Abstract
Background
Revascularisation is the gold standard therapy for patients with critical limb ischaemia (CLI). In over 30% of patients who are not suitable for or have failed previous revascularisation therapy (the 'no‐option' CLI patients), limb amputation is eventually unavoidable. Preliminary studies have reported encouraging outcomes with autologous cell‐based therapy for the treatment of CLI in these 'no‐option' patients. However, studies comparing the angiogenic potency and clinical effects of autologous cells derived from different sources have yielded limited data. Data regarding cell doses and routes of administration are also limited.
Objectives
To compare the efficacy and safety of autologous cells derived from different sources, prepared using different protocols, administered at different doses, and delivered via different routes for the treatment of 'no‐option' CLI patients.
Search methods
The Cochrane Vascular Information Specialist (CIS) searched the Cochrane Vascular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, Embase Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), and trials registries (16 May 2018). Review authors searched PubMed until February 2017.
Selection criteria
We included randomised controlled trials (RCTs) involving 'no‐option' CLI patients comparing a particular source or regimen of autologous cell‐based therapy against another source or regimen of autologous cell‐based therapy.
Data collection and analysis
Three review authors independently assessed the eligibility and methodological quality of the trials. We extracted outcome data from each trial and pooled them for meta‐analysis. We calculated effect estimates using a risk ratio (RR) with 95% confidence interval (CI), or a mean difference (MD) with 95% CI.
Main results
We included seven RCTs with a total of 359 participants. These studies compared bone marrow‐mononuclear cells (BM‐MNCs) versus mobilised peripheral blood stem cells (mPBSCs), BM‐MNCs versus bone marrow‐mesenchymal stem cells (BM‐MSCs), high cell dose versus low cell dose, and intramuscular (IM) versus intra‐arterial (IA) routes of cell implantation. We identified no other comparisons in these studies. We considered most studies to be at low risk of bias in random sequence generation, incomplete outcome data, and selective outcome reporting; at high risk of bias in blinding of patients and personnel; and at unclear risk of bias in allocation concealment and blinding of outcome assessors. The quality of evidence was most often low to very low, with risk of bias, imprecision, and indirectness of outcomes the major downgrading factors.
Three RCTs (100 participants) reported a total of nine deaths during the study follow‐up period. These studies did not report deaths according to treatment group.
Results show no clear difference in amputation rates between IM and IA routes (RR 0.80, 95% CI 0.54 to 1.18; three RCTs, 95 participants; low‐quality evidence). Single‐study data show no clear difference in amputation rates between BM‐MNC‐ and mPBSC‐treated groups (RR 1.54, 95% CI 0.45 to 5.24; 150 participants; low‐quality evidence) and between high and low cell dose (RR 3.21, 95% CI 0.87 to 11.90; 16 participants; very low‐quality evidence). The study comparing BM‐MNCs versus BM‐MSCs reported no amputations.
Single‐study data with low‐quality evidence show similar numbers of participants with healing ulcers between BM‐MNCs and mPBSCs (RR 0.89, 95% CI 0.44 to 1.83; 49 participants) and between IM and IA routes (RR 1.13, 95% CI 0.73 to 1.76; 41 participants). In contrast, more participants appeared to have healing ulcers in the BM‐MSC group than in the BM‐MNC group (RR 2.00, 95% CI 1.02 to 3.92; one RCT, 22 participants; moderate‐quality evidence). Researchers comparing high versus low cell doses did not report ulcer healing.
Single‐study data show similar numbers of participants with reduction in rest pain between BM‐MNCs and mPBSCs (RR 0.99, 95% CI 0.93 to 1.06; 104 participants; moderate‐quality evidence) and between IM and IA routes (RR 1.22, 95% CI 0.91 to 1.64; 32 participants; low‐quality evidence). One study reported no clear difference in rest pain scores between BM‐MNC and BM‐MSC (MD 0.00, 95% CI ‐0.61 to 0.61; 37 participants; moderate‐quality evidence). Trials comparing high versus low cell doses did not report rest pain.
Single‐study data show no clear difference in the number of participants with increased ankle‐brachial index (ABI; increase of > 0.1 from pretreatment), between BM‐MNCs and mPBSCs (RR 1.00, 95% CI 0.71 to 1.40; 104 participants; moderate‐quality evidence), and between IM and IA routes (RR 0.93, 95% CI 0.43 to 2.00; 35 participants; very low‐quality evidence). In contrast, ABI scores appeared higher in BM‐MSC versus BM‐MNC groups (MD 0.05, 95% CI 0.01 to 0.09; one RCT, 37 participants; low‐quality evidence). ABI was not reported in the high versus low cell dose comparison.
Similar numbers of participants had improved transcutaneous oxygen tension (TcO₂) with IM versus IA routes (RR 1.22, 95% CI 0.86 to 1.72; two RCTs, 62 participants; very low‐quality evidence). Single‐study data with low‐quality evidence show a higher TcO₂ reading in BM‐MSC versus BM‐MNC groups (MD 8.00, 95% CI 3.46 to 12.54; 37 participants) and in mPBSC‐ versus BM‐MNC‐treated groups (MD 1.70, 95% CI 0.41 to 2.99; 150 participants). TcO₂ was not reported in the high versus low cell dose comparison.
Study authors reported no significant short‐term adverse effects attributed to autologous cell implantation.
Authors' conclusions
Mostly low‐ and very low‐quality evidence suggests no clear differences between different stem cell sources and different treatment regimens of autologous cell implantation for outcomes such as all‐cause mortality, amputation rate, ulcer healing, and rest pain for 'no‐option' CLI patients. Pooled analyses did not show a clear difference in clinical outcomes whether cells were administered via IM or IA routes. High‐quality evidence is lacking; therefore the efficacy and long‐term safety of autologous cells derived from different sources, prepared using different protocols, administered at different doses, and delivered via different routes for the treatment of 'no‐option' CLI patients, remain to be confirmed.
Future RCTs with larger numbers of participants are needed to determine the efficacy of cell‐based therapy for CLI patients, along with the optimal cell source, phenotype, dose, and route of implantation. Longer follow‐up is needed to confirm the durability of angiogenic potential and the long‐term safety of cell‐based therapy.
Plain language summary
Cell‐based therapy using different sources and different treatment regimens for 'no‐option' CLI patients
Background
Critical limb ischaemia (CLI) is characterised by severe leg pain on walking and at rest and hard‐to‐heal wounds, which may lead to disability and death. The procedure that aims to improve blood flow to the affected limb, known as 'revascularisation', is the gold standard therapy. However, 25% to 40% of people with CLI are not suitable for or have failed previous revascularisation therapy. Therefore, for these patients, the only option for relieving pain and stopping wound infection from spreading is limb amputation. These patients are commonly referred to as 'no‐option' CLI patients.
Cell‐based therapy is increasingly recognised as a promising novel treatment for CLI. Most of the data for this novel approach have been obtained from studies based on patients' own cells, also known as 'autologous cells'. However, current data on the efficacy of autologous cells are limited because available information about the sources used to obtain these cells (e.g. bone marrow, peripheral blood), the doses used (e.g. high or low cell dose), and the method of cell administration selected (e.g. cell injection into muscles or into blood vessels) is limited. In this review, we evaluated the efficacy and safety of autologous cell‐based therapy derived from different sources and prepared as different treatment regimens for 'no‐option' CLI patients.
Study characteristics and key results
We analysed the findings of seven randomised controlled trials (RCTs) involving 359 CLI patients revealed by our literature search, which was current to 16 May 2018.
We evaluated two main sources of stem cell treatment, namely, 'bone marrow‐mononuclear cells (BM‐MNCs)' and 'mobilised peripheral blood stem cells (mPBSCs)'. Limited data suggest that BM‐MNCs or mPBSCs resulted in similar rates of limb amputation and death. Also, the two cell sources appeared to yield similar numbers of patients with improved rest pain, ulcer healing, and lower limb blood flow parameters as measured via the ankle‐brachial index (ABI). However, data from one RCT show that mPBSC implantation resulted in improved transcutaneous oxygen tension (TcO₂) readings when compared to BM‐MNC. Data from one RCT show no clear difference in amputation rates between patients receiving high cell dose and low cell dose, and no difference in clinical outcomes whether patients received cell doses via intramuscular or intra‐articular routes. Study authors reported no significant short‐term adverse effects attributed to autologous cell implantation.
Quality of the evidence
The quality of evidence for all outcomes varied but was mostly low to very low owing to limitations in study design and lack of data for several important outcomes. Taken together, there is insufficient high‐quality evidence to assess the effects of using a particular source or treatment regimen of cell‐based therapy for CLI in clinical practice. Larger trials with longer follow‐up are needed to evaluate the long‐term benefits and safety of various cell‐based products for patients with CLI.
Summary of findings
Background
Description of the condition
Peripheral arterial disease (PAD) affects 3% to 10% of the population (Norgren 2010). It is a global health problem that is associated with significant morbidity and mortality attributed to intermittent claudication and critical limb ischaemia (CLI). Intermittent claudication is the most common presentation of PAD and is generally managed conservatively. Critical limb ischaemia, the severe form of PAD, is characterised by rest pain, ulceration, and gangrene. Revascularisation therapy via the surgical or endovascular approach is the gold standard treatment for severe PAD, provided with the aim of improving blood flow to the affected extremity. However, this treatment modality cannot be applied to over 30% of patients owing to excessive anaesthetic and operative risks and unfavourable vascular involvement (Sasajima 1997). Moreover, revascularisation therapy is likely to be futile in the presence of extensive atherosclerotic plaque and low rates of long‐term vessel patency in severe PAD (Conrad 2011). Hence, many patients are reliant on medical therapy that may halt disease progression only temporarily, leaving limb amputation as the only remaining option for relief from pain or gangrene (Botti 2012). Of note, after one year from diagnosis, limb amputation is unavoidable in 30% of patients with CLI (Norgren 2007). An estimated 120 to 150 amputations are performed per million people per year, and one‐quarter of these patients require long‐term institutional care or professional assistance at home (Norgren 2007). There is a critical need to develop novel strategies to promote vascular regeneration or neovascularisation in patients with CLI who are not suitable for conventional treatments, to reduce physical disability, mortality, and socioeconomic burden.
Description of the intervention
Although initial clinical studies on cell‐based therapy have been encouraging, current evidence from large‐scale randomised controlled clinical trials (RCTs) comparing active treatment versus placebo is limited, leading to a previous Cochrane review concluding that there was "insufficient evidence to support cell therapy in clinical practice" (Moazzami 2014).
To date, the types of cell‐based products used for implantation in CLI patients have been derived from bone marrow‐mononuclear cells (BM‐MNCs) (Durdu 2006; Miyamoto 2006; Tateishi‐Yuyama 2002), peripheral blood‐mononuclear cells (PB‐MNCs) (Huang 2004; Kawamura 2006; Lenk 2005; Matsui 2003), granulocyte colony‐stimulating factor (G‐CSF)‐mobilised PB‐MNCs (mPBSCs) (Huang 2005; Huang 2007; Ishida 2005), CD34 antigen‐positive mononuclear cells (MNCs) (Inaba 2002; Kawamoto 2009), CD133 antigen‐positive MNCs (Burt 2010), and BM‐mesenchymal stem cells (BM‐MSCs) (Dash 2009; Lu 2011).
Cell implantation procedures are generally safe and well tolerated, as has been described in extensive clinical studies involving patients with PAD that utilised stem cells derived from various sources (Benoit 2013; Liew 2016; Liu 2015; Sun 2015). Bone marrow is the most common source of stem cells in clinical trials involving cell‐based therapy. However, mobilised stem cells from patients' peripheral blood after administration of G‐CSF (mPBSCs) are now preferred over bone marrow stem cells owing to relative ease of collection and avoidance of anaesthesia and pain associated with bone marrow biopsy (Fadilah 2013). Apart from bone marrow and peripheral blood, mesenchymal stem cells (MSCs) can be isolated from various adult human tissues (e.g. adipose tissue, skeletal muscles, tendons, nerves, cartilages) or neonatal tissues (placenta and umbilical cord blood). The safety and efficacy of MSCs isolated from adipose tissue (AT‐MSCs) in a small series of CLI patients have been reported (Lee 2012). However, apart from bone marrow, peripheral blood, and adipose tissue sources, MSCs derived from other human tissues have not been tested in CLI patients.
Mononuclear cells and stem cells derived from different sources may lead to different clinical outcomes in patients with PAD. Stem cells obtained from different sources may vary in biological (plasticity, self‐renewal, differentiation, homing, migration, secretion of trophic factors) and immunological (modulation of immune response) properties. This may be attributed to the inherent biological properties of the stem cells or to changes to the cells that may occur during cell enrichment and culture. For example, G‐CSF injection used to mobilise bone marrow‐derived progenitor cells may significantly enhance the formation of several growth factors involved in vascular repair (Huang 2007). In addition, the apheresis procedure results in transient cleavage of chemokine receptors expressed on cell surfaces, causing uncoupling from the bone marrow stroma (Honold 2006). Implantation of cells into the lower limb of CLI patients can be performed via several routes including intramuscular, intra‐arterial, or a combination of both, although it is yet unclear which method is superior (Gu 2008; Klepanec 2012; Van Tongeren 2008). Intramuscular administration is usually performed through multiple injections at the level of gastrocnemius muscles, and intra‐arterial infusion is usually performed via the femoral artery. Limited data from two RCTs have not shown superiority of either route (Gu 2008; Klepanec 2012).
How the intervention might work
To date, the mechanisms by which implanted cells improve clinical outcomes in patients with PAD are still unclear. Experimental animal studies indicate that bone marrow‐derived cells contribute to vascular and muscle regeneration by physically integrating into the tissues, by secreting growth factors, or by both means (Fadini 2007; Honold 2006). Adult bone marrow stem cells with angiogenic potential such as endothelial progenitor cells (EPCs) and MSCs have the capability to stimulate formation of new blood vessels by differentiating into endothelial cells and vascular smooth muscle (Schatteman 2004), and by stimulating endothelial cell proliferation and migration (Pittenger 1999; Reyes 2002). EPCs also exert direct angiogenic action through their ability to secrete paracrine mediators (Jarajapu 2010). Furthermore, MSCs support neo‐angiogenesis by releasing soluble factors to stimulate EPC sprouting from pre‐existing blood vessels (Cobellis 2010; Jarajapu 2010). Therefore, cell implantation into ischaemic limbs may promote neo‐angiogenesis by providing precursor cells capable of vascular transdifferentiation, and by supplying multiple angiogenic cytokines, growth factors, and homing signals for mural cells or pericytes for microvascular stabilisation (Benoit 2013; Kaelin 2008). The combination of these mechanisms is responsible for augmenting vascular repair and ameliorating tissue perfusion, leading to reversal of ischaemia in the affected limb.
Why it is important to do this review
It is important to determine if different sources or methods of MNCs and stem cell preparations have different effects on clinical outcomes following implantation into CLI patients; and whether a combination versus a single type of MNC or stem cell treatment improves ischaemic symptoms and survival among these patients. To date, data comparing the angiogenic potency of autologous cells derived from different sources are limited. Moreover, direct comparison of different autologous cell types shows conflicting results (Liew 2016). One study showed that BM‐MNCs were associated with significant improvement in lower limb perfusion when compared to PB‐MNCs (Tateishi‐Yuyama 2002); another study showed similar outcomes in both treatment groups (Onodera 2011). Additionally, other sources of stem cells such as placenta or stored autologous cord blood might become available. It is not yet known whether cells from these sources would be as effective as cells derived from bone marrow or peripheral blood for treating CLI patients. Furthermore, thus far no safety data have been published by head‐to‐head RCTs comparing a particular cell‐based therapy versus another type of cell‐based therapy. The individual RCTs comparing cell treatment and non‐cell treatment presented in previous reviews have not reported significant procedure‐related complications or adverse biochemical and immunological effects related to cell implantation in CLI patients (Liu 2015; Teraa 2013; Wang 2014).
Up‐to‐date synthesised evidence on optimal cell sources, cell dose, and administration protocols for the treatment of 'no‐option' CLI patients is required to guide clinical practice and direct future research. The current proposed meta‐analysis aims to attain comprehensive insight into the optimal cell‐based treatment program for patients with CLI.
Objectives
To compare the efficacy and safety of autologous cells derived from different sources, prepared using different protocols, administered at different doses, and delivered via different routes for the treatment of 'no‐option' CLI patients.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We excluded cluster‐randomised trials (owing to difficulties in adjusting for the unit of analysis) and cross‐over studies (owing to a possible 'contaminating' effect of one intervention on another).
Types of participants
Study participants were adult patients with the diagnosis of CLI who were not candidates for revascularisation therapy and did not show any improvement in response to best standard medical therapy. We included in the review all causes of CLI such as atherosclerosis, Buerger's disease, acute embolism, and others. We applied no age restriction.
Types of interventions
Intervention: administration to CLI patients of autologous MNCs or stem cells obtained from a particular source, prepared using a particular protocol, administered at a particular dose, and delivered via a particular route.
Comparison: administration to CLI patients of autologous MNCs or stem cells obtained from any other source of MNCs or stem cells, prepared using any other protocol, administered at any other dose, and delivered via any other route.
We did not compare administration of autologous MNCs or stem cells to patients with CLI against no cell therapy, control, standard therapy, or best medical practice because this approach would overlap that used in another Cochrane review (Moazzami 2014).
Types of outcome measures
Primary outcomes
All‐cause mortality
Amputation rate
Wound/ulcer healing as determined by the number of ulcers healed and the change in ulcer size
Secondary outcomes
Reduction in rest pain as assessed by a validated visual analogue scale (VAS) or analgesic requirement (rest pain score)
Improvement in lower limb perfusion as measured by improvement in ankle‐brachial index (ABI)
Improvement in lower limb perfusion as measured by improvement in transcutaneous oxygen tension (TcO₂)
Improvement in ischaemic symptoms as assessed by improvement in pain‐free walking distance (PFWD) and pain‐free walking time (PFWT)
Improvement in vascularity and blood supply to the ischaemic limb as measured by the numbers of newly formed collaterals in the lower limbs
-
Adverse effects and safety
Adverse effects included an inflammatory reaction at the stem cell implantation site (grade I to IV), cardiovascular abnormalities, or thromboembolic complications
Safety was measured as the rate of adverse events and the rate of withdrawal
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) first searched the following databases for relevant trials on 17 February 2017: the Cochrane Vascular Specialised Register; and the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library, via the Cochrane Register of Studies Online. See Appendix 1 for details of the search strategy used to search CENTRAL.
The CIS also searched the following trials databases on 17 February 2017 for details of ongoing and unpublished studies using the terms "(critical ischemia or critical ischaemia)" and "cell": the World Health Organization International Clinical Trials Registry (http://apps.who.int/trialsearch/); ClinicalTrials.gov (clinicaltrials.gov/); and the International Standard Randomized Controlled Trials Number (ISRCTN) Register (isrctn.com/).
The CIS performed a top‐up search of the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and trials registries, on 16 May 2018. See Appendix 2 for details of the search strategies used.
Review authors' searches
The review authors searched PubMed until February 2017 using the strategy shown in Appendix 3.
Searching other resources
To identify further eligible studies, we inspected the reference lists of relevant articles that we had retrieved via the search strategies outlined above and from relevant Cochrane reviews that assessed cell‐based treatments as interventions.
Data collection and analysis
Selection of studies
Two review authors (NAI and MKAAH) independently screened the titles and abstracts of articles retrieved in the first round of the search, aiming to exclude articles that were clearly irrelevant. After completing the initial step of screening, we had obtained a list of articles that appeared to be relevant to our review. Two review authors (SFAW and NAM) independently assessed the short‐listed articles in greater detail by using the abstract and full text to identify eligible articles. In instances of disagreements between review authors on article selection, a third review author (NML) acted as an arbiter.
We accepted published and unpublished studies in full article and abstract forms, as long as assessment of risk of bias was possible and relevant data were available. When required, we would contact authors of unpublished studies and studies available only as abstracts to request further information.
We screened for duplicate publications of the same trial, and we contacted the trial authors for clarification when necessary.
Data extraction and management
Three review authors (SFAW, NAI, and MKAAH) independently extracted and coded all data for each included study using a proforma designed specifically for this review. We extracted the following information from each study: study design, participants, setting, sample size, nature of intervention, comparison, outcomes, methods (unit of allocation and analysis), and results. We screened for duplicate entry of patients, when possible, by matching the initial number of patients recruited against the total number along each step in the conduct of the study. If we discovered a discrepancy (e.g. if the total number in a later stage of the study exceeded the initial number), we attempted to look for an explanation within the article (e.g. multiple enrolment of the same patient at different hospital admissions). We contacted study authors for clarification if necessary. We compared data in duplicate publications against data from all versions to avoid duplicate extraction.
We resolved disagreements among the review authors through discussion leading to a consensus.
Assessment of risk of bias in included studies
Three review authors (NAI, SFAW, and NML) independently assessed each included study using Cochrane's tool for assessing risk of bias to address six specific domains (Higgins 2011).
Sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective outcome reporting.
Other issues (e.g. extreme baseline imbalance).
We made a judgement on each of the criteria above as to whether the study was at high, low, or unclear risk of bias. We assessed blinding for each category of outcomes (objective and subjective) separately when possible. We completed a 'Risk of bias' table for each eligible study and resolved disagreement among review authors through discussion leading to a consensus. We presented an overall assessment of the risk of bias using the 'Risk of bias' graph and the 'Risk of bias' summary.
Measures of treatment effect
For dichotomous data (amputation rate and ulcer healing rate, numbers of patients with improvement in blood flow parameters, and number of limbs with new collaterals), we used risk ratio (RR) to measure outcome estimates on the same scale. For continuous data (rest pain score, ABI score, TcO₂ reading, PFWD, PFWT), we pooled measures at a similar time point using mean difference (MD). If pooled analyses were not possible, we reported results of the studies individually.
Unit of analysis issues
We used each individual patient as our unit of analysis when possible. However, some studies reported their results using the limbs as the unit of analysis without adjusting results to account for non‐independence between two limbs of the same patient. None of the studies reported the number of patients with one or two limbs included in the analysis, making it impossible for us to adjust the results. We therefore reported the results of those studies unadjusted but undertook a sensitivity analysis when we encountered a mixture of studies with patients and limbs as the unit of analysis, to assess the impact of pooled results after exclusion of studies that reported results using the limbs as the unit of analysis.
We did not include cluster‐RCTs and cross‐over studies.
Dealing with missing data
We assessed the dropout rate of each study and determined whether an intention‐to‐treat analysis was performed. To assess whether the dropout rate was worrisome, we inspected event rates for intervention and comparison groups. We then used a 'worst‐case scenario' method for the primary outcomes (Guyatt 1993). For instance, with negatively worded outcomes (such as mortality), for a trial that favoured the intervention group we assumed that all dropouts from the intervention group had developed the outcome, and that all dropouts from the comparison group had not developed the outcome. We then analysed the results to see if such an assumption changed the direction of the results (e.g. from favouring the intervention group to favouring the comparison group). If so, we considered the dropout rate to be worrisome and made a corresponding note in the table that corresponded to characteristics of the study and its accompanying risk of bias assessment table under the heading of 'Incomplete outcome data'. We made the reverse assumption when a trial favoured the comparison group.
Assessment of heterogeneity
We assessed all included studies in terms of their clinical and methodological characteristics, including the following.
Aetiology of the disease.
Baseline characteristics of participants (age, gender, race and ethnicity, comorbidity group).
Nature of the intervention (different regimens of implantation, different types of cells, different preparations and doses of cells implanted).
Types of co‐interventions.
Duration of follow‐up period.
Methodological quality (as detailed in the assessment of risk of bias section, e.g. studies at high risk of bias, which were defined as studies with unclear or no allocation concealment; studies in which participants, caregivers, or investigators were not blinded, or in which blinding was unclear).
We visually inspected the forest plots for any evidence of heterogeneity of treatment effects. We used the I² statistic to measure inconsistency in results (Deeks 2011), with a value greater than 50% indicating substantial statistical heterogeneity. If we found significant statistical heterogeneity but considered the studies suitable for a meta‐analysis based on clinical and methodological characteristics, we then used the random‐effects model to provide the pooled effect estimates.
Assessment of reporting biases
We specifically assessed publication bias in our review using a funnel plot if 10 or more studies were included in the analysis. If publication bias was implied by significant asymmetry of the funnel plot, we would have included a statement in our results with a corresponding note of caution in our discussion.
Data synthesis
We used Review Manager to perform meta‐analysis of the included studies (RevMan 5.3). We used a fixed‐effect model unless we found significant heterogeneity, in which case we employed the strategies as outlined in the previous section on assessment of heterogeneity. For data management, we followed the strategies detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Our primary data analyses followed the intention‐to‐treat principle, namely, we used the original number of participants allocated to each study arm as the denominator in subsequent analyses.
Subgroup analysis and investigation of heterogeneity
Had sufficient data been available, we would have performed the following subgroup analyses for studies describing patients:
with different severity of CLI (e.g. rest pain vs tissue loss);
with different aetiology of CLI (e.g. atherosclerosis obliterans (ASO) vs thromboangiitis obliterans (TAO));
with and without significant comorbidity (e.g. smoking, diabetes, hypercholesterolaemia, hypertension);
with different age groups;
with different genders;
with different races or ethnicities;
injected with cells obtained via different preparation techniques (e.g. fresh vs cultured, non‐selected vs selected);
injected with different doses of cells;
injected with single versus a combination of cell‐based products; and
implanted via different routes;
or when:
the intervention was administered with and without co‐intervention; or
studies were undertaken in patients with different follow‐up periods.
Sensitivity analysis
We performed sensitivity analysis for each outcome that we extracted and pooled from a mixture of studies with patients and limbs as the unit of analysis to assess the impact of the pooled results after exclusion of studies that reported results using the limbs as the unit of analysis.
Had sufficient data been available, we would have performed the following additional sensitivity analyses to assess the impact of excluding studies based on the following criteria.
Significant or worrisome dropout rates, as defined under the heading Dealing with missing data.
Significant methodological issues identified in the assessment of risk of bias. For the purpose of this systematic review, we took the following criteria to indicate a significant risk of bias: studies with unclear or no allocation concealment; and studies in which participants, caregivers, or investigators were not blinded, or in which blinding was unclear.
'Summary of findings'
We presented in 'Summary of findings' (SoF) tables the main findings of this review concerning quality of evidence, magnitude of effects of the interventions examined, and sum of available data on the primary outcomes in the review, namely, all‐cause mortality, amputation rate, and wound/ulcer healing, as well as the major secondary outcomes (i.e. reduction in rest pain and improvement in lower limb perfusion as measured by ABI and TcO₂) (Schünemann 2011), according to Higgins 2011. We used the web‐based GRADEpro software (gdt.guidelinedevelopment.org) to generate the SoF table (Schünemann 2011a). In generating the SoF table, we took the median control group event rate for the outcome as showing 'moderate risk'.
Results
Description of studies
A summary of our search results is shown in Figure 1.
1.
