Abstract
Background
Parastomal herniation is a common problem following formation of a stoma after both elective and emergency abdominal surgery. Symptomatic hernias give rise to a significant amount of patient morbidity, and in some cases mortality, and therefore may necessitate surgical treatment to repair the hernial defect and/or re‐site the stoma. In an effort to reduce this complication, recent research has focused on the application of a synthetic or biological mesh, inserted during stoma formation to help strengthen the abdominal wall.
Objectives
The primary objective was to evaluate whether mesh reinforcement during stoma formation reduces the incidence of parastomal herniation. Secondary objectives included the safety or potential harms or both of mesh placement in terms of stoma‐related infections, mesh‐related infections, patient‐reported symptoms/postoperative quality of life, and re‐hospitalisation/ambulatory visits.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2018, Issue 1), Ovid MEDLINE (1970 to 11 January 2018), Ovid Embase (1974 to 11 January 2018), and Science Citation Index Expanded (1970 to 11 January 2018). To identify ongoing studies, we also searched the metaRegister of Controlled Trials (mRCT) on 11 January 2018.
Selection criteria
We considered for inclusion all randomised controlled trials (RCTs) of prosthetic mesh (including biological/composite mesh) placement versus a control group (no mesh) for the prevention of parastomal hernia.
Data collection and analysis
Two review authors independently assessed the studies identified by the literature search for potential eligibility. We obtained the full articles for all studies that potentially met the inclusion criteria and included all those that met the criteria. Any differences in opinion between review authors were resolved by consensus. We pooled study data into a meta‐analysis. We assessed heterogeneity by calculation of I2 and expressed results for each variable as a risk ratio (RR) with corresponding 95% confidence intervals (CI). We expressed continous outcomes as mean difference (MD) with corresponding 95% CIs.
Main results
We included 10 RCTs involving a total of 844 participants. The primary outcome was overall incidence of parastomal herniation. Secondary outcomes were rate of reoperation at 12 months, operative time, postoperative length of hospital stay, stoma‐related infections, mesh‐related infections, quality of life, and rehospitalisation rate. We judged the risk of bias across all domains to be low in six trials. We judged four trials to have an overall high risk of bias.
The overall incidence of parastomal hernia was less in participants receiving a prophylactic mesh compared to those who had a standard ostomy formation (RR 0.53, 95% CI 0.43 to 0.66; 10 studies, 771 participants; I2 = 69%; low‐quality evidence). In absolute numbers, the incidence of parastomal hernia was 22 per 100 participants (18 to 27) receiving prophylactic mesh compared to 41 per 100 participants having a standard ostomy formation. There were no differences in the need for reoperation (RR 0.90, 95% CI 0.50 to 1.64; 9 studies, 757 participants; I2 = 0%; low‐quality evidence); operative time (MD ‐6.50 (min), 95% CI ‐18.24 to 5.24; 6 studies, 671 participants; low‐quality evidence); postoperative length of hospital stay (MD ‐0.95 (days), 95% CI ‐2.03 to 0.70; 4 studies, 500 participants; moderate‐quality evidence); or stoma‐related infections (RR 0.89, 95% CI 0.32 to 2.50; 6 studies, 472 participants; I2 = 0%; low‐quality evidence) between the two groups.
We were unable to analyse mesh‐related infections, quality of life, and rehospitalisation rate due to sparse data or because the outcome was not reported in the included studies.
Authors' conclusions
This Cochrane Review included 10 RCTs with a total of 844 participants. The review demonstrated a reduction in the incidence of parastomal hernia in people who had a prophylactic synthetic mesh placed at the time of the index operation compared to a standard ostomy formation. However, our confidence in this estimate is low due to the presence of a large degree of clinical heterogeneity, as well as high variability in follow‐up duration and technique of parastomal herniation detection. We found the rate of stoma‐related infection to be similar in both the intervention and control groups.
Plain language summary
Does prophylactic mesh placement around a stoma prevent the development of future hernia and general patient discomfort?
Review question
This review looked at whether placing a mesh (foreign material) around a stoma (a surgically created opening in the bowel and abdominal wall to allow the diversion of faeces) at the time of stoma formation affected whether study participants developed a hernia (a protrusion of the abdominal contents through a weakness within the abdominal wall) around the stoma. We also aimed to judge whether there were any risks or complications associated with mesh placement compared to no mesh.
Background
Hernia formation around a stoma affects up to 50% of people undergoing formation of a stoma. The hernia might enlarge over time, which can cause considerable patient discomfort which in turn may lead patients to restrict their work and other physical activities. Reoperation and cosmetic concerns may also arise.
Study characteristics
Following our data search in January 2018, we included 10 trials with a total of 844 participants, which we assessed using the standard Cochrane Review protocol. The trials compared the incidence of hernia development around a stoma between a group having a mesh placement at the time of stoma formation and a control group having a conventional stoma formation without mesh placement.
Key results
We found that mesh placement around the stoma at the time of stoma formation reduces the incidence of future hernia formation. The participants having a mesh fitted had a similar level of complications as those not having a mesh.
Quality of evidence
We found low‐quality evidence favouring the insertion of a mesh into people having a stoma.
Summary of findings
Background
Establishing a stoma (colostomy or ileostomy) may be necessary following colorectal surgery, and roughly 100,000 people in the USA and 20,000 in England require an operation that results in a colostomy or ileostomy each year (Goldberg 2010; Harris 2005). A stoma can either be permanent (e.g. following removal of the rectum) or temporary (e.g. a defunctioning ileostomy or colostomy to protect an anastomosis and prevent anastomotic leakage by diverting the faecal stream). However, recent evidence has indicated that not all temporary stomas are reversed, with 3% to 25% becoming permanent (Sier 2015).
A parastomal hernia (PH) is a type of incisional hernia of the gastrointestinal tract that occurs in relation to a previously constructed stoma. Most PHs develop within the first few years after construction of the stoma, however they can occur up to 20 years later (Carne 2003; Londono‐Schimmer 1994). Parastomal hernia remains a relatively common complication amongst stoma patients, with reported incidences between 2% and 56%, depending on the type of stoma and the length of follow‐up (Carne 2003; Shabbir 2010). Although PHs can be asymptomatic (Pearl 1989), many cause considerable morbidity, with up to a third requiring surgical intervention for complications such as pain, bowel obstruction, and fistulation (Jänes 2004a; Jänes 2009; Wara 2011). Quality of life has been shown to be significantly impaired in people with a PH, with those affected experiencing social isolation and social restriction (Gooszen 2000).
Parastomal hernia can be repaired surgically, either through an open or laparoscopic approach (Hansson 2009; Israelsson 2008; López‐Cano 2009; Pastor 2009). Open surgical techniques include relocation of the stoma, direct repair of the fascial defect, and repair using a prosthetic mesh. However, results to date when these techniques are used have been disappointing, with reported re‐recurrence rates of 30% to 76% (Amin 2001; Burns 1970; Hansson 2009; Kronborg 1974; Rubin 1994; Sjodahl 1988; Williams 1990). While the short‐term results of laparoscopic repair appear to be promising (with reported re‐recurrence rates of less than 2%) (Hansson 2009), patient selection remains an issue, with those having extensive intra‐abdominal adhesions considered not suitable for this approach (Pilgrim 2010).
Due to the frequency of parastomal herniation and the relatively limited success of repair, attention has focused on preventing PH at the time of stoma formation. Techniques such as placement of the stoma through the rectus abdominis muscle have traditionally been thought to reduce the incidence of herniation (Eldrup 1982; Sjodahl 1988; Stephenson 2010), and are often performed routinely. More recently, mesh reinforcement of the stoma has been advocated to further decrease the incidence of hernia formation (Ellis 2010; Hammond 2008; Jänes 2004a; Jänes 2004b; Jänes 2009; Serra‐Aracil 2009), although safety concerns persist regarding the use of prosthetic material in a contaminated surgical field (Tam 2010). Issues such as mesh infection, ostomy stenosis, mesh erosion, seroma formation, and the formation of adhesions have previously prevented surgeons from utilising this technique (Steele 2003).
Description of the condition
Development of a parastomal hernia remains relatively common in people undergoing colorectal surgery (Carne 2003; Shabbir 2010). Although many PHs remain asymptomatic, presenting symptoms range from unacceptable cosmesis and poorly fitting stoma devices to bowel strangulation, ischaemia, and obstruction (Carne 2003). Repair rates vary (Carne 2003; Israelsson 2005; Tam 2010), with surgical repair associated with its own risk of morbidity. Cost‐analysis has shown that the repair itself can be expensive, and continued high re‐recurrence rates after surgical repair exacerbate this (Carne 2003; Israelsson 2005; Tam 2010).
Description of the intervention
A prosthetic mesh is placed circumferentially adjacent to the stoma at the time of its formation. The mesh can be inserted intraperitoneally, under the externus fascia (called sublay), or on top of the externus fascia (called onlay). This can be achieved via either an open, Ellis 2010; Hammond 2008; Jänes 2004a; Jänes 2004b; Jänes 2009; Serra‐Aracil 2009, or laparoscopic approach (López‐Cano 2009; Lopez‐Cano 2012). The material used may vary; both prosthetic, Vijayasekar 2008, and biological/composite, Wijeyekoon 2010, mesh materials have been described.
How the intervention might work
A prosthetic mesh placed at the time of stoma formation may act as a mechanical buttress, increasing the strength of the abdominal wall at a site of potential weakness and thus preventing future hernia formation. Studies have shown that placement of such a mesh may reduce the incidence of PH (Hammond 2008; Jänes 2009; Serra‐Aracil 2009; Tam 2010). Mesh placement at the time of index surgery has been considered relatively safe, with complication rates of less than 5% (Tam 2010).
Why it is important to do this review
In colorectal surgery, stomas are often constructed on a temporary basis, sometimes to protect a downstream colorectal anastomosis with future reversal intended. Indications for permanent stoma formation persist, however, in individuals with cancer (i.e. abdominoperineal excision of the rectum), inflammatory bowel disease (e.g. total colectomy), and functional bowel surgery. Some patients, many of whom are young, are required to live a significant proportion of their lives with a permanent stoma.
Parastomal herniation often leads to significant lifetime morbidity, and therefore the current emphasis on prevention is particularly important. Traditional revision surgery (aponeurotic repair or relocation) rarely offers a robust long‐term solution, and associated re‐recurrence rates are greater than 30% (Tekkis 1999).
Mesh reinforcement of stomas to prevent parastomal herniation seems to be an intuitive way to address this problem and has been advocated in several prospective observational studies (Berger 2008; Gögenur 2006; Israelsson 2005; Marimuthu 2006; Vijayasekar 2008). Nevertheless, the uptake of this technique has been limited to date. Despite current evidence, the perceived lack of robustness of observational studies and uncertainty regarding the applicability of results from small cohorts may have contributed to the lack of dissemination of the technique. A meta‐analysis of data from available randomised controlled trials would improve the precision of any beneficial treatment effect and would enhance the power of studies to identify adverse outcomes associated with this procedure.
Objectives
The primary objective was to evaluate whether mesh reinforcement during stoma formation reduces the incidence of parastomal herniation. Secondary objectives included the safety or potential harms or both of mesh placement in terms of stoma‐related infections, mesh‐related infections, patient‐reported symptoms/postoperative quality of life, and re‐hospitalisation/ambulatory visits.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of parallel design, irrespective of blinding, sample size, publication status, or language. Cluster RCTs were not considered for inclusion, as this research modality would not have been suited for answering the research question. We excluded quasi‐randomised studies and other study designs in the presence of RCTs due to the potential for bias (Figure 1) (Gurusamy 2009; Higgins 2011).
1.
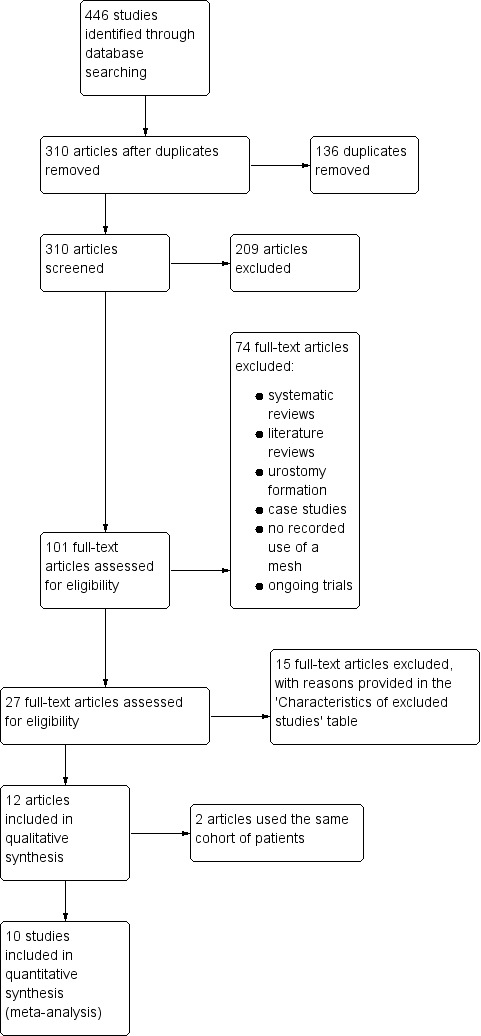
Study flow diagram.
Types of participants
We included all individuals of any age receiving a permanent or temporary abdominal wall stoma for colorectal (ileostomy or colostomy) operations in the elective and emergency setting, regardless of the underlying indication for surgery. We included participants with intraoperative faecal contamination in this review.
Types of interventions
We considered any form of mesh reinforcement of the stoma site at the index operation, regardless of type of mesh, type of stoma, anatomical plane of placement, and experience of the operating surgeon. We included both laparoscopic and open approaches in this review.
Types of outcome measures
Primary outcomes
Overall incidence of Parastomal Hernias at a minimum of 6 months' postsurgery, with or without placement of a parastomal mesh support at the time of the index operation
Secondary outcomes
Reoperation rate at 12 months
Operative time for index operation (minutes)
Postoperative length of hospital stay (days)
Stoma‐related infections that develop from 2 to 30 days' postoperatively
Mesh‐related infection from 2 to 30 days' postoperatively
Patient‐reported symptoms and postoperative quality of life (i.e. difficulty with bag application, leakage of stoma bag contents, nausea, vomiting, abdominal bloating, and parastomal discomfort)
Rehospitalisations/ambulatory visits required for parastomal hernia problems/treatment
Search methods for identification of studies
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs with no language restriction. The first reference to parastomal herniation in the literature appeared in 1974 (Lynne 1974), hence searches were commenced from 1970 onwards. We searched the following electronic databases to identify potential studies:
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2018, Issue 1, 11 January 2018) (Appendix 1);
Ovid MEDLINE (January 1970 to 11 January 2018) (Ovid MEDLINE) (Appendix 2);
Ovid Embase (January 1974 to 11 January 2018) (Ovid Embase) (Appendix 3); and
Science Citation Index Expanded (1970 to 11 January 2018) (Science Citation Index Expanded) (Appendix 4).
We also searched the metaRegister of Controlled Trials (mRCT) for ongoing trials on 11 January 2018. This register includes the ISRCTN Register, the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Register Platform (ICTRP).
Searching other resources
Two review authors (HGJ and JC) screened proceedings and abstracts of relevant meetings (from 1980 to 2018) for presentations not yet in print. These included the annual meetings of the Association of Coloproctology of Great Britain and Ireland, European Association of Coloproctology, American Society of Colon and Rectal Surgeons, Royal Society of Medicine (coloproctology section), British Society of Gastroenterology, and American Gastroenterology Association. Furthermore, two review authors (HGJ and JC) reviewed other sources such as dissertation abstracts.
