Abstract
Background
Mirror therapy is used to improve motor function after stroke. During mirror therapy, a mirror is placed in the person's midsagittal plane, thus reflecting movements of the non‐paretic side as if it were the affected side.
Objectives
To summarise the effectiveness of mirror therapy compared with no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke. We also aimed to assess the effects of mirror therapy on activities of daily living, pain, and visuospatial neglect.
Search methods
We searched the Cochrane Stroke Group's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, AMED, PsycINFO and PEDro (last searched 16 August 2017). We also handsearched relevant conference proceedings, trials and research registers, checked reference lists, and contacted trialists, researchers and experts in our field of study.
Selection criteria
We included randomised controlled trials (RCTs) and randomised cross‐over trials comparing mirror therapy with any control intervention for people after stroke.
Data collection and analysis
Two review authors independently selected trials based on the inclusion criteria, documented the methodological quality, assessed risks of bias in the included studies, and extracted data. We assessed the quality of the evidence using the GRADE approach. We analysed the results as standardised mean differences (SMDs) or mean differences (MDs) for continuous variables, and as odds ratios (ORs) for dichotomous variables.
Main results
We included 62 studies with a total of 1982 participants that compared mirror therapy with other interventions. Of these, 57 were randomised controlled trials and five randomised cross‐over trials. Participants had a mean age of 59 years (30 to 73 years). Mirror therapy was provided three to seven times a week, between 15 and 60 minutes for each session for two to eight weeks (on average five times a week, 30 minutes a session for four weeks).When compared with all other interventions, we found moderate‐quality evidence that mirror therapy has a significant positive effect on motor function (SMD 0.47, 95% CI 0.27 to 0.67; 1173 participants; 36 studies) and motor impairment (SMD 0.49, 95% CI 0.32 to 0.66; 1292 participants; 39 studies). However, effects on motor function are influenced by the type of control intervention. Additionally, based on moderate‐quality evidence, mirror therapy may improve activities of daily living (SMD 0.48, 95% CI 0.30 to 0.65; 622 participants; 19 studies). We found low‐quality evidence for a significant positive effect on pain (SMD −0.89, 95% CI −1.67 to −0.11; 248 participants; 6 studies) and no clear effect for improving visuospatial neglect (SMD 1.06, 95% CI −0.10 to 2.23; 175 participants; 5 studies). No adverse effects were reported.
Authors' conclusions
The results indicate evidence for the effectiveness of mirror therapy for improving upper extremity motor function, motor impairment, activities of daily living, and pain, at least as an adjunct to conventional rehabilitation for people after stroke. Major limitations are small sample sizes and lack of reporting of methodological details, resulting in uncertain evidence quality.
Plain language summary
Mirror therapy for improving movement after stroke
Review question Does mirror therapy improve movement, the performance of daily activities, pain, and lack of attention to and awareness of the affected field of vision (visuospatial neglect) after stroke.
Backround Paralysis of the arm or leg is common after stroke and frequently causes problems with activities of daily living such as walking, dressing, or eating. Mirror therapy (MT) is a rehabilitation therapy in which a mirror is placed between the arms or legs so that the image of a moving non‐affected limb gives the illusion of normal movement in the affected limb. By this setup, different brain regions for movement, sensation, and pain are stimulated. However, the precise working mechanisms of mirror therapy are still unclear. We conducted a search for literature in various databases and extracted the data of relevant studies.
Search date This review identified studies up to 16 August 2017.
Study characteristics We found 62 relevant studies, of which 57 randomly allocated participants to receive either MT or a control therapy (randomised controlled trials) and five provided both therapies to all participants, but in random order (cross‐over trials). The studies involved a total of 1982 participants with a mean age of 59 years (30 to 73 years) after stroke. Mirror therapy was provided three to seven times a week, between 15 and 60 minutes for each session for two to eight weeks (on average five times a week, 30 minutes a session for four weeks).
Key results At the end of treatment, mirror therapy moderately improved movement of the affected upper and lower limb and the ability to carry out daily activities for people within and also beyond six months after the stroke. Mirror therapy reduced pain after stroke, but mainly in people with a complex regional pain syndrome. We found no clear effect for visuospatial neglect. The beneficial effects on movement were maintained for six months, but not in all study groups. No adverse effects were reported.
Quality of the evidence The studies provide moderately‐reliable evidence that MT improves movement (motor function, motor impairment) and the performance of daily activities. However, there was only low reliability that MT decreases pain and visuospatial neglect. This may be due to the small number of studies. Further research is needed, with larger methodologically‐sound studies.
Summary of findings
Summary of findings for the main comparison. Mirror therapy compared to all other interventions: primary and secondary outcomes for improving motor function after stroke.
| Mirror therapy compared to all other interventions: primary and secondary outcomes for improving motor function after stroke | |||||
|
Participants: people with paresis of the upper or lower limb, or both, caused by stroke Setting: inpatient and outpatient Intervention: mirror therapy Control: no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | ||||
| Control | Mirror therapy versus all other interventions | ||||
| Motor function at the end of intervention phase: all outcome measures | The mean motor function at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor function at the end of intervention phase ‐ all studies in the intervention groups was 0.47 SDs higher (0.27 to 0.67 higher) | 1173 (36 RCTs) | ⊕⊕⊕⊝ Moderatea | SMD 0.47, 95% CI 0.27 to 0.67; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Motor impairment at the end of intervention phase: all outcome measures | The mean motor impairment at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor impairment at the end of intervention phase ‐ all studies in the intervention groups was 0.49 SDs higher (0.32 to 0.66 higher) | 1292 (39 RCTs) | ⊕⊕⊕⊝ Moderatea |
SMD 0.49, 95% CI 0.32 to 0.66; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Fugl‐Meyer Assessment upper extremity at the end of intervention phase | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the control groups was NA | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the intervention groups was 4.32 pointshigher (2.46 to 6.19 higher) | 898 (28 RCTs) | ⊕⊕⊝⊝ Lowa,b | MD 4.32, 95% CI 2.46 to 6.19; the minimum important difference is approximately 5.25 |
| Activities of daily living at the end of intervention phase: all studies | The mean activities of daily living at the end of intervention phase ‐ all studies in the control groups was NA | The mean activities of daily living at the end of intervention phase ‐ all studies in the intervention groups was 0.48 SDs higher (0.29 to 0.67 higher) | 622 (19 RCTs) | ⊕⊕⊕⊝ Moderatea | SMD 0.48, 95% CI 0.30 to 0.65; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase: all studies | The mean pain at the end of intervention phase ‐ all studies in the control groups was NA | The mean pain at the end of intervention phase ‐ all studies in the intervention groups was 0.89 SDs lower (1.67 to 0.11 lower) | 248 (6 RCTs) | ⊕⊕⊝⊝ Lowb,c | SMD −0.89, 95% CI −1.67 to −0.11; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase after excluding studies with CRPS | The mean pain at the end of intervention phase ‐ studies without CRPS in the control groups was NA | The mean pain at the end of intervention phase ‐ studies without CRPS in the intervention groups was 0.23 SDs lower (0.53 lower to 0.08 higher) | 176 (4 RCTs) |
⊕⊕⊕⊝ Moderateb | SMD −0.23, 95% CI −0.53 to 0.08; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Visuospatial neglect at the end of intervention: all studies | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the control groups was NA | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the intervention groups was 1.06SDs higher (0.10 lower to 2.23 higher) | 175 (5 RCTs) | ⊕⊕⊝⊝ Lowb,c | SMD 1.06, 95% CI −0.10 to 2.23; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NA: not applicable; SD: standard deviation; SMD: standardised mean difference; MD: mean difference; CRPS: complex regional pain syndrome | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded due to several ratings in one or more items with high or unknown risk of bias. bDowngraded because 95% CI contains effect size of no difference and the minimum important difference. cDowngraded due to unexplained heterogeneity.
Background
Description of the condition
Cerebrovascular diseases, taken together with ischaemic heart diseases, are the leading causes of death worldwide. Stroke is one of the leading causes of long‐term disability, particularly in high‐ and middle‐income countries (Murray 2013). Immediately after stroke onset, approximately 80% of survivors have an upper or lower limb motor impairment (Barker 1997; Jorgensen 1995; Nakayama 1994). Full upper limb function is achieved by nearly 80% of people with mild paresis, but only by 20% of people with severe paresis of the upper limb (Nakayama 1994). Of those people with an initial plegic upper limb, only half regain some motor function in the paretic upper limb six months later (Kwakkel 2003). Two‐thirds of people with lower limb impairment are not able to walk independently soon after their stroke, and after rehabilitation only half have independent walking function (Jorgensen 1995). The initial severity of upper and lower extremity paresis is one of the most important predictors of long‐term functional recovery after stroke (Hendricks 2002; Jorgensen 1995; Nakayama 1994), but variability is high, possibly influenced by therapeutic interventions.
Up to 50% of people experience pain of the upper extremity during the first 12 months post‐stroke, especially shoulder pain and complex regional pain syndrome‐type I (CRPS‐type I) (Jönsson 2006; Kocabas 2007; Lundström 2009; Sackley 2008). Pain after stroke may restrict activities of daily living and reduce quality of life (Jönsson 2006; Lindgren 2007).
Additionally, about 40% of people with an acute right hemispheric and 20% of people with a left hemispheric stroke present a unilateral neglect (Ringman 2004), especially visuospatial neglect. After three months a unilateral neglect was present in about 15% of people with a right and 5% of people with a left hemispheric stroke (Ringman 2004). Besides the spatial attention deficits, neglect is a negative factor for functional recovery (Farnè 2004; Katz 1999), and was found to be associated with a reduced health‐related quality of life (Franceschini 2010).
Effective training strategies to promote motor recovery and activities of daily living, to reduce pain or visuospatial neglect or both are therefore needed to reduce the burden of stroke.
Description of the intervention
Evidence suggests that effective therapeutic interventions for regaining motor function should potentially focus on the practice of functional tasks (Van Peppen 2004). However, task‐oriented training strategies, such as constraint‐induced movement therapy (Corbetta 2015; French 2016; Liepert 1998; Miltner 1999; Taub 1993), require some degree of voluntary movement, and are therefore not applicable for people with severe paresis after stroke. Novel training strategies for this patient population use electromechanical training devices (Mehrholz 2015; Mehrholz 2017), electrical muscle stimulation (Hatem 2016; Urton 2007), or repetitive passive or assistive movement stimulation (Feys 2004; Platz 2005).
As an alternative treatment approach, mirror therapy has been proposed as potentially beneficial (Ramachandran 1994). In contrast to other interventions, which employ somatosensory input to assist motor recovery (Feys 2004), mirror therapy is based on visual stimulation. During mirror therapy, a mirror is placed in the person's midsagittal plane, thus reflecting the non‐paretic side as if it were the affected side (Ramachandran 1995). By this setup, movements of the non‐paretic limb create the illusion of normal movements of the paretic limb (Deconinck 2015). One of the advantages of mirror therapy is the relatively easy administration and the possibility of self‐administered home therapy, even for people with severe motor deficits. Clinical studies reported effects of mirror therapy on pain reduction in arm amputees or CRPS‐type I (Ramachandran 1995; Ramachandran 1996; Thieme 2016). Furthermore, mirror therapy was claimed to alleviate hemiparesis after stroke (Ramachandran 1994), which was confirmed in a pilot study (Altschuler 1999).
Recently, some authors have described 'mirror‐like' video or computer‐graphic setups, where a video or computer‐graphic image of the moving limb is presented as if it were the opposite one (Adamovich 2009; Eng 2007; Gaggioli 2004; Hoermann 2017; In 2012; Laver 2017; Morganti 2003).
How the intervention might work
The concept of mirror therapy has been substantiated neurophysiologically. There is long‐standing evidence that observation of movements and performance of the observed actions share similar cortical motor areas (Grèzes 2001). Movement mirroring (i.e. the inversion of the visual feedback) leads to an additional activation of the hemisphere contralateral to the perceived limb laterality (Deconinck 2015; Dohle 2004; Matthys 2009; Shinoura 2008). The mirror illusion may increase cortico‐muscular excitability (Fukumura 2007; Garry 2005; Kang 2011; Kang 2012). However, the precise mechanisms of the effect of mirror therapy in people with stroke remain speculative. As the visual image of the paretic limb is perceived similarly to the person's own moving limb (Dohle 2004), the mirror illusion might prevent or reverse a learned non‐use of the paretic limb (Liepert 1995). Also, by modulation of the cortico‐muscular excitability, mirror therapy might directly stimulate motor recovery. Finally, mirror therapy was regarded as a variant of motor imagery training, which is based on repetitive imagination and mental rehearsal of motor tasks (Miltner 1998; Stevens 2003). Behavioural studies suggest that the experience of agency (the attribution of visual images of body parts as being controlled by oneself) relies on a tight temporal coupling of the visual feedback of active, but not passive, movements (Longo 2009). It is this active performance that seems to distinguish mirror therapy from movement observation therapy (Wang 2013b).
Imaging studies further suggest that mirrored computer‐graphic images are processed similarly to those of real movements (Adamovich 2009; Dohle 2011), as long as the temporal and spatial consistency with real movements does not fall below certain thresholds (Franck 2001). Thus, even technically‐generated images of a human moving limb can be integrated into the body scheme with the same sense of agency as during 'real' mirroring.
Regarding non‐motor symptoms, some studies also found significant effects of mirror therapy on somatosensory impairment after stroke (Acerra 2007; Dohle 2009). Cortical effects might be different from those for rehabilitation of motor function (Fritzsch 2014). Besides, mirror therapy was proposed to reduce unilateral visuospatial neglect after stroke (Dohle 2009). The strong visual stimulus of watching self‐induced movements in the neglected hemifield was postulated to be responsible for this effect. However, this could only be confirmed if the mirror was placed in the affected, rather than the non‐affected, side of the body (Ramachandran 1999).
Finally, mirror therapy was found to be effective in reducing pain in different conditions (Bowering 2013; Thieme 2016). It is hypothesised that mirror therapy may normalise central sensory processing by providing a physiological image of the affected limb (McCabe 2003).
Why it is important to do this review
Since the first publication of our Cochrane Review, a number of new clinical studies about mirror therapy after stroke have been published. An update of the review is therefore required in order to provide a current estimation of the available evidence and to address limitations found in the original review.
Objectives
To summarise the effectiveness of mirror therapy compared with no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke. We also aimed to assess the effects of mirror therapy on activities of daily living, pain, and visuospatial neglect.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cross‐over RCTs comparing mirror therapy (provided by a mirror or a simultaneous video or virtual setup) with any other therapy modality, no therapy, or sham therapy. If we included cross‐over RCTs, we only analysed the first period as a parallel‐group trial.
Types of participants
We included studies examining participants with a paresis of the upper or lower limb, or both, caused by stroke (all types, severity and stages of stroke) aged over 18 years. If we identified studies with mixed populations of people with neurological conditions, we included those studies if separate data for people with stroke were available.
Types of interventions
Mirror therapy is defined as an intervention that uses a mirror to create a reflection of the non‐paretic upper or lower limb, thus giving the person visual feedback of normal movement of the paretic limb. Using this setup, different variations in the experimental protocol are possible (Bieniok 2011; Dohle 2005). We included studies that used direct mirroring of movement of any regimen and variation (i.e. including video or virtual reality settings). However, we only included those studies where the regimen and delivery of mirror therapy could be identified. Furthermore, for studies with a combination of mirror therapy and other therapies in the experimental condition, we only included studies where a minimum of 50% of the experimental intervention time was applied for mirror therapy.
The control arm of the study could include a no‐treatment group, usual or standard practice, or any other control treatment (i.e. placebo or sham therapy). We excluded studies where the influence of mirror therapy could not be isolated due to the comparison of different mirror therapy regimens or delivery. We contacted trialists if the regimen or delivery (or both) of mirror therapy or the control intervention was unclear.
Types of outcome measures
We evaluated outcome measures post‐intervention and at follow‐up after six months or longer.
Primary outcomes
The primary outcome was motor function. Due to the wide variety of outcome measures, we selected outcome measures to facilitate quantitative pooling. If more than one outcome measure was available we prioritised measures as follows:
Upper limb and hand motor function: Action Research Arm Test (Lyle 1981), Wolf Motor Function Test (Wolf 2001), Motor Assessment Scale ‐ upper limb and hand function or both (Carr 1985), Manual Function Test (Miyamoto 2009), Box and Bock Test (Mathiowetz 1985).
Lower limb motor function: Motor Assessment Scale ‐ Items 4 or 5 (or both) (Carr 1985), Berg Balance Scale (Berg 1992).
Global motor function: Motor Assessment Scale (Carr 1985), Rivermead Motor Assessment Scale (Collen 1991).
However, if these scales were not available, we accepted other measurements that evaluate motor function.
Secondary outcomes
Secondary outcomes included measures of motor impairment (upper limb motor impairment: Fugl‐Meyer Assessment ‐ upper limb or hand function or both (Fugl‐Meyer 1975); Brunnstrom Stages of the Upper Extremity (Brunnstrom 1966); Motricity Index ‐ arm score, muscle or grip strength (Demeurisse 1980)); lower limb motor impairment: Fugl‐Meyer Assessment ‐ lower limb function (Fugl‐Meyer 1975); Brunnstrom Stages of the Lower Extremity (Brunnstrom 1966), activities of daily living (e.g. Functional Independence Measure: Keith 1987), Barthel Index: Mahoney 1965)); pain (Visual Analogue Scale or Numeric Rating Scale), and visuospatial neglect. We also searched for reported adverse effects (e.g. swelling) and dropout rate.
Search methods for identification of studies
See the 'Specialised register' section in the Cochrane Stroke Group module. We searched for relevant trials in all languages and arranged translation of trial reports where necessary.
Electronic searches
We searched the Cochrane Stroke Group's Trials Register (last searched on 16 August 2017); Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) in the Cochrane Library (last searched on 16 August 2017); MEDLINE Ovid (1946 to August 2017); Embase Ovid (1974 to August 2017); Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO; 1982 to August 2017); Allied and Complementary Medicine (AMED Ovid; 1985 to August 2017); PsycINFO Ovid (1806 to August 2017); and the Physiotherapy Evidence Database (PEDro; searched August 2017).
We developed the MEDLINE search strategy for this review with the assistance of the Cochrane Stroke Group's Information Specialist and adapted it to search the other databases (Appendix 1; Appendix 2). We included all languages, and imposed no date limits. As the subject area of this review is quite specific, we did not include a trials filter to maximise the sensitivity of the search.
We also searched ongoing trials and research registers:
ISRCTN Registry (www.isrctn.com/, searched December 2016);
ClinicalTrials.gov (www.clinicaltrials.gov/, searched December 2016);
StrokeTrials Registry (www.strokecenter.org/trials/, searched December 2016);
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/, searched December 2016);
Searching other resources
In an effort to identify further published, unpublished and ongoing trials not available in the major databases, we:
-
handsearched the following conference proceedings:
Deutsche Gesellschaft für Neurologie (2008 to 2016);
Deutsche Gesellschaft für Neurorehabilitation (2000, 2001, 2003, 2005, 2007, 2009, 2010, 2012, 2013, 2014, 2016);
Deutsche Gesellschaft für Neurotraumatologie und klinische Neurorehabilitation (2005, 2007, 2009, 2010, 2014, 2016);
European Stroke Conference (2001 to 2015);
European Congress of Neurorehabilitation (2011, 2013, 2015);
World Congress of Neurorehabilitation (1999, 2002, 2006, 2010, 2012, 2014, 2016);
World Congress of Physical Therapy (2003, 2007, 2011, 2015);
World Stroke Congress (2000, 2004, 2008, 2010, 2012, 2014);
screened reference lists of all relevant articles and books;
contacted trialists, experts, researchers and commercial companies (Reflex Pain Management Ltd) in our field of study to obtain information of unpublished studies and studies not available in the electronic databases;
searched System for Information on Grey Literature in Europe (OpenSIGLE ‐ www.opengrey.eu/, searched December 2016); and
searched the REHABDATA database (www.naric.com/research/rehab, searched December 2016).
Data collection and analysis
Selection of studies
Two of three review authors (HT, NM and CD) independently screened titles of the references identified from the electronic database searches and ruled out obviously irrelevant references. We obtained abstracts or full texts, or both, of the remaining studies and used our inclusion criteria (types of studies, types of participants, types of interventions and outcome measures) to assess whether they were eligible for inclusion. We resolved disagreements by discussion. If the inclusion of a study was unclear due to missing information, we tried to contact the authors of the studies for further details. Otherwise, we listed the study as 'awaiting classification'.
Data extraction and management
Two of three review authors (HT, NM and CD) independently extracted trial and outcome data of the included trials using a checklist. Because two of the review authors (HT, CD) are principal investigators of included trials, other authors (JB, JM) did the data extraction of those study. The checklists for data extraction contained:
methods of randomisation;
methods of concealment of allocation;
blinding;
use of an intention‐to‐treat (ITT) analysis (all participants initially randomised were included in the analysis in their originally‐allocated groups);
adverse events;
dropouts for all reasons;
imbalance of important prognostic factors;
participants (country, number of participants, age, gender, type of stroke, time since stroke onset to study entry);
inclusion and exclusion criteria;
details of interventions in treatment and control groups;
outcomes;
time points of measurement.
We tried to establish all unclear characteristics of the studies by contacting the trial co‐ordinator or principal investigator. We checked the extracted data for agreement between review authors and entered the data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
We used the 'Risk of bias' assessment tool according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to assess the adequacy of methods for sequence generation (selection bias), concealment of allocation (selection bias), completeness of outcome data or handling of incomplete outcome data (attrition bias), and blinding of assessors (detection bias) (Higgins 2011).
We did not integrate blinding of therapists and participants as an item in the 'Risk of bias' assessment, since this appeared not to be possible for the type of interventions in this review.
We resolved disagreements in methodological assessment by consulting a third review author (MP, JM or JB), and reached consensus through discussion. If an article did not contain information on any methodological criteria, we contacted the study authors for additional information. If no further information was available, we rated the criteria as 'unclear'.
GRADE and 'Summary of findings' table
We assessed the quality of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), for the following main outcomes of analysis: motor function, motor impairment, Fugl‐Meyer Assessment, activities of daily living, pain, pain at the end of intervention phase after excluding studies with CRPS, and visuospatial neglect, each at the end of the intervention phase. We presented key findings of the review, including a summary of the amount of data, the magnitude of the effect size, and the overall quality of the evidence, in Table 1.
Measures of treatment effect
The primary and secondary outcome variables of interest were continuous outcomes. We entered data of post‐intervention assessment and follow‐up assessment at six months as means and standard deviations (SDs) and calculated the standardised mean difference (SMD) or mean difference (MD) with 95% confidence intervals (CIs) for each trial. We pooled data through calculation of the overall SMD/MD and 95% CI. For dichotomous data (adverse events, dropouts) we calculated odds ratios (ORs) between groups.
Unit of analysis issues
We considered randomised cross‐over trials prior to cross‐over and analysed only the first intervention phase.
Dealing with missing data
We contacted study authors if appropriate data for analysis were not adequately reported. If study authors did not respond within one month after contact, we tried to contact them at least once more. If data were not sufficient to decide on inclusion or exclusion of studies, we rated the studies as 'awaiting classification'. If data were insufficient for meta‐analysis, we excluded the studies from meta‐analysis. If we were unable to get the missing data for participants who dropped out, we only analysed the participants for which we had data. However, we considered an ITT analysis as part of the 'Risk of bias' assessment and performed a sensitivity analysis in which we excluded studies with no or unreported ITT analysis. We also conducted a sensitivity analysis, excluding studies with missing methodological data (therefore rated as 'unclear' risk of bias).
Assessment of heterogeneity
We evaluated clinical heterogeneity through reported clinical and methodological diversity, variability of participants, interventions, and outcomes in an additional table. We used the I2 statistic to assess heterogeneity. We used a random‐effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity, we did not violate the preconditions of a fixed‐effect model approach.
Assessment of reporting biases
We tried to minimise reporting bias through an extensive search of databases, handsearching of references lists and conference abstracts, and by contacting study authors, trialists, and experts in the field for other unpublished or ongoing trials. We also conducted a sensitivity analysis, excluding studies of low methodological quality.
Data synthesis
Where possible, we conducted a pooled analysis of primary outcomes (motor function) and secondary outcomes (motor impairment, activities of daily living, pain, visuospatial neglect, dropout rate) as described above, using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis to establish the effectiveness of mirror therapy focused on upper or lower extremity. We also investigated heterogeneity regarding time since stroke. We performed a subgroup analysis separating participants in an acute/subacute stage from those in a chronic stage after stroke; the cut‐off point for separating these subgroups was six months after stroke. We also investigated heterogeneity by the type of control intervention used. We separated subgroups using no (additional) control intervention, another control intervention, and sham intervention with restricted view on the paretic extremity.
Sensitivity analysis
We conducted a sensitivity analysis to test the robustness of the results, removing studies that we assessed to be of lower or ambiguous methodological quality (studies with risk of bias for at least one method of sequence generation, concealment of allocation, ITT analysis, or blinded assessors). We also reanalysed the data by removing cross‐over RCTs.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, Characteristics of ongoing studies and Table 2.
1. Characteristics of participants of included studies.
| Study ID | Mean age | Sex | Side of paresis | Time since stroke | Type of stroke | |||
| Years | Women | Men | Left | Right | Mean time | Ischaemic | Haemorrhagic | |
| Acerra 2007 | 68 | 22 | 18 | 16 | 24 | 5.3 days | 40 | 0 |
| Alibakhshi 2016 | 50.9 | 9 | 15 | 15 | 9 | n/r | n/r | n/r |
| Altschuler 1999 | 58.2 | 4 | 5 | 8 | 1 | 4.8 years | n/r | n/r |
| Amasyali 2016 | 58.8 | 11 | 13 | 8 | 16 | 5.3 months | 24 | 0 |
| Arya 2015 | 45.6 | 8 | 25 | 7 | 26 | 12.9 months/12.3 months. | 17 | 16 |
| Arya 2017 | 46.4 | 6 | 30 | 16 | 20 | 15.9 months | 17 | 9 |
| Bae 2012 | 53.9 | 7 | 13 | 13 | 7 | 4.6 months | 9 | 11 |
| Bahrami 2013 | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Cacchio 2009a | 58.4 | 26 | 22 | 34 | 14 | 5 months | 35 | 13 |
| Cacchio 2009b | 62 | 13 | 11 | 15 | 9 | 15.7 months | 19 | 5 |
| Cha 2015 | 58.7 | 17 | 19 | n/r | n/r | 1.8 months | n/r | n/r |
| Cho 2015 | 59.3 | 12 | 15 | 14 | 13 | 13.2 months/15.5 months | 17 | 10 |
| Colomer 2016 | 53.5 | 5 | 26 | 24 | 7 | 551 days | 23 | 8 |
| Dalla Libera 2015 | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Dohle 2009 | 56.5 | 10 | 26 | 25 | 11 | 27 days | 48 | 0 |
| Geller 2016 | n/r | 3 | 3 | n/r | n/r | n/r | n/r | n/r |
| Gurbuz 2016 | 60.9 | 14 | 17 | 14 | 17 | 44.3 days | 25 | 6 |
| Hiragami 2012 | 67.5 | 6 | 8 | 6 | 8 | 47 days | 9 | 5 |
| In 2012 | 63.9 | 8 | 11 | 9 | 10 | 14.1 months | 10 | 9 |
| In 2016 | 55.9 | 10 | 15 | 13 | 12 | 13.1 days | 16 | 9 |
| Invernizzi 2013 | 66.6 | 9 | 17 | 13 | 13 | 23 days | 26 | 0 |
| Ji 2014a | 52.6 | 13 | 22 | 14 | 21 | 8.9 months | 19 | 16 |
| Kawakami 2015 | 64.1 | 24 | 43 | 35 | 32 | 32.3 days | 28 | 39 |
| Kim 2014 | 55.8 | 9 | 14 | 13 | 10 | 34.5 days | 14 | 9 |
| Kim 2015a | 57.7 | 9 | 20 | 20 | 9 | 404.4 days | 14 | 15 |
| Kim 2016 | 49.1 | 9 | 16 | 16 | 9 | n/r | 8 | 17 |
| Kojima 2014 | 69.1 | 3 | 10 | 5 | 8 | 78.8 days | 10 | 3 |
| Kumar 2013 | 57.3 | 8 | 22 | n/r | n/r | n/r | n/r | n/r |
| Kuzgun 2012 | 61.4 | 10 | 10 | 10 | 10 | n/r | n/r | n/r |
| Lee 2012 | 57.1 | 11 | 15 | 11 | 15 | 3.6 months | n/r | n/r |
| Lee 2016 | 54.7 | 13 | 14 | 8 | 19 | 39.6 months | 8 | 20 |
| Lim 2016 | 64.9 | 21 | 39 | 31 | 29 | 52 days | 19 | 41 |
| Lin 2014a | 55 | 11 | 32 | 22 | 21 | 19.6 months | 20 | 28 |
| Manton 2002 | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Marquez 2012 | 68.7 | 8 | 7 | 9 | 6 | 24.3 days | 10 | 5 |
| Michielsen 2011 | 57 | 20 | 20 | 28 | 12 | 4.6 years | 28 | 12 |
| Mirela 2015 | 57.5 | 8 | 7 | 5 | 10 | 53.2 days | 15 | 0 |
| Mohan 2013 | 63 | 10 | 12 | 6 | 16 | 6.4 days | 14 | 8 |
| Moustapha 2012 | 53.5 | 4 | 4 | 4 | 4 | 4.5 months | n/r | n/r |
| Nagapattinam 2015 | 44.9 | 20 | 40 | n/r | n/r | 4.2 months | 60 | 0 |
| Pandian 2014 | 63.4 | 20 | 28 | 37 | 11 | 2 days | 26 | 22 |
| Park 2015a | 56.3 | 13 | 17 | 14 | 16 | 20.9 months | 16 | 14 |
| Park 2015b | 60 | 15 | 15 | 17 | 13 | 8.2 months | 17 | 13 |
| Piravej 2012 | 56 | 19 | 21 | 25 | 15 | 7.2 months | 27 | 13 |
| Rajappan 2016 | 58 | 9 | 21 | 3 | 27 | 5 months | 20 | 10 |
| Rehani 2015 | 56.3 | n/r | n/r | n/r | n/r | 83.9 days | n/r | n/r |
| Rodrigues 2016 | 57.5 | 6 | 10 | 11 | 5 | 34.8 months | 16 | 0 |
| Rothgangel 2004 | 73.4 | 10 | 6 | 8 | 8 | 9.5 months | 16 | 0 |
| Salhab 2016 | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Samuelkamaleshkumar 2014 | 51.2 | 4 | 16 | 9 | 11 | 4.1weeks | 14 | 6 |
| Schick 2017 | 63 | 13 | 19 | 15 | 17 | 50 days | 27 | 5 |
| Seok 2010 | 51.4 | 22 | 18 | n/r | n/r | 4.0 months | n/r | n/r |
| Sütbeyaz 2007 | 63.4 | 17 | 23 | 27 | 13 | 3.7 months | 33 | 7 |
| Tezuka 2006 | 63.7 | 9 | 6 | 6 | 9 | 32.7 days | n/r | n/r |
| Thieme 2013 | 67.2 | 25 | 35 | 37 | 23 | 45 days | 45 | 15 |
| Tyson 2015 | 64 | 34 | 60 | 56 | 38 | 29 days | 76 | 18 |
| Wang 2015 | 64.9 | 40 | 50 | 39 | 51 | 63.7 days | 57 | 33 |
| Wu 2013 | 54.2 | 10 | 23 | 18 | 15 | 20.6 months | 20 | 13 |
| Yavuzer 2008 | 63.3 | 17 | 19 | 21 | 19 | 5.5 months | 29 | 7 |
| Yoon 2014 | 57.8 | 10 | 16 | 15 | 11 | 22.7 days | 16 | 10 |
| Yun 2011 | 63.3 | 21 | 39 | 31 | 29 | 25.8 days | 46 | 14 |
| Zacharis 2014 | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
n/r: not reported
Results of the search
We identified 33 new studies from the updated search of the Cochrane Stroke Group's Trials Register. We also identified 8879 references from other electronic databases and 14 references from other sources. After excluding all duplicate references we identified 3588 references from the updated search in all electronic databases (5408 with references in the first version of this review). Two review authors (HT, NM or CD) identified 519 possibly eligible studies (652 with studies in the first version of this review). We discarded 470 studies (599 with studies in the first version of this review). There was insufficient information to determine inclusion eligibility for six trials (Amimoto 2008; ISRCTN40903497; Magni 2014; May 2011; Wang 2013a; Yeldan 2015), but we failed to get in contact with the authors, so the studies are listed as 'awaiting classification' (see Characteristics of studies awaiting classification). We also identified 15 ongoing trials (see Characteristics of ongoing studies). We therefore include 49 new studies (62 with studies in the first version of this review) in this updated version of the review (see Figure 1).
