 101.4 Regional 156.6 Inf’ 116.4 Regional 187.8 |
* |
| Naik 2017 | IANB (2.0 mL) using the following:
|
Lip and associated tissues | 184.7 357.8 |
± 39.10 ± 58.8 |
| Nordenram 1990 | Maxillary BI (0.6 mL) of:
|
Maxillary soft tissues | Elderly = 168.0 Young = 174.2 Elderly = 102.2 Young = 97.3 Elderly = 167.4 Young = 171.0 |
± 42.8 ± 53.9 ± 48.9 ± 56.8 ± 77.0 ± 53.7 |
| Odabas 2012 | Maxillary BI (1.8 mL) of:
|
Upper lip | 140.69 117.52 |
± 49.76 ± 42.99 |
| Oliveira 2004 | BI (1.8 mL) and PI (0.35 mL) of:
|
Upper lip | 238.5** 227.5** |
168.0‐308.0†† 159.0‐273.0†† |
| Pellicer‐Chover 2013 | IANB (1.8 mL) and BI (1.8 mL) of:
|
Lower lip and tongue | 316.5 250.3 |
± 30.1 ± 48.3 |
| Porto 2007 | IANB and BI (minimum of 3.6 mL) of:
|
Lower lip | 208.2 222 |
53.4 57.6 |
| Ram 2006 | IANB and maxillary BI (up to 1 cartridge) of:
|
Maxillary and mandibular soft tissues | 180 206 |
± 49.2 ± 44.4 |
| Sancho‐Puchades 2012 | IANB and BI (1.8 mL) of:
|
Lower lip and tongue | Lip 289.6 621.2 Tongue 238.1 512.1 |
± 82.0 ± 148.4 ± 67.9 ± 127.3 |
| Sherman 1954 | Mandibular and maxillary injections (1.1‐2.2 mL) of:
|
Maxillary and mandibular soft tissues | Inf' = 150** Conduction = 195** Inf' = 165** Conduction = 195** |
* |
| Sierra Rebolledo 2007 | IANB (1.8 mL) and BI (1.8 mL) of:
|
Lower lip | 168.20 220.8 |
± 10.77 ± 13.81 |
| Stibbs 1964 | "Mandibular" injection and maxillary/mandibular infiltration (varying volumes) of:
|
Maxillary and mandibular soft tissues | Mand' 205.50 Inf' 177.83 Other 168.21 Mand' 224.48 Inf' 191.79 Other 180.64 |
Mand' ± 5.08† Inf' ± 7.32† Other ± 7.86† Mand' ± 5.74† Inf' ± 6.31 Other ± 9.27† |
| Tofoli 2003 | IANB (1.8 mL) of:
|
Lower lip | 264 260 |
± 37† ± 45† |
| Weil 1961 | Infiltration and mandibular injections (1 or more cartridges) of:
|
Maxillary and mandibular soft tissues | Inf' 132.56 Mand' 193.11 Inf' 184.03 Mand' 255.50 |
± 10.69 ± 9.14 ± 10.37 ± 9.66 |
* Not available; ** median; *** lower‐upper quartiles; † standard error; †† range; ††† 90% range; # unsure if measurement is standard error or standard deviation.
BI = buccal infiltration; IANB = inferior alveolar nerve block; Inf' = infiltration injection; Mand' = mandibular; Max' = maxillary; PI = palatal infiltration.
5. Orphan studies (success).
| Study | Comparison | Outcome | Data |
| Abdulwahab 2009 | Mandibular BI (0.9 mL) of 1 of the following solutions:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 4% articaine, 1:100,000 epinephrine BIs = 7/18 4% articaine, 1:200,000 epinephrine BIs = 6/18 4% prilocaine, 1:200,000 epinephrine BIs = 4/18 3% mepivacaine, no vasoconstrictor BIs = 6/18 0.5% bupivacaine, 1:200,000 epinephrine BIs = 2/18 |
| Aggarwal 2009 | IANB of 1.8 mL of 2% lidocaine, 1:200,000 epinephrine, followed by 1 of the following:
|
Success of subjective soft tissue anaesthesia Success of anaesthesia during endodontic access cavity preparation and instrumentation in teeth with irreversible pulpitis |
Soft tissue anaesthesia success 4% articaine, 1:200,000 epinephrine IANB/BI/LIs = 30/31 2% lidocaine, 1:200,000 epinephrine IANB/BI/LIs = 30/31 Clinical anaesthetic success 4% articaine, 1:200,000 epinephrine IANB/BI/LIs = 14/30 2% lidocaine, 1:200,000 epinephrine IANB/BI/LIs = 7/30 |
| Aggarwal 2014 | IANB using 1.8 mL of 1 of the following:
|
Success of soft tissue anaesthesia Success of pulpal anaesthesia during endodontic access cavity preparation and instrumentation in teeth with irreversible pulpitis |
Soft tissue anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 30/31 2% lidocaine, 1:200,000 epinephrine IANBs = 32/32 Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 3/30 2% lidocaine, 1:200,000 epinephrine IANBs = 5/32 |
| Aggarwal 2017 | IANB using 1.8 mL of 1 of the following:
|
Success of subjective soft tissue anaesthesia Success of pulpal anaesthesia during endodontic access cavity preparation and instrumentation in teeth with irreversible pulpitis |
Soft tissue anaesthesia success 2% lidocaine, 1:200,000 epinephrine IANBs = 31/32 4% articaine, 1:100,000 epinephrine IANBs = 30/31 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 30/34 Clinical anaesthesia success 2% lidocaine, 1:200,000 epinephrine IANBs = 3/32 4% articaine, 1:100,000 epinephrine IANBs = 2/31 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 2/34 |
| Albertson 1963 | Injections (not specified) of 1 of the following:
|
Success of anaesthesia during various dental procedures (not stated) |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine Injections = 64/110 2% mepivacaine, 1:20,000 levonordefrin Injections = 99/113 |
| Allegretti 2016 | IANB using 3.6 mL of 1 of the following:
|
Success of subjective soft tissue anaesthesia Success of anaesthesia during pulpectomy in mandibular first and second molars with irreversible pulpitis |
Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 22/22 4% articaine, 1:100,000 epinephrine IANBs = 22/22 2% mepivacaine, 1:100,000 epinephrine IANBs = 22/22 Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 7/22 4% articaine, 1:100,000 epinephrine IANBs = 11/22 2% mepivacaine, 1:100,000 epinephrine IANBs = 4/22 |
| Arrow 2012 | IANB or mandibular BI (up to 2.2 mL) using 1 of the following:
|
Success of anaesthesia during paediatric restorative procedures |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 17/29 BIs = 5/27 4% articaine, 1:100,000 epinephrine IANBs = 19/27 BIs = 7/28 |
| Atasoy Ulusoy 2014 | Maxillary BI of 1.5 mL of 1 of the following:
|
Success of anaesthesia during endodontic access cavity preparation and instrumentation in teeth with irreversible pulpitis |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine BIs = 8/25 4% articaine 1:100,000 epinephrine bitartrate BIs = 9/25 |
| Berberich 2009 | Intraoral, IONBs of 1.8 mL of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:50,000 epinephrine IONBs = 40/40 3% mepivacaine, no vasoconstrictor IONBs = 40/40 |
| Bouloux 1999 | Patients received the following injections:
with either:
|
Success of soft tissue anaesthesia using a probe |
Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 20/23 0.5% bupivacaine, 1:200,000 epinephrine IANB/BIs = 18/23 |
| Bradley 1969 | Infiltration or "mandibular" injection (1.8 mL) of 1 of the following:
|
Success of anaesthesia during various dental procedures including restorative, surgical, root extirpation, and miscellaneous procedures (data for those injections of 1.8 mL presented) |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine Infiltrations = 40/53 Mandibular = 31/42 3% mepivacaine, no vasoconstrictor Infiltrations = 27/36 Mandibular = 33/39 |
| Chilton 1971 | Infiltration (of at least 1.5 mL) and IANB (of at least 1.8 mL), which may include supplemental injections of 1 of the following:
|
Success of anaesthesia during endodontic and periodontal procedures |
Clinical anaesthesia success (periodontal) 2% lidocaine, 1:100,000 epinephrine Infiltrations = 57/61 IANBs = 31/43 4% prilocaine, 1:200,000 epinephrine Infiltrations = 61/69 IANBs = 28/35 4% prilocaine, no epinephrine Infiltrations = 50/66 IANBs = 26/40 Clinical anaesthesia success (endodontic) 2% lidocaine, 100,000 epinephrine Infiltrations = 61/69 IANBs = 21/31 4% prilocaine, 1:200,000 epinephrine Infiltrations = 52/65 IANBs = 24/33 4% prilocaine, no epinephrine Infiltrations = 45/65 IANBs = 23/34 |
| Cohen 1993 | IANB using 1.8 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with DDM Success of anaesthesia during pulpotomy in teeth with irreversible pulpitis |
Pulp anaesthesia success (DDM) 2% lidocaine, 1:100,000 epinephrine IANBs = 17/27 3% mepivacaine, no vasoconstrictor IANBs 21/34 Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs 15/27 3% mepivacaine, no vasoconstrictor IANBs 19/34 |
| Elbay 2016 | IANB using 0.9 mL of 1 of the following:
|
Success of anaesthesia during pulpotomy in mandibular primary molars with irreversible pulpitis Success of soft tissue anaesthesia using a probe |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 17/30 3% mepivacaine, no vasoconstrictor IANBs = 15/30 Soft tissue anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 28/30 3% mepivacaine, no vasoconstrictor IANBs = 24/30 |
| Epstein 1965 | Maxillary BI (1.2 mL) and IANB (1.5 mL) of 1 of the following:
|
Success of anaesthesia during restorative dentistry or "other" procedures |
Clinical anaesthesia success (restorative) 2% lidocaine, 1:100,000 epinephrine BIs = 59/63 IANBs = 49/57 4% prilocaine, no vasoconstrictor BIs = 71/73 IANBs = 52/53 Clinical anaesthesia success (other procedures) 2% lidocaine, 1:100,000 epinephrine BIs = 2/2 IANBs = 2/2 4% prilocaine, no vasoconstrictor BIs= 8/8 IANBs = 1/1 |
| Epstein 1969 | Maxillary BI (average = 1.2 mL) and IANB (average = 1.4 mL) of 1 of the following:
|
Success of anaesthesia during extraction or restorative dentistry procedures |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine BIs = 108/115 IANBs = 65/82 4% prilocaine, 1:200,000 epinephrine BIs = 125/135 IANBs = 62/74 4% prilocaine, no vasoconstrictor BIs = 119/128 IANBs = 67/76 |
| Haase 2008 | IANB (1.8 mL) of 4% articaine, 1:100,000 epinephrine followed by additional BI (1.8 mL) of either:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 4% articaine, 1:100,000 epinephrine IANB/BIs = 64/73 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 52/73 |
| Hellden 1974 | IANB (1.8 mL) and local infiltration (1.8 mL) of 1 of the following:
|
Success of anaesthesia during surgical removal of lower third molar teeth |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANB/BIs = 123/140 3% mepivacaine plain IANB/BIs = 106/140 |
| Kammerer 2012 | IANB and an additional buccal nerve block using up to 2.2 mL of 1 of the following:
|
Success of anaesthesia during extraction of posterior, mandibular teeth |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine IANB/BIs = 32/41 4% articaine, no vasoconstrictor IANB/BIs = 27/47 |
| Kammerer 2014 | BI of:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 4% articaine, no vasoconstrictor BIs = 4/10 4% articaine, 1:400,000 epinephrine BIs = 10/10 |
| Kanaa 2012 | Maxillary BI (2.0 mL) of the following:
Patients for extraction received a supplementary palatal injection of 0.2 mL 2% lidocaine, 1:80,000 epinephrine |
Success of anaesthesia during extraction or pulp extirpation in teeth with irreversible pulpitis |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine BIs = 33/50 2% lidocaine, 1:80,000 epinephrine BIs = 29/50 |
| Katz 2010 | Maxillary BI using 1.8 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 4% prilocaine, 1:200,000 epinephrine BIs = 28/30 4% prilocaine, no vasoconstrictor BIs = 24/30 |
| Khoury 1991 | Various types of injections, using varying volumes (most were 2.0 mL with a range of 0.8 mL‐5.0 mL ‐ further injections of 0.5 mL‐2.0 mL were injected if required) of 1 of the following:
|
Success of anaesthesia during surgical procedures |
Clinical anaesthesia success 3% prilocaine, 0.03IU felypressin Injections = 207/364 4% articaine, 1:100,000 epinephrine Injections = 298/408 4% articaine, 1:200,000 epinephrine Injections = 269/382 2% lidocaine, 1:100,000 epinephrine Injections = 242/363 |
| Knoll‐Kohler 1992a | Maxillary BI using 0.5 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:50,000 epinephrine BIs = 10/10 2% lidocaine, 1:200,000 epinephrine BIs = 6/10 |
| Lawaty 2010 | Maxillary BI using 1.8 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine BIs = 26/30 2% mepivacaine, 1:20,000 levonordefrin BIs = 27/30 |
| McLean 1993 | IANB of 1.8 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester Subjective success of soft tissue anaesthesia |
Pulp anaesthesia success (EPT) 4% prilocaine, no vasoconstrictor IANBs = 17/30 3% mepivacaine, no vasoconstrictor IANBs = 13/30 Soft tissue anaesthesia success 4% prilocaine, no vasoconstrictor IANBs = 30/30 3% mepivacaine, no vasoconstrictor IANBs = 30/30 2% lidocaine, 1:100,000 epinephrine IANBs = 30/30 |
| Mittal 2015 | Maxillary BI of 1 of the following:
|
Soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:80,000 epinephrine BIs = = 0/52 4% articaine, 1:100,000 epinephrine BIs = 1/52 |
| Moore 1983 | Maxillary and mandibular dental block and infiltration using 2 cartridges (2 × 1.8 mL) for each procedure using 1 of the following:
|
Success of anaesthesia during non‐surgical and surgical endodontic treatment |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine Injections = 8/16 0.5% bupivacaine, 1:200,000 epinephrine Injections = 12/16 |
| Mumford 1961 | "Regional" and infiltration injections (1.5 and 1.0 mL, respectively) of 1 of the following:
|
Success of anaesthesia during routine tooth cavity preparation |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine Infiltrations = 40/50 Regional = 43/50 3% mepivacaine, no epinephrine Infiltrations = 42/50 Regional = 42/50 |
| Nordenram 1990 | Maxillary BI of 0.6 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:80,000 epinephrine BIs = 38/40 3% mepivacaine, no vasoconstrictor BIs = 34/40 3% prilocaine, 0.03 IU/mL felypressin BIs = 34/40 |
| Odabas 2012 | Maxillary BI using 1.8 mL of 1 of the following:
|
Success of pulpal anaesthesia during operative dentistry procedures in deciduous teeth |
Clinical anaesthesia success 4% articaine, 1:200,000 epinephrine BIs = 50/50 3% mepivacaine, no epinephrine BIs = 50/50 |
| Parirokh 2015 | IANB (1.8 mL) using the following:
|
Success of soft tissue anaesthesia (subjectively measured) |
Soft tissue anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 29/29 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 30/30 |
| Porto 2007 | IANB and BI (a minimum of 3.6 mL in total) using 1 of the following:
|
Success of pulpal anaesthesia (Endofrost) Success of anaesthesia during extraction of lower third molars (tested by recording teeth requiring re‐anaesthesia) |
Pulp anaesthesia success (Endofrost) 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 28/35 2% mepivacaine, 1:100,000 epinephrine IANB/BIs = 29/35 Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 32/25 2% mepivacaine, 1:100,000 epinephrine IANB/BIs = 33/25 |
| Ram 2006 | IANB and maxillary BI (up to 1 cartridge) using the following:
|
Success of anaesthesia during paediatric dental procedures |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 53/62 4% articaine, 1:200,000 epinephrine IANB/BIs = 54/62 |
| Sadove 1962 | Various types of dental block and infiltration, using varying volumes of 1 of the following:
|
Success of pulpal anaesthesia during restorative and surgical procedures |
Clinical anaesthesia success (surgery) 2% lidocaine, 1:100,000 epinephrine Injections = 119/148 2% mepivacaine, 1:20,000 levonordefrin Injections = 102/130 Clinical anaesthesia success (restorative) 2% lidocaine, 1:100,000 epinephrine Injections = 23/26 2% mepivacaine, 1:20,000 levo‐nordefrin Injections = 39/39 |
| Sampaio 2012 | IANB using 3.6 mL of 1 of the following:
|
Success of pulpal anaesthesia during access cavity preparation and instrumentation Success of pulpal anaesthesia tested with an electric pulp tester |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 14/35 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 8/35 Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine IANBs = 15/35 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 7/35 |
| Sherman 1954 | Mandibular and maxillary injections using 1.1 mL‐2.2 mL of 1 of the following:
|
Pulpal anaesthesia during operative dentistry procedures |
Clinical anaesthesia success 2% lidocaine, 1:50,000 adrenaline BIs = 84/100 2% lidocaine, 1:100,000 adrenaline BIs = 88/100 |
| Sherman 2008 | Gow‐Gates IANB and maxillary BI of 1 of the following:
|
Success of pulpal anaesthesia tested with Endo‐Ice |
Pulp anaesthesia success (Endo‐Ice) 4% articaine with 1:100,000 epinephrine IANB/BIs = 19/20 2% lidocaine with 1:100,000 epinephrine IANB/BIs = 19/20 |
| Sood 2014 | IANB (1.8 mL) of:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 50/50 4% articaine, 1:100,000 epinephrine IANBs = 50/50 |
| Srisurang 2011 | Maxillary BI (0.9 mL) and PI (0.3 mL) of:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine BI/PIs = 15/16 4% articaine, 1:100,000 epinephrine BI/PIs = 16/16 2% mepivacaine, 1:100,000 epinephrine BI/PIs = 16/16 |
| Stibbs 1964 | Various mandibular and maxillary injections and varying volumes of 1 of the following:
|
Success of pulpal anaesthesia during "restorative operations" |
Clinical anaesthesia success 2% mepivacaine, 1:20,000 levonordefrin Infiltrations = 90/99 Mandibular = 97/107 2% lidocaine, 1:50,000 epinephrine Infiltrations = 90/102 Mandibular = 96/114 |
| Vahatalo 1993 | Maxillary BI 0.6 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:80,000 epinephrine BIs = 20/20 4% articaine, 1:200,000 epinephrine BIs = 20/20 |
| Vilchez‐Perez 2012 | BI (0.9 mL) of 1 of the following:
|
Success of subjective soft tissue anaesthesia Success of pulpal anaesthesia tested with an electric pulp tester |
Soft tissue anaesthesia success 4% articaine, 1:200,000 epinephrine BIs = 16/20 0.5% bupivacaine, 1:200,000 epinephrine BIs = 16/20 Pulp anaesthesia success (EPT) 4% articaine, 1:200,000 epinephrine BIs = 20/20 0.5% bupivacaine, 1:200,000 epinephrine BIs = 18/20 |
| Vreeland 1989 | IANB of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine 1:100,000 epinephrine IANBs = 30/30 2% lidocaine, 1:200,000 epinephrine IANBs = 30/30 |
| Weil 1961 | Mandibular and maxillary injections using 1 or more cartridges, if required, of 1 of the following:
|
Success of anaesthesia during operative dentistry procedures |
Clinical anaesthesia success 3% mepivacaine, no vasoconstrictor Mandibular = 89/91 Infiltration = 77/88 2% mepivacaine, 1:20,000 levo‐nordefrin Mandibular = 30/31 Infiltration = 39/40 |
| Yilmaz 2011 | IANB and maxillary BI (1.0 mL) of 1 of the following:
|
Success of soft tissue anaesthesia (probing buccal and lingual to the tooth in question) |
Soft tissue anaesthesia success 4% articaine, 100,000 epinephrine IANBs = 46/47 BIs = 32/32 3% prilocaine, 0.03 IU/mL felypressin IANBs = 42/42 BIs = 36/36 |
BI = buccal infiltration; DDM = dichlorodifluoromethane; EPT = electric pulp tester; Gow‐Gates = Gow‐Gates injection (Gow‐Gates 1973); IANB = inferior alveolar nerve block; IONB = infraorbital nerve block; LI = lingual infiltration; PI = palatal infiltration.
Unit of analysis issues
The studies identified were a combination of parallel and cross‐over studies. Therefore, to pool data for both types of studies, we performed the meta‐analysis in several stages.
We performed a meta‐analysis on parallel‐group studies only, using the ‘inverse variance’ method to generate odds ratios. We used a fixed‐effect analysis or random‐effects analysis model depending on whether there were signs of statistical heterogeneity from the I² and P value. From these values, we generated logs of the OR and standard errors (SEs).
We used Microsoft Excel to generate the log of the OR and associated SEs for cross‐over studies from the studies' paired data, if available.
We completed the meta‐analysis using Review Manager (RevMan 2014) by entering the generic inverse variance data of logs of the OR and associated SEs from both types of studies using the 'inverse variance' method. We used a fixed‐effect or random‐effects analysis model depending on whether there were signs of statistical heterogeneity from the I² and P value (P ≤ 0.05, I² ≥ 50% (substantial heterogeneity)).
When paired data were not available, we used data from cross‐over studies in the analysis as if they were derived from parallel studies to estimate the overall effect of interest in the meta‐analysis. The confidence intervals were wider when we used this approach; therefore we performed a sensitivity analysis while removing the data from cross‐over studies from the meta‐analysis, when present.
We assessed statistical heterogeneity by calculating the 'Q' statistic and I² (Higgins 2011a).
Dealing with missing data
When data were unclear or missing, we contacted study authors to clarify the data. In circumstances for which clarification was not possible, we assessed the effect of including these studies by performing sensitivity analysis.
Assessment of heterogeneity
We planned to assess sources of heterogeneity between studies by performing sensitivity analyses and meta‐analysis regression (STATA 13) while exploring, quantifying, and controlling for this factor whenever it was possible to do so. Our planned analyses for heterogeneity are outlined below.
Participant characteristics
Participants undergoing treatment or volunteers
Treatment characteristics
Clinical procedure carried out
Type of local anaesthetic administered
Dosage of local anaesthetic given
Concentration of local anaesthetic used
Number of similar injections given
Number of injection techniques applied
Types of injection techniques used
Study design characteristics
Randomization
Allocation concealment
Blinding
Completeness of follow‐up
Simulated scenario studies using a cross‐over design and evaluating carry‐over effects
Length of study
Source of funding
We considered the following subgroups for analysis.
Tooth type
Presence of inflammation (pulpitis)
Tissue type anaesthetized
Treatment type
Type of injection
Age of participant
Type of study (treatment vs simulated scenario)
Pharmaceutical company sponsorship
When we identified other important sources of heterogeneity during the course of the review, we explored and identified these as post hoc analyses.
Assessment of reporting biases
We planned to assess the possibility of publication bias and other possible biases related to the size of trials via graphical methods, the Begg and Mazumdar adjusted rank correlation test (Begg 1994), and the regression asymmetry test (Egger 1997).
Data synthesis
We collated data into evidence tables, grouped according to local anaesthetic. We formulated a descriptive summary to determine the quality of data, checking further for study variations in terms of study characteristics, quality, and results. This assisted us in confirming the suitability of further synthesis methods, including possible meta‐analysis.
We used fixed‐effect or random‐effects meta‐analyses as appropriate, based on the 'Q' statistic (P < 0.10) to combine quantitative data. For continuous data, we expressed pooled outcomes as pooled MDs with their associated 95% CIs. For binary data, these were predominantly pooled ORs or RRs and associated 95% CIs.
Subgroup analysis and investigation of heterogeneity
We grouped outcomes according to which dental tissues required anaesthesia.
Studies testing healthy pulps and hard and soft tissues (e.g. extractions).
Studies testing healthy pulps (e.g. cavity preparations).
Studies testing diseased pulps with irreversible pulpitis.
Studies testing different individual dental tissues, when their results were pooled.
Studies in which it was unclear exactly which dental tissues required anaesthesia (e.g. endodontic treatment when necrotic or inflamed pulps may have been treated).
Studies in which healthy pulps were tested in simulated scenarios.
Studies in which diseased pulps with irreversible pulpitis were tested in simulated scenarios.
Studies in which soft tissues were tested in simulated scenarios.
In addition, we conducted a subgroup analysis of those studies chosen for meta‐analysis to see if it was appropriate to combine studies concerned with anaesthesia in the maxilla, the mandible, or both jaws pooled/when the jaw tested was not clear.
We combined the results of trials only if levels of clinical heterogeneity were low to ensure that effects measured were meaningful. We assessed statistical heterogeneity by calculating the 'Q' statistic and I² (Higgins 2011a). We performed analysis using Review Manager (RevMan 2014).
Sensitivity analysis
We performed sensitivity analyses to investigate the robustness of results of our primary outcomes. We did this to explore the influence of study quality in terms of those factors influencing bias: generation and concealment of the randomisation sequence, blinding, attrition bias, reporting bias, or other bias. We also explored the influence of cross‐over studies, for which paired data were not available, on the same outcome.
'Summary of findings' tables and GRADE
We used the GRADE approach to assess the quality of evidence related to each of the outcomes. We used the GRADE profiler (GRADEpro GDT) to import data from RevMan 2014 and to create 'Summary of findings' tables for the eight major comparisons in this review.
4% articaine, 1:100,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine (Table 1).
3% prilocaine, 0.03 IU felypressin versus 2% lidocaine, 1:100,000 epinephrine (Table 2).
4% articaine, 1:200,000 epinephrine versus 4% articaine, 1:100,000 epinephrine (Table 3).
4% prilocaine plain versus 2% lidocaine, 1:100,000 epinephrine (Table 4).
4% articaine, 1:200,000 epinephrine versus 0.5% bupivacaine, 1:200,000 epinephrine (Table 5).
0.5% bupivacaine, 1:200,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine (Table 6).
4% articaine, 1:100,000 epinephrine versus 2% mepivacaine, 1:100,000 epinephrine (Table 7).
2% mepivacaine, 1:100,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine (Table 8).
Summary of findings for the main comparison. 4% articaine, 1:100,000 epinephrine compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia.
| 4% articaine, 1:100,000 epinephrine compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested Settings: university departments in Brazil (n = 2), India (n = 1), and USA (n = 4) Intervention: 4% articaine, 1:100,000 epinephrine Comparison: 2% lidocaine, 1:100,000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 2% lidocaine, 1:100,000 epinephrine | 4% articaine, 1:100,000 epinephrine | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of diseased pulps with irreversible pulpitis) Absence of pain ('0' on a visual or verbal analogue scale. Scales of 0‐3, 0‐4, 0‐10, and Heft‐Parker VAS) Follow‐up: from 10 minutes post injection to end of the clinical procedure | Moderatea | RR 1.6 (1.1 to 2.32) | 203 participants, 203 interventions (4 studies) | ⊕⊕⊝⊝ lowb,c | Duration of follow‐up not reported (estimated to be < 1 hour) | |
| 309 per 1000 | 494 per 1000 (340 to 717) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) Heft‐Parker VAS (0‐170 millimetres) Follow‐up: 0‐1 minute following needle insertion |
Mean pain on injection in the lidocaine group was 34.92 mm | Mean pain on injection in the articaine group was 4.74 mm higher (1.98 mm lower to 11.46 mm higher) | 157 participants, 314 interventions (3 studies) | ⊕⊕⊕⊝ moderatec | ||
|
Adverse effects: pain following injection Heft‐Parker VAS (0‐170 millimetres) Follow‐up: measured at the time anaesthesia wore off |
Mean pain following injection in the lidocaine group was 18.54 mm | Mean pain following injection in the articaine group was 6.41 mm higher (1.01 mm to 11.8 mm higher). | 156 participants, 309 interventions (3 studies) | ⊕⊕⊕⊝ moderatec | Exact times of follow‐up not reported | |
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: not applicable |
Not measured | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to study limitations (unclear risks of selection and detection bias). cDowngraded one level owing to imprecision (small total sample size).
Summary of findings 2. 3% prilocaine, 0.03 IU felypressin compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia.
| 3% prilocaine, 0.03 IU felypressin compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested Settings: university departments in Germany Intervention: 3% prilocaine, 0.03 IU felypressin Comparison: 2% lidocaine, 1:100,000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 2% lidocaine, 1:100,000 epinephrine | 3% prilocaine, 0.03 IU felypressin | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) Absence of pain Follow‐up: not reported | Moderatea | RR 0.86 (0.79 to 0.95) | 907 participants, 907 interventions (2 studies) | ⊕⊕⊕⊝ moderateb | Duration of follow‐up not reported (estimated to be < 2 hours) | |
| 763 per 1000 | 656 per 1000 (603 to 725) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) VAS Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain following injection VAS Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: not applicable |
Not measured | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to study limitations (unclear risk of attrition bias in one study, and both trials have unclear methods of randomization sequence generation and allocation concealment).
Summary of findings 3. 4% articaine, 1:200,000 epinephrine compared with 4% articaine, 1:100,000 epinephrine for dental anaesthesia.
| 4% articaine, 1:200,000 epinephrine compared with 4% articaine, 1:100,000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested Settings: university departments in Brazil (n = 1), Germany (n = 2), and USA (n = 2; pain on injection/pain following injection and allergy) Intervention: 4% articaine, 1:200,000 epinephrine Comparison: 4% articaine, 1:100,000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 4% articaine, 1:100,000 epinephrine | 4% articaine, 1:200,000 epinephrine | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) Absence of pain Follow‐up: from 5 minutes post injection to end of the clinical procedure | Moderatea | RR 0.85 (0.71 to 1.02) | 930 participants, 930 interventions (3 studies) | ⊕⊝⊝⊝ very lowb,c,d | Duration of follow‐up not reported (estimated to be < 1 hour) | |
| 940 per 1000 | 799 per 1000 (667 to 959) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) Heft‐Parker VAS (0‐170 millimetres) Follow‐up: 0‐1 minute following needle insertion |
See comment | See comment | 86 participants, 172 interventions (1 study) | See comment | Orphan study | |
|
Adverse effects: pain following injection Heft‐Parker VAS (0‐170 millimetres) Follow‐up: measured at the time anaesthesia wore off |
See comment | See comment | 86 participants, 172 interventions (1 study) | See comment | Orphan study. Exact time of follow‐up not reported | |
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: 0‐24 hours |
See comment | See comment | Not estimable | 63 participants, 187 interventions (1 study) | See comment | 1 case of urticaria occurred ‐ unclear which local anaesthetic this occurred with |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to study limitations (unclear risk of attrition bias in one trial; unclear risks of selection bias in two trials). cDowngraded one level owing to inconsistency (substantial, unexplained heterogeneity). dDowngraded one level owing to imprecision (95% CI includes no effect and an appreciable benefit for 4% articaine, 1:100,000 epinephrine)
Summary of findings 4. 4% prilocaine plain compared with 2% lidocaine 1:100, 000 epinephrine for dental anaesthesia.
| 4% prilocaine plain compared with 2% lidocaine 1:100, 000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested Settings: private practice and a hospital setting in USA Intervention: 4% prilocaine plain Comparison: 2% lidocaine 1:100, 000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 2% lidocaine 1:100, 000 epinephrine | 4% prilocaine plain | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) Absence of pain ("complete anaesthesia") Follow‐up: 5‐30 minutes | Moderatea | RR 0.86 (0.75 to 0.99) | 228 participants, 228 interventions (2 studies) | ⊕⊕⊝⊝ lowb,c | Duration of follow‐up reported only for bone study | |
| 828 per 1000 | 712 per 1000 (621 to 820) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) VAS Follow‐up: See comment |
Not measured | |||||
|
Adverse effects: pain following injection VAS Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: See comment |
See comment | 1 case of prolonged anaesthesia recorded | Not estimable | 0 participants (0 studies) | See comment | No clinical studies met outcome definition Unable to confirm if prolonged anaesthesia = paraesthesia and how long this lasted |
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: not applicable |
Not measured | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to study limitations (unclear methods of randomization sequence generation and allocation concealment). cDowngraded one level owing to imprecision (small total sample size).
Summary of findings 5. 4% articaine, 1:200,000 epinephrine compared with 0.5% bupivacaine, 1:200,000 epinephrine for dental anaesthesia.
| 4% articaine, 1:200,000 epinephrine compared with 0.5% bupivacaine, 1:200,000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies where dental local anaesthesia was tested Settings: university departments in Spain (n = 2) and USA (n = 1; pain on injection) Intervention: 4% articaine, 1:200,000 epinephrine Comparison: 0.5% bupivacaine, 1:200,000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 0.5% bupivacaine, 1:200,000 epinephrine | 4% articaine, 1:200,000 epinephrine | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) Absence of pain Follow‐up: from 10 minutes post injection to the end of the clinical procedure | Moderatea | OR 0.87 (0.27 to 2.83) | 37 participants, 74 interventions (2 studies) | ⊕⊕⊝⊝ lowb,c | Duration of follow‐up not reported for both studies (estimated to be < 1 hour) | |
| 481 per 1000 | 446 per 1000 (200 to 724) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) VAS. scale of 0‐100 Follow‐up: 0‐30 seconds following needle insertion |
See comment | See comment | 18 participants, 36 interventions (1 study) | See comment | Orphan study. Unclear whether data relate to just solution deposition. Standand deviations not reported | |
|
Adverse effects: pain following injection VAS Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: not applicable |
Not measured | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; OR = odds ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to study limitations (trials had unclear or high risk of bias related to methods of randomization sequence generation and allocation concealment, and one study had high risk of bias for blinding of participants and personnel and incomplete outcome data (high attrition rate of 46%)). cDowngraded one level owing to imprecision (small total sample size, and 95% confidence interval includes no effect and an appreciable benefit for both solutions).
Summary of findings 6. 0.5% bupivacaine, 1:200,000 epinephrine compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia.
| 0.5% bupivacaine, 1:200,000 epinephrine compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested Settings: university departments in Australia (n = 1) and USA (n = 3, including speed of onset (1) and pain on injection (1)) Intervention: 0.5% bupivacaine, 1:200,000 epinephrine Comparison: 2% lidocaine, 1:100,000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 2% lidocaine, 1:100,000 epinephrine | 0.5% bupivacaine, 1:200,000 epinephrine | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) Absence of pain Follow‐up: from 10 minutes post injection to the end of the clinical procedure | Moderatea | OR 0.58 (0.07 to 5.12) | 31 participants, 62 interventions (2 studies) | ⊕⊕⊝⊝ lowb,c | Duration of follow‐up not reported for both studies (estimated to be < 1 hour) | |
| 611 per 1000 | 477 per 1000 (99 to 889) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: See comment |
See comment | See comment | Not estimable | 100 participants, 100 interventions (1 study) | See comment | Orphan study. Duration of follow‐up not reported |
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) VAS. scale of 0‐100 Follow‐up: 0‐30 seconds following needle insertion |
See comment | See comment | 18 participants, 36 interventions (1 studies) | See comment | Orphan study. Unclear whether data relate to just solution deposition. Standand deviations not reported | |
|
Adverse effects: pain following injection VAS Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: not applicable |
Not measured | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; OR = odds ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to study limitations (unclear methods of randomization sequence generation). cDowngraded one level owing to imprecision (small total sample size, and 95% confidence interval includes no effect and an appreciable benefit for both solutions).
Summary of findings 7. 4% articaine, 1:100,000 epinephrine compared with 2% mepivacaine, 1:100,000 epinephrine for dental anaesthesia.
| 4% articaine, 1:100,000 epinephrine compared with 2% mepivacaine, 1:100,000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested Settings: university departments in Brazil (n = 1), Saudi Arabia (n = 1), and Thailand (n = 1) Intervention: 4% articaine, 1:100,000 epinephrine Comparison: 2% mepivacaine, 1:100,000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 2% mepivacaine, 1:100,000 epinephrine | 4% articaine, 1:100,000 epinephrine | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) Absence of pain Follow‐up: 10‐20 minutes | Moderatea | OR 3.82 (0.61 to 23.82) | 110 participants, 130 interventions (2 studies) | ⊕⊕⊝⊝ lowb,c | ||
| 931 per 1000 | 996 per 1000 (912 to 1000) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) VAS. scale of 0‐100 Follow‐up: 0‐40 seconds and 0‐60 seconds following needle insertion |
See comment | See comment | 147 participants, 147 interventions (2 studies) | See comment | Unclear whether data relate to just solution deposition in both studies | |
|
Adverse effects: pain following injection VAS Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: not applicable |
Not measured | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to study limitations (unclear risks of bias (methods of randomization sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessors)). cDowngraded one level owing to imprecision (small total sample size).
Summary of findings 8. 2% mepivacaine, 1:100,000 epinephrine compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia.
| 2% mepivacaine, 1:100,000 epinephrine compared with 2% lidocaine, 1:100,000 epinephrine for dental anaesthesia | ||||||
| Patient or population: participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested Settings: university departments in Brazil (n = 2) and Saudi Arabia (n = 1) Intervention: 2% mepivacaine, 1:100,000 epinephrine Comparison: 2% lidocaine, 1:100,000 epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 2% lidocaine, 1:100,000 epinephrine | 2% mepivacaine, 1:100,000 epinephrine | |||||
| Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of diseased pulps with irreversible pulpitis) Absence of pain Follow‐up: from 10 minutes or 14 minutes post injection to the end of the clinical procedure | Moderatea | RR 1.16 (0.25 to 5.45) | 68 participants, 68 interventions (2 studies) | ⊕⊕⊝⊝ lowb,c | Duration of follow‐up not reported (estimated to be < 1 hour) | |
| 231 per 1000 | 268 per 1000 (58 to 1000) | |||||
|
Speed of onset of anaesthesia Time from injection to complete anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Duration of anaesthesia Time from onset of anaesthesia to loss of anaesthesia, measured in minutes Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: pain on injection (solution deposition) VAS. scale of 0‐100 Follow‐up: 0‐1 minute following needle insertion |
See comment | See comment | 48 participants, 48 interventions (1 study) | See comment | Orphan study. Unclear whether data relate to just solution deposition | |
|
Adverse effects: pain following injection VAS Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: paraesthesia following injection Number of participants Follow‐up: not applicable |
Not measured | |||||
|
Adverse effects: allergy to local anaesthetic Number of participants Follow‐up: not applicable |
Not measured | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; VAS = visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aLittle variation in baseline risks across studies. bDowngraded one level owing to inconsistency (wide variation in point estimates and substantial unexplained heterogeneity). cDowngraded one level owing to imprecision (small total sample size, and 95% CI includes no effect and an appreciable benefit for both solutions).
When assessing the quality of evidence for each outcome including pooled data from RCTs, we downgraded evidence from 'high quality' by one level for serious (or by two levels for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Two review authors (GST and AM) independently assessed the quality of evidence for each outcome. When we were unable to come to an agreement on assessment of quality, we (GST and AM) resolved disagreements initially by mutual discussion. When a difference of opinion could not be resolved, we involved a third review author ‐ John Meechan (JM).
We included in the 'Summary of findings' tables the following outcomes for a variety of local anaesthetic comparisons.
Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method, or by measuring pulpal anaesthesia via an electric pulp tester or cold stimulus.
Speed of onset and duration of anaesthesia.
Adverse effects: local and systemic.
Results
Description of studies
Results of the search
Our search identified 1601 citations from MEDLINE, 2791 from Embase, 1351 from CENTRAL, 2544 from CINAHL PLUS, 595 from Web of Science, and 2566 from other electronic sources, yielding a total of 11,448 citations. We performed searches in other Internet databases and identified 2566 citations (Australian New Zealand Clinical Trials Registry (ANZCTR); IndMED;Ingenta Connect; KoreaMED; Panteleimon). From all these databases, we found 3148 citations to be duplicates and 7903 to be irrelevant studies or studies that were not RCTs.
Other sources revealed 255 citations from bibliographies/reference lists; 56 from conference proceedings, of which 16 were available only as an abstract; and 63 from handsearched journals. From these, we found 39 to be duplicate citations and 73 to be irrelevant studies or studies that were not RCTs.
Searching for unpublished dissertations on Internet databases (OpenSIGLE) and other resources (metaRegister of Controlled Trials; National Research Register Archive; UK Clinical Research Network (UKCRN) Study Portfolio) revealed two additional studies (searched in December 2013). These were found on the National Research Register Archive. After communication, we excluded one because it was not completed (the study author is an author of this review ‐ JM) and we excluded the second (author ‐ Simpson E) because it included participants treated under sedation. Since the time this database was archived, the original references have no longer been available for referencing.
We repeated the searches at regular intervals up to 31 January 2018.
Removal of irrelevant or non‐randomized controlled trials and duplicates and screening by their titles resulted in 659 articles. We screened all of these using their abstracts, which led to exclusion of 317 and further screening of 342 full‐text articles. This relatively large number comprised relevant studies, older articles with vague titles and no abstract, a large number of non‐English titles, and articles that initially appeared to be testing different outcomes but may have been testing our primary objectives.
We located 56 conference proceedings, of which 39 abstracts were published as full‐text articles at a later date; one had been published in full in the conference proceeding. We had identified these through our database searches and handsearches. Of 16 unpublished abstracts, we deemed three to be relevant. We located one recently and placed it in the category of 'Ongoing studies' (see Characteristics of ongoing studies) (Sheikh 2014), we emailed one study author to request data (Caicedo 1996), and we found that another study author was deceased (Iqbal 2009).
We entered 34 studies under Characteristics of studies awaiting classification. These studies require translation or further information from study authors.
We attempted to contact authors by email for clarification of study methods and to obtain data. We emailed the authors of 103 studies to request further information and found that 20 provided no means of contact. The authors of 73 studies replied to our queries, and the authors of 30 studies did not reply. For 18 studies, we made initial contact with study authors but received no replies to further emails. We found that the authors of four studies were deceased (Albertson 1963; Chilton 1971; Fertig 1968; Nespeca 1976).
We described the included studies under Characteristics of included studies.
We used 123 articles (19,223 participants) for qualitative analysis and determined that 68 of these (6615 participants) were suitable for quantitative analysis. Many studies compared more than two formulations of local anaesthetic and reported numerous outcomes including success and onset and duration of local anaesthesia in different tissues. This meant that we found more comparisons and outcomes than individual studies. Only 68 studies were suitable for meta‐analysis because 57 were classified as orphan studies and 80 provided data that were not usable in meta‐analysis for certain comparisons and outcomes. We summarized in Table 9; Table 10; Table 11; Table 12; Table 13; and Table 14 data for primary outcomes that were not included in meta‐analysis. Adverse effects were rare and were difficult to compare; we summarized in Table 15 data that were not suitable for meta‐analysis.
6. Cross‐over and parallel studies (success: raw data not available/not usable).
| Study | Comparison | Outcome | Data |
| Allegretti 2016 | IANB of 3.6 mL of 1 of the following:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis, tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine IANBs = 14/22 4% articaine, 1:100,000 epinephrine IANBs = 14/22 2% mepivacaine, 1:100,000 epinephrine IANBs = 15/22 |
| Atasoy Ulusoy 2014 | Maxillary BI of 1.5 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with Endo‐Ice in teeth with irreversible pulpitis |
Pulp anaesthesia success (EPT) 4% articaine, 1:100,000 epinephrine BIs = 25/25 4% articaine, 1:100,000 epinephrine bitartrate BIs = 25/25 |
| Berberich 2009 | Intraoral, IONB of 1.8 mL of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:50,000 epinephrine IONBs = 40/40 2% lidocaine, 1:100,000 epinephrine IONBs = 40/40 3% mepivacaine, no vasoconstrictor IONBs = 40/40 |
| Bhagat 2014 | IANB using (volume not stated) 1 of the following:
|
Success of anaesthesia during surgical extraction of mandibular third molars 1. VAS (0‐10) 2. Faces Pain Scale (Wong 1988) |
Clinical anaesthesia success (VAS) 2% lidocaine, 1:100,000 epinephrine IANBs = 3.16 ± 2.053* 4% articaine, 1:100,000 epinephrine IANBs = 2.19 ± 1.543* Clinical anaesthesia success (Faces Pain Scale) 2% lidocaine, 1:100,000 epinephrine IANBs = 3.10 ± 1.750* 4% articaine, 1:100,000 epinephrine IANBs = 2.32 ± 1.351* |
| Chapman 1988 | IANB (2.0 mL) and BI (1.0 mL) of either:
|
Success of anaesthesia during surgical extraction of mandibular third molars |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANB/BIs = 20/20 0.5% bupivacaine, 1:200,000 epinephrine IANB/BIs = 20/20 |
| Cohen 1993 | IANB using 1.8 mL of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 27/27 3% mepivacaine, no vasoconstrictor IANBs = 34/34 |
| Dagher 1997 | IANB using 1.8 mL of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:50,000 epinephrine IANBs = 30/30 2% lidocaine, 1:80,000 epinephrine IANBs = 30/30 2% lidocaine, 1:100,000 epinephrine IANBs = 30/30 |
| Elbay 2016 | IANB using 0.9 mL of 1 of the following:
|
Success of clinical anaesthesia during extraction of mandibular primary molars |
Clinical anaesthetic success 2% lidocaine, 1:80,000 epinephrine IANBs = 10/30 3% mepivacaine, no vasoconstrictor IANBs = 10/30 |
| Fernandez 2005 | IANB (1.8 mL) of each of the following:
|
Success of soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 39/39 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 39/39 |
| Gazal 2015 | IANB (1.8 mL) of 2% mepivacaine, 1:100,000 epinephrine, followed by a BI (1.8 mL) of 1 of the following solutions:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% mepivacaine, 1:100,000 epinephrine IANB/BIs = 23/23 4% articaine, 1:100,000 epinephrine IANB/BIs = 23/23 |
| Gregorio 2008 | Mandibular block (1.8 mL) and local infiltration (0.9 mL) of 1 of the following:
|
Success of clinical anaesthesia during surgical removal of mandibular third molars |
Clinical anaesthesia success 4% articaine, 1:200,000 epinephrine IANB/BIs = 49/50 0.5% bupivacaine, 1:200,000 epinephrine IANB/BIs = 43/50 |
| Hersh 1995 | IANB (1.8 mL) of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 14/20 4% prilocaine, no vasoconstrictor IANBs = 14/19 3% mepivacaine, no vasoconstrictor IANBs = 17/21 |
| Hinkley 1991 | IANB (1.8 mL) of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 4% prilocaine, 1:200,000 epinephrine IANBs = 13/28 2% mepivacaine, 1:20,000 levonordefrin IANBs = 16/28 2% lidocaine, with 1:100,000 epinephrine IANBs = 15/28 |
| Hosseini 2016 | Maxillary BI (1.8 mL) of the following:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine BIs = 13/23 4% articaine, 1:100,000 epinephrine BIs = 16/24 |
| Jain 2016 | IANB and BI (1.7 mL) of the following:
|
Success of anaesthesia during surgical extraction of mandibular third molars (VAS 0‐10) |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 2.6 ± 1.06* 4% articaine, 1:100,000 epinephrine IANB/BIs = 1.31 ± 0.87* |
| Kambalimath 2013 | IANB and BI (volume not stated) of the following:
|
Success of anaesthesia during surgical extraction of mandibular third molars |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 26/30 4% articaine, 1:100,000 epinephrine IANB/BIs = 29/30 |
| Kammerer 2014 | BI (1.7 mL) of:
|
Success of pulpal anaesthesia tested with an electric pulp tester Success of soft tissue anaesthesia (VAS = 0‐10) |
Pulp anaesthesia success (EPT) 4% articaine, 1:100,000 epinephrine BIs = 10/10 4% articaine, 1:200,000 epinephrine BIs = 10/10 4% articaine, 1:400,000 epinephrine BIs = 10/10 Soft tissue anaesthesia success 4% articaine, no vasoconstrictor BIs = 1.4 ± 0.9* 4% articaine, 1:100,000 epinephrine BIs = 6.2 ± 3* 4% articaine, 1:200,000 epinephrine BIs = 6.4 ± 1.9* 4% articaine, 1:400,000 epinephrine BIs = 6.8 ± 2* |
| Kanaa 2012 | BI (2.0 mL) of:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis, tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 4% articaine, 1:100,000 epinephrine BIs = 38/50 2% lidocaine,1:80,000 epinephrine BIs = 35/50 |
| Karm 2017 | IANB and BI (1.8 mL) of:
|
Success of anaesthesia during impacted, mandibular, third molar removal (0‐100 mm VAS) Total volume of anaesthetic solution used (mL) Operator’s overall satisfaction (Likert scale: 1‐5) Participant’s overall satisfaction (Likert scale: 1‐5) |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANB/BIs = 13.7 ± 1.9 mm* 2% lidocaine, 1:200,000 epinephrine IANB/BIs = 20.0 ± 2.5 mm* Total volume of anaesthetic solution used 2% lidocaine, 1:80,000 epinephrine IANB/BIs = 3.6 ± 0.1* 2% lidocaine, 1:200,000 epinephrine IANB/BIs = 3.6 ± 0.2* Operator’s overall satisfaction 2% lidocaine, 1:80,000 epinephrine IANB/BIs = 3.9 ± 0.9* 2% lidocaine, 1:200,000 epinephrine IANB/BIs = 3.8 ± 1.0* Participant’s overall satisfaction 2% lidocaine, 1:80,000 epinephrine IANB/BIs = 3.6 ± 0.1* 2% lidocaine, 1:200,000 epinephrine IANB/BIs = 3.7 ± 0.1* |
| Keskitalo 1975 | IANB and BI (3.6 mL initially) of 1 of the following:
|
Success of anaesthesia during impacted, mandibular, third molar removal |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANB/BIs = 163/188 3% prilocaine, 0.03 IU/mL felypressin IANB/BIs = 138/191 |
| Knoll‐Kohler 1992b | Maxillary BI using 0.5 mL of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine BIs = 12/12 4% articaine, 1:100,000 epinephrine BIs = 12/12 |
| Kolli 2017 | Maxillary BI (1.7 mL) of:
(epinephrine concentrations assumed) Maxillary BI/PI (1.7 mL in total) of:
|
Success of anaesthesia during extraction of primary maxillary molars
|
Faces Pain Scale ‐ Revised 2% lidocaine, 1:80,000 epinephrine BIs = 2.67 ± 1.91* 4% articaine, 1:100,000 epinephrine BIs = 1.20 ± 1.34* 2% lidocaine, 1:80,000 epinephrine BI/PIs = 0.73 ± 1.11* FLACC Scale 2% lidocaine, 1:80,000 epinephrine BIs = 2.17 ± 1.46* 4% articaine, 1:100,000 epinephrine BIs = 1.27 ± 1.28* 2% lidocaine, 1:80,000 epinephrine BI/PIs = 0.80 ± 0.84* |
| Kramer 1958 | Mandibular and maxillary injections using 1 cartridge, or more if required, of 1 of the following:
|
Success of pulpal anaesthesia during operative dentistry procedures |
Clinical anaesthesia success 2% lidocaine, 1:50,000 epinephrine Maxillary = 86% Mandibular = 82.5% 2% lidocaine, 1:100,000 epinephrine Maxillary = 80.2% Mandibular = 76% |
| Malamed 2000a | Standard infiltration or nerve block of the following mean volumes: Simple procedures:
Complex procedures:
|
Success of anaesthesia during various dental procedures |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine Simple procedures = 0.4 cm (range 0‐8 cm) Complex procedures = 0.6 cm (range 0‐8.7 cm) 2% lidocaine, 1:100,000 epinephrine Simple procedures = 0.6 cm (range 0‐9.8 cm) Complex procedures = 0.7 cm (range 0‐7.7 cm) |
| Malamed 2000b | Standard infiltration or nerve block of the following mean volumes: Simple procedures:
Complex procedures:
|
Success of anaesthesia during various dental procedures |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine Simple procedures = 0.5 cm (range 0‐5.5 cm) Complex procedures = 1.1 cm (range 0‐0.25 cm) 2% lidocaine, 1:100,000 epinephrine Simple procedures = 0.7 cm (range 0‐3.0 cm) Complex procedures = 2.3 cm (range 0‐4.5 cm) |
| Maniglia‐Ferreira 2009 | IANB (1 cartridge) of 1 of the following:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis |
Clinical anaesthesia success 2% mepivacaine, 1:100,000 epinephrine IANBs = 2.8 cartridges 4% articaine, 1:100,000 epinephrine IANBs = 2.6 cartridges |
| Maruthingal 2015 | Mandibular BI (1.7 mL) of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester Success of soft tissue anaesthesia |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine BIs = 17/32 4% articaine, 1:100,000 epinephrine BIs = 28/32 Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine BIs = 32/32 4% articaine, 1:100,000 epinephrine BIs = 32/32 |
| McLean 1993 | IANB of 1.8 mL of 1 of the following:
|
Success of soft tissue anaesthesia |
Soft tissue anaesthesia success 3% mepivacaine, no vasoconstrictor IANBs = 30/30 2% lidocaine, 1:100,000 epinephrine IANBs = 30/30 |
| Mittal 2015 | Maxillary BI of 1 of the following:
|
Success of anaesthesia during extraction of primary maxillary molars 1. Wong‐Baker FACES Pain Rating Scale 2. Modified Behaviour Pain Scale (Taddio 1994)
|
Clinical anaesthesia success (Wong‐Baker FACES pain rating scale) 2% lidocaine, 1:80,000 epinephrine BIs = 1.88 ± 1.688* 4% articaine, 1:100,000 epinephrine BIs = 1.31 ± 1.13* Modified Behaviour Pain Scale 2% lidocaine, 1:80,000 epinephrine BIs:
4% articaine, 1:100,000 epinephrine BIs:
|
| Moore 2007 | Maxillary BI (buccal and palatal if required) using 1 of the following:
|
Success of anaesthesia during periodontal surgery |
Clinical anaesthesia success 4% articaine, 1:200,000 epinephrine BIs = 42/42 4% articaine, 1:100,000 epinephrine BIs = 42/42 |
| Nabeel 2014 | Maxillary BI (1.7 mL) using the following:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine BIs = 33/38 4% articaine, 1:100,000 epinephrine BIs = 35/38 |
| Naik 2017 | IANB (2 mL) using the following:
|
Success of anaesthesia during surgical extraction of mandibular third molars (volume of solution in mL) |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 2.2 ± 0.56 mL* 4% articaine, 1:100,000 epinephrine IANBs = 2.0 ± 0.14 mL* |
| Ozec 2010 | Maxillary BI (1.7 mL) using 1 of the following:
|
Success of soft tissue anaesthesia (Heft‐Parker VAS) |
Soft tissue anaesthesia success 4% articaine, 1:200,000 epinephrine BIs = 75.53 ± 49.78 mm*** 4% articaine, 1:100,000 epinephrine BIs = 57.20 ± 46.69 mm*** |
| Parirokh 2015 | IANB (1.8 mL) using the following:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis (VAS score of zero or mild pain ≤ 54 mm on a Heft‐Parker visual analogue scale) |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 7/29 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 6/30 |
| Pellicer‐Chover 2013 | IANB (1.8 mL) and BI (1.8 mL) using the following:
|
Success of anaesthesia during impacted, mandibular, third molar removal (no discomfort, or slight discomfort but not requiring additional anaesthesia) |
Clinical anaesthetic success 0.5% bupivacaine, 1:200,000 epinephrine IANB/BIs = 20/36 4% articaine, 1:100,000 epinephrine IANB/BIs = 30/36 |
| Poorni 2011 | IANB (1.8 mL) using 1 of the following:
or a BI (1.8 mL) using:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis |
Clinical anaesthetic success 4% articaine 1:100,000 epinephrine IANBs = 39/52 2% lidocaine, 1:100,000 epinephrine IANBs = 36/52 4% articaine, 1:100,000 epinephrine BIs = 36/52 |
| Ruprecht 1991 | Maxillary BI (0.5 mL) of 1 of the following:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success 4% articaine, 1:100,000 epinephrine BIs = 10/10 2% lidocaine, 1:100,000 epinephrine BIs = 10/10 |
| Sampaio 2012 | IANB using 3.6 mL of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 35/35 0.5% bupivacaine, 1:200,000 epinephrine IANBs = 35/35 |
| Santos 2007 | IANB (1.8 mL) and mandibular BI (0.9 mL) of 1 of the following:
|
Success of anaesthesia during extraction (3‐point scale: 1‐3) |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine IANB/BIs (with osteotomy) = 1.04 ± 0.04** IANB/BIs (without osteotomy) = 1.00 ± 0.00** 4% articaine, 1:200,000 epinephrine IANB/BIs (with osteotomy) = 1.17 ± 0.08** IANB/BIs (without osteotomy) = 1.11 ± 0.08** |
| Sherman 2008 | Gow‐Gates IANB and maxillary BI of 1 of the following:
|
Success of pulpal anaesthesia during pulpotomy |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine Gow‐Gates = 9/10 Max' infiltration = 10/10 2% lidocaine, 1:100,000 epinephrine Gow‐Gates = 8/11 Max' infiltration = 8/9 |
| Sierra Rebolledo 2007 | IANB (1.8 mL) and BI (1.8 mL) of 1 of the following:
|
Success of anaesthesia during tooth removal (visual analogue scale from 0‐100 mm) |
Clinical anaesthetic success (means and standard deviations) 4% articaine, 1:100,000 epinephrine IANB/BIs = 13.81 mm ± 3.012 mm* 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 12.83 mm ± 3.186 mm* |
| Silva 2012 | IANB (3.6 mL) and mandibular BI (0.9 mL) of 1 of the following:
|
Success of anaesthesia during tooth extraction (volume of local anaesthetic solution) |
Clinical anaesthesia success (means and standard deviations) 4% articaine, 1:100,000 epinephrine IANB/BIs = 5.76 ± 1.09 mL* 2% lidocaine, 1:100,000 epinephrine IANB/BIs = 6.12 ± 0.96 mL* |
| Sood 2014 | IANB (1.8 mL) of:
|
Success of anaesthesia during pulp extirpation Success of pulpal anaesthesia in teeth with irreversible pulpitis, tested with an electric pulp tester |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANBs = 41/50 4% articaine, 1:100,000 epinephrine IANBs = 44/50 Pulp anaesthesia success (EPT) 2% lidocaine, 1:80,000 epinephrine IANBs = 29/50 4% articaine, 1:100,000 epinephrine IANBs = 38/50 |
| Tortamano 2009 | IANB (3.6 mL) of:
|
Success of pulpal anaesthesia tested with an electric pulp tester |
Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine IANBs = 14/20 4% articaine, 1:100,000 epinephrine IANBs = 13/20 |
| Trieger 1979 | IANB and infiltration anaesthesia, using variable volumes of:
Note ‐ some patients received a general anaesthetic, and injections were given at the end of surgery. |
Success of anaesthesia during tooth extraction (dose/quadrant) |
Clinical anaesthesia success 0.5% bupivacaine, 1:200,000 epinephrine IANB/BIs = 11.95 mg 3% mepivacaine, no epinephrine IANB/BIs = 68.18 mg |
| Visconti 2016 | IANB (1.8 mL or 3.6 mL) of:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis (4‐point scale: 0‐4) Success of pulpal anaesthesia in teeth with irreversible pulpitis, tested with an electric pulp tester Success of soft tissue anaesthesia |
Clinical anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs (1.8 mL) = 0/21 2% mepivacaine, 1:100,000 epinephrine IANBs (1.8 mL) = 6/21 Pulp anaesthesia success (EPT) 2% lidocaine, 1:100,000 epinephrine IANBs (1.8 mL) = 7/21 IANBs (3.6 mL) = 7/14 2% mepivacaine, 1:100,000 epinephrine IANBs (1.8 mL) = 11/21 IANBs (3.6 mL) = 7/10 Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 21/21 2% mepivacaine, 1:100,000 epinephrine IANBs = 21/21 |
| Wali 2010; | IANB of 1 of the following:
|
Success of soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:100,000 epinephrine IANBs = 30/30 2% lidocaine, 1:50,000 epinephrine IANBs = 30/30 |
| Yadav 2015 | IANB (1.8 mL) followed by BI (0.9 mL) and LI (0.9 mL) of the following:
|
Success of pulpal anaesthesia in teeth with irreversible pulpitis (VAS score of zero or mild pain ≤ 54 mm on a Heft‐Parker visual analogue scale) |
Clinical anaesthesia success 2% lidocaine, 1:80,000 epinephrine IANB/BI/LIs = 8/25 4% articaine, 1:100,000 epinephrine IANB/BI/LIs = 16/25 |
| Yared 1997 | IANB (3.6 mL) of 1 of the following:
|
Success of subjective soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:50,000 epinephrine IANBs = 30/30 2% lidocaine, 1:80,000 epinephrine IANBs = 30/30 2% lidocaine, 1:100,000 epinephrine IANBs = 30/30 |
| Yilmaz 2011 | IANB and maxillary BI of 1.0 mL of the following:
|
Success of anaesthesia during pulpotomy |
Clinical anaesthesia success 4% articaine, 1:100,000 epinephrine IANBs = 44/47 BIs = 9/32 3% prilocaine, 0.03 IU/mL felypressin IANBs = 39/42 BIs = 31/36 |
| Yonchak 2001 | Mandibular BI (1.8 mL) of 1 of the following:
|
Success of soft tissue anaesthesia |
Soft tissue anaesthesia success 2% lidocaine, 1:50,000 epinephrine BIs = 40/40 2% lidocaine, 1:100,000 epinephrine BIs = 40/40 |
* = mean ± standard deviation (SD); ** = mean ± standard error of the mean (SEM); ** = unsure if SD or SEM.
BI = buccal infiltration; EPT = electric pulp tester; IANB = inferior alveolar nerve block; IONB = infraorbital nerve block; VAS = visual analogue scale; Faces Pain Scale ‐ Revised = a modified version of the Faces Pain Scale (Hicks 2001); PI = palatal infiltration.
