Abstract
Background
Systemic corticosteroid therapy is central to the management of acute asthma. The use of inhaled corticosteroids (ICS) may also be beneficial in this setting.
Objectives
To determine the benefit of ICS for the treatment of patients with acute asthma managed in the emergency department (ED).
Search methods
We identified controlled clinical trials from the Cochrane Airways Group specialised register of controlled trials. Bibliographies from included studies, known reviews, and texts also were searched. The latest search was September 2012.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs. Studies were included if patients presented to the ED or its equivalent with acute asthma, and were treated with ICS or placebo, in addition to standard therapy. Two review authors independently selected potentially relevant articles, and then independently selected articles for inclusion. Methodological quality was independently assessed by two review authors. There were three different types of studies that were included in this review: 1) studies comparing ICS vs. placebo, with no systemic corticosteroids given to either treatment group, 2) studies comparing ICS vs. placebo, with systemic corticosteroids given to both treatment groups, and 3) studies comparing ICS alone versus systemic corticosteroids. For the analysis, the first two types of studies were included as separate subgroups in the primary analysis (ICS vs. placebo), while the third type of study was included in the secondary analysis (ICS vs. systemic corticosteroid).
Data collection and analysis
Data were extracted independently by two review authors if the authors were unable to verify the validity of extracted information. Missing data were obtained from the authors or calculated from other data presented in the paper. Where appropriate, individual and pooled dichotomous outcomes were reported as odds ratios (OR) with 95% confidence intervals (CIs). Where appropriate, individual and pooled continuous outcomes were reported as mean differences (MD) or standardized mean differences (SMD) with 95% CIs. The primary analysis employed a fixed‐effect model and a random‐effects model was used for sensitivity analysis. Heterogeneity is reported using I‐squared (I2) statistics.
Main results
Twenty trials were selected for inclusion in the primary analysis (13 paediatric, seven adult), with a total number of 1403 patients. Patients treated with ICS were less likely to be admitted to hospital (OR 0.44; 95% CI 0.31 to 0.62; 12 studies; 960 patients) and heterogeneity (I2 = 27%) was modest. This represents a reduction from 32 to 17 hospital admissions per 100 patients treated with ICS in comparison with placebo. Subgroup analysis of hospital admissions based on concomitant systemic corticosteroid use revealed that both subgroups indicated benefit from ICS in reducing hospital admissions (ICS and systemic corticosteroid versus systemic corticosteroid: OR 0.54; 95% CI 0.36 to 0.81; 5 studies; N = 433; ICS versus placebo: OR 0.27; 95% CI 0.14 to 0.52; 7 studies; N = 527). However, there was moderate heterogeneity in the subgroup using ICS in addition to systemic steroids (I2 = 52%). Patients receiving ICS demonstrated small, significant improvements in peak expiratory flow (PEF: MD 7%; 95% CI 3% to 11%) and forced expiratory volume in one second (FEV1: MD 6%; 95% CI 2% to 10%) at three to four hours post treatment). Only a small number of studies reported these outcomes such that they could be included in the meta‐analysis and most of the studies in this comparison did not administer systemic corticosteroids to either treatment group. There was no evidence of significant adverse effects from ICS treatment with regard to tremor or nausea and vomiting. In the secondary analysis of studies comparing ICS alone versus systemic corticosteroid alone, heterogeneity among the studies complicated pooling of data or drawing reliable conclusions.
Authors' conclusions
ICS therapy reduces hospital admissions in patients with acute asthma who are not treated with oral or intravenous corticosteroids. They may also reduce admissions when they are used in addition to systemic corticosteroids; however, the most recent evidence is conflicting. There is insufficient evidence that ICS therapy results in clinically important changes in pulmonary function or clinical scores when used in acute asthma in addition to systemic corticosteroids. Also, there is insufficient evidence that ICS therapy can be used in place of systemic corticosteroid therapy when treating acute asthma. Further research is needed to clarify the most appropriate drug dosage and delivery device, and to define which patients are most likely to benefit from ICS therapy. Use of similar measures and reporting methods of lung function, and a common, validated, clinical score would be helpful in future versions of this meta‐analysis.
Plain language summary
Early use of inhaled corticosteroids in the emergency department treatment of acute asthma
Asthma is one of the most common chronic diseases in the world. It is estimated that 300 million people of all ages, and all ethnic backgrounds, suffer from asthma, with 1 in every 250 deaths worldwide attributed to asthma. In an asthma attack, the airways (passages to the lungs) narrow from muscle spasm and swelling (inflammation). Corticosteroid drugs can be used to reduce the swelling. Corticosteroids can be inhaled, or taken systemically by mouth (orally) or through a drip into the veins (intravenously).
Standard treatment for asthma attacks is to administer beta2‐agonists (to open up the airways) and systemic corticosteroids (to reduce the inflammation). The purpose of this review was to determine if the use of inhaled corticosteroid (ICS) agents is beneficial in emergency department treatment settings. A total of 90 studies were identified for this review; 20 were deemed relevant and selected for inclusion (13 paediatric, 7 adult), with a total number of 1403 patients.
This review found that inhaled corticosteroids used alone or in combination with systemic corticosteroids helped to relieve asthma attacks, were well tolerated and had few side effects. However, the most effective drug and dosage are unclear. The studies in the review included a variety of ICSmedications: beclomethasone (Beclovent/Becloforte/QVAR), budesonide (Pulmicort), dexamethasone sodium phosphate, fluticasone propionate (Flovent or Flixotide), Flunisolide (Aerobid) and triamcinolone (Azmacort). The review also found that ICS administered in this setting resulted in fewer hospital admissions. There was a reduction from 32 to 17 hospital admissions per hundred patients treated with ICS agents compared with placebo. At this time there is insufficient evidence to support using ICS agents alone as a replacement for systemic corticosteroid therapy in acute asthma attacks
However, there are many unanswered questions about the use of ICS in the emergency department treatment setting. Future research should focus on optimal dosage, dosage frequency and delivery device, identification of effective ICS agents, clearly defined outcomes (such as admissions criteria, pulmonary function testing and follow‐up after discharge from emergency departments).
Summary of findings
Background
Description of the condition
Acute asthma exacerbation is a common presenting complaint to the emergency department (ED). In the US, acute asthma exacerbations account for almost two million ED visits per year (Mannino 1998). Approximately 10% to 20% of these patients will require admission to the hospital, and, of those discharged from the ED after apparently successful treatment, approximately 10% to 20% will relapse within two weeks (Griswold 2005; Rowe 2008a; Rowe 2010). Several national (Boulet 2000; BTS 1997; BTS/SIGN 2011; NAEPP 1997; EPR3 2007) and international (GINA 2011; Masoli 2004; NHLBI/WHO 1995) guidelines have been produced for the management of acute asthma.
Description of the intervention
There is general agreement that bronchodilators and systemic corticosteroids are first‐line agents for acute asthma. Beta2‐agonists are used to provide rapid symptom relief, whereas corticosteroids are used to counter airway inflammation and hasten resolution of the asthma exacerbation. There remain numerous controversies regarding the optimal agent, dose, frequency of delivery and route of delivery for both bronchodilators and corticosteroids in the acute setting. Current practice patterns usually include the use of beta2‐agonists via a nebuliser or metered‐dose inhaled (MDI) and spacer and oral or intravenous (IV) corticosteroids administered early in the ED treatment of acute asthma (Griswold 2005). While inhaled corticosteroids (ICS) are used more commonly after ED discharge, their use is uncommon in the ED setting (Barnes 1995; BTS/SIGN 2011).
How the intervention might work
ICS have the potential to be of benefit in the acute setting. They have been shown to be effective alternatives to oral corticosteroids in long‐term asthma therapy, where they can reduce or even eliminate oral corticosteroid requirements (Barnes 1995). Potential advantages of ICS in acute asthma therapy might include fewer systemic side effects, direct delivery to the airways, and a greater efficacy in reducing airway reactivity and oedema either alone or in addition to systemic corticosteroids (Gibbs 2000; Rodrigo 1998). Furthermore, ancillary evidence from studies of patients with croup suggests that ICS agents may act on the airways over the short term to improve outcomes (Ausejo 1999).
Why it is important to do this review
Only a limited number of trials have examined the use of ICS in acute asthma and they have yielded inconsistent results. The previous version of this systematic review (Edmonds 2003) concluded that "inhaled steroids reduced hospital admissions in patients with acute asthma, but it is unclear if there is a benefit of ICS when used in addition to systemic corticosteroids. There is insufficient evidence that ICS therapy results in clinically important changes in pulmonary function or clinical scores when used in acute asthma. Similarly, there is insufficient evidence that ICS alone is as effective as systemic corticosteroid. Further research is needed to clarify if there is a benefit of ICS when used in addition to systemic corticosteroid." The 2012 update of the review evaluated these conclusions in relation to randomised controlled trials (RCTs) published since the publication of the previous version of the review.
Separate reviews are available in The Cochrane Library for: increased versus stable doses of ICS for exacerbations of chronic asthma in adults and children (Quon 2010), early ED treatment of acute asthma with systemic corticosteroids (Rowe 2008) and ICS for acute asthma following ED discharge (Edmonds 2009).
Objectives
To determine the effect of ICS therapy on outcomes in the ED treatment for acute asthma. The two comparisons were:
ICS versus placebo;
ICS versus systemic corticosteroids.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs or quasi‐RCTs (e.g. allocation on days of the week or flipping a coin).
Types of participants
We included studies involving patients presenting to an ED or its equivalent. We included trials involving participants from other settings if the people enrolled at the ED were reported separately (e.g., if stratified randomisation was employed). Studies recruiting paediatric or adult participants were reviewed, and this designation formed one of the subgroup analyses. Studies of young children (< two years of age) with bronchiolitis or viral‐induced wheeze were excluded.
Types of interventions
Patients must have been randomised to receive either single‐ or multiple‐dose ICS early in their ED treatment. 'Inhaled corticosteroid' administration was defined as any corticosteroid agent administered by MDI, dry powder inhaler, or nebuliser in the ED. We included trials where people may also have received additional asthma medications (such as systemic corticosteroid and beta2‐agonists by any route, ipratropium bromide, theophylline compounds, magnesium sulphate or anti‐histamines). Data for these co‐interventions were recorded or requested from the authors who were available for contact, where it was not reported in the articles.
We included the following comparisons:
-
ICS versus placebo:
with concomitant systemic corticosteroids in both groups;
with no concomitant systemic corticosteroids in either group;
ICS versus systemic corticosteroids.
Types of outcome measures
Primary outcomes
Admission to hospital (based on the criteria reported in the manuscript).
Secondary outcomes
Pulmonary function tests (absolute and percent predicted peak expiratory flow (PEF) and forced expiratory volume in one second (FEV1));
Adverse effects;
Physiological outcomes (e.g., clinical scores, pulse rate, respiratory rate, arterial oxygen saturation, blood pressure, arterial pH, etc.).
Several studies included a clinical score, such as the pulmonary index or pulmonary index score (PIS), and others that are scoring systems to evaluate patients with acute asthma. These scores were generally a composite score assessing a number of physical examination parameters often including heart rate, respiratory rate, presence and severity of wheezing, accessory muscle use and severity of dyspnoea. (Becker 1984)
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched all records in the CAGR coded as 'asthma'. For the review version up to 2005 we used the search strategy in Appendix 2, while for the 2012 update, we used the strategy described in Appendix 3. We also conducted a search of ClinicalTrials.gov. All databases were searched from their inception with no restriction on language of publication.
The latest search was conducted in September 2012.
Searching other resources
For the 2005 version, additional efforts to locate potential trials were as follows:
reference lists of all available primary studies and review articles were reviewed to identify potentially relevant citations;
inquiries were made regarding other published or unpublished trials known or supported by the authors of the primary studies so that these results could be included in this review;
we contacted scientific advisors of the various pharmaceutical industries that manufacture known ICS agents (AstraZeneca: budesonide; GlaxoSmithKline: fluticasone and beclomethasone; Forest laboratories: flunisolide) to request any unpublished, or interim results on relevant research;
we handsearched abstracts from the Society for Academic Emergency Medicine (1997 to 2000, published in Academic Emergency Medicine), the American College of Chest Physicians (1995 to 2000, published in Chest) the British Thoracic Society (published in Thorax) and the American Thoracic Society (1997 to 1999 published in American Journal of Respiratory and Critical Care Medicine) meetings;
we made personal contact with colleagues, collaborators and other trialists working in the field of asthma was made to identify potentially relevant studies.
In 2012 we checked the reference sections of included papers to search for additional potentially relevant trials.
Data collection and analysis
Selection of studies
Prior to the 2012 update two review authors (MLE, BHR) independently examined the references returned by searches to identify potentially relevant trials for full review. No specific blinding techniques were used (Jadad 1996). In the 2012 update this process was completed by SJM and MLE.
Data extraction and management
Prior to the 2012 update data extraction was performed independently by two review authors (BHR, MLE), and the authors of trials were contacted to provide missing data where possible. In some cases, expansion of graphic representations of data from the manuscripts was used to estimate missing data. The data were checked and entered into Review Manager (RevMan 2011) by one review author. In the 2012 update data extraction was performed by SJM and checked by MLE, and entered into RevMan (RevMan 2011) by SJM and checked by MLE.
Assessment of risk of bias in included studies
The risk of bias of included studies was assessed using the Cochrane Collaboration's risk of bias tool (Higgins 2011). Two review authors (MLE and SJM) assessed the risk of bias for all included studies with regards to random sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting. Each item was assessed as high, low or unclear risk of bias along with relevant information reported in the randomised controlled trial.
Measures of treatment effect
For dichotomous variables, data are expressed as odds ratios (OR) with 95% confidence intervals (CI). Data for continuous variables were reported as mean differences (MD) with 95% CIs.
Unit of analysis issues
The unit of analysis was the patient.
Dealing with missing data
If outcome data or information on trial design was missing, we attempted to contact authors for clarification. We reported intention‐to‐treat analyses.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of forest plots. The I2 statistic was also considered and interpreted in relation to the following guidance:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity (Higgins 2011).
The Chi2 test was similarly considered (P ‐value < 0.10); however, we regarded the I2 statistic as our primary measure of heterogeneity.
Assessment of reporting biases
We planned to evaluate publication bias using visual inspection of funnel plots if there was an adequate number of trials aggregated in the analyses (more than 10). However, we recognised that an asymmetric funnel plot can reflect heterogeneity, outcome reporting bias and small study effects and is therefore not necessarily a reflection of publication bias. A sufficient number of trials in the analysis provided the opportunity to include funnel plots for the primary analyses, and they are presented in Figure 1 and Figure 2.
1.
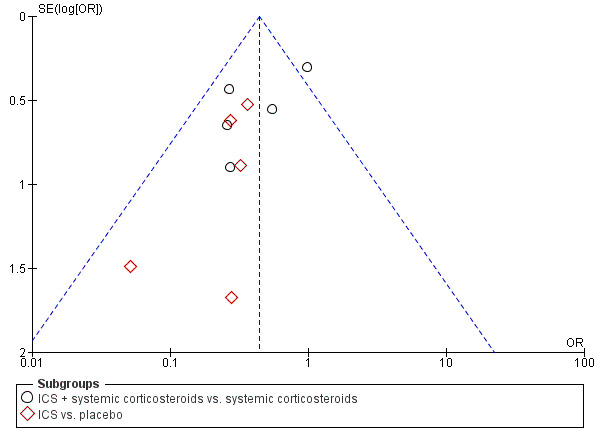
Funnel plot of comparison: 1 ICS therapy, outcome: 1.1 Hospital admission.
Data synthesis
We combined trials were combined using RevMan (RevMan 2011). Continuous variables were combined using a MD or standardised mean difference (SMD) and reported together with a 95% CI. We combined dichotomous variables using an OR with 95% CI.
Subgroup analysis and investigation of heterogeneity
We specified the following three specific subgroups a priori:
adults compared to children;
severe asthma compared to those with less severe asthma (categorised by % predicted PEF), and by the placebo group hospital admissions);
high versus low dose (high dose was defined as 2 mg or more beclomethasone dipropionate (BDP) equivalence).
Sensitivity analysis
We conducted sensitivity analyses based on methodological quality and fixed‐ versus random‐effects models.
Results
Description of studies
Results of the search
The initial search in 1998 identified 352 articles. There were a total of 187 original citations; 15 articles were deemed potentially relevant by one or both of the two review authors, with substantial agreement (kappa = 0.78) between the two review authors. These 15 articles were retrieved and the full‐text manuscripts were reviewed for inclusion. From the full text, using specific criteria, two review authors independently selected trials for inclusion in the review. Five articles were identified by both review authors for inclusion, with 100% simple agreement and a kappa of 1.0. Six further articles were identified using other methods (one by author contact, two by searching abstracts from recent meetings and three in the updates from the register or computerised searches), which were selected for inclusion for a total of 11 articles; seven in the primary analysis and four in the secondary analysis.
In the update search in April 1999 we identified 42 articles with 33 original citations. One of these articles was selected for inclusion in the secondary analysis (Volovitz 1998). In an update search in February 2001 using EMBASE and MEDLINE, two further trials were selected for inclusion, one in the primary analysis (Singhi 1999), and one in the secondary analysis (Devidayal 1999). One further article was added from searching abstracts for inclusion in the secondary analysis (Schuh 2000). An update search in April 2002 identified one further trial for inclusion (Tsai 2001). An update search in February 2003 did not find any new studies for inclusion.
The update search in February 2005 identified five full trials for inclusion. Three of these trials were included in the primary analysis (Milani 2004; Rodrigo 2003; Sharma 2003) and three trials were included in the secondary analysis (Macias 2003; Milani 2004; Rodrigo 2005). One trial (Milani 2004) had three treatment arms (ICS, systemic corticosteroid and placebo) and so contributed to both the primary and secondary analyses. Six trials have been published in abstract form only; author contact was unsuccessful. Three were judged as potentially eligible for inclusion in the primary analysis (Agarwal 2003; Blandon 2004; Olaivar 1999) and two for inclusion in the secondary analysis (Acun 2003; Sari 2004). Three of these were included in the 2012 update (Blandon 2004; Olaivar 1999; Sari 2004).
In the 2012 update 1240 trial reports were identified (Figure 2) and this produced a further 16 included studies to bring the total to 32. The new studies were Ancheta 2008 (24 patients), Bateman 2006 (115 patients), Bautista 1994 (30 patients), Belda 2007 (39 patients), Blandon 2004 (86 patients), Estrada 2005 (100 patients), Go 2010 (33 patients), Nuhoglu 2005 (26 patients), Olaivar 1999 (55 patients), Rahman 2008 (100 patients), Razi 2012 (100 patients), Sari 2004 (76 patients), Schuh 2006 (69 patients), Sekerel 2005 (67 patients), Starobin 2008 (33 patients) and Upham 2011 (180 patients). This added a further 1173 (49%) patients to the 1201 already in the review, bringing the total to 2374.
2.
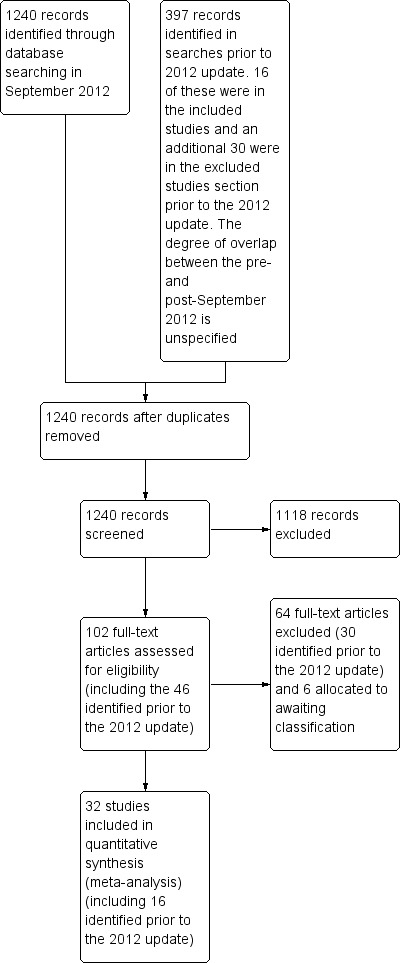
Study flow diagram.
Included studies
All 32 studies were published after 1992. There were studies from all over the world; five were from centres in Canada; four from the Philippines; three each from the US, Uruguay, India and Turkey; two each from South Africa, Israel and Mexico; and one from each of Indonesia, Brazil and Taiwan. Twenty studies were included in the primary analysis that compared ICS versus placebo; seven studies (Bateman 2006; Guttman 1997; Nuhoglu 2005; Razi 2012; Starobin 2008; Sung 1998; Upham 2011) compared ICS versus placebo with both groups receiving systemic corticosteroid and 13 studies compared ICS versus placebo with systemic corticosteroid withheld from both treatment groups (Afilalo 1999; Blandon 2004; Bautista 1994; Estrada 2005; Milani 2004; Olaivar 1999; Pansegrouw 1992; Rodrigo 1998; Rodrigo 2003; Sekerel 2005; Sharma 2003; Singhi 1999; Tsai 2001).
Fourteen studies compared ICS versus systemic corticosteroid (Ancheta 2008; Belda 2007; Devidayal 1999; Go 2010; Macias 2003; Milani 2004; Scarfone 1995; Schuh 2000; Schuh 2006; Volovitz 1998; Rahman 2008; Rodrigo 2005; Sari 2004; Starobin 2008). Two of the studies (Milani 2004; Starobin 2008) had three treatment arms (ICS, systemic corticosteroid and placebo) and so were included in both comparisons. Details of the characteristics of all three comparisons can be found in Table 3; Table 4 and Table 5.
1. Summary of included trials: ICS versus placebo, both groups receiving oral corticosteroids.
| Study | N on ICS | N on placebo | Age group | Delivery device | ICS dose | Hospital admissions reported? |
| Bateman 2006 | 58 | 57 | Adults | * | Budesonide 320 μg/puff 2 puffs q 5 min x 2; total dose 1280 μg; LOW | N |
| Guttman 1997 | 30 | 30 | Adults | MDI plus chamber | Beclomethasone dipropionate 1 mg at 0, 30 min, 1, 2, 4, 6, 8 h; total dose 7 mg; HIGH | Y |
| Nuhoglu 2005 | 12 | 14 | Children | Nebuliser | Budesonide 1 mg; LOW | N |
| Razi 2012 | 50 | 50 | Children | Nebuliser | Budesonide 1 mg q 20 min x 3; total dose 3 mg; HIGH | Y |
| Starobin 2008 | 26 | 23 | Adults | Nebuliser | Fluticasone 0.5 mg; LOW | Y |
| Sung 1998 | 24 | 20 | Children | Nebuliser | Budesonide 2 mg; HIGH | Y |
| Upham 2011 | 91 | 89 | Children | Nebuliser | Budesonide 2 mg; HIGH | Y |
* Denotes uncertainty.
Dose equivalency used: high ≥ beclomethasone dipropionate 2 mg (e.g. budesonide 1.5 mg, fluticasone 1.5 mg, triamcinolone 5 mg).
2. Summary of included trials: ICS versus placebo, systemic corticosteroids withheld from both treatment groups.
| Study | N on ICS | N on placebo | Age group | Delivery device | ICS dose | Hospital admissions reported? |
| Afilalo 1999 | 28 | 26 | Adults | MDI | Beclomethasone 1 mg at 0, 30 min, 1, 2, 4 h; total dose 5 mg; HIGH | Y |
| Blandon 2004 | 40 | 46 | Children | Nebuliser | Budesonide 550 μg x 1 dose; LOW | N |
| Bautista 1994 | * | * | 30 Children | * | Dose not stated | N |
| Estrada 2005 | 50 | 50 | Children | Nebuliser | Fluticasone 500 μg/dose x 3 doses; total dose 1500 μg; HIGH | Y |
| Milani 2004 | 17 | 15 | Children | Nebuliser | Budesonide 2 mg; HIGH | Y |
| Olaivar 1999 | 33 | 32 | Children | Nebuliser | Budesonide 0.5 mg q 20 min x 3; total dose 1.5 mg; HIGH | N |
| Pansegrouw 1992 | 20 | 20 | Adults | MDI | Beclomethasone 200 μg; LOW | N |
| Rodrigo 1998 | 47 | 47 | Adults | MDI+spacer | Flunisolide 1 mg q 10 min x 3 hours; total dose 18 mg; HIGH | Y |
| Rodrigo 2003 | 58 | 62 | Adults | MDI+spacer | Fluticasone 1 mg q 10 min x 3 hours; total dose 18 mg; HIGH | Y |
| Sekerel 2005 | 33 | 34 | Children | Nebuliser | Budesonide 1 mg q 1 h x 3; total dose 3 mg; HIGH | Y |
| Sharma 2003 | 28 | 29 | Children | MDI+spacer | Beclomethasone 100 μg q 20 min x 3 prn; total dose 300 μg; LOW | N |
| Singhi 1999 | 30 | 30 | Children | MDI+spacer | Budesonide 400 μg q 30 min x 3; total dose 1200 μg; LOW | Y |
| Tsai 2001 | 12 | 12 | Children | Nebuliser | Budesonide 0.05 mg/kg, maximum 2 mg; HIGH | N |
* Denotes uncertainty.
