Abstract
Background
Around 30% of people who are admitted to hospital with aneurysmal subarachnoid haemorrhage (SAH) will rebleed in the initial month after the haemorrhage if the aneurysm is not treated. The two most commonly used methods to occlude the aneurysm for prevention of rebleeding are microsurgical clipping of the neck of the aneurysm and occlusion of the lumen of the aneurysm by means of endovascular coiling. This is an update of a systematic review that was previously published in 2005.
Objectives
To compare the effects of endovascular coiling versus neurosurgical clipping in people with aneurysmal SAH on poor outcome, rebleeding, neurological deficit, and treatment complications.
Search methods
We searched the Cochrane Stroke Group Trials Register (March 2018). In addition, we searched CENTRAL (2018, Issue 2), MEDLINE (1966 to March 2018), Embase (1980 to March 2018), US National Institutes of Health Ongoing Trials Register (March 2018), and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (last searched March 2018). We also contacted trialists.
Selection criteria
We included randomised trials comparing endovascular coiling with neurosurgical clipping in people with SAH from a ruptured aneurysm.
Data collection and analysis
Two review authors independently extracted data, and assessed trial quality and risk of bias using the GRADE approach. We contacted trialists to obtain missing information. We defined poor outcome as death or dependence in daily activities (modified Rankin scale 3 to 6 or Glasgow Outcome Scale (GOS) 1 to 3). In the special worst‐case scenario analysis, we assumed all participants in the group with better outcome with missing follow‐up information had a poor outcome and those in the other group with missing data a good outcome.
Main results
We included four randomised trials involving 2458 participants (range per trial: 20 to 2143 participants). Evidence is mostly based on the largest trial. Most participants were in good clinical condition and had an aneurysm on the anterior circulation. None of the included trials was at low risk of bias in all domains. One trial was at unclear risk in one domain, two trials at unclear risk in three domains, and one trial at high risk in one domain.
After one year of follow‐up, 24% of participants randomised to endovascular treatment and 32% of participants randomised to the surgical treatment group had poor functional outcome. The risk ratio (RR) of poor outcome (death or dependency) for endovascular coiling versus neurosurgical clipping was 0.77 (95% confidence interval (CI) 0.67 to 0.87; 4 trials, 2429 participants, moderate‐quality evidence), and the absolute risk reduction was 7% (95% CI 4% to 11%). In the worst‐case scenario analysis for poor outcome, the RR for endovascular coiling versus neurosurgical clipping was 0.80 (95% CI 0.71 to 0.91), and the absolute risk reduction was 6% (95% CI 2% to 10%). The RR of death at 12 months was 0.80 (95% CI 0.63 to 1.02; 4 trials, 2429 participants, moderate‐quality evidence). In a subgroup analysis of participants with an anterior circulation aneurysm, the RR of poor outcome was 0.78 (95% CI 0.68 to 0.90; 2 trials, 2157 participants, moderate‐quality evidence), and the absolute risk decrease was 7% (95% CI 3% to 10%). In subgroup analysis of those with a posterior circulation aneurysm, the RR was 0.41 (95% CI 0.19 to 0.92; 2 trials, 69 participants, low‐quality evidence), and the absolute decrease in risk was 27% (95% CI 6% to 48%). At five years, 28% of participants randomised to endovascular treatment and 32% of participants randomised to surgical treatment had poor functional outcome. The RR of poor outcome for endovascular coiling versus neurosurgical clipping was 0.87 (95% CI 0.75 to 1.01, 1 trial, 1724 participants, low‐quality evidence). At 10 years, 35% participants allocated to endovascular and 43% participants allocated to surgical treatment had poor functional outcome. At 10 years RR of poor outcome for endovascular coiling versus neurosurgical clipping was 0.81 (95% CI 0.70 to 0.92; 1 trial, 1316 participants, low‐quality evidence). The RR of delayed cerebral ischaemia at two to three months for endovascular coiling versus neurosurgical clipping was 0.84 (95% CI 0.74 to 0.96; 4 trials, 2450 participants, moderate‐quality evidence). The RR of rebleeding for endovascular coiling versus neurosurgical clipping was 1.83 (95% CI 1.04 to 3.23; 4 trials, 2458 participants, high‐quality evidence) at one year, and 2.69 (95% CI 1.50 to 4.81; 1 trial, 1323 participants, low‐quality evidence) at 10 years. The RR of complications from intervention for endovascular coiling versus neurosurgical clipping was 1.05 (95% CI 0.44 to 2.53; 2 trials, 129 participants, low‐quality evidence).
Authors' conclusions
The evidence in this systematic review comes mainly from one large trial, and long‐term follow‐up is available only for a subgroup of participants within that trial. For people in good clinical condition with ruptured aneurysms of either the anterior or posterior circulation the data from randomised trials show that, if the aneurysm is considered suitable for both neurosurgical clipping and endovascular coiling, coiling is associated with a better outcome. There is no reliable trial evidence that can be used directly to guide treatment in people with a poor clinical condition.
Plain language summary
Endovascular coiling versus neurosurgical clipping for people with aneurysmal subarachnoid haemorrhage
Review question We reviewed the outcome after endovascular coiling compared with neurosurgical clipping after a subarachnoid haemorrhage.
Background Bleeding under the surface membrane of the brain is called a subarachnoid haemorrhage. The bleeding usually comes from the rupture of a weak spot in an artery carrying blood to the brain. This weak spot is like a small balloon, which is called an aneurysm. The outcome after subarachnoid haemorrhage from an aneurysm is generally poor: a third of all people die within three months, and one of every five people remains dependent on someone else for help with every day activities such as walking, dressing, bathing, and taking care of one's own affairs. One of the risks in people with subarachnoid haemorrhage is rebleeding. There are two ways to try and prevent this: neurosurgical clipping of the neck of the aneurysm in an operation or blocking the aneurysm from inside by endovascular coiling.
Study characteristics In March 2018, we searched for randomised controlled trials (RCTs, clinical studies where people are randomly put into one of two or more treatment groups) comparing endovascular coiling with neurosurgical clipping for subarachnoid haemorrhage. We found one new RCT and additional data for previously identified RCTs, allowing us to include four RCTs involving 2458 participants.
Key results The data from RCTs showed that the number of people who survived and were independent in their daily living was higher after endovascular coiling than after neurosurgical clipping, if both treatment options were possible. Risk of rebleeding was higher in people treated with endovascular coiling. The evidence came mainly from one large trial.
Quality of the evidence We judged that there is sufficient evidence to guide treatment for people in a relatively good condition whose aneurysm is considered suitable for both neurosurgical clipping and endovascular treatment. There is no reliable trial evidence that can be used directly to guide treatment in people with a poor clinical condition.
Summary of findings
Summary of findings for the main comparison. Endovascular coiling compared with neurosurgical clipping for subarachnoid haemorrhage.
| Endovascular coiling compared with neurosurgical clipping for subarachnoid haemorrhage | ||||||
|
Patient or population: people with subarachnoid haemorrhage from a ruptured intracranial aneurysm Settings: tertiary care Intervention: endovascular coiling of aneurysm Comparison: neurosurgical clipping of aneurysm | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Neurosurgical clipping | Endovascular coiling | |||||
| Poor outcome: death or dependence in daily activities (12 months) | Study population | RR 0.77 (0.67 to 0.87) | 2429 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 366 per 1000 | 281 per 1000 (245 to 318) | |||||
| Poor outcome (death or dependence) (10 years) | Study population | RR 0.81 (0.70 to 0.92) | 1316 (1 RCT) | ⊕⊕⊝⊝a,b Low | Based on subgroup of participants in 1 large RCT only | |
| 430 per 1000 | 348 per 1000 (301 to 395) | |||||
| Death from any cause (12 months) | Study population | RR 0.80 (0.63 to 1.02) | 2429 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 154 per 1000 | 123 per 1000 (97 to 157) | |||||
| Delayed cerebral ischaemia (2–3 months) | Study population | RR 0.84 (0.74 to 0.96) | 2450 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 384 per 1000 | 322 per 1000 (284 to 368 ) | |||||
| Rebleeding postprocedure up to 1 year | Study population | RR 1.83 (1.04 to 3.23) | 2458 (4 RCTs) | ⊕⊕⊕⊕ High | — | |
| 21 per 1000 | 38 per 1000 (21 to 67) | |||||
| Rebleeding postprocedure up to 10 years | Study population | RR 2.69 (1.50 to 4.81) | 1323 (1 RCT) | ⊕⊕⊝⊝a,b Low | Based on 1 large RCT only | |
| 22 per 1000 | 61 per 1000 (34 to 109) | |||||
| Complications from the intervention | Study population | RR 1.05 (0.44 to 2.53) | 129 (2 RCTs) | ⊕⊕⊝⊝c Low | Based on 2 small RCTs only | |
| 235 per 1000 |
246 per 1000 (103 to 594) |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived from the studies included in the meta‐analysis. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to indirectness of evidence: participants in poor condition on admission under‐represented in the largest RCT.
bDowngraded one level due to risk of bias: long‐term outcome data available for only a subgroup of participants.
cDowngraded two levels due to risk of bias: underpowered due to data availability from only two small trials, unclear definition of complication from intervention.
Background
This systematic review of randomised trials compared outcome after subarachnoid haemorrhage (SAH) for people treated with endovascular coiling versus neurosurgical clipping. It is the first update of a review published in 2005 (van der Schaaf 2005), which was preceded by a published protocol (Algra 2001).
Description of the condition
SAH is a subset of stroke with an incidence of around 9 per 100,000 people per year (de Rooij 2007). It occurs in relatively young people: half the people are younger than 55 years of age (de Rooij 2007), and it carries a poor prognosis. A third of all people die within three months of the haemorrhage, and one of every five people remains dependent on the care of others for activities of daily living (Nieuwkamp 2009). Because of the poor outcome after the haemorrhage and the young age at which it occurs, the loss of productive life years from SAH is as large as that from ischaemic stroke, which is the most common subset of stroke (Johnston 1998). In 85% of people with SAH, the cause is rupture of an intracranial aneurysm. About 15% of people with aneurysmal SAH die before reaching the hospital (Huang 2002). Those who survive the initial hours after the haemorrhage are at risk of rebleeding until the aneurysm is occluded. Although since the late 1990s the time delay to occlusion of the aneurysm has decreased considerably, around 15% of people still rebleed in hospital (Vergouwen 2016).
Description of the intervention
Both neurosurgical clipping and endovascular coiling are common methods for treating intracranial aneurysms. Neurosurgical clipping of an aneurysm requires opening of the skull (craniotomy). During the operation, a metal clip is placed over the neck of the aneurysm to occlude the blood flow to the aneurysm but to preserve it through the adjacent normal arteries. Endovascular treatment of a ruptured aneurysm is performed by advancing a catheter up to the parent artery of the aneurysm. Metal coils are then deposited into the aneurysm sac through a microcatheter arresting intra‐aneurysmal blood flow and inducing thrombus formation, which occludes the aneurysm. Other endovascular treatment techiques such as stent‐ or balloon‐assisted coiling are also being used, but we only studied simple coiling in this review.
How the intervention might work
The main target of the intervention is to occlude the blood flow to the ruptured aneurysm to prevent rebleeding. However, both neurosurgical clipping and endovascular coiling are associated with significant intervention‐related mortality and morbidity. Aneurysm clipping is a major neurosurgical operation necessitating craniotomy, but the treatment result is considered to be durable. Endovascular treatment is a less invasive intervention than neurosurgical clipping. Major concerns associated with endovascular coiling include incomplete obliteration of the aneurysm and durability of the treatment result over long‐term follow‐up.
Why it is important to do this review
Prospective randomised trials have examined the effects of endovascular coiling versus neurosurgical clipping in SAH. A previous version of this review only included short‐term and one‐year follow‐up data of the treatment effects (van der Schaaf 2005), and publication of long‐term follow‐up data necessitated the update of the review. This synthesis also includes assessment of evidence quality using the GRADE process.
Objectives
To compare the effects of endovascular coiling versus neurosurgical clipping in people with aneurysmal SAH on poor outcome, rebleeding, neurological deficit, and treatment complications.
Methods
Criteria for considering studies for this review
Types of studies
We sought all randomised trials that compared endovascular coiling of intracranial aneurysms versus neurosurgical clipping. We only included studies with adequate allocation concealment.