1.
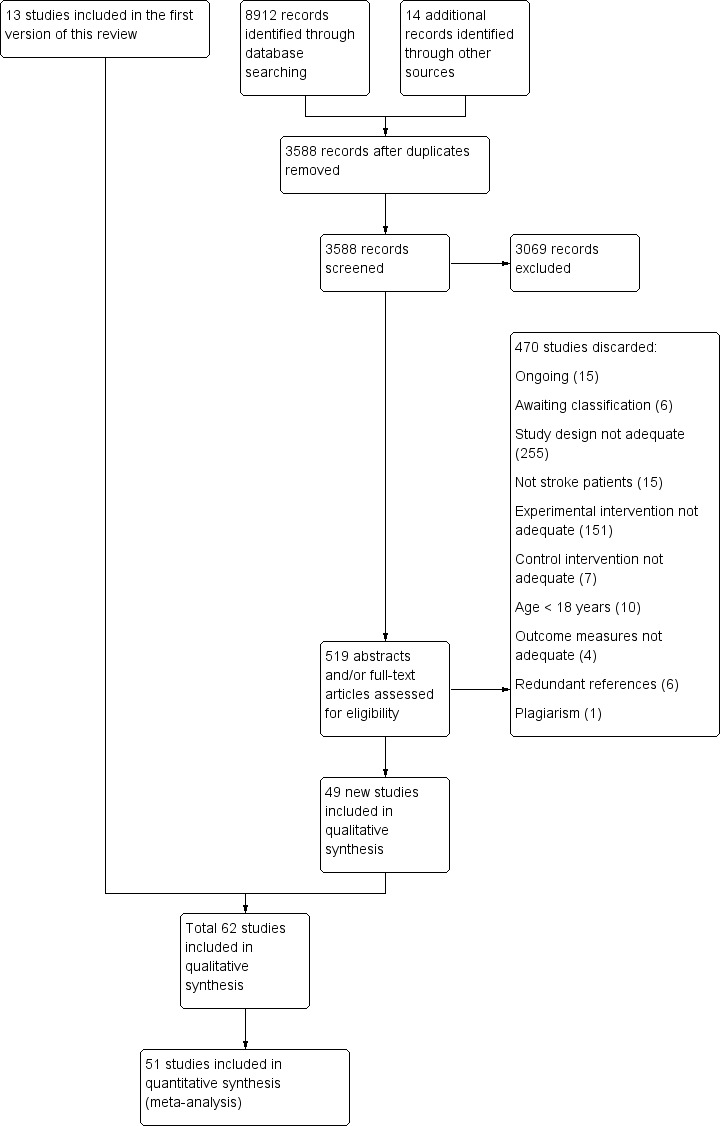
Study flow diagram of updated search and selection process
Included studies
Sixty‐two trials met the inclusion criteria of our review (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Moustapha 2012; Nagapattinam 2015; Pandian 2014; Park 2015a; Park 2015b; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Salhab 2016; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011; Zacharis 2014) (see Characteristics of included studies).
We now exclude one study which we had included in the first version of this review, as only 15% of the experimental intervention was spent in mirror therapy (Ietswaart 2011).
Because the two groups in Rothgangel 2004 received significantly different treatment sessions, we decided to split the data and analyse them separately (outpatient group: Rothgangel 2004a, and inpatient group: Rothgangel 2004b).
Design
Fifty‐seven studies were RCTs with a parallel‐group design (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Nagapattinam 2015; Pandian 2014; Park 2015a; Park 2015b; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011; Zacharis 2014), and five studies used a cross‐over design with random allocation to the order of treatment (Altschuler 1999; Kojima 2014; Moustapha 2012; Salhab 2016; Tezuka 2006).
Sample Size
The 62 studies included a total of 1982 participants. Individual sample sizes of identified trials ranged from six (Geller 2016) to 94 (Tyson 2015). A detailed description of individual sample sizes can be found in Characteristics of included studies.
Participants
Not all studies provided data on characteristics of participants. Detailed descriptions of participant characteristics are given in Table 2.
The mean age of participants in the included studies was 59 years, with a range from 30 years (Moustapha 2012) to 78 years (Tezuka 2006). There were more participants with a hemiparesis of the left side (53%). There were more men (60%) than women (40%). Twenty‐four studies included participants after their first‐ever stroke. Mean time post‐stroke ranged between five days (Acerra 2007), and five years (Altschuler 1999). Twenty‐nine studies included participants in the acute or subacute phase after stroke (within six months post‐stroke) and 21 trials included participants in the chronic phase (more than six months). Among those participants with known aetiology, 67% had an ischaemic and 33% a haemorrhagic stroke.
Fifty‐two studies provided information on the study setting: 39 inpatient rehabilitation settings or hospitals; three inpatient and outpatient rehabilitation settings; four home settings; two inpatient and home setting; and four outpatient settings (Table 3). The included studies were conducted in 21 different countries.
2. Characteristics of interventions of included studies.
| Study ID | Extremity | Mirror therapy variation | Control intervention | Type of movements | Minutes per session | Sessions per week | Total duration (weeks) | Total amount of therapy (minutes) | Setting |
| Acerra 2007 | Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Functional motor tasks (i.e. with objects); motor co‐ordination tasks; sensory discrimination tasks; grip strength; active range of motion |
20 to 30 | 7 | 2 | 280 ‐ 420 | Inpatient hospital |
| Alibakhshi 2016 | Upper extremity | Bilateral activities | Bilateral activities without mirror | n/r | 30 | 5 | 3 | 450 | Inpatient hospital |
| Altschuler 1999 | Upper extremity | Bilateral activities | Bilateral activities; transparent plastic between limbs | Proximal and distal movements | 15 (2 times a day) | 12 | 4 (1st period) | 720 | n/r |
| Amasyali 2016 | Upper extremity | Activities of the unaffected limb | 1. EMG‐triggered electrostimulation; 2. control group: no additional therapy | Wrist, hand flexion, extension and forearm circumduction, and supination–pronation | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre |
| Arya 2015 | Upper extremity | Activities of the unaffected limb | Conventional therapy based on Brunnstrom and Bobath principles | Task‐based mirror therapy: finger dexterity, mass grasp/finger flexion, release/finger extension, wrist dorsiflexion, and forearm supination by using objects and practising tasks | 45 | 5 | 8 | 1800 | Inpatient hospital, home after discharge |
| Arya 2017 | Lower extremity | Activities of the unaffected limb | Conventional motor therapy based on neurophysiological approaches | Activity‐based MT: ball‐rolling, rocker‐board and pedaling | 60 | n/r | 3 ‐ 4 (30 session) | 1800 | Inpatient rehabilitation centre |
| Bae 2012 | Upper extremity | Bilateral activities | Activities of the non‐paretic arm, without mirror | Flexion/extension of the shoulder, radial/ulnar deviation and pro‐/supination of the forearm, flexion/extension of the fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre |
| Bahrami 2013 | Upper and lower extremity | Activities of the unaffected limbs | Routine programme (physiotherapy and neuromuscular stimulation) | Range of motion of the healthy limbs | 30 | 5 | 4 | 600 | n/r |
| Cacchio 2009a | Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 1st 2 weeks; 60 last 2 weeks |
5 | 4 | 900 | Inpatient and outpatient rehabilitation centre |
| Cacchio 2009b | Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror (control group 1); imagination of movements of the affected limb (control group 2) |
Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 | Daily | 4 | 840 | Inpatient and outpatient rehabilitation centre |
| Cha 2015 | Lower extremity | Activities of the unaffected limb + rTMS | Activities of the unaffected limb; covered mirror + rTMS | Flexing and extending the hip, knee, and ankle at a self‐selected speed under supervision but without additional verbal feedback | 20 | 5 | 4 | 400 | n/r |
| Cho 2015 | Upper extremity | Activities of the unaffected limb + tDCS /anode attached over primary motor cortex | Activities of the unaffected limb; covered mirror + tDCS | Pronation, supination, flexion, and extension of both wrists, flexion and extension of the fingers, and flexion and extension of the elbows (10 sets, 20 repetitions per motion and set, 2 min rest between sets) | 20 | 3 | 6 | 360 | n/r |
| Colomer 2016 | Upper extremity | Activities of the unaffected limb | Passive mobilisation of the affected limb | Flexion and extension of shoulder, pronation and supination of forearm, gross and fine motor movements of wrist, hand and fingers (also with objects) | 45 | 3 | 8 | 1080 | Outpatient rehabilitation centre |
| Dalla Libera 2015 | Upper extremity | 10 Hz TMS applied by 8‐coil on the ipsilesional somatosensory cortex, followed by MT | TMS only | n/r | 30 | 3 | 4 | 360 | n/r |
| Dohle 2009 | Upper extremity | Bilateral activities | Bilateral activities; without mirror | Execution of arm, hand and finger postures | 30 | 5 | 6 | 900 | Inpatient rehabilitation centre |
| Geller 2016 | Upper extremity | Bilateral and unilateral activities | Traditional occupational therapy | n/r | 30 | 5 | 6 | 900 | Home setting |
| Gurbuz 2016 | Upper extremity | Activities of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion and extension of wrist and finger | 20 | 5 | 4 | 400 | Inpatient rehabilitation centre |
| Hiragami 2012 | Upper extremity | Bilateral activities | No additional therapy | Supination and eversion of the forearm, flexion and extension of the wrist and finger, grasp a block | 30 | 6 or 7 | 4 | 720 ‐ 840 | Inpatient Hospital |
| In 2012 | Upper extremity | Bilateral activities; virtual mirror on a screen; arm projected by a camera | Bilateral activities; without mirror (screen was off) | 1st week: wrist flexion/ extension, forearm pro‐/supination, clenching and opening the hand, 2nd week gross motor tasks, 3rd and 4th week fine motor tasks; 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre |
| In 2016 | Lower extremity | Uni‐ and bilateral activities; virtual mirror on the screen, leg projected by a camera | Uni‐ and bilateral activities; without mirror (screen was off) | 1st week: dorsiflexion and plantarflexion (lifting of the heel) of the unaffected ankle; adduction and abduction of forefoot and rear foot; and adduction and abduction of the hip (moving the knees inward and outward), 2nd week mimicked the movements (1st week) of the unaffected lower limb on the monitor with the affected lower limb, 3rd dorsiflexion, adduction and abduction of the unaffected ankle; plantar flexion, adduction and abduction of the ankle; and adduction and abduction of the hip; 4th week: complex movements and different tasks (remote control with up and down buttons); 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 5 | 600 | Inpatient rehabilitation centre |
| Invernizzi 2013 | Upper extremity | Movements of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist, pro‐ /supination of the forearm, self selected speed, no additional verbal feedback | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient rehabilitation centre |
| Ji 2014a | Upper extremity | Experimental 1: MT: Movements of the unaffected limb + rTMS; Experimental 2: MT: Movements of the unaffected limb | Activities of the unaffected limb, covered mirror | Experimental 1: finger flexion and extension + 10Hz rTMS on lesioned hemisphere; Experimental 2: finger flexion and extension | 15 | 5 | 6 | 450 | University hospital |
| Kawakami 2015 | Lower extremity | Bilateral activities and activities of the unaffected limb | 4 control groups: (1) EMG triggered electrical muscle stimulation; (2) electrical muscle stimulation; (3) repetitive facilitation exercises; (4) passive and active‐assistive range of motion exercises | Dorsiflexion of the ankle joint, stepping over, and abduction/adduction of the hip joint) | 20 | 7 | 4 | 560 | Inpatient rehabilitation centre |
| Kim 2014 | Upper extremity | Bilateral activities + FES | Bilateral activities + FES; covered mirror | Extension of wrist and fingers to lift of the hand from an FES switch, at the same time attempt to extend affected hand supported by electrical stimulation (20 Hz), pulse rate 300 μs, individual intensity for muscle contraction and complete extension | 30 | 5 | 4 | 600 | University hospital |
| Kim 2015a | Upper extremity | Bilateral activities + FES | No additional therapy | 2 experimental groups: (1) EMG‐triggered FES (due to unaffected limb) of affected wrist extension + physiological and object‐related movements; (2) FES of affected wrist extension + physiological and object‐related movements | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre |
| Kim 2016 | Upper extremity | Activities of the unaffected limb | Conventional therapy | Arm bicycling, peg board exercise, skateboard‐supported exercises on a tabletop, donut on base putty kneading, double curved arch, bimanual placing cone, block stacking, graded pinch exercise, plastic cone stacking, shoulder curved arch | 30 | 5 | 4 | 300 | Outpatient hospital |
| Kojima 2014 | Upper extremity | Bilateral activities + EMTS | No additional therapy | Extension of wrist and fingers to reach EMG threshold on 50 ‐ 70% of maximum wrist extension, neuromuscular stimulation 10 seconds symmetrical biphasic pulses at 50 Hz, pulse width 200 μs, followed by 20 seconds of rest to assist full range of motion; bimanual wrist and finger extension during 'on' and 'off' period, difficulty of exercises dependent upon participants’ levels of functioning with regard to wrist and finger flexion and extension or thumb opposition | 20 (2 times a day) | 5 | 4 | 800 | Inpatient rehabilitation centre |
| Kumar 2013 | Lower extremity | Activities of the unaffected limb | No additional therapy | Flexion/ extension of the knee and ankle; self‐selected speed; under supervision | 2 times daily for 15 minutes | 5 | 2 | 300 | n/r |
| Kuzgun 2012 | Upper extremity | n/r | No additional therapy | Wrist extension | 4 times daily for 15 minutes | 5 | 4 | 1200 | n/r |
| Lee 2012 | Upper extremity | Bilateral activities | No additional therapy | Lifting both arms, flexion/ extension of the elbow, pronation of the forearm, wrist extension, internal/ external rotation of the wrist, clenching and opening the fist, tapping on the table; self‐performed; supervision of a guardian | 2 times daily for 25 minutes | 5 | 4 | 1000 | Inpatient rehabilitation ward |
| Lee 2016 | Lower extremity | Bilateral activities + NMES | Conventional therapy | Dorsiflexion movements of the ankle | n/r | 5 | 4 | n/r | Rehabilitation hospital |
| Lim 2016 | Upper extremity | Bilateral activities | Bilateral activities, covered mirror | Task‐oriented MT: forearm pronation‐supination and wrist flexion/extension, finger flexion‐extension, counting numbers, tapping, and opposing; simple manipulating tasks (such as picking up coins and beans, flipping over cards); complicated tasks (plugging and unplugging pegboards, drawing simple figures, and colouring) | 20 | 5 | 4 | 400 | Inpatient rehabilitation ward |
| Lin 2014a | Upper extremity | Experimental 1: MT: Bilateral activities; Experimental 2: MT and sensory electrical stimulation by a mesh‐glove | Task‐oriented training | Transitive movements (e.g. gross motor tasks, such as reaching out to put a cup on a shelf, or fine motor tasks, such as picking up marbles); intransitive movements (e.g. gross motor movements, such as pronation and supination, or fine motor movements, such as finger opposition) | 60 | 5 | 4 | 1200 | In‐ and outpatient setting |
| Manton 2002 | Upper extremity | n/r | n/r; transparent plastic between limbs | n/r | n/r | n/r | 4 | n/r | Home |
| Marquez 2012 | Lower extremity | Bilateral activities | 1: Bilateral activities, covered mirror; 2: Routine therapy | Alternate dorsiflexion and plantarflexion in both ankles as best as possible, self‐paced speed | 15 | 5 | 3 | 225 | Inpatient rehabilitation unit |
| Michielsen 2011 | Upper extremity | Bilateral activities | Bilateral activities | Exercises based on the Brunnstrom phases of motor recovery; functional tasks (i.e. with objects) | 60 | 1 (under supervision) + 5 (at home) | 6 | 2160 | Home |
| Mirela 2015 | Upper extremity | Bilateral activities | No additional therapy | Flexion and extension of shoulder, elbow, wrist and finger, prone‐supination of the forearm | 30 | 5 | 6 | 900 | Inpatient |
| Mohan 2013 | Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Lying position: hip‐knee‐ankle flexion, with the hip and knee placed in flexion, moving the knee inward and outward, hip abduction with external rotation followed by hip adduction with internal rotation; sitting position: Hip‐knee‐ankle flexion, knee extension with ankle dorsiflexion, knee flexion beyond 90 °; each exercise 2 sets of 10 repetitions | 60 | 6 | 2 | 720 | Inpatient rehabilitation |
| Moustapha 2012 | Upper extremity | Bilateral activities | Landscape images were shown to participants, they should try to describe the images, without movements | Finger and hand movements | 30 | 5 | 1 | 150 | n/r |
| Nagapattinam 2015 | Upper extremity | Bilateral activities | functional electrical stimulation, covered mirror | Experimental 1: wrist and finger extension, grasping and releasing a bottle; Experimental 2: combined MT and functional electrical stimulation | 30 | 6 | 2 | 360 | Hospital |
| Pandian 2014 | Upper extremity | Bilateral activities, therapist supported if patients were not able to move paretic limb | Bilateral activities, covered mirror | Flexion and extension movements of wrist and fingers | 60 | 5 | 4 | 1200 | inpatient rehabilitation and home training after discharge |
| Park 2015a | Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Pronation and supination of the forearm and the flexion and extension movements of the wrist and fingers; 5 sets each motion, 30 repetitions per set | 30 | 5 | 4 | 600 | Inpatient |
| Park 2015b | Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Task‐oriented activities consisted with reaching, grasping, lifting and releasing objects | n/r | 5 | 6 | n/r | Rehabilitation unit |
| Piravej 2012 | Upper extremity | Not stated | Same tasks; covered mirror | Task‐oriented activities consisting of grasping and releasing objects | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre |
| Rajappan 2016 | Upper extremity | bilateral activities | Same tasks; covered mirror | Finger and wrist movements, grasping different objects | 30 | 5 | 4 | 600 | Nursing homes |
| Rehani 2015 | Upper extremity | Bilateral activities | Motor relearning programme | Hand‐opening, wrist flexion/ extension, forearm pronation/ supination, hand sliding on surface | n/r | 6 | 4 | n/r | Outpatient |
| Rodrigues 2016 | Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Task‐orientend activities consisted with manipulating objects | 60 | 3 | 4 | 720 | Home |
| Rothgangel 2004a | Upper extremity | Bilateral activities (hypotone muscles); unilateral activities (hypertone muscles) | Bilateral activities; without mirror | Gross motor arm and hand movements; functional activities (i.e. with objects); fine motor activities (i.e. with objects) | 30 | Total number of sessions: 17 | 5 | 510 | Outpatient centre |
| Rothgangel 2004b | See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | 30 | Total number of sessions: 37 | 5 | 1110 | Inpatient rehabilitation centre |
| Salhab 2016 | Lower extremity | MT + Electrical stimulation | Conventional therapy | n/r | 50 | 4 | 2 | 400 | n/r |
| Samuelkamaleshkumar 2014 | Upper extremity | Activities of the unaffected limb | No additional therapy | Wrist flexion, extension, radial and ulnar deviation, circumduction, fisting, releasing, abduction, and adduction of all fingers; activities such as squeezing a ball, stacking rings, flipping cards, placing pegs on a board | 2 times for 30 | 5 | 3 | 900 | Inpatient rehabilitation centre |
| Schick 2017 | Upper extremity | Bilateral activities | Electromyographic‐triggered muscular electrical stimulation | Grasping movements in combination with electromyographic‐triggered muscular electrical stimulation | 30 | 5 | 3 | 450 | 3 inpatient rehabilitation centres |
| Seok 2010 | Upper extremity | Activities of the unaffected limb | No therapy | 5 movements of wrist and fingers, each 6 minutes | 30 | 5 | 4 | 500 | Inpatient rehabilitation centre |
| Sütbeyaz 2007 | Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Dorsiflexion movements of the ankle | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre |
| Tezuka 2006 | Upper extremity | Activities of the unaffected limb; affected limb passively moved by therapist | Activities of the unaffected limb; affected limb passively moved by therapist; without mirror | 13 kinds of movements, i.e. flexion/extension of wrist, pinching fingers, gripping ball | 10 to 15 | Daily | 4 (1st period) | 280 to 420 | Inpatient rehabilitation centre |
| Thieme 2013 | Upper extremity | Bilateral activities | Bilateral activities; covered mirror | 1st week: isolated movements of fingers, wrist, lower arm, elbow and shoulder in all degrees of freedom, up to 50 repetitions per series, up to 4 series; 2nd to 5th week: additional movements, object‐related movements; adapted by therapists according to patients’ abilities; Experimental 1 and control in group setting 2 ‐ 6 participants | 30 | 3 ‐ 5 | 4 ‐ 5 | 600 | Inpatient rehabilitation centre |
| Tyson 2015 | Upper extremity | Not stated; self‐performed, daily checking by therapist | Lower limb activities; without a mirror | n/r | 30 | 5 | 4 | 600 | 12 inpatient stroke services |
| Wang 2015 | Upper extremity | n/r | 1: no additional therapy; 2: electromyographic biofeedback | n/r | n/r | n/r | n/r | n/r | n/r |
| Wu 2013 | Upper extremity | Bilateral activities | Usual occupational therapy | Transitive movements: fine motor tasks of squeezing sponges, placing pegs in holes, flipping a card, gross motor tasks (reaching out for touch); intransitive movements (repetitive wrist flexion/extension, finger opposition, forearm pro‐/supination) | 60 | 5 | 4 | 1200 | 4 hospitals |
| Yavuzer 2008 | Upper extremity | Bilateral activities | Bilateral activities; nonreflecting side of the mirror | Flexion/extension of wrist and fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre |
| Yoon 2014 | Upper extremity | Activities of the unaffected limb | 1: constraint induced movement therapy (6 hours/day) + palliative rehabilitation programme + self‐exercise; 2: palliative rehabilitation programme + self‐exercise | Flexion/extension of the shoulder, elbow, wrist, finger, and pronation/supination of the forearm | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre |
| Yun 2011 | Upper extremity | Experimental 1: activities of the unaffected limb Experimental 2: activities of the unaffected limb and additionally neuromuscular electrical stimulation of the affected arm |
Neuromuscular electrical stimulation of finger and wrist extensors of the affected arm | Flexion/extension of wrist and fingers | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre |
| Zacharis 2014 | n/r | n/r | n/r | n/r | 30 | Total: 20 ‐ 24 | 8 | 600 ‐ 720 | n/r |
EMG: electromyography ETMS: electromyography‐triggered neuromuscular stimulation FES: functional electrical stimulation Hz: hertz MT: mirror therapy NMES: neuromuscular electrical stimulation n/r: not reported rTMS: repetitive transcranial magnetic stimulation tDCS: transcranial direct current stimulation TMS: transcranial magnetic stimulation μs: microsiemens
Inclusion and exclusion criteria of studies are listed in Characteristics of included studies.
Interventions
Characteristics of interventions are summarised in Table 3. All except two included studies (In 2012; In 2016), provided mirror therapy using a mirror or a mirror box in the midsagittal plane between the upper or lower limbs. Thus the mirror reflected movements of the non‐affected side as if these movements were executed with the affected side. In 2012 and In 2016 used a virtual reflection setting where the affected extremity was placed under a screen while the non‐affected extremity was placed under a camera. The screen displayed the mirrored picture of the unaffected limb.
Ten studies examined the effects of mirror therapy for the lower extremity (Arya 2017; Cha 2015; In 2016; Kawakami 2015; Kumar 2013; Lee 2016; Marquez 2012; Mohan 2013; Salhab 2016; Sütbeyaz 2007); all other studies examined the effects of mirror therapy for the upper extremity.
Eleven studies used a combination of mirror therapy and other interventions. Kim 2014, Kim 2015a, Lee 2016, and Yun 2011 integrated a combination of mirror therapy with functional or neuromuscular electrical stimulation, Kojima 2014 and Schick 2017 with electromyographic‐triggered electrical muscle stimulation, and Lin 2014a combined mirror therapy with electrical sensory stimulation using a mesh‐glove. Mirror therapy was further combined with transcranial direct current stimulation (Cho 2015), or transcranial magnetic stimulation (Cha 2015; Dalla Libera 2015; Ji 2014a). If studies used two experimental groups, we combined both intervention groups for analysis.
Mirror therapy was provided for between three and seven days a week, and for between two and eight weeks. Each session lasted between 15 and 60 minutes. The total time for experimental intervention was between 225 and 2160 minutes.
Rothgangel 2004 included 16 participants and randomised them to mirror therapy or bilateral arm training. However, six of the participants were treated in an outpatient rehabilitation centre, and 10 in an inpatient care facility, which led to a significant difference in treatment time: the outpatient group received 17 treatment sessions of 30 minutes each; the inpatient group received 37 treatment sessions of 30 minutes each. Because these two groups are considerably different in total treatment time, we decided to analyse them separately (outpatient group: Rothgangel 2004a, and inpatient group: Rothgangel 2004b).
In 29 studies participants performed bilateral movements, moving the affected limb behind the mirror as best they could. In 22 studies participants only moved the unaffected side while looking in the mirror. In two studies participants performed both uni‐ and bilateral movements (In 2016; Kawakami 2015). In Rothgangel 2004 participants with muscle hypotonia had to move the affected arm as best they could; participants with muscle hypertonia should only move the unaffected arm while looking into the mirror. In two studies, a therapist passively moved the affected arm behind the mirror according to the movements of the unaffected one (Pandian 2014; Tezuka 2006).
In 11 studies the control group received no additional intervention other than standard rehabilitation. Twenty‐two studies used a form of sham therapy where the reflecting side of the mirror was covered, or the non‐reflecting side of the mirror was placed in the direction of the unaffected arm while practising. Eleven studies provided interventions with an unrestricted view of the affected side using the same training as in the experimental groups but without a mirror or with a plexiglas between limbs. Eighteen studies used other interventions in the control groups: electromyographic‐triggered muscle stimulation (Amasyali 2016; Kawakami 2015; Schick 2017; Wang 2015); (functional) electrical muscle stimulation (Kawakami 2015; Nagapattinam 2015; Yun 2011); conventional therapy (Arya 2015; Arya 2017; Geller 2016; Kim 2016; Salhab 2016; Wu 2013); motor imagery (Cacchio 2009b); passive mobilisation of the affected limb (Colomer 2016; Kawakami 2015); transcranial magnetic stimulation (Dalla Libera 2015); task‐oriented training (Lin 2014a); motor relearning programme (Rehani 2015); lower limb activities (Tyson 2015); or constraint‐induced movement therapy (Yoon 2014). In one study a therapist passively moved the affected arm according to the movements of the unaffected one, but without a mirror between limbs (Tezuka 2006). If studies integrated two control groups we combined both groups for analysis (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9). However, for testing the influence of different control treatments, we analysed single control groups in a subgroup analysis. Based on the difference of using a covered mirror, another intervention without mirror (also transparent plexiglas), or no additional therapy, we performed a subgroup analysis differentiating the effects of types of control intervention (covered mirror versus another intervention with unrestricted view versus no additional therapy) (Analysis 3.1).
1.1. Analysis.
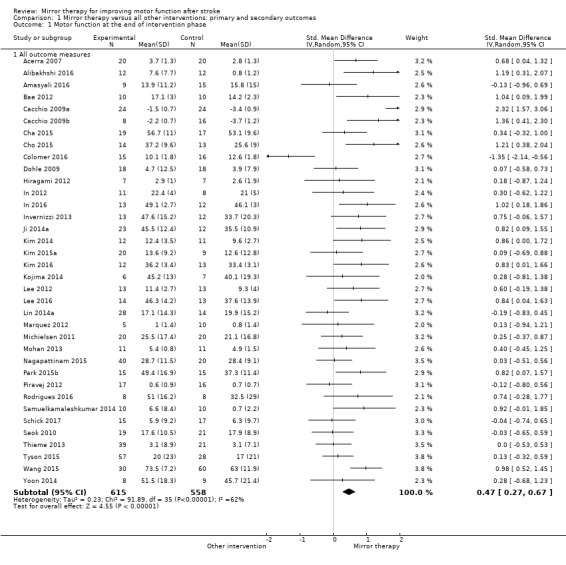
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 1 Motor function at the end of intervention phase.
1.2. Analysis.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 2 Motor impairment at the end of intervention phase.
1.3. Analysis.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase.
1.4. Analysis.
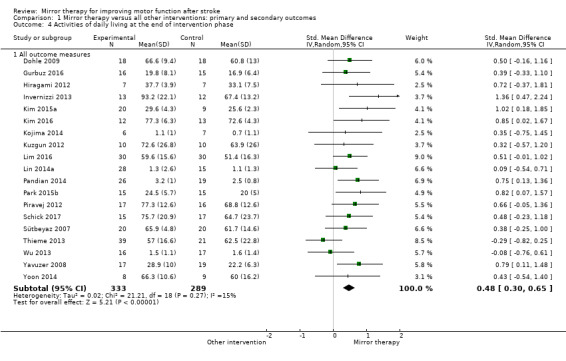
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 4 Activities of daily living at the end of intervention phase.
1.5. Analysis.
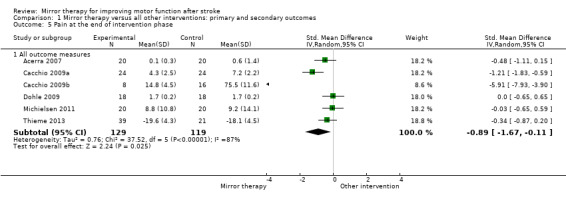
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 5 Pain at the end of intervention phase.
1.6. Analysis.
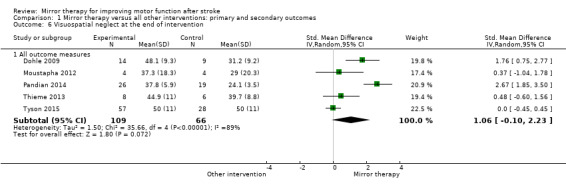
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 6 Visuospatial neglect at the end of intervention.
