Abstract
Background
Hypoxic‐ischaemic encephalopathy (HIE) is a serious birth complication affecting term and late preterm newborns. Although therapeutic hypothermia (cooling) has been shown to be an effective therapy for neonatal HIE, many cooled infants have poor long‐term neurodevelopmental outcomes. In animal models of neonatal encephalopathy, inhaled xenon combined with cooling has been shown to offer better neuroprotection than cooling alone.
Objectives
To determine the effects of xenon as an adjuvant to therapeutic hypothermia on mortality and neurodevelopmental morbidity, and to ascertain clinically important side effects of xenon plus therapeutic hypothermia in newborn infants with HIE. To assess early predictors of adverse outcomes and potential side effects of xenon.
Search methods
We used the standard strategy of the Cochrane Neonatal Review Group to search the Cochrane Library (2017, Issue 8), MEDLINE (from 1966), Embase (from 1966), and PubMed (from 1966) for randomised controlled and quasi‐randomised trials. We also searched conference proceedings and the reference lists of cited articles. We conducted our most recent search in August 2017.
Selection criteria
We included all trials allocating term or late preterm encephalopathic newborns to cooling plus xenon or cooling alone, irrespective of timing (starting age and duration) and concentrations used for xenon administration.
Data collection and analysis
Two review authors independently assessed results of searches against predetermined criteria for inclusion, assessed risk of bias, and extracted data. We performed meta‐analyses using risk ratios (RRs), risk differences (RDs), and number needed to treat for an additional beneficial outcome (NNTB) with 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs) for continuous data.
Main results
A single randomised controlled trial enrolling 92 participants was eligible for this review. Researchers have not reported long‐term neurodevelopmental outcomes, including the primary outcome of this review ‐ death or long‐term major neurodevelopmental disability in infancy (18 months to three years of age). Cooling plus xenon was not associated with reduced mortality at latest follow‐up, based upon low quality evidence. Investigators noted no substantial differences between groups for other secondary outcomes of this review, such as biomarkers of brain damage assessed with magnetic resonance imaging and occurrence of seizures during primary hospitalisation. Available data do not show an increased adverse event rate in the cooling plus xenon group compared with the cooling alone group.
Authors' conclusions
Current evidence from one small randomised controlled pilot trial is inadequate to show whether cooling plus xenon is safe or effective in near‐term and term newborns with HIE. Further trials reporting long‐term neurodevelopmental outcomes are needed.
Plain language summary
Cooling plus inhaled xenon for newborns with hypoxic‐ischaemic encephalopathy
Review question: How does cooling plus inhaled xenon compare with cooling alone for improving survival and development of newborn babies who may have suffered from lack of oxygen at birth?
Background: Hypoxic‐ischaemic encephalopathy, or HIE, is a brain injury caused by oxygen deprivation to the brain during birth (birth asphyxia). Hypoxic‐ischaemic encephalopathy is a leading cause of death or severe impairment among infants. Therapeutic hypothermia (cooling) is a treatment option available to reduce the chances of severe brain damage when an infant's body temperature is reduced shortly after birth. Although cooling has been shown to be an effective therapy for neonatal HIE, half of treated newborn babies still die or face neurodevelopmental sequelae later in life. Evidence indicates that inhaled xenon, an odourless gas, in combination with body cooling, can help to improve survival and development at 18 to 36 months.
Study characteristics: This review found a single randomised controlled trial that examined the short‐term effects of cooling plus xenon for infants with HIE.
Key results: This trial enrolled 92 participants. Cooling plus xenon did not improve clinical outcomes before discharge from the hospital compared with cooling alone. Data on long‐term development were not provided.
Quality of evidence: Current low quality evidence is inadequate to show whether cooling plus xenon improves survival and development of newborn babies with HIE. Evidence is up‐to‐date as of August 2017.
Summary of findings
for the main comparison.
| Cooling plus xenon compared with cooling alone for newborns with hypoxic‐ischaemic encephalopathy | ||||||
|
Patient or population: late preterm or term newborns with hypoxic‐ischaemic encephalopathy Settings: neonatal intensive care unit Intervention: cooling plus xenon Comparison: cooling alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cooling alone | Risk with cooling plus xenon | |||||
| Death or major neurodevelopmental disability in infancy | No data | No data | No data | No data | Absence of evidence | |
| Mortality at latest reported age | Study population |
RR 1.22 (0.56 to 2.67) |
92 (1 RCT) | ⊕⊕⊝⊝ Low | Single study Unblinded trial |
|
| 196 per 1000 | 239 per 1000 | |||||
|
Major neurodevelopmental disability in infancy |
No data | No data | No data | No data | Absence of evidence | |
|
Major neurodevelopmental disability at school age |
No data | No data | No data | No data | Absence of evidence | |
| Cerebral palsy in infancy | No data | No data | No data | No data | Absence of evidence | |
|
Developmental delay or intellectual impairment in infancy |
No data | No data | No data | No data | Absence of evidence | |
| Blindness vision in infancy | No data | No data | No data | No data | Absence of evidence | |
| Sensorineural deafness in infancy requiring amplification | No data | No data | No data | No data | Absence of evidence | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Intrapartum asphyxia is the third leading cause of child death globally (Liu 2015). It is estimated that each year, over 0.7 million affected newborns die and 1.15 million develop acute disordered brain function known as 'hypoxic‐ischaemic encephalopathy (HIE)' (Lee 2013). HIE, which is one of the most common causes of childhood neurodisability worldwide, results in considerable psychosocial and economic impact for families and society (Lawn 2014). Induced therapeutic hypothermia (body cooling) has emerged as an effective neuroprotective strategy for term and late preterm newborns with moderate to severe HIE. However, in the developed world, half of treated infants still die or face neurodevelopmental sequelae later in life (Jacobs 2013).
Human and animal studies have demonstrated that the basic cascade of brain injury related to hypoxic‐ischaemic insults typically occurs in distinct phases (Hassell 2015); in the acute phase, the culmination of energy failure, acidosis, glutamate release, lipid peroxidation, and the toxic effect of nitric oxide leads to cell death via necrosis and activates apoptotic cascades (Ferriero 2004). After partial recovery and a latent phase that lasts up to six hours, secondary deterioration occurs. This secondary phase is characterised by cytotoxic oedema, excitotoxicity, and secondary energy failure with nearly complete loss of mitochondrial activity (Douglas‐Escobar 2015). In newborns with moderate to severe HIE, this secondary phase of injury is typically associated with clinical deterioration and increased seizure activity. Magnetic resonance spectroscopy (MRS), which is the most accurate quantitative magnetic resonance biomarker in the neonatal period for prediction of neurodevelopmental outcome after HIE (Thayyil 2010), shows that this secondary phase is generally accompanied by a second lactate elevation (Barkovich 1995). A tertiary phase involves active pathological processes that occur for months after a hypoxic‐ischaemic insult, including late cell death, remodelling of the injured brain, and astrogliosis due to persistent inflammation and epigenetic changes (Fleiss 2012). It is the time period following resuscitation, before the secondary phase of injury, that provides a potential window for neuroprotection or diminution of injury.
Description of the intervention
Xenon is an odourless, dense, noble gas that has been approved as an inhalational anaesthetic in adults. Xenon has a rapid onset of action via inhalation and is eliminated unchanged via the lungs within minutes of cessation of delivery. Upon administration, xenon rapidly decreases amplitude‐integrated electroencephalographic (aEEG) background activity (Sabir 2016), which is consistent with clinical findings that demonstrate its anticonvulsant and electroencephalographic (EEG) suppressant effects in infants with HIE (Azzopardi 2013). Small and large preclinical studies have evaluated its potential as a neuroprotective agent, when inhaled at a subanaesthetic concentration of 50% (Dingley 2006; Ma 2005). In animal models of moderate HIE, xenon significantly reduced brain injury and had an additive neuroprotective effect when combined with cooling immediately after the insult (Chakkarapani 2010; Dingley 2008; Liu 2015; Thoresen 2009). This benefit was sustained with complete restoration of long‐term functional outcomes and improved regional histopathology (Hobbs 2008).
The optimal timing, dose, and duration of xenon inhalation have not yet been established. Xenon has been shown to be neuroprotective in neonatal rats when administered before (Ma 2006), during (Ma 2005), and after a hypoxic insult (Dingley 2006). When administered immediately or within hours after a hypoxic‐ischaemic event, xenon had a significant effect at concentrations of 40% (Xe40%) and greater (Ma 2005). When combined with therapeutic hypothermia in a hypoxic‐ischaemic pig model, xenon was effective and safe in concentrations up to 70% and for as long as 24 hours (Chakkarapani 2010; Dingley 2008; Faulkner 2011). Although Xe70% has been suggested to be more neuroprotective than Xe50%, most preclinical studies used concentrations ≤ 50% that induced sedation and allowed administration of substantial concentrations of oxygen but did not result in respiratory depression (Dingley 2008).
In the field of adult critical care medicine, the cardiovascular, analgesic, and safety profile of xenon has been thoroughly evaluated (Dingley 2001; Rossaint 2003; Sanders 2005). In the paediatric population, however, its safety has not yet been assessed systematically. In a piglet model of HIE that closely resembles perinatal asphyxia, xenon together with therapeutic hypothermia improved cardiovascular stability and reduced the requirement for inotropes (Chakkarapani 2012). Investigators noted no increase in oxygen requirements, no cuffed tracheal tube complications, and no stridor or extubation delays either during or after xenon delivery (Chakkarapani 2010). Despite their favourable short‐term safety profile, considerable controversy surrounds the lasting effects of anaesthetic agents on the developing brain in general (Sun 2010). Animal studies have demonstrated that general anaesthetic agents produce accelerated apoptosis and cause adverse effects on cognition and behaviour (Andropoulos 2017; Jevtovic‐Todorovic 2013). Xenon acts mainly by inhibiting the N‐methyl‐D‐aspartate (NMDA) receptor, but in contrast to other inhalation anaesthetic agents, xenon lacks dopamine‐releasing properties and is not associated with an increase in neuroapoptosis (Faulkner 2011; Sabir 2013).
