Abstract
Background
This is an updated version of the original Cochrane Review published in the Cochrane Library 2013, Issue 9. Despite good evidence for the health benefits of regular exercise for people living with or beyond cancer, understanding how to promote sustainable exercise behaviour change in sedentary cancer survivors, particularly over the long term, is not as well understood. A large majority of people living with or recovering from cancer do not meet current exercise recommendations. Hence, reviewing the evidence on how to promote and sustain exercise behaviour is important for understanding the most effective strategies to ensure benefit in the patient population and identify research gaps.
Objectives
To assess the effects of interventions designed to promote exercise behaviour in sedentary people living with and beyond cancer and to address the following secondary questions: Which interventions are most effective in improving aerobic fitness and skeletal muscle strength and endurance? Which interventions are most effective in improving exercise behaviour amongst patients with different cancers? Which interventions are most likely to promote long‐term (12 months or longer) exercise behaviour? What frequency of contact with exercise professionals and/or healthcare professionals is associated with increased exercise behaviour? What theoretical basis is most often associated with better behavioural outcomes? What behaviour change techniques (BCTs) are most often associated with increased exercise behaviour? What adverse effects are attributed to different exercise interventions?
Search methods
We used standard methodological procedures expected by Cochrane. We updated our 2013 Cochrane systematic review by updating the searches of the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, Embase, AMED, CINAHL, PsycLIT/PsycINFO, SportDiscus and PEDro up to May 2018. We also searched the grey literature, trial registries, wrote to leading experts in the field and searched reference lists of included studies and other related recent systematic reviews.
Selection criteria
We included only randomised controlled trials (RCTs) that compared an exercise intervention with usual care or 'waiting list' control in sedentary people over the age of 18 with a homogenous primary cancer diagnosis.
Data collection and analysis
In the update, review authors independently screened all titles and abstracts to identify studies that might meet the inclusion criteria, or that could not be safely excluded without assessment of the full text (e.g. when no abstract is available). We extracted data from all eligible papers with at least two members of the author team working independently (RT, LS and RG). We coded BCTs according to the CALO‐RE taxonomy. Risk of bias was assessed using the Cochrane's tool for assessing risk of bias. When possible, and if appropriate, we performed a fixed‐effect meta‐analysis of study outcomes. If statistical heterogeneity was noted, a meta‐analysis was performed using a random‐effects model. For continuous outcomes (e.g. cardiorespiratory fitness), we extracted the final value, the standard deviation (SD) of the outcome of interest and the number of participants assessed at follow‐up in each treatment arm, to estimate the standardised mean difference (SMD) between treatment arms. SMD was used, as investigators used heterogeneous methods to assess individual outcomes. If a meta‐analysis was not possible or was not appropriate, we narratively synthesised studies. The quality of the evidence was assessed using the GRADE approach with the GRADE profiler.
Main results
We included 23 studies in this review, involving a total of 1372 participants (an addition of 10 studies, 724 participants from the original review); 227 full texts were screened in the update and 377 full texts were screened in the original review leaving 35 publications from a total of 23 unique studies included in the review. We planned to include all cancers, but only studies involving breast, prostate, colorectal and lung cancer met the inclusion criteria. Thirteen studies incorporated a target level of exercise that could meet current recommendations for moderate‐intensity aerobic exercise (i.e.150 minutes per week); or resistance exercise (i.e. strength training exercises at least two days per week).
Adherence to exercise interventions, which is crucial for understanding treatment dose, is still reported inconsistently. Eight studies reported intervention adherence of 75% or greater to an exercise prescription that met current guidelines. These studies all included a component of supervision: in our analysis of BCTs we designated these studies as 'Tier 1 trials'. Six studies reported intervention adherence of 75% or greater to an aerobic exercise goal that was less than the current guideline recommendations: in our analysis of BCTs we designated these studies as 'Tier 2 trials.' A hierarchy of BCTs was developed for Tier 1 and Tier 2 trials, with programme goal setting, setting of graded tasks and instruction of how to perform behaviour being amongst the most frequent BCTs. Despite the uncertainty surrounding adherence in some of the included studies, interventions resulted in improvements in aerobic exercise tolerance at eight to 12 weeks (SMD 0.54, 95% CI 0.37 to 0.70; 604 participants, 10 studies; low‐quality evidence) versus usual care. At six months, aerobic exercise tolerance was also improved (SMD 0.56, 95% CI 0.39 to 0.72; 591 participants; 7 studies; low‐quality evidence).
Authors' conclusions
Since the last version of this review, none of the new relevant studies have provided additional information to change the conclusions. We have found some improved understanding of how to encourage previously inactive cancer survivors to achieve international physical activity guidelines. Goal setting, setting of graded tasks and instruction of how to perform behaviour, feature in interventions that meet recommendations targets and report adherence of 75% or more. However, long‐term follow‐up data are still limited, and the majority of studies are in white women with breast cancer. There are still a considerable number of published studies with numerous and varied issues related to high risk of bias and poor reporting standards. Additionally, the meta‐analyses were often graded as consisting of low‐ to very low‐certainty evidence. A very small number of serious adverse effects were reported amongst the studies, providing reassurance exercise is safe for this population.
Keywords: Female, Humans, Male, Cancer Survivors, Exercise, Habits, Sedentary Behavior, Breast Neoplasms, Breast Neoplasms/rehabilitation, Colorectal Neoplasms, Colorectal Neoplasms/rehabilitation, Exercise Tolerance, Exercise Tolerance/physiology, Health Promotion, Muscle Strength, Neoplasms, Neoplasms/rehabilitation, Patient Compliance, Patient Compliance/statistics & numerical data, Prostatic Neoplasms, Prostatic Neoplasms/rehabilitation, Randomized Controlled Trials as Topic, Time Factors
Plain language summary
Interventions for promoting habitual exercise in people living with and beyond cancer
The issue Being regularly active can bring a range of health benefits for people living with and beyond cancer, including improved quality of life and physical function. Being physically active might also reduce the risk of cancer recurrence and of dying from cancer. Because most cancer survivors are not regularly physically active, there is a need to understand how best to promote and sustain physical activity in this population.
The aim of the review To understand what are the most effective ways to improve and sustain exercise behaviour in people living with and beyond cancer.
Study characteristics We included only studies that compared an exercise intervention with a usual care comparison or 'waiting list' control. Only studies that included sedentary people over the age of 18 with the same cancer diagnosis were eligible. Participants must have been allocated to exercise or usual care at random. We searched for evidence from research databases from 1946 to May 2018.
What are the main findings? We included 23 studies involving 1372 participants in total. Evidence suggests that exercise studies that incorporate an element of supervision can help cancer survivors. However, we still have a poor understanding of how to promote exercise long term (over six months). There is some concern that research is not being reported as clearly as it should be. We found that setting goals, graded physical activity tasks and providing instructions on how to perform the exercises could help people to do beneficial amounts of exercise. In addition, we found some evidence that in people who do meet recommended exercise levels, get fitter for up to six months.
Quality of the evidence The main problems that we found regarding the quality of studies in this review included: not knowing how study investigators conducted randomisation for the trials and not knowing whether investigators who were doing trial assessments knew to which group the person they were assessing had been randomly assigned. The quality of the evidence from these studies was found to be low due to the majority of the trials often containing a low number of participants.