Data collection and analysis
Selection of studies
Three review authors (HGJ, JB, and JC) independently assessed the studies identified by the literature search for potential eligibility. We obtained full articles for all studies that potentially met the inclusion criteria and included all those that met the criteria. Any differences in opinion between review authors at this stage were resolved by discussion and consensus. Studies were included in the review irrespective of whether measured outcomes were reported on. Excluded trials and reasons for their exclusion are provided in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (JC and HGJ) independently extracted the following data from included trials.
Population characteristics (sex, age, disease aetiology)
Interventions (experimental and control regimens randomised)
Outcomes (parastomal hernia rate, reoperation rate, stoma‐related infection, operative time, length of hospital stay, operative technique, and type of mesh used)
We independently assessed the methodological quality of the trials without the masking of trial names. Any differences in opinion were resolved through discussion and consensus among all review authors. We contacted the study authors regarding any unclear or missing information. We did not identify any studies that potentially shared the same participants.
A minimum follow‐up period of six months from the time of the index operation was necessary to assess the presence of a PH. We took data on the incidence of PH at 12 months, otherwise we used data on the longest follow‐up period reported. The presence of PH should have been assessed either with radiological investigation (i.e. either ultrasonography or computed tomography (CT)), defined as any intraabdominal content protruding along the ostomy, or with clinical examination.
We would consider the objective measurements of complications for meta‐analysis if the authors had used a validated tool (i.e. the Clavien‐Dindo classification of postoperative complications or a validated quality of life questionnaire) (Dindo 2004).
Assessment of risk of bias in included studies
We used the Cochrane ‘Risk of bias’ tool to assess the risk of bias of the included trials as specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed risk of bias with regard to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective reporting bias
Early stopping
We judged each domain as low, high, or unclear risk of bias according to criteria used in the Cochrane ‘Risk of bias’ tool (see Appendix 5) as specified in Section 8.5.d of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We determined the overall risk of bias across all domains in a study by the following assessment.
Low risk of bias: low risk of bias across all domains
Unclear risk of bias: unclear risk of bias for one or more domains
High risk of bias: high risk of bias for one or more key domains
A summary of the assessment of risk of bias is shown in Figure 2.
2.
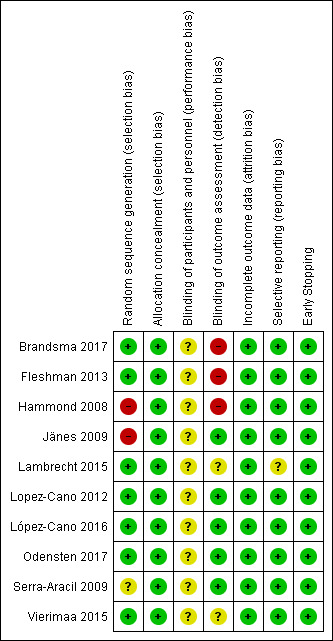
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We calculated risk ratios (RR) and 95% confidence intervals (CIs) for dichotomous outcomes. For continuous outcomes, we calculated mean differences (MD) and 95% CIs, processing continuous variables using the mean and standard deviation values. We considered a P value less than 0.05 as significant. We used weighted mean difference (WMD) in situations where outcome measurements in all studies were made on the same scale. We used the standardised mean difference (SMD) when the outcomes were measured on different scales.
Unit of analysis issues
The unit of analysis was each participant recruited into the trials.
Dealing with missing data
Where data were missing, insufficient, or unclear, we attempted to contact study authors for further information. If data were missing due to participants dropping out of the studies (and despite attempts to contact the primary authors we were unable to obtain information on reasons for the dropout), we conducted an intention‐to‐treat analysis and considered dropouts as successful rehabilitation when they occurred. For those data derived from completers only, we conducted best‐ (missing participants did not suffer event)/worst‐ (missing participants did suffer event) case scenario sensitivity analyses to assess the impact of missing data on the estimates of effect.
Assessment of heterogeneity
We assessed clinical heterogeneity in terms of participant characteristics (age, sex) and interventions (type of operation, surgical approach, mesh type). We assessed statistical heterogeneity using the Chi2 test with a P value of less than 0.10, and quantified the level of heterogeneity using the I2 statistic following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions:
0% to 30%: might not be important;
30% to 60%: may represent moderate heterogeneity;
60% to 90%: may represent substantial heterogeneity;
more than 90%: considerable heterogeneity (Deeks 2011).
In the case of substantial or considerable heterogeneity, we attempted to identify the possible sources of the heterogeneity.
Assessment of reporting biases
Reporting bias can lead to overly optimistic estimates of intervention effects. Funnel plots allow a visual assessment and statistical analysis of whether small‐study effects are present in the meta‐analysis. This assessment is only recommended where 10 or more studies are included. (Sterne 2011)
Data synthesis
We used Review Manager 5 software to analyse the data (RevMan 2014). For dichotomous outcomes, we pooled data in meta‐analyses using the Mantel‐Haenszel approach (fixed‐effect model); for continuous outcomes we used the inverse variance method (fixed‐effect model). If we deemed homogeneity between studies to be invalid (with considerable heterogeneity (I2 > 90%)), we instead adopted a random‐effects model after exploring the causes of the heterogeneity. We applied a fixed‐effect model if fewer than four studies were included in a meta‐analysis (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
To explore whether the operative technique affected the effectiveness of the intervention, we performed the following subgroup analyses, presented in Table 1:
Summary of findings for the main comparison. Prosthetic mesh placement compared with standard treatment for the prevention of parastomal hernia.
|
Participants or population: individuals having a stoma formation Settings: hospital, operating theatre Intervention: prophylactic stomal mesh reinforcement when forming a stoma Comparison: standard stoma formation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No prosthetic mesh placement | Prosthetic mesh placement | ||||
|
Overall incidence of parastomal hernia (follow‐up: 6 to 24 months) |
41 per 100 | 22 per 100 (18 to 27) | RR 0.53 (0.43 to 0.66) | 771 (10 studies) |
low1,2 |
|
Reoperation rate (follow‐up: 6 to 12 months) |
5 per 100 | 5 per 100 (3 to 8) | RR 0.90 (0.50 to 1.64) | 757 (9 studies) |
low3,4 |
|
Operative time for index procedure (follow‐up: N/A) |
66 to 356 minutes |
7 minutes faster (18 minutes faster to 5 minutes longer) |
MD ‐6.50 (‐18.24 to 5.24) |
671 (6 studies) |
low 4,5 |
|
Postoperative length of stay (follow‐up: N/A) |
9 to 17.5 days |
1 day shorter stay (2 days shorter to 1 day longer) |
MD ‐0.95 (‐2.03 to 0.70) | 500 (4 studies) |
moderate4 |
|
Stoma‐related infection (follow‐up: 6 to 24 months) |
3 per 100 | 3 per 100 (1 to 8) | RR 0.89 (0.32 to 2.50) |
472 (6 studies) |
low4,6 |
|
Mesh‐related infection (follow‐up: 12 to 60 months) |
‐ | ‐ | ‐ | 128 (4 studies) |
‐ |
|
Patient‐reported quality of life (follow‐up: 12 to 24 months) |
‐ | ‐ | ‐ | 263 (2 studies) |
‐ |
|
Rehospitalisations/ambulatory visits required for parastomal hernia problems/treatment (follow‐up: N/A) |
Not reported in any of the included studies | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; N/A: not available; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded one level due to heterogeneity. 2Downgraded one level due to high risk of detection and selection bias. 3Downgraded one level due to high risk of selection bias. 4Downgraded one level due to inconsistency of results. 5Substantial clinical heterogeneity. 6Downgraded one level due to high risk of detection bias.
laparoscopic versus open surgery;
sublay mesh versus intraperitoneal mesh.
We had planned to perform subgroup analyses on loop versus end stomas, and intraoperative contamination versus clean surgery, but the available data did not permit these.
Sensitivity analysis
We used a sensitivity analysis to assess the impact of specific trials (e.g. those with doubts about the randomisation process or those characterised by a high risk of bias) by excluding them from the meta‐analysis (if at least two RCTs were available). Such analyses can help test the robustness of meta‐analysis findings, especially in the presence of result uncertainty (e.g. in the presence of substantial between‐study heterogeneity). We performed a sensitivity analysis to determine the:
worst‐case scenario: all the participants who dropped out of the control arm were presumed to have no evidence of PH at maximal follow‐up, where participants in the intervention arm were presumed to have developed a PH;
best‐case scenario: all the participants who dropped out of the control arm were presumed to have evidence of PH at maximal follow‐up, where participants in the intervention arm were presumed not to have developed a PH.
We presented the best‐case and worst‐case scenarios in Table 1.
Summary of findings
We assessed the quality of evidence of all outcomes using the GRADE approach (Schünemann 2009), including any subgroup and sensitivity analyses, and presented this in Table 1.
The GRADE system classifies the quality of evidence in one of four grades:
high quality: further research is very unlikely to change our confidence in the estimate of effect;
moderate quality: further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate;
low quality: further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate;
very low: any estimate of effect is very uncertain.
The quality of evidence can be downgraded by one (serious concern) or two (very serious concerns) levels for the following reasons: high risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes), imprecision (wide confidence intervals), and risk of publication bias.
Results
Description of studies
See: Characteristics of included studies
Results of the search
The search identified 446 eligible studies, of which 136 were duplicates and removed. We excluded 209 studies because they did not meet the inclusion criteria after screening of the abstracts. We considered 101 study reports potentially eligible for inclusion, for which we sought the full texts. Of these, we excluded 74 studies for various reasons, including studies reporting use of mesh in the repair, as opposed to prevention, of parastomal hernia, and studies not being RCT's. Further 15 studies were excluded for various reasons, outlined in the Characteristics of excluded studies section. Two review authors (HGJ and JC) evaluated conference abstracts and other sources such as dissertation abstracts to identify eligible trials for inclusion. No further trials were found for inclusion. Finally, we included 10 studies (encompassing 12 references) in this review (Figure 1).
Included studies
Ten studies met the eligibility criteria, of which one study (two references) reported the same patient cohort, but outcomes were assessed after 1 month and 12 months from the time of intervention (Jänes 2004a), and 60 months from the time of intervention (Jänes 2009). A summary of all included studies is provided in the Characteristics of included studies table. A total of 835 participants contributed to four comparable outcomes (the incidence of parastomal hernia at maximal follow‐up, reoperation, stoma‐related infections, and operative time).
Brandsma 2017 published results from the PREVENT trial, aiming to assess the incidence of parastomal herniation, operating time, postoperative morbidity, and quality of life in people undergoing an elective open formation of a permanent end‐colostomy. There were a 146 participants with an intention‐to‐treat (ITT) analysis. A 12‐month follow‐up was reported. The presence of PH was assessed through clinical examination and a subsequent CT scan in the presence of clinical suspicion. Secondary endpoints included morbidity, reoperation rates, operative time, length of hospital stay, pain, quality of life, mortality, and cost‐effectiveness.
Fleshman 2013 aimed to assess the safety and efficacy of the placement of a porcine‐derived acellular dermal matrix (PADM, Stratice Recontructive Tissue Matrix, LifeCell Corporation, Branchburg, NJ, USA) around the stoma of people having a single, permanent ileostomy or colostomy compared to a control group having a conventional stoma formation. The number of participants randomised into the trial was 120, the ITT was 113, and PH rates were reported in 102 participants. The presence of a PH was assessed through clinical examination and a CT scan. Other listed outcomes included early and late stoma‐related events (i.e. pain, stenosis, leak, and obstruction), operative time, reoperation rates, and quality of life assessment in those with the PADM mesh compared to the control group.
Hammond 2008 randomised 20 participants having a defunctioning loop enterostomy as part of an elective procedure into either having a conventional stoma formation (through the rectus sheath) or a stoma formation with the placement of a Permacol mesh (Tissue Science Laboratories, Aldershot, UK). All 20 participants had outcomes reported. Eight of the 20 participants had their stoma reversed at a median of six months post‐index procedure. The main outcome assessment was the presence of a PH, which was assessed through the use of a questionnaire, clinical assessment, and ultrasound assessment in 16 of the 20 participants. Follow‐up was for a maximum of 12 months. Other listed outcomes included reoperation rates, postoperative white cell count (WCC), erythrocyte sedimentation rate (ESR), and C‐reactive protein levels (CRP), as well as a non‐structured patient questionnaire on stoma‐related symptoms.
Jänes and colleagues, Jänes 2004a; Jänes 2009, randomised 54 participants who were scheduled for either a permanent ileostomy or colostomy formation. Outcomes were available for all 54 participants. Participants were randomised into two groups, one for standard rectus abdominis stoma formation, and the other for the same stoma technique but with the placement of a synthetic Vypro mesh (Ethicon, Norderstedt, Germany). Patients scheduled for a loop enterostomy and those with a short life expectancy were excluded. The primary outcome measure was the presence of a PH at 1 and 12 months, Jänes 2004a, and 60 months, Jänes 2009, from the index procedure. Other listed outcomes included reoperation rates, mortality rates, wound and mesh‐specific infections.
Lambrecht 2015 undertook a prospective RCT that randomised 58 participants undergoing an open Hartmann's procedure or abdominoperineal excision for rectal cancer. Primary outcome was reported for all 58 participants. The trial aimed to assess the clinical and radiological development of PH with or without the use of a polypropylene mesh. Secondary outcomes included stoma‐related complications, such as infection, retraction, fistulation, and the need for reoperation. Furthermore, they presented a Cox regression analysis aimed at identifying secondary risk factors leading to PH.
Lopez‐Cano 2012 aimed to assess whether there was a reduced incidence of PH when a PROCEED mesh (Ethicon, UK) was placed laparoscopically during the formation of a sigmoid end colostomy, compared to a conventional non‐reinforced procedure. The authors utilised their own surgical technique in placing the mesh in an intraperitoneal fashion (López‐Cano 2009). A total of 36 participants were randomised in the trial, with outcomes available for 33. An assessment of PH was made using an abdominal CT scan at 12 months, which was performed by a radiologist blinded to the procedure used. Other listed outcomes included perioperative complications including ostomy‐specific complications, wound infections, and other body system complications such as heart failure or lower respiratory tract infections. Reoperation rates were also reported.
Another RCT was published in 2016, López‐Cano 2016, asking the same clinical question, but using a modified Sugarbaker technique rather than cutting a hole into the centre of the mesh as in their earlier trial. Again, participants requiring a permanent end colostomy after an abdominoperineal excision were selected; a total of 52 participants were randomised in this trial, with outcomes available from all 52. The presence of PH was determined using a CT scan and a radiologist blinded to the procedure at 12 months. Other listed outcomes included mortality rates, mesh infection, wound dehiscence, reoperation rates, length of stay in hospital, and surgical time.
Serra‐Aracil 2009 performed a prospective RCT of 54 participants having either an elective conventional sigmoid end colostomy through the rectus sheeth, or the same procedure with the addition of an Ultrapro mesh (Ethicon, UK). Outcomes were available for all 54 participants. The presence of a PH was assessed through the use of abdominal CT scan at one month after the index operation, then at six‐month intervals. Signs of mesh complications and parastomal complications were noted at this point. The clinical follow‐up lasted for a median of 29 months. Other listed outcomes included mortality, wound infection, mesh intolerance, operative time, and reoperation rates.