A major disadvantage of xenon is that it is difficult to use in clinical practice owing to its scarcity (0.0087 ppm in air) and high costs, along with the need for closed‐circuit delivery (including cuffed tubes) and recycling systems.
How the intervention might work
Xenon is an NMDA receptor antagonist that prevents postsynaptic binding of the excitatory neurotransmitter glutamate (Franks 1998). It competitively binds to the glycine site of the receptor by interacting with the aromatic ring of phenylalanine (Armstrong 2012; Dickinson 2007). Researchers have demonstrated the neuroprotective properties of xenon in cell culture (Petzelt 2003), in a rodent model of hypoxia‐ischaemia (Dingley 2008; Hobbs 2008; Ma 2005; Thoresen 2009; Zhuang 2012), and in a neonatal pig model of global hypoxia‐ischaemia (Chakkarapani 2010; Faulkner 2011). Apart from its blocking effect on NMDA receptors, additional neuroprotective mechanisms have been identified. Xenon activates two species of potassium channels including the inwardly rectifying KATP channel, as reported in Bantel 2010, and the two pore domain K+channels studied by Gruss 2004, both of which have been linked to neuroprotection. Other actions include inhibition of the calcium/calmodulin‐dependent protein kinase II (Petzelt 2001), as well as activation of the antiapoptotic effectors Bcl‐XL and Bcl‐2 (Ma 2007). Furthermore, xenon increases the production of hypoxia‐inducible factor 1 alpha (HIF‐1 alpha) and its downstream effectors erythropoietin, vascular endothelial growth factor, and glucose transporter 1 protein, which can interrupt the apoptotic pathway (Ma 2009).
Through inhibition of NMDA receptors and reduction of apoptotic cell death, xenon is believed to exert most of its neuroprotective properties in the early and late phases of reperfusion injury.
Why it is important to do this review
Cooling has been shown to be an effective therapy for neonatal HIE. However, the rate of death and disability remains at about 50% in treated infants, necessitating the development of additional neuroprotective therapies. This is the first systematic review conducted to assess the evidence for xenon as an adjuvant to therapeutic hypothermia for newborns with HIE.
Objectives
To determine the effects of xenon as an adjuvant to therapeutic hypothermia on mortality and neurodevelopmental morbidity, and to ascertain clinically important side effects of xenon plus therapeutic hypothermia in newborn infants with HIE. To assess early predictors of adverse outcomes and potential side effects of xenon.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐RCTs that compare cooling plus xenon versus cooling alone.
Types of participants
-
Newborn infants at 35 weeks’ gestation or greater with:
-
evidence of peripartum asphyxia, with each enrolled infant satisfying at least one of the following criteria.
Apgar score ≤ 5 at 10 minutes.
Mechanical ventilation or resuscitation at 10 minutes.
Cord pH < 7.1, or arterial pH < 7.1, or base deficit ≥ 12 within 60 minutes of birth.
-
evidence of encephalopathy according to Sarnat staging (Finer 1981; Sarnat 1976).
Stage 1 (mild): hyperalertness, hyperreflexia, dilated pupils, tachycardia, absence of seizures.
Stage 2 (moderate): lethargy, hyperreflexia, miosis, bradycardia, seizures, hypotonia with weak suck, and Moro.
Stage 3 (severe): stupor, flaccidity, small to mid‐position pupils that react poorly to light, decreased stretch reflexes, hypothermia, and absent Moro.
induced therapeutic hypothermia treatment (whole body or selective head cooling to 32°C to 34°C) initiated within six hours after birth; and
no major congenital abnormalities recognisable at birth.
-
Types of interventions
Inhaled xenon (irrespective of timing and concentrations used) as an adjuvant to therapeutic hypothermia versus therapeutic hypothermia alone, based on the following prespecified definitions.
-
Therapeutic hypothermia.
Standard therapeutic hypothermia (whole body or selective head cooling to 32°C to 34°C initiated within six hours after birth and continued for 72 hours before slow rewarming.
-
Xenon administration.
Irrespective of timing (starting age and duration) and concentrations used.
Types of outcome measures
Primary outcomes
The primary outcome measure was death or long‐term major neurodevelopmental disability in infancy (18 months to three years of age) defined as the following.
Cerebral palsy (CP), graded according to the Gross Motor Function Classification System of Palisano 1997 for children two years of age and younger.
Developmental delay (Bayley or Griffith assessment more than two standard deviations (SD) below the mean).
Intellectual impairment (intelligence quotient (IQ) more than two SD below the mean).
Blindness (vision < 6/60 in both eyes).
Sensorineural deafness requiring amplification.
Secondary outcomes
Secondary outcomes included the following.
Mortality (all‐cause mortality at latest reported age).
-
Major neurodevelopmental disability:
in infancy (18 months to three years of age); and
at school age (> five years).
-
Major neurodevelopmental disability in infancy (18 months to three years of age) consists of the following components.
CP, graded according to the Gross Motor Function Classification System of Palisano 1997 for children two years of age and younger.
-
Developmental delay or intellectual impairment.
Bayley or Griffith assessment more than two SD below the mean or intellectual impairment (IQ more than two SD below mean).
Neuromotor development (Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI)) assessed in survivors.
Mental development (Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI)) assessed in survivors.
Blindness (vision < 6/60 in both eyes).
Sensorineural deafness requiring amplification.
-
Cognitive and educational outcomes in survivors over five years of age.
IQ and/or indices of educational achievement measured by a validated assessment tool including school examination results.
-
Additional predictors of neurodevelopmental outcome.
Severity of encephalopathy at enrolment (Sarnat staging) (Finer 1981; Sarnat 1976).
-
Severity of EEG abnormality at enrolment.
Severe: isoelectric or burst‐suppression pattern.
Moderate: low voltage or discontinuous background.
Mild: electrographic seizures, dysmaturity.
-
Seizures.
Seizures during initial hospitalisation.
Seizures or need for anticonvulsants at follow‐up.
-
Magnetic resonance imaging (MRI) abnormalities during primary hospitalisation.
Moderate or severe abnormalities in the basal ganglia or thalamus, severe white matter lesions, or abnormalities in the posterior limb of the internal capsule (Rutherford 2010).
-
Potential adverse effects of xenon therapy during or immediately after administration.
-
Heart rate.
Sinus bradycardia (heart rate < 80 beats/min).
Sinus tachycardia (heart rate > 180/min).
Prolonged QT interval.
Major arrhythmia (requiring medical intervention or cessation of xenon therapy, or both).
-
Blood pressure.
Hypotension (mean arterial pressure (MAP) < 40 mmHg).
Need for inotrope support.
-
Respiratory impairment.
Pneumonia.
Pulmonary air leak.
Pulmonary haemorrhage.
Persistent pulmonary hypertension (PPHN) (diagnosed clinically or by echocardiogram).
-
Cuffed endotracheal tube complications.
Extubation stridor.
Skin rashes.
-
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials (RCTs) or quasi‐RCTs and considered only parallel‐group trials. We applied no language, publication year, or publication status restrictions.
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group. We undertook a comprehensive search of the following electronic sources.
We used MeSH terms and keywords to search the following.
MEDLINE (1966 to 01 August 2017).
Embase (1966 to 01 August 2017).
Cochrane Library (01 August 2017; 2017, Issue 8).
We used keywords (to retrieve e‐publications and items not indexed in MEDLINE).
PubMed (1966 to 01 August 2017).
Others.
Conference proceedings of the Perinatal Society of Australia and New Zealand (from 2005 to 01 August 2017).
Conference proceedings of the Pediatric Academic Societies (from 2000 to 01 August 2017).
We have presented in Appendix 1 the full search strategies for each database. We screened the reference lists of any cited articles.
Searching other resources
We searched clinical trial registries for ongoing and recently completed trials (e.g. ClinicalTrials.gov (clinicaltrials.gov), World Health Organization International Trials Registry and Platform, International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.isrctn.com/)).
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group as described below.
Selection of studies
Two review authors (CR and JC) independently searched and identified eligible trials that met the inclusion criteria using Covidence, which is an online screening and data extraction tool used for Cochrane Reviews (Covidence 2017). First, we screened titles and abstracts to identify potentially relevant citations; then we retrieved the full text of all potentially relevant articles. We independently assessed the eligibility of studies in accordance with the specified inclusion criteria. We reviewed studies for relevance based on study design and types of participants, interventions, and outcome measures. We resolved disagreements by discussion and, if necessary, by consultation with a third review author (PD).
We have provided details of studies excluded from the review in the Characteristics of excluded studies table, along with reasons for exclusion. We contacted trial authors if details of primary trials were unclear.
Data extraction and management
Two review authors (CR and JC) separately extracted, assessed, and coded all data for each study using an online screening and data extraction tool for Cochrane Reviews (Covidence 2017). One review author (CR) checked exported data using Review Manager 5 (RevMan 5) software (Review Manager 2014). A third review author addressed disagreements.
Assessment of risk of bias in included studies
Two review authors (CR and JC) independently assessed the risk of bias (as low, high, or unclear) of all included trials using the Cochrane 'Risk of bias' tool (Higgins 2011) for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third review author. See Appendix 2 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using standard methods of the Cochrane Neonatal Review Group. We analysed results of studies using RevMan 5 (Review Manager 2014), and we presented results as risk ratios (RRs), risk differences (RDs), number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH) for categorical variables. We used mean differences (MDs) for continuous variables. We reported 95% confidence intervals (95% CIs) for all estimates.
Unit of analysis issues
We included all RCTs and quasi‐RCTs. We took into account the level at which randomisation occurred, including cross‐over trials, cluster‐randomised trials, and multiple observations for the same outcome.