What are the conclusions? The main conclusions from this review are that exercise is generally safe for cancer survivors. We have a better understanding of how to encourage cancer survivors to meet current exercise recommendations. However, there is still a lack of evidence of how to encourage exercise in cancer survivors over six months.
Summary of findings
Summary of findings for the main comparison. Exercise interventions compared to usual care for promoting habitual exercise in people living with and beyond cancer to improve aerobic exercise tolerance.
| Exercise interventions compared to usual care for promoting habitual exercise in people living with and beyond cancer to improve aerobic exercise tolerance | ||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Anticipated absolute effects* (95% CI) | |
| Risk with usual care | Risk difference with exercise interventions | |||
| Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) | 604 (10 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | The mean aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) was 0 | SMD 0.54 higher (0.37 higher to 0.70 higher) |
| Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) | 201 (4 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | The mean aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) was 0 | SMD 0.85 higher (0.56 higher to 1.14 higher) |
| Aerobic exercise tolerance (all cancers: 6 months) | 591 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | The mean aerobic exercise tolerance (all cancers: 6 months) was 0 | SMD 0.56 higher (0.39 higher to 0.72 higher) |
| Aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up) | 441 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 | The mean aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up) was 0 | SMD 0.57 higher (0.22 higher to 0.93 higher) |
| Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up) | 357 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | The mean aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 0.53 higher (0.01 higher to 1.04 higher) |
| Aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up) | 155 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | The mean aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 0.70 higher (0.37 higher to 1.03 higher) |
| Aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up) | 92 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 5 | The mean aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 1.07 higher (0.26 higher to 1.89 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SMD: standarised mean difference | ||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate‐certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low‐certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low‐certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1 Some concerns with high number of participants lost to follow‐up, selective reporting of data and other risks of bias 2 Concerns over number of small studies included with positive results 3 Low number of participants in the studies overall and large confidence intervals 4 Some concerns over variations in effect sizes, the test for heterogeneity is significant and I2 value is high (> 50) 5 Some concerns over the variations in effect sizes.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2013, Issue 9) Bourke 2013.
Description of the condition
Cancer is a major public health issue. In 2015, there were 17.5 million cases of cancer globally, 8.7 million deaths and the disease is estimated to be responsible for 208 million disability adjusted life years (Global Burden of Disease Cancer Collaboration 2017). Age‐standardised cancer mortality rates are decreasing (in the Western hemisphere), which is encouraging progress (Hashim 2016). However, although increasing numbers of cancer survivors live longer, this does not equate to living well. Survivors face a multitude of unique, debilitating health problems, even after treatment with curative intent. These range from an increased risk of recurrent cancers (Low 2014), persistent symptoms such as fatigue (Low 2014), ongoing poor health and well‐being (Elliott 2011), and mental health comorbidity (Nakash 2014). The burden of these problems can lead to negative impacts on health‐related quality of life (HRQoL) (Corner 2013). Throughout this review, the term we define as 'cancer survivor' is synonymous with someone 'living with and beyond cancer', in accordance with the Macmillan Cancer Support definition (Macmillan Cancer Support 2011).
Description of the intervention
The goal of any exercise intervention is to offer a sustained physiological challenge that, over time, will induce a spectrum of beneficial cardiovascular, respiratory, musculoskeletal, neurological, metabolic adaptations as well as bringing a host of psychosocial benefits. In the context of living with and beyond cancer, such adaptations underpin improvements in cancer‐related fatigue, HRQoL and physical function (Mishra 2014; Stout 2017). The UK Chief Medical Officer recommends that in adults, weekly activity should add up to at least 150 minutes of moderate intensity aerobic activity, performed in bouts of 10 minutes or longer (Department of Health 2011), with similar international recommendations for cancer survivors (Rock 2012). For example, this could translate to 30 minutes of aerobic activity that raises heart rate and breathing rate, five times per week. Alternatively, 75 minutes of vigorous intensity aerobic activity spread across the week has been suggested to confer similar benefit (Schmitz 2010a).
We have deliberately chosen the term 'habitual' over 'regular' to reflect the intention to assess which interventions could both A) improve and B) sustain exercise behaviour. 'Regular exercise' can be applied to both short‐term and long‐term contexts, where as a 'habitual' exerciser indicates a sustained and regular pattern of behaviour. Whilst 'habitual' refers to the process of behavioural 'habit forming' and an automaticity of behaviour (Gardner 2011; Verplanken 2009), we recognise there are other theoretical principals underpinning physical activity behaviour (Kwasnica 2016).
How the intervention might work
Encouraging people to participate in regular exercise from a background of an inactive lifestyle is difficult, requiring attention to important psychosocial and behavioural influences (Kampshoff 2014; Ormel 2017). A major challenge is to provide a support structure for physical activity until it becomes a pattern of sustained healthy behaviour. Randomised controlled trials (RCTs) in cancer survivors have assessed a number of exercise interventions, with the aim of promoting short‐ and long‐term habitual exercise. A wide range of approaches have been investigated; including supervised exercise and home‐based exercise (Bourke 2014), and inclusive of group counselling sessions, (Rogers 2015). Tailored exercise interventions commonly comprise aerobic exercise training, strength training or a combination of both, with or without behaviour change support. Behaviour change theory within exercise interventions is often viewed as essential, with the UK Medical Research Council (MRC) recommending the use of theory in intervention development for complex interventions to help improve behaviour change (Craig 2008). However, the application of behaviour change theory or specific behaviour change techniques is often generally poor, unclear and not clearly examined for impact of effectiveness.
Why it is important to do this review
The majority of people living with and beyond cancer are not regularly active, with estimates ranging from less than 10% to 20% to 30% of cancer survivors meeting the physical activity guidelines (Garcia 2014). There are a number of important beneficial effects of exercise participation in cancer survivors reported from RCTs including improved HRQoL, reduced fatigue and improved physical function, (Bourke 2014; Dittus 2017; Meneses‐Echavez 2015; Mishra 2012a; Mishra 2012b; Stout 2017). However, the original review (Bourke 2013) found that most of the current evidence comes from studies with short‐term interventions and follow‐up. Understanding which interventions are most efficacious in supporting the maintenance of long‐term exercise behaviour is critical not just because of the HRQoL benefits (Bourke 2012a), but multiple observational reports link being regularly active to reduced chances of dying from cancer after diagnosis (Li 2016).
The original review showed that there is a poor understanding of how to encourage people living with and beyond cancer to meet current exercise recommendations (Bourke 2013). Poor study reporting standards was a pervasive issue e.g. failure to report adherence data. However, there were some useful data regarding the use of behaviour change techniques (BCTs). An updated review can firstly, offer insight as to whether interventions being tested in contemporary studies are mapping to the existing international recommendations i.e. the American Cancer Society (ACS) guidance (i.e. provided by Rock 2012). Secondly, this will allow us to evaluate if there have been any improvements in the quality of intervention reporting around specifics of set prescriptions (i.e. frequency, intensity, duration etc). Thirdly, and critically, we can use a larger data set from our updated searches to assess if both the quality of reporting of exercise adherence has improved and if there is more to learn about how to promote and sustain better adherence to exercise behaviour interventions in previously inactive cancer survivors.