7. Adverse events.
| Adverse event | Method of measurement | Results | Statistical tests if reported |
| Pain on injection | |||
| Abdulwahab 2009 | 0–100 mm VAS (0 = no pain, 100 = worst pain ever) |
BI (0.9 mL) of:
|
Pain ratings were similar for all test anaesthetic formulations as compared with those for 2% lidocaine with 1:100,000 epinephrine (ANOVA, P = 0.19) |
| Aggarwal 2014 | 170 mm Heft‐Parker VAS:
|
IANB (1.8 mL) of: 2% lidocaine 1:80,000 epinephrine
2% lidocaine 1:200,000 epinephrine
|
There was no significant difference in injection pain of 2% lidocaine, 1:80,000 and 2% lidocaine, 1:200,000 solutions (P > 0.05) |
| Arrow 2012 | Faces Pain Scale ‐ Revised, dichotomized into ‘no or mild pain’ = 0 and ‘moderate to severe pain’ = 1 |
IANB or BI (up to 2.2 mL) of: 2% lidocaine, 1:80,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
There were no statistically significant differences between formulations with the test carried out (Faces: P = 0.65) |
| Batista da Silva 2010 | 100 mm VAS ranging from 0 = "no pain" to 100 = "unbearable pain" |
Mental nerve blocks (0.6 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
There was no significant difference (P > 0.05) between solutions regarding injection pain |
| Berberich 2009 | Pain scale:
|
Intraoral IONB (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine
2% lidocaine, 1:50,000 epinephrine
3% mepivacaine, no vasoconstrictor
|
There were no significant differences (P > 0.05) among the 3 anaesthetic formulations |
| Caldas 2015 | 10 cm VAS (0 = no pain, and 10 = the most severe pain) |
BI (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine
2% lidocaine, 1:200,000 epinephrine
|
There was no difference between formulations for pain during anaesthetic injection (P > 0.05) |
| Chilton 1971 | Numbers of adverse events listed (pooled ‐ exact type not stated) |
Infiltration (1.5 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
IANB (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
|
No statistical significance between solutions, although slightly more occurred with lidocaine |
| Elbay 2016 | The Face, Legs, Activity, Cry, Consolability (FLACC) Behavioural Pain Assessment Scale, each given a pain score of 0–2, for a total behavioural pain score in the range of 0–10, as follows:
|
IANB (0.9 mL) of: 2% lidocaine, 1:80,000 epinephrine
3% mepivacaine, no vasoconstrictor
|
Pain‐related behaviour differed significantly as 2% lidocaine with 1:80,000 epinephrine produced less pain during injection than plain mepivacaine (P = 0.015) There was no statistically significant difference between solutions in pain scores during injection for ‘mild’ or ‘moderate’ pain (P = 0.275, P = 0.084, respectively) |
| Epstein 1969 | Numbers of local adverse events listed (pooled ‐ unclear about exact types of adverse effects) |
BI (1.2 mL) and IANB (1.4 mL) of: Local side effects 2% lidocaine, 100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
|
Not reported |
| Evans 2008 | Heft‐Parker VAS (170 mm line with various descriptive terms)
|
Maxillary BI (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine Needle insertion (mean ± SD)
Needle placement (mean ± SD)
Solution deposition (mean ± SD)
4% articaine, 1:100,000 epinephrine Needle insertion (mean ± SD)
Needle placement (mean ± SD)
Solution deposition (mean ± SD)
|
There were no significant differences (P > 0.05) between the 2 anaesthetic solutions for any phases of the injection Needle insertion Lateral incisor: P = 0.9934 First molar: P = 0.9555 Needle placement Lateral incisor: P = 0.9943 First molar: P = 0.8731 Solution deposition Lateral incisor: P = 0.5378 First molar: P = 0.4405 |
| Forloine 2010 | Heft‐Parker VAS (170 mm line)
|
High‐tuberosity maxillary second division nerve blocks (4.0 mL) of: 2% lidocaine, 1:100,000 epinephrine Needle insertion
Needle placement
Solution deposition
3% mepivacaine, no vasoconstrictor Needle insertion
Needle placement
Solution deposition
|
There was no significant difference (P > 0.05) between the 2 solutions |
| Gangarosa 1967 | Numbers of adverse events listed |
Mandibular block and infiltration (volume not stated) of each of the following:
No adverse events were reported |
Not applicable |
| Gazal 2017 | 0–100 mm VAS (0 = no pain and 100 = unbearable pain) |
Maxillary BI (1.4 mL) and PI (0.4 mL) Post‐buccal infiltration 2% mepivacaine, 1:100,000 epinephrine
4% articaine, 1:100,000 epinephrine
Post‐palatal infiltration 2% mepivacaine, 1:100,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
Post‐buccal infiltration: P < 0.001 Post‐palatal infiltration: P = 0.19 |
| Gregorio 2008 | Numbers of adverse events listed |
IANB (1.8 mL) and local infiltration (0.9 mL) of:
No adverse events were reported |
Not applicable |
| Haase 2008 | Heft‐Parker VAS (170 mm line)
|
IANB of 4% articaine, 1:100,000 epinephrine (1.8 mL), followed by additional BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine (mean ± SD)
2% lidocaine, 1:100,000 epinephrine (mean ± SD)
|
There were no significant differences (P > 0.05) between the 2 anaesthetic solutions for any phases of the injection Needle insertion: P = 0.95 Needle placement: P = 0.99 Solution deposition: P > 0.99 |
| Hellden 1974 | Numbers of adverse events listed |
IANB (1.8 mL) and local infiltration (1.8 mL) of:
No adverse events were reported |
Not applicable |
| Hosseini 2016 | Adverse events |
Maxillary BI (1.8 mL) of the following:
There were no adverse events |
Not applicable |
| Jaber 2010 | 100 mm visual analogue scale with endpoints marked ‘no pain’ (0 mm) and ‘unbearable pain’ (100 mm) |
BI (0.9 mL) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
LIs (0.9 mL) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
1 BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
Dummy LI of:
|
No significant differences were noted between drugs and methods of administration Lingual penetration (dummy LI) was more comfortable than lingual infiltration (student’s paired t–test P < 0.01) |
| Jain 2016 | VAS from 0 (no pain) to 10 (worst pain imaginable) |
IANB and BI (1.7 mL in total) of: 2% lidocaine, 1:100,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
The difference was not significant (P = 0.393) |
| Kammerer 2012 | VAS from 0 (no pain) to 10 (worst pain) |
IANB and buccal nerve block (up to 2.2 mL) of: 4% articaine, 1:100,000 epinephrine
4% articaine, no vasoconstrictor
|
The difference was not significant (P = 0.647) |
| Kanaa 2006 | 100 mm VAS with endpoints tagged no pain (0 mm) and unbearable pain (100 mm) |
Mandibular BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
There was no significant difference in injection discomfort between treatments (P = 0.320) |
| Kanaa 2012 | 100 mm VAS with endpoints tagged no pain (0 mm) and unbearable pain (100 mm) |
Maxillary BI (2.0 mL) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:80,000 epinephrine
Patients for extraction received a supplementary palatal injection of 0.2 mL 2% lidocaine, 1:80,000 epinephrine |
Articaine buccal infiltrations were more comfortable than lidocaine buccal infiltrations (P = .026) |
| Kolli 2017 | Adverse events |
Maxillary BI (1.7 mL) of:
(epinephrine concentrations assumed) There were no adverse events |
Not applicable |
| Kramer 1958 | Numbers of adverse events listed (pooled) |
Mandibular and maxillary injections (1 or more cartridges) of: 2% lidocaine, 1:50,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| McEntire 2011 | Heft‐Parker VAS (170 mm line)
|
Mandibular BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine Needle insertion
Needle placement
Solution deposition
4% articaine, 1:200,000 epinephrine Needle insertion
Needle placement
Solution deposition
|
There was no significant difference (P > 0.05) between the 2 solutions for pain of injection |
| Mikesell 2005 | Heft‐Parker VAS (170 mm line)
|
IANB (1.8 mL) of: 2% lidocaine with 1:100,000 epinephrine Solution deposition
4% articaine with 1:100,000 epinephrine Solution deposition
|
There was no significant difference (P > 0.05) between the 2 solutions |
| Moore 2006 | Numbers of local adverse events (sharp injection pain) listed |
IANB (1.7 mL) or BI (1.0 mL) of:
Events that did occur were as follows: IANB
Infiltration
|
No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Moore 2007 | Numbers of participants experiencing pain on injection were listed |
Maxillary BI (buccal and palatal if required, and variable volumes) of: 4% articaine, 1:200,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Mumford 1961 | Numbers of adverse events listed |
"Regional" (1.5 mL) and infiltration injections (1.0 mL) of:
No adverse events reported |
Not applicable |
| Nordenram 1990 | Numbers of adverse events listed |
Maxillary BI (0.6 mL) of:
No adverse events occurred |
Not applicable |
| Nydegger 2014 | Heft‐Parker VAS (170 mm line)
|
Mandibular BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine Needle insertion
Needle placement
Solution deposition
4% prilocaine, 1:200,000 epinephrine Needle insertion
Needle placement
Solution deposition
|
There were no significant differences (P > .05) among the anaesthetic formulations within each injection phase |
| Odabas 2012 | Taddio's Scale was used for objective evaluation of children
Wong–Baker FACES Pain Rating Scale |
Maxillary BI (1.8 mL) of: 4% articaine, 1:200,000 epinephrine
3% mepivacaine, no epinephrine
|
No significant difference was found between objective evaluations (Taddio's Scale) during injection or between first and second evaluation periods Wong‐Baker FACES Pain Rating Scale showed children reacted positively to injections of both solutions immediately after receiving anaesthetic solutions. No significant difference was found between solutions (P = 0.07) |
| Oliveira 2004 | VAS ranging measured in cm, from 0 = ‘no pain’ to 10 = ‘worst pain imaginable’ following injection of the palate . |
Maxillary BI (1.8 mL) and PI (0.35 mL) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
There was no difference between articaine and lidocaine (P = 0.45) |
| Ram 2006 | Taddio's Scale was used for objective evaluation of children
Wong‐Baker FACES Pain Rating Scale |
IANB and BI (up to 1 cartridge) of: 2% lidocaine, 1:100,000 epinephrine
4% articaine, 1:200,000 epinephrine
|
There was no difference in subjective evaluation (Wong–Baker FPS) of pain reaction between lidocaine and articaine between boys and girls when maxillary infiltration or mandibular block techniques were used. Ninety‐eight per cent of scores ≤ 3 were recorded when either method was used and for either solution No significant difference was found between solutions in the objective evaluation (according to Taddio’s Scale) during injection or between maxillary infiltrations or mandibular blocks |
| Robertson 2007 | Heft‐Parker VAS (170 mm line)
|
Mandibular BI (1.8 mL) of: 4% articaine with 1:100,000 epinephrine (mean ± SD)
2% lidocaine with 1:100,000 epinephrine (mean ± SD)
|
There were no significant differences between the 2 anaesthetic formulations in terms of this variable Needle insertion P = 0.9795 Needle placement P = 1.0 Solution deposition P = 0.9999 |
| Santos 2007 | Numbers of adverse events listed |
IANB (1.8 mL) and mandibular BI (0.9 mL) of:
No adverse reactions occurred with each local anaesthetic solution intraoperatively |
Not applicable |
| Sherman 1954 | Numbers of adverse events listed |
Mandibular and maxillary injections (1.1‐2.2 mL) of:
There were no adverse events |
Not applicable |
| Srisurang 2011 | 100 mm VAS (no pain = 0 mm, worst pain imaginable = 100 mm) |
Maxillary BI (0.9 mL) and PI (0.3 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% articaine, 1:100,000 epinephrine
2% mepivacaine, 1:100,000 epinephrine
|
There was no statistically significant difference between the 3 local anaesthetic solutions for buccal or palatal injection |
| Yilmaz 2011 | Signs of discomfort measured as a surrogate marker for the presence or absence of pain
|
IANB and BI (1.0 mL) of: 4% articaine, 100,000 epinephrine Maxilla
Mandible
3% prilocaine, 0.03 IU/mL felypressin Maxilla
Mandible
|
More pain was present with maxillary infiltration than with inferior alveolar nerve blocks Pain following injections of both solutions was also statistically significant (P < 0.05) – twice as many responses to maxillary prilocaine than articaine. There were no pain‐related behaviours among inferior alveolar nerve block patients |
| Postoperative injection pain, swelling, and bruising | |||
| Abdulwahab 2009 | Numbers of adverse events listed |
Mandibular BI (0.9 mL) During testing session
Follow‐up (24 hours after testing)
|
“Minor in number and not dependent on local anesthetic formulation” |
| Albertson 1963 | Numbers of adverse events listed |
Injections (unspecified in terms of technique and volume) of: 2% mepivacaine, 1:20,000 levonordefrin
2% lidocaine, 1:100,000 epinephrine
Total numbers of participants assessed were not clear (dropouts, etc). Totals are based on those whose success was measured |
None reported |
| Arrow 2012 | Numbers of adverse events listed |
BI (up to 2.2 mL) of: 4% articaine, 1:100,000 epinephrine
Other solutions and injections produced no pain at injection sites |
Tests of association between postoperative complications and local anaesthetic technique and local anaesthetic type were not statistically significant |
| Batista da Silva 2010 | Postoperative pain: 100 mm VAS ranging from 0 = "no pain" to 100 = "unbearable pain" |
Mental nerve blocks (0.6 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
There was no significant difference (P > 0.05) between solutions regarding injection pain and postoperative pain |
| Berberich 2009 | Pain following injection (after numbness wore off and each morning on arising for 3 days)
Facial bruising: numbers of adverse events, pooled for all 3 solutions |
IONBs (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
2% lidocaine, 1:50,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
3% mepivacaine, no vasoconstrictor Day 0 (day of injection)
Day 1
Day 2
Day 3
Facial bruising = 2/120 total injections |
There were no significant differences (P > .05) among the 3 anaesthetic formulations Moderate pain was reported by only 1 patient when the anaesthesia wore off, which decreased during the next 3 days |
| Bradley 1969 | Numbers of adverse events listed |
Infiltration or "mandibular" injection (0.8‐3.6 mL) of: 2% lidocaine, 1:100,000 epinephrine Infiltration
Mandibular
3% mepivacaine, no vasoconstrictor Infiltration
Mandibular
|
None reported |
| Caldas 2015 | 10 cm VAS (0 = no pain, and 10 = the most severe pain) |
BI (1.8 mL) of:
|
For pain after injection, there was a difference between 2% lidocaine with 1:200,000 epinephrine and 2% lidocaine and 1:100,000 epinephrine 24 hours later (P = 0.001) |
| Chilton 1971 | Numbers of adverse events listed (pooled ‐ exact type not stated) |
Infiltration (1.5 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
IANB (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine,no epinephrine
|
No statistical significance between solutions, although slightly more occurred with lidocaine |
| Elbay 2016 | Numbers of adverse events listed |
IANB (0.9 mL) of: 2% lidocaine, 1:80,000 epinephrine Pulpotomy
Extraction
3% mepivacaine, no vasoconstrictor Pulpotomy
Extraction
|
There was no statistically significant difference in postoperative pain between the 2 local anaesthetics (P = 0.130) |
| Epstein 1965 | Numbers of adverse events listed |
BI (1.2 mL) and IANB (1.5 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, no vasoconstrictor
|
Not reported |
| Epstein 1969 | Numbers of local adverse events listed (pooled ‐ unclear of exact types of adverse effects) |
BI (1.2 mL) and IANB (1.4 mL) of: Local side effects 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
|
Not reported |
| Evans 2008 | Heft‐Parker VAS (170 mm line with various descriptive terms)
|
Maxillary BI (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine Day 0 (day of injection: mean ± SD)
Day 1 (mean ± SD)
Day 2 (mean ± SD)
Day 3 (mean ± SD)
Swelling = 2/80 (1 lateral incisor and 1 molar) Bruising = 0/80 4% articaine, 1:100,000 epinephrine Day 0 (day of injection: mean ± SD)
Day 1 (mean ± SD)
Day 2 (mean ± SD)
Day 3 (mean ± SD)
Swelling = 1/80 (1 molar) Bruising = 1/80 (lateral incisor) |
P values for lidocaine vs articaine comparisons Day 0 Lateral incisor = 0.0049‡ First molar = 0.0035‡ Day 1 Lateral incisor = 0.2888§ First molar = 0.2506§ Day 2 Lateral incisor = 0.0617§ First molar = 1.0000§ Day 3 Lateral incisor = 0.3432§ First molar = 1.0000§ ‡There was a significant difference (P < 0.05) between anaesthetic solutions §There was no significant difference (P > 0.05) between solutions |
| Forloine 2010 | Heft‐Parker VAS (170 mm line)
|
High‐tuberosity maxillary second division nerve blocks (4.0 mL) of: 2% lidocaine, 1:100,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
3% mepivacaine, no vasoconstrictor Day 0 (day of injection)
Day 1
Day 2
Day 3
|
There was no significant difference between the 2 anaesthetic formulations |
| Gangarosa 1967 | Numbers of adverse events listed |
Mandibular block and infiltration (volume not stated) of each of the following:
No adverse events reported |
Not applicable |
| Gregorio 2008 | Numbers of adverse events listed |
IANB (1.8 mL) and local infiltration (0.9 mL) of:
No adverse events reported |
Not applicable |
| Haase 2008 | Heft‐Parker VAS (170 mm line)
|
IANB of 4% articaine, 1:100,000 epinephrine (1.8 mL) and an additional BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine (mean pain ± SD)
Swelling = 4/73 Bruising = 2/73 2% lidocaine, 1:100,000 epinephrine (mean pain ± SD)
Swelling = 3/73 Bruising = 2/73 |
Results showed no significant differences (P > 0.05) between anaesthetic formulations. Day 0: P > 0.99 Day 1: P > 0.99 Day 2: P > 0.99 Day 3: P > 0.99 |
| Hellden 1974 | Numbers of adverse events listed |
IANB (1.8 mL) and local infiltration (1.8 mL) of:
No adverse events reported |
Not applicable |
| Hosseini 2016 | Adverse events. |
Maxillary BI (1.8 mL) of the following:
There were no adverse events |
Not applicable |
| Jain 2016 | VAS from 0 (no pain) to 10 (worst pain imaginable) |
IANB and BI (1.7 mL in total) of: 2% lidocaine, 1:100,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
The difference was significant (P = 0.039) |
| Kammerer 2012 | Adverse events |
IANB and buccal nerve block (up to 2.2 mL) of:
No adverse events reported |
Not applicable |
| Karm 2017 | 100 mm VAS from “minimum” = no pain at all (left end) to “maximum” = maximum imaginable pain (right end) |
IANB and BI (1.8 mL in total) of: 2 hours post injection 2% lidocaine, 1:80,000 epinephrine
2% lidocaine, 1:200,000 epinephrine
4 hours post injection 2% lidocaine, 1:80,000 epinephrine
2% lidocaine, 1:200,000 epinephrine
6 hours post injection 2% lidocaine, 1:80,000 epinephrine
2% lidocaine, 1:200,000 epinephrine
|
P = 0.405 P = 0.433 P = 0.267 |
| Kolli 2017 | Adverse events |
Maxillary BI (1.7 mL) of:
(epinephrine concentrations assumed) There were no adverse events |
Not applicable |
| Kramer 1958 | Numbers of adverse events listed (pooled) |
Mandibular and maxillary injections (1 or more cartridges) of: 2% lidocaine, 1:50,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| Malamed 2000b | Numbers of local adverse events listed |
Infiltration or nerve block (1.9‐2.6 mL depending on solution and complexity of procedure) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| McEntire 2011 | Heft‐Parker VAS (170 mm line)
|
Mandibular BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
4% articaine, 1:200,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
For both solutions
|
There was no significant difference (P > 0.05) between the 2 solutions |
| Mikesell 2005 | Heft‐Parker VAS (170 mm line)
Episodes of soreness and swelling |
IANB (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
4% articaine, 1:100,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
|
P values for lidocaine vs articaine comparison: Day 0: P = 0.1746 Day 1: P = 0.2756 Day 2: P = 0.0236 Day 3: P = 0.0458 There was no significant difference between the 2 formulations for the day of injection and the first post‐injection day. Articaine had statistically higher pain ratings for days 2 and 3 There was no significant difference (P < 0.05) between the 2 formulations (soreness and swelling, on each day) |
| Moore 2006 | Numbers of local adverse events (pain following injection) listed |
IANB (1.7 mL) or BI (1.0 mL) of:
Events that did occur were as follows: IANB
Infiltration
|
No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Moore 2007 | Numbers of participants experiencing pain on injection, swelling, and bruising |
Maxillary BI (buccal and palatal if required, and variable volumes) of: 4% articaine, 1:200,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Mumford 1961 | Numbers of adverse events listed |
"Regional" (1.5 mL) and infiltration injections (1.0 mL) of:
No adverse events reported |
Not applicable |
| Nordenram 1990 | Numbers of adverse events listed |
Maxillary BI (0.6 mL) of:
No adverse events occurred |
Not applicable |
| Nydegger 2014 | Pain following injection, tested after numbness wore off and each morning, on rising, for 3 days Heft‐Parker VAS (170 mm line)
|
Mandibular BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine Day 0
Day 1
Day 2
Day 3
4% prilocaine, 1:200,000 epinephrine Day 0
Day 1
Day 2
Day 3
|
Articaine was significantly more painful than prilocaine (P = 0.0014) on day 1 |
| Odabas 2012 | Taddio's Scale was used for objective evaluation of children
Wong‐Baker FACES Pain Rating Scale |
BI (1.8 mL) of: 4% articaine, 1:200,000 epinephrine Pain after 1 hour
Pain after 2 hours
3% mepivacaine, no epinephrine Pain after 1 hour
Pain after 2 hours
|
No significant difference was found between objective evaluation (Taddio's Scale) during injection and first and second evaluation periods Wong‐Baker FACES Pain Rating Scale showed children reacted positively to injections of both solutions by phone 1 hour (P = 0.89) and 2 hours after (P = 0.77) injection |
| Ram 2006 | Taddio's Scale was used for objective evaluation of children
Wong‐Baker FACES Pain Rating Scale |
IANB and BI (up to 1 cartridge) of: 2% lidocaine, 1:100,000 epinephrine (Wong–Baker FPS) Pain after 1 hour
Pain after 2 hours
4% articaine, 1:200,000 epinephrine (Wong–Baker FPS) Pain after 1 hour
Pain after 2 hours
|
There was no difference in subjective evaluation (Wong–Baker FPS) of pain reaction between lidocaine and articaine between boys and girls when maxillary infiltration or mandibular block techniques were used No significant difference was found between solutions in objective evaluation (according to Taddio’s Scale) during injection or between maxillary infiltrations or mandibular blocks |
| Robertson 2007 | Heft‐Parker VAS (170 mm line)
|
Mandibular BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine (mean ± SD)
Swelling = 2/56 Bruising = 0/56 2% lidocaine, 1:100,000 epinephrine (mean ± SD)
Swelling = 3/59 Bruising = 1/59 |
There were no significant differences (P > 0.05) between anaesthetic formulations for post‐injection pain: Day 0: P = .9976 Day 1: P = .9841 Day 2: P = .9957 Day 3: P = 1.0000 |
| Santos 2007 | Numbers of adverse events listed |
IANB (1.8 mL) and mandibular BI (0.9 mL) of:
No adverse reactions occurred with each local anaesthetic solution intraoperatively or postoperatively |
Not applicable |
| Sherman 1954 | Numbers of adverse events listed |
Mandibular and maxillary injections (1.1‐2.2 mL) of: 2% lidocaine, 1:50,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| Stibbs 1964 | Numbers of adverse events listed (pooled) |
"Mandibular" injections and infiltrations (varying volumes) of: 2% mepivacaine, 1:20,000 levonordefrin
2% lidocaine, 1:50,000 epinephrine
|
Not reported |
| Trullenque‐Eriksson 2011 | Numbers of local adverse events |
IANB and mandibular BI (1.8 mL) of: 0.5% bupivacaine, 1:200,000 epinephrine
4% articaine, 1:200,000 epinephrine
42.1% had ≥ 1 adverse event (figure includes both local anaesthetics) |
Not reported |
| Yilmaz 2011 | Adverse event frequency was measured at 24 hours and 7 days after the procedure |
IANB and maxillary infiltration (1.0 mL) of: 4% articaine, 1:100,000 epinephrine
3% prilocaine, 0.03 IU/mL felypressin
|
Not reported |
| Other local adverse events. | |||
| Abdulwahab 2009 | Numbers of adverse events (results for each solution were pooled) |
Mandibular BI (0.9 mL) Follow‐up (24 hours after testing)
|
"Minor in number and not dependent on local anaesthetic formulation" |
| Allegretti 2016 | Adverse effects were recorded if present |
IANB (3.6 mL) of:
No local adverse events reported |
Not applicable |
| Arrow 2012 | Numbers of adverse events listed |
IANB (up to 2.2 mL) of: 2% lidocaine, 1:80,000 epinephrine
BI of: 2% lidocaine, 1:80,000 epinephrine
IANB of: 4% articaine, 1:100,000 epinephrine
Episodes of aching jaw occurred in 2 participants = (2 articaine and 2 lidocaine) |
Tests of association between postoperative complications and different formulations were not statistically significant |
| Atasoy Ulusoy 2014 | Numbers of adverse events listed |
Maxillary BI (1.5 mL) of:
No local adverse events were reported during the investigation |
Not reported |
| Batista da Silva 2010 | Postoperative complications (24 hours later) |
Mental nerve block (0.6 mL) of:
No local adverse effect other than pain was reported by any participants |
Not applicable |
| Chilton 1971 | Numbers of adverse events listed (pooled ‐ exact type not stated) |
Infiltration (1.5 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
IANB (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine,no epinephrine
|
No statistical significance between solutions, although slightly more occurred with lidocaine |
| Colombini 2006 | Assessment of mouth opening at suture removal (5 days postoperatively), measured as a percentage of preoperative mouth opening Total amount of rescue medication taken Numbers of local adverse events listed |
IANB (1.8 mL) and local infiltration (0.9 mL) of: 2% mepivacaine, 1:100,000 epinephrine (mean ± SEM)
4% articaine, 1:100,000 epinephrine (mean ± SEM)
No adverse reactions were reported during surgery and during the first postoperative hour |
There was no significant difference in mouth opening at suture removal compared with preoperative measures for both treatment groups (P > 0.05) There was no statistically significant difference concerning the total amount of rescue analgesic medication (paracetamol) ingested by patients (P > 0.05) Not applicable |
| Elbay 2016 | Numbers of adverse events listed |
IANB (0.9 mL) of: 2% lidocaine, 1:80,000 epinephrine
3% mepivacaine, no vasoconstrictor
|
There was no statistically significant difference in bleeding following extraction between the 2 anaesthetic solutions (P = 0.102) |
| Epstein 1965 | Numbers of local adverse events listed |
BI (1.2 mL) and IANB (1.5 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, no vasoconstrictor
|
Not reported |
| Epstein 1969 | Numbers of local adverse events listed (pooled ‐ unclear of exact types of adverse effects) |
BI (1.2 mL) and IANB (1.4 mL) Local side effects 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
|
Not reported |
| Forloine 2010 | Numbers of adverse events listed |
High‐tuberosity maxillary second division nerve blocks (4.0 mL) of: 2% lidocaine, 1:100,000 epinephrine
3% mepivacaine, no vasoconstrictor
|
Not reported |
| Gangarosa 1967 | Amount of bleeding (surgical cases only)
|
Mandibular block and infiltration (volume not stated) of each of the following:
Exact data for each solution not reported |
Not applicable |
| Gregorio 2008 | Assessment of mouth opening at suture removal (7 days postoperatively), measured as a percentage of preoperative mouth opening Surgeon's assessment of quality of wound healing at suture removal (7 days postoperatively). Three‐point scale 1 = normal healing 2 = delayed healing 3 = complicated healing due to alveolitis |
IANB and local infiltration of: 4% articaine, 1:200,000 epinephrine Mouth opening Without osteotomy
With osteotomy
Wound healing Without osteotomy
With osteotomy
0.5% bupivacaine, 1:200,000 epinephrine Mouth opening Without osteotomy
With osteotomy
Wound healing Without osteotomy
With osteotomy
|
Mouth opening at suture removal for patients with surgery not requiring osteotomy was not significant (P > .05), whereas with those requiring osteotomy it was significant (P < .05) The quality of wound healing was similar for both local anaesthetics, with or without osteotomy (P > .05) |
| Hellden 1974 | Numbers of adverse events listed |
IANB (1.8 mL) and local infiltration (1.8 mL) of:
No adverse events reported |
Not applicable |
| Hosseini 2016 | Adverse events |
Maxillary BI (1.8 mL) of the following:
There were no adverse events |
Not applicable |
| Kammerer 2012 | Postoperative pain. (VAS from 0 (no pain) to 10 (worst pain)) Bleeding complications |
IANB and buccal nerve block (up to 2.2 mL) of: 4% articaine, 1:100,000 epinephrine
4% articaine, no vasoconstrictor
No data for bleeding complications were reported |
The difference was not significant (P = 0.96) |
| Karm 2017 | Perioperative bleeding (Likert scale: scored 1–5: 1 = “a little bleeding” and 5 = “very much bleeding”) |
IANB and BI (1.8 mL in total) Perioperative bleeding 2% lidocaine, 1:80,000 epinephrine
2% lidocaine, 1:200,000 epinephrine
Alveolar osteitis
Inflammation at injection site
|
No significant differences between groups (P = .206) There was no statistically significant difference between groups in frequency of these adverse events (P = 1.000) There was no statistically significant difference between groups in frequency of these adverse events (P = 1.000) |
| Keskitalo 1975 | Numbers of adverse events listed |
IANB and BI (3.6 mL) of:
No differences between solutions in terms of ability to open mouth, as well as swelling, dry socket, and postoperative bleeding |
Not reported |
| Khoury 1991 | Numbers of adverse events listed (pooled) |
"Conduction" and infiltration injections (varying volumes) of:
Uncomplicated wound healing was observed In > 91% of cases in both upper and lower jaw in all groups. There were no differences in occurrence of dry socket between all groups |
Not reported |
| Kolli 2017 | Adverse events |
Maxillary BI (1.7 mL) of:
(epinephrine concentrations assumed) There were no adverse events |
Not applicable |
| Kramer 1958 | Numbers of adverse events listed (pooled) |
Mandibular and maxillary injections (1 or more cartridges) of: 2% lidocaine, 1:50,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| Laskin 1977 | Not reported |
IANB and BI (1.8 mL) of:
No side effects were reported |
Not applicable |
| Linden 1986 | Numbers of local adverse events listed |
In the mandible, IANB (2.7 mL), lingual and long buccal injections, while in the maxilla, posterior superior alveolar nerve block, local and palatal infiltration (1.8 mL)
The bupivacaine group demonstrated more bleeding during surgery in 11 of 20 patients |
Not reported |
| Malamed 2000a | Numbers of local adverse events listed |
Infiltration or nerve block (2.5‐4.5 mL depending on solution and complexity of procedure) of:
No serious adverse events occurred. Minor events included postprocedural pain, facial oedema, infection, gingivitis, and transient paraesthesia They occurred in low numbers in both groups |
Not reported |
| Malamed 2000b | Numbers of local adverse events listed |
Infiltration or nerve block (1.9‐2.6 mL depending on solution and complexity of procedure) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| Mikesell 2005 | Numbers of other local adverse events listed |
IANB (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
4% articaine, 1:100,000 epinephrine Day 0 (day of injection)
Day 1
Day 2
Day 3
|
There was no significant difference (P < 0.05) between the 2 formulations on each day |
| Moore 2006 | Numbers of local adverse events listed |
IANB (1.7 mL) or maxillary BI (1.0 mL) of:
Adverse events No serious adverse events occurred. Events that did occur were as follows: IANB
Infiltration
|
No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Moore 2007 | Numbers of adverse events listed |
Maxillary BI (buccal and palatal if required, and variable volumes) of:
(possibly related to the solutions used. These were not specifically detailed in the results) Events that did occur were as follows: 4% articaine, 1:100,000 epinephrine
4% articaine, 1:200,000 epinephrine
|
No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Mumford 1961 | Numbers of adverse events listed |
"Regional" (1.5 mL) and infiltration injections (1.0 mL) of:
No local adverse events were reported |
Not applicable |
| Naik 2017 | Analgesic medication consumed |
IANB (2 mL) of: 2% lidocaine, 1:80,000 epinephrine
4% articaine, 1:100,000 epinephrine
|
The difference was statistically significant (P < 0.001) |
| Nespeca 1976 | Numbers of adverse events listed |
IANB and infiltration injection (1.5‐2.0 mL) of:
There were no local adverse events with either solution |
Not applicable |
| Nordenram 1990 | Numbers of adverse events listed |
BI (0.6 mL) of:
There were no adverse events with any solution |
Not applicable |
| Nydegger 2014 | Numbers of adverse events listed |
Mandibular BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine Tenderness
Subjective swelling
4% prilocaine, 1:200,000 epinephrine Tenderness
Subjective swelling
|
Not reported |
| Odabas 2012 | Numbers of adverse events listed |
Maxillary injections (1.8 mL) of:
Similar for both solutions:
|
No statistically significant differences between solutions |
| Pässler 1996 | Numbers of adverse events listed |
Injections (2.0‐4.0 mL) of: 2% lidocaine, 1:100,000 epinephrine Blood filling of tooth socket after extraction
Haemorrhage during apicectomy
3% prilocaine, felypressin (0.03 IU) Blood filling of tooth socket after extraction
Haemorrhage following apicectomy
|
Not reported |
| Pellicer‐Chover 2013 | Bleeding during the procedure Postoperative pain, VAS 0‐10 (means) Postoperative analgesia duration (means and SDs) Postoperative analgesia (number needing rescue medication) |
IANB (1.8 mL) and BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine
0.5% bupivacaine, 1:200,000 epinephrine
|
Bleeding during the procedure: P = 0.000 Postoperative pain: P = 0.072 Postoperative analgesia duration: P = 0.363 Postoperative analgesia (number needing rescue medication): P = 0.836 |
| Porto 2007 | Postoperative pain (100 mm VAS) |
IANB and BI (3.6 mL) of: 2% lidocaine, 1:100,000 epinephrine
2% mepivacaine, 1:100,000 epinephrine
|
P = 0.4607 |
| Ram 2006 | Numbers of adverse events listed |
IANB and BI (up to 1 cartridge) of: 2% lidocaine, 1:100,000 epinephrine
4% articaine, 1:200,000 epinephrine
|
No statistically significant differences between solutions |
| Sancho‐Puchades 2012 | Postoperative pain (100 mm VAS) Amount of rescue analgesic medication needed during first 4 postoperative days |
IANB and BI (1.8 mL) of: 4% articaine, 1:200,000 epinephrine Postoperative pain
Mean number of rescue analgesic tablets
0.5% bupivacaine, 1:200,000 epinephrine Postoperative pain
Mean number of rescue analgesic tablets
|
Postoperative VAS of pain varied significantly across time (P = 0.017). The bupivacaine group had lower pain scores during day 1, being statistically significant at 2:00 PM (P = 0.011) and 4:00 PM (P = 0.007) There were no statistically significant differences between total intake of rescue analgesics during the first 4 postoperative days (P > 0.05) |
| Santos 2007 | Numbers of adverse events listed |
IANB (1.8 mL) and mandibular BI (0.9 mL) of:
No adverse reactions occurred with each local anaesthetic solution intraoperatively or postoperatively |
Not applicable |
| Sherman 1954 | Numbers of adverse events listed |
Mandibular and maxillary injections (1.1‐2.2 mL) of:
No adverse reactions were observed |
Not applicable |
| Thakare 2014 | Time to first rescue analgesic medication |
Maxillary BI (1.4 mL) of: 4% articaine, 1:200,000 epinephrine
0.5% bupivacaine, 1:200,000 epinephrine
|
P < 0.0001 |
| Trieger 1979 | Numbers of adverse events listed |
IANB and infiltration (varying volumes) of:
There were no side effects or complications with either solution |
Not applicable |
| Trullenque‐Eriksson 2011 | Numbers of local adverse events |
IANB and mandibular BI (1.8 mL) of: 0.5% bupivacaine, 1:200,000 epinephrine
4% articaine, 1:200,000 epinephrine
42.1% of patients had at least one adverse event (figure includes both local anaesthetics). |
Not applicable |
| Weil 1961 | Numbers of adverse events listed |
Mandibular and maxillary injections (1 cartridge or more) of:
Local postoperative side effects were few in number (exact number not stated) and were possibly due to needle trauma |
Not reported |
| Yilmaz 2011 | Adverse event frequency was measured at 24 hours and 7 days after the procedure |
IANB (1.0 mL) and maxillary infiltration (1.0 mL) of: 4% articaine, 100,000 epinephrine Self‐inflicted soft tissue injury
3% prilocaine, 0.03 IU/mL felypressin Self‐inflicted soft tissue injury
|
Not reported |
| Systemic adverse events | |||
| Abdulwahab 2009 | Numbers of systemic adverse events (results for each solution were pooled) |
Mandibular BI (0.9 mL) Follow‐up (24 hours after testing)
|
“Minor in number and not dependent on local anaesthetic formulation" |
| Albertson 1963 | Numbers of adverse events listed |
Injections (unspecified in terms of technique and volume) of: 2% mepivacaine, 1:20,000 levonordefrin
2% lidocaine, 1:100,000 epinephrine
Total number of participants assessed is not clear (dropouts, etc.). Totals are based on those for whom success was measured |
None reported |
| Allegretti 2016 | Adverse effects were recorded if present |
IANB (3.6 mL) of:
No systemic adverse effects reported |
Not applicable |
| Atasoy Ulusoy 2014 | Heart rates of patients were measured with a pulse oximeter during root canal procedures |
Maxillary BI (1.5 mL) of:
No systemic adverse events were reported during the investigation |
There was no significant difference between solutions regarding heart rate measurements during root canal treatment (P > 0.05) Heart rates during treatment of palatal root canals were significantly higher than during treatment of mesiobuccal and distobuccal canals with both solutions (P < 0.0001) |
| Batista da Silva 2010 | Postoperative complications (24 hours later) |
Mental nerve block (0.6 mL) of:
No systemic adverse effect other than pain was reported by any participants |
Not applicable |
| Bortoluzzi 2009 | Blood pressure and heart rate were measured at 0, 3, and 15 minutes after injection |
Mandibular BI (0.18 mL) of:
No differences were found between solutions |
When solutions were compared, no differences were found at the times measured |
| Bouloux 1999 | Cardiovascular responses as well as systemic adverse effects were assessed |
IANB and infiltration (4.4 mL), or BI and greater palatine nerve block (2.2 mL) of:
No signs or symptoms of central nervous system or cardiovascular toxicity were seen |
There were no differences in cardiovascular responses between solutions; a statistically significant decrease in mean heart rate occurred between 15 and 30 minutes with bupivacaine (P = 0.002) and lidocaine (P = 0.007) |
| Bradley 1969 | Numbers of adverse events listed |
Infiltration or "mandibular" injection (0.8‐3.6 mL) of: 2% lidocaine, 1:100,000 epinephrine Infiltration
"Mandibular" injections
3% mepivacaine, no vasoconstrictor Infiltration
"Mandibular" injections
Unclear whether these symptoms were related to the injection, the local anaesthetic used, or the anxiety of patients: "the systemic and local postoperative reactions recorded could be attributed (respectively) to the high proportion of emotionally nervous subjects" |
Not applicable |
| Caldas 2015 | Blood pressure, partial oxygen concentration, and heart rate were measured at each of 3 sessions in 3 periods: 5 minutes before anaesthetic administration, during anaesthetic injection, and immediately after injection |
BI (1.8 mL) of:
|
Oxygen saturation: no variation in initial observed levels, which remained at around 96% of saturation Heart rate: no significant variations that could interfere with study results There were no statistically significant differences in systolic blood pressure (Friedman, P = 0.33), diastolic blood pressure (Friedman, P = 0.1505), heart rate (Friedman, P = 0.9464), and oxygen saturation (Friedman, P = 0.9297) with each local anaesthetic during and after local anaesthesia |
| Chilton 1971 | Numbers of systemic adverse events listed (pooled) |
BI (1.5 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
IANB (1.8 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
Most were syncope reactions |
There was no statistical significance between solutions, although slightly more occurred with lidocaine |
| Colombini 2006 | Numbers of systemic adverse events listed |
IANB (1.8 mL) and local infiltration (0.9 mL) of:
No adverse reactions were reported during surgery and during the first postoperative hour |
No statistically significant difference in blood pressure, heart rate, or oxygen saturation was seen before and during surgery, and after suture, for both groups (P > 0.05). Data were presented only in graphs |
| Epstein 1965 | Numbers of systemic adverse events listed |
Maxillary BI (1.2 mL) and IANB (1.5 mL) of: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, no vasoconstrictor
|
Not reported |
| Epstein 1969 | Numbers of systemic adverse events listed |
Maxillary BI (1.2 mL) and IANB (1.5 mL) Systemic side effects 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, 1:200,000 epinephrine
4% prilocaine, no epinephrine
|
Not reported |
| Forloine 2010 | Numbers of systemic adverse events listed |
High‐tuberosity maxillary second division nerve block (4.0 mL) of: 2% lidocaine, 1:100,000 epinephrine
3% mepivacaine, no vasoconstrictor
|
Not reported |
| Gangarosa 1967 | Numbers of systemic adverse events listed |
Mandibular block and infiltration (volume not stated) of each of the following: 2% lidocaine, 1:100,000 epinephrine
4% prilocaine, no vasoconstrictor
|
Not reported |
| Gregorio 2008 | Numbers of systemic adverse events listed, as well as assessments of systolic, diastolic, mean arterial pressure, heart rate, and oxygen saturation |
IANB (1.8 mL) and local infiltration (0.9 mL) of: 4% articaine, 1:200,000 epinephrine
0.5% bupivacaine, 1:200,000 epinephrine
There were no differences measured for the following parameters between solutions:
apart from diastolic and mean arterial pressures for surgery with osteotomy |
No significant differences between systolic, diastolic, and mean arterial pressure during surgery without osteotomy (P > 0.05) For surgery with osteotomy, there were statistically significant differences in diastolic (64 mmHg and 68 mmHg, respectively, P = 0.001) and mean arterial pressures (86 mmHg and 89 mmHg, respectively; P = 0.031) for pooled data from all surgical phases Heart rate was not influenced by the local anaesthetic used (P > 0.05) No statistically significant difference was seen between solutions for oxygen saturation during surgery with or without osteotomy (P > 0.05). The solution used did not influence the results of oximetry (P > 0.05) |
| Hellden 1974 | Numbers of systemic adverse events listed |
IANB (1.8 mL) and local infiltration (1.8 mL) of:
No adverse events were reported |
Not applicable |
| Hosseini 2016 | Adverse events |
Maxillary BI (1.8 mL) of the following:
There were no adverse events |
Not applicable |
| Kammerer 2012 | Adverse events |
IANB and buccal nerve block (up to 2.2 mL) of:
No adverse events reported |
Not applicable |
| Kammerer 2014 | Heart rate, systolic and diastolic blood pressures, and oxygen saturation measured |
Maxillary BI (1.7 mL) of:
No systemic side effects or complications were detected in any groups. Heart rate, blood pressure, and oxygen saturation were not affected |
Not applicable |
| Karm 2017 | Numbers of systemic adverse events listed Vital signs |
IANB and BI (1.8 mL in total) of: 2% lidocaine, 1:80,000 epinephrine
2% lidocaine, 1:200,000 epinephrine
2% lidocaine, 1:80,000 epinephrine
2% lidocaine, 1:200,000 epinephrine
|
There was no statistically significant difference between groups in terms of the frequency of these adverse events (P = 1.0) Systolic blood pressure P < 0.002 Diastolic blood pressure P < 0.205 Heart rate P < 0.010 |
| Khoury 1991 | Blood pressure and heart rate measured |
"Conduction" and infiltration anaesthesia (varying volumes) of:
No differences were measured between solutions |
Not reported |
| Kolli 2017 | Heart rate |
Maxillary BI (1.7 mL) of:
(epinephrine concentrations assumed) Maxillary BI/PI (1.7 mL in total) of: Heart rate (means ± SD) Baseline
During
After
There were no adverse events |
P = 0.26 P = 0.08 P = 0.56 |
| Kramer 1958 | Numbers of adverse events listed (pooled) |
Mandibular and maxillary injections (1 or more cartridges) of: 2% lidocaine, 1:50,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| Lasemi 2015 | Heart rate Systolic blood pressure Diastolic blood pressure |
IANB (volume not stated) of: 4% articaine, 1:100,000 epinephrine Heart rate (beats per minute):
Systolic blood pressure (mm of mercury):
Diastolic blood pressure (mm of mercury):
4% articaine, 1:200,000 epinephrine Heart rate (beats per minute):
Systolic blood pressure (mm of mercury):
Diastolic blood pressure (mm of mercury):
|
For both local anaesthetics: Heart rate during treatment: P = 0.6 Heart rate after 5 minutes: P = 0.8 Systolic blood pressure during treatment: P = 0.9 Systolic blood pressure after 5 minutes: P = 0.4 Diastolic blood pressure during treatment: P = 0.9 Diastolic blood pressure after 5 minutes: P = 0.8 |
| Laskin 1977 | Numbers of systemic adverse events listed |
IANB and BI (1.8 mL) of:
No adverse events were seen |
Not applicable |
| Malamed 2000a | Numbers of systemic adverse events listed |
Infiltration or nerve block (2.5‐4.5 mL depending on solution and complexity of procedure) of:
No serious systemic adverse events occurred |
Not reported |
| Malamed 2000b | Numbers of systemic adverse events listed |
Infiltration or nerve block (1.9‐2.6 mL depending on solution and complexity of procedure) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
Vital signs: Slight increases were seen in supine blood pressure with articaine, as compared with a slight decrease overall. These changes were not clinically significant and produced no adverse effects |
Not reported |
| Martinez‐Rodriguez 2012 | Numbers of systemic adverse events listed |
IANB (1.8 mL) and mandibular BI (0.9 mL) of: 4% articaine, 1:100,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| Mikesell 2005 | Numbers of adverse events listed |
IANB (1.8 mL) of:
No systemic adverse events occurred |
Not applicable |
| Mittal 2015 | Blood pressure and heart rate were measured Stage 1: before injection (average of 4 readings taken at 2‐minute intervals for 8 minutes before administration of anaesthetic injection) Stage 2: taken 5 minutes after injection, before the start of extraction (average of readings taken at 15‐second intervals) Stage 3: taken during extraction (average of readings taken at 15‐second intervals) |
Maxillary BI (1.8 mL lidocaine, 1.7 mL lidocaine) of: 2% lidocaine, 1:80,000 epinephrine Heart rate (mean beats per minute ± SD)
Systolic blood pressure (mean mm of mercury ± SD)
Diastolic blood pressure (mean mm of mercury ± SD)
4% articaine, 1:100,000 epinephrine Heart rate (mean beats per minute ± SD)
Systolic blood pressure (mean mm of mercury ± SD)
Diastolic blood pressure (mean mm of mercury ± SD)
|
Heart rate Student t‐test found no statistically significant difference in mean heart rate values with either local anaesthetic (P> 0.05) Systolic blood pressure Student t‐test found no statistically significant difference between the 2 local anaesthetics (P > 0.05) Diastolic blood pressure There was no statistically significant difference between local anaesthetics (P > 0.05) |
| Moore 2006 | Numbers of systemic adverse events were listed and blood pressure and heart rate were measured 5 minutes before injection, immediately after injection, and on completion of treatment |
IANB (1.7 mL) or maxillary BI (1.0 mL) of: 4% articaine, 1:200,000 epinephrine (A200) IANB heart rate (mean beats per minute ± SD)
Infiltration heart rate (mean beats per minute ± SD)
IANB systolic blood pressure (mean mm of mercury ± SD)
Infiltration systolic blood pressure (mean mm of mercury ± SD)
IANB diastolic blood pressure (mean mm of mercury ± SD)
Infiltration diastolic blood pressure (mean mm of mercury ± SD)
Mean values for vital signs were similar for all solutions and were not clinically significant. Adverse events 4% articaine, 1:100,000 epinephrine (A100) IANB heart rate (mean beats per minute ± SD)
Infiltration heart rate (mean beats per minute ± SD)
IANB systolic blood pressure (mean mm of mercury ± SD)
Infiltration systolic blood pressure (mean mm of mercury ± SD)
IANB diastolic blood pressure (mean mm of mercury ± SD)
Infiltration diastolic blood pressure (mean mm of mercury ± SD)
Mean values for vital signs were similar for all solutions and were not clinically significant Adverse events No serious adverse events occurred 4% articaine, no vasoconstrictor (Aw/O) IANB heart rate (mean beats per minute ± SD)
Infiltration heart rate (mean beats per minute ± SD)
IANB systolic blood pressure (mean mm of mercury ± SD)
Infiltration systolic blood pressure (mean mm of mercury ± SD)
IANB diastolic blood pressure (mean mm of mercury ± SD)
Infiltration diastolic blood pressure (mean mm of mercury ± SD)
Mean values for vital signs were similar for all solutions and were not clinically significant Adverse events No serious adverse events occurred. Events that did occur were as follows: IANB
Infiltrations
|
¶ P < 0.01 compared with pre injection (t = 0 minutes)
# P < 0.01 compared with Aw/O at the same time
** P < 0.05 compared with Aw/O at the same time
†† P < 0.05 compared with pre injection (t = 0 minutes) Inferior alveolar nerve block A100 and A200 groups' heart rate increased 5 minutes post injection (A100 increased 3.5 beats/min, P = 0.0051; A200 increased 2.6 beats/min, P = 0.0064). The A200 treatment group showed a decrease in heart rate at completion (A200 decreased 2.4 beats/min, P = 0.0421) No difference was seen for the pairwise treatment comparison of A100 and A200 groups’ heart rates from baseline to post injection; There was a difference when A100 and Aw/O groups were compared (P = 0.0005) and when A200 and Aw/O groups (P = 0.0016) were compared post injection The A100 treatment group was the only one that showed a statistically significant decrease in systolic blood pressure at completion (A100 decreased 2.6 mmHg, P = 0.0153) Both A100 and A200 treatment groups showed small (2‐4 mmHg) but statistically significant decreases in diastolic blood pressure at 5 minutes post injection (P = 0.0002 and 0.0062, respectively), but with diastolic blood pressure, all 3 solutions showed a significant decrease at completion Maxillary infiltration The Aw/O treatment group’s heart rate decreased significantly (3.6 beats/min) compared with the preinjection heart rate immediately post injection (P = 0.0013) There was a significant increase in heart rate when A100 was compared with Aw/O at post‐injection dose (P = 0.0150) There was a significant decrease in pulse rate for all solutions at completion of the study (P = 0.0034 for Aw/O, P = 0.0025 for A100, P = 0.0009 for A200) There was a significant decrease in diastolic blood pressure for all 3 groups 10 minutes post injection (P = 0.0079 for Aw/O, P < 0.0001 for A100, P < 0.0001 for A200) There was a significant decrease in diastolic blood pressure from baseline to completion for the Aw/O treatment group (P = 0.0046) A significant decrease in systolic blood pressure occurred with all 3 groups 10 minutes post injection (P = 0.0041 for Aw/O, P = 0.0065 for A100, P = 0.0003 for A200). A significant decrease in systolic blood pressure at completion of the testing occurred with 2 solutions (P = 0.0487 for A100, P = 0.0333 for A200) Adverse events No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Moore 2007 | Vital signs (blood pressure, pulse, and respiratory rate) measured:
Adverse events |
Maxillary BI (buccal and palatal if required, and variable volumes) of: 4% articaine, 1:200,000 epinephrine (A200) Heart rate (mean beats/min ± SD)
Systolic blood pressure (mean mm of mercury ± SD)
Diastolic blood pressure (mean mm of mercury ± SD)
Respiratory rate (mean beats per minute ± SD)
Adverse events Events that did occur were as follows:
4% articaine, 1:100,000 epinephrine (A100) Heart rate (mean beats/min ± SD)
Systolic blood pressure (mean mm of mercury ± SD)
Diastolic blood pressure (mean mm of mercury ± SD)
Respiratory rate (mean beats per minute ± SD)
Adverse events Events that did occur were as follows:
|
* P < 0.01 compared with pre injection
† P < 0.05 compared with pre injection There were only 2 statistically significant differences in cardiovascular and respiratory functions following local anaesthetic administration
for the decrease in pulse rate from pre to post treatment (6.8 beats/min and 3.7 beats/min (P = 0.0433). In each surgical session, statistically significant findings were found Heart rate: pre injection to completion showed a decrease for A200: P = 0.0013 A100: P < 0.0001 Diastolic blood pressure A100: decreased from pre to post injection (P = 0.0003) A200 and A100: increased from pre injection to completion of surgery (P = 0.0303 and P = 0.0162, respectively) Systolic blood pressure: An increase was seen from pre injection to completion of surgery for A200 (P = 0.0220) and A100 (P = 0.0118) Adverse events: No statistically significant differences occurred between solutions in terms of numbers of adverse events |
| Mumford 1961 | Numbers of systemic adverse events listed |
"Regional" (1.5 mL) and infiltration injections (1.0 mL) of: 2% lidocaine, 1:80,000 epinephrine
3% mepivacaine, no epinephrine
|
Not reported |
| Nespeca 1976 | Numbers of systemic adverse events listed |
IANB and infiltration injection (1.5‐2.0 mL) of:
No systemic adverse events occurred |
Not applicable |
| Nordenram 1990 | Numbers of systemic adverse events listed |
Maxillary BI (0.6 mL) of:
No systemic adverse events occurred |
Not applicable |
| Odabas 2012 | Measurements of blood pressure, heart rate, and oxygen saturation |
Maxillary injection (1.8 mL) of:
Similar measurements for both solutions (results presented graphically) |
No statistically significant differences between solutions (P = 0.72) |
| Pellicer‐Chover 2013 | Systolic blood pressure Diastolic blood pressure Cardiac rate |
IANB (1.8 mL) and BI (1.8 mL) of: 4% articaine, 1:100,000 epinephrine
0.5% bupivacaine, 1:200,000 epinephrine
|
No statistically significant differences between solutions:
|
| Ram 2006 | Numbers of systemic adverse events listed |
IANB and maxillary BI (up to 1 cartridge) of:
No systemic adverse effects occurred |
Not applicable |
| Robertson 2007 | Numbers of systemic adverse events listed |
Mandibular BI (1.8 mL) of:
No systemic adverse effects occurred |
Not applicable |
| Sancho‐Puchades 2012 | Systolic and diastolic arterial pressure, heart rate, and oxygen saturation Adverse reactions during surgery or during first postoperative week |
IANB and BI (1.8 mL in total) of: 4% articaine, 1:200,000 epinephrine
0.5% bupivacaine, 1:200,000 epinephrine
|
Statistically significant higher levels of systolic blood pressure were seen in the articaine group (P = 0.013), which varied significantly across time (P = 0.024) Diastolic blood pressure was similar between groups (P = 0.320), with no significant changes over time (P = 0.090) Oxygen saturation did not differ significantly between groups (P = 0.194) with no significant changes over time (P = 0.199) Heart rate varied significantly between groups over time (P = 0.036) and was higher in the articaine group at tissue incision and bone removal |
| Santos 2007 | Numbers of systemic adverse events listed as well as measurements of systolic, diastolic, and mean arterial pressures and heart rate |
IANB (1.8 mL) and mandibular BI (0.9 mL) of:
No adverse reactions occurred with each local anaesthetic solution intraoperatively and postoperatively. For systolic, diastolic, and mean arterial pressures, no hypertensive peak was observed during all steps of treatment |
There were no significant differences between solutions when measuring:
Oxygen saturation increased immediately after first cartridge of articaine (P < 0.05) was given. This remained until the end with surgery without osteotomy (data not shown), although it was not dependent on the local anaesthetic used (P > 0.05) Data were presented on graphs |
| Sherman 1954 | Numbers of systemic adverse events listed |
Mandibular and maxillary injections (1.1‐2.2 mL) of: 2% lidocaine, 1:50,000 epinephrine
2% lidocaine, 1:100,000 epinephrine
|
Not reported |
| Stibbs 1964 | Numbers of systemic adverse events listed |
Various mandibular and BI (varying volumes) of: 2% mepivacaine, 1:20,000 levonordefrin
2% lidocaine, 1:50,000 epinephrine
|
Not reported |
| Trieger 1979 | Numbers of systemic adverse events listed |
IANB and BI (varying volumes) of:
No systemic adverse effects occurred |
Not applicable |
| Trullenque‐Eriksson 2011 | Number of systemic adverse events and measurement of patients’ vital signs with a blood pressure monitor, and pulse and oxygen saturation with a pulse oximeter |
IANB and mandibular BI (1.8 mL) of: 0.5% bupivacaine, 1:200,000 epinephrine
4% articaine, 1:200,000 epinephrine
42.1% of patients had at least 1 adverse event (figure includes both local anaesthetics) |
No statistically significant differences were found for blood pressure, pulse, or bleeding during surgery The only significant differences for oxygen saturation were found at initial and final measurements, but not between measurements after administration of anaesthesia or changes in oxygen saturation |
| Vilchez‐Perez 2012 | Numbers of systemic adverse events including haemodynamic parameters (heart rate, systolic blood pressure, diastolic blood pressure, and oxygen saturation) were recorded |
BI (0.9 mL) of:
No complications were reported with either solution |
No statistically significant differences were found for either anaesthetic solution during the intervals under study (ANOVA test P > 0.05) |
| Weil 1961 | Numbers of systemic adverse events listed |
Mandibular and maxillary injections (1 cartridge or more) of:
Few systemic adverse reactions. Most were attributed to apprehension rather than toxicity. Average incidence of lack of systemic reactions for each formulation ranged from 96.92 ± 1.52 to 100% |
No significant differences in local or systemic tolerance were found between solutions |
| Yilmaz 2011 | Adverse event frequency was measured at 24 hours and 7 days after the procedure |
IANB and BI (1.0 mL) of: 4% articaine, 100,000 epinephrine IANB
Maxillary infiltration
3% prilocaine, 0.03 IU/mL felypressin IANB
Maxillary infiltration
|
Not reported |
A100 = 4% articaine, 1:100,000 epinephrine ; A200 = 4% articaine, 1:200,000 epinephrine; ANOVA = analysis of variance; Aw/O = 4% articaine with no vasoconstrictor; BI = buccal infiltration; Faces Pain Scale Revised = a modified version of the Faces Pain Scale (Hicks 2001); Heft‐Parker VAS = Heft‐Parker visual analogue scale (Heft 1984); Hg = mercury; IANB = inferior alveolar nerve block; IONB = infraorbital nerve block; L80 = 2% lidocaine, 1:80,000 epinephrine; LA = local anaesthetic; LI = lingual infiltration; PI = palatal infiltration; SD = standard deviation; T = Taddio's Scale (Taddio 1994); VAS = visual analogue scale; Wong–Baker FPS = Wong‐Baker FACES Pain Rating Scale (Wong 1988).
# unsure if measurement is standard error or standard deviation; ## unsure if measurements are means and standard errors, or standard deviations.
The flow diagram for studies from start to finish is shown in Figure 1.
1.
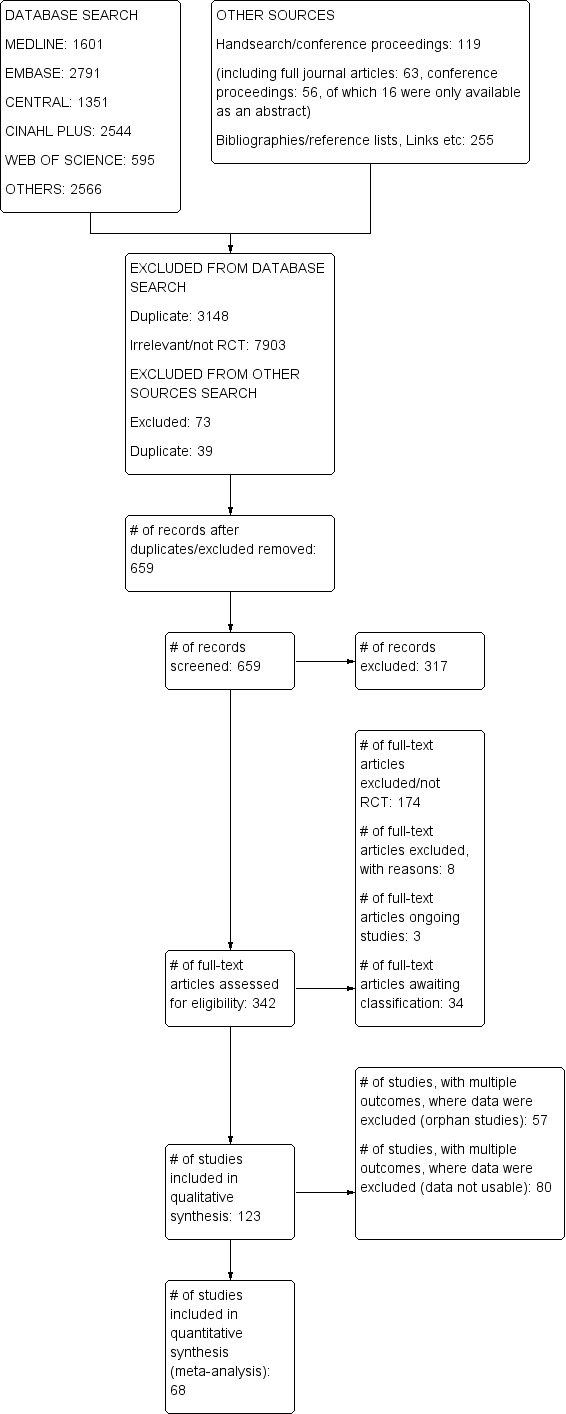
Study flow diagram.
Included studies
We considered only commercially available formulations used for dental anaesthesia, leading to inclusion of studies comparing outcomes for different formulations of lidocaine, articaine, mepivacaine, prilocaine, and bupivacaine. We identified a total of 123 RCTs (19,223 participants) that met our inclusion criteria and were published in peer‐reviewed journals.
Types of interventions
We investigated the success of dental anaesthesia among participants in studies that used clinical or simulated scenario testing.
For clinical studies, we classified outcomes by the dental tissues that were anaesthetized and tested (pulp, hard and soft tissues combined, healthy pulps, pulps with signs and symptoms of irreversible pulpitis; as well as individual tissues that underwent different dental interventions followed by pooling of results and tissues for which testing was unclear). These studies looked at pain associated with a variety of dental interventions including:
extraction/surgical treatment (30 studies);
endodontic treatment of teeth with irreversible pulpitis (20 studies);
surgical and non‐surgical root canal treatment (Moore 1983);
surgical periodontal treatment (Moore 2007);
restorative procedures including cavity preparation and crown preparation in vital teeth (8 studies);
various treatments for which results were pooled (9 studies); and
treatment for which the exact clinical procedure was not specified (Albertson 1963).
Simulated scenario testing of success involved testing one or more dental tissues per study, although clinical testing of local anaesthetic success may also have been performed.
The tissues tested were:
healthy pulps, using a cold stimulus (Porto 2007; Sancho‐Puchades 2012);
healthy pulps, using an electric pulp tester (38 studies);
diseased pulps with irreversible pulpitis, using a cold stimulus (Atasoy Ulusoy 2014; Cohen 1993; Sherman 2008);
diseased pulps with irreversible pulpitis, using an electric pulp tester (Allegretti 2016; Kanaa 2012; Sampaio 2012; Sood 2014; Tortamano 2009; Visconti 2016);
soft tissues, using an appropriate method (33 studies); and
soft tissues, using an electric pulp tester (Haas 1990; Haas 1991).
Eleven studies did not assess local anaesthetic success (Costa 2005; Donaldson 1987; Fertig 1968; Kalia 2011; Lasemi 2015; Linden 1986; Martinez‐Rodriguez 2012; Nespeca 1976; Oliveira 2004; Tofoli 2003; Tortamano 2013).
The speed of onset of anaesthesia was measured in various ways.
Healthy pulps, using a cold stimulus (Sancho‐Puchades 2012).
Healthy pulps, using an electric pulp tester (35 studies).
Soft tissues, using an appropriate method (46 studies).
Various dental tissues, using a clinical procedure (Kramer 1958; Mumford 1961; Nespeca 1976).
Method of testing was not clear (Nespeca 1976), but it was assumed to be onset of soft tissue anaesthesia (Bradley 1969; Gangarosa 1967; Silva 2012; Thakare 2014), or it was not a conventional technique (Gazal 2017).
Duration of anaesthesia was measured in several ways.
Healthy pulps, using an electric pulp tester (17 studies).
Soft tissues, using an appropriate method (45 studies).
Various dental tissues, using a clinical procedure (Mumford 1961; Weil 1961).
Method of testing was not clear (Khoury 1991; Thakare 2014).
Types of injections
Types of injection used in each study included:
inferior alveolar nerve blocks (27 studies);
inferior alveolar nerve blocks and buccal infiltrations using the same local anaesthetic formulation for both injections (23 studies);
inferior alveolar nerve blocks and a different local anaesthetic formulation for the infiltrations (Aggarwal 2009; Haase 2008);
maxillary infiltrations (29 studies);
mandibular infiltrations (9 studies);
a mixture of mandibular and maxillary infiltrations (Haas 1990; Haas 1991; Kramer 1958); and
a mixture of separate dental blocks and infiltration anaesthesia (24 studies).
We found one study that used each of the following techniques: a mental block (Batista da Silva 2010), an infraorbital block (Berberich 2009), a palatal‐anterior superior alveolar nerve block (Burns 2004), and a high‐tuberosity maxillary second nerve block (Forloine 2010).
Two studies did not specify the type of injection technique used (Albertson 1963; Pässler 1996).
The volume of solution used for each injection ranged from 0.18 mL in Bortoluzzi 2009 to over 4.5 mL in Silva 2012, although this volume could have been greater in studies that used variable amounts of local anaesthetic.
Locations of studies
The 123 studies were conducted in 19 countries, which included USA (43 studies); Brazil (18 studies); India (16 studies); Germany and Spain (six studies each); Turkey (five); UK, Australia, Canada, and Iran (four studies each); Sweden (three studies); Lebanon and Saudi Arabia (two studies each); and Finland, Israel, Moldova, Thailand, Pakistan, and Republic of Korea (one study each). All were single‐centre studies, apart from Karm 2017 and two multi‐centre trials (Malamed 2000a; Malamed 2000b), although these were possibly documenting the same study. However, attempts to contact the first study author to confirm this were unsuccessful.
All studies were conducted in a university or hospital setting, apart from two studies that were conducted in private practice (Chilton 1971; Fertig 1968), one study that took place in a specialist endodontic practice (Cohen 1993), one study that was undertaken in both hospital and private practice (Gangarosa 1967), and one study that was conducted at a military base (Nespeca 1976).
Types of study design
We identified 54 RCTs that used a parallel design. Of these, 10 looked at purely clinical outcomes (Bradley 1969; Hosseini 2016; Kolli 2017; Lima 2009; Malamed 2000a; Malamed 2000b; Nabeel 2014; Pässler 1996; Srinivasan 2009; Yadav 2015), four looked at purely simulated scenario outcomes (Fertig 1968; Hersh 1995; Martinez‐Rodriguez 2012; Srisurang 2011), and 40 looked at both clinical and simulated scenario outcomes.
We identified 68 RCTs that applied a cross‐over design. Of these, two looked at purely clinical outcomes (Moore 2007; Thakare 2014), 48 looked at purely simulated scenario outcomes, and 18 looked at both clinical and simulated scenario outcomes.
One study compared local anaesthesia success in participants having teeth extracted but it was not clear whether the study used a parallel or cross‐over design (Keskitalo 1975). Attempts to contact the first study author to clarify this were unsuccessful.
Types of participants
A total of 19,223 participants were recruited to the 123 studies. Numbers in each study ranged from 10 in Ruprecht 1991 to 3703 in Kramer 1958. The ages of participants ranged from four years in Malamed 2000a and Malamed 2000b to 81 years in Nordenram 1990. One hundred eleven studies stated an average age, a range of ages, or both. However, 12 studies gave no indication of the age of participants (Albertson 1963; Cohen 1993; Fertig 1968; Gangarosa 1967; Hosseini 2016; Kalia 2011; Kramer 1958; Martinez‐Rodriguez 2012; Sadove 1962; Sherman 2008; Stibbs 1964; Weil 1961), although when we communicated with the study author, we discovered that one of these ‐ Cohen 1993 ‐ involved mainly adults. Ninety‐five studies had a varying mixture of male and female participants, six had only male participants (Gazal 2015; Gazal 2017; Kammerer 2014; Knoll‐Kohler 1992a; Knoll‐Kohler 1992b; Ruprecht 1991), and 22 gave no indication of the male‐to‐female ratio.
When studies defined their measurement of anaesthetic success differently than we did or presented findings in a unit other than percentage or number of successful outcomes, we recalculated these values when possible (Table 16). Alternatively, we sought data that would allow us to do these calculations, if they were not available. This also applied to other aspects of the paper that needed clarification.
8. Definitions of success, if changed, for each study and data used.
| Abdulwahab 2009 | Soft tissue data (success and onset) requested, but not possible to obtain from first study author. Available data for success using pulp testing, when additional studies were not available to perform meta‐analysis, are included in the table of orphan studies, Table 13. For comparisons of different solutions when meta‐analysis was possible, we requested paired data, but it was not possible to obtain them. Therefore, study data were treated as parallel study data. Mandibular first molar data were used for pulpal anaesthetic success and are presented in Analysis 1.2,Analysis 3.2,Analysis 6.2,Analysis 13.1,Analysis 16.1,Analysis 19.1, and Analysis 18.1 |
| Aggarwal 2009 | Three participants (1 from each group) were eliminated owing to lack of soft tissue anaesthesia. These were excluded from the study’s results but have been included in our calculation of success of soft tissue anaesthesia Pulpal anaesthesia success was defined as "no pain" and "faint, weak, or mild pain", but we classed only "no pain” as successful (raw data obtained from study authors) Quote (from correspondence): "Three out of 24 patients (12%) in control IANB group, 7 out of 30 patients (23%) in IANB and lidocaine infiltration group and 14 out of 30 patients (47%) in IANB and articaine infiltration group had no pain (HP‐VAS ‘0 mm’)" Only data for additional lidocaine or articaine injections have been used (placebo excluded). Available data are included in the table of orphan studies, Table 13 |
| Aggarwal 2014 | Pulpal anaesthesia success was defined as "no pain" and "faint, weak, or mild pain", but we classed only "no pain" as successful (raw data obtained from study authors) Quote (from correspondence): "Out of 30 patients receiving lidocaine, 1:80,000 epinephrine, 3 patients had no pain (HP VAS score of 0), whereas in patients receiving lidocaine, 1:200,000 epinephrine 5 patients (out of 32) had no pain" Of the original 63 patients, 1 patient receiving 2% lidocaine, 1:80,000 epinephrine did not have profound lip numbness at 15 minutes and was excluded from the study. For the review, this patient was included as a failure in the 2% lidocaine, 1:80,000 epinephrine group, when failure of pulpal and soft tissue anaesthesia was calculated (i.e. group size was still 31 participants. Available data are included in the table of orphan studies, Table 13 |
| Aggarwal 2017 | In this study, success during access cavity preparation and instrumentation in teeth with irreversible pulpitis was defined as no pain or mild pain (0 or ≤ 54 mm on a VAS, respectively). Only patients with a VAS of 0 were classed as successful for this systematic review. Also, participants excluded owing to absence of lip numbness were classed as failures. Pulpal and soft tissue success data are included in the table of orphan studies, Table 13 |
| Albertson 1963 | Only grade A anaesthesia (complete absence of pain) was classed as successful anaesthesia. Grade B and grade C were classed as failure. Available data are included in the table of orphan studies, Table 13 |
| Allegretti 2016 | In this study, success for pulpectomy included patients who had no pain (0) or mild, bearable pain (1). Only patients with scores of 0 were classed as successful anaesthesia in the review. Data for success of clinical pulpal anaesthesia for 2% lidocaine, 1:100,000 epinephrine vs 4% articaine, 1:100,000 epinephrine were used for meta‐analysis and are presented in Analysis 1.1. Data for 2% mepivacaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine are presented in Analysis 8.1. Data for 2% mepivacaine, 1:100,000 epinephrine vs 4% articaine, 1:100,000 epinephrine are presented in Table 13 Subjective soft tissue success data are presented in Analysis 1.3 and Analysis 7.2, apart from the data for 2% lidocaine, 1:100,000 epinephrine vs 2% mepivacaine, 1:100,000 epinephrine, which are presented in Table 14 (local anaesthetics have 100% success in all studies in that analysis) Simulated scenario pulpal anaesthesia success data are included in Table 14, as a negative response to electric pulp testing is not a reliable indicator of pulpal anaesthesia |
| Arrow 2012 | Available data are included in the table of orphan studies (Table 13) and were obtained from the study author. Data for IANBs and mandibular infiltrations are presented. One hundred fourteen outcomes were scheduled to be recorded, but because of failure of 1 visit, only 113 were recorded. Of these interventions, 111 recorded children's response to treatment (confirmed by the study author) |
| Ashraf 2013 | Although the study looked at the success of supplemental injections following initial anaesthetic failure, success data for the initial IANB for each solution could be used in this review. The exact numbers of successful injections for pulpal and soft tissue anaesthesia for each local anaesthetic were not given. Attempts were made to get both pulpal and soft tissue anaesthesia data, but we were unable to contact the study author; therefore no data could be used |
| Atasoy Ulusoy 2014 | Clinical, pulpal anaesthetic success was defined as "no pain" or "weak/mild" at various stages of treatment including endodontic access and instrumentation of each root canal in the paper. It was defined as "no pain" during access cavity preparation for this review Pulpal anaesthetic success, when measured by simulated scenario testing, was defined as no response to cold stimuli (Endo‐Ice), 10 minutes after local anaesthetic administration. Available data for clinical, pulpal anaesthetic success are included in the table of orphan studies, Table 13, and data for simulated scenario testing of pulps are included in Table 14 |
| Batista da Silva 2010 | Pulpal anaesthetic success values were shown only graphically in the original research paper, but the study author supplied actual numerical values for success via email correspondence,.as well as paired data. Mandibular second premolar data are included in Analysis 1.2 |
| Berberich 2009 | Definitions of success from the study were used. Paired data for comparisons in this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Maxillary canine data were used for pulpal anaesthetic success and are presented in Analysis 10.1,Analysis 14.1, and Analysis 13.1. Soft tissue anaesthesia success data are presented in the table of orphan studies, Table 13 (2% lidocaine, 1:50,000 epinephrine vs 3% mepivacaine plain), and in Table 14 (local anaesthetics have 100% success in all studies in that analysis) |
| Bhagat 2014 | Success for each local anaesthetic group was presented as mean VAS scores in the study. Also, only IANBs appear to have been used for extractions, as there was no mention of buccal infiltrations administered. An attempt to obtain VAS = 0 data and to clarify the injections given were unsuccessful, as no contact could be made with the study author. Data are presented in Table 14 |
| Bortoluzzi 2009 | Tests for soft tissue success (VAS = 0) were done at 3 minutes and at 15 minutes and are presented as such. However local anaesthetic could have been successful at 3 minutes, at 15 minutes, at both times, or at neither time. Therefore data were requested from the study author to allow soft tissue success at any time during the first 15 minutes. Test 1 (a little scrub over the anaesthetized area with a standardized piece of cotton) data were used, as they are similar to patients' self‐reported anaesthesia of the lip. Available data are presented in Analysis 7.2 |
| Bouloux 1999 | No pain on the global pain scale was classed as anaesthetic success (a little, some, a lot, and worst possible were classed as failure) and used in this review after raw data were obtained from the study author. Success defined as 0 on a VAS (0–100 mm scale) was not used, as success for each solution was less than success on the global pain scale, which suggested that some patients with single‐figure VAS scores said they had no pain on the global pain scale. Success was measured in terms of patients, not teeth, as each patient needed either 2 or 4 teeth extracted (i.e.data were pooled for both jaws) Although paired data for this cross‐over study were available for meta‐analysis, paired data from the other study it could be combined with ‐ Laskin 1977 ‐ were not available. Therefore, study data from this latter study were treated as if they were parallel study data and were combined with paired data from this study, as detailed in Unit of analysis issues, allowing Laskin 1977 to be removed in a sensitivity analysis. The data are presented in Analysis 6.1. Data for soft tissue anaesthetic success, tested with a probe, are presented in the table of orphan studies, Table 13 |
| Bradley 1969 | A variety of treatments were carried out, and their results were pooled. Injections were described as infiltrations and "mandibular" injections. It was assumed that infiltration data could reflect injections into the maxilla and mandible, while with "mandibular injections", it was assumed that these were IANBs Only combined data for 1.8 mL injections were used, as 1.8 mL is a standard volume of anaesthetic to inject, while the 0.8–3.6 mL data contain much greater variation in volume. Success was classed as grade A only. Grade B and grade C were classed as failure. Available data are included in the table of orphan studies, Table 13 |
| Burns 2004 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Right maxillary central incisor data were used for pulpal anaesthetic success and are presented in Analysis 13.1 |
| Caldas 2015 | Paired data for this cross‐over study were not available for meta‐analysis, although success for each formulation was 100%. Therefore, study data were treated as parallel study data. Right maxillary canine data are presented in Analysis 12.1 |
| Chapman 1988 | The method of success measurement was not stated Quote: "Satisfactory depth of anaesthesia was established within a further five minutes with both agents" Data are not usable and are presented in Table 14 |
| Chilton 1971 | Only those injections graded as "complete" were classed as successful (complete but wore off, partial no reinjection, partial reinjection were excluded). Injections were classed as infiltrations and IANBs rather than mandibular/maxillary (i.e. some infiltrations may have been mandibular injections). Available data for periodontal and endodontic treatment are included in the table of orphan studies, Table 13, apart from periodontal treatment using inferior alveolar nerve blocks and infiltrations, comparing 2% lidocaine, 1:100,000 epinephrine vs 4% prilocaine, no vasoconstrictor, when meta‐analysis was possible (Analysis 4.1) |
| Claffey 2004 | In this study, success was defined as the ability to access and instrument the teeth with no pain or mild pain (0 or ≤ 54 mm on a VAS, respectively). Only patients with VAS of 0 were classed as successful for the systematic review 5 patients were excluded from the lidocaine group and 2 from the articaine group, as they failed to achieve lip anaesthesia. These were also counted as failures when overall success was calculated. Data are presented in Analysis 1.1. The final figures for soft tissue anaesthesia success are therefore based on 79 rather than 72 patients (confirmed by the study author). Soft tissue data are presented in Analysis 1.3 |
| Cohen 1993 | Definition of success and data from the study were used. Data for mandibular molars for simulated scenario pulp anaesthesia and anaesthesia during pulpotomy are included in the table of orphan studies, Table 13. Data for simulated scenario soft tissue anaesthesia are included in Table 14 (local anaesthetics have 100% success in all studies in that analysis) |
| Colombini 2006 | Success was classed as "no perceived pain during the surgical procedures". Data for extraction of mandibular third molar teeth are presented in Analysis 7.1 |
| Costa 2005 | Anaesthetic success was not measured ‐ only onset and duration of pulpal anaesthesia |
| Dagher 1997 | Success of pulpal anaesthesia: Only mandibular, first molar data were used for the review. Soft tissue anaesthesia: Subjective feeling of soft tissue numbness was used for the review. Definitions of success from the study were used. Paired data for were not available for meta‐analysis. Therefore, study data were treated as parallel study data and are presented in Analysis 9.1,Analysis 10.1, and Analysis 11.1. The data for soft tissue anaesthetic success are presented in Table 14 (local anaesthetics have 100% success in all studies in that analysis) |
| Donaldson 1987 | Anaesthetic success was not measured ‐ only onset and duration of pulpal anaesthesia |
| Elbay 2016 | For clinical anaesthesia, success was classed as no pain (mild discomfort, moderate pain, and severe discomfort and/or pain were classed as failure). For extractions, the data for success during extraction were used (data for probing were used for soft tissue success, and data for gingival elevation were not used). Only an inferior alveolar nerve block was used; therefore the long buccal nerve may not have been anaesthetized. This may have reduced any differences between the 2 solutions tested rather than show their true differences. Study data could not be combined with the data from Hellden 1974 for this reason. For pulpotomies, participants who had no pain during every part of the procedure were classed as successful; these data were requested from the study author but were not obtained. Paired data for this cross‐over study were not available for meta‐analysis Data for success of clinical anaesthesia during pulpotomies (removal of coronal pulp) and for soft tissue anaesthesia are presented in Table 13, and data for success during extractions are presented in Table 14 |
| Epstein 1965 | Complete anaesthesia was classed as successful (complete but wore off; partial or failure was classed as failure). Overall impression was also recorded, but this looked at other factors such as haemostasis and side effects and did not specifically look at anaesthetic success. Available data for restorative treatment and "other" procedures (endodontic and periodontal) are included in the table of orphan studies, Table 13, apart from extractions using inferior alveolar nerve blocks and infiltrations comparing 2% lidocaine, 1:100,000 epinephrine vs 4% prilocaine, no vasoconstrictor when meta‐analysis was possible. Data are presented in Analysis 4.1 |
| Epstein 1969 | Complete anaesthesia was classed as successful (complete but wore off; partial or failure was classed as failure) General impression was also recorded, but this looked at other factors such as haemostasis and side effects and did not specifically look at anaesthetic success. Available data for combined restorative treatment and extractions are included in the table of orphan studies, Table 13 |
| Evans 2008 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 1.2 |
| Fernandez 2005 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Data for first molar teeth simulated scenario pulpal anaesthetic success were used and are presented in Analysis 6.2. Data for simulated scenario soft tissue anaesthetic success are presented in Table 14 (both local anaesthetics have 100% success in all studies in that analysis) |
| Fertig 1968 | Anaesthetic success was not measured ‐ only duration of soft tissue anaesthesia |
| Forloine 2010 | Success of soft tissue anaesthesia could not be presented, as it was not known which local anaesthetic groups those participants who were re‐appointed following initial soft tissue anaesthesia failure and then went on to have success a second time belonged to. Overall success for pulpal anaesthesia could not be re‐calculated for the same reason; therefore the pulpal anaesthetic success quoted in the original journal article for first molar is presented in Analysis 13.1. Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data |
| Gangarosa 1967 | Unable to use anaesthetic success data for each solution, as available data were difficult to interpret. The total number of participants in each group was not the same as the figures attached to the graphs in the results section. Therefore no data were used |
| Gazal 2015 | Pulpal anaesthetic success data are presented in Table 14, as data were not usable |
| Gazal 2017 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Data for extraction of various maxillary teeth are presented in Analysis 7.1 |
| Gregorio 2008 | Quote: "the percentage of people who required complementary anaesthetic infiltration was significantly higher (P < .05) for B200 (14%) than for A200 (2%)" This allowed anaesthetic success to be calculated but calculations may have included those participants who required no additional local anaesthetic to complete treatment, but still felt pain. As we could not make contact with the study author to obtain paired data for this cross‐over study, we were not able to use the study for meta‐analysis. Data for anaesthetic success are therefore presented in Table 14 |
| Gross 2007 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 6.2 |
| Haas 1990 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Data for pulpal anaesthetic success are presented in Analysis 18.1. Data for soft tissue anaesthetic success were excluded, as testing was carried out with an electric pulp tester |
| Haas 1991 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Data for pulpal anaesthetic success are presented in Analysis 18.1. Data for soft tissue anaesthetic success were excluded, as testing was carried out with an electric pulp tester |
| Haase 2008 | Paired data for this cross‐over study were not available for meta‐analysis. The study involved both test groups receiving 4% articaine with 1:100,000 epinephrine IANB, followed by a buccal infiltration of 4% articaine, 1:100,000 epinephrine or 2% lidocaine, 1:100,000 epinephrine. First molar data were used for pulpal anaesthetic success and are included in the table of orphan studies, Table 13 |
| Hellden 1974 | "Good” anaesthesia was classed as success (when treatment could be carried out without any additional injection and no pain was felt). "Acceptable" or "poor" anaesthesia was classed as failure. Available data are included in the table of orphan studies, Table 13 |
| Hersh 1995 | For soft tissue anaesthetic success, participants who did not rate numbness at 50 mm or greater on the 1‐100 mm scale (1 = "not numb" and 100 = "completely numb") by 45 minutes after the injection were considered as "injection technique failures". Success was graded as < 50 on the 1‐100 mm scale; therefore available data are included in Table 14 |
| Hinkley 1991 | Two of 30 patients achieved lip numbness after 20 minutes and were excluded from the data analysis, although the study author was unsure which solution this occurred with. Therefore data for soft tissue anaesthesia could not be calculated. Overall success for pulpal anaesthesia could not be re‐calculated for the same reason, and paired data were not available for meta‐analysis. Pulpal anaesthetic success as quoted in the original journal article for the first molar is presented in Table 14 |
| Hosseini 2016 | In this study, success was defined as the ability to access and instrument the teeth with no pain or mild pain (0 or < 54 mm on a VAS, respectively). We attempted to contact the study author for the numbers of participants with a VAS of zero, but we were unsuccessful. Data are presented in Table 14 |
| Jaber 2010 | Data for the right mandibular incisor and 1.8 mL buccal injection were used, as this is where the injection was given. After communication with the study author, success was defined as 2 successive 80 readings on the electric pulp tester and no sustained anaesthesia, as reported in the study. Paired data for pulpal anaesthetic success were obtained from the study author and are presented in Analysis 1.2 |
| Jain 2016 | Success for each local anaesthetic group was presented as a mean VAS score in the study. An attempt to obtain zero VAS data was unsuccessful, as no contact could be made with the study author. Data are presented in Table 14 |
| Kambalimath 2013 | Success for each local anaesthetic group was defined as no pain during surgery or a short duration of pain sensation when a tooth was sectioned. An attempt to obtain data for participants who had no pain was unsuccessful, as no contact could be made with the study author. Paired data for this cross‐over study were not available for meta‐analysis. Data are presented in Table 14 |
| Kammerer 2012 | The study author was contacted to obtain the numbers of patients who had a VAS of 0, which was the criterion chosen for success. This differed slightly from those who did not need an additional injection, as reported in the study (some of these patients may have had the procedure completed with a small amount of discomfort, despite receiving no extra injection). Available data are included in the table of orphan studies, Table 13 |
| Kammerer 2014 | Definition of success and data from the study were used. Soft tissue anaesthetic success was measured on a VAS (0‐10) and was reported as mean values. Data for patients with a VAS of 0 were not available from the study author Data for pulpal anaesthetic success for 4% articaine, 1:100,000 epinephrine vs 4% articaine; 1:200,000 epinephrine 4% articaine, 1:100,000 epinephrine vs 4% articaine plain; and 4% articaine, 1:200,000 epinephrine vs 4% articaine plain, when meta‐analyses were possible, are presented in Analysis 3.2, Analysis 20.1, and Analysis 21.1, respectively. Data for pulpal anaesthetic success for 4% articaine plain vs 4% articaine, 1:400,000 are included in the table of orphan studies, Table 13. Other comparisons, including continuous data for soft tissue success, are included in Table 14 |
| Kanaa 2006 | The definition of success and data from the study were used. For soft tissue success, lip anaesthesia data were chosen and are presented in Analysis 1.3. Paired data for pulpal anaesthetic success were obtained from the study author and are presented in Analysis 1.2 |
| Kanaa 2012 | Only teeth deemed successfully anaesthetized after pulp testing were included in the clinical portion (extraction/pulp extirpation) of the study. For the review, overall success was calculated as the proportion of anaesthetized teeth from the total number of study participants entering each study group ‐ not from only those who tested negatively with the electric pulp tester. Extraction and pulp extirpation success data were combined. Available data are included in the table of orphan studies, Table 13. Simulated scenario pulpal anaesthesia success data are included in Table 14, as a negative response to electric pulp testing is not a reliable indicator of pulpal anaesthesia |
| Karm 2017 | Success for each local anaesthetic group was presented as a mean VAS score in the study. An attempt to obtain zero VAS data was unsuccessful, as no contact could be made with the study author. Data are presented in Table 14 |
| Katz 2010 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Data for pulpal anaesthetic success for first molar teeth are presented in Analysis 4.2 and Analysis 19.1. First molar data for pulpal anaesthetic success for 4% prilocaine with no vasoconstrictor vs 4% prilocaine, 1:200,000 epinephrine are presented in the table of orphan studies, Table 13 |
| Keskitalo 1975 | The study looked at extraction of wisdom teeth including for patients who received 1 local anaesthetic or another (parallel comparison), while some patients had bilateral extractions when a different local anaesthetic was used for each side. Therefore, the study was a mixture of parallel and cross‐over comparisons. The original data could not be obtained; therefore the study data could not be used for meta‐analysis and are included in Table 14 |
| Khoury 1991 | The definition of success and data from the study were used. Available data are included in the table of orphan studies, Table 13, apart from data comparing 2% lidocaine, 1:100,000 epinephrine vs 3% prilocaine, 0.03 IU felypressin and 4% articaine, 1:100,000 epinephrine vs 4% articaine, 1:200,000 epinephrine, when meta‐analyses are possible. Data for these are presented in Analysis 2.1 and Analysis 3.1, respectively |
| Knoll‐Kohler 1992a | Definitions of success for the right maxillary incisor from the study were used. Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Data for pulpal anaesthetic success for 2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine and for 2% lidocaine, 1:100,000 epinephrine vs 2% lidocaine, 1:200,000 epinephrine and 1:100,000 epinephrine are presented in Analysis 10.1 and Analysis 12.1, respectively. Data for 2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:200,000 epinephrine are included in the table of orphan studies, Table 13 |
| Knoll‐Kohler 1992b | Data for pulpal anaesthetic success were not usable (100% success for local anaesthetics for all studies; data could not be added to Analysis 1.2, because the data could not be entered as logs of the OR and associated SE using the 'inverse variance' method) and are presented in Table 14 |
| Kolli 2017 | Success for each local anaesthetic group was presented in the study as a mean score from the Faces Pain Scale ‐ Revised. An attempt to obtain "no pain" data was unsuccessful, as no contact could be made with the study author. Data are presented in Table 14 |
| Kramer 1958 | Figure 1 and Figure 2 in the study gave graphical representation of success in terms of percentages only, but the number of successful injections and the totals from which the percentages were calculated were not stated. These could be calculated from the induction time data in Table 1 (number of injections), which should represent induction times for patients who had grade A potency (success). However the total number of injections calculated from this (2163) for maxillary injections was different from the 2128 figure stated in Figure 1. The same applies for mandibular injections (1670 vs 1575, stated in Figure 2). Therefore success data were presented in Table 14 as percentages |
| Laskin 1977 | For this systematic review, success was classed as no additional local anaesthetic required after mandibular nerve block and long buccal nerve injection (the further dose of 1.8 mL was deemed failure if used). The paper states that the procedure was painless if no additional local anaesthetic was used Paired data for this cross‐over study were not available for meta‐analysis. Therefore, data from this study were treated as if they were parallel study data and were combined with the paired data from Bouloux 1999, as detailed in Unit of analysis issues, allowing this study to be removed from a sensitivity analysis. Data for success of anaesthesia during extraction are presented in Analysis 6.1 |
| Lawaty 2010 | Paired data for this cross‐over study were not available for meta‐analysis. First molar pulpal anaesthetic success data are presented in the table of orphan studies, Table 13 |
| Lima 2009 | Teeth extracted with no pain was the criterion chosen for anaesthetic success. Success was tested after 5 minutes and 10 minutes. The data for 10 minutes were used, as they yielded the maximum success rate, allowing maximum diffusion of local anaesthetic (only 1 buccal injection was given, no palatal). Data are presented in Analysis 3.1 |
| Linden 1986 | Success was not measured |
| Malamed 2000a | The results section combines different procedures requiring anaesthesia of different tissues and different injections. Raw data would be needed to calculate success (scores of 0), but attempts to contact the study author were unsuccessful. Available data (patient VAS scores in cm, on a scale of 0‐10) are included in Table 14 |
| Malamed 2000b | The data from this study were possibly derived from the Malamed 2000a study. Attempts to contact the study author to confirm this were unsuccessful. Available data (patient VAS scores in cm, on a scale of 0‐10) are included in Table 14 |
| Maniglia‐Ferreira 2009 | Data for success in the study were presented as the average number of cartridges of local anaesthetic required to obtain anaesthesia, or were rated as excellent (although a VAS was used). Attempts to contact the study author for those patients who had a VAS score of 0 during treatment were unsuccessful. Data (average number of cartridges used) are included in Table 14 |
| Martinez‐Rodriguez 2012 | Success was not measured |
| Maruthingal 2015 | Although the study was described as a prospective randomized double‐blind cross‐over trial, the order of local anaesthetic administration appeared to be pre‐determined for every participant. Clarification was sought from the study author, but contact by email could not be made. Paired data for this cross‐over study were not available for meta‐analysis. Data are presented in Table 14 |
| Mason 2009 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data for pulpal anaesthetic success were used for pulpal anaesthetic success and are presented in Analysis 10.1,Analysis 13.1, and Analysis 14.1 |
| McEntire 2011 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 3.2 |
| McLean 1993 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 4.2 and Analysis 13.1. First molar data are presented for 4% prilocaine with no vasoconstrictor vs 3% mepivacaine, no vasoconstrictor in the table of orphan studies, Table 13 Subjective soft tissue anaesthesia success data are presented in Table 14 for 2% lidocaine, 1:100,000 epinephrine vs 3% mepivacaine plain (local anaesthetics have 100% success in all studies in that analysis) and in the table of orphan studies, Table 13, for the other comparisons (orphan study) |
| Mikesell 2005 | Success of soft tissue and pulpal anaesthesia was based on the original number of participants in each group (57). Some of the participants who failed to develop soft tissue anaesthesia initially were re‐appointed and successfully achieved anaesthesia at the second visit. Therefore they were classed as overall failures and were deducted from the totals of success for pulpal anaesthesia. Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Subjective soft tissue and first molar pulpal anaesthetic success data are presented in Analysis 1.2 and Analysis 1.3 |
| Mittal 2015 | Success of anaesthesia during extraction of primary maxillary molars was not usable, and data are presented in Table 14 Success of soft tissue anaesthesia following probing is included in the table of orphan studies, Table 13 |
| Moore 1983 | Success of anaesthesia was pooled for both dental arches and procedures (access/instrumentation, canal obturation, apicoectomy + retrofilling). The study author was asked for individual data, but these were not available. Satisfactory and unsatisfactory anaesthesia were classed as failure. Only excellent anaesthesia was classed as successful and was judged by "Clinician decisions for lack or near lack of response to treatment", which may not necessarily mean pain‐free treatment. Available data are included in the table of orphan studies, Table 13 |
| Moore 2006 | One patient withdrew consent after the first drug treatment session, following testing with 4% articaine, 1:100,000 epinephrine. Sixty‐two patients completed all sessions of the study protocol; therefore success was re‐calculated after communication with the study author. Paired data were not available for meta‐analysis for IANBs and infiltrations. Therefore, study data were treated as parallel study data. Data for pulpal anaesthetic success are presented in Analysis 3.2, Analysis 20.1, and Analysis 21.1 |
| Moore 2007 | Failure was defined as the need to administer an alternative anaesthetic agent for pain control or visualization of the surgical field, which may mean that a minor degree of pain was present during the procedure. Data for meta‐analysis were not usable. Therefore data for success of anaesthesia during periodontal surgery are presented in Table 14 |
| Mumford 1961 | Injections were described as infiltrations and regional injections. It was assumed that infiltration data could be obtained from injections into the maxilla and mandible; for regional injections, it was assumed that these were IANBs. Pulpal anaesthesia success data for male and female patients were combined. Data are included in the table of orphan studies, Table 13 |
| Nabeel 2014 | Success was defined in the study as no pain or mild pain (0 to 3 on the VAS). However, despite emailing the study author for numbers of participants with scores of only zero on the VAS, it was not possible to make contact. Data for maxillary first premolars are presented in Table 14 |
| Naik 2017 | The volume of anaesthetic solution used during surgery for each local anaesthetic group was used to demonstrate success. Data are presented in Table 14 |
| Nespeca 1976 | Success was not measured |
| Nordenram 1990 | Data for elderly patients and young patients were combined. Paired data were not available for meta‐analysis. Data for success of pulpal anaesthesia (tested with an electric pulp tester) are presented in the table of orphan studies, Table 13 |
| Nydegger 2014 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Mandibular first molar data for pulpal anaesthetic success are presented in Analysis 23.1 |
| Odabas 2012 | Success was defined by the study author, following correspondence, as follows: "No pain and feeling numbness. During dental treatment, when children did not feel pain from their treated teeth and told us of feeling numbness of their lip, we considered anaesthesia complete" Data are presented in the table of orphan studies, Table 13 |
| Oliveira 2004 | Success was not measured |
| Ozec 2010 | Results were represented graphically rather than as numbers, and it was not possible to contact the study author to confirm figures. Paired data for this cross‐over study were not available for meta‐analysis. Soft tissue success data (average VAS scores) are presented in Table 14 |
| Parirokh 2015 | Success during access cavity preparation and instrumentation in teeth with irreversible pulpitis was defined as ability to access and instrument teeth without pain or with mild pain. The contact author was emailed for raw data (patients who experienced no pain), but it was not possible to make contact. Data for this outcome and for success of soft tissue anaesthesia are presented in Table 14 and Table 13, respectively |
| Pässler 1996 | Success was estimated from graphs, as numerical data were not available. Part 1 success data were not used, as 1 of the local anaesthetic formulations contained norepinephrine (not commercially available). Part 2 success – criterion for success was not stated but was likely to be absence of pain, which was the criterion chosen for success. Data for extraction and apicectomy were pooled. Part 3 success – criterion chosen for success was no pain (both partial success (additional LA given and then no pain) and failure (unable to anaesthetize) were classed as failure) Data for part 2 and part 3 are presented in Analysis 2.1 and Analysis 3.1, respectively |
| Pellicer‐Chover 2013 | Success was graded as no discomfort and as slight discomfort but not requiring additional anaesthesia. The contact author was emailed for raw data (patients who experienced no discomfort), but it was not possible to make contact. Paired data for this cross‐over study were not available for meta‐analysis. Data are presented in Table 14 |
| Poorni 2011 | The study author was emailed for data regarding success of pulpal anaesthesia, as the study classes success as < mild pain, rather than no pain, on the Heft‐Parker VAS. After making contact with the study author, I was unable to make email contact again to obtain the data. Therefore data are included in Table 14. Soft tissue data are presented in Analysis 1.3 |
| Porto 2007 | Clinical success was determined by the number of re‐anaesthesia cases for each solution; patients not needing re‐anaesthesia may have felt pain but may not have received further local anaesthetic. Paired data for this cross‐over study were not available for meta‐analysis. Data for success of pulpal anaesthesia (cold test) and anaesthesia during extraction of lower third molars are included in the table of orphan studies, Table 13 |
| Ram 2006 | "No pain during drilling" (communication with study author) was the criterion for success. Available data are included in the table of orphan studies, Table 13 |
| Robertson 2007 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 1.2 |
| Ruprecht 1991 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. The data for 4% articaine with 1:100,000 epinephrine vs 4% articaine with 1:200,000 epinephrine are presented in Analysis 3.2, and for 2% lidocaine with 1:100,000 epinephrine vs 4% articaine with 1:200,000 epinephrine in Analysis 16.1. Data for pulpal anaesthetic success for 2% lidocaine with 1:100,000 epinephrine vs 4% articaine with 1:100,000 epinephrine were not usable (100% success for local anaesthetics for all studies; data could not be added to Analysis 1.2 because the data could not be entered as logs of the OR and associated SE using the 'inverse variance' method) and are presented in Table 14 |
| Sadove 1962 | "Grade A: profound anaesthesia" was classed as success (patient did not experience any discomfort). "Grade B: adequate anaesthesia" and "grade C: inadequate anaesthesia" were classed as failure. Only data for 2% lidocaine with 1:100,000 epinephrine and 2% mepivacaine with 1:20,000 levonordefrin were used, as 2% mepivacaine, no vasoconstrictor and 2% lidocaine, no vasoconstrictor are not commercially available. Available data are included in the table of orphan studies, Table 13 |
| Sampaio 2012 | In the study, clinical anaesthesia was considered successful when the dentist accessed the pulp chamber without the patient reporting pain (pain score of 0 or 1). For the systematic review, only a score of 0 was classed as success. Data for this were obtained from the study author: "Eight (8) patients from the bupivacaine group did not report any pain (score of zero), while twenty (20) reported mild pain (score of 1). Fourteen (14) patients from the lidocaine group did not report any pain (score of zero), while eight (8) reported mild pain (score of 1)" Available data for pulpal anaesthesia during access cavity preparation and instrumentation and pulp testing using an electric pulp tester are included in the table of orphan studies, Table 13. Data for soft tissue anaesthesia are included in Table 14 (both local anaesthetics have 100% success in all studies in that analysis) |
| Sancho‐Puchades 2012 | Anaesthetic success was classified as no pain during extraction. Only success for anaesthesia during extraction was entered, as the data for overall success for testing with tetrafluoroethane were not provided in the journal article. Only failure data at each stage of the extraction, when re‐injection was required, were given. The contact author was emailed, but it was not possible to make contact. Paired data for clinical anaesthetic success (global pain judged by the patient) are presented in Analysis 5.1 |
| Santos 2007 | As paired data were not available for meta‐analysis, means of anaesthetic success measured on a 3‐point scale (continuous data) are presented in Table 14. The study author was emailed, but it was not possible to make contact |
| Sherman 1954 | Only grade A anaesthesia was classed as success. Grade B and grade C were classed as failure. Mandibular and maxillary injections for both operators were combined. Data (VAS) are included in the table of orphan studies, Table 13 |
| Sherman 2008 | Data for success during pulpotomy included cases for which pain on the VAS was mild. Despite making contact with the study author, individual success data (VAS scores of 0) for each local anaesthetic solution were not available. Data for success during pulpotomy are included in Table 14. Data for success when testing with Endo‐Ice are included in the table of orphan studies, Table 13, because it became an orphan study, and the other study that measured success, Tortamano 2009, used an electric pulp tester |
| Sierra Rebolledo 2007 | Success of anaesthesia was presented as average VAS values or need for re‐injection. The contact author was emailed for data on those with no pain during extraction and for clarification of patient numbers, but it was not possible to make contact. Paired data were not available for meta‐analysis. Available data (VAS) are included in Table 14 |
| Silva 2012 | Total volume of anaesthetic solution used during surgery was used to demonstrate success. The contact author was emailed for raw data, but it was not possible to make contact; therefore data were entered into Table 14 |
| Sood 2014 | Success during pulp extirpation was defined as when the pulp chamber was accessed with no pain or mild, bearable pain reported by the patient (pain score of 0 or 1). The contact author was emailed for raw data (patient scoring just 0; no pain), but it was not possible to make contact; therefore data were entered into Table 14, while data for success of soft tissue anaesthesia were entered into Table 13. Simulated scenario pulpal anaesthesia success data are included in Table 14, as a negative response to electric pulp testing is not a reliable indicator of pulpal anaesthesia |
| Srinivasan 2009 | Success defined as no pain or mild pain. However data in the study allowed re‐calculation of success using the criterion of no pain as success. Combined maxillary first premolar and first molar data are presented in Analysis 1.1 |
| Srisurang 2011 | Paired data for this cross‐over study were not available for meta‐analysis. Data for pulpal anaesthetic success are presented in the table of orphan studies, Table 13, apart from the data for 2% lidocaine, 1:100,000 epinephrine vs 4% articaine, 1:100,000 epinephrine, which are presented in Analysis 1.2 |
| Stibbs 1964 | Success was classed as grade A anaesthesia only. Grade B and grade C were classed as failure. It was assumed that "mandibular" injections were IANBs, and that "infiltrations" could be injections in the maxilla and mandible. It is not clear whether "other" injections included supplemental injections; therefore the data were excluded Data for 2% lidocaine, 1:50,000 epinephrine vs 2% mepivacaine, 1:20,000 levonordefrin (Neo‐Cobefrin) are included in the table of orphan studies, Table 13 (2% procaine/1.5% tetracaine, 1:20,000 levonordefrin is not commercially available) |
| Thakare 2014 | Although intraoperative and postoperative pain were measured, it is not clear whether results presented (VAS) pertain to intraoperative or postoperative pain. The study author was emailed, but we were unable to maintain contact, although we were initially successful. Data have not been used |
| Tofoli 2003 | Success was not measured |
| Tortamano 2009 | In the study, success for pulpectomy included patients who had no pain (0) or mild, bearable pain (1). Only patients with a score of 0 were classed as successful anaesthesia in the review. Data for this were obtained from the study author: "Ten patients from the articaine group did not report any pain (score of zero), while three reported mild pain (score of 1). Six patients from the lidocaine group did not report any pain (score of zero), while three reported mild pain (score of 1)." Data for success of clinical pulpal anaesthesia were used for meta‐analysis and are presented in Analysis 1.1. Subjective soft tissue success data are presented in Analysis 1.3. Simulated scenario pulpal anaesthesia success is included in Table 14, as a negative response to electric pulp testing is not a reliable indicator of pulpal anaesthesia |
| Tortamano 2013 | Success was not measured |
| Trieger 1979 | Although the study looked at postoperative analgesia, anaesthetic success measured in terms of the volume injected per quadrant to obtain local anaesthesia was presented in Figure 1 in the study. From this, the success rate for a specific volume could be calculated. It was not possible to determine if success was based on no pain. Some patients were given a general anaesthetic and received injections at the end of surgery. Data for anaesthetic success are presented in Table 14 |
| Trullenque‐Eriksson 2011 | Success was classed as no need for local anaesthetic reinforcement. The study author was contacted to determine whether patients who had no reinforcement had any pain Quote: "Surgery was only carried out if the anaesthesia had been successful. Some of them may have felt discomfort due to the force applied in more complicated extractions but not pain as such" Paired data were provided for meta‐analysis and are presented in Analysis 5.1 |
| Vahatalo 1993 | Definitions of success from the study were used. Pulpal anaesthesia data could not be entered and are included in the table of orphan studies, Table 13 |
| Vilchez‐Perez 2012 | Raw data obtained from the study author. For pulpal anaesthesia, peak rate of anaesthetic success was chosen, which occurred for articaine and bupivacaine at 10 and 15 minutes, respectively. For soft tissue anaesthesia, peak rate of anaesthetic success was chosen for the lip mucosa. After making contact with the study author, I was unable to make email contact again to obtain paired data. Available data are included in the table of orphan studies, Table 13 |
| Visconti 2016 | Clinical success of pulpal anaesthesia included teeth for which pain was rated as 0 = no pain and 1 = mild pain, on a 4‐point scale. The study author was emailed for details of those participants who had scores of only zero. Data for success using 3.6 mL of solution (the same volume used in the study by Allegretti 2016) are presented in Analysis 8.1 Success of pulpal anaesthesia upon testing with an electric pulp tester is unreliable in teeth with irreversible pulpitis. Therefore, the data are presented in Table 14, along with subjective soft tissue success data (local anaesthetics have 100% success in all studies in that analysis) |
| Vreeland 1989 | Only first molar data for 2% lidocaine, 1:100,000 epinephrine vs 2% lidocaine, 1:200,000 epinephrine were used, as 4% lidocaine, 1:100,000 epinephrine is not commercially available. Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 12.1. Subjective soft tissue data are included in the table of orphan studies, Table 13 |
| Wali 2010 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 10.1. Subjective soft tissue data are presented in Table 14 (local anaesthetics have 100% success in all studies in that analysis). Only data for solutions administered as 1.8 mL were used (2% lidocaine, 1:100,000 epinephrine and 2% lidocaine, 1:50,000 epinephrine) |
| Weil 1961 | Injections were described as infiltrations and mandibular injections. It was assumed that infiltration data could be obtained from injections in the maxilla and the mandible, while with mandibular injections, it was assumed that these were inferior alveolar blocks. Only grade A anaesthesia was classed as success. Grade B and grade C were classed as failure. Available data (VAS) are included in the table of orphan studies, Table 13 |
| Yadav 2015 | In this study, success was defined as the ability to access and instrument the teeth with no pain or mild pain (0 or ≤ 54 mm on a VAS, respectively). Only patients with a VAS of zero were classed as successful for this systematic review. The contact author was emailed for data for those participants with a VAS of zero, but it was not possible to make contact. Paired data were not available for meta‐analysis. Data are presented in Table 14 |
| Yared 1997 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. First molar data were used for pulpal anaesthetic success and are presented in Analysis 9.1,Analysis 10.1, and Analysis 11.1. Subjective soft tissue success data are presented in Table 14 (local anaesthetics have 100% success in all studies in that analysis) |
| Yilmaz 2011 | For pulpal anaesthesia, the clinical procedures performed were divided up into individual stages: use of a high‐speed handpiece, use of a low‐speed handpiece, removal of coronal pulp, restoration of the tooth. Pain (failure) could have occurred at any stage in different patients or at more than 1 stage. The first major pain event would have occurred at entry to the pulp with a high‐speed handpiece; therefore this stage was used to determine success/failure. Original data would be needed to determine overall success for the whole clinical intervention, as failure may have occurred at any stage of the procedure, but we were unable to contact the study author. Available data (VAS) are therefore included in Table 14. Subjective soft tissue success data are included in the table of orphan studies, Table 13 |
| Yonchak 2001 | Paired data for this cross‐over study were not available for meta‐analysis. Therefore, study data were treated as parallel study data. Lateral incisor data were used for pulpal anaesthetic success and are presented in Analysis 10.1. Subjective soft tissue success data are presented in Table 14 (local anaesthetics have 100% success in all studies in that analysis) |
Faces Pain Scale ‐ Revised = a modified version of the Faces Pain Scale (Hicks 2001); HP‐VAS = Heft‐Parker visual analogue scale; IANB = inferior alveolar nerve block; LA = local anaesthetic; VAS = visual analogue scale.
Excluded studies
We excluded eight clinical studies that initially appeared to be suitable for inclusion in the review because studies were non‐randomized (Cowan 1964; Cowan 1968; Hassan 2011; Raab 1990; Shruthi 2013), the solutions tested were not commercially available (Adler 1969), or solutions were compared against a placebo ‐ as in Kanaa 2009 ‐ or against sedation that was used in the study ‐ as in Caruso 1989. We described these reasons for exclusion in the Characteristics of excluded studies.