Dealing with missing data
We planned to request additional data from the authors of each trial if data on important outcomes were missing or needed clarification. When data were still missing, we planned to examine the effects of losses by performing sensitivity analysis. We performed these analyses by intention‐to‐treat.
Assessment of heterogeneity
We assessed statistical heterogeneity by examining the I² statistic ‐ a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than to sampling error (Higgins 2011). We applied I² statistic cutoffs and labels for heterogeneity as follows.
Less than 25%: no heterogeneity.
25% to 49%: heterogeneity.
50% to 74%: moderate heterogeneity.
≥ 75%: high heterogeneity.
We considered statistical heterogeneity to be substantial when the I² statistic value was greater than 50%. In addition, we employed the Chi² test of homogeneity to determine the strength of evidence that heterogeneity is genuine. We explored clinical variation across studies by comparing the distribution of important participant factors among trials and trial factors (randomisation concealment, blinding of outcome assessment, loss to follow‐up, treatment type, and co‐interventions). We considered a threshold P value less than 0.1 as an indicator of important heterogeneity (genuine variation in effect sizes).
Assessment of reporting biases
We planned to use funnel plots to assess small‐study effects. Owing to several possible explanations for funnel plot asymmetry, we planned to interpret results carefully (Sterne 2011).
Data synthesis
We planned to perform statistical analyses using standard methods of the Cochrane Neonatal Review Group. We used RevMan 5 software with the fixed‐effect model for meta‐analysis (Review Manager 2014). We used standardised mean differences (SMDs) to combine trials that measured the same outcome using different methods. We used weighted mean differences (WMDs) with 95% CIs for outcomes measured on a continuous scale.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
Death (as above).
Major neurodevelopmental disability (as above).
Each component of major neurodevelopmental disability (as above).
Two review authors (CR and JC) independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool (GRADEpro GDT) to create Table 1 to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence using one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
-
Severity of HIE.
-
Based on Sarnat score (Finer 1981; Sarnat 1976).
Mild versus moderate/severe.
-
Based on EEG or aEEG at baseline.
Mild (electrographic seizures, dysmaturity) versus moderate/severe (low voltage or discontinuous background/isoelectric or burst‐suppression pattern).
-
-
Xenon administration.
Concentration: < 30% versus ≥ 30%.
Starting age: < six hours versus ≥ six hours after insult.
Duration: < 12 hours versus ≥ 12 hours.
-
Gestational age.
Late preterm (35 0/7 through 36 6/7 gestational weeks) versus term infants (≥ 37 0/7 gestational weeks).
-
Quality of outcome assessment.
High quality (≥ 18 months with formal psychological testing and review by developmental paediatrician for diagnosis of cerebral palsy (CP)) versus lower quality.
Sensitivity analysis
We conducted sensitivity analyses to explore the effects of methodological quality of trials and to ascertain whether studies at high risk of bias overestimate the effects of treatment.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We assessed 89 titles and abstracts in electronic format. We assessed two studies as relevant and determined that one study met the criteria for inclusion (Figure 1, Study flow diagram).
1.
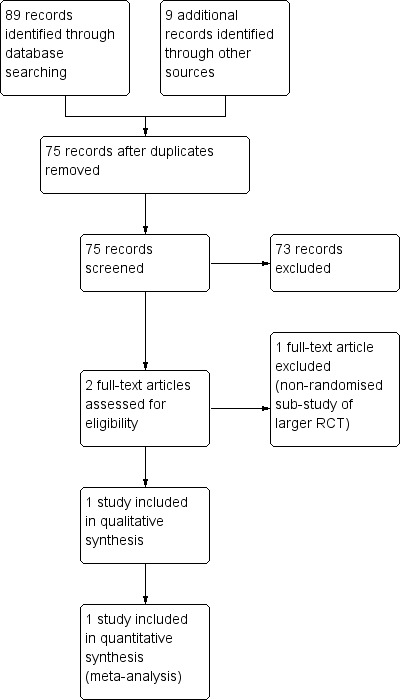
Study flow diagram.
Included studies
Azzopardi 2016
Azzopardi 2016 is a proof‐of‐concept RCT conducted at four neonatal intensive care units (NICUs) in the UK between January 2012 and September 2014. Researchers randomised 92 infants at gestational age 36 weeks or greater born with evidence of peripartum hypoxia‐ischaemia (based upon Apgar score ≤ 5 at 10 minutes after birth, continued need for resuscitation 10 minutes after birth, or acidosis within 1 hour of birth), moderate to severe encephalopathy, and moderately or severely abnormal background activity or seizures as shown by amplitude‐integrated EEG. Trialists cooled 46 infants in the intervention group to a target rectal temperature of 33.5°C and provided them with 30% xenon through an uncuffed endotracheal tube connected to a recirculating device developed for the trial. Investigators commenced xenon immediately after randomisation and continued this for 24 hours. Forty‐six infants in the control group received standard care and were cooled to a target rectal temperature of 33.5°C with servo‐controlled equipment. Researchers started whole‐body cooling within six hours of birth and continued this for 72 hours. The primary outcome for the study was a reduction in the lactate‐to‐N‐acetyl aspartate ratio in the thalamus and preserved fractional anisotropy in the posterior limb of the internal capsule, measured by magnetic resonance spectroscopy and magnetic resonance imaging, respectively, within 15 days of birth. Investigators performed prespecified subgroup analyses of the primary outcome by severity of the abnormality on aEEG at randomisation, time from birth to start of xenon therapy, and the relation between these measures and neurological findings at discharge. Secondary outcomes included maximum Thompson HIE score, neurological examination at discharge from the treatment centre, occurrence of seizures, intracranial haemorrhage, persistent hypotension, pulmonary haemorrhage, pulmonary hypertension, prolonged blood coagulation time, thrombocytopaenia, major venous thrombosis, cardiac arrhythmia, culture‐proven late‐onset sepsis, necrotising enterocolitis, pneumonia, pulmonary air leak, anuria or oliguria, age at which full oral feeding was achieved, duration of hospital stay, and grade of abnormalities on visual analysis of MRI. Study authors recorded serious adverse events and included death, hypertension (mean blood pressure > 85 mmHg), hypotension (mean blood pressure < 25 mmHg), cardiac arrhythmia (severe bradycardia (heart rate < 60 beats per minute) or ventricular arrhythmia), and inability to achieve adequate ventilation despite appropriate adjustment of ventilator settings. They assessed the primary outcome in 41 (89%) of 46 infants in the cooling plus xenon group and in 37 (80%) of 46 infants in the cooling only group. Lactate‐to‐N‐acetyl aspartate ratio in the thalamus and fractional anisotropy values in the posterior limb of the internal capsule were similar in the two groups. The thalamic geometric mean ratio of lactate to N‐acetyl aspartate was 1.09 (95% CI 0.90 to 1.32), and the mean difference in fractional anisotropy was –0.01 (95% CI –0.03 to 0.02). Nine (20%) infants in the cooling only group and 11 (24%) infants in the cooling plus xenon group died. Exclusion of deaths from the analysis did not significantly affect results.
Excluded studies
We excluded from this review one potentially relevant study (Azzopardi 2013), which was a nested, non‐randomised substudy of a larger RCT (Azzopardi 2016).
Risk of bias in included studies
We judged the included study to be at low risk of bias overall. See the risk of bias graph (Figure 2) and summary (Figure 3).
2.
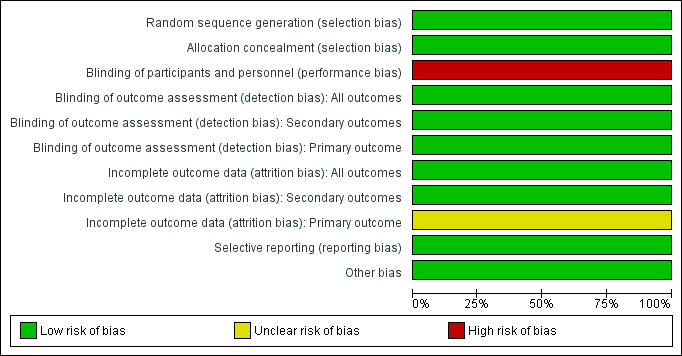
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
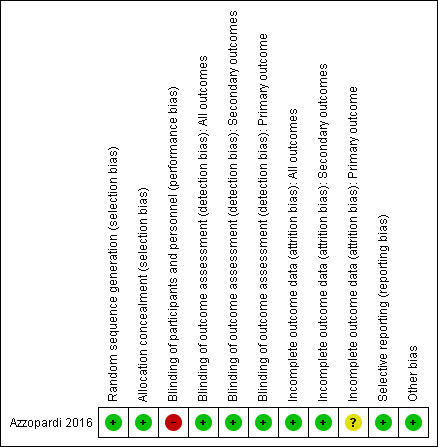
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators used a computer‐generated randomisation sequence. They concealed allocation to treatment by using a central, web‐based system with telephone backup (low risk of bias).
Blinding
This was an open‐label trial. Masking of investigators and parents to allocation was not feasible because of the need for a special ventilator to administer xenon (high risk of bias). However, investigators who assessed the primary outcome were masked to treatment allocation (low risk of bias). Unblinded assessors assessed secondary outcomes, but only two of these outcomes were prone to detection bias (Thompson score and neurological assessment at discharge; low risk of bias).
Incomplete outcome data
Investigators presented a complete flow chart for all screened and randomised infants. They randomised a total of 92 infants, 78 (85%) of whom were available for assessment of the primary outcome ‐ 41 (89%) of 46 in the cooling plus xenon group and 37 (80%) of 46 in the cooling alone group. Five (5%) infants did not have MRI scans done, although they were alive at discharge ‐ two (4%) in the cooling plus xenon group and three (7%) in the cooling alone group. Researchers assessed secondary outcomes completely. We judged the risk of attrition bias for all outcomes as low. An additional nine of 92 (9.7%) infants died before discharge and were excluded from the analysis ‐ three of 46 (6.5%) in the cooling plus xenon group and six of 46 (13.0%) in the cooling only group. However, it remains unclear at which postnatal age these deaths occurred (before or after the prespecified 15‐day window for MRI). Therefore, we decided to rate the risk of attrition bias for the primary outcome as unclear.