In the UK, the Independent Cancer Taskforce strategy document sets out a number of initiatives to achieve world class outcomes in cancer; ensuring survivors have the best possible quality of life and improving rates of mortality. Promoting habitual exercise participation could help to accomplish these high priority agendas within the UK.
Objectives
Primary objective
To assess the effects of interventions designed to promote exercise behaviour in sedentary people living with and beyond cancer.
Secondary objectives
To address the following questions.
Which interventions are most effective in improving aerobic fitness and skeletal muscle strength and endurance?
What adverse effects are attributed to different exercise interventions?
Which interventions are most effective in improving exercise behaviour amongst patients with different cancers?
Which interventions are most likely to promote long‐term (12 months or longer) exercise behaviour?
What frequency of contact with exercise professionals and/or healthcare professionals is associated with increased exercise behaviour?
What theoretical basis is most often associated with increased exercise behaviour?
What behaviour change techniques are most often associated with increased exercise behaviour?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that allocated participants or clusters of participants by a random method to an exercise‐promoting intervention compared with usual care or 'waiting list' control. We included studies conducted both during and after primary treatment or during active monitoring. Only interventions that included a component targeted at increasing aerobic exercise and/or resistance exercise behaviour were included in this review. We did not include studies of heterogeneous cancer cohorts (i.e. participants with different primary cancer sites). We did not include studies in 'at risk' populations (i.e. studies involving individuals who have risk factors for cancer but who have not yet been diagnosed with the disease) that addressed primary prevention research questions.
Types of participants
We included only studies involving adults (18 years of age or older) who had a sedentary lifestyle or physically inactive at baseline (i.e. not undertaking 30 minutes or more of exercise of at least moderate intensity, three days per week, or 90 minutes in total of moderate intensity exercise per week). Participants must have been histologically or clinically diagnosed with cancer regardless of sex, tumour site, tumour type, tumour stage and type of anticancer treatment received. We excluded studies directed specifically at end‐of‐life‐care patients and individuals who were currently hospital inpatients.
Types of interventions
For the purposes of this review, the phrases 'exercise' and 'physical activity' were used interchangeably. Definitions of exercise, related terms and nomenclature that describe the performance of exercise must adhere to principles of science and must satisfy the Système International d'Unités (SI), which was adopted universally in 1960. Hence, we referred to the appropriate, combined definition that applies to all situations: 'A potential disruption to homeostasis by muscle activity that is either exclusively or in combination, concentric, eccentric or isometric' (Winter 2009). Investigators must have reported the frequency, duration and intensity of aerobic exercise behaviour or frequency, intensity, type, sets and repetitions of resistance exercise behaviour that was prescribed in the intervention.
We acknowledge that the maximal aerobic capacity (V̇O2max)/peak is often the most informative metric for setting aerobic exercise intensity; however, given the nature of the population involved (elderly, potentially with multiple comorbidities), it is often difficult to conduct maximal testing protocols to prescribe intensity on the basis of this measures because of the requirements for medically qualified staff to be present during assessment. As such, for reasons of pragmatism, we accepted that exercise intensity is more frequently reported in cancer the cohorts in terms of age‐predicted maximum heart rate(HRmax) or Borg Rating of Perceived Exertion (RPE) (Borg 1982). The interventions in this review were categorised as achieving a mild (less than 60% HRmax/10 RPE or less), moderate (60% to 84% HRmax/11 to 14 RPE) or vigorous (85% HRmax or more/15 RPE or more) exercise intensity.
Types of outcome measures
Primary outcomes
Aerobic exercise behaviour as measured by:
exercise frequency (number of bouts per week);
exercise duration (total minutes of exercise achieved);
exercise intensity (e.g. % HRmax, RPE);
estimated energy expenditure from free‐living physical activity (e.g. from accelerometer readings (where available));
adherence to the exercise intervention (% of exercise sessions completed/attended); total duration of intervention when ≥75% adherence is achieved (in weeks);
total duration of sustained exercise behaviour meeting American Cancer Society guidelines for exercise in people living with and beyond cancer (Rock 2012; i.e. aim to exercise at least 150 minutes per week, with at least two days per week of strength training).
Resistance exercise behaviour as measured by:
exercise frequency (number of bouts per week);
exercise intensity (e.g. % of 1 repetition max or % of body mass);
type of exercise (e.g. free weights, body weight exercise);
repetitions;
sets.
Secondary outcomes
Change in aerobic fitness or exercise tolerance (maximal or submaximal when measured directly or by a standard field test).
Change in skeletal muscle strength and endurance.
Adverse effects.
study recruitment rate.
Intervention attrition rate.
Interventions were judged as successful in achieving exercise goals if investigators reported at least 75% adherence over a given follow‐up period as done in the original review (Bourke 2013). Data on compliance with the intervention were quantified in terms of number of prescribed exercise sessions completed as a proportion of the total set. The intervention must have included at least six weeks of follow‐up. Interventions were described according to whether they reported being based on a behaviour change theory e.g. control theory, social cognitive theory; (Bandura 2000; Bandura 2002; Carver 1982. This relates to the National Institute for Health and Clinical Excellence (NICE) guidance for behaviour change, which recommends that clinicians should be explicit about the theoretical constructs on which interventions are based (NICE 2007). Interventions were also coded using the ‘Coventry, Aberdeen & London-Refined’ (CALO‐RE) taxonomy (Michie 2011). This is a validated taxonomy of behaviour change techniques (BCTs) that can be used to help people change their exercise behaviour. Coding interventions according to this taxonomy allows for a better understanding of which techniques are employed by current interventions and how they are related to short‐ and longer‐term exercise behaviour change.
Search methods for identification of studies
Electronic searches
The searches were run for the original review from inception to August 2012. The subsequent searches from the following electronic databases were run from August 2012 up to 3 May 2018. We carried out the following searches:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 5) in The Cochrane Library;
MEDLINE via OVID August 2012 to April week 4 2018;
Embase via OVID August 2012 to 2018 week 18;
AMED (Allied and Alternative Medicine Database; covers occupational therapy, physiotherapy and complementary medicine) August 2012 to May 2018;
CINAHL (Cumulative Index to Nursing and Allied Health Literature) August 2012 to May 2018;
PsycINFO (Database of the American Psychological Association) August 2012 to May 2018;
SportDiscus (Sports Evidence Database) August 2012 to April 2017;
PEDro (Physiotherapy Evidence Database) August 2012 to April 2017.
The search strategies are presented in the Appendices, with both the 2018 updated strategy and previous 2012 strategy reported. CENTRAL search strategy is presented in Appendix 1 and the MEDLINE search strategy in Appendix 2. For databases other than MEDLINE, we adapted the search strategy accordingly: Embase (Appendix 3), AMED (Appendix 4), CINAHL (Appendix 5) PsycINFO (Appendix 6) PEDro (Appendix 7) SportsDiscus (Appendix 8).
The search strategies were developed with the Cochrane Gynaecological Cancer Group Information Specialist and included MeSH and text word terms as appropriate.