Types of participants
People with aneurysmal SAH in whom the haemorrhage was documented by either computed tomography (CT) scan or magnetic resonance imaging (MRI), or by the presence of xanthochromia in the cerebral spinal fluid in cases with a negative head CT; in whom the presence of an intracranial aneurysm had been demonstrated before randomisation by catheter angiography, CT angiography or magnetic resonance angiography; and whose aneurysm had been judged suitable for both neurosurgical clipping and endovascular coiling were included in the analysis. Initially we intended to exclude people who were treated more than 14 days after SAH. However, since the ISAT (International Subarachnoid Aneurysm Trial) trial included participants who were treated until 28 days after SAH, we changed this criterion to exclude people who were treated at more than 28 days after SAH (ISAT 2002).
Types of interventions
Endovascular treatment of ruptured intracranial aneurysm with detachable coils and neurosurgical clipping.
Types of outcome measures
To provide an intention‐to‐treat analysis, we aimed to extract from each trial the outcome at the end of the follow‐up period for all participants who were originally allocated to each treatment group.
Primary outcomes
Poor outcome: death or dependence in daily activities (defined as modified Rankin scale 3 to 6 or Glasgow Outcome Scale (GOS) 1 to 3).
Secondary outcomes
Death from any cause.
Delayed cerebral ischaemia (DCI), where episodes of clinical deterioration for which no other cause than DCI was found were considered probable ischaemia; episodes with clinical deterioration; and CT or MRI‐confirmed cerebral infarction were classified as definite ischaemia.
Rebleeding, where a sudden deterioration leading to death without confirmation of rebleeding by CT, MRI or postmortem examination was considered a probable rebleeding; a sudden clinical deterioration with rebleeding confirmed by CT or postmortem was classified as a definite rebleeding.
Complications from the intervention (endovascular coiling or neurosurgical clipping), defined as a clinical deterioration observed during the intervention procedure or within 24 hours after the intervention.
Search methods for identification of studies
See the 'Specialised register' section in the Cochrane Stroke Group module. We aimed to identify all relevant randomised trials regardless of language or publication status (published, unpublished, in press, or in progress) and arranged translation of relevant papers published in languages where necessary.
Electronic searches
We identified relevant trials in the Cochrane Stroke Group Trials Register, which was last searched by the Cochrane Stroke Information Specialist in March 2018. In addition, we searched the following electronic databases:
CENTRAL (2018, Issue 2) in the Cochrane Library (2018, Issue 2; Appendix 1);
MEDLINE Ovid (1966 to 26 March 2018; Appendix 2);
Embase Ovid (1980 to 26 March 2018; Appendix 3).
We used the search strategy developed for MEDLINE with the assistance of the Cochrane Stroke Group Information Specialist and modified it to suit other databases. We searched the following ongoing trials and research registers:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; last searched 27 March 2018; Appendix 4);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch; last searched 27 March 2018; Appendix 4).
Searching other resources
In an effort to identify additional relevant published and unpublished studies, we contacted trialists and scanned the reference list of all relevant publications
Data collection and analysis
Selection of studies
Two review authors (original version of the review: IvdS and GJER; updated version of the review: AL and MV) independently reviewed the studies identified by the search for their relevance using the selection criteria. We resolved disagreements through discussion.
Data extraction and management
Two review authors (original version of the review: IvdS and MV; updated version of the review: AL and MV) independently extracted details of method of randomisation, inclusion and exclusion criteria, blinding of outcome assessment, prognostic factors for outcome (clinical condition on admission, site and size of aneurysm, and time interval between SAH and treatment allocation), the definition of outcome measures, and the number of participants who were excluded or lost to follow‐up. Furthermore, we assessed whether intention‐to‐treat analysis was possible from the published data and if treatment groups were comparable with regard to major prognostic risk factors. In addition, we recorded duration of follow‐up, the numbers of deaths and participants with poor outcome (dependent in daily life) at the time points used by the trialists, the number of participants with DCI or rebleeding at the time points used by the trialists, complications from the intervention, and technical results of the intervention in terms of degree of obliteration of the aneurysm. Where there was disagreement, both review authors reassessed and discussed the article in question together until they reached consensus.
Assessment of risk of bias in included studies
Two review authors (AL and MV) independently assessed the risk of bias in the included studies according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using the Cochrane tool for assessing risk of bias. We assessed the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other relevant biases.
We judged each domain for included studies as low, unclear, or high risk and provided information from the study report or obtained straight from the trialists together with a justification for our judgement in the 'Risk of bias' tables. Where there was disagreement, both authors reassessed and discussed the article in question together until they reached consensus.
Measures of treatment effect
We analysed the primary and other outcomes according to the intention‐to‐treat principle. However, in one study it was unclear whether the published results represented intention‐to‐treat or treatment received, and so we performed a sensitivity analysis excluding this study (Li 2012). We calculated an estimate of the treatment effect across trials (risk ratio (RR) with 95% confidence interval (CI)) using standard methods for the main outcome measures. We also calculated absolute risk differences with 95% CI.
In addition, we assessed the number of participants with rebleeding from the target aneurysm at more than one year after the SAH (as a number per patient‐years of follow‐up).
We also assessed the results of treatment in terms of degree of occlusion of the aneurysm directly after treatment and at a follow‐up period of one year. We categorised occlusion after endosaccular packing as:
100% occlusion with coils filling the neck;
90% to 100% occlusion with neck remnant, and:
less than 90% occlusion, which included any contrast filling within the dome of the aneurysm.
If data on angiographic follow‐up were given for both endovascular and surgically treated participants, we compared the proportions of incompletely occluded aneurysms at the end of the follow‐up period.
Other prespecified analyses were:
timing of the intervention as early (within three days after onset of the SAH); intermediate (three to 10 days after onset of the SAH); or late (more than 10 days after the SAH);
timing of the follow‐up period, with trials categorised according to time of outcome assessment between one to three months; three to six months; and six to 12 months;
methodological quality of trials with:
exclusion of studies with insufficient information on inclusion and exclusion criteria;
exclusion of studies with insufficient data on method of randomisation;
exclusion of studies with insufficient data on blinding of outcome assessment;
exclusion of studies with insufficient data on the number of participants who were excluded or lost to follow‐up;
exclusion of studies with insufficient data on the definition of outcome events; and
-
exclusion of studies with insufficient data on the following prognostic factors:
clinical condition on admission;
site and size of the aneurysm; and
time interval between the SAH and treatment allocation.
For DCI and rebleeding, we performed separate analyses for the combination of probable and definite episodes and for definite episodes alone. We compared the number of participants with rebleeding per patient‐year of follow‐up for the period more than one year after the initial haemorrhage.
Unit of analysis issues
The individual participant was the unit of analysis.
Dealing with missing data
If any participants were excluded or lost to follow‐up from the analyses, or if any of the necessary data were not available from the publication, we sought further information by contacting the trialists. If the primary analysis suggested a beneficial effect of the main outcome but follow‐up was not complete because data were missing from participants excluded after randomisation or who were lost to follow‐up, we performed a special worst‐case scenario analysis in which participants in the group with better outcome with missing follow‐up information were assumed to have had a poor outcome and those in the other group with missing data a good outcome.
Assessment of heterogeneity
We assessed the statistical validity of aggregating the trials with Chi2 test statistics for heterogeneity and by calculating the I2 statistic. Substantial heterogeneity was defined as an I2 statistic greater than 50%. (Higgins 2011). We used the Peto method to calculate a weighted estimate of the treatment effects across trials (APT 1994).
Assessment of reporting biases
As we only included four studies in this review, we did not produce funnel plots to analyse reporting bias.
Data synthesis
Two review authors (AL and MV) extracted data, which one author (AL) compiled and entered into Review Manager 5 (Review Manager 2014). We calculated the RR and absolute risk difference using Review Manager 5, in accordance with Cochrane guidelines (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for aneurysm location (anterior versus posterior circulation, and a separate analysis for basilar artery aneurysms) and timing of the intervention. We used the I2 statistic to assess heterogeneity.
Sensitivity analysis
We planned a sensitivity analysis if it was unclear in any of the included studies whether an intention‐to‐treat analysis had been performed or if the analysis was based on treatment received. For the sensitivity analysis, we only included trials with an intention‐to‐treat analysis.
GRADE and 'Summary of findings' table
Two review authors (AL and MV) assessed the quality of the evidence generated from the review according to the GRADE approach. We presented the main results in a 'Summary of findings' table. We initially considered the quality of the evidence to be high because of the study design (randomised controlled trial). We subsequently downgraded this depending on whether there were study limitations; whether the results were inconsistent, imprecise, the evidence was indirect, or there was publication bias. The 'Summary of findings' table presented the following outcomes: poor outcome at 12 months, poor outcome at 10 years, death from any cause at 12 months, DCI at two to three months, rebleeding at one year, rebleeding at 10 years, and complications from the intervention.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
The summary of the search results is presented in a PRISMA study flow diagram (Figure 1). In total, we screened 2185 records. At the time of the original review, we included three RCTs (Brilstra 2000; ISAT 2002; Koivisto 2000: three records and one unpublished dataset). For this updated review, we identified four additional potentially eligible studies (BRAT 2012; ISAT‐2; Li 2012; Wadd 2015), and new long‐term follow‐up data for one of the previously included RCTs (ISAT 2002: four new records). We identified one additional record of the study of Koivisto 2000. We included one new RCT in the present review (Li 2012: one record), we excluded one study because it was not an RCT (BRAT 2012: three records), we moved one study to Studies awaiting classification pending additional data from the study authors (Wadd 2015: one record), and one study is still ongoing without published results to date (ISAT‐2: one record).
1.
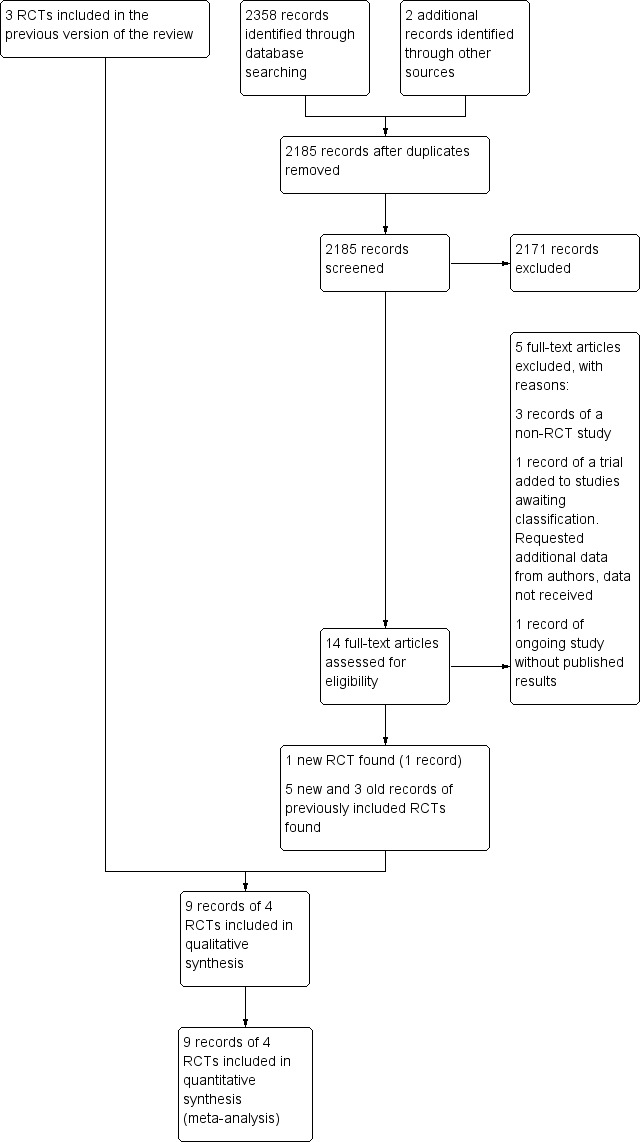
Study flow diagram. RCT: randomised controlled trial.
Included studies
We included four trials: three published, RCTs of endovascular coiling versus neurosurgical clipping for people with aneurysmal SAH (ISAT 2002; Koivisto 2000; Li 2012): and one unpublished, unconfounded controlled trial of a series of 20 people randomly allocated to either endovascular or surgical treatment (Brilstra 2000). These trials recruited participants in the years between 1994 and 2009.
The meta‐analysis included 2458 randomised participants: 1229 in the endovascular treatment group and 1229 in the surgical treatment group. The largest trial was ISAT 2002, which recruited 2143 participants. The other trials recruited 20 (Brilstra 2000), 109 (Koivisto 2000), and 186 (Li 2012) participants. The mean age of the participants in each trial ranged from 49.5 to 54 years. In all trials, randomisation was done within 28 days of the participant's ictus.
