Abstract
Background
Placing a small volume of colostrum directly onto the buccal mucosa of preterm infants during the early neonatal period may provide immunological and growth factors that stimulate the immune system and enhance intestinal growth. These benefits could potentially reduce the risk of infection and necrotising enterocolitis (NEC) and improve survival and long‐term outcome.
Objectives
To determine if early (within the first 48 hours of life) oropharyngeal administration of mother’s own fresh or frozen/thawed colostrum can reduce rates of NEC, late‐onset invasive infection, and/or mortality in preterm infants compared with controls. To assess trials for evidence of safety and harm (e.g. aspiration pneumonia). To compare effects of early oropharyngeal colostrum (OPC) versus no OPC, placebo, late OPC, and nasogastric colostrum.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8), MEDLINE via PubMed (1966 to August 2017), Embase (1980 to August 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to August 2017). We also searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry (www.whoint/ictrp/search/en/), and the ISRCTN Registry), conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials. We performed the last search in August 2017. We contacted trial investigators regarding unpublished studies and data.
Selection criteria
We searched for published and unpublished randomised controlled trials comparing early administration of oropharyngeal colostrum (OPC) versus sham administration of water, oral formula, or donor breast milk, or versus no intervention. We also searched for studies comparing early OPC versus early nasogastric or nasojejunal administration of colostrum. We considered only trials that included preterm infants at < 37 weeks' gestation. We did not limit the review to any particular region or language.
Data collection and analysis
Two review authors independently screened retrieved articles for inclusion and independently conducted data extraction, data analysis, and assessments of 'Risk of bias' and quality of evidence. We graded evidence quality using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. We contacted study authors for additional information or clarification when necessary.
Main results
We included six studies that compared early oropharyngeal colostrum versus water, saline, placebo, or donor, or versus no intervention, enrolling 335 preterm infants with gestational ages ranging from 25 to 32 weeks' gestation and birth weights of 410 to 2500 grams. Researchers found no significant differences between OPC and control for primary outcomes ‐ incidence of NEC (typical risk ratio (RR) 1.42, 95% confidence interval (CI) 0.50 to 4.02; six studies, 335 infants; P = 0.51; I² = 0%; very low‐quality evidence), incidence of late‐onset infection (typical RR 0.86, 95% CI 0.56 to 1.33; six studies, 335 infants; P = 0.50; I² = 0%; very low‐quality evidence), and death before hospital discharge (typical RR 0.76, 95% CI 0.34 to 1.71; six studies, 335 infants; P = 0.51; I² = 0%; very low‐quality evidence). Similarly, meta‐analysis showed no difference in length of hospital stay between OPC and control groups (mean difference (MD) 0.81, 95% CI ‐5.87 to 7.5; four studies, 293 infants; P = 0.65; I² = 49%). Days to full enteral feeds were reduced in the OPC group with MD of ‐2.58 days (95% CI ‐4.01 to ‐1.14; six studies, 335 infants; P = 0.0004; I² = 28%; very low‐quality evidence).
The effect of OPC was uncertain because of small sample sizes and imprecision in study results (very low‐quality evidence).
No adverse effects were associated with OPC; however, data on adverse effects were insufficient, and no numerical data were available from the included studies.
Overall the quality of included studies was low to very low across all outcomes. We downgraded GRADE outcomes because of concerns about allocation concealment and blinding, reporting bias, small sample sizes with few events, and wide confidence intervals.
Authors' conclusions
Large, well‐designed trials would be required to evaluate more precisely and reliably the effects of oropharyngeal colostrum on important outcomes for preterm infants.
Keywords: Humans; Infant, Newborn; Administration, Oral; Colostrum; Colostrum/immunology; Enterocolitis, Necrotizing; Enterocolitis, Necrotizing/immunology; Enterocolitis, Necrotizing/prevention & control; Hospital Mortality; Immunity, Mucosal; Immunity, Mucosal/immunology; Infant, Premature; Infant, Premature/immunology; Length of Stay; Mouth Mucosa; Mouth Mucosa/immunology; Oropharynx; Randomized Controlled Trials as Topic; Sepsis; Sepsis/prevention & control
Plain language summary
Maternal colostrum provided into the mouth of preterm babies to prevent complications and improve outcomes
Review question
Does providing a very small volume of maternal colostrum into the mouth of preterm babies (oropharyngeal colostrum (OPC)) prevent complications and improve health outcomes?
Background
Placing a small volume of colostrum ‐ the first milk produced by the mother during the first few days of life ‐ directly onto the inside of the cheeks of preterm infants may provide immunological and growth factors that stimulate the immune system and enhance growth of the intestine. These benefits could potentially reduce infections, including severe infections in the intestine known as necrotising enterocolitis (NEC), thereby improving survival and long‐term outcomes.
Study characteristics
We searched for both published and unpublished studies comparing oropharyngeal colostrum versus a control such as water, placebo, or no oral priming. We included only clinical trials reporting outcomes in preterm babies (< 37 weeks' gestation). The evidence is up‐to‐date as of August 2017. We did not limit the review to any particular region or language.
Key results
Six studies were eligible for inclusion, involving 335 preterm infants with gestational ages ranging from 25 to 32 weeks' gestation and birth weights of 410 to 2500 grams. Reviewers noted no differences between OPC and control for rate of NEC, infection, or death before hospital discharge. Similarly, they observed no difference in length of hospital stay between OPC and control babies. Infants who received OPC achieved full milk feeds on average 2.5 days earlier than those given placebo or no intervention. However, included studies were small, data were insufficient, and study designs were not ideal. Combining study data did not provide sufficient evidence to recommend the use of colostrum for oral priming to prevent complications in preterm infants. Five of the included studies reported no harms (adverse effects); however, no numerical data are available from these studies. Included studies were of very low quality; therefore the effects of OPC remain uncertain.
Conclusions
Larger, better quality clinical trials would be needed to evaluate more precisely and reliably the effects of OPC on important outcomes for preterm infants. .
Summary of findings
Summary of findings for the main comparison. Oropharyngeal colostrum (OPC) compared to control (water, saline, or no intervention) in preterm infants.
| Oropharyngeal colostrum (OPC) compared to control (water, saline, or no intervention) in preterm infants | ||||||
| Patient or population: preterm infants Setting: neonatal intensive care unit Intervention: oropharyngeal colostrum (OPC) Comparison: control (water, saline, or no intervention) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control (water, saline, or no intervention) | Risk with oropharyngeal colostrum (OPC) | |||||
| Incidence of necrotising enterocolitis (NEC) | Study population | RR 1.42 (0.50 to 4.02) | 335 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | ||
| 3 per 100 | 4 per 100 (2 to 12) | |||||
| Incidence of late‐onset sepsis | Study population | RR 0.86 (0.56 to 1.33) | 335 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 20 per 100 | 17 per 100 (11 to 26) | |||||
| Death before discharge to home | Study population | RR 0.76 (0.34 to 1.71) | 335 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWd,e,f | ||
| 6 per 100 | 5 per 100 (2 to 10) | |||||
| Days to full enteral feed | Mean time to full enteral feed was 10 to 25 days | MD 2.58 days lower (4.01 lower to 1.14 lower) | ‐ | 335 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWb,d,e | A lower score indicates a better outcome |
| Length of hospital stay (days) | Mean length of hospital stay was 47 to 86 days | MD 0.81 days higher (5.87 lower to 7.5 higher) | ‐ | 293 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWa,g,h | A lower score indicates a better outcome |
| Pneumonia | Study population | RR 2.08 (0.54 to 8.06) | 57 (3 RCTs) | ⊕⊕⊝⊝ LOWd,f | ||
| 6 per 100 (3 to 52) |
7 per 100 (1 to 45) | |||||
| Reported adverse effects | No pre‐defined adverse effects have been described by any studies. Adverse effects were narratively reported as no adverse effects with the intervention. No numerical data were provided. One study reported "no recorded episodes of apnea, bradycardia, desaturation or other adverse effects" but without defining the adverse effects. A second study stated that "no adverse events were noted", and another mentioned in the method section that "apnea, bradycardia events and aspiration pneumonia were charted according to unit policy" | ‐ | 335 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWa,d,i,j | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NEC: necrotising enterocolitis; OPC: oropharyngeal colostrum; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStudies with the highest weight involved concern about allocation concealment and blinding.
bSmall sample size. The confidence interval was wide and crossed the line of no effect.
cThe study with the highest weight involved concern about method of randomisation.
d75% of the included studies were not blinded.
eTwo studies involved concerns about allocation concealment.
fSmall sample size, variable effect size, and confidence interval crossing the line of no effect.
gTwo studies involved concerns about blinding, and two provided incomplete outcome data (attrition bias).
hI² was 47% ‐ not high enough to lower our confidence. Heterogeneity could be explained as one study had lower participant gestational age and birth weight.
iA narrative report was provided without a clear statement of adverse effect; estimates are not precise.
jNeither definitions of adverse effects nor methods used in monitoring were reported.
Background
Description of the condition
Preterm birth (before 37 weeks' gestation) is one of the most significant issues associated with perinatal health care (WHO 2012). Complications of preterm birth contributed to approximately 50% of neonatal deaths in 2016 and are the leading cause of death before five years of age (Blencowe 2012; UN IGME 2017). The rate of preterm birth is increasing by an average of 1% per year worldwide; the World Health Organization (WHO) estimated 12.9 million preterm births (9.6% of total live births) in 2005 (Beck 2009), and 15 million (11.1% of total live births) in 2010 (Blencowe 2013). Despite substantial advances in neonatal care, mortality and morbidity remain high in this population (Goldenberg 2008; Slattery 2002).
Necrotising enterocolitis (NEC), a multi‐factorial, life‐threatening inflammatory condition of the gastrointestinal tract, affects 0.3% to 2.4% per 1000 live births, with 70% of cases occurring in preterm infants (Hunter 2008; Thompson 2008). NEC is a complex process involving inflammation and bacterial invasion of the immature mucosa. Hypoperfusion of the bowel, use of antibiotics, and delay in commencing enteral feeding act synergistically to promote intestinal atrophy and abnormal bacterial colonisation of the bowel (Rodriguez 2015a; Westerbeek et al 2006). Late‐onset infection (LOI), defined as a blood culture‐positive microbial infection after 72 hours of life (Stoll 2002), is associated with a high burden of morbidity and mortality in preterm infants. Despite a variety of infection control measures and the use of antibiotics, preterm infants remain at high risk for infection and NEC, both of which are associated with poor neurodevelopmental and growth outcomes (Stoll 2004). These conditions are also linked to prolonged hospital stays and substantial increases in the cost of care for both hospitals and families (Bisquera 2002; Johnson 2013).
It has been suggested that oropharyngeal colostrum (OPC) is a continuation of the exposure of the foetal oropharynx to growth and protective biofactors of the amniotic fluid during foetal life. Colostrum, the fluid secreted by the mammary glands over the first few postnatal days, is rich in biological protective factors that are present in higher concentrations in the colostrum of mothers who have delivered preterm infants (Araújo 2005; Wheeler 2007). Colostrum may act via different mechanisms: as a local barrier that prevents adhesion of microbes to the mucosa, modulating cytokine interaction with oropharyngeal‐associated lymphoid tissues and facilitating the absorption of immune factors by buccal mucosa; through pre‐biotic and anti‐inflammatory actions; via antioxidant properties of lactoferrin; and by stimulation of intestinal growth and repair (Rodriguez 2009).