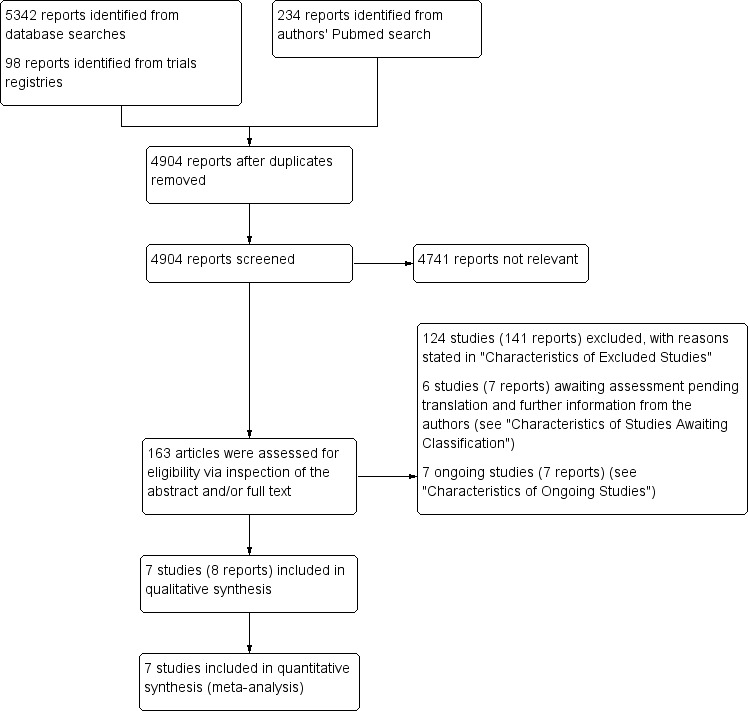
Study flow diagram.
Results of the search
We included in this review seven RCTs from eight published articles (Gu 2008; Huang 2007; Klepanec 2012; Losordo 2012; Lu 2011; Van Tongeren 2008; Zhang 2009). We excluded 124 studies (141 reports) on the basis of study design, characteristics of participants, types of interventions, characteristics of cell treatment, and absence of outcome data (see Characteristics of excluded studies). We identified six studies (seven reports) awaiting classification (Gurunathan 2009; Korymasov 2009; Molavi 2016; NCT00595257; NCT00987363; NCT02993809): three studies are clinical trial protocols without outcome data (NCT00595257; NCT00987363; NCT02993809), one pilot study provided preliminary reporting of outcome data based on small sample size (Molavi 2016), one article provided incomplete reporting of outcome data (Gurunathan 2009), and one article written in the Russian language could not be assessed fully by the time of writing the review, and we are still awaiting its translated full‐text version (Korymasov 2009). (See Characteristics of studies awaiting classification.) We identified seven ongoing studies (seven reports) (NCT00311805; NCT00753025; NCT01257776; NCT01408381; NCT01446055; NCT01745744; NCT02454231. (See Characteristics of ongoing studies.) Recent review articles and meta‐analyses did not yield additional relevant clinical trials. We did not identify new trials by scanning the reference lists of included clinical trials.
Included studies
Characteristics of included studies
We included seven RCTs that were conducted in four countries, including China (four studies), USA, Netherlands, and Slovakia (one study each). Six studies were single‐centre RCTs, and one was a multi‐centre RCT (see Characteristics of included studies).
Characteristics of participants
The included studies were conducted between 2003 and 2010 and enrolled a total of 359 participants. The number of participants recruited in the individual RCTs ranged from 16 in Losordo 2012 to 150 in Huang 2007. The mean age of participants ranged from 61.8 years to 69.8 years. Atherosclerosis obliterans and thromboangiitis obliterans were the two most common aetiologies of CLI. Most studies reported the baseline demographics of participants, including age and gender, comorbidities (diabetes mellitus, renal failure, cerebral and coronary artery disease), and severity of limb ischaemia as comparable between groups. One study recruited only diabetic patients (Lu 2011). In two studies, researchers used each of the two limbs as one experimental arm, resulting in administration of the two interventions to two different limbs of the same patient (Lu 2011). Median follow‐up ranged from one to twelve months after cell implantation.
Characteristics of cell treatment
The included RCTs used essentially two sources of autologous stem cells: bone marrow and peripheral blood. Five of the seven included RCTs used mononuclear cells (MNCs) harvested and separated manually from bone marrow by density gradient centrifugation on Ficoll‐Hypaque and implanted into affected limbs without prior manipulation; study authors denoted these as BM‐MNCs. Three RCTs used progenitor cells isolated from patients' peripheral blood via leukapheresis following administration of 5 μg/kg/d of G‐CSF for four or five days (termed 'mobilised PBSCs', or 'mPBSCs') for implantation (Huang 2007; Losordo 2012; Zhang 2009). One RCT further enriched the leukapheresis product (mPBSC) for CD34+ cells using a magnetic cell selection system before implantation of CD34+ cells (Losordo 2012). Bone marrow cells were cultured to generate mesenchymal stem cells (MSCs), and one RCT used these cells (BM‐MSCs) (Lu 2011).
One RCT evaluated the effect of cell dose on clinical outcomes. In this study, researchers randomised patients and treated them with 1 × 10⁶ (high dose) and 1 × 10⁵ (low dose) autologous CD34+ cells/kg (Losordo 2012). No standard definition of high versus low cell dose is provided in the literature. However, we considered a standard definition not critical for determining clinical outcomes in the present review because only a single RCT performed this comparison, and pooled analysis was not feasible.
Intramuscular (IM) cell implantation was the route most commonly employed. Other methods of cell administration were intra‐arterial (IA) ‐ in Klepanec 2012 ‐ and combined IM plus IA ‐ in Van Tongeren 2008. IM cell implantation was usually performed via multiple injections into the gastrocnemius muscle, and IA cell infusion was usually done through the femoral artery of the affected lower limb.
Trials provided four major categories of interventions.
Comparison between BM‐MNCs (104 participants) and mPBSCs (98 participants) in two RCTs (Huang 2007; Zhang 2009).
Comparison between BM‐MNCs (20 participants) and BM‐MSCs (21 participants) in one RCT (Lu 2011).
Comparison between high cell dose (9 participants) and low cell dose (7 participants) implanted into the affected limb in one RCT (Losordo 2012).
Comparison between IM (52 participants) and IA or IA plus IM (48 participants) cell implantation in three RCTs (Gu 2008; Klepanec 2012; Van Tongeren 2008).
Assessment of outcomes
For efficacy analysis, the participants in the seven included RCTs were pooled in groups according to source, type, dose, and route of delivery of MNCs/stem cells. We extracted the outcome data from individual RCTs and incorporated them into the meta‐analysis according to the following groups: BM‐MNCs versus mPBSCs, BM‐MNCs versus BM‐MSCs, high cell dose versus low cell dose, and IM implantation route versus IA infusion route.
Most included RCTs assessed four efficacy outcomes almost exclusively, including amputation rate (six studies), rest pain (six studies), ulcer healing (three studies), and ABI (six studies).
All RCTs assessed subjective symptoms (rest pain) and objective surrogate indexes of blood flow in the lower limbs (wound healing, ABI, TcO₂, PFWD, PFWT) before cell implantation and regularly thereafter, ranging from two weeks to twelve months. The researchers determined the number and nature of amputations at the end of the study. They determined wound or ulcer healing weekly by (1) ulcer healing rate (number of participants whose ulcers healed divided by the total number of participants with ulcers in a particular group) and (2) changes from baseline in ulcer size and area as measured by grid maps, acetate tracings, and digital planimetry. Trialists assessed rest pain using two types of numerical rating pain scales ranging from 0 (completely resolved without analgesics) to 4 points (severe pain unresolved with analgesics) (Lu 2011), or from 1 (least pain) to 10 points (greatest pain) on a visual analogue scale (VAS). They measured resting ABI using a laser Doppler at room temperature according to standard protocol. Study authors measured TcO₂ with a TCM400 Mk2 monitor (Klepanec 2012), and they assessed walking capacity reflected by a change in walking distance (metres), walking duration (minutes), and walking speed (miles per hour or mph) at a constant speed on a treadmill, or at the same ground with no inclination. Investigators determined collateral vessel formation of the lower limbs using magnetic resonance angiography (MRA) (Lu 2011), or via subtraction angiography (Klepanec 2012), before and at three to six months after cell implantation. They assessed angiographic scores of blood vessel images as +0 (no collateral formation), +2 (moderate collateral circulation), and +3 (abundant collateral circulation) (Drescher 2006; Klepanec 2012). They paid specific attention to detecting potential adverse effects resulting from cell harvesting and implantation during follow‐up visits.
Excluded studies
In total, we excluded 141 reports of 124 studies on the basis of study design, patient characteristics, types of interventions, characteristics of cell treatment, and incomplete outcome data (see Characteristics of excluded studies).
Forty RCTs investigated effects of cell‐based treatment versus standard medical therapy (SMT), including BM‐MNCs versus SMT (Amann 2008; Arai 2006; Barć 2006; Benoit 2011; Dou 2015; Gu 2017;Guo 2018; Iafrati 2016; Li 2013; NCT00539266; NCT01049919; NCT01245335; Peeters 2016; Pignon 2017; Prochazka 2010; Tateishi‐Yuyama 2002; Teraa 2014; Teraa 2015; Walter 2011; Zhou 2017), BM‐MSCs versus SMT (Dash 2009; Debin 2008), concentrated bone marrow aspirate versus SMT (Murphy 2017; NCT00434616; Wang 2017), peripheral blood‐derived stem cell therapy versus SMT (Doudar 2013; Huang 2005; Mohammadzadeh 2013; NCT00922389; Niven 2017;Ohtake 2017; Ozturk 2012; Szabo 2013), Ixmyelocel‐T versus SMT (Powell 2012), Rexmyelocel‐T versus SMT (NCT03174522), CD133+ cells versus SMT (Zhang 2016), aldehyde dehydrogenase (ALDH) bright cells versus SMT (Perin 2017), vascular endothelial growth factor (VEGF) 165 gene‐modified PB CD34+ cells versus SMT (Zhou 2017a), granulocyte macrophage‐stimulating factor (GM‐CSF) versus placebo (NCT03304821), and angiogenic cell precursors (ACPs) versus SMT (NCT01584986).
Forty‐two studies were single‐arm studies without a comparator/control arm. These studies investigated the safety and efficacy of bone marrow cells (Chochola 2008; Cobellis 2010; Duong 2008; Franz 2011; Gabr 2011; Heo 2016;Higashi 2004; Iso 2010; Kolvenbach 2010; Kondo 2018;Maione 2013; Malyar 2014; Matoba 2009; Motukuru 2008; Murphy 2011; NCT00306085; Nizankowski 2005; Ponemone 2017; Ruiz‐Salmeron 2011; Saito 2007; Schiavetta 2012; Wester 2008; Yanishi 2017), PBSCs (Hoshino 2007; Ishida 2005; Kawamoto 2009; Kinoshita 2012; Lara‐Hernandez 2010; Madaric 2016), PB‐MNCs (Amato 2012; Moriya 2009), adipose tissue stem cells (Bura 2014; Darinskas 2017;Kondo 2016; Lee 2012), BM‐MSCs plus BM‐MNCs (Lasala 2010), BM‐MSCs (Mohamed 2017), peripheral blood CD133+ cells (Arici 2015; Burt 2010), circulating blood‐derived progenitor cells (Frogel 2017; Lenk 2005), and venous endothelial and smooth muscle cells, called MultiGeneAngio (MGA) (Grossman 2016).
Eleven studies were comparative non‐randomised studies. These studies investigated the effects of BM‐MNCs versus mPBSCs (Dubsky 2013; Gu 2007; Kamata 2007; Matsui 2003), BM‐MNCs versus control (Bartsch 2007; Cobellis 2008; Idei 2011; Napoli 2008), PB‐MNCs versus control (De Angelis 2014), circulating blood‐derived progenitor cells versus control (Nemcova 2017), and low versus high cell dose used for implantation (Gu 2006).
In three studies, the study populations were not CLI patients (Bing 2009; Holzinger 1994; Subramaniyam 2009).
Nine studies used allogeneic mesenchymal stem cells (MSCs) instead of autologous MSCs (Das 2013; Du 2017;Gupta 2013; Gupta 2017; Majumdar 2015;NCT02336646; NCT03339973; Wang 2018;Wijnand 2018).
In one study, the agent used in the intervention group was not a cell‐based product (Rajagopalan 2003).
Three studies investigated the therapeutic effects of growth factors (GM‐CSF and G‐CSF) instead of cell‐based products (Choi 2012; Poole 2013; Zafarghandi 2010).
Two studies compared cell treatment versus treatment with non‐cell‐based products (Takagi 2011; Wang 2014).
Two studies investigated specialised cell‐based products derived via specific isolation and culture methods (Kirana 2012; Perin 2011). Significant variation in the final product injected into participants precluded inclusion of these studies in our systematic review.
Three studies co‐administered two different sources of autologous stem cells into the affected limb: BM‐MNCs combined with mPBSCs (Zhao 2008), BM‐MSCs with BM‐MNCs versus BM‐MNCs alone (Harunarashid 2016), and venous endothelial cells (ECs) combined with venous smooth muscle cells (SMCs) (Flugelman 2017).
One study compared the clinical outcomes of CLI patients treated with one type of stem cell (BM‐MNC) that was separated via two different methods (Hernandez 2007).
One study compared the clinical outcomes of CLI patients treated with BM‐MNCs versus mPBSCs by performing a pooled analysis using data from two previous cohort studies (Onodera 2011).
Two studies investigated the count and phenotype of cells used for implantation and did not study the clinical outcomes after cell implantation (Capiod 2009; Smadja 2012).
One study investigated the thrombogenicity of the transplanted cell and did not study clinical outcomes after cell implantation (Tournois 2015).
One study investigated the number of endothelial progenitor cells (EPCs) in PAD patients and did not study clinical outcomes after cell implantation (Afan 2015).
One study investigated the level of asymmetric dimethylarginine (ADMA) and changes in oxidative stress in patients with CLI after BM‐MNC therapy and did not study clinical outcomes of cell treatment (Madaric 2017).
One study investigated the safety and efficacy of hyaluronic acid (HA) combined with BM‐MNCs for patients with PAD (NCT03214887).
Risk of bias in included studies
2.
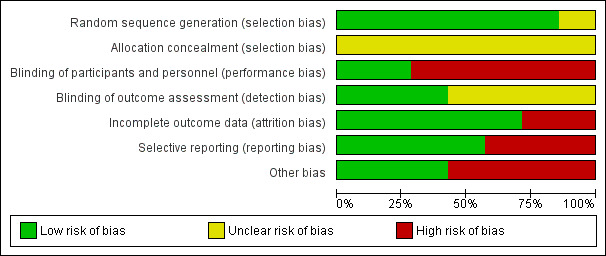
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
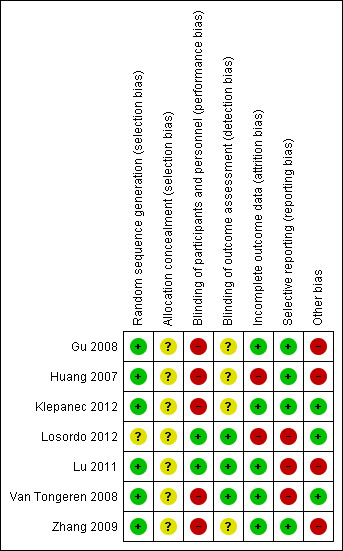
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged six studies as having low risk of bias for random sequence generation (Gu 2008; Huang 2007; Klepanec 2012; Lu 2011; Van Tongeren 2008; Zhang 2009). One RCT had unclear risk of bias for random sequence generation, as study authors did not explicitly state the method of sequence generation used (Losordo 2012). We graded allocation concealment as causing unclear risk in all studies because papers provided insufficient information.
Blinding
Five studies had high risk of performance bias. In two RCTs, both physicians and patients were unblinded to the different techniques used in autologous cell collection (Huang 2007; Zhang 2009), and in three studies, both physicians and patients were unblinded to the different routes of cell implantation employed (Gu 2008; Klepanec 2012; Van Tongeren 2008). We deemed the remaining two included studies to have low risk of performance bias (Losordo 2012; Lu 2011).
Four studies had unclear detection bias, as study authors did not mention whether outcome assessors were blinded to patients' assigned groups (Gu 2008; Huang 2007; Klepanec 2012; Zhang 2009). We deemed the remaining three included studies to have low risk of detection bias (Losordo 2012; Lu 2011; Van Tongeren 2008).
Incomplete outcome data
One study reported that six participants (two in one group and four in another) were excluded before the implantation and 10 participants (four in one group and six in another) discontinued after amputation (Huang 2007). Another study reported that eight participants (28.6%) did not complete the 12‐month study period (Losordo 2012). We judged both studies to have high risk of attrition bias owing to unequal numbers of dropouts between groups and high overall dropout rates. The remaining studies had low risk of bias in this domain.
Selective reporting
We judged three studies as having high risk of reporting bias because study authors provided incomplete outcome information (Losordo 2012; Lu 2011; Van Tongeren 2008); we considered the remaining studies to have low risk of reporting bias.
Other potential sources of bias
We screened for other potential sources of bias including extreme baseline imbalance and unit of analysis issues. We identified four studies with unit of analysis issues (Gu 2008; Huang 2007; Lu 2011; Zhang 2009). We judged the remaining included studies to have low risk of other potential sources of bias. Cell doses for MNCs and stem cells cannot be equated, as < 0.01% of MNCs consisted of stem cells. Therefore, an analysis of cell doses would have been intrinsically biased.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. BM‐MNCs compared to mPBSCs for critical lower limb ischaemia.
| Is BM‐MNC implantation more effective than mPBSC implantation for reducing all‐cause mortality, amputation rate, number of participants with any reduction in rest pain score, and number of healing ulcers and for improving lower limb perfusion in people with critical lower limb ischaemia? | ||||||
|
Patient or population: people with critical lower limb ischaemia Setting: hospital Intervention: BM‐MNCs Comparison: mPBSCs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with mPBSCs | Risk with BM‐MNCs | |||||
| All‐cause mortality Assessed during in‐patient stay |
See comments. | See comments. | Not estimable | 202 (2 RCTs) |
See comments. | No deaths were reported by the 2 studies in this comparison (Huang 2007; Zhang 2009). |
| Amputation rate Assessed during in‐patient stay |
Study population | RR 1.54 (0.45 to 5.24) |
150 (1 RCT) |
⊕⊕⊝⊝ LOWa,b |
||
| 53 per 1000 | 81 per 1000 (24 to 276) | |||||
| Moderate** | ||||||
| 53 per 1000 | 81 per 1000 (24 to 276) |
|||||
| Wound/ulcer healing: number of participants with healing ulcers Assessed during in‐patient stay |
Study population | RR 0.89 (0.44 to 1.83) |
49 (1 RCT) |
⊕⊕⊝⊝ LOWa,b |
||
| 407 per 1000 | 363 per 1000 (179 to 746) |
|||||
| Moderate** | ||||||
| 407 per 1000 | 363 per 1000 (179 to 746) |
|||||
| Reduction in rest pain: number of participants with any reduction in rest pain score Assessed during in‐patient stay |
Study population | RR 0.99 (0.93 to 1.06) | 104 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ||
| 977 per 1000 | 968 per 1000 (909 to 1000) |
|||||
| Moderate** | ||||||
| 977 per 1000 | 968 per 1000 (909 to 1000) | |||||
| Improvement in lower limb perfusion: number of participants with increased ABI Assessed at 4 weeks |
Study population | RR 1.00 (0.71 to 1.40) | 104 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | ||
| 568 per 1000 | 568 per 1000 (403 to 795) | |||||
| Moderate** | ||||||
| 568 per 1000 | 568 per 1000 (369 to 744) | |||||
| Improvement in lower limb perfusion: TcO₂ reading in mmHg | Mean TcO₂ reading in mmHg was 4.68. | Mean TcO₂ reading in the intervention group was 1.7 mmHg more (0.41 more to 2.99 more). | ‐ | 150 (1 RCT) |
⊕⊕⊝⊝ LOWa,b | The study included in this comparison reported the TcO₂ reading in mmHg, not the number of participants with improvement in lower limb perfusion, which was our preferred outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** We took the median control group event rate for the outcome as "moderate risk". ABI: ankle‐brachial index; BM‐MNCs: bone marrow‐mononuclear cells; CI: confidence interval; mPBSCs: mobilised peripheral blood stem cells; RCT: randomised controlled trial; RR: risk ratio; TcO₂: transcutaneous oxygen tension. | ||||||
|
GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aThe single included study had unclear risk of selection bias and high risk of performance bias (patients and personnel were not blinded). Quality of evidence was downgraded by one level. bThe 95% CI is wide, as it ranges from substantial benefits favouring BM‐MNCs to substantial benefits favouring mPBSCs. Quality of evidence was downgraded by one level.
Summary of findings 2. BM‐MNCs compared to BM‐MSCs for critical lower limb ischaemia.
| Is BM‐MNC implantation more effective than BM‐MSC implantation for reducing all‐cause mortality, amputation rate, number of participants with any reduction in rest pain score, and number of healing ulcers and for improving lower limb perfusion in patients with critical lower limb ischaemia? | ||||||
| Patient or population: people with critical lower limb ischaemia Setting: hospital Intervention: BM‐MNCs Comparison: BM‐MSCs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with BM‐MSCs | Risk with BM‐MNCs | |||||
| All‐cause mortality Assessed during in‐patient stay |
See comments. | See comments. | Not estimable | 37 (1 RCT) | See comments. | No deaths were reported by the single study included in this comparison (Lu 2011). |
| Amputation rate Assessed during in‐patient stay |
See comments. | See comments. | Not estimable | 37 (1 RCT) | See comments. | No amputations were reported by the single study included in this comparison (Lu 2011). |
| Wound/ulcer healing: number of participants with healing ulcers | Study population | RR 2.00 (1.02 to 3.92) | 22 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ||
| 455 per 1000 | 909 per 1000 (464 to 1000) | |||||
| Moderate** | ||||||
| 455 per 1000 | 909 per 1000 (464 to 1000) | |||||
| Reduction in rest pain: rest pain score | Mean rest pain score was 1.4. | Mean rest pain score in the intervention group was similar (0) (0.61 fewer to 0.61 more). | ‐ | 37 (1 RCT) | ⊕⊕⊕⊝ MODERATEb | The study included in this comparison reported the rest pain score, not the number of participants with improved rest pain, which was our preferred outcome. |
| Improvement in lower limb perfusion: ABI score | Mean ABI score was 0.12. | Mean ABI score in the intervention group was 0.05 more (0.01 more to 0.09 more). | ‐ | 37 (1 RCT) | ⊕⊕⊝⊝ LOWc,d | The study included in this comparison reported the ABI score, not the number of participants with improvement in lower limb perfusion, which was our preferred outcome. |
| Improvement in lower limb perfusion: TcO₂ reading in mmHg | Mean TcO₂ reading in mmHg was 16.4. | Mean TcO₂ reading in the intervention group was 8 mmHg more (3.46 more to 12.54 more). | ‐ | 37 (1 RCT) | ⊕⊕⊝⊝ LOWd,e | The study included in this comparison reported the TcO₂ reading in mmHg, not the number of participants with improvement in lower limb perfusion, which was our preferred outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** We took the median control group event rate for the outcome as "moderate risk". ABI: ankle‐brachial index; BM‐MNCs: bone marrow mononuclear cells; BM‐MSCs: bone marrow mesenchymal stem cells; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; TcO₂: transcutaneous oxygen tension. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aThe effect estimate of a single small study ranges from slight benefit to substantial benefit favouring BM‐MSCs. Quality of evidence was downgraded by one level. bThe effect estimate of a single small study ranges from substantial benefit favouring BM‐MNCs to substantial benefit favouring BM‐MSCs. Quality of evidence was downgraded by one level. cABI score is not a clinical outcome, and its correlation with clinical symptoms and functions is unclear. Quality of evidence was downgraded by one level. dThe effect estimate of a single small study ranges from slight to substantial benefit for the intervention group in terms of the outcome measured (ABI score and TcO₂ reading, respectively). Quality of evidence was downgraded by one level for each outcome concerned. eTcO₂ reading is not a clinical outcome, and its correlation with clinical symptoms and functions is unclear. Quality of evidence was downgraded by one level.
Summary of findings 3. High cell dose compared to low cell dose for critical lower limb ischaemia.
| Is high cell dose more effective than low cell dose for reducing all‐cause mortality, amputation rate, number of participants with any reduction in rest pain score, and number of healing ulcers and for improving lower limb perfusion in patients with critical lower limb ischaemia? | ||||||
| Patient or population: people with critical lower limb ischaemia Setting: hospital Intervention: high cell dose Comparison: low cell dose | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with low cell dose | Risk with high cell dose | |||||
| All‐cause mortality Assessed during in‐patient stay |
See comments. | See comments. | Not estimable | 16 (1 RCT) | See comments. | No deaths were reported by the single RCT included in this comparison (Losordo 2012). |
| Amputation rate Assessed during in‐patient stay (Losordo 2012) |
Study population | RR 3.21 (0.87 to 11.90) | 16 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | ||
| 222 per 1000 | 713 per 1000 (193 to 1000) | |||||
| Moderate** | ||||||
| 222 per 1000 | 713 per 1000 (193 to 1000) | |||||
| Wound ulcer healing | See comments. | See comments. | See comments. | See comments. | See comments. | This outcome was not reported by the single RCT included in this comparison (Losordo 2012). |
| Reduction in rest pain | See comments. | See comments. | See comments. | See comments. | See comments. | This outcome was not reported by the single RCT included in this comparison (Losordo 2012). |
| Improvement in lower limb perfusion: ABI | See comments. | See comments. | See comments. | See comments. | See comments. | This outcome was not reported by the single RCT included in this comparison (Losordo 2012). |
| Improvement in lower limb perfusion: TcO₂ | See comments. | See comments. | See comments. | See comments. | See comments. | This outcome was not reported by the single RCT included in this comparison (Losordo 2012). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** We took the median control group event rate for the outcome as "moderate risk". ABI: ankle‐brachial index; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; TcO₂: transcutaneous oxygen tension. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aThe 95% CI of the effect estimate is extremely wide, covering both sides of the no‐effect line. Quality of evidence was downgraded by two levels on the basis of imprecision. bThe single included study had unclear risk of selection bias and high risk of selection bias and unclear risk of performance and detection biases, attrition bias, and reporting bias. Quality of evidence was downgraded by one level.