Searching other resources
We used snowballing, by searching reference lists of retrieved articles and published reviews on the topic.
We expanded the database search by identifying additional relevant studies for this review, including unpublished studies and references in the grey literature. This was done by searching the OpenGrey database (www.opengrey.eu/), which includes technical or research reports, doctoral dissertations, conference papers and other types of grey literature. We also searched the following clinical trials web pages.
World Health Organisation apps.who.int/trialsearch/Default.aspx
National cancer institute www.cancer.gov/about‐cancer/treatment/clinical‐trials/search
Furthermore, we wrote to Cancer Research UK (CRUK), Macmillan Cancer Support, the World Cancer Research Fund (WCRF), Worldwide Cancer Research , the American Association for Cancer Research (AACR), the American Cancer Society (ACS) and the American Society of Clinical Oncology (ASCO) to enquire about relevant unpublished papers.
Data collection and analysis
Since publication of the previous version of this review, we have included the use of the GRADE assessment to assess the quality of the evidence and produced a Table 1.
Selection of studies
We imported results from each database into the reference management software package Endnote, from which we removed duplicates. After training on the first 100 references retrieved from two different databases to ensure a consistent approach, two review authors (RT and HQ) worked independently to screen all titles and abstracts to identify studies that met the inclusion criteria, or that could not be safely excluded without assessment of the full text (e.g. when no abstract was available). Disagreements were resolved by discussion with another review author (LB). Full texts were retrieved for these articles.
After training was provided to ensure a consistent approach to study assessment and data abstraction, two review authors worked independently to assess the retrieved full texts (RT and HQ). We linked together multiple publications and reports on the same study. Studies that appeared to be relevant but were excluded at this stage are listed in the 'Characteristics of excluded studies' table. We resolved disagreements by discussion with other group members. We attempted to contact study corresponding authors if we could not access a full text (e.g. if only an abstract was available), if we required more information to determine whether a study could be included (e.g. to determine baseline exercise behaviour of a cohort), or if we required supplementary information about an already eligible study (please also see Excluded studies).
Data extraction and management
Review authors (RT and LB) extracted the following data using the same data extraction form used in the original review and entered data into RevMan 5.3 (Review Manager 2014).
Study details: author; year; title; journal; research question/study aim; country where the research was carried out; funding source; recruitment source (e.g. consecutive sampling from outpatient appointments; advertising in the community; convenient sample from support groups); inclusion and exclusion criteria; study design (cluster RCT, non‐cluster RCT, single centre or multi‐centre); sample size; number of participants per arm; length of follow‐up; description of usual care.
Intervention details: categorisation of intervention (e.g. supervised, independent, educational); setting (e.g. dedicated exercise facility, community, home); exercise prescription components (e.g. aerobic exercise, resistance exercise, stretching); theoretical basis, behaviour change techniques (using CALO‐RE taxonomy), frequency of contact with an exercise professional and or healthcare professional; instructions to controls.
Participant characteristics: primary cancer diagnosis; any cancer treatment currently undertaken; metastatic disease status; age; sex; body mass index (BMI); ethnicity; reported comorbidities.
Resulting exercise behaviour: method of measuring exercise (e.g. self‐report questionnaire). Numbers of participants randomly assigned and assessed at specified follow‐up points. Frequency, duration, intensity of aerobic exercise achieved; frequency, intensity, type, sets and repetitions of resistance exercise achieved; total duration of the intervention; total duration of sustained meaningful exercise behaviour as a result of the intervention and whether the Rock 2012 guidelines were met, adherence to the intervention; rate of attrition and adverse effects reported.
Resulting change in other outcomes: changes in aerobic fitness and estimated energy expenditure from free‐living physical activity.
Three members of the group worked independently (RT, RG and LS) to extract data from all eligible papers using the data collection form. Data were entered into the Cochrane's statistical software, Review Manager 2014, by one review author and checked by a second review author.
Assessment of risk of bias in included studies
Risk of bias and methodological quality were assessed in accordance with Cochrane's tool for assessing risk of bias (Higgins 2011). The tool includes the following seven domains:
sequence generation (method of randomisation);
allocation concealment (selection bias);
blinding (masking) of participants and personnel (detections bias);
blinding (masking) of outcome assessors (detection bias);
incomplete outcome data;
selective outcome reporting;
other sources of bias.
However, we did not include blinding to group allocation, as it is not possible (e.g. in a supervised exercise setting) to blind participants to an intervention while promoting exercise behaviour. Two review authors (RT and RG) independently applied the 'Risk of bias' tool, and differences were resolved by discussion with a third review author (LB). We summarised results in both a 'Risk of bias' graph and a 'Risk of bias' summary. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias. We contacted study authors to ask for additional information or for further clarification of study methods if any doubt surrounded potential sources of bias. Individual 'Risk of bias' items can be seen in Appendix 9.
Measures of treatment effect
For the purposes of this review, all exercise behaviour was synthesised as specified in the primary outcomes. For comparison of measures of change in fitness levels or estimated energy expenditure from free‐living physical activity, please see the section on 'Continuous data' in Data synthesis.
Unit of analysis issues
We did not include any cross‐over trials in this review because of the high risk of contamination. It can be very difficult to “wash out” exercise behaviour. Cancer survivors in particular can be a highly‐motivated cohort, and significant contamination has been reported even in conventional RCT settings (Courneya 2003; Mock 2005). Hence this learning effect distorts results. Furthermore, asking individuals to revert to sedentary behaviour could be considered unethical (Das 2012). Therefore, any cross‐over trials identified were rejected at the title and abstract screening stage.
Dealing with missing data
We assessed missing data and dropout rates for each of the included studies and reported the numbers of participants included in the final analysis as a proportion of all participants included in the study. We assessed the extent to which studies conformed to an intention‐to‐treat analysis.
Assessment of heterogeneity
Consistency of results was assessed visually and through examination of the I2 statistic, a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error.An I2 greater than or equal to 50% was considered significant heterogeneity. We addressed this by performing a sensitivity analysis that excluded any heterogeneous trials. We supplemented this with a test of homogeneity to determine the strength of evidence that the heterogeneity was genuine. When significant statistical heterogeneity was detected, differences in characteristics of the studies or other factors were explored as possible sources of explanation. Any differences were summarised in a narrative synthesis.
Assessment of reporting biases
Publication bias
We intended to examine funnel plots corresponding to meta‐analysis of the primary outcomes to assess the potential for small study effects such as publication bias if a sufficient number of studies (i.e. more than 10) was identified. However, this was not the case; therefore this step was not included in the analysis.
Data synthesis
Continuous data
For continuous outcomes (e.g. cardiorespiratory fitness), we extracted the final value, the standard deviation (SD) of the outcome of interest and the number of participants assessed at endpoint for each treatment arm at the end of follow‐up, to estimate standardised mean differences (SMD) between treatment arms.
Dichotomous outcomes
For dichotomous outcomes (e.g. adverse effects, deaths), if it was not possible to use a hazard ratio (HR), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, to estimate a risk ratio (RR).
Meta‐analysis
When possible, and if appropriate, we performed a meta‐analysis of review outcomes. If statistical heterogeneity was noted, a meta‐analysis was performed using a random‐effects model. We planned to use a fixed‐effect model if no significant statistical heterogeneity was observed.