Description of the intervention
Oropharyngeal administration of the mother’s own colostrum to her preterm infant consists of placing a small amount (0.1 to 0.5 mL) of colostrum directly onto the buccal mucosa at least once and usually repeatedly within the first 48 hours of life. Oropharyngeal administration can be performed by instilling colostrum inside each cheek via syringe, or by gently painting the colostrum over the tongue, around the gums, and along the lips using a sterile swab soaked with 0.1 to 0.5 mL of colostrum, or any other means such that fluid is absorbed by the buccal mucosa.
How the intervention might work
Human colostrum and milk are known to contain significant levels of anti‐infective agents (cytokines, lactoferrin, lysozymes, and immunoglobulin A (IgA)) (Radillo 2013). Together, many of these chemokines and trophic agents protect the infant from infection, stimulate development of the gastrointestinal tract, and modulate the immune system of the infant (Chirico 2008). Many of these factors are present in higher concentrations in the colostrum secreted by mothers who have delivered preterm infants (Araújo 2005; Wheeler 2007). The oral cavity is a predominant site of microbial colonisation, and the oral mucosa is an important interface between microbiota, immunologically active factors in colostrum, and the infant’s immune system. When administered directly onto the oropharyngeal mucosa, colostrum may provide benefit by acting in three ways: (1) by stimulating the oropharyngeal‐associated lymphoid tissue system, (2) by promoting systemic absorption of protective factors through the buccal mucosa, inducing systemic immune responses, and (3) by acting as a barrier, blocking microbial adhesion to the mucosa (Rodriguez 2009). In addition, the high concentration of growth factors in colostrum, such as epidermal growth factor, may enhance intestinal growth and development (Ballard 2013; Chang 2002). Substantial evidence indicates that colostrum is a rich source of growth factors, immunoglobulins, lactoferrin, cytokines, and other immunological active factors (Montagne 1999; Ustundag 2005; Walker 2010). Few studies support OPC, although existing studies have suggested that it is a safe, feasible prophylactic measure against sepsis, NEC, and ventilator‐associated pneumonia (Gephart 2014; Lee 2015; Rodriguez 2010; Seigel 2013). Such immune‐inflammatory modulation and improved bowel growth may reduce rates of LOI and NEC, potentially improving survival and neurodevelopmental outcomes in preterm infants.
Why it is important to do this review
In the first few days of life, OPC is a novel, low‐cost, simple to administer intervention that may reduce NEC and sepsis. A systematic review of the evidence, including potential harms, is required before recommendations can be made for or against its use. This systematic review analysed the existing literature to collate current evidence to determine if early (first 48 hours) OPC is safe and feasible, and whether it affects important clinical outcomes in preterm infants.
Objectives
To determine if early (within the first 48 hours of life) oropharyngeal administration of mother’s own fresh or frozen/thawed colostrum can reduce rates of NEC, late‐onset invasive infection, or mortality in preterm infants compared with controls. To assess trials for evidence of safety and harm (e.g. aspiration pneumonia). To compare effects of early oropharyngeal colostrum (OPC) versus no OPC, placebo, late OPC, and nasogastric colostrum.
Methods
Criteria for considering studies for this review
Types of studies
We included all published randomised trials for which the unit of randomisation was the infant, and cluster‐randomised trials for which the unit of randomisation was the neonatal unit. We excluded quasi‐randomised and non‐randomised trials such as controlled before‐and‐after studies. We did not limit the review to any particular region or language.
Types of participants
We included preterm infants (at less than 37 weeks' gestation) receiving care in any neonatal unit.
Types of interventions
We included the following interventions.
Oropharyngeal administration of mother's own fresh or frozen/thawed colostrum to preterm infants in the first 48 hours of life, irrespective of when enteral feeding is initiated, what type of milk is used for enteral feeding, or which feed advancement regimen is applied.
Instillation of the colostrum inside the cheeks of the infant by oral syringe or by gentle application over the tongue, around the gums, and along the lips using a swab or sponge soaked with a small amount of colostrum (0.1 to 0.5 mL), at least once and usually repeatedly in the first 48 hours of life.
Any technique of oropharyngeal administration, such as instillation by syringe, direct application to the oral mucosa by swab, or any other means such that the fluid could be absorbed by the buccal mucosa.
We considered studies comparing early administration of oropharyngeal colostrum versus sham administration of water, oral formula, or donor breast milk, or no intervention. We also considered studies comparing OPC versus nasogastric or nasojejunal administration of colostrum.
We planned to perform three comparisons.
Early oropharyngeal colostrum versus sham administration of water, oral formula, or donor breast milk, or no intervention.
Early oropharyngeal colostrum versus early nasogastric or nasojejunal administration of colostrum.
Early oropharyngeal colostrum versus late (after 48 hours) oropharyngeal colostrum .
This review identified only studies that compared early oropharyngeal colostrum versus sham administration of water, normal saline, oral formula, or donor breast milk, or versus no intervention.
Types of outcome measures
Primary outcomes
Incidence of NEC (Bell's stage 2 or 3) until discharge to home (Walsh 1986)
Incidence of microbiologically confirmed late‐onset invasive infection until discharge to home, defined as a blood or cerebrospinal fluid culture positive for microbial infection after 72 hours of life (Stoll 2002)
Death before discharge to home
Secondary outcomes
Pneumonia (chest X‐ray changes/treated with at least five days of antibiotics before discharge to home)
Chronic lung disease (defined as the need for oxygen supplementation at 36 weeks' postmenstrual age)
Retinopathy of prematurity (all stages and severe stage > 2) (ICCROP 2005)
Death in the first year of life
Neurodevelopmental outcome at 18 to 24 months assessed by clinician or parent‐reported questionnaire
Formally reported adverse effects (e.g. aspiration, gagging/choking on administration, bradycardia, desaturation, increase in oxygen requirement, disturbances in vital signs) between start of the intervention and discharge home
Weight gain from birth to discharge home (using weight percentiles or Z‐scores), time to regain birth weight
Length of hospital stay (days) from birth to discharge home
Days to full enteral feeds
Days of parenteral nutrition before discharge to home
Days of antibiotic therapy before discharge to home
Receiving any breast milk at discharge to home
Receiving only breast milk (and not formula) at discharge to home
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialised register).
Electronic searches
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) in the Cochrane Library; MEDLINE via PubMed (1966 to August 2017); Embase (1980 to August 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to August 2017) using the following search terms: (colostrum), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/), and the ISRCTN Registry).
Searching other resources
We examined reference lists of included studies and previous reviews. We searched proceedings of annual meetings of the Paediatric Academic Societies (1993 to 2017), the European Society for Paediatric Research (1995 to 2017), the Royal College of Paediatrics and Child Health (2000 to 2017), the Perinatal Society of Australia and New Zealand (2000 to 2017), and the National Association of Neonatal Nurses.
Trials reported only as abstracts were eligible if sufficient information to fulfil the inclusion criteria was available from the report or upon contact with study authors.
We also searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group.
Selection of studies
We followed the standard processes recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Two review authors screened the title and abstract of all citations identified through the search and independently assessed the full text of all articles selected by the principal review author to determine which studies were eligible for inclusion. We resolved disagreements by discussion until we reached consensus.
We contacted study authors for additional information or clarification when necessary.
Data extraction and management
Two review authors independently extracted and compared data; we resolved discrepancies through discussion and by consultation with the third review author.
We used a modified Cochrane standard data collection sheet to extract the following data from each study.
Study ID and contact details.
Method (design, duration of study, sequence generation, allocation concealment, blinding).
Participants (total number, gestational age, sex, country, socioeconomic and ethnic groups, diagnosis, status).
Intervention (number, time, technique, dose and duration, any additional interventions).
Outcomes (time of outcome, reporting method, effect size).
We contacted study authors for additional information when required.
We have presented the included studies in Characteristics of included studies tables.
Assessment of risk of bias in included studies
Two review authors (NA and SO) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2017).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor (JD). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed treatment effects in individual trials using Review Manager 5.3 (Review Manager 2014); we have reported risk ratios and risk differences for dichotomous data and mean differences for continuous data, with respective 95% confidence intervals. We also reported the number needed to treat for an additional beneficial outcome or an additional harmful outcome for analyses with statistically significant differences in the risk difference.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. An infant was considered only once per analysis.
Dealing with missing data
We contacted investigators when we identified important missing data (in the outcomes) or unclear data. We performed intention‐to‐treat analyses.
Assessment of heterogeneity
We observed heterogeneity between effect sizes of the included studies by inspecting the forest plot and by using the Chi² test and I² for heterogeneity (with P < 0.1).
We quantified inconsistency across studies to determine whether heterogeneity was present and assessed its impact on the meta‐analysis using the equation I² = [(Q ‐ df)/Q] × 100%, where Q is the Chi² statistic and df is its degrees of freedom (Higgins 2011). We used the percentage of variability in effect estimates to describe inconsistency between trials that was due to heterogeneity rather than to chance. We followed the guidelines recommended by the Cochrane Neonatal Review Group for interpreting the I² statistic: < 25% = none, 25% to 49% = low, 50% to 74% = moderate, and > 75% = high heterogeneity. If we detected moderate or high heterogeneity (I² > 50%), we explored possible causes (e.g. differences in study design, participants, interventions, definitions, measurement of outcome assessments).
Assessment of reporting biases
Although we planned to use a funnel plot to assess potential reporting bias, we did not do this, as we identified fewer than 10 trials.
Data synthesis
We performed the meta‐analysis using Review Manager 5.3 and the fixed‐effect model for meta‐analyses (Review Manager 2014). For dichotomous data, we used Mantel‐Haenszel for estimates of typical risk ratio. For continuous data, we used the inverse variance method for estimates of mean difference. We reported all estimates with respective 95% confidence intervals.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
Incidence of NEC (Bell's stage 2 or 3) until discharge to home (Walsh 1986).
Incidence of microbiologically confirmed late‐onset invasive infection until discharge to home.
Death before discharge to home.
Length of hospital stay (days) from birth to discharge to home.
Days to full enteral feed.
Pneumonia.
Formally reported adverse effects between start of the intervention and discharge to home.
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a 'Summary of findings' table to report the quality of the evidence.
The GRADE approach yields an assessment of the quality of a body of evidence by one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
When substantial heterogeneity existed, we tested potential causes through subgroup and sensitivity analyses.
If data were available, we planned to perform the following subgroup analyses.
Infants born at < 30 weeks' gestation.
Infants born at < 1500 grams.
Infants who were small for gestational age at birth (i.e. birth weight < 10th centile).
Sensitivity analysis
We planned to perform sensitivity analyses to determine if findings were affected by including only studies using adequate methods (low risk of bias).