Selective reporting
The study protocol is available, and study authors reported all prespecified primary and secondary outcomes (low risk of bias).
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
See: Table 1
Comparison 1: cooling plus xenon versus cooling alone (all infants)
See Table 1.
-
Primary outcome.
Death or major neurodevelopmental disability: the included study did not report this outcome.
-
Secondary outcomes.
-
Mortality (Analysis 1.1, Figure 4).
At latest follow‐up: 11 (24%) infants in the cooling plus xenon group and nine (20%) infants in the cooling alone group died before discharge from the hospital. Cooling plus xenon was not associated with reduced mortality at the latest reported age (risk ratio (RR) 1.22, 95% confidence interval (CI) 0.56 to 2.67; risk difference (RD) 0.04, 95% CI ‐0.12 to 0.21; one study and 92 infants; low qualilty evidence).
-
Major neurodevelopmental disability.
In infancy: the included study did not report this outcome.
At school age: the included study did not report this outcome.
-
Each component of major neurodevelopmental disability in infancy.
Cerebral palsy: the included study did not report this outcome.
Developmental delay or intellectual impairment: the included study did not report this outcome.
Blindness vision: the included study did not report this outcome.
Sensorineural deafness requiring amplification: the included study did not report this outcome.
-
Cognitive and educational outcomes in survivors over five years old.
IQ and/or indices of educational achievement measured using a validated assessment tool including school examination results: the included study did not report this outcome.
-
Additional predictors of neurodevelopmental outcome.
Severity of encephalopathy at enrolment: the included study did not report this outcome.
Severity of EEG abnormality at enrolment: the included study did not report this outcome.
Seizures during initial hospitalisation: the included study did not report this outcome.
Seizures or need for anticonvulsants at follow‐up: the included study did not report this outcome.
Magnetic resonance imaging: the included study did not report this outcome.
-
Adverse events: data show no increase in adverse event rates in the cooling plus xenon group compared with the cooling alone group.
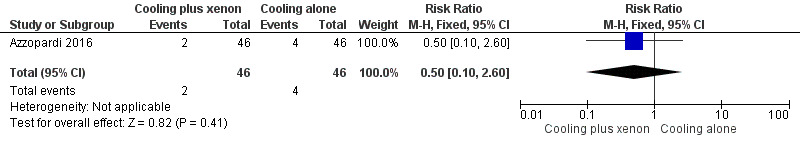
Cardiac arrhythmia (heart rate < 80 beats per minute; Analysis 1.2, Figure 5): two (4%) infants in the cooling plus xenon group and four (9%) infants in the cooling alone group were noted to have cardiac arrhythmias (RR 0.50, 95% CI 0.10 to 2.60; RD ‐0.04, 95% CI ‐0.14 to 0.06; one study and 92 infants).
Persistent hypotension (mean arterial pressure < 40 mmHg; Analysis 1.3, Figure 6): mean arterial pressure dropped persistently in 31 (67%) infants in the cooling plus xenon group and in 29 (63%) infants in the cooling alone group (RR 1.07, 95% CI 0.79 to 1.44; RD 0.04, 95% CI ‐0.15 to 0.24; one study and 92 infants).
Respiratory impairment (pneumonia, pulmonary air leak, pulmonary haemorrhage, persistent pulmonary hypertension; Analysis 1.4, Figure 7): eight (17%) infants in the cooling plus xenon group and seven (15%) infants in the cooling alone group were affected by respiratory complications (RR 1.14, 95% CI 0.45 to 2.89; RD 0.02, 95% CI ‐0.13 to 0.17; one study and 92 infants).
Cuffed endotracheal tube complications: uncuffed endotracheal tubes were used in the included study.
Skin rashes: the included study did not report this outcome.
-
1.1. Analysis.
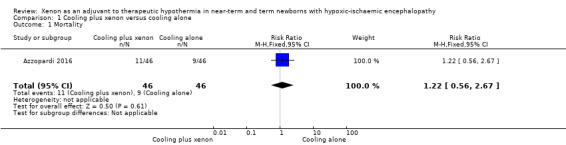
Comparison 1 Cooling plus xenon versus cooling alone, Outcome 1 Mortality.
4.
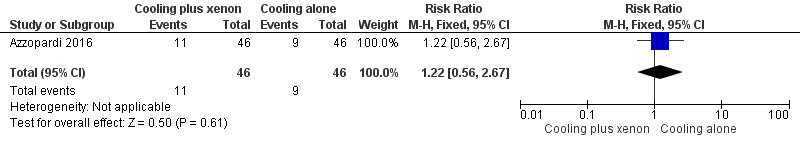
Forest plot of comparison: 1 Cooling plus xenon versus cooling alone, outcome: 1.1 Mortality.
1.2. Analysis.
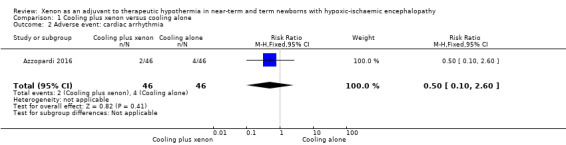
Comparison 1 Cooling plus xenon versus cooling alone, Outcome 2 Adverse event: cardiac arrhythmia.
5.
Forest plot of comparison: 1 Cooling plus xenon versus cooling alone, outcome: 1.2 Adverse event: cardiac arrhythmia.
1.3. Analysis.
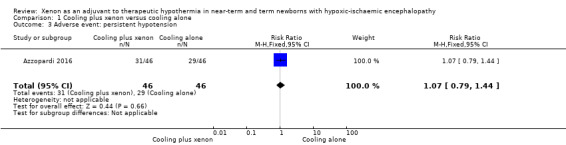
Comparison 1 Cooling plus xenon versus cooling alone, Outcome 3 Adverse event: persistent hypotension.
6.
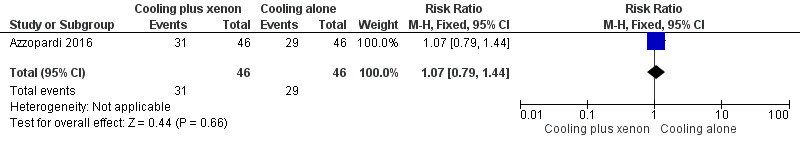
Forest plot of comparison: 1 Cooling plus xenon versus cooling alone, outcome: 1.3 Adverse event: persistent hypotension.
1.4. Analysis.
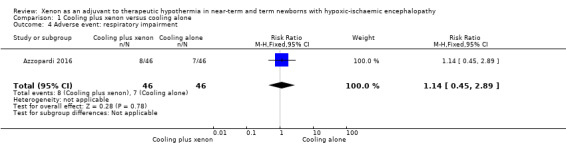
Comparison 1 Cooling plus xenon versus cooling alone, Outcome 4 Adverse event: respiratory impairment.
7.
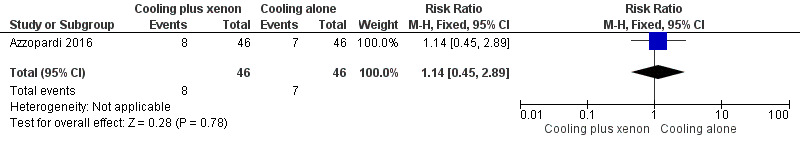
Forest plot of comparison: 1 Cooling plus xenon versus cooling alone, outcome: 1.4 Adverse event: respiratory impairment.
Comparisons 2 to 6 (subgroup analyses)
Comparison 2: cooling plus xenon versus cooling alone: subgroup analysis by baseline severity of encephalopathy
Death or major neurodevelopmental disability: the included study did not report this outcome.
-
Mortality at latest follow‐up (Analysis 2.1, Figure 8).
Mild encephalopathy (Thompson score 0 to 10): none of the five infants with mild encephalopathy in the cooling plus xenon group and none of the two infants with mild encephalopathy in the cooling alone group died.
Moderate/severe encephalopathy (Thompson score 11 to 22): nine (20%) of 44 infants with moderate/severe encephalopathy in the cooling plus xenon group and 11 (27%) of 41 infants with moderate/severe encephalopathy in the cooling alone group died. Among infants with moderate/severe encephalopathy, cooling plus xenon was not associated with reduced mortality at the latest reported age (risk ratio (RR) 1.31, 95% CI 0.61 to 2.84; risk difference (RD) 0.06, 95% CI ‐0.12 to 0.24; one study and 92 infants; low quality evidence).
Major neurodevelopmental disability: the included study did not report this outcome.
2.1. Analysis.
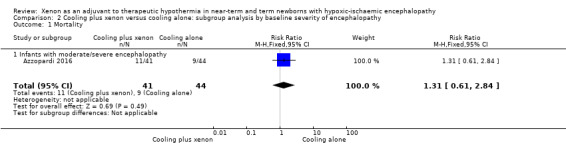
Comparison 2 Cooling plus xenon versus cooling alone: subgroup analysis by baseline severity of encephalopathy, Outcome 1 Mortality.
8.
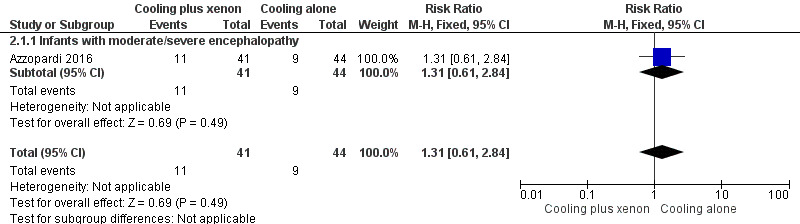
Forest plot of comparison: 2 Cooling plus xenon versus cooling alone: subgroup analysis by baseline severity of encephalopathy, outcome: 2.1 Mortality.