When possible, all data extracted were those relevant to an intention‐to‐treat analysis in which participants were analysed in groups to which they were assigned. We noted the time points at which outcomes were collected and reported.
Subgroup analysis and investigation of heterogeneity
If a sufficient number of studies were identified, we performed subgroup analyses for the following.
Cancer site.
Type of intervention (i.e. supervised, home‐based, etc).
Age of individuals (i.e. elderly versus non‐elderly).
Current treatment (currently undergoing treatment versus not currently undergoing treatment).
Participants with metastatic disease (metastatic cohort versus non‐metastatic cohort).
Accordance with behaviour change theory.
Interventions in obese individuals (mean body mass index (BMI) of intervention group > 30 kg/m2 versus mean BMI of intervention group < 30 kg/m2).
Sensitivity analysis
Methodological strength was judged using Cochrane's tool for assessing risk of bias to identify studies of high and low quality (Higgins 2011). Sensitivity analyses were performed with the studies of low quality excluded.
Summary of findings
To assess the overall quality of the evidence for each outcome of the meta‐analysis, we employed the GRADE approach. The GRADE profile (https://gradepro.org) enabled us to import data directly from Review Manager 5.3 to create Table 1. These tables provide outcome‐specific information concerning the overall certainty of the evidence from studies included in the meta‐analysis. Risk of bias, inconsistency of the data, the preciseness of the data publication bias and the indirectness of the data were all considered in assessing the quality of the data.
We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The following outcomes were included in the 'Summary of findings' table.
Aerobic exercise tolerance (all cancers: eight to 12 weeks of follow‐up)
Aerobic exercise tolerance (all cancers: eight to 12 weeks of follow‐up sensitivity analysis)
Aerobic exercise tolerance (all cancers: six months of follow‐up)
Aerobic exercise tolerance (breast cancer: eight to 12 weeks of follow‐up)
Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: eight to 12 weeks of follow‐up)
Aerobic exercise tolerance (all cancers: supervised exercise: eight to 12 weeks of follow‐up)
Aerobic exercise tolerance (all cancers: home‐based exercise: eight to 12 weeks of follow‐up)
Results
Description of studies
Please see Table 2, 'Summary of included studies'. See 'Characteristics of included studies'; 'Characteristics of excluded studies'; 'Characteristics of studies awaiting classification'; and 'Characteristics of ongoing studies'.
1. Summary of included studies.
| Study | Exercise components | n | Meets Rock et al guidelines? | Adherence summary | At least 75% adherence? | High risk of bias? | Change in AET reported? | Adverse effects |
| Cadmus 2009 | Aerobic | 37, 38 (intervention vs control) | 33% reported 150 minutes/week of moderate intensity aerobic exercise at an average of 76% HR, for six months | 75% of women were doing between 90 and 119 minutes of moderate intensity aerobic activity per week at six months | Yes; for up to 119 minutes per week | No | Not reported | Five of the 37 women randomly assigned to exercise experienced an adverse effect; two were related to the study (plantar fasciitis) |
| Daley 2007a | Aerobic | 34, 36, 38 (intervention, sham, control, respectively) |
No | 77% of the exercise therapy; attended 70% (at least 17 of 24 sessions) or more of sessions | Unclear | Yes; outcome assessors were not blinded to participants’ group allocation | Yes | Three withdrawals in the intervention group: unclear as to why this occurred. Some withdrawals because of medical complications in placebo and control arms but unclear whether study related |
| Drouin 2005 | Aerobic | 13 intervention, 8 placebo stretching controls | Unclear | Participants in the intervention group averaged 3.6 days per week of aerobic exercise over an 8‐week period | Unclear | No | Yes | None reported |
| Kaltsatou 2011 | Aerobic | 14, 13 (intervention vs control) | Unclear | Not reported | Not reported | Yes; method of measuring exercise and adherence not reported | Not reported | None reported |
| Kim 2006 | Aerobic | 22,19 (intervention vs control). | No | Average weekly frequency of exercise was 2.4 ± 0.6 sessions, and average duration of exercise within prescribed target HR was 27.8 ± 8.1 minutes per session. Overall adherence was 78.3% ± 20.1% | Yes | Yes; data missing for 45% of the cohort | Yes | Reasons for withdrawal included personal problems (n = 2), problems at home (n = 2), problems related to chemotherapy (n = 3), thrombophlebitis in the lower leg (n = 2), non-exercise‐related injuries (n = 1), and death (n = 1). Unclear to which arm of the study these date relate |
| Pinto 2003 | Aerobic | 12, 12 (intervention vs control) |
Unclear | Participants attended a mean of 88% of the 36‐session supervised exercise programme | Yes | Yes; 38% lost to follow‐up. Exercise tolerance test was performed but no control group comparison data were reported | Yes | None reported; however, it is unclear why the six controls dropped out |
| Pinto 2005 | Aerobic | 43, 43 (intervention vs control) | Unclear | At week 12, intervention participants reported a mean of 128.53 minutes/week of moderate intensity exercise. However, no changes were reported in the accelerometer data in the intervention group (change score = ‐0.33 kcal/hour) | Less than 75% of the intervention group was meeting the prescribed goal after week 4 | Yes; significantly more control group participants were receiving hormone treatment. Accelerometer data do not support the self‐reported physical activity behaviour | Yes | Not clear whether chest pain was related to exercise in dropout whose participation was terminated |
| Pinto 2011 | Aerobic | 20, 26 (intervention vs control) | Three‐day PAR questionnaire indicates that 64.7% of the intervention group and 40.9% of the control group were achieving the guidelines at three months | Correlation between self‐reported moderate intensity exercise and accelerometer data at three‐month follow‐up, when the only significant between‐group change is reported: r = 0.32 | No | Yes; accelerometer data were not reported; also, cited correlation is weak (0.32). Further, substantial contamination was noted in the control group | Yes | One cancer recurrence in the control group at three months |
| Bourke 2011a | Aerobic and resistance | 9, 9 (intervention vs control) | Six weeks of resistance exercise twice a week | 90% attendance at the supervised sessions. 94% of independent exercise sessions were completed | Yes | No | Yes | One stroke in the intervention group, unrelated to the exercise programme |
| Hayes 2009 | Aerobic and resistance | 16, 16 (intervention vs control) | Unclear | Most women (88%) allocated to the intervention group participated in 70% or more of scheduled supervised exercise sessions | Unclear | Yes; adherence data on unsupervised aspect of the intervention are not clear | No | None reported |
| McKenzie 2003 | Aerobic and resistance | 7,7 (intervention vs control) | No | Unclear | Unclear | Yes; adherence to exercise not reported | Not reported | None reported |