Results
Description of studies
Results of the search
We identified 14 study reports for full text screening (Alvarez 2016; Glass 2017; JPRN‐UMIN000022923; Lee 2015; McFadden 2011 (see McFadden 2012); McFadden 2012; Montgomery 2008; NCT02912585; Rodriguez 2008 (see Rodriguez 2011); Rodriguez 2011; Rodriguez 2015b; Romano‐Keeler 2016; Sohn 2015; Zhang 2017). Of these, after translation of the abstract of the report from Spanish, we found that one study was a non‐randomised trial (Alvarez 2016). Similarly, Montgomery 2008 was an observational study assessing the feasibility of oropharyngeal administration of mother's colostrum. Rodriguez 2015b and JPRN‐UMIN000022923 were protocols for ongoing studies; we have described both under Characteristics of ongoing studies. Two were additional reports; one of these ‐ McFadden 2011 was the conference abstract for McFadden 2012, a PhD thesis that we have included in this review. Rodriguez 2008 was the PhD thesis for Rodriguez 2011, which we have included in this review. (See Figure 1.) Two trials fulfilled all inclusion criteria for the review except that colostrum was given after 48 hours of life (Lee 2015; Zhang 2017). We have described these studies in the section on Excluded studies and in the Characteristics of excluded studies table. Six trials fulfilled the inclusion criteria of the review protocol: Glass 2017; McFadden 2012; NCT02912585; Rodriguez 2011; Romano‐Keeler 2016; Sohn 2015. We conducted the last search in August 2017.
1.
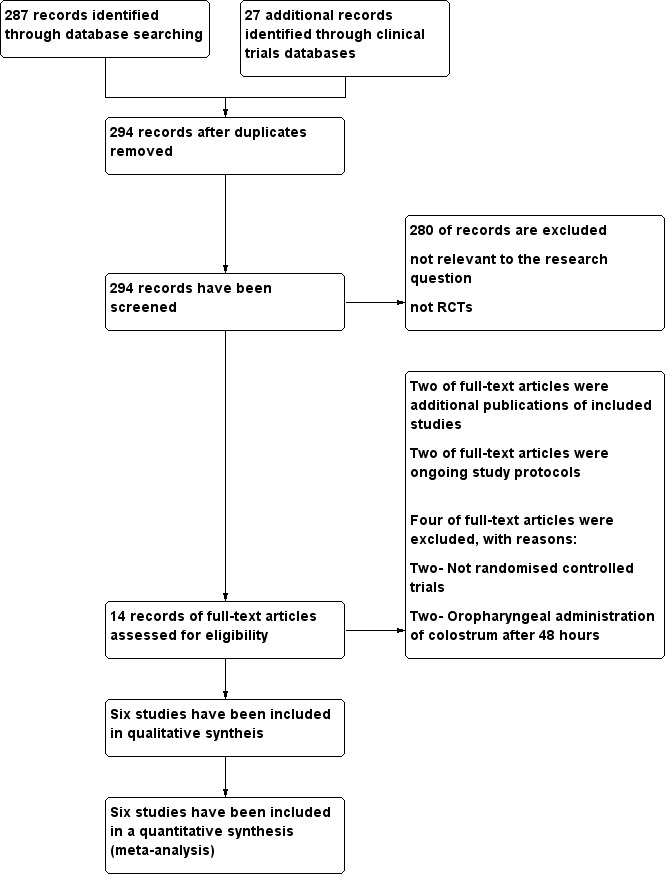
Study flow diagram.
Included studies
We included six studies in the review: Glass 2017; McFadden 2012; NCT02912585; Rodriguez 2011; Romano‐Keeler 2016; Sohn 2015. All included studies compared administration of early oropharyngeal colostrum versus sham administration of water, placebo, or donor breast milk, or no intervention. Five studies were published and one study was described in an unpublished report that we obtained from the study author (NCT02912585). We have presented features of these studies in the Characteristics of included studies tables.
All included studies were small, single‐centre trials involving a total of 335 infants with sample sizes ranging from 12 preterm infants in Sohn 2015 to 149 in NCT02912585. Four studies pre‐specified prematurity with birth weight < 1500 grams as an inclusion criterion (Glass 2017; NCT02912585; Rodriguez 2011; Sohn 2015). Two studies included only infants who were mechanically ventilated (McFadden 2012; Sohn 2015). The gestational age of included infants ranged from 25 to 32 weeks' gestation with birth weights between 410 and 2500 grams (see Table 2).
1. Characteristics of participants in the included studies.
| Criteria | Rodriguez 2011 | McFadden 2012 | Sohn 2015 | Romano‐Keeler 2016 | NCT02912585 | Glass 2017 |
| Number of participants | 16 | 27 | 12 | 99 | 149 | 30 |
| Gestational age (weeks) | 25‐28 | 27‐32 | 25‐30 | 28‐31 | 26‐31 | 27‐29 |
| Birth weight (grams) | 410‐1250 | 590‐2530 | 490‐1300 | 905‐1602 | 787‐1217 | 1020‐1169 |
Interventions and comparisons
All six included studies commenced oropharyngeal administration of mother's own colostrum or placebo within 48 hours of birth. Four studies administered 0.2 mL colostrum (or placebo) via syringe: 0.1 mL on each side of the oropharynx (NCT02912585; Rodriguez 2011; Romano‐Keeler 2016; Sohn 2015). Two studies, both open‐label trials, used 0.2 mL colostrum for oral care administered via "gentle swab along the inside of the mouth" ‐ in McFadden 2012 ‐ or with a cotton‐tipped applicator ‐ in Glass 2017. Two studies did not use placebo and provided no additional intervention to participants randomised to the control group (Romano‐Keeler 2016; Sohn 2015). Three studies administered sterile water to infants in the control group (Glass 2017; NCT02912585; Rodriguez 2011), and one study included two control groups ‐ one receiving sterile water and the other normal saline (McFadden 2012). Researchers administered control interventions in a manner similar to administration of colostrum to the intervention group.
Outcomes
All included studies reported the primary outcomes of the review (NEC, LOI, and death before discharge to home) (Glass 2017; McFadden 2012; NCT02912585; Rodriguez 2011; Romano‐Keeler 2016; Sohn 2015). Four studies defined NEC as Bell's stage 2 or 3 (Glass 2017; NCT02912585; Romano‐Keeler 2016; Sohn 2015), whereas two reports provided no specific diagnostic criteria (McFadden 2012; Rodriguez 2011). Three studies defined LOI as clinical signs and a positive blood culture (Glass 2017; NCT02912585; Sohn 2015). Glass 2017 provided additional criteria for defining LOI (onset after day three of life and antibiotic therapy for at least five days), and three studies did not provide a pre‐determined definition (McFadden 2012; Rodriguez 2011; Romano‐Keeler 2016). Included studies variably reported secondary outcomes. All included studies followed up on participants until hospital discharge, and no studies reported any later outcomes.
Excluded studies
We excluded two studies because investigators provided oropharyngeal colostrum after 48 hours of life (Characteristics of excluded studies). Lee 2015 was a double‐blind, placebo‐controlled RCT that included 48 infants born at < 28 weeks' gestation who were randomised to receive 0.2 mL of their mother's colostrum or sterile water (control) via the oropharyngeal route every three hours for three days. However, most of the infants included in this study received colostrum after 48 hours of life; thus we excluded this study from the analysis. Similarly, Zhang 2017 was a double‐blind, placebo‐controlled trial including 64 very low birth weight infants (birth weight < 1500 grams) that compared administration of 0.1 mL of mother's colostrum to each side of the cheek versus similar administration of normal saline. Mean age at the first dose of colostrum or normal saline was > 48 hours in both groups; hence we did not include this study in the review.
Risk of bias in included studies
Included studies were of variable quality (see Risk of bias in included studies; Figure 2; Figure 3). All studies stated that treatment was allocated randomly; however two reports did not specify the method used to generate the random sequence (McFadden 2012; Sohn 2015). Similarly, two studies did not mention allocation concealment methods (NCT02912585; Romano‐Keeler 2016), and Glass 2017 reported that the allocation method was not applicable. Only three studies were blinded and used opaque syringes to deliver treatment (McFadden 2012; NCT02912585; Rodriguez 2011). Study authors reported outcomes for most infants in all studies, except Glass 2017, which did not report the outcomes of 13 infants.
2.
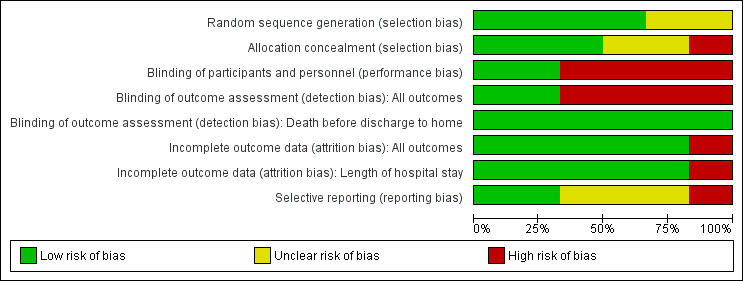
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
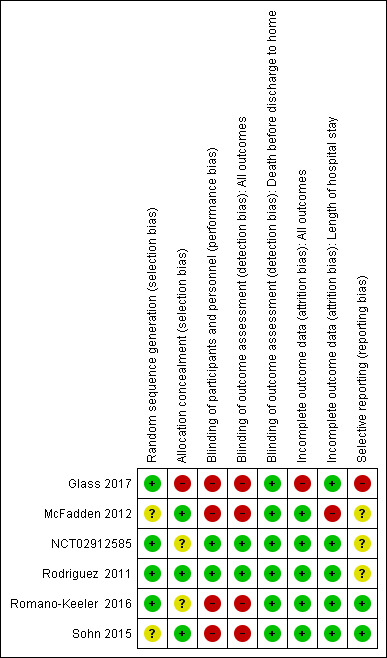
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Primary outcomes
Incidence of necrotising enterocolitis
All included studies reported the incidence of NEC in 335 infants. Meta‐analysis did not show an effect on the risk of NEC (typical risk ratio (RR) 1.42, 95% confidence interval (CI) 0.50 to 4.02; six studies, 335 infants; P = 0.51; Analysis 1.1; Figure 4). Two studies had no cases of NEC (Rodriguez 2011; McFadden 2012), so the estimate is based on four studies including 290 participants. The typical risk difference was 0.01 (95% CI ‐0.03 to 0.06). We noted no evidence of heterogeneity between studies (I² = 0%). The quality of evidence was very low owing to imprecision and high to unclear risk of bias.
1.1. Analysis.
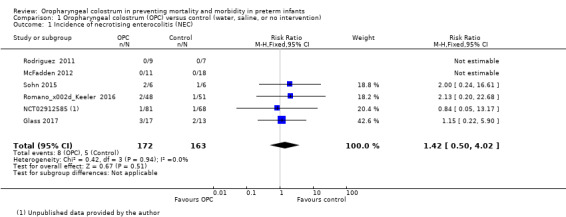
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 1 Incidence of necrotising enterocolitis (NEC).
4.
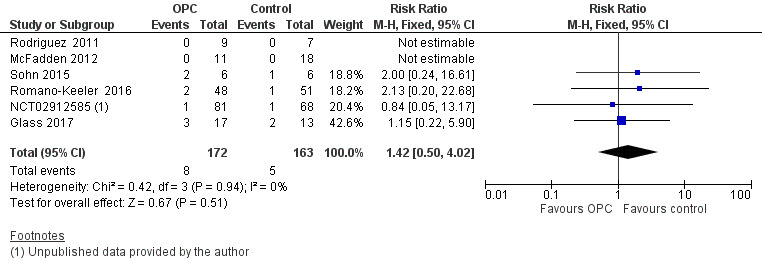
Forest plot of comparison: 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), outcome: 1.1 Incidence of necrotising enterocolitis.
Incidence of late‐onset infection
All included studies reported the incidence of LOI in 335 infants. Meta‐analysis did not show an effect on the risk of LOI (typical RR 0.86, 95% CI 0.56 to 1.33; six studies, 335 infants; P = 0.50; Analysis 1.2; Figure 5). We found no evidence of heterogeneity between studies (I² = 0%). The quality of evidence was very low owing to imprecision and high to unclear risk of bias.
