Abstract
Background
Beta thalassaemia is a common inherited blood disorder. The need for frequent blood transfusions in this condition poses a difficult problem to healthcare systems. The most common cause of morbidity and mortality is cardiac dysfunction from iron overload. The use of iron chelation therapy has reduced the severity of systemic iron overload but specific, non‐toxic treatment is required for removal of iron from the myocardium.
Objectives
To assess the effects of calcium channel blockers combined with standard iron chelation therapy in people with transfusion‐dependent beta thalassaemia on the amount of iron deposited in the myocardium, on parameters of heart function, and on the incidence of severe heart failure or arrhythmias and related morbidity and mortality.
Search methods
We searched the Cochrane Haemoglobinopathies Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched ongoing trials databases, and the reference lists of relevant articles and reviews.
Date of last search: 24 February 2018.
Selection criteria
We included randomised controlled trials of calcium channel blockers combined with standard chelation therapy compared with standard chelation therapy alone or combined with placebo in people with transfusion‐dependent beta thalassaemia.
Data collection and analysis
Two authors independently applied the inclusion criteria for the selection of trials. Two authors assessed the risk of bias of trials and extracted data and a third author verified these assessments. The authors used the GRADE system to assess the quality of the evidence.
Main results
Two randomised controlled trials (n = 74) were included in the review; there were 35 participants in the amlodipine arms and 39 in the control arms. The mean age of participants was 24.4 years with a standard deviation of 8.5 years. There was comparable participation from both genders. Overall, the risk of bias in included trials was low. The quality of the evidence ranged across outcomes from low to high, but the evidence for most outcomes was judged to be low quality.
Cardiac iron assessment, as measured by heart T2*, did not significantly improve in the amlodipine groups compared to the control groups at six or 12 months (low‐quality evidence). However, myocardial iron concentration decreased significantly in the amlodipine groups compared to the control groups at both six months, mean difference ‐0.23 mg/g (95% confidence interval ‐0.07 to ‐0.39), and 12 months, mean difference ‐0.25 mg/g (95% confidence interval ‐0.44 to ‐0.05) (low‐quality evidence). There were no significant differences between treatment and control groups in serum ferritin (low‐quality evidence), liver T2* (low‐quality evidence), liver iron content (low‐quality evidence) and left ventricular ejection fraction (low‐quality evidence). There were no serious adverse events reported in either trial; however, one trial (n = 59) reported mild adverse events, with no statistically significant difference between groups (low‐quality evidence).
Authors' conclusions
The available evidence does not clearly suggest that the use of calcium channel blockers is associated with a reduction in myocardial iron in people with transfusion‐dependent beta thalassaemia, although a potential for this was seen. There is a need for more long‐term, multicentre trials to assess the efficacy and safety of calcium channel blockers for myocardial iron overload, especially in younger children. Future trials should be designed to compare commonly used iron chelation drugs with the addition of calcium channel blockers to investigate the potential interplay of these treatments. In addition, the role of baseline myocardial iron content in affecting the response to calcium channel blockers should be investigated. An analysis of the cost‐effectiveness of the treatment is also required.
Plain language summary
Calcium channel blockers for preventing heart dysfunction related with iron overload in transfusion‐dependent beta thalassaemia
Review question
We reviewed the evidence on whether drugs can prevent heart dysfunction due to excessive iron deposits in the hearts of people with beta thalassaemia who receive regular blood transfusions.
Background
Beta thalassaemia is a common inherited blood disorder that causes anaemia. People with this disorder need frequent blood transfusions which result in excess iron deposited in the heart causing it to be damaged. Complications related to the heart are the most common cause of death and disability in these individuals. High levels of iron in the heart strongly predicts subsequent heart failure. It is common practice to give drugs to reduce iron in the body (known as chelators) but there is no specific treatment for reducing iron deposited in the heart and protecting it from damage. Drugs that block calcium channels in the heart have been shown to reduce iron entering into this organ. However, little is known about how effective and safe these drugs are in people with beta thalassaemia.
Search date
The evidence is current to 24 February 2018.
Trial characteristics
We included two trials (74 participants) in the review, an earlier pilot study and the later larger study. Participants had beta thalassaemia, significant iron overload, and were receiving standard chelation treatment; they had an average age of 24 years. They were randomly selected to be treated for 12 months with either amlodipine (a calcium channel blocker) in addition to their chelation drugs or with chelation drugs alone (in the earlier trial) or with chelation drugs together with a placebo (dummy drug with no active medication) in the later trial.
Key results
Although there was no significant decrease in the amount of iron in the heart (main outcome) seen after 12 months of treatment with amlodipine when measured in one way, there was a significant decrease after 12 months of treatment using another measurement. There was no difference in other outcomes such as iron levels in the blood or the liver or in a further measure of heart function. Even though no serious adverse events were noted, further trials are needed to assess the safety of this treatment. Further research with larger, long‐term trials is needed.
Quality of the evidence
Overall, the trials included in this review appeared to be well run. However, the main outcome was reported differently in both trials and several other outcomes were missing details. The quality of evidence was low for all outcomes.
Summary of findings
Summary of findings for the main comparison. Amlodipine plus standard chelation therapy versus standard chelation therapy with or without placebo.
| Amlodipine compared with control for myocardial iron overload in transfusion‐dependent beta thalassaemia | ||||||
|
Patient or population: aged 6 years or older with transfusion‐dependent beta thalassaemia Settings: any Intervention: oral amlodipine 5 mg once daily (reduced to 2.5 mg once daily if adverse effects) plus standard chelation therapy Comparison: standard chelation therapy with or without placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard chelation therapy with or without placebo | Amlodipine 5 mg once daily plus standard chelation therapy | |||||
|
Mean change in cardiac T2* (msec) Follow‐up: at 12 months |
The mean absolute change in cardiac T2* across the control groups was ‐1.50 m/s. | The mean absolute change in cardiac T2* in the intervention group was 0.27 m/s lower (2.66 lower to 2.11 higher). | N/A | 74 (2 studies) |
⊕⊕⊖⊖ low1,2 |
No statistically significant difference in cardiac T2* between the amlodipine group and the control group. |
|
Mean change in MIC (mg/g) Follow‐up: at 12 months |
The mean absolute change in MIC across the control groups was ‐0.041 mg/g. | The mean absolute change in MIC in the intervention group was 0.25 mg/g lower (0.44 lower to 0.05 lower). | N/A | 74 (2 studies) |
⊕⊕⊖⊖ low1,2 |
Statistically significant decrease in MIC in the amlodipine group compared to the control group. |
|
Mean change in serum ferritin ng/mL Follow‐up: at 12 months |
The mean absolute change in serum ferritin across the control groups was ‐36.24 ng/mL. | The mean absolute change in serum ferritin in the intervention group was 247.78 ng/mL lower (741.61 lower to 246.05 higher). | N/A | 71 (2 studies) |
⊕⊕⊖⊖ low1,2 |
No statistically significant difference in serum ferritin between the amlodipine group and the control group. |
|
Mean change in liver T2* (m/s) Follow‐up: at 12 months |
The mean absolute change in liver T2* across the control groups was 0.96 m/s. | The mean absolute change in liver T2* in the intervention group was 1.24 m/s lower (2.76 lower to 0.27 higher). | N/A | 74 (2 studies) |
⊕⊕⊖⊖ low1,2 |
No statistically significant difference in liver T2* between the amlodipine group and the control group. |
|
Mean change in LIC (mg/g) Follow‐up: at 12 months |
The mean absolute change in LIC in the control group was 2.86 mg/g. | The mean absolute change in LIC in the intervention group was 2.01 mg/g lower (4.84 lower to 0.82 lower). | N/A | 59 (1 study) |
⊕⊕⊖⊖ low2, 3 |
Statistically significant decrease in LIC in the amlodipine group compared to the control group. |
|
Mean change in LVEF (%) Follow‐up: at 12 months |
The mean absolute change in LVEF cross the control groups was ‐0.63%. | The mean absolute change in LVEF in the intervention group was 0.40% lower (2.97 lower to 2.17 higher). | N/A | 72 (2 studies) |
⊕⊕⊖⊖ low1,2 |
No statistically significant difference in LVEF between the amlodipine group and the control group. |
|
Adverse events Follow‐up: at 12 months |
There were no events in the control group. | There were 4 events in the intervention group. |
RR 8.71 (95% CI 0.49 to 154.89) |
59 (1 study) |
⊕⊕⊖⊖ low2, 3 |
Amlodipine dose was reduced in 2 participants due to oedema in the lower extremities and in 1 participant due to dizziness. 1 participant discontinued amlodipine use due to a mild cutaneous allergy. There was 1 death reported due to liver cirrhosis that existed prior to trial enrolment. |
| *The authors calculated the assumed risk as the mean of the effect size of the control group in each study. The corresponding risk (and it's 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; MIC: myocardial iron concentration; LIC: liver iron concentration; LVEF: left ventricular ejection fraction. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once for risk of bias: unclear information on random sequence generation and allocation concealment and lack of blinding
2. Downgraded once due to indirectness as the results are not applicable to the wider population as both trials were conducted in Brazil, included mainly adolescents and young adults and results pertain only to the calcium channel blocker amlodipine at a dose and frequency of 5 mg once daily.
3. Downgraded once due to wide CIs so uncertainty about results.
Background
Please see the glossary for an explanation of terms used in this review (Appendix 1).
Description of the condition
Beta thalassaemia is a common inherited blood disorder that results from genetic mutations. It is associated with severe anaemia that requires frequent blood transfusions, often on a monthly or bi‐monthly basis. Beta thalassaemia trait (minor), disease (major) and beta thalassaemia of intermediate severity (Hb E) are prevalent in the Indian sub‐continent, Southeast and Central Asia, Southern China, the Mediterranean, North Africa, and the Middle East. In these regions, the World Health Organisation (WHO) estimates a carrier frequency of between 1% and 20% (Weatherall 2001). Population movements have resulted in the introduction of thalassaemia genes into Northern Europe, North America and Australia, where this disease was once very rare. Indigenous populations in Northern Europe have a thalassaemia carrier rate of 0.1% (Modell 2007), but the carrier rate in immigrant populations within European countries is much higher (e.g. 0.68% in immigrants in Belgium) (Angastiniotis 2013). However, while the number of thalassaemia cases has been declining progressively in some parts of the world such as the USA and Europe due to effective prevention methods (Cao 2002), the situation in low‐ and middle‐income countries is different. According to a public health review conducted in 2008, there are over 25,000 annual births of infants with transfusion‐dependent beta thalassaemia worldwide with most births occurring in low‐ to middle‐income countries (Modell 2008). According to the same study, more than 22,000 individuals die annually due to not receiving transfusions.
Transfusion‐dependent beta thalassaemia presents a difficult challenge to health systems, especially in low‐ and middle‐income countries. According to an estimate for the Eastern Mediterranean region, the annual cost of adequately treating an adolescent with beta thalassaemia can be around USD 6000 (Alwan 1997). Globally, only 11.7% of those who require blood transfusions receive them (Modell 2008). The problem with repeated blood transfusions is that people who receive them develop an excess of iron in their bodies. This is known as haemosiderosis or iron overload. In fact, most individuals receiving transfusions will die from iron overload unless they receive treatment with iron chelating drugs which remove the excess iron from the body (Rund 2005). Unfortunately, only 39% of those transfused people receive adequate chelation therapy (Modell 2008).
Iron overload results in an abnormal deposition of iron in tissues. Free iron in tissues leads to the generation of reactive oxygen species (ROS) that damage organs such as the liver, pancreas and heart (Livrea 1996). Increased iron uptake by myocardial cells can cause cardiomyopathy (a weakening of the heart muscle or its function), pulmonary hypertension, heart failure, arrhythmias, pericarditis and myocarditis (Buja 1971; Engle 1964; Kirk 2012). Once it is clinically obvious that heart failure is present, myocardial function may decrease rapidly, leading to death (Gujja 2010). In fact, iron overload cardiomyopathy and its related complications are responsible for nearly 70% of deaths in people with thalassaemia (Borgna‐Pignatti 2004; Charafeddine 2008; Modell 2000; Olivieri 1994). It has been shown that cardiac iron overload can occur even in children under 10 years of age, despite receiving chelation therapy (Borgna‐Pignatti 2014).
