Abstract
Background
Acute asthma is a common cause of presentations to acute care centres, such as the emergency department (ED), and while the majority of patients can be discharged, relapse requiring additional medical care is common. Systemic corticosteroids are a major part in the treatment of moderate to severe acute asthma; however, there is no clear evidence regarding the most effective route of administration for improving outcomes in patients discharged from acute care.
Objectives
To examine the effectiveness and safety of a single dose of intramuscular (IM) corticosteroids provided prior to discharge compared to a short course of oral corticosteroids in the treatment of acute asthma patients discharged from an ED or equivalent acute care setting.
Search methods
The Cochrane Airways Group conducted searches of the Cochrane Airways Group Register of Trials, most recently on 14 March 2018. In addition in April 2017 we completed an extensive search of nine electronic databases including Medline, Embase, EBM ALL, Global Health, International Pharmaceutical Abstracts, CINAHL, SCOPUS, Proquest Dissertations and Theses Global, and LILACS. Furthermore, we searched the grey literature to identify any additional studies.
Selection criteria
We included randomized controlled trials or controlled clinical trials if they compared the effectiveness of intramuscular (IM) versus oral corticosteroids to treat paediatric or adult patients presenting with acute asthma to an ED or equivalent acute care setting. Two independent reviewers assessed study eligibility and study quality. We resolved disagreements via a third party and assessed risk of bias using the Cochrane 'Risk of bias' tool. We assessed the quality of the evidence using GRADE.
Data collection and analysis
For dichotomous outcomes, we calculated individual and pooled statistics as risk ratios (RRs) with 95% confidence intervals (CIs) using a random‐effects model. We reported continuous outcomes using mean difference (MD) or standardised mean difference (SMD) with 95% CIs using a random‐effects model. We reported heterogeneity using I² and Cochran Q statistics. We used standard procedures recommended by Cochrane.
Main results
Nine studies involving 804 participants (IM = 402 participants; oral = 402 participants) met our review inclusion criteria. Four studies enrolled children (n = 245 participants), while five studies enrolled adults (n = 559 participants). All of the studies recruited participants presenting to an ED, except one study which recruited participants attending a primary care clinic. All of the paediatric studies compared intramuscular (IM) dexamethasone to oral prednisone/prednisolone. In the adult studies, the IM corticosteroid provided ranged from methylprednisolone, betamethasone, dexamethasone, or triamcinolone, while the regimen of oral corticosteroids provided consisted of prednisone, methylprednisolone, or dexamethasone. Only five studies were placebo controlled. For the purposes of this review, we did not take corticosteroid dose equivalency into account in the analysis. The most common co‐intervention provided to participants during the acute care visit included short‐acting beta₂‐agonists (SABA), methylxanthines, and ipratropium bromide. In some instances, some studies reported providing some participants with supplemental oral or IV corticosteroids during their stay in the ED. Co‐interventions provided to participants at discharge consisted primarily of SABA, methylxanthine, long‐acting beta₂‐agonists (LABA), and ipratropium bromide. The risk of bias of the included studies ranged from unclear to high across various domains. The primary outcome of interest was relapse to additional care defined as an unscheduled visit to a health practitioner for worsening asthma symptoms, or requiring subsequent treatment with corticosteroids which may have occurred at any time point after discharge from the ED.
We found intramuscular and oral corticosteroids to be similarly effective in reducing the risk for relapse (RR 0.94, 95% CI 0.72 to 1.24; 9 studies, 804 participants; I² = 0%; low‐quality evidence). We found no subgroup differences in relapse rates between paediatric and adult participants (P = 0.71), relapse occurring within or after 10 days post‐discharge (P = 0.22), or participants with mild/moderate or severe exacerbations (P = 0.35). While we found no statistical difference between participants receiving IM versus oral corticosteroids regarding the risk for adverse events (RR 0.83, 95% CI 0.64 to 1.07; 5 studies, 404 participants; I² = 0%; moderate‐quality evidence), an estimated 50 fewer patients per 1000 receiving IM corticosteroids reported experiencing adverse events (95% from 106 fewer to 21 more). We found inconsistent reporting of specific adverse events across the studies. There were no differences in the frequency of specific adverse events including nausea and vomiting, pain, swelling, redness, insomnia, or personality changes. We did not seek additional adverse events data.
Participants receiving IM corticosteroids or oral corticosteroids both reported decreases in peak expiratory flow (MD −7.78 L/min, 95% CI −38.83 L/min to 23.28 L/min; 4 studies, 272 participants; I² = 33%; moderate‐quality evidence), similar symptom persistence (RR 0.41, 95% CI 0.14 to 1.20; 3 studies, 80 participants; I² = 44%; low‐quality evidence), and 24‐hour beta‐agonist use (RR 0.54, 95% CI 0.21 to 1.37; 2 studies, 48 participants; I² = 0%; low‐quality evidence).
Authors' conclusions
There is insufficient evidence to identify whether IM corticosteroids are more effective in reducing relapse compared to oral corticosteroids among children or adults discharged from an ED or equivalent acute care setting for acute asthma. While we found no statistical differences, patients receiving IM corticosteroids reported fewer adverse events. Additional studies comparing the effectiveness of IM versus oral corticosteroids could provide further evidence clarity. Furthermore, there is a need for studies comparing different IM corticosteroids (e.g. IM dexamethasone versus IM methylprednisone) and different oral corticosteroids (e.g. oral dexamethasone versus oral prednisone), with consideration for dosing and pharmacokinetic properties, to better identify the optimal IM or oral corticosteroid regimens to improve patient outcomes. Other factors, such as patient preference and potential issues with adherence, may dictate practitioner prescribing.
Plain language summary
Intramuscular versus oral corticosteroids for acute asthma
Review question
We examined the effectiveness of an injection of corticosteroids compared to corticosteroids taken by mouth to improve outcomes among patients who presented to an emergency department or similar acute care setting with acute asthma.
Background
Asthma attacks result from airway passages to the lungs becoming constricted due to inflammation, resulting in wheezing, coughing and difficulty breathing. People experiencing asthma attacks often go to emergency departments. Corticosteroids, which are powerful anti‐inflammatory agents, are the treatment cornerstone of asthma exacerbations, and have been shown to be effective in improving lung function and reducing hospitalisations in patients with asthma. At discharge, patients are commonly provided with corticosteroids to reduce the chance of returning to the emergency department due to worsening asthma symptoms. Corticosteroids may be provided via a single injection under the skin into the muscle ('intramuscular') or as tablets to take home, and it is currently unclear which regimen of corticosteroids is more effective at improving outcomes for patients following discharge from the emergency department.
Search date
We conducted our most recent search in March 2018.
Study characteristics
We included nine studies that compared the effectiveness of an intramuscular injection compared to corticosteroid tablets in patients presenting to an ED or similar acute care setting with acute asthma. The studies enrolled a total of 804 paediatric and adult participants. Most studies investigated the injectable corticosteroids dexamethasone or methylprednisolone compared to the corticosteroid tablets prednisone or methylprednisolone.
Study funding sources
Most studies did not report sources of funding (5 studies). Two studies received funding from general health research grants. One study was funded by a pharmaceutical company (Pfizer); however, reported that the company was not involved in any aspect of the study or manuscript preparation. One study reported being unfunded.
Key results
Intramuscular injections of corticosteroids appear to be as effective as corticosteroids tablets in preventing relapse. We did not find any differences in the risk of relapse between participants receiving intramuscular injections and corticosteroid tablets. Although not all studies reported adverse effects in their study groups, we found no differences between participants receiving intramuscular injections and corticosteroid tablets. At follow‐up, we found no differences in pulmonary function tests between participants who had received an intramuscular injection or corticosteroid tablets. In the studies that reported symptom scores and duration, we did not identify any differences between participants receiving corticosteroids by injection or by tablets.
Quality of the evidence
The quality of the evidence regarding the effectiveness of intramuscular injections of corticosteroids in improving health outcomes ranged from low to moderate. We had only moderate confidence about the estimated effects of intramuscular steroids on hospital admissions, improvement in respiratory function and relapse because of the risk of bias among included studies.
Summary of findings
Summary of findings for the main comparison. Intramuscular corticosteroids compared to Oral corticosteroids for acute asthma.
| Intramuscular corticosteroids compared to Oral corticosteroids for acute asthma | |||||
| Patient or population: patients with acute asthma Settings: Acute care settings Intervention: Intramuscular corticosteroids Comparison: Oral corticosteroids | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Oral corticosteroids | Intramuscular corticosteroids | ||||
| Relapse | 201 per 1000 | 12 fewer per 1000 (from 56 fewer to 48 more) | RR 0.94 (0.72 to 1.24) | 804 (9 studies) | ⊕⊕⊝⊝ low1,2 |
| Relapse within 10 days post‐discharge | 154 per 1000 |
40 fewer per 1000 (from 75 fewer to 11 more) |
RR 0.74 (0.51 to 1.07) | 742 (7 studies) | ⊕⊕⊕⊝ moderate1 |
| Relapse occurring after 10 days post‐discharge | 245 per 1000 | 2 fewer per 1000 (from 64 fewer to 81 more) | RR 0.99 (0.74 to 1.33) | 556 (5 studies) | ⊕⊕⊝⊝ low1,2 |
| Adverse events | 294 per 1000 | 50 fewer per 1000 (from 106 fewer to 21 more) | RR 0.83 (0.64 to 1.07) | 404 (5 studies) | ⊕⊕⊝⊝ low1,3 |
| Pulmonary function: Peak expiratory flow | The mean pulmonary function: peak expiratory flow ranged across control groups from 304 to 419 litres/min | The mean pulmonary function: peak expiratory flow in the intervention groups was 7.78 liters/min lower (38.83 lower to 23.28 higher) | 272 (4 studies) | ⊕⊕⊕⊝ moderate2 | |
| Symptom persistence | 537 per 1000 | 317 fewer per 1000 (from 461 fewer to 107 more) | RR 0.41 (0.14 to 1.2) | 80 (3 studies) | ⊕⊕⊝⊝ low1,3 |
| 24‐hour beta agonist use | 375 per 1000 | 172 fewer per 1000 (from 296 fewer to 139 more) | RR 0.54 (0.21 to 1.37) | 48 (2 studies) | ⊕⊕⊝⊝ low1,3 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded 1 level for risk of bias. Majority of studies received an unclear risk of bias for random sequence generation and selective outcome reporting 2 Downgraded 1 level for imprecision including wide confidence intervals (including both benefit, harm, and no effect) 3 Downgraded 1 level for Imprecision including wide confidence intervals (including both benefit, harm, and no effect) and few events
Background
Description of the condition
Asthma is a chronic inflammatory disease of the airways of the lungs that affects both children and adults. It is estimated that 300 million people worldwide have asthma (Croisant 2014). In the United States, the prevalence of asthma has increased from 7.3% to 8.2% from 2001 to 2009 (Croisant 2014). Acute asthma, characterised by worsening cough, wheezing, shortness of breath, or chest tightness, is a common cause of presentation to the hospital emergency department (ED) or similar acute care settings. Most patients with acute asthma presenting to the ED can be safely managed with interventions including systemic corticosteroids, anticholinergics, or beta₂‐agonists, and discharged home (Rowe 2009). However, approximately 10% to 18% (Emerman 1999; Emerman 2001; Rowe 2015; Topal 2014), and up to 31% (Ducharme 1993), of patients will relapse and return to the ED or other acute care settings with acute exacerbations of asthma within the following four weeks. In the US, it is estimated that the economic cost of asthma in 2007 was approximately USD 56 billion due to medical costs and missed work and school days (CDC 2011). Identifying effective treatment options to help patients manage their symptoms after discharge from acute care and reducing the proportion of patients who relapse are important management issues designed to improve health outcomes for patients with asthma.
Description of the intervention
Systemic corticosteroids are potent general anti‐inflammatory agents for the treatment of asthma (Alangari 2014). When given in the ED, systemic corticosteroids can reduce hospitalisations and improve lung function in patients with acute asthma (Rowe 2001). A Cochrane Review reported significant decreases in symptom scores when patients were given systemic corticosteroids at or following discharge from the ED (i.e. an IM injection at discharge, or oral corticosteroids to be taken at home over the subsequent 3 to 8 days). Heterogeneity in outcome reporting prohibited meaningful pooling (Rowe 2007a). Treatment with systemic corticosteroids at discharge has also been shown to prevent relapse (Rowe 2007a). Current guidelines recommend systemic corticosteroids at ED discharge for all but the mildest presentations of acute asthma in an effort to reduce future relapse (GINA 2017). While systemic corticosteroids can effectively mitigate asthma relapses, the optimal route of administration is less clear.
How the intervention might work
At discharge from the ED or acute care setting, systemic corticosteroids may be administered via intramuscular (IM) or oral routes. A single dose of IM corticosteroids has long‐acting pharmacokinetic properties with fewer side effects associated with nausea/vomiting; however, adverse events associated with the IM injection (i.e. pain and swelling around the injection site) are well known to occur (Lahn 2004). Oral corticosteroids have short‐acting properties, and patients are typically provided with a short course of oral corticosteroids for five to seven days (GINA 2017). While no injection is needed, side effects associated with oral corticosteroids often include nausea and vomiting, and adherence/compliance with oral corticosteroid regimens is often sub‐optimal (Ducharme 2011). Although IM corticosteroids represent an alternative treatment option for patients with intolerance to oral agents or patients where adherence/compliance concerns exist, it is unclear whether IM corticosteroids are as effective as oral corticosteroids in mitigating relapse.
Why it is important to do this review
The effectiveness of systemic corticosteroids is known (Rowe 2007a), and widely accepted by clinicians; however, whether patients benefit more or less from IM or oral corticosteroids is less clear. This review provides supplemental information with another review of different oral corticosteroid regimens for acute asthma (Normansell 2016). An umbrella review reported no differences in relapse events in adults after treatment with IM or oral corticosteroids for acute asthma (Krishnan 2009); however, this review was limited to English‐language studies, and since its publication no other systematic reviews have been conducted that have used an extensive literature search to synthesize all of the available evidence from studies that have compared IM to oral corticosteroids.
Objectives
To examine the effectiveness and safety of a single dose of intramuscular (IM) corticosteroids provided prior to discharge compared to a short course of oral corticosteroids in the treatment of acute asthma patients discharged from an ED or equivalent acute care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT) in this systematic review. We also included studies reported in full text, those published as an abstract only, and unpublished data in our search criteria.