| Musanti 2012 | Aerobic and resistance | Flexibility group (n = 13), aerobic group (n = 12), resistance group (n = 17), aerobic and resistance group (n = 13) | 12 weeks of resistance exercise two or three times per week | Mean percentages of adherence were as follows: flexibility = 85%, aerobic = 81%, resistance = 91% and aerobic plus resistance = 86% | Unclear | Yes; a significant number of dropouts belonged to the resistance exercise group (n = 8/13). Only 50% of activity logs were returned | Yes | Adverse effects were reported in two women during the study. In both cases, the women developed tendonitis: one in the shoulder and the other in the foot. Both had a history of tendonitis, and both received standard treatment |
| Perna 2010 | Aerobic and resistance | 51 participants in total. Numbers randomly assigned to each arm are unclear | Three months of resistance exercise three times per week | Women assigned to the structured intervention completed an average of 83% of their scheduled hospital‐based exercise sessions (only 4 weeks in duration), and 76.9% completed all 12 sessions. Home‐based component (8 weeks in duration) | Unclear | Yes; numbers randomly assigned to intervention and control groups are unclear, as are numbers completing in each arm | Not reported | Unclear |
| al‐Majid 2015 | Aerobic | 7,7 (intervention vs control) | No | Adherence to per‐protocol exercise sessions was very high, ranging between 95% and 97%. | Yes | No | Yes | None reported |
| Bourke 2014 | Aerobic and resistance | 25,25 (intervention vs control) | Yes; 6 weeks of resistance exercise | Adherence was 94% for the supervised and 82% of the prescribed independent exercise sessions over the first 12 week. | Yes | Yes incomplete outcome data at 6 months. | Yes | None reported |
| Campbell 2017 | Aerobic | 10 in exercise intervention, 9 in delayed exercise control | 150 minutess per week of moderate‐vigorous aerobic exercise for 24 weeks. | Participants attended 88% of supervised gym sessions (mean 1.8 sessions/ week and 87.5 minutes/week), and participants met 82% of the prescribed exercise targets (mean intensity 74.5% HRR). Home session completion was 87% (mean 2.4 sessions/week and 101.5 minutes/week), and participants met 87% of the prescribed exercise targets (mean intensity 73.5% HRR) | Yes | Yes; Low study recruitment rate. | Yes | None reported |
| Cantarero‐Villanueva 2012b | Aerobic | 33,33 (intervention vs control) | Three sixty minute sessions per week for 8 weeks. | All intervention group completed more than 85% of the 24 water exercise sessions, showing a high adherence rate to the program. | Yes | No | Not reported | One participant in the intervention dropped out due to a recurrence of breast cancer during the program. Three women reported a transient increase of oedema, and four women noted an increase in fatigue immediately after the beginning of the first session, which improved in the next few days. These women did not dropout of the study. No other adverse effects were reported. |
| Cavalheri 2017 | Aerobic and resistance | 9, 8 (intervention vs control) | Yes; six weeks of resistance exercise. | Nine of the participants randomised to the EG, four (44%) adhered to exercise training by completing 15 or more training sessions (i.e.,≥60%). | No | Yes; missing patient data in both arms with no reasons given. | Yes | One participant completed four sessions and another completed six sessions. Both stopped training as they felt unwell. They completed some of the post‐intervention assessments and were later diagnosed with a primary cancer other than lung cancer. |
| Kim 2017 | Aerobic and resistance | 15, 15 (intervention vs control) | Three sixty minute sessions per week for 12 weeks. | Vague statement: Two participants did not fulfil the required exercise | Unclear | Yes; Age differences between groups in baseline demographics were present. Adherence data is vague. | Not reported | None reported |
| Mohamady 2017 | Aerobic | 15, 15 (intervention vs control) | No | Unclear | Unclear | Yes; No adherence data. | Unclear | Unclear |
| Rogers 2015 | Aerobic | 110, 112 (intervention vs control) | Yes | Adherence to the intervention was 98 % for supervised exercise sessions, 96 % for update sessions, and 91 % for discussion group sessions. | Yes | Yes; differences in objective and subjective measures of physical activity reported | Yes | Related expected adverse events in the intervention group included back or lower extremity musculoskeletal pain or injury (n = 14), heart rate monitor rash (n = 1), fall while walking (n = 1), breast reconstruction (n = 3), and chest pain during treadmill fitness test (n = 1) |
| Scott 2013 | Aerobic and resistance | 47, 43 (intervention vs control) | Yes, six weeks of resistance exercise. | Adherence for the intervention group was 80% | Yes | No | Yes | None reported. |
| Thomas 2013 | Aerobic | 35, 30 (intervention vs control) | Yes | The exercise goal was 150 minutes/week of moderate intensity aerobic exercise; 33% of women achieved this amount. 57% of women achieved 80% of the exercise goal or 120 minutes/week, and 75% of women achieved 90 minutes/week. | No | Yes; not all outcomes were reported and low recruitment rate. | Not reported | None reported. |
| Irwin 2015 | Aerobic and resistance | 61, 60 (intervention vs control) | Yes | Women randomly assigned to exercise also reported their exercise prospectively in daily activity logs and reported an average 119 minutes per week of aerobic exercise, with an average of 70% of strength‐training sessions completed. Women randomly assigned to exercise increased their physical activity by an average 159 minutes per week, compared with 49 minutes per week in the usual‐care group. | No | No | Yes | 5 participants had to discontinue the use of Atromatise inhibitors. |
AET = aerobic exercise tolerance.
Results of the search
Figure 1 illustrates the process of the literature search and study selection for the review. The updated search identified 5442 unique records from databases searched. In addition, we identified 2750 records from grey literature and 'snowballing' techniques for this update. Given that the details of prescribed exercise are rarely reported in manuscript abstracts (e.g. frequency, intensity, duration of exercise prescription), this led to evaluation of a large number of manuscripts at full text stage (n = 227). From these full‐text articles, 212 manuscripts were excluded, leaving 15 publications from 10 unique studies included in the review (total unique studies = 23). Reasons for excluding these 212 publications and a subset of the original review total (n = 377) are covered in the Excluded studies section below.
1.
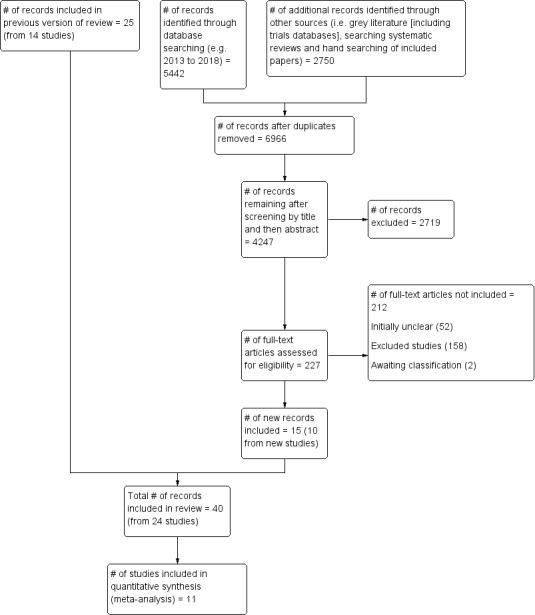
PRISMA flow diagram.
Included studies
This update identified 15 publications from 10 new studies, which when combined with the studies from the original review equates to 40 publications in total from 23 studies. (al‐Majid 2015; Bourke 2014; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Drouin 2005; Hayes 2009; Irwin 2015; Kaltsatou 2011; Kim 2006; Kim 2017; McKenzie 2003; Mohamady 2017; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). One study (Bourke 2014) was a efficacy study, following on from a previous feasibility study (Bourke 2011a) from the original review.