Description of the intervention
The focus of this intervention review is the use of L‐type calcium channel blockers (L‐TCC blockers) for the prevention of cardiovascular dysfunction in people with transfusion‐dependent beta thalassaemia. Oral L‐TCC blockers are drugs that are conventionally indicated for the treatment of arrhythmia (Grace 2000) and high blood pressure (Guazzi 1983) depending on whether the drugs have higher affinity for the heart (the phenylalkylamines such as verapamil), or for the blood vessels (the dihydropyridine drugs such as nifedipine or amlodipine). These drugs block voltage‐dependent Ca2+ channels in cardiac and vascular smooth muscle. Therefore, when electrical stimulation (depolarization) occurs, these drugs prevent Ca2+ entry into muscle cells in the cardiovascular system. This relaxes muscle cells in blood vessels, enlarging the vessel diameter, and causes a decrease in vessel resistance that lowers the blood pressure. Blockade of Ca2+ entry into cells also reduces the contractility of the heart (leading to reduced oxygen consumption) as well as suppressing abnormal rhythms. Based on these physiologic effects, calcium channel blockers are approved for treating hypertension and arrhythmias (Singh 1986).
Many of the known adverse reactions of calcium channel blockers are related to their vasodilatory effects, such as hypotension, flushing, dizziness, and headache (Russell 1988). Drugs classified as dihydropyridines frequently cause oedema and ankle swelling after long‐term use. The phenylalkylamine drug, verapamil, often causes constipation because of its blockade of calcium channels in intestinal smooth muscle cells. Verapamil and diltiazem are also responsible for many drug interactions with agents that are metabolized by the liver such as antibiotics (Henneman 2012), the lipid‐lowering statin drugs (Zhelyazkova‐Savova 2014) and grapefruit juice (Sica 2006). In addition, short‐acting dihydropyridine drugs, such as nifedipine, can exacerbate angina by diverting blood flow from less perfused areas of the heart (Egstrup 1993) or by the reactive activation of the sympathetic nervous system that increases heart rate and cardiac oxygen consumption (Ferguson 1989; Wenzel 1997).
The use of iron chelating drugs is standard in people with beta thalassaemia who receive blood transfusions. The goal of this therapy is to prevent iron‐mediated damage to organs such as the heart and liver. Iron chelator drugs have selectivity and affinity for Fe3+ ion that allows the formation of a stable ligand‐metal complex (Hider 2003). This mechanism effectively removes free iron from circulation. General indications for initiating chelation therapy include age greater than two to four years, more than 20 to 25 red blood cell (RBC) units transfused, a serum ferritin level greater than 1000 μg/dL and a liver iron concentration (LIC) greater than 3 mg iron/gm dry weight as measured by liver biopsy or by noninvasive hepatic T2*MRI (Rachmilewitz 2011). Chelators that are currently in use include desferrioxamine (DFO), deferiprone (DFP) and deferasirox (DFX), which are the subject of other Cochrane Reviews (Fisher 2013a; Fisher 2013b; Meerpohl 2017). Administration of DFO is through the subcutaneous or intravenous route. This drug used to be the standard of care for nearly 40 years until it fell out of favour due to its cost, difficult administration and significant side effects such as rash, heart failure and haematologic toxicity (Alpendurada 2012; Cappellini 2007). The orally administered agent DFP was introduced in the 1990s with the hope that it would have better efficacy and safety than DFO. However, serious side effects such as neutropenia and agranulocytosis have been reported with DFP, which warrant weekly blood counts that pose an inconvenience for patients (Cohen 2003). In 2005, DFX was introduced as a once‐daily oral iron chelator. The adverse effect profile of DFX is somewhat better than previous agents (Cappellini 2006), although further research is needed to define its role in reducing cardiac iron (Kwiatkowski 2011).
How the intervention might work
There is some understanding of how free iron damages the heart. Several mechanisms have been proposed to explain how this damage is caused (Hershko 2010; Murphy 2010), including damage to cell membranes by lipid peroxidation, mitochondrial damage and disruption of the electron transport chain (Link 1994; Link 2001), interference with electrical function, including ryanodine release channel interference (Kim 1995; Wood 2005), promotion of cardiac fibrosis (Buja 1971), and altered gene expression (Eaton 2002). It has been shown that non‐protein bound or free iron may be rapidly taken up by isolated myocardial cells (Link 1985). However, the mechanism by which iron enters the myocardial cells in the first place is not clearly understood. Growing evidence from animal studies suggests the use of certain ion channels for this purpose. Reports indicate that the uptake of ferrous iron (Fe2+) may take place through L‐TCCs (Oudit 2006; Tsushima 1999), divalent metal transporter 1 (DMT1) (Ludwiczek 2007; Oudit 2003) and T‐type calcium channels (Kumfu 2012). A potential separate pathway for uptake of ferric (Fe3+) ions may exist (Kumfu 2013). The role of calcium channels in iron uptake by myocardial cells was further explained by one study in which researchers showed that iron uptake by a rat heart was increased by the L‐TCC agonist, Bay K 8644 and was inhibited by the L‐TCC blocker, nifedipine (Tsushima 1999). Furthermore, Oudit demonstrated that treatment with L‐TCC blockers such as amlodipine and verapamil could lead to the inhibition of L‐TCC current in myocardial cells as well as decreased oxidative stress and improved survival in iron‐loaded mice (Oudit 2003). Based on this evidence, it may be reasonable to suspect that drugs that block L‐TCCs in the heart may reduce the uptake of iron by heart cells and delay or prevent iron overload‐related damage.
Why it is important to do this review
Beta thalassaemia is a common inherited blood disorder particularly in low‐ and middle‐income countries. In people with this condition, iron overload‐related cardiomyopathy and heart failure is a major cause of morbidity and mortality. Although it has been shown by some researchers that chelating agents can significantly improve myocardial iron content and left ventricular function (Cassinerio 2012; Maggio 2012), these drugs are expensive and have toxic side effects (Xia 2013). It is important to evaluate the role of interventions, such as calcium channel blockers, that may be used as adjuvants with chelation therapy for the prevention of iron deposition in the heart. There are no guidelines or recommendations for the use of calcium channel blockers in beta thalassaemia. Therefore, we were motivated to conduct a systematic review of randomised controlled trials (RCTs) to benefit the care of people with beta thalassaemia by reviewing the evidence on the effects of calcium channel blockers on myocardial iron deposition.
Objectives
To assess the effects of calcium channel blockers combined with standard iron chelation therapy in people with transfusion‐dependent beta thalassaemia on the amount of iron deposited in the myocardium, on parameters of heart function, and on the incidence of severe heart failure or arrhythmias and related morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs were considered for this review.
Types of participants
We included trials of participants with beta thalassaemia (six years of age and older) with a constant transfusion need, and who have been receiving regular chelation therapy for at least one year prior to enrolment in the trial. We excluded trials of people with alpha thalassaemia or beta thalassaemia without a constant transfusion need, other iron overload conditions, trials of children under six years of age, or those individuals not receiving regular chelation therapy with recommended drugs of adequate doses for at least one year prior to enrolment in the trial. Any trial setting (health‐care centre or community) was considered appropriate for inclusion.
Types of interventions
Experimental intervention
Calcium channel blockers with standard chelation therapy. We included trials in which calcium channel blockers were regularly and consistently administered according to a pre‐determined dosage and frequency (at least once daily) along with standard chelation therapy. The minimum dosage used was at least equal to the manufacturer's suggested minimum dosage for anti‐hypertensive purposes and the duration of therapy was clearly stated (at least one month).
Comparator intervention
Standard chelation therapy alone or standard chelation therapy with placebo. The three chelating agents currently available are desferrioxamine DFO, DFP and DFX. We included trials in which participants were receiving chelation therapy (monotherapy or combination therapy) on a regular and consistent basis for at least one year before enrolment in the clinical trial. In addition, we only included trials that clearly stated cumulative dosage or its equivalent, average frequency of administration or total duration of chelation or both.
Types of outcome measures
None of the outcome measures formed part of the eligibility criteria for including trials in the review.
Primary outcomes
-
Cardiac iron assessment by
T2* cardiac magnetic resonance (CMR) measurements (m/s)
myocardial iron content (MIC) (post hoc change)
Cardiac mortality (measured as time‐to‐event)
-
Cardiac function assessments
N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and B‐type natriuretic peptide (BNP) levels
echocardiographic estimation of left ventricular ejection fraction (LVEF)
echocardiographic estimation of left and right ventricular filling pressures
estimation of LVEF by CMR
estimation of left and right ventricular volumes by CMR
estimation of left and right ventricular strain and strain rate by CMR tagging
estimation of left and right ventricular volume and function by myocardial tissue phase mapping
estimation of left and right ventricular volume and function by radionuclide ventriculography during exercise
exercise stress testing
Secondary outcomes
-
Electrocardiographic abnormalities (measured as time‐to‐event)
supraventricular arrhythmias
electrocardiographic findings that suggest right‐sided heart involvement (S1Q3 pattern and right‐axis deviation) (e.g. repolarization abnormalities and relative bradycardia)
new‐onset T‐wave inversion beyond lead V1 and a consistent decrease in QRS height
-
Measures of iron overload
serum ferritin (ng/mL)
iron levels in biopsies of liver (LIC) and other tissue (mg/g dry weight)
tissue iron assessment by superconducting quantum interference device (SQUID) (mg/g liver wet weight)
tissue (other than cardiac) iron assessment by magnetic resonance imaging (MRI) (m/s)
Mean reduction in dose of chelation therapy (mg/kg bodyweight)
Self‐reported quality of life (QoL) measured using validated instruments for heart failure such as the Chronic Heart Failure Assessment Tool, the Left Ventricular Disease Questionnaire (LVDQ), the Quality of Life in Severe Heart Failure Questionnaire, etc.
Compliance with calcium channel blocker and chelation treatment (measured by the number of people in each arm that show adequate level of adherence to treatment (intake or application of iron chelator or calcium channel blocker, or both, for five or more days per week))
-
Adverse effects of calcium channel blockers
somnolence
dizziness
headache
abdominal pain
nausea
flushing
hypotension
ankle swelling
oedema
fatigue
palpitations
unanticipated adverse events as reported in the primary studies
Cost of intervention per year
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
We identified relevant trials from the Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: (thalassaemia OR (haemoglobinopathies AND general)) AND calcium channel blocker).
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of theCochrane Library) and weekly searches of MEDLINE. Unpublished work was identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Health Research Council Meetings; and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders website.
Date of the latest search: 15 March 2018.
We also searched the following trial registries for ongoing clinical trials (last searched on 24 February 2018) (Appendix 2).
Searching other resources
Reference lists of all identified papers were screened to identify potentially relevant citations. Experts were contacted for updates on unpublished or ongoing work.
Data collection and analysis
Selection of studies
Two authors (AS and NA) independently screened all titles and abstracts of papers identified by the search strategies for relevance. At the screening stage, we excluded only clearly irrelevant citations. After we obtained the full text versions of all potentially relevant trials, two authors (AS and NA) independently assessed the eligibility of trials for inclusion. Although we had planned to resolve any disagreements on the eligibility of trials through discussion and consensus or, if necessary, through a third party (BH), no disagreements arose on the selection of trials. Four authors (AS, NA, BH and SC) are current investigators on one potentially relevant trial, but this trial does not have results available for inclusion at this time (Hasan 2014). We documented reasons for exclusion.
Data extraction and management
Two authors (AS and NA) used a standard data collection form developed at the start of the review to independently extract the data. One author (JKD) verified the data collection. In general, we extracted two sets of data as follows (in addition to the assessment of risk of bias in included trials):
trial characteristics ‐ setting, year of publication, population characteristics, characteristics of intervention, details of comparator treatments, and characteristics of outcome measures;
results ‐ primary and secondary outcomes indicated in the review question; we documented reasons when screened or included trials did not contribute data on main outcomes and considered the potential for selective reporting. One of the authors (AS) contacted primary investigators for additional data and details of the correspondences were recorded.
We resolved any disagreements on the extracted data by discussion. The eligible time periods for endpoint analysis were decided to be at least one month after calcium channel blocker treatment. However, we did not encounter any trials where the time period reported was less than six months. Therefore, we analysed outcomes at six and 12 months.
Assessment of risk of bias in included studies
Two authors (AS and NA) independently assessed the risk of bias in the included trials. We conducted the evaluation using a form designed on the domains described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). These domains include:
sequence generation (e.g. whether randomisation was adequate);
allocation concealment (e.g. whether the allocation was adequately concealed);
blinding of participants, personnel and outcome assessors;
incomplete outcome data (e.g. whether attrition and exclusion were reported);
selective outcome reporting;
other sources of bias.