Summary of findings 4. IM injection compared to IA injection for critical lower limb ischaemia.
| Is IM cell implantation more effective than IA cell implantation for reducing all‐cause mortality, amputation rate, number of participants with any reduction in rest pain score, and number of healing ulcers and for improving lower limb perfusion in patients with critical lower limb ischaemia? | ||||||
| Patient or population: people with critical lower limb ischaemia Setting: hospital Intervention: IM injection Comparison: IA injection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with IA injection | Risk with IM injection | |||||
| All‐cause mortality Assessed during in‐patient stay |
See comments. | See comments. | See comments. | 95 (3 RCTs) | See comments. | Three RCTs with a total of 100 participants reported 9 deaths (Gu 2008; Klepanec 2012; Van Tongeren 2008). None of the 3 studies reported all‐cause mortality according to treatment group. |
| Amputation rate Assessed during in‐patient stay |
Study population | RR 0.80 (0.54 to 1.18) | 95 (3 RCTs) | ⊕⊕⊕⊝ LOWa,b | ||
| 500 per 1000 | 400 per 1000 (270 to 590) | |||||
| Moderate** | ||||||
| 636 per 1000 | 509 per 1000 (344 to 751) | |||||
| Wound/ulcer healing: number of participants with healing ulcer | Study population | RR 1.13 (0.73 to 1.76) | 41 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | ||
| 619 per 1000 | 700 per 1000 (452 to 1000) | |||||
| Moderate** | ||||||
| 619 per 1000 | 699 per 1000 (452 to 1000) | |||||
| Reduction in rest pain: number of participants with reduction in rest pain score | Study population | RR 1.22 (0.91 to 1.64) | 32 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | ||
| 765 per 1000 | 933 per 1000 (696 to 1000) | |||||
| Moderate** | ||||||
| 765 per 1000 | 933 per 1000 (696 to 1000) | |||||
| Improvement in lower limb perfusion: number of participants with increased ABI | Study population | RR 0.93 (0.43 to 2.00) | 35 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c,d | ||
| 444 per 1000 | 413 per 1000 (191 to 889) | |||||
| Moderate** | ||||||
| 444 per 1000 | 41 3 per 1000 (191 to 889) |
|||||
| Improvement in lower limb perfusion: number of participants with improved TcO₂ reading | Study population | RR 1.22 (0.86 to 1.72) | 62 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWb,e,f | ||
| 613 per 1000 | 748 per 1000 (527 to 1000) | |||||
| Moderate** | ||||||
| 603 per 1000 | 735 per 1000 (518 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** We took the median control group event rate for the outcome as "moderate risk". ABI: ankle‐brachial index; CI: confidence interval; IA: intra‐arterial; IM: intramuscular; RCT: randomised controlled trial; RR: risk ratio; TcO₂: transcutaneous oxygen tension. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aAll three included studies had unclear risk of selection bias and high risk of performance biases (participants and personnel were not blinded). Quality of evidence was downgraded by one level. bThe 95% CI for the effect estimate from a single small study ranges from an effect size that clearly favours IM injection to an effect size that clearly favours IA injection. Quality of evidence was downgraded by one level on the basis of imprecision. cThe single included study had unclear risk of selection bias and high risk of performance bias (participants and personnel were not blinded). Quality of evidence was downgraded by one level. dAn improvement in ABI might not correlate strongly with an improvement in clinical symptoms and functions. Quality of evidence was downgraded by one level. eThe two included studies had unclear risk of selection bias and high risk of performance bias (participants and personnel were not blinded). Quality of evidence was downgraded by one level. fAn improvement in TcPO₂ reading might not correlate with an improvement in clinical symptoms and functions. Quality of evidence was downgraded by one level.
Primary outcomes
1. All‐cause mortality
Three of the nine included RCTs involving a total of 100 CLI participants reported a total of nine deaths (9%) during the study follow‐up period (Gu 2008; Klepanec 2012; Van Tongeren 2008). None of the included studies reported all‐cause mortality according to treatment group. Causes of death were heart failure in four participants (Gu 2008; Klepanec 2012), myocardial infarction in two participants (Klepanec 2012; Van Tongeren 2008), and respiratory tract infection in three participants (Klepanec 2012; Van Tongeren 2008).
2. Amputation rate
Comparison 1: BM‐MNCs vs mPBSCs
A single study in this comparison showed no clear difference in amputation rates between the two groups (risk ratio (RR) 1.54, 95% confidence interval (CI) 0.45 to 5.24; 150 participants; low‐quality evidence) (Analysis 1.1) (Huang 2007).
1.1. Analysis.
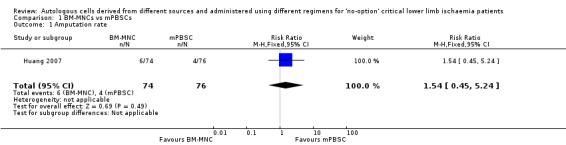
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 1 Amputation rate.
Comparison 2: BM‐MNCs vs BM‐MSCs
Only a single study (37 participants) performed this comparison, reporting zero cases of amputation in both groups (Analysis 2.1) (Lu 2011).
2.1. Analysis.
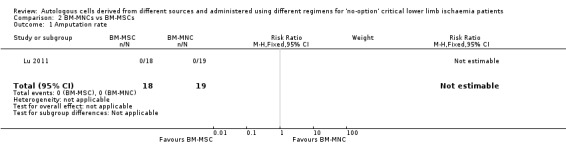
Comparison 2 BM‐MNCs vs BM‐MSCs, Outcome 1 Amputation rate.
Comparison 3: high cell dose vs low cell dose
The only study in this comparison showed no clear difference in amputation rates between high cell dose and low cell dose groups (RR 3.21, 95% CI 0.87 to 11.90; 16 participants; very low‐quality evidence) (Analysis 3.1) (Losordo 2012).
3.1. Analysis.
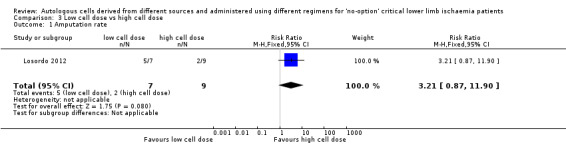
Comparison 3 Low cell dose vs high cell dose, Outcome 1 Amputation rate.
Comparison 4: route of implantation: IM vs IA
Three studies involving a total of 95 CLI participants showed no clear difference in amputation rates between the different routes of cell implantation (RR 0.80, 95% CI 0.54 to 1.18; 95 participants; I² = 48%; low‐quality evidence) (Analysis 4.1) (Gu 2008; Klepanec 2012; Van Tongeren 2008).
4.1. Analysis.
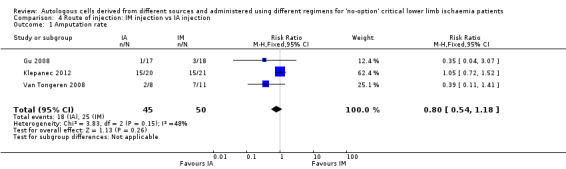
Comparison 4 Route of injection: IM injection vs IA injection, Outcome 1 Amputation rate.
3. Wound/ulcer healing
Included studies reported wound/ulcer healing as the number of participants with improvement or healing of ulcers or change in ulcer size.
Comparison 1: BM‐MNCs vs mPBSCs
The single study in this comparison showed no clear difference in numbers of participants with healing ulcers between the two treatment groups (RR 0.89, 95% CI 0.44 to 1.83; 49 participants; low‐quality evidence) (Analysis 1.2) (Huang 2007).
1.2. Analysis.
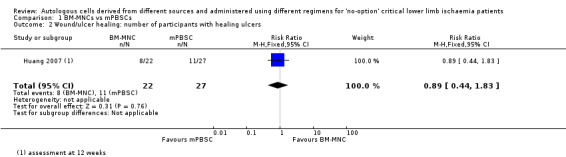
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 2 Wound/ulcer healing: number of participants with healing ulcers.
For the change in ulcer size outcome, Huang 2007 reported no clear change between the two treatment groups (mean difference (MD) ‐1.06 cm², 95% CI ‐5.35 to 3.23; 49 participants) (Analysis 1.3).
1.3. Analysis.
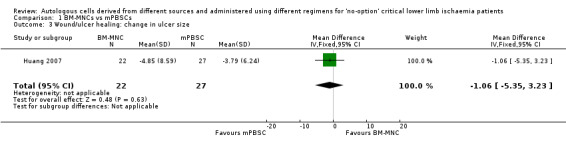
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 3 Wound/ulcer healing: change in ulcer size.
Comparison 2: BM‐MNCs vs BM‐MSCs
The only study in this comparison showed that the number of participants with healing ulcers was higher in the BM‐MSC group than in the BM‐MNC group (RR 2.00, 95% CI 1.02 to 3.92; 22 participants; P = 0.04; moderate‐quality evidence) (Analysis 2.2) (Lu 2011).
2.2. Analysis.
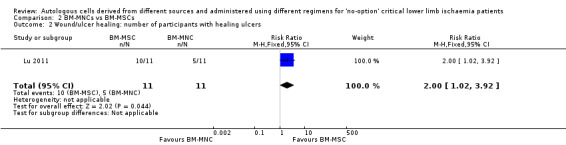
Comparison 2 BM‐MNCs vs BM‐MSCs, Outcome 2 Wound/ulcer healing: number of participants with healing ulcers.
Comparison 3: high cell dose vs low cell dose
The single study in this comparison did not report on this outcome (Losordo 2012).
Comparison 4: route of implantation: IM vs IA
A single included study showed no clear difference in the numbers of participants with healing ulcers between IM and IA cell implantation (RR 1.13, 95% CI 0.73 to 1.76; 41 participants; low‐quality evidence) (Analysis 4.2) (Klepanec 2012).
4.2. Analysis.
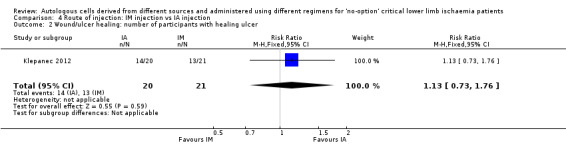
Comparison 4 Route of injection: IM injection vs IA injection, Outcome 2 Wound/ulcer healing: number of participants with healing ulcer.
Secondary outcomes
1. Reduction in rest pain
Reduction in rest pain is reported either as the number of participants with reduction in rest pain score or as a mean reduction in rest pain score.
Comparison 1: BM‐MNCs vs mPBSCs
Two studies involving a total of 202 CLI participants reported rest pain score of any magnitude at three to six months from baseline for participants treated with either BM‐MNCs or mPBSCs (Huang 2007; Zhang 2009). We could not pool data obtained from Huang 2007 with data from Zhang 2009 because Huang 2007 reported rest pain data as mean reduction in rest pain score, but Zhang 2009 reported data as the number of participants with reduction in rest pain. Zhang 2009 showed no clear difference in pain relief between the two treatment groups (RR 0.99, 95% CI 0.93 to 1.06; 104 participants; moderate‐quality evidence) (Analysis 1.4). In contrast, Huang 2007 showed a reduction in rest pain score 12 weeks after mPBSC injection (MD ‐0.57, 95% CI ‐0.90 to ‐0.24; 150 participants) (Analysis 1.5).
1.4. Analysis.
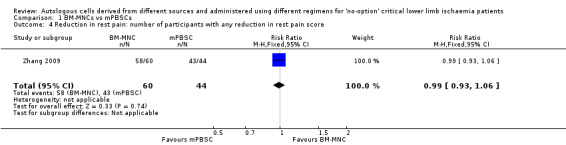
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 4 Reduction in rest pain: number of participants with any reduction in rest pain score.
1.5. Analysis.
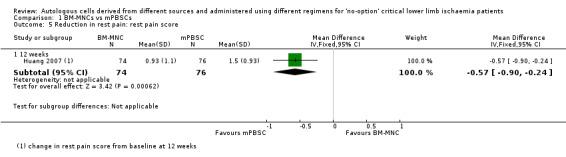
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 5 Reduction in rest pain: rest pain score.
Comparison 2: BM‐MNCs vs BM‐MSCs
The only study in this comparison did not show a difference in pain relief in ischaemic limbs between the two groups (MD 0.00, 95% CI ‐0.61 to 0.61; 37 participants; moderate‐quality evidence) (Analysis 2.3) (Lu 2011).
2.3. Analysis.
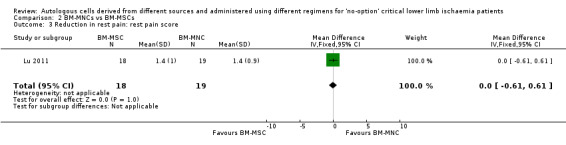
Comparison 2 BM‐MNCs vs BM‐MSCs, Outcome 3 Reduction in rest pain: rest pain score.
Comparison 3: high cell dose vs low cell dose
Losordo 2012 did not report on this outcome.
Comparison 4: route of implantation: IM vs IA
A single included study showed no difference in the numbers of participants with reduction in rest pain between IA and IM routes of implantation (RR 1.22, 95% CI 0.91 to 1.64; 32 participants; low‐quality evidence) (Analysis 4.3) (Gu 2008).
4.3. Analysis.
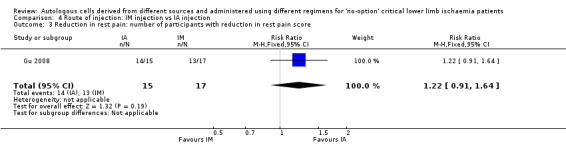
Comparison 4 Route of injection: IM injection vs IA injection, Outcome 3 Reduction in rest pain: number of participants with reduction in rest pain score.
2. Improvement in lower limb perfusion as measured by improvement in ankle‐brachial pressure index (ABI)
Dormandy 2000 defined improvement in ABI as an increase in ABI values greater than 0.1 in the treated limb from baseline value according to the standard assessment of interventional therapy for PAD. Pooled estimates of ABI values extracted from individual RCTs are presented as RRs of the number of participants with improvement in ABI or MD of the ABI score after intervention from baseline.
Comparison 1: BM‐MNCs vs mPBSCs
Two studies reported change in ABI at 4 and 12 weeks from baseline in 202 participants treated with either BM‐MNCs or mPBSCs (Huang 2007; Zhang 2009). Pooled analysis was not feasible owing to differences in units used to report a change in ABI in these studies. Huang 2007 showed that improvement in ABI was better in the mPBSC group than in the BM‐MNC group (MD ‐0.06, 95% CI ‐0.09 to ‐0.03; 150 participants; Analysis 1.7), and Zhang 2009 showed no improvement in ABI between the two treatment groups (RR 1.00, 95% CI 0.71 to 1.40; 104 participants; moderate‐quality evidence; Analysis 1.6).
1.7. Analysis.
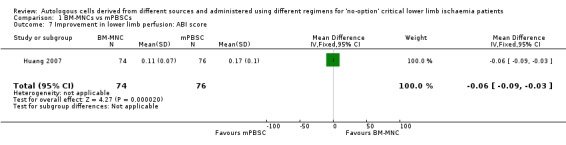
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 7 Improvement in lower limb perfusion: ABI score.
1.6. Analysis.
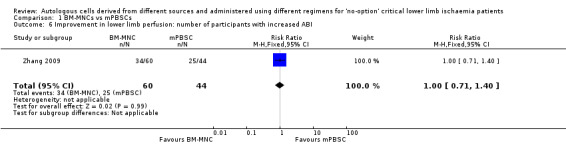
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 6 Improvement in lower limb perfusion: number of participants with increased ABI.
Comparison 2: BM‐MNCs vs BM‐MSCs
Only a single study performed this comparison. Lu 2011 showed that participants who received BM‐MSCs had higher ABI than those given BM‐MNCs (MD 0.05, 95% CI 0.01 to 0.09; 37 participants; low‐quality evidence) (Analysis 2.4).
2.4. Analysis.
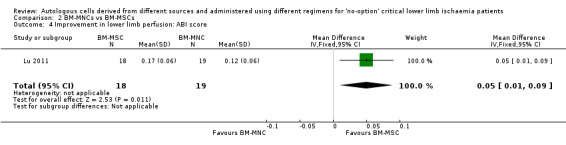
Comparison 2 BM‐MNCs vs BM‐MSCs, Outcome 4 Improvement in lower limb perfusion: ABI score.
Comparison 3: high cell dose vs low cell dose
Losordo 2012 did not report on this outcome.
Comparison 4: route of implantation: IM vs IA
Gu 2008 did not show a clear difference in the number of participants with improvement in ABI following treatment via the IA or IM route of implantation (RR 0.93, 95% CI 0.43 to 2.00; 35 participants; very low‐quality evidence) (Analysis 4.4).
4.4. Analysis.
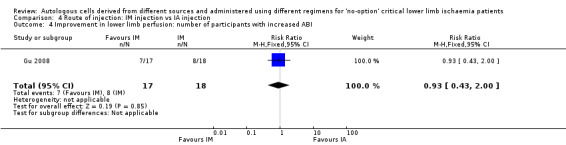
Comparison 4 Route of injection: IM injection vs IA injection, Outcome 4 Improvement in lower limb perfusion: number of participants with increased ABI.
Klepanec 2012, who reported ABI score, did not show a clear difference in ABI scores between different routes of implantation (MD ‐0.17, 95% CI ‐0.37 to 0.03; 27 participants) (Analysis 4.5).
4.5. Analysis.
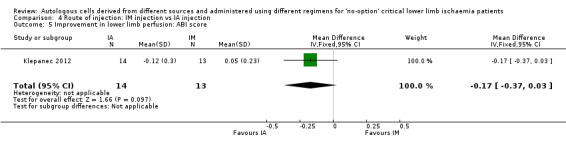
Comparison 4 Route of injection: IM injection vs IA injection, Outcome 5 Improvement in lower limb perfusion: ABI score.
3. Improvement in lower limb perfusion as measured by improvement in transcutaneous oxygen tension (TcO₂)
We have presented pooled estimates of TcO₂ values extracted from individual RCTs as RR of the number of participants with improvement in TcO₂ readings or mean difference (MD) in the TcO₂ reading from baseline.
Comparison 1: BM‐MNCs vs mPBSCs
One study involving a total of 150 CLI participants reported an increase in TcO₂ readings in the mPBSC group compared with the BM‐MNC group (MD 1.70 mmHg, 95% CI 0.41 to 2.99; 150 participants; low‐quality evidence) (Analysis 1.8) (Huang 2007).
1.8. Analysis.
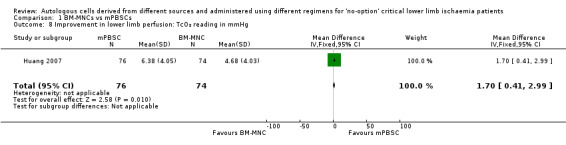
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 8 Improvement in lower limb perfusion: TcO₂ reading in mmHg.
Comparison 2: BM‐MNCs vs BM‐MSCs
Only a single study performed this comparison (Lu 2011). This study showed an increase in TcO₂ readings in the BM‐MSC group compared with the BM‐MNC group (MD 8.00 mmHg, 95% CI 3.46 to 12.54; 37 participants; low‐quality evidence) (Analysis 2.5).
2.5. Analysis.
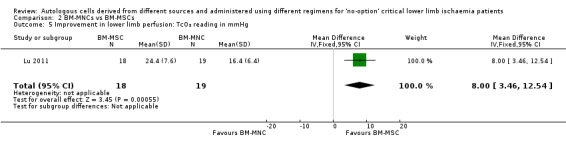
Comparison 2 BM‐MNCs vs BM‐MSCs, Outcome 5 Improvement in lower limb perfusion: TcO₂ reading in mmHg.
Comparison 3: high cell dose vs low cell dose
Losordo 2012 did not report on this outcome.
Comparison 4: route of implantation: IM vs IA
Pooled analysis of two studies did not show a difference in the numbers of participants with improved TcO₂ readings between IM and IA cell implantation groups (RR 1.22, 95% CI 0.86 to 1.72; 62 participants; I² = 0%; very low‐quality evidence) (Analysis 4.6) (Gu 2008; Klepanec 2012).
4.6. Analysis.
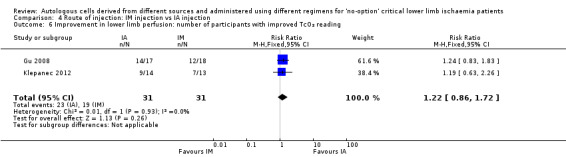
Comparison 4 Route of injection: IM injection vs IA injection, Outcome 6 Improvement in lower limb perfusion: number of participants with improved TcO₂ reading.
4. Improvement in ischaemic symptoms as assessed by improvement in pain‐free walking distance (PFWD) and pain‐free walking time (PFWT)
Only two studies reported PFWD or PFWT (Huang 2007; Lu 2011).
Comparison 1: BM‐MNCs vs mPBSCs
The only study that performed this comparison did not show a clear difference in mean PFWD between the two treatment groups (MD 33.05 metres, 95% CI ‐37.69 min to 103.79 min; 150 participants) (Analysis 1.9) (Huang 2007).
1.9. Analysis.
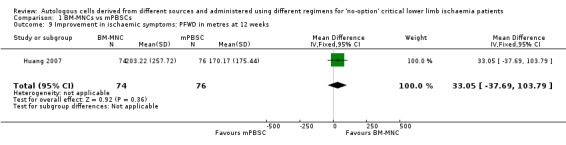
Comparison 1 BM‐MNCs vs mPBSCs, Outcome 9 Improvement in ischaemic symptoms: PFWD in metres at 12 weeks.
Comparison 2: BM‐MNCs vs BM‐MSCs
The only study that performed this comparison did not show a clear difference in mean PFWT between the two treatment groups (MD 0.6 min, 95% CI ‐0.01 min to 1.21 min; 37 participants) (Analysis 2.6) (Lu 2011).
2.6. Analysis.
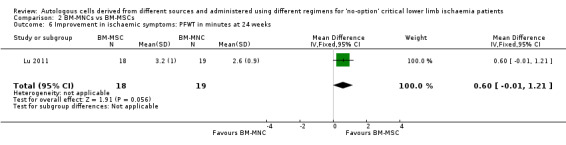
Comparison 2 BM‐MNCs vs BM‐MSCs, Outcome 6 Improvement in ischaemic symptoms: PFWT in minutes at 24 weeks.
5. Improvement in vascularity and blood supply to the ischaemic limb as measured by collateral vessel formation
CLI participants in the included studies were subjected to angiography before and at three to six months after cell implantation to identify new collateral vessel formation in treated and control limbs.
Comparison 1: BM‐MNCs vs PBSCs
Huang 2007 and Zhang 2009 did not report on this outcome.
Comparison 2: BM‐MNCs vs BM‐MSCs
The only study that performed this comparison showed a higher number of participants with new collaterals at 24 weeks in the BM‐MSC‐treated group than in the BM‐MNC‐treated group (RR 1.98, 95% CI 1.12 to 3.49; 37 participants) (Analysis 2.7) (Lu 2011).
2.7. Analysis.
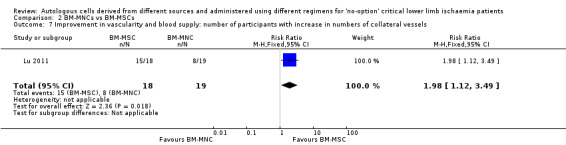
Comparison 2 BM‐MNCs vs BM‐MSCs, Outcome 7 Improvement in vascularity and blood supply: number of participants with increase in numbers of collateral vessels.
Comparison 3: high cell dose vs low cell dose
Losordo 2012 did not report on this outcome.
Comparison 4: route of implantation: IM vs IA
The only study in this comparison showed no difference in the numbers of participants with collateral formation between different routes of cell implantation (RR 0.91, 95% CI 0.40 to 2.11; 15 participants) (Analysis 4.7) (Gu 2008).
4.7. Analysis.
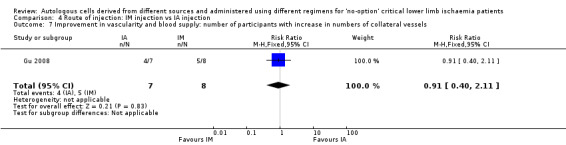
Comparison 4 Route of injection: IM injection vs IA injection, Outcome 7 Improvement in vascularity and blood supply: number of participants with increase in numbers of collateral vessels.
6. Adverse events and safety
None of the included RCTs reported adverse events for the intervention and control groups separately, hence we were unable to undertake meta‐analyses for this outcome. Nonetheless, most of the studies (six out of seven RCTs) reported that in general, cell‐based therapy was well tolerated without significant and severe adverse events related to bone marrow aspiration and cell implantation into the affected limb. The individual studies reported no infection and no biochemical or immunological reactions among participants who had received cell implantation. However, reported data on the long‐term safety of cell‐based therapy were lacking.
As pooled analyses were not possible for this outcome, we describe adverse effects reported by the individual studies according to the following comparisons.
Comparison 1: BM‐MNCs vs mPBSCs
One study reported that cell implantation in both groups did not induce local inflammatory reaction or oedema of the lower limbs (Huang 2007). Study results show that the most common adverse events during G‐CSF mobilisation in mPBSC were bone pain (13.2%; 10/76) and lassitude (5.3%; 4/76), but no participants were required to withdraw from the mobilisation procedure or required special treatment. No implantation‐related complications were observed through electrocardiograms, liver and kidney function tests, and urine tests during the 12‐week follow‐up period. Researchers followed up 43 participants in the mPBSC group and 41 in the BM‐MNC group over a 12‐month period and noted no implantation‐related complications during this time. Another study reported no significant adverse effects in all participants (Zhang 2009).
Comparison 2: BM‐MNCs vs BM‐MSCs
One of the three RCTs that used BM‐MSCs reported detailed data on the safety profile of cell‐based treatment (Lu 2011). Study authors observed minimal bleeding at the posterior iliac crest after bone marrow aspiration in one and two participants in the BM‐MSC and BM‐MNC groups, respectively. In addition, three limbs in the BM‐MSC group and two in the BM‐MNC group were reported as painful in the short term after cell implantation (Lu 2011).
Comparison 3: high cell dose vs low cell dose
One RCT reported 60 serious adverse events (SAEs) in 22 participants (78.6%), 59 of which occurred after IM cell implantation, and one during G‐CSF mobilisation (Losordo 2012). Only two SAEs were considered possibly study‐related: one participant developed moderate hypotension and another experienced worsening ischaemia of the implanted limb. One participant in the control (placebo) group developed acute myocardial infarction four to five months after randomisation. Adverse events did not necessitate withdrawal of participants from the study.
Comparison 4: route of implantation: IM vs IA
Individual studies reported no adverse events related to implantation of cells and no difference in the occurrence of adverse events among participants who received cell treatment via IM and IA routes, indicating that both routes of cell delivery were safe and feasible (Gu 2008; Klepanec 2012; Van Tongeren 2008). Eight deaths due to cardiovascular and respiratory events were not related to cell implantation. Meta‐analysis was not feasible, as these studies did not provide comparative adverse events data for the different routes of cell implantation.
Subgroup analysis
We were unable to perform any subgroup analyses for each outcome describing participants with different severity and aetiology of CLI, participants with or without significant comorbidity, and participants of different age groups, gender, and ethnicity, as we could not obtain separate data according to these pre‐specified criteria from the included studies under each comparison.
Sensitivity analysis
We were unable to perform sensitivity analyses for significant dropout rates and significant methodological issues identified in the assessment of risk of bias, as insufficient data were available.
We conducted sensitivity analyses for the relevant outcomes to identify if pooled estimates of outcomes would change with inclusion and exclusion of studies in which limbs rather than participants were used as units of analysis.
Outcome: amputation rate
Comparison: IM versus IA implantation: results show no substantial change after one study that used limbs as the unit of analysis ‐ Gu 2008 ‐ was excluded (before exclusion: RR 0.80, 95% CI 0.54 to 1.18; three RCTs, 95 participants; I² = 48%; after exclusion: RR 0.86, 95% CI 0.59 to 1.26; two RCTs, 60 participants; I² = 61%) (Analysis 4.1).
Outcome: improvement in lower limb perfusion as measured by improvement in transcutaneous oxygen tension (TcO₂)
Number of participants with improved TcO₂ reading
Comparison: IM versus IA implantation: results show no substantial change after one study that used limbs as the unit of analysis ‐ Gu 2008 ‐ was excluded (before exclusion: RR 1.22, 95% CI 0.86 to 1.72; two RCTs, 62 participants; I² = 0%; after exclusion: RR 1.19, 95% CI 0.63 to 2.26; one RCT, 27 participants) (Analysis 4.6).
Discussion
Summary of main results
The main findings of the review show no clear differences in almost all comparisons between different cell sources and implantation regimens for the major clinical outcomes of all‐cause mortality, amputation rate, improvement in rest pain, number of participants with healing ulcer, and improvement in ankle‐brachial index (ABI). However, underpowered analyses for all comparisons precluded any clear and firm conclusions.