For the 2018 update, we sent an additional 112 emails to request unpublished information for manuscripts that were unclear in reporting relative to our inclusion and exclusion criteria. We were able to include an additional seven published manuscripts and to exclude an additional eight published manuscripts on the basis of information received in correspondence from authors.
Only randomised controlled trials (RCTs) were included in the review. All included studies used a parallel‐group design with baseline assessment and follow‐up of 12 months maximum. All included studies were conducted using participant‐level randomisation. The format of reporting precluded data extraction for meta‐analytical combination in two studies (Drouin 2005; Pinto 2003). Sample size ranged from 14 to 222, with a total of 1372 participants included in this review (mean age range 51 to 72).
Participants
Twenty of the included trials were on breast cancer survivors (al‐Majid 2015; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Daley 2007a; Drouin 2005; Hayes 2009; Irwin 2015; Kaltsatou 2011; Kim 2006; Kim 2017; McKenzie 2003; Mohamady 2017; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2005; Rogers 2015; Scott 2013; Thomas 2013); only two studies involved colorectal cancer (Bourke 2011a; Pinto 2011), one prostate cancer (Bourke 2014,) and one lung cancer (Cavalheri 2017). Of these studies, 12 included participants who were currently undergoing active treatment inclusive of hormone‐based therapy (al‐Majid 2015; Bourke 2014; Cadmus 2009; Daley 2007a; Drouin 2005; Irwin 2015; Kim 2006; Mohamady 2017; Musanti 2012; Perna 2010; Pinto 2005; Scott 2013). We found only one study that reported data from participants with metastatic disease (Bourke 2014), and six studies that were conducted in obese cohorts (i.e. mean BMI > 30 kg/m2; (Cadmus 2009; Drouin 2005; Mohamady 2017; Rogers 2015; Scott 2013; Thomas 2013). The majority of participants were white, and only five studies reported data from an ethnically diverse sample (al‐Majid 2015; Irwin 2015; Perna 2010; Rogers 2015; Thomas 2013). Comorbidities at baseline were largely unclear or unreported.
Interventions
Type of exercise
Fourteen studies prescribed exclusively aerobic exercise (al‐Majid 2015; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Daley 2007a; Drouin 2005; Kaltsatou 2011; Kim 2006; Mohamady 2017; Pinto 2003; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013); the remaining RCTs used a mix of aerobic and resistance training (no exclusively resistance training studies met our inclusion criteria). Ten studies used a combination of supervised and home‐based exercise (Bourke 2011a; Bourke 2014; Cadmus 2009; Campbell 2017; Hayes 2009; Irwin 2015; Kim 2006; Perna 2010; Pinto 2003; Rogers 2015), four studies opted to use an exclusively home‐based design (Drouin 2005; Musanti 2012; Pinto 2005; Pinto 2011), and 10 studies were exclusively supervised studies (al‐Majid 2015; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Kaltsatou 2011; Kim 2017; McKenzie 2003; Mohamady 2017; Scott 2013; Thomas 2013).
Exercise sessions and the role of exercise professionals and healthcare professionals
Contact with exercise professionals or study researchers ranged from two to three weekly supervised sessions (Rogers 2015), to weekly phone calls after an initial one‐to‐one exercise consultation (Pinto 2005; Pinto 2011). Most commonly however, supervised sessions were offered two to three times per week. Of note, seven studies (Drouin 2005; Kaltsatou 2011; Kim 2006; McKenzie 2003; Pinto 2003; Pinto 2005; Pinto 2011), placed restrictions on the control group regarding exercise behaviour during the course of the study, usually taking the form of direct instruction to refrain from changing exercise behaviour. However, the 2018 update found no additional studies that placed restrictions on the control group, usual activities were encouraged. Contact with healthcare professionals was not frequent amongst the studies, with three studies having healthcare professionals carry out medical assessments for eligibility (Cantarero‐Villanueva 2012b; Kim 2017; Mohamady 2017), two studies having oncologists refer the participants onto the study, but it was not stated explicitly if they delivered any aspects of the intervention (al‐Majid 2015; Campbell 2017).
Level of exercise and adherence
Thirteen studies incorporated prescriptions that would meet the Rock 2012 recommendations for aerobic exercise (i.e.150 minutes per week); (Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Pinto 2011; Rogers 2015) or resistance exercise (i.e. resistance training strength training exercises at least two days per week); (Bourke 2011a; Bourke 2014; Cavalheri 2017; Irwin 2015; Kim 2017; Musanti 2012; Perna 2010; Scott 2013). However, only eight of these studies reported 75% adherence to these guidelines, (Bourke 2011a; Bourke 2014; Campbell 2017; Cantarero‐Villanueva 2012b; Irwin 2015; Kim 2017; Rogers 2015; Scott 2013).
Theoretical basis
Of the interventions provided, only six were explicitly based on a theoretical model (Daley 2007a; Musanti 2012; Perna 2010; Pinto 2005; Pinto 2011; Rogers 2015); the trans‐theoretical model was most common, followed by social cognitive theory and exercise, and self‐esteem theory. Only one intervention from the 2018 update was found to be based on a theoretical model (Rogers 2015).
Behaviour change techniques (BCT) and adherence
Full details of intervention BCT coding according to the CALO‐RE taxonomy for the previous review (Bourke 2013) and the 2018 update can be seen in Table 3 and Table 4 (respectively). In the previous review, there was a lack of identified studies that met the Rock 2012 guidelines. For this updated version of the review, our searches found more instances of studies (eight in total) that meet the 150 minutes per week or two strength sessions per week Rock 2012 target. Also, there were other studies with lower exercise targets but good adherence (i.e. over 75%). Hence, we presented BCTs in a hierarchy format: Tier 1 and Tier 2. Tier 1 BCTs are presented from interventions that set prescriptions which meet the Rock 2012 target and achieved 75% or more adherence. Tier 2 BCTs are presented from interventions that reported good adherence (i.e. 75% or more) but set prescriptions that are below the 150 minutes per week Rock 2012 target. BCTs reported in the eight Tier 1 trials are presented in Table 5. It is notable that four of these studies incorporated both a supervised and an independent exercise component as part of their intervention and four were exclusively supervised, with all placing no restrictions on the control group in terms of exercise behaviour. Six studies were included in Tier 2 BCTs and reported adherence of 75% or greater to a specified exercise aerobic prescription which was lower than the targets set in the Rock 2012 guidelines (al‐Majid 2015; Bourke 2011a; Bourke 2014; Cadmus 2009; Kim 2017; Scott 2013). BCTs reported in Tier 2 studies are presented in Table 6.