1.2. Analysis.
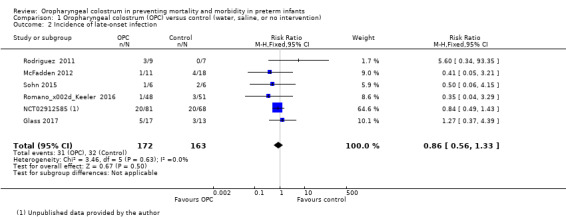
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 2 Incidence of late‐onset infection.
5.
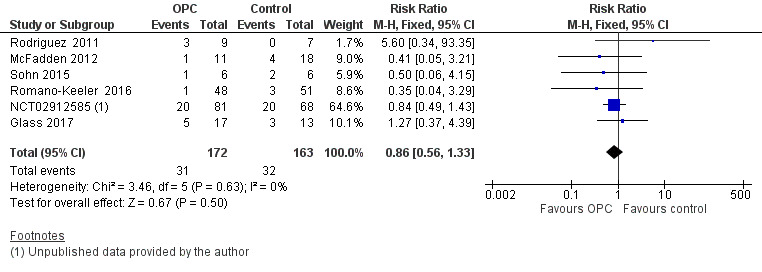
Forest plot of comparison: 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), outcome: 1.2 Incidence of late‐onset infection.
Death before discharge to home
All included studies reported death before discharge home in 335 infants. Meta‐analysis did not show an effect on the risk of death before discharge home (typical RR 0.76, 95% CI 0.34 to 1.71; six studies, 335 infants; P = 0.51; Analysis 1.3; Figure 6). One study did not report death in any of the enrolled infants (Glass 2017), so the estimate is based on five studies in 305 infants. No evidence suggests heterogeneity between studies (I² = 0%). The quality of evidence was very low owing to imprecision and high to unclear risk of bias.
1.3. Analysis.
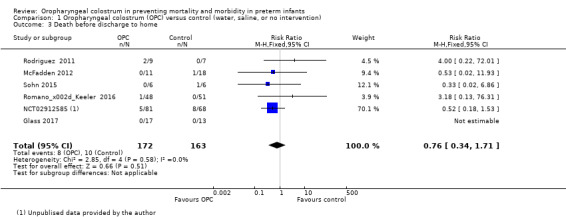
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 3 Death before discharge to home.
6.
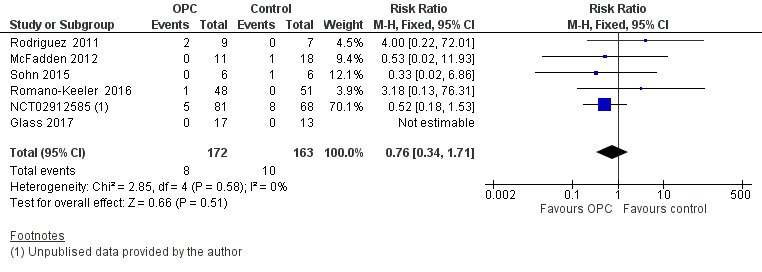
Forest plot of comparison: 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), outcome: 1.3 Death before discharge to home.
Secondary outcomes
Days to full enteral feeds
All included studies reported days to full enteral feeds in 335 infants. Two studies reported that infants who received oropharyngeal colostrum established full enteral feeds more quickly (NCT02912585; Rodriguez 2011). The other included studies did not show any statistically significant difference in this outcome. Meta‐analysis of the six included studies showed that infants who received OPC within 48 hours of birth achieved full enteral feeds earlier than those given placebo or no intervention (mean difference (MD) ‐2.58, 95% CI ‐4.01 to ‐1.14; P = 0.0004; Analysis 1.4; Figure 7). Heterogeneity was moderate (I² = 53%) between studies. The quality of evidence was very low owing to imprecision, high to unclear risk of bias, and moderate heterogeneity.
1.4. Analysis.
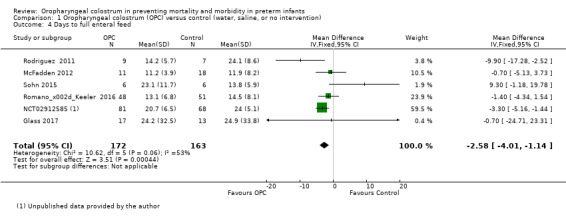
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 4 Days to full enteral feed.
7.
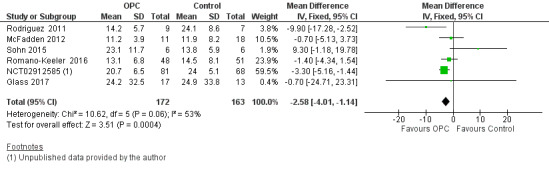
Forest plot of comparison: 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), outcome: 1.4 Days to full enteral feed.
Length of hospital stay
Four studies reported length of hospital stay in 293 infants (McFadden 2012; NCT02912585; Rodriguez 2011; Romano‐Keeler 2016). Meta‐analysis did not show an effect in the two groups (MD 0.81, 95% CI ‐5.87 to 7.50; P = 0.81; Analysis 1.5). Heterogeneity was moderate (I² = 49%) across studies. We retrospectively explored this heterogeneity and identified one study that included infants with a larger birth weight (McFadden 2012). Exclusion of this study reduced heterogeneity to I² = 12% and did not change the estimated effect in the meta‐analysis for length of hospital stay. The quality of evidence was very low owing to imprecision, high to unclear risk of bias, and moderate heterogeneity.
1.5. Analysis.
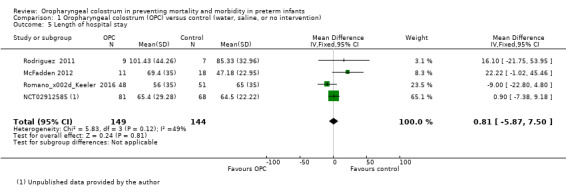
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 5 Length of hospital stay.
Pneumonia
Meta‐analysis did not show an effect on pneumonia (typical RR 2.08, 95% CI 0.54 to 8.06; three studies, 57 infants; P = 29) (McFadden 2012; Rodriguez 2011; Sohn 2015). We found no evidence of heterogeneity between studies (I² = 17%) (Analysis 1.6). The quality of evidence was very low owing to imprecision and performance and selection bias.
1.6. Analysis.
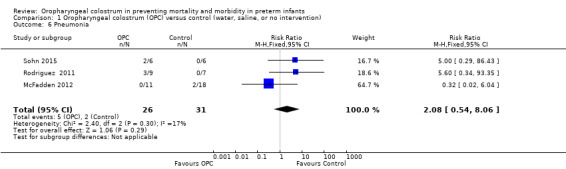
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 6 Pneumonia.
Formally reported adverse effects (e.g. aspiration, gagging/choking on administration, bradycardia, desaturation, increase in oxygen requirement, disturbances in vital signs) between start of the intervention and discharge to home
Five studies mentioned adverse events (Glass 2017; McFadden 2012; NCT02912585; Rodriguez 2011; Romano‐Keeler 2016), but no studies reported the occurrence of any adverse events (additional tables; Table 3). Researchers reported adverse events as not occurring with the intervention and provided no numerical data. The quality of evidence was very low owing to imprecision and high to unclear risk of bias.
2. Adverse effects.
| Study ID | Outcome | Definition | Comments |
| Rodriguez 2011 | No adverse effects reported | No pre‐defined adverse effects described in the study | "All subjects tolerated the intervention and there were no recorded episodes of apnea, bradycardia, hypotension, desaturation or other adverse effects during the treatment protocol" No numerical data were provided |
| McFadden 2012 | No adverse effects reported | No pre‐defined adverse effects described in the study | Adverse effects were narratively reported as no adverse effects with the intervention. No numerical data were provided |
| Sohn 2015 | No adverse effects reported | No pre‐defined adverse effects described in the study | No data or comment in the report |
| Romano‐Keeler 2016 | No adverse effects reported | No pre‐defined adverse effects described in the study | "No adverse events were noted among patients in either group during the course of the study" No numerical data were provided |
| NCT02912585 | No adverse effects reported | No pre‐defined adverse effects described in the study | "The subjects tolerated the intervention and there were no adverse effects during the protocol treatment" No numerical data were provided |
| Glass 2017 | No adverse effects reported | Infants monitored for apnoea, bradycardia, and aspiration pneumonia | "There were no apneic, bradycardic events reported with oropharyngeal colostrum or sterile water administration" |
Chronic lung disease
Three studies reported chronic lung disease in 57 infants (McFadden 2012; Rodriguez 2011; Sohn 2015). One study provided the definition of chronic lung disease (CLD) (oxygen required at 36 weeks' corrected gestational age, or at discharge, if sooner) (Sohn 2015). Meta‐analysis did not show an effect on the incidence of CLD (typical RR 0.85, 95% CI 0.60 to 1.20; three studies, 57 infants; P = 0.84; Analysis 1.8). We noted no evidence of heterogeneity between studies (I² = 0%). Another study reported bronchopulmonary dysplasia as an outcome but did not define the diagnostic criteria used (NCT02912585). Including this study in the meta‐analysis did not change the result significantly (typical RR 0.85, 95% CI 0.60 to 1.20; four studies, 206 infants; P = 0.37). Also, we noted no evidence of heterogeneity between studies (I² = 0%). The quality of evidence was very low owing to imprecision and performance and reporting bias.
1.8. Analysis.
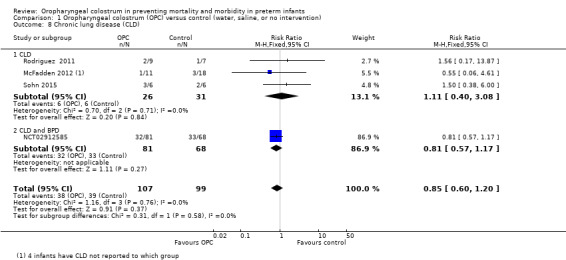
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 8 Chronic lung disease (CLD).
Days of antibiotic therapy before discharge to home
Three studies reported days of antibiotic therapy in 141 infants (McFadden 2012; Romano‐Keeler 2016; Sohn 2015). Meta‐analysis did not show an effect of administration of oropharyngeal colostrum on the number of days of antibiotic therapy before discharge home (MD 1.69, 95% CI ‐4.00 to 7.39; P = 0.65; Analysis 1.9). One trial found that infants who received oral care with saline or sterile water (control) required fewer days of antibiotic therapy when compared with those who received oral care with colostrum (McFadden 2012). Heterogeneity (I² = 91%) between studies was very high. We retrospectively explored this heterogeneity and identified that one study enrolled infants with a larger birth weight (McFadden 2012). Excluding this study removed the heterogeneity but did not change the effect estimate. The quality of evidence was very low owing to imprecision, performance and reporting bias, and high heterogeneity.
1.9. Analysis.
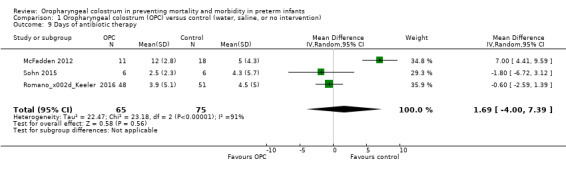
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 9 Days of antibiotic therapy.