Comparison 3: cooling plus xenon versus cooling alone: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings
Death or major neurodevelopmental disability: the included study did not report this outcome.
-
Mortality at latest follow‐up (Analysis 3.1, Figure 9).
Mild aEEG abnormality at baseline: none of the included infants in the cooling plus xenon group and none of the infants in the cooling only group had signs of mild aEEG abnormality at baseline.
Moderate/severe aEEG abnormality at baseline: 11 (24%) of 46 infants with moderate/severe aEEG abnormalities in the cooling plus xenon group and nine (20%) of 46 infants with moderate/severe aEEG abnormalities in the cooling alone group died. Among infants with moderate/severe aEEG abnormalities at baseline, cooling plus xenon was not associated with reduced mortality at the latest reported age (risk ratio (RR) 1.22, 95% CI 0.56 to 2.67; risk difference (RD) 0.04, 95% CI ‐0.12 to 0.21; one study and 92 infants; low quality evidence).
Major neurodevelopmental disability: the included study did not report this outcome
3.1. Analysis.
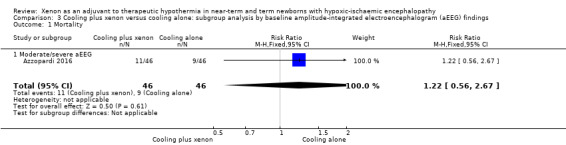
Comparison 3 Cooling plus xenon versus cooling alone: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 1 Mortality.
9.
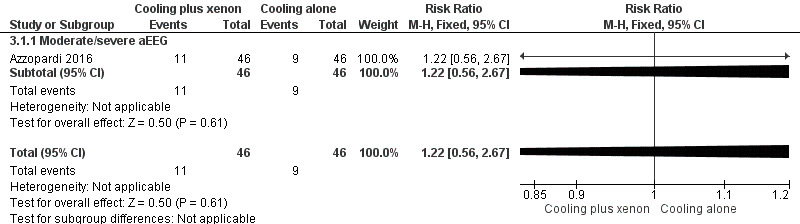
Forest plot of comparison: 3 Cooling plus xenon versus cooling alone: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, outcome: 3.1 Mortality.
Comparison 4: cooling plus xenon versus cooling alone: subgroup analysis by xenon concentration
Death or major neurodevelopmental disability: the included study did not report this outcome.
Mortality at the latest follow‐up: the included study did not report this outcome.
Major neurodevelopmental disability: the included study did not report this outcome.
Comparison 5: cooling plus xenon versus cooling alone: subgroup analysis by gestational age
Death or major neurodevelopmental disability: the included study did not report this outcome.
-
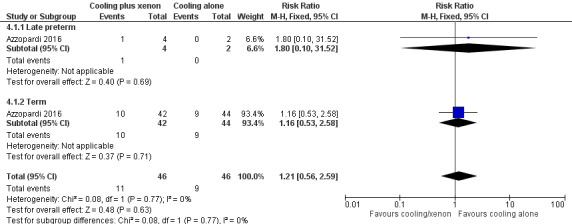
Mortality (Analysis 4.1, Figure 10).
Late preterm infants: one (25%) of four late preterm infants in the cooling plus xenon group and none of two late preterm infants in the cooling alone group died. Among late preterm infants with HIE, cooling plus xenon was not associated with reduced mortality at the latest reported age (risk ratio (RR) 1.80, 95% confidence interval (CI) 0.10 to 31.52; risk difference (RD) 0.25, 95% CI ‐0.33 to 0.83; one study and 92 infants).
Term infants: 10 (24%) of 42 term infants in the cooling plus xenon group and nine (20%) of 44 term infants in the cooling alone group died. Among term infants with HIE, cooling plus xenon was not associated with reduced mortality at the latest reported age (risk ratio (RR) 1.16, 95% CI 0.53 to 2.58; risk difference (RD) 0.03, 95% CI ‐0.14 to 0.21; one study and 92 infants; low quality evidence).
Major neurodevelopmental disability: the included study did not report this outcome.
4.1. Analysis.
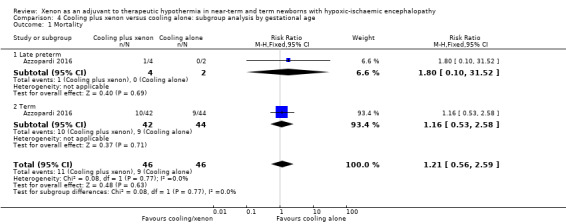
Comparison 4 Cooling plus xenon versus cooling alone: subgroup analysis by gestational age, Outcome 1 Mortality.
10.
Forest plot of comparison: 4 Cooling plus xenon versus cooling alone: subgroup analysis by gestational age, outcome: 4.1 Mortality.
Comparison 6: cooling plus xenon versus cooling alone: subgroup analysis by quality of follow‐up
Death or major neurodevelopmental disability: the included study did not report this outcome.
Mortality at the latest follow‐up: the included study did not report this outcome.
Major neurodevelopmental disability: the included study did not report this outcome.
Discussion
Summary of main results
This review identified a single randomised controlled open‐label trial looking at the neuroprotective short‐term effects of xenon in combination with therapeutic hypothermia after birth asphyxia. The trial randomised 92 newborns with moderate to severe HIE to either cooling plus xenon or cooling alone. The primary outcome ‐ reduction in the lactate‐to‐N‐acetyl aspartate ratio in the thalamus and in preserved fractional anisotropy in the posterior limb of the internal capsule measured with magnetic resonance spectroscopy and magnetic resonance imaging, respectively ‐ was not significantly different between the two groups. Long‐term neurodevelopmental outcomes, such as the primary outcome of this review, were not reported. Researchers found no substantial differences between groups for other secondary outcomes, such as mortality and occurrence of seizures during primary hospitalisation. Available data do not show an increased adverse event rate in the cooling plus xenon group compared with the cooling alone group.
Overall completeness and applicability of evidence
The neuroprotective effects of xenon as an adjuvant to cooling have been evaluated only in a single randomised controlled trial. This trial enrolled a small number of participants and did not report long‐term neurodevelopmental outcomes. Moreover, infants in the intervention group received a relatively low xenon concentration of 30%, and it may well be that use of higher xenon concentrations (≥ 40%) is effective in reducing brain injury after hypoxic‐ischaemic encephalopathy (HIE), as suggested by preclinical studies. Thus, current evidence is inadequate to determine whether xenon therapy for newborns with HIE is safe or effective. Because of its high costs and complex use of xenon in clinical practice, applicability of evidence is restricted to high‐resource settings.
Quality of the evidence
We assessed the quality of evidence for death, major neurodevelopmental disability, and each component of major neurodevelopmental disability (Table 1). We were able to include only one randomised controlled trial (RCT) in this review; therefore we were not able to assess the level of evidence for inconsistency. However, the included trial reported short‐term outcomes for the control group (cooling alone) similar to those seen in the cooled groups of prior cooling trials (Azzopardi 2014; Gluckman 2005; Shankaran 2005). Owing to the small sample included and the research question addressed, we downgraded the level of evidence for imprecision and indirectness. Although the included trial was at low risk of bias, we judged the overall quality of evidence as low.
Potential biases in the review process
We are aware of no bias in our review process.
Agreements and disagreements with other studies or reviews
We are aware of no other systematic reviews on this topic.
Authors' conclusions
Implications for practice.
Currently available evidence does not support the routine use of xenon as a neuroprotective agent for newborns with HIE and suggests that this practice should be limited to RCTs.
Implications for research.
The biological plausibility of using xenon to prevent HIE injury has been well established in preclinical studies (Dingley 2006; Ma 2005), and available clinical data have not raised major safety concerns related to use of xenon in newborn infants (Azzopardi 2016). Further large trials may be justified and should focus on effects of various xenon concentrations and timing regimens on long‐term neurodevelopmental outcomes.
Acknowledgements
None.
Appendices
Appendix 1. Search strategy
MEDLINE (Ovid)
Asphyxia neonatorum/
Hyoxia‐Ischemia, Brain/
exp Anoxia/
exp Hypothermia, Induced/ OR hypothermia/
Xenon/
(birth or newborn* or neonat* or infan* or gestation* or near‐term or term or perinatal or prematur* or pre‐term or preterm or low‐birth‐weight or LBW or VLBW or ("35" adj5 week*) or ("36" adj5 week*) or ("37" adj5 week*) or ("38" adj5 week*) or ("39" adj5 week*) or ("40" adj5 week*) or ("41" adj5 week*) or ("42" adj5 week*) or ("43" adj5 week*) or ("44" adj5 week*)).af.
(2 or 3) and 4 and 5 and 6
1 and 4 and 5
7 or 8
exp animals/ not human*.sh
9 not 10
Embase (Ovid)
(birth or newborn* or neonat* or infan* or gestation* or near‐term or term or perinatal or prematur* or pre‐term or preterm or low‐birth‐weight or LBW or VLBW or ("35" adj5 week*) or ("36" adj5 week*) or ("37" adj5 week*) or ("38" adj5 week*) or ("39" adj5 week*) or ("40" adj5 week*) or ("41" adj5 week*) or ("42" adj5 week*) or ("43" adj5 week*) or ("44" adj5 week*)).af.
hypoxic ischaemic encephalopathy/
exp asphyxia/
brain hypoxia
(neonatal adj asphyxial adj seizure*).tw,kw,hw.
xenon/
hypothermia/
induced hypothermia
(2 or 3 or 4) and 6 and (7 or 8) and 1
5 and 6 and (7 or 8)
9 or 10
exp ANIMAL/ not human*.sh.