In the included trials, SAH was confirmed either by CT or lumbar puncture, and aneurysms were confirmed by CT‐angiography or angiography. After obtaining informed consent, all participants with a ruptured aneurysm that was considered suitable for both neurosurgical clipping and endovascular coiling were included if the clinical condition justified treatment. The maximum delay between SAH and treatment was three days in Koivisto 2000, five days in Brilstra 2000, and 28 days in ISAT 2002. Li 2012 did not give the maximum time to treatment, but mean time to treatment was three days.
ISAT 2002 excluded people if they were already participating in another trial. Koivisto 2000 defined exclusion criteria for participant and aneurysm characteristics. They excluded people older than 75 years, with a large haematoma necessitating operation or having a mass effect causing neurological deficit, or with a history of any previous operation for the same aneurysm. Furthermore, they gave exclusion criteria for the aneurysm concerning size, shape, and relationship to adjacent vessel. Brilstra 2000 excluded people with a fusiform, traumatic, or dissecting aneurysm. Li 2012 included all participants with aneurysmal SAH.
Outcome measures and follow‐up duration
In ISAT 2002, the primary outcome measure was the proportion of participants with a modified Rankin scale score of 3 to 6 (dependency or death) at one year. Secondary outcome measures were rebleeding, quality of life at one year, proportion of participants with epilepsy, cost‐effectiveness, and neuropsychological outcomes. Accrual to ISAT was stopped prematurely, before the planned sample size had been achieved, on the basis of an interim analysis. The Data Monitoring Committee analysed the data on 29 April 2002 and advised the Steering Committee, on the basis of the result, to stop recruitment. The Steering Committee met on 2 May 2002 and decided that recruitment should stop but that follow‐up must continue. Recruitment ceased immediately (ISAT 2002). Follow‐up continued and data from five‐year, 10‐year, and 18‐year follow‐ups have been published (Molyneux 2005; Molyneux 2009; Molyneux 2015).
In Koivisto 2000, the primary outcome measurements were 12‐month functional outcome and 12‐month neuropsychological and radiological outcomes. The 12‐month functional outcome was defined by the GOS, trichotomised into good or moderate recovery (GOS 5 and 4), severe disability and vegetative state (GOS 3 and 2), and death (GOS 1).
In Brilstra 2000, outcome measures were the proportion of participants with a Rankin score of 3 to 6 (dependency or death), the rates of rebleeding and DCI, and the rate of procedural complications at three months.
In Li 2012, outcome measures were 12‐month functional outcome (modified Rankin scale), 12‐month case fatality, 12‐month rebleeding rate and rate of DCI and rate of non‐complete obliteration of aneurysm within 12 months.
Excluded studies
We excluded one non‐randomised trial comparing endovascular coiling and neurosurgical clipping (BRAT 2012). This trial, which recruited people with SAH between 2003 and 2007, had several methodological flaws including inadequate allocation concealment: the treatment allocation was performed by alternating on 1:1 ratio (McDougall 2012) (Characteristics of excluded studies table).
Risk of bias in included studies
2.
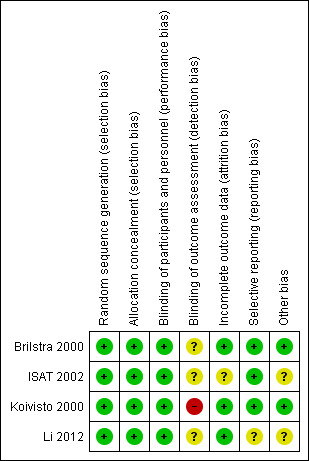
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
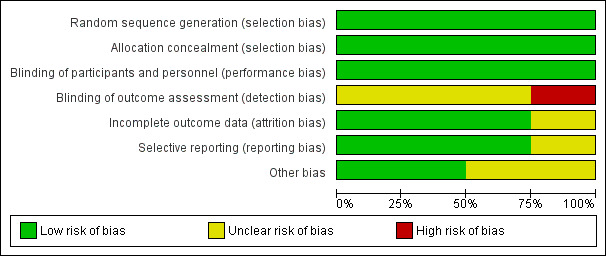
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Two trials used sealed envelopes as the method of randomisation (Brilstra 2000; Koivisto 2000). Brilstra 2000 used a computer‐generated list and the sealed envelopes were not within reach of the treating physician.
ISAT 2002 used a minimisation algorithm to ensure balance between the two groups based on clinical grade, size and location of aneurysm, and extent of extravasated blood on CT, and made allocations by telephone call to a central randomisation service.
Li 2012 used a computer‐generated randomisation schedule.
We assessed the risk of selection bias as low for all included trials.
In the included trials, the prognostic factors of sex, age, and clinical condition on admission were balanced. Aneurysm location was similar for the treatment groups within each trial. Aneurysm size was balanced in three trials, but aneurysm size per treatment group was not available for Li 2012. However, in ISAT 2002, the prognostic determinant time between randomisation and first procedure (i.e. the time between SAH and treatment) differed slightly but statistically significantly between the coiled and clipped participants. For participants allocated to endovascular treatment the mean interval was 1.1 days (interquartile range IQR 0 to 1; range 0 to 30), and for participants allocated to neurosurgical treatment the mean interval was 1.7 days (IQR 0 to 2; range 0 to 41) (ISAT 2002).
Blinding
Due to the nature of the interventions, it was not possible to blind the interventionists, participants, or care personnel to the interventions. However, we have judged that the risk of performance bias to be low in the included studies.
ISAT 2002 collected clinical outcome measures using a validated postal questionnaire mailed to the participants. A single neurosurgeon primarily responsible for treatment or the principal investigator of the study evaluated the 12‐month functional outcome in Koivisto 2000. Brilstra 2000 interviewed participants or their carers by telephone to assess functional outcome three months after SAH and a neurologist or by a neurosurgeon who had not operated on the participant assessed 12‐month functional outcome at the outpatients clinic. Li 2012 assessed 12‐month outcome in outpatient clinic visits or by structured telephone interview with participants or close relatives.
ISAT 2002 defined DCI as a clinical diagnosis. In Koivisto 2000, DCI was not CT or MRI confirmed, but the diagnosis was based upon clinical signs of ischaemic neurological deficit. Brilstra 2000 confirmed DCI by CT or MRI. Li 2012 confirmed DCI by CT. In three trials, rebleeding had to be confirmed by CT (Brilstra 2000; ISAT 2002; Koivisto 2000), but Li 2012 did not state this. Li 2012 only reported rebleeding within 12 months after haemorrhage.
We judged the risk of detection bias to be high in Koivisto 2000, and unclear in Brilstra 2000, ISAT 2002, and Li 2012.
Incomplete outcome data
ISAT 2002 sought the main outcome measure at two months and one year for all participants, and annually thereafter for some participants. At one‐year follow‐up, the vital status was known for all included participants. For eight coiled participants (endovascular coiling group) and seven clipped participants (neurosurgical clipping group), the disability status was missing at two‐month follow‐up. At one‐year follow‐up, the disability status was missing for 10 endovascular participants and 15 neurosurgical participants. Long‐term follow‐up results (five and 10 years) were only available for part of the original ISAT cohort: annual follow‐ups were continued in all UK and eight non‐UK centres. At five years, functional outcome was available for 867 participants in the endovascular coiling group and 857 participants in the neurosurgical clipping group; and mortality was available for 1046 participants in the endovascular coiling group and 1041 participants in the neurosurgical clipping group. At 10 years, functional outcome was available for 666 participants in the endovascular coiling group and for 650 participants in the neurosurgical clipping group, with 10‐year mortality data available for 809 participants in the endovascular coiling group and 835 participants in the neurosurgical clipping group.
Koivisto 2000 assessed clinical and neuropsychological outcomes after three and 12 months. No participants were lost to follow‐up. Mean follow‐up was 39 months (standard deviation (SD) 18 months).
Brilstra 2000 assessed the main outcome measures at three months and no participants were lost to follow‐up at that time. Mean duration of follow‐up was 25 months (SD 22 months). At 12‐month follow‐up, eight coiled participants (endovascular coiling group) and eight clipped participants (neurosurgical clipping group) were available for analysis. There was no information on vital status for two participants in the endovascular coiling group and two participants in the neurosurgical clipping group.
Li 2012 assessed outcomes at 12 months and functional outcome was available for all surviving participants.
ISAT 2002 reported the angiographic occlusion on the first follow‐up angiography performed after the procedure for 881/988 participants allocated to endovascular treatment and alive after one year, and for 450/965 participants allocated to surgical treatment alive after one year. In the endovascular coiling group, timing of follow‐up angiography was before discharge in 28 participants, before two months in 80 participants, between two to 12 months in 690 participants, between one and two years in 58 participants, and after two years in 25 participants. MRI angiography was used in 47 participants. In the neurosurgical clipping group, timing of follow‐up angiography was before discharge in 142 participants, before two months in 61 participants, between two to 12 months in 199 participants, and between one and five years in 48 participants.
Koivisto 2000 gave the primary (direct post treatment) and final (after one‐year follow‐up) angiographic results of endovascular and neurosurgical treatment of the ruptured aneurysms.
Brilstra 2000 had direct post‐treatment information of completeness of occlusion after treatment for all participants; there was angiographic follow‐up information for only one of the clipped participants and for six of the eight participants who survived six months after the SAH.
Li 2012 had 12‐month angiographical follow‐up for all surviving participants. They provided only the rate of non‐complete aneurysm obliteration.
In summary, we judged the risk of attrition bias to be unclear in ISAT 2002, and low in Brilstra 2000, Koivisto 2000, and Li 2012.
Selective reporting
The risk for selective reporting in ISAT 2002, which provided a protocol (and reported outcomes as specified in the protocol), was low.
We did not identify the protocol for Brilstra 2000, but obtained information via personal communication that indicated that all intended outcomes were reported. We judged the risk for selective reporting to be low.
Koivisto 2000 published a report including the study protocol and intended outcomes, and we judged the risk of bias to be low.
Li 2012 have not published a protocol. A published report suggested that all intended outcomes were reported. We contacted the study authors for clarification, but are awaiting a response. We judged the risk of reporting bias as unclear.
Other potential sources of bias
Most of the evidence came from the largest trial (ISAT 2002). During recruitment of participants in ISAT, only 22% of people with SAH treated within the participating centres were enrolled in the study. In addition, people with SAH in poor clinical condition on admission were under‐represented in the ISAT cohort as 88% of the included participants were in good clinical condition (Grade 1‐2 on World Federation of Neurological Surgeons Subarachnoid Haemorrhage grading scale). Thus, the results of the review are only applicable to people with SAH who are in relatively good condition on admission.
It was unclear whether the published results represented intention‐to‐treat or treatment received in Li 2012. We requested missing data from the trialists but are awaiting a response.
We assessed the risk of other types of bias to be low in two trials (Brilstra 2000; Koivisto 2000), and unclear in the other two trials (ISAT 2002; Li 2012).
Effects of interventions
See: Table 1
See Table 1.
Poor outcome: death or dependence in daily activities
The weighted relative risk reduction for endovascular coiling versus neurosurgical clipping at two‐ to three‐month follow‐up was 29% (RR 0.71, 95% CI 0.63 to 0.81; 3 trials; 2257 participants; Analysis 1.1). The absolute risk reduction was 10% (95% CI 7% to 14%).
1.1. Analysis.
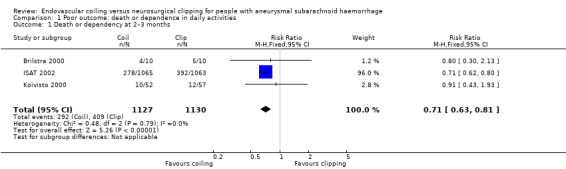
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 1 Death or dependency at 2–3 months.
At one year, 295/1217 (24%) participants randomised to endovascular treatment and 383/1212 (32%) participants randomised to the surgical treatment group had poor functional outcome (Analysis 1.2). All trials adequately reported on functional outcome at 12‐month follow‐up. The reduction in the weighted RR for endovascular coiling versus neurosurgical clipping was 23% (RR 0.77, 95% CI 0.67 to 0.87; 4 trials; moderate‐quality evidence). The absolute risk reduction was 7% (95% CI 4% to 11%); this means that for every 14 (95% CI 9 to 25) participants who were allocated to be coiled instead of clipped, one poor outcome was prevented. In the worst‐case scenario, the relative risk reduction of endovascular coiling versus neurosurgical clipping was 20% (RR 0.80, 95% CI 0.71 to 0.91; 4 trials; 2458 participants; Analysis 1.3), and the absolute risk reduction was 6% (95% CI 3% to 10%).