Types of participants
We considered for inclusion in the review studies that included children or adults presenting to a hospital ED or other equivalent acute care setting with an uncomplicated exacerbation of asthma. For the purposes of this review, we defined 'uncomplicated exacerbations' as patients whose acute exacerbation of asthma was the primary reason for presentation to the ED, with no coexisting complications (e.g. no evidence of pneumonia, pneumothorax, etc.). The asthma diagnosis had to be made either using international/national clinical guidelines, or spirometric criteria, or both. We excluded from this review studies that focused on corticosteroid treatment in hospitalised participants. We only included studies that assessed patients with acute asthma who were treated and discharged from the ED or other urgent care/acute care setting. None of the included studies enrolled participants with both chronic obstructive pulmonary disease (COPD) and asthma; however, we had determined a priori that if this were to be the case, we would only include these studies if they provided results of asthma participants separately from COPD participants, or if the study population of COPD participants consisted of less than 20% of the total population.
Types of interventions
We included studies that compared a single dose of IM corticosteroids given prior to discharge versus a short course (one to 14 days) of oral corticosteroids. There were no restrictions on the IM or oral corticosteroids used, or the dosage. The single dose of IM corticosteroids may have been administered to participants at any point prior to discharge from the ED or other equivalent acute care setting. The short course of oral corticosteroids may have been started at any point prior to discharge or have been provided to participants to be taken over a period of a few days (one to 14 days) post‐discharge. We did not set any restrictions on the type of co‐interventions participants could receive (e.g. beta₂‐agonists, systemic or inhaled corticosteroids (ICS), anticholinergics, theophylline compounds, or antihistamines) during their stay in the ED/acute care setting; however, we did not permit other prescriptions of corticosteroids.
Types of outcome measures
Primary outcomes
The primary dichotomous outcome was relapse to additional care defined as an unscheduled visit to a health practitioner for worsening asthma symptoms, or requiring subsequent treatment with corticosteroids, which may have occurred at any time point after discharge from the ED. We also accepted the occurrence of relapse at any point as well as whether the occurrence of relapse was reported via self‐report or verification via health records.
Secondary outcomes
The occurrence of serious adverse events (e.g. hospitalisation; intensive care unit (ICU) admission; death; relapse for visits other than worsening symptoms of asthma).
Adverse events (e.g. pain, cellulitis/abscess, gastrointestinal bleeding, vomiting, behavioural/mental health exacerbations, abdominal pain, insomnia, hyperphagia/weight gain, skin eruptions, etc.).
Continuous data from pulmonary function testing (including peak expiratory flow (PEF), absolute/per cent change in PEF from baseline; forced expiratory volume in one second (FEV₁), and absolute/per cent change in FEV₁ from baseline).
Continuous data from symptom scores, measured via validated scales.
Duration of symptoms (days until symptom free).
Descriptive analysis of compliance/adherence with oral (corticosteroid or placebo) treatment.
Quality of life measures measured via validated scale.
The number of beta₂‐agonists doses taken by participants within a 24‐hour period of discharge.
Reporting one or more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register (CATR), which is maintained by the Information Specialist for the Cochrane Airways Group. The CATR contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, through the Cochrane Register of Studies Online (http://crso.cochrane.org/).
Weekly searches of MEDLINE Ovid SP.
Weekly searches of Embase Ovid SP.
Monthly searches of PsycINFO Ovid SP.
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature).
Monthly searches of AMED EBSCO (Allied and Complementary Medicine).
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the CATR are identified through search strategies based on the scope of the Cochrane Airways Group. Details of these strategies, as well as a list of handsearched conference proceedings, are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review. This search was completed on 14 March 2018.
In addition, an expert medical librarian (SC) conducted a supplemental search of nine electronic databases including MEDLINE (Appendix 3), Embase (Appendix 4), CINAHL (Appendix 5), Proquest Dissertation Abstracts and Theses Global (Appendix 6), SCOPUS (Appendix 7), PROSPERO (Appendix 8), the Cochrane Library (Appendix 9), and LILACS (Appendix 10) using controlled vocabulary and key words.
We also conducted an extensive search of the grey literature including ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), Google Scholar, bibliographies of included studies and relevant reviews, and SCOPUS forward search of a sentinel paper; and a handsearch of medical conference abstracts from 2008 to 2017 including the Canadian Journal of Emergency Medicine, Academic Emergency Medicine, and Annals of Emergency Medicine.
We conducted all searches without any restrictions on language or publication status.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We also searched relevant manufacturers' web sites for study information.
We searched for errata or retractions from included studies published in full text on PubMed and planned to report the date this was done within the review.
Data collection and analysis
Selection of studies
Two review authors (SWK and EC) screened the titles and abstracts of the search results independently and coded them as either potentially eligible or ineligible. We then retrieved the full‐text study reports of all potentially eligible studies and two review authors (SWK and EC) independently screened them for inclusion and recorded the reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third review author (CVR or BHR). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we piloted on at least one included study in the review. Two review authors (SWK and EC) independently extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number, mean age, age range, sex, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of study authors.
Two review authors (SWK and EC) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if an included study did not report outcome data in a usable way. We resolved disagreements by consensus or by involving a third review author (CVR or BHR). One review author (SWK) transferred data into the Review Manager 5 (RevMan 5) file (Review Manager 2014). To ensure that the data was entered correctly, a second review author (EC) verified the extracted data for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (SWK and EC) assessed risk of bias independently for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author (CVR or BHR). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as high risk, low risk, or unclear risk and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias relates to unpublished data or correspondence with an author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that may have contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and justify any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
Dichotomous data are reported as relative risk (RR) values and continuous data are reported as mean difference (MD) or standardised mean difference (SMD), as appropriate. If we combined data from rating scales in a meta‐analysis, we ensured we entered them with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only when meaningful; that is, if the treatments, participants, outcomes and the underlying interventions were similar enough for pooling to make sense.
We provided a narrative description of skewed data reported as medians and interquartile ranges (IQR).
In individual studies with multiple arms, we included only the study arms assessing IM and oral corticosteroids. If we combined two comparison groups (e.g. IM corticosteroids versus different regimens of oral corticosteroids) in the same meta‐analysis, we either combined the active study arms or halved the control group to avoid double‐counting.
If a study reported outcomes at multiple time points, we used the last time point measured.
We conducted an 'as reported' and intention‐to‐treat (ITT) analysis of the primary outcome. We assessed the secondary outcomes using an 'as reported' analysis.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of individuals who relapsed rather than number of relapses per individual).
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. if we identified a study as an abstract only). Where this was not possible and the missing data had the potential to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity we reported it and explored the possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We were unable to pool more than 10 studies, so we were unable to create a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes including: all relapse, relapse within 10 days post‐discharge, relapse after 10 days post‐discharge, adverse events, PEF/FEV₁, symptom scores, and beta₂‐agonist use in a 24‐hour period. We used the five GRADE considerations (i.e. risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and used GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We examined potential sources of heterogeneity in the primary outcome in the following subgroup analyses.
Children (zero to 18 years of age) versus adults (18 years of age and older) to examine any potential age‐specific treatment effects of IM or oral corticosteroids.
Relapse occurring within 10 days and over 10 days post‐discharge.
Low versus moderate versus high exacerbation severity based on the pulmonary function taken at the time of the participant's presentation to the ED, prior to treatment with a bronchodilator.
Co‐interventions received (ICS versus ICS corticosteroids/long‐acting beta₂‐agonists (LABA)).
We used the formal test for subgroup interactions in RevMan 5 (Review Manager 2014). We restricted the subgroup analysis to relapse.
Sensitivity analysis
We carried out the following sensitivity analyses, by removing the following types of studies from the primary outcome analyses.
Studies that we consider to be at high risk of bias based on the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Studies in which the duration of oral corticosteroid treatment was less than five days.
The results from fixed‐effect models were compared with the random‐effects models for the main outcome.
Studies in which supplemental corticosteroids were provided to the patients in the ED as a co‐intervention.
Results
Description of studies
Please see Characteristics of included studies for additional information on the included studies.
Results of the search
The literature searches identified 912 studies (see Figure 1). Removing duplicates resulted in 685 potentially eligible studies overall. We selected 20 studies for full‐text review, while we excluded the remaining 665 studies, based on lack of relevance. We excluded 11 studies after full‐text review (see Excluded studies). We included the remaining nine studies in the review. We identified one of the eligible studies by grey literature search (Al‐Wahadneh 2006). A search of errata or retractions in September 2017 did not identify any retracted publications. We included insufficient studies in this review for an assessment of publication bias (i.e. by a funnel plot). We made every effort to locate all available studies through an extensive search of the literature, with no limitations set on year of publication or language of publication.
1.
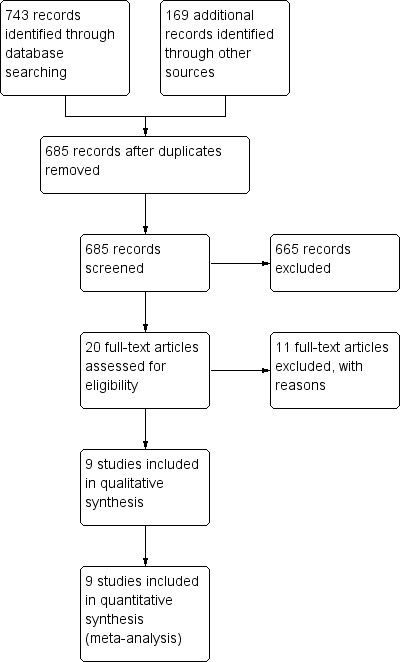
Study flow diagram.
Included studies
Publication
The first study to compare the effectiveness of IM versus oral corticosteroids in participants with acute asthma discharged from an acute care setting was published in 1988 (Hoffman 1988) while the most recent study was published in 2007 (Gordon 2007). The majority of studies were published in the United States (Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Schuckman 1998), while the remaining studies were published in Canada (Chan 2001), Jordan (Al‐Wahadneh 2006), and Taiwan (Lee 1993). All of the studies were RCTs published in peer‐reviewed journals.
Participants/Setting
The nine studies enrolled a total of 804 participants. Overall, the studies were small clinical trials, with a range of 17 to 187 participants across the studies. Five of the studies enrolled fewer than 90 participants each (Al‐Wahadneh 2006; Gries 2000; Hoffman 1988; Klig 1997; Lee 1993). Five of the included studies enrolled adults (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998), while the remaining four enrolled children (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). The age of eligible children included in the studies ranged from 9 months to 14 years (Al‐Wahadneh 2006), 18 months to 7 years (Gordon 2007), 6 months to 7 years (Gries 2000), and 3 to 16 years (Klig 1997). Among the adult studies, the mean ages of participants ranged from 31 to 42 years old (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998), while the mean age of children ranged from 3 to 6.8 years old (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). Most of the included studies recruited more male participants, except in three studies where women comprised over 50% of participants (Chan 2001; Lahn 2004; Schuckman 1998). Only three studies reported smoking history (Chan 2001; Lahn 2004; Schuckman 1998). Chan 2001 reported that current smokers made up 31% to 33% of the participants in the IM and oral corticosteroid groups respectively. Tobacco use ranged from 35% to 38% of participants randomised to the IM and oral corticosteroid groups respectively in Lahn 2004, while Schuckman 1998 reported a range of 64% to 74% of participants randomised to receive IM or oral corticosteroids had ever smoked. All of the studies recruited participants with acute asthma presenting to an ED, except one study which recruited participants with acute asthma attending a primary care clinic (Gries 2000). We attempted to estimate and categorize exacerbation severity among the included studies based on the reported baseline pulmonary function of the included studies (Table 2). We considered studies that reported a baseline average PEF of greater than 200 L/min of the groups to be mild/moderate (Chan 2001; Lahn 2004; Lee 1993; Schuckman 1998). We considered the exacerbation severity of patients enrolled in Hoffman 1988 to be severe, as the baseline PEF of included patients was less than 150 L/min. Four studies did not report baseline pulmonary function and so exacerbation severity could not be estimated (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997).
1. Exacerbation severity.
| Studies | Pulmonary function: Eligibility criteria | Exacerbation severity |
| Al‐Wahadneh 2006 | Severity estimated using modified scoring system based on GINA guidelines. Reported to enrolling patients with mild‐moderate exacerbations, however baseline pulmonary function of the groups was not reported. | Unable to assess |
| Chan 2001 | Reported mean baseline PEF greater than 200 L/min: IM group: 270 L/min (SD: 103); oral group: 261 L/min (SD: 104). | Mild/moderate |
| Gordon 2007 | Reported to enrolling patients identified as moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess |
| Gries 2000 | Applied adapted exacerbation severity score (unspecified). Reported to enrolling patients rated as mild/moderate, however baseline pulmonary function of the groups was not reported. | Unable to assess |
| Hoffman 1988 | Reported baseline mean PEF of enrolled patients of less than 150 L/min: IM group: 129 L/min (SD:14); oral group: 141 L/min (SD: 14). | Severe |
| Klig 1997 | Exacerbation severity estimated via pulmonary index score. Study reported to enrolling patients with mild/moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess |
| Lahn 2004 | Eligibility criteria required patients to have a PEFR of ≤ 70% predicted with a minimum PEFR of ≥ 40%. Reported PEF of enrolled patients was ≥ 200 L/min: IM group: 205 L/min (SD: 70); oral group: 209 L/min (SD: 72). | Mild/moderate |
| Lee 1993 | Reported mean baseline PEF of ≥ 200 L/min: IM group: 210 L/min (SD: 30); oral group: 208 L/min (SD: 26). | Mild/moderate |
| Schuckman 1998 | Reported mean baseline PEF of ≥ 200 L/min: IM group: 243.6 L/min (SD: 64); oral group: 244.7 L/min (SD: 83). | Mild/moderate |
Abbreviations:
GINA = Global Initiative for Asthma; PEF = Peak expiratory flow; PEFR = Peak expiratory flow rate; IM = intramuscular; SD = standard deviation
Intervention
All of the paediatric studies provided their participants with a single dose of IM dexamethasone (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997), while the IM corticosteroid provided in adult studies ranged from methylprednisolone (Hoffman 1988; Lahn 2004), to betamethasone (Chan 2001), dexamethasone (Lee 1993), and triamcinolone (Schuckman 1998). All of the participants randomised to receive IM corticosteroids received a single dose of IM corticosteroids prior to discharge. Only five studies were placebo controlled, in which all participants receiving IM corticosteroids were also provided with a course of oral placebo tablets (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998). There was significant variation between the IM corticosteroid used, as well as the dosing. We provide additional information on the IM corticosteroids used in Characteristics of included studies and Table 3. We estimated corticosteroid equivalency to methylprednisolone using an equivalence converter (www.medcalc.com/steroid.html). Overall, the median dose was 78 mg with a range of 40 mg to 120 mg (Table 3).