11 not 12
Cochrane Library (Wiley)
MeSH descriptor: [Asphyxia Neonatorum] this term only
Asphyxia* or Hypoxia or Hypoxic or Hypoxemia or Hypoxaemia or Ischemia or Ischaemia or Ischemic or Ischaemic or anoxia or anoxic (Word variations have been searched)
MeSH descriptor: [Hypoxia‐Ischemia, Brain] this term only
MeSH descriptor: [Anoxia] explode all trees
MeSH descriptor: [Hypothermia, Induced] explode all trees
MeSH descriptor: [Hypothermia] this term only
Hypothermia or Cooling (Word variations have been searched)
MeSH descriptor: [Xenon] this term only
Xenon or Xe (Word variations have been searched)
(35 or 36 or 37 or 38 or 39 or 40 or 41 or 42) next week* (Word variations have been searched)
birth or newborn* or neonat* or infan* or gestation* or near‐term or "near term" or term or perinatal or prematur* or pre‐term or preterm or "pre term" or "low birth weight" or "low birth‐weight" or LBW or VLBW (Word variations have been searched)
MeSH descriptor: [Infant] explode all trees
(2 or 3 or 4) and (5 or 6 or 7) and (8 or 9) and (10 or 11 or 12)
(1) and (5 or 6 or 7) and (8 or 9)
13 or 14
PubMed
(birth OR newborn* OR neonat* OR infan* OR gestation* OR near‐term OR term OR perinatal OR prematur* OR pre‐term OR preterm OR "pre term" OR "low birth weight" OR "low birth‐weight" OR LBW OR VLBW OR ((35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42) AND weeks)) AND (Asphyxia* OR Hypoxia OR Hypoxic OR Hypoxemia OR Hypoxaemia OR Ischemia OR Ischaemia OR Ischemic OR Ischaemic) AND (Hypothermia OR Cooling) AND (Xenon OR Xe) AND (NOTNLM OR publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb] OR indatareview[sb] OR pubstatusaheadofprint)
Appendix 2. ʿRisk of bias' tool
We will evaluate the following issues and will enter findings into the ʿRisk of bias' table.
1. Sequence generation (checking for possible selection bias)
Was the allocation sequence adequately generated?
For each included study, we will categorise the method used to generate the allocation sequence as follows.
Low risk (any truly random process, e.g. random number table; computer random number generator).
High risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number).
Unclear risk.
2. Allocation concealment (checking for possible selection bias)
Was allocation adequately concealed?
For each included study, we will categorise the method used to conceal the allocation sequence as follows.
Low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes).
High risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth).
Unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias)
Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we will categorise the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes. We will categorise the methods according to the following.
Low risk, high risk, or unclear risk for participants.
Low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias)
Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we will categorise the methods used to blind outcome assessment. We will assess blinding separately for different outcomes or classes of outcomes. We will categorise the methods as follows.
Low risk for outcome assessors.
High risk for outcome assessors.
Unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data including attrition and exclusions from the analysis. We will note whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information is reported or supplied by the trial authors, we will re‐include missing data in the analyses. We will categorise methods as one of the following.
Low risk (< 20% missing data).
High risk (≥ 20% missing data).
Unclear risk.
6. Selective reporting bias
Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found. We will assess methods as one of the following.
Low risk (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported).
High risk (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported).
Unclear risk.
7. Other sources of bias
Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we will describe any important concerns we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We will assess whether each study was free of other problems that could put it at risk of bias as follows.
Low risk.
High risk.
Unclear risk.
If needed, we plan to explore the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Cooling plus xenon versus cooling alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.56, 2.67] |
| 2 Adverse event: cardiac arrhythmia | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.60] |
| 3 Adverse event: persistent hypotension | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.44] |
| 4 Adverse event: respiratory impairment | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.45, 2.89] |
Comparison 2. Cooling plus xenon versus cooling alone: subgroup analysis by baseline severity of encephalopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.84] |
| 1.1 Infants with moderate/severe encephalopathy | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.84] |
Comparison 3. Cooling plus xenon versus cooling alone: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.56, 2.67] |
| 1.1 Moderate/severe aEEG | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.56, 2.67] |
Comparison 4. Cooling plus xenon versus cooling alone: subgroup analysis by gestational age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.56, 2.59] |
| 1.1 Late preterm | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.10, 31.52] |
| 1.2 Term | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.53, 2.58] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azzopardi 2016.
| Methods | Proof‐of‐concept, randomised, open‐label, parallel‐group trial done at 4 neonatal intensive care units in the United Kingdom | |
| Participants |
Included infants born between 36 and 43 weeks' gestational age with signs of moderate to severe encephalopathy and moderately or severely abnormal background activity for at least 30 minutes, or seizures as shown by amplitude‐integrated EEG, with 1 of the following: Apgar score ≤ 5 at 10 minutes after birth, continued need for resuscitation 10 minutes after birth, or acidosis within 1 hour of birth Excluded infants were older than 6 hours when cooling was started or were older than12 hours at randomisation. Infants were excluded if their oxygen requirement was greater than 60%, if they needed nitric oxide inhalation or ventilation with a high‐frequency oscillator, if they needed extracorporeal membrane oxygenation, or if they had major congenital abnormalities Total number of participants: N = 92 Baseline characteristics: ‐ Mean (SD) gestational age at delivery (weeks): cooling plus xenon = 39.8 (1.3), cooling alone = 39.8 (1.3) ‐ Mean (SD) birthweight (g): cooling plus xenon 3392 (685), cooling alone = 3213 (448) ‐ Male sex (n): cooling plus xenon = 26 (57%), cooling alone = 21 (46%) ‐ Median (IQR) Apgar at 10 minutes: cooling plus xenon = 5 (3 to 6), cooling alone = 5 (4 to 7) ‐ Median (IQR) cord or first blood pH: cooling plus xenon = 6.9 (6.8 to 7.1), cooling alone = 6.9 (6.7 to 7.0) ‐ Median (IQR) age cooling commenced (hours): cooling plus xenon = 0.2 (0.0 to 1.5), cooling only = 0.3 (0.0 to 0.8) ‐ Mild HIE at trial entry (n): cooling plus xenon = 5 (11%), cooling alone = 2 (4%) ‐ Moderate HIE at trial entry (n): cooling plus xenon = 21 (46%), cooling alone = 30 (65%) ‐ Severe HIE at trial entry (n): cooling plus xenon = 20 (43%), cooling alone = 14 (30%) ‐ Moderate EEG/aEEG abnormality at trial entry (n): cooling plus xenon = 6 (13%), cooling alone = 7 (15%) ‐ Severe EEG/aEEG abnormality at trial entry (n): cooling plus xenon = 40 (87%), cooling alone = 39 (85%) |
|
| Interventions |
Standard care (n = 46): whole body cooling to a target rectal temperature of 33.5°C for 72 hours starting within 6 hours of birth. If cooling equipment was not available at the referring hospital, passive cooling was commenced and active cooling was started by the transport team and continued during transport to the treatment centre Intervention (n = 46): cooled infants in the inhaled xenon group received 30% xenon through an uncuffed endotracheal tube connected to a recirculating device developed for this trial. Xenon was commenced immediately after randomisation and continued for 24 hours Intervention characteristics: ‐ Mean (SD) xenon concentration (%): 32.2 (6.9) ‐ Median (IQR) starting age of xenon administration (hours): 10 (8.2 to 11.2) ‐ Median (IQR) duration of xenon administration (hours): 24 (24 to 24) |
|
| Outcomes |
Primary: reduction in the lactate‐to‐N‐acetyl aspartate ratio in the thalamus on MRS or preserved FA in the posterior limb of the internal capsule, measured by magnetic resonance spectroscopy and MRI, respectively, within 15 days of birth Secondary: maximum Thompson HIE score, neurological examination at discharge from treatment centre, occurrence of seizures, intracranial haemorrhage, persistent hypotension, pulmonary haemorrhage, pulmonary hypertension, prolonged blood coagulation time, thrombocytopaenia, major venous thrombosis, cardiac arrhythmia, culture‐proven late‐onset sepsis, necrotising enterocolitis, pneumonia, pulmonary air leak, anuria or oliguria, age at which full oral feeding was achieved, duration of hospital stay, grade of abnormalities on visual analysis of MRI |
|
| Identification | ClinicalTrials.gov: NCT00934700 ISRCTN: ISRCTN08886155 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate, with a computer‐generated randomisation sequence. Assignment done through a secure web‐based system with telephone backup |
| Allocation concealment (selection bias) | Low risk | Adequate, central allocation system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was unblinded with regards to intervention allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Detection bias judged as low risk for primary outcome and as low risk for secondary outcomes (see below) |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Assessors of outcomes unblinded to treatment allocation; however, only 2 secondary outcomes reliant on assessors (Thompson score and neurological assessment at discharge). The other outcomes are less likely to be prone to bias |
| Blinding of outcome assessment (detection bias) Primary outcome | Low risk | Investigators who assessed the primary outcome were masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete flow chart of all screened and randomised infants available. Fourteen (15.2%) infants were excluded after randomisation, nine (9.7%) of whom died before discharge |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Complete assessment of secondary outcomes (92/92) |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | The primary outcome was assessed in 41 (89%) of 46 infants in the cooling plus xenon group and in 37 (80%) of 46 infants in the cooling only group. In 2/46 (4.3%) infants in the cooling plus xenon group and in 3/46 (6.5%) infants in the cooling alone group, MRI scans were not done, although infants were alive at discharge. An additional 9/92 (9.7%) patients died before discharge and were excluded from the analysis ‐ 3/46 (6.5%) in the cooling plus xenon group and 6/46 (13.0%) in the cooling only group. However, it remains unclear at which postnatal age these deaths occurred (before or after the prespecified 15‐day window for the MRI). Therefore, we decided to rate the risk of attrition bias as "unclear" |
| Selective reporting (reporting bias) | Low risk | Study protocol available online; all prespecified primary and secondary outcomes reported |
| Other bias | Low risk | None apparent |
aEEG: amplitude‐integrated electroencephalogram.