Vierimaa 2015 published results of a prospective, multicentre RCT of participants undergoing laparoscopic abdominoperineal excision of rectum and end colostomy formation for rectal cancer. Seventy‐five participants were randomised in the trial (37 assigned to receive a prophylactic parastomal mesh and 38 assigned as controls), with an ITT of 70 participants and full outcomes available on 67 participants. A dual component intraperitoneal onlay mesh was used consisting of 88% polyvinylidene fluoride and 12% polypropylene (DynaMesh‐IPOM, FEG Textiltechnik mbH, Aachen, Germany). The primary aim of the study was to assess the incidence of clinically and radiologically detected parastomal hernias at 12 months, as well as their extent. Secondary outcome measures included mortality rates, length of hospital stay, operative time, CRP and WCC, pneumonia/urinary infections, blood loss, reoperation rates, as well as stoma‐related morbidity (primarily pain and colostomy‐related problems).
Odensten 2017 published an RCT of 232 participants scheduled to have a permanent colostomy. The study randomised 118 participants to have a standard formation of a colostomy, and 114 participants to have a lightweight polypropylene mesh placed in the sublay position at the time of surgery. Outcomes with radiological follow‐up were available on 198 participants, and outcomes of clinical examination at 12 months were available on 211 participants. The primary aim of the study was to assess the incidence of clinically and radiologically detected parastomal hernias at 12 months. Secondary outcomes included the development of surgical (wound infection, stoma complications, reoperation) and non‐surgical complications (cardiac events, pneumonia, urinary infection) as well as reoperation rates, length of hospital stay, and operative time.
Excluded studies
A number of studies were excluded for reasons including not utilising a mesh, the study was on urostomy formation, and the study was not an RCT (Figure 1). The 16 excluded studies that were examined in detail are listed in the Characteristics of excluded studies section. The main reason for exclusion of these 16 studies was that the study design was not an RCT, and were made up of prospective and retrospective observational studies.
We identified seven ongoing studies on clinicaltrials.gov, for which the data were not available from the authors (Correa 2014; Demartines 2017; Garcia‐Urena 2017; Harb‐de la Rosa 2017; Prudhomme 2017; Uyanik 2017), and therefore listed as ongoing (see Characteristics of ongoing studies).
Risk of bias in included studies
Results of the 'Risk of bias' assessment are provided in the Characteristics of included studies table. See Figure 2 for a 'Risk of bias' summary of our judgements about each 'Risk of bias' item for each included study, and Figure 3 for a 'Risk of bias' graph which illustrates our judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
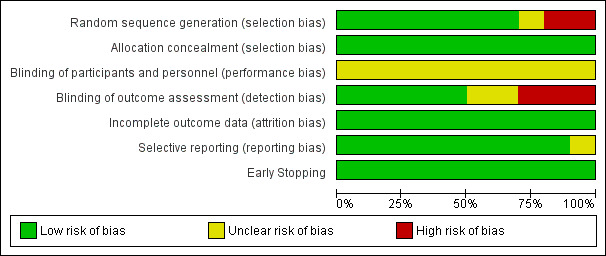
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We judged the overall risk of bias to be low in six trials (Lambrecht 2015; Lopez‐Cano 2012; López‐Cano 2016; Odensten 2017; Serra‐Aracil 2009; Vierimaa 2015), as we judged all domains to be at low risk of bias apart from the performance bias, which we determined could not be avoided. The overall risk of bias was high in four trials (Brandsma 2017; Fleshman 2013; Hammond 2008; Jänes 2009), as they had high risk of bias in at least one domain.
Allocation
We concluded that all ten of the included studies had adequate evidence to demonstrate a low risk of selection bias. The patient population that was selected did vary between studies, with seven studies choosing to exclude emergency ostomy formation (Brandsma 2017; Hammond 2008; Lambrecht 2015; Lopez‐Cano 2012; López‐Cano 2016; Odensten 2017; Vierimaa 2015). Only one study included patients having a defunctioning loop stoma (Hammond 2008), whilst the other nine studies only included patients having a permanent end colostomy. We concluded that these were reasonable allocations.
Four of the studies utilised an envelope randomisation process (Hammond 2008; Jänes 2009; Odensten 2017; Serra‐Aracil 2009), whilst block randomisation, Fleshman 2013, and computerised randomisation were also used (Brandsma 2017; Lambrecht 2015; Lopez‐Cano 2012; López‐Cano 2016; Vierimaa 2015).
Blinding
As it was not possible to blind the surgeon as to which technique was being used, we judged all studies to have an unclear risk of performance bias. Six trials demonstrated low detection bias (Jänes 2009; Lopez‐Cano 2012; López‐Cano 2016; Odensten 2017; Serra‐Aracil 2009; Vierimaa 2015). This was achieved either by using radiological examination to determine the presence of a PH, or blinded clinical assessment (Jänes 2009). The other studies could not adequately demonstrate the absence of bias when evaluating the participant for the presence of a PH.
Incomplete outcome data
All 10 studies were able to demonstrate low attrition bias. Fifty‐four participants in total were lost from the ITT population, but there was a clear explanation of participant disposition in all the studies.
Selective reporting
We considered nine of the studies to have a low risk of reporting bias. Lambrecht 2015 changed the method of hernia diagnosis from clinical to radiological during the study period. With regards to post‐operative complications, these were not fully reported by all the studies, but all other outcomes were otherwise thoroughly reported with no evidence of selective reporting.
Other potential sources of bias
One study reported early stopping (Jänes 2009), where an independent observer deemed it unethical to continue the trial before full recruitment due to the lower rates of PH reported in the mesh group. The reason for the early stopping is only mentioned in the paper presenting the preliminary data (Jänes 2004a). As the early stopping was due to the reduced rate of PH in the intervention arm (which was clearly documented), we considered the study to be at low risk of bias for this domain. The studies by Serra‐Aracil 2009 and López‐Cano 2016 were per‐protocol analyses, not ITT analyses.
Effects of interventions
Summary of findings 2. Prosthetic mesh placement compared with standard treatment for the prevention of parastomal hernia: subgroup and sensitivity analyses.
|
Participants or population: individuals having a stoma formation Settings: hospital, operating theatre Intervention: prophylactic stomal mesh reinforcement when forming a stoma Comparison: standard stoma formation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No prosthetic mesh placement | Prosthetic mesh placement | ||||
|
Subgroup analysis: Incidence of parastomal hernia at 12 months |
45 per 100 | 21 per 100 (13 to 35) | RR 0.47 (0.29 to 0.78) |
592 (7 studies) |
low1,2 |
|
Subgroup analysis:
Incidence of parastomal hernia (laparoscopic surgery) (follow‐up: 6 to 24 months) |
65 per 100 | 42 per 100 (31 to 57) | RR 0.64 (0.47 to 0.87) |
153 (3 studies) |
low1,2 |
|
Subgroup analysis:
Incidence of parastomal hernia (open surgery) (follow‐up: 6 to 24 months) |
39 per 100 | 18 per 100 (13 to 24) | RR 0.46 (0.34 to 0.62) |
517 (6 studies) |
moderate1 |
|
Subgroup analysis:
Incidence of parastomal hernia (sublay (extraperitoneal) mesh subgroup) (follow‐up: 6 to 24 months) |
34 per 100 | 16 per 100 (12 to 22) | RR 0.48 (0.36 to 0.64) |
619 (7 studies) |
moderate1 |
|
Subgroup analysis:
Incidence of parastomal hernia (intraperitoneal mesh subgroup) (follow‐up: 6 to 24 months) |
67 per 100 | 51 per 100 (37 to 71) | RR 0.76 (0.55 to 1.06) |
101 (2 studies) |
moderate3 |
|
Incidence of parastomal hernia (worst‐case scenario sensitivity analysis) (follow‐up: 6 to 24 months) |
38 per 100 | 19 per 100 (11 to 31) | RR 0.49 (0.30 to 0.81) |
835 (10 studies) |
low1,2 |
|
Incidence of parastomal hernia (best‐case scenario sensitivity analysis) (follow‐up: 6 to 24 months) |
46 per 100 | 18 per 100 (12 to 28) | RR 0.40 (0.26 to 0.61) |
835 (10 studies) |
low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded one level due to heterogeneity. 2Downgraded one level due to high risk of detection and selection bias. 3Downgraded one level due to inconsistency.
We included 10 studies involving a total of 844 participants in this Cochrane Review and contributing to four comparable outcomes: incidence of PH at maximal follow‐up, incidence of surgical intervention for a complication, incidence of mesh‐specific complications, and operative time. The study by Jänes 2009 included 36 participants from the original Jänes 2004a study. All the data analysed were from the published literature, and no further data were obtained.
1. Primary outcome
1.1 Incidence of parastomal herniation
We included 10 studies with a total of 771 participants (adequate follow‐up data were not available for 64 participants). There was a statistically significant reduction in the risk of PH in participants receiving a prophylactic mesh compared to those who had a standard ostomy formation (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.43 to 0.66; 10 studies, 771 participants; I2 = 69%) (Analysis 1.1) (Figure 4). The substantial statistical heterogeneity for this outcome (I2 = 69%, P = 0.005) may be explained by pronounced clinical heterogeneity in the trials. For example, Hammond 2008 reported no incidence of PH at maximal follow‐up in the intervention arm, whereas Vierimaa 2015 describes a rate of 53% in this group. This clinical heterogeneity is a result of the way the presence of PH was measured, where the former utilised clinical examination, and the latter used CT scanning. Other factors affecting heterogeneity include the use of laparoscopic surgery, Lopez‐Cano 2012; López‐Cano 2016; Vierimaa 2015, or open surgery (Brandsma 2017; Hammond 2008; Jänes 2009; Lambrecht 2015; Odensten 2017; Serra‐Aracil 2009).
1.1. Analysis.
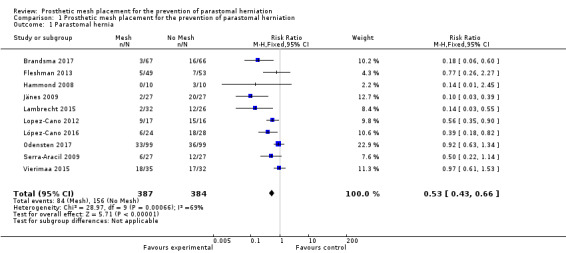
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 1 Parastomal hernia.
4.
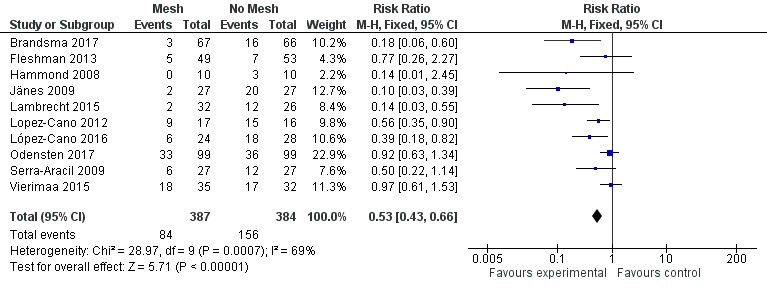
Forest plot of presence of parastomal hernia at maximal follow‐up.
There was a wide range of incidence of PH in the control groups (13.2% to 93.8%), and the length of follow‐up was variable, with seven studies collecting data at 12 months' postintervention (Brandsma 2017; Jänes 2009; Lopez‐Cano 2012; López‐Cano 2016; Odensten 2017; Serra‐Aracil 2009; Vierimaa 2015). One study presented data ranging from 6 to 12 months' postintervention (Hammond 2008), and the study by Fleshman 2013 presented data from 24 months of follow‐up. (See Characteristics of included studies)
Eight studies used some form of radiological assessment of PH in their follow‐up, with four studies using this as the primary tool to detect the presence of a PH (Lopez‐Cano 2012; López‐Cano 2016; Serra‐Aracil 2009; Vierimaa 2015), five studies using mostly clinical assessments (Brandsma 2017; Fleshman 2013; Hammond 2008; Jänes 2009; Lambrecht 2015), and Odensten 2017 using both. Brandsma 2017 and Fleshman 2013 utilised radiological assessment, but only to confirm the findings of the clinical examination. Lambrecht 2015 used radiological assessment to measure the stomal aperture, in a deviation from their original protocol. They found no correlation with the clinical diagnosis of PH, and did not use the data in their conclusions.
Vierimaa 2015 reported no difference in the incidence of PH at one‐year follow‐up on radiological assessment (48.6% intervention versus 46.9% control, P = 0.322), but a significant reduction in the presence of a clinically palpable hernia (14.7% intervention versus 37.5% control, P = 0.049).
We considered the GRADE classification of the quality of evidence to be low due to risk of bias (detection and selection bias in some of the studies) and the presence of heterogeneity (Table 1).
A subgroup analysis of the incidence of parastomal herniation in studies reporting follow‐up after 12 months showed similar results (RR 0.47, 95% CI 0.29 to 0.78; 7 studies, 592 participants; I2 = 74%) (Analysis 1.2).
1.2. Analysis.
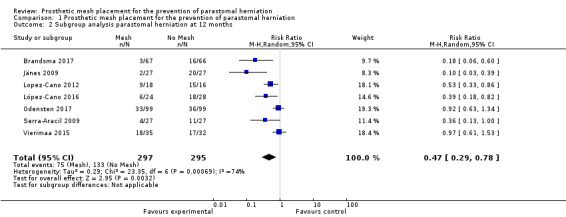
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 2 Subgroup analysis parastomal herniation at 12 months.
2. Secondary outcomes
2.1 Reoperation rate at 12 months
Nine of the 10 studies reported the reoperation rate, finding no statistically significant difference between the experimental and control groups (RR 0.90, 95% CI 0.50 to 1.64; 9 studies, 757 participants; I2 = 0%) (Analysis 1.3) (Figure 5). Vierimaa 2015 did not report data on surgical re‐intervention. There was no evidence of heterogeneity in the data (I2 = 0%, P = 0.53). We considered the GRADE quality of evidence to be low due to inconsistency of the results and risk of bias (Table 1).
1.3. Analysis.
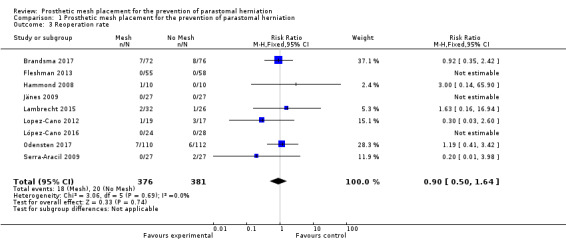
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 3 Reoperation rate.
5.
Forest plot of reoperation rate.
2.2 Operative time for index procedure
Six of the 10 included studies measured operative time for the index procedure (Brandsma 2017; Fleshman 2013; López‐Cano 2016; Odensten 2017; Serra‐Aracil 2009; Vierimaa 2015). Fleshman 2013, López‐Cano 2016, Serra‐Aracil 2009, and Vierimaa 2015 found no significant difference between groups, whereas Brandsma 2017 and Odensten 2017 found a significant increase in the operative time in participants receiving a mesh. Unusually, Vierimaa 2015 and Odensten 2017 describe prolonged operative times of between five and seven hours in both control and experimental groups, compared to one hour in the study by Fleshman 2013. When the operative times for all three studies were combined, there was no significant effect (MD ‐6.50, 95% CI ‐18.24 to 5.24; 6 studies, 671 participants) (Analysis 1.4) (Figure 6). We considered the GRADE quality of evidence to be low due to risk of bias and clinical heterogeneity (Table 1).
1.4. Analysis.
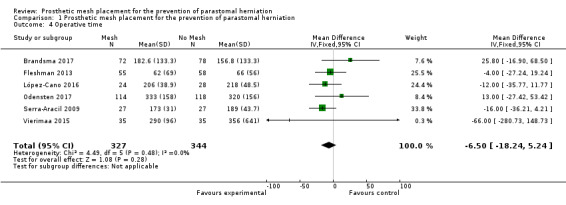
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 4 Operative time.