Based on these domains, we categorised trials as having either a 'low', 'unclear', or 'high' risk of bias. We did not exclude any trial as a result of a rating of 'unclear' or 'high' risk of bias. Disagreements between authors during the evaluation of the risk of bias in individual trials were resolved through discussion. We have presented the evaluation of the risk of bias in the included trials in tabular form as 'Risk of bias' tables (Characteristics of included studies). Since we included only two trials in the analysis, we did not conduct a sensitivity analysis based on our risk of bias assessment.
Measures of treatment effect
We obtained individual participant data (IPD) from the principal investigator of both included trials.
We used the statistical package for social sciences (SPSS) version 16.0 to obtain the mean change from baseline at six and 12 months and the standard deviations (SDs) for continuous outcomes such as heart and liver T2* CMR measurements, MIC (post hoc change), LIC, serum ferritin, and left ventricular ejection fraction (LVEF). We tested whether data were normally distributed using the Kolmogorov‐Smirnov test. The IPD from the later trial were not normally distributed (Fernandes 2016), but the relatively large sample size was sufficient to enable the central limit theorem to hold approximately so that we may assume normality in the distribution of the means (Higgins 2008). This implies that the mean of the outcome measurements is approximately normally distributed with SD given by the standard error.
We analysed the data for these outcomes using the most up‐to‐date version of Review Manager software (Review Manager 2014) to obtain the mean difference (MD) for these outcomes with 95% confidence intervals (CIs) as measures of uncertainty.
For the continuous outcomes, we presented relative effect as MD with the 95% CIs. For the dichotomous outcome (adverse events) we presented the relative risk (RR) with the 95% CIs. For future updates of the review, we will extract hazard ratios (HR) with their 95% CIs for the time‐to‐event outcomes of cardiac mortality and new‐onset electrophysiologic abnormalities. If reports do not provide HRs, we will use indirect estimation methods described by Parmar and Williamson to calculate them (Parmer 1998; Williamson 2002).
Unit of analysis issues
There were no cluster‐randomised trials or cross‐over trials included in the current version of the review. If we identify such designs in trials that are eligible for inclusion in the future, we will proceed as follows. We will only include cross‐over trials if there is a sufficient washout period between the treatment arms. We will use methods recommended by Elbourne to analyse data from such trials using paired analysis (Elbourne 2002). In the case of cluster randomised trials, we will calculate the effective sample size and conduct analysis based on methods recommended by Donner (Donner 2002). We will consider the risk of unit of analysis error caused by repeated observations on participants as outlined in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted the principal investigator to obtain IPD for both included trials. Intention‐to‐treat analysis was not conducted since there was no significant loss‐to‐follow‐up.
In future updates, if important information is missing from the published full text of included trials or if the data are unclear, we will contact the primary investigators to obtain the necessary data.
Assessment of heterogeneity
We tested for heterogeneity between trial results using the I² statistic which describes the percentage of total variation across studies that are caused by heterogeneity rather than by chance (Higgins 2002; Higgins 2003). We used the following ranges for the I² statistic to determine heterogeneity:
0 to 40%: might not be important;
30 to 60%: may represent moderate heterogeneity;
50 to 90%: may represent substantial heterogeneity;
75 to 100%: considerable heterogeneity.
Assessment of reporting biases
We made a full attempt at identifying unpublished trials and aimed to minimise any potential publication bias by using a comprehensive search strategy. We had planned to assess publication bias if the included number of trials was greater than 10; however, we were unable to make this assessment as only two trials were included in the review.
For the included trials, we compared the 'Methods' section of the full text to the 'Results' section to determine if all outcomes that were measured were reported. We sought the protocol of the included trials from clinical trial registers in order to identify any potential reporting bias.
Data synthesis
We used the RevMan software for quantitative analysis (Review Manager 2014). We used the fixed‐effect model for all analyses except for outcomes with I² greater than 40%, where we investigated the sources of heterogeneity and used a random‐effects analysis.
Subgroup analysis and investigation of heterogeneity
For future updates of the review, if reported data are available, we plan to conduct the following subgroup analyses and also use these to investigate heterogeneity:
calcium channel blocker used (e.g. nifedipine versus amlodipine);
cumulative type and dosage of chelation administered;
age (6 to 11 years of age, 12 to 18 years of age, over 18 years of age);
total transfusions received by participants;
co‐morbid conditions (diabetes mellitus, hypothyroidism, hepatitis B and C).
For most outcomes we did not identify significant heterogeneity using the I² statistic (Higgins 2003). We used a random‐effects model where there was high heterogeneity, I² over 40%.
Sensitivity analysis
For future updates of the review, if a sufficient number of trials are included, we plan to test the robustness of their results with the following sensitivity analyses:
trials of different designs (e.g. cross‐over and cluster randomised trials);
assessment of risk of bias (evaluating only trials of low risk of bias);
publication status (unpublished and published trials).
Summary of findings table
A summary of findings table was developed with the following characteristics based on published recommendations (Schünemann 2011).
Participant population: participants with transfusion‐dependent beta thalassaemia (age six years and above)
Setting: any
Intervention: calcium channel blocker and standard chelation therapy
Comparator: standard chelation therapy alone or standard chelation therapy plus placebo
-
Outcomes
mean change in cardiac T2* values (m/s)
mean change in MIC (mg/g)
mean change in serum ferritin ng/mL
mean change in liver T2* (m/s)
mean change in LIC (mg/g)
mean change in LVEF estimated by echocardiography (%)
adverse events
We used the grading of recommendations assessment, development and evaluation (GRADE) approach to assess the quality of the body of evidence; GRADE is used to grade the quality of evidence and the strength of recommendations in healthcare. The GRADE system classifies the quality of evidence in one of four levels ‐ high, moderate, low, and very low. This is based on the following parameters: trial limitations; inconsistency of results; indirectness of evidence; and imprecision and reporting bias (GRADE Working Group 2004).
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
Results
Description of studies
Results of the search
There were 839 records identified from the search. After screening of titles, abstracts and full texts, eight trials were excluded and four trials were identified for potential inclusion in our review. Please see the trial flow diagram, adapted from the PRISMA statement, for a summary of these results (Figure 1).
1.
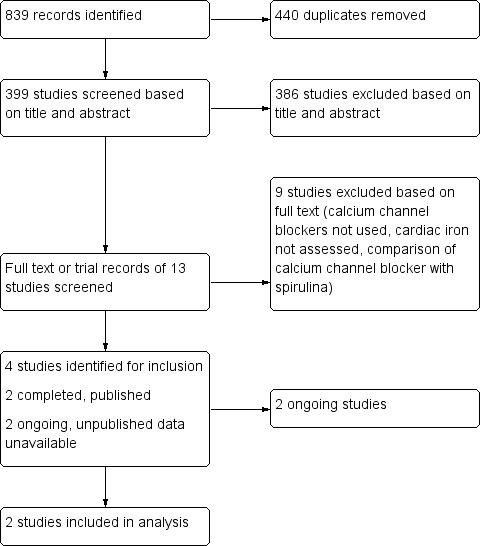
Study flow diagram. Adapted from PRISMA (Liberati 2009)
Of the four trials identified for inclusion, only two were included in the review (Fernandes 2013; Fernandes 2016). Of the remaining two trials, one is in the process of seeking publication (Hasan 2014) and one is an ongoing trial (NCT02474420). Several co‐authors on this review are investigators on one of these trials (Hasan 2014). We are awaiting peer‐review of our work prior to its inclusion in this version of the review. Therefore, we have listed this trial as ongoing. One trial is currently underway in Canada and, based on our correspondence with the primary investigator there are no results available at this time (NCT02474420). Details of these two trials are available at ClinicalTrials.gov and have been included in a table (Characteristics of ongoing studies). We anticipate inclusion of these trials in the next update of this review.
Included studies
The total number of participants from the two included trials is 74 (35 in the intervention and 39 in the control groups); full details of the participants, intervention and outcomes are included in tables (Characteristics of included studies).
Design
Both included trials were RCTs with a parallel design lasting 12 months. The earlier trial used a 1:2 allocation to intervention or control, respectively (Fernandes 2013), whereas the later trial used a 1:1 allocation ratio (Fernandes 2016). The later trial also stratified participants into two subgroups based on their baseline MIC (Fernandes 2016). The "reduction subgroup" had participants with MIC above the normal human threshold (MIC > 0.59 mg/g dry weight or T2* < 35 m/s). The "prevention subgroup" had MIC below the normal human threshold (MIC≤ 0.59 mg/g or T2* ≥ 35 m/s).
The earlier trial was a single‐centre, open‐label and single‐blind pilot trial (Fernandes 2013), but the later follow‐on trial was multicenter and double‐blind (Fernandes 2016). Both trials took place in Brazil under the same principal investigator.
Study participants
There were 15 participants in the earlier trial (Fernandes 2013), five of whom were randomised to the intervention arm and 10 to the comparator arm. In the later trial there were a total of 62 participants that were equally randomised to the two arms; however, one participant from the treatment arm and two from the placebo arm were excluded, leaving 59 participants who provided data for analysis (Fernandes 2016).
Both children and adults were eligible for inclusion in the trials; the mean age in the earlier trial was 29 years and in the later trial it was 23.4 years (Fernandes 2013; Fernandes 2016). The gender split was fairly even; 60% males in the earlier trial and 51% in the later trial. Both trials reported no statistically significant differences in baseline characteristics between groups in terms of clinical parameters such as history of splenectomy, haemoglobin concentration, serum ferritin and LVEF (Fernandes 2013; Fernandes 2016).
Interventions
Both trials used oral amlodipine 5 mg once daily (which was reduced to 2.5 mg once daily if side effects were observed) in addition to standard chelation treatment in the intervention arm (Fernandes 2013; Fernandes 2016). The pilot trial compared this treatment to standard chelation treatment alone (Fernandes 2013). The later trial gave participants in the control arm a placebo in addition to standard chelation therapy (Fernandes 2016).
Outcomes
The primary outcomes assessed in the two trials differed. In the earlier trial the primary outcome assessed was heart T2* values at 12 months (Fernandes 2013). In the later trial, the primary outcome was the change in MIC from baseline and the effect of treatment on the prevention and reduction subgroups at 12 months (Fernandes 2016). Both trials reported data for the change from baseline for heart T2*, MIC, LVEF, serum ferritin and liver T2* at both six and 12 months; they also provided information on adverse events and changes in chelation therapy (Fernandes 2013; Fernandes 2016). Additionally, the later trial reported LIC at six and 12 months (Fernandes 2016).
Excluded studies
Nine trials were excluded based on a review of full texts (Characteristics of excluded studies). Seven of these trials were excluded because the intervention studied was not a calcium channel blocker (Cappellini 2006; Chirnomas 2009; NCT02173951; Lai 2010; Neufeld 2012; NCT00115349; NCT01927913). One trial was excluded because it compared calcium channel blockers to spirulina which are cyanobacteria (NCT02671695).
One trial was non‐randomised and assessed the efficacy of nifedipine (a calcium channel blocker) in increasing the urinary excretion of iron; there are no published results available and the trial was assessed using the information available online (NCT00712738).
Ongoing studies
We identified two ongoing trials. One of these is being undertaken in Canada and has an estimated completion date in 2018 (NCT02474420). It is described as a randomised, open‐label trial of parallel design. Participants are 18 years and older with a primary diagnosis of transfusion‐dependent thalassaemia and evidence of moderate cardiac iron overload, on a stable dose of DFX therapy for more than three months. The intervention group receive a combination of DFX and amlodipine (2.5 mg once daily and titrated up to 10 mg per day based on tolerability). The control group receive DFX based on the standard of care by the treating physician. The main outcome is change in cardiac T2* MRI, assessed at baseline and 12 months post‐treatment.
A second open‐label, single‐blinded, randomised controlled pilot trial conducted in a tertiary care hospital in Pakistan is pending publication (Hasan 2014). It used a parallel design with 1:1 allocation. The trial included 20 participants on chelation therapy aged between six and 20 years of age with transfusion‐dependent beta thalassaemia major. Participants in the intervention arm (n = 10) received oral amlodipine 10 mg once daily in addition to standard chelation treatment; those in the control arm (n = 10) received standard chelation treatment alone. Laboratory values such as ferritin levels, MRI T2* assessment of cardiac iron as well as echocardiographic assessment of cardiac volumes, function and strain analysis were assessed at baseline and then (following randomisation) at six and 12 months. The primary outcome assessed was change in heart T2* values at six and 12 months from baseline. Secondary outcomes included assessments at six and 12 months for the change from baseline in the following parameters: absolute left ventricular size, LVEF, strain and strain rate, echocardiographic assessment of diastolic function, MRI evaluation of liver iron content by T2*.
Risk of bias in included studies
In general, we judged there to be an overall low risk of bias in the included trials. The risk assessment for allocation, blinding, attrition, and selective reporting has been summarised for each trial in risk of bias sections (Characteristics of included studies).