Dose equivalency used: high ≥ beclomethasone dipropionate 2 mg (e.g. budesonide mg, fluticasone 1.5 mg, triamcinolone 5 mg).
3. Summary of included studies: ICS versus systemic corticosteroids.
| Study | N on ICS | N on oral corticosteroids | Age group | Delivery device | ICS dose | Systemic corticosteroid dose and mode of delivery | Hospital admissions reported? |
| Ancheta 2008 | 12 | 12 | Children | MDI+spacer | Fluticasone 125 μg/puff 4 puffs q 20 min x 3; total dose 1500 μg; HIGH | Hydrocortisone 4 mg/kg to maximum 200 mg; IV | Y |
| Belda 2007 | 19 | 20 | Adults | MDI | Fluticasone 16 puffs; total dose 4000 μg; HIGH | Prednisone 30 mg; oral | N |
| Devidayal 1999 | 41 | 39 | Children | Nebuliser | Budesonide 800 μg x 3 doses; total dose 2400 μg; HIGH | Prednisolone 2 mg/kg; oral | Y |
| Go 2010 | 16 | 17 | Adults | Nebuliser | Dose not stated | Hydrocortisone 250 mg; IV | N |
| Macias 2003 | 45 | 90 | Children | MDI+spacer | Triamcinolone 600 μg; LOW | Prednisone 2 mg/kg; oral | Y |
| Milani 2004 | 17 | 17 | Children | Nebuliser | Budesonide 2 mg; HIGH | Prednisone 1 mg/kg; oral | Y |
| Rahman 2008 | * | * | 100 Adults |
MDI+spacer | Budesonide 3000 μg/h x 2 hours; total dose 6000 μg; HIGH | "systemic corticosteroid" 500 mg; IV | N |
| Rodrigo 2005 | 60 | 61 | Adults | MDI+spacer | Fluticasone 500 μg q10 min x 3 hours; total dose 9000 μg; HIGH | Hydrocortisone 500 mg; IV | Y |
| Sari 2004 | 38 | 38 | Adults | Nebuliser | Fluticasone 500 μg; LOW | Methylprednisolone 125 mg; IV | N |
| Scarfone 1995 | 62 | 66 | Children | Nebuliser | Dexamethasone 1.5 mg/kg; HIGH* | Prednisone 2 mg/kg; oral | Y |
| Schuh 2000 | 52 | 51 | Children | MDI+spacer | Fluticasone 2000 μg | Prednisolone syrup 2 mg/kg to maximum 60 mg; oral | Y |
| Schuh 2006 | 35 | 34 | Children | MDI+spacer | Fluticasone 2000 μg; HIGH | Prednisolone syrup 2 mg/kg to maximum 60 mg; oral | Y |
| Starobin 2008 | 24 | 23 | Adults | Nebuliser | Fluticasone 500 μg; LOW | Methylprednisolone 125 mg; IV | Y |
| Volovitz 1998 | 11 | 11 | Children | Turbohaler | Budesonide 1600 μg; HIGH | Prednisolone 2 mg/kg; oral | Y |
* Denotes uncertainty.
Dose equivalency used: high ≥ beclomethasone dipropionate 2 mg (e.g. budesonide 1.5 mg, fluticasone 1.5 mg, triamcinolone 5 mg).
Populations
In the comparison ICS versus placebo, 13 of the studies involved children (Bautista 1994; Blandon 2004; Estrada 2005; Milani 2004; Nuhoglu 2005; Olaivar 1999; Razi 2012; Sekerel 2005; Sharma 2003; Singhi 1999; Sung 1998; Tsai 2001; Upham 2011), and seven involved adults (Afilalo 1999; Bateman 2006; Guttman 1997; Pansegrouw 1992; Rodrigo 1998; Rodrigo 2003; Starobin 2008). In the adult studies, the populations varied from only those with severe asthma (forced expiratory volume in one second (FEV1) < 40% to 50% predicted or investigator‐assigned severity (Bateman 2006; Rodrigo 1998; Rodrigo 2005; Upham 2011), to only those with mild to moderate asthma (FEV1 = 40% to 70% predicted (Afilalo 1999; Guttman 1997). All of the paediatric studies excluded patients with very severe asthma (pulmonary index > 13 or equivalent), and four excluded those with only mild asthma (pulmonary index < 8 or equivalent).
In the studies comparing ICS versus systemic corticosteroid, eight involved children (Ancheta 2008; Devidayal 1999; Macias 2003; Milani 2004; Scarfone 1995; Schuh 2000; Schuh 2006; Volovitz 1998) and six involved adults (Belda 2007; Go 2010; Rahman 2008; Rodrigo 2005; Sari 2004; Starobin 2008).
Most of the studies in the secondary analysis comparing ICS versus systemic corticosteroid involved patients with mild to moderate exacerbations, although three included children with moderate to severe asthma exacerbations (Ancheta 2008; Macias 2003; Schuh 2000) and one included adults with severe asthma (Rodrigo 2005).
Interventions
ICS were administered early in the course of ED treatment; usually at the time of the first beta2‐agonist treatment. Total doses ranged from low (BDP 200 μg; Pansegrouw 1992) to very high (flunisolide 18 mg; Rodrigo 1998). The route of administration was via nebuliser or MDI with spacer in the paediatric studies, and predominantly via MDI with spacer in all the adult studies. In the analysis of ICS versus systemic corticosteroid, the doses of ICS were generally moderate to high. The dose, frequency and agents used in this review varied widely; however, there appears to be evidence of effect despite this heterogeneity. For example, various ICS agents were used (budesonide most often {14 studies}), the median single dose was 900 µg, the median frequency of treatment was 2 activations, and the median cumulative dose was 2 mg over up to 6 hours of observation. Delivery was most commonly by nebuliser or MDI with spacer. The dose, frequency and agents for the trials using each comparison are listed separately in Table 3, Table 4 and Table 5.
Co‐interventions
All studies gave beta2‐agonists to participants, although the type varied. Systemic corticosteroids were administered to both the experimental and control groups in six studies (Bateman 2006; Guttman 1997; Nuhoglu 2005; Razi 2012; Sung 1998; Upham 2011); however, Razi 2012 gave intramuscular systemic corticosteroids (rather than using the oral or intra‐venous routes suggested in guidelines (BTS/SIGN 2011)). Fourteen studies compared ICS versus placebo with systemic corticosteroid withheld from both treatment groups (Afilalo 1999; Bautista 1994; Blandon 2004; Estrada 2005; Milani 2004; Olaivar 1999; Pansegrouw 1992; Rodrigo 1998; Rodrigo 2003; Sekerel 2005; Sharma 2003; Singhi 1999; Starobin 2008; Tsai 2001). In one study, systemic corticosteroids and aminophylline were administered to patients who failed to improve after two hours of treatment, while maintaining the study blinding (Singhi 1999).
Fourteen studies compared ICS with systemic corticosteroid (Ancheta 2008; Belda 2007; Devidayal 1999; Go 2010; Macias 2003; Milani 2004; Rahman 2008; Rodrigo 2005; Sari 2004; Scarfone 1995; Schuh 2000; Schuh 2006; Starobin 2008; Volovitz 1998). Two of the studies (Milani 2004; Starobin 2008) had three treatment arms (ICS, systemic corticosteroid and placebo) and so were included in both the primary and secondary analyses. Ipratropium bromide was given in a number of the studies to all included patients.
Outcomes
Outcomes were measured at different time points. Most trials included pulmonary function tests or a clinical score (in paediatric studies), and hospital admissions. The criteria for admission, and the timing of admission decisions, varied among the trials, with only one trial reporting pre‐specified admission criteria (Singhi 1999). Reporting of symptom scores and adverse effects also were variable, and further information about adverse effects had to be provided by authors. Vital signs were reported frequently or were requested from the authors in the initial version of this review, when not reported.
Excluded studies
Sixty‐five studies failed to meet the eligibility criteria of this review and the reasons for their exclusion are provided in Characteristics of excluded studies. The primary reasons for exclusion are as follows: patients were hospitalised rather than treated just in the ED (18 (28%)), patients with stable asthma 10 (15%), comparison between systemic corticosteroids versus placebo nine (14%), outpatient treatment of acute asthma eight (12%), treatment of acute asthma after ED four (6%), non‐randomised studies five (8%), review three (5%), dose comparison in ICS two (3%), combination therapy (corticosteroids plus beta2‐agonists versus beta2‐agonists alone) two (3%), combination therapy (corticosteroids + beta2‐agonists versus placebo) one (2%), IV corticosteroids one (2%), prevention of ER visits one (2%) and delivery of ICS one (2%).
Risk of bias in included studies
Full details of the risk of bias can be found in Characteristics of included studies. A graphical display of our judgements can be found in Figure 3 and Figure 4.
3.
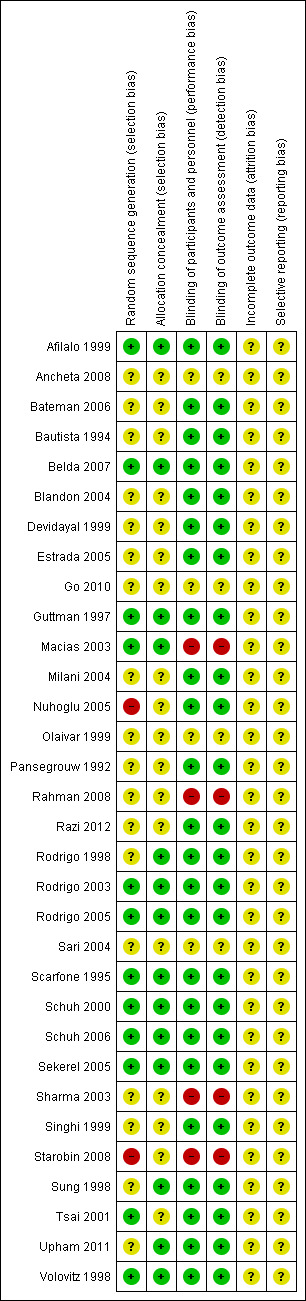
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
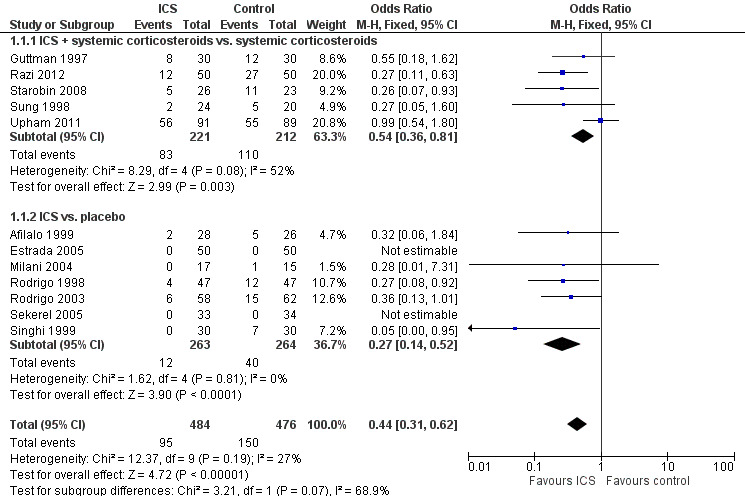
Forest plot of comparison: 1 ICS versus placebo, outcome: 1.1 Admission to hospital.
Allocation
The quality of reported randomisation was variable. Less than half of the 32 studies were judged as low risk of bias. Eleven (34%) studies were assessed as low risk of selection bias (Afilalo 1999; Belda 2007; Guttman 1997; Macias 2003; Rodrigo 2003; Rodrigo 2005; Scarfone 1995; Schuh 2000; Schuh 2006; Sekerel 2005; Volovitz 1998), while two (6%) were judged to be at high risk of selection bias (Nuhoglu 2005; Starobin 2008). The remaining 19 studies were at unclear risk on this respect.
Blinding
Twenty‐four trials (75%) were assessed as low risk of performance and detection bias (Afilalo 1999; Bateman 2006; Bautista 1994; Belda 2007; Blandon 2004; Devidayal 1999; Estrada 2005; Guttman 1997; Milani 2004; Nuhoglu 2005; Pansegrouw 1992; Razi 2012; Rodrigo 1998; Rodrigo 2003; Rodrigo 2005; Scarfone 1995; Schuh 2000; Schuh 2006; Sekerel 2005; Singhi 1999; Sung 1998; Tsai 2001; Upham 2011; Volovitz 1998). Four (13%) were regarded as unclear in terms of risk of performance and detection bias (Ancheta 2008; Go 2010; Olaivar 1999; Sari 2004) and four were assessed as high risk of bias (Macias 2003; Rahman 2008; Sharma 2003; Starobin 2008).
Incomplete outcome data
It was unclear if any of the studies encountered attrition. However, as these trials are very short we evaluated trials where no patients were reported as having been withdrawn to be at no higher risk of bias than those where it was reported that several failed to complete the trial. In acute asthma trials it is conceivable that all participants will complete the trial and this may not be reported explicitly.
Selective reporting
In all 32 included studies reporting bias was judged to be unclear. There was no apparent indication of selective reporting in any of the trials; however, hospital admissions were reported in 12 of the 20 trials comparing ICS versus placebo and in 10 of the 14 trials in the secondary analysis where ICS alone was compared to systemic corticosteroid alone.
Effects of interventions
Summary of findings for the main comparison. ICS versus placebo.
| ICS versus placebo | ||||||
|
Patient or population: people with acute asthma
Settings: emergency department
Intervention: ICS therapy Control: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ICS therapy | |||||
| Hospital admission | 316 per 1000 | 169 per 1000 (125 to 220) | OR 0.44 (0.31 to 0.61) | 959 (12 studies) | ⊕⊕⊝⊝ low1 | There was conflicting evidence from the studies of ICS in addition to systemic corticosteroids (I2 = 52%) |
| FEV1 at 1 hour | The mean FEV1 ranged from 1.41 to 2.17 L | The mean FEV1 at 1 hour in the intervention groups was 0.28 L higher (0.22 lower to 0.77 higher) | MD 0.28 (95% CI ‐0.22 to 0.77) |
248 (4 studies) | ⊕⊕⊝⊝ low2 | |
| FEV1 at 3 to 4 hours | The mean FEV1 ranged from 1.7 to 2.1 L | The mean FEV1 at 3 to 4 hours in the intervention groups was 0.15 L higher (0.09 lower to 0.39 higher) | MD 0.15 (95% CI ‐0.09 to 0.39) |
319 (4 studies) | ⊕⊕⊕⊝ moderate3 | |
| Adverse effects ‐ nausea/vomiting | 64 per 1000 | 21 per 1000 (2 to 178) | OR 0.32 (0.03 to 3.18) | 94 (1 study) | ⊕⊕⊝⊝ low4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in one second; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Point deducted in hospital admissions due to variability in risk of bias among contributing trials and a point deducted due to heterogeneity.
2 Point deducted in FEV1 at 1 hour due to variability in risk of bias among contributing trials, and an additional point deducted for the very high level of heterogeneity (I2 = 90%).
3 Point deducted in FEV1 at 3 to 4 hours due to heterogeneity (I2 = 55%).
4 2 points deducted due to wide CI and only one study contributing to outcome.
Summary of findings 2. ICS versus systemic corticosteroids.
| ICS versus systemic corticosteroids | ||||||
| Patient or population: people with acute asthma Settings: emergency department Intervention: ICS versus systemic corticosteroid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ICS versus systemic corticosteroid | |||||
| Hospital admission | 181 per 1000 | 110 per 1000 (52 to 215) | OR 0.56 (0.25 to 1.24) | 763 (10 studies) | ⊕⊕⊝⊝ low1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Variability in risk of bias among included studies and 61% I2 heterogeneity with regard to hospital admissions in ICS versus systemic corticosteroid comparison.
Inhaled corticosteroids versus placebo
Twelve studies involving 959 patients compared ICS with placebo (N = 484 ICS treated, N = 475 placebo) in our primary outcome, admission to hospital.
Admission to hospital
Admission to hospital is reported in Analysis 1.1 and indicates a significant reduction in hospital admissions in patients treated with ICS (OR 0.44; 95% CI 0.31 to 0.62; 12 studies; N = 960) and the heterogeneity (I2 = 27%) was modest. Closer inspection of Analysis 1.1 reveals that both subgroups ‐ ICS plus systemic corticosteroids versus systemic corticosteroids (OR 0.54; 95% CI 0.36 to 0.81; 5 studies; N = 433) and ICS versus placebo (OR 0.27; 95% 0.14 to 0.52; 7 studies; N = 527) ‐ indicate benefit from ICS in reducing hospital admissions (Figure 4). However, there was heterogeneity within the subgroup of trials using ICS plus systemic corticosteroids (I2 = 52%), and using a random effects model the confidence interval widens to OR 0.46 (95% CI 0.24 to 0.88). The two newest trials showed divergent results, but Razi 2012 is not yet fully reported. The large reduction in hospital admissions found in Razi 2012 contrasts with Upham 2011, the most recent fully reported study, which did not find a reduction in hospital admissions. The distribution of these effects is shown in the funnel plot in Figure 1.
1.1. Analysis.
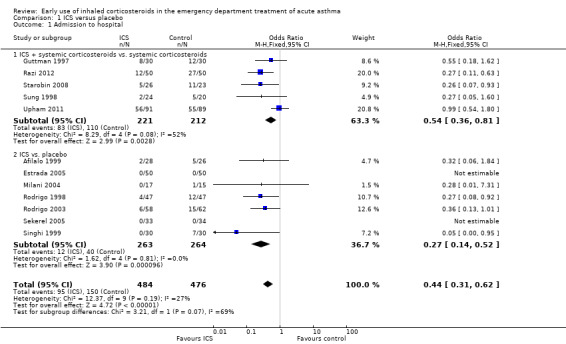
Comparison 1 ICS versus placebo, Outcome 1 Admission to hospital.
Three additional randomised studies were identified that we could not include in the meta‐analysis (as the number of participants in each group with the outcome were not reported). A summary of the results is included below to ensure that they are available to provide context for the above data. In Bautista 1994 (30 children) hospital admissions were reported as 13% in the budesonide plus beta2‐agonist group whereas in the group receiving beta2‐agonists alone it was 73% and is therefore consistent with the effect observed in Analysis 1.1. In Bateman 2006 "treatment failure" was reported, which was defined as the need for additional asthma treatment or hospitalisation. The data for hospitalisations could not be extracted separately but treatment failure was reported in 10% of those receiving ICS and 16% of those receiving placebo, in keeping with the findings of the included studies. However, in Olaivar 1999, a low dose of budesonide was compared to placebo, with both groups receiving beta2‐agonists, and they found no difference in the number of "good responders" between the groups.
Pulmonary function tests
A variety of pulmonary function tests were recorded during the ED stay (absolute and % predicted PEF and FEV1 over a range of time points) and they are reported separately in Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16 and Analysis 1.17. Results were pooled at one, two, three to four, and five hours after the start of treatment.
1.2. Analysis.
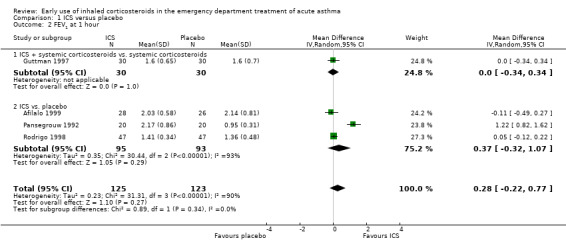
Comparison 1 ICS versus placebo, Outcome 2 FEV1 at 1 hour.
1.3. Analysis.
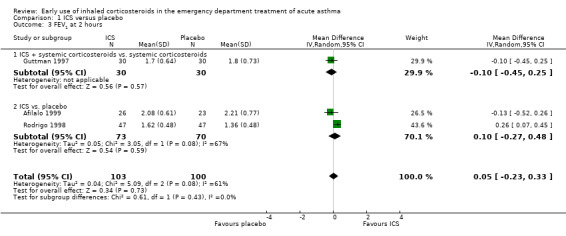
Comparison 1 ICS versus placebo, Outcome 3 FEV1 at 2 hours.
1.4. Analysis.
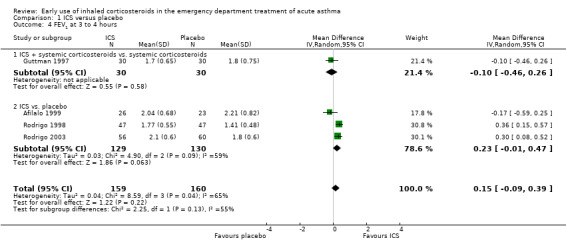
Comparison 1 ICS versus placebo, Outcome 4 FEV1 at 3 to 4 hours.
1.5. Analysis.
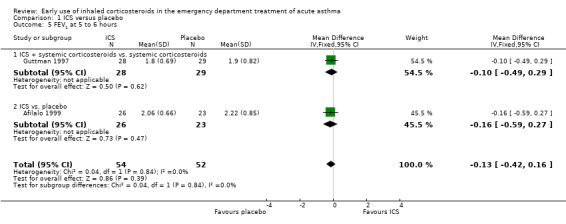
Comparison 1 ICS versus placebo, Outcome 5 FEV1 at 5 to 6 hours.
1.6. Analysis.
Comparison 1 ICS versus placebo, Outcome 6 FEV1 (% predicted) at 1 hour.
1.7. Analysis.
Comparison 1 ICS versus placebo, Outcome 7 FEV1 (% predicted) at 2 hours.
1.8. Analysis.
Comparison 1 ICS versus placebo, Outcome 8 FEV1 (% predicted) at 3 to 4 hours.
1.9. Analysis.
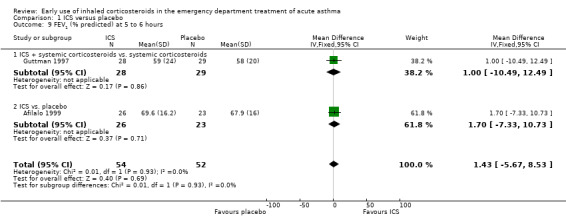
Comparison 1 ICS versus placebo, Outcome 9 FEV1 (% predicted) at 5 to 6 hours.
1.10. Analysis.
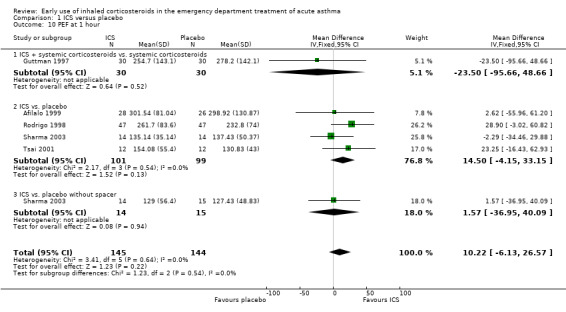
Comparison 1 ICS versus placebo, Outcome 10 PEF at 1 hour.
1.11. Analysis.
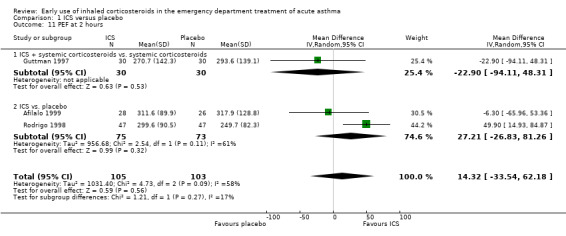
Comparison 1 ICS versus placebo, Outcome 11 PEF at 2 hours.
1.12. Analysis.
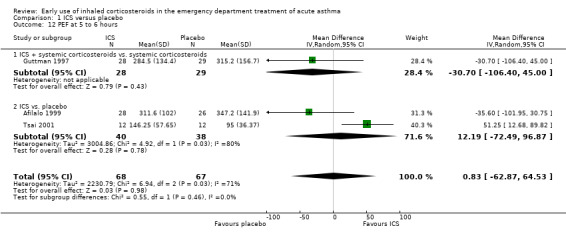
Comparison 1 ICS versus placebo, Outcome 12 PEF at 5 to 6 hours.
1.13. Analysis.
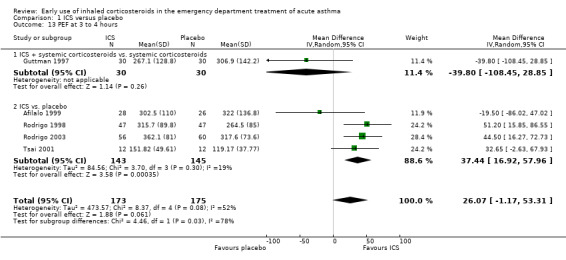
Comparison 1 ICS versus placebo, Outcome 13 PEF at 3 to 4 hours.
1.14. Analysis.
Comparison 1 ICS versus placebo, Outcome 14 PEF (% predicted) at 1 hour.
1.15. Analysis.
Comparison 1 ICS versus placebo, Outcome 15 PEF (% predicted) at 2 hours.