1.2. Analysis.
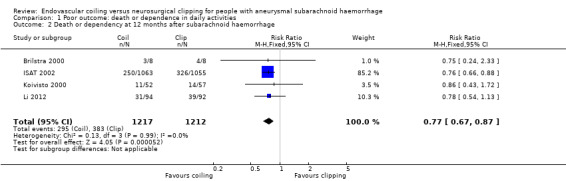
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 2 Death or dependency at 12 months after subarachnoid haemorrhage.
1.3. Analysis.
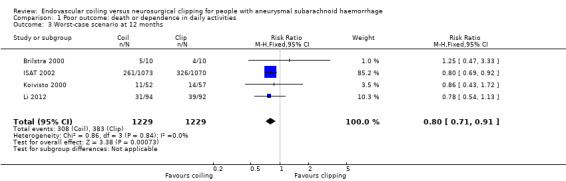
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 3 Worst‐case scenario at 12 months.
At five years, 241/867 (28%) participants randomised to endovascular treatment and 273/857 (32%) participants randomised to surgical treatment had poor functional outcome. The relative risk reduction was 13% (RR 0.87, 95% CI 0.75 to 1.01; 1 trial; Analysis 1.4) and the absolute risk reduction (ARR) was 4% (ARR –0.04, 95% CI –0.08 to 0.00).
1.4. Analysis.
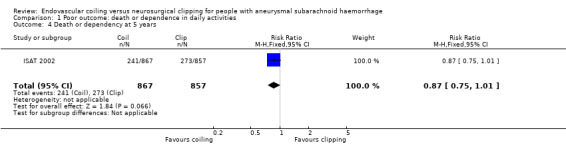
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 4 Death or dependency at 5 years.
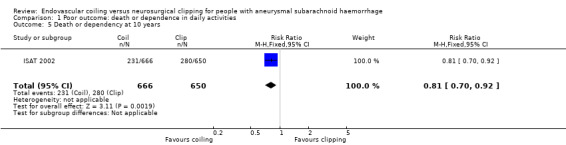
At 10 years, 231/666 (35%) participants allocated to endovascular treatment and 280/650 (43%) participants allocated to surgical treatment had poor functional outcome. The relative risk reduction was 19% (RR 0.81, 95% CI 0.70 to 0.92; 1 trial; 1316 participants; low‐quality evidence) and the absolute risk reduction was 8% (95% CI 3 to 14%).
Death from any cause
The relative risk reduction in death for endovascular coiling versus neurosurgical clipping at two or three months was 12% (RR 0.88, 95% CI 0.66 to 1.18; 3 trials; 2257 participants; Analysis 2.1). The absolute risk reduction was 1% (95% CI –1% to 3%). In the endovascular treatment group, 104/1217 (9%) participants had died from any cause within one year compared with 130/1212 (11%) participants allocated to the surgical treatment group. The relative risk reduction in deaths at one‐year follow‐up for endovascular treatment compared with neurosurgical treatment was 20% (RR 0.80, 95% CI 0.63 to 1.02; 4 trials; 2429 participants; moderate‐quality evidence; Analysis 2.2). The absolute risk reduction was 2% (95% CI 0% to 5%). At five years, 112/1046 (11%) participants in the endovascular treatment group and 144/1041 (14%) participants in the neurosurgical clipping group had died. The relative risk reduction for death at five years was 13% (RR 0.77, 95% CI 0.61 to 0.98; 1 trial; Analysis 2.3). At 10 years, 135/809 (17%) participants in the endovascular treatment group and 178/835 (21%) participants in the neurosurgical clipping group had died (relative risk reduction: 22%; RR 0.78, 95% CI 0.64 to 0.96; 1 study; Analysis 2.4).
2.1. Analysis.
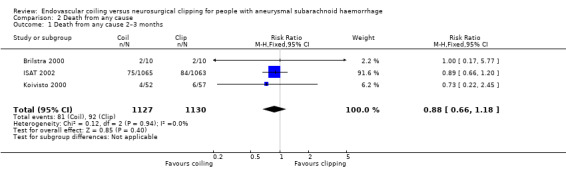
Comparison 2 Death from any cause, Outcome 1 Death from any cause 2–3 months.
2.2. Analysis.
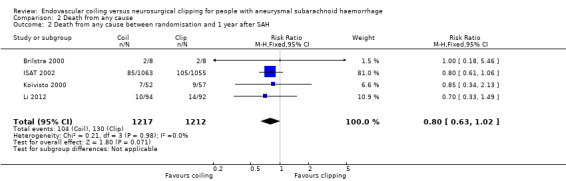
Comparison 2 Death from any cause, Outcome 2 Death from any cause between randomisation and 1 year after SAH.
2.3. Analysis.
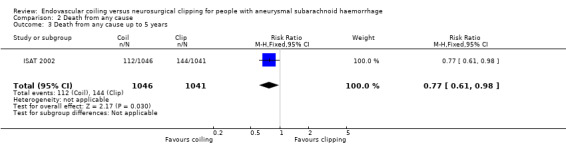
Comparison 2 Death from any cause, Outcome 3 Death from any cause up to 5 years.
2.4. Analysis.
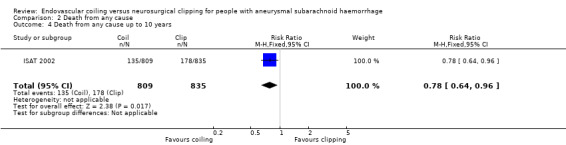
Comparison 2 Death from any cause, Outcome 4 Death from any cause up to 10 years.
Delayed cerebral ischaemia
DCI at two to three months after SAH was observed in 292/1225 (24%) participants allocated to the endovascular treatment group and in 349/1225 (28%) participants allocated to the surgical treatment group. The weighted relative risk reduction of endovascular coiling versus neurosurgical clipping was 16% (RR 0.84, 95% CI 0.74 to 0.96; 4 trials; moderate‐quality evidence; Analysis 3.1). The absolute risk reduction was 4% (95% CI 0% to 7%).
3.1. Analysis.
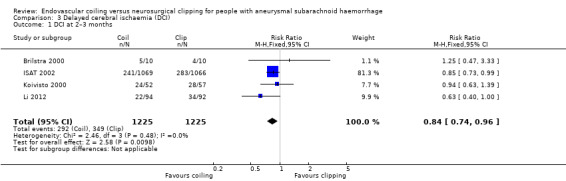
Comparison 3 Delayed cerebral ischaemia (DCI), Outcome 1 DCI at 2–3 months.
Rebleeding
Nineteen of 1135 (1.7%) participants allocated to endovascular treatment and 30/1137 (2.6%) participants allocated to neurosurgical clipping experienced rebleeding before treatment. The risk for preprocedural rebleeding did not significantly differ between endovascular coiling and neurosurgical clipping groups (RR 0.64, 95% CI 0.37 to 1.12; Analysis 4.1). With regard to post‐procedural rebleeding up to one year after treatment, the relative risk of rebleeding was higher for endovascular treatment: 32/1229 (2.6%) participants allocated to endovascular treatment and 17/1137 (1.4%) participants allocated to neurosurgical clipping had an episode of rebleeding. The RR was 1.83 (95% CI 1.04 to 3.23; 4 trials; high‐quality evidence; Analysis 4.2). The absolute increase in risk was 1% (95% CI 0% to 2%). At a follow‐up period of one (ISAT 2002) to three months (Brilstra 2000; Koivisto 2000), the relative risk for postprocedural rebleeding was 2.66 (95% CI 0.71 to 10.00) for endovascular coiling versus neurosurgical clipping. The absolute increase in risk was 0% (95% CI 0% to 1%).
4.1. Analysis.
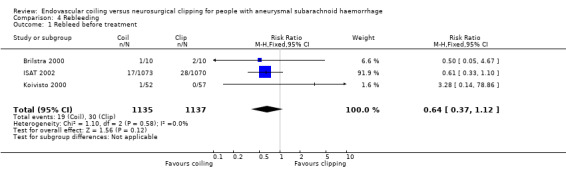
Comparison 4 Rebleeding, Outcome 1 Rebleed before treatment.
4.2. Analysis.
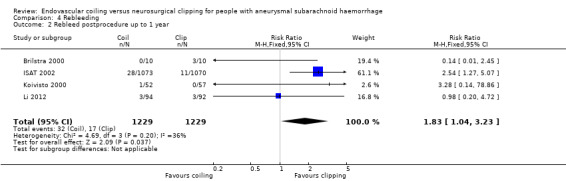
Comparison 4 Rebleeding, Outcome 2 Rebleed postprocedure up to 1 year.
Complications from intervention
Koivisto 2000 reported information on technical failure and clinical deterioration within 24 hours of the intervention. Brilstra 2000 reported on complications from the intervention, defined as clinical deterioration within 24 hours after the intervention. Complications occurred in 8/62 (13%) participants (13%) in the endovascular coiling group and in 8/67 (12%) participants in the neurosurgical clipping group. The weighted relative risk increase with endovascular coiling versus neurosurgical clipping was 5% (RR 1.05, 95% CI 0.44 to 2.53; 2 trials; Analysis 5.1). The absolute risk increase was 1% (95% CI –10% to 12%).
5.1. Analysis.
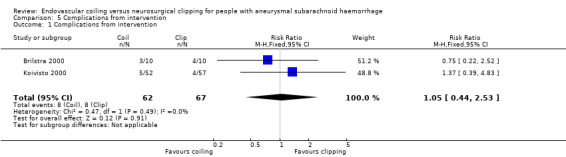
Comparison 5 Complications from intervention, Outcome 1 Complications from intervention.
Neither ISAT 2002 nor Li 2012 reported information on complications from the interventions.
Death or rebleeding at more than one year after the subarachnoid haemorrhage
Death
In ISAT 2002, 27 participants randomised to endovascular coiling and 39 participants randomised to neurosurgical clipping died between one and five years. In addition, 23 participants randomised to endovascular coiling and 34 participants randomised to neurosurgical clipping died between five and 10 years of follow‐up. In the survival analysis, the proportion of participants alive decreased by 3% in the four‐year period after the first year for endovascular participants and by 4% for the neurosurgical participants. This corresponded to a death rate of 7.6 per 1000 patient‐years for participants in the endovascular coiling group and 10.2 per 1000 patient‐years for participants in the neurosurgical clipping group.
In Koivisto 2000, two participants allocated to endovascular treatment and one participant allocated to surgical treatment died after one year. In survival analyses, the proportion of participants alive decreased by 5% for participants in the endovascular coiling group in the three‐and‐half year period after the first year of follow‐up and by 7% for participants in the neurosurgical clipping group. This corresponded to a death rate of 14.6 per 1000 patient‐years for participants in the endovascular coiling group and 20.7 per 1000 patient‐years for participants in the neurosurgical clipping group.
In Brilstra 2000, a total of 8.7 patient‐years of follow‐up were available for the participant in the endovascular coiling group and a total of 19.6 patient‐years were available for the participants in the neurosurgery group after the one‐year follow‐up period. None of the participants died during this follow‐up.
Rebleeding
In ISAT 2002, 10 participants had a rebleeding from the target aneurysm between one and five years of follow‐up (8447 patient‐years' follow‐up in the endovascular coiling group and 8177 patient‐years' follow‐up in the neurosurgical clipping group). Seven of these participants were in the endovascular coiling group and three were in the neurosurgical clipping group. Between five and 10 years of follow‐up, three additional participants in the endovascular coiling group and one participant in the neurosurgical clipping group had a rebleeding from the target aneurysm. Additionally, three participants had a rebleeding after one year from another aneurysm but the trialists provided no information on the treatment modality of the target aneurysm for these participants. The RR for postprocedural rebleeding in the endovascular coiling group was 2.75 (95% CI 1.51 to 5.02) at five years' follow‐up and 2.69 (95% CI 1.50 to 4.81) at 10 years' follow‐up. Absolute risk increase was 3% (95% CI 1% to 5%) at five years and 4% (95% CI 2% to 6%) at 10 years.
Koivisto 2000 and Brilstra 2000 reported no episodes of rebleeding more than one year after SAH. The mean follow‐up period after one year was 27 months in Koivisto 2000 and 13 months in Brilstra 2000.
Degree of occlusion after endovascular coiling and neurosurgical clipping
ISAT 2002 gave direct post‐treatment results and angiographic occlusion on the first follow‐up angiography performed after the procedure. Direct post‐treatment information was based upon the first procedure actually performed, not the original allocation. Of the 1095 participants in the endovascular coiling group, endovascular coiling failed in 81 (7.4%) participants. In 22/1012 (2.2%) participants in whom the first treatment was neurosurgical, clipping was not completed or not attempted in 35 participants (3.5%). The aneurysm was successfully wrapped in 14 of these 35 participants. The angiographic occlusion on the first follow‐up angiography performed after the procedure was reported for 881/988 eligible participants allocated to endovascular treatment and for 450/965 eligible participants allocated to surgical treatment. Occlusion was complete in 66% of participants in the endovascular coiling group and 82% of the participants in the neurosurgical clipping group; a 90% to 100% occlusion of the aneurysm had occurred in a further 26% of participants in the endovascular coiling group and 12% of participants in the neurosurgery group; there was incomplete occlusion (less than 90%) in 7.8% of the participants in the endovascular coiling group and 5.6% of participants in the neurosurgery group (Table 2).
