Abstract
Background
The management of postoperative pain and recovery is still unsatisfactory in a number of cases in clinical practice. Opioids used for postoperative analgesia are frequently associated with adverse effects, including nausea and constipation, preventing smooth postoperative recovery. Not all patients are suitable for, and benefit from, epidural analgesia that is used to improve postoperative recovery. The non‐opioid, lidocaine, was investigated in several studies for its use in multimodal management strategies to reduce postoperative pain and enhance recovery. This review was published in 2015 and updated in January 2017.
Objectives
To assess the effects (benefits and risks) of perioperative intravenous (IV) lidocaine infusion compared to placebo/no treatment or compared to epidural analgesia on postoperative pain and recovery in adults undergoing various surgical procedures.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, and reference lists of articles in January 2017. We searched one trial registry contacted researchers in the field, and handsearched journals and congress proceedings. We updated this search in February 2018, but have not yet incorporated these results into the review.
Selection criteria
We included randomized controlled trials comparing the effect of continuous perioperative IV lidocaine infusion either with placebo, or no treatment, or with thoracic epidural analgesia (TEA) in adults undergoing elective or urgent surgery under general anaesthesia. The IV lidocaine infusion must have been started intraoperatively, prior to incision, and continued at least until the end of surgery.
Data collection and analysis
We used Cochrane's standard methodological procedures. Our primary outcomes were: pain score at rest; gastrointestinal recovery and adverse events. Secondary outcomes included: postoperative nausea and postoperative opioid consumption. We used GRADE to assess the quality of evidence for each outcome.
Main results
We included 23 new trials in the update. In total, the review included 68 trials (4525 randomized participants). Two trials compared IV lidocaine with TEA. In all remaining trials, placebo or no treatment was used as a comparator. Trials involved participants undergoing open abdominal (22), laparoscopic abdominal (20), or various other surgical procedures (26). The application scheme of systemic lidocaine strongly varies between the studies related to both dose (1 mg/kg/h to 5 mg/kg/h) and termination of the infusion (from the end of surgery until several days after).
The risk of bias was low with respect to selection bias (random sequence generation), performance bias, attrition bias, and detection bias in more than 50% of the included studies. For allocation concealment and selective reporting, the quality assessment yielded low risk of bias for only approximately 20% of the included studies.
IV Lidocaine compared to placebo or no treatment
We are uncertain whether IV lidocaine improves postoperative pain compared to placebo or no treatment at early time points (1 to 4 hours) (standardized mean difference (SMD) −0.50, 95% confidence interval (CI) −0.72 to −0.28; 29 studies, 1656 participants; very low‐quality evidence) after surgery. Due to variation in the standard deviation (SD) in the studies, this would equate to an average pain reduction of between 0.37 cm and 2.48 cm on a 0 to 10 cm visual analogue scale . Assuming approximately 1 cm on a 0 to 10 cm pain scale is clinically meaningful, we ruled out a clinically relevant reduction in pain with lidocaine at intermediate (24 hours) (SMD −0.14, 95% CI −0.25 to −0.04; 33 studies, 1847 participants; moderate‐quality evidence), and at late time points (48 hours) (SMD −0.11, 95% CI −0.25 to 0.04; 24 studies, 1404 participants; moderate‐quality evidence). Due to variation in the SD in the studies, this would equate to an average pain reduction of between 0.10 cm to 0.48 cm at 24 hours and 0.08 cm to 0.42 cm at 48 hours. In contrast to the original review in 2015, we did not find any significant subgroup differences for different surgical procedures.
We are uncertain whether lidocaine reduces the risk of ileus (risk ratio (RR) 0.37, 95% CI 0.15 to 0.87; 4 studies, 273 participants), time to first defaecation/bowel movement (mean difference (MD) −7.92 hours, 95% CI −12.71 to −3.13; 12 studies, 684 participants), risk of postoperative nausea (overall, i.e. 0 up to 72 hours) (RR 0.78, 95% CI 0.67 to 0.91; 35 studies, 1903 participants), and opioid consumption (overall) (MD −4.52 mg morphine equivalents , 95% CI −6.25 to −2.79; 40 studies, 2201 participants); quality of evidence was very low for all these outcomes.
The effect of IV lidocaine on adverse effects compared to placebo treatment is uncertain, as only a small number of studies systematically analysed the occurrence of adverse effects (very low‐quality evidence).
IV Lidocaine compared to TEA
The effects of IV lidocaine compared with TEA are unclear (pain at 24 hours (MD 1.51, 95% CI −0.29 to 3.32; 2 studies, 102 participants), pain at 48 hours (MD 0.98, 95% CI −1.19 to 3.16; 2 studies, 102 participants), time to first bowel movement (MD −1.66, 95% CI −10.88 to 7.56; 2 studies, 102 participants); all very low‐quality evidence). The risk for ileus and for postoperative nausea (overall) is also unclear, as only one small trial assessed these outcomes (very low‐quality evidence). No trial assessed the outcomes, 'pain at early time points' and 'opioid consumption (overall)'. The effect of IV lidocaine on adverse effects compared to TEA is uncertain (very low‐quality evidence).
Authors' conclusions
We are uncertain whether IV perioperative lidocaine, when compared to placebo or no treatment, has a beneficial impact on pain scores in the early postoperative phase, and on gastrointestinal recovery, postoperative nausea, and opioid consumption. The quality of evidence was limited due to inconsistency, imprecision, and study quality. Lidocaine probably has no clinically relevant effect on pain scores later than 24 hours. Few studies have systematically assessed the incidence of adverse effects. There is a lack of evidence about the effects of IV lidocaine compared with epidural anaesthesia in terms of the optimal dose and timing (including the duration) of the administration. We identified three ongoing studies, and 18 studies are awaiting classification; the results of the review may change when these studies are published and included in the review.
Plain language summary
Intravenous infusion of lidocaine starting at the time of surgery for reduction of pain and improvement of recovery after surgery
Background
The most common problems immediately following surgery under general anaesthesia are pain, nausea and vomiting, delirium and slow or no movement of food through the digestive system. Opioid medications given to reduce postoperative pain may also be associated with nausea and constipation, also preventing a smooth recovery. It is of interest for patients and clinicians to reduce or prevent these complications leading to an early recovery so that patients can leave hospital earlier. One option for pain relief after surgery is epidural analgesia, where an opioid or local anaesthetic such as lidocaine is injected into the space surrounding the spinal cord. Not all patients may be suited to epidural analgesia, and so additional options such as intravenous non‐opioid analgesic medications that enable a rapid recovery are required.
The aim of this review was to assess the benefits and risks of intravenous infusion of lidocaine in patients undergoing various surgical procedures. Lidocaine is a medication used to numb tissue in a specific area.
Study characteristics
This review was published in 2015, and updated in 2017. We found 68 randomized controlled studies (RCTs), (clinical studies where people are randomly put into one of two or more treatment groups), with results from a total of 4525 participants. RCTs are used because they provide the most reliable evidence.
Intravenous lidocaine was compared with placebo or standard care in 66 of the studies, and with thoracic (chest area of spine) epidural analgesia in two studies. (A placebo is an inactive substance or procedure given to a participant in a medical trial to compare its effects with those of a real drug or other intervention). Lidocaine infusion was started during the surgery, before the first cut, and continued to at least the end of surgery. The included studies were moderately well conducted.
Key results
We are uncertain whether lidocaine infusion reduces pain, one to four hours after surgery when compared to placebo or usual care (29 studies, over 1600 participants). There was probably no difference in pain at 24 hours (33 studies, 1847 participants) and at 48 hours (24 studies, 1404 participants) between participants in the lidocaine and the placebo group. We are uncertain whether lidocaine infusion improves recovery of bowel function, with a reduction in the time to first defaecation or bowel movements (12 studies, 684 participants), and reduced risk of stopping the passage of food in the gut (4 studies, 273 participants). We are also uncertain whether lidocaine reduces postoperative nausea (35 studies, 1903 participants), and the requirement for opioids for pain relief (40 studies, 2201 participants). Only a limited number of studies systematically analysed adverse effects of intravenous lidocaine infusion. The side effects of intravenous lidocaine were unclear.
In the two studies that investigated intravenous lidocaine compared to epidural analgesia (102 participants), the effect on pain at 24 and 48 hours, and on the time to first bowel movement, remains unclear. The effect of lidocaine on the risk of stopping the passage of food in the gut and for postoperative nausea is also unclear, as only one small trial assessed these outcomes. Neither study investigated the effect on pain immediately after surgery, or on opioid consumption. Both studies looked at adverse effects associated with lidocaine, but the effect is uncertain.
Quality of the evidence
We rated the quality of evidence for most outcomes as very low. This was because of inconsistent findings across studies and the fact that the evidence came from small studies that were of moderate design quality or a limited number of studies. The quality of the evidence for minimal or no effect on pain at 24 and 48 hours was moderate quality. The studies involved a variety of surgical procedures. The dose of lidocaine used, and how long it was delivered for after the end of surgery, also varied between studies.
Summary of findings
Background
During the perioperative period alterations in haemodynamic, endocrine, metabolic and immune responses occur. Inflammatory processes are especially important for structural and functional wound repair. Conversely, excessive stimulation of the inflammatory response may lead to tissue damage (for example, reperfusion injury after cardiothoracic surgery), chronic postoperative pain, acute respiratory distress syndrome, systemic inflammatory response syndrome, and multiple organ failure. Typical and more common problems in the postoperative recovery are acute postoperative pain, postoperative nausea and vomiting (PONV), hypercoagulation, paralytic ileus, and postoperative cognitive dysfunction (Cassuto 2006). Fast‐track protocols aim to prevent or reduce these postoperative complications, facilitating early recovery. Evidence suggests that pain and paralytic ileus, causing a prolonged hospital stay, are major cost drivers in the postoperative period (Kehlet 2008).
Description of the condition
Local anaesthetics administered via the epidural route may reduce the catabolic stress response (Holte 2002); and provide sufficient pain therapy with a reduced need for opioids. Therefore, amongst other effects, an effective epidural analgesia can reduce the risk of developing a paralytic ileus, thus enabling enhanced recovery after surgery. However, recent research has demonstrated that systemic absorption of local anaesthetic, and not just drug interactions with the dorsal root ganglion neurons, also plays an important role in this protective action. Direct systemic (intravenous; IV) administration of lidocaine leading to low plasma levels in the range of 0.5 μg/mL to 5 μg/mL, which are comparable to concentrations after epidural administration, have been shown to achieve protective effects (Collinsworth 1974; Mayumi 1983).
Description of the intervention
Lidocaine (lignocaine), developed in 1948, is the first amino amide‐type short‐acting local anaesthetic. Originally it was used mainly via the IV route as an antiarrhythmic drug. Lidocaine has a very short half‐life and is therefore the local anaesthetic of choice for continuous IV administration. Drug accumulation because of delayed elimination due to hepatic or renal insufficiency might be a safety concern, limiting its usefulness in the perioperative setting. However, since only low plasma levels are required, in contrast to the therapy of chronic pain diseases like neuropathic pain syndromes, major complications following a continuous lidocaine infusion will not be expected.
How the intervention might work
Research in other pain entities, for example, peripheral neuropathic pain and complex regional pain syndromes, has shown that IV lidocaine administration produces prolonged analgesic effects (Kingery 1997). Inhibition of spontaneous impulse generation from injured peripheral nerves and dorsal root ganglions proximal to the injured fibres (Devor 1992a), as well as suppression of polysynaptic reflexes in the spinal dorsal horn (Woolf 1985), have been proposed as underlying mechanisms. Pain in the perioperative context is principally inflammatory pain, but could also be neuropathic or based on hyperalgesia. All these entities have been shown to be ameliorated by the administration of IV lidocaine (Koppert 2004). The anti‐inflammatory effects of local anaesthetic mediated through interactions with polymorphonuclear cells (Hollmann 2000a), and the inhibition of G protein‐coupled receptors (Hollmann 2000a; Hollmann 2001; Hollmann 2002; Hollmann 2005), may play a crucial role for the observed effects in the perioperative setting. Especially for the recovery of the gastrointestinal function and the prevention of the development of a paralytic ileus, which is thought to be the result of neurogenic inflammation, the anti‐inflammatory action of IV lidocaine can be beneficial (Herroeder 2007). Altogether, numerous clinically relevant outcomes may be influenced by IV administration of lidocaine; these include wound healing, analgesia, coagulation, postoperative cognitive dysfunction, paralytic ileus, and lung protection (Hollmann 2000b).
Why it is important to do this review
Epidural anaesthesia was once thought to be an anaesthetic strategy that improves outcomes after major surgery to a greater extent than has been confirmed recently (Popping 2014). However, recent evidence questions the risk‐benefit ratio for some patients and types of surgery (e.g. laparoscopic procedures, lower abdominal surgery or patients without pre‐existing lung disease). Serious neurologic complications after placement of an epidural catheter seem to occur more frequently than originally thought (Christie 2007; Cook 2009; Popping 2008). Thus, a growing number of patients and anaesthesiologists perform a proper risk‐benefit analysis in individual cases and also decide against epidural analgesia for some types of surgery except open thoracotomy and major abdominal surgery. This is notable with patients after coronary stenting, as they receive anticoagulant therapy and thus require careful assessment as to the risks and benefits of administering a regional anaesthetic technique. Nowadays anaesthesiologists are facing increasing numbers of these patients. In addition, for numerous types of surgery (e.g. surgeries involving the head), neuraxial techniques are not feasible at all. Therefore, alternative therapeutic interventions for optimal perioperative care are desirable. By characterizing the effects of IV lidocaine in the perioperative setting, lidocaine may be shown to offer a safe and alternative strategy for improving the perioperative outcome for patients unwilling or unable to receive epidural anaesthesia.
In spite of numerous preclinical studies in favour of systemic lidocaine, large published trials testing these effects in humans are not available. However, the number of rather small clinical studies has increased in recent years. Some of these trials have already been summarized in six systematic reviews (Chang 2017; Marret 2008; McCarthy 2010; Sun 2012; Vigneault 2011), including the original version of the current review (Kranke 2015). The current review update includes all new studies published until January 2017 and is the most comprehensive systematic review to date on the use of perioperative lidocaine for postoperative pain and recovery in adults.
Objectives
To assess the effects (benefits and risks) of perioperative intravenous (IV) lidocaine infusion compared to placebo/no treatment or compared to epidural analgesia on postoperative pain and recovery in adults undergoing various surgical procedures.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) that evaluated the effect of perioperative lidocaine infusions versus no treatment, placebo treatment or versus epidural analgesia on relevant clinical outcomes.
We excluded cross‐over trials, since this study design is not relevant for the current review.
Types of participants
We included results obtained in adult (over 18 years) participants, independent of sex, undergoing any elective or urgent surgical procedure on any body part(s), and only if the procedure required general anaesthesia. Specifially, we excluded participants undergoing:
any kind of emergency procedure, and
minor surgical procedures, which are sometimes conducted using local or regional anaesthesia alone and do not provide a control event rate being high enough to demonstrate an effect of the investigated intervention.
Types of interventions
We included all studies comparing the effect of continuous perioperative lidocaine infusion, either with no treatment or placebo treatment, or with epidural analgesia. The IV lidocaine infusion must have been started intraoperatively (with or without an IV bolus) prior to incision and continued until the end of surgery. In trials of this intervention, standard care to enhance the postoperative recovery after surgery should also be provided.
Types of outcome measures
We analysed the following outcome measures.
Primary outcomes
Pain score at rest (0 to 10 cm, 0 to 100 mm visual analogue scale (VAS), numeric rating scale (NRS)), at 'early', 'intermediate', and 'late time points'
Gastrointestinal recovery: postoperative ileus (dichotomous), time to first defaecation/bowel movement (hours), time to first flatus (hours), and time to first bowel sounds (hours)
Adverse events (dichotomous; e.g. death, arrhythmias, other heart rate disorders or any sign of lidocaine toxicity)
Secondary outcomes
Length of hospital stay (inpatient ‐ days; outpatient ‐ minutes)
Functional postoperative neuropsychological status scales (e.g. quality of recovery (QoR) score or Mini Mental State Examination (MMSE))
Surgical complications (dichotomous; postoperative infections, thromboembolism, wound breakdown, etc.)
Patient satisfaction (0 to 10 cm VAS, 0 to 100 mm VAS, 0 to 10 NRS)
Cessation of the intervention (dichotomous; termination of the study before completion)
We investigated two separate outcomes for postoperative nausea and vomiting (PONV): First, postoperative nausea including PONV, if nausea was not separately reported in the study (referred to below as 'nausea') and, second, postoperative vomiting, both at 'early time points' (dichotomous; in postanaesthesia care unit (PACU)) and 'overall'
Intraoperative opioid consumption (remifentanil was separated from all other opioids due to an exceptional mode of action)
Postoperative opioid consumption, 'in PACU' and 'overall' (in mg morphine equivalents (MEQ)
When we reported 'early time points' for pain, nausea, vomiting, and opioid consumption, this referred to trials in which the outcome was reported approximately within the time period one to four hours postoperatively, or in the PACU. When we reported the 'intermediate' and 'late time points' for pain, this referred to pain ratings at 24 hours and 48 hours after surgery, respectively. In case of nausea, vomiting, and opioid consumption, 'overall' meant data that covered the time intervals from 0 to 24 hours, 0 to 48 hours, or 0 to 72 hours. We also accepted data that reported these outcomes for an interval from PACU (1 to 4 hours) to 24 hours, to 48 hours, or to 72 hours. If studies reported these outcomes for the 0 to 24‐hour time interval and later, we decided to analyse only the 0 to 24‐hour time interval. If studies did not explicitly report the time interval at which the outcome was documented, we grouped these data into the 'overall' category.
Search methods for identification of studies
Electronic searches
The search for the original review was performed in May 2014 (Kranke 2015), and the search for the update was performed in January 2017. We performed a further search in February 2018. We have added the February 2018 results to 'Studies awaiting classification' and we will incorporate them into the review at the next update.
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011). We did not apply restrictions to language or publication status. We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1).
MEDLINE (Ovid SP, 1966 to 25 January 2017).
Embase (Ovid SP, 1980 to 25 January 2017).
CINAHL (EBSCO host, 1982 to 25 January 2017).
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other databases listed. Where appropriate, we expanded the search strategy with search terms for identifying RCTs. All search strategies can be found in Appendix 1, Appendix 2, Appendix 3, Appendix 4.
We searched the American Society of Anesthesiologists (ASA) proceedings for relevant abstracts (16 March 2017).
We scanned the trial registry, Clinical Trials.gov for ongoing and unpublished trials to 16 March 2017 (ClinicalTrials.gov).
We developed the search strategy in consultation with the Information Specialist. We contacted researches in the field.
Searching other resources
We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials.
When necessary, we contacted trial authors for additional information.
Data collection and analysis
Selection of studies
Three review authors (original review: SW, JJ; update: SW, AH, YJ) independently scanned the titles retrieved by the initial search to exclude irrelevant trials. Then two review authors (original review: SW, JJ; update: AH, YJ) identified the studies that might be included in this review using a standardized study eligibility form developed by the authors (Appendix 5). If there were differences, we included a third review (original review: PK; update: SW) as arbiter. If necessary, we retrieved additional missing data and information about ongoing trials.
We resolved all differences by discussion among the authors. A PRISMA flow chart was prepared (Moher 2009).
Data extraction and management
Two authors (original review: SW as tandem with JJ, AS, LHJE, KH, DMP, MWH; update: AH, YJ) extracted the data using standardized data extraction forms developed by the authors (Appendix 6). If necessary, we retrieved additional data that were missing in published trials and information about ongoing trials by contacting the authors of the studies. We resolved all differences by discussion among the review authors at each step of data extraction.
Assessment of risk of bias in included studies
Two review authors (original review: SW, JJ; update: AH, YJ) independently performed the study quality assessment using a critical appraisal form provided by the Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group with minor modifications (Appendix 7). We resolved any disagreements by discussion between the review authors, with a further review author acting as arbiter (original review: PK; update: SW).
We assessed the risk of bias of included studies using the Cochrane 'Risk of bias' tool (Higgins 2011). The standard domains include random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; and any other bias. Details of the risk of bias assessment were reported in the 'Critical Appraisal Form' (Appendix 7). We judged each component as being either low risk of bias, high risk of bias, or unclear. We included a 'Risk of bias' table as part of the table 'Characteristics of included studies' and a 'Risk of bias summary', which details all of the judgements we made for all included studies in the review.
Measures of treatment effect
For dichotomous outcomes, we obtained the risk ratio (RR) from the intervention and control group event rates. For continuous data, we obtained the mean difference (MD) from the difference between the intervention and control group mean values with associated standard deviations (SDs) if all studies measured data on the same scale. We used the standardized mean difference (SMD) when the studies all assessed the same outcome but measured it in a variety of ways (for example, studies measuring pain scores on different scales (visual analogue scale (VAS) 0 to 10 cm, 0 to 100 mm; numeric rating scale (NRS) 1 to 10)). We performed back‐transformation of SMD values into absolute values on a scale between 0 to 10 cm (VAS) to facilitate clinical interpretation. We used the smallest as well as the largest SD from the control groups of the pooled studies for back‐transformation (SMD * SD) to reflect the range of possible effects.
We transformed all opioid quantities into IV morphine equivalents (MEQ, mg) as described in the anatomic therapeutic chemical (ATC)/defined daily dose (DDD) Index (www.whocc.no/atc_ddd_index).
Unit of analysis issues
Multiple‐armed studies
We had planned to overcame a unit of analysis error for studies that contributed multiple comparisons by combining groups (by using the appropriate formula for adding SDs when required) to create a single pair‐wise comparison, if the presented data in the trials allow us to do so (Higgins 2011). Up to this update there were no studies with multiple comparisons of interest for this review.
Cluster‐randomized trials
We planned to include cluster‐randomized trials in the analyses along with individually‐randomized trials. However, for the present review we did not identify any relevant cluster‐randomized trials.
Dealing with missing data
If we encountered missing data, we contacted the relevant authors to obtain further information. If we obtained data, we included the data in the analyses. If data were missing, we included data in the analysis only on those participants whose results were known; we performed a complete‐case analysis. We subsequently excluded studies with incomplete reporting of their study flow or disputable exclusions in a sensitivity meta‐analysis to assess bias (Table 3). We considered the potential impact of the missing data on the results in the interpretation of the results of the review.
1. Sensitivity analyses ‐ risk of bias (incomplete outcome data).
| All studies | Without high/unclear risk of bias studies (incomplete outcome data) | ||||
| Outcome | Statistical method | Studies | Effect estimate | Studies | Effect estimate |
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | SMD (IV, Random, 95% CI) | 29 | −0.50 (−0.72 to −0.28) | 17 | −0.45 (−0.77 to −0.14) |
| Pain score, rest, 'intermediate time points' (24 hrs) | SMD (IV, Random, 95% CI) | 33 | −0.14 (−0.25 to −0.04) | 18 | −0.12 (−0.26 to 0.01) |
| Pain score, rest, 'late time points' (48 hrs) | SMD (IV, Random, 95% CI) | 24 | −0.11 (−0.25 to 0.04) | 11 | −0.06 (−0.27 to 0.15) |
| Postoperative ileus (dichotomous) | RR (MH, Random, 95% CI) | 4 | 0.37 (0.15 to 0.87) | 4 | 0.37 (0.15 to 0.87) |
| Time to first defaecation/bowel movement (hrs) | MD (IV, Random, 95% CI) | 12 | −7.92 (−12.71 to −3.13) | 8 | −7.5 (−14.38 to −0.63) |
| Time to first flatus (hrs) | MD (IV, Random, 95% CI) | 13 | −4.09 (−6.30 to −1.87) | 9 | −3.98 (−7.03 to −0.93) |
| Time to first bowel sounds (hrs) | MD (IV, Random, 95% CI) | 2 | −6.08 (−13.77 to 1.60) | 2 | −6.08 (−13.77 to 1.60) |
| Length of hospital stay (days) | MD (IV, Random, 95% CI) | 32 | −0.37 (−0.60 to −0.15) | 17 | −0.23 (−0.49 to 0.02) |
| Length of hospital stay (outpatient surgery, mins) | MD (IV, Random, 95% CI) | 3 | −10.81 (−36.93 to 15.31) | 3 | −10.81 (−36.93 to 15.31) |
| Surgical complications ‐ anastomotic leak | RR (MH, Random, 95% CI) | 3 | 0.61 (0.08 to 4.80) | 3 | 0.61 (0.08 to 4.80) |
| Surgical complications ‐ bleeding | RR (MH, Random, 95% CI) | 3 | 1.79 (0.41 to 7.89) | 3 | 1.79 (0.41 to 7.89) |
| Surgical complications ‐ postoperative infection | RR (MH, Random, 95% CI) | 5 | 1.64 (0.41 to 6.52) | 4 | 1.19 (0.25 to 5.67) |
| Patient satisfaction | MD (IV, Random, 95% CI) | 6 | 0.76 (0.46 to 1.06) | 2 | 0.59 (−0.09 to 1.26) |
| Postoperative nausea, early (PACU) | RR (MH, Random, 95% CI) | 8 | 0.72 (0.53 to 0.98) | 7 | 0.66 (0.47 to 0.91) |
| Postoperative nausea, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 35 | 0.78 (0.67 to 0.91) | 19 | 0.87 (0.72 to 1.06) |
| Postoperative vomiting, early (PACU) | RR (MH, Random, 95% CI) | 4 | 0.49 (0.16 to 1.48) | 3 | 0.75 (0.15 to 3.80) |
| Postoperative vomiting, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 19 | 0.83 (0.63 to 1.08) | 7 | 0.88 (0.58 to 1.31) |
| Intraoperative opioid consumption (MEQ, mg) | MD (IV, Random, 95% CI) | 18 | −2.14 (−3.87 to −0.40) | 10 | −1.52 (−4.13 to 1.09) |
| Intraoperative opioid consumption with remifentanil (MEQ, mg) | MD (IV, Random, 95% CI) | 6 | −14.17 (−35.27 to 6.92) | 5 | −16.08 (−41.41 to 9.25) |
| Postoperative opioid consumption, PACU (MEQ, mg) | MD (IV, Random, 95% CI) | 21 | −3.10 (−3.87 to −2.32) | 12 | −2.59 (−3.76 to −1.42) |
| Postoperative opioid consumption, overall (MEQ, mg) | MD (IV, Random, 95% CI) | 40 | −4.52 (−6.25 to −2.79) | 25 | −2.84 (−4.45 to −1.22) |
Acronyms and abbreviations used in the table:
CI = confidence interval, hrs = hours, IV = inverse variance, MD = mean difference, MEQ = morphine equivalent dose, mins = minutes, MH = Mantel Haenszel, PACU = post anaesthesia care unit, RR = risk ratio, SMD = standardized mean difference
We calculated missing SDs from standard errors (SEs) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If data were reported as median with interquartile range and the distribution of the data was symmetrical (median = mean), we used the median directly in the meta‐analysis and calculated the SD from the interquartile range, in accordance with Higgins 2011. For asymmetric data (median ≠ mean) we proceeded as described for symmetric data and addressed the impact of all median data by performing sensitivity analyses (Table 4).
2. Sensitivity analyses ‐ median + interquartile range.
| Mean + SD and median + IQR values | Only mean + SD values | ||||
| Outcome | Statistical method | Studies | Effect estimate | Studies | Effect estimate |
| Pain score , rest, 'early time points' (1 hr to 4 hrs, PACU) | SMD (IV, Random, 95% CI) | 29 | −0.50 (−0.72 to −0.28) | 23 | −0.64 (−0.89 to −0.38) |
| Pain score , rest, 'intermediate time points' (24 hrs) | SMD (IV, Random, 95% CI) | 33 | −0.14 (−0.25 to −0.04) | 27 | −0.16 (−0.29 to −0.04) |
| Pain score , rest, 'late time points' (48 hrs) | SMD (IV, Random, 95% CI) | 24 | −0.11 (−0.25 to 0.04) | 20 | −0.12 (−0.29 to 0.04) |
| Time to first defaecation/bowel movement (hrs) | MD (IV, Random, 95% CI) | 12 | −7.92 (−12.71 to −3.13) | 7 | −6.03 (−10.98 to −1.08) |
| Time to first flatus (hrs) | MD (IV, Random, 95% CI) | 13 | −4.09 (−6.30 to −1.87) | 10 | −4.40 (−6.30 to −2.50) |
| Time to first bowel sounds (hrs) | MD (IV, Random, 95% CI) | 2 | −6.08 (−13.77 to 1.60) | 2 | −6.08 (−13.77 to 1.60) |
| Length of hospital stay (days) | MD (IV, Random, 95% CI) | 32 | −0.37 (−0.60 to −0.15) | 16 | −0.32 (−0.54 to −0.10) |
| Length of hospital stay (outpatient surgery, mins) | MD (IV, Random, 95% CI) | 3 | −10.81 (−36.93 to 15.31) | 0 | Not estimable |
| Patient satisfaction | MD (IV, Random, 95% CI) | 6 | 0.76 (0.46 to 1.06) | 1 | 0.30 (−0.21 to 0.81) |
| Intraoperative opioid consumption (MEQ, mg) | MD (IV, Random, 95% CI) | 18 | −2.14 (−3.87 to −0.40) | 13 | −2.32 (−4.33 to −0.32) |
| Intraoperative opioid consumption with remifentanil (MEQ, mg) | MD (IV, Random, 95% CI) | 6 | −14.17 (−35.27 to 6.92) | 4 | −20.45 (−52.10 to 11.19) |
| Postoperative opioid consumption, PACU (MEQ, mg) | MD (IV, Random, 95% CI) | 21 | −3.10 (−3.87 to −2.32) | 15 | −2.88 (−3.80 to −1.96) |
| Postoperative opioid consumption, overall (MEQ, mg) | MD (IV, Random, 95% CI) | 40 | −4.52 (−6.25 to −2.79) | 28 | −4.64 (−6.72 to −2.56) |
Acronyms and abbreviations used in this table:
CI = confidence interval, hrs = hours, IQR = interquartile range, IV = inverse variance, MD = mean difference, MEQ = morphine equivalent dose, mins = minutes, PACU = post anaesthesia care unit, SD = standard deviation, SMD = standardized mean difference
Assessment of heterogeneity
We assessed the clinical and methodological differences of included studies. We used clinical judgement, not heterogeneity statistics, to decide whether we could combine the studies.
We reported statistical heterogeneity using the Chi² test and the I² statistic. We calculated both for each of the outcomes listed in the 'Types of outcome measures' section. We declared statistical heterogeneity if P < 0.1 for the Chi² statistic and I2 ≥ 30%. We classified heterogeneity following the interpretation specified within the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Briefly, we determined heterogeneity as not important for I² of 0% to 40%, as moderate for I² of 30% to 60%, as substantial for I² of 50% to 90%, and as considerable for I² of 75% to 100% (Higgins 2011).
We further calculated the 95% prediction intervals (PIs) to understand the impact of heterogeneity on a range of true treatment effects in future studies (see Data synthesis). In case of heterogeneity, a PI covers a wider range than a CI (IntHout 2016). Consequently, in case of a statistically significant effect (all values of the 95% CI are on the same side of the null), the corresponding 95% PI may indicate that values are possible on both sides of the null (IntHout 2016). In this case, the conclusion based on the CI is not warranted. We used the R package 'meta' (version 4.8‐1) to calculate 95% PIs (Schwarzer 2007).
Assessment of reporting biases
We created contour‐enhanced funnel plots as plots of the trial's effect estimates against the precision (inverse of the SE of the estimate) including contour lines corresponding to perceived ‘milestones’ of statistical significance (P = 0.01, 0.05, 0.1) for outcomes having 10 or more included studies. We used the funnel plot primarily as a visual aid for detecting reporting bias and small‐study effects. In addition to funnel plots, we further explored the relation of the treatment effect and study size by regression analysis by method of moments using an arcsine transformation for RR (Rücker 2008), and weighted regression for MD/SMD (Egger 1997). We performed sensitivity analyses by using the trim and fill method to identify and correct for funnel plot asymmetry arising from publication bias (Duval 2000). We reported the estimated number of missing studies and the adjusted intervention effects derived by performing the meta‐analyses, including the filled studies. We performed explorative analyses of reporting bias (funnel plot asymmetry) with the R package 'metasens' (version 0.3‐1), an add‐on package for 'meta' (Schwarzer 2007).
Data synthesis
We used a random‐effects model to analyse data. This allowed unconditional inference of how large the average true effect is in the population of all possible studies (Hedges 1998). We used Review Manager 5 for statistical modelling using inverse variance weighting summary of continuous outcomes and using Mantel‐Haenszel methods for dichotomous outcomes, all presented with 95% CIs (Review Manager 2014). We considered dichotomous outcomes with the range of the 95% CIs not crossing 1 and continuous outcomes with the range of the 95% CIs not crossing 0 as significant effect estimates. The CI is an index of precision (based on the SE) that tells us how precisely we have estimated the mean effect size and as such, it is a property of the sample and strongly driven by the number of studies in the analysis (Borenstein 2017).
We additionally calculated the 95% PI which is an index of dispersion (based on the SD) that tells us how widely the mean effects vary across populations (Borenstein 2017). Reporting a PI in addition to the summary estimate and CI illustrate which range of true mean effects can be expected in future settings and is helpful in the clinical interpretation of heterogeneity (IntHout 2016). We restricted the calculation of a 95% PI to meta‐analyses with ≥ 4 studies (≥ 200 participants), since the interval would be imprecise when a summary estimate was based on only a few small studies (IntHout 2016). We used the R package 'meta' (version 4.8‐1) to calculate 95% PIs (Schwarzer 2007).
We analysed four time‐to‐event outcomes: time to defaecation/bowel movement, time to flatus, time to bowel sounds, and time to hospital discharge. We treated these time‐to‐event outcomes as continuous variables and used the MD. We did not use survival analysis methods since there was no censoring (all outcomes were known within hours to days).
As this systematic review was planned to include studies of IV lidocaine versus an inactive (placebo or no treatment) comparator and studies of IV lidocaine versus an active (for example, epidural) comparator, we independently analysed effect estimates for lidocaine versus placebo or no treatment and lidocaine versus an epidural. If feasible, in future updated versions of this review we will estimate mixed direct‐indirect comparisons of the two interventions using random‐effects model meta‐regression.
Subgroup analysis and investigation of heterogeneity
We gave consideration to the magnitude of clinical and methodological heterogeneity. To evaluate the effects of clinical heterogeneity (specified by statistical heterogeneity with an I2 ≥ 30% in the meta‐analysis), we performed subgroup analyses calculating the RR or MD/SMD in conjunction with the corresponding CI for each subgroup. We used a random‐effects model heterogeneity I² statistic to compare subgroups.
We analysed the data concerning the following subgroups.
Type of surgery (open abdominal, laparoscopic abdominal, and other surgery; Table 5).
Time and dosing of IV lidocaine administration (Table 6).
3. Subgroup analyses ‐ type of surgery.
| Main meta‐analyses | Open abdominal surgery | Laparoscopic surgery | Other surgery | Test for subgroup difference (P) | |||
| Outcome | n |
Effect estimate (I2) |
n |
Effect estimate (I2) |
n |
Effect estimate (I2) |
|
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | 8 | −0.54 (−0.82 to −0.26) (51%) |
10 | −0.78 (−1.34 to −0.21) (89%) |
11 | −0.21 (−0.44 to 0.02) (56%) |
0.07 |
| Pain score, rest, 'intermediate time points' (24 hrs) | No subgroup analysis performed (I2 < 30%) | ||||||
| Pain score, rest, 'late time points' (48 hrs) | 7 | 0.03 (−0.17 to 0.23) (0%) |
7 | −0.30 (−0.74 to 0.13) (74%) |
10 | −0.10 (−0.27 to 0.08) (20%) |
0.35 |
| Postoperative ileus (dichotomous) | No subgroup analysis performed (I2 < 30%) | ||||||
| Time to first defaecation/bowel movement (hrs) | 6 | −7.09 (−10.33 to −3.86) (0%) |
5 | −6.23 (−18.07 to 5.62) (85%) |
1 | −6.10 (−24.49 to 12.29) NE |
0.41 |
| Time to first flatus (hrs) | 6 | −4.49 (−7.38 to −1.60) (6%) |
5 | −3.07 (−8.28 to 2.15) (78%) |
2 | −2.15 (−3.56 to −0.74) (0%) |
0.36 |
| Time to first bowel sounds (hrs) | 1 | −10.00 (−17.13 to −2.87) NE |
1 | −2.16 (−9.30 to 4.98) NE |
0 | NE | 0.13 |
| Length of hospital stay (days) | 6 | −0.59 (−0.99 to −0.18) (27%) |
12 | −0.15 (−0.58 to 0.28) (77%) |
14 | −0.48 (−0.84 to −0.11) (69%) |
0.32 |
| Length of hospital stay (outpatient surgery, mins) | 0 | NE | 3 | −10.81 (−36.93 to 15.31) | 0 | NE | NE |
| Surgical complications ‐ anastomotic leak | No subgroup analysis performed (I2 < 30%) | ||||||
| Surgical complications ‐ bleeding | No subgroup analysis performed (I2 < 30%) | ||||||
| Surgical complications ‐ postoperative infection | No subgroup analysis performed (I2 < 30%) | ||||||
| Patient satisfaction | No subgroup analysis performed (I2 < 30%) | ||||||
| Postoperative nausea, early (PACU) | No subgroup analysis performed (I2 < 30%) | ||||||
| Postoperative nausea, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | No subgroup analysis performed (I2 < 30%) | ||||||
| Postoperative vomiting, early (PACU) | No subgroup analysis performed (I2 < 30%) | ||||||
| Postoperative vomiting, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | No subgroup analysis performed (I2 < 30%) | ||||||
| Intraoperative opioid consumption (MEQ, mg) | 7 | −1.93 (‐4.61 to 0.75) (78%) |
3 | −0.71 (−7.95 to 6.53) (93%) |
8 | −2.03 (−4.14 to 0.07) (40%) |
0.94 |
| Intraoperative opioid consumption with remifentanil (MEQ, mg) | No subgroup analysis performed (I2 < 30%) | ||||||
| Postoperative opioid consumption, PACU (MEQ, mg) | 5 | −3.03 (−4.82 to −1.23) (0%) |
7 | −3.84 (−4.57 to −3.11) (0%) |
9 | −2.66 (−4.19 to −1.13) (61%) |
0.33 |
| Postoperative opioid consumption, overall (MEQ, mg) | 11 | −3.56 (−6.76 to −0.35) (40%) |
16 | −4.85 (−7.46 to −2.23) (77%) |
13 | −5.54 (−9.35 to −1.72) (77%) |
0.71 |
Acronyms and abbrviations used in this table:
hrs = hours, MEQ = morphine equivalent dose, mins = minutes, NE = not estimable, PACU =post anaesthesia care unit
4. Subgroup analyses ‐ time and dosing of lidocaine.
| Main meta‐analyses | < 2 mg/kg/hr until end of surgery to PACU | ≥ 2 mg/kg/hr until end of surgery to PACU | < 2 mg/kg/hr for ≥ 24 hrs | ≥ 2 mg/kg/hr for ≥ 24 hrs | Test for subgroup difference (P) | ||||
| Outcome | n |
Effect estimate (I2) |
n |
Effect estimate (I2) |
n |
Effect estimate (I2) |
n |
Effect estimate (I2) |
|
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | 8 | −0.36 (−0.70 to −0.02) (67%) |
21 | −0.54 (−0.82 to −0.27) (82%) |
0.42 | ||||
| Pain score, rest, 'intermediate time points' (24 hrs) | No subgroup analysis performed (I2 < 30%) | ||||||||
| Pain score, rest, 'late time points' (48 hrs) | 5 | −0.15 (−0.39 to 0.09) (0%) |
13 | −0.18 (−0.34 to −0.02) (12%) | 5 | 0.03 (−0.45 to 0.51) (78%) | 1 | 0.11 (−0.39 to 0.61) NE |
0.66 |
| Postoperative ileus (dichotomous) | No subgroup analysis performed (I2 < 30%) | ||||||||
| Time to first defaecation/bowel movement (hrs) | 4 | −7.06 (−11.37 to −2.75) (10%) | 3 | −7.27 (−13.54 to −1.00) (0%) | 4 | −6.97 (−20.09 to 6.16) (86%) |
1 | −20.00 (−50.62 to 10.62) NE |
0.62 |
| Time to first flatus (hrs) | 4 | −5.72 (−9.58 to −1.87) (28%) | 4 | −3.63 (−6.07 to −1.20) (64%) | 4 | −0.43 (−9.46 to 8.61) (84%) | 1 | −6.50 (−17.05 to 4.05) NE |
0.65 |
| Time to first bowel sounds (hrs) | 1 | −10.00 (−17.13 to −2.87) NE |
1 | −2.16 (−9.30 to 4.98) NE |
0 | NE | 0 | NE | 0.13 |
| Length of hospital stay (days) | 7 | −0.51 (−0.84 to −0.19) (2%) | 16 | −0.26 (‐0.50 to −0.03) (58%) | 7 | −0.25 (−1.04 to 0.54) (83%) | 2 | −1.29 (−4.47 to 1.89) (94%) |
0.59 |
| Length of hospital stay (outpatient surgery, mins) | 1 | −44.00 (−75.57 to −12.43) NE |
2 | −2.97 (−11.33 to 5.39) (0%) |
0 | NE | 0 | NE | 0.01 |
| Surgical complications ‐ anastomotic leak | No subgroup analysis performed (I2 < 30%) | ||||||||
| Surgical complications ‐ bleeding | No subgroup analysis performed (I2 < 30%) | ||||||||
| Surgical complications ‐ postoperative infection | No subgroup analysis performed (I2 < 30%) | ||||||||
| Patient satisfaction | No subgroup analysis performed (I2 < 30%) | ||||||||
| Postoperative nausea, early (PACU) | No subgroup analysis performed (I2 < 30%) | ||||||||
| Postoperative nausea, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | No subgroup analysis performed (I2 < 30%) | ||||||||
| Postoperative vomiting, early (PACU) | No subgroup analysis performed (I2 < 30%) | ||||||||
| Postoperative vomiting, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | No subgroup analysis performed (I2 < 30%) | ||||||||
| Intraoperative opioid consumption (MEQ, mg) | 7 | −0.68 (−3.51 to 2.15) (80%) | 11 | −3.11 (−5.74 to −0.47) (79%) | 0.22 | ||||
| Intraoperative opioid consumption with remifentanil (MEQ, mg) | No subgroup analysis performed (I2 < 30%) | ||||||||
| Postoperative opioid consumption, PACU (MEQ, mg) | 6 | −3.55 (−5.43 to −1.67) (63%) | 15 | −3.02 (−3.86 to −2.18) (30%) | 0.61 | ||||
| Postoperative opioid consumption, overall (MEQ, mg) | 8 | −2.33 (−5.05 to −0.13) (59%) | 21 | −7.41 (−10.91 to −3.91) (76%) | 8 | −2.88 (−6.25 to 0.49) (72%) | 3 | −3.90 (−10.18 to 2.38) (18%) |
0.14 |
Acronyms and abbreviations used in this table:
hrs = hours, MEQ = morphine equivalent dose, mins = minutes, NE = not estimable, PACU =post anaesthesia care unit
Tests on subgroup differences are based on the assumption that the tau2 (between‐study heterogeneity) varies across the subgroups. We used the R package 'metafor' (Viechtbauer 2010), to estimate the individual tau2s of the subgroups (multivariate meta‐analysis models) and tested if they have a common value (likelihood ratio test; Table 7). We rejected the null hypothesis for P < 0.05. We considered subgroup analyses to be exploratory and we did not adjust for multiplicity.
5. Subgroup analyses with independent tau2 (type of surgery).
| Outcome |
Meta‐regression model (random‐effects model, tau2 estimator: REML) |
Open abdominal surgery | Laparoscopic surgery | Other surgery |
Test of moderators (P) |
Likelihood ratio test (P) |
|||
| n |
Effect estimate (tau2) |
n |
Effect estimate (tau2) |
n |
Effect estimate (tau2) |
||||
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | Individual tau2 | 8 | −0.55 (−0.83 to −0.27) (0.08) |
10 | −0.78(−1.35 to −0.21) (0.76) |
11 | −0.21 to (−0.44 0.03) (0.09) |
0.07 | 0.017 |
| Pain score, rest, 'late time points' (48 hrs) | Individual tau2 | 7 | 0.03 (−0.17 to 0.23) (0.00) |
7 | −0.03 (−0.73 to 0.12) (0.24) |
10 | −0.10 (−0.27 to 0.08) (0.02) |
0.34 | 0.049 |
| Time to first defaecation/bowel movement (hrs) | Individual tau2 | 6 | −8.16 (−12.44 to −3.87) (5.94) |
5 | −6.22 (−18.42 to 5.98) (127.06) |
1 | −6.10 (−24.49 to 12.29) (0.00) |
0.94 | 0.097 |
| Time to first flatus (hrs) | Individual tau2 | 6 | −4.36 (−6.99 to −1.72) (0.00) |
5 | −2.55 (−9.31 to 4.21) (47.86) |
2 | −2.15 (−3.56 to −0.74) (0.00) |
0.35 | 0.234 |
| Length of hospital stay (days) | Individual tau2 | 6 | −0.62 (−1.07 to −0.18) (0.11) |
12 | −0.16 (−0.66 to 0.33) (0.43) |
14 | −0.47 (−0.83 to −0.12) (0.18) |
0.39 | 0.592 |
| Intraoperative opioid consumption (MEQ, mg) | Individual tau2 | 7 | −2.00 (−4.30 to 0.30) (4.34) |
3 | 0.04 (−11.99 to 12.08) (107.18) |
8 | −1.86 (−3.34 to −0.38) (0.55) |
0.95 | 0.027 |
| Postoperative opioid consumption, PACU (MEQ, mg) | Individual tau2 | 5 | −3.03 (−4.82 to −1.23) (0.00) |
7 | −3.84 (−4.57 to −3.11) (0.00) |
9 | −2.71 (−4.32 to −1.09) (3.45) |
0.37 | 0.211 |
| Postoperative opioid consumption, overall (MEQ, mg) | Individual tau2 | 11 | −3.43 (−6.01 to −0.85) (3.08) |
16 | −5.78 (−9.33 to −2.23) (32.11) |
13 | −6.42 (−11.60 to −1.24) (50.54) |
0.43 | 0.285 |
Subgroup‐analyses are based on multivariate meta‐analysis models (method: REML, R package 'metafor'; Viechtbauer 2010). We tested for subgroup differences (test of moderators). Tests on subgroup differences are based on the assumption that the tau2 (between‐study heterogeneity) varies across the subgroups. We estimated the tau2s and tested if they have a common value (likelihood ratio test). We rejected the null hypothesis for P < 0.05.
Acronyms and abbreviations used in this table:
MEQ = morphine equivalent dose, PACU =post anaesthesia care unit, REML = restricted maximum likelihood approach
We further calculated 95% PIs to understand the impact of heterogeneity on range of true treatment effects in future studies (see Data synthesis).
'Summary of findings' tables and GRADE
We used the principles of the GRADE working system to assess the quality of the body of evidence associated with patient‐relevant outcomes for both comparisons (lidocaine versus placebo, lidocaine versus thoracic epidural analgesia (TEA)) in our review (Guyatt 2008); and constructed two 'Summary of findings' tables for the following outcomes using GRADEpro software (GRADEpro GDT).
Pain scores: pain ('early', i.e. 0 to 4 hours, and in the PACU), pain ('intermediate', i.e. 24 hours), and pain ('late', i.e. 48 hours).
Gastrointestinal recovery: postoperative ileus and time to first defaecation/bowel movement.
Adverse effects.
Postoperative nausea ('overall', i.e. 0 to 24 hours, 0 to 48 hours, 0 to 72 hours).
Postoperative opioid consumption 'overall'.
With the GRADE approach we appraised the quality of evidence on the basis of the extent to which one can be confident that the estimate of effect reflects the item assessed. The quality of the body of evidence reflects within‐study risk of bias (methodological quality), indirectness, heterogeneity of data (inconsistency), imprecision of effect estimates, and risk of publication bias.
For risk of bias, we downgraded the quality by one level (serious) if the risk of bias (selection bias, blinding, attrition bias) was sufficiently significant to affect the robustness of the estimated effect in sense of a changed clinical conclusion. We tested the robustness of the effect estimates in sensitivity analyses for selection bias, blinding, and attrition bias by excluding studies which we assessed as high or unclear risk of bias for the respective domains (Guyatt 2011a).
We judged the quality of evidence for indirectness as adequate if the outcome data were based on direct comparisons of interest, on the population of interest, and on the outcome of interest (Guyatt 2011b), not surrogate markers. Otherwise, we downgraded for inconsistency by one level.
To judge for imprecision and inconsistency, we examined the 95% CI and the sample size (Guyatt 2011c), as well as the 95% PI (IntHout 2016; Riley 2011). The 95% PI helps in the clinical interpretation of the between‐study heterogeneity by estimating which true mean treatment effects can be expected in future studies. If the 95% PI covered the range (clinical relevance) of the 95% CI we assumed no relevant between‐study heterogeneity and examined the extent of the CI and the sample size to judge for imprecision. If the CI was narrow and the total number of participants was large enough (≥ 400 participants for MD/SMD, ≥ 1000 participants for RR), we judged precision as adequate. We downgraded the quality of evidence for imprecision by one level if the CI around the effect size was large (e.g. including appreciable benefit or harm or including clinical relevance and non‐relevance) and/or when the number of participants was insufficient (< 400 participants). If the 95% PI was significantly wider than the random‐effects 95% CI, we assumed between‐study heterogeneity and downgraded for inconsistency. If the wider 95% PI crossed the line of identity in contrast to the 95% CI and the PI around the effect size was large (i.e. clinical relevance and non‐relevance), we additionally downgraded for imprecision. If the wider 95% PI and the 95% CI both lie on the same side of, or both crossed the line of identity, and the PI around the effect size was large (i.e. clinical relevance and non‐relevance), we also downgraded for imprecision.
For publication bias (Guyatt 2011d), we downgraded the quality of evidence by one level if the statistical test for funnel plot asymmetry suggested publication bias and the adjustment for small‐study effects, as assessed by Duval and Tweedie’s trim and fill analysis (Duval 2000), changed the conclusion. If the 95% PI was larger than the 95% CI and we had already downgraded for inconsistency, we did not downgrade for publication bias. True heterogeneity may be a source of funnel plot asymmetry (Higgins 2011).
The GRADE assessment resulted in one of four levels of 'quality' of the evidence, and these expressed our confidence in the estimate of effect (Balshem 2011).
High quality: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: we are moderately confident in the effect estimate, and the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: our confidence in the effect estimate is limited, and the true effect may be substantially different from the estimate of the effect.
Very low quality: we have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect.
Sensitivity analysis
We performed sensitivity meta‐analyses, excluding studies at high or unclear risk of bias in the evaluated domains for selection bias (random sequence generation and allocation concealment; Table 8), blinding (participants, personnel, and outcome assessment; Table 9), and incomplete outcome data (Table 3), to judge the robustness of the summary statistics.
Since we included all trials, even if they reported their data as median plus interquartile range (IQR), we performed sensitivity meta‐analyses using only trials which presented data as mean plus SD to judge the robustness of the estimated effect (Table 4).
We tested robustness of the effect estimates with regard to the model (random‐effects versus fixed‐effect model; Table 10).
We identified several studies with suspected variance reporting (unrealistically small SDs) during the update of this review (see Effects of interventions). We added studies with suspected variance reporting to the meta‐analyses of relevant outcomes to explore the impact on the effect estimates (Table 11).
6. Sensitivity analyses ‐ risk of bias (selection bias).
| All studies | Without high/unclear risk of bias studies (selection bias) | ||||
| Outcome | Statistical method | Studies | Effect estimate | Studies | Effect estimate |
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | SMD (IV, Random, 95% CI) | 29 | −0.50 (−0.72 to −0.28) | 6 | −0.23 (−0.51 to 0.05) |
| Pain score, rest, 'intermediate time points' (24 hrs) | SMD (IV, Random, 95% CI) | 33 | −0.14 (−0.25 to −0.04) | 6 | 0.09 (−0.13 to 0.30) |
| Pain score, rest, 'late time points' (48 hrs) | SMD (IV, Random, 95% CI) | 24 | −0.11 (−0.25 to 0.04) | 3 | 0.03 (−0.24 to 0.29) |
| Postoperative ileus (dichotomous) | RR (MH, Random, 95% CI) | 4 | 0.37 (0.15 to 0.87) | 0 | Not estimable |
| Time to first defaecation/bowel movement (hrs) | MD (IV, Random, 95% CI) | 12 | −7.92 (−12.71 to −3.13) | 2 | −6.18 (−11.19 to −1.18) |
| Time to first flatus (hrs) | MD (IV, Random, 95% CI) | 13 | −4.09 (−6.30 to −1.87) | 2 | −3.27 (−6.33 to −0.21) |
| Time to first bowel sounds (hrs) | MD (IV, Random, 95% CI) | 2 | −6.08 (−13.77 to 1.60) | 0 | Not estimable |
| Length of hospital stay (days) | MD (IV, Random, 95% CI) | 32 | −0.37 (−0.60 to −0.15) | 3 | −0.13 (−0.70 to 0.44) |
| Length of hospital stay (outpatient surgery, mins) | MD (IV, Random, 95% CI) | 3 | −10.81 (−36.93 to 15.31) | 3 | −10.81 (−36.93 to 15.31) |
| Surgical complications ‐ anastomotic leak | RR (MH, Random, 95% CI) | 3 | 0.61 (0.08 to 4.80) | 0 | Not estimable |
| Surgical complications ‐ bleeding | RR (MH, Random, 95% CI) | 3 | 1.79 (0.41 to 7.89) | 0 | Not estimable |
| Surgical complications ‐ postoperative infection | RR (MH, Random, 95% CI) | 5 | 1.64 (0.41 to 6.52) | 0 | Not estimable |
| Patient satisfaction | MD (IV, Random, 95% CI) | 6 | 0.76 (0.46 to 1.06) | 2 | 0.59 (−0.09 to 1.26) |
| Postoperative nausea, early (PACU) | RR (MH, Random, 95% CI) | 8 | 0.72 (0.53 to 0.98) | 3 | 0.64 (0.30 to 1.37) |
| Postoperative nausea, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 35 | 0.78 (0.67 to 0.91) | 8 | 0.99 (0.69 to 1.42) |
| Postoperative vomiting, early (PACU) | RR (MH, Random, 95% CI) | 4 | 0.49 (0.16 to 1.48) | 2 | 0.39 (0.11 to 1.38) |
| Postoperative vomiting, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 19 | 0.83 (0.63 to 1.08) | 3 | 1.33 (0.50 to 3.53) |
| Intraoperative opioid consumption (MEQ, mg) | MD (IV, Random, 95% CI) | 18 | −2.14 (−3.87 to −0.40) | 3 | −3.28 (−6.56 to −0.00) |
| Intraoperative opioid consumption with remifentanil (MEQ, mg) | MD (IV, Random, 95% CI) | 6 | −14.17 (−35.27 to 6.92) | 2 | −9.53 (−59.18 to 40.12) |
| Postoperative opioid consumption, PACU (MEQ, mg) | MD (IV, Random, 95% CI) | 21 | −3.10 (−3.87 to −2.32) | 6 | −2.69 (−4.13 to −1.24) |
| Postoperative opioid consumption, overall (MEQ, mg) | MD (IV, Random, 95% CI) | 40 | −4.52 (−6.25 to −2.79) | 10 | −2.74 (−5.60 to 0.13) |
Acronyms and abbreviations used in this table:
CI = confidence interval, IV = inverse variance, MD = mean difference, MEQ = morphine equivalent dose, MH = Mantel Haenszel, PACU = postanaesthesia care unit, RR = risk ratio, SMD = standardized mean difference
7. Sensitivity analyses ‐ risk of bias (blinding).
| All studies | Without high/unclear risk of bias studies (blinding) | ||||
| Outcome | Statistical method | Studies | Effect estimate | Studies | Effect estimate |
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | SMD (IV, Random, 95% CI) | 29 | −0.50 (−0.72 to −0.28) | 21 | −0.62 (−0.88 to −0.35) |
| Pain score, rest, 'intermediate time points' (24 hrs) | SMD (IV, Random, 95% CI) | 33 | −0.14 (−0.25 to −0.04) | 22 | −0.19 (−0.33 to −0.05) |
| Pain score, rest, 'late time points' (48 hrs) | SMD (IV, Random, 95% CI) | 24 | −0.11 (−0.25 to 0.04) | 19 | −0.17 (−0.31 to −0.04) |
| Postoperative ileus (dichotomous) | RR (MH, Random, 95% CI) | 4 | 0.37 (0.15 to 0.87) | 2 | 0.55 (0.16 to 1.88) |
| Time to first defaecation/bowel movement (hrs) | MD (IV, Random, 95% CI) | 12 | −7.92 (−12.71 to −3.13) | 5 | −8.87 (−20.51 to 2.78) |
| Time to first flatus (hrs) | MD (IV, Random, 95% CI) | 13 | −4.09 (−6.30 to −1.87) | 6 | −3.63 (−6.59 to −0.67) |
| Time to first bowel sounds (hrs) | MD (IV, Random, 95% CI) | 2 | −6.08 (−13.77 to 1.60) | 1 | −2.16 (−9.30 to 4.98) |
| Length of hospital stay (days) | MD (IV, Random, 95% CI) | 32 | −0.37 (−0.60 to −0.15) | 19 | −0.32 (−0.59 to −0.04) |
| Length of hospital stay (outpatient surgery, mins) | MD (IV, Random, 95% CI) | 3 | −10.81 (−36.93 to 15.31) | 1 | −4.00 (−12.64 to 4.64) |
| Surgical complications ‐ anastomotic leak | RR (MH, Random, 95% CI) | 3 | 0.61 (0.08 to 4.80) | 1 | 1.00 (0.07 to 15.26) |
| Surgical complications ‐ bleeding | RR (MH, Random, 95% CI) | 3 | 1.79 (0.41 to 7.89) | 1 | Not estimable |
| Surgical complications ‐ postoperative infection | RR (MH, Random, 95% CI) | 5 | 1.64 (0.41 to 6.52) | 3 | 0.69 (0.11 to 4.33) |
| Patient satisfaction | MD (IV, Random, 95% CI) | 6 | 0.76 (0.46 to 1.06) | 5 | 1.00 (0.63 to 1.37) |
| Postoperative nausea, early (PACU) | RR (MH, Random, 95% CI) | 8 | 0.72 (0.53 to 0.98) | 6 | 0.75 (0.53 to 1.05) |
| Postoperative nausea, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 35 | 0.78 (0.67 to 0.91) | 23 | 0.78 (0.66 to 0.93) |
| Postoperative vomiting, early (PACU) | RR (MH, Random, 95% CI) | 4 | 0.49 (0.16 to 1.48) | 3 | 0.52 (0.16 to 1.68) |
| Postoperative vomiting, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 19 | 0.83 (0.63 to 1.08) | 15 | 0.70 (0.50 to 0.96) |
| Intraoperative opioid consumption (MEQ, mg) | MD (IV, Random, 95% CI) | 18 | −2.14 (−3.87 to −0.40) | 13 | −1.86 (−3.74 to 0.02) |
| Intraoperative opioid consumption with remifentanil (MEQ, mg) | MD (IV, Random, 95% CI) | 6 | −14.17 (−35.27 to 6.92) | 5 | −16.08 (−41.41 to 9.25) |
| Postoperative opioid consumption, PACU (MEQ, mg) | MD (IV, Random, 95% CI) | 21 | −3.10 (−3.87 to −2.32) | 18 | −2.93 (−3.75 to −2.11) |
| Postoperative opioid consumption, overall (MEQ, mg) | MD (IV, Random, 95% CI) | 40 | −4.52 (−6.25 to −2.79) | 24 | −7.29 (−10.38 to −4.19) |
Acronyms and abbreviations used in this table: CI = confidence interval, IV = inverse variance, MD = mean difference, MEQ = morphine equivalent dose, MH = Mantel Haenszel, PACU = postanaesthesia care unit, RR = risk ratio, SMD = standardized mean difference
8. Sensitivity analyses ‐ random‐effects versus fixed‐effect model.
| Random‐effects model | Fixed‐effect model | ||||
| Outcome | Statistical method | Studies | Effect estimate | Studies | Effect estimate |
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | SMD (IV, Random, 95% CI) | 29 | −0.50 (−0.72 to −0.28) | 29 | −0.40 (−0.50 to −0.30) |
| Pain score, rest, 'intermediate time points' (24 hrs) | SMD (IV, Random, 95% CI) | 33 | −0.14 (−0.25 to −0.04) | 33 | −0.13 (−0.22 to −0.04) |
| Pain score, rest, 'late time points' (48 hrs) | SMD (IV, Random, 95% CI) | 24 | −0.11 (−0.25 to 0.04) | 24 | −0.09 (−0.19 to 0.02) |
| Postoperative ileus (dichotomous) | RR (MH, Random, 95% CI) | 4 | 0.37 (0.15 to 0.87) | 4 | 0.35 (0.15 to 0.82) |
| Time to first defaecation/bowel movement (hrs) | MD (IV, Random, 95% CI) | 12 | −7.92 (−12.71 to −3.13) | 12 | −6.01 (−8.53 to −3.49) |
| Time to first flatus (hrs) | MD (IV, Random, 95% CI) | 13 | −4.09 (−6.30 to −1.87) | 13 | −3.63 (−4.59 to −2.68) |
| Time to first bowel sounds (hrs) | MD (IV, Random, 95% CI) | 2 | −6.08 (−13.77 to 1.60) | 2 | −6.09 (−11.13 to −1.04) |
| Length of hospital stay (days) | MD (IV, Random, 95% CI) | 32 | −0.37 (−0.60 to −0.15) | 32 | −0.21 (−0.30 to −0.12) |
| Length of hospital stay (outpatient surgery, mins) | MD (IV, Random, 95% CI) | 3 | −10.81 (−36.93 to 15.31) | 3 | −5.66 (−13.74 to 2.43) |
| Surgical complications ‐ anastomotic leak | RR (MH, Random, 95% CI) | 3 | 0.61 (0.08 to 4.80) | 3 | 0.58 (0.08 to 4.24) |
| Surgical complications ‐ bleeding | RR (MH, Random, 95% CI) | 3 | 1.79 (0.41 to 7.89) | 3 | 1.86 (0.43 to 8.05) |
| Surgical complications ‐ postoperative infection | RR (MH, Random, 95% CI) | 5 | 1.64 (0.41 to 6.52) | 5 | 1.69 (0.53 to 5.33) |
| Patient satisfaction | MD (IV, Random, 95% CI) | 6 | 0.76 (0.46 to 1.06) | 6 | 0.76 (0.46 to 1.06) |
| Postoperative nausea, early (PACU) | RR (MH, Random, 95% CI) | 8 | 0.72 (0.53 to 0.98) | 8 | 0.72 (0.53 to 0.99) |
| Postoperative nausea, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 35 | 0.78 (0.67 to 0.91) | 35 | 0.77 (0.68 to 0.88) |
| Postoperative vomiting, early (PACU) | RR (MH, Random, 95% CI) | 4 | 0.49 (0.16 to 1.48) | 4 | 0.51 (0.18 to 1.44) |
| Postoperative vomiting, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random, 95% CI) | 19 | 0.83 (0.63 to 1.08) | 19 | 0.78 (0.60 to 1.01) |
| Intraoperative opioid consumption (MEQ, mg) | MD (IV, Random, 95% CI) | 18 | −2.14 (−3.87 to −0.40) | 18 | −1.05 (−1.47 to −0.62) |
| Intraoperative opioid consumption with remifentanil (MEQ, mg) | MD (IV, Random, 95% CI) | 6 | −14.17(−35.27 to 6.92) | 6 | −13.68 (−33.53 to 6.17) |
| Postoperative opioid consumption, PACU (MEQ, mg) | MD (IV, Random, 95% CI) | 21 | −3.10 (−3.87 to −2.32) | 21 | −3.14 (−3.67 to −2.61) |
| Postoperative opioid consumption, overall (MEQ, mg) | MD (IV, Random, 95% CI) | 40 | −4.52 (−6.25 to −2.79) | 40 | −1.52 (−2.14 to −0.90) |
Acronyms and abbreviations used in this table: CI = confidence interval, hrs = hours, IV = inverse variance, MD = mean difference, MEQ = morphine equivalent dose, MH = Mantel Haenszel, PACU = postanaesthesia care unit, mins = minutes, RR = risk ratio, SMD = standardized mean difference
9. Sensitivity analyses ‐ with studies with 'suspected variance reporting'.
| Without suspicious studies | With suspicious studies | ||||
| Outcome | Statistical method | Studies | Effect estimate | Studies | Effect estimate |
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | SMD (IV, Random, 95% CI) | 29 | −0.50 (−0.72 to −0.28) | 37 | −0.88 (−1.18 to −0.57) |
| Pain score, rest, 'intermediate time points' (24 hrs) | SMD (IV, Random, 95% CI) | 33 | −0.14 (−0.25 to −0.04) | 41 | −0.29 (−0.44 to −0.15) |
| Pain score, rest, 'late time points' (48 hrs) | SMD (IV, Random, 95% CI) | 24 | −0.11 (−0.25 to 0.04) | 30 | −0.22 (−0.40 to −0.03) |
| Time to first defaecation/bowel movement (hrs) | MD (IV, Random, 95% CI) | 12 | −7.92 (−12.71 to −3.13) | 14 | −7.09 (−10.06 to −4.11) |
| Time to first flatus (hrs) | MD (IV, Random, 95% CI) | 13 | −4.09 (−6.30 to −1.87) | 16 | −5.02 (−7.73 to −2.31) |
| Time to first bowel sounds (hrs) | MD (IV, Random, 95% CI) | 2 | −6.08 (−13.77 to 1.60) | 4 | −4.28 (−10.32 to 1.76) |
| Postoperative opioid consumption, PACU (MEQ, mg) | MD (IV, Random, 95% CI) | 21 | −3.10 (−3.87 to −2.32) | 25 | −3.51 (−4.88 to −2.15) |
| Postoperative opioid consumption, overall (MEQ, mg) | MD (IV, Random, 95% CI) | 40 | −4.52 (−6.25 to −2.79) | 43 | −4.81 (−6.55 to −3.07) |
Acronyms and abbreviations used in this table: CI = confidence interval, hrs = hours, IV = inverse variance, MD = mean difference, MEQ = morphine equivalent dose, PACU = postanaesthesia care unit, SMD = standardized mean difference
We considered sensitivity analyses to be exploratory and we did not adjust for multiplicity.
Results
Description of studies
Results of the search
The results of the literature search process are graphically presented in a PRISMA flow chart (Figure 1). We performed the electronic searches for the first review on 12 February 2013, and on 15 May 2014 (Kranke 2015). We performed the updated search on 25 January 2017. We re‐ran the search in February 2018, but we have not yet fully incorporated these results in the review (Studies awaiting classification). In summary, we identified 5224 records by database searching, 4162 for the first review, 901 in the updated search, and 161 for the top‐up search. We identified an additional 798 and 53 records in 2014 and 2017, respectively, by searching other sources (ASA; ClinicalTrials.gov), abstracts and handsearching the reference lists of the included articles. We did not find any additional studies by contacting experts in the field. After we removed duplicate studies, at least two review authors (original review: SW, JJ; update; SW, AH, YJ) reviewed the remaining 3611 records. Of those 3611 records, we excluded 3489 by reading the title or abstract. We reviewed the remaining 122 records: we included 76 records, which could be assigned to 68 studies. Forty‐five of these 68 studies were already subject to the published review (Kranke 2015), we added 23 of those 68 studies to this current update. We included these 68 studies in the synthesis of this review.
1.
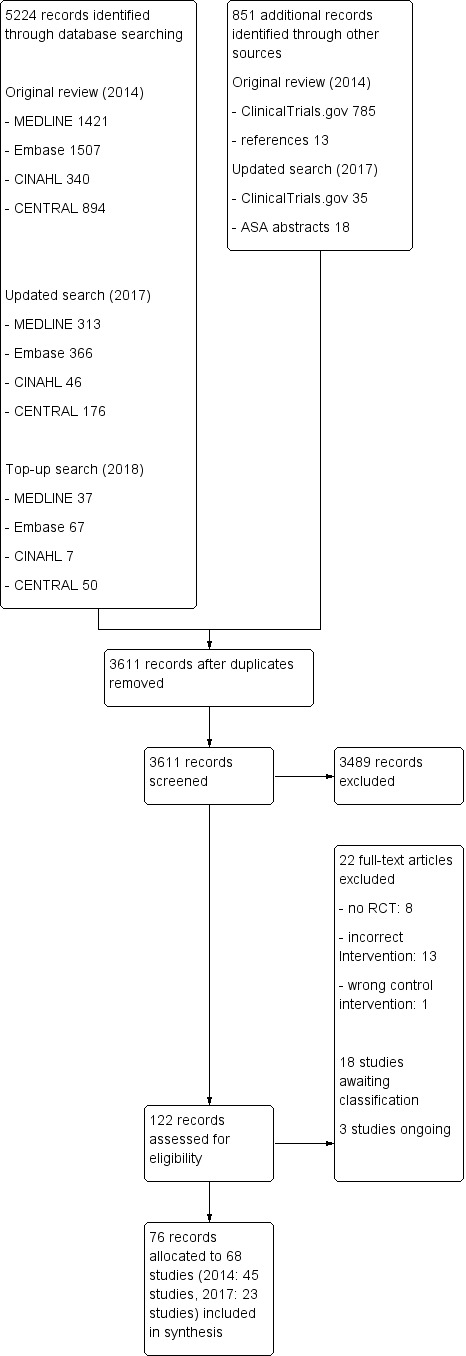
Study flow diagram.