6.
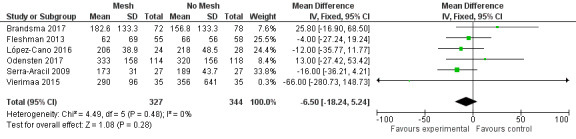
Forest plot of operative time.
2.3 Postoperative length of hospital stay
Four of the 10 included studies with a total of 500 participants measured the length of hospital stay following the index procedure. There were no significant differences between the two groups in any of these trials (MD ‐0.66, 95% CI ‐2.03 to 0.70, 4 studies, 500 participants) (Analysis 1.5). Brandsma 2017 described no difference between the two groups (12.1 days for the mesh group versus 13.8 days for the control group, P = 0.31). López‐Cano 2016 found no difference between the experimental and control groups (9 days versus 8 days, P = 0.64), with similar results from Odensten 2017 (12 days in the control group versus 12 days in the experimental group, P = 0.792). Vierimaa 2015 described a similar duration of hospital stay with no significant difference between the groups (10.2 days versus 9.5 days, P = 0.781). We considered the GRADE quality of evidence to be moderate (Table 1).
1.5. Analysis.
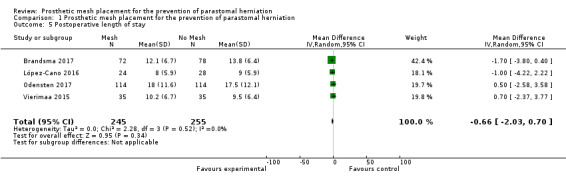
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 5 Postoperative length of stay.
2.4 Stoma‐related infections
Six studies with a total of 472 participants reported stoma‐related infections. There were no differences between the control and experimental groups. Serra‐Aracil 2009 reported two episodes of peristomal infection (one in the mesh group and one in the control group), and necrosis of the stoma was recorded in two participants, one in each group. After three months, Brandsma 2017 found no difference in stoma‐specific infections between the two groups (three participants from the control arm versus one participant from the experimental arm). Fleshman 2013 did not report specifically on stoma‐related infections. Jänes 2009 did not provide any details regarding postoperative complications, noting only the fact that there was no fistulation or stenosis‐associated complications with the mesh. Lopez‐Cano 2012 reported zero ostomy‐related complications in both the control and mesh group. Lambrecht 2015 reported a greater number of stoma‐related complications in the control group versus the mesh group, but no statistical difference was found (21% versus 9%, P = 0.446). Vierimaa 2015 reported no difference in ostomy‐related complications between the experimental and control groups. This includes data on stoma detachment (0% versus 2.9% in the intervention and control groups, respectively, P > 0.99), mucosal ischaemia (11.4% versus 8.6%, P > 0.99), intestinal ischaemia (0% versus 8.6%, P = 0.239), although the number of participants analysed was very small (n < 5). López‐Cano 2016 reported two participants with partial dehiscence of the colostomy in the experimental group compared to 0 in the control group, although this was not reported as significant (8.3% versus 0%, P = 0.21). Neither Hammond 2008 nor Odensten 2017 mentioned stoma‐specific complications (Table 1).
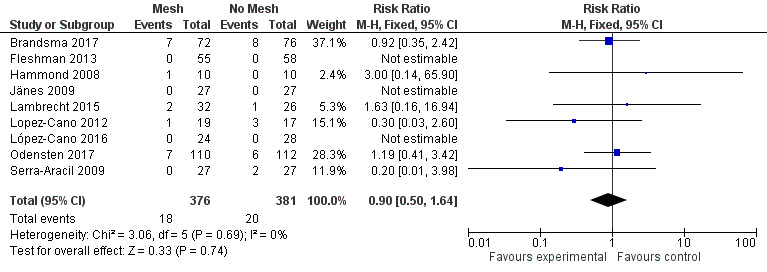
There was a large degree of variation in the reporting of stoma‐specific infections, which included superficial and deep infections, cellulitis, and fistulation. We therefore compared only clearly defined deep or superficial stoma‐related infections that developed from 2 to 30 days' postoperatively. There was no difference between the control and mesh groups (RR 0.89, 95% CI 0.32 to 2.50; 6 studies, 472 participants; I2 = 0%) (Analysis 1.6) (Figure 7). We considered the GRADE quality of evidence to be low due to inconsistency of the results and detection bias in some trials.
1.6. Analysis.
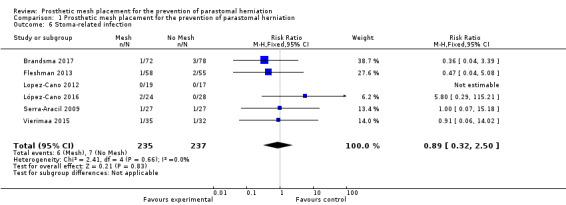
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 6 Stoma‐related infection.
7.
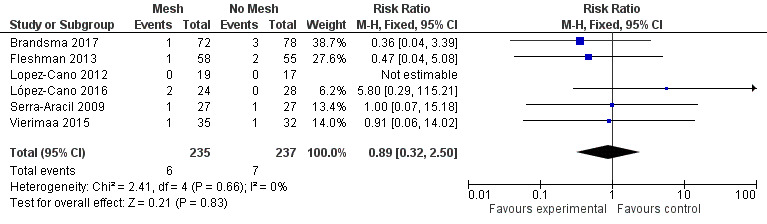
Forest plot of stoma‐related infection.
2.5 Mesh‐related infection
Four of the 10 included studies reported mesh‐related infections, with the others failing to distinguish 'stoma‐related infections' from 'mesh‐related infections'. Brandsma 2017, Lopez‐Cano 2012, and López‐Cano 2016 reported no mesh‐related infections at 12 months' postsurgery. Jänes 2009 reported no mesh infections after five years of follow‐up (Table 1) . Only one study reported mesh‐related infections, in 3 of 27 participants (11.1%), where no surgical intervention was required (Serra‐Aracil 2009). Consequently, we did not present an analysis of this outcome.
2.6 Patient‐reported symptoms/postoperative quality of life
Two studies included data on postoperative quality of life or patient symptoms (Brandsma 2017; Fleshman 2013). Brandsma 2017 demonstrated no difference between the two study groups in seven domains used to assess chronic pain (von Korff score), or eight domains assessing quality of life (36‐Item Short Form Health Survey). Fleshman 2013 used a stoma‐specific quality of life score on all participants (Prieto 2005). At 24 months, there was no difference in the mean (standard deviation) quality of life score between the control and intervention groups, respectively (80.8 (21.8) versus 65.5 (19.4), P = 0.22).
In summary, neither study showed any difference for this outcome between the control and intervention groups. Given the presence of substantial clinical heterogeneity between studies and the fact that that the reported data were based upon two different questionnaires measuring quality of life, we did not present an analysis of this outcome (Table 1).
2.7 Rehospitalisations/ambulatory visits
None of the included studies measured re‐hospitalisation/ambulatory visits for treatment, therefore we were unable to conduct an analysis of this outcome.
3. Subgroup analysis
Laparoscopic versus open surgery
Three studies evaluated a laparoscopic placement of the mesh in the intervention arm (Lopez‐Cano 2012; López‐Cano 2016; Vierimaa 2015), while six studies used the open approach (Brandsma 2017; Hammond 2008; Jänes 2009; Lambrecht 2015; Odensten 2017; Serra‐Aracil 2009). One study used both methods (Fleshman 2013), and was therefore excluded from the subgroup analysis. When we analysed data from the laparoscopic subgroup, we found no significant difference in the incidence of PH at 12 months between the control and intervention groups (RR 0.64, 95% CI 0.47 to 0.87; 3 studies, 153 participants; I2 = 63%) (Analysis 1.7) (Figure 8). When we considered the open‐surgery subgroup, there was a significant effect in favour of the mesh intervention with no significant statistical heterogeneity (RR 0.46, 95% CI 0.34 to 0.62; 6 studies, 517 participants; I2 = 79%) (Analysis 1.7). When the open technique was compared to a laparoscopic placement of the mesh, there was a significant advantage to the open technique in terms of reducing the rate of PH (18% (open) versus 43% (laparoscopic), P < 0.001). We considered the GRADE quality of evidence to be moderate in the open‐surgery subgroup due to the inclusion of studies with significant reporting bias, but low in the laparoscopic subgroup due to the inclusion of studies with significant reporting bias and the presence of heterogeneity (Table 2).
1.7. Analysis.
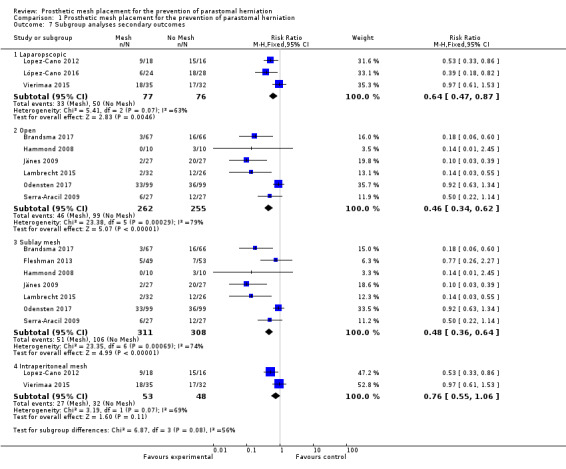
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 7 Subgroup analyses secondary outcomes.
8.
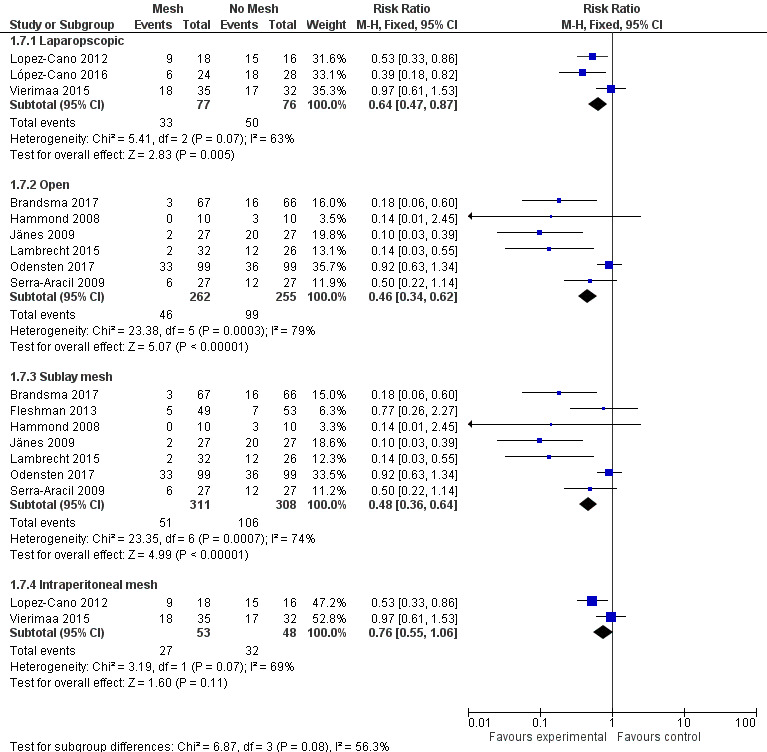
Subgroup analysis of laparoscopic and open surgery.
Intraoperative contamination versus clean surgery
All ten included studies excluded patients undergoing emergency surgery or those patients with intraoperative contamination. Therefore a subgroup analysis was not possible.
Sublay mesh versus intraperitoneal mesh
Seven studies used an extraperitoneal (sublay) mesh (Brandsma 2017; Fleshman 2013; Hammond 2008; Jänes 2009; Lambrecht 2015; Odensten 2017; Serra‐Aracil 2009), whilst three used an intraperitoneal mesh (Fleshman 2013; López‐Cano 2016; Vierimaa 2015). None of the 10 included studies utilised an onlay mesh. We excluded Fleshman 2013 from the subgroup analysis because a modified Sugarbaker technique was used that was not comparable with the other two trials employing an intraperitoneal technique. The extraperitoneal technique was associated with a significant reduction in PH compared to the control (RR 0.48, 95% CI 0.36 to 0.64; 7 studies, 619 participants; I2 = 74%) (Analysis 1.7), whilst there was no significant difference when the mesh was placed in the intraperitoneal position (RR 0.76, 95% CI 0.55 to 1.06; 2 studies, 101 participants; I2 = 69%) (Analysis 1.7). We considered the GRADE quality of evidence to be moderate in both the sublay and intraperitoneal technique subgroups due to the inclusion of studies with significant reporting bias (Table 2).
Loop versus end stomas
We had planned to perform subgroup analyses on loop versus end stomas, but the available data did not permit this.
4. Sensitivity analysis
We attempted to perform a sensitivity analysis on the primary and secondary outcomes. The length of reported follow‐up was highly variable between the studies. We performed a sensitivity analysis to analyse the rate of PH in studies with 12 months' follow‐up data (Figure 9). Seven studies were included in the sensitivity analysis (Brandsma 2017; Jänes 2009; Lopez‐Cano 2012; López‐Cano 2016; Odensten 2017; Serra‐Aracil 2009; Vierimaa 2015), with previously published data from Jänes 2004a. There was still evidence of a significant benefit in using a prophylactic mesh in these participants, with the presence of heterogeneity seen between the trials (RR 0.47, 95% CI 0.29 to 0.78; 7 studies, 592 participants; I2 = 74%). We considered the GRADE quality of evidence to be low due to risk of bias and clinical heterogeneity (Table 2).
9.
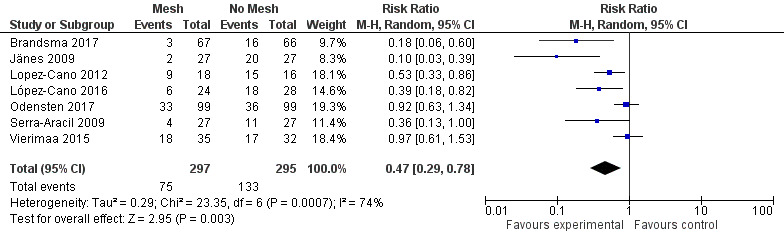
Parastomal herniation at 12 months.
We performed sensitivity analyses exploring worst‐ and best‐case scenarios. In a worst‐case scenario, all the participants who dropped out of the control arm had no evidence of PH at maximal follow‐up, whereas participants in the intervention arm developed a PH. The opposite was assumed when considering the best‐case scenario. Figure 10 demonstrates the 'worst' case (RR 0.49, 95% CI 0.30 to 0.81; 10 studies, 835 participants; I2 = 77%) (Analysis 1.8), and Figure 11 demonstrates the 'best' case (RR 0.40, 95% CI 0.26 to 0.61; 10 studies, 835 participants; I2 = 67%) (Analysis 1.9). These results demonstrate that both worst‐ and best‐case scenarios favour the intervention. However, we considered the GRADE quality of evidence to be low due to significant risk of bias and the presence of heterogeneity (Table 2).
10.
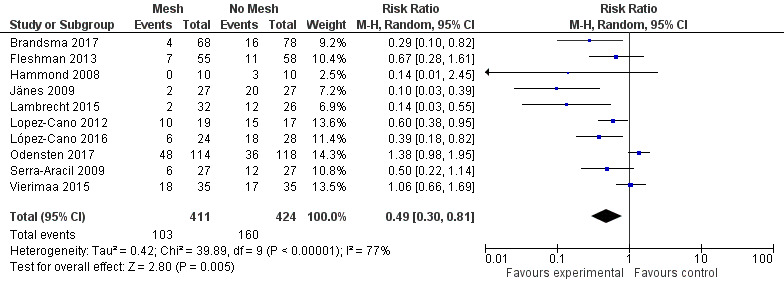
Worst‐case scenario incidence of parastomal hernia.