1. Angiographic occlusion on follow‐up angiography during first year post‐treatment.
| Number of participants per treatment | Extent of occlusion | ||
| 100% | 90% to 100% | < 90% | |
| ISAT 2002 | |||
| Endovascular coiling: 881 | 584 (66%) | 228 (26%) | 69 (8%) |
| Neurosurgical clipping: 450 | 370 (82%) | 55 (12%) | 25 (6%) |
| Koivisto 2000 | |||
| Endovascular coiling: 52 | 40 (77%) | 10 (19%) | 2 (4%) |
| Neurosurgical clipping: 57 | 49 (86%) | 7 (12%) | 1 (2%) |
| Total | |||
| Endovascular coiling: 933 | 624 (67%) | 238 (26%) | 71 (8%) |
| Neurosurgical clipping: 507 | 419 (83%) | 62 (12%) | 26 (5%) |
Koivisto 2000 provided the direct post‐treatment results of treatment for all participants, and one‐year follow‐up angiographic results. In participants in the endovascular coiling group, there was direct post‐treatment complete obliteration in 50% of participants compared with 74% in the neurosurgical clipping group. In 35% of participants in the endovascular coiling group, there was an occlusion of 90% to 100% compared with 16% of participants in the neurosurgical group; and in 15% of participants in the endovascular coiling group the aneurysm was less than 90% occluded compared with 11% of participants in the neurosurgical clipping group. After one year, there was complete occlusion in 77% of participants in the endovascular coiling group and 86% of participants in the neurosurgical clipping group; 90% to 100% occlusion of the aneurysm in 19% of participants in the endovascular coiling group and 12% of participants in the neurosurgical clipping group; and incomplete occlusion (less than 90%) in 4% of participants in the endovascular coiling group and 2% of participants in the neurosurgical clipping group.
Brilstra 2000 did not provide angiographic follow‐up for all participants but gave immediate post‐treatment information for all participants. Neurosurgical clipping of the aneurysm was not feasible in one participant and was incomplete in another participant. Immediate postcoiling angiography showed complete occlusion in five participants and 90% to 99% occlusion in the other five participants in the endovascular coiling group.
Li 2012 reported angiographical outcomes at 12 months for all surviving participants. There was incomplete occlusion of the treated aneurysm in 35% of participants in the endovascular coiling group and 16% of participants in the neurosurgery group.
On comparison of incomplete obliteration (less than 100% obliteration) for coiled versus clipped participants during one‐year follow‐up, the relative risk increase was 2.02 (95% CI 1.65 to 2.47; 3 trials; 1626 participants; Analysis 6.1) and the absolute increase in risk was 17% (95% CI 12% to 22%). The RR for obliteration less than 90% was 1.43 (95% CI 0.93 to 2.21; 2 trials; 1440 participants; Analysis 6.2) and the absolute increase in risk was 2% (95% CI 0% to 5%).
6.1. Analysis.
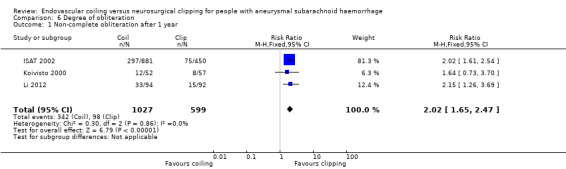
Comparison 6 Degree of obliteration, Outcome 1 Non‐complete obliteration after 1 year.
6.2. Analysis.
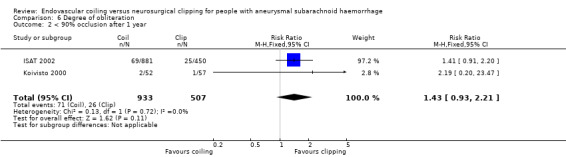
Comparison 6 Degree of obliteration, Outcome 2 < 90% occlusion after 1 year.
Additional analysis
The timing of the intervention was early in Koivisto 2000 (treatment within three days); early or intermediate in Brilstra 2000; and early, intermediate, or late in ISAT 2002. Li 2012 did not give the exact timing of interventions; we requested the details but have not received a reply.
In Li 2012, it was not clear if analyses were done according to the intention‐to‐treat principle; therefore, we performed a sensitivity analysis excluding this study. The results of this analysis were essentially the same as those of the main analysis: the RR for poor outcome was 0.76 (95% CI 0.66 to 0.88; Analysis 7.1).
7.1. Analysis.
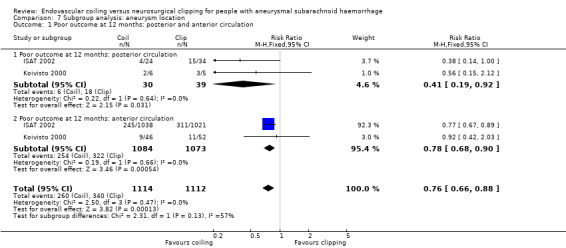
Comparison 7 Subgroup analysis: aneurysm location, Outcome 1 Poor outcome at 12 months: posterior and anterior circulation.
A subgroup analysis for basilar aneurysms could not be performed since only data for all posterior circulation aneurysms combined were available. Therefore, we performed an analysis for all posterior circulation aneurysms and all anterior circulation aneurysms for which information was available (for ISAT 2002 and Koivisto 2000). Brilstra 2000 included no participants with posterior circulation. For participants with a posterior circulation aneurysm, the RR of poor outcome was 0.41 (95% CI 0.19 to 0.92; 69 participants; Analysis 7.1), and the absolute decrease in risk was 27% (95% CI 6% to 48%). For participants with an anterior circulation aneurysm, the RR was 0.78 (95% CI 0.68 to 0.90; Analysis 7.1), and the absolute risk decrease was 7% (95% CI 3% to 10%).
Discussion
Summary of main results
The aggregation of the results of all identified randomised trials on endovascular coiling versus neurosurgical clipping treatment in participants with SAH from a ruptured aneurysm showed a reduction of poor outcome after treatment by endovascular coiling compared with neurosurgical clipping at one year, even in the special worst‐case scenario analysis. At five‐ and 10‐year follow‐up, the difference between endovascular coiling and neurosurgical clipping was smaller, and no longer statistically significant. The smaller effect size may be explained by the competing risk from other cardiovascular diseases (Rinkel 2011). The absence of a statistically significant effect may be explained by the fact that long‐term outcome data were only available for a subset of the original ISAT cohort, resulting in less statistical power. Endovascular coiling was associated with reduced mortality at one, five, and 10 years, although the difference at one year was not statistically significant. Participants in the endovascular coiling group had a higher risk of postprocedural rebleeding at one, five, and 10 years. Risk for DCI at two to three months was lower in participants in the endovascular coiling group.
Overall completeness and applicability of evidence
This review represents the results of one large trial and three much smaller trials. The results were largely dependent on the largest trial (ISAT 2002), which was stopped prematurely on the basis of an interim analysis, because results were weighted to number of participants and events in each trial. However, the results of the smaller trials were in the same direction for the primary outcome measure and there was no evidence of heterogeneity in any of the analyses.
The intervention groups in all four included trials were well balanced regarding baseline characteristics. Peri‐ and postprocedural management is probably also similar between the two treatment strategies except for the use of platelet aggregation inhibitors. Aspirin is often prescribed after coiling of aneurysms. In a systematic review, antiplatelet therapy reduced the occurrence of DCI and tended to improve overall outcome (Dorhout Mees 2003). One study of 19 centres representing 1422 (66%) participants in the ISAT trial found that only two of the centres (8% of coiled participants) routinely prescribed antiplatelet drugs during coiling and six centres (24% of coiled participants) did so after coiling. There was no evidence that antiplatelets during or after endovascular coiling improved outcome (van den Bergh 2009).
We intended to perform a subgroup analysis for participants with a ruptured aneurysm of the basilar artery. However, no specified information on main outcome measures was available for this subgroup of participants. In our subgroup analysis for all posterior circulation aneurysms, the relative risk of poor outcome showed a trend towards decrease for endovascular coiling, but was not statistically significant.
Another type of patient that was under‐represented in the included trials were people with aneurysms of the middle cerebral artery (MCA). These aneurysms were often considered not suitable for endovascular coiling when the included trials were recruiting participants. The total number of people with aneurysms from the MCA was still considerable and the results in people with MCA aneurysms were in the same direction as in people with aneurysms at other sites. Therefore, it seems reasonable to assume that the results of this review also hold true for people with MCA aneurysms whose anatomy makes them suitable for either endovascular or surgical treatment. People in poor clinical condition were also under‐represented in the trials, since all three trials only included participants when clinical condition justified aneurysm treatment and if informed consent was obtained, which is more difficult in people with a poor clinical condition. Because a poor clinical condition at time of admission to hospital is an important predictor for poor outcome, the results of this review cannot be directly applied to people in poor clinical condition on admission.
Late spontaneous reopening of the aneurysm may lead to insufficient protection against rebleedings in the future. A disadvantage of endovascular coiling is that aneurysms are more often incompletely treated (90% to 100% obliteration) and carry a higher risk for reopening than clipped aneurysms. The higher risk of rebleeding at five and 10 years in participants randomised to endovascular treatment in ISAT indicates that coiled aneurysms are more susceptible to rebleeding than clipped ones. The clinical relevance of this late reopening is uncertain given the de novo formation of aneurysms on locations other than the original aneurysm site. In follow‐up studies of people treated for an aneurysm, the rate of development of new aneurysms ranged from 0.2% to 2% per year (David 1999; Juvela 1993; Juvela 2001; Lindgren 2016; Tsutsumi 2001; Wermer 2005a). The SAHs occurring from these new aneurysms may outnumber those resulting from reopened aneurysms (Wermer 2005b).
Moreover, since the previous version of this review (van der Schaaf 2005), endovascular coiling has become the first occlusive treatment option to be considered in most centres treating aneurysmal SAH. Today, endovascular coiling is performed not only by radiologists but also by neurosurgeons and neurologists. This may have led to physicians with less experience performing endovascular coiling in hospital settings with less experience. One study indeed showed differences in rates of complications according to the type of physician performing the procedure (Fennell 2016). Technical development has also made new endovascular treatments available, in addition to endovascular coiling, such as flow‐diverting stents (Ding 2011) and WEB (Wowen EndoBridge) devices (Çinar 2013), but there are no randomised trials that compare these treatment modalities with neurosurgical clipping for ruptured intracranial aneurysms.
Quality of the evidence
We analysed data from four trials involving 2458 randomised participants: 1229 in the endovascular coiling group and 1229 in the neurosurgical clipping group. Included trials took place between 1994 and 2009. We judged all trials to have at least one domain where risk of bias was unclear, and in one study the risk of detection bias was high (Figure 2; Figure 3). However, we did not detect important heterogeneity between the results of the studies in any of the outcomes. We could not assess publication bias due to low number of included trials. Although publication bias may exist, it is unlikely that a large unpublished randomised controlled study exists that would alter our findings. Evidence was mainly based on the largest included trial and long‐term follow‐up was available only for a subgroup of participants within that trial. Thus, we judged evidence of the outcomes with long‐term follow‐up (poor outcome, rebleeding) to be of low quality. We judged the evidence of the outcomes with short‐to‐intermediate follow‐up to be of moderate (poor outcome, death from any cause, DCI) or high (rebleeding) quality.
Potential biases in the review process
Two review authors independently performed study selection, data extraction, and quality assessment in order to reduce bias and subjectivity. We attempted to identify all RCTs of potential relevance to the review. We did not perform a funnel‐plot analysis, as we only identified four eligible RCTs.