Allocation
Randomisation
We judged one trial to have a low risk of bias as the randomisation sequence was computer‐generated (Fernandes 2016). We judged the second trial to have an unclear risk of bias because, although the trial is described as randomised, the trial report did not describe how the sequence was generated (Fernandes 2013).
Concealment of allocation sequence
We judged one trial to have a low risk of bias as the assignments were made by a central pharmacy (Fernandes 2016). We judged the second trial to have an unclear risk of bias because there was no information on how the randomisation sequence was concealed (Fernandes 2013).
Blinding
We judged the earlier trial to have a high risk of performance bias because it was open label, but a low risk of detection bias for MRI exams because the readers of these assessments were blinded to treatment allocation (Fernandes 2013). We judged the later trial to have a low risk of both performance and detection biases as participants, healthcare personnel and MRI readers were all blinded to treatment allocation (Fernandes 2016).
Incomplete outcome data
We judged the earlier trial to have a low risk of attrition bias as outcome data were available for all participants except one in the amlodipine group (Fernandes 2013). This participant died before the 12‐month follow‐up.
We also judged the later trial to have a low risk of attrition bias (Fernandes 2016). This trial performed an intention‐to‐treat analysis. One participant was excluded from the amlodipine arm due to the lack of follow‐up MRIs. In the control arm, one participant was lost to follow‐up and another excluded due to a diagnosis other than thalassaemia major. Although trial investigators did not report myocardial T2* values, we had access to the the IPD and were able to obtain the heart T2* values.
Selective reporting
We judged both trials to have a low risk of reporting bias as all intended primary and secondary outcomes were reported. In addition, we had access to IPD from both trials.
Other potential sources of bias
We did not identify other potential sources of bias in the included trials.
Effects of interventions
See: Table 1
Two trials (n = 74) were included in our meta‐analyses (Fernandes 2013, Fernandes 2016). For most outcomes, we did not identify significant heterogeneity using the I² statistic (Higgins 2003).
A negative MD indicates that the mean change from baseline in the intervention group is larger than the control group. Therefore, if there is an increase in heart T2* from baseline, a decrease in MIC from baseline, or an increase in LVEF from baseline in the intervention group compared to the control group, the MD for these estimates would be a negative number and the magnitude of the number would indicate the magnitude of the effect size, i.e. a large negative number favours the intervention group.
The quality of the evidence has been graded for those outcomes included in the summary of findings table. For the definitions of these gradings, please refer to the summary of findings tables (Table 1).
Primary outcome measures
1. Cardiac iron assessment by T2* cardiac magnetic resonance (CMR) measurements (m/s)
a. Heart T2* (m/s)
At the six‐month time‐point, the MD for the mean change from baseline in heart T2* was estimated to be ‐3.91 m/s (95% CI ‐9.65 to 1.83) (Analysis 1.1; Figure 2) which was not a statistically significant change. At 12 months, there was an increase in heart T2* in the amlodipine group compared with the control group, but this was also not statistically significant, MD ‐0.27 m/s (95% CI ‐2.66 to 2.11) (Analysis 1.1; Figure 2). We judged that the quality of evidence for this outcome was low at both six and 12 months.
1.1. Analysis.

Comparison 1 Amlodipine versus control, Outcome 1 Heart T2* (m/s) (change from baseline).
2.

Forest plot of comparison: 1 Amlodipine versus control, outcome: 1.1 Heart T2* (m/s) (change from baseline).
For the mean change from baseline in heart T2* at six months, we identified significant heterogeneity, I² = 82%, that could not be readily explained (Analysis 1.1). We used random‐effects model for this analysis. We investigated this heterogeneity as follows. We checked the data to ensure they were correct. We could not explore heterogeneity by conducting subgroup analyses as there were insufficient data available to compare treatment effects in subgroups such as type of chelation treatment used or dose of amlodipine administered. One explanation for the heterogeneity may be an interaction between treatment effects and baseline MIC. The later Fernades trial found that the effect of treatment was affected by baseline MIC (P = 0.005 for interaction) and thus stratified participants into the reduction subgroup (baseline MIC > 0.59) and prevention subgroup (baseline MIC ≦ 0.59) (Fernandes 2016). We had access to the IPD for the 2013 trial in which all participants except one participant had a baseline MIC > 0.59 (Fernandes 2013). This made it difficult to perform a meaningful subgroup analysis across both trials.
We could not perform a sensitivity analysis because there were only two trials included.
b. MIC (mg/g)
When combining data at six months, the difference between groups in the mean change from baseline in MIC was estimated to be ‐0.23 mg/g (95% CI ‐0.39 to ‐0.07) (Analysis 1.2; Figure 3). This represented a statistically significant decrease in MIC in the amlodipine group compared with the control group. At 12 months, the statistically significant decrease in MIC in the amlodipine group compared with the control group was maintained, MD ‐0.25 mg/g (95% CI ‐0.44 to ‐0.05) (Analysis 1.2; Figure 3). We judged that the quality of evidence for this outcome was low at both six and 12 months.
1.2. Analysis.
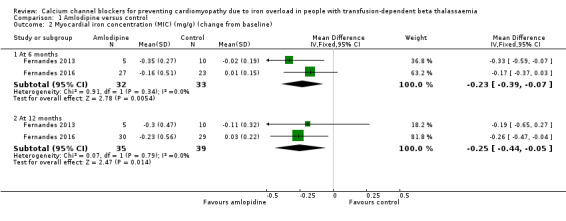
Comparison 1 Amlodipine versus control, Outcome 2 Myocardial iron concentration (MIC) (mg/g) (change from baseline).
3.
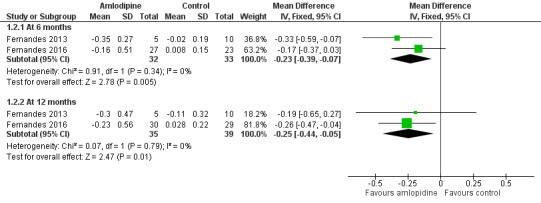
Forest plot of comparison: 1 Amlodipine versus control, outcome: 1.2 Myocardial iron concentration (MIC) (mg/g) (change from baseline).
2. Cardiac mortality
There was no reported occurrences of cardiovascular complications in either trial.
3. Cardiac function assessments
a. N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and B‐type natriuretic peptide (BNP) levels (pg/mL)
This outcome was not assessed by the included trials.
b. Echocardiographic estimation of LVEF (%)
The difference between groups in the mean change from baseline in LVEF at six months was estimated to be MD ‐0.02 % (95% CI ‐2.49 to 2.45) (Analysis 1.3). This represented an increase in LVEF in the amlodipine group compared with the control group that was not statistically significant. At 12 months the increase in LVEF in the amlodipine group compared with the control group was also not statistically significant, MD ‐0.40 % (95% CI ‐2.97 to 2.17) (Analysis 1.3). We judged that the quality of evidence for this outcome was low at both six and 12 months.
1.3. Analysis.
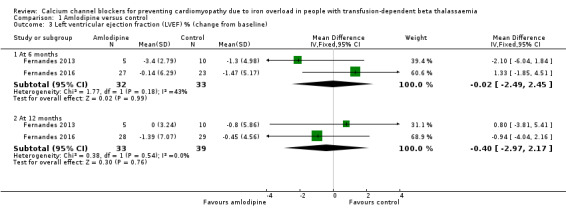
Comparison 1 Amlodipine versus control, Outcome 3 Left ventricular ejection fraction (LVEF) % (change from baseline).
c. Echocardiographic estimation of left and right ventricular filling pressures (mm Hg)
This outcome was not assessed by the included trials.
d. Estimation of LVEF by CMR (%)
This outcome was not assessed by the included trials.
e. Estimation of left and right ventricular volumes by CMR (mL)
This outcome was not assessed by the included trials.
f. Estimation of left and right ventricular strain (%) and strain rate (s‐1) by CMR tagging
This outcome was not assessed by the included trials.
g. Estimation of left and right ventricular volume (mL) and function (%) by myocardial tissue phase mapping
This outcome was not assessed by the included trials.
h. Estimation of left and right ventricular volume (mL) and function (%) by radionuclide ventriculography during exercise
This outcome was not assessed by the included trials.
i. Exercise stress testing
This outcome was not assessed by the included trials.
j. Other echocardiographic assessments
Outcomes under this category were not assessed by the included trials.
Secondary outcome measures
1. Electrocardiographic abnormalities (measured as time‐to‐event)
a. Supraventricular arrhythmias
This outcome was not assessed by the included trials.
b. Electrocardiographic findings that suggest right‐sided heart involvement (S1Q3 pattern and right‐axis deviation)
This outcome was not assessed by the included trials.
c. New‐onset T‐wave inversion beyond lead V1 and a consistent decrease in QRS height
This outcome was not assessed by the included trials.
2. Measures of iron overload
a. Serum ferritin (ng/mL)
The difference in mean change from baseline in serum ferritin at six months was estimated to be MD ‐390.54 ng/ml (95% CI ‐819.00 to 37.93) which represented a non‐significant decrease in serum ferritin in the amlodipine group compared with the control group (Analysis 1.4). At 12 months, the difference in the mean change from baseline in serum ferritin was also not statistically significant, MD ‐247.78 ng/mL (95% CI ‐741.61 to 246.05) (Analysis 1.4). We judged that the quality of evidence for this outcome was low at both six and 12 months.
1.4. Analysis.
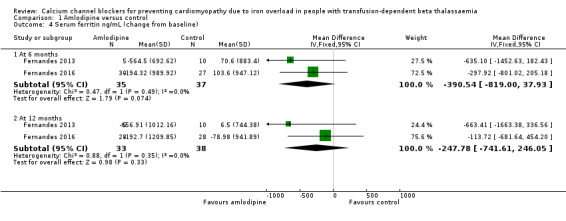
Comparison 1 Amlodipine versus control, Outcome 4 Serum ferritin ng/mL (change from baseline).
b. Iron levels in biopsies of liver and other tissue
This outcome was not assessed by the included trials.
c. Tissue iron assessment by superconducting quantum interference device (SQUID)
This outcome was not assessed by the included trials.
d. Tissue (other than cardiac) iron assessment by magnetic resonance imaging (MRI) (m/s)
i. Liver T2* (m/s)
When data from both included trials are combined at six months, the difference in mean change from baseline in liver T2* was estimated to be MD ‐0.71 m/s (95% CI ‐1.61 to 0.18) which represented a non‐statistically significant increase in liver T2* in the amlodipine group compared with the control group (Analysis 1.5). At 12 months, the result again favoured amlodipine but was still not statistically significant, MD ‐1.24 m/s (95% CI ‐2.76 to 0.27) (Analysis 1.5). We judged that the quality of evidence for this outcome to be low at both six and 12 months.
1.5. Analysis.

Comparison 1 Amlodipine versus control, Outcome 5 Liver T2* (m/s) (change from baseline).
ii. LIC (mg/g)
This outcome was only reported by one trial (n = 59) (Fernandes 2016).
At six months, the difference in mean change from baseline in LIC was estimated to be MD ‐1.64 mg/g (95% CI ‐3.96 to 0.68) which represented a decrease in LIC in the amlodipine group compared with the control group that was not statistically significant (Analysis 1.6). At 12 months, the difference between groups in the mean change from baseline in LIC was again not statistically significant, MD ‐2.01 mg/g (95% CI ‐4.84 to 0.82) (Analysis 1.6). We judged that the quality of evidence for this outcome was low
1.6. Analysis.

Comparison 1 Amlodipine versus control, Outcome 6 Liver iron concentration (LIC) (mg/g) (change from baseline).
e. Mean reduction in dose of chelation therapy
This specific outcome was not assessed by either trial. However, changes in chelation therapy were addressed in the reports of both trials. The earlier trial did not report analysable data, but stated that there were no significant changes in chelation therapy (type of chelator used or dose) in the treatment and control groups across the trial (Fernandes 2013).
The investigators from the later trial provided us with IPD on type and dose of chelation drugs used in the trial (Fernandes 2016). Of the 59 participants in the trial, there were 30 participants on DFX, 11 on DFP, seven on DFO, eight on a combination regimen of oral DFP and subcutaneous DFO, and three participants on combinations of oral chelators or DFX plus DFO categorized as ‘other’. The small number of participants on each type of chelator precluded the application of a statistical test to compare amlodipine and placebo arms.