Of note, most of the included trials were not specifically designed to compare adverse events or long‐term safety between the various sources and types of cell‐based regimens. Hence review authors found a paucity of comparative adverse event data between treatment groups, confounded by short follow‐up duration. Nonetheless, the randomised controlled trials (RCTs) included in this review support the short–term safety and feasibility of autologous implantation of stem cells obtained from bone marrow or peripheral blood into ischaemic limbs. The most common adverse events following bone marrow harvesting were short‐term episodes of slight pain, bleeding, and haematoma at the bone marrow aspiration site that did not require specific intervention. Apart from granulocyte colony‐stimulating factor (G‐CSF)‐induced transient bone pain that did not require special treatment, mobilisation of mobilised peripheral blood stem cells (mPBSCs) was well tolerated. Researchers reported no local adverse reactions at the cell implantation site. Individual studies reported no infection and no biochemical or immunological reactions among participants following cell implantation.
Although each individual RCT described no difference in the occurrence of adverse events related to route of implantation and cell dose, the small sample size and lack of comparative data in these studies precluded any convincing conclusions on the overall safety of cell‐based therapy. In addition, evidence on the long‐term safety profile of different types of cell‐based products, namely, bone marrow‐mononuclear cells (BM‐MNCs), bone marrow‐mesenchymal stem cells (BM‐MSCs), mPBSCs, and peripheral blood‐mononuclear cells (PB‐MNCs), is lacking.
Overall completeness and applicability of evidence
In terms of the applicability of our review, the current mainstay of treatment for critical limb ischaemia (CLI) has been endovascular or surgical revascularisation and pharmacological therapy. However, the presence of significant comorbidities and distal vessel disease renders many patients unsuitable for revascularisation, often resulting in amputation (Liew 2016). Exploring new strategies for revascularisation therapy is thus of major importance. The most recent published clinical practice guideline did not include cell‐based therapy as one of the limb salvage treatment modalities (NGC 2012). Currently, cell‐based therapy is at a preliminary stage of use or is being utilised mainly in research.
Each RCT included in this review contained a small sample size ranging from 16 to 150 participants and a relatively short follow‐up period (range 1 to 12 months). The number of included RCTs was very small (seven), and comparisons for each outcome included only one to three studies.
Moreover, substantial variation in patient selection processes was evident, even within the relatively small population group (Sultan 2014). In particular, the criteria used to define 'CLI' and 'no‐option' patients lacked uniformity. Trials examined varying degrees of lower limb ischaemia, ranging from intermittent claudication to non‐healing ulcers and gangrene. Differences in the severity of peripheral arterial disease (PAD) may partly explain the differences in changes in blood flow parameters between these studies. The review includes a range of aetiologies for CLI, namely, atherosclerosis, Buerger's disease, acute embolism, and others. However, subanalysis for outcomes according to severity and aetiology of CLI was not possible, as individual RCTs did not provide such data. Risk factors such as diabetes mellitus and smoking that are known to cause worsening and progression of lower limb ischaemia were inhomogeneous between the included studies. For example, one RCT included only diabetic patients (Lu 2011), and another RCT excluded patients with poorly controlled diabetes mellitus, or those with proliferative diabetic retinopathy (Huang 2007).
Lack of uniformity in assessment, analysis, and reporting of efficacy endpoints between the individual RCTs precluded combining efficacy data for meta‐analysis, so much so that the findings of this systematic review are based in part on results derived from one or two studies. For example, there were differences in the methods and units used to calculate, document, and report objective outcomes such as ABI and ulcer healing. Investigators used several different scoring systems to measure and interpret subjective outcomes including patient‐perceived rest pain and pain‐free walking distance (PFWD) and pain‐free walking time (PFWT) in individual RCTs. Moreover, the timing of assessment of outcomes varied between studies. These important variations in efficacy data between studies resulted in insufficient data that could be pooled for meta‐analysis. This was particularly important for comparisons performed with low number of patients in each arm.
The present systematic review is not able to demonstrate differences in efficacy and safety between the various sources and types of cell‐based products for the following reasons. Most published trials on this topic compared cell‐based treatment versus conventional, non‐cell‐based therapy. Our search revealed limited published RCTs that performed head‐to‐head comparisons between cells derived from different sources, prepared via different processing protocols, and administered through different doses and routes. We analysed two RCTs comparing BM‐MNCs versus mPBSCs (Huang 2007; Zhang 2009), as well as one RCT comparing BM‐MNCs versus BM‐MSCs (Lu 2011). For the comparison BM‐MNCs versus mPBSCs, meta‐analysis was not feasible because of differences in the units used to report outcomes between the two cell treatment groups.
Limited evidence suggests clear differences in any clinical outcome whether cells were administered at high or low cell doses. Our analysis based on data from a single study shows no clear difference in amputation rates between patients who received high and low doses of CD34+ cells. However, this finding should be interpreted cautiously because of the small sample size and the phenotype of cells used in this study. This study used mobilised PB CD34+ cells (Losordo 2012), whereas many studies examining cell therapy for CLI patients had used unselected bone marrow‐derived cells (BM‐MNCs). Of note, different cell sources and phenotypes may contain different proportions of stem cells with angiogeneic potential.
Most included trials implanted the cells into the gastrocnemius muscle (intramuscular (IM) route). Only three RCTs involving 100 participants directly compared the efficacy of IM and IA routes of cell administration (Gu 2008; Klepanec 2012; Van Tongeren 2008). Meta‐analysis of these trials failed to show superiority of either route; however, the accuracy of this finding is confounded by the small sample size.
All‐cause mortality from three RCTs was 9%. However, in view of baseline high risk factors among CLI patients and the limitations of the individual RCTs included in our review, it is not possible to attribute the cause of death to cell treatment. Of note, all RCTs included in this review reported no significant adverse effects related to cell‐based treatment. However, none of these trials performed head‐to‐head comparison of safety data between treatment groups. Moreover, the long‐term safety of cell‐based therapy could not be determined, as the follow‐up period for most RCTs was relatively short, ranging from one to twelve months. Head‐to‐head comparisons between CLI patients treated with different cell‐based regimens and given long‐term follow‐up are warranted to confirm the safety and durability of the efficacy of cell‐based therapy for these patients.
Additionally, the relatively short follow‐up period of the included studies has limited the usefulness of this review in informing practice.
Quality of the evidence
Overall, we noted a mixed quality of evidence for different outcome‐comparison combinations, and we concluded that overall the quality of evidence presented was very low to moderate (Table 1; Table 2; Table 3; Table 4). Major factors that led to downgrading of the quality of evidence were risk of bias issues, imprecision, and indirectness of evidence. Major methodological limitations in most studies included unclear randomisation methods and/or allocation concealment, as well as lack of blinding of participants and insufficient outcome data. It is important to note that single studies or small numbers of studies with limited participants contributed to all outcomes, resulting in underpowered analyses with wide 95% confidence intervals. Overall, high‐quality evidence needed to assess the effects of utilisation of a particular source or treatment regimen of cell‐based therapy for CLI in clinical practice is lacking.
Potential biases in the review process
We performed a comprehensive search covering multiple major databases to look for published and unpublished studies in full text or abstract format, and we screened for relevant studies among 4904 citations. We believe that the scope of our search was sufficiently complete.
It is possible that we may not have identified still unpublished trials. In particular, small studies reporting negative findings may not have been published as full articles. Moreover, several phase 3 trials are currently ongoing. We identified seven ongoing RCTs (NCT00311805; NCT00753025; NCT01257776; NCT01408381; NCT01446055; NCT01745744; NCT02454231), and we discovered six clinical trials classified as 'awaiting classification' (Gurunathan 2009; Korymasov 2009; Molavi 2016; NCT00595257; NCT00987363; NCT02993809), the eligibility criteria of which have yet to be determined. Results of these trials will likely provide efficacy data on cell‐based therapy derived from different sources, administered via different doses, and delivered via different routes.
We have excluded a substantial number of clinical trials related to cell‐based treatment for patients with PAD, as most of these were single‐arm studies (42 trials) or were non‐randomised (11 trials). To date, the number of high‐quality trials conducted to compare different types of cell products and treatment regimens is limited.
Four RCTs reported their results using limbs rather than patients as the unit of analysis and reported unadjusted results (Gu 2008; Huang 2007; Lu 2011; Zhang 2009). We could not obtain sufficient data from the primary studies to adjust for clustering effects, and this might have led to a biased estimate in our results for certain limb‐related outcomes.
Researchers described one RCT as a "controlled clinical case analysis", and indicated that patients were randomly assigned to two groups (BM‐MNCs vs mPBSCs) (Zhang 2009). Study authors did not mention the true nature of randomisation. Ideally we would have performed a sensitivity analysis to investigate the effects of randomisation on estimates of outcome; however, this was the only study reporting these effects on outcomes, so we could not perform a sensitivity analysis.
Our sensitivity analyses performed to assess the impact of excluding such studies show that in most cases, these exclusions did not change the pooled estimates substantially.
We were unable to perform sensitivity analyses for significant or worrisome dropout rates and significant methodological issues identified during assessment of risk of bias, as insufficient data were available.
Agreements and disagreements with other studies or reviews
To date, apart from the current systematic review, limited published meta‐analyses have included RCTs that directly compared the effectiveness of cell‐based products derived from different sources, prepared using different regimens, and delivered via different routes. Most published meta‐analyses compared cell therapy versus conventional treatment (non‐cell‐based therapy) and also included non‐RCTs. Direct comparison of the efficacy of different cell sources and types reported by individual RCTs has yielded inconsistent results. Hence, the efficacy and safety of different cell‐based products remain to be determined.
Three previous meta‐analyses involving 510 (twelve RCTs), 276 (seven RCTs), and 373 (seven RCTs) PAD patients, respectively, underlined the promising results of cell‐based therapy compared to conventional therapy (non‐cell‐based therapy) (Liu 2012; Teraa 2013; Wen 2011). In contrast to the current review, these previously published meta‐analyses combined and analysed patients treated with cells from different sources and different types of cells (BM‐MNCs, BM‐MSCs, mPBSCs, Ixmyelocel‐T) and collectively reported them as bone marrow‐derived cell therapy.
Two recent meta‐analyses attempted to compare outcomes for patients receiving different cell types versus non‐cell‐based therapy (Liew 2016; Liu 2015). In Liu 2015, the subgroup analysis based on stem cell sources showed significant improvement in amputation rates, ulcer healing, and ABI in patients implanted with bone marrow‐derived cells or peripheral blood‐derived cells compared to controls. However, in contrast to our review, Liu 2015 included non‐randomised studies in its meta‐analysis (Cobellis 2008; Dubsky 2013). Review authors compared clinical outcomes of cell treatment groups versus non‐cell treatment groups and did not perform head‐to‐head comparisons between cells derived from different sources or implanted via different implantation regimens. Furthermore, review authors pooled and analysed outcome data from studies using different types of cells, for example, the bone marrow cell group consisted of patients treated with allogeneic MSCs (Gupta 2013), BM‐MNCs (Walter 2011), and tissue repair cells (Powell 2012), and the peripheral blood cell group consisted of patients treated with VesCell ‐ as in Szabo 2013 ‐ and with mPBSCs (Huang 2005; Mohammadzadeh 2013; Ozturk 2012).
Liew 2016 concluded that patients treated with PB‐MNCs and bone marrow concentrate had a reduced rate of major amputation compared to those given other cell types (i.e. PB‐MNCs, BM‐MSCs, BM‐MNCs). However, in contrast to our analysis, review authors did not perform head‐to‐head comparisons between a particular cell‐based product and another cell‐based product. Furthermore, review authors arrived at their conclusions upon analysing pooled data from studies that used different types of cells, namely, mPBSCs, VesCell (Szabo 2013), and CD133+ cells (Raval 2014), and they combined and analysed data collectively as PB‐MNCs, while pooling, combining, and analysing collectively as BM‐MSCs allogeneic MSCs (Gupta 2013), autologous MSCs (Lu 2011), and tissue repair cells (Powell 2012). As with meta‐analysis performed by Liu 2015, differences in the types of cell products used for implantation may have had an important impact on clinical outcomes.
A large meta‐analysis involving almost 700 'no‐option' CLI patients from 37 clinical trials comparing the efficacy of BM‐MNC and mPBSC treatment included uncontrolled and non‐randomised trials, rendering the results to have high risk of bias (Fadini 2010).
A Cochrane review that was first published in 2011 and was updated in 2014 includes two small RCTs involving 57 patients comparing IM autologous BM‐MNCs and conventional therapy; the review authors concluded that evidence was insufficient to support intramuscular cell treatment in patients with CLI (Moazzami 2014). Review authors did not perform a meta‐analysis because the two studies included in that review differed with regards to types of cells implanted (BM‐MNCs vs mPBSCs) and assessment of outcomes (Barć 2006; Huang 2005); hence the conclusion regarding efficacy of cell treatment compared to conventional treatment was based on data extracted from individual studies with very small sample sizes.
Previous meta‐analyses reported that autologous bone marrow‐derived stem cells and G‐CSF‐mobilised PBSCs (mPBSCs) given via IM or IA implantation into the ischaemic limbs appeared to be relatively safe, and side effects were generally mild and transient (Liew 2016; Liu 2015; Sun 2015; Teraa 2013). Only two RCTs previously reported in detail comparisons of adverse events between cell treatment and non‐cell treatment groups (Li 2013; Teraa 2014). These studies showed no significant differences in the incidence of adverse events between autologous BM‐MNCs and control interventions. Adverse events most commonly reported in the BM‐MNC group were short‐term episodes of slight pain, bleeding, and haematoma at the bone marrow aspiration site that did not require specific intervention. Adverse events most commonly noted in the mPBSC group were bone pain (13.2%) and lassitude (5.3%) associated with G‐CSF administration, which were transient and did not require discontinuation from G‐CSF therapy and PBSC mobilisation (Huang 2007). In conclusion, most published clinical trials in people with CLI have presented a reassuring short‐term safety profile but sparse data on the long‐term safety of cell‐based therapy.
Authors' conclusions
Implications for practice.
Evidence of mostly low and very low quality shows no clear differences between different sources, cell doses, and implantation routes for cell‐based therapy among patients with CLI in terms of mortality, amputation rate, rest pain, ulcer healing, and lower limb perfusion. Cell therapy appeared to be well tolerated without serious adverse events arising from cell harvesting from bone marrow or peripheral blood and cell implantation into the ischaemic limb. Overall the efficacy and long‐term safety of autologous cells derived from different sources, prepared using different protocols, administered at different doses, and delivered via different routes for treatment of 'no‐option' CLI patients, remain to be confirmed. Underpowered analyses have precluded any firm conclusions that may influence practice.
Implications for research.
Authors of the current review could not draw definitive conclusions on the efficacy of different sources, types, and treatment regimens of cell‐based therapy for CLI patients, and high‐quality clinical trials are urgently needed to answer these clinical questions. In particular, the long‐term safety of cell‐based products derived from different sources, prepared using different protocols, and administered at different doses and via different routes, needs to be determined. Future clinical trials should consider the following factors.
Larger sample size with adequate blinding of study population and personnel.
Head‐to‐head comparison between cell‐based treatment derived from different sources via different cell preparation protocols, routes of cell delivery, cell doses, and implantation regimens.
Determination of clinical endpoints according to underlying aetiologies, risk factors (smoking, diabetes, age group, and gender), and severity of PAD, with separate analyses and reporting of results according to these subgroups.
Long‐term follow‐up to evaluate the durability of angiogenic potential and the long‐term safety of cell‐based therapy.
Use of angiogenic growth factors, cell carriers, and other adjunctive therapies in combination with cell‐based treatment.
Comparison of the safety and efficacy of allogeneic cell therapy versus autologous cell therapy.
Comparison of the cost of therapy amongst different cell‐based treatment modalities.
Acknowledgements
We would like to acknowledge the Cochrane Vascular Group managing and coordinating editors and reviewers, and the Dean of the Faculty of Medicine and Director of Hospital Chanselor Tuanku Muhriz for their support.
Appendices
Appendix 1. CENTRAL search strategy, 17 February 2017
| Search run on Fri Feb 17 2017 | ||
| #1 | MESH DESCRIPTOR Arteriosclerosis | 868 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 71 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 619 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 724 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 712 |
| #7 | MESH DESCRIPTOR Ischemia | 789 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2201 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9119 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 7966 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 3371 |
| #12 | (claudic* or IC):TI,AB,KY | 3063 |
| #13 | (isch* or CLI):TI,AB,KY | 23713 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 10 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 95 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 145 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 77 |
| #19 | ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 1008 |
| #20 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1107 |
| #21 | MESH DESCRIPTOR Iliac Artery | 144 |
| #22 | MESH DESCRIPTOR Popliteal Artery | 278 |
| #23 | MESH DESCRIPTOR Femoral Artery | 810 |
| #24 | MESH DESCRIPTOR Tibial Arteries | 33 |
| #25 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 1157 |
| #26 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 | 43742 |
| #27 | MESH DESCRIPTOR Stem Cell Transplantation EXPLODE ALL TREES | 1531 |
| #28 | MESH DESCRIPTOR Hematopoietic Stem Cell Mobilization EXPLODE ALL TREES | 259 |
| #29 | MESH DESCRIPTOR Stem Cells EXPLODE ALL TREES | 690 |
| #30 | MESH DESCRIPTOR Bone Marrow Cells EXPLODE ALL TREES | 1484 |
| #31 | ((mononuclear or endothelial or mesenchymal) near3 cell*):TI,AB,KY | 5253 |
| #32 | ((stem or progenitor or precursor or therap*) near3 cell*):TI,AB,KY | 12700 |
| #33 | ((embryo* or fetal or foetal or umbilical or marrow or cord) near5 cell*):TI,AB,KY | 2023 |
| #34 | (BM‐MNC* or PB‐MNC* or M‐PBNNC* or AT‐MSC*):TI,AB,KY | 42 |
| #35 | MESH DESCRIPTOR Transplantation, Autologous | 1297 |
| #36 | #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 | 18669 |
| #37 | #26 AND #36 | 1264 |
Appendix 2. Trials databases searches, 17 February 2017
Clinicaltrials.gov 69 studies found for: (bone marrow OR stem cells) AND (critical limb ischemia)
WHO Clinical trials register 17 records for 17 trials found for: (ischemia or ischaemia) and cells in Title and Cells in intervention
ISRCTN Register 4 results for: (ischemia or ischaemia) and cells
Appendix 3. Authors' PubMed search strategy, February 2017
| #1 | Search (Peripheral arterial disease[Title/Abstract] OR Peripheral vascular disease[Title/Abstract] OR chronic limb ischemia[Title/Abstract] OR critical limb ischemia[Title/Abstract] OR CLI[Title/Abstract] OR PAD[Title/Abstract] OR limb ischaemia[Title/Abstract]) | 35028 |
| #2 | Search (limb arteriosclerosis obliterans [Title/Abstract] OR foot ulcer [Title/Abstract] OR diabetic foot [Title/Abstract] OR arteriosclerosis obliterans [Title/Abstract] OR thromboangiitis obliterans [Title/Abstract] OR buerger's disease [Title/Abstract]) | 9809 |
| #3 | Search Peripheral Vascular Diseases [MeSH Terms] | 48342 |
| #4 | Search Peripheral Arterial Diseases [MeSH Terms] | 4259 |
| #5 | Search #1 OR #2 OR #3 OR #4 | 85418 |
| #6 | Search (mononuclear cells[Title/Abstract] OR mesenchymal stem cells[Title/Abstract] OR therapeutic angiogenesis[Title/Abstract] OR bone marrow transplantation[Title/Abstract] OR adult stem cells[Title/Abstract]) | 117626 |
| #7 | SearchLeukocytes, Mononuclear [MeSH Terms] | 526581 |
| #8 | Search Mesenchymal Stromal Cells[MeSH Terms] | 24202 |
| #9 | Searchbone marrow transplantation[MeSH Terms] | 42775 |
| #10 | Search stem cells[MeSH Terms] | 167737 |
| #11 | Search #6 OR #7 OR #8 OR #9 OR #10 | 747219 |
| #12 | Search #5 AND #11 | 1488 |
| #13 | Search randomized controlled trial [pt] | 428782 |
| #14 | Search controlled clinical trial [pt] | 515129 |
| #15 | Search randomized [tiab] | 400989 |
| #16 | Search placebo [tiab] | 182281 |
| #17 | Search drug therapy [sh] | 1902784 |
| #18 | Search randomly [tiab] | 266458 |
| #19 | Search trial [ti] | 162684 |
| #20 | Search #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 | 2588730 |
| #21 | Search (animals [mh] NOT humans [mh]) | 4299695 |
| #22 | Search #20 NOT #21 | 2322098 |
| #23 | Search #12 AND #22 | 234 |
Appendix 4. Database searches, 16 May 2018
| Source | Search strategy | Hits retrieved |
| 1. VASCULAR REGISTER IN CRSW | #1 Stem Cell OR Autologous cells OR Bone Marrow Cells AND INREGISTER #2 2017 or 2018 AND INREGISTER #3 #1 AND #2 |
1 |
| 2. CENTRAL via CRSO | #1 MESH DESCRIPTOR Arteriosclerosis 927 #2 MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 76 #4 MESH DESCRIPTOR Atherosclerosis 963 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 804 #6 MESH DESCRIPTOR Intermittent Claudication 805 #7 MESH DESCRIPTOR Ischemia 1354 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2660 #9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY 11705 #10 ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 10331 #11 ((peripheral near3 dis*)):TI,AB,KY 4618 #12 (claudic* or IC):TI,AB,KY 3969 #13 (isch* or CLI):TI,AB,KY 30894 #14 arteriopathic:TI,AB,KY 7 #15 dysvascular*:TI,AB,KY 17 #16 (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 124 #17 (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 210 #18 ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 106 #19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 1481 #20 MESH DESCRIPTOR leg EXPLODE ALL TREES 2784 #21 MESH DESCRIPTOR Iliac Artery 158 #22 MESH DESCRIPTOR Popliteal Artery 300 #23 MESH DESCRIPTOR Femoral Artery 894 #24 MESH DESCRIPTOR Tibial Arteries 36 #25 ((((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ))):TI,AB,KY 1661 #26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 57950 #27 MESH DESCRIPTOR Stem Cell Transplantation EXPLODE ALL TREES 1753 #28 MESH DESCRIPTOR Hematopoietic Stem Cell Mobilization EXPLODE ALL TREES 278 #29 MESH DESCRIPTOR Stem Cells EXPLODE ALL TREES 735 #30 MESH DESCRIPTOR Bone Marrow Cells EXPLODE ALL TREES 1589 #31 (((mononuclear or endothelial or mesenchymal) near3 cell*)):TI,AB,KY 7061 #32 (((stem or progenitor or precursor or therap*) near3 cell*)):TI,AB,KY 17942 #33 (((embryo* or fetal or foetal or umbilical or marrow or cord) near5 cell*)):TI,AB,KY 3057 #34 ((BM‐MNC* or PB‐MNC* or M‐PBNNC* or AT‐MSC*)):TI,AB,KY 65 #35 MESH DESCRIPTOR Transplantation, Autologous 1432 #36 #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 25398 #37 #26 AND #36 1796 #38 01/01/2017 TO 16/05/2018:CD 257803 #39 #37 AND #38 520 |
520 |
| 3. Clinicaltrials.gov | Peripheral arterial disease OR Peripheral Vascular Diseases OR lower limb ischaemia | Stem Cell OR Autologous cells OR Bone Marrow Cells | Start date on or after 01/01/2017 | Last update posted on or before 03/05/2019 | 8 |
| 4. ICTRP Search Portal | Peripheral arterial disease OR Peripheral Vascular Diseases OR lower limb ischaemia | Stem Cell OR Autologous cells OR Bone Marrow Cells | 01/01/2017 to 03/05/2019 | 0 |
| 5. MEDLINE | 1. ARTERIOSCLEROSIS/ 2. exp ARTERIOLOSCLEROSIS/ 3. Arteriosclerosis Obliterans/ 4. ATHEROSCLEROSIS/ 5. Arterial Occlusive Diseases/ 6. Intermittent Claudication/ 7. ISCHEMIA/ 8. Peripheral Vascular Diseases/ 9. (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 10. ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 11. (peripheral adj3 dis*).ti,ab. 12. (claudic* or IC).ti,ab. 13. (isch* or CLI).ti,ab. 14. arteriopathic.ti,ab. 15. dysvascular*.ti,ab. 16. (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 17. (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 18. (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 19. ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 20. exp LEG/bs [Blood Supply] 21. Iliac Artery/ 22. Popliteal Artery/ 23. Femoral Artery/ 24. Tibial Arteries/ 25. ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 26. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. exp Stem Cell Transplantation/ 28. exp Bone Marrow Cells/ 29. ((mononuclear or endothelial or mesenchymal) adj3 cell*).ti,ab. 30. ((stem or progenitor or precursor or therap*) adj3 cell*).ti,ab. 31. ((embryo* or fetal or foetal or umbilical or marrow or cord) adj5 cell*).ti,ab. 32. (BM‐MNC* or PB‐MNC* or M‐PBNNC* or AT‐MSC*).ti,ab. 33. Transplantation, Autologous/ 34. exp Hematopoietic Stem Cell Mobilization/ 35. exp Stem Cells/ 36. or/27‐35 37. 26 and 36 38. randomized controlled trial.pt. 39. controlled clinical trial.pt. 40. randomized.ab. 41. placebo.ab. 42. drug therapy.fs. 43. randomly.ab. 44. trial.ab. 45. groups.ab. 46. or/38‐45 47. exp animals/ not humans.sh. 48. 46 not 47 49. 37 and 48 50. (2017* or 2018*).ed. 51. 49 and 50 |
526 |
| 6. EMBASE | 1 arteriosclerosis/ 14659 2 exp arteriolosclerosis/ 486 3 peripheral occlusive artery disease/ 21901 4 atherosclerosis/ 113655 5 peripheral occlusive artery disease/ 21901 6 intermittent claudication/ 6103 7 ischemia/ 59992 8 exp peripheral vascular disease/ 1280555 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 192387 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 144769 11 (peripheral adj3 dis*).ti,ab. 43541 12 (claudic* or IC).ti,ab. 52623 13 (isch* or CLI).ti,ab. 399372 14 arteriopathic.ti,ab. 82 15 dysvascular*.ti,ab. 177 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 684 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2162 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1461 19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 9348 20 iliac artery/ 9843 21 popliteal artery/ 5196 22 femoral artery/ 20875 23 tibial artery/ 2263 24 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10857 25 or/1‐24 1556237 26 exp stem cell transplantation/ 128994 27 exp stem cell/ 300361 28 exp bone marrow cell/ 85006 29 ((mononuclear or endothelial or mesenchymal) adj3 cell*).ti,ab. 317089 30 ((stem or progenitor or precursor or therap*) adj3 cell*).ti,ab. 415578 31 ((embryo* or fetal or foetal or umbilical or marrow or cord) adj5 cell*).ti,ab. 232042 32 (BM‐MNC* or PB‐MNC* or M‐PBNNC* or AT‐MSC*).ti,ab. 994 33 autotransplantation/ 15144 34 or/26‐33 860956 35 25 and 34 82480 36 randomized controlled trial/ 454282 37 controlled clinical trial/ 415959 38 random$.ti,ab. 1164643 39 randomization/ 69778 40 intermethod comparison/ 224992 41 placebo.ti,ab. 222171 42 (compare or compared or comparison).ti. 334278 43 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1614974 44 (open adj label).ti,ab. 62586 45 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 157598 46 double blind procedure/ 123039 47 parallel group$1.ti,ab. 19496 48 (crossover or cross over).ti,ab. 71898 49 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 248541 50 (assigned or allocated).ti,ab. 289586 51 (controlled adj7 (study or design or trial)).ti,ab. 261038 52 (volunteer or volunteers).ti,ab. 171731 53 trial.ti. 213834 54 or/36‐53 3469338 55 35 and 54 15884 56 (2017* or 2018*).em. 3418276 57 55 and 56 2878 58 from 57 keep 2001‐2878 878 |
2878 |
| 7. CINAHL | S47 S45 AND S46 53 S46 EM 2017 OR EM 2018 350,115 S45 S32 AND S44 628 S44 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 338,202 S43 MH "Random Assignment" 37,958 S42 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" 32,619 S41 MH "Crossover Design" 11,154 S40 MH "Factorial Design" 916 S39 MH "Placebos" 8,339 S38 MH "Clinical Trials" 93,234 S37 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,438 S36 TX crossover OR "cross‐over" 14,482 S35 TX random* 217,617 S34 TX trial* 248,860 S33 TX "latin square" 142 S32 S23 AND S31 2,772 S31 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 43,836 S30 (MH "Bone Marrow Transplantation, Autologous") 147 S29 TX BM‐MNC* or PB‐MNC* or M‐PBNNC* or AT‐MSC* 6,548 S28 TX ((embryo* or fetal or foetal or umbilical or marrow or cord) n5 cell*) 5,617 S27 TX ((stem or progenitor or precursor or therap*) n3 cell*) 27,759 S26 TX ((mononuclear or endothelial or mesenchymal) n3 cell*) 8,446 S25 (MH "Stem Cells+") 10,218 S24 (MH "Hematopoietic Stem Cell Transplantation") 4,000 S23 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 (85,427 S22 (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )) 1,080 S21 (MH "Tibial Arteries") 145 S20 (MH "Femoral Artery") 1,201 S19 (MH "Popliteal Artery") 360 S18 (MH "Iliac Artery") 458 S17 TX ((iliac or femoral or popliteal or femoro* or fempop* or crural) n3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 941 S16 TX ((lower n3 extrem*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 121 S15 TX (limb n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 273 S14 TX (leg n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 123 S13 TX dysvascular* 172 S12 TX arteriopathic 10 S11 TX isch* or CLI 39,225 S10 TX claudic* or IC 5,793 S9 TX peripheral ADJ3 dis* 0 S8 TX ((arter* or vascular or vein* or veno* or peripher*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 12,600 S7 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD 26,253 S6 (MH "Peripheral Vascular Diseases+") 10,351 S5 (MH "Ischemia") 3,352 S4 (MH "Intermittent Claudication") 851 S3 (MH "Arterial Occlusive Diseases") 1,603 S2 (MH "Atherosclerosis") 3,297 S1 (MH "Arteriosclerosis") 4,827 |
53 |
| 8. AMED | 1 arteriosclerosis/ 78 2 atherosclerosis/ 219 3 intermittent claudication/ 73 4 ischemia/ 262 5 exp peripheral vascular disease/ 116 6 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 802 7 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 458 8 (peripheral adj3 dis*).ti,ab. 435 9 (claudic* or IC).ti,ab. 1024 10 (isch* or CLI).ti,ab. 1663 11 arteriopathic.ti,ab. 1 12 dysvascular*.ti,ab. 57 13 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 21 14 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 32 15 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 25 16 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 54 17 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 109 18 or/1‐17 4307 19 exp stem cell transplantation/ 54 20 ((mononuclear or endothelial or mesenchymal) adj3 cell*).ti,ab. 554 21 ((stem or progenitor or precursor or therap*) adj3 cell*).ti,ab. 360 22 ((embryo* or fetal or foetal or umbilical or marrow or cord) adj5 cell*).ti,ab. 295 23 (BM‐MNC* or PB‐MNC* or M‐PBNNC* or AT‐MSC*).ti,ab. 87 24 or/19‐23 1074 25 18 and 24 104 26 exp CLINICAL TRIALS/ 3738 27 RANDOM ALLOCATION/ 314 28 DOUBLE BLIND METHOD/ 653 29 Clinical trial.pt. 1210 30 (clinic* adj trial*).tw. 5364 31 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2816 32 PLACEBOS/ 585 33 placebo*.tw. 3094 34 random*.tw. 17431 35 PROSPECTIVE STUDIES/ 1072 36 or/26‐35 22400 37 25 and 36 17 38 ("2017" or "2018").yr. 1412 39 37 and 38 0 |
0 |
Data and analyses
Comparison 1. BM‐MNCs vs mPBSCs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation rate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.45, 5.24] |
| 2 Wound/ulcer healing: number of participants with healing ulcers | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.44, 1.83] |
| 3 Wound/ulcer healing: change in ulcer size | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐5.35, 3.23] |
| 4 Reduction in rest pain: number of participants with any reduction in rest pain score | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 5 Reduction in rest pain: rest pain score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 12 weeks | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐0.90, ‐0.24] |
| 6 Improvement in lower limb perfusion: number of participants with increased ABI | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.71, 1.40] |
| 7 Improvement in lower limb perfusion: ABI score | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.09, ‐0.03] |
| 8 Improvement in lower limb perfusion: TcO₂ reading in mmHg | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.41, 2.99] |
| 9 Improvement in ischaemic symptoms: PFWD in metres at 12 weeks | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 33.05 [‐37.69, 103.79] |
Comparison 2. BM‐MNCs vs BM‐MSCs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation rate | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Wound/ulcer healing: number of participants with healing ulcers | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.02, 3.92] |
| 3 Reduction in rest pain: rest pain score | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.61, 0.61] |
| 4 Improvement in lower limb perfusion: ABI score | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.01, 0.09] |
| 5 Improvement in lower limb perfusion: TcO₂ reading in mmHg | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [3.46, 12.54] |
| 6 Improvement in ischaemic symptoms: PFWT in minutes at 24 weeks | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.01, 1.21] |
| 7 Improvement in vascularity and blood supply: number of participants with increase in numbers of collateral vessels | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.12, 3.49] |
Comparison 3. Low cell dose vs high cell dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation rate | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.87, 11.90] |
Comparison 4. Route of injection: IM injection vs IA injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation rate | 3 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.18] |
| 2 Wound/ulcer healing: number of participants with healing ulcer | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.73, 1.76] |
| 3 Reduction in rest pain: number of participants with reduction in rest pain score | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.64] |
| 4 Improvement in lower limb perfusion: number of participants with increased ABI | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 2.00] |
| 5 Improvement in lower limb perfusion: ABI score | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.37, 0.03] |
| 6 Improvement in lower limb perfusion: number of participants with improved TcO₂ reading | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.86, 1.72] |
| 7 Improvement in vascularity and blood supply: number of participants with increase in numbers of collateral vessels | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.40, 2.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gu 2008.