1.16. Analysis.
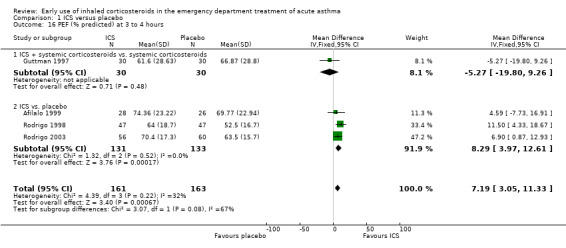
Comparison 1 ICS versus placebo, Outcome 16 PEF (% predicted) at 3 to 4 hours.
1.17. Analysis.
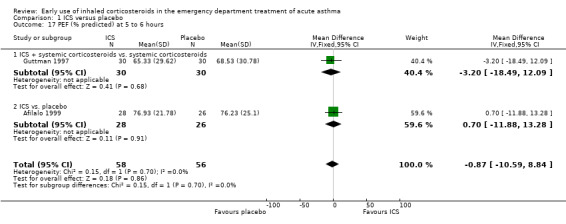
Comparison 1 ICS versus placebo, Outcome 17 PEF (% predicted) at 5 to 6 hours.
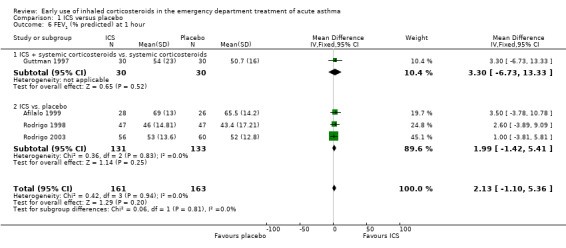
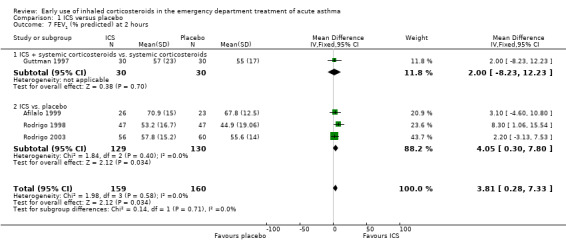
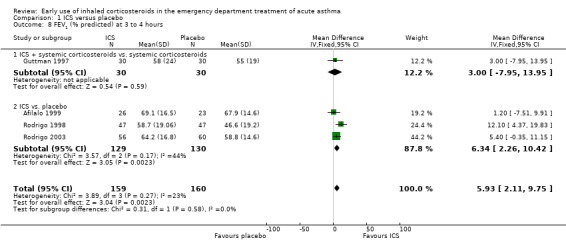
Pooled results showed a benefit of ICS therapy on % predicted FEV1 at two and four hours post treatment (two hours MD 3.81; 95% CI 0.28 to 7.33; 4 studies; N = 319; Analysis 1.7; four hour MD 5.93; 95% CI 2.11 to 9.75; 4 studies; N = 319; Analysis 1.8), without significant visual or statistical heterogeneity (two hours: I2 = 0%; four hours: I2 = 23%). There was a trend towards benefit at one hour that was not statistically significant. At six hours post treatment, there was no significant difference between the treatments; the largest study with the most marked benefit of ICS therapy followed patients for only three hours so did not contribute to the six‐hour analyses. Analysis of absolute FEV1 showed no statistically significant difference between treatments but there was marked heterogeneity. This was most marked at one hour (I2 = 90%). Despite adjusting for the baseline difference in the trial with the largest difference, significant heterogeneity remained. This heterogeneity was also present at two‐hour (I2 = 61%) and 3‐ to 4‐hour analyses (I2 = 65%).
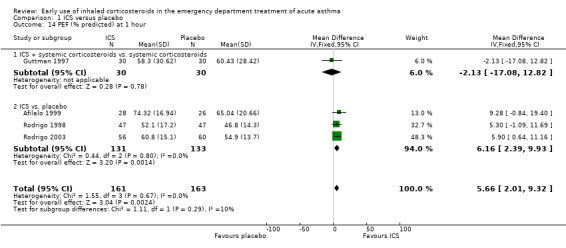
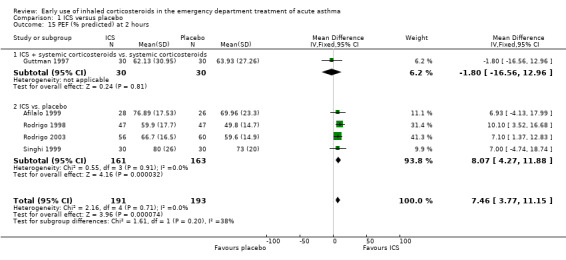
In the analyses of % predicted PEF, there was a small, statistically significant benefit of ICS therapy at both one and two hours (one hour MD 5.66; 95% CI 2.01 to 9.32; 4 studies; N = 324; Analysis 1.14; two hour MD 7.46; 95% CI 3.77 to 11.15; 5 studies; N = 384; Analysis 1.15). There was no statistically significant heterogeneity in the analyses for % predicted PEF (P > 0.1 at all time intervals). Heterogeneity was present in the results for absolute PEF in both the two and three‐ to four‐hour analyses, with no significant differences between the groups when the random‐effects model was used.
Eight additional relevant randomised studies were identified that we could not include in the meta‐analysis and a summary of the results are reported below to provide context for the above data. In Bateman 2006 (115 patients) data were reported for FEV1 with respect to change from baseline (whereas the analyses in this review focus on absolute levels at fixed time points or in terms of % predicted) and there were no significant differences between the two groups (budesonide/formoterol versus formoterol alone). Estrada 2005 (100 children) similarly reported no significant difference in improvement between fluticasone plus salbutamol versus salbutamol alone; Olaivar 1999 (65 children) also reported no significant difference between budesonide and placebo in change in FEV1. Sekerel 2005 (67 children) reported levels of improvement in FEV1 from baseline for the treatment and control groups, and the difference between the two groups was not significant (P = 0.24). The randomised trial by Blandon 2004 (86 children) reported no significant differences between the two groups (nebulised budesonide plus albuterol versus albuterol alone) in terms of improvement in PEF; as these data reflect change in PEF rather than absolute values they were not included in the meta‐analysis.
However, three further studies reporting change in PEF scores have reported an advantage for ICS. Bautista 1994 (30 children) reported "budesonide + beta2‐agonist improves the PEF (49.1 to 173) and Pulmonary Index Score at 1 hour (P < 0.05) compared with those given beta2‐agonists alone (41.6 to 77.5) with no untoward drug reactions among the subjects in the combination group". Nuhoglu 2005 (26 children) reported a significant difference between budesonide versus placebo (with both groups receiving parenteral methylprednisolone) with regard to their baseline/one hour after treatment change in PEF (P = 0.0155); however, it should be noted that there was considerable difference between the two groups in baseline mean PEF L/min (150.00 ± 32.19 SD in the budesonide group and 192.86 ± 58.63 SD in the placebo arm). Starobin 2008 (49 adult patients) also found that with respect to the before and after ED treatment percentage of PEF improvement in a comparison between fluticasone plus methylprednisolone versus methylprednisolone alone there was a significantly superior improvement with the combined intervention (MD 4.4; 95% CI 1.74 to 7.06).
It is therefore difficult to provide a clear overall conclusion with respect to pulmonary function data considering trials that cannot be incorporated into the meta‐analysis. Five studies (Bateman 2006; Blandon 2004; Estrada 2005; Olaivar 1999; Sekerel 2005) (433 patients in total) report no significant advantage, whereas three studies (Bautista 1994; Nuhoglu 2005; Starobin 2008) (105 patients in total) report additional benefit with ICS.
Clinical scores
A range of different clinical scores, including the pulmonary index, PIS and other novel scores (including combinations of measures of accessory muscle use, respiratory rate, wheezing, retractions, dyspnoea and oxygen saturation) were combined using an SMD technique and random‐effects model. At three to four hours there was a modest, statistically significant difference favouring ICS (SMD 0.33; 95% CI 0.05 to 0.62; 4 studies; N = 198; Analysis 1.19), with no significant heterogeneity (I2 = 0%).
1.19. Analysis.
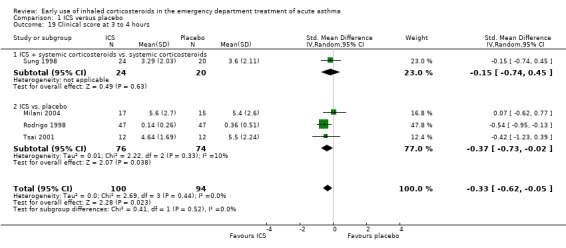
Comparison 1 ICS versus placebo, Outcome 19 Clinical score at 3 to 4 hours.
However at an earlier time point (one to two hours) there appeared to be no benefit from ICS with regard to clinical scores (SMD ‐0.34; 95% CI ‐0.60 to ‐0.07; 4 studies; N = 176; Analysis 1.18)
1.18. Analysis.
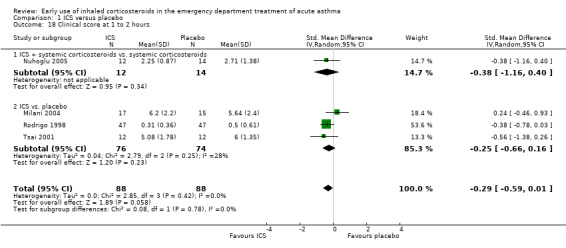
Comparison 1 ICS versus placebo, Outcome 18 Clinical score at 1 to 2 hours.
Three additional studies were identified that were relevant to this outcome but reported change scores that could not be included in the meta‐analysis. Razi 2012 (100 children) observed a significant improvement in PIS with regard to change from baseline to 120 minutes between budesonide versus placebo (P = 0.026). Upham 2011 (179 children) reported no difference between budesonide and placebo with respect to change in asthma scores from baseline and two hours (P = 0.64). Starobin 2008 (49 adult patients) reported a dyspnoea score (baseline dyspnoea index), which was scored from 0 (no dyspnoea) to 3 (severe dyspnoea), and did not find a difference between the group receiving fluticasone plus IV methylprednisolone versus the group receiving IV methylprednisolone alone.
Vital signs
Data for heart rate, respiratory rate, oxygen saturation and systolic blood pressure were pooled (Analysis 1.20). There was no significant difference between the two groups with regards to oxygen saturation (MD ‐0.18; 95% CI ‐0.66 to 0.31; 5 studies; N = 301). There was also no significant difference between the two groups in respiratory rate (MD 0.57; 95% CI ‐1.69 to 2.83; 3 studies; N = 198) and systolic blood pressure (MD ‐0.32; 95% CI ‐6.00 to 5.36; 3 studies; N = 128). However, there was a small difference with respect to heart rate, with a higher level in the group treated with ICS (MD 3.99; 95% CI 0.59 to 7.39; 5 studies; N = 363)
1.20. Analysis.
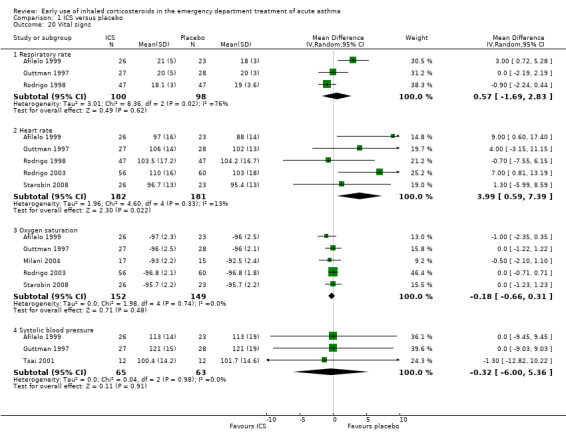
Comparison 1 ICS versus placebo, Outcome 20 Vital signs.
Four additional randomised studies were identified that we could not include in the meta‐analyses; the results are included below to provide context for the above data. In Bateman 2006 (115 patients) data were reported in insufficient detail to be accommodated in the meta‐analyses for vital signs, electrocardiograph (ECG) parameters, respiratory rate and oxygen saturation. There were no significant differences between the two groups apart from with respect to heart rate where a lower maximum value was observed in the budesonide group (P = 0.026), but the absolute difference was small (91.6 versus 94.3 beats per minute). With regard to SaO2 levels, Estrada 2005 (100 children) reported no significant difference between fluticasone plus salbutamol versus salbutamol alone and Razi 2012 (100 children) observed no significant difference in SaO2 levels between budesonide versus placebo; these data were not reported in sufficient detail to be included in the meta‐analyses.
Upham 2011 (180 children) reported no significant difference between budesonide and placebo with respect to change in respiratory rate, heart rate or SaO2 levels from baseline and two hours; the data were reported as medians and interquartile ranges and therefore could not be included in the meta‐analyses.
The narrative inclusion of these additional studies is broadly consistent with the results presented in Analysis 1.20 with regard to oxygen saturation. In terms of heart rates the finding reported in Bateman 2006 is inconsistent with the overall findings reported in Analysis 1.20. However, the differences found in heart rate were small.
Adverse effects
Six studies reported no significant adverse effects of the treatments (Afilalo 1999; Bautista 1994; Estrada 2005Guttman 1997; Starobin 2008; Sung 1998). Eight studies did not report any adverse effect data (Blandon 2004; Nuhoglu 2005; Olaivar 1999; Pansegrouw 1992; Razi 2012; Sekerel 2005; Singhi 1999; Tsai 2001). Five studies reported a number of minor adverse events, with no significant differences between the groups (Bateman 2006; Milani 2004; Rodrigo 1998; Rodrigo 2003; Upham 2011). One study reported an increase in hypokalaemia in the treatment group (Sharma 2003) over the first hour of treatment, although the difference was small. There were few data in the two pre‐specified outcomes of nausea/vomiting and tremor, and no significant differences were found between the groups in these outcomes.
Subgroup analyses
Age group
There was no clear evidence of a difference in hospital admissions between children (OR 0.52; 95% CI 0.33 to 0.80; 7 studies N = 583) and adults (OR 0.35; 95% CI 0.20 to 0.60; 5 studies; N = 377) (Analysis 1.23).
1.23. Analysis.
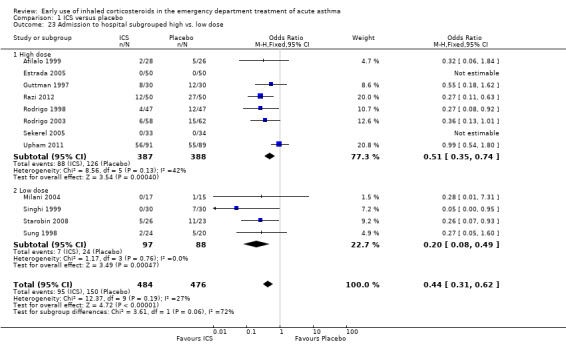
Comparison 1 ICS versus placebo, Outcome 23 Admission to hospital subgrouped high vs. low dose.
Dosage
This analysis was included to compare high‐dose versus low‐dose therapy (high‐dose therapy defined as 2 mg or greater of BDP equivalent dosing). The small number of trials and different protocols did not permit meaningful comparison of the dose of ICS use and comparisons should be interpreted with a degree of caution. The high‐ versus low‐dose analysis (Analysis 1.23) indicated that a significant effect was obtained in favour of ICS in both subgroups. In the high‐dose subgroup there were eight studies (Afilalo 1999; Estrada 2005; Guttman 1997; Razi 2012; Rodrigo 1998; Rodrigo 2003; Sekerel 2005; Upham 2011) (OR 0.51; 95% CI 0.35 to 0.74; 8 studies; 775 patients) and in the low‐dose subgroup there were four studies (Milani 2004; Singhi 1999; Starobin 2008; Sung 1998) (OR 0.20; 95% CI 0.08 to 0.49; 4 studies; 185 patients). The basis for our grouping on high and low dose for different ICS is based on Colice 2000.
Delivery devices
We performed a post‐hoc comparison of the studies using a nebuliser to deliver ICS and those that reported using an MDI and spacer. The results of this analysis for hospital admissions are shown in Analysis 1.24, and there was no significant difference between the two methods of delivery. There was a significant reduction in the risk of admission with nebuliser (OR 0.53; 95% CI 0.35 to 0.82) and with MDI and spacer (OR 0.32; 95% CI 0.18 to 0.59), and the test for differences between the two subgroups was negative (I2 = 45.6%).
1.24. Analysis.
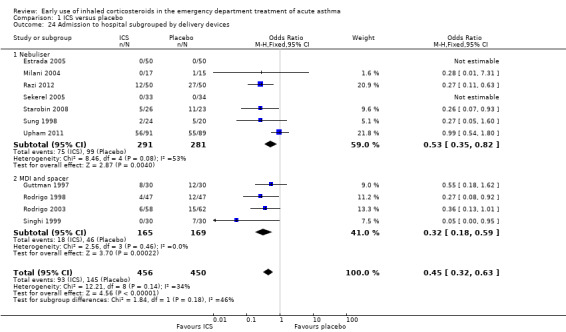
Comparison 1 ICS versus placebo, Outcome 24 Admission to hospital subgrouped by delivery devices.
Protocols
Thirteen studies compared ICS versus placebo with no systemic corticosteroids given in either treatment group (Afilalo 1999; Bautista 1994; Blandon 2004; Estrada 2005; Milani 2004; Olaivar 1999; Pansegrouw 1992; Rodrigo 1998; Rodrigo 2003; Sekerel 2005; Sharma 2003; Singhi 1999; Tsai 2001) and seven studies compared ICS plus systemic corticosteroid versus systemic corticosteroid alone (Bateman 2006; Guttman 1997; Nuhoglu 2005; Razi 2012; Starobin 2008; Sung 1998; Upham 2011). With respect to admissions (Analysis 1.1), this comparison confirms an advantage for ICS in this outcome overall (OR 0.44; 95% CI 0.31 to 0.62; 12 studies; 960 patients) and the heterogeneity (I2 = 27%) was modest. The subgroups in Analysis 1.1 by protocol (whether systemic corticosteroid were given as standard therapy or not) reveals similar benefits in the two subgroups. For ICS plus systemic corticosteroids versus systemic corticosteroids there is a clear benefit for ICS (OR 0.54; 95% CI 0.36 to 0.81) and similarly in the ICS versus placebo subgroup (OR 0.27; 95% CI 0.14 to 0.52) there is a significant benefit from ICS in terms of hospital admissions. However, the large reduction in hospital admissions reported from Razi 2012, contrasts with the results from Upham 2011, the most recent fully reported study, resulting in considerable heterogeneity in this subgroup (I2 = 52%).
Inhaled corticosteroids versus systemic corticosteroids
Fourteen trials compared ICS versus systemic corticosteroid: 10 contributed to this analysis. Eight were paediatric studies (N = 595): Ancheta 2008 (24 children); Devidayal 1999 (80 children); Macias 2003 (135 children); Milani 2004 (34 children); Scarfone 1995 (128 children); Schuh 2000 (103 children); Schuh 2006 (69 children) and Volovitz 1998 (22 children). A further two were with adults (N = 168): Rodrigo 2005 (121 adults) and Starobin 2008 (47 patients). Analysis 2.1 (OR 0.56; 95% 0.25 to 1.24; 10 studies; N = 763) indicated no clear advantage for either ICS or systemic corticosteroid in terms of hospital admissions (Figure 5). However, there was very high level of heterogeneity (I2= 61%) and this result should be interpreted with degree of caution.
2.1. Analysis.
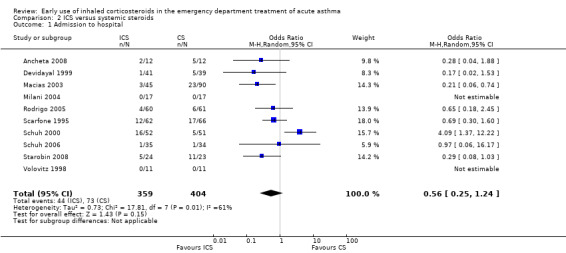
Comparison 2 ICS versus systemic steroids, Outcome 1 Admission to hospital.
5.
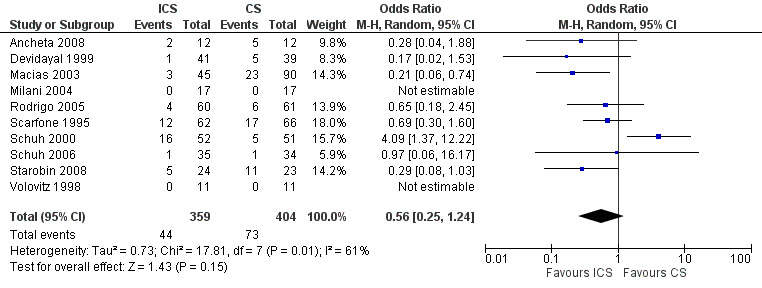
Forest plot of comparison: 2 ICS versus systemic steroids, outcome: 2.1 Admission to hospital.
No admissions data were reported in Belda 2007, Rahman 2008 or Sari 2004. Go 2010 (33 adults) reported that their ICS group had a significantly higher number of admissions than their IV hydrocortisone group, but numbers were not included in the published abstract so could not be incorporated in the meta‐analysis.
Discussion
Summary of main results
This systematic review examined the available clinical evidence for the use of ICS in the ED management of acute asthma. The primary meta‐analysis was based on 20 studies that included 1403 patients. The pooled results showed a beneficial effect of ICS therapy compared to placebo in preventing hospital admission, with a significant (OR 0.44; 95% CI 0.31 to 0.62) reduction in admission following the administration of ICS in the ED. Given a hospital admission level of 32% in the placebo group, approximately eight patients would require ICS treatment to prevent one admission (95% CI 6 to 14) The review shows a beneficial effect of ICS versus placebo in preventing hospital admission even in the studies where systemic corticosteroid was administered to all patients as standard therapy; however,disparate results from the new studies added in the 2012 update for this subgroup have contributed to increased heterogeneity.
Only a small number of trials reported data on pulmonary function tests in a manner that could be included in the meta‐analysis; however, the effects of ICS on measurement of lung function found in the review were small. The minimum difference in pulmonary function tests that is considered clinically significant has been widely debated. This value remains unclear, not identified through empirical studies, and much of this research is based on stable asthma. However, a minimum improvement of 10% to 12% in FEV1, or approximately 30 L/minute in PEF (Karras 2000; Tiffany 1993), is likely to be necessary to demonstrate an important clinical difference. Based on these guidelines, the improvement of 6% in FEV1 at three to four hours, or 7% in the PEF would be of questionable clinical importance. There was heterogeneity in the results of the absolute FEV1 and PEF. This may have been due to baseline differences in pulmonary function tests between the groups, which were statistically and clinically significant. Other possible causes include differences in the populations, interventions, designs and methods of measuring the pulmonary function tests. However, the small number of studies did not permit meaningful analysis of these differences.
The pulmonary index (a clinical score) has been shown to correlate with pulmonary function test results including FEV1, FEV1/FVC and FEV25‐75%, and with hospital admission (Becker 1984). Five of the trials used clinical scores that were quite similar to the pulmonary index and reported them in a manner they could be included in the review. A modest, statistically significant improvement in clinical score between the groups was demonstrated at three to four hours (SMD ‐0.33; 95% CI ‐0.62 to ‐0.05). This difference would represent an insignificant clinical change for most patients. For example, from the cited studies, this would represent an improvement from 0.1 points in clinical index (Rodrigo 1998) to 0.8 (Sung 1998) in the PIS. Moreover, in view of the use of an SMD measure to combine different scores, and the small magnitude of the difference, this result and any conclusions based on it should be viewed with caution.
Overall completeness and applicability of evidence
Very few adverse effects of ICS therapy were reported in any of the studies, and this was confirmed with corresponding authors. Most importantly, no increase in cough or bronchospasm, occasionally attributed to ICS therapy (Passalacqua 2000), was observed. The lack of effect on vital signs also supports the safety of ICS therapy.
Twelve of the twenty published trials did not show a beneficial effect of ICS on the primary outcome of the trial. Nonetheless, the possibility of publication bias in favour of ICS remains. However, a comprehensive search strategy was conducted using a systematic strategy. Attempts to find unpublished trials were also made, including extensive correspondence with the authors of the studies included in the 2003 review as well as other experts in the field, searching of abstracts from recent conferences and contact with the pharmaceutical companies that manufacture ICS. Four unpublished trials were identified and included in the review (Bautista 1994; Blandon 2004; Olaivar 1999; Razi 2012); although we were only successful in contacting one of the authors (Razi 2012). While the results of the unpublished studies appear consistent with those of the published studies, many of the details of these studies are missing. One of these studies comparing ICS versus placebo (Bautista 1994) reported a significantly decreased number of hospital admissions and improved pulmonary function tests in the group treated with ICS, while one abstract stated that the group treated with ICS had a trend towards improvement in % predicted FEV1 that was not statistically significant (Olaivar 1999). The third study found no significant differences between the treatment groups (Blandon 2004) in PEF. All of these studies were relatively small, and these results appear consistent with those of the published studies.
Six studies remain in the awaiting assessment section of this review: four of these studies related to the primary analysis. Three have published in abstract form only, and it is unclear whether the patients included in the studies were treated in the ED or after hospital admission (Agarwal 2003; Agarwal 2005; Agarwal 2009). The one published study was unobtainable (Ambrosio 1997).