EEG: electroencephalogram.
FA: fluorescent antibody.
HIE: hypoxic‐ischaemic encephalopathy.
IQR: interquartile ratio.
MRI: magnetic resonance imaging.
MRS: magnetic resonance spectroscopy.
SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Azzopardi 2013 | Nested, non‐randomised substudy of larger randomised controlled trial (Azzopardi 2016) |
Characteristics of ongoing studies [ordered by study ID]
NCT01545271.
| Trial name or title | Xenon and Cooling Therapy in Babies at High Risk of Brain Injury Following Poor Condition at Birth: Randomised Pilot Study (The CoolXenon2 Study) |
| Methods | Randomised controlled single‐centre pilot study in UK |
| Participants |
Includes: infants born at ≥ 36 weeks’ gestation WITH clinical evidence of peripartum hypoxia‐ischaemia (Apgar score ≤ 5 at 10 minutes, continued need for resuscitation at 10 minutes, or severe acidosis (pH < 7 or base deficit ≥ 16 mmol/L in cord blood or arterial/venous blood within 60 minutes of birth)) AND abnormal amplitude‐integrated electroencephalogram background AND moderate or severe encephalopathy (Sarnat criteria) with 1 of hypotonia, abnormal reflexes, absent or weak suck, clinical seizures, or a combination. For xenon therapy, infants must be intubated with normal partial pressure of CO2 (pCO2), positive end‐expiratory pressure < 6 cm H2O and fraction of inspired oxygen (FiO2) < 0.40, seizures under control, weighing > 2.3 kg and < 5 hours old, with birthweight greater than the second percentile for age, with no major congenital anomalies, and haemodynamically stable with no evidence of infection Excludes: infants considered futile and infants not meeting above criteria |
| Interventions |
Standard care: cooling to 33.5°C body temperature, starting within 3 hours after birth Intervention: cooling to 33.5°C body temperature plus xenon gas at 50% concentration for 18 hours, started within 5 hours after birth |
| Outcomes |
Primary outcomes: amplitude‐integrated electroencephalogram (aEEG) grading: number of hours after birth when aEEG voltage has reached normal or discontinuous normal pattern. Brain MRI findings before 2 weeks of age Secondary outcomes: not provided |
| Starting date | May 2012 |
| Contact information | Marianne Thoresen, MD |
| Notes | NCT01545271 |
NCT02071394.
| Trial name or title | Xenon and Cooling Therapy in Babies at High Risk of Brain Injury Following Poor Condition at Birth: A Randomised Pilot Outcomes Study (CoolXenon3 Study) |
| Methods | Randomised controlled pilot outcomes study at 2 centres in the UK |
| Participants |
Includes: infants born at ≥ 36 weeks' gestation WITH clinical evidence of peripartum hypoxia‐ischaemia (Apgar score ≤ 5 at 10 minutes, continued need for resuscitation at 10 minutes, or severe acidosis (pH < 7 or base deficit ≥ 16 mmol/L in cord blood or arterial/venous blood within 60 minutes of birth)) AND abnormal amplitude‐integrated electroencephalogram background AND moderate or severe encephalopathy (Sarnat criteria) with 1 of hypotonia, abnormal reflexes, absent or weak suck, clinical seizures, or a combination For xenon therapy, infants must be intubated with normal partial pressure of CO2 (pCO2), positive end‐expiratory pressure < 8 cm H2O and fraction of inspired oxygen (FiO2) < 0.40, seizures under control, < 5 hours old, with birthweight greater than the second percentile for age, with no major congenital anomalies, and haemodynamically stable Excludes: infants older than 3 hours of age when cooling started, infants considered futile, and infants not meeting above criteria |
| Interventions |
Standard care: cooling to 33.5°C body temperature, starting within 3 hours after birth Intervention: cooling to 33.5°C body temperature plus xenon gas at 50% concentration for 18 hours, started within 5 hours after birth |
| Outcomes |
Primary outcomes: death and moderate or severe disability (Bayley III) at 18 months of age Secondary outcomes: brain MRI within 2 weeks of birth and before hospital discharge, amplitude‐integrated electroencephalogram (aEEG) grading within 1 week of birth, number of hours after birth when aEEG voltage has reached a normal or discontinuous normal pattern, Dubovitz score within 7 days, number of normal infants (Bayley III composite score ≥ 85 and no neurosensory disability) at 18 to 24 months of age |
| Starting date | March 2014 |
| Contact information | Marianne Thoresen, MD |
| Notes | NCT02071394 |
aEEG: amplitude‐integrated electroencephalogram.
CO2: carbon dioxide.
FiO2: fraction of inspired oxygen.
MRI: magnetic resonance imaging.
pCO2: partial pressure of CO2.
Differences between protocol and review
None.
Contributions of authors
Christoph Rüegger: responsible for all aspects of review ‐ search; data abstraction, entry, and analysis; manuscript; and editing of review.
Peter Davis: manuscript preparation; critical review of manuscript.
Jeanie Cheong: data abstraction and entry; manuscript preparation; critical review of manuscript.
Sources of support
Internal sources
The Royal Women's Hospital, Melbourne, Australia.
External sources
The Swiss National Science Foundation (Early Postdoc Mobility Fellowship P2ZHP3_161749 to CMR), Switzerland.
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
Christoph Rüegger: nothing to declare.
Peter Davis: nothing to declare.
Jeanie Cheong: nothing to declare.
New
References
References to studies included in this review
Azzopardi 2016 {published data only}
- Azzopardi D, Robertson NJ, Bainbridge A, Cady E, Charles‐Edwards G, Deierl A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY‐Xe): a proof‐of‐concept, open‐label, randomised controlled trial. Lancet Neurology 2016;15(2):145‐53. [DOI: 10.1016/S1474-4422(15)00347-6; PUBMED: 26708675] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Azzopardi 2013 {published data only}
- Azzopardi D, Robertson NJ, Kapetanakis A, Griffiths J, Rennie JM, Mathieson SR, et al. Anticonvulsant effect of xenon on neonatal asphyxial seizures. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(5):F437‐9. [DOI: 10.1136/archdischild-2013-303786; PUBMED: 23572341] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01545271 {published data only}
- NCT01545271. Xenon and cooling therapy in babies at high risk of brain injury following poor condition at birth [Xenon and cooling therapy in babies at high risk of brain injury following poor condition at birth: a randomised pilot study]. clinicaltrials.gov/show/NCT01545271 (first received 06 March 2012).
NCT02071394 {published data only}
- NCT02071394. Xenon and cooling therapy in babies at high risk of brain injury following poor condition at birth [Xenon and cooling therapy in babies at high risk of brain injury following poor condition at birth: a randomised pilot outcomes study (COOLXENON3 Study)]. clinicaltrials.gov/show/NCT02071394 (first received 25 February 2014).
Additional references
Andropoulos 2017
- Andropoulos DB, Greene MF. Anesthesia and developing brains ‐ implications of the FDA warning. New England Journal of Medicine 2017;376(10):905‐7. [DOI: 10.1056/NEJMp1700196; PUBMED: 28177852] [DOI] [PubMed] [Google Scholar]
Armstrong 2012
- Armstrong SP, Banks PJ, McKitrick TJ, Geldart CH, Edge CJ, Babla R, et al. Identification of two mutations (F758W and F758Y) in the N‐methyl‐D‐aspartate receptor glycine‐binding site that selectively prevent competitive inhibition by xenon without affecting glycine binding. Anesthesiology 2012;117(1):38‐47. [DOI: 10.1097/ALN.0b013e31825ada2e; PUBMED: 22634870] [DOI] [PubMed] [Google Scholar]
Azzopardi 2014
Bantel 2010
- Bantel C, Maze M, Trapp S. Noble gas xenon is a novel adenosine triphosphate‐sensitive potassium channel opener. Anesthesiology 2010;112(3):623‐30. [DOI: 10.1097/ALN.0b013e3181cf894a; PUBMED: 20179498] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkovich 1995
- Barkovich AJ, Westmark K, Partridge C, Sola A, Ferriero DM. Perinatal asphyxia: MR findings in the first 10 days. AJNR. American Journal of Neuroradiology 1995;16(3):427‐38. [PUBMED: 7793360] [PMC free article] [PubMed] [Google Scholar]
Chakkarapani 2010
- Chakkarapani E, Dingley J, Liu X, Hoque N, Aquilina K, Porter H, et al. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Annals of Neurology 2010;68(3):330‐41. [DOI: 10.1002/ana.22016; PUBMED: 20658563] [DOI] [PubMed] [Google Scholar]
Chakkarapani 2012
- Chakkarapani E, Thoresen M, Liu X, Walloe L, Dingley J. Xenon offers stable haemodynamics independent of induced hypothermia after hypoxia‐ischaemia in newborn pigs. Intensive Care Medicine 2012;38(2):316‐23. [DOI: 10.1007/s00134-011-2442-7; PUBMED: 22160201] [DOI] [PubMed] [Google Scholar]
Covidence 2017 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 17 February 2017. Melbourne, Australia: Veritas Health Innovation, 2017.