Of the 68 included studies, one trial was published in Persian (Soltani 2013), and one in Portugese (Oliveira 2015); all other studies were published in English. We obtained only the abstract of the full text from Ismail 2008, despite requesting a full copy of the paper from the authors and the journal.
Included studies
We included 68 trials in this review. Five trials were published as a full text publication and as a poster abstract (Choi GJ 2016; Choi KW 2016; Lee 2011; Terkawi 2014; Yang 2014). Two studies published a secondary report with follow up data (Peng 2016; Terkawi 2014). For one study a correction note was available (Weinberg 2016). The included studies were published between 1985 (Cassuto 1985); and 2017 (Xu 2017). A detailed description of the trials can be found in the Characteristics of included studies. These RCTs include data on 4525 participants, 2254 of which received intravenous (IV) lidocaine and 2271 received a control treatment.
Comparators
In 63 trials, participants in the comparator arm received placebo treatment with saline; in three trials participants received no treatment (Choi SJ 2012; Kim HJ 2014; Lauwick 2008). In two trials thoracic epidural analgesia with bupivacaine and hydromorphone (Swenson 2010), or morphine (Wongyingsinn 2011), was used as a comparator.
Surgical procedures
In 22 trials, open abdominal surgery was performed, i.e. abdominal hysterectomy (Bryson 2010; Grady 2012; Oliveira 2015; Samimi 2015; Wang 2015; Xu 2017; Yardeni 2009); cholecystectomy (Cassuto 1985; Rimbäck 1990; Wallin 1987); colorectal surgery (Herroeder 2007; Kuo 2006; Staikou 2014; Swenson 2010); caesarean delivery (El‐Tahan 2009); mixed major open abdominal procedures (Baral 2010; Koppert 2004; Sridhar 2015; Zengin 2015); and radical retropubic prostatectomy (Groudine 1998; Maquoi 2016; Weinberg 2016).
In 20 trials, laparoscopic surgical procedures were conducted, i.e. laparoscopic cholecystectomy (Jain 2015; Lauwick 2008; Ortiz 2016; Saadawy 2010; Wu 2005; Yang 2014); laparoscopic colectomy (Ahn 2015; Kaba 2007; Kim HO 2014; Tikuisis 2014; Wongyingsinn 2011); laparoscopic gastrectomy (De Oliveira 2014; Kim TH 2013; Yon 2014); laparoscopic fundoplication (Dale 2016); laparoscopic prostatectomy (Lauwick 2009); laparoscopic appendectomy (Kim TH 2011); laparoscopic renal surgery (Wuethrich 2012); and ambulatory laparoscopic gynaecological surgery (De Oliveira 2012; Dewinter 2016).
The remaining 26 studies looked at various other surgical procedures, i.e. cardiac surgery (Insler 1995; Kasten 1986; Kim HJ 2014; Lee 2011; Mathew 2009; Mitchell 1999; Mitchell 2009; Wang 2002); breast surgery (Choi SJ 2012; Grigoras 2012; Terkawi 2014); thoracic surgery (Cui 2010); video‐assisted thoracoscopic surgery (Slovack 2015); spine surgery (Chen 2015; Farag 2013); supratentorial tumour surgery (Peng 2016); endoscopic sinus surgery (Omar 2013); hip arthroplasty (Martin 2008); inguinal herniorrhaphy (Kang 2011); ophthalmologic surgery (Soltani 2013); tonsillectomy (Striebel 1992); lumbar discectomy (Ismail 2008; Kim KT 2014); thyroidectomy (Choi GJ 2016; Choi KW 2016); and ambulatory surgery (McKay 2009).
Details on lidocaine administration (dose and timing)
A summary of details of lidocaine administration for each study is presented in Table 12. Briefly, systemic lidocaine administration was initiated up to 30 minutes before induction, at induction, or after induction of anaesthesia, or at the latest 30 minutes before skin incision. In five studies the exact intraoperative starting time point of lidocaine administration was not reported (De Oliveira 2012; De Oliveira 2014; Grady 2012; Ortiz 2016; Soltani 2013). However, we were able to obtain this information by contacting the authors of four of these studies (De Oliveira 2012; De Oliveira 2014; Grady 2012; Ortiz 2016).
10. Study drug administration.
| Study ID | Surgical procedure | Start infusion | End infusion | Duration of infusion | Bolus dose | Infusion dose | Total dose |
| Ahn 2015 | Laparoscopic colectomy | 2 mins before intubation | End of the operaton | 216.60 mins (surgery) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Baral 2010 | Abdominal surgery | 30 mins before skin incision | 1 hr after the end of surgery | 157.80 min (infusion) | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Bryson 2010 | Abdominal hysterectomy | Prior to induction | Skin closure | 105.0 mins (anaesthesia) | 1.5 mg/kg | 3 mg/kg/hr | NA |
| Cassuto 1985 | Cholecystectomy | 30 mins before skin incision | 24 hrs postop | 105 mins (surgery) + 30 mins (prior) + 24 hrs (postop) | 100 mg | 2 mg/min | NA |
| Chen 2015 | Spine surgery | After induction of anaesthesia | End of surgery | 129.2 mins (surgery) | 1 mg/kg | 1.5 mg/kg/hr | NA |
| Choi SJ 2012 | Breast plastic surgeries | 30 mins before skin incision | Skin closure | 295 mins (surgery) + 30 mins (prior) | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Choi GJ 2016 | Elective total thyroidectomy | Prior to anaesthesia | End of surgery | 135 mins (anaesthesia) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Choi KW 2016 | Thyroidectomy | Immediately after induction | Extubation | 148.9 mins (anaesthesia) | 2 mg/kg | 3 mg/kg/hr | NA |
| Cui 2010 | Thoracic surgery | At induction | Skin closure | 244 mins (anaesthesia) | No bolus | 33 µg/kg/mins | NA |
| Dale 2016 | Laparoscopic fundoplication | At induction | 24 hrs after start of continuous infusion | 24 hrs | 1 mg/kg | 2 mg/kg/hr | NA |
| De Oliveira 2012 | Outpatient laparoscopic surgery | Prior to induction | End of the surgical procedure | 105.5 mins (time of induction to skin incision) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| De Oliveira 2014 | Laparoscopic bariatric surgery | Prior to induction | End of the surgical procedure | 144 mins (surgery) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Dewinter 2016 | Laparoscopic sterilisation in women | At induction | 30 mins after arrival at PACU | 77 mins | 1.5 mg/kg | 1.5 mg/kg/hr | 240 mg |
| El‐Tahan 2009 | Caesarean delivery | 30 mins before induction | 60 mins after skin closure | 43.2 mins (anaesthesia) + 60 mins (postop) | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Farag 2013 | Spine surgery | At induction | Discharge from the PACU or a maximum of 8 hrs | 8.5 hrs | No bolus | 2 mg/kg/hr | NA |
| Grady 2012 | Abdominal hysterectomy | At induction | 24 hours postop | NA | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Grigoras 2012 | Surgery for breast cancer | Prior to induction | 60 mins after skin closure | 60.6 mins (surgery) + 60 mins (postop) | 1.5 mg/kg | 1.5 mg/kg/hr | 328.1 mg |
| Groudine 1998 | Radical retropubic prostatectomy | Prior to induction | 60 mins after skin closure | NA | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Herroeder 2007 | Colorectal surgery | Prior to induction | 4 hours postop | 194.3 mins (surgery) + NA (induction to skin incision) + 4 hrs (postop) | 1.5 mg/kg | 2 mg/mins | NA |
| Insler 1995 | CABG | After induction of anaesthesia and before surgical incision | Up to 48 hours in the ICU unless discharged earlier | NA | 1.5 mg/kg | 30 μg/kg/min | NA |
| Ismail 2008 | Lumbar discectomy | 30 mins before induction | Until 10 mins after extubation | NA | 1.5 mg/kg | 1.5 mg/kg | NA |
| Jain 2015 | Laparoscopic cholecystectomy | 10 mins prior to induction | End of first postop hr, max. 180 mins | NA | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Kaba 2007 | Laparoscopic colectomy | At induction | 24 hrs postop | 169 mins (anaesthesia) + 24 hrs (postop) | 1.5 mg/kg | 2 mg/kg/hr intraop and 1.33 mg/kg/h for 24 hrs postop | NA |
| Kang 2011 | Inguinal herniorrhaphy | 2 mins before induction | End of the surgical procedure | 66.03 mins (anaesthesia) + 2 mins (before induction) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Kasten 1986 | CABG | 2 mins before induction | NA | NA | 3 mg/kg | 0.05 mg/kg/min | NA |
| Kim TH 2011 | Laparoscopic appendectomy | 2 mins before induction | End of the surgical procedure | 70.0 mins (anaesthesia) or 55.0 (surgery) + 2 mins (before induction) | 1.5 mg/kg | 2 mg/kg/hr | 240.3 mg |
| Kim TH 2013 | Laparoscopic gastrectomy | Preop | End of the surgical procedure | 324 mins (anaesthesia) or 282.06 mins (surgery) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Kim HJ 2014 | Coronary artery bypass graft | Before induction | 24 hrs after end of surgery | 339 mins (anaesthesia) + 24 hrs | 1.5 mg/kg | 2 mg/kg/hr | 3917 mg |
| Kim HO 2014 | Laparoscopic colectomy | Prior to incision | After 24 hrs | 24 hrs | 1 mg/kg | 1 mg/kg/hr | NA |
| Kim KT 2014 | Elective one‐level laminectomy and discectomy | Preop | End of surgery | 110 min (surgery) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Koppert 2004 | Major abdominal surgery | 30 mins before skin incision | 1 hr after the end of surgery | 6.2 hrs (infusion) | 1.5 mg/kg | 5 mg/kg/hr | NA |
| Kuo 2006 | Surgery for colon cancer | 30 mins before surgery | End of the surgical procedure | 157.8 mins (surgery) + 30 min (before surgery) | 2 mg/kg | 3 mg/kg/hr | NA |
| Lauwick 2008 | Outpatient laparoscopic cholecystectomy | At induction | End of the surgical procedure | 60 mins (surgery) + NA (induction to skin incision) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Lauwick 2009 | Laparoscopic prostatectomy | At induction | End of the surgical procedure | 262.5 mins (surgery) + NA (induction to skin incision) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Lee 2011 | Off‐pump coronary artery bypass graft surgery | At induction | End of the surgical procedure | 208.9 mins (surgery) + NA (induction to skin incision) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Maquoi 2016 | Prostatectomy | Before induction | 24 hrs postop | 173 mins (anaesthesia) + 24 hrs | 1.5 mg/kg | 2 mg/kg/hr during surgery, then 1.33 mg/kg/hr | NA |
| Martin 2008 | Hip arthroplasty | 30 mins before skin incision | 1 hr after the end of surgery | NA | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Mathew 2009 | Cardiac surgery | After induction | 48 hrs postop | NA | 1 mg/kg | 4 mg/min for 1 hr, 2 mg/min for the second hr, 1 mg/min for the rest | NA |
| McKay 2009 | Outpatient surgery | After induction | 1 hr after arrival in the PACU | NA | 1.5 mg/kg | 2 mg/kg/hr | 517 mg |
| Mitchell 1999 | Cardiac surgery | At induction | 48 hrs postop | NA | 1 mg/kg | 240 mg over the first hr and 120 mg over the second hr, and then 60 mg/h thereafter if the patient was receiving lidocaine | NA |
| Mitchell 2009 | Cardiac surgery | At induction | Total of 12 hours | NA | 1 mg/kg | 2 mg/min for 2 hrs, and 1 mg/min thereafter | NA |
| Oliveira 2015 | Hysterectomy | At induction | End of surgery | 145.1 mins (anaesthesia) | No bolus | 2 mg/kg/hr | NA |
| Omar 2013 | Functional endoscopic sinus surgery | After induction | End of the surgical procedure | 87 mins (anaesthesia) or 62 mins (surgery) | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Ortiz 2016 | Laparoscopic cholecystectomy | Before incision | 1 hr after end of surgery | 105.23 mins (surgery) + 1 hr | 1.5 mg/kg | 3 mg/kg/hr | NA |
| Peng 2016 | Supratentorial tumour surgery | After induction | End of surgery | 254 mins (surgery) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Rimbäck 1990 | Cholecystectomy | Prior to induction | 24 hrs postop | 109 mins (surgery) + NA (induction to skin incision) + 24 hrs (postop) | 100 mg | 3 mg/min | NA |
| Saadawy 2010 | Laparoscopic cholecystectomy | Prior to induction | End of the surgical procedure | 80.3 mins (surgery) + NA (induction to skin incision) | 2 mg/kg | 2 mg/kg/hr | NA |
| Samimi 2015 | Abdominal hysterectomy | 30 mins before incision | 1 hr after surgery | 30 mins + 95 min (surgery) + 60 mins | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Slovack 2015 | VATS | At induction | End of the surgical procedure | NA | 1.5 mg/kg | 3 mg/min if the patient’s total body weight was more than 70 kg or 2 mg/min if weight was less than 70 kg | 239.6 mg |
| Soltani 2013 | Ophthalmologic surgeries | NA | Intraoperatively | No bolus | 2.5 mg/kg/hr | ||
| Sridhar 2015 | Open abdominal surgery | Time of intubation | 1 hr after surgery | 145.8 mins (surgery) + 60 min | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Staikou 2014 | Large bowel surgery | Before induction | Before skin suturing | 122 mins | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Striebel 1992 | Tonsillectomy | 30 mins before skin incision | 24 hrs | 57 mins (surgery) + 30 min (before skin incision) + 24 hrs (postop) | 1.5 mg/kg | 2 mg/kg/hr over 6 hrs and 0.5 mg/kg/hr for another 18 hrs | NA |
| Swenson 2010 | Colon resection | Prior to induction | Until the day after return of bowel function or fifth postop day | 69 hrs 54 mins (infusion) | No bolus | 11 patients: 2 mg/min in patients < 70 kg, 3 mg/min in patients > 70 kg, and 11 patients: 1 mg/min in patients < 70 kg, 2 mg/min in patients > 70 kg | NA |
| Terkawi 2014 | Breast cancer surgery | Before induction | 2 hrs after arrival in PACU or at discharge from PACU | 85 mins | 1.5 mg/kg, max. 150 mg | 2 mg/kg/h, max 200 mg/hr | NA |
| Tikuisis 2014 | Laparoscopic colon resection | Prior to induction | 24 hrs postop | 115 mins (anaesthesia) + 24 hrs (postop) | 1.5 mg/kg | 2 mg/kg/hr during surgery, 1 mg/ kg/hr for 24 hrs | NA |
| Wallin 1987 | Cholecystectomy | 30 mins before skin incision | 24 hrs postop | 110 mins (surgery) + 30 min (before skin incision) + 24 hrs | 100 mg | 2 mg/min | NA |
| Wang 2002 | CABG | At the opening of the pericardium | End of the surgical procedure | NA | 1.5 mg/kg, second dose (4 mg/kg) was administered to the priming solution of CPB |
4 mg/min | NA |
| Wang 2015 | Hysterectomy | 10 mins prior to induction | Discharge from the operating room | 152.3 (anaesthesia) + 10 mins | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Weinberg 2016 | Radical retropubic prostatectomy | Before induction | End of surgery | NA | 1.5 mg/kg | 1.5 mg/kg/hr | NA |
| Wongyingsinn 2011 | Laparoscopic colorectal surgery | Prior to induction | 48 hrs postop | 220 mins (surgery) + NA (induction to skin incision) + 48 (postop) | 1.5 mg/kg, max: 100 mg | 2 mg/kg/hr during surgery, 1 mg/kg/hr for 48 hrs | NA |
| Wu 2005 | Laparoscopic cholecystectomy | 30 mins before skin incision | End of the surgical procedure | 81.4 mins (surgery) + 30 mins (before incision) | No bolus | 3 mg/kg/hr | NA |
| Wuethrich 2012 | Laparoscopic transperitoneal renal surgery | At induction | 24 hrs postop | 293 mins (anaesthesia) + 24 hrs (postop) | 1.5 mg/kg | 2 mg/kg/hr during surgery, 1.3 mg/kg/hr for 24 hrs | NA |
| Xu 2017 | Abdominal hysterectomy | 10 mins before induction | Wound closure | 118.7 mins (anaesthesia) | 1.5 mg/kg | 1.5 mg/kg/hr | 185.7 mg |
| Yang 2014 | Laparoscopic cholecystectomy | 2 mins before induction | End of the surgical procedure | 65 mins (anaesthesia) + 2 mins (before induction) | 1.5 mg/kg | 2 mg/kg/hr | 228.71 mg |
| Yardeni 2009 | Transabdominal hysterectomy | 20 mins before skin incision | End of the surgical procedure | 109 mins (surgery) + 20 mins (before skin incision) | 2 mg/kg | 1.5 mg/kg/hr | NA |
| Yon 2014 | Subtotal gastrectomy | Preop (protocol: 2 mins before intubation) | End of surgery | 271.27 mins (surgery) | 1.5 mg/kg | 2 mg/kg/hr | NA |
| Zengin 2015 | Laparotomy | At induction | Wound closure | 114.1 mins (surgery) | 1.0 mg/kg | 2 mg/kg/hr | NA |
Acronyms and abbreviations used in this table:
CABG = coronary artery bypass graft , CPB = cardiopulmonary bypass, hr = hour, ICU = intensive care unit, min = minute, NA = not available, preop = preoperatively, postop =postoperatively, VATS = Video‐assisted thoracoscopic surgery
In 62 studies IV lidocaine administration was initiated with a bolus dose of 1 mg/kg to 3 mg/kg of body weight or 100 mg lidocaine, 1.5 mg/kg being the most common dose, used in 69% of the included trials. In six studies lidocaine administration was started without a bolus dose (Cui 2010; Farag 2013; Oliveira 2015; Soltani 2013; Swenson 2010; Wu 2005).
The lidocaine infusion dose varied between studies from 1 mg/kg/h to 5 mg/kg/h. In 36 studies, the continuous infusion of lidocaine was delivered with a rate of ≥ 2 mg/kg/h , whereas an infusion rate of < 2 mg/kg/h was used in another 22 studies (Baral 2010; Cassuto 1985; Chen 2015; Choi SJ 2012; Cui 2010; Dewinter 2016; El‐Tahan 2009; Grigoras 2012; Groudine 1998; Herroeder 2007; Insler 1995; Ismail 2008; Jain 2015; Kim HO 2014; Martin 2008; Omar 2013; Sridhar 2015; Wallin 1987; Wang 2015; Weinberg 2016; Xu 2017; Yardeni 2009). In the remaining 10 trials, a higher infusion dose (≥ 2 mg/kg/h) was used during the first study period followed by continuous infusion < 2 mg/kg/h during the second study period (Kaba 2007; Maquoi 2016; Mathew 2009; Mitchell 1999; Mitchell 2009; Striebel 1992; Swenson 2010; Tikuisis 2014; Wongyingsinn 2011; Wuethrich 2012).
The continuous lidocaine infusion was terminated either at the end of the surgical procedure or with skin closure (Ahn 2015; Bryson 2010; Chen 2015; Choi GJ 2016; Choi KW 2016; Choi SJ 2012; Cui 2010; De Oliveira 2012; De Oliveira 2014; Kang 2011; Kim KT 2014; Kim TH 2011; Kim TH 2013; Kuo 2006; Lauwick 2008; Lauwick 2009; Lee 2011; Oliveira 2015; Omar 2013; Peng 2016; Saadawy 2010; Slovack 2015; Soltani 2013; Staikou 2014; Wang 2002; Wang 2015; Weinberg 2016; Wu 2005; Xu 2017; Yang 2014; Yardeni 2009; Yon 2014; Zengin 2015); 30 minutes after arrival at the postanaesthesia care unit (PACU) (Dewinter 2016); one hour after the end of surgery/skin closure (Baral 2010; El‐Tahan 2009; Grigoras 2012; Groudine 1998; Koppert 2004; Martin 2008; Ortiz 2016; Samimi 2015; Sridhar 2015); but a maximum 180 minutes in total (Jain 2015); one hour after arrival in the PACU (McKay 2009); two hours after arrival in the PACU or at discharge from the PACU (Terkawi 2014); four hours postoperatively (Herroeder 2007); up to eight hours postoperatively (or at PACU discharge, whichever occurred earlier) (Farag 2013); after a total of 12 hours (Mitchell 2009); after a total of 24 hours (Dale 2016; Kim HO 2014); 24 hours postoperatively (Cassuto 1985; Grady 2012; Kaba 2007; Kim HJ 2014; Maquoi 2016; Rimbäck 1990; Striebel 1992; Tikuisis 2014; Wallin 1987; Wuethrich 2012); 48 hours postoperatively (Insler 1995; Mathew 2009; Mitchell 2009; Wongyingsinn 2011); or on the day of return of bowel function, or on the fifth postoperative day at the latest (Swenson 2010). Two studies did not report the exact time point for stopping the lidocaine infusion (Ismail 2008; Kasten 1986).
Inclusion and exclusion criteria
The studies varied strongly in their inclusion and exclusion criteria of participants (see Characteristics of included studies). The proportion of male and female participants varied in the studies. In 18 trials the proportion of female participants was more than 75% (Baral 2010; Bryson 2010; Choi GJ 2016; Choi KW 2016; Choi SJ 2012; De Oliveira 2012; De Oliveira 2014; Dewinter 2016; El‐Tahan 2009; Grady 2012; Grigoras 2012; Jain 2015; Oliveira 2015; Saadawy 2010; Samimi 2015; Wang 2015; Xu 2017; Yardeni 2009). In nine trials male participants counted for more than 75% (Groudine 1998; Insler 1995; Koppert 2004; Lauwick 2009; Maquoi 2016; McKay 2009; Mitchell 2009; Wang 2002; Weinberg 2016). In five trials (Dale 2016; Kim KT 2014; Lauwick 2008; Staikou 2014; Swenson 2010), there was an imbalance of the gender distribution between the experimental and the control groups (> 20 %). We were unable to identify the gender distribution in four trials (Ismail 2008; Kasten 1986; Soltani 2013; Terkawi 2014).
Study conduct (location)
We noted geographical variability among the studies. Eighteen of the 68 included trials were conducted in either the USA, Canada or South America (Bryson 2010; De Oliveira 2012; De Oliveira 2014; Farag 2013; Grady 2012; Groudine 1998; Insler 1995; Kasten 1986; Lauwick 2008; Lauwick 2009; Mathew 2009; McKay 2009; Oliveira 2015; Ortiz 2016; Slovack 2015; Swenson 2010; Terkawi 2014; Wongyingsinn 2011); 24 trials in Asia (Ahn 2015; Baral 2010; Chen 2015; Choi GJ 2016; Choi KW 2016; Choi SJ 2012; Cui 2010; Jain 2015; Kang 2011; Kim HJ 2014; Kim HO 2014; Kim KT 2014; Kim TH 2011; Kim TH 2013; Kuo 2006; Lee 2011; Peng 2016; Sridhar 2015; Wang 2002; Wang 2015; Wu 2005; Xu 2017; Yang 2014; Yon 2014); 15 trials in Europe (Cassuto 1985; Dewinter 2016; Grigoras 2012; Herroeder 2007; Kaba 2007; Koppert 2004; Maquoi 2016; Martin 2008; Rimbäck 1990; Staikou 2014; Striebel 1992; Tikuisis 2014; Wallin 1987; Wuethrich 2012; Zengin 2015); seven trials in the Middle East (El‐Tahan 2009; Ismail 2008; Omar 2013; Saadawy 2010; Samimi 2015; Soltani 2013; Yardeni 2009); and four in New Zealand or Australia (Dale 2016; Mitchell 1999; Mitchell 2009; Weinberg 2016).
Study sample size
The overall sample size ranged from 20 randomized participants (Cassuto 1985; Kasten 1986), to 277 randomized participants (Mathew 2009). Sixteen trials did not report any sample size calculation for their primary outcome (Baral 2010; Cassuto 1985; Chen 2015; Cui 2010; Groudine 1998; Kasten 1986; Mitchell 1999; Ortiz 2016; Rimbäck 1990; Samimi 2015; Soltani 2013; Sridhar 2015; Striebel 1992; Wallin 1987; Wang 2002; Wang 2015). There is a possibility that these 16 trials may have been underpowered. All other trials reported undertaking a sample size calculation and the primary endpoints are listed in the Characteristics of included studies.
Source of funding
Financial support was provided by institutional or departmental or ministerial sources, or combinations thereof, in 38 of the 68 included trials (Ahn 2015; Bryson 2010; Chen 2015; Choi GJ 2016; Choi KW 2016; Cui 2010; De Oliveira 2012; Farag 2013; Grady 2012; Herroeder 2007; Kaba 2007; Kang 2011; Kim HJ 2014; Kim HO 2014; Kim TH 2011; Kim TH 2013; Koppert 2004; Kuo 2006; Lauwick 2008; Lauwick 2009; Martin 2008; Mathew 2009; McKay 2009; Mitchell 1999; Mitchell 2009; Ortiz 2016; Staikou 2014; Tikuisis 2014; Wallin 1987; Wang 2002; Wang 2015; Weinberg 2016; Wongyingsinn 2011; Wu 2005; Wuethrich 2012; Xu 2017; Yang 2014; Yon 2014). Three trials explicitly stated that there was no funding (Dale 2016; Peng 2016; Zengin 2015). All other trials did not mention the source of funding in their publications. None of the trials reported funding by industry.
Reported outcomes
Sixty‐five studies reported at least one outcome of interest for this review. Two trials did not contribute any appropriate outcome (Kasten 1986; Wang 2015); and from another trial only the abstract from the full text, with insufficient details was available (Ismail 2008). Additional outcomes that were reported in the included studies but were not of interest for this review are listed in the Characteristics of included studies.
Excluded studies
We excluded 22 studies after reviewing the full texts, for the reasons referred to in Figure 1 and described in more detail in the Characteristics of excluded studies. Three studies either did not have a control group and were not RCTs, or investigated only one cohort of participants, all receiving lidocaine (Bartlett 1961; De Clive‐Lowe 1958; Knight 1980). We identified four studies as review articles (Marret 2008; McCarthy 2010; Sun 2012; Vigneault 2011); and one as a referenced article of the original review from McCarthy and colleagues (Joppich 2010). The remaining 13 excluded studies did not describe an intervention which fitted the inclusion criteria of this review. Six of these studies administered lidocaine after, and not during the surgical procedure (Birch 1987; Cepeda 1996; Chia 1998; Couceiro 2015; Harvey 2009; Perniola 2014). In one study participants received lidocaine as a repeated bolus and not as a continuous infusion (De Kock 1994). In two studies the infusion was stopped before the end of the surgical procedure (Hans 2010; Juarez‐Pichardo 2009). In one study lidocaine was administered during surgery but not until skin closure (Rinne 1998). In another study lidocaine was given as part of a multimodal drug regime and compared to fentanyl (Feld 2003). Another trial compared lidocaine infusion to magnesium infusion and therefore did not have a control group relevant for the purpose of this review (Olivares 2012). In one trial ketamine was added to lidocaine infusion (Zhu 2015), and in another trial remifentanil was administered in the control group (Kavak 2014).
Ongoing studies
We identified three ongoing studies which we plan to include in future updates of this review (NCT02059902; NCT02607488; NCT02862769) .
See Characteristics of ongoing studies for more details
Studies awaiting classification
There were 21 records that we allocated to 18 studies awaiting classification (Cho 2014; Choi 2017; Dewinter 2017; Horvat 2014; Jendoubi 2017; Kendall 2017; Khalili 2017a; Khalili 2017b; Kim 2017; Kim 2018; Lee 2017; Metha 2017; NCT02257346; Rahaymeh 2016; Sherif 2017; Song 2017; Van Den Heuvel 2016; Yoo 2016). We identified 6 of those studies during the January 2017 search, and 12 of those 21 records during the top‐up search in February 2018.
See Characteristics of studies awaiting classification for more details.
Risk of bias in included studies
We estimated the risk of bias in each of the included studies as described in the 'Risk of bias' tables (Characteristics of included studies). The results of the quality assessments are graphically presented in Figure 2. The overall risk of bias concerning selection bias (random sequence generation), performance bias, attrition bias, detection bias and other bias revealed low risk of bias in more than 50% of the included studies (Figure 3). For allocation concealment and selective reporting the quality assessment yielded low risk of bias for only approximately 20% of the included studies.
2.
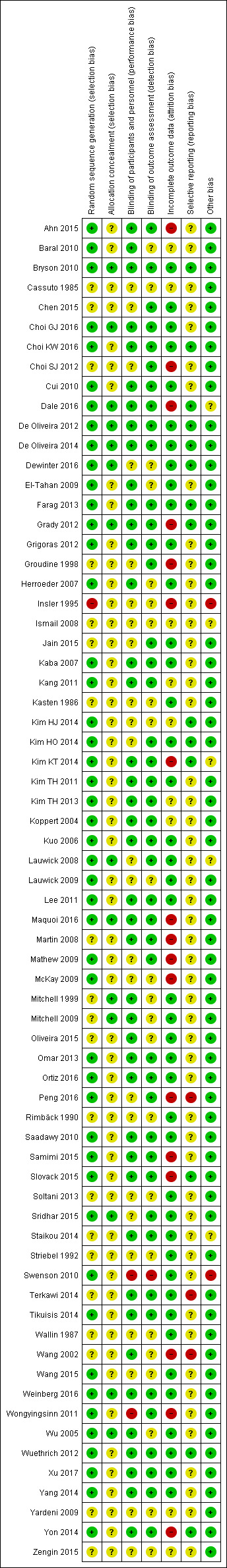
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
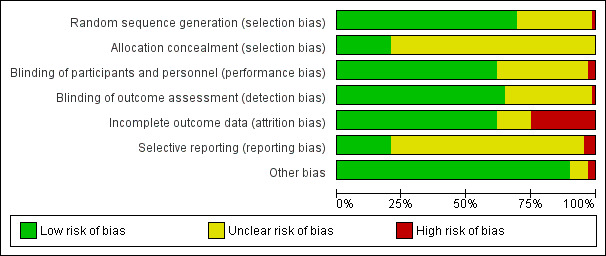
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Please note that we could not perform an unambiguous critical appraisal for Ismail 2008, since we obtained only the study's abstract. Due to insufficient information, we classified this study for all domains as unclear risk of bias.
Allocation
Random sequence generation
Forty‐seven trials described using random number tables or a computer random number generator for sequence generation and we deemed them to be at low risk of bias for this domain. One trial generated the allocation sequence based on the date of admission and we classified it at high risk of bias as the sequence generation process involved a non‐random component (Insler 1995). All other trials did not report sufficient information about the sequence generation process and we judged them at unclear risk of bias (Cassuto 1985; Chen 2015; Choi SJ 2012; Groudine 1998; Ismail 2008; Jain 2015; Kasten 1986; Martin 2008; Mitchell 1999; Mitchell 2009; Oliveira 2015; Rimbäck 1990; Soltani 2013; Staikou 2014; Striebel 1992; Terkawi 2014; Wallin 1987; Wang 2002; Yardeni 2009; Zengin 2015).
Allocation concealment
Adequate allocation concealment was described for 14 trials. Of these 14 trials, nine reported using 'SNOSE' (sequentially numbered AND opaque AND sealed envelopes) (Choi GJ 2016; Dale 2016; De Oliveira 2012; De Oliveira 2014; Dewinter 2016; Grady 2012; Lauwick 2008; Sridhar 2015; Weinberg 2016); two trials reported central allocation (Bryson 2010; Maquoi 2016); and three trials used sequentially numbered drug containers of identical appearance (Mitchell 1999; Mitchell 2009; Wu 2005). We classified all of these trials as being at a low risk of bias for this domain. All other 54 trials did not describe the method used for allocation concealment in sufficient detail (e.g. incomplete 'SNOSE' statements) and we judged them at unclear risk of bias.
Blinding
Blinding of participants and personnel
Eleven trials reported that participants and personnel were blinded to group allocation (Baral 2010; Dale 2016; Farag 2013; Kang 2011; Kim TH 2013; Koppert 2004; Oliveira 2015; Saadawy 2010; Tikuisis 2014; Wuethrich 2012; Yang 2014). Twenty‐one trials explicitly reported that either a nurse, a clinician or a non‐attending anaesthetist were unblinded to prepare the syringes containing the study drug, but these persons were not involved further in participant management or evaluation. Additionally, it was stated that all other personnel and participants were blinded (Ahn 2015; Cui 2010; Choi GJ 2016; Choi KW 2016; De Oliveira 2012; De Oliveira 2014; El‐Tahan 2009; Grigoras 2012; Herroeder 2007; Kim KT 2014; Kim TH 2011; Lee 2011; Maquoi 2016; Martin 2008; Ortiz 2016; Samimi 2015; Slovack 2015; Staikou 2014; Wang 2002; Xu 2017; Yon 2014). Ten trials reported on using pharmacy prepared study drugs of identical appearance to avoid unblinding (Bryson 2010; Grady 2012; Kaba 2007; Kuo 2006; Mitchell 1999; Mitchell 2009; Omar 2013; Terkawi 2014; Weinberg 2016; Wu 2005). We deemed all of these 42 trials at low risk of bias concerning blinding of participants and personnel.
Thirteen trials did not report any statement on blinding of participants and personnel (Cassuto 1985; Ismail 2008; Jain 2015; Kasten 1986; Mathew 2009; McKay 2009; Peng 2016; Rimbäck 1990; Soltani 2013; Striebel 1992; Wallin 1987; Wang 2015; Zengin 2015). We did not accept statements such as 'double‐blind study' or 'performed in a double‐blind manner' as adequate for low risk of bias in this domain. Therefore, we judged 13 trials at unclear risk of bias.
We allocated a further 11 trials to unclear risk of bias. In five of these cases, the attending anaesthetist was unblinded (Choi SJ 2012; Groudine 1998; Kim HJ 2014; Kim HO 2014; Lauwick 2008); in another five trials (Chen 2015; Dewinter 2016; Lauwick 2009; Sridhar 2015; Yardeni 2009), there was no adequate information about blinding of the study staff or at least personnel before outcome assessment. In the study from Insler 1995, the study drugs were prepared by the pharmacy. It is not clear from the description who was responsible for randomization and who informed the pharmacy how to prepare the study drugs (i.e. which number referred to which group). Therefore, it is unclear if blinding of personnel and participants was adequate.
The two trials that offered thoracic epidural analgesia (TEA) in the comparator arm were not able to sufficiently blind participants and personnel due to the study design. We therefore classified both at high risk of bias (Swenson 2010; Wongyingsinn 2011).
Blinding of outcome assessment
Statements that outcome assessors were blinded to participant group allocation were reported in 44 of the 68 included trials. We classified them at low risk of bias for this domain. Swenson 2010, offered TEA in the comparator arm and did not report blinding of the outcome assessors. Therefore, we classified this trial at high risk of bias. The remaining 23 trials did not provide any statement on blinding of outcome assessment. We judged these trials at unclear risk of bias (Baral 2010; Cassuto 1985; Dewinter 2016; El‐Tahan 2009; Herroeder 2007; Insler 1995; Ismail 2008; Kasten 1986; Kim HJ 2014; Lauwick 2009; McKay 2009; Mitchell 1999; Mitchell 2009; Oliveira 2015; Rimbäck 1990; Soltani 2013; Striebel 1992; Wallin 1987; Wang 2002; Wang 2015; Wu 2005; Yardeni 2009; Zengin 2015).
Incomplete outcome data
In total, we judged 42 studies at low risk of attrition bias. We classified 19 trials, reporting no exclusion or withdrawals of participants at low risk of bias (Choi GJ 2016; El‐Tahan 2009; Grigoras 2012; Jain 2015; Kasten 1986; Kim TH 2011; Kuo 2006; Lauwick 2009; Omar 2013; Rimbäck 1990; Soltani 2013; Sridhar 2015; Staikou 2014; Striebel 1992; Wallin 1987; Wang 2015; Wu 2005; Xu 2017; Yang 2014). Twenty‐three trials reported the number of participants being withdrawn or excluded from the study from each group along with the reasons (Bryson 2010; Cui 2010; Chen 2015; Choi KW 2016, De Oliveira 2012; De Oliveira 2014; Dewinter 2016; Farag 2013; Herroeder 2007; Kaba 2007; Kim HO 2014; Lauwick 2008; Lee 2011; Mitchell 1999; Mitchell 2009; Oliveira 2015; Ortiz 2016; Saadawy 2010; Swenson 2010; Terkawi 2014; Tikuisis 2014; Weinberg 2016; Wuethrich 2012). Farag 2013 additionally reported an intention‐to‐treat analysis including all enrolled participants.
Four trials provided no statement as to whether the presented results were for all participants who entered the trial (Baral 2010; Cassuto 1985; Ismail 2008; Zengin 2015). One trial described the excluded participants without uncovering their group assignment (Yardeni 2009). In one study it remained unclear if the reported reasons were related to true outcome (Kim HJ 2014). Kang 2011, Kim TH 2013 and Koppert 2004, described excluded participants, along with the reasons for their exclusion and reported on their replacement by other participants who fitted the inclusion criteria. We assumed (no response upon request to the authors) that the replacement did not fulfil criteria for adequate randomization, allocation and blinding and thus it had an impact on relevant outcomes. We classified these nine trials as being of unclear risk of bias.
Overall, we judged 17 studies to have a high risk of attrition bias. Three trials described the use of "last observed carried forward" (LOCF) ‐ a method for imputing missing data (Ahn 2015; Kim KT 2014; Yon 2014). In the trial from Dale 2016, three participants were withdrawn due to lidocaine toxicity, but the results were reported for all participants. Two trials did not report the reasons for withdrawal or exclusion of participants (Choi SJ 2012; McKay 2009). Grady 2012 reported on excluded participants along with reasons; however, the numbers of participants allocated to the groups, and finally analysed, were unclear. Four trials reported reasons for dropouts or exclusions which were likely to have an impact on relevant outcomes (Groudine 1998; Insler 1995; Maquoi 2016; Wang 2002). Martin 2008 reported on exclusion of two participants in the lidocaine group, who wanted to leave the study in the PACU due to extreme pain. Mathew 2009 described exclusions and dropouts. However, the dropout rate was high (23% experimental/26% control) and the reasons for missing data might be related to true outcome. Peng 2016 reported a large dropout rate. Reasons for missing data have been reported but were not the same in the two publications of this study. Samimi 2015 stated that data were available from 109 participants but presented results were obtained from 116 or 117 patients. Slovack 2015 presented high dropout rates at PACU and it is unclear if these participants were missing at random. Wongyingsinn 2011 reported on exclusion of one participant in the lidocaine group from the analysis because of an unknown drug reaction.
Selective reporting
For 14 trials a published trial protocol was available before participants' enrolment. The primary outcomes of the14 studies were reported in the corresponding protocols (Bryson 2010; Choi KW 2016; Dale 2016; De Oliveira 2012; De Oliveira 2014; Dewinter 2016; Farag 2013; Grady 2012; Kim HJ 2014; Kim HO 2014; Kim KT 2014; Slovack 2015; Wuethrich 2012; Yon 2014); we therefore classified these 14 trials at low risk of bias.
Another nine trials published a study protocol, and each study's primary and secondary outcomes relevant for the current review were reported in this published protocol (Choi GJ 2016; Kaba 2007; Kim TH 2011; Kim TH 2013; Maquoi 2016; Ortiz 2016; Swenson 2010; Wongyingsinn 2011; Yang 2014). However, these protocols were retrospectively registered. Therefore, we judged these studies to be at an unclear risk of bias for the domain 'selective reporting'. Lee 2011 reported publication of a study protocol on www.cris.cdc.go.kr. There was no English version of the protocol available. Since we could not judge for this domain, we classified this trial at unclear risk of bias. The remaining 41 included trials provided no reference to a trial registry or published study protocol and we classified these studies at unclear risk of bias.
We classified three trials at high risk of reporting bias. Wang 2002 defined distinct outcomes in two out of nine tests as the hurdle for "cognitive dysfunction"; it seems not entirely plausible that this hurdle had been set/defined prior to study conduct (no mention of a trial registration beforehand). It is very unlikely that, based on the pre‐existing work, only neuropsychological test performance was considered as a relevant outcome. Peng 2016 published a prospectively registered protocol. However, all data that are important for the current review have not been prespecified and have separately been published in a secondary findings report. Terkawi 2014 has a retrospectively registered protocol where postoperative pain was defined as a secondary outcome. In the final study report, pain was presented as the primary outcome.
Other potential sources of bias
We classified 61 of the 68 studies as low risk since these trials appeared to be free of other bias.
Five trials had unclear risk of bias. Dale 2016 described early stopping due to futility, having recruiting 24 participants instead of 36 estimated participants. Additionally, female gender was imbalanced between the groups. More females might have an impact on relevant outcomes. We could not assess Ismail 2008 adequately since only the abstract from the full text publication was available. Kim KT 2014 reported that the control group included more females, which could have influenced the occurrence of postoperative nausea and vomiting (PONV). Staikou 2014 reported more females in the experimental group. Lauwick 2008 included a greater proportion of males in the control group which might have an impact on relevant outcomes.
We judged two trials to be at high risk of other bias. One study used lidocaine as an anti‐arrhythmic drug during surgery in the placebo group, when ventricular ectopic beats or fibrillation occurred (Insler 1995). This may have influenced the study outcome. Swenson 2010 had a potential source of bias related to the intervention regimen since 50% of the participants received a higher lidocaine dose than the other trial participants. Additionally, ASA scores and gender were imbalanced between groups.
Effects of interventions
Summary of findings for the main comparison. Intravenous (IV) lidocaine compared to placebo or no treatment in patients undergoing any elective or urgent surgical procedure under general anaesthesia.
| IV Lidocaine compared to placebo or no treatment in patients undergoing any elective or urgent surgical procedure under general anaesthesia | |||||||
| Patient or population: adult patients undergoing any elective or urgent surgical procedure under general anaesthesia Settings: Asia (24 trials); USA, Canada, and South America (18 trials); Europe (15 trials); Middle East (7 trials); New Zealand and Australia (4 trials) Intervention: IV lidocaine Comparison: placebo or no treatment | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Prediction interval (95% PI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with placebo or no treatment | Corresponding risk with IV lidocaine | ||||||
| 1. Pain (VAS 0 to 10 cm, 0 to 100 mm, NRS 0 to 10) | Pain score at rest, 'early time points' (1 hto 4 hpostoperatively, or in the PACU) | ‐ | (1.61 lower to 0.62 higher) | 1656 (29 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | A SMD of 0.50 fewer is equivalent to a range of 0.37 cm fewer (SD = 0.74 cm) to 2.48 cm fewer (SD = 4.95 cm) on a VAS 0 to 10 cm scale in the intervention group. The range of mean effects that can be expected in a future study (95% PI) includes both benefit and harmk. |
|
| The standardized mean pain score in the intervention group was 0.50 lower (0.72 lower to 0.28 lower) | |||||||
| Pain score at rest, 'intermediate time points' (24 hpostoperatively) | ‐ | (0.44 lower to 0.16 higher) | 1847 (33 RCTs) | ⊕⊕⊕⊝ Moderatee,f,g,h,i | A SMD of 0.14 fewer is equivalent to a range of 0.10 cm fewer (SD = 0.74 cm) to 0.48 cm fewer (SD = 3.42 cm) on a VAS 0 to 10 cm scale in the intervention group. The range of mean effects that can be expected in a future study (95% PI) is clinically not relevantk. |
||
| The standardized mean pain score in the intervention group was 0.14 lower (0.25 lower to 0.04 lower) | |||||||
| Pain score at rest, 'late time points' (48 hpostoperatively) | ‐ | (0.60 lower to 0.38 higher) | 1404 (24 RCTs) | ⊕⊕⊕⊝ Moderatee,f,g,h,i | A SMD of 0.11 fewer is equivalent to a range of 0.08 cm fewer (SD = 0.7 cm) to 0.42 cm fewer (SD = 3.8 cm) on a VAS 0 to 10 cm scale in the intervention group. The range of mean effects that can be expected in a future study (95% PI) is clinically not relevantk. |
||
| The standardized mean pain score in the intervention group was 0.11 lower (0.25 lower to 0.04 higher) | |||||||
| 2. Gastrointestinal recovery |
Postoperative ileus (dichotomous) The number of participants with postoperative ileus |
RR 0.37 (0.15 to 0.87) | (0.05 lower to 2.43 higher) | 273 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The range of mean effects that can be expected in a future study (95% PI) includes both benefit and harm. | |
| 131 per 1000 | 48 per 1000 (20 to 114) | ||||||
| Time to first defaecation/bowel movement (h) | ‐ | (22.19 h shorter to 6.36 h longer) | 684 (12 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The range of mean effects that can be expected in a future study (95% PI) includes both benefit and clinical non‐relevance. | ||
| The mean time to first defaecation/bowel movement in the control group ranged from 24 h to 94 h | The mean time to first defaecation/bowel movement in the intervention group was 7.92 h shorter (12.71 h shorter to 3.13 h shorter) | ||||||
|
3. Adverse events (e.g. the number of participants that died, or had arrhythmias, other heart rate disorders, or showed any signs of lidocaine toxicity) |
See comment | See comment | ‐ | ‐ | See comment | ⊕⊝⊝⊝ Very Lowj | Adverse events that were investigated in a few trials are death, arrhythmia, light‐headedness, perioral numbness, and dizziness. The effect of lidocaine on these adverse effects is uncertain. |
| 4. Postoperative nausea, 'overall' (0 to 24 h, to 48 h, to 72 h) | 350 per 1000 | 273 per 1000 (235 to 319) | RR 0.78 (0.67 to 0.91) | (0.49 lower to 1.23 higher) | 1903 (35 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | The range of mean effects that can be expected in a future study (95% PI) includes both benefit and clinical non‐relevance. |
| 5. Postoperative opioid consumption, 'overall' (MEQ, mg) | The mean postoperative opioid consumption in the control group ranged from 1.13 mg to 233.93 mg | The mean postoperative opioid consumption in the intervention group was 4.52 mg lower (6.25 mg lower to 2.79 mg lower) | ‐ | (12.03 mg lower to 3.00 mg higher) | 2201 (40 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c,d | The range of mean effects that can be expected in a future study (95% PI) includes both benefit and clinical non‐relevance. |
|
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MEQ: morphine equivalents; NRS: numeric rating scale; PACU: postanaesthesia care unit; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference; SD: standard deviation; VAS: visual analogue scale | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngrading for study limitations: substantial information is derived from studies at high or unclear risk of bias (selection bias, blinding, attrition bias). Exclusion of high and unclear risk of bias studies affected the robustness of the estimated effect.
bDowngrading for inconsistency: the 95% PI is significantly wider than the 95% CI (we assume between‐study heterogeneity).
cDowngrading for imprecision: we downgraded for imprecision due to the fact that the 95% PI crosses the line of identity in contrast to the 95% CI.
dPublication bias: test for publication bias suggested funnel plot asymmetry and trim and fill analysis changed the conclusion. We did not downgrade for publication bias since we have already downgraded for inconsistency (true heterogeneity may be a source of funnel plot asymmetry).
eStudy limitations: substantial information is derived from studies at high or unclear risk of bias (selection bias, blinding, attrition bias). However, exclusion of high and unclear risk of bias studies did not affect the robustness of the estimated effect (95% CI: clinical non‐relevant range of effects).
fInconsistency: the 95% PI is wider than the 95% CI (we assume between‐study heterogeneity), but the range of effects lie in areas of clinical non‐relevance. Therefore, we did not downgrade for inconsistency.
gImprecision: we did not downgrade for imprecision since the 95% PI and the 95% CI around the effect size are narrow (precise result with no clinical relevance).
hPublication bias: test for publication bias suggested funnel plot asymmetry but trim and fill analysis did not change the conclusion (95% CI: clinical non‐relevant range of effects).
iDowngrading for study limitations and publication bias: we downgraded by one level for the combination of study limitations and funnel plot asymmetry, because of the uncertain risk of bias domains for over half of the studies and the evidence for publication bias shown by funnel plot asymmetry.
jThere are few trials investigating adverse events with a great heterogeneity in the investigated adverse events and with a lack of systematic assessment and reporting of adverse events which limits quality of evidence. Data of adverse events were not pooled in any meta‐analysis. Downgrading for inconsistency, imprecision, and study quality.
kClinical relevance is assumed if the minimally important difference on the 0 to 10 cm pain scale is approximately 1 cm.
Summary of findings 2. Intravenous (IV) lidocaine compared to thoracic epidural analgesia (TEA) in patients undergoing any elective or urgent surgical procedure under general anaesthesia.
| IV lidocaine compared to TEA in adult patients undergoing any elective or urgent surgical procedure under general anaesthesia | |||||||
| Patient or population: adult patients undergoing any elective or urgent surgical procedure under general anaesthesia Settings: USA and Canada (two trials) Intervention: IV lidocaine Comparison: TEA | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Prediction interval (95% PI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with placebo or no treatment | Corresponding risk with IV lidocaine | ||||||
| 1. Pain (VAS 0 to 10 cm, 0 to 100 mm, NRS 0 to 10 | Pain score at rest, 'early time points' (1 hto 4 hpostoperatively, or in the PACU) | ‐ | ‐ | (0 RCTs) | ‐ | No trial assessed this outcome. | |
| See comment | See comment | ||||||
|
Pain score at rest, 'intermediate time points' (24 hpostoperatively) (VAS 0 to 10 cm) |
‐ | Not estimable* | 102 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The estimated effect (95% CI) includes both benefit and harmg. | ||
| The mean pain score 'intermediate time points' ranged across control groups from 0 to 3.3 cm | The mean pain score 'intermediate time points' in the intervention group was 1.51 cm higher (0.29 lower to 3.32 higher) | ||||||
|
Pain score at rest 'late time points' (48 hpostoperatively) (VAS 0 to 10 cm) |
‐ | Not estimable* | 102 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The estimated effect (95% CI) includes both benefit and harmg. | ||
| The mean pain score 'late time points' ranged across control groups from 0 to 2.7 cm | The mean pain score 'late time points' in the intervention group was 0.98 cm higher (1.19 lower to 3.16 higher) | ||||||
| 2. Gastrointestinal recovery |
Postoperative ileus (dichotomous) The number of participants with postoperative ileus |
Not estimable | Not estimable* | 60 (1 RCT) |
⊕⊝⊝⊝ Very lowa,d | Only one small trial assessed this outcome. | |
| Two out of 30 participants in the control group and one out of 30 in the lidocaine group had postoperative ileus | |||||||
| Time to bowel movements (h) | ‐ | Not estimable* | 102 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,e | The estimated effect (95% CI) includes both benefit and harm. | ||
| The mean time to first bowel movements (h) ranged across control groups from 39 h to 72 h | The mean time to first bowel movements (h) in the intervention group was 1.66 h shorter (10.88 shorter to 7.56 longer) | ||||||
|
3. Adverse events (e.g. the number of participants that died, or had arrhythmias, other heart rate disorders, or showed any signs of lidocaine toxicity) |
See comment | See comment | ‐ | ‐ | See comment | ⊕⊝⊝⊝ Very lowf | All adverse events that are reported in the individual studies were listed in Table 14. |
| 4. Postoperative nausea, 'overall' (0 to 24 h, to 48 h, to 72 h) | 17 out of 30 participants in the control group and 11 out of 30 in the lidocaine group had nausea | Not estimable | Not estimable* | 60 (1 RCT) |
⊕⊝⊝⊝ Very lowa,d | Only one small trial assessed this outcome. | |
| 5. Postoperative opioid consumption, 'overall' (MEQ, mg) | See comment | See comment | ‐ | ‐ | (0 RCTs) | ‐ | No trial assessed this outcome. |
|
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MEQ: morphine equivalents; NRS: numeric rating scale; PACU: postanaesthesia care unit; RCT: randomized controlled trial; VAS: visual analogue scale | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
* The estimate of the PI is imprecise when based on only a few studies with small sample size (IntHout 2016). In this case, we did not provide the 95% PI.
aDowngrading for study limitations: substantial information is derived from studies at high or unclear risk of bias (selection bias, blinding, attrition bias). Exclusion of high and unclear risk of bias studies affected the robustness of the estimated effect.
bDowngrading for inconsistency: between‐study heterogeneity was high for this outcome.
cDowngrading for imprecision: we downgraded for imprecision due to the fact that the 95% CI around the effect size was large.
dDowngrading for imprecision: we double‐downgraded for imprecision since information is derived from only one small trial.
eDowngrading for imprecision: we double‐downgraded for imprecision since the 95% CI around the effect size was large, including benefit and harm. There is a high uncertainty associated with this effect estimate.
fThere is great heterogeneity in the investigated adverse events in the individual trials with a lack of systematic assessment and reporting of adverse events which limits quality of evidence.
gClinical relevance is assumed if the minimally important difference on the 0 to 10 cm pain scale is approximately 1 cm.
1. IV Lidocaine versus placebo
The first comparison analysed IV lidocaine versus placebo. For this comparison, we identified 66 trials (Ahn 2015; Baral 2010; Bryson 2010; Cassuto 1985; Chen 2015; Choi GJ 2016; Choi KW 2016; Choi SJ 2012; Cui 2010; Dale 2016; De Oliveira 2012; De Oliveira 2014; Dewinter 2016; El‐Tahan 2009; Farag 2013; Grady 2012; Grigoras 2012; Groudine 1998; Herroeder 2007; Insler 1995; Ismail 2008; Jain 2015; Kaba 2007; Kang 2011; Kasten 1986; Kim HJ 2014; Kim HO 2014; Kim KT 2014; Kim TH 2011; Kim TH 2013; Koppert 2004; Kuo 2006; Lauwick 2008; Lauwick 2009; Lee 2011; Maquoi 2016; Martin 2008; Mathew 2009; McKay 2009; Mitchell 1999; Mitchell 2009; Oliveira 2015; Omar 2013; Ortiz 2016; Peng 2016; Rimbäck 1990; Saadawy 2010; Samimi 2015; Slovack 2015; Soltani 2013; Sridhar 2015; Staikou 2014; Striebel 1992; Terkawi 2014; Tikuisis 2014; Wallin 1987; Wang 2002; Wang 2015; Weinberg 2016; Wu 2005; Wuethrich 2012; Xu 2017; Yang 2014; Yardeni 2009; Yon 2014; Zengin 2015).
Important note to the current update ‐ 'studies with suspected variance reporting'
All included studies of this review showed a small sample size and therefore, we expected large variances for some continuous outcomes such as pain, gastrointestinal recovery, and opioid consumption. As an example of the latter (Moore 2011), it was reported that the standard deviation (SD) of the mean opioid consumption often had the same size as the mean when the sample size of trials was small (20 to 30 patients per group). During the update of this review we noted that several studies reported very small variances for different continuous outcomes relevant to this review. We assumed that these small variances may have been derived from a misinterpretation of a standard error (SE) as a SD, since SDs and SEs are occasionally confused in the reports of studies, and the terminology is used inconsistently (Higgins 2011). Unfortunately, in other studies, it was not clear from the description what was actually reported.
Small variances result in larger standardized mean differences (SMDs) compared to large variances. Therefore, these studies with 'suspected (small) variance reporting' may lead to an overestimation of treatment effects and systematically introduce bias into the meta‐analyses.
We contacted the authors of all studies with suspected variance reporting of relevant outcomes to clarify the issue (Choi GJ 2016; Kuo 2006; Peng 2016; Saadawy 2010; Samimi 2015; Terkawi 2014; Tikuisis 2014; Weinberg 2016; Wu 2005; Xu 2017; Yang 2014; Zengin 2015). Unfortunately, only two authors responded to our requests (Weinberg 2016; Xu 2017), and only Weinberg 2016 solved the issue satisfactorily. In this case, the authors erroneously reported in the figure legend to pain and opioid consumption the use of mean and SD. Indeed, mean and 95% confidence intervals (CIs) were shown. We corrected the values and included this study in the analyses.
Finally, we decided to omit all studies with suspected variance reporting (and unsolved status) for the outcomes pain, gastrointestinal recovery, and postoperative opioid consumption from the relevant meta‐analyses. The 'omitted' studies with suspected variance reporting are listed under the relevant outcomes below in the 'Effects of interventions' section. Sensitivity analyses, including studies with suspected variance reporting were performed and reported in Table 11 to demonstrate the impact on effect estimates.
Primary outcomes
1. Postoperative pain
In total, 42 studies provided data on postoperative pain; we omitted eight due to suspected variance reporting. The remaining 34 trials that contributed to our meta‐analysis used different scores when reporting on postoperative pain. Thirteen studies asked the participants about pain on a visual analogue scale (VAS) from 0 to 10 cm (Bryson 2010; Choi SJ 2012; Herroeder 2007; Insler 1995; Kim HO 2014; Koppert 2004; McKay 2009; Omar 2013; Ortiz 2016; Saadawy 2010; Slovack 2015; Weinberg 2016; Yardeni 2009); in 12 studies a VAS from 0 to 100 mm was used (Ahn 2015; Cassuto 1985; Grigoras 2012; Kaba 2007; Kang 2011; Kim KT 2014; Kim TH 2011; Kim TH 2013; Maquoi 2016; Martin 2008; Striebel 1992; Yon 2014); and in nine studies the trialists used a numeric rating scale (NRS) from 0 to 10 (Choi KW 2016; Dewinter 2016; Farag 2013; Grady 2012; Lauwick 2008; Oliveira 2015; Staikou 2014; Terkawi 2014; Wuethrich 2012).
Pain score at rest, 'early time points' (1 hour to 4 hours postoperatively, or in the PACU)
Thirty‐seven trials reported pain score data at early time points postoperatively (1 to 4 hours, or in the PACU) (Ahn 2015; Bryson 2010; Cassuto 1985; Choi GJ 2016; Choi KW 2016; Dewinter 2016; Farag 2013; Grady 2012; Grigoras 2012; Herroeder 2007; Insler 1995; Kaba 2007; Kang 2011; Kim KT 2014; Kim TH 2011; Kim TH 2013; Kuo 2006; Lauwick 2008; Maquoi 2016; McKay 2009; Omar 2013; Ortiz 2016; Saadawy 2010; Samimi 2015; Slovack 2015; Staikou 2014; Striebel 1992; Terkawi 2014; Tikuisis 2014; Weinberg 2016; Wu 2005; Wuethrich 2012; Xu 2017; Yang 2014; Yardeni 2009; Yon 2014; Zengin 2015); we omitted eight of these 37 trials due to suspected variance reporting (Choi GJ 2016; Kuo 2006; Samimi 2015; Tikuisis 2014; Wu 2005; Xu 2017; Yang 2014; Zengin 2015). In the remaining 29 trials, involving 1656 participants (37% of the total participants included in this review), 829 participants received the intervention and 827 participants received a placebo treatment. The meta‐analysis of the early pain score data showed reduced pain ratings in the lidocaine group compared to the control group (SMD −0.50, 95% confidence interval (CI) −0.72 to −0.28; I2 = 79%; 29 studies, 1656 participants; Analysis 1.1). A SMD of 0.50 fewer in the average pain score of the intervention group is equivalent to an average pain reduction (mean difference (MD)) in the order of 0.37 cm to 2.48 cm on a VAS 0 to 10 cm scale, depending on the variance of the study. However, the 95% prediction intervals (PIs) included both appreciable benefit and harm (95% PI −1.61 to 0.62; Table 13).
1.1. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 1 Pain score at rest, 'early time points' (1 h to 4 h, PACU).
11. Heterogeneity/prediction intervals/publication bias.
| Outcome | Statistical method | Studies | Effect estimate (95% CI) | Prediction interval (95% PI) | I2 | Adjusted effect estimate (trim and fill), (number of added studies) |
| Pain score, rest, 'early time points' (1 hr to 4 hrs, PACU) | SMD (IV, Random) | 29 | −0.50 (−0.72 to −0.28) | (−1.61 to 0.62) | 79% | −0.26 (−0.52 to −0.004), (6)* |
| Pain score, rest, 'intermediate time points' (24 hrs) | SMD (IV, Random) | 33 | −0.14 (−0.25 to −0.04) | (−0.44 to 0.16) | 20% | 0.007 (−0.12 to 0.13), (11)* |
| Pain score, rest, 'late time points' 48 hrs) | SMD (IV, Random) | 24 | −0.11 (−0.25 to 0.04) | (−0.60 to 0.38) | 42% | −0.015 (−0.17 to 0.14), (4)* |
| Time to first defaecation/bowel movement (hrs) | MD (IV, Random) | 12 | −7.92 (−12.71 to −3.13) | (−22.19 to 6.36) | 62% | −4.06 (−9.07 to 0.95), (4) |
| Time to first flatus (hrs) | MD (IV, Random) | 13 | −4.09 (−6.30 to −1.87) | (−10.431 to 2.26) | 63% | −3.63(−5.88 to −1.37), (1) |
| Length of hospital stay (days) | MD (IV, Random) | 32 | −0.37 (−0.60 to −0.15) | (−1.26 to 0.52) | 69% | −0.19 (−0.42 to −0.04), (8)* |
| Postoperative nausea, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random) | 35 | 0.78 (0.67 to 0.91) | (0.49 to 1.23) | 22% | 0.87 (0.74 to 1.03), (9)* |
| Postoperative vomiting, overall (0 to 24 hrs, to 48 hrs, to 72 hrs) | RR (MH, Random) | 19 | 0.83 (0.63 to 1.08) | (0.62 to 1.10) | 0% | 0.89 (0.69 to 1.15), (3) |
| Intraoperative opioid consumption (MEQ, mg) | MD (IV, Random) | 18 | −2.14 (−3.87 to −0.40) | (−8.13 to 3.86) | 80% | −2.10 (−3.83 to −0.38), (1) |
| Postoperative opioid consumption, PACU (MEQ, mg) | MD (IV, Random) | 21 | −3.10 (−3.87 to −2.32) | (−5.43 to −0.77) | 40% | −2.91 (−3.72 to −2.11), (2) |
| Postoperative opioid consumption, overall (MEQ, mg) | MD (IV, Random) | 40 | −4.52 (−6.25 to −2.79) | (−12.03 to 3.00) | 73% | −1.09 (−2.97 to 0.79), (16)* |
Acronyms and abbreviations used in this table:
CI = confidence interval, hr = hour, IV = inverse variance, MD = mean difference, MEQ =morphine equivalent dose, MH = Mantel Haenszel, PACU = postanaesthesia care unit, PI = prediction interval, RR = risk ratio, SMD = standardized mean difference
We analysed all studies with 10 or more studies for funnel plot asymmetry. The asterisk (*) indicates that we rejected the null hypothesis of funnel plot asymmetry (P < 0.1).
In consideration of the high statistical heterogeneity (I² = 79%), we performed preplanned subgroup analyses according to the type of surgery (open abdominal, laparoscopic abdominal, other surgery) and the lidocaine infusion dose (infusion dose < 2 mg/kg/h and ≥ 2 mg/kg/h) used in the individual trials. Heterogeneity was not reduced below an I2 of 50% in any of the subgroups and the tests for subgroup difference did not reach statistical significance (Table 5; Table 6). However, the different tau2s of the surgical subgroups might have contributed to the failure to identify surgical procedures as having different effect estimates (P = 0.017; Table 7).
Exclusion of one outlier study (Saadawy 2010), reduced the I2 from 79% to 61% and the estimated effect to a SMD of −0.39 with a 95% CI reaching from −0.56 to −0.23.
Six trials reported pain scores as median with interquartile range (IQR) (Choi KW 2016; Lauwick 2008; Maquoi 2016; Omar 2013; Striebel 1992; Wuethrich 2012). A sensitivity analysis excluding all trials reporting data as median did not affect the overall result of the estimated effect (Table 4).
For this outcome, we classified 23, eight, and 12 trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effect (95% CI) of the sensitivity meta‐analysis on selection bias included the line of no effect (Table 8), however, the estimated effects (95% CI) for the sensitivity analyses on blinding and attrition bias remained robust (Table 3; Table 9).
The contour‐enhanced funnel plot and the linear regression test suggested funnel plot asymmetry and the trim and fill sensitivity analysis (with k = 6 studies added) changed the conclusion (the lower 95% CI boundary reached the line of no effect; Table 13).
We graded the quality of evidence for the outcome, 'pain score at rest (early time points)' as very low (we downgraded for study limitations, inconsistency and imprecision); we did not downgrade for publication bias since we had already downgraded for inconsistency (true heterogeneity may be a source of funnel plot asymmetry; Table 1).
Pain score at rest, 'intermediate time points' (24 hours postoperatively)
Forty‐one trials reported pain score data at intermediate time points postoperatively (24 hours) (Ahn 2015; Bryson 2010; Cassuto 1985; Choi GJ 2016; Choi KW 2016; Choi SJ 2012; Dewinter 2016; Farag 2013; Grady 2012; Grigoras 2012; Herroeder 2007; Insler 1995; Kaba 2007; Kang 2011; Kim HO 2014; Kim KT 2014; Kim TH 2011; Kim TH 2013; Koppert 2004; Kuo 2006; Lauwick 2008; Maquoi 2016; Martin 2008; McKay 2009; Oliveira 2015; Ortiz 2016; Saadawy 2010; Samimi 2015; Slovack 2015; Staikou 2014; Striebel 1992; Terkawi 2014; Tikuisis 2014; Weinberg 2016; Wu 2005; Wuethrich 2012; Xu 2017; Yang 2014; Yardeni 2009; Yon 2014; Zengin 2015); we omitted eight of these 41 trials due to suspected variance reporting (Choi GJ 2016; Kuo 2006; Samimi 2015; Tikuisis 2014; Wu 2005; Xu 2017; Yang 2014; Zengin 2015). In the remaining 33 trials, involving 1847 participants (41% of the total participants included in this review), 921 participants received the intervention and 926 participants received a placebo treatment. Meta‐analysis revealed reduced pain ratings in the lidocaine group compared to the control group (SMD −0.14, 95% CI −0.25 to −0.04; I2 = 20%; 33 studies, 1847 participants; Analysis 1.2), however, the effect lacks clinical relevance. A SMD of 0.14 fewer in the average pain score of the intervention group is equivalent to an average pain reduction (MD) in the order of 0.48 cm to 0.10 cm on a VAS 0 to 10 cm scale depending on the variance of the study. The 95% PI crossed the line of identity and the range of true mean effects mostly remained in areas of clinical non‐relevance (95% PI −0.44 to 0.16; Table 13).
1.2. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 2 Pain score at rest, 'intermediate time points' (24 h).
Since we considered statistical heterogeneity of the meta‐analysis as not important (I2 = 20%), we did not perform any subgroup analyses.
Six trials reported pain scores as median with IQR (Choi KW 2016; Koppert 2004; Lauwick 2008; Maquoi 2016; Striebel 1992; Wuethrich 2012). However, a sensitivity analysis excluding all trials reporting data as median did not affect the overall estimated effect on pain score at intermediate postoperative time points (Table 4).
For this outcome, we classified 27, 11, and 15 trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) for the sensitivity meta‐analyses on selection and attrition bias included the line of no effect (Table 3; Table 8). However, the estimated effect (95% CI) for the sensitivity analysis on blinding remained robust (Table 9). The 95% CIs of all sensitivity analyses remained in areas of clinical non‐relevance.
The contour‐enhanced funnel plot and the linear regression test suggested funnel plot asymmetry but trim and fill sensitivity analysis (with k = 11 studies added) did not change the conclusion (the 95% CI crossed the line of no effect, but remained in areas of clinical non‐relevance; Table 13).
We graded the quality of evidence for the outcome, 'pain score at rest (intermediate time points)' as moderate (we combined the downgrade for study limitations and publication bias by one level); the 95% CIs (main meta‐analysis and sensitivity analyses) and the 95% PI were narrow and the range of true mean effects remained in areas of clinical non‐relevance, therefore, we did not downgrade for inconsistency and imprecision (Table 1).
Pain score at rest, 'late time points' (48 hours)
Thirty trials reported pain score data at late time points postoperatively (48 hours) (Ahn 2015; Bryson 2010; Choi GJ 2016; Choi KW 2016; Choi SJ 2012; Farag 2013; Grady 2012; Grigoras 2012; Herroeder 2007; Insler 1995; Kaba 2007; Kang 2011; Kim HO 2014; Kim KT 2014; Kim TH 2011; Kim TH 2013; Koppert 2004; Kuo 2006; Maquoi 2016; Martin 2008; Slovack 2015; Staikou 2014; Terkawi 2014; Wu 2005; Wuethrich 2012; Xu 2017; Yang 2014; Yardeni 2009; Yon 2014; Zengin 2015); we omitted six of these 30 trials due to suspected variance reporting (Choi GJ 2016; Kuo 2006; Wu 2005; Xu 2017; Yang 2014; Zengin 2015). In the remaining 24 trials, involving 1404 participants (31% of the total participants included in this review), 697 participants received the intervention and 707 participants received a placebo treatment. The meta‐analysis revealed no difference between the lidocaine and the control group with respect to pain scores at late time points (SMD −0.11, 95% CI −0.25 to 0.04; I2 = 42%; 24 studies, 1404 participants; Analysis 1.3). A SMD of 0.11 fewer in the average pain score of the intervention group is equivalent to an average pain reduction (MD) in the order of 0.42 cm to 0.08 cm on a VAS 0 to 10 cm scale, depending on the variance of the study. The 95% PI was larger than the 95% CI but the range of true mean effects mostly remained in areas of clinical non‐relevance (95% PI −0.60 to 0.38; Table 13).
1.3. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 3 Pain score at rest, 'late time points' (48 h).
The preplanned subgroup analyses on type of surgery and lidocaine regimen (dose and timing) did not explain heterogeneity for all subgroups and tests for subgroup differences did not reach statistical significance (Table 5; Table 6). However, the different tau2s of the surgical subgroups might have contributed to the failure to identify surgical procedures as having different effect estimates (P = 0.049; Table 7). None of the estimated effects of the different subgroups were of clinical relevance.
Four trials reported pain scores as median with IQR (Choi KW 2016; Koppert 2004; Maquoi 2016; Wuethrich 2012). A sensitivity analysis excluding all trials reporting data as median did not affect the overall result for the estimated effect on pain score at late postoperative time points (Table 4).