Receiving any breast milk at discharge to home
One study reported on 99 infants receiving any breast milk at discharge home (Romano‐Keeler 2016). The study author divided this outcome between those on unfortified breast milk and those on fortified breast milk. Results show no statistically significant differences between OPC and control groups for either type of feeding, that is, receiving any fortified breast milk at discharge (RR 0.67, 95% CI 0.44 to 1.02; one study, 99 infants; P = 0.06) and receiving any unfortified breast milk at discharge (RR 0.98, 95% CI 0.89 to 1.07; one study, 99 infants; P = 0.60). However, combining these outcomes revealed an effect (P = 0.04) of OPC on infants receiving any breast milk (fortified or unfortified) compared with controls. The quality of evidence was very low because data were obtained from only one unblinded study with a small sample size.
Receiving only breast milk (and not formula) at discharge to home
One study reported on infants receiving only breast milk at discharge to home (Romano‐Keeler 2016). Researchers reported no effect of OPC on infants receiving only breast milk (unfortified) at discharge (RR 0.98, 95% CI 0.89 to 1.07; one study, 99 infants; P = 0.60). The quality of evidence was very low because data were obtained from only one unblinded study with a small sample size.
Days of parenteral nutrition before discharge to home
Two studies reported days of parenteral nutrition in 179 infants before discharge to home (Glass 2017; NCT02912585). Meta‐analysis showed no difference in days of parenteral nutrition between infants who received oropharyngeal colostrum and those given control interventions (MD 0.37, 95% CI ‐1.78 to 2.52; two studies, 179 infants; P = 0.7; Analysis 1.11). We found no evidence of heterogeneity between studies (I² = 0%). The quality of evidence was very low owing to imprecision and performance and reporting bias.
1.11. Analysis.
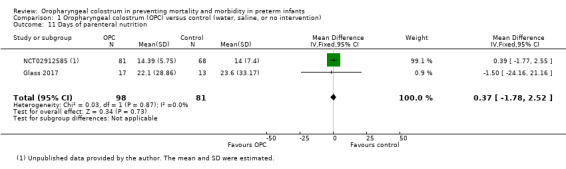
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 11 Days of parenteral nutrition.
Weight gain from birth to discharge home (using weight percentiles or Z‐scores), time to regain birth weight
One unpublished study reported the outcome of weight at discharge home in 149 infants (NCT02912585). This study showed no effect of OPC on weight at discharge (MD ‐15.00, 95% CI ‐50.83 to 20.83; P = 0.60). The quality of evidence was very low owing to imprecision, unclear selection and reporting bias, and the fact that data were obtained from only one study.
Retinopathy of prematurity (ROP)
Two studies reported ROP in 165 infants (NCT02912585; Rodriguez 2011 as reported in Rodriguez 2008). Meta‐analysis did not show a statistically significant effect of the intervention on the incidence of ROP (typical RR 0.98, 95% CI 0.33 to 2.94; two studies, 165 infants; P = 0.98; Analysis 1.13). We noted no heterogeneity between studies (I² = 0%). The quality of evidence was very low owing to imprecision and performance and reporting bias.
1.13. Analysis.
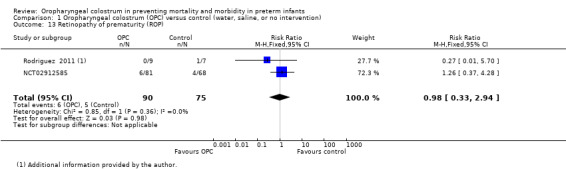
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 13 Retinopathy of prematurity (ROP).
Death in the first year of life
None of the included studies reported death in the first year of life.
Neurodevelopmental outcome at 18 to 24 months assessed by clinician or parent‐reported questionnaire
None of the included studies reported this outcome.
Discussion
Summary of main results
Review authors identified six randomised controlled trials (RCTs) that were eligible for inclusion in this review. These studies reported a range of outcomes. All six studies reported the three primary outcomes in this review, and available trial data do not provide evidence that oropharyngeal administration of colostrum compared with placebo (saline or sterile water) or no intervention reduces the risk of late‐onset infection (LOI), necrotising enterocolitis (NEC), or death before discharge home in preterm infants. However, all six studies were small and included a total of 335 participants; this is likely to be insufficient to demonstrate small but clinically important effects in these important outcomes. One ongoing study (see Characteristics of ongoing studies) aims to recruit and follow up 489 extremely preterm infants with birth weight < 1250 grams, within the infant's first 96 hours of life (Rodriguez 2015b). When available, including the findings of this study in the meta‐analysis may alter the estimates of effects and the conclusions.
Infants who received oropharyngeal colostrum established full enteral feeding sooner than those who received placebo or no intervention. Despite this, the included studies do not show consistent evidence of effect on length of hospital stay. In addition, we considered several secondary outcomes in this review, and the included studies do not demonstrate any effects on risk of pneumonia, chronic lung disease, or retinopathy of prematurity. They also show no differences in weight at discharge, days of antibiotic therapy, days on parenteral nutrition, or chances of receiving any breast milk at the time of discharge. None of the included studies followed up with participants beyond hospital discharge, and no data were available to assess the effect of the intervention on death by one year of age nor on neurodevelopmental outcome at 18 to 24 months of age.
These studies narratively reported adverse events and described no adverse events related to administration of oropharyngeal colostrum or placebo.
Overall completeness and applicability of evidence
Most participants were very or extremely preterm infants with very low birth weight. None of the included studies specified as exclusion criteria small for gestational age or in utero compromise such as absent end‐diastolic flow on maternal dopplers. One study reported that five of 30 participants were small for gestational age (Glass 2017). Most studies included ventilated infants, and only Rodriguez 2011 specified the need for "vasopressor medications at a dosage of > 10microg/kg/min" as an exclusion criterion. Therefore, review findings should be applicable to most preterm and very low birth weight babies.
Quality of the evidence
The GRADE quality of evidence was low and was downgraded to very low owing to concerns about allocation concealment and blinding in the highest weighted studies, concerns about incomplete outcome data, small sample sizes with few events, and wide confidence intervals crossing the line of no effect for almost all outcomes (see Table 1). Studies showed heterogeneity in days to full feeds, which could be due to variability between studies in the definition of time to reach full enteral feeds (100 to 150 mL/kg/d). Although results show a statistically significant effect and a moderate effect size, the confidence interval was very wide (‐4.01 to ‐1.14).
Potential biases in the review process
Our main concern with the review process is the possibility of publication bias, as we identified insufficient studies to prepare a funnel plot to assess this. We attempted to minimise this bias by screening the reference lists of included trials and by searching for abstracts and proceedings of major perinatal conferences. Another major concern was incomplete reporting of results. We endeavoured to minimise this by contacting study authors when needed, several of whom provided missing data that we have reported in the review. Although most data included in the analyses were derived from study reports (published or unpublished) and additional information was provided by study authors, NCT02912585 presented continuous outcomes as median and interquartile range (IQR), and mean and standard deviation (SD) were not available on request. We therefore assumed a normal distribution and estimated mean ± SD using the formula: IQR = approximately 1.35 of the SD (Cochrane Handbook for Systematic Reviews of Interventions; Section 7.7.3.5; Higgins 2011a).
Agreements and disagreements with other studies or reviews
This review focuses on oropharyngeal administration of mother's own colostrum within the first 48 hours of life and, to the best of our knowledge, is the first review based on Cochrane systematic methods. Gephart 2014 conducted a systematic review to assess the effect of oral therapy with colostrum compared with no colostrum in sick neonates; findings of this review were generally consistent with our review findings. Review authors reported lack of strong evidence to support the proposed clinical benefits of early colostrum oral care in sick neonates; they cited earlier studies supporting the safety and feasibility of the use of colostrum, but these studies used different study design methods, including RCTs, observational, cross‐sectional, and longitudinal studies, and clinical audits.
Authors' conclusions
Implications for practice.
Limited available evidence currently suggests that oropharyngeal administration of mother's colostrum starting within the first 48 hours of life does not reduce the risk of NEC, late‐onset infection, or death until discharge in preterm infants, including very preterm, very low birth weight infants. This approach can shorten the time taken to achieve full enteral feeds but does not reduce the duration of hospital stay. Results now awaited from an ongoing study may alter these conclusions.
Implications for research.
Although it is biologically plausible that oropharyngeal administration of mother's colostrum can reduce the risk of late‐onset infection and NEC in preterm infants, additional, larger RCTs are required to conclusively answer these questions. These trials need to be adequately powered to assess effects of the intervention on clinically relevant outcomes, including late‐onset infection and NEC. A priori agreements on dose and procedure for administration of oropharyngeal colostrum, inclusion of the most immature and smallest (including growth‐restricted) infants with other intensive care needs (such as mechanical ventilation and inotropic support), and well‐defined, objective, and clinically relevant outcome measures will enable wider application of the evidence to groups at greatest risk. In addition, trials should aim to assess long‐term outcomes, principally mortality and neurodevelopment.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2019 | Amended | Plain language summary title added |
Acknowledgements
The methods section of this review is based on a standard template used by the Cochrane Neonatal Review Group.
We acknowledge Colleen Ovelman, Managing Editor, and Yolanda Brosseau, Trials Search Co‐ordinator, of the Cochrane Neonatal Review Group, for assisting us with the literature search.
We thank Dr Nancy Rodriguez, Dr Sandra Cesario, Dr Barbara McFadden, Dr Mark Underwood, Dr James Wynn, Dr Kirsten Glass, and Dr Daniela Mota Ferreira for providing extra information and data for this review.
Appendices
Appendix 1. Cochrane Neonatal standard search strategy
The search term 'colostrum' was used in combination with the following searches in the different databases.
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: ((exp infant) OR (infan* OR newborn or neonat* OR premature or very low birth weight or low birth weight or VLBW or LBW).mp AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp
CINAHL: (infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared pre‐specified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (when it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (when not all of the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of necrotising enterocolitis (NEC) | 6 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.50, 4.02] |
| 2 Incidence of late‐onset infection | 6 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 3 Death before discharge to home | 6 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.34, 1.71] |
| 4 Days to full enteral feed | 6 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐2.58 [‐4.01, ‐1.14] |
| 5 Length of hospital stay | 4 | 293 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐5.87, 7.50] |
| 6 Pneumonia | 3 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.54, 8.06] |
| 7 Reported adverse effects | Other data | No numeric data | ||
| 8 Chronic lung disease (CLD) | 4 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 8.1 CLD | 3 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.40, 3.08] |
| 8.2 CLD and BPD | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.17] |
| 9 Days of antibiotic therapy | 3 | 140 | Mean Difference (IV, Random, 95% CI) | 1.69 [‐2.00, 7.39] |
| 10 Receiving any breast milk at discharge to home. | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Fortified breast milk | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.02] |
| 10.2 Unfortified breast milk | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.07] |
| 10.3 Any breast milk (fortified or unfortified) | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.38, 0.97] |
| 11 Days of parenteral nutrition | 2 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐1.78, 2.52] |
| 12 Weight at discharge to home | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | 24.5 [‐69.66, 118.66] |
| 13 Retinopathy of prematurity (ROP) | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.33, 2.94] |
1.7. Analysis.
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 7 Reported adverse effects.
| Reported adverse effects | |||
|---|---|---|---|
| Study | Outcome | Definition | Comments |
| Glass 2017 | No adverse effects reported. | Infants were monitored for apnea, bradycardia and aspiration pneumonia. | "There were no apenic, bradycardic events reported with oropharyngeal colostrum or sterile water administration." |
| McFadden 2012 | No adverse effects have been reported | No pre‐defined adverse effects have been described in the study | Adverse effects were narratively reported as no adverse effects with the intervention. No numerical data were provided |
| NCT02912585 | No adverse effects have been reported | No pre‐defined adverse effects have been described in the study | "The subjects tolerated the intervention and there were no adverse effects during the protocol treatment. No numerical data provided." |
| Rodriguez 2011 | No adverse effects have been reported | No pre‐defined adverse effects have been described in the study | "All subjects tolerated the intervention and there were no recorded episodes of apnea, bradycardia, hypotension, desaturation or other adverse effects during the treatment protocol". No numerical data were provided |
| Romano‐Keeler 2016 | No adverse effects have been reported | No pre‐defined adverse effects have been described in the study | "No adverse events were noted among patients in either group during the course of the study". No numerical data were provided |
| Sohn 2015 | No adverse effects have been reported | No pre‐defined adverse effects have been described in the study | No data or comment in the report. |
1.10. Analysis.
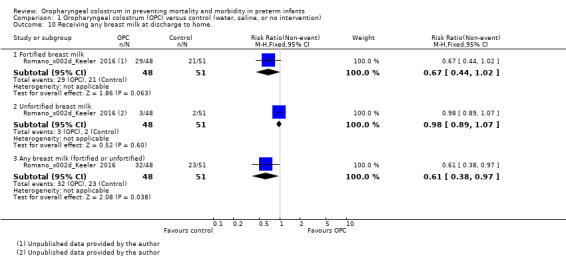
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 10 Receiving any breast milk at discharge to home..