Dickinson 2007
- Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, et al. Competitive inhibition at the glycine site of the N‐methyl‐D‐aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology 2007;107(5):756‐67. [DOI: 10.1097/01.anes.0000287061.77674.71; PUBMED: 18073551] [DOI] [PubMed] [Google Scholar]
Dingley 2001
- Dingley J, King R, Hughes L, Terblanche C, Mahon S, Hepp M, et al. Exploration of xenon as a potential cardiostable sedative: a comparison with propofol after cardiac surgery. Anaesthesia 2001;56(9):829‐35. [PUBMED: 11531666] [DOI] [PubMed] [Google Scholar]
Dingley 2006
- Dingley J, Tooley J, Porter H, Thoresen M. Xenon provides short‐term neuroprotection in neonatal rats when administered after hypoxia‐ischaemia. Stroke 2006;37(2):501‐6. [DOI: 10.1161/01.STR.0000198867.31134.ac; PUBMED: 16373643] [DOI] [PubMed] [Google Scholar]
Dingley 2008
- Dingley J, Hobbs C, Ferguson J, Stone J, Thoresen M. Xenon/hypothermia neuroprotection regimes in spontaneously breathing neonatal rats after hypoxic‐ischaemic insult: the respiratory and sedative effects. Anesthesia and Analgesia 2008;106(3):916‐23. [DOI: 10.1213/ane.0b013e3181618669] [DOI] [PubMed] [Google Scholar]
Douglas‐Escobar 2015
- Douglas‐Escobar M, Weiss MD. Hypoxic‐ischaemic encephalopathy: a review for the clinician. JAMA Pediatrics 2015;169(4):397‐403. [DOI: 10.1001/jamapediatrics.2014.3269; PUBMED: 25685948] [DOI] [PubMed] [Google Scholar]
Faulkner 2011
- Faulkner S, Bainbridge A, Kato T, Chandrasekaran M, Kapetanakis AB, Hristova M, et al. Xenon augmented hypothermia reduces early lactate/N‐acetylaspartate and cell death in perinatal asphyxia. Annals of Neurology 2011;70(1):133‐50. [DOI: 10.1002/ana.22387; PUBMED: 21674582] [DOI] [PubMed] [Google Scholar]
Ferriero 2004
- Ferriero DM. Neonatal brain injury. New England Journal of Medicine 2004;351(19):1985‐95. [DOI: 10.1056/NEJMra041996; PUBMED: 15525724] [DOI] [PubMed] [Google Scholar]
Finer 1981
- Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL. Hypoxic‐ischaemic encephalopathy in term neonates: perinatal factors and outcome. Journal of Pediatrics 1981;98(1):112‐7. [PUBMED: 7452386] [DOI] [PubMed] [Google Scholar]
Fleiss 2012
- Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy?. Lancet Neurology 2012;11(6):556‐66. [DOI: 10.1016/S1474-4422(12)70058-3; PUBMED: 22608669] [DOI] [PubMed] [Google Scholar]
Franks 1998
- Franks NP, Dickinson R, Sousa SL, Hall AC, Lieb WR. How does xenon produce anaesthesia?. Nature 1998;396(6709):324. [DOI: 10.1038/24525; PUBMED: 9845069] [DOI] [PubMed] [Google Scholar]
Gluckman 2005
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 14 September 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gruss 2004
- Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two‐pore‐domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Molecular Pharmacology 2004;65(2):443‐52. [DOI: 10.1124/mol.65.2.443; PUBMED: 14742687] [DOI] [PubMed] [Google Scholar]
Hassell 2015
- Hassell KJ, Ezzati M, Alonso‐Alconada D, Hausenloy DJ, Robertson NJ. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(6):F541‐52. [DOI: 10.1136/archdischild-2014-306284; PUBMED: 26063194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hobbs 2008
- Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long‐term functional and histopathologic neuroprotection after neonatal hypoxia/ischaemia. Stroke 2008;39(4):1307‐13. [DOI: 10.1161/STROKEAHA.107.499822; PUBMED: 18309163] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jevtovic‐Todorovic 2013
- Jevtovic‐Todorovic V, Absalom AR, Blomgren K, Brambrink A, Crosby G, Culley DJ, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. British Journal of Anaesthesia 2013;111(2):143‐51. [DOI: 10.1093/bja/aet177; PUBMED: 23722106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2014
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Lancet Every Newborn Study Group. Every Newborn: progress, priorities, and potential beyond survival. Lancet 2014;384(9938):189‐205; Erratum in: Lancet 2014;384(9938):132. [DOI: 10.1016/S0140-6736(14)60496-7; 24853593] [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, et al. Intrapartum‐related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatric Research 2013;74(Suppl 1):50‐72. [DOI: 10.1038/pr.2013.206; PUBMED: 24366463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2015
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000‐13, with projections to inform post‐2015 priorities: an updated systematic analysis. Lancet 2015;385(9966):430‐40; Erratum in: Lancet; 2015;385(9966):420. [DOI: 10.1016/S0140-6736(14)61698-6; 25280870] [DOI] [PubMed] [Google Scholar]
Ma 2005
- Ma D, Hossain M, Chow A, Arshad M, Battson RM, Sanders RD, et al. Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Annals of Neurology 2005;58(2):182‐93. [DOI: 10.1002/ana.20547; PUBMED: 16049939] [DOI] [PubMed] [Google Scholar]
Ma 2006
- Ma D, Hossain M, Pettet GK, Luo Y, Lim T, Akimov S, et al. Xenon preconditioning reduces brain damage from neonatal asphyxia in rats. Journal of Cerebral Blood Flow and Metabolism 2006;26(2):199‐208. [DOI: 10.1038/sj.jcbfm.9600184; PUBMED: 16034370] [DOI] [PubMed] [Google Scholar]
Ma 2007
- Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, et al. Xenon mitigates isoflurane‐induced neuronal apoptosis in the developing rodent brain. Anesthesiology 2007;106(4):746‐53. [DOI: 10.1097/01.anes.0000264762.48920.80; PUBMED: 17413912] [DOI] [PubMed] [Google Scholar]
Ma 2009
- Ma D, Lim T, Xu J, Tang H, Wan Y, Zhao H, et al. Xenon preconditioning protects against renal ischaemic‐reperfusion injury via HIF‐1alpha activation. Journal of the American Society of Nephrology 2009;20(4):713‐20. [DOI: 10.1681/ASN.2008070712; PUBMED: 19144758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palisano 1997
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology 1997;39(4):214‐23. [PUBMED: 9183258] [DOI] [PubMed] [Google Scholar]
Petzelt 2001
- Petzelt CP, Kodirov S, Taschenberger G, Kox WJ. Participation of the Ca(2+)‐calmodulin‐activated Kinase II in the control of metaphase‐anaphase transition in human cells. Cell Biology International 2001;25(5):403‐9. [DOI: 10.1006/cbir.2000.0646; PUBMED: 11401327] [DOI] [PubMed] [Google Scholar]
Petzelt 2003
- Petzelt C, Blom P, Schmehl W, Müller J, Kox WJ. Prevention of neurotoxicity in hypoxic cortical neurons by the noble gas xenon. Life Sciences 2003;72(17):1909‐18. [PUBMED: 12597990] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rossaint 2003
- Rossaint R, Reyle‐Hahn M, Schulte Am Esch J, Scholz J, Scherpereel P, Vallet B, et al. Multicenter randomised comparison of the efficacy and safety of xenon and isoflurane in patients undergoing elective surgery. Anesthesiology 2003;98(1):6‐13. [PUBMED: 12502972] [DOI] [PubMed] [Google Scholar]
Rutherford 2010
- Rutherford M, Malamateniou C, McGuinness A, Allsop J, Biarge MM, Counsell S. Magnetic resonance imaging in hypoxic‐ischaemic encephalopathy. Early Human Development 2010;86(6):351‐60. [DOI: 10.1016/j.earlhumdev.2010.05.014; PUBMED: 20541877] [DOI] [PubMed] [Google Scholar]
Sabir 2013
- Sabir H, Bishop S, Cohen N, Maes E, Liu X, Dingley J, et al. Neither xenon nor fentanyl induces neuroapoptosis in the newborn pig brain. Anesthesiology 2013;119(2):345‐57. [DOI: 10.1097/ALN.0b013e318294934d; PUBMED: 23591070] [DOI] [PubMed] [Google Scholar]
Sabir 2016
- Sabir H, Wood T, Gill H, Liu X, Dingley J, Thoresen M. Xenon depresses aEEG background voltage activity whilst maintaining cardiovascular stability in sedated healthy newborn pigs. Journal of the Neurological Sciences 2016;363:140‐4. [DOI: 10.1016/j.jns.2016.02.051; PUBMED: 27000239] [DOI] [PubMed] [Google Scholar]
Sanders 2005
- Sanders RD, Ma D, Maze M. Xenon: elemental anaesthesia in clinical practice. British Medical Bulletin 2005;71:115‐35. [DOI: 10.1093/bmb/ldh034; PUBMED: 15728132] [DOI] [PubMed] [Google Scholar]
Sarnat 1976
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology 1976;33(10):696‐705. [PUBMED: 987769] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shankaran 2005
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Sun 2010
- Sun L. Early childhood general anaesthesia exposure and neurocognitive development. British Journal of Anaesthesia 2010;105(Suppl 1):i61‐8. [DOI: 10.1093/bja/aeq302; PUBMED: 21148656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thayyil 2010
- Thayyil S, Chandrasekaran M, Taylor A, Bainbridge A, Cady EB, Chong WK, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta‐analysis. Pediatrics 2010;125(2):e382‐95. [DOI: 10.1542/peds.2009-1046; PUBMED: 20083516] [DOI] [PubMed] [Google Scholar]
Thoresen 2009
- Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J. Cooling combined with immediate or delayed xenon inhalation provides equivalent long‐term neuroprotection after neonatal hypoxia‐ischemia. Journal of Cerebral Blood Flow and Metabolism 2009;29(4):707‐14. [DOI: 10.1038/jcbfm.2008.163; PUBMED: 19142190] [DOI] [PubMed] [Google Scholar]
Zhuang 2012
- Zhuang L, Yang T, Zhao H, Fidalgo AR, Vizcaychipi MP, Sanders RD, et al. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Critical Care Medicine 2012;40(6):1724‐30. [DOI: 10.1097/CCM.0b013e3182452164; PUBMED: 22610177] [DOI] [PubMed] [Google Scholar]