2. Study characteristics of included studies.
| Studies | Location/setting | Co‐interventions | Corticosteroid doses and durations | Methyprednisolone equivalency | Relapse outcome |
| Al‐Wahadneh 2006 | Jordan, ED | Provided in ED: not stated Provided at discharge: SABA |
Dexamethasone (IM) 1.7 mg/kg Mean dose: 24 mg Single dose Prednisolone (oral) 2 mg/kg/day for 5 days Mean dose: 19.2 mg per day Total dose: 96 mg |
IM Methylprednisone equivalency: 120 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 76.8 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 1/16 Day 21 Oral group 3/14 Day 21 |
| Chan 2001 | Canada, ED | Provided in ED: SABA, methylxanthines, supplemental oral/IV corticosteroids Provided at discharge: Methylxanthines, unspecified inhaled beta₂‐agonists, and ICS |
Betamethasone (IM) 12 mg Single dose. Received placebo capsules over 7 days. Prednisone (oral) 50 mg a day for 7 days. Received a single placebo injection Total dose: 350 mg |
IM Methylprednisone equivalency: 72 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 280 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 12/86 Day 7 Oral group 19/82 Day 7 |
| Gordon 2007 | United States, Pediatric ED | Provided in ED: SABA, ipratropium bromide. IV corticosteroids for patients who vomited oral corticosteroids. Provided at discharge: Inhaled beta₂‐agonists and ICS |
Dexamethasone (IM) 0.6 mg/kg (max 16 mg) Single dose Prednisolone (oral) 2 mg/kg (max 50 mg) daily for 5 days Total: 250 mg |
IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12‐36 hours) Oral Methylprednisone equivalency: 200 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 8/69 Day 4 Oral group 11/73 Day 4 IM group 15/68 Day 14 Oral group 16/73 Day 14 |
| Gries 2000 | United States, Tertiary medical center | Provided in ED: SABA Provided at discharge: SABA |
Dexamethasone (IM) Patients 6 to 12 months old received 16 mg. Patients 13 to 35 months old received 24 mg. Children ≥ 36 months received 36 mg. Single dose Prednisone (oral) 2 mg/kg a day for 5 days Total dose: unclear |
IM Methylprednisone equivalency: Unable to assess Oral Methylprednisone equivalency: Unable to assess |
IM group 1/15 Day 28 Oral group 3/17 Day 28 |
| Hoffman 1988 | United States, ED | Provided in ED: SABA, epinephrine, methylxanthines, IV corticosteroids Provided at discharge: Methylxanthine and inhaled beta₂‐agonists |
Methylprednisonlone (IM) 80 mg Single dose. Received placebo capsules for 7 days Methylprednisolone (oral) Tapering dose over 7 days. Total dose: 216 mg Received placebo injection |
IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 216 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 0/8 day Day 7 Oral group 2/10 Day 7 |
| Klig 1997 | United States, Pediatric ED | Provided in ED: SABA Provided at discharge: SABA |
Dexamethasone (IM) 0.3 mg/kg Total dose: 15 mg Single dose Prednisone (oral) 2 mg/kg a day for 3 days Total dose: 100 mg |
IM Methylprednisone equivalency: 75 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 0/21 Day 5 Oral group 0/21 Day 5 |
| Lahn 2004 | United States, ED | Provided in ED: inhaled beta₂‐agonists, IV corticosteroids Provided at discharge: SABA |
Methylprednisolone (IM) 160 mg Single dose. Received placebo capsules for 8 days Methylprednisolone (oral) Tapering dose over 8 days. Total dose: 160 mg (tapering dose 32 mg day 1). Received placebo injection |
IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 13/92 Day 10 Oral group 12/88 Day 10 IM group 17/92 Day 21 Oral group 20/88 Day 21 |
| Lee 1993 | Taiwan, ED | Provided in ED: SABA, methylxanthines Provided at discharge: Methylxanthine and inhaled beta₂‐agonists |
Dexamethasone (IM) 10 mg Single dose. Received placebo capsules for 7 days Dexamethasone (oral) Tapering dose over 7 days. 11.75 mg total. Received placebo injection |
IM Methylprednisone equivalency: 50 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 58.8 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 1/17 Day 7 Oral group 0/19 Day 7 |
| Schuckman 1998 | United States, ED | Provided in ED: SABA, oral/IV corticosteroids Provided at discharge: SABA, antibiotics, ICS, cromolyn sodium, ipratropium bromide |
Triamcinolone (IM) 40 mg Single dose. Received placebo capsules for 5 days Prednisone (oral) 40 mg a day for 5 days. Total dose: 160 mg Received placebo injection |
IM Methylprednisone equivalency: 40 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 160 mg Duration: intermediate half‐life (12 to 36 hours) |
IM group 7/78 Day 7 Oral group 11/76 Day 7 |
ED = emergency department; SABA = short‐acting beta₂‐agonists; LABA = long‐acting beta₂‐agonists; IV = intravenous; IM = intramuscular; ICS = inhaled corticosteroids
Comparison
All of the paediatric studies compared IM dexamethasone to oral prednisone/prednisolone (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). The oral corticosteroid regimen provided in the adult studies consisted of either prednisone (Chan 2001; Schuckman 1998), methylprednisolone (Hoffman 1988; Lahn 2004), or dexamethasone (Lee 1993). The paediatric studies tended to provide participants a shorter course of oral corticosteroids, lasting from three (Klig 1997) to five days (Al‐Wahadneh 2006; Gordon 2007; Gries 2000). The course of oral corticosteroids tended to last slightly longer in the adult studies, ranging from five (Schuckman 1998), seven (Hoffman 1988; Lee 1993), to eight days (Lahn 2004). Six studies provided a consistent dose of oral corticosteroids (Al‐Wahadneh 2006; Chan 2001; Gordon 2007; Gries 2000; Klig 1997; Schuckman 1998) while three studies provided a tapering dose (Hoffman 1988; Lahn 2004; Lee 1993). All participants randomised to receive oral corticosteroids were provided with their medications prior to discharge which was to be taken at home over the next 3 to 8 days. Only five studies were placebo controlled, in which all participants receiving oral corticosteroids were provided with IM placebo (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998). We present additional information on the oral corticosteroids provided in Characteristics of included studies and Table 3. We estimated corticosteroid equivalency to methylprednisolone using an equivalence converter (www.medcalc.com/steroid.html). Overall, the median dose was 116 mg with a range of 58.8 mg to 280 mg (Table 3).
Co‐interventions
The co‐interventions provided varied among the included studies (see Characteristics of included studies and Table 3). In some cases, all participants received standardised co‐interventions (Al‐Wahadneh 2006; Gordon 2007; Hoffman 1988; Gries 2000; Klig 1997; Lee 1993; Schuckman 1998), while another study reported that participants received non‐standardized co‐interventions at the discretion of the attending physicians (Chan 2001). The most common co‐interventions provided to participants during the acute care visit included: SABA (Chan 2001; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Lee 1993; Schuckman 1998), methylxanthines (Chan 2001; Hoffman 1988; Lee 1993), and ipratropium bromide (Gordon 2007). In some instances, some studies reported providing participants with supplemental oral (Chan 2001; Schuckman 1998) or IV (Chan 2001; Hoffman 1988; Schuckman 1998) corticosteroids during their stay in the ED. Gordon 2007 provided participants with IV corticosteroids if they vomited the oral corticosteroids while in the ED. Co‐interventions provided to participants at discharge consisted primarily of SABA (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997; Lahn 2004; Schuckman 1998), methylxanthine (Chan 2001; Hoffman 1988; Lee 1993), LABA (Chan 2001), and unspecified inhaled beta₂‐agonists (Hoffman 1988). Supplemental corticosteroids provided at discharge were limited across the included studies. Chan 2001 and Gordon 2007 reported that the attending ED physician decided whether or not to provide participants with ICS at discharge. Participants in Lahn 2004 received ICS at discharge if they were using them previously.
Outcomes
All included studies reported relapse to additional care, with a measurement range of four to 28 days after discharge. Seven studies assessed relapse within 10 days post‐discharge (Chan 2001; Gordon 2007; Hoffman 1988; Klig 1997; Lahn 2004; Lee 1993; Schuckman 1998), while five studies measured relapse occurring more than 10 days post‐discharge (Al‐Wahadneh 2006; Chan 2001; Gordon 2007; Gries 2000; Lahn 2004). Three studies assessed relapse at two different time points after discharge (Chan 2001; Gordon 2007; Lahn 2004). In comparison to relapse, we found infrequent reporting and inconsistent measurement of our proposed secondary outcomes.
Excluded studies
We excluded 11 studies from this review, mostly due to inappropriate study design (not an RCT) (Andrews 2012; Droszcz 1981; Droszcz 1985; Ducharme 2004; Green 1995; Hofmann 2008; Kelso 2014; Ozpenpe 2011; Razi 2006; Watnick 2016; White 2010) (See Characteristics of excluded studies).
Risk of bias in included studies
Overall, the risk of bias of the included studies ranged from unclear to high and none of the studies had an overall low risk of bias (see Figure 2; Figure 3). Of the nine studies assessed, we rated four as having high risk of bias (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997), while we assessed the remaining studies as having an unclear risk of bias (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998).
2.
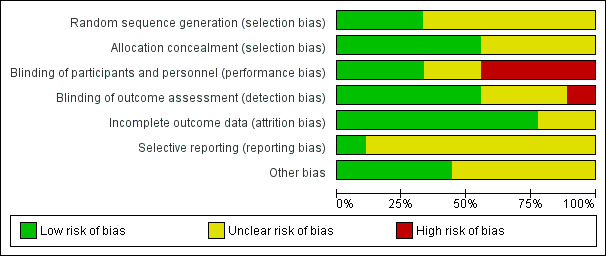
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
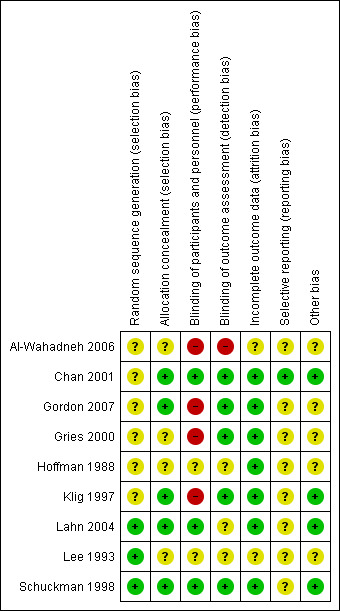
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies provided adequate information on randomisation to allow for an assessment of low risk of bias for sequence generation (Lahn 2004; Lee 1993; Schuckman 1998). We considered the remaining studies to be at unclear risk of bias due to missing information on randomisation of participants (Al‐Wahadneh 2006; Chan 2001; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997). Five studies had a low risk of bias with regard to allocation concealment (Chan 2001; Gordon 2007; Klig 1997; Lahn 2004; Schuckman 1998), while the remaining studies did not provide enough information to make a clear judgement on the risk of bias on allocation concealment (Al‐Wahadneh 2006; Gries 2000; Hoffman 1988; Lee 1993).
Blinding
Given the feasibility of employing a placebo‐controlled study design, and that many outcomes of interest were collected via participant self‐report, we considered the primary and secondary outcomes of interest may have been susceptible to potential performance and detection bias. We considered three studies to have adequately blinded participants and personnel (Chan 2001; Lahn 2004; Schuckman 1998). Four studies did not blind participants or personnel, and we judged them to be at high risk of bias (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). We considered two studies to be at unclear risk of bias due to a lack of information provided (Hoffman 1988; Lee 1993). Five studies reported adequate blinding of outcome assessors (Chan 2001; Gordon 2007; Gries 2000; Klig 1997; Schuckman 1998). We considered one study to be at high risk of bias because they did not blind outcome assessors (Al‐Wahadneh 2006), while we considered three studies to be at unclear risk of bias due to a lack of available information on the blinding of outcome assessors (Hoffman 1988; Lahn 2004; Lee 1993).
Incomplete outcome data
We assessed two studies as having an unclear risk of bias due to a lack of information on attrition (Al‐Wahadneh 2006; Lee 1993). We assessed the remaining studies as having a low risk of bias (Chan 2001; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Schuckman 1998).
Selective reporting
We considered only one study to be at low risk of bias for reporting bias; we received a study protocol upon request from the study authors (Chan 2001). We considered the remaining studies at be at unclear risk of reporting bias due to the lack of an available protocol (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Lee 1993; Schuckman 1998).
Other potential sources of bias
We considered four studies to be at low risk of bias for other potential sources of bias (Chan 2001; Klig 1997; Lahn 2004; Schuckman 1998). Three studies reported their source of funding in the included article (Chan 2001; Lahn 2004; Schuckman 1998), while we found additional information regarding study funding in one study (Klig 1997). Two studies received funding from general health research grants (Chan 2001; Schuckman 1998). One study received pharmaceutical company (Pfizer) sponsorship but reported that Pfizer was not involved in any aspect of the study or manuscript preparation (Lahn 2004). One study was unfunded (Klig 1997). We considered five studies to be at unclear risk of bias because the studies did not state their source of funding (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Hoffman 1988; Lee 1993). We did not consider any study to be at high risk of bias for other potential sources of bias.
Effects of interventions
See: Table 1
See Table 1 for a summary of the main comparisons. Insufficient reporting prevented meaningful meta‐analysis of several proposed secondary outcomes, including symptom scores; relapse requiring hospitalisation; and quality of life; and specific adverse events such as pain; swelling; redness/bruising; personality changes; and insomnia.
Primary Outcome
Relapse
We detected no difference in the proportion of patients who relapsed between participants receiving IM versus oral corticosteroids (RR 0.94, 95% CI 0.72 to 1.24; 9 studies, 804 participants = 804; I² = 0%; Analysis 1.1) with low heterogeneity (I² = 0%). An ITT analysis revealed similar results (RR 0.95, 95% CI 0.72 to 1.26; 9 studies, 821 participants; I² = 0%; Analysis 1.2).
1.1. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 1 Relapse.
1.2. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 2 Relapse intention to treat.
Subgroup analysis
A subgroup analysis comparing the effectiveness of IM versus oral corticosteroids to reduce relapse in paediatric (RR 0.86, 95% CI 0.48 to 1.53; 4 studies, 245 participants; I² = 0%) or adult (RR 0.97, 95% CI 0.71 to 1.33; 5 studies, 559 participants; I² = 0%) participants revealed no subgroup differences (P = 0.71; Analysis 1.3). In addition, we found no subgroup differences between studies assessing relapse within 10 days (RR 0.74, 95% CI 0.51 to 1.07; 7 studies, 742 participants; I² = 0%) or greater than 10 days post‐discharge (RR 0.99, 95% CI 0.74 to 1.33; 5 studies, 556 participants; I² = 0%) (P = 0.22; Analysis 1.4). It is important to note, however, that for the relapse greater than 10 days post‐discharge subgroup, it is unclear whether this includes relapses occurring within 10 days. We found no subgroup differences (P = 0.35; Analysis 1.5) in relapse between studies estimated to have enrolled participants with mild/moderate (RR 0.98, 95% CI 0.71 to 1.34; 4 studies, 539 participants; I² = 0%) versus severe exacerbations (RR 0.24, 95% CI 0.01 to 4.47; 1 study, 18 participants; I² = 100%). We could not complete the proposed co‐interventions subgroup comparing the effects of ICS versus ICS/LABA on relapse as planned due to a lack of studies providing information on the use of ICS and ICS/LABA agents as co‐interventions.