1.12. Analysis.
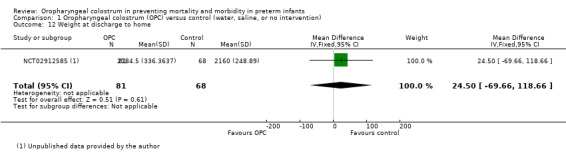
Comparison 1 Oropharyngeal colostrum (OPC) versus control (water, saline, or no intervention), Outcome 12 Weight at discharge to home.
Characteristics of studies
Characteristics of included studies [author‐defined order]
Glass 2017.
| Methods | Open label, placebo‐controlled, randomised, controlled trial | |
| Participants | 30 infants (17 intervention and 13 control) Inclusion criteria: birth weight < 1500 grams, born in the same hospital Exclusion criteria: infants with major congenital anomalies or chromosomal syndromes incompatible with life, infants of mothers not willing to provide colostrum for their infant in the first week of life, infants of mothers with known human immunodeficiency virus, hepatitis B, or hepatitis C Setting: NICU, Children's Hospital, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania, USA From January 2011 to January 2016 |
|
| Interventions | With a cotton‐tipped applicator, 0.2 mL of mother's own colostrum was applied to the infant's oropharyngeal mucosa every 3 hours for 5 days from day 2 to day 7 of life (intervention) vs 0.2 mL sterile water delivered in a similar manner (control) | |
| Outcomes | Change in salivary secretory IgA concentration from baseline to 2 weeks of age Necrotising enterocolitis (Bell's stage 2 or 3) Culture‐positive sepsis Time to full enteral feeding (defined as 140 mL/kg/d) |
|
| Funding source | Children’s Miracle Network Research Grant National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under award number 1R01DK099350. USA |
|
| Notes | Results were initially available as a conference abstract, and further information was provided by Dr Karen Glass. Data included in the review included information from the subsequent publication ‐ Glass 2017 ‐ and information provided by Dr Glass | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number generation"; additional information provided by the study author |
| Allocation concealment (selection bias) | High risk | "Not applicable"; additional information provided by the study author |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The trial was not blinded |
| Blinding of outcome assessment (detection bias) Death before discharge to home | Low risk | The trial was not blinded. but death is unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 participants were excluded and were not analysed. This was determined through additional information provided by the study author |
| Incomplete outcome data (attrition bias) Length of hospital stay | Low risk | This outcome has not been reported in this trial |
| Selective reporting (reporting bias) | High risk | Proposed outcomes as given in the study protocol (clinicaltrials.gov NCT01443091) were reported. However, the estimated sample size in the protocol was 60 infants, although the report included only 30 infants. No explanation for this was provided |
McFadden 2012.
| Methods | Open‐label, randomised, controlled trial | |
| Participants | 29 infants (11 intervention and 18 control) Inclusion criteria: gestational age 26 to 34 weeks, admission to NICU, intubation and mechanical ventilation, support with nasal continuous positive airway pressure (NCPAP) Exclusion criteria: age > 24 hours, major congenital anomalies, infants diagnosed with infection in the first 24 hours of life or born to mothers with active infection, parental refusal, mothers not speaking English, mothers not wishing to breastfeed Setting: The Woman’s Hospital of Texas, Houston, Texas, USA. From August 2011 to January 2012 |
|
| Interventions | Oral care (gentle swirl of swab along inside of mouth ‐ wiping cheeks, tongue, palate, and lips every 3 to 6 hours, or more often as indicated) using colostrum/human milk (intervention) or sterile water (control A) or normal saline (control B) | |
| Outcomes | Oral colonisation (oral culture) and time to oral colonisation Necrotising enterocolitis (diagnostic criteria not specified) Ventilator‐associated pneumonia (diagnosed by increasing oxygen or ventilatory requirement and X‐ray changes) Duration of antibiotics (days) Days to reach full enteral feeds Length of hospital stay (days) Chronic lung disease (diagnostic criteria not specified) |
|
| Funding source | The Woman’s Hospital of Texas, USA | |
| Notes | No protocol was available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Once consent was signed, a study envelope was drawn for randomization and a study number was assigned" No other detail of the randomisation methods were given |
| Allocation concealment (selection bias) | Low risk | "by drawing an envelope with the designation sealed inside" and "Once consent was signed, a study envelope was drawn for randomization and a study number was assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded "Staff were informed whether the subject was assigned as control group A (sterile water) or B (normal saline) or group C (treatment: colostrum/human milk), assigned group was documented on the patient kardex in the chart and a sign placed on the infants chart to indicate study participation and the assigned group" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded "Staff were informed whether the subject was assigned as control group A (sterile water) or B (normal saline) or Group C (treatment: colostrum/human milk), assigned group was documented on the patient kardex in the chart and a sign placed on the infants chart to indicate study participation and the assigned group" |
| Blinding of outcome assessment (detection bias) Death before discharge to home | Low risk | The study was not blinded, but death is unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three infants were still in the hospital when the study was completed and were not included in the analysis |
| Incomplete outcome data (attrition bias) Length of hospital stay | High risk | Three infants were still in the hospital when the study was completed and were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
NCT02912585.
| Methods | Double‐blinded, randomised, placebo‐controlled trial | |
| Participants | 149 infants (81 intervention and 68 control) Inclusion criteria: birth weight < 1500 grams, gestational age < 34 weeks Exclusion criteria: congenital anomalies, gastrointestinal disorders, maternal history of substance abuse, positive HIV status Setting: NICU, Clinics Hospital of Federal University of Uberlandia, Minas Gerais, Uberlandia, Brazil. From 15 July 2013 to 15 July 2015 |
|
| Interventions | Oropharyngeal administration of own mother's colostrum (intervention) vs sterile water (control) using the same protocol as in Rodriguez 2011 | |
| Outcomes | Incidence of proven sepsis (late‐onset sepsis) defined as bacterial growth in blood culture in a neonate with signs of clinical sepsis Serum and urine IgA levels Death before discharge Necrotising enterocolitis (Bell's stage 2 or 3) Bronchopulmonary dysplasia (diagnostic criteria not specified) Retinopathy of prematurity (grade 3) Length of hospital stay (days) |
|
| Funding source | Supported by The Minas Gerais State Research Support Foundation (FAPEMIG), Brazil | |
| Notes | This study is not published yet but has been submitted for publication by the Journal of Pediatrics. Results and further information provided by Dr Daniela Marques de Lima Mota Ferreira (April 2017) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generation. Additional information provided by study author: "a randomization schedule of 30 infants by computer considering 15 number 1 (colostrum group) and 15 number 2 (placebo group)" |
| Allocation concealment (selection bias) | Unclear risk | No details were provided regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study reported: "Clinical and research staff remained unaware of study group assignments, except one independent research member, from the human milk bank, who prepared the colostrum and placebo syringes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study reported: "Clinical and research staff remained unaware of study group assignments, except one independent research member, from the human milk bank, who prepared the colostrum and placebo syringes" |
| Blinding of outcome assessment (detection bias) Death before discharge to home | Low risk | The study reported: "Clinical and research staff remained unaware of study group assignments, except one independent research member, from the human milk bank, who prepared the colostrum and placebo syringes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study reported: "Thirty two randomized to colostrum group were excluded because own mother’s colostrum were not available" These 32 infants were excluded from the final analysis of data. Additional information regarding this group was provided by the study author, and intention‐to‐treat analysis was conducted in the meta‐analysis |
| Incomplete outcome data (attrition bias) Length of hospital stay | Low risk | The study reported: "Thirty two randomized to colostrum group were excluded because own mother’s colostrum were not available" These 32 infants were excluded from the final analysis of data. Additional information regarding this group was provided by the study author, and intention‐to‐treat was conducted in the meta‐analysis |
| Selective reporting (reporting bias) | Unclear risk | The trial has not been published. Data have been provided by the trial’s investigator on request. Pre‐specified primary outcomes within the study protocol were reported (protocol was available at clinicaltrials.gov: NCT01776268). Secondary outcomes were not listed in the protocol |
Rodriguez 2011.
| Methods | Blinded, placebo‐controlled, randomised, controlled trial | |
| Participants | 16 infants (9 intervention, 7 control) Inclusion criteria: birth weight < 1000 grams and/or gestation < 28 weeks; appropriate weight for gestational age Exclusion criteria: presence of congenital anomalies, gastrointestinal or renal disorders, receipt of vasopressor medications at a dosage > 10 mcg/kg/min, maternal chorioamnionitis, history of substance abuse, positive HIV status Setting: Level III neonatal unit, NorthShore University Hospital, Chicago, Illinois, USA |
|
| Interventions | 0.2 mL of own mother's colostrum (intervention) vs sterile water (control). Using a syringe, 0.1 mL was administrated by placing the tip of the syringe into the infant’s mouth, alongside the right buccal cavity, and directing it posteriorly towards the oropharynx over a period of at least 2 minutes. An additional 0.1 mL was administered similarly on the left side. The procedure was started within 48 hours of life and was carried out every 2 hours for 48 consecutive hours |
|
| Outcomes | Secretory immunoglobulin A, lactoferrin, and interleukin‐10 Necrotising enterocolitis (diagnostic criteria not specified) Chronic lung disease (diagnostic criteria not specified) Death before discharge Pneumonia and bacteraemia Retinopathy of prematurity (data provided by Dr Nancy Rodreiguez; diagnostic criteria not specified) Days to full enteral feeds, days to full per oral feeds, and days to full per oral feeds since start of enteral feeds Length of hospital stay (days) Corrected gestational age at discharge (weeks) |
|
| Funding source | This study was funded by the National Institutes of Health and by Medela, Inc., USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Additional information was provided by the investigator: "we used a simple randomization process (as described in Nursing Research; Principles and Methods. Polit & Hungler 1987 Chapter 8. Experiments and quasi‐experiments, p 123). Random assignment was carried out by writing the word "experimental" on nine slips of paper and the word "control" on 9 additional pieces of paper. The eighteen pieces of paper were placed in a box. On a separate piece of paper, the numbers one through eighteen were listed. The box was shaken, and then each of these pieces of paper [was] withdrawn, one at a time, and placed next to a number in consecutive order. The final list of eighteen numbers, which would correspond to the eighteen potential subjects, had either "experimental" or "control" written next to the number, which would correspond to the group assignment for each potential subject. The pieces of paper were withdrawn from the box by the principal investigator with a research nurse serving as a witness. The randomization process took place before any subjects were enrolled in the study" |
| Allocation concealment (selection bias) | Low risk | The randomisation process took place before any participants were enrolled in the study "This list of randomized group assignment was placed in a locked cabinet in a locked room" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "were covered with an opaque tape as a blinding procedure" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "were covered with an opaque tape as a blinding procedure" and "specimens were collected after the syringes were prepared" |
| Blinding of outcome assessment (detection bias) Death before discharge to home | Low risk | The study was blinded, and death is unlikely is to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study author provided us with additional information, and intention‐to‐treat analysis was conducted in this review |
| Incomplete outcome data (attrition bias) Length of hospital stay | Low risk | One control infant was excluded as this infant received colostrum in error, and data for 2 were excluded rather than used in an intention‐to‐treat analysis. The study author provided us with additional information regarding that infant, and intention‐to‐treat analysis was conducted in this review |
| Selective reporting (reporting bias) | Unclear risk | Outcomes described in the methods were reported in the results. The study protocol is not available |
Romano‐Keeler 2016.