A secondary analysis was performed including 14 studies where ICS alone was compared to systemic corticosteroid alone. Owing to the diverse outcomes reported in the studies, only hospital admissions were compared (with 10 studies reporting hospital admissions). There was significant heterogeneity between the studies for hospital admissions. The small number of studies, and the small number of outcomes amenable to pooling, precluded further investigation of the sources of the heterogeneity. Pooling of hospital admission data using the random‐effects model resulted in an OR of 0.56 (95% CI 0.25 to 1.24). This result does not exclude the possibility of either treatment being significantly better (or worse) than the other. In addition, many of these studies included patients with relatively mild asthma. Two unpublished studies are awaiting assessment for inclusion in the secondary analysis (Acun 2003; Jerez 2002); both were relatively small and did not report significant differences between the treatment groups in the published abstracts in clinical outcomes.
Quality of the evidence
The quality of reported randomisation was variable and less than half of the 32 studies were judged as low risk of bias. Fifty‐nine per cent of trials were at unclear risk of selection bias while two (6%) were judged to be at high risk of selection bias. The majority (75%) of trials were at low risk of performance and detection bias while four were assessed as high risk of bias. Overall we felt that the evidence was of moderate quality and the effect that risks of bias had on our confidence in the treatment effects can be seen in Table 1 and Table 2.
Potential biases in the review process
As with most systematic reviews there is a risk of publication bias with regard to the identification of unpublished negative trials, and therefore the effect of ICS therapy may be overestimated and conversely the possibility of failure to identify unpublished positive trials may underestimate the treatment effect. Having said that, the review was based on a comprehensive search with a systematic strategy to minimise the risk of bias. We believe we have identified the majority of the research considering the questions addressed by the review.
We are also conscious of the risk of study selection bias. However, each phase of the review was conducted by two independent review authors, and we feel confident that the studies excluded were assessed against consistent and relevant criteria.
Agreements and disagreements with other studies or reviews
The 2012 update provides further support for the findings of the earlier systematic review (Edmonds 2003). In Edmonds 2003, there was a beneficial effect of ICS compared to placebo when used early in the ED treatment of acute asthma, but it was not conclusive if there was benefit when ICS is used in addition to systemic corticosteroid therapy, which would be considered the standard of care (BTS/SIGN 2011). The 2012 update shows a statistically significant benefit of ICS therapy when used in addition to systemic corticosteroid therapy, but there is statistical heterogeneity in this subgroup which may reflect the clinical heterogeneity among the trials . Despite the addition of more studies to the secondary analysis, it remains unclear whether ICS could be used in place of systemic corticosteroid in the ED treatment of acute asthma, as there is marked heterogeneity between the studies that could not be explained on the basis of obvious differences in the study characteristics.
The 2012 update reports additional subgroup analyses comparing the results in adults and children, high and low doses of ICS and nebulised versus MDI and spacer delivery. All of these subgroups now demonstrate significant reductions in hospital admissions and there are no significant differences between the subgroups.
Authors' conclusions
Implications for practice.
There is insufficient evidence that ICS therapy alone can be used to replace systemic corticosteroid therapy, therefore systemic corticosteroids should not be withheld from patients with acute asthma presenting to the ED.
ICS therapy decreases hospital admissions in patients compared to treatment with placebo, and may be considered in addition to systemic corticosteroid treatment (although the most recent evidence is conflicting).
ICS are well tolerated with few short term side effects across a wide variety of doses.
ICS appear to decrease hospital admissions to a similar degree in both children and adults
Implications for research.
There are many unanswered questions about the use of ICS in the ED treatment of acute asthma and we believe the following merit further research:
additional studies are required to determine the optimal dose and delivery device, type of ICS and frequency of administration of ICS;
studies investigating the effect of ICS based on prior ICS and beta2‐agonist use are needed. One study suggested a marked benefit of ICS therapy in patients who had been unresponsive to beta2‐agonists in the hours prior to ED presentation (Pansegrouw 1992). Further research is needed to confirm the validity of this subgroup;
a large randomised controlled trial would be less susceptible to the effect of marked baseline pulmonary function test differences between the treatment groups, which was observed in some of these small studies;
future research should focus on clearly defined outcomes, with specific criteria for admission, relapse, timing and type of pulmonary function testing, and length of follow‐up in the ED and after discharge.
What's new
| Date | Event | Description |
|---|---|---|
| 13 August 2018 | Amended | Nine trials identified from an update search of the Cochrane Airways Group Register of trials as being potentially eligible for inclusion in a future update. Studies added to Studies awaiting classification. |
History
Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 28 September 2012 | New search has been performed | Update. Ten new studies were added to the primary analysis, which now shows a clear benefit of ICS use in the ED treatment of asthma. |
| 28 September 2012 | New citation required and conclusions have changed | Clear benefit of inhaled steroids in the ED treatment of acute asthma. |
| 23 July 2008 | Amended | Converted to new review format. |
| 13 September 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The original version of this review in 2000 included the following acknowledgment: the authors wish to acknowledge the assistance of Stephen Milan, Anna Bara and Jane Dennis of the Cochrane Airways Group (CAG) for their assistance in the original review and Toby Lasserson and librarian Elizabeth Stovold for their assistance in the updates. We would also like to acknowledge the assistance of the corresponding authors: Drs M. Afilalo, A. Guttman and G. Rodrigo for providing additional information about the design of their studies, feedback on the data abstracted and additional data analyses, to Drs. T. Klassen and B. Volovitz for providing original data and feedback about abstracted data, and to Dr. R. Scarfone for providing additional information about the design of his study. Drs A. Guttman and B. Volovitz also provided additional references. In addition, we would like to thank Dr Yevgeny Filanovsky for providing translation of the article by Latysheva et al and Dr Molly Gong for providing translation of the article by Yang et al. Finally, we would like to thank the reviewers for their comprehensive appraisal of this review and Professor Paul Jones (past CAG Co‐ordinating Editor) and Dr Christopher Cates (current CAG Co‐ordinating Editor) for their helpful suggestions.
In 2012 the authors responsible for the update of this review would particularly like to acknowledge the excellent support and assistance from Emma Welsh, Liz Stovold and Emma Jackson of the Cochrane Airways Review group, together with the greatly appreciated guidance from Chris Cates (Cochrane Airways Review Group Coordinating Editor). We are most grateful to Jos Verbeek, Alexey Seniukovich and Chao Liu for assistance with the translation of clinical trial reports. The support provided by librarians Judith Scammel, Jane Appleton and Hilary Garrett at St Georges University London is also greatly appreciated. As well, we would like to thank the corresponding authors Drs J. Belda and D. Starobin for providing further information about their studies, and Dr C. Razi for providing unpublished data from his study.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (T he Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Database searches pre‐2005
The Cochrane Airways Group Asthma and Wheez* register was searched using the following terms:
[Emerg* OR acute OR status] AND [dexa* OR deca* OR fluticasone OR Flovent OR beclomethasone OR Becloforte OR budesonide OR Pulmicort OR flunisolide OR Aerobid OR Bronalide OR triamcinalone OR Beclovent OR Azmacort OR Vanceril OR Becotide OR Flixotide OR Aerobec]
Appendix 3. Database search strategies 2005‐2012
Cochrane Airways Group Register (CAGR)
(emergenc* or acute* or status or sever* or exacerbat* or hospital* or intensiv* or admit* or admission or discharg*) and ((steroid* or corticosteroid* or glucocorticoid* or fluticasone or flovent or flixotide or beclomethasone or beclometasone or becloforte or becotide or QVAR or budesonide or pulmicort or flunisolide or aerobid or bronalide or triamcinolone or kenalog or beclovent or azmacort or vanceril or aerobec or ciclesonide or Alvesco) and (inhal* or nebuli* or aerosol*))
[Search was limited to records coded as 'asthma']
Clinicaltrials.gov
steroid | Interventional Studies | acute asthma budesonide| Interventional Studies | acute asthma fluticasone| Interventional Studies | acute asthma
Data and analyses
Comparison 1. ICS versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admission to hospital | 12 | 960 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.62] |
| 1.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 5 | 433 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.36, 0.81] |
| 1.2 ICS vs. placebo | 7 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.14, 0.52] |
| 2 FEV1 at 1 hour | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.22, 0.77] |
| 2.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.34, 0.34] |
| 2.2 ICS vs. placebo | 3 | 188 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.32, 1.07] |
| 3 FEV1 at 2 hours | 3 | 203 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.23, 0.33] |
| 3.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.45, 0.25] |
| 3.2 ICS vs. placebo | 2 | 143 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.48] |
| 4 FEV1 at 3 to 4 hours | 4 | 319 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.09, 0.39] |
| 4.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 4.2 ICS vs. placebo | 3 | 259 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.01, 0.47] |
| 5 FEV1 at 5 to 6 hours | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.42, 0.16] |
| 5.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.49, 0.29] |
| 5.2 ICS vs. placebo | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.59, 0.27] |
| 6 FEV1 (% predicted) at 1 hour | 4 | 324 | Mean Difference (IV, Fixed, 95% CI) | 2.13 [‐1.10, 5.36] |
| 6.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐6.73, 13.33] |
| 6.2 ICS vs. placebo | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | 1.99 [‐1.42, 5.41] |
| 7 FEV1 (% predicted) at 2 hours | 4 | 319 | Mean Difference (IV, Fixed, 95% CI) | 3.81 [0.28, 7.33] |
| 7.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐8.23, 12.23] |
| 7.2 ICS vs. placebo | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | 4.05 [0.30, 7.80] |
| 8 FEV1 (% predicted) at 3 to 4 hours | 4 | 319 | Mean Difference (IV, Fixed, 95% CI) | 5.93 [2.11, 9.75] |
| 8.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.95, 13.95] |
| 8.2 ICS vs. placebo | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | 6.34 [2.26, 10.42] |
| 9 FEV1 (% predicted) at 5 to 6 hours | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | 1.43 [‐5.67, 8.53] |
| 9.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐10.49, 12.49] |
| 9.2 ICS vs. placebo | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐7.33, 10.73] |
| 10 PEF at 1 hour | 5 | 289 | Mean Difference (IV, Fixed, 95% CI) | 10.22 [‐6.13, 26.57] |
| 10.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐23.5 [‐95.66, 48.66] |
| 10.2 ICS vs. placebo | 4 | 200 | Mean Difference (IV, Fixed, 95% CI) | 14.50 [‐4.15, 33.15] |
| 10.3 ICS vs. placebo without spacer | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.57 [‐36.95, 40.09] |
| 11 PEF at 2 hours | 3 | 208 | Mean Difference (IV, Random, 95% CI) | 14.32 [‐33.54, 62.18] |
| 11.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐22.90 [‐94.11, 48.31] |
| 11.2 ICS vs. placebo | 2 | 148 | Mean Difference (IV, Random, 95% CI) | 27.21 [‐26.83, 81.26] |
| 12 PEF at 5 to 6 hours | 3 | 135 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐62.87, 64.53] |
| 12.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐30.70 [‐106.40, 45.00] |
| 12.2 ICS vs. placebo | 2 | 78 | Mean Difference (IV, Random, 95% CI) | 12.19 [‐72.49, 96.87] |
| 13 PEF at 3 to 4 hours | 5 | 348 | Mean Difference (IV, Random, 95% CI) | 26.07 [‐1.17, 53.31] |
| 13.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐39.80 [‐108.45, 28.85] |
| 13.2 ICS vs. placebo | 4 | 288 | Mean Difference (IV, Random, 95% CI) | 37.44 [16.92, 57.96] |
| 14 PEF (% predicted) at 1 hour | 4 | 324 | Mean Difference (IV, Fixed, 95% CI) | 5.66 [2.01, 9.32] |
| 14.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.13 [‐17.08, 12.82] |
| 14.2 ICS vs. placebo | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | 6.16 [2.39, 9.93] |
| 15 PEF (% predicted) at 2 hours | 5 | 384 | Mean Difference (IV, Fixed, 95% CI) | 7.46 [3.77, 11.15] |
| 15.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐16.56, 12.96] |
| 15.2 ICS vs. placebo | 4 | 324 | Mean Difference (IV, Fixed, 95% CI) | 8.07 [4.27, 11.88] |
| 16 PEF (% predicted) at 3 to 4 hours | 4 | 324 | Mean Difference (IV, Fixed, 95% CI) | 7.19 [3.05, 11.33] |
| 16.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐19.80, 9.26] |
| 16.2 ICS vs. placebo | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | 8.29 [3.97, 12.61] |
| 17 PEF (% predicted) at 5 to 6 hours | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐10.59, 8.84] |
| 17.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐18.49, 12.09] |
| 17.2 ICS vs. placebo | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐11.88, 13.28] |
| 18 Clinical score at 1 to 2 hours | 4 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.59, 0.01] |
| 18.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.16, 0.40] |
| 18.2 ICS vs. placebo | 3 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.66, 0.16] |
| 19 Clinical score at 3 to 4 hours | 4 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.62, ‐0.05] |
| 19.1 ICS + systemic corticosteroids vs. systemic corticosteroids | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.74, 0.45] |
| 19.2 ICS vs. placebo | 3 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| 20 Vital signs | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Respiratory rate | 3 | 198 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐1.69, 2.83] |
| 20.2 Heart rate | 5 | 363 | Mean Difference (IV, Random, 95% CI) | 3.99 [0.59, 7.39] |
| 20.3 Oxygen saturation | 5 | 301 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.66, 0.31] |
| 20.4 Systolic blood pressure | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐4.00, 5.36] |
| 21 Adverse effects | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 Nausea/vomiting | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.18] |
| 21.2 *Tremor | 3 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.84, 2.45] |
| 22 Admission to hospital subgrouped children vs. adults | 12 | 960 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.62] |
| 22.1 Adults | 5 | 377 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.20, 0.60] |
| 22.2 Children | 7 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.80] |
| 23 Admission to hospital subgrouped high vs. low dose | 12 | 960 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.62] |
| 23.1 High dose | 8 | 775 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.74] |
| 23.2 Low dose | 4 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.08, 0.49] |
| 24 Admission to hospital subgrouped by delivery devices | 11 | 906 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.63] |
| 24.1 Nebuliser | 7 | 572 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.35, 0.82] |
| 24.2 MDI and spacer | 4 | 334 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.59] |
1.21. Analysis.
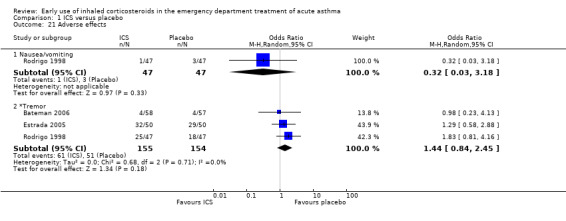
Comparison 1 ICS versus placebo, Outcome 21 Adverse effects.
1.22. Analysis.
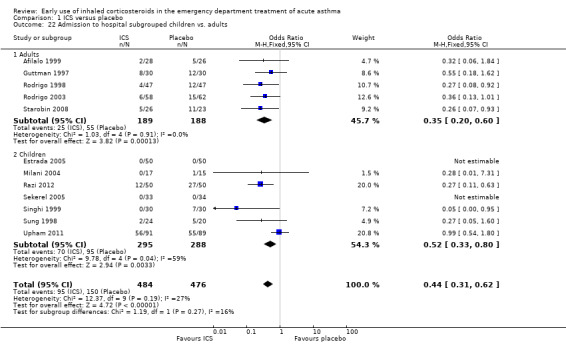
Comparison 1 ICS versus placebo, Outcome 22 Admission to hospital subgrouped children vs. adults.
Comparison 2. ICS versus systemic steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admission to hospital | 10 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.24] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afilalo 1999.
| Methods | Design: randomised controlled trial Method of randomisation: computer‐generated block randomisation Means of allocation concealment: randomisation code concealed Blinding: double‐blind, placebo controlled Withdrawal/drop‐outs: 2 withdrawn from the BDP group (1 because of deterioration, 1 because patient discharged self early), 3 withdrawn from the placebo group (1 because of deterioration, 2 because patient discharged self early) | |
| Participants | Eligible: 193 Randomised: 54 (28/26) Completed: 49 (26/23) Sex (M/F): BDP 8/20, placebo 12/14 Asthma diagnosis: physician diagnosis Inclusion criteria: FEV1 40‐69% predicted, ≥ 18 years of age, able to perform spirometry, informed consent Major exclusions: in extremis, long‐term systemic corticosteroid treatment (> 1 month) within 6 months of presentation, use of high‐dose ICS (> 1 mg/day), use of systemic corticosteroid within previous 2 months Baseline FEV1% (SD): BDP52 (9), placebo 51 (10) | |
| Interventions | Setting: ED at an urban teaching hospital in Canada Intervention: BDP by MDI 1 mg at 0, 30 min, 1 h, 2 h, 4 h (total 5 mg) versus placebo MDI at the same time intervals Standard of care: salbutamol 2.5 mg by wet nebuliser prior to each MDI treatment. Oxygen at an FiO2 of 0.35 was given at the physician's discretion | |
| Outcomes | Primary outcome was improvement in FEV1% predicted. Secondary outcome was hospitalisations. Other outcomes included other PFTs, vital signs, and Borg dyspnoea scale | |
| Notes | The author was contacted and provided additional information about the study and further data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation list and medications provided by pharmaceutical company |
| Allocation concealment (selection bias) | Low risk | Randomisation code concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind and the MDIs were identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 withdrawn from the BDP group (1 because of deterioration, 1 because patient discharged self early), 3 withdrawn from the placebo group (1 because of deterioration, 2 because patient discharged self early) |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Ancheta 2008.
| Methods | Design: randomised controlled trial | |
| Participants | Children with severe asthma exacerbations. 12 in each group Baseline lung function: mean PEF (SD): fluticasone 40.22 (8.23), hydrocortisone 39.10 (9.17) Mean baseline MPIS (SD): fluticasone 12.75 (1.06), hydrocortisone 13.08 (1.16) |
|
| Interventions | Intervention: fluticasone propionate (1500 μg total) 125 μg/actuation 4 actuations via MDI and spacer administered every 20 min for 3 doses Control: single dose of IV hydrocortisone (4 mg/kg; maximum of 200 mg) |
|
| Outcomes | Baseline to 6 h change (for PEF and MPIS). No numerical data included for the baseline to 6 h change for MPIS as reported narratively, % admitted and adverse events | |
| Notes | ICS versus systemic corticosteroid subgroup Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (abstract) |
| Allocation concealment (selection bias) | Unclear risk | Unclear (abstract) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial reported as double blind, although unclear how this may have been maintained in practice with inhaled versus IV interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial reported as double blind, although unclear how this may have been maintained in practice with inhaled versus IV interventions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No patients were withdrawn from the trial |
| Selective reporting (reporting bias) | Unclear risk | Details of data recorded at all time points not included in trial report |
Bateman 2006.
| Methods | Design: randomised controlled trial | |
| Participants | Budesonide/formoterol (N = 58) Formoterol (N = 57) Mean age (years): budesonide/formoterol 45.9 (range 13 to 78), formoterol 43.9 (range 12 to 72) Sex (M/F): budesonide/formoterol 22/36, formoterol 20/37 Baseline lung function; FEV1 (L): budesonide/formoterol 1.12 (range 0.6–1.9), formoterol 1.15 (range 0.7–2.0) Baseline lung function; FEV1 (% predicted): budesonide/formoterol 40 (range 26–55), formoterol 41 (range 30–55) Inclusion criteria: all patients were required to have asthma, as defined by the American Thoracic Society criteria (including symptoms of wheeze, episodic cough and dyspnoea), with a pre‐bronchodilator FEV1 measured on arrival in the acute setting ≥ 30% and ≤ 55% of predicted normal. In addition, patients had to have a relative lack of reversibility, as demonstrated by their FEV1 improving by 8% or less of predicted normal, 10 min after receiving salbutamol 400 μg from a pressurised MDI Major exclusions: acute severe asthma (defined as an inability to generate an FEV1 value, an FEV1 of less than 30% predicted, or asthma requiring transfer to an intensive care unit on initial assessment); use of ICS within the 8 h preceding the baseline measurements; receipt of oral or other systemic corticosteroids in the 48 h before the baseline measurements; beta‐blocker therapy (including eye drops); any significant disease or concomitant disorder; and known sensitivity to the study medication or lactose. Patients ≥ 45 years of age with a history of ≥ 10 pack‐years of smoking were also excluded from the study |
|
| Interventions | Intervention: budesonide/formoterol; 320/9 μg, 2 inhalations at t = ‐5 min and a further 2 inhalations at t = 0 min (total dose 1280/36 μg), plus 2 inhalations of formoterol placebo containing lactose at t = ‐5 min and at t = 0 min Control: formoterol 9 μg, 2 inhalations at t = ‐5 min and a further 2 inhalations at t = 0 min (total dose 36 μg), plus 2 inhalations of budesonide/formoterol placebo containing lactose at t = ‐5 min and at t = 0 min) Also: oral prednisolone; 5 mg per tablet, 12 tablets (total dose 60 mg)) was administered to all patients 90 min after they received the second dose of study medication Both groups received salbutamol 400 μg by MDI prior to randomisation During the treatment period, patients were not permitted to receive any asthma medication other than the investigational product, although oxygen therapy was allowed. Other medication considered necessary for the patient's safety and well‐being, and early withdrawal from the study, were permitted at the discretion of the investigator |
|
| Outcomes | Lung function (FEV1 mean % change from baseline at 3, 15, 60, 90 and 180 min), RR, treatment success, treatment failure and effectiveness of medication, adverse events, clinical laboratory data and other safety evaluations | |
| Notes | ICS versus placebo, both received systemic corticosteroid and LABA Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear – method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Unclear – method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double‐dummy, randomised, parallel‐group multicentre study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 patient, who was randomised into the formoterol group, discontinued the study because of worsening asthma. The full analysis set comprised all randomised patients |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Bautista 1994.
| Methods | Design: randomised controlled trial | |
| Participants | 30 children aged 6 to 18 years with no intake of corticosteroids in the 4 weeks prior to the study | |
| Interventions | Intervention: budesonide + beta2‐agonist at 20‐min intervals (up to 3 doses) Control: beta2‐agonist inhalation at 20‐min intervals (up to 3 doses) |
|
| Outcomes | PEF and PIS at 0, 30 and 60 min at 48 h on follow‐up, hospital admissions | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Conference abstract with limited information |
| Allocation concealment (selection bias) | Unclear risk | Conference abstract with limited information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported as double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conference abstract with limited information |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract with limited information |
Belda 2007.
| Methods | Design: randomised controlled trial | |
| Participants | Moderate‐severe asthma. 45 recruited (6 drop‐outs). Fluticasone: 19, prednisone: 20 Age (mean (range)) (years): fluticasone: 39 (20 to 69), prednisone: 34 (19 to 68) Sex (M/F): fluticasone: 4/15, prednisone: 7/13 Baseline lung function; Mean (SD) FEV1 (L): fluticasone: 2.11 (0.74), prednisone: 2.09 (0.98) Baseline lung function; Mean (SD) FEV1 (% predicted): fluticasone: 69 (19), prednisone: 62 (20) Inclusion criteria: patients aged 16 to 65 years with a moderate to severe asthma exacerbation (but not life threatening). Asthma diagnosis from current or previous history of chest tightness, wheezing, dyspnoea or cough in association with variable airflow limitation (documented from either methacholine airway hyper‐responsiveness if FEV1 was ≥ 70% of predicted value, or 12% increases in FEV1 after inhaled salbutamol 200 μg if FEV1 was < 70% Exclusion criteria: smokers or ex‐smokers within the last year, treatment with oral or IV corticosteroids, cromoglycate, nedocromil, theophylline, allergen desensitisation injections and leukotriene antagonists at any time in 4 weeks prior to the study Patients with life‐threatening exacerbations of asthma or other serious medical conditions were excluded (e.g. heart disease, gastrointestinal, liver or renal disease), and those with chest diseases that could interfere with study outcomes |
|
| Interventions | Run in: 15 min with nebulised salbutamol and oxygen to see if suitable for study Intervention: fluticasone (16 puffs: total 4000 μg/day) (by MDI) + placebo of prednisone Control: prednisone (30 mg/day) + placebo of fluticasone (by MDI) Reported as: ongoing therapy with LABAs was permitted but was balanced between 2 groups in block randomisation |
|
| Outcomes | Spirometry, induced sputum for differential cell counts, albumin and alpha2‐macroglobulin levels and blood eosinophils, interleukin‐5 and granulocyte macrophage colony‐stimulating factor levels were obtained before treatment and at 2, 6 and 24 h after treatment | |
| Notes | ICS versus systemic corticosteroid subgroup Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Codified by hospital pharmacy department who packed and blinded medications |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 excluded because sputum samples unsuitable for processing (2 in each group). 2 excluded because chest radiograph infiltrates were compatible with pneumonia |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Blandon 2004.
| Methods | Design: randomised controlled trial | |
| Participants | Children (aged 7 to 17 years) with moderate acute asthma: 40 randomised to budesonide + albuterol, 46 to albuterol alone Age (mean (SD)) (years): budesonide + albuterol: 10.04 (2.31), albuterol 10. 71 (2.06) Sex (M/F): budesonide + albuterol: 21/19, albuterol: 26/20 |
|
| Interventions | Intervention: budesonide 550 μg + albuterol 0.15 mg/kg dose x 1 dose Control: albuterol 0.15 mg/kg x 1 dose |
|
| Outcomes | Symptoms and PEF (% change) before and after treatment | |
| Notes | ICS versus placebo Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (abstract – limited information) |
| Allocation concealment (selection bias) | Unclear risk | Unclear (abstract – limited information) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear (abstract – limited information) |
| Selective reporting (reporting bias) | Unclear risk | Unclear (abstract – limited information). Peak flow % change reported but no data presented on symptoms |
Devidayal 1999.