1.7. Analysis.
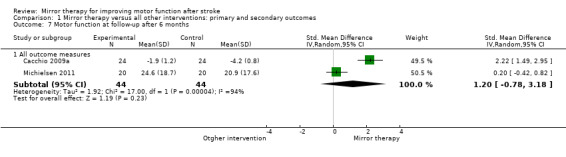
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 7 Motor function at follow‐up after 6 months.
1.8. Analysis.
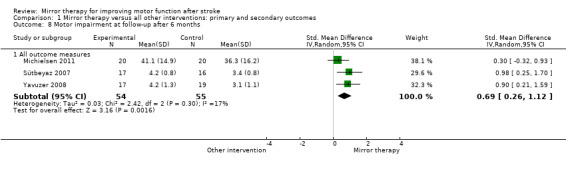
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 8 Motor impairment at follow‐up after 6 months.
1.9. Analysis.
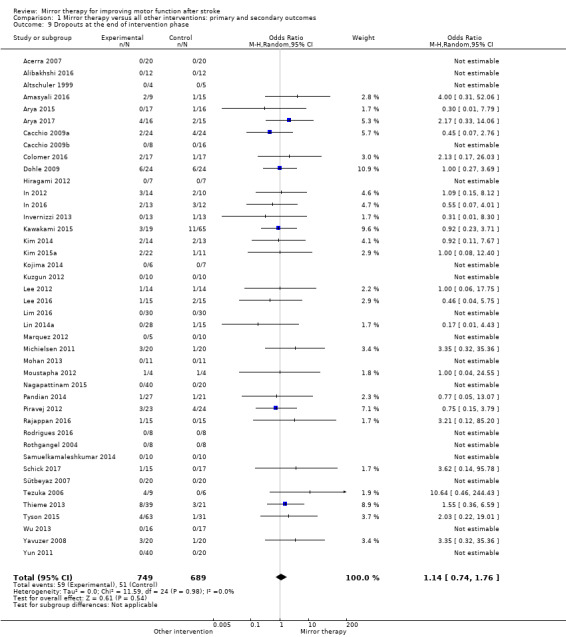
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 9 Dropouts at the end of intervention phase.
3.1. Analysis.
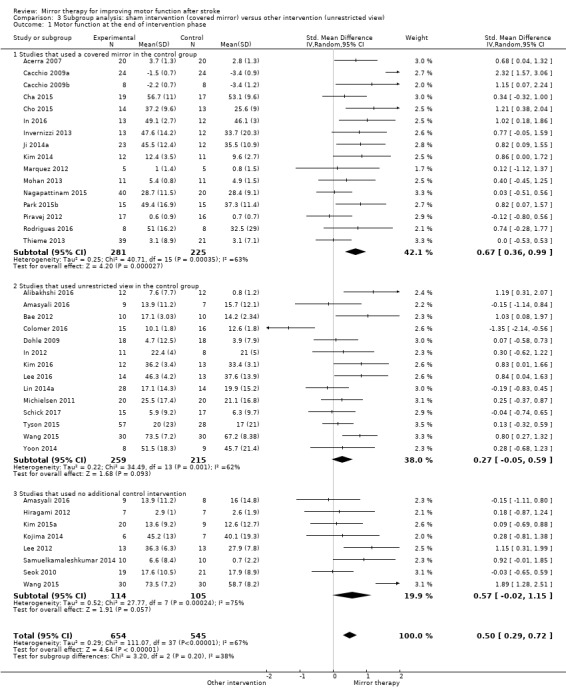
Comparison 3 Subgroup analysis: sham intervention (covered mirror) versus other intervention (unrestricted view), Outcome 1 Motor function at the end of intervention phase.
Outcome
The included studies used a number of different outcomes. A description of the outcome measures used can be found in Characteristics of included studies.
Primary outcome: motor function
For analysis of our primary outcome of motor function we used the Motor Assessment Scale Item 7 (Acerra 2007; Marquez 2012; Piravej 2012), the Box and Block Test (Alibakhshi 2016; Amasyali 2016; Cho 2015; Kim 2015a; Lin 2014a; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a), the Action Research Arm Test (Dohle 2009; Geller 2016; Invernizzi 2013; Kim 2016; Michielsen 2011; Nagapattinam 2015; Thieme 2013; Tyson 2015), the Wolf Motor Function Test (functional ability) (Cacchio 2009a; Cacchio 2009b; Colomer 2016; Hiragami 2012; Kojima 2014; Yoon 2014), the Manual Function Test (Bae 2012; In 2012; Kim 2014; Lee 2012; Park 2015b; Seok 2010), the Berg Balance Scale (Cha 2015; In 2016; Lee 2016), the Brunnel Balance Assessment (Mohan 2013), the CAHAI (Rehani 2015), and the TEMPA (Rodrigues 2016).
Secondary outcomes: motor impairment, activities of daily living, pain and visuospatial neglect
For analysing motor impairment we used the Fugl‐Meyer score (Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009;Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lim 2016; Lin 2014a; Michielsen 2011; Mirela 2015; Mohan 2013; Park 2015a; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Tezuka 2006; Thieme 2013; Wang 2015; Wu 2013; Yoon 2014; Yun 2011), the Brunnstrom stages of motor recovery (Piravej 2012; Sütbeyaz 2007; Yavuzer 2008), muscle or grip strength (Acerra 2007; Lee 2016; Marquez 2012), the Motricity Index (Invernizzi 2013; Tyson 2015), and the Manual Muscle Test (Seok 2010).
In our pooled analysis of the secondary outcome activities of daily living we used the Functional Independence Measure (Dohle 2009; Geller 2016; Hiragami 2012; Invernizzi 2013; Kim 2015a; Kim 2016; Pandian 2014; Park 2015a; Park 2015b; Sütbeyaz 2007; Yavuzer 2008), the Barthel Index (Kuzgun 2012; Lim 2016; Piravej 2012; Schick 2017; Thieme 2013 ; Yoon 2014), and the Motor Activity Log (amount of use) (Kojima 2014; Lin 2014a; Wu 2013).
For the analysis of the secondary outcome of pain we included the measurement of pain at rest (Acerra 2007; Cacchio 2009b; Michielsen 2011), and during movement (Cacchio 2009a; Dohle 2009). The investigators used Numerical Rating Scales between 0 and 10 (Acerra 2007), Visual Analogue Scales between 0 and 10 (Cacchio 2009a), or between 0 mm and 100 mm (Cacchio 2009b; Michielsen 2011), or the pain section of the Fugl‐Meyer Assessment, normalised on the average score for each item (0 to 2; 2 indicating no pain) (Dohle 2009, Thieme 2013).
Visuospatial neglect as an outcome was analysed using the Star Cancellation Test (Moustapha 2012; Pandian 2014; Thieme 2013; Tyson 2015), and a self‐developed score (Dohle 2009).
Follow‐up assessment
For analysis of sustained treatment effects for our primary outcome of motor function, we used only the data of follow‐up assessments after six months (Cacchio 2009a; Michielsen 2011), as well as for motor impairment (Michielsen 2011; Sütbeyaz 2007; Yavuzer 2008).
Adverse effects
Twenty‐one studies explicitly reported the assessment of adverse effects (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Colomer 2016; Hiragami 2012; Invernizzi 2013; Kojima 2014; Kuzgun 2012; Lin 2014a; Marquez 2012; Mohan 2013; Rodrigues 2016; Nagapattinam 2015; Schick 2017; Sütbeyaz 2007; Tyson 2015; Wu 2013; Yavuzer 2008; Zacharis 2014). No adverse events were reported.
Excluded studies
We discarded 470 studies following consideration of abstracts, full texts or both (see: Characteristics of excluded studies). In the Excluded studies section, we mention only those studies that might in a superficial view appear to meet the eligibility criteria and those studies that we classified as well‐known and likely to be considered relevant by some readers (Characteristics of excluded studies).
Risk of bias in included studies
All details about the methodological quality of the included studies using the 'Risk of bias' assessment tool (Higgins 2011) are provided in Characteristics of included studies and Figure 2.
2.
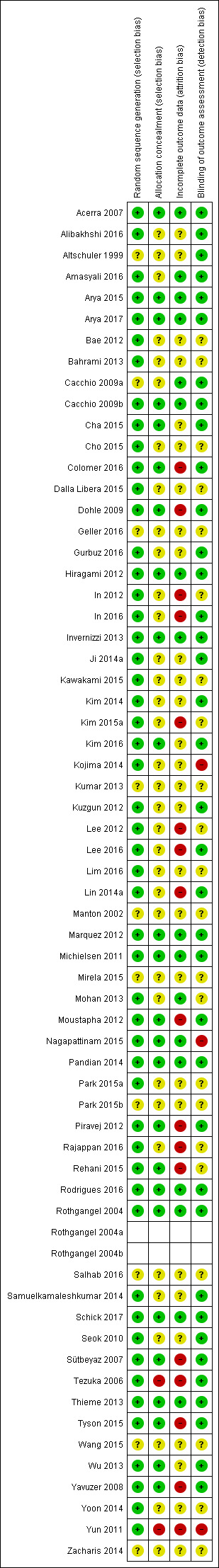
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We emailed all trialists of the included studies to clarify some methodological or design issues, or both. Most trialists provided at least some of the requested information. Two review authors (from HT, NM, CD, JB or JM) independently evaluated the methodological quality of the studies. The assessing authors discussed all disagreements and resolved them by contacting another author or by obtaining additional information through contact with the principal investigator of the study.
Allocation
Fifty‐two studies used adequate randomisation procedures, and were therefore at low risk of bias (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Moustapha 2012; Nagapattinam 2015; Pandian 2014; Park 2015a; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011). We were not able to rate risk of bias for 10 trials due to missing information about the sequence generation process (Altschuler 1999; Cacchio 2009a; Geller 2016; Kumar 2013; Manton 2002; Mirela 2015; Park 2015b; Salhab 2016; Wang 2015; Zacharis 2014). Five studies used a cross‐over design with random allocation to the order of treatment (Altschuler 1999; Kojima 2014; Moustapha 2012; Salhab 2016; Tezuka 2006). We only analysed the first treatment period as a parallel‐group design in these five studies. Eight studies used block randomisation methods (Cacchio 2009b; Hiragami 2012; Kojima 2014; Lin 2014a; Mohan 2013; Piravej 2012; Sütbeyaz 2007; Yavuzer 2008). One study randomly allocated ability‐matched pairs to treatment groups (Manton 2002).
Twenty‐five studies used an adequate concealment of allocation, and we therefore considered them to be at low risk of bias (Acerra 2007; Arya 2015; Arya 2017; Cacchio 2009b; Cha 2015; Colomer 2016; Dohle 2009; Hiragami 2012; Invernizzi 2013; Kim 2016; Marquez 2012; Michielsen 2011; Moustapha 2012; Pandian 2014; Piravej 2012; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Nagapattinam 2015; Schick 2017; Sütbeyaz 2007; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008). There was no description of the allocation concealment process, so we rated 35 trials at unclear risk of bias (Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Bae 2012; Bahrami 2013; Cacchio 2009a; Cho 2015; Dalla Libera 2015; Geller 2016; Gurbuz 2016; In 2012; In 2016; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Mirela 2015; Mohan 2013; Park 2015a; Park 2015b; Rajappan 2016; Salhab 2016; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015; Yoon 2014; Zacharis 2014). Two studies were at high risk of bias because the authors of the trials confirmed that no concealment of allocation process had occurred (Tezuka 2006; Yun 2011). The methods used for concealment of allocation are presented in Characteristics of included studies.
Blinding
We rated 37 studies at low risk of bias, since at least the primary outcome measures were assessed by people blinded to group allocation (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Cha 2015; Colomer 2016; Dohle 2009; Gurbuz 2016; Hiragami 2012; In 2016; Invernizzi 2013; Ji 2014a; Kim 2014; Kim 2016; Kuzgun 2012; Lee 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Moustapha 2012; Pandian 2014; Piravej 2012; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008). In 22 studies the process of blinding was not described (Bae 2012; Bahrami 2013; Cho 2015; Dalla Libera 2015; Geller 2016; In 2012; Kawakami 2015; Kim 2015a; Kumar 2013; Lee 2012; Lim 2016; Manton 2002; Mirela 2015; Mohan 2013; Park 2015a; Park 2015b; Rajappan 2016; Rehani 2015; Salhab 2016; Wang 2015; Yoon 2014; Zacharis 2014). In three trials the study authors stated that the assessors of the primary outcome measure were not blinded, so we considered them to be at high risk of bias (Kojima 2014; Nagapattinam 2015; Yun 2011)
Incomplete outcome data
Seventeen studies conducted an ITT analysis that included incomplete outcome data (Acerra 2007; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Hiragami 2012; Invernizzi 2013; Marquez 2012; Michielsen 2011; Mohan 2013; Nagapattinam 2015; Pandian 2014; Rodrigues 2016; Rothgangel 2004; Schick 2017; Thieme 2013). No description of handling incomplete outcome data was available in 28 studies, and we considered them to be at unclear risk of bias for this domain (Alibakhshi 2016; Altschuler 1999; Bae 2012; Bahrami 2013; Cha 2015; Cho 2015; Dalla Libera 2015; Geller 2016; Gurbuz 2016; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lim 2016; Manton 2002; Mirela 2015; Park 2015a; Park 2015b; Salhab 2016; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015; Wu 2013; Yoon 2014; Zacharis 2014). Seventeen studies reported that no ITT analysis was performed, and we rated them at high risk of bias (Colomer 2016; Dohle 2009; In 2012; In 2016; Kim 2015a; Lee 2012; Lee 2016; Lin 2014a; Moustapha 2012; Piravej 2012; Rajappan 2016; Rehani 2015; Sütbeyaz 2007; Tezuka 2006; Tyson 2015; Yavuzer 2008; Yun 2011)
Selective reporting
We did not evaluate studies for selective reporting.
Other potential sources of bias
Twenty studies did not report whether or not participants dropped out during the intervention. In the remaining 42 studies, 109 participants dropped out, which is a rate of 5.5%. Seventeen studies reported no dropouts during the intervention period, 17 trialists reported dropout rates of 15% or less, and in eight studies the dropout rate was above 15%. Fifty‐nine participants dropped out of the experimental groups and 51 participants dropped out of the control groups, giving balanced dropout rates between groups. A detailed description of study characteristics can be found in Characteristics of included studies.
Effects of interventions
See: Table 1
Comparison 1: Mirror therapy versus all other interventions
Outcome 1.1: Motor function at the end of the intervention phase
We included 36 studies in a pooled analysis of motor function after study end, with a total of 615 participants in the intervention and 558 in the control groups in the post‐assessment data analysis (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Bae 2012; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Kim 2014; Kim 2015a; Kim 2016;Kojima 2014; Lee 2012; Lee 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Park 2015b; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Nagapattinam 2015; Seok 2010; Thieme 2013; Tyson 2015; Wang 2015; Yoon 2014). Mirror therapy had a statistically significant effect on motor function in participants after stroke compared with all other types of interventions (SMD 0.47, 95% CI 0.27 to 0.67; 1173 participants; 36 studies; I2 = 62%; Analysis 1.1).
Based on our sensitivity analysis for the influence of trial methodology, we found robust effects on motor function except for concealment of allocation. By analysing only those studies with adequate methods of concealment, the effect on motor function was not significant (Analysis 5.1). We therefore downgraded the quality of evidence to moderate, due to several ratings of unclear risk of bias.
5.1. Analysis.

Comparison 5 Sensitivity analysis by trial methodology, Outcome 1 Motor function at the end of intervention.
Outcome 1.2: Motor impairment at the end of intervention phase
We included 39 studies in a pooled analysis of motor impairment after study end, with a total of 672 participants in the intervention and 620 in the control groups in the post‐assessment data analysis (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Cho 2015; Colomer 2016; Dohle 2009;Gurbuz 2016; In 2012; Invernizzi 2013; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lin 2014a; Lim 2016; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yun 2011; Yoon 2014). Mirror therapy has a statistically significant effect on motor impairment in participants after stroke compared with all other types of interventions (SMD 0.49, 95% CI 0.32 to 0.66; 1292 participants; 39 studies; I2 = 53%; Analysis 1.2). The quality of evidence for motor impairment was moderate.
The effect was robust even after excluding studies with no or inadequate methods of allocation concealment (Analysis 5.2)
5.2. Analysis.
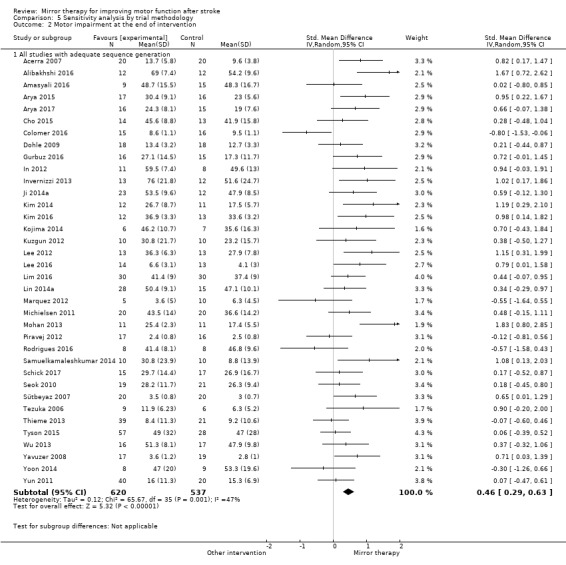
Comparison 5 Sensitivity analysis by trial methodology, Outcome 2 Motor impairment at the end of intervention.
Outcome 1.3: Fugl‐Meyer Assessment for the upper extremity at the end of intervention phase
Since 29 studies used the Fugl‐Meyer Asssessment for analysing treatment effects on motor impairment, we analysed the effect on motor impairment for this outcome measure, using mean differences. We included 28 studies in a pooled analysis on Fugl‐Meyer Assessment for the upper extremity after study end, with a total of 463 participants in the intervention and 435 in the control groups in the post‐assessment data analysis (Alibakhshi 2016; Amasyali 2016; Arya 2015; Cho 2015; Colomer 2016; Dohle 2009; Gurbuz 2016; In 2012; Kim 2014; Kim 2015a; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lin 2014a; Lim 2016; Michielsen 2011; Mirela 2015; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Tezuka 2006; Thieme 2013; Wang 2015; Wu 2013; Yun 2011; Yoon 2014). Mirror therapy had a statistically significant effect on Fugl‐Meyer‐Assessment in participants after stroke compared with all other types of interventions (MD 4.32, 95% CI 2.46 to 6.19; 898 participants; 28 studies; I2 = 77%; Analysis 1.3). We rated the evidence for this outcome as of low quality.
Outcome 1.4: Activities of daily living at the end of the intervention phase
We included 19 studies in the analysis of the outcome of activities of daily living (Dohle 2009; Gurbuz 2016; Hiragami 2012; Invernizzi 2013; Kim 2014; Kim 2015a; Kojima 2014; Kuzgun 2012; Lim 2016; Lin 2014a; Pandian 2014; Park 2015a; Piravej 2012; Schick 2017; Sütbeyaz 2007; Thieme 2013; Wu 2013; Yavuzer 2008; Yoon 2014). These studies included 333 participants in the intervention and 289 in the control groups. Mirror therapy had a statistically significant effect on activities of daily living for participants with stroke, compared with all other interventions (SMD 0.48, 95% CI 0.30 to 0.65; 622 participants; 19 studies; I2 = 15%; Analysis 1.4). We rated the evidence for this secondary outcome as of moderate quality.
Outcome 1.5: Pain at the end of the intervention phase
For analysing the effects of mirror therapy on pain at the end of the intervention, we included six studies presenting data on pain at rest or during movement (Acerra 2007; Cacchio 2009a; Cacchio 2009b; Dohle 2009; Michielsen 2011; Thieme 2013). These studies included 129 participants in the intervention and 119 in the control groups. Mirror therapy had a statistically significant effect on pain reduction for participants after stroke, compared with all other interventions (SMD −0.89, 95% CI −1.67 to −0.11; 248 participants; 6 studies; I2 = 87%; Analysis 1.5). We rated the quality of the evidence for this secondary outcome pain as low.
However, two studies only included participants after stroke with a diagnosis of CRPS‐type I, which might have influenced the effects of the intervention (Cacchio 2009a; Cacchio 2009b). We therefore performed a post hoc sensitivity analysis and removed the studies that only included participants with CRPS after stroke. After removing those two studies, we were left with four studies with 97 participants in the intervention and 79 in the control groups (Acerra 2007; Dohle 2009; Michielsen 2011; Thieme 2013). We found no statistically significant effect on pain for mirror therapy compared with all other interventions in this subgroup (SMD −0.23, 95% CI −0.53 to 0.08; 176 participants; 4 studies; I2 = 0%; Analysis 6.1).
6.1. Analysis.
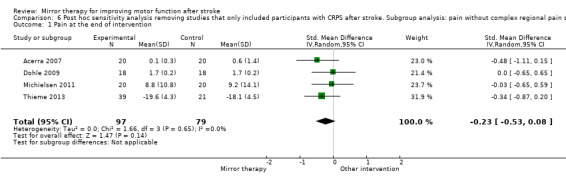
Comparison 6 Post hoc sensitivity analysis removing studies that only included participants with CRPS after stroke. Subgroup analysis: pain without complex regional pain syndrome (CRPS), Outcome 1 Pain at the end of intervention.
Outcome 1.6: Visuospatial neglect at the end of the intervention
Five studies reported outcome on visuospatial neglect (Dohle 2009; Moustapha 2012; Pandian 2014; Thieme 2013; Tyson 2015). These studies included 109 participants in the intervention and 66 in the control groups. Based on these data, we found a statistically non‐significant effect of mirror therapy versus all other interventions on visuospatial neglect after stroke (SMD 1.06, 95% CI −0.10 to 2.23; 175 participants; 5 studies; I2 = 89%; Analysis 1.6). We rated the quality of evidence for this secondary outcome as low.
Outcome 1.7: Motor function at follow‐up after six months
Two studies provided data on motor function at a follow‐up period of six months (Cacchio 2009a; Michielsen 2011). These studies included 44 participants each in the experimental and control groups. At follow‐up after six months from the end of intervention, mirror therapy had a statistically non‐significant effect on motor impairment in people after stroke, compared with all other interventions (SMD 1.20, 95% CI −0.78 to 3.18; 88 participants; 2 studies; I2 = 94%; Analysis 1.7).
Outcome 1.8: Motor impairment at follow‐up after six months
Three studies provided data on motor impairment at a follow‐up period of six months (Michielsen 2011; Sütbeyaz 2007; Yavuzer 2008). These studies included 54 participants in the experimental and 55 in the control groups. At follow‐up after six months from the end of intervention, mirror therapy had a statistically significant effect on motor function in people after stroke, compared with all other interventions (SMD 0.69, 95% CI 0.26 to 1.12; 109 participants; 3 studies; I2 = 17%; Analysis 1.8).
Outcome 1.9: Dropouts at the end of intervention phase
We included 42 studies that provided data for the dropout rate at the end of the intervention phase in this analysis (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Kawakami 2015; Kim 2014; Kim 2015a; Kojima 2014; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Moustapha 2012; Pandian 2014; Piravej 2012; Rajappan 2016; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Nagapattinam 2015; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008; Yun 2011). We found a statistically non‐significant effect for dropping out in the mirror‐therapy groups compared with the control groups (OR 1.14, 95% CI 0.74 to 1.76; 1438 participants; 42 studies; I2 = 0%; Analysis 1.9).
Comparison 2: Subgroup analysis ‐ upper versus lower extremity
Outcome 2.1: Motor function at the end of the intervention phase
We performed a subgroup analysis for those studies examining mirror therapy for the upper extremity (subgroup 2.1.1) and lower extremity (subgroup 2.1.2) (Analysis 2.1). We included 31 studies in the analysis of motor function after mirror therapy for the upper extremity. Studies included 553 participants in the experimental and 495 in the control groups (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Bae 2012; Cacchio 2009a; Cacchio 2009b; Cho 2015; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; Invernizzi 2013; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Lee 2012; Lin 2014a; Michielsen 2011; Park 2015b; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Seok 2010; Nagapattinam 2015; Thieme 2013; Tyson 2015; Wang 2015; Yoon 2014). We found a statistically significant effect of mirror therapy on motor function of the upper extremity for people after stroke compared to all other interventions (SMD 0.46, 95% CI 0.23 to 0.69; 1048 participants; 31 studies; I2 = 66%; Analysis 2.1.1).
2.1. Analysis.
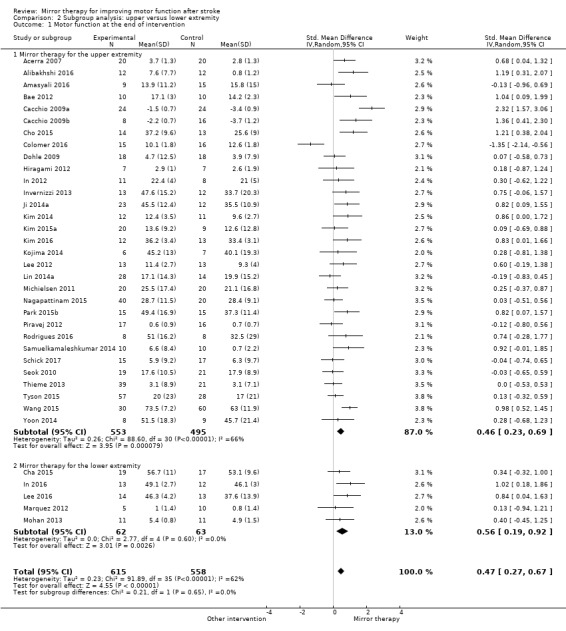
Comparison 2 Subgroup analysis: upper versus lower extremity, Outcome 1 Motor function at the end of intervention.
Five studies with 62 participants in the experimental and 63 in the control groups were included in the analysis for the lower extremity (Cha 2015; In 2016; Lee 2016; Marquez 2012; Mohan 2013). The positive effect of mirror therapy on motor function of the lower extremity for people after stroke compared with all other interventions was statistically significant (SMD 0.56, 95% CI 0.19 to 0.92; 125 participants; 5 studies; I2 = 0%; Analysis 2.1.2). There was a statistically non‐significant difference between subgroups.
Comparison 3: Subgroup analysis ‐ sham intervention (covered mirror) versus other intervention (unrestricted view) versus no intervention
We found two different groups of control interventions. In all studies, participants in the control group performed the same movements as participants in the experimental groups. However, in one type of control intervention the view of the affected side was obscured with a covered mirror, or with the non‐reflective side of the mirror (sham intervention). In the other type of control intervention participants had an unrestricted view of both; the unaffected and the affected limb (other intervention). Because we believed that this may have influenced the effect of therapy, we performed a subgroup analysis, differentiating between these two types of studies.
Outcome 3.1: Motor function at the end of the intervention phase
Sixteen studies with the outcome of motor function used a covered mirror in the control group (Acerra 2007; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; In 2016; Invernizzi 2013; Ji 2014a; Kim 2014; Marquez 2012; Mohan 2013; Nagapattinam 2015; Park 2015b; Piravej 2012; Rodrigues 2016; Thieme 2013). These studies included 281 participants in the intervention and 225 in the control groups. For this subgroup we found a statistically significant effect of mirror therapy on motor function after stroke (SMD 0.67, 95% CI 0.36 to 0.99; 506 participants; 16 studies; I2 = 63%).
Fourteen studies with an intervention using an unrestricted view in the control groups, thus providing a view of both limbs, were analysed in this subgroup (Alibakhshi 2016; Amasyali 2016; Bae 2012; Colomer 2016; Dohle 2009; In 2012; Kim 2016; Lee 2016; Lin 2014a; Michielsen 2011; Schick 2017; Tyson 2015; Wang 2015; Yoon 2014). These studies included 259 participants in the experimental and 215 in the control groups. The effect of mirror therapy on motor function after stroke in these studies was not statistically significant (SMD 0.27, 95% CI −0.05 to 0.59; 474 participants; 14 studies; I2 = 62%).
We included eight studies with no additional control therapy in this analysis (Amasyali 2016; Hiragami 2012; Kim 2015a; Kojima 2014; Lee 2012; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015). Studies included 114 participants in the experimental and 105 in the control groups. This subgroup showed no statistically significant effect in favour of mirror therapy (SMD 0.57, 95% CI −0.02 to 1.15; 219 participants; 8 studies; I2 = 75%; Analysis 3.1).
However, subgroup differences did not demonstrate statistical significance.
Comparison 4: Subgroup analysis: subacute versus chronic stage after stroke
In this subgroup analysis we differentiated between studies that included participants within six months (subacute stage) and those at more than six months after stroke (chronic stage). Eighteen studies with participants in the subacute stage after stroke were included in this analysis. We found a statistically significant effect of mirror therapy compared to all other interventions for this subgroup (SMD 0.45, 95% CI 0.18 to 0.73; 596 participants; 18 studies; I2 = 59%). Fourteen studies in this analysis included participants in the chronic phase after stroke. The effect on motor function was also significant for this subgroup (SMD 0.43, 95% CI 0.06 to 0.81; 398 participants; 14 studies; I2 = 68%; Analysis 4.1). Subgroup difference did not demonstrate statistical significance.
4.1. Analysis.
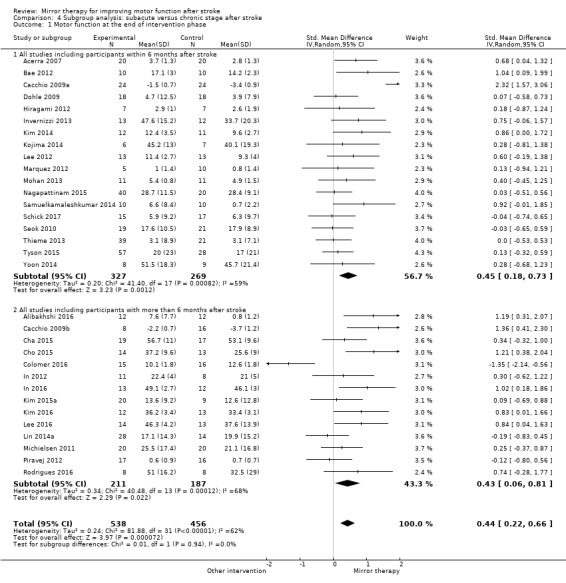
Comparison 4 Subgroup analysis: subacute versus chronic stage after stroke, Outcome 1 Motor function at the end of intervention phase.
Comparison 5: Sensitivity analysis by trial methodology
We tested the robustness of the results by analysing only RCTs and excluding randomised cross‐over trials, and by using specific methodological variables that could influence the observed treatment effects (randomisation procedure, concealment of allocation, blinding of assessors and ITT analysis; Analysis 5.1).
Outcome 5.1: Motor function at the end of the intervention phase
All studies without randomised cross‐over trials
We included 35 studies in a subgroup analysis of all studies without randomised cross‐over trials. The studies included 609 participants in the experimental and 551 in the control groups. Based on this analysis, mirror therapy had a statistically significant effect on motor function in people after stroke, compared to all other treatments (SMD 0.47, 95% CI 0.27 to 0.68; 1160 participants; 35 studies; I2 = 63%; Analysis 5.1.1).
All studies with adequate sequence generation
We analysed 33 studies with 546 participants in the intervention and 459 in the control groups in this subgroup analysis of studies that we rated as having adequate sequence generation. We found a statistically significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.36, 95% CI 0.19 to 0.54; participants = 1005; studies = 33; I2 = 45%; Analysis 5.1.2)
All studies with adequate concealed allocation
We analysed 16 studies as having used an adequate method of allocation concealment. These studies included 313 participants in the experimental and 259 in the control groups. Based on this analysis, we found a non‐significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.21, 95% CI −0.04 to 0.47; 572 participants; 16 studies; I2 = 51%; Analysis 5.1.3).