| Methods | Open‐label, randomised, controlled trial | |
| Participants | 99 infants (48 intervention and 51 control) Inclusion criteria: gestational age < 32 weeks Exclusion criteria: refusal to participate, enrolment in competing studies, Spanish‐speaking only Setting: NICU, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tennessee, USA From February 2013 to July 2014 |
|
| Interventions | Oral priming with mother's colostrum ‐ 0.1 mL colostrum administered to each cheek every 6 hours for 5 days started in the first 48 hours of life (intervention) compared with no oral priming with mother's colostrum (control) | |
| Outcomes | Salivary immuno‐peptides and oral microbiota Necrotising enterocolitis (Bell's stage 2 or 3) Late‐onset bacteraemia Days to enteral feeds at 100 mL/kg/d Days of antimicrobial exposure Length of hospital stay (days) Type of feeds at discharge |
|
| Funding source | Vanderbilt University Medical Center and Thrasher Fund and NIH/NIGMS (GM106143 (to JLW)), USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "preterm infants (< 32 weeks' completed gestation) were randomised by a numeric list generated a priori to receive oral priming with mother’s colostrum or no oral priming" |
| Allocation concealment (selection bias) | Unclear risk | No details were provided regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded "Participating staff members were not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded "Participating staff members were not blinded", and assessors were not described so presumably were also part of the clinical team |
| Blinding of outcome assessment (detection bias) Death before discharge to home | Low risk | The study was not blinded, but death is unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised infants were included in the final analysis |
| Incomplete outcome data (attrition bias) Length of hospital stay | Low risk | Length of hospital stay was available for all participants |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results. The protocol is available at clinicaltrials.gov (NCT01776268) |
Sohn 2015.
| Methods | Open‐label, randomised, controlled trial | |
| Participants | 12 participants (6 intervention and 6 control) Inclusion criteria: birth weight < 1500 grams, age < 7 days, intubated within 48 hours of birth, maternal colostrum available Exclusion criteria: a lethal medical condition Setting: NICU, University of California Davis Children’s Hospital, Sacramento, California, USA. From November 2013 to October 2014 |
|
| Interventions | 0.2 mL of the mother’s colostrum via sterile syringe into the baby’s oral cavity (0.1 mL into each buccal pouch) every 2 hours for 46 hours. The comparison group received routine care | |
| Outcomes | Oral microbiota Necrotising enterocolitis (Bell's stage 2 or 3) Days to full enteral feeding Duration of antibiotics (days) Ventilator‐associated pneumonia (diagnosed by positive culture on endotracheal aspirate) Other pneumonia Chronic lung disease (defined as oxygen requirement at 36 weeks' corrected gestational age, or discharge, if sooner) Early‐ and late‐onset sepsis (diagnosed by positive blood cultures) Death before discharge |
|
| Funding source | University of California Davis, USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "neonates were randomly assigned to the colostrum group", but no details were given on how this was done |
| Allocation concealment (selection bias) | Low risk | "neonates were randomly assigned to the colostrum group using sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) Death before discharge to home | Low risk | The study was not blinded, but death is unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised infants were included in the final analysis |
| Incomplete outcome data (attrition bias) Length of hospital stay | Low risk | This outcome was not reported in this study |
| Selective reporting (reporting bias) | Low risk | The study protocol was available at clinicaltrials.gov (NCT02306980). The pre‐specified and expected outcomes of interest were reported for all participants, except length of stay, which was not reported |
IgA: immunoglobulin A.
NCPAP: nasal continuous positive airway pressure.
NICU: neonatal intensive care unit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2016 | This study was found to be a non‐randomised trial upon translation of the report from Spanish |
| Lee 2015 | The mean age at which the study intervention was started was > 48 hours Double‐blind, placebo‐controlled, randomised controlled trial conducted in the NICU, Seoul National University Children's Hospital, in Seoul, Korea, including 48 infants born at < 28 weeks' gestation. Every 3 hours for 72 hours, 0.2 mL of colostrum or placebo (distilled water) was administered via the oropharyngeal route using opaque syringes. Reported outcomes included necrotising enterocolitis (defined as Bell's stage 2 or 3), which occurred in 4 of 24 in the colostrum group and in 6 of 24 in the control group; and proven sepsis, which occurred in 11 of 24 in the intervention group and in 14 of 24 in the control group. Three of the 24 infants in the intervention group died as compared to 6 of 24 in the control group. However, this study is not included in the review, as the study intervention was given after 48 hours of age to most participants |
| Montgomery 2008 | This was an observational study conducted to determine the feasibility of oropharyngeal administration to very low birth weight infants |
| Zhang 2017 | The mean age at which the study intervention was started was > 48 hours in both groups Double‐blind, placebo‐controlled, randomised controlled trial conducted in the NICU, Children's Hospital, Fudan University, in Shanghai, China. The study included infants with birth weight < 1500 grams who were transferred into the hospital within 24 hours after birth and parents who agreed to provide colostrum. Infants with a life‐threatening condition and any disease that influenced enteral feeding were excluded. Those in the intervention group received 0.1 mL of colostrum in each side of the cheek, and those in the control group received normal saline in a similar manner. Outcomes reported included necrotising enterocolitis (defined as Bell's stage 2 or 3) and late‐onset sepsis (defined as "bacterial growth occurred in at least one blood culture and symptoms of clinical sepsis"). NEC was reported in 1 out of 32 infants in the intervention group and in 5 out of 32 in the control group, and late‐onset sepsis occurred in 4 out of 32 in the intervention group and in 8 out of 32 in the control group. However, this study was excluded from the review as infants in both groups received the first dose of study medication (colostrum or normal saline) after 48 hours of life |
NEC: necrotising enterocolitis.
NICU: neonatal intensive care unit.
Characteristics of ongoing studies [ordered by study ID]
JPRN‐UMIN000022923.
| Trial name or title | The effect of oropharyngeal colostrum administration in preterm infants |
| Methods | Parallel, double‐blinded, randomised controlled trial |
| Participants | Inclusion criteria: neonates born before 32 weeks' gestation Exclusion criteria: infants who cannot obtain colostrum within 72 hours, infants with congenital malformations, infants with chromosomal abnormalities Setting: neonatal unit of Okayama University Hospital Pediatrics, Okayama, Japan |
| Interventions | Intervention: 0.2 mL of mother's colostrum administered from 72 hours after birth every 3 hours for 120 hours Control: similar administration of saline |
| Outcomes | Serum IgA, weight gain, length of hospital stay, carriage rate of MRSA, septicaemia |
| Starting date | Not yet recruiting |
| Contact information | Dr Yoshuke Wahio, 2‐5‐1,Shikatacho, Kitaku Okayamcity, Okayama, 700‐8558, Japan email: wxy‐kk@hotmail.co.jp |
| Notes |
Rodriguez 2015b.
| Trial name or title | Oropharyngeal administration of mother’s colostrum, health outcomes of preterm infants: study protocol for a randomised controlled trial |
| Methods | Prospective, multi‐centre, double‐blind, placebo‐controlled, randomised trial |
| Participants | Extremely preterm infants with birth weight < 1250 grams, within the infant’s first 96 hours of life Setting: 5 NICUs in hospitals within the United States 1. NorthShore University HealthSystem, Evanston, Illinois 2. Betty H. Cameron Women & Children's Hospital, New Hanover Regional Medical Center, Wilmington, North Carolina 3. St. Joseph's Children's Hospital, Paterson, New Jersey 4. Advocate Children's Hospital, Park Ridge, Illinois 5. Morristown Medical Center, Morristown, New Jersey Estimated enrolment: 622 participants, with an estimated dropout rate of 20%, allowing completed analysis of 489 participants |
| Interventions | Intervention group: oropharyngeal administration of mother's colostrum 1. Initial treatment with 0.2 mL of colostrum administered per dose followed by buccal swabbing for 10 seconds. The procedure is repeated 2‐hourly for 48 hours 2. Extended treatment period beginning immediately after completion of initial treatment. The procedure is repeated every 3 hours until the infant reaches 32 weeks' corrected gestational age Comparison: sterile water given by the same procedure for colostrum |
| Outcomes |
Primary outcomes 1. Late‐onset sepsis defined as new onset of at least 2 clinical symptoms with a positive blood culture (noted after day of life 3) and identification of an organism known to be a cause of sepsis rather than a contaminant 2. NEC defined according to modified Bell's criteria stage > 2 with clinical signs and radiological evidence including any of the following: pneumatosis intestinalis or portal venous gas with or without pneumoperitoneum 3. Death Secondary outcomes Faecal microbiota, urinary biomarker of oxidative stress, concentrations of urinary lactoferrin |
| Starting date | November 2013 |
| Contact information | Nancy Rodriguez; nrodriguez@northshore.org NorthShore University HealthSystem, Evanston, IL, USA |
| Notes | This trial is actively recruiting |
IgA: immunoglobulin A.
MRSA: methicillin‐resistant Staphylococcus aureus.
NICU: neonatal intensive care unit.
Contributions of authors
Dr Nasuf wrote the protocol, and Drs Dorling and Ojha reviewed and edited it. Drs Nasuf and Ojha independently determined the eligibility of identified studies, assessed the methodological quality of included trials, and extracted and analysed the data. Dr Nasuf entered the data into RevMan, created the SoF table, wrote to study authors for additional information, obtained additional data from the included studies, and updated the search. Drs Nasuf and Ojha wrote the manuscript. Dr Dorling checked data extraction, resolved differences in opinion, and reviewed and edited the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded by a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
The review authors have no conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Glass 2017 {published and unpublished data}
- Glass KM, Doheny K. Oral care with colostrum increases salivary secretory IGA in very low birth weight infants [Oropharyngeal colostrum for immune stimulation in very low birth weight infants]. Pediatric Academic Societies (PAS) Annual Meeting; 2015 April 25‐28; San Diego (CA). 2015.