| Methods | Design: randomised controlled trial Method of randomisation: not stated Means of allocation concealment: identical drug packages, but concealment not described Blinding: double‐blind, double‐dummy Withdrawals/drop‐outs: none | |
| Participants | Eligible: 110 Randomised: 80 (41/39) Completed: 80 (41/39) Sex (M/F): budesonide group: 76%/24%, placebo group: 74%/26% Asthma diagnosis: doctor's diagnosis Inclusion criteria: ≥ 1 attack of acute asthma requiring bronchodilators within the previous 6 months, evidence of wheezing and hyperinflation, age 2 to 12 years Major exclusions: presence of other significant acute or chronic diseases, use of oral or ICS within the previous 24 h Baseline PEF: budesonide 64%, placebo 62% | |
| Interventions | Setting: paediatric ED in Chandigarh, India Intervention: budesonide group received budesonide 800 μg by nebuliser every 30 min for 3 doses and oral placebo, while the prednisolone group received prednisolone 2 mg/kg once orally, and nebulised placebo All patients received nebulised salbutamol 0.15 mg/kg every 30 min for 3 doses. If the response after 3 doses of salbutamol was inadequate, subjects received salbutamol 3 mg/kg hourly, IV hydrocortisone and aminophylline If there was no response to this therapy, patients were given IV magnesium sulphate 50 mg/kg and were admitted to intensive care | |
| Outcomes | Outcomes included a respiratory distress score, PIS, PEF, SaO2, RR, HR, duration in emergency/hospital and need for hospitalisation | |
| Notes | This trial was only included in the secondary analysis comparing ICS versus systemic corticosteroids New reference in 2003 update: the author was not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No report of patients being withdrawn from trial |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Estrada 2005.
| Methods | Design: randomised controlled trial | |
| Participants | Children with moderate acute asthma Randomised: salbutamol/fluticasone: 50, salbutamol: 50 Age (mean (SD)) (years): salbutamol/fluticasone: 9.8 (2.4), salbutamol: 9.9 (2.6) Sex (M/F): salbutamol/fluticasone: 25/25, salbutamol: 32/18 Baseline lung function; mean SaO2 % median (range): salbutamol/fluticasone: 93 (91–95), salbutamol: 93 (90–95) Baseline lung function; mean % predicted PEF1 (SD): median (range): salbutamol/fluticasone: 71.2 (54.8–83.9), salbutamol: 72.3 (55.6–85.8) Inclusion criteria: patients with acute moderate asthma requiring nebulised treatment, who did had not used inhaled or oral corticosteroids or sodium cromoglycate in the 2 weeks previous to the study, had no clinical evidence or history of any systemic disease or abnormal cardiac, liver or renal or neurological function |
|
| Interventions | Intervention: patients were randomised to 1 of 3 treatment groups. In 2 groups, different dosing regimens of salbutamol/fluticasone were compared to the third arm where salbutamol alone was given. For the review, the arm receiving the higher dose of fluticasone was included as the intervention group. In this group, patients received salbutamol 30 μL/kg and fluticasone 500 μg/dose x 3 doses, each combined dose administered every 15 min Control: 3 doses of salbutamol 30 μL/kg per dose x 3 doses, each dose administered every 15 min Evaluation was performed within 10 min immediately after patients arrived to the ED Medications were given via a Plarre nebuliser (salbutamol and fluticasone nebulised together) |
|
| Outcomes | Pulse oximetry (SaO2), PEF and a Wood et al clinical scale were monitored as efficacy outcomes at baseline (pre‐dose) and at 15, 30, 45, 60, 90 and 120 min after the first nebulisation The clinical evaluation was performed according to a scale developed and validated by Wood et al. It evaluates SaO2 (%), cyanosis, respiratory sounds, accessory muscle use, wheezing and alertness. A maximum score of 10 is obtainable for the most severe episodes, whereas a minimum score of 0 reflects normal, physiological, parameters |
|
| Notes | ICS versus placebo Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Patient eligibility and randomisation was performed by 1 of the investigators who did not participate in therapy or clinical evaluations |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears no patients were withdrawn |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Go 2010.
| Methods | Design: randomised controlled trial | |
| Participants | Age: 18 to 60 years Randomised: ICS 16, systemic corticosteroid 17 Groups were reported to be comparable in terms of demographic variables except for oxygen saturation and corticosteroid use at baseline Inclusion criteria: adults with known bronchial asthma Exclusion criteria: status asthmaticus, COPD or corticosteroid intake in past 2 weeks |
|
| Interventions | Intervention: inhaled fluticasone (dose not stated) via ultrasonic nebuliser every 15 min with inhaled short‐acting beta2‐agonists Control: hydrocortisone 250 mg/mL IV single dose with inhaled short‐acting beta2‐agonists |
|
| Outcomes | Baseline demographic and clinical factors including vital signs, wheeze, RR, oxygen saturation, baseline PEF and mean rate of change in PEF post intervention | |
| Notes | ICS versus systemic corticosteroid comparison Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Conference abstract – very limited information. It is stated that the patients were randomised to treatment |
| Allocation concealment (selection bias) | Unclear risk | Conference abstract – very limited information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Conference abstract – very limited information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference abstract – very limited information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conference abstract – very limited information |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract – very limited information |
Guttman 1997.
| Methods | Design: randomised controlled trial Method of randomisation: computer‐generated randomisation Means of allocation concealment: randomisation sequence concealed Blinding: double‐blind, placebo controlled Withdrawal/drop‐outs: 3 patients in BDP group and 1 in placebo group released themselves prior to completion, and 1 in the placebo group was withdrawn early because of deterioration in respiratory status. 2 patients initially randomised were later found to meet exclusion criteria and were excluded from the analyses | |
| Participants | Eligible: 60 Randomised: 30/30 (2 others excluded after randomisation) Completed: 27/28 Sex (M/F): BDP 16/14, placebo 16/14 Asthma diagnosis: as per American Thoracic Society criteria, FEV1 < 70% predicted Inclusion criteria: having at least 1 indication for IV corticosteroids, defined as: FEV1 < 40% predicted; chronic corticosteroid therapy (> 1 month) within 6 months of presentation; high‐dose ICS (> 1 mg/day) on presentation: and a short course of oral corticosteroids (< 1 month) within 2 months of presentation Major exclusions: in extremis, history of coronary artery disease, documented pneumonia, previously enrolled Baseline FEV1% mean (SD): BDP 32.4 (9.1), placebo 30.6 (9.1) | |
| Interventions | Setting: ED at an urban teaching hospital in Canada Intervention: BDP 1 mg by MDI plus spacer at 0, 0.5, 1, 2, 4, 6, 8 (total 7 mg) versus placebo MDI at same time intervals Standard of care: all patients received methylprednisolone 80 mg IV at 0 h and 40 mg IV at 6 h, salbutamol 2.5 mg by wet nebuliser at 0, 0.5, 1, 2, 4, 6, 8, 10 h. If FEV1 was < 40% predicated at 0, 0.5 h, salbutamol 5.0 mg was given instead of 2.5 mg Aminophylline and ipratropium were not permitted | |
| Outcomes | Primary outcome was the change in % predicted FEV1 Other outcomes included other PFTs, Borg Dyspnoea Scale, vital signs, serum potassium and hospital admissions | |
| Notes | The author was contacted and provided an additional reference, as well as additional information about the study and more data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 patients in BDP group and 1 in placebo group released themselves prior to completion, and 1 in the placebo group was withdrawn early because of deterioration in respiratory status. 2 patients initially randomised were later found to meet exclusion criteria and were excluded from the analyses |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Macias 2003.
| Methods | Design: randomised controlled trial Method of randomisation: randomisation table Means of allocation concealment: sealed packets Blinding: none Description of withdrawals/drop‐outs: 3 patients were excluded after randomisation because they did not meet the inclusion criteria | |
| Participants | Eligible: unclear Randomised: unclear Completed: (inhaled/oral/IV corticosteroids): 45/45/45 Sex (M/F): inhaled 32/13, oral 25/20, IV 26/19 Age (mean (SD)) (years): inhaled 9.5 (2.4), oral 10.8(3.0), IV 9.5(2.6) Asthma diagnosis: at least 1 prior episode of wheezing Inclusion criteria: at least 6 years of age, Wood score of 4 or 5, 1 or more prior episodes of wheezing Exclusion criteria: saw a doctor for asthma or was on corticosteroids within the week prior to presentation, unable to use MDI, no phone Baseline severity; mean PEF (SD): inhaled 45.3 (13.7), oral 43.5 (19.5), IV 40.6 (15.8) | |
| Interventions | Setting: 2 paediatric ED in the US (Texas, Colorado) Intervention: this study had 3 treatment arms. Group 1 received triamcinolone 600 μg by MDI with spacer. Group 2 received prednisone 2 mg/kg orally, with a maximum dose of 80 mg. Group 3 received IV methylprednisolone 2 mg/kg, with a maximum dose of 80 mg. All patients received nebulised albuterol 0.15 mg/kg (maximum 5 mg) every 4 h, and ipratropium bromide 0.5 mg nebulised every 4 h. In addition, if deemed necessary by the attending physicians, patients received either oral prednisone or IV methylprednisolone prior to randomisation | |
| Outcomes | The primary outcome was admission to hospital. Other outcomes included number of albuterol treatments given, oxygen saturation and PEF | |
| Notes | This trial was only included in the secondary analysis comparing ICS versus systemic corticosteroid. The results for hospital admissions were combined for the groups using IV and oral corticosteroids and compared to the group using ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by random number table |
| Allocation concealment (selection bias) | Low risk | Sealed packets |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 patients were excluded after randomisation because they did not meet the inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Milani 2004.
| Methods | Design: randomised controlled trial Method of randomisation: not stated Means of allocation concealment: not stated Withdrawals/drop‐outs: 1 patient in the placebo/placebo group was excluded from the study at 60 min because of worsening clinical status and was admitted Blinding: double‐blind, placebo controlled | |
| Participants | Eligible: unclear Randomised: (placebo/inhaled/oral corticosteroids): 15/17/17 Completed: placebo 14, inhaled 17, oral 17 Sex (M/F): placebo 9/6, inhaled 8/9, oral 8/9 Age (mean (SD)) (months): placebo 49.6 (15.6), inhaled 51.1 (18.7), oral 47.3 (16.3) Asthma diagnosis: ≥ 3 previous episodes, current episode lasting ≥ 6 h Inclusion criteria: moderate acute asthma defined as audible wheeze, use of accessory muscles, increased RR and an inability either to walk or to speak more than 3 to 5 words per breath Exclusion criteria: chronic or acute cardiopulmonary disease, use of systemic corticosteroids within the past 14 days, use of ICS within the past 72 h, severe exacerbations, other previous medical conditions | |
| Interventions | Setting: patients with acute asthma who sought treatment at the paediatric walk‐in clinic at 1 hospital in Brazil Intervention: this trial had 3 treatment groups. Group 1 received inhaled placebo and oral placebo. Group 2 received inhaled placebo and oral prednisone 1 mg/kg. Group 3 received nebulised budesonide 2 mg and oral placebo All patients received nebulised salbutamol 0.15 mg/kg. This was repeated if the patients' severity scores or oxygen saturations worsened. After 4 h' observation, patients were discharged with a prescription for inhaled bronchodilators | |
| Outcomes | Primary outcome were clinical severity scores and oxygen saturations. The clinical severity score was calculated based on: RR, wheezing, retraction, dyspnoea and oxygen saturation | |
| Notes | This trial was included in July 2005 in both the primary and secondary analyses. The author was not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 patient in the placebo/placebo group was excluded from the study at 60 min because of worsening clinical status and was admitted |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Nuhoglu 2005.
| Methods | Design: randomised controlled trial | |
| Participants | 26 children with moderate asthma Randomised: nebulised budesonide 12, placebo 14 Age (mean ± SD) (years): nebulised budesonide 7.90 ± 2.34, placebo 9.36 ± 2.55 Sex (M/F): nebulised budesonide 7/5, placebo 8/6 Baseline PEF L/Min (mean ± SD): nebulised budesonide 150.00 ± 32.19, placebo 192.86 ± 63 Baseline PIS (mean ± SD): nebulised budesonide 6.08 ± 1.00, placebo 6.07 ± 1.44 Inclusion criteria: patients aged 5 to 15 years, with a diagnosis of asthma, with a PIS ≥ 3 and ≤ 6, and who were able to use a peak flow meter |
|
| Interventions | Intervention: nebulised budesonide 1 mg Control: placebo (nebulised saline) Both groups received 3 consecutive doses of nebulised salbutamol 0.15 mg/kg/dose and 1 dose of IM methylprednisolone 1 mg/kg/dose |
|
| Outcomes | PIS and peak flow (before and after at 0 and 1 h) | |
| Notes | ICS versus placebo, both groups received systemic corticosteroid Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Reported as "the randomisation was performed according to the patients' social security number. Odd numbers got into Group I, even numbers got into Group II." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, and it was reported that PIS and PEFs were measured by a paediatrician blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Olaivar 1999.
| Methods | Design: randomised controlled trial | |
| Participants | 65 children aged 5 to 18 years. All had baseline FEV1 < 70% of predicted Randomised: nebulised budesonide + terbutaline 33, nebulised terbutaline 32 "Decision to admit, discharge and prescribe steroids was based on the Philippine consensus" |
|
| Interventions | Intervention: nebulised budesonide (0.5 μg) + terbutaline (2.5 mg) x 3 doses, given 20 min apart Control: nebulised placebo + terbutaline (2.5 mg) x 3 doses, given 20 min apart |
|
| Outcomes | FEV1 20 min after nebulisation completed and on second, third and fourth hour from start of treatment Patients were classified as good responders if FEV1 returned to > 80% of predicted and this response was sustained for 4 h (or poor if response was not prompt and sustained and FEV1 remained < 80% of predicted) |
|
| Notes | ICS versus placebo comparison Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Conference abstract – limited details reported |
| Allocation concealment (selection bias) | Unclear risk | Conference abstract – limited details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Conference abstract – limited details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference abstract – limited details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conference abstract – limited details reported |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract – limited details reported |
Pansegrouw 1992.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group study Method of randomisation: randomisation stated, method not described Means of allocation concealment: unclear Blinding: placebo inhaler Withdrawal/drop‐outs: 3 patients in the placebo group were withdrawn after developing life‐threatening asthma and were replaced | |
| Participants | Eligible: 40 (consecutive) Randomised: 20/20 Completed: 20/20 Sex: not stated Asthma diagnosis: "atopic asthmatics" defined as: I) a previous history of episodic increase in airway hyperirritability presenting as cough and/or increased sputum production and/or bronchospasm, which was reversible either with medications or spontaneously; and ii) an increased blood or sputum eosinophil count and/or increased serum immunoglobulin E levels and positive reactions to allergens on either skin tests or radio‐allergosorbent tests Inclusion criteria: "acute resistant asthma" defined as no response to an inhaled fenoterol, with no improvement clinically or in flow/volume curve measurements Other inclusion criteria were: FEV1 and FVC < 70% predicted, age 18 to 70 years, and misuse of beta2‐agonist bronchodilator inhalers during the present asthma attack Exclusion criteria: left ventricular failure, infective lung disease, degenerative lung disease and pulmonary embolism Baseline FEV1: BDP group approximately 1.2 L/min, placebo group approximately 0.9 L/min | |
| Interventions | Setting: 2 EDs in Bloemfontein, South Africa Intervention: both groups received an initial dose of fenoterol 400 μg by MDI. 5 min later the intervention group received BDP 200 μg by MDI, while the placebo group received placebo MDI. 5 min later both groups received 400 μg formoterol by MDI | |
| Outcomes | Primary outcome was FEV1, measured on presentation; prior to BDP/placebo inhalation; prior to repeat fenoterol inhalation; and at 5, 15, 30, 45 and 60 min after the second fenoterol inhalation Other outcomes included clinical evaluation of symptoms | |
| Notes | ICS versus placebo. The author did not respond to requests for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 patients in the placebo group were withdrawn after developing life‐threatening asthma and were replaced in the trial |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Rahman 2008.
| Methods | Design: randomised controlled trial | |
| Participants | 100 consecutive participants selected for the study but no details on how many per group | |
| Interventions | Intervention: BDP 2 puffs at 10‐min intervals (3000 μg/hour) delivered by MDI into spacer for 120 min along with oxygen supplement and salbutamol nebulisation Control: IV corticosteroid 500 mg (type not specified) along with oxygen supplement and salbutamol nebulisation |
|
| Outcomes | RR, HR, PEF measured immediately before starting treatment and at 30 min intervals for 2 h | |
| Notes | Trial added in 2012 update. ICS versus systemic corticosteroid comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as single blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Described as single blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Razi 2012.
| Methods | Design: randomised controlled trial | |
| Participants | 100 children aged 6 months to 6 years Inclusion criteria: children admitted to the ED with acute wheezing with a history of recurrent wheezing, PIS 7 and 13 Major exclusions: systemic corticosteroid use in the previous 30 days; chronic lung, cardiac, hepatic or renal disease; immune deficiency; suspected croup or foreign body From communication with the author, there were no significant differences between the groups in their baseline characteristics |
|
| Interventions | Intervention: 3 doses of budesonide 1 mg/2 mL with salbutamol 0.15 mg/kg/dose (maximum 5 mg) driven by 100% oxygen at a flow of 6 L/min at 0, 20 and 40 min Control: normal saline (2 mL) in addition to salbutamol 0.15 mg/kg/dose (maximum 5 mg) for 3 doses at the same time intervals (information provided by author) Both groups received 1 dose of IM methylprednisolone 1 mg/kg/dose at the initial of the treatment, ipratropium bromide, and additional doses of salbutamol at 80, 120 and 180 min |
|
| Outcomes | Changes in PIS from 0 to 120 min, discharge rates at 120, 180 and 240 min, hospitalisations and SaO2 | |
| Notes | ICS versus placebo, both groups received IM systemic corticosteroid Trial added in 2012 update. No results reported at clinicaltrials.gov/ct2/show/NCT00733317 but results were obtained directly from the trialist |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as randomised |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition bias unclear |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Rodrigo 1998.
| Methods | Design: randomised, controlled trial Method of randomisation: central randomisation. Method not described Means of allocation concealment: randomisation list concealed from all study personnel Blinding: double‐blind, placebo controlled Withdrawal/drop‐outs: none | |
| Participants | Eligible: 94 Randomised: 47/47 Completed: 47/47 Age; (mean (SD)) (years): treatment 31.1 (9.7), control 33.8 (9.7) Sex (M/F): treatment 32%/68%, control 36%/64% Asthma diagnosis: criteria for asthma of the American Thoracic Society Inclusion criteria: ages 18 to 50 years, FEV1 and PEF < 50% predicted, informed consent Major exclusions: chronic cough; cardiac, hepatic, renal, or other medical diseases; pregnancy Baseline FEV1% (SD): treatment 27.6 (10.3), control 25.9 (8.3) | |
| Interventions | Setting: ED of a hospital in Montevideo, Uruguay Intervention: treatment group received salbutamol 400 μg by MDI with spacer, followed by flunisolide, 1 mg every 10 min by MDI with spacer for 3 h (total dose of flunisolide 18 mg) Standard of care: control group received salbutamol 400 μg every 10 min by MDI and spacer, followed by placebo MDI with spacer | |
| Outcomes | Primary outcome change in FEV1. Other outcomes included other PFTs, vital signs; a clinical index (assessing the presence of dyspnoea, accessory‐muscle use and wheezing); and symptoms including nausea, palpitations, tremor, anxiety and headache | |
| Notes | The author was contacted and provided additional information about the study and more data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Low risk | Randomisation list concealed from all study personnel |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No indication of patients being withdrawn from the trial |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Rodrigo 2003.
| Methods | Design: randomised, double‐blind, placebo controlled trial Method of randomisation: random number table Means of allocation concealment: opaque envelopes containing identical canisters Blinding: double‐blind, placebo controlled Withdrawals/drop‐outs: 2 patients were withdrawn early from the ICS group, and 3 from the control group, because they did not meet the inclusion criteria | |
| Participants | Eligible: unclear Randomised: 58/62 Completed: 56/60 Age (mean (SD)) (years): treatment 34.0 (10.4), control 33.7 (11.0) Sex (M/F): treatment 36%/64%, control 37%/63% Asthma diagnosis: criteria of American Thoracic Society Inclusion criteria: age 18 to 50 years, FEV1 and PEF < 50% predicted Major exclusions: fever; history of chronic cough; heart, liver, renal or other disease; pregnancy Baseline FEV1% mean (SD): treatment 24.9 (10.8), control 25.1 (9.0) | |
| Interventions | Setting: ED of a hospital in Montevideo, Uruguay Intervention: this study had 3 treatment arms. Group 1 received fluticasone 1000 μg, albuterol 400 μg, and ipratropium 84 μg every 10 min by MDI with spacer for 3 h. Group 2 received albuterol 400 μg and ipratropium 84 μg every 10 min by MDI with spacer for 3 h. Group 3 received fluticasone 1000 μg and albuterol 400 μg every 10 min by MDI with spacer for 3 h. For this review, group 1 was included as the treatment group (received ICS) and group 2 was included as the control group (did not receive ICS, but identical other treatments) Standard of care: systemic corticosteroids were withheld until discharge or admission to hospital. Discharged patients received prednisone 60 mg daily for 7 days, admitted patients received IV corticosteroids. Oxygen was administered if oxygen saturations were < 92% | |
| Outcomes | Primary outcome was admission to hospital and improvement in PFTs. Admission was determined by senior ED staff without knowledge of the treatment allocation Other outcomes included vital signs and side effects | |
| Notes | This trial was included in July 2005. The author was not contacted. ICS versus placebo comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by random number table |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes containing identical canisters |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 patients were withdrawn early from the ICS group, and 3 from the control group, because they did not meet the inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Rodrigo 2005.
| Methods | Design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: random number table Means of allocation concealment: opaque envelopes containing identical canisters and syringes Withdrawals/drop‐outs: 8 patients in the ICS treatment group, and 7 patients in the IV hydrocortisone group were withdrawn early because they did not meet the inclusion criteria | |
| Participants | Eligible: unclear
Randomised: 60/61
Completed: 52/54
Age (mean (SD)) (years): ICS 33.9 (8.8), IV hydrocortisone: 33.2 (8.8)
Sex (M/F): ICS 16/36, IV hydrocortisone 15/39 Asthma diagnosis: diagnostic criteria of acute asthma of the Global Strategy of Asthma Management and prevention report Inclusion criteria: aged 18 to 50 years, PEF or FEV1 < 50% predicted Major exclusions: fever, history of other significant medical diseases, pregnancy Baseline PEF (%): ICS 32.9 (8.2), IV hydrocortisone 33.8 (7.2) |
|
| Interventions | Setting: ED of a hospital in Montevideo, Uruguay Intervention 1: fluticasone by MDI with spacer, 500 μg every 10 min for 3 h (total dose 9000 μg) Intervention 2: hydrocortisone 500 mg IV at start of trial Both groups received albuterol by MDI with spacer 600 μg every 10 min for 3 h, and Ipratropium bromide 84 μg every 10 min for 3 h. Systemic corticosteroids were withheld until patients were discharged from the ED, at the end of the study, when all patients were started on prednisone 60 mg orally every day for 7 days | |
| Outcomes | Primary outcomes were hospital admissions and PFTs. Need for admission was determined by senior ED staff at 180 min, without knowledge of the treatment group Other outcomes included a clinical score and symptoms | |
| Notes | This trial was included in July 2005. The author was not contacted. ICS versus systemic corticosteroid comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by random number table |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes containing identical canisters and syringes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 patients in the ICS treatment group, and 7 patients in the IV hydrocortisone group were withdrawn early because they did not meet the inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Sari 2004.
| Methods | Design: randomised controlled trial | |
| Participants | Patients with severe asthma Randomised: nebulised fluticasone 38, IV methylprednisolone 38 |
|
| Interventions | Intervention: combination nebulised fluticasone 0.5 mg plus salbutamol 2.5 mg Control: methylprednisolone IV 125 mg plus nebulised salbutamol 2.5 mg |
|
| Outcomes | PEF and symptom scores for 6 h | |
| Notes | ICS versus systemic corticosteroid comparison Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear – conference abstract with very limited information in report |
| Allocation concealment (selection bias) | Unclear risk | Unclear – conference abstract with very limited information in report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear – conference abstract with very limited information in report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear – conference abstract with very limited information in report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear – conference abstract with very limited information in report |
| Selective reporting (reporting bias) | Unclear risk | Unclear – conference abstract with very limited information in report |
Scarfone 1995.