1.8. Analysis.
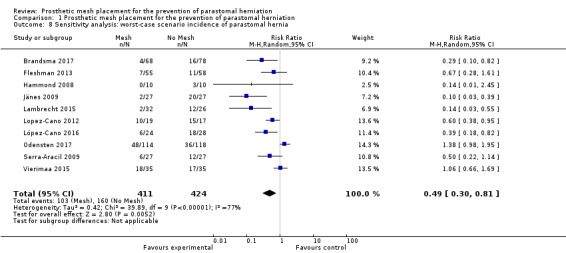
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 8 Sensitivity analysis: worst‐case scenario incidence of parastomal hernia.
11.
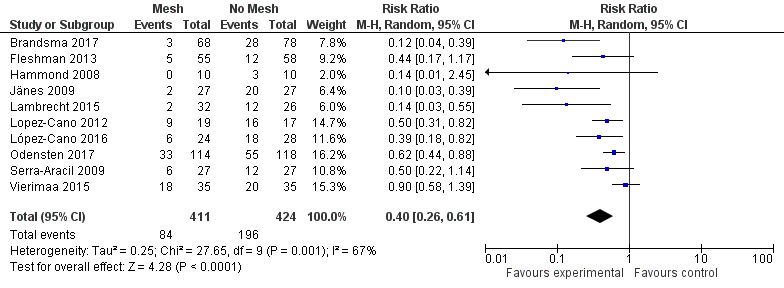
Best‐case scenario incidence of parastomal hernia.
1.9. Analysis.
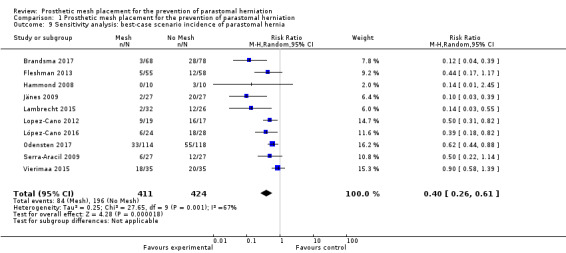
Comparison 1 Prosthetic mesh placement for the prevention of parastomal herniation, Outcome 9 Sensitivity analysis: best‐case scenario incidence of parastomal hernia.
Discussion
Summary of main results
We included 10 RCTs in the review comparing prosthetic mesh placement at the time of initial stoma formation versus standard stoma formation alone. All trials compared the incidence of PH development between the two groups, and nine of the 10 studies had a minimum of 12 months' follow‐up. Seven of the trials demonstrated a reduction in PH development within the intervention arm (Brandsma 2017; Jänes 2009; Lambrecht 2015; Lopez‐Cano 2012; López‐Cano 2016; Serra‐Aracil 2009; Vierimaa 2015), which translated to a reduction in PH development within the mesh group on meta‐analysis of 771 participants (RR 0.53, 95% CI 0.43 to 0.66; 10 studies, 771 participants; I2 = 69%). This effect was also seen when we performed the best‐ and worst‐case scenario subgroup analysis. When we undertook a subgroup analysis of extraperitoneal sublay versus intraperitoneal operative technique, only the sublay technique showed evidence of efficacy in preventing PH (RR 0.66, 95% CI 0.52 to 0.84; 9 studies, 612 participants; I2 = 61%).
Nine of the 10 included studies assessed surgical re‐intervention (757 participants), finding no significant difference between the control and intervention arms at meta‐analysis (RR 0.90, 95% CI 0.50 to 1.64; 9 studies, 757 participants; I2 = 0%). Meanwhile, stoma‐specific infections were reported in 8 studies (472 participants), with no reported differences between the control and intervention groups (RR 0.89, 95% CI 0.32 to 2.50; 6 studies, 472 participants; I2 = 0%).
Six studies assessed operative time as an individual outcome, two of which reported a significant increase in operative time in the intervention arm (Brandsma 2017; Odensten 2017). Overall, there were no significant differences between the intervention and control arms at combined meta‐analysis (MD ‐6.50, 95% CI ‐18.24 to 5.24; 6 studies, 671 participants; I2 = 0%). Finally, two of the included studies assessed postoperative quality of life measures, and although individually no study reported a significant difference between the control and intervention arms, combined analysis was not possible due to the variety of individual tools used to assess this outcome within each individual paper.
Overall completeness and applicability of evidence
Placement of a prosthetic mesh at the time of stoma formation remains an area of controversy in colorectal surgery. This review has shown that there appears to be evidence that mesh placement at the time of index procedure reduces the incidence of parastomal hernia formation following surgery. The review also demonstrated that the placement of a mesh is safe; is associated with few perioperative complications; and has no significant impact on the length of surgery.
Quality of the evidence
We considered the overall quality of the evidence, as defined by the GRADE classification, to be low to moderate for all four main outcomes (incidence of PH at maximal follow‐up, reoperation rate, operative time, and stoma‐related infection). We downgraded the evidence for incidence of PH due to heterogeneity and inconsistency of results. The evidence for stoma‐related complications and mesh‐related infections was also downgraded, for similar reasons. Meanwhile, we deemed the quality of the evidence related to patient‐reported symptoms and quality of life measures to be very low, based almost entirely on the lack of published data in this area.
We identified 10 studies suitable for inclusion in the review, with an average of 84 participants per trial (range 10 to 232). There was some evidence of clinical heterogeneity between the studies, in particular regarding the operative technique (laparoscopic versus open and sublay versus intraperitoneal); the way the presence of a hernia was detected (clinically/radiologically); and the types of complications that were reported. Also, the choice of mesh type differed between studies, with eight studies opting for a synthetic mesh and the others utilising a biological acellular derma matrix (Fleshman 2013; Hammond 2008). The indication and type of stoma formed also varied across the trials, with the majority of studies assessing mesh placement in the setting of an end colostomy, while others also included participants receiving an ileostomy, Fleshman 2013; Jänes 2009, or a defunctioning stoma (Hammond 2008). Furthermore, the surgical technique used to place the mesh differed between the included studies. Extraperitoneal mesh insertion can include placement both under the externus fascia (sublay) or on top of the externus fascia (onlay). Seven studies described an open sublay placement, and three a laparoscopic onlay intraperitoneal technique (Lopez‐Cano 2012; López‐Cano 2016; Vierimaa 2015), with the most recent trial using a laparoscopic modified Sugarbaker approach (López‐Cano 2016), in contrast to their earlier work (Lopez‐Cano 2012). In addition to these differences, the experience of the operating surgeon performing the procedure was not widely discussed and is another area of potential heterogeneity amongst the data set. None of the included trials utilised a mesh on top of the externus fascia (onlay technique). The only evidence for the use of an onlay mesh in the literature was from small prospective cohort studies that suggested possible benefits of its use (Bayer 1986; Berger 2008; Gögenur 2006).
Clinical follow‐up and participant assessment also varied between the studies. Firstly, length of participant follow‐up varied considerably, from six months, in Hammond 2008, to more than five years, in Jänes 2009. As a result of this wide variation, we considered a minimum follow‐up period of 12 months to be necessary for inclusion in combined analysis in order to determine reliable rates of herniation in the control and intervention arms. The way in which PHs were assessed differed between the included studies, with some studies choosing to rely on clinical examination alone, and others choosing a combination of clinical examination along with radiological assessment or radiological assessment alone (Lopez‐Cano 2012; López‐Cano 2016; Serra‐Aracil 2009; Vierimaa 2015). Information to assess the presence of detection bias was also limited in some of the studies, with only five studies demonstrating adequate detail on how either the radiologist or clinical assessor was blinded at the time of assessment (Jänes 2009; López‐Cano 2016; Odensten 2017; Serra‐Aracil 2009; Vierimaa 2015).
Reporting of stoma‐related infections suffered from heterogeneity in reporting, and a wide variation in the rates of infection were seen. Some studies reported no identified cases (Jänes 2009; Lopez‐Cano 2012), while others show this figure to be as high as 23% (Brandsma 2017). Consequently, while statistical analysis of the available data shows no difference between the control and intervention arms, interpretation of the data should be undertaken with caution. Three of the included studies covered postoperative quality of life and patient symptoms (Brandsma 2017; Fleshman 2013; Vierimaa 2015), but as each used individual tools and scoring systems to assess this variable, comparison between the studies was not possible.
Potential biases in the review process
This review was limited by the relatively small amount of published material in the field of study. We identified several ongoing RCTs, but the preliminary results were not available for us to evaluate. The review was limited by significant clinical and statistical heterogeneity between the ten included studies. This heterogeneity included variability in type of mesh used, surgical technique, and methods of outcome reporting. The absence of relevant information on blinding, generation of allocation sequence, type of randomisation, allocation concealment, reasons for withdrawals and those lost to follow‐up will also compromise the wider applicability of these studies. Finally, the overall length of follow‐up (maximum 60 months) limited the evidence on long‐term efficacy and safety of the mesh placement, and therefore caution is advised in extrapolating the results of this review to the long term.
Agreements and disagreements with other studies or reviews
There are six recent systematic reviews assessing the use of prophylactic mesh insertion in relation to stomas, four of which include RCTs alone (Patel 2016; Shabbir 2012; Tam 2010; Wijeyekoon 2010), and two including a mixture of RCT and observational study designs (Fortelny 2015; Helgstrand 2008). All six reviews report similar findings to those found in this review, agreeing that placement of a mesh at the time of index surgery relates to a reduction in incidence of parastomal hernia, and possibly a reduced necessity for recurrent surgical intervention (Helgstrand 2008; Fortelny 2015; Patel 2016; Shabbir 2012; Tam 2010; Wijeyekoon 2010). Similar concerns regarding heterogeneity, relatively small numbers of participants, and short follow‐up periods have led to a more guarded view in recommending widespread uptake of the technique. This is particularly true when considering mesh material and whether the results of studies that used a biological mesh, Fleshman 2013; Hammond 2008, are comparable to the other studies where synthetically constructed ones were used. Broadly speaking, the length of follow‐up in studies using synthetic material was longer (Jänes 2004a; Jänes 2009), and failed to show any difference between groups in mesh‐related complications, and the temptation is to suggest that they are comparable.
All included studies except Hammond 2008 used the same method for estimating appropriate sample size. Hammond and colleagues did not estimate an appropriate sample size due to the small phase I nature of their study. The authors of the other studies worked out an appropriate sample size in order to achieve a significance (alpha) of 0.05 and a power (beta) of 0.9 (90%) (Jänes 2009; Lambrecht 2015; Lopez‐Cano 2012; López‐Cano 2016; Odensten 2017; Serra‐Aracil 2009; Vierimaa 2015). They all suggested a sample size of between 17 and 67 for each arm, however Fleshman 2013 hypothesised that 110 participants per arm would be needed, and Odensten 2017 calculated that a total sample size of 220 would be needed.
In addition, there are multicentre RCTs that are either ongoing or recently published (Correa 2014; Garcia‐Urena 2017), and long‐term follow‐up data from the currently published PREVENT trial should be included in future updates of this review (Brandsma 2017).
Seven ongoing studies are assessing the role of prophylactic mesh placement in relation to stoma formation (Correa 2014; Demartines 2017; Garcia‐Urena 2017; Harb‐de la Rosa 2017; Prudhomme 2017; Uyanik 2017; Tabusa 2018). The majority of these trials are RCT in design, are currently in the recruitment phase, and are either exclusively, (Demartines 2017; Harb‐de la Rosa 2017; Prudhomme 2017; Uyanik 2017; Tabusa 2018), or partially, (Correa 2014; Garcia‐Urena 2017), aimed at assessing the effect of mesh insertion for the prevention of PHs.
Interestingly, the National Institute for Health Research (NIHR) has recently funded the UK Cohort study to Investigate the prevention of Parastomal Hernia (CIPHER) (Tabusa 2018) . This study plans to focus on interview and observation techniques to understand the components of how stomas are formed in an attempt to assess any important steps in preventing PH development. The authors then plan for this to lead to the development of a questionnaire that patients can complete in order to elicit symptoms associated with PHs, which can then be used to accurately detect PH during phase B of the study. While this study is of cohort design and not specifically related to mesh placement in PH prevention, the focus on a symptom‐based approach should add further evidence, particularly in terms of a quality of life perspective.
Authors' conclusions
Implications for practice.
The review concludes that placement of a prosthetic mesh at the time of stoma formation at index surgery is safe and reduces the incidence of parastomal hernia development, although we considered the overall quality of the evidence to be low, and the largest trial, which was of good quality, demonstrated no advantage to using a mesh. There is no significant increase in operative time or any increase in reoperation rate with this intervention, and we found the rate of stoma‐related infection to be similar in both the intervention and control groups.
Based on the results of this review, we judge that the placement of a prosthetic mesh at the time of stoma formation is a safe and feasible option for surgeons to consider at the time of surgery. The current body of evidence would limit the scope of recommended practice to elective colorectal procedures where faecal contamination is limited, as all included studies failed to assess the technique after emergency surgical procedure or in those with heavy contamination. All but one included study assessed mesh placement in relation to an end (as compared to loop) ostomy. With limited data on the benefits of mesh placement in relation to loop stomas, it would therefore appear sensible at present to limit the widespread practice of mesh placement to end ostomy formation alone until future data provide more insight into this area. The available data demonstrated the greatest advantage when the open, extraperitoneal (sublay) approach for mesh placement was utilised, and this should be considered by the operating surgeon.
In summary, the current evidence provided by this review would support the uptake of prophylactic mesh placement in conjunction with elective end‐stoma formation using an open, extraperitoneal technique.
Implications for research.
While the authors feel that an accurate overall picture of both the clinical incidence and implications on parastomal hernia development remain poorly documented within the literature, there is a growing body of evidence within this area that is gradually improving our overall understanding. Within the last 12 months alone there have been a number of large systematic reviews or meta‐analyses published addressing this issue (Cross 2017; López‐Cano 2017; Patel 2017; Pianka 2017). All of these reviews have a broadly similar conclusion in that they all support the finding in this review that prophylactic mesh insertion prevents the rate of parastomal hernia. In addition, as was the case in this review, a high risk of bias was reported in some cases (Patel 2017; Pianka 2017), and while all the reviews suggest there was no difference in mesh‐ or stoma‐related complications between control and treatment arms, patient‐reported symptoms and quality of life aspects were poorly captured overall.
Although this review has indicated an advantage in the use a prophylactic mesh in terms of reducing the incidence of parastomal hernia formation, the main body of evidence relates to elective surgery when forming an end stoma. Future research exploring the role of a prophylactic mesh placement during emergency surgery (particularly in terms of safety) should therefore focus on whether mesh placement may have a role in this setting, especially given a reported higher possible rate of parastomal hernia formation amongst this group (Arumugam 2003). Similarly, future research that investigates the role of mesh placement in relation to temporary and loop stomas (with particular focus on cost‐effectiveness) would likely add significant value to the current body of evidence.
While this review appears to have found no evidence to support the role of laparoscopic or pre‐peritoneal mesh placement, there remains a relative lack of evidence to assess these techniques fully. The number of participants undergoing a laparoscopic approach (n = 153) was less than a third of those undergoing open mesh placement (n = 517). Similarly, the number of participants undergoing an extraperitoneal approach for mesh placement (n = 619) was more than six times those undergoing a pre‐peritoneal approach (n = 101). A larger body of evidence assessing these techniques is therefore likely to add value in assessing the relative effectiveness compared to an open, extraperitoneal placement approach.