Overall, chelator doses were decreased in 27% of the participants in the amlodipine arm and 14% of the participants in the placebo arm. Of the participants on DFX, four participants in the amlodipine group (23%) and five in the placebo group (38%) had their chelation dose reduced at 12 months. For participants on DFP, two in the amlodipine group (40%) and none in the placebo group had in their chelation dose reduced at 12 months. For DFO, none of the participants had their dose reduced at 12 months. Among participants on a combination regimen of oral DFP and subcutaneous DFO, one in the amlodipine group (33%) and three in the placebo group (60%) had their dose reduced at 12 months. One participant who received amlodipine in the category of ‘other’ chelators had the dose reduced at 12 months.
3. Self‐reported QoL measured using validated instruments for heart failure
This outcome was not assessed by the included trials.
4. Compliance with calcium channel blocker and chelation treatment
This outcome was not assessed by the included trials.
5. Adverse effects of calcium channel blocker
The earlier trial reported that there were no serious adverse events experienced by participants taking amlodipine (Fernandes 2013). There was no significant reduction in systolic blood pressure related to amlodipine. In one participant, the amlodipine dose was reduced by half (2.5 mg per day) due to development of lower extremity oedema. One death was reported in the amlodipine group secondary to advanced liver cirrhosis that was present in the participant prior to enrolment in the trial.
The second trial reported that there were no serious adverse events in participants taking amlodipine (Fernandes 2016). However, mild adverse events (ankle edema, dizziness, mild cutaneous allergy) were reported with no statistically significant difference between the amlodipine and control arms. We calculated the risk of any adverse event in the amlodipine arm compared with placebo, RR 8.71 (95% CI 0.49 to 154.89) (Analysis 1.7). In the amlodipine arm, in three participants the dose was reduced to 2.5 mg per day; two of these due to the development of ankle oedema and one due to dizziness. These adverse effects resolved after dose reduction. In addition, one participant stopped taking the medication after the development of a mild cutaneous allergic reaction but continued to participate in the study. We judged that the quality of evidence for this outcome was low at both six and 12 months.
1.7. Analysis.

Comparison 1 Amlodipine versus control, Outcome 7 Adverse events.
6. Cost of intervention per year
This outcome was not assessed by the included trials.
Discussion
Summary of main results
Two randomised controlled trials (n = 74) are included in this review. Both included trials were conducted by the same investigators and include a pilot trial (Fernandes 2013) followed by a larger multicentre trial (Fernandes 2016). We were given access to the IPD for both trials. The mean (SD) age of participants was 24.4 (8.5) years and approximately 52% of all participants were male. Primary outcomes are presented in the tables (Table 1).
The review's primary outcome of change in MIC was reported as heart T2* values (cardiac magnetic resonance measurements) and MIC. Based on our analysis, heart T2* at six months and 12 months did not show a statistically significant increase in the amlodipine group compared with the control group, MD at six months ‐3.91 m/s (95% CI ‐9.65 to 1.83) and at 12 months, MD ‐0.27 m/s (95% CI ‐2.66 to 2.11) (Analysis 1.1). The MIC showed a statistically significant decrease in the amlodipine group compared with the control group both at six months, MD ‐0.23 mg/g (95% CI ‐0.39 to ‐0.07), and at 12 months, MD ‐0.25 mg/g (95% CI ‐0.44 to ‐0.05) (Analysis 1.2). The fact that the heart T2* comparison did not approach statistical significance while the corresponding MIC changes were statistically significant may be explained by the non‐linear relationship between heart T2* and MIC as well the interaction of treatment effects with baseline MIC (i.e. participants with high baseline MIC experienced greater treatment effects than those with lower baseline MIC values).
For the larger trial, participants were categorized into prevention or reduction subgroups based on whether their baseline MIC was below or above the normal human threshold, respectively (Fernandes 2016). Only participants in the reduction subgroup showed a significant reduction in their median MIC after 12 months of treatment with amlodipine compared with placebo. This finding implies that there has to be significant iron overload at baseline for amlodipine to have any benefit. We were unable to conduct a similar subgroup meta‐analysis for the earlier trial because (based on IPD) all except one participant would be classified in the reduction subgroup (Fernandes 2013).
Assessment of cardiac function was reported as echocardiographic estimation of LVEF. There was a statistically non‐significant increase in LVEF in the amlodipine arm compared with the control arm at six months, MD ‐0.02 % (95% CI ‐2.49 to 2.45) and at 12 months, MD ‐0.40 % (95% CI ‐2.97 to 2.17) (Analysis 1.3).
Secondary measures of iron overload such as serum ferritin and LIC were also assessed. Serum ferritin showed a statistically non‐significant decrease in the amlodipine group compared to the control group both at six months, MD ‐390.54 ng/mL (95% CI ‐819.00 to 37.93) and at 12 months, MD ‐247.78 ng/mL (95% CI ‐741.61 to 246.05) (Analysis 1.4). Liver T2* values also increased in the amlodipine group compared to the control group at six months, MD ‐0.71 m/s (95% CI ‐1.61 to 0.18) and at 12 months, MD ‐1.24 m/s (95% CI ‐2.76 to 0.27), but these differences were not statistically significant (Analysis 1.5). Only the later trial measured LIC (Fernandes 2016) and showed a statistically non‐significant decrease in the amlodipine arm compared with the control arm at six months, MD ‐1.64 mg/g (95% CI ‐3.96 to 0.68) and at 12 months, MD ‐2.01 mg/g (95% CI ‐4.84 to 0.82) (Analysis 1.6).
One death was reported in the amlodipine arm of the earlier trial, but was thought to be related to pre‐existing liver cirrhosis (Fernandes 2013). There were no serious adverse events reported by the second trial (Fernandes 2016); and analysis of mild adverse events yielded a statistically non‐significant result, RR 8.71 (95% CI 0.49 to 154.89) (Analysis 1.7).
Overall completeness and applicability of evidence
Overall sample size for our analysis was small (n = 74) with only two trials included. Participants studied were mainly adolescents and young adults (mean (SD) 24.4 years) with good gender distribution (52% male). Both trials were conducted in Brazil and may not be applicable to other populations. The results pertain only to the calcium channel blocker amlodipine at a dose and frequency of 5 mg once daily (reduced to 2.5 mg once daily for adverse effects).
Both trials excluded participants with severe left ventricular dysfunction since these individuals may experience higher adverse effects from the use of a calcium channel blocker (Fernandes 2013; Fernandes 2016). The participants in both trials were expected to remain on a stable chelation regimen for the duration of the trial. This may have inadvertently excluded individuals with poorly‐controlled iron overload since these people are more likely to switch to combination chelation therapy or use increasingly higher dosages of chelation drugs.
The review's primary outcome of MIC was addressed by both trials. However, it was reported as mean cardiac T2* values by the pilot trial (Fernandes 2013) and as median MIC by the follow‐up trial (Fernandes 2016). We were able to obtain the IPD for both trials to assess the mean change from baseline for relevant outcomes and analyse the data for MDs. Data for outcomes such as LVEF that were not included in the published reports were also available for analysis through the IPD. However, there were several outcomes that were not assessed and are likely to be important to individuals with this condition and to clinicians who evaluate the response to treatment. These outcomes include changes in quality of life, dose reduction in chelation therapy, measures of cardiac function such as ventricular strain patterns and the incidence of electrocardiographic abnormalities.
There were insufficient data reported to conduct a subgroup analysis for the type of chelation therapy used. Importantly, some chelation drugs are more efficacious than others and combination therapy may be more effective than monotherapy so the relative prevalence of each type of chelation therapy in the intervention and control groups should affect the outcomes but randomisation ensures that these characteristics are balanced in both groups.
Outcomes are also likely to be affected by the severity of baseline myocardial iron overload as indicated in the larger trial (Fernandes 2016), but there was insufficient variation in baseline MIC in the smaller trial to conduct a subgroup analysis (Fernandes 2013). In the later trial, stratification was done after randomisation which further underpowered the study (Fernandes 2016). In addition, the participants in this trial had a near normal baseline MIC with median values of 34.1 m/s (95% CI 30.9 to 36.4 m/s) (Fernandes 2016). They used a T2* threshold of 35 m/s to stratify participants into reduction and prevention subgroups. This is opposed to the commonly‐used risk threshold of 20 m/s that predicts significant cardiomyopathy (Anderson 2001; Wang 2010). Although there is some evidence that even a small reduction in MIC improves cardiac outcomes (Voskaridou 2012), it may not be clinically significant for most individuals with significant iron overload.
Quality of the evidence
As only two small trials were included in the review, the overall quality of the evidence for all outcomes reported was low quality, due to risks of bias, imprecision (wide CIs), and indirectness (not applicable to wider population). The quality of evidence was improved by our access to the IPD. Both trials were conducted by the same investigators and the larger follow‐up trial was multicentre. Further trials from different investigators are required to verify the results.
Potential biases in the review process
Most of the review authors are also investigators on a currently ongoing trial (Hasan 2014). When this is included the review, authors who are not involved in the trial will be responsible for assessments and data extraction to avoid possible bias.
Agreements and disagreements with other studies or reviews
We did not identify any observational or non‐randomised studies on the use of calcium channel blockers in humans for the reduction of MIC. However, evidence from the two included trials in our review correlates well with findings from animal studies.
Based on the proposed mechanism of action of L‐type calcium channel blockers on myocardial tissue, the effects of amlodipine should be specific to the myocardium with minimal effects on liver iron content which was consistent with the findings of our review (Oudit 2003; Oudit 2006; Tsushima 1999). An increase in LVEF was observed concomitant with decreases in MIC which is consistent with reports of heart T2* and MIC being used as reliable prognostic indicators of cardiac dysfunction (Carpenter 2011; Kirk 2009; Pennell 2014).
The larger trial reported that participants with high MIC at baseline benefited from treatment with amlodipine, but in those with normal MIC at baseline there was no change with amlodipine (Fernandes 2016). These findings are not readily explainable from our current understanding of the interaction of calcium channel blockers and myocardial iron. It is plausible that iron content in the heart needs to be above a certain threshold before L‐type calcium channels become a significant pathway for iron entry and their blockade becomes clinically important. Other pathways for iron entry into the heart have been described, such as divalent metal transporter 1 (DMT1) (Ludwiczek 2007; Oudit 2003) and T‐type calcium channels (Kumfu 2012), and it remains to be seen whether they play a significant role in myocardial iron overload, perhaps at lower MIC levels.
Authors' conclusions
Implications for practice.
The use of calcium channel blockers such as amlodipine is common in the treatment of systemic hypertension, but its application in reducing myocardial iron overload is a novel concept that is in preliminary stages of research. Although amlodipine has shown promise in animal studies, currently there is insufficient evidence to determine whether amlodipine is effective in preventing cardiomyopathy due to iron overload in people with transfusion‐dependent beta thalassaemia.
Implications for research.
There is potential for calcium channel blockers such as amlodipine to reduce myocardial iron overload in people with transfusion‐dependent thalassaemia and randomised controlled trials are warranted. Although the results of the included trials are consistent, there needs to be corroboration from larger studies and different investigators. Multicentre collaborative trials are needed especially in those parts of the world where thalassaemia is prevalent. As significant myocardial iron overload may develop during childhood (Wood 2004; Wood 2008), trials in the paediatric population should be conducted to assess for safety and efficacy. Trials with extended follow‐up should be conducted to determine if the effects of calcium channel blockers are sustained beyond 12 months.
Recent studies indicate that chelation drugs have varying efficacy in reducing cardiac iron (Pepe 2013; Xia 2013). Future trials should be designed to compare the effects of calcium channel blockers with different chelation regimens. Clinically relevant outcomes such as dose reduction in standard chelation and quality of life parameters should also be investigated. In addition, the role of baseline myocardial iron content in affecting the response to calcium channel blockers should be investigated. Determining the cost of intervention is also important for individuals with the condition, clinicians and healthcare policy makers to determine if the use of calcium channel blockers is cost‐effective.
Since myocardial T2* values and myocardial iron content may have a non‐linear relationship, it is important to report both outcomes. This is also true for liver T2* and liver iron concentration.