All studies with adequate intention‐to‐treat (ITT) analysis
We included 12 studies in our analysis of studies with an adequate ITT analysis. Based on our analysis of 204 participants in the experimental and 184 in the control groups with post‐intervention data, mirror therapy had a significant effect on motor function compared with all other interventions (SMD 0.55, 95% CI 0.14 to 0.95; 388 participants; 12 studies; I2 = 70%; Analysis 5.1.4).
All studies with blinded assessors
In this analysis we included 25 studies with 437 participants in the experimental and 383 in the control groups. Mirror therapy had a statistically significant positive effect on motor function compared with all other interventions (SMD 0.44, 95% CI 0.17 to 0.70; 820 participants; 25 studies; I2 = 69%; Analysis 5.1.5).
Outcome 5.2: Motor impairment at the end of the intervention phase
All studies with adequate sequence generation
We analysed 36 studies with 620 participants in the intervention and 537 in the control groups in this subgroup analysis of studies that we rated as having adequate sequence generation. We found a statistically significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.46, 95% CI 0.29 to 0.63; 1157 participants; 36 studies; I2 = 47%; Analysis 5.2).
Discussion
Summary of main results
The main purpose of this review was to evaluate the effect of mirror therapy for improving motor function, motor impairment, activities of daily living, and reducing pain and visuospatial neglect for people after stroke. We included 62 studies (57 RCTs and five randomised cross‐over studies), with a total of 1982 included participants that compared mirror therapy with other interventions. We found moderate‐quality evidence that mirror therapy improves motor function and motor impairment and activities of daily living. Furthermore, with low‐quality evidence we found reduced pain after stroke and improved motor impairment six months after the end of the intervention. However, after excluding studies that included participants with a complex regional pain syndrome only, we found no statistically significant effect on pain, based on moderate‐quality evidence. Results for motor function after six months and for visuospatial neglect were not statistically significant and were of low‐quality evidence. Acceptability of the intervention was high, without significantly more dropouts from the intervention groups compared with control groups, and with no reported adverse events during or after mirror therapy.
Fifty‐two of the included studies evaluated the effect of mirror therapy on motor function of the upper extremity, and 10 studies evaluated the effect of mirror therapy on the lower extremity. Mirror therapy was effective in improving both upper and lower limb motor function.
Based on a subgroup analysis, we found statistically significant effects on motor function in those studies that compared mirror therapy with a sham intervention using a covered mirror (thus avoiding any view of the affected limb), but not in studies that used unrestricted view (no mirror or a transparent plexiglas) or no additional intervention in the control groups. However, there were no statistically significant differences between subgroups with different control interventions.
In a further subgroup analysis, we compared studies that included participants in the acute/subacute phase after stroke (within six months after stroke) and participants in the chronic phase (more than six months after stroke). Mirror therapy was effective for both subgroups of participants.
Overall completeness and applicability of evidence
Based on the available and included evidence, we were able to answer the research question, especially for the outcomes of motor function and motor impairment for the upper and lower extremity, as well as activities of daily living and pain. However, for visuospatial neglect, the number of studies and participants was low, so we could draw no final conclusion. Furthermore, we found some indications for a selective effect of mirror therapy on pain in participants with CRPS. However, this is based on only two studies, so we could draw no final conclusion. The positive results for motor impairment were consistent with follow‐up assessment after six months, but not for motor function. The results are limited because our subgroup analysis indicates evidence of a greater effect of mirror therapy on motor function when compared with a sham intervention (using a covered mirror) than when compared with other (using unrestricted view) or no interventions. The positive effects in this review therefore at least indicate that mirror therapy as an adjunct to routine therapy can improve motor function for people after stroke. Furthermore, the effect on motor function was statistically significant both in acute/subacute and in chronic participants.
One of the potential advantages of mirror therapy compared with other interventions may be due to the possibility of training by moving the unaffected arm, or both arms, while looking in the mirror. Even people with severe paresis could therefore practise on their own without a therapist. Furthermore, mirror therapy could be applied at home, at least after inpatient training, as evaluated in five studies (Arya 2015; Manton 2002; Michielsen 2011; Pandian 2014; Rodrigues 2016).
Quality of the evidence
We used several methodological domains (adequate sequence generation, adequate concealment of allocation, adequate handling of missing outcome data and blinding of assessors) to assess the risks of bias in the included studies. We assessed nine studies as having unclear sequence generation. We found 33 studies with no or unclear use of concealed allocation of participants to study groups, 40 studies with no or unclear use of an adequate handling of missing outcome data, and 24 studies with no or unclear blinding of assessors. Results must therefore be interpreted with caution due to risks of bias. On this basis, we downgraded the quality of the evidence.
Some of the analyses showed significant heterogeneity. However, in the case of motor function and motor impairment this was no longer present when we excluded from the analysis those studies with unclear methods of sequence generation.
In order to test for potential biases through methodological issues, we performed a sensitivity analysis excluding randomised cross‐over studies, studies with unclear adequacy of sequence generation, studies with inadequate concealment of allocation, studies not providing adequate handling of missing outcome data, and studies that did not use assessors blinded to the intervention. Based on these sensitivity analyses, the effects of mirror therapy on motor function for people after stroke were robust, except for studies with adequate methods of allocation concealment. For those studies, the effects on motor function, but not motor impairment, did not demonstrate statistical significance.
Additionally, overall limitations of the included studies were the small sample sizes of most studies and differences in study participants (e.g. severity of motor impairment) and therapy delivery between the studies (i.e. amount and frequency of the treatment period).
Potential biases in the review process
Through an extensive searching process, we are confident that we have identified all relevant studies in the field. However, there remains a risk of publication bias towards a selection of positive results. Furthermore, there is a small possibility of additional (published or unpublished) studies that we did not identify. As stated above, there was heterogeneity between studies in terms of trial design (i.e. parallel‐group and cross‐over trials, duration of follow‐up and selection criteria for participants), characteristics of participants (i.e. severity of motor impairment and time since stroke onset) and characteristics of interventions (i.e. total amount of time of therapy, percentage of the intervention dedicated to mirror therapy only, and therapy for upper or lower extremity). We also identified methodological limitations of studies. However, as stated above, a sensitivity analysis of methodological limitations and participant characteristics revealed the robustness of the results across the stated potential confounding factors, except for concealment of allocation. Blinding of therapists and participants would be an additional item in the 'Risk of bias' assessment, but we decided not to integrate this item, since blinding of therapists or participants appears not to be not practicable for the type of intervention in this review.
Agreements and disagreements with other studies or reviews
The results of this review are in line with the results of other reviews (Ezendam 2009; Rothgangel 2011). These reviews were systematic in terms of their methods. However, they had more limited search strategies, only included studies that were published before 2009, and did not use a pooled analysis of identified studies. A narrative review also describes positive effects of mirror therapy after stroke (Ramachandran 2009).
Authors' conclusions
Implications for practice.
The results of this review indicate that there is moderate evidence for the effectiveness of mirror therapy for people after stroke in terms of improving motor function and motor impairment of the upper and lower extremity, as well as improving activities of daily living. The effects on motor function were more prominent when mirror therapy was compared to sham interventions. Mirror therapy could be applied as an additional intervention in the rehabilitation of people after stroke, but no clear conclusion could be drawn if mirror therapy replaced other interventions for improving motor function of the arm or leg, or both. No clear implication could be drawn for visuospatial neglect, since the positive results did not demonstrate statistical significance. Significant effects on pain were present in studies that included only participants with a CRPS‐type I after stroke. For this subgroup of people, mirror therapy may therefore be an effective intervention for reducing pain.
Implications for research.
The existing studies suggest an effect of mirror therapy after stroke, but they suffer from methodological problems such as small sample sizes and lack of proper reporting. There is thus an urgent need for well‐designed and properly‐reported multicentre randomised controlled studies with large sample sizes in order to provide a high level of evidence. Specifically, these studies should not deliver mirror therapy as an adjunct, but should compare it to other routinely‐applied therapies. Further research should also address specific questions about the optimal dose, frequency, and duration of mirror therapy. Studies should answer questions about the effects of mirror therapy according to the extent of motor impairment, and should also focus on people with impairments other than motor impairments after stroke, such as pain and visuospatial neglect. Finally, it is important to update this review regularly in order to include studies that are ongoing at the time of publication.
What's new
| Date | Event | Description |
|---|---|---|
| 27 September 2017 | New search has been performed | Based on an updated search we include 49 new studies, bringing the total number of included studies to 62 (1982 participants) in this updated review. In this updated review we:
|
| 27 September 2017 | New citation required and conclusions have changed | We added a new outcome (motor impairment) and the quality of evidence to our conclusion. Based on this, we found moderate evidence that mirror therapy is effective in improving motor function and motor impairment after stroke. Furthermore, we found moderate evidence for improved activities of daily living after mirror therapy following stroke. |
Acknowledgements
We thank Brenda Thomas and Josh Cheyne for their help with developing and running the search strategies, and Hazel Fraser for providing us with relevant trials from the Cochrane Stroke Group Trials Register and giving us helpful support. We also thank Gaby Voigt for providing us with many helpful studies and Luara Ferreira dos Santos for performing literature searches. We thank all authors and investigators who provided us with additional information and data on their studies. Finally, we owe thanks to the reviewers of this review who provided several helpful suggestions, especially Odie Geiger as Consumer Reviewer.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE Ovid
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ 2. brain injuries/ or brain injury, chronic/ 3. (stroke$ or cva or poststroke or post‐stroke).tw. 4. (cerebrovasc$ or cerebral vascular).tw. 5. (cerebral or cerebellar or brain$ or vertebrobasilar).tw. 6. (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy).tw. 7. 5 and 6 8. (cerebral or brain or subarachnoid).tw. 9. (haemorrhage or hemorrhage or haematoma or hematoma or bleed$).tw. 10. 8 and 9 11. exp hemiplegia/ or exp paresis/ 12. (hempar$ or paretic or paresis or hemipleg$ or brain injur$).tw. 13. Gait Disorders, Neurologic/ 14. 1 or 2 or 3 or 4 or 7 or 10 or 11 or 12 or 13 15. exp Upper Extremity/ 16. (upper limb$ or upper extremit$ or arm or shoulder or hand or axilla or elbow$ or forearm$ or finger$ or wrist$).tw. 17. exp Lower Extremity/ 18. (lower limb$ or lower extremit$ or buttock$ or foot or feet or hip or hips or knee or knees or leg or legs or thigh$ or ankle$ or heel$ or toe or toes).tw. 19. 15 or 16 or 17 or 18 20. Illusions/ 21. (mirror$ or visual$ or virtual$).tw. 22. (visual adj5 (reflection or illusion or feedback or therapy)).tw. 23. ((limb$ or arm or leg) adj5 (reflect or reflection or illusion)).tw. 24. 20 or 21 or 22 or 23 25. 14 and 19 and 24
Appendix 2. Adapted search strategies for other electronical databases
CENTRAL
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] or [mh ^"brain injuries"] or [mh ^"brain injury, chronic"]
#2 (stroke or poststroke or post‐stroke or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab,kw (Word variations have been searched)
#3 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw (Word variations have been searched)
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab,kw (Word variations have been searched)
#5 [mh ^hemiplegia] or [mh paresis] or [mh "gait disorders, neurologic"]
#6 (hempar* or hemipleg* or brain next injur*):ti,ab,kw (Word variations have been searched)
#7 {or #1‐#6}
#8 [mh "upper extremity"]
#9 (upper limb* or upper extremit* or arm or shoulder or hand or axilla or elbow* or forearm* or finger* or wrist*):ti,ab,kw (Word variations have been searched)
#10 [mh "lower extremity"]
#11 (lower limb* or lower extremit* or buttock* or foot or feet or hip or hips or knee or knees or leg or legs or thigh* or ankle* or heel* or toe or toes):ti,ab,kw (Word variations have been searched)
#12 {or #8‐#11}
#13 [mh illusions]
#14 (mirror* or visual* or virtual*):ti,ab,kw (Word variations have been searched)
#15 ((limb* or arm or leg) near/5 (mirror* or reflect* or reflection or illusion or visual* or virtual*)):ti,ab,kw (Word variations have been searched)
#16 {or #13‐#15}
#17 #7 and #12 and #16
Embase Ovid
1. cerebrovascular disease/ or brain disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. exp hemiplegia/ or exp paresis/ or neurologic gait disorder/
6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
7. or/1‐6
8. exp arm/ or limb/
9. (upper limb$ or upper extremit$ or arm or shoulder or hand or axilla or elbow$ or forearm$ or finger$ or wrist$).tw.
10. exp leg/
11. (lower lib$ or lower extremit$ or buttock$ or foot or feet or hip or hips or knee or knees or leg or legs or thigh$ or ankle$ or heel$ or toe or toes).tw.
12. or/8‐11
13. exp illusion/
14. (mirror$ or visual$ or virtual$).tw.
15. ((limb$ or arm or leg) adj5 (mirror$ or reflect$ or reflection or illusion or visual$ or virtual$)).tw.
16. or/13‐15
17. 7 and 12 and 16
18. limit 17 to yr="2011 ‐Current"
CINAHL Ebsco
(MH "Cerebrovascular Disorders+") or (MH "stroke patients") or (MH "stroke units")
TI ( stroke or poststroke or post‐stroke or cerebrovasc* or cerebral vasc or cva) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or cerebral vasc or cva)
TI ( brain* or cerebr* or cerebell* or vertebrobasilar ) or AB ( brain* or cerebr* or cerebell* or vertebrobasilar )
TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or apoplexy* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or apoplexy* )
3 and 4
TI ( brain* or cerebr* or cerebell* or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or subarachnoid )
TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
6 and 7
(MH "Hemiplegia")
TI ( hemipleg* or hemipar* or paresis or paretic or brain injur* ) or AB ( hemipleg* or hemipar* or paresis or paretic or brain injur* )
(MH "Brain Injuries")
1 or 2 or 5 or 8 or 9 or 10 or 11
(MH "Upper Extremity+")
TI ( upper limb* or upper extremit* or arm or shoulder or hand or axilla or elbow* or forearm* or finger* or wrist* ) or AB ( upper limb* or upper extremit* or arm or shoulder or hand or axilla or elbow* or forearm* or finger* or wrist* )
(MH "Lower Extremity+")
TI ( lower limb* or lower extremit* or buttock* or foot or feet or hip or hips or knee or knees or leg or legs or thigh* or ankle* or heel* or toe or toes ) or AB ( lower limb* or lower extremit* or buttock* or foot or feet or hip or hips or knee or knees or leg or legs or thigh* or ankle* or heel* or toe or toes )
13 or 14 or 15 or 16
(MH "Illusions+")
(MH "Reflection")
TI ( mirror* or video* or virtual* ) and AB ( mirror* or video* or virtual* )
TI ( reflect or reflection or illusion or visual feedback ) or AB ( reflect or reflection or illusion or visual feedback )
18 or 19 or 20 or 21
12 and 17 and 22
AMED Ovid
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h? ematoma$ or bleed$)).tw.
5. hemiplegia/ or exp gait disorders/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. axilla/ or buttocks/ or exp extremities/
9. (upper limb$ or upper extremit$ or arm or shoulder or hand or axilla or elbow$ or forearm$ or finger$ or wrist$).tw.
10. (lower limb$ or lower extremit$ or buttock$ or foot or feet or hip or hips or knee or knees or leg or legs or thigh$ or ankle$ or heel$ or toe or toes).tw.
11. 8 or 9 or 10
12. perception/ or visual perception/
13. (mirror$ or visual$ or virtual$).tw.
14. ((limb$ or arm or leg) adj5 (mirror$ or reflect$ or reflection or illusion or visual$ or virtual$)).tw.
15. 12 or 13 or 14
16. 7 and 11 and 15
PsychInfo
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. "arm (anatomy)"/ or "elbow (anatomy)"/ or "hand (anatomy)"/ or "shoulder (anatomy)"/ or wrist/
9. (upper limb$ or upper extremit$ or arm or shoulder or hand or axilla or elbow$ or forearm$ or finger$ or wrist$).tw.
10. "leg (anatomy)"/ or ankle/ or "feet (anatomy)"/ or knee/ or restless leg syndrome/ or thigh/
11. (lower limb$ or lower extremit$ or buttock$ or foot or feet or hip or hips or knee or knees or leg or legs or thigh$ or ankle$ or heel$ or toe or toes).tw.
12. 8 or 9 or 10 or 11
13. mirror image/ or visual perception/
14. (mirror$ or visual$ or virtual$).tw.
15. ((limb$ or arm or leg) adj5 (mirror$ or reflect$ or reflection or illusion or visual$ or virtual$)).tw
16. 13 or 14 or 15
17. 7 and 12 and 16
18. limit 17 to yr="2011 ‐Current"
PEDro
<mirror*> in “Abstract and Title” field, "Intervention" field, <upper arm, shoulder or shoulder girdle> in "Body part" field, <neurology> in "Subdiscipline" field, and <clinical trial> in "Method"
<mirror*> in “Abstract and Title” field, "Intervention" field, <forearm & elbow> in "Body part" field, <neurology> in "Subdiscipline" field, and <clinical trial> in "Method"
<mirror*> in “Abstract and Title” field, "Intervention" field, <hand & wrist> in "Body part" field, <neurology> in "Subdiscipline" field, and <clinical trial> in "Method"
<mirror*> in “Abstract and Title” field, "Intervention" field, <thigh or hip> in "Body part" field, <neurology> in "Subdiscipline" field, and <clinical trial> in "Method"
<mirror*> in “Abstract and Title” field, "Intervention" field, <lower leg or knee> in "Body part" field, <neurology> in "Subdiscipline" field, and <clinical trial> in "Method"
<mirror*> in “Abstract and Title” field, "Intervention" field, <foot or ankle> in "Body part" field, <neurology> in "Subdiscipline" field, and <clinical trial> in "Method"
Appendix 3. Search straetgies for study registers
ISRCTN Registry (www.isrctn.com/)
1. mirror therapy
2. mirror AND stroke
ClinicalTrials.gov (www.clinicaltrials.gov/)
1. Condition/ Disease: stroke; Other term: mirror
2. Condition/ Disease: cerebrovascular accident; Other term: mirror
StrokeTrials Registry (www.strokecenter.org/trials/)
1. keywords: mirror
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/)
1. mirror therapy
2. stroke AND mirror
OpenSIGLE ‐ System for Information on Grey Literature in Europe (www.opengrey.eu/)
1. mirror therapy
REHABDATA database (www.naric.com/research/rehab)
1. mirror therapy
Data and analyses
Comparison 1. Mirror therapy versus all other interventions: primary and secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Motor function at the end of intervention phase | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All outcome measures | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 2 Motor impairment at the end of intervention phase | 39 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All outcome measures | 39 | 1292 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.32, 0.66] |
| 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase | 28 | 898 | Mean Difference (IV, Random, 95% CI) | 4.32 [2.46, 6.19] |
| 4 Activities of daily living at the end of intervention phase | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All outcome measures | 19 | 622 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.30, 0.65] |
| 5 Pain at the end of intervention phase | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 All outcome measures | 6 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.67, ‐0.11] |
| 6 Visuospatial neglect at the end of intervention | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 All outcome measures | 5 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.10, 2.23] |
| 7 Motor function at follow‐up after 6 months | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 All outcome measures | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.78, 3.18] |
| 8 Motor impairment at follow‐up after 6 months | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 All outcome measures | 3 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.26, 1.12] |
| 9 Dropouts at the end of intervention phase | 42 | 1438 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.74, 1.76] |
Comparison 2. Subgroup analysis: upper versus lower extremity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Motor function at the end of intervention | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 1.1 Mirror therapy for the upper extremity | 31 | 1048 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.23, 0.69] |
| 1.2 Mirror therapy for the lower extremity | 5 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.92] |
Comparison 3. Subgroup analysis: sham intervention (covered mirror) versus other intervention (unrestricted view).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Motor function at the end of intervention phase | 36 | 1199 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.29, 0.72] |
| 1.1 Studies that used a covered mirror in the control group | 16 | 506 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.36, 0.99] |
| 1.2 Studies that used unrestricted view in the control group | 14 | 474 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.05, 0.59] |
| 1.3 Studies that used no additional control intervention | 8 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.02, 1.15] |
Comparison 4. Subgroup analysis: subacute versus chronic stage after stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Motor function at the end of intervention phase | 32 | 994 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.22, 0.66] |
| 1.1 All studies including participants within 6 months after stroke | 18 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.18, 0.73] |
| 1.2 All studies including participants with more than 6 months after stroke | 14 | 398 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.06, 0.81] |
Comparison 5. Sensitivity analysis by trial methodology.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Motor function at the end of intervention | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies without randomised cross‐over trials | 35 | 1160 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.68] |
| 1.2 All studies with adequate sequence generation | 33 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.19, 0.54] |
| 1.3 All studies with adequate concealed allocation | 16 | 572 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 1.4 All studies with adequate handling of incomplete outcome data | 12 | 388 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.14, 0.95] |
| 1.5 All studies with blinded assessors | 25 | 820 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.17, 0.70] |
| 2 Motor impairment at the end of intervention | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies with adequate sequence generation | 36 | 1157 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.29, 0.63] |
Comparison 6. Post hoc sensitivity analysis removing studies that only included participants with CRPS after stroke. Subgroup analysis: pain without complex regional pain syndrome (CRPS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at the end of intervention | 4 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acerra 2007.
| Methods | RCT | |
| Participants | Country: Australia Setting: inpatient Age: adults (mean age: 68 years) Sample size: 40 participants (20 in each group) Sex: 22 women, 18 men Inclusion criteria: acute stroke (< 2 weeks) Exclusion criteria: previous stroke; vision or hearing impairment; acute trauma or impairment of the limbs; inability to sit supported in a high‐backed chair for < 1 hour; MMSE < 22/30; major comorbidities |
|
| Interventions | 2 arms
1 and 2: 5 days a week, 20 to 30 minutes for 2 weeks; additional usual rehabilitation programme Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 2 weeks of treatment and 1 month after treatment
|
|
| Notes | Unpublished data We used means and SDs of Item 7 of the mAS, and combined the scores on pain intensity of shoulder and hand Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Generated list was used by an independent person for group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results were analysed on an ITT basis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Alibakhshi 2016.
| Methods | RCT | |
| Participants | Country: Iran Setting: inpatient hospital Age: adults (mean age: 50.9 years) Sample size: 24 participants (12 in each group, no dropouts published) Sex: 9 women, 15 men Inclusion criteria: stroke > 6 months, ability to understand treatment guidelines Exclusion criteria: any structural abnormalities that prevent the execution, any cognitive or perceptual deficit that can affect the implementation of treatment, visual deficits |
|
| Interventions | 2 arms
1 and 2: 3 weeks, 5 days a week, 30 minutes a day Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, immediately after treatment and 1 month after treatment
|
|
| Notes | Published and unpublished information Funding source: Neuromuscular Rehabilitation Research Centre ‐ Semnan University of Medical Sciences Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with odd‐ and even‐numbered cards |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Altschuler 1999.
| Methods | Randomised cross‐over trial | |
| Participants | Country: USA Setting: not stated Age: adults (mean age: 58.2 years) Sample size: 9 participants (9 in each group) Sex: 4 women, 5 men Inclusion criteria: at least 6 months post‐stroke Exclusion criteria: not stated |
|
| Interventions | 2 arms
Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 2, 4, 6 and 8 weeks
|
|
| Notes | Data not included in the analysis Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Amasyali 2016.
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehablitation centre Age: adults (mean age: 58.8 years) Sample size: 24 participants (9 in experimental group (2 dropped out at follow‐up assessment); 8 in control group 1; 7 in control group 2 (1 dropped out at follow‐up assessment) Sex: 11 women, 13 men Inclusion criteria: ischaemic stroke during the previous 12 months, between 20 and 85 years old, could understand simple verbal instructions (MMSE > 21), BRS between stage 2 and 5 for the hand, mAS < 3 Exclusion criteria: not stated |
|
| Interventions | 3 arms 1, 2 and 3: conventional physiotherapy programme
1, 2 and 3: 3 weeks, 5 days a week, 2 hours a day 1 and 2: additional 30 minutes a day Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 3 weeks of treatment and 3 months after treatment
|
|
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed as assigned to groups (authors' statement) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Arya 2015.
| Methods | RCT | |
| Participants | Country: India Setting: inpatient hospital, home after discharge Age: adults (mean age: 45.6 years) Sample size: 33 participants (17 in experimental group, 16 in control group, 1 dropout) Sex: 8 women, 25 men Inclusion criteria: aged < 60 years, single unilateral stroke with hemiparesis, more than 24 weeks post‐stroke, able to understand instructions, Brunnstrom recovery stage of arm (BRS‐A) 2 or above Exclusion criteria: associated neurological complications, severe perceptual and visual deficits (as evaluated by the National Institutes of Health Stroke Subscales and clinical tests: copying and drawing, line‐bisection, cancellation tasks, and functional performance), shoulder subluxation, uncontrolled medical illness |
|
| Interventions | 2 arms 1 and 2: usual occupational therapy using principles of Brunnstrom and Bobath approaches
1: 8 weeks, 5 days a week, 45 minutes MT, additional 45 minutes usual occupational therapy 2: 8 weeks, 5 days a week, 90 minutes usual occupational therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 8 weeks of treatment
|
|
| Notes | Information partly based on authors' information Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement), computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by numbered sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT using 'last measure carried forward' method |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Arya 2017.
| Methods | RCT | |
| Participants | Country: India Setting: inpatient rehabilitation centre Age: adults (mean age: 46.4 years) Sample size: 36 participants (19 in experimental group, 17 in control group; 6 dropouts) Sex: 6 women, 30 men Inclusion criteria: post‐stroke hemiparesis due to unilateral stroke; post‐stroke duration > 6 months; paresis of either right or left side; age range between 30 and 60 years; functional ambulation classification (FAC) level 2 and above; ability to walk for a distance of at least 10 metres without any orthosis and walking device Exclusion criteria: any other associated neurological disorder; severe cognitive, perceptual and visual deficits (evaluated by the National Institutes of Health Stroke Subscales and copying, drawing, line bisection, cancellation, and functional tasks); cardiovascular instability; any musculoskeletal disorder affecting locomotion |
|
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1: 3 to 4 weeks, 30 sessions, 30 minutes MT and 30 minutes conventional rehabilitation programme 2: 3 to 4 weeks, 30 sessions, 60 minutes conventional rehabilitation programme Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 3 months
|
|
| Notes | Information based on abstract and authors' information, full‐text publication received in 2017 Funding source: Pandit Deendayal Upadhayaya National Institute for Persons with Physical Disabilities, 4 VD Marg, New Delhi‐110002, India Declarations of trialists’ interests: no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement), computer‐generated (SPSS software) random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes (authors' statement) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT using 'last measure carried forward' method (authors' statement) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors, participants, and therapists were blinded to group allocation (authors' statement) |
Bae 2012.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 53.9 years) Sample size: 20 (10 in each group; no dropouts published) Sex: 7 women, 13 men Inclusion criteria: onset of stroke within 6 months Exclusion criteria: did not understand treatment method of the study, MMSE < 16, visual impairment, damage on musculoskeletal system or peripheral nerve on paretic side, mAS score > 2, Brunnstrom recovery stage 1, 5 or 6 |
|
| Interventions | 2 arms 1 and 2: usual rehabilitation treatment and additional:
1 and 2: 4 weeks, 5 days a week, 30 minutes MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by cards composed of odd and even numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Bahrami 2013.
| Methods | RCT | |
| Participants | Country: Iran Setting: not stated Age: adults (age not stated) Sample size: 50 participants (25 in each group, no dropouts published) Sex: not stated Inclusion criteria: 1st unilateral stroke (ischaemic or haemorrhagic verified by CT‐scan or MRI), between 1 month and 1 year after stroke, Brunnstrom recovery stages 1 ‐ 3 Exclusion criteria: severe cognitive deficit, severe aphasia, visual deficits, dementia, not able to understand instructions, did not participate in 4 sessions or 2 consecutive sessions |
|
| Interventions | 2 arms 1 and 2: physiotherapy and neuromuscular stimulation
1 and 2: 20 sessions, 3 to 5 days a week, 30 minutes 1: 20 sessions, 3 to 5 days a week, additional 30 minutes MT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after the 5th, 10th, and 15th session
|
|
| Notes | Information based on abstract, partly translated from Persian language Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by coin tossing |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Cacchio 2009a.
| Methods | RCT | |
| Participants | Country: Italy Seting: inpatient and outpatient rehabilitation centre Age: adults (mean age: 58.4 years) Sample size: 48 participants (24 in each group; 6 dropped out post‐treatment, 3 more dropped out after 6 months) Sex: 26 women, 22 men Inclusion criteria: hemiparesis after first‐ever ischaemic or haemorrhagic stroke; during 1st 6 months post‐stroke; diagnosed with CRPS‐type 1 with a VAS pain score > 4 cm Exclusion criteria: intra‐articular injection into the affected shoulder during the previous 6 months or use of systemic corticosteroids during the previous 4 months; presence of another explanation of pain; prior surgery to shoulder or neck; serious uncontrolled medical conditions; global aphasia or cognitive impairments; visual impairments which might interfere with the aims of the study; evidence of recent alcohol or drug abuse; or severe depression |
|
| Interventions | 2 arms: 4‐week conventional stroke rehabilitation programme and additional:
1 and 2: 5 days a week, 30 minutes of therapy for the 1st 2 weeks; and 5 days a week, 60 minutes of therapy for the last 2 weeks Date of intervention: October 2000 to December 2006 |
|
| Outcomes | Outcomes were recorded at baseline, 1 week after the intervention period and after 6 months
|
|
| Notes | Published and unpublished data Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results were analysed on an ITT basis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Cacchio 2009b.