There remained a relative lack of data in several areas assessed under the scope of this review. This included the documentation of mesh‐related complications, which was generally lacking in the body of evidence examined, which led to mesh‐related infections being used as a more specific outcome. Only two of the included studies considered quality of life measures, both of which used a different measure to assess this index. Future high‐quality research using a standard measure of quality of life to limit clinical heterogeneity would therefore be needed to better define the effect of mesh placement on these factors. Finally, none of the included studies assessed re‐hospitalisation or ambulatory visits as a recorded outcome. As recurrent hospital attendances for symptoms related to surgery remain a common cause of patient anxiety and incur significant financial costs, future evidence highlighting this aspect in particular would add further value to the uptake of prophylactic mesh placement.
In summary, while there is a growing volume of data supporting the use of prophylactic mesh insertion to reduce rates of parastomal hernia, it is interesting to note that the uptake of the procedure as a routine part of stoma formation remains limited (Blake 2017). While this may be related to some of the quality issues with existing data as identified by this review, it is also possible that there may be other factors at play, including the potential lack of awareness of the issue and surgeon anxiety.
What's new
| Date | Event | Description |
|---|---|---|
| 18 May 2018 | Amended | Text on differences between onlay and sublay mesh added to the review. |
| 11 January 2018 | Amended | New randomised controlled trial added ‐ no changes to the overall findings. Brandsma 2016 now a reference included in the study Brandsma 2017 |
History
Protocol first published: Issue 12, 2010 Review first published: Issue 7, 2018
| Date | Event | Description |
|---|---|---|
| 12 October 2017 | Amended | Further alterations, and discrepancies corrected. |
| 11 September 2017 | Amended | 'Risk of bias' updated. 'Risk of bias' across all domains added. MECIR guidelines met. |
| 19 March 2017 | Amended | Edits throughout manuscript |
| 11 March 2017 | New search has been performed | New randomised controlled trial added (López‐Cano 2016). Conclusions unchanged. |
| 1 November 2016 | Amended | Worst‐ and best‐case scenarios added, as well as other changes requested by the editorial team. |
| 5 September 2016 | Amended | Random‐effects model, sensitivity testing, and subgroup analysis |
| 1 September 2016 | New search has been performed | Brandsma 2016 paper added to replace the pilot data. |
| 24 April 2016 | New citation required but conclusions have not changed | Three new randomised controlled trials added to the review. Conclusions unchanged. |
| 12 January 2016 | New search has been performed | Searches repeated. |
Acknowledgements
The authors would like to thank Mr Peter Billings, who passed away during the writing of this review. His dedication to teaching as well as his leadership was an inspiration to a generation of junior doctors who had the pleasure of working for him.
The authors would also like to thank Professor Norman S Williams, MS, FRCS, FMedSci, and Wijeyekoon SP, Gurusamy K, El‐Gendy K, and Chan CL for allowing us to use their protocol as a template.
The authors would like to thank the Cochrane Colorectal Cancer Group editorial office (Henning Keinke Andersen and Sara Hallum) for valuable input and continuous support, and editors and peer referees for careful evaluation of this review.
Appendices
Appendix 1. Cochrane Library search strategy
The Cochrane Library (CLib 75 hits), CENTRAL (issue 11 of 12, December 2016): 67 hits
Search History
#1 MeSH descriptor: [Enterostomy] explode all trees
#2 MeSH descriptor: [Surgical Stomas] explode all trees
#3 (colostom* or ileostom* or enterostom* or stom* or ostom* or parastom*):ti,ab,kw
#4 (#1 or #2 or #3)
#5 MeSH descriptor: [Hernia, Abdominal] explode all trees
#6 hernia*:ti,ab,kw
#7 (#5 or #6)
#8 MeSH descriptor: [Surgical Mesh] explode all trees
#9 MeSH descriptor: [Prosthesis Implantation] explode all trees
#10 MeSH descriptor: [Absorbable Implants] explode all trees
#11 MeSH descriptor: [Bioprosthesis] explode all trees
#12 (mesh* or prosthesis* or implant*):ti,ab,kw
#13 (#8 or #9 or #10 or #11 or #12)
#14 (#4 and #7 and #13)
Appendix 2. MEDLINE (Ovid) search strategy
MEDLINE (Epub Ahead of print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and 1946 to present): 57 hits
Search History
1. exp Enterostomy/
2. exp Surgical Stomas/
3. (colostom* or ileostom* or enterostom* or stom* or ostom* or parastom*).mp.
4. 1 or 2 or 3
5. exp Hernia, Abdominal/
6. hernia*.mp.
7. 5 or 6
8. exp Surgical Mesh/
9. exp Prosthesis Implantation/
10. exp Absorbable Implants/
11. exp Bioprosthesis/
12. (mesh* or prosthesis* or implant*).mp.
13. 8 or 9 or 10 or 11 or 12
14. 4 and 7 and 13
15. randomized controlled trial.pt.
16. controlled clinical trial.pt.
17. randomized.ab.
18. placebo.ab.
19. clinical trial as topic.sh.
20. randomly.ab.
21. trial.ti.
22. 15 or 16 or 17 or 18 or 19 or 20 or 21
23. exp animals/ not humans.sh.
24. 22 not 23
25. 14 and 24
Appendix 3. Embase (Ovid) search strategy
EMBASE (OVID) ‐ January 1974 to January 2018
EMBASE (Ovid, 1974 to week 50): 68 hits
Search History
1. exp enterostomy/
2. *colon pouch/
3. *stoma bag/
4. (colostom* or ileostom* or enterostom* or stom* or ostom* or parastom*).ti.
5. 1 or 2 or 3 or 4
6. exp hernia/
7. hernia*.ti.
8. 6 or 7
9. exp surgical mesh/
10. *implantation/
11. *prosthesis/
12. (mesh* or prosthesis* or implant*).ti.
13. 9 or 10 or 11 or 12
14. 5 and 8 and 13
15. CROSSOVER PROCEDURE.sh.
16. DOUBLE‐BLIND PROCEDURE.sh.
17. SINGLE‐BLIND PROCEDURE.sh.
18. (crossover* or cross over*).ti,ab.
19. placebo*.ti,ab.
20. (doubl* adj blind*).ti,ab.
21. allocate*.ti,ab.
22. trial.ti.
23. RANDOMIZED CONTROLLED TRIAL.sh.
24. random*.ti,ab.
25. 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.)
27. 25 not 26
28. 14 and 27
Appendix 4. Science Citation Index Expanded search strategy
Science Citation Index Expanded (1970 to 2018): 213 hits
Search History
#1 Topic=(colostom* or ileostom* or enterostom* or stom* or ostom* or parastom*)
#2 Topic=(hernia*)
#3 Topic=(mesh* or prosthesis* or implant*)
#4 Topic=(random* OR controlled OR RCT OR placebo OR trial OR group* OR trial*)
#5 (#1 AND #2 AND #3 AND #4)
Appendix 5. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of ‘low risk’ of bias | The investigators describe a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘high risk’ of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information about the sequence generation process to permit judgement of low or high risk |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | |
| Criteria for a judgement of ‘low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of ‘high risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, such as if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature, or handling of incomplete outcome data | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of ‘low risk’ of bias | Any of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of low or high risk. It is likely that the majority of studies will fall into this category. |
|
Early stopping Bias due to problems not covered elsewhere in the table | |
| Criteria for a judgement of ‘low risk’ of bias | Sample size calculation was reported, and the trial was not stopped or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was low. |
| Criteria for the judgement of ‘high risk’ of bias | Sample size calculations were not reported, and it is not clear whether the trial was stopped early. |
| Criteria for the judgement of ‘unclear risk’ of bias | The trial was stopped early because of an informal stopping rule, or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high. |
Data and analyses
Comparison 1. Prosthetic mesh placement for the prevention of parastomal herniation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parastomal hernia | 10 | 771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.43, 0.66] |
| 2 Subgroup analysis parastomal herniation at 12 months | 7 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.78] |
| 3 Reoperation rate | 9 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.50, 1.64] |
| 4 Operative time | 6 | 671 | Mean Difference (IV, Fixed, 95% CI) | ‐6.50 [‐18.24, 5.24] |
| 5 Postoperative length of stay | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐2.03, 0.70] |
| 6 Stoma‐related infection | 6 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.32, 2.50] |
| 7 Subgroup analyses secondary outcomes | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Laparopscopic | 3 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.87] |
| 7.2 Open | 6 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.62] |
| 7.3 Sublay mesh | 7 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.64] |
| 7.4 Intraperitoneal mesh | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.06] |
| 8 Sensitivity analysis: worst‐case scenario incidence of parastomal hernia | 10 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| 9 Sensitivity analysis: best‐case scenario incidence of parastomal hernia | 10 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.26, 0.61] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brandsma 2017.
| Methods | Randomised trial, computer randomisation | |
| Participants | 146 (mean age= 63) Male 92:58 Female | |
| Interventions | Placement of retromuscular lightweight polypropylene mesh around a colostomy vs no mesh | |
| Outcomes | After 12 months of follow‐up: incidence of parastomal herniation, operation time, postoperative morbidity, pain and quality of life | |
| Length of Follow Up | Maximum 12 months | |
| Notes | The PREVENT trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by computer, and an interactive voice response system was used. |
| Allocation concealment (selection bias) | Low risk | Computerised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The assessment of the presence of PH is made through clinical examination. The authors state that CT scan assessment would be done on all suspected PH, but only 16 were performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 months' data were available with the full publication in 2016. 13 out of 150 participants not included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of reporting bias. Complications well documented. |
| Early Stopping | Low risk | Comment: Sample size calculation given. No early stopping reported. |
Fleshman 2013.
| Methods | Randomised trial. Block randomisation using 160 equally weighted blocks of 2 treatments with a blocking factor of 4 | |
| Participants | 113 (mean age = 60 years, +/‐ 14 years (1 SD)) Male 59:54 Female | |
| Interventions | In the experimental group (n = 49), a 6 x 6 cm or 8 x 8 cm porcine‐derived acellular dermal matrix was inserted between the anterior and posterior rectal sheath, with a cruciate incision in the centre. The control group (n = 53) had a traditional end colostomy/ileostomy in the same position. | |
| Outcomes | The presence of a parastomal hernia was assessed using clinical examination, or CT scan if there was clinical doubt. Other outcomes included safety and stoma‐related quality of life. | |
| Length of Follow Up | Maximum 24 months | |
| Notes | Data from 12 months' follow‐up sought but not received from author. 5 participants from the control group and 7 participants from the intervention group lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised trial (1:1 randomisation) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was conducted centrally by using 160 equally weighted blocks of 2 treatments with a blocking factor of 4. Patients and staff members who performed the assessments were blinded as to assignment" Comment: Probably occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "of the 13 hernias that occurred, 11 were confirmed by CT scan and 2 were confirmed operatively." Comment: No standardised, predefined way of assessing the presence of a PH, although assessors were blinded to the procedure performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐month PH data on 45 of 113 participants not included in the final analysis. The author has adequately justified participants excluded in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: No selective reporting identified. |
| Early Stopping | Low risk | Comment: Sample size calculation given. No early stopping reported. |
Hammond 2008.
| Methods | Randomised trial. Envelope randomisation | |
| Participants | 20 (mean age control = 50 years (22 to 70 years), mean age intervention = 43 (21 to 69 years)), Male 7:13 Female | |
| Interventions | Conventional loop ileostomy via a trephine incision (n = 10) vs loop ileostomy with a 10 x 10 cm Permacol mesh with a 2‐centimetre cylindrical defect cut in the centre (n = 10) | |
| Outcomes | The incidence of parastomal hernia was assessed by ultrasound scan (16 of 20 trial participants). Incidence of infection and seroma assessed by laboratory tests and ultrasound scan. Incidence of patient symptoms assessed by a questionnaire. | |
| Length of Follow Up | Maximum 12 months (if no reversal) Maximum 6.5 months (if stoma reversed) |
|
| Notes | No participants lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: Participants were randomised using consecutively numbered, sealed envelopes. No random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered sealed envelopes" Comment: Probably occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "underwent a clinical examination for signs of a parastomal hernia" Comment: No evidence of assessor blinding when performing clinical examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up. No complete 12‐month data. Appropriately outlined follow‐up regimen |
| Selective reporting (reporting bias) | Low risk | Quote: "Patients were followed‐up until the time of stoma reversal or, in the event of the stoma not being reversed, until 12 months after stoma formation." Comment: No selective reporting. All participants and outcomes accounted for. |
| Early Stopping | Low risk | No early stopping |
Jänes 2009.
| Methods | Randomised trial. Envelope randomisation | |
| Participants | 54 (mean age control group = 71 (67 to 76 years), invervention group = 70 (64 to 75 years)), Male 31:23 Female | |
| Interventions | Participants received either a traditional end colostomy (n = 21) or had a Vypro mesh placed dorsal to the rectus abdominis muscle and anterior to the posterior rectal sheath (n = 15). | |
| Outcomes | Development of parastomal hernia at 5 years from index surgery by clinical examination | |
| Length of Follow Up | Mean 65.2 months (range 57 to 83 months) | |
| Notes | Mortality data from Jänes 2009 were only available for a 12‐month follow‐up (Jänes 2004a), therefore subsequent data may not be relevant in the assessment of this review. 6 participants from the control group and 12 from the intervention group lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: Participants were randomised using consecutively numbered, sealed envelopes. No random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered sealed envelopes" Comment: Probably occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were examined after 1 month, 12 months, and 5 years by an investigator blinded to the actual randomisation. They were then examined straining in both an erect and a supine position." Comment: Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Those lost to follow‐up were accounted for. |
| Selective reporting (reporting bias) | Low risk | We identified no selective reporting. |
| Early Stopping | Low risk | Comment: Sample size calculation was included. Trial was stopped early on ethical grounds due to statistical testing favouring the mesh. |
Lambrecht 2015.
| Methods | 2‐centre randomised trial | |
| Participants | 58 (mean age control group = 63 +/‐ 4.1 years, intervention group = 64 +/‐ 4.0 years), Male 43:15 Female | |
| Interventions | Placement of retromuscular synthetic mesh vs no mesh at the time of end‐colostomy formation in people with rectal cancer undergoing open pelvic surgery | |
| Outcomes | Primary outcomes were incidence of parastomal hernia detected by clinical examination and routine CT. Secondary outcomes were stoma complications and reoperation rates. | |
| Length of Follow Up | Median follow‐up of the study was 40 months (range 3 to 87 months), data at 12 months sought but not recieved from the authors. | |
| Notes | 18 participants, 6 and 12 in the control and intervention groups respectively, died during follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation was completed in blocks of 6 and sealed in numbered envelopes. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed prior to surgery via sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of the surgeon performing the surgery was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of the examining doctor at follow‐up appointments was not stated, although the reporting radiologist assessing participant CT scans was blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Median follow‐up is reasonable at 40 months. No participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Method of hernia diagnosis changed from clinical diagnosis to radiological during the study period. |
| Early Stopping | Low risk | The sample size calculation is included, and there is no evidence of early stopping. |
Lopez‐Cano 2012.
| Methods | Randomised trial. Computerised randomisation | |
| Participants | 36 (mean age control group = 66 +/‐ 13.9 years, intervention group = 72 +/‐ 7.6 years), Male 18:18 Female | |
| Interventions | Participants were randomised into a group undergoing placement of a large‐pore lightweight mesh in the intraperitoneal/onlay position at the time of surgery (n = 19) or to a control group (no mesh) (n = 17). | |
| Outcomes | Incidence of parastomal hernia at 12 months (CT detected) and subcutaneous fat thickness at 12 months (CT measured) | |
| Length of Follow Up | 12 months | |
| Notes | 1 participant from the control group and 1 participant from the intervention group were lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed prior to surgery. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The reporting radiologist assessing participant CT scans was blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 of the 44 randomised participants were not included in the final analysis. Comment: Clear explanation of incomplete follow‐up data |
| Selective reporting (reporting bias) | Low risk | We identified no selective reporting. |
| Early Stopping | Low risk | Comment: Sample size calculation included. No evidence of early stopping |
López‐Cano 2016.