Ongoing studies
We identified three ongoing studies as abstracts when handsearching journals (Caicedo 1996; Iqbal 2009; Sheikh 2014), although they have not yet been published. We will attempt to contact these study authors (attempts so far have been unsuccessful). We described these studies under Characteristics of ongoing studies.
Studies awaiting classification
We found 34 studies that are still awaiting classification. These were published in Japanese (Manabe 2005; Oka 1990; Ouchi 2008; Shimada 2002), Korean (Im 2010; Lee 2004), or Chinese journals (27 studies), or we obtained full‐text articles too late to include these studies in the review (da Silva‐Junior 2017). The Chinese studies were identified in four systematic reviews (Su 2014a; Su 2014b; Su 2016; Xiao 2010), but we have not been able to obtain them. We will make a further attempt and will translate these papers, if obtained, along with the Japanese and Korean studies, before we decide to include or exclude them from this review. When possible, we described these studies under Characteristics of studies awaiting classification.
Risk of bias in included studies
Most of the included studies had risk of bias that was low or unclear. Most had unclear risk of bias because journal articles presented information that was unclear, and because contact could not be made with study authors. When contact with study authors was made, most studies were confirmed as having low risk of bias. A few instances of high risk of bias were noted; these were related to random sequence generation and allocation concealment (Maruthingal 2015; Trieger 1979; Trullenque‐Eriksson 2011), blinding of participants and personnel administering local anaesthetic (Trullenque‐Eriksson 2011), blinding of outcome assessment (Maruthingal 2015; Naik 2017; Trieger 1979), and incomplete outcome data (Albertson 1963; Arrow 2012; Chilton 1971; Epstein 1965; Epstein 1969; Kammerer 2014; Knoll‐Kohler 1992a; Moore 2006; Sadove 1962; Stibbs 1964; Trullenque‐Eriksson 2011; Weil 1961).
We have shown the proportion of studies with low, high, and unclear risk of bias in Figure 2. We have displayed the risk of bias summary in Figure 3. The Characteristics of included studies table details the risk of bias of each study.
2.
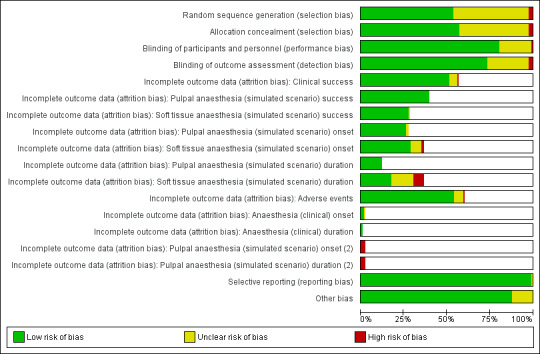
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
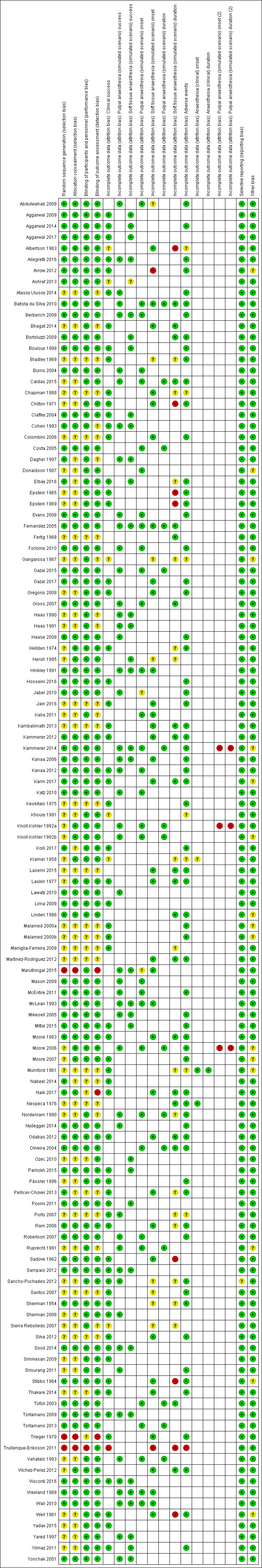
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We have provided below a summary of the risk of bias of included studies.
Allocation
For random sequence generation, we graded 66 studies as having low risk of bias, 54 as having unclear risk of bias, and three as having high risk of bias (Maruthingal 2015; Trieger 1979; Trullenque‐Eriksson 2011). Most studies used a computer programme to generate the randomization sequence, but others used random number tables, an online random number generator, tossing a coin, and randomly picking a card, envelope, or masked local anaesthetic cartridge. Those with high risk of bias had a predetermined order for local anaesthetic allocation (Maruthingal 2015), used the alphabet based on the family name of each participant (Trieger 1979), or allowed clinicians to have some choice of the local anaesthetic used (Trullenque‐Eriksson 2011).
For allocation concealment, we graded 70 studies as having low risk of bias, 50 as having unclear risk of bias, and three as having high risk of bias (Maruthingal 2015; Trieger 1979; Trullenque‐Eriksson 2011).
Blinding
We graded most of the included studies as having low or unclear risk of bias for blinding of participants and personnel (performance bias) and for blinding of outcome assessment (detection bias). We graded 99 studies as having an adequate risk of bias for blinding of participants and personnel, 23 as having unclear risk, and one as having high risk (Trullenque‐Eriksson 2011).
We graded 99 studies as having low risk of bias for blinding of outcome assessment and 30 as having unclear risk. We graded three studies as having high risk of bias for blinding of outcome assessment when no attempt was made to blind the local anaesthetic used (Maruthingal 2015; Naik 2017; Trieger 1979).
The description of the blinding technique varied between studies, with some including very detailed descriptions (Mason 2009), and others mentioning that the study was blinded only in the abstract (Sierra Rebolledo 2007). A few studies described the coding of local anaesthetic but offered no explanation of the coding system used (i.e. it could have included simple coding of two or more letters or numbers, which if used for certain local anaesthetics with obvious differences in their properties could allow determination of the identity of each of the local anaesthetics used).
Incomplete outcome data
Only a few studies had any serious omissions of data. High risk of bias was judged to have occurred when there had been a high attrition rate (> 20%), especially if this was seen more in one group than in another. We graded 117 studies as having outcomes with low risk of bias and 23 as having outcomes with unclear of risk of bias.
We graded 12 studies as having outcomes with high risk of bias. One study had a very high attrition rate of 47% (Keskitalo 1975), and another study had a marked attrition rate (Khoury 1991). For Keskitalo 1975, 141 cases were not included because teeth were not suitable for the study, possibly following further radiographic examination. Groups were still balanced after their removal and reasons for removal were similar, so we graded risk of bias for this study as low. Khoury 1991 did not include data for 282 participants. Reasons for dropouts and whether dropouts were equal among groups were not clear, so we graded risk of bias as unclear.
Some studies excluded participants who had been anaesthetized with inferior alveolar nerve block if lower lip soft tissue anaesthesia had not been achieved. These participants were re‐appointed and the inferior alveolar block was repeated; if successful a second time, this approach was classified as successful. If after a further injection participants still were not experiencing lower lip anaesthesia, some were excluded completely or were replaced with new participants and testing was repeated. When details of those excluded were available, we classed them as failures and also for any subsequent clinical procedure or simulated scenario test that was completed. It was not always possible to take this approach. For Forloine 2010, a cross‐over study, we found that it was not possible to calculate overall failure rates, but as loss of participants was balanced across groups, we graded risk of bias as low. Although recalculation was not possible for the cross‐over study Sierra Rebolledo 2007, the final numbers seemed to be greater in one group than in the other. As the reasons for this were not clear, we graded risk of bias as unclear. In the parallel study Ashraf 2013, it also was not clear from which group participants had been removed; therefore we graded risk of bias for this study as unclear.
Owing to the nature of the studies included in this review, we had to assess risk of attrition bias for several different outcomes within the same study in most cases. Therefore we added rows to the risk of bias tables under Characteristics of included studies.
We graded risk of attrition bias for the success of local anaesthesia, measured by the absence of pain during a procedure assessed on a visual analogue scale (VAS) or by other appropriate method as low in 63 studies, unclear in six studies, and high in one study (Trullenque‐Eriksson 2011). Clinical success was not assessed in 53 studies. We graded risk of attrition bias as unclear for a variety of reasons including the numbers of participants entering the trials (Albertson 1963; Gangarosa 1967), numbers completing the trial (Sierra Rebolledo 2007), clear numbers, number of participants tested with each local anaesthetic not stated (Ashraf 2013; Kramer 1958), and a high dropout rate occurred resulting in groups of similar size when it was not clear if the groups were equal in size at the start of the trial (Khoury 1991). In the only study with high risk of bias (Trullenque‐Eriksson 2011), 46% of participants from each group were excluded for a variety of reasons.
We graded risk of attrition bias for the success of pulpal anaesthesia, measured by an electric pulp tester or a cold stimulus, as low in 49 studies, unclear in zero studies, and high in zero studies. Pulpal anaesthesia, measured by an electric pulp tester or a cold stimulus, was not assessed in 74 studies.
We graded risk of attrition bias for the success of soft tissue anaesthesia, measured by the absence of pain during a procedure assessed on a VAS or by other appropriate method including using an electric pulp tester ‐ in Haas 1990 and Haas 1991 ‐ as low in 34 studies, unclear in one study (Ashraf 2013), and high in zero studies. Soft tissue success was not assessed in 88 studies. In the only study with unclear risk of bias (Ashraf 2013), six participants who did not report lip numbness were excluded from the study. However it was not clear from the journal article which groups these participants were excluded from, as they should have been classed as failures.
We graded risk of attrition bias for the onset of pulpal anaesthesia, measured by an electric pulp tester or a cold stimulus, as low in 32 studies, unclear in two studies, and high in three studies. Onset of pulpal anaesthesia was not assessed in 89 studies. We graded risk of attrition bias as unclear for two studies because journal articles did not state the numbers of participants assessed for each local anaesthetic solution (Jaber 2010; Maruthingal 2015). We graded studies as having high risk of bias owing to the small numbers of participants assessed and differences in group sizes in two studies (Kammerer 2014: 4/10 for 4% articaine, no vasoconstrictor vs 10/10 for other formulations; and Knoll‐Kohler 1992a: 6/10 for 2% lidocaine, 1:200,000 epinephrine vs 10/10 for other formulations). These two studies each tested three local anaesthetics, so when group sizes were equal, we graded outcomes as having low risk of bias. We graded Moore 2006 as having high risk of bias owing to the relatively small number of participants and differences in group sizes between 4% articaine, no vasoconstrictor; 4% articaine, 1:100,000 epinephrine; and 4% articaine, 1:100,000 epinephrine groups. We graded outcomes for these latter two groups, when numbers of participants were better balanced, as having low risk of bias.
We graded risk of attrition bias for the onset of soft tissue anaesthesia, measured by self‐assessment or following gingival probing, as low in 35 studies, unclear in eight studies, and high in two studies. Onset of soft tissue anaesthesia was not assessed in 78 studies. Gross 2007 measured onset only at 15 minutes following injection; therefore we did not assess data. We graded risk of attrition bias as unclear for outcomes from eight studies because journal articles did not state the numbers of participants assessed for each local anaesthetic solution (Abdulwahab 2009; Bradley 1969; Gangarosa 1967; Hersh 1995; Sancho‐Puchades 2012; Santos 2007; Sherman 1954) or because the number of participants completing the trial was not clear (Sierra Rebolledo 2007). In the two studies with high risk of bias, high dropout rates of up to 29% in Arrow 2012 and 46% in Trullenque‐Eriksson 2011 were seen in some groups; these may underestimate the true dropout rates.
We graded risk of attrition bias for the duration of pulpal anaesthesia, measured by an electric pulp tester or a cold stimulus, as low in 15 studies, unclear in zero studies, and high in three studies. Duration of pulpal anaesthesia was not assessed in 108 studies. We graded outcomes as being at high risk of bias because of the small numbers of participants assessed and differences in group size between the two studies (Kammerer 2014: 4/10 for 4% articaine, no vasoconstrictor vs 10/10 for other formulations; and Knoll‐Kohler 1992a: 6/10 for 2% lidocaine, 1:200,000 epinephrine vs 10/10 for other formulations). Each of these two studies tested three local anaesthetics, and for outcomes with equal group sizes, we graded them as having low risk of bias. We graded Moore 2006 as having high risk of bias owing to the relatively small number of participants and differences in group sizes between 4% articaine, no vasoconstrictor; 4% articaine, 1:100,000 epinephrine; and 4% articaine, 1:100,000 epinephrine groups. We graded outcomes between these latter local anaesthetics, when numbers of participants were better balanced, as having low risk of bias.
We graded the risk of attrition bias for the duration of soft tissue anaesthesia, measured by self‐assessment or following gingival probing, as low in 21 studies, unclear in 16 studies, and high in eight studies. Onset of soft tissue anaesthesia was not assessed in 78 studies. We rated outcomes from studies that did not state the numbers of participants assessed as having unclear risk of attrition bias. For eight studies (Albertson 1963; Chilton 1971; Epstein 1965; Epstein 1969; Sadove 1962; Stibbs 1964; Trullenque‐Eriksson 2011; Weil 1961), we graded risk of attrition bias as high because dropouts for each local anaesthetic solution were high in number and numbers were variable among the different groups included in the study. The exact reason why the expected number of participants was not assessed was unknown, although it could have been lack of compliance with reporting the time anaesthesia completely disappeared, or it could have been due to side effects. The estimated percentage dropout may be an underestimate, as soft tissue success, on which this could be calculated, often was not known and would be greater than clinical anaesthetic success, for which researchers often provided the only data available to estimate attrition bias.
We graded the risk of attrition bias for adverse events as low in 66 studies, unclear in seven studies, and high in one study. Adverse events were not assessed in 49 studies. In studies for which the numbers of participants assessed were not stated (Albertson 1963; Chapman 1988; Gangarosa 1967; Khoury 1991; Kramer 1958; Mumford 1961; Porto 2007), we graded risk of bias as unclear. In the only study with an outcome graded as having high risk of bias, a high dropout rate of up to 46% was seen in some groups, and this may underestimate the true dropout rate (Trullenque‐Eriksson 2011).
We graded risk of attrition bias for the onset of anaesthesia, measured by self‐assessment of pain during a clinical procedure, as low in two studies (Mumford 1961; Nespeca 1976), unclear in one study, and high in zero studies. Onset of anaesthesia was not assessed in 120 studies. We graded risk of attrition bias as unclear for one study because the journal article did not clearly state the number of participants assessed for each local anaesthetic solution (Kramer 1958).
We graded risk of attrition bias for the duration of anaesthesia, measured by self‐assessment of pain during a clinical procedure, as low in one study (Mumford 1961), unclear in zero studies, and high in zero studies. We did not assess data from Weil 1961, as measurement of duration ended when the clinical procedure was completed (i.e. before pain was experienced). Duration of anaesthesia was not assessed in 122 studies.
Selective reporting
We graded all included studies as having low risk of reporting bias, apart from one, which we graded as having unclear risk because researchers did not provide details of pulpal anaesthesia onset times (Sancho‐Puchades 2012).
Other potential sources of bias
Eleven studies received funding or were supplied with local anaesthetic from the solution's manufacturer (Arrow 2012; Donaldson 1987; Gangarosa 1967; Karm 2017; Knoll‐Kohler 1992b; Linden 1986; Moore 2006; Moore 2007; Ruprecht 1991; Stibbs 1964; Weil 1961). Three other studies may have received funding from local anaesthetic manufacturers (Malamed 2000a; Malamed 2000b; Mumford 1961). Three studies had authors who had an association with the trial's sponsors, which in each case was declared (Kammerer 2014; Moore 2006; Moore 2007).
The potential for introducing bias was unknown; therefore we graded the risk of bias as unclear (in most cases, all solutions were provided by the same manufacturer rather than a single local anaesthetic provided by one manufacturer and other product provided by a rival manufacturer).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Clinical outcomes
4% articaine, 1:100,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method
We pooled the results of four studies measuring the success of local anaesthesia of maxillary and mandibular posterior teeth with irreversible pulpitis, requiring endodontic access and instrumentation (Analysis 1.1), measured using a VAS (no pain). The four pooled, parallel studies included 203 participants (203 episodes of dental anaesthesia) in total (Allegretti 2016; Claffey 2004; Srinivasan 2009; Tortamano 2009). Data for Srinivasan 2009 were for maxillary buccal infiltrations for first premolars and first molars, while data from the mandibular studies used an inferior alveolar nerve block injection (IANB) for first molar and second molar teeth (Allegretti 2016); second premolar, first molar, second molar, and third molar teeth (Tortamano 2009); and first premolar, second premolar, first molar, and second molar teeth (Claffey 2004). Their pooling favoured articaine over lidocaine (risk ratio (RR) 1.60, 95% confidence interval (CI) 1.10 to 2.32), with little heterogeneity between studies (P = 0.33, I² = 13%). Pooling of just the three mandibular studies using an IANB suggested no evidence of a difference between formulations (RR 1.32, 95% CI 0.81 to 2.16), as well as no heterogeneity (P = 0.42, I² = 0%). The test for subgroup differences revealed evidence of moderate heterogeneity (P = 0.16, I² = 49%).
1.1. Analysis.
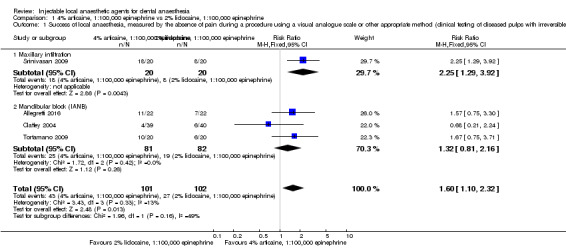
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of diseased pulps with irreversible pulpitis).
We downgraded the outcome from high to low quality owing to imprecision (sample size of 203 participants/episodes of anaesthesia and 70 events) and study limitations (one trial ‐ Srinivasan 2009 ‐ having unclear risks of selection bias). For the three mandibular studies using an IANB, we downgraded the outcome from high to moderate quality owing to imprecision (sample size of 163 participants/episodes of anaesthesia and 44 events).
Primary outcome 3: adverse effects: local and systemic
We pooled the results of three studies measuring pain on injection for local anaesthesia of maxillary and mandibular posterior teeth (Analysis 1.8), measured using a Heft‐Parker VAS. The three pooled, cross‐over studies included 157 participants (314 episodes of dental anaesthesia) in total (Evans 2008; Mikesell 2005; Robertson 2007). Data were included for pain during injection of an IANB in Mikesell 2005 and following maxillary and mandibular buccal infiltrations, respectively (Evans 2008; Robertson 2007). All infiltrations were adjacent to first molar teeth, and pain was measured only during the deposition of local anaesthetic. Pooling suggested no evidence of a difference between lidocaine and articaine (mean difference (MD) 4.74 mm, 95% CI ‐1.98 to 11.46 mm), with no heterogeneity between studies (P = 0.51, I² = 0%). The test for subgroup differences also revealed evidence of no heterogeneity (P = 0.51, I² = 0%).
1.8. Analysis.
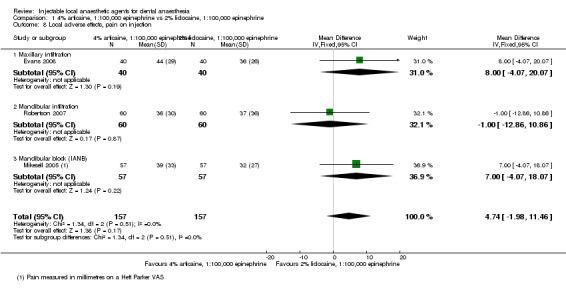
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 8 Local adverse effects, pain on injection.
We conducted a sensitivity analysis (Table 17) that excluded one study in which topical anaesthetic was not used before injection (Robertson 2007), which suggested no evidence of a difference between formulations (MD 7.46 mm, 95% CI ‐0.70 to 15.61 mm) with no heterogeneity between studies (P = 0.90, I² = 0%).
9. Sensitivity analysis (local anaesthetic success, with and without cross‐over studies with no paired data, and with and without studies with high risk of bias).
| Outcomes following removal of cross‐over studies without paired data | ||||||||
| Outcome | With all studies included | With cross‐over studies removed | ||||||
| Studies | Participants (events) | Effect estimate | Heterogeneity | Studies | Participants | Effect estimate | Heterogeneity | |
| Analysis 1.2 | Abdulwahab 2009; Batista da Silva 2010; Evans 2008; Jaber 2010; Kanaa 2006; Mikesell 2005; Robertson 2007; Srisurang 2011 | 309 (586) | OR 2.71, 95% CI 1.74 to 4.22 | P = 0.45, I² = 0% | Batista da Silva 2010; Jaber 2010; Kanaa 2006; Srisurang 2011 | 134 (236) | OR 3.28, 95% CI 1.23 to 8.80 | P = 0.46, I² = 0% |
| Analysis 1.3 | Allegretti 2016; Claffey 2004; Kanaa 2006; Mikesell 2005; Poorni 2011; Tortamano 2009 | 355 (443) | RR 1.03, 95% CI 0.99 to 1.07 | P = 0.3, I² = 17% | Allegretti 2016; Claffey 2004; Poorni 2011; Tortamano 2009 | 179 (267) | RR 1.02, 95% CI 0.98 to 1.07 | P = 0.47, I² = 0% |
| Analysis 1.6 | Bhagat 2014; Kalia 2011; Kambalimath 2013; Kanaa 2006; Martinez‐Rodriguez 2012; Silva 2012 | 637 (818) | MD ‐0.23 minutes, 95% CI ‐0.45 to ‐0.01 minutes | P = 0.00001, I² = 87% | Bhagat 2014; Martinez‐Rodriguez 2012 | 456 (456) | MD ‐0.18 minutes, 95% CI ‐0.30 to ‐0.07 minutes | P = 0.23, I² = 29% |
| Analysis 4.4 | Chilton 1971; McLean 1993 | 406 (436) | MD 0.02 minutes, 95% CI ‐0.10 to 0.14 minutes | P = 0.51, I² = 0% | Chilton 1971 (orphan) | 376 (376) | MD 0.02 minutes, 95% CI ‐0.10 to 0.14 minutes | Not applicable |
| Analysis 6.1 | Bouloux 1999; Laskin 1977 | 31 (62) | OR 0.58, 95% CI 0.07 to 5.12 | P = 0.17, I² = 47% | Bouloux 1999 (orphan) | 23 (46) | OR 0.14, 95% CI 0.01 to 2.77 | Not applicable |
| Analysis 6.4 | Fernandez 2005; Laskin 1977; Moore 1983 | 79 (126) | MD 0.02 minutes, 95% CI ‐1.07 to 1.10 minutes | P = 0.06, I² = 64% | Moore 1983 (orphan) | 32 (32) | MD ‐0.90 minutes, 95% CI ‐1.96 to 0.16 minutes | Not applicable |
| Analysis 6.5 | Fernandez 2005; Gross 2007; Laskin 1977; Linden 1986; Moore 1983; Nespeca 1976 | 232 (332) | MD 222.88 minutes, 95% CI 135.99 to 309.76 minutes | P < 0.00001, I² = 92% | Moore 1983; Nespeca 1976 | 132 (132) | MD 261.07 minutes, 95% CI 195.96 to 326.18 minutes | P = 0.12, I² = 53% |
| Analysis 7.2 | Allegretti 2016; Bortoluzzi 2009 | 68 (92) | RR 1.07, 95% CI 0.73 to 1.59 | P = 0.06, I² = 72% | Allegretti 2016 (orphan) | 44 (44) | RR 1.00, 95% CI 0.92 to 1.09 | Not applicable |
| Analysis 19.3 | Chilton 1971; Hinkley 1991 | 421 (449) | MD ‐0.01 minutes, 95% CI ‐0.14 to 0.11 minutes | P = 0.86, I² = 0% | Chilton 1971 (orphan) | 393 (393) | MD ‐0.01 minutes, 95% CI ‐0.14 to 0.11 minutes | Not applicable |
| Outcomes following removal of studies with high risk of bias | ||||||||
| Outcome | With all studies included | With high risk of bias studies removed | ||||||
| Studies | Participants (events) | Effect estimate | Heterogeneity | Number of studies | Participants (events) | Effect estimate | Heterogeneity | |
| Analysis 5.1 | Sancho‐Puchades 2012; Trullenque‐Eriksson 2011 | 37 (74) | OR 0.87, 95% CI 0.27 to 2.83 | P = 0.18, I² = 44% | Sancho‐Puchades 2012 (orphan) | 18 (36) | OR 2.00, 95% CI 0.37 to 10.92 | Not applicable |
| Analysis 5.2 | Gregorio 2008; Trullenque‐Eriksson 2011 | 69 (138) | MD ‐0.85 minutes, 95% CI ‐1.26 to ‐0.44 minutes | P = 0.98, I² = 0% | Gregorio 2008 (orphan) | 50 (100) | MD ‐0.85 minutes, 95% CI ‐1.27 to ‐0.43 minutes | Not applicable |
| Analysis 5.3 | Trullenque‐Eriksson 2011; Vilchez‐Perez 2012 | 39 (78) | MD ‐172.61 minutes, 95% CI ‐239.69 to ‐105.53 minutes | P = 1.0, I² = 0% | Vilchez‐Perez 2012 (orphan) | 20 (40) | MD ‐172.55 minutes, 95% CI ‐249.73 to ‐95.37 minutes | Not applicable |
| Outcomes following removal of studies in which topical anaesthetic was not used before injection | ||||||||
| Analysis 1.8 | Evans 2008; Mikesell 2005; Robertson 2007 | 157 (314) | MD 4.74 mm, 95% CI ‐1.98 to 11.46 mm | P = 0.51 I² = 0% | Evans 2008; Mikesell 2005 | 97 (194) | MD 7.46 mm, 95% CI ‐0.70 to 15.61 mm) | P = 0.90 I² = 0% |
We downgraded the outcome one level from high to moderate quality owing to imprecision (95% CI includes no effect and an appreciable benefit for 2% lidocaine, 1:100,000 epinephrine/sample size of 157 participants/314 episodes of anaesthesia).
We pooled the results of three studies measuring the pain following injection for local anaesthesia of maxillary and mandibular posterior teeth (Analysis 1.9), measured using a Heft‐Parker VAS. The three pooled, cross‐over studies included 156 participants (309 episodes of dental anaesthesia) in total (Evans 2008; Mikesell 2005; Robertson 2007). Data were included for pain following injection of an IANB in Mikesell 2005 and following maxillary and mandibular buccal infiltrations, respectively (Evans 2008; Robertson 2007). All infiltrations were adjacent to first molar teeth, and peak pain data from the day of the injection were used. Pooling suggested that injection of lidocaine resulted in less pain than articaine (MD 6.41 mm, 95% CI 1.01 to 11.80 mm), with little heterogeneity between studies (P = 0.24, I² = 30%). The test for subgroup differences also revealed evidence of moderate heterogeneity (P = 0.24, I² = 30.3%).
1.9. Analysis.
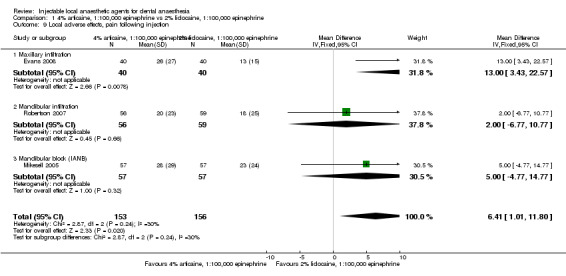
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 9 Local adverse effects, pain following injection.
We downgraded the outcome one level from high to moderate quality owing to imprecision (sample size of 156 participants/309 episodes of anaesthesia).
Other adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies. We have summarized the data for these outcomes in Table 15.
We have summarized the above outcomes in Table 1.
3% prilocaine, 0.03 IU felypressin versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method
We pooled the results of two parallel studies (Analysis 2.1) measuring the success of local anaesthesia of teeth/dental tissues requiring surgical procedures (Khoury 1991), or those requiring extraction/apicectomy, measured by the absence of pain (Pässler 1996). The two studies included 907 participants (907 episodes of dental anaesthesia) in total. Data were pooled from the Pässler 1996 study, testing the anterior part of the mouth (injection type not stated), and from the Khoury 1991 study, using combined data for infiltration anaesthesia and IANB while testing a selection of teeth and dental tissues (not stated). Pooling favoured lidocaine over prilocaine (RR 0.86, 95% CI 0.79 to 0.95), with evidence of no heterogeneity between studies (P = 0.59, I² = 0%).
2.1. Analysis.
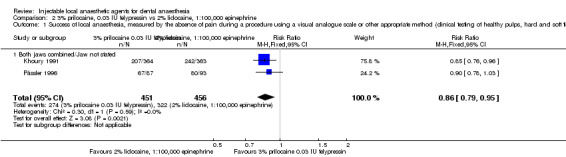
Comparison 2 3% prilocaine, 0.03 IU felypressin vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues).
We downgraded the outcome one level from high to moderate quality owing to study limitations, including an unclear risk of attrition bias (Khoury 1991), and the fact that both trials reported unclear methods of randomization sequence generation and allocation concealment. We have summarized this outcome in Table 2.
4% articaine, 1:200,000 epinephrine versus 4% articaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method
We pooled the results of three parallel studies measuring the success of local anaesthesia during surgical procedures, including extractions and apicectomies, measured by the absence of pain (Analysis 3.1). The three pooled studies included 930 participants (930 episodes of dental anaesthesia) in total (Khoury 1991; Lima 2009; Pässler 1996). Data for Lima 2009, were for maxillary third molars using infiltration anaesthesia, data from Khoury 1991 were for various types of teeth, and Pässler 1996 tested mandibular anterior and premolar teeth, although these latter two studies used multiple injection techniques and did not state the exact methods applied. Pooling suggested no evidence of a difference between formulations of articaine (RR 0.85, 95% CI 0.71 to 1.02), with substantial heterogeneity between studies (P = 0.04; I² = 68%). The test for subgroup differences also revealed substantial heterogeneity (P = 0.05, I² = 68%).
3.1. Analysis.
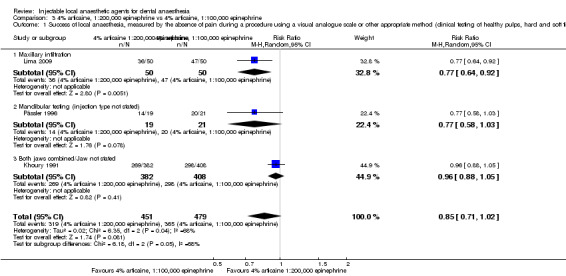
Comparison 3 4% articaine, 1:200,000 epinephrine vs 4% articaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues).
We downgraded the outcome three levels from high to very low quality because of study limitations (unclear risk of attrition bias ‐ Khoury 1991) and because two trials ‐ Khoury 1991 and Pässler 1996 ‐ had unclear risks of selection bias, imprecision (95% CI includes no effect and an appreciable benefit for 4% articaine, 1:100,000 epinephrine), and inconsistency (substantial, unexplained heterogeneity). We have summarized this outcome in Table 3.
4% prilocaine plain versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method
We pooled the results of two parallel studies measuring the success of local anaesthesia during periodontal procedures (Chilton 1971), as well as extractions (Epstein 1965), measured by the absence of pain (Analysis 4.1). The two pooled studies included 228 participants (228 episodes of dental anaesthesia) in total. Data for both studies were for various types of teeth using maxillary infiltration anaesthesia as well as IANB, and in the case of Chilton 1971, it was not specified whether infiltrations were confined to the maxilla. Pooling favoured lidocaine over prilocaine (RR 0.86, 95% CI 0.75 to 0.99) with low heterogeneity between studies (P = 0.37; I² = 5%). Pooling of just IANB data suggested no evidence of a difference between formulations (RR 0.96, 95% CI 0.73 to 1.26), with moderate heterogeneity (P = 0.18, I² = 43%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.55, I² = 0%).
4.1. Analysis.
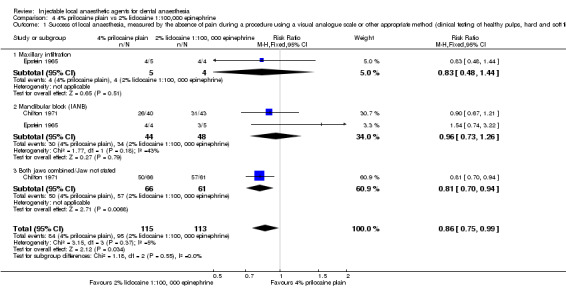
Comparison 4 4% prilocaine plain vs 2% lidocaine 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues).
We downgraded the outcome two levels from high to low quality because of study limitations, including that both trials reported unclear methods of randomization sequence generation and allocation concealment and imprecision (sample size of 228 participants and 179 events). For the two mandibular studies using an IANB, we downgraded the outcome from high to low quality for the same reasons (sample size in this case was 92 participants/episodes of anaesthesia and 64 events). We have summarized these outcomes in Table 4.
0.5% bupivacaine, 1:200,000 epinephrine versus 4% articaine, 1:200,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method
We pooled the results of two cross‐over studies measuring the success of local anaesthesia during extraction of lower third molar teeth, measured by the absence of pain (Analysis 5.1). The two pooled studies included 37 participants (74 episodes of dental anaesthesia) in total (Sancho‐Puchades 2012; Trullenque‐Eriksson 2011). Data were included for third molar teeth using IANB and mandibular buccal infiltration. Pooling suggested no evidence of a difference between articaine and bupivacaine (odds ratio (OR) 0.87, 95% CI 0.27 to 2.83), with moderate heterogeneity between studies (P = 0.18, I² = 44%).
5.1. Analysis.
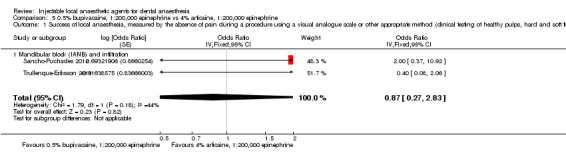
Comparison 5 0.5% bupivacaine, 1:200,000 epinephrine vs 4% articaine, 1:200,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded the cross‐over study Trullenque‐Eriksson 2011 because this study had high risk of selection, performance, and attrition bias, which meant that the cross‐over study Sancho‐Puchades 2012 became an orphan study (OR 2.00, 95% CI 0.37 to 10.92).
We downgraded the outcome two levels from high to low quality, first because of study limitations, as the two trials had unclear ‐ as in Sancho‐Puchades 2012 ‐ or high risk ‐ as in Trullenque‐Eriksson 2011 ‐ of bias related to methods of randomization sequence generation and allocation concealment. In addition, Trullenque‐Eriksson 2011 had high risk of bias for blinding of participants and personnel, provided incomplete outcome data (high attrition rate of 46%), and showed imprecision (sample size of 37 participants/74 episodes of anaesthesia, 95% confidence interval includes no effect and an appreciable benefit for both solutions). We have summarized this outcome in Table 5.
0.5% bupivacaine, 1:200,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, hard and soft tissues, tested clinically by extraction of third molar teeth (Analysis 6.1). The two pooled studies included 31 participants (62 episodes of dental anaesthesia) in total (Bouloux 1999; Laskin 1977). Data were included for mandibular third molars alone using an IANB and buccal infiltration (Laskin 1977), and for mandibular and maxillary third molars using an IANB and buccal infiltration, or greater palatine nerve block and buccal infiltration, respectively (Bouloux 1999). Pooling suggested no evidence of a difference between lidocaine and bupivacaine (OR 0.58, 95% CI 0.07 to 5.12), with evidence of moderate heterogeneity between studies (P = 0.17, I² = 47%). The test for subgroup differences revealed evidence of moderate heterogeneity (P = 0.17, I² = 47%).
6.1. Analysis.
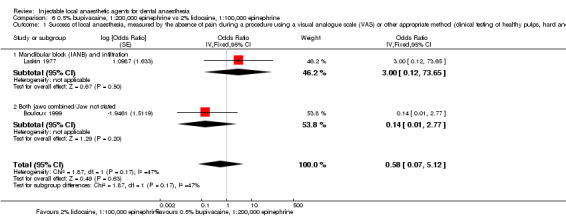
Comparison 6 0.5% bupivacaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (clinical testing of healthy pulps, hard and soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded the cross‐over study Laskin 1977, for which paired data were not available, which meant that Bouloux 1999 became an orphan study (OR 0.14, 95% CI 0.01 to 2.77).
We downgraded the outcome two levels from high to low quality because of study limitations, with one trial ‐ Laskin 1977 ‐ reporting unclear methods of randomization sequence generation and imprecision (95% confidence interval includes no effect and an appreciable benefit for both solutions, sample size of 31 participants/62 episodes of anaesthesia and 25 events). We have summarized this outcome in Table 6.
4% articaine, 1:100,000 epinephrine versus 2% mepivacaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method
We pooled the results of one cross‐over study ‐ Colombini 2006 ‐ and one parallel study ‐ Gazal 2017 ‐ measuring the success of local anaesthesia during extraction of lower third molar teeth and various maxillary teeth, respectively (Analysis 7.1), measured by the absence of pain. The two pooled studies included 110 participants (130 episodes of dental anaesthesia) in total. Data were included for IANB and mandibular buccal infiltration (Colombini 2006), as well as for maxillary, buccal, and palatal infiltrations (Gazal 2017). Pooling suggested no evidence of a difference between mepivacaine and articaine (OR 3.82, 95% CI 0.61 to 23.82), with no heterogeneity between studies (P = 0.86, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.86, I² = 0%).
7.1. Analysis.
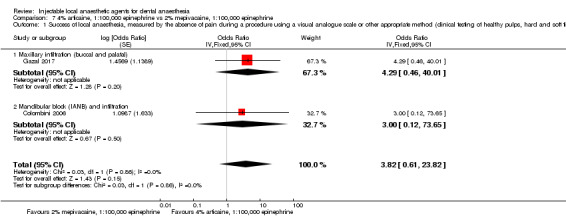
Comparison 7 4% articaine, 1:100,000 epinephrine vs 2% mepivacaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues).
We downgraded the outcome two levels from high to low quality, first owing to study limitations (the study Colombini 2006 had unclear risks of bias related to methods of randomization sequence generation, allocation concealment, and blinding of participants, personnel, and outcome assessment). There was also imprecision (95% CI includes no effect and an appreciable benefit for 4% articaine, 1:100,000 epinephrine, with sample size of 110 participants/130 episodes of anaesthesia). We have summarized this outcome in Table 7.
2% mepivacaine, 1:100,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method
We pooled the results of two studies measuring the success of local anaesthesia of mandibular, molar teeth with irreversible pulpitis, requiring endodontic access and instrumentation (Analysis 8.1), measured by the absence of pain. The two pooled, parallel studies included 68 participants (68 episodes of dental anaesthesia) in total (Allegretti 2016; Visconti 2016). Data from Allegretti 2016 were for IANB (3.6 mL) for mandibular first and second molars, and data from Visconti 2016 were for IANB (3.6 mL) for mandibular first molars. Pooling suggested no evidence of a difference between formulations (RR 1.16, 95% CI 0.25 to 5.45), with substantial heterogeneity between studies (P = 0.09, I² = 65%).
8.1. Analysis.
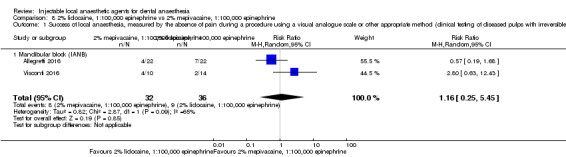
Comparison 8 2% lidocaine, 1:100,000 epinephrine vs 2% mepivacaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of diseased pulps with irreversible pulpitis).
We downgraded the outcome two levels from high to low quality owing to imprecision (sample size of 68 participants/68 episodes of anaesthesia, 95% CI includes no effect and an appreciable benefit for both formulations) and inconsistency (wide variation in point estimates and substantial, unexplained heterogeneity). We have summarized this outcome in Table 8.
Other outcomes (including simulated scenario testing)
4% articaine, 1:100,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
We pooled the results of seven cross‐over studies and one parallel study (Srisurang 2011), measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 1.2). The eight pooled studies included 309 participants (586 episodes of dental anaesthesia) in total (Abdulwahab 2009; Batista da Silva 2010; Evans 2008; Jaber 2010; Kanaa 2006; Mikesell 2005; Robertson 2007; Srisurang 2011). Data were for first premolars (Srisurang 2011), as well as for first molars (Evans 2008), using maxillary buccal infiltration, mandibular buccal infiltration (Abdulwahab 2009; Kanaa 2006; Robertson 2007), and IANB (Mikesell 2005); for second premolars using mental blocks (Batista da Silva 2010); and for central incisors using mandibular labial and lingual infiltrations (Jaber 2010). Pooling favoured articaine over lidocaine (OR 2.71, 95% CI 1.74 to 4.22), with evidence of no heterogeneity between studies (P = 0.45, I² = 0%). Pooling of just the two maxillary buccal infiltration studies ‐ Evans 2008 and Srisurang 2011 ‐ suggested no evidence of a difference between formulations (OR 1.41, 95% CI 0.54 to 3.73) and provided evidence of no heterogeneity between studies (P = 0.61, I² = 0%). Pooling of just the three mandibular buccal infiltration studies ‐ Abdulwahab 2009,Kanaa 2006, and Robertson 2007 ‐ also favoured articaine over lidocaine (OR 4.88, 95% CI 2.30 to 10.37) with evidence of no heterogeneity between studies (P = 0.60, I² = 0%). The test for subgroup differences also revealed evidence of little heterogeneity (P = 0.24, I² = 27%).
1.2. Analysis.
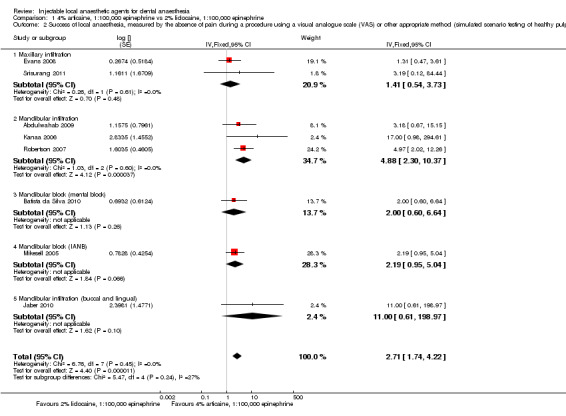
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We conducted a sensitivity analysis (Table 17) that excluded four cross‐over studies without paired data (Abdulwahab 2009; Evans 2008; Mikesell 2005; Robertson 2007), which favoured articaine over lidocaine (OR 3.28, 95% CI 1.23 to 8.80), with evidence of no heterogeneity between studies (P = 0.46, I² = 0%).
We noted study limitations (unclear methods of randomization sequence generation and allocation concealment) in one study (Srisurang 2011). We also noted indirectness (success defined as only one ‐ in Abdulwahab 2009 ‐ or two ‐ in Batista da Silva 2010,Evans 2008,Kanaa 2006, and Robertson 2007 ‐ negative responses to maximal electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum electric pulp tester values). Therefore, we downgraded the outcome two levels from high to low quality.
We pooled the results of six studies measuring the success of local anaesthesia of soft tissues, as self‐reported by participants (Analysis 1.3). Two pooled, cross‐over studies ‐ Kanaa 2006 and Mikesell 2005 ‐ and four parallel studies ‐ Allegretti 2016,Claffey 2004,Poorni 2011, and Tortamano 2009) included 355 participants (443 episodes of dental anaesthesia) in total. Data from these studies were for anaesthesia of the lower lip using IANB (Allegretti 2016; Claffey 2004; Mikesell 2005; Poorni 2011; Tortamano 2009), or using buccal infiltration (Kanaa 2006). Pooling suggested no evidence of a difference between formulations (RR 1.03, 95% CI 0.99 to 1.07), with little heterogeneity between studies (P = 0.30, I² = 17%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.33, I² = 0%).
1.3. Analysis.
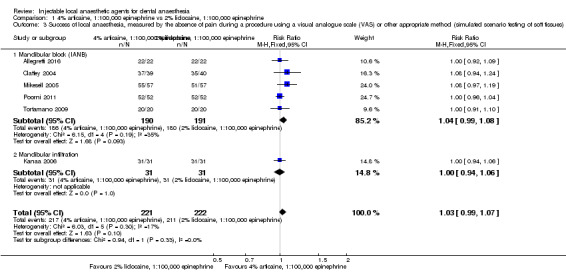
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 3 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded two cross‐over studies (Kanaa 2006; Mikesell 2005), whose data were not paired. This also suggested no evidence of a difference between lidocaine and articaine (RR 1.02, 95% CI 0.98 to 1.07; P = 0.47, I² = 0%).
We downgraded the outcome one level from high to moderate quality owing to indirectness (soft tissue anaesthesia is a poor indicator of pulp and hard tissue anaesthesia).
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of six cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 1.4). The six pooled studies included 202 participants (402 episodes of dental anaesthesia) in total (Evans 2008; Kalia 2011; Knoll‐Kohler 1992b; Robertson 2007; Ruprecht 1991; Tortamano 2013). Data were included for central incisors ‐ in Knoll‐Kohler 1992b and Ruprecht 1991 ‐ and for first molars ‐ in Evans 2008 ‐ using maxillary infiltration; for first molars using mandibular buccal infiltration (Robertson 2007), for mandibular molars using IANB (Tortamano 2013), and for a variety of maxillary and mandibular teeth using various injections whose outcomes were combined (Kalia 2011). Pooling suggested no evidence of a difference between lidocaine and articaine (MD ‐0.63 minutes, 95% CI ‐1.69 to 0.42 minutes), with evidence of substantial heterogeneity between studies (P = 0.002, I² = 73%). Pooling of just maxillary infiltration data suggested no evidence of a difference between the two formulations (MD 0.45 minutes, 95% CI ‐1.10 to 2.00 minutes), with moderate heterogeneity (P = 0.2, I² = 38%). The test for subgroup differences revealed substantial heterogeneity (P = 0.001, I² = 81%).
1.4. Analysis.
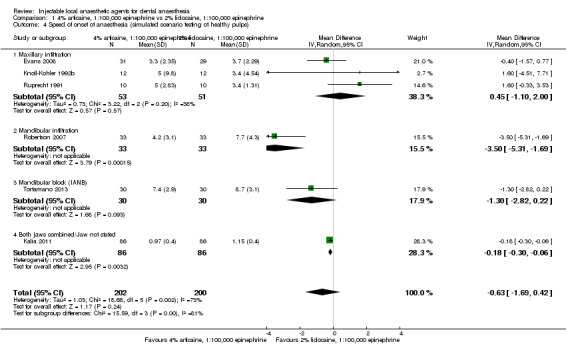
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 4 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to study limitations (unclear risks of selection (Kalia 2011; Knoll‐Kohler 1992b; Ruprecht 1991); performance and detection bias (Kalia 2011; Ruprecht 1991); imprecision (95% CI includes no effect and an appreciable benefit for articaine); inconsistency (not all confidence intervals overlap, substantial heterogeneity, and wide variation in point estimates); and indirectness (pulp testing repeated at intervals that are large compared with the onset times measured, and onset may occur at less than maximum electric pulp tester values).
We pooled the results of three cross‐over studies measuring the duration of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 1.5). The three pooled studies included 52 participants (104 episodes of dental anaesthesia) in total (Knoll‐Kohler 1992b; Ruprecht 1991; Tortamano 2013). Data were included for central incisors using maxillary labial infiltration (Knoll‐Kohler 1992b; Ruprecht 1991); and for mandibular molars using IANB (Tortamano 2013). Pooling suggested no evidence of a difference between lidocaine and articaine (MD 21.87 minutes, 95% CI ‐10.96 to 54.71 minutes), with evidence of considerable heterogeneity between studies (P < 0.0008, I² = 86%). Pooling of just maxillary infiltration data also suggested no evidence of a difference between the two formulations (MD 5.50 minutes, 95% CI ‐11.33 to 22.33 minutes), with no heterogeneity (P = 1.00, I² = 0%). The test for subgroup differences revealed considerable heterogeneity (P = 0.0002, I² = 93%).
1.5. Analysis.
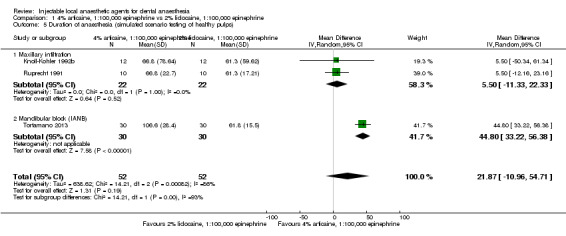
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 5 Duration of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to study limitations (two studies ‐ Knoll‐Kohler 1992b and Ruprecht 1991 ‐ had unclear risks of bias for random sequence generation, and one study ‐ Ruprecht 1991 ‐ had unclear risks of bias related to methods of allocation concealment and blinding of outcome assessment, imprecision (sample size of 52 participants/104 episodes of anaesthesia and 95% CI includes no effect and an appreciable benefit for both formulations), and inconsistency (not all confidence intervals overlap, considerable unexplained heterogeneity, and wide variation in point estimates). Indirectness (clinical anaesthesia may be present at less than maximum pulp tester readings) was also present .
We pooled the results of six studies measuring the onset of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 1.6). The four pooled, cross‐over studies ‐ Kalia 2011,Kanaa 2006,Kambalimath 2013, and Silva 2012 ‐ and two parallel studies ‐ Bhagat 2014 and Martinez‐Rodriguez 2012 ‐ included 637 participants (818 episodes of dental anaesthesia) in total. Data were included for subjective testing of soft tissues using mandibular buccal infiltration (Kanaa 2006), IANB (Bhagat 2014), IANB supplemented with buccal infiltration (Kambalimath 2013; Martinez‐Rodriguez 2012; Silva 2012), and a variety of injections whose outcomes were combined (Kalia 2011). Times for Silva 2012 were assumed to be for soft tissues (lower lip, measured subjectively) because of their speed of onset. Pooling favoured articaine over lidocaine (MD ‐0.23 minutes, 95% CI ‐0.45 to ‐0.01 minutes), with evidence of considerable heterogeneity (P = 0.00001, I² = 87%). Pooling of data for just IANB supplemented with buccal infiltration favoured articaine over lidocaine (MD ‐0.11 minutes, 95% CI ‐0.20 to ‐0.03 minutes), with evidence of no heterogeneity (P = 0.42, I² = 0%). The test for subgroup differences revealed evidence of considerable heterogeneity (P = 0.00001, I² = 92%).
1.6. Analysis.
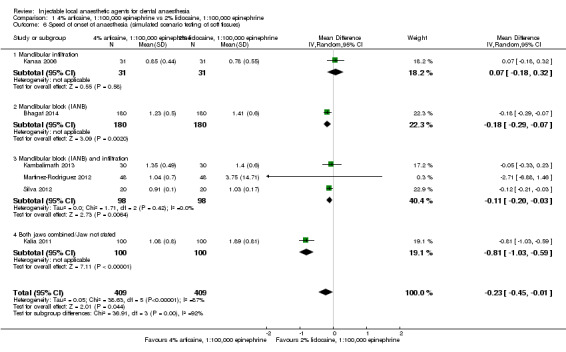
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 6 Speed of onset of anaesthesia (simulated scenario testing of soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded four cross‐over studies whose data were not paired (Kalia 2011; Kanaa 2006; Kambalimath 2013; Silva 2012). Pooling favoured articaine over lidocaine (MD ‐0.18 minutes, 95% CI ‐0.30 to ‐0.07 minutes; P = 0.23, I² = 29%).
We downgraded the outcome three levels from high to very low quality owing to study limitations (unclear risks of selection and detection bias in Bhagat 2014,Kalia 2011,Kambalimath 2013,Martinez‐Rodriguez 2012, and Silva 2012, and unclear risk of performance bias in Kambalimath 2013,Martinez‐Rodriguez 2012, and Silva 2012), inconsistency (not all confidence intervals overlap and considerable heterogeneity is evident), and indirectness (soft tissue anaesthesia is a poor indicator of onset of clinical anaesthesia).
We pooled the results of two studies measuring the duration of local anaesthesia of mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 1.7). Pooled parallel studies included 422 participants (422 episodes of dental anaesthesia) in total (Bhagat 2014; Martinez‐Rodriguez 2012). Data were included for IANB supplemented with buccal infiltration (Martinez‐Rodriguez 2012), and with IANB alone (Bhagat 2014). Pooling favoured articaine over lidocaine (MD 56.88 minutes, 95% CI 44.08 to 69.69 minutes), with evidence of no heterogeneity (P = 0.43, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.43, I² = 0%).
1.7. Analysis.
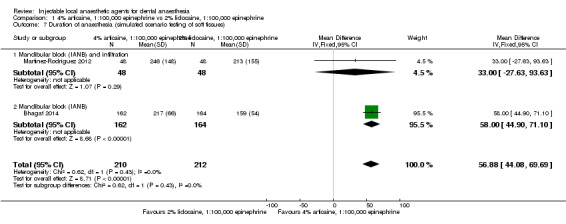
Comparison 1 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 7 Duration of anaesthesia (simulated scenario testing of soft tissues).
We downgraded the outcome two levels from high to low quality owing to study limitations (unclear risks of selection and detection bias in both studies and unclear risk of performance bias in one study ‐ Martinez‐Rodriguez 2012) and indirectness (soft tissue anaesthesia is a poor indicator of duration of clinical anaesthesia).
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
3% prilocaine, 0.03 IU felypressin versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
No data from the included studies were available for meta‐analysis.
Primary outcome 2: speed of onset and duration of anaesthesia (data for onset and duration were not included in the meta‐analysis)
No data from the included studies were available for meta‐analysis.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% articaine 1:200,000 epinephrine versus 4% articaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of five cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested using an electric pulp tester (Analysis 3.2). The five pooled studies included 248 participants (496 episodes of dental anaesthesia) in total (Abdulwahab 2009; Kammerer 2014; McEntire 2011; Moore 2006; Ruprecht 1991). Data were included for first molar teeth using mandibular buccal infiltration (Abdulwahab 2009; McEntire 2011); for central incisor teeth using maxillary labial infiltration (Kammerer 2014; Ruprecht 1991); for canine teeth using IANB (Moore 2006); and for first premolar teeth using maxillary buccal infiltration (Moore 2006). Pooling suggested no evidence of a difference between formulations of articaine (RR 0.97, 95% CI 0.87 to 1.08), with evidence of no heterogeneity between studies (P = 0.87, I² = 0%). Pooling of just the three maxillary infiltration studies also suggested no evidence of a difference between formulations (RR 0.99, 95% CI 0.92 to 1.06) and no heterogeneity between studies (P = 0.98, I² = 0%). Pooling of just the two mandibular buccal infiltration studies also suggested no evidence of a difference between the formulations (RR 0.88, 95% CI 0.70 to 1.10), with no heterogeneity between studies (P = 0.96, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.43, I² = 0%).
3.2. Analysis.
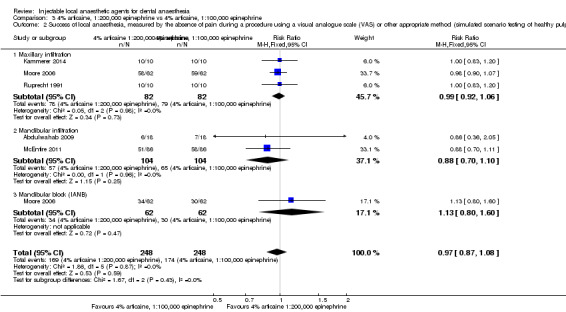
Comparison 3 4% articaine, 1:200,000 epinephrine vs 4% articaine, 1:100,000 epinephrine, Outcome 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to study limitations (unclear risks of random sequence generation in Moore 2006 and Ruprecht 1991; and of allocation concealment and detection bias in Ruprecht 1991). Indirectness was also present (success defined as only one in Abdulwahab 2009 and Kammerer 2014, as two in McEntire 2011, or as three in Moore 2006 negative responses to the maximal electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum electric pulp tester values).
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of five cross‐over studies measuring onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 3.3). The five pooled studies included 162 participants (322 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006; Ruprecht 1991; Tofoli 2003; Tortamano 2013). Data were included for central incisors (Kammerer 2014; Ruprecht 1991), as well as for first premolars (Moore 2006), using maxillary infiltration, and for canines (Moore 2006), first premolars (Tofoli 2003), and mandibular molars (Tortamano 2013), using IANB. Pooling suggested no evidence of a difference between formulations of articaine (MD 0.15 minutes, 95% CI ‐0.42 to 0.73 minutes), with no heterogeneity between studies (P = 0.99, I² = 0%). Pooling of just maxillary infiltration data suggested no evidence of a difference between the two formulations (MD 0.02 minutes, 95% CI ‐0.69 to 0.73 minutes), with no heterogeneity between studies (P = 0.91, I² = 0%). Pooling of just IANB data also suggested no evidence of a difference between the two formulations (MD 0.41 minutes, 95% CI ‐0.58 to 1.40 minutes), with no heterogeneity between studies (P = 0.98, I² = 0%). The test for subgroup differences revealed no heterogeneity between studies (P = 0.52, I² = 0%).
3.3. Analysis.
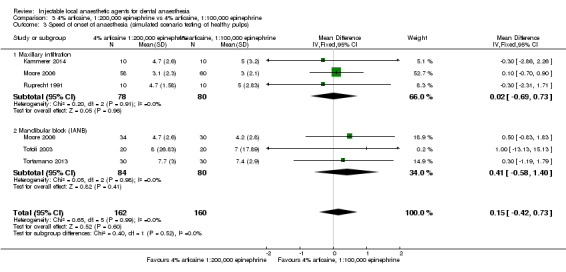
Comparison 3 4% articaine, 1:200,000 epinephrine vs 4% articaine, 1:100,000 epinephrine, Outcome 3 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to imprecision (95% CI includes no effect and appreciable benefit for 4% articaine, 1:100,000 epinephrine, with sample size of 162 participants/322 episodes of anaesthesia) and indirectness (pulp testing is repeated at intervals that are large compared with the onset times measured, and clinical anaesthesia may have been present at less than maximum electric pulp tester values). We also noted study limitations (unclear risks of random sequence generation in Moore 2006 and Ruprecht 1991, and allocation concealment and detection bias in Ruprecht 1991).
We pooled the results of five cross‐over studies measuring the duration of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 3.4). The five pooled studies included 162 participants (322 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006; Ruprecht 1991; Tofoli 2003; Tortamano 2013). Data were included for central incisors ‐ Kammerer 2014 and Ruprecht 1991 ‐ and first premolars ‐ Moore 2006 ‐ using maxillary infiltration, and for canines (Moore 2006), first premolars (Tofoli 2003), and mandibular molars (Tortamano 2013) using IANB. Pooling favoured 4% articaine, 1:100,000 epinephrine over 4% articaine, 1:200,000 epinephrine (MD ‐8.98 minutes, 95% CI ‐15.17 to ‐2.79 minutes), with evidence of little heterogeneity between studies (P = 0.39, I² = 5%). Pooling of just the maxillary infiltration data suggested no evidence of a difference between the two formulations (MD ‐6.62 minutes, 95% CI ‐13.68 to 0.44 minutes), with little heterogeneity between studies (P = 0.21, I² = 35%). Pooling of just IANB data favoured 4% articaine with 1:100,000 epinephrine over 4% articaine with 1:200,000 epinephrine (MD ‐16.80 minutes, 95% CI ‐29.65 to ‐3.95 minutes), with no heterogeneity between studies (P = 0.86, I² = 0%). The test for subgroup differences revealed moderate heterogeneity (P = 0.17, I² = 46%).
3.4. Analysis.
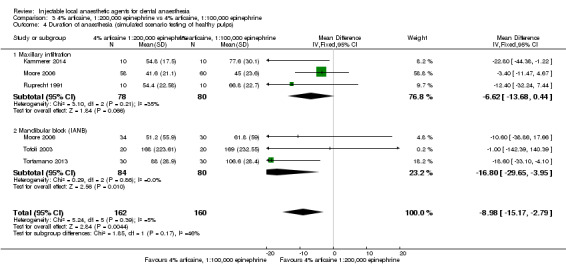
Comparison 3 4% articaine, 1:200,000 epinephrine vs 4% articaine, 1:100,000 epinephrine, Outcome 4 Duration of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to imprecision (sample size of 162 participants/322 episodes of anaesthesia), study limitations (unclear risks of random sequence generation in Moore 2006 and Ruprecht 1991), allocation concealment and detection bias (Ruprecht 1991), and indirectness (clinical anaesthesia may have been present at less than maximum electric pulp tester values).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% prilocaine plain versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested using an electric pulp tester (Analysis 4.2). The two pooled studies included 60 participants (120 episodes of dental anaesthesia) in total (Katz 2010; McLean 1993). Data were included for first molars using maxillary buccal infiltration (Katz 2010), as well as IANB (McLean 1993). Pooling suggested no evidence of a difference between formulations (RR 0.93, 95% CI 0.75 to 1.17), with evidence of no heterogeneity between studies (P = 0.76, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.77, I² = 0%).
4.2. Analysis.
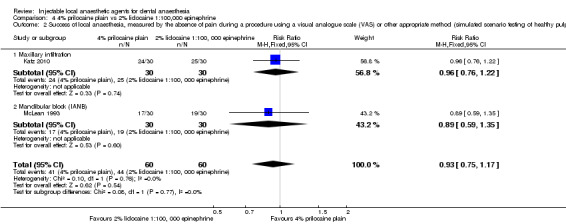
Comparison 4 4% prilocaine plain vs 2% lidocaine 1:100,000 epinephrine, Outcome 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (sample size of 120 episodes of anaesthesia/85 events) and indirectness (success defined in one study ‐ Katz 2010 ‐ as only two negative responses to the maximal electric pulp tester output for 10 minutes not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 4.3). The two pooled studies included 52 participants (103 episodes of dental anaesthesia) in total (Katz 2010; McLean 1993). Data were included for first molars using maxillary infiltration (Katz 2010), as well as IANB (McLean 1993). Pooling suggested no evidence of a difference between formulations (MD ‐0.96 minutes, 95% CI ‐2.87 to 0.95 minutes), with evidence of no heterogeneity between studies (P = 0.68, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.68, I² = 0%).
4.3. Analysis.
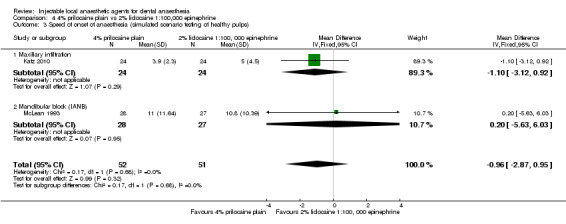
Comparison 4 4% prilocaine plain vs 2% lidocaine 1:100,000 epinephrine, Outcome 3 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (95% CI includes no effect and an appreciable benefit for both formulations, sample size of 52 participants/103 episodes of anaesthesia) and indirectness (pulp testing is repeated at intervals that are large compared with onset times measured, and clinical anaesthesia may be present at less than maximum pulp tester readings).
We pooled the results of two studies measuring the onset of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 4.4). Pooled cross‐over study ‐ McLean 1993 ‐ and parallel study ‐ Chilton 1971 ‐ data included 406 participants (436 episodes of dental anaesthesia) in total. Testing was done by using a gingival stick or subjective testing, depending on which occurred first, in McLean 1993. It was assumed that subjective anaesthesia would occur before anaesthesia using gingival sticks. Data were included for subjective testing of soft tissues using IANB and infiltration when the jaw was not stated in Chilton 1971. Pooling suggested no evidence of a difference between formulations (MD 0.02 minutes, 95% CI ‐0.10 to 0.14 minutes), with evidence of no heterogeneity between studies (P = 0.51, I² = 0%). The test for subgroup differences revealed little heterogeneity (P = 0.27, I² = 18%).
4.4. Analysis.
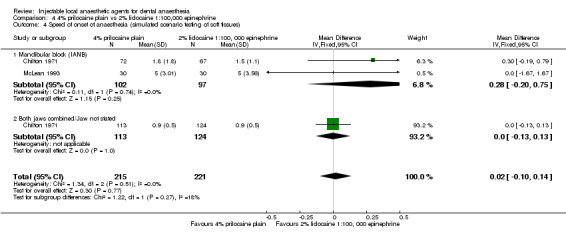
Comparison 4 4% prilocaine plain vs 2% lidocaine 1:100,000 epinephrine, Outcome 4 Speed of onset of anaesthesia (simulated scenario testing of soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded the cross‐over study McLean 1993, whose data were not paired, which meant that Chilton 1971 became an orphan study (MD 0.02 minutes, 95% CI ‐0.10 to 0.14 minutes).
We downgraded the outcome two levels from high to low quality owing to study limitations (unclear methods of randomization sequence generation and allocation concealment in Chilton 1971 and indirectness; soft tissue anaesthesia is a poor indicator of onset of clinical anaesthesia).
We pooled the results of three parallel studies measuring the duration of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 4.5). The three pooled studies included 698 participants (698 episodes of dental anaesthesia) in total (Chilton 1971; Epstein 1965; Epstein 1969). Data were included for maxillary buccal infiltration (Epstein 1965; Epstein 1969), IANB (Chilton 1971; Epstein 1965; Epstein 1969), and infiltration for which the jaw was not stated (Chilton 1971). Pooling favoured lidocaine over prilocaine (MD ‐33.95 minutes, 95% CI ‐48.05 to ‐19.84 minutes), with evidence of substantial heterogeneity between studies (P = 0.02, I² = 64%). Lidocaine was also favoured over prilocaine when just maxillary infiltration data were pooled (MD ‐47.36 minutes, 95% CI ‐63.24 to ‐31.49 minutes), with no heterogeneity between studies (P = 0.78, I² = 0%). Lidocaine was also favoured over prilocaine when just IANB data were pooled (MD ‐21.09 minutes, 95% CI ‐37.23 to ‐4.94 minutes), with moderate heterogeneity between studies (P = 0.13, I² = 52%). The test for subgroup differences revealed substantial heterogeneity (P = 0.04, I² = 69,8%).
4.5. Analysis.
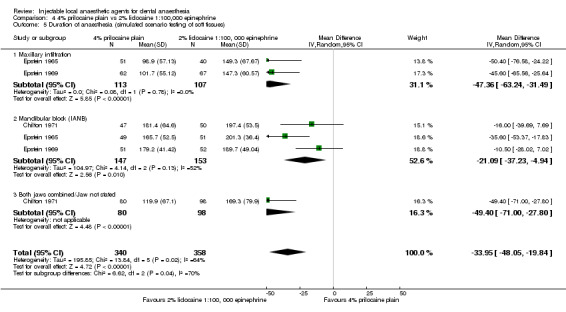
Comparison 4 4% prilocaine plain vs 2% lidocaine 1:100,000 epinephrine, Outcome 5 Duration of anaesthesia (simulated scenario testing of soft tissues).
We downgraded the outcome three levels from high to very low quality because of study limitations (all three studies reported unclear methods of randomization sequence generation and allocation concealment and had high risk of attrition bias), indirectness (soft tissue anaesthesia is a poor indicator of duration of clinical anaesthesia), and inconsistency (with substantial unexplained heterogeneity).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
0.5% bupivacaine, 1:200,000 epinephrine versus 4% articaine, 1:200,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
No data from the included studies were available for meta‐analysis.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 5.2). Pooled studies included 69 participants (138 episodes of dental anaesthesia) in total (Gregorio 2008; Trullenque‐Eriksson 2011). Data were included for subjective testing of soft tissues using IANB and additional infiltration. Pooling favoured articaine over bupivacaine (MD ‐0.85 minutes, 95% CI ‐1.26 to ‐0.44 minutes), with evidence of no heterogeneity between studies (P = 0.98, I² = 0%).
5.2. Analysis.
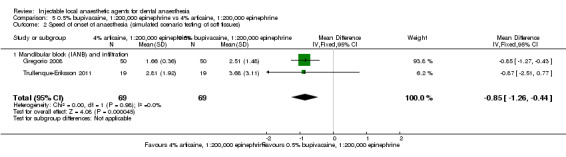
Comparison 5 0.5% bupivacaine, 1:200,000 epinephrine vs 4% articaine, 1:200,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of soft tissues).
We performed a sensitivity analysis (Table 17) that excluded Trullenque‐Eriksson 2011, which had high risk of selection, performance, and attrition bias; this resulted in the cross‐over study Gregorio 2008 becoming an orphan study (MD ‐0.85 minutes, 95% CI ‐1.27 to ‐0.43 minutes).
We downgraded the outcome three levels from high to very low quality. There were study limitations, as the included trials had unclear ‐ in Gregorio 2008 ‐ or high risk ‐ in Trullenque‐Eriksson 2011 ‐ of bias related to randomization sequence generation and allocation concealment. In addition, one study had high risk of bias, with blinding of participants and personnel and incomplete outcome data (high attrition rate of 46%) (Trullenque‐Eriksson 2011). Imprecision (sample size of 69 participants/138 episodes of anaesthesia) and indirectness (soft tissue anaesthesia is a poor indicator of onset of clinical anaesthesia) were also present.
We pooled the results of two cross‐over studies measuring duration of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 5.3). The two pooled studies included 39 participants (78 episodes of dental anaesthesia) in total (Trullenque‐Eriksson 2011; Vilchez‐Perez 2012). Data were included for maxillary buccal infiltration (Vilchez‐Perez 2012), as well as IANB (Trullenque‐Eriksson 2011). Pooling favoured bupivacaine over articaine (MD ‐172.61 minutes, 95% CI ‐239.69 to ‐105.53 minutes), with no heterogeneity between studies (P = 1.00, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 1.0, I² = 0%).
5.3. Analysis.
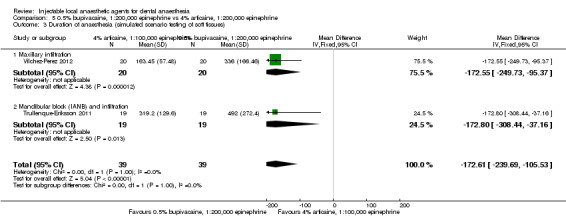
Comparison 5 0.5% bupivacaine, 1:200,000 epinephrine vs 4% articaine, 1:200,000 epinephrine, Outcome 3 Duration of anaesthesia (simulated scenario testing of soft tissues).
We performed a sensitivity analysis (Table 17) that excluded Trullenque‐Eriksson 2011, which had high risk of selection, performance, and attrition bias; this resulted in the cross‐over study Vilchez‐Perez 2012, becoming an orphan study (MD ‐172.55 minutes, 95% CI ‐249.73 to ‐95.37 minutes).
We downgraded the outcome three levels from high to very low quality. There were study limitations, as the included trials had unclear ‐ Vilchez‐Perez 2012 ‐ or high risk ‐ Trullenque‐Eriksson 2011 ‐ of bias related to randomization sequence generation. In addition, one study had high risk of bias related to allocation concealment, blinding of participants and personnel, and incomplete outcome data (high attrition rate of 46%) (Trullenque‐Eriksson 2011). Imprecision (sample size of 39 participants/78 episodes of anaesthesia) and indirectness (soft tissue anaesthesia is a poor indicator of duration of clinical anaesthesia) were present.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
0.5% bupivacaine, 1:200,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of three cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 6.2). The three pooled studies included 90 participants (180 episodes of dental anaesthesia) in total (Abdulwahab 2009; Fernandez 2005; Gross 2007). Data were included for maxillary and mandibular first molars using maxillary buccal infiltration (Gross 2007), mandibular buccal infiltration (Abdulwahab 2009), and IANB (Fernandez 2005). Pooling suggested no evidence of a difference between lidocaine and bupivacaine (RR 0.80, 95% CI 0.62 to 1.05), with no heterogeneity between studies (P = 0.92, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.92, I² = 0%).
6.2. Analysis.
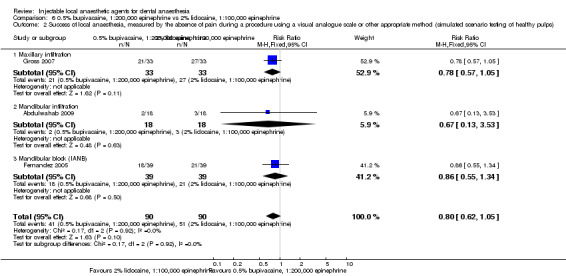
Comparison 6 0.5% bupivacaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (90 participants/180 episodes of anaesthesia and 92 events, and 95% CI includes no effect and an appreciable benefit for lidocaine) and indirectness (success defined in one study ‐ Abdulwahab 2009 ‐ as only one negative response and in another study ‐ Gross 2007 ‐ as only two negative responses to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 6.3). The two pooled studies included 63 participants (116 episodes of dental anaesthesia) in total (Fernandez 2005; Gross 2007). Data were included for first molars using maxillary buccal infiltration (Gross 2007), as well as IANB (Fernandez 2005). Pooling favoured 2% lidocaine, 1:100,000 epinephrine over 0.5% bupivacaine, 1:200,000 epinephrine (MD 3.32 minutes, 95% CI 0.27 to 6.37 minutes), with no heterogeneity between studies (P = 0.97, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.97, I² = 0%).
6.3. Analysis.
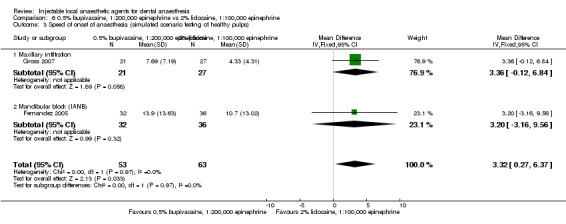
Comparison 6 0.5% bupivacaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 3 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (sample size of 63 participants/116 episodes of anaesthesia) and indirectness (pulp testing is repeated at intervals that are large compared with onset times measured, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
We pooled the results of three studies measuring the speed of onset of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 6.4). The pooled, parallel study Moore 1983 and the cross‐over studies Fernandez 2005 and Laskin 1977 included 79 participants (126 episodes of dental anaesthesia) in total. The infiltrations used were assumed to be pooled from both jaws in the parallel study, and IANBs were used in the cross‐over studies, with ‐ Laskin 1977 ‐ and without ‐ Fernandez 2005 ‐ an additional buccal infiltration. Testing was done by using subjective self‐reporting of onset in all three studies. Pooling suggested no evidence of a difference between lidocaine and bupivacaine (MD 0.02 minutes, 95% CI ‐1.07 to 1.10 minutes), with evidence of substantial heterogeneity between studies (P = 0.06, I² = 64%). The test for subgroup differences revealed moderate heterogeneity (P = 0.06, I² = 64%).
6.4. Analysis.
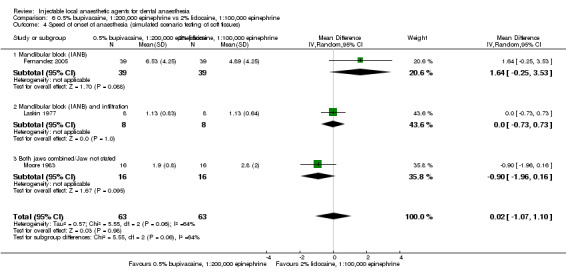
Comparison 6 0.5% bupivacaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 4 Speed of onset of anaesthesia (simulated scenario testing of soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded the cross‐over studies without paired data (Fernandez 2005; Laskin 1977), which meant that the parallel study Moore 1983 became an orphan study (MD ‐0.90 minutes, 95% CI ‐1.96 to 0.16 minutes).
We downgraded the outcome three levels from high to very low quality because of study limitations, with one trial ‐ Laskin 1977 ‐ reporting unclear methods of randomization sequence generation, imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations, sample size of 79 participants/126 episodes of anaesthesia), indirectness (soft tissue anaesthesia is a poor indicator of onset of clinical anaesthesia), and inconsistency (substantial heterogeneity).
We pooled the results of six studies measuring the duration of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 6.5). The two pooled, parallel studies (Moore 1983; Nespeca 1976), along with four cross‐over studies (Fernandez 2005; Gross 2007; Laskin 1977; Linden 1986), included 232 participants (332 episodes of dental anaesthesia) in total. Testing was done by using subjective self‐reporting of duration in all six studies. Data were included for maxillary (Gross 2007; Moore 1983), mandibular (Laskin 1977), and buccal infiltrations, as well as for IANBs ‐ Fernandez 2005 ‐ and infiltrations that were assumed to be pooled from both jaws (Linden 1986; Nespeca 1976). Pooling favoured bupivacaine over lidocaine (MD 222.88 minutes, 95% CI 135.99 to 309.76 minutes), with evidence of considerable heterogeneity between studies (P < 0.00001, I² = 92%). Bupivacaine was also favoured over lidocaine when the combined mandibular and maxillary infiltration data were pooled (MD 224.26 minutes, 95% CI 47.01 to 401.50 minutes), with considerable heterogeneity (P = 0.01, I² = 84%). Pooling just the maxillary infiltration data suggested no evidence of a difference between lidocaine and bupivacaine (MD 109.52 minutes, 95% CI ‐39.40 to 258.44 minutes), with substantial heterogeneity (P = 0.03, I² = 78%). The test for subgroup differences revealed evidence of considerable heterogeneity (P = 0.03, I² = 62%).
6.5. Analysis.
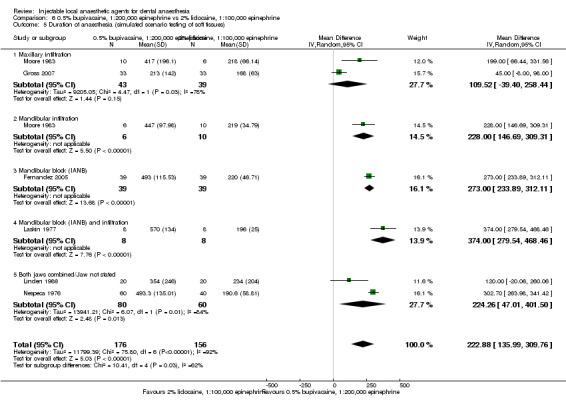
Comparison 6 0.5% bupivacaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 5 Duration of anaesthesia (simulated scenario testing of soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded the four cross‐over studies without paired data (Fernandez 2005; Gross 2007; Laskin 1977; Linden 1986), which left two parallel studies (Moore 1983; Nespeca 1976). Pooling favoured bupivacaine over lidocaine (MD 261.07 minutes, 95% CI 195.96 to 326.18 minutes; P = 0.12, I² = 53%).
We downgraded the outcome three levels from high to very low quality because of study limitations, including reporting unclear methods of randomization sequence generation (Laskin 1977; Nespeca 1976), allocation concealment, and unclear methods of blinding of participants, personnel, and outcome assessors (Nespeca 1976). Imprecision (sample size of 232 participants/332 episodes of anaesthesia), indirectness (soft tissue anaesthesia is a poor indicator of duration of clinical anaesthesia), and inconsistency (not all confidence intervals overlap, substantial heterogeneity, and wide variation of point estimates) were also present.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experience: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% articaine, 1:100,000 epinephrine versus 2% mepivacaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by using self‐reported patient pain or anaesthesia
We pooled the results of two studies measuring the success of local anaesthesia of soft tissues, self‐reported by participants (Analysis 7.2). The pooled, cross‐over study ‐ Bortoluzzi 2009 ‐ and the parallel study ‐ Allegretti 2016 ‐ included 68 participants (92 episodes of dental anaesthesia) in total. Data for these studies were for anaesthesia of the lower lip using IANB ‐ Allegretti 2016 ‐ or buccal infiltration ‐ Bortoluzzi 2009. Pooling suggested no evidence of a difference between formulations (RR 1.07, 95% CI 0.73 to 1.59), with substantial heterogeneity between studies (P = 0.06, I² = 72%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.38, I² = 0%).
7.2. Analysis.
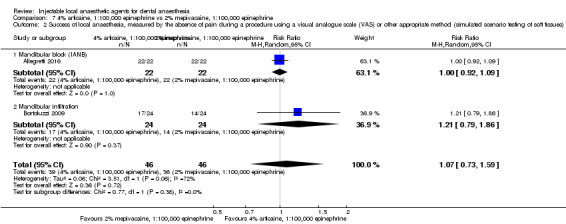
Comparison 7 4% articaine, 1:100,000 epinephrine vs 2% mepivacaine, 1:100,000 epinephrine, Outcome 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of soft tissues).
We conducted a sensitivity analysis (Table 17) that excluded the cross‐over study Bortoluzzi 2009, whose data were not paired, which resulted in Allegretti 2016 becoming an orphan study (RR 1.00, 95% CI 0.92 to 1.09).
We downgraded the outcome three levels from high to very low quality owing to imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations, sample size of 68 participants/92 episodes of anaesthesia and 75 events), indirectness (soft tissue anaesthesia is a poor indicator of pulp and hard tissue anaesthesia), and inconsistency (substantial heterogeneity).
Primary outcome 2: speed of onset and duration of anaesthesia
No data from the included studies were available.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
2% lidocaine, 1:100,000 epinephrine versus 2% mepivacaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
No data from the included studies were available for meta‐analysis.
Primary outcome 2: speed of onset and duration of anaesthesia
No data from the included studies were available.
Primary outcome 3: adverse effects: local and systemic
No studies were used in meta‐analyses. We have summarized orphan study data in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
2% lidocaine, 1:50,000 epinephrine versus 2% lidocaine, 1:80,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of mandibular teeth with healthy pulps, tested using an electric pulp tester (Analysis 9.1). The two pooled studies included 60 participants (120 episodes of dental anaesthesia) in total (Dagher 1997; Yared 1997). Data were included for first molars using mandibular buccal infiltration (Dagher 1997), as well as IANB (Yared 1997). Pooling suggested no evidence of a difference between formulations of lidocaine (RR 0.81, 95% CI 0.65 to 1.01), with evidence of no heterogeneity between studies (P = 0.86, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.88, I² = 0%).
9.1. Analysis.
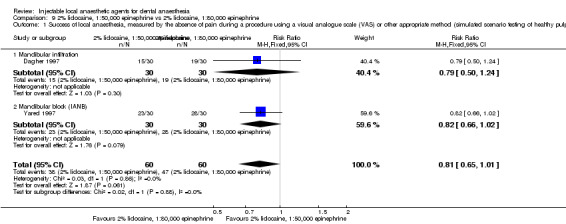
Comparison 9 2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:80,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations, including one trial (Yared 1997), which reported unclear methods of randomization sequence generation, and another trial (Dagher 1997), which reported unclear methods of blinding of outcome assessors; both described unclear methods of allocation concealment. Imprecision (95% confidence interval includes no effect and an appreciable benefit for 2% lidocaine, 1:80,000 epinephrine, sample size of 60 participants/120 episodes of anaesthesia/85 events) and indirectness (clinical anaesthesia may be present at less than maximum pulp tester readings) were also present.
Primary outcome 2: speed of onset and duration of anaesthesia
No data from the included studies were available for meta‐analysis.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
2% lidocaine, 1:50,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of seven cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 10.1). The seven pooled studies included 210 participants (420 episodes of dental anaesthesia) in total (Berberich 2009; Dagher 1997; Knoll‐Kohler 1992a; Mason 2009; Wali 2010; Yared 1997; Yonchak 2001). Data were included for first molars using mandibular buccal infiltration (Dagher 1997), IANB (Wali 2010; Yared 1997), and maxillary buccal infiltration (Mason 2009). Data were also included for central incisors using maxillary labial infiltration (Knoll‐Kohler 1992a), lateral incisors using mandibular labial infiltration (Yonchak 2001), and canine teeth using infraorbital block (Berberich 2009). Pooling suggested no evidence of a difference between formulations of lidocaine (RR 0.99, 95% CI 0.88 to 1.12), with evidence of no heterogeneity between studies (P = 0.90, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.63, I² = 0%).
10.1. Analysis.
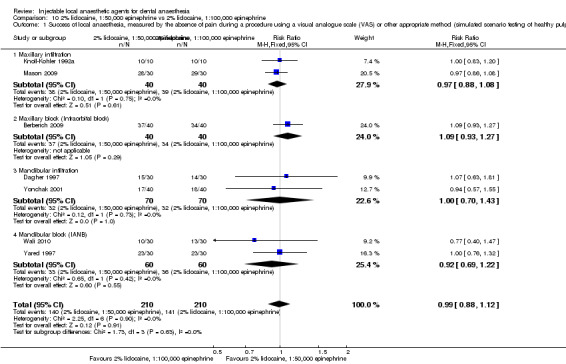
Comparison 10 2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
Pooling of just the two maxillary buccal infiltration studies suggested no evidence of a difference between solutions (RR 0.97, 95% CI 0.88 to 1.08), with no heterogeneity between studies (P = 0.75, I² = 0%). Pooling of just the two mandibular buccal infiltration studies suggested no evidence of a difference between solutions (RR 1.00, 95% CI 0.70 to 1.43), with no heterogeneity between studies (P = 0.73, I² = 0%). Pooling of just the two IANB studies suggested no evidence of a difference between solutions (RR 0.92, 95% CI 0.69 to 1.22), with evidence of no heterogeneity (P = 0.42, I² = 0%).
We downgraded the outcome three levels from high to very low quality owing to study limitations, including two trials reporting unclear methods of randomization sequence generation (Knoll‐Kohler 1992a; Yared 1997), two trials reporting unclear methods of allocation concealment (Dagher 1997; Yared 1997), and one trial having unclear risk of bias for outcome assessment (Dagher 1997). Imprecision (sample size of 210 participants/420 episodes of anaesthesia and 282 events) was present. Indirectness (success defined in two studies ‐ Berberich 2009 and Mason 2009 ‐ as only two negative responses, and in one study ‐ Knoll‐Kohler 1992a ‐ as only one negative response to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings) was also present.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of four cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 10.2). The four pooled studies included 92 participants (184 episodes of dental anaesthesia) in total (Berberich 2009; Knoll‐Kohler 1992a; Mason 2009; Wali 2010). Data were included for lateral incisors and first molars using maxillary buccal infiltration (Knoll‐Kohler 1992a; Mason 2009), canines using infraorbital nerve block (Berberich 2009), and first molars using IANB (Wali 2010). Pooling suggested no evidence of a difference between formulations (MD ‐0.44 minutes, 95% CI ‐1.66 to 0.79 minutes), with evidence of no heterogeneity between studies (P = 0.90, I² = 0%). Pooling of just maxillary infiltration data also suggested no evidence of a difference between formulations (MD ‐0.75 minutes, 95% CI ‐3.04 to 1.54 minutes), with no heterogeneity (P = 0.91, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.75, I² = 0%).