| Methods | RCT | |
| Participants | Country: Italy Setting: inpatient and outpatient rehabilitation centre Age: adults (mean age: 62 years) Sample size: 24 participants (8 in each group) Sex: 13 women, 11 men Inclusion criteria: 1st ischaemic or haemorrhagic stroke (> 6 months); diagnosis of CRPS‐type 1 (pain VAS > 4 cm) Exclusion criteria: intra‐articular shoulder injection in the previous 6 months or systemic corticosteroid in the previous 4 months; another obvious explanation for pain; prior surgery to shoulder or neck region; serious uncontrolled medical conditions; global aphasia or cognitive impairments interfering with understanding instructions, motor testing and treatment; visual impairments interfering with aims of the study; evidence of recent alcohol or drug abuse; or severe depression |
|
| Interventions | 3 arms
1, 2 and 3: 5 days a week; 30 minutes of therapy for 4 weeks Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after the intervention period
|
|
| Notes | Published and unpublished data; we only analysed the 1st intervention period (4 weeks); we combined groups 2 and 3 into 1 control group Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation; cards composed with random‐numbers method |
| Allocation concealment (selection bias) | Low risk | A therapist not involved in the treatments opened sealed envelopes and assigned appointments according to treatment group (authors' statement) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results were analysed on an ITT basis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Cha 2015.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: not stated Age: adults (mean age: 58.7 years) Sample size: 36 participants (19 in experimental group, 17 in control group, no dropouts published) Sex: 17 women, 19 men Inclusion criteria: stroke onset duration of > 6 months; no neurological deficits in the cerebellum or the brainstem; no hemineglect or visual field deficits; no cognitive problems (> 24 points in the MMSE); independent walking (with or without walking aids) Excludion criteria: not stated |
|
| Interventions | 2 arms
1 and 2: 4 weeks, 5 days a week, 40 minutes (20 minutes rTMS and 20 minutes MT or sham therapy) Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after the 4 weeks of therapy:
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by blindly drawing 1 card out of an envelope containing 2 cards that were each marked as experimental group and control group |
| Allocation concealment (selection bias) | Low risk | Concealment by sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated; no dropouts |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Cho 2015.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: not stated Age: adults (mean age: 59.3 years) Sample size: 27 participants (14 in experimental group, 13 in control group, no dropouts published) Sex: 12 women, 15 men Inclusion criteria: stroke with hemiplegic symptoms, a score of 24 or higher on the MMSE‐K, stroke onset more than 6 months earlier Exclusion criteria: orthopaedic or neurological disease history |
|
| Interventions | 2 arms 1 and 2: tDCS 1: MT: participants performed movements of both upper limbs, 10 sets, 20 repetitions of each motion, 2‐minute rest between sets 2: sham therapy: participants performed the same exercises with non‐reflective surface between limbs 1 and 2: 6 weeks, 3 days a week, 20 minutes tDCS + 5 minutes rest + 20 minutes MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after the 6 weeks of therapy
|
|
| Notes | Funding source: Wonkwang Health Science University Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Colomer 2016.
| Methods | RCT | |
| Participants | Country: Spain Setting: outpatient rehabilitation centre Age: adults (mean age: 53.5 years) Sample size: 34 (17 in experimental group (2 dropped out); 16 in control group (1 dropped out)) Sex: 5 women, 26 men Inclusion criteria: stroke > 6 months, BRS 1 or 2, FM‐UE < 19, sensory impairment assessed by clinical examination, able to maintain sitting position for at least 60 minutes, MMSE > 23 Exclusion criteria: impaired comprehension that hindered understanding of instructions (Mississippi Aphasia screening < 45), upper limb pain that limited participation in rehabilitation protocol, spatial neglect, self‐awareness disorder, emotional circumstances that impeded adequate collaboration |
|
| Interventions | 2 arms 1 and 2: usual physical therapy:
1 and 2: 8 weeks, 5 days a week, 60 minutes each, additional 3 days a week, 45 minutes a session MT or passive mobilisation Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 8 weeks of intervention
|
|
| Notes | Published information Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes and independent investigator |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis was performed |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Dalla Libera 2015.
| Methods | RCT | |
| Participants | Country: Switzerland Setting: not stated Age: adults (age not stated) Sample size: 10 participants (no dropouts published) Sex: not stated Inclusion criteria: 3 months after stroke; severe disability (NIHSS 10 ‐ 14), hand paresis Exclusion criteria: not stated |
|
| Interventions | 2 arms 1 and 2: TMS: double‐pulse TMS through a figure‐eight focal coil for bilateral intracortical inhibition in primary motor at rest and during movement preparation
1 and 2: 4 weeks, 3 days a week, 15 minutes TMS 1: additional 15 minutes MT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after therapy period
|
|
| Notes | Information based on authors' information and abstract Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Dohle 2009.
| Methods | RCT | |
| Participants | Country: Germany Setting: inpatient rehabiltation centre Age: aduts (mean age: 56.5 years) Sample size: 48 participants (24 in each group, 12 dropped out) Sex: 10 women, 26 men Inclusion criteria: first‐ever ischaemic stroke in the territory of the middle cerebral artery; not more than 8 weeks post‐stroke; between 25 and 80 years old; able to follow therapy instructions; capable of participating in 30‐minute daily therapy sessions Exclusion criteria: experienced previous stroke; major haemorrhagic changes; increased intracranial pressure; hemicraniectomy or orthopaedic, rheumatologic, or other diseases interfering with their ability to sit or to move either upper limb |
|
| Interventions | 2 arms
1 and 2: 5 days a week; 30 minutes of therapy for 6 weeks Date of intervention: October 2004 to April 2006 |
|
| Outcomes | Outcomes were recorded at baseline and after the intervention
|
|
| Notes | Published and unpublished data; we extracted the motor section of the FM‐UE (without reflex activity, 0 to 60) Funding source: rehabilitation research network (refonet) of the German Pension Scheme Rhineland Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed, numbered envelopes were created |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were broken after study inclusion |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors of primary outcome were blinded to group allocation |
Geller 2016.
| Methods | RCT | |
| Participants | Country: USA Setting: outpatient (at home) Age: adults (34 to 73 years old) Sample size: 6 participants (4 in 2 experimental groups, 2 in control group; dropouts not published) Sex: 3 women, 3 men Inclusion criteria: first‐time unilateral stroke occurring at least 3 months prior with FMA‐UE scores between 10 and 50 Exclusion criteria: not stated |
|
| Interventions | 3 arms 1 ‐ 3 : occupational therapy (OT)
1 ‐ 3: 6 weeks, 2 times a week OT in the clinic 1 ‐ 3: 6 weeks, 5 days a week, 30‐minute home programme bimanual MT, unimanual MT or traditional OT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded
|
|
| Notes | Information based on abstract Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Gurbuz 2016.
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehabiltation centre Age: adults (mean age: 60.9 years) Sample size: 31 (16 in experimental group, 15 in control group, no dropouts published) Sex: 14 women, 17 men Inclusion criteria: unilateral hemiplegia due to first‐ever stroke (verified by CT or MRI); < 6 months; BRS for the upper extremity between I and IV; MMSE 24 and above; lack of excessive spasticity in the joints of the affected upper extremity (stage 2 and below according to the mAS) Exclusion criteria: joint movement limitations in the healthy upper extremity; a visual field defect or neglect syndrome; and those who had previously undergone a rehabilitation programme |
|
| Interventions | 2 arms 1 and 2: upper extremity rehabilitation programme
1 and 2: 4 weeks, 5 times a week, 60 to 120 minutes upper extremity rehabilitation programme 1 and 2: 4 weeks, 5 times a week, 20 minutes MT or sham therapy Date of intervention: July 2013 to July 2014 |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated; no dropouts published |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Hiragami 2012.
| Methods | RCT | |
| Participants | Country: Japan Setting: inpatient hospital Age: adults (mean age: 67.5 years) Sample size: 14 participants (7 in each group, no dropouts published) Sex: 6 women, 8 men Inclusion criteria: 1st episode of stroke with hemiparesis or second episode of stroke with no upper limb motor dysfunction after 1st stroke, > 1 month since stroke, Brunnstrom recovery stage finger 1 ‐ 5, no severe cognitive disorders (MMSE score ≥ 24, and item score of consciousness, gaze, visual fields, language, attention of National Institutes of Health Stroke scale = 0) Exclusion criteria: hypertonia of upper limb, limitation in range of motion of upper limb, other diseases interfering with ability to move upper limbs |
|
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme (physiotherapy, occupational therapy)
1 and 2: 4 weeks, 6 ‐ 7 days a week, daily 2 hours 1: additional 30 minutes MT Date of intervention: October 2010 to March 2011 |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent author who drew sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts and group changes |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
In 2012.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 63.9 years) Sample size: 24 participants (14 in experimental group, 10 in control group; 5 dropouts) Sex: 8 women, 11 men Inclusion criteria: onset of stroke at least 6 months prior to study, able to understand and follow simple verbal instructions, MMSE > 21, Brunnstrom stages 1 ‐ 4 Exclusion criteria: apraxia, hemineglect, orthopaedic conditions or digital neuropathy in upper extremities |
|
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme
1 and 2: 4 weeks, 5 days a week, 30 minutes additional virtual reality (VR) reflection therapy or additional sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
In 2016.
| Methods | RCT | |
| Participants | Country: Republic of Korea Seting: inpatient rehabilitation centre Age: adults (mean age: 55.9 years) Sample size: 30 participants (15 in experimental group and 15 in control group; 5 dropouts) Sex: 10 women, 15 men Inclusion criteria: onset of stroke at least 6 months prior to study; were able to understand and follow simple verbal instructions; had a MMSE score over 21; had a Brunnstrom score between stages I and IV Exclusion criteria: had no apraxia or hemineglect; had no orthopaedic and neurologic conditions such as fractures and digital neuropathy on their lower extremities |
|
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme
1 and 2: 4 weeks, 5 days a week, 30 minutes conventional stroke rehabilitation programme 1 and 2: 4 weeks, 5 days a week, 30 minutes additional virtual reality (VR) reflection therapy or additional sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks:
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to the participants’ groups |
Invernizzi 2013.
| Methods | RCT | |
| Participants | Country: Italy Setting: inpatient rehabilitation centre Age: adults (mean age: 66.6 years) Sample size: 26 (13 in each group; 1 dropped out) Sex: 9 women, 17 men Inclusion criteria: hemiplegia after 1st stroke (diagnosed by CT scan) within 4 weeks post‐stroke, absence of severe attentive deficits, presence of movement in shoulder/elbow/hand with Motricity score < 77; Exclusion criteria: haemorrhagic stroke, global aphasia and cognitive impairments that interfere with study or treatment participation (MMSE < 22), concomitant cns‐ or pns‐disorder or myopathia |
|
| Interventions | 2 arms: usual rehabilitation programme 1 hour, 5 times a week, additional:
1 and 2: 5 days a week, 30 minutes of MT or sham therapy for 1st 2 weeks, 60 minutes of MT or sham therapy for the last 2 weeks Date of intervention: October 2009 to August 2011 |
|
| Outcomes | Outcomes were recorded and reported at baseline and after 4 weeks
|
|
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed as allocated (authors' information) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Ji 2014a.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: university hospital Age: adults (mean age: 52.6 years) Sample size: 35 participants (12 in experimental group 1, 11 in experimental group 2, 12 in control group, no dropouts published) Sex: 13 women, 22 men Inclusion criteria: hemiparesis by stroke Exclusion criteria: not stated |
|
| Interventions | 3 arms 1, 2 and 3: traditional physiotherapy
1, 2, and 3: 6 weeks, 5 days a week, 30 minutes a session physiotherapy 1, 2, and 3: additional 15 minutes a day MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 6 weeks of therapy
|
|
| Notes | Information based on published article Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number blocks |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Kawakami 2015.
| Methods | RCT | |
| Participants | Country: Japan Setting: inpatient rehabilitation centre Age: adults (mean age: 64.1 years) Sample size: 81 participants (19 in group 1 (3 dropped out), 25 in group 2 (6 dropped out), 17 in group 3 (2 dropped out),11 in group 4 (2 dropped out), 9 in group 5 (1 dropped out)) Sex: 24 women, 43 men Inclusion criteria: hemiplegia following initial supratentorial stroke, admitted to a convalescent rehabilitation ward Exclusion criteria: time to admission from the onset is within 14 days, difficult communication due to severe cognitive disorder, comorbidity index of 4 or higher, necessity of high‐level consideration and caution for rehabilitation, and scores of hip‐flexion, knee‐extension, and foot‐pat items of the Stroke Impairment Assessment Set (SIAS) lower than 2 |
|
| Interventions | 5 arms 1 to 5: standard rehabilitation programme
1 to 5: 4 weeks, 1 hour a day standard rehabilitation programme 1 to 5: 20 minutes within conventional physiotherapy Date of intervention: September 2009 to July 2011 |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | Information based on published article Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Kim 2014.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: university hospital Age: adults (mean age: 55.8 years) Sample size: 27 (14 in experimental group, 13 in control group, 4 dropouts) Sex: 9 women, 14 men Inclusion criteria: onset of stroke within 6 months, MMSE > 21, FMA upper extremity score < 44, Brunnstrom recovery stage 1 ‐ 4, absence of orthopaedic disease in the upper extremity, no visual perception disorder (unilateral neglect, hemianopsia, apraxia), no pacemaker, no anticonvulsant medication, medically stable condition Exclusion criteria: not stated |
|
| Interventions | 2 arms 1 and 2: usual rehabilitation treatment
1 and 2: 60 minutes/day, 5 times/week, 4 weeks usual rehabilitation treatment 1 and 2: additional 5 days a week, 30 minutes a day, 4 weeks MT or sham therapy Date of intervention: 1 July to 31 July 2013 |
|
| Outcomes | Outcomes were recorded and reported at baseline (t1), after 4 weeks of treatment (t2)
|
|
| Notes | Funding source: Sahmyook University Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Outcome assessors were blinded to group allocation |
Kim 2015a.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 57.7 years) Sample size: 33 participants (20 in 2 experimental groups, 9 in control group; 4 dropouts) Sex: 9 women, 20 men Inclusion criteria: onset of stroke > 6 months, MMSE > 25, absence of cognitive problems, BRS 1 – 4, and the ability to understand the purpose of the study Exclusion criteria: impaired vision, cognitive problems such as a severe decline in cognition or aphasia that would prevent normal progress in the experiment, neurological or musculoskeletal (fracture or balance‐related) disorders not caused by stroke, hemineglect |
|
| Interventions | 3 arms 1, 2 and 3: conventional rehabilitation programme
1, 2 and 3: 4 weeks, 5 days a week, 30 minutes a day 1 and 2: additional 4 weeks, 5 days per week, 30 minutes a session Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts not analysed |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Kim 2016.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: outpatient hospital Age: adults (mean age: 49.1 years) Sample size: 25 participants (12 in experimental group, 13 in control group, no dropouts published) Sex: 9 women, 16 men Inclusion criteria: hemiplegia due to stroke, stroke > 6 months. MMSE > 24, understanding the procedure and purpose of the study, volunteer participation in the study Exclusion criteria: not stated |
|
| Interventions | 2 arms
1and 2: 4 weeks, 5 days a week, 30 minutes a day MT or control intervention Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by dice with odd and even numbers |
| Allocation concealment (selection bias) | Low risk | Allocation by throwing dice after inclusion in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information, no drop‐outs |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Kojima 2014.
| Methods | Randomised cross‐over trial | |
| Participants | Country: Japan Setting: inpatient rehabilitation centre Age: adults (mean age: 69.1 years) Sample size: 13 participants (6 in group 1, 7 in group 2, no dropouts) Sex: 3 women, 10 men Inclusion criteria: hemiparesis caused by a single stroke, between 30 and 180 days post‐stroke, MMSE > 20, palpable contraction of paretic wrist and finger extensors, detectable EMG signal (> 5 V) from those muscles Exclusion criteria: cardiac pacemaker; serious contractures or pain in the shoulder, elbow or wrist; shoulder subluxation; severe cognitive impairment or severe aphasia; inability to give informed consent; engagement in any other experimental studies |
|
| Interventions | 2 arms 1 and 2: standard physiotherapy and occupational therapy
1 and 2: 8 weeks, 5 days a week, 2 hours a day physiotherapy and occupational therapy 1 and 2: 4 weeks, 5 days a week, two 20‐minute sessions a day; group 1: additional ETMS‐MT for the 1st 4 weeks, group 2: additional ETMS‐MT for the second 4 weeks Date of intervention: November 2009 to May 2012 |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks and 8 weeks of therapy
|
|
| Notes | Based on published and unpublished information Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by permuted block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | High risk | Assessor not blinded to group allocation |
Kumar 2013.
| Methods | RCT | |
| Participants | Country: India Setting: not stated Age: adults (mean age: 57.3 years) Sample size: 30 (15 in each group, no dropouts) Sex: 8 women, 22 men Inclusion criteria: 1st stroke (ischaemic or haemorrhagic), unilateral stroke with hemiparesis, Brunnstrom recovery stage 2 ‐ 4, age > 25 years, ambulatory before stroke, able to understand simple verbal instructions Exclusion criteria: severe cognitive disorder, previous stroke, orthopaedic or rheumatologic problems restricting lower limbs, other diseases that interfere with ability to sit or moving lower limbs |
|
| Interventions | 2 arms 1 and 2: conventional physical therapy and
1 and 2: 40 ‐ 45 minutes/day for 10 days conventional physical therapy 1: twice daily for 15 minutes for 10 days Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 5 and 10 days
|
|
| Notes | Unpublished data Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Kuzgun 2012.
| Methods | RCT | |
| Participants | Country: Turkey Setting: not stated Age: adults (mean age: 61.4 years) Sample size: 20 participants (10 in experimental group, 10 in control group, no dropouts published) Sex: 10 women, 10 men Inclusion criteria (information based on translation): 1st stroke < 8 weeks; Brunnstrom recovery stages 1 ‐ 4 Exclusion criteria (information based on translation): previously received treatment/rehabilitation; mAS > 3; pain in the paretic side; cognitive impairments; vision impairments/neglect |
|
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1 and 2: 4 weeks, 5 days a week, daily 1 ‐ 2 hours 1: additional 15 minutes , 4 times daily MT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy:
|
|
| Notes | Information based on an abstract; partly translated; not possible to contact author Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by envelope method Comment: information based on translation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated (no dropouts) Comment: information based on translation |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded Comment: information based on translation |
Lee 2012.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 57.1 years) Sample size: 28 (14 in each group; 2 dropped out) Sex: 11 women, 15 men Inclusion criteria: stroke within last 6 months, able to understand and follow the instructions (MMSE > 21), Brunnstrom recovery stages upper limb 1 ‐ 4 Exclusion criteria: orthopaedic disorders, apraxia, hemineglect, upper‐limb fracture, peripheral nerve injury, participation in other studies or rehabilitation programmes, participation rate < 80% |
|
| Interventions | 2 arms 1 and 2: usual rehabilitation programme
1 and 2: 75 minutes, 5 times/week 1: 1st 4 weeks, 5 days/week, 25 minutes twice a day MT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded and reported at baseline and after 1 day after therapy period
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not analysed |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Lee 2016.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: rehabilitation hospital Age: adults (mean age: 54.7 years) Sample size: 30 participants (15 in experimental group (1 dropped out), 15 in control group (2 dropped out)) Sex: 13 women, 14 men Inclusion criteria: stroke diagnosed by a neurologist using computed tomography or magnetic resonance imaging, hemiplegia for > 6 months after stroke onset, active ankle dorsiflexion ROM > 10 °, ability to walk > 10 metres independently, MMSE > 21, no visual problems, no adverse effects from NMES, absence of use of any medication that could affect balance or gait Exclusion criteria: uncontrolled blood pressure or angina, history of seizure, pacemaker use, musculoskeletal problems of the lower extremity, any intervention other than conventional therapy |
|
| Interventions | 2 arms 1 and 2: conventional physiotherapy
1 and 2: 4 weeks, 5 days a week, 1 hour a day 1: additional 4 weeks, 5 days a week MT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and 1 day after therapy period
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | 2 assessors were blinded to group allocation |
Lim 2016.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehablitation centre Age: adults (mean age: 64.9 years) Sample size: 60 (30 in each group, no dropouts) Sex: 21 women, 39 men Inclusion criteria: hemiplegia due to stroke within 6 months, Korean version of MMSE > 24, BRS upper extremity of 3 to 4 Exclusion criteria: musculoskeletal disease, neglect, mental illness |
|
| Interventions | 2 arms
1 and 2: 4 weeks, 5 days a week, 20 minutes/day MT or sham therapy Date of intervention: February to May 2012 |
|
| Outcomes | Outcomes were recorded at baseline and after therapy period
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated (no dropouts) |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Lin 2014a.
| Methods | RCT | |
| Participants | Country: Taiwan Setting: inpatient and outpatient Age: adults (mean age: 55 years) Sample size: 43 participants (14 in experimental group 1, 14 in experimental group 2, 15 in control group, 1 dropout) Sex: 11 women, 32 men Inclusion criteria: ischaemic or haemorrhagic stroke of at least 6 months duration, Brunnstrom stage 3 or above in the arm Exclusion criteria: severe spasticity in any joints of the affected arm (modified AS ≤ 2), serious cognitive deficits (MMSE score > 24), serious vision or visual perception deficits (score of 0 on the best gaze and visual subtest of the National Institutes of Health Stroke Scale), history of other neurologic, neuromuscular, or orthopaedic disease, participation in other studies concurrent with this study |
|
| Interventions | 3 arms
1, 2, and 3: 4 weeks, 5 days a week, 1½ hours daily Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after therapy
|
|
| Notes | Funding source: National Health Research Institutes, National Science Council, Healthy Ageing Research Center at Chang Gung University, Taiwan Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned , stratified into 4 strata according to the side of lesion and the level of motor impairment |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts not analysed |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Manton 2002.
| Methods | RCT | |
| Participants | Country: USA Setting: home Age: adults (age not stated) Sample size: 10 participants Sex: not stated Inclusion criteria: 6 months or more post‐cerebrovascular accident Exclusion criteria: not stated |
|
| Interventions | 2 arms
1 and 2: 4 weeks Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at pretreatment, mid‐treatment, post‐treatment and after 3 months
|
|
| Notes | Abstract data only; not included in the analysis due to insufficient data Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Ability‐matched pairs were created and randomly assigned to groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Marquez 2012.
| Methods | RCT | |
| Participants | Country: Australia Setting: inpatient rehabilitation unit Age: adults (mean age: 68.7 years) Sample size: 15 participants (5 in experimental group, 10 in 2 control groups, no dropouts) Sex: 8 women, 7 men Inclusion criteria: first‐ever neurological injury < 8 weeks, affected dorsiflexion strength of < Grade 3, ambulatory prior to admission Exclusion criteria: impaired cognition (MoCA < 21), peripheral neuropathy, impaired ROM of the intact lower limb, medically unfit for rehabilitation |
|
| Interventions | 3 arms 1, 2 and 3: individual physiotherapy sessions
1, 2 and 3: 3 weeks, 5 days a week, 45 minutes a day individual physiotherapy 1 and 2: 15 minutes MT or sham therapy during the individual physiotherapy session Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 3 weeks of therapy, and 6 weeks after the intervention
|
|
| Notes | Based on unpublished information, only stroke patients included Funding source: National Stroke Foundation, Australia Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by independent person |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data collected, reported and analysed as allocated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Michielsen 2011.
| Methods | RCT | |
| Participants | Country: Netherlands Setting: home Age: adults (mean age: 57 years) Sample size: 40 participants (20 in each group,; 4 dropped out during intervention period, 4 more dropped out after 6 months) Sex: 20 women, 20 men Inclusion criteria: knowledge of Dutch language, Brunnstrom score upper extremity between 3 and 5; home dwelling status; at least 1 year post‐stroke Exclusion criteria: neglect; comorbidities that influenced upper extremity usage; history of multiple strokes |
|
| Interventions | 2 arms
1 and 2: once a week physiotherapeutic supervision for 60 minutes; 5 times a week, 60 minutes of practice at home for 6 weeks Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, post‐treatment and after 6 months
|
|
| Notes | Published and unpublished data Funding source: Fonds NutsOhra [SNO‐T‐0602‐23]; Innovatiefonds Zorgverzekeraars [06‐262]; Wetenschappelijk College Fysiotherapie [WU/2007/07] and Hersenstichting Nederland [15F07.54] Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Participants received group allocation after baseline measurement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results were analysed on an ITT basis (multiple imputation) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Mirela 2015.
| Methods | RCT | |
| Participants | Country: Romania Setting: inpatient Age: adults (mean age: 57.5 years) Sample size: 15 participants (7 in experimental group, 8 in control group, no dropouts published) Sex: 8 women, 7 men Inclusion criteria: hemiplegia following a 1st stroke (documented by CT scan), time from stroke between 1 to 3 months, without severe attention deficit Exclusion criteria: global aphasia and cognitive impairments that might interfere with understanding instructions for testing, concomitant progressive central or peripheral nervous system disorders |
|
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme (neuro‐rehabilitation technique, electrical stimulation and occupational therapy)
1 and 2: 6 weeks, 5 times a week, 30 minutes a session conventional stroke rehabilitation programme 1: additional 30 minutes of MT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and 1 day after therapy
|
|
| Notes | Funding source: not financed Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Mohan 2013.
| Methods | RCT | |
| Participants | Country: India Setting: inpatient rehabilitation centre Age: adults (mean age: 63 years) Sample size: 22 participants (11 in each group, no dropouts published) Sex: 10 women, 12 men Inclusion criteria: 1st episode of unilateral stroke with hemiparesis (onset ≤ 2 weeks), able to understand and follow simple verbal instructions, Brunnstrom recovery stage 2 and above, no severe cognitive disorders that would interfere with the study’s purpose (MMSE score > 23), stable medical condition to allow participation in the study, ambulatory before stroke Exclusion criteria: neglect, Pusher syndrome, visual deficits, and history of multiple stroke, or comorbidities that influenced lower extremity usage |
|
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme: neurodevelopmental facilitation techniques, sensory motor re‐education, active exercises, mobility training, balance, and gait training
1 and 2: 2 weeks, 6 days a week, 60 minutes a day and additional 30 minutes of MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 2 weeks of therapy
|
|
| Notes | Funding source: not financed (according to authors) Declarations of trialists’ interests: there are no conflicts of interest (according to authors) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) by block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed as intended |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Moustapha 2012.
| Methods | Randomised cross‐over trial | |
| Participants | Country: France Setting: not stated Age: adults (mean age: 53.5 years) Sample size: 8 participants (4 in each group, 2 dropouts) Sex: 4 women, 4 men Inclusion criteria: neglect (according to Negligence Evaluation Battery) secondary to a unilateral stroke of the right hemisphere Exclusion criteria: other concomitant cerebral injuries, Illetrism or cognitive dysfunction altering comprehension |
|
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes a day; 1: MT for 5 consecutive days, 1 session a day, after 10 days sham therapy for 5 consecutive days; 2: same protocol as group 1, but participants received sham therapy before MT Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded before and after each session
|
|
| Notes | Based on unpublished information Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by randomised‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by drawing lots |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis was performed |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Nagapattinam 2015.
| Methods | RCT | |
| Participants | Country: India Seting: hospital Age: adults (mean age: 44.9 years) Sample size: 60 participants (40 in 2 experimental groups, 20 in control group, 1 dropout) Sex: 20 women, 40 men Inclusion criteria: unilateral hemiplegic stroke, between 6 weeks and 6 months post‐stroke, ischaemic stroke, age 18 to 60 years, both men and women, BRS 2 ‐ 5, modified AS ≥ 1, voluntary extension of wrist and fingers of at least 10 ° from the resting position Exclusion criteria: > 60 years of age, BRS 1 or 6, wrist and/or finger contracture, cardiac pacemaker or other metal implants, significant visual, auditory and cognitive impairment |
|
| Interventions | 3 arms 1, 2 and 3: conventional therapy
1, 2 and 3: 2 weeks, 6 days a week, 30 minutes daily MT, MT + FES, or FES Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 2 weeks of therapy
|
|
| Notes | Based on published information Funding source: not stated Declarations of trialists’ interests: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by cards composed of odd and even numbers |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data were collected and analysed as allocated |
| Blinding of outcome assessment (detection bias) primary outcome | High risk | Assessors were not blinded to group allocation |
Pandian 2014.
| Methods | RCT | |
| Participants | Country: India Setting: inpatient rehabilitation centre and home training after discharge Age: adults (mean age: 63.4 years) Sample size: 48 participants (27 in experimental group, 21 in control group, 2 dropouts) Sex: 20 women, 28 men Inclusion criteria: stroke patients with thalamic and parietal lobe lesions within 48 hours of stroke onset who had upper limb weakness, provided informed consent Exclusion criteria: Glasgow Coma Scale score < 7, unco‐operative patients |
|
| Interventions | 2 arms 1 and 2: home programme
1: 4 weeks, 5 days a week, 1 hour a day MT and 1 hour limb activation 2: 4 weeks, 5 days a week, 1 hour a day sham therapy and 1 hour limb activation Date of intervention: January 2011 to August 2013 |
|
| Outcomes | Outcomes were recorded at baseline and at 1, 3 and 6 months
|
|
| Notes | Published and unpublished information Funding source: Christian Medical College, Department of Neurology, India, Intramural research fund Declarations of trialists’ interests: there are no conflicts of interest to the manuscript; full disclosures at http://n.neurology.org/content/83/11/1012/tab‐article‐info |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed ('last observation carried forward' method) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Park 2015a.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient Age: adults (mean age: 56.3 years) Sample size: 30 participants (15 in each group) Sex: 13 women, 17 men Inclusion criteria: diagnosis of hemiplegia due to stroke of at least a 6‐month duration, scores of ≥ 24 points on the MMSE‐Korean (MMSE‐K; no difficulty with cognitive functions), Brunnstrom’s upper extremity stage IV, no difficulties with perceptual abilities including hemineglect based on the MVPT, voluntary consent to participate in the study Exclusion criteria: not stated |
|
| Interventions | 2 arms 1 and 2: conventional occupational therapy
1 and 2: 4 weeks, 5 days a week, 30 minutes MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after therapy
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random card selection |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Park 2015b.
| Methods | RCT | |
| Participants | Country: South Korea Setting: rehabilitation unit Age: adults (mean age: 60 years) Sample size: 30 participants (15 in experimental group, 15 in control group, no dropouts published) Sex: 15 women, 15 men Inclusion criteria: stroke > 3 months identifiable by CT or MRI, no cognitive dysfunction that would interfere with the study purpose as indicated by a MMSE‐K > 24, no perceptual disorder or unilateral neglect that would have interfered with the study purpose as indicated by the MVPT, Brunnstrom score between stages I – IV for the UE Exclusion criteria: aphasia, vision or hearing disorders, or had had MT previously |
|
| Interventions | 2 arms
1 and 2: 6 weeks, 5 days a week task‐oriented MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and immediately after treatment and 1 month after treatment
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Piravej 2012.
| Methods | RCT | |
| Participants | Country: Thailand Setting: inpatient rehabilitation centre Age: adults (mean age: 56 years) Sample Size: 47 participants (20 in each group; 7 dropped out) Sex: 19 women, 21 men Inclusion criteria: 1st stroke hemiparesis onset more than 3 months, age > 18 years, able to follow 2‐step command, upper extremity Brunnstrom stage between 1 and 4, able to sit with or without support more than 30 minutes, cognitive function evaluated by MMSE ≥ 24, no previous disease of the hemiparetic side Exclusion criteria: unstable medical conditions, sensory or global aphasia, severe spasticity (mAS > 3), neglect of the hemiparetic side |
|
| Interventions | 2 arms
1 and 2: 30 minutes/session, 10 sessions Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and at the end of 2 weeks, 4 weeks and 12 weeks
|
|
| Notes | Published and unpublished data Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by blocked randomisation, computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Central randomisation by a third party |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts not analysed |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Rajappan 2016.
| Methods | RCT | |
| Participants | Country: Malaysia Setting: nursing homes Age: adults (mean age: 58 years) Sample size: 30 participants (15 in each group: 1 dropped out from the experimental group) Sex: 9 women, 21 men Inclusion criteria: men and women, age 50 to 70 years, 1st episode of unilateral stroke with hemiparesis, 2 to 12 months post‐stroke, diagnosis of stroke with involvement of middle cerebral artery on MRI or CT scan by neurologist Exclusion criteria: MMSE < 24, uncontrolled systemic hypertension, perceptual or apraxic deficits, visual deficit such as homonymous hemianopia, reflex sympathetic dystrophy, severe shoulder subluxation, contracture in the affected upper limb and botox injection within past 6 months to the affected upper limb |
|
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1 and 2: 4 weeks, 5 days a week, 1 hour a day conventional rehabilitation programme 1 and 2: additional 30 minutes a day MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | Information based on unpublished data Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in the analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Rehani 2015.