| Methods | Randomised trial. Computerised randomisation | |
| Participants | 52 (mean age control group = 67.3 +/‐ 13.6 years, intervention group = 70.5 +/‐ 9.5 years), Male 37:11 Female | |
| Interventions | Participants were randomised into a group undergoing placement of a flexible composite prosthetic mesh with a modified Sugarbaker technique at the time of surgery (n = 24) or to a control group (no mesh) (n = 28). | |
| Outcomes | Incidence of parastomal hernia at 12 months (CT detected) | |
| Length of Follow Up | Median follow‐up of the study was 26 months (range 13 to 38 months). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed prior to surgery. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The reporting radiologist assessing participant CT scans was blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We identified no selective reporting. |
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of reporting bias |
| Early Stopping | Low risk | Comment: No evidence of early stopping |
Odensten 2017.
| Methods | Randomised trial. Envelope randomisation | |
| Participants | 232 (mean age control group = 65.9 +/‐ 15.6 years, intervention group = 66.6 +/‐ 12.9 years), Male 135:97 Female | |
| Interventions | Conventional end colostomy (n = 118) vs end colostomy plus a lightweight polypropylene mesh (n = 114) | |
| Outcomes | Incidence of parastomal hernia at 12 months (CT and clinically detected). Early and late complications | |
| Length of Follow Up | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes prepared by an outside institution |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed prior to surgery. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical postoperative assessment made by a surgeon not involved in the primary procedure. It was not clear if the reporting radiologists were also blinded to the procedure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Transparent reporting of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of reporting bias |
| Early Stopping | Low risk | Comment: No evidence of early stopping |
Serra‐Aracil 2009.
| Methods | Randomised trial. Envelope randomisation | |
| Participants | 54 (mean age control group = 67.2 +/‐ 9.7 years, intervention group = 67.5 +/‐ 8.8 years), Male 35:13 Female | |
| Interventions | Conventional end colostomy (n = 27) vs end colostomy plus a lightweight mesh (Ultrapro) (n = 27) | |
| Outcomes | Incidence of parastomal hernia, mortality, wound infection, colostomy necrosis, mesh intolerance, need for re‐intervention. The presence of a parastomal hernia was determined by a CT scan. | |
| Length of Follow Up | Median 29 months (range 13 to 49 months) | |
| Notes | Data from 12‐month follow‐up sought and received from author. No participants reported lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information given. |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelope technique" Comment: Probably occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Abdominal CT controls were performed every 6 months by a radiologist blind to the technique used to identify possible subclinical PH." Comment: Radiological examination of PH has less risk of detection bias than clinical examination. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of reporting bias |
| Early Stopping | Low risk | Comment: Sample size calculation included. No evidence of early stopping |
Vierimaa 2015.
| Methods | Prospective, multicentre, randomised controlled clinical trial | |
| Participants | 70 (mean age control group = 65.1 +/‐ 11.7 years, intervention group = 67.1 +/‐ 10.7 years), Male 37:33 Female | |
| Interventions | Placement of a dual component, intraperitoneal onlay mesh vs no mesh | |
| Outcomes | Incidence of clinically and radiologically detected parastomal hernia and their extent 12 months after surgery. Stoma‐related morbidity and need for surgical repair | |
| Length of Follow Up | Maximum 12 months | |
| Notes | 12‐month outcome data published within study. 2 participants from the control group and 2 participants from the intervention group were lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised allocation |
| Allocation concealment (selection bias) | Low risk | Quote: "The operating surgeon was informed regarding the treatment allocation in the operating room before starting the operation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not possible to blind the surgeon as to which procedure was being performed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of examining doctor at follow‐up, as well as radiologist assessing 12‐month CT scan was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on 13 of the 83 randomised participants were not available for analysis. Comment: Clear explanation for incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of reporting bias |
| Early Stopping | Low risk | Comment: Sample size calculation included. No evidence of early stopping |
CT: computed tomography PH: parastomal hernia SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Craft 2008 | This is not a randomised controlled trial and does not evaluate the prevention of parastomal hernia. |
| Deol 2003 | This is a paper on the repair, not prevention of, parastomal hernias. There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
| Guzman‐Valdivia 2008 | This is a paper on the repair of parastomal hernia. There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
| Halabi 2013 | This paper is on the repair, not prevention of, parastomal hernias. This is not a randomised controlled trial. |
| Hamada 2012 | This paper compares rates of parastomal herniation in end colostomies in totally extraperitoneal vs transabdominal pre‐peritoneal approach to stomas formation. There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
| Hansson 2007 | This is a paper on repair of parastomal hernia. This is not a randomised controlled trial. |
| Hansson 2009 | This is a paper on the repair, not prevention of, parastomal hernias. There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
| Hansson 2012 | There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
| Hiles 2009 | This small systematic review demonstrated some evidence for the use of biological mesh in the repair of parastomal hernia, but it is not a randomised controlled trial. |
| Lee 2014 | This is not a randomised controlled trial on the prevention of parastomal hernias. |
| Linn 2010 | This paper evaluates the complications of parastomal hernia repair. This is not a randomised controlled trial. |
| Mancini 2007 | This is a case series of parastomal hernia repairs. There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
| Shah 2013 | A collection of different techniques for repair of parastomal hernias and their outcomes. This is not a randomised controlled trial. |
| Smart 2011 | A review of methods of parastomal hernia repair and outcomes. There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
| Stephenson 2010 | This paper evaluates the repair of parastomal hernia. There is no evidence for the prevention of parastomal herniation. This is not a randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
Correa 2014.
| Trial name or title | Stoma‐Const |
| Methods | Scandinavian RCT comparing mesh repair of fascia with 2 surgical/anatomical methods to prevent parastomal hernia formation |
| Participants | 240 |
| Interventions | N/A |
| Outcomes | N/A |
| Starting date | Ongoing |
| Contact information | eva.angenete@vgregion.se |
| Notes |
NCT01694238. 24 September 2012 www.trialsjournal.com/content/pdf/1745‐6215‐15‐254.pdf |
Demartines 2017.
| Trial name or title | Stomaplasty ring (Koring) for prevention of parastomal hernia (StoKo) |
| Methods | Primary outcome measures: parastomal hernia rate [ Time Frame: at 12 months ] [ Designated as safety issue: No ] Evaluated by abdominal CT and clinical examination, number of participants with parastomal hernia |
| Participants | N/A |
| Interventions | N/A |
| Outcomes | N/A |
| Starting date | Not yet recruiting |
| Contact information | dieter.hahnloser@chuv.ch |
| Notes | none |
Garcia‐Urena 2017.
| Trial name or title | Prophylactic mesh to reduce the incidence of ventral hernia |
| Methods | Spanish RCT comparing prophylactic midline mesh vs no prophylactic mesh cover for emergency or elective colorectal surgery |
| Participants | 112 |
| Interventions | N/A |
| Outcomes | N/A |
| Starting date | June 2009 |
| Contact information | N/A |
| Notes | NCT01788826 |
Harb‐de la Rosa 2017.
| Trial name or title | Prevention of parastomal hernia by mesh placement |
| Methods | Primary outcome measures: reduction in the incidence of parastomal hernia [ Time Frame: 18 months ] [ Designated as safety issue: No ] Assessed by physical examination |
| Participants | N/A |
| Interventions | N/A |
| Outcomes | N/A |
| Starting date | N/A |
| Contact information | axh311@med.miami.edu |
| Notes | none |
Prudhomme 2017.
| Trial name or title | Primary prevention of peristomal hernias via parietal prostheses (GRECCAR 07) |
| Methods | Presence/absence of a peristomal hernia [ Time Frame: 24 months ] |
| Participants | N/A |
| Interventions | N/A |
| Outcomes | N/A |
| Starting date | Recruiting |
| Contact information | michel.prudhomme@chu‐nimes.fr |
| Notes | NCT01380860 |
Tabusa 2018.
| Trial name or title | The CIPHER study: UK Cohort study to Investigate the prevention of Parastomal Hernia |
| Methods | Observational; Design type: Cohort study |
| Participants | N/A |
| Interventions | N/A |
| Outcomes | N/A |
| Starting date | Recruiting |
| Contact information | cipher‐study@bristol.ac.uk |
| Notes | ISRCTN17573805 |
Uyanik 2017.
| Trial name or title | Role of Prosthetic Mesh in Preventing Parastomal Hernias (RPMPPH) |
| Methods | Compare the incidence of parastomal hernias between groups [ Time Frame: During the monitoring period of 1 year ] [ Designated as safety issue: Yes ] Monitoring will be realised with clinical controls (after 15 days and 2, 6, 12 months) and with an abdominal CT in the first year. The expected result is the statistically significant reduction in the incidence of parastomal hernias in people undergoing elective laparoscopy‐assisted colorectal surgery. |
| Participants | N/A |
| Interventions | N/A |
| Outcomes | N/A |
| Starting date | Recruiting |
| Contact information | ouyanik@santpau.cat |
| Notes | none |
CT: computed tomography
N/A: not available
RCT: randomised controlled trial
Differences between protocol and review
We removed the incidence of parastomal herniation in different types of ostomies (e.g. ileostomy versus colostomy) as a secondary objective and as a secondary outcome from the main review. The authors believed that this was not relevant in determining the effectiveness of this intervention.
Contributions of authors
HGJ, MR, and JC developed the search strategies and screened the titles and abstracts of all studies identified by the search, overseen by PC and PB. HGJ, MR, and JC designed the data extraction form and extracted full texts of the included studies. HGJ, OA, and JB checked the extracted data for accuracy against the trial reports. HGJ and BC carried out the statistical analyses. HGJ and MR drafted the full review, overseen and edited by PC, while all authors added valuable comments and participated in the revision of the draft review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
The review authors declare that all analyses and interpretations reflect their opinions; no company was involved in the analysis or interpretation of data, or in the writing of this systematic review.
Deceased
New
References
References to studies included in this review
Brandsma 2017 {published data only}
- Brandsma HT, Hansson BM, Aufenacker TJ, Geldere D, Lammeren FMV, Mahabier C, et al. Prophylactic mesh placement during formation of an end‐colostomy reduces the rate of parastomal hernia: short‐term results of the Dutch PREVENT‐trial. Annals of Surgery 2017;265(4):663‐9. [DOI] [PubMed] [Google Scholar]
- Brandsma HT, Hansson BM, Aufenacker TJ, Geldere D, Lammeren FM, Mahabier C, et al. Prophylactic mesh placement to prevent parastomal hernia, early results of a prospective multicentre randomized trial. Hernia 2016;20(4):535‐41. [DOI] [PubMed] [Google Scholar]
Fleshman 2013 {published data only}
- Fleshman JW, Beck DE, Hyman N, Wexner SD, Bauer J, George V. A prospective, multicenter, randomized, controlled study of non‐cross‐linked porcine acellular dermal matrix fascial sublay for parastomal reinforcement in patients undergoing surgery for permanent abdominal wall ostomies. Diseases of the Colon & Rectum 2014; Vol. 57, issue 5:623‐31. [DOI] [PubMed]
Hammond 2008 {published data only}
- Hammond T, Huang A, Prosser K, Frye J, Williams N. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia 2008;12(5):475‐81. [DOI] [PubMed] [Google Scholar]
Jänes 2009 {published data only}
- Jänes A, Cengiz Y, Israelsson L. A randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. British Journal of Surgery 2004;91(3):280‐2. [DOI] [PubMed] [Google Scholar]
- Jänes A, Cengiz Y, Israelsson L. Preventing parastomal hernia with a prosthetic mesh: a 5‐year follow‐up of a randomized study. World Journal of Surgery 2009;33(1):118‐23. [DOI] [PubMed] [Google Scholar]
Lambrecht 2015 {published data only}
- Lambrecht JR, Larsen SG, Reiertsen O, Vaktskjold A, Julsrud L, Flatmark K. Prophylactic mesh at end‐colostomy construction reduces parastomal hernia rate: a randomized trial. Colorectal Disease 2015;17:O191‐7. [DOI] [PubMed] [Google Scholar]
Lopez‐Cano 2012 {published data only}
- Lopez‐Cano M, Lozoya‐Trujillo R, Quiroga S, Sanchez J, Vallribera F, Mart M, et al. Use of a prosthetic mesh to prevent parastomal hernia during laparoscopic abdominoperineal resection: a randomized controlled trial. Hernia 2012;16(6):661‐7. [DOI] [PubMed] [Google Scholar]
López‐Cano 2016 {published data only}
- López‐Cano M, Serra‐Aracils X, Mora L, Sánchez‐García JL, Jiménez‐Gómez LM, Martí M, et al. Preventing parastomal hernia using a modified Sugarbaker technique with composite mesh during laparoscopic abdominoperineal resection: a randomized controlled trial. Annals of Surgery 2016;264(6):923‐8. [DOI] [PubMed] [Google Scholar]
Odensten 2017 {published data only}
- Odensten C, Strigård K, Rutegård J, Dahlberg M, Ståhle U, Gunnarsson U, et al. Use of prophylactic mesh when creating a colostomy does not prevent parastomal hernia: a randomized controlled trial ‐ STOMAMESH. Annals of Surgery 2017 Oct 23 [Epub ahead of print]. [DOI: 10.1097/SLA.0000000000002542] [DOI] [PMC free article] [PubMed]
Serra‐Aracil 2009 {published data only}
- Serra‐Aracil X, Bombardo‐Junca J, Moreno‐Matias J, Darnell A, Mora‐Lopez L, Alcantara‐Moral M, et al. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Annals of Surgery 2009;249(4):583‐7. [DOI] [PubMed] [Google Scholar]
Vierimaa 2015 {published data only}
- Vierimaa M, Klintrup K, Biancari F, Victorzon M, Carpelan‐Holmstrom M, Kossi J, et al. Prospective, randomized study on the use of a prosthetic mesh for prevention of parastomal hernia of permanent colostomy. Diseases of the Colon & Rectum 2015;58:943‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Craft 2008 {published data only}
- Craft RO, Huguet KL, McLemore EC, Harold KL. Laparoscopic parastomal hernia repair. Hernia 2008;12(2):137‐40. [DOI] [PubMed] [Google Scholar]
Deol 2003 {published data only}
- Deol ZK, Shayani V. Laparoscopic parastomal hernia repair. Archives of Surgery 2003;138(2):203‐5. [DOI] [PubMed] [Google Scholar]
Guzman‐Valdivia 2008 {published data only}
- Guzman‐Valdivia G, Guerrero TS, Laurrabaquio HV. Parastomal hernia repair using mesh and an open technique. World Journal of Surgery 2008;32(3):465‐70. [DOI] [PubMed] [Google Scholar]
Halabi 2013 {published data only}
- Halabi WJ, Jafari MD, Carmichael JC, Nguyen VQ, Mills S, Phelan M, et al. Laparoscopic versus open repair of parastomal hernias: an ACS‐NSQIP analysis of short‐term outcomes. Surgical Endoscopy 2013;27(11):4067‐72. [DOI] [PubMed] [Google Scholar]
Hamada 2012 {published data only}
- Hamada M, Ozaki K, Muraoka G, Kawakita N, Nishioka Y. Permanent end‐sigmoid colostomy through the extraperitoneal route prevents parastomal hernia after laparoscopic abdominoperineal resection. Diseases of Colon & Rectum 2012;55(9):963‐9. [DOI] [PubMed] [Google Scholar]
Hansson 2007 {published data only}
- Hansson BM, Hingh IH, Bleichrodt RP. Laparoscopic parastomal hernia repair is feasible and safe: early results of a prospective clinical study including 55 consecutive patients. Surgical Endoscopy 2007;21(6):989‐93. [DOI] [PubMed] [Google Scholar]
Hansson 2009 {published data only}
- Hansson BM, Bleichrodt RP, Hingh IH. Laparoscopic parastomal hernia repair using a keyhole technique results in a high recurrence rate. Surgical Endoscopy 2009;23(7):1456‐9. [DOI] [PubMed] [Google Scholar]
Hansson 2012 {published data only}
- Hansson BM, Slater NJ, Velden AS, Groenewoud HMM, Buyne OR, Hingh IH, et al. Surgical techniques for parastomal hernia repair: a systematic review of the literature. Annals of Surgery 2012;255(4):685‐95. [DOI] [PubMed] [Google Scholar]
Hiles 2009 {published data only}
- Hiles M, Record RRD, Altizer AM. Are biologic grafts effective for hernia repair?: a systematic review of the literature. Surgical Innovation 2009;16(1):26‐37. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee L, Saleem A, Landry T, Latimer E, Chaudhury P, Feldman LS. Cost effectiveness of mesh prophylaxis to prevent parastomal hernia in patients undergoing permanent colostomy for rectal cancer. Journal of the American College of Surgeons 2014;218(1):82‐91. [DOI] [PubMed] [Google Scholar]
Linn 2010 {published data only}
- Linn JG, Mikami DJ. Parastomal hernia repair. Journal of Long‐Term Effects of Medical Implants 2010;20(2):133‐8. [DOI] [PubMed] [Google Scholar]
Mancini 2007 {published data only}
- Mancini GJ, McClusky DA, Khaitan L, Goldenberg EA, Heniford BT, Novitsky YW, et al. Laparoscopic parastomal hernia repair using a nonslit mesh technique. Surgical Endoscopy 2007;21(9):1487‐91. [DOI] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah NR, Craft RO, Harold KL. Parastomal hernia repair. Surgical Clinics of North America 2013;93(5):1185‐98. [DOI] [PubMed] [Google Scholar]
Smart 2011 {published data only}
- Smart NJ, Velineni R, Khan D, Daniels IR. Parastomal hernia repair outcomes in relation to stoma site with diisocyanate cross‐linked acellular porcine dermal collagen mesh. Hernia 2011;15(4):433‐7. [DOI] [PubMed] [Google Scholar]
Stephenson 2010 {published data only}
- Stephenson BM, Evans MD, Hilton J, McKain ES, Williams GL. Minimal anatomical disruption in stoma formation: the lateral rectus abdominis positioned stoma (LRAPS). Colorectal Disease 2010;12(10):1049‐52. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Correa 2014 {unpublished data only}
- Correa MA, Erestam S, Haglind E, Ekelund J, Angerås U, Rosenberg J, et al. Stoma‐Const ‐ the technical aspects of stoma construction: study protocol for a randomised controlled trial. Trials 2014; Vol. 15:254. [DOI: 10.1186/1745-6215-15-254] [DOI] [PMC free article] [PubMed]
Demartines 2017 {unpublished data only}
- NCT02489175. Stomaplasty Ring (KoringTM) for Prevention of Parastomal Hernia (StoKo) [International Prospective Randomized Multicenter Trial to Analyze the Stomaplasty Ring (KoringTM) for Prevention of Parastomal Hernia]. clinicaltrials.gov/ct2/show/record/NCT02489175 (First received 17 June 2015).