10.2. Analysis.
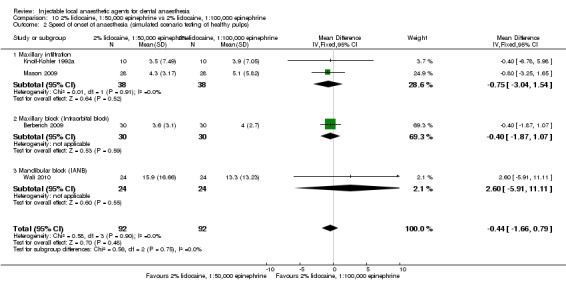
Comparison 10 2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to study limitations (one study ‐ Knoll‐Kohler 1992a ‐ reported an unclear method of randomization of sequence generation), imprecision (95% CI includes no effect and an appreciable benefit for both solutions, sample size of 92 participants/184 episodes of anaesthesia), and indirectness (pulp testing is repeated at intervals that are large compared with the onset times measured, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
2% lidocaine, 1:80,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 11.1). The two pooled studies included 60 participants (120 episodes of dental anaesthesia) in total (Dagher 1997; Yared 1997). Data were included for first molars using mandibular buccal infiltration (Dagher 1997), as well as IANB (Yared 1997). Pooling favoured 2% lidocaine with 1:80,000 epinephrine over 2% lidocaine with 1:100,000 epinephrine (RR 1.27, 95% CI 1.01 to 1.59), with no heterogeneity between studies (P = 0.64, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.68, I² = 0%).
11.1. Analysis.
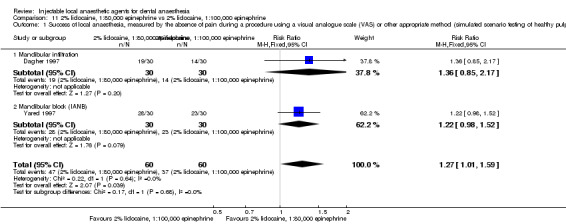
Comparison 11 2% lidocaine, 1:80,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome by three levels from high to very low quality because of study limitations, including one trial that reported unclear methods of randomization sequence generation (Yared 1997), one trial reporting unclear methods of blinding of outcome assessors (Dagher 1997), and both trials describing unclear methods of allocation concealment. Imprecision was present (sample size of 60 participants/120 episodes of anaesthesia and 84 events) as was indirectness (clinical anaesthesia may be present at less than maximum pulp tester readings).
Primary outcome 2: speed of onset and duration of anaesthesia
No data from the included studies were available for meta‐analysis.
Primary outcome 3: adverse effects: local and systemic
No data from the included studies were available.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
2% lidocaine, 1:200,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of three cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 12.1). The three pooled studies included 70 participants (140 episodes of dental anaesthesia) in total (Caldas 2015; Knoll‐Kohler 1992a; Vreeland 1989). Data were included for lateral incisors and canine teeth using maxillary labial infiltration (Caldas 2015; Knoll‐Kohler 1992a), as well as for first molar teeth using IANB (Vreeland 1989), using different volumes of local anaesthetic (1.8 mL of 2% lidocaine, 1:100,000 epinephrine vs 3.6 mL of 2% lidocaine, 1:200,000 epinephrine). Pooling suggested no evidence of a difference between formulations of lidocaine (RR 0.89, 95% CI 0.63 to 1.26), with evidence of substantial heterogeneity between studies (P = 0.03, I² = 72%). Pooling of just the maxillary infiltration data also suggested no evidence of a difference between formulations (RR 0.80, 95% CI 0.33 to 1.95), with considerable heterogeneity between studies (P = 0.0005, I² = 92%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.65, I² = 0%).
12.1. Analysis.
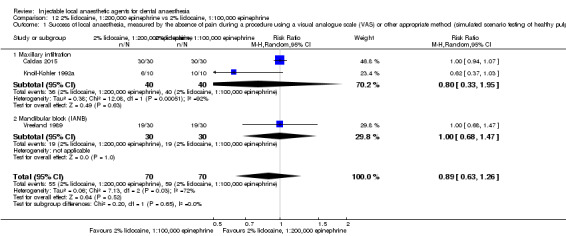
Comparison 12 2% lidocaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations, including reporting unclear methods of randomization sequence generation ‐ Caldas 2015 and Knoll‐Kohler 1992a ‐ and allocation concealment ‐ Caldas 2015. Imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations, sample size of 70 participants/140 episodes of anaesthesia and 114 events) and inconsistency (substantial heterogeneity) were present, as was indirectness (success defined in one study ‐ Knoll‐Kohler 1992a ‐ as only one negative response, and in another study ‐ Caldas 2015 ‐ as two responses, to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 2: speed of onset and duration of anaesthesia
No data from the included studies were suitable for meta‐analysis. We have summarized the data for these outcomes in Table 9Table 10 and Table 11.
Primary outcome 3: adverse effects: local and systemic
No data from the included studies were available.
Secondary outcome 1: participants' experience: these include but are not limited to preference, overall experience
No data from the included studies were available.
3% mepivacaine plain versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of six cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 13.1). The six pooled studies included 208 participants (416 episodes of dental anaesthesia) in total (Abdulwahab 2009; Berberich 2009; Burns 2004; Forloine 2010; Mason 2009; McLean 1993). Data were included for first molars using maxillary buccal infiltration (Mason 2009), mandibular buccal infiltration (Abdulwahab 2009), IANB (McLean 1993), and high‐tuberosity maxillary second division nerve block (Forloine 2010). Data were also included for canine teeth using infraorbital blocks (Berberich 2009), as well as for central incisors using palatal‐anterior superior alveolar injections (Burns 2004). Pooling suggested no evidence of a difference between lidocaine and mepivacaine (RR 0.92, 95% CI 0.83 to 1.02), with evidence of moderate heterogeneity between studies (P = 0.09, I² = 48%). The test for subgroup differences revealed evidence of little heterogeneity (P = 0.2, I² = 32%).
13.1. Analysis.
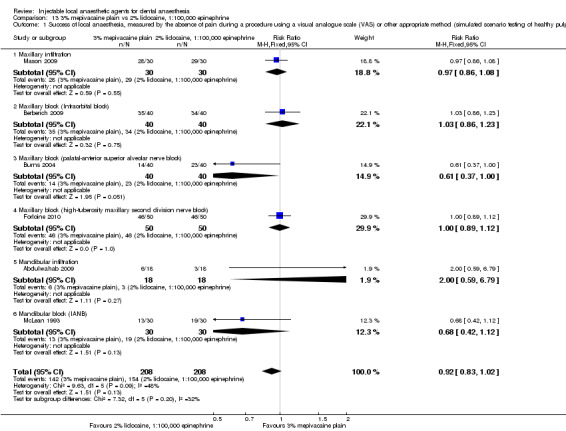
Comparison 13 3% mepivacaine plain vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (sample size of 208 participants/416 episodes of anaesthesia and 296 events) and indirectness (success defined in three studies ‐ Berberich 2009; Burns 2004; Mason 2009 ‐ as only two negative responses, and in one study ‐ Abdulwahab 2009 ‐ as only one negative response to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of three cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 13.2). The three pooled studies included 85 participants (170 episodes of dental anaesthesia) in total (Berberich 2009; Mason 2009; McLean 1993). Data were included for first molars using maxillary buccal infiltration (Mason 2009), canines using infraorbital nerve block (Berberich 2009), and first molars using IANB (McLean 1993). Pooling favoured mepivacaine over lidocaine (MD ‐1.23 minutes, 95% CI ‐2.31 to ‐0.16 minutes), with evidence of no heterogeneity between studies (P = 0.88, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.88, I² = 0%).
13.2. Analysis.
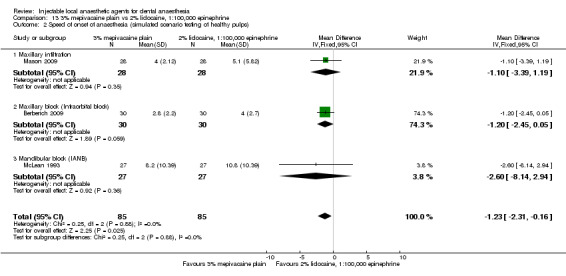
Comparison 13 3% mepivacaine plain vs 2% lidocaine, 1:100,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (sample size of 85 participants/170 episodes of anaesthesia) and indirectness (pulp testing is repeated at intervals that are large compared with onset times measured, and clinical anaesthesia may be present at less than maximum pulp tester readings)
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
3% mepivacaine plain versus 2% lidocaine, 1:50,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of maxillary teeth with healthy pulps, tested with an electric pulp tester (Analysis 14.1). The two pooled studies included 70 participants (140 episodes of dental anaesthesia) in total (Berberich 2009; Mason 2009). Data were included for first molars using maxillary buccal infiltration (Mason 2009), and for canine teeth using infraorbital block (Berberich 2009). Pooling suggested no evidence of a difference between lidocaine and mepivacaine (RR 0.97, 95% CI 0.88 to 1.07), with evidence of no heterogeneity between studies (P = 0.58, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.59, I²= 0%).
14.1. Analysis.
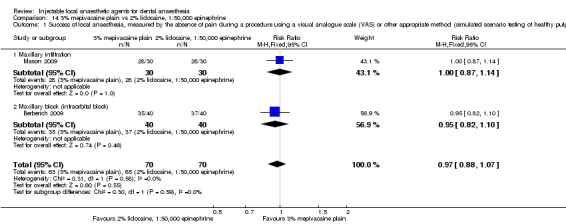
Comparison 14 3% mepivacaine plain vs 2% lidocaine, 1:50,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (sample size of 70 participants/140 episodes of anaesthesia and 128 events) and indirectness (success defined in two studies ‐ Berberich 2009; Mason 2009 ‐ as only two negative responses to maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 14.2). The two pooled studies included 58 participants (116 episodes of dental anaesthesia) in total (Berberich 2009; Mason 2009). Data were included for first molars using maxillary buccal infiltration (Mason 2009), and for canines using infraorbital nerve block (Berberich 2009). Pooling suggested no evidence of a difference between formulations (MD ‐0.56 minutes, 95% CI ‐1.54 to 0.42 minutes), with evidence of no heterogeneity between studies (P = 0.62, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.62, I² = 0%).
14.2. Analysis.
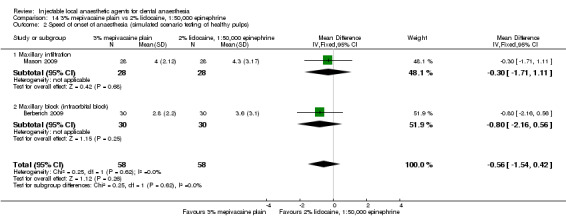
Comparison 14 3% mepivacaine plain vs 2% lidocaine, 1:50,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (95% CI includes no effect and an appreciable benefit for 3% mepivacaine plain, sample size of 58 participants/116 episodes of anaesthesia) and indirectness (pulp testing is repeated at intervals that are large compared with onset times measured, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 3: adverse effects: local and systemic
No data from the included studies were available.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
2% mepivacaine, 1:20,000 levonordefrin versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
No data from the included studies were available for meta‐analysis. We have summarized the data for this outcome in Table 14.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two parallel studies measuring the duration of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 15.1). The two pooled studies included 458 participants (458 episodes of dental anaesthesia) in total (Albertson 1963; Sadove 1962). Types and specific sites of injection were not stated. Pooling suggested no evidence of a difference between lidocaine and mepivacaine (MD 4.43 minutes, 95% CI ‐10.63 to 19.48 minutes), with evidence of no heterogeneity between studies (P = 0.80, I² = 0%).
15.1. Analysis.
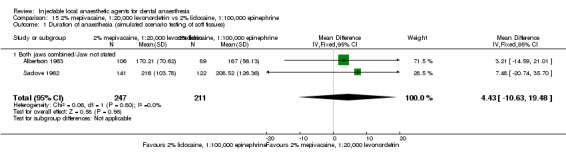
Comparison 15 2% mepivacaine, 1:20,000 levonordefrin vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Duration of anaesthesia (simulated scenario testing of soft tissues).
We downgraded the outcome three levels from high to very low quality because of study limitations (both studies had high risk of attrition bias), imprecision (95% confidence interval includes no effect and an appreciable benefit for both solutions), and indirectness (soft tissue anaesthesia is a poor indicator of duration of clinical anaesthesia).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experience: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% articaine, 1:200,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 16.1). The two pooled studies included 28 participants (56 episodes of dental anaesthesia) in total (Abdulwahab 2009; Ruprecht 1991). Data were included for first molars using mandibular buccal infiltration (Abdulwahab 2009), and for central incisors using maxillary labial infiltration (Ruprecht 1991). Pooling suggested no evidence of a difference between lidocaine and articaine (RR 1.33, 95% CI 0.33 to 5.36), with evidence of substantial heterogeneity between studies (P = 0.02, I²= 81%). The test for subgroup differences revealed evidence of little heterogeneity (P = 0.27, I² = 17%).
16.1. Analysis.
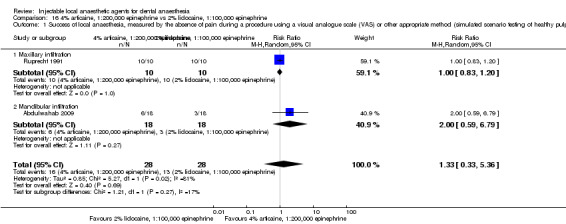
Comparison 16 4% articaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to study limitations, including one trial ‐ Ruprecht 1991 ‐ that reported unclear methods of randomization sequence generation, allocation concealment, and blinding of outcome assessors. Imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations, sample size of 28 participants/56 episodes of anaesthesia and 29 events), inconsistency (substantial heterogeneity), and indirectness (success defined in one study ‐ Abdulwahab 2009 ‐ as only one negative response to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings) were also present.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 16.2). The two pooled studies included 40 participants (80 episodes of dental anaesthesia) in total (Ruprecht 1991; Tortamano 2013). Data were included for central incisors using maxillary labial infiltration (Ruprecht 1991), and mandibular molars using IANB (Tortamano 2013). Pooling suggested no evidence of a difference between lidocaine and articaine (MD 0.19 minutes, 95% CI ‐2.06 to 2.45 minutes), with evidence of substantial heterogeneity between studies (P = 0.02, I² = 80%). The test for subgroup differences revealed substantial heterogeneity (P = 0.02, I² = 80%).
16.2. Analysis.
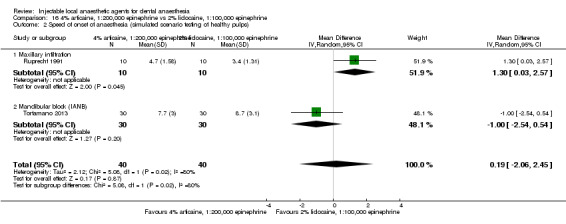
Comparison 16 4% articaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations (unclear risks of selection and detection bias ‐ Ruprecht 1991, imprecision (95% confidence interval includes no effect and an appreciable benefit for both solutions, sample size of 40 participants/80 episodes of anaesthesia), indirectness (pulp testing is repeated at intervals that are large compared with the onset times measured, with clinical anaesthesia possibly present at less than maximum pulp tester readings), and inconsistency (substantial heterogeneity).
We pooled the results of two cross‐over studies measuring the duration of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 16.3). The two pooled studies included 40 participants (80 episodes of dental anaesthesia) in total (Ruprecht 1991; Tortamano 2013). Data were included for central incisors using maxillary labial infiltration (Ruprecht 1991), and for mandibular molars using IANB (Tortamano 2013). Pooling suggested no evidence of a difference between lidocaine and articaine (MD 10.33 minutes, 95% CI ‐22.08 to 42.74 minutes), with evidence of considerable heterogeneity between studies (P = 0.002, I² = 89%). The test for subgroup differences revealed considerable heterogeneity (P = 0.002, I² = 89%).
16.3. Analysis.
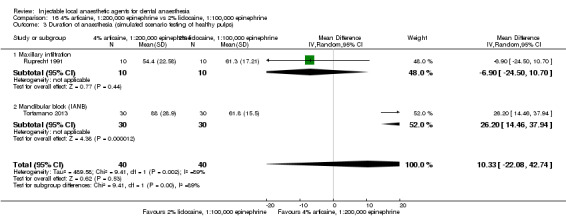
Comparison 16 4% articaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 3 Duration of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations, as one trial had unclear risks of selection, performance, and detection bias (Ruprecht 1991). Imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations, sample size of 40 participants/80 episodes of anaesthesia), inconsistency (considerable heterogeneity), and indirectness (clinical anaesthesia may be present at less than maximum pulp tester readings) were also present.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% articaine, 1:100,000 epinephrine versus 2% lidocaine, 1:80,000 epinephrine
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
No data from the included studies were available for meta‐analysis. We have summarized the data for this outcome in Table 14.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two studies measuring the onset of local anaesthesia of mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 17.1). The cross‐over study ‐ Arrow 2012 ‐ and the parallel study ‐ Naik 2017 ‐ included 116 participants (125 episodes of dental anaesthesia) in total. Data were included for subjective testing of soft tissues using IANB. Pooling favoured articaine over lidocaine (MD ‐0.78 minutes, 95% CI ‐1.04 to ‐0.52 minutes), with evidence of little heterogeneity (P = 0.26, I² = 21%).
17.1. Analysis.
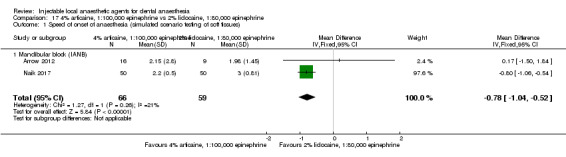
Comparison 17 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:80,000 epinephrine, Outcome 1 Speed of onset of anaesthesia (simulated scenario testing of soft tissues).
We downgraded the outcome three levels from high to very low quality owing to study limitations (unclear risk of performance and high risk of detection bias (Naik 2017), and high risk of attrition bias (Arrow 2012)), imprecision (sample size of 116 participants/125 episodes of anaesthesia), and indirectness (soft tissue anaesthesia is a poor indicator of onset of clinical anaesthesia).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% articaine, 1:200,000 epinephrine versus 4% prilocaine, 1:200,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of three cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 18.1). The three pooled studies included 97 participants (194 episodes of dental anaesthesia) in total (Abdulwahab 2009; Haas 1990; Haas 1991). Data were included for mandibular first molars (Abdulwahab 2009), and for mandibular and maxillary second molars (Haas 1991), using buccal infiltration, and for mandibular and maxillary canine teeth using buccal infiltration (Haas 1990). Pooling suggested no evidence of a difference between prilocaine and articaine (RR 1.15, 95% CI 0.93 to 1.41), with no heterogeneity between studies (P = 0.80, I² = 0%). No evidence of a difference was seen between formulations for maxillary infiltration (RR 1.03, 95% CI 0.83 to 1.28, P = 0.78, I² = 0%) and mandibular infiltration (RR 1.29, 95% CI 0.89 to 1.87, P = 0.93, I² = 0%). The test for subgroup differences also revealed little heterogeneity (P = 0.31, I² = 5%).
18.1. Analysis.
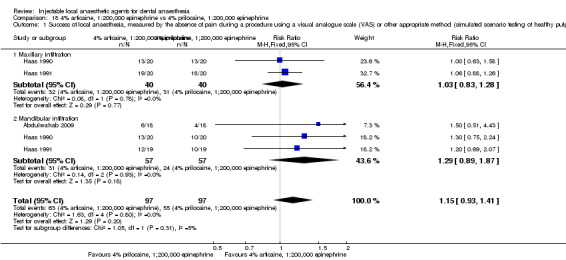
Comparison 18 4% articaine, 1:200,000 epinephrine vs 4% prilocaine, 1:200,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations, including unclear randomization sequence generation, allocation concealment, and blinding of outcome assessors (Haas 1990; Haas 1991). Indirectness (success defined in three studies ‐ Abdulwahab 2009; Haas 1990; Haas 1991 ‐ as only one negative response to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings) and imprecision (95% CI includes no effect and suggests an appreciable benefit for 4% articaine, 1:200,000 epinephrine, sample size of 97 participants/194 episodes of anaesthesia/118 events) were also present.
Primary outcome 2: speed of onset and duration of anaesthesia
No data from the included studies were available for meta‐analysis. We summarized the data for these outcomes in Table 9 and Table 10
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% prilocaine, 1:200,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 19.1). The two pooled studies included 48 participants (96 episodes of dental anaesthesia) in total (Abdulwahab 2009; Katz 2010). Data were included for first molars using maxillary ‐ Katz 2010 ‐ and mandibular ‐ Abdulwahab 2009 ‐ buccal infiltration. Pooling suggested no evidence of a difference between lidocaine and prilocaine (RR 1.14, 95% CI 0.91 to 1.43), with no heterogeneity between studies (P = 0.76, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.80, I² = 0%).
19.1. Analysis.
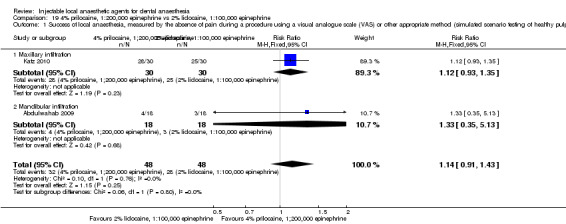
Comparison 19 4% prilocaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (95% CI includes no effect and suggests an appreciable benefit for 4% prilocaine, 1:200,000 epinephrine, sample size of 49 participants/96 episodes of anaesthesia/60 events) and indirectness (success defined in both studies as only two negative responses to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 19.2). The two pooled studies included 39 participants (76 episodes of dental anaesthesia) in total (Hinkley 1991; Katz 2010). Data were included for first molars using maxillary buccal infiltration (Katz 2010), as well as for IANB (Hinkley 1991). Pooling suggested no evidence of a difference between lidocaine and prilocaine (MD ‐1.19 minutes, 95% CI ‐3.08 to 0.70 minutes), with no heterogeneity between studies (P = 0.37, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.37, I² = 0%).
19.2. Analysis.
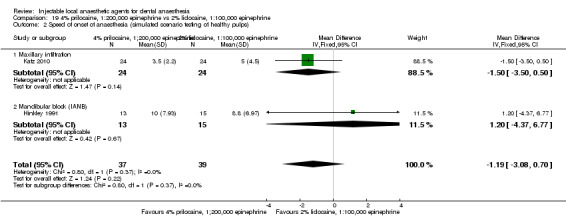
Comparison 19 4% prilocaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality because of imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations, sample size of 39 participants/76 episodes of anaesthesia) and indirectness (pulp testing is repeated at intervals that are large compared with onset times measured, with clinical anaesthesia possibly present at less than maximum pulp tester readings).
We pooled the results of two studies measuring the speed of onset of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 19.3). Pooled results of a parallel study ‐ Chilton 1971 ‐ and a cross‐over study ‐ Hinkley 1991 ‐ included 421 participants (449 episodes of dental anaesthesia) in total. Infiltrations were assumed to be pooled from both jaws in the parallel study, with IANB used in the cross‐over study. Testing was done by using a gingival stick or subjective testing, depending on which occurred first, in the study by Hinkley 1991. It was assumed that subjective anaesthesia would occur before anaesthesia using gingival sticks. Subjective testing was used in the other study (Chilton 1971). Pooling suggested no evidence of a difference between lidocaine and prilocaine (MD ‐0.01 minutes, 95% CI ‐0.14 to 0.11 minutes), with evidence of no heterogeneity between studies (P = 0.86, I² = 0%). Pooling of just IANB data also suggested no evidence of a difference between lidocaine and prilocaine (MD ‐0.10 minutes, 95% CI ‐0.43 to 0.24 minutes), with no heterogeneity between studies (P = 0.83, I² = 0%). The test for subgroup differences revealed no heterogeneity (P = 0.61, I² = 0%).
19.3. Analysis.
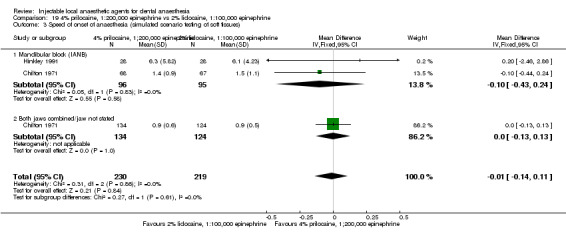
Comparison 19 4% prilocaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 3 Speed of onset of anaesthesia (simulated scenario testing of soft tissues).
We carried out a sensitivity analysis (Table 17) that excluded the cross‐over study Hinkley 1991, whose data were not paired, which resulted in Chilton 1971 becoming an orphan study (MD ‐0.01, 95% CI ‐0.14 to 0.11).
We downgraded the outcome two levels from high to low quality because of study limitations, as one trial ‐ Chilton 1971 ‐ had unclear risks of selection bias, and because of indirectness (soft tissue anaesthesia is a poor indicator of onset of clinical anaesthesia).
We pooled the results of two studies measuring the duration of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 19.4). The two pooled, parallel studies included 533 participants (533 episodes of dental anaesthesia) in total (Chilton 1971; Epstein 1969). Data were included for subjective soft tissue anaesthesia using maxillary buccal infiltration (Epstein 1969), IANB (Chilton 1971; Epstein 1969), and pooled infiltrations from either jaw (Chilton 1971). Pooling suggested no evidence of a difference between lidocaine and prilocaine (MD ‐11.80 minutes, 95% CI ‐27.76 to 4.16 minutes), with evidence of substantial heterogeneity between studies (P = 0.05, I² = 61%). Pooling of just IANB data also suggests no evidence of a difference between lidocaine and prilocaine (MD 2.19 minutes, 95% CI ‐12.26 to 16.65 minutes), with no heterogeneity between studies (P = 0.49, I² = 0%). The test for subgroup differences revealed substantial heterogeneity (P = 0.03, I² = 73%).
19.4. Analysis.
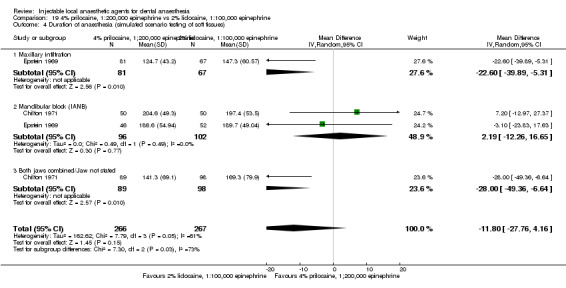
Comparison 19 4% prilocaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine, Outcome 4 Duration of anaesthesia (simulated scenario testing of soft tissues).
We downgraded the outcome three levels from high to very low quality because of study limitations, with both trials having unclear methods of randomization sequence generation and allocation concealment, and high risk of attrition bias. Imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations), indirectness (soft tissue anaesthesia is a poor indicator of duration of clinical anaesthesia), and inconsistency (substantial heterogeneity and wide variation of point estimates) were also present.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis were completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% articaine plain versus 4% articaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 20.1). The two pooled studies included 134 participants (268 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006). Data were included for maxillary central incisor teeth ‐ Kammerer 2014 ‐ and for first premolars ‐ Moore 2006 ‐ using maxillary buccal infiltration, and for mandibular canine teeth using IANB (Moore 2006). Pooling favoured 4% articaine, 1:100,000 epinephrine over 4% articaine plain (RR 0.61, 95% CI 0.38 to 0.97), with substantial heterogeneity between studies (P = 0.03, I² = 71%). Pooling of just the maxillary infiltration data suggested no evidence of a difference between formulations of articaine (RR 0.64, 95% CI 0.34 to 1.19, P = 0.08, I² = 68%). The test for subgroup differences revealed no heterogeneity (P = 0.65, I² = 0%).
20.1. Analysis.
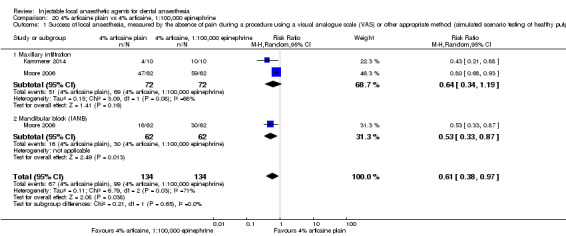
Comparison 20 4% articaine plain vs 4% articaine, 1:100,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to imprecision (sample size of 134 participants/268 episodes of anaesthesia and 166 events) and indirectness (success defined in one study ‐ Kammerer 2014 ‐ as only one negative response, and in another study ‐ Moore 2006 ‐ as only three negative responses to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings). Inconsistency (substantial heterogeneity) was also present. Study limitations were evident with one study (Moore 2006), which had unclear risk of selection bias.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 20.2). The two pooled studies included 100 participants (167 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006). Data were included for central incisors ‐ Kammerer 2014 ‐ and for first premolars ‐ Moore 2006 ‐ using maxillary buccal infiltration, and for canines using IANB (Moore 2006). Pooling suggested no evidence of a difference between formulations of articaine (MD 0.13 minutes, 95% CI ‐0.54 to 0.80 minutes), with evidence of no heterogeneity between studies (P = 0.52, I² = 0%). Pooling of just maxillary infiltration data suggested no evidence of a difference between formulations of articaine (MD 0.14, 95% CI ‐0.61 to 0.88, P = 0.26, I² = 22%). The test for subgroup differences revealed no heterogeneity (P = 0.97, I² = 0%).
20.2. Analysis.
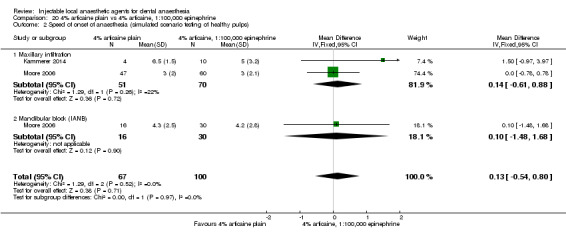
Comparison 20 4% articaine plain vs 4% articaine, 1:100,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations, with both trials having high risk of attrition bias and one ‐ Moore 2006 ‐ having unclear risk of selection bias. Imprecision was present (95% confidence interval includes no effect and an appreciable benefit for both solutions, sample size of 100 participants/167 episodes of anaesthesia), as was indirectness (pulp testing is repeated at intervals that are large compared with onset times measured, and clinical anaesthesia may be present at less than maximum pulp tester readings).
We pooled the results of two cross‐over studies measuring the duration of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 20.3). The two pooled studies included 100 participants (167 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006). Data were included for central incisors ‐ Kammerer 2014 ‐ and for first premolars ‐ Moore 2006 ‐ using maxillary buccal infiltration, and for canines using IANB (Moore 2006). Pooling favoured 4% articaine, 1:100,000 epinephrine over 4% articaine plain (MD ‐37.08 minutes, 95% CI ‐60.95 to ‐13.21 minutes), with substantial heterogeneity between studies (P = 0.004, I² = 82%). Pooling of just maxillary infiltration data also favoured 4% articaine with 1:100,000 epinephrine over 4% articaine plain (MD ‐45.85 minutes, 95% CI ‐76.25 to ‐15.45 minutes, P = 0.003, I² = 89%). The test for subgroup differences revealed moderate heterogeneity (P = 0.12, I² = 58%).
20.3. Analysis.
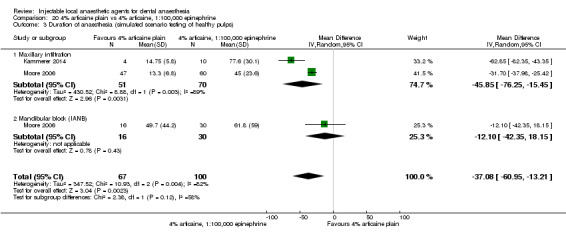
Comparison 20 4% articaine plain vs 4% articaine, 1:100,000 epinephrine, Outcome 3 Duration of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations, with both trials having high risk of attrition bias and one study having unclear risk of selection bias, imprecision (sample size of 100 participants/167 episodes of anaesthesia), inconsistency (substantial heterogeneity), and indirectness (clinical anaesthesia may be present at less than maximum pulp tester readings) (Moore 2006).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% articaine plain versus 4% articaine, 1:200,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 21.1). The two pooled studies included 134 participants (268 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006). Data were included for maxillary first premolars (Moore 2006), and for central incisors (Kammerer 2014), using maxillary buccal infiltration, and for mandibular canine teeth using IANB (Moore 2006). Pooling suggested no evidence of a difference between formulations of articaine (RR 0.58, 95% CI 0.33 to 1.01), with substantial heterogeneity between studies (P = 0.006, I² = 80%). Pooling of just maxillary study data also suggested no evidence of a difference between formulations of articaine (RR 0.64, 95% CI 0.34 to 1.22, P = 0.07, I² = 69%). The test for subgroup differences revealed no heterogeneity (P = 0.44, I² = 0%).
21.1. Analysis.
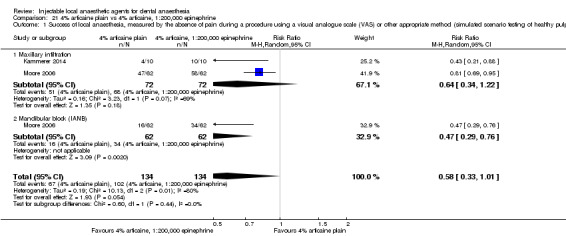
Comparison 21 4% articaine plain vs 4% articaine, 1:200,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality owing to imprecision (95% confidence interval includes no effect and an appreciable benefit for 4% articaine, 1:200,000 epinephrine, sample of 134 participants/268 episodes of anaesthesia and 169 events) and indirectness (success defined in one study ‐ Kammerer 2014 ‐ as only one negative response, and in another study ‐ Moore 2006 ‐ as only three negative responses to the maximum electric pulp tester output not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings). Inconsistency (substantial, unexplained heterogeneity) and study limitations (one study ‐ Moore 2006 ‐ had unclear risk of selection bias) were also present.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two cross‐over studies measuring the onset of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 21.2). The two pooled studies included 102 participants (169 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006). Data were included for central incisors (Kammerer 2014), and for first premolars (Moore 2006), using maxillary buccal infiltration, and for canines using IANB (Moore 2006). Pooling suggested no evidence of a difference between formulations of articaine (MD 0.03 minutes, 95% CI ‐0.66 to 0.71 minutes), with evidence of little heterogeneity between studies (P = 0.23, I² = 32%). Pooling of just maxillary infiltration data also suggested no evidence of a difference between formulations (MD 0.14 minutes, 95% CI ‐0.63 to 0.91 minutes, P = 0.11, I² = 61%). The test for subgroup differences revealed no heterogeneity (P = 0.53, I² = 0%).
21.2. Analysis.
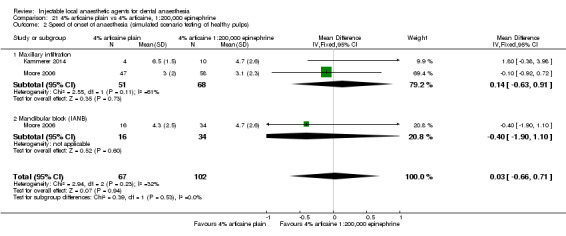
Comparison 21 4% articaine plain vs 4% articaine, 1:200,000 epinephrine, Outcome 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations (both trials had high risk of attrition bias, and one ‐ Moore 2006 ‐ had unclear risk of selection bias) and imprecision (95% confidence interval includes no effect and an appreciable benefit for both formulations, sample size of 102 participants/169 episodes of anaesthesia). Indirectness (pulp testing is repeated at intervals that are large compared with the onset times measured, and clinical anaesthesia may be present at less than maximum pulp tester readings) was also present.
We pooled the results of two cross‐over studies measuring the duration of local anaesthesia of maxillary and mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 21.3). The two pooled studies included 102 participants (169 episodes of dental anaesthesia) in total (Kammerer 2014; Moore 2006). Data were included for central incisors (Kammerer 2014), and for first premolars (Moore 2006), using maxillary buccal infiltration, and for canines using IANB (Moore 2006). Pooling favoured 4% articaine, 1:200,000 epinephrine over 4% articaine plain (MD ‐28.36 minutes, 95% CI ‐42.06 to ‐14.65 minutes), with evidence of substantial heterogeneity between studies (P = 0.04, I² = 70%). Pooling of just maxillary infiltration data also favoured 4% articaine, 1:200,000 epinephrine over 4% articaine plain (MD ‐32.88 minutes, 95% CI ‐44.12 to ‐21.65 minutes, P = 0.09, I² = 65%).The test for subgroup differences revealed substantial heterogeneity (P = 0.05, I² = 75%).
21.3. Analysis.
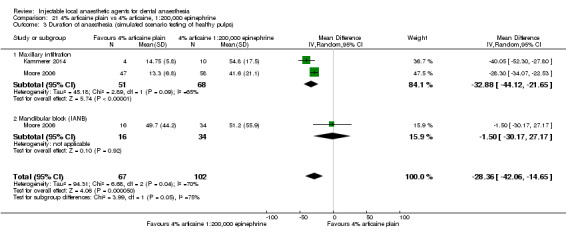
Comparison 21 4% articaine plain vs 4% articaine, 1:200,000 epinephrine, Outcome 3 Duration of anaesthesia (simulated scenario testing of healthy pulps).
We downgraded the outcome three levels from high to very low quality because of study limitations (both trials had high risk of attrition bias, and one study ‐ Moore 2006 ‐ had unclear risk of selection bias), imprecision (sample size of 102 participants/169 episodes of anaesthesia), inconsistency (substantial heterogeneity), and indirectness (clinical anaesthesia may be present at less than maximum pulp tester readings).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% prilocaine, 1:200,000 epinephrine versus 4% prilocaine plain
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
No data from the included studies were available for meta‐analysis. We have summarized the data for this outcome in Table 13.
Primary outcome 2: speed of onset and duration of anaesthesia
We pooled the results of two parallel studies measuring the duration of local anaesthesia of maxillary and mandibular soft tissues, using the simulated scenario testing of soft tissues (Analysis 22.1). The two pooled studies included 506 participants (506 episodes of dental anaesthesia) in total. Testing was done by using subjective self‐reporting for maxillary infiltration (Epstein 1969), IANB (Chilton 1971; Epstein 1969), or buccal infiltration data combined from both jaws (Chilton 1971). Pooling favoured 4% prilocaine, 1:200,000 epinephrine over 4% prilocaine plain (MD 18.78 minutes, 95% CI 9.02 to 28.54 minutes), with evidence of no heterogeneity between studies (P = 0.62, I² = 0%). The test for subgroup differences revealed evidence of no heterogeneity (P = 0.70, I² = 0%).
22.1. Analysis.
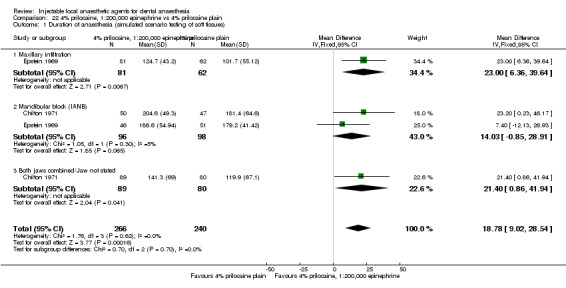
Comparison 22 4% prilocaine, 1:200,000 epinephrine vs 4% prilocaine plain, Outcome 1 Duration of anaesthesia (simulated scenario testing of soft tissues).
We downgraded the outcome two levels from high to low quality owing to study limitations (both studies had unclear methods of randomization sequence generation and allocation concealment and high risk of attrition bias) and indirectness (soft tissue anaesthesia is a poor indicator of duration of clinical anaesthesia).
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
4% prilocaine, 1:200,000 epinephrine versus 4% articaine, 1:100,000 epinephrine
Primary outcome 1: success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester
We pooled the results of two cross‐over studies measuring the success of local anaesthesia of mandibular teeth with healthy pulps, tested with an electric pulp tester (Analysis 23.1). The two pooled studies included 78 participants (156 episodes of dental anaesthesia) in total (Abdulwahab 2009; Nydegger 2014). Data were included for mandibular first molars using buccal infiltration. Pooling favoured 4% articaine, 1:100,000 epinephrine over 4% prilocaine, 1:200,000 epinephrine (RR 1.74, 95% CI 1.16 to 2.60), with evidence of no heterogeneity between studies (P = 0.99, I² = 0%).
23.1. Analysis.
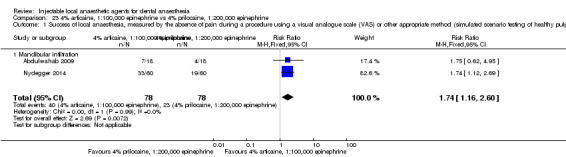
Comparison 23 4% articaine, 1:100,000 epinephrine vs 4% prilocaine, 1:200,000 epinephrine, Outcome 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps).
We downgraded the outcome two levels from high to low quality owing to imprecision (sample size of 78 participants/156 episodes of anaesthesia and 63 events). Indirectness (success defined in one study ‐ Abdulwahab 2009 ‐ as one negative response in 20 minutes, and in another study ‐ Nydegger 2014 ‐ as only two negative responses to maximum electric pulp tester output during the study not sustained over a period typical of a clinical procedure, with clinical anaesthesia possibly present at less than maximum pulp tester readings) was also present.
Primary outcome 2: speed of onset and duration of anaesthesia
No data from the included studies were available for meta‐analysis. We have summarized the data for this outcome in Table 9.
Primary outcome 3: adverse effects: local and systemic
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore no meta‐analysis was completed. We have summarized the data for these outcomes in Table 15.
Secondary outcome 1: participants' experiences: these include but are not limited to preference, overall experience
No data from the included studies were available.
Other comparisons with 100% success in all studies
Primary outcome 1: success of local anaesthesia, measured by the absence of pain during a procedure using a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus.
A number of outcomes measured the success of soft tissue anaesthesia using subjective self‐reporting, when all studies reported 100% success for each formulation of local anaesthetic. These outcomes did not require meta‐analysis to determine that there was no difference in efficacy between them. The outcomes and studies are listed below.
2% lidocaine, 1:100,000 epinephrine versus 2% lidocaine, 1:80,000 epinephrine
Mandibular buccal infiltration (Dagher 1997): 30/30 vs 30/30
IANB (Yared 1997): 30/30 vs 30/30
2% lidocaine, 1:100,000 epinephrine versus 2% lidocaine, 1:50,000 epinephrine
Infraorbital nerve block (Berberich 2009): 40/40 vs 40/40
Mandibular buccal infiltration (Dagher 1997): 30/30 vs 30/30; Yonchak 2001: 40/40 vs 40/40
IANB (Yared 1997): 30/30 vs 30/30; Wali 2010: 30/30 vs 30/30
2% lidocaine, 1:50,000 epinephrine versus 2% lidocaine, 1:80,000 epinephrine
Mandibular buccal infiltration (Dagher 1997): 30/30 vs 30/30
IANB (Yared 1997): 30/30 vs 30/30
2% lidocaine, 1:100,000 epinephrine versus 3% mepivacaine plain
Infraorbital nerve block (Berberich 2009): 40/40 vs 40/40
IANB (Cohen 1993): 27/27 vs 34/34; McLean 1993: 30/30 vs 30/30
2% lidocaine, 1:100,000 epinephrine versus 0.5% bupivacaine, 1:200,000 epinephrine
IANB (Sampaio 2012): 35/35 vs 35/35; Fernandez 2005: 39/39 vs 39/39
2% lidocaine, 1:100,000 epinephrine versus 2% mepivacaine, 1:100,000 epinephrine
IANB (Allegretti 2016): 22/22 vs 22/22; Visconti 2016: 21/21 vs 21/21
Discussion
Summary of main results
The main aim of this systematic review was to evaluate the success, speed of onset, duration, and incidence of systemic and local adverse effects among patients using different local anaesthetic formulations for dental anaesthesia.
We included 123 studies (19,223 participants recruited) in the review, of which we pooled the data from 68 studies (6615 participants) for meta‐analysis for the primary outcomes of success, onset, and duration of local anaesthesia. Data unsuitable for meta‐analyses were derived from orphan studies (57 studies), or from those that had unusable data or paired data from cross‐over studies that were not available (80 studies). The quality of outcomes ranged from moderate to very low.
Success of anaesthesia
For outcomes for which clinical study data were pooled, three comparisons showed one formulation to be superior to another when the success of anaesthesia was measured. Researchers found that 4% articaine, 1:100,000 epinephrine was superior to 2% lidocaine, 1:100,000 epinephrine when root canal treatment was performed in teeth with irreversible pulpitis. Evidence showed no difference when inferior alveolar nerve block injections (IANBs) were used to test the same formulations. When surgical procedures and surgical procedures/periodontal treatment were performed, 2% lidocaine, 1:100,000 epinephrine was superior to 3% prilocaine, 0.03 IU felypressin and 4% prilocaine plain, respectively. However, researchers found no evidence of a difference when IANBs were used in testing 2% lidocaine, 1:100,000 epinephrine versus 4% prilocaine plain.
Studies provided no evidence of a difference between 4% articaine, 1:100,000 epinephrine and both 2% mepivacaine, 1:100,000 and 4% articaine, 1:200,000 epinephrine for extracting teeth and performing surgical procedures, respectively, and between 0.5% bupivacaine, 1;200,000 epinephrine and both 4% articaine, 1:200,000 epinephrine and 2% lidocaine, 1:100,000 epinephrine for extracting teeth. Results showed no evidence of a difference between 2% lidocaine, 1:100,000 epinephrine and 2% mepivacaine, 1:100,000 when root canal treatment was performed in teeth with irreversible pulpitis.
For outcomes that pooled data from simulated scenario studies, we often downgraded quality owing to indirectness. We did this because the criteria for success in studies testing pulpal anaesthesia with an electric pulp tester or cold stimulus failed to replicate the duration of painful stimulation found in a clinical study, and because electric pulp testing may have underestimated successful anaesthesia. We also downgraded self‐assessed, soft tissue anaesthesia, as it is a poor indicator of clinical anaesthetic success.
Onset and duration of anaesthesia
No clinical studies met our outcome definition. We downgraded the quality ratings of simulated scenario testing of these outcomes owing to indirectness. We did this because self‐assessed soft tissue anaesthesia was a poor indicator of clinical anaesthesia, and because the intervals between testing, when an electric pulp tester was used to measure onset of anaesthesia, were relatively long when compared with the onset times measured. Also, electric pulp testing may have underestimated successful anaesthesia. When testing involved a simulated scenario, the speed of onset for the different local anaesthetics was within clinically acceptable times, while the duration of each local anaesthetic solution was variable, making them suitable for different applications.
Adverse effects
When 4% articaine, 1:100,000 epinephrine and 2% lidocaine, 1:100,000 epinephrine were compared, results showed no difference in pain on injection, while the injection of lidocaine resulted in less pain than articaine following the disappearance of anaesthesia, although clinically the difference was minor. Apart from this comparison, unwanted effects were rare. We were unable to combine data for these outcomes because of the different ways that adverse effects were measured in each study.
Participants' experience of the procedures carried out
Participants' experience of procedures was not assessed owing to lack of data.
Overall completeness and applicability of evidence
We identified 123 studies, conducted in a range of settings in 19 different countries, of which 68 were suitable for meta‐analysis. Despite a thorough, structured search of bibliographic databases, handsearching of journals and bibliographies, and a search of other resources, three other published systematic reviews revealed 27 journal articles that we had not identified (Su 2014a; Su 2014b; Su 2016). These were almost certainly found in Chinese databases (the Chinese BioMedical Literature Database and the China National Knowledge Infrastructure), which were referenced in the three reviews, and to which we did not have access. We have included them in the Characteristics of studies awaiting classification table, and when this review is updated, we will locate, translate, and include these journal articles. Another published systematic review, Xiao 2010, referenced a further six Chinese parallel trials that were cited in Chinese. An attempt will be made to locate and translate them. Their inclusion may introduce more bias into the review, as the systematic reviews that have assessed these studies ‐ Su 2014a and Su 2014b ‐ have, with few exceptions ‐ Chen 2004 and Shi 2002 ‐ reported unclear risks of all types of bias during their assessment. Lack of access to foreign databases and problems of language may limit the number of studies that can be included in systematic reviews. However, these problems are not unique to this review, and the review authors are not aware at present of any other source of studies that could be included in this review for quantitative and qualitative assessment.
Of the 68 cross‐over studies identified, three had their paired success data presented in 2 × 2 tables that could be combined with data from parallel studies (Arrow 2012; Porto 2007; Sancho‐Puchades 2012). We attempted to contact authors of the remaining cross‐over studies to request paired data. Of these, four study authors provided the data for five studies (Batista da Silva 2010; Bouloux 1999; Jaber 2010; Kanaa 2006; Trullenque‐Eriksson 2011). Two further studies ‐ Colombini 2006 and Laskin 1977 ‐ had success data showing that the events in each local anaesthetic group differed by one (19/20 vs 20/20 and 7/8 vs 8/8, respectively); therefore we were able to calculate the paired values. When no events were observed in one of the trial arms (Bouloux 1999; Colombini 2006; Jaber 2010; Kanaa 2006; Laskin 1977), cell counts of zero occurred when paired data were used. Therefore we adopted the principle recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b) and added 0.5 to each cell value in the 2 × 2 table, to allow entry into Microsoft Excel. We could not use data from the remaining cross‐over studies in this way; instead we treated them as parallel studies and included them in the meta‐analysis.
For the outcomes of onset and duration of local anaesthesia, the data were continuous and were again present in parallel and cross‐over trials. To include in the meta‐analysis a cross‐over study with continuous data, it is necessary to have the mean difference (A – B) and its standard error, preferably with the mean and standard error of each of A and B. This information was not available; therefore, we treated the cross‐over studies as if they were parallel studies in the meta‐analysis.
For both dichotomous and continuous data, it is possible to estimate the overall effect of interest in the meta‐analysis by incorporating cross‐over study data as if they came from parallel studies, but the standard errors are wider, and hence the confidence intervals are wider than they would be if the cross‐over studies were recognized as such. For this reason, we conducted a sensitivity analysis after removing data from cross‐over studies from the meta‐analysis for dichotomous and continuous data, when present.
We could not use data from 80 studies for some analyses for a variety of reasons.
For dichotomous data:
criteria for local anaesthetic success included no pain and mild pain, when it was impossible to calculate the success for just no pain;
data calculations were unclear;
data were presented as continuous data;
testing methods were not reported; and
the study provided a mixture of parallel and cross‐over data (Keskitalo 1975).
For continuous data:
standard deviations or standard errors were not reported; and
it was unclear whether a standard error or a standard deviation was reported in the journal article.
We have listed the data for these and orphan studies in Table 9Table 10Table 11Table 12Table 13 and Table 14.
We originally asked a broad question rather than a focused one because we did not fully know the scope of our search. The 15 commercially available local anaesthetics that are available for dental use gave rise to an enormous number of different comparisons. If the comparisons are grouped, depending on the tissues or methods of testing used, and are further divided into jaw type as in Table 18, more than 2000 different comparisons were possible. However, some of the local anaesthetic formulations would not be suitable for certain clinical uses (e.g. bupivacaine, which is long‐acting, would not be used for dental procedures that have a short duration). The scope of this work is huge and may be thought of as too great to be managed in a single systematic review when attempts are made to compare all commercially available local anaesthetics.
10. Studies showing success grouped according to local anaesthetic used, testing method, and subgroup.
| Comparison: lidocaine vs lidocaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
2% lid' 1:80,000 vs 2% lid' 1:200,000 Karm 2017 |
||
| Healthy pulps (clinical testing) |
2% lid' 1:50,000 vs 2% lid' 1:100,000 Kramer 1958 |
2% lid' 1:50,000 vs 2% lid' 1:100,000 Kramer 1958 |
2% lid' 1:50,000 vs 2% lid' 1:100,000 Sherman 1954 |
| Diseased pulps with irreversible pulpitis (clinical testing) |
2% lid' 1:80,000 vs 2% lid' 1:200,000 Aggarwal 2014 |
||
| Healthy pulps (simulated scenario testing) |
2% lid' 1:50,000 vs 2% lid' 1:100,000 Berberich 2009; Knoll‐Kohler 1992a; Mason 2009 2% lid' 1:50,000 vs 2% lid' 1:200,000 Knoll‐Kohler 1992a 2% lid' 1:100,000 vs lid' 1:200,000 Caldas 2015; Knoll‐Kohler 1992a |
2% lid' 1:50,000 vs 2% lid' 1:80,000 Dagher 1997; Yared 1997 2% lid' 1:50,000 vs 2% lid' 1:100,000 Dagher 1997; Wali 2010; Yared 1997; Yonchak 2001 2% lid' 1:80,000 vs 2% lid' 1:100,000 Dagher 1997; Yared 1997 2% lid' 1:100,000 vs 2% lid' 1:200,000 Vreeland 1989 |
|
| Soft tissues (simulated scenario testing) |
2% lid' 1:50,000 vs 2% lid' 1:100,000 Berberich 2009 |
2% lid' 1:50,000 vs 2% lid' 1:80,000 Dagher 1997; Yared 1997 2% lid' 1:80,000 vs 2% lid' 1:100,000 Dagher 1997; Yared 1997 2% lid' 1:50,000 vs 2% lid' 1:100,000 Dagher 1997; Wali 2010; Yared 1997; Yonchak 2001 2% lid' 1:100,000 vs 2% lid' 1:200,000 Vreeland 1989 2% lid' 1:80,000 vs 2% lid' 1:200,000 Aggarwal 2014 |
|
| Comparison: lidocaine vs mepivacaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
2% lid' 1:80,000 vs 3% mep' plain Elbay 2016; Hellden 1974 2% lid' 1:100,000 vs 2% mep' 1:100,000 Porto 2007 |
2% lid' 1:100,000 vs 2% mep' 1:20,000 Sadove 1962 |
|
| Healthy pulps (clinical testing) |
2% lid' 1:50,000 vs 2% mep' 1:20,000 Stibbs 1964 2% lid' 1:80,000 vs 3% mep' plain Mumford 1961 |
2% lid' 1:50,000 vs 2% mep' 1:20,000 Stibbs 1964 2% lid' 1:80,000 vs 3% mep' plain Mumford 1961 2% lid' 1:100,000 vs 2% mep' 1:20,000 Sadove 1962 |
|
| Diseased pulps with irreversible pulpitis (clinical testing) |
2% lid' 1:100,000 vs 2% mep' 1:100,000 Allegretti 2016; Visconti 2016 2% lid' 1:100,000 vs 3% mep' plain Cohen 1993 2% lid' 1:80,000 vs 3% mep' plain Elbay 2016 |
||
| Different tissues pooled (clinical testing) |
2% lid' 1:100,000 vs 3% mep' plain Bradley 1969 |
2% lid' 1:100,000 vs 3% mep' plain Bradley 1969 |
|
| Tissues, when tissues tested were unclear (clinical testing) |
2% lid' 1:100,000 vs 3% mep' plain Albertson 1963 2% lid' 1:100,000 vs 2% mep' 1:20,000 Albertson 1963 |
||
| Healthy pulps (simulated scenario testing) |
2% lid 1:100,000 vs 3% mep' plain Berberich 2009; Burns 2004; Forloine 2010; Mason 2009 2% lid' 1:50,000 vs 3% mep' plain Berberich 2009; Mason 2009 2% lid' 1:80,000 vs 3% mep' plain Nordenram 1990 2% lid' 1:100,000 vs 2% mep' plain 1:20,000 Lawaty 2010 2% lid' 1:100,000 vs 2% mep' 1:100,000 Srisurang 2011 |
2% lid' 1:100,000 vs 3% mep' plain Abdulwahab 2009; Cohen 1993; McLean 1993 2% lid' 1:100,000 vs 2% mep' 1:20,000 Hinkley 1991 2% lid' 1:100,000 vs 2% mep' 1:100,000 Porto 2007 |
|
| Diseased pulps with irreversible pulpitis (simulated scenario testing) |
2% lid' 1:100,000 vs 2% mep' 1:100,000 Allegretti 2016; Visconti 2016 |
||
| Soft tissues (simulated scenario testing) |
2% lid' 1:50,000 vs 3% mep' plain Berberich 2009 2% lid 1:100,000 vs 3% mep' plain Berberich 2009; Forloine 2010 |
2% lid' 1:100,000 vs 2% mep' 1:100,000 Allegretti 2016; Visconti 2016 2% lid' 1:100,000 vs 3% mep' plain Abdulwahab 2009; Cohen 1993; Hersh 1995; McLean 1993 2% lid' 1:100,000 vs 2% mep' 1:20,000 Hersh 1995; Hinkley 1991 2% lid' 1:80,000 vs 3% mep' plain Elbay 2016 |
|
| Comparison: lidocaine vs articaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
2% lid' 1:80,000 vs 4% art' 1:100,000 Kolli 2017 |
2% lid' 1:100,000 vs 4% art' 1:100,000 Bhagat 2014; Jain 2016; Kambalimath 2013; Sierra Rebolledo 2007; Silva 2012 2% lid' 1:80,000 vs 4% art' 1:100,000 Naik 2017 |
2% lid' 1:100,000 vs 4% art' 1:100,000 Khoury 1991 2% lid' 1:100,000 vs 4% art' 1:200,000 Khoury 1991 |
| Healthy pulps (clinical testing) |
2% lid' 1:80,000 vs 4% art' 1:100,000 Arrow 2012 |
2% lid' 1:100,000 vs 4% art' 1:200,000 Ram 2006 |
|
| Diseased pulps with irreversible pulpitis (clinical testing) |
2% lid' 1:100,000 vs 4% art' 1:100,000 Nabeel 2014; Srinivasan 2009 2% lid' 1:80,000 vs 4% art' 1:100,000 Hosseini 2016 |
2% lid' 1:200,000 vs 4% art' 1:200,000 Aggarwal 2009 2% lid' 1:100,000 vs 4% art' 1:100,000 Allegretti 2016; Ashraf 2013; Claffey 2004; Poorni 2011; Tortamano 2009 2% lid' 1:200,000 vs 4% art' 1:100,000 Aggarwal 2017 2% lid' 1:80,000 vs 4% art' 1:100,000 Sood 2014; Yadav 2015 |
2% lid' 1:100,000 vs 4% art' 1:100,000 Sherman 2008 |
| Different tissues pooled (clinical testing) |
2% lid' 1:80,000 vs 4% art' 1:100,000 Kanaa 2012 |
2% lid' 1:100,000 vs 4% art' 1:100,000 Malamed 2000a; Malamed 2000b |
|
| Healthy pulps (simulated scenario testing) |
2% lid' 1:80,000 vs 4% art' 1:100,000 Kanaa 2012 2% lid' 1:100,000 vs 4% art' 1:100,000 Evans 2008; Knoll‐Kohler 1992b; Ruprecht 1991; Srisurang 2011 2% lid' 1:100,000 vs 4% art' 1:200,000 Ruprecht 1991 2% lid' 1:80,000 vs 4% art' 1:200,000 Vahatalo 1993 |
2% lid' 1:100,000 vs 4% art' 1:100,000 Abdulwahab 2009; Batista da Silva 2010; Haase 2008; Jaber 2010; Kanaa 2006; Maruthingal 2015; Mikesell 2005; Robertson 2007 2% lid' 1:100,000 vs 4% art' 1:200,000 Abdulwahab 2009 |
2% lid' 1:100,000 vs 4% art' 1:100,000 Sherman 2008 |
| Diseased pulps with irreversible pulpitis (simulated scenario testing) |
2% lid' 1:100,000 vs 4% art' 1:100,000 Allegretti 2016; Tortamano 2009 2% lid' 1:80,000 vs 4% art' 1:100,000 Sood 2014; |
||
| Soft tissues (simulated scenario testing) |
2% lid' 1:200,000 vs 4% art' 1:200,000 Aggarwal 2009; 2% lid' 1:100,000 vs 4% art' 1:100,000 Abdulwahab 2009; Allegretti 2016; Ashraf 2013; Claffey 2004; Hersh 1995; Kanaa 2006; Maruthingal 2015; Mikesell 2005; Poorni 2011; Tortamano 2009 2% lid' 1:100,000 vs 4% art' 1:200,000 Abdulwahab 2009 2% lid' 1:80,000 vs 4% art' 1:100,000 Sood 2014; Yadav 2015 |
||
| Comparison: lidocaine vs prilocaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
2% lid' 1:100,000 vs 4% pril' plain Epstein 1965 |
2% lid' 1:100,000 vs 4% pril' plain Chilton 1971; Epstein 1965 2% lid' 1:100,000 vs 4% pril' 1:200,000 Chilton 1971 2% lid' 1:80,000 vs 3% pril' 0.03IU fely' Keskitalo 1975 |
2% lid' 1:100,000 vs 3% pril' 0.03IU fely' Khoury 1991; Pässler 1996 2% lid' 1:100,000 vs 4% pril' plain Chilton 1971 2% lid' 1:100,000 vs 4% pril' 1:200,000 Chilton 1971 |
| Healthy pulps (clinical testing) |
2% lid' 1:100,000 vs 4% pril' plain Epstein 1965 |
2% lid' 1:100,000 vs 4% pril' plain Epstein 1965 |
|
| Different tissues, pooled (clinical testing) |
2% lid' 1:100,000 vs 4% pril' plain Epstein 1969 2% lid' 1:100,000 vs 4% pril' 1:200,000 Epstein 1969 |
2% lid' 1:100,000 vs 4% pril' plain Epstein 1969; Gangarosa 1967 2% lid' 1:100,000 vs 4% pril' 1:200,000 Epstein 1969 |
2% lid' 1:100,000 vs 4% pril' plain Gangarosa 1967 |
| Tissues, when tissues tested were unclear (clinical testing) |
2% lid' 1:100,000 vs 4% pril' plain Chilton 1971; 2% lid' 1:100,000 vs 4% pril' 1:200,000 Chilton 1971 |
2% lid' 1:100,000 vs 4% pril' plain Chilton 1971 2% lid' 1:100,000 vs 4% pril' 1:200,000 Chilton 1971 |
|
| Healthy pulps (simulated scenario testing) |
2% lid' 1:100,000 vs 4% pril' plain Katz 2010 2% lid' 1:100,000 vs 4% pril' 1:200,000 Katz 2010 2% lid' 1:80,000 vs 3% pril' 0.03IU fely' Nordenram 1990 |
2% lid' 1:100,000 vs 4% pril' plain McLean 1993 2% lid' 1:100,000 vs 4% pril' 1:200,000 Abdulwahab 2009; Hinkley 1991 |
|
| Soft tissues (simulated scenario testing) |
2% lid' 1:100,000 vs 4% pril' plain Hersh 1995; McLean 1993 2% lid' 1:100,000 vs 4% pril' 1:200,000 Abdulwahab 2009; Hinkley 1991 |
||
| Comparison: lidocaine vs bupivacaine | |||
| Maxilla | Mandible | Both jaws/not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Bouloux 1999 |
2% lid' 1:80,000 vs 0.5% bup' 1:200,000 Chapman 1988 2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Bouloux 1999; Laskin 1977 |
|
| Diseased pulps with irreversible pulpitis (clinical testing) |
2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Sampaio 2012 2% lid' 1:200,000 vs 0.5% bup' 1:200,000 Aggarwal 2017 2% lid' 1:80,000 vs 0.5% bup' 1:200,000 Parirokh 2015 |
||
| Different tissues, pooled (clinical testing) |
2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Moore 1983 |
||
| Healthy pulps (simulated scenario testing) |
2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Gross 2007 |
2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009; Fernandez 2005; Sampaio 2012 |
|
| Soft tissues (simulated scenario testing) |
2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009; Fernandez 2005; Sampaio 2012 2% lid' 1:80,000 vs 0.5% bup' 1:200,000 Parirokh 2015 |
2% lid' 1:100,000 vs 0.5% bup' 1:200,000 Bouloux 1999 |
|
| Comparison: articaine vs articaine | |||
| Maxilla | Mandible | Both jaws/not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
4% art' 1:100,000 vs 4% art' 1:200,000 Lima 2009; Moore 2007 4% art' 1:100,000 vs 4% art' plain Moore 2007 4% art' 1:200,000 vs 4% art' 1:200,000 Moore 2007 |
4% art' 1:100,000 vs 4% art' plain Kammerer 2012 4% art' 1:100,000 vs 4% art' 1:200,000 Pässler 1996; Santos 2007 |
4% art' 1:100,000 vs 4% art' 1:200,000 Khoury 1991 |
| Diseased pulps with irreversible pulpitis (clinical testing) |
4% art' 1:100,000 vs 4% art' 1:100,000 bitartrate Atasoy Ulusoy 2014 |
||
| Healthy pulps (simulated scenario testing) |
4% art' 1:100,000 vs 4% art' 1:200,000 Kammerer 2014; Moore 2006; Ruprecht 1991 4% art' 1:100,000 vs 4% art' plain Kammerer 2014; Moore 2006 4% art' 1:200,000 vs 4% art' plain Kammerer 2014; Moore 2006 4% art' 1:100,000 vs 4% art' 1:400,000 Kammerer 2014 4% art' 1:400,000 vs 4% art' plain Kammerer 2014 4% art' 1:200,000 vs 4% art' 1:400,000 Kammerer 2014 |
4% art' 1:100,000 vs 4% art' 1:200,000 Abdulwahab 2009; McEntire 2011; Moore 2006 4% art' 1:100,000 vs 4% art' plain Moore 2006 4% art' 1:200,000 vs 4% art' plain Moore 2006 4% art' 1:100,000 vs 4% art' 1:100,000 bitartrate Atasoy Ulusoy 2014 |
|
| Soft tissues (simulated scenario testing) |
4% art' 1:100,000 vs 4% art' 1:200,000 Kammerer 2014; Ozec 2010 4% art' 1:100,000 vs 4% art' plain Kammerer 2014; Moore 2006 4% art' 1:200,000 vs 4% art' plain Kammerer 2014; Moore 2006 4% art' 1:100,000 vs 4% art' 1:400,000 Kammerer 2014 4% art' 1:400,000 vs 4% art' plain Kammerer 2014 4% art' 1:200,000 vs 4% art' 1:400,000 Kammerer 2014 |
4% art' 1:100,000 vs 4% art' 1:200,000 Abdulwahab 2009 |
|
| Comparison: articaine vs prilocaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
4% art' 1:100,000 vs 3% pril' 0.03IU fely' Khoury 1991 4% art' 1:200,000 vs 3% pril' 0.03IU fely' Khoury 1991 |
||
| Diseased pulps with irreversible pulpitis (clinical testing) |
4% art' 1:100,000 vs 3% pril' 0.03IU fely' Yilmaz 2011 |
4% art' 1:100,000 vs 3% pril' 0.03IU fely' Yilmaz 2011 |
|
| Healthy pulps (simulated scenario testing) |
4% art' 1:200,000 vs 4% pril' 1:200,000 Donaldson 1987; Haas 1990; Haas 1991 |
4% art' 1:200,000 vs 4% pril' 1:200,000 Donaldson 1987; Haas 1990; Haas 1991 4% art 1:100,000 vs 4% pril 1:200,000 Abdulwahab 2009; Nydegger 2014 |
|
| Soft tissues (simulated scenario testing) |
4% art' 1:200,000 vs 4% pril' 1:200,000 Haas 1990; Haas 1991 4% art' 1:100,000 vs 3% pril' 0.03IU fely' Yilmaz 2011 |
4% art' 1:200,000 vs 4% pril' 1:200,000 Haas 1990; Haas 1991 4% art' 1:100,000 vs 3% pril' 0.03IU fely Yilmaz 2011 4% art' 1:100,000 vs 4% pril' 1:200,000 Abdulwahab 2009 |
|
| Comparison: articaine vs mepivacaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
4% art' 1:100,000 vs 2% mep' 1:100,000 Gazal 2017 |
4% art' 1:100,000 vs 2% mep' 1:100,000 Colombini 2006 |
|
| Healthy pulps (clinical testing) |
4% art' 1:200,000 vs 3% mep' plain Odabas 2012 |
||
| Diseased pulps with irreversible pulpitis (clinical testing) |
4% art' 1:100,000 vs 2% mep' 1:100,000 Allegretti 2016; Maniglia‐Ferreira 2009 |
||
| Healthy pulps (simulated scenario testing) |
4% art' 1:100,000 vs 2% mep' 1:100,000 Srisurang 2011 |
4% art' 1:100,000 vs 2% mep' 1:100,000 Gazal 2015 4% art' 1:200,000 vs 3% mep' plain Abdulwahab 2009 4% art 1:100,000 vs 3% mep plain Abdulwahab 2009 |
|
| Diseased pulps with irreversible pulpitis (simulated scenario testing) |
4% art' 1:100,000 vs 2% mep' 1:100,000 Allegretti 2016; |
||
| Soft tissues (simulated scenario testing) |
4% art' 1:100,000 vs 2% mep' 1:100,000 Allegretti 2016; Bortoluzzi 2009 4% art' 1:200,000 vs 3% mep' plain Abdulwahab 2009 4% art' 1:100,000 vs 3% mep' plain Abdulwahab 2009 |
||
| Comparison: articaine vs bupivacaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
4% art' 1:200,000 vs 0.5% bup' 1:200,000 Gregorio 2008; Sancho‐Puchades 2012; Trullenque‐Eriksson 2011 4% art' 1:100,000 vs 0.5% bup' 1:200,000 Pellicer‐Chover 2013 |
||
| Diseased pulps with irreversible pulpitis (clinical testing) |
4% art' 1:100,000 vs 0.5% bup' 1:200,000 Aggarwal 2017 |
||
| Healthy pulps (simulated scenario testing) |
4% art' 1:200,000 vs 0.5% bup' 1:200,000 Vilchez‐Perez 2012 |
4% art' 1:200,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009; Sancho‐Puchades 2012 4% art' 1:100,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009 |
|
| Soft tissues (simulated scenario testing) |
4% art' 1:200,000 vs 0.5% bup' 1:200,000 Vilchez‐Perez 2012 |
4% art' 1:200,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009 4% art' 1:100,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009 |
|
| Comparison: prilocaine vs mepivacaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps (simulated scenario testing) |
3% mep' plain vs 3% pril' 0.03 IU fely' Nordenram 1990 |
2% mep' 1:20,000 vs 4% pril' 1:200,000 Hinkley 1991 3% mep' plain vs 4% pril' plain McLean 1993 3% mep' plain vs 4% pril' 1:200,000 Abdulwahab 2009 |
|
| Soft tissues (simulated scenario testing) |
3% mep' plain vs 4% pril' plain Hersh 1995; McLean 1993 2% mep' 1:20,000 vs 4% pril' 1:200,000 Hersh 1995; Hinkley 1991 3% mep' plain vs 4% pril' 1:200,000 Abdulwahab 2009 |
||
| Comparison: prilocaine vs prilocaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
4% pril' plain vs 4% pril' 1:200,000 Chilton 1971 |
4% pril' plain vs 4% pril' 1:200,000 Chilton 1971 |
|
| Different tissues, pooled (clinical testing) |
4% pril' plain vs 4% pril' 1:200,000 Epstein 1969 |
4% pril' plain vs 4% pril' 1:200,000 Epstein 1969 |
|
| Tissues, where the tissues tested were unclear (clinical testing) |
4% pril' plain vs 4% pril' 1:200,000 Chilton 1971 |
4% pril' plain vs 4% pril' 1:200,000 Chilton 1971 |
|
| Healthy pulps (simulated scenario testing) |
4% pril' plain vs 4% pril' 1:200,000 Katz 2010 |
||
| Comparison: prilocaine vs bupivacaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps (simulated scenario testing) |
4% pril' 1:200,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009 |
||
| Soft tissues (simulated scenario testing) |
4% pril' 1:200,000 vs 0.5% bup' 1:200,000 Abdulwahab 2009 |
||
| Comparison: mepivacaine vs bupivacaine | |||
| Maxilla | Mandible | Both jaws/Not stated | |
| Healthy pulps, hard and soft tissues (clinical testing) |
3% mep' plain vs 0.5% bup' 1:200,000 Trieger 1979 |
||
| Healthy pulps (simulated scenario testing) |
3% mep' plain vs 0.5% bup' 1:200,000 Abdulwahab 2009 |
||
| Soft tissues (simulated scenario testing) |
3% mep' plain vs 0.5% bup' 1:200,000 Abdulwahab 2009 |
||
| Comparison: mepivacaine vs mepivacaine | |||
| Maxilla | Mandible | Both jaws/not stated | |
| Healthy pulps (clinical testing) |
3% mep' plain vs 2% mep' 1:20,000 Weil 1961 |
3% mep' plain vs 2% mep' 1:20,000 Weil 1961 |
|
| Tissues, when tissues tested were unclear (clinical testing) |
3% mep' plain vs 2% mep' 1:20,000 Albertson 1963 |
||
art' = articaine; BI = buccal infiltration; bup' = bupivacaine; conc' = concentration; fely' = felypressin; IANB = inferior alveolar nerve block; lid' = lidocaine; mep' = mepivacaine; pril = prilocaine.
We graded no outcomes as high quality, four outcomes as moderate quality, and most outcomes as low (23) or very low quality (30). Therefore, the evidence for evaluating dental local anaesthesia in this review is very limited and should be interpreted with caution. Remaining evidence is available only in the form of orphan studies, or lacks the appropriate data to make more definitive conclusions possible.
Quality of the evidence
Using the GRADE approach resulted in four outcomes rated as moderate quality (Analysis 1.3; Analysis 1.8; Analysis 1.9; Analysis 2.1), 23 rated as low quality, and 30 rated as very low quality.
Study limitations were present in 41 outcomes, and downgrading occurred if there was high risk of bias or if unclear risks existed that may have had an impact on the outcomes. The most common reasons for this were noted in studies with unclear risk of randomization sequence generation, concealment of the allocation process, and blinding of participants, personnel, and outcome assessors. A small number of studies provided incomplete outcome data.
Eleven studies had received industry sponsorship, although we did not downgrade them owing to publication bias, as the sponsors manufactured both control and test formulations.
We often downgraded outcomes owing to imprecision because of the small overall numbers of participants and events. For dichotomous outcomes, only five out of 26 outcomes had over 300 successful dental anaesthesia events, and for continuous outcomes, only nine out of 31 outcomes had over 400 episodes of dental anaesthesia.
We downgraded outcomes owing to indirectness when measuring anaesthetic success, onset, and duration with an electric pulp tester. We did this because testing of pulp anaesthesia in this way required the maximum reading of an electric pulp tester as a sign of complete anaesthesia. Two studies have validated this (Certosimo 1996; Dreven 1987). However, clinical anaesthesia may still be present at lower readings than the maximum available, Therefore, onset times clinically may in fact be shorter than those obtained with an electric pulp tester. Clinical success and duration figures may also be greater than those measured by this method of testing, for the same reasons.
For pulpal anaesthesia onset, the shortest frequency of testing was one minute. As the onset of a number of local anaesthetic formulations was less than five minutes, this was regarded as a fairly insensitive way of determining anaesthesia onset. However, apart from a direct clinical intervention (Kramer 1958; Mumford 1961; Nespeca 1976), which would involve stimulating dental tissues for several minutes for painful procedures before the start of clinical anaesthesia, it would be difficult to overcome this problem or suggest a better way of testing.
When measuring pulpal anaesthesia success with an electric pulp tester, many studies set their criterion for success as obtaining a negative response to the maximal output of the pulp tester within a set period of time, then maintaining this negative response for a period of time similar to the duration of a clinical procedure. However, other studies required only one (Abdulwahab 2009; Haas 1990; Haas 1991; Kammerer 2014; Kanaa 2012; Knoll‐Kohler 1992a; Knoll‐Kohler 1992b; Nordenram 1990; Srisurang 2011; Vahatalo 1993), two (Allegretti 2016; Batista da Silva 2010; Berberich 2009; Burns 2004; Caldas 2015; Costa 2005; Evans 2008; Forloine 2010; Gross 2007; Kanaa 2006; Katz 2010; Lawaty 2010; Maruthingal 2015; Mason 2009; McEntire 2011; Nydegger 2014; Oliveira 2004; Robertson 2007; Visconti 2016; Yonchak 2001), or three ‐ Moore 2006 ‐ consecutive negative responses to classify the anaesthetic as successful. As a result of this, the outcomes containing these studies were downgraded one level.
We did not include the outcome of anaesthetic success for diseased pulps with irreversible pulpitis, as a negative response to pulp testing is not a reliable indicator of pulpal anaesthesia (Dreven 1987).
We downgraded the outcomes of soft tissue anaesthesia success (Analysis 1.3; Analysis 7.2), onset (Analysis 1.6; Analysis 4.4; Analysis 5.2; Analysis 6.4; Analysis 17.1; Analysis 19.3), and duration (Analysis 1.7; Analysis 4.5; Analysis 5.3; Analysis 6.5; Analysis 15.1; Analysis 19.4; Analysis 22.1), as subjective self‐assessed soft tissue anaesthesia alone is a poor indicator of clinical anaesthetic success.
We also downgraded many studies owing to inconsistency (high, unexplained heterogeneity). When possible, we attempted to investigate the cause of this by examining the factors mentioned in Assessment of heterogeneity.
Owing to the limited number of high and moderate outcomes, and the large numbers of low and very low quality outcomes presented in this review, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate for all measured outcomes,
Potential biases in the review process
There were no marginal decisions related to included studies and analysis of data that could have impacted this review. Types of interventions (infiltration and block anaesthesia), types of studies (parallel and cross‐over), and subgroups used (maxillary, mandibular, both jaws combined/jaws not stated) related to primary injections of local anaesthetic were provided in all studies found in our searches. The only factor that may have excluded some data was our primary outcome: "success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus".
A number of studies defined success as the absence of pain or the presence of mild pain, which still allowed clinical procedures to be performed, albeit painfully. Study authors took the view that these findings are important to document in studies. In practice, it is common to experience a small degree of pain, despite using local anaesthetic, and still complete a dental procedure. However for this review, and from a patient perspective, any pain felt during a procedure when local anaesthetics were compared would be regarded as failure. Therefore, we used only the complete absence of pain ("no pain" or "0" on a VAS) to indicate local anaesthetic success. Outcomes of "no pain" and "0" on a VAS allow data to be pooled from different studies for meta‐analysis, whereas outcomes of success from studies that include mild pain cannot be combined so easily, if mild pain is defined differently in each study. This resulted in the exclusion of data for 10 studies (Hosseini 2016; Kambalimath 2013; Maniglia‐Ferreira 2009; Nabeel 2014; Parirokh 2015; Pellicer‐Chover 2013; Poorni 2011; Sherman 2008; Sood 2014; Yadav 2015).
Although the outcomes in our final review were slightly different from those defined in our protocol, changes were made to clarify the outcomes. Classifying outcomes in relation to the anaesthetized tissues under investigation and the method of testing used may have reduced the number of studies included in each comparison. However, the tissues and testing used were so different that review authors thought this was essential, as the individual outcomes would not be comparable. Changes did not result in any changes to studies nor to data included in the review.
A cross‐over study design is often used when local anaesthetics are tested with some form of simulated scenario method, such as testing pulpal anaesthesia with an electric pulp tester. Alternatively, clinical dentistry may be performed using the same study design provided identical treatment can be provided in both arms of the study (e.g. extraction of similarly positioned third molar teeth).
The ideal approach for meta‐analysis using dichotomous data from cross‐over studies is to use their paired data (Elbourne 2002), which requires that success and failure for both arms of the study, for each individual, must be known. These data are rarely reported, and in this review only three cross‐over studies reported the data in 2 × 2 tables to allow meta‐analyses (Arrow 2012; Porto 2007; Sancho‐Puchades 2012). Contacting authors for this missing data resulted in data provided for only five further studies (Batista da Silva 2010; Bouloux 1999; Jaber 2010; Kanaa 2006; Trullenque‐Eriksson 2011). This meant that the anaesthetic success data for other cross‐over studies could not have been pooled for meta‐analyses using paired data.
For meta‐analysis of cross‐over studies with continuous data, it is necessary to have the mean difference between groups and its standard error, preferably along with the mean and standard error of each group. As these data were not available, we could not pool the data in that way for a number of studies.
An alternative approach is to treat cross‐over studies as if they were parallel studies in the meta‐analysis. It is possible to estimate the overall effect of interest using this approach in the meta‐analysis, but the standard errors are larger and hence the confidence intervals wider than they would be if cross‐over studies were recognized as such. We made the decision to do this while acknowledging this fact, but we also performed a sensitivity analysis, while removing the data from cross‐over studies from the meta‐analysis to assess the effect of their removal.
A further complication of using cross‐over data is that success and failure data are needed for calculation of logs of odds ratios and hence for meta‐analysis. Solutions that are 100% successful and therefore have 0% failure cannot have their data entered into the formula for calculation of odds ratios, as the numerator or denominator of the formula may contain 0, depending on which study is the control or experimental solution. This prevents their calculation in Microsoft Excel or using any other mathematical software. This would introduce bias into a review, as studies in which one or both solutions were 100% effective could not be included in meta‐analyses. Although we were unable to obtain paired data for many studies, in those studies with 100% success for one solution, the paired data could be calculated. However, for the reasons stated above, their data could not be entered, unless the principle of adding 0.5 to each of the cells in the 2 × 2 table was applied (Higgins 2011b). We needed this to make this adjustment for only five studies, in three analyses: Jaber 2010 and Kanaa 2006 in Analysis 1.2, Bouloux 1999, and Laskin 1977 in Analysis 6.1, and Colombini 2006 in Analysis 7.1.
For those studies in which the success for both groups in a comparison was 100%, we entered data into the appropriate analyses. When all studies in an analysis had 100% success for both solutions, we did not complete meta‐analysis. We entered the results of these studies, which looked at just the outcome of soft tissue anaesthesia success, at the end of Effects of interventions and in Table 14. We summarized in Table 14 the data from two studies ‐ Knoll‐Kohler 1992b and Ruprecht 1991 ‐ that were meant to be added to an existing analysis (Analysis 1.2) measuring the success of pulpal anaesthesia using an electric pulp tester, when both local anaesthetics had 100% success. We did this because the data could not be entered as logs of the odds ratio (OR) and associated standard error (SE), using the 'inverse variance' method.
We reported on selection bias related to baseline characteristics of the groups being investigated. For sequence generation, among studies having low risk of bias (66), we needed clarification from their authors regarding the exact methods used to generate a random sequence in 49 studies. Although randomization was often referred to, the basic method of sequence generation was often missing, such as the use of computer software or random selection of local anaesthetic cartridges from a container. The main source of bias for this review was seen in studies for which risk was graded as high and studies for which the risk was unclear. In analyses containing any of the 54 studies with unclear risk of bias, the effect of this is unknown. Analyses containing data from these studies may have risk of selection bias, although the significance of this is unknown. One study used in meta‐analysis had high risk of bias (Trullenque‐Eriksson 2011), which means that we downgraded Analysis 5.1,Analysis 5.2, and Analysis 5.3 owing to study limitations.
For implementation of the randomization sequence (allocation concealment) in studies having low risk of bias (70), we needed clarification from study authors regarding the exact methods used to conceal a randomization sequence in 51 studies. Often, small but important details were missed in the report, such as how the sequence was kept hidden, and when it was eventually revealed. Therefore, Analysis 5.1,Analysis 5.2, and Analysis 5.3 have study limitations due to the inclusion of Trullenque‐Eriksson 2011, which had high risk of bias; however, in those analyses containing any of the 50 studies with unclear risk of bias, the effect of this is unknown.
For performance bias (blinding of study participants and personnel), among those having low risk of bias (99), we needed clarification from study authors regarding exact methods used in 26 studies. For data analysis, one study had high risk of bias (Trullenque‐Eriksson 2011). This means that Analysis 5.1,Analysis 5.2, and Analysis 5.3 have study limitations, and in those analyses containing any of the 23 studies with unclear risk of bias, the effect of this is unknown.
For blinding of outcome assessors among studies having low risk of bias (90), we needed clarification from study authors regarding exact methods used to blind outcome assessors in 25 studies. During meta‐analysis, one study with high risk of bias was used (Naik 2017), which means that Analysis 17.1 has study limitations, and in those analyses containing any of the 30 studies with unclear risk of bias, the effect of this is unknown.
For randomization and blinding, the following numbers of journal articles were deficient in their reporting, which meant that we needed to seek clarification from study authors.
Randomization sequence generation: 103/123 (84%).
Randomization allocation concealment: 101/123 (82%).
Blinding of participants and personnel: 49/123 (40%).
Blinding of outcome assessors: 37/123 (30%).
These figures were surprisingly high, as 44 studies were published in journals endorsing the CONSORT guidelines for reporting of randomized trials, although some studies may have been published before the journal adopted these guidelines. Of the 49 journals represented in this review, 11 endorsed the CONSORT guidelines.
We rated the risk of attrition bias as low in 118 studies, unclear in 23 studies, and high in 12 studies.
An unclear level of reporting bias occurred in one study (Sancho‐Puchades 2012), which was used for analysis owing to missing pulpal anaesthesia onset data.
We included in Analysis 4.5,Analysis 5.1,Analysis 5.2,Analysis 5.3,Analysis 15.1,Analysis 17.1,Analysis 19.4,Analysis 20.2,Analysis 20.3,Analysis 21.2,Analysis 21.3, and Analysis 22.1 studies that were graded as having high risk of bias.
Agreements and disagreements with other studies or reviews
Following our structured search, we identified nine other systematic reviews in peer‐reviewed journals. Six compared articaine and lidocaine (Brandt 2011; Katyal 2010; Kung 2015; Paxton 2010; Su 2016; Xiao 2010), one compared bupivacaine with lidocaine (Su 2014a), one compared lidocaine and mepivacaine (Su 2014b), and one compared a variety of local anaesthetics and techniques to enhance local anaesthesia using an inferior alveolar nerve block for teeth with irreversible pulpitis (Corbella 2017). We identified in our search the studies included in these reviews. However, we did not include some owing to differing inclusion criteria such as looking at postoperative anaesthesia, using non‐commercially available local anaesthetic solutions, and using supplemental anaesthetic techniques. A number of studies included in these systematic reviews had been screened as part of this review, but we did not include them because they did not appear to be randomized controlled trials (RCTs), or because specific data were not available (e.g. missing data for participants who had scores of zero when visual analogue scale scores were used (no pain)).