1.3. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 3 Subgroup analysis: children versus adults.
1.4. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge.
1.5. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 5 Subgroup analysis: mild/moderate versus severe exacerbations.
Sensitivity analysis
We conducted a sensitivity analysis in which we removed from the meta‐analysis the four studies considered to be at high risk of bias (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). We identified no difference in relapse proportions between participants receiving IM or oral corticosteroids (RR 0.97, 95% CI 0.71 to 1.33; 5 studies, 559 participants; I² = 0%; Analysis 1.6). An additional sensitivity analysis, in which studies providing participants with an oral corticosteroid regimen of less than five days (Klig 1997), revealed no difference in relapse rates between participants receiving IM or oral corticosteroids (RR 0.94, 95% CI 0.72 to 1.24; 8 studies, 762 participants; I² = 0%; Analysis 1.7). Finally, fixed effects revealed similar findings to that of random effects (RR 0.91, 95% CI 0.69 to 1.19; 9 studies, 804 participants; I² = 0%; Analysis 1.8). Removing studies in which patients received additional corticosteroids as a co‐intervention during the patients' stay in the ED revealed no differences in relapse rates between participants receiving IM or oral corticosteroids (RR 0.76, 95% CI 0.45 to 1.29; 5 studies, 320 participants; I² = 0%; Analysis 1.9).
1.6. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 6 Sensitivity analysis: risk of bias.
1.7. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days.
1.8. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 8 Sensitivity analysis: fixed effects.
1.9. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 9 Sensitivity analysis: corticosteroids in ED.
Secondary outcomes
Serious adverse effects
Insufficient reporting of serious adverse events prohibited any meaningful meta‐analysis. Only one study reported on participants relapsing and requiring hospitalisation or ICU admission by 14‐day follow‐up; however, the effect estimate is imprecise (RR 1.43, 95% CI 0.33 to 6.16; 1 study, 141 participants; Analysis 1.10) (Gordon 2007).
1.10. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 10 Serious adverse events; hospitalization following discharge.
Adverse events
Across the included studies we found poor documentation and inconsistent measurement of adverse events associated with the use of IM and oral corticosteroids, resulting in few meaningful comparisons. Overall, a meta‐analysis did not reveal whether participants receiving IM corticosteroids are more or less likely to experience any adverse events compared to participants receiving oral corticosteroids (RR 0.83, 95% CI 0.64 to 1.07; 5 studies, 404 participants; I² = 0%; Analysis 1.11). In some cases, the occurrence of specific adverse events was reported in greater detail. We found no differences in the frequency of nausea and vomiting among participants receiving either IM or oral corticosteroids (RR 0.56, 95% CI 0.09 to 3.59; 3 studies, 320 participants; I² = 58%; Analysis 1.12). We found insufficient evidence to conclusively state whether there is a difference in the occurrence of insomnia (RR 1.74, 95% CI 0.61 to 4.97; 1 study, 171 participants; I² = 0%; Analysis 1.13) or personality changes (RR 0.82, 95% CI 0.56 to 1.19; 1 study, 30 participants; I² = 0%; Analysis 1.14) among participants receiving IM or oral corticosteroids. Some studies reported the occurrence of side effects associated with the IM corticosteroid injection. Two studies strictly reported on the occurrence of pain, swelling, and redness at the injection site among participants receiving IM corticosteroids only, as these studies did not utilize a IM placebo control group (Gordon 2007; Gries 2000). Gordon 2007 reported the proportion of paediatric participants experiencing discomfort, swelling, and redness following the IM corticosteroid injection was 1.6%, 6.5%, and 1.6% respectively. Gries 2000 reported that no paediatric participants receiving IM corticosteroids experienced any other side effects besides personality changes. Two placebo‐controlled studies compared the occurrence of pain, swelling, and redness between participants receiving IM corticosteroids compared to participants receiving oral corticosteroids who received an IM placebo injection. Overall, the imprecision of the estimate precluded identifying a difference in the occurrence of pain (RR 1.75, 95% CI 0.32 to 9.66; 2 studies, 181 participants; I² = 8%; Analysis 1.15), swelling (RR 4.76, 95% CI 0.57 to 39.84; 2 studies, 180 participants; I² = 0%; Analysis 1.16), or redness (RR 13.50, 95% CI 0.77 to 235.63; 2 studies, 175 participants; I² = 0%; Analysis 1.17) between participants receiving IM corticosteroids versus IM placebo.
1.11. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 11 Adverse events.
1.12. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 12 Adverse events: nausea/vomiting/GI distress.
1.13. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 13 Adverse events: insomnia.
1.14. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 14 Adverse events: personality changes.
1.15. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 15 Adverse events: pain.
1.16. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 16 Adverse events: swelling.
1.17. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 17 Adverse events: redness.
Pulmonary function
None of the included studies assessing a paediatric population collected PEF or FEV₁ measures. Four studies assessed PEF among an adult population following discharge from the ED. We found no differences in PEF between participants receiving IM versus oral corticosteroids at follow‐up (MD −7.78 L/min, 95% CI −38.83 L/min to 23.28 L/min; 4 studies, 272 participants; I² = 33%; Analysis 1.18). Only one study reported on FEV₁/FVC (%), and it reported no significant differences between participants receiving IM versus oral corticosteroids (MD −1.00, 95% CI −12.44 to 10.44; 1 study, 36 participants; I² = 0%; Analysis 1.19). No studies assessed per cent change in baseline PEF following discharge from the ED.
1.18. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 18 Pulmonary function: peak expiratory flow (L/min).
1.19. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 19 Pulmonary function: FEV₁/FVC (%).
Symptom scores
None of the included studies assessed symptom scores following discharge from the ED using validated scales. One study reported symptom scores for shortness of breath, cough, wheeze, activity limitation, and sleep disturbance at days 7 and 21 post‐discharge using an unspecified tool, in which participants were asked to give a score between 1 (indicating 'not present') to 10 (indicating 'most severe') for each symptom (Chan 2001). At day 21 post‐discharge, symptom scores between participants receiving IM versus oral corticosteroids were similar for each symptom including: shortness of breath (IM 3.8 (SD 2.4) n = 48; oral: 3.8 (SD 2.4) n = 58), cough (IM 2.8 (SD 2.2) n = 48; oral 3.4 (SD 2.4) n = 58), wheeze (IM 3.4 (SD 2.5) n = 48; oral 3.4 (SD 2.3) n = 58), activity limitation (IM 2.1 (SD 2.3) n = 48; oral 2.2 (SD 2.1) n = 58), and sleep disturbance (IM 2.9 (SD 2.7) n = 48; oral 2.8 (SD 2.7) n = 58). The study reported no significant differences in symptoms' scores between patients receiving IM or oral corticosteroids (P value not reported).
Duration of symptoms
None of the included studies reported the duration for which symptoms occurred following discharge from the ED. Three studies reported whether participants were still experiencing non‐specific symptoms at the time of follow‐up, which was assessed at day 5 (Al‐Wahadneh 2006; Gries 2000) and between 5 to 7 days post‐discharge (Hoffman 1988). We found no significant differences in the presence of symptoms at follow‐up between participants receiving IM or oral corticosteroids (RR 0.41, 95% CI 0.14 to 1.20; 3 studies, 80 participants; I² = 44%; Analysis 1.20). Three studies reported whether participants were still experiencing cough (RR 0.69, 95% CI 0.17 to 2.73; 3 studies, 178 participants; I² = 50%; Analysis 1.21) or wheezing (RR 0.59, 95% CI 0.14 to 2.52; 3 studies, 177 participants; I² = 53%; Analysis 1.22) at the time of follow‐up but no differences between participants receiving IM versus oral corticosteroids were found. As previously noted, while Chan 2001 reported symptom scores for shortness of breath, cough, wheeze, activity limitation, and sleep disturbances at days 7 and 21 post‐discharge, the study did not assess whether there was a significant difference in symptom scores between the IM and oral corticosteroid groups at days 7 and 21.
1.20. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 20 Symptom persistence.
1.21. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 21 Symptom persistence: cough.
1.22. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 22 Symptom persistence: wheezing.
Compliance with oral medications
Three adult studies reported compliance with the oral corticosteroid treatment regimens. Although the overall compliance with the prescribed oral corticosteroid regimen varied, the reported adherence was high, with a low of 66.7% (n = 6/9; Hoffman 1988), to 94% (n = 64/68; Schuckman 1998), and 100% (n = 84/84; Chan 2001). Only two paediatric studies examined compliance with oral corticosteroids. Al‐Wahadneh 2006 reported that eight parents reported difficulties in providing their children with the oral corticosteroid tablets and four children (n = 4/14) missed between 50% and 75% of their doses. Gries 2000 reported that 41% (n = 7/17) of patients did not comply with their prescribed oral prednisone regimen, with three participants missing more than 75% of their oral prednisone doses, while another four children did not take one‐third of their medication.
Beta agonist use
Two studies reported the use of beta₂‐agonists among participants 24 hours after discharge from the ED. We found no differences in beta₂‐agonists use 24 hours after discharge from the ED among participants receiving either IM or oral corticosteroids (RR 0.54, 95% CI 0.21 to 1.37; 2 studies, 48 participants; I² = 0%; Analysis 1.23).
1.23. Analysis.
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 23 24‐hour beta agonist use.
Discussion
Summary of main results
Systemic corticosteroids are an effective treatment in reducing relapses in patients with acute asthma after discharge from an emergency department (ED) or equivalent acute care setting (Krishnan 2009; Rowe 2007a). While systemic corticosteroids are commonly provided to prevent admission and reduce the risk of relapse, the optimal route of administration is unclear.
This systematic review set out to compare the effectiveness of a single dose of intramuscular (IM) corticosteroids to a regimen of oral corticosteroids to prevent relapse among children and adult patients discharged from an ED or equivalent acute care setting for acute asthma. Utilizing methods to reduce publication and selection bias, we identified nine trials involving 804 participants comparing a single dose of IM to oral corticosteroids in adult and paediatric participants with acute asthma.
Overall, this review could not identify a statistically significant difference in relapses among participants receiving IM versus oral corticosteroids. Furthermore, we identified no subgroup differences between children and adults, relapse occurring within or after 10 days post‐discharge, or participants presenting to the ED with a mild/moderate versus severe exacerbation. The effectiveness of IM versus oral corticosteroids to mitigate relapse remained consistent following several sensitivity analyses, including fixed versus random effects, variations in study quality, and variations in duration of oral corticosteroid regimens. Regarding other important clinical outcomes, the risk for adverse events among participants receiving IM corticosteroids ranged from a 36% decrease up to a 7% increase. Neither corticosteroid regimen demonstrated superiority with respect to pulmonary function measures, symptom persistence, or need for beta₂‐agonists following discharge.
With a similar risk for relapse, the decision to provide patients with either IM or oral corticosteroids at discharge will likely involve other considerations such as patient preference, palatability, socio‐economic conditions, ability to afford prescriptions, side‐effect profile, and clinician estimation of patient adherence. This may vary between paediatric and adult patients. For patients who may have difficulties complying with an oral corticosteroid or patients who have previously experienced nausea or vomiting with oral corticosteroids, IM corticosteroids appear to provide physicians with an effective alternative. Unfortunately, a lack of reporting of many secondary outcomes of interest severely limited the number of studies that could be included in each meta‐analysis. While all of the included studies reported on relapse outcomes, the majority of secondary outcomes were reported sparingly across the included studies, and this limited the ability of this review to make any meaningful conclusions regarding the overall effectiveness of IM corticosteroids.
Overall completeness and applicability of evidence
We believe the overall completeness of the review to be moderate. We conducted an extensive search of the literature which identified nine studies including 804 participants. Among the nine studies, five enrolled adults (n = 559) (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998) while four studies involved children (n = 245) (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997).
Overall, the paediatric studies tended to focus on young children, with the mean ages of the IM and oral corticosteroid groups ranging from 3 to 7 years old. The age of eligible children included in the studies ranged from 9 months to 14 years (Al‐Wahadneh 2006), 18 months to 7 years (Gordon 2007), 6 months to 7 years (Gries 2000), and 3 to 16 years (Klig 1997). It is important for future studies assessing the effectiveness of corticosteroids in children with acute asthma to attempt to ensure that the included patients had a true diagnosis of asthma: among the included children studies, only two of the studies reported only enrolling children with prior episodes of respiratory illness treated with bronchodilators (Gordon 2007; Klig 1997).
Within the paediatric studies, there is a paucity of studies on systemic corticosteroids in children aged 7 to 17 years old. Given the evidence that pre‐school‐aged children with acute asthma/wheezing may not respond to corticosteroids due to fewer airway eosinophils (Castro‐Rodriguez 2016), it is possible that the effectiveness of the IM or oral corticosteroid treatments may have been impacted by the age of the enrolled patients, which would affect the generalisability of this review. To our knowledge, efforts were made to avoid the inclusion of patients with a diagnosis of bronchiolitis in the included paediatric studies. This contamination, however, would have a conservative effect by decreasing the chance of detecting a difference.
In the adult studies, the age of eligible participants ranged from over the age of 18 (Chan 2001), 15 to 55 (Hoffman 1988), 18 to 45 (Lahn 2004), 16 to 60 (Lee 1993), and 18 to 50 years old (Schuckman 1998). While the majority of studies included young and middle‐aged adults, only one study included patients over the age of 60 (Chan 2001). As a result, the results of this review may not be applicable to the teenage (up to age 14) and older (> 60 years) patients with acute asthma. In addition, the effectiveness of corticosteroids in the paediatric studies may be underestimated due to the age of the participants included in the paediatric studies. All of the included studies enrolled patients presenting with acute asthma to the ED, except Gries 2000 which enrolled patients in an paediatric medical clinic. Overall, the applicability of our review focuses primarily on very young (0 to 7 years old) and young/middle‐aged adults with acute asthma presenting to the ED with acute asthma in North America (United States/Canada). More research is needed in acute care centres across the world. In particular, additional paediatric (enrolling school aged children) or elderly studies are needed to better understand the effectiveness of IM versus oral corticosteroids in those patient populations.