| Methods | RCT | |
| Participants | Country: India Setting: outpatient Age: adults (mean age: 54.8/57.9 years) Sample size: 20 participants (6 in experimental group, 6 in control group, 8 dropped out) Sex: not stated Inclusion criteria: age 45 to 65 years, 1st episode of ischaemic and haemorrhagic stroke, stroke between 1 to 6 months, men and women, MMSE > 23, BRS 4 and 5 Exclusion criteria: any musculoskeletal disorders, neurological disorder other than stroke, visual impairment, systemic disease, non‐cooperative patients, psychological problems |
|
| Interventions | 2 arms 1 and 2: conventional therapy
1 and 2: 4 weeks, 6 days a week, 30 minutes a day conventional therapy 1 and 2: additional 30 minutes a day MT or MRP Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | Information based on unpublished data Funding source: not stated Declarations of trialists’ interests: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent investigator |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Rodrigues 2016.
| Methods | RCT | |
| Participants | Country: Brazil Setting: home Age: adults (mean age: 57.5 years) Sample size: 16 participants (8 in each group: no dropouts published) Sex: 6 women, 10 men Inclusion criteria: stroke > 6 months, spasticity < 3 modified AS for horizontal shoulder adductors, elbow flexors, and wrist and finger flexors; FM‐UE score 30 ‐ 49 points Exclusion criteria: other neurological diseases, orthopaedic upper limb problems which interfered with their activity level, uncontrolled shoulder pain, significant uncorrectable visual impairment, aphasia or difficulty understanding simple tasks, visual hemineglect, those who were receiving other upper‐limb interventions |
|
| Interventions | 2 arms
1 and 2: 4 weeks, 3 days a week, 1 hour a day MT or sham therapy Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 4 weeks of therapy, and 2 weeks after training
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation by independent person and stapled, sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT was performed (authors' information) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Outcome measures were videotaped and rated by a trained physiotherapist blinded to the group allocation |
Rothgangel 2004.
| Methods | RCT; 2 baseline subgroups | |
| Participants | Country: Netherlands Setting: inpatient and outpatient rehabilitation centre Age: adults (mean age: 73.4 years) Sample size: 16 participants (6 in the outpatient centre group (Rothgangel 2004a), 10 in the inpatient rehabilitation group (Rothgangel 2004b) Sex: 10 women, 6 men Inclusion criteria: 1st stroke in the territory of the middle cerebral artery; minimum 3 months post‐stroke; minimum score of 1 in the ARAT Exclusion criteria: bilateral stroke; severe neglect; severe visual impairments |
|
| Interventions | 2 arms
1 and 2: day hospital group (6 participants): 17 treatments during 5 weeks for 30 minutes each; inpatient rehabilitation group (10 participants): 37 treatments during 5 weeks for 30 minutes each Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, in the middle of the treatment, after 5 weeks of treatment and 10 weeks after baseline
|
|
| Notes | Due to sufficient differences in treatment intensity, we analysed both experimental and both control groups separately Significant differences in baseline characteristics (age, ARAT, participant‐specific problem scale) Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Participants received group allocation after baseline measurement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed as allocated to groups. No dropouts |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Rothgangel 2004a.
| Methods | RCT; subgroup: outpatient centre | |
| Participants | see Rothgangel 2004 | |
| Interventions | see Rothgangel 2004 | |
| Outcomes | see Rothgangel 2004 | |
| Notes | see Rothgangel 2004 | |
Rothgangel 2004b.
| Methods | RCT; subgroup: inpatient rehabilitation | |
| Participants | see Rothgangel 2004 | |
| Interventions | see Rothgangel 2004 | |
| Outcomes | see Rothgangel 2004 | |
| Notes | see Rothgangel 2004 | |
Salhab 2016.
| Methods | Randomised cross‐over trial | |
| Participants | Country: Lebanon/USA Setting: not stated Age: adults (age not stated) Sample size: 18 participants (9 in experimental group, 9 in control group, no dropouts published) Sex: not stated Inclusion criteria: stroke (subacute stage) Exclusion criteria: not stated |
|
| Interventions | 2 arms
1 and 2: 2 weeks, 4 times a week, 50 minutes MT + ES or conventional therapy; followed by 2 weeks vice versa Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 1st 2 weeks, and immediately after the last 2 weeks, and 4 weeks after end of training
|
|
| Notes | Information based on abstract Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Samuelkamaleshkumar 2014.
| Methods | RCT | |
| Participants | Country: India Seting: inpatient rehabilitation centre Age: adults (mean age: 51.2 years) Sample size: 20 participants (10 in each group, no dropouts published) Sex: 4 women, 16 men Inclusion criteria: aged between 18 and 60 years, first‐time ischaemic or haemorrhagic stroke of the middle cerebral artery tertiary, occurring < 6 months before the start of the study, Brunnstrom recovery stages I to IV for the arm and hand, MMSE > 24 Exclusion criteria: not stated |
|
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation 1: additional MT: participants performed unilateral movements while watching in the mirror 2: additional sham therapy: participants performed the same exercises as in MT group using the non‐reflecting surface of the mirror 1 and 2: 3 weeks, 5 days a week, 6 hours conventional stroke rehabilitation 1 and 2: 3 weeks, 5 days a week, 2 x 30 minutes additional MT or sham therapy a day Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after therapy
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Schick 2017.
| Methods | RCT | |
| Participants | Country: Austria/Germany Setting: 3 inpatient rehabilitation centres Age: adults (mean age: 63 years) Sample size: 32 participants (15 in experimental group, 17 in control group, 2 dropouts) Sex: 13 women, 19 men Inclusion criteria: had suffered their 1st ischaemic or haemorrhagic stroke within 6 months prior to entering the study, had severe (FM‐UE ≥ 18 ≤ 33 points) or very severe arm paresis (FM‐UE ≤ 17 points) as assessed with the Fugl‐Meyer Assessment, had arm/hand function that could be electrically stimulated and EMG‐triggered pulses that could be elicited, reported to have been independent in their activities of daily living before stroke, reported to have had full functionality of their upper extremities before the stroke, and were able to understand study tasks and test instructions Exclusion criteria: were pregnant, had an implanted cardiac pacemaker, defibrillator, brain stimulation, drug pump, or metal implant, had wounds, thrombosis, or phlebitis in the stimulation area; severe forms of Dupuytren’s contracture, dementia and concomitant severe neurological diseases; or profound neurocognitive deficits |
|
| Interventions | 2 arms 1 and 2: conventional therapy
1 and 2: 3 weeks, 5 days a week conventional therapy 1 and 2: 3 weeks, 5 days a week, 30 minutes a day EMG‐MES + MT or EMG‐MES Date of intervention: September 2013 to August 2014 |
|
| Outcomes | Outcomes were recorded at baseline and after therapy
|
|
| Notes | Abstract published in 2015, full‐text publication received in 2017 Funding source: not stated Declarations of trialists' interests: the first author was employed by MED‐EL after the end of study (STILLWELL, one of the distributors and developers of the stimulation device which was used in the study) and gives seminars for EMG‐triggered multichannel electrostimulation; the senior author delivers seminars and has authored two manuals on MT; the other authors declared no potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence and block randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes and independent investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data included as intended |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor were blinded to group allocation |
Seok 2010.
| Methods | RCT | |
| Participants | Country: South Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 51.4 years) Sample size: 40 participants (19 in mirror therapy group, 21 in control group) Sex: 22 women, 18 men Inclusion criteria: stroke within 6 months Exclusion criteria: not able to understand treatment instructions; communication difficulties due to aphasia; MMSE < 15 points; musculoskeletal or neurological damage of the unaffected upper extremity; modified AS of 3 or more points; Brunnstrom stage of recovery (arm) of 1 or more than 5 points |
|
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes of therapy for 4 weeks Date of intervention: September 2008 to February 2009 |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of treatment
|
|
| Notes | Published data only, extracted in part on the basis of an unauthorised, automatic translation of the original publication in Korean; Significant difference in MFT between groups at baseline measurement Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Sütbeyaz 2007.
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehabilitation centre Age: adults (mean age: 63.4 years) Sample size: 40 participants (20 in each group; 7 dropped out at 6 months follow‐up) Sex: 17 women, 23 men Inclusion criteria: 1st unilateral stroke during previous 12 months; a score of 1 or 2 in the Brunnstrom stages of lower extremity; ambulatory before stroke Exclusion criteria: severe cognitive disorders |
|
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes of therapy for 4 weeks Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 4 weeks and after 6 months
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, computer‐generated allocation of blocks |
| Allocation concealment (selection bias) | Low risk | The physicians who assessed potential participants to determine eligibility did not know to which group the participants would be allocated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Tezuka 2006.
| Methods | Randomised cross‐over trial | |
| Participants | Country: Japan Setting: inpatient rehabilitation centre Age: adults (mean age: 63.7 years) Sample size: 15 participants (9 in mirror therapy group; 6 dropped out, 4 during the 1st interval) Sex: 9 women, 6 men Inclusion criteria: people admitted or planned to be admitted to rehabilitation ward on the hospital due to post‐stroke hemiparesis; within 1 month post‐stroke; informed consent was obtained from the participant and their family Exclusion criteria: higher brain dysfunction |
|
| Interventions | 2 arms
1 and 2: 10 to 15 minutes a day for 4 weeks, followed by 4 weeks vice versa Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
|
|
| Notes | We only analysed the 1st intervention period of 4 weeks Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation to groups |
| Allocation concealment (selection bias) | High risk | Stated by authors (unpublished information) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Stated by authors (unpublished information) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Thieme 2013.
| Methods | RCT | |
| Participants | Country: Germany Setting: inpatient rehabilitation centre Age: adults (mean age: 67.2 years) Sample size: 60 participants (21 in the mirror therapy group intervention, 18 in the mirror therapy single therapy, 21 in the sham group; 11 dropped out in the intervention period) Sex: 25 women, 35 men Inclusion criteria: 1st supratentorial stroke within the previous 3 months; aged between 18 and 80 years; clinically diagnosed severe hemiparesis or hemiplegia of the distal upper limb with MRC grading of 0 or 1 of wrist and finger extensors Exclusion criteria: visual impairments that may limit participation in mirror therapy; severe cognitive and/or language deficits which preclude participants from following instructions in the group training protocol; other neurological or musculoskeletal impairments of the upper extremity not due to stroke; severe neglect (head is not turned to the affected side due to instruction) |
|
| Interventions | 3 arms 1, 2 and 3: standard rehabilitation programme, additional:
1, 2 and 3: 5 weeks, additional 20 sessions, 30 minutes MT, MT group or sham therapy Date of intervention: April 2009 to July 2011 |
|
| Outcomes | Outcomes assessed before and after treatment, and 7 months after treatment
|
|
| Notes | Published and unpublished data Funding source: Klinik Bavaria Kreischa, Germany Declarations of trialists’ interests: the first author received and will receive honorarium for presentations and seminars on MT; the other authors declared no potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent person |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was performed ('last observation carried forward' method) |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors of primary outcome (motor function) were blinded to group allocation |
Tyson 2015.
| Methods | RCT | |
| Participants | Country: UK Setting: 12 inpatient stroke services Age: adults (mean age: 64 years) Sample size: 94 participants from 12 sites: 63 in experimental group (6 dropped out), 31 in control group (3 dropped out) Sex: 34 women, 60 men Inclusion criteria: stroke at least 1 week previously and inpatient in a stroke rehabilitation unit, no premorbid conditions limiting upper or lower limb function, sufficient cognitive and communication to give consent, medically stable and able to participate in rehabilitation, upper or lower limb weakness which limits activity Exclusion criteria: not stated |
|
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1 and 2: 4 weeks, 7 days a week, 30 minutes a day MT or lower‐limb exercises Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, after 4 weeks of therapy, and 8 weeks after baseline
|
|
| Notes | Published and unpublished data, full‐text publication received in 2016 Funding source: National Institute for Health Research under its Research for Patient Benefit (RfPB) Programme Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation by an independent web‐based randomisation service |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessor was blinded to group allocation |
Wang 2015.
| Methods | RCT | |
| Participants | Country: China Setting: not stated Age: adults (mean age: 64.9 years) Sample size: 90 participants (30 in experimental group, 60 in 2 control groups, no dropouts) Sex: 40 women, 50 men Inclusion criteria: 1st ischaemic or haemorrhagic stroke (CT or MRI); neurological deficit; aged 30 to 75 years; unilateral paralysis of upper limb; stable vital signs; mental health; normal intelligence; no significant cognitive dysfunction; MMSE > 24; middle school education and above; no visual impairment; no aphasia and dementia; can execute instructions Exclusion criteria: unstable condition; severe disease or infection of heart; liver or kidney; other complicated diseases which could affect motor function |
|
| Interventions | 3 arms 1, 2 and 3: routine rehabilitation and task‐oriented training
1, 2 and 3: 8 weeks, 6 days a week, 60 minute routine rehabilitation 1: 8 weeks, 6 days a week, 30 minutes additional mirror therapy 2: 8 weeks, 6 days a week, 20 minutes additional EMGBF Date of intervention: March 2012 to June 2014 |
|
| Outcomes | Outcomes were recorded at baseline and after therapy
|
|
| Notes | Information based on abstract; extracted in part on the basis of an unauthorised, automatic translation of the original publication in Chinese Funding source: Changsha Economics Office Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Wu 2013.
| Methods | Multicentre RCT; stratified by lesion side and motor impairment level | |
| Participants | Country: Taiwan Setting: 4 hospitals Age: adults (mean age: 54.2 years) Sample size: 33 (16 in the MT group, 17 in the control group; 12 lost to 6 months follow‐up) Sex: 10 women, 23 men Inclusion criteria: 1st unilateral ischaemic or haemorrhagic cerebrovascular accident before > 6 months, mild to moderate motor impairment (FM‐UE 26‐56), mild spasticity (mAS < 3), able to understand and follow the instructions (MMSE > 24); Exclusion criteria: participation in another study or experimental rehabilitation project < 6 months, serious visual or visual perception impairment (e.g. neglect and poor visual fields) assessed by NIHSS, severe neuropsychologic, neuromuscular or orthopaedic disease |
|
| Interventions | 2 arms: usual rehabilitation programme, additional:
1: 4 weeks, 5 days a week, 60 minutes a day of MT, followed by 30 minutes task‐oriented training 2: 4 weeks, 5 days a week, 90 minutes a day Date of intervention: not stated |
|
| Outcomes | Outcomes were recorded at baseline, and after 4 weeks and 6 months after treatment
|
|
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by randomly‐selected numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Yavuzer 2008.
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehabilitation centre Age: adults (mean age: 63.3 years) Sample size: 40 participants (20 in each group; 4 dropped out at 6 months follow‐up) Sex: 17 women, 19 men Inclusion criteria: 1st unilateral stroke during previous 12 months; a Brunnstrom recovery stage between 1 and 4 of the upper extremity; able to understand and follow simple instructions Exclusion criteria: severe cognitive disorders (MMSE < 24) |
|
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes of therapy for 4 weeks Date of intervention: February 2006 to April 2006 |
|
| Outcomes | Outcomes were recorded at baseline, after 4 weeks and after 6 months
|
|
| Notes | We combined the Brunnstrom stages of upper extremity and hand into 1 item using raw data Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, computer‐generated allocation of blocks |
| Allocation concealment (selection bias) | Low risk | The physicians who assessed potential participants to determine eligibility did not know to which group the participants would be allocated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in the analysis |
| Blinding of outcome assessment (detection bias) primary outcome | Low risk | Assessors were blinded to group allocation |
Yoon 2014.
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 57.8 years) Sample size: 26 participants (8 in experimental group, 9 each in 2 control groups, no dropouts published) Sex: 10 women, 16 men Inclusion criteria: hemiplegia due to stroke < 6 weeks after onset, no past history of stroke, able to perform an active extension of the affected wrist and more than 2 fingers at an angle of > 10 ° and an active abduction of the affected thumb at an angle of > 10 °, capable of simple communication, can receive care by guardians or caregivers, able to maintain a sitting position for > 30 minutes Exclusion criteria: people with depression who were unable to co‐operate in the treatment, not able to perform active task training due to musculoskeletal problems, spasticity of mAS II or higher, complex regional pain syndrome or secondary adhesive capsulitis |
|
| Interventions | 3 arms 1, 2 and 3: conventional therapy
1, 2 and 3: 2 weeks, 5 days a weeks, 40 minutes a day of conventional therapy
Date of intervention: October 2012 to May 2013 |
|
| Outcomes | Outcomes were recorded at baseline and after 2 weeks of therapy
|
|
| Notes | Funding source: 2‐year research grant of Pusan National University, Republik of Korea Declarations of trialists’ interests: there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random cards with numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
Yun 2011.
| Methods | RCT | |
| Participants | Country: South Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 63.3 years) Sample size: 60 participants (40 in 2 experimental groups, 20 in control group, no dropouts published) Sex: 21 women, 39 men Inclusion criteria: 1st unilateral stroke; Brunnstrom recovery stage I ‐ IV; MMSE > 21 Exclusion criteria: unco‐operative due to cognitive impairment; medically unstable; neurologic deficit; neglect |
|
| Interventions | 3 arms 1, 2 and 3: conventional rehabilitation programme, additional
1, 2 and 3: 3 weeks, 5 days a week, 30 minutes MT, MT + NMES, or sham therapy Date of intervention: March 2009 to March 2010 |
|
| Outcomes | Outcomes were recorded at baseline and after 3 weeks of treatment
|
|
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling number table as stated by authors (unpublished information) |
| Allocation concealment (selection bias) | High risk | Stated by authors (unpublished information) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Stated by authors (unpublished information) |
| Blinding of outcome assessment (detection bias) primary outcome | High risk | Assessors were not blinded; stated by authors (unpublished information) |
Zacharis 2014.
| Methods | RCT | |
| Participants | Country: Greece Setting: not stated Age: adults (age not stated) Sample size: 30 participants (15 in experimental group, 15 in control group, no dropouts published) Sex: not stated Inclusion criteria: > 4 weeks after stroke, upper limb plegia (Motricity Index ≤ 77) Exclusion criteria: not stated |
|
| Interventions | 2 arms 1 and 2: routine rehabilitation treatment
1 and 2: 8 weeks (20 ‐ 24 sessions) 1: additional 30 minutes MT a day Date of intervention: March 2013 to November 2013 |
|
| Outcomes | Outcomes were recorded at baseline and after 8 weeks of therapy
|
|
| Notes | Information based on an abstract Funding source: not stated Declarations of trialists’ interests: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) primary outcome | Unclear risk | Not stated |
ABILHAND: a measure of manual ability for adults with upper limb impairment ADL: activities of daily living ARAT: Action Research Arm Test AS: Ashworth Scale BBT: Box and Block Test BF‐FES: biofeedback functional electrical stimulation BI: Barthel Index BRS: Brunnstrom Recovery Stages CAHAI: Chedoke Arm and Hand Activity Inventory CIMT: Constraint‐Induced Movement Therapy CRPS‐type 1: complex regional pain syndrome ‐ type I CT: computerised tomography EMG: electromyography EMGBF: electromyographic biofeedback FAC: Functional Ambulatory Categories FES: functional electrical stimulation FIM: Functional Independence Measure FM/FMA: Fugl‐Meyer Assessment FM‐UE: Fugl‐Meyer Assessment upper extremity FM‐LE: Fugl‐Meyer Assessment lower extremity FRT: functional reach test GAS: goal attainment scaling iEMG: integrated electro‐myogram ITT: intention‐to‐treat JTHFT: Jebson Taylor Hand Function Test LBT: Line Bisection Test MAL: Motor Activity Log MAS: Motor Assessment Scale mAS: modified Ashworth Scale MBI: modified Barthel index MCSI: modified composite spasticity index MFT: Manual Function Test MI‐UL: Motricity Index ‐ upper limb MMSE: Mini Mental State Examination MMT: Manual Muscle Test MoCA: Montreal Cognitive Assessment MRC: Medical Research Council MRI: magnetic resonance imaging MT: mirror therapy MVPT: Motor‐free Visual Perception Test NIHSS: National Institutes of Health Stroke Scales NMES: neuromuscular electrical stimulation NRS: Numeric Rating Scale NSA: Nottingham Sensory Assessment QOM: quality of movement QST: quantitative sensory testing RASP: Rivermead Assessment of Somatosensory Performance RCT: randomised controlled trial ROM: range of motion rTMS: repetitive transcranial magnetic stimulation SCT: Star Cancellation Test SD: standard deviation SIS: Stroke Impact Scale SSQOL: Stroke‐specific quality of life scale tDCS: transcranial direct current stimulation TEMPA: Upper Extremity Performance Evaluation Test for the Elderly (English title) TS: Tardieu Scale TUG: timed‐up and go test UEFI: Upper Extremity Functional Index UEFT: Upper Extremity Function Test Stroke‐ULAM: Stroke Upper‐Limb Activity Monitor VAS: Visual analogue scale WMFT: Wolf Motor Function Test WMFT/FA: Wolf Motor Function Test ‐ functional ability WMFT/PT: Wolf Motor Function Test ‐ performance time
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altschuler 2005 | Study of healthy people |
| Dohle 2009b | Study did not use relevant outcomes |
| Ietswaart 2011 | Only 10% of the experimental intervention was used for mirror therapy |
| Jax 2012 | Mirror therapy is also part of the control intervention |
| Ji 2014b | No relevant outcomes included |
| Kim 2015b | The paper was withdrawn permanently, because of plagiarism (Wu 2013) |
| Lee 2014 | Intervention was not mirror therapy as defined in this review |
| Lin 2014b | Control intervention included mirror therapy |
| Moseley 2004 | Study did not include people after stroke |
| Radajewska 2013 | Randomisation procedure not adequate |
| Ramachandran 1999 | Study is not a RCT |
| Selles 2014 | Time spent in mirror therapy did not reach inclusion criteria |
| Stevens 2003 | Study is not an RCT |
| Vural 2016 | Randomisation procedure not adequate |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Amimoto 2008.
| Methods | Randomised cross‐over trial |
| Participants | Country: Japan Sample size: 14 participants Inclusion criteria: 4 months and longer after stroke |
| Interventions | 2 arms
|
| Outcomes |
|
| Notes | We were not able to include this trial because of unclear outcome of motor function |
ISRCTN40903497.
| Methods | RCT |
| Participants | Country: Canada Inclusion criteria: adults, people with stroke, with normal vision, admitted to the rehabilitation programme at Toronto Rehabilitation Institute |
| Interventions | 2 arms
|
| Outcomes | Outcomes: not stated |
| Notes | Information based on www.isrctn.com/ISRCTN40903497 |
Magni 2014.
| Methods | RCT |
| Participants | Country: not published Sample size: 10 (5 in experimental group, 5 in control group) Inclusion criteria: chronic stroke, paresis of the upper limb, aged 40 ‐ 75 Exclusion criteria: not published |
| Interventions | 2 arms
1 and 2: 6 weeks, 24 minutes twice a day |
| Outcomes | Outcomes were recorded:
|
| Notes | Information based on abstract |
May 2011.
| Methods | Cohort study (randomisation not published) |
| Participants | Country: not published Sample size: 42 participants (21 in experimental group, 21 in control group) Inclusion criteria: stroke, paresis of the lower limb Exclusion criteria: not published |
| Interventions | 2 arms 1 and 2: conventional therapy programme
1 and 2: 4 weeks, 5 days a week, 60 to 120 minutes conventional therapy programme 1: 4 weeks, 5 days a week, 30 minutes MT |
| Outcomes | Outcomes were recorded at baseline, after therapy period, and 12 weeks
|
| Notes | Information based on abstract |
Wang 2013a.
| Methods | Cohort study (randomisation not published) |
| Participants | Country: not published Inclusion criteria: stroke, hemiplegia Exclusion criteria: not published |
| Interventions | 2 arms 1 and 2: conventional therapy programme
1 and 2: 4 weeks |
| Outcomes | Outcomes were recorded at baseline, and 2 weeks and 4 weeks after therapy period
|
| Notes | Information based on abstract |
Yeldan 2015.
| Methods | Cohort study (randomisation not published) |
| Participants | Country: Turkey Sample size: 8 participants (4 in experimental group, 4 in control group) Inclusion criteria: diagnosis of a stroke (within 1 month), partial anterior circulation infarction (PACI), upper extremity motor functional level according to Brunnstrom stages between 1 and 4, and no musculoskeletal injury history in the affected upper extremity Exclusion criteria: a residual upper extremity deficit from a previous stroke, intolerance to upright position, visual problem, cognitive deficit preventing them from following instructions, and unilateral neglect preventing them from being able to view the mirror |
| Interventions | 2 arms 1 and 2: neurodevelopmental treatment
|
| Outcomes | Outcomes were recorded at baseline and after therapy period
|
| Notes |
ARAT: Action Research Arm Test BBS: Berg balance scale BI: Barthel Index FIM: functional independence measure FM/FMA: Fugl‐Meyer Assessment FM‐UE: Fugl‐Meyer Assessment upper extremity MI: Motricity Index MT: Mirror therapy RCT: Randomised controlled trial STEF: Simple Test for Evaluating Hand Function WMFT: Wolf Motor Function Test
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000121763.
| Trial name or title | Developing new ways to minimise disability after stroke, a randomized controlled trial of Functional Electrical Stimulation (FES) of the arm and mirror therapy |
| Methods | RCT |
| Participants | Country: New Zealand Inclusion criteria: over 18 years, admitted to Waikato Hospital with a confirmed diagnosis of stroke, living in the community within the Hamilton area on admission to hospital, a score of greater than 16/30 on MoCA, ARAT score of < 30/57 Exclusion criteria: severe cognitive impairment (< 16/30 on the MoCA), severe or unstable cardiovascular disease (i.e. unstable angina, pacemaker fitted, dysrhythmia other than controlled atrial fibrillation), near‐terminal disease (including advanced lung, heart, kidney, liver failure resistant to medical management), acute musculoskeletal disorder |
| Interventions | 3 arms 1, 2 and 3: task‐specific training, additional
1, 2 and 3: 4 ‐ 6 weeks, 2 times a day 30 minutes of task‐specific training 1, 2 and 3: 4 ‐ 6 weeks, 2 times a day 30 minutes of MT, MT + FES or FES |
| Outcomes | Outcomes will be recorded at baseline and after therapy period:
|
| Starting date | Not stated |
| Contact information | Principal Investigator: Dr John Parsons, The University of Auckland, Auckland, New Zealand, Email: j.parsons@auckland.ac.nz |
| Notes | Estimated sample size: 100 participants (in 2 experimental groups and 1 control group) www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000121763 |
ChiCTR‐IOR‐16008137.
| Trial name or title | Graded motor imagery based on mirror neuron on rehabilitative training for stroke patients: a BOLD‐fMRI study |
| Methods | RCT |
| Participants | Country: China Inclusion criteria: participants signed informed consent, unconscious obstacles, condition is relatively stable, no obvious lack of eyesight, aged 40 ‐ 75 years, no history of cerebrovascular disease, with cerebral infarction diagnosis standards, the course in 2 weeks to 3 months, right‐handed, left hemiplegia, no metal implants in the body, no MRI testing taboo, NIHSS score > 4 minutes, paresis test positive, muscle strength level 1 ‐ 3 Exclusion criteria: people who are not diagnosed with cerebral infarction by imaging, the acute stage of cerebrovascular diseases, unstable vital signs, persons with serious mental illness, with understanding disabilities who cannot meet the test, persons with serious heart, liver and kidney dysfunction, those who have contraindications to MRI examination |
| Interventions | 2 arms 1 and 2: routine training
|
| Outcomes | Outcomes:
|
| Starting date | Not stated |
| Contact information | Principal Investigator: Tu Wenzhan, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, China Email: tuwenzhan@163.com |
| Notes | Estimated sample size: 30 participants (in experimental group and in control group) www.chictr.org.cn/showprojen.aspx?proj=13608 |
DRKS00009288.
| Trial name or title | Zentrale Gesichtslähmung nach Schlaganfall: eine randomisierte, kontrollierte Studie [German] |
| Methods | RCT |
| Participants | Country: Germany Inclusion criteria: 1st episode of stroke with facial paresis (within 7 days to 6 months), 18 years of age and over Exclusion criteria: Speech disorder, which prevents understanding of the questionnaires, pre‐existing brain lesions, degenerative diseases of the brain |
| Interventions | 4 arms 1 to 4: training of oral motor skills (MMT)
1 to 4: 3 weeks, 4 days a week, 30 minutes a day MMT 1 and 2: 3 weeks, 4 days a week, 30 minutes a day additional MMT or additional MT |
| Outcomes | Outcomes will be recorded at baseline and after treatment
|
| Starting date | October 2015 |
| Contact information | Moritz Klinik Bad Klosterlausnitz, Hermann‐Sachse Strasse 46, 07639 Bad Klosterlausnitz, Deutschland |
| Notes | Estimated sample size: 80 participants www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00009288 |
IRCT201504224787N5.
| Trial name or title | The effect of mirror therapy on motor ability of patients after stroke |
| Methods | RCT |
| Participants | Country: Iran Inclusion criteria: people with age between 30 to 65 years, with the 1st‐ever stroke that are diagnosed by neurologists and confirmed by computed tomography or magnetic resonance imaging, 1 month has passed since their stroke, 1 to 4 point from Brunnstrom scale, not to have severe cognitive disorders and be able follow simple verbal instructions (MMSE score > 24). Exclusion criteria: participants excluded if they do not participate in 4 sessions intermittently or 2 sessions constantly in intervention |
| Interventions | 3 arms 1, 2 and 3: conventional rehabilitation programme
|
| Outcomes | Outcomes will be recorded before intervention and the end of 5, 10, 15, and 20 sessions
|
| Starting date | 23 July 2015 |
| Contact information | Principal Investigator: Tahereh Khaleghdoost Mohammadi, Physiotherapy centre for elderly and handicap city of Rasht, Rasht, Iran Email: khaleghdoost@gums.ac.ir |
| Notes | Estimated sample size: 93 participants (in 1 experimental group and 2 control groups) www.irct.ir/searchresult.php?id=4787&number=5 |
NCT01010607.
| Trial name or title | Use of tendon vibration and mirror for the improvement of upper limb function and pain reduction |
| Methods | RCT |
| Participants | Country: Israel Inclusion criteria: stroke; 18 to 85 years of age; stroke onset between 1 month and 1 year ago; NIHSS 3 to 15 on study admission; affected upper‐limb function 10% to 90% on Fugl‐Meyer Scale; ability to understand instructions and to move the unaffected limb freely Exclusion criteria: severe cognitive impairment; severe aphasia; severe neglect that impairs ability to understand instructions or to execute tasks |
| Interventions | 3 arms
1, 2 and 3: 10 sessions, 30 minutes each |
| Outcomes | Outcomes will be assessed after treatment and 3 months after treatment
|
| Starting date | September 2009 |
| Contact information | Principal Investigator: Elior Moreh, MD, Hadassah University Hospital, Jerusalem, Israel Email: elior@hadassah.org.il |
| Notes | Estimated enrolment: 30 clinicaltrials.gov/ct2/show/NCT01010607 |
NCT01724164.