One systematic review ‐ Xiao 2010 ‐ found that for teeth with irreversible pulpitis, articaine anaesthetic success was superior to lidocaine when both jaws were combined (risk ratio (RR) 1.33, 95% confidence interval (CI) 1.23 to 1.44), and when maxillary anaesthesia was used (RR 1.65, 95% CI 1.38 to 1.98), but success was similar for mandibular anaesthesia (RR 1.28, 95% CI 0.97 to 1.69). Kung 2015 also found for teeth with irreversible pulpitis that articaine was more likely to achieve successful anaesthesia than lidocaine formulations for combined maxillary and mandibular injections (odds ratio (OR) 2.21, 95% CI 1.41 to 3.47) and for combined mandibular injections (OR 2.20, 95% CI1.40 to 3.44). This review also found no differences between formulations when used for maxillary infiltration (OR 3.99, 95% CI 0.50 to 31.62) or for mandibular block anaesthesia (OR 1.44, 95% CI 0.87 to 2.38). Su 2016 also favoured 4% articaine with 1:100,000 epinephrine over 2% lidocaine with 1:100,000 epinephrine in terms of success rates of anaesthesia for teeth with irreversible pulpitis (RR 1.10, 95% CI 1.10 1.19).
Despite differences in inclusion criteria, definitions of success, and anaesthetic formulations used, the Xiao 2010, Kung 2015, and Su 2016 reviews had similar results to ours. The Brandt 2011 review showed no evidence of a difference between formulations in terms of success in teeth with irreversible pulpitis (OR 1.61, 95% CI 0.74 to 3.53). This may have been due to inclusion of data from different studies and different types of included data. The Corbella 2017 review showed no evidence of a difference between formulations in terms of success when an inferior alveolar nerve block was used for teeth with irreversible pulpitis (RR 1.00, 95% CI 0.88 to 1.15, respectively). Results were similar to the results of this review, despite inclusion of data from additional studies.
When comparing 2% lidocaine, 1;100,000 epinephrine against 4% articaine, 1;100,000 epinephrine for pulpal anaesthesia, the Katyal 2010 review favoured articaine (RR 1.31, 95% CI of 1.12 to 1.54), as did the Paxton 2010 review, which was also available as the study author's master's thesis online (9.21% greater proportion of success, 95% CI 2.56% to 15.58%). The Brandt 2011 systematic review also showed the superiority of articaine over lidocaine for pulpal anaesthesia (OR 2.44, 95% CI 1.59 to 3.76). These findings were similar to ours.
In the Katyal 2010 review, the pain score (VAS) for 4% articaine, 1;100,000 epinephrine was similar to that for 2% lidocaine, 1;100,000 epinephrine during solution injection (mean difference (MD) ‐2.49, 95% CI ‐14.49 to 9.52) but favoured articaine in the Su 2016 review (MD ‐0.67, 95% CI ‐1.26 ‐0.08); these results differed from the findings of this review, possibly because the data for 4% articaine, 1;100,000 epinephrine from the study by Evans 2008 included in the review by Katyal 2010 were incorrect (mean = 22, rather than 44 in the journal article), and only data from an orphan study were used (Kanaa 2012) in the Su 2016 review.
In the Katyal 2010 review, injections of 4% articaine, 1;100,000 epinephrine resulted in a higher pain score (VAS) than injections of 2% lidocaine, 1;100,000 epinephrine at the injection site, when the local anaesthetic wore off (MD 6.49, 95% CI 0.02 to 12.96). Despite identical data, minor differences from this review (MD 6.41, 95% CI 1.01 to 11.80) occurred because the Katyal 2010 review used a 'random‐effects' analysis model, as there were signs of statistical heterogeneity (I² = 30%), whereas we used a 'fixed‐effect' analysis model in this review, as this level of heterogeneity might not be important.
The Su 2014a systematic review included a comparison assessing healthy pulps tested with an electric pulp tester, and showed that 0.5% bupivacaine, 1:200,000 epinephrine was less successful than 2% lidocaine, 1:100,000 epinephrine (OR 0.39, 95% CI 0.27 to 0.57). Our review showed no evidence of a difference, and this may be related to our definitions of success. There was no evidence of a difference in pulpal anaesthesia onset times between these formulations (MD 4.13, 95% CI ‐0.26 to 8.51), which differed from this review (MD 3.32, 95% CI 0.27 to 6.37), because that review pooled different teeth, rather than using the data for first molar teeth. Bupivacaine had a longer pulpal anaesthesia duration time than lidocaine (MD 102.59, 95% CI 87.49 to117.68). However, although this outcome used pulpal anaesthesia duration data (Fernandez 2005), the other study used soft tissue duration data (Moore 1983).
The systematic review of mepivacaine and lidocaine, when comparing pulpal anaesthetic success, reported similar findings to this review (Su 2014b). No evidence showed a difference between 3% mepivacaine plain and 2% lidocaine, 1:100,000 epinephrine (OR 0.71, 95% CI 0.51 to 1.00) or 2% lidocaine, 1:50,000 epinephrine (OR 0.82, 95% CI 0.58 to 1.17). The same was true of onset times.
When compared with 2% lidocaine, 1:100,000 epinephrine pulpal anaesthesia onset times were quicker with 3% plain mepivacaine (MD ‐1.13, 95% CI ‐ 1.77 to ‐0.49), but no evidence suggested a difference with 2% mepivacaine with 1:20,000 levonordefrin (MD 0.20, 95% CI ‐2.87 to 3.27). When compared with 2% lidocaine, 1:50,000 epinephrine, 3% plain mepivacaine had a quicker pulpal anaesthesia onset time (MD ‐0.83, 95% CI ‐1.40 to ‐0.26), although our review found no evidence of a difference between formulations. This may be related to the data included, as numerous teeth were investigated.
Authors' conclusions
Implications for practice.
We do not have sufficient high‐quality evidence to determine whether one formulation of local anaesthetic is more effective than another. The quality of our evidence ranged from very low to moderate. Only four outcomes were graded as moderate quality.
Only three outcomes showed one formulation to be superior to another when the success of anaesthesia was measured. Researchers found that 4% articaine, 1:100,000 epinephrine was superior to 2% lidocaine, 1:100,000 epinephrine when root canal treatment was performed in teeth with irreversible pulpitis (inferior alveolar nerve block injections (IANBs) showed no evidence of a difference). Study results showed that 2% lidocaine, 1:100,000 epinephrine was superior to 3% prilocaine, 0.03 IU felypressin and 4% prilocaine plain when surgical procedures and surgical procedures/periodontal treatment respectively, were performed. IANBs showed no evidence of a difference when 2% lidocaine, 1:100,000 epinephrine was compared with 4% prilocaine plain.
The only other outcomes testing clinical success showed no evidence of a difference between 4% articaine, 1:100,000 epinephrine and both 2% mepivacaine, 1:100,000 and 4% articaine, 1:200,000 epinephrine when teeth were extracted and surgical procedures were performed, respectively, nor between 0.5% bupivacaine, 1:200,000 epinephrine and both 4% articaine, 1:200,000 epinephrine and 2% lidocaine, 1:100,000 epinephrine when teeth were extracted. There was no evidence of a difference between 2% lidocaine, 1:100,000 epinephrine and 2% mepivacaine, 1:100,000 when root canal treatment was performed in teeth with irreversible pulpitis.
A large number of included trials were simulated scenario studies, which were often downgraded in quality owing to indirectness, because the testing method failed to adequately mimic what occurs in clinical practice. Therefore, their results should be interpreted with caution.
Implications for research.
More studies are required that have clear reporting, low risk of bias, and an adequate sample size. Furthermore, studies should employ common validated methods with clinical outcome measures, when possible, and should provide data in a format that will allow meta‐analysis.
Although studies in most comparisons showed consistent agreement in the size and direction of their effects, some showed differences between subgroups (injection types), which may be a reflection of differences in diffusion and retention of the bolus of the local anaesthetic solution when delivered in different ways. Any true differences between injection types were difficult to determine owing to the small sample sizes and therefore large confidence intervals present. For the same reasons, and because of the limited number of studies for some outcomes, it was not possible to determine whether results of any studies were outliers. This emphasises the importance of a sufficient sample size when further research is planned.
In our search, we found a substantial number of simulated scenario trials testing healthy pulps with an electric pulp tester. Although this type of study is convenient to carry out and provides a validated method of testing (Certosimo 1996; Dreven 1987), clinical anaesthesia may be present at values less than a maximum pulp tester reading, which is a common criterion for success. Also, for many clinical procedures, only a clinical intervention can be used to test the oral tissues anaesthetized. These tissues may be more successfully anaesthetized or less successfully anaesthetized than pulpal tissues tested with an electric pulp tester. The same applies to testing of soft tissues, as soft tissue anaesthesia does not necessarily reflect successful clinical anaesthesia, clinical onset, or clinical duration. However, despite the advantage of clinical procedures to test different formulations, certain outcomes such as pulpal onset and duration could be ethically measured only using a cold test or an electric pulp tester, as the alternative is to start treatment in initially unanaesthetized patients. Despite this, a few studies did adopt this latter method for measurement (Kramer 1958; Mumford 1961), although this method is unlikely to be adopted in current research.
Better reporting of randomized controlled trials is required. Although several journals have adopted the CONSORT standards, the basic information required for critical appraisal was often missing from journal articles. This occurred most commonly with randomization sequence generation and concealment and blinding of patients, personnel, and outcome assessors. Randomization is easy to perform, but actual reporting of the method used (e.g. toss of a coin, use of a computer programme) was missing in a surprisingly large number of studies. Despite this, we often were able to clarify the method used by contacting the trial author.
In older studies, blinding of local anaesthetic cartridges was poorly performed or was poorly reported, although actual masking of cartridges is relatively easy to perform.
Criteria for success varied between studies. For simulated scenario studies that tested pulps, this varied from one negative response to an electric pulp tester during the testing session (Abdulwahab 2009; Haas 1990; Haas 1991; Kammerer 2014; Kanaa 2012; Knoll‐Kohler 1992a; Knoll‐Kohler 1992b; Nordenram 1990; Srisurang 2011; Vahatalo 1993), to a sustained negative response for up to 60 minutes (Fernandez 2005; Haase 2008; Mikesell 2005; Wali 2010).
Differences in the criteria for success were also seen in clinical studies. Successful local anaesthesia could be classed as no pain experienced during a clinical procedure, or as no pain or mild pain experienced when a procedure could still be completed although pain was felt. Although treatment can be completed when patients experience mild pain, we took the view that successful local anaesthesia should include only those instances in which no pain is experienced. Patients receiving dental treatment do not want to experience pain, and dentists want the same for their patients; therefore including mild pain as successful may be misleading. However in practice, a number of patients can experience pain while treatment is completed. Therefore, it is important to publish separately the results for study participants experiencing no pain or mild pain.
Criteria for success should be consistent between studies to reduce clinical heterogeneity. Testing in simulated scenario studies should be performed over a period similar to that seen in dental treatment. Journals publishing local anaesthesia research could set guidelines for this.
Some studies that gave IANB injections had participants eliminated or re‐appointed for repeat testing if soft tissue anaesthesia was not achieved. This ensured that different local anaesthetics were compared for their anaesthetic properties rather than introducing other factors responsible for failure (e.g. differences in anatomy). This would seem reasonable, but dentists and patients may be unaware that repeat injections were given when success rates were stated. A local anaesthetic may fail for many reasons, and separating these out to allow better comparison of just the properties of different local anaesthetics may result in reporting of success rates that may not be achievable in clinical practice, especially when less strict criteria for success are applied.
Reporting of cross‐over studies was the same as for parallel studies, in most cases using simple success and failure percentages with few exceptions (Arrow 2012; Porto 2007; Sancho‐Puchades 2012). For these three studies, paired data were presented that made meta‐analysis possible. Failure to publish cross‐over data in this way and to obtain paired data after contacting study authors meant that many of these cross‐over studies could not be used for meta‐analysis by this method. Therefore, data in these studies should be comprehensively reported as paired data for inclusion in meta‐analyses.
Outcomes reported in trials were varied, making combining data for meta‐analysis difficult. Standardized sets of outcomes, or "core outcome sets", need to be developed as recommended in the COMET (Core Outcome Measures in Effectiveness Trials) initiative.
A poor response rate when study authors were contacted should also be mentioned. Unfortunately, this has resulted in meta‐analyses that could not be completed and risk of bias that could not be clarified, leading to grading of a large number of studies as having unclear risk.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Co‐ordinating Editor |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 7, 2018
| Date | Event | Description |
|---|---|---|
| 1 September 2008 | Amended | Converted to new review format |
Acknowledgements
We would like to thank Andrew Smith (Content and Co‐ordinating Editor); Nathan Pace (Statistical Editor); Derek Richards, Paul Ashley, and Regina El Dib (Peer Reviewers); and Janet Wale (Consumer Editor) for their help and editorial advice during preparation of this systematic review.
We would like to thank Mona Nasser for her help with the review, and we would like to thank Elham Sassani, Soti Petraki, Luca Moranzoni, Luis Ferrandez Escarabajal, Shima Mahdavi‐Izadi, and Margarita Baulina for translation of non‐English journal articles.
We would like to thank Dr. François Curtin for providing statistical advice related to meta‐analysis using dichotomous and continuous cross‐over data.
We would like to thank Karen Hovhannisyan from the Cochrane Anaesthesia, Critical and Emergency Care Group for assistance with the search strategy. We also would like to thank Mathew Zacharias, Jörg Eberhard, Richard Niederman, Derek Richards, Janet Wale, and Mark Edward for help and editorial advice provided during preparation of the protocol for this systematic review (St George 2007).
Appendices
Appendix 1. Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Anesthesia, Local explode all trees #2 MeSH descriptor Anesthetics, Local explode all trees #3 ((an?est* or analg*) near local) #4 (an?est* near (solution* or agent*)) #5 (#1 OR #2 OR #3 OR #4) #6 (dent* or pulp*):ti,ab #7 MeSH descriptor Oral Surgical Procedures explode all trees #8 MeSH descriptor Surgery, Oral explode all trees #9 MeSH descriptor Dentistry explode all trees #10 (#6 OR #7 OR #8 OR #9) #11 (#5 AND #10)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. ((an?est* or analg*) adj3 local).mp. 2. (an?est* adj3 (solution* or agent*)).mp. 3. exp Anesthesia‐Local/ or exp Anesthetics‐Local/ 4. 1 or 2 or 3 5. (dent* or pulp*).ti,ab. 6. exp v/ or exp Dentistry‐Operative/ or Surgery‐Oral/ or Dentistry/ 7. 6 or 5 8. 4 and 7 9. exp Anesthesia‐Dental/ 10. 8 or 9 11. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 12. 11 and 10
Appendix 3. Search strategy for Embase (Ovid SP)
1. exp local anesthetic agent/ or exp local anesthesia/ 2. ((an?est* or analg*) adj3 local).mp. 3. (an?est* adj3 (solution* or agent*)).mp. 4. 1 or 2 or 3 5. (dent* or pulp*).ti,ab. 6. exp oral surgery/ or exp dental surgery/ or dentistry/ 7. 6 or 5 8. 4 and 7 9. exp dental anesthesia/ 10. 8 or 9 11. (RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/ or (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER* or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab.) not (animals not (humans and animals)).sh. 12. 11 and 10
Appendix 4. Seartch strategy for CINAHL PLUS (EBSCOhost)
S1 (MM "Anesthesia, Local") or (MH "Anesthetics, Local+") S2 TX ((an?est* or analg*) and local) S3 (an?est* and (solution* or agent*)) S4 S1 or S2 or S3 S5 TX ( (dent* or pulp*) ) or AB ( (dent* or pulp*) ) S6 (MH "Surgery, Oral+") or (MH "Dentistry, Operative+") S7 (MH "Dentistry") S8 S5 or S6 or S7 S9 S4 and S8 S10 (MM "Anesthesia, Dental") S11 S9 or S10 S12 (MM "Random Assignment") or (MH "Clinical Trials+") S13 AB (random* or placebo) S14 TI trial* S15 (MM "Double‐Blind Studies") or (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies") S16 S12 or S13 or S14 or S15 S17 S11 and S16
Appendix 5. Search strategy for Web of Science
#1 TS=((an?est* or analg*) SAME local) or TS=(an?est* SAME (solution* or agent*)) #2 TS=(dent* or pulp*) or TS=(Surgery SAME Oral) or TS=(Dentistry SAME Operative) #3 #2 AND #1 #4 TS=(random* or placebo*) or TI=trial* or TS=((Doubl* or Sinlg*?or Tripl*) SAME blind) #5 #4 AND #3
Appendix 6. Data collection form
| Bibliographic reference: | |||
| Authors: | |||
| Medline journal ID: | |||
| Year of publication: | |||
| Country where performed: | |||
| Language: | |||
| Source of funding: | |||
| Type of study: | RCT | CCT | Non‐randomized |
| Experimental trial? | Patient treatment trial? | ||
| Comments on study design: | |||
| METHOD OF RANDOMIZATION | Generation of random number sequence: Method of concealment: |
||
| Quality of concealment of random allocation | A. Concealment was adequate B. Methods of concealment were unclear C. Allocation concealment was inadequate D. Allocation was not concealed |
||
| Inclusion/Exclusion criteria | Inclusion and exclusion criteria were clearly defined in the text? | ||
| Patients taking medication that alter pain perception excluded? | |||
| Inclusion/Exclusion criteria: Inclusion Exclusion | |||
| Age | Age range: (or mean age + standard deviation) | ||
| Blinding | Yes | No | Unclear |
| Participant blinded? | |||
| Physician blinded? | |||
| Outcome assessor blinded? | |||
| Were the administrator and the outcome assessor the same person? | |||
| INTERVENTION | |||
| Treatment group 1 | Treatment group 2 | Treatment group 3 | |
| Local anaesthetic (specify type) | |||
| Local anaesthetic (concentration) | |||
| Dose (volume) | |||
| Vasoconstrictor (specify type) | |||
| Vasoconstrictor (concentration) | |||
| No. of injections | |||
| Technique | |||
| Needle gauge | |||
| Type of syringe used | |||
| Duration of injection or rate of injection | |||
| Topical anaesthetic used? (specify type) | |||
| Topical anaesthetic (duration) | |||
| Quantity of topical anaesthetic used | |||
| Concentration of topical anaesthetic used | |||
| Intra‐individual (cross‐over design) or parallel? | |||
| If cross‐over, time between injections | |||
| COMMENT ON TREATMENT | |||
| PARTICIPANTS | |||
| Number of eligible participants | Number enrolled in study | ||
| Number of males | Number of females | ||
| Statistics | |||
| No. of participants recruited to Group 1 | No. of participants completing study in Group 1 | ||
| No. of participants recruited to Group 2 | No. of participants completing study in Group 2 | ||
| No. of participants recruited to Group 3 | No. of participants completing study in Group 3 | ||
| No. of participants recruited to Group 4 | No. of participants completing study in Group 4 | ||
|
Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals |
|||
| Treatment and control groups were adequately described at entry? | |||
| SAMPLE SIZE AND STATISTICS | |||
| Size | |||
| Methods used to estimate sample size (statistical power) | |||
| Statistical method used | |||
| Unit of analysis | |||
| Use of intention‐to‐treat analysis | |||
| OUTCOMES | |||
| Calibration of examiners? | |||
| Number of examiners | |||
| Pulpal anaesthesia | |||
| Method of testing |
EPT thermal other (model) (hot/cold?) (type) |
||
| Teeth tested | |||
| Teeth Isolated? | |||
| Frequency of testing | |||
| Number of repeat readings to confirm anaesthesia | |||
| Criteria for success | |||
| Teeth tested before local anaesthesia given? (state no. of times) | |||
| Control teeth used during experiment? | |||
| Control teeth tested before LA given? | |||
| Speed of onset | |||
| Speed of onset statistics | |||
| Anaesthetic success | |||
| Anaesthetic success statistics | |||
| Duration | |||
| Duration statistics | |||
| Soft tissue anaesthesia | |||
| Method of measurement | |||
| Soft tissues tested? (state location) | |||
| Frequency of testing | |||
| Number of repeat readings to confirm anaesthesia | |||
| Tissues tested before local anaesthesia given? (state no. of times) | |||
| Control site used during experiment? | |||
| Control site tested before LA given? | |||
| Speed of onset | |||
| Speed of onset statistics | |||
| Anaesthetic success | |||
|
Anaesthetic success statistics |
|||
| Duration | |||
| Duration statistics | |||
| Local anaesthesia during an operative procedure | |||
| Diagnosis | Secure? | Insecure? | Unclear? |
| Method of testing | |||
| Procedure(s) carried out | |||
| Criteria for success | |||
| Teeth tested | |||
| Statistics | |||
| Onset | |||
| Onset statistics | |||
| Anaesthetic success | |||
| Anaesthetic success statistics | |||
| Duration | |||
| Duration statistics | |||
| Duration of procedure (+ range) | |||
| Adverse effects: pain on injection | |||
| Method of measurement | |||
| Results | |||
| Pain on injection statistics | |||
| Adverse effects: pain following injection | |||
| Method of measurement | |||
| Frequency of testing | |||
| Results | |||
| Pain following injection statistics | |||
| Other adverse effects: | |||
| CHANGES IN PROTOCOL: | |||
| CONTACT WITH STUDY AUTHOR: | |||
| OTHER COMMENTS ON THIS STUDY: | |||
Data and analyses
Comparison 1. 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of diseased pulps with irreversible pulpitis) | 4 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.10, 2.32] |
| 1.1 Maxillary infiltration | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.29, 3.92] |
| 1.2 Mandibular block (IANB) | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.81, 2.16] |
| 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 8 | (Fixed, 95% CI) | 2.71 [1.74, 4.22] | |
| 2.1 Maxillary infiltration | 2 | (Fixed, 95% CI) | 1.41 [0.54, 3.73] | |
| 2.2 Mandibular infiltration | 3 | (Fixed, 95% CI) | 4.88 [2.30, 10.37] | |
| 2.3 Mandibular block (mental block) | 1 | (Fixed, 95% CI) | 2.00 [0.60, 6.64] | |
| 2.4 Mandibular block (IANB) | 1 | (Fixed, 95% CI) | 2.19 [0.95, 5.04] | |
| 2.5 Mandibular infiltration (buccal and lingual) | 1 | (Fixed, 95% CI) | 11.00 [0.61, 198.97] | |
| 3 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of soft tissues) | 6 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 3.1 Mandibular block (IANB) | 5 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.99, 1.08] |
| 3.2 Mandibular infiltration | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.94, 1.06] |
| 4 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 6 | 402 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.69, 0.42] |
| 4.1 Maxillary infiltration | 3 | 104 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐1.10, 2.00] |
| 4.2 Mandibular infiltration | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐5.31, ‐1.69] |
| 4.3 Mandibular block (IANB) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.82, 0.22] |
| 4.4 Both jaws combined/Jaw not stated | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.30, ‐0.06] |
| 5 Duration of anaesthesia (simulated scenario testing of healthy pulps) | 3 | 104 | Mean Difference (IV, Random, 95% CI) | 21.87 [‐10.96, 54.71] |
| 5.1 Maxillary infiltration | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 5.5 [‐11.33, 22.33] |
| 5.2 Mandibular block (IANB) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 44.8 [33.22, 56.38] |
| 6 Speed of onset of anaesthesia (simulated scenario testing of soft tissues) | 6 | 818 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.45, ‐0.01] |
| 6.1 Mandibular infiltration | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.32] |
| 6.2 Mandibular block (IANB) | 1 | 360 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.29, ‐0.07] |
| 6.3 Mandibular block (IANB) and infiltration | 3 | 196 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.20, ‐0.03] |
| 6.4 Both jaws combined/Jaw not stated | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.03, ‐0.59] |
| 7 Duration of anaesthesia (simulated scenario testing of soft tissues) | 2 | 422 | Mean Difference (IV, Fixed, 95% CI) | 56.88 [44.08, 69.69] |
| 7.1 Mandibular block (IANB) and infiltration | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 33.0 [‐27.63, 93.63] |
| 7.2 Mandibular block (IANB) | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 58.00 [44.90, 71.10] |
| 8 Local adverse effects, pain on injection | 3 | 314 | Mean Difference (IV, Fixed, 95% CI) | 4.74 [‐1.98, 11.46] |
| 8.1 Maxillary infiltration | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐4.07, 20.07] |
| 8.2 Mandibular infiltration | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐12.86, 10.86] |
| 8.3 Mandibular block (IANB) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐4.07, 18.07] |
| 9 Local adverse effects, pain following injection | 3 | 309 | Mean Difference (IV, Fixed, 95% CI) | 6.41 [1.01, 11.80] |
| 9.1 Maxillary infiltration | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [3.43, 22.57] |
| 9.2 Mandibular infiltration | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.77, 10.77] |
| 9.3 Mandibular block (IANB) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐4.77, 14.77] |
Comparison 2. 3% prilocaine, 0.03 IU felypressin vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) | 2 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.95] |
| 1.1 Both jaws combined/Jaw not stated | 2 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.95] |
Comparison 3. 4% articaine, 1:200,000 epinephrine vs 4% articaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) | 3 | 930 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.02] |
| 1.1 Maxillary infiltration | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.64, 0.92] |
| 1.2 Mandibular testing (injection type not stated) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.03] |
| 1.3 Both jaws combined/Jaw not stated | 1 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.05] |
| 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 5 | 496 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 2.1 Maxillary infiltration | 3 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.06] |
| 2.2 Mandibular infiltration | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.10] |
| 2.3 Mandibular block (IANB) | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.80, 1.60] |
| 3 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 5 | 322 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.42, 0.73] |
| 3.1 Maxillary infiltration | 3 | 158 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.69, 0.73] |
| 3.2 Mandibular block (IANB) | 3 | 164 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.58, 1.40] |
| 4 Duration of anaesthesia (simulated scenario testing of healthy pulps) | 5 | 322 | Mean Difference (IV, Fixed, 95% CI) | ‐8.98 [‐15.17, ‐2.79] |
| 4.1 Maxillary infiltration | 3 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐6.62 [‐13.68, 0.44] |
| 4.2 Mandibular block (IANB) | 3 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐16.80 [‐29.65, ‐3.95] |
Comparison 4. 4% prilocaine plain vs 2% lidocaine 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.75, 0.99] |
| 1.1 Maxillary infiltration | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.44] |
| 1.2 Mandibular block (IANB) | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 1.3 Both jaws combined/Jaw not stated | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.94] |
| 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.17] |
| 2.1 Maxillary infiltration | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.22] |
| 2.2 Mandibular block (IANB) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.35] |
| 3 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐2.87, 0.95] |
| 3.1 Maxillary infiltration | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐3.12, 0.92] |
| 3.2 Mandibular block (IANB) | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐5.63, 6.03] |
| 4 Speed of onset of anaesthesia (simulated scenario testing of soft tissues) | 2 | 436 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.10, 0.14] |
| 4.1 Mandibular block (IANB) | 2 | 199 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.20, 0.75] |
| 4.2 Both jaws combined/Jaw not stated | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.13, 0.13] |
| 5 Duration of anaesthesia (simulated scenario testing of soft tissues) | 3 | 698 | Mean Difference (IV, Random, 95% CI) | ‐33.95 [‐48.05, ‐19.84] |
| 5.1 Maxillary infiltration | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐47.36 [‐63.24, ‐31.49] |
| 5.2 Mandibular block (IANB) | 3 | 300 | Mean Difference (IV, Random, 95% CI) | ‐21.09 [‐37.23, ‐4.94] |
| 5.3 Both jaws combined/Jaw not stated | 1 | 178 | Mean Difference (IV, Random, 95% CI) | ‐49.40 [‐69.00, ‐27.80] |
Comparison 5. 0.5% bupivacaine, 1:200,000 epinephrine vs 4% articaine, 1:200,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) | 2 | Odds Ratio (Fixed, 95% CI) | 0.87 [0.27, 2.83] | |
| 1.1 Mandibular block (IANB) and infiltration | 2 | Odds Ratio (Fixed, 95% CI) | 0.87 [0.27, 2.83] | |
| 2 Speed of onset of anaesthesia (simulated scenario testing of soft tissues) | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.26, ‐0.44] |
| 2.1 Mandibular block (IANB) and infiltration | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.26, ‐0.44] |
| 3 Duration of anaesthesia (simulated scenario testing of soft tissues) | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐172.61 [‐239.69, ‐105.53] |
| 3.1 Maxillary infiltration | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐172.55 [‐249.73, ‐95.37] |
| 3.2 Mandibular block (IANB) and infiltration | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐172.8 [‐308.44, ‐37.16] |
Comparison 6. 0.5% bupivacaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) | 2 | Odds Ratio (Fixed, 95% CI) | 0.58 [0.07, 5.12] | |
| 1.1 Mandibular block (IANB) and infiltration | 1 | Odds Ratio (Fixed, 95% CI) | 3.00 [0.12, 73.65] | |
| 1.2 Both jaws combined/Jaw not stated | 1 | Odds Ratio (Fixed, 95% CI) | 0.14 [0.01, 2.77] | |
| 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (simulated scenario testing of healthy pulps) | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.62, 1.05] |
| 2.1 Maxillary infiltration | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.05] |
| 2.2 Mandibular infiltration | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.53] |
| 2.3 Mandibular block (IANB) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.55, 1.34] |
| 3 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | 3.32 [0.27, 6.37] |
| 3.1 Maxillary infiltration | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 3.36 [‐0.12, 6.84] |
| 3.2 Mandibular block (IANB) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐3.16, 9.56] |
| 4 Speed of onset of anaesthesia (simulated scenario testing of soft tissues) | 3 | 126 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐1.07, 1.10] |
| 4.1 Mandibular block (IANB) | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 1.64 [‐0.25, 3.53] |
| 4.2 Mandibular block (IANB) and infiltration | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.73, 0.73] |
| 4.3 Both jaws combined/Jaw not stated | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.96, 0.16] |
| 5 Duration of anaesthesia (simulated scenario testing of soft tissues) | 6 | 332 | Mean Difference (IV, Random, 95% CI) | 222.88 [135.99, 309.76] |
| 5.1 Maxillary infiltration | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 109.52 [‐39.40, 258.44] |
| 5.2 Mandibular infiltration | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 228.0 [146.69, 309.31] |
| 5.3 Mandibular block (IANB) | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 273.0 [233.89, 312.11] |
| 5.4 Mandibular block (IANB) and infiltration | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 374.0 [279.54, 468.46] |
| 5.5 Both jaws combined/Jaw not stated | 2 | 140 | Mean Difference (IV, Random, 95% CI) | 224.26 [47.01, 401.50] |
Comparison 7. 4% articaine, 1:100,000 epinephrine vs 2% mepivacaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of healthy pulps, hard and soft tissues) | 2 | Odds Ratio (Fixed, 95% CI) | 3.82 [0.61, 23.82] | |
| 1.1 Maxillary infiltration (buccal and palatal) | 1 | Odds Ratio (Fixed, 95% CI) | 4.29 [0.46, 40.01] | |
| 1.2 Mandibular block (IANB) and infiltration | 1 | Odds Ratio (Fixed, 95% CI) | 3.00 [0.12, 73.65] | |
| 2 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of soft tissues) | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.73, 1.59] |
| 2.1 Mandibular block (IANB) | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 2.2 Mandibular infiltration | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.79, 1.86] |
Comparison 8. 2% lidocaine, 1:100,000 epinephrine vs 2% mepivacaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method (clinical testing of diseased pulps with irreversible pulpitis) | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.25, 5.45] |
| 1.1 Mandibular block (IANB) | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.25, 5.45] |
Comparison 9. 2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:80,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.01] |
| 1.1 Mandibular infiltration | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.24] |
| 1.2 Mandibular block (IANB) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.02] |
Comparison 10. 2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 7 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 1.1 Maxillary infiltration | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.08] |
| 1.2 Maxillary block (Infraorbital block) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.27] |
| 1.3 Mandibular infiltration | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] |
| 1.4 Mandibular block (IANB) | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.22] |
| 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 4 | 184 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐1.66, 0.79] |
| 2.1 Maxillary infiltration | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐3.04, 1.54] |
| 2.2 Maxillary block (Infraorbital block) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.87, 1.07] |
| 2.3 Mandibular block (IANB) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐5.91, 11.11] |
Comparison 11. 2% lidocaine, 1:80,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.01, 1.59] |
| 1.1 Mandibular infiltration | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.85, 2.17] |
| 1.2 Mandibular block (IANB) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.98, 1.52] |
Comparison 12. 2% lidocaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.26] |
| 1.1 Maxillary infiltration | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.33, 1.95] |
| 1.2 Mandibular block (IANB) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.68, 1.47] |
Comparison 13. 3% mepivacaine plain vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 6 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| 1.1 Maxillary infiltration | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.08] |
| 1.2 Maxillary block (Infraorbital block) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.23] |
| 1.3 Maxillary block (palatal‐anterior superior alveolar nerve block) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.37, 1.00] |
| 1.4 Maxillary block (high‐tuberosity maxillary second division nerve block) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.89, 1.12] |
| 1.5 Mandibular infiltration | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.59, 6.79] |
| 1.6 Mandibular block (IANB) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.12] |
| 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 3 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐1.23 [‐2.31, ‐0.16] |
| 2.1 Maxillary infiltration | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐3.39, 1.19] |
| 2.2 Maxillary block (Infraorbital block) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.45, 0.05] |
| 2.3 Mandibular block (IANB) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐8.14, 2.94] |
Comparison 14. 3% mepivacaine plain vs 2% lidocaine, 1:50,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 1.1 Maxillary infiltration | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.87, 1.14] |
| 1.2 Maxillary block (infraorbital block) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.54, 0.42] |
| 2.1 Maxillary infiltration | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.71, 1.11] |
| 2.2 Maxillary block (infraorbital block) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.16, 0.56] |
Comparison 15. 2% mepivacaine, 1:20,000 levonordefrin vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of anaesthesia (simulated scenario testing of soft tissues) | 2 | 458 | Mean Difference (IV, Fixed, 95% CI) | 4.43 [‐10.63, 19.48] |
| 1.1 Both jaws combined/Jaw not stated | 2 | 458 | Mean Difference (IV, Fixed, 95% CI) | 4.43 [‐10.63, 19.48] |
Comparison 16. 4% articaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.33, 5.36] |
| 1.1 Maxillary infiltration | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.83, 1.20] |
| 1.2 Mandibular infiltration | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.59, 6.79] |
| 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐2.06, 2.45] |
| 2.1 Maxillary infiltration | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.03, 2.57] |
| 2.2 Mandibular block (IANB) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.54, 0.54] |
| 3 Duration of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 10.33 [‐22.08, 42.74] |
| 3.1 Maxillary infiltration | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐6.90 [‐24.50, 10.70] |
| 3.2 Mandibular block (IANB) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 26.20 [14.46, 37.94] |
Comparison 17. 4% articaine, 1:100,000 epinephrine vs 2% lidocaine, 1:80,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Speed of onset of anaesthesia (simulated scenario testing of soft tissues) | 2 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.04, ‐0.52] |
| 1.1 Mandibular block (IANB) | 2 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.04, ‐0.52] |
Comparison 18. 4% articaine, 1:200,000 epinephrine vs 4% prilocaine, 1:200,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 3 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.41] |
| 1.1 Maxillary infiltration | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.28] |
| 1.2 Mandibular infiltration | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.89, 1.87] |
Comparison 19. 4% prilocaine, 1:200,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.91, 1.43] |
| 1.1 Maxillary infiltration | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.93, 1.35] |
| 1.2 Mandibular infiltration | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.35, 5.13] |
| 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐3.08, 0.70] |
| 2.1 Maxillary infiltration | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐3.50, 0.50] |
| 2.2 Mandibular block (IANB) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐4.37, 6.77] |
| 3 Speed of onset of anaesthesia (simulated scenario testing of soft tissues) | 2 | 449 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.14, 0.11] |
| 3.1 Mandibular block (IANB) | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.43, 0.24] |
| 3.2 Both jaws combined/jaw not stated | 1 | 258 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.13, 0.13] |
| 4 Duration of anaesthesia (simulated scenario testing of soft tissues) | 2 | 533 | Mean Difference (IV, Random, 95% CI) | ‐11.80 [‐27.76, 4.16] |
| 4.1 Maxillary infiltration | 1 | 148 | Mean Difference (IV, Random, 95% CI) | ‐22.60 [‐39.89, ‐5.31] |
| 4.2 Mandibular block (IANB) | 2 | 198 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐12.26, 16.65] |
| 4.3 Both jaws combined/Jaw not stated | 1 | 187 | Mean Difference (IV, Random, 95% CI) | ‐28.0 [‐49.36, ‐6.64] |
Comparison 20. 4% articaine plain vs 4% articaine, 1:100,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.38, 0.97] |
| 1.1 Maxillary infiltration | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.19] |
| 1.2 Mandibular block (IANB) | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.87] |
| 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 167 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.54, 0.80] |
| 2.1 Maxillary infiltration | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.61, 0.88] |
| 2.2 Mandibular block (IANB) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.48, 1.68] |
| 3 Duration of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 167 | Mean Difference (IV, Random, 95% CI) | ‐37.08 [‐60.95, ‐13.21] |
| 3.1 Maxillary infiltration | 2 | 121 | Mean Difference (IV, Random, 95% CI) | ‐45.85 [‐76.25, ‐15.45] |
| 3.2 Mandibular block (IANB) | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐12.10 [‐42.35, 18.15] |
Comparison 21. 4% articaine plain vs 4% articaine, 1:200,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.01] |
| 1.1 Maxillary infiltration | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.22] |
| 1.2 Mandibular block (IANB) | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.76] |
| 2 Speed of onset of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 169 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.66, 0.71] |
| 2.1 Maxillary infiltration | 2 | 119 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.63, 0.91] |
| 2.2 Mandibular block (IANB) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.90, 1.10] |
| 3 Duration of anaesthesia (simulated scenario testing of healthy pulps) | 2 | 169 | Mean Difference (IV, Random, 95% CI) | ‐28.36 [‐42.06, ‐14.65] |
| 3.1 Maxillary infiltration | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐32.88 [‐44.12, ‐21.65] |
| 3.2 Mandibular block (IANB) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐30.17, 27.17] |
Comparison 22. 4% prilocaine, 1:200,000 epinephrine vs 4% prilocaine plain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of anaesthesia (simulated scenario testing of soft tissues) | 2 | 506 | Mean Difference (IV, Fixed, 95% CI) | 18.78 [9.02, 28.54] |
| 1.1 Maxillary infiltration | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | 23.0 [6.36, 39.64] |
| 1.2 Mandibular block (IANB) | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | 14.03 [‐0.85, 28.91] |
| 1.3 Both jaws combined/Jaw not stated | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | 21.40 [0.86, 41.94] |
Comparison 23. 4% articaine, 1:100,000 epinephrine vs 4% prilocaine, 1:200,000 epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method (simulated scenario testing of healthy pulps) | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.16, 2.60] |
| 1.1 Mandibular infiltration | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.16, 2.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdulwahab 2009.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 18 enrolled, 18 completing the study. Mean age 24.9 years, ranging from 18 to 53 years. 6 male, 12 female Inclusion criteria
Exclusion criteria
They asked female participants of childbearing age to verify the specific birth control method they or their partner had used (such as abstinence, use of oral contraceptives, or use of other devices or methods) for at least 1 month before and during participation in the study. They required that female participants of childbearing potential receive negative results on a urine pregnancy test before receiving test medications |
|
| Interventions | Buccal infiltration (0.9 mL) opposite mandibular first molars using 1 of the following solutions:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars Soft tissue anaesthesia (self‐reported: no change in sensation, slight feeling of numbness, moderate but not complete feeling of numbness, or complete numbness on one side of the mouth)
Soft tissues tested: soft tissues on the injected side Adverse events (96/96)
|
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly assigned participants to one of six treatment sequence allocations (6 × 6 Latin square design)" Quote (from correspondence): "Six sequences were created to assure that each formulation was administered only once per patient and that each formulation would be given during each of the six sessions. After establishing the six sequences via a Latin square, a random assignment was made in 3 blocks of six determined by using a random number chart" |
| Allocation concealment (selection bias) | Low risk | Quote: "He placed cartridges in coded envelopes numbered for treatment sequence. To ensure blinding, neither the research assistant, the administrator nor the patient had knowledge of the formulation used" Quote (from correspondence): "The investigator designed the study and prepared blinded cartridges. He was not present for the LA administration of subjects or for the data collection. Another person, the clinician, administered the local anaesthetic and performed the EPT testing. A research assistant recorded data and monitored the project regarding timing, etc" Comment: participants and personnel would not be able to identify the local anaesthetic used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "One of the authors (S.B.) removed the manufacturers’ labels from the dental cartridges containing the six study formulations so that they were identical in appearance" "To ensure blinding, neither the research assistant, the administrator nor the patient had knowledge of the formulation used" Quote (from correspondence): "The investigator designed the study and prepared blinded cartridges. He was not present for the LA administration of subjects or for the data collection. Another person, the clinician, administered the local anaesthetic and performed the EPT testing. A research assistant recorded data and monitored the project regarding timing, etc" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To ensure blinding, neither the research assistant, the administrator nor the patient had knowledge of the formulation used" Comment: Although blinding of the research assistant, the administrator, and the patient was ensured, the person administering the injections and assessing outcomes is referred to as the clinician Quote (from correspondence): "The investigator designed the study and prepared blinded cartridges. He was not present for the LA administration of subjects or for the data collection. Another person, the clinician, administered the local anaesthetic and performed the EPT testing. A research assistant recorded data and monitored the project regarding timing, etc" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 28 occasions (on those experiencing successful anaesthesia). The number assessed in each group was reasonably well balanced with some minor differences, and the reason that assessment was not possible was the same for all groups. Therefore risk of attrition bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Comment: numbers of participants assessed were not reported and individual onset data were not available for each solution. Therefore risk of attrition bias was graded as unclear |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Quote: "We found A100 to provide the highest degree of numbness and B200 to provide the lowest" for soft tissue anaesthesia Comment: Exact data were requested from first study author, but none were received |
| Other bias | Low risk | Comment: no other bias present |
Aggarwal 2009.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: hospital (India) Participants: 87 enrolled, 84 completing the study (3 excluded as did not experience lip anaesthesia). Mean age 29 years, ranging from 23 to 37 years. 44 male, 40 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of 2% lidocaine, 1:200,000 epinephrine, followed by 1 of the following solutions:
Although a volume of 1.7 mL was used for each injection, the true volume was 1.8 mL, which included the small amount used for aspiration (e.g. "All patients received standard IANB injections using 1.8 mL of 2% lidocaine with 1:200,000 epinephrine") |
|
| Outcomes |
Clinical anaesthesia during endodontic access cavity preparation and instrumentation in teeth with irreversible pulpitis
Type of treatment: endodontic treatment of teeth with irreversible pulpitis Teeth tested: mandibular first molars and second molars Soft tissue anaesthesia (self‐reported)
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "The patients were randomly allocated to the treatment groups with the help of an online random generator which use permuted block randomization protocol (stratified) (randomization.com)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The solutions were masked with an opaque label and were randomly assigned a three‐digit alpha‐numeric value. Only the alpha‐numeric values were recorded on the data sheets to blind the experiment" Quote (from correspondence): "The code was broken at the end of the study and just before compilation/evaluation of results" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The solutions were masked with an opaque label and were randomly assigned a three‐digit alpha‐numeric value" "Only the alpha‐numeric values were recorded on the data sheets to blind the experiment" Comment: although participants and local anaesthetic administrators would know when no injection (control injection) was given, the only 2 comparisons for which data were to be used were blinded. Participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the alpha‐numeric values were recorded on the data sheets to blind the experiment" Comment: outcome assessor is the same person who administered the injections. Although this person would know when no injection was given, the identities of the articaine and lidocaine cartridges for infiltration, for which data would be used, were unknown owing to masking. Outcomes are patient‐reported outcomes (the outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: numbers in each group who were tested were equal following removal of those with failed lip anaesthesia |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Quote: "After 15 minutes of the initial IANB, each patient was asked if his/her lip was numb. If profound lip numbness was not recorded within 15 minutes, the block was considered unsuccessful, and the patients were excluded from the study" Comment: patients excluded were accounted for and used for calculation of soft tissue success |
| Selective reporting (reporting bias) | Low risk | Comment: outcome data were reported on, although exact data for pulpal anaesthesia success were not reported ‐ only the statistics |
| Other bias | Low risk | Comment: no other bias present |
Aggarwal 2014.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (India) Participants: 63 enrolled, 62 completing the study. Mean age 26 years, ranging from 20 to 31 years (2% lidocaine, 1:80,000 epinephrine), and mean age 27 years, ranging from 21 to 37 years (2% lidocaine, 1:200,000 epinephrine). 35 male, 27 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks of 1.8 mL of:
|
|
| Outcomes |
Clinical anaesthesia during endodontic access cavity preparation and instrumentation in teeth with irreversible pulpitis
Type of treatment: endodontic treatment of teeth with irreversible pulpitis Teeth tested: mandibular first and second molars Soft tissue anaesthesia (self‐reported)
Soft tissues tested: lower lip Adverse events reported (63/63)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allocated to 2 treatment groups with the help of an online random generator using permuted block stratified randomization protocol (randomization.com)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The solutions were given an alphanumeric code, and the syringes were masked with an opaque label marked with the code. Only the code and the primary/ secondary outcomes were recorded to blind the operator. The code was broken only after completion of the study" Quote (from correspondence): "The sequence was concealed in an opaque envelope. Before initiating the treatment, the sequence was opened by a dental assistant, who loaded the cartridge in the syringe according to the sequence only. The cartridges were masked with a label with alpha‐numeric code to blind the operator and the patient" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The solutions were given an alphanumeric code, and the syringes were masked with an opaque label marked with the code. Only the code and the primary/secondary outcomes were recorded to blind the operator" Quote (from correspondence): "The sequence was concealed in an opaque envelope. Before initiating the treatment, the sequence was opened by a dental assistant, who loaded the cartridge in the syringe according to the sequence only. The cartridges were masked with a label with alpha‐numeric code to blind the operator and the patient" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The solutions were given an alphanumeric code, and the syringes were masked with an opaque label marked with the code. Only the code and the primary/ secondary outcomes were recorded to blind the operator" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Quote: "After 15 min, each patient was asked whether his/ her lip was numb. If profound lip numbness was not recorded, the block was considered unsuccessful, and the patients were excluded from the study" Comment: the only patient excluded was accounted for, classed as a failure, and used for calculation of clinical success |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Quote: "After 15 min, each patient was asked whether his/ her lip was numb. If profound lip numbness was not recorded, the block was considered unsuccessful, and the patients were excluded from the study" Comment: the only patient excluded was accounted for and was used for calculation of soft tissue success |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Aggarwal 2017.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (India) Participants: 97 enrolled, 91 completing the study. Mean age 34 years, ranging from 27 to 47 years. 57 male, 34 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) using the following:
|
|
| Outcomes |
Clinical anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: mandibular molars Soft tissue anaesthesia (subjective testing)
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated to three treatment groups (lidocaine, articaine, and bupivacaine). The allocation was randomized using an online random generator (randomization.com) using a permuted block stratified randomization protocol" |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were allocated to three treatment groups (lidocaine, articaine, and bupivacaine). The allocation was randomized using an online random generator (randomization.com) using a permuted block stratified randomization protocol" Comment: detailed method not reported Quote (from correspondence): "The sequence was concealed in an opaque envelope. The sequence was opened just before the treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A trained dental assistant loaded the local anesthetic solutions in masked disposable syringes and coded them (three digit alpha‐numeric) for treatment sequence" "To ensure blinding, neither the operator nor the assistant had knowledge of the solution tested" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A trained dental assistant loaded the local anesthetic solutions in masked disposable syringes and coded them (three digit alpha‐numeric) for treatment sequence" "To ensure blinding, neither the operator nor the assistant had knowledge of the solution tested" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients were excluded following re‐calculation of success. Outcome data were complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: one patient each from the lidocaine and articaine groups and 4 patients from the bupivacaine group did not present lip numbness at 15 minutes and were excluded from the study. However, these were classed as failures in this review; therefore no participants were excluded. Outcome data were complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Albertson 1963.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: numbers enrolled and completing the study not clear (266; 223 without 3% mepivacaine, no vasoconstrictor) Age of participants and male:female ratio not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Injections (not specified) of:
|
|
| Outcomes |
Clinical anaesthesia
Soft tissue anaesthesia
Apart from success, methods were not reported Type of treatment: not stated (possibly surgery?). Teeth/soft tissues tested: not reported Adverse effects reported (223/223?) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In general, the method of Weil et al (2) was used (2 = Weil et al (1961). Clinical evaluation of mepivacaine hydrochloride by a new method" JADA 63:26‐32) Method was as follows: "Solutions.....were supplied in identical dental cartridges marked only by a control number printed on each cartridge. At least three different code numbers were assigned to each local anaesthetic solution. All the cartridges under test were mixed indiscriminately with cartridges of the control solution, in cans of 50" Comment: only 2% lidocaine, 1:100,000 epinephrine and 2% mepivacaine, 1:20,000 levo‐nordefrin were randomized. The solution of 3% mepivacaine, no vasoconstrictor was used as a comparison, without randomization; therefore data for this were not used |
| Allocation concealment (selection bias) | Low risk | Quote: "In general, the method of Weil et al (2) was used" (2 = Weil et al (1961). Clinical evaluation of mepivacaine hydrochloride by a new method" JADA 63:26‐32) Method used was as follows: "Solutions.....were supplied on identical dental cartridges marked only by a control number printed on each cartridge. At least three different code numbers were assigned to each local anaesthetic solution. All the cartridges under test were mixed indiscriminately with cartridges of the control solution, in cans of 50" Comment: only 2% lidocaine, 1:100,000 epinephrine and 2% mepivacaine, 1:20,000 levo‐nordefrin were randomized. The solution of 3% mepivacaine, no vasoconstrictor was used as a comparison, without randomization; therefore data for this were not used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The solutions, supplied in 1.8‐ml. Cartridges, were identified only by code numbers" Method used was as follows: "Solutions.....were supplied on identical dental cartridges marked only by a control number printed on each cartridge. At least three different code numbers were assigned to each local anaesthetic solution. All the cartridges under test were mixed indiscriminately with cartridges of the control solution, in cans of 50" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The solutions, supplied in 1.8‐mL cartridges, were identified only by code numbers" Comment: Having a limited number of code numbers may allow identification of a solution by personnel recording the outcomes if they also administered injections, and if the properties of the solutions were markedly different. However, properties of the 2 solutions did not allow identification, and outcomes were patient‐reported outcomes (the outcome assessor was the patient); therefore risk of bias was graded as low, as identification of the local anaesthetic by participants and personnel recording outcomes was not possible |
| Incomplete outcome data (attrition bias) Clinical success | Unclear risk | Comment: unsure whether some patients were excluded from calculation of anaesthetic success, as the number of participants at the start of the study was not stated |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: of the total number of participants recruited who were tested, some did not have onset of soft tissue anaesthesia measured (lidocaine: 3/110 (3%), mepivacaine: 2/113 (2%)). Dropout rates were minor and balanced between groups. Therefore risk of attrition bias has been graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Comment: of the total number of participants recruited who had successful anaesthesia and therefore had onset measured, some did not have duration of soft tissue anaesthesia measured (lidocaine: 18/107 (17%), mepivacaine: 5/111 (5%)). No dropouts would occur if the numbers of participants having duration measured were equal to those having soft tissue onset measured, assuming that all those who should have had onset measured, did. However, dropout rates of up to 17% were seen, based on those who had onset of soft tissue onset measured. Therefore attrition bias has been graded as high risk, because if dropout rates were based on soft tissue success, which was not measured, they may be higher still |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: as the number of participants assessed was unclear, risk of bias was also graded as unclear |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Allegretti 2016.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Brazil) Participants: 66 enrolled, 66 completing the study, with a mean age of 28.7 years (articaine)/30.3 years (lidocaine)/33.9 years (mepivacaine). 25 males and 41 females Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (3.6 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during pulpectomy of teeth with irreversible pulpitis
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first and second molars Soft tissue anaesthesia
Soft tissues tested: lower lip Adverse events were recorded if present (66/66) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure a blind test, 2 cartridges (3.6 mL) of each anesthetic solution were sealed in envelopes. At the time of application, the researcher randomly selected 1 of the envelopes and consecutively administered the 2 anesthetic injections" |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure a blind test, 2 cartridges (3.6 mL) of each anesthetic solution were sealed in envelopes. At the time of application, the researcher randomly selected 1 of the envelopes and consecutively administered the 2 anesthetic injections" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To ensure a blind test, 2 cartridges (3.6 mL) of each anesthetic solution were sealed in envelopes. At the time of application, the researcher randomly selected 1 of the envelopes and consecutively administered the 2 anesthetic injections" Comment: despite no details of the blinding method, risk of bias was graded as low, as identification of the local anaesthetic by participants is unlikely. Also, a pre‐determined method for administration was used by personnel, which minimized variation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Electrical stimulation of the tooth pulp (RMS) and the pulpectomy (CEA) were performed by different professionals to ensure that the anesthetic solution remained unknown, thereby maintaining the double‐blind nature of the study" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Arrow 2012.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel (technique) and cross‐over (local anaesthetic type) study design | |
| Participants |
Location: university (Australia) Participants: 57 enrolled, 56 completing all parts of the study. Mean age 12.7 years, ranging from 5.9 to 16.9 years. 21 male, 36 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block or mandibular infiltration (up to 2.2 mL) using the following:
|
|
| Outcomes |
Clinical anaesthesia during paediatric restorative procedures
Teeth tested: second deciduous molar, first permanent molar, and second permanent molar Soft tissue anaesthesia
Soft tissues tested: tongue and lower lip Adverse events reported (113/114)
|
|
| Notes | Industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were allocated to LA technique (IANB or BI) and then to LA type (lignocaine or articaine) using a two‐stage computer generated random permuted block design. The first stage was used to assign administration technique and the second stage for assignment of anaesthetic agent to be used at the first visit (each participant required two visits to complete the course of care for the study). The clinicians were advised to use clinical judgement to determine which side of the jaw to treat first and treatment visits were spaced at least one week apart" |
| Allocation concealment (selection bias) | Low risk | Quote: "Once a participant was registered into the trial, the clinic personnel contacted the central trial coordinator who, using a table of random numbers, selected the block for the first stage allocation of the LA technique to be used on the patient. The coordinator then used the second random block to allocate the anaesthetic drug for use at the first visit. The central trial coordinator maintained a register of trial participants and the random assignments. The coding for the anaesthetic agents was kept locked by the lead researcher at the central coordinating centre" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The clinician administering the anaesthetic, the chairside assistant and the patient receiving the anaesthetic and his ⁄ her parent were all ‘blind’ to the anaesthetic agent, but not to the LA technique. Each clinic was issued with two 2.2 ml cartridges of local anaesthetic (test and control) in sealed envelopes with the manufacturer’s label removed and re‐labelled with a researcher‐generated six‐digit code. The coding for the anaesthetic agents was kept locked by the lead researcher at the central coordinating centre" Comment: impossible to blind technique used, although for this review only similar techniques were compared. Participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinician administering the anaesthetic, the chairside assistant and the patient receiving the anaesthetic and his ⁄ her parent were all ‘blind’ to the anaesthetic agent, but not to the LA technique. Each clinic was issued with two 2.2 ml cartridges of local anaesthetic (test and control) in sealed envelopes with the manufacturer’s label removed and re‐labelled with a researcher generated six‐digit code. The coding for the anaesthetic agents was kept locked by the lead researcher at the central coordinating centre" Comment: impossible to blind technique used, although for this review only similar techniques were compared. Some outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: one patient was excluded but accounted for (did not attend second appointment). One hundred fourteen outcomes were scheduled to be recorded, but owing to failing 1 visit, only 113 were recorded. Of these interventions, 111 recorded the children's response to treatment (confirmed by the study author). This did not result in a large difference between groups. Therefore risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | High risk | Comment: for onset of soft tissue anaesthesia, the number of participants for whom this was recorded was 9/29 (IANB lidocaine), 13/28 (BI lidocaine), 16/29 (IANB articaine), and 7/28 (BI articaine). Onset may have been measured in those with successful clinical anaesthesia but could have been measured in those with unsuccessful clinical anaesthesia (i.e. soft tissues were anaesthetised but pulps were not). Unfortunately the exact dropout rate cannot be calculated (following communication with the study author) Of the total number of participants recruited who had successful anaesthesia, some did not have onset of soft tissue anaesthesia measured:
The dropout rate in one group was as high as 29%. However, the true dropout rate could be calculated only if those having soft tissue success were known, and it is likely to be higher than the figures calculated. Therefore attrition bias was rated as high risk |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: one patient was excluded but accounted for (did not attend second appointment). One hundred fourteen outcomes were scheduled to be recorded, but owing to failing 1 visit, only 113 were recorded. This did not result in a large difference between groups; therefore risk of bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Comment: the study author thanked Septodont Australia, which facilitated the supply of some local anaesthetic cartridges used in the study, although this was relatively minor funding |
Ashraf 2013.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Iran) Participants: 125 enrolled, 125 completing the study. Age ranging from 20 to 60 years. Male:female ratio not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.5 mL) and long buccal infiltration (0.3 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: first molars, second molars Soft tissue anaesthesia on questioning
Soft tissues tested: lower lip |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Initially, the patients were divided into 2 groups of men and women, who were then classified randomly into 2 subgroups of lidocaine or articaine by using random allocation software. One blinded nurse enrolled all participants and assigned them to intervention" |
| Allocation concealment (selection bias) | Low risk | Quote: "There were equal numbers of lidocaine and articaine cartridges available that had been covered and given a code. Another nurse in the department was aware of the codes and gave out the cartridges randomly and in equal numbers according to the subgroups of lidocaine or articaine. There was 1 code for each of the 2 cartridges packed together because the block and infiltration injections were supposed to be administered by using the same anaesthetic" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "There were equal numbers of lidocaine and articaine cartridges available that had been covered and given a code. Another nurse in the department was aware of the codes and gave out the cartridges randomly and in equal numbers according to the subgroups of lidocaine or articaine. There was 1 code for each of the 2 cartridges packed together because the block and infiltration injections were supposed to be administered by using the same anaesthetic" Comment: Patients and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "There were equal numbers of lidocaine and articaine cartridges available that had been covered and given a code. Another nurse in the department was aware of the codes and gave out the cartridges randomly and in equal numbers according to the subgroups of lidocaine or articaine. There was 1 code for each of the 2 cartridges packed together because the block and infiltration injections were supposed to be administered by using the same anaesthetic" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Unclear risk | Exact numbers of successful injections for pulpal and soft tissue anaesthesia for each local anaesthetic were not given. Therefore risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Unclear risk | Quote: "Patients who did not report lip numbness were excluded from the study, and their cartridges were replaced. Those who reported lip numbness were studied for data analyses" Comment: six patients did not experience lip numbness after the IANB. However it is not clear from the journal article which group they were from, as they should have been classed as failures |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Atasoy Ulusoy 2014.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Turkey) Participants: 50 enrolled, 50 completing the study. Mean age 30.5 years (4% articaine, 1:100,000 epinephrine) and 30.7 years (4% articaine, 1:100,000 epinephrine bitartrate). 24 male, 26 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.5 mL) of 1 of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with Endo‐Ice (Coltene/Whaledent)
Pulpal anaesthesia during endodontic access cavity preparation and instrumentation in teeth with irreversible pulpitis
Type of treatment: endodontic treatment of teeth with irreversible pulpitis Teeth tested: maxillary first molars Adverse events and heart rate were measured (50/50) |
|
| Notes | No funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For the randomization process, the two anaesthetic formulations were randomly assigned 4‐digit numbers from a random table by a graduate student who was not involved in the trial. The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered" Comment: detailed methods were not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "For the randomization process, the two anaesthetic formulations were randomly assigned 4‐digit numbers from a random table by a graduate student who was not involved in the trial. The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered" "Only the random numbers were recorded on the data collection sheets" Comment: detailed methods were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the random numbers were recorded on the data collection sheets. Patients were blinded to the type of anaesthetic solution" Comment: despite no details of the blinding method, risk of bias was graded as low, as identification of the local anaesthetic by participants is unlikely. Also, a pre‐determined method for administration was used by personnel, which minimized variation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Only the random numbers were recorded on the data collection sheets. Patients were blinded to the type of anaesthetic solution" Comment: no details of the blinding method were reported, and it is not clear whether the person recording the patient outcomes was a different person than the person administering the local anaesthetic, who may have been able to influence the participant's response (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Batista da Silva 2010.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 40 enrolled, 40 completing the study. Ages ranging from 18 to 35 years (no mean). 20 male, 20 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Incisive/mental nerve blocks (0.6 mL) of 1 of the following solutions:
|
|
| Outcomes |
Pulpal anaesthesia tested using an electric pulp tester
Teeth tested: right mandibular lateral incisors, canines, first premolars, second premolars, and contralateral canines Soft tissue anaesthesia tested by asking volunteers to palpate the inferior lip
Soft tissues tested: lower lip on the affected side Adverse events reported (80/80)
|
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Volunteers randomly received two incisive/mental nerve blocks according to the technique described by Malamed (10) at 2 separate appointments spaced at least 2 weeks apart in a repeated‐measures design" Quote (from correspondence): "The randomization was performed prior to the study by using an Excel sheet in order to sort the injection sequence. Volunteers were assigned to the injection code in the first visit" |
| Allocation concealment (selection bias) | Low risk | Quote: "Volunteers randomly received two incisive/mental nerve blocks according to the technique described by Malamed (10) at 2 separate appointments spaced at least 2 weeks apart in a repeated‐measures design" Quote (from correspondence): "The participants and also the clinician were not aware of the cartridges since those cartridges were colour coded (no brand or other names on them). Those responsible for all analysis (statistics, graphics, etc) just received the data described by colour codes. After the end of all procedure, the main investigator revealed the codes and the name of colours in the graphics were changed for the real names of solutions" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: All pulp testing was performed by a trained person who was blinded to the anaesthetic solutions administered Quote (from correspondence): "The participants and also the clinician were not aware of the cartridges since those cartridges were colour coded (no brand or other names on them)" Comment: coding the cartridges of each formulation with the same colour could allow identification of a solution by the personnel administering injections in a cross‐over study if properties of the solutions were markedly different. Participants may comment about long duration, poor anaesthesia, etc., at their second visit. However, the properties of the 2 solutions would not allow identification, and a pre‐determined method for administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the pulp testing was performed by a trained person who was blinded to the anaesthetic solutions administered" Quote (from correspondence): "Those responsible for all analysis (statistics, graphics, etc.) just received the data described by colour codes" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant); therefore risk of bias was graded as low, as identification of the local anaesthetic by participants is unlikely |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 50 occasions with mandibular second premolar teeth (on those experiencing successful anaesthesia: 28 cases of lidocaine, 32 cases of articaine) and was confirmed by the study author. Both groups were equally balanced; therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: all 40 participants (80 episodes of successful anaesthesia) had onset of soft tissue anaesthesia measured (confirmed by study author) |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: onset of pulpal anaesthesia was tested on 50 occasions with mandibular second premolar teeth (on those experiencing successful anaesthesia: 28 cases of lidocaine, 32 cases of articaine) and was confirmed by the study author. Both groups were equally balanced; therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: all 40 participants (80 episodes of successful anaesthesia) had duration of soft tissue anaesthesia measured (confirmed by study author) |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Berberich 2009.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 40 enrolled, 40 completing the study. Mean age 26 years, ranging from 23 to 33 years. 34 male, 6 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intraoral infraorbital nerve blocks of 1 cartridge (1.8 mL; confirmed by study author) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary anterior teeth, premolars, and first molars Soft tissue anaesthesia (palpation of soft tissues)
Soft tissues tested: lip, side of nose, and lower eyelid Adverse effects reported (120/120)
(scale: 0 = no pain, 1 = mild pain that was recognizable but not discomforting, 2 = moderate pain that was discomforting but bearable, 3 = severe pain that caused considerable discomfort and was difficult to bear)
|
|
| Notes | Non‐industry funding (confirmed by study author) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, the 3 anaesthetic formulations were randomly assigned 4‐digit numbers from a random number table. Each subject was randomly assigned to 1 of the 3 anaesthetic formulations to determine which anaesthetic was to be administered at each appointment" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthetic formulations ........ were masked with opaque labels, and the cartridge caps and plungers were masked with a black felt tip marker. The corresponding 4‐digit codes were written on each cartridge label" Comment: participants and personnel would not be able to identify the local anaesthetic used. Risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the random numbers were recorded on the data collection and post‐injection survey sheets to blind the experiment" "Trained personnel, who were blinded to the type of injection technique used, performed all preinjection and post‐injection tests" Quote (from correspondence): "The master code list was not available to the investigator. The data sheets to record the pulp test results only had the random number on each sheet for each random number/subject" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: thirty sets of available matched pair data (from participants experiencing anaesthetic success) were used to assess onset of pulpal anaesthesia. Local anaesthetic groups were balanced. Therefore risk was graded as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Bhagat 2014.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (India) Participants: 209 male, 151 female
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (volume not stated) using the following:
|
|
| Outcomes |
Clinical anaesthesia during surgical removal of mandibular third molars
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomized, double‐blinded, controlled clinical trial comparing the efficacy of 4% articaine (Articaine 4% Inibsa®, Inibsa, Barcelona, Spain) and 2% lignocaine for the surgical removal of the mandibular third molar" Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A randomized, double‐blinded, controlled clinical trial comparing the efficacy of 4% articaine (Articaine 4% Inibsa®, Inibsa, Barcelona, Spain) and 2% lignocaine for the surgical removal of the mandibular third molar" Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A randomized, double‐blinded, controlled clinical trial comparing the efficacy of 4% articaine (Articaine 4% Inibsa®, Inibsa, Barcelona, Spain) and 2% lignocaine for the surgical removal of the mandibular third molar" Comment: despite no details of the blinding method, risk of bias was graded as low, as identification of the local anaesthetic by participants is unlikely. Also, a pre‐determined method of administration was used by personnel, which minimized variation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A randomized, double‐blinded, controlled clinical trial comparing the efficacy of 4% articaine (Articaine 4% Inibsa®, Inibsa, Barcelona, Spain) and 2% lignocaine for the surgical removal of the mandibular third molar" Comment: no details of the blinding method were reported, and it is not clear if the person recording participants' outcomes was a different person than the person administering the local anaesthetic, who may have been able to influence the participant's response (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: soft tissue duration was tested on 164/180 participants in the lidocaine group and 162/180 participants in the articaine group. As the groups were balanced, risk was graded as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Bortoluzzi 2009.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 25 enrolled, 24 completing the study. Mean age 22.6 ± 2.3 years. 10 male, 14 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Mandibular buccal infiltration (0.18 mL) using 1 of the following solutions:
|
|
| Outcomes |
Soft tissue anaesthesia (VAS ranging from zero (deep or total anaesthesia with no sensibility) to 10 (no anaesthesia or lower lip with normal sensibility)
Soft tissues tested: centre of the lower lip Adverse events (48/50)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated through a raffle to receive the anaesthetic ME (Drug 1) or AR (Drug 2)" Quote (from correspondence): the patients "just picked up a card yellow (drug 1) or green (drug 2)" "It was done at the same time as the first injection" |
| Allocation concealment (selection bias) | Low risk | Quote: "Both patient and operator were blind (double‐blind) to which anaesthetic were receiving or using. For this, in a separate room and under aseptic conditions, the commercial anaesthetic solutions were transferred from the original container to disposable insulin syringes in an amount of 0.18 mL (10% of an anaesthetic cartridge) (authors 1&2)" Quote (from correspondence): "The second research assistant kept a research instrument in order to collect data. A third research assistant conducted the injections. Both assistants didn't know which drug was to be administered, since I prepared the insulin syringes with the anaesthetic solutions in a separated room. With time they tried to guess which drug was being administered but it was only supposition. Patients and assistants had no access to the anaesthetic packs, garbage, or other information that could reveal the drugs" "This code was maintained during all research" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both patient and operator were blind (double‐blind) to which anaesthetic were receiving or using. For this, in a separate room and under aseptic conditions, the commercial anaesthetic solutions were transferred from the original container to disposable insulin syringes in an amount of 0.18 mL (10% of an anaesthetic cartridge) (authors 1&2)" Comment: participants and personnel would not be able to identify the local anaesthetic used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both patient and operator were blind (double‐blind) to which anaesthetic were receiving or using. For this, in a separate room and under aseptic conditions, the commercial anaesthetic solutions were transferred from the original container to disposable insulin syringes in an amount of 0.18 mL (10% of an anaesthetic cartridge) (authors 1&2)" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was npt possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Quote: "One subject was excluded due to a possibly delayed‐type hypersensitivity to articaine", but accounted for. Groups remained equal in numbers; therefore risk was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Quote: "One subject was excluded due to a possibly delayed‐type hypersensitivity to articaine", but accounted for. Duration of soft tissue anaesthesia measured for all 24 participants (confirmed by study author). Groups remained balanced; therefore risk was graded as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "One subject was excluded due to a possibly delayed‐type hypersensitivity to articaine", but accounted for. Groups remained equal in numbers; therefore risk was graded as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Bouloux 1999.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Australia) Participants: 23 enrolled, 23 completing the study. Mean age 24 years, ranging from 18 to 41 years. 9 male, 14 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Patients received the following injections:
with either:
|
|
| Outcomes |
Clinical anaesthesia during third molar removal
Soft tissue anaesthesia
Tissues tested: mucosa adjacent to tooth Adverse effects reported (46/46)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The choice of local anaesthetic and the side to be operated on was decided by the toss of a coin" |
| Allocation concealment (selection bias) | Low risk | Quote: "The choice of local anaesthetic and the side to be operated on was decided by the toss of a coin" Quote (from correspondence): "The randomization of the side to be operated and the choice of local anaesthetic were both made with a coin toss on the same day as the procedure several hours before the patient arrived. This was done by a research coordinator. The operator (myself) was blinded to the local anaesthetic but was informed of the side to be operating on" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The dental anaesthetic cartridges (2.2 mL) used were marked only as ‘A’ or ‘B’" Quote (from correspondence): "The labels were removed from the cartridges by the research coordinator and were supplied to the investigator (myself) only labelled as A or B. The outcome assessor was myself and I was blinded to all data except surgical side" Comment: labelling all cartridges containing similar local anaesthetic with a similar code (A or B) may allow identification of a solution by personnel recording the outcomes and the administrator in a cross‐over study if he or she also recorded outcomes, if properties of the solutions were markedly different. However, properties of the 2 solutions did not allow identification (only success ‐ not duration ‐ was measured). Outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person. Identification of the local anaesthetic by participants was not possible. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The dental anaesthetic cartridges (2.2 mL) used were marked only as ‘A’ or ‘B’" Quote (from correspondence): "The labels were removed from the cartridges by the research coordinator and were supplied to the investigator (myself) only labelled as A or B. The outcome assessor was myself and I was blinded to all data except surgical side" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Bradley 1969.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Australia) Participants: 254 enrolled, 254 completing the study. Ages ranging from 5 to 14 years. 131 male, 123 female Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Infiltration or "mandibular" injection of the following solutions:
Volume of solution was observed to range from 0.8 mL to 3.6 mL, with 1.8 mL given in:
|
|
| Outcomes |
Clinical anaesthesia during various dental procedures including restorative (65%), surgical (19%), root extirpation (10%), miscellaneous (5%)
Soft tissue anaesthesia
Teeth/soft/hard tissues tested: All tissues were tested, depending on what procedure was being performed Adverse effects reported (254/254) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The drug used for each injection was administered in a randomized double‐blind procedure" Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods not reported. Although participants were unlikely to identify the local anaesthetic because it was contained in a syringe, there was no mention of whether the administrator was blinded, or whether a specific pre‐determined method was used to inject the solution and minimize variation. Therefore risk was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods not reported Comment: no details of the blinding method reported; not clear if the person recording participants' outcomes was a different person than the person administering the local anaesthetic, who may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Comment: the number of participants who had onset measured is not known. Therefore, attrition bias was judged as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants who had duration measured is not known. Therefore, attrition bias was judged as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; success outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Burns 2004.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 40 enrolled, 40 completing the study. Average age 27 years, ranging from 19 to 47 years. 20 male, 20 female Inclusion criteria: All participants were in good health (written health history and oral questioning) and were not taking any medication that would alter pain perception Exclusion criteria: not reported |
|
| Interventions | Palatal‐anterior superior alveolar injections (1.4 mL) of either:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary central incisors, lateral incisors, and canines |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, we randomly assigned the two anaesthetic solutions six‐digit numbers from a random number table. We assigned the random numbers to a subject to designate which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Each solution had a six‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Two blinded cartridges of the same anaesthetic solution were placed in letter‐sized envelopes that were labelled with the six‐digit code, so the code would not have to be broken in the event of a broken or dropped cartridge. Only the random numbers were recorded on the data collection sheets to further blind the experiment" Comment: participants and personnel would not be able to identify the local anaesthetic used. Risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two blinded cartridges of the same anaesthetic solution were placed in letter‐sized envelopes that were labelled with the six‐digit code, so the code would not have to be broken in the event of a broken or dropped cartridge. Only the random numbers were recorded on the data collection sheets to further blind the experiment" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset presented as a range of values for participants with successful anaesthesia |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Caldas 2015.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 30 enrolled, 30 completing the study. Mean age of females 22.6 ± 3.7 years and of males 24.3 ± 4.7 years. 12 male, 18 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.8 mL) using the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary right canine Soft tissue anaesthesia
Soft tissues tested: vestibular mucosa Adverse events (60/60)
(scale: 0 = no pain, 10 = most severe pain)
|
|
| Notes | Non‐industry funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Volunteers were submitted to two more clinical sessions, with a previously defined randomized order for the application of both tested solutions and with a minimum interval of two weeks between anesthesias" Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Volunteers were submitted to two more clinical sessions, with a previously defined randomized order for the application of both tested solutions and with a minimum interval of two weeks between anesthesias" Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigator‐operator was not involved in the evaluation of anesthetic parameters, characterizing a double‐blind study" Comment: despite no details of the blinding method, risk of bias was graded low, as identification of the local anaesthetic by participants was unlikely. Also, a pre‐determined method for administration was used by personnel, which minimized variation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigator‐operator was not involved in the evaluation of anesthetic parameters, characterizing a double‐blind study" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants is unlikely. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Chapman 1988.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Australia) Participants: 20 enrolled, 20 completing the study. Mean age 22 years, ranging from 17 to 33 years. 14 male, 6 female Inclusion criteria: not stated, although healthy patients requiring removal of both impacted mandibular third molar teeth participated in the study Exclusion criteria: not stated |
|
| Interventions | Inferior alveolar nerve block (2.0 mL) and buccal infiltration (1.0 mL) of either:
|
|
| Outcomes |
Clinical anaesthesia during extraction of teeth
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lower lip/mental region. Methods of testing unclear Adverse effects related to extractions were reported (number assessed not clear)
|
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The order of use of anaesthetics was randomly selected before the first operation" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The order of use of anaesthetics was randomly selected before the first operation" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The survey was conducted as a double‐blind cross‐over study" Comment: no details of the blinding method were reported (bupivacaine was loaded into a 10‐mL syringe), although identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The survey was conducted as a double‐blind cross‐over study" Comment: no details of the blinding method were reported, and it is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, who may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants who had duration measured was not reported. This was probably measured by participants at home, but it is not clear whether all participants provided data. Therefore, attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: the number of participants who had adverse events measured is not known. It is not clear whether all participants returned to provide the data. Therefore, attrition bias is graded as unclear. Data were not used for meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Chilton 1971.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: private practice (United States of America) Participants: 821 enrolled, 821 completing the study. Average age 39 years. 304 male, 517 female. 424 (52%) required periodontal treatment; 397 (48%) required endodontic treatment Inclusion criteria: none Exclusion criteria
|
|
| Interventions | Infiltration: average volume for periodontal procedures = 1.5 mL (greater volume for endo procedures). Inferior alveolar nerve block: average volume for periodontal procedures = 1.8 mL (including supplemental injections) of either:
|
|
| Outcomes |
Clinical anaesthesia during endodontic and periodontal procedures (821/821)
Hard/soft tissues tested: various Soft tissue anaesthesia
Soft tissues tested: those relevant to the type of injection Adverse effects were measured (821/821) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Cartridges of local anaesthetic were provided by the manufacturer and coded randomly with a sealed copy of the code" Comment: method of randomization not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Cartridges of local anaesthetic were provided by the manufacturer and coded randomly with a sealed copy of the code" Comment: method of randomization not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Cartridges of local anaesthetic were provided by the manufacturer and coded randomly with a sealed copy of the code" Comment: despite limited details of the blinding method, risk of bias was graded as low, as identification of the local anaesthetic by participants and personnel is unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Cartridges of local anaesthetic were provided by the manufacturer and coded randomly with a sealed copy of the code" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient); therefore risk of bias was graded as low, as identification of the local anaesthetic by participants and personnel recording the outcomes is unlikely |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: the true dropout rate could be calculated only if those having soft tissue success were known, as successful soft tissue anaesthesia is required to measure onset. Soft tissue anaesthesia may have been present in those who had failure of anaesthesia during endodontic and periodontal treatment, or may have been absent, meaning it was not measured. As this measurement was performed in a clinic, immediately before treatment, the only minor differences in proportions between groups would be due to differences in soft tissue success. Therefore attrition bias has been graded as low risk |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Comment: of the total number of participants recruited who had onset of soft tissue anaesthesia measured, some did not have duration of soft tissue anaesthesia measured Inferior alveolar nerve block
Infiltration
For duration of soft tissue anaesthesia, no dropouts would occur if the number of participants having duration measured was equal to the number having soft tissue onset measured. However, dropout rates of up to 35% were seen. This was probably due to lack of compliance of patients returning postcards with time when "sense of numbness" disappeared. Therefore attrition bias was graded as high risk |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Claffey 2004.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: 25 male, 47 female
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (2.2 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: mandibular first premolars, second premolars, first molars, second molars Soft tissue anaesthesia on questioning
Soft tissues tested: lower lip |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was randomly assigned a five‐digit random number to determine which anaesthetic solution was administered" Quote (from correspondence): "Each solution had a five‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The appropriate five digit random number was placed on a label, which was affixed to the outside of the Luer‐Lok syringe. Only the random number was used on the data collection sheets to further blind the experiment" Comment: participants and personnel would not be able to identify the local anaesthetic used. Risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The appropriate five digit random number was placed on a label, which was affixed to the outside of the Luer‐Lok syringe. Only the random number was used on the data collection sheets to further blind the experiment" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Quote: "At 15‐min post‐injection, the patient was questioned regarding lip numbness. If profound lip numbness was not recorded, the block was considered missed and the patient was eliminated from the study" "A total of 7 patients, two using the articaine solution and five using the lidocaine solution, did not have profound lip numbness at 15 min and were not included in the data analysis of the 72 patients. The number of these missed blocks was not statistically different between the articaine and lidocaine solutions (P = 0.43). One hundred percent of the subjects used for data analysis had subjective lip anaesthesia with either the articaine and lidocaine solutions" Comment: patients excluded were accounted for and used for calculation of overall failure |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Cohen 1993.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: endodontic specialist practice (United States of America) Participants
Proportion of male and female patients, age and initial pain not reported Quote (from correspondence): "We did not record age or sex for the purposes of the study. The overwhelming number of our patients are adults past school age" Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during pulpotomy in teeth with irreversible pulpitis
Pulpal anaesthesia tested with dichlorodifluoromethane
Teeth tested: mandibular molars Soft tissue anaesthesia on questioning (61/61)
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly, 27 subjects were injected with 2% lidocaine HCI with 1:100,000 epinephrine and 34 subjects were injected with 3% mepivacaine HCI with no vasoconstrictor" Quote (from correspondence): "Forty sealed envelopes for each of the two treatment modalities were prepared. At each case an envelope was opened. Thus the treatment choice was decided by lottery" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomly, 27 subjects were injected with 2% lidocaine HCI with 1:100,000 epinephrine and 34 subjects were injected with 3% mepivacaine HCI with no vasoconstrictor" Quote (from correspondence): "Forty sealed envelopes for each of the two treatment modalities were prepared. At each case an envelope was opened. Thus the treatment choice was decided by lottery" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "There was no blinding. Since we were following our normal protocol for treatment of emergencies in our office, the patients were not informed that we were involved in a study" Comment: despite no attempt to blind the local anaesthetic cartridges, risk of bias was graded as low, as identification of the local anaesthetic by participants is unlikely. Also, a pre‐determined method of administration was used by personnel, which minimized variation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (from correspondence): "There was no blinding. Since we were following our normal protocol for treatment of emergencies in our office, the patients were not informed that we were involved in a study" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by the local anaesthetic administrator. Identification of the local anaesthetic by participants is unlikely, and whether the clinician recording the outcomes influenced patients is not clear. Therefore risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Colombini 2006.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 20 enrolled, 20 completing the study. Mean age 23.1 years, ranging from 18 to 37 years. 13 male, 7 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) and local infiltration (0.9 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during impacted lower third molar removal (40/40)
Teeth tested: mandibular third molars Soft tissue anaesthesia (loss of sensibility of the soft tissues)
Soft tissues tested: inferior lip, tongue, and mucosa Adverse effects were reported (40/40) |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For local anaesthesia, in the first appointment the patients were randomly selected to receive either 2% mepivacaine or 4% articaine (both with 1:100,000 epinephrine). In the second appointment, the local anaesthetic not used previously was then administered in a crossed manner" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "For local anaesthesia, in the first appointment the patients were randomly selected to receive either 2% mepivacaine or 4% articaine (both with 1:100,000 epinephrine). In the second appointment, the local anaesthetic not used previously was then administered in a crossed manner" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a double‐blind study; neither the surgeon nor the patients were aware of the local anaesthetic being tested at the 2 different appointments" Comment: no details of the blinding method were reported, and it is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This was a double‐blind study; neither the surgeon nor the patients were aware of the local anaesthetic being tested at the 2 different appointments" Comment: no details of the blinding method were reported, and it is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, who may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Costa 2005.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 20 enrolled, 20 completing the study. Ages ranging from 18 to 31 years. 5 male, 15 female Inclusion criteria: healthy individuals with 3 maxillary posterior teeth on the same side with initial stage occlusal caries or indication for occlusal sealant Exclusion criteria: none |
|
| Interventions | Maxillary buccal infiltration (of 1.8 mL) of each of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
This was performed while patients were having restorative dentistry treatment of low capacity Teeth tested: maxillary posterior teeth |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The tooth that would be treated randomly received 1.8 ml of one of three local anaesthetics" Quote (from correspondence): "Three cartridges for local anaesthetic (2% lidocaine with 1:100.000 epinephrine; 4% articaine with 1:200.000 epinephrine and 4% articaine with 1:100.000 epinephrine) were placed in 20 sealed envelopes, so we had one envelope for each patient. The anaesthetic was always administered by the same researcher, who placed the hand inside the envelope and randomly chose one cartridge to be used in each session, leaving the remaining cartridges inside the envelope to be used in the next sessions, until the last application of sealant in the last tooth. The tooth where the sealant was going to be applied in each appointment was also chosen randomly" |
| Allocation concealment (selection bias) | Low risk | Quote: "The tooth that would be treated randomly received 1.8 ml of one of three local anaesthetics" Quote (from correspondence): "Three cartridges for local anaesthetic (2% lidocaine with 1:100.000 epinephrine; 4% articaine with 1:200.000 epinephrine and 4% articaine with 1:100.000 epinephrine) were placed in 20 sealed envelopes, so we had one envelope for each patient. The anaesthetic was always administered by the same researcher, who placed the hand inside the envelope and randomly chose one cartridge to be used in each session, leaving the remaining cartridges inside the envelope to be used in the next sessions, until the last application of sealant in the last tooth. The tooth where the sealant was going to be applied in each appointment was also chosen randomly" Quote (from correspondence): “In the Costa research where there were 3 cartridges inside the envelopes, some masking tape was put around the cartridges after they were used in order to identify appointment 1,2, or 3, and they were transferred to another envelope that had the number of the patient. These envelopes were only opened at the end of the experiments by a third researcher, too. The ink was removed with 70% alcohol and thus could see the identification of articaine solution with 1:100,000 or 1:200,000 epinephrine. The lidocaine solution presented rubber different colour (orange)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "In this case, only one cartridge per appointment was used and the infiltration injection delivered by myself in all cases. All cartridges were masked and in every experiment I chose one randomly from an envelope before using it to administer the injection to that patient. After that, I left the workstation and immediately after the researcher (Costa) would enter to apply the electric tests and sealant" Comment: identification of the local anaesthetic by participants and administrator was not possible, and a pre‐determined method for administration was used by personnel, which minimized variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "In this case, only one cartridge per appointment was used and the infiltration injection delivered by myself in all cases. All cartridges were masked and in every experiment I chose one randomly from an envelope before using it to administer the injection to that patient. After that, I left the workstation and immediately after the researcher (Costa) would enter to apply the electric tests and sealant" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete (confirmed by study author) |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete (confirmed by study author) |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Dagher 1997.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Lebanon) Participants: 30 enrolled, 30 completing the study. Mean age 32 years, range 22 to 50 years. 22 male, 8 female Inclusion criteria
Exclusion criteria: none stated |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of each of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molar, first premolar, and lateral incisor Soft tissue anaesthesia (feeling of numbness/response to mucosal sticks)
Soft tissues tested: labial and lingual to the premolar and buccal to the first molar |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequence of solution administration was determined randomly" Comment: exact method of generation of randomized sequence not stated Quote (from correspondence: conversation with author, P. Machtou): "Randomization sequence was generated from random number tables" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The sequence of solution administration was determined randomly" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All of the injections were given blindly by one operator" Comment: despite no details of the blinding method, risk of bias was graded as low, as identification of the local anaesthetic by participants is unlikely. Also, a pre‐determined method for administration was used by personnel, which minimized variation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All preinjection and post‐injection tests were done by a trained person who was blinded to the solutions injected" Comment: no details of the blinding method were reported, and it is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, who may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Donaldson 1987.