The type of IM agents used, as well as the doses employed, varied considerably across studies. For example, IM agents included methylprednisolone (Hoffman 1988; Lahn 2004), betamethasone (Chan 2001), dexamethasone (Lee 1993), and triamcinolone (Schuckman 1998); they also varied in dose. It is possible that the variation in the dosing and IM agents used could have impacted the effectiveness of this corticosteroid comparison. Moreover, the co‐interventions were poorly reported; and, given the age of some of the studies, the use of these agents in clinical practice likely has been stopped (e.g. metaproterenol, aminophylline, etc.).
Some newer agents, which are now the mainstay of chronic asthma treatment such as ICS and ICS/LABA agents, were either not prescribed or not reported in sufficient detail among the included studies. A recent study found that patients receiving ICS/LABA at presentation to the ED were at the highest risk of relapse following discharge (Rowe 2015) and there is evidence that ICS agents, when added to oral (and presumably IM) systemic corticosteroids at discharge, may reduce relapse (Rowe 1999). Among patients presenting with acute asthma who are already receiving ICS agents, fewer relapses occurred when ICS/LABA was substituted for ICS agents in addition to oral (and presumably IM) systemic corticosteroids at discharge (Rowe 2007b). A lack of reporting on the use of ICS and ICS/LABA agents limited the ability of this review to estimate the impact of these agents on the efficacy of IM or oral corticosteroids.
This review did not attempt to control for corticosteroid equivalency of the varying corticosteroids regimens used across the studies. It is possible that the dose and pharmacokinetic properties of various prescribed corticosteroids could have impacted the effectiveness of the IM or oral corticosteroids. Other studies have compared the effectiveness of oral prednisone compared to oral dexamethasone (which has a longer half‐life) to improve outcomes in children with acute asthma (Paniagua 2017; Normansell 2016). A recently published Cochrane Review reported insufficient evidence to identify whether lower or shorter doses of oral corticosteroids were any less effective than longer or higher doses and recommended additional large high‐quality clinical trials (Normansell 2016). Additional studies comparing the effectiveness of varying IM corticosteroids (e.g. IM dexamethasone versus IM methylprednisone) and the impact of dosing/pharmacokinetics are needed.
Quality of the evidence
The overall risk of bias of the included studies ranged from unclear to high, with no studies being assessed as having a low risk of bias. Four high risk of bias studies were not placebo controlled trials (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). The remaining studies had an overall unclear risk of bias (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998). The majority of studies did not adequately describe their method of randomisation and all but Chan 2001 had an unclear risk of bias for selective outcome reporting due to a lack of an available protocol. Using GRADE, the overall quality of the outcomes ranged from low to moderate. We reduced to low quality the primary outcomes of relapse as well as relapse after 10 days due to overall unclear to high risk of bias of the studies, and imprecision due to wide confidence intervals including both benefit, harm, and no effect. We assessed the quality of the subgroup analyses for relapse to be low due to the low number of available patients and wide confidence intervals. We judged the quality of the outcome adverse events to be low quality due to the overall unclear to high risk of bias of the included studies and imprecision due to few events. We considered both 'symptom persistence' and '24 hour beta₂‐agonists use' to be low quality due the overall unclear to high risk of bias of the included studies, as well as few events. We assessed the 'outcome of relapse within 10 days' as being of moderate quality due to the overall unclear to high risk of bias of the studies. We reduced 'peak expiratory flow' to moderate quality due to imprecision of the results.
Potential biases in the review process
While there is always a potential for screening and study selection bias, we took several steps to attenuate this risk. An expert health sciences librarian conducted a comprehensive search of the literature, including the CATR, a separate search of nine electronic databases, and a detailed search of the grey literature. Indeed, we identified one study from the grey literature (Al‐Wahadneh 2006). We placed no limits with regard to language, date of publication, or status of publication on any of the searches. We translated and screened all foreign language articles identified. If the information provided in the text did not allow for a clear inclusion/exclusion decision, we attempted to contact study authors to clarify the information provided in the text. Two review authors independently completed study selection, including both title and abstract screening and full‐text review. Despite our efforts, we recognize that some studies could have been missed. Unfortunately, due to an insufficient number of included studies, publication bias could not be assessed as planned (Higgins 2011). As such, the risk of publication bias should be considered unclear.
Agreements and disagreements with other studies or reviews
Only two reviews have been published comparing the effectiveness of IM versus oral corticosteroids in patients presenting to an acute care centre with acute asthma (Krishnan 2009; Rowe 2017). The results of this review are similar to a previously published umbrella review which compared the effectiveness of IM versus oral corticosteroids in adults (Krishnan 2009). The review identified five adult studies (all five of which were included in this review) and reported no significant difference in relapse rates in adult participants receiving IM versus oral corticosteroids for acute asthma after discharge from an ED. Unlike the current review, Krishnan 2009 did not include paediatric studies. In addition, a recently published review compared the effectiveness of various regimens of systemic corticosteroids to reduce relapse in adults with acute asthma (Rowe 2017). Unlike the current review which strictly included studies comparing IM versus oral corticosteroids, Rowe 2017 included any studies comparing IM or oral corticosteroids to other oral corticosteroid or placebo regimens and conducted indirect comparisons using a Bayesian network analysis. The review reported that both IM and long‐course oral corticosteroids were effective in reducing relapse, with a risk of bias ability of best analysis identifying IM corticosteroids as having the highest risk of bias of being the most effective treatment option compared to short‐course or long‐course oral corticosteroids. Unlike the current review, Rowe 2017 did not exclusively compare the effectiveness of IM versus oral corticosteroids to mitigate relapse.
Authors' conclusions
Implications for practice.
For adults and children who have presented to the ED or equivalent acute care settings and who are suitable for discharge, a single dose of IM corticosteroids or a short dose of oral corticosteroids are both effective treatments to mitigate relapse events. We noted no differences with regards to pulmonary function, symptom persistence, or beta‐agonist use following treatment with either IM or oral corticosteroids. This review did not control for corticosteroid equivalency in the analysis; however, both arms used a moderate to high dose of intermediate‐duration agents. The review is also unable to reach conclusions regarding specific IM or oral corticosteroid treatments based on dosing or pharmacokinetic properties.
There is some evidence to suggest IM corticosteroids may decrease the risk of adverse events by as much as 36%, compared to a 7% potential increase; however, IM delivery is painful and can cause inflammation or infection (or both) at the site of injection.
Implications for research.
Additional comparative effectiveness studies of IM versus oral corticosteroids to improve patient outcomes are needed to better inform clinicians and patients about how best to optimise patient care. In particular, we recommend additional studies enrolling school‐aged children to better understand the effectiveness of IM versus oral corticosteroids in this age group.
Additional comparative effectiveness studies using different IM corticosteroids (e.g. IM dexamethasone versus IM methylprednisone) and different oral corticosteroids (e.g. oral dexamethasone versus oral prednisone), with consideration for dosing and pharmacokinetic properties, are needed to explore the optimal IM or oral corticosteroid regimens to improve patient outcomes.
We strongly recommend comprehensive and standardized outcome reporting including clearly defined criteria and adjudication of health services outcomes (e.g. relapses resulting in primary care or ED visits, hospitalisation, death), side effects, pulmonary function, and symptom prevalence in future studies.
Compliance with the provided oral corticosteroid regimens was infrequently documented across the studies, and compliance, or lack thereof, could have impacted the results of this review. It is important that future comparative studies document compliance with oral corticosteroid regimens.
Future research needs to consider and address potential sources of bias, including randomisation, concealment of allocation, blinded outcome assessment, placebo‐controlled, avoidance of selective outcome reporting, and complete documentation of follow‐up.
Future research needs to better document the occurrence of adverse events associated with IM and oral corticosteroids, in particular adverse events associated with IM corticosteroids, which have been reported less extensively than those associated with oral corticosteroids, and range from pain, redness and swelling to rarer adverse events including avascular necrosis and hypothalamic/pituitary axis suppression.
Future research needs to examine the effects of repeat treatments of IM or oral corticosteroids among paediatric or adult patients with frequent exacerbations.
Given the importance of ICS agents in the chronic treatment of asthma, either alone or in combination with other agents, it is important that future research clearly documents the co‐interventions (e.g. ICS, ICS/LABA, monoclonal antibodies, etc.) provided to patients either in the ED, or at discharge, so that researchers may better understand the impact of these co‐interventions on the efficacy of IM or oral corticosteroids' reports on the pre‐ED and post‐ED use of all other anti‐inflammatory treatments (e.g. ICS, ICS/LABA, monoclonal antibodies, etc.).
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
The review authors would like to thank Drs Chan, Gallagher, Klig, Razi, and Schuckman for their correspondence and clarification of their study procedures. The authors would like to thank Dr Tariq AlShawabkeh for his assistance with preparing the manuscript. This study was supported by a grant from the Canadian Institutes of Health Research (CIHR). The study was supported with in‐kind resources by the Emergency Medicine Research Group (EMeRG) affiliated with the Department of Emergency Medicine, University of Alberta. Ms Cross was supported by an Alberta Innovates Health Solutions (AIHS) Summer Studentship Award (Edmonton, Alberta). Dr Villa‐Roel is supported by CIHR in partnership with the Knowledge Translation branch. Dr Rowe’s research is supported by a Tier I Canada Research Chair in Evidence‐based Emergency Medicine from CIHR through the Government of Canada (Ottawa, Ontario).
The National Institute for Health Research (NIHR) supported this project, via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the protocol authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health in the UK or CIHR or the University of Alberta in Canada.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Search frequency |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify studies for the Cochrane Airways Trials Register
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify studies in other electronic databases
Appendix 2. Search strategy to identify relevant studies from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 prednis*
#6 methylprednis*
#7 dexamethasone
#8 cortisone
#9 hydrocortisone*
#10 medrol
#11 solumedrol
#12 solu‐medrol
#13 betamethasone
#14 triamcinolone
#15 steroid* or corticosteroid* or glucocorticoid*
#16 MeSH DESCRIPTOR Dexamethasone
#17 MeSH DESCRIPTOR Prednisolone Explode All
#18 MeSH DESCRIPTOR Prednisone
#19 MeSH DESCRIPTOR Cortisone
#20 MeSH DESCRIPTOR Injections, Intramuscular
#21 intramuscular* OR intra* NEXT muscular*
#22 IM:ti,ab
#23 injection*
#24 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19
#25 #20 or #21 or #22 or #23
#26 #4 AND #24 AND #25
[Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma]
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to April 2017> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. asthma*.mp. or exp Asthma/ [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2. Respiratory Sounds/ 3. wheez*.mp. 4. Bronchial Spasm/ 5. bronchospas$.mp. 6. bronchoconstrict*.mp. 7. exp Bronchoconstriction/ 8. (bronch$ adj3 constrict$).mp. 9. Bronchial Hyperreactivity/ 10. Respiratory Hypersensitivity/ 11. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp. 12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 13. exp Adrenal Cortex Hormones/ 14. exp Glucocorticoids/ 15. exp Prednisone/ 16. exp Methylprednisolone/ 17. corticosteroid*.mp. 18. glucocortico*.mp. 19. cortico‐steroid*.mp. 20. prednis*.mp. 21. deltason.mp. 22. prelone.mp. 23. orapred.mp. 24. pediapred.mp. 25. deltasone.mp. 26. betamethasone.mp. 27. solumedrol.mp. 28. medrol.mp. 29. dexamethasone.mp. 30. methylpred*.mp. 31. solucortef.mp. 32. decadron.mp. 33. triamcinolone.mp. 34. kenalog*.mp. 35. trivaris.mp. 36. or/13‐35 37. exp Injections/ or inject*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 38. (intramuscul* or IM or repository or intra muscul*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 39. 37 or 38 40. oral*.mp. 41. ((drug administration or drug delivery).mp. or exp Drug Administration Routes/) and po.mp. 42. exp Administration, Oral/ 43. 40 or 41 or 42 44. (randomi?ed or randomi?ed).ab,ti. 45. placebo*.ti,ab. 46. dt.fs. 47. randomly.ti,ab. 48. trial*.ti,ab. 49. groups.ti,ab. 50. clinical trial/ 51. rct.ti,ab. 52. 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 53. animals/ 54. humans/ 55. 53 not (53 and 54) 56. 52 not 55 57. 12 and 36 and 39 and 43 and 56
Appendix 4. Embase search strategy
Database: Embase <1974 to 2017 April 17> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1. asthma*.mp. or exp asthma/ [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] 2. exp abnormal respiratory sound/ 3. wheez*.mp. 4. bronchial spasm/ 5. bronchospas$.mp. 6. bronchoconstrict*.mp. 7. exp bronchoconstriction/ 8. (bronch$ adj3 constrict$).mp. 9. bronchial hyperreactivity/ 10. exp respiratory tract allergy/ 11. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp. 12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] 13. exp acute disease/ or (acute or exacerbat* or emergen*).mp. 14. 12 and 13 15. exp corticosteroids/ 16. corticosteroid*.mp. 17. glucocortico*.mp. 18. cortico‐steroid*.mp. 19. prednis*.mp. 20. deltason.mp. 21. prelone.mp. 22. orapred.mp. 23. pediapred.mp. 24. deltasone.mp. 25. betamethasone.mp. 26. solumedrol.mp. 27. medrol.mp. 28. dexamethasone.mp. 29. methylpred*.mp. 30. solucortef.mp. 31. decadron.mp. 32. triamcinolone.mp. 33. kenalog*.mp. 34. trivaris.mp. 35. or/15‐34 36. exp Injection/ or inject*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] 37. (intramuscul* or IM or repository or intra muscul*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] 38. 36 or 37 39. ((drug administration or drug delivery).mp. or exp drug administration route/) and oral*.ti,ab. 40. ((drug administration or drug delivery).mp. or exp drug administration route/) and po.ti,ab. 41. exp oral drug administration/ 42. 39 or 40 or 41 43. clinical trial.pt. or randomi?ed.ab,ti. 44. placebo*.ti,ab. 45. dt.fs. 46. randomly.ti,ab. 47. trial*.ti,ab. 48. groups.ti,ab. 49. controlled clinical trial/ 50. rct.ti,ab. 51. 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 52. animals/ 53. humans/ 54. 52 not (52 and 53) 55. 51 not 54 56. 14 and 35 and 38 and 42 and 55
Appendix 5. CINAHL search strategy
1. (MH "Asthma+") or (MH "Bronchoconstriction") or (MH "Bronchial Spasm") or asthma* or wheez* or ((bronchia* or respiratory or airway* or lung$) N3 (hypersensitiv* or hyperreactiv* or allerg* or insufficiency or contrict*))
2. (MH "Disease Exacerbation") or (severe or acute* or emergen* or exacerbat*)
3. S1 and S2
4. (MH "Injections, Intramuscular+") or "intra muscular" or "intramuscul* or repository
5. (MH "Administration, Oral+") or (oral N3 (admins*" or delivery or drug or pharmasceutical*))
6. S4 or S5
7. (MH "Adrenal Cortex Hormones+") or glucocorticosteroid* or "cortico steroid*" prednis* or "adrenal cortex hormone*"
8. (corticosteroid* OR "steroids, cortico*" or prelone or orapred or pediapred or deltasone or solumedrol or medrol or betamethasone or dexamethasone or methylpred or solucortef or decahedron or triamcinolone or kenalog or trivaris)
9. S7 OR S8
10. S3 AND S6 AND S9
Appendix 6. Proquest Dissertations and Theses Global search strategy
(all(asthma* AND (emergency OR emergent OR emergencies OR acute* OR exacerbate*)) AND all(corticosteroid* OR "steroids, cortico*" OR corticosteroid* OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris) AND (oral OR ((im OR po) AND (drug* OR delivery OR administration OR route*)) OR intramuscul*)) AND all(randomiz* OR randomis* OR "clinical trial*" OR randomly OR placebo* OR "controlled trial*" OR RCT*)
Appendix 7. SCOPUS search strategy
1. (asthma*)
2. (emergency OR emergent OR emergencies OR acute* OR exacerbate* )
3. (corticosteroid* OR "steroids,cortico*" OR corticosteroid* OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris)