| Methods | Study design: stated as randomised Method of randomisation: not stated Blinding: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: China No. of participants: 32, 16 in intramuscular (IM) group, 16 in intra‐arterial (IA) group Mean age (SD): 69.5 (total) Gender: 20 male, 12 female Thirty‐two patients (35 lower limbs) who were admitted into the Department of Vascular Surgery of Xuanwu Hospital in Beijing, for chronic ischaemic limbs from March 2003 to April 2004, were studied. Follow‐up period: not stated |
|
| Interventions | Group 1 (16 participants with 18 affected limbs) received implantation of autologous BM‐MNCs by IM injection into affected limbs. Group 2 (16 participants with 17 affected limbs) received implantation of autologous BM‐MNCs by IA injection into affected limbs. |
|
| Outcomes | Clinical symptoms, physical examinations, and other vascular assessments
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 32 patients with lower limb ischaemia were divided into two groups by randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding appears very unlikely, as one group had injection into the muscles, while the other had injection from a percutaneous retrograde contralateral femoral. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the outcome assessor was blinded to participants' assigned groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Low risk | We did not identify any reporting bias. |
| Other bias | High risk | We noted unit of analysis issues, as the outcome of ABI was reported with the lower limbs rather than patients as the unit of analysis, and study authors did not adjust their data to account for the effect of clustering. |
Huang 2007.
| Methods | Study design: stated as randomised Method of randomisation: not stated Blinding: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: China No. of participants: 150, 76 in mPBSC group, 74 in BM‐MNC group Mean age (SD): 67 (9) in mPBSC group, 67 (11) in BM‐MNC group Gender: 47 male, 29 female in mPBSC group; 51 male, 23 female in BM‐MNC group Patients qualified for cell implantation in group A (active treatment) and group B (control) if they were diagnosed with LASO, with no improvement after a at least 3 months of treatment with adapted drugs, including urokinase, prostaglandin E1, heparin, or pentoxifylline, as described previously. Requisite haemodynamic deficits included resting ABI < 0.9 in the affected limb on 2 consecutive examinations performed at least 1 week apart. Patients with proliferative retinopathy, evidence of malignant disorder during the past 5 years, hypercoagulable states, gangrene above the ankle, and/or severe coronary, cerebral, and renal vascular disease were excluded Follow‐up period: 12 months |
|
| Interventions | Randomised (1:1) to implantation of mPBSCs (group A, active treatment, 76 participants) or BM‐MNCs (group B, control, 74 participants) Route of delivery: IM implantation |
|
| Outcomes | Primary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They were randomised (1:1) to implantation of mPBSCs or BM‐MNCs. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding appeared very unlikely, as 1 group underwent bone marrow aspiration, and the other group received subcutaneous G‐CSF followed by apheresis. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the outcome assessor was blinded to participants' assigned groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six participants (2 from the intervention group and 4 from the comparison group) were excluded before implantation, and 10 participants (4 from the intervention group and 6 from the comparison group) discontinued owing to limb amputation after implantation. Although total loss to follow‐up (10.6%) was not by itself a major concern, the unequal numbers of withdrawals between the 2 groups posed a concern; therefore this study was accorded high risk for incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | We did not identify any reporting bias. |
| Other bias | High risk | There were unit of analysis issues as some outcomes (ulcer healing, ABI) were reported using the limbs rather than patients as the unit of analysis, and the study authors did not adjust their data to account for the effect of clustering. |
Klepanec 2012.
| Methods | Study design: stated as randomised clinical study Method of randomisation: not stated Blinding: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Slovakia No. of participants: 41, 20 in intra‐arterial (IA) group, 21 in intramuscular (IM) group Mean age (SD): 66 (11) in IA group, 66 (10) in IM group Gender: 18 male, 2 female in IA group; 17 male, 4 female in IM group Between October 2009 and August 2010, 41 patients with advanced CLI (Rutherford category 5 or 6) after failed or impossible revascularisation were randomised to application of 40 mL of bone marrow concentrate via the local IM route (n = 21) or via selective IA infusion (n = 20). Inclusion criteria
Exclusion criteria
Follow‐up period: 6 months |
|
| Interventions | This study compared the therapeutic effects of IM (Group A) and IA (Group B) delivery of bone marrow cells (BMCs). | |
| Outcomes | Primary endpoints: limb salvage and wound healing Secondary endpoints: changes in TcO₂, Rutherford category, quality‐of‐life questionnaire (EQ5D), ABI, amputation rate, pain score (0–10) |
|
| Notes | This study was sponsored by a grant from European Regional Development Funding (ITMS code: 26240220023). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…..randomised to application of 40 ml of bone marrow concentrate via the local IM route (n = 21) or via selective IA infusion (n = 20)" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding appeared very unlikely, as in Group A cells were administered into the muscles of affected limbs, and in Group B IA injection of cells was undertaken from a percutaneous retrograde contralateral femoral approach or an antegrade femoral approach at the site of arterial occlusion of the affected limb. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the outcome assessor was blinded to assigned participant groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for, for all outcomes. |
| Selective reporting (reporting bias) | Low risk | We did not identify any reporting bias. |
| Other bias | Low risk | None identified |
Losordo 2012.
| Methods | Study design: prospective double‐blind randomised placebo‐controlled clinical pilot study Method of randomisation: not stated Blinding: double‐blind Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: United States No. of participants: 28, 7 in low‐dose group, 9 in high‐dose group, 12 in control group Mean age (SD): 61.8 (13.9) in low‐dose group, 69.7 (10.9) in high‐dose group, 67.1 (14.2) in control group Gender: 5 male, 2 female in low‐dose group; 8 male, 1 female in high‐dose group; 6 male, 6 female in control group Inclusion criteria: male or female patients aged ≥ 21 years with Rutherford category 4 or 5 CLI and no suitable revascularisation options; demonstrated atherosclerosis with stenosis (> 70%) or occlusion (100%) of a major vessel and absolute ankle pressure in the affected limb < 60 mmHg or reduced toe pressure < 40 mmHg or abnormal photoplethysmography; diagnosis of microvascular insufficiency Exclusion criteria: thromboangiitis obliterans (Buerger's disease) allowed if arterial insufficiency in the lower extremity was the result of a non‐atherosclerotic disorder, including but not limited to advanced scleroderma (CREST syndrome); advanced CLI (Rutherford category 6); expected amputation within 4 weeks of screening; clinical evidence of sepsis; advanced AV block or New York Heart Association class III or class IV heart failure; myocardial infarction within 3 months; clinically successful aortic or lower extremity arterial surgery; percutaneous revascularisation; lumbar sympathectomy within 3 months preceding screening Follow‐up period: 12 months |
|
| Interventions | Route of delivery: IM injection Treatment groups: 1. Low dose (1 × 10⁵ auto‐CD34+ cells/kg, 7 participants) 2. High dose (1 × 10⁶ auto‐CD34+ cells/kg, 9 participants) 3. Control: 12 participants |
|
| Outcomes | Safety 1. Primary endpoint of this exploratory study was safety of the IM injection of auto‐CD34+ cells 2. Follow‐up period: at 2, 4, 6, 8, and 12 weeks and at 6 and 12 months Efficacy 1. Assessment of limb salvage, occurrence of amputation, nature of amputation (toe or transmetatarsal, below or above the knee, preserving or not preserving function), and time to amputation were recorded during the 12‐month follow‐up period. |
|
| Notes | Baxter Healthcare Corporation. This study was supported in part by grants from the NIH (HL‐53354, HL‐77428, HL‐63414, HL‐80137, HL95874, HLPO1‐108795, HL‐57516). The control group is not included in the analysis of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 28 subjects were randomised 1:1:1…" Comments: the method of sequence generation was not explicitly stated. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigator, subject, study site personnel, core laboratory(ies), blinded study statistician, and all sponsor and clinical research organisation personnel remained blinded to all treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator, subject, study site personnel, core laboratory(ies), blinded study statistician, and all sponsor and clinical research organisation personnel remained blinded to all treatments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eight participants (28.6%) (4 in the high‐dose group and 4 in the control group) did not complete the 12‐month study period. |
| Selective reporting (reporting bias) | High risk | Rest pain, ABI, and ulcer size cannot be incorporated into the meta‐analysis, as data provided were insufficient. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Lu 2011.
| Methods | Study design: double‐blind randomised controlled trial Method of randomisation: randomisation table Blinding: double‐blind Exclusions post randomisation: not stated Losses to follow‐up: 4 of the 41 enrolled participants left this trial because 3 died from sudden cardiac death and 1 from severe pulmonary infection within 4 weeks after they were enrolled into the trial. |
|
| Participants | Country: China No. of participants: 41 Mean age: 64 Gender: 19 male, 22 female Inclusion criteria
Exclusion criteria
Follow‐up period: 24 weeks |
|
| Interventions | 3 groups: BM‐MSCs 20 limbs BM‐MNCs 21 limbs Normal saline (NS) 41 limbs |
|
| Outcomes | Primary outcome: safety and feasibility of treatment, defined as improvements in rest pain, PFWT, ulcer healing rate, limb salvage rate, ABI, and TcO₂ and enhancement of vessel formation as judged by the MRA | |
| Notes | This study was supported by the Clinical Research Fund of Southwest Hospital, Third Military Medical University. The study authors thank all patients and investigators who participated in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to groups A and B via a randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | It was not stated whether allocation was performed independently from sequence generation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The procedures of this clinical trial are presented, and patients and investigators were blinded to the treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors stated that "..... investigators were blinded to the treatments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts in the study. |
| Selective reporting (reporting bias) | High risk | Data for ABI in the control group were not provided. |
| Other bias | High risk | There were unit of analysis issues, as the allocation was made at the level of the legs rather than the participants, and study authors did not adjust their data to account for the effect of clustering. |
Van Tongeren 2008.
| Methods | Study design: stated as randomised Method of randomisation: random number table Blinding: not stated Exclusions post randomisation: not stated |
|
| Participants | Country: Netherlands No. of participants: 27, 12 in IA + IM group, 15 in intramuscular (IM) group Mean age (SD): 66.9 (16.3) in IA + IM group, 69.8 (12.4) in IM group Gender: 9 male, 3 female in IA + IM group; 10 male, 5 female in IM group Technical options for revascularisation by percutaneous transluminal angioplasty (PTA) or reconstructive surgery were extensively evaluated and disqualified. Patients were eligible for implantation of autologous bone marrow‐derived mononuclear cells if they suffered from CLI (ischaemic rest pain or ulcers), or if they had persistent (> 12 months) profound disabling claudication and a maximum walking distance of 100 metres. Life expectancy should be at least 1 year. Patients with a history of malignant disease in the 5 years before treatment were excluded. Mean follow‐up: 24 ± 8 months |
|
| Interventions | 27 participants were treated with combined IA + IM (n = 12) or sole IM (n = 15) administration of autologous BMCs. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of administration was randomly assigned to participants via a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to enable an evaluation on whether allocation was made independently from sequence generation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding appears very unlikely, as 1 group had injection into the muscles, and the other had injection from a percutaneous retrograde contralateral femoral. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two evaluating radiologists and 2 vascular surgeons were blinded for preprocedural and postprocedural angiographies. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants (1 in each group) were excluded. One participant died early in the study, and another did not attend follow‐up. |
| Selective reporting (reporting bias) | High risk | Apart from amputation rate, the study author did not report other key outcomes in sufficient detail. |
| Other bias | Low risk | None identified |
Zhang 2009.
| Methods | Study design: controlled clinical case analysis Method of randomisation: not stated Blinding: not stated Exclusions post randomisation: not stated |
|
| Participants | Country: China No. of participants: 52, 30 in BM‐MNC group, 22 in PBSC group Mean age (SD): 69 (6) total Gender: 27 male, 25 female 52 patients with diabetic lower limb ischaemia Follow‐up period: not stated |
|
| Interventions | Treatment group (30): bone marrow stem cell Intervention (22): peripheral blood stem cell |
|
| Outcomes |
|
|
| Notes | The article was reported in Chinese and was translated into English for the purposes of the current review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...., were randomly divided into two groups....." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding appears very unlikely, as 1 group had blood drawn from the bone marrow, and the other had blood drawn from the peripheral blood. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the outcome assessor was blinded to participants' assigned groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are accounted for for all outcomes. |
| Selective reporting (reporting bias) | Low risk | We did not identify any reporting bias. |
| Other bias | High risk | There were unit of analysis issues as allocation was made at the level of the legs rather than patients, and study authors did not adjust their data to account for the effect of clustering. |
ABI: ankle‐brachial pressure index. AV: atrioventricular. BM‐MNCs: bone marrow‐mononuclear cells. BM‐MSCs: bone marrow‐mesenchymal cells. BMCs: bone marrow cells. CLI: critical limb ischaemia. CREST: calcinosis, Raynaud phenomenon, oesophageal dysmotility, sclerodactyly, and telangiectasia. EQ5D: EuroQoL–5 Dimensional forma. G‐CSF: granulocyte colony‐stimulating factor. IA: intra‐arterial. IM: intramuscular. LASO: limb arteriosclerosis obliterans. mPBSCs: mobilised peripheral blood stem cells. MRA: magnetic resonance angiography. NS: normal saline. PB‐MNCs: peripheral blood‐mononuclear cells. PBSCs: peripheral blood stem cells. PFWD: pain‐free walking distance. PFWT: pain‐free walking time. RCT: randomised controlled trial. SD: standard deviation. TASC: TransAtlantic Intersociety Consensus. TBI: toe‐brachial index. TcO₂: transcutaneous oxygen tension. VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afan 2015 | This study investigated the number of endothelial progenitor cells (EPCs) in PAD patients and did not study clinical outcomes after cell implantation. |
| Amann 2008 | This is a randomised, double‐blind, placebo‐controlled phase 3 study for autologous bone marrow cell transplantation in critical limb ischaemia. |
| Amato 2012 | This is a pilot single‐arm study investigating the therapeutic effects of peripheral blood mononuclear cells (PB‐MNCs) in CLI patients. |
| Arai 2006 | This is an RCT investigating BM‐MNCs plus conventional drug therapy compared with conventional treatment in PAD patients. |
| Arici 2015 | This is a prospective single‐centre non‐randomised single‐arm study assessing the safety and effectiveness of highly purified CD133+ autologous stem cells in CLI. |
| Bartsch 2007 | This is a non‐randomised study investigating the therapeutic effects of IM + IA implantation of BM‐MNCs in CLI patients. |
| Barć 2006 | This is an RCT investigating BM‐MNC therapy compared with conventional therapy for CLI patients. |
| Benoit 2011 | This is an RCT investigating BMCs compared with sham injection in CLI patients. |
| Bing 2009 | This study investigated the effects of BM‐MSCs on patients with diabetic foot only, not CLI patients. |
| Bura 2014 | This study investigated the feasibility and safety of intramuscular injections of autologous cultured adipose‐derived stroma/stem cells and included no control group. |
| Burt 2010 | This study investigated the safety and feasibility of autologous CD133+ cells and included no control group. |
| Capiod 2009 | This study compared the characteristics of bone marrow and peripheral blood mononuclear cells for CLI and did not report clinical outcomes. |
| Chochola 2008 | This is a single‐arm study investigating the safety and feasibility of IA BM‐MNC injection in CLI patients. |
| Choi 2012 | This is an RCT conducted to determine the effectiveness of G‐CSF in stimulating angiogenesis in patients with CLI. Abstract only |
| Cobellis 2008 | This is a non‐randomised study investigating the long‐term effects of repeated autologous transplantation of bone marrow cells in patients affected by peripheral arterial disease. |
| Cobellis 2010 | This is a case report showing the beneficial effects of vascular endothelial growth factor secreted from stromal cells in supporting endothelial cell functions in a patient with CLI. |
| Darinskas 2017 | This is a pilot study using stromal vascular fraction cells for the treatment of CLI. |
| Das 2013 | This is a phase 1 single‐arm study investigating the safety and efficacy of allogeneic BM‐MSCs in CLI patients. |
| Dash 2009 | This is an RCT investigating BM‐MSCs compared with standard wound care in patients with lower limb ischaemia. |
| De Angelis 2014 | This is a prospective non‐randomised study investigating the efficacy of peripheral blood mononuclear cells (PM‐MNCs) in patients with CLI. |
| Debin 2008 | This is a randomised trial investigating the efficacy and safety of autologous BM‐MSCs compared with control interventions in patients with lower limb ischaemia. |
| Dou 2015 | This study assessed the long‐term effectiveness and safety of autologous BM‐MNC transplantation compared with standard medical therapies in patients with critical diabetic lower arteriosclerosis obliterans (ASO). |
| Doudar 2013 | This is a randomised trial investigating the safety and efficacy of PB‐MNCs compared with control interventions in patients with chronic lower limb ischaemia. |
| Du 2017 | This clinical trial studied the clinical efficacy of implanted umbilical cord MSCs combined with bone marrow stem cells compared with implanted bone marrow stem cells for treatment of lower limb ischaemia. |
| Dubsky 2013 | This non‐randomised study compared the effects of BM‐MNCs vs mPBSCs vs standard medical therapy for diabetic patients with CLI. |
| Duong 2008 | This is a multi‐centre phase 1 non‐randomised study including a single group treated with BM‐MNCs. |
| Flugelman 2017 | Study participants received venous endothelial cells (ECs) combined with venous smooth muscle cells (SMCs). |
| Franz 2011 | This single‐arm study investigated the therapeutic effects of IM + IA implantation of BM‐MNCs in CLI. |
| Frogel 2017 | This is a pilot open‐label study of treatment progenitor cells derived from peripheral blood for CLI patients. |
| Gabr 2011 | This is a single‐arm prospective study using intramuscular injection of unfractionated autologous BM‐MNCs in CLI patients. |
| Grossman 2016 | This is a phase 1 safety, dose‐escalating, non‐randomised, open‐label study of autologous, fully differentiated venous endothelial and smooth muscle cells called MultiGeneAngio (MGA) for claudication due to peripheral artery disease. |
| Gu 2006 | This is a non‐randomised study comparing cell dosage for treatment of CLI patients. |
| Gu 2007 | This is a non‐randomised study comparing BM‐MNCs vs mPBSCs for treatment of CLI patients. |
| Gu 2017 | Study participants receive 2 injections of G‐CSF (300 µg) before BM‐MNC transplantation. |
| Guo 2018 | In this retrospective study, participants were treated with smoking cessation and either aspirin (100 mg/d) alone or aspirin and ABMMNC injection according to participant preference. |
| Gupta 2013 | This randomised placebo‐controlled phase 1/2 study assessed the safety and efficacy of allogeneic BM‐MSCs in CLI patients. |
| Gupta 2017 | This phase 2, non‐randomised, multi‐centre, dose‐finding study assessed the efficacy of allogeneic BM‐MSCs given to CLI patients with Buerger's disease. |
| Harunarashid 2016 | Study participants received both BM‐MSCs with BM‐MNCs or BM‐MNCs alone. |
| Heo 2016 | This single‐arm study used autologous BM‐MNCs for CLI. |
| Hernandez 2007 | This non‐randomised study investigated the therapeutic effects of BM‐MNC implantation in CLI patients. |
| Higashi 2004 | This phase 1 single‐arm clinical trial was conducted to determine the effect of autologous bone marrow‐mononuclear cell (BM‐MNC) implantation on endothelial dysfunction in patients with limb ischaemia. |
| Holzinger 1994 | This study investigated the therapeutic effects of BM‐MNC implantation in patients with chronic arterial occlusive disease or venous post‐thrombotic syndrome, not CLI. |
| Hoshino 2007 | This single‐arm study investigated the therapeutic effects of G‐CSF mobilised PBSCs in diabetic patients on haemodialysis with limb ischaemia. |
| Huang 2005 | This randomised study was conducted to investigate the efficacy of PB‐MNCs vs control interventions in patients with CLI. |
| Iafrati 2016 | This randomised placebo‐controlled study was conducted to assess the safety of autologous bone marrow concentrate in patients with CLI. |
| Idei 2011 | This non‐randomised trial was undertaken to investigate the effects of bone marrow‐mononuclear cells (BM‐MNCs) implanted in patients with CLI. |
| Ishida 2005 | This single‐arm study was conducted to investigate the therapeutic effects of G‐CSF mobilised PBSCs in CLI patients. |
| Iso 2010 | This single‐arm study investigated the therapeutic effects of BM‐MNCs in CLI patients. |
| Kamata 2007 | This non‐randomised study compared the therapeutic effects of BM‐MNCs and PB‐MNCs in ischaemic digits of patients with connective tissue diseases. |
| Kawamoto 2009 | This single‐arm study investigated the safety and feasibility of CD34+ cell therapy in CLI patients. |
| Kinoshita 2012 | This study investigated the therapeutic effects of G‐CSF mobilised PBSCs in CLI and included no control group. |
| Kirana 2012 | This study used specialised cultured cell products (CD90+ tissue repair cells) that have been expanded from autologous bone marrow. |
| Kolvenbach 2010 | This single‐arm study investigated the safety and feasibility of intraoperative stem cell treatment in combination with bypass surgery and/or interventional treatment in CLI patients. |
| Kondo 2016 | This single‐arm, non‐randomised, open‐label, historically controlled study was designed to assess the safety and feasibility of intramuscular injection of ADRCs for treatment of patients with CLI. |
| Kondo 2018 | This retrospective, observational, non‐controlled study included no‐option CLI patients who had BM‐MNC implantation performed. |
| Lara‐Hernandez 2010 | This prospective pilot study investigated the safety and efficacy of therapeutic angiogenesis in patients with CLI. |
| Lasala 2010 | This phase 1 non‐randomised single‐arm study assessed the safety and efficacy of combined BM‐MNCs plus BM‐MSCs in CLI patients. |
| Lee 2012 | This study investigated the safety and effects of adipose tissue‐derived stem cells and included no control group. |
| Lenk 2005 | This single‐arm study investigated the safety and therapeutic effects of circulating blood‐derived progenitor cells in CLI patients. |
| Li 2013 | This single‐blinded study investigated the effects of BM‐MNCs compared with control interventions in patients with CLI. |
| Madaric 2016 | This study investigated factors predictive of the effects of BMCs on progression of advanced CLI in patients with CLI receiving autologous BMCs (SmartPreP2) by local intramuscular (n = 32) or intra‐arterial (n = 30) application. |
| Madaric 2017 | This study investigated levels of asymmetrical dimethylarginine (ADMA) and changes in oxidative stress in patients with CLI after BM‐MNC therapy and did not study the clinical outcomes of cell treatment. |
| Maione 2013 | This pilot non‐randomised non‐controlled phase 1 study assessed the effects of bone marrow cells in CLI patients. |
| Majumdar 2015 | This phase 2 study investigated allogeneic mesenchymal stromal cells in CLI. |
| Malyar 2014 | This non‐randomised single‐arm study evaluated the effects of autologous BM‐MNCs in severe PAD. |
| Matoba 2009 | This single‐arm study investigated the therapeutic effects of BM‐MNCs in CLI patients. |
| Matsui 2003 | Study participants with bilateral ischaemic limbs received both autologous mononuclear bone marrow and peripheral blood mononuclear cells. |
| Mohamed 2017 | This is a phase 1b, non‐randomised, uncontrolled, open‐label, dose‐escalation study. |
| Mohammadzadeh 2013 | This study investigated the efficacy and safety of transplanted mPBSCs compared with control interventions in diabetic patients with CLI. |
| Moriya 2009 | This study is a retrospective analysis of the therapeutic effects of PB‐MNCs for limb ischaemia. |
| Motukuru 2008 | This single‐arm study investigated the efficacy of IM implantation of BM‐MNCs in CLI. |
| Murphy 2011 | This non‐randomised single‐arm study assessed the safety and efficacy of autologous BM‐MNCs. |
| Murphy 2017 | This double‐blinded, placebo‐controlled trial was designed to assess the safety and efficacy of autologous concentrated bone marrow aspirate vs placebo. |
| Napoli 2008 | This non‐randomised study compared the effects of BM‐MNCs plus antioxidant/L‐arginine vs antioxidant/L‐arginine only. |
| NCT00306085 | This is a non‐randomised study. |
| NCT00434616 | This multi‐centre randomised placebo‐controlled double‐blind clinical study investigated autologous BMC implantation vs saline injection. |