For this outcome, we classified 21, five, and 13 trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The 95% CIs of all sensitivity analyses remained in areas of clinical non‐relevance (Table 3; Table 8; Table 9).
The contour‐enhanced funnel plot and the linear regression test suggested funnel plot asymmetry but trim and fill sensitivity analysis (with k = 4 studies added) did not change the conclusion (the 95% CI remained in areas of clinical non‐relevance; Table 13).
We graded the quality of evidence for the outcome, 'pain score at rest (late time points)' as moderate (we combined the downgrade for study limitations and publication bias by one level); the 95% CIs (main meta‐analysis and sensitivity analyses) and the 95% PI were narrow and the range of true mean effects remained in areas of clinical non‐relevance, therefore, we did not downgrade for inconsistency and imprecision (Table 1).
2. Gastrointestinal recovery
Postoperative ileus (dichotomous)
Four trials, with a total of 273 participants (6% of the total participants included in the review), reported the incidence of postoperative ileus (Farag 2013; Herroeder 2007; Kim HO 2014; Tikuisis 2014). The intervention group consisted of 136 participants and 137 participants received placebo treatment. Postoperative ileus occurred in 4.4% of participants in the lidocaine group and in 13.1% of participants in the control group. Lidocaine reduced the risk for postoperative ileus when compared to placebo (risk ratio (RR) 0.37, 95% CI 0.15 to 0.87; I2 = 0%; 4 studies, 273 participants; Analysis 1.4). However, the 95% PI included both appreciable benefit and harm (95% PI 0.05 to 2.43).
1.4. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 4 Postoperative ileus (dichotomous).
For this outcome, we classified all trials as unclear risk of selection bias, two trials as unclear risk of blinding, and none of the trials as unclear risk for attrition bias (Figure 2); we excluded these trials in the sensitivity meta‐analyses. The estimated effect (95% CI) for the sensitivity meta‐analysis on blinding included the line of no effect (Table 9).
We graded the quality of evidence for the outcome, 'postoperative ileus' as very low (downgraded for study limitations, inconsistency, and imprecision; Table 1).
Time to first defaecation/bowel movement (hours)
Fourteen trials reported data on time to first defaecation or bowel movement in hours postoperatively (Choi SJ 2012; Groudine 1998; Herroeder 2007; Kaba 2007; Kim HO 2014; Kim TH 2011; Koppert 2004; Lauwick 2009; Maquoi 2016; Rimbäck 1990; Sridhar 2015; Tikuisis 2014; Wuethrich 2012; Zengin 2015); we omitted two of these 14 trials due to suspected variance reporting (Tikuisis 2014; Zengin 2015). In the remaining 12 trials, involving 684 participants (15% of the total participants included in this review), 340 participants received the intervention and 344 participants received a placebo treatment. The meta‐analysis revealed that lidocaine reduced the time (hours) to first defaecation/bowel movement compared to control with moderate heterogeneity (MD −7.92, 95% CI −12.71 to −3.13; I2 = 62%; 12 studies, 684 participants; Analysis 1.5). The 95% PI crossed the line of identity and the range of true mean effects ranged from benefit to areas of clinical non‐relevance (95% PI −22.19 to 6.36; Table 13).
1.5. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 5 Time to first defaecation/bowel movement (h).
The preplanned subgroup analyses on type of surgery and lidocaine regimen (dose and timing) did not explain heterogeneity for all subgroups and tests for subgroup differences did not reach statistical significance (Table 5; Table 6).
Five trials reported this outcome as median with IQR (Kaba 2007; Kim HO 2014; Kim TH 2011; Koppert 2004; Maquoi 2016). The estimated effect remained robust in a sensitivity analysis when we excluded these trials (Table 4).
For this outcome, we classified 10, seven, and four trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effect (95% CI) for the sensitivity meta‐analysis on blinding included the line of no effect (Table 9), however, the estimated effects (95% CI) for the sensitivity analyses on selection and attrition bias remained robust (Table 3; Table 8).
The contour‐enhanced funnel plot and the linear regression test did not suggest funnel plot asymmetry but trim and fill sensitivity analysis (with k = 3 studies added) changed the conclusion (95% CI crossed the line of no effect; Table 13).
We graded the quality of evidence for the outcome, 'time to defaecation/bowel movement' as very low (downgraded for study limitations, inconsistency, and imprecision; Table 1).
Time to first flatus (hours)
Sixteen trials reported this outcome in hours postoperatively (Choi SJ 2012; Groudine 1998; Herroeder 2007; Kaba 2007; Kang 2011; Kim HO 2014; Kuo 2006; Lauwick 2009; Maquoi 2016; Rimbäck 1990; Saadawy 2010; Sridhar 2015; Staikou 2014; Wu 2005; Wuethrich 2012; Xu 2017); we omitted three of these 16 trials due to suspected variance reporting (Kuo 2006; Wu 2005; Xu 2017). In the remaining 13 trials, involving 785 participants (17% of the total participants included in this review), 390 participants received the intervention and 395 participants received a placebo treatment. The meta‐analysis revealed that the lidocaine infusion shortened the time to first flatus with substantial heterogeneity (MD −4.09, 95% CI −6.30 to −1.87; I2 = 63%; 13 studies, 785 participants; Analysis 1.6). The 95% PI crossed the line of identity and the range of true mean effects ranged from benefit to areas of clinical non‐relevance (95% PI −10.43 to 2.26; Table 13).
1.6. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 6 Time to first flatus (h).
The preplanned subgroup analyses on type of surgery and lidocaine regimen (dose and timing) did not explain heterogeneity for all subgroups and tests for subgroup differences did not reach statistical significance (Table 5; Table 6).
Three trials reported this outcome as median with IQR (Kaba 2007; Kang 2011; Kim HO 2014); and a sensitivity analysis excluding all trials reporting data as median did not affect the overall result for the estimated effect on time to first flatus (Table 4).
For this outcome, we classified 11, seven, and four trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all three domains remained robust (Table 3; Table 8; Table 9).
The contour‐enhanced funnel plot and the linear regression test did not suggest funnel plot asymmetry and trim and fill sensitivity analysis (with k = 1 studies added) did not change the conclusion (Table 13).
Time to first bowel sounds (hours)
Four trials reported this outcome as time to first bowel sounds in days or hours after surgery (Herroeder 2007; Xu 2017; Yang 2014; Zengin 2015); we omitted two of these four trials due to suspected variance reporting (Xu 2017; Zengin 2015). In the remaining two trials, involving 110 participants (2% of the total participants included in this review), 57 participants received the intervention and 53 participants received a placebo treatment. The pooled meta‐analysis of these two trials revealed no significant effect for lidocaine to shorten the time to first bowel sounds with substantial heterogeneity (MD −6.08, 95% CI −13.77 to 1.60; I2 = 57%; 2 studies, 110 participants; Analysis 1.7).
1.7. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 7 Time to first bowel sounds (h).
All data were presented as mean ± SD.
For this outcome, we classified two, one, and no trials as unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all the domains remained robust (Table 3; Table 8; Table 9).
3. Adverse events
A detailed description of all adverse events/side effects reported in the included trials is listed in Table 14.
12. Adverse events.
| Study ID | Type of adverse event/side effect ‐ lidocaine group | Type of adverse event/side effect ‐ control group | No adverse events/side effects detectable (statement) |
| Ahn 2015 | NA | NA | “Not one patient had a postoperative complication related to lidocaine infusion.” |
| Baral 2010 | Light headache (3), cardiac arrhythmias (0), perioral numbness (0), hypotension (0) | Cardiac arrhythmias (0), perioral numbness (0), hypotension (0) | NA |
| Bryson 2010 | Light‐headedness, tinnitus, dysgeusia, etc. (11) | Light‐headedness, tinnitus, dysgeusia, etc. (21) | NA |
| Cassuto 1985 | Light‐headedness (1) | Light‐headedness (1) | "No adverse reactions to lidocaine were observed." |
| Chen 2015 | NA | NA | NA |
| Choi SJ 2012 | Dizziness 24 hrs/ 48 hrs/ 72 hrs (1/1/1), itching 24 hrs/ 48 hrs/ 72 hrs (8/3/0), respiratory repression 24 hrs/ 48 hrs/ 72 hrs (0/0/0) |
Dizziness 24 hrs/ 48 hrs/ 72 hrs (3/2/1), itching 24 hrs/ 48 hrs/ 72 hrs (6/2/1), respiratory repression 24 hrs/ 48 hrs/ 72 hrs (0/0/0) |
NA |
| Choi GJ 2016 | NA | NA | “There were no adverse events related to the investigational procedure or systemic administration of lidocaine such as arrhythmia, perioral numbness, visual disturbance, metal taste, or light‐headedness.” |
| Choi KW 2016 | NA | NA | "In addition, none of the patients showed symptoms or signs associated with lidocaine toxicity during the perioperative period." |
| Cui 2010 | Drowsiness (0), metal taste (0), perioral numbness (0), visual disturbances (0) | Drowsiness (0), metal taste (0), perioral numbness (0), visual disturbances (0) | "No patient reported any side effect of lidocaine toxicity." |
| Dale 2016 | Severe bradycardia (1), perioral paraesthesia (1), restless legs (1) | Severe bradycardia (0), perioral paraesthesia (0), restless legs (0) | NA |
| De Oliveira 2012 | NA | NA | "We did not observe any potential cardiovascular or neurological side effects associated with the infusion of systemic lidocaine in our investigation." |
| De Oliveira 2014 | NA | NA | "We did not observe any potential cardiovascular or neurological side effects associated with the infusion of systemic lidocaine in our investigation." |
| Dewinter 2016 | NA | NA | “Both groups did not differ with respect to the incidence of other AEs. No patient receiving lidocaine reported subjective symptoms of local anesthetic systemic toxicity.” |
| El‐Tahan 2009 | Perioperative arrhythmia (0), light‐headedness (0), headache (0), perioral numbness (0), tunnel vision (0), seizures (0) | Perioperative arrhythmia (0), light‐headedness (0), headache (0), perioral numbness (0), tunnel vision (0), seizures (0) | "There were no reported serious side effects during the study." |
| Farag 2013 | Pneumonia (0), respiratory failure (0), cardiac arrest (0), arrhythmia (0), heart failure (0), stroke (0), intravascular coagulopathy (0), thromboembolism (0), delirium (0), monoplegia (0), upper gastrointestinal bleeding (0), sepsis (0), readmission (2) | Pneumonia (0), respiratory failure (0), cardiac arrest (0), arrhythmia (0), heart failure (0), stroke (0), intravascular coagulopathy (0), thromboembolism (0), delirium (0), monoplegia (1), upper gastrointestinal bleeding (0), sepsis (0), readmission (3) | NA |
| Grady 2012 | NA | NA | NA |
| Grigoras 2012 | NA | NA | "No side effects related to lidocaine were observed." |
| Groudine 1998 | NA | NA | "No patient experienced identifiable adverse events related to the lidocaine infusion." |
| Herroeder 2007 | NA | NA | NA |
| Insler 1995 | Death (1), myocardial infarction (0) | Death (1), myocardial infarction (0) | NA |
| Ismail 2008 | NA | NA | NA |
| Jain 2015 | Drowsiness (3) | Drowsiness (0) | “None of the patients complained of lignocaine‐related side effects such as perioral numbness or metallic taste. The incidence of light‐headedness and nausea was comparable in both the groups. Three patients in Group B demonstrated drowsiness in the postoperative period lasting between 10 and 17 mins.” |
| Kaba 2007 | NA | NA | NA |
| Kang 2011 | NA | NA | NA |
| Kasten 1986 | NA | NA | NA |
| Kim TH 2011 | NA | NA | "There was no adverse effect from intravenous lidocaine throughout the study." |
| Kim TH 2013 | NA | NA | "In our study, no neuropsychiatric events were observed throughout the process." |
| Kim HJ 2014 | NA | NA | “No specific complication or side effect regarding lidocaine or dexmedetomidine was reported.” |
| Kim HO 2014 | Hospital mortality (0) | Hospital mortality (0) | “There were no significant lidocaine‐related adverse events during our trial.” |
| Kim KT 2014 | NA | NA | “There were no side effects from the lidocaine, such as arrhythmia, hypotension, and hypersensitivity.” |
| Koppert 2004 | NA | NA | "No anaesthesiologist noted adverse events related to the lidocaine infusion during surgery. Furthermore, no patient after having regained consciousness complained of lidocaine‐related side effects such as perioral numbness or metallic taste. The incidences of drowsiness, light‐headedness, and nausea were comparable in the lidocaine and control groups." |
| Kuo 2006 | Bradycardia (3) | Bradycardia (0) | "No patient experienced an identifiable adverse event related to IV lidocaine infusion." |
| Lauwick 2008 | NA | NA | NA |
| Lauwick 2009 | Bleeding (1), sepsis (1), chest infection (1) | Bladder leakage (1) | NA |
| Lee 2011 | Atrial fibrillation (9), other arrhythmia (7), myocardial infarction (0), death (0) | Atrial fibrillation (5), other arrhythmia (10), myocardial infarction (0), death (0) | "All patients started on lidocaine completed their full course of drug and did not experience any adverse events related to the local anaesthetic, such as severe bradycardia (< 40 beats min‐1), asystole, or neurological symptoms." |
| Maquoi 2016 | NA | NA | NA |
| Martin 2008 | NA | NA | "No patient reported lidocaine toxicity side effects and no adverse events were reported in both groups" |
| Mathew 2009 | Serious adverse events (12.3%), no detailed description | Serious adverse events (10.2%), no detailed description | "Adverse events were not significantly different between treatment groups." |
| McKay 2009 | Dizziness and visual disturbances (1) | NA | "There were no serious adverse events recorded." |
| Mitchell 1999 | Death (1) | Death (1) | NA |
| Mitchell 2009 | Death due to multiorgan failure (3) and acute graft occlusion (1) | Death (0) | NA |
| Oliveira 2015 | NA | NA | NA |
| Omar 2013 | Hypotension (0) | Hypotension (0) | NA |
| Ortiz 2016 | NA | NA | "There was no arrhythmia or adverse effect occurrence." |
| Peng 2016 | Hypertension (3), coronary heart disease (0) | Hypertension (4), coronary heart disease (0) | “There were no seizures or other symptoms of potential lidocaine toxicity found in patients who received lidocaine infusion. There was no significant difference in the number of cases complicated by hypertension, tachycardia, dysphoria, or PONV between the normal saline group and the lidocaine group.” |
| Rimbäck 1990 | Sedation (2) | NA | "No adverse reactions to lidocaine were reported." |
| Saadawy 2010 | NA | NA | NA |
| Samimi 2015 | NA | NA | "...also none of the patients experienced lidocaine‐related adverse effects." |
| Slovack 2015 | Confusion (1), sedation (2), light‐headedness/dizziness (0), blurred vision (0), hypotension (0), respiratory depression (0), pruritus (0) | Confusion (0), sedation (0), light‐headedness/dizziness (0), blurred vision (1), hypotension (1), respiratory depression (0), pruritus (0) | NA |
| Soltani 2013 | NA | NA | NA |
| Sridhar 2015 | NA | NA | NA |
| Staikou 2014 | Transient confusion in PACU (1), bradycardia requiring treatment (0) | Transient confusion in PACU (0), bradycardia requiring treatment (0) | NA |
| Striebel 1992 | NA | NA | No signs of urticaria, dermatitis, asthma bronchiale, anaphylactic shock, restlessness, anxiety, lalopathy, tinnitus, metallic taste, dizziness, visual disturbance, and tremor. |
| Swenson 2010 | Wound infection (0), anaemia (1), anxiety (1), supraventricular tachycardia (3), back pain (0), bradycardia (0), confusion (2), decreased oxygen saturation level (1), dizziness/light‐headedness (1), fever (1), hyperglycaemia (3), hypertension (3), itching (3), lower extremity numbness (1), intravascular device infection (0), syncope (0), arrhythmia severe (1), confusion severe (1), facial numbness severe (1), shortness of breath (1) | Wound infection (1), anaemia (1), anxiety (0), supraventricular tachycardia (1), back pain (1), bradycardia (1), confusion (0), decreased oxygen saturation level (0), dizziness/light‐headedness (1), fever (1), hyperglycaemia (0), hypertension (0), itching (3), lower extremity numbness (6), intravascular device infection (1), syncope (1), arrhythmia severe (1), confusion severe (0), facial numbness severe (0), shortness of breath (0) | NA |
| Terkawi 2014 | NA | NA | "...no toxicity cases were reported in our cohort...." |
| Tikuisis 2014 | Light‐headedness (0), perioral numbness (0), metallic taste (0), dizziness (0), and visual disturbances (0) | NA | "Lidocaine‐associated haemodynamic changes such as severe hypotension, bradycardia, and arrhythmia were not observed in any lidocaine group patient during surgery." |
| Wallin 1987 | Drowsiness (2) | NA | "Aside from drowsiness in two patients of the lidocaine group, no side effects due to possible lidocaine overdosage were reported." |
| Wang 2002 | Death (2) | Death (4) | NA |
| Wang 2015 | NA | NA | NA |
| Weinberg 2016 | Pruritus (6), dizziness (14), visual disturbances (4), perioral numbness (2), muscle weakness (1), constipation (4) | Pruritus (9), dizziness (20), visual disturbances (6), perioral numbness (2), muscle weakness (3), constipation (10) | NA |
| Wongyingsinn 2011 | NA | NA | "No patients showed signs of lidocaine toxicity in the postoperative period." |
| Wu 2005 | NA | NA | "No patient experienced an identifiable adverse event related to the lidocaine infusion, except that an occasional arrhythmia with stable vital signs was noted in one patient in both groups." |
| Wuethrich 2012 | Light‐headedness (0), drowsiness (0), perioral numbness (0), visual disturbances (0), metal taste (0), pathological cardiac rhythm disturbances (0), and seizures (0) | NA | "No postoperative complications and no adverse events related to systemic administration of lidocaine were observed." |
| Xu 2017 | NA | NA | NA |
| Yang 2014 | Blurred vision (0), hearing problems (0), peripheral paraesthesia (0), dizziness (0), uncontrolled muscle contraction (0), convulsions (0), hypotension (0), bradycardia (0), headache (0), and itching (0) | NA | NA |
| Yardeni 2009 | NA | NA | NA |
| Yon 2014 | Shivering (0), tinnitus (0) | Shivering (1), tinnitus (0) | NA |
| Zengin 2015 | Pruritus (1) | Pruritus (4) | NA |
Acronyms and abbreviations used in this table:
AE = adverse events, IV = intravenous, NA = not available, PACU = postanaesthesia care unit, PONV = postoperative nausea and vomiting
Fifty studies gave a statement on adverse events. Of these 50, 23 trials reported there were no significant adverse events during the study (Ahn 2015; Choi GJ 2016; Choi KW 2016; Cui 2010; De Oliveira 2012; De Oliveira 2014; El‐Tahan 2009; Grigoras 2012; Groudine 1998; Kim HJ 2014; Kim HO 2014; Kim KT 2014; Kim TH 2011; Kim TH 2013; Martin 2008; Omar 2013; Ortiz 2016; Samimi 2015; Striebel 1992; Terkawi 2014; Tikuisis 2014; Wuethrich 2012; Yang 2014). The other 27 trials (Baral 2010; Bryson 2010; Cassuto 1985; Choi SJ 2012; Dale 2016; Dewinter 2016; Farag 2013; Insler 1995; Jain 2015; Koppert 2004; Kuo 2006; Lauwick 2009; Lee 2011; Mathew 2009; McKay 2009; Mitchell 1999; Mitchell 2009; Peng 2016; Rimbäck 1990; Slovack 2015; Staikou 2014; Wallin 1987; Wang 2002; Weinberg 2016; Wu 2005; Yon 2014; Zengin 2015), reported the occurrence of adverse events, e.g. light‐headedness (Bryson 2010; Cassuto 1985), arrhythmia (Lee 2011; Wu 2005), or perioral numbness (Weinberg 2016).
Four trials, including participants undergoing cardiac surgeries, reported that participants died during the study period (Insler 1995; Mitchell 1999; Mitchell 2009; Wang 2002). Of those, Mitchell 2009 reported that four participants died within the lidocaine group, whereas no participant died within the control group. The trial authors claimed that no participant died during the lidocaine infusion and none of these events could be plausibly linked to lidocaine administration. Two trials reported hospital mortality (Kim HJ 2014), and death (Lee 2011), but nil participants died in both groups.
The 50 trials which gave a statement on adverse events showed a great variance in their data presentation, e.g. from a short conclusion (e.g. Martin 2008), to a detailed summary table with statement of numbers of adverse events (e.g. El‐Tahan 2009; Farag 2013). We did not perform a meta‐analysis due to the great heterogeneity of the presented data on adverse events.
The remaining 16 trials did not comment on the occurrence of adverse events or lidocaine‐related side effects (Chen 2015; Grady 2012; Herroeder 2007; Ismail 2008; Kaba 2007; Kang 2011; Kasten 1986; Lauwick 2008; Maquoi 2016; Oliveira 2015; Saadawy 2010; Soltani 2013; Sridhar 2015; Wang 2015; Xu 2017; Yardeni 2009).
We graded the quality of evidence for the outcome 'adverse events' as very low (lack of systematic assessment and reporting of adverse events in the individual studies; Table 1).
Secondary outcomes
1. Length of hospital stay (days)
Length of hospital stay (days) ‐ inpatient
The outcome, 'length of hospital stay in hours or days after inpatient surgery' was reported by 32 studies (Ahn 2015; Chen 2015; Choi KW 2016; Choi SJ 2012; Dale 2016; De Oliveira 2014; Farag 2013; Groudine 1998; Herroeder 2007; Insler 1995; Kaba 2007; Kang 2011; Kim HJ 2014; Kim HO 2014; Kim KT 2014; Kim TH 2011; Kim TH 2013; Koppert 2004; Kuo 2006; Lauwick 2009; Martin 2008; Mathew 2009; Mitchell 1999; Mitchell 2009; Terkawi 2014; Tikuisis 2014; Wang 2002; Weinberg 2016; Wuethrich 2012; Yang 2014; Yon 2014; Zengin 2015), including 2077 participants (46% of the total participants included in the review). From these, 1032 participants received the intervention and 1045 served as a control. The combined meta‐analysis revealed that lidocaine shortened the time of hospital stay (days) compared to the control intervention and substantial heterogeneity was noted (MD −0.37, 95% CI −0.60 to −0.15; I2 = 69%; 32 studies, 2077 participants; Analysis 1.8). The 95% PI crossed the line of identity and the range of true mean effects ranged from benefit to harm (95% PI −1.26 to 0.52; Table 13).
1.8. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 8 Length of hospital stay (days).
The preplanned subgroup analyses on type of surgery and lidocaine regimen (dose and timing) did not explain heterogeneity for all subgroups and tests for subgroup differences did not reach statistical significance (Table 5; Table 6).
Altogether, 16 trials reported length of hospital stay as median with IQR (Ahn 2015; Choi KW 2016; De Oliveira 2014; Farag 2013; Herroeder 2007; Kaba 2007; Kang 2011; Kim HJ 2014; Kim HO 2014; Kim KT 2014; Kim TH 2011; Mathew 2009; Mitchell 1999; Terkawi 2014; Wuethrich 2012; Yon 2014). A sensitivity meta‐analysis, excluding all trials reporting data as median, did not affect the overall result for the estimated effect on length of hospital stay (Table 4).
For this outcome, we classified 29, 13, and 15 trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) for the sensitivity meta‐analyses on selection and attrition bias included the line of no effect (Table 3; Table 8), however, the estimated effect (95% CI) for the sensitivity analysis on blinding remained robust (Table 9).
The contour‐enhanced funnel plot and the linear regression test suggested funnel plot asymmetry but trim and fill sensitivity analysis (with k = 8 studies added) did not change the conclusion (Table 13).
Length of hospital stay (minutes) ‐ outpatient
Three trials reported length of hospital stay in minutes for participants undergoing outpatient surgery (De Oliveira 2012; Dewinter 2016; Lauwick 2008), including 191 participants (4% of the total participants included in the review). Of these trials, 95 participants received the intervention and 96 served as a control. The combined meta‐analysis revealed no difference between lidocaine and control treatment in terms of shortening the time of hospital stay (minutes) and substantial heterogeneity was noted (MD −10.81, 95% CI −36.93 to 15.31; I2 = 71%; 3 studies, 191 participants; Analysis 1.9).
1.9. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 9 Length of hospital stay (outpatient surgery, mins).
All data were presented as median and IQR.
For this outcome, we only classified two trials as unclear risk of blinding (Figure 2); we excluded these trials in the sensitivity meta‐analysis. The estimated effect (95% CI) of the sensitivity meta‐analysis for blinding remained robust (Table 9).
2. Functional postoperative neuropsychological status scales
Nine trials reported this outcome (Bryson 2010; Chen 2015; Choi KW 2016; De Oliveira 2012; De Oliveira 2014; Mitchell 1999; Mitchell 2009; Peng 2016; Wang 2002); however, since different neuropsychological scales were used, we could not combine the results in a quantitative meta‐analysis.
The trial from Bryson 2010, analysed the quality of recovery (QoR) score from 0 to 18 at 6 hours, 24 hours, 48 hours and seven days. De Oliveira 2012 reported on QoR‐40 at 24 hours but only provided values for subcomponents and no global score. De Oliveira 2014 and Choi KW 2016 also reported on QoR‐40 scores on postoperative day one. We did not perform any meta‐analyses since only two studies reported the same outcome at the same time points.
Chen 2015 and Peng 2016 both reported results of the Mini Mental State Examination (MMSE), but at different time points (preoperative and after three days; and preoperative, at 24 hours, after one week, one month, three months and six months, respectively). Peng 2016 additionally reported on the information‐memory‐concentration‐test (IMCT), Hamilton rating scale for anxiety (HAMA) and Hamilton rating scale for depression (HRSD).
Both trials from Mitchell and colleagues analysed participants using a set of neurophysiological tests as self‐rating inventories for memory (Mitchell 1999; Mitchell 2009); and inventories measuring depression and anxiety (Mitchell 1999).
Wang 2002 performed a battery of nine postoperative neuropsychological tests analysing, for instance, mental control, visual retention, and paired associate verbal learning.
3. Surgical complications
Eight trials reported surgical complications, which are described in detail below (Farag 2013; Groudine 1998; Herroeder 2007; Kim HJ 2014; Kim HO 2014; Lauwick 2009; Tikuisis 2014; Wuethrich 2012). We performed a meta‐analysis if the number of studies reporting the outcome was three or more.
Lauwick 2009 reported a combined number of cases for infection, bleeding and bladder leak. The authors provided the number of cases for each single complication on request.
The risk for an anastomotic leak was reported by three trials (Herroeder 2007; Kim HO 2014; Tikuisis 2014), including 188 participants (4% of the total participants included in the review). From these, 93 participants received the intervention and 95 served as a control. Kim HO 2014 reported no events in either group. An anastomotic leak occurred in 1.08% of participants in the lidocaine group and in 2.11% of participants of the placebo‐treated control group. The results of the meta‐analysis revealed no evidence of effect for lidocaine to reduce or enhance the risk for an anastomotic leak (RR 0.61, 95% CI 0.08 to 4.80; I2 = 0%; 3 studies, 188 participants; Analysis 1.10). For this outcome, we classified all, two, and nil trials as unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded all these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all the domains remained robust (Table 3; Table 8; Table 9).
1.10. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 10 Surgical complications ‐ anastomotic leak.
Three studies reported data on bleeding as a surgical complication (Farag 2013; Kim HO 2014; Lauwick 2009), including 222 participants (5% of the total participants included in the review). From these, 109 participants received the intervention and 113 served as a control. Farag 2013 reported no events in both groups. Bleeding occurred in 3.67% and 1.77% of the participants in the lidocaine and control group, respectively. The meta‐analysis revealed no difference in the risk for bleeding between the lidocaine and control group (RR 1.79, 95% CI 0.41 to 7.89; I2 = 0%; 3 studies, 222 participants; Analysis 1.11). For this outcome, we classified all, two, and nil trials as unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded all these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all the domains remained robust (Table 3; Table 8; Table 9).
1.11. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 11 Surgical complications ‐ bleeding.
Five trials, including 352 participants (8% of the total participants included in the review), reported data on postoperative infections (Farag 2013; Kim HJ 2014; Lauwick 2009; Tikuisis 2014; Wuethrich 2012). From these, 175 participants received the intervention and 177 served as a control. Postoperative infections occurred in 2.86% of participants in the lidocaine group and in 1.13% of participants of the placebo‐treated control group. The results of the analysis revealed no evidence of effect for lidocaine infusion to reduce or enhance postoperative infection rates and no statistical heterogeneity was found (RR 1.64, 95% CI 0.41 to 6.52; I2 = 0%; 5 studies, 352 participants; Analysis 1.12). For this outcome, we classified all trials, two trials, and one trial as unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded all these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all the domains remained robust (Table 3; Table 8; Table 9).
1.12. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 12 Surgical complications ‐ postoperative infection.
Two trials reported urinary retention as a surgical complication (Farag 2013; Tikuisis 2014), but only a small proportion (2 out of 57; and 2 out of 30, respectively) of participants in the control group were affected.
Kim HJ 2014 additionally reported on events of myocardial ischaemia (none of 36 in the lidocaine group, 1 out of 38 in the control group), pleural effusion (4 out of 36 in the lidocaine group, 2 out of 38 in the control group), pulmonary consolidation (2 out of 36 in the lidocaine group, 1 out of 38 in the control group) and neurologic deterioration (no patients in either group).
Kim HO 2014 investigated additional complications such as chylous ascites (7 out of 32 in the lidocaine group, 3 out of 36 in the control group) and wound discharge (1 out of 32 in the lidocaine group, none of 36 in the control group).
One trial reported that no thromboembolic disease occurred in either arm following complex spine surgery (Farag 2013). Another trial reported deep vein thrombosis following radical retropubic prostatectomy in two out of 19 participants in the lidocaine group and in two out of 20 participants in the control group (Groudine 1998)
One trial reported wound healing disturbances in two cases after colorectal surgery (Herroeder 2007). One participant in the control group developed a subphrenic abscess, whereas one participant receiving lidocaine showed minor signs of skin wound irritation.
The trial from Wuethrich 2012 further reported one participant in the lidocaine group with complication after renal surgery and need for pyelonephrostomy; and one participant in the control group who developed postoperative delirium.
4. Patient satisfaction
Six trials reported the outcome, 'patient satisfaction' on a NRS or Likert scale ranging from 0 to 10 (Ahn 2015; Choi GJ 2016; Dewinter 2016; Kim KT 2014; Kim TH 2013; Yon 2014), including 306 participants (7% of the total participants included in the review). Of these trials, 151 participants received the intervention and 155 served as a control. The combined meta‐analysis revealed higher satisfaction scores in participants receiving lidocaine compared to control treatment (MD 0.76, 95% CI 0.46 to 1.06; I2 = 0%; 6 studies, 306 participants; Analysis 1.13). The 95% PI remained above zero and showed that lidocaine will be beneficial for patient satisfaction when applied in at least 95% of the individual study settings (95% PI 0.34 to 1.18).
1.13. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 13 Patient satisfaction.
Five trials reported data as median and IQR (Ahn 2015; Choi GJ 2016; Kim KT 2014; Kim TH 2013; Yon 2014). The effect estimate of the remaining one trial was not robust with a 95% CI including zero (Table 4).
For this outcome, we classified four, one, and four trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) for the sensitivity meta‐analyses on selection and attrition bias included the line of no effect (Table 3; Table 8), however, the estimated effect (95% CI) for the sensitivity analysis on blinding remained robust (Table 9).
5. Cessation of the intervention
No study considered cessation of the intervention as a study endpoint.
6. Postoperative nausea and vomiting
Postoperative nausea, 'early time points' (or in the PACU)
Eight trials, involving 511 participants (11% of the total participants included in the review) reported nausea (or PONV) at early time points (De Oliveira 2014; Farag 2013; Grady 2012; Grigoras 2012; Lauwick 2008; Omar 2013; Soltani 2013; Terkawi 2014). Of these, 255 participants received the intervention and 256 served as a control. Postoperative nausea occurred in 19.2% of participants in the lidocaine group and in 26.6% of participants in the control group. The results of the meta‐analysis showed that the perioperative lidocaine administration reduced nausea compared to control treatment (RR 0.72, 95% CI 0.53 to 0.98; I2 = 0%; 8 studies, 511 participants; Analysis 1.14). The 95% PI crossed the line of identity and the range of true mean effects ranged from benefit to areas of clinical non‐relevance (95% PI 0.49 to 1.06).
1.14. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 14 Postoperative nausea, 'early time points' (PACU).
For this outcome, we classified five, two, and one trial(s) as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) for the sensitivity meta‐analyses on selection bias and blinding included the line of no effect (Table 8; Table 9), however, the estimated effect (95% CI) for the sensitivity analysis on attrition bias remained robust (Table 3).
Postoperative nausea, 'overall' (0 to 24 hours, to 48 hours, or to 72 hours)
Thirty‐five studies, including 1903 participants (42% of the total participants included in the review), reported nausea (or PONV) 'overall' (Ahn 2015; Baral 2010; Cassuto 1985; Choi GJ 2016; Choi KW 2016; Choi SJ 2012; Dale 2016; Dewinter 2016; Farag 2013; Grady 2012; Kaba 2007; Kang 2011; Kim HO 2014; Kim KT 2014; Kim TH 2011; Kim TH 2013; Koppert 2004; Kuo 2006; Lauwick 2008; Lauwick 2009; Maquoi 2016; McKay 2009; Oliveira 2015; Rimbäck 1990; Saadawy 2010; Samimi 2015; Slovack 2015; Terkawi 2014; Tikuisis 2014; Weinberg 2016; Wu 2005; Wuethrich 2012; Yang 2014; Yon 2014; Zengin 2015). Altogether, 950 participants received the intervention treatment and 953 received either placebo treatment or were untreated. Postoperative nausea occurred in 26.9% of participants in the lidocaine group and in 34.9% of participants in the control group. The meta‐analysis revealed a reduced risk of nausea overall for participants in the lidocaine group when compared to control (RR 0.78, 95% CI 0.67 to 0.91; I2 = 22%; 35 studies, 1903 participants; Analysis 1.15). However, the 95% PI crossed the line of identity and the range of true mean effects ranged from benefit to areas of clinical non‐relevance (95% PI 0.49 to 1.23; Table 13).
1.15. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 15 Postoperative nausea, 'overall' (0 to 24 h, to 48 h, to 72 h).
For this outcome, we classified 27, 12, and 16 trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) for the sensitivity meta‐analyses on selection and attrition bias included the line of no effect (Table 3; Table 8), however, the estimated effect (95% CI) for the sensitivity analysis on blinding remained robust (Table 9).
The contour‐enhanced funnel plot and the linear regression test suggested funnel plot asymmetry and trim and fill sensitivity analysis (with k = 9 studies added) changed the conclusion (the 95% CI crossed the line of identity; Table 13).
We graded the quality of evidence for the outcome 'postoperative nausea, overall' as 'very low' (downgraded for study limitations, inconsistency, and imprecision; we did not downgrade for publication bias since we had already downgraded for inconsistency (true heterogeneity may be a source of funnel plot asymmetry; Table 1).
Postoperative vomiting, 'early time points' (or in the PACU)
Postoperative vomiting was reported at early postoperative time points in four trials (De Oliveira 2014; Farag 2013; Grady 2012; Soltani 2013), including 305 participants (7% of the total participants included in the review). Postoperative vomiting at 'early' postoperative time points occurred in 2.6% of participants in the intervention group and in 5.8% of participants in the placebo‐treated group. There was no difference in the risk for postoperative vomiting at early postoperative time points between the lidocaine and the control group (RR 0.49, 95% CI 0.16 to 1.48; I2 = 0%; 4 studies, 305 participants; Analysis 1.16).
1.16. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 16 Postoperative vomiting, 'early time points' (PACU).
For this outcome, we classified two, one, and one trial(s) as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all the domains remained robust (Table 3; Table 8; Table 9).
Postoperative vomiting, 'overall' (0 to 24 hours, to 48 hours, or to 72 hours)
Postoperative vomiting (overall) was reported in 19 trials (Ahn 2015; Cassuto 1985; Choi GJ 2016; Choi SJ 2012; Dale 2016; Farag 2013; Grady 2012; Kang 2011; Kim KT 2014; Kim TH 2013; Koppert 2004; McKay 2009; Rimbäck 1990; Saadawy 2010; Samimi 2015; Tikuisis 2014; Wuethrich 2012; Yang 2014; Yon 2014), including 1026 participants (23% of the total participants included in the review). Overall, vomiting occurred in 15.6% of participants in the lidocaine group and in 20.1% of participants in the control group after surgery. There was no difference in the risk for postoperative vomiting overall between the lidocaine and the control group (RR 0.83, 95% CI 0.63 to 1.08; I2 = 0%; 19 studies, 1026 participants; Analysis 1.17).
1.17. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 17 Postoperative vomiting, 'overall' (0 to 24 h, to 48 h, to 72 h).
For this outcome, we classified 16, four, and 12 trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses on selection and attrition bias remained robust (Table 3; Table 8), however, the effect estimate (95% CI) of the sensitivity meta‐analysis on blinding did not cross the line of no effect (Table 9).
The contour‐enhanced funnel plot and the linear regression test did not suggest funnel plot asymmetry and trim and fill sensitivity analysis (with k = 3 studies added) did not change the conclusion (Table 13).
7. Intraoperative opioid consumption
Intraoperative opioid consumption (MEQ, mg)
Eighteen trials, including 1116 participants (25% of the participants in this review), reported intraoperative opioid requirements which could be included in the analysis (Bryson 2010; Farag 2013; Grady 2012; Grigoras 2012; Kaba 2007; Maquoi 2016; Martin 2008; McKay 2009; Omar 2013; Rimbäck 1990; Saadawy 2010; Samimi 2015; Slovack 2015; Terkawi 2014; Wallin 1987; Wang 2002; Wuethrich 2012; Yardeni 2009). Most of the studies intraoperatively applied fentanyl (Bryson 2010; Omar 2013; Rimbäck 1990; Saadawy 2010; Samimi 2015; Slovack 2015; Terkawi 2014; Wallin 1987; Wang 2002; Wuethrich 2012; Yardeni 2009); three trials administered sufentanil (Kaba 2007; Maquoi 2016; Martin 2008); one trial applied intraoperative morphine (Grigoras 2012); one trial reported the use of morphine and fentanyl (McKay 2009); and in two trials the intraoperative opioid consumption was reported in IV morphine equivalents without stating the opioid that was used (Farag 2013; Grady 2012). From these 18 trials, 556 participants received the intervention and 560 served as a control. The combined meta‐analysis revealed that lidocaine reduced the amount of intraoperative opioid use (MEQ, mg) compared to the control intervention and substantial heterogeneity was noted (MD −2.14, 95% CI −3.87 to −0.40; I2 = 80%; 18 studies, 1116 participants; Analysis 1.18). The 95% PI crossed the line of identity and the range of true mean effects ranged from benefit to harm (95% PI −8.13 to 3.86; Table 13).
1.18. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 18 Intraoperative opioid consumption (MEQ, mg).
The preplanned subgroup analyses on type of surgery and lidocaine regimen (dose and timing) did not explain heterogeneity for all subgroups and tests for subgroup differences did not reach statistical significance (Table 5; Table 6). However, the different tau2s of the surgical subgroups might have contributed to the failure to identify surgical procedures as having different effect estimates (P = 0.027; Table 7).
Five trials reported intraoperative opioid consumption as median with IQR (Farag 2013; Grady 2012; Maquoi 2016; Martin 2008; Terkawi 2014). A sensitivity meta‐analysis excluding all trials reporting data as median did not affect the overall result for the estimated effect on intraoperative opioid consumption (Table 4).
For this outcome, we classified 15, five, and eight trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analysis. The estimated effects (95% CI) for the sensitivity meta‐analyses on selection bias, blinding and attrition bias included the line of no effect (Table 3; Table 8; Table 9).
The contour‐enhanced funnel plot and the linear regression test did not suggest funnel plot asymmetry and trim and fill sensitivity analysis (with k = 1 study added) did not change the conclusion (Table 13).
Intraoperative remifentanil consumption (µg)
Due to the exceptional mode of action (short half‐life) known for remifentanil in contrast to all other opioids used, we analysed trials which applied remifentanil in a separate meta‐analysis. Six trials, including 490 participants (11% of the participants in this review), reported intraoperative opioid requirements which could be included in the analysis (Choi KW 2016; De Oliveira 2012; De Oliveira 2014; Kim HJ 2014; Lee 2011; Xu 2017). From these, 241 participants received the intervention and 249 served as a control. The combined meta‐analysis revealed no significant difference between the lidocaine and control treatment with respect to a reduction in the consumption of intraoperative remifentanil (µg) (MD −14.17, 95% CI −35.27 to 6.92; I2 = 5%; 6 studies, 490 participants; Analysis 1.19).
1.19. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 19 Intraoperative remifentanil consumption (µg).
Two trials reported intraoperative opioid consumption as median with IQR (De Oliveira 2012; De Oliveira 2014). A sensitivity meta‐analysis excluding all trials reporting data as median did not affect the overall result for the estimated effect on intraoperative remifentanil consumption (Table 4).
For this outcome, we classified four, one, and one trial(s) as unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all the domains remained robust ( Table 3; Table 8; Table 9).
8. Opioid consumption during the postoperative period
Postoperative opioid consumption (in the PACU) (MEQ, mg)
Twenty‐five trials (Ahn 2015; Bryson 2010; Choi GJ 2016; Choi KW 2016; Cui 2010; De Oliveira 2012; De Oliveira 2014; Farag 2013; Grady 2012; Grigoras 2012; Groudine 1998; Kaba 2007; Kang 2011; Kim TH 2013; Koppert 2004; Lauwick 2008; Martin 2008; McKay 2009; Peng 2016; Saadawy 2010; Slovack 2015; Terkawi 2014; Weinberg 2016; Xu 2017; Yang 2014), reported postoperative opioid consumption during the PACU (0 to 2 hours, 0 to 4 hours postoperatively). We omitted four of these 25 trials due to suspected variance reporting (Choi GJ 2016; Peng 2016; Xu 2017; Yang 2014). From the remaining 21 trials, involving 1219 participants (27% of the participants in this review), 611 participants received the intervention and 608 served as a control. Of all trials reporting data on postoperative opioid consumption, 12 trials applied morphine for postoperative pain relief (Bryson 2010; Cui 2010; Farag 2013; Grady 2012; Grigoras 2012; Groudine 1998; Koppert 2004; Martin 2008; McKay 2009; Saadawy 2010; Slovack 2015; Weinberg 2016); nine trials applied fentanyl (Ahn 2015; Choi GJ 2016; Choi KW 2016; Kang 2011; Kim TH 2013; Lauwick 2008; Terkawi 2014; Xu 2017; Yang 2014); two hydromorphone (De Oliveira 2012; De Oliveira 2014); one trial offered sufentanil (Peng 2016); and one piritramide (Kaba 2007). A detailed description of the opioid medication for pain relief as well as information regarding concurrent medication (if stated within the study) is listed within the Characteristics of included studies.
The meta‐analysis of data on opioid consumption (MEQ, mg) during PACU revealed that lidocaine reduced the opioid consumption compared to control with moderate heterogeneity between the studies (MD −3.10, 95% CI −3.87 to −2.32; I2 = 40%; 21 studies, 1219 participants; Analysis 1.20). The 95% PI remained below zero (95% PI −5.43 to −0.77) indicating that lidocaine will be beneficial and reduce opioid consumption when applied in at least 95% of the individual study settings (Table 13).
1.20. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 20 Postoperative opioid consumption, PACU (MEQ, mg).
The preplanned subgroup analyses on type of surgery and lidocaine regimen (dose and timing) did not explain heterogeneity for all subgroups and tests for subgroup differences did not reach statistical significance (Table 5; Table 6).
Six trials reported opioid consumption in PACU as median with IQR (Cui 2010; De Oliveira 2012; De Oliveira 2014; Grady 2012; Kaba 2007; Martin 2008). A sensitivity meta‐analysis excluding all trials reporting data as median did not affect the overall result for the estimated effect on opioid consumption in PACU (Table 4).
For this outcome, we classified 15, three, and nine trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analyses. The estimated effects (95% CI) of the sensitivity meta‐analyses for all the domains remained robust (Table 3; Table 8; Table 9).
The contour‐enhanced funnel plot and the linear regression test did not suggest funnel plot asymmetry and trim and fill sensitivity analysis (with k = 2 studies added) did not change the conclusion (Table 13).
Postoperative opioid consumption, 'overall' (MEQ, mg)
Forty‐three trials (Ahn 2015; Bryson 2010; Cassuto 1985; Choi GJ 2016; Choi KW 2016; Cui 2010; Dale 2016; De Oliveira 2012; De Oliveira 2014; Dewinter 2016; Farag 2013; Grady 2012; Grigoras 2012; Groudine 1998; Herroeder 2007; Insler 1995; Jain 2015; Kaba 2007; Kang 2011; Kim HO 2014; Kim KT 2014; Kim TH 2013; Koppert 2004; Lauwick 2008; Lauwick 2009; Maquoi 2016; Martin 2008; McKay 2009; Oliveira 2015; Ortiz 2016; Rimbäck 1990; Saadawy 2010; Samimi 2015; Slovack 2015; Soltani 2013; Striebel 1992; Terkawi 2014; Wallin 1987; Weinberg 2016; Wu 2005; Wuethrich 2012; Xu 2017; Yon 2014), presented data on cumulative or total postoperative opioid consumption after surgery (0 to 24 hours, 0 to 48 hours, 0 to 72 hours).
Of all trials reporting data on postoperative opioid consumption, 17 trials applied morphine for postoperative pain relief (Bryson 2010; Cui 2010; Farag 2013; Grady 2012; Grigoras 2012; Groudine 1998; Koppert 2004; Lauwick 2009; Martin 2008; McKay 2009; Oliveira 2015; Ortiz 2016; Saadawy 2010; Samimi 2015; Slovack 2015; Weinberg 2016; Wuethrich 2012); 11 trials applied fentanyl (Ahn 2015; Choi GJ 2016; Choi KW 2016; Dale 2016; Insler 1995; Kang 2011; Kim KT 2014; Kim TH 2013; Terkawi 2014; Xu 2017; Yon 2014); seven trials applied meperidine/pethidine (Cassuto 1985; Kim HO 2014; Rimbäck 1990; Soltani 2013; Striebel 1992; Wallin 1987; Wu 2005); one hydromorphone (De Oliveira 2012); one trial offered tramadol (Dewinter 2016); one pentazocine (Jain 2015); one oxycodone (Lauwick 2008); and three piritramide (Herroeder 2007; Kaba 2007; Maquoi 2016). One study did not report which opioid was used (De Oliveira 2014). A detailed description of the opioid medication for pain relief as well as information regarding concurrent medication (if stated within the study) is listed within the Characteristics of included studies.
We omitted three of the 43 trials due to suspected variance reporting (Choi GJ 2016; Samimi 2015; Xu 2017). From the remaining 40 trials, involving 2201 participants (49% of the participants in this review), 1091 participants received the intervention and 1110 served as a control. The random‐effects meta‐analysis of combined data on total or cumulative postoperative opioid consumption (MEQ, mg) showed that lidocaine reduced opioid consumption compared to control with substantial between‐study heterogeneity (MD −4.52, 95% CI −6.25 to −2.79; I2 = 73%; 40 studies, 2201 participants; Analysis 1.21). The 95% PI crossed the line of identity and the range of true mean effects ranged from benefit to areas of clinical non‐relevance (95% PI −12.03 to 3.00; Table 13). A fixed‐effect meta‐analysis revealed a lower MD in the opioid consumption (MEQ, mg) compared to the random‐effects meta‐analysis result (MD −1.52, 95% CI −2.14 to −0.90; Table 10).
1.21. Analysis.
Comparison 1 Intravenous (IV) lidocaine versus placebo, Outcome 21 Postoperative opioid consumption, overall (MEQ, mg).
The preplanned subgroup analyses on type of surgery and lidocaine regimen (dose and timing) did not explain heterogeneity for all subgroups and tests for subgroup differences did not reach statistical significance (Table 5; Table 6).
Twelve trials reported cumulative opioid consumption as median with IQR (Cui 2010; De Oliveira 2012; De Oliveira 2014; Dewinter 2016; Farag 2013; Grady 2012; Kaba 2007; Kim HO 2014; Lauwick 2009; Maquoi 2016; Martin 2008; Ortiz 2016). A sensitivity meta‐analysis excluding all trials reporting data as median did not affect the overall result for the estimated effect on cumulative opioid consumption (Table 4).
For this outcome, we classified 30, 16, and 15 trials as high or unclear risk of selection bias, blinding, and attrition bias (Figure 2), respectively; we excluded these trials in the sensitivity meta‐analysis. The estimated effect (95% CI) for the sensitivity meta‐analysis on selection bias included the line of no effect (Table 8), however, the estimated effects (95% CI) for the sensitivity analyses on blinding and attrition bias remained robust ( Table 3; Table 9).
The contour‐enhanced funnel plot and the linear regression test suggested funnel plot asymmetry and the trim and fill sensitivity analysis (with k = 16 studies added) changed the conclusion (the 95% CI crossed the line of no effect; Table 13).
We graded the quality of evidence for the outcome, 'postoperative opioid consumption' as very low (downgraded for study limitations, inconsistency, and imprecision); we did not downgrade for publication bias since we had already downgraded for inconsistency (true heterogeneity may be a source of funnel plot asymmetry; Table 1).
2. IV Lidocaine versus thoracic epidural analgesia (TEA)
The second comparison analysed IV lidocaine versus TEA. For this comparison, we were able to identify two studies (Swenson 2010; Wongyingsinn 2011). Due to the low number of identified studies analysing the effect of systemic lidocaine compared to TEA, the summarized effects for each outcome in this comparison are only of very low evidence.
Primary outcomes
1. Postoperative pain ‐ IV lidocaine versus TEA
Pain score at rest (VAS 0 to 10 cm), 'early time points' (0 to 4 hours, or in the PACU) ‐ IV lidocaine versus TEA
No trial assessed this outcome.
Pain score at rest (VAS 0 to 10 cm), 'intermediate time points' (24 hours) ‐ IV lidocaine versus TEA
Two trials, including 102 participants (2% of the participants included in this review), reported this outcome (Swenson 2010; Wongyingsinn 2011). Wongyingsinn 2011 reported pain for two subgroups with respect to different surgical procedures, i.e. colonic resection and rectal resection. We reported both subgroups in the meta‐analysis as separate studies. In total 52 participants received the intervention and 50 received the TEA comparator. The analysis revealed no evidence of effect for lidocaine to reduce pain intensity at rest compared to the TEA group (and thus also no superiority of TEA) and substantial heterogeneity (MD 1.51, 95% CI −0.29 to 3.32; I² = 85%; 2 studies, 102 participants; Analysis 2.1). The results of this analysis were only of limited evidence, as both trials reported data as median with IQR and we classified both trials (at least for two domains of the quality assessment) as high risk of bias. Furthermore, in one trial there were missing participant data which may not be missing at random (Wongyingsinn 2011).
2.1. Analysis.
Comparison 2 Intravenous (IV) lidocaine versus thoracic epidural analgesia (TEA), Outcome 1 Pain score (VAS 0 to 10 cm) at rest, 'intermediate time points' (24 h).
We graded the quality of evidence for the outcome, 'pain score at rest (intermediate time points)' as very low (we downgraded for study limitations, inconsistency, and imprecision; Table 2).
Pain score at rest (VAS 0 to 10 cm), late time points (48 hours) ‐ IV lidocaine versus TEA
The same trials, including 102 participants (2% of the participants in this review), as for 'pain at 24 hours' reported this outcome in the same fashion as described above (Swenson 2010; Wongyingsinn 2011). We found no evidence of effect for IV lidocaine compared to TEA on pain reduction (and thus also no superiority of TEA) and substantial heterogeneity (MD 0.98, 95% CI −1.19 to 3.16; I² = 88%; 2 studies, 102 participants; Analysis 2.2). Since both trials reported data as median with IQR and we classified both trials (at least for two domains of the quality assessment) as high risk of bias, the results of this analysis were only of limited evidence. In addition, in one trial there were missing participant data which may not be at random (Wongyingsinn 2011).
2.2. Analysis.
Comparison 2 Intravenous (IV) lidocaine versus thoracic epidural analgesia (TEA), Outcome 2 Pain score (VAS 0 to 10 cm) at rest, 'late time points' (48 h).
We graded the quality of evidence for the outcome, 'pain score at rest (late time points)' as very low (we downgraded for study limitations, inconsistency, and imprecision; Table 2).
2. Gastrointestinal recovery
Postoperative ileus (dichotomous) ‐ IV lidocaine versus TEA
Only one trial reported postoperative ileus with one out of 30 participants in the lidocaine group and two out of 30 participants in the TEA group without significant difference between the groups (P = 0.129; Wongyingsinn 2011). We assessed the study from Wongyingsinn 2011 as high risk for blinding and attrition bias.
We graded the quality of evidence for the outcome, 'postoperative ileus' as very low (we downgraded for study limitations and double‐downgraded for imprecision; Table 2).
Time to first defaecation/bowel movement (hours) ‐ IV lidocaine versus TEA
Two trials, including 102 participants (2% of the total participants in the review), reported this outcome as time to first bowel movement in hours after surgery (Swenson 2010; Wongyingsinn 2011). Wongyingsinn 2011 reported this outcome for two subgroups in regard to different surgical interventions (e.g. primary colonic anastomosis and rectal anastomosis). We reported both subgroups in the meta‐analysis as separate studies. In total, 52 participants received IV lidocaine and 50 received TEA. We found no evidence of effect for lidocaine compared to TEA to shorten the time to first bowel movement, and thus also no superiority of TEA (MD −1.66, 95% CI −10.88 to 7.56; I² = 0%; 2 studies, 102 participants; Analysis 2.3). Swenson 2010 reported the data as median with IQR. In terms of risk of bias, we classified both trials (at least for two domains of the quality assessment) as high risk of bias.
2.3. Analysis.
Comparison 2 Intravenous (IV) lidocaine versus thoracic epidural analgesia (TEA), Outcome 3 Time to first bowel movement (h).
We graded the quality of evidence for the outcome, 'time to defaecation/bowel movement' as very low (we downgraded for study limitations, inconsistency, and imprecision; Table 2).
Time to first flatus (hours) ‐ IV lidocaine versus TEA
Two trials reported time to first flatus (Swenson 2010; Wongyingsinn 2011). However, Swenson 2010 reported this outcome as median and IQR with highly asymmetric distribution, whereby these data could not be transformed into mean plus SD for the analysis. The other study reported time to first flatus for both subgroups (primary anastomosis and primary ileostomy) without significant difference between the lidocaine and the TEA group.
Time to first bowel sounds (hours) ‐ IV lidocaine versus TEA
Neither trial reported this outcome for the comparison of systemic lidocaine versus TEA.
3. Adverse events ‐ IV lidocaine versus TEA
One trial reported there were no significant lidocaine‐associated adverse events during the study (Wongyingsinn 2011). The other study reported a detailed summary table with a number of monitored adverse events (Swenson 2010), which we have integrated in the 'Adverse events' table (Table 14). However, the trial authors reported no significant difference in the occurrence of adverse events between the lidocaine and the TEA group.
Secondary outcomes
1. Length of hospital stay (days) ‐ IV lidocaine versus TEA
The outcome, 'length of hospital' stay in days after surgery was reported by two studies, including 102 participants (Swenson 2010; Wongyingsinn 2011). We found no evidence of effect for lidocaine on the length of hospital stay compared to TEA, and thus also no superiority of TEA (MD −0.02, 95% CI −0.38 to 0.33; I² = 0%; 2 studies, 102 participants; Analysis 2.4). Both trials reported this outcome as median with IQR. In terms of risk of bias, we classified both trials (at least for two domains of the quality assessment) as high risk of bias.
2.4. Analysis.
Comparison 2 Intravenous (IV) lidocaine versus thoracic epidural analgesia (TEA), Outcome 4 Length of hospital stay (days).
2. Functional postoperative neuropsychological status scales ‐ IV lidocaine versus TEA
Neither trial reported this outcome for the comparison of systemic lidocaine versus TEA.
3. Surgical complications ‐ IV lidocaine versus TEA
One trial reported surgical complications, in particular the number of participants with urinary retention, bleeding per rectum, and exudate from stroma (Wongyingsinn 2011). The trial authors detected no significant difference between either group.
4. Patient satisfaction ‐ IV lidocaine versus TEA
Neither trial reported this outcome for the comparison of systemic lidocaine versus TEA.
5. Cessation of the intervention ‐ IV lidocaine versus TEA
Neither study considered cessation of the intervention as a study endpoint.
6. Postoperative nausea and vomiting ‐ IV lidocaine versus TEA
Postoperative nausea and postoperative vomiting were both reported in two studies (Swenson 2010; Wongyingsinn 2011). The time points at which nausea and vomiting was reported varied between the studies from 72 hours up to 5 days postoperatively. However, a monitoring period of five days postsurgery for PONV seems unreliable to detect primary effects associated with the intervention. Therefore, we did not take these data into consideration (Swenson 2010). Wongyingsinn 2011 reported 11 out of 30 participants in the lidocaine group and 17 out of 30 in the TEA group with nausea. For vomiting, the trial authors reported 18 out of 30 participants in the lidocaine group, and 12 out of 30 in the TEA group. The detected differences did not reach statistical significance. We assessed the study from Wongyingsinn 2011 as high risk of blinding and attrition bias.
We graded the quality of evidence for the outcome, 'postoperative nausea, (overall)' as very low (we downgraded for study limitations and double‐downgraded for imprecision; Table 2).
7. Intraoperative opioid consumption ‐ IV lidocaine versus TEA
Two trials, including 100 participants, reported intraoperative opioid consumption (Swenson 2010; Wongyingsinn 2011). The data were reported as mean with SD. During general anaesthesia both studies intraoperatively applied fentanyl and Swenson 2010 additionally applied morphine. All opioid quantities were transformed into IV MEQ (mg) as described in detail in the anatomic therapeutic chemical (ATC)/defined daily dose (DDD) Index (www.whocc.no/atc_ddd_index). No evidence of effect was found for lidocaine to reduce intraoperative opioid consumption compared to TEA, and thus also no superiority of TEA (MD 7.27, 95% CI −13.92 to 28.47; I² = 91%; 2 studies, 100 participants; Analysis 2.5).
2.5. Analysis.
Comparison 2 Intravenous (IV) lidocaine versus thoracic epidural analgesia (TEA), Outcome 5 Intraoperative opioid consumption (MEQ, mg).
8. Opioid consumption during the postoperative period ‐ IV lidocaine versus TEA
Two trials reported postoperative opioid consumption (Swenson 2010; Wongyingsinn 2011). However, both trials applied postoperative analgesia in the control and intervention group by different routes (IV versus TEA). In this case, we could not compare and analyse data for opioid consumption.
Discussion
Summary of main results
The effect estimates of the meta‐analysis on pain at early time points (1 to 4 hours, and in the postanaesthesia care unit (PACU)) reveals that participants undergoing any elective surgery under general anaesthesia who received perioperative lidocaine treatment have on average less pain than participants in the placebo control group. Average pain reduction was in the order of 0.37 cm to 2.48 cm on a visual analogue scale (VAS) 0 to 10 cm scale (standardized mean difference (SMD) −0.50), depending on the variance of the study and with a precision ranging from 0.20 cm to 3.56 cm lower pain scores (95% confidence interval (CI) −0.72 to −0.28). Due to heterogeneity, the dispersion (95% prediction interval (PI)) of the true mean effects in the population is far greater than estimated by the random‐effects meta‐analysis, including both benefit and harm (95% PI −1.61 to 0.62). Therefore, we graded the quality of evidence as very low, since we are uncertain about this effect estimate and the true effect may be significantly different from the estimated effect (Table 1).
In contrast to the original review (Kranke 2015), we were no longer able to demonstrate a significant subgroup difference for different surgical procedures (open abdominal, laparoscopic abdominal, and other surgery) with regard to pain at early time points. However, the different tau2s of the surgical subgroups might have contributed to the failure to identify surgical procedure as having different effect estimates.
At 24 hours and at later postoperative time points, perioperative lidocaine has probably no clinically relevant effect on postoperative pain (Table 1). Average pain reduction (back‐transformed mean difference (MD)) at 24 hours and 48 hours postoperatively ranged from 0.10 cm to 0.48 cm (SMD −0.14) and from 0.08 cm to 0.42 cm (SMD −0.11), respectively. Taking precision (95% CI) and dispersion (95% PI) into account, estimated mean effects remained in a range of clinically non‐relevant pain scores varying around the null effect.
We omitted several studies with suspected small variance reporting in the current update from the meta‐analyses for pain and other continuous outcomes. The effect estimates for pain, at all three time points, dropped down due to that omission.
The random‐effects meta‐analysis on postoperative ileus suggested that lidocaine reduced the risk for this complication compared to control treatment (risk ratio (RR) 0.37, 95% CI 0.15 to 0.87). Taking into account the uncertainty associated with this effect estimate, we cannot conclude that lidocaine has beneficial effects in all settings. We graded the quality of evidence as very low for postoperative ileus (Table 1).
The same applied for the other patient‐relevant endpoints, namely 'time to first defaecation/bowel movement' (MD −7.92 (h), 95% CI −12.71 to −3.13), 'postoperative nausea, overall' (RR 0.78, 95% CI 0.67 to 0.91), and 'postoperative opioid consumption, overall' (MD −4.52 (mg, morphine equivalents (MEQ)), 95% CI −6.25 to −2.79). Although random‐effects meta‐analyses suggested, on average, beneficial effects for participants receiving lidocaine, the intervention may not always be beneficial in an individual setting, considering the variation of effects in the population. Therefore, we graded the quality of evidence for all three outcomes as very low (Table 1).
In comparison to the original review (see 'Summary of findings tables' 1 and 2 in Kranke 2015), the level of the quality of evidence has changed for all GRADE‐relevant outcomes (Table 1). As we introduced the PI with this update to support the clinical interpretation of the results in the light of substantial heterogeneity, the assessment of the quality of the evidence was significantly influenced. Thus, the confidence in the effect estimates has increased for pain at 24 hours and 48 hours postoperatively to a moderate level, whereas the confidence in the effect estimates of all other outcomes has diminished to very low levels.
With a random‐effects meta‐analysis, we also found evidence of positive effects for additional primary and secondary outcomes not included in the 'Summary of findings' table. Lidocaine shortened the time to first flatus, reduced the length of hospital stay, the risk for postoperative nausea (in the PACU), and the need for intraoperative opioid consumption. However, the range of effects that can be expected in future studies (taking existing heterogeneity into account) indicated that lidocaine may not always be beneficial in an individual setting. In contrast, for patient satisfaction and for postoperative opioid consumption in the PACU, results were consistent. Lidocaine will be beneficial for patients with on average 0.76 higher satisfaction scores (numeric rating scale (NRS) 0 to 10) and on average 3.10 mg (MEQ) lower opioid consumption in the PACU, when applied in at least 95% of the individual study settings.
For the outcomes, 'time to bowel sound', 'length of hospital stay (ambulatory setting)', 'surgical complications (anastomotic leak, bleeding, postoperative infection)', and 'vomiting (in the PACU and overall after surgery)' we found no difference between lidocaine and control treatment. However, with exception of vomiting (overall) the meta‐analyses were based on only a few studies and the effect estimates may be too imprecise to suggest lack of effect.
This review illustrates that there are no major adverse events due to systemic lidocaine administration in the perioperative setting reported in 68 small randomized controlled trials (RCTs). Four trials reported mortality during the study. However, all claimed that postoperative death in the lidocaine group was not associated with the intervention. In general, there was great heterogeneity in the investigated adverse events in the individual trials, with a lack of systematic assessment and reporting of adverse events. Effects of lidocaine on adverse events remained unclear and we graded the quality of evidence for all three outcomes as very low (Table 1).
The second comparison analysed in this review was intravenous (IV) lidocaine versus thoracic epidural analgesia (TEA). For this comparison, we were able to identify two studies. Due to the low number of identified studies analysing the effect of systemic lidocaine compared to thoracic epidural analgesia, the summarized effects of each outcome for this comparison are only of very low‐quality evidence (Table 2). In general, we were not able to identify any evidence of effect in terms of postoperative pain, functional gastrointestinal recovery, ileus, length of hospital stay, and nausea or vomiting.
Overall completeness and applicability of evidence
For this review we examined data from 68 trials, including 4525 participants undergoing various elective surgical procedures under general anaesthesia. Of these 68 trials, 66 analysed systemic lidocaine versus placebo or no treatment, whereas only two trials compared IV lidocaine with epidural analgesia. Results of the latter have to be considered with caution due to imprecision of the effect estimates (small sample size). For the comparison, IV lidocaine versus placebo, we identified a sufficient number of studies for most of the clinically relevant outcomes to evaluate the review question, e.g. more than 1000 participants for the outcomes: pain (early, intermediate, and late), length of hospital stay, postoperative nausea (overall), vomiting (overall), and intraoperative and postoperative opioid consumption (in the PACU and overall). The gathered evidence for these outcomes is based on at least a significant sample size. However, the individual sample sizes of the included trials were small, for which reason external validity and generalizations of the estimated treatment effects may be limited. The overall number of participants for the outcomes: postoperative ileus, functional gastrointestinal recovery, surgical complications, and vomiting (early) was rather low. Therefore, confidence in the body of evidence with respect to sample size is limited for these. Most of the studies investigated adverse events in their study protocol, however, most of them without systematic assessment.
As far as the clinical applicability of these results are concerned, it is reassuring that despite the encouraging effects of lidocaine administration in the administered doses (˜1.5 mg/kg of body weight as bolus and ˜2 mg/kg of body weight as continuous infusion) in the investigated cohort of participants, this intervention did not produce (reporting of) relevant clinical side effects. However, since no phase III registration trials aiming at labelling this new indication of IV lidocaine are included in this systematic review, we should be cautious regarding the extrapolation of these results to any (minor) side effects. However, it is plausible that major adverse events would have been detected even without explicit mentioning of quality control measures, such as audits and inspections more prevalent in controlled study scenarios, that should lead to the labelling of a new indication. Further, we cannot make any conclusions regarding the tolerability in patients with compromised liver or renal function.
The resulting clinical question and implication is whether these effects are worth the efforts associated with this intervention.
To address this question, it is useful to bear in mind that under conditions of clinical trials and meta‐analyses (Block 2003; Hughes 2014; Wu Cohen 2005), and clinical audits (Popping 2008; Toren 2009), the benefit of neuraxial techniques (e.g. epidural analgesia) over an opioid‐based patient‐controlled analgesia ‐ although usually considered superior in terms of pain relief ‐ is in the range of 1 to 2 points on a 0 to 10 VAS, depending on the specified pain outcome. Based on the current findings, lidocaine could not reach pain reduction in the range of clinical relevance of approximately 1 cm at intermediate and late time points. But at early time points, lidocaine may exert effects of clinical relevance, at least in some settings. However, uncertainty with this estimated effect is currently high.
The fact that the baseline pain score (control group) in most of the trials was moderate does not mean that there was no noxious stimulus, and therefore no sensitivity in the analysis, but means that the other analgesic treatments also worked (in the control group). Nonetheless, and despite the fact that the control group also could have as much analgesia as required, we see some effects on pain 'early' and nausea and/or vomiting. Clearly, this could also be interpreted that opioids simply provoke nausea and vomiting (and the latter can also be controlled by giving anti‐emetics).
Overall, the range of surgical procedures in the included studies of this review was broad. We were able to perform subgroup analyses for several outcomes with between‐study heterogeneity: pain (early and late), time to first defaecation/bowel movement, flatus, length of hospital stay, and intraoperative and postoperative opioid consumption (in the PACU and overall); and to analyse studies focusing on open abdominal surgery and laparoscopic abdominal surgery, separated from all other surgeries. In the original review in 2015, subgroup analysis revealed benefit of the intervention for laparoscopic abdominal followed by open abdominal surgeries, but not for the category other surgeries with respect to pain relief at early time points (P = 0.04; Kranke 2015). In the current update, we were not able to demonstrate this subgroup difference (P = 0.07), and heterogeneity could not be explained for the outcome, 'pain (early)'. The different tau2s of the surgical subgroups might have contributed to the failure to identify surgical procedures as having different effect estimates. However, the tendency of benefit (laparoscopic > open > others) remained. The subgroup analyses on the type of surgery did not sufficiently explain heterogeneity or did not reach statistically significant subgroup differences for any other outcome in the current update.
At the protocol stage, we had closely chosen the inclusion criteria regarding the intervention with respect to start and duration of the administration of lidocaine to minimize clinical heterogeneity. We wanted, for example, not to include studies in the review that administered lidocaine only as a single dose, at the end of anaesthesia, to suppress an extubation response (Haldar 2016). These studies are not aimed at improving postoperative recovery and, in that setting, the lidocaine application regimen is not appropriate to establish postoperative effects. Nonetheless, the application scheme of systemic lidocaine in the perioperative setting strongly varies between the studies related to both dose and timing of the infusion. We discriminated between studies applying low (< 2 mg/kg/h) and high (≥ 2 mg/kg/h) lidocaine doses in combination with either short duration (until end of surgery or until PACU) or long duration (≥ 24 hours postoperatively) of the infusion. With this allocation, we performed subgroup analyses for the same outcomes with between‐study heterogeneity mentioned above. However, subgroup analyses on the application regimen of lidocaine did not sufficiently explain heterogeneity or did not reach statistically significant subgroup differences for any outcome in the current update. Although there is a clinical rationale to expect a different magnitude of the intervention effects in different surgical populations, or with different doses of lidocaine, we were not able to explain heterogeneity satisfactorily or show any clinically relevant (and statistically significant) difference between study groups. We assume that clinical heterogeneity in the individual studies, caused for example, by different anaesthesia regimen (with or without opioid supplementation) or even varying modalities in postoperative pain relief, may act as latent effect modifiers.