| Trial name or title | Robot‐ versus mirror‐assisted motor interventions in rehabilitating upper‐limb motor and muscle performance and daily functions post‐stroke: a comparative effectiveness study |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: willing to provide written informed consent, > 6 months onset of unilateral stroke, an initial 25 ‐ 56 or 18 ‐ 50 scores of the FM‐UE, sufficient cognitive ability (MMSE ≧ 24 points), without upper limb fracture within 3 months, 40 years to 75 years of age Exclusion criteria: recurrence of stroke or seizure episode during the intervention, occurrence of serious or continuous pain on affected upper extremity, history of other neurological disease or severe orthopaedic condition |
| Interventions | 5 arms
1 to 5: 4 weeks, 5 days a week, 1 hour MT, RR, RR with FES, CR, or RR‐PI and ½ hour functional training a day |
| Outcomes | Outcomes:
|
| Starting date | August 2011 |
| Contact information | Principal Investigator: Keh‐chung Lin, ScD, School of Occupational Therapy, College of Medicine, National Taiwan University |
| Notes | Estimated sample size: 100 participants (in 3 experimental groups and 2 control groups) clinicaltrials.gov/ct2/show/record/NCT01655446 clinicaltrials.gov/ct2/show/record/NCT01724164 |
NCT02254616.
| Trial name or title | Hybrid approach to mirror therapy and transcranial direct current stimulation for stroke recovery: a follow‐up study on brain reorganisation, motor performance of upper extremity, daily function, and activity participation |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: 1st episode of stroke in cortical regions, time since stroke > 6 months, initial motor part of UE of FMA score ranging from 24 to 52, indicating moderate to mild movement impairment, no severe spasticity in any joints of the affected arm (mAS ≤ 2), no serious cognitive impairment (i.e. MMSE score ≧ 24), willing to sign the informed consent form Exclusion criteria: visual/attention impairments that might interfere with seeing mirror illusion, including hemineglect/hemianopsia, major health problems or poor physical conditions that might limit participation, currently participating in any other research, previous brain neurosurgery, metallic implants within the brain |
| Interventions | 4 arms
1: 2 weeks, 20 minutes tDCS, 40 minutes MT, 30 minutes functional training; 2 weeks 60 minutes MT and 30 minutes functional training 2: 2 weeks, 20 minutes sham tDCS, 40 minutes MT, 30 minutes functional training; 2 weeks 60 minutes MT and 30 minutes functional training 3: 4 weeks, 60 minutes MT and 30 minutes functional training |
| Outcomes | Outcomes will be recorded at baseline, after 2 weeks, after 4 weeks, 16 weeks, and 28 weeks
|
| Starting date | August 2014 |
| Contact information | Principal Investigator: Ching‐Yi Wu, ScD, Chang Gung Memorial Hospital, Taipei City,Taiwan Email: cywu@mail.cgu.edu.tw |
| Notes | Estimated sample size: 80 participants (in 3 experimental groups and 1 control group) clinicaltrials.gov/ct2/show/record/NCT02254616 |
NCT02276729.
| Trial name or title | A pilot randomised controlled trial (RCT) of mirror box therapy in upper limb rehabilitation with sub‐acute stroke patients |
| Methods | RCT |
| Participants | Country: UK Inclusion criteria: 18 years and over; newly‐admitted inpatient of the rehabilitation ward; diagnosis of CVA in the last 3 months resulting in upper‐limb motor loss; able to follow 2‐part spoken or written commands in the English language; upper‐limb therapy designated as a main portion of goal directed treatment programme; consent to take part in the study Exclusion criteria: not stated |
| Interventions | 2 arms 1 and 2: standard occupational therapy for upper limb rehabilitation
1 and 2: 6 weeks, 3 ‐ 5 sessions a week of approximately 45 minutes OT |
| Outcomes | Outcomes will be recorded at baseline and after treatment and 3 and 6 months after treatment
|
| Starting date | April 2015 |
| Contact information | Principal Investigator: Alison Porter‐Armstrong, DPhil, University of Ulster, Belfast, Northern Ireland Email: a.porter@ulster.ac.uk |
| Notes | Estimated sample size: 50 participants (25 in experimental group and 25 in control group) clinicaltrials.gov/show/NCT02276729 |
NCT02319785.
| Trial name or title | Effects of robot‐assisted combined therapy in upper limb rehabilitation in stroke patients |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: 1st‐ever unilateral stroke with more than 3 months onset, an initial UE subsection of the Fugl‐Meyer Assessment score of 18 to 56 indicating moderate to severe and moderate upper‐limb movement impairment, no excessive spasticity in any of the joints of the affected upper limb (shoulder, elbow, wrist, fingers), be able to follow study instructions and perform study tasks, and willing to provide written informed consent Exclusion criteria: with neural or psychological medical problem that may influence the study, with severe joint pain, with upper limb fracture within 3 months, participation in any experimental rehabilitation or drug studies during the study period; and refusing to provide written informed consent |
| Interventions | 6 arms 1 to 6: OT, additional
1 to 6: 4 weeks, 5 days a week, 90 minutes OT |
| Outcomes | Outcomes will be recorded within 3 days and immediately after therapy period
|
| Starting date | August 2014 |
| Contact information | Principal Investigator: Chia‐Yi Lee, MD, Cathay General Hospital, Taipei City, Taiwan |
| Notes | Estimated sample size: 120 participants (in 3 experimental groups and 3 control groups) clinicaltrials.gov/ct2/show/record/NCT02319785 |
NCT02432755.
| Trial name or title | Effects of home‐based mirror therapy combined with task‐oriented training for patients with stroke: a randomised controlled trial |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: diagnosed as having a unilateral stroke, at least 3 months after stroke onset, from 20 to 80 years of age, having completed acute rehabilitation care or discharged home, a baseline score of the Fugl‐Meyer Assessment (FMA) of 20 to 60, able to follow the therapy instructions (cognition status will be measured by the Montreal Cognitive Assessment), capable of participating in therapy and assessment sessions Exclusion criteria: neglect, global or receptive aphasia, major medical problems, comorbidities that influenced UE usage or caused severe pain |
| Interventions | 3 arms
1 and 2: 4 weeks (12 sessions), 3 days a week, 30 minutes MT followed by 30 minutes TOT |
| Outcomes | Outcomes will be recorded at baseline, immediately after treatment, and 3 months follow‐up
|
| Starting date | March 2016 |
| Contact information | Principal investigator: not stated, Chang Gung Memorial Hospital, Taipei City, Taiwan Email: not stated |
| Notes | Estimated sample size: 90 participants (in 2 experimental groups and 1 control group) ClinicalTrials.gov/show/NCT02432755 |
NCT02548234.
| Trial name or title | Effect of mirror therapy versus bilateral arm training for rehabilitation after chronic stroke: a pilot randomised controlled trial |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: > 6 months after onset of an ischaemic or haemorrhage stroke, no excessive spasticity on any joints of the affected arm, aged 21 years and older Exclusion criteria: history of stroke or other neurologic, neuromuscular, or orthopaedic disease; participation in other experimental rehabilitation or drug studies concurrent with this study |
| Interventions | 2 arms 1 and 2: home programme
1 and 2: 4 weeks, 3 days a week, 1½ hours/day MT or bilateral arm training 1 and 2: 4 weeks, 5 days a week, ½ hour home programme |
| Outcomes | Outcomes will be assessed within 4 weeks (± 3 days) after intervention
|
| Starting date | September 2015 |
| Contact information | Principal Investigator: Keh‐chung Lin, ScD, School of Occupational Therapy, College of Medicine, National Taiwan University |
| Notes | Estimated sample size: 60 participants (in experimental group and in control group) clinicaltrials.gov/ct2/show/record/NCT02548234 |
NCT02776306.
| Trial name or title | Effects of mirror box therapy on neuroplasticity and functional outcome in hemiparetic upper limb post‐stroke |
| Methods | Randomised cross‐over trial |
| Participants | Country: UK Inclusion criteria: 18 years to 105 years, hemiparetic upper limb post‐stroke, capable of providing informed consent, intact vision: if diagnosis of peripheral field defect, participant should be able to compensate for it Exclusion criteria: any contraindication to MRI scanning, clinically‐significant psychiatric disorder (e.g. depression), pre‐existing neurological or psychiatric disease that could confound the study results |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline, after 3 weeks, and 6 weeks
|
| Starting date | April 2016 |
| Contact information | Principal Investigator: Iris Grunwald, Anglia Ruskin University, Cambridge, England |
| Notes | Estimated sample size: 40 participants (in experimental group and in control group) clinicaltrials.gov/ct2/show/record/NCT02776306 |
NCT02778087.
| Trial name or title | Self‐directed box (mirror) therapy after stroke: a dosing study |
| Methods | RCT |
| Participants | Country: USA Inclusion criteria: adults status post‐stroke (ischaemic or haemorrhagic) between the ages 18 and 85 years, receiving inpatient rehabilitation, using the impaired arm, ability to lift and release a wash cloth off a table with any means of prehension in either the sitting or standing positions, a score > 21/30 on the MMSE, ability to consent Exclusion criteria: serious visual or visual‐perceptual deficits, neuropsychological impairments, or orthopaedic conditions that would prevent participation in the BT protocol as determined by the treatment team, involvement in another study protocol related to motor function after stroke, anticipated length of stay > 2 weeks, < 6 months post‐stroke |
| Interventions | 3 arms 1 to 3: treatment as usual
1: Additional 30 minutes MT a day (twice for 15 minutes) 2: Additional 60 minutes MT a day (4 times for 15 minutes) |
| Outcomes | Outcomes will be recorded at pre‐ and post‐intervention up to 12 months
|
| Starting date | January 2017 |
| Contact information | Principal Investigator: Glenn Gillen, EdD, OTR, Columbia University, New York City, USA E‐mail: dmn12@cumc.columbia.edu (Dawn M. Nilsen EdD, OTR) |
| Notes | Estimated sample size: 45 participants (in 2 experimental groups and 1 control group) ClinicalTrials.gov/show/NCT02778087 |
NCT02827864.
| Trial name or title | Efficacy and time dependent effects of transcranial direct current stimulation (tDCS) combined with mirror therapy for rehabilitation after subacute and chronic stroke |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: experienced a 1st‐ever unilateral stroke with stroke onset ≥ 1 week, UE‐FMA score between 18 and 56, able to follow instructions to perform the tasks (MMSE ≥ 24) Exclusion criteria: participants are currently involved in other rehabilitation or drug research trial(s), have neurological or psychological disorders other than stroke, have joint contracture or excessive spasticity of the paretic upper limb that prohibits them performing the tasks, received Botulinum toxin injections 3 months prior to enrolment, have unstable cardiovascular status such as uncontrolled hypertension or New York Heart Association (NYHA) Class III/IV heart failure, have contradictions to tDCS including a history of epilepsy, migraine headache, uncontrolled medical status, being pregnant, having a pacemaker, or metal implanted in their head or body, have a history of drug or alcohol abuse, skin lesions on the electrode sites, brain tumour, brain injury, arteriovenous malformation (AVM), had brain surgery, other brain diseases (such as intracranial hypertension or cerebral oedema), or being not suitable for using tDCS by the physician's assessment. |
| Interventions | 3 arms
1: 4 weeks, 5 days a week, 90 minutes: 20 minutes tDCS, 20 minutes MT + sham tDCS, 20 minutes MT without tDCS, 30 minutes functional task practice 2: 4 weeks, 5 days a week, 90 minutes: 20 minutes sham tDCS, 20 minutes MT + tDCS, 20 minutes MT without tDCS, 30 minutes functional task practice |
| Outcomes | Primary outcomes will be recorded at baseline, and after 4 weeks therapy period
|
| Starting date | August 2016 |
| Contact information | Contact: Ching‐Yi Wu, ScD, Chang Gung Memorial Hospital, Taipei City, Taiwan E.mail: cywu@mail,cgu.edu.tw |
| Notes | Estimated sample size: 99 participants (in 2 experimental groups and 1 control group) ClinicalTrials.gov/show/NCT02827864 |
NCT02871700.
| Trial name or title | Comparative efficacy study of action observation therapy and mirror therapy after stroke: rehabilitation outcomes and neural mechanisms by MEG |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: diagnosed as having a unilateral stroke, 1 to 6 months after stroke onset, from 20 to 80 years of age, a baseline score of the Fugl‐Meyer Assessment (FMA) of 20 to 60, able to follow the study instructions (measured by the MoCA), capable of participating in therapy and assessment sessions Exclusion criteria: people with global or receptive aphasia, severe neglect, major medical problems, or comorbidities that influenced UE usage or cause severe pain |
| Interventions | 3 arms
1: 3 weeks, 15 sessions 2: 3 weeks, 15 sessions, 60 minutes MT |
| Outcomes | Outcomes will be recorded at baseline and after treatment and 3 months after treatment
|
| Starting date | August 2016 |
| Contact information | Contact: Yu‐Wei Hsieh, PhD, Chang Gung Memorial Hospital, Taipei City, Taiwan E‐mail: ywhsieh@mail.cgu.edu.tw |
| Notes | Estimated sample size: 90 participants (60 in 2 experimental groups and 30 in control group) clinicaltrials.gov/ct2/show/record/NCT02871700 |
ABILHAND: a measure of manual ability for adults with upper limb impairment ARAT: Action Resarch Arm Test AROM: active range of motion BBT: Box and Block test CAHAI: Chedoke arm and hand activity inventory EQ‐5D‐5L: health questionnaire for adults FES: Functional electrical stimulation FAM: Functional Assessment Measure FIM: Functional Independence Measure FM‐UE: Fugl‐Meyer Assessment‐ upper extremity gWMFT: Graded Wolf Motor Function Test Hz: Hertz JTHFT: Jebson Taylor Hand Function Test MAL: Motor Activity Log MAS: Motor Assessment Scale mAS: Modified Ashworth Scale mBI: Modified Barthel Index MMSE: Mini Mental State Examination MoCA: Montreal cognitive assessment MRC: Medical research council Scale for Muscle Strength MRI: magnetic resonance imaging mRS: Modified Rankin scale MT: Mirror therapy NEADL: Nottingham Extended Activities of Daily Living Scale NIHSS: National Institutes of Health Stroke Scales NMES: Neuromuscular Electrical Stimulation OT: occupational therapy QMI: Questionnaire upon Mental Imagery RCT: randomised controlled trial rNSA: Revised Nottingham sensory assessment RR: robotic rehabilitation tDCS: Transcranial direct current stimulation UE: upper extremity (WHOQOL)‐BREF: World Health Organization Quality of Life WMFT: Wolf Motor Function Test
Differences between protocol and review
We added the outcome 'motor impairment' for this update and separated outcome measures for motor function and motor impairment.
We undertook a new subgroup analysis: subacute versus chronic stage after stroke: the cut‐off point between both subgroups was six months after stroke onset.
We added a further database for searching ongoing studies: International Clinical Trials Registry Platform (ICTRP).
We had previously planned to perform a subgroup analysis comparing studies that included participants with different severities of motor impairment. Based on the baseline data for motor function, we were not able to clearly differentiate studies based on this criterion. Most studies included participants with mixed severities of motor impairments. Due to these problems of differentiation, we decided not to do this subgroup analysis.
Two studies only included people after stroke with a diagnosis of CRPS‐type I, which might have influenced the effects of the intervention (Cacchio 2009a; Cacchio 2009b). We therefore performed a post hoc sensitivity analysis by removing these studies; this was not planned in the protocol.
We only included studies with a minimum amount of 50% mirror therapy in the experimental intervention.
Contributions of authors
Holm Thieme (HT), Christian Dohle (CD), and Nadine Morkisch (NM) were involved in all stages of the review and contributed to the conception and design of the review. Bernhard Borgetto (BB) contributed to the conception and design of the review and was involved in interpreting the results. Jan Mehrholz (JM) was involved in methodological planning and conducting the review, statistical analysis of outcome data, and interpreting the results. Johann Behrens (JB) and Marcus Pohl (MP) were involved in extracting data, assessing the methodological quality of selected studies, and interpreting the results. All authors approved the protocol and the final review.
Sources of support
Internal sources
Erste Europäische Schule für Physiotherapie, Ergotherapie und Logopädie, Klinik Bavaria Kreischa, Germany.
Klinik Bavaria Kreischa, Germany.
SRH Hochschule Gera, Germany.
Martin‐Luther‐Universität Halle‐Wittenberg, Germany.
Median Klinik Berlin‐Kladow, Germany.
External sources
-
BMBF, Germany.
The research is funded by the Bundesministerium für Bildung und Forschung (01KG1025).
-
BMBF, Germany.
This research is funded by the Bundesminiterium für Bildung und Forschung (01KG1514).
Declarations of interest
Holm Thieme (HT) is an author of an included study on the effect of mirror therapy after stroke. He was not involved in checking this trial for eligibility, extracting data or assessing the methodological quality of this study. He has received and will receive honorarium for presentations and seminars on mirror therapy.
Christian Dohle (CD) is author of two included studies on the effect of mirror therapy after stroke. He was not involved in checking these trials for eligibility, extracting data or assessing the methodological quality of the studies. He has received and will receive honorarium for presentations and seminars on mirror therapy.
Christian Dohle (CD) and Nadine Morkisch (NM) are authors of corresponding therapy manuals (Bieniok 2011; Morkisch 2015).
Jan Mehrholz: None known
Marcus Pohl: Marcus Pohl (MP) is an author of an included study on the effect of mirror therapy after stroke. He was not involved in checking this trial for eligibility, extracting data or assessing the methodological quality of this study.
Johann Behrens: Johann Begrens (JB) is an author of an included study on the effect of mirror therapy after stroke. He was not involved in checking this trial for eligibility, extracting data or assessing the methodological quality of this study.
Bernhard Borgetto: None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Acerra 2007 {unpublished data only}
- Acerra NE. Is early post‐stroke upper limb mirror therapy associated with improved sensation and motor recovery? A randomised‐controlled trial [PhD thesis]. Sensorimotor Dysfunction in CRPS1 and Stroke: Characteristics, Prediction and Intervention. Brisbane, Australia: University of Queensland, 2007. [Google Scholar]
Alibakhshi 2016 {published data only}
- Alibakhshi H, Samaei A, Khalili MA, Siminghalam M. A comparative study on the effects of mirror therapy and bilateral arm training on hand function of chronic hemiparetic patients. Koomesh Journal of Semnan University of Medical Sciences 2016;17(3):589‐95. [Google Scholar]
Altschuler 1999 {published data only}
- Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1999;353(9169):2035‐6. [PUBMED: 10376620] [DOI] [PubMed] [Google Scholar]
Amasyali 2016 {published data only}
- Amasyali SY, Yaliman A. Comparison of the effects of mirror therapy and electromyography‐triggered neuromuscular stimulation on hand functions in stroke patients [Inmeli Hastalarda Ayna Tedavisi ile Elektromyografik Elektrostimulasyonun El Fonksiyonlari Uzerine Olan Etkilerinin Karsilastirilmasi]. International Journal of Rehabilitation Research 2016;39(4):302‐7. [DOI] [PubMed] [Google Scholar]
Arya 2015 {published data only}
- Arya KN, Pandian S, Kumar D, Puri V. Task‐based mirror therapy augmenting motor recovery in poststroke hemiparesis: a randomized controlled trial. Journal of Stroke and Cerebrovascular Diseases 2015;24(8):1738‐48. [DOI] [PubMed] [Google Scholar]
Arya 2017 {published and unpublished data}
- Arya KN, Pandian S, Kumar V. Effect of activity‐based mirror therapy on lower limb motor‐recovery and gait in stroke: a randomized controlled trial. Neuropsychological Rehabilitation 2017;27:1‐18. [DOI] [PubMed] [Google Scholar]
Bae 2012 {published data only}
- Bae SH, Jeong WS, Kim KY. Effects of mirror therapy on subacute stroke patients' brain waves and upper extremity functions. Journal of Physical Therapy Science 2012;24(11):1119‐22. [Google Scholar]
Bahrami 2013 {published data only}
- Bahrami M, Mazloom, Hasanzadeh F, Ghandehari K. The effect of mirror therapy on self care of patients with stroke. Journal of Mazandaran University of Medical Sciences 2013;23(107):84‐95. [Google Scholar]
Cacchio 2009a {published and unpublished data}
- Cacchio A, Blasis E, Blasis V, Santilli V, Spacca G. Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabilitation and Neural Repair 2009;23(8):792‐9. [PUBMED: 19465507] [DOI] [PubMed] [Google Scholar]
Cacchio 2009b {published and unpublished data}
Cha 2015 {published data only}
- Cha H‐G, Kim M‐K. Therapeutic efficacy of low frequency transcranial magnetic stimulation in conjunction with mirror therapy for sub‐acute stroke patients. Journal of Magnetics 2015;20(1):52‐6. [Google Scholar]
Cho 2015 {published data only}
- Cho HS, Cha HG. Effect of mirror therapy with tDCS on functional recovery of the upper extremity of stroke patients. Journal of Physical Therapy Science 2015;27(4):1045‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colomer 2016 {published data only}
- Colomer C, Noé E, Llorens R. Mirror therapy in chronic stroke survivors with severely impaired upper limb function: a randomized controlled trial. European Journal of Physical and Rehabilitation Medicine 2016;52(3):271‐8. [PubMed] [Google Scholar]
Dalla Libera 2015 {published data only}
- Dalla Libera D, Regazzi S, Fasoletti C, Dinacci Ruggieri D, Rossi P. Beneficial effect of transcranic magnetic stimulation combined with mirror therapy in stroke patients: a pilot study in neurorehabilitative setting. Brain Stimulation 2015;8(2):377. [Google Scholar]
Dohle 2009 {published and unpublished data}
- Dohle C, Pullen J, Nakaten A, Kust J, Rietz C, Karbe H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabilitation and Neural Repair 2009;23(3):209‐17. [PUBMED: 19074686] [DOI] [PubMed] [Google Scholar]
Geller 2016 {published data only}
- Geller D, Nilsen D, Lew S, Gillen G, Bernardo M. Home mirror therapy: a randomized controlled pilot study comparing unimanual and bimanual mirror therapy for improved upper limb function post‐stroke. Archives of Physical Medicine and Rehabilitation 2016;97:e4. [DOI: 10.1016/j.apmr.2016.08.008] [DOI] [PubMed] [Google Scholar]
Gurbuz 2016 {published data only}
- Gurbuz N, Afsar SI, Ayaş S, Cosar SN. Effect of mirror therapy on upper extremity motor function in stroke patients: a randomized controlled trial. Journal of Physical Therapy Science 2016;28(9):2501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiragami 2012 {published data only}
- Hiragami SY, Sato Y, Harada K, Kagawa K. The effect of mirror therapy on finger motor dysfunction after stroke. Journal of Japanese Society of Physical Therapy 2012;39(5):330‐7. [Google Scholar]
In 2012 {published data only}
- In TS, Jung KS, Lee WL, Song CH. Virtual reality reflection therapy improves motor recovery and motor function in the upper extremities of people with chronic stroke. Journal of Physical Therapy Science 2012;24(4):339‐43. [Google Scholar]
In 2016 {published data only}
- In TS, Lee K, Song CH. Virtual reality reflection therapy improves balance and gait in patients with chronic stroke: randomized controlled trials. Medical Science Monitor 2016;22:4046‐53. [DOI: 10.12659/MSM.898157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Invernizzi 2013 {published data only}
- Invernizzi M, Negrini S, Carda S, Lanzotti L, Cisari C, Baricich A. The value of adding mirror therapy for upper limb motor recovery of subacute stroke patients: a randomized controlled trial. European Journal of Physical and Rehabilitation Medicine 2013;49(3):311‐7. [PubMed] [Google Scholar]
Ji 2014a {published data only}
- Ji S‐G, Cha H‐G, Kim M‐K. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation combined with mirror therapy. Journal of Magnetics 2014;19(1):28‐31. [Google Scholar]
Kawakami 2015 {published data only}
- Kawakami K, Miyasaka H, Nonoyama S, Hayashi K, Tonogai Y, Tanino G, et al. Randomized controlled comparative study on effect of training to improve lower limb motor paralysis in convalescent patients with post‐stroke hemiplegia. Journal of Physical Therapy Science 2015;27(9):2947‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim H, Lee G, Song C. Effect of functional electrical stimulation with mirror therapy on upper extremity motor function in poststroke patients. Journal of Stroke and Cerebrovascular Diseases 2014;23(4):655‐61. [PUBMED: 23867040] [DOI] [PubMed] [Google Scholar]
Kim 2015a {published data only}
- Kim JH, Lee BH. Mirror therapy combined with biofeedback functional electrical stimulation for motor recovery of upper extremities after stroke: a pilot randomized controlled trial. Occupational Therapy International 2015;22(2):51‐60. [DOI] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim K, Lee S, Kim D, Lee K, Kim Y. Effects of mirror therapy combined with motor tasks on upper extremity function and activities daily living of stroke patients. Journal of Physical Therapy Science 2016;28(2):483‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kojima 2014 {published data only}
- Kojima K, Ikuno K, Morii Y, Tokuhisa K, Morimoto S, Shomoto K. Feasibility study of a combined treatment of electromyography‐triggered neuromuscular stimulation and mirror therapy in stroke patients: a randomized crossover trial. Neurorehabilitation 2014;34(2):235‐44. [DOI] [PubMed] [Google Scholar]
Kumar 2013 {unpublished data only}
- Kumar PV. Effect of functional electrical stimulation with mirror therapy on upper extremity motor function in poststroke patients [PhD thesis]. Karnataka, Bangalore, India: Rajiv Gandhi University of Health Sciences, 2013. [Google Scholar]
Kuzgun 2012 {published data only}
- Kuzgun S, Ozgen M, Armagan O, TascIoglu F, Baydemir C. The efficacy of mirror therapy combined with conventional stroke rehabilitation program on motor and functional recovery [İnme rehabilitasyon programı ile kombine edilen ayna tedavisinin motor ve fonksiyonel iyileşme üzerine etkinliğinin araştırılması]. Türk Beyin Damar Hastalıkları Dergisi [Turkish Journal of Cardiovascular Diseases] 2012;18(3):77‐82. [Google Scholar]
Lee 2012 {published data only}
- Lee MM, Cho HY, Song CH. The mirror therapy program enhances upper‐limb motor recovery and motor function in acute stroke patients. American Journal of Physical Medicine and Rehabilitation 2012;91(8):689‐96. [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee D, Lee G, Jeong J. Mirror therapy with neuromuscular electrical stimulation for improving motor function of stroke survivors: a pilot randomized clinical study. Technology and Health Care 2016;24(4):503‐11. [DOI] [PubMed] [Google Scholar]
Lim 2016 {published data only}
- Lim K‐B, Lee H‐J, Yoo J, Yun H‐J, Hwang H‐J. Efficacy of mirror therapy containing functional tasks in poststroke patients. Annals of Rehabilitation Medicine 2016;40(4):629‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2014a {published data only}
- Lin KC, Huang PC, Chen YT, Wu CY, Huang WL. Combining afferent stimulation and mirror therapy for rehabilitating motor function, motor control, ambulation, and daily functions after stroke. Neurorehabilitation and Neural Repair 2014;28(2):153‐62. [DOI] [PubMed] [Google Scholar]
Manton 2002 {published data only (unpublished sought but not used)}
- Manton JC, Hanson C. The effects of a new treatment for survivors of stroke six months or more post‐cerebrovascular accident. Physical Therapy 2002;82(5):Abstract PL‐RR‐142‐F. [Google Scholar]
Marquez 2012 {published data only}
- Marquez JL, Hollingsworth SE, Lancaster M. It's all just stroke and mirrors! The clinical implementation of mirror therapy to restore lower limb function and mobility following stroke and traumatic brain injury. Cerebrovascular Diseases 2012;33 Suppl 2:843. [Google Scholar]
Michielsen 2011 {published and unpublished data}
- Michielsen ME, Selles RW, Geest JN, Eckhardt M, Yavuzer G, Stam HJ, et al. Motor recovery and cortical reorganisation after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabilitation and Neural Repair 2011;25(3):223‐33. [DOI: 10.1177/1545968310385127; PUBMED: 21051765] [DOI] [PubMed] [Google Scholar]
Mirela 2015 {published data only}
- Mirela CL, Matei D, Ignat B, Popescu CD. Mirror therapy enhances upper extremity motor recovery in stroke patients. Acta Neurologica Belgica 2015;115(4):597‐603. [DOI] [PubMed] [Google Scholar]
Mohan 2013 {published data only}
- Mohan U, Babu SK, Kumar KV, Suresh BV, Misri ZK, Chakrapani M. Effectiveness of mirror therapy on lower extremity motor recovery, balance and mobility in patients with acute stroke: a randomized sham‐controlled pilot trial. Annals of Indian Academy of Neurology 2013;16(4):634‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moustapha 2012 {published and unpublished data}
- Moustapha A, Rousseaux M. Immediate effects of mirror therapy on spatial neglect. Annals of Physical and Rehabilitation Medicine 2012;55(S1):e197. [Google Scholar]
Nagapattinam 2015 {published data only}
- Nagapattinam S, Babu KV, Kumar NS, Ayyappan VR. Effect of task specific mirror therapy with functional electrical stimulation on upper limb function for subacute hemiplegia. International Journal of Physiotherapy 2015;2(5):840‐9. [Google Scholar]
Pandian 2014 {published data only}
- Pandian JD, Arora R, Kaur P, Sharma D, Vishwambaran DK, Arima H. Mirror therapy in unilateral neglect after stroke (MUST trial): a randomized controlled trial. Neurology 2014;83(11):1012‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2015a {published data only}
- Park JY, Chang M, Kim KM, Kim HJ. The effect of mirror therapy on upper‐extremity function and activities of daily living in stroke patients. Journal of Physical Therapy Science 2015;27(6):1681‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2015b {published data only}
- Park Y, Chang M, Kim KM, An DH. The effects of mirror therapy with tasks on upper extremity function and self‐care in stroke patients. Journal of Physical Therapy Science 2015;27(5):1499‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piravej 2012 {published and unpublished data}
- Piravej K, Champaiboon J, Sontim W, Ruengyoo R. Effect of mirror therapy in recovering upper limb strength and function in chronic stroke patients. Neurorehabilitation and Neural Repair 2012;26(6):(Abst.126). [Google Scholar]
Rajappan 2016 {published data only}
- Rajappan R, Abudaheer S, Selvaganapathy K, Gokanadason D. Effect of mirror therapy on hemiparetic upper extremity in subacute stroke patients. Indian Journal of Physical Therapy 2016;2(6):1041‐6. [Google Scholar]
Rehani 2015 {published data only}
- Rehani P, Kumari R, Midha D. Effectiveness of motor relearning programme and mirror therapy on hand functions in patients with stroke‐a randomized clinical trial. International Journals of Therapies and Rehabilitation Research 2015;4(3):20‐4. [Google Scholar]
Rodrigues 2016 {published data only}
- Rodrigues LC, Farias NC, Gomes RP, Michaelsen SM. Feasibility and effectiveness of adding object‐related bilateral symmetrical training to mirror therapy in chronic stroke: a randomized controlled pilot study. Physiotherapy Theory and Practice 2016;32(2):83‐91. [DOI] [PubMed] [Google Scholar]
Rothgangel 2004 {published data only}
- Rothgangel AS, Morton AR, Hout JWE, Beurskens AJHM. Phantoms in the brain: mirror therapy in chronic stroke patients; a pilot study. Nederlands Tijdschrift voor Fysiotherapie 2004;114(2):36‐40. [ISSN: 0377‐208X] [Google Scholar]
Rothgangel 2004a {published data only}
- Rothgangel AS, Morton AR, Hout JWE, Beurskens AJHM. Phantoms in the brain: mirror therapy in chronic stroke patients; a pilot study. Nederlands Tijdschrift voor Fysiotherapie 2004;114(2):36‐40. [Google Scholar]
Rothgangel 2004b {published data only}
- Rothgangel AS, Morton AR, Hout JWE, Beurskens AJHM. Phantoms in the brain: mirror therapy in chronic stroke patients: a pilot study. Nederlands Tijdschrift voor Fysiotherapie 2004;114(2):36‐40. [Google Scholar]
Salhab 2016 {published data only}
- Salhab G, Sarraj AR, Saleh S. Mirror therapy combined with functional electrical stimulation for rehabilitation of stroke survivors' ankle dorsiflexion. IEEE xplore digital library, 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2016. [DOI] [PubMed]
Samuelkamaleshkumar 2014 {published data only}
- Samuelkamaleshkumar S, Reethajanetsureka S, Pauljebaraj P, Benshamir B, Padankatti SM, David JA. Mirror therapy enhances motor performance in the paretic upper limb after stroke: a pilot randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2014;95(11):2000‐5. [DOI] [PubMed] [Google Scholar]
Schick 2017 {published data only}
- Schick T, Schlake HP, Kallusky J, Hohlfeld G, Steinmetz M, Tripp F, et al. Synergy effects of combined multichannel EMG‐triggered electrical stimulation and mirror therapy in subacute stroke patients with severe or very severe arm/hand paresis. Restorative Neurology and Neuroscience 2017;35(3):319‐32. [DOI] [PubMed] [Google Scholar]
Seok 2010 {published data only}
- Seok H, Kim SH, Jang YW, Lee JB, Kim SW. Effect of mirror therapy on recovery of upper limb function and strength in subacute hemiplegia after stroke. Journal of Korean Academy of Rehabilitation Medicine 2010;34:508‐12. [Google Scholar]
Sütbeyaz 2007 {published data only}
- Sütbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower‐extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(5):555‐9. [PUBMED: 17466722] [DOI] [PubMed] [Google Scholar]
Tezuka 2006 {published and unpublished data}
- Tezuka Y, Fujiwara M, Kikuchi K, Ogawa S, Tokunaga N, Ichikawa A, et al. Effect of mirror therapy for patients with post‐stroke paralysis of upper limb: randomized cross‐over study. Journal of Japanese Physical Therapy Association 2006;33(2):62‐8. [Google Scholar]
Thieme 2013 {published and unpublished data}
- Thieme H, Bayn M, Wurg M, Zange C, Pohl M, Behrens J. Mirror therapy for patients with severe arm paresis after stroke‐ a randomized controlled trial. Clinical Rehabilitation 2013;27(4):314‐24. [DOI] [PubMed] [Google Scholar]
Tyson 2015 {published and unpublished data}
- Tyson S, Wilkinson J, Thomas N, Selles R, McCabe C, Tyrrell P, et al. Phase II pragmatic randomized controlled trial of patient‐led therapies (mirror therapy and lower‐limb exercises) during inpatient stroke rehabilitation. Neurorehabilitation and Neural Repair 2015;29(9):818‐26. [DOI] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang L‐J, Chen L‐Z, Ou Y, Guo L, Hao D, Chen S‐S, et al. Effects of mirror visual feedback and electromyographic biofeedback on upper extremity function in hemiplegics after stroke. Chinese Journal of Rehabilitation Theory and Practice 2015;21(2):202‐6. [Google Scholar]
Wu 2013 {published data only}
- Wu C‐Y, Huang P‐C, Chen Y‐T, Lin K‐C, Yang H‐W. Effects of mirror therapy on motor and sensory recovery in chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2013;94(6):1023‐30. [DOI] [PubMed] [Google Scholar]
Yavuzer 2008 {published data only}
- Yavuzer G, Selles R, Sezer N, Sutbeyaz S, Bussmann JB, Koseoglu F, et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2008;89(3):393‐8. [PUBMED: 18295613] [DOI] [PubMed] [Google Scholar]
Yoon 2014 {published data only}
- Yoon JA, Koo BI, Shin MJ, Shin YB, Ko HY, Shin YI. Effect of constraint‐induced movement therapy and mirror therapy for patients with subacute stroke. Annals of Rehabilitation Medicine 2014;38(4):458‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2011 {published and unpublished data}
- Yun GJ, Chun M‐H, Parl JY, Kim BR. The synergic effects of mirror therapy and neuromuscular electrical stimulation for hand function in stroke patients. Annals of Rehabilitation Medicine 2011;35:316‐21. [PUBMED: 22506139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zacharis 2014 {published data only}
- Zacharis D, Moumtzi E, Terzis N, Roussos N, Patatoukas D. The use of mirror therapy in stroke patients with hemiplegic upper limb: a randomized controlled trial. Annals of Physical and Rehabilitation Medicine 2014;57:e27. [Google Scholar]
References to studies excluded from this review
Altschuler 2005 {published data only}
- Altschuler EL. Interaction of vision and movement via a mirror. Perception 2005;34:1153‐5. [PUBMED: 16245491] [DOI] [PubMed] [Google Scholar]
Dohle 2009b {published data only}
- Dohle C, Kaick S, Görtner H, Schnellenbach I. [Kombination von funktioneller Elektrostimulation und Spiegeltherapie]. Gemeinsame Jahrestagung der DGNR/DGNKN. Berlin, 2009.