| NCT00539266 | This clinical trial compared BM‐MNCs vs placebo in patients with limb ischaemia. |
| NCT00922389 | This clinical trial compared peripheral blood‐derived stem cells vs standard therapy in patients with CLI. |
| NCT01049919 | This clinical trial compared autologous concentrated bone marrow aspirate vs placebo in patients with CLI. |
| NCT01245335 | This clinical trial compared bone marrow aspirate concentrate vs placebo in patients with CLI. |
| NCT01584986 | This randomised open‐label clinical study assessed the safety and efficacy of autologous angiogenic cell precursors (ACPs) compared with SMT. |
| NCT02336646 | This randomised clinical trial compared allogeneic MSCs vs placebo in patients with CLI. |
| NCT03174522 | This clinical trial compared Rexmyelocel‐T vs placebo in patients with CLI. |
| NCT03214887 | This clinical trial investigated the safety and efficacy of hyaluronan (HA) combined with BM‐MNCs for PAD. |
| NCT03304821 | This double‐blind placebo‐controlled randomised clinical study examined whether 3 weeks of 3‐times‐a‐week injection of GM‐CSF would improve measures of ischaemia in patients with intermittent claudication compared with placebo. |
| NCT03339973 | This clinical trial is investigating the efficacy and safety of 1 dose of allo‐APZ2‐PAOD administered intramuscularly into the affected lower leg of patients with peripheral arterial occlusive disease. |
| Nemcova 2017 | This study was conducted to evaluate serum levels of angiogenic cytokines in diabetic patients with CLI treated by ACT vs patients treated by PTA. |
| Niven 2017 | This pilot open‐label study examined treatment with progenitor cells for peripheral vascular disease. |
| Nizankowski 2005 | This non‐randomised single‐arm study investigated the safety and therapeutic effects of BM‐MNC implantation in CLI patients. |
| Ohtake 2017 | This prospective phase 1/2 interventional clinical trial examined autologous granulocyte colony‐stimulating factor (G‐CSF)‐mobilised CD34+ cell transplantation in HD patients with CLI. |
| Onodera 2011 | This non‐randomised trial compared BM‐MNCs vs mPB‐MNCs in CLI patients. |
| Ozturk 2012 | This prospective randomised study evaluated the therapeutic effects of mobilised peripheral blood mononuclear cells vs control interventions in type 2 diabetic patients with CLI. |
| Peeters 2016 | This study investigated quality of life between BM‐MNCs and placebo in patients with severe limb ischaemia. |
| Perin 2011 | This study used specialised cell products (aldehyde dehydogenase bright cells) that had been isolated from autologous bone marrow via immunomagmetic beads and cell sorters. |
| Perin 2017 | This study randomised participants 1:1 to receive aldehyde dehydogenase bright (ALDHbr) cells or placebo. |
| Pignon 2017 | This multi‐centre randomised controlled double‐blind clinical trial compared BM‐MNCs vs control interventions in patients with CLI. |
| Ponemone 2017 | This open‐label single‐arm feasibility study evaluated the safety and therapeutic effectiveness of autologous bone marrow cell (aBMC) concentrate. |
| Poole 2013 | This RCT investigated the effects of granulocyte macrophage colony‐stimulating factor (GM‐CSF), not cell therapy, in patients with intermittent claudication |
| Powell 2012 | This RCT assessed the safety and efficacy of patient‐specific multi‐cellular cell products (Ixmyecel‐T) that had been expanded from autologous bone marrow in an automated closed‐culture system. |
| Prochazka 2010 | This randomised study investigated major limb amputation between participants given autologous bone marrow cells compared to standard care for CLI and foot ulcer. |
| Rajagopalan 2003 | This study assessed the effects of vascular endothelial growth factor, not cell therapy, in PAD patients. |
| Ruiz‐Salmeron 2011 | This non‐randomised single‐arm study demonstrated neoangiogenesis after intra‐arterial infusion of autologous BM‐MNCs in diabetic patients with CLI. |
| Saito 2007 | This single‐arm study investigated the therapeutic effects of IM implantation of BM‐MNCs in CLI patients. |
| Schiavetta 2012 | This multi‐centre prospective not‐controlled phase 2 study examined bone marrow stem cells for no‐option CLI patients. |
| Smadja 2012 | This phase 1 non‐randomised study assessed the numbers of endothelial progenitor cells in peripheral blood and bone marrow cells. Results include no clinical outcome data. |
| Subramaniyam 2009 | This study included patients with intermittent claudication and excluded patients with CLI or rest pain. Also, patients received subcutaneous GM‐CSF, not stem cell treatment. |
| Szabo 2013 | This RCT assessed the safety and efficacy of a specialised cultured cell product (VesCell) that had been expanded from autologous peripheral blood. |
| Takagi 2011 | This study compared the therapeutic effects of autologous bone marrow‐derived stem cells vs basic fibroblast growth factor. |
| Tateishi‐Yuyama 2002 | This RCT investigated BM‐MNCs vs placebo and BM‐MNCs vs PB‐MNCs in CLI patients. |
| Teraa 2014 | This study investigated BM‐MNCs vs placebo in patients with CLI. |
| Teraa 2015 | This randomised double‐blind placebo‐controlled clinical trial compared BM‐MNCs vs placebo in patients with limb ischaemia. |
| Tournois 2015 | This phase 1 and 2 clinical trial examined bone marrow‐CTPs (cell therapy products) and CTPs obtained by cytapheresis (peripheral blood‐CTPs). |
| Walter 2011 | This multi‐centre phase 2 trial with a double‐blind randomised design evaluated the safety and feasibility BM‐MNCs vs control interventions in patients with CLI. |
| Wang 2014 | This RCT assessed the efficacy and safety of the combination of mPBSC and Panax notoginseng saponins (PNS) for treatment of CLI. |
| Wang 2017 | This multi‐centre randomised double‐blind placebo‐controlled trial was designed to assess the efficacy of intramuscular injections of cBMA for promoting amputation‐free survival in patients with poor‐option CLI. |
| Wang 2018 | Study participants received intramuscular injections of allogeneic MSCs (CHAMP; n = 16) or autogenous concentrated bone marrow aspirate. |
| Wester 2008 | This pilot study assessed the effects of autologous BM‐MNCs in CLI patients. |
| Wijnand 2018 | This study used allogeneic mesenchymal stem cells for CLI. |
| Yanishi 2017 | This single‐arm study used BM‐MNCs for CLI. |
| Zafarghandi 2010 | This study investigated the therapeutic effects of granulocyte colony‐stimulating factor (G‐CSF) administration following implantation of autologous BM‐MNCs for patients with lower limb ischaemia. Both control and intervention groups received BM‐MNCs. |
| Zhang 2016 | This prospective non‐randomised trial evaluated the efficacy and immune‐regulatory impact of intra‐arterial infusion of autologous CD133+ cells for diabetic patients with PAD. |
| Zhao 2008 | Study participants received both autologous mononuclear bone marrow and peripheral blood mononuclear cells. |
| Zhou 2017 | This randomised open parallel‐control clinical study compared BM‐MNC with SMT. |
| Zhou 2017a | This prospective single‐centre open‐label randomised controlled clinical trial compared peripheral blood CD34+ cells transfected with vascular endothelial growth factor 165 (VEGF165) vs SMT. |
aBMC: autologous bone marrow cell. ABMMNCs:autologous bone marrow‐derived mononuclear cells. ACPs: angiogenic cell precursors. ACT: autologous cell therapy. ADRCs: adipose‐derived regenerative cells. ALDHbr: aldehyde dehydogenase bright. ASO: arteriosclerosis obliterans. BMCs: bone marrow cells. BM‐MNCs: bone marrow‐mononuclear cells. BM‐MSCs: bone marrow‐mesenchymal stem cells. cBMAs: concentrated bone marrow aspirate. CLI: critical limb ischaemia. CTPs: cell therapy products. ECs: endothelial cells. EPCs: endothelial progenitor cells. G‐CSF: granulocyte colony‐stimulating factor. GM‐CSF: granulocyte‐macrophage colony‐stimulating factor. HA: hyaluronan. HD: haemodialysis. IA: intra‐arterial. IM: intramuscular. MGA: MultiGeneAngio. mPB‐MNCs: mobilised peripheral blood mononuclear cells. mPBSCs: mobilised peripheral blood stem cells. MSCs: mesenchymal stem cells. PAD: peripheral arterial disease. PB‐MNCs: peripheral blood mononuclear cells. PBSCs: peripheral blood stem cells. PM‐MNCs: peripheral blood mononuclear cells. PNS: Panax notoginseng saponins. PTA: percutaneous transluminal angioplasty. RCT: randomised controlled trial. SMCs: smooth muscle cells. SMT: Standard Medical Treatment. VEGF: vascular endothelial growth factor.
Characteristics of studies awaiting assessment [ordered by study ID]
Gurunathan 2009.
| Methods | Multi‐centre RCT |
| Participants | Sixty patients with lower limb CLI as per the Rutherford classification 4 or 5 were randomised to 2 arms |
| Interventions | IM injections (n = 30) IA + IM injections (n = 30) |
| Outcomes |
|
| Notes | Abstract, incomplete reporting of outcome data |
Korymasov 2009.
| Methods | Randomised double‐blind placebo‐controlled study |
| Participants | Patients were subdivided into 3 groups, each consisting of 14 participants. |
| Interventions | Group 1: participants received autologous progenitor cells CD133+ Group 2: participants were given the leucocytic fraction of the marrow (CD34+) Group 3: participants (comparison group) received normal saline as a placebo |
| Outcomes | Clinical outcomes were evaluated according to the Rutherford scale, which revealed that group 1 and group 2 participants given the cellular material exhibited a statistically significant improvement in their clinical condition. Distance of pain‐free walk was noted to statistically significantly increase in group 2 and group 2 participants. |
| Notes | This article was written in the Russian language could not be assessed fully, as we are still awaiting its translated full‐text version. |
Molavi 2016.
| Methods | Allocation: randomised controlled trial Endpoint classification: safety study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures: side effects Secondary outcome measures:
|
| Notes |
NCT00595257.
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: Injection of BMAC into ischaemic limb. Intervention: device: centrifuge, laboratory, tabletop (SmartPReP2 BMAC System) Active comparator: injection and infusion of BMAC into ischaemic lower limb. Intervention: device: centrifuge, laboratory, tabletop (SmartPReP2 BMAC System) |
| Outcomes | Primary outcome measures: avoidance of amputation (time frame: 60 days) Secondary outcome measures: measurement of haemodynamic response (time frame: 60 days) |
| Notes | Clinical trial protocol without outcome data. According to ClincialTrials.gov, this study has been completed. |
NCT00987363.
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Notes | Clinical trial protocol without outcome data. According to ClincialTrials.gov, this study has been completed. |
NCT02993809.
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria: limb ischaemia patients (e.g. arteriosclerosis obliterans, diabetic critical limb ischemia, thromboangiitis obliterans)
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Notes | According to ClincialTrials.gov, this study is not yet open for participant recruitment. |
ABI: ankle‐brachial pressure index. ALT: alanine aminotransferase. AngioRNM: magnetic resonance angiography. AngioTC: computed tomography angiography. ASA: American Society of Anesthesiologists. AST: aspartate aminotransferase. BM‐ECs: bone marrow endothelial cells. BM‐MNCs: bone marrow‐mononuclear cells. BMACs: bone marrow cells. BMT: bone marrow transplant. CLI: critical limb ischaemia. COX: cyclo‐oxygenase. EF: ejection fraction. G‐CSF: granulocyte colony‐stimulating factor. Hb: haemoglobin. HbA1c: glycosylated haemoglobin. HBV: hepatitis B virus. HCV: hepatitis C virus. HIV: human immunodeficiency virus. IA: intra‐arterial. IM: intramuscular. INR: international normalised ratio. IRB/IEC: institutional review board/independent ethics committee. MDS: myelodysplastic syndrome. MI: myocardial infarction. NHL: non‐Hodgkin lymphoma. NYHA: New York Heart Association. PAP: Papanicolaou test. PB‐MNCs: peripheral blood‐mononuclear cells. PFWD: pain‐free walking distance. PRPE: platelet‐rich plasma extract. PSA: prostate‐specific antigen. PTT: partial thromboplastin time. PVD: peripheral vascular disease. RCT: randomised controlled trial. TASC: TransAtlantic Intersociety Consensus. TBI: toe‐brachial index. TcO₂: transcutaneous oxygen tension. TcPO₂: transcutaneous oxygen. VAS: visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
NCT00311805.
| Trial name or title | Autologous CD34+ stem cell injection for severe intermittent claudication (leg pain) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (patient, investigator) Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Biological: autologous stem cells (CD34+), intramuscular injections |
| Outcomes | Primary outcome measures: safety of intramuscular administration of CD34‐positive cells (time frame: all) Secondary outcome measures: functional improvement (time frame: week 12, month 6, month 12) |
| Starting date | April 2006 |
| Contact information | |
| Notes | Primary completion date: December 2012 (final data collection date for primary outcome measure) |
NCT00753025.
| Trial name or title | Autologous bone marrow for lower extremity ischemia treating |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Biological: injection of isolated CD 133+ cells |
| Outcomes | Primary outcome measure: increase in painless walking distance |
| Starting date | September 2008 |
| Contact information | |
| Notes | Primary completion date: September 2008 (final data collection date for primary outcome measure) |
NCT01257776.
| Trial name or title | Human adipose derived mesenchymal stem cells for critical limb ischemia (CLI) in diabetic patients |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: none (open label) Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Biological: autologous adipose‐derived mesenchymal stem cells |
| Outcomes | Primary outcome measures: angiographic assessment of neovasculogenesis (angiogenesis plus arteriogenesis) (time frame: 6 months) Secondary outcome measures: ankle‐brachial index (time frame: 1 month, 6 months, 12 months); University of Texas Classification at target limb (time frame: 1 month, 6 months, 12 months) |
| Starting date | December 2010 |
| Contact information | |
| Notes | Primary completion date: August 2015 (final data collection date for primary outcome measure) |
NCT01408381.
| Trial name or title | Intra‐arterial Infusion of autologous bone marrow mononuclear cells in non‐diabetic patients with critical limb ischaemia (CLI) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: none (open label) Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Biological: intra‐arterial infusion of autologous bone marrow mononuclear cells |
| Outcomes | Primary outcome measures: adverse events (time frame: 6 months)
Secondary outcome measures:
|
| Starting date | April 2006 |
| Contact information | |
| Notes | Primary completion date: December 2012 (final data collection date for primary outcome measure) |
NCT01446055.
| Trial name or title | Safety and efficacy study of autologous BM‐MNC processed by two methods for treating patients with chronic limb ischemia |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: single‐blind (patient) Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: autologous BM‐MNC is enriched with ResQ process (an automatic cell separator). Then the cell product is implanted into the ischaemic limbs of a patient Active comparator: a conventional method based on Ficoll cell separation is used to process bone marrow |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | October 2011 |
| Contact information | Contact: Yongquan Gu, MD; 13910002909; gu‐yq@263.net |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently on clinical trials.gov. |
NCT01745744.
| Trial name or title | Application of cell regeneration therapy with mesenchymal stem cells from adipose tissue in critical chronic ischaemic syndrome of lower limbs (CLI) in non‐diabetic patients |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: low dose
Experimental: high dose
No intervention: control
|
| Outcomes | Primary outcome measures: numbers of adverse events and serious adverse events Secondary outcome measures: evolution of chronic critical ischaemia parameters: ankle‐brachial index, transcutaneous oxygen tension, degree of Rutherford‐Becker, larger ulcer size (as Texas classification), twin perimeter, scores for pain and intermittent claudication (walking test) |
| Starting date | February 2011 |
| Contact information | |
| Notes | This study is ongoing but is not recruiting participants. |
NCT02454231.
| Trial name or title | Monocentric trial: stem cell emergency life threatening limbs arteriopathy (SCELTA) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: peripheral blood EPC injection Active comparator: bone marrow MNC injection |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | September 2009 |
| Contact information | Contact: Enrico Maggi, professor; +39 055/2751802; enrico.maggi@unifi.it Contact: Francesco Annunziato, professor; +39 0552758337; francesco.annunziato@unifi.it |
| Notes | Primary completion date: May 2015 (final data collection date for primary outcome measure) |
ABI: ankle‐brachial pressure index. ALT: alanine aminotransferase. APTT: activated partial thromboplastin time. AST: aspartate aminotransferase. BM‐MNCs: bone marrow‐mononuclear cells. BMI: body mass index. CLI: critical limb ischaemia. ECG: electrocardiography. EPC: endothelial progenitor cell. HbA1c: glycosylated haemoglobin. HIV: human immunodeficiency virus. IM: intramuscular. MNC: mononuclear cell. NYHA: New York Heart Association. PAD: peripheral arterial disease. PFWD: pain‐free walking distance. pCO₂: partial pressure of carbon dioxide. pO₂: partial pressure of oxygen. RCT: randomised controlled trial. TASC: TransAtlantic Intersociety Consensus.. TBI: toe‐brachial index. TcO₂: transcutaneous oxygen tension.
Differences between protocol and review
1. Under types of outcome measures, we have amended the primary outcomes to include amputation rate and deleted amputation‐free survival because only one RCT reported on amputation‐free survival (Losordo 2012), and most included studies used amputation rate and not amputation‐free survival as the primary clinical endpoint. The 'number of newly formed collaterals in the lower limbs as analysed by angiography' has been added to the list of secondary outcomes. 2. We have revised the section under Unit of analysis issues to incorporate statements on some studies that reported their results using limbs rather than patients as the unit of analysis. 3. Unit of analysis issues ‐ We have inserted sensitivity analyses for relevant outcomes to address the issues related to discrepancies in the unit of analysis used by studies for the same comparison group. 4. To reflect the importance of outcomes, we have made wound/ulcer healing a primary outcome and reduction in rest pain a secondary outcome.
Contributions of authors
SFAW: designed the project and review, searched the PubMed database, undertook data extraction and analysis, performed statistical analysis, and wrote the review. NAI: searched the PubMed database, undertook data extraction and analysis, assessed risk of bias in the included studies, and prepared tables and figures. WFWJ: reviewed the results and edited the manuscript. NAM: contributed to extracting and interpreting the data and assessed risk of bias in the included studies. MKAAH: searched the databases. HH: reviewed the findings of the analysis. NML: contributed to designing the review, analysing data, performing statistical analyses, assessing risk of bias in the included studies, and reviewing and editing the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
SFAW: none known. NAI: none known. WFWJ: none known. NAM: none known. MKAAH: none known. HH: none known. NML: none known.
New
References
References to studies included in this review
Gu 2008 {published data only}
- Gu YQ, Zhang J, Guo LR, Qi LX, Zhang SW, Xu J, et al. Transplantation of autologous bone marrow mononuclear cells for patients with lower limb ischemia. Chinese Medical Journal 2008;121(11):963‐7. [PUBMED: 18706241] [PubMed] [Google Scholar]
Huang 2007 {published data only}
- Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. Randomised comparison of G‐CSF‐mobilized peripheral blood mononuclear cells versus bone marrow‐mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thrombosis and Haemostasis 2007;98(6):1335‐42. [PUBMED: 18064333] [DOI] [PubMed] [Google Scholar]
Klepanec 2012 {published data only}
- Klepanec A, Mistrik M, Altaner C, Valachovicova M, Olejarova I, Slysko R, et al. No difference in intra‐arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplantation 2012;21(9):1909‐18. [PUBMED: 22472173] [DOI] [PubMed] [Google Scholar]
- Madaric J, Klepanec A, Mistrik M, Altaner C, Valachovicova M, Necpal R, et al. Autologous bone marrow cells transplantation in patients with advanced critical limb ischemia: no difference in intra‐arterial and intramuscular application. Journal of the American College of Cardiology 2011;57(14):E1473. [Google Scholar]
Losordo 2012 {published data only}
- Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circulation. Cardiovascular Interventions 2012;5(6):821‐30. [PUBMED: 23192920] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2011 {published data only}
- Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow‐derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double‐blind, randomized, controlled trial. Diabetes Research and Clinical Practice 2011;92(1):26‐36. [PUBMED: 21216483] [DOI] [PubMed] [Google Scholar]
Van Tongeren 2008 {published data only}
- Tongeren RB, Hamming JF, Fibbe WE, Weel V, Frerichs SJ, Stiggelbout AM, et al. Intramuscular or combined intramuscular/intra‐arterial administration of bone marrow mononuclear cells: a clinical trial in patients with advanced limb ischemia. Journal of Cardiovascular Surgery 2008;49(1):51‐8. [PUBMED: 18212687] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang HF, Zhao ZG, Zhang CL, Yuan HJ, Wang YF. Autologous transplantation of bone marrow stem cells versus peripheral blood stem cells for treatment of diabetic lower limb ischemia: a comparative study in 52 cases. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13(6):1109‐12. [Google Scholar]
References to studies excluded from this review
Afan 2015 {published data only}
- Afan S, Bitterli L, Buhler S, DiSanto S, Zwahlen M, Schmidlin K, et al. Endothelial progenitor cells as biological marker of peripheral arterial disease. Vasa‐European Journal of Vascular Medicine 2015;44(Suppl 88):59‐60. [DOI] [PubMed] [Google Scholar]
Amann 2008 {published data only}
- Amann B, Ludemann C, Ruckert R, Lawall H, Liesenfeld B, Schneider M, et al. Design and rationale of a randomized, double‐blind, placebo‐controlled phase III study for autologous bone marrow cell transplantation in critical limb ischemia: the BONe Marrow Outcomes Trial in Critical Limb Ischemia (BONMOT‐CLI). VASA. Zeitschrift fur Gefasskrankheiten 2008;37(4):319‐25. [PUBMED: 19003741] [DOI] [PubMed] [Google Scholar]
Amato 2012 {published data only}
- Amato B, Compagna R, Della Corte GA, Martino G, Bianco T, Coretti G, et al. Peripheral blood mono‐nuclear cells implantation in patients with peripheral arterial disease: a pilot study for clinical and biochemical outcome of neoangiogenesis. BMC Surgery 2012;12(Suppl 1):S1. [PUBMED: 23173612] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Arai 2006 {published data only}
- Arai M, Misao Y, Nagai H, Kawasaki M, Nagashima K, Suzuki K, et al. Granulocyte colony‐stimulating factor: a noninvasive regeneration therapy for treating atherosclerotic peripheral artery disease. Circulation Journal 2006;70(9):1093‐8. [PUBMED: 16936417] [DOI] [PubMed] [Google Scholar]
Arici 2015 {published data only}
- Arici V, Perotti C, Fabrizio C, Fante C, Ragni F, Alessandrino F, et al. Autologous immuno magnetically selected CD133+ stem cells in the treatment of no‐option critical limb ischemia: clinical and contrast enhanced ultrasound assessed results in eight patients. Journal of Translational Medicine 2015;13:342. [PUBMED: 26526721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barć 2006 {published data only}
- Barć P, Skóra J, Pupka A, Turkiewicz D, Dorobisz AT, Garcarek J, et al. Bone‐marrow cells in therapy of critical limb ischaemia of lower extremities ‐ own experience. Acta Angiologica 2006;12(4):155‐66. [Google Scholar]
Bartsch 2007 {published data only}
- Bartsch T, Brehm M, Zeus T, Kogler G, Wernet P, Strauer BE. Transplantation of autologous mononuclear bone marrow stem cells in patients with peripheral arterial disease (the TAM‐PAD study). Clinical Research in Cardiology 2007;96(12):891‐9. [PUBMED: 17694378] [DOI] [PubMed] [Google Scholar]
Benoit 2011 {published data only}
- Benoit E, O'Donnell TF Jr, Iafrati MD, Asher E, Bandyk DF, Hallett JW, et al. The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: implications for clinical trial design. Journal of Translational Medicine 2011;9:165. [PUBMED: 21951607] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafrati MD, Hallett JW, Geils G, Pearl G, Lumsden A, Peden E, et al. Early results and lessons learned from a multicenter, randomized, double‐blind trial of bone marrow aspirate concentrate in critical limb ischemia. Journal of Vascular Surgery 2011;54(6):1650‐8. [PUBMED: 22019148] [DOI] [PubMed] [Google Scholar]
Bing 2009 {published data only}
- Bing C, De‐bin L, Zi‐wen L, You‐zao J, Fu‐hua W, Qi‐nan W, et al. Autologous bone marrow mesenchymal stem cell transplantation for treatment of diabetic foot following amplification in vitro. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13:6227‐30. [Google Scholar]
Bura 2014 {published data only}
- Bura A, Planat‐Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, et al. Phase I trial: the use of autologous cultured adipose‐derived stroma/stem cells to treat patients with non‐revascularizable critical limb ischemia. Cytotherapy 2014;16(2):245‐57. [PUBMED: 24438903] [DOI] [PubMed] [Google Scholar]
Burt 2010 {published data only}
- Burt RK, Testori A, Oyama Y, Rodriguez HE, Yaung K, Villa M, et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplantation 2010;45(1):111‐6. [PUBMED: 19448678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Capiod 2009 {published data only}
- Capiod JC, Tournois C, Vitry F, Sevestre MA, Daliphard S, Reix T, et al. Characterization and comparison of bone marrow and peripheral blood mononuclear cells used for cellular therapy in critical leg ischaemia: towards a new cellular product. Vox Sanguinis 2009;96(3):256‐65. [PUBMED: 19207166] [DOI] [PubMed] [Google Scholar]
Chochola 2008 {published data only}
- Chochola M, Pytlik R, Kobylka P, Skalicka L, Kideryova L, Beran S, et al. Autologous intra‐arterial infusion of bone marrow mononuclear cells in patients with critical leg ischemia. International Angiology 2008;27(4):281‐90. [PUBMED: 18677289] [PubMed] [Google Scholar]
Choi 2012 {published data only}
- Choi ET, Geraghty P, Cooke C, Schechtman K, Link D, Chambers CM, Veeraswamy RK. Stem cell mobilization to treat severe peripheral artery disease (STEMPAD). Vascular Annual Meeting 2012:LB7.