- Glass KM, Greecher CP, Doheny KK. Oropharyngeal administration of colostrum increases salivary secretory IgA levels in very low‐birth‐weight infants. American Journal of Perinatology 2017;34(14):1389‐95. [DOI: 10.1055/s-0037-1603655; PUBMED: 28575910] [DOI] [PMC free article] [PubMed] [Google Scholar]
McFadden 2012 {published and unpublished data}
- McFadden B. Oral colonization in the preterm neonate: effect of oral care [Dissertation]. Denton, Texas: Texas Woman's University, 2012. [Available from ProQuest Dissertations & Theses A&I. (1336635115)] [Google Scholar]
- McFadden BE. Oral colonization in the preterm neonate: effect of oral care. 41st Biennial Convention; 2011 10/29‐11/2; Grapevine(TX). 2011. [https://stti.confex.com/stti/bc41/webprogram/Paper49723.html]
NCT02912585 {unpublished data only}
- NCT02912585. Oropharyngeal administration of colostrum [Oropharyngeal administration of colostrum and prevention of infections in very low birthweight infants]. clinicaltrials.gov/show/NCT02912585 (first received 27 July 2016).
Rodriguez 2011 {published data only}
- Rodriguez NA. A randomized, placebo‐controlled study of the oropharyngeal administration of own mother's colostrum during the first days of life: effects on immune function of extremely low birth weight infants [PhD dissertation]. Chicago (IL): Rush University, 2008. [Google Scholar]
- Rodriguez NA, Groer MW, Zeller JM, Engstrom JL, Fogg L, Du H, et al. A randomized controlled trial of the oropharyngeal administration of mother’s colostrum to extremely low birth weight infants in the first days of life. Neonatal Intensive Care 2011;24(4):31‐35. [Google Scholar]
Romano‐Keeler 2016 {published and unpublished data}
- Romano‐Keeler J, Azcarate‐Peril MA, Weitkamp JH, Slaughter JC, McDonald WH, Meng S, et al. Oral colostrum priming shortens hospitalization without changing the immunomicrobial milieu. Journal of Perinatology 2017;37(1):36‐41. [DOI: 10.1038/jp.2016.161; PUBMED: 27684425] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sohn 2015 {published data only}
- Sohn K, Kalanetra KM, Mills DA, Underwood MA. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. Journal of Perinatology 2016;36(2):106‐11. [DOI: 10.1038/jp.2015.157; PUBMED: 26658119] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 2016 {published data only}
- Alvarez EM, Cabanillas MVJ, Caballero M, Lozano JM. Effect of oropharyngeal colostrum administration on immunoglobulin A levels in preterm newborns [Efectos de la administración de calostro orofaríngeo en recién nacidos prematuros sobre los niveles de inmunoglobulina A]. Nutricion Hospitalaria 2016;33(2):232‐8. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics 2015;135(2):e357‐66. [DOI: 10.1542/peds.2014-2004; PUBMED: 25624376] [DOI] [PubMed] [Google Scholar]
Montgomery 2008 {published data only}
- Montgomery D, Schmutz N, Baer VL, Rogerson R, Wheeler R, Rowley AM, et al. Effects of instituting the "BEST Program" (Breast Milk Early Saves Trouble) in a level III NICU. Journal of Human Lactation 2008;24(3):248‐51. [10.1177/0890334408316080; PUBMED: 18689711] [DOI] [PubMed] [Google Scholar]
Zhang 2017 {published data only}
- Zhang Y, Ji F, Hu X, Cao Y, Latour JM. Oropharyngeal colostrum administration in very low birth weight infants: a randomized controlled trial. Pediatric Critical Care Medicine 2017;18(9):869‐75. [DOI: 10.1097/pcc.0000000000001221; PUBMED: 28617764] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
JPRN‐UMIN000022923 {published data only}
- JPRN‐UMIN000022923. The effect of oropharyngeal colostrum administration in preterm infants. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000022923 (first received 29 June 2016).
Rodriguez 2015b {published data only}
- Rodriguez NA, Vento M, Claud EC, Wang CE, Caplan MS. Oropharyngeal administration of mother's colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial. Trials 2015;16(1):453. [DOI: 10.1186/s13063-015-0969-6; PUBMED: 26458907] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Araújo 2005
- Araújo ED, Gonçalves AK, Cornetta MDC, Cunha H, Cardoso ML, Morais SS, et al. Evaluation of the secretory immunoglobulin A levels in the colostrum and milk of mothers of term and pre‐term newborns. Brazilian Journal of Infectious Diseases 2005;9(5):357‐62. [PUBMED: 16410886] [PubMed] [Google Scholar]
Ballard 2013
- Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatric Clinics of North America 2013;60(1):49‐74. [DOI: 10.1016/j.pcl.2012.10.002; PUBMED: 23178060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2009
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010; Vol. 88, issue 1:31‐8. [DOI: 10.2471/BLT.08.062554; PUBMED: 20428351] [DOI] [PMC free article] [PubMed]
Bisquera 2002
- Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics 2002;109(3):423‐8. [PUBMED: 11875136] [DOI] [PubMed] [Google Scholar]
Blencowe 2012
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379(9832):2128‐72. [DOI: 10.1016/S0140-6736(12)60820-4; PUBMED: 22682464] [DOI] [PubMed] [Google Scholar]
Blencowe 2013
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reproductive Health 2013;10(Suppl 1):S2. [DOI: 10.1186/1742-4755-10-S1-S2; PUBMED: 24625129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2002
- Chang CJ, Chao JC. Effect of human milk and epidermal growth factor on growth of human intestinal Caco‐2 cells. Journal of Pediatric Gastroenterology and Nutrition 2002;34(4):394‐401. [PUBMED: 11930096] [PubMed] [Google Scholar]
Chirico 2008
- Chirico G, Marzollo R, Cortinovis S, Fonte C, Gasparoni A. Antiinfective properties of human milk. Journal of Nutrition 2008;138(9):1801S‐6S. [PUBMED: 18716190] [DOI] [PubMed] [Google Scholar]
Gephart 2014
- Gephart SM, Weller M. Colostrum as oral immune therapy to promote neonatal health. Advances in Neonatal Care 2014;14(1):44‐51. [DOI: 10.1097/ANC.0000000000000052; PUBMED: 24472888] [DOI] [PubMed] [Google Scholar]
Goldenberg 2008
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371(9606):75‐84. [DOI: 10.1016/S0140-6736(08)60074-4; PUBMED: 18177778] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 05 February 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2017
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Hunter 2008
- Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatric Research 2008;63(2):117‐23. [DOI: 10.1203/PDR.0b013e31815ed64c; PUBMED: 18091350] [DOI] [PubMed] [Google Scholar]
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI: 10.1001/archopht.123.7.991; PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
Johnson 2013
- Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. The cost of morbidities in very low birth weight infants. Journal of Pediatrics 2013;162(2):243‐9. [DOI: 10.1016/j.jpeds.2012.07.013; PUBMED: 22910099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montagne 1999
- Montagne P, Cuillière ML, Molé C, Béné MC, Faure G. Immunological and nutritional composition of human milk in relation to prematurity and mother's parity during the first 2 weeks of lactation. Journal of Pediatric Gastroenterology and Nutrition 1999;29(1):75‐80. [PUBMED: 10400108] [DOI] [PubMed] [Google Scholar]
Radillo 2013
- Radillo O, Norcio A, Addobbati R, Zauli G. Presence of CTAK/CCL27, MCP‐3/CCL7 and LIF in human colostrum and breast milk. Cytokine 2013;61(1):26‐8. [DOI: 10.1016/j.cyto.2012.09.001; PUBMED: 23040056] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez 2009
- Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. Journal of Perinatology 2009;29(1):1‐7. [DOI: 10.1038/jp.2008.130; PUBMED: 18769379] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2010
- Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother's colostrum to extremely low‐birth‐weight infants. Advances in Neonatal Care 2010;10(4):206‐12. [DOI: 10.1097/ANC.0b013e3181e94133; PUBMED: 20697221] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2015a
- Rodriguez NA, Caplan MS. Oropharyngeal administration of mother's milk to prevent necrotizing enterocolitis in extremely low‐birth‐weight infants: theoretical perspectives. Journal of Perinatal & Neonatal Nursing 2015;29(1):81‐90. [DOI: 10.1097/jpn.0000000000000087; PUBMED: 25633403] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seigel 2013
- Seigel JK, Smith PB, Ashley PL, Cotten CM, Herbert CC, King BA, et al. Early administration of oropharyngeal colostrum to extremely low birth weight infants. Breastfeeding Medicine 2013;8(6):491‐95. [DOI: 10.1089/bfm.2013.0025; PUBMED: 23805944] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slattery 2002
- Slattery MM, Morrison JJ. Preterm delivery. Lancet 2002;360(9344):1489‐97. [DOI: 10.1016/S0140-6736(02)11476-0; PUBMED: 12433531] [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285‐91. [PUBMED: 12165580] [DOI] [PubMed] [Google Scholar]
Stoll 2004
- Stoll BJ, Hansen NI, Adams‐Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low‐birth‐weight infants with neonatal infection. JAMA 2004;292(19):2357‐65. [DOI: 10.1001/jama.292.19.2357; PUBMED: 15547163] [DOI] [PubMed] [Google Scholar]
Thompson 2008
- Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs 2008;68(9):1227‐38. [PUBMED: 18547133] [DOI] [PubMed] [Google Scholar]
UN IGME 2017
- UNICEF, WHO, World Bank, UN‐DESA Population Division. Estimates Developed by the UN Inter‐agency Group for Child Mortality Estimation. Levels & Trends in Child Mortality Report 2017. who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2017/en/ 2017. Accessed July 2017.
Ustundag 2005
- Ustundag B, Yilmaz E, Dogan Y, Akarsu S, Canatan H, Halifeoglu I, et al. Levels of cytokines (IL‐1beta, IL‐2, IL‐6, IL‐8, TNF‐alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediators of Inflammation 2005;2005(6):331‐6. [PUBMED: 16489252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2010
- Walker A. Breast milk as the gold standard for protective nutrients. Journal of Pediatrics 2010;156(2 Suppl):S3‐7. [DOI: 10.1016/j.jpeds.2009.11.021; PUBMED: 20105662] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179‐201. [PUBMED: 3081865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westerbeek et al 2006
- Westerbeek EA, Berg A, Lafeber HN, Knol J, Fetter WP, Elburg RM. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clinical Nutrition 2006;25(3):361‐8. [DOI: 10.1016/j.clnu.2006.03.002; PUBMED: 16677741] [DOI] [PubMed] [Google Scholar]
Wheeler 2007
- Wheeler TT, Hodgkinson AJ, Prosser CG, Davis SR. Immune components of colostrum and milk ‐ a historical perspective. Journal of Mammary Gland Biology and Neoplasia 2007;12(4):237‐47. [DOI: 10.1007/s10911-007-9051-7; PUBMED: 17992474] [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization, March of Dimes, The Partnership for Maternal, Newborn & Child Health, Save the Children. Born too soon: the global action report on preterm birth. who.int/maternal_child_adolescent/documents/born_too_soon/en/ 2012. Accessed May 2015.
References to other published versions of this review
Nasuf 2015
- Nasuf AW, Ojha S, Dorling J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants (Protocol). Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2015, issue 10. [DOI: 10.1002/14651858.CD011921; CD011921] [DOI] [PMC free article] [PubMed]