Acknowledgements
We would like to thank Tracey Remmington and Nikki Jahnke (Cochrane Cystic Fibrosis and Genetic Disorders Group) for their assistance throughout the preparation of this manuscript, Raid Amin (Department of Mathematics and Statistics, University of West Florida) for his expertise in statistical analysis, and Mathew B. Steiner (Division of Pediatric Cardiology, Nemours Childrens Specialty Care) for his expertise in assessment of diastolic function. We would like to extend a special thanks to Juliano L. Fernandes (Jose Michel Kalaf Research Institute, Brazil) who provided access to the individual patient data for both included trials.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Glossary of terms
| Term | Meaning |
| agranulocytosis | agranulocytosis means a failure of the bone marrow to make sufficient white blood cells, especially neutrophils, that fight infections |
| arrhythmias | arrhythmia is a condition in which the heart beats with an irregular or otherwise abnormal rhythm |
| cardiac fibrosis | cardiac fibrosis refers to the abnormal proliferation of cells called fibroblasts in the heart muscle; this causes the heart muscle to become stiffer or less compliant than normal which affects its ability as an efficient pump |
| cardiomyopathy | cardiomyopathy is a weakening of the heart muscle or a decrease in its effectiveness at pumping blood |
| divalent metal transporter | the divalent metal transporter is a protein located in the membrane of cells; it transports metal ions with the charge of 2+ (e.g. calcium (Ca2+), iron (Fe2+) across the cell membrane |
| ferric ions | ferric is the term used for iron ions that have an oxidation number or charge of 3+; they are denoted as Fe3+ |
| ferrous iron | ferrous is the term used for iron ions that have an oxidation number or charge of 2+; they are denoted as Fe2+ |
| haematologic toxicity | haematologic toxicity refers to damage to the bone marrow that results in decreased production of red blood cells, white blood cells and platelets |
| hypotension | a decrease in blood pressure to below normal values |
| iron‐mediated | brought about by iron |
| ligand‐metal complex | when a ligand (any ion or molecule with a functional group) binds with a metal atom, such as iron, the resulting compound is called a ligand‐metal complex |
| mitochondrial damage | damage to mitochondria which are microscopic components of cells that are responsible for producing energy‐containing molecules that can be used for driving chemical reactions in the cell |
| myocardial | relating to the heart muscle |
| myocarditis | inflammation of the heart muscle |
| neutropenia | an abnormally low number of neutrophils, which are a specific kind of white blood cells |
| noninvasive hepatic tests | diagnostic tests that do not require the use of instruments to enter the body are known as noninvasive tests; hepatic relates to the liver. Therefore, 'noninvasive hepatic tests' refers to diagnostic tests for liver disorders that do not require instruments to be entered into the individual's body |
| oedema | commonly referred to as swelling; it is the abnormal accumulation of fluid inside or around tissues |
| pericarditis | inflammation of the membrane that encloses the heart |
| pulmonary hypertension | an abnormal increase in blood pressure inside the pulmonary artery that carries blood from the right side of the heart to the lungs |
| reactive oxygen species | chemically reactive molecules that contain oxygen and, through their interaction with other molecules, can cause damage to cell structures; also abbreviated to ROS |
| ryanodine release channels | proteins located inside the cell that are involved in the transport of calcium ions (Ca2+) into and out of calcium storing bodies inside the cell |
| somnolence | either a strong desire to sleep, a state of near‐sleep or a condition of sleeping for abnormally long duration |
| sympathetic nervous system | one of the two main divisions of the autonomic nervous system that regulate the body's involuntary actions (the other is called the parasympathetic nervous system) and the one which is primarily responsible for fight‐or‐flight responses of the body that are facilitated by hormones such as adrenaline |
Appendix 2. Electronic searches
| Database / Resource | Search terms | Date last searched |
| Current Controlled Trials (www.controlled‐trials.com) |
thalassaemia OR thalassemia OR (iron overload) OR haemosiderosis OR hemosiderosis | 24 February 2018 |
| ClinicalTrials.gov (www.clinicaltrials.gov) |
thalassaemia OR thalassemia OR iron overload OR haemosiderosis OR hemosiderosis | 24 February 2018 |
| ICTRP (www.who.int/ictrp/en/) |
thalassaemia OR thalassemia OR iron overload OR haemosiderosis OR hemosiderosis | 24 February 2018 |
Data and analyses
Comparison 1. Amlodipine versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart T2* (m/s) (change from baseline) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 months | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐3.91 [‐9.65, 1.83] |
| 1.2 At 12 months | 2 | 74 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.66, 2.11] |
| 2 Myocardial iron concentration (MIC) (mg/g) (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 6 months | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.39, ‐0.07] |
| 2.2 At 12 months | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.44, ‐0.05] |
| 3 Left ventricular ejection fraction (LVEF) % (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 months | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐2.49, 2.45] |
| 3.2 At 12 months | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.97, 2.17] |
| 4 Serum ferritin ng/mL (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 6 months | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐390.54 [‐819.00, 37.93] |
| 4.2 At 12 months | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐247.78 [‐741.61, 246.05] |
| 5 Liver T2* (m/s) (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 6 months | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.61, 0.18] |
| 5.2 At 12 months | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐2.76, 0.27] |
| 6 Liver iron concentration (LIC) (mg/g) (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.64 [‐3.96, 0.68] |
| 6.2 At 12 months | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐2.01 [‐4.84, 0.82] |
| 7 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Mild adverse events at 12 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.71 [0.49, 154.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fernandes 2013.
| Methods | Randomised controlled trial. Design: parallel group. Duration: 12 months. Location: single centre in Brazil. |
|
| Participants |
Inclusion criteria: thalassaemia major, at least 7 years of age (in order to comply with MRI examinations), regularly transfused, iron overload with no prospect of changing chelation therapy in the coming 6 months after enrolment, had a record of good compliance with chelation therapy. Exclusion criteria: significant left ventricular dysfunction (ejection fraction < 35%), renal insufficiency, advanced atrioventricular conduction disturbances, and formal contraindications to MRI examinations. 15 participants were selected for randomisation from a pool of 60 individuals who had undergone repeated MRI examinations over the preceding 5 years. Total cohort N = 15, mean (SD) age 28.3 (4.9) years, 60% male. Intervention group n = 5, mean (SD) age 31.2 (3.9) years, 60% male. Mean (SD) baseline characteristics: haemoglobin 9.4 (0.65) g/dL; heart T2* 21.7 (7.2) m/s; MIC 1.19 (0.67) mg/g; LVEF 66.5 (9.2) %; serum ferritin 1110 (837) ng/mL; liver T2* 5.47 (2.97) m/s; LIC 6.5 (4.3) mg/g. Control group: n =10, mean (SD) age 26.8 (4.8) years, 60 % male. Mean (SD) baseline characteristics: haemoglobin 9.8 (0.29) g/dL; heart T2* 25.1 (8.8) m/s; MIC 1.07 (0.55) mg/g; LVEF 66.6 (6.7) %; serum ferritin 1602 (1084) ng/mL; liver T2* 5.38 (4.36) m/s; LIC 9.5 (7.9) mg/g. |
|
| Interventions |
Intervention group: oral amlodipine 5 mg once daily in addition to standard chelation therapy. Control group: standard chelation therapy alone. |
|
| Outcomes |
Primary outcome: difference in heart T2* values (m/s) between groups at 12 months. Secondary outcomes: heart T2* values (m/s) at 6 months, serum ferritin (ng/dL) at 6 and 12 months, LIC (mg of Fe/gm of dry weight of liver) at 6 and 12 months. |
|
| Notes | Baseline MIC and liver T2* values were not included in the published report. We obtained these values from individual patient data provided by the principal investigator. P values for type of chelation therapy used were not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Readers of MRI were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the amlodipine group died before 12‐month follow up so data for this participant were not available for analysis at 12 months. |
| Selective reporting (reporting bias) | Low risk | All intended primary and secondary outcome data were reported. |
| Other bias | Low risk | None identified. |
Fernandes 2016.
| Methods | Randomised controlled trial. Design: parallel group. Duration: 12 months. Location: multicentre in Brazil. |
|
| Participants |
Inclusion criteria: people with thalassaemia major, at least 6 years of age, regular transfusions for 2 or more years (total lifetime red blood cell transfusions above 20 units). Exclusion criteria: change in chelation therapy expected or scheduled in the next 12 months, presence of advanced clinical heart failure or ejection fraction > 35%, presence of a contraindication to use of MRI. 62 participants were enrolled from a pool of 77 screened individuals. 1 participant from the placebo arm was excluded because of a diagnosis other than thalassaemia major and a second was lost to follow‐up. One patient was excluded from the amlodipine arm due to lack of follow‐up MRI examinations. This left 59 participants for analysis, 30 in the amlodipine arm and 29 in the placebo arm. Particpants were stratified into two subgroups based on their baseline myocardial T2* values: reduction group (T2* < 35 m/s) and prevention group (T2* > 35 m/s). Within the reduction subgroup, 15 participants were randomised to the amlodipine arm and 15 to the placebo arm. There were no statistically significant differences in baseline characteristics between these arms. Similarly, within the prevention subgroup, 15 participants were randomised to the amlodipine arm and 14 to the placebo arm. Again, there were no statistically significant differences in the baseline characteristics between these 2 arms. Total cohort N = 59, mean (SD) age 23.4 (9) years, 51% male. Intervention group n = 30, mean (SD) age 27.7 (7.7) years, 43% male. Mean (SD) baseline characteristics: haemoglobin 9.4 (1) g/dL; heart T2* 29.37 (12.81) m/s; MIC 1.48 (2.37) mg/g; LVEF 67.73 (5.46) %; serum ferritin 2682.34 (1908.64) ng/mL; liver T2* 5.11 (4.58) m/s; LIC 12.97 (10.29) mg/g. Control group n = 29, mean (SD) age 23.5 (10.2) years, 59 % male. Mean (SD) baseline characteristics: haemoglobin 9.5 (1) g/dL; heart T2* 31.24 (SD) 9.42 m/s; MIC 0.92 (0.88) mg/g; LVEF 67.07 (6.64) %; serum ferritin 2662.6 (2840.61) ng/mL; liver T2* 5.23 (4.7) m/s; LIC 10.53 (7.3) mg/g. |
|
| Interventions |
Intervention group: oral amlodipine 5 mg once daily in addition to standard chelation therapy. Control group: placebo in addition to standard chelation therapy. |
|
| Outcomes |
Primary outcome: change in MIC from baseline and a comparison of the effect of treatment between the prevention and reduction subgroups at 12 months. Secondary outcomes: assessment of MIC at 6 months, change in LIC at 12 months, serum ferritin, LVEF and incidence of adverse events at 6 and 12 months. |
|
| Notes | All outcomes were reported as median values in the published report. We obtained mean values from individual patient data provided by the principal investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Assignments made by central pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and health personnel involved in diagnosis, exam interpretation, and treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A central core lab concealed to treatment allocation and identity of the participant, performed interpretation of the all MRI images using a dedicated workstation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed; 1 participant excluded from the amlodipine arm due to lack of follow‐up MRIs; in the placebo arm, 1 participant lost to follow up and another excluded due to diagnosis other than thalassaemia major. Myocardial T2* values were not reported as the investigators uncovered a nonlinear relationship between MIC and myocardial T2*, and MIC was considered more clinically applicable. However, since we had access to the the individual patient data, we were able to obtain the heart T2* values. |
| Selective reporting (reporting bias) | Low risk | All intended primary and secondary outcome data were reported. |
| Other bias | Low risk | None identified. |
LIC: liver iron concentration LVEF: left ventricular ejection fraction MIC: myocardial iron concentration MRI: magnetic resonance imaging SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cappellini 2006 | Intervention other than calcium channel blocker. |
| Chirnomas 2009 | Intervention other than calcium channel blocker. |
| Lai 2010 | Intervention other than calcium channel blocker. |
| NCT00115349 | Intervention other than calcium channel blocker (trial terminated). |
| NCT00712738 | Not a randomised controlled trial. |
| NCT01927913 | Intervention other than calcium channel blocker (trial withdrawn). |
| NCT02173951 | Intervention other than calcium channel blocker. |
| NCT02671695 | Comparator arm other than standard chelation therapy (Spirulina). |
| Neufeld 2012 | Intervention other than calcium channel blocker. |
Characteristics of ongoing studies [ordered by study ID]
Hasan 2014.