- NCT00797056. Stem cell mobilization by G‐CSF to treat severe peripheral artery disease. clinicaltrials.gov/ct2/show/NCT00797056 (first received 25 November 2008).
Cobellis 2008 {published data only}
- Cobellis G, Silvestroni A, Lillo S, Sica G, Botti C, Maione C, et al. Long‐term effects of repeated autologous transplantation of bone marrow cells in patients affected by peripheral arterial disease. Bone Marrow Transplantation 2008;42(10):667‐72. [PUBMED: 18695661] [DOI] [PubMed] [Google Scholar]
Cobellis 2010 {published data only}
- Cobellis G, Botti C, Taddeo A, Silvestroni A, Lillo S, Ponte A, et al. Successful bone marrow transplantation reveals the lack of endothelial progenitor cells mobilization in a patient with critical limb ischemia: a case report. Transplantation Proceedings 2010;42(7):2816‐20. [PUBMED: 20832596] [DOI] [PubMed] [Google Scholar]
Darinskas 2017 {published data only}
- Darinskas A, Paskevicius M, Apanavicius G, Vilkevicius G, Labanauskas L, Ichim TE, et al. Stromal vascular fraction cells for the treatment of critical limb ischemia: a pilot study. Journal of Translational Medicine 2017;15(1):143. [PUBMED: 28629476] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Das 2013 {published data only}
- Das AK, Bin Abdullah BJ, Dhillon SS, Vijanari A, Anoop CH, Gupta PK. Intra‐arterial allogeneic mesenchymal stem cells for critical limb ischemia are safe and efficacious: report of a phase I study. World Journal of Surgery 2013;37(4):915‐22. [PUBMED: 23307180] [DOI] [PubMed] [Google Scholar]
Dash 2009 {published data only}
- Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Research 2009;12(5):359‐66. [PUBMED: 19929258] [DOI] [PubMed] [Google Scholar]
De Angelis 2014 {published data only}
- Angelis B, Gentile P, Orlandi F, Bocchini I, Pasquali C, Agovino A, et al. Limb rescue: a new autologous‐peripheral blood mononuclear cells technology in critical limb ischemia and chronic ulcers. Tissue Engineering. Part C, Methods 2014;21(5):423‐35. [PUBMED: 25341088] [DOI] [PubMed] [Google Scholar]
Debin 2008 {published data only}
- Debin L, Youzhao J, Ziwen L, Xiaoyan L, Zhonghui Z, Bing C. Autologous transplantation of bone marrow mesenchymal stem cells on diabetic patients with lower limb ischemia. Journal of Medical Colleges of PLA 2008;23(2):106‐15. [Google Scholar]
Dou 2015 {published data only}
- Dou Y, Zhao J, Zhang L, Wang X, Yuan H, Yuan C. A follow‐up study on autologous bone marrow mononuclear cells transplantation for critical lower arteriosclerosis obliterans in diabetic patients. Zhongguo Xiu fu Chong Jian Wai ke za Zhi = Zhongguo Xiufu Chongjian Waike Zazhi = Chinese Journal of Reparative and Reconstructive Surgery 2015;29(7):893‐8. [PUBMED: 26540987] [PubMed] [Google Scholar]
Doudar 2013 {published data only}
- Doudar N, El AM, Abdel SS. Stem cell implantation in the treatment of peripheral vascular disease. Vox Sang 2013;Suppl 1(105):290. [Google Scholar]
Du 2017 {published data only}
- Du JW, Wu T, Zhang K, Su BY, Lu CP, Wang WC, et al. Umbilical cord mesenchymal stem cells combined with bone marrow stem cells for treatment of lower limb ischemia [脐带间充质干细胞联合骨髓干细胞治疗下肢缺血]. Chinese Journal of Tissue Engineering Research 2017;21(1):82‐6. [Google Scholar]
Dubsky 2013 {published data only}
- Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, Sixta B, et al. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes/Metabolism Research and Reviews 2013;29(5):369‐76. [PUBMED: 23390092] [DOI] [PubMed] [Google Scholar]
Duong 2008 {published data only}
- Duong Van Huyen JP, Smadja DM, Bruneval P, Gaussem P, Dal‐Cortivo L, Julia P, et al. Bone marrow‐derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Modern Pathology 2008;21(7):837‐46. [PUBMED: 18487998] [DOI] [PubMed] [Google Scholar]
Flugelman 2017 {published data only}
- Flugelman MY, Halak M, Yoffe B, Schneiderman J, Rubinstein C, Bloom AI, et al. Phase Ib safety, two‐odse study of MultiGeneAngio in patients with chronic critical limb ischemia. Molecular Therapy 2017;25(3):816‐25. [PUBMED: 28143739] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franz 2011 {published data only}
- Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Journal of Vascular Surgery 2009;50(6):1378‐90. [PUBMED: 19837539] [DOI] [PubMed] [Google Scholar]
- Franz RW, Shah KJ, Johnson JD, Pin RH, Parks AM, Hankins T, et al. Short‐ to mid‐term results using autologous bone‐marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Vascular and Endovascular Surgery 2011;45(5):398‐406. [PUBMED: 21669864] [DOI] [PubMed] [Google Scholar]
Frogel 2017 {published data only}
- Frogel M, Niven MJ, Galili O, Sivak G, Kafri E, Moshe M, et al. Adult stem/progenitor cells derived from peripheral blood as a personalized treatment for critical limb ischemia (CLI). Vascular 2017;25(2 Suppl 1):88. [Google Scholar]
Gabr 2011 {published data only}
- Gabr H, Hedayet A, Imam U, Nasser M. Limb salvage using intramuscular injection of unfractionated autologous bone marrow mononuclear cells in critical limb ischemia: a prospective pilot clinical trial. Experimental and Clinical Transplantation 2011;9(3):197‐202. [PUBMED: 21649569] [PubMed] [Google Scholar]
Grossman 2016 {published data only}
- Grossman PM, Mohler ER 3rd, Roessler BJ, Wilensky RL, Levine BL, Woo EY, et al. Phase I study of multi‐gene cell therapy in patients with peripheral artery disease. Vascular Medicine (London, England) 2016;21(1):21‐32. [PUBMED: 26584888] [DOI] [PubMed] [Google Scholar]
Gu 2006 {published data only}
- Gu Y, Zhang J, Qi L. Effective autologous bone marrow stem cell dosage for treatment of severe lower limb ischemia. Zhongguo Xiu fu Chong Jian Wai ke za Zhi = Zhongguo Xiufu Chongjian Waike Zazhi = Chinese Journal of Reparative and Reconstructive Surgery 2006;20(5):504‐6. [PUBMED: 16752834] [PubMed] [Google Scholar]
Gu 2007 {published data only}
- Gu Y, Zhang J, Qi L. [Comparative study on autologous implantation between bone marrow stem cells and peripheral blood stem cells for treatment of lower limb ischemia]. Zhongguo Xiu fu Chong Jian Wai ke za Zhi = Zhongguo Xiufu Chongjian Waike Zazhi = Chinese Journal of Reparative and Reconstructive Surgery 2007;21(7):675‐8. [PUBMED: 17694651] [PubMed] [Google Scholar]
Gu 2017 {published data only}
- Gu Y, Guo L, Guo J, Dardik A, Zhang S, Tong Z, et al. Granulocyte colony‐stimulating factor improves the efficacy of autologous bone marrow‐derived mononuclear cell transplantation treatment for lower limb ischemia. International Angiology 2017;36(4):346‐53. [PUBMED: 27958690] [DOI] [PubMed] [Google Scholar]
Guo 2018 {published data only}
- Guo J, Guo L, Cui S, Tong Z, Dardik A, Gu Y. Autologous bone marrow‐derived mononuclear cell therapy in Chinese patients with critical limb ischemia due to thromboangiitis obliterans: 10‐year results. Stem Cell Research & Therapy 2018;9(1):43. [PUBMED: 29471870] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. Journal of Translational Medicine 2013;11:143. [PUBMED: 23758736] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2017 {published data only}
- Gupta PK, Chullikana A, Rajkumar M, Krishna M, Dutta S, Sarkar U, et al. Role of bone marrow derived allogeneic mesenchymal stromal cells (Stempeucel) in critical limb ischemia due to Buerger's disease‐efficacy and safety results of non‐randomized, open label, multicentric, dose ranging, phase II study in India. Cytotherapy 2014;16(4):S80‐1. [Google Scholar]
- Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S. Administration of adult human bone marrow‐derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger's disease: phase II study report suggests clinical efficacy. Journal of Vascular Surgery 2017;66(4):1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S, et al. Administration of adult human bone marrow‐derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger's disease: phase II study report suggests clinical efficacy. Stem Cells Translational Medicine 2017;6(3):689‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harunarashid 2016 {published data only}
- Harunarashid H, Mohd Idris M, Chin S, Mohamad Yusoff F, Shahari S, Amran@Azman N, et al. Combination BM‐MSC with BM‐MNC is better than BM‐MNC alone in resolution of large ischemic ulcers: a phase II/III clinical randomised study. 22nd Annual ISCT Meeting 2016;18(6 Suppl):S21. [Google Scholar]
Heo 2016 {published data only}
- Heo SH, Park YS, Kang ES, Park KB, Do YS, Kang KS, et al. Early results of clinical application of autologous whole bone marrow stem cell transplantation for critical limb ischemia with Buerger's disease. Scientific Reports 2016;6:19690. [PUBMED: 26791280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2007 {published data only}
- Hernandez P, Cortina L, Artaza H, Pol N, Lam RM, Dorticos E, et al. Autologous bone‐marrow mononuclear cell implantation in patients with severe lower limb ischaemia: a comparison of using blood cell separator and Ficoll density gradient centrifugation. Atherosclerosis 2007;194(2):e52‐6. [PUBMED: 16982058] [DOI] [PubMed] [Google Scholar]
Higashi 2004 {published data only}
- Higashi Y, Kimura M, Hara K, Noma K, Jitsuiki D, Nakagawa K, et al. Autologous bone‐marrow mononuclear cell implantation improves endothelium‐dependent vasodilation in patients with limb ischemia. Circulation 2004;109(10):1215‐8. [PUBMED: 15007007] [DOI] [PubMed] [Google Scholar]
Holzinger 1994 {published data only}
- Holzinger C, Zuckermann A, Kopp C, Schollhammer A, Imhof M, Zwolfer W, et al. Treatment of non‐healing skin ulcers with autologous activated mononuclear cells. European Journal of Vascular Surgery 1994;8(3):351‐6. [PUBMED: 8013688] [DOI] [PubMed] [Google Scholar]
Hoshino 2007 {published data only}
- Hoshino J, Ubara Y, Hara S, Sogawa Y, Suwabe T, Higa Y, et al. Quality of life improvement and long‐term effects of peripheral blood mononuclear cell transplantation for severe arteriosclerosis obliterans in diabetic patients on dialysis. Circulation Journal 2007;71(8):1193‐8. [PUBMED: 17652880] [DOI] [PubMed] [Google Scholar]
Huang 2005 {published data only}
- Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony‐stimulating factor‐mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 2005;28(9):2155‐60. [PUBMED: 16123483] [DOI] [PubMed] [Google Scholar]
- NCT00730561. Hematopoietic stem cell transplantation for the treatment of limb ischemia and diabetic neuropathy. clinicaltrials.gov/ct2/show/NCT00730561 (first received 8 August 2008).
Iafrati 2016 {published data only}
- Iafrati MD, O'Donnell Jr TF, Perler B, Illig KA, Hallett J, Woo K, et al. SS03. Bone marrow aspirate concentrate in critical limb ischemia: results of an abridged prospective randomized pivotal trial in no option CLI. Abstracts of the 2016 Vascular Annual Meeting, The Society for Vascular Surgery 2016;63(6 Suppl):47S. [Google Scholar]
Idei 2011 {published data only}
- Idei N, Soga J, Hata T, Fujii Y, Fujimura N, Mikami S, et al. Autologous bone‐marrow mononuclear cell implantation reduces long‐term major amputation risk in patients with critical limb ischemia: a comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circulation. Cardiovascular Interventions 2011;4(1):15‐25. [PUBMED: 21205941] [DOI] [PubMed] [Google Scholar]
Ishida 2005 {published data only}
- Ishida A, Ohya Y, Sakuda H, Ohshiro K, Higashiuesato Y, Nakaema M, et al. Autologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemia. Circulation Journal 2005;69(10):1260‐5. [PUBMED: 16195628] [DOI] [PubMed] [Google Scholar]
Iso 2010 {published data only}
- Iso Y, Soda T, Sato T, Sato R, Kusuyama T, Omori Y, et al. Impact of implanted bone marrow progenitor cell composition on limb salvage after cell implantation in patients with critical limb ischemia. Atherosclerosis 2010;209(1):167‐72. [PUBMED: 19748620] [DOI] [PubMed] [Google Scholar]
Kamata 2007 {published data only}
- Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, Muroi K, et al. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology (Oxford, England) 2007;46(5):882‐4. [PUBMED: 17309890] [DOI] [PubMed] [Google Scholar]
Kawamoto 2009 {published data only}
- Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, et al. Intramuscular transplantation of G‐CSF‐mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single‐blinded, dose‐escalation clinical trial. Stem Cells 2009;27(11):2857‐64. [PUBMED: 19711453] [DOI] [PubMed] [Google Scholar]
Kinoshita 2012 {published data only}
- Kinoshita M, Fujita Y, Katayama M, Baba R, Shibakawa M, Yoshikawa K, et al. Long‐term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor‐mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis 2012;224(2):440‐5. [PUBMED: 22877866] [DOI] [PubMed] [Google Scholar]
Kirana 2012 {published data only}
- Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, Lammers D, et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. International Journal of Clinical Practice 2012;66(4):384‐93. [PUBMED: 22284892] [DOI] [PubMed] [Google Scholar]
- NCT01065337. Induced wound healing by application of expanded bone marrow stem cells in diabetic patients with critical limb ischemia. clinicaltrials.gov/ct2/show/NCT01065337 (first received 9 February 200).
Kolvenbach 2010 {published data only}
- Kolvenbach R, Kreissig C, Cagiannos C, Afifi R, Schmaltz E. Intraoperative adjunctive stem cell treatment in patients with critical limb ischemia using a novel point‐of‐care device. Annals of Vascular Surgery 2010;24(3):367‐72. [PUBMED: 19896796] [DOI] [PubMed] [Google Scholar]
Kondo 2016 {published data only}
- Kondo K, Hayashida R, Shibata R, Murohara T. Therapeutic angiogenesis for critical limb ischemia by implantation of autologous adipose‐derived regenerative cells: a clinical pilot study. Circulation 2016;134(Suppl 1):A18595. [Google Scholar]
Kondo 2018 {published data only}
- Kondo K, Yanishi K, Hayashida R, Shintani S, Shibata R, Murotani K, et al. Long‐term clinical outcomes survey of bone marrow‐derived cell therapy in critical limb ischemia in Japan. Circulation Journal 2018;82(4):1168‐78. [PUBMED: 29386474] [DOI] [PubMed] [Google Scholar]
Lara‐Hernandez 2010 {published data only}
- Lara‐Hernandez R, Lozano‐Vilardell P, Blanes P, Torreguitart‐Mirada N, Galmes A, Besalduch J. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Annals of Vascular Surgery 2010;24(2):287‐94. [PUBMED: 20142004] [DOI] [PubMed] [Google Scholar]
Lasala 2010 {published data only}
- Lasala GP, Silva JA, Gardner PA, Minguell JJ. Combination stem cell therapy for the treatment of severe limb ischemia: safety and efficacy analysis. Angiology 2010;61(6):551‐6. [PUBMED: 20498146] [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, et al. Safety and effect of adipose tissue‐derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circulation Journal 2012;76(7):1750‐60. [PUBMED: 22498564] [DOI] [PubMed] [Google Scholar]
Lenk 2005 {published data only}
- Lenk K, Adams V, Lurz P, Erbs S, Linke A, Gielen S, et al. Therapeutical potential of blood‐derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. European Heart Journal 2005;26(18):1903‐9. [PUBMED: 15855189] [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li M, Zhou H, Jin X, Wang M, Zhang S, Xu L. Autologous bone marrow mononuclear cells transplant in patients with critical leg ischemia: preliminary clinical results. Experimental and Clinical Transplantation 2013;11(5):435‐9. [PUBMED: 23477421] [DOI] [PubMed] [Google Scholar]
Madaric 2016 {published data only}
- Madaric J, Klepanec A, Valachovicova M, Mistrik M, Bucova M, Olejarova I, et al. Characteristics of responders to autologous bone marrow cell therapy for no‐option critical limb ischemia. Stem Cell Research & Therapy 2016;7(1):116. [PUBMED: 27530339] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madaric 2017 {published data only}
- Madaric J, Valachovicova M, Paulis L, Pribojova J, Mateova R, Sebekova K, et al. Improvement in asymmetric dimethylarginine and oxidative stress in patients with limb salvage after autologous mononuclear stem cell application for critical limb ischemia. Stem Cell Research & Therapy 2017;8(1):165. [PUBMED: 28697789] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maione 2013 {published data only}
- Maione C, Botti C, Coppola CA, Silvestroni C, Lillo S, Schiavone V, et al. Effect of autologous transplantation of bone marrow cells concentrated with the MarrowXpress system in patients with critical limb ischemia. Transplantation Proceedings 2013;45(1):402‐6. [PUBMED: 23375329] [DOI] [PubMed] [Google Scholar]
Majumdar 2015 {published data only}
- Majumdar AS, Balasubramanian S, Thej C, Rajkumar M, Krishna M, Dutta S, et al. A first of its kind phase II clinical trial in critical limb ischemia patients using bone marrow derived, pooled, allogeneic mesenchymal stromal cells (Stempeucel). Cytotherapy 2015; Vol. 17:S84.
Malyar 2014 {published data only}
- Malyar NM, Radtke S, Malyar K, Arjumand J, Horn PA, Kröger K, et al. Autologous bone marrow mononuclear cell therapy improves symptoms in patients with end‐stage peripheral arterial disease and reduces inflammation‐associated parameters. Cytotherapy 2014;16(9):1270‐9. [DOI] [PubMed] [Google Scholar]
Matoba 2009 {published data only}
- Matoba S, Tatsumi T, Murohara T. Long‐term clinical outcome after intramuscular implantation of bone barrow mononuclear cells (therapeutic angiogenesis by cell transplantation [TACT] Trial) in patients with chronic limb ischemia. American Heart Journal 2009;50(1):233. [DOI] [PubMed] [Google Scholar]
Matsui 2003 {published data only}
- Matsui K, Murakami Y, Yoshioka T, Muroi K, Kasuda H, Shimpo M, et al. Therapeutic angiogenesis by transplantation of autologous bone marrow and peripheral blood mononuclear cells in patients with peripheral arterial disease. International Journal of Angiology 2003;12:155–61. [Google Scholar]
Mohamed 2017 {published data only}
- Mohamed S, McInerney V, Dunne A, Hayat A, Krawczyk J, Naughton S, et al. Autologous mesenchymal stem cells as a novel therapy for no‐option critical limb ischemia: preliminary results of a phase 1 study. Cytotherapy 2017;19(5):S198. [Google Scholar]
- Mohamed S, McInerney V, Dunne A, Hayat A, Krawczyk J, Naughton S, et al. Autologous mesenchymal stem cells as a novel therapy for no‐option critical limb ischemia: preliminary results of a phase 1 study. Irish Journal of Medical Science 2017;186(Suppl 2):S82. [Google Scholar]
Mohammadzadeh 2013 {published data only}
- Mohammadzadeh L, Samedanifard SH, Keshavarzi A, Alimoghaddam K, Larijani B, Ghavamzadeh A, et al. Therapeutic outcomes of transplanting autologous granulocyte colony‐stimulating factor‐mobilised peripheral mononuclear cells in diabetic patients with critical limb ischaemia. Experimental and Clinical Endocrinology & Diabetes 2013;121(1):48‐53. [PUBMED: 23329572] [DOI] [PubMed] [Google Scholar]
Moriya 2009 {published data only}
- Moriya J, Minamino T, Tateno K, Shimizu N, Kuwabara Y, Sato Y, et al. Long‐term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circulation. Cardiovascular Interventions 2009;2(3):245‐54. [PUBMED: 20031722] [DOI] [PubMed] [Google Scholar]
Motukuru 2008 {published data only}
- Motukuru V, Suresh KR, Vivekanand V, Raj S, Girija KR. Therapeutic angiogenesis in Buerger's disease (thromboangiitis obliterans) patients with critical limb ischemia by autologous transplantation of bone marrow mononuclear cells. Journal of Vascular Surgery 2008;48(6 Suppl):53S‐60S; discussion 60S. [PUBMED: 19084740] [DOI] [PubMed] [Google Scholar]
Murphy 2011 {published data only}
- Murphy MP, Lawson JH, Rapp BM, Dalsing MC, Klein J, Wilson MG, et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation‐free survival in patients with critical limb ischemia. Journal of Vascular Surgery 2011;53(6):1565‐74.e1. [PUBMED: 21514773] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2017 {published data only}
- Murphy MP, Ross CB, Kibbe M, Kelso R, Sharafuddin M, Tzeng E, et al. Intramuscular injection of autologous bone marrow cells to prevent amputation in critical limb ischemia: the results of the phase III MOBILE trial. Abstracts of the 2017 Vascular Annual Meeting 2017;65(6 Suppl):131S‐2S. [Google Scholar]
Napoli 2008 {published data only}
- Napoli C, Farzati B, Sica V, Iannuzzi E, Coppola G, Silvestroni A, et al. Beneficial effects of autologous bone marrow cell infusion and antioxidants/L‐arginine in patients with chronic critical limb ischemia. European Journal of Cardiovascular Prevention and Rehabilitation 2008;15(6):709‐18. [PUBMED: 19050436] [DOI] [PubMed] [Google Scholar]
NCT00306085 {published data only}
- Cobellis G, Botti C, Taddeo A, Silvestroni A, Lillo S, Ponte A, et al. Successful bone marrow transplantation reveals the lack of endothelial progenitor cells mobilization in a patient with critical limb ischemia: a case report. Transplantation Proceedings 2010;42(7):2816‐20. [PUBMED: 20832596] [DOI] [PubMed] [Google Scholar]
- Maione C, Botti C, Coppola CA, Silvestroni C, Lillo S, Schiavone V, et al. Effect of autologous transplantation of bone marrow cells concentrated with the MarrowXpress system in patients with critical limb ischemia. Transplantation Proceedings 2013;45(1):402‐6. [PUBMED: 23375329] [DOI] [PubMed] [Google Scholar]
- NCT00306085. Autologous bone marrow cell treatment in peripheral atherosclerosis. clinicaltrials.gov/ct2/show/study/NCT00306085 (first received 22 March 2006).
- Napoli C, Farzati B, Sica V, Iannuzzi E, Coppola G, Silvestroni A, et al. Beneficial effects of autologous bone marrow cell infusion and antioxidants/L‐arginine in patients with chronic critical limb ischemia. European Journal of Cardiovascular Prevention and Rehabilitation 2008;15(6):709‐18. [PUBMED: 19050436] [DOI] [PubMed] [Google Scholar]
NCT00434616 {published data only}
- NCT00434616. Autologous bone marrow stem cell transplantation for critical, limb‐threatening ischemia (BONMOT). clinicaltrials.gov/ct2/show/NCT00434616 (first received 13 February 2007).
NCT00539266 {published data only}
- NCT00539266. Autologous bone marrow‐derived mononuclear cells for therapeutic arteriogenesis in patients with limb ischemia (ABC). clinicaltrials.gov/ct2/show/NCT00539266 (first received October 2007).
NCT00922389 {published data only}
- NCT00922389. A clinical trial on diabetic foot using peripheral blood derived stem cells for treating critical limb ischemia. clinicaltrials.gov/ct2/show/NCT00922389 (first received 17 June 2009).
NCT01049919 {published data only}
- NCT01049919. Safety and efficacy study of autologous concentrated bone marrow aspirate (cBMA) for critical limb ischemia (CLI) (MOBILE). clinicaltrials.gov/ct2/show/NCT01049919 (first received 15 January 2010).
NCT01245335 {published data only}
- NCT01245335. Bone marrow aspirate concentrate (BMAC) for treatment of critical limb ischemia (CLI). clinicaltrials.gov/ct2/show/NCT01245335 (first received 22 November 2010).
NCT01584986 {published data only}
- NCT01584986. Autologous angiogenic cell precursors (ACPs) for the treatment of peripheral artery disease. clinicaltrials.gov/ct2/show/NCT01584986 (first received 25 April 2012).
NCT02336646 {published data only}
- NCT02336646. Cell therapy with mesenchymal stem cell in ischemic limb disease. clinicaltrials.gov/ct2/show/NCT02336646 (first received 13 January 2015).
NCT03174522 {published data only}
- NCT03174522. The efficacy and safety of Rexmyelocel‐T to treat ischemic ulcers in subjects with CLI Rutherford category 5 and DM. clinicaltrials.gov/ct2/show/NCT03174522 (first received 2 June 2017).
NCT03214887 {published data only}
- NCT03214887. Autologous BMMNC combined with HA therapy for PAOD. clinicaltrials.gov/ct2/show/NCT03214887 (first received 12 July 2017).
NCT03304821 {published data only}
- NCT03304821. Granulocyte‐macrophage stimulating factor (GM‐CSF) in peripheral arterial disease. clinicaltrials.gov/ct2/show/NCT03304821 (first received 9 October 2017).
NCT03339973 {published data only}
- NCT03339973. Allogeneic ABCB5‐positive stem cells for treatment of PAOD. clinicaltrials.gov/ct2/show/NCT03339973 (first received 13 November 2017).
Nemcova 2017 {published data only}
- Nemcova A, Jirkovska A, Dubsky M, Bem R, Fejfarova V, Pysna A, et al. Serum levels of angiogenic cytokines in the assessment of vasculogenesis after autologous cell therapy in diabetic patients with critical limb ischaemia. Diabetologia. 2017; Vol. 60:S468.