| Methods | Design: randomised controlled trial Method of randomisation: computer generated, blocks of 10 Means of allocation concealment: allocation list kept by pharmacy, who provided study drugs according to the list Blinding: double‐blind, double‐dummy Withdrawal/drop‐outs: 17 patients were excluded after randomisation: pneumonia (7), missing scheduled albuterol treatment (3), vomiting study medication (5), parental withdrawal (1) or equipment failure (1) | |
| Participants | Eligible: unclear; convenience sample Randomised: 62/66 Completed: 56/55 Age (mean (SD)) (months): dexamethasone 64 (39), prednisone 55 (36). Range: 1 to 17 Sex (M/F): dexamethasone 57%/43%, prednisone 62%/38% Asthma diagnosis: 1 prior episode wheezing, doctor's diagnosis Inclusion criteria: moderate exacerbation, defined by PIS > 8 Major exclusions: severe exacerbation, defined by PIS > 13 or oxygen saturation < 88%, inhaled or systemic corticosteroids use in the preceding 72 h, vomiting oral study drug x 2, requiring more frequent albuterol than every 30 min or deteriorating status or missing a scheduled albuterol dose by > 10 min, previous enrolment, pneumonia, bronchiolitis or croup Baseline PIS (median): dexamethasone 10, prednisone 11 | |
| Interventions | Setting: ED at a Children's Hospital in US Intervention 1: nebulised dexamethasone 1.5 mg/kg x 1 Intervention 2: oral prednisone 2 mg/kg x 1 Both groups received nebulised albuterol 15 mg/kg every 30 min x 3 doses then every 40 min as needed to a maximum of 6 doses, and supplemental oxygen to keep O2 saturation > 92%. On discharge all patients were given a prescription for prednisone 2 mg/kg in 2 divided doses for 5 days, and oral or inhaled albuterol 3 times per day for 7 days. Co‐interventions such as extra doses of beta2‐agonists, theophylline or ipratropium bromide were not permitted | |
| Outcomes | Hospital admissions, change in PIS, time to discharge, vomiting and relapse to additional care after discharge PIS calculated from scoring RR, wheezing, inspiratory/expiratory ratio, accessory muscle use and oxygen saturation | |
| Notes | This trial was only included in the secondary analysis comparing ICS versus systemic corticosteroids. The author was contacted and provided additional information about the study design; however, the data were not available for further analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation list kept by pharmacy, who provided study drugs according to the list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17 patients were excluded after randomisation: pneumonia (7), missing scheduled albuterol treatment (3), vomiting study medication (5), parental withdrawal (1) or equipment failure (1) |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Schuh 2000.
| Methods | Design: randomised controlled trial Method of randomisation: blocked randomisation code from computer‐generated list Means of allocation concealment: sealed sequential packets Blinding: double‐blind, double‐dummy Withdrawal/drop‐outs: 1 in fluticasone group (FEV1% too high after 1 treatment), 2 from prednisone group (1 because of repeated vomiting of oral drug, 1 became acutely ill within 1 h after treatment with prednisone) | |
| Participants | Eligible: 148 Randomised: 103 (52/51) Completed: 100 (51/49) Age (mean (SD)) (years): fluticasone 9.3 (3.3), prednisone 9.5 (3.2) Sex (M/F): fluticasone 49%/51%, prednisone 69%/31% Asthma diagnosis: prior episodes of wheezing, baseline FEV1% < 60% predicted Inclusion criteria: age ≥ 5, FEV1 < 60% predicted, presentation between 8 AM and 5 PM, ability to use inhaler and do PFTs reliably Major exclusions: requiring immediate IV corticosteroids or intubation, use of oral corticosteroids within 7 days, or current use of moderate or high dose ICS Baseline FEV1% mean (SD): fluticasone 35.8 (8.5), prednisone 34.4 (9.8) | |
| Interventions | Setting: ED at a Children's hospital in Canada Intervention: fluticasone group received 2 mg fluticasone by MDI with spacer at start of study and oral placebo; prednisone group received 2 mg/kg prednisone orally and placebo by MDI Standard of care: both groups received nebulised albuterol 0.15 mg/kg at time ‐20, 0, 20, 40, 60, 80 and 140 min, and 250 μg nebulised ipratropium bromide at time = 0. Oxygen was given at 6 to 7 L/min with treatments | |
| Outcomes | Primary outcome was the change in % predicted FEV1. Other outcomes included other PFT results, hospital admission, clinical response, asthma relapse and vital signs | |
| Notes | This trial was included in the ICS versus systemic corticosteroid comparison in September 2000; the author was not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sealed sequential packets |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 patient in fluticasone group (FEV1% too high after 1 treatment), 2 from prednisone group (1 because of repeated vomiting of oral drug, 1 became acutely ill within 1 h after treatment with prednisone) |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Schuh 2006.
| Methods | Design: randomised controlled trial | |
| Participants | Children with mild to moderate asthma Randomised: fluticasone 35, placebo 34 Age (mean (SD)) (years): fluticasone 9.0 (2.6), placebo 9.2 (3.4) Sex (M/F): fluticasone 25/10, placebo 20/14 Baseline lung function; mean % predicted FEV1 (SD): fluticasone 63.0 (10.8), placebo 61.5 (10.7) Inclusion criteria: children aged 5 to 17 years, diagnosed with acute asthma by the ED paediatrician, with FEV1 50% to 79% predicted on ED presentation Exclusion criteria: children presenting with a first episode of wheezing, persistent vomiting, airway instability, those who had received oral corticosteroids within 7 days, co‐existent cardiopulmonary/neuromuscular disease, varicella contact within 21 days of the study entry, previous intensive care unit treatment for asthma, inability to communicate in English |
|
| Interventions | Intervention: fluticasone propionate 2 mg (8 inhalations, 250 μg each) via MDI/VHC with a mouthpiece and prednisolone placebo syrup in the ED Control: 8 inhalations of fluticasone placebo and 2 mg/kg (maximum 60 mg) of active prednisolone syrup Co‐interventions: all children received nebulised albuterol (dose of 0.15 mg/kg in first 10 patients, then decreased to 0.075 mg/kg because of tremor and tachycardia noted in the first 10 patients attributed to the high‐efficiency nebuliser) and ipratropium bromide, 250 μg per dose, with a mouthpiece 20 min before the experimental therapy, immediately after the initial dose of the experimental therapy (0 min), and at 60, 120, 180 and 240 min |
|
| Outcomes | Change in % predicted FEV1 from baseline to 240 min and 48 h in the 2 groups, RR and oxygen saturation, hospital admissions, adverse events | |
| Notes | ICS versus systemic corticosteroid subgroup Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme, random block sizes of 10 |
| Allocation concealment (selection bias) | Low risk | The research pharmacist supplied the corresponding MDI/syrup in a double‐dummy setup to the study nurses who enrolled participants and delivered the experimental therapy. Randomisation codes were secured in the pharmacy until enrolment and analysis decisions had been completed. The study nurses were unable to determine which intervention a given patient had received |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 children performed spirometry at 240 min, 30 in the intervention group and 30 in the control group (of 35 and 34 enrolled) 1 parent changed her mind regarding participation at 120 min, 5 children vomited the syrup and were too nauseated for FEV1 assessment, and 3 children were too sleepy to cooperate with FEV1 assessment |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Sekerel 2005.
| Methods | Design: randomised controlled trial | |
| Participants | Children with mild to moderate asthma Randomised: budesonide 33, placebo 34 Age (mean (SD)) (years): budesonide 10.0 (1.72), placebo 10.5 (2.33) Sex (M/F): budesonide 24/9, placebo 21/13 Baseline lung function mean (SD) % predicted FEV1: budesonide 77.7 (6.89), placebo 81.7 (9.91) Inclusion criteria: children with asthma exacerbations (defined as the presence of all of the following: 1) increased frequency of cough, wheeze and/or dyspnoea; 2) at least 2‐fold increase in mean daily bronchodilator use for > 24 h; and 3) > 15% decrease in FEV1 if spirometric evaluation was possible at baseline) and an FEV1 between 70% to 90% of personal best after 3 nebulised salbutamol doses. They also had to have had their FEV1 measured during the past 6 months when they were well Exclusion criteria: current use of ICS exceeding 800 mg/day or any change in their dose of ICS in the prior 2 months, any systemic corticosteroid in the past 6 months, any hospitalisation within 1 year, hypoxia (documented oxygen saturation of < 90%), presence of concurrent pulmonary disease, history of non‐compliance |
|
| Interventions | All participants received nebulised salbutamol (0.15 mg/kg, maximum 5 mg) every 20 min for 3 doses prior to enrolment. FEV1 was performed 20 min after the third dose of salbutamol to determine eligibility for enrolment Intervention: budesonide (1 mg/2mL) with salbutamol (0.15 mg/kg/ dose, maximum 5 mg) every hour for 3 h Control: normal saline (2 mL) as well as salbutamol every hour for 3 doses |
|
| Outcomes | The primary outcome measure was the proportion of children requiring systemic corticosteroid intervention The secondary outcome measures were FEV1, PEF, rescue beta2‐agonist use and hospitalisations |
|
| Notes | ICS versus placebo comparison Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A unit nurse, who was not involved in the study design and analysis, randomised children using a table of random numbers (blocks of 6) |
| Allocation concealment (selection bias) | Low risk | Identical numbered plastic syringes were provided, each covered with aluminium foil and containing either budesonide (1 mg/2 mL) or preservative‐free normal saline (2 mL), which served as a placebo. The content of the 2 types of syringes appeared identical. The investigators and the patients were unaware of group assignments and syringe contents The randomisation list was concealed in sealed opaque envelopes until data analysis was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No patients were reported as being withdrawn |
| Selective reporting (reporting bias) | Unclear risk | No indication of reporting bias |
Sharma 2003.
| Methods | Design: randomised controlled trial Method of randomisation: not stated Means of allocation concealment: not stated Withdrawals/drop‐outs: not stated Blinding: none | |
| Participants | Eligible: not stated
Randomised: 57 (group 1: 14, group 2: 14, group 3: 15, group 4: 14)
Completed: 57
Age range (years): 5 to 12
Sex (M/F): 29/28 Asthma diagnosis: unclear Inclusion criteria: history of cough, breathlessness with wheezing, RR > 30 per min, in ED for asthma Major exclusions: tuberculosis, history suggestive of tuberculosis Baseline PEF mean (SD): treatment 92 (48), control 92 (35) |
|
| Interventions | Setting: patients attending outpatient or casualty department for acute asthma at a hospital in India Patients were randomised to 1 of 4 groups: group 1: BDP 100 μg plus salbutamol 200 μg via MDI plus spacer; group 2: BDP 100 μg plus salbutamol 200 μg via MDI; group 3: salbutamol 200 μg via MDI plus spacer; group 4: salbutamol via MDI. The treatment was repeated every 20 min for a total of 3 treatments as needed | |
| Outcomes | Clinical parameters recorded included PEF, HR, RR, blood pressure, wheezing and retractions. Laboratory parameters recorded included serum glucose, sodium, potassium and arterial blood gases | |
| Notes | This trial was added in July 2005; the author was not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No indication of patients being withdrawn from trial |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Singhi 1999.
| Methods | Design: randomised, double‐blind, placebo‐controlled study Method of randomisation: not described Concealment: identical drug packages, but concealment not described | |
| Participants | Eligible: unclear; convenience sample Randomised: 60/60 Completed: 60/60 Age (mean (SD)) (years): budesonide 7.8 (2.5), placebo 8.1 (2.9) Sex (M/F): budesonide 19/11, placebo 20/10 Asthma diagnosis: at least 1 prior attack of asthma within the past 6 months Major exclusions: severe asthma; patients who had received corticosteroids in the preceding 72 h; and those with pneumonia, pertussis, measles, suspected foreign body, or any other chronic disease Baseline PEF mean (SD) (%): budesonide group 55 (26), placebo group 53 (20) | |
| Interventions | Setting: ED at a Children's Hospital in India Intervention 1: 400 μg budesonide x 3 doses through MDI and spacer Intervention 2: placebo through MDI and spacer Both groups received nebulised salbutamol 0.15 mg/kg every 30 min x 3 doses then every 30 min as needed, and supplemental oxygen. If there was an inadequate response, co‐interventions such as extra doses of beta2‐agonists, theophylline or corticosteroids were given while blinding was maintained | |
| Outcomes | Change in RR, oxygen saturation, % predicted PEF (in those who could perform it), respiratory distress score, and respiratory distress grade (mild, moderate, severe) | |
| Notes | New reference in 2003. The author was not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No indication of patients being withdrawn from the trial but baseline PEF was available for only 28 in the intervention group and 28 patients in the control group |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Starobin 2008.
| Methods | Design: randomised controlled trial, 3 arms | |
| Participants | Randomised: nebulised fluticasone 24, IV methylprednisolone 23, combined 26 Age (mean ± SD) (years): nebulised fluticasone 37.9 ± 16.8, IV methylprednisolone 47 ± 14.6, combined 48.2 ± 17.2 Sex (M/F): nebulised fluticasone 11/13, IV methylprednisolone 9/14, combined 15/11 Baseline PEF mean ± SD (% predicted): nebulised fluticasone 42 ± 27.5, IV methylprednisolone 35.2 ± 21.3, combined 38.9 ± 18.0 Inclusion criteria: ED patients aged 18 to 75 years with acute asthma, history of atopic or idiosyncratic asthma with any grade of severity Exclusion criteria: chronic use of systemic corticosteroids for severe asthma; history of severe cardiac, renal or liver disease; tracheostomy; requiring mechanical ventilation |
|
| Interventions | Group 1 patients (Flixotide group) received inhalation of Flixotide Nebules® (fluticasone propionate) (Glaxo Wellcome, Australia) 2 mL (0.5 mg) Group 2 patients (the Solumedrol® group) received IV infusion of Solumedrol (methylprednisolone) 125 mg within the first 30 min after admission Group 3 patients (combined group) were treated by both routes of corticosteroids All patient received oxygen at a rate of 5 L/min, salbutamol 0.5 mL plus ipratropium bromide 1 mg plus 2 mL of normal saline 0.9% every 20 min during the first hour of ED treatment Decisions regarding admission to the internal medicine ward, intensive care unit or discharge, as well as additional treatment decisions were made independently by the local ED staff |
|
| Outcomes | Primary outcome: hospital admissions. Other outcomes included PEF, oxygen saturation, HR and dyspnoea score, measured before and 2 h after ED treatment was initiated. Corticosteroids were continued for 1 week following the ED visit according to the protocol arm, and hospital admission/discharge rate, ED re‐admissions in the week after enrolment and other major events related to asthma were recorded. (Only the 0‐ and 2‐h outcomes contribute to this review) It is stated there were no serious adverse event, although 1 patient (treatment group not stated) required mechanical ventilation and 1 patient from group 3 (both ICS and systemic corticosteroid) returned to the ED during the first week of the study |
|
| Notes | ICS versus systemic corticosteroid subgroup, and ICS versus placebo (systemic corticosteroid in both groups) Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Reported as "random consecutive case fashion to one of three protocol arms" |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Sung 1998.
| Methods | Design: randomised controlled trial Method of randomisation: block randomisation in groups of 4 to 6 stratified by age (6 months to < 5 years, and > 5 years) and by concurrent use of ICS Means of allocation concealment: randomisation sequence concealed to all study personnel, identical drug packages Blinding: double‐blind, identical packages of study drugs made indistinguishable from each other and identified only by a study number Withdrawal/drop‐outs: all subjects accounted for | |
| Participants | Eligible: 82 referred. 38 excluded for: PIS < 5 (32), declined (2), congenital heart disease (1), chicken pox exposure (1), assessed too late (1), ICS use (1) Randomised: 44 (24/20) Completed: 44 Age (mean) (years): study group 4.4, control group 3.5. Range: 6 months to 18 years Sex (M/F): 80%/20% Asthma diagnosis: wheezing on at least 2 prior occasions Inclusion criteria: PIS ≥ 5 and ≤ 11 Major exclusion criteria: acute exacerbation ≤ 2 weeks prior, use of systemic glucocorticoids < 1 month prior, previous enrolment, presence of any underlying disease that could affect the patient's cardiopulmonary status Baseline PIS median (range): budesonide 7 (6 to 8.8), placebo 7 (6 to 8) | |
| Interventions | Setting: tertiary care paediatric hospital in Canada
Study group received nebulised budesonide 2 mg x 1 dose Control group received normal saline placebo Both groups were given oral prednisone 1 mg/kg (maximum 50 mg) x 1 dose and salbutamol 0.15 mg/kg x 3 doses then every hour x 4 h. Supplemental oxygen was given as needed to maintain O2 saturation > 92% |
|
| Outcomes | Primary outcome was PIS, which was correlated with FEV1/FVC in asthmatic children > 6 years. Secondary outcomes included oxygen saturation, HR and co‐interventions including the use of extra doses of salbutamol, ipratropium bromide use and IV glucocorticoid use. Other outcomes included admission, length of hospitalisation and relapse of wheezing | |
| Notes | The author was contacted and provided additional data from the study. ICS versus placebo, both groups received systemic corticosteroid | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of how the random sequence was generated are not available in the trial report. Block randomisation in groups of 4 to 6 stratified by age (6 months to < 5 years, and > 5 years) and by concurrent use of ICS |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence concealed to all study personnel, identical drug packages |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No indication of patients being withdrawn from trial |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Tsai 2001.
| Methods | Design: randomised controlled trial Method of randomisation: random number allocation Means of allocation concealment: not stated Blinding: double‐blind, double‐dummy Withdrawals/drop‐outs: 4 patients excluded because the ambient nitric oxide levels were too high (the primary outcome), 2 patients were excluded because of disease deterioration | |
| Participants | Eligible: unclear Randomised: 30 Completed: 24 (12/12) Sex: not stated Asthma diagnosis: previous diagnosis of allergic asthma Inclusion criteria: age 6 to 17 years, allergic asthma Exclusion criteria: acute febrile respiratory infection, on corticosteroids or leukotriene D4 receptor agonists within 4 weeks Baseline PEF: treatment group 110 L/min, control group 89 L/min | |
| Interventions | Setting: ED at a Children's Hospital in Taiwan Intervention group was given nebulised terbutaline 0.1 mg/kg per dose at 0 a 6 h plus budesonide 0.05 mg/kg, maximum 2 mg, at 0 h, while the control group received the same dose of terbutaline plus nebulised placebo | |
| Outcomes | Outcomes evaluated included exhaled nitric oxide levels, PIS, PEF and blood pressure | |
| Notes | This trial was added in July 2002. ICS versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number allocation but unclear from trial report whether the sequence was produced by computer or tables |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 patients excluded because the ambient nitric oxide levels were too high (the primary outcome), 2 patients were excluded because of disease deterioration |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Upham 2011.
| Methods | Design: randomised controlled trial | |
| Participants | Randomised: budesonide 91, placebo 89 Age (mean (range)) (years): budesonide 6.2 (5.4 to 7.0), placebo 6.3 (5.5 to 7.1) Sex (M/F): budesonide 64/27, placebo 54/35 Reported as: "116 patients (64%) presented in the moderate severity range (asthma score 8–11), and 62 patients (34%) presented in the severe (12–15) range." General point. Reported as: "Baseline demographic and clinical characteristics were similar between treatment groups (Table 2). BUD subjects had a slightly higher rate of prior hospitalisation and number of nebulized albuterol treatments prior to ED arrival" Inclusion criteria: children 2 to 18 years of age, with a history of asthma defined by at least 2 prior episodes of treatment with bronchodilators, and an asthma score of ≥ 8 who were prescribed systemic corticosteroid were eligible for inclusion. Screening was limited to patients triaged to the highest 2 acuity categories of a 4‐level triage system. The asthma score used was reported by Qureshi et al (Qureshi 1998), and was chosen due to its similarity to the PIS, age‐specific RR and excellent inter‐rater reliability Exclusion criteria: any use of systemic corticosteroid in the prior 30 days; chronic lung disease, sickle cell anaemia, immunodeficiency, cardiac disease requiring surgery or medications, known renal or hepatic dysfunction, impending respiratory failure, altered level of consciousness, exposure to varicella in the prior 21 days, suspected foreign body or croup; had been enrolled in the study previously; or had an adverse drug reaction or allergy to the study drugs |
|
| Interventions | Intervention: budesonide inhalation suspension 2 mg, nebulised Control: placebo All patients received standard acute asthma therapy while consent was being obtained, which included albuterol (3.75 mg for patients weighing 10 to 20 kg, 5 mg for those > 20 kg); ipratropium bromide (500 μg); and prednisolone, prednisone, or methylprednisolone (2 mg/kg to a maximum of 60 mg) |
|
| Outcomes | Primary outcome: difference in median asthma scores between study groups 2 h after intervention Secondary outcomes: hospital admissions to an inpatient floor or observation unit, HR, RR and oxygen saturation |
|
| Notes | Include ‐ ICS versus placebo (systemic corticosteroid in both groups) Trial added in 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were stratified by age category (2 to 8 and 9 to 18 years) and use of ICS at study entry. Enrolment occurred in each strata until that strata was filled |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes that were pre‐randomised in variable‐sized blocks |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study medication was prepared by a respiratory therapist out of sight of other staff, the patient, and the patient's family, and was placed into a shielded nebulisation chamber. Nebulised medications were administered by a respiratory therapist not involved in asthma scoring, treatment decisions or disposition decisions. The investigators recorded which treatment the study physicians felt the patient received at the time of the patient's final disposition, and they did not seem to be able to distinguish between the |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators recorded which treatment the study physicians felt the patient received at the time of the patient's final disposition, and they did not seem to be able to distinguish between the 2 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All 91 patients randomised to budesonide received budesonide, and 89 were evaluable for the primary outcome (1 patient was discharged before the 2‐h evaluation, and 1 did not have a 2‐h score). 88 of 89 patients randomised to normal sterile saline placebo received normal sterile saline placebo (1 patient was inadvertently started on standard therapy without placebo), and 81 of those 89 patients were evaluable for the primary outcome. 2 patients in the normal sterile saline group withdrew from the study: 1 for feeling "jittery" and 1 for unspecified reasons at the guardian's request. 5 additional patients in the normal sterile saline group could not be evaluated for the primary outcome because they were admitted to the inpatient ward before the 2‐h evaluation. 2 patients in the budesonide group and 4 patients in the normal sterile saline group were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Volovitz 1998.