| Methods | Randomized controlled simulated scenario trial (treatment carried out but anaesthesia determined with a pulp tester), cross‐over study design | |
| Participants |
Location: university (Canada) Participants: 81 enrolled, 71 completing the study. Mean age 20.91 years. 23 male, 48 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) or maxillary infiltration (0.6 mL) of 1 of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: not stated |
|
| Notes | Industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized into two groups" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized into two groups" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Cartridges were blinded so that neither the patient nor the investigator was aware of which product was being given (Fig. 3)" Comment: a photograph of the coded cartridge is shown in the journal article, which would prevent participants, personnel, and outcome assessors from identifying the local anaesthetic used. Risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Cartridges were blinded so that neither the patient nor the investigator was aware of which product was being given (Fig. 3)" Comment: a photograph of the coded cartridge is shown in the journal article, which would prevent participants, personnel, and outcome assessors from identifying the local anaesthetic used. Risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: of 142 possible episodes of anaesthesia, onset was measured only in those with successful pulpal anaesthesia (134 times). Therefore, 3% (1/38) of prilocaine infiltrations, 6% (2/33) of prilocaine IANBs, 5% (2/38) of articaine infiltrations, and 9% (3/33) of articaine IANBs were not measured. Attrition bias was graded as low risk, as losses were balanced across groups and for the same reasons |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Comment: Study was supported by Astra Pharmaceuticals |
Elbay 2016.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Turkey) Participants: 60 enrolled, 60 completing the study Pulpotomy:
Extraction:
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (0.9 mL) of each of the following:
|
|
| Outcomes |
Clinical anaesthesia during extraction or pulpotomy
These were recorded for:
Teeth tested: mandibular primary molars Soft tissue anaesthesia
Soft tissues tested: relevant soft tissues (success) and lower lip and soft tissues (duration) Adverse events reported (120/120)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The local anesthetic used in a patient at the first appointment was randomly selected using a computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The local anesthetic used in a patient at the first appointment was randomly selected using a computer‐generated list" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A dental assistant put the anesthetic solution in the device, so both the practitioner and the rater were blinded to the local anesthetic solution being tested" Comment: participants and personnel would not be able to identify the local anaesthetic used. Risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A dental assistant put the anesthetic solution in the device, so both the practitioner and the rater were blinded to the local anesthetic solution being tested" "A single practitioner who had 6 months of experience using the CCDS performed all injections and operations and a single rater who was not the practitioner evaluated the anesthetic solutions" Comment: outcomes were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants who had duration measured was not reported. This was probably measured by participants at home, but it is not clear whether all participants provided data. Therefore, attrition bias is graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Epstein 1965.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: hospital (United States of America) Participants: 420 enrolled, 420 completing the study (277 without 3% prilocaine, 1:300,000 epinephrine). Mean age 33 years, range 10 to 75 years. 128 male, 255 female Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Maxillary buccal infiltration (1.2 mL) and inferior alveolar nerve block (1.5 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during extraction (18/18), restorative dentistry (246/246) or other procedures (13/13) (total = 277/277)
Teeth tested: various (individual teeth not stated) Soft tissue anaesthesia
Soft tissues tested: lower lip and adjacent hard/soft tissues Adverse effects were reported (278/278?) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The solutions were distributed in a completely randomized sequence" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The solutions were distributed in a completely randomized sequence" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In the present study, the anaesthetic cartridges were coded by the manufacturer. A sealed copy of the code was provided to the investigator" Comment: participants and personnel would not be able to identify the local anaesthetic used. Risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In the present study, the anaesthetic cartridges were coded by the manufacturer. A sealed copy of the code was provided to the investigator" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording the outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Comment: of the total number of participants recruited who had complete, completely worn off, or partial anaesthesia, when soft tissue anaesthesia may occur, some did not have duration of soft tissue anaesthesia measured: Inferior alveolar nerve block
Infiltration
For duration of soft tissue anaesthesia, the dropout rate could be calculated only if those having soft tissue success were known. No dropouts would occur if the number of participants having duration measured was equal to the number having soft tissue anaesthetic success. Soft tissue anaesthesia may have been present in those who had failure of anaesthesia during treatment, or may have been absent, meaning it was not measured. However, even with these difficulties in measuring attrition rate, dropout rates of up to 41% were seen. Therefore attrition bias has been graded as high risk |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: the total number of injections administered is mentioned throughout the journal article (277 for the solutions commercially available). However in Table 9, which presents data related to adverse events, the total is 278, which is possibly due to a typographical error. However, all patients appear to have been assessed; therefore risk was graded as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Epstein 1969.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: hospital (United States of America) Participants: 816 enrolled, 816 completing the study (610 participants, not including the 4% prilocaine, 1:300,000 epinephrine group). Median age 32 years. 272 male, 544 female Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Maxillary buccal infiltration (average = 1.2 mL) and inferior alveolar nerve block (average = 1.4 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during extraction or restorative dentistry, including restorations, endodontic and periodontal procedures (610/610)
Teeth tested: various (individual teeth not stated) Soft tissue anaesthesia
Soft tissues tested: relevant soft tissues Adverse effects were reported (599/610) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eight hundred and sixteen injections were administered from single‐coded cartridges, about equally divided among the four solutions in randomized sequence" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eight hundred and sixteen injections were administered from single‐coded cartridges, about equally divided among the four solutions in randomized sequence" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthetic cartridges were coded by the manufacturer, and a sealed copy of the code was provided" Comment: participants and personnel would not be able to identify the local anaesthetic used. Risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The anaesthetic cartridges were coded by the manufacturer, and a sealed copy of the code was provided" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Comment: of the total number of participants recruited who had complete, completely worn off, or partial anaesthesia, when soft tissue anaesthesia may occur, some did not have duration of soft tissue anaesthesia measured Inferior alveolar nerve block
Infiltration
For duration of soft tissue anaesthesia, the dropout rate could be calculated only if those having soft tissue success were known. No dropouts would occur if the number of participants having duration measured was equal to the number having soft tissue anaesthetic success. Soft tissue anaesthesia may have been present in those who had failure of anaesthesia during treatment, or may have been absent, meaning it was not measured. However, even with these difficulties in measuring attrition rate, dropout rates of up to 51% were seen. Therefore attrition bias has been graded as high risk |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Dropouts were few and occurred in similar numbers over all groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Evans 2008.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 80 enrolled, 80 completing the study Lateral incisor:
First molar:
Inclusion criteria: All participants were in good health and were not taking any medication that would alter pain perception Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.8 mL) of each of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary lateral incisors and first molars Adverse effects were reported (lateral incisors and first molars: 80/80)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The two anaesthetic solutions were randomly assigned six‐digit numbers from a random number table. The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Each solution had a six‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "The two anaesthetic solutions were randomly assigned six‐digit numbers from a random number table. The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The lidocaine and articaine cartridges were masked with opaque labels, and the cartridge caps and plungers were masked with a black felt‐tip marker. The corresponding six‐digit codes were written on each cartridge label" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The lidocaine and articaine cartridges were masked with opaque labels, and the cartridge caps and plungers were masked with a black felt‐tip marker. The corresponding six‐digit codes were written on each cartridge label" "Trained personnel who were blinded to the anaesthetic solutions administered all preinjection and post‐injection tests" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 60 occasions (for those experiencing successful anaesthesia: 29 cases of lidocaine, 31 cases of articaine). As numbers assessed were balanced across groups, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Fernandez 2005.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 39 enrolled, 39 completing the study. Mean age 24 years, ranging from 20 to 30 years. 26 male, 13 female Inclusion criteria: Participants were in good health and were not taking any medications that would alter their perception of pain Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of each of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular lateral incisor, first premolar, second premolar, first molar, second molar Soft tissue anaesthesia tested by pinching/palpating lip + completing post‐injection questionnaire
Soft tissues tested: lower lip |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The two anaesthetic solutions were randomly assigned six digit numbers from a random number table. Each subject was randomly assigned to one of the two solutions to determine which anaesthetic solution was to be administered at each appointment" "Forty IAN block injections were administered on the right side and 38 injections were administered on the left side. The same side randomly chosen for the first injection was used again for the second injection" Quote (from correspondence): "Each solution had a six‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "The two anaesthetic solutions were randomly assigned six digit numbers from a random number table. Each subject was randomly assigned to one of the two solutions to determine which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Masking the appropriate cartridges with opaque tape, which were labelled with the six‐digit numbers, blinded the anaesthetic solutions administered" Comment: Participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the random numbers were recorded on the data collection and post‐injection survey sheets to blind the experiment" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 68 occasions (for those experiencing successful pulpal anaesthesia: 36 cases of lidocaine, 32 cases of articaine). As numbers assessed were balanced across groups, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: duration of pulpal anaesthesia was tested on 68 occasions (for those experiencing successful pulpal anaesthesia: 36 cases of lidocaine, 32 cases of articaine). As numbers assessed were balanced across groups, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Fertig 1968.
| Methods | Randomized controlled clinical trial (treatment carried out but soft tissue duration determined in a simulated scenario). Study design not reported, although appears to be a parallel design from the data presented | |
| Participants |
Location: private practice (United States of America) Participants: 79 enrolled, 79 completing the study (62 excluding 4% prilocaine, 1:300,000 epinephrine). Mean age, age range, and male:female ratio not reported Inclusion criteria: none reported Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of 1 of the following:
|
|
| Outcomes |
Soft tissue anaesthesia tested by the patient reporting disappearance of anaesthesia
Soft tissues tested: soft tissues on injected side |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The solutions were randomly assigned to all patients for whom local anaesthesia was indicated for a particular endodontic procedure or for periodontic surgery" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The solutions were randomly assigned to all patients for whom local anaesthesia was indicated for a particular endodontic procedure or for periodontic surgery" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: exact method of blinding not stated Comment: despite no details of the blinding method, risk of bias was graded as low, as identification of the local anaesthetic by participants is unlikely. Also, a pre‐determined method of administration was not used by personnel, which would minimize variation. Therefore, risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: exact method of blinding not stated Comment: no details of the blinding method were reported, and it is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, who may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Forloine 2010.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 50 enrolled, 50 completing the study. Mean age 25 years, ranging from 18 to 57 years. 27 male, 23 female Inclusion criteria: Participants were in good health Exclusion criteria
|
|
| Interventions | High‐tuberosity maxillary second division nerve blocks (4.0 mL) of 1 of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary molars, premolars, canines, lateral incisors, and central incisors Soft tissue anaesthesia (participants questioned regarding subjective numbness)
Soft tissues tested: lip, side of nose, and lower eyelid Adverse effects were reported (100/100)
|
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, the 2 anaesthetic formulations were randomly assigned 5‐digit numbers from a random number table. Each subject was randomly assigned to the right or left side. The order of the anaesthetic solutions was also randomly assigned to determine which solutions were to be administered at each appointment" Quote (from correspondence): "Each solution had a five‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the experiment, the 2 anaesthetic formulations were randomly assigned 5‐digit numbers from a random number table. Each subject was randomly assigned to the right or left side. The order of the anaesthetic solutions was also randomly assigned to determine which solutions were to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each syringe was masked with an opaque label, and the corresponding 5‐digit code was written on each label" "Only the random numbers were recorded on the data collection and post‐injection survey sheets to blind the experiment" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the random numbers were recorded on the data collection and post‐injection survey sheets to blind the experiment" "Trained personnel who were blinded to the type of anaesthetic solution used performed all preinjection and post‐injection tests" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Quote: "If the subject did not obtain any signs of subjective anaesthesia after 20 minutes, the block was considered a failure, and the subject was dismissed and reappointed for 1 week later" "Twelve percent (6 of 50) of the subjects did not achieve soft issue anaesthesia within 20 minutes of the injection but did achieve soft tissue anaesthesia at a subsequent appointment. Five subjects (3 lidocaine and 2 mepivacaine) were eliminated from the study because they did not attain soft tissue anaesthesia after 2 attempts. Five additional subjects were recruited to replace these subjects" Comment: two attempts were made to anaesthetize some participants, and additional participants were recruited when a second attempt to anaesthetize them also failed. It was not possible to re‐calculate success accounting for these participants. However, the numbers involved were small compared with total group sizes, and those eliminated were well balanced across groups. Therefore risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 92 occasions (for those experiencing successful anaesthesia: 46 cases of lidocaine, 46 cases of mepivacaine). As numbers assessed were equal across groups, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Gangarosa 1967.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: hospital/private practice (United States of America) Participants: 542 enrolled, 542 completing the study? Mean age, age range, and male:female ratio not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Mandibular block and infiltration (volume not stated) of each of the following:
|
|
| Outcomes |
Clinical anaesthesia during various general practice, oral surgery, and periodontal procedures
Teeth tested: not reported Soft tissue anaesthesia
Soft tissues tested: not reported Adverse effects were reported (number assessed not clear) |
|
| Notes | Industry and non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each cartridge of anaesthetic was supplied in a randomly numbered coin‐envelope" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each cartridge of anaesthetic was supplied in a randomly numbered coin‐envelope" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthetics were kindly supplied in blinded cartridges by Astra Pharmaceuticals, Inc" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The anaesthetics were kindly supplied in blinded cartridges by Astra Pharmaceuticals, Inc" Comment: limited details of the blinding method were reported, and it is not clear whether the person recording participant outcomes was a different person from the one administering the local anaesthetic, who may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Unclear risk | Comment: total number of participants is not the same as those in Figures 1 and 2 attached to the graphs in the journal article. Therefore some participants may have been excluded, but this is not clear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Comment: the number of participants who had onset of soft tissue anaesthesia measured was not stated; therefore risk of bias was rated as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants who had duration of soft tissue anaesthesia measured was not stated; therefore risk of attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: the number of participants who had adverse events measured was not stated; therefore risk of attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Quote: "The anaesthetics were kindly supplied in blinded cartridges by Astra Pharmaceuticals, Inc" |
Gazal 2015.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Saudi Arabia) Participants: 25 enrolled, 23 completing the study. Mean age 29.9 years, ranging from 17 to 60 years. 25 male, 0 female (determined following correspondence) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) of 2% mepivacaine, 1:100,000 epinephrine, followed by mandibular buccal infiltration (1.8 mL) of 1 of the following solutions:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was achieved by an independent researcher (KHA)" Quote (from correspondence): "For allocation of the participants, a computer‐generated list of random numbers was used by the study coordinator, who was not involved in the treatments or assessments" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was achieved by an independent researcher (KHA)" Quote (from correspondence): "The treatment alternative was placed in envelopes, numbered in accordance with the randomization list and concealed. An independent dental assistant consequently revealed the allocation and made preparation for local anesthetic injection" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both volunteers and the researcher testing anesthetic effectiveness (American Medical Association) were not aware to which local anesthetic buccal infiltration regimen was administered" Quote (from correspondence): "The local anesthetic cartilages were covered with opaque stickers to hide the type of local anesthetic which will be used. Dental Surgeon and assessors involved in treatment were blinded to which type of local anesthetic the patient was allocated" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both volunteers and the researcher testing anesthetic effectiveness (American Medical Association) were not aware to which local anesthetic buccal infiltration regimen, was administered" Quote (from correspondence): "The local anesthetic cartilages were covered with opaque stickers to hide the type of local anesthetic which will be used. Dental Surgeon and assessors involved in treatment were blinded to which type of local anesthetic the patient was allocated" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Quote: "Two volunteers were excluded due to faint following first local anesthetic IANB injection (one volunteer from mepivacaine regimen and one from articaine regimen) and were excluded consequently according to study protocol and official clearances" Comment: patients excluded were accounted for, were used for calculation of pulp anaesthesia success, and were balanced across groups |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Quote: "Two volunteers were excluded due to faint following first local anesthetic IANB injection (one volunteer from mepivacaine regimen and one from articaine regimen) and were excluded consequently according to study protocol and official clearances" Comment: patients excluded were accounted for, were few, and were balanced across groups |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Quote: "Two volunteers were excluded due to faint following first local anesthetic IANB injection (one volunteer from mepivacaine regimen and one from articaine regimen) and were excluded consequently according to study protocol and official clearances" Comment: patients excluded were accounted for, were few, and were balanced across groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Gazal 2017.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Saudi Arabia) Participants: 94 enrolled, 90 completing the study. Age ranging from 16 to 70 years. All participants were male Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.4 mL) and palatal infiltration (0.4 mL) using the following:
|
|
| Outcomes |
Clinical anaesthesia during extraction of teeth
Teeth tested: various maxillary teeth Soft tissue anaesthesia
Soft tissues tested: adjacent soft tissues in the maxilla Adverse effects were reported (90/90)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Prior to the study, a researcher allocated the sequence of patient identity numbers to either the test or control group" Comment: detailed methods not reported. Following contact with study author, it was confirmed that "Slips of paper with test group or control group were placed in opaque envelopes and sealed. This was done by a secretary who was not associated with the study" Envelopes were then randomly chosen and allocated to each patient by the main study author |
| Allocation concealment (selection bias) | Low risk | Quote: "Slips of paper with 4% articaine (test group) or 2% mepivacaine (control group) were placed in opaque envelopes and sealed by a secretary who was not associated with the study. These envelopes had been numbered sequentially on their outside with the patient identity number and were attached to the patient's dental hospital treatment record" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both patients and the researcher testing anesthetic effectiveness were not aware to which local anesthetic BI regimen was administered" Comment: detailed methods not reported. Following contact with the study author, it was determined that the cartridges were masked and the syringe was loaded by a dental assistant. Participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both patients and the researcher testing anesthetic effectiveness were not aware to which local anesthetic BI regimen was administered" Comment: detailed methods not reported. Following contact with the study author, it was confirmed that the assessor was not present when the injections were administered. In addition, the cartridges were masked. Outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Gregorio 2008.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 50 enrolled, 50 completing the study. Mean age 21.84 ± 0.65 years, ranging from 18 to 35 years. 21 male, 29 female Inclusion criteria: good health and not taking any medication that would alter pain perception Exclusion criteria: references given for eligibility/exclusion criteria within the study |
|
| Interventions | Inferior alveolar nerve block (1.8 mL) and local infiltration (0.9 mL) of each of the following:
|
|
| Outcomes |
Clinical anaesthesia during surgical removal of lower third molars
Patients were divided into 2 categories:
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: inferior lip, corresponding half of the tongue, and mucosa Adverse effects were reported (100/100) |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For local anaesthesia, in the first appointment, the patients randomly received A200 or B200 solutions" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "For local anaesthesia, in the first appointment, the patients randomly received A200 or B200 solutions" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This was a double‐blind study, that is, neither the surgeon nor the patients were aware of the local anaesthetic being used at the two different appointments, since the labels of both anaesthetics were pulled off and the cartridges were coded by someone not directly involved in data collection prior to the patient visit" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This was a double‐blind study, that is, neither the surgeon nor the patients were aware of the local anaesthetic being used at the two different appointments, since the labels of both anaesthetics were pulled off and the cartridges were coded by someone not directly involved in data collection prior to the patient visit" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Gross 2007.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 65 enrolled, 65 completing the study Lateral incisor:
First molar:
Inclusion criteria: good health and not taking any medication that would alter pain perception Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.8 mL) of each of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary lateral incisors and first molars Soft tissue anaesthesia tested by palpation
Soft tissues tested: upper lip and buccal gingiva |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, the two anaesthetic solutions were randomly assigned four‐digit numbers from a random number table. The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered and which side (right or left) was to be used at each appointment" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the experiment, the two anaesthetic solutions were randomly assigned four‐digit numbers from a random number table. The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered and which side (right or left) was to be used at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each anaesthetic cartridge had its label removed and was masked with an opaque label. The random number was written on the label. Only the random numbers were recorded on the data collection sheets to further blind the experiment" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each anaesthetic cartridge had its label removed and was masked with an opaque label. The random number was written on the label. Only the random numbers were recorded on the data collection sheets to further blind the experiment" "Trained personnel, who were blinded to the anaesthetic solutions, administered all pre‐injection and post‐injection tests" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 48 occasions on first molar teeth (for those experiencing successful anaesthesia: 27 cases of lidocaine, 21 cases of bupivacaine). As numbers were reduced in both groups for the same reason and were fairly balanced across groups, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Haas 1990.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Canada) Participants: 20 enrolled, with 20 completing the study. Mean age 25 years, ranging from 22 to 32 years. Male:female ratio not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Mandibular and maxillary infiltration (1.5 mL) of each of the following:
|
|
| Outcomes |
Pulpal anaesthesia and soft tissue anaesthesia (both tested with an electric pulp tester)
Teeth tested: all maxillary and mandibular canine teeth |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was double blind, with the order of drug administration randomized" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This study was double blind, with the order of drug administration randomized" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This study was double blind, with the order of drug administration randomized" "The cartridges were covered with an adhesive paper label, leaving only a 4 mm window adjacent to the cap to allow visualization of the aspiration results, yet concealing the type of anaesthetic. The cartridge was loaded by a nurse assistant so that neither the subject nor the dentist administering the anaesthetic was aware of which preparation was being injected" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This study was double blind, with the order of drug administration randomized" "The cartridges were covered with an adhesive paper label, leaving only a 4 mm window adjacent to the cap to allow visualization of the aspiration results, yet concealing the type of anaesthetic. The cartridge was loaded by a nurse assistant so that neither the subject nor the dentist administering the anaesthetic was aware of which preparation was being injected" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants was not possible. It is not clear whether the person recording participant outcomes was blinded and was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Haas 1991.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Canada) Participants: 20 enrolled, 20 completing the study. Mean age 26 years, ranging from 23 to 41 years. Male:female ratio not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Mandibular and maxillary infiltration (1.5 mL) of each of the following:
|
|
| Outcomes |
Pulpal anaesthesia and soft tissue anaesthesia (tested with an electric pulp tester)
Teeth tested: all maxillary and mandibular second molar teeth |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was double blind, with the order of drug administration randomized" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This study was double blind, with the order of drug administration randomized" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This study was double blind, with the order of drug administration randomized" "The cartridges were covered with an adhesive paper label, leaving only a 4‐mm window adjacent to the cap to allow visualization of the aspiration results, yet concealing the type of anaesthetic. The cartridge was loaded by a nurse assistant so that neither the subject nor the dentist administering the anaesthetic was aware of which preparation was being injected" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This study was double blind, with the order of drug administration randomized" "The cartridges were covered with an adhesive paper label, leaving only a 4 mm window adjacent to the cap to allow visualization of the aspiration results, yet concealing the type of anaesthetic. The cartridge was loaded by a nurse assistant so that neither the subject nor the dentist administering the anaesthetic was aware of which preparation was being injected" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants was not possible. It is not clear whether the person recording participant outcomes was blinded and was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Haase 2008.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 73 enrolled, 73 completing the study. Mean age 27 years, ranging from 20 to 36 years. 46 male, 27 female Inclusion criteria: in good health and not taking any medications that would alter their perception of pain Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
followed by additional mandibular buccal infiltration (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars Adverse effects were reported (146/146)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, we randomly assigned to the two anaesthetic solutions six‐digit numbers from a random number table. In addition, we randomly assigned each subject to each of the two formulations to determine which anaesthetic formulation was to be administered at each appointment" Quote (from correspondence): "Each solution had a six‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the experiment, we randomly assigned to the two anaesthetic solutions six‐digit numbers from a random number table. In addition, we randomly assigned each subject to each of the two formulations to determine which anaesthetic formulation was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We recorded only the random numbers on the data collection sheets to further blind the experiment" "Research personnel masked the lidocaine and articaine cartridges with opaque labels and the cartridge caps and rubber plungers with a black felt‐tip marker. The research personnel wrote the corresponding six‐digit codes on each cartridge label" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "We recorded only the random numbers on the data collection sheets to further blind the experiment" "Research personnel masked the lidocaine and articaine cartridges with opaque labels and the cartridge caps and rubber plungers with a black felt‐tip marker. The research personnel wrote the corresponding six‐digit codes on each cartridge label" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Hellden 1974.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Sweden) Participants: 420 enrolled, 420 completing the study. 280 excluding 0.25% bupivacaine, 1:200,000 epinephrine. Mean age 26.7 ± 0.6 years (standard error). 198 male, 222 female Inclusion criteria: healthy outpatients Exclusion criteria: none reported |
|
| Interventions | Mandibular block (1.8 mL) and local infiltration (1.8 mL) of each of the following:
An additional 1.8 mL was used if supplemental anaesthesia was required |
|
| Outcomes |
Clinical anaesthesia during surgical removal of lower third molars
Teeth tested (and adjacent soft and hard tissues): mandibular third molars Soft tissue anaesthesia tested by self‐assessment
Soft tissues tested: lower lip and adjacent soft tissues Adverse effects were reported (280/280) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The test solutions were delivered as a random series of code‐marked cartridges of 1.8 mL" Comment: exact method of generation of randomized sequence not stated Quote (from correspondence): "Sorry. I cannot answer your question. The 'Bofors coordinating person' (pharmacist + statistician) was (now dead) extremely strict" |
| Allocation concealment (selection bias) | Low risk | Quote: "The test solutions were delivered as a random series of code‐marked cartridges of 1.8 ml" Comment: exact method of allocation concealment not stated Quote (from correspondence): "The nurses followed a consecutive list/table (from Bofors) telling which one of the 'code‐numbered boxes' they should 'serve' the surgeon. Thus, neither the nurse nor the surgeon had any knowledge about the type of anaesthetics that was used in the individual case" "The surgeon had to use the substance that was served" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was performed as a double blind test" "The test solutions were delivered as a random series of code‐marked cartridges of 1.8 ml. Three cartridges of each anaesthetic type were marked with the same code and corresponded to one of the patients and to one of the operators. In this way each operator treated an equal number of patients from each test group" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was performed as a double blind test" "The test solutions were delivered as a random series of code‐marked cartridges of 1.8 ml. Three cartridges of each anaesthetic type were marked with the same code and corresponded to one of the patients and to one of the operators. In this way each operator treated an equal number of patients from each test group" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the exact number of participants who had duration of soft tissue anaesthesia measured is not clear. It is likely that it would have been possible to measure this for all participants, but the compliance of participants in returning questionnaires was not mentioned in the study. Attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Hersh 1995.
| Methods | Randomized controlled simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: 60 enrolled, 60 completing the study
Inclusion criteria: had to be in good general health and to have no contraindications to local anaesthetics or vasoconstrictors Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks.(1.8 mL) of:
|
|
| Outcomes |
Soft tissue anaesthesia (visual analogue scale: 100 mm bar connecting the words "not numb" and "completely numb")
Soft tissues tested: lower lip and tongue |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study participants were randomly assigned to receive a single cartridge (1.8 mL) of 2 percent lido‐epi, 3 percent mepivacaine or 4 percent prilocaine" Quote (from correspondence): "Randomization I believe was in blocks of three" |
| Allocation concealment (selection bias) | Low risk | Quote: "Study participants were randomly assigned to receive a single cartridge (1.8 mL) of 2 percent lido‐epi, 3 percent mepivacaine or 4 percent prilocaine" Quote (from correspondence): "Randomization code broken at end of study and after all queries addressed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To maintain double‐blind conditions, we instructed a dental assistant who was not directly involved in the study to remove the product identification label from each cartridge before loading it into a syringe" Quote (from correspondence): "Label of identifying local anaesthetic removed by research assistant and replaced by code # which she kept. Person injecting and subject blinded to treatment. Randomization code broken at end of study and after all queries addressed" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To maintain double‐blind conditions, we instructed a dental assistant who was not directly involved in the study to remove the product identification label from each cartridge before loading it into a syringe" Quote (from correspondence): "Label of identifying local anaesthetic removed by research assistant and replaced by code # which she kept. Person injecting and subject blinded to treatment. Randomzation code broken at end of study and after all queries addressed" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Comment: the number of participants who had onset of anaesthesia measured was not stated; therefore risk of attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants who had duration of anaesthesia measured was not stated; therefore risk of attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Hinkley 1991.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 30 enrolled, 28 completing the study. Mean age 27 years, ranging from 23 to 42 years. 19 male, 11 female Inclusion criteria: in good health and not taking any medications that would alter pain perception Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars, first premolars, and lateral incisors Soft tissue anaesthesia (participant felt numbness upon sticking of the alveolar mucosa with a sharp explorer)
Tissues tested: lower lip, tongue, and mucosa |
|
| Notes | Non‐industry funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each subject randomly received each anaesthetic solution on three successive appointments spaced at least 1 week apart" "The subjects were randomly assigned to one of six letter (ABC) combinations to determine the sequence of solution administration" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "The three cartridges for each subject were placed in an autoclave bag with the numbers recorded on the outside showing the injection order" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each anaesthetic cartridge label was removed and masked with tape. A four‐digit random number, corresponding to the letter designation, was written on each cartridge" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All pre‐ and post‐injection tests were done by trained personnel who were blinded to the solutions injected" "Each anaesthetic cartridge label was removed and masked with tape, A four‐digit random number, corresponding to the letter designation, was written on each cartridge" Quote (from correspondence): "The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Quote: "Two of 30 subjects achieved lip numbness only after 20 min and were excluded from the data analysis. All of the remaining 28 subjects had subjective lip and tongue numbness" Comment: it was not stated which solution this was with, or whether the other 2 solutions were tested. The study author was contacted, but the identity of the solutions used for the 2 cases of failed lip anaesthesia was not known. However, as the study used a cross‐over design, the groups remained balanced. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Quote: "Two of 30 subjects achieved lip numbness only after 20 min and were excluded from the data analysis. All of the remaining 28 subjects had subjective lip and tongue numbness" Comment: it was not stated which solution this was with, or whether the other 2 solutions were tested. The study author was contacted, but the identity of the solutions used for the 2 cases of failed lip anaesthesia was not known. However, as the study used a cross‐over design, the groups remained balanced. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 44 occasions on first molar teeth (for those experiencing successful anaesthesia: 15 cases of lidocaine, 16 cases of mepivacaine, and 13 cases of prilocaine). As numbers were reduced in all groups for the same reasons and were fairly balanced across groups, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Quote: "Two of 30 subjects achieved lip numbness only after 20 min and were excluded from the data analysis. All of the remaining 28 subjects had subjective lip and tongue numbness" Comment: it was not stated which solution this was with, or whether the other 2 solutions were tested. The study author was contacted, but the identity of the solutions used for the 2 cases of failed lip anaesthesia was not known. However, as the study used a cross‐over design, the groups remained balanced. Therefore risk of bias was graded as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Hosseini 2016.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (Iran) Participants: 50 enrolled, 47 completing the study. Mean age/age range not stated. Proportion of male and female patients not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.8 mL) using the following:
|
|
| Outcomes |
Pulpal anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: maxillary first molars Adverse events reported (47/50) |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly divided into two groups of 25 patients each. In order to randomize the patients, the number of patients in each group were written on paper and kept in a sealed box. The practitioner who administrated the local anesthesia chose one of the papers and based on the number, the patient was assigned to one of the groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomly divided into two groups of 25 patients each. In order to randomize the patients, the number of patients in each group were written on paper and kept in a sealed box. The practitioner who administrated the local anesthesia chose one of the papers and based on the number, the patient was assigned to one of the groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A trained dental assistant loaded the local anesthetic solutions in masked disposable syringes and coded them (three digit alpha‐numeric) for treatment sequence" "To ensure blinding, neither the operator nor the assistant had knowledge of the solution tested" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A trained dental assistant loaded the local anesthetic solutions in masked disposable syringes and coded them (three digit alpha‐numeric) for treatment sequence" "To ensure blinding, neither the operator nor the assistant had knowledge of the solution tested" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: only patients for whom a different, definitive diagnosis was determined during treatment were excluded (23 assessed in the lidocaine group (1 pulp was not exposed, another pulp was necrotic) and 24 assessed in the articaine group (pulp not exposed in 1 case)). As numbers were reduced in both groups for similar reasons and were fairly balanced across groups, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: only patients for whom a different, definitive diagnosis was determined during treatment were excluded (23 assessed in the lidocaine group (1 pulp was not exposed, another pulp was necrotic) and 24 assessed in the articaine group (pulp not exposed in 1 case)). As numbers were reduced in both groups for similar reasons and were fairly balanced across groups, risk of attrition bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Jaber 2010.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United Kingdom) Participants: 31 enrolled, 31 completing the study. Mean age 24.4 years, standard deviation 4.4 years. 11 male, 20 female Inclusion criteria: healthy adult volunteers 18 years of age and older Exclusion criteria
|
|
| Interventions | Injections were given as:
of the following
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular central incisor and contralateral mandibular lateral incisor Adverse effects reported (62/62)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Local anaesthetic regimens were applied in randomized order determined by a web‐based program (http://department.obg.cuhk.edu.hk/researchsupport/random_integer.asp)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Local anaesthetic regimens were applied in randomized order determined by a web‐based program (http://department.obg.cuhk.edu.hk/researchsupport/random_integer.asp)" Quote (from correspondence): "The researcher recording the outcome measures who also did the data analyses was blinded till the last data collection – he was given the code after completion of data collection" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Dummy injections were administered to blind the volunteers to the method of anaesthesia used" Comment (from correspondence): there was no blinding for participants and personnel to the type of local anaesthetic used Comment: despite no blinding of participants and personnel administering the local anaesthetic, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore, risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Efficacy of anaesthesia was determined by electronic pulp testing (Analytic Technology) by an investigator blinded to the injections administered" Quote (from correspondence): "The researcher recording the outcome measures who also did the data analyses was blinded till the last data collection – he was given the code after completion of data collection" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Unclear risk | Comment: exact number of participants having onset of pulpal anaesthesia measured was not stated. Data were not used in meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Jain 2016.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (India) Participants: 70 enrolled, 70 completing the study. Age ranging from 18 to 45 years. Proportion of male and female patients not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block and buccal infiltration (1.7 mL in total) using the following:
|
|
| Outcomes |
Clinical anaesthesia during surgical removal of mandibular third molars
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: inferior lip, corresponding half of the tongue, and buccal mucosa Adverse effects were reported
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly administered one of the two local anesthetics" Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly administered one of the two local anesthetics" Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The anesthetic used was unknown for the patient and the observer who performed the measurements" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation, or if they were blinded. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The anesthetic used was unknown for the patient and the observer who performed the measurements" Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (patient‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Kalia 2011.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (India) Participants: 100 enrolled, 100 completing the study. Mean age/age range not stated. 51 male, 49 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks, inferior alveolar nerve blocks and long buccal nerve blocks, infraorbital and greater palatine nerve blocks (volumes not stated) using the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: various pairs of mandibular and maxillary teeth Soft tissue anaesthesia
Soft tissues tested: lip, buccal mucosa, tongue and palate (subjective), and attached gingiva, 7 mm from gingival margin (objective) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "100 individuals participated in this single centre, randomized, controlled, single blind, single operator, cross over study design" Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "100 individuals participated in this single centre, randomized, controlled, single blind, single operator, cross over study design" Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "100 individuals participated in this single centre, randomized, controlled, single blind, single operator, cross over study design" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method for administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "100 individuals participated in this single centre, randomized, controlled, single blind, single operator, cross over study design" Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia tested on 172 occasions on teeth (for those experiencing successful anaesthesia: 86 cases of lidocaine, 86 cases of articaine). As numbers were reduced in both groups for the same reasons and are exactly balanced across groups, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Kambalimath 2013.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (India) Participants: 38 enrolled, 30 completing the study. Mean age 25.8 years, ranging from 18 to 48 years. 13 male, 17 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block and buccal infiltration (volume not stated) using the following:
|
|
| Outcomes |
Clinical anaesthesia during surgical removal of mandibular third molars
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lower lip and adjacent soft tissues Adverse effects were reported (60/76)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For local anesthesia, in the first appointment the patients were randomly selected to receive either 2 % lidocaine (Lignospan, Indore, India) or 4% Articaine (Articaine 4% Septanest, Indore, India) both with 1:100,000 epinephrine. In the second appointment, the local anesthetic not used previously was then administered in a crossed manner" Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "For local anesthesia, in the first appointment the patients were randomly selected to receive either 2 % lidocaine (Lignospan, Indore, India) or 4% Articaine (Articaine 4% Septanest, Indore, India) both with 1:100,000 epinephrine. In the second appointment, the local anesthetic not used previously was then administered in a crossed manner" Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a double‐blind study; neither the surgeon nor the patients were aware of the local anesthetic being tested at the two different appointments" Comment: detailed methods not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation, or if they were blinded. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This was a double‐blind study; neither the surgeon nor the patients were aware of the local anesthetic being tested at the two different appointments" Comment: detailed methods not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: originally 38 patients were scheduled for treatment, but 8 were withdrawn (1 owing to transient inferior alveolar nerve paraesthesia, 1 because of transient paraesthesia of the lingual nerve, and 6 because of voluntary dropout from the study). Because the study had a cross‐over design, the reduction in numbers across groups and reasons for reduction were identical. Therefore risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: originally 38 patients were scheduled for treatment, but 8 were withdrawn (1 owing to transient inferior alveolar nerve paraesthesia, 1 because of transient paraesthesia of the lingual nerve, and 6 because of voluntary dropout from the study). Because the study had a cross‐over design, the reduction in numbers across groups and reasons for the reduction were identical. Therefore risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: originally 38 patients were scheduled for treatment, but 8 were withdrawn (1 owing to transient inferior alveolar nerve paraesthesia, 1 because of transient paraesthesia of the lingual nerve, and 6 because of voluntary dropout from the study). Because the study had a cross‐over design, the reduction in numbers across groups and the reasons for reduction were identical. Therefore risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: originally 38 patients were scheduled for treatment, but 8 were withdrawn (1 owing to transient inferior alveolar nerve paraesthesia, 1 because of transient paraesthesia of the lingual nerve, and 6 because of voluntary dropout from the study). Because the study had a cross‐over design, the reduction in numbers across groups and the reasons for reduction were identical. Therefore risk of attrition bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Kammerer 2012.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Moldova) Participants: 88 enrolled, 88 completing the study. Mean age 36.7 years, ranging from 18 to 80 years. 43 male, 45 female Inclusion criteria: all who required single tooth extractions in the mandibular arch Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks and additional buccal nerve blocks using a variable amount (2.2 mL was available in each syringe) of:
|
|
| Outcomes |
Clinical anaesthesia during extraction of mandibular posterior teeth (88/88)
Teeth tested: mandibular posterior teeth Soft tissue anaesthesia
Soft tissues tested: vestibular mucosa and oral gingivae Adverse effects were reported (88/88)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Equal randomization was achieved with the use of a computer‐generated random number list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Equal randomization was achieved with the use of a computer‐generated random number list" "A dental nurse gave the different solutions in identical syringes (2 mL) marked with the patient’s randomization number only. The blinding was rendered when evaluating the data. The same LA was used in second and repeated injections" Quote (from correspondence): "The list was organized by a nurse only. It was not shown to any clinician. She chose the solution and gave it to the assistant helping the respective dentist" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A dental nurse gave the different solutions in identical syringes (2 mL) marked with the patient’s randomization number only. The blinding was rendered when evaluating the data. The same LA was used in second and repeated injections" Comment: participants and personnel would not be able to identify the local anaesthetic used. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A dental nurse gave the different solutions in identical syringes (2 mL) marked with the patient’s randomization number only. The blinding was rendered when evaluating the data. The same LA was used in second and repeated injections" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: duration of soft tissue anaesthesia was tested on 70 occasions (for those experiencing successful anaesthesia: 34 cases of 4% articaine, 1:100,000 epinephrine and 36 cases of 4% articaine, no vasoconstrictor). Because the reduction in numbers across groups was well balanced and reasons were identical, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Kammerer 2014.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Germany) Participants: 10 enrolled, 10 completing the study. Mean age 30 years ranging from 24 to 34 years. 10 men and 0 women Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.7 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: right maxillary central incisors Soft tissue anaesthesia
Soft tissues tested: gingivae around each tooth Adverse effects reported (40/40)
|
|
| Notes | No funding reported. One of the study authors is a member of the scientific advisory board of the local anaesthetic manufacturer, 3M ESPE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: detailed method not reported Quote (from correspondence): "For randomization, the old program 'Clinstat' was used (MS‐DOS). The injections were carried out as indicated by the program" |
| Allocation concealment (selection bias) | Low risk | Comment: detailed method not reported Quote (from correspondence): "For randomization, the old program 'Clinstat' was used (MS‐DOS). The injections were carried out as indicated by the program" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All solutions were supplied by 3M ESPE (Seefeld, Germany) and delivered in similar coded glass carpules containing 1.7 ml colorless fluid" "In order to obtain a double‐blinded design, the code on the carpule was noted for each injection and unblinded after the whole study was completed" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All solutions were supplied by 3M ESPE (Seefeld, Germany) and delivered in similar coded glass carpules containing 1.7 ml colorless fluid" "In order to obtain a double‐blinded design, the code on the carpule was noted for each injection and unblinded after the whole study was completed" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group (4% articaine, 1:100,000 epinephrine vs 4% articaine, 1:200,000 epinephrine). No patients were excluded. Outcome data were complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: duration of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group (4% articaine, 1:100,000 epinephrine vs 4% articaine, 1:200,000 epinephrine). No patients were excluded. Outcome data were complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset (2) | High risk | Comment: onset of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group except 4% articaine, no vasoconstrictor, when only 4/10 were measured (those who achieved anaesthetic success). Risk of bias was rated as high owing to differences in numbers and small numbers measured |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration (2) | High risk | Comment: duration of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group except 4% articaine, no vasoconstrictor, when only 4/10 were measured (those who achieved anaesthetic success). Risk of bias was rated as high owing to differences in numbers and small numbers measured |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Comment: one of the study authors is a member of the scientific advisory board of the local anaesthetic manufacturer, 3M ESPE |
Kanaa 2006.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United Kingdom) Participants: 31 enrolled, 31 completing the study. Mean age 22.8 years, ranging from 20 to 30 years of age; standard deviation 2.1 years. 15 male, 16 female Inclusion criteria: healthy adult volunteers between 20 and 30 years of age Exclusion criteria: none reported |
|
| Interventions | Mandibular buccal infiltration (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars Soft tissue anaesthesia
Soft tissues tested: lower lip and lingual mucosa Adverse effects reported (62/62)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was determined using a computer‐generated sequence of random numbers by one of the authors who was not involved in delivering the local anaesthetic" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was determined using a computer‐generated sequence of random numbers by one of the authors who was not involved in delivering the local anaesthetic" Quote (from correspondence): "The researcher recording the outcome measures who also did the data analyses was blinded till the last data collection – he was given the code after completion of data collection" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigator who enrolled the volunteers was blinded to the order of injection" "Both the volunteer and the investigator of anaesthetic efficacy were blinded to the drug being used" Quote (from correspondence): "Volunteers always had the same type of injection and did not see the solution. Administrator was not blinded" Comment: despite no blinding of the local anaesthetic administrator, identification of the local anaesthetic by participants was unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both the volunteer and the investigator of anaesthetic efficacy were blinded to the drug being used" Quote (from correspondence): "The outcome measurer was not in the room during LA administration and was blinded (did not get the code broken till study completed)" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Kanaa 2012.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United Kingdom) Participants: 100 enrolled, 73 completing the study. Mean age 33.4 years, ranging from 16 to 62 years of age. Standard deviation 10.6 years. 66 male, 34 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (2.0 mL) of the following:
Patients for extraction received a supplementary palatal injection of 0.2 mL 2% lidocaine, 1:80,000 epinephrine |
|
| Outcomes |
Clinical anaesthesia during extraction or pulp extirpation
Tissues tested: pulp (+ bone and gingivae in the case of extractions) Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary teeth Adverse effects reported (100/100)
|
|
| Notes | No funding reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of drug allocation was determined by a web‐based program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization codes were held by researchers (JGM and JMW) who were responsible for syringe preparation but had no involvement in drug administration or in assessing outcomes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Blinding of drugs was achieved by drawing local anaesthetic solutions from their 2.2‐mL cartridges into coded 2.5 mL sterile standard syringes" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinding of drugs was achieved by drawing local anaesthetic solutions from their 2.2‐mL cartridges into coded 2.5 mL sterile standard syringes" "Randomization codes were held by researchers (JGM and JMW) who were responsible for syringe preparation but had no involvement in drug administration or in assessing outcomes" Comment: Outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Quote: "Patients who did not secure successful pulpal anaesthesia within 10 minutes were withdrawn from the trial, categorized as failure of pulp anaesthesia, and managed according to the local best clinical practice, with further supplementary injections as needed" Comment: participants who were excluded were accounted for, which allowed overall failure to be calculated |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 73 occasions (for those experiencing successful anaesthesia: 38 cases of 4% articaine, 1:100,000 epinephrine and 35 cases of lidocaine, 1:80,000 epinephrine). Because the reduction in numbers across groups was well balanced and the reasons identical, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Karm 2017.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Republic of Korea) Participants: 65 enrolled, 51 completing the study. Mean age 24.1 ± 5.0 (SD) years. 34 male, 31 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block and buccal infiltration (1.8 mL in total) using the following:
|
|
| Outcomes |
Clinical anaesthesia (success) during surgical removal of mandibular third molars
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lower lip, corresponding half of the tongue, and mucosa Other adverse events (102/102)
|
|
| Notes | Industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The statistician randomly assigned the participants using the block randomization method with SAS (SAS Institute, Cary, NC)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The statistician delivered a list of random assignment codes to the pharmacy packager" Comment: No details were given of where the key to the coding was stored Quote (from correspondence): "An independent statistician generated random codes and provided them to the factory of Huons company. The company's random assignment officer removed the labels from both products and labeled them the same while keeping a thorough secret. Random numbers and information needed for clinical trials were written on the label. Boxed and provided to research institutions (hospitals).The research institute provided a local anesthetic cartridge to the operator while maintaining double blindness" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This study was double blinded; neither the operator nor the participant was aware of which anesthetic was administered" "2% lidocaine with 1:200,000 epinephrine…. and 2% lidocaine with 1:80,000 epinephrine…. were packaged so that they could not be recognized and were distributed to the trial institutes" Quote (from correspondence): "An independent statistician generated random codes and provided them to the factory of Huons company. The company's random assignment officer removed the labels from both products and labeled them the same while keeping a thorough secret. Random numbers and information needed for clinical trials were written on the label. Boxed and provided to research institutions (hospitals).The research institute provided a local anesthetic cartridge to the operator while maintaining double blindness" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This study was double blinded; neither the operator nor the participant was aware of which anesthetic was administered" "2% lidocaine with 1:200,000 epinephrine…. and 2% lidocaine with 1:80,000 epinephrine…. were packaged so that they could not be recognized and were distributed to the trial institutes" Quote (from correspondence): "An independent statistician generated random codes and provided them to the factory of Huons company. The company's random assignment officer removed the labels from both products and labeled them the same while keeping a thorough secret. Random numbers and information needed for clinical trials were written on the label. Boxed and provided to research institutions (hospitals). The research institute provided a local anesthetic cartridge to the operator while maintaining double blindness" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Quote: "Ten participants dropped out because of randomization error. Four participants were omitted because the study drugs were not administered or the inclusion or exclusion criteria were violated" Comment: study used a cross‐over design. Despite errors producing dropouts, the reduction in numbers across groups resulted in study numbers that were still above the sample size required (23), with groups exactly balanced and reasons for reduction in numbers identical. Risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Quote: "Ten participants dropped out because of randomization error. Four participants were omitted because the study drugs were not administered or the inclusion or exclusion criteria were violated" Comment: study used a cross‐over design. Despite errors producing dropouts, the reduction in numbers across groups resulted in study numbers that were still above the sample size required (23), with groups exactly balanced and reasons for reduction in numbers identical. Risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Quote: "Ten participants dropped out because of randomization error. Four participants were omitted because the study drugs were not administered or the inclusion or exclusion criteria were violated" Comment: study used a cross‐over design. Despite errors producing dropouts, the reduction in numbers across groups resulted in study numbers that were still above the sample size required (23), with groups exactly balanced and reasons for reduction in numbers identical. Risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "Ten participants dropped out because of randomization error. Four participants were omitted because the study drugs were not administered or the inclusion or exclusion criteria were violated" Comment: study used a cross‐over design. Despite errors producing dropouts, the reduction in numbers across groups resulted in study numbers that were still above the sample size required (23), with groups exactly balanced and reasons for reduction in numbers identical. Risk of attrition bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Comment: study was supported by Huons Co. Ltd. Pharmaceutical Company |
Katz 2010.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 60 enrolled, 60 completing the study
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration using 1.8 mL of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary first molars and lateral incisors |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment was begun, the 3 anaesthetic solutions were randomly assigned 4‐ digit numbers from a random number table generated by Microsoft Office Excel (Microsoft Corporation, Redmond, Wash). The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the experiment was begun, the 3 anaesthetic solutions were randomly assigned 4‐ digit numbers from a random number table generated by Microsoft Office Excel (Microsoft Corporation, Redmond, Wash). The random numbers were assigned to a subject to designate which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The 2% lidocaine cartridges .......... were masked with opaque labels, and the cartridge caps and plungers were masked with a black felt tip marker. Corresponding 4‐digit codes were written on each cartridge label" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The 2% lidocaine cartridges ........... were masked with opaque labels, and the cartridge caps and plungers were masked with a black felt tip marker. Corresponding 4‐digit codes were written on each cartridge label." "Trained personnel,who were blinded to the anaesthetic solutions, administered all preinjection and post‐injection tests" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 72 occasions with first molar teeth (for those experiencing successful anaesthesia (matched pairs): 24 cases of 2% lidocaine, 1:100,000 epinephrine, 24 cases of 4% prilocaine, 1:200,000 epinephrine and 24 cases of 4% prilocaine, no vasoconstrictor). Because numbers were reduced across groups and reasons for reduction were identical, risk of bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Keskitalo 1975.
| Methods | Randomized controlled clinical trial, part parallel and part cross‐over study design | |
| Participants |
Location: university (Sweden) Participants: 439 enrolled, 298 completing the study. 379 teeth were removed. Age ranged from 18 to 62 years. 193 teeth were removed from males, 186 teeth from females Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Inferior alveolar nerve block and buccal infiltration (3.6 mL initially) of:
|
|
| Outcomes |
Clinical anaesthesia during extraction of impacted mandibular third molars
Teeth tested: mandibular third molars Adverse events reported (379/379) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The anaesthetic agents were randomly varied between the two operations in the bilateral cases. In the unilateral cases the anaesthetic agents were randomly varied between the patients" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The anaesthetic agents were randomly varied between the two operations in the bilateral cases. In the unilateral cases the anaesthetic agents were randomly varied between the patients" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The investigation was planned as a double blind study" Comment: detailed methods not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation, or if they were blinded. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The investigation was planned as a double blind study" Comment: detailed methods were not reported. It is not clear whether the person recording participants’ outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Quote: "141 cases were not included" Comment: these were accounted for and were due to teeth not having closed apices, administrative reasons, or teeth not likely to produce postoperative symptoms. This is high (47%), as only 298 cases were enrolled in the trial. However, most of these were initially entered into the study but were removed from the trial before treatment was performed, probably following radiographic examination when incomplete apices were detected. Because numbers across groups were reduced and reasons for reduction were balanced, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "141 cases were not included" Comment: these were accounted for and were due to teeth not having closed apices, administrative reasons, or teeth not likely to produce postoperative symptoms. This is high (47%), as only 298 cases were enrolled in the trial. However, most of these were initially entered into the study but were removed from the trial before treatment was performed, probably following radiographic examination when incomplete apices were detected. Because numbers across groups were reduced and the reasons for reduction were balanced, risk of attrition bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Khoury 1991.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel design | |
| Participants |
Location: university (Germany) Participants: 1700 enrolled, 1518 completing the study. Participants aged 18 years and older. 755 males, 763 females completed the study Inclusion criteria: none reported Exclusion criteria: contraindications to using the different local anaesthetic solutions, mentioned in the local anaesthetic packaging insert |
|
| Interventions | Varying doses of local anaesthetic were given depending on the procedure undertaken. Techniques used were described as "conduction and infiltration anaesthesia". Most used volumes of 2.0 mL, with a range from 0.8 mL to 5.0 mL. Further injections of 0.5 mL to 2.0 mL were given if required:
|
|
| Outcomes |
Clinical anaesthesia during surgical procedures (1518/1700)
Hard and soft tissues tested: various Adverse events reported (1518/1700) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the similar looking 2 mL ampoules did not bear the name of the anaesthetic but consecutive numbers. Detailed methods were not reported. The sequence of numbering is not clear, but it may have allowed identification of the formulations used if properties between the local anaesthetics were markedly different and all ampoules of a formulation were labelled in a similar way (e.g. 1 formulation was labelled with even numbers and the other formulation was labelled with even numbers). However, properties of the 2 solutions did not allow identification. Identification of the local anaesthetic by participants was not possible. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Unclear risk | Comment: data for 282 patients were not included, which represents 17% of those enrolled. Reasons for the dropouts and whether these were equal amongst groups were not clear, although the final numbers in groups were not too dissimilar. Risk of bias was therefore graded as unclear |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: data for 282 patients were not included, which represents 17% of those enrolled. Reasons for the dropouts and whether these were equal amongst groups were not clear, although the numbers in groups were not too dissimilar. Risk of bias was therefore graded as unclear |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Knoll‐Kohler 1992a.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Germany) Participants: 10 enrolled, 10 completing the study. Aged 26 years ± 1 year. 10 male, 0 female Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions | Maxillary buccal infiltration injections using 0.5 mL of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: right maxillary incisor |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "0.5 ml of one of the anaesthetic solutions compiled in Table I was injected into the mucobuccal aspect adjacent to the apex of the maxillary right incisor in a random manner with a double‐blind crossover design" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "0.5 ml of one of the anaesthetic solutions compiled in Table I was injected into the mucobuccal aspect adjacent to the apex of the maxillary right incisor in a random manner with a double‐blind crossover design" "The ampoules were coded with serial numbers" "After data collection the code was broken for statistical analysis" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigation was carried out as a double‐blind study with coded cartridges" "The ampoules were coded with serial numbers" "After data collection the code was broken for statistical analysis" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigation was carried out as a double‐blind study with coded cartridges" "The ampoules were coded with serial numbers" "After data collection the code was broken for statistical analysis" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group (2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine). No patients were excluded. Outcome data were complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: duration of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group (2% lidocaine, 1:50,000 epinephrine vs 2% lidocaine, 1:100,000 epinephrine). No patients were excluded. Outcome data were complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset (2) | High risk | Comment: onset of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group except 2% lidocaine, 1:200,000 epinephrine, when only 6/10 were measured (those who achieved anaesthetic success). Risk of bias was rated as high owing to differences in numbers assessed and the few participants involved. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration (2) | High risk | Comment: duration of pulpal anaesthesia was tested on all 10 participants in each local anaesthetic group except 2% lidocaine, 1:200,000 epinephrine, when only 6/10 were measured (those who achieved anaesthetic success). Risk of bias was rated as high owing to differences in numbers assessed and the few participants involved. Data were not used for meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Knoll‐Kohler 1992b.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Germany) Participants: 12 enrolled, 12 completing the study. Aged 26 years ± 1 year. 12 male, 0 female Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions | Maxillary buccal infiltration injection (0.5 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: right maxillary incisor |
|
| Notes | Industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thereafter, 0.5 ml of one of the anaesthetic solutions........were injected into the mucobuccal aspect adjacent to the apex of the maxillary right incisor in a random manner using a double blind crossover design" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Thereafter, 0.5 ml of one of the anaesthetic solutions........were injected into the mucobuccal aspect adjacent to the apex of the maxillary right incisor in a random manner using a double blind crossover design" "The ampoules were coded with serial numbers" "After data collection the code was broken for statistical analysis" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The ampoules were coded with serial numbers" "After data collection the code was broken for statistical analysis" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The ampoules were coded with serial numbers" "After data collection the code was broken for statistical analysis" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Espe GmbH & Co KG (Seefeld, Germany) was responsible for preparation and supply of the anaesthetic solutions |
Kolli 2017.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (India) Participants: 90 enrolled, 90 completing the study. Mean age 9.74 ± 1.9 years. 45 male, 45 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.7 mL) of:
Epinephrine concentrations assumed to be 1:80,000 for lidocaine (same as control injection, below) and 1:100,000 epinephrine for articaine (most common formulation), as these were not included in the journal article. Attempts to clarify this were unsuccessful, as contact with the study author via email was unsuccessful. Maxillary buccal/palatal infiltration (1.7 mL in total) of:
|
|
| Outcomes |
Clinical anaesthesia during extraction of primary maxillary molars
Teeth tested: primary first or second maxillary molars Adverse effects were reported (90/90)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The treatment allocation was predetermined by generating randomization list using GraphPad StatMate version 1.01i (GraphPad Software, Inc., Armonk, NY: IBM Corp). Children were allocated sequentially into one of the three groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The treatment allocation was predetermined by generating randomization list using GraphPad StatMate version 1.01i (GraphPad Software, Inc., Armonk, NY: IBM Corp). Children were allocated sequentially into one of the three groups" Comment: method used for concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "An experienced pediatric dentist performed all the injections who was blinded to the anesthetic solutions while another experienced pediatric dentist performed the extraction procedure" Comment: detailed methods not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An experienced pediatric dentist performed all the injections who was blinded to the anesthetic solutions while another experienced pediatric dentist performed the extraction procedure" Comment: detailed methods not reported. Some outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Kramer 1958.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Canada) Participants: 3703 injections given, although the numbers of participants in each group (success) were not known. Mean age and range and male:female ratio not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Mandibular and maxillary buccal injections (1 or more cartridges if required) of:
|
|
| Outcomes |
Clinical anaesthesia during operative dentistry procedures
Teeth tested: not stated Soft tissue anaesthesia from time of injection to time participant reported soft tissues returning to normal, or was given a postcard to record duration
Soft tissues tested: relevant soft tissues, depending on injection and jaw Adverse events reported (3703/3703) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These seven test solutions were issued at random from a central dispensary" "The dental assistant issuing the solutions maintained a record so that each solution was distributed equally to all operators" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "These seven test solutions were issued at random from a central dispensary and were identified only by a code in which the identifying digit was placed in a certain location in a varying four digit number. None of the operators knew the identity of the compound being used when he received a prepared syringe" "The dental assistant issuing the solutions maintained a record so that each solution was distributed equally to all operators" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "These seven test solutions were issued at random from a central dispensary and were identified only by a code in which the identifying digit was placed in a certain location in a varying four digit number. None of the operators knew the identity of the compound being used when he received a prepared syringe" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Seven test solutions were issued at random from a central dispensary and were identified only by a code in which the identifying digit was placed in a certain location in a varying four digit number. None of the operators knew the identity of the compound being used when he received a prepared syringe" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Unclear risk | Comment: the total number of participants was 3703 according to the journal article. The percentages of participants having successful anaesthesia were given, but not the numbers in each group; therefore it was impossible to determine whether there had been any dropouts. Attrition bias was therefore graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the total number of participants was 3703 according to the journal article. The number of participants having duration of soft tissue anaesthesia measured was 2434, but without information on how many in each group had successful soft tissue anaesthesia, it was impossible to determine whether there had been any dropouts. Attrition bias was therefore graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: the total number of participants was 3703 according to the journal article. The number of participants having numbers of adverse events measured was not stated; therefore it was impossible to determine whether there had been any dropouts. Attrition bias was therefore graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Anaesthesia (clinical) onset | Unclear risk | Comment: the total number of participants was 3703 according to the journal article. The number of participants having onset of pulpal anaesthesia measured was 3061, but without information on how many in each group had successful anaesthesia, it was impossible to determine whether there had been any dropouts. Attrition bias was therefore graded as unclear. Data were not used for meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Lasemi 2015.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Iran) Participants: 20 enrolled, 20 completing the study. Mean age 38.3 ± 11.3 years, ranging from 18 to 50 years. 20 male, 20 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (volume not stated) using the following:
|
|
| Outcomes |
Soft tissue anaesthesia
Soft tissues tested: lower lip Other adverse events (40/40)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The procedures were performed during 2 separate appointments. In the first session, the side of the mouth for administering the IANB (right or left) and the type of anesthetic solution (A100 and A200) (Primacaine, Pierre Rolland, Bordeaux, France) were chosen randomly" Comment: detailed methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The procedures were performed during 2 separate appointments. In the first session, the side of the mouth for administering the IANB (right or left) and the type of anesthetic solution (A100 and A200) (Primacaine, Pierre Rolland, Bordeaux, France) were chosen randomly" Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The surgeon and patient were blinded about the type of anesthetic solution administered" Comment: detailed methods not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was not used by personnel to minimize variation. Therefore, risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The surgeon and patient were blinded about the type of anesthetic solution administered" Comment: detailed methods not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no participants excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Laskin 1977.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 25 enrolled, 25 completing the study. 50 teeth were reported. Age ranging from 18 to 35 years old, with mean age of 23 years. 11 males, 14 females Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions | 1.8 mL for each quadrant: mandibular nerve block (1.6 mL), long buccal nerve (0.2 mL) initially, then a further dose of up to 1.8 mL was administered if required of:
|
|
| Outcomes |
Clinical anaesthesia during extraction
Teeth tested: impacted mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lower lip Adverse events reported (16/16) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The first six preparations were administered randomly without knowledge of what the syringe contained; the seventh preparation was known and was reserved for use in anaesthetic failures. Random sampling was used for determination of which side of the jaw was treated at the first appointment" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "The local anaesthetics were supplied by the pharmacy, prepackaged, and labelled for each patient, following a random pattern that had been predetermined and unknown to the operator" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each patient had a specific 'number' and the drugs were identified as 'number' right and 'number' left. The local anaesthetics were supplied by the pharmacy, prepackaged, and labelled for each patient, following a random pattern that had been predetermined and unknown to the operator" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each patient had a specific 'number' and the drugs were identified as 'number' right and 'number' left. The local anaesthetics were supplied by the pharmacy, prepackaged, and labelled for each patient, following a random pattern that had been predetermined and unknown to the operator" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Lawaty 2010.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 60 enrolled, 60 completing the study
Inclusion criteria: in good health and not taking any medication that would alter pain perception Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary first molars and lateral incisors |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 2 anaesthetic solutions were randomly assigned 4‐digit numbers from a random number table. Each subject was randomly assigned to the right or left side infiltration grouping. The order of the anaesthetic solutions was also randomly assigned to determine which solutions were to be administered at each appointment" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "The 2 anaesthetic solutions were randomly assigned 4‐digit numbers from a random number table. Each subject was randomly assigned to the right or left side infiltration grouping. The order of the anaesthetic solutions was also randomly assigned to determine which solutions were to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: The local anaesthetic cartridges "were masked with opaque labels. The corresponding 4‐digit codes were written on each cartridge label" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the random numbers were recorded on the data collection sheets to help blind the experiment" "Trained personnel, who were blinded to the anaesthetic solutions, administered all preinjection and post‐injection tests" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no participants excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Lima 2009.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (Brazil) Participants: 100 enrolled, 100 completing the study (200 teeth), with age ranging from 15 to 46 years. Male:female ratio not reported, although confirmed as 50 male and 50 female by the first study author following email communication Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Maxillary buccal infiltration (1.8 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during each surgical phase of extraction (presence or absence of pain)
Teeth tested: maxillary third molars |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "The randomization was done in "blocks" of predetermined size, 25 patients per group. We used a program which selected in 5 of 5 patients per group, where the size of each group was 25 patients" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "The surgeon did not know the patients nor the data and operated five patient groups selected by the program and coordinated by staff (interns)" "The syringes were sealed with tape, preventing the visualization of the applicator, only the appraiser who prepared the syringes knew the division of anaesthesia and groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "The syringes were sealed with tape, preventing the visualization of the applicator, only the appraiser who prepared the syringes knew the division of anaesthesia and groups. The patient also had no knowledge of type of anaesthetic used. Only one person made all anaesthesia to avoid variation or deviation of the anaesthetic technique" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "The syringes were sealed with tape, preventing the visualization of the applicator, only the appraiser who prepared the syringes knew the division of anaesthesia and groups. The patient also had no knowledge of type of anaesthetic used. Only one person made all anaesthesia to avoid variation or deviation of the anaesthetic technique" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Linden 1986.
| Methods | Randomized controlled simulated scenario trial (following a clinical intervention), cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 20 enrolled, 20 completing the study. Age ranging from 20 to 65 years of age. Male:female ratio not reported Inclusion criteria: none reported Exclusion criteria
|
|
| Interventions | One or more of the following injections:
using either 1.5 Carpule (2.7 mL) for block injections or 1 Carpule (1.8 mL) for other injections (including palatal blocks) of 1 of the following solutions:
|
|
| Outcomes |
Soft tissue anaesthesia following periodontal surgery
Soft tissues tested: relevant soft tissues Adverse effects reported (38/40)
|
|
| Notes | Industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Identical Carpules were used and placed in containers labelled A or B. 8 of each were removed and placed in identical envelopes with a coded number on the outside, which was not available to the investigator" Each patient was assigned 2 envelopes "The second party then randomly assigned the anaesthetic given, the quadrant to be treated surgically, and the order of the surgeries (i.e. left or right side), so that the first anaesthetic given to a patient and order of the surgeries varied" Quote (from correspondence): “We had a third party not involved randomize the anaesthetic given which was placed in a sterile bag, (blank Carpules) so we didn't know what we were giving the patient. In addition, the quadrant was also randomly assigned until the patient was seated. In summary we didn't know until we were given the instructions of the quadrant, side of the mouth, anaesthetic blank Carpules and location to treat before the patient arrived" "The entire process was randomized by a independent third party who literally pulled numbers blindly out of a box with anaesthetic, locations or quadrants, and order" |
| Allocation concealment (selection bias) | Low risk | Quote: "Identical Carpules were used and placed in containers labelled A or B. 8 of each were removed and placed in identical envelopes with a coded number on the outside, which was not available to the investigator" Each patient was assigned 2 envelopes "The second party then randomly assigned the anaesthetic given, the quadrant to be treated surgically, and the order of the surgeries (i.e. left or right side), so that the first anaesthetic given to a patient and order of the surgeries varied" Quote (from correspondence): "We had a third party not involved randomize the anaesthetic given which was placed in a sterile bag, (blank Carpules) so we didn't know what we were giving the patient" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical Carpules were used and placed in containers labelled A or B. 8 of each were removed and placed in identical envelopes with a coded number on the outside, which was not available to the investigator" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Identical Carpules were used and placed in containers labelled A or B. 8 of each were removed and placed in identical envelopes with a coded number on the outside, which was not available to the investigator" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: one patient failed to respond when asked for preference of local anaesthetic solution. This was balanced across groups because the study used a cross‐over design. Also, haemostasis was assessed during surgery. Therefore risk of bias was graded as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Quote: "Cooke Waite donated a small amount of money to our dental clinic. There were no stipends or bonuses" |
Malamed 2000a.
| Methods | Randomized controlled clinical trial, parallel study design, carried out at 27 sites | |
| Participants |
Location: United States of America, United Kingdom Participants: 1325 enrolled, 1325 completing the study:
Inclusion criteria: not reported Exclusion criteria
|
|
| Interventions | Standard infiltration or nerve block of the following mean volumes:
2.5 mL ± 0.07 SEM of 4% articaine, 1:100,000 epinephrine (675) 2.6 mL ± 0.09 SEM of 2% lidocaine, 1:100,000 epinephrine (338)
4.2 mL ± 0.15 SEM of 4% articaine, 1:100,000 epinephrine (207) 4.5 mL ± 0.21 SEM of 2% lidocaine, 1:100,000 epinephrine (105) |
|
| Outcomes |
Clinical anaesthesia during various procedures
"Simple" group included single extractions with no complications, routine operative procedures, single apical resections, single crown procedures "Complex" group included multiple extractions, multiple crowns, bridge procedures or both, multiple apical resections, alveolectomies; mucogingival operations, other osseous surgical procedures Teeth tested: not reported Adverse events reported (1323/1325) |
|
| Notes | No funding stated. Study authors thanked Septodont | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We randomized subjects in a 2:1 ratio to receive articaine or lidocaine" Comment: the difference in group size was deliberate and was related to safety issues. Exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "We randomized subjects in a 2:1 ratio to receive articaine or lidocaine" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method for administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods not reported. The person recording participants’ outcomes also administered the local anaesthetic, so they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: data were missing for 2 participants. As missing data were minor, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data were missing for 2 participants. As missing data were minor, risk of bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Unsure whether study was sponsored by Septodont |
Malamed 2000b.
| Methods | Randomized controlled clinical trial, parallel study design, carried out at 7 sites | |
| Participants |
Location: United States of America and United Kingdom Participants: 70 enrolled, 70 completing the study
Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Standard infiltration or nerve block of the following mean volumes:
1.9 mL ± 0.10 SEM of 4% articaine, 1:100,000 epinephrine (43) 1.9 mL ± 0.23 SEM of 2% lidocaine, 1:100,000 epinephrine (18)
2.5 mL ± 0.43 SEM of 4% articaine, 1:100,000 epinephrine (7) 2.6 mL ± 0.00 SEM of 2% lidocaine, 1:100,000 epinephrine (2) |
|
| Outcomes |
Clinical anaesthesia during various procedures
"Simple" group included single extractions with no complications, routine operative procedures, single apical resections, single crown procedures "Complex" group included multiple extractions, multiple crowns, bridge procedures or both, multiple apical resections, alveolectomies; mucogingival operations, other osseous surgical procedures Teeth tested: not reported Adverse events reported along with vital signs (70/70) |
|
| Notes | No funding reported. Study authors thanked Septodont | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All subjects were randomized in a 2:1 ratio to receive articaine or lidocaine, with the paediatric population ultimately receiving the anaesthetics in a 2.5:1 ratio" Comment: the difference in group size was deliberate and was related to safety issues. Exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All subjects were randomized in a 2:1 ratio to receive articaine or lidocaine, with the pediatric population ultimately receiving the anaesthetics in a 2.5:1 ratio" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods not reported. It is not clear whether the person recording participants’ outcomes also administered the local anaesthetic, as he or she may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Unsure whether study was sponsored by Septodont |
Maniglia‐Ferreira 2009.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Brazil) Participants: 60 enrolled, 60 completing the study. Age ranging from 21 to 48 years. Male:female ratio not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1 cartridge) of:
|
|
| Outcomes |
Clinical anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: mandibular molars Soft tissue anaesthesia
Soft tissues tested: unclear. Lower lip? |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to three groups of 20 participants each" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to three groups of 20 participants each" Comment: methods of concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel, to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. It is not clear whether the person recording participants’ outcomes also administered the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants who had duration of anaesthesia measured was not stated; therefore risk of attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Martinez‐Rodriguez 2012.
| Methods | Randomized controlled simulated scenario (before and following a clinical intervention), parallel study design | |
| Participants |
Location: university (Spain) Participants: 96 enrolled, 96 completing the study (48 in each group). Mean age or range of ages and male:female ratio not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) and mandibular buccal infiltration (0.9 mL) of:
|
|
| Outcomes |
Soft tissue anaesthesia (self‐reported)
Soft tissues tested: lower lip Teeth extracted: mandibular third molars Adverse effects were reported (96/96)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "....designed as a parallel, simple blind, single‐site study with randomization in four‐element blocks or two treatments" "6 blocks of 4 possible treatments were established; Test‐A and reference‐B (Table 2)" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "....designed as a parallel, simple blind, single‐site study with randomization in four‐element blocks or two treatments" "6 blocks of 4 possible treatments were established; Test‐A and reference‐B (Table 2)" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was open to the investigators and blind to the patients" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study was open to the investigators and blind to the patients" Comment: detailed methods were not reported. It is not clear whether the person recording participants’ outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Maruthingal 2015.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (India) Participants: 32 enrolled, 32 completing the study. Mean age 18.2 years ranging from 15 to 35 years. 7 male, 25 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Mandibular buccal infiltration (1.7 mL) using the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars Soft tissue anaesthesia
Soft tissues tested: lip and lingual mucosa |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Study was designed as a prospective randomized double‐blind crossover trial" "They were treated as Group I to receive 2% lidocaine with 1:100,000 epinephrine……in the first visit and the same individuals were treated as Group II to receive 4% articaine with 1:100,000 epinephrine……local anesthesia in the second visit" Comment: although the trial was described as randomized, the order of local anaesthetics administered was pre‐determined for everyone in a non‐randomized way. Attempts were made to contact the study author to clarify this, but no contact could be made |
| Allocation concealment (selection bias) | High risk | Quote: "Study was designed as a prospective randomized double‐blind crossover trial" "They were treated as Group I to receive 2% lidocaine with 1:100,000 epinephrine……in the first visit and the same individuals were treated as Group II to receive 4% articaine with 1:100,000 epinephrine……local anesthesia in the second visit" Comment: although the trial was described as randomized, the order of local anaesthetics administered was pre‐determined for everyone in a non‐randomized way. Attempts were made to contact the study author to clarify this, but no contact could be made |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the subjects, and the dentist and dental nurse were blinded for the drug being used and had no involvement with testing the outcome" "They were treated as Group I to receive 2% lidocaine with 1:100,000 epinephrine……in the first visit and the same individuals were treated as Group II to receive 4% articaine with 1:100,000 epinephrine……local anesthesia in the second visit" Comment: the order of local anaesthetics administered was pre‐determined for everyone in a non‐randomized way; therefore the local anaesthetic used would be known. A pre‐determined method of administration was used by personnel to minimize variation. Despite no blinding, identification of the local anaesthetic by participants would be possible only if they were informed of the order of formulations administered. Attempts were made to contact the study author to clarify this, but no contact could be made. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Both the subjects, and the dentist and dental nurse were blinded for the drug being used and had no involvement with testing the outcome" "They were treated as Group I to receive 2% lidocaine with 1:100,000 epinephrine……in the first visit and the same individuals were treated as Group II to receive 4% articaine with 1:100,000 epinephrine ……local anesthesia in the second visit" Comment: the order of local anaesthetics administered was pre‐determined for everyone in a non‐randomized way; therefore the local anaesthetic used would be known, although a separate outcome assessor was used. Attempts were made to contact the study author to clarify this, but no contact could be made. Risk of bias was graded as high |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Unclear risk | Comment: the number of participants who had onset of pulpal anaesthesia measured is not clear. Numbers of participants for lidocaine and articaine were likely to be 17 and 28, respectively, based on those who had successful pulpal anaesthesia. As this could not be clarified by the study author, risk of attrition bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Mason 2009.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 60 enrolled, 60 completing the study
Inclusion criteria: in good health and not taking any medication that would alter pain perception Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary first molars and lateral incisors |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, the three anaesthetic solutions were randomly assigned to designate which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Each solution had a random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the experiment, the three anaesthetic solutions were randomly assigned to designate which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: The cartridges "were masked with opaque labels, and the cartridge caps and plungers were masked with a black felt tip marker. The corresponding random code number was written on each cartridge label" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trained personnel, who were blinded to the anaesthetic solutions, administered all preinjection and post‐injection tests" "Only the random numbers were recorded on the data‐collection sheets to further blind the experiment" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 84 occasions on first molar teeth and on 84 occasions on lateral incisors (for those experiencing successful anaesthesia (matched pairs): 28 cases of 2% lidocaine, 1:100,000 epinephrine; 28 cases of 2% lidocaine, 1:50,000 epinephrine; and 28 cases of 3% mepivacaine, no vasoconstrictor). As numbers were reduced in all groups equally and for the same reasons, risk of bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
McEntire 2011.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 86 enrolled, 86 completing the study. Mean age 26 years, ranging from 18 to 43 years. 43 male, 43 female Inclusion criteria: in good health and not taking any medication that would alter pain perception Exclusion criteria
|
|
| Interventions | Mandibular buccal infiltration (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars Adverse events reported (172/172)
|
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 2 anaesthetic formulations were randomly assigned 6‐digit numbers from a random number table. Each subject was randomly assigned to each of the 2 anaesthetic formulations to determine which formulation was to be administered at each appointment" Quote (from correspondence): "Each solution had a six‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "The 2 anaesthetic formulations were randomly assigned 6‐digit numbers from a random number table. Each subject was randomly assigned to each of the 2 anaesthetic formulations to determine which formulation was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthetic cartridges were masked with opaque labels, and the corresponding 6‐digit codes were written on each cartridge" "Only the random numbers were recorded on the data collection sheets to further blind the experiment" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trained personnel who were blinded to the anaesthetic formulations administered all preinjection and post‐injection tests" "Only the random numbers were recorded on the data collection sheets to further blind the experiment" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 90 occasions (for those experiencing successful anaesthesia (matched pairs): 45 cases of 4% articaine, 1:100,000 epinephrine and 45 cases of 4% articaine, 1:200,000 epinephrine). As numbers were reduced in both groups equally and for the same reasons, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
McLean 1993.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 30 enrolled, 30 completing the study. Mean age 28 years, ranging from 24 to 43 years. 24 male, 6 female Inclusion criteria: in good health and not taking any medication that would alter pain perception Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars, first premolars, and lateral incisors Soft tissue anaesthesia sticking the alveolar mucosa (labial and lingual to the premolar and buccal to the first molar) with a sharp explorer
Soft tissues tested: lower lip/tongue (participant felt numbness) and labial and lingual to the premolar and buccal to the first molar (mucosal sticks) |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomly assigned to one of six letter (ABC) combinations to determine the sequence of solution administration" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "A four‐digit random number, corresponding to the letter designation, was written on each cartridge, and the three cartridges for each subject were placed in an autoclave bag with the numbers recorded on the outside showing the injection order" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an autoclave bag for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment etc was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each anaesthetic cartridge label was removed and masked with tape. A four‐digit random number, corresponding to the letter designation, was written on each cartridge, and the three cartridges for each subject were placed in an autoclave bag with the numbers recorded on the outside showing the injection order" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All pre‐ and post‐injection tests were done by trained personnel who were blinded to the solutions injected" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 82 occasions (for those experiencing successful anaesthesia: 27 cases of 2% lidocaine, 1:100,000 epinephrine; 28 cases of 4% prilocaine, no vasoconstrictor; and 27 cases of 3% mepivacaine, no vasoconstrictor). As numbers in both groups were well balanced and were reduced for the same reasons, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Mikesell 2005.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 57 enrolled, 57 completing the study. Mean age 28 years, ranging from 19 to 60 years. 30 male, 27 female Inclusion criteria: in good health and not taking any medications that would alter their perception of pain Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular second molars, first molars, second premolars, first premolars, lateral incisors, and central incisors Soft tissue anaesthesia
Soft tissues tested: lower lip and tongue Adverse events reported (114/114)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 57 blinded subjects randomly received an IAN block injection of either an articaine or lidocaine solution at two separate appointments" "Before the experiment, the two anaesthetic solutions were randomly assigned six‐digit numbers from a random number table. Each subject was randomly assigned to one of the two solutions to determine which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Each solution had a six‐digit random number for each subject and for each solution. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "The 57 blinded subjects randomly received an IAN block injection of either an articaine or lidocaine solution at two separate appointments" "Before the experiment, the two anaesthetic solutions were randomly assigned six‐digit numbers from a random number table. Each subject was randomly assigned to one of the two solutions to determine which anaesthetic solution was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an autoclave bag for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment etc was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthetic solutions administered were blinded by masking the appropriate cartridges with opaque labels, which were labelled with the six‐digit numbers" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the random numbers were recorded on the data collection and post‐injection survey sheets to blind the experiment" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Quote: "If profound lip numbness was not recorded within 15 min, the block was considered unsuccessful; the subject was then reappointed" "A total of eight patients, two using the articaine solution and six using the lidocaine solution, did not have profound lip numbness at 15 min (unsuccessful blocks) and were reappointed. One hundred percent of the subjects used for data analysis had profound lip anaesthesia with both the articaine and lidocaine solutions" Comment: participants who were re‐appointed had successful pulpal anaesthesia; therefore it was possible to re‐calculate overall success |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Quote: "If profound lip numbness was not recorded within 15 min, the block was considered unsuccessful; the subject was then reappointed" "A total of eight patients, two using the articaine solution and six using the lidocaine solution, did not have profound lip numbness at 15 min (unsuccessful blocks) and were reappointed. One hundred percent of the subjects used for data analysis had profound lip anaesthesia with both the articaine and lidocaine solutions" Comment: participants who were re‐appointed had failed lip anaesthesia; therefore it was possible to re‐calculate overall success |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: two participants using articaine and 6 using lidocaine were re‐allocated, after initial failure of lip anaesthesia. Adverse events in the journal article represent those from participants initially having successful anaesthesia and 8 participants following re‐allocation. The numbers re‐allocated were relatively low in number; therefore as numbers in both groups were well balanced and reduced for the same reasons, risk of bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Mittal 2015.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (India) Participants: 104 enrolled, 104 completing the study. Age ranging from 5 to 12 years. 68 male, 36 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration using the following:
|
|
| Outcomes |
Clinical anaesthesia during extraction of primary maxillary molars
Teeth/soft tissues tested: primary maxillary molars Soft tissue anaesthesia
Soft tissues tested: soft tissues, buccal and palatal to the tooth to be extracted Adverse events reported (104/104)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomly selected employing the envelope method to receive buccal infiltration using either 1.8 ml lidocaine HC1 two percent with epinephrine 1:80,000 (Lignospan special, Septodont, Saint‐Maur‐des‐Fosses, France) (Group A) or 1.7 ml articaine HC1 four percent with epinephrine 1:100,000 (Septanest, Septodont, France; Group B)" Quote (from correspondence): "The name of the anesthetic agent to be used was written on multiple slips (equal number of slips for both the agents), which were kept in an envelope. Patient picked one of the slips without seeing the name, the slips were folded" |
| Allocation concealment (selection bias) | Low risk | Quote: "Children were randomly selected employing the envelope method to receive buccal infiltration using either 1.8 ml lidocaine HC1 two percent with epinephrine 1:80,000 (Lignospan special, Septodont, Saint‐Maur‐des‐Fosses, France) (Group A) or 1.7 ml articaine HC1 four percent with epinephrine 1:100,000 (Septanest, Septodont, France; Group B)" Quote (from correspondence): "The name of the anesthetic agent to be used was written on multiple slips (equal number of slips for both the agents), which were kept in an envelope. Patient picked one of the slips without seeing the name, the slips were folded" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The clinician administering the anesthetic, chairside assistant, patient receiving the anesthetic, and his/her parent were all blinded to the anesthetic agent being used" Quote (from correspondence): "The cartridge or the Wand assembly was loaded by a person different from clinician, assistant, patient or parent. There is a very slight colour difference between the two anesthetic cartridges. The clinician could see the cartridge if he saw carefully. The cartridge was loaded and given to him just before injection" Comment: identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Facial display followed Craig’s behavioral description of facial actions. Only two of the four of Craig’s most descriptive facial actions were evident (eyebrow bulge or eye squeeze), as the mouth was open and the nose was partly covered by the operator’s hand during the procedure. These behavioural parameters were evaluated during the extraction procedure by a trained dental assistant who did not participate in the treatment and was blind to the agent being used" Comment: outcomes were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Moore 1983.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States) Participants: 32 enrolled, 32 completing the study. 14 male, 18 female. Mean age of bupivacaine patients: 40.3 years, ranging from 21 to 64 years. Mean age of lidocaine patients: 41.4 years, ranging from 22 to 66 years Inclusion criteria: none reported Exclusion criteria
|
|
| Interventions | Maxillary and mandibular injections (blocks and infiltrations), 2 cartridges (2 × 1.8 mL) used for each procedure of:
|
|
| Outcomes |
Clinical anaesthesia during non‐surgical and surgical endodontic treatment
Teeth tested: maxillary or mandibular teeth, but no specific details Soft tissue anaesthesia
Soft tissues tested: lip Adverse events reported (32/32)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "We used a random number table and sealed envelopes to assure blinding" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "We used a random number table and sealed envelopes to assure blinding" "Double‐blind conditions were maintained by coding identically appearing unlabeled cartridges of the two agents. The code was available in a sealed envelope if needed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind conditions were maintained by coding identically appearing unlabeled cartridges of the two agents. The code was available in a sealed envelope if needed" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Double‐blind conditions were maintained by coding identically appearing unlabeled cartridges of the two agents. The code was available in a sealed envelope if needed" Comment: identification of the local anaesthetic by participants and personnel was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Moore 2006.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants
One person withdrew, who had successfully received an injection of 4% articaine, 1:100,000 epinephrine in trial 1 and trial 2 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Either inferior alveolar nerve block (1.7 mL; trial 1) or maxillary buccal infiltration anaesthesia (1.0 mL; trial 2) using:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Note: 189 is the total number of expected measurements, but owing to 1 dropout in each trial, the number of participants was reduced in each group by 1, except the 4% articaine, 1:100,000 epinephrine group, for which measurements had already been recorded before dropout Teeth tested: mandibular canines (trial 1) and maxillary first premolars (trial 2) Adverse events reported (187/189 in trial 1, 187/189 in trial 2) |
|
| Notes | Industry funded (first study author is a paid consultant, and another study author is an employee of the study sponsor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the first treatment visit, we enrolled subjects and assigned them to a randomized sequence for drug allocation" Quote (from correspondence): "Randomized by sponsor, sealed in a box labelled with subject code and not visually different" |
| Allocation concealment (selection bias) | Low risk | Quote: "At the first treatment visit, we enrolled subjects and assigned them to a randomized sequence for drug allocation" "The study sponsor (Novocol Pharmaceutical, Cambridge, Ontario, Canada) prepared identical‐appearing dental cartridges of the three study formulations and coded them properly to ensure blinded administration" Quote (from correspondence): "Randomized by sponsor, sealed in a box labelled with subject code and not visually different" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study sponsor (Novocol Pharmaceutical, Cambridge, Ontario, Canada) prepared identical‐appearing dental cartridges of the three study formulations and coded them properly to ensure blinded administration" Comment: participants and personnel would not be able to identify the local anaesthetic used, and a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study sponsor (Novocol Pharmaceutical, Cambridge, Ontario, Canada) prepared identical‐appearing dental cartridges of the three study formulations and coded them properly to ensure blinded administration" Comment: identification of the local anaesthetic by participants and personnel was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Quote: "One subject was not included in the second and third treatment session owing to an error in the EPT protocol. At the conclusion of the trial, data were available for 63 subjects who received A100, 62 who received A200 and 62 who received Aw/o" Comment: excluded participant accounted for. Different groups were well balanced. Therefore risk was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 165 occasions in the maxilla (for those experiencing successful anaesthesia: 58 cases of 4% articaine, 1:200,000 epinephrine; 60 cases of 4% articaine, 1:100,000 epinephrine; and 47 cases of 4% articaine, with no vasoconstrictor) Onset of pulpal anaesthesia was tested on 80 occasions in the mandible (for those experiencing successful anaesthesia: 34 cases of 4% articaine, 1:200,000 epinephrine; 30 cases of 4% articaine, 1:100,000 epinephrine; and 16 cases of 4% articaine, with no vasoconstrictor) As numbers in all groups were well balanced and were reduced for the same reasons, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: duration of pulpal anaesthesia was tested on 165 occasions in the maxilla (for those experiencing successful anaesthesia: 58 cases of 4% articaine, 1:200,000 epinephrine; 60 cases of 4% articaine, 1:100,000 epinephrine; and 47 cases of 4% articaine, with no vasoconstrictor) Duration of pulpal anaesthesia was tested on 80 occasions in the mandible (for those experiencing successful anaesthesia: 34 cases of 4% articaine, 1:200,000 epinephrine; 30 cases of 4% articaine, 1:100,000 epinephrine; and 16 cases of 4% articaine, with no vasoconstrictor) As numbers in all groups were well balanced and were reduced for the same reasons, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "One subject was not included in the second and third treatment session owing to an error in the EPT protocol. At the conclusion of the trial, data were available for 63 subjects who received A100, 62 who received A200 and 62 who received Aw/o" Comment: excluded participant accounted for. Different groups were well balanced. Therefore risk was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset (2) | High risk | Comment: onset of pulpal anaesthesia was tested on 165 occasions in the maxilla (for those experiencing successful anaesthesia: 58 cases of 4% articaine, 1:200,000 epinephrine; 60 cases of 4% articaine, 1:100,000 epinephrine; and 47 cases of 4% articaine, with no vasoconstrictor) Onset of pulpal anaesthesia was tested on 80 occasions in the mandible (for those experiencing successful anaesthesia: 34 cases of 4% articaine, 1:200,000 epinephrine; 30 cases of 4% articaine, 1:100,000 epinephrine; and 16 cases of 4% articaine, with no vasoconstrictor) Onset of pulpal anaesthesia was measured in similar numbers of participants in each local anaesthetic group except 4% articaine, no vasoconstrictor, when only 47/62 were measured in the maxilla and 16/62 in the mandible (those who achieved anaesthetic success). Risk of bias was rated as high owing to differences in numbers measured in each group |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration (2) | High risk | Comment: duration of pulpal anaesthesia was tested on 165 occasions in the maxilla (for those experiencing successful anaesthesia: 58 cases of 4% articaine, 1:200,000 epinephrine; 60 cases of 4% articaine, 1:100,000 epinephrine; and 47 cases of 4% articaine, with no vasoconstrictor) Duration of pulpal anaesthesia was tested on 80 occasions in the mandible (for those experiencing successful anaesthesia: 34 cases of 4% articaine, 1:200,000 epinephrine; 30 cases of 4% articaine, 1:100,000 epinephrine; and 16 cases of 4% articaine, with no vasoconstrictor) Duration of pulpal anaesthesia was measured in similar numbers of participants in each local anaesthetic group, except 4% articaine, no vasoconstrictor, when only 47/62 were measured in the maxilla and 16/62 in the mandible (those who achieved anaesthetic success). Risk of bias was rated as high owing to differences in numbers measured in each group |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Quote: industry funded (first study author is a paid consultant, and another study author is an employee of the study sponsor) |
Moore 2007.