4. (randomiz* OR randomis* OR "clinical trial*" OR randomly OR placebo* OR "controlled trial*" OR rct*)
5. ("intra muscul*" OR intramusc* OR inject* OR (im W/5 (drug OR adminst* OR deliver* OR route* ))
6. (oral* OR "by mouth" OR ( op W/5 ( drug OR adminst* OR deliver* OR route* ))
7. 1 AND 2 AND 3 AND 4 AND 5 AND 6
Appendix 8. PROSPERO search strategy
1. (corticosteroid* OR "steroids, cortico*" OR corticosteroid* OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris)
2. asthma*
3. emergency OR emergent OR emergencies OR acute* OR exacerbate*
4. (oral OR ((im OR po) AND (drug* OR delivery OR administration OR route*)) OR intramuscular* or intra muscular* or repository)
5. #4 and #3 and #1 and #2
6. (randomiz* OR randomis* OR "clinical trial*" OR randomly OR placebo* OR "controlled trial*" OR RCT*)
7. #5 and #6
Appendix 9. Cochrane Library search strategy
1. (asthma* and (emergency or emergent or emergencies or acute* or exacerbate*))
2. (oral or (PO and (drug* or delivery or administration or route*))) and (intramuscul* or "intra muscl*" or (IM and (drug* or delivery or administration or route*)))
3. (corticosteroid* or "steroids, cortico*" or corticosteroid* or prelone or orapred or pediapred or deltasone or solumedrol or medrol or betamethasone or dexamethasone or methylpred or solucortef or decahedron or triamcinolone or kenalog* or trivaris)
4. (randomiz* or randomis* or "clinical trial*" or randomly or placebo* or "controlled trial*" or RCT*)
5. #1 and #2 and #3 and #4
Appendix 10. LILACS search strategy
1. (asthma* and (acute or exacerbat* and emerg*)
2. (oral or (po and (drug* or administration or route* or delivery)))
3. (intramuscular or intra‐muscular or (IM and (drug* or administration or route* or delivery)))
4. (corticosteroid* OR "steroids cortico*" OR "cortico steroid*" OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris)
5. #1 AND #2 AND #3 AND #4
Data and analyses
Comparison 1. Intramuscular versus oral corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 2 Relapse intention to treat | 9 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
| 3 Subgroup analysis: children versus adults | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 3.1 Children | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 3.2 Adults | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Within 10 days post‐discharge | 7 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 4.2 Greater than 10 days post‐discharge | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 5 Subgroup analysis: mild/moderate versus severe exacerbations | 5 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| 5.1 Mild/moderate exacerbations | 4 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 5.2 Severe exacerbations | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.47] |
| 6 Sensitivity analysis: risk of bias | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days | 8 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 8 Sensitivity analysis: fixed effects | 9 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.19] |
| 9 Sensitivity analysis: corticosteroids in ED | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.29] |
| 10 Serious adverse events; hospitalization following discharge | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Adverse events | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| 12 Adverse events: nausea/vomiting/GI distress | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.59] |
| 13 Adverse events: insomnia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Adverse events: personality changes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Adverse events: pain | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.32, 9.66] |
| 16 Adverse events: swelling | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.76 [0.57, 39.84] |
| 17 Adverse events: redness | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 13.5 [0.77, 235.63] |
| 18 Pulmonary function: peak expiratory flow (L/min) | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐38.83, 23.28] |
| 19 Pulmonary function: FEV₁/FVC (%) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Symptom persistence | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| 21 Symptom persistence: cough | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.73] |
| 22 Symptom persistence: wheezing | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.52] |
| 23 24‐hour beta agonist use | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Wahadneh 2006.
| Methods | A prospective, randomised, non‐blinded clinical trial Comparison of IM dexamethasone to oral prednisolone Randomisation and allocation concealment methods were reported but methods were not described |
|
| Participants | Patients with known history of asthma who presented to the ED with a moderate asthma exacerbation who did not require admission on presentation and had no evidence of varicella exposure or fever and did not take any systemic corticosteroids within 2 weeks of the start of the study A locally modified scoring system based on GINA guidelines for the management of asthma was used to evaluate the clinical response at presentation, and 5 days after treatment. Ages: enrolled patients 9 months to 14 years. Mean age of IM corticosteroid group: 79.7 months. Mean age of oral corticosteroid group: 45.4 months Reported enrolling patients with mild‐moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Set in Jordan Sex: 20 men, 9 women |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Authors did not respond to requests for clarification. No registered protocol was identified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 16): "Patients were randomised to receive either a single Intramuscular (IM) dose of dexamethasone acetate (1.7 mg/kg) or prednisolone taken orally each day for 5 days (2 mg/kg//day)." |
| Allocation concealment (selection bias) | Unclear risk | Study did not comment on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported as a non‐blinded study. Quote (p. 16): "The study is prospective randomised and non‐blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reported as a non‐blinded study. Quote (p. 16): "The study is prospective randomised and non‐blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Source of funding not provided. Baseline imbalance detected, dexamethasone group significantly older than prednisolone group. Quote (p. 16): "Males out‐numbered females in both groups (13:4 in the dexamethasone group, and 7:5 in the second group). Patients in dexamethasone acetate group were older than those in prednisolone group (P = 0.007), which can risk of bias ably be explained by the small‐sized sample and the random assignment of patients to either group." |
Chan 2001.
| Methods | A prospective, randomised, double‐blinded, controlled clinical trial Comparison of IM betamethasone versus oral prednisone Randomisation was reported but method was not described. Allocation concealment: pharmacy provided randomised, sequentially numbered, sealed kits that contained an injection of betamethasone 2 mL and 7 placebo capsules, or an injection of saline 2 mL and 7 capsules of prednisone 50 mg each |
|
| Participants | Patients who presented to the ED with an exacerbation of asthma and had no contraindication to prednisone or betamethasone and were likely to be discharged after treatment. The diagnosis of asthma was made clinically by the ED physician. Patients who received oral or parenteral corticosteroids within 24 hours of enrolment were not excluded from study. Ages: enrolled patients 18 years and above. Mean age of IM corticosteroid group: 31.6 years (SD: 13.1). Mean age of oral corticosteroids group: 21.1 years (SD: 10.7) Exacerbation severity not discussed. Exacerbation severity estimated as mild/moderate based on baseline PEF Set in Canada Sex: 77 men, 94 women |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No registered protocol was identified; however study authors were contacted and provided the study protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 148): "The hospital pharmacy provided sequentially numbered, sealed kits that were randomised to contain either ...." |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled central allocation. Drug kits were sealed and sequentially numbered. Quote (p. 148): "The hospital pharmacy provided sequentially numbered, sealed kits that were randomised to contain either an injection of betamethasone 2 mL and seven placebo capsules, or an injection of saline 2 mL and seven capsules containing prednisone 50 mg each." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded study. Drug kits opened by nurse not involved in study patient. Medication names were obscured so neither staff or patients knew medication name. Quote (p. 149): "After consent was obtained, a kit was opened by a nurse who was not involved with the study patient. This nurse drew up the 2 mL solution and placed a translucent sleeve over the syringe to obscure the medication but to allow the graduations to be seen. The primary nurse involved with the patient was given the covered syringe and capsules, and administered the intramuscular injection." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment completed by study coordinator blinded to treatment allocation. All instances of suspected relapses reviewed by investigators before un‐blinding. Quote (p. 149): "Patients were contacted by telephone on day 7 and 21 by a study coordinator blinded to the treatment allocation, and asked if they sought treatment for their asthma since the initial ED visit." "Before un‐blinding, all instances of suspected relapse were reviewed by two of the investigators." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 149). Excluded patients balanced between groups. Quote (p. 149‐50): "During the study, 176 patients with acute asthma agreed to participate in the trial. After random assignment, five patients were subsequently excluded: three patients required hospitalisation before ED discharge; one was discovered to be on chronic prednisone; and one refused the injection." "At day 7, three patients were lost to follow‐up ‐ two in the prednisone group and one in the betamethasone group. By day 21, 12 patients were lost to follow‐up ‐ four in the prednisone group and eight in the betamethasone group. At the completion of the study, follow‐up information was available for 159 patients (93%)." |
| Selective reporting (reporting bias) | Low risk | Protocol made available to authors by study investigators (protocol not registered). All pre‐selected outcomes reported. |
| Other bias | Low risk | Study appears to be free of other sources of bias. Source of funding stated. Quote (p. 152): "Supported by a grant by Research and Development of the University of Calgary, Calgary, Alberta." |
Gordon 2007.
| Methods | A prospective, randomised, non‐blinded controlled clinical trial Comparison of a single dose of IM dexamethasone versus oral prednisolone Randomization was reported but method was not described. Allocation concealment method was accomplished using sequentially numbered opaque study packets containing group assignments. |
|
| Participants | Patients with known history of asthma who presented to the ED with moderate asthma exacerbation who did not require admission on presentation. Asthma was defined as at least 2 prior episodes of respiratory illness treated with bronchodilators. Clinical asthma score adapted from a previous pulmonary score was used to evaluate asthma children aged from 5 to 17 years measuring: respiratory rate, retractions and wheezing. Reported enrolling patients with moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Ages: enrolled patients 18 months to 7 years. Median age of IM corticosteroid group: 38 months (IQR: 29‐59). Median age of oral corticosteroid group: 42 months (IQR: 28‐60.5) Patients who received IV therapy (not due to vomiting but to severity of asthma) were excluded from study. Set in United States Sex: 110 men, 71 women |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Authors were not contacted No registered protocol was identified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 521): "After written informed consent was obtained, we randomised patients to 1 of 2 treatment groups in blocks of 6, 8, or 10." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment ensured via sequentially numbered opaque packets containing group assignments kept in ED and opened by physician immediately after enrolment. Quote (p. 522): "Allocation concealment was maintained by use of sequentially numbered opaque study packets containing group assignments, which were kept in the ED and opened by the treating physician immediately after enrolment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study. Quote (p. 522): "Treating physicians were not masked to study group." "At the time of disposition from the ED or 3 hours after enrolment for those patients still in the ED, physicians not blinded to group assignment performed a standardized repeat physical examination including the assessment of a second asthma score." Quote (p. 527): "The only people masked to group assignment in this study were the investigators who assigned follow‐up scores at the 4‐day return visit." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment completed via physician blinded to group assignment and not involved in patient's care on the initial visit. Quote (p. 523): "At the 4‐day follow‐up visit, a physician masked to group assignment and not involved in the patient's care on the initial visit performed a standard examination, including assignment of an asthma score. Physicians and guardians were instructed not to engage in any conversation before completion of the physical examination. After completing and recording this physical examination, the physician administered a structured interview regarding interim use of bronchodilators and ICS, guardian's perception of bronchodilators and ICS, guardian's perception of clinical improvement, need for further medical care since enrolment, and compliance with oral steroid regimen." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 523). Excluded patients balanced between groups. Quote (p. 523, 525‐6): "Of 194 randomised patients, 13 were subsequently excluded from the study (7 in the dexamethasone arm and 6 in the prednisolone arm) for the following reasons: 6 had initial asthma scores of only 2, 4 had previously participated, 1 had only one prior episode of wheezing, 1 had taken prednisolone within the previous month, and 1 patient was randomised to the dexamethasone group, but inappropriately given prednisolone in the ED. The guardians of 2 included patients randomised to dexamethasone withdrew their consent for further participation during the ED visit (one before drug administration, one afterward)." |
| Selective reporting (reporting bias) | Unclear risk | No available protocol |
| Other bias | Unclear risk | Source of funding was not stated. |
Gries 2000.
| Methods | A prospective, randomised, non‐blinded controlled clinical trial with blinded outcome assessors Comparison of single dose of IM dexamethasone versus oral prednisolone Randomization and allocation concealment were reported but methods were not discussed. |
|
| Participants | Patients with known history of asthma who presented to the ED with mild to moderate asthma exacerbation who did not require admission on presentation Asthma was defined as recurrent coughing, wheezing, or shortness of breath responsive to corticosteroids or β₂‐agonists. Asthma exacerbation was defined as increased asthma signs and symptoms unresponsive to the patient’s routine asthma medications and additional β₂‐agonist therapy. Clinical asthma score was defined as a composite score of wheeze and cough scores ranging from 0 to 8. Reported to enrolling patients with mild‐moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Ages: enrolled patients 6 months to 7 years. Mean age of IM corticosteroid group: 38 months (SD: 18). Mean age of oral corticosteroid group: 36 months (SD: 22) Patients were excluded from study if they received IV corticosteroids on the ED initial visit. Set in United States Sex: 23 men, 9 women |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Authors were not contacted. No registered protocol was identified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 299): "Once enrolled in the study, patients were randomised to receive either a single IM dose of dexamethasone acetate (Dalalone, Forest Pharmaceuticals, 16 mg/mL) or prednisone (either suspension 3 mg/mL or tablets—patient’s choice) taken orally each day for 5 days." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study. Quote (p. 300‐1): "The study investigators were blinded to the patient’s treatment arm. Patients and parents were not blinded. The nurses in the paediatric clinic who administered the IM injections did not discuss the patients with the study investigators." "Investigator blinding was achieved in all but 4 patients. For 2 of these children, the nurses told an investigator that they had administered a shot, and the other 2 children disclosed what they had received (one IM Dex and one oral Pred). A second investigator, who was still blinded, completed the assessments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group assignment. Quote (p. 300): "The study investigators were blinded to the patient’s treatment arm" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study attrition provided. Excluded patients balanced between groups. Quote: (p. 300): "From September 1996 through February 1997, we approached 35 children (age range, 8 months to 7 years) and their legal guardians concerning enrolment, and all but 2 agreed to participate in the study. Sixteen children received IM Dex, and 17 were randomised to receive oral Pred. One child, a 16‐month‐old (randomised to IM Dex), was withdrawn on her second study day for treatment of a persistent cough by an emergency department physician who noted no wheezing or respiratory distress but gave her an additional IM injection of dexamethasone." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
Hoffman 1988.