| Methods | Design: randomised controlled trial Method of randomisation: computer generated Means of allocation concealment: sealed envelopes Blinding: double‐blind, double‐dummy Withdrawal/drop‐outs: 1 patient excluded because of pneumonia, and another for non‐compliance | |
| Participants | Eligible: unclear
Randomised: 24
Completed: 11/11
Sex (M/F): intervention 73%/37%, control 64%/46%
Asthma diagnosis: moderately severe attack with PEF1 35‐75% predicted and PIS 8 to 13
Inclusion criteria: PEF 35‐75% and PIS 8 to 13, aged 6 to 16 years
Major exclusions: presence of acute febrile illness, regular use of ICS, cromolyn, nedocromil sodium or theophylline in past 2 weeks
Baseline FEV1%: FEV1 not done Baseline PEF% mean (SD): budesonide 54.40 (10.25), prednisolone 61.67 (10.73) Baseline PIS mean (SD): budesonide 8.75 (1.22), prednisolone 8.67 (1.12) |
|
| Interventions | Interventions: ED at a paediatric Hospital in Israel Intervention 1: single‐dose budesonide 1600 μg by Turbohaler Intervention 2: prednisolone 2 mg/kg orally Both groups received terbutaline 5 mg by nebuliser or 0.5 mg by Turbohaler at start of trial Intervention 1 group was discharged on budesonide 200 μg 4 times daily by Turbohaler, reduced by 25% every second day, and placebo tablets. From the eighth day, they continued on 200 μg twice daily x 2 weeks Intervention 2 group was discharged on prednisolone 2 mg/kg/day, reduced by 25% every second day, and placebo Turbohaler | |
| Outcomes | Outcomes evaluated in the ED included PEF, PIS and vital signs | |
| Notes | This trial was only included in the secondary analysis comparing ICS versus systemic corticosteroid. The author was contacted and provided additional data from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported as double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study personnel responsible for outcome assessment indicates the risk of detection bias would be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 patient excluded because of pneumonia, and 1 for non‐compliance |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
BDP: beclomethasone dipropionate; COPD: chronic obstructive pulmonary disease; ED: emergency department; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HR: heart rate; ICS: inhaled corticosteroid; IM: intramuscular; IV: intravenous; LABA: long‐acting beta2‐agonist; M/F: male/female; MDI: metered‐dose inhaler; MPIS: modified pulmonary index score; PEF: peak expiratory flow; PEF: peak expiratory flow rate; PFT: pulmonary function test; PIS: Pulmonary Index Score; RR: respiratory rate; SD: standard deviation; VHC: valved holding chamber.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2010 | Study compared combination therapy with inhaled corticosteroids + long‐acting beta2‐agonists versus placebo |
| Balanag 2003 | RCT comparing combination therapy with budesonide plus formoterol versus salbutamol alone |
| Balanag 2006 | Study compared combination therapy with a long‐acting beta2‐agonist + ICS versus a short‐acting beta2‐agonist alone |
| Bilancia 1998 | Study included only hospitalised patients |
| Brenner 2000 | Study compared ICS versus placebo, with both groups receiving systemic corticosteroids, in the treatment of acute asthma after ED discharge |
| Camargo 2000 | Study compared ICS versus placebo, with both groups receiving systemic corticosteroids, in the treatment of asthma exacerbations after ED discharge |
| Chhabra 1994 | Study compared sequential treatment with beta2‐agonists alone with beta2‐agonists plus ICS in chronic asthma |
| Clarke 2007 | Study involved only patients with chronic asthma |
| Connett 1994 | RCT of systemic corticosteroids versus placebo |
| Crain 1998 | Review of the study by Pauwels 1997 |
| Cueva 1975 | Study investigated the effects of chronic beclomethasone use on pulmonary function tests and adrenal function in chronic asthma |
| Curtis 1995 | Study involved only hospitalised patients |
| da Silva 2007 | Study involved children under 3 years of age hospitalised with episodes of wheezing, who did not have a clear diagnosis of asthma |
| Decimo 2009 | Study compared fluticasone versus budesonide in the outpatient treatment of asthma exacerbations in children, with no placebo group |
| Di Franco 2006 | Study randomised clinic patients with an asthma exacerbation to outpatient treatment with ICS versus oral corticosteroids |
| Ediger 2001 | RCT including patients with asthma and COPD, who were all admitted to hospital |
| Ediger 2006 | Study randomised outpatients with asthma exacerbations at a clinic to treatment with ICS alone, ICS plus systemic corticosteroid, or systemic corticosteroid alone |
| Fitzgerald 2000 | Study comparing ICS versus systemic corticosteroid in the treatment of asthma exacerbations after ED discharge |
| Francis 1997 | Study investigated the outpatient treatment of asthma exacerbations with ICS versus systemic corticosteroid in children under 4 years of age |
| Frye 1988 | Letter regarding intravenous corticosteroid use in acute asthma |
| Higenbottam 2000 | Study included only patients admitted to hospital |
| Joubert 1985 | Study used patients with chronic asthma with simulated acute attacks |
| La Rosa 1997 | Study compared ICS versus placebo in the outpatient treatment of acute asthma. Systemic corticosteroids were withheld from both treatment groups |
| Lai 2005 | All patients in this study were hospitalised |
| Latysheva 1996 | Non‐randomised study that compared betamethasone with dexamethasone in asthma and other allergic conditions |
| Lee‐Wong 2002 | All the patients in this study were hospitalised |
| Leuppi 2002 | Study investigated the use of single high dose of budesonide versus doubling of standard dose budesonide in induced asthma exacerbations, with no placebo group |
| Lim 1996 | Study included only hospitalised patients |
| Lin 1999 | RCT of systemic corticosteroids versus placebo |
| Lipworth 1997 | Review article on acute asthma |
| Littenberg 1986 | RCT of systemic corticosteroids versus placebo |
| Manjra 2000 | Study investigated the use of ICS versus systemic corticosteroid in the outpatient treatment of acute asthma |
| Mannan 2008 | Study investigated patient initiated increase in the baseline dose of inhaled corticosteroids to prevent ED visits and oral corticosteroid use |
| Matthews 1999 | Study involved only children who were hospitalised with acute asthma |
| McEvoy 1977 | All patients in this study were hospitalised, and were enrolled after 2 days of hospitalisation if they had not responded satisfactorily to intravenous aminophylline and beta2‐agonists |
| Mendes 2008 | Study included outpatients with mild stable asthma |
| Mitchell 1995 | Study involved only hospitalised patients |
| Nana 1998a | Study compared budesonide and prednisolone in the treatment of asthma exacerbations after ED discharge |
| Nuhoglu 2001 | Study compared high‐dose ICS versus medium‐dose ICS plus systemic corticosteroid in the outpatient treatment of asthma exacerbations |
| Pauwels 1997 | Study involved long acting beta2‐agonists and budesonide use in chronic asthma |
| Pierson 1974 | Study investigated the use of ICS in acute asthma |
| Postma 2006 | Study included outpatients with asthma exacerbations induced by withdrawal of ICS |
| Razi 2008 | Trial compared different methods of giving ICS, but all patients received ICS |
| Rivera 1999 | Not a clinical trial |
| Rodrigo 1994 | RCT of systemic corticosteroids versus placebo |
| Salmeron 1989 | Study compared the use of ICS versus placebo in chronic asthma |
| Sano 2000 | Study included only children less than 24 months old admitted to hospital for wheezing episodes |
| Scarfone 1993 | RCT of systemic corticosteroids versus placebo |
| Schneider 1988 | RCT of systemic corticosteroids versus placebo |
| Sereda 2004 | Study included only admitted patients |
| Singhi 1995 | Review of the study by Scarfone 1995 |
| Stein 1990 | RCT of systemic corticosteroids versus placebo |
| Storr 1987 | RCT of systemic corticosteroids versus placebo |
| Tal 1990 | RCT of systemic corticosteroids versus placebo |
| Verona 1998 | Study compared ICS versus systemic corticosteroid in the outpatient treatment of asthma exacerbations |
| Wen 2008 | Not a randomised trial, and involved children hospitalised with asthma |
| Wendel 1996 | All of the patients randomised in this study were all admitted to hospital |
| Winter 1997 | All of the patients included in this study were hospitalised |
| Wolfson 1994 | RCT of systemic corticosteroids versus placebo |
| Yang 2000 | All of the patients included in this study were all admitted to hospital |
| Yashina 2001 | Study investigated the outpatient treatment of asthma exacerbations. There were 3 treatment arms, with varying types and dose of ICS given in all 3 treatment arms |
| Yi 2003 | Study recruited patients both from the ED and hospitalised patients with acute asthma |
| Zhen 2003 | Not an RCT. Allocation was by order of administration |
| Zhou 2000 | This study investigated the use of a spacer in the delivery of beclomethasone in chronic asthma therapy |
COPD: chronic obstructive pulmonary disease; ED: emergency department; ICS: inhaled corticosteroid; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Acun 2003.
| Methods | Randomised controlled trial |
| Participants | 42 children with moderately severe asthma. Aged 6 months and 15 years (total sample). The baseline characteristics of the 2 groups were reported as similar |
| Interventions | Intervention: on first and second days: nebulised budesonide 2000 μg/day. On third and fourth days: 1000 μg/day Control: on first and second days: prednisolone 2 mg/kg/day. On third and fourth days: 1 mg/kg/day Both groups received salbutamol 1.2 mg/kg/day on first day, and on the second day 0.6 mg/kg/day. On the third and fourth days and on both groups received 0.3 mg/kg/day |
| Outcomes | Hospital length of stay. PIS (pre‐ and post‐treatment), heart rate and oxygen saturation levels |
| Notes | Unclear whether patients were hospitalised |
Agarwal 2003.
| Methods | Randomised controlled trial |
| Participants | 114 patients, 15 and 45 years of age with acute moderate exacerbations of asthma |
| Interventions | budesonide 800 μg with MDI and spacer versus placebo at 30‐min intervals for 3 doses |
| Outcomes | PEF, length of stay |
| Notes | Study reported as conference abstract. Unclear whether patients were hospitalised. Awaiting further clarification from author |
Agarwal 2005.
| Methods | Randomised controlled trial |
| Participants | 90 patients, 15 and 45 years of age with acute moderate exacerbations of asthma |
| Interventions | Fluticasone 500 μg with MDI and spacer versus placebo at 30‐min intervals for 3 doses |
| Outcomes | PEF, length of stay |
| Notes | Study reported as conference abstract. Unclear whether all patients were hospitalised. Awaiting further clarification from author |
Agarwal 2009.
| Methods | Randomised controlled trial |
| Participants | 70 patients, 15 and 45 years of age with acute moderate exacerbations of asthma |
| Interventions | Fluticasone 500 μg + salmeterol 50 μg with MDI and spacer versus placebo at 30‐min intervals for 3 doses |
| Outcomes | PEF, length of stay |
| Notes | Study reported as conference abstract. Unclear whether all patients were hospitalised. Awaiting further clarification from author |
Akhtaruzzaman 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Alangari 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ambrosio 1997.
| Methods | Randomised controlled trial |
| Participants | Adults with acute exacerbations of asthma |
| Interventions | Terbutaline + budesonide versus terbutaline alone given through nebulisation at 15‐min intervals for 3 doses |
| Outcomes | Admissions, PEF, adverse effects and vital signs |
| Notes | Trial report unobtainable |
Arulparithi 2015.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Chen 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Demirca 2015.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Jerez 2002.
| Methods | Randomised controlled trial |
| Participants | 15 patients, mean age 38 years with acute asthma |
| Interventions | Fluticasone 4000 μg versus oral prednisolone 30 mg |
| Outcomes | FEV1 and eosinophils in differential cell counts |
| Notes | Unclear whether all patients were hospitalised |
Ndeezi 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Razi 2017.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Sampayo 2017.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Silverman 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
FEV1: forced expiratory volume in 1 second; MDI: metered‐dose inhaler; PEF: peak expiratory flow rate; PIS: Pulmonary Index Score.
Differences between protocol and review
In the 2012 update of this review heterogeneity was assessed mainly in relation to the I2 statistic. Risk of bias is assessed in accordance with Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A post‐hoc subgroup analysis comparing delivery with nebuliser and MDI with spacer was performed following the suggestion of a peer reviewer.
Contributions of authors
In the original version of the review:
Edmonds ML: initiated the review, wrote the protocol, performed searches, performed quality assessments, entered data and performed analysis, and primary author of review and updated versions. Camargo CA Jr: protocol development, methodological input, statistical support and assumed major editorial role. Pollack CV Jr: protocol development and manuscript review. Rowe BH: co‐authored protocol, performed selection for inclusion and quality assessment, data extraction and data entry, manuscript review, conversion to RevMan 4 and assigned editor for ARG.
In the 2012 revision of this review: Milan SJ and Edmonds M independently selected trials for inclusion from initial searches. Edmonds M and Milan SJ selected trials for inclusion from full trial reports. Milan SJ updated the 'Risk of bias' tables for trials already included in the review and similarly for any new trials identified in the update. Milan SJ updated the results section and this was checked by Edmonds M. Milan SJ and Edmonds M jointly completed the restructuring of the remaining areas of the review and in updated the text. Rowe BH: review and in updated the text, assigned editor for ARG.
Sources of support
Internal sources
Department of Emergency Medicine, University of Alberta, Edmonton, AB, Canada.
National Institute for Health Research (SJM), UK.
External sources
National Institutes of Health (HL‐03533) Bethesda, MD (Dr. CA Camargo, Jr), USA.
Canadian Institutes of Health Research (CIHR), Ottawa, ON (BH Rowe), Canada.
Declarations of interest
The review authors who have been involved in this review have done so without any known conflicts of interest. They are not involved with the primary studies. Drs. Camargo, Pollack have received unrestricted educational grants for research from AstraZeneca, Boehringer‐Ingelheim, Forest, GlaxoSmithKline, Merck and Sepracor. In the past three years, Dr. Rowe has participated in trials sponsored by GSK and MedImmune Inc. None of the authors, however, are considered paid consultants by any pharmaceutical company that produces ICS agents.
Edited (no change to conclusions)
References
References to studies included in this review
Afilalo 1999 {published and unpublished data}
- Afilalo M, Guttman A, Colacone A, Dankoff J, Tselios C, Stern E, et al. Efficacy of inhaled steroids (beclomethasone dipropionate) for treatment of mild to moderately severe asthma in the emergency department: a randomized clinical trial. Annals of Emergency Medicine 1999;33(3):304‐9. [DOI] [PubMed] [Google Scholar]
Ancheta 2008 {published data only}
- Ancheta VA, Jiao AGQ, Erguiza GSD, Arellano MA, Dizon CC, Catacutan MS. Comparison of inhaled fluticasone propionate versus intravenous hydrocortisone in the treatment of severe asthma exacerbation in children aged 6‐18 years. Chest 2008; Vol. 134:24002s.
Bateman 2006 {published data only}
- Bateman ED, Fairall L, Lombardi DM, English R. Budenoside/formoterol and formoterol provide similar rapid relief in patients with acute asthma showing refractoriness to salbutamol. Respiratory Research 2006;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bautista 1994 {published data only}
- Bautista MS, Balderas J. The efficacy of combined inhaled steroids and beta‐2 agonist in the treatment of acute asthma in children 6‐18 years of age versus inhaled beta‐2 agonist alone. European Respiratory Journal 1994; Vol. 7 Suppl 18:93s.
Belda 2007 {published data only}
- Belda J, Margarit G, Martinez C, Bellido‐Casado J, Casan P, Torrejon M, et al. Anti‐inflammatory effects of high‐dose inhaled fluticasone versus oral prednisone in asthma exacerbations. European Respiratory Journal 2007;30(6):1143‐9. [DOI] [PubMed] [Google Scholar]
Blandon 2004 {published data only}
- Blandon MV, Rosas MA, Rio BE, Sienra JJ. Comparison of effectiveness between nebulized budesonide plus albuterol versus albuterol alone in children with moderate acute asthma. Journal of Allergy and Clinical Immunology 2004;113 Suppl(2):S118. [Google Scholar]
Devidayal 1999 {published data only}
- Devidayal, Singhi S, Kumar L, Jayshree M. Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatrica 1999;88:835‐40. [DOI] [PubMed] [Google Scholar]
Estrada 2005 {published data only}
- Estrada‐Reyes E, Rio‐Navarro BE, Rosas‐Vargas MA, Nava‐Ocampo AA. Co‐administration of salbutamol and fluticasone for emergency treatment of children with moderate acute asthma. Pediatric Allergy & Immunology 2005;16(7):609‐14. [DOI] [PubMed] [Google Scholar]
Go 2010 {published data only}
- Go J. Comparison of intravenous hydrocortisone versus inhaled fluticasone in adult acute asthma a randomized controlled trial. Respirology 2009; Vol. 14 Suppl 3:A247.
- Go J, Ong E, Koh A. Comparison of intravenous hydrocortisone versus inhaled fluticasone in adult acute asthma a randomized controlled trial. Congress of the Asian Pacific Society of Respirology, Manila 2010.
Guttman 1997 {published and unpublished data}
- Guttman A, Afilalo M, Colacone A, Kreisman H, Dankoff J. The effects of combined intravenous and inhaled steroids (beclomethasone diproprionate) for the emergency treatment of acute asthma. Academic Emergency Medicine 1997;4:100‐6. [DOI] [PubMed] [Google Scholar]
Macias 2003 {published data only}
- Macias CG, Felner EI, Gan V. Inhaled corticosteroids may be superior to systemic corticosteroids in children with moderate‐to‐severe acute asthma. Pediatrics 2003;16(3):121‐8. [Google Scholar]
Milani 2004 {published data only}
- Milani GK, Rosario Filho NA, Riedi CA, Figueiredo BC. Nebulized budesonide to treat acute asthma in children. Jornal de Pediatria 2004;80(2):106‐12. [PubMed] [Google Scholar]
Nuhoglu 2005 {published data only}
- Nuhoglu Y, Atas E, Nuhoglu C, Iscan M, Ozcay S. Acute effect of nebulized budesonide in asthmatic children. Journal of Investigational Allergology & Clinical Immunology 2005;15(3):197‐200. [PubMed] [Google Scholar]
Olaivar 1999 {published data only}
- Olaivar EA, Tuazon AO, Martirez T, Gaerlan EB. Nebulized budesonide in the management of acute asthma. American Journal of Respiratory and Critical Care Medicine 1999;159(3):A142. [Google Scholar]
Pansegrouw 1992 {published data only}
- Pansegrouw DF. Acute resistant asthma caused by excessive beta‐2‐adrenoceptor agonist inhalation and reversed by inhalation of beclomethasone. South African Medical Journal 1992;82:179‐82. [PubMed] [Google Scholar]
Rahman 2008 {published data only}
- Rahman M, Hossain A, Hossain DA. Comparative study of inhaled corticosteroid with systemic corticosteroids in the management of acute asthma at emergency department. Respirology 2007; Vol. 12 Suppl 4:A146.
- Rahman MM, Hossain DA, Hossain A. Comparative study of inhaled corticosteroid with systemic corticosteroid in the management of acute asthma at emergency department. Respirology 2008; Vol. 13 Suppl 5:A131.
Razi 2012 {unpublished data only}
- Razi CH. Budesonide for emergency treatment of acute wheezing in children. clinicaltrials.gov/ct2/show/NCT00733317 (accessed 16 September 2012).
Rodrigo 1998 {published and unpublished data}
- Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. American Journal of Respiratory and Critical Care Medicine 1998;157:698‐703. [DOI] [PubMed] [Google Scholar]
Rodrigo 2003 {published data only}
- Rodrigo GJ, Rodrigo C. Triple inhaled drug protocol for the treatment of acute severe asthma. Chest 2003;123:1908‐15. [DOI] [PubMed] [Google Scholar]
Rodrigo 2005 {published data only}
- Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. American Journal of Respiratory and Critical Care Medicine 2005;171:1231‐6. [DOI] [PubMed] [Google Scholar]
Sari 2004 {published data only}
- *Sari A, Wiyono WH, Yunus F, Jusuf A, Hupudio H. Efficacy of nebulized fluticasone compared with intravenous methylprednisolone in controlling severe acute asthma. Respirology 2004;9(Suppl):A92. [Google Scholar]
Scarfone 1995 {published and unpublished data}
- Scarfone RJ, Loiselle JM, Wiley JF II, Decker JM, Henretig FM, Joffe MD. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Annals of Emergency Medicine 1995;26:480‐6. [DOI] [PubMed] [Google Scholar]
Schuh 2000 {published data only}
- Schuh S, Reisman J, Alshehri M, Dupuis A, Corey M, Arseneault R, et al. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. New England Journal of Medicine 2000;343(10):689‐94. [DOI] [PubMed] [Google Scholar]
Schuh 2006 {published data only}
- Schuh S, Dick PT, Stephens D, Hartley M, Khaikin S, Rodrigues L, et al. High‐dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics 2006;118(2):644‐50. [DOI] [PubMed] [Google Scholar]
Sekerel 2005 {published data only}
- Sekerel BE, Sackesen C, Tuncer A, Adalioglu G. The effect of nebulized budesonide treatment in children with mild to moderate exacerbations of asthma. Acta Paediatrica 2005;94(10):1372‐7. [DOI] [PubMed] [Google Scholar]
Sharma 2003 {published data only}
- Sharma S, Godatwar P, Kulkarni LR. Salbutamol or beclomethasone diproprionate in asthma. Indian Journal of Pediatrics 2003;70(2):129‐32. [DOI] [PubMed] [Google Scholar]
Singhi 1999 {published data only}
- Singhi SC, Banerjee S, Nanjundaswamy HM. Inhaled budesonide in acute asthma. Journal of Paediatric Child Health 1999;35:483‐7. [DOI] [PubMed] [Google Scholar]
Starobin 2008 {published data only}
- Starobin D, Bolotinsky L, Or J, Fink G, Shtoeger Z. Efficacy of nebulized fluticasone propionate in adult patients admitted to the emergency department due to bronchial asthma attack. Israel Medical Association Journal 2008;10(9‐9):568‐71. [PubMed] [Google Scholar]
Sung 1998 {published and unpublished data}
- Sung L, Osmond MH, Klassen TP. Randomized, controlled trial of inhaled budesonide as an adjunct to oral prednisone in acute asthma. Academic Emergency Medicine 1998;5:209‐13. [DOI] [PubMed] [Google Scholar]
Tsai 2001 {published data only}
- Tsai YG, Lee MY, Yang KD, Chu DM, Yuh YS, Hung CH. A single dose of nebulized budesonide decreases exhaled nitric oxide in children with acute asthma. Journal of Pediatrics 2001;139(3):433‐7. [DOI] [PubMed] [Google Scholar]
Upham 2011 {published data only}
- Upham BD, Mollen CJ, Scarfone RJ, Seiden J, Chew A, Zorc JJ. Nebulized budesonide added to standard pediatric emergency department treatment of acute asthma: a randomized, double‐blind trial. Academic Emergency Medicine 2011;18(7):665‐73. [DOI] [PubMed] [Google Scholar]
Volovitz 1998 {published and unpublished data}
- Volovitz B, Bentur L, Finkelstein Y, Mansour Y, Shalitin S, Nussinovitch, et al. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: a controlled comparative study with oral prednisolone. Journal of Allergy and Clinical Immunology 1998;102:605‐9. [DOI] [PubMed] [Google Scholar]
- Volovitz B, Bentur L, Finkelstein Y, Nussinovitch M, Mansour Y, Shalitin S, et al. Effectiveness and safety of inhaled budesonide in controlling acute asthma attacks in children ‐ a controlled study compared to oral prednisolone in the emergency department, and evaluation of efficacy in 150 outpatient children. Journal of Allergy and Clinical Immunology 1998:S10 Abstract 43. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2010 {published data only}
- Agarwal SK, Sharma S. Effect of fluticasone/formoterol pressurized metered‐dose inhaler (pMDI) in early management of acute exacerbations of asthma. American Journal of Respiratory and Critical Care Medicine 2010; Vol. 181:A5659.
- Agarwal SK, Sharma S. Utility of inhaled corticosteroids (fluticasone/formoterol) by pressurized metered‐dose inhaler for the early management of acute exacerbations of asthma. European Respiratory Society Annual Congress; 2010 Sept 18‐22; Barcelona 2010:E5476.
Balanag 2003 {published data only}
- Balanag VM, Yunus F, Tang PC, Jorup C. Budesonide/formoterol in a single inhaler is as effective and well tolerated as salbutamol in relieving acute asthma in adults and adolescents. European Respiratory Journal 2003;22(Suppl 45):P2836. [Google Scholar]
Balanag 2006 {published data only}
- Balanag VM, Yunus F, Yang P‐C, Jorup C. Efficacy and safety of budesonide/formoterol compared with salbutamol in the treatment of acute asthma. Pulmonary Pharmacology & Therapeutics 2006;19(2):139‐47. [DOI] [PubMed] [Google Scholar]
Bilancia 1998 {published data only}
- Bilancia R, Caputo F, Margiotta D, Sebastio G, Balacco D, Mastrandrea L. Oral metil prednisolone vs fluticasone propionate in the treatment of patients hospitalized for middle exacerbation of asthma. ALA/ATS Abstracts. 1998; Vol. A403.
Brenner 2000 {published data only}
- Brenner BE, Chavda KK, Camargo CAJ. Randomized trial of inhaled flunisolide versus placebo among asthmatic patients discharged from the emergency department. Annals of Emergency Medicine 2000;36:417‐26. [DOI] [PubMed] [Google Scholar]
Camargo 2000 {published data only}
- Camargo CJ, on behalf of the MARC investigators. Randomized trial of medium‐dose fluticasone vs. placebo after an emergency department visit for acute asthma. AAAAI 56th Annual Meeting 2000.
Chhabra 1994 {published data only}
- Chhabra SK. A comparison of inhaled salbutamol with a combination of salbutamol and beclomethasone dipropionate in moderately severe asthma. Indian Journal of Chest Diseases and Allied Science 1994; Vol. 36, issue 3:119‐24. [PubMed]
Clarke 2007 {published data only}
- Clarke GW, Greenaway SD, James WY, Adcock G, Siew LQ, O'Connor BJ. The acute effect of inhaled steroids on airway mucosal flow in asthma. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California, USA. Poster #B60. 2007.
Connett 1994 {published data only}
- Connett GJ, Warde C, Wooler E, Lenney W. Prednisolone and salbutamol in the hospital treatment of acute asthma. Archives of Diseases of Childhood 1994;70:170‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crain 1998 {published data only}
- Crain EF. Effect of inhaled formoterol and budesonide on exacerbations of asth (N Engl J Med 336:1405‐1411, 1997). Emergency and Office Pediatrics 1998;11(2):76‐77. [Google Scholar]
Cueva 1975 {published data only}
- Cueva JV, Sucilla H, Gorocica D, Cicero R, Durazo F. Beclomethasone dipropionate ‐ adrenal function and acute asthma. Postgraduate Medical Journal 1975;51(Suppl 4):87. [PubMed] [Google Scholar]
Curtis 1995 {published data only}
- Curtis P. Comparison of prednisolone and nebulised budesonide in acute asthma in children: a pilot study. European Respiratory Journal 1995;8(Suppl 19):470s. [Google Scholar]
da Silva 2007 {published data only}
- Silva ML. Effectiveness and safety of budesonide nebulised in control of acute crisis of wheezing in children under three years not responsive to fenoterol [Dissertation] [Efetividade e segurança da budesonida nebulizada no controle da crise aguda de sibilância em crianças menores de três anos não responsivas ao fenoterol]. Universidade Estadual Paulista. Faculdade de Medicina de Botucatu. 2007. 152p.