In most of the trials, participants in both groups can have as much postoperative analgesia as they need. In consequence, all effects of lidocaine on pain, nausea, vomiting, and gastrointestinal recovery which were assessed at later (≥ 24 hours) postoperative time points might be influenced by postoperative analgesia requirements. In the end, this is what we see in the current review update. Lidocaine may have some effect at 'early' time points on outcomes such as pain, nausea, vomiting, and opioid consumption in the PACU. But at later time points, when lidocaine is probably no longer effective and participants in the lidocaine group also need additional analgesia, the effect of lidocaine disappeared.
Geographically, reasonable variability among the studies was noted. Twenty‐four trials were conducted in Asia, 18 in the USA, Canada, or South America, 15 trials in Europe, seven trials in the Middle East, and four in New Zealand or Australia. We have noticed a strong increase in the number of trials (from 11 to 24) conducted in Asia since the original search in 2014, with 13 newly included trials in the current update. Therefore, 56% of the newly included information in this update came from Asian studies. Altogether, the results of this review are based on different countries worldwide, with different models of healthcare delivery, which supports the generalization of the findings of this review.
Quality of the evidence
The overall methodological quality of the 68 included studies was moderate, with an overall low risk of bias concerning selection bias (random sequence generation), performance bias, attrition bias, and detection bias in more than 50% of the included studies. For allocation concealment and selective reporting, the quality assessment yielded low risk of bias for only approximately 20% of the included studies. For this kind of intervention trial, the best practice to ensure allocation concealment and blinding of key personnel, are sequentially numbered, pharmaceutically prepared containers of the study drug and placebo with identical appearance. This was done by only three included trials. In terms of selective reporting, only 14 trials published a trial protocol before participants' enrolment and the primary outcomes of the studies have been reported in the corresponding protocols. For each outcome, we performed sensitivity analyses for the domains, selection bias, blinding, and attrition bias, including only trials at low risk of bias for the respective domains. Sensitivity analysis altered the robustness of the estimated effects (clinical relevance) for the outcomes: pain (early), postoperative ileus, time to defaecation/bowel movement, length of hospital stay, patient satisfaction, nausea (early and overall), vomiting (overall), and intraoperative and postoperative opioid consumption (overall). The effect estimates for the outcomes: pain (intermediate and late), and postoperative opioid consumption (in the PACU) remained robust. However, only the latter indicated a beneficial effect for lidocaine.
We did not downgrade any of the GRADE‐relevant outcomes for indirectness. In all cases we have investigated the comparisons of interest, in the population of interest, and did not use any surrogate parameters as outcome measures.
A major limitation of this review is the large and unexplained heterogeneity between studies. Accordingly, we downgraded the quality of evidence for most of the outcomes for inconsistency. The preplanned subgroup analysis, according to different surgical procedures and different lidocaine application regimens, were not successful in explaining heterogeneity. With the current update, we have introduced the 95% PI to enhance the understanding of the uncertainty about whether the intervention works or not in 95% of settings, in the light of between‐study heterogeneity. The reporting of a 95% PI, in addition to the summary estimate and the 95% CI, illustrates which range of true mean effects can be expected in future trials (IntHout 2016). For the GRADE‐relevant outcomes of the comparison, 'lidocaine versus placebo/no treatment' (Table 1), most of the 95% PIs revealed a wider range of expected mean treatment effects than the 95% CIs, and thus lead to different conclusions for pain (early), postoperative ileus, time to defaecation/bowel movement, nausea (overall), and postoperative opioid consumption (overall). Only for pain (intermediate and late) the range of expected effects remained in areas of clinical non‐relevance and the conclusion did not change. For the second comparison, 'lidocaine versus TEA', we did not calculate 95% PIs since the intervals were imprecise due to a limited number of studies (Table 2).
Despite the fact that for several outcomes, more than 1000 participants could be analysed, we downgraded the quality of evidence for most of the outcomes for imprecision (Table 1). The decision to downgrade for imprecision, although the outcomes had a sufficient number of participants and the 95% CIs were narrow and located on one side of the null (precise), was based on the fact that the 95% PI in such cases overlapped the line of identity. This indicates that lidocaine may actually be ineffective in some settings.
We analysed all outcomes with more than 10 trials for publication bias (funnel plot asymmetry) with visual assessment by contour‐enhanced funnel plots, regression analysis, and trim and fill sensitivity analysis. We found funnel plot asymmetry for several outcomes and even trim and fill sensitivity analyses changed the conclusion. Since most of these outcomes were characterized by between‐study heterogeneity (95% PI > 95% CI), for which we downgraded due to inconsistency, we did not further downgrade for publication bias. It is known that true heterogeneity may be a reason for funnel plot asymmetry and we can not exclude this as a possible reason for asymmetry (Higgins 2011).
In summary, we have very low confidence in most of the effect estimates obtained for the GRADE‐relevant outcomes (Table 1; Table 2). However, we are moderately confident that lidocaine has no beneficial effect on reduction of pain scores later than 24 hours postoperatively compared to placebo treatment (Table 1).
Potential biases in the review process
This review was performed according to procedures described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We systematically searched ClinicalTrials.gov, congress proceedings, and reference lists of included and excluded trials and reviews, without language restriction. The review group consists of several experts in the field (PK, LE, MH, KH) who are in contact with those continuing clinical research in the field. We contacted further trialists who published the study protocol on ClinicalTrials.gov, and asked for the actual status, and whether there are data available for inclusion in this review. In this way, we were able to include one trial which is finished but unpublished at submission of the original review (Slovack 2015). The search was independently performed by at least two review authors in two steps. First, they screened the title and abstracts; and in a second step, they reviewed in detail potentially relevant full texts of trials. Thus, we can be confident that we have identified all relevant studies. We attempted to conduct a comprehensive search for studies, but the fact that we have not yet incorporated 18 studies, may be a source of potential bias (Studies awaiting classification).
Two review authors independently performed assessment of methodological quality and data abstraction. Published reports did not always provide sufficient information for quality judgement or to abstract the data for quantitative analysis in this review. In such cases we contacted study authors, but some information is still outstanding as of the publication date of this review. In particular, for one trial we were only able to review the abstract, and repeated requests to the authors, as well as to the journal, were unsuccessful. Thus quality assessment and data abstraction for this trial is still lacking.
Several studies reported their data as median rather than as mean, and the distribution was reported as interquartile range (IQR). We included these data (with symmetric and asymmetric distribution) and approximated to mean and standard deviation (SD) by using the calculation described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed sensitivity analyses, excluding trials that reported data as median with IQR. Exclusion of these trials did not affect the robustness of the estimated effects of any outcome.
During the update of this review, we noted that several studies reported very small variances for different continuous outcomes, such as pain, gastrointestinal recovery, and opioid consumption. Moore 2011 recently analysed the outcome 'opioid consumption' in detail and reported that the SD of the mean opioid consumption often had the same size as the mean when the sample size of trials was small (20 to 30 patients per group). We identified studies with variances for pain 'early' as low as SD = 0.1 cm with a mean of 3 cm (n = 26) and for opioid consumption 'overall' a SD = 1.5 mg morphine with a mean of 17 mg morphine (n = 39). We assumed that these small variances may have been derived from a misinterpretation of a standard error (SE) as a SD, since SDs and SEs are occasionally confused in the reports of studies, and the terminology is used inconsistently (Higgins 2011). Unfortunately, in other studies, it was not clear from the description what was actually reported. Small variances result in larger standardized mean differences (SMDs) compared to large variances. Therefore, these studies with 'suspected (small) variance reporting' may lead to an overestimation of treatment effects and systematically introduce bias into the meta‐analyses. We contacted the authors of all studies with suspected variance reporting in relevant outcomes to clarify the issue. Unfortunately, only Weinberg 2016 has solved the issue satisfactorily. In this case, the authors have committed an error in the report. We corrected the values and included this study into analyses. Finally, we decided to omit all other studies with suspected variance reporting (and unsolved status) for the outcomes 'pain', 'gastrointestinal recovery', and 'postoperative opioid consumption' from the relevant meta‐analyses. The effect estimates for pain, at all three time points, significantly dropped down due to that omission (Table 11).
We used the principles of the GRADE system to assess the quality of the body of evidence. At the protocol stage we planned to present results on pain scores and gastrointestinal recovery within 'Summary of findings' tables. We decided post‐analysis, during preparation of the original review (Kranke 2015), to additionally present nausea as an outcome of public interest, as well as the results of the different surgical subgroups (open abdominal, laparoscopic abdominal, and other surgeries) for the outcome, pain 'early', to reflect the specific benefit of lidocaine in the early postoperative period for abdominal surgery participants. In the current update, we were no longer able to demonstrate a significant subgroup difference for the different surgical procedures (open abdominal, laparoscopic abdominal, and other surgery) with regard to pain at 'early time points'; we decided not to focus on these subgroups as done in the original review.
Agreements and disagreements with other studies or reviews
At the time of submission of our protocol (Selig 2012), there were three systematic reviews addressing similar questions (Marret 2008; McCarthy 2010; Vigneault 2011); and one article was published as a referenced review to the original of McCarthy and colleagues (Joppich 2010). In 2012, another meta‐analysis was published, which analyses perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery (Sun 2012). Updating this review in 2107, we identified another systematic review (Chang 2017), dealing with the effect of perioperative lidocaine infusion on acute and chronic pain after breast surgery.
Marret 2008 searched three databases (MEDLINE, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL)) and included RCTs comparing continuous IV lidocaine infusion, during and after abdominal surgery, with placebo. The review authors selected eight RCTs, including 320 participants, which were published between 1985 and 2007. They scored quality assessment using the Oxford Quality Score, based on randomization, double‐blinding and follow‐up. Outcome measures were: duration of ileus, length of hospital stay, postoperative pain, and incidence of nausea and vomiting. The authors concluded that continuous IV administration of lidocaine, during and after abdominal surgery, improves patient rehabilitation and shortens hospital stay. We included all eight trials included by Marret 2008 in this review; the conclusions in Marret 2008 were more beneficial compared to the present review update. The quality of evidence was, as in the present Cochrane Review, limited by inconsistency (high heterogeneity) of the effect estimates.
McCarthy 2010 searched three databases (MEDLINE, CINAHL, and the Cochrane Library) from 1966 to 2009 and included all randomized controlled comparisons of lidocaine infusion with placebo in the surgical setting, and reported on postoperative analgesia and other aspects of participants' recovery from surgery. The review authors selected 16 RCTs, including 764 participants. They assessed the quality of all included studies using the Modified Oxford Scale. Outcome measures were: postoperative pain intensity, analgesic requirements, return of bowel function, length of hospital stay, intraoperative anaesthetic requirements, and adverse events. As a conclusion, the authors stated that lidocaine infusion in the perioperative period is safe and has clear advantages in participants undergoing abdominal surgery. From the 16 trials included in the McCarthy 2010 review, we included 15 in the present Cochrane Review. We excluded one study from our analysis, since lidocaine was given only in the postoperative period (Harvey 2009). The results for postoperative pain and hospital length of stay were more beneficial in the McCarthy 2010 review compared to the results presented in the current updated Cochrane Review.
Vigneault 2011 performed a systematic search using four databases (MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and SCOPUS) and grey literature. The review included all RCTs that used a placebo or any comparator, and evaluated IV lidocaine during general anaesthesia for any type of surgery. The review authors included 29 studies,, involving a total of 1754 participants. Two review authors evaluated the methodological quality of the included studies using an adaptation of the scale used by Cochrane, and the Jadad scale. Primary outcomes were: pain control, and opioid requirement. Secondary outcomes were: mortality, length of stay, ileus recovery time, nausea/vomiting, and adverse events. The review authors stated that abdominal surgery was strongly associated with benefit, and they further concluded that the incidence of adverse cardiac and neurologic events was comparable between both groups. From the 29 trials included in this review, we included 26 in the present Cochrane Review, and reasonably excluded the remaining three (Juarez‐Pichardo 2009; Knight 1980; Rinne 1998; see Characteristics of excluded studies). The presented results were more beneficial compared to the results presented in the current Cochrane Review update.
Sun 2012 systematically searched MEDLINE (1966 to 2010), CINAHL, the Cochrane Central Register of Controlled Trials (CENTRAL), and SCOPUS. The review authors included all RCTs of systemic administration of lidocaine for postoperative analgesia and recovery after abdominal surgery in adults. They performed quality assessment using a Modified 7‐point 4‐item Oxford Scale. They included 21 trials with 1108 participants in this review. Outcome measures were: opioid consumption, postoperative pain intensity, opioid‐related side effects, time to first flatus, time to first bowel movement, and length of hospital stay. The review authors concluded that perioperative systemic lidocaine may be a useful adjunct for postoperative pain management. From 21 included trials, we included 15 in the present Cochrane Review, and excluded the remaining six (Birch 1987; Cepeda 1996; Chia 1998; De Kock 1994; Harvey 2009; Juarez‐Pichardo 2009), for the reasons detailed in the 'Characteristics of excluded studies' tables. The presented results were more beneficial compared to the results presented in the current Cochrane Review update.
Chang 2017 performed a systematic search of four databases (MEDLINE, Embase, the Cochrane Library, and San Antonio Breast Cancer Symposium abstracts) in June 2015. The review authors included four RCTs with 167 participants comparing the effects of IV lidocaine with placebo or any other medications in patients undergoing breast surgery. We also included these four studies in the present Cochrane Review. Outcomes were: postoperative pain scores and analgesic consumption, as well as chronic postmastectomy pain. As a conclusion, the review authors stated that no significant benefits for pain relief are indicated using lidocaine.
For all comparative reviews, it has to be emphasized that their meta‐analyses results, when looking at effect estimates and 95% CIs, appear in similar or slightly more beneficial ranges to the ones found in the current Cochrane Review update. However, when considering not only precision (95% CI) but also dispersion (95% PI) of the mean effect estimates, the 95% PI crossed the line of identity in most cases (except for patient satisfaction and postoperative opioid consumption in PACU). Taking into account the 95% PIs and the GRADE assessment reported in this version of the review, quality of evidence is very low for all GRADE‐relevant outcomes, with the exception of pain (intermediate and late). For the latter, we are moderately confident that lidocaine has no effect on pain scores after 24 hours postoperatively. The focus of the current Cochrane Review update and the interpretive approach is different from all other systematic reviews mentioned above. Additionally, the more up‐to‐date search, the greater number of included trials, and the broader range of included surgery types, improved the precision and the external validity of the present review. On top of that, the present review analysed publication bias, imprecision, and inconsistency for each outcome, and provides sufficient background information to the study's details.
Authors' conclusions
Implications for practice.
In this systematic review update we found evidence of very low quality for an effect of intravenous (IV) lidocaine, compared to placebo or no treatment, on 'pain score at rest (early time points)', 'postoperative ileus', and 'time to first defaecation/bowel movement', 'postoperative nausea (overall)', and 'postoperative opioid consumption (overall)'. However, we have very little confidence in the estimated mean effects and the true effects may be substantially different from these, including ranges of clinical non‐relevance or even harm. In contrast, we found evidence of moderate quality for 'pain score at rest (at 24 hours and 48 hours)'. We are moderately confident that the true effects are close to the estimated mean effects, which are all in clinically non‐relevant ranges. The effect of IV lidocaine on adverse effects compared to placebo treatment is uncertain, as only a small number of studies systematically analysed the occurrence of adverse effects. The 18 studies in 'Characteristics of studies awaiting classification' may alter the conclusions of the review once we assess them.
The described effects on postoperative pain, when compared to placebo, are most obvious and evident in the immediate postoperative period (standardized mean difference (SMD) −0.5, which corresponds to a range of 0.37 cm to 2.48 cm on a visual analogue scale (VAS) 0 to 10 cm scale), defined as one to four hours postoperatively for the purpose of this review. Since the effect of lidocaine on 'pain at early time points', based on the current review, is associated with high uncertainty, we cannot currently give an answer as to in which settings lidocaine may be beneficial.
The described effects in the early postoperative phase may be considered relevant if conditions are prevalent that worsen the risk‐benefit ratio of more invasive treatments such as (thoracic) epidural analgesia or peripheral regional analgesia techniques. Such conditions include hereditary or acquired coagulation disorders, and treatment with anticoagulants resulting in absolute or relative contraindications to perform central neuraxial blocks. This may also include conditions with less precisely defined risk, e.g. patients receiving low molecular weight heparin (LMWH) in the presence of additional drugs interfering with coagulation (e.g. acetylsalicylic acid); or LMWH plus the presence of renal or liver diseases.
Further, the provision of epidural analgesia, e.g. for major abdominal surgery, may not be possible in distinct groups of patients or individual patients. Since the likelihood for complications is increased with prolonged and multiple attempts to perform regional techniques, such an intervention may also be considered appropriate if the insertion of a more invasive (neuraxial) analgesia technique has failed.
Since risk‐perception is highly subjective, the method of IV lidocaine may also be offered to patients who express fears in conjunction with potential complications of epidural analgesia, such as deep epidural infection, epidural bleeding, and temporary or persistent neurological sequelae (Popping 2008; Popping 2012).
Implications for research.
As almost all included studies analysed a small sample size (fewer than 200 participants), ideally larger trials would be necessary to reach confidence in the estimate of effects for all outcomes with very low quality evidence and to avoid the overestimation of the pleiotropic effects of perioperatively administered lidocaine on postoperative outcomes.
So far, we are not able to make any assumptions regarding the most appropriate dosing, timing (including the duration of administration) and the type of surgery that is most promising for this perioperative technique. However, the results are based on indirect comparisons of cohorts of participants studied in different heterogeneous clinical trials and settings. For this reason, clinical trials investigating a dose‐response and multiple surgical categories within one trial would be warranted to further elucidate and gain insights into these issues based on direct comparisons.
Upcoming indications of neuraxial analgesia include the treatment and secondary prevention of deep vein thrombosis, pulmonary embolism and non‐valvular atrial fibrillation, as well as postoperative use to prevent thromboembolism. As far as future indications of the investigated interventions are concerned, there are hints to assume that the likelihood of contraindications to apply central neuraxial analgesia or deep peripheral nerve blocks (e.g. paravertebral blocks, psoas compartment blocks) will increase. This is, amongst other reasons, due to the further spread of the use of direct (new) oral anticoagulants for various indications, e.g. rivaroxaban or apixaban. Unlike warfarin, these substances so far cannot be antagonized, rendering a regional analgesia technique in various surgical settings impossible. In this patient cohort described above, lidocaine may represent an alternative to neuraxial or regional analgesia. Thus, future studies may concentrate on participants unable (or even unwilling) to receive neuraxial or regional anaesthesia.
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2018 | Amended | Acknowledgement section updated |
History
Protocol first published: Issue 2, 2012 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 25 January 2017 | New citation required but conclusions have not changed | The results of the meta‐analyses have remained similar to the original review, but the interpretation (GRADE) has changed methodically. We have introduced the 95% prediction interval (PI) to understand the uncertainty of the mean effect estimates associated with heterogeneity. The conclusion has changed for all GRADE‐relevant outcomes, and we graded quality of evidence as very low for: pain (early), postoperative ileus, time to first defaecation/bowel movement, adverse events, postoperative nausea (overall), and opioid consumption (overall). Quality of evidence was moderate for pain at 24 hours and at 48 hours and we are moderately confident that lidocaine has no effect on pain later than 24 hours. In contrast to the original review (Kranke 2015), we were no longer able to demonstrate a significant subgroup difference for the different surgical subgroups investigating pain (early). |
| 25 January 2017 | New search has been performed | We updated the search to January 2017. We found 23 new trials that we incorporated into this update and identified a further six trials that we have placed in ‘Studies awaiting classification’. We ran a top‐up search in February 2018, and added 12 trial reports to the six studies already in 'Studies awaiting classification' (Choi 2017; Dewinter 2017; Jendoubi 2017; Kendall 2017; Khalili 2017a; Khalili 2017b; Kim 2017; Kim 2018; Lee 2017; Metha 2017; Sherif 2017; Song 2017). We will incorporate these studies when we next update the review. We have omitted data of up to eight studies per meta‐analysis of continuous outcomes (pain, gastrointestinal recovery, and opioid consumption) from a total of 12 studies with suspected variance reporting. The list of authors has changed. Peter Kranke moves from first to last author (contact author). Stephanie Weibel is the new first author. Johanna Jokinen left the review team and Yvonne Jelting and Antonia Helf are newly added. |
Acknowledgements
We would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia, Critical and Emergency Care Group (ACE), formerly Cochrane Anaesthesia Review Group (CARG)), Mathew Zacharias (Content Editor), Marialena Trivella (Statistical Editor), Jane Ballantyne and William Zempsky (Peer Reviewers), and Janet Wale (Consumer Editor) for their help and editorial advice during the preparation of the protocol for the review (Selig 2012); and Karen Hovhannisyan (ACE Information Specialist) for performing the professional literature search.
We would like to thank Andy Smith (Content Editor), Vibeke E Horstmann (Statistical Editor), Andrew Moore and Marc Gentili (Peer Reviewers), and Janet Wale (Consumer Editor) for their help and editorial advice during the preparation of the review (Kranke 2015).
We would like to thank Andrew Smith (content editor), Vibeke E Horstmann (statistical editor), Marc Gentili,Tim R McCormick (peer reviewers), Janet Wale (consumer editor) for their help and editorial advice during the preparation of the updated systematic review.
We would like to thank Elisabeth Friedrich‐Würstlein (University library, Wuerzburg) for her assistance during the literature search and organizing full texts of the relevant publications.
We thank Bita Mesgarpour for translation and data abstraction of the included Persian study (Ismail 2008); and Taru Jokinen for helping with the translation of Spanish articles during the search process.
We thank Wolfgang Viechtbauer, author of the R metafor package, for advice on multivariate meta‐analytic methods for independent tau2 estimation in subgroups.
We would like to explicitly thank Laurence Weinberg for the willingness to answer all of our open questions on his manuscript patiently until the end (Weinberg 2016). He was the only author that showed this commitment.
We would like to thank Johanna Jokinen for her contribution to the original review (Kranke 2015).
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Lidocaine] explode all trees #2 lidocain* or Lignocain* or Xylocain*:ti,ab #3 #1 or #2 #4 MeSH descriptor: [Pain, Postoperative] explode all trees #5 MeSH descriptor: [Postoperative Care] explode all trees #6 MeSH descriptor: [Postoperative Complications] this term only #7(postoperative near (pain* or recovery)):ti,ab #8 #4 or #5 or #6 or #7 #9 #3 and #8, in Trials
Appendix 2. MEDLINE (Ovid SP) search strategy
1 Lidocaine/ or (lidocain* or Lignocain* or Xylocain*).mp. 2 exp Pain, Postoperative/ or exp Postoperative Care/ or Postoperative Complications/ or Pain/ or ((post operative or postoperative) adj6 (pain* or recovery)).ti,ab. 3 ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 4 1 and 2 and 3
Appendix 3. Embase (Ovid SP) search strategy
1 lidocaine/ or (lidocain* or Lignocain* or Xylocain*).ti,ab. 2 postoperative pain/ or postoperative care/ or postoperative complication/dt, pc, rh or pain/pc or ((post operative or postoperative) adj6 (pain* or recovery)).ti,ab. 3 (controlled study.ab. or random*.ti,ab. or trial*.ti.) not (animal not human).sh. 4 1 and 2 and 3
Appendix 4. CINAHL (EBASCO host) search strategy
S1( MM "Lidocaine") OR ( lidocain* or Lignocain* or Xylocain* ) S2((MH "Postoperative Pain") OR (MH "Postoperative Complications/RH/TH/PC/DT") OR (MH "Postoperative Care") OR (MH "Pain/PC")) OR AB ((post operative or postoperative) N3 (pain* or recovery)) S3 S1 AND S2
Appendix 5. Study eligibility form
| Author (Year) | ||
| Journal | ||
| Title | ||
| Relevant study design | RCT | Yes/No/Unclear |
| Relevant population | Adults of any age and either sex undergoing any surgical procedure | Yes/No/Unclear |
| Relevant intervention | Experimental group must have received an intravenous lidocaine infusion, that had to be started prior to surgical incision and to be continued at least until the end of surgery | Yes/No/Unclear |
| If you have not answered Yes to all of the questions, please exclude the study. If you answered Yes to all questions, please continue to data extraction form and critical appraisal. | ||
Appendix 6. Data extraction form
| Author (year) | ||
| Journal/Source | ||
| Title | ||
| Study design | RCT | |
| Population | Experimental | Control |
| Age | ||
| Gender distribution | ||
| Weight | ||
| ASA physical status | ||
| Duration of anaesthesia | ||
| Duration of surgery | ||
| Main surgical procedure | ||
| Intervention | Experimental | Control |
| Duration of lidocaine infusion (if stated) | ||
| Dose of lidocaine infusion and bolus | ||
| Total administered dose of lidocaine infusion (if stated) | ||
| Starting point of administration before the operation (if stated) | ||
| Ending point of administration after the operation (if stated) | ||
| Dichotomous outcome data | Experimental (n) | Control (n) |
| Number of participants with postoperative ileus | ||
| Number of participants with occurrence of postoperative nausea (including PONV) or vomiting (early and overall) | ||
| Number of participants with surgical complications | ||
| Number of participants with cessation of the intervention | ||
| Number of participants with adverse effects following the lidocaine infusion (e.g. arrhythmia) |
| Continuous outcome data (unit of measurement) | Experimental (n) | Control (n) | ||
| n | Mean (SD) | n | Mean (SD) | |
| Functional gastrointestinal recovery (e.g. time to defaecation) | ||||
| Pain scores (e.g. VAS) | ||||
| Length of hospital stay | ||||
| Functional postoperative neuropsychological status scales | ||||
| Intraoperative opioid consumption | ||||
| Postoperative opioid consumption (early and overall) | ||||
| Patient satisfaction | ||||
Primary outcomes are written in bold letters.
Appendix 7. Critical appraisal form
| Author (Year) | ||
| Journal | ||
| Title | ||
| Random sequence generation | ||
| We considered sequence generation as adequate if it was generated by a random system (for example, computer, random number table algorithm, tossing of a coin). We considered sequence generation inadequate if a non‐random system was used (for example, names, dates). | ||
| State here method used to generate allocation and reasons for grading: | Low risk/High risk/Unclear | |
| Allocation concealment | ||
| We considered concealment adequate if an acceptable method, such as a central allocation system, sequentially numbered opaque sealed envelopes (SNOSE), or an on‐site locked computer, was used to ensure that the group assignment was not revealed to participant recruiters, investigators or participants prior to the final allocation in the respective group. We considered concealment to be inadequate if it allowed the participant recruiters, investigators or participants to know the treatment allocation in advance. | ||
| State here method used to conceal allocation and reasons for grading: | Low risk/High risk/Unclear | |
| Performance bias (blinding of participants and personnel) | ||
| We considered blinding adequate if participants and persons responsible for participants' care (for example, anaesthetists and nurses) were all blinded to the intervention. We considered blinding inadequate if participants and personnel were not blinded to the intervention and if outcomes were likely to be influenced. | ||
| State here method used for blinding: | Low risk/High risk/Unclear | |
| Detection bias (blinding of outcome assessors) | ||
| We considered blinding adequate if outcome assessors were all blinded to the intervention. We considered blinding inadequate if outcome assessors were not blinded to the intervention and if outcomes were likely to be influenced. | ||
| State here method used for blinding: | Low risk/High risk/Unclear | |
| Incomplete outcome data | ||
| We considered incomplete outcome data as adequately addressed if there were either no missing outcome data, or reasons for missing outcome data were described and unlikely to influence outcome analysis, or missing data had been inputted using appropriate methods. We considered incomplete outcome data as not adequately addressed if reasons for missing outcome data were likely to be related to true outcome, or reasons for missing outcome data were not described, or in the event of inappropriate application of simple imputation (for example, last observed carried forward (LOCF) analysis). | ||
| Are incomplete outcome data adequately addressed? | Low risk/High risk/Unclear | |
| Selective outcome reporting | ||
| We considered reports of a study to be free of suggestion or selective outcome reporting if a prospectively registered study protocol was available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review had been reported in the prespecified way. We considered reports not free of selective reporting if not all of the study's prespecified primary outcomes had been reported or if secondary outcomes in the protocol were chosen as primary outcomes in the study report due to statistical significance. We classified studies with retrospectively registered protocols or without protocols as unclear risk of bias. | ||
| Is the study free of selective outcome reporting? | Low risk/High risk/Unclear | |
| Other bias | ||
| We inspected studies for other potential threats to validity such as early stopping or baseline imbalances. | Low risk/High risk/Unclear | |
Data and analyses
Comparison 1. Intravenous (IV) lidocaine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score at rest, 'early time points' (1 h to 4 h, PACU) | 29 | 1656 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.72, ‐0.28] |
| 1.1 open abdominal surgery | 8 | 448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.82, ‐0.26] |
| 1.2 laparoscopic abdominal surgery | 10 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.34, ‐0.21] |
| 1.3 other surgery | 11 | 690 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.44, 0.02] |
| 2 Pain score at rest, 'intermediate time points' (24 h) | 33 | 1847 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.25, ‐0.04] |
| 3 Pain score at rest, 'late time points' (48 h) | 24 | 1404 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.25, 0.04] |
| 4 Postoperative ileus (dichotomous) | 4 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.15, 0.87] |
| 5 Time to first defaecation/bowel movement (h) | 12 | 684 | Mean Difference (IV, Random, 95% CI) | ‐7.92 [‐12.71, ‐3.13] |
| 6 Time to first flatus (h) | 13 | 785 | Mean Difference (IV, Random, 95% CI) | ‐4.09 [‐6.30, ‐1.87] |
| 7 Time to first bowel sounds (h) | 2 | 110 | Mean Difference (IV, Random, 95% CI) | ‐6.08 [‐13.77, 1.60] |
| 8 Length of hospital stay (days) | 32 | 2077 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.60, ‐0.15] |
| 9 Length of hospital stay (outpatient surgery, mins) | 3 | 191 | Mean Difference (IV, Random, 95% CI) | ‐10.81 [‐36.93, 15.31] |
| 10 Surgical complications ‐ anastomotic leak | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.80] |
| 11 Surgical complications ‐ bleeding | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.41, 7.89] |
| 12 Surgical complications ‐ postoperative infection | 5 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.41, 6.52] |
| 13 Patient satisfaction | 6 | 306 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.46, 1.06] |
| 14 Postoperative nausea, 'early time points' (PACU) | 8 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.98] |
| 15 Postoperative nausea, 'overall' (0 to 24 h, to 48 h, to 72 h) | 35 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.91] |
| 16 Postoperative vomiting, 'early time points' (PACU) | 4 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.48] |
| 17 Postoperative vomiting, 'overall' (0 to 24 h, to 48 h, to 72 h) | 19 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.08] |
| 18 Intraoperative opioid consumption (MEQ, mg) | 18 | 1116 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐3.87, ‐0.40] |
| 19 Intraoperative remifentanil consumption (µg) | 6 | 490 | Mean Difference (IV, Random, 95% CI) | ‐14.17 [‐35.27, 6.92] |
| 20 Postoperative opioid consumption, PACU (MEQ, mg) | 21 | 1219 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐3.87, ‐2.32] |
| 21 Postoperative opioid consumption, overall (MEQ, mg) | 40 | 2201 | Mean Difference (IV, Random, 95% CI) | ‐4.52 [‐6.25, ‐2.79] |
Comparison 2. Intravenous (IV) lidocaine versus thoracic epidural analgesia (TEA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10 cm) at rest, 'intermediate time points' (24 h) | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 1.51 [‐0.29, 3.32] |
| 2 Pain score (VAS 0 to 10 cm) at rest, 'late time points' (48 h) | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 0.98 [‐1.19, 3.16] |
| 3 Time to first bowel movement (h) | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐10.88, 7.56] |
| 4 Length of hospital stay (days) | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.38, 0.33] |
| 5 Intraoperative opioid consumption (MEQ, mg) | 2 | 100 | Mean Difference (IV, Random, 95% CI) | 7.27 [‐13.92, 28.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 2015.
| Methods | Prospective, randomized, placebo‐controlled study. Double‐blind. The purpose of this study was to evaluate the effectiveness of IV lidocaine in reducing postoperative pain for laparoscopic colectomy patients. The study was conducted in Korea. Date not published. |
|
| Participants | Number assessed for eligibility: 52 Number randomized: 50→ 25:25 Number analysed: 50→ 25:25 Inclusion criteria Adult patients scheduled to undergo a laparoscopic colectomy, age range 20 to 65 years Exclusion criteria Severe underlying cardiovascular, renal, or hepatic disease, allergic to local anaesthesia, weight < 45 kg or > 100 kg, patients who received opioid or nonsteroidal anti‐inflammatory drugs during the prior week or were taking these drugs chronically as a pain treatment, history of previous abdominal surgery Baseline details Experimental group (n = 25) Mean age (years): 64.48, SD = 11.68 M = 44%, F = 56% Mean weight (kg): 58.87, SD = 8.40 ASA I/II/III: 9:13:3 Mean duration of anaesthesia (mins): N/A Mean duration of surgery (mins): 216.60, SD = 56.29 Main surgical procedures (n): Laparoscopic colectomy (25) Control group (n = 25) Mean age (years): 66.20, SD = 8.88 M = 32%, F = 68% Mean weight (kg): 61.13, SD = 11.47 ASA I/II/III: 8:12:5 Mean duration of anaesthesia (mins): N/A Mean duration of surgery (mins): 204.20, SD = 75.69 Main surgical procedures (n): laparoscopic colectomy (25) |
|
| Interventions |
Experimental group (25 patients) Group L received IV lidocaine. Two minutes before orotracheal intubation, patients in group L received an IV bolus of lidocaine, 1.5 mg/kg. After induction of anaesthesia, lidocaine (2 mg/kg/hr) was continuous infused during the operation. Control group (25 patients) Group C received normal saline as a placebo. Patients in group C received an IV normal saline bolus and then received the same amount of a continuous infusion of normal saline as that of group L. |
|
| Outcomes | The primary endpoint of the study was pain score at 2 hrs. Dichotomous ‐ Postoperative nausea and vomiting (exact time point not mentioned (overall)). Continuous
|
|
| Notes |
Medication "All patients received fentanyl via PCA postoperatively. Postoperative nausea and vomiting were treated with 4 mg of intravenous ondansetron. " Anaesthesia All patients received the same anaesthetic protocol. Funding Ministry of Education, Science, and Technology |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomization into 1 of the 2 groups was based on a random table generated using PASS 11 (NCSS, Kaysville, Utah, USA). The randomization sequence was generated by a statistician who was not otherwise involved with the study.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “the details of the series were unknown to the investigators, and the group assignments were kept in sealed envelopes, each bearing only the case number on the outside.” Not explicitly mentioned opaque and sequentially envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “in order to keep the anesthesiologist ‘‘blind’’ to the patients’ assigned group, lidocaine or normal saline (placebo) was prepared in a syringe and a bottle that was only labeled with a case number. The preparations for the bolus and continuous infusion were arranged by an additional investigator who read the card.” Quote: “all parties involved, including the patients, surgeon, anesthesiologists, and investigator collecting the data, were unaware of the study drugs or the patients’ group assignment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “VAS scores were collected by 1 blinded investigator who had more than 2 years of experience interviewing patients regarding postoperative pain.” Quote:“all parties involved, including the patients, surgeon, anesthesiologists, and investigator collecting the data, were unaware of the study drugs or the patients’ group assignment.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 4%/12% Quote: “we used an intention‐to‐treat strategy—that is, all participants were included in the analysis regardless of whether they had completed the study. Missing data were completed using a last‐observed carried‐forward (LOCF) analysis.” Quote: “four patients had incomplete data because 1 patient in group L and 1 patient in group C were treated with other drugs to control shivering and because 2 patients in group C discontinued the study after stopping the patient‐controlled analgesics because of nausea induced by the fentanyl infusion.” The imputation method (LOCF) was inappropriate and may introduce bias to relevant outcomes. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Baral 2010.
| Methods | Randomized, placebo‐controlled trial. Participants and personnel were blinded. No statement on blinding of outcome assessors. The study aimed to assess the effectiveness of perioperative intravenous lidocaine infusion on postoperative pain intensity and analgesic requirement in patients undergoing major upper abdominal surgery. The study was conducted in Nepal. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 60→ 30:30 Number analysed: N/A, probably 30:30 Inclusion criteria Patients undergoing major upper abdominal surgery under general anaesthesia, 18 to 60 years, ASA I to II. Exclusion criteria Emergency surgery, hepatic or renal dysfunction, cardiac dysrhythmias/atrioventricular block, surgery > 3 hrs, hypersensitivity/allergy to study medication. Baseline details Experimental group (n = 30) Mean age (years): 36.8 M = 10%, F = 90% Mean weight (kg): 50.17 ASA I/II: 23:7 Mean duration of anaesthesia (mins): 63.13 Main surgical procedures (n): open cholecystectomy (26), open cholecystectomy with common bile duct exploration (4), partial gastrectomy (0) Control group (n = 30) Mean age (years): 35.63 M = 16.6%, F = 83.3% Mean weight (kg): 50.4 ASA I/II: 25:5 Mean duration of anaesthesia (mins): 70.17 Main surgical procedures (n): open cholecystectomy (26), open cholecystectomy with common bile duct exploration (3), partial gastrectomy (1) |
|
| Interventions |
Experimental group (30 patients) Lidocaine 2.0% (intravenous bolus 1.5 mg/kg followed by an infusion of 1.5 mg/kg/hr). The infusion started 30 mins before skin incision and stopped 1 hr after the end of surgery. Control group (30 patients) Patients received normal saline according to randomization. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication "A patient with VAS score of more than four was treated with injection of diclofenac sodium 75 mg IM If the patient’s VAS remained more than four even after 30 minutes of injection diclofenac sodium then injection tramadol 100 mg IV was given as rescue analgesic. Further and subsequent doses of diclofenac were allowed after an interval of 6 hours without exceeding a total dose of 225 mg in 24 hours." Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated codes…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...opaque envelopes…" Not explicitly mentioned sequentially numbered and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the anaesthesiologist, the surgeon, and the nursing staff all were kept unaware about the group allocation". Due to adequate blinding of personnel participants cannot know the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate: unclear. No statement on complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bryson 2010.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The purpose of this trial was to determine if intravenous lidocaine limited to the intraoperative period reduces length of hospital stay and improves functional recovery following abdominal hysterectomy. The study was conducted in Canada from June 2007 to October 2008 (NCT00382499). |
|
| Participants | Number assessed for eligibility: 279 Number randomized: 93→ 46:47 Number analysed: 44:46 Inclusion criteria Women, abdominal hysterectomy, ASA I to II Exclusion criteria ASA III, IV, and V, BMI < 18.5 or > 30 kg*m‐2, unable to use PCA, liver dysfunction, creatinine clearance < 50 ml*mins‐1, seizure disorder, hypersensitivity/allergy to amide‐type local anaesthetics study medication, chronic pain, opioid use more than once per week. Baseline details Experimental group (n = 44) Mean age (years): 46.3 M = 0%, F = 100% Mean weight (kg): 70.4 ASA I/II: 13:31 Mean duration of anaesthesia (mins): 105 Main surgical procedures: abdominal hysterectomy Control group (n = 46) Mean age (years): 45.4 M = 0%, F = 100% Mean weight (kg): 69.7 ASA I/II: 18:28 Mean duration of anaesthesia (mins): 108 Main surgical procedures: abdominal hysterectomy |
|
| Interventions |
Experimental group (46 patients) Lidocaine subjects received prior to induction of anaesthesia an intravenous bolus of 1.5 mg/kg followed by an infusion of 3 mg/kg/hr until skin closure. Control group (47 patients) Control subjects received matching placebo. |
|
| Outcomes | The primary endpoint of the study was length of hospital stay. Dichotomous
Continuous
|
|
| Notes |
Medication "All patients received antiemetic prophylaxis with dexamethasone 8 mg and ondansetron 4 mg. All wounds were infiltrated with 20 ml of 0.25% bupivacaine with adrenaline at skin closure. Postoperatively, all patients received celecoxib 200 mg po q12hr and acetaminophen 650 mg po q4hr until hospital discharge. Intravenous patient‐controlled morphine was prescribed with the following settings: boluses of 0.02 mg/kg, no continuous infusion, and a one‐hour maximum of 0.16 mg/kg/hr. Intravenous analgesia was discontinued when the patient tolerated a clear fluid diet. Morphine 5‐10 mg po q4hr prn was ordered for pain that was not controlled with celecoxib and acetaminophen." Anaesthesia All patients received a standardized balanced general anaesthetic. Funding "Trial expenses were funded by the Chair’s Research Fund, Department of Anesthesiology, University of Ottawa. Dr. Bryson was supported by the Ottawa Hospital Anesthesia Alternate Funds Association." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table. |
| Allocation concealment (selection bias) | Low risk | Central allocation. Quote: "campus‐specific randomization schedules were held by the research pharmacist at each campus. Study medications …prepared by the pharmacist in identical syringes labelled only with the patient’s unique study number". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "research personnel, patients, and attending anaesthesiologists were blinded to the contents of the syringes". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome measures were recorded by study personnel blinded to treatment allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 4%:2%. Quote: "five patients could not be contacted for follow‐up 7 days after surgery." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary outcomes that are of interest in the review have been reported. NCT00382499 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Cassuto 1985.
| Methods | Randomized, placebo‐controlled trial. No statement on sequence generation, allocation concealment and blinding. The study analysed the efficacy of a continuous low‐dose intravenous infusion of lidocaine on postoperative pain and the requirements for postoperative analgesics in patients after cholecystectomy. The study was conducted in Sweden. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 20→ 10:10 Number analysed: N/A, probably 10:10 Inclusion criteria Adult women/man, cholecystectomy Exclusion criteria Patients with hepatic, renal, or cardiovascular disease were excluded. Baseline details Experimental group (n = 10) Median age (years): 44 M = 60%, F = 40% Median weight (kg): 72 ASA I/II: N/A Mean duration of surgery (mins): 105 Main surgical procedures: cholecystectomy Control group (n = 10) Median age (years): 55 M = 50%, F = 50% Median weight (kg): 70 ASA I/II: N/A Mean duration of surgery (mins): 112 Main surgical procedures: cholecystectomy |
|
| Interventions |
Experimental group (10 patients) Half an hour before skin incision a bolus of lidocaine 100 mg was given followed by continuous infusion of lidocaine 2 mg/min for 24 hours postoperatively. Control group (10 patients) A placebo group received normal saline. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication "No meperidine was administered before the first pain assessment. When patients complained of pain they were given injections of 50 mg of meperidine intramuscularly until pain was relieved. Each patient's requirements for meperidine were recorded for 48 hr after surgery." Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No statement on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No statement on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate: unclear. There is no statement as to whether the presented results are for all patients who entered the trial or otherwise. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Chen 2015.
| Methods | Randomized, controlled trial. No exact statement on blinding of personnel. This study aimed to evaluate the effects of lidocaine treatment on cognitive impairment in aged patients undergoing spine surgery and to explore the underlying mechanism. The study was conducted in China from September 2013 to February 2015. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 87→ N/A:N/A Number analysed: 80→ 40:40 Inclusion criteria ASA I to II, > 65 years old, scheduled for spine surgery Exclusion criteria Mini‐Mental State Examination score < 23 before surgery, history of neurological diseases (including Alzheimer’s disease and stroke history), psychological disorder, and drug or alcohol abuse, history of diabetes mellitus, severe hypertension, severe anaemia, hepatic or renal dysfunction; unwillingness to comply with the protocol or procedures, inability to speak and read Chinese Baseline details Experimental group (n = 40) Mean age(± SD) (years): 71.3 ± 2.0 M = 57.5%, F = 42.5% Mean weight (± SD) (kg): 64.7 ± 4.3 ASA I/II: 16:24 Mean duration of anaesthesia (± SD) (mins): N/A Mean duration of surgery (± SD) (mins): 129.2 ± 7.4 Main surgical procedures (n): spine surgery (40) Control group (n = 40) Mean age (± SD) (years): 71.8 ± 1.9 M = 62.5%, F = 37.5% Mean weight (± SD) (kg): 63.8 ± 4.3 ASA I/II: 18:22 Mean duration of anaesthesia (± SD) (mins): N/A Mean duration of surgery (± SD) (mins): 128.3 ± 7.3 Main surgical procedures (n): spine surgery (40) |
|
| Interventions |
Experimental group (40 patients) Patients in the experimental group received a bolus of 1 mg/kg of lidocaine over 5 minutes administered after induction of anaesthesia and followed by a continuous infusion at 1.5 mg/kg/hr until the end of the surgery. Control group (40 patients) Normal saline administered as a bolus and an infusion with the same volume and rate changes as the lidocaine group. |
|
| Outcomes | The primary endpoint of the study were functional postoperative neuropsychological status scales. Dichotomous No dichotomous outcomes reported. Continuous
|
|
| Notes | ‐ Small trial sample size (< 200 patients) ‐ Power analysis not performed Medication N/A Anaesthesia All patients were anaesthetized using standard protocols. Funding This work was supported by grants from the Shandong Province Science and Technology Program. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly allocated…” No exact statement on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “both patients and the psychometrician were blinded to the treatment and group.” No statement on blinding before outcome assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “all study personnel were blinded to the results of the laboratory analysis.” Quote: “both patients and the psychometrician were blinded to the treatment and group.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate overall: 8% Quote: “seven patients were excluded because of refusal to neuropsychological evaluation after operation.” |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Choi GJ 2016.
| Methods | Randomized, controlled trial. Double‐blind. The purpose of this study was to evaluate the effects of intravenous lidocaine on pain following thyroidectomy. The study was conducted in Korea. Date not published. In the protocol it is stated that the study started in July 2011 and was planned to be completed in December 2014 (not verified). (NCT01608360) |
|
| Participants | Number assessed for eligibility: 62 Number randomized: 56→ 28:28 Number analysed: 56→ 28:28 Inclusion criteria 18 to 65 years of age, scheduled for elective total thyroidectomy Exclusion criteria body weight < 45 kg or > 100 kg; severe respiratory, renal, or hepatic disease; psychological disorders; history of allergies to local anaesthetics; preoperative use of analgesics, modified radical neck dissection for lateral neck lymph node metastasis Baseline details Experimental group (n = 28) Mean age (years): 49.89, SD = 8.48 M = 28.6%, F = 71.4% Mean weight (kg): 58.62, SD = 7.95 ASA I/II/III: 20:7:1 Duration of anaesthesia (mins) (median): 135.00, IQR (112.25 ‐ 170.00). Duration of surgery (mins) (media, IQR): 100.00, IQR (86.25 ‐140.00) Main surgical procedures (n): elective total thyroidectomy (28) Control group (n = 28) Mean age (years): 50.61, SD = 15.02 M = 17.9%, F = 82.1% Mean weight (kg): 58.16, SD = 7.50 ASA I/II/III: 22:4:2 Duration of anaesthesia (mins) (median): 135.00, IQR (120.00 ‐ 182.50) Duration of surgery (mins) (median): 107.50, IQR (90.00 ‐ 152.50) Main surgical procedures (n): elective total thyroidectomy (28) |
|
| Interventions |
Experimantal group (28 patients) Just prior to anaesthesia, patients in the lidocaine group received an intravenous bolus infusion of 1.5 mg/kg of lidocaine followed by a continuous infusion of 2 mg/kg/hr lidocaine until the end of surgery. Control group (28 patients) Patients in the control group received normal saline according to the same method. |
|
| Outcomes | The primary endpoint of the study was pain at PACU. Dichotomous
Continuous
|
|
| Notes |
Medication All patients received ramosetron prior to the end of surgery. No additional analgesics or premedication were administered. For postoperative pain control, a fentanyl PCA was provided. Metoclopramide was used as the initial antiemetic rescue medication. Rescue medication was offered for persistent nausea with a NRS ≥ 4. For nausea scores < 4, rescue medication was administered when requested. Ondansetron 4 mg was administered as a second antiemetic, at the investigator’s discretion. Anaesthesia The anaesthesia regime was standardized in both groups. Funding Ministry of Education, Science and Technology |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “blocks of four were generated using the Wei’s Urn model, and randomization was performed using PASSTM 11 software (NCSS, Kaysville, UT, USA). The randomization code was generated by a statistician who was not otherwise involved in the study.” |
| Allocation concealment (selection bias) | Low risk | Quote: “different researchers completed group randomization and drug preparation, and these researchers were not involved in perioperative management or data collection.” Quote: “researcher A prepared sequentially numbered and sealed opaque envelopes containing patient group information.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “researcher A delivered the allocation envelope to Researcher B, who opened and returned the envelope after identifying the assigned group, and then prepared trial medication (or placebo control) in a syringe pump labeled only with a case number. […] Researcher B delivered the prepared syringe pump to the anesthesiologist, who was not involved in the study.” Quote: “all surgical procedures were performed by an experienced endocrine surgeon who was unaware of patient group.” “All patients, investigators, and medical staff were blinded to group assignments during patient hospitalization.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “rescue analgesics and antiemetics were administered by nursing staff following direction by an investigator responsible for post‐operative data collection.” Quote: “a trained investigator, who was not involved in the perioperative patient management, was responsible for data collection during the postoperative period. All patients, investigators, and medical staff were blinded to group assignments during patient hospitalization.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or exclusions after randomization and no missing data |
| Selective reporting (reporting bias) | Unclear risk | There is a retrospective registered protocol available (NCT01608360, registered May 25, 2012; study start: July 2011). In the protocol, the primary outcome was pain at 2 hours. Secondary outcomes were among others pain at 4, 8, 12, 24, 48 hours. In the publication, the primary outcome was pain at all time points. The other secondary outcomes in the protocol were the same as in the publication. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Choi KW 2016.
| Methods | Randomized, controlled study. Double‐blinded. The purpose of this study was to evaluate the effect of intravenously administered lidocaine on the quality of recovery and on acute and chronic postoperative pain after robot‐assisted thyroidectomy. The study was conducted in the Korea from July 2013 to January 2015. (NCT01907997) |
|
| Participants | Number assessed for eligibility: 94 Number randomized: 90→ 45:45 Number analysed: 84→ 41:43 Inclusion criteria ASA I to II, scheduled to undergo elective robot‐assisted thyroidectomy Exclusion criteria history of chronic pain, chronic use of analgesics, allergy to local anaesthetics, severe cardiopulmonary, hepatic or renal disease, diabetes, and neuropsychiatric disease Baseline details Experimental group (n = 41) Mean age (years): 34.0, SD = 7.3 M = 9.8%, F = 90.2% Mean weight (kg): 58.7, SD = 8.4 ASA I/II: 39:2 Mean duration of anaesthesia (mins): 148.9, SD = 54.1 Mean duration of surgery (mins): 121.6, SD = 54.2 Main surgical procedures (n): thyroidectomy (41) Control group (n = 43) Mean age (years): 34.4, SD = 8.4 M = 2.3%, F = 97.7% Mean weight (kg): 58.0, SD = 9.0 ASA I/II: 37:6 Mean duration of anaesthesia (mins): 152.4, SD = 57.4 Mean duration of surgery (mins): 125.9, SD = 54.1 Main surgical procedures (n): thyroidectomy (43) |
|
| Interventions |
Experimantal group (41 patients) In the lidocaine group (Group L), 0.1 ml/kg of 2% lidocaine (2 mg/kg) was infused intravenously for 10 mins immediately after anaesthesia induction, and then, it was continuously infused at a rate of 0.15 ml/kg/hr of 2 % lidocaine (3 mg/kg/hr) until the patients were extubated. Control group (43 patients) The control group (Group C) received the same volumes of 0.9% normal saline during the same time periods. |
|
| Outcomes | The primary endpoint of the study were functional postoperative neuropsychological status scales (QoR‐40). Dichotomous
Continuous
|
|
| Notes |
Medication Before the end of the operation, patients received propacetamol 2 g for postoperative pain control and ramosetron 0.3 mg to prevent PONV. On the ward, 200 mg of oral ibuprofen was routinely administered three times per day to all of the patients until they were discharged. If a patient’s vascular endothelial growth factor score for pain was greater than four or if the patient requested an analgesic, he/she was intravenously administered fentanyl (50 µg) in the PACU or tramadol (25 mg) on the ward. Metoclopramide was administered as a rescue antiemetic agent if a patient suffered severe nausea or retching/vomiting. Anaesthesia The anaesthesia regime was standardized in both groups. Funding Ministry of Science, ICT and Future planning |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…the principal investigator (J.H.L.) randomly allocated the patients to either the control or the lidocaine group, using a randomization sequence generated by the web site www.randomizer.org/form.htm.” |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the other investigators, including the anesthesiologists responsible for the patients’ intraoperative care, the surgeons, and the nursing staffs, and the patients were blinded with regard to the groups to which the patients were assigned during the entire study period.” Quote: “an anesthetic nurse, who did not participate in the study, prepared the 2% lidocaine or the 0.9% normal saline in 50‐ml syringes in accordance with the principal investigator’s instructions. These injections were administered to the patients by the attending anesthesiologists who did not know the patients’ group allocations.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the other investigators, including the anesthesiologists responsible for the patients’ intraoperative care, the surgeons, and the nursing staffs, and the patients were blinded with regard to the groups to which the patients were assigned during the entire study period.” Quote: “all of the preoperative and postoperative data for this study were obtained by one investigator who was unaware of the groups to which the patients had been allocated.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 10%:5% One patient in the control group did not receive the allocated intervention because of conversion to open radical neck dissection and another one was lost to follow up after three months. Four patients in the intervention group didn't receive the allocated intervention due to decline to participate (n = 1), conversion to modified open radical neck dissection (n = 2) or surgical complication (n = 1). Reasons for missing data are unlikely to be related to true outcome (before start of the intervention). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01907997) as well as one conference abstract (Lee 2015). All of the study’s prespecified primary outcomes that are of interest in the review have been reported in the prespecified way. The protocol was prospectively registered (July 2013). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Choi SJ 2012.
| Methods | Randomized, controlled trial. No information provided on random sequence generation and allocation concealment. Participants and outcome assessors were blinded. Anaesthesiologists were unblinded. The study aimed to examine whether intraoperative systemic lidocaine may present beneficial effects on the recovery of bowel function, pain intensity, and analgesic consumption in patients undergoing various breast plastic surgeries. The study was conducted in Korea. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 60→ 30:30 Number analysed: (30:28:26)/(30:27:22) at 24 hrs: 48 hrs: 72 hrs postoperatively. Inclusion criteria Female patients, aged 20 to 60 years, ASA I to II, elective breast plastic surgeries. Exclusion criteria Severe hepatic, renal, cardiac, respiratory, or endocrine diseases, morbid obesity, or allergies to local anaesthetics. Episodes of intraoperative hypotension (mean BP < 60 mmHg) or bradycardia (heart rate < 40 beats/min), arrhythmia or urticaria associated with lidocaine infusion were also criteria for exclusion. Baseline details Experimental group (n = 30) Mean age (years): 41 M = 0%, F = 100% Mean weight (kg): 56 ASA I/II: N/A Mean duration of surgery (min): 295 Main surgical procedures (n): Augumentation mammaplasty (8), reduction mammaplasty (1), tissue expander removal + augmentation mammaplasty (single/both, 3:4), breast reconstruction with flap (10), mastectomy with implant (4) Control group (n = 30) Mean age (years): 40 M = 0%, F = 100% Mean weight (kg): 55 ASA I/II: N/A Mean duration of surgery (min): 288 Main surgical procedures (n): augumentation mammaplasty (5), reduction mammaplasty (1), tissue expander removal + augmentation mammaplasty (single/both, 5:2), breast reconstruction with flap (11), mastectomy with implant (6) |
|
| Interventions |
Experimental group (30 patients) 1.5 mg/kg bolus of lidocaine approximately 30 min before incision followed by continuous infusion of lidocaine (1.5 mg/kg/hr) until skin closure. Control group (30 patients) The control group was untreated. |
|
| Outcomes | The primary endpoint of the study was restoration of bowel function after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "No supplemental opioid was given to patients in either group during the maintenance of anaesthesia. For postoperative pain control, pethidine 0.5 mg/kg was provided within 30 min of the end of surgery and supplemented after recovery as needed with further boluses of 0.25 mg/kg at 20‐30 min intervals. Upon arrival to the post anaesthetic care unit (PACU), patients were connected to an intravenous patient controlled analgesic system (IVPCA) with fentanyl 1,500 μg and ketorolac 180 mg in 64 ml of saline (100 ml of total volume) to deliver a bolus of 1 ml of the above analgesics with a lockout time of 15 min and a basal rate of 1 ml/hr. After transfer to the ward, all patients received IVPCA, and extra rescue medications such as pethidine or NSAID according to body weight, if required." Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " …patients…were randomly and equally divided to two groups". No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: " participating patients … were all blinded to the patient's group assignment". "For the safety of patients, anaesthesiologists involved in the anaesthetic managements were not blinded to the groups. However, they were not involved in further management of postoperative pain control or data collection associated with this study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...surgeons, and medical investigators who were involved in the data collection, were all blinded to the patient’s group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): at 48 hrs: 7%:10%; at 72 hrs: 13%:27% Outcome data 48 hrs and 72 hrs after surgery were incomplete. Withdrawals were not described. High dropout rate at 72 hrs. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Cui 2010.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. This study evaluated the effects of systemic administration of lidocaine on postoperative pain and morphine requirements after propofol‐remifentanil‐based anaesthesia in patients undergoing thoracic surgery. The study was conducted in China from 1 January to 31 July 2008. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 45 → 22:23 Number analysed: 20:20 Inclusion criteria Patients (18 to 65 years) undergoing thoracic surgery of at least 3 to 6 hrs, ASA I to II Exclusion criteria Chronic pain, analgesics or opioids 7 days before surgery, drug or alcohol abuse, psychiatric disorder or obesity, cardiovascular disorder, central nervous disease they could communicate with the investigator, contraindications to propofol, opioids, and lidocaine; they had contraindications to the self‐administration of morphine (PCA device), their intra‐operative time lasted more than 6 hrs or their immediate extubation was not planned after surgery. Baseline details Experimental group (n = 20) Mean age (years): 54 M = 65%, F = 35% Mean weight (kg): 65 ASA I/II: 6:14 Mean duration of anaesthesia (min): 244 Main surgical procedures (n): pulmonary lobectomy (7), oesophagectomy (9), cardiectomy (4) Control group (n = 20) Mean age (years): 40 M = 0%, F = 100% Mean weight (kg): 55 ASA I/II: N/A Mean duration of anaesthesia (min): 288 Main surgical procedures (n): pulmonary lobectomy (6), oesophagectomy (9), cardiectomy (5) |
|
| Interventions |
Experimantal group (20 patients) No bolus; lidocaine was given as a continuous infusion (33 µg/kg/min) from induction of anaesthesia until skin closure. Control group (20 patients) The control group received the same volume normal saline. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication "During the preoperative anaesthetic evaluation, patients were instructed in the use of the PCA pump, the four‐point verbal rating scale (VRS‐4, 0 = no pain, 1 = slight pain, 2 = moderate pain, 3 = intense or severe pain) and the 100 mm VAS for pain (from 0 = no pain to 100 = worst pain), and were premedicated with 10 mg diazepam orally on the evening before surgery. Postoperative pain was treated with morphine. At the patient’s demand, boluses of morphine (1.0 to 2.0 mg, 2 min intervals) were given to keep the VRS‐4 score less than 2 and Riker’s sedation–agitation status less than 5 during the period immediately after general anaesthesia. Subsequently, 2 hrs after tracheal extubation, patients were connected to a PCA device set to deliver 1.0 mg morphine as an intravenous bolus with a 5min lockout interval, and this PCA regimen was continued for 48 hrs after completion of surgery." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "The present work was supported by the following grants: Clinical‐Basic Medicine Cooperation Fund of Capital Medical University, Research Fund of the Beijing Friendship Hospital, National Natural Science Foundation of China (30670782 and 30871219), Beijing Natural Science Foundation (5072008), Key Scientific Developing Programme of Beijing Municipal Commission of Education (KZ200810025012), Beijing Municipal Programme for Hundred‐Thousand‐Ten Thousand Excellent Talents of the New Century (Li J), and the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of Beijing Municipality (PHR200906116)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…a random‐number table was generated to specify the group each patient would be assigned upon entry into the trial." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...envelope containing the group assignment was prepared." Not clear if envelopes were sequentially numbered, sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…a nurse who was not involved in the patient’s evaluation opened the envelope and prepared remifentanil, lidocaine and physiological saline solution syringes." Quote: "the investigators involved in patient management or data collection were not aware of the group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the investigators involved in patient management or data collection were not aware of the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 9%:13% Quote: "...five patients were excluded from this research (three in the control group and two in the lidocaine group) because the duration of the operation exceeded 6 hrs." |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Dale 2016.
| Methods | Randomized, controlled trial. Double‐blinded. This study aimed to determine if intravenous lidocaine infusion reduces postoperative pain intensity following laparoscopic fundoplication surgery and to also validate the safety of intravenous lidocaine at the dose tested. The study was conducted in the Sydney Adventist Hospital, Wahroonga, Sydney, Australia. Date not published. The registered protocol states a recruitment period from May to December 2013 (early stopping). (ACTRN12613000440729) |
|
| Participants | Number assessed for eligibility: 33 Number randomized: 24→ 12:12 Number analysed: 24→ 12:12 Inclusion criteria All adults (age >18 years) undergoing laparoscopic fundoplication surgery by a single surgeon Exclusion criteria Allergies to local anaesthetics, chronic use of analgesics or corticosteroids, impaired hepatic function (any single liver function test ≥ 20 % normal reference range), epilepsy or other seizure disorder, severe cardiac failure (left ventricular ejection fraction ≤ 0.35) or cardiac arrhythmias and pregnancy Baseline details Experimental group (n = 12) Mean age (years): 68.5, SD = 10.17 M = 25%, F = 75% Mean weight (kg): N/A ASA I/II: N/A (given as mean) Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 68.83, SD = 20.76 Main surgical procedures (n): laparoscopic fundoplication (12) Control group (n = 12) Mean age (years): 66.5, SD = 11.39 M = 50%, F = 50% Mean weight(kg): N/A ASA I/II: N/A (given as mean) Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 64.50, SD = 19.58 Main surgical procedures (n): laparoscopic fundoplication (12) |
|
| Interventions |
Experimental group (12 patients) The patients in the intervention group received 1 mg/kg IV lidocaine bolus at induction, followed immediately by an infusion at 2 mg/kg/hr for 24 hours. Control group (12 patients) The patients in the control group were treated likewise using 0.9 % sodium chloride in a double‐blind fashion. |
|
| Outcomes | The primary endpoint of the study was pain (NRS). Dichotomous
Continuous
|
|
| Notes |
Medication Intraoperatively, all patients received IV granisetron 3 mg and dexamethasone 8 mg as prophylaxis against nausea and vomiting and parecoxib 40 mg for analgesia. Postoperative analgesia was commenced with fentanyl 1 µg kg−1 IV at the cessation of the remifentanil infusion. The diaphragmatic crura and port sites were infiltrated with 20 ml ropivacaine 0.2% by the surgeon. A PCA device administering IV fentanyl was provided (10 µg/ml, 10 µg bolus, 5 minute lockout, no background) and PCA usage was recorded. Fentanyl PCA was discontinued if nausea was reported by the patient. Acetaminophen (1 g IV every 6 hours) and indomethacin (100 mg per rectum every 12 hours) were administered to provide multi‐modal analgesia. Rescue antiemetics (ondansetron 4 mg sublingual and droperidol 0.5 mg/kg IV) were offered to any patient who experienced nausea or vomiting. Anaesthesia The anaesthesia regime was standardized in both groups. Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomly assigned to study groups in fixed blocks of 12 using a computer‐generated table of random numbers through the use of the randomization.com program. No stratification was used.” |
| Allocation concealment (selection bias) | Low risk | Quote: “the randomization schedule was stored in a locked cupboard that was only accessible by the randomization authority (thus concealed from all care providers and other research personnel). When a patient was recruited into the study, the randomization authority would prepare the appropriate study drug. The study drug was given to the anesthetist accompanied by a sealed, opaque, tamper‐proof envelope containing the treatment allocation. This envelope was kept in the patient file at all times in case serious adverse event required the knowledge of treatment allocation. Envelopes were examined at the completion of the trial to ensure that they were unopened.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the lidocaine and placebo study drug were visually identical. No patient, research nurse, investigator, or any other medical or nursing staff was aware of the treatment assignments for the duration of the study.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “no patient, research nurse, investigator, or any other medical or nursing staff was aware of the treatment assignments for the duration of the study.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 0%:0% Three patients in the intervention group did not complete the study. Quote: “the three patients who did not complete the study were withdrawn due to adverse events suspicious of lidocaine toxicity (treatment allocation was not known until after withdrawal from the trial).” It is not clear from the description how missing data from patients who did not complete the study were handled (imputation method not described). The reason for missing outcome data is likely related to true outcome. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified primary outcomes that are of interest in the review have been reported. Pain scores at 32 and 36 hours were planned to be examined but were only reported until 30 hours in the study publication. Nausea and vomiting are presented dichotomously instead of continuously. The secondary endpoint “oxycodone consumption” was prespecified but not reported in the end. The deviations from protocol and study report were minimal and did not bias the conclusion of the study. The study has been prospectively registered (ACTRN12613000440729) on 17 April 2013. |
| Other bias | Unclear risk | Early stopping. Quote: “the study would be stopped early when there was sufficient evidence to claim superiority (net benefit) or inferiority (net harm), or futility (little chance of achieving statistical significance) if the futility index was found to be > 0.8. […] A planned interim analysis was performed when recruitment was 66% complete. At this point, the trial was stopped early on the basis of futility, prior to reaching the target sample size of 36 patients (18 per group).” The gender was imbalanced between the groups (more female patients in experimental group may influence relevant outcomes). |
De Oliveira 2012.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The objective in the current study was to examine the effect of systemic lidocaine on postoperative quality of recovery in patients undergoing outpatient laparoscopic surgery. The study was conducted in the USA from November 2010 to September 2011 (NCT01250002). |
|
| Participants | Number assessed for eligibility: 88 Number randomized: 70 → 35:35 Number analysed: 31:32 Inclusion criteria Healthy females undergoing outpatient gynaecological laparoscopy. Exclusion criteria Patients with a history of allergy to local anaesthetics, chronic use of an opioid analgesic, corticosteroid use, and/or pregnant subjects were not enrolled. Reason for exclusion from the study after study drug administration was conversion from a laparoscopic to an open incision. Baseline details Experimental group (n = 31) Mean age (years): 37.2 M = 0%, F = 100% Mean BMI (kg/m2): 26.3 ASA I/II: 14:17 Mean duration of surgery (min): 105.5 Main surgical procedures (n): salpingo‐oophorectomy (14), cystectomy (8), tubal ligation (2), diagnostic laparoscopy (7) Control group (n = 32) Mean age (years): 39.1 M = 0%, F = 100% Mean BMI (kg/m2): 24.7 ASA I/II: 14:18 Mean duration of surgery (min): 105 Main surgical procedures (n): salpingo‐oophorectomy (13), cystectomy (13), tubal ligation (2), diagnostic laparoscopy (4) |
|
| Interventions |
Experimental group (31 patients) Patients received a 1.5 mg/kg bolus of lidocaine before induction of anaesthesia followed by a 2 mg/kg/hr infusion until the end of the surgical procedure. Control group (32 patients) Control patients received the same volume of saline. |
|
| Outcomes | The primary endpoint of the study was the Quality of Recovery‐40 questionnaire at 24 hrs after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "Hydromorphone 0.4 mg IV was administered every 5 minutes to maintain an NRS pain score < 4 of 10. In cases of postoperative nausea or vomiting, subjects received 10 mg IV metoclopramide, followed by 5 mg IV prochlorperazine if necessary. At the end of the procedure with the removal of the laparoscopic instruments, the remifentanil infusion was discontinued and the subjects received IV ketorolac 30 mg and ondansetron 4 mg. In cases of postoperative nausea or vomiting, subjects received 10 mg IV metoclopramide, followed by 5 mg IV prochlorperazine if necessary." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Supported by departmental funds" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated table of random numbers…" |
| Allocation concealment (selection bias) | Low risk | Quote: "group assignments were sealed in sequentially numbered opaque envelopes that were opened by a research nurse not involved with the patient care or data collection…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…blind subjects enrolled in the study…", "The same nurse prepared syringes labelled with study drug to blind … anaesthesia providers and investigators collecting the data." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "subjects were contacted by telephone 24 hours after the procedure by an investigator unaware of group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 11%:9% Three patients converted to open procedure. Four patients were lost during follow‐up. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way. (NCT01250002) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
De Oliveira 2014.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The main objective of this study was to evaluate the effect of systemic intraoperative lidocaine on postoperative quality of recovery when compared to saline after laparoscopic bariatric surgery. The study was conducted in the USA from August 2010 to October 2012 (NCT01180660). |
|
| Participants | Number assessed for eligibility: 62 Number randomized: 51 → 25:26 Number analysed: 24:26 Inclusion criteria Obese M or female undergoing laparoscopic gastric reduction surgery. Exclusion criteria History of allergy to local anaesthetics, chronic use of an opioid analgesic, corticosteroid, and/or pregnant subjects were not enrolled. Reason for exclusion from the study following study drug administration was conversion from a laparoscopic to an open incision. Baseline details Experimental group (n = 24) Median age (years): 44 M = 17%, F = 83% Median BMI (kg/m2): 47 ASA I/II: 7:17 Median duration of surgery (min): 144 Main surgical procedures (n): Roux‐en‐y gastric bypass (19), gastric sleeve (5) Control group (n = 26) Median age (years): 42 M = 12%, F = 88% Median BMI (kg/m2): 48 ASA I/II: 9:17 Median duration of surgery (min): 146 Main surgical procedures (n): Roux‐en‐y gastric bypass (19), gastric sleeve (7) |
|
| Interventions |
Experimental group (24 patients) Patients received lidocaine as a 1.5 mg/kg bolus before induction of anaesthesia followed by a 2 mg/kg/hr infusion until the end of the surgical procedure. Control group (26 patients) Control patients received the same volume of saline. |
|
| Outcomes | The primary endpoint of the study was the QoR‐40 questionnaire at 24 hrs after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "Hydromorphone 0.4 mg IV was administered every 5 mins to maintain a NRS pain score less than 4 out of 10. In cases of postoperative nausea or vomiting, subjects received 10 mg IV metoclopramide. Before PACU discharge, subjects were started on a PCA intravenous pump set to deliver 1 mg of intravenous morphine equivalent, no basal rate and lockout time of 10 min. Subjects also received 30 mg of intravenous ketorolac every 6 for 24 hrs. Total postoperative opioid consumption (24 hrs) was calculated in equivalent doses of intravenous morphine." Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized using a computer‐generated table of random numbers…" |
| Allocation concealment (selection bias) | Low risk | Quote: "group assignments were sealed in sequentially numbered opaque envelope that were opened by a research nurse not involved with the patient care or data collection after the subject provided written informed consent." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the same nurse prepared syringes labelled with study drug to blind subjects enrolled in the study, anaesthesia providers, and investigators collecting the data" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the same nurse prepared syringes labelled with study drug to blind subjects enrolled in the study, anaesthesia providers, and investigators collecting the data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 4%:0% One protocol violation (conversion to open surgery). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way. (NCT01180660) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Dewinter 2016.