| Methods | A prospective, randomised, double‐blinded controlled clinical trial Comparison of IM methylprednisolone versus oral methylprednisolone Randomization and allocation concealment were reported but methods were not described. |
|
| Participants | Patients who presented to the ED with an acute asthma and were discharged after ED treatment Inclusion criteria required eligible patients to fulfil the American Thoracic Society for the diagnosis of asthma. Ages: enrolled patients 15 to 55 years. Mean age of IM corticosteroid group: 42 years (SEM: 4). Mean age of oral corticosteroid group: 37 years (SEM:4) Exacerbation severity not discussed. Exacerbation severity estimated as severe based on baseline PEF Patients who received corticosteroids at the time of presentation were excluded from study. Patients who needed admission or remained in ED for more than 6 hours were excluded from study. Set in United States Sex: 11 men, 7 women |
|
| Interventions |
|
|
| Outcomes | Follow‐up between days 5 and 7
|
|
| Notes | Authors did not respond to requests for clarification. No registered protocol was identified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 11): "All subjects were given an IV injection of 4 mg/kg of methylprednisolone sodium succinate, then randomised in double‐blind fashion to receive either an IM injection of methylprednisolone sodium acetate..." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind study. Oral placebo and oral methylprednisolone was indistinguishable from each other but unsure if IM placebo and IM methylprednisolone was indistinguishable. Quote (p. 11): "All subjects were given an IV injection of 4 mg/kg of methylprednisolone sodium succinate, then randomised in double‐blind fashion to receive either an IM injection of methylprednisolone sodium acetate..." "Oral placebo and oral methylprednisolone were indistinguishable, and the dosage and tapering schedule were the same as in our previous study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing patients balanced between groups. Quote (p. 12): "Follow‐up was obtained by return visit in seven of eight patients in group 1, and in nine of ten patients in group 2." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No source of funding provided |
Klig 1997.
| Methods | A prospective, randomised, non‐blinded clinical trial with blinded outcome assessors Comparison of IM dexamethasone versus oral prednisone Randomization was stated but method was not described. Allocation concealment was accomplished using sealed opaque packets. |
|
| Participants | Patients, who had 2 prior episodes of wheezing and were treated with beta‐2‐adrenergic agents, presented to ED with mild to moderate wheezing and did not require admission to hospital Enrolment of patients was based on the pulmonary index (0 to 3), assessment of a clinical score for asthma, and pulse oximetry on arrival to ED. Ages: enrolled patients 3 to 16 years. Mean age of IM corticosteroid group: 82 months (SD: 46). Mean age of oral corticosteroid group: 63 months (SD: 36) Reported enrolling patients with mild‐moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Patients were excluded from study if they received corticosteroids within the last month or were hospitalised for asthma treatment within the last 2 months prior to the study. Set in United States Sex: 24 men, 18 women |
|
| Interventions |
|
|
| Outcomes | Followed up on day 5 either by assessment in out‐patient clinic or via telephone interview
|
|
| Notes | Author was contacted and provided clarification on allocation concealment and source of funding No registered protocol was identified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Study authors confirmed via personal communication that randomisation was in blocks. Quote (p. 421): "Patients were randomly assigned by sealed packets to receive either oral prednisone tablets 2 mg/kg or IM dexamethasone 0.3 mg/kg." Personal communication: "Randomization was in blocks." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment ensured via sealed packets. Study authors confirmed via personal communication that the sealed packets were opaque. Quote (p. 421): "Patients were randomly assigned by sealed packets to receive either oral prednisone tablets 2 mg/kg or IM dexamethasone 0.3 mg/kg." Personal communication: "Sealed packets were opaque" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study. Quote (p. 421): "The assigned corticosteroid was administered in an unblinded manner immediately after enrolment in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. Stated that Investigators (Physicians) were blinded to patient's treatment. Quote (p. 422): "Patient follow‐up was conducted on the fifth day after discharge from the PED by a physician blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient exclusions balanced between groups. Quote (p. 422): "Forty‐four patients participated in the pilot study of whom 23 received a single dose of IM dexamethasone and 21 received 3 days of oral prednisone. Two patients from the IM dexamethasone group were admitted to the hospital shortly after enrolment and therefore were removed from the study. The remaining 42 patients were discharged to home, resulting in an even distribution between the IM dexamethasone and oral prednisone groups." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Study authors confirmed via personnel communication that the study was not funded. Personal communication: "Study was funded via supplies only (decadron from Merck Pharmaceuticals)." |
Lahn 2004.
| Methods | A prospective, randomised, double blinded, placebo‐controlled clinical trial Comparison of single dose of IM methylprednisolone versus oral methylprednisolone Randomisation was accomplished using computer‐generated random set of numbers (20 blocks). Allocation concealment was maintained by research pharmacist using computer generated set of numbers to package medications in balanced blocks (20). Intramuscular injection (methylprednisolone and placebo) with similar appearances and covered content were prepared and administered by a nurse who was not involved in the study. Oral methylprednisolone and placebo were identical in appearance and were given in identical containers. |
|
| Participants | Patients diagnosed with asthma (based on the American Thoracic Society Guidelines (1962), PEF ≤ 70% predicted during the ED visit with a minimum PEF ≥ 40% predicted, and included both clinical symptoms and physical examination findings), who presented with an asthma exacerbation and were expected to be discharged after the ED treatment Exacerbation severity not discussed. Exacerbation severity estimated as mild/moderate based on baseline PEF Ages: enrolled patients 18 to 45 years. Mean age of IM corticosteroid group: 33 years (SD: 8). Mean age of oral corticosteroid group: 33 years (SD: 8) Patients who received systemic corticosteroids (within 1 month prior to the study), theophylline or inhaled anticholinergic agents were excluded from study. Set in United States Sex: 56 men, 131 women |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Authors were contacted, but were unable to provide protocol No registered protocol was identified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation completed via block randomised computer generated numbers. Quote (p. 363): "The medication was prepared and block‐randomised by a research pharmacist who used a computer‐generated set of random numbers to package the medications in balanced blocks of 20 (i.e., each block of 20 medication packets contained 10 packets of oral methylprednisolone plus an IM placebo and 10 packets of IM methylprednisolone plus oral placebo." |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled central allocation. Quote (p. 363): "The randomisation code was held by the pharmacist and was not broken during the course of the study." "To the best of our knowledge, allocation concealment was maintained." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded study. Corticosteroids administered to patients via a study nurse not involved with the patient. Intramuscular injection was administered in a private setting with no one else present. Nurse instructed not to provide patients or staff information on contents of the syringe. Nurse covered the syringe so that other staff or patients could not see what was in the syringe. Oral placebo and corticosteroids was identical in appearance. Quote (p. 363): "The injection was reconstituted and administered by an ED nurse who was not blinded to the treatment but who had no involvement in any aspect of the study. This individual was instructed not to provide the patient, physician, or study personnel with any information about the contents of the syringe. Although the placebo and methylprednisolone injections were similar in appearance, the nurse also was instructed not to allow anyone to see the contents of the syringe. The injection was administered in a private setting with no one else present. The oral methylprednisolone and oral placebo were identical in appearance and were given to patients in identical containers. To the best of our knowledge, allocation concealment was maintained." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 364). Excluded patients balanced between groups. Quote (p. 364): "One hundred ninety patients were entered into the study over a 60‐month period from November 1997 to November 2002. As shown in the diagram of the Consolidated Standards of Reporting trials, three patients (all in the oral methylprednisolone/IM placebo group) were removed after study entry due to protocol violations. One patient’s asthma was too severe to be discharged safely from the ED, a second patient did not receive a ‐agonist prescription at ED discharge, and a third patient was instructed by the primary physician to discontinue the study medication at day 5. Seven patients (IM administration group, three patients; oral administration group, four patients) were lost to follow‐up and were excluded from the primary efficacy analysis. The remaining 180 patients, 92 of whom received IM methylprednisolone plus oral placebo, and 88 of whom received oral methylprednisolone plus IM placebo, completed the protocol and were available for follow‐up at 10 days. All patients reached for follow‐up at 10 days were successfully contacted again for follow‐up at 21 days. No patients who were lost to follow‐up at 10 days were available for follow‐up at 21 days." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Study funded by pharmaceutical company. Authors state that pharmaceutical company just provided financial support, and was not involved in the design, execution, data analysis, or manuscript preparation. Quote (p. 362): "This research was funded in part by an unrestricted grant from Pharmacia & Upjohn (currently, Pfizer), who, other than providing financial support, were not involved in any way with the design, execution, data analysis, or manuscript preparation." |
Lee 1993.
| Methods | A prospective, randomised, double blinded, placebo‐controlled clinical trial Comparison of single dose of IM dexamethasone versus oral dexamethasone Randomization was accomplished using computer‐generated random set of numbers (9 blocks). Allocation concealment was reported but method was not described. |
|
| Participants | Patients diagnosed with asthma (based on the American Thoracic Society criteria, 1962), presented to ED with acute asthma and did not require hospital admission Exacerbation severity not discussed. Exacerbation severity estimated as mild/moderate based on baseline PEF Ages: enrolled patients 16 to 60 years. Mean age of IM corticosteroid group: 37 years (SD: 4). Mean age of oral corticosteroid group: 40 years (SD: 4) Patients who received systemic corticosteroids prior to enrolment in the study were excluded. Set in Taiwan Sex: 20 men, 16 women |
|
| Interventions | Patients were divided into 3 groups (A, B, and C)
Tapering of dexamethasone was as follows: 3.0 mg day 1 and 2, 2.0 mg day 3, 1.5 mg day 4, 1.0 mg day 5, 0.75 mg day 6 and 0.5 mg day 7)
|
|
| Outcomes | Primary and secondary outcomes were not defined.
|
|
| Notes | Attempts to contact the authors failed No registered protocol was identified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised via block randomised computer generated numbers. Quote (p. 26): "The subjects were randomly assigned into one of three groups using a double blind model. Randomisation was by means of a set of computer‐generated set of random‐numbers in blocks of nine." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded study. Oral corticosteroids and oral placebo were indistinguishable from each other but no information provided as to whether IM corticosteroids were indistinguishable from IM placebo. Quote (p. 26): "The subjects were randomly assigned into one of three groups using a double blind model." "The oral dexamethasone and oral placebo were indistinguishable." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on outcome assessment blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not report attrition/exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Source of funded not provided. |
Schuckman 1998.
| Methods | A prospective, randomised, double‐blinded, placebo‐controlled clinical trial Comparison of IM triamcinolone versus oral prednisone Randomization was accomplished using computer‐generated random numbers. Allocation concealment was reported and discussed as pharmacy controlled by using computerized generated set of numbers to package medications and placebo into sequentially numbered kits. |
|
| Participants | Patients presented to the ED with an asthma exacerbation that had an initial PEF < 350 L/min and did not require admission to hospital on ED presentation The diagnosis of asthma was according to the American Thoracic Society criteria (1987) Patients were excluded from study if had received corticosteroids within 2 weeks prior to ED presentation or were unable or unwilling to attend follow‐up evaluation. Ages: Enrolled patients 18 to 50 years. Mean age of IM corticosteroid group: 31 years (SD: 9). Mean age of oral corticosteroid group: 32 years (SD: 9) Exacerbation severity not reported. Exacerbation severity estimated as mild/moderate based on baseline PEF Set in United States Sex: 47 mean, 107 women |
|
| Interventions |
|
|
| Outcomes | Patients were followed up 7 to 10 days after ED enrolment.
|
|
| Notes | Author was contacted and provided clarification on the methodology of blinding and outcome data. Was unable to provide copy of the study protocol. No registered protocol was identified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation completed via computer generated set of random numbers. Quote (p. 334): "A computer‐generated set of random numbers was used by the hospital pharmacy to package the active medications and placebos into sequentially numbered kits." |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled allocation concealment. Medications and placebos packaged in sequentially numbered kits. Quote (p. 334): "A computer‐generated set of random numbers was used by the hospital pharmacy to package the active medications and placebos into sequentially numbered kits." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study. Study authors confirmed via personnel communication that the study medications were indistinguishable from each other. Personal communication: "Syringes were prefilled and tablets looked similar for placebo and prednisone. Each patient received injection and pill bottle. They brought their bottle to follow‐up." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (physicians) were blinded to the patient's group assignment. Quote (p. 335): "Physicians evaluating patients at follow‐up remained blinded to drug group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 337). Excluded patients balanced between groups. Quote (p. 335‐6): "We approached 186 patients who were potentially eligible; 15 (9%) refused to participate, and 3 (2%) resided out of town and could not return for follow‐up. A total of 168 patients were enrolled, 82 in the triamcinolone group and 86 in the prednisone group. Fourteen patients were withdrawn from analysis: 6 (3 in each group) for protocol violations because the patient was older than 50 years of age, and 8 (1 in the triamcinolone group and 7 in the prednisone group) because they were lost to follow‐up. The final study population was 154 patients, 78 in the triamcinolone group and 76 in the prednisone group." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No other potential source of bias found. Source of funding provided. Quote (p. 333): "Research supported by the Summa Health System Foundation." |
Abbreviations
ED: emergency department
FEV1: forced expiratory volume
FVC: forced vital capacity
ICS: inhaled corticosteroids
IM: intramuscular
MDI: metered dose inhaler
PEF: peak expiratory flow
IV: intravenous
SD: standard deviation
SEM: standard error of mean
IQR: interquartile range
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2012 | Did not compare IM versus oral corticosteroids |
| Droszcz 1985 | Not an RCT/CCT |
| Ducharme 2004 | Not an RCT/CCT |
| Green 1995 | Did not provide a single dose of intramuscular corticosteroids |
| Hofmann 2008 | Did not compare IM versus oral corticosteroids |
| Kelso 2014 | Not an RCT/CCT |
| Ozpenpe 2011 | Patients not discharged from ED |
| Razi 2006 | Did not provide a single dose of intramuscular corticosteroids |
| Watnick 2016 | Not an RCT/CCT |
| White 2010 | Not an RCT/CCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Droszcz 1981.
| Methods | No abstract for full publication available. Unable to assess |
| Participants | No abstract for full publication available. Unable to assess |
| Interventions | No abstract for full publication available. Unable to assess |
| Outcomes | No abstract for full publication available. Unable to assess |
| Notes | No abstract for full publication available. Unable to make assessment on study eligibility |
Differences between protocol and review
One change from the initial protocol and the final review included the databases' search by our health librarian, which was modified to include the Cochrane Library and PROSPERO, while EBM reviews, Global Health, and International Pharmaceutical abstracts were dropped prior to the conduct of the search. In response to peer review, we added an additional sensitivity analysis, in which studies that provided patients with additional corticosteroids as a co‐intervention were excluded from the analysis. Due to the variance in the type of IM and oral corticosteroid, as well as dosage, corticosteroid equivalency to methylprednisolone was estimated using an equivalence converter.