Decimo 2009 {published data only}
- Decimo F, Maiello N, Miraglia Del Giudice M, Amelio R, Capristo C, et al. High‐dose inhaled flunisolide versus budesonide in the treatment of acute asthma exacerbations in preschool‐age children. International Journal of Immunopathology & Pharmacology 2009; Vol. 22:363‐70. [0394‐6320] [DOI] [PubMed]
Di Franco 2006 {published data only}
- Franco A, Bacci E, Laura Bartoli M, Cianchetti S, Dente FL, Taccola M, et al. Inhaled fluticasone propionate is effective as well as oral prednisone in reducing sputum eosinophilia during exacerbations of asthma which do not require hospitalization. Pulmonary Pharmacology & Therapeutics 2006; Vol. 19, issue 5:353‐60. [DOI] [PubMed]
Ediger 2001 {published data only}
- Ediger D, Uzaslan EK, Yuksel EG, Coskun E, Ozyardimei N, Ege E. Clinical effectiveness of nebulized budesonide in the treatment of acute asthma and exacerbations of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 2001;18 Suppl 3:146s. [Google Scholar]
Ediger 2006 {published data only}
- Ediger D, Coskun F, Kunt Uzaslan E, Gurdal Yuksel E, Karadag M, Ege E, et al. Clinical effectiveness of nebulised budesonide in the treatment of acute asthma attacks. Tuberkuloz ve Toraks 2006;54(2):128‐36. [PubMed] [Google Scholar]
Fitzgerald 2000 {published data only}
- Fitzgerald JM, Shragge DL, Haddon JMF, Jennings B, Lee J, Bai A, et al. A randomized, controlled trial of high does, inhaled budesonide versus oral prednisone in patients discharged from the emergency department following an acute asthma exacerbation. Canadian Respiratory Journal 2000;7(1):61‐7. [DOI] [PubMed] [Google Scholar]
- Grunfeld AF, Bai A, Shragge DL, Haddon JMF, Lee J, Jennings B, et al. High dose inhaled budesonide versus prednisone in patients with acute asthma discharged from the emergency department. Clinical and Investigative Medicine 1997;20(4):S15. [Google Scholar]
Francis 1997 {published data only}
- Francis P, Geelhoed G, Harris MA, Morton J, Efthimiou J, Barnacle H. Effect of nebulised fluticasone propionate 1 mg twice daily compared with oral prednisolone in pre‐school children aged 48 months or less with an acute exacerbation of asthma. European Respiratory Journal 1997;10 Suppl 25:275s. [Google Scholar]
Frye 1988 {published data only}
- Frye CB. Corticosteroid of choice in the treatment of acute asthma. Drug Intelligence and Clinical Pharmacy 1988;22:407. [Google Scholar]
Higenbottam 2000 {published data only}
- Higenbottam TW, Britton J, Lawrence D, Connolly CK, Harrison NK, Eastham HM, et al. Comparison of nebulised budesonide and prednisolone in severe asthma exacerbation in adults. Biodrugs 2000; Vol. 14, issue 4:247‐54. [EMBASE 2000392806]
Joubert 1985 {published data only}
- Joubert JR, Burger G, Shephard E. Inhalation therapy during acute asthma: the role of a combined steroid and beta‐stimulant preparation. South African Medical Journal 1985;68:381‐4. [PubMed] [Google Scholar]
Lai 2005 {published data only}
- Lai S‐T, Hua Y‐M, Lai Y‐S, Chen C‐J, Hung C‐H. Comparison of nebulized budesonide with intravenous dexamethasone in the treatment of young children hospitalized with acute asthma. Journal of Medical Sciences 2005;25(5):223‐8. [Google Scholar]
La Rosa 1997 {published data only}
- Rosa M, Ranno C, Mandarà G, Barbato A, Biraghi M. Double‐blind study of inhaled salbutamol versus salbutamol plus high‐dose flunisolide in exacerbation of bronchial asthma: a pilot study. Pediatric Asthma, Allergy & Immunology 1997;11(1):23‐30. [Google Scholar]
Latysheva 1996 {published data only}
- Latysheva TV, Ilina NI. Experience in using Celeston (betamethasone) in emergency states in allergology. Anesteziologiia i Reanimatologiia 1996;3:48‐50. [PubMed] [Google Scholar]
Lee‐Wong 2002 {published data only}
- Lee‐Wong M, Dayrit FM, Kohli AR, Acquah S, Mayo PH. Comparison of high‐dose inhaled flunisolide to systemic corticosteroids in severe adult asthma. Chest 2002;122(4):1208‐13. [DOI] [PubMed] [Google Scholar]
Leuppi 2002 {published data only}
- Leuppi JD, Sownie SR, Salome CM, Jenkins CR, Woolcock AJ. A single high dose of inhaled corticosteroids: a possible treatment of asthma exacerbations. Swiss Medical Weekly 2002;132(1‐2):7‐11. [DOI] [PubMed] [Google Scholar]
Lim 1996 {published data only}
- Lim TK. Comparison of inhaled fluticasone propionate and oral prednisolone in the treatment of acute severe asthma. American Journal of Respiratory Critical Care Medicine 1996;153(4 part 2):A340. [Google Scholar]
Lin 1999 {published data only}
- Lin RY, Pesola GR, Bakalchuk L, Heyl GT, Dow AM, Tenenbaum C, et al. Rapid improvement of peak flow in asthmatic patients treated with parenteral methylprednisolone in the emergency department: a randomized controlled study. Annals of Emergency Medicine 1999;33:487‐94. [DOI] [PubMed] [Google Scholar]
Lipworth 1997 {published data only}
- Lipworth BJ. Treatment of acute asthma. Lancet 1997;350(Suppl II):18‐23. [DOI] [PubMed] [Google Scholar]
Littenberg 1986 {published data only}
- Littenberg B, Gluck E. A controlled trial of methyl‐prednisolone in the emergency treatment of acute asthma. New England Journal of Medicine 1986;314:150‐2. [DOI] [PubMed] [Google Scholar]
Manjra 2000 {published data only}
- Manjra AI, Price J, Lenney W, Hughes S, Barnacle H. Efficacy of nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respiratory Medicine 2000;94:1206‐14. [DOI] [PubMed] [Google Scholar]
Mannan 2008 {published data only}
Matthews 1999 {published data only}
- Mathews EE, Curtis PD, McLain BI, Morris LS, Turbitt ML. Nebulized budesonide versus oral steroid in severe exacerbations of childhood asthma. Acta Paediatrica 1999;88:841‐3. [DOI] [PubMed] [Google Scholar]
McEvoy 1977 {published data only}
- McEvoy JDS, Edwards RL. Beclomethasone dipropionate in acute asthma. Australian & New Zealand Journal of Medicine 1977; Vol. 7:665. [0004‐8291]
Mendes 2008 {published data only}
Mitchell 1995 {published data only}
- Mitchell CA, Alpers JH, Morton SM, Baggoley CJ, Croker WD, Walsh AJ, et al. Comparison of nebulized budesonide with oral prednisolone in the treatment of severe acute asthma. European Respiratory Journal 1995;8 Suppl 19:490S. [Google Scholar]
Nana 1998a {published data only}
- Nana A, Youngchaiyud P, Charoenratanakul S, Boe J, Löfdahl C‐G, Selroos O, et al. High‐dose inhaled budesonide may substitute for oral therapy after and acute asthma attack. Journal of Asthma 1998;35(8):647‐55. [DOI] [PubMed] [Google Scholar]
Nuhoglu 2001 {published data only}
- Nuhoglu Y, Bahceciler NN, Barlan IB, Basaran MM. The effectiveness of high‐dose inhaled budesonide therapy in the treatment of acute asthma exacerbations in children. Annals of Allergy, Asthma and Immunology 2001;86:318‐22. [DOI] [PubMed] [Google Scholar]
Pauwels 1997 {published data only}
- Pauwels RA, Löfdahl C‐G, Postma DS, Tattersfield AE, O'Byrne PO, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine 1997;337(20):1405‐11. [DOI] [PubMed] [Google Scholar]
Pierson 1974 {published data only}
- Pierson WE, Bierman CW, Kelley VC. A double blind trial of corticosteroid treatment in status asthmaticus. Pediatrics 1974;54(3):282‐8. [PubMed] [Google Scholar]
Postma 2006 {published data only}
- Postma DS, Ind PW, Magnussen H, Berge M. A double‐blind, randomized, controlled study comparing the efficacy and safety of inhaled ciclesonide with oral prednisolone in patients with an asthma exacerbation. Proceedings of the American Thoracic Society 2006:A74.
- Postma S, Arshad H, Hamelmann H, B. Efficacy of inhaled ciclesonide compared with oral prednisolone in the treatment of asthma exacerbations. European Respiratory Journal 2006; Vol. 28:206.
Razi 2008 {published data only}
Rivera 1999 {published data only}
- Rivera JMO. High dose corticosteroids in spray in the treatment of serious bronchial asthma [Spanish]. Alergologia e Immunologia Clinica 1999;14(4):277‐81. [Google Scholar]
Rodrigo 1994 {published data only}
- Rodrigo C, Rodrigo G. Early administration of hydrocortisone in the emergency room treatment of acute asthma: a controlled clinical trial. Respiratory Medicine 1994;88:755‐61. [DOI] [PubMed] [Google Scholar]
Salmeron 1989 {published data only}
- Salmeron S, Guerin J‐C, Godard P, Renon D, Henry‐Amar M, Duroux P, et al. High doses of inhaled corticosteroids in unstable chronic asthma. American Review of Respiratory Disease 1989;140:167‐71. [DOI] [PubMed] [Google Scholar]
Sano 2000 {published data only}
- Sano F, Cortez GK, Sole D, Naspitz CK. Inhaled budesonide for the treatment of acute wheezing and dyspnea in children up to 24 months old receiving intravenous hydrocortisone. Journal of Allergy and Clinical Immunology 2000;105(4):699‐703. [DOI] [PubMed] [Google Scholar]
Scarfone 1993 {published data only}
- Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics 1993;92:513‐8. [PubMed] [Google Scholar]
Schneider 1988 {published data only}
- Schneider SM, Pipher A, Britton HL, Borok Z, Harcup CH. High‐dose methylprednisolone as initial therapy in patients with acute bronchospasm. Journal of Asthma 1988;25:189‐93. [DOI] [PubMed] [Google Scholar]
Sereda 2004 {published data only}
- Sereda VP, Svistov AS. Efficacy of nebulized budesonide in severe exacerbation of bronchial asthma [Effektivnost nebulizirovannogo budesonide pritiazhelom obostrenii bronkhial'noi astmy]. Klinicheskaia Meditsina 2004;82(6):61‐6. [PubMed] [Google Scholar]
Singhi 1995 {published data only}
- Singhi S. Steroids in acute asthma: oral or nebulized?. Indian Pediatrics 1996;33:262‐3. [PubMed] [Google Scholar]
Stein 1990 {published data only}
- Stein LM, Cole RP. Early administration of corticosteroids in emergency room treatment of acute asthma. Annals of Internal Medicine 1990;112:822‐7. [DOI] [PubMed] [Google Scholar]
Storr 1987 {published data only}
- Storr J, Barrel E, Barry W, Lenney W, Hatcher G. Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet 1987;8538:879‐81. [DOI] [PubMed] [Google Scholar]
Tal 1990 {published data only}
- Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics 1990;86:350‐6. [PubMed] [Google Scholar]
Verona 1998 {published data only}
- Verona E, Ahel V, Williams L, Medley HV. A multicentre, randomized, double‐blind, double‐dummy, parallel group study comparing efficacy and safety of fluticasone propionate 500μg BID delivered by metered dose inhaler and spacer device with oral soluble prednisolone. American Journal of Respiratory and Critical Care Medicine 1998;157(3):A711. [Google Scholar]
Wen 2008 {published data only}
- Wen CJ, Lai ST, Deng YC, Hung CH. Plasma levels of leukotriene E4 and 9alpha, 11 beta‐PGF2 in asthmatic young children during exacerbation and convalescence. Journal of Medical Sciences 2008;28(6):233‐7. [Google Scholar]
Wendel 1996 {published data only}
- Wendel PJ, Ramin SM, Barnett‐Hamm C, Rowe TF, Cunningham FG. Asthma treatment in pregnancy: a randomized controlled study. American Journal of Obstetrics and Gynecology 1996;175:150‐4. [DOI] [PubMed] [Google Scholar]
Winter 1997 {published data only}
- Winter JH, Dhillon DP, Winter JE, Feeney Y, Allen D, Maslen T. Effect of the early substitution of nebulised fluticasone propionate 2 mg bd for oral prednisolone 50 mg od in adults during the early recovery period of an acute exacerbation. European Respiratory Journal 1997;10(Suppl 25):174S. [Google Scholar]
Wolfson 1994 {published data only}
- Wolfson DH, Nypaver MM, Blaster M, Hogan A, Evans R, Davis T. A controlled trial of methylprednisolone in the early emergency department treatment of acute asthma in children. Pediatric Emergency Care 1994;10:335‐8. [DOI] [PubMed] [Google Scholar]
Yang 2000 {published data only}
- Yang S, Feng E, Suo Y. Inhaled budesonide for severe asthma at high altitude. Chinese Journal of Tuberculosis and Respiratory Diseases 2000;23(10):613‐6. [PubMed] [Google Scholar]
Yashina 2001 {published data only}
- Yashina LO, Gorovenko NG, Gogunska IV. Efficacy of high doses of inhaled corticosteroids in treatment of asthma exacerbation. Ukrainian Journal of Pulmonology 2001;3:21‐5. [Google Scholar]
Yi 2003 {published data only}
- Yi TY, Li JM, Li QL, Zhi AI, Yi J. Budesonide in combination with salbutamol in the treatment of 61 cases of childhood bronchial asthma during acute episodes. Herald of Medicine 2003; Vol. 22, issue 8:540‐1.
Zhen 2003 {published data only}
- Zhen YZ. Observation on effect of treating acute episode of infant asthma by budesonide suspension driven by oxygen. Journal of Practical Medical Techniques 2003;10:1021‐2. [Google Scholar]
Zhou 2000 {published data only}
- Zhou ZY, Wang ZF, Chen J, Chen Y, Wang CZ. Treatment of severe asthma with large dosage of inhaled beclometasone. Acta Academiae Medicine Militaris Tertiae 2000; Vol. 6:591‐3.
References to studies awaiting assessment
Acun 2003 {published data only}
- *Acun C, Tomac N, Sogut S, Demirel F, Yuksel B. A comparison of inhaled budesonide and oral prednisolone for children with acute asthma. European Respiratory Journal 2003;22 Suppl 45:P887. [Google Scholar]
Agarwal 2003 {published data only}
- *Agarwal SK. Utility of aerosolized budesonide therapy in acute moderate exacerbations of asthma. ERS Annual Congress. 2003:P1836.
- Agarwal K. Aerosolized budesonide therapy in acute moderate exacerbations of Indian asthmatics. European Respiratory Journal 2004; Vol. 24:343s.
- Agarwal SK. Aerosolized budesonide therapy in acute moderate exacerbations of asthma. Chest 2004; Vol. 126:815S.
Agarwal 2005 {published data only}
- Agarwal SK, Gupta S. Aerosolized fluticasone propionate therapy in acute moderate exacerbations of asthma. European Respiratory Society Annual Congress 2002;20:P397. [Google Scholar]
- Agarwal SK, Singh D, Singh V. Utility of aerosolized fluticasone therapy in the early management of acute moderate exacerbations of asthma in the emergency room. Respirology 2005; Vol. 10:A115.
Agarwal 2009 {published data only}
- Agarwal SK, Arshad N. Aerosolized fluticasone therapy in acute moderate exacerbations of asthma. Chest 2008; Vol. 134:44001s.
- Agarwal SK, Arshad N. Utility of high dose inhaled fluticasone therapy for the early management of acute exacerbations of asthma. 19th European Respiratory Society Annual Congress; 2009, Sept 12‐15. Vienna 2009:E4352.
Akhtaruzzaman 2014 {published data only}
- Akhtaruzzaman M, Ahmed SU, Hoque MA, Choudhury AM, Hossain MA, Islam MN, et al. Effects of nebulized budesonide as an adjunct to standard treatment of asthma exacerbations: a randomized, double‐blind, placebo‐controlled trial. Mymensingh Medical Journal : MMJ 2014;23(3):418‐425. [PubMed] [Google Scholar]
Alangari 2014 {published data only}
- Alangari AA, Malhis N, Mubasher M, Al‐Ghamedi N, Al‐Tannir M, Riaz M, et al. Asthma diagnosis and treatment‐1012. The efficacy of budesonide in the treatmetn of acute asthma in children: A double‐blind, randomized, controlled trial. World Allergy Organization Journal. 2013; Vol. 6:12. [DOI] [PMC free article] [PubMed]
- Alangari AA, Malhis N, Mubasher M, Al‐Ghamedi N, Al‐Tannir M, Riaz M, et al. Budesonide nebulization added to systemic prednisolone in the treatment of acute asthma in children: a double‐blind, randomized, controlled trial. Chest 2014;145(7):772‐8. [DOI] [PubMed] [Google Scholar]
Ambrosio 1997 {published data only}
- Ambrosio MI, Ramiro A, Dyseng J. A comparative study on terbutaline + budesonide versus terbutaline alone given through nebulization in the treatment of acute exacerbations of asthma in adults. Philippine Scientific Journal 1997; Vol. 6:42‐7.
Arulparithi 2015 {published data only}
- Arulparithi CS, Babu TA, Ravichandran C, Santhanam I, Sathyamurthi B, Parivathini S, et al. Efficacy of nebulised budesonide versus oral prednisolone in acute severe asthma. Indian Journal of Pediatrics 2015;82(4):328‐32. [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen AH, Chen RC, Zhan JY, Hunag S, Lin YH, Chen DH, et al. The efficacy of nebulized budesonide in acute moderate to severe exacerbations of asthma in children. Zhonghua jie he he hu xi za zhi [Chinese journal of tuberculosis and respiratory diseases] 35(4):269‐74. [PubMed] [Google Scholar]
- Chen AH, Zeng GQ, Chen RC, Zhan JYSun LH, Hunag SK, et al. Effects of nebulized high‐dose budesonide on moderate‐to‐severe acute exacerbation of asthma in children: a randomized, double‐blind, placebo‐controlled study. Respirology 2013;18(0):47‐52. [DOI] [PubMed] [Google Scholar]
Demirca 2015 {published data only}
- Demirca BP, Cagan H, Kiykim A, Arig U, Arpa M, Tulunay A, et al. Nebulized fluticasone propionate, a viable alternative to systemic route in the management of childhood moderate asthma attack: A double‐blind, double‐dummy study. Respiratory Medicine 2015;109(9):1120‐5. [DOI] [PubMed] [Google Scholar]
Jerez 2002 {published data only}
- Jerez FR, Brufal M, Bellido J, Margarit G, Torrejon M, Belda J, et al. Inhaled corticosteroids in the treatment of acute asthma. European Respiratory Society Annual Congress; 2002, Sept 14‐18; Stockholm 2002:P1917.
Ndeezi 2014 {published data only}
- Ndeezi G, Tumwine JK, Østergaard MS. Acute respiratory infections and asthma in U‐5 children; improved treatment to reduce morbidity and mortality in Uganda. 7th International Primary Care Respiratory Group (IPCRG) World Conference Athens 21 ‐ 24 May, 2014. 2014; Vol. 0:OOR‐075.
Razi 2017 {published data only}
- Razi CH, Corut N, Andiran N. Budesonide reduces hospital admission rates in preschool children with acute wheezing. Pediatric Pulmonology 2017;52(6):720‐728. [DOI] [PubMed] [Google Scholar]
Sampayo 2017 {published data only}
- Sampayo EM, Mazer‐Amirshahi M, Camp EA, Zorc JJ. Initiation of an inhaled corticosteroid during a pediatric emergency visit for asthma: a randomized clinical trial. Annals of Emergency Medicine 2017;70(3):331‐37. [DOI] [PubMed] [Google Scholar]
Silverman 2010 {published data only}
- Silverman R, Khan F, Jean‐Francois A, Pearson D, Wallman A, Declaro D, et al. Emergency department use of nebulized budesonide as an adjunct to standard therapy in acutely ill adults with refractory asthma: A randomized, doubleblind, placebo‐controlled trial. Academic Emergency Medicine 2010;17(0):S115. [Google Scholar]
Additional references
Ausejo 1999
- Ausejo M, Saenz A, Ba'Pham, Kellner JD, Johnson DW, Moher D, et al. Evaluating the effectiveness of glucocorticoids in the treatment of croup: a meta‐analysis of randomized controlled trials. BMJ 1999;319:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 1995
- Barnes PJ. Inhaled glucocorticoids for asthma. New England Journal of Medicine 1995;332(13):868‐75. [DOI] [PubMed] [Google Scholar]
Becker 1984
- Becker AB, Nelson NA, Simons ER. The Pulmonary Index. Assessment of a clinical score for asthma. American Journal of Diseases of Children 1984;138:574‐6. [DOI] [PubMed] [Google Scholar]
Boulet 2000
- Boulet L‐P, Becker A, Berube D, Beveridge RC, Ernst P, on behalf of the Canadian Asthma Consensus Group. Canadian asthma consensus report 1999. Canadian Medical Association Journal 1999;161 Suppl(11):S1‐61. [PMC free article] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. The British guidelines on asthma management: 1995 review and position statement. Thorax 1997; Vol. 52:S1‐S20.
BTS/SIGN 2011
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. May 2008. Revised Jan 2012. www.sign.ac.uk/guidelines/fulltext/101/index.html (accessed 16 September 2012).
Colice 2000
- Colice GL. Comparing inhaled corticosteroids. Respiratory Care 2000;45(7):846–53. [PubMed] [Google Scholar]
Edmonds 2009
- Edmonds M, Brenner BE, Camargo CA, Rowe BH. Inhaled steroids for acute asthma following emergency department discharge. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002316] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPR3 2007
- Expert Panel Report 3 (EPR‐3). Guidelines for the Diagnosis and Management of Asthma‐Summary Report. Journal of Allergy and Clinical Immunology 2007;120:S94‐138. [DOI] [PubMed] [Google Scholar]
Gibbs 2000
- Gibbs MA, Camargo CA Jr, Rowe BH, Silverman RA. Therapeutic controversies in severe acute asthma. Academic Emergency Medicine 2000;7(7):800‐15. [DOI] [PubMed] [Google Scholar]
GINA 2011
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. December 2011. www.ginasthma.org/guidelines‐gina‐report‐global‐strategy‐for‐asthma.html (accessed 16 September 2012).
Griswold 2005
- Griswold SK, Nordstrom CR, Clark S, Gaeta TJ, Price ML, Camargo CA Jr. Asthma exacerbations in North American adults: Who are the "frequent fliers" in the emergency department?. Chest 2005;127:1579‐1586. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jadad 1996
- Jadad A, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Karras 2000
- Karras DJ, Sammon ME, Terregino CA, Lopez BL, Griswold SK, Arnold GK. Clinically meaningful changes in quantitative measure of asthma severity. Academic Emergency Medicine 2000;7(4):327‐34. [DOI] [PubMed] [Google Scholar]
Mannino 1998
- Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, et al. Surveillance for asthma ‐ United States, 1960‐1995. Morbidity and Mortality Weekly Report 1998;47:1‐27. [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59(5):469‐78. [DOI] [PubMed] [Google Scholar]
NAEPP 1997
- National Heart Lung and Blood Institute. Expert Panel Report II: guidelines for the diagnosis and management of asthma, 1997. www.nhlbi.nih.gov/guidelines/archives/epr‐2/index.htm.
NHLBI/WHO 1995
- National Heart, Lung and Blood Institute. NHLBI/WHO Workshop Report. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention 1995. Bethesda, MD: National Institutes of Health, 1995. [Google Scholar]
Passalacqua 2000
- Passalacqua G, Albano M, Canonica GW, Bachert C, Cauwenberge P, Davies RJ, et al. Inhaled and nasal corticosteroids: safety aspects. Allergy 2000;55(1):16‐33. [DOI] [PubMed] [Google Scholar]
Quon 2010
- Quon BS, FitzGerald JM, Lemière C, Shahidi N, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007524.pub3] [DOI] [PubMed] [Google Scholar]
Qureshi 1998
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect ofnebulized ipratropium on the hospitalization ratesof children with asthma. N Engl J Med 1998;339:1030‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rowe 2008
- Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD002178] [DOI] [Google Scholar]
Rowe 2008a
- Rowe BH, Villa‐Roel C, Sivilotti ML, Lang E, Borgundvaag B, Worster A, Walker A, Ross S. . Relapse after emergency department discharge for acute asthma.. Acad Emerg Med. 2008;15(8):709‐17. [DOI] [PubMed] [Google Scholar]
Rowe 2010
- Rowe BH, Villa‐Roel C, Abu‐Laban RB, Stenstrom R, Mackey D, Stiell IG, et al. Admissions to Canadian hospitals for acute asthma: a prospective, multicentre study. Canadian Respiratory Journal 2010;17:25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiffany 1993
- Tiffany BR, Berk WA, Todd IK, White SR. Magnesium bolus or infusion fails to improve expiratory flow in acute asthma exacerbations. Chest 1993;104(3):831‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Edmonds 2002
- Edmonds ML, Camargo CA Jr, Pollack CV, Rowe BH. The effectiveness of inhaled corticosteroids in the emergency department treatment of acute asthma: a meta‐analysis. Annals of Emergency Medicine 2002;40(2):145‐54. [DOI] [PubMed] [Google Scholar]
Edmonds 2003
- Edmonds M, Camargo CA Jr, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD002308] [DOI] [PubMed] [Google Scholar]