| Methods | Randomized controlled clinical trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 42 enrolled, 42 completing the study. Mean age 46.3 (SD ± 9.7), ranging from 22 to 65 years. 26 male, 16 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (buccal and palatal if required) techniques using the following:
Volumes varied depending on procedure (minimum used, with a maximum of 4 cartridges) |
|
| Outcomes |
Clinical anaesthesia during periodontal surgery
Teeth tested: maxillary teeth/soft tissues Adverse events reported (84/84) |
|
| Notes | Industry funded (1 study author is an employee of the study sponsor) (first study author was a paid consultant for the study sponsor in a previous study (Moore 2006), although this is not declared in the current study) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized to one of the two drug sequence groups: A200 at the first surgical appointment and A100 at the second surgical appointment or A100 at the first surgical appointment and A200 at the second surgical appointment" Comment: exact method of generation of randomized sequence not reported Quote (from correspondence): "Randomized by sponsor, sealed in a box, labelled with subject code and not visually different" |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were randomized to one of the two drug sequence groups: A200 at the first surgical appointment and A100 at the second surgical appointment or A100 at the first surgical appointment and A200 at the second surgical appointment" Quote (from correspondence): "Randomized by sponsor, sealed in a box, labelled with subject code and not visually different" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "Cartridges were unlabelled and dispensed in an investigator container with only subject number" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Cartridges were unlabelled and dispensed in an investigator container with only subject number" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Comment: industry funded (1 study author is an employee of the study sponsor) (first study author was a paid consultant for the study sponsor in a previous study (Moore 2006), although this is not declared in the current study) |
Mumford 1961.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United Kingdom) Participants: 300 enrolled, 300 completing the study (200 without 2% mepivacaine, 1:80,000 epinephrine). Mean age 21.3 to 25.4 years, ranging from 11 to 59 years. Exact number of male and female participants not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | "Regional" and infiltration injections (1.5 and 1.0 mL, respectively) of:
|
|
| Outcomes |
Clinical anaesthesia during routine tooth cavity preparation
Teeth tested: maxillary lateral incisors, canines, first premolars, mandibular molars Soft tissue anaesthesia
Soft tissues tested: soft tissues relevant to the procedure Adverse events reported (number assessed not clear)
|
|
| Notes | Possibly industry funded, as study authors thank Bayer and Astrapharm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The materials were randomized and coded for double blind testing" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The materials were randomized and coded for double blind testing" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The materials were randomized and coded for double blind testing" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The materials were randomized and coded for double blind testing" Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: of 300 participants, 209 were given postcards for soft tissue duration. Of these, 192 were returned. It is not clear how many postcards were given to each local anaesthetic group's participants, or why these were not given to every participant. Therefore risk was graded as unclear |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: of 300 participants, 209 were given postcards to record adverse events. Of these, 192 were returned. It is not clear how many postcards were given to each local anaesthetic group's participants, or why these were not given to every participant. Therefore risk was graded as unclear |
| Incomplete outcome data (attrition bias) Anaesthesia (clinical) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 82 occasions for those experiencing successful anaesthesia for infiltration (40 cases of 2% lidocaine, 1:80,000 epinephrine; 42 cases of 3% mepivacaine, no epinephrine) and on 85 occasions for IANBs (43 cases of 2% lidocaine, 1:80,000 epinephrine; 42 cases of 3% mepivacaine, no epinephrine). As numbers in both groups were well balanced and were reduced for the same reasons, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Anaesthesia (clinical) duration | Low risk | Comment: duration of pulpal anaesthesia was tested on 80 occasions for those experiencing successful anaesthesia for infiltration (39 cases of 2% lidocaine, 1:80,000 epinephrine; 41 cases of 3% mepivacaine, no epinephrine) and on 84 occasions for IANBs (42 cases of 2% lidocaine, 1:80,000 epinephrine; 42 cases of 3% mepivacaine, no epinephrine) Of the total number of participants recruited who had successful pulpal anaesthesia, a few did not have duration of pulpal anaesthesia measured:
As numbers in both groups were well balanced and were reduced for the same reasons, risk of bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Comment: possibly industry funded, as study authors thank Bayer and Astrapharm |
Nabeel 2014.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (Pakistan) Participants: 76 enrolled, 76 completing the study. Age ranging from 18 to 67 years. 33 male, 43 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.7 mL) using the following:
|
|
| Outcomes |
Pulpal anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: maxillary first premolars |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were assigned group 1 or 2 by using a computer‐generated list of random numbers with randomization ratio of 1:1 produced by random allocation software (version 1.0)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "They were assigned group 1 or 2 by using a computer‐generated list of random numbers with randomization ratio of 1:1 produced by random allocation software (version 1.0)" Comment: detailed methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear if a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Naik 2017.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (India) Participants: 49 male, 51 female
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (2 mL) using the following:
Followed by 0.5 mL long buccal infiltration for extraction and measurement of success/duration (confirmed by study author) |
|
| Outcomes |
Clinical anaesthesia during surgical removal of third molars
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lip and associated tissues Adverse effects (100/100)
|
|
| Notes | No funding reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated to one of two possible treatment groups according to a randomized list on the visit for surgery" Comment: detailed methods not reported Quote (from correspondence): "We had prepared 2 small chits one with lignocaine and one with articaine. We would pick one chit and allot the patient to whichever group it came" |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were allocated to one of two possible treatment groups according to a randomized list on the visit for surgery" Comment: detailed methods not reported Quote (from correspondence): "We had prepared 2 small chits one with lignocaine and one with articaine. We would pick one chit and allot the patient to whichever group it came" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "As articaine 4% is available in only 2ml cartridges in India, before the administration of local anesthetic, it was aspirated in a plastic syringe from the articaine cartridges (SEPTANEST® 4%, SEPTODONT) whereas lignocaine was aspirated in a plastic syringe from lignocaine vials (LIGNOX 2% A, INDOCO REMEDIES LTD). The patients were thus blinded with respect to the type of local anaesthetic treatment given on each occasion" Quote (from correspondence): "Only the patients were blinded" Comment: participants would not be able to identify the local anaesthetic used. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Study author was emailed to see if this was done, but no reply. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "As articaine 4% is available in only 2ml cartridges in India, before the administration of local anesthetic, it was aspirated in a plastic syringe from the articaine cartridges (SEPTANEST® 4%, SEPTODONT) whereas lignocaine was aspirated in a plastic syringe from lignocaine vials (LIGNOX 2% A, INDOCO REMEDIES LTD). The patients were thus blinded with respect to the type of local anaesthetic treatment given on each occasion" Quote (from correspondence): "Outcome assessor was not blinded" Comment: if the outcome assessor was not blinded, he or she may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as high |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Nespeca 1976.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: military base (United States of America) Participants: 143 enrolled, 143 completing the study (100, excluding 0.25% bupivacaine, 1:200,000 epinephrine, which is not commercially available). Mean age 26 years, ranging from 16 to 65 years. Numbers of male and female participants not reported Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions | Inferior alveolar nerve block and infiltration injections (1.5 to 2.0 mL) of:
|
|
| Outcomes |
Cinical anaesthesia during maxillofacial procedures
Teeth/soft tissues tested: not stated Soft tissue anaesthesia: method of testing not stated: lip numbness, sensitivity in the vestibular gum
Soft tissues tested: soft tissues relevant to the procedure Adverse events reported (100/100)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The proposed agents....were randomly selected by the dental assistant, who blindly chose a lettered marker corresponding to one of the six agents" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The proposed agents....were randomly selected by the dental assistant, who blindly chose a lettered marker corresponding to one of the six agents" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The operator was not aware of the contents of the anaesthetic syringe" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The operator was not aware of the contents of the anaesthetic syringe" Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Anaesthesia (clinical) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Nordenram 1990.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Sweden) Participants: 40 enrolled, 40 completing the study. Mean age 71 years, ranging from 65 to 81 years (elderly group); mean age 24 years, ranging from 17 to 33 years (young group). 19 male, 21 female Inclusion criteria
Exclusion criteria: not stated |
|
| Interventions | Maxillary buccal infiltration of 0.6 mL of 1 of the following:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: central incisors, lateral incisors, cuspids Soft tissue anaesthesia
Soft tissues tested: upper lip Adverse effects were reported (120/120) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was carried out in a double‐blind design according to a randomized pattern" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study was carried out in a double‐blind design according to a randomized pattern" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was carried out in a double‐blind design according to a randomized pattern" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method for administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study was carried out in a double‐blind design according to a randomized pattern" Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia measured on 106 occasions (for those experiencing successful anaesthesia: 38 cases of 2% lidocaine, 1:80,000 epinephrine; 34 cases of 3% mepivacaine, no epinephrine; and 34 cases of 3% prilocaine, 0.03 IU/mL felypressin). As numbers in the groups were well balanced and were reduced for the same reasons, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: the number of participants who had the duration of pulpal anaesthesia measured was not stated but was probably the same as for onset of pulpal anaesthesia, as this was recorded at the same visit as onset. As numbers in the groups were well balanced and were reduced for the same reasons, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comments: the number of participants in each group who had the duration of soft tissue anaesthesia measured was not stated. Therefore risk of bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Nydegger 2014.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 60 enrolled, 60 completing the study. Mean age 26 years, ranging from 20 to 38 years. 30 male, 30 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Buccal infiltration (1.8 mL) opposite the mandibular first molars using:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars Adverse events reported (120/120)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, the 3 anesthetic formulations were randomly assigned 6‐digit numbers from a random number table. Each subject was randomly assigned to each of the 3 anesthetic formulations to determine which formulation was to be administered at each appointment" Quote (from correspondence): "We did use a computer to assign the anesthetic solutions to the subjects" |
| Allocation concealment (selection bias) | Low risk | Quote: "A master list with the 6‐digit numbers and the order in which the subject received the anesthetic formulations was accessible to a research assistant who prepared the anesthetic formulations for injection. Only the random numbers were recorded on the data collection sheets to further blind the experiment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Formulations were loaded by trained personnel into a separate, sterile 5‐mL Luer‐Lok disposable syringe (Becton‐Dickinson & Co, Rutherford, NJ) by aspirating the standard cartridge contents into an appropriate 6‐digit, labelled syringe. A master list with the 6‐digit numbers and the order in which the subject received the anesthetic formulations was accessible to a research assistant who prepared the anesthetic formulations for injection. Only the random numbers were recorded on the data collection sheets to further blind the experiment" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A master list with the 6‐digit numbers and the order in which the subject received the anesthetic formulations was accessible to a research assistant who prepared the anesthetic formulations for injection. Only the random numbers were recorded on the data collection sheets to further blind the experiment" "Trained personnel, who were blinded to the anesthetic formulations, administered all preinjection and postinjection tests" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Odabas 2012.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Turkey) Participants: 50 enrolled, 50 completing the study. 25 male, 25 female. Mean age of patients 11.3 years, ranging from 7 to 13 years Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration, using 1 cartridge (1.8 mL) of:
|
|
| Outcomes |
Clinical anaesthesia while performing operative dentistry procedures in deciduous teeth
Soft tissue anaesthesia
Soft tissues tested: upper lip Adverse events reported (100/100)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomized, double‐blind, split‐mouth design was used" "For local infiltration anaesthesia, in the first appointment, subjects were randomly selected to receive either a cartridge of Articaine 4% with 1:200,000 epinephrine.....or mepivacaine 3%" Quote (from correspondence): "Our nurse randomly selected a masked cartridge from a box and gave it to the operator. They were coded. We gave code 1 to articaine, code 2 to mepivacaine. In the first visit, the nurse recorded which code was used then at the second visit she gave the other coded cartridge. The cartridge was chosen randomly. Interestingly, when we checked the order of administration (first or second visit) we found equality for both solutions" |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomized, double‐blind, split‐mouth design was used" "For local infiltration anaesthesia, in the first appointment, subjects were randomly selected to receive either a cartridge of Articaine 4% with 1:200,000 epinephrine.....or mepivacaine 3%" Quote (from correspondence): "Our nurse randomly selected a masked cartridge from a box and gave it to the operator. They were coded. We gave code 1 to articaine, code 2 to mepivacaine. In the first visit, the nurse recorded which code was used then at the second visit she gave the other coded cartridge. The cartridge was chosen randomly. Interestingly, when we checked the order of administration (first or second visit) we found equality for both solutions" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Trained personnel, who were blind to the anaesthetic solutions, administered all preinjection and post‐injection tests" Quote (from correspondence): "We used our dental nurse for this procedure. She prepared the cartridges of local anaesthetic. We masked the cartridges with tape. They were coded. We gave code 1 to articaine, code 2 to mepivacaine" Comment: labelling all cartridges containing the same local anaesthetic with the same number could allow identification of a solution by personnel administering injections in a cross‐over study if the properties of the solutions were markedly different. Patients may comment about long duration, poor anaesthesia, etc., at their second visit. However, the properties of the 2 solutions would not allow identification, and a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trained personnel, who were blind to the anaesthetic solutions, administered all preinjection and post‐injection tests" Quote (from correspondence): "We used our dental nurse for this procedure. She prepared the cartridges of local anaesthetic. We masked the cartridges with tape. They were coded. We gave code 1 to articaine, code 2 to mepivacaine" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator (confirmed in correspondence). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete. Onset of anaesthesia was measured for all 50 participants (confirmed by study author) |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete. Duration of anaesthesia was measured for all 50 participants (confirmed by study author) |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Oliveira 2004.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 20 enrolled, 20 completing the study. Mean age 26 years, ranging from 20 to 39 years. 4 male, 16 female Inclusion criteria: healthy adults not taking any pain perception‐altering medication Exclusion criteria: none reported |
|
| Interventions | Maxillary infiltration buccally (1.8 mL) and palatally (0.35 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: right maxillary canines Soft tissue anaesthesia
Soft tissues tested: upper lip Adverse events reported (40/40)
|
|
| Notes | Non‐industry funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "During two separate appointments the subjects randomly received an infiltration" Quote (from correspondence): "The order of administration was randomized by a coin toss, previously to the beginning of the study. Each volunteer that entered the study was assigned to a number in the list, in sequence, following the order of entrance in the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "During two separate appointments the subjects randomly received an infiltration" Quote (from correspondence): "The cartridges were coded (nail polish with different colours) by a person not related to the study. The codes were opened after statistical analysis, which was performed by another researcher, not involved in the administration or in the pulp testing. The researchers and the subjects could just see the colours of the cartridges, with no knowledge of their content" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "The cartridges were coded (nail polish with different colours) by a person not related to the study. The codes were opened after statistical analysis, which was performed by another researcher, not involved in the administration or in the pulp testing. The researchers and the subjects could just see the colours of the cartridges, with no knowledge of their content" Comment: disguising the cartridges of each formulation with the same nail polish could allow identification of a solution by personnel administering injections in a cross‐over study if the properties of the solutions were markedly different. Patients may comment about long duration, poor anaesthesia, etc., at their second visit. However, the properties of the 2 solutions would not allow identification, and a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "The cartridges were coded (nail polish with different colours) by a person not related to the study. The codes were opened after statistical analysis, which was performed by another researcher, not involved in the administration or in the pulp testing. The researchers and the subjects could just see the colours of the cartridges, with no knowledge of their content" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator (confirmed in correspondence). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded (confirmed by study author); outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded (confirmed by study author); outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded (confirmed by study author); outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Ozec 2010.
| Methods | Randomized controlled simulated scenario trial, intraindividual study design | |
| Participants |
Location: university (Turkey) Participants: 30 enrolled, 30 completing the study. Mean age 22.6, ranging from 21 to 27 years. 14 male, 16 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.7 mL) using the following:
|
|
| Outcomes |
Soft tissue anaesthesia
Tissues tested: palatal tissues of maxillary first molars and first premolars |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 30 volunteers were divided randomly into 2 groups" "The teeth were randomized according to epinephrine doses. In the second group the same procedure was applied to the first molars" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The 30 volunteers were divided randomly into 2 groups" "The teeth were randomized according to epinephrine doses. In the second group the same procedure was applied to the first molars" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "All local anaesthetic injections were given by the same surgeon (U.T.), who had no involvement in assessing outcome" "The volunteers and the investigators of anaesthetic outcome were blinded to the epinephrine dose used" Comment:detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The volunteers and the investigators of anaesthetic outcome were blinded to the epinephrine dose used" Comment: detailed methods were not reported. Outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Parirokh 2015.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Iran) Participants
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) using the following:
|
|
| Outcomes |
Pulpal anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: first or second mandibular molars Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients who agreed to participate in the study were randomly divided into two groups of 30 patients each. Patients were randomly assigned to the groups by selecting a sealed opaque envelope with the group number concealed inside it" |
| Allocation concealment (selection bias) | Low risk | Quote: "All patients who agreed to participate in the study were randomly divided into two groups of 30 patients each. Patients were randomly assigned to the groups by selecting a sealed opaque envelope with the group number concealed inside it" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the clinician who administered the anesthetic solution was aware of the type of anesthetic technique used" Comment: detailed methods were not reported. The clinician administering the injections was aware of the formulation injected. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two clinicians performed the clinical procedures, one administered the IANB injection and the other prepared the endodontic access cavity 15 minutes following the injection. Only the clinician who administered the anesthetic solution was aware of the type of anesthetic technique used" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: one patient dropped out of the 2% lidocaine, 1:80,000 epinephrine group. As the 2 groups were still well balanced, risk of bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: one patient dropped out of the 2% lidocaine, 1:80,000 epinephrine group. As the 2 groups were still well balanced, risk of bias was rated as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Pellicer‐Chover 2013.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Spain) Participants: 36 enrolled, 36 completing the study. Mean age 23.1 ± 6 years, ranging from 18 to 37 years. 12 male, 24 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) and buccal infiltration (1.8 mL) using the following:
|
|
| Outcomes |
Clinical anaesthesia during surgical removal of third molars
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lower lip and tongue Adverse effects (72/72)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The articaine and bupivacaine carpules (1.8 ml) were marked as “1” or “2” by an individual unrelated to the study. The local anesthetic used and the side of the intervention were allotted randomly using a predefined random numbers table and enclosed in envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The articaine and bupivacaine carpules (1.8 ml) were marked as “1” or “2” by an individual unrelated to the study. The local anesthetic used and the side of the intervention were allotted randomly using a predefined random numbers table and enclosed in envelopes" Comment: clarification of the method of concealment needed; unsure whether coding the cartridges as 1 or 2 is related to their order of use or is used as an identifier. In this latter case, it would be possible to determine the identity of the local anaesthetic used through their differing properties |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The articaine and bupivacaine carpules (1.8 ml) were marked as '1' or '2' by an individual unrelated to the study. The local anesthetic used and the side of the intervention were allotted randomly using a predefined random numbers table and enclosed in envelopes" Comment: coding the cartridges as 1 or 2 may allow identification of a solution by personnel administering injections in a cross‐over study if the properties of the solutions were markedly different. Patients may comment about long duration, poor anaesthesia, etc., at their second visit. The properties of these 2 solutions may have allowed identification (related to duration). However it is not clear whether this occurred or whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The articaine and bupivacaine carpules (1.8 ml) were marked as '1' or '2' by an individual unrelated to the study. The local anesthetic used and the side of the intervention were allotted randomly using a predefined random numbers table and enclosed in envelopes" Comment: it is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes) if the identity of the local anaesthetic had been determined. Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comments: the number of participants in each group who had the duration of soft tissue anaesthesia measured was not stated (patients were asked to record the time of recovery ‐ unsure of compliance). Therefore risk of bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Poorni 2011.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: dental college and hospital (India) Participants: 156 enrolled, 156 completing the study. 90 male, 60 female. Age (mean ± standard deviation in years):
Inclusion criteria
Exclusion criteria
|
|
| Interventions | 1.8 mL of 1 of the following:
|
|
| Outcomes |
Clinical anaesthesia during pulpotomy of teeth with irreversible pulpitis
Teeth tested: mandibular molar teeth Soft tissue anaesthesia (self‐reported)
Soft tissues tested: lower lip |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For allocation of the subjects, a computer‐generated list of random numbers was used with a randomization ratio of 1:1:1 by using random allocation software (version 1.0, May 2004)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation sequence was concealed from the researchers who were a part of the study to reduce selection bias" Quote (from correspondence): "A case sheet was filled for every patient by the operator who enrolled the patients. The case sheet had a column which carried the group name to which the patient belonged to. Hence the sequence was concealed to the clinicians administering LA and recording outcomes" "The sequence was generated and was available to only one person and [was] hidden" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All local anaesthetic injections were given by a single operator who was not a part of the study process. This operator had no involvement with the study outcome. The trial adhered to established procedures to maintain separation among the operators" Quote (from correspondence): "The cartridges were concealed from the patients as they were masked" Comment: participants and personnel would not be able to identify the local anaesthetic used, and a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All local anaesthetic injections were given by a single operator who was not a part of the study process. This operator had no involvement with the study outcome. The trial adhered to established procedures to maintain separation among the operators" Quote (from correspondence): "The cartridges were hidden before the assessor entered" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Porto 2007.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 35 enrolled, 35 completing the study. Age ranging from 13 to 27 years. 10 male, 25 female Inclusion criteria
Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve block and buccal infiltration (minimum of 3.6 mL in total) of:
|
|
| Outcomes |
Clinical anaesthesia during extraction of lower third molars
Teeth/soft tissues tested: mandibular wisdom teeth and associated soft tissues Pulpal anaesthesia
Teeth tested: mandibular wisdom teeth Soft tissue anaesthesia tested by recording the time of return of normal sensation
Soft tissues tested: lower lip Adverse effects (number assessed was unclear)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Two groups were established (n = 35 each) on a randomized basis (by allotment), according to the anaesthetic solution" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Two groups were established (n = 35 each) on a randomized basis (by allotment), according to the anaesthetic solution" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants in each group who had the duration of soft tissue anaesthesia measured was not stated. Therefore risk of bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: the number of participants in each group who had adverse events measured was not stated. Therefore risk of bias was graded as unclear. Data were not used for meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Pässler 1996.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (Germany) Participants: 3 parts to the study, 2 suitable for this review:
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Second part: injections of 1 cartridge of either 2 mL (extractions) or 4 mL (apicectomies) of 1 of the following:
Third part: Injections of 1.7 mL of either:
|
|
| Outcomes |
Clinical anaesthesia during tooth removal and apicectomy
Teeth/tissues tested: second part: apicectomy ‐ anterior teeth, extraction ‐ not stated. Third part: extraction of mandibular anterior and premolar teeth Adverse effects were reported (220/220) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The distribution of the patients was carried out according to a randomization code which was known during the double‐blind experiment, by only the investigator" Comment: detailed method not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The distribution of the patients was carried out according to a randomization code which was known during the double‐blind experiment, by only the investigator" An assistant prepared the syringes, and vials were labelled and concealed Comment: detailed method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: "The distribution of the patients was carried out according to a randomization code which was known during the double‐blind experiment, by only the investigator" An assistant prepared the syringes, and vials were labelled and concealed Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: An assistant prepared the syringes, and vials were labelled and concealed. Exact details of blinding were not given Comment: the outcome is a participant‐reported outcome (outcome assessor is the participant) and was recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Ram 2006.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Israel) Participants: 62 enrolled, 62 completing the study. Mean age 8.4 years, ranging from 5 to 13 years. 28 male, 34 female Inclusion criteria
Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve block and maxillary buccal infiltration (up to 1 cartridge) using the following:
|
|
| Outcomes |
Clinical anaesthesia during paediatric operative dental procedures
Teeth tested: not stated Soft tissue anaesthesia
Soft tissues tested: not stated Adverse events reported (124/124)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random cross‐over design was used and each child served as his or her own control" "Each patient was randomly assigned to receive either lidocaine HCl 2% with 1:100 000 epinephrine ....... or articaine HCl 4% with 1:200 000 epinephrine ........ for the first visit, with the other solution administered during the second visit" Quote (from correspondence): "Closed envelopes were kept by the dental assistant (one in Jerusalem and other in Tel Aviv), inside there was written: lidocaine or articaine. The envelopes were mixed up before starting the study, and no one knew what was inside the envelope. The dental assistant (who was the only one who gave the operator the syringe) was the only one who knew which solution was delivered, and of course that she wrote the solution in a special file in order to know which solution should be administered in the second visit" |
| Allocation concealment (selection bias) | Low risk | Quote: "A random cross‐over design was used and each child served as his or her own control" "Each patient was randomly assigned to receive either lidocaine HCl 2% with 1:100 000 epinephrine ....... or articaine HCl 4% with 1:200 000 epinephrine ........ for the first visit, with the other solution administered during the second visit" Quote (from correspondence): "Closed envelopes were kept by the dental assistant (one in Jerusalem and other in Tel Aviv), inside there was written: lidocaine or articaine. The envelopes were mixed up before starting the study, and no one knew what was inside the envelope. The dental assistant (who was the only one who gave the operator the syringe) was the only one who knew which solution was delivered, and of course that she wrote the solution in a special file in order to know which solution should be administered in the second visit" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "The only person who knew which local anaesthesia was delivered was the dental assistant. The cartridge was 'hidden' in the syringe with aluminium foil, therefore no one other that the dental assistant knew which local anaesthetic solution was delivered" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A trained dental assistant, who did not participate in the treatment and was blinded to the agent being used, recorded the behavioural parameters in each centre" Quote (from correspondence): "The only person who knew which local anaesthesia was delivered was the dental assistant. The cartridge was 'hidden' in the syringe with aluminium foil, therefore no one other that the dental assistant knew which local anaesthetic solution was delivered" Comment: identification of the local anaesthetic by personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the numbers of participants in each group who had the duration of anaesthesia measured were not stated. Therefore, risk of bias was graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; success outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Robertson 2007.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 60 enrolled, 60 completing the study. Mean age 27 years, ranging from 19 to 51 years. 26 men and 34 women Inclusion criteria: in good health and not taking any medications that would alter the perception of pain Exclusion criteria
|
|
| Interventions | Mandibular buccal infiltration (1.8 mL) of either:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars, second molars, first premolars, and second premolars Adverse events reported (120/120)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, we randomly assigned the two anaesthetic formulations six‐digit numbers from a random number table. We randomly assigned each subject to one of the two formulations to determine which anaesthetic formulation was to be administered at each appointment" Quote (from correspondence): "Each solution had a six‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the experiment, we randomly assigned the two anaesthetic formulations six‐digit numbers from a random number table. We randomly assigned each subject to one of the two formulations to determine which anaesthetic formulation was to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To further blind the experiment, we recorded only the random numbers on the data collection sheets" "We masked the lidocaine and articaine cartridges with opaque labels and wrote the corresponding six‐digit codes on each cartridge" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To further blind the experiment, we recorded only the random numbers on the data collection sheets" "We masked the lidocaine and articaine cartridges with opaque labels and wrote the corresponding six‐digit codes on each cartridge" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: only those participants with successful pulpal anaesthesia could have pulpal onset measured. Study authors used 33 matched pairs from the 2 groups, so both groups were equal in size. As the groups were equal in size, risk of attrition bias was graded as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Ruprecht 1991.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Germany) Participants: 10 enrolled, 10 completing the study. Age ranging from 25 ± 5 years. 10 male, 0 female Inclusion criteria
Exclusion criteria: none stated |
|
| Interventions | Maxillary labial infiltrations (0.5 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary central incisors |
|
| Notes | Industry funding (local anaesthetic provided by Espe) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: detailed method not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: detailed method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: vials were coded by consecutive numbers. Detailed methods were not reported. Labelling all cartridges containing the same local anaesthetic with consecutive numbers could allow identification of a solution by personnel administering injections in a cross‐over study if the properties of the solutions were markedly different. It would depend on how the cartridges were numbered (e.g. 1 to 10 for one solution and 11 to 20 for another solution, etc.). Participants may comment about long duration, poor anaesthesia, etc., at their second visit. However, the properties of these solutions would not allow identification by participants and personnel, and a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: vials were coded by consecutive numbers. Detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Comment: industry funding, as local anaesthetic provided by Espe |
Sadove 1962.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: The journal article says approximately 700 completed the study. Actual total is 687 (343, excluding those not commercially available). Age range and mean age not reported. Male:female ratio not reported Inclusion criteria: not reported Exclusion criteria
|
|
| Interventions | Various types of dental block and infiltration of:
|
|
| Outcomes |
Pulpal anaesthesia tested during restorative and surgical procedures
Teeth tested: various Soft tissue anaesthesia
Soft tissues tested: various |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each solution in cartridges were assigned three different code numbers, and these cartridges were packed and used at random" |
| Allocation concealment (selection bias) | Low risk | Quote: "Each solution in cartridges were assigned three different code numbers, and these cartridges were packed and used at random" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This rigidly controlled double‐blind investigation was designed to eliminate all possible bias by using specially manufactured, coded dental anaesthetic cartridges, a sealed coding system, and a statistical evaluation of the collected data" "All the anaesthetic cartridges were identical in appearance, had no markings except for the numerical code" "Each solution in cartridges were assigned three different code numbers, and these cartridges were packed and used at random" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This rigidly controlled double‐blind investigation was designed to eliminate all possible bias by using specially manufactured, coded dental anaesthetic cartridges, a sealed coding system, and a statistical evaluation of the collected data." "All the anaesthetic cartridges were identical in appearance, had no markings except for the numerical code" "Each solution in cartridges were assigned three different code numbers, and these cartridges were packed and used at random" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: of the total number of participants recruited who had profound and adequate clinical anaesthesia, some did not have onset of soft tissue anaesthesia measured: lidocaine: ‐4/157 (% N/A), mepivacaine: ‐6/151 (% N/A) Negative values were obtained for dropouts (i.e. numbers of participants having soft tissue onset measured were greater than the numbers having clinical anaesthetic success measured). This is to be expected. The dropout rate, if present, could be calculated only if participants having soft tissue success were known. Soft tissue anaesthesia may have been present in those who had failure of clinical anaesthesia, or it may have been absent, meaning that it was not measured. As this measurement was performed in a clinic immediately before treatment, and as groups were fairly well balanced in numbers, it is highly unlikely that there was any significant attrition bias. Therefore risk of bias has been graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Comment: of the total number of participants recruited who had profound and adequate clinical anaesthesia, some did not have duration of soft tissue anaesthesia measured: lidocaine: 39/161 (24%), mepivacaine: 16/157 (10%) No dropouts would occur if the numbers of participants who had duration measured were equal to the numbers having soft tissue onset measured, assuming there were no incomplete onset data. However, even with these difficulties in measuring attrition rate, dropout rates of up to 24% were seen, which are likely to be conservative estimates if true soft tissue success figures are higher. Therefore risk of bias has been graded as high |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Sampaio 2012.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Brazil) Participants: 32 male, 38 female
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (3.6 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during access cavity preparation and instrumentation
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars and second molars Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Seventy adult patients (n = 70) were included in this prospective, randomized, double‐blind clinical study. To ensure the blindness of the study, 2 cartridges (3.6 mL) of either anaesthetic solution were sealed in envelopes. At the time of application, the senior researcher who administered the 2 consecutive anaesthesia injections chose 1 of the envelopes at random" |
| Allocation concealment (selection bias) | Low risk | Quote: "Seventy adult patients (n = 70) were included in this prospective, randomized, double‐blind clinical study. To ensure the blindness of the study, 2 cartridges (3.6 mL) of either anaesthetic solution were sealed in envelopes. At the time of application, the senior researcher who administered the 2 consecutive anaesthesia injections chose 1 of the envelopes at random" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Seventy adult patients (n = 70) were included in this prospective, randomized, double‐blind clinical study. To ensure the blindness of the study, 2 cartridges (3.6 mL) of either anaesthetic solution were sealed in envelopes. At the time of application, the senior researcher who administered the 2 consecutive anaesthesia injections chose 1 of the envelopes at random" Quote (from correspondence): "I was administering the anaesthetic injections in all cases and the cartridges were masked. However, as it could still be possible (unlikely but possible) to identify the rubber bungs, we have always used a different researcher to deliver the electric test and pulpectomy, to eliminate this risk" Comment: although the bung of the cartridge may have been visible and allowed the person administering the local anaesthetic to identify the solution, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Electric pulp stimulations to assess pulpal anaesthesia and the pulpectomy were performed by a different professional to guarantee that the anaesthetic solution remained unknown, thus maintaining the double blindness of the study" Quote (from correspondence): "We have always used a different researcher to deliver the electric test and pulpectomy, to eliminate this risk. The other researcher (Sampaio) was not present during the anaesthetic procedure and only 10 minutes after the anaesthetic procedure was completed (and I had left the workstation) Sampaio would enter to carry on the electric tests and pulpectomy. Therefore, the patients, as well as the post‐graduation student who was administering the electric tests and making the pulpectomy (Sampaio), were not aware of the identity of the cartridges" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Sancho‐Puchades 2012.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Spain) Participants: 20 enrolled, 18 completing the study. Mean age 23.8 years (SD 5.0 years; ranging from 18 to 35 years). 7 male, 11 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL: 1.3 mL injected at the mandibular foramen, 0.5 mL injected on withdrawal) and buccal infiltration (0.9 mL) of:
A further inferior alveolar nerve block (1.8 mL) was given if thermal testing was positive on the mandibular second molar on the injected side Additional intraligamental injections (0.2 mL) or inferior alveolar nerve blocks (1.8 mL) were given if pain was felt during surgery |
|
| Outcomes |
Clinical anaesthesia during extraction of lower third molars
Teeth/soft tissues tested: mandibular wisdom teeth and associated hard/soft tissues Pulpal anaesthesia tested with tetrafluoroethane (cold test)
Teeth tested: mandibular wisdom teeth Soft tissue anaesthesia
Soft tissues tested: lower lip.and tongue (+ retromolar trigone mucosa with onset) Adverse events reported (36/36)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study design: triple‐blind crossover randomized clinical trial" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Study design: triple‐blind crossover randomized clinical trial" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study design comprised a triple‐blind scheme. All anaesthetic Carpules were equally manufactured and were encoded. The patient, the surgeon and the statistician who performed the data analysis did not know which anaesthetic solution had been used" Comment: despite the method of encoding not being reported, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study design comprised a triple‐blind scheme. All anaesthetic Carpules were equally manufactured and were encoded. The patient, the surgeon and the statistician who performed the data analysis did not know which anaesthetic solution had been used" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: only 18 participants completed the study from 20 who started it, as 2 of the participants were withdrawn from the study because they did not attend the second surgical appointment. Excluded participants were accounted for when success was calculated. Because the study used a cross‐over design, groups remained exactly balanced. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: only 18 participants completed the study from 20 who started it, as 2 of the participants were withdrawn from the study because they did not attend the second surgical appointment. Excluded participants were accounted for when success was calculated. Because the study used a cross‐over design, groups remained exactly balanced. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Comment: the number of participants in each group who had the onset of soft tissue anaesthesia measured was not stated. As it is not clear how many participants had successful soft tissue anaesthesia, so that onset could be measured, risk of attrition bias has been graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants in each group who had the duration of soft tissue anaesthesia measured was not stated. It is not clear how many participants were compliant with reporting the duration. Therefore risk of attrition bias has been graded as unclear |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: only 18 participants completed the study from 20 who started it, as 2 of the participants were withdrawn from the study because they did not attend the second surgical appointment. Excluded participants were accounted for when success was calculated. Because the study used a cross‐over design, groups remained exactly balanced. Therefore, risk of attrition bias was graded as low |
| Selective reporting (reporting bias) | Unclear risk | Comment: all expected outcomes, apart from onset data, were reported. The study author could not be contacted. Therefore risk was rated as unclear |
| Other bias | Low risk | Comment: no other bias present |
Santos 2007.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 50 enrolled, 50 completing the study. Mean age 21.8 years, ranging from 18 to 40 years. 18 male and 32 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) and mandibular buccal infiltration (0.9 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during tooth removal
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: inferior lip, tongue, and mucosa Adverse effects were reported (100/100) |
|
| Notes | No funding was reported, but the study authors thanked Dixtal Biomédica Ind e Com Ltda, Marília/SP, Brazil, for providing the DX2010 monitoring system | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For local anaesthesia, in the first appointment, the patients randomly received A100 or A200. In the second appointment, the local anaesthetic not used previously was administered in a crossed manner" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "For local anaesthesia, in the first appointment, the patients randomly received A100 or A200. In the second appointment, the local anaesthetic not used previously was administered in a crossed manner" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a double‐blind study; that is, neither the surgeon nor the patients were aware of the local anaesthetic being used at the 2 different appointments" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This was a double‐blind study; that is, neither the surgeon nor the patients were aware of the local anaesthetic being used at the 2 different appointments" Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Comment: the number of participants in each group who had the onset of soft tissue anaesthesia measured was not stated. As it is not clear how many participants had successful soft tissue anaesthesia, so that onset could be measured, risk of attrition bias has been graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; success outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Sherman 1954.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: 191 enrolled, 191 completing the study. 700 injections given in total. Age ranging from 9 to 75 years. 63 male, 128 female Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Mandibular and maxillary injections:
of 1 of the following solutions:
2% procaine, 1:50,000 epinephrine used as a standard |
|
| Outcomes |
Clinical anaesthesia during operative dentistry procedures
Teeth tested: various Soft tissue anaesthesia
Soft tissues tested: various Adverse events reported (200/200) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A double code system was used, so that each solution was represented by two different code numbers. The cartridges were packaged in boxes of six, so that each box contained cartridges representing all six solutions" "the cartridges were selected by dental assistants who loaded the syringes for the operators" |
| Allocation concealment (selection bias) | Low risk | Quote: "A double code system was used, so that each solution was represented by two different code numbers. The cartridges were packaged in boxes of six, so that each box contained cartridges representing all six solutions" "the cartridges were selected by dental assistants who loaded the syringes for the operators" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In order to make this study as objective as possible, the six test solutions were placed in identical cartridges and codified. Thus, the characteristic metal or rubber caps and the distinctive Coloured plungers were not present" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"In order to make this study as objective as possible, the six test solutions were placed in identical cartridges and codified. Thus, the characteristic metal or rubber caps and the distinctive Coloured plungers were not present" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Comment: the number of participants in each group who had the onset of soft tissue anaesthesia measured was not stated. As it is not clear how many participants had successful soft tissue anaesthesia, so that onset could be measured, risk of attrition bias has been graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Comment: the number of participants in each group who had the duration of soft tissue anaesthesia measured was not stated. As it is not clear how many participants had successful soft tissue anaesthesia, so that duration could be measured, risk of attrition bias has been graded as unclear. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; success outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Sherman 2008.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: 42 enrolled, 40 completing the study. Age and age range not reported
Inclusion criteria
Exclusion criteria: none reported |
|
| Interventions | Gow‐Gates alveolar nerve block and maxillary buccal infiltration of 1 of the following:
|
|
| Outcomes |
Clinical anaesthesia during pulpotomy of teeth with irreversible pulpitis
Pulpal anaesthesia
Teeth tested: maxillary and mandibular posterior teeth |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Preceding the experiment, the 2 anaesthetic solutions were randomly assigned 3‐digit numbers from a random number table. The random numbers were subsequently assigned to a subject designating which anaesthetic solution the patient was to receive" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Preceding the experiment, the 2 anaesthetic solutions were randomly assigned 3‐digit numbers from a random number table. The random numbers were subsequently assigned to a subject designating which anaesthetic solution the patient was to receive" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Preceding the experiment, the 2 anaesthetic solutions were randomly assigned 3‐digit numbers from a random number table. The random numbers were subsequently assigned to a subject designating which anaesthetic solution the patient was to receive" "The cartridges of anaesthetic solution were 'blinded' by completely masking the aluminium caps with a permanent black marker and masking the appropriate cartridges with an opaque label" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Preceding the experiment, the 2 anaesthetic solutions were randomly assigned 3‐digit numbers from a random number table. The random numbers were subsequently assigned to a subject designating which anaesthetic solution the patient was to receive" "The cartridges of anaesthetic solution were 'blinded' by completely masking the aluminium caps with a permanent black marker and masking the appropriate cartridges with an opaque label" Comment: Outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Quote: "One patient with LE and one with AE did not have a negative response to cold stimuli at the 15‐minute mark and were not included in this study" Comment: one patient dropped out of each group. As the 2 groups were still equal in size and reasons for dropping out were the same (still positive to the cold test following local anaesthesia), risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; success outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Sierra Rebolledo 2007.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Spain) Participants: 30 enrolled, 27(?) completing the study. Mean age 23.72 years, ranging from 18 to 36 years. 13 male, 17 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) and buccal infiltration (1.8 mL) of either:
|
|
| Outcomes |
Clinical anaesthesia during tooth removal
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to one of the two anaesthetic groups" "The anaesthetic techniques were performed on a random basis by one of the two operators" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to one of the two anaesthetic groups" "The anaesthetic techniques were performed on a random basis by one of the two operators" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Unclear risk | Quote: "three were excluded from the study: one due to the development of transient inferior alveolar nerve paraesthesia, another because of transient paraesthesia of the lingual nerve, and the third as a result of voluntary dropout from the study" Comment: results were based only on those who were not excluded. However, it is not clear from the journal article how the final figures for those completing the study were derived. Although 3 participants dropped out, these were in the lidocaine group and there were 6 dropouts in this group. Six dropouts would occur only if the study used a parallel design and 2 teeth in each of the 3 dropouts would have been extracted. Risk of attrition bias was therefore graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Unclear risk | Quote: "three were excluded from the study: one due to the development of transient inferior alveolar nerve paraesthesia, another because of transient paraesthesia of the lingual nerve, and the third as a result of voluntary dropout from the study" Comment: results were based only on those who were not excluded. However, it is not clear from the journal article how the final figures for those completing the study were derived. Although 3 participants dropped out, these were in the lidocaine group and there were 6 dropouts in this group. Six dropouts would occur only if the study used a parallel design and 2 teeth in each of the 3 dropouts should have been extracted. Risk of attrition bias was therefore graded as unclear |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Unclear risk | Quote: "three were excluded from the study: one due to the development of transient inferior alveolar nerve paraesthesia, another because of transient paraesthesia of the lingual nerve, and the third as a result of voluntary dropout from the study" Comment: results were based only on those who were not excluded. However, it is not clear from the journal article how the final figures for those completing the study were derived. Although 3 participants dropped out, these were in the lidocaine group and there were 6 dropouts in this group. Six dropouts would occur only if the study used a parallel design and 2 teeth in each of the 3 dropouts should have been extracted. Risk of attrition bias was therefore graded as unclear |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Silva 2012.
| Methods | Randomized controlled clinical and simulated scenario trial, intraindividual study design, although the study is described by the authors as using "a prospective, randomized, controlled, parallel group" design | |
| Participants |
Location: university (Brazil) Participants: 24 enrolled, 20 completing the study. Mean age 23.25 ± 3.94 years, ranging from 18 to 30 years. 6 men and 18 women Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (3.6 mL) and mandibular buccal infiltration (0.9 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during tooth removal
Teeth tested: mandibular third molars Soft tissue anaesthesia
Soft tissues tested: not stated Adverse effects were reported (40/48)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The choice of the first side to be operated and the group of anaesthetic solutions used had been randomly distributed, after a random drawing using the envelope method" Comment: exact method of generation of randomized sequence not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The choice of the first side to be operated and the group of anaesthetic solutions used had been randomly distributed, after a random drawing using the envelope method" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. It is not clear whether the person recording participant outcomes was a different person than the one administering the local anaesthetic, as they may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as unclear |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: only 20 participants completed the study from 24 who started it, as 2 were excluded from the analysis because of an incomplete pain diary form, and the other 2 did not return for the second surgery. Excluded participants were accounted for when success was calculated. Because the study used a cross‐over design, groups remained exactly balanced. Therefore, risk of attrition bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: only 20 participants completed the study from 24 who started it, as 2 were excluded from the analysis because of an incomplete pain diary form, and the other 2 did not return for the second surgery. Excluded participants were accounted for when success was calculated. Because the study used a cross‐over design, groups remained exactly balanced. Therefore, risk of attrition bias was graded as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: only 20 participants completed the study from 24 who started it, as 2 were excluded from the analysis because of an incomplete pain diary form, and the other 2 did not return for the second surgery. Excluded participants were accounted for when adverse events were studied. Because the study used a cross‐over design, groups remained exactly balanced. Therefore, risk of attrition bias was graded as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Sood 2014.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (India) Participants: 100 enrolled, 100 completing the study. Age ranging from 18 to 50 years. 47 male, 53 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during pulpectomy of teeth with irreversible pulpitis
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first premolars, second premolars, first molars, second molars, and third molars Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "1 cartridge [1.8 mL] of either anesthetic solution was sealed in envelopes. At the time of application, one researcher, who administered the anesthesia injections, chose one of the envelopes at random" |
| Allocation concealment (selection bias) | Low risk | Quote: "1 cartridge [1.8 mL] of either anesthetic solution was sealed in envelopes. At the time of application, one researcher, who administered the anesthesia injections, chose one of the envelopes at random" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To ensure the blindness of the study, the label on the cartridges was removed and the cartridges were coded" Comment: participants and personnel would not be able to identify the local anaesthetic used.. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To ensure the blindness of the study, the label on the cartridges was removed and the cartridges were coded" "Electric pulp stimulations to assess pulpal anesthesia were performed by a colleague to guarantee that the anesthetic solution remained unknown and thus maintain the double blindness of the study" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Srinivasan 2009.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (India) Participants: 40 enrolled, 40 completing the study First premolar:
First molar:
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal infiltration (1.7 mL) of 2 of the following:
|
|
| Outcomes |
Clinical anaesthesia during access cavity preparation in teeth with irreversible pulpitis
Teeth tested: maxillary first premolars and first molars |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These 40 patients were randomly divided into 4 study groups" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "These 40 patients were randomly divided into 4 study groups" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All the patients and investigator were blinded to the type of anaesthetic solution used" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore, risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A single operator gave all local anaesthetic injections using standard dental aspirating syringe fitted with a 27‐gauge, 1.5‐inch needle and this operator had no involvement with testing the outcome" "All the patients and investigator were blinded to the type of anaesthetic solution used" Comment: the outcome is a patient‐reported outcome (outcome assessor is the patient) and was recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Srisurang 2011.
| Methods | Randomized controlled simulated scenario trial, parallel study design | |
| Participants |
Location: university (Thailand) Participants: 33 enrolled, 33 completing the study (48 teeth extracted). Mean age 18.2 years, ranging from 13 to 45 years. Male:female ratio not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary buccal (0.9 mL) and palatal (0.3 mL) infiltrations of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary lateral incisors, canines, first and second premolars, and first molars Soft tissue anaesthesia
Adverse effects (48/48)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each patient was randomly assigned to one of the following three anesthetic solution groups: 2% lidocaine (lidocaine HCl 2%; Cook‐Waite, Abbott Laboratories, KS, USA), 2% mepivacaine (Scandonest 2% special; Septodont, Kent, UK) or 4% articaine (Ubistesin 3M ESPE; ESPE Platz, Seefeld, Germany), all with 1:100 000 epinephrine" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each patient was randomly assigned to one of the following three anesthetic solution groups: 2% lidocaine (lidocaine HCl 2%; Cook‐Waite, Abbott Laboratories, KS, USA), 2% mepivacaine (Scandonest 2% special; Septodont, Kent, UK) or 4% articaine (Ubistesin 3M ESPE; ESPE Platz, Seefeld, Germany), all with 1:100 000 epinephrine" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All cartridges were unidentified, with their stickers removed and the volume of the solution labelled with a permanent marker" Comment: exact details of blinding methods were not reported. However, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All cartridges were unidentified, with their stickers removed and the volume of the solution labelled with a permanent marker" "Soft tissues: One trained person, blinded to the anesthetic solutions, performed all pre‐injection and post‐injection tests" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Stibbs 1964.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: 751 enrolled, 751 completing the study (512 excluding 2% procaine/1.5% tetracaine, 1:20,000 levonordefrin). Age and sex distribution not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Various mandibular and infiltration injections of 1 of the following (varying volumes):
|
|
| Outcomes |
Pulpal anaesthesia during restorative procedures
Teeth tested: not reported Soft tissue anaesthesia
Soft tissues tested: not reported Adverse events reported (512/512) |
|
| Notes | Industry funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The numbered cartridges were jumbled and packed in lots of 50 in sealed cans, thus assuring randomized use" |
| Allocation concealment (selection bias) | Low risk | Quote: "The numbered cartridges were jumbled and packed in lots of 50 in sealed cans, thus assuring randomized use" "The identity of each code was not revealed until the data were to be assembled for analysis" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Three sterile isotonic local anesthetic solutions were provided in identical dental cartridges. Each cartridge was identified only by a control number. To assure the blindness of the study, three different numbers were assigned to each solution which made a total of nine code numbers. The identity of each code was not revealed until the data were to be assembled for analysis" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Three sterile isotonic local anesthetic solutions were provided in identical dental cartridges. Each cartridge was identified only by a control number. To assure the blindness of the study, three different numbers were assigned to each solution which made a total of nine code numbers. The identity of each code was not revealed until the data were to be assembled for analysis" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: of the total number of participants recruited who had successful anaesthesia, some did not have onset of soft tissue anaesthesia measured: mandibular injection:
infiltration:
For onset of soft tissue anaesthesia, small values and even negative values were obtained for dropouts (i.e. numbers of participants having onset measured were greater than numbers having anaesthetic success measured). This is to be expected. However, the dropout rate if present could be calculated only if those having soft tissue success were known. Soft tissue anaesthesia may have been present in those who had failure of anaesthesia during endodontic and periodontal treatment, or it may have been absent, meaning that it was not measured. As this measurement was performed in a clinic immediately before treatment, and as groups were fairly well balanced in numbers, it is highly unlikely that there was any attrition bias. Therefore risk of attrition bias has been graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Comment: of the total number of participants recruited who had successful anaesthesia, some did not have duration of soft tissue anaesthesia measured: mandibular injection:
infiltration:
No dropouts would occur if the numbers of participants having duration measured were equal to the numbers having soft tissue onset measured, assuming there were no incomplete onset data. However, even with these difficulties in measuring attrition rate, dropout rates of up to 21% were seen, which are likely to be conservative estimates if true soft tissue success figures are higher. Therefore risk of attrition bias has been graded as high |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; success outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Study was supported by a grant from Cook‐Waite Laboratories, Inc. |
Thakare 2014.
| Methods | Randomized controlled clinical trial, cross‐over study design | |
| Participants |
Location: hospital (India) Participants: 40 enrolled, 40 completing the study (160 teeth in total). Ages ranging from 10 to 18 years (no mean). Male:female ratio not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary labial infiltration (1.4 mL) of either:
|
|
| Outcomes |
Clinical anaesthesia during premolar removal
Teeth tested: maxillary and mandibular premolars (one side of the face) Adverse effects were reported (160/160)
|
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A computer‐generated list was used to allocate each patient into either 4% articaine or 0.5% bupivacaine groups" Comment: exact method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A computer‐generated list was used to allocate each patient into either 4% articaine or 0.5% bupivacaine groups" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: self‐assessment of onset of soft tissue anaesthesia may have been the method used. No patients were excluded. Outcome data were complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Tofoli 2003.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 20 enrolled, 20 completing the study. Mean age 23 years, ranging from 20 to 35 years. 7 male, 13 female Inclusion criteria: healthy individuals who did not use any medication 1 week before or during the experiment, having the right inferior first premolars free of caries and restorations Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: right mandibular first premolars Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | Study authors acknowledge the financial support of CNPQ‐PIBIC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Both solutions were randomly applied to the subjects at 2 different sessions" Quote (from correspondence): "The order of administration was randomized by a tossed coin, prior to the beginning of the study. Each volunteer that entered the study was assigned to a number in the list, in sequence, following the order of entrance in the study (Heads: code 1; Tails: code 2. Code 1: First anaesthesia: blue solution; Code 2: First anaesthesia: red solution)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Both solutions were randomly applied to the subjects at 2 different sessions" Quote (from correspondence): "The solutions were administered by a senior [researcher]; a clinician previously trained to use the pulp tester evaluated anaesthesia parameters. Another [researcher] performed the statistical analysis before the codes were revealed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In this double blind random study, the solutions were codified by an individual involved neither in the administration of the anaesthetic solutions nor in pulp testing procedures" Quote (from correspondence): "The identification on the cartridges was removed with alcohol and gauze, and each solution was assigned a colour (a strip of adhesive tape). This procedure was conducted by a person not involved in the administration or evaluation of anaesthesia parameters (pulp testing and statistical analysis). Therefore, the person who administered the solutions, the one that evaluated the anaesthesia parameters and the volunteers were able just to see the colour assigned to the solutions (tape strip)" Comment: disguising the cartridges of each formulation with the same coloured tape could allow identification of a solution by personnel administering injections in a cross‐over study if the properties of the solutions were markedly different. Participants may comment about long duration, poor anaesthesia, etc., at their second visit. However, the properties of the 2 solutions are unlikely to allow identification, and a pre‐determined method of administration was used by personnel, to minimize variation. Therefore, risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In this double blind random study, the solutions were codified by an individual involved neither in the administration of the anaesthetic solutions nor in pulp testing procedures" Quote (from correspondence): "The identification on the cartridges was removed with alcohol and gauze, and each solution was assigned a colour (a strip of adhesive tape). This procedure was conducted by a person not involved in the administration or evaluation of anaesthesia parameters (pulp testing and statistical analysis). Therefore, the person who administered the solutions, the one that evaluated the anaesthesia parameters and the volunteers were able just to see the colour assigned to the solutions (tape strip)" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded (confirmed by study author); outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded (confirmed by study author); outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded (confirmed by study author); outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: study authors acknowledge the financial support of CNPQ‐PIBIC |
Tortamano 2009.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Brazil) Participants: 40 enrolled, 40 completing the study, with mean age of 29.9 years (articaine)/34.1 years (lidocaine). 16 males and 24 females Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (3.6 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during pulpectomy of teeth with irreversible pulpitis
Teeth tested: mandibular second premolars, first molars, second molars, and third molars Pulpal anaesthesia tested with an electric pulp tester
Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure the blindness of the study, 2 cartridges (3.6 mL) of either anaesthetic solution were sealed in envelopes" "At the time of application, the senior researcher, who administered the 2 consecutive anaesthesia injections, chose 1 of the envelopes at random" |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure the blindness of the study, 2 cartridges (3.6 mL) of either anaesthetic solution were sealed in envelopes" "At the time of application, the senior researcher, who administered the 2 consecutive anaesthesia injections, chose 1 of the envelopes at random" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To ensure the blindness of the study, 2 cartridges (3.6 mL) of either anaesthetic solution were sealed in envelopes" Quote (from correspondence): "Apart from placing the cartridges inside the envelopes and sealing them, we also masked (painted) the cartridges. However, during the pilot tests, we realized that, as the rubber of the cartridges had different colours, it might be possible still to identify the cartridges. Therefore, we did not mention the painted cartridges and decided to have a different researcher performing the electric tests and making the pulpectomy, so that we could ensure that the testing was blind and relevant" Comment: although the bung of the cartridge may have been visible and allowed the person administering the local anaesthetic to identify the solution, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To ensure the blindness of the study, 2 cartridges (3.6 mL) of either anaesthetic solution were sealed in envelopes" "Electric pulp stimulations to assess pulpal anaesthesia were performed by a postgraduate student to guarantee that the anaesthetic solution remained unknown and thus maintain the double‐blindness of the study" Quote (from correspondence): "Apart from placing the cartridges inside the envelopes and sealing them, we also masked (painted) the cartridges. However, during the pilot tests, we realized that, as the rubber of the cartridges had different colours, it might be possible still to identify the cartridges. Therefore, we did not mention the painted cartridges and decided to have a different researcher performing the electric tests and making the pulpectomy, so that we could ensure that the testing was blind and relevant" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Tortamano 2013.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Brazil) Participants: 30 enrolled, 30 completing the study. Mean age 24.63 years, ranging from 18 to 40 years. 15 male, 15 female Inclusion criteria
Exclusion criteria: taking medication that can potentially interact with any of the anaesthetics used in the study |
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular molars |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 30 blinded subjects randomly received an IAN block injection..." "Three cartridges of each local anesthetic solution were sealed in 30 envelopes (one for each patient). During application, the main investigator who administered the three injections (one per appointment) randomly removed one cartridge from the envelope. Only one cartridge was randomly chosen and administered per appointment. The initial tooth to be restored was randomly selected" Quote (from correspondence): "Although the cartridges were all painted with black ink, the rubber in lidocaine solution is orange which, if observed against a bright light, could eventually be identified. To avoid this risk, the main investigator (myself) personally took out of the envelope (blind) one of the cartridges, inserted it into the carpule syringe and applied the injection on the patient. Then, I would leave the patient to the Graduate student, who applied all the electric tests" "The remaining cartridges would stay in the same envelope, ready to be randomly selected and used in the next appointment of that specific patient. For cross check, the used cartridge was identified (e.g. 'C1' referred to 'appointment 1') and placed in another brown envelope, with the same patient identification number. The same procedure was used in appointment 2 and 3" |
| Allocation concealment (selection bias) | Low risk | Quote: "Three cartridges of each local anesthetic solution were sealed in 30 envelopes (one for each patient). During application, the main investigator who administered the three injections (one per appointment) randomly removed one cartridge from the envelope. Only one cartridge was randomly chosen and administered per appointment. The initial tooth to be restored was randomly selected" Quote (from correspondence): "Although the cartridges were all painted with black ink, the rubber in Lidocaine solution is orange which, if observed against a bright light, could eventually be identified. To avoid this risk, the main investigator (myself) personally took out of the envelope (blind) one of the cartridges, inserted it into the carpule syringe and applied the injection on the patient. Then, I would leave the patient to the Graduate student, who applied all the electric tests" "The remaining cartridges would stay in the same envelope, ready to be randomly selected and used in the next appointment of that specific patient. For cross check, the used cartridge was identified (e.g. 'C1' referred to 'appointment 1') and placed in another brown envelope, with the same patient identification number. The same procedure was used in appointment 2 and 3" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "These tests were conducted by a blinded researcher to ensure that the anesthetic solution remained unknown, thus maintaining the double‐blindness of the study" Quote (from correspondence): "Although the cartridges were all painted with black ink, the rubber in Lidocaine solution is orange which, if observed against a bright light, could eventually be identified. To avoid this risk, the main investigator (myself) personally took out of the envelope (blind) one of the cartridges, inserted it into the carpule syringe and applied the injection on the patient. Then, I would leave the patient to the Graduate student, who applied all the electric tests" Comment: although the bung of the cartridge may have been visible and allowed the person administering the local anaesthetic to identify the solution, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore, risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "These tests were conducted by a blinded researcher to ensure that the anesthetic solution remained unknown, thus maintaining the double‐blindness of the study" Quote (from correspondence): "Although the cartridges were all painted with black ink, the rubber in lidocaine solution is orange which, if observed against a bright light, could eventually be identified. To avoid this risk, the main investigator (myself) personally took out of the envelope (blind) one of the cartridges, inserted it into the carpule syringe and applied the injection on the patient. Then, I would leave the patient to the Graduate student, who applied all the electric tests" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Trieger 1979.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: 69 enrolled, 69 completing the study. Age ranging from 14 to 55 years. Male:female ratio not reported Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions | Mandibular nerve block and infiltration anaesthesia, using variable volumes of:
Note ‐ Some patients received a general anaesthetic, and injections were given at the end of surgery |
|
| Outcomes |
Clinical anaesthesia and postoperative analgesia during extraction
Teeth tested: mandibular third molars and teeth requiring bone removal at time of extraction Soft tissue anaesthesia
Soft tissues tested: those at the site of extraction Adverse effects were reported (54/54) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Assignment to the three drug groups was randomized based on the alphabet" Quote (from correspondence): "Random sampling was done by dividing up the alphabet into three segments and assigning each patient to a group, based on the family name of each subject. For example: a to i made one group; j to r another and s to z a third" Comment: the randomisation process, which was based on the alphabet, resulted in imbalance in group size:
|
| Allocation concealment (selection bias) | High risk | Quote: "Assignment to the three drug groups was randomized based on the alphabet" Quote (from correspondence): "Random sampling was done by dividing up the alphabet into three segments and assigning each patient to a group, based on the family name of each subject. For example: a to i made one group; j to r another and s to z a third" "Once that selection was made the dental assistant was requested to put the specific disposable loaded syringe on the surgical tray for the surgeon to administer" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (from correspondence): "Blinding as to which anaesthetic was used for a given case was not possible since the dental assistant who was directed to provide the drug, the surgeon and the recorder all were aware. However the patient was unaware as to which agent was used" Comment: participants undergoing testing were blinded but the clinician administering local anaesthetic was not. Identification of the local anaesthetic by participants is unlikely. It is not clear whether a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (from correspondence): "Blinding as to which anaesthetic was used for a given case was not possible since the dental assistant who was directed to provide the drug, the surgeon and the recorder all were aware. However the patient was unaware as to which agent was used" Comment: detailed methods were not reported. The person recording participant outcomes knew the identity of the formulations and may have been able to influence participants' responses (participant‐reported outcomes). Therefore, risk of bias was graded as high |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; success outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; success outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Trullenque‐Eriksson 2011.
| Methods | Randomized controlled clinical and simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Spain) Participants: 35 enrolled, 19 completing the study. Mean age 24.47 years. 6 male and 13 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block and mandibular buccal infiltration (1.8 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during tooth removal
Teeth/soft tissues tested: mandibular third molars and adjacent tissues .Soft tissue anaesthesia: Lip numbness was self‐reported; sensitivity in the vestibular gum was measured with a sharp instrument
Soft tissues tested: inferior alveolar nerve: lip; buccal nerve: vestibular gum Adverse events reported (38/70)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were randomly administered one of the two local anaesthetics in the first surgery, and the other one in the following" Quote (from correspondence): "Several surgeons participated in our study. They were told to randomly choose an anaesthetic for the first surgery. They were not offered both anaesthetics in a container to blindly pick one. Both were in the same container (drawer) but they were able to see their choice. The observer however was not able to see what anaesthetic had been chosen / was being used" |
| Allocation concealment (selection bias) | High risk | Quote: "The patients were randomly administered one of the two local anaesthetics in the first surgery, and the other one in the following" Quote (from correspondence): "Several surgeons participated in our study. They were told to randomly choose an anaesthetic for the first surgery. They were not offered both anaesthetics in a container to blindly pick one. Both were in the same container (drawer) but they were able to see their choice. The observer however was not able to see what anaesthetic had been chosen / was being used" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The anaesthetic used was unknown for the patient and the observer who performed the measurements. At the time of the surgery this information was only known by the surgeon who administered the anaesthesia and the surgeon who assisted him, who recorded the anaesthetic and dose in the patient’s medical history and a collection sheet in an opaque envelope, which were not consulted until the data analysis." "The double‐blind contributed to avoid bias, as the observer and the patient ignored the anaesthetic used in each surgery" Comment: identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was not used by personnel to minimize variation. The surgeon administering local anaesthetic was not blinded and was allowed to vary the dose depending on what he or she thought was necessary. Therefore, risk of bias was graded as high |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The anaesthetic used was unknown for the patient and the observer who performed the measurements. At the time of the surgery this information was only known by the surgeon who administered the anaesthesia and the surgeon who assisted him, who recorded the anaesthetic and dose in the patient’s medical history and a collection sheet in an opaque envelope, which were not consulted until the data analysis" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | High risk | Quote: "Of the thirty‐five patients selected, nineteen were included in the study" Quote (from correspondence): "Due to not fulfilling inclusion criteria:
Comment: although accounted for, 46% were excluded and reasons for exclusion varied. Therefore risk of attrition bias was graded as high |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | High risk | Quote: "Of the thirty‐five patients selected, nineteen were included in the study" Quote (from correspondence): "Due to not fulfilling inclusion criteria:
Comment: although accounted for, 46% were excluded and reasons for exclusion varied. Therefore risk of attrition bias was graded as high |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Quote: "Of the thirty‐five patients selected, nineteen were included in the study" Quote (from correspondence): "Due to not fulfilling inclusion criteria:
Comment: although accounted for, 46% were excluded and reasons for exclusion varied. Therefore risk of attrition bias was graded as high |
| Incomplete outcome data (attrition bias) Adverse events | High risk | Quote: "Of the thirty‐five patients selected, nineteen were included in the study" Quote (from correspondence): “Due to not fulfilling inclusion criteria:
Comment: although accounted for, 46% were excluded and reasons for exclusion varied. Therefore risk of attrition bias was graded as high |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Vahatalo 1993.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Finland) Participants: 20 enrolled, 20 completing the study. Mean age 23.8 years. 8 male, 12 female Inclusion criteria
Exclusion criteria: none reported |
|
| Interventions | Maxillary buccal infiltration (0.6 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: maxillary lateral incisors |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study protocol was double‐blind. The code was not broken until the statistical analysis of the data. The dental assistant was the only person aware of which preparation was being injected" Quote (from correspondence): "The one and only research nurse loaded 1 ml tuberculin syringes by aspiration with 0.6ml test solution from commercially available cartridges" "She had lists of tested study persons who were coded and was aware what solution (A or B) used in second visit" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study protocol was double‐blind. The code was not broken until the statistical analysis of the data. The dental assistant was the only person aware of which preparation was being injected" Quote (from correspondence): "The one and only research nurse loaded 1 ml tuberculin syringes by aspiration with 0.6ml test solution from commercially available cartridges" "She had lists of tested study persons who were coded and was aware what solution (A or B) used in second visit" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Vilchez‐Perez 2012.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Spain) Participants: 33 enrolled, 20 completing the study. Mean age 22.75 years (SD = 2.15). 5 male, 15 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Maxillary labial infiltration (0.9 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: right and left maxillary lateral incisors Soft tissue anaesthesia
Soft tissues tested:
Adverse events reported (40/40)
|
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was based on a sequence generated by Laboratorios Inibsa, Barcelona, Spain" Quote (from correspondence): "Randomization was based on a sequence generated by Laboratorios Inibsa, Barcelona, Spain. There was an individual envelope for each volunteer with information about which solution (solution A or solution B) had to be infiltrated in each side (right side or left side). The solutions A or B were different in each envelope. The list of treatment implemented (articaine or bupivacaine) was saved by Laboratorios Inibsa" Comment: exact method of generation of randomized sequence needing clarification |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was based on a sequence generated by Laboratorios Inibsa, Barcelona, Spain" Quote (from correspondence): "Randomization was based on a sequence generated by Laboratorios Inibsa, Barcelona, Spain. There was an individual envelope for each volunteer with information about which solution (solution A or solution B) had to be infiltrated in each side (right side or left side). The solutions A or B were different in each envelope. Both solutions were encoded so that the surgeon performing the anaesthesia infiltration, the monitor recording the variables and the volunteer could not identify the anaesthetic solution used. The code of solutions was given to us after the statistical analysis by Laboratorios Inibsa" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both solutions were encoded so that the surgeon performing the anaesthesia infiltration, the monitor recording the variables and the volunteer could not identify the anaesthetic solution used" Quote (from correspondence): "The code of solutions was given to us after the statistical analysis by Laboratorios Inibsa" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore, risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both solutions were encoded so that the surgeon performing the anaesthesia infiltration, the monitor recording the variables and the volunteer could not identify the anaesthetic solution used" Quote (from correspondence): "The code of solutions was given to us after the statistical analysis by Laboratorios Inibsa" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Visconti 2016.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Brazil) Participants
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks (1.8 mL or 3.6 mL) using the following:
|
|
| Outcomes |
Clinical anaesthesia during access cavity preparation in teeth with irreversible pulpitis
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular molars Soft tissue anaesthesia
Soft tissues tested: lower lip |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blinding was achieved as follows: 3 cartridges (1.8 mL each) of each anesthetic solution were sealed in 42 envelopes by the first author. The senior researcher, who was not involved in the endodontic procedure, administered the anaesthesia injection after choosing 1 of the envelopes at random" |
| Allocation concealment (selection bias) | Low risk | Quote: "Blinding was achieved as follows: 3 cartridges (1.8 mL each) of each anesthetic solution were sealed in 42 envelopes by the first author. The senior researcher, who was not involved in the endodontic procedure, administered the anaesthesia injection after choosing 1 of the envelopes at random" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Blinding was achieved as follows: 3 cartridges (1.8 mL each) of each anesthetic solution were sealed in 42 envelopes by the first author. The senior researcher, who was not involved in the endodontic procedure, administered the anaesthesia injection after choosing 1 of the envelopes at random" Quote (from correspondence): "We assembled 21 envelopes with three cartridges of 2% lidocaine with 1:100,000 epinephrine another 21 envelopes with three cartridges of 2% mepivacaine with 1:100,000 epinephrine. The 42 envelopes were stored in a box where the anesthesia applicator (senior operator) randomly, took one of the envelopes. It should be noted that all injections were administered by the same operator (senior operator), since the electrical tests and the opening were performed by another operator. So the patient was blind, as well as the operator of the electrical tests. The only person who knows the anesthesia was the senior operator (who did the injections)" Comment: identification of the local anaesthetic by participants is unlikely. Although the operator administering the local anaesthetic knew the identity of the formulation used, a pre‐determined method of administration was used by the operator to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Electric pulp stimulations to assess pulpal anesthesia and the pulpectomy were performed by a postgraduate student to guarantee that the anesthetic solution remained unknown and thus maintain the double‐blindness of the study. All pre‐injection and post‐injection tests were conducted by trained personnel who were blinded to the anesthetic volumes administered" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: only 32 participants were tested clinically from 42 who started it, as 3 were eliminated from the mepivacaine group and 7 were eliminated from the lidocaine group following failure of anaesthesia tested with the electric pulp tester. Excluded participants were accounted for when success was calculated; groups remained balanced (18 vs 14) and reasons for dropout were the same. Therefore risk of attrition bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Vreeland 1989.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 30 enrolled, 30 completing the study. Mean age 25.5 years, ranging from 22 to 32 years. 27 male, 3 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars, canines, and lateral incisors Soft tissue anaesthesia (alveolar mucosal sticks labial and lingual to the test canine and buccal to the test molar; patient was asked if the lip and tongue were numb)
Soft tissues tested: soft tissues labial and lingual to the test canine and buccal to the test contralateral canine/first molar |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each subject was randomly assigned to one of six letter combinations in order to determine the sequence of solution administration. A four digit random number was assigned prior to the experiment for each subject and recorded on a master code list" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Each subject was randomly assigned to one of six letter combinations in order to determine the sequence of solution administration. A four digit random number was assigned prior to the experiment for each subject and recorded on a master code list" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetics solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All injections, as timed with a watch, took 2 min to complete so the subject was unaware of which solution he or she received" Comment: the participant may be aware of a difference in injections, as double the volume of 2% lidocaine, 1:200,000 epinephrine was injected, which may feel different. However, the participant would not necessarily know the identity of the formulation at each visit. The clinician delivering this solution would know which solution was being injected, but a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All pre‐ and post‐injection tests were done by trained personnel who had no knowledge of the solutions injected" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 52 occasions (for those not experiencing anaesthetic failure: 25 cases of 2% lidocaine, 1:100,000 epinephrine (1.8 mL) and 27 cases of 2% lidocaine, 1:200,000 epinephrine (3.6 mL)). Because the reduction in numbers across groups was well balanced and the reasons identical, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Wali 2010.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (United States of America) Participants: 30 enrolled, 30 completing the study. Mean age 28 years, ranging from 22 to 44 years. 22 male and 8 female Inclusion criteria: in good health and not taking any medications that would alter the perception of pain Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve blocks of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars, first premolars, lateral incisors Soft tissue anaesthesia (patient was asked if his/her lip was numb)
Soft tissues tested: lower lip |
|
| Notes | Non‐industry funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 3 solutions were randomly assigned 4‐digit numbers from a random number table. Each subject was randomly assigned to the right or left side for the set of injections. The order of the anaesthetic solutions was also randomly assigned to determine which solutions were to be administered at each appointment" Quote (from correspondence): "Each solution had a four‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "The 3 solutions were randomly assigned 4‐digit numbers from a random number table. Each subject was randomly assigned to the right or left side for the set of injections. The order of the anaesthetic solutions was also randomly assigned to determine which solutions were to be administered at each appointment" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The 3 solutions were randomly assigned 4‐digit numbers from a random number table" "An opaque tape was placed on each syringe, and the corresponding 4‐digit code number was written on the tape" Comment: the identity of 3.6 mL of 2% lidocaine with 1:50,000 epinephrine solution would be clear to the patient and clinician, as it would require the injection rate to be twice as fast (all injections were given over 2 minutes). However, these data were not used in this review. Participants and personnel would not be able to identify the other local anaesthetics used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the random numbers were recorded on the data collection and post‐injection survey sheets to help blind the experiment" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) onset | Low risk | Comment: onset of pulpal anaesthesia was tested on 48 occasions for molar teeth, excluding 3.6 mL of 2% lidocaine, 1:50,000 epinephrine (for those not experiencing anaesthetic failure = 24 in each group). Because the reduction in numbers across groups was equal and the reasons identical, risk of attrition bias was rated as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: no patients excluded; outcome data complete |
| Other bias | Low risk | Comment: no other bias present |
Weil 1961.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (United States of America) Participants: 592 enrolled, 592 completing the study (252, excluding those not commercially available). Mean age and range and male:female ratio not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Mandibular and maxillary injections (1 cartridge or more if required) of:
Not commercially available:
|
|
| Outcomes |
Clinical anaesthesia during operative dentistry procedures
Soft tissue anaesthesia
Soft tissues tested: relevant soft tissues, depending on injection and jaw Teeth tested: not stated Adverse events reported (252/252) |
|
| Notes | Industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Solutions.....were supplied in identical dental cartridges marked only by a control number printed on each cartridge. At least three different code numbers were assigned to each local anaesthetic solution. All the cartridges under test were mixed indiscriminately with cartridges of the control solution, in cans of 50" Comment: exact method of generation of randomized sequence not reported (probably drawing from the can). There was a large difference in group size (71 vs 181); this may indicate a problem with the randomization process. Therefore, risk of bias was rated as unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Solutions.....were supplied in identical dental cartridges marked only by a control number printed on each cartridge. At least three different code numbers were assigned to each local anaesthetic solution. All the cartridges under test were mixed indiscriminately with cartridges of the control solution, in cans of 50" "The key to the code of control numbers was kept by the administrator in a sealed envelope until the data from the cards were tabulated and analysed" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Solutions.....were supplied in identical dental cartridges marked only by a control number printed on each cartridge. At least three different code numbers were assigned to each local anaesthetic solution. All the cartridges under test were mixed indiscriminately with cartridges of the control solution, in cans of 50" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore, risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Solutions.....were supplied on identical dental cartridges marked only by a control number printed on each cartridge. At least three different code numbers were assigned to each local anaesthetic solution. All the cartridges under test were mixed indiscriminately with cartridges of the control solution, in cans of 50" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) onset | Low risk | Comment: of the total number of participants tested, some did not have onset of soft tissue anaesthesia measured: mandibular injection:
infiltration:
For onset of soft tissue anaesthesia, values of zero or only small numbers were obtained for dropouts. However, the dropout rate if present could be calculated only if those having soft tissue success were known. Soft tissue anaesthesia may have been present in those who had failure of anaesthesia or may have been absent, meaning that it was not measured. However, as the number measured is very similar to the total number enrolled, and any dropouts in both groups would be due to failure of local anaesthetic, risk of bias has been graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) duration | High risk | Comment: of the total number of participants recruited who had onset of soft tissue anaesthesia measured, some did not have duration of soft tissue anaesthesia measured: mandibular injection:
infiltration:
For duration of soft tissue anaesthesia, no dropouts would occur if the numbers of participants having duration measured were equal to the numbers having soft tissue onset measured. However, dropout rates of up to 31% were seen and were based on those who had onset of soft tissue anaesthesia measured. Therefore risk of bias has been graded as high because if dropout rates were based on soft tissue success, they might be higher still. Data were not used for meta‐analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Unclear risk | Supported by a grant from Cook‐Waite |
Yadav 2015.
| Methods | Randomized controlled clinical trial, parallel study design | |
| Participants |
Location: university (India) Participants: 150 enrolled, 150 completing the study. Age ranging from 20 to 35 years. 78 male, 72 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | Inferior alveolar nerve block (1.8 mL) followed by buccal (0.9 mL) and lingual (0.9 mL) infiltrations using the following:
Other participants (100) had oral ketorolac (10 mg) with and without buccal and lingual infiltrations |
|
| Outcomes |
Clinical anaesthesia during access cavity preparation and instrumentation in teeth with irreversible pulpitis
Teeth tested: mandibular first and second molars |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients were randomly divided into 2 major groups" Comment: detailed method not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All patients were randomly divided into 2 major groups" Comment: detailed method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The labels of the solutions were removed, and unique 3‐digit numeric values were coded on them; the results were recorded according to those values only" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The labels of the solutions were removed, and unique 3‐digit numeric values were coded on them; the results were recorded according to those values only" Comment: the outcome is a patient‐reported outcome (outcome assessor is the patient). Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Yared 1997.
| Methods | Randomized controlled simulated scenario trial, cross‐over study design | |
| Participants |
Location: university (Lebanon) Participants: 30 enrolled, 30 completing the study. Mean age 32 years, ranging from 22 to 50 years. 22 male, 8 female Inclusion criteria: in good health and not taking any medications that would alter pain perception Exclusion criteria: none reported |
|
| Interventions | Inferior alveolar nerve blocks (3.6 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular first molars, first premolars, lateral incisors Soft tissue anaesthesia
Soft tissues tested: labial and lingual to the premolar and buccal to the first molar |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each subject randomly received each anaesthetic solution on three successive appointments at least 1 week apart" "The sequence of solution administration was determined randomly" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each subject randomly received each anaesthetic solution on three successive appointments at least 1 week apart" "The sequence of solution administration was determined randomly" Comment: exact method of concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "These solutions were designated by D, E, and F, respectively. The sequence of solution administration was determined randomly, and all the injections were given blindly by one operator" Comment: disguising the cartridges of each formulation with the same code (D, E, and F) could allow identification of a solution by personnel administering injections in a cross‐over study if the properties of the solutions were markedly different. Patients may comment about long duration, poor anaesthesia, etc., at their second visit. However, the properties of the 3 solutions are unlikely to allow identification, and a pre‐determined method of administration was used by personnel to minimize variation. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All preinjection and post‐injection tests were done by a trained person who was blinded to the solutions injected" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Yilmaz 2011.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design | |
| Participants |
Location: university (Turkey) Participants: 162 enrolled, 157 completing the study. Mean age 7.2 years, standard deviation = 0.6 years. 81 male, 81 female Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions | Inferior alveolar nerve block and maxillary buccal infiltration (1.0 mL) of:
|
|
| Outcomes |
Clinical anaesthesia during pulpotomy
Teeth tested: maxillary and mandibular deciduous posterior teeth Soft tissue anaesthesia
Soft tissues tested: relevant soft tissues, depending on tooth and jaw Adverse events reported (157/162) |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 162 children were randomly divided into two equal groups" Comment: exact method of generation of randomized sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The 162 children were randomly divided into two equal groups" Comment: exact method of generation of concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both local anaesthetic agents were administered either as a maxillary infiltration or a mandibular block by a paediatric dentist who was blinded to the type of local anaesthetic that was injected" Comment: detailed methods were not reported. Despite no details of the blinding method, identification of the local anaesthetic by participants is unlikely. A pre‐determined method of administration was used by personnel to minimize variation. Therefore, risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both local anaesthetic agents were administered either as a maxillary infiltration or a mandibular block by a paediatric dentist who was blinded to the type of local anaesthetic that was injected" Comment: outcomes are patient‐reported outcomes (outcome assessor is the patient) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore, risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Clinical success | Low risk | Comment: a small number (5) were excluded from the study for the same reason: discomfort following maxillary injection (4 with articaine and 1 with prilocaine). However the groups were still well balanced; therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: a small number (5) were excluded from the study for the same reason: discomfort following maxillary injection (4 with articaine and 1 with prilocaine). However the groups were still well balanced; therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: a small number (5) were excluded from the study for the same reason: discomfort following maxillary injection (4 with articaine and 1 with prilocaine). However the groups were still well balanced; therefore risk of bias was graded as low |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
Yonchak 2001.
| Methods | Randomized controlled simulated scenario trial, parallel and cross‐over study design | |
| Participants |
Location: university (United States of America) Participants Cross‐over: 40 enrolled, 40 completed the study. Mean age 26 years, ranging from 21 to 34 years. 30 male, 10 female Parallel: 40 enrolled, 40 completing the study. Mean age 26 years, ranging from 20 to 34 years. 30 male, 10 female Inclusion criteria: in good health and not taking any medications that would alter pain perception Exclusion criteria: none reported |
|
| Interventions | Cross‐over: mandibular labial infiltration (1.8 mL) of:
Parallel: mandibular lingual infiltration (1.8 mL) of:
|
|
| Outcomes |
Pulpal anaesthesia tested with an electric pulp tester
Teeth tested: mandibular lateral incisors, central incisors, and canines Soft tissue anaesthesia
Soft tissues tested: lip (cross‐over study only) |
|
| Notes | Non‐industry funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the experiment, the 2 anaesthetic solutions were randomly assigned 5‐digit numbers from a random number table. Each subject was randomly assigned to 1 of the 2 solutions to determine the sequence of the injections" Quote (from correspondence): "Each solution had a five‐digit random number for each subject and for each solution, and for each side. This was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the experiment, the 2 anaesthetic solutions were randomly assigned 5‐digit numbers from a random number table. Each subject was randomly assigned to 1 of the 2 solutions to determine the sequence of the injections" Quote (from correspondence): "Concealment was achieved by having an experimenter label the cartridges with the random number so neither the operator, patient, or pulp tester knew which of the anaesthetic solutions were used. The cartridges with the random numbers were placed in an envelope for Subject 1, 2, 3, etc. and which random number was to be used for the first appointment was written on the outside. The master code list was not available to the investigator. The coding was broken at the end of the study by our statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each anaesthetic cartridge (1:100,000 or 1:50,000) was masked with a white opaque label and numbered to determine the order of anaesthetic administration. The random numbers were recorded on the cartridges and the data collection sheets to blind the experiment" Comment: participants and personnel would not be able to identify the local anaesthetic used. Therefore risk of bias was graded as low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each anaesthetic cartridge (1:100,000 or 1:50,000) was masked with a white opaque label and numbered to determine the order of anaesthetic administration. The random numbers were recorded on the cartridges and the data collection sheets to blind the experiment" Comment: outcomes are participant‐reported outcomes (outcome assessor is the participant) and were recorded by a different person than the local anaesthetic administrator. Identification of the local anaesthetic by participants and personnel recording outcomes was not possible. Therefore risk of bias was graded as low |
| Incomplete outcome data (attrition bias) Pulpal anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Incomplete outcome data (attrition bias) Soft tissue anaesthesia (simulated scenario) success | Low risk | Comment: no patients excluded; outcome data complete |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
| Other bias | Low risk | Comment: no other bias present |
We suspected that two studies may be from the same clinical trial (Malamed 2000a; Malamed 2000b), but we were unable to contact the study author to confirm this. Until we receive clarification from the study author that they are, we have assumed for the review that they are different trials. Neither was used in the meta‐analysis.