Agreements and disagreements with other studies or reviews
We identified other systematic reviews on this topic that included only prospective trials (Lanzino 2013), or prospective trials combined with non‐randomised studies (Falk Delgado 2017; Fotakopoulos 2017; Li 2013; Xia 2017). One review of prospective trials found that the risk of poor outcome at one year after endovascular coiling and clipping of a ruptured aneurysm was lower after endovascular coiling (RR 0.75, 95% CI 0.65 to 0.87) (Lanzino 2013), which is in line with our results. Another review found that the risk of poor outcome was higher after clipping (odds ratio (OR) 1.25, 95% CI 1.12 to 1.40), but there was no difference in mortality (OR 1.07, 95% CI 0.88 to 1.30). Risk of rebleeding was lower after clipping (OR 0.43, 95% CI 0.28 to 0.66) (Li 2013). One review comparing clipping versus endovascular coiling in people with ruptured anterior circulation aneurysms found no difference in terms of operative mortality (OR 0.80, 95% CI 0.31 to 2.08), permanent neurological deficit (OR 1.42, 95% CI 0.85 to 2.39), late mortality (OR 0.97, 95% CI 0.42 to 2.25), or the need for reintervention (OR 0.86, 95% CI 0.25 to 2.95) (Fotakopoulos 2017). Results from a review comparing rates of independent outcome in people with a ruptured aneurysm treated with coiling or clipping favoured coiling after intermediate follow‐up (OR 0.80, 95% CI 0.68 to 0.94, OR less than 1 favours coiling) and long‐term follow‐up (OR 0.81, 95% CI 0.71 to 0.93, OR less than 1 favours coiling) (Falk Delgado 2017). One review focusing on people with high‐grade SAH found that coiling was associated with higher mortality (OR 0.55, 95% CI 0.41 to 0.75, OR less than 1 favours clipping), but found no differences in rates of complications including rebleeding (OR 0.62, 95% CI 0.30 to 1.29), ischaemic infarct (OR 0.76, 95% CI 0.45 to 1.29), shunt‐dependent hydrocephalus (OR 1.33, 95% CI 0.52 to 3.40), or good outcome (OR 1.44, 95% CI 0.95 to 2.36) (Xia 2017). The differences in comparison to our results were most likely due to inclusion of non‐randomised data and restricting the analyses to subgroups of participants.
Authors' conclusions
Implications for practice.
The results of this review mainly draw on evidence from people in good clinical condition after subarachnoid haemorrhage (SAH). Data from the randomised trials show that participants in relatively good condition whose aneurysm is considered suitable for both neurosurgical clipping and endovascular treatment, are likely to have a better functional outcome with endovascular coiling. Middle cerebral artery (MCA) aneurysms were under‐represented in the included trials, but it is plausible that for MCA aneurysms suitable for both treatment methods, endovascular coiling is associated with a better outcome. For people with SAH in poor clinical condition, there is no reliable randomised evidence comparing the potential harms and benefits of endovascular coiling versus neurosurgical clipping. It should also be noted that this review provides evidence of the benefit of simple endovascular coiling only, because there are no randomised data for balloon‐ or stent‐assisted coiling or for other endovascular procedures (e.g. WEB (Wowen EndoBridge) devices and flow‐diverting stents) to treat ruptured intracranial aneurysms.
Implications for research.
There is no reliable trial evidence that can be used directly to guide treatment in people with a poor clinical condition. Trials comparing endovascular coiling, neurosurgical clipping, and treatment with advanced endovascular techniques (e.g. flow‐diverting stents and WEB‐devices) are required to extend the evidence to people and interventions that were not part of the four included trials. The ongoing ISAT‐2 trial may provide evidence to support treatment decisions for subgroups of participants with ruptured aneurysms that were not well represented in ISAT, as well as for the use of advanced endovascular techniques. In addition, data from consecutive patient series would allow an assessment of the results of the treatment of ruptured intracranial aneurysms in current clinical practice, outside the constraints of a randomised trial.
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2018 | New citation required and conclusions have changed | We included new data; conclusions revised |
| 26 March 2018 | New search has been performed | We included one additional trial (186 participants): the review now has four included studies involving 2458 participants; we updated the searches, the review text and references, and added a 'Summary of findings' table |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 19 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Drs EH Brilstra and WJJ van Rooij for their contributions to the development of the protocol for this review.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2) (searched 26 March 2017)
#1MeSH descriptor: [Subarachnoid Hemorrhage] this term only #2MeSH descriptor: [Intracranial Hemorrhages] this term only #3MeSH descriptor: [Cerebral Hemorrhage] this term only #4MeSH descriptor: [Intracranial Aneurysm] this term only #5MeSH descriptor: [Rupture, Spontaneous] this term only #6#4 and #5 #7MeSH descriptor: [Aneurysm, Ruptured] this term only #8MeSH descriptor: [Brain] explode all trees #9MeSH descriptor: [Meninges] explode all trees #10#8 or #9 #11#7 and #10 #12(subarachnoid or arachnoid) near/6 (haemorrhage* or hemorrhage* or bleed* or blood*) #13MeSH descriptor: [Vasospasm, Intracranial] this term only #14(cerebral or intracranial or cerebrovascular) near/6 (vasospasm or spasm) #15(brain or cereb* or intracranial) near/3 aneurysm* near/3 ruptur* #16SAH #17#1 or #2 or #3 or #6 or #11 or #12 or #13 or #14 or #15 or #16 #18MeSH descriptor: [Embolization, Therapeutic] this term only #19MeSH descriptor: [Endovascular Procedures] this term only #20MeSH descriptor: [Prostheses and Implants] this term only #21MeSH descriptor: [Blood Vessel Prosthesis] this term only #22MeSH descriptor: [Vascular Surgical Procedures] this term only #23MeSH descriptor: [Blood Vessel Prosthesis Implantation] this term only #24coil* or hydrocoil* or Guglielmi* #25#18 or #19 or #20 or #21 or #22 or #23 or #24 #26MeSH descriptor: [Neurosurgical Procedures] this term only #27MeSH descriptor: [Craniotomy] this term only #28MeSH descriptor: [Neurosurgery] this term only #29MeSH descriptor: [Aneurysm] this term only and with qualifier(s): [Surgery ‐ SU] #30MeSH descriptor: [Aneurysm, Ruptured] this term only and with qualifier(s): [Surgery ‐ SU] #31MeSH descriptor: [Intracranial Aneurysm] this term only and with qualifier(s): [Surgery ‐ SU] #32MeSH descriptor: [Subarachnoid Hemorrhage] this term only and with qualifier(s): [Surgery ‐ SU] #33clip* #34#26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 #35#17 and #25 and #34
Appendix 2. MEDLINE Ovid (1966 to 26 March 2018)
1. Subarachnoid Hemorrhage/ 2. intracranial hemorrhages/ or cerebral hemorrhage/ or vasospasm, intracranial/ 3. Intracranial Aneurysm/ 4. Rupture, Spontaneous/ 5. 3 and 4 6. Aneurysm, Ruptured/ 7. exp brain/ 8. 6 and 7 9. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 10. ((brain or cereb$ or intracranial) adj3 aneurysm$ adj3 ruptur$).tw. 11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 12. sah.tw. 13. 1 or 2 or 5 or 8 or 9 or 10 or 11 or 12 14. Embolization, Therapeutic/ or endovascular procedures/ 15. "prostheses and implants"/ or blood vessel prosthesis/ 16. vascular surgical procedures/ or blood vessel prosthesis implantation/ 17. (coil$ or hydrocoil$ or Guglielmi$).tw. 18. or/14‐17 19. 13 and 18 20. neurosurgical procedures/ or craniotomy/ 21. Neurosurgery/ 22. aneurysm/su or aneurysm, ruptured/su or intracranial aneurysm/su 23. Subarachnoid Hemorrhage/su [Surgery] 24. clip$.tw. 25. or/20‐24 26. 19 and 25 27. Randomized Controlled Trials as Topic/ 28. random allocation/ 29. Controlled Clinical Trials as Topic/ 30. control groups/ 31. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 32. double‐blind method/ 33. single‐blind method/ 34. Research Design/ 35. randomized controlled trial.pt. 36. controlled clinical trial.pt. 37. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 38. random$.tw. 39. (controlled adj5 (trial$ or stud$)).tw. 40. (clinical$ adj5 trial$).tw. 41. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 42. (surgical adj5 (group$ or subject$ or patient$)).tw. 43. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 44. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 45. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 46. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 47. controls.tw. 48. trial.ti. 49. or/27‐48 50. 26 and 49
Appendix 3. Embase Ovid (1980 to 26 March 2018)
1. subarachnoid hemorrhage/ 2. brain hemorrhage/ or brain vasospasm/ or intracranial aneurysm/ or brain artery aneurysm/ 3. brain artery aneurysm rupture/ 4. aneurysm rupture/ and exp brain/ 5. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 6. ((brain or cereb$ or intracranial) adj3 aneurysm$ adj3 ruptur$).tw. 7. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 8. sah.tw. 9. or/1‐8 10. Artificial Embolism/ or coil embolization/ 11. blood vessel prosthesis/ 12. endovascular surgery/ 13. endovascular coiling/ 14. (coil$ or hydrocoil$ or Guglielmi$).tw. 15. or/10‐14 16. aneurysm surgery/ or aneurysm clip/ 17. subarachnoid hemorrhage/su 18. brain hemorrhage/su or brain vasospasm/su or intracranial aneurysm/su or brain artery aneurysm/su or aneurysm rupture/su 19. neurosurgery/ or craniotomy/ 20. clip/ or clip$.tw. 21. or/16‐20 22. 9 and 15 and 21 23. Randomized Controlled Trial/ 24. Randomization/ 25. Controlled Study/ 26. control group/ 27. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/ 28. Double Blind Procedure/ 29. Single Blind Procedure/ or triple blind procedure/ 30. "types of study"/ 31. (random$ or RCT$).tw. or trial.ti. 32. (controlled adj5 (trial$ or stud$)).tw. 33. (clinical$ adj5 trial$).tw. 34. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 35. (surgical adj5 (group$ or subject$ or patient$)).tw. 36. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 37. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 38. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 39. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 40. controls.tw. 41. or/23‐40 42. 22 and 41
Appendix 4. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy:
ClinicalTrials.gov: subarachnoid AND (coiling OR clipping)
WHO International Clinical Trials Registry Platform: subarachnoid AND (coiling OR clipping)
Data and analyses
Comparison 1. Poor outcome: death or dependence in daily activities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency at 2–3 months | 3 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.63, 0.81] |
| 2 Death or dependency at 12 months after subarachnoid haemorrhage | 4 | 2429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.87] |
| 3 Worst‐case scenario at 12 months | 4 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.91] |
| 4 Death or dependency at 5 years | 1 | 1724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.01] |
| 5 Death or dependency at 10 years | 1 | 1316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.92] |
1.5. Analysis.
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 5 Death or dependency at 10 years.
Comparison 2. Death from any cause.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause 2–3 months | 3 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.18] |
| 2 Death from any cause between randomisation and 1 year after SAH | 4 | 2429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.02] |
| 3 Death from any cause up to 5 years | 1 | 2087 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 4 Death from any cause up to 10 years | 1 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
Comparison 3. Delayed cerebral ischaemia (DCI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DCI at 2–3 months | 4 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.96] |
Comparison 4. Rebleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rebleed before treatment | 3 | 2272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.37, 1.12] |
| 2 Rebleed postprocedure up to 1 year | 4 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.04, 3.23] |
| 3 Rebleed postprocedure up to 3 months | 3 | 2272 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.71, 10.00] |
| 4 Rebleed postprocedure up to 5 years | 1 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.51, 5.02] |
| 5 Rebleed postprocedure up to 10 years | 1 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.50, 4.81] |
4.3. Analysis.
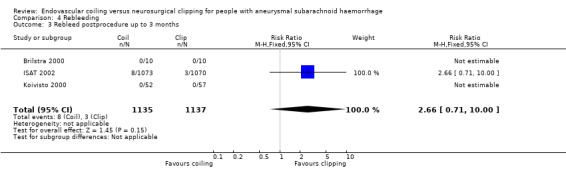
Comparison 4 Rebleeding, Outcome 3 Rebleed postprocedure up to 3 months.
4.4. Analysis.
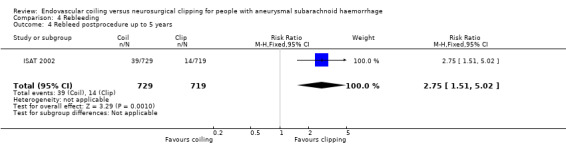
Comparison 4 Rebleeding, Outcome 4 Rebleed postprocedure up to 5 years.
4.5. Analysis.
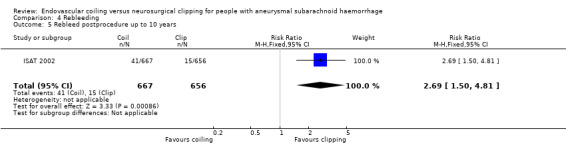
Comparison 4 Rebleeding, Outcome 5 Rebleed postprocedure up to 10 years.