Contributions of authors
Scott W Kirkland (SWK) contributed to the development of the protocol, performed the grey literature search, selection of studies, quality assessment, data extraction, data analysis and manuscript preparation.
Elfriede Cross (EC) contributed to the development of the protocol, performed the grey literature search, selection of studies, quality assessment, data extraction, data analysis and manuscript preparation/editing.
Sandra Campbell (SC) edited the protocol, developed the search terms, performed the literature search and assisted with manuscript preparation/editing.
Cristina Villa‐Roel (CVR) edited the protocol, and assisted with the selection of studies, quality assessment, data extraction, data analysis, and manuscript preparation/editing.
Brian H Rowe (BHR) initiated the review, contributed to the development of the protocol, assisted with data analysis, and manuscript preparation/editing.
Sources of support
Internal sources
-
Department of Emergency Medicine, University of Alberta, Alberta, Canada.
The Emergency Medicine Research Group provided resources in kind
Emergency Strategic Clinical Network (SWK), AB, Canada.
External sources
-
Canadian Institutes of Health Research (CIHR), Canada.
The CIHR funded the completion of this Cochrane Review. The CIHR was not involved in the preparation of this protocol and will not be involved in any aspect of the conduct and reporting of the review.
Declarations of interest
SWK is supported by the Partnerships in Research Innovation in Health Services (PRIHS) Choosing Wisely Project Health. SWK has no known conflicts of interest.
EC is supported by the Department of Emergency Medicine, University of Alberta. EC has no known conflicts of interest.
SC has no known conflicts of interest.
CVR is supported by the Emergency Medicine Research Group (EMeRG). CVR has no known conflicts of interest.
BHR is supported by a Tier I Canada Research Chair in Evidence‐based Emergency Medicine from CIHR through the Government of Canada (Ottawa, Ontario). Several reviews in which he is a co‐author are cited in this review; however, no primary studies from his Emergency Medicine Research Group (EMeRG) were included.
New
References
References to studies included in this review
Al‐Wahadneh 2006 {published data only}
- Al‐Wahadneh A, Jboor S, Dahabreh M. A comparison of efficacy and safety of a single dose of intramuscular dexamethasone acetate with oral prednisolone in the management of asthma exacerbations in children. Journal of the Royal Medical Services 2006;13(1):15‐8. [Google Scholar]
Chan 2001 {published data only}
- Chan JS, Cowie RL, Lazarenko GC, Little C, Scott S, Ford GT. Comparison of intramuscular betamethasone and oral prednisone in the prevention of relapse of acute asthma. Canadian Respiratory Journal 2001;8(3):147‐52. [PUBMED: 11420590] [DOI] [PubMed] [Google Scholar]
Gordon 2007 {published data only}
- Gordon S, Tompkins T, Dayan PS. Randomized trial of single‐dose intramuscular dexamethasone compared with prednisolone for children with acute asthma. Pediatric Emergency Care 2007;23(8):521‐7. [DOI: 10.1097/PEC.0b013e318128f821] [DOI] [PubMed] [Google Scholar]
Gries 2000 {published data only}
- Gries DM, Moffitt DR, Pulos E, Carter ER. A single dose of intramuscularly administered dexamethasone acetate is as effective as oral prednisone to treat asthma exacerbations in young children. Journal of Pediatrics 2000;136(3):298‐303. [DOI: 10.1067/mpd.2000.103353] [DOI] [PubMed] [Google Scholar]
Hoffman 1988 {published data only}
- Hoffman IB, Fiel SB. Oral versus repository corticosteroid therapy in acute asthma. Chest 1988;93(1):11‐3. [PUBMED: 3275525] [DOI] [PubMed] [Google Scholar]
Klig 1997 {published data only}
- Klig JE, Hodge D, Rutherford MW. Symptomatic improvement following emergency department management of asthma: a pilot study of intramuscular dexamethasone versus oral prednisone. Journal of Asthma 1997;34(5):419‐25. [PUBMED: 9350159] [DOI] [PubMed] [Google Scholar]
Lahn 2004 {published data only}
- Lahn M, Bijur P, Gallagher EJ. Randomized clinical trial of intramuscular vs oral methylprednisolone in the treatment of asthma exacerbations following discharge from an emergency department. Chest 2004;126(2):362‐8. [DOI: 10.1378/chest.126.2.362] [DOI] [PubMed] [Google Scholar]
Lee 1993 {published data only}
- Lee CH, Lee CJ, Lan RS, Tsai YH, Chiang YC, Wang WJ, et al. Repository dexamethasone in the treatment of acute bronchial asthma. Changgeng Yi Xue Za Zhi [Chang Gung Medical Journal] 1993;16(1):25‐9. [PUBMED: 8490772] [PubMed] [Google Scholar]
Schuckman 1998 {published data only}
- Schuckman H, DeJulius DP, Blanda M, Gerson LW, DeJulius AJ, Rajaratnam M. Comparison of intramuscular triamcinolone and oral prednisone in the out‐patient treatment of acute asthma: a randomized controlled trial. Annals of Emergency Medicine 1998;31(3):333‐8. [PUBMED: 9506490] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2012 {published data only}
- Andrews AL, Wong KA, Heine D, Scott RW. A cost‐effectiveness analysis of dexamethasone versus prednisone in pediatric acute asthma exacerbations. Academic Emergency Medicine 2012;19(8):943‐8. [DOI: 10.1111/j.1553-2712.2012.01418.x] [DOI] [PubMed] [Google Scholar]
Droszcz 1985 {published data only}
- Droszcz W, Piotrowska B. Comparison of oral prednisolone and intramuscular depot triamcinolone in patients with severe chronic asthma. Thorax 1985;40(3):207‐8. [PUBMED: PMC460032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducharme 2004 {published data only}
- Ducharme FM, Rowe BH. Intramuscular versus oral methylprednisolone in asthma. Lancet 2004;364(9450):2000‐1. [DOI: 10.1016/S0140-6736(04)17526-0] [DOI] [PubMed] [Google Scholar]
Green 1995 {published data only}
- Green SS, Lamb GC, Schmitt S, Kaufman J. Oral versus repository corticosteroid therapy after hospitalization for the treatment of asthma. Journal of Allergy and Clinical Immunology 1995;95(1):15‐22. [PUBMED: 7822659 ] [DOI] [PubMed] [Google Scholar]
Hofmann 2008 {published data only}
- Hofmann M, Rodríguez JE, Klatt C. What is the best treatment for an adult whose asthma exacerbation has not completely responded to 5 days of oral corticosteroids?. Evidence‐Based Practice 2008;11(8):4. [Google Scholar]
Kelso 2014 {published data only}
- Kelso JM. Question 1: prednisolone or dexamethasone for acute exacerbations of asthma: do they have similar efficacy in the management of exacerbations of childhood asthma?. Pediatrics 2014;134:S179. [DOI: 10.1542/peds.2014-1817AAAA] [DOI] [PubMed] [Google Scholar]
Ozpenpe 2011 {published data only}
- Ozpenpe O, Aydogan M, Iraneci R. Comparison of the efficacy and side effects of intramuscular, intravenous and oral methylprednisolone used in the acute exacerbation of childhood asthma: A randomized clinical trial. Allergy 2011;66(S94):668. [DOI: 10.1111/j.1398-9995.2011.02650.x] [DOI] [Google Scholar]
Razi 2006 {published data only}
- Razi E, Moosavi GA. A comparative efficacy of oral prednisone with intramuscular triamcinolone in acute exacerbation of asthma. Iran Journal of Allergy, Asthma and Immunology 2006;5(1):17‐22. [DOI: ] [PubMed] [Google Scholar]
Watnick 2016 {published data only}
- Watnick CS, Fabbri D, Arnold DH. Single‐dose oral dexamethasone is effective in preventing relapse after acute asthma exacerbations. Annals of Allergy, Asthma & Immunology 2016;116(2):171‐2. [DOI: 10.1016/j.anai.2015.11.015] [DOI] [PubMed] [Google Scholar]
White 2010 {published data only}
- White S. BET 2 Dexamethasone versus prednisolone in asthma. Emergency Medicine Journal 2010;27(9):716‐7. [DOI: 10.1136/emj.2010.101212] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Droszcz 1981 {published data only}
- Droszcz W, Lech B, Piotrowska B, Garlicki A. Clinical evaluation of betamethasone depot. Pneumonologia Polska 1981;49(8‐9):645‐8. [PUBMED: 7029485] [PubMed] [Google Scholar]
Additional references
Alangari 2014
- Alangari AA. Corticosteroids in the treatment of acute asthma. Annals of Thoracic Medicine 2014;9(4):187‐92. [10.4103/1817‐1737.140120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro‐Rodriguez 2016
- Castro‐Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta‐analysis. Pediatric Pulmonology 2016;51(8):868‐76. [DOI: 10.1002/ppul.23429] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2011
- CDC. Asthma in the U.S. CDC Vital Signs. www.cdc.gov/vitalsigns/asthma. May 2011.
Croisant 2014
- Croisant S. Epidemiology of asthma: prevalence and burden of disease. Advances in Experimental Medicine and Biology 2014;795:17‐29. [DOI: 10.1007/978-1-4614-8603-9_2] [DOI] [PubMed] [Google Scholar]
Ducharme 1993
- Ducharme FM, Kramer MS. Relapse following emergency treatment for acute asthma: can it be predicted or prevented?. Journal of Clinical Epidemiology 1993;46(12):1395‐402. [PUBMED: 8263566] [DOI] [PubMed] [Google Scholar]
Ducharme 2011
- Ducharme FM, Zemek RL, Chalut D, McGillivray D, Noya FJ, Resendes S, et al. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. American Journal of Respiratory and Critical Care Medicine 2011;183(2):195‐203. [DOI: 10.1164/rccm.201001-0115OC] [DOI] [PubMed] [Google Scholar]
Emerman 1999
- Emerman CL, Woodruff PG, Cydulka RK, Gibbs MA, Pollack CV Jr, Camargo CA Jr. Prospective multicenter study of relapse following treatment for acute asthma among adults presenting to the emergency department. MARC investigators. Multicenter Asthma Research Collaboration. Chest 1999;115(4):919‐27. [PUBMED: 10208187] [DOI] [PubMed] [Google Scholar]
Emerman 2001
- Emerman CL, Cydulka RK, Crain EF, Rowe BH, Radios MS, Camargo CA Jr, et al. Prospective multicenter study of relapse after treatment for acute asthma among children presenting to the emergency department. Journal of Pediatrics 2001;138(3):318‐24. [DOI: 10.1067/mpd.2001.111320] [DOI] [PubMed] [Google Scholar]
GINA 2017
- Global Initiative for Asthma. Global Initative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. ginasthma.org/2017‐gina‐report‐global‐strategy‐for‐asthma‐management‐and‐prevention/ (accessed 12 September 2016).
GRADEpro GDT [Computer program]
- McMaster University (developed by EvidencePrime). GRADEpro GDT. Version accessed 24 November 2016. Hamilton (ON): McMaster University (developed by EvidencePrime), 2015.
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Krishnan 2009
- Krishnan JA, Davis SQ, Naureckas ET, Gibson P, Rowe BH. An umbrella review: corticosteroid therapy for adults with acute asthma. American Journal of Medicine 2009;122(11):977‐91. [DOI: 10.1016/j.amjmed.2009.02.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Normansell 2016
- Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD011801.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paniagua 2017
- Paniagua N, Lopez R, Muñoz N, Tames M, Mojica E, Arana‐Arri E, et al. Randomized trial of dexamethasone versus prednisone for children with acute asthma exacerbations. Journal of Pediatrics 2017;191:190‐6.e1. [DOI: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowe 1999
- Rowe BH, Travers AH, Holroyd BR, Kelly KD, Bota GW. Nebulized ipratropium bromide in acute pediatric asthma: does it reduce hospital admissions among children presenting to the emergency department?. Annals of Emergency Medicine 1999;34(1):75‐85. [PUBMED: 10381998] [DOI] [PubMed] [Google Scholar]
Rowe 2001
- Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002178] [DOI] [PubMed] [Google Scholar]
Rowe 2007a
- Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000195.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2007b
- Rowe BH, Wong E, Blitz S, Diner B, Mackey D, Ross S, et al. Adding long‐acting beta‐agonists to inhaled corticosteroids after discharge from the emergency department for acute asthma: a randomized controlled trial. Academic Emergency Medicine 2007;14(10):833‐40. [DOI: 10.1197/j.aem.2007.06.020] [DOI] [PubMed] [Google Scholar]
Rowe 2009
- Rowe BH, Voaklander DC, Wang D, Senthilselvan A, Klassen TP, Marrie TJ, et al. Asthma presentations by adults to emergency departments in Alberta, Canada: a large population‐based study. Chest 2009;135(1):57‐65. [DOI: 10.1378/chest.07-3041] [DOI] [PubMed] [Google Scholar]
Rowe 2015
- Rowe BH, Villa‐Roel C, Majumdar SR, Abu‐Laban RB, Aaron SD, Stiell IG, et al. Rates and correlates of relapse following ED discharge for acute asthma: a Canadian 20‐site prospective cohort study. Chest 2015;147(1):140‐9. [DOI: 10.1378/chest.14-0843] [DOI] [PubMed] [Google Scholar]
Rowe 2017
- Rowe BH, Kirkland SW, Vandermeer B, Campbell S, Newton A, Ducharme FM, et al. Prioritizing systemic corticosteroid treatments to mitigate relapse in adults with acute asthma: A systematic review and network meta‐analysis. Academic Emergency Medicine 2017;24(3):371‐81. [DOI: 10.1111/acem.13107] [DOI] [PubMed] [Google Scholar]
Topal 2014
- Topal E, Gücenmez OA, Harmanci K, Arga M, Derinoz O, Turktas I. Potential predictors of relapse after treatment of asthma exacerbations in children. Annals of Allergy, Asthma and Immunology 2014;112(4):361‐4. [DOI: 10.1016/j.anai.2014.01.025] [DOI] [PubMed] [Google Scholar]