Niven 2017 {published data only}
- Niven MJ, Sivak G, Kafri E, Moshe M, Galili O, Frogel M, et al. Adult stem/progenitor cells as a personalised treatment for peripheral vascular disease. Diabetologia. 2017; Vol. 60:S31.
Nizankowski 2005 {published data only}
- Nizankowski R, Petriczek T, Skotnicki A, Szczeklik A. The treatment of advanced chronic lower limb ischaemia with marrow stem cell autotransplantation. Kardiologia Polska 2005;63(4):351‐60; discussion 361. [PUBMED: 16273471] [PubMed] [Google Scholar]
Ohtake 2017 {published data only}
- Ohtake T, Mochida Y, Ishioka K, Oka M, Maesato K, Moriya H, et al. Effect of autologous G‐CSF‐mobilized CD34+ cell transplantation in hemodialysis patients with critical limb ischemia. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii309. [Google Scholar]
Onodera 2011 {published data only}
- Onodera R, Teramukai S, Tanaka S, Kojima S, Horie T, Matoba S, et al. Bone marrow mononuclear cells versus G‐CSF‐mobilized peripheral blood mononuclear cells for treatment of lower limb ASO: pooled analysis for long‐term prognosis. Bone Marrow Transplantation 2011;46(2):278‐84. [PUBMED: 20479708] [DOI] [PubMed] [Google Scholar]
Ozturk 2012 {published data only}
- Ozturk A, Kucukardali Y, Tangi F, Erikci A, Uzun G, Bashekim C, et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. Journal of Diabetes and Its Complications 2012;26(1):29‐33. [PUBMED: 22240264] [DOI] [PubMed] [Google Scholar]
Peeters 2016 {published data only}
- Peeters Weem SMO, Teraa M, Ruijter HM, Borst GJ, Verhaar MC, Moll FL. Quality of life after treatment with autologous bone marrow derived cells in no option severe limb ischemia. European Journal of Vascular and Endovascular Surgery 2016;51(1):83‐9. [DOI] [PubMed] [Google Scholar]
Perin 2011 {published data only}
- Perin EC, Silva G, Gahremanpour A, Canales J, Zheng Y, Cabreira‐Hansen MG, et al. A randomized, controlled study of autologous therapy with bone marrow‐derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheterization and Cardiovascular Interventions 2011;78(7):1060‐7. [PUBMED: 21594960] [DOI] [PubMed] [Google Scholar]
Perin 2017 {published data only}
- Perin EC, Murphy M, Cooke JP, Moye L, Henry TD, Bettencourt J, et al. Rationale and design for PACE: patients with intermittent claudication injected with ALDH bright cells. American Heart Journal 2014;168(5):667‐73. [PUBMED: 25440794] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Murphy MP, March KL, Bolli R, Loughran J, Yang PC, et al. Evaluation of cell therapy on exercise performance and limb perfusion in peripheral artery disease: the CCTRN PACE Trial (patients with intermittent claudication injected with ALDH bright cells). Circulation 2017;135(15):1417‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pignon 2017 {published data only}
- NCT00904501. Bone marrow autograft in limb ischemia (BALI). clinicaltrials.gov/ct2/show/study/NCT00904501 (first received 19 May 2009).
- Pignon B, Sevestre MA, Kanagaratnam L, Pernod G, Stephan D, Emmerich J, et al. Autologous bone marrow mononuclear cell implantation and its impact on the outcome of patients with critical limb ischemia: results of a randomized, double‐blind, placebo‐controlled trial. Circulation Journal 2017;81(11):1713‐20. [PUBMED: 28603176] [DOI] [PubMed] [Google Scholar]
Ponemone 2017 {published data only}
- Ponemone V, Gupta S, Sethi D, Suthar M, Sharma M, Powell RJ, et al. Safety and effectiveness of bone marrow cell concentrate in the treatment of chronic critical limb ischemia utilizing a rapid point‐of‐care system. Stem Cells International 2017;2017:4137626. [PUBMED: 28194186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poole 2013 {published data only}
- Poole J, Mavromatis K, Binongo JN, Khan A, Li Q, Khayata M, et al. Effect of progenitor cell mobilization with granulocyte‐macrophage colony‐stimulating factor in patients with peripheral artery disease: a randomized clinical trial. JAMA 2013;310(24):2631‐9. [PUBMED: 24247554] [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 2012 {published data only}
- Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, et al. Cellular therapy with Ixmyelocel‐T to treat critical limb ischemia: the randomized, double‐blind, placebo‐controlled RESTORE‐CLI trial. Molecular Therapy 2012;20(6):1280‐6. [PUBMED: 22453769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochazka 2010 {published data only}
- Prochazka V, Gumulec J, Jaluvka F, Salounova D, Jonszta T, Czerny D, et al. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplantation 2010;19(11):1413‐24. [PUBMED: 20529449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajagopalan 2003 {published data only}
- Rajagopalan S, Mohler E 3rd, Lederman RJ, Saucedo J, Mendelsohn FO, Olin J, et al. Regional angiogenesis with vascular endothelial growth factor (VEGF) in peripheral arterial disease: design of the RAVE trial. American Heart Journal 2003;145(6):1114‐8. [PUBMED: 12796772] [DOI] [PubMed] [Google Scholar]
Ruiz‐Salmeron 2011 {published data only}
- Ruiz‐Salmeron R, Cuesta‐Diaz A, Constantino‐Bermejo M, Perez‐Camacho I, Marcos‐Sanchez F, Hmadcha A, et al. Angiographic demonstration of neoangiogenesis after intra‐arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplantation 2011;20(10):1629‐39. [PUBMED: 22289660] [DOI] [PubMed] [Google Scholar]
Saito 2007 {published data only}
- Saito Y, Sasaki K, Katsuda Y, Murohara T, Takeshita Y, Okazaki T, et al. Effect of autologous bone‐marrow cell transplantation on ischemic ulcer in patients with Buerger's disease. Circulation Journal 2007;71(8):1187‐92. [PUBMED: 17652879] [DOI] [PubMed] [Google Scholar]
Schiavetta 2012 {published data only}
- Schiavetta A, Maione C, Botti C, Marino G, Lillo S, Garrone A, et al. A phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the Naples and Pietra Ligure Evaluation of Stem Cells study. Stem Cells Translational Medicine 2012;1(7):572‐8. [PUBMED: 23197862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smadja 2012 {published data only}
- Smadja DM, Duong‐van‐Huyen JP, Dal Cortivo L, Blanchard A, Bruneval P, Emmerich J, et al. Early endothelial progenitor cells in bone marrow are a biomarker of cell therapy success in patients with critical limb ischemia. Cytotherapy 2012;14(2):232‐9. [PUBMED: 22040109] [DOI] [PubMed] [Google Scholar]
Subramaniyam 2009 {published data only}
- Subramaniyam V, Waller EK, Murrow JR, Manatunga A, Lonial S, Kasirajan K, et al. Bone marrow mobilization with granulocyte macrophage colony‐stimulating factor improves endothelial dysfunction and exercise capacity in patients with peripheral arterial disease. American Heart Journal 2009;158(1):53‐60.e1. [PUBMED: 19540392] [DOI] [PubMed] [Google Scholar]
Szabo 2013 {published data only}
- Szabo GV, Kovesd Z, Cserepes J, Daroczy J, Belkin M, Acsady G. Peripheral blood‐derived autologous stem cell therapy for the treatment of patients with late‐stage peripheral artery disease ‐ results of the short‐ and long‐term follow‐up. Cytotherapy 2013;15(10):1245‐52. [PUBMED: 23993298] [DOI] [PubMed] [Google Scholar]
Takagi 2011 {published data only}
- Takagi G, Miyamoto M, Tara S, Takagi I, Takano H, Yasutake M, et al. Controlled‐release basic fibroblast growth factor for peripheral artery disease: comparison with autologous bone marrow‐derived stem cell transfer. Tissue Engineering. Part A 2011;17(21‐22):2787‐94. [PUBMED: 21810028] [DOI] [PubMed] [Google Scholar]
Tateishi‐Yuyama 2002 {published data only}
- Tateishi‐Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone‐marrow cells: a pilot study and a randomised controlled trial. Lancet 2002;360(9331):427‐35. [PUBMED: 12241713] [DOI] [PubMed] [Google Scholar]
Teraa 2014 {published data only}
- Teraa M, Fledderus JO, Rozbeh RI, Leguit RJ, Verhaar MC. Bone marrow microvascular and neuropathic alterations in patients with critical limb ischemia. Circulation Research 2014;114(2):311‐4. [PUBMED: 24218170] [DOI] [PubMed] [Google Scholar]
Teraa 2015 {published data only}
- Sprengers RW, Moll FL, Teraa M, Verhaar MC. Rationale and design of the JUVENTAS trial for repeated intra‐arterial infusion of autologous bone marrow‐derived mononuclear cells in patients with critical limb ischemia. Journal of Vascular Surgery 2010;51(6):1564‐8. [PUBMED: 20488328] [DOI] [PubMed] [Google Scholar]
- Teraa M, Sprengers RW, Schutgens RE, Slaper‐Cortenbach IC, Graaf Y, Algra A, et al. Effect of repetitive intra‐arterial infusion of bone marrow mononuclear cells in patients with no‐option limb ischemia: the randomized, double‐blind, placebo‐controlled rejuvenating endothelial progenitor cells via transcutaneous intra‐arterial supplementation (JUVENTAS) trial. Circulation 2015;131(10):851‐60. [PUBMED: 25567765] [DOI] [PubMed] [Google Scholar]
Tournois 2015 {published data only}
- Tournois C, Pignon B, Sevestre MA, Djerada Z, Capiod JC, Poitevin G, et al. Critical limb ischemia: thrombogenic evaluation of two autologous cell therapy products and biologic profile in treated patients. Transfusion 2015;55(11):2692‐701. [PUBMED: 26222701] [DOI] [PubMed] [Google Scholar]
Walter 2011 {published data only}
- NCT00282646. Safety and feasibility study of autologous bone marrow cell transplantation in patients with PAOD. clinicaltrials.gov/ct2/show/study/NCT00282646 (first received 27 January 2006).
- Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schluter M, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized‐start, placebo‐controlled pilot trial (PROVASA). Circulation. Cardiovascular Interventions 2011;4(1):26‐37. [PUBMED: 21205939] [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang X, Jiang L, Wang X, Yin F, Li G, Feng X, et al. Combination of autologous transplantation of G‐CSF‐mobilized peripheral blood mononuclear cells and Panax notoginseng saponins in the treatment of unreconstructable critical limb ischemia. Annals of Vascular Surgery 2014;28(6):1501‐12. [PUBMED: 24632316] [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang SK, Green L, Babbey C, Wilson M, Motaganahalli R, Fajardo A, et al. Ethnic minorities with critical limb ischemia derive equal amputation risk reduction from autologous cell therapy compared to Caucasians. Journal of Vascular Surgery 2017;65(6):113S. [DOI] [PubMed] [Google Scholar]
- Wang SK, Green LA, Motaganahalli RL, Wilson MG, Fajardo A, Murphy MP. Rationale and design of the MarrowStim PAD Kit for the treatment of critical limb ischemia in subjects with severe peripheral arterial disease (MOBILE) trial investigating autologous bone marrow cell therapy for critical limb ischemia. Journal of Vascular Surgery 2017;65(6):1850‐7.e2. [PUBMED: 28390770] [DOI] [PubMed] [Google Scholar]
Wang 2018 {published data only}
- Wang SK, Green LA, Drucker NA, Motaganahalli RL, Fajardo A, Murphy MP. Rationale and design of the Clinical and Histologic Analysis of Mesenchymal stromal cells in amPutations (CHAMP) trial investigating the therapeutic mechanism of mesenchymal stromal cells in the treatment of critical limb ischemia. Journal of Vascular Surgery 2018;68(1):176‐81.e1. [PUBMED: 29395424] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wester 2008 {published data only}
- Wester T, Jorgensen JJ, Stranden E, Sandbaek G, Tjonnfjord G, Bay D, et al. Treatment with autologous bone marrow mononuclear cells in patients with critical lower limb ischaemia. A pilot study. Scandinavian Journal of Surgery 2008;97(1):56‐62. [PUBMED: 18450207] [DOI] [PubMed] [Google Scholar]
Wijnand 2018 {published data only}
- NCT03042572. Allogeneic mesenchymal stromal cells for angiogenesis and neovascularization in no‐option ischemic limbs (SAIL). clinicaltrials.gov/ct2/show/NCT03042572 (first received 3 February 2017).
- Wijnand JGJ, Teraa M, Gremmels H, Rhijn‐Brouwer FCC, Borst GJ, Verhaar MC. Rationale and design of the SAIL trial for intramuscular injection of allogeneic mesenchymal stromal cells in no‐option critical limb ischemia. Journal of Vascular Surgery 2018;67(2):656‐61. [PUBMED: 29242062] [DOI] [PubMed] [Google Scholar]
Yanishi 2017 {published data only}
- Yanishi K, Nakanishi N, Zen K, Ogata T, Nakamura T, Yamano T, et al. Long‐term clinical outcome of therapeutic angiogenesis by cell transplantation in patients with critical limb ischemia. European Heart Journal. 2017;38(Suppl 1):1080. [Google Scholar]
Zafarghandi 2010 {published data only}
- Zafarghandi MR, Ravari H, Aghdami N, Namiri M, Moazzami K, Taghiabadi E, et al. Safety and efficacy of granulocyte‐colony‐stimulating factor administration following autologous intramuscular implantation of bone marrow mononuclear cells: a randomized controlled trial in patients with advanced lower limb ischemia. Cytotherapy 2010;12(6):783‐91. [PUBMED: 20078390] [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang X, Lian W, Lou W, Han S, Lu C, Zuo K, et al. Transcatheter arterial infusion of autologous CD133(+) cells for diabetic peripheral artery disease. Stem Cells International 2016;2016:6925357. [PUBMED: 26981134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2008 {published data only}
- Zhao ZG, Yuan HJ, Zhang HF, Zhang CL, Wang YF, Ma SP, et al. Combined transplantation of autologous peripheral blood and bone marrow stem cells for the treatment of diabetic lower limb ischaemia: randomized controlled trial. [Chinese]. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(8):1464‐6. [Google Scholar]
Zhou 2017 {published data only}
- Zhou HM, Liu F, Yang AG, Guo YQ, Zhou YR, Gu YQ, et al. Efficacy, safety and influencing factors of intra‐calf muscular injection of bone marrow mononuclear cells in the treatment of type 2 diabetes mellitus‐induced lower extremity vascular disease. Experimental and Therapeutic Medicine 2017;14(5):5177‐85. [PUBMED: 29201234] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2017a {published data only}
- Zhou CH, Xu LL, Hao XX, Sun XJ, Guo MJ, Liu B. Autologous CD34+ cell transplantation promotes angiogenesis in older adult patients with atherosclerotic ischemia: study protocol for a prospective, single‐center, open‐label, randomized controlled clinical trial [外周血CD34+细胞自体移植促进老年动脉粥样性缺血肢体的血管新生:前瞻性、单中心、开放性、随机对照临床试验方案]. Chinese Journal of Tissue Engineering Research 2017;21(13):1998‐2002. [Google Scholar]
References to studies awaiting assessment
Gurunathan 2009 {published data only}
- Gurunathan Mani S, Raju R, Kuppu Sampath V. Multicentre randomised clinical trial on the role of autologous bone‐marrow aspirate‐concentrate (BMAC)/CD34/(EPC) in non‐reconstructable critically ischaemic limbs. The Vascular Society of Great Britain & Ireland Yearbook 2009. 2009:56.
Korymasov 2009 {published data only}
- Korymasov EA, Tiumina OV, Aiupov AM, Kazantsev AV, Rossiev VA, Mikheev GV, et al. Use of autologous progenitor cells of the bone marrow in treatment of patients with lower‐limb atherosclerosis obliterans. Angiologiia i Sosudistaia Khirurgiia = Angiology and Vascular Surgery 2009;15(3):28‐31. [PUBMED: 20092179] [PubMed] [Google Scholar]
Molavi 2016 {published data only}
- Molavi B, Zafarghandi MR, Aminizadeh E, Hosseini SE, Mirzayi H, Arab L, et al. Safety and efficacy of repeated bone marrow mononuclear cell therapy in patients with critical limb ischemia in a pilot randomized controlled trial. Archives of Iranian Medicine 2016;19(6):388‐96. [PUBMED: 27293053] [PubMed] [Google Scholar]
- NCT01480414. Side effects of 4 times bone marrow mononuclear transplantation in patients with ischemic lower limb. clinicaltrials.gov/ct2/show/NCT01480414 (first received 28 November 2011).
NCT00595257 {published data only}
- NCT00595257. Feasibility study of autologous bone marrow aspirate concentrate for treatment of CLI. clinicaltrials.gov/ct2/show/study/NCT00595257 (first received 16 January 2008).
NCT00987363 {published data only}
- NCT00987363. Intraarterial infusion of autologous bone marrow in diabetic patients with chronic ischemia of lower limbs (CLI) no revascularization. clinicaltrials.gov/ct2/show/study/NCT00987363 (first recieved 30 September 2009).
NCT02993809 {published data only}
- NCT02993809. Autologous transplantation of BM‐ECs with platelet‐rich plasma extract for the treatment of critical limb ischemia. clinicaltrials.gov/ct2/show/study/NCT02993809 (first received 15 December 2016).
References to ongoing studies
NCT00311805 {published data only}
- NCT00311805. Autologous CD34+ stem cell injection for severe intermittent claudication (leg pain). clinicaltrials.gov/ct2/show/study/NCT00311805 (first received 6 April 2006).
NCT00753025 {published data only}
- NCT00753025. Autologous bone marrow for lower extremity ischemia treating. clinicaltrials.gov/ct2/show/study/NCT00753025 (first received 16 September 2008).
NCT01257776 {published data only}
- NCT01257776. Human adipose derived mesenchymal stem cells for critical limb ischemia (CLI) in diabetic patients. clinicaltrials.gov/ct2/show/study/NCT01257776 (first received 10 December 2010).
NCT01408381 {published data only}
- NCT01408381. Intra‐arterial infusion of autologous bone marrow mononuclear cells in non‐diabetic patients with critical limb ischemia (CLI). clinicaltrials.gov/ct2/show/study/NCT01408381 (first received 3 August 2011).
NCT01446055 {published data only}
- NCT01446055. Safety and efficacy study of autologous BM‐MNC processed by two methods for treating patients with chronic limb ischemia. clinicaltrials.gov/ct2/show/study/NCT01446055 (first received 4 October 2011).
NCT01745744 {published data only}
- NCT01745744. Application of cell regeneration therapy with mesenchymal stem cells from adipose tissue in critical chronic ischemic syndrome of lower limbs (CLI) in nondiabetic patients. clinicaltrials.gov/ct2/show/study/NCT01745744 (first received 10 December 2012).
NCT02454231 {published data only}
- NCT02454231. Monocentric trial: stem cell emergency life threatening limbs arteriopathy (SCELTA). clinicaltrials.gov/ct2/show/NCT02454231 (first received 27 May 2015).
Additional references
Benoit 2013
- Benoit E, O'Donnell TF, Patel AN. Safety and efficacy of autologous cell therapy in critical limb ischemia: a systematic review. Cell Transplantation 2013;22(3):545‐62. [DOI] [PubMed] [Google Scholar]
Botti 2012
- Botti C, Maione C, Coppola A, Sica V, Cobellis G. Autologous bone marrow cell therapy for peripheral arterial disease. Stem Cells and Cloning: Advances and Applications 2012;5:5‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conrad 2011
- Conrad MF, Crawford RS, Hackney LA, Paruchuri V, Abularrage CJ, Patel VI, et al. Endovascular management of patients with critical limb ischemia. Journal of Vascular Surgery 2011;53(4):1020‐5. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9. Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dormandy 2000
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC). Journal of Vascular Surgery 2000;31(1 Pt 2):S1‐S296. [PUBMED: 10666287] [PubMed] [Google Scholar]
Drescher 2006
- Drescher R, Haller S, Koster O, Rothenburg T, Titschert A. Standard‐protocol moving‐table magnetic resonance angiography for planning of interventional procedures in patients with peripheral vascular occlusive disease. Clinical Imaging 2006;30(6):382‐7. [PUBMED: 17101406] [DOI] [PubMed] [Google Scholar]
Durdu 2006
- Durdu S, Akar AR, Arat M, Sancak T, Eren NT, Ozyurda U. Autologous bone‐marrow mononuclear cell implantation for patients with Rutherford grade II‐III thromboangiitis obliterans. Journal of Vascular Surgery 2006;44(4):732‐9. [DOI] [PubMed] [Google Scholar]
Fadilah 2013
- Fadilah SA, Mohd‐Razif MI, Seery ZA, Nor‐Rafeah T, Wan‐Fariza WJ, Habsah A, et al. Predictors of the yield of mobilized peripheral blood CD34+ cells in HLA‐matched sibling donor. Transfusion and Apheresis Science 2013;49(3):583‐9. [PUBMED: 24012241] [DOI] [PubMed] [Google Scholar]
Fadini 2007
- Fadini GP, Agostini C, Sartore S, Avogaro A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis 2007;194(1):46‐54. [DOI] [PubMed] [Google Scholar]
Fadini 2010
- Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta‐analysis and systematic review of the literature. Atherosclerosis 2010;209(1):10‐7. [PUBMED: 19740466] [DOI] [PubMed] [Google Scholar]
Guyatt 1993
- Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence‐Based Medicine Working Group. JAMA 1993;270(21):2598‐601. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
Honold 2006
- Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, et al. Effects of granulocyte colony stimulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arteriosclerosis Thrombosis and Vascular Biology 2006;26(10):2238‐43. [DOI] [PubMed] [Google Scholar]
Huang 2004
- Huang PP, Li SZ, Han MZ, Xiao ZJ, Yang RC, Qui LG, et al. Autologous transplantation of peripheral blood stem cells as an effective therapeutic approach for severe arteriosclerosis obliterans of lower extremities. Thrombosis and Haemostasis 2004;91(3):606‐9. [DOI] [PubMed] [Google Scholar]
Inaba 2002
- Inaba S, Egashira K, Komori K. Peripheral‐blood or bone‐marrow mononuclear cells for therapeutic angiogenesis?. Lancet 2002;360(9350):2083. [DOI] [PubMed] [Google Scholar]
Jarajapu 2010
- Jarajapu YP, Grant MB. The promise of cell‐based therapies for diabetic complications challenges and solutions. Circulation Research 2010;106(5):854‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaelin 2008
- Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular Cell 2008;30(4):393‐402. [DOI] [PubMed] [Google Scholar]
Kawamura 2006
- Kawamura A, Horie T, Tsuda I, Abe Y, Yamada M, Egawa H, et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. Journal of Artificial Organs 2006;9(4):226‐33. [DOI] [PubMed] [Google Scholar]
Liew 2016
- Liew A, Bhattacharya V, Shaw J, Stansby G. Cell therapy for critical limb ischemia: a meta‐analysis of randomized controlled trials. Angiology 2016;67(5):444‐555. [PUBMED: 26195561] [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu FP, Dong JJ, Sun SJ, Gao WY, Zhang ZW, Zhou XJ, et al. Autologous bone marrow stem cell transplantation in critical limb ischemia: a meta‐analysis of randomized controlled trials. Chinese Medical Journal 2012;125(23):4296‐300. [PUBMED: 23217403] [PubMed] [Google Scholar]
Liu 2015
- Liu Y, Xu Y, Fang F, Zhang J, Guo L, Weng Z. Therapeutic efficacy of stem cell‐based therapy in peripheral arterial disease: a meta‐analysis. PloS One 2015;10(4):e0125032. [PUBMED: 25923119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miyamoto 2006
- Miyamoto K, Nishigami K, Nagaya N, Akutsu K, Chiku M, Kamei M, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation 2006;114(24):2679‐84. [DOI] [PubMed] [Google Scholar]
Moazzami 2014
- Moazzami K, Moazzami B, Roohi A, Nedjat S, Dolmatova E. Local intramuscular transplantation of autologous mononuclear cells for critical lower limb ischaemia. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD008347.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NGC 2012
- National Guideline Clearinghouse (NGC). Lower limb peripheral arterial disease: diagnosis and management. National Institute for Health and Clinical Excellence (NICE) 2012 Aug. 28, issue Clinical guideline no. 147.
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45(Suppl S):S5‐67. [DOI] [PubMed] [Google Scholar]
Norgren 2010
- Norgren L, Hiatt WR, Dormandy JA, Hirsch AT, Jaff MR, Diehm C, et al. The next 10 years in the management of peripheral artery disease: Perspectives from the 'PAD 2009' conference. European Journal of Vascular and Endovascular Surgery 2010;40:375‐80. [DOI] [PubMed] [Google Scholar]
Pittenger 1999
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284(5411):143‐7. [DOI] [PubMed] [Google Scholar]
Raval 2014
- Raval AN, Schmuck EG, Tefera G, Leitzke C, Ark CV, Hei D, et al. Bilateral administration of autologous CD133+ cells in ambulatory patients with refractory critical limb ischemia: lessons learned from a pilot randomized, double‐blind, placebo‐controlled trial. Cytotherapy 2014;16(12):1720‐32. [PUBMED: 25239491] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2002
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. Journal of Clinical Investigation 2002;109(3):337‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sasajima 1997
- Sasajima T, Kubo Y, Inaba M, Goh K, Azuma N. Role of infrainguinal bypass in Buerger's disease: an eighteen‐year experience. European Journal of Vascular and Endovascular Surgery 1997;13(2):186‐92. [DOI] [PubMed] [Google Scholar]
Schatteman 2004
- Schatteman GC, Awad O. Hemangioblasts, angioblasts, and adult endothelial cell progenitors. The Anatomical Record Part A. Discoveries in Molecular, Cellular, and Evolutionary Biology 2004;276(1):13‐21. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH, on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11. Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sultan 2014
- Sultan S, Hynes N. Critical appraisal of stem cell therapy in peripheral arterial disease: do current scientific breakthroughs offer true promise or false hope?. Journal of Biomedical Science and Engineering 2014;7:75‐85. [Google Scholar]
Sun 2015
- Sun X, Ying J, Wang Y, Li W, Wu Y, Yao B, et al. Meta‐analysis on autologous stem cell transplantation in the treatment of limb ischemia. International Journal of Clinical and Experimental Medicine 2015;8(6):8740‐8. [PUBMED: 26309525] [PMC free article] [PubMed] [Google Scholar]
Teraa 2013
- Teraa M, Sprengers RW, Graaf Y, Peters CE, Moll FL, Verhaar MC. Autologous bone marrow‐derived cell therapy in patients with critical limb ischemia: a meta‐analysis of randomized controlled clinical trials. Annals of Surgery 2013;258(6):922‐9. [PUBMED: 23426345] [DOI] [PubMed] [Google Scholar]
Wen 2011
- Wen Y, Meng L, Gao Q. Autologous bone marrow cell therapy for patients with peripheral arterial disease: a meta‐analysis of randomized controlled trials. Expert Opinion on Biological Therapy 2011;11(12):1581‐9. [PUBMED: 21973046] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abdul Wahid 2013
- Abdul Wahid SF, Ismail NA, Abdul Hamid MKA, Harunarashid H, Idris MAM, Muhamad NA, Lai NM. Different sources of autologous mononuclear cells and stem cells for critical lower limb ischaemia. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010747] [DOI] [PMC free article] [PubMed] [Google Scholar]