| Methods | Randomized, controlled trial. Double‐blinded. The aim of this study to find out if the perioperative administration of lidocaine reduces postoperative pain in women undergoing laparoscopic sterilisation in day‐case surgery. The study was conducted in Leuven, Belgium, from November 2011 to May 2015. (EUDRACT 2011‐001315‐31) |
|
| Participants | Number assessed for eligibility: 116 Number randomized: 80→ 40:40 Number analysed: 79→ 39:40 Inclusion criteria ASA I to II, aged 18 years and older Exclusion criteria Hypersensitivity to lidocaine, ASA physical status III and IV, chronic opioid use, liver disease (total bilirubin ≥ 2 mg/dL), renal impairment (estimated glomerular filtration rate ≤ 60 ml/min/1.73m2), and epilepsy Baseline details Experimental group (n = 39) Age (years) (median): 37, range (19‐47) M = 0%, F = 100% Weight (kg) (median): 69, range (46‐108) ASA I/II: 26:13 Duration of anaesthesia (min) (median): N/A Duration of surgery (min) (median): 57, range (42‐101) Main surgical procedures (n): laparoscopic sterilization in women (39) Control group (n = 40) Age (years) (median): 40, range (27‐46) M = 0%, F = 100% Weight (kg) (median): 65, range (45‐100) ASA I/II: 29:11 Duration of anaesthesia (min): N/A Duration of surgery (min) (median): 54, range (36‐91) Main surgical procedures (n): laparoscopic sterilization in women (40) |
|
| Interventions |
Experimental group (39 patients) Patients in the L group were given an IV bolus injection of lidocaine 1.5 mg/kg at induction of anaesthesia followed by a continuous infusion of 1.5 mg/kg/hr, which was continued until 30 minutes after arrival at the PACU. Control group (40 patients) Patients in the P group were given equal volumes of saline. |
|
| Outcomes | The primary endpoint of the study was pain > 3 (NRS 0 to 10). Dichotomous
Continuous
|
|
| Notes |
Medication Patients were premedicated with alprazolam (0.5 mg orally) 1 hour before surgery. For postoperative pain control, paracetamol (15 mg/kg) and ketorolac (0.5 mg/kg) were administered after induction of anaesthesia. Dexamethasone 5 mg IV, droperidol 1.25 mg IV, and ondansetron 4 mg IV were given as antiemetic prophylaxis also at induction. Postoperative nausea and vomiting was treated with alizapride 50 mg IV. If the NRS exceeded 3, patients received piritramide boli of 2 mg IV. Pain medication prescribed at discharge was paracetamol 1 g by mouth 3 per day, ibuprofen 400 mg by mouth 3 per day, and tramadol hydrochloride 50 mg by mouth maximally 4 per day. The patients were recommended to take paracetamol and ibuprofen in a fixed scheme and tramadol only for breakthrough pain. Anaesthesia The anaesthesia regime was standardized in both groups Funding: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…patients were randomly assigned to either the lidocaine group (L group) or the placebo group (P group) using a computer‐generated random table…” |
| Allocation concealment (selection bias) | Low risk | Quote: “allocation concealment was ensured by enclosing assignment in sealed, opaque, sequentially numbered envelopes, which were opened only after arrival of the patient in the operation room.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “the study medication was prepared by an independent anesthetist not involved in the treatment or follow‐up of the study patients.” No explicit statement on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate: 3%:0% One patient in the intervention group was excluded from analysis due to a change in type of operation. Missing outcome data from one patient are unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way. The study has been prospectively registered on 26/07/2011 (EUDRACT 2011‐001315‐31). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
El‐Tahan 2009.
| Methods | Randomized, placebo‐controlled trial. No statement on allocation concealment. Participants, personnel, and outcome assessors were blinded. The study aimed to evaluate the effects of perioperative lidocaine on the haemodynamic and hormonal responses for caesarean delivery. The study was conducted in Saudi Arabia. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 90 → 45:45 Number analysed: 45:45 Inclusion criteria Women (ASA I and II) with uncomplicated, singleton pregnancy of at least 36 weeks of gestation, who refused regional anaesthesia and were scheduled for elective caesarean delivery under general anaesthesia. Exclusion criteria History of cardiac, liver, or kidney diseases; allergy to amide local anaesthetics; epilepsy; those taking cardiovascular medications; those with pregnancy‐induced hypertension; evidence of intrauterine growth restriction or foetal compromise. Baseline details Experimental group (n = 45) Mean age (years): 28.1 M = 0%, F = 100% Mean weight (kg): 75.3 ASA I/II: N/A Mean duration of anaesthesia (min): 43.2 Main surgical procedures: caesarean section Control group (n = 45) Mean age (years): 26.5 M = 0%, F = 100% Mean weight (kg): 75.4 ASA I/II: N/A Mean duration of anaesthesia (min): 40.8 Main surgical procedures: caesarean section |
|
| Interventions |
Experimental group (45 patients) Patients received an i.v. bolus of 1.5 mg/kg lidocaine 1.5% infused for 10 min, at 30 min before induction of anaesthesia, followed by constant infusion at 1.5 mg/kg/hrs of the same solution continued until 60 min after skin closure. Control group (45 patients) Placebo identical setting. |
|
| Outcomes | The primary endpoint of the study was post‐induction BP. Dichotomous
Continuous
|
|
| Notes |
Medication "Hydromorphone 0.4 mg IV was administered every 5 min to maintain a NRS pain score less than 4 out of 10. In cases of postoperative nausea or vomiting, subjects received 10 mg IV metoclopramide. Before PACU discharge, subjects were started on a PCA intravenous pump set to deliver 1 mg of intravenous morphine equivalent, no basal rate and lockout time of 10 min. Subjects also received 30 mg of intravenous ketorolac every 6 for 24 hrs. Total postoperative opioid consumption (24 hrs) was calculated in equivalent doses of intravenous morphine." Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the subjects were allocated randomly to two groups using a computer‐generated randomization code." |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " another anaesthesiologist, who was blinded to the study solution, gave the anaesthetic and was instructed to avoid using local anaesthetics, and a third performed the assessments. All staff in the operating room were unaware of the randomization code." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "...and a third performed the assessments." No statement on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Farag 2013.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The authors tested the primary hypothesis that perioperative IV lidocaine administration during spine surgery (and in the PACU for no more than 8 hrs) decreases pain and/or opioid requirements in the initial 48 postoperative hours. The study was conducted in the USA from September 2009 to October 2011 (NCT00840996). |
|
| Participants | Number assessed for eligibility: 2578 Number randomized: 116 → 58:58 Number analysed: 57:58 Inclusion criteria ASA Status I to III patients, between the ages of 18 and 75 yrs, who were scheduled for elective multilevel spine surgery with or without instrumentation. Exclusion criteria Contraindication to lidocaine, such as those with substantial hepatic impairment (alanine aminotransferase or aspartate transaminase more than twice normal), renal impairment (serum creatinine >2 mg/dl), seizure disorder requiring medication within 2 yrs, and/or planned epidural anaesthesia or analgesia. Baseline details Experimental group (n = 57) Mean age (years): 58 M = 61.4%, F = 38.6% Mean BMI (kg/m2): 29 ASA II/III + IV: 45:16 Case duration (min): 280 Superior vertebral region (n): cervical (28), thoracic (7), lumbosacral (25) Control group (n = 58) Mean age (years): 54 M = 60.3%, F = 39.7% Mean BMI (kg/m2): 30 ASA II/III+IV: 24:24 Case duration (min): 259 Superior vertebral region (n): cervical (26), thoracic (1), lumbosacral (31) |
|
| Interventions |
Experimental group (57 patients) Patients received IV lidocaine (2 mg/kg/hr) with maximum of 200 mg/hr starting at induction of anaesthesia and continuing until discharge from the PACU or a maximum of 8 hrs. Control group (58 patients) Control patients received an equal volume of saline placebo. |
|
| Outcomes | The primary endpoint of the study was reduction in pain/morphine requirements. Dichotomous
Continuous
|
|
| Notes |
Medication "Postoperatively, pain was treated with PCA with morphine sulfate at a concentration of 1 mg/ml, with a demand dose of 1 mg and a lockout interval of 10 min. Comparable doses of fentanyl or hydromorphone were used on patients unable to tolerate morphine. Bolus doses of opioid were provided if additional analgesia was required. Patients were transitioned to oral opioids on the first POD according to the pain management protocol at our institution." Anaesthesia The anaesthesia regime was not standardized ("Anesthetic, fluid, and transfusion management was at the discretion of the attending anesthesiologist.") Funding "Support was provided solely from institutional and/or departmental sources." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to one of two groups using a reproducible set of computer‐generated random numbers…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...that were maintained in sequentially numbered opaque envelopes until just before induction of anaesthesia." Not mentioned if envelopes were sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "investigators, clinicians, and patients were all fully blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "investigators, clinicians, and patients were all fully blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 2%:0% Patients with protocol violations (one in each group) were included in an intention‐to‐treat analysis; one patient was withdrawn from the study (rescheduled for outpatient surgery). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary outcomes that are of interest in the review have been reported in the prespecified way. (NCT00840996) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Grady 2012.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The study tested the hypothesis that perioperative IV lidocaine and/or ketamine in patients undergoing open abdominal hysterectomy improves rehabilitation as measured by a 6‐minute walk distance (6‐MWD) on the second postoperative morning. The study was conducted in the USA from September 2008 to October 2010 (NCT00721110). |
|
| Participants | Number assessed for eligibility: 212 Number randomized: 62 → ?:? (numbers in the reported flow diagram unclear) Number analysed: ?:? (numbers in the reported flow diagram unclear) Inclusion criteria ASA I to III patients between the ages of 18 and 75 years who were scheduled for elective open abdominal hysterectomy for fibroid disease or uterine myomectomy. Exclusion criteria Chronic pain at any site requiring treatment, contraindication to ketamine or lidocaine, history of significant axis I psychiatric disease, substantial hepatic (alanine aminotransferase or aspartate aminotransferase > 2 times normal) or renal (serum creatinine > 2 mg/dL) impairment, seizure disorder requiring medication within the previous 2 years, and planned spinal or epidural anaesthesia or analgesia. Baseline details Experimental group (n = 31) Mean age (years): 46 M = 0%, F = 100% Mean BMI (kg/m²): 29 ASA I/II/III: 0:87:13 Duration of anaesthesia (min): N/A Main surgical procedure: open abdominal hysterectomy for fibroid disease or uterine myomectomy Control group (n = 31) Mean age (years): 47 M = 0%, F = 100% Mean BMI (kg/m²): 29 ASA I/II/III: 6:81:13 Duration of anaesthesia (min): N/A Main surgical procedure: open abdominal hysterectomy for fibroid disease or uterine myomectomy |
|
| Interventions |
Experimental group (31 patients) Lidocaine was given as a bolus (1.5 mg/kg) at induction of anaesthesia, followed by lidocaine infusion of 2 mg/kg/hr for the first 2 hours, and then 1.2 mg/kg/hrs for 24 postoperative hours. Control group (31 patients) Placebo treatment. |
|
| Outcomes | The primary endpoint of the study was 6‐MWD. Dichotomous
Continuous
|
|
| Notes |
Medication "Initial postoperative pain was treated with bolus IV morphine and then with IV patient‐controlled morphine (bolus = 1 mg, lockout interval = 6 minutes, basal rate = 0). Fentanyl or hydromorphone was substituted in morphine‐intolerant patients. Patients transitioned on the first POD to oral acetaminophen 325 mg with oxycodone 5 mg every 4 hours as needed." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Supported by internal funding." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment assignments were based on computer‐generated, randomized assignments." |
| Allocation concealment (selection bias) | Low risk | Quote: "...maintained in sequentially numbered, sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "study medications were prepared by our hospital pharmacy, and all clinicians and investigators were blinded to treatment." Due to adequate blinding of personnel patients have to be unaware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "study medications were prepared by our hospital pharmacy, and all clinicians and investigators were blinded to treatment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate unclear. Two patients withdrew before they received interventions and were thus excluded from the study based on the modified intention‐to‐treat principle. From the study report it is unclear how many participants were allocated to the groups, excluded post‐allocation and were finally analysed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way. (NCT00721110) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Grigoras 2012.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The aim of this study was to evaluate the impact of intravenous lidocaine on acute and persistent post‐surgical pain, analgesic requirements, and sensation abnormalities in patients undergoing surgery for breast cancer. The study was conducted in Ireland from December 2008 to December 2009. |
|
| Participants | Number assessed for eligibility: 36 Number randomized: 36 → 17:19 Number analysed: 17:19 Inclusion criteria Women, breast cancer surgery, ASA I to II. Exclusion criteria Preexisting malignancy, chronic infection, pain conditions, pregnancy, diabetes, thyroid disorder, severe cardiac, renal or hepatic disease, previous breast surgery other than biopsy, psychiatric illness, neurological disease, contraindications for lidocaine or morphine use, and patient refusal. Baseline details Experimental group (n = 17) Mean age (years): 55.9 M = 0%, F = 100% Mean weight (kg): 70.8 ASA I/II: 6:11 Duration of surgery (min): 60.6 Main surgical procedure (n): mastectomy with axillary node clearance (3), wide local excision with sentinel lymph node mapping (14) Control group (n = 19) Mean age (years): 56.8 M = 0%, F = 100% Mean weight (kg): 68.1 ASA I/II: 8:11 Duration of surgery (min): 71.2 Main surgical procedure (n): mastectomy with axillary node clearance (5), wide local excision with sentinel lymph node mapping (14) |
|
| Interventions |
Experimental group (17 patients) Before induction of general anaesthesia, patients received a bolus of intravenous lidocaine 1.5 mg/kg followed by a continuous infusion of lidocaine 1.5 mg/kg/hr until 1 hr of skin closure. Control group (19 patients) The control group received an equal volume of saline. |
|
| Outcomes | The primary endpoint of the study was persistent post‐surgical pain at 3 month after breast surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "Intraoperative analgesia in both groups consisted of paracetamol IV 1 g, diclofenac IV 75 mg, and morphine sulphate PRN IV. Morphine was administered after induction of general anaesthesia and titrated according to patient response to surgical stimuli. Postoperatively, patients in both groups received a standard analgesic regimen (morphine sulphate by patient controlled analgesia pump, 1 mg maximally every 5 minutes; diclofenac sodium, 50 mg PO/PR, 12 hourly PRN; paracetamol, 1 g PO/PR, 6 hourly PRN; tramadol 100 mg intramuscular/by mouth PRN as rescue medication)" Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated to 1 of 2 groups based on computer generated code" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…sequentially numbered opaque envelopes." Not mentioned that envelopes were sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "on the morning of surgery an anaesthetist who was not involved in the patient’s evaluation opened the envelope... None of the investigators involved in patients management were aware of the group assignment." Due to adequate blinding of personnel patients have to be unaware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "none of the investigators involved in … data collection were aware of the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "each patient was assessed 3 month after surgery." No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Groudine 1998.
| Methods | Randomized, placebo‐controlled trial. No information provided on random sequence generation and allocation concealment. Participants and outcome assessors were blinded. Anaesthesiologists were unblinded. This study examined whether many of the beneficial effects on bowel function seen with epidural lidocaine are also present when the drug is given parenterally in patients undergoing radical retropubic prostatectomy. The study was conducted in the USA from May 1995 to August 1996. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 40 → 20:20 Number analysed: 18:20 Inclusion criteria Male patients undergoing radical retropubic prostatectomy, ASA I to II. Exclusion criteria Preexisting disorder of the gastrointestinal tract; used enemas, opioids, or anticholinergic medication chronically; or were ASA physical status III or more. Baseline details Experimental group (n = 18) Mean age (years): 64.4 (mean age of all experimental and control patients) M = 100%, F = 0% Mean weight (kg): N/A Mean ASA: 2.2 Duration of surgery (min): N/A Main surgical procedure: radical retropubic prostatectomy Control group (n = 20) Mean age (years): 64.4 (mean age of all experimental and control patients) M = 100%, F = 0% Mean weight (kg): N/A Mean ASA: 2.3 Duration of surgery (min): N/A Main surgical procedure: radical retropubic prostatectomy |
|
| Interventions |
Experimental group (18 patients) Before induction of general anaesthesia, patients received a bolus of intravenous lidocaine 1.5 mg/kg followed by a continuous infusion of lidocaine 1.5 mg/kg/hr until the patients arrived in PACU. The infusion was terminated 60 min after skin closure. Control group (20 patients) Control patients received an equal volume of saline. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication "Ketorolac (30 mg IV) was initiated for all patients in the PACU unless there was a contraindication (history of peptic ulcer disease, renal disease, or concerns about postoperative haemostasis) and continued (15 mg IV) every 6 hrs if needed for pain control. Morphine was used for breakthrough pain and for those patients not receiving ketorolac." Anaesthesia The anaesthesia regime was not standardized ("flexibility in opioid use"). Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…a number from 1 to 40 was randomly drawn. Even‐numbered patients received lidocaine. Odd‐numbered patients were assigned to the control group." Insufficient information about the sequence generation process provided. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the nursing staff, surgeons, and patients were all blinded."; "…anaesthesiologists were not blinded and were not involved in any of the data collection…" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "pain scores and inquiries about first flatus and bowel movements were all made by a nurse blinded only to the patient´s lidocaine status." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 10%:0% Quote: "two patients from the lidocaine group were excluded from analysis. One patient withdrew his consent before induction. The other patient had multiple surgical complications….The remaining 38 patients completed the study." The reason for missing data (due to surgical complications) is likely to have an impact on relevant outcomes. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Herroeder 2007.
| Methods | Randomized, placebo‐controlled trial. No information provided on allocation concealment. Participants and personnel were blinded. No statement on blinding of outcome assessors. This study aimed to evaluate beneficial effects of systemic lidocaine and to provide insights into underlying mechanisms in patients undergoing colorectal surgery. The study was conducted in Germany from September 2002 to December 2004. |
|
| Participants | Number assessed for eligibility: 77 Number randomized: 66 → 33:33 Number analysed: 31:29 Inclusion criteria Patients undergoing colorectal surgery, not willing or unable to receive an epidural catheter, ASA I to III, 18 to 75. Exclusion criteria Known allergies to local anaesthetics, chronic use of analgesics or corticosteroids, underlying inflammatory bowel disease (Crohn’s disease or ulcerative colitis), prolonged postoperative ventilatory support, impaired liver function, and severe cardiac arrhythmia. Baseline details Experimental group (n = 31) Mean age (years): 56.13 M = 61.3%, F = 38.7% Mean weight (kg): 75.88 ASA I/II/III: 2:21:8 Duration of surgery (min): 194.3 Main surgical procedure (n): Ileocecal resection (2), hemicolectomy (6), subtotal colectomy (0), proctocolectomy (1), sigmoid resection (12), anterior rectum resection (5), rectum extirpation (3), others (2) Control group (n = 29) Mean age (years): 56.93 M = 51.7%, F = 48.3% Mean weight (kg): 73.59 ASA I/II/III: 3:23:3 Duration of surgery (min): 210.5 Main surgical procedure (n): Ileocecal resection (0), hemicolectomy (9), subtotal colectomy (1), proctocolectomy (3), sigmoid resection (8), anterior rectum resection (4), rectum extirpation (2), others (2) |
|
| Interventions |
Experimental group (31 patients) Before induction of general anaesthesia, an intravenous lidocaine bolus (1.5 mg/kg) was administered followed by a continuous lidocaine infusion (2 mg/min) until 4 hours postoperatively. Control group (29 patients) Patients in the control group were treated likewise with normal saline. |
|
| Outcomes | The primary endpoint of the study was length of hospital stay. Dichotomous ‐ Surgical complications (anastomotic leak, gastrointestinal atonia defined as postoperative ileus > 5 days, wound healing disturbances) ‐ Adverse events (hospital mortality, morbidity) Continuous
|
|
| Notes |
Medication "After surgery, patients were transferred to the postoperative anaesthesia care unit (PACU) and discharged not earlier than 30 minutes after completion of lidocaine/saline treatment. PCA devices were adjusted to a demand dose of 2 mg piritramide and a lockout period of 10 minutes without basal infusion. Additionally, after transfer to the ward, 1 g metamizol or in case of contraindications 1 g paracetamol was given every 6 hours." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Supported in part by the Medical Faculty, University of Heidelberg, Germany (F203699 to S.P. and M.W.H; F206639 to S.H. and M.W.H.) and by institutional money from the Department of Anesthesiology, University of Heidelberg, Germany." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to either lidocaine or placebo treatment using the following multi step protocol to minimize effects of type and length of surgery. Dependent on the surgical procedure performed, patients were allocated to 2 different groups (colectomy vs. rectum resection). Each group was subdivided into blocks consisting of 6 patients." |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients in the control group were treated likewise using NaCl 0.9% in a double‐blinded fashion." "The study medication was prepared by an anaesthesiologist not involved in further treatment of the patients. The anaesthesia team and all other staff involved in patient care were blinded to study group assignments. " |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 6%:12% Quote: "because of intraoperative hypothermia, 2 patients in each group required prolonged ventilatory support and had to be excluded during the postoperative course. In addition, 2 patients of the control group dropped (unknown drug abuse)." |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Insler 1995.
| Methods | Randomized, placebo‐controlled trial. Sequence generation based on date of admission. No information provided on allocation concealment. Participants and personnel were blinded. No statement on blinding of outcome assessors. This study was designed to evaluate whether a continuous low‐dose lidocaine infusion reduces postoperative pain and anxiety in patients undergoing CABG and to retrospectively examine time to extubation, ICU stay, and hospital length of stay. The study was conducted in the USA. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 100 → 50:50 Number analysed: 44:45 Inclusion criteria Hospital patients undergoing first‐time CABG Exclusion criteria Patients > 75 years, hepatic dysfunction, vitamin K deficiency, serum albumin < 3.0 mg/dl, serum bilirubin > 2.0 mg/dl. Renal impairment, serum creatinine > 1.8 mg/dl, severe left ventricular dysfunction, concomitant valvular surgery, CABG reoperation, patients with pacemakers or atrial and/or ventricular arrhythmias Baseline details Experimental group (n = 44) Mean age (years): 62.65 (mean age of all experimental and control patients) M = 81%, F = 19% Mean weight (kg): 64.5 to 85.3 kg (range of weight for all experimental and control patients) ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure: CABG Control group (n = 45) Mean age (years): 62.65 (mean age of all experimental and control patients) M = 75%, F = 25% Mean weight (kg): 64.5 to 85.3 kg (range of weight for all experimental and control patients) ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure: CABG |
|
| Interventions |
Experimental group (44 patients) An infusion was begun after induction of anaesthesia and before surgical incision. An intravenous dose of 1.5 mg/kg was administered over a 10‐minute period, followed by an infusion of 30 µg/kg/min throughout surgery and for up to 48 hours in the ICU unless discharged earlier. Control group (45 patients) The control group received a placebo substitute. |
|
| Outcomes | The primary endpoint of the study was postsurgical pain. Dichotomous
Continuous
|
|
| Notes |
Medication "If the patient experienced pain, residual neuromuscular blockade or anxiety as evidenced by hypertension (systolic blood pressure greater than 150 mmHg), or tachycardia (heart rate greater than 100 beats per minute), if a conscious patient was unable to maintain a sustained 5‐second head lift, or if a patient experienced a direct communication of pain or discomfort in response to questioning, he or she was treated with intravenous fentanyl and/or midazolam via the following standardized regimen. Fentanyl was administered an 250‐1xg intravenous increments every 15 minutes until a total of I mg was reached. If these demonstrated features were still evident, then intravenous midazolam was administered in 0.5‐mg increments every 5 minutes until the patient was judged comfortable according to the previously cited criteria or a total of 5 mg was reached. If a hyperdynamic situation persisted, then propranolol was administered in 0.25‐rag intravenous increments until the situation abated or a total of 1.0 mg was administered." Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "a double‐blinded, randomized, and prospective approach." "Patients accepted into the study were numbered sequentially 1 through 100." |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…pharmacy‐prepared lidocaine infusion (L) in an 8 mg/ml concentration, or placebo substitute (P), numbered 1 through 100, was sent to the operating room on the day of surgery…" It is not clear who was responsible for randomisation and informed the pharmacy how to prepare the study drugs (i. e. which number referred to which group). Therefore, it is unclear if blinding of personnel and participants was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 12%:10% Eleven patients were excluded and reasons were described. Reasons for exclusion (e.g. ventricular arrhythmia) may be related to the intervention. One patient in the lidocaine group was excluded due to death (multi‐organ system failure). |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | High risk | Patients in the placebo group received an intravenous bolus of lidocaine (1.5 mg/kg) if ventricular ectopy or fibrillation occurred during surgery (CABG). |
Ismail 2008.
| Methods | Randomized, placebo‐controlled trial. No statement on blinding of participants, personnel, and outcome assessors within the abstract. This study was designed to assess the effect of lidocaine infusion on perioperative stress response, propofol and alfentanil consumption intraoperatively, recovery characteristics and postoperative analgesia during total intravenous anaesthesia in patients undergoing discectomy. The study was conducted in Egypt. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: N/A → 30:30 Number analysed: N/A Inclusion criteria Patients scheduled to undergo lumbar discectomy. Exclusion criteria N/A Baseline details Experimental group (n = 30) Mean age (years): N/A M = N/A, F = N/A Mean weight (kg): N/A ASA I/II: N/A Duration of anaesthesia (min): N/A Main surgical procedure (n): lumbar discectomy Control group (n = 30) Mean age (years): N/A M = N/A, F = N/A Mean weight (kg): N/A ASA I/II: N/A Duration of anaesthesia (min): N/A Main surgical procedure (n): lumbar discectomy |
|
| Interventions |
Experimental group (30 patients) Thirty minutes before anaesthesia induction, the patients in the lidocaine group received lidocaine bolus i.v. injection of 1.5 mg/kg, followed by an i.v. infusion of 1.5 mg/kg/hr until 10 minutes after extubation. Control group (30 patients) Thirty minutes before anaesthesia induction, the control group received a 0.1 ml/kg i.v. bolus of 0.9% saline, followed by a constant infusion at 0.1 ml/kg/hr continued until 10 minutes after extubation. |
|
| Outcomes | The authors stated within the abstract "Hemodynamic variables, plasma cortisol, propofol and alfentanil consumption, postoperative pain scores and analgesic rescue requirement were recorded." No detailed information provided within the abstract. | |
| Notes | Abstract only. No response on full text request from the authors as well as the journal. No relevant data for this review.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "sixty patients undergoing lumbar discectomy were randomly allocated to receive lidocaine (Lidocaine group) or saline (Control group)…". No method of randomization described within the abstract. |
| Allocation concealment (selection bias) | Unclear risk | No statement within the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No statement within the abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement within the abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement within the abstract. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Unclear risk | There is no assessment possible due to publication; in abstract form only. |
Jain 2015.
| Methods | Randomized, controlled trial. No exact statement on blinding. The aim of this study was to investigate whether IV perioperative lignocaine (bolus and infusion) would be able to produce both the effects simultaneously in elective laparoscopic cholecystectomies. The study was conducted in India. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 60→ 30:30 Number analysed: 60→ 30:30 Inclusion criteria ASA I to II, age between 20 and 50 years and weighing between 40 and 70 kg, undergoing elective laparoscopic cholecystectomy (non‐malignant) Exclusion criteria cardio‐respiratory, renal, hepatic or endocrine disease, predicted difficult tracheal intubation; whenever the surgical procedure necessitated the conversion of laparoscopic to open cholecystectomy or surgical time exceeded 180 min, patients were excluded from the study Baseline details Experimental group (n = 30) Mean age (years): 34.97, SD = 11.06 M = 0%, F = 100% Mean weight (kg): 53.90, SD = 9.06 ASA I/II (n): N/A Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 54.80, SD = 9.14 Main surgical procedures (n): laparoscopic cholecystectomy (30) Control group (n = 30): Mean age (years): 34.43, SD = 9.71 M = 0%, F = 100% Mean weight (kg): 52, SD = 10.31 ASA I/II (n): N/A Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 53.37, SD = 8.47 Main surgical procedures (n): laparoscopic cholecystectomy (30) |
|
| Interventions |
Experimantal group (30 patients): In Group Lidocaine, patients received ten min prior to induction preservative free lignocaine 2 % 1.5 mg/kg IV bolus (made to a volume of 6 ml with normal saline) administered over a period of 10 min and thereafter an infusion at a rate of 1.5 mg/kg/hr (pre‐diluted in normal saline made to a volume of 6 ml/hr). It was continued till the end of first post‐operative hour. The maximum duration of infusion was kept to 180 min (including 1 hr post‐operative infusion) as a safeguard against potential lignocaine toxicity. Control group (30 patients) Ten min prior to induction of anaesthesia patients received 6 ml normal saline as bolus over 10 min, followed by 6 ml/hr infusion. It was continued till the end of 1st post‐operative hour. The maximum duration of infusion was kept to 180 min (including 1 hr post‐operative infusion) as a safeguard against potential lignocaine toxicity. |
|
| Outcomes | The primary endpoint of the study was MAP (mmHg). Dichotomous
Continuous
|
|
| Notes |
Medication All patients were premedicated with injection midazolam 0.025 mg/kg IV, injection ketorolac 0.5 mg/kg IM (maximum of 30 mg), and injection ondansetron 0.1 mg/kg IV. First dose of ketorolac 0.5 mg/kg (maximum 30 mg) IM was administered when the NRS ≥ 4 was reported by the patient. Subsequently, if NRS was ≥ 4, the patient received injection ketorolac IM 6 hourly. Despite administration of ketorolac, if patient reported NRS ≥ 4, then injection pentazocine 0.25 mg/kg was administered. Anaesthesia The anaesthesia regime was standardized in both groups. Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “the patients were randomly divided (by chit‐ in‐ a‐ box technique).” There is insufficient information to decide whether this technique provided adequate randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “all the cases were done by same surgeon and anaesthesia given by the same team. […] Surgeon and the nursing staff in the recovery room were also blinded about the patient's group.” It is not explicitly stated that the attending anaesthetist and the patient were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “data collection was done by a team member who was blinded to the group of patient.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are no missing data. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kaba 2007.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The authors tested the hypothesis that perioperative lidocaine infusion facilitates acute rehabilitation protocol in patients undergoing laparoscopic colectomy. The study was conducted in Belgium from January 2003 until December 2004 (NCT00330941). |
|
| Participants | Number assessed for eligibility: 58 Number randomized: 45 → 22:23 Number analysed: 20:20 Inclusion criteria Patients scheduled to undergo laparoscopic colectomy, ASA I to III, age < 70 years Exclusion criteria Age greater than 70 years, history of gastroduodenal peptic ulcer or renal failure (contraindications to the use of nonsteroidal anti‐inflammatory drugs), hepatic insufficiency, psychiatric disorder, steroid treatment, or chronic treatment with opioid Baseline details Experimental group (n = 20) Mean age (years): 57 M = 75%, F = 25% Mean weight (kg): 77 ASA I/II/III: 7:10:3 Duration of anaesthesia (min): 169 Main surgical procedure (n): right/left colectomy (3:17), inflammatory bowel disease (1), poly resection (3), dolichosigmoid (2), diverticulitis (14) Control group (n = 20) Mean age (years): 52 M = 55%, F = 45% Mean weight (kg): 73 ASA I/II/III: 7:12:1 Duration of anaesthesia (min): 170 Main surgical procedure (n): right/left colectomy (6:14), inflammatory bowel disease (4), poly resection (5), dolichosigmoid (0), diverticulitis (11) |
|
| Interventions |
Experimental group (20 patients) Bolus injection of 1.5 mg/kg lidocaine at induction of anaesthesia, then a continuous infusion of 2 mg/kg/hr intraoperatively and 1.33 mg/kg/hr for 24 hrs postoperatively. Control group (20 patients) Patients received an equal volume of saline. |
|
| Outcomes | The primary endpoint of the study was anticipated time for recovery of bowel gastrointestinal function (flatus and defaecation). Dichotomous
Continuous
|
|
| Notes |
Medication "Postoperative analgesia was provided in both groups by the combination of the paracetamol (acetaminophen) precursor propacetamol (Pro‐Dafalgan®; UPSA Medica, Waterloo, Belgium; 2 g propacetamol = 1 g paracetamol), 2 g intravenously 30 min before the end of surgery and then every 6 hrs, and ketorolac, 30 mg intravenously every 8 hrs. PCA with piritramide (Dipidolor®; Janssen Pharmaceutica, Beerse, Belgium), a synthetic opioid, was used as rescue medication (bolus = 1 mg, lockout interval = 5 min, no basal infusion). Twenty‐four hours after the end of surgery, the intravenous infusion of lidocaine or placebo was stopped, and analgesia was provided with oral paracetamol, 1 g every 6 hrs; diclofenac (a nonsteroidal antiinflammatory drug), 75 mg twice daily; and 100 mg tramadol, if necessary." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Supported in part by a Clinical Research Grant granted to Dr. Kaba by the CHU de Liège, Liège, Belgium. Dr. Sessler is supported by grant No. GM 061655 from the National Institutes of Health, Bethesda, Maryland; the Gheens Foundation, Louisville, Kentucky; the Joseph Drown Foundation, Los Angeles, California; and the Commonwealth of Kentucky Research Challenge Trust Fund, Louisville, Kentucky." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated to two groups based on computer‐generated codes…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: " codes that were maintained in sequentially numbered opaque envelopes." Not mentioned that envelopes were sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "allocation envelopes were opened by a pharmacy staff member who then prepared either 2% lidocaine or saline in coded 50‐ml syringes.", "The anaesthesiologist in charge of the case was unaware of the patient’s group assignment; the study was thus fully double blinded." "The surgeons were unaware of the patient’s group assignment." Patients could not be aware of group assignment due to adequate blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the clinical personnel recording these data were not aware of the patient’s group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 9%:13% Quote: "of the 45 patients enrolled, 5 patients (3 in the control group and 2 in the lidocaine group) were eliminated from the study because the surgeon decided to convert their surgeries to laparotomies…" |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and all of the study's primary and secondary outcomes that are of interest in the review have been reported in the protocol. However, the protocol was retrospectively registered (May 2006). Participant enrolment (January 2003 to December 2004). (NCT00330941) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kang 2011.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. This study evaluated the effectiveness of intravenous lidocaine to reduce postoperative pain in inguinal herniorrhaphy patients. The study was conducted in Korea from December 2009 to September 2010. |
|
| Participants | Number assessed for eligibility: 87 Number randomized: 64 → 32:32 Number analysed: 32:32 Inclusion criteria Inguinal herniorrhaphy patients aged 18 to 65 years. Exclusion criteria Patients who weighed < 45 kg or > 100 kg, had severe underlying cardiovascular (especially atrioventricular block), renal or hepatic disease and were allergic to local anaesthetics were excluded. Patients were also excluded if they had received opioids or non‐steroidal anti‐inflammatory drugs within the previous one week or were taking these drugs chronically as pain treatment. Baseline details Experimental group (n = 32) Median age (years): 35.5 M = 69%, F = 31% Median weight (kg): 67 ASA I/II/III: 21:/6:/5 Duration of anaesthesia (min): 66.03 Main surgical procedure: unilateral inguinal hernia surgery Control group (n = 32) Median age (years): 34.5 M = 63%, F = 37% Median weight (kg): 66 ASA I/II/III: 25:4:3 Duration of anaesthesia (min): 63.38 Main surgical procedure: unilateral inguinal hernia surgery |
|
| Interventions |
Experimental group (32 patients) The lidocaine group received an intravenous bolus of 1.5 mg/kg lidocaine 2 min before orotracheal intubation followed by a continuous infusion of 2 mg/kg/hr. The intravenous infusion of lidocaine was started immediately and continued during the operation. Control group (32 patients) Control patients received an intravenous normal saline bolus injection followed by infusion of normal saline. |
|
| Outcomes | The primary endpoint of the study was the VAS pain score 2 hrs after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "The mode of post‐operative analgesia was continuous infusion of 0.1 µg/kg per hr fentanyl plus, by pushing a button on the PCA system, on‐demand release of a 0.1 µg/kg bolus (total regimen of 100 ml of fentanyl); the PCA had a lockout period of 15 min. In the case of a persistent VAS pain score > 30 mm, an additional rescue analgesia dose of 50 µg fentanyl was injected intravenously by an investigator to lower the VAS pain score to < 30 mm. Post‐operative nausea and vomiting were treated with 4 mg intravenous ondansetron as required." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "This research was supported by Chung‐Ang University Research Grants in 2010." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was based on computerized random‐number generation." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the group assignments were kept in a set of sealed envelopes, each bearing only the case number on the outside." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all patients, surgeons, anaesthesiologists and the investigator collecting data were unaware of patient´s group assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all patients, surgeons, anaesthesiologists and the investigator collecting data were unaware of patient´s group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate (experimental/control): 9%:13% Quote: "one patient in the control group was excluded from the study at the conclusion of the operation as meperidine was required to treat post‐operative shivering. Another patient who fit the inclusion criteria replaced this patient." We assume that the replacing patient was not randomized based on the description (no response from the authors upon request). Replacement may have an impact on relevant outcomes. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kasten 1986.
| Methods | Randomized, placebo‐controlled trial. No statement on random sequence generation and allocation concealment. No statement on blinding of participants, personnel, and outcome assessors. This study evaluates lidocaine as a supplement to fentanyl for the prevention of haemodynamic abnormalities during CABG surgery. The study was conducted in the USA. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 20 → 10:10 Number analysed: 10:10 Inclusion criteria Adult male, 45 to 65 yrs old, weight 60 to 100 kg, angiographically proven coronary artery disease, currently receiving a ß‐blocking drug were scheduled for elective CABG surgery. Exclusion criteria Left main coronary artery disease; ejection fraction of less than 45%; type II or III atrioventricular block; active seizure disorder; lidocaine allergy; hepatic disease; or cimetidine therapy. Baseline details Experimental group (n = 10) Mean age (years): 58.1 M, F (%): N/A Mean weight (kg): 76.8 ASA I/II: N/A Duration of anaesthesia (min): N/A Main surgical procedure: CABG surgery Control group (n = 10) Mean age (years): 55.1 M, F (%): N/A Mean weight (kg): 74.7 ASA I/II: N/A Duration of anaesthesia (min): N/A Main surgical procedure: CABG surgery |
|
| Interventions |
Experimental group (10 patients) Patients received intravenous lidocaine, 3 mg/kg, followed by an infusion of 0.05 mg/kg/min, as an adjunct to fentanyl. The infusion was started 2 min before tracheal intubation and continued until CPB was instituted and then restarted after conclusion of CPB when the patient was haemodynamically stable. Control group (10 patients) Patients received saline in addition to fentanyl. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication N/A Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No statement on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No statement on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals described. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kim HJ 2014.
| Methods | Randomized, controlled trial. Single‐blinded. The aim of this study was to compare the effect of lidocaine and dexmedetomidine infusion during off‐pump CABG. The study was conducted in Korea from September 2012 to August 2013. (NCT01688648) |
|
| Participants | Number assessed for eligibility: 174 Number randomized: 160→ 40:40:40:40 Number analysed: 153→ 36:40:39:38 Four groups, two not of interest (dexmedetomidine, lidocaine and dexmedetomidine (combined)) Inclusion criteria Patients undergoing off‐pump CABG by a single surgical team Exclusion criteria Surgery with pre‐planned CPB; patients diagnosed with arrhythmia with medication or pacemaker; unexpected conversion to CPB during the surgery Baseline details Experimental group (n = 36) Age (years) (median): 67, IQR (61 ‐ 72) M = 69.4%, F = 30.6% Mean weight (kg): 66, SD = 11 ASA I/II (n): N/A Mean duration of anaesthesia (min): 339, SD = 52 Mean duration of surgery (min): 283, SD = 50 Main surgical procedures (n): CABG (36) Control group (n = 38) Age (years) (median): 65, IQR (57 ‐ 72) M = 73.7%, F = 26.3% Mean weight (kg): 67, SD = 9 ASA I/II (n): N/A Mean duration of anaesthesia (min): 302, SD = 52 Mean duration of surgery (min): 247, SD = 50 Main surgical procedures (n): CABG (38) |
|
| Interventions |
Experimental group (36 patients) For the lidocaine infusion group (Group LIDO), lidocaine was infused at a dose of 2 mg/kg/hr from the start of anaesthesia induction after a bolus dose of 1.5 mg/kg. Both lidocaine and dexmedetomidine were infused until 24 hrs after the end of surgery on (POD 1). Control group (38 patients) Neither lidocaine nor dexmedetomidine was infused in the control group. |
|
| Outcomes | The primary endpoint of the study were cTnI levels. Dichotomous
Continuous
|
|
| Notes |
Medication Packed red blood cells were transfused when the hematocrit level was less than 25 % during the surgery. In all groups, remifentanil was infused at a dose range of 0.05 – 0.30 μg/kg/min during the surgery. Anaesthesia: The anaesthesia regime was standardized in both groups. Funding No external fund received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “subjects were randomly assigned at a 1:1:1:1 allocation ratio into one of four groups, Group LIDO, Group DEX, Group Combined or control group, using the random numbers generated by an internet‐based computer program (www.randomizer.org) […].” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] and sealed envelope technique.” Not explicitly mentioned sequentially numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “while the anesthesiologists were not blinded to the study drug, the participants, surgeon, and data analyst were kept blinded to the assigned group.” The attending anaesthesiologists were not blinded. It is unclear if outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate (LIDO/DEX/COMB/Control): 10%:0%:3%:5% Quote: “of the 160 patients initially randomized, four patients were excluded from the study due to unexpected conversion to surgery with CPB. Three patients were further excluded from the analysis because there was a missing laboratory value. We analyzed 36 patients in Group LIDO, 40 in Group DEX, 39 in the Group Combined, and 38 in the control group.” Reasons for missing outcome data are explained in the text. According to the flow diagram, the seven missing patients were excluded because of unexpected conversion to surgery with CBP and not due to missing laboratory values. It is not clear from the description, if patients refused blood draw or follow up. It remains unclear if reasons for missing outcome data are related to true outcome. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest have been reported in the prespecified way (NCT01688648). The secondary outcomes blood sodium, potassium and calcium level as well as hypokalaemia and the incidence of arrhythmia have been prespecified but have not been reported in the publication (these outcomes are not of interest for the review). The study has been prospectively registered (first received: 10 September 2012). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kim HO 2014.
| Methods | Randomized, controlled trial. Single‐blinded. The aim of this study was to evaluate the tolerability of early oral feeding following laparoscopic colorectal cancer surgery and the effects of intravenous lidocaine. The study was conducted at Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea between April 2011 and June 2012. (NCT01346917) |
|
| Participants | Number assessed for eligibility: 129 Number randomized: 77→ 38:39 Number analysed: 68→ 32:36 Inclusion criteria Men and non‐pregnant women aged 18 years or older who were scheduled to undergo elective laparoscopic colorectal cancer surgery Exclusion criteria Allergy to local anaesthetic agents or severe cardiovascular, pulmonary, hepatic or renal diseases that could interfere with study outcomes, ASA IV, peritoneal carcinomatosis, open conversion or a stoma created for faecal diversion Baseline details Experimental group (n = 32) Mean age (years): 60.9, SD = 10.6 M = 71.9%, F = 28.1% Mean weight (kg): N/A ASA I/II /III (n): 11:20:1 Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): N/A Main surgical procedures (n): laparoscopic colectomy (32) Control group (n = 36) Mean age (years): 60.1, SD = 11.1 M = 63.9%, F = 36.1% Mean weight (kg): N/A ASA I/II /III (n): 19:15:2 Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): N/A Main surgical procedures (n): laparoscopic colectomy (36) |
|
| Interventions |
Experimental group (32 patients) A loading dose of 1 mg/kg lidocaine was administered prior to skin incision, and a continuous dose of 1 mg/kg/hr lidocaine with 90 mg ketorolac (NSAID) (in normal saline, total 240 ml) was administered for 24 hrs. Control group (36 patients) Patients randomized to the placebo control group received a loading dose of 5 ml saline and a continuous dose of 90 mg ketorolac in 240 ml saline. |
|
| Outcomes | The primary endpoint of the study was the prevalence of postoperative nausea/vomiting and intolerance to early oral feeding caused by nausea/vomiting. Dichotomous
Continuous
|
|
| Notes |
Medication All patients received standard mechanical bowel preparation with polyethylene glycol. Glycopyrolate IM injection was used as a preoperative medication for all patients. After surgery, glycopyrrolate and pyridostigmine were administered to reverse the residual neuromuscular blockade. Epidural anaesthesia or other procedures for pain control were not performed in either group. During continuous infusion of 90 mg ketorolac for 24 hrs, relaxation therapy was administered initially for pain management followed by 25 to 50 mg meperidine by slow intravenous injection as needed for pain relief. After removal of the continuous infusion of ketorolac, patients initially received NSAIDs followed by meperidine for pain. Anaesthesia The anaesthesia regime was standardized in both groups. Funding Medical research funds from Kangbuk Samsung Hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were assigned by computerized blocked randomization (block size was four) after stratification by surgical site (right colectomy versus left colectomy and anterior resection) and method (conventional laparoscopic versus hand‐assisted laparoscopic surgery).” |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “this study was single‐blinded with regard to subjects. The study medication was prepared by an anaesthesiologist not involved in further treatment of the patients. Surgeons, patients and the researcher who collected the clinical data in the surgical ward were blinded to study group assignments during the postoperative course. The anaesthesiologist participated in this study was the only person who was not blinded.” “This trial was single‐blinded with regard to subjects because the total dose of lidocaine was heterogeneous among the subjects. Lidocaine was prepared and administered by an anaesthesiologist who participated in this trial. However, this trial was somewhat double‐blind because postoperative management and data collection on the outcome measures were performed by surgeons and the researcher, respectively, who were blinded to study group assignments during the postoperative course.” It is not clear from the description if the attending anesthesiologist who performed anaesthesia was blinded to the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “surgeons, patients and the researcher who collected the clinical data in the surgical ward were blinded to study group assignments during the postoperative course.” “All VAS scoring was performed by the attending nurse who was unaware of the ongoing study.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 16%:8% Quote: “nine patients had to be excluded during the perioperative course.” Six patients from the experimental group were excluded (decline to participate (n=2), ileostomy formation (n=3), open conversion (n=1)) and three from the control group (carcinomatosis (n=1) and ileostomy formation (n=2)). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01346917). All of the study’s prespecified primary outcomes that are of interest in the review have been reported in the prespecified way. The protocol was prospectively registered (April 2011). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kim KT 2014.
| Methods | Randomized, controlled trial. Double‐blinded. The aim of this study was to evaluate the analgesic effect of lidocaine infusion on postoperative pain after lumbar microdiscectomy. The study was conducted in Korea from March 2011 to April 2012. (NCT01319682) |
|
| Participants | Number assessed for eligibility: 66 Number randomized: 51→ 25:26 Number analysed: 51→ 25:26 Inclusion criteria Adult patients, presence of a lumbar disc herniation on magnetic resonance imaging and persistent radiating pain in the leg after 6 weeks of conservative treatment Exclusion criteria Less than 45 kg or more than 100 kg; history of prior spinal surgery at the same level; severe underlying respiratory, renal, hepatic, or cardiologic disease; history of allergic reactions to local anaesthetics, evidence of previous opioid usage or a psychiatric medical history Baseline details Experimental group (n = 25) Age (years) (median): 52.00, IQR (44.50 ‐ 57.50) M = 52%, F = 48% Mean weight (kg): 65.05, SD = 7.08 ASA I/II /III (n): 3:19:3 Mean duration of anaesthesia (min): N/A Duration of surgery (min) (median): 110.00, IQR (80.0 ‐ 140.00) Main surgical procedures (n): elective one‐level laminectomy and discectomy (25) Control group (n = 26) Age (years) (median): 48.00, IQR (41.00 ‐ 56.00) M = 30.8%, F = 69.2% Mean weight (kg): 64.62, SD = 9.50 ASA I/II /III (n): 5:19:2 Mean duration of anaesthesia (± SD) (min): N/A Duration of surgery (min) (median): 107.50, IQR (55.00 ‐ 135.00) Main surgical procedures (n): elective one‐level laminectomy and discectomy (26) |
|
| Interventions |
Experimental group (25 patients) Preoperatively and throughout the surgery, patients assigned to Group L received an IV bolus injection of 1.5 mg/kg lidocaine followed by a continuous infusion of 2 mg/kg/hr. Control group (26 patients) Group C received the same amount of normal saline injection as a placebo. |
|
| Outcomes | The primary endpoint of the study was VAS (0 to 100mm) pain score at 4 hrs after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication The patients did not receive premedication. No additional analgesics were injected during the surgery. Patients received postoperative fentanyl‐PCA (continuous infusion of 0.1 µg/kg/hr, total regimen of 100 ml and a 0.1 µg/kg bolus with a lockout interval of 15 min). In the case of persistent pain exceeding a visual analogue scale (VAS) score of 30 mm, an additional 50 mg of fentanyl was IV injected by an investigator until the pain was relieved to a level falling below a VAS pain score of 30 mm. Anaesthesia The anaesthesia regime was standardized in both groups. Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomization into one of the two groups was based on a random table generated using an R‐program. Block randomization with a block size of four or six and equal allocation was used to prevent imbalances in treatment assignments. The randomization sequence was generated by a statistician who was not involved with the study.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “ […] and the group assignments were kept in sealed envelopes, each bearing only the case number on the outside.” Not explicitly mentioned opaque and sequentially numbered envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the decision to enroll or exclude patients was made by the investigator, who did not otherwise participate in conducting the study and data collection.” Quote: “to keep the anesthesiologist ‘‘blind’’ to the patients’ assigned group, the patients were given lidocaine or normal saline without labels. Preparation of the bolus and continuous infusion was arranged by an additional investigator reading the card.” Quote: “all parties involved, including the patients, the surgeon, the anesthesiologists, and the investigator preparing drugs and collecting data were unaware of the study drugs or the patients’ group assignment, with the exception of the study coordinator (HK).” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “all parties involved, including the patients, the surgeon, the anesthesiologists, and the investigator preparing drugs and collecting data were unaware of the study drugs or the patients’ group assignment, with the exception of the study coordinator (HK).” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 0%:0% Quote: “four patients dropped out during the study. Three patients in Group C and one in Group L were treated by other pain killers, because of PONV that was unresponsive to antiemetic treatment and likely induced by fentanyl injection.” Quote: “missing data were completed using a last observation carried forward analysis.” Reasons for dropout were reliably described. Data from patients who dropped out are likely to influence outcomes of interest due to LOCF analysis. The imputation method (LOCF) was inappropriate and may introduce bias to relevant outcomes. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified primary outcomes that are of interest in the review have been reported in the prespecified way. (NCT01319682) The outcomes ‘pain at 2 and 8 hours’ and the outcomes ‘satisfaction’, ‘PONV’ and ‘length of hospital stay’ have not been prespecified. The protocol was prospectively registered (March 2011). |
| Other bias | Unclear risk | The gender was imbalanced between the groups (more female patients in control group may influence occurrence of PONV). |
Kim TH 2011.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The aim of this study was to compare the analgesic effect of intravenous lidocaine injection to that of intraperitoneal lidocaine instillation in patients who were undergoing laparoscopic appendectomy. The study was conducted in Korea from March 2009 until December 2009 (ACTRN12610000649011). |
|
| Participants | Number assessed for eligibility: 83 Number randomized: 68 → 22:21, (25: intraperitoneal instillation of lidocaine) Number analysed: 22:21 Inclusion criteria Patients (age range = 18 to 65) who underwent laparoscopic appendectomy for unperforated appendicitis. Exclusion criteria Body weight below 45 kg or greater than 100 kg, a history of severe underlying cardiovascular, pulmonary, renal, or hepatic disease, and an allergic reaction to local anaesthetics. Baseline details Experimental group (n = 22) Mean age (years): 38.5 M = 41%, F = 59% Mean BMI (kg/m2): 22.9 ASA I/II/III: 18:3:1 Duration of anaesthesia (min): 70 Main surgical procedure: laparoscopic appendectomy Control group (n = 21) Mean age (years): 32 M = 48%, F = 52% Mean BMI (kg/m2): 23.9 ASA I/II/III: 15:3:3 Duration of anaesthesia (min): 64 Main surgical procedure: laparoscopic appendectomy |
|
| Interventions |
Experimental group (22 patients) Patients received an intravenous bolus injection of 1.5 mg/kg lidocaine followed by a continuous infusion of 2 mg/kg/hr and normal saline intraperitoneal instillation 2 min before orotracheal intubation till end of surgery. Control group (21 patients) Patients received intravenous normal saline and an intraperitoneal instillation of normal saline was applied. |
|
| Outcomes | The primary endpoint of the study was the VAS pain score 2 hrs after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "To control postoperative pain, intravenous fentanyl with a computerized intravenous PCA system was used. The mode of PCA was a bolus of 0.1 µg/kg, a lockout interval of 15 min, and a continuous infusion of 0.1 µg/kg/hr (total regimen: 10 µg/kg/100 ml). The patients were taught to push the button of the PCA system to get a bolus of drug each time pain occurred. In the case of persistent pain greater than a visual analogue scale (VAS) pain score of 30 mm, an additional 50 µg of fentanyl was injected intravenously by the investigator until the pain was relieved to a level below a VAS pain score of 30 mm. No other analgesics such as NSAIDs or acetaminophens were included." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A100054)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization into one of the three groups was based on Excel (Microsoft Corp., Redmond, WA) random number generation." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...the numbers were contained in a set of sealed envelopes." Not mentioned sequentially numbered and opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "in order to keep the surgeon and the anaesthesiologist ‘‘blind’’ to the patient’s group, the patients were given lidocaine or normal saline as placebo, unlabeled, by an investigator who read the card." Patients could not be aware of group assignment due to adequate blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "two investigators who were blinded to the details of the study collected the postoperative data." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and all of the study´s primary and secondary outcomes that are of interest in the review have been reported in the protocol. However, the protocol was retrospectively registered (August 2010). First participant enrolment (March 2010). (ACTRN12610000649011) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kim TH 2013.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The aim of this study was to assess the effect of intravenous lidocaine injection on postoperative pain in patients who had undergone laparoscopy‐assisted distal gastrectomy. The study was conducted in Korea from March 2011 to December 2011 (ACTRN12612000007831). |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 34 → 17:17 Number analysed: 17:17 Inclusion criteria Patients who required laparoscopic gastrectomy for preoperatively diagnosed early gastric cancer. Exclusion criteria Advanced renal and pulmonary disease, heart failure, and hypersensitivity to lidocaine were excluded; those who were required to convert to laparotomies. Baseline details Experimental group (n = 17) Median age (years): 59 M = 65%, F = 35% Mean weight (kg): 63.66 ASA I/II/III: 1:14:2 Duration of anaesthesia (min): 324 Main surgical procedure: laparoscopy‐assisted distal gastrectomy Control group (n = 17) Median age (years): 62 M = 59%, F = 41% Mean weight (kg): 64.38 ASA I/II/III: 1:14:2 Duration of anaesthesia (min): 308.94 Main surgical procedure: laparoscopy‐assisted distal gastrectomy |
|
| Interventions |
Experimental group (17 patients) Patients received an intravenous bolus injection of 1.5 mg/kg lidocaine followed by a continuous infusion of 2 mg/kg/hr (preoperatively and throughout the surgery). Control group (17 patients) Control patients received the same amount of normal saline injection as placebo. |
|
| Outcomes | The primary endpoint of the study was the VAS pain score 2 hrs after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "The PCA regimen contained 20 µg/kg fentanyl in 100 ml of solution. The PCA system was programmed to administer a basal flow of 1 ml/hour and a PCA level of 1ml/dose with a lockout interval of 15 minutes. In the case of persistent pain exceeding a visual analogue scale (VAS) pain score of 30 mm, an additional 50 µg of fentanyl was intravenously injected by an investigator until the pain was relieved to a level falling below a VAS pain score of 30 mm." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (grant 2012R1A1A1003700)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...based on a computer‐generated random table. Block randomization was used in order to prevent imbalances in treatment assignments." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...group assignments were kept in sealed envelopes…" Not mentioned sequentially numbered and opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all parties involved, including the patients, the surgeon, the anaesthesiologists, and the investigator collecting the data, were unaware of the study drugs or the patients’ group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all parties involved, including the patients, the surgeon, the anaesthesiologists, and the investigator collecting the data, were unaware of the study drugs or the patients’ group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate (experimental/control): 0%:0% Quote: "one patient in Group I was excluded from this study as laparoscopic‐assisted distal gastrectomy was converted to laparotomy because of technical failure of the laparoscopic apparatus. One patient in Group C was excluded as the patient required meperidine because of postoperative shivering". "...in each group was one patient excluded post‐allocation. Subsequently, two patients who fulfilled our inclusion criteria replaced this excluded patients…" We assume that the replacing patient was not randomized based on the description (no response from the authors upon request). Replacement may have an impact on relevant outcomes. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and all of the study´s primary and secondary outcomes that are of interest in the review have been reported in the protocol. However, the study was retrospectively registered (January 2012). First participant enrolment (March 2011). (ACTRN12612000007831) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Koppert 2004.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The objective in this study was to determine the time course of the analgesic and antihyperalgesic mechanisms of perioperative lidocaine administration in patients undergoing major abdominal surgery. The study was conducted in Germany. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 40 → 20:20 Number analysed: 20:20 Inclusion criteria Patients undergoing major abdominal surgery. Exclusion criteria Immediate tracheal extubation after surgery was not planned, when they regularly took analgesics or had taken opioids or anti‐arrhythmic drugs within 1 wk of surgery, when they had a history of drug or alcohol abuse, or when there were contraindications to the self‐administration of opioids. Baseline details Experimental group (n = 20) Mean age (years): 58 M = 80%, F = 20% Mean weight (kg): 75.6 ASA I/II/III: 2:12:6 Duration of infusion (hrs): 6.2 Main surgical procedure (n): prostatectomy with lymph node dissection (10), cystectomy with lymph node dissection (2), abdominal nephrectomy with lymph node dissection (2), colectomy with lymph node dissection (3), lymph node dissection (3) Control group (n = 20) Mean age (years): 56 M = 80%, F = 20% Mean weight (kg): 76.8 ASA I/II/III: 3:13:4 Duration of infusion (hrs): 6.2 Main surgical procedure (n): prostatectomy with lymph node dissection (9), cystectomy with lymph node dissection (3), abdominal nephrectomy with lymph node dissection (3), colectomy with lymph node dissection (2), lymph node dissection (3) |
|
| Interventions |
Experimental group (20 patients): Lidocaine 2% (bolus injection of 1.5 mg/kg in 10 min followed by an IV infusion of 1.5 mg/kg/hr). The infusion started 30 min before skin incision and was stopped 1 hr after the end of surgery. Control group (20 patients) Control patients were treated with saline. |
|
| Outcomes | The primary endpoint of the study was PCA morphine consumption over the initial 72 hrs. Dichotomous
Continuous
|
|
| Notes |
Medication "During the first postoperative hours, pain intensity was evaluated every 15 min. If pain intensity exceeded 4 (out of 10), PCA was started, and the time between skin closure and the first PCA request was noted. The PCA settings were a demand dose of 2 mg of morphine hydrochloride and a lockout of 10 min, with no continuous rate provided. If the pain intensity exceeded 6 (out of 10) for at least 30 min, the demand dose was doubled for at least 12 hrs. Patients were monitored for sedation via a four‐point categorical scale (0, alert; 1, sleepy but arousable; 2, stupor; 3, coma) and for episodes of desaturation via pulse oximetry. After discontinuation of the PCA pump, morphine consumption and the time and number of positive and negative PCA requests were recorded via dedicated software." Anaesthesia The anaesthesia regime was not standardized ("The maintenance of anesthesia was left to the discretion of each anesthesiologist, with the exception of the administration of opioids.") Funding "This work was supported by the Deutsche Forschungsgemeinschaft (SFB 353)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization of the study medication (lidocaine versus saline) was performed with computer‐generated codes." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...maintained in sequentially numbered, opaque envelopes. Additional envelopes were provided if patients had to be excluded after recruitment and randomization." Not mentioned that envelopes were sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients of the control group received an infusion of saline in an equal manner.", "The anaesthesiologist, the surgeon, and the nursing staff were all blinded to the group allocations." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...the patients were observed by nursing staff members who was blinded to the treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate (experimental/control): 0%:0% Quote: "three patients were excluded during the study because of hypothermia that required prolonged mechanical ventilation (n = 2, one in each group) or because of surgical complications that required another procedure on the second POD (n = 1 patient from the control group). They were replaced according to the previously described procedure. Finally, 8 women and 32 men finished the study protocol;" We assume that the replacing patient was not randomized based on the description (no response from the authors upon request). Replacement may have an impact on relevant outcomes. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kuo 2006.
| Methods | Randomized, placebo‐controlled trial. No statement on allocation concealment. No statement on blinding of personnel. Participants and outcome assessors were blinded. The study compared TEA and IV lidocaine regarding their effects on cytokines, pain and bowel function after colonic surgery. The study was conducted in Taiwan from December 2003 to November 2004. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 60 → 20:20:20 Number analysed: 20:20:20 Inclusion criteria Patients, ASA I or II, aged 40 to 80 yrs, and undergoing elective surgery for colon cancer. Exclusion criteria Systemic diseases, such as diabetes mellitus, or hypertension, or received opioids or nonsteroidal anti‐inflammatory drugs within one week of surgery, were excluded. Patients likely to have received blood transfusion during the perioperative period were excluded. Baseline details Experimental group I (n = 20) Median age (years): 63 M = 50%, F = 50% Mean weight (kg): 61.5 ASA I/II: N/A Duration of operation (min): 157.8 Main surgical procedure: colonic surgery Experimental group II (n = 20) Median age (years): 63 M = 55%, F = 45% Mean weight (kg): 60.1 ASA I/II: N/A Duration of operation (min): 153.5 Main surgical procedure: colonic surgery Control group (n = 20) Median age (years): 62 M = 60%, F = 40% Mean weight (kg): 61.6 ASA I/II: N/A Duration of operation (min): 150.8 Main surgical procedure: colonic surgery |
|
| Interventions |
Experimental group I (20 patients) Patients in Group IV received 2 mg/kg for 10 min and then 3 mg/kg/hr i.v. and normal saline via the epidural catheter. Drugs were started 30 min before surgery and the infusion maintained throughout the surgical procedure. Experimental group II (20 patients) Patients of Group TEA received lidocaine 2 mg/kg for 10 min and then 3 mg/kg/hr via the epidural catheter and an equal volume of normal saline through i.v. drugs were started 30 min before surgery and the infusion maintained throughout the surgical procedure. Control group (20 patients) The control group received normal saline via both routes. |
|
| Outcomes | The primary endpoint of the study was PCEA consumption. Dichotomous
Continuous
|
|
| Notes |
Medication "All patients received balanced salt solution at a rate of 6 ml/kg/hr during surgery and 2 ml/kg/hr after operation. Patients likely to have received blood transfusion during the perioperative period were excluded. At the end of surgery, residual neuromuscular block was antagonized with edrophonium(0.8 mg/kg) and atropine (0.01 mg/kg). On arrival at the PACU, all patients were connected with the PCEA pump with morphine (0.1 mg/ml) in 100 ml of ropivacaine 0.2%. They received PCEA solution 10 ml at the first trigger and then 4 ml per delivery (lockout time was 15 min without a 4 hr limitation or continuous background infusion)." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "This work was supported by a grant from National Science Council (NSC 92‐2314‐B‐016‐057) of Taiwan, Republic of China and C.Y. Foundation for Advancement of Education, Sciences and Medicine." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...anaesthesiologist nurse randomly allocated the patients to one of the three groups using a computer program." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the study drugs (lidocaine and saline) were prepared by the hospital pharmacy in identical containers." No information about coding. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients of the control group (Group C, n = 20) received normal saline via both the peripheral i.v. line and the epidural catheter.", "The study drugs (lidocaine and saline) were prepared by the hospital pharmacy in identical containers." Due to adequate random sequence generation and identical containers prepared by the hospital pharmacy blinding is ensured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all observations were double‐blinded and made by a study nurse." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Lauwick 2008.
| Methods | Randomized, controlled trial. No statement on blinding of participants. The anaesthesiologists were unblinded. The outcome assessors were blinded. The purpose of this study was to determine whether intraoperative lidocaine infusion reduces opioid consumption in the PACU in patients undergoing laparoscopic cholecystectomy. The study was conducted in Canada from May 2007 to February 2008. |
|
| Participants | Number assessed for eligibility: 63 Number randomized: 50 → 25:25 Number analysed: 25:24 Inclusion criteria Outpatient laparoscopic cholecystectomy. Exclusion criteria: Age < 18 yrs or > 85 yrs, ASA physical status III and greater, history of hepatic, renal or cardiac failure, organ transplant, diabetes, morbid obesity (BMI > 40 kg/m2), chronic use of opioids, allergy to local anaesthetics, or inability to comprehend pain assessment. Baseline details Experimental group (n = 25) Mean age (years): 50.2 M = 20%, F = 80% Mean weight (kg): 66.9 ASA I/II: 17:8 Duration of surgery (min): 60 Main surgical procedure: laparoscopic cholecystectomy Control group (n = 24) Mean age (years): 53.8 M = 48%, F = 52% Mean weight (kg): 75 ASA I/II: 11:14 Duration of surgery (min): 70 Main surgical procedure: laparoscopic cholecystectomy |
|
| Interventions |
Experimental group (25 patients) At induction of anaesthesia the lidocaine group received fentanyl 1.5 µg/kg and a bolus of lidocaine 1.5 mg/kg followed by a continuous infusion of lidocaine 2 mg/kg/hr until the end of surgery. Control group (24 patients) At induction of anaesthesia the control group received fentanyl 3 µg/kg. |
|
| Outcomes | The primary endpoint of the study was fentanyl consumption. Dichotomous
Continuous
|
|
| Notes |
Medication "No supplemental opioids were given during surgery. All patients received acetaminophen, ketorolac, dexamethasone, droperidol and local anaesthetics in the skin incision. Patients received fentanyl and ondansetron in the PACU. Before induction of anaesthesia, patients in the control group received fentanyl 3.0 µg/kg iv, while patients in the lidocaine group received fentanyl 1.5 µg/kg iv. No supplemental fentanyl was given to patients in either group during maintenance of anaesthesia. Ketorolac 15 mg and droperidol 0.625 mg were also given intravenously. Ten millilitres of bupivacaine 0.25% with epinephrine was injected into the surgical incisions. According to study protocol, the PACU nursing staff administered fentanyl 25 µg iv boluses for postoperative pain relief, to be administered every five minutes up to a maximum of 200 µg/hr only if the VRS score for pain (0–10 scale, where 0 = no pain, and 10 = excruciating pain) was > 3, at rest. Ondansetron 2 mg iv was prescribed for persistent nausea (lasting > five minutes) or vomiting, and it could be repeated up to four times over a three‐hour period if necessary." Anaesthesia The anaesthesia regime was not fully standardized. The control group received more fentanyl. Funding "This work was supported by internal funds, Department of Anesthesia, McGill University Health Centre." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned, using a computer‐generated randomization schedule, into two groups of 25 patients…" |
| Allocation concealment (selection bias) | Low risk | Quote: "...sequentially numbered sealed brown envelopes…" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The anaesthesiologist was not blinded, but (quote:) "the anaesthesia record was not made available to the recovery room nurse, to avoid bias.", "...the anaesthesiologists (S.L. and F.C.) who executed the study protocol, were not involved in either the preoperative or the postoperative data collection." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...monitored and recorded by nurses who were blinded to the randomization sequence."; "The anaesthesia record was not made available to the recovery room nurse, to avoid bias." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 0%:4% Quote: "One patient in the control group was excluded from the analysis because his surgery was converted from laparoscopy to laparotomy." |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Unclear risk | The study groups differ with a greater proportion of males (48% versus 20%) in the control group. |
Lauwick 2009.