In the Characteristics of included studies, the number of participants tested is included in brackets after each local anaesthetic in the Interventions section and after each outcome in the Outcomes section. This latter figure is the ratio of those actually tested against the original number eligible for testing.
AE = articaine; AR = articaine; ASA= American Society of Anaesthesiologists; Aw/o = articaine with no vasoconstrictor; BI = buccal infiltration; bpm = beats per minute; CNPQ‐PIBIC = Conselho Nacional de Desenvolvimento Científico e Tecnológico ‐ Programa Institucional de Bolsas de Iniciação Científica; EPT = electric pulp tester; FLACC = Face, Legs, Activity, Cry, Consolability Scale; FPS‐R = Faces Pain Scale ‐ Revised; HCI = hydrochloride; Hg = mercury; HP VAS = Heft‐Parker visual analogue scale; IAN = inferior alveolar nerve; IANB = inferior alveolar nerve block; IU = international units; JADA = Journal of the American Dental Association; LA = local anaesthetic; LE = lidocaine; ME = mepivacaine; N/A = not applicable; PDL = periodontal ligament; SD = standard deviation; SEM = standard error of the mean; V = volt.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 1969 | Although a randomized trial, only optical isomers of mepivacaine were compared |
| Caruso 1989 | Some of the participants were sedated |
| Cowan 1964 | This study compared procaine, lidocaine, mepivacaine, and prilocaine but was not a randomized controlled trial. This study is referenced in Cowan 1968 as a double‐blind randomized study. However, there was no mention of this in the 1964 paper. The summary describes the study as a series of injections |
| Cowan 1968 | The study was not a randomized controlled study |
| Hassan 2011 | The study was not randomized |
| Kanaa 2009 | Although both groups of participants had an identical inferior alveolar nerve block initially, an additional buccal infiltration of 4% articaine, 1:100,000 epinephrine was compared with a dummy buccal infiltration |
| Raab 1990 | The study was double‐blind but was not randomized |
| Shruthi 2013 | The study was referred to as a randomized clinical trial by the study authors. However, the abstract states, "This study was done on 50 subjects; 25 of them received 4% articaine HCl with 1:100,000 epinephrine, and the next 25 received 2% lignocaine HCl with 1:100,000 epinephrine", which implies that randomization of participants into each local anaesthetic group did not occur. The study fails to mention that participants, personnel, and assessors were blinded and does not describe the method of injection used, although this is likely to be IANB and BI. The author of the study was emailed for clarification, but no contact could be made |
BI = buccal infiltration; IANB = inferior alveolar nerve block.
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2004.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
da Silva‐Junior 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Dong 2010.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Ge 2005.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Guo 2014.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
He 2010.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Huang 2011.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Im 2010.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Jin 2005.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Lee 2004.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Li 2005.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Liang 2001.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Liao 2004.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Liu 2010.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Luo 2009.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Manabe 2005.
| Methods | Randomized controlled simulated scenario trial |
| Participants |
Location: university (Japan) Participants: 194 fifth grade students who had been recorded from 2002 to 2003 |
| Interventions | Inferior alveolar nerve blocks (1.35 mL) of 1 of the following:
|
| Outcomes | Soft tissue anaesthesia
Soft tissues tested: tongue, lower lip, and gingiva Post‐anaesthetic complications such as pain at the injection site and/or difficulty opening the mouth |
| Notes | Not yet assessed |
Oka 1990.
| Methods | Randomized controlled clinical and simulated scenario trial, parallel study design |
| Participants |
Location: university (Japan) Participants: not yet assessed |
| Interventions | Injections of 1 of the following:
|
| Outcomes | Adequacy of anaesthesia during tooth extraction
Teeth tested: not stated Influence of each local anaesthetic on haemodynamics, local ischaemias, bleeding was measured |
| Notes | Not yet assessed |
Ouchi 2008.
| Methods | Randomized controlled simulated scenario trial, parallel study design |
| Participants |
Location: university (Japan) Participants: 19 healthy volunteers |
| Interventions | Inferior alveolar nerve blocks (1.6 mL) of 1 of the following:
|
| Outcomes | Pulpal anaesthesia (tested using an electric pulp tester)
Teeth tested: lateral incisors, premolars, and molars Soft tissue anaesthesia
Soft tissues tested: lower lip Adverse events
|
| Notes | Not yet assessed |
Qiu 2007.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Qiu 2011.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Shi 2002.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Shimada 2002.
| Methods | Randomized controlled simulated scenario trial, parallel study design, carried out at 7 sites |
| Participants |
Location: university (Japan) Participants: 231 |
| Interventions | Infiltration and block anaesthesia of 1 of the following:
|
| Outcomes | Outcomes
Teeth tested: not stated Soft tissue anaesthesia
Soft tissues tested: not stated Local and systemic adverse reactions were measured |
| Notes | Not yet assessed |
Wang 2009.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Wu 2005.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Xie 2008.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Xing 2005.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Xu 1991.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Xu 2008.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Xu 2013.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Xuan 2007.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Zhang 2005.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Zhang 2009.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Zhou 2011.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Zhou 2013.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Characteristics of ongoing studies [ordered by study ID]
Caicedo 1996.
| Trial name or title | Evaluation of Three Anesthetic Solutions Using Two Local Anesthesia Techniques |
| Methods | Randomized controlled double‐blind simulated scenario cross‐over study? |
| Participants | 30 |
| Interventions | Akinosi and alveolar mandibular conventional blockade technique (AMCB) of 1 of the following:
|
| Outcomes |
Soft tissue anaesthesia
Soft tissues tested: lip and tongue Pulpal anaesthesia
Teeth tested: not determined |
| Starting date | Not determined |
| Contact information | Ricardo Caicedo (ri.caicedo@louisville.edu) |
| Notes | Available only as an abstract; unpublished as a full paper. Study author has been contacted for details of the trial |
Iqbal 2009.
| Trial name or title | Comparison of Anaesthetic Efficacy of Articaine and Lidocaine for Inferior Alveolar Nerve Blocks With Buccal Infiltration in Patients With Irreversible Pulpitis |
| Methods | Randomized double‐blinded parallel clinical study |
| Participants | 31 emergency patients |
| Interventions | 1.8 mL of 4% articaine, 1:100,000 epinephrine and 1.8 mL of 2% lidocaine, 1:100,000 epinephrine were given in 1 of the following combinations:
|
| Outcomes | Anaesthetic success (ability to access and instrument the root canal without pain ‐ VAS score of zero) Patients who reported inadequate lip and tongue numbness and/or painful response to Endo Ice were excluded from the study Teeth tested: mandibular molars with irreversible pulpitis |
| Starting date | Not determined |
| Contact information | First study author deceased |
| Notes | An attempt will be made to contact one of the other study authors |
Sheikh 2014.
| Trial name or title | Preliminary Comparison of Missed Blocks With 4% Articaine and 2% Lidocaine Both With 1:100,000 Epinephrine on Inferior Alveolar Nerve Block Injections |
| Methods | Double‐blind randomized controlled clinical trial |
| Participants | Not reported |
| Interventions | Inferior alveolar nerve blocks using a conventional approach of:
|
| Outcomes | Soft tissue anaesthetic success
Soft tissues tested: lower lip and alveolar mucosa |
| Starting date | Not determined |
| Contact information | Not determined |
| Notes | An attempt will be made to contact study authors |
AMCB = alveolar mandibular conventional blockade; VAS = visual analogue scale.
Differences between protocol and review
We modified the title of the review from "Injectable local anaesthetics agents for operative dental anaesthesia" to “Injectable local anaesthetic agents for dental anaesthesia". Originally the word "operative" was meant to be used in relation to a surgical or non‐surgical operation or intervention. As the word "operative" may confuse the reader into thinking that included studies relate only to operative dentistry and treatment of diseased teeth, we removed the word "operative" from the title.
We replaced the second author of the protocol (St George 2007), Sela Hussain, with Alyn Morgan for the full review. Also, we recruited two other authors ‐ Yuan‐Ling Ng and Aviva Petrie ‐ to help with data handling and statistical analysis issues (pooling of cross‐over study data). Contact details for David Moles have changed.
We updated the Background section of the main text to include more recent references to studies and up‐to‐date headings.
We included the following explanation of why the review was needed in the Why it is important to do this review section: "We are conducting this systematic review to determine which local anaesthetic solution is most successful for dental interventions owing to the current popularity of some formulations, such as those of articaine, for which growing evidence suggests that they provide more successful anaesthesia than other formulations. A rigorous systematic review of the success rate of local anaesthesia is needed to inform evidence‐based practice. This review will consider only injectable agents used for dental block or infiltration, while excluding supplemental injections."
We replaced the word "experimental", used to describe studies for which outcomes were measured when treatment was not performed, with the words "simulated scenario".
In Objectives, we removed the first line, "To determine what is the most effective local anaesthetic formulation for dental anaesthesia."
In Objectives, we changed the wording of our primary objectives from:
"Our primary objectives were to test
the adequacy of anaesthesia in patients when using different local anaesthetic formulations for operative dental anaesthesia;
the speed of onset and duration of anaesthesia in patients when using different local anaesthetic formulations for operative dental anaesthesia;
systemic and local adverse effects associated with dental local anaesthetic."
to:
"Our primary objectives were to compare the success of anaesthesia, the speed of onset and duration of anaesthesia, and systemic and local adverse effects amongst different local anaesthetic formulations for dental anaesthesia. We define success of anaesthesia as absence of pain during a dental procedure, or a negative response to electric pulp testing or other simulated scenario tests. We define dental anaesthesia as anaesthesia given at the time of any dental intervention"
In Objectives, we modified the primary outcome definitions:
"We define adequacy of anaesthesia as the absence of pain during a dental procedure, or a negative response to electric pulp testing" changed to "We define success of anaesthesia as absence of pain during a dental procedure, or a negative response to electric pulp testing or other simulated scenario tests".
"We define operative dental anaesthesia as anaesthesia at the time of an operative intervention" changed to "We define dental anaesthesia as anaesthesia given at the time of any dental intervention".
In Objectives, the secondary objective of "participants' experience of the procedures carried out" and in Secondary outcomes, the outcome of "participants' experiences: these include but are not limited to preference and overall experience" was due to be assessed. However, because of lack of data, we did not report these.
In Objectives, we removed the secondary objective of "the influence of modifying factors on efficacy of local anaesthetic formulations", and in Secondary outcomes the outcome of "modifying factors: influence on efficacy of local anaesthetic solutions", as these were wrongly inserted into the two relative sections of the protocol in error.
In the Types of studies section, we added "When paired data, or data from the first period, were not available, we treated the data from cross‐over studies as if derived from a parallel study, then performed sensitivity analysis with cross‐over data removed."
In the Types of participants section, we changed "We included male and female adults and children, who were undergoing dental procedures, or volunteers who took part in experimental studies where dental local anaesthesia was tested." to "We included participants regardless of age and gender who were undergoing dental procedures and volunteers who took part in simulated scenario studies in which dental local anaesthesia was tested."
In the Types of interventions section, we originally wrote "Only infiltration and block anaesthesia will be considered". To clarify that supplemental anaesthesia was not to be considered, we changed this to "We considered only primary infiltration and block anaesthesia and did not consider supplemental anaesthesia". Also, we added a paragraph giving examples of local anaesthetic formulations:
"Examples of commercial local anaesthetic solutions considered for inclusion in the review include:
2% lidocaine (with no epinephrine, 1:50,000 epinephrine, 1:80,000 epinephrine, 1:100,000 epinephrine, or 1:200,000 epinephrine);
4% articaine hydrochloride (HCl) (with no epinephrine, 1:100,000 epinephrine, 1:100,000 epinephrine, or 1:400,000 epinephrine);
3% prilocaine HCl (with 0.03 international units/mL (IU/mL) octapressin);
4% prilocaine HCl (with no epinephrine, or 1:200,000 epinephrine);
2% mepivacaine (with 1:20,000 levonordefrin or 1:100,000 epinephrine);
3% mepivacaine (with no epinephrine); and
0.5% bupivacaine (with 1:200,000 epinephrine)".
In Primary outcomes, we changed the wording of our primary outcomes from:
"success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus
speed of onset and duration of anaesthesia
adverse effects: local and systemic"
to:
"Success of local anaesthesia, measured by the absence of pain during a procedure via a visual analogue scale (VAS) or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia by an electric pulp tester or cold stimulus.
Speed of onset (from time of injection to complete anaesthesia) and duration (time from onset until anaesthesia disappeared) of anaesthesia, measured by the absence of pain during a procedure seen on a VAS or other appropriate method, including self‐reported patient pain or anaesthesia, or measurement of pulpal anaesthesia by an electric pulp tester or cold stimulus.
-
Adverse effects: local and systemic, when the cause of the harmful effect is attributed to the local anaesthetic formulation, including:
pain on injection (solution deposition), measured on a VAS;
pain following injection, measured by VAS;
paraesthesia following injection; and
allergy to local anaesthetic".
We also added that the outcomes were classified separately into the oral tissues tested or the testing method used.
"Outcomes were classified separately by the oral tissues tested or the testing method used, which included the following.
-
Clinical testing of:
healthy pulps ‐ hard and soft tissues;
healthy pulps;
diseased pulps with irreversible pulpitis;
different tissues, pooled; and
tissues, when tissues tested were unclear.
-
Simulated scenario testing of:
healthy pulps;
diseased pulps with irreversible pulpitis; and
soft tissues".
which was also mentioned in Subgroup analysis and investigation of heterogeneity.
We further modified the original primary outcome of success to one of the following outcomes, depending on the test method used, in the Effects of interventions section.
"Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale (VAS) or other appropriate method.
Success of local anaesthesia, by measurement of pulpal anaesthesia using an electric pulp tester.
Success of local anaesthesia, measured by using self‐reported patient pain or anaesthesia."
We did this to clarify the test method used.
In Types of interventions, one injection technique was to be compared against another injection technique. However after starting the review, we realized that this was the topic of a different review.
We had planned to search the Community of Science database but chose not to for the final review. A number of databases had their names modified while the review was performed. We updated these.
In the Selection of studies section, we changed "Two authors (GStG and SH) will independently assess the quality of the chosen randomized controlled trials" to "Two review authors (GST and AM) independently read all titles and abstracts of publications retrieved through our search".
In the Data extraction and management section, we changed "Two authors will carry out the data abstraction (GStG and SH)." to "Two review authors carried out the data abstraction independently (GST and AM)".
In the Measures of treatment effect section, we changed "For binary data, we expressed pooled outcomes as pooled odds ratios (OR) and associated 95% confidence intervals (CIs)." to "For binary data, we expressed pooled outcomes as pooled odds ratios (ORs), risk ratios (RRs), and associated 95% confidence intervals (CIs)".
"When a data and analysis had only one included study (orphan study), it was not entered into a data and analysis table. Instead, the outcome was placed in the appropriate additional table (Table 9; Table 10; Table 11; Table 12; Table 13). When an orphan study was the sole study entered into a subgroup, its data were still analysed if data were available from other studies included in other subgroups in the data and analysis table".
We added details to the Unit of analysis issues section to clarify how cross‐over study data would be handled.
"The studies identified were a combination of parallel and cross‐over studies. Therefore, to pool data for both types of studies, we performed the meta‐analysis in several stages.
We performed a meta‐analysis on parallel‐group studies only, using the ‘inverse variance’ method to generate odds ratios. We used a fixed‐effect analysis or random‐effects analysis model depending on whether there were signs of statistical heterogeneity from the I² and P value. From these values, we generated logs of the OR and standard errors (SEs).
We used Microsoft Excel to generate the log of the OR and associated SEs for cross‐over studies from the studies' paired data, if available.
We completed the meta‐analysis using Review Manager (RevMan 2014) by entering the generic inverse variance data of logs of the OR and associated SEs from both types of studies using the 'inverse variance' method. We used a fixed‐effect or random‐effects analysis model depending on whether there were signs of statistical heterogeneity from the I² and P value (P ≤ 0.05, I² ≥ 50% (substantial heterogeneity))".
When paired data were not available, the data from cross‐over studies were used in the analysis as if they were from parallel studies, to estimate the overall effect of interest in the meta‐analysis. Owing to the confidence intervals being wider when this approach is used, a sensitivity analysis was performed while removing the data from cross‐over studies from the meta‐analysis, when present.
In Data synthesis we originally wrote "For binary data, these were predominately pooled OR and associated 95% CI." We changed this to "For binary data, we expressed pooled outcomes as pooled odds ratios (ORs), risk ratios (RRs), and associated 95% confidence intervals (CIs)".
In Subgroup analysis and investigation of heterogeneity we planned to consider a number of subgroups for analysis:
tooth type;
presence of inflammation (pulpitis);
tissue type anaesthetized;
treatment type;
type of injection;
age of patient;
type of study (treatment versus experimental); and
pharmaceutical company sponsorship.
For the final review, we grouped outcomes depending on which dental tissues required anaesthesia, and a subgroup analysis was conducted during meta‐analysis to look at the following subgroups: maxillary infiltration, maxillary block (Infraorbital block), maxillary block (palatal‐anterior superior alveolar nerve block), maxillary block (high‐tuberosity maxillary second division nerve block), mandibular infiltration, mandibular infiltration (buccal and lingual), mandibular block (IANB), mandibular block (mental block), mandibular block (IANB) and infiltration, mandibular testing (injection type not stated), or both jaws combined/jaw not stated.
The statistical software originally stated in the protocol for this was STATA 7, which we changed to STATA 13 following a number of updates.
Adverse effects were rare and were difficult to compare owing to differing ways of measuring each outcome and lack of raw data related to cross‐over studies; therefore we completed meta‐analysis for pain on injection (Analysis 1.8) and post‐injection pain (Analysis 1.9), when data were available. We summarized the data for other adverse effects in Table 15. No data were available from the included studies for the secondary outcome of patient experience.
We performed Sensitivity analysis to explore the influence of study quality on our primary outcome of success of local anaesthesia in terms of those factors influencing bias: generation and concealment of the randomization sequence; blinding, attrition bias, reporting bias, or other bias as planned; and the influence of cross‐over studies, when paired data were not available, on the same outcome.
We planned to investigate the possibility of publication bias but found insufficient studies to allow this.
We added a section in Data collection and analysis to describe the methods used to assess the quality of the evidence.
We planned to use the kappa statistic to assess agreement between authors, but this was not required.
We added a section entitled "Summary of findings tables and GRADE" detailing use of the GRADE approach to assess the quality of evidence and which outcomes were to be placed in the 'Summary of findings' tables:
"We used the GRADE approach to assess the quality of evidence related to each of the outcomes. We used the GRADE profiler (GRADEpro GDT) to import data from RevMan 2014 and to create 'Summary of findings' tables for the eight major comparisons in this review.
4% articaine, 1:100,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine (Table 1).
3% prilocaine, 0.03 IU felypressin versus 2% lidocaine, 1:100,000 epinephrine (Table 2).
4% articaine, 1:200,000 epinephrine versus 4% articaine, 1:100,000 epinephrine (Table 3).
4% prilocaine plain versus 2% lidocaine, 1:100,000 epinephrine (Table 4).
4% articaine, 1:200,000 epinephrine versus 0.5% bupivacaine, 1:200,000 epinephrine (Table 5).
0.5% bupivacaine, 1:200,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine (Table 6).
4% articaine, 1:100,000 epinephrine versus 2% mepivacaine, 1:100,000 epinephrine (Table 7).
2% mepivacaine, 1:100,000 epinephrine versus 2% lidocaine, 1:100,000 epinephrine (Table 8)".
When assessing the quality of evidence for each outcome, which included pooled data from RCTs, we downgraded evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Two review authors (GST and AM) independently assessed the quality of evidence for each outcome. When we were unable to come to an agreement on assessment of quality, we (GST and AM) resolved disagreements initially by mutual discussion. When a difference of opinion could not be resolved, we involved a third review author ‐ John Meechan (JM).
We included the following outcomes, for a variety of local anaesthetic comparisons, in the 'Summary of findings' tables.
Success of local anaesthesia, measured by the absence of pain during a procedure using a visual analogue scale or other appropriate method, or measurement of pulpal anaesthesia using an electric pulp tester or cold stimulus.
Speed of onset and duration of anaesthesia.
Adverse effects: local and systemic".
Throughout the review, we carried out minor modifications of text in the Methods section.
Contributions of authors
Geoffrey St George (GST), Alyn Morgan (AM), John Meechan (JM), David R Moles (DM), Aviva Petrie (AP), Ian Needleman (IN), Yuan‐Ling Ng (Y‐LNg).
Conceiving the review: GST. Co‐ordinating the review: GST. Undertaking manual searches: GST, AM. Screening search results: GST, AM. Organizing retrieval of papers: GST, AM. Screening retrieved papers against inclusion criteria: GST, AM. Appraising quality of papers: GST, AM. Abstracting data from papers: GST, AM. Writing to authors of papers for additional information: GST. Providing additional data about papers: GST. Obtaining and screening data on unpublished studies: GST, AM. Managing data for the review: GST, AM. Entering data into Review Manager (RevMan 2014): GST, AM. Analysing RevMan statistical data: GST, DM, AP, Y‐LNg. Performing other statistical analysis not using RevMan: GST, DM, AP, Y‐LNg. Performing double entry of data: (data entered by person one: GST; data entered by person two: AM). Interpreting data: GST, AM, IN, DM, AP, JM. Performing statistical analysis: GST, DM, AP, Y‐LNg. Writing the review: GST, AM, IN, DM, JM, Y‐LNg. Securing funding for the review: GST. Performing previous work that was the foundation of the present study: GST, JM. Serving as guarantor for the review (one review author): GST. Taking responsibility for reading and checking the review before submission: primarily GST, although all review authors were consulted.
Sources of support
Internal sources
New Source of support, Other.
-
Eastman Dental Hospital and Institute, UK.
Library facilities, Internet access to journal databases and e‐Journals
External sources
No sources of support supplied
Declarations of interest
Geoffrey St George: none known.
Alyn Morgan: none known.
John Meechan previously received research funding from Septodont, Astra, and Dentsply. At present, he has no research funding from any companies and will not be receiving any funding for this review. He was an author on three of the primary studies included in this review (Jaber 2010; Kanaa 2006; Kanaa 2012). Data for these studies were extracted by GSG and AM.
David R Moles: none known.
Ian Needleman: none known.
Yuan‐Ling Ng: none known.
Aviva Petrie: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abdulwahab 2009 {published data only}
- Abdulwahab M, Boynes S, Moore P, Seifikar S, Al‐Jazzaf A, Alshuraidah A, et al. The efficacy of six local anesthetic formulations used for posterior mandibular buccal infiltration anesthesia. Journal of the American Dental Association 2009;140(8):1018‐24. [PUBMED: 19654255] [DOI] [PubMed] [Google Scholar]
Aggarwal 2009 {published data only}
- Aggarwal V, Jain A, Kabi D. Anesthetic efficacy of supplemental buccal and lingual infiltrations of articaine and lidocaine after an inferior alveolar nerve block in patients with irreversible pulpitis. Journal of Endodontics 2009;35(7):925‐9. [PUBMED: 19567309] [DOI] [PubMed] [Google Scholar]
Aggarwal 2014 {published data only}
- Aggarwal V, Singla M, Miglani S, Kohli S. Comparison of the anaesthetic efficacy of epinephrine concentrations (1:80,000 and 1:200,000) in 2% lidocaine for inferior alveolar nerve block in patients with symptomatic irreversible pulpitis: a randomized, double‐blind clinical trial. International Endodontic Journal 2014;47(4):373‐9. [PUBMED: 23895176] [DOI] [PubMed] [Google Scholar]
Aggarwal 2017 {published data only}
- Aggarwal V, Singla M, Miglani S. Comparative evaluation of anesthetic efficacy of 2% lidocaine, 4% articaine, and 0.5% bupivacaine on inferior alveolar nerve block in patients with symptomatic irreversible pulpitis: a prospective, randomized, double‐blind clinical trial. Journal of Oral & Facial Pain and Headache 2017;31(2):124‐8. [DOI: 10.11607/ofph.1642] [DOI] [PubMed] [Google Scholar]
Albertson 1963 {published data only}
- Albertson GL. Mepivacaine HCl in dental surgery. Dental Progress 1963;4(1):56‐9. [Google Scholar]
Allegretti 2016 {published data only}
- Allegretti CE, Sampaio RM, Horliana AC, Armonia PL, Rocha RG, Tortamano IP. Anesthetic efficacy in irreversible pulpitis: a randomized clinical trial. Brazilian Dental Journal 2016;27(4):381‐6. [EMBASE: 614935231] [DOI] [PubMed] [Google Scholar]
Arrow 2012 {published data only}
- Arrow P. A comparison of articaine 4% and lignocaine 2% in block and infiltration analgesia in children. Australian Dental Journal 2012;57(3):325‐33. [PUBMED: 22924356 ] [DOI] [PubMed] [Google Scholar]
Ashraf 2013 {published data only}
- Ashraf H, Kazem M, Dianat O, Noghrehkar F. Efficacy of articaine versus lidocaine in block and infiltration anesthesia administered in teeth with irreversible pulpitis: a prospective, randomized, double‐blind study. Journal of Endodontics 2013;39(1):6‐10. [PUBMED: 23228249] [DOI] [PubMed] [Google Scholar]
Atasoy Ulusoy 2014 {published data only}
- Atasoy Ulusoy Öİ, Alaçam T. Efficacy of single buccal infiltrations for maxillary first molars in patients with irreversible pulpitis: a randomized controlled clinical trial. International Endodontic Journal 2014;47(3):222‐7. [PUBMED: 23656209] [DOI] [PubMed] [Google Scholar]
Batista da Silva 2010 {published data only}
- Batista da Silva C, Berto LA, Volpato MC, Ramacciato JC, Motta RH, Ranali J, et al. Anesthetic efficacy of articaine and lidocaine for incisive/mental nerve block. Journal of Endodontics 2010;36(3):438‐41. [PUBMED: 20171359] [DOI] [PubMed] [Google Scholar]
Berberich 2009 {published data only}
- Berberich G, Reader A, Drum M, Nusstein J, Beck M. A prospective, randomized, double‐blind comparison of the anesthetic efficacy of two percent lidocaine with 1:100,000 and 1:50,000 epinephrine and three percent mepivacaine in the intraoral, infraorbital nerve block. Journal of Endodontics 2009;35(11):1498‐504. [PUBMED: 19840637] [DOI] [PubMed] [Google Scholar]
Bhagat 2014 {published data only}
- Bhagat MJ, Narayan V, Muthusekhar MR, Jain AR. Comparative study of the anesthetic efficacy of 4% articaine versus 2% Lignocaine in the inferior alveolar nerve block during the surgical extraction of impacted mandibular third molars. Universal Research Journal of Dentistry 2014;4(2):108‐14. [DOI: 10.4103/2249-9725.132975] [DOI] [Google Scholar]
Bortoluzzi 2009 {published data only}
- Bortoluzzi MC, Manfro R, Kafer GC, Busetti LF. Comparative study of the efficacy of articaine and mepivacaine: a double‐blind, randomized, clinical trial. Internet Journal of Dental Science 2009;7(1):14p. [MEDLINE: ] [Google Scholar]
Bouloux 1999 {published data only}
- Bouloux GF, Punnia‐Moorthy A. Bupivacaine versus lidocaine for third molar surgery: a double‐blind, randomized, crossover study. Journal of Oral & Maxillofacial Surgery 1999;57(5):510‐4; discussion 515. [PUBMED: 10319823] [DOI] [PubMed] [Google Scholar]
Bradley 1969 {published data only}
- Bradley DJ, Martin ND. Clinical evaluation of mepivacaine and lidocaine. Australian Dental Journal 1969;14(6):377‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burns 2004 {published data only}
- Burns Y, Reader A, Nusstein J, Beck M, Weaver J. Anesthetic efficacy of the palatal‐anterior superior alveolar injection. Journal of the American Dental Association 2004;135(9):1269‐76. [PUBMED: 15493391] [DOI] [PubMed] [Google Scholar]
Caldas 2015 {published data only}
- Caldas CS, Bergamaschi C, Succi G, Motta R, Ramacciato JC. Clinical evaluation of different epinephrine concentrations for local dental anesthesia. Revista Dor 2015;16(1):1‐5. [DOI: 10.5935/1806-0013.20150001] [DOI] [Google Scholar]
Chapman 1988 {published data only}
- Chapman PJ. A controlled comparison of effectiveness of bupivacaine for post‐operative pain control. Australian Dental Journal 1988;33(4):288‐90. [PUBMED: 3075452] [DOI] [PubMed] [Google Scholar]
Chilton 1971 {published data only}
- Chilton NW. Clinical evaluation of prilocaine hydrochloride 4 percent solution with and without epinephrine. Journal of the American Dental Association 1971;83(1):149‐54. [PUBMED: 5281166] [DOI] [PubMed] [Google Scholar]
Claffey 2004 {published data only}
- Claffey E, Reader A, Nusstein J, Beck M, Weaver J. Anesthetic efficacy of articaine for inferior alveolar nerve blocks in patients with irreversible pulpitis. Journal of Endodontics 2004;30(8):568‐71. [PUBMED: 15273637] [DOI] [PubMed] [Google Scholar]
Cohen 1993 {published data only}
- Cohen HP, Cha BY, Spangberg LS. Endodontic anesthesia in mandibular molars: a clinical study. Journal of Endodontics 1993;19(7):370‐3. [PUBMED: 8245762] [DOI] [PubMed] [Google Scholar]
Colombini 2006 {published data only}
- Colombini BL, Modena KC, Calvo AM, Sakai VT, Giglio FP, Dionisio TJ, et al. Articaine and mepivacaine efficacy in postoperative analgesia for lower third molar removal: a double‐blind, randomized, crossover study. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics 2006;102(2):169‐74. [PUBMED: 16876058] [DOI] [PubMed] [Google Scholar]
Costa 2005 {published data only}
- Costa CG, Tortamano IP, Rocha RG, Francischone CE, Tortamano N. Onset and duration periods of articaine and lidocaine on maxillary infiltration. Quintessence International 2005;36(3):197‐201. [PUBMED: 15887505] [PubMed] [Google Scholar]
Dagher 1997 {published data only}
- Dagher FB, Yared GM, Machtou P. An evaluation of 2% lidocaine with different concentrations of epinephrine for inferior alveolar nerve block. Journal of Endodontics 1997;23(3):178‐80. [PUBMED: 9594760] [DOI] [PubMed] [Google Scholar]
Donaldson 1987 {published data only}
- Donaldson D, James‐Perdok L, Craig BJ, Derkson GD, Richardson AS. A comparison of Ultracaine DS (articaine HCl) and Citanest forte (prilocaine HCl) in maxillary infiltration and mandibular nerve block. Journal (Canadian Dental Association) 1987;53(1):38‐42. [PUBMED: 3545401] [PubMed] [Google Scholar]
Elbay 2016 {published data only}
- Elbay ÜŞ, Elbay M, Kaya E, Yıldırım S. Effects of two different anesthetic solutions on injection pain, efficacy, and duration of soft‐tissue anesthesia with inferior alveolar nerve block for primary molars. Journal of Clinical Pediatric Dentistry 2016;40(6):456‐63. [EMBASE: 616640618] [DOI] [PubMed] [Google Scholar]
Epstein 1965 {published data only}
- Epstein S. Clinical comparison of a new local anesthetic, propitocaine, with lidocaine. Journal of Oral Therapeutics & Pharmacology 1965;2(3):161‐70. [PUBMED: 5321605] [PubMed] [Google Scholar]
Epstein 1969 {published data only}
- Epstein S. Clinical study of prilocaine with varying concentrations of epinephrine. Journal of the American Dental Association 1969;78(1):85‐90. [PUBMED: 5248313] [DOI] [PubMed] [Google Scholar]
Evans 2008 {published data only}
- Evans G, Nusstein J, Drum M, Reader A, Beck M. A prospective, randomized, double‐blind comparison of articaine and lidocaine for maxillary infiltrations. Journal of Endodontics 2008;34(4):389‐93. [PUBMED: 18358883] [DOI] [PubMed] [Google Scholar]
Fernandez 2005 {published data only}
- Fernandez C, Reader A, Beck M, Nusstein J. A prospective, randomized, double‐blind comparison of bupivacaine and lidocaine for inferior alveolar nerve blocks. Journal of Endodontics 2005;31(7):499‐503. [PUBMED: 15980707] [DOI] [PubMed] [Google Scholar]
Fertig 1968 {published data only}
- Fertig JW, Chilton NW. A dental local anaesthetic study. Fixed model, two‐way layout design. Archives of Oral Biology 1968;13(12):1477‐89. [PUBMED: 4885681] [DOI] [PubMed] [Google Scholar]
Forloine 2010 {published data only}
- Forloine A, Drum M, Reader A, Nusstein J, Beck M. A prospective, randomized, double‐blind comparison of the anesthetic efficacy of two percent lidocaine with 1:100,000 epinephrine and three percent mepivacaine in the maxillary high tuberosity second division nerve block. Journal of Endodontics 2010;36(11):1770‐7. [PUBMED: 20951285] [DOI] [PubMed] [Google Scholar]
Gangarosa 1967 {published data only}
- Gangarosa LP, Halik FJ. A clinical evaluation of local anaesthetic solutions containing graded epinephrine concentrations. Archives of Oral Biology 1967;12(5):611‐21. [PUBMED: 5336946] [DOI] [PubMed] [Google Scholar]
Gazal 2015 {published data only}
- Gazal G, Alharbi AM, Al‐Samadani K, Kanaa MD. Articaine and mepivacaine buccal infiltration in securing mandibular first molar pulp anesthesia following mepivacaine inferior alveolar nerve block: a randomized, double‐blind crossover study. Saudi Journal of Anaesthesia 2015;9(4):397‐403. [EMBASE: 2015443802] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gazal 2017 {published data only}
- Gazal G, Alharbi R, Fareed WM, Omar E, Alolayan AB, Al‐Zoubi H, et al. Comparison of onset anesthesia time and injection discomfort of 4% articaine and 2% mepivacaine during teeth extractions. Saudi Journal of Anaesthesia 2017;11(2):152‐7. [EMBASE: 615606206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregorio 2008 {published data only}
- Gregorio LV, Giglio FP, Sakai VT, Modena KC, Colombini BL, Calvo AM, et al. A comparison of the clinical anesthetic efficacy of 4% articaine and 0.5% bupivacaine (both with 1:200,000 epinephrine) for lower third molar removal. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics 2008;106(1):19‐28. [PUBMED: 18420431 ] [DOI] [PubMed] [Google Scholar]
Gross 2007 {published data only}
- Gross R, McCartney M, Reader A, Beck M. A prospective, randomized, double‐blind comparison of bupivacaine and lidocaine for maxillary infiltrations. Journal of Endodontics 2007;33(9):1021‐4. [PUBMED: 17931925] [DOI] [PubMed] [Google Scholar]
Haas 1990 {published data only}
- Haas DA, Harper DG, Saso MA, Young ER. Comparison of articaine and prilocaine anesthesia by infiltration in maxillary and mandibular arches. Anesthesia Progress 1990;37(5):230‐7. [PUBMED: 2096746] [PMC free article] [PubMed] [Google Scholar]
Haas 1991 {published data only}
- Haas DA, Harper DG, Saso MA, Young ER. Lack of differential effect by Ultracaine (articaine) and Citanest (prilocaine) in infiltration anaesthesia. Journal (Canadian Dental Association). 1991;57(3):217‐23. [PUBMED: 2043997] [PubMed] [Google Scholar]
Haase 2008 {published data only}
- Haase A, Reader A, Nusstein J, Beck M, Drum M. Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block.[erratum appears in J Am Dent Assoc. 2008 Oct;139(10):1312]. Journal of the American Dental Association 2008;139(9):1228‐35. [PUBMED: 18762633] [DOI] [PubMed] [Google Scholar]
Hellden 1974 {published data only}
- Hellden L, Blomberg S, Woxberg B, Ohman A. A controlled trial of a long‐acting local anaesthetic (Marcaine) in oral surgery for relief of post‐operative pain. Svensk Tandlakaretidskrift 1974;67(4):223‐8. [PUBMED: 4606108] [PubMed] [Google Scholar]
Hersh 1995 {published data only}
- Hersh EV, Hermann DG, Lamp CJ, Johnson PD, MacAfee KA. Assessing the duration of mandibular soft tissue anesthesia. Journal of the American Dental Association 1995;126(11):1531‐6. [PUBMED: 7499650] [DOI] [PubMed] [Google Scholar]
Hinkley 1991 {published data only}
- Hinkley SA, Reader A, Beck M, Meyers WJ. An evaluation of 4% prilocaine with 1:200,000 epinephrine and 2% mepivacaine with 1:20,000 levonordefrin compared with 2% lidocaine with 1:100,000 epinephrine for inferior alveolar nerve block. Anesthesia Progress 1991;38(3):84‐9. [PUBMED: 1814249] [PMC free article] [PubMed] [Google Scholar]
Hosseini 2016 {published data only}
- Hosseini HR, Parirokh M, Nakhaee N, Abbott PV, Samani S. Efficacy of articaine and lidocaine for buccal infiltration of first maxillary molars with symptomatic irreversible pulpitis: a randomized double‐blinded clinical trial. Iranian Endodontic Journal 2016;11(2):79‐84. [DOI: 10.7508/iej.2016.02.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaber 2010 {published data only}
- Jaber A, Whitworth JM, Corbett IP, Al‐Baqshi B, Kanaa MD, Meechan JG. The efficacy of infiltration anaesthesia for adult mandibular incisors: a randomised double‐blind cross‐over trial comparing articaine and lidocaine buccal and buccal plus lingual infiltrations. British Dental Journal 2010;209(9):E16. [PUBMED: 20953168] [DOI] [PubMed] [Google Scholar]
Jain 2016 {published data only}
- Jain NK, John RR. Anesthetic efficacy of 4% articaine versus 2% lignocaine during the surgical removal of the third molar: a comparative prospective study. Anesthesia Essays and Researches 2016;10(2):356‐61. [DOI: 10.4103/0259-1162.171445] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalia 2011 {published data only}
- Kalia V, Supreet KR. Comparative evaluation of onset and duration of anesthesia of 4% articaine versus 2% lidocaine with epinephrine 1:100,000 during exodontia. Indian Journal of Comprehensive Dental Care 2011;1(1):19‐24. [2231‐6973] [Google Scholar]
Kambalimath 2013 {published data only}
- Kambalimath DH, Dolas RS, Kambalimath HV, Agrawal SM. Efficacy of 4% articaine and 2% lidocaine: a clinical study. Journal of Maxillofacial and Oral Surgery 2013;12(1):3‐10. [DOI: 10.1007/s12663-012-0368-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kammerer 2012 {published data only}
- Kammerer PW, Palarie V, Daublander M, Bicer C, Shabazfar N, Brullmann D, et al. Comparison of 4% articaine with epinephrine (1:100,000) and without epinephrine in inferior alveolar block for tooth extraction: double‐blind randomized clinical trial of anesthetic efficacy. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2012;113(4):495‐9. [PUBMED: 22676931] [DOI] [PubMed] [Google Scholar]
Kammerer 2014 {published data only}
- Kammerer PW, Seeling J, Alshihri A, Daublander M. Comparative clinical evaluation of different epinephrine concentrations in 4% articaine for dental local infiltration anesthesia. Clinical Oral Investigations 2014;18(2):415‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kanaa 2006 {published data only}
- Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine and lidocaine mandibular buccal infiltration anesthesia: a prospective randomized double‐blind cross‐over study. Journal of Endodontics 2006;32(4):296‐8. [PUBMED: 16554198] [DOI] [PubMed] [Google Scholar]
Kanaa 2012 {published data only}
- Kanaa MD, Whitworth JM, Meechan JG. A comparison of the efficacy of 4% articaine with 1:100,000 epinephrine and 2% lidocaine with 1:80,000 epinephrine in achieving pulpal anesthesia in maxillary teeth with irreversible pulpitis. Journal of Endodontics 2012;38(3):279‐82. [PUBMED: 22341059] [DOI] [PubMed] [Google Scholar]
Karm 2017 {published data only}
- Karm MH, Park FD, Kang M, Kim HJ, Kang JW, Kim S, et al. Comparison of the efficacy and safety of 2% lidocaine HCl with different epinephrine concentration for local anesthesia in participants undergoing surgical extraction of impacted mandibular third molars. Medicine 2017;96(21):e6753. [EMBASE: 616768530] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2010 {published data only}
- Katz S, Drum M, Reader A, Nusstein J, Beck M. A prospective, randomized, double‐blind comparison of 2% lidocaine with 1:100,000 epinephrine, 4% prilocaine with 1:200,000 epinephrine, and 4% prilocaine for maxillary infiltrations. Anesthesia Progress 2010;57(2):45‐51. [PUBMED: 20553134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keskitalo 1975 {published data only}
- Keskitalo E, Persson G. Complications after removal of mandibular third molars with special reference to local anaesthetics with different vasoactive properties. Odontologisk Revy 1975;26(2):149‐64. [PUBMED: 1097978] [PubMed] [Google Scholar]
Khoury 1991 {published data only}
- Khoury F, Hinterthan A, Schurmann J, Arns H. Clinical comparative study of local anesthetics. Random double blind study with four commercial preparations. Deutsche Zahnarztliche Zeitschrift 1991;46(12):822‐4. [PUBMED: 1817898] [PubMed] [Google Scholar]
Knoll‐Kohler 1992a {published data only}
- Knoll‐Kohler E, Fortsch G. Pulpal anesthesia dependent on epinephrine dose in 2% lidocaine. A randomized controlled double‐blind crossover study. Oral Surgery, Oral Medicine, Oral Pathology 1992;73(5):537‐40. [PUBMED: 1518636] [DOI] [PubMed] [Google Scholar]
Knoll‐Kohler 1992b {published data only}
- Knoll‐Kohler E, Rupprecht S. Articaine for local anaesthesia in dentistry. A lidocaine controlled double blind cross‐over study. European Journal of Pain 1992;13(2):59‐63. [EMBASE: 1992242721] [Google Scholar]
Kolli 2017 {published data only}
- Kolli NK, Nirmala SV, Nuvvula S. The effectiveness of articaine and lidocaine single buccal infiltration versus conventional buccal and palatal injection using lidocaine during primary maxillary molar extraction: a randomized control trial. Anesthesia Essays and Researches 2017;11(1):160‐4. [PUBMED: 28298777] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 1958 {published data only}
- Kramer WS. A comparative clinical evaluation of some commonly used local anesthetic compounds. Journal of the American Dental Association 1958;56(6):820‐30. [PUBMED: 13538629] [DOI] [PubMed] [Google Scholar]
Lasemi 2015 {published data only}
- Lasemi E, Sezavar M, Habibi L, Hemmat S, Sarkarat F, Nematollahi Z. Articaine (4%) with epinephrine (1:100,000 or 1:200,000) in inferior alveolar nerve block: effects on the vital signs and onset, and duration of anesthesia. Journal of Dental Anesthesia and Pain Medicine 2015;15(4):201‐5. [DOI: 10.17245/jdapm.2015.15.4.201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laskin 1977 {published data only}
- Laskin JL, Wallace WR, DeLeo B. Use of bupivacaine hydrochloride in oral surgery ‐ a clinical study. Journal of Oral Surgery 1977;35(1):25‐9. [PUBMED: 264254] [PubMed] [Google Scholar]
Lawaty 2010 {published data only}
- Lawaty I, Drum M, Reader A, Nusstein J. A prospective, randomized, double‐blind comparison of 2% mepivacaine with 1:20,000 levonordefrin versus 2% lidocaine with 1:100,000 epinephrine for maxillary infiltrations. Anesthesia Progress 2010;57(4):139‐44. [PUBMED: 21174567] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lima 2009 {published data only}
- Lima JL Jr, Dias‐Ribeiro E, Araujo TN, Ferreira‐Rocha J, Honfi ES Jr, Sarmento CFDM, et al. Evaluation of the buccal vestibule‐palatal diffusion of 4% articaine hydrochloride in impacted maxillary third molar extractions. Medicina Oral, Patologia Oral y Cirugia Bucal 2009;14(3):E129‐32. [PUBMED: 19242392] [PubMed] [Google Scholar]
Linden 1986 {published data only}
- Linden ET, Abrams H, Matheny J, Kaplan AL, Kopczyk RA, Jasper SJ Jr. A comparison of postoperative pain experience following periodontal surgery using two local anesthetic agents. Journal of Periodontology 1986;57(10):637‐42. [PUBMED: 3534212] [DOI] [PubMed] [Google Scholar]
Malamed 2000a {published data only}
- Malamed SF, Gagnon S, Leblanc D. Efficacy of articaine: a new amide local anesthetic. Journal of the American Dental Association 2000;131(5):635‐42. [PUBMED: 10832257] [DOI] [PubMed] [Google Scholar]
Malamed 2000b {published data only}
- Malamed SF, Gagnon S, Leblanc D. A comparison between articaine HCl and lidocaine HCl in pediatric dental patients. Pediatric Dentistry 2000;22(4):307‐11. [PUBMED: 10969438] [PubMed] [Google Scholar]
Maniglia‐Ferreira 2009 {published data only}
- Maniglia‐Ferreira C, Almeida‐Gomes F, Carvalho‐Sousa B, Barbosa AVH, Lins Carla CSA, Souza Fabricio D, et al. Clinical evaluation of the use of three anesthetics in endodontics. Acta Odontologica Latinoamericana 2009;22(1):21‐6. [PUBMED: 19601492] [PubMed] [Google Scholar]
Martinez‐Rodriguez 2012 {published data only}
- Martinez‐Rodriguez N, Barona‐Dorado C, Martin‐Ares M, Cortes‐Breton‐Brinkman J, Martinez‐Gonzalez JM. Evaluation of the anaesthetic properties and tolerance of 1:100,000 articaine versus 1:100,000 lidocaine. A comparative study in surgery of the lower third molar. Medicina Oral, Patologia Oral y Cirugia Bucal 2012;17(2):345‐51. [PUBMED: 22143691] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maruthingal 2015 {published data only}
- Maruthingal S, Mohan D, Maroli RK, Alahmari A, Alqahtani A, Alsadoon M. A comparative evaluation of 4% articaine and 2% lidocaine in mandibular buccal infiltration anesthesia: a clinical study. Journal of International Society of Preventive & Community Dentistry 2015;5(6):463–9. [DOI: 10.4103/2231-0762.167717] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2009 {published data only}
- Mason R, Drum M, Reader A, Nusstein J, Beck M. A prospective, randomized, double‐blind comparison of 2% lidocaine with 1:100,000 and 1:50,000 epinephrine and 3% mepivacaine for maxillary infiltrations. Journal of Endodontics 2009;35(9):1173‐7. [PUBMED: 19720211 ] [DOI] [PubMed] [Google Scholar]
McEntire 2011 {published data only}
- McEntire M, Nusstein J, Drum M, Reader A, Beck M. Anesthetic efficacy of 4% articaine with 1:100,000 epinephrine versus 4% articaine with 1:200,000 epinephrine as a primary buccal infiltration in the mandibular first molar. Journal of Endodontics 2011;37(4):450‐4. [PUBMED: 21419288] [DOI] [PubMed] [Google Scholar]
McLean 1993 {published data only}
- McLean C, Reader A, Beck M, Meryers WJ. An evaluation of 4% prilocaine and 3% mepivacaine compared with 2% lidocaine (1:100,000 epinephrine) for inferior alveolar nerve block. Journal of Endodontics 1993;19(3):146‐50. [PUBMED: 8509754] [DOI] [PubMed] [Google Scholar]
Mikesell 2005 {published data only}
- Mikesell P, Nusstein J, Reader A, Beck M, Weaver. A comparison of articaine and lidocaine for inferior alveolar nerve blocks. Journal of Endodontics 2005;31(4):265‐70. [PUBMED: 15793381] [DOI] [PubMed] [Google Scholar]
Mittal 2015 {published data only}
- Mittal M, Sharma S, Kumar A, Chopra R, Srivastava D. Comparison of anesthetic efficacy of articaine and lidocaine during primary maxillary molar extractions in children. Pediatric Dentistry 2015;37(7):520‐24. [PubMed] [Google Scholar]
Moore 1983 {published data only}
- Moore PA, Dunsky JL. Bupivacaine anesthesia ‐ a clinical trial for endodontic therapy. Oral Surgery, Oral Medicine, Oral Pathology 1983;55(2):176‐9. [PUBMED: 6340015] [DOI] [PubMed] [Google Scholar]
Moore 2006 {published data only}
- Moore PA, Boynes SG, Hersh EV, DeRossi SS, Sollecito TP, Goodson JM, et al. The anesthetic efficacy of 4 percent articaine 1:200,000 epinephrine: two controlled clinical trials. Journal of the American Dental Association 2006;137(11):1572‐81. [PUBMED: 17082284] [DOI] [PubMed] [Google Scholar]
Moore 2007 {published data only}
- Moore PA, Doll B, Delie RA, Hersh EV, Korostoff J, Johnson S, et al. Hemostatic and anesthetic efficacy of 4% articaine HCl with 1:200,000 epinephrine and 4% articaine HCl with 1:100,000 epinephrine when administered intraorally for periodontal surgery. Journal of Periodontology 2007;78(2):247‐53. [PUBMED: 17274713] [DOI] [PubMed] [Google Scholar]
Mumford 1961 {published data only}
- Mumford JM, Geddes IC. Trial of carbocaine in conservative dentistry. British Dental Journal 1961;110:92. [Google Scholar]
Nabeel 2014 {published data only}
- Nabeel M, Ahmed A, Sikander M. A comparison of the anesthetic efficacy of lidocaine and articaine for buccal infiltration in patients with acute irreversible pulpitis in maxillary first premolars. Pakistan Oral & Dental Journal 2014;34(4):714‐16. [100212933] [Google Scholar]
Naik 2017 {published data only}
- Naik VG, Rai KK, Adhyaru P. A comparison of efficacy of articaine versus lignocaine in inferior alveolar nerve block during surgical extraction of impacted lower third molar ‐ a randomized single blind prospective study. World Journal of Pharmaceutical and Medical Research 2017;3(1):167‐72. [2455‐3301] [Google Scholar]
Nespeca 1976 {published data only}
- Nespeca JA. Clinical trials with bupivacaine in oral surgery. Oral Surgery, Oral Medicine, Oral Pathology 1976;42(3):301‐7. [PUBMED: 1067530 ] [DOI] [PubMed] [Google Scholar]
Nordenram 1990 {published data only}
- Nordenram A, Danielsson K. Local anaesthesia in elderly patients. An experimental study of oral infiltration anaesthesia. Swedish Dental Journal 1990;14(1):19‐24. [PUBMED: 2363109] [PubMed] [Google Scholar]
Nydegger 2014 {published data only}
- Nydegger B, Nusstein J, Reader A, Drum M, Beck M. Anesthetic comparisons of 4% concentrations of articaine, lidocaine, and prilocaine as primary buccal infiltrations of the mandibular first molar: a prospective randomized, double‐blind study. Journal of Endodontics 2014;40(12):1912‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Odabas 2012 {published data only}
- Odabas ME, Cinar C, Deveci C, Alacam A. Comparison of the anesthetic efficacy of articaine and mepivacaine in pediatric patients: a randomized, double‐blind study. Pediatric Dentistry 2012;34(1):42‐5. [PUBMED: 22353456] [PubMed] [Google Scholar]
Oliveira 2004 {published data only}
- Oliveira PC, Volpato MC, Ramacciato JC, Ranali J. Articaine and lignocaine efficiency in infiltration anaesthesia: a pilot study. British Dental Journal 2004;197(1):45‐6; discussion 33. [PUBMED: 15243610 ] [DOI] [PubMed] [Google Scholar]
Ozec 2010 {published data only}
- Ozec I, Tasdemir U, Gumus C, Solak O. Is it possible to anesthetize palatal tissues with buccal 4% articaine injection?. Journal of Oral and Maxillofacial Surgery 2010;68(5):1032‐7. [PUBMED: 20223573] [DOI] [PubMed] [Google Scholar]
Parirokh 2015 {published data only}
- Parirokh M, Yosefi MH, Nakhaee N, Abbott PV, Manochehrifar H. The success rate of bupivacaine and lidocaine as anesthetic agents in inferior alveolar nerve block in teeth with irreversible pulpitis without spontaneous pain. Restorative Dentistry & Endodontics 2015;40(2):155‐60. [PUBMED: 25984478] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pässler 1996 {published data only}
- Pässler L, Wickede J. Articain, lidocain, prilocain und vasokonstriktorische zusätze‐untersuchungen zur verminderung des anästhesierisikos. Quintessenz 1996;47:669‐78. [Google Scholar]
Pellicer‐Chover 2013 {published data only}
- Pellicer‐Chover H, Cervera‐Ballester J, Sanchis‐Bielsa JM, Peñarrocha‐Diago MA, Peñarrocha‐Diago M, García‐Mira B. Comparative split‐mouth study of the anesthetic efficacy of 4% articaine versus 0.5% bupivacaine in impacted mandibular third molar extraction. Journal of Clinical and Experimental Dentistry 2013;5(2):e66‐71. [PUBMED: 24455059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poorni 2011 {published data only}
- Poorni S, Veniashok B, Senthilkumar AD, Indira R, Ramachandran S. Anesthetic efficacy of four percent articaine for pulpal anesthesia by using inferior alveolar nerve block and buccal infiltration techniques in patients with irreversible pulpitis: a prospective randomized double‐blind clinical trial. Journal of Endodontics 2011;37(12):1603‐7. [PUBMED: 22099890] [DOI] [PubMed] [Google Scholar]
Porto 2007 {published data only}
- Porto GG, Vasconcelos BC, Gomes AC, Albert D. Evaluation of lidocaine and mepivacaine for inferior third molar surgery. Medicina Oral, Patologia Oral y Cirugia Bucal 2007;12(1):E60‐4. [PUBMED: 17195831] [PubMed] [Google Scholar]
Ram 2006 {published data only}
- Ram D, Amir E. Comparison of articaine 4% and lidocaine 2% in paediatric dental patients. International Journal of Paediatric Dentistry 2006;16(4):252‐6. [PUBMED: 16759322] [DOI] [PubMed] [Google Scholar]
Robertson 2007 {published data only}
- Robertson D, Nusstein J, Reader A, Beck M, McCartney M. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth.. Journal of the American Dental Association 2007;138(8):1104‐12. [PUBMED: 17670879] [DOI] [PubMed] [Google Scholar]
Ruprecht 1991 {published data only}
- Ruprecht S, Knoll‐Kohler E. A comparative study of equimolar solutions of lidocaine and articaine for anesthesia. A randomized double‐blind cross‐over study. Schweizer Monatsschrift fur Zahnmedizin 1991;101(10):1286‐90. [PUBMED: 1947972] [PubMed] [Google Scholar]
Sadove 1962 {published data only}
- Sadove MS, Vernino D, Lock F, Kolodny S. An evaluation of mepivacaine hydrochloride. Journal of oral surgery, anesthesia, and hospital dental service 1962;20(5):399‐404. [PubMed] [Google Scholar]
Sampaio 2012 {published data only}
- Sampaio RM, Carnaval TG, Lanfredi CB, Ratto Tempestini Horliana AC, Rocha RG, Tortamano IP. Comparison of the anesthetic efficacy between bupivacaine and lidocaine in patients with irreversible pulpitis of mandibular molar. Journal of Endodontics 2012;38(5):594‐7. [PUBMED: 22515885] [DOI] [PubMed] [Google Scholar]
Sancho‐Puchades 2012 {published data only}
- Sancho‐Puchades M, Vilchez‐Perez MA, Valmaseda‐Castellon E, Paredes‐Garcia J, Berini‐Aytes L, Gay‐Escoda C. Bupivacaine 0.5 % versus articaine 4 % for the removal of lower third molars. A crossover randomized controlled trial. Medicina Oral, Patologia Oral y Cirugia Bucal 2012;17(3):e462‐8. [PUBMED: 22143739] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2007 {published data only}
- Santos CF, Modena KC, Giglio FP, Sakai VT, Calvo AM, Colombini BL, et al. Epinephrine concentration (1:100,000 or 1:200,000) does not affect the clinical efficacy of 4% articaine for lower third molar removal: a double‐blind, randomized, crossover study. Journal of Oral & Maxillofacial Surgery 2007;65(12):2445‐52. [PUBMED: 18022467] [DOI] [PubMed] [Google Scholar]
Sherman 1954 {published data only}
- Sherman H, Fiasconaro JE, Chilton NW. A comparison of the clinical effectiveness of the higher potency local anesthetics in operative dentistry. Journal of the American Dental Association 1954;48(2):151‐7. [PUBMED: 13128982] [DOI] [PubMed] [Google Scholar]
Sherman 2008 {published data only}
- Sherman MG, Flax M, Namerow K, Murray PE. Anesthetic efficacy of the Gow‐Gates injection and maxillary infiltration with articaine and lidocaine for irreversible pulpitis. Journal of Endodontics 2008;34(6):656‐9. [PUBMED: 18498883] [DOI] [PubMed] [Google Scholar]
Sierra Rebolledo 2007 {published data only}
- Sierra Rebolledo A, Delgado Molina E, Berini Aytis L, Gay Escoda C. Comparative study of the anesthetic efficacy of 4% articaine versus 2% lidocaine in inferior alveolar nerve block during surgical extraction of impacted lower third molars. Medicina Oral, Patologia Oral y Cirugia Bucal 2007;12(2):E139‐44. [PUBMED: 17322803] [PubMed] [Google Scholar]
Silva 2012 {published data only}
- Silva LCF, Santos TS, Santos JASS, Maia MC, Mendonca CG. Articaine versus lidocaine for third molar surgery: A randomized clinical study. Medicina Oral, Patologia Oral y Cirugia Bucal 2012;17(1):e140‐5. [PUBMED: 22157664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sood 2014 {published data only}
- Sood R, Manoj‐Kumar H, Shetty S. Comparison of anesthetic efficacy of 4% articaine with 1:100,000 epinephrine and 2% lidocaine with 1:80,000 epinephrine for inferior alveolar nerve block in patients with irreversible pulpitis. Journal of Clinical and Experimental Dentistry 2014;6(5):e520‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasan 2009 {published data only}
- Srinivasan N, Kavitha M, Loganathan CS, Padmini G. Comparison of anesthetic efficacy of 4% articaine and 2% lidocaine for maxillary buccal infiltration in patients with irreversible pulpitis. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics 2009;107(1):133‐6. [PUBMED: 19101495] [DOI] [PubMed] [Google Scholar]
Srisurang 2011 {published data only}
- Srisurang S, Narit L, Prisana P. Clinical efficacy of lidocaine, mepivacaine, and articaine for local infiltration. Journal of Investigative and Clinical Dentistry 2011;2(1):23‐8. [EMBASE: 25427324] [DOI] [PubMed] [Google Scholar]
Stibbs 1964 {published data only}
- Stibbs GD, Korn JH. An evaluation of the local anaesthetic mepivacaine hydrochloride, in operative dentistry. Journal of Prosthetic Dentistry 1964;14(2):355‐64. [Google Scholar]
Thakare 2014 {published data only}
- Thakare A, Bhate K, Kathariya R. Comparison of 4% articaine and 0.5% bupivacaine anesthetic efficacy in orthodontic extractions: prospective, randomized crossover study. Acta Anaesthesiologica Taiwanica 2014;52(2):59‐63. [EMBASE: 2014469720] [DOI] [PubMed] [Google Scholar]
Tofoli 2003 {published data only}
- Tofoli GR, Ramacciato JC, Oliveira PC, Volpato MC, Groppo FC, Ranali J. Comparison of effectiveness of 4% articaine associated with 1: 100,000 or 1: 200,000 epinephrine in inferior alveolar nerve block. Anesthesia Progress 2003;50(4):164‐8. [PUBMED: 14959904] [PMC free article] [PubMed] [Google Scholar]
Tortamano 2009 {published data only}
- Tortamano IP, Siviero M, Costa CG, Buscariolo IA, Armonia PL. A comparison of the anesthetic efficacy of articaine and lidocaine in patients with irreversible pulpitis. Journal of Endodontics 2009;35(2):165‐8. [PUBMED: 19166765] [DOI] [PubMed] [Google Scholar]
Tortamano 2013 {published data only}
- Tortamano IP, Siviero M, Lee S, Sampaio RM, Simone JL, Rocha RG. Onset and duration period of pulpal anesthesia of articaine and lidocaine in inferior alveolar nerve block. Brazilian Dental Journal 2013;24(4):371‐4. [EMBASE: 24173259] [DOI] [PubMed] [Google Scholar]
Trieger 1979 {published data only}
- Trieger N, Gillen GH. Bupivacaine anesthesia and post‐operative analgesia in oral surgery. Anesthesia Progress 1979;26(1):20‐3. [PUBMED: 295581] [PMC free article] [PubMed] [Google Scholar]
Trullenque‐Eriksson 2011 {published data only}
- Trullenque‐Eriksson A, Guisado‐Moya B. Comparative study of two local anesthetics in the surgical extraction of mandibular third molars: bupivacaine and articaine. Medicina Oral, Patologia Oral y Cirugia Bucal 2011;16(3):e390‐6. [PUBMED: 21196829] [DOI] [PubMed] [Google Scholar]
Vahatalo 1993 {published data only}
- Vahatalo K, Antila H, Lehtinen R. Articaine and lidocaine for maxillary infiltration anesthesia. Anesthesia Progress 1993;40(4):114‐6. [PUBMED: 7943919] [PMC free article] [PubMed] [Google Scholar]
Vilchez‐Perez 2012 {published data only}
- Vilchez‐Perez MA, Sancho‐Puchades M, Valmaseda‐Castellon E, Paredes‐Garcia J, Berini‐Aytes L, Gay‐Escoda C. A prospective, randomized, triple‐blind comparison of articaine and bupivacaine for maxillary infiltrations. Medicina Oral, Patologia Oral y Cirugia Bucal 2012;17(2):325‐30. [PUBMED: 22143708] [DOI] [PMC free article] [PubMed] [Google Scholar]
Visconti 2016 {published data only}
- Visconti RP, Tortamano IP, Buscariolo IA. Comparison of the anesthetic efficacy of mepivacaine and lidocaine in patients with irreversible pulpitis: a double‐blind randomized clinical trial. Journal of Endodontics 2016;42(9):1314‐19. [PUBMED: 27475099 ] [DOI] [PubMed] [Google Scholar]
Vreeland 1989 {published data only}
- Vreeland DL, Reader A, Beck M, Meyers W, Weaver J. An evaluation of volumes and concentrations of lidocaine in human inferior alveolar nerve block. Journal of Endodontics 1989;15(1):6‐12. [PUBMED: 2607268] [DOI] [PubMed] [Google Scholar]
Wali 2010 {published data only}
- Wali M, Drum M, Reader A, Nusstein J. Prospective, randomized single‐blind study of the anesthetic efficacy of 1.8 and 3.6 milliliters of 2% lidocaine with 1:50,000 epinephrine for inferior alveolar nerve block. Journal of Endodontics 2010;36(9):1459‐62. [PUBMED: 20728709] [DOI] [PubMed] [Google Scholar]
Weil 1961 {published data only}
- Weil C, Santangelo C, Welham FS, Yackel RF. Clinical evaluation of mepivacaine hydrochloride by a new method. Journal of the American Dental Association 1961;63:26‐32. [PUBMED: 13783827] [DOI] [PubMed] [Google Scholar]
Yadav 2015 {published data only}
- Yadav M, Grewal MS, Grewal S, Deshwal P. Comparison of preoperative oral ketorolac on anesthetic efficacy of inferior alveolar nerve block and buccal and lingual infiltration with articaine and lidocaine in patients with irreversible pulpitis: a prospective, randomized, controlled, double‐blind study. Journal of Endodontics 2015;41(11):1773‐7. [EMBASE: 616595072] [DOI] [PubMed] [Google Scholar]
Yared 1997 {published data only}
- Yared GM, Dagher FB. Evaluation of lidocaine in human inferior alveolar nerve block. Journal of Endodontics 1997;23(9):575‐8. [PUBMED: 9587285] [DOI] [PubMed] [Google Scholar]
Yilmaz 2011 {published data only}
- Yilmaz Y, Eyuboglu O, Keles S. Comparison of the efficacy of articaine and prilocaine local anaesthesia for pulpotomy of maxillary and mandibular primary molars. European Journal of Paediatric Dentistry 2011;12(2):117‐22. [PUBMED: 21668284] [PubMed] [Google Scholar]
Yonchak 2001 {published data only}
- Yonchak T, Reader A, Beck M, Clark K, Meyers WJ. Anesthetic efficacy of infiltrations in mandibular anterior teeth. Anesthesia Progress 2001;48(2):55‐60. [PUBMED: 11515948] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adler 1969 {published data only}
- Adler R, Aler G, Aberg G. Effects of optically active isomers and racemate of mepivacaine (Carbocaine) in dental anaesthesia. Svensk Tandlakaretidskrift 1969;62(8):501‐4. [PUBMED: 4911578] [PubMed] [Google Scholar]
Caruso 1989 {published data only}
- Caruso JM, Brokaw WC, Blanton EE. Bupivacaine and lidocaine compared for postoperative pain control. General Dentistry 1989;37(2):148‐51. [PUBMED: 2599329] [PubMed] [Google Scholar]
Cowan 1964 {published data only}
- Cowan A. Minimum dosage technique in the clinical comparison of representative modern local anesthetic agents. Journal of Dental Research 1964;43:1228‐49. [PUBMED: 14238893] [DOI] [PubMed] [Google Scholar]
Cowan 1968 {published data only}
- Cowan A. Further clinical evaluation of prilocaine (Citanest), with and without epinephrine. Oral Surgery, Oral Medicine, Oral Pathology 1968;26(3):304‐11. [PUBMED: 4875791] [DOI] [PubMed] [Google Scholar]
Hassan 2011 {published data only}
- Hassan S, Sripathi Rao BH, Sequeria J, Rai G. Efficacy of 4% articaine hydrochloride and 2% lignocaine in the extraction of maxillary premolars for orthodontic reasons. Annals of Maxillofacial Surgery 2011;1(1):14‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanaa 2009 {published data only}
- Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. International Endodontic Journal 2009;42(3):238‐46. [PUBMED: 19228214] [DOI] [PubMed] [Google Scholar]
Raab 1990 {published data only}
- Raab WH, Muller R, Muller HF. Comparative investigations of anesthetic activity of 2‐ and 4% articain. Quintessenz 1990;41(7):1207‐16. [PubMed] [Google Scholar]
Shruthi 2013 {published data only}
- Shruthi R, Kedarnath N, Mamatha N, Rajaram P, BhadraShetty D. Articaine for surgical removal of impacted third molar; a comparison with lignocaine. Journal of International Oral Health 2013;5(1):48‐53. [PUBMED: 24155578 ] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chen 2004 {published data only}
- Chen XM, Shi ZD, Huang DM, Zeng HB, Wang XY, Ding Y. Anesthetic efficacy of 2% mepivacaine in conservative dentistry. West China Journal of Stomatology 2004;22(5):390‐2. [PubMed] [Google Scholar]
da Silva‐Junior 2017 {published data only}
- Silva‐Junior GP, Almeida Souza LM, Groppo FC. Comparison of articaine and lidocaine for buccal infiltration after inferior alveolar nerve block for intraoperative pain control during impacted mandibular third molar surgery. Anesthesia Progress 2017;64(2):80‐4. [PUBMED: 28604089] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dong 2010 {published data only}
- Dong HD, Liu Q. The pulpal anesthetic efficacy of the scandonest on maxillary pulpitis molars. Journal of Practical Stomatology 2010;26:609‐11. [Google Scholar]
Ge 2005 {published data only}
- Ge XJ, Li X, Wang XY. Comparison between scandonest and lidocaine in the endodontic treatment of the mandibular posterior teeth. Chinese Remedies and Clinics 2005;15:956‐7. [Google Scholar]
Guo 2014 {published data only}
- Guo SL, Yang WD, Jiang WM. Comparison of anesthetic efficacy of 2% lidocaine and 4% articaine in patients with irreversible pulpitis in maxillary teeth. Jilin Medical Journal 2014;35:1795‐7. [Google Scholar]
He 2010 {published data only}
- He P, Wang JR. Application of scandonest in endodontic treatment. Stomatology 2010;30:511‐2. [Google Scholar]
Huang 2011 {published data only}
- Huang YD, Xia H, Li XD, Yang XZ, Pei ZQ, Xia X. A comparison of the clinical anesthetic efficacy of articaine infiltration and lidocaine blocking for microport extraction of impacted mandibular molar. West China Journal of Stomatology 2011;29(3):268‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Im 2010 {published data only}
- Im TY, Hwang KG, Park CJ, Kim KS, Oh Y, Han JY, et al. Randomized, double‐blind, comparative clinical trial on the efficacy of 4% articaine and 2% lidocaine in inferior alveolar nerve block anesthesia. Journal of the Korean Dental Society of Anesthesiology 2010;10(1):1‐6. [DOI: 10.17245/jkdsa.2010.10.1.1] [DOI] [Google Scholar]
Jin 2005 {published data only}
- Jin XD, Wang GL. Efficacy of mepivacaine hydrochloride with epinephrine in endodontic treatment. Guangdong Medical Journal 2005;26:694‐5. [Google Scholar]
Lee 2004 {published data only}
- Lee WY, Park CJ, Seo KS, Kim HJ, Yum KW. Comparative study for the anesthetic efficacy between articaine HCl and lidocaine HCl during the surgical extraction of bilateral mandibular impacted third molars. Journal of the Korean Dental Society of Anesthesiology 2004;4(1):13‐6. [DOI: 10.17245/jkdsa.2004.4.1.13] [DOI] [Google Scholar]
Li 2005 {published data only}
- Li SY, Liu Y, Liu RS. Clinical evaluation of the anesthetic effect of mepivacaine hydrochloride and adrenaline injection during pulpal treatment in the elderly. Chinese Journal of Geriatric Dentistry 2005;3:78‐80. [Google Scholar]
Liang 2001 {published data only}
- Liang ZD, Hu YL, Guan SN, Li L, He KX. Observation of anesthetic efficacy of articaine in endodontic treatment. Journal of Guangxi Medical University 2001;18:529‐30. [Google Scholar]
Liao 2004 {published data only}
- Liao MH. Observation of anesthetic efficacy of articaine in pulp inactivation. Journal of Youjiang Medical College for Nationalities 2004;4:504‐5. [Google Scholar]
Liu 2010 {published data only}
- Liu MZ. Application of mepivacaine hydrochloride injection in tooth extraction and endodontic treatment. Chinese Journal of Nursing Education 2010;7:378‐9. [Google Scholar]
Luo 2009 {published data only}
- Luo LC, Li H. Comparison of anesthetic effect between scandonest and lidocaine in the extraction of the upper molar. Hainan Medical Journal 2009;20:204‐5. [Google Scholar]
Manabe 2005 {published data only}
- Manabe Y, Seto M, Koyanagi N, Tominaga S, Taniguchi S. An evaluation of 3% mepivacaine compared with 2% lidocaine (1:80,000 epinephrine) for the inferior alveolar nerve block. [Japanese]. Journal of Japanese Dental Society of Anesthesiology 2005;33(3):369‐72. [EMBASE: 2005356341] [Google Scholar]
Oka 1990 {published data only}
- Oka S. Effect of the concentration of the vasoconstrictor in local anesthetic on local anesthesia and hemodynamics. Journal of Japanese Dental Society of Anesthesiology 1990;1:43‐66. [Google Scholar]
Ouchi 2008 {published data only}
- Ouchi K, Sunada K. Randomized controlled study of felypressin‐propitocaine and mepivacaine for inferior alveolar nerve block. [Japanese]. Journal of Japanese Dental Society of Anesthesiology 2008;36(3):263‐8. [EMBASE: 2008395088] [Google Scholar]
Qiu 2007 {published data only}
- Qiu YY, Zhou TQ. Comparison of efficacy between two kinds of anesthesia in pulpectomy. Modern Hospital 2007;7:30‐1. [Google Scholar]
Qiu 2011 {published data only}
- Qiu H, Wang RY. Anesthetic efficacy of articaine for premolars in the mandibular in endodontic treatment. Hebei Medical Journal 2011;33:2494. [Google Scholar]
Shi 2002 {published data only}
- Shi ZD, Wang XY, Chen ZY, Yang XM, Chen XM, Xu BH, et al. Multi‐center randomized double‐blinded controlled clinical trial on local anesthetic efficacy of scandonest 2% special and its safety. Chinese Journal of Evidence‐Based Medicine 2002;2:86‐91. [Google Scholar]
Shimada 2002 {published data only}
- Shimada M, Miyawaki T, Takada K, Misaki T, Oka S, Yoshimura S, et al. The clinical anesthetic efficacy of 3% mepivacaine in infiltration and block anesthesia: a comparative double blind study with 2% lidocaine hydrochloride containing 1:80,000 epinephrine. [Japanese]. Journal of Japanese Dental Society of Anesthesiology 2002;30(1):48‐61. [EMBASE: 2002060162] [Google Scholar]
Wang 2009 {published data only}
- Wang LC, Zheng J, Zhang FY. Analysis of efficacy of articaine in irreversible pulpitis. Journal of Modern Medicine and Health 2009;25:850. [Google Scholar]
Wu 2005 {published data only}
- Wu YN, Yang SQ, Zhu QP, Wang JH, Guo W, Fu CF. Effects of three local anesthesia agents during procedures of pulpectomy. Stomatology 2005;25:291‐2. [Google Scholar]
Xie 2008 {published data only}
- Xie GY. Observation of the local anesthesia effect for aged acute pulpitis. Journal of Practical Medical Techniques 2008;15:3171‐2. [Google Scholar]
Xing 2005 {published data only}
- Xing XY, Xing WB, Zhang QS. Clinical evaluation of the effect of scandonest (mepivacaine hydrochloride) used in dentistry. Journal of Tianjin Medical University 2005;11:243‐5. [Google Scholar]
Xu 1991 {published data only}
- Xu SS, Qian CH. Clinical research of bupivacaine in endodontic treatment. Journal of Comprehensive Stomatology 1991;7:113‐4. [Google Scholar]
Xu 2008 {published data only}
- Xu X, Ji HH, Yang Y, Cao XM, Gao YG. Application and therapeutic effect of articaine in the dental treatment. Chinese Journal of Modern Drug Application 2008;2:11‐2. [Google Scholar]
Xu 2013 {published data only}
- Xu XY. Anesthetic efficacy of local infiltration anesthesia with primacaine adrenaline and inferior alveolar nerve block anesthesia with lidocaine hydrochloride in patients with acute pulpitis in mandibular. National Medical Frontiers of China 2013;8:87‐8. [Google Scholar]
Xuan 2007 {published data only}
- Xuan GJ. Application of scandonest in teeth preparation of vital pulp. Journal of Clinical Stomatology 2007;123:190‐1. [Google Scholar]
Zhang 2005 {published data only}
- Zhang L, Lin XF, Chen G, Li Y. Clinical observation of local anesthesia in dentistry for articaine. Chinese Journal of School Doctor 2005;19:308‐9. [Google Scholar]
Zhang 2009 {published data only}
- Zhang DH. Observation of clinical efficacy of articaine in endodontic treatment. Medical Journal of Chinese People's Health 2009;21:2378‐9. [Google Scholar]
Zhou 2011 {published data only}
- Zhou Q. Observation of mepivacaine hydrochloride with epinephrine solutions in inferior alveolar nerve block anesthesia. Modern Practical Medicine 2011;23:1257‐9. [Google Scholar]
Zhou 2013 {published data only}
- Zhou Y, You GL, Qiu SB, et al. Comparison of anesthetic effects of articaine and lidocaine in acute attack of chronic pulpitis odontotrypy. Chinese Pharmacy 2013;24:4543‐4. [Google Scholar]
References to ongoing studies
Caicedo 1996 {published data only}
- Caicedo R, Helo JF, Lopez J. Evaluation of three anesthetic solutions using two local anesthesia techniques. Journal of Endodontics 1996;22(4):209. [Google Scholar]
Iqbal 2009 {published data only}
- Iqbal MK, Greenberg BD, Wang D, Hersh EV, Pinto A. Comparison of anesthetic efficacy of articaine and lidocaine for inferior alveolar nerve blocks with buccal infiltrations in patients with irreversible pulpitis. Journal of Endodontics 2009;35(3):448. [Google Scholar]
Sheikh 2014 {published data only}
- Sheikh S. Preliminary comparison of missed blocks with 4% articaine and 2% lidocaine both with 1:100,000 epinephrine on inferior alveolar nerve block injections. American Association of Oral and Maxillofacial Surgeons (AAOMS) Conference 2014 September 8‐13. 2014:e51‐2.
Additional references
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
Brandt 2011
- Brandt RG, Anderson PF, McDonald NJ, Sohn W, Peters MC. The pulpal anesthetic efficacy of articaine versus lidocaine in dentistry a meta‐analysis. Journal of the American Dental Association 2011;142(5):493‐504. [DOI] [PubMed] [Google Scholar]
Certosimo 1996
- Certosimo AJ, Archer RD. A clinical evaluation of the electric pulp tester as an indicator of local anesthesia. Operative Dentistry 1996;21(1):25‐30. [PubMed] [Google Scholar]
Clark 1999
- Clark S, Reader A, Beck M, Meyers WJ. Anesthetic efficacy of the mylohyoid nerve block and combination inferior alveolar nerve/mylohyoid nerve block. Oral Surgery, Oral Medicine, Oral Pathology 1999;87:557‐63. [PUBMED: 10348512] [DOI] [PubMed] [Google Scholar]
Corbella 2017
- Corbella S, Taschieri S, Mannocci F, Rosen E, Tsesis I, Fabbro M. Inferior alveolar nerve block for the treatment of teeth presenting with irreversible pulpitis: a systematic review of the literature and meta‐analysis. Quintessence International 2017;48(1):69‐82. [121334449] [DOI] [PubMed] [Google Scholar]
Dreven 1987
- Dreven LJ, Reader A, Beck M, Meyers WJ, Weaver J. An evaluation of an electric pulp tester as a measure of analgesia in human vital teeth. Journal of Endodontics 1987;13(5):233‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Garisto 2010
- Garisto GA, Gaffen AS, Lawrence HP, Tenenbaum HC, Haas DA, Garisto Gabriella A, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States.[Erratum appears in J Am Dent Assoc. 2010 Aug;141(8):944]. Journal of the American Dental Association 2010;141(7):836‐44. [DOI] [PubMed] [Google Scholar]
Gow‐Gates 1973
- Gow‐Gates GA. Mandibular conduction anesthesia: a new technique using extraoral landmarks. Oral Surgery, Oral Medicine, Oral Pathology 1973;36(3):321‐8. [PUBMED: 4516460] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gray 1987
- Gray RJ, Lomax AM, Rood JP. Periodontal ligament injection: with or without a vasoconstrictor?. British Dental Journal 1987;162:263‐5. [PUBMED: 3472559] [DOI] [PubMed] [Google Scholar]
Haas 1995
- Haas DA, Lennon D. A 21 year retrospective study of reports of paraesthesia following local anaesthetic administration. Journal (Canadian Dental Association) 1995;61:319‐30. [PUBMED: 7736335] [PubMed] [Google Scholar]
Hargreaves 2001
- Hargreaves KM. Neurochemical factors in injury and inflammation in orofacial tissues. In: Lavigne GJ, Lund JP, Sessle BJ, Dubner R editor(s). Orofacial Pain: Basic Sciences to Clinical Management. Chicago: Quintessence, 2001. [Google Scholar]
Heft 1984
- Heft MW, Parker SR. An experimental basis for revising the graphic rating scale for pain. Pain 1984;19(2):153‐61. [PUBMED: 6462727] [DOI] [PubMed] [Google Scholar]
Hicks 2001
- Hicks C, Baeyer C, Spafford P, Korlaar I, Goodenough B. The faces pain scale–revised: toward a common metric in pediatric pain measurement. Pain 2001;93:173–83. [PUBMED: 11427329] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillerup 2006
- Hillerup S, Jensen R. Nerve injury caused by mandibular block analgesia. International Journal of Oral and Maxillofacial Surgery 2006;35:437‐43. [PUBMED: 16343853] [DOI] [PubMed] [Google Scholar]
Katyal 2010
- Katyal V, Katyal Vandana. The efficacy and safety of articaine versus lignocaine in dental treatments: a meta‐analysis. Journal of Dentistry 2010;38(4):307‐17. [DOI] [PubMed] [Google Scholar]
Kaufman 1984
- Kaufman E, Weinstein P, Milgrom P. Difficulties in achieving local anesthesia. Journal of the American Dental Association 1984;108:205‐8. [PUBMED: 6584494] [DOI] [PubMed] [Google Scholar]
Kung 2015
- Kung J, McDonagh M, Sedgley C. Does articaine provide an advantage over lidocaine in patients with symptomatic irreversible pulpitis? A systematic review and meta‐analysis. Journal of Endodontics 2015;41(11):1784‐94. [DOI] [PubMed] [Google Scholar]
Malamed 1982
- Malamed SF. The periodontal ligament (pdl) injection: an alternative to inferior alveolar nerve block. Oral Surgery, Oral Medicine, Oral Pathology 1982;53:117‐21. [PUBMED: 6949113] [DOI] [PubMed] [Google Scholar]
Meechan 1997
- Meechan JG. Plasma potassium changes in hypertensive patients undergoing oral surgery with local anesthetics containing epinephrine. Anesthesia Progress 1997;44:106‐9. [PUBMED: 9481971] [PMC free article] [PubMed] [Google Scholar]
Meechan 2001
- Meechan JG, Cole B, Welbury RR. The influence of two different dental local anaesthetic solutions on the haemodynamic responses of children undergoing restorative dentistry: a randomised, single‐blind, split‐mouth study. British Dental Journal 2001;190:502‐4. [PUBMED: 11384023] [DOI] [PubMed] [Google Scholar]
Meechan 2002
- Meechan JG, Ledvinka JIM. Pulpal anaesthesia for mandibular central incisor teeth: a comparison of infiltration and intraligamentary injections. International Endodontic Journal 2002;35:629‐34. [PUBMED: 12190903] [DOI] [PubMed] [Google Scholar]
Meechan 2010
- Meechan JG. Infiltration anesthesia in the mandible. Dental Clinics of North America 2010;54(4):621‐9. [DOI] [PubMed] [Google Scholar]
Microsoft Excel [Computer program]
- Microsoft. Microsoft Office Excel 2007. Redmond, Washington: Microsoft, 2007.
Paxton 2010
- Paxton K, Thome DE. Efficacy of articaine formulations: quantitative reviews. Dental Clinics of North America 2010;54(4):643‐53. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rood 1972
- Rood JP. Ocular complication of inferior dental nerve block. British Dental Journal 1972;132:23‐4. [PUBMED: 4501854] [DOI] [PubMed] [Google Scholar]
Rood 1976
- Rood JP. Inferior alveolar nerve blocks. The use of 5% lignocaine. British Dental Journal 1976;140:413‐4. [PUBMED: 1067102] [DOI] [PubMed] [Google Scholar]
Rood 1977
- Rood JP. The nerve supply of the mandibular incisor region. British Dental Journal 1977;143:227‐30. [Google Scholar]
STATA 13 [Computer program]
- StataCorp. Stata Statistical Software: Release 13. Version 13. College Station, TX, USA: StataCorp, 2013.
STATA 7 [Computer program]
- StataCorp. Stata Statistical Software: Release 7. Version 7. College Station, TX, USA: StataCorp, 2000.
Su 2014a
- Su N, Wang H, Zhang S, Liao S, Yang S, Huang Y. Efficacy and safety of bupivacaine versus lidocaine in dental treatments: a meta‐analysis of randomised controlled trials. International Dental Journal 2014;64(1):34‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2014b
- Su N, Liu Y, Yang X, Shi Z, Huang Y. Efficacy and safety of mepivacaine compared with lidocaine in local anaesthesia in dentistry: a meta‐analysis of randomised controlled trials. International Dental Journal 2014;64(2):96‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2016
- Su NC, Li CJ, Wang H, Shen JF, Liu WJ, Kou L. Efficacy and safety of articaine versus lidocaine for irreversible pulpitis treatment: a systematic review and meta‐analysis of randomised controlled trials. Australian Endodontic Journal 2016;42(1):4‐15. [DOI] [PubMed] [Google Scholar]
Taddio 1994
- Taddio A, Nulman I, Goldbach M, Ipp M, Koren G. Use of lidocaine‐prilocaine cream for vaccination pain in infants. Journal of Pediatrics 1994;124(4):643‐8. [DOI] [PubMed] [Google Scholar]
White 1998
- White JJ, Reader A. Beck M, Meyers WJ. The periodontal ligament injection: a comparison of the efficacy in human maxillary and mandibular teeth. Journal of Endodontics 1998;14:508‐14. [PUBMED: 3255778] [DOI] [PubMed] [Google Scholar]
Wong 1988
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatric Nursing 1988;14(1):9‐17. [PubMed] [Google Scholar]
Xiao 2010
- Xiao JL, Li YL, Ma B, Peng H, Shi QL, Yu ZH. Anesthetic efficacy of articaine versus lidocaine for irreversible pulpitis: a meta‐analysis. [Chinese]. Chinese Journal of Evidence‐Based Medicine 2010;10(9):1058‐62. [EMBASE: 2010601600] [Google Scholar]
References to other published versions of this review
St George 2007
- George G, Morgan A, Meechan J, Moles DR, Needleman I, Ng. Injectable local anaesthetic agents for operative dental anaesthesia. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006487] [DOI] [PMC free article] [PubMed] [Google Scholar]