| Trial name or title | Effect of L‐type calcium channel blocker (amlodipine) on myocardial iron deposition in patients with thalassaemia with moderate‐to‐severe myocardial iron deposition. |
| Methods | Randomised controlled trial (open label, single blinded). Design: parallel group. Duration: 12 months. Location: single centre, a tertiary care hospital in Pakistan. |
| Participants |
Inclusion criteria: age ≥ 6 and ≤ 20 years, ≥ 10 blood transfusions in a lifetime, transfusion need ≥180 mL/kg/year, serum ferritin ≥1000 μg/dL, deemed eligible for standard chelation therapy (by treating haematologist), individuals who have been on a stable chelation regimen for ≥6 months. Exclusion criteria: known hypersensitivity to amlodipine or history of developing tetany after use of a calcium channel blocker, known sinoatrial nodal disease or aortic stenosis, known severe myocardial dysfunction (LVEF of ≤4 SD for age even without symptoms), signs and symptoms of heart. failure, T2* value of <4 m/s on cardiac MRI, systolic blood pressure ≤2 SD for age (systemic hypotension) at the time of enrolment, previously diagnosed significant congenital heart diseases or acquired heart diseases other than thalassaemia, known contraindications to MRI, known pregnancy. Total cohort N = 21, 62 % male. Intervention group n =10, median age 18.0 years (IQR 15.8 ‐ 19.3), 50 % male. Control group n = 11, median age 16.0 years (IQR 13.0 ‐ 18.0), 73% male. |
| Interventions |
Intervention group: oral amlodipine 10 mg once daily in addition to standard chelation therapy. Control group: standard chelation therapy alone. |
| Outcomes |
Primary outcome: difference in heart T2* values (m/s) between groups at 12 months. Secondary outcomes: effects on systolic and diastolic function, absolute left ventricular size, ejection fraction, strain and strain rate, echocardiographic assessment of diastolic function, MRI evaluation of liver iron content by T2*. |
| Starting date | |
| Contact information | |
| Notes | Completed November 2015; awaiting peer review and publication. |
NCT02474420.
| Trial name or title | The use of the calcium channel blocker amlodipine as an adjuvant treatment to iron chelation for the prevention of iron overload cardiomyopathy in patients with thalassaemia (CANALI). |
| Methods | Allocation: randomised. Endpoint classification: safety/efficacy study. Intervention model: parallel assignment. Masking: single blind (outcomes assessor). Location: Canada. |
| Participants |
Inclusion criteria: diagnosis of transfusion‐dependent thalassaemia being followed by a thalassaemia comprehensive care clinic; age 18 years or older; on a stable dose of deferasirox for over 3 months; evidence on cardiac MRI of mild to moderate cardiac iron overload (T2* < 20 m/s but ≥10 m/s) as measured within 3 months prior to randomisation; preserved LVEF > 55% as measured by cardiac MRI; agreeable to use an approved method of contraception if female of childbearing potential for the entire duration of the trial. Exclusion criteria: serum ferritin < 500 ng/mL at screening; LIC > 30 mg/gm measured by liver R2 MRI (FerriScan); congestive heart failure, severe refractory hypotension (less than 90 mmHg systolic), currently taking any calcium channel blockers, pregnancy or nursing, considered by principal investigator to be unfit for the trial based on medical review, physical examination or screening investigations, non availability of permanent address, hypersensitivity to amlodipine or other dihydropyridine. |
| Interventions |
Intervention group: deferasirox iron chelation therapy plus amlodipine; amlodipine titrated up to 10 mg daily or maximum tolerated dose, whichever comes first. Control group: deferasirox iron chelation therapy and standard of care by the treating physician. |
| Outcomes |
Primary outcome: change in cardiac T2* as determined by MRI at 12 months following randomisation. Secondary outcomes: change in LVEF (%) as determined by MRI at 12 months following randomisation. |
| Starting date | June 2015. |
| Contact information | Kevin H.M. Kuo, MD, MSc, FRCPC, Assistant Professor, University Health Network, Toronto. |
| Notes | Completion date: June 2018. |
LIC: liver iron concentration LVEF: left ventricular ejection fraction MRI: magnetic resonance imaging SD: standard deviation IQR: inter‐quartile range
Differences between protocol and review
For the assessment of cardiac iron deposition, we had planned to include heart T2* as the only variable. However, in a post hoc change, we also included myocardial iron content (MIC) as an outcome. Heart T2* and MIC have an inverse curvilinear relationship (Carpenter 2011). It is easier to follow the linear changes in MIC in the clinical setting (higher value indicates greater risk of cardiac dysfunction) just as liver T2* is often reported as liver iron concentration (LIC).
Contributions of authors
AS and NA independently applied the inclusion criteria for including studies into this review. AS and NA independently assessed the trial quality and extracted data. JD verified the data. AS drafted the review text, NA, BH, SC and JD provided reviews and suggestions to the draft.
Sources of support
Internal sources
-
Division of Women and Child Health, Aga Khan University Hospital, Pakistan.
Salary
-
Department of Paediatrics and Child Health, Aga Khan University, Pakistan.
Salary
-
Department of Cardiology, Boston Children's Hospital, USA.
Salary
-
Pediatric Residency, University of Florida, USA.
Stipend
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Alina Sadaf, Steve Colan, Najveen Alvi and Babar Hasan are investigators in an RCT that meets inclusion criteria for this review although results of the trial are not yet available.
The remaining author (Jai Das) declares no known conflicts of interest.
New
References
References to studies included in this review
Fernandes 2013 {published and unpublished data}
- Fernandes JL, Sampaio EF, Fertrin K, Coelho OR, Logetto S, Piga A, et al. Amlodipine reduces cardiac iron overload in patients with thalassemia major: a pilot trial. American Journal of Medicine 2013;126:834‐7. [CFGD Register: TH148] [DOI] [PubMed] [Google Scholar]
Fernandes 2016 {published data only}
- Fernandes JL, Loggetto SR, Veríssimo MPA, Fertrin KY, Baldanzi GR, Fioravante LAB, et al. A randomised trial of amlodipine in addition to standard chelation therapy in patients with thalassemia major. Blood 2016;128(12):1555‐61. [DOI: 10.1182/blood-2016-06-721183; PUBMED: 27412888 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cappellini 2006 {published data only}
- Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, et al. A phase 3 study of deferasirox (ICL670), a once‐daily oral iron chelator, in patients with beta‐thalassemia. Blood 2006;107(9):3455‐62. [DOI] [PubMed] [Google Scholar]
Chirnomas 2009 {published data only}
- Chirnomas D, Smith AL, Braunstein J, Finkelstein Y, Pereira L, Bergmann AK, et al. Deferasirox pharmacokinetics in patients with adequate versus inadequate response. Blood 2009;114(19):4009‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2010 {published data only}
- Lai ME, Grady RW, Vacquer S, Pepe A, Carta MP, Bina P, et al. Increased survival and reversion of iron‐induced cardiac disease in patients with thalassemia major receiving intensive combined chelation therapy as compared to desferoxamine alone. Blood Cells, Molecules and Diseases 2010;45(2):136‐9. [DOI] [PubMed] [Google Scholar]
NCT00115349 {published data only}
- NCT00115349. Combination therapy compared with single‐drug therapy in patients with cardiac diseases [Thalassemia clinical research network ‐ Cardiac L1/DFO Trial]. clinicaltrials.gov/ct2/show/NCT00115349 Date first received: 21 June 2005.
NCT00712738 {unpublished data only}
- NCT00712738. Oral nifedipine to treat iron overload [A trial of oral nifedipine for the treatment of iron overload]. clinicaltrials.gov/ct2/show/NCT00712738 Date first received: 09 July 2008.
NCT01927913 {published data only}
- NCT01927913. Treatment of iron overload requiring chelation therapy [A 48‐week, open‐label, 2‐arm, parallel‐group, randomised exploratory study to assess liver iron concentration measured by FerriScan® (R2) magnetic resonance imaging in B‐thalassemia subjects administered SPD602 (SSP‐004184AQ) or Exjade® (Deferasirox) for treatment of chronic transfusional iron overload]. clinicaltrials.gov/ct2/show/NCT01927913 Date first received: 20 August 2013.
NCT02173951 {published data only}
- NCT02173951. An algorithm to start iron chelation in minimally transfused young beta‐thalassemia major patients [A decisional algorithm to start iron chelation in minimally transfused young beta‐thalassemia major patients naive to iron chelation therapy. A comparative randomised prospective Study]. clinicaltrials.gov/ct2/show/NCT02173951 Date first received: 23 June 2014.
NCT02671695 {published data only}
- El‐Haggar, SM. Effect of Spirulina Compared to Amlodipine on Cardiac Iron Overload in Children With Beta Thalassemia. www.clinicaltrials.gov Date first received: 2 February, 2016.
Neufeld 2012 {published data only}
- Neufeld EJ, Galanello R, Viprakasit V, Aydinok Y, Piga A, Harmatz P, et al. A phase 2 study of the safety, tolerability, and pharmacodynamics of FBS0701, a novel oral iron chelator, in transfusional iron overload. Blood 2012;119(14):3263‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Hasan 2014 {unpublished data only}
- Alvi N, Tipoo F, Rizvi A, Fadoo Z, Imran A, Sadaf A, et al. Effect of L‐type calcium channel blocker (amlodipine) on myocardial iron deposition in patients with thalassemia major: a randomised control trial. Haematologica. Conference: 21st congress of the European Hematology Association. Denmark 2016;101(S1):848‐9. [CFGD Register: TH137b] [Google Scholar]
- Shakoor A, Zahoor M, Sadaf A, Alvi N, Fadoo Z, Rizvi A, et al. Effect of L‐type calcium channel blocker (amlodipine) on myocardial iron deposition in patients with thalassaemia with moderate‐to‐severe myocardial iron deposition: protocol for a randomised, controlled trial. BMJ (Clinical Research Ed.) 2014;4(12):e005360. [CENTRAL: 1051146; CFGD Register: TH137a; CRS: 5500133000000011; JID:: 101552874; PMCID:: PMC4265146; PUBMED: 25492271] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor A, Zahoor M, Sadaf A, Alvi N, Fadoo Z, Rizvi A, et al. Effect of L‐type calcium channel blocker (amlodipine) on myocardial iron deposition in patients with thalassaemia with moderate‐to‐severe myocardial iron deposition: protocol for a randomised, controlled trial. BMJ Open 2014; Vol. 4:e005360. [DOI: 10.1136/bmjopen-2014-005360] [DOI] [PMC free article] [PubMed]
NCT02474420 {unpublished data only}
- NCT02474420. Amlodipine as adjuvant treatment to iron chelation for prevention of cardiac iron overload in thalassemia patients (CANALI) [The use of the calcium channel blocker amlodipine as an adjuvant treatment to iron chelation for the prevention of iron overload cardiomyopathy in patients with thalassemia]. clinicaltrials.gov/ct2/show/NCT02474420 Date first received: 11 June 2015.