| Methods | Randomized, placebo‐controlled trial. No statement on blinding of participants and outcome assessors. The anaesthesiologists were blinded. This study was performed to assess the effect of intra‐ and postoperative lidocaine infusion on postoperative functional walking capacity, as a measure of surgical recovery in patients undergoing laparoscopic prostatectomy. The study was conducted in Canada from May 2007 to February 2008. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 40 → 20:20 Number analysed: 20:20 Inclusion criteria Male patients undergoing laparoscopic prostatectomy. Exclusion criteria ASA physical status ≥ 4, history of hepatic, renal, or cardiac failure, organ transplant, insulin‐dependent diabetes mellitus, morbid obesity (BMI > 40 kg m²), chronic use of opioids, allergy to local anaesthetics, or inability to comprehend pain assessments. Baseline details Experimental group (n = 20) Mean age (years): 60 M = 100%, F = 0% Mean weight (kg): 79 ASA I/II/III: 5:14:1 Duration of surgery (min): 262.5 Main surgical procedure: laparoscopic prostatectomy Control group (n = 20) Mean age (years): 59 M = 100%, F = 0% Mean weight (kg): 82 ASA I/II/III: 10:7:3 Duration of surgery (min): 240 Main surgical procedure: laparoscopic prostatectomy |
|
| Interventions |
Experimental group (20 patients) At induction of anaesthesia, the lidocaine group received an i.v. bolus injection of lidocaine 1.5 mg/kg up to a maximum of 100 mg, followed by a continuous infusion of lidocaine 2 mg/kg/hr until the end of surgery. Control group (20 patients) Patients in the control group received an equivalent volume of saline 0.9%. |
|
| Outcomes | The primary endpoint of the study was functional walking capacity. Dichotomous
Continuous
|
|
| Notes |
Medication "PCA morphine (1 mg bolus, 7 min lockout) was started in PACU and continued for 48 hrs. Patients also received acetaminophen 1.0 g 6 hourly and naproxen 500 mg 12 hourly for the first 72 hrs. Once PCA morphine was discontinued, patients were offered oxycodone 5–10 mg 4 hourly if the VAS (0 = no pain and 10 = excruciating pain) was > 3 at rest. Ondansetron 2 mg i.v. was prescribed for persistent nausea (lasting > 5 min) or vomiting." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Dr S. Lauwick is a recipient of a clinical fellowship in anaesthesia for minimally invasive surgery from the Steinberg‐Bernstein Centre for Minimally Invasive Surgery and the Montreal General Hospital Foundation, and a clinical research grant from the CHU of LIEGE, Belgium. This work was supported by internal funds, Department of Anesthesia, McGill University Health Centre." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…patients were randomly assigned (using a computer‐generated randomization schedule…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "….sealed brown envelopes…" Not mentioned that envelopes were sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the anaesthesiologists (S.L. and F.C.) who executed the study protocol were blinded to the group allocation and were not involved in preoperative or postoperative data collection." No statement on blinding of participants and other personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. No exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Lee 2011.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. This study was designed to assess whether a continuous intravenous lidocaine infusion reduced myocardial injury in patients undergoing off‐pump CABG surgery. The study was conducted in Korea from October 2008 to August 2009 (Lee ‐ KCT0000012). |
|
| Participants | Number assessed for eligibility: 184 Number randomized: 109 → 53:56 (49:50 received allocated intervention) Number analysed: 49:50 Inclusion criteria Patients aged 18–80 yrs undergoing elective off‐pump coronary artery bypass under general anaesthesia. Exclusion criteria Exclusion criteria were contraindications to lidocaine or opioid use, pulmonary or hepatic diseases, psychiatric disorders, recent (within 14 days) myocardial infarction, unstable angina with elevated creatinine kinase myocardial band or TnI, elevated serum creatinine (115 mmol/l) before surgery, left ventricular ejection fraction < 50%, and patients undergoing emergency or repeat operations, or concomitant valvular or aortic surgery. Baseline details Experimental group (49) Median age (years): 63 M = 67.3%, F = 32.7% Mean BMI (kg/m2): 24.9 ASA I/II: N/A Duration of anaesthesia (min): 279.1 Main surgical procedure (n): off‐pump CABG surgery Control group (50) Median age (years): 66 M = 64%, F = 36% Mean BMI (kg/m2): 24.9 ASA I/II: N/A Duration of anaesthesia (min): 282.5 Main surgical procedure (n): off‐pump CABG surgery |
|
| Interventions |
Experimental group (49 patients) Patients received lidocaine 2% with a 1.5 mg/kg intravenous bolus at induction of anaesthesia followed by a 2.0 mg/kg/hr intravenous infusion intraoperatively in the lidocaine group. Control group (50 patients) Patients received an equal volume of saline. |
|
| Outcomes | The primary endpoint of the study was serum troponin I (TnI) at 24 hrs. Dichotomous
Continuous
|
|
| Notes |
Medication N/A Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated to two groups based on computer‐generated codes..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...were maintained in sequentially numbered opaque envelopes." Not mentioned that envelopes were sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "on the morning of surgery and before induction of anaesthesia, the allocation envelope was opened by a nurse or anaesthetist with no involvement in patient management, who then prepared either 2% lidocaine or saline in coded 50 ml syringes." Due to adequate randomization blinding of other personnel and participants is ensured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "none of the anaesthetists involved in patient management or data collection was aware of the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control) after received intervention: 0%/0% Ten patients were excluded before the intervention was started and reasons for exclusion were described. Quote: Quote: "ten of these patients were excluded after enrolment and not included in data analyses, nine due to changes in surgical schedules, and one due to a change in procedure..." |
| Selective reporting (reporting bias) | Unclear risk | The study was registered at http://cris.cdc.go.kr (Lee ‐ KCT0000012). However, the data set was not available in English and we can not judge for selective reporting. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Maquoi 2016.
| Methods | Randomized, controlled trial. Double‐blind. The aim of this study was to find out if TAP block and intravenous lignocaine improve post‐operative analgesia after open prostatectomy and whether one of the two techniques was superior to the other. The study was conducted in Belgium from October 2010 to September 2013. (EudraCT:2010‐018321‐20) |
|
| Participants | Number assessed for eligibility: 247 Number randomized: 129→ N/A:N/A:N/A Number analysed: 101→ 33:34:34 Three groups, one not of interest (TAP block) Inclusion criteria patients undergoing open prostate surgery Exclusion criteria age lower than 18 years, BMI less than 20 or above 30 kg/m2, obstructive sleep apnoea syndrome, history of liver or renal insufficiency, seizures, second or third degree atrioventricular block and any contraindication to the anaesthetic protocol of the study Baseline details Experimental group (n = 33) Mean age (years): 62, SD = 8 M = 100%, F = 0% Mean weight (kg): 80, SD = 17 ASA I/II (%): 35:65 Mean duration of anaesthesia (min): 173, SD = 76 Mean duration of surgery (min): 133, SD = 58 Main surgical procedures (n): prostatectomy (33) Control group (n = 34) Mean age (years): 65, SD = 11 M = 100%, F = 0% Mean weight (kg): 82, SD = 17 ASA I/II (%): 20:80 Mean duration of anaesthesia (min): 150, SD = 51 Mean duration of surgery (min): 112, SD = 44 Main surgical procedures (n): prostatectomy (34) |
|
| Interventions |
Experimental group (33 patients) Patients assigned to the intravenous lignocaine group received a 1.5 mg/kg bolus of 2% intravenous lignocaine before induction of anaesthesia followed by a continuous infusion of 2 mg/kg/hr until the end of surgery. The lignocaine infusion was then continued at 1.33 mg/kg/hr until the end of the 24th post‐operative hour. A bilateral ‘sham block’ with normal saline was also performed in this group. Control group (34 patients) Finally, in the placebo group, saline was used both for the TAP block and the intravenous infusion. |
|
| Outcomes | The primary endpoint of the study was the piritramide consumption after 48 hrs. Dichotomous
Continuous
|
|
| Notes |
Medication They were premedicated orally with 0.5 mg of alprazolam, 50 mg of hydroxyzine and 0.5 mg of atropine 1 hr before surgery. A 2 g loading dose of paracetamol was given intravenously 1 hr before the anticipated end of surgery. In the recovery room, intravenous piritramide was titrated if necessary to achieve a numeric rating scale for rest pain ≤ 3 out of 10. On the ward, patients received 1 g of intravenous paracetamol every 6 hrs until the end of the study period. PCA with piritramide was used as a rescue medication (bolus = 1 mg, lockout time = 5 min, no basal infusion). Boluses of 100 mg of tramadol were allowed every 6 hrs in case of persistent pain (VAS ≥ 30 mm out of 100 mm) despite appropriate use of the PCA pump. Alizapride 50 mg or dehydrobenzperidol 0.625 mg was used in case of nausea or vomiting. Anaesthesia The anaesthesia regime was standardized in both groups. Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “[…] were randomly allocated to one of the three study groups according to a computer‐generated list (Graphpad online randomizer, Graphpad software, San Diego, CA, USA).” |
| Allocation concealment (selection bias) | Low risk | Quote: “the list was kept at the anaesthetic secretariat. When patients arrived in the anaesthetic room a research nurse called the anaesthetic secretariat to get a randomization number.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “[the research nurse] prepared three 50 ml syringes for continuous i.v. infusion and two 20 ml syringes for the TAP block. These syringes were respectively labelled ‘STUDY: iv infusion at ... ml/hr’ and ‘STUDY: TAP’. Patients, anaesthetists and caregivers remained fully blinded to the randomization.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “post‐operative opioid requirements, pain score and recovery of bowel function were recorded during the ward round by anaesthetists not involved in the study. Treatment assignments were not unblinded before the data collection was completed.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 % of all patients dropped out of analysis (high dropout, 129 randomized, 101 analysed). The reasons for missing data are not completely clear and are not described in detail (protocol violation n = 11, withdraw of consent n = 4, incomplete results n = 13). Reasons for missing data are likely to be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and the study’s prespecified primary outcome has been reported in the prespecified way (EudraCT: 2010‐018321‐20). Nonetheless, the protocol has been retrospectively registered (25 August 25) after the study was completed (2013). Nausea and vomiting are mentioned as a secondary outcome in the protocol but were not analysed in the study. Instead, the number of participants requesting antiemetics was reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Martin 2008.
| Methods | Randomized, placebo‐controlled trial. Sequence generation based on date of admission. Participants, personnel, and outcome assessors were blinded. This study aimed to evaluate whether a continuous intravenous low‐dose lidocaine infusion reduced postoperative pain and modified nociceptive pain threshold after total hip arthroplasty. The study was conducted in France from January 2006 to March 2007. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 60 → 30:30 Number analysed: 28:30 Inclusion criteria Hip arthroplasty, M and F Exclusion criteria Anterior surgical approach; regional anaesthesia; contraindications for lidocaine or morphine use; severe cardiac, renal or hepatic diseases; and preoperative use of analgesics (corticosteroids or opioid). Baseline details Experimental group (n = 28) Mean age (years): 64 M = 46%, F = 54% Mean weight (kg): 73 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure: total hip arthroplasty Control group (n = 30) Mean age (years): 62 M = 33%, F = 67% Mean weight (kg): 70 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure: total hip arthroplasty |
|
| Interventions |
Experimental group (28 patients) Patients received lidocaine 1% with a 1.5 mg/kg intravenous bolus in 10 min followed by a 1.5 mg/kg/hr intravenous infusion. These regimens were started 30 min before surgical incision and stopped 1 hr after skin closure. Control group (30 patients) Control patients received saline. |
|
| Outcomes | The primary endpoint of the study was PCA morphine consumption over 24 hrs. Dichotomous No outcomes reported. Continuous
|
|
| Notes |
Medication "Postoperative analgesia was provided in both groups only with IV patient controlled morphine. No others co‐analgesics were prescribed. After the patient arrived in the PACU, pain was evaluated every 5 min using a 4‐point verbal rating scale for pain (0 = no pain; 1 = slight pain; 2 = moderate pain; 3 = intense or severe pain). If the score was greater than 2, patients under 65 yrs received morphine 3 mg while older patients were given 2 mg, every 5 min, if permitted according to the respiration rate (respiratory rate > 10 breaths/min) and sedation score (score < 1), until a verbal rating scale score of 0 or 1 had been achieved. PCA was stopped in both groups at the 48th hour, and further analgesia was provided by combination of paracetamol, nonsteroidal anti‐inflammatory drugs and subcutaneous morphine as needed." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Support was provided solely from institutional and/or departmental sources." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...a randomization list balanced by centre was established and each center enrolled patients and assigned treatments consecutively." It is not clear from the description how the randomization list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...an envelope containing the group assignment was prepared, sealed, and sequentially numbered." Not mentioned that envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "in the control group, patients were given equal volumes of saline.", "On the morning of surgery and before induction of anaesthesia, a “blinded” nurse prepared lidocaine or saline solution syringes. None of the other investigators involved in patient management or data collection were aware of the group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "on the morning of surgery and before induction of anaesthesia, a “blinded” nurse prepared lidocaine or saline solution syringes. None of the other investigators involved in patient management or data collection were aware of the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 7%:0% Quote: "of sixty patients included, two were excluded in the lidocaine group. They decided to leave the study in the PACU because of extreme pain." Missing outcome data may introduce bias to relevant outcomes of interest. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mathew 2009.
| Methods | Randomized, placebo‐controlled trail. No statement on blinding of personnel. Participants and outcome assessors were blinded. This study assessed the potential of intravenously administered lidocaine to reduce postoperative cognitive dysfunction following cardiac surgery employing cardiopulmonary bypass. The study was conducted in the USA from March 1999 to April 2003. |
|
| Participants | Number assessed for eligibility: 2681 Number randomized: 277 → 133:144 Number analysed: 88:94 Inclusion criteria Patients scheduled to undergo CABG and/or an open chamber procedure with CPB. Exclusion criteria Patients undergoing circulatory arrest or had a history of symptomatic cerebrovascular disease (e.g. stroke with a residual deficit), psychiatric illness (any clinical diagnoses requiring therapy), renal failure (serum creatinine > 2 mg/dl), liver disease (liver function tests > 1.5 times the upper limit of normal), higher alcohol consumption (> 2 drinks/day), or were unable to read or had less than a seventh grade education. Baseline details Experimental group (n = 114) Mean age (years): 61.7 M = 72.8%, F = 27.2% Mean weight (kg): 86.1 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure (n): CABG (51), CABG with valve (22), valve (40), other (1) Control group (n = 127) Mean age (years): 61.4 M = 66.9%, F = 33.1% Mean weight (kg): 81.6 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure (n): CABG (52), CABG with valve (23), valve (47), other (5) |
|
| Interventions |
Experimental group (114 patients) Lidocaine was administered after induction of anaesthesia as a 1 mg/kg bolus followed by a continuous infusion (4 mg/min for 1 hr, 2 mg/min for the second hr, 1 mg/min for the rest) through 48 hours postoperatively. Control group (127 patients) Placebo bolus and infusion for 48 hrs. |
|
| Outcomes | The primary endpoint of the study was incidence of cognitive deficit. Dichotomous
Continuous
|
|
| Notes |
Medication N/A Anaesthesia Standardization of the anaesthesia regime is unclear. Funding "Supported in part by grants #9970128N (Dr. Newman) from the American Heart Association, Dallas, TX, USA, #M01‐RR‐30 from the National Institutes of Health, Washington, D.C., USA, and by the Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Department of Anesthesiology, Duke University Medical Center, Durham, NC, USA." Conflict Of Interest "Dr. Laskowitz is a consultant for Biosite Diagnostics." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a group assignment schedule was prepared using a randomization function in sedation‐agitation status…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...and stored in consecutively numbered sealed envelopes until allocation." Not mentioned if envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...identical volume and rate changes as the treatment group such that blinding was preserved." No statement on blinding of personnel and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "experienced psychometricians blinded to the treatment group examined subjects..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate after received intervention (experimental/control): 23%:26% Exclusions, withdrawals, and dropouts were described. It is unclear from the description whether the reasons (e.g. lack of interest, health, other) may be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
McKay 2009.
| Methods | Randomized, placebo‐controlled trial. No statement on allocation concealment. No statement on blinding of participants, personnel, and outcome assessors. This trial evaluated whether systemic lidocaine would reduce pain and time to discharge in ambulatory surgery patients. The study was conducted in the USA from August 2004 to August 2006. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 67 → N/A Number analysed: 29:27 Inclusion criteria Patients 18 to 75 yrs of age (ASA physical status I to III) presenting for outpatient surgery under general anaesthesia. Exclusion criteria N/A Baseline details Experimental group (n = 29) Mean age (years): 43 M = 83%, F = 17% Mean weight (kg): 81 ASA I/II: N/A Duration of anaesthesia (min): N/A Main surgical procedure (n): laparoscopic general (11), open general (3), endocrine and breast (7), laparoscopic gynaecology (4), minor gynaecology (2), urology (0), plastics (2), minor ortho (0), minor ear, nose, throat (0) Control group (n = 27) Mean age (years): 46 M = 78%, F = 22% Mean weight (kg): 81 ASA I/II: N/A Duration of anaesthesia (min): N/A Main surgical procedure (n): laparoscopic general (13), open general (3), endocrine and breast (5), laparoscopic gynaecology (2), minor gynaecology (0), urology (1), plastics (1), minor ortho (1), minor ear, nose, throat (1) |
|
| Interventions |
Experimental group (29 patients) At induction of anaesthesia, all patients received 1.5 mg/kg of lidocaine by slow IV push. The lidocaine infusion (2 mg/kg/hr) was started immediately after induction of anaesthesia and continued until 1 hr after arrival in the PACU. Control group (27 patients) The control group received saline as placebo. |
|
| Outcomes | The primary endpoint of the study was time to PACU discharge readiness. Dichotomous
Continuous
|
|
| Notes |
Medication "Pain was assessed at rest by a visual analogue scale every 15 min and treated with either fentanyl (0.5 – 1 µg/kg) or morphine (0.01 – 0.02 mg/kg) when pain was more than 3 on a visual analogue scale of 0–10 (0 = no pain, 10 = more pain imaginable). Nausea was assessed at 15‐min intervals and treated with ondansetron or if persistent with promethazine or diphenhydramine." Anaesthesia Anaesthetic management during surgery was standardized for opioid use, ketorolac and prophylaxis for postoperative nausea and/or vomiting. Funding "Supported by departmental funding." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were assigned in a double‐blind 1:1 ratio using a computer‐generated randomization list…" |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…double‐blind…". No detailed information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate overall: 16% Quote: "based on an expected withdrawal rate of 20%, 67 patients were enrolled in the trial." Only 56 were analysed, 11 excluded. Excluded patients were not described. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mitchell 1999.
| Methods | Randomized, placebo‐controlled trial. No detailed information on random sequence generation and allocation concealment provided. Participants and personnel were blinded. No statement on blinding of outcome assessors. This study investigated cerebral protection by lidocaine during cardiac operations. The study was conducted in New Zealand. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 65 → 33:32 Number analysed: 28:27 (outcome: length of hospital stay) Inclusion criteria Patients undergoing left heart valve procedures. Exclusion criteria The exclusion criteria were as follows: age outside the 20‐ to 70‐year range; any current neurologic disorder; a first or most commonly used language other than English; residence outside the greater Auckland area; and any past history of adverse reactions to lidocaine. Baseline details Experimental group (n = 28) Mean age (years): 56.9 M = 60.7%, F = 39.3% Mean BMI (kg/m2): 25.3 ASA I/II: N/A Duration of CPB (min): 129.3 Main surgical procedure (n): aortic valve replacement (20), mitral valve replacement (6), dual valve replacement (2), valve plus coronary grafts (13), redo operation (7), ascending aorta atheroma (1) Control group (n = 27) Mean age (years): 54.4 M = 51.9%, F = 58.1% Mean BMI (kg/m2): 28.5 ASA I/II: N/A Duration of CPB (min): 109.5 Main surgical procedure (n): aortic valve replacement (15), mitral valve replacement (9), dual valve replacement (3), valve plus coronary grafts (5), redo operation (4), ascending aorta atheroma (3) |
|
| Interventions |
Experimental group (28 patients) Patients received a 1 mg/kg “bolus” over 5 minutes, followed by 240 mg over the first hour and 120 mg over the second hour, and then 60 mg/hr thereafter if the patient was receiving lidocaine. The trial infusion was begun at induction of anaesthesia and continued for 48 hours. Control group (27 patients) To preserve double blinding, the laboratory also reported sham levels for placebo patients. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication N/A Anaesthesia "Any departure from this standard protocol was recorded." Funding "This work was supported by grants from the English Freemasons of New Zealand and the Health Research Council of New Zealand." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were block randomized by surgeon…". No more information provided. |
| Allocation concealment (selection bias) | Low risk | Quote: "The medication was repackaged by a pharmaceutical laboratory into coded vials." To preserve double blinding, the laboratory also reported sham levels for placebo patients." We assumed that allocation concealment and blinding occurred in the same way as in Mitchell 2009. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the medication was repackaged by a pharmaceutical laboratory into coded vials." To preserve double blinding, the laboratory also reported sham levels for placebo patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 15%:16% Quote: "ten of the 65 consented patients did not enter the review phase of the trial." Reasons for exclusion for all patients were described in the text. It is unclear from the description whether the reasons (e.g. postoperative complications) may be related to true outcome (neurophysiological testing). However, the relevant outcome for the current review (length of hospital stay) remains unaffected. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mitchell 2009.
| Methods | Randomized, placebo‐controlled trial. No detailed information on random sequence generation and allocation concealment provided. Participants and personnel were blinded. No statement on blinding of outcome assessors. This study aimed to test the benefit of a 12‐hour infusion of lidocaine in a broader group of cardiac surgery patients, including those undergoing CABG surgery. The study was conducted in New Zealand. Date not published. |
|
| Participants | Number assessed for eligibility: 639 Number randomized: 158 → 81:77 Number analysed: 80:77 (outcome: length of hospital stay), 59:59 (analysed at 10 weeks), 54:53 (analysed at 25 weeks) Inclusion criteria Adult patients (20 to 75 years old) undergoing CABG (with or without cardiopulmonary bypass), valve surgery, or combined procedures; resident in the greater Auckland area, English speaker, no preexisting cerebral dysfunction, no history of sensitivity to lidocaine, and no condition the procedural anaesthesiologist would normally consider to be a contraindication to lidocaine administration. Exclusion criteria N/A Baseline details Experimental group (n = 81) Mean age (years): 61.5 M = 74.1%, F = 25.9% Mean weight (kg): 82.9 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure (n): aortic valve replacement (3), mitral valve replacement (5), aortic valve replacement + mitral valve replacement (1), CABG on pump (58), off‐pump CABG (10), valve plus CABG (4) Control group (n = 77) Mean age (years): 58.1 M = 81.8%, F = 18.2% Mean weight (kg): 83.2 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure (n): aortic valve replacement (3), mitral valve replacement (1), aortic valve replacement + mitral valve replacement (0), CABG on pump (54), off‐pump CABG (8), valve plus CABG (11) |
|
| Interventions |
Experimental group (81 patients) The infusion was started at induction of anaesthesia with a “bolus” of 1 mg/kg over 5 minutes followed by 2 mg/min for 2 hours, and 1 mg/min thereafter, for a total of 12 hours. Control group (77 patients) Control patients received saline as placebo. |
|
| Outcomes | The primary endpoint of the study was neurocognitive deficit. Dichotomous
Continuous
|
|
| Notes |
Medication N/A Anaesthesia The anaesthesia regime was not standardized. ("There was no attempt to rigidly standardize the anesthetic technique, but practice among anesthesiologists was confluent, and no significant changes occurred over the course of the study") Funding "This work was supported by medical equipment grant AP72364 from the Lottery Grants Board of New Zealand, grants 81354 and 81399 from the Auckland Medical Research Foundation, and by a grant from the English Freemasons of New Zealand." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a collaborator who had no other role in the trial generated a block‐randomized sequence of allocations." No detailed information provided. |
| Allocation concealment (selection bias) | Low risk | Quote: "this sequence was concealed from the patients, all medical staff in contact with the patients, and from all other trial collaborators.", "Trial solutions...were repackaged into generic vials by a licensed pharmaceutical company." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "trial solutions...were repackaged into generic vials by a licensed pharmaceutical company." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 1%:0% Withdrawals, losses during follow‐up etc. were described. It is unclear from the description whether the reasons (e.g. postoperative complications) may be related to true outcome (neurophysiological testing). However, the relevant outcome for the current review (length of hospital stay) remains unaffected. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Oliveira 2015.
| Methods | Randomized, controlled trial. Double‐blinded. The purpose of this study was to evaluate the effect of intravenous lidocaine on pain severity and plasma IL‐6 after hysterectomy. The study was conducted in Sao Paulo, Brazil. Date not published. |
|
| Participants | Number assessed for eligibility: 80 Number randomized: 46→ 24:22 Number analysed: 40→ 20:20 Inclusion criteria ASA I and II, women among 18 and 60 years, submitted to elective total hysterectomy Exclusion criteria Cardiac arrhythmia, myocardiopathy, alteration of cardiac conduction; electrolyte disturbance; acid‐base disorder; hypersensitivity to lidocaine; psychiatric disease, hepatic, respiratory disorder or cancer; patients who received any type of analgesic in the week prior to surgery and who received blood products during the study period Baseline details Experimental group (n = 20) Mean age(years): 44.1, SD = 6.6 M = 0%, F = 100% Mean weight (kg): 72.2, SD = 13.7 ASA I/II (n): N/A Mean duration of anaesthesia (min): 145.1, SD = 51.8 Mean duration of surgery (min): 102.6 , SD = 49.4 Main surgical procedures (n): hysterectomy (20) Control group (n = 20) Mean age (years): 42.9, SD = 5.7 M = 0%, F = 100% Mean weight (kg): 74.2, SD = 12.6 ASA I/II (n): N/A Mean duration of anaesthesia (min): 124.0, SD = 438 Mean duration of surgery (min): 93.0, SD = 48.2 Main surgical procedures (n): hysterectomy (20) |
|
| Interventions |
Experimental group (20 patients) Patients in the lidocaine group received 2 mg/kg/hr lidocaine whose infusion was started at the time of induction of anaesthesia and maintained until the end of the operation. Control group (20 patients) Patients in the control group received 0.9 % saline solution in equal volume. |
|
| Outcomes | The primary endpoint of the study was not stated but power analysis was performed for pain. Dichotomous
Continuous
|
|
| Notes |
Medication Midazolam was given at a dose of 15 mg intravenously one hour before anaesthesia. During the surgical procedure, additional doses of opioids or other analgesics were used. Prophylaxis for nausea and vomiting was not performed. For postoperative analgesia, morphine (5 mg) was administered subcutaneously as needed by a nurse. Anaesthesia The anaesthesia regime was standardized in both groups. Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “the patients were divided into two groups of equal size by lot. The random distribution was done through the G1 and G2 records, placed in sealed envelopes, prepared before the beginning of the study and opened approximately 30 minutes before anesthesia by a physician, who prepared the venous solution and identified it as the patient's number according to the envelopes.” Insufficient information for judgement on adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “…placed in sealed envelopes…” Not explicitly mentioned SNOSE. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the solution was given to another anesthesiologist who was not aware of the content of the prepared solutions. The responding researcher was not aware of the group chosen by the end of the study.” Patients could not know group allocation, either. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 17%:9% Four participants in the experimental group and two in the control group were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry or study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Omar 2013.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded.
This study hypothesized that lidocaine may be effective in producing controlled hypotension in patients undergoing functional endoscopic sinus surgery. The study was conducted in Saudi Arabia from October 2011 to December 2012. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 48 → 24:24 Number analysed: 24:24 Inclusion criteria ASA I to II adults (age 18 to 50) planned to undergo functional endoscopic sinus surgery. Exclusion criteria Patients with hepatic, renal, cardiovascular, neuromuscular, or hematological disorders were excluded. Patients on anticoagulant, opioid, or sedative drugs were also excluded. Baseline details Experimental group (n = 24) Mean age (years): 36.7 M = 54.1%, F = 46.9% Mean BMI (kg/m2): 27.9 ASA I/II: 15:9 Duration of anaesthesia (mins): 87 Main surgical procedure: functional endoscopic sinus surgery Control group (n = 24) Mean age (years): 36.3 M = 58.3%, F = 41.7% Mean BMI (kg/m2): 26.7 ASA I/II: 17:7 Duration of anaesthesia (min): 99 Main surgical procedure: functional endoscopic sinus surgery |
|
| Interventions |
Experimental group (24 patients) Patients received a bolus with 1.5 mg/kg lidocaine (1% solution) after endotracheal intubation, continuous infusion with a rate of 1.5 mg/kg/hr (0.15 ml/kg/hr). On conclusion of surgery, the study medications and sevoflurane were discontinued. Control group (24 patients) Control patients received an equal volume of saline. |
|
| Outcomes | The primary endpoint of the study was surgery field quality. Dichotomous
Continuous
|
|
| Notes |
Medication "If pain VAS score was 1–4, 30 mg of IV ketorolac was given. If pain score > 4 or if the pain was not relieved by ketorolac, fentanyl 0.5 µg/kg was given. Ondansetron 4mg IV was given as a rescue antiemetic in case of PONV. Phenylephrine was used in PACU with the same doses used intraoperatively to treat hypotension." Anaesthesia The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "immediately after endotracheal intubation, patients were randomly assigned to 2 equal groups using computerized randomization tables…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…in closed envelopes…" Not mentioned sequentially numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the hospital pharmacists who were not involved in the study prepared the study medications in 4 different coded syringes…", "...the attendant anaesthesiologist who was blinded to group allocation." Patients could not know group allocation due to adequate blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "pain was assessed in PACU by a nurse who was blinded to group allocation…" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ortiz 2016.
| Methods | Multicentric, double‐blind, randomized, and placebo‐controlled trial. This trial aimed to compare postoperative analgesia, opioid consumption, duration of ileus and hospital stay, and cytokine levels in patients undergoing laparoscopic cholecystectomies who received intravenous lidocaine in comparison with a control group. The study was conducted in Brazil from July 2013 to February 2014. (NCT02363699) |
|
| Participants | Number assessed for eligibility:87 Number randomized: 44→ 22:22 Number analysed: 43→ 21:22 Inclusion criteria patients scheduled to undergo elective laparoscopic cholecystectomy, 18 years and older, ASA I and II Exclusion criteria Patients older than 75 years, patients with heart disease, and patients with history of kidney failure, liver failure, psychiatric disorder, chronic use of opioids, or medications that could cause induction of liver enzymes (anticonvulsants) were not included in the study. In addition, the presentation of adverse effects during the intervention or postoperative complications, and the conversion to open surgery were used as exclusion criteria. Baseline details Experimental group (n = 22) Mean age (years): 43.77, SD = 12.55 M = 22,7%, F = 77,3% Mean weight(kg): 76.91, SD = 16.41 ASA I/II (n): NA Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 105.23, SD = 38.25 Main surgical procedures (n): elective laparoscopic cholecystectomy (21) Control group (n = 22) Mean age (years): 46.09, SD = 11.50 M = 40,9%, F = 59,1% Mean weight (kg): 86.86, SD = 19.76 ASA I/II (n): NA Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 112.50, SD = 47.58 Main surgical procedures (n): elective laparoscopic cholecystectomy (22) |
|
| Interventions |
Experimental group (22 patients) Lidocaine was administered in bolus of 1.5 mg/kg at the start of the procedure and maintained at a dose of 3 mg/kg/h until 1 hour after the end of the surgery. A solution of 0.3 % lidocaine was used, so that the infusion rate was equal to the patient's weight. Answer from author upon request: lidocaine infusion started before incision Control group (22 patients) Saline solution was administered in the control group with the same infusion rates |
|
| Outcomes | The primary endpoint of the study was pain within the first 24 hrs. Dichotomous
Continuous
|
|
| Notes |
Medication During surgery, all patients received 4 mg ondansetron for prophylaxis of nausea and vomiting and dipyrone 30 mg/kg, 30 minutes before the end of the procedure. Postoperatively, patients received dipyrone 1 g IV every 6 hours and ondansetron 8 mg IV every 8 hrs. For patients who reported pain at rest equal or greater than 4, morphine titration was started with 1 mg increments every 5 minutes until the pain was reported as less than 4. At this point, patients were encouraged to manage their own medication. The patient‐controlled analgesia pumps were programmed to bolus of 4 ml (morphine solution 0.5 mg/ml) followed by 8 minutes of security lock between doses. The maximum dose in 4 hrs was 30 mg. No continuous maintenance dose of morphine was used in the postoperative period. Fifteen percent increases in mean arterial pressure or heart rate values greater than 100 beats/min, with bispectral index between 40 and 60, allowed for supplementary administration of 5 μg sufentanil. Anaesthesia The anaesthesia regime was standardized in both groups. Funding No external funding source was used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomization was performed by a computer program (random.org) that generated a random number sequence from 1 to 44, divided into 2 columns. “ |
| Allocation concealment (selection bias) | Unclear risk | Quote: “an employee of the Surgical Center pharmacy, previously trained for that, appointed each column with a group (lidocaine and placebo) and stored the results in 44 envelopes. “ From the description of the text is it unclear if the allocation was fully concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “this same collaborator was responsible for preparing the solutions according to the dilution protocol. These solutions were kept in identical color and volume containers for both groups and were provided just before anesthetic induction.” Quote: “The study was double blind. Patients were not informed about the solution they were receiving. Likewise, both the research team and the auxiliary anesthetists were unaware of which group each patient belonged to.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the study was double blind. Patients were not informed about the solution they were receiving. Likewise, both the research team and the auxiliary anesthetists were unaware of which group each patient belonged to.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 5%:0% Quote: “the patients who had hospital discharge before 24 hours were asked to wait until the final pain assessment and blood collection. In this way, we avoided losses and all the data were included in the analysis.” Quote: “the lidocaine group lost one patient who had his surgery converted due to problems during the procedure. All patients who started receiving lidocaine continued the infusion according to the protocol.” Reasons for missing data (1 patient) are unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and the primary outcome as well as some secondary outcomes have been reported. The secondary outcomes “length of hospital stay” and “time to first flatus” have not been prespecified. The protocol has been retrospectively registered (4 February 2015) (NCT02363699). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Peng 2016.
| Methods | Randomized, controlled trial. Double‐blinded. The aim of this study was to test the hypothesis that intraoperative infusion of lidocaine would improve postoperative analgesia in patients following supratentorial tumour surgery in a randomized controlled trial. The study was conducted at Beijing Tiantan Hospital, Capital Medical University, Beijing, China from September 2009 to March 2012. (NCT00975910) |
|
| Participants | Number assessed for eligibility: 100 Number randomized: 94→ 46:48 Number analysed: 80→ 40:40 Inclusion criteria Scheduled for elective supratentorial craniotomy; age 18 to 65 years; ASA I or II; BMI < 30; no history of systemic malignant tumours, diabetes, psychiatric disorders, alcohol abuse, or drug abuse; sufficient education to complete preoperative neuropsychological tests; cooperative and able to give informed consent in person Exclusion criteria Preoperative Mini‐Mental State Examination score of < 2 4, vascular surgery Baseline details Experimental group (n = 40) Mean age (years): 45, SD = 9 M = 50%, F = 50% Mean weight (kg): 64, SD = 10 ASA I/II (n): 30:10 Mean duration of anaesthesia (min): 307, SD = 90 Mean duration of surgery (min): 254, SD = 87 Main surgical procedures (n): supratentorial tumour surgery (40) Control group (n = 40) Mean age (years): 44, SD = 10 M = 47.5%, F = 52.5% Mean weight (kg): 66, SD = 13 ASA I/II (n): 31:9 Mean duration of anaesthesia (min): 303, SD = 78 Mean duration of surgery (min): 246, SD = 75 Main surgical procedures (n): supratentorial tumour surgery (40) |
|
| Interventions |
Experimental group (40 patients) Patients in the lidocaine group, received lidocaine as an intravenous bolus (1.5 mg/kg) after anaesthesia induction followed immediately by infusion at 2 mg/kg/hr in a normal saline vehicle until the end of surgery. Control group (40 patients) The normal saline group received the same volume of 0.9 % saline at the same rate. |
|
| Outcomes | The primary endpoint of the study was not stated but power analysis was performed for postoperative cognitive dysfunction. Dichotomous
Continuous
|
|
| Notes |
Medication: None of the patients in either group were using opioids before the surgery. All patients were premedicated with midazolam (0.05 mg/kg) intravenously 15 minutes before induction. Sufentanil (0.1 to 0.2 mg/kg) was injected to attenuate potent stress responses induced by noxious stimuli at certain time points during surgery, including scalp incision and skull drilling. A PCA electronic pump was connected to a peripheral venous catheter at the end of surgery, it was filled with sufentanil (100 mg) and ondansetron (16 mg) diluted in 100mL of normal saline (background infusion rate, bolus dose, and lock out time were set at 2 ml/hr, 0.5 ml, and 15 min, respectively). The patients were not alert enough to use the PCA pump in PACU. The patients were treated with additional tramadol if they had mild to moderate pain in the PACU. Patients would have been treated with additional sufentanil if they had severe pain in the PACU; no patients had severe pain in the PACU and they only received the background sufentanil delivered by the continuous PCA pump infusion. Anaesthesia: The anaesthesia regime was standardized in both groups. Funding: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomization was performed through a computer‐produced randomized control table.” |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "a research nurse gave the participants an equal volume of lidocaine or saline from a coded vial according to the randomized control table. The research team that collected and analyzed the data was blinded to the treatment allocation.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The research team that collected and analyzed the data was blinded to the treatment allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 15%:20% Quote: “fourteen of 94 patients did not complete the study and their data were not analyzed; 4 patients either died or had tumor recurrence, 10 patients were lost to follow‐up before 6 months. The proportion of patients who did not complete the study was not different between groups (P = 0.77). “ Quote: “the reasons for exclusion of the 14 patients were: 5 patients remained intubated, 8 patients were not alert enough to assess a NRS pain score until they were transferred out of PACU, and 1 patient had dysphoria.” Large dropout rate. Reasons for missing data have been reported but are not the same in the two publications. |
| Selective reporting (reporting bias) | High risk | A prospectively registered study protocol is available (NCT00975910, 9 November 2009). Quote: “there were discrepancies between the registered study (NCT00975910) and the final study that were not corrected on the site “clinicaltrials.gov,” These differences were due to miscommunication between those conducting the study and those who planned and reported the study. These discrepancies included the inclusion age, the neuropsychological‐ cognitive tests used and the time points for postoperative cognitive testing. All of these changes in the study protocol occurred before the first patient was included in the study and no change in protocol was made after the study began. The neuropsychological‐cognitive tests were carried out by an independent blinded group; this was a major contributor to the discrepancy in the neuropsychological‐cognitive tests planned and performed. However, the method of lidocaine administration, anesthesia induction and maintenance, monitoring and patient selection were the same as in the registered protocol. The substituted tests were similar to the ones originally planned and the changes from the preplanned protocol are not likely to have influenced the outcome.“ All data that are important for the current review have not been prespecified in the protocol and have separately been published in a secondary findings report. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Rimbäck 1990.
| Methods | Randomized, placebo‐controlled trial. No detailed information on random sequence generation provided. No statement on allocation concealment. No statement on blinding of participants, personnel, and outcome assessors. This study analysed the effects of continuous intravenous infusion of lidocaine on postoperative paralytic ileus in cholecystectomized patients. The study was conducted in Sweden. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 30 → 15:15 Number analysed: 15:15 Inclusion criteria Patients scheduled for elective cholecystectomy. Exclusion criteria Patients using laxatives or drugs known to affect gastrointestinal motility and patients with a history of gastrointestinal disease or complications to surgery were excluded. When the possibility of pregnancy could not be minimized, the patient was not included. Patients in whom the markers had not reached segment 3 before the last radiograph were excluded from calculations of colonic transit time. Baseline details Experimental group (n = 15) Mean age (years): 55 M = 33.3%, F = 66.7% Mean weight (kg): 70 ASA I/II: N/A Duration of surgery (min): 109 Main surgical procedure: cholecystectomy Control group (n = 15) Mean age (years): 51 M = 40%, F = 60% Mean weight (kg): 73 ASA I/II: N/A Duration of surgery (min): 104 Main surgical procedure: cholecystectomy |
|
| Interventions |
Experimental group (15 patients) Patients received an IV bolus injection of 100 mg lidocaine 30 min before induction of anaesthesia followed by a continuous IV infusion of lidocaine (3 mg/min) for 24 hrs after surgery. Control group (15 patients) Control patients received an equal volume of saline. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication "Postoperative pain relief was achieved by IM injections of meperidine" Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Supported by grants from Bohuslandstinget, the Medical Society of Gothenburg, the Swedish Society of Medical Sciences, and the Swedish Medical Research Council (grant No. 09072)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to receive double‐blind IV bolus injections of 100 mg lidocaine." No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…double‐blind study…". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. No exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Saadawy 2010.
| Methods | Randomized, placebo‐controlled trial. No statement on allocation concealment. Participants, personnel, and outcome assessors were blinded. This study aimed at evaluating and comparing the effects of magnesium and lidocaine on pain, analgesic requirements, bowel function, and quality of sleep in patients undergoing a laparoscopic cholecystectomy. The study was conducted in Saudi Arabia. Date not published. |
|
| Participants | Number assessed for eligibility: 138 Number randomized: 125 → 42:40 (43: intravenous infusion of magnesium sulfate) Number analysed: 40:40 Inclusion criteria ASA I to II patients, scheduled for elective laparoscopic cholecystectomy. Exclusion criteria Major hepatic, renal, or cardiovascular dysfunction, especially atrioventricular block, a history of myopathy, or previous treatment with calcium‐channel blockers. Moreover, patients were excluded if they had received opioids or non‐steroidal anti‐inflammatory drugs within one week, or for chronic pain treatment. Baseline details Experimental group (n = 40) Mean age (years): 41.2 M = 15%, F = 85% Mean weight (kg): 80.1 ASA I/II: 31:9 Duration of surgery (min): 80.3 Main surgical procedure: laparoscopic cholecystectomy Control group (n = 40) Mean age (years): 42.1 M = 20%, F = 80% Mean weight (kg): 77.9 ASA I/II: 27:13 Duration of surgery (min): 79.5 Main surgical procedure: laparoscopic cholecystectomy |
|
| Interventions |
Experimental group (40 patients) Patients received an i.v. bolus of 2 mg/kg lidocaine, followed by a continuous i.v. infusion of 2 mg/kg/hr. Bolus doses were given over 15 min before induction of anaesthesia, followed by an i.v. infusion through the surgery. Control group (40 patients) Control patients received i.v. saline. |
|
| Outcomes | The primary endpoint of the study was morphine consumption within 24 hrs after surgery. Dichotomous
Continuous
|
|
| Notes |
Medication "The PCA settings were a demand dose of 1 mg of morphine i.v. and a lockout of 10 min, with no background infusion. The time to first request of PCA and the total morphine consumption were recorded at 2 hrs in the PACU and after 24 hrs. Morphine was the only painkiller prescribed for postoperative pain control, and no other sedatives or analgesics were administered during the first 24 hrs." Anaesthesia The anaesthesia regime was standardized in both groups Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to one of three groups (n = 40 each) using a computer‐generated table." |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all the parties involved, including the patients, surgeon, anaesthesiologist, nurses, and the data collecting research assistant, were unaware of the study drugs or patient assignment to different groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all the parties involved, including the patients, surgeon, anaesthesiologist, nurses, and the data collecting research assistant, were unaware of the study drugs or patient assignment to different groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 5%:0% Exclusions were described and reasons (unable to use PCA, conversion of procedure) are unrelated to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Samimi 2015.
| Methods | Randomized, controlled trial. Double‐blind. The aim of this study was to compare the efficacy of intravenous and intraperitoneal injection of lidocaine and normal saline in relieving postoperative pain after elective abdominal hysterectomy. The study was conducted in Iran. Date not published. The study period lasted 24 months. |
|
| Participants | Number assessed for eligibility: 130 Number randomized: 117→ N/A:N/A:N/A Number analysed: 109→ 36:35:38 Three groups, one not of interest (intraperitoneal administered group) Inclusion criteria Patients 35 to 65 years undergoing elective abdominal hysterectomy, ASA I to II Exclusion criteria Operation duration more than three hours, diagnosis of cancer, need for additional surgery, substance abuse, chronic pain syndromes, allergy to study medications, severe psychologic, hepatic, renal and cardiac diseases and any incision other than Pfannenstiel Baseline details Experimental group (n = 39) Mean age (years): 46.2, SD = 12.9 M = 0%, F = 100% Mean weight (kg): 63.3, SD = 7.10 ASA I/II (n): N/A Mean duration of anaesthesia (min): 105, SD = 23.28 Mean duration of surgery(min): 95, SD = 20.70 Main surgical procedures (n): elective abdominal hysterectomy (39) Control group (n = 38): (one patient missing) Mean age (years): 48.2, SD = 11.2 M = 0%, F = 100% Mean weight (kg): 62.30, SD = 7.18 ASA I/II (n): N/A Mean duration of anaesthesia (min): 105, SD = 11.04 Mean duration of surgery (min): 92, SD = 21.20 Main surgical procedures (n): elective abdominal hysterectomy (38) |
|
| Interventions |
Experimental group (39 patients) Patients in group IV (intravenous injection group) received lidocaine, (1.5 mg/kg bolus injection) 30 minutes before incision and then a continuous lidocaine infusion until 1 hour after the end of surgery and before closure of wound 50 cc normal saline intraperitoneally. Control group (38 patients) Group P (normal saline injection group) received a bolus dose of normal saline with equal volume with bolus dose of lidocaine in IV group patients and a continuous infusion of normal saline until 1 hour postoperatively. At the end of surgery and before wound closure 50 cc normal saline was administered into intraperitoneal cavity. |
|
| Outcomes | The primary endpoint of the study was not stated and power analysis was not performed. Dichotomous
Continuous
|
|
| Notes |
Medication The patients received 2 mg midazolam intravenously. At the end of surgery neostigmine 0.05 mg/kg and atropine 0.02 mg/kg were used for antagonizing neuromuscular block. After transferring to the postoperative anaesthesia care unit (PACU), all the patients received diclofenac suppository 100 mg. Postoperative pain was treated with morphine 2 mg intravenously when the patients asked for an analgesic or her VAS was ≥ 4. Anaesthesia The anaesthesia regime was standardized in both groups. Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomly allocated to 1 of 3 treatment groups using computer randomization number generation.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “…and then were enveloped and sealed and patient′s code was recorded on it.” Not explicitly mentioned SNOSE. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the study medications were prepared by an anesthesiologist who did not otherwise participate in the study.” Quote: “the envelopes were opened in operation room 1 hour before starting of induction of anesthesia by an anesthesiologist who was blinded to patient′s study group and type of solution. All of the nurses and patients were blinded to the type of solution and to the patient′s study group allocation.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “…by an assistant who was blinded to the detail of study.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate overall: 7% Quote: “during the study, 8 of them excluded from postoperative data analysis: 5 because of the operation was lasted more than 3 hours and 3 because of need to additional procedures, and we completed the study with 109 patients (IV group = 36, IP group = 35 and P group = 38).” It is stated that data were incomplete due to exclusion of eight patients (n=109 remaining) but data from 116 and 117 participants were presented, respectively. It is unclear how these data were analysed. One patient in the baseline characteristics and for continuous outcome data (n = 116) is missing without explanation. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry or study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Slovack 2015.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. This trial aimed to determine if infusing lidocaine during video‐assisted thoracoscopic surgery lowers postoperative opioid consumption and improves pain control. The study was conducted in Canada from April 2010 to February 2013 (NCT01277835). |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 48 → 24:24 Number analysed: 19:17 (analysed at PACU), 16:17 (analysed at 24 hrs), 14:13 (analysed at 48 hrs), 6:7 (analysed at 72 hrs) Inclusion criteria Men and women aged 18 to 75 years, scheduled for video‐assisted thoracoscopic surgery, and evaluated as ASA I‐III at their preoperative assessment clinic visit. Exclusion criteria
Baseline details Experimental group (n = 19) Mean age (years): 58.2 M = 68.5%, F = 31.5% Mean weight (kg): 81.3 ASA I/II/III: 0:8:11 Duration of surgery (min): N/A Main surgical procedure: video‐assisted thoracoscopic surgery Control group (n = 17) Mean age (years): 63.5 M = 47.1%, F = 52.9% Mean weight (kg): 84.2 ASA I/II/III: 0:8:9 Duration of surgery (min): N/A Main surgical procedure: video‐assisted thoracoscopic surgery |
|
| Interventions |
Experimental group (19 patients) The lidocaine treatment group received a 1.5 mg/kg bolus of intravenous lidocaine that began on induction and before incision, followed by an infusion of 3 mg/min if the patient’s total body weight was more than 70 kg or 2 mg/min if weight was less than 70 kg. All infusions were discontinued at the completion of the surgical procedure. Control group (17 patients) The placebo control group received an intravenous normal saline bolus as well as a normal saline infusion to simulate the study drug. |
|
| Outcomes | The primary endpoint of the study was PCA morphine consumption. Dichotomous
Continuous
|
|
| Notes |
Medication "Antiemetic medications and neuromuscular reversal agents were administered at the discretion of each attending anaesthesiologist. Patients were transferred to the post anaesthetic care unit where nursing staff were instructed to administer morphine to keep the patients’ NRS less than four, and the amount of morphine was recorded. PCA morphine, 2 mg bolus and 7 minute lockout, was set up half an hour prior to patient discharge from recovery, with instructions to increase morphine to 2.5 mg bolus if NRS pain greater than six lasted for more than 30 minutes Acetaminophen 975 mg was regularly scheduled for 48 hours postoperatively." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "There are no sources of funding or conflict of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization software was used to generate 1:1 allocation to placebo or treatment groups…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...and sealed into individual opaque envelopes by a research associate not involved in recruitment, data collection, drug preparation or analysis." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the attending anaesthesiologist, surgeon, and nursing staff were all blinded to the treatment groups." and "The study drug was prepared by a physician not involved in the case, labelled ‘study drug’ and given to the attending anaesthesiologist to administer." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nursing stuff collected the data. Quote: "The attending anaesthesiologist, surgeon, and nursing staff were all blinded to the treatment groups." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): at PACU: 21%:30%, at 24 hrs: 33%:30%, at 48 hrs: 42%:46%, at 72 hrs: 75%:71% Quote: "forty‐eight patients consented to be included in the study, and 36 were included in the statistical analysis. Of the other 12 patients, 7 were excluded because the procedure was converted to open, 1 because the lidocaine infusion was not connected, 1 because information on the ward was not filled out, and 3 decided to be excluded for personal reasons." The dropout rates at PACU are very high and it is unclear whether these participants were missing at random. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way. (NCT01277835) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Soltani 2013.
| Methods | Randomized, placebo‐controlled trial. No detailed information on random sequence generation provided. No statement on allocation concealment. No statement on blinding of personnel and outcome assessors. This study evaluated the effects of intravenous infusion of lidocaine on the need for anaesthetics during the operation and analgesics after the operation in patients undergoing ophthalmologic surgeries. The study was conducted in Iran during 2011. |
|
| Participants | Number assessed for eligibility: 80 Number randomized: 80 → 40:40 Number analysed: 40:40 Inclusion criteria Ophthalmologic surgeries such as cataract, dacryocystorhinostomy, keratoplasty, retinal detachment repair, eyelid repair; ASA status: Class I and II, Age: 18 to 70 year Exclusion criteria Any change in management of patient’s surgery or anaesthesia which resulted in change in protocol such as change of technique or type of surgery, abnormal bleeding, oculo‐cardiac reflex which needs pharmacologic intervention. Baseline details Experimental group (n = 40) Mean age (years): N/A M, F (%): N/A Mean weight (kg): 66.2 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure: ophthalmologic surgeries Control group (n = 40) Mean age (years): N/A M, F (%): N/A Mean weight (kg): 68.2 ASA I/II: N/A Duration of surgery (min): N/A Main surgical procedure: ophthalmologic surgeries |
|
| Interventions |
Experimental group (40 patients) Lidocaine (2.5 mg/kg/hr) was infused intraoperatively. Control group (40 patients) Normal saline as placebo. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication Pethidine for pain management (VAS > 4), metoclopramide for nausea management (VAS > 5) Anaesthesia N/A Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No statement on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No explicit statement on blinding of participants and personnel. Quote: “it was a double blind clinical trial conducted in Feiz hospital in 2010.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry. No published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Sridhar 2015.
| Methods | Randomized, controlled trial. Double‐blinded. This study was done to assess the antiinflammatory activity and bowel function hastening properties of IV lignocaine. The study was conducted in the Department of General Surgery in a tertiary care hospital in South India. Date not published. The length of the study period was 24 months. |
|
| Participants | Number assessed for eligibility: 176 Number randomized: 134→ 67:67 Number analysed: 134→ 67:67 Inclusion criteria Above 18 years of age, of either gender, undergoing elective open abdominal surgeries, ASA I, II and III Exclusion criteria Patients sensitive to lignocaine, suffering from cardiovascular diseases, on beta blocker drugs, on opioid drugs for prolonged period, and with functional bowel disorders Baseline details Experimental group (n = 67) Mean age (years): 49.2, SD = 12.8 M = 71,6%, F = 28,4% Mean weight (kg): 43.66, SD = 8.19 ASA I/II/III (n): 39:27:1 Mean duration of anaesthesia (min): N/A Mean duration of surgery (hrs): 2.43, SD = 0.63 Main surgical procedures (n): gastrectomy/ gastrojejunostomy (24), abdominoperineal resection/ anterior resection (11), cholecystectomy/common bile duct exploration and other biliary surgeries (13), others (19) Control group (n = 67) Mean age (years): 52.5, SD = 15.7 M = 64,2%, F = 35,8% Mean weight (kg): 42.43, SD = 7.94 ASA I/II/III (n): 31:29:7 Mean duration of anaesthesia (min): N/A Mean duration of surgery (hrs): 2.40, SD = 0.75 Main surgical procedures (n): gastrectomy/ gastrojejunostomy (24), abdominoperineal resection/ anterior resection (12), cholecystectomy/common bile duct exploration and other biliary surgeries (8), others (23) |
|
| Interventions |
Experimental group (67 patients) Patients in the interventional group received 2 % IV lignocaine as a bolus dose of 1.5 mg/kg at the time of intubation followed by a continuous infusion at a rate of 1.5 mg/kg/hr in the intraoperative period, and continued until 1 hr post‐surgery. Control group (67 patients) Patients in the control group received an IV bolus followed by continuous infusion of 0.9 % normal saline at a volume and rate similar to lignocaine until 1 hr post‐surgery. |
|
| Outcomes | The primary endpoint of the study was not stated and power analysis was not performed. Dichotomous
Continuous
|
|
| Notes |
Medication Postoperative analgesia was administered through a PCA pump delivering IV morphine bolus dose of 1 mg with each demand. Lockout period of 15 min was used to avoid overdosage. Anaesthesia The anaesthesia regime was standardized in both groups. Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomized into two groups to receive i.v. lignocaine infusion or normal saline (placebo) infusion by block randomization (block size of 10) using Microsoft Excel 2007 (Microsoft Corp, Redmond, WA, USA).” |
| Allocation concealment (selection bias) | Low risk | Quote: “location concealment was performed using serially numbered opaque‐sealed envelope technique.” SNOSE was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “Envelopes were opened on the day of surgery outside the operating room by an anaesthetist not involved in the research, and the drug solution (L or S) was prepared based on the patient’s body weight and administered as an infusion during the surgery.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The patients and the investigator who assessed the outcome (pain, ileus) post‐operatively were blinded.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry or study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Staikou 2014.
| Methods | Randomized, controlled trial. Double‐blind. The aim of the study was to compare the effects of IV lidocaine with epidural lidocaine and placebo on postoperative pain, analgesic consumption, and return of bowel function. The study was conducted in Greece between December 2011 and February 2013. |
|
| Participants | Number assessed for eligibility: 77 Number randomized: 60→ 20:20:20 Number analysed: 60→ 20:20:20 Three groups, one not of interest (epidural) Inclusion criteria Patients with bowel cancer, ASA I and II, aged between 40 and 85 years old, scheduled for open, major, large bowel surgery (right or left hemicolectomy, sigmoidectomy, low anterior and abdominoperineal resection) via a midline abdominal incision Exclusion criteria Patient’s refusal or contraindication to the epidural technique/use of local anaesthetics, cardiovascular disease/arrhythmias/conduction abnormalities, significant renal or hepatic impairment, insulin‐dependent diabetes mellitus, central nervous system disease, chronic pain, depression, use of drugs acting on the central nervous system or analgesics during the previous two weeks, and drug/alcohol abuse Baseline details Experimental group (n = 20) Mean age (years): 73.6, SD = 7.5 M = 60%, F = 40% Mean weight (kg): 75.6, SD = 13.57 ASA I/II (n): N/A Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 102.25, SD = 20.51 Main surgical procedures (n): large bowel surgery (20) Control group (n = 20) Mean age (years): 74.4, SD = 8 M = 80%, F = 20% Mean weight (kg): 76.6, SD = 15.4 ASA I/II (n): N/A Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 132, SD = 63.57 Main surgical procedures (n): large bowel surgery (20) |
|
| Interventions |
Experimental group (20 patients) The patients of the IVL group received a bolus dose of 1.5 mg/kg lidocaine IV and an equal volume of NS epidurally, followed by a continuous IV infusion of lidocaine at 2 mg/kg/hr and a continuous epidural infusion of NS at the same rate. The bolus doses, immediately followed by the infusions, were given after the confirmation of correct placement of the epidural catheter (test dose), before the induction of general anaesthesia. Control group (20 patients) The C group received NS bolus epidurally and intravenously, followed by NS infusions epidurally and IV at volumes and rates calculated as if containing lidocaine at the aforementioned doses. |
|
| Outcomes | The primary endpoint of the study was pain. Dichotomous
Continuous
|
|
| Notes |
Medication At the completion of abdominal wall closure, before the start of skin suturing, the infusions were terminated and a bolus of 10 ml of ropivacaine 0.2 %. (20 mg) and 1 mg of morphine were given epidurally to all patients. Atropine 1 mg and neostigmine 2.5 mg IV were given for reversal of neuromuscular blockade before tracheal extubation. After emergence of anaesthesia, the patients were transferred to the PACU and a PCEA pump containing ropivacaine 2 mg/ml and morphine 0.1 mg/ml was connected to the epidural catheter. The pump released 4 ml of solution per delivery, had a lockout interval of 20 min, and had no continuous background infusion. The use of the pump would be started at PACU if the level of analgesia was inadequate. Paracetamol up to 4 g/day and lornoxicam up to 16 mg/day were allowed for rescue analgesia, if needed. Anaesthesia The anaesthesia regime was standardized in both groups. Funding Departmental only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was performed by the use of sealed envelopes describing the group of assignment.” It is not clear if randomization is adequate. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was performed by the use of sealed envelopes describing the group of assignment.” Not mentioned SNOSE. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Prefilled identical syringes containing NS or lidocaine were used. The syringes were prepared by an independent investigator, who was not further involved in the study, i.e., data collection, or analysis.” Quote: “all syringes seemed identical to the blinded anesthesiologist who administered them to the patients.” “Both at PACU and surgical ward, the patients were observed by the nursing staff, blinded to the treatment.” All patients received an epidural catheter and a peripheral vein cannula. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The intensity of pain at rest and during cough was assessed at 1, 2, 4, 12, 24, and 48 hrs postoperatively with the NRS (0 = no pain, 10 = worst possible pain) by study team members blinded to the group allocation.” “Both at PACU and surgical ward, the patients were observed by the nursing staff, blinded to the treatment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry or study protocol. |
| Other bias | Unclear risk | Imbalance between groups: Gender (more females in the experimental group) |
Striebel 1992.
| Methods | Randomized, placebo‐controlled trial. No detailed information on adequate sequence generation and allocation concealment provided. No statement on blinding of participants, personnel, and outcome assessors. This study investigates postoperative pain management using intravenous lidocaine infusion in patients undergoing elective tonsillectomy. The study was conducted in Germany. Date not published. |
|
| Participants | Number assessed for eligibility: 42 Number randomized: 40 → 20:20 Number analysed: 20:20 Inclusion criteria ASA I patients undergoing elective tonsillectomy. Exclusion criteria Intolerance/allergy to any medications. Baseline details Experimental group (n = 20) Mean age (years): 32.2 M = 75%, F = 25% Mean weight (kg): 66.5 ASA I/II: N/A Duration of surgery (min): 57 Main surgical procedure: tonsillectomy Control group (n = 20) Mean age (years): 31.09 M = 55%, F = 45% Mean weight (kg): 77.7 ASA I/II: N/A Duration of surgery (min): 64 Main surgical procedure: tonsillectomy |
|
| Interventions |
Experimental group (20 patients) Patients received an infusion of lidocaine at a dose of 1.5 mg/kg body weight (over 10 min) 30 min before the beginning of surgery, followed by 2 mg/kg/hr over 6 hrs and 0.5 mg/kg/hr for another 18 hrs. Control group (20 patients) The control group received identical volumes of 0.9% NaCl solution. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication Patients reporting pain were treated on request with 25 mg pethidine (intravenous) Anaesthesia It is unclear if the anaesthesia regime was standardized Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No statement on adequate sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No statement on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry. No published study protocol |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Swenson 2010.
| Methods | Randomized, controlled trial. No blinding of participants, personnel, and outcome assessors. This study compared postoperative epidural analgesia and IV infusion of local anaesthetic on ileus duration and hospital stay in patients after colon surgery. The study was conducted in the USA from April 2005 to July 2006 (NCT00600158). |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 45 → 24:21 Number analysed: 22:20 Inclusion criteria Patients aged 18 to 75 years of ASA I to III, scheduled for elective colon resection. Exclusion criteria Allergy to local anaesthetics, myocardial infarction within 6 months before surgery, liver disease (aspartate aminotransferase, alanine aminotransferase, or bilirubin 92.5 times the upper limit of normal), renal impairment (creatinine clearance 60 ml/min), systemic corticosteroid use, chronic use of opiates, unwillingness or contraindication to epidural analgesia, pregnancy, or active breast‐feeding. Baseline details Experimental group (n = 22) Median age (years): 52 M = 45%, F = 55% Median BMI (kg/m2): 25 ASA I/II/III: 1:14:7 Duration of surgery (min): 181 Main surgical procedure (n): subtotal colectomy (2), total abdominal colectomy (0), low‐anterior resection/abdominal perineal resection/ileal pouch‐anal anastomosis (20), lyses of adhesion, small‐bowel resection with primary anastomosis and ileostomy (0), closure of end ileostomy with bowel resection (0) Control (epidural) group (n = 20) Median age (years): 49 M = 80%, F = 20% Median BMI (kg/m2): 28 ASA I/II/III: 1:18:0 Duration of surgery (min): 175 Main surgical procedure (n): subtotal colectomy (4), total abdominal colectomy (1), low‐anterior resection/abdominal perineal resection/ileal pouch‐anal anastomosis (12), lyses of adhesion, small‐bowel resection with primary anastomosis and ileostomy (1), closure of end ileostomy with bowel resection (1) |
|
| Interventions |
Experimental group (22 patients) Before induction of general anaesthesia, patients received IV lidocaine (11 patients: 2 mg/min in patients < 70 kg, 3 mg/min in patients > 70 kg, and 11 patients: 1 mg/min in patients < 70 kg, 2 mg/min in patients > 70 kg). The day after return of bowel function, the lidocaine infusion was turned off. If flatus had not occurred on the fifth POD, IV lidocaine was discontinued. Control group (20 patients) Patients received an epidural analgesia (bupivacaine 0.125% and hydromorphone 6 Kg/ml were started at 10 ml/hr within 1 hr of the end of surgery). |
|
| Outcomes | The primary endpoint of the study was time to first bowel movement. Dichotomous
Continuous
|
|
| Notes |
Medication "When fentanyl was used rather than morphine, it was converted to morphine equivalents using a conversion ratio of 100 µg fentanyl = 10 mg morphine." "In the recovery area, pain was assessed using an 11‐point verbal scale (0 to 10) every 15 min, and scores greater than 3 were treated with either fentanyl 50 µg every 10 min or morphine 4 mg every 20 min as needed. After transfer to the ward, all patients received PCA for breakthrough pain. Initial PCA setting included morphine 2 mg IV demand dose with 6‐min lockout interval (10 mg/hr maximum). Fentanyl was used in an appropriate dose if the patient reported an allergy to morphine." Anaesthesia The anaesthesia regime was standardized. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patient assignments were generated using a published table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...stored in sealed envelopes before initiation of the study protocol." Not mentioned sequentially numbered and opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible due to study design. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding possible due to study design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 8%:5% Withdrawals were described. Quote: "if therapy outside the standard protocol was required, the patient was withdrawn from the study and followed in an intent‐to‐treat manner for assessment of primary outcomes." |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and all of the study's prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way. However, trial registry occurred retrospectively (registered January 2008, study completed July 2006). (NCT00600158) |
| Other bias | High risk | Fifty percent of the patients in the lidocaine group received higher doses of lidocaine infusion than the other 50%. The authors used two different local anaesthetics: bupivacaine for epidural administration and lidocaine for IV administration. The starting time of local anaesthetics infusion was different between the groups (lidocaine: before induction of general anaesthesia, Epidural: within 1 hr of the end of surgery). There were differences in the proportion of female patients (20% in the epidural group and 55% in the IV lidocaine group; P value = 0.021) and distribution of ASA scores (P value = 0.014: the IV lidocaine arm included all the ASA III patients). |
Terkawi 2014.
| Methods | Randomized, controlled trial. Double‐blinded. The aim of this study was to assess the effects of intravenous (IV) lidocaine infusion on the early postoperative recovery profile [opiate consumption, pain scores, fatigue, PONV, and length of stay] of patients undergoing breast cancer surgery The study was conducted at the University of Virginia Health System, USA between January 2009 and June 2013. (NCT01204242) |
|
| Participants | Number assessed for eligibility: 120 (estimated by the authors) Number randomized: 80→ 40:40 Number analysed: 71→ 37:34 Inclusion criteria Aged 18 to 80 years, with ASA Physical Status I, II, or III Exclusion criteria Allergy to local anaesthetics, fentanyl, or morphine; myocardial infarction within six months; profoundly decreased left ventricular function (ejection fraction < 40%) or high‐grade arrhythmias; severe liver disease (aspartate aminotransferase or alanine transaminase or bilirubin > 2.5 times the upper limit of normal); renal impairment (creatinine clearance < 60 ml/min); pregnancy or breastfeeding; and enrolment in another clinical trial within the last 30 days (except blood draw studies, surgical technique studies, or questionnaire studies) Baseline details Experimental group (n = 37) Mean age (years): 53, SD = 13.14 M = N/A%, F = N/A% Mean weight (kg): N/A ASA I/II (n): N/A Mean duration of anaesthesia (mins): N/A Duration of surgery (min) (median): 167, IQR (131 ‐ 216) Main surgical procedures (n): breast cancer surgery (37) Control group (n = 34) Mean age(years): 54, SD = 11.13 M = N/A%, F = N/A% Mean weight (kg): N/A ASA I/II (n): N/A Mean duration of anaesthesia (min): N/A Duration of surgery (min) (median): 161, IQR (122.5 ‐ 250) Main surgical procedures (n): breast cancer surgery (34) |
|
| Interventions |
Experimental group (37 patients) Lidocaine was administered as a bolus to all patients before anaesthetic induction, at a dose of up to 1.5 mg/kg, with a maximum of 150 mg (ie, patients 100 kg and above received a fixed dose of 150 mg). This was to prevent the possibility of overdose because of changes in body composition in obese patients. This bolus was followed by a lidocaine infusion at 2 mg/kg/hr (to an upper limit of 200 mg/hr) until 2 hours after arrival in the PACU, or PACU discharge, whichever was earlier. If the patient left PACU before 2 hours, study drug infusion was terminated. Control group (34 patients) 0.9% NaCl (normal saline) |
|
| Outcomes | The primary endpoint of the study was postoperative pain at rest and postoperative opioid consumption. Dichotomous
Continuous
|
|
| Notes |
Medication Intraoperative analgesia was limited to fentanyl IV (5 μg/kg maximum). Antiemetic prophylaxis was given according to Apfel’s recommendations. Pain was assessed every 15 minutes, and patients with scores greater than 3 were treated with either fentanyl 50 μg every 10 minutes or morphine 4 mg every 20 minutes as needed. Nausea was assessed at 15‐minute intervals and treated using ondansetron 4 mg IV first, followed by doses of promethazine 6.25 mg IV every 20 minutes as needed. Postoperative analgesia was not standardized. Anaesthesia All patients received general anaesthesia. At the discretion of the attending anaesthesiologist were choice of induction drug and the use of premedication and muscle relaxant. Funding N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “subjects were randomized to 1 of 2 study drug arms.” No statement on type of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the anesthesia provider was given a blinded infusion bag that contained lidocaine 8 mg/ml or 0.9% NaCl (normal saline), which was prepared in the pharmacy.” Quote: “the patient and research team remained blinded until after all data were analyzed.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the patient and research team remained blinded until after all data were analyzed.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 15%:8% Nine patients did not complete the study (six in the control group, three in the intervention group). The reasons in the control were: patient changed her mind (n = 1), protocol violation (n=1), codeine allergy (n = 1). The reasons in the intervention group were: patient changed her mind (n = 2), protocol violation (n = 3), codeine allergy (n = 1). Quote: “if the patient left PACU before 2 hours, study drug infusion was terminated and the subject analyzed per intention to treat.” |
| Selective reporting (reporting bias) | High risk | The study protocol as well as a congress abstract is available (NCT01204242). The study was registered retrospectively (15 September 2010, start of recruitment: August 2009). Postoperative pain was defined as a secondary outcome in the protocol but was analysed as primary outcome in the final publication. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Tikuisis 2014.