Comparison 5. Complications from intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications from intervention | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.44, 2.53] |
Comparison 6. Degree of obliteration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐complete obliteration after 1 year | 3 | 1626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.65, 2.47] |
| 2 < 90% occlusion after 1 year | 2 | 1440 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.93, 2.21] |
Comparison 7. Subgroup analysis: aneurysm location.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor outcome at 12 months: posterior and anterior circulation | 2 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.66, 0.88] |
| 1.1 Poor outcome at 12 months: posterior circulation | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.92] |
| 1.2 Poor outcome at 12 months: anterior circulation | 2 | 2157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.68, 0.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brilstra 2000.
| Methods | Method of randomisation: sealed envelopes Blinding of outcome assessment: no Analysis: intention to treat Excluded participants: 9 prior to randomisation Cross‐over cases: no Losses to follow‐up: at 1‐year follow‐up: 2 participants in the endovascular coiling group and 2 participants in the neurosurgical clipping group Definition of outcomes: stated |
|
| Participants | Location: University Medical Centre Utrecht and St Elisabeth Hospital Tilburg, The Netherlands Coiling: 10 (men: 3 (30%)) Clipping: 10 (men: 3 (30%)) Age range: 35–75 years Entry criteria: documented aneurysmal SAH by either CT or DSA within the preceding 4 days, clinical state justifying treatment, aneurysm suitable for both treatment modalities Comparability of treatment groups: good for major prognostic factors Clinical grade on admission:
Aneurysm location:
|
|
| Interventions | Endovascular coiling Neurosurgical clipping |
|
| Outcomes | Clinical outcomes: dependency and death at 1‐year follow‐up (Rankin score 3–6), rebleeding, epilepsy, quality of life at 1 year and neuropsychological outcomes Additional measures: cost‐effectiveness |
|
| Notes | Exclusion criteria: the logistic conditions for early operation could not be fulfilled Follow‐up duration: 3 months and 1 year |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Allocation concealment performed by sealed envelopes that were not within reach of the treating physician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants of personnel was not possible due to the characteristics of the interventions. However, review authors judged that the risk of performance bias was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants or their carers interviewed by telephone to assess functional outcome 3 months after SAH and 12‐month functional outcome assessed at outpatients clinic by a neurologist or by a neurosurgeon who had not operated on the participant. Unclear whether blinding was performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete for all outcomes |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but it was clear that the obtained data include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Other sources of bias not identified |
ISAT 2002.
| Methods | Method of randomisation: minimisation algorithm by telephone call to central randomisation service Blinding of outcome assessment: unblinded interim data Analysis: intention to treat Excluded participants: 7416 excluded prior to randomisation Cross‐over cases: 48 participants Losses to follow‐up: at 1‐year follow‐up: vital status known for all participants, for 10 participants in the endovascular coiling group and 15 participants in the neurosurgical clipping group the disability status was missing. Definition of outcomes: stated |
|
| Participants | Location: 43 major neurosurgical centres (international) Coiling: 1073 (men:women ratio: 0.6) Clipping: 1070 (men:women ratio: 0.6) Age range: 18–87 years Entry criteria: documented aneurysmal SAH by either CT or LP within the preceding 28 days, clinical state justifying treatment, aneurysm suitable for both treatment modalities Comparability of treatment groups: good for major prognostic factors (except for a significant difference in the time interval between SAH and treatment) Clinical grade on admission:
Aneurysm location:
|
|
| Interventions | Endovascular coiling Neurosurgical clipping |
|
| Outcomes | Clinical outcomes: dependency and death at 1, 5, and 10 years' follow‐up (modified Rankin Scale score of 3–6), rebleeding at 1, 5, and 10 years, proportion of participants with epilepsy, quality of life at 1 year, and neuropsychological outcomes Additional measures: cost‐effectiveness |
|
| Notes | Exclusion criteria: refused informed consent, if participating in another randomised trial of a treatment for SAH Follow‐up duration: 2 months, 1 year, 5 years, 10 years Accrual to ISAT was stopped prematurely, before the planned sample size had been achieved, on the basis of an interim analysis. The Data Monitoring Committee analysed the data on 29 April 2002 and advised the Steering Committee, on the basis of the result, to stop recruitment. The Steering Committee met on 2 May 2002 and decided that recruitment should stop but that follow‐up must continue. Recruitment ceased immediately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All random assignments were done through a 24‐h [hour] telephone randomisation service, provided by the Clinical Trial Service Unit at the University of Oxford. Key baseline data were recorded before the treatment allocation was issued. A minimisation algorithm was used to ensure balance between the two groups." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants of personnel was not possible due to the characteristics of the interventions. However, review authors judged that the risk of performance bias was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Data were collected by a validated method by use of a postal questionnaire mailed to the patient with a Euroqol Health state questionnaire and a questionnaire concerning employment status, further hospital admissions, or any episodes of rebleeding." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcome (functional outcome) at 1 year was available for almost all participants at 1 year. However, long‐term follow‐up data were available for only a proportion of the participants. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary and secondary outcomes reported |
| Other bias | Unclear risk | During recruitment of ISAT, only 22% of participants with SAH treated within the participating centres were enrolled to the study, and 78% were excluded. Most included participants had aneurysm in the anterior circulation. People with SAH in poor clinical condition on admission are under‐represented in the ISAT cohort, which may be considered as a threat to external validity of this trial. |
Koivisto 2000.
| Methods | Method of randomisation: sealed envelopes Blinding of outcome assessment: no Analysis: intention to treat Excluded participants: 131 before randomisation, 2 after randomisation Cross‐over cases: 16 Losses to follow‐up: at 1 year: no losses to follow‐up Definition of outcomes: stated |
|
| Participants | Location: Kuopio University Hospital, Kuopio, Finland Coiling: 52 (men:women ratio: 0.5) Clipping: 57 (men:women ratio: 0.4) Age range: 14–75 years Entry criteria: informed consent, SAH from a ruptured aneurysm suitable for both endovascular and neurosurgical treatment (based on diagnostic angiographic determinants), SAH in the preceding 3 days Comparability of treatment groups: good for major prognostic factors Clinical grade on admission:
Aneurysm location:
|
|
| Interventions | Endovascular coiling Neurosurgical clipping |
|
| Outcomes | Clinical outcomes: 12‐month clinical (good or moderate recovery: GOS 5 and 4; severe disability and vegetative state: GOS 3 and 2; death: GOS 1), neuropsychological and radiological outcomes Endpoints:
|
|
| Notes | Exclusion criteria: aged > 75 years, presence of large haematoma necessitating surgery, mass effect causing neurological deficit, previous surgery for the ruptured aneurysm Follow‐up duration: 3 months and 1 year |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | To avoid selection bias, random assignment was performed separately for people with a Hunt and Hess grade of I–II, for those with a grade of III, and for those with a grade of IV–V. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants or personnel was not possible due to the characteristics of the interventions. However, review authors judged that the risk of performance bias was low. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Clinical outcome at 3 months after treatment was evaluated by the neurosurgeon primarily responsible for treatment or the principal investigator of the study. The 12‐month outcome was evaluated by a single neurosurgeon. The last outcome data obtained by telephone interview were evaluated by a single neurosurgeon or by a single trained nurse." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | Other sources of bias were not identified. |
Li 2012.
| Methods | Method of randomisation: computer‐generated randomisation sequence Blinding of outcome assessment: no Analysis: treatment received Excluded participants: unclear Cross‐over cases: no Losses to follow‐up at 1 year: none Definition of outcomes: stated |
|
| Participants | Location Fengxian District Central Hospital, Shanghai, China Coiling: 94 (men: 68, 72%) Clipping: 92 (men: 62, 67%) Mean age: coiling: 55 years, clipping: 54 years Entry criteria: informed consent, SAH from a ruptured aneurysm Comparability of treatment groups good for major prognostic factors, aneurysm size not given Clinical grade on admission
Aneurysm location:
|
|
| Interventions | Endovascular coiling Neurosurgical clipping |
|
| Outcomes | Clinical outcomes: 12‐month functional outcome (modified Rankin scale), 12‐month case fatality, 12‐month rebleeding rate, rate of delayed cerebral ischaemia, and rate of non‐complete obliteration of aneurysm within 12 months | |
| Notes | Time to randomisation, maximum time between SAH and treatment, and aneurysm size not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence was used |
| Allocation concealment (selection bias) | Low risk | Quote: "…were enrolled into the study and assigned (according to a computer‐generated randomization schedule) to undergo either endovascular coiling or surgical clipping treatment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants or personnel was not possible due to the characteristics of the interventions. However, review authors judged that the risk of performance bias was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Clinical follow‐up was performed in both groups during outpatient clinic visits. A structured telephone interview was performed with outpatients or family who missed the clinic visits; a close relative was contacted in cases where the patient was unavailable." It was unclear whether interviewers were blinded to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. Published report suggested that all intended outcomes were reported, but we contacted study authors for clarification. Risk of reporting bias judged as unclear |
| Other bias | Unclear risk | It was unclear whether the published results represented intention‐to‐treat or treatment received. We sought missing data from the trialists but are awaiting response. |
ACA: anterior cerebral artery; Acom: anterior communicating artery; CT: computed tomography; DSA: digital subtraction angiography; ICA: internal carotid artery; LP: lumbar puncture; MCA: middle cerebral artery; Pcom: posterior communicating artery; SAH: subarachnoid haemorrhage; WFNS: World Federation of Neurological Surgeons subarachnoid haemorrhage grading scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| BRAT 2012 | Inadequate methodological quality:
|
Characteristics of studies awaiting assessment [ordered by study ID]
Wadd 2015.
| Methods | Method of randomisation: unknown Blinding of outcome assessment: no Analysis: treatment received Excluded participants: no Cross‐over cases: unknown Losses to follow‐up at 1 year: none Definition of outcomes: stated |
| Participants | Location Lahore General Hospital, Lahore, Pakistan Coiling: 70 (men 28, 40%) Clipping: 70 (men 28, 40%) Median age: coiling 53 years, clipping 51 years Entry criteria: SAH from a ruptured anterior circulation aneurysm, WFNS 1–3, aged 14–60 years. Giant aneurysms (> 2.5 cm) and aneurysms with broad neck (> 5 mm) were excluded. Comparability of treatment groups good for major prognostic factors, aneurysm size not given Clinical grade on admission
Aneurysm location: not given |
| Interventions | Endovascular treatment by coils Surgical treatment by clips |
| Outcomes | Outcome at 12 months (poor/favourable) |
| Notes | Study authors contacted for missing data in August 2017, but no data received. |
SAH: subarachnoid haemorrhage; World Federation of Neurological Surgeons subarachnoid haemorrhage grading scale.
Characteristics of ongoing studies [ordered by study ID]
ISAT‐2.
| Trial name or title | International Subarachnoid Aneurysm Trial 2 |
| Methods | Method of randomisation: centralised minimisation procedure via web platform Minimisation criteria to be balanced between groups: age ≥ 70 years, WFNS grade > III, aneurysm size > 3 cm, posterior circulation location of aneurysm |
| Participants | Target sample size: 1896 participants, expected loss to follow‐up < 10% Listed location countries: Canada, Spain, US Inclusion criteria
Exclusion criteria
|
| Interventions | Surgical management of the ruptured aneurysm Endovascular management (including use of adjunctive techniques such as flow‐diverting stents and WEB devices in addition to coiling) |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | October 2012 |
| Contact information | clinicaltrials.gov/ct2/show/record/NCT01668563 |
| Notes | Estimated primary completion date: 2023 |
AVM: arteriovenous malformation; mRS: modified Rankin scale; SAH: subarachnoid haemorrhage; WEB: Wowen EndoBridge; WFNS: World Federation of Neurological Surgeons subarachnoid haemorrhage grading scale.
Contributions of authors
AL: extracted data from the studies included in the review; appraised the quality of studies; analysed and interpreted data; and wrote the updated version of the review.
MDIV: extracted data from the studies included in the review; appraised the quality of studies; analysed and interpreted data; and revised the updated version of the review.
IvdS: extracted data from the studies included in the review; prepared the analysis; and wrote the first drafts of the review.
AA: participated in writing the grant application; developed the protocol; appraised the quality of studies; analysed and interpreted data; and participated in writing the review.
MW: participated in writing the review.
MJC: participated in revising the text of the review.
GJER: participated in developing the protocol; extracted data; appraised the quality of studies; analysed and interpreted data; wrote the review and entered the review into Review Manager 5. Dr Rinkel is the guarantor for this review.
Declarations of interest
AL: none known.
MDIV: none known.
IvdS: none known.
AA: none known.
MW: none known.