Additional references
Alpendurada 2012
- Alpendurada F, Smith GC, Carpenter JP, Nair SV, Tanner MA, Banya W, et al. Effects of combined deferiprone with deferoxamine on right ventricular function in thalassaemia major. Journal of Cardiovascular Magnetic Resonance 2012;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alwan 1997
- Alwan A, Modell B. Community control of genetic and congenital disorders. Eastern Mediterranean region office technical publication series 24. Alexandria, VA: World Health Organization, 1997. [Google Scholar]
Anderson 2001
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2‐star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European Heart Journal 2001;22(23):2171‐9. [DOI] [PubMed] [Google Scholar]
Angastiniotis 2013
- Angastiniotis M, Corrons JLV, Soteriades ES, Eleftheriou A. The impact of migrations on the health services for rare diseases in Europe: the example of haemoglobin disorders. Scientific World Journal 2013;2013:Article ID: 727905. [DOI: 10.1155/2013/727905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borgna‐Pignatti 2004
- Borgna‐Pignatti C, Rugolotto S, Stefano P, Zhao H, Cappellini MD, Vecchio GC, et al. Survival and complications in patients with thalassaemia major treated with transfusion and deferoxamine. Haematologica 2004;89(10):1187‐93. [PubMed] [Google Scholar]
Borgna‐Pignatti 2014
- Borgna‐Pignatti C, Meloni A, Guerrini G, Gulino L, Filosa A, Ruffo GB, et al. Myocardial iron overload in thalassaemia major. How early to check?. British Journal of Haematology 2014;164(4):579‐85. [DOI] [PubMed] [Google Scholar]
Buja 1971
- Buja LM, Roberts WC. Iron in the heart: etiology and clinical significance. American Journal of Medicine 1971;51(2):209‐21. [DOI] [PubMed] [Google Scholar]
Cao 2002
- Cao A, Galanello R. Effect of consanguinity on screening for thalassemia. New England Journal of Medicine 2002;347:1200‐2. [DOI] [PubMed] [Google Scholar]
Cappellini 2007
- Cappellini MD, Bejaoui M, Agaoglu L, Porter J, Coates T, Jeng M, et al. Prospective evaluation of patient‐reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with beta‐thalassemia. Clinical Therapeutics 2007;29(5):909‐17. [DOI] [PubMed] [Google Scholar]
Carpenter 2011
- Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, Noronha SV, et al. On T2* magnetic resonance and cardiac iron. Circulation 2011;123(14):1519‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassinerio 2012
- Cassinerio E, Roghi A, Pedrotti P, Brevi F, Zanaboni L, Graziadei G, et al. Cardiac iron removal and functional cardiac improvement by different iron chelation regimens in thalassemia major patients. Annals of Hematology 2012;91(9):1443‐9. [DOI] [PubMed] [Google Scholar]
Charafeddine 2008
- Charafeddine K, Isma'eel H, Charafeddine M, Inati A, Koussa S, Naja M, et al. Survival and complications of beta‐thalassaemia in Lebanon: a decade’s experience of centralized care. Acta Haematologica 2008;120(2):112‐6. [DOI: 10.1159/000171088] [DOI] [PubMed] [Google Scholar]
Cohen 2003
- Cohen AR, Galanello R, Piga A, Sanctis V, Tricta F. Safety and effectiveness of long‐term therapy with the oral iron chelator deferiprone. Blood 2003;102(5):1583‐7. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomised trials.. Statistics in Medicine 2002;21(19):2071‐80. [DOI] [PubMed] [Google Scholar]
Eaton 2002
- Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radical Biology & Medicine 2002;32(9):833‐40. [DOI] [PubMed] [Google Scholar]
Egstrup 1993
- Egstrup K, Andersen PE. Transient myocardial ischaemia during nifedipine therapy in stable angina pectoris, and its relation to coronary collateral flow and comparison with metoprolol. American Journal of Cardiology 1993;71(2):177‐83. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues.. International Journal of Epidemiology Feb 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Engle 1964
- Engle MA, Erlandson M, Smith CH. Late cardiac complications of chronic, severe, refractory anaemia with haemochromatosis. Circulation 1964;30:698‐705. [DOI] [PubMed] [Google Scholar]
Ferguson 1989
- Ferguson DW, Hayes DW. Nifedipine potentiates cardiopulmonary baroreflex control of sympathetic nerve activity in healthy humans: direct evidence from microneurographic studies. Circulation 1989;80(2):285‐98. [DOI] [PubMed] [Google Scholar]
Fisher 2013a
- Fisher SA, Brunskill SJ, Doree C, Gooding S, Chowdhury O, Roberts DJ. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion‐dependent thalassaemia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004450.pub3] [DOI] [PubMed] [Google Scholar]
Fisher 2013b
- Fisher SA, Brunskill SJ, Doree C, Chowdhury O, Gooding S, Roberts DJ. Oral deferiprone for iron chelation in people with thalassaemia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004839.pub3] [DOI] [PubMed] [Google Scholar]
Grace 2000
- Grace AA, Camm AJ. Voltage‐gated calcium‐channels and antiarrhythmic drug action. Cardiovascular Research 2000;45(1):43‐51. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations.. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guazzi 1983
- Guazzi MD, Polese A, Fiorentini C, Bartorelli A, Moruzzi P. Treatment of hypertension with calcium antagonists. Review. Hypertension 1983;5(4 Pt 2):1185‐90. [DOI] [PubMed] [Google Scholar]
Gujja 2010
- Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. Journal of the American College of Cardiology 2010;56(13):1001‐12. [DOI: 10.1016/j.jacc.2010.03.083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henneman 2012
- Henneman A, Thornby KA. Risk of hypotension with concomitant use of calcium‐channel blockers and macrolide antibiotics. American Journal of Health‐System Pharmacy 2012;69(12):1038‐43. [DOI] [PubMed] [Google Scholar]
Hershko 2010
- Hershko C. Pathogenesis and management of iron toxicity in thalassaemia. Annals of the New York Academy of Sciences 2010;1202:1‐9. [DOI: 10.1111/j.1749-6632.2010.05544.x] [DOI] [PubMed] [Google Scholar]
Hider 2003
- Hider RC, Liu ZD. Emerging understanding of the advantage of small molecules such as hydroxypyridinones in the treatment of iron overload. Current Medicinal Chemistry 2003;10(12):1051‐64. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP, White IR, Anzures‐Cabrera J. Meta‐analysis of skewed data: combining results reported on log‐transformed or raw scales. Statistics in Medicine 2008;27(29):6072‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kim 1995
- Kim E, Giri SN, Pessah IN. Iron(II) is a modulator of ryanodine‐sensitive calcium channels of cardiac muscle sarcoplasmic reticulum. Toxicology and Applied Pharmacology 1995;130(1):57‐66. [DOI] [PubMed] [Google Scholar]
Kirk 2009
- Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009;120(20):1961‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirk 2012
- Kirk P, Sheppard M, Carpenter J, Anderson L, He T, Noronha S, et al. Fibrosis in cardiac siderosis: update on historical perspectives. Haematologica 2012;97 Suppl 1:382. [Google Scholar]
Kumfu 2012
- Kumfu S, Chattipakorn S, Chinda K, Fucharoen S, Chattipakorn N. T‐type calcium channel blockade improves survival and cardiovascular function in thalassaemic mice. European Journal of Haematology 2012;88(6):535‐48. [DOI: 10.1111/j.1600-0609.2012.01779.x] [DOI] [PubMed] [Google Scholar]
Kumfu 2013
- Kumfu S, Chattipakorn S, Fucharoen S, Chattipakorn N. Ferric iron uptake into cardiomyocytes of β‐thalassaemic mice is not through calcium channels. Drug and Chemical Toxicology 2013;36(3):329‐34. [DOI: 10.3109/01480545.2012.726625] [DOI] [PubMed] [Google Scholar]
Kwiatkowski 2011
- Kwiatkowski JL. Real‐world use of iron chelators. Hematology. American Society of Hematology, Education Program 2011;1:451‐8. [DOI: 10.1182/asheducation-2011.1.451] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Link 1985
- Link G, Pinson A, Hershko C. Heart cells in culture: a model of myocardial iron overload and chelation. Journal of Laboratory and Clinical Medicine 1985;106(2):147‐53. [PubMed] [Google Scholar]
Link 1994
- Link G, Pinson A, Hershko C. Ability of the orally effective iron chelators dimethyl‐ and diethyl‐hydroxypyrid‐4‐one and of deferoxamine to restore sarcolemmal thiolic enzyme activity in iron‐loaded heart cells. Blood 1994;83(9):2692‐7. [PubMed] [Google Scholar]
Link 2001
- Link G, Konijn AM, Breuer W, Cabantchik ZI, Hershko C. Exploring the “iron shuttle” hypothesis in chelation therapy: effects of combined deferoxamine and deferiprone treatment in hypertransfused rats with labelled iron stores and in iron‐loaded rat heart cells in culture. Journal of Laboratory and Clinical Medicine 2001;138(2):130‐8. [DOI] [PubMed] [Google Scholar]
Livrea 1996
- Livrea MA, Tesoriere L, Pintaudi AM, Calabrese A, Maggio A, Freisleben HJ, et al. Oxidative stress and antioxidant status in beta‐thalassaemia major: iron overload and depletion of lipid‐soluble antioxidants. Blood 1996;88(9):3608‐14. [PubMed] [Google Scholar]
Ludwiczek 2007
- Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter‐1. Nature Medicine 2007;13(4):448‐54. [DOI] [PubMed] [Google Scholar]
Maggio 2012
- Maggio A, Vitrano A, Lucania G, Capra M, Cuccia L, Gagliardotto F, et al. Long‐term use of deferiprone significantly enhances left‐ventricular ejection function in thalassemia major patients. American Journal of Hematology 2012;87(7):732‐3. [DOI] [PubMed] [Google Scholar]
Meerpohl 2017
- Meerpohl JJ, Antes G, Rücker G, Fleeman N, Motschall E, Niemeyer CM, et al. Deferasirox for managing iron overload in people with thalassaemia. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD007476.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Modell 2000
- Modell B, Khan M, Darlison M. Survival in beta‐thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051‐2. [DOI] [PubMed] [Google Scholar]
Modell 2007
- Modell B, Darlison M, Birgens H, Cario H, Faustino P, Giordano PC, et al. Epidemiology of haemoglobin disorders in Europe: an overview. Scandinavian Journal of Clinical & Laboratory Investigation 2007;67(1):39‐70. [DOI] [PubMed] [Google Scholar]
Modell 2008
- Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organization 2008;86:480‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2010
- Murphy CJ, Oudit GY. Iron‐overload cardiomyopathy: pathophysiology, diagnosis, and treatment. Journal of Cardiac Failure 2010;16(11):888‐900. [DOI: 10.1016/j.cardfail.2010.05.009] [DOI] [PubMed] [Google Scholar]
Olivieri 1994
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, et al. Survival in medically treated patients with homozygous beta‐thalassaemia. New England Journal of Medicine 1994;331(9):574‐8. [DOI] [PubMed] [Google Scholar]
Oudit 2003
- Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, et al. L‐type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron‐overload cardiomyopathy. Nature Medicine 2003;9(9):1187‐94. [DOI] [PubMed] [Google Scholar]
Oudit 2006
- Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH. Role of L‐type Ca2+ channels in iron transport and iron‐overload cardiomyopathy. Journal of Molecular Medicine 2006;84(5):349‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmer 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pennell 2014
- Baksi AJ, Penell DJ. T2* imaging of the heart: methods, applications, and outcomes. Topics in Magnetic Resonance Imaging 2014;23(1):13‐20. [DOI] [PubMed] [Google Scholar]
Pepe 2013
- Pepe A, Meloni A, Rossi G, Cuccia L, D'Ascola GD, Santodirocco M, et al. Cardiac and hepatic iron and ejection fraction in thalassemia major: multicentre prospective comparison of combined deferiprone and deferoxamine therapy against deferiprone or deferoxamine monotherapy. Journal of Cardiovascular Magnetic Resonance 2013;15(1):1. [DOI: 10.1186/1532-429X-15-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rachmilewitz 2011
- Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood 2011;118(13):3479‐88. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rund 2005
- Rund D, Rachmilewitz E. Beta‐thalassemia. New England Journal of Medicine 2005;353(11):1135‐46. [DOI] [PubMed] [Google Scholar]
Russell 1988
- Russell RP. Side effects of calcium channel blockers. Hypertension 1988;11:1142. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sica 2006
- Sica DA. Interaction of grapefruit juice and calcium channel blockers. American Journal of Hypertension 2006;19:768‐73. [DOI] [PubMed] [Google Scholar]
Singh 1986
- Singh BN. The mechanism of action of calcium antagonists relative to their clinical applications. British Journal of Clinical Pharmacology 1986;21 Suppl 2:109‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsushima 1999
- Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Modulation of iron uptake in heart by L‐type Ca2+ channel modifiers: possible implications in iron overload. Circulation Research 1999;84(11):1302‐9. [DOI] [PubMed] [Google Scholar]
Voskaridou 2012
- Voskaridou E1, Ladis V, Kattamis A, et al. A national registry of haemoglobinopathies in Greece: deducted demographics, trends in mortality and affected births.. Annals of Hematology Sept 2012;91(9):1451‐8. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang ZJ, Fischer R, Chu Z, Mahoney DH Jr, Mueller BU, Muthupillai R. Assessment of cardiac iron by MRI susceptometry and R2* in patients with thalassemia. Magnetic Resonance Imaging 2010;28(3):363‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weatherall 2001
- Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bulletin of the World Health Organization 2001;79:704‐12. [PMC free article] [PubMed] [Google Scholar]
Wenzel 1997
- Wenzel RR, Allegranza G, Binggeli C, Shaw S, Weidmann P, Luscher TF, et al. Differential activation of cardiac and peripheral sympathetic nervous system by nifedipine: role of pharmacokinetics. Journal of the American College of Cardiology 1997;29(7):1607‐14. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [DOI] [PubMed] [Google Scholar]
Wood 2004
- Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion‐dependent thalassemia and sickle cell disease. Blood 2004;103(5):1934–6. [DOI] [PubMed] [Google Scholar]
Wood 2005
- Wood JC, Enriquez C, Ghugre N, Otto‐Duessel M, Aguilar M, Nelson MD, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassaemia. Annals of the New York Academy of Sciences 2005;1054:386‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood JC, Origa R, Agus A, Matta G, Coates TD, Galanello R. Onset of cardiac iron loading in pediatric patients with thalassemia major. Haematologica 2008;93(6):917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2013
- Xia S, Zhang W, Huang L, Jiang H. Comparative efficacy and safety of deferoxamine, deferiprone and deferasirox on severe thalassemia: a meta‐analysis of 16 randomised controlled trials. PLoS One 2013;8(12):e82662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhelyazkova‐Savova 2014
- Zhelyazkova‐Savova M, Gancheva S, Sirakova S. Potential statin‐drug interactions: prevalence and clinical significance. Springerplus 2014;3:168. [DOI] [PMC free article] [PubMed] [Google Scholar]