| Methods | Randomized, placebo‐controlled trial. No statement on allocation concealment. Participants, personnel, and outcome assessors were blinded. This clinical trial evaluated the impact of IV lidocaine on the quality of post‐operative analgesia after hand‐assisted laparoscopic colon surgery. The study was conducted in Lithuania from March 2010 to March 2012. |
|
| Participants | Number assessed for eligibility: 64 Number randomized: 64 → 32:32 Number analysed: 30:30 Inclusion criteria Adult patients 18 to 75 years old, ASA I to III, with normal cognitive function, with colon cancer were scheduled for an elective laparoscopic colon resection. Exclusion criteria Severe hepatic, renal, cardiac, respiratory, and endocrine disease and history of alcohol or drug addiction, those taking analgesics pre‐operatively and those with allergy to local anaesthetic. Baseline details Experimental group (n = 30) Mean age (years): 57.2 M = 60%, F = 40% Mean weight (kg): 73 ASA I/II/III: 19:7:4 Duration of anaesthesia (min): 115 Main surgical procedure: hand‐assisted laparoscopic hemicolectomy Control group (n = 30): Mean age (years): 56 M = 63.3%, F = 36.7% Mean weight (kg): 75.53 ASA I/II/III: 21:5:4 Duration of anaesthesia (min): 114.33 Main surgical procedure: hand‐assisted laparoscopic hemicolectomy |
|
| Interventions |
Experimental group (30 patients) Patients received an IV bolus of lidocaine 1.5 mg/kg was given (maximum 100 mg) just before the induction of anaesthesia, followed by an IV infusion of lidocaine 2 mg/kg/hr during the entire surgical procedure. The dose of lidocaine was then lowered to 1 mg/kg/hr in PACU and continued for the first 24 hrs. Control group (30 patients) Control patients received the same amount of pre‐operative bolus and continuous infusion of normal saline during surgery and for 24 hrs after the operation. |
|
| Outcomes | The primary endpoints of the study were intensity of pain and ketorolac consumption. Dichotomous
Continuous
|
|
| Notes |
Medication "Twenty‐four‐hour post‐operative analgesia in the recovery area was maintained by continuous infusion of 0.1 µg/kg/hr fentanyl." "Patients with a VAS score > 3 were treated with IV ketorolac 30 mg as needed. Ketorolac consumption was registered." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "This study was supported by the Institute of Oncology, Vilnius University, Vilnius, Lithuania (research support)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were allocated to lidocaine group or placebo group before surgery using a computer‐generated randomization list of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients and those who gathered data (treating surgeons, anaesthesiologist, and nurse) were blinded to study allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients and those who gathered data (treating surgeons, anaesthesiologist, and nurse) were blinded to study allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 6%:6% Quote: "four patients had to be excluded from final analysis because hand‐assisted laparoscopic colon surgery was converted to laparotomy." |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry. No published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Wallin 1987.
| Methods | Randomized, placebo‐controlled trial. No detailed information on adequate sequence generation and allocation concealment provided. No statement on blinding of participants, personnel, and outcome assessors. The present study investigated the effects of a continuous IV infusion of lidocaine on the sympathoadrenal stress response to surgery in patients scheduled for elective cholecystectomy. The study was conducted in Sweden. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 38 → 18:20 Number analysed: 18:20 Inclusion criteria Patients scheduled for elective cholecystectomy. Exclusion criteria Patients with hepatic, renal, cardiovascular, or hormonal diseases. Baseline details Experimental group (n = 18) Mean age (years): 54 M = 33.3%, F = 66.7% Mean weight (kg): 69 ASA I/II: N/A Duration of surgery (min): 110 Main surgical procedure: cholecystectomy Control group (n = 20) Mean age (years): 49 M = 30%, F = 70% Mean weight (kg): 72 ASA I/II: N/A Duration of surgery (min): 102 Main surgical procedure: cholecystectomy |
|
| Interventions |
Experimental group (18 patients) Patients received an IV bolus injection of lidocaine (100 mg; 20 mg/ml) was given 30 min before the skin incision, followed by a continuous IV infusion at 2 mg/min (2 g lidocaine in 500 ml physiologic saline) that was continued for 24 hrs after completion of surgery. Control group (20 patients) Patients received a similar infusion with saline. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication "Patients complaining of pain postoperatively were given as many IM injections of 50 mg meperidine as necessary for relief of pain." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "This study was supported by grants from Bohuslandstinget, the ASTRA Research Foundation, the Medical Society of Göteborg, and the Swedish Society of Medicine." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "38 patients scheduled for elective cholecystectomy who were randomly assigned to two groups…" No method described. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the infusions were given in a double‐blind manner." Insufficient information to permit judgment "Yes" or "No". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the infusions were given in a double‐blind manner." Insufficient information to permit judgment "Yes" or "No". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry. No published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Wang 2002.
| Methods | Randomized, placebo‐controlled trial. No detailed information on adequate sequence generation and allocation concealment provided. Participants and personnel were blinded. No statement on blinding outcome assessors. The study investigated the effect of lidocaine on the incidence of cognitive dysfunction in the early postoperative period after cardiac surgery. The study was conducted in China from September 1997 to August 2001. |
|
| Participants | Number assessed for eligibility: 165 Number randomized: 118 → 57:61 Number analysed: 43:45 Inclusion criteria Patients undergoing elective coronary artery bypass surgery with CPB. Exclusion criteria Other simultaneous surgery (e.g. valvular replacement); previous cardiac surgery; history of neurological or psychiatric disorders; suspected history of adverse reactions to lidocaine; age > 70 yrs; preoperative left ventricular ejection fraction < 35%; preoperative biochemical evidence of renal dysfunction (indicated by a serum creatinine concentration more than 177 mol/L (2.0 mg/dl)) or active hepatic disease; and no sufficient education to complete preoperative neuropsychological tests. Baseline details Experimental group (n = 43) Mean age (years): 57.8 M = 97.7%, F = 2.3% Mean weight (kg): 72.7 ASA I/II: N/A Duration of CPB (mins): 149.9 Main surgical procedure: CPB Control group (n = 45) Mean age (years): 59.3 M = 97.8%, F = 2.2% Mean weight (kg): 71.5 ASA I/II: N/A Duration of CPB (mins): 132.2 Main surgical procedure: CPB |
|
| Interventions |
Experimental group (43 patients) Lidocaine was delivered as a bolus of 1.5 mg/kg over 5 min at the opening of the pericardium and was followed by continuous infusion at 4 mg/min until the end of the operation. Another dose of lidocaine (4 mg/kg) was administered to the priming solution of CPB. Control group (45 patients) In the placebo group, normal saline was administered in the same volume and rate as that of 2% lidocaine. |
|
| Outcomes |
Dichotomous
Continuous
|
|
| Notes |
Medication N/A Anaesthesia The anaesthesia regime was not standardized. Funding "Supported by Grant 96‐1‐264 for scientific research from the Ministry of Public Health of the People’s Republic of China." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in a prospective, randomized, and double‐blinded manner, the patients were divided into two groups." No method described. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the medication (2% lidocaine or normal saline) was prepared and coded by an anaesthesiologist who did not participate in anaesthesia and neuropsychological testing." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information on blinding of outcome assessors to permit judgement of "Yes" or "No". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 25%:26% Withdrawals and exclusions were described but reasons for exclusion (noncerebral postoperative complications, death) would have significantly altered neuropsychological performance. Quote: "eighteen patients refused postoperative testing, although their postoperative recovery was uneventful; of these, 10 (16.4%) were in the placebo group and 8 (14.0%) were in the lidocaine group. Six patients had noncerebral postoperative complications that would have significantly altered neuropsychological performance; of these two (3.3%) were in the placebo group and four (7.0%) were in the lidocaine group. Six patients died in the early postoperative period; of these four (6.6%) were in the placebo group and two (3.5%) were in the lidocaine group." Reasons for missing outcome data may have an influence on at least one outcome of interest. |
| Selective reporting (reporting bias) | High risk | It seems not reliable that the threshold for two out of nine tests as defining the hurdle for "cognitive dysfunction" had been set/defined prior to study conduct (no mention of a trial registration beforehand). It is very unlikely, that—based on the preexisting work—only neuropsychological test performance was considered a relevant outcome. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Wang 2015.
| Methods | Randomized, controlled trial. Blinding unclear. The aim of this study was to investigate if lidocaine has a beneficial effect on anti‑cell‑mediated immunity during the postoperative period in patients with cervical cancer undergoing radical hysterectomy. The study was conducted in China between August 2013 and January 2014. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 30→ 15:15 Number analysed: 30→ 15:15 Inclusion criteria Aged between 25 and 65 years old, undergoing radical hysterectomy Exclusion criteria Weight < 45 kg or > 65 kg; a history of allergies to local anaesthetics, bradycardia or heart block; severe respiratory, renal or hepatic disease, previous history of opioid medication use or a psychiatric medical history Baseline details Experimental group (n = 15) Mean age (years): 44.2, SD = 11.8 M = 0%, F = 100% Mean weight (kg): 56.0, SD = 6.5 ASA I/II (n): 11:4 Mean duration of anaesthesia (min): 152.3, SD = 14.1 Mean duration of surgery (min): 132.3, SD = 25.1 Main surgical procedures (n): hysterectomy (15) Control group (n = 15) Mean age (years): 48.6, SD = 5.6 M = 0%, F = 100% Mean weight (kg): 56.9, SD = 7.6 ASA I/II (n): 10:5 Mean duration of anaesthesia (min): 158.0, SD = 16.9 Mean duration of surgery (min): 129.3, SD = ± 24.4 Main surgical procedures (n): hysterectomy (15) |
|
| Interventions |
Experimental group (15 patients) The patients assigned to the lidocaine group received an intravenous bolus infusion of 1.5 mg/kg lidocaine 10 min prior to the induction of anaesthesia, followed by continuous infusion at 1.5 mg/kg/hr until discharge from the operating room. Control group (15 patients) The patients in the control group received the same volume of normal saline. |
|
| Outcomes | The primary endpoint of the study was not reported and power analysis was not performed. Dichotomous No outcomes reported Continuous
|
|
| Notes |
Medication Premedication 0.1 mg/kg midazolam Anaesthesia The anaesthesia regime was standardized in both groups. Funding This study was supported by the Qilu Hospital Science Research Foundation (grant no. 26010175616032), and in part, by Shandong Provincial Natural Science Foundation, China. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “all participants were randomized into two groups, according to a computer‑generated random number table […].” |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “the solutions were prepared in a 20 cc syringe and labeled only with a case number by a nurse in a blinded‑manner.” No statement on blinding of participants and other research team. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessment. All outcomes were dependent on blood values. It remains unclear who assessed the blood draws. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “all 30 patients recruited in the present study completed the study.” No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry or study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Weinberg 2016.
| Methods | Randomized, controlled trial. Double‐blinded. The aim of this study was to test whether intra‐operative intravenous lidocaine combined with a postoperative 24 hrs subcutaneous lidocaine infusion, would decrease pain and hospital stay after radical retropubic prostatectomy. The study was conducted at the Austin hospital and the Box Hill hospital in Australia. Date not published. |
|
| Participants | Number assessed for eligibility: 86 Number randomized: 76→ 38:38 Number analysed: 75→ 37:38 Inclusion criteria Men scheduled for open retropubic radical prostatectomy, who were older than 18 years and ASA physical status < 4 Exclusion criteria Intolerance to opioids local anaesthetics or non‐steroidal anti‐inflammatory drugs; second or third degree heart block, sino‐atrial block without pacemaker; prescribed Class 1 anti‐arrhythmic drugs or amiodarone; epilepsy, seizures, cognitive impairment, or craniotomy within the last five years; myasthenia gravis; pre‐operative morphine consumption > 3 mg/hr orally or > 1 mg/hr intravenously, for more than one month; creatinine > 200 µmol/l; bilirubin > 30 µmol/l or alkaline phosphatase > 300 iu/l; or alanine transaminase > 50 iu/l or albumin < 25 g/dl; platelets < 150 x 109/l or prothrombin time > 14 s; or activated partial thromboplastin time > 35 s or fibrinogen < 2 g/l Baseline details Experimental group (n = 37) Mean age (years): 61.1, SD = 6.3 M = 100%, F = 0% Mean weight (kg): 85.2, SD = 14.1 ASA I/II (n): 24:13 Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): N/A Main surgical procedures (n): radical retropubic prostatectomy (37) Control group (n = 38) Mean age (years): 60.0, SD = 7.6 M = 100%, F = 0% Mean weight (kg): 82.9, SD = 11.9 ASA I/II (n): 26:12 Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): N/A Main surgical procedures (n): radical retropubic prostatectomy (38) |
|
| Interventions |
Experimental group (37 patients) Before induction of anaesthesia, 0.075 ml/kg lidocaine (2%) was injected intravenously over three minutes, which then was infused at 0.075 ml/kg/hr until the end of surgery. The intravenous lidocaine infusion was stopped after tracheal extubation. In recovery, a subcutaneous cannula in the upper arm or abdomen was inserted, and the allocated 0.075 ml/kg/hr subcutaneous infusion of lidocaine was started. The subcutaneous infusion was discontinued at 24 postoperative hours. Control group (38 patients) Before induction of anaesthesia, 0.075 ml/kg saline was injected intravenously over three minutes, which then was infused at 0.075 ml/kg/hr until the end of surgery. The intravenous infusion was stopped after tracheal extubation. In recovery, a subcutaneous cannula in the upper arm or abdomen was inserted, and the allocated 0.075 ml/kg/hr subcutaneous infusion was started. The subcutaneous infusion was discontinued at 24 postoperative hours. |
|
| Outcomes | The primary endpoint of the study was postoperative hospital stay. Dichotomous
Continuous
|
|
| Notes |
Medication: Dexamethasone 8 mg and ondansetron 8 mg intravenously was injected for antiemetic prophylaxis. Paracetamol 1 g intravenously and ketorolac 30 mg was given intramuscularly about 30 min before the end of surgery and the fentanyl infusion was stopped. Participants used morphine PCA with a 1 mg bolus and a 5‐min lockout. Staff could treat pain scores > 6 mm with a 0.05 – 0.10 mg/kg intravenous bolus of morphine, supplemented by a 20 min infusion of tramadol 100 mg and followed as necessary with intravenous ketamine, loaded at 0.1 mg/kg and maintained at 0.05 – 0.20 mg/kg/hr. Intravenous metoclopramide 20 mg or droperidol 2.5 mg were used to treat nausea or vomiting. The subcutaneous infusion of lidocaine/saline was discontinued at 24 postoperative hours, after which participants could take oral oxycodone 10 – 20 mg every four hours as required for discharge. Anaesthesia The anaesthesia regime was standardized in both groups. Funding Funding was provided by a Pfizer Australian and New Zealand College of Anaesthetists Research Fellow grant, and an Australian and New Zealand College of Anaesthetists Academic Enhancement grant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “an independent statistician generated a computerized sequence of 76 allocation codes, 38 for each group (www.randomization.com).” |
| Allocation concealment (selection bias) | Low risk | Quote: “pharmacy staff sealed the allocation codes into sequentially numbered opaque envelopes. The sequence was decoded after we had analysed the data.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the study participants, surgeons, anaesthetists, nurses, and all perioperative staff were blinded to treatment assignments. On the day of surgery, an independent clinical pharmacist prepared 50‐ml and 200‐ml infusions for intra‐operative and postoperative use, respectively, labelled “2% lignocaine or saline”, which contained lidocaine 2% or saline 0.9%.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the study participants, surgeons, anaesthetists, nurses, and all perioperative staff were blinded to treatment assignments.” “The sequence was decoded after we had analysed the data.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “we excluded one man in the lidocaine group whose operation was cancelled after anaphylaxis to cephalosporin antibiotic, given on induction of anaesthesia. We analysed the outcomes for another man in the lidocaine group who was not given intra‐operative and postoperative infusions after lidocaine 1 g was inadvertently injected before anaesthetic induction.” Upon request for data on pain and opioid consumption (presented in figures) the author provided us individual patient data for both outcomes. Missing data at different time points were due to losses of follow up ("no pain score recorded by the pain service"). Reasons for missing data are unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry or study protocol. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Wongyingsinn 2011.
| Methods | Randomized, controlled trial. No blinding of participants and personnel. Outcome assessors were blinded. This study compared the effect of intraoperative and postoperative IV lidocaine infusion with TEA on postoperative restoration of bowel function in patients undergoing laparoscopic colorectal resection using an Enhanced Recovery Program. The study was conducted in Canada from July 2009 to June 2010 (NCT01155440). |
|
| Participants | Number assessed for eligibility: 75 Number randomized: 62 → 31:31 Number analysed: 30:30 Inclusion criteria Patients scheduled for elective laparoscopic colorectal surgery, ASA I to III. Exclusion criteria Allergy to lidocaine, contraindication to have TEA, chronic treatment with opioid, inability to communicate in either French or English or to understand the purpose of the study, severe physical disability, or metastatic carcinoma. Baseline details Experimental group (n = 30) Mean age (years): 58 M = 63.3%, F = 36.7% Mean weight (kg): 80 ASA I/II/III: 9:20:1 Duration of surgery (min): 220 Main surgical procedure (n): right hemicolectomy (9), left hemicolectomy (4), sigmoid resection (4), anterior resection (3), low anterior resection (6), proctocolectomy (4) Control (epidural) group (n = 30) Mean age (years): 61 M = 63.3%, F = 36.7% Mean weight (kg): 74 ASA I/II/III: 12:14:4 Duration of surgery (min): 213 Main surgical procedure (n): right hemicolectomy (10), left hemicolectomy (3), sigmoid resection (2), anterior resection (7), low anterior resection (6), proctocolectomy (2) |
|
| Interventions |
Experimental group (30 patients) Patients received a bolus of lidocaine 1.5 mg/kg (maximum, 100 mg) just before the induction of anaesthesia, followed by an IV infusion of lidocaine 2 mg/kg per hour for the whole surgical procedure. The infusion was then decreased to 1 mg/kg per hour in the PACU and continued 48 hrs postoperative. Control (epidural) group (30 patients) Control patients received TEA. The neural blockade was maintained during surgery with additional infusion of 5 to 8 ml/hr of bupivacaine 0.25%. A continuous epidural analgesia with bupivacaine 0.1% and morphine 0.02 mg/ml was started in the PACU and continued for 48 hrs on the surgical ward. |
|
| Outcomes | The primary endpoint of the study was bowel movement. Dichotomous
Continuous
|
|
| Notes |
Medication "As a rescue analgesia, patients in the IL group received PCA using IV morphine for 48 hrs. The PCA was set up at 1 to 2 mg every 7 min with no background infusion and was increased if the VRS at rest exceeded 4 at rest.", "If the VRS (in the TEA group) at rest exceeded 4, the rate of epidural infusion was increased by increments of 1 ml to a maximum of 15 ml/hr. No rescue analgesia with systemic morphine was used.", " If the VRS at rest in both groups exceeds 4 at 48 hours after surgery, TEA or IL infusion would continue, and VRS reassessed every 2 hours.", "In both groups, multimodal analgesia included 500 mg of naproxen twice a day and acetaminophen 1 g 4 times a day for up to 5 days. Both epidural and lidocaine with PCA were discontinued 48 hrs after surgery if VRS at rest was less than 4, and oral oxycodone 5 to 10 mg was then provided every 4 hrs as breakthrough medication." "Prevention of PONV was achieved with droperidol 0.625 mg and dexamethasone 8 mg." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Funding for the study was provided by the Department of Anesthesia, McGill University Health Centre, Montreal, Quebec, Canada." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients assignments were generated using a published table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...sealed in a brown envelope…" Not mentioned sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel possible due to study design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all the postoperative data were collected daily by the research assistant unaware of the hypothesis." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 3%:3% Quote: "two patients had to be excluded from final analysis: 1 patient in the TEA group for conversion to laparotomy, and 1 patient in the IL group for unknown drug reaction." The exclusion due to unknown drug reaction may influence the results of the study. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and all of the study's prespecified primary outcomes that are of interest in the review have been reported in the prespecified way. However, trial registry occurred retrospectively (registered October 2010, study start date June 2009, study completion October 2011). (NCT01155440) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Wu 2005.
| Methods | Randomized, placebo‐controlled trial. Participants and personnel were blinded. No statement on blinding outcome assessors. The present study evaluated the interaction of dextromethorphan and IV lidocaine on pain management after laparoscopic cholecystectomy. The study was conducted in China. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 100 → 25:25:25:25 Number analysed: 25:25:25:25 Inclusion criteria ASA physical status I or II patients scheduled for elective laparoscopic cholecystectomy. Exclusion criteria Clinically diagnosed acute pancreatitis, were scheduled to undergo any surgical procedure expected to produce more trauma than laparoscopic cholecystectomy alone, had acute preoperative pain other than biliary colic, required chronic pain treatment, or had current or recent cancer or any condition that would contraindicate participation in a surgical study of this nature. Patients with contraindications for lidocaine or who had received opioids or nonsteroidal antiinflammatory drugs within 1 wk were excluded. Baseline details Experimental group (n = 25) Mean age (years): 51.8 M = 40%, F = 60% Mean weight (kg): 61.8 ASA I/II: N/A Duration of surgery (min): 81.4 Main surgical procedure: laparaoscopic cholecystectomy Control group (n = 25) Mean age (years): 51.4 M = 44%, F = 56% Mean weight (kg): 60.8 ASA I/II: N/A Duration of surgery (min): 81.0 Main surgical procedure: laparaoscopic cholecystectomy |
|
| Interventions | Patients were randomized in to four equal groups to receive either:
Experimental group (25 patients) Patients received 20 mg of chlorpheniramine IM and IV lidocaine 3 mg/kg/hr; all treatments were administered 30 min before skin incision, and lidocaine or normal saline was infused with a pump throughout the surgery. Control group (25 patients) Patients of the control group received 20 mg of chlorpheniramine IM and an equal IV volume of normal saline. |
|
| Outcomes | The primary endpoint of the study was meperidine consumption. Dichotomous
Continuous
|
|
| Notes |
Medication "For all patients, general anaesthesia was induced with IV fentanyl (2 µg/kg), ...", "No additional opioids were given during the operation.", "A meperidine (1 mg/kg) IM injection was used for postoperative pain relief, if requested, because it has been widely used for pain relief after laparoscopic cholecystectomy in our country. In most laparoscopic cholecystectomy patients, one or two doses of meperidine can provide adequate pain relief, so this treatment was preferable to PCA." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Supported, in part, by grants from Armed Forces Taoyuan General Hospital (AFTYGH‐9327)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the study was double‐blind and randomized with a computer program." |
| Allocation concealment (selection bias) | Low risk | Quote: "the study drugs…were prepared by the hospital pharmacy in identical containers marked with … consecutive numbers." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study drugs…were prepared by the hospital pharmacy in identical containers marked with … consecutive numbers." Due to adequate randomization and pharmacy‐prepared vials blinding of personnel and participants was ensured. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry. No published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Wuethrich 2012.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. The objective of this study was to evaluate the effect of systemic lidocaine on the length of hospital stay, readiness for discharge, opioid requirement, bowel function and inflammatory and stress response after laparoscopic renal surgery. The study was conducted in Switzerland from July 2009 to February 2011 (NCT00789620). |
|
| Participants | Number assessed for eligibility: 70 Number randomized: 65 → 33:32 Number analysed: 32:32 Inclusion criteria ASA I to III and laparoscopic transperitoneal renal surgery under general anaesthesia. Exclusion criteria Liver insufficiency, steroid therapy, chronic opioid therapy, known allergy to lidocaine, a pre‐existing disorder of the gastrointestinal tract, an atrio‐ventricular block grade II to III, congestive heart failure, a long QT syndrome and pregnancy. Baseline details Experimental group (n = 32) Mean age (years): 50.6 M = 50%, F = 50% Mean weight (kg): 77.4 ASA I/II/III: 12:16:4 Duration of anaesthesia (min): 293 Main surgical procedure (n): pyeloplasty (6), adrenalectomy (10), partial nephrectomy (5), nephrectomy (8), others (3) Control group (n = 32) Mean age (years): 52.3 M = 47%, F = 53% Mean weight (kg): 73.3 ASA I/II/III: 11:17:4 Duration of anaesthesia (min): 287 Main surgical procedure (n): pyeloplasty (6), adrenalectomy (7), partial nephrectomy (6), nephrectomy (10), others (3) |
|
| Interventions |
Experimental group (32 patients) Lidocaine was given as a 1.5 mg/kg bolus during induction of anaesthesia, followed by a continuous intraoperative infusion of 2 mg/kg/hr. At the end of surgery, the dose was reduced to 1.3 mg/kg/hr for the following 24 hrs, after which the infusion was discontinued. Control group (32 patients) Control patients received NaCl 0.9%. |
|
| Outcomes | The primary endpoint of the study was length of hospital stay. Dichotomous
Continuous
|
|
| Notes |
Medication "Supplemental intravenous morphine was given by the nurses upon request if the patients reported a pain score at rest of 4 or more. Morphine was given intravenously during the first 24 hrs on the intermediate care unit in boluses of 2 mg, with a minimum interval of 10min between two doses. On day 2, morphine was given subcutaneously in a dose of 7.5 mg, at minimal intervals of 3h between two doses. Morphine requirement was recorded at 2 and 6 hrs postoperatively (POD 0) and at 09:00, 13:00 and 19:00 hrs on postoperative days 1 and 2. No other analgesics were used." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "Financial support and sponsorship: support was provided solely from departmental sources." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to receive either a lidocaine or saline infusion by computer‐generated randomization following the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statements." |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthesiologist in charge, the surgeon, the nursing staff and the patients were blind to the group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "These variables were recorded by research personnel, blind to the allocation…" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (experimental/control): 3%:0% quote: "Of the 65 patients enrolled, one patient was excluded because the surgeon decided to convert to an open procedure." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way. (NCT00789620) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Xu 2017.
| Methods | Randomized, controlled trial. Double‐blinded. The aim of this study was to investigate whether lidocaine combined with dexmedetomidine infusion was superior in controlling pain and recovery of bowel function. The study was conducted in China from March 2013 to August 2014. |
|
| Participants | Number assessed for eligibility: 320 Number randomized: 240→ 60:60:60:60 Number analysed: 240→ 60:60:60:60 Four groups, two not of interest (dexmedetomidine, lidocaine and dexmedetomidine combined) Inclusion criteria ASA I and II, 40 to 65 years old women scheduled for elective abdominal hysterectomy with general anaesthesia Exclusion criteria < 50 kg or > 65 kg, history of allergy to local anaesthetics, severe respiratory, renal or hepatic disease, preoperative opioids medication and psychiatric medical history, severe arterial hypotension (MAP < 60 mmHg) or bradycardia (< 40 bpm), arrhythmia, urticaria due to lidocaine and dexmedetomidine infusion during the surgery Baseline details Experimental group (n = 60) Mean age (years): 45.2, SD = 6.7 M = 0%, F = 100% Mean weight (kg): 59.2, SD = 6.5 ASA I/II (n): NA Mean duration of anaesthesia (min): 118.7, SD = 11.2 Mean duration of surgery (min): NA Main surgical procedures (n): elective abdominal hysterectomy (60) Control group (n = 60) Mean age (years): 46.5, SD = 4.4 M = 0%, F = 100% Mean weight (kg): 58.9, SD = 6.3 ASA I/II (n): NA Mean duration of anaesthesia (min): 120.5, SD = 10.8 Mean duration of surgery (min): N/A Main surgical procedures (n): elective abdominal hysterectomy (60) |
|
| Interventions |
Experimental group (60 patients) IV bolus of lidocaine (2%) 1.5 mg/kg made to 20 ml with normal saline and 20 ml normal saline, respectively, over 10 min before induction of anaesthesia, followed by a continuous IV infusion of lidocaine 1.5 mg/kg made up to 20 ml and 20 ml normal saline every hour until abdominal wound closure, respectively. Control group (60 patients) IV bolus of 20 ml normal saline and 20 ml normal saline, respectively, over 10 min before induction of anaesthesia, followed by a continuous IV infusion 20 ml and 20 ml normal saline every hour until abdominal wound closure, respectively. |
|
| Outcomes | The primary endpoint of the study was VAS pain score. Dichotomous No outcomes reported Continuous
|
|
| Notes |
Medication All patients received intramuscular phenobarbital (0.5 mg) before induction of anaesthesia. Dezocine 0.1 mg/kg and ondansetron 0.1 mg/kg were given intravenously 30 min before the end of surgery. Patients were connected to an IV patient‐controlled analgesic system (IVPCA) with 0.3 µg/kg/h fentanyl and granisetron hydrochloride 6 mg (100 ml of total volume) to deliver a bolus of 0.075 µg/kg of the analgesics with a lockout time of 15 min at the end of surgery. For persistent pain with VAS > 3, an additional 25 µg of fentanyl was administered until the pain was VAS < 3. Anaesthesia The anaesthesia regime was standardized in both groups. Funding The study was supported with science and technology key project of Anhui Province. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were assigned into one of four groups using computer‐generated random numbers […].” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] in sequentially numbered opaque envelopes […].” Not mentioned sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “investigators, clinicians and patients were all fully blinded to treatment allocation. The drug solutions were prepared by an anesthesiologist who also was not involved in the management of the case.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “[…] by an anesthesiologist who was not involved in the study.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Unclear risk | There is no registered protocol or reference to a trial registry. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yang 2014.
| Methods | Randomized, placebo‐controlled trial. Participants, personnel, and outcome assessors were blinded. This trial evaluated IP lidocaine administration and intravenous (IV) lidocaine infusion for postoperative pain control after laparoscopic cholecystectomy. The study was conducted in Korea from May 2011 to May 2012. |
|
| Participants | Number assessed for eligibility: 83 Number randomized: 72 → 26:24:22 (22: lidocaine IP) Number analysed: 26:24:22 (22: lidocaine IP) Inclusion criteria Adult patients, 18 to 65 yrs, (ASA) physical status I and II, undergoing elective laparoscopic cholecystectomy. Exclusion criteria In receipt of analgesics or sedatives 24 hrs before scheduled surgery; spillage or cholelithiasis with known common bile duct pathology; body weight < 45 kg or > 100 kg; underlying severe systemic disease; history of abdominal surgery, a chronic pain disorder other than gallbladder disease or allergy to lidocaine. Baseline details Experimental group (n = 26) Mean age (years): 48.5 M = 38%, F = 62% Mean weight (kg): 62.35 ASA I/II/III: 21:4:1 Duration of anaesthesia (min): 65 Main surgical procedure: laparoscopic cholecystectomy Control group (n = 24) Mean age (years): 48.0 M = 50%, F = 50% Mean weight (kg): 66.38 ASA I/II/III: 18:3:3 Duration of anaesthesia (min): 63.5 Main surgical procedure: laparoscopic cholecystectomy |
|
| Interventions | Patients were randomized in to three groups to receive either:
Experimental group (26 patients) Patients received an IV bolus injection of lidocaine (1.5 mg/kg) 2 min before orotracheal intubation. This was followed by a continuous IV lidocaine infusion at 2 mg/kg/hr during the operation. Control group (24 patients) Patients in the control group received the same volume of saline. |
|
| Outcomes | The primary endpoint of the study was pain score, VAS 2 hrs. Dichotomous
Continuous
|
|
| Notes |
Medication "To control the severity of postoperative pain, IV fentanyl (15 mg/kg, mixed with normal saline, total 100 ml) contained in a computerized IV PCA system was used. The mode of PCA was set to a bolus of 0.1 mg/kg, a lock‐out interval of 15 min and a continuous infusion of 0.1 mg/kg per hr. Patients were taught to push the button of the PCA system to receive a bolus of drug at the first onset of pain. A 10‐point visual analogue scale (VAS) of pain severity was used to assess pain levels in patients, with 0 denoting the patient was pain free, and 10 denoting that the patient was in intolerable pain. If the VAS score was > 3 despite the bolus, an additional 50 mg of fentanyl was administered IV until the pain was below a VAS score of 3." Anaesthesia The anaesthesia regime was standardized in both groups. Funding "This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1003700)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization of patients into one of the three study groups was performed using Excel software…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the numbers determining group assignment were written on cards within a set of sealed envelopes…" Not mentioned sequentially numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "details of the series, which were generated by a statistician who did not otherwise participate in this study (C.W.B.),were unknown to the investigators or patients.", "...the surgeon and the anaesthesiologist were blind to the patient’s group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "one research nurse, who was blinded to the details of the study, collected the postoperative data." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "no patient was withdrawn from the study.". |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was retrospectively registered. (NCT01608373) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yardeni 2009.
| Methods | Randomized, placebo‐controlled trial. No detailed information on adequate sequence generation and allocation concealment provided. The anaesthesiologist in charge was blinded. No statement on blinding of participants and outcome assessors. The study focused on the effects of pre‐incisional and intraoperative IV lidocaine on pain intensity and immune reactivity in the postoperative period in patients undergoing abdominal hysterectomy. The study was conducted in Israel. Date not published. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 65 → 32:33 Number analysed: 30:30 Inclusion criteria Female patients (ASA physical status I to II) scheduled for transabdominal hysterectomy, age 45 to 70. Exclusion criteria Hypertension, arrhythmia, diabetes, and patients with previous medication with immunosuppressive drugs, nonsteroidal anti‐inflammatory drugs, or steroids. Baseline details Experimental group (n = 30) Mean age (years): 55.9 M = 0%, F = 100% Mean weight (kg): 71.2 ASA I/II: N/A Duration of surgery (min): 109 Main surgical procedure: abdominal hysterectomy Control group (n = 30) Mean age (years): 53.4 M = 0%, F = 100% Mean weight (kg): 70.0 ASA I/II: N/A Duration of surgery (min): 106 Main surgical procedure: abdominal hysterectomy |
|
| Interventions |
Experimental group (32 patients) Patients received IV lidocaine (bolus 2 mg/kg, continuous infusion 1.5 mg/kg/hr), starting 20 min before the beginning of surgery and continued during the operation. Control group (33 patients) Patients in the second group were given an equal volume of saline infusion. |
|
| Outcomes |
Dichotomous no outcomes reported. Continuous
|
|
| Notes |
Medication "On arrival to the PACU, patients of both groups were connected to a PCEA pump and received an initial loading dose of 3–5 ml 0.1% bupivacaine 2 g/ml fentanyl and a bolus of 3 ml 0.1% bupivacaine 2 g/ml fentanyl on demand (lockout time 10 min), with continuous background infusion of 6 ml/hr. The total doses of intraoperative fentanyl and PCEA during the 24 hrs after surgery for both groups are detailed in Table 1. Postoperative analgesia was given only by PCEA to avoid nonsteroidal anti‐inflammatory drugs or opiates that may have affected the study outcome. Anaesthesia: The anaesthesia regime was standardized in both groups. Funding No funding mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "at the preoperative anesthesiology visit, the patients were randomly assigned to 1 of 2 perioperative pain management techniques:…" No method described. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...anaesthesiologist who did not participate in the study was instructed to prepare 2% lidocaine or saline solution in a syringe pump labelled number 1 or 2, respectively, and hand it to the anaesthesiologist in charge without notifying him of the content." In consideration of the fact that "label 1" and "label 2" are not true random numbers for each patient, we judged that this is not sufficient as an adequate blinding method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate (experimental/control): 6%:9% Exclusions were described. Group assignment unclear. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry. No published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yon 2014.
| Methods | Randomized, controlled trial. Double‐blinded. The aim of this study was to assess the effect of intraoperative systemic lidocaine infusion in patients who underwent subtotal gastrectomy. The study was conducted in Korea between May 2012 and March 2013. (ACTRN12612000545864) |
|
| Participants | Number assessed for eligibility: 40 Number randomized: 36→ 17:19 Number analysed: 36→ 17:19 Inclusion criteria Adult patients (age 18 to 80 years) with subtotal gastrectomy Exclusion criteria Weight less than 45 kg or more than 100 kg; severe underlying respiratory, renal or hepatic disease; history of allergies to local anaesthetics, evidence of previous opioid medication or psychiatric medical history Baseline details Experimental group (n = 17) Age (years) (median): 59.00, IQR (57.00 – 66.50) M = %, F = % 1 patient missing male/female (n) (10:6) Mean weight (kg): 63.56, SD = 11.36 ASA I/II/III (n): 2:13:2 Mean duration of anaesthesia (min): 316.00, SD = 43.58 Mean duration of surgery (min): 271.47, SD = 33.11 Main surgical procedures (n): subtotal gastrectomy (17) Control group (n = 19) Age (years) (median): 66.00, IQR (59.00 – 72.00) M = 63.2%, F = 36.8% Mean weight (kg): 61.56, SD = 7.83 ASA I/II/III (n): 1:17:1 Mean duration of anaesthesia (min): 331.74, SD = 56.45 Mean duration of surgery (min): 291.32, SD = 50.47 Main surgical procedures (n): subtotal gastrectomy (19) |
|
| Interventions |
Experimental group (17 patients) Patients assigned to the lidocaine group received an intravenous bolus infusion of 1.5 mg/kg of lidocaine followed by a continuous infusion of 2 mg/kg/hr (preoperatively and throughout surgery). Control group (19 patients) Patients in the placebo group received the same amount of normal saline. |
|
| Outcomes | The primary endpoint of the study was pain score. Dichotomous
Continuous
|
|
| Notes |
Medication The patients were not premedicated. No additional analgesics were injected during surgery. To control postoperative pain, intravenous fentanyl was administered with the use of a PCA system. The mode of PCA was a 0.3 µg/kg bolus with a lockout interval of 15 minutes, continuous infusion and 0.2 µg/kg/hr (total regimen of 100 ml) of fentanyl. In the case of persistent pain exceeding a visual analogue scale (VAS) pain score of 30 mm, an additional 50 µg of fentanyl (rescue) was intravenously injected by an investigator until the pain was relieved to a level falling below a VAS pain score of 30 mm. Anaesthesia The anaesthesia regime was standardized in both groups. Funding This work was supported by the 2012 Inje University research grant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “random assignment was based on a random table generated using PASS software version 11 (NCSS). We used block randomization with a block size of 4 and equal allocation to prevent imbalances in treatment assignments. The randomization sequence was generated by a statistician who was not otherwise involved with the study.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “the details of the series were unknown to the investigators, and the group assignments were kept in sealed envelopes, each bearing only the case number on the outside.” Not explicitly mentioned that the envelopes were opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “to keep the anesthesiologist blind to the patients’ assigned group, lidocaine or placebo were prepared in a syringe and a bottle labelled only with a case number. The preparations of bolus and continuous infusions were arranged by an additional investigator (H.S.Y.) who read the card.” Quote: “all parties involved, including the patients, the surgeon, the anesthesiologists and the investigator (J.H.Y.) collecting the data were unaware of the study drugs or the patients’ group assignment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The VAS scores were collected by 1 blinded investigator (J.H.Y.) with more than 2 years of experience interviewing patients about postoperative pain.” Quote: “all parties involved, including the patients, the surgeon, the anesthesiologists and the investigator (J.H.Y.) collecting the data were unaware of the study drugs or the patients’ group assignment.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate (experimental/control): 0%:0% Quote: “we used an intention‐to‐treat strategy — that is, all participants were included in the analysis irrespective of whether they had completed the study. Missing data were completed using a last observation carried forward analysis.” Quote: “data were incomplete for 3 patients. One patient in the lidocaine group and 1 patient in the placebo group were treated with other painkillers for PONV that was unresponsive to antiemetic treatment and likely induced by fentanyl infusion. One patient in the placebo group received meperidine owing to postoperative shivering. Despite incomplete data, these 3 patients were included in our analysis according to the intention‐to‐treat principle.” Reasons for missing data are likely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12612000545864). It has been prospectively registered (22 May 2012, study start: May 2012). The primary and two secondary outcomes have been reported in the prespecified way. All other secondary outcomes were not prespecified but were not judged as selective reporting. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Zengin 2015.
| Methods | Randomized, controlled trial. Blinding unclear. The purpose of this study was to evaluate and compare the effects of preoperative oral pregabalin and perioperative intravenous lidocaine infusion on postoperative morphine requirement, adverse effects, patients’ satisfaction, mobilization, time to first defaecation and time to discharge in patients undergoing laparotomy. The study was conducted in Turkey between November 2010 and April 2011. |
|
| Participants | Number assessed for eligibility: N/A Number randomized: 80→ 20:20:20:20 Number analysed: (80)→ (20):(20):(20):(20) (not clearly stated) Four groups, two not of interest (pregabalin, pregabalin+ lidocaine) Inclusion criteria ASA I and II, 18 to 65 years of age and elective laparotomy Exclusion criteria ASA ≥ III, liver or kidney failure, chronic pain, epilepsy or other neurological disease or a history of allergy to one of the study drugs Baseline details Experimental group (n = 20) Mean age (years): 51.1, SD = 26.2 M = 55%, F = 45% Mean weight (kg): 72.2, SD = 25.5 ASA I/II (n): N/A Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 114.1, SD = ± 89.8 Main surgical procedures (n): laparotomy (20) Control group (n = 20) Mean age (years): 53.2, SD = 15.7 M = 50%, F = 50% Mean weight (kg): 70.0, SD = ± 16.7 ASA I/II (n): N/A Mean duration of anaesthesia (min): N/A Mean duration of surgery (min): 101.0, SD = 78.0 Main surgical procedures (n): laparotomy (20) |
|
| Interventions |
Experimental group (20 patients) Group L (lidocaine) patients ingested placebo capsules 12 hrs before surgery and on the morning of surgery, and received a bolus injection of 1.0 mg/kg lidocaine at induction of anaesthesia, then a continuous infusion with a Braun Perfusor infusion pump at a rate of 2 mg/kg/hr during the operation until skin closure. Control group (20 patients) In group C (control‐placebo), patients ingested placebo capsules 12 hrs before the operation and on the morning of the operation, and received saline infusion perioperatively. |
|
| Outcomes | The primary endpoint of the study was not defined. Power analysis was performed for postoperative morphine requirement. Dichotomous
Continuous
|
|
| Notes |
Medication Postoperatively, intravenous morphine was administered until visual analogue scale (VAS) scores were < 30, and intravenous PCA consisting of 1 mg/ml morphine solution with 1.5 mg bolus dose and 8 min lockout interval was started in the recovery room. Anaesthesia The anaesthesia regime was standardized in both groups. Funding None. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomization was performed with sealed envelope method before the day of surgery when patients arrived to the ward reception area.” It is not clear from the description how the randomization was performed (e. g. shuffling envelopes). |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “[…] [T]he present prospective, randomized, placebo‐controlled and double‐blinded study […].” No explicit statement how participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate unclear. There is no statement as to whether the presented results are for all participants who were randomized. |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry or study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Acronyms and abbreviations used in these tables
ASA = American Society of Anesthesiologists Physical Status, BMI = body mass index, BP = blood pressure, CABG = coronary artery bypass grafting, CPB = cardiopulmonary bypass, CRP = C‐reactive protein, cTnI = cardiac troponin I, F = female, hrs= hours, ICU = intensive care unit, IL = interleukin, IM = intramuscular, IP = intraperitoneal; IQR = interquartile range, IV = intravenous, LOCF = last observation carried forward, LVEF = left ventricular ejection fraction, M = male, MAP = mean arterial pressure, mins = minutes, MWD = minute‐walk distance, n = number of participants, N/A = not available, NRS = normal rating scale, NSAID = non‐steroid anti‐inflammatory drug, PACU = postanaesthesia care unit, PCA = patient‐controlled analgesia, PCEA = patient‐controlled epidural analgesia, pm = afternoon, POD = postoperative day, PONV = postoperative nausea and vomiting, PO/PR = by mouth/by rectum, PRN = as needed, QoR = Quality of Recovery; SD = standard deviation, SE = standard error, SEM = standard error of mean, SNOSE = sequentially numbered opaque sealed envelopes, TAP = transverse abdominal plane, TEA = thoracic epidural analgesia, Tnl = troponin I, VAS = visual analogue scale, VRS = verbal rating scale, yrs = years, IL‐1RA = Interleukin 1 receptor antagonist
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bartlett 1961 | No control group, not a randomized controlled trial |
| Birch 1987 | Lidocaine was given only after the operation |
| Cepeda 1996 | Lidocaine was given as patient‐controlled analgesia only after the operation |
| Chia 1998 | Lidocaine was given only after the operation |
| Couceiro 2015 | Lidocaine was given after incision |
| De Clive‐Lowe 1958 | No control group, not a randomized controlled trial |
| De Kock 1994 | Lidocaine was given as repeated bolus, not as continuous infusion |
| Feld 2003 | Lidocaine was only one of a group of non‐opioid drugs which were compared with fentanyl |
| Hans 2010 | Infusion was only given over the first 30 minutes |
| Harvey 2009 | Lidocaine was given for 24 hours only after the operation |
| Joppich 2010 | Review article (relating to McCarthy 2010; no secondary publication) |
| Juarez‐Pichardo 2009 | Lidocaine infusion was terminated 10 minutes before end of surgery |
| Kavak 2014 | Wrong control intervention (remifentanil) |
| Knight 1980 | No control group, not a randomized controlled trial |
| Marret 2008 | Review article |
| McCarthy 2010 | Review article |
| Olivares 2012 | No control group, the other group received magnesium |
| Perniola 2014 | Licocaine was given after incision |
| Rinne 1998 | Infusion was started after skin incision |
| Sun 2012 | Review article |
| Vigneault 2011 | Review article |
| Zhu 2015 | Wrong intervention (additional ketamine infusion in intervention group) |
Characteristics of studies awaiting assessment [ordered by study ID]
Cho 2014.
| Methods | Randomized controlled trial. No statement on blinding. We evaluate the effects of perioperative infusion of lidocaine and dexmedetomidine IV on postoperative pain control and analgesics consumption after laparoscopic cholecystectomy. The study was conducted in the Republic of Korea. It is not stated when the study was conducted. Trial identifier: n/a |
| Participants | Sample size: 84 (3 groups, n = 28, respectively) Three groups, one not of interest (dexmedetomidine) Inclusion criteria Aged 20 to 60 years, elective LC Exclusion criteria N/A |
| Interventions |
Experimental group (28 patients) The patients in group L received an IV lidocaine bolus of 1.5 mg/kg and then continuous infusion of 2 mg/kg/h. Bolus doses were given during 10 minutes before the induction of anaesthesia, followed by continuous infusion until end of the surgery. Control group (28 patients) The group N received saline by same method as group L (bolus of 1.5 mg/kg and then continuous infusion of 2 mg/kg/h). Bolus doses were given during 10 minutes before the induction of anaesthesia, followed by continuous infusion until end of the surgery. |
| Outcomes | VAS pain score during first 24 h after LC, postoperative analgesics consumption (amount of fentanyl consumption in PACU) were evaluated during 24 h after the surgery. |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding This work is supported by the 2011 Inje University research grant. Notes Only abstract available |
Choi 2017.
| Methods | Randomized, double‐blinded, controlled trial. The aim of this randomized, double‐blinded, controlled study was to evaluate the effect of IV administered lidocaine on the QoR and on acute and chronic postoperative pain after robot‐assisted thyroidectomy. The study was conducted in the Republic of Korea between July 2013 and January 2015. Trial identifier: NCT01907997 |
| Participants | Sample size: 90 (2 groups, n = 45, respectively) Inclusion criteria The patients were aged between 20 and 65 years, had ASA grades of 1 or 2, and were scheduled to undergo elective robot‐assisted thyroidectomy. Exclusion criteria History of chronic pain, chronic use of analgesics, allergy to local anaesthetics, severe cardiopulmonary, hepatic or renal disease, diabetes, and neuropsychiatric disease. |
| Interventions |
Experimental group (45 patients) In the lidocaine group (Group L), 0.1 mL/kg of 2% lidocaine (2 mg/kg) was infused IV for 10 mins immediately after anaesthesia induction, and then, it was continuously infused at a rate of 0.15 mL/kg/h of 2% lidocaine (3 mg/kg/h) until the patients were extubated. Control group (45 patients) The control group (Group C) received the same volumes of 0.9% normal saline during the same time periods. |
| Outcomes | QoR‐40, pain at admission to and discharge of PACU, at 24 and 48 hours, analgesic consumption, chronic postsurgical pain |
| Notes | Small trial sample size (< 200 patients) Anaesthesia Anaesthesia was induced using IV propofol (1.5 mg/kg) and remifentanil (1 µg/kg). Endotracheal intubation was performed after sufficient muscle relaxation had been achieved by administering 0.6 mg/kg of rocuronium. Anaesthesia was maintained with sevoflurane and remifentanil. Funding This work supported by a research grant. Notes N/A |
Dewinter 2017.
| Methods | Randomized, double‐blinded, controlled trial. The authors hypothesized that in adolescent and adult patients undergoing posterior spinal arthrodesis, a perioperative lidocaine infusion would reduce opioid requirements during the first 24 postoperative h. The study was conducted in Belgium between September 2013 and July 2015. Trial identifier: EUDRACT 2012‐005264‐98 |
| Participants | Sample size: 70 (2 groups, n = 35, respectively) Inclusion criteria ASA I–III and an age between 12 and 18 yrs. Eight months after the beginning of the study, the Ethics Committee approved a modification of the inclusion criteria (EC OG032, 23 December 2013) so that patients up to 75 yrs could be included. This modification became necessary to increase the number of eligible patients. Exclusion criteria Hypersensitivity to lidocaine, liver disease (defined as total serum bilirubin 2 mg/dl), renal impairment (defined as Glomerular Filtration Rate 60 ml/min/1.73 m2), cardiac arrhythmias, epilepsy, intellectual disability and preoperative chronic medication with strong opioids (e.g. morphine or transdermal fentanyl). |
| Interventions |
Experimental group (35 patients) Patients in the lidocaine group were given an IV bolus injection of lidocaine 1.5 mg/kg at induction of anaesthesia and then a continuous infusion of 1.5 mg/kg/h which was continued until six h after arrival at the PACU. Control group (35 patients) Patients in the placebo group received equivalent volumes of saline using the identical application scheme. |
| Outcomes | Morphine consumption during 24 h, pain up to three days, PONV, SF‐12, adverse events, cytokines |
| Notes | Small trial sample size (< 200 patients) Anaesthesia All patients received a standardized anaesthesia technique including premedication with alprazolam one h before surgery. Induction of anaesthesia was performed with a TCI with propofol with a targeted effective plasma concentration of 5 µg/mL, remifentanil (0.5 mg/kg/min) and cisatracurium (0.15 mg/kg). After tracheal intubation, anaesthesia was maintained with an IV infusion (TCI) of propofol and remifentanil. The doses of both agents were titrated at the discretion of the anaesthetist. Funding N/A Notes N/A |
Horvat 2014.
| Methods | Pilot study, randomized, placebo controlled. No statement on blinding. The aim of present study was to compare its effects on patients scheduled for nephrectomy regarding pain and cytokine production. The study was conducted in Croatia. It is not stated when the study was conducted. Trial identifier: n/a |
| Participants | Sample size: 20 Inclusion criteria Patients scheduled for nephrectomy Exclusion criteria N/A |
| Interventions |
Experimental group Lidocaine infusion 1.5 mg/kg/h for a period of 4 h Control group Normal 0.9% saline infusion for a period of 4 h |
| Outcomes | Postoperative pain (at rest and in coughing) (VAS) at 1, 4, 24, 48 h after surgery, plasma concentration of glucose, C‐reactive protein, interleukins (IL‐1 and IL‐6), tumour necrosis factor alpha (TNF‐alpha), leucocyte count |
| Notes | Small trial sample size (< 200 patients). Anaesthesia The anaesthesia regime was standardized Funding N/A Notes Only abstract available |
Jendoubi 2017.
| Methods | Randomized, double‐blinded, controlled trial. To compare the effects of perioperative IV lidocaine and ketamine on morphine requirements, pain scores, QoR, and chronic pain after open nephrectomy. The study was conducted in Tunisia. Date not specified. Trial identifier: NCT02653651 |
| Participants | Sample size: 63 (3 groups, n = 21, respectively) Only two groups are of interest for this review. Inclusion criteria Age ≥ 18 years and ASA I or II Exclusion criteria Known allergy to any of the study medications, an inability to understand the use of patient‐controlled analgesia, renal (serum creatinine > 2 mg/dl) or hepatic (alanine aminotransferase or aspartate aminotransferase > 2 times normal) dysfunction, a severe cardiovascular disorder (ejection fraction < 30%), ASA ≥ 3, history of chronic pain, epilepsy, psychiatric disorders, chronic use of opioids or alcohol, and drug abuse. |
| Interventions |
Experimental group (21 patients) Lidocaine group received an IV lidocaine bolus of 1.5 mg/kg (0.075 mL/kg of lidocaine 2%) at the induction of anaesthesia, followed by a continuous infusion of 1 mg/kg/h intraoperatively and for 24 h postoperatively. Control group (21 patients) The control group received an equal volume of normal saline 0.9%. |
| Outcomes | Morphine consumption, VAS pain scores, time to the first passage of flatus and faeces, postoperative nausea and vomiting, 6‐min walk distance (6MWD) at discharge, and the incidence of chronic neuropathic pain using the “Neuropathic Pain Questionnaire” at 3 months. |
| Notes | Small trial sample size (< 200 patients). Anaesthesia General anaesthesia was induced with propofol 2–3 mg/kg, fentanyl 3 μg/kg, cisatracurium 0.15 mg/kg and maintained by boluses of fentanyl 1 μg/kg every 30 mins and inhaled sevoflurane 1 minimum alveolar concentration in 50% oxygen/air. Funding Nil Notes N/A |
Kendall 2017.
| Methods | Randomized, double‐blinded, controlled trial. To compare the incidence in postsurgical persistent pain following breast cancer surgery in women receiving IV lidocaine compared to saline using validated pain instruments in accordance with the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations. The study was conducted in the USA. Date not specified. Trial identifier: N/A |
| Participants | Sample size: 148 (2 groups) Inclusion criteria N/A Exclusion criteria N/A |
| Interventions |
Experimental group (N/A patients) 1.5 mg/kg bolus of IV lidocaine followed by a 2 mg/kg/h infusion Control group (N/A patients) Normal saline at the same bolus and infusion rate |
| Outcomes | QoR, pain burden, opioid consumption at 24 h |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
Khalili 2017a.
| Methods | Randomized, double‐blinded, controlled trial (published in Persian). This study aimed to compare two methods of IV lidocaine and intramuscular piroxicam on postoperative pain in lower abdominal surgery. It is not stated where the study was conducted. Date not specified. Trial identifier: N/A |
| Participants | Sample size: 96 (3 groups, n = 21, respectively) Only two groups are of interest for this review. Inclusion criteria N/A Exclusion criteria N/A |
| Interventions |
Experimental group (21 patients) 2 mL intramuscular normal saline and 5 mL IV lidocaine 2% Control group (21 patients) 2 mL intramuscular and 5 mL IV normal saline |
| Outcomes | Postoperative pain at 24 h, the first time of receiving analgesia and the doses of analgesia |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
Khalili 2017b.
| Methods | Randomized, double‐blinded, controlled trial (published in Persian). The aim of this study was to evaluate the effect of IV lidocaine in comparison to intraperitoneal lidocaine on postsurgical pain in laparoscopic cholecystectomy. The study was conducted in Iran between 2013 and 2014. Trial identifier: N/A |
| Participants | Sample size: 96 (3 groups, n = 21, respectively) Only two groups are of interest for this review. Inclusion criteria Patients with ASA I and II considered to undergo laparoscopic cholecystectomy Exclusion criteria N/A |
| Interventions |
Experimental group (21 patients) IV lidocaine Control group (21 patients) IV normal saline |
| Outcomes | Pain, pethidine consumption |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
Kim 2017.
| Methods | Randomized, double‐blinded, controlled trial. The authors aimed to compare the effects of intraoperative lidocaine and magnesium on postoperative functional recovery and chronic pain after mastectomy due to breast cancer. The study was conducted in the Republic of Korea between July 2014 and July 2015. Trial identifier: NCT02185859 |
| Participants | Sample size: 126 (3 groups, n = 42, respectively) Only two groups are of interest for this review. Inclusion criteria ASA 1‐2, aged between 20 and 65 years, scheduled to undergo a mastectomy under general anaesthesia before enrolment. Only female patients were enrolled. Exclusion criteria Patients experiencing pain due to any cause or who were taking analgesics were excluded from this clinical trial. Additionally, patients with a BMI > 30 kg/m2, severe heart, kidney, or liver disease, a psychiatric or neurological disorder, contraindications, or allergic responses to lidocaine or magnesium were excluded from participation. |
| Interventions |
Experimental group (42 patients) Lidocaine (lidocaine hydrochloride) was administered at 2 mg/kg for 15 minutes immediately after induction, followed by infusion at 2 mg/kg/h. Control group (42 patients) Patients in group C were administered and infused with the same volume of saline. |
| Outcomes | QoR‐40 survey, pain scales, length of hospital stay, and the short‐form McGill pain questionnaire (SFMPQ) at postoperative 1 month and 3 months |
| Notes | Small trial sample size (< 200 patients) Anaesthesia Patients were administered 0.2 mg glycopyrrolate IV, and anaesthesia was induced with a bolus administration of 1.5‐2 mg/kg of propofol and 1‐2 mg/kg of remifentanil; anaesthesia was maintained using 4% to 7% desflurane with an adjuvant infusion of 0.05 ± 0.2 mg kg/min of remifentanil. Funding Nil Notes N/A |
Kim 2018.
| Methods | Randomized, double‐blinded, controlled trial. The authors compared the QoR‐40 scores of female patients who received IV lidocaine, magnesium, and saline during thyroidectomy to investigate their effects on comprehensive recovery from anaesthesia. The study was conducted in the Republic of Korea between December 2013 and October 2014. Trial identifier: NCT02018276 |
| Participants | Sample size: 135 (3 groups, n = 45, respectively) Only two groups are of interest for this review. Inclusion criteria Female patients 20–65 years of age and ASA I or II scheduled to undergo open thyroidectomy under general anaesthesia Exclusion criteria Patients who had been experiencing pain before surgery for any reason and those taking analgesics were excluded. Additional exclusion criteria were as follows: pregnancy or breast feeding; BMI > 30 kg/m2; significant heart, kidney, or liver disease; psychiatric or neurological disorders; and contraindications or hypersensitivity reactions to lidocaine or magnesium. |
| Interventions |
Experimental group (40 patients) Immediately after intubation, lidocaine was administered at 2 mg/kg for 15 minutes, followed by 2 mg/kg/h infusions. The study drug was discontinued just before transfer to the PACU. Control group (37 patients) The saline administered to the patients in group C was infused at the same rate. |
| Outcomes | QoR‐40 survey, pain, analgesic consumption |
| Notes | Small trial sample size (< 200 patients) Anaesthesia Anaesthesia was induced by a bolus administration of propofol (1–2 mg/kg) and remifentanil (1–2 μg/kg). Anaesthesia was conducted with 4% to 7% desflurane with adjuvant IV infusion of remifentanil (0.05 to 0.1 μg/kg/min). Funding Departmental funding only Notes N/A |
Lee 2017.
| Methods | Randomized, double‐blinded, controlled trial. The aim of this prospective, randomized, double‐blind, placebo‐controlled study was to evaluate the analgesic effect of IV lidocaine on postoperative pain in bimaxillary surgery. The study was conducted in the Republic of Korea between July 2015 and November 2015. Trial identifier: KCT0001574 |
| Participants | Sample size: 52 (2 groups, n = 26, respectively) Inclusion criteria Patients aged 19–64 years scheduled to receive bimaxillary surgery and agree to participate to the study projects. Exclusion criteria Patients with problems related to osteogenesis, congenital malformations, or a history of maxillofacial trauma, who weighed < 45 kg or > 100 kg; had an ASA grade > 3; had severe underlying cardiovascular (especially atrioventricular block), renal, or hepatic disease; hypertension; arteriosclerosis; heart failure; hyperthyroidism; diabetes mellitus; or were allergic to local anaesthetics, had received opioids or nonsteroidal anti‐inflammatory drugs within the previous 1 week, or were taking these drugs chronically. |
| Interventions |
Experimental group (26 patients) Two minutes before nasotracheal intubation, patients assigned to group L received an IV bolus infusion of 1.5 mg/kg of lidocaine, followed by 2 mg/kg/h lidocaine continuous infusion during the operation. Control group (26 patients) Patients in group C received the same amount of saline. |
| Outcomes | Pain intensity VAS was used at 2, 4, 8, 12, 24, and 48 h after surgery). Rescue ketorolac use was measured in the first 4, 4 to 8, 8 to 24, and 24 to 48 h after surgery. Total ketorolac consumption (the sum of rescue and eight‐hourly fixed schedule ketorolac injection), white blood cell count, neutrophil count, and postoperative swelling were recorded. |
| Notes | Small trial sample size (< 200 patients). Anaesthesia All patients received the same anaesthetic protocol. The patients did not receive premedication, and anaesthesia was induced with IV administration of 2 mg/kg propofol and 0.6 mg/kg rocuronium. Anesthesia was maintained using 2% to 3% sevoflurane in 1.5 L/min N2O and 1.5 L/min O2. Funding None Notes N/A |
Metha 2017.
| Methods | Randomized, double‐blinded, controlled trial. To analyse risk versus benefit of using intraoperative bolus of IV lignocaine (1.5 mg/kg) followed by constant rate (1.5 mg/kg/h) lignocaine for intraoperative and postoperative analgesic requirement in lower abdominal gynaecologic oncology surgeries. It is not stated where the study was conducted. Date not specified. Trial identifier: N/A |
| Participants | Sample size: 60 (2 groups, n = N/A) Inclusion criteria ASA I and II women scheduled for elective lower abdominal gynaecologic oncology surgeries Exclusion criteria N/A |
| Interventions |
Experimental group (N/A patients) IV lignocaine 1.5 mg/kg bolus over 5 minutes preinduction followed by a continuous lignocaine infusion of 1.5 mg/kg/h until the end of surgery. Control group (N/A patients) Saline in a similar manner (control group). |
| Outcomes | Sedation, postoperative nausea and vomiting and need of anti‐emetic drugs, bowel mobility (passage of flatus and motion), incidence of pruritus, need of anti‐histaminic drugs, length of hospital stay, and postoperative analgesic requirement. |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
NCT02257346.
| Methods | Randomized, double‐blinded, controlled trial. The aim of this study is to investigate whether lidocaine, given during and immediately after a patient undergoes a caesarean section, will help and improve a mother's overall recovery experience, as well as positively influence bonding with her new baby. The study was conducted in the USA. It is not clear when the study was conducted. Trial identifier: NCT02257346 |
| Participants | Estimated sample size: 90 Inclusion criteria Pregnant women aged 18 years and older, ASA II, English speaking, scheduled for caesarean delivery Exclusion criteria Allergy to local anaesthetics, chronic opioid use, greater than two prior caesarean deliveries, prior myomectomy, prior classical caesarean incision, BMI greater than 40, history of cardiac disease |
| Interventions |
Experimental group Lidocaine infusion was administered immediately after delivery of the foetus and continued through one hour into recovery period (IV lidocaine 1.5 mg/kg bolus dose and 2 mg/kg/h infusion). Control group Normal Saline was administered as a placebo immediately after delivery of the foetus and continued through one hour into recovery period. |
| Outcomes | Primary outcome: QoR‐40 on the day after surgery Secondary outcomes: total opioid consumption for the first 24 h after delivery, percentage of time a patient spent in skin‐to‐skin contact with their newborn in the first 24 hours |
| Notes |
Anaesthesia N/A Funding N/A Notes Only protocol available |
Rahaymeh 2016.
| Methods | Prospective, randomized, double‐blind study. The aim of this study was to examine the effect of lidocaine infusion in acute pain control and postoperative morphine consumption. The study was conducted in Jordan. It is not stated when the study was conducted. Trial identifier: N/A |
| Participants | Sample size: 40 Inclusion criteria ASA I and II patients aged 25 to 45 years who were scheduled to undergo major abdominal surgeries Exclusion criteria Patients with history of drug or alcohol abuse and patients with uncontrolled medical conditions (hypertension, diabetes mellitus and ischaemic heart disease) |
| Interventions |
Experimental group Lidocaine 2%, 1.5 mg/kg IV bolus 30 mins before induction, followed by an infusion of 1.5 mg/kg/h Control group Saline was given |
| Outcomes | Pain score (VAS) at 2, 4, 24 h (data presented graphically), average morphine consumption (2, 4, 8, 12, 24, 48 h) (mg) , nausea and vomiting, side effects (nausea circum‐oral tingling, sedation, light‐headedness, tinnitus or metallic taste), lidocaine concentration |
| Notes | Small trial sample size (< 200 patients) Anaesthesia The anaesthesia regime was standardized Funding N/A Notes The full text is available. We contacted the authors for information on intervention details in order to include or exclude the study, but we received no answer. |
Sherif 2017.
| Methods | Randomized, controlled trial. No statement on blinding. Postoperative pain control for morbidly obese patients represents a challenge because of their sensitivity towards opioid‐induced respiratory depression. We elected both dexmedetomidine and xylocaine (lidocaine) continuous infusions as adjuvants because they lack respiratory depression side effect. It is not stated where the study was conducted. Date not specified. Trial identifier: N/A |
| Participants | Sample size: 150 (3 groups, n = 50, respectively) Only two groups are of interest for this review. Inclusion criteria Patients with ASA physical status Exclusion criteria N/A |
| Interventions |
Experimental group (N/A patients) Lidocaine 2 mg/kg bolus over 10 minutes followed by 1.5 mg/kg/h continuous infusion during the whole operation period Control group (N/A patients) Saline in a similar manner (control group) |
| Outcomes | The total morphine consumption was designed to be the primary outcome variable, pain score, and QoR‐40 was set as secondary outcome variables. Pain score was measured by NRS, while the QoR score was estimated by the QoR‐40 questionnaire |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
Song 2017.
| Methods | Randomized, double‐blinded, controlled trial. To assess the effect of perioperative IV lidocaine infusion on pain intensity, bowel function and cytokine response after laparoscopic cholecystectomy. It is not stated where the study was conducted. Date not specified. Trial identifier: N/A |
| Participants | Sample size: 80 (2 groups, n = N/A) Inclusion criteria N/A Exclusion criteria N/A |
| Interventions |
Experimental group (N/A patients) Bolus injection of 1.5 mg/kg lidocaine at induction of anaesthesia, then a continuous infusion of 2 mg/kg/h until the end of surgery Control group (N/A patients) An equal volume of saline |
| Outcomes | Blood cytokines were measured at scheduled times within 48 h. Pain scores, opioid consumption, time to first flatus and time to first bowel movement |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
Van Den Heuvel 2016.
| Methods | Single centre, double‐blind, randomized, controlled, clinical trial. The aim of this study was to evaluate the impact of IV lidocaine on the neuroinflammatory response during oncologic breast surgery. The study was conducted in the Netherlands. It is not stated when the study was conducted. Trial identifier: N/A |
| Participants | Sample size: 16 Inclusion criteria N/A Exclusion criteria N/A |
| Interventions |
Experimental group Before induction of anaesthesia, patients received a bolus of IV lidocaine 1.5 mg/kg/h followed by continuous infusion of 2 mg/kg/h until one postoperative hour Control group Saline in an equivalent volume (control group) |
| Outcomes | Pain (NRS 0 to 10) preoperative and 4 h postoperative, cytokine plasma level (TNF‐alpha, interleukin‐6 (IL‐6), IL‐8, IL‐1b, IL‐1RA, IL‐10 plasma levels), perioperative sufentanil consumption, NRS scores, and perioperative consumption of dipidolor and diclofenac |
| Notes | Small trial sample size ( < 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
Yoo 2016.
| Methods | Prospective, double‐blind clinical trial. This study aimed to compare the effects of intraoperative lidocaine and magnesium on the postoperative functional recovery and chronic pain after mastectomy. The study was conducted in the Republic of Korea. It is not stated when the study was conducted. Trial identifier: n/a |
| Participants | Sample size: 126 Three groups, one not of interest (magnesium) Inclusion criteria Mastectomy Exclusion criteria N/A |
| Interventions |
Experimental group Lidocaine was administered at 2 mg/kg for 15 minutes immediately after induction, followed by infusions of 2 mg/kg/h. Control group Controls received the same volume of saline. |
| Outcomes | QoR‐40 survey, pain scales, length of hospital stay and the short‐form McGill pain questionnaire on postoperative one month and three months |
| Notes | Small trial sample size (< 200 patients) Anaesthesia N/A Funding N/A Notes Only abstract available |
Acronyms and abbreviations used in these tables
ASA = American Society of Anesthesiologists Physical Status, BMI = body mass index, h = hour, IL = Interleukin, IV = intravenous, LC = laparoscopic cholecystectomy, N/A = not applicable/not available, NRS = numerical rating scale, PACU = postanaesthesia care unit, QoR = quality of recovery, TCI = target controlled infusion, TNF = tumour necrosis factor, VAS = visual analogue scale, IL‐1RA = Interleukin 1 receptor antagonist
Characteristics of ongoing studies [ordered by study ID]
NCT02059902.
| Trial name or title | Continuous lidocaine infusion for management of perioperative burn pain |
| Methods | Randomized, double‐blinded, controlled trial. The design of this study will examine if lidocaine will reduce the pain scores and narcotic utilisation in patients undergoing surgical procedures for burn injuries. The study is conducted in the USA. |
| Participants | Estimated enrolment: 36 Inclusion criteria Burns patient ≥ 18 years of age, scheduled to go to operating room for excision and/or grafting procedure Exclusion criteria Burns patient < 18 years of age, intubated patient on sedation drip, prolonged hypotension defined as systolic blood pressure < 90 mm/Hg for greater than 30 minutes in the preoperative area, severe underlying cardiovascular disease (documented ejection fraction < 40%), documented conduction block, bradycardia or active congestive heart failure, documented active gastritis or ulcers, previous steroid medication history if documented adrenal insufficiency, patient with documented liver disease, patient with epilepsy or known seizure disorder, pregnant women |
| Interventions |
Experimental Lidocaine (preoperative = 1.5 kg/mg over a minimum of 30 minutes; perioperative = 2.0 mg/kg/h; postoperative = 1.5 kg/mg/h), infusion runs for a total of 24 hours Control Normal saline (bolus followed by continuous infusion), infusion runs for a total of 24 hours |
| Outcomes | Primary outcome: narcotic consumption |
| Starting date | September 2012 (study has been completed) |
| Contact information | William Mohr, MD, Sandi Wewerka, MPH, Regions Hospital, Saint Paul, Minnesota, USA, 55101 |
| Notes | N/A |
NCT02607488.
| Trial name or title | Perioperative systemic lidocaine for enhanced bowel recovery after bariatric surgery: a dose dependent study |
| Methods | Randomized, double‐blinded, controlled trial. The aim of this study is to investigate whether perioperative administration of low rather than high intravenous infusion rates of lidocaine can achieve early postoperative restoration of bowel motility at lower plasma levels. The study is conducted in Egypt. |
| Participants | Estimated enrolment: 180 Inclusion criteria 18 years to 65 years, obese patients with a body mass index equal or greater than 35 kg/m2, ASA II and III, patients scheduled for laparoscopic bariatric surgery under general anaesthesia Exclusion criteria History of significant cardiac, respiratory, hepatic, renal disease, history of an atrioventricular block grade II to III, long QT‐syndrome, pre‐existing disorder of the gastrointestinal tract, patients with history of alcohol or drug abuse, allergy to amide local anaesthetics, history of epilepsy, pregnancy, patients receiving cardiovascular medications, steroids or patients receiving opioid analgesic medication within 24 hours before the operation, conversion from a laparoscopic to an open laparotomy |
| Interventions |
Experimental (lidocaine 1%) Participants will receive an intravenous bolus of 0.1 mL/kg of lidocaine 1.5% solution followed by a continuous infusion 0.1 mL/kg/h of lidocaine 1% solution which will be continued for 24 hours after surgery. Experimental (lidocaine 1.5%) Participants will receive an intravenous bolus of 0.1 mL/kg of lidocaine 1.5% solution followed by a continuous infusion 0.1 mL/kg/h of lidocaine 1.5% solution which will be continued for 24 hours after surgery. Experimental (lidocaine 2%) Participants will receive an intravenous bolus of 0.1 mL/kg of lidocaine 1.5% solution followed by a continuous infusion 0.1 mL/kg/h of lidocaine 2% solution which will be continued for 24 hours after surgery. Control Participants will receive an intravenous bolus of 0.1 mL/kg of saline 0.9% solution followed by a continuous infusion 0.1 mL/kg/h of Saline 0.9% which will be continued for 24 hours after surgery. All medications in the study protocol will be based on the dosing body weight (IBW + 0.4 × (actual body weight−IBW). |
| Outcomes |
Primary outcome Postoperative recovery of bowel function (times to first passage of flatus, first defecation, and tolerating liquids measured in hours from the end of surgery) Secondary outcomes
|
| Starting date | The study started in November 2015 (currently recruiting) |
| Contact information | Mohamed R El Tahan, MD, mohamedrefaateltahan@yahoo.com; Samah El Kenany, MD, sk_20022000@yahoo.com |
| Notes | N/A |
NCT02862769.
| Trial name or title | The role of intra‐operative lidocaine infusion in preventing chronic post surgical pain after video assisted thoracoscopic surgery |
| Methods | Randomized, double‐blinded, controlled trial. The aim of this study is to evaluate the impact of intravenous lidocaine on acute and chronic post surgical pain on patients undergoing VATS. The study is conducted in Canada. |
| Participants | Estimated enrolment: 120 Inclusion criteria 18 years to 75 years, VATs for lobectomy, understanding of English (reading, writing and speaking), written consent for being involved in this study Exclusion criteria Chronic pain including fibromyalgia, patients using opioids (more than 80 mg equivalent of oral morphine/day for > 60 days), major depression, received or going to receive chemotherapy or radiotherapy, pregnant |
| Interventions |
Experimental First group (lidocaine group) will include those who receive a intraoperative lidocaine infusion (induction bolus dose of 1.5 mg/kg body weight followed by a continuous lidocaine infusion) Control The second group will include those who receive a intraoperative placebo (induction bolus dose of 1.5 mg/kg body weight of lidocaine followed by a continuous saline infusion at the same rate as the lidocaine infusion |
| Outcomes | Primary outcome: chronic pain post‐VATS at 3 and 6 months Secondary outcomes: opioid requirement (1 h, 6 h, 24 h and 48 h), pain score for acute postoperative pain, mean pain scores at 3 and 6 months, pain interference at 3 and 6 months |
| Starting date | Estimated: January 2017 (not yet open for participant recruitment) |
| Contact information | Qutaiba Tawfic Hamodi, qutaiba.Tawfic@lhsc.on.ca |
| Notes | N/A |
Acronyms and abbreviations used in this table
ASA = American Society of Anesthesiologists Physical Status, h = hours, IBW = ideal body weight, mins = minutes, N/A = not applicable, VATS = video‐assisted thoracic surgery
Differences between protocol and review
Changes to the authors of the review since publication of the protocol (Selig 2012)
In 2015 (Kranke 2015)
Two authors (C Selig and N Hahn) were no longer involved with the review and were removed from the list of authors.