Garcia‐Urena 2017 {unpublished data only}
- NCT01788826. Prophylactic Mesh to Reduce The Incidence of Ventral Hernia [Randomized Clinical Trial to Evaluate the Use of a Prophylactic Polypropylene Mesh To Reduce the Incidence of Ventral Hernias in Colorectal Surgery]. clinicaltrials.gov/ct2/show/record/NCT01788826 (First received 11 February 2013).
Harb‐de la Rosa 2017 {unpublished data only}
- NCT02404545. Prevention of Parastomal Hernia by Mesh Placement [A Randomized Study of the Utility of Composite Mesh Placement to Prevent Parastomal Hernia in Patients Undergoing Urinary Diversion With Ileal Conduit]. clinicaltrials.gov/ct2/show/record/NCT02404545 (First received 26 March 2015).
Prudhomme 2017 {unpublished data only}
- NCT01380860. Primary Prevention of Peristomial Hernias Via Parietal Prostheses [Primary Prevention of Peristomial Hernias Via Parietal Prostheses: a Randomized, Multicentric Study]. clinicaltrials.gov/ct2/show/record/NCT01380860 (First received 22 June 2011).
Tabusa 2018 {unpublished data only}
Uyanik 2017 {unpublished data only}
- NCT01955278. Role of Prosthetic Mesh in Preventing Parastomal Hernias [Prospective Randomized Study of the Role of Prosthetic Mesh in Preventing Parastomal Hernias in Patients With Definitive End Colostomy]. clinicaltrials.gov/ct2/show/record/NCT01955278 (First received 11 September 2013).
Additional references
Amin 2001
- Amin SN, Armitage NC, Abercrombie JF, Scholefield JH. Lateral repair of parastomal hernia. Annals of the Royal College of Surgeons of England 2001;83(3):206‐8. [PMC free article] [PubMed] [Google Scholar]
Arumugam 2003
Bayer 1986
- Bayer I, Kyzer S, Chaimoff CH. A new approach to primary strengthening of colostomy with Marlex mesh to prevent paracolostomy hernia. Surgery, Gynecology & Obstetrics 1986;163(6):579‐80. [PubMed] [Google Scholar]
Berger 2008
- Berger D. Prevention of parastomal hernias by prophylactic use of a specially designed intraperitoneal onlay mesh (Dynamesh IPST). Hernia 2008;12(3):243‐6. [DOI] [PubMed] [Google Scholar]
Blake 2017
- Blake PA, Williams GL, Stephenson BM. Prophylactic mesh to prevent parastomal hernia: further questions need answering. Techniques in Coloproctology 2017;21:481. [DOI] [PubMed] [Google Scholar]
Burns 1970
- Burns FJ. Complications of colostomy. Diseases of the Colon & Rectum 1970;13(6):448‐50. [DOI] [PubMed] [Google Scholar]
Carne 2003
- Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. British Journal of Surgery 2003;90(7):784‐93. [DOI] [PubMed] [Google Scholar]
Cross 2017
- Cross A, Buchwald P, Frizelle F, Eglinton T. Meta‐analysis of prophylactic mesh to prevent parastomal hernia. British Journal of Surgery 2017;104:179‐86. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dindo 2004
- Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eldrup 1982
- Eldrup J, Wied U, Bischoff N, Pedersen VM. Post‐colostomy hernias. Incidence and relation to placing of the stoma. Ugeskrift for Læger 1982;144(50):3742‐3. [PubMed] [Google Scholar]
Ellis 2010
- Ellis CN. Short‐term outcomes with the use of bioprosthetics for the management of parastomal hernias. Diseases of the Colon & Rectum 2010;53(3):279‐83. [DOI] [PubMed] [Google Scholar]
Fortelny 2015
- Fortelny RH, Hofmann A, May C, Köckerling F, BioMesh Study Group. Prevention of a parastomal hernia by biological mesh reinforcement. Frontiers in Surgery 2015;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2010
- Goldberg M, Aukett LK, Carmel J, Fellows J, Pittman J. Management of the patient with a fecal ostomy: best practice guideline for clinicians. Journal of Wound, Ostomy and Continence Nursing 2010;37(6):596‐8. [DOI] [PubMed] [Google Scholar]
Gooszen 2000
- Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG. Quality of life with a temporary stoma. Diseases of the Colon & Rectum 2000;43(5):650‐5. [DOI] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Gögenur 2006
- Gögenur I, Mortensen J, Harvald T, Rosenberg J, Fischer A. Prevention of parastomal hernia by placement of a polypropylene mesh at the primary operation. Diseases of the Colon & Rectum 2006;49(8):1131‐5. [DOI] [PubMed] [Google Scholar]
Harris 2005
- Harris DA, Egbeare D, Jones S, Benjamin H, Woodward A, Foster ME. Complications and mortality following stoma formation. Annals of the Royal College of Surgeons England 2005;87(6):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helgstrand 2008
- Helgstrand F, Gögenur I, Rosenberg J. Prevention of parastomal hernia by the placement of a mesh at the primary operation. Hernia 2008;12(6):577‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Israelsson 2005
- Israelsson LA. Preventing and treating parastomal hernia. World Journal of Surgery 2005;29(8):1086‐9. [DOI] [PubMed] [Google Scholar]
Israelsson 2008
- Israelsson LA. Parastomal hernias. Surgical Clinics of North America 2008;88(1):113‐25, ix. [DOI] [PubMed] [Google Scholar]
Jänes 2004a
- Jänes A, Cengiz Y, Israelsson LA. Randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. British Journal of Surgery 2004;91(3):280‐2. [DOI] [PubMed] [Google Scholar]
Jänes 2004b
- Jänes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh. Archives of Surgery 2004;139(12):1356‐8. [DOI] [PubMed] [Google Scholar]
Kronborg 1974
- Kronborg O, Kramhoft J, Backer O, Sprechler M. Late complications following operations for cancer of the rectum and anus. Diseases of the Colon & Rectum 1974;17(6):750‐3. [DOI] [PubMed] [Google Scholar]
Londono‐Schimmer 1994
- Londono‐Schimmer EE, Leong AP, Phillips RK. Life table analysis of stomal complications following colostomy. Diseases of the Colon & Rectum 1994;37(9):916‐20. [DOI] [PubMed] [Google Scholar]
Lynne 1974
- Lynne CM, Politano VA, Cohen RL. Parastomal hernia causing anuria: unusual complication of ileal conduit diversion. Urology 1974;4(5):603‐4. [DOI] [PubMed] [Google Scholar]
López‐Cano 2009
- López‐Cano M, Lozoya‐Trujillo R, Espin‐Basany E. Prosthetic mesh in parastomal hernia prevention. Laparoscopic approach. Diseases of the Colon & Rectum 2009;52(5):1006‐7. [DOI] [PubMed] [Google Scholar]
López‐Cano 2017
- López‐Cano M, Brandsma HT, Bury K, Hansson B, Kyle‐Leinhase I, Alamino JG, et al. Prophylactic mesh to prevent parastomal hernia after end colostomy: a meta‐analysis and trial sequential analysis. Hernia 2017;21:177‐89. [DOI] [PubMed] [Google Scholar]
Marimuthu 2006
- Marimuthu K, Vijayasekar C, Ghosh D, Mathew G. Prevention of parastomal hernia using preperitoneal mesh: a prospective observational study. Colorectal Diseases 2006;8(8):672‐5. [DOI] [PubMed] [Google Scholar]
Pastor 2009
- Pastor DM, Pauli EM, Koltun WA, Haluck RS, Shope TR, Poritz LS. Parastomal hernia repair: a single centre experience. Journal of the Society of Laparoendoscopic Surgeons 2009;13(2):170‐5. [PMC free article] [PubMed] [Google Scholar]
Patel 2016
- Patel SV, Zhang L, Chadi SA, Wexner SD. Prophylactic mesh to prevent parastomal hernia: a meta‐analysis of randomized controlled studies. Techniques in Coloproctology 2016;1:1‐9. [DOI] [PubMed] [Google Scholar]
Patel 2017
- Patel S, Zhang L, Chadi S, Wexner S. Prophylactic mesh to prevent parastomal hernia: a meta‐analysis of randomized controlled studies. Techniques in Coloproctology 2017;21:5‐13. [DOI] [PubMed] [Google Scholar]
Pearl 1989
- Pearl RK. Parastomal hernias. World Journal of Surgery 1989;13(5):569‐72. [DOI] [PubMed] [Google Scholar]
Pianka 2017
- Pianka F, Probst P, Keller A, Saure D, Grummich K, Buchler M, et al. Prophylactic mesh placement for the PREvention of paraSTOmal hernias: The PRESTO systematic review and meta‐analysis. PLOS ONE 2017;9:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilgrim 2010
- Pilgrim CH, McIntyre R, Bailey M. Prospective audit of parastomal hernia: prevalence and associated co‐morbidities. Diseases of the Colon & Rectum 2010;53(1):71‐6. [DOI] [PubMed] [Google Scholar]
Prieto 2005
- Prieto L, Thorsen H, Juul K. Development and validation of a quality of life questionnaire for patients with colostomy or ileostomy. Health and Quality of Life Outcomes 2005;3(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubin 1994
- Rubin MS, Schoetz DJ Jr, Matthews JB. Parastomal hernia. Is stoma relocation superior to fascial repair?. Archives of Surgery 1994;129(4):413‐8; discussion 418‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shabbir 2010
- Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Disease 2010;12(10):958‐64. [DOI] [PubMed] [Google Scholar]
Shabbir 2012
- Shabbir J, Chaudhary BN, Dawson R. A systematic review on the use of prophylactic mesh during primary stoma formation to prevent parastomal hernia formation. Colorectal Disease 2012;14(8):931‐6. [DOI] [PubMed] [Google Scholar]
Sier 2015
- Sier MF, Gelder L, Ubbink DT, Bemelman WA, Oostenbroek RJ. Factors affecting timing of closure and non‐reversal of temporary ileostomies. International Journal of Colorectal Disease 2015;30(9):1185‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sjodahl 1988
- Sjodahl R, Anderberg B, Bolin T. Parastomal hernia in relation to site of the abdominal stoma. British Journal of Surgery 1988;75(4):339‐41. [DOI] [PubMed] [Google Scholar]
Steele 2003
- Steele SR, Lee P, Martin MJ, Mullenix PS, Sullivan ES. Is parastomal hernia repair with polypropylene mesh safe?. American Journal of Surgery 2003;185(5):436‐40. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tam 2010
- Tam KW, Wei PL, Kuo LJ, Wu CH. Systematic review of the use of a mesh to prevent parastomal hernia. World Journal of Surgery 2010;34(11):2723‐9. [DOI] [PubMed] [Google Scholar]
Tekkis 1999
- Tekkis PP, Kocher HM, Payne JG. Parastomal hernia repair: modified Thorlakson technique, reinforced by polypropylene mesh. Diseases of the Colon & Rectum 1999;42(11):1505‐8. [DOI] [PubMed] [Google Scholar]
Vijayasekar 2008
- Vijayasekar C, Marimuthu K, Jadhav V, Mathew G. Parastomal hernia: is prevention better than cure? Use of preperitoneal polypropylene mesh at the time of stoma formation. Techniques in Coloproctology 2008;12(4):309‐13. [DOI] [PubMed] [Google Scholar]
Wara 2011
- Wara P, Andersen LM. Long‐term follow‐up of laparoscopic repair of parastomal hernia using a bilayer mesh with a slit. Surgical Endoscopy 2011;25(2):526‐30. [DOI] [PubMed] [Google Scholar]
Wijeyekoon 2010
- Wijeyekoon SP, Gurusamy K, El‐Gendy K, Chan CL. Prevention of parastomal herniation with biologic/composite prosthetic mesh: a systematic review and meta‐analysis of randomized controlled trials. Journal of the American College of Surgeons 2010;211(5):637‐45. [DOI] [PubMed] [Google Scholar]
Williams 1990
- Williams JG, Etherington R, Hayward MW, Hughes LE. Paraileostomy hernia: a clinical and radiological study. British Journal of Surgery 1990;77(12):1355‐7. [DOI] [PubMed] [Google Scholar]