Ietswaart 2011 {published data only}
- Ietswaart M, Johnston M, Dijkerman HC, Joice S, Scott CL, MacWalter RS, et al. Mental practice with motor imagery in stroke recovery: randomized controlled trial of efficacy. Brain 2011;134:1373‐86. [PUBMED: 21515905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jax 2012 {published data only}
- Jax S. Benefits of mirror‐therapy for hemiparesis following stroke are reduced with increased engagement of contralesional hemisphere. Archives of Physical Medicine and Rehabilitation 2012;93(10):E21. [Google Scholar]
Ji 2014b {published data only}
- Ji S‐G, Cha H‐G, Kim M‐K, Lee C‐R. The effect of mirror therapy integrating functional electrical stimulation on the gait of stroke patients. Journal of Physical Therapy Science 2014;26(4):497‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015b {published data only}
- Kim D, Lim J. Effect of task‐based mirror therapy and task‐based therapy on motor function and sensory recovery of the upper extremity and daily living function in chronic stroke patients. Journal of the Korean Society of Integrative Medicine 2015;3(3):17‐24. [Google Scholar]
Lee 2014 {published data only}
- Lee D, Lee M, Lee K, Song C. Asymmetric training using virtual reality reflection equipment and the enhancement of upper limb function in stroke patients: a randomized controlled trial. Journal of Stroke and Cerebrovascular Diseases 2014;23(6):1319‐26. [DOI] [PubMed] [Google Scholar]
Lin 2014b {published data only}
- Lin KC, Chen YT, Huang PC, Wu CY, Huang WL, Yang HW, et al. Effect of mirror therapy combined with somatosensory stimulation on motor recovery and daily function in stroke patients: a pilot study. Journal of the Formosan Medical Association 2014;113(7):422‐8. [DOI] [PubMed] [Google Scholar]
Moseley 2004 {published data only}
- Moseley GL. Graded motor imagery is effective for long‐standing complex regional pain syndrome: a randomised controlled trial. Pain 2004;108:192‐8. [PUBMED: 15109523] [DOI] [PubMed] [Google Scholar]
Radajewska 2013 {published and unpublished data}
- Radajewska A, Opara JA, Kucio C, Blaszczyszyn M, Mehlich K, Szczygiel J. The effects of mirror therapy on arm and hand function in subacute stroke in patients. International Journal of Rehabilitation Research 2013;36(3):268‐74. [DOI] [PubMed] [Google Scholar]
Ramachandran 1999 {published data only}
- Ramachandran VS, Altschuler EL, Stone L, Al‐Aboudi M, Schwartz E, Siva N. Can mirrors alleviate visual hemineglect?. Medical Hypotheses 1999;52(4):303‐5. [DOI] [PubMed] [Google Scholar]
Selles 2014 {published data only}
- Selles RW, Michielsen ME, Bussmann JB, Stam HJ, Hurkmans HL, Heijnen I, et al. Effects of a mirror‐induced visual illusion on a reaching task in stroke patients: implications for mirror therapy training. Neurorehabilitation & Neural Repair 2014;28(7):652‐9. [DOI] [PubMed] [Google Scholar]
Stevens 2003 {published data only}
- Stevens JA, Stoykov ME. Using motor imagery in the rehabilitation of hemiparesis. Archives of Physical Medicine and Rehabilitation 2003;84(7):1090‐2. [PUBMED: 12881842] [DOI] [PubMed] [Google Scholar]
Vural 2016 {published data only}
- Vural SP, Yuzer GFN, Ozcan DS, Ozbudak SD, Ozgirgin N. Effects of mirror therapy in stroke patients with complex regional pain syndrome type 1: a randomized controlled study. Archives of Physical Medicine and Rehabilitation 2016;97(4):575‐81. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Amimoto 2008 {published data only (unpublished sought but not used)}
- Amimoto K, Matsuda T, Watanabe S. The effect of mirror therapy on the lower limb function of chronic hemiplegic patients. International Journal of Stroke 2008;3 Suppl 1:336‐7 (Abstract PO02‐274). [Google Scholar]
ISRCTN40903497 {published data only}
- ISRCTN40903497. Mirror therapy for the lower extremity after stroke. www.isrctn.com/ISRCTN40903497 (date first received 22 October 2015).
Magni 2014 {published data only}
- Magni E, Hochsprung A, Izquierdo Ayuso G. On‐going clinical trials efficacy of mirror therapy on upper limb motor recovery in chronic stroke patients: a pilot study. Journal of Stroke 2014;9:332–45. [Google Scholar]
May 2011 {published data only}
- May HI, Ozdolap S, Sarikaya S, Ortancil O. The effect of mirror therapy on lower extremity motor function and ambulation in poststroke patients [Inmeli hastalarda ayna tedavisinin alt ekstremite motor fonksiyonuna ve ambulasyona etkisi]. Zonguldak Karaelmas Üniversitesi 2011.
Wang 2013a {published data only}
- Wang W, Ma YW, Yang W. Mirror therapy on upper extremity function in patients with cerebral apoplexy hemiplegia and hand function. Journal of Dalian Medical University 2013;35(6):600‐2. [Google Scholar]
Yeldan 2015 {published data only}
- Yeldan I, Huseyinsinoglu BE, Akinci B, Tarakci E, Baybas S, Ozdincler AR. The effects of very early mirror therapy on functional improvement of the upper extremity in acute stroke patients. Journal of Physical Therapy Science 2015;27(11):3519‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12613000121763 {published data only}
- ACTRN12613000121763. Developing new ways to minimise disability after stroke, a randomised controlled trial of functional electrical stimulation (FES) of the arm and mirror therapy. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000121763 (date first received 1 February 2013).
ChiCTR‐IOR‐16008137 {published data only}
- ChiCTR‐IOR‐16008137. Graded motor imagery based on mirror neuron on rehabilitative training for stroke patients: a BOLD‐fMRI study. www.chictr.org.cn/showprojen.aspx?proj=13608 (date first received 22 March 2016).
DRKS00009288 {published data only}
- DRKS00009288. Central facial paralysis after stroke: a randomized controlled trial [Zentrale Gesichtslähmung nach Schlaganfall: eine randomisierte, kontrollierte Studie]. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00009288 (date first received 28 August 2015).
IRCT201504224787N5 {published data only}
- IRCT201504224787N5. The effect of mirror therapy on motor ability of patients after stroke. www.irct.ir/searchresult.php?id=4787&number=5 (date first received 1 August 2015).
NCT01010607 {published data only}
- NCT01010607. Use of tendon vibration and mirror for the improvement of upper limb function and pain reduction. clinicaltrials.gov/show/NCT01010607 (date first received 10 November 2009).
NCT01724164 {published data only}
- NCT01724164. Robot‐versus mirror‐assisted motor interventions in rehabilitating upper‐limb motor and muscle performance and daily functions poststroke: a comparative effectiveness study. clinicaltrials.gov/ct2/show/record/NCT01724164 (date first received 9 November 2012).
NCT02254616 {published data only}
- NCT02254616. Hybrid approach to mirror therapy and transcranial direct current stimulation for stroke recovery: a follow up study on brain reorganization, motor performance of upper extremity, daily function, and activity participation. clinicaltrials.gov/ct2/show/record/NCT02254616 (date first received 2 October 2014).
NCT02276729 {published data only}
- NCT02276729. A pilot randomized controlled trial (RCT) of mirror box therapy in upper limb rehabilitation with sub‐acute stroke patients. clinicaltrials.gov/show/NCT02276729 (date first received 28 October 2014 ).
NCT02319785 {published data only}
- NCT02319785. Effects of robot‐assisted combined therapy in upper limb rehabilitation in stroke patients. clinicaltrials.gov/ct2/show/record/NCT02319785 (date first received 18 December 2014).
NCT02432755 {published data only}
- NCT02432755. Effects of home‐based mirror therapy combined with task‐oriented training for patients with stroke: a randomized controlled trial. clinicaltrials.gov/show/NCT02432755 (date first received 4 May 2015).
NCT02548234 {published data only}
- NCT02548234. Effect of mirror therapy versus bilateral arm training for rehabilitation after chronic stroke: a pilot randomized‐controlled trial. clinicaltrials.gov/ct2/show/record/NCT02548234 (date first received 14 September 2015).
NCT02776306 {published data only}
- NCT02776306. Effects of mirror box therapy on neuroplasticity and functional outcome in hemiparetic upper limb post stroke. clinicaltrials.gov/ct2/show/record/NCT02776306 (date first received 18 May 2015).
NCT02778087 {published data only}
- NCT02778087. Self‐directed box (mirror) therapy after stroke: a dosing study. ClinicalTrials.gov/show/NCT02778087 (date first received 19 May 2016).
NCT02827864 {published data only}
- NCT02827864. Efficacy and time dependent effects of transcranial direct current stimulation (tDCS) combined with mirror therapy for rehabilitation after subacute and chronic stroke. clinicaltrials.gov/show/NCT02827864 (date first received 11 July 2016).
NCT02871700 {published data only}
- NCT02871700. Comparative efficacy study of action observation therapy and mirror therapy after Stroke: rehabilitation outcomes and neural mechanisms by MEG. clinicaltrials.gov/ct2/show/record/NCT02871700 (date first received 18 August 2016).
Additional references
Adamovich 2009
- Adamovich SV, August K, Merians A, Tunik E. A virtual reality‐based system integrated with fMRI to study neural mechanisms of action observation‐execution: a proof of concept study. Restorative Neurology and Neuroscience 2009;27(3):209‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 1997
- Barker WH, Mullooly JP. Stroke in a defined elderly population, 1967‐1985. A less lethal and disabling but no less common disease. Stroke 1997;28(2):284‐90. [PUBMED: 9040676] [DOI] [PubMed] [Google Scholar]
Berg 1992
- Berg KO, Wood‐Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Canadian Journal of Public Health = Revue Canadienne de Santé Publique 1992;83(Suppl 2):S7‐11. [PUBMED: 1468055] [PubMed] [Google Scholar]
Bieniok 2011
- Bieniok A, Govers J, Dohle C. Mirror therapy in neurorehabilitation [Spiegeltherapie in der Neurorehabilitation]. Spiegeltherapie in der Neurorehabilitation. Vol. 2, Idstein: Schulz‐Kirchner Verlag, 2011. [Google Scholar]
Bowering 2013
- Bowering KJ, O'Connell NE, Tabor A, Catley MJ, Leake HB, Moseley GL, et al. The effects of graded motor imagery and its components on chronic pain: a systematic review and meta‐analysis. Journal of Pain 2013;14(1):3‐13. [DOI] [PubMed] [Google Scholar]
Brunnstrom 1966
- Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Physical Therapy 1966;46:357‐75. [PUBMED: 590725] [DOI] [PubMed] [Google Scholar]
Carr 1985
- Carr J, Shepherd R. Investigation of a new Motor Assessment Scale. Physical Therapy 1985;65:175‐80. [PUBMED: 3969398] [DOI] [PubMed] [Google Scholar]
Collen 1991
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13(2):50‐4. [DOI] [PubMed] [Google Scholar]
Corbetta 2015
- Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint‐induced movement therapy for upper extremities in people with stroke. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD004433.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deconinck 2015
- Deconinck FJ, Smorenburg AR, Benham A, Ledebt A, Feltham MG, Savelsbergh GJ. Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabilitation and Neural Repair 2015;29(4):349‐61. [DOI] [PubMed] [Google Scholar]
Demeurisse 1980
- Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. European Neurology 1980;19:382‐9. [PUBMED: 7439211] [DOI] [PubMed] [Google Scholar]
Dohle 2004
- Dohle C, Kleiser R, Seitz RJ, Freund HJ. Body scheme gates visual processing. Journal of Neurophysiology 2004;91(5):2376‐9. [PUBMED: 14681333] [DOI] [PubMed] [Google Scholar]
Dohle 2005
- Dohle C, Nakaten A, Püllen J, Rietz C, Karbe H. Basic mechanisms and application of mirror training [Grundlagen und Anwendung des Spiegeltrainings]. In: Minkwitz K, Scholz E editor(s). Standardisierte Therapieverfahren und Grundlagen des Lernens in der Neurologie. Idstein, Germany: Schulz‐Kirchner‐Verlag, 2005. [Google Scholar]
Dohle 2011
- Dohle C, Stephan KM, Valvoda JT, Hosseiny O, Tellmann L, Kuhlen T, et al. Representation of virtual arm movements in precuneus. Experimental Brain Research 2011;208(4):543‐55. [DOI: 10.1007/s00221-010-2503-0] [DOI] [PubMed] [Google Scholar]
Eng 2007
- Eng K, Siekierka E, Pyk P, Chevrier E, Hauser Y, Cameirao M, et al. Interactive visuo‐motor therapy system for stroke rehabilitation. Medical and Biological Engineering and Computing 2007;45(9):901‐7. [PUBMED: 17687578] [DOI] [PubMed] [Google Scholar]
Ezendam 2009
- Ezendam D, Bongers RM, Jannink MJ. Systematic review of the effectiveness of mirror therapy in upper extremity function. Disability and Rehabilitation 2009;31:2135‐49. [PUBMED: 19903124] [DOI] [PubMed] [Google Scholar]
Farnè 2004
- Farnè A, Buxbaum LJ, Ferraro M, Frassinetti F, Whyte J, Veramonti T, et al. Patterns of spontaneous recovery of neglect and associated disorders in acute right brain‐damaged patients. Journal of Neurology, Neurosurgery, and Psychiatry 2004;75(10):1401‐10. [PUBMED: 15377685] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feys 2004
- Feys H, Weerdt W, Verbeke G, Steck GC, Capiau C, Kiekens C, et al. Early and repetitive stimulation of the arm can substantially improve the long‐term outcome after stroke: a 5‐year follow‐up study of a randomized trial. Stroke 2004;35(4):924‐9. [PUBMED: 15001789] [DOI] [PubMed] [Google Scholar]
Franceschini 2010
- Franceschini M, Porta F, Agosti M, Massucci M, ICR2 group. Is health‐related quality of life of stroke patients influenced by neurological impairments at one year after stroke?. European Journal of Physical and Rehabilitation Medicine 2010;46(3):389‐99. [PUBMED: 20927005] [PubMed] [Google Scholar]
Franck 2001
- Franck N, Farrer C, Georgieff N, Marie‐Cardine M, Dalery J, D'Amato T, et al. Defective recognition of one's own actions in patients with schizophrenia. American Journal of Psychiatry 2001;158(3):454‐9. [PUBMED: 11229988] [DOI] [PubMed] [Google Scholar]
French 2016
- French B, Thomas LH, Coupe J, McMahon NE, Connell L, Harrison J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD006073.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fritzsch 2014
- Fritzsch C, Wang J, Dos Santos LF, Mauritz KH, Brunetti M, Dohle C. Different effects of the mirror illusion on motor and somatosensory processing. Restorative Neurology and Neuroscience 2014;32(2):269‐80. [DOI] [PubMed] [Google Scholar]
Fugl‐Meyer 1975
- Fugl‐Meyer AR, Jaasko L, Leyman IL, Olsson S, Steglind S. The post‐stroke hemiplegic patient. I. A method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine 1975;7:13‐31. [PUBMED: 1135616] [PubMed] [Google Scholar]
Fukumura 2007
- Fukumura K, Sugawara K, Tanabe S, Ushiba J, Tomita Y. Influence of mirror therapy on human motor cortex. International Journal of Neuroscience 2007;117(7):1039‐48. [PUBMED: 17613113] [DOI] [PubMed] [Google Scholar]
Gaggioli 2004
- Gaggioli A, Morganti F, Walker R, Meneghini A, Alcaniz M, Lozano JA, et al. Training with computer‐supported motor imagery in post‐stroke rehabilitation. Cyberpsychology and Behavior 2004;7(3):327‐32. [PUBMED: 15257833] [DOI] [PubMed] [Google Scholar]
Garry 2005
- Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Experimental Brain Research 2005;163(1):118‐22. [PUBMED: 15754176] [DOI] [PubMed] [Google Scholar]
Grèzes 2001
- Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta‐analysis. Human Brain Mapping 2001;12(1):1‐19. [PUBMED: 11198101 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatem 2016
- Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Frontiers in Human Neuroscience 2016;10(442):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendricks 2002
- Hendricks HT, Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Archives of Physical Medicine and Rehabilitation 2002;83(11):1629‐37. [PUBMED: 12422337] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoermann 2017
- Hoermann S, Ferreira Dos Santos L, Morkisch N, Jettkowski K, Sillis M, Devan H, et al. Computerised mirror therapy with augmented reflection technology for early stroke rehabilitation: clinical feasibility and integration as an adjunct therapy. Disability and Rehabilitation 2017;39(15):1503‐14. [DOI] [PubMed] [Google Scholar]
Jorgensen 1995
- Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation 1995;76(1):27‐32. [PUBMED: 7811170] [DOI] [PubMed] [Google Scholar]
Jönsson 2006
- Jönsson AC, Lindgren I, Hallström B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: a population based study focusing on patients' perspectives. Journal of Neurology, Neurosurgery, and Psychiatry 2006;77(5):590‐5. [PUBMED: 16354737] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2011
- Kang YJ, Ku J, Kim HJ, Park HK. Facilitation of corticospinal excitability according to motor imagery and mirror therapy in healthy subjects and stroke patients. Annals of Rehabilitation Medicine 2011;35(6):747‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2012
- Kang YJ, Park HK, Kim HJ, Lim T, Ku J, Cho S, et al. Upper extremity rehabilitation of stroke: facilitation of corticospinal excitability using virtual mirror paradigm. Journal of Neuroengineering and Rehabilitation 2012;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 1999
- Katz N, Hartman‐Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Archives of Physical Medicine and Rehabilitation 1999;80(4):379‐84. [PUBMED: 10206598] [DOI] [PubMed] [Google Scholar]
Keith 1987
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Advances in Clinical Rehabilitation 1987;1:6‐18. [PUBMED: 3503663] [PubMed] [Google Scholar]
Kocabas 2007
- Kocabas H, Levendoglu F, Ozerbil OM, Yuruten B. Complex regional pain syndrome in stroke patients. International Journal of Rehabilitation Research 2007;30(1):33‐8. [PUBMED: 17293718] [DOI] [PubMed] [Google Scholar]
Kwakkel 2003
- Kwakkel G, Kollen BJ, Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34(9):2181‐6. [PUBMED: 12907818] [DOI] [PubMed] [Google Scholar]
Laver 2017
- Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD008349.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liepert 1995
- Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalography and Clinical Neurophysiology 1995;97(6):382‐6. [DOI] [PubMed] [Google Scholar]
Liepert 1998
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, et al. Motor cortex plasticity during constraint‐induced movement therapy in stroke patients. Neuroscience Letters 1998;250(1):5‐8. [PUBMED: 9696052] [DOI] [PubMed] [Google Scholar]
Lindgren 2007
- Lindgren I, Jönsson AC, Norrving B, Lindgren A. Shoulder pain after stroke: a prospective population‐based study. Stroke 2007;38(2):343‐8. [PUBMED: 17185637] [DOI] [PubMed] [Google Scholar]
Longo 2009
- Longo MR, Haggard P. Sense of agency primes manual motor responses. Perception 2009;38(1):69‐78. [PUBMED: 19323137] [DOI] [PubMed] [Google Scholar]
Lundström 2009
- Lundström E, Smits A, Terént A, Borg J. Risk factors for stroke‐related pain 1 year after first‐ever stroke. European Journal of Neurology 2009;16(2):188‐93. [PUBMED: 19138338] [DOI] [PubMed] [Google Scholar]
Lyle 1981
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research 1981;4:483‐92. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PUBMED: 14258950] [PubMed] [Google Scholar]
Mathiowetz 1985
- Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. American Journal of Occupational Therapy 1985;39(6):386‐91. [PUBMED: 3160243] [DOI] [PubMed] [Google Scholar]
Matthys 2009
- Matthys K, Smits M, Geest JN, Lugt A, Seurinck R, Stam HJ, et al. Mirror‐induced visual illusion of hand movements: a functional magnetic resonance imaging study. Archives of Physical Medicine and Rehabilitation 2009;90(4):675‐81. [PUBMED: 19345786] [DOI] [PubMed] [Google Scholar]
McCabe 2003
- McCabe CS, Haigh RC, Ring EF, Halligan PW, Wall PD, Blake DR. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology 2003;42(1):97‐101. [PUBMED: 12509620] [DOI] [PubMed] [Google Scholar]
Mehrholz 2015
- Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot‐assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD006876.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehrholz 2017
- Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical‐assisted training for walking after stroke. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD006185.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miltner 1998
- Miltner R, Simon U, Netz J, Hömberg V. Motor imagery in the therapy of patients with central motor deficit [Bewegungsvorstellung in der Therapie von Patienten mit Hirninfarkt]. Funktionelle Bildgebung und Physiotherapie. Hippocampus Verlag, 1998. [Google Scholar]
Miltner 1999
- Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E. Effects of constraint‐induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke 1999;30(3):586‐92. [PUBMED: 10066856] [DOI] [PubMed] [Google Scholar]
Miyamoto 2009
- Miyamoto S, Kondo T, Suzukamo Y, Michimata A, Izumi S. Reliability and validity of the Manual Function Test in patients with stroke. American Journal of Physical Medicine & Rehabilitation 2009;88(3):247‐55. [PUBMED: 19106794] [DOI] [PubMed] [Google Scholar]
Morganti 2003
- Morganti F, Gaggioli A, Castelnuovo G, Bulla D, Vettorello M, Riva G. The use of technology‐supported mental imagery in neurological rehabilitation: a research protocol. Cyberpsychology and Behavior 2003;6(4):421‐7. [PUBMED: 14511455] [DOI] [PubMed] [Google Scholar]
Morkisch 2015
- Morkisch N, Dohle C. Berlin mirror therapy protocol ‐ a scientifically evaluated manual to perform mirror therapy. BeST Berliner Spiegeltherapieprotokoll‐ Ein Wissenschaftlich Evaluiertes Manual zur Durchführung der Spiegeltherapie. Bad Honneff: Hippocampus Verlag, 2015. [Google Scholar]
Murray 2013
- Murray CJ, Lopez AD. Measuring the global burden of disease. New England Journal of Medicine 2013;369(5):448‐57. [DOI] [PubMed] [Google Scholar]
Nakayama 1994
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation 1994;75(4):394‐8. [PUBMED: 8172497] [DOI] [PubMed] [Google Scholar]
Platz 2005
- Platz T, Eickhof C, Kaick S, Engel U, Pinkowski C, Kalok S, et al. Impairment‐oriented training or Bobath therapy for severe arm paresis after stroke: a single‐blind, multicentre randomized controlled trial. Clinical Rehabilitation 2005;19(7):714‐24. [PUBMED: 16250190] [DOI] [PubMed] [Google Scholar]
Ramachandran 1994
- Ramachandran VS. Phantom limbs, neglect syndromes, repressed memory, and Freudian psychology. International Review of Neurobiology 1994;37:291‐333. [PUBMED: 7883483 ] [DOI] [PubMed] [Google Scholar]
Ramachandran 1995
- Ramachandran VS, Rogers‐Ramachandran D, Cobb S. Touching the phantom limb. Nature 1995;377(6549):489‐90. [PUBMED: 7566144 ] [DOI] [PubMed] [Google Scholar]
Ramachandran 1996
- Ramachandran VS, Rogers‐Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proceedings of the Royal Society B: Biological Sciences 1996;263(1369):377‐86. [PUBMED: 8637922] [DOI] [PubMed] [Google Scholar]
Ramachandran 2009
- Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain 2009;132:1693‐710. [PUBMED: 19506071] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringman 2004
- Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology 2004;63(3):468‐74. [PUBMED: 15304577] [DOI] [PubMed] [Google Scholar]
Rothgangel 2011
- Rothgangel AS, Braun SM, Beurskens AJ, Seitz RJ, Wade DT. The clinical aspects of mirror therapy in rehabilitation: a systematic review of the literature. International Journal of Rehabilitation Research 2011;34(1):1‐13. [PUBMED: 21326041] [DOI] [PubMed] [Google Scholar]
Sackley 2008
- Sackley C, Brittle N, Patel S, Ellins J, Scott M, Wright C, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke 2008;39(12):3329‐34. [PUBMED: 18787199] [DOI] [PubMed] [Google Scholar]
Shinoura 2008
- Shinoura N, Suzuki Y, Watanabe Y, Yamada R, Tabei Y, Saito K, et al. Mirror therapy activates outside of cerebellum and ipsilateral M1. NeuroRehabilitation 2008;23(3):245‐52. [PUBMED: 18560141] [PubMed] [Google Scholar]
Taub 1993
- Taub E, Miller NE, Novack TA, Cook EW 3rd, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Archives of Physical Medicine and Rehabilitation 1993;74(4):347‐54. [PUBMED: 8466415] [PubMed] [Google Scholar]
Thieme 2016
- Thieme H, Morkisch N, Rietz C, Dohle C, Borgetto B. The efficacy of movement representation techniques for treatment of limb pain: a systematic review and meta‐analysis. Journal of Pain 2016;17(2):167‐80. [PUBMED: 26552501] [DOI] [PubMed] [Google Scholar]
Urton 2007
- Urton ML, Kohia M, Davis J, Neill MR. Systematic literature review of treatment interventions for upper extremity hemiparesis following stroke. Occupational Therapy International 2007;14(1):11‐27. [PUBMED: 17623376] [DOI] [PubMed] [Google Scholar]
Van Peppen 2004
- Peppen RP, Kwakkel G, Wood‐Dauphinee S, Hendriks HJ, Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what's the evidence?. Clinical Rehabilitation 2004;18(8):833‐62. [PUBMED: 15609840] [DOI] [PubMed] [Google Scholar]
Wang 2013b
- Wang J, Fritzsch C, Bernarding J, Krause T, Mauritz K‐H, Brunetti M, et al. Cerebral activation evoked by the mirror illusion of the hand in stroke patients compared to normal subjects. Neurorehabilitation 2013;33(4):593‐603. [DOI] [PubMed] [Google Scholar]
Wolf 2001
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke 2001;32:1635‐9. [PUBMED: 11441212] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Thieme 2010
- Thieme H, Mehrholz J, Pohl M, Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD008449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thieme 2012
- Thieme H, Mehrholz J, Pohl M, Behrens J, Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD008449.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