Two new authors (J Jokinen and S Weibel) were added to the authors list and contributed to the review, as described in the Contributions of authors section.
For the current update:
Peter Kranke moved from first to last author (contact author). Stephanie Weibel became the new first author.
Johanna Jokinen left the review team.
Yvonne Jelting and Antonia Helf were newly added.
Differences in the methods used between the protocol (Selig 2012), and the review (Kranke 2015)
Criteria for considering studies for this review: we added the following to the review 'The IV lidocaine infusion, must have been started intraoperatively (with or without an IV bolus) prior to incision and continued until the end of surgery.' In the protocol we only described 'to have been continued postoperatively'.
At the protocol stage we planned to include quasi‐RCTs if it were found that few RCTs were available for meta‐analysis. However, in the review we did not include quasi‐RCTs due to the large number of available RCTs, which present the best available evidence, regarding the topic of interest.
We did not pre‐specify in the protocol for this review at which time periods the relevant outcomes of this review should be analysed. Based on pharmacodynamic and pharmacokinetic considerations and the logic in many included trials in which the postoperative observation period was divided into at least two distinct time intervals, we decided to subdivide outcome reporting for pain, postoperative opioid consumption, nausea and vomiting, etc. into different postoperative time points (e.g. 'early' and 'late'/'overall') to cover most of the reported data adequately.
In the protocol we planned to include pain data reported on VAS 0 to 100 mm scale. Due to the large proportion of data reported on other scales, we decided to include all pain data presented on a VAS 0 to 100 mm scale, NRS 0 to 10 and VRS 0 to 10 (0 = no pain, 10 = worst pain), and VAS 0 to 10 cm.
We broadened the outcome 'time to first bowel sounds' and included data on 'time to first bowel movement'.
The outcomes 'intraoperative and postoperative opioid requirements' were not previously considered in the published protocol. However, after intensive study of the relevant published trials dealing with perioperative lidocaine infusion for reduction of postoperative pain, we recognized that this outcome was widely analysed within the studies and we believe that opioid consumption is another relevant outcome to understand the effect of lidocaine in the perioperative setting since it may also affect the postoperative recovery and occurrence of side effects, e.g. ileus, nausea or vomiting.
Measurement of treatment effects: in the protocol we planned for data on pain scores, neuropsychological status or patient satisfaction that are reported on disparate scales, to calculate the standardized mean difference (SMD) obtained from the MD and SD. In the published review we combined, for the outcome 'pain', all data presented on either VAS 0 to 10 cm scale, VAS 0 to 100 mm, NRS 0 to 10, or VRS 0 to 10 (0 = no pain, 10 = worst pain) and transformed the first three into VAS 0 to 10 cm and presented the effect estimates as MD.
Dealing with missing data: in the protocol we planned to perform complete‐case analyses if there were exclusions/dropouts in the study flow. We intended to perform sensitivity analyses by inputting missing data (best case and worst case) in instances of more than trivial missing data. To the review we added the following statement which explains the handling of missing data which are obviously not crucial for the overall estimation of the treatment effect: 'If data were missing due to random events and the impact of missing data was considered marginal, we included data in the analysis only on those participants whose results were known. Studies with incomplete reporting of their study flow or disputable exclusions were subsequently excluded in a sensitivity meta‐analysis to assess bias. The potential impact of the missing data on the results was considered in the interpretation of the results of the review.'
In the protocol we did not pre‐specify that we will include median values and IQR. However, during data extraction we recognized that the data in large part were reported as median and IQR. Since we wanted to include as much data as possible, we calculated in the review the mean and SD from median and IQR in accordance with Higgins 2011. To assess the impact of the median data on the summary statistics, we performed a sensitivity analysis.
Assessment of heterogeneity: in the protocol we planned to perform meta‐regressions to explain heterogeneity. In the present review, we did not perform these calculations.
'Summary of findings' table: at the protocol stage we planned to present results on pain scores and gastrointestinal recovery within 'Summary of findings' tables. We decided post‐analysis to additionally present nausea as an outcome of public interest. We presented further the results of the different surgical subgroups (open abdominal, laparoscopic abdominal, and other surgeries) for the outcome 'pain (early)' to reflect the specific benefit for abdominal surgery patients.
Sensitivity analysis: in the protocol we planned to perform a sensitivity analysis using the inverse variance weighted fixed‐effect model. Due to the large heterogeneity observed between the studies the random‐effects model fits much better than the fixed‐effect model. Therefore, we did not perform this sensitivity analysis.
Sensitivity analysis: we analysed the impact of data reported as median and IQR on the overall effect estimation to each outcome to judge the robustness of the summary statistics.
Sensitivity analysis: we planned in the protocol to perform a sensitivity analysis including only low risk of bias studies to test the robustness of the summary statistics. Since only few studies received an overall low risk classification, we reconsidered that point in the review and proceeded to exclude the high risk of bias studies to judge the robustness of the summary statistics.
Differences in the methods used between the published review (Kranke 2015), and the updated review
We have changed the title from 'Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery' to 'Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults' to reflect the focus on the adult population.
We have made changes to the list of primary outcomes: due to the high priority of adverse effects in the investigation of medical interventions, we moved the outcome 'adverse events' from secondary to primary outcomes.
We separated the outcome 'time to first bowel movement/sounds' into 'time to first bowel movement' and 'time to first bowel sounds'. We combined the outcomes 'time to first defaecation' and 'time to first bowel movement' into one outcome named as 'time to first defaecation/bowel movement' since both outcomes measure the same clinical condition.
We have made changes to the 'Summary of findings' table (GRADE‐relevant outcomes). We have reduced the number of 'Summary of findings' tables to one per comparison and created the following outcome categories: pain (early, intermediate, and late time points), gastrointestinal recovery (postoperative ileus, time to first defaecation/bowel movement), adverse events, postoperative nausea (overall), postoperative opioid consumption (overall). The outcomes 'time to first bowel sound', 'time to first flatus', and 'postoperative nausea (early)' are no longer GRADE‐relevant outcomes.
We performed meta‐analyses for the following new outcomes due to availability of more than three studies: length of hospital stay (outpatient surgery, mins), surgical complications (anastomotic leak), surgical complications (bleeding), patient satisfaction, and intraoperative remifentanil consumption.
We omitted studies with suspected small variance reporting for the outcomes: pain, gastrointestinal recovery, and opioid consumption.
Assessment of risk of other bias: we no longer assessed a lack of sample size calculation in trials as high risk of other bias.
In contrast to the original review, we calculated the SMD as summary statistics for all pain outcomes since several different scales were used in the individual trials. The use of SMD as summary statistics was originally described in the protocol.
We changed 'dealing with missing data' to the method described in the original protocol.
We introduced the 95% prediction interval (PI) to understand the uncertainty associated with an intervention about whether an intervention works or not in the light of between‐study heterogeneity.
We used the Mantel‐Haenszel method for RRs instead of inverse variance weighting.
We have changed the 'Summary of findings' table with respect to presented outcomes (we removed subgroups for pain 'early' and added adverse events plus postoperative opioid consumption 'overall') and to the approach for assessing inconsistency and imprecision (see 95% PI).
Sensitivity analysis (fixed‐effect model): as described in the protocol, we included in the current update sensitivity analyses using the fixed‐effect model.
Sensitivity analysis (risk of bias): as in the protocol described, we performed in the current update sensitivity analyses, including only low risk of bias studies to test the robustness of the summary statistics.
Sensitivity analysis (suspected variance reporting): we added studies with suspected variance reporting to the meta‐analyses of relevant outcomes to explore the impact on the effect estimates in sensitivity meta‐analyses.
Contributions of authors
Stephanie Weibel (SW), Yvonne Jelting (YJ), Nathan Leon Pace (NLP), Antonia Helf (AH), Leopold HJ Eberhart (LE), Klaus Hahnenkamp (KH), Markus W Hollmann (MH), Daniel M Poepping (DP), Alexander Schnabel (AS), Peter Kranke (PK)
(Original review: Kranke 2015)
Conceiving the review: AS, PK
Co‐ordinating the review: original review ‐ PK; update ‐ SW, PK
Undertaking manual searches: original review ‐ JJ, SW; update ‐ YJ, SW
Screening search results: original review ‐ JJ, SW; update ‐ SW, YJ, AH
Organizing retrieval of papers: original review ‐ JJ, SW, PK; update ‐ SW, YJ, PK
Screening retrieved papers against inclusion criteria: original review ‐ JJ, SW; update ‐ SW, YJ, AH
Appraising quality of papers: original review ‐ JJ, SW; update ‐ SW; YJ, AH
Abstracting data from papers: original review ‐ SW, JJ, PK, DP, LE, AS, KH, MH; update ‐ SW, YJ, AH
Writing to authors of papers for additional information ‐ original review: SW; update ‐ SW, YJ
Providing additional data about papers: SW
Obtaining and screening data on unpublished studies: SW
Data management for the review: SW
Entering data into Review Manager 5 (RevMan 5; Review Manager 2014): SW
RevMan statistical data: SW, NLP
Other statistical analysis not using RevMan: original review ‐ PK, NLP; update ‐ SW, NLP
Double entry of data: original review ‐ (data entered by person one: SW; data checked by person two: JJ); update ‐ data entered by person one: SW; data checked by person two: YJ)
Interpretation of data: original review ‐ PK, SW, LE, MH, KH, DP, NLP; update ‐ PK, SW, LE, MH, KH, DP, NLP, AS, YJ, AH
Statistical inferences: original review ‐NLP, PK, SW; update ‐ NLP, PK, SW
Writing the review: original review: SW, PK, JJ, MH; update ‐ SW, PK, YJ, AH
Securing funding for the review: PK
Performing previous work that was the foundation of the present study: original review ‐ MH, KH, PK; update: PK
Guarantor for the review (one author): PK
Person responsible for reading and checking review before submission: PK
Sources of support
Internal sources
Departmental resources only, Germany.
External sources
No sources of support supplied
Declarations of interest
Stephanie Weibel: none known.
Yvonne Jelting: none known.
Nathan Leon Pace: has no conflict of interest regarding the topic of this review. Nathan L Pace has received payment for development of educational presentations (Barash, Cullen, Stoelting Clinical Anesthesia 8th edition) and provided consultancy (St Marks Hospital, Salt Lake City, UT) on topics not related to the current review. He has received supplements to attend Cochrane meetings. He also has stocks and shares in companies which have no interest in the topic of this review (TIAA‐CREF, Fidelity, Vanguard, USAA, Morgan Stanley).
Antonia Helf: none known.
Leopold HJ Eberhart: has no conflict of interest regarding the topic of this review. Leopold HJ Eberhart has received lecture fees (from Baxter GmbH and Fresenius GmbH), payment for lectures (from Grünenthal GmbH, Baxter GmbH and Fresenius, GmbH) and has provided consultancy (for Grünenthal GmbH, Baxter GmbH, ratiopharm GmbH) for topics not related to the current review. He holds a board membership (with Grünenthal GmbH Deutschland) who do not have an interest in the topic of this review.
Klaus Hahnenkamp: is working in this research area and has participated in a clinical study that is relevant for this systematic review (Herroeder 2007). Critical appraisal and data extraction were done by JJ and SW.
Markus W Hollmann: is working in this research area and has participated in a clinical study that is relevant for this systematic review (Herroeder 2007). Critical appraisal and data extraction were done by JJ and SW.
Daniel M Poepping: none known.
Alexander Schnabel: none known.
Peter Kranke: has no conflict of interest regarding the topic of this review. Peter Kranke has received lecture fees (from FreseniusKabi, MSD, Ratiopharm, Covidien) and has provided consultancy (to MSD, FreseniusKabi, Ratiopharm, Covidien) on topics not related to the current review. He has been involved in the conduct of Phase II and phase III clinical trials not related to the current review.
Edited (no change to conclusions)
References
References to studies included in this review
Ahn 2015 {published data only}
- Ahn E, Kang H, Choi GJ, Park YH, Yang SY, Kim BG, et al. Intravenous lidocaine for effective pain relief after a laparoscopic colectomy: a prospective, randomized, double‐blind, placebo‐controlled study. International Surgery 2015;100(3):394‐401. [PUBMED: 25785316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baral 2010 {published data only}
- Baral BK, Bhattarai BK, Rahman TR, Singh SN, Regmi R. Perioperative intravenous lidocaine infusion on postoperative pain relief in patients undergoing upper abdominal surgery. Nepal Medical College Journal 2010;12(4):215‐20. [PUBMED: 21744761] [PubMed] [Google Scholar]
Bryson 2010 {published data only}
- Bryson GL, Charapov I, Krolczyk G, Taljaard M, Reid D. Intravenous lidocaine does not reduce length of hospital stay following abdominal hysterectomy. Canadian Journal of Anaesthesia 2010;57(8):759‐66. [PUBMED: 20532723] [DOI] [PubMed] [Google Scholar]
- NCT00382499. Intraoperative lidocaine infusion for analgesia (ILIA). clinicaltrials.gov/ct2/show/NCT00382499 (first received 29 September 2006).
Cassuto 1985 {published data only}
- Cassuto J, Wallin G, Högström S, Faxén A, Rimbäck G. Inhibition of postoperative pain by continuous low‐dose intravenous infusion of lidocaine. Anesthesia and Analgesia 1985;64(10):971‐4. [PUBMED: 3898920] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen K, Wei P, Zheng Q, Zhou J, Li J. Neuroprotective effects of intravenous lidocaine on early postoperative cognitive dysfunction in elderly patients following spine surgery. Medical Science Monitor 2015;21:1402‐7. [1643‐3750; PUBMED: 25975969] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi GJ 2016 {published data only}
- Choi GJ, Kang H, Ahn EJ, Oh JI, Baek CW, Jung YH, et al. Clinical efficacy of intravenous lidocaine for thyroidectomy: a prospective, randomized, double‐blind, placebo‐controlled trial. World Journal of Surgery 2016;40(12):2941‐7. [1432‐2323; PUBMED: 27379388] [DOI] [PubMed] [Google Scholar]
- NCT01608360. The effect of intravenous lidocaine on pain after thyroidectomy: a prospective, randomized, double‐blind, placebo‐controlled study. clinicaltrials.gov/show/NCT01608360 (first received 31 May 2012).
- Oh J, Choi GJ, Kang H, Baek C, Jung YH, Ko JS. Clinical efficacy of intravenous lidocaine for thyroidectomy: a prospective, randomized, double‐blind, placebo‐controlled trial. Anesthesia and Analgesia 2016;123((3 Suppl 2)):573. [EMBASE: 612648853; 1526‐7598] [DOI] [PubMed] [Google Scholar]
Choi KW 2016 {published data only}
- Choi KW, Nam KH, Lee JR, Chung WY, Kang SW, Joe YE, et al. The effects of intravenous lidocaine infusions on the quality of recovery and chronic pain after robotic thyroidectomy: A randomized, double‐blinded, controlled study. World Journal of Surgery 2017;41(5):1305‐12. [1432‐2323; PUBMED: 27896411] [DOI] [PubMed] [Google Scholar]
- Lee J, Choi KW, Choi SH, Min KT. Effect of systemic lidocaine on quality of recovery after robot‐assisted thyroidectomy. Regional Anesthesia and Pain Medicine. 2015; Vol. 1:e138. [EMBASE: 72027298; 1098‐7339]
- NCT01907997. Effects of systemic lidocaine on postoperative quality of recovery after robot‐assisted thyroidectomy. clinicaltrials.gov/show/NCT01907997 (first received 25 July 2013).
Choi SJ 2012 {published data only}
- Choi SJ, Kim MH, Jeong HY, Lee JJ. Effect of intraoperative lidocaine on anesthetic consumption, and bowel function, pain intensity, analgesic consumption and hospital stay after breast surgery. Korean Journal of Anesthesiology 2012;62(5):429‐34. [PUBMED: 22679539] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cui 2010 {published data only}
- Cui W, Li Y, Li S, Wang R, Li J. Systemic administration of lidocaine reduces morphine requirements and postoperative pain of patients undergoing thoracic surgery after propofol‐remifentanil‐based anaesthesia. European Journal of Anaesthesiology 2010;27(1):41‐6. [PUBMED: 19478674] [DOI] [PubMed] [Google Scholar]
Dale 2016 {published data only}
- ACTRN12613000440729. Intravenous lignocaine infusion for analgesia following laparoscopic hiatus hernia repair: a double‐blinded, randomised, placebo‐controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364024 (first received 13 April 2013).
- Dale GJ, Phillips S, Falk GL. The analgesic efficacy of intravenous lidocaine infusion after laparoscopic fundoplication: a prospective, randomized, double‐blind, placebo‐controlled trial. Local and Regional Anesthesia 2016;9:87‐93. [PUBMED: 27980437] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Oliveira 2012 {published data only}
- Oliveira Jr GS, Fitzgerald P, Streicher LF, Marcus RJ, McCarthy RJ. Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic surgery. Anesthesia and Analgesia 2012;115(2):262‐7. [PUBMED: 22584558] [DOI] [PubMed] [Google Scholar]
- NCT01250002. Lidocaine and outpatient gynecologic laparoscopy [The effect of perioperative systemic lidocaine on quality of recovery after outpatient gynecologic laparoscopy]. clinicaltrials.gov/ct2/show/NCT01250002 (first received 30 November 2010).
De Oliveira 2014 {published data only}
- Oliveira Jr GS, Duncan K, Fitzgerald P, Nader A, Gould RW, McCarthy RJ. Systemic lidocaine to improve quality of recovery after laparoscopic bariatric surgery: A randomized double‐blinded placebo‐controlled trial. Obesity Surgery 2014;24(2):212‐8. [PUBMED: 24036842] [DOI] [PubMed] [Google Scholar]
- NCT01180660. IV Lidocaine on postoperative pain and QOR on morbid obese patients undergoing bypass surgery [The effect of systemic intraoperative lidocaine on postoperative pain and quality of recovery on morbid obese patients undergoing laparoscopic gastric bypass surgery]. clinicaltrials.gov/ct2/show/NCT01180660 (first received 12 August 2010).
Dewinter 2016 {published data only}
- Dewinter GBE, Teunkens A, Vermeulen K, Al Tmimi L, Velde M, Rex S. Systemic lidocaine fails to improve postoperative pain, but reduces time to discharge readiness in patients undergoing laparoscopic sterilization in day‐case surgery: a double‐blind, randomized, placebo‐controlled trial. Regional Anesthesia and Pain Medicine 2016;41(3):362‐7. [EMBASE: 610227896; 1098‐7339] [DOI] [PubMed] [Google Scholar]
- EudraCT 2011‐001315‐31. Analgesia with continuous IV‐infusion of lidocaine during the perioperative period in patients undergoing laparoscopic sterilization. www.clinicaltrialsregister.eu/ctr‐search/trial/2011‐001315‐31/BE (first received 21 September 2011).
El‐Tahan 2009 {published data only}
- El‐Tahan MR, Warda OM, Diab DG, Ramzy EA, Matter MK. A randomized study of the effects of perioperative i.v. lidocaine on hemodynamic and hormonal responses for cesarean section. Journal of Anesthesia 2009;23(2):215‐21. [PUBMED: 19444560] [DOI] [PubMed] [Google Scholar]
Farag 2013 {published data only}
- Farag E, Ghobrial M, Sessler DI, Dalton JE, Liu J, Lee JH, et al. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology 2013;119(4):932‐40. [PUBMED: 23681143] [DOI] [PubMed] [Google Scholar]
- NCT00840996. Perioperative intravenous lidocaine or epidural anesthesia on outcomes in complex spine surgery. clinicaltrials.gov/ct2/show/NCT00840996 (first received 11 February 2009).
Grady 2012 {published data only}
- Grady MV, Mascha E, Sessler DI, Kurz A. The effect of perioperative intravenous lidocaine and ketamine on recovery after abdominal hysterectomy. Anesthesia and Analgesia 2012;115:1078‐84. [PUBMED: 23011561] [DOI] [PubMed] [Google Scholar]
- NCT00721110. Lidocaine and ketamine in abdominal surgery. clinicaltrials.gov/ct2/show/NCT00721110 (first received 23 July 2008).
Grigoras 2012 {published data only}
- Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clinical Journal of Pain 2012;28(7):567‐72. [PUBMED: 22699129 ] [DOI] [PubMed] [Google Scholar]
Groudine 1998 {published data only}
- Groudine SB, Fisher HA, Kaufman RP, Patel MK, Wilkins LJ, Mehta SA, et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesthesia and Analgesia 1998;86(2):235‐9. [PUBMED: 9459225] [DOI] [PubMed] [Google Scholar]
Herroeder 2007 {published data only}
- Herroeder S, Pecher S, Schönherr ME, Kaulitz G, Hahnenkamp K, Friess H, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double‐blinded, randomized, placebo‐controlled trial. Annals of Surgery 2007;246(2):192‐200. [PUBMED: 17667496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Insler 1995 {published data only}
- Insler SR, O'Connor M, Samonte AF, Bazaral MG. Lidocaine and the inhibition of postoperative pain in coronary artery bypass patients. Journal of Cardiothoracic and Vascular Anesthesia 1995;9(5):541‐6. [PUBMED: 8547556] [DOI] [PubMed] [Google Scholar]
Ismail 2008 {published data only}
- Ismail SA. Effects of systemic lidocaine infusion during lumbar discectomy under total intravenous anesthesia. Egyptian Journal of Anaesthesia 2008;24:39‐46. [CN‐00802801] [Google Scholar]
Jain 2015 {published data only}
- Jain S, Khan RM. Effect of peri‐operative intravenous infusion of lignocaine on haemodynamic responses to intubation, extubation and post‐operative analgesia. Indian Journal of Anaesthesia 2015;59(6):342‐7. [0019‐5049; PUBMED: 26195829] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaba 2007 {published data only}
- Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME, Lamy ML, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology 2007;106(1):11‐8. [PUBMED: 17197840] [DOI] [PubMed] [Google Scholar]
- NCT00330941. Intravenous lidocaine and acute rehabilitation. clinicaltrials.gov/ct2/show/NCT00330941 (first received 29 May 2006).
Kang 2011 {published data only}
- Kang H, Kim BG. Intravenous lidocaine for effective pain relief after inguinal herniorrhaphy: a prospective, randomized, double‐blind, placebo‐controlled study. Journal of International Medical Research 2011;39(2):435‐45. [PUBMED: 21672347] [DOI] [PubMed] [Google Scholar]
Kasten 1986 {published data only}
- Kasten GW, Owens E. Evaluation of lidocaine as an adjunct to fentanyl anesthesia for coronary artery bypass graft surgery. Anesthesia and Analgesia 1986;65(5):511‐5. [PUBMED: 3516016] [PubMed] [Google Scholar]
Kim HJ 2014 {published data only}
- Kim HJ, Kim WH, Kim G, Kim E, Park MH, Shin BS, et al. A comparison among infusion of lidocaine and dexmedetomidine alone and in combination in subjects undergoing coronary artery bypass graft: a randomized trial. Contemporary Clinical Trials 2014;39(2):303‐9. [1559‐2030; PUBMED: 25447444] [DOI] [PubMed] [Google Scholar]
- NCT01688648. Comparison between lidocaine, dexmedetomidine, and their combined infusion in subjects undergoing coronary artery bypass graft [The comparison of antiarrhythmic and myocardial protective effect between lidocaine, dexmedetomidine, and their combined infusion in subjects undergoing coronary artery bypass Graft]. clinicaltrials.gov/show/NCT01688648 (first received 29 September 2012).
Kim HO 2014 {published data only}
- Kim HO, Lee SR, Choi WJ, Kim H. Early oral feeding following laparoscopic colorectal cancer surgery. ANZ Journal of Surgery 2014;84(7‐8):539‐44. [1445‐2197; PUBMED: 24612414] [DOI] [PubMed] [Google Scholar]
- NCT01346917. Effect of intravenous lidocaine on the tolerability of early oral feeding after laparoscopic colorectal surgery [Prospective randomized controlled study for the effect of intravenous lidocaine on the tolerability of early oral feeding after laparoscopic colorectal surgery in patients with colorectal cancer]. clinicaltrials.gov/show/NCT01346917 (first received 3 May 2011).
Kim KT 2014 {published data only}
- Kim KT, Cho DC, Sung JK, Kim YB, Kang H, Song KS, et al. Intraoperative systemic infusion of lidocaine reduces postoperative pain after lumbar surgery: a double‐blinded, randomized, placebo‐controlled clinical trial. Spine Journal: Official Journal of the North American Spine Society 2014;14(8):1559‐66. [1878‐1632; PUBMED: 24216403] [DOI] [PubMed] [Google Scholar]
- NCT01319682. The effect of intravenous lidocaine on pain after lumbar spinal fusion [Intravenous lidocaine for effective pain relief after posterior lumbar spinal fusion: a prospective, randomized, double‐blind, placebo‐controlled study]. clinicaltrials.gov/show/NCT01319682 (first received 22 March 2011).
Kim TH 2011 {published data only}
- ACTRN12610000649011. The effect of intravenous lidocaine infusion and intraperitoneal lidocaine instillation on the pain after laparoscopic appendectomy [In patients undergoing laparoscopic appendectomy, is intravenous lidocaine infusion and intraperitoneal lidocaine instillation more effective than a placebo in reducing post‐operative pain?]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335814 (first received 9 August 2010).
- Kim TH, Kang H, Hong JH, Park JS, Baek CW, Kim JY, et al. Intraperitoneal and intravenous lidocaine for effective pain relief after laparoscopic appendectomy: a prospective, randomized, double‐blind, placebo‐controlled study. Surgical Endoscopy 2011;25(10):3183‐90. [PUBMED: 21487863] [DOI] [PubMed] [Google Scholar]
Kim TH 2013 {published data only}
- ACTRN12612000007831. The effect of intravenous lidocaine infusion on the pain after laparoscopic assisted distal gastrectomy [In patients undergoing laparoscopic assisted distal gastrectomy, is intravenous lidocaine infusion effective in reducing post‐operative pain?]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000007831 (first received 16 December 2011).
- Kim TH, Kang H, Choi YS, Park JM, Chi KC, Shin HY, et al. Pre‐ and intraoperative lidocaine injection for preemptive analgesics in laparoscopic gastrectomy: A prospective, randomized, double‐blind, placebo‐controlled study. Journal of Laparoendoscopic and Advanced Surgical Techniques 2013;23(8):663‐8. [PUBMED: 23614819] [DOI] [PubMed] [Google Scholar]
Koppert 2004 {published data only}
- Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesthesia and Analgesia 2004;98(4):1050‐5. [PUBMED: 15041597] [DOI] [PubMed] [Google Scholar]
Kuo 2006 {published data only}
- Kuo CP, Jao SW, Chen KM, Wong CS, Yeh CC, Sheen MJ, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. British Journal of Anaesthesia 2006;97(5):640‐6. [PUBMED: 16952918] [DOI] [PubMed] [Google Scholar]
Lauwick 2008 {published data only}
- Lauwick S, Do JK, Michelagnoli G, Mistraletti G, Feldman L, Fried G, et al. Intraoperative infusion of lidocaine reduces postoperative fentanyl requirements in patients undergoing laparoscopic cholecystectomy. Canadian Journal of Anesthesia 2008;55(11):754‐60. [PUBMED: 19138915] [DOI] [PubMed] [Google Scholar]
Lauwick 2009 {published data only}
- Lauwick S, Kim DJ, Mistraletti G, Carli F. Functional walking capacity as an outcome measure of laparoscopic prostatectomy: the effect of lidocaine infusion. British Journal of Anaesthesia 2009;103(2):213‐9. [PUBMED: 19443419] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- KCT0000012. Effect of palonosetron on postoperative nausea and vomiting (comparison with ondansetron and ramosetron) [Effect of palonosetron on patient‐controlled analgesia related nausea and vomiting after thyroidectomy (comparison with ondansetron and ramosetron)]. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=947 (first received 26 April 2010).
- Kim WJ, Lee EH, Sim JY, Choi IC. Impact of intravenous lidocaine on myocardial injury during off pump coronary artery surgery. Journal of Cardiothoracic and Vascular Anesthesia 2010;24:S63‐4. [Google Scholar]
- Lee EH, Lee HM, Chung CH, Chin JH, Choi DK, Chung HJ, et al. Impact of intravenous lidocaine on myocardial injury after off pump coronary artery surgery. British Journal of Anaesthesia 2011;106(4):487‐93. [PUBMED: 21343159] [DOI] [PubMed] [Google Scholar]
Maquoi 2016 {published data only}
- EUDRACT 2010‐018321‐20. Transversus abdominis plane block, intravenous lignocaine or placebo for postoperative analgesia after open prostate surgery: a randomized controlled trial. www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐018321‐20/BE (first received 4 November 2015).
- Maquoi I, Joris JL, Dresse C, Vandenbosch S, Venneman I, Brichant JF, et al. Transversus abdominis plane block or intravenous lignocaine in open prostate surgery: a randomized controlled trial. Acta Anaesthesiologica Scandinavica 2016;60(10):1453‐60. [1399‐6576; PUBMED: 27507582] [DOI] [PubMed] [Google Scholar]
Martin 2008 {published data only}
- Martin F, Cherif K, Gentili ME, Enel D, Abe E, Alvarez JC, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology 2008;109:118‐23. [PUBMED: 18580181] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathew 2009 {published data only}
- Mathew JP, Mackensen GB, Phillips‐Bute B, Grocott HP, Glower DD, Laskowitz DT, et al. Randomized, double‐blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 2009;40(3):880‐7. [PUBMED: 19164788] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2009 {published data only}
- McKay A, Gottschalk A, Ploppa A, Durieux ME, Groves DS. Systemic lidocaine decreased the perioperative opioid analgesic requirements but failed to reduce discharge time after ambulatory surgery. Anesthesia and Analgesia 2009;109(6):1805‐8. [PUBMED: 19923506] [DOI] [PubMed] [Google Scholar]
Mitchell 1999 {published data only}
- Mitchell SJ, Pellett O, Gorman DF. Cerebral protection by lidocaine during cardiac operations. Annals of Thoracic Surgery 1999;67(4):1117‐24. [PUBMED: 10320260] [DOI] [PubMed] [Google Scholar]
Mitchell 2009 {published data only}
- Mitchell SJ, Merry AF, Frampton C, Davies E, Grieve D, Mills BP, et al. Cerebral protection by lidocaine during cardiac operations: a follow‐up study. Annals of Thoracic Surgery 2009;87(3):820‐5. [PUBMED: 19231397] [DOI] [PubMed] [Google Scholar]
Oliveira 2015 {published data only}
- Oliveira CM, Sakata RK, Slullitel A, Salomao R, Lanchote VL, Issy AM. Effect of intraoperative intravenous lidocaine on pain and plasma interleukin‐6 in patients undergoing hysterectomy. Revista Brasileira de Anestesiologia 2015;65(2):92‐8. [0034‐7094; PUBMED: 25740274] [DOI] [PubMed] [Google Scholar]
Omar 2013 {published data only}
- Omar AM. Can systemic lidocaine be used in controlled hypotension? A double‐blinded randomized controlled study in patients undergoing functional endoscopic sinus surgery. Egyptian Journal of Anaesthesia 2013;29(4):295‐300. [Google Scholar]
Ortiz 2016 {published data only}
- NCT02363699. Endovenous lidocaine and serum cytokines concentration [Effect of endovenous lidocaine on analgesia and serum cytokines concentration: randomized and double‐blind trial]. clinicaltrials.gov/show/NCT02363699 (first received 16 February 2015).
- Ortiz MP, Godoy MC, Schlosser RS, Ortiz RP, Godoy JP, Santiago ES, et al. Effect of endovenous lidocaine on analgesia and serum cytokines: double‐blinded and randomized trial. Journal of Clinical Anesthesia 2016;35:70‐7. [1873‐4529; PUBMED: 27871598] [DOI] [PubMed] [Google Scholar]
Peng 2016 {published data only}
- NCT00975910. Effect of lidocaine in antiarrhythmic dosage on postoperative cognitive dysfunction in supratentorial craniotomy. clinicaltrials.gov/show/NCT00975910 (first received 14 September 2009).
- Peng Y, Zhang W, Kass IS, Han R. Lidocaine reduces acute postoperative pain after supratentorial tumor surgery in the PACU: a secondary finding from a randomized, controlled trial. Journal of Neurosurgical Anesthesiology 2016;28(4):309‐15. [1537‐1921; PUBMED: 26397235] [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhang W, Zhou X, Ji Y, Kass IS, Han R. Lidocaine did not reduce neuropsychological‐cognitive decline in patients 6 months after supratentorial tumor surgery: A randomized, controlled trial. Journal of Neurosurgical Anesthesiology 2016;28(1):6‐13. [1537‐1921; PUBMED: 26083427] [DOI] [PubMed] [Google Scholar]
Rimbäck 1990 {published data only}
- Rimbäck G, Cassuto J, Tollesson PO. Treatment of postoperative paralytic ileus by intravenous lidocaine infusion. Anesthesia and Analgesia 1990;70(4):414‐9. [PUBMED: 2316883] [PubMed] [Google Scholar]
Saadawy 2010 {published data only}
- Saadawy IM, Kaki AM, Abd El Latif AA, Abd‐Elmaksoud AM, Tolba OM. Lidocaine vs. magnesium: effect on analgesia after a laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2010;54(5):549‐56. [PUBMED: 19919581] [DOI] [PubMed] [Google Scholar]
Samimi 2015 {published data only}
- Samimi S, Taheri A, Davari Tanha F. Comparison between intraperitoneal and intravenous lidocaine for postoperative analgesia after elective abdominal hysterectomy, a double‐blind placebo controlled study. Journal of Family and Reproductive Health 2015;9(4):193‐8. [1735‐8949; PUBMED: 27047566] [PMC free article] [PubMed] [Google Scholar]
Slovack 2015 {unpublished data only}
- NCT01277835. Intravenous lidocaine infusion during video‐assisted thoracic procedures for improved pain control [Intravenous lidocaine infusion during VATS procedures reduces postoperative analgesic requirements]. clinicaltrials.gov/ct2/show/NCT01277835 (first received 17 January 2011 ).
- Slovack M, Taylor B, Bryce R, Ong D. Does intravenous lidocaine infusion during video‐assisted thoracoscopic surgery reduce postoperative analgesia? A randomized controlled study. Canadian Journal of Anaesthesia 2015;62(6):676‐7. [DOI] [PubMed] [Google Scholar]
Soltani 2013 {published data only}
- Soltani HA, Nasr M, Siadat ZD. Effects of lidocaine on reducing the need for anesthetic drugs during ophthalmologic surgeries. Journal of Isfahan Medical School 2013;31(224):41‐9. [Google Scholar]
Sridhar 2015 {published data only}
- Sridhar P, Sistla SC, Ali SM, Karthikeyan VS, Badhe AS, Ananthanarayanan PH. Effect of intravenous lignocaine on perioperative stress response and post‐surgical ileus in elective open abdominal surgeries: a double‐blind randomized controlled trial. ANZ Journal of Surgery 2015;85(6):425‐9. [1445‐2197; PUBMED: 25078385] [DOI] [PubMed] [Google Scholar]
Staikou 2014 {published data only}
- Staikou C, Avramidou A, Ayiomamitis GD, Vrakas S, Argyra E. Effects of intravenous versus epidural lidocaine infusion on pain intensity and bowel function after major large bowel surgery: a double‐blind randomized controlled trial. Journal of Gastrointestinal Surgery 2014;18(12):2155‐62. [1873‐4626; PUBMED: 25245767] [DOI] [PubMed] [Google Scholar]
Striebel 1992 {published data only}
- Striebel HW, Klettke U. Is intravenous lidocaine infusion suitable for postoperative pain management?. Schmerz 1992;6(4):245‐50. [PUBMED: 18415635] [DOI] [PubMed] [Google Scholar]
Swenson 2010 {published data only}
- NCT00600158. Effects of intravenous local anesthetic on bowel function after colectomy. clinicaltrials.gov/ct2/show/NCT00600158 (first received 24 January 2008 ).
- Swenson BR, Gottschalk A, Wells LT, Rowlingson JC, Thompson PW, Barclay M, et al. Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: a randomized clinical trial. Regional Anesthesia and Pain Medicine 2010;35(4):370‐6. [PUBMED: 20588151] [DOI] [PubMed] [Google Scholar]
Terkawi 2014 {published data only}
- NCT01204242. IV lidocaine for patients undergoing primary breast cancer surgery: effects on postoperative recovery and cancer recurrence. clinicaltrials.gov/show/NCT01204242 (first received 17 September 2010).
- Terkawi A, Durieux M, Gottschalk A, Brenin D, Tiouririne M. Effect of intravenous lidocaine on postoperative recovery of patients undergoing mastectomy: A double‐blind, placebo‐controlled randomized trial. Regional Anesthesia and Pain Medicine 2014;39(6):472‐7. [1098‐7339; PUBMED: 25275577] [DOI] [PubMed] [Google Scholar]
- Terkawi AS, Brenin D, Durieux ME, Blum FE, Tiouririne M. Effect of intravenous lidocaine on postoperative recovery in patients undergoing breast surgery: A double‐blinded, placebo randomized controlled trial. Anesthesia and Analgesia 2014;118((5 Suppl 1)):S208. [EMBASE: 71590951] [Google Scholar]
- Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post‐mastectomy chronic pain: a double‐blind, placebo‐controlled randomized trial. Pain Physician 2015;18(2):E139‐46. [2150‐1149; PUBMED: 25794212] [PubMed] [Google Scholar]
Tikuisis 2014 {published data only}
- Tikuisis R, Miliauskas P, Samalavicius NE, Zurauskas A, Samalavicius R, Zabulis V. Intravenous lidocaine for post‐operative pain relief after hand‐assisted laparoscopic colon surgery: A randomized, placebo‐controlled clinical trial. Techniques in Coloproctology 2014;18(4):373‐80. [PUBMED: 24030782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallin 1987 {published data only}
- Wallin G, Cassuto J, Hogstrom S, Linden I, Faxen A, Rimback G, et al. Effects of lidocaine infusion on the sympathetic response to abdominal surgery. Anesthesia and Analgesia 1987;66(10):1008‐13. [PUBMED: 3631561] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesthesia and Analgesia 2002;95(5):1134‐41. [PUBMED: 12401580] [DOI] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang HL, Yan HD, Liu YY, Sun BZ, Huang R, Wang XS, et al. Intraoperative intravenous lidocaine exerts a protective effect on cell‐mediated immunity in patients undergoing radical hysterectomy. Molecular Medicine Reports 2015;12(5):7039‐44. [1791‐3004; PUBMED: 26299324] [DOI] [PubMed] [Google Scholar]
Weinberg 2016 {published data only}
- Anonymous. Correction. Erratum for: A randomised controlled trial of peri‐operative lidocaine infusions for open radical prostatectomy [Anaesthesia, 2016, 71, (405‐410), 10.1111/anae.13368]. Anaesthesia 2016;71(10):1249‐50. [0003‐2409; PUBMED: 27611054] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg L, Rachbuch C, Ting S, Howard W, Yeomans M, Gordon I, et al. A randomised controlled trial of peri‐operative lidocaine infusions for open radical prostatectomy. [Erratum appears in Anaesthesia. 2016;71(10):1249‐50; PMID: 27611054]. Anaesthesia 2016;71(4):405‐10. [1365‐2044; PUBMED: 26749026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wongyingsinn 2011 {published data only}
- NCT01155440. Bowel function after laparoscopic colon surgery: effect of IV lidocaine. clinicaltrials.gov/ct2/show/NCT01155440 (first received 1 July 2010).
- Wongyingsinn M, Baldini G, Charlebois P, Liberman S, Stein B, Carli F. Intravenous lidocaine versus thoracic epidural analgesia: a randomized controlled trial in patients undergoing laparoscopic colorectal surgery using an enhanced recovery program. Regional Anesthesia and Pain Medicine 2011;36(3):241‐8. [PUBMED: 21519309] [DOI] [PubMed] [Google Scholar]
Wu 2005 {published data only}
- Wu CT, Borel CO, Lee MS, Yu JC, Liou HS, Yi HD, et al. The interaction effect of perioperative cotreatment with dextromethorphan and intravenous lidocaine on pain relief and recovery of bowel function after laparoscopic cholecystectomy. Anesthesia and Analgesia 2005;100(2):448‐53. [PUBMED: 15673874] [DOI] [PubMed] [Google Scholar]
Wuethrich 2012 {published data only}
- NCT00789620. Effects of intravenous lidocaine on transperitoneal laparoscopic urological surgery [Effects of intravenous lidocaine on transperitoneal laparoscopic urological surgery: a prospective, randomised, placebo controlled, double‐blind, phase III study]. clinicaltrials.gov/ct2/show/NCT00789620 (first received 13 November 2008).
- Wuethrich PY, Romero J, Burkhard FC, Curatolo M. No benefit from perioperative intravenous lidocaine in laparoscopic renal surgery: a randomised, placebo‐controlled study. European Journal of Anaesthesiology 2012;29(11):537‐43. [PUBMED: 22907609] [DOI] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu SQ, Li YH, Wang SB, Hu SH, Ju X, Xiao JB. Effects of intravenous lidocaine, dexmedetomidine and their combination on the postoperative pain and recovery of bowel function in patients undergoing abdominal hysterectomy. Minerva Anestesiologica 2017;83(7):685‐94. [1827‐1596; PUBMED: 28094477] [DOI] [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Choi YS, Gang H. Intraperitoneal and intravenous lidocaine for pain relief after laparoscopic cholecystectomy: A prospective randomized double‐blind placebo‐controlled study. Surgical Endoscopy and Other Interventional Techniques. 2014; Vol. 28:265. [EMBASE: 71479921; 0930‐2794]
- Yang SY, Kang H, Choi GJ, Shin HY, Baek CW, Jung YH, et al. Efficacy of intraperitoneal and intravenous lidocaine on pain relief after laparoscopic cholecystectomy. Journal of International Medical Research 2014;42(2):307‐19. [PUBMED: 24648482] [DOI] [PubMed] [Google Scholar]
Yardeni 2009 {published data only}
- Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesthesia and Analgesia 2009;109(5):1464‐9. [PUBMED: 19843784] [DOI] [PubMed] [Google Scholar]
Yon 2014 {published data only}
- ACTRN12612000545864. The effect of intravenous lidocaine infusion on the pain after subtotal gastrectomy [In patients undergoing subtotal gastrectomy, is intravenous lidocaine infusion effective in reducing post‐operative pain?]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362535&isReview=true (first received 22 May 2012).
- Yon JH, Choi GJ, Kang H, Park JM, Yang HS. Intraoperative systemic lidocaine for pre‐emptive analgesics in subtotal gastrectomy: a prospective, randomized, double‐blind, placebo‐controlled study. Canadian Journal of Surgery 2014;57(3):175‐82. [1488‐2310; PUBMED: 24869609] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zengin 2015 {published data only}
- Zengin SU, Saracoglu A, Eti Z, Umuroglu T, Gogus FY. The effects of preoperative oral pregabalin and perioperative intravenous lidocaine infusion on postoperative morphine requirement in patients undergoing laparatomy. Pain Research and Management 2015;20(4):179‐82. [1918‐1523; PUBMED: 25950425] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bartlett 1961 {published data only}
- Bartlett EE, Hutserani O. Xylocaine for the relief of postoperative pain. Anesthesia and Analgesia 1961;40:296‐304. [PUBMED: 14448503] [PubMed] [Google Scholar]
Birch 1987 {published data only}
- Birch K, Jørgensen J, Chraemmer‐Jørgensen B, Kehlet H. Effect of i.v. lignocaine on pain and the endocrine metabolic responses after surgery. British Journal of Anesthesia 1987;59(6):721‐4. [PUBMED: 3606915] [DOI] [PubMed] [Google Scholar]
Cepeda 1996 {published data only}
- Cepeda MS, Delgado M, Ponce M, Cruz CA, Carr DB. Equivalent outcomes during postoperative patient‐controlled intravenous analgesia with lidocaine plus morphine versus morphine alone. Anesthesia and Analgesia 1996;83(1):102‐6. [PUBMED: 8659717] [DOI] [PubMed] [Google Scholar]
Chia 1998 {published data only}
- Chia YY, Tan PH, Wang KY, Liu K. Lignocaine plus morphine in bolus patient‐controlled intravenous analgesia lacks post‐operative morphine‐sparing effect. European Journal of Anaesthesiology 1998;15(6):664‐8. [PUBMED: 9884851] [DOI] [PubMed] [Google Scholar]
Couceiro 2015 {published data only}
- Couceiro TC, Lima LC, Burle LM, Valenca MM. Intravenous lidocaine for postmastectomy pain treatment: randomized, blind, placebo controlled clinical trial. Brazilian Journal of Anesthesiology 2015;65(3):207‐12. [PUBMED: 25925033 ] [DOI] [PubMed] [Google Scholar]
De Clive‐Lowe 1958 {published data only}
- Clive‐Lowe SG, Desmond J, North J. Intravenous lignocaine anaesthesia. Anaesthesia 1958;13(2):138‐46. [PUBMED: 13521304] [DOI] [PubMed] [Google Scholar]
De Kock 1994 {published data only}
- Kock M, Lavandhomme P, Scholtes JL. Intraoperative and postoperative analgesia using intravenous opioid, clonidine and lignocaine. Anaesthesia and Intensive Care 1994;22(1):15‐21. [PUBMED: 8160943] [DOI] [PubMed] [Google Scholar]
Feld 2003 {published data only}
- Feld JM, Laurito CE, Beckerman M, Vincent J, Hoffman WE. Non‐opioid analgesia improves pain relief and decreases sedation after gastric bypass surgery. General Anesthesia 2003;50(4):336‐41. [PUBMED: 12670809] [DOI] [PubMed] [Google Scholar]
Hans 2010 {published data only}
- Hans GA, Lauwick SM, Kaba A, Bonhomme V, Struys MM, Hans PC, et al. Intravenous lidocaine infusion reduces bispectral index‐guided requirements of propofol only during surgical stimulation. British Journal of Anaesthesia 2010;105(4):471‐9. [PUBMED: 20650919] [DOI] [PubMed] [Google Scholar]
Harvey 2009 {published data only}
- Harvey KP, Adair JD, Isho M, Robinson R. Can intravenous lidocaine decrease postsurgical ileus and shorten hospital stay in elective bowel surgery? A pilot study and literature review. American Journal of Surgery 2009;198(2):231‐6. [PUBMED: 19285304] [DOI] [PubMed] [Google Scholar]
Joppich 2010 {published data only}
- Joppich R. Thema: impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery ‐ a systemic review of randomized controlled trials. Perioperative Medizin 2010;2:209‐10. [EMBASE: 360114423] [Google Scholar]
Juarez‐Pichardo 2009 {published data only}
- Juarez‐Pichardo JS, Avila‐Lopez A, Serrano‐Herrera MA. Preventive postoperative analgesia with dexmetomidine iv compared to lidocaine iv in cholecystectomy. Revista Mexicana de Anestesiología 2009;32(2):81‐8. [EMBASE: 355200435] [Google Scholar]
Kavak 2014 {published data only}
- Kavak Akelma F, Ergil J, Ozkan D, Akinci M, Ozmen M, Gumus H. A comparison of the effects of intraoperative esmolol and lidocaine infusions on postoperative analgesia. Anestezi Dergisi 2014;22(1):25‐31. [EMBASE: 372676615] [Google Scholar]
Knight 1980 {published data only}
- Knight PR, Kroll DA, Nahrwold ML, Denlinger JK, Kirsh M, Welter LO, et al. Comparison of cardiovascular responses to anesthesia and operation when intravenous lidocaine or morphine sulfate is used as adjunct to diazepam‐nitrous oxide anesthesia for cardiac surgery. Anesthesia and Analgesia 1980;59(2):130‐9. [PUBMED: 7189333] [PubMed] [Google Scholar]
Marret 2008 {published data only}
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta‐analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. British Journal of Surgery 2008;95(11):1331‐8. [PUBMED: 18844267] [DOI] [PubMed] [Google Scholar]
McCarthy 2010 {published data only}
- McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010;70(9):1149‐63. [PUBMED: 20518581] [DOI] [PubMed] [Google Scholar]
Olivares 2012 {published data only}
- Olivares AC, Ahuactzin TR, Marquez IV. Preventive analgesia with lidocaine in intravenous infusion vs sulfate of magnesium for postoperative pain management. British Journal of Anesthesia 2012;108:ii258. [EMBASE: 70719377] [Google Scholar]
Perniola 2014 {published data only}
- Perniola A, Magnuson A, Axelsson K, Gupta A. Intraperitoneal local anesthetics have predominant local analgesic effect: a randomized, double‐blind study. Anesthesiology 2014;121(2):352‐61. [PUBMED: 24758776 ] [DOI] [PubMed] [Google Scholar]
Rinne 1998 {published data only}
- Rinne T, Kaukinen S. Does lidocaine protect the heart during coronary revascularisation?. Acta Anaesthesiologica Scandinavica 1998;42(8):936‐40. [PUBMED: 9773138] [DOI] [PubMed] [Google Scholar]
Sun 2012 {published data only}
- Sun Y, Li T, Wang N, Yun Y, Gan TJ. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta‐analysis of randomized controlled trials. Diseases of the Colon and Rectum 2012;55(11):1183‐94. [PUBMED: 23044681] [DOI] [PubMed] [Google Scholar]
Vigneault 2011 {published data only}
- Vigneault L, Turgeon AF, Cote D, Lauzier F, Zarychanski R, Moore L, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: A meta‐analysis of randomized controlled trials. Canadian Journal of Anesthesia 2011;58(1):22‐37. [PUBMED: 21061107] [DOI] [PubMed] [Google Scholar]
Zhu 2015 {published data only}
- Zhu M, Li Y, Wan Z, Zhang D, Wang X. Effects of small‐dose lidocaine combined with ketamine on early postoperative cognitive function in elderly patients undergoing gastrointestinal tumor surgery. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University 2015;35(7):1076‐8, 1 p following 1078. [1673‐4254; PUBMED: 26198966] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cho 2014 {published data only}
- Cho K, Lee W, Lee JH, Kim MH, Lee KM. Perioperative infusion of lidocaine vs. dexmedetomidine; effect on reduction of postoperative analgesics consumption after laparoscopic cholecystectomy. European Journal of Anaesthesiology 2014;31:228. [0265‐0215] [Google Scholar]
Choi 2017 {published data only}
- Choi K, Nam K, Lee J, Chung W, Kang S, Joe Y, et al. The effects of intravenous lidocaine infusions on the quality of recovery and chronic pain after robotic thyroidectomy: a randomized, double‐blinded, controlled study. World Journal of Surgery 2017;41:1305‐12. [PUBMED: 27896411] [DOI] [PubMed] [Google Scholar]
Dewinter 2017 {published data only}
- Dewinter G, Moens P, Fieuws S, Vanaudenaerde B, Velde M, Rex S. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double‐blind, randomized, placebo‐controlled trial. British Journal of Anaesthesia 2017;118:576‐85. [PUBMED: 28403408] [DOI] [PubMed] [Google Scholar]
Horvat 2014 {published data only}
- Horvat M, Sotosek Tokamdzic V, Dvornik S, Frkovic V, Azman J, et al. Perioperative intravenous lidocaine effects on postoperative pain and cytokine production in patients undergoing nephrectomy‐a pilot study. Regional Anesthesia and Pain Medicine 2014;1:e223‐4. [1098‐7339] [Google Scholar]
Jendoubi 2017 {published data only}
- Jendoubi A, Ben Naceur I, Marzougui Y, Kouka J, Ghedira S, Houissa M. A comparison of lidocaine and ketamine in acute and chronic pain after open nephrectomy: a prospective, double‐blind, randomized, placebo controlled study. Regional Anesthesia and Pain Medicine 2015;1:e80‐1. [1098‐7339] [Google Scholar]
- Jendoubi A, Naceur IB, Bouzouita A, Trifa M, Ghedira S, Chebil M, et al. A comparison between intravenous lidocaine and ketamine on acute and chronic pain after open nephrectomy: a prospective, double‐blind, randomized, placebo‐controlled study. Saudi Journal of Anaesthesia 2017;11:177‐84. [PUBMED: 28442956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall 2017 {published data only}
- Kendall MC, McCarthy RJ, Panaro S, Goodwin E, Bialek JM, Nader A, et al. The effect of intraoperative systemic lldocaine on postoperative persistent pain using initiative on methods, measurement, and pain assessment in clinical trials criteria assessment following breast cancer surgery: a randomized, double‐blind, placebo‐controlled trial. Pain Practice 2017;10:10. [PUBMED: 28691269] [DOI] [PubMed] [Google Scholar]
Khalili 2017a {published data only}
- Khalili G, Attari MA, Rahnama H. Comparative study of the effects of pre‐emptive use of intravenous lidocaine and intramuscular piroxicam on muscular pain after lower abdominal surgery under the general anesthesia. Journal of Isfahan Medical School 2017;34:1481‐6. [614786865] [Google Scholar]
Khalili 2017b {published data only}
- Khalili G, Sadeghifar M, Attari MA. Evaluation of the effect of intravenous and intraperitoneal lidocaine on pain intensity in comparison to control group in laparoscopic cholecystectomy. Journal of Isfahan Medical School 2017;35:927‐33. [618692163] [Google Scholar]
Kim 2017 {published data only}
- Kim M, Lee K, Park S, Kim S, Park H, Yoo Y. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients undergoing breast cancer surgery: a prospective, randomized, double‐blind, comparative clinical trial. PLOS ONE 2017;12(3):e0173026. [PUBMED: 28253307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2018 {published data only}
Lee 2017 {published data only}
- Lee U, Choi YJ, Choi GJ, Kang H. Intravenous lidocaine for effective pain relief after bimaxillary surgery. Clinical Oral Investigations 2017;21:2645‐52. [PUBMED: 28168381] [DOI] [PubMed] [Google Scholar]
Metha 2017 {published data only}
- Metha M, Trivedi B, Sharma K. A prospective randomised placebo controlled study to analyze effects of constant rate intravenous infusion of lignocaine on peri‐operative analgesia verses similar rate normal saline infusion. Regional Anesthesia and Pain Medicine. Forty‐Second Annual Regional Anesthesiology and Acute Pain Medicine Meeting, ASRA; 2017 April 6‐8; San Francisco (CA). 2017. [619776930]
NCT02257346 {published data only}
- NCT02257346. Intravenous lidocaine and quality of recovery after cesarean delivery [The effect of perioperative systemic lidocaine on quality of recovery after cesarean delivery]. clinicaltrials.gov/show/NCT02257346 (first received 6 October 2014).
Rahaymeh 2016 {published data only}
- Rahaymeh J, Alkhalaileh M, Meqbel A, Almajali B, Dabas RA. Effect of preoperative lidocaine infusion on postoperative morphine requirements in major laparotomy surgery. Rawal Medical Journal 2016;41(3):312‐5. [0303‐5212] [Google Scholar]
Sherif 2017 {published data only}
- Sherif A. The impact of continuous infusion adjutants on the quality of recovery after laproscopic barbaric surgery: dexmedetomidine versus lidocaine: a double‐blinded, randomized, placebo‐controlled trial. Regional Anesthesia and Pain Medicine 2016;41((5 Suppl 1)):e95. [1532‐8651] [Google Scholar]
- Sherif AA, Elsersy HE. The impact of dexmedetomidine or xylocaine continuous infusion on opioid consumption and recovery after laparoscopic sleeve gastrectomy. Minerva Anestesiologica 2017;83:1274‐82. [DOI] [PubMed] [Google Scholar]
Song 2017 {published data only}
- Song X, Sun Y, Zhang X, Li T, Yang B. Effect of perioperative intravenous lidocaine infusion on postoperative recovery following laparoscopic cholecystectomy ‐ a randomized controlled trial. International Journal of Surgery 2017;45:8‐13. [PUBMED: 28705592] [DOI] [PubMed] [Google Scholar]
Van Den Heuvel 2016 {published data only}
- Heuvel S, Berkum B, Markerink H, Wal S, Ronday M, Plat J, et al. Intravenous lidocaine reduces perioperative opioid administration and postoperative TNF‐a during oncologic breast surgery. Pain Practice. 2016; Vol. 16:134. [1533‐2500]
Yoo 2016 {published data only}
- Kim MH, Lee KY, Yoo YC, Kim YH, Min NH. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients. Anesthesiology. 2016:A2190. [DOI] [PMC free article] [PubMed]
- Yoo YC, Kang HJ. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients undergoing breast cancer surgery: a randomized double‐blind clinical trial. Anesthesia and Analgesia 2016;123((3 Suppl 2)):484‐5. [1526‐7598] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02059902 {published data only}
- NCT02059902. Continuous lidocaine infusion for management of perioperative burn pain. clinicaltrials.gov/show/NCT02059902 (first received 11 February 2014).
NCT02607488 {published data only}
- NCT02607488. Perioperative systemic lidocaine for enhanced bowel recovery after bariatric surgery [Perioperative systemic lidocaine for enhanced bowel recovery after bariatric surgery: a dose dependent study]. clinicaltrials.gov/show/NCT02607488 (first received 18 November 2015).
NCT02862769 {published data only}
- NCT02862769. Intra‐operative lidocaine infusion in preventing CPSP Post VATs [The role of Intra‐operative lidocaine infusion in preventing chronic post surgical pain after video assisted thoracoscopic surgery]. clinicaltrials.gov/show/NCT02862769 (first received 11 August 2016).
Additional references
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779 ] [DOI] [PubMed] [Google Scholar]
Block 2003
- Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA Jr, Wu CL. Efficacy of postoperative epidural analgesia: a meta‐analysis. JAMA 2003;290(18):2455‐63. [PUBMED: 14612482] [DOI] [PubMed] [Google Scholar]
Borenstein 2017
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. [DOI: 10.1002/jrsm.1230; PUBMED: 28058794] [DOI] [PubMed] [Google Scholar]
Cassuto 2006
- Cassuto J, Sinclair R, Bonderovic M. Anti‐inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiologica Scandinavica 2006;50(3):265‐82. [PUBMED: 16480459] [DOI] [PubMed] [Google Scholar]
Chang 2017
- Chang YS, Liu CL, Liu TP, Yang PS, Chen MJ, Cheng AP. Effect of perioperative intravenous lidocaine infusion on acute and chronic pain after breast surgery: A meta‐analysis of randomized controlled trials. Pain Practice 2017;17(3):336‐43. [PUBMED: 26913591] [DOI] [PubMed] [Google Scholar]
Christie 2007
- Christie IW, McCabe S. Major complications of epidural analgesia after surgery: results of a six‐year survey. Anaesthesia 2007;62(4):335‐41. [PUBMED: 17381568] [DOI] [PubMed] [Google Scholar]
Collinsworth 1974
- Collinsworth KA, Kalman SM, Harrison DC. The clinical pharmacology of lidocaine as an antiarrhythmic drug. Circulation 1974;50(6):1217‐30. [PUBMED: 4609637] [DOI] [PubMed] [Google Scholar]
Cook 2009
- Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. British Journal of Anaesthesia 2009;102(2):179‐90. [PUBMED: 19139027] [DOI] [PubMed] [Google Scholar]
Devor 1992a
- Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain 1992;48(2):261‐8. [PUBMED: 1589245] [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455–63. [PUBMED: 10877304] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical research edition) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903 ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Haldar 2016
- Haldar R, Dubey M, Rastogi A, Singh PK. Intravenous lignocaine to blunt extubation responses: a double‐edged sword. American Journal of Therapeutics 2016;23(2):e646‐8. [PUBMED: 25807045] [DOI] [PubMed] [Google Scholar]
Hedges 1998
- Hedges LV, Vevea JL. Fixed‐ and random‐effects models in meta‐analysis. Psychological Methods 1998;3(4):486‐504. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hollmann 2000a
- Hollmann MW, Fischer LG, Byford AM, Durieux ME. Local anesthetic inhibition of m1 muscarinic acetylcholine signaling. Anesthesiology 2000;93(2):497‐509. [PUBMED: 10910501] [DOI] [PubMed] [Google Scholar]
Hollmann 2000b
- Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication?. Anesthesiology 2000;93(3):858‐75. [PUBMED: 10969322] [DOI] [PubMed] [Google Scholar]
Hollmann 2001
- Hollmann MW, Wieczorek KS, Berger A, Durieux ME. Local anesthetic inhibition of G protein‐coupled receptor signaling by interference with galpha(q) protein function. Molecular Pharmacology 2001;59(2):294‐301. [PUBMED: 11160866] [DOI] [PubMed] [Google Scholar]
Hollmann 2002
- Hollmann MW, McIntire WE, Garrison JC, Durieux ME. Inhibition of mammalian Gq protein function by local anesthetics. Anesthesiology 2002;97(6):1451‐7. [PUBMED: 12459671] [DOI] [PubMed] [Google Scholar]
Hollmann 2005
- Hollmann MW, Strumper D, Herroeder S, Durieux ME. Receptors, G proteins, and their interactions. Anesthesiology 2005;103(5):1066‐78. [PUBMED: 16249682] [DOI] [PubMed] [Google Scholar]
Holte 2002
- Holte K, Kehlet H. Epidural anaesthesia and analgesia ‐ effects on surgical stress responses and implications for postoperative nutrition. Clinical Nutrition (Edinburgh, Scotland) 2002;21(3):199‐206. [PUBMED: 12127927] [DOI] [PubMed] [Google Scholar]
Hughes 2014
- Hughes MJ, Ventham NT, McNally S, Harrison E, Wigmore S. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta‐analysis. JAMA Surgery 2014;149(12):1224‐30. [DOI: 10.1001/jamasurg.2014.210; PUBMED: 25317633] [DOI] [PubMed] [Google Scholar]
IntHout 2016
- IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open 2016;6(7):e010247. [DOI: 10.1136/bmjopen-2015-010247; PUBMED: 27406637] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehlet 2008
- Kehlet H. Postoperative ileus ‐ an update on preventive techniques. Nature Clinical Practice. Gastroenterology & Hepatology 2008;5(10):552‐8. [PUBMED: 18695704] [DOI] [PubMed] [Google Scholar]
Kingery 1997
- Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain 1997;73(2):123‐39. [PUBMED: 9415498] [DOI] [PubMed] [Google Scholar]
Mayumi 1983
- Mayumi T, Dohi S, Takahashi T. Plasma concentrations of lidocaine associated with cervical, thoracic, and lumbar epidural anesthesia. Anesthesia and Analgesia 1983;62(6):578‐80. [PUBMED: 6846880] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting Items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [PUBMED: 19621072 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2011
- Moore RA, Ni Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [PUBMED: 21445017] [DOI] [PubMed] [Google Scholar]
Popping 2008
- Popping DM, Zahn PK, Aken HK, Dasch B, Boche R, Pogatzki‐Zahn EM. Effectiveness and safety of postoperative pain management: a survey of 18 925 consecutive patients between 1998 and 2006 (2nd revision): a database analysis of prospectively raised data. British Journal of Anaesthesia 2008;101(6):832‐40. [PUBMED: 18945716] [DOI] [PubMed] [Google Scholar]
Popping 2012
- Popping DM, Wenk M, Aken H K. Neurologic complications after epidural analgesia. Anasthesiol Intensivmed Notfallmed Schmerzther 2012;47(5):336‐43; 344. [PUBMED: 22628030] [DOI] [PubMed] [Google Scholar]
Popping 2014
- Popping DM, Elia N, Aken HK, Marret E, Schug SA, Kranke P, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta‐analysis of randomized controlled trials. Annals of Surgery 2014;259(6):1056‐67. [PUBMED: 24096762] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI: 10.1136/bmj.d549; PUBMED: 21310794] [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746–63. [PUBMED: 17592831 ] [DOI] [PubMed] [Google Scholar]
Schwarzer 2007
- Schwarzer G. meta: An R package for meta‐analysis. R News 2007;7(3):40‐5. [Google Scholar]
Toren 2009
- Toren P, Ladak S, Ma C, McCluskey S, Fleshner N. Comparison of epidural and intravenous patient controlled analgesia in patients undergoing radical cystectomy. The Canadian Journal of Urology 2009;16(4):4716‐20. [PUBMED: 19671221] [PubMed] [Google Scholar]
Viechtbauer 2010
- Viechtbauer W. Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software 2010;36(3):1‐48. [Google Scholar]
Woolf 1985
- Woolf CJ, Wiesenfeld‐Hallin Z. The systemic administration of local anaesthetics produces a selective depression of C‐afferent fibre evoked activity in the spinal cord. Pain 1985;23(4):361‐74. [PUBMED: 3937116] [DOI] [PubMed] [Google Scholar]
Wu Cohen 2005
- Wu CL, Cohen SR, Richman JM, Rowlingson AJ, Courpas GE, Cheung K, et al. Efficacy of postoperative patient‐controlled and continuous infusion epidural analgesia versus intravenous patient‐controlled analgesia with opioids: a meta‐analysis. Anesthesiology 2005;103(5):1079‐88; 1109‐10. [PUBMED: 16249683] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kranke 2015
- Kranke P, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD009642.pub2] [DOI] [PubMed] [Google Scholar]
Selig 2012
- Selig C, Schnabel A, Hollmann MW, Hahnenkamp K, Eberhart LHJ, Poepping D, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009642] [DOI] [PubMed] [Google Scholar]
Weibel 2016
- Weibel S, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. British Journal of Anaesthesia 2016;116(6):770‐83. [PUBMED: 27199310] [DOI] [PubMed] [Google Scholar]