MJC: was on the Executive Committee for ISAT, but was not involved in decisions about the eligibility or quality of ISAT for this review.
GJER: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Brilstra 2000 {unpublished data only}
- Brilstra EH, Lusseveld E, on behalf of the SCATO Study Group. Early embolization with coils in patients scheduled for delayed operation after aneurysmal subarachnoid hemorrhage: a randomized pilot study. Data on file.
ISAT 2002 {published data only}
- Dorhout Mees SM, Kerr RS, Rinkel GJE, Algra A, Molyneux AJ. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the International Subarachnoid Aneurysm Trial (ISAT). Journal of Neurology 2012;259(4):679‐83. [DOI: 10.1007/s00415-011-6243-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux A, Birks J, Clarke A, Kerr RSC. The durability of endovascular coiling versus neurological clipping of ruptured cerebral aneurysms: 18 year follow‐up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015;385:691‐7. [DOI: 10.1016/S0140-6736(14)60975-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267‐74. [DOI] [PubMed] [Google Scholar]
- Molyneux A, Kerr R, Yu L, Clarke M, Sneade M, Yarnold J, et al. International Subarachnoid Aneurysm Trial (ISAT). A multi‐centre randomised trial comparing neurosurgical clipping with endovascular coiling in 2143 patients with ruptured intracranial aneurysms: overall effect on survival, dependency, seizures, re‐bleeding, subgroup analyses, and frequency of angiographic aneurysm occlusion. Lancet 2005; Vol. 366:809‐17. [DOI] [PubMed]
- Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependency and standardized mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarahcnoid Aneurysm Trial (ISAT): long‐term follow‐up. Lancet Neurology 2009;8:427‐33. [DOI: 10.1016/S1474-4422(09)70080-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh WM, Kerr RC, Algra A, Rinkel GJE, Molyneux AJ, for the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. Effect of antiplatelet therapy for endovascular coiling in aneurysmal subarachnoid hemorrhage. Stroke 2009;40:1969‐72. [DOI: 10.1161/STROKEAHA.108.528802] [DOI] [PubMed] [Google Scholar]
Koivisto 2000 {published and unpublished data}
- Koivisto T. Prospective outcome study of aneurysmal subarachnoid hemorrhage: endovascular versus surgical therapy. Kuopio University Publications D. Medical Sciences 2002;284:1‐149. [ISBN 951‐781‐884‐X; ISSN 1235‐0303] [Google Scholar]
- Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000;31(10):2369‐77. [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only (unpublished sought but not used)}
- Li ZQ, Wang QH, Chen G, Quan Z. Outcomes of endovascular coiling versus surgical clipping in the treatment of ruptured intracranial aneurysms. Journal of International Medical Research 2012;40:2145‐51. [DOI: 10.1177/030006051204000612] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
BRAT 2012 {published data only}
- McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, et al. The Barrow Ruptured Aneurysm Trial. Journal of Neurosurgery 2012;116:135‐44. [DOI: 10.3171/2011.8.JNS101767] [DOI] [PubMed] [Google Scholar]
- Spetzler RF, McDougall CG, Albuquerque FC, Zabramski JM, Hills, NK, Partovi S, et al. The Barrow Ruptured Aneurysm Trial: 3‐year results. Journal of Neurosurgery 2013;119:146‐57. [DOI: 10.3171/2013.3.JNS12683] [DOI] [PubMed] [Google Scholar]
- Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, et al. The Barrow Ruptured Aneurysm Trial: 6‐year results. Journal of Neurosurgery 2015;123:609‐17. [CENTRAL: CN‐01109008; DOI: 10.3171/2014.9.JNS141749] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Wadd 2015 {published data only (unpublished sought but not used)}
- Wadd HI, Haroon A, Habibullah, Ansari S, Mukhtar S, Rashid U, et al. Aneurysmal subarachnoid hemorrhage: outcome of aneurysm clipping versus coiling in anterior circulation aneurysm. Journal of the College of Physicians and Surgeons Pakistan 2015;25:798‐801. [11.2015/JCPSP.798801.] [PubMed] [Google Scholar]
References to ongoing studies
ISAT‐2 {published data only}
- Darsaut TE, Jack AS, Kerr RS, Raymond J. International Subarachnoid Aneurysm Trial ‐ ISAT part II: study protocol for a randomized controlled trial. Trials 2013; Vol. 156. [DOI: 10.1186/1745-6215-14-156] [DOI] [PMC free article] [PubMed]
Additional references
APT 1994
- Antiplatelet Trialist's Collaboration. Collaborative overview of trials of antiplatelet therapy ‐ I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81‐106. [PMC free article] [PubMed] [Google Scholar]
David 1999
- David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow‐up review of surgically treated aneurysms. Neurosurgery 1999;91(3):396‐401. [DOI] [PubMed] [Google Scholar]
de Rooij 2007
- Rooij NK, Linn FHH, Plas JA, Algra A, Rinkel GJE. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. Journal of Neurology, Neurosurgery and Psychiatry 2007;78(12):1365‐72. [DOI: 10.1136/jnnp.2007.117655] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ding 2011
- Ding YH, Lewis DA, Kadirvel R, Dai D, Kallmes DF. The Wowen EndoBridge: a new aneurysm occlusion device. American Journal of Neuroradiology 2011;32(3):607‐11. [DOI: 10.3174/ajnr.A2399] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorhout Mees 2003
- Dorhout Mees SM, Rinkel GJ, Hop JW, Algra A, Gijn J. Antiplatelet therapy in aneurysmal subarachnoid hemorrhage: a systematic review. Stroke 2003;34:2285‐9. [DOI] [PubMed] [Google Scholar]
Falk Delgado 2017
- Falk Delgado A, Andersson T, Falk Delgado A. Clinical outcome after surgical clipping or endovascular coiling for cerebral aneurysms: a pragmatic meta‐analysis of randomized and non‐randomized trials with short‐ and long‐term follow‐up. Journal of Neurointerventional Surgery 2017;9(3):264‐77. [DOI: 10.1136/neurintsurg-2016-012292] [DOI] [PubMed] [Google Scholar]
Fennell 2016
- Fennell VS, Martirosyan NL, Palejwala SK, Lemole M, Dumont TM. Morbidity and mortality of patients with endovascularly treated intracerebral aneurysms: does physician specialty matter?. Journal of Neurosurgery 2016;124:13‐7. [DOI: 10.3171/2014.11.JNS141030] [DOI] [PubMed] [Google Scholar]
Fotakopoulos 2017
- Fotakopoulos G, Tsianaka E, Fountas K, Makris D, Spyrou M, Hernesniemi J. Clipping versus coiling in anterior circulation ruptured intracranial aneurysms: a meta‐analysis. World Neurosurgery 2017;104:482‐8. [DOI: 10.1016/j.wneu.2017.05.040] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook For Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huang 2002
- Huang J, Gelder JM. The probability of sudden death from rupture of intracranial aneurysms: a meta‐analysis. Neurosurgery 2002;51(5):1101‐5. [PUBMED: 12383354] [DOI] [PubMed] [Google Scholar]
Johnston 1998
- Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998;50(5):1413‐8. [DOI] [PubMed] [Google Scholar]
Juvela 1993
- Juvela S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: a long‐term follow‐up study. Journal of Neurosurgery 1993;79(2):174‐82. [DOI] [PubMed] [Google Scholar]
Juvela 2001
- Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms. A long term follow‐up study. Stroke 2001;32(2):485‐91. [DOI] [PubMed] [Google Scholar]
Lanzino 2013
- Lanzino G, Murad MH, d'Urso PI, Rabinstein AA. Coil immobilization versus clipping for ruptured intracranial aneurysms: a meta‐analysis of prospective controlled published studies. American Journal of Neuroradiology 2013;34(9):1764‐8. [DOI: 10.3174/ajnr.A3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li H, Wang H, Rong X, Yin Z, Milgrom D, Shi X, et al. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta‐analysis. Stroke 2013;44:29‐37. [DOI: 10.1161/STROKEAHA.112.663559] [DOI] [PubMed] [Google Scholar]
Lindgren 2016
- Lindgren A, Räisänen S, Björkman J, Tattari H, Huttunen J, Huttunen T, et al. De novo aneurysm formation in carriers of saccular intracranial aneurysm disease in Eastern Finland. Stroke 2016;47:1213‐8. [DOI: 10.1161/STROKEAHA.115.012573] [DOI] [PubMed] [Google Scholar]
McDougall 2012
- McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, et al. The Barrow Ruptured Aneurysm Trial. Journal of Neurosurgery 2012;116:135‐44. [DOI: 10.3171/2011.8.JNS101767] [DOI] [PubMed] [Google Scholar]
Molyneux 2005
- Molyneux A, Kerr R, Yu L, Clarke M, Sneade M, Yarnold J, et al. International Subarachnoid Aneurysm Trial (ISAT). A multi‐centre randomised trial comparing neurosurgical clipping with endovascular coiling in 2143 patients with ruptured intracranial aneurysms: overall effect on survival, dependency, seizures, re‐bleeding, subgroup analyses, and frequency of angiographic aneurysm occlusion. Lancet 2005; Vol. 366:809‐17. [DOI] [PubMed]
Molyneux 2009
- Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependency and standardized mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarahcnoid Aneurysm Trial (ISAT): long‐term follow‐up. Lancet Neurology 2009;8:427‐33. [DOI: 10.1016/S1474-4422(09)70080-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molyneux 2015
- Molyneux A, Birks J, Clarke A, Kerr RSC. The durability of endovascular coiling versus neurological clipping of ruptured cerebral aneurysms: 18 year follow‐up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015;385:691‐7. [DOI: 10.1016/S0140-6736(14)60975-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieuwkamp 2009
- Nieuwkamp DJ, Setz LE, Algra A, Linn FH, Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta‐analysis. Lancet Neurology 2009;8(7):635‐42. [DOI: 10.1016/S1474-4422(09)70126-7] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rinkel 2011
- Rinkel GJ, Algra A. Long‐term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurology 2011;10(4):349‐56. [DOI: 10.1016/S1474-4422(11)70017-5] [DOI] [PubMed] [Google Scholar]
Tsutsumi 2001
- Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms. Results of long‐term follow‐up angiography. Stroke 2001;32(5):1191‐4. [DOI] [PubMed] [Google Scholar]
van den Bergh 2009
- Bergh WM, Kerr RC, Algra A, Rinkel GJE, Molyneux AJ, for the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. Effect of Antiplatelet Therapy for Endovascular Coiling in Aneurysmal Subarachnoid Hemorrhage. Stroke 2009;40:1969‐72. [DOI: 10.1161/STROKEAHA.108.528802] [DOI] [PubMed] [Google Scholar]
Vergouwen 2016
- Vergouwen MD, Jong‐Tien‐Fa AV, Algra A, Rinkel GJ. Time trends in causes of death after aneurysmal subarachnoid hemorrhage: a hospital‐based study. Neurology 2016;86(1):59‐63. [DOI: 10.1212/WNL.0000000000002239] [DOI] [PubMed] [Google Scholar]
Wermer 2005a
- Wermer MJ, Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ, ASTRA Study Group. Follow‐up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain 2005;128:2421‐9. [DOI: 10.1093/brain/awh587] [DOI] [PubMed] [Google Scholar]
Wermer 2005b
- Wermer MJ, Rinkel GJ, Greebe P, Albrecht KW, Dirven CM, Tulleken CA. Recurrence of subarachnoid hemorrhage after treatment for ruptured aneurysms: patient characteristics and outcome. Neurosurgery 2005;56(2):197‐204. [DOI] [PubMed] [Google Scholar]
Xia 2017
- Xia ZW, Liu XM, Wang JY, Cao H, Chen FH, Huang J, et al. Coiling is not superior to clipping in patients with high‐grade aneurysmal subarachnoid hemorrhage: systematic review and meta‐analysis. World Neurosurgery 2017;98:411‐20. [DOI: 10.1016/j.wneu.2016.11.032] [DOI] [PubMed] [Google Scholar]
Çinar 2013
- Çinar C, Oran İ, Bozkaya H, Ozgiray E. Endovascular treatment of ruptured blister‐like aneurysms with special reference to the flow‐diverting strategy. Neuroradiology 2013;55(4):441‐7. [DOI: 10.1007/s00234-013-1136-y] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Algra 2001
- Algra A, Brilstra EH, Clarke M, Gijn J, Molyneux A, Rinkel GJ, et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD003085] [DOI] [PubMed] [Google Scholar]
van der Schaaf 2005
- Schaaf I, Algra A, Wermer M, Molyneux A, Clarke M, Gijn J, et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003085.pub2] [DOI] [PubMed] [Google Scholar]