2. Original review Behaviour change components.
| Behaviour change technique | Bourke 2011a |
Cadmus 2009 YALE |
Daley 2007a | Drouin 2005 | Hayes 2009 | Kaltsatou 2011 | McKenzie 2003 | Musanti 2012 | Perna 2010 | Kim 2006 | Pinto 2003 | Pinto 2005 | Pinto 2011 |
| Theory | TTM | EXSEM | TTM | TTM | TTM SCT | ||||||||
| 1. Provide Info on consequences of behaviour in general | X | X | X | X | |||||||||
| 2. Provide Info on consequences of behaviour to the individual | |||||||||||||
| 3. Provide Info about others' approval | |||||||||||||
| 4. Provide normative info about others' behaviour | |||||||||||||
| Programme set goal | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 5. Goal setting (behaviour) | X | X | X | X | X | X | |||||||
| 6. Goal setting (outcome) | |||||||||||||
| 7. Action planning | |||||||||||||
| 8. Barrier identification/Problem solving | X | X | X | X | X | ||||||||
| 9. Setting of graded tasks | X | X | X | X | X | X | X | X | X | ||||
| 10. Prompt review of behavioural goals | X | X | |||||||||||
| 11. Prompt review of outcome goals | |||||||||||||
| 12. Prompt rewards contingent on effort or progress towards goal | X | X | X | ||||||||||
| 13. Provide rewards contingent on successful behaviour | X | ||||||||||||
| 14. Shaping | |||||||||||||
| 15. Prompt generalisation of a target behaviour | X | X | X | X | |||||||||
| 16. Prompt self‐monitoring of behaviour | X | X | X | X | X | X | X | X | X | ||||
| 17. Prompt self‐monitoring of behavioural outcome | X | X | X | X | X | X | |||||||
| 18. Prompt focus on past success | X | ||||||||||||
| 19. Feedback on performance provided | X | X | X | X | |||||||||
| 20. Information provided on where and when to perform behaviour | X | X | |||||||||||
| 21. Instruction provided on how to perform the behaviour | X | X | X | X | X | X | X | X | X | ||||
| 22. Modelling/Demonstration of behaviour | X | X | X | ||||||||||
| 23. Teaching to use prompts/cues | X | X | X | ||||||||||
| 24. Environmental restructuring | X | X | |||||||||||
| 25. Agreement on behavioural contract | X | ||||||||||||
| 26. Prompt practise | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 27. Use of follow‐up prompts | X | ||||||||||||
| 28. Facilitating social comparison | |||||||||||||
| 29. Planning social support/social change | X | X | X | ||||||||||
| 30. Prompt identification as role model/position advocate | |||||||||||||
| 31. Prompt anticipated regret | |||||||||||||
| 32. Fear arousal | |||||||||||||
| 33. Prompt self‐talk | |||||||||||||
| 34. Prompt use of imagery | |||||||||||||
| 35. Relapse prevention/coping planning | X | X | |||||||||||
| 36. Stress management/emotional control training | X | ||||||||||||
| 37. Motivational interviewing | |||||||||||||
| 38. Time management | |||||||||||||
| 39. General communication skills training | |||||||||||||
| 40. Stimulation of anticipation of future rewards |
EXSEM = exercise self‐esteem model; SCT = social cognitive theory; TTM = trans‐theoretical model.
3. 2018 Update Behaviour change components.
| Behaviour change technique | al‐Majid 2015 | Bourke 2014 | Campbell 2017 | Cantarero‐Villanueva 2012b | Cavalheri 2017 | Irwin 2015 | Kim 2017 | Mohamady 2017 | Rogers 2015 | Scott 2013 | Thomas 2013 |
| Theory | SCT | ||||||||||
| 1. Provide Info on consequences of behaviour in general | x | x | |||||||||
| 2. Provide Info on consequences of behaviour to the individual | |||||||||||
| 3. Provide Info about others' approval | |||||||||||
| 4. Provide normative info about others' behaviour | |||||||||||
| Programme set goal | x | x | x | x | x | x | x | x | x | x | x |
| 5. Goal setting (behaviour) | x | x | |||||||||
| 6. Goal setting (outcome) | |||||||||||
| 7. Action planning | |||||||||||
| 8. Barrier identification/Problem solving | x | x | |||||||||
| 9. Setting of graded tasks | x | x | x (implicit) | x | x | x | x | x | x | x | |
| 10. Prompt review of behavioural goals | x | ||||||||||
| 11. Prompt review of outcome goals | |||||||||||
| 12. Prompt rewards contingent on effort or progress towards goal | x | ||||||||||
| 13. Provide rewards contingent on successful behaviour | |||||||||||
| 14. Shaping | |||||||||||
| 15. Prompt generalisation of a target behaviour | x (from linked paper Gilbert 2016* | x | |||||||||
| 16. Prompt self‐monitoring of behaviour | |||||||||||
| 17. Prompt self‐monitoring of behavioural outcome | x | x | x | ||||||||
| 18. Prompt focus on past success | |||||||||||
| 19. Feedback on performance provided | x | ||||||||||
| 20. Information provided on where and when to perform behaviour | x | x | |||||||||
| 21. Instruction provided on how to perform the behaviour | x | x | x | ||||||||
| 22. Modelling/Demonstration of behaviour | x | ||||||||||
| 23. Teaching to use prompts/cues | |||||||||||
| 24. Environmental restructuring | |||||||||||
| 25. Agreement on behavioural contract | |||||||||||
| 26. Prompt practise | x | x | |||||||||
| 27. Use of follow‐up prompts | |||||||||||
| 28. Facilitating social comparison | |||||||||||
| 29. Planning social support/social change | x | ||||||||||
| 30. Prompt identification as role model/position advocate | |||||||||||
| 31. Prompt anticipated regret | |||||||||||
| 32. Fear arousal | |||||||||||
| 33. Prompt self‐talk | |||||||||||
| 34. Prompt use of imagery | |||||||||||
| 35. Relapse prevention/coping planning | x | ||||||||||
| 36. Stress management/emotional control training | |||||||||||
| 37. Motivational interviewing | |||||||||||
| 38. Time management | |||||||||||
| 39. General communication skills training | |||||||||||
| 40. Stimulation of anticipation of future rewards |
4. Tier 1 BCTs ‐ trials which had 75% adherence to the Rock resistance or aerobic guidelines.
| BCT | Bourke 2014 | Campbell 2017 | Cantarero‐Villanueva 2012b | Bourke 2011a | Rogers 2015 | Scott 2013 | Kim 2017 | Irwin 2015 | |
| Resistance | Aerobic | Aerobic | Resistance | Aerobic | Resistance | Resistance | Aerobic | Frequency of BCTs | |
| Programme set goal | x | x | x | x | x | x | x | x | 8 |
| 9. Setting of graded tasks | x | x | x | x | x | x | x | 7 | |
| 21. Instruction provided on how to perform behaviour | x | x | x | 3 | |||||
| 26. Prompt practise | x | x | 2 | ||||||
| 5. Goal setting (outcome) | x | x | 2 | ||||||
| 8. Barrier identification/problem solving | x | x | 2 | ||||||
| 1. Provide information on consequences of behaviour in general | x | x | 2 | ||||||
| 15. Prompt generalisation of a target behaviour | x | x | 2 | ||||||
| 17. Prompt self‐monitoring of behavioural outcome | x | x | 2 | ||||||
| 20. Information provided on where and when to perform behaviour | x | x | 2 | ||||||
| 19. Feedback on performance provided | x | 1 | |||||||
| 22. Modelling/demonstration of behaviour | x | 1 | |||||||
| 12. Prompt rewards contingent on effort or progress towards goal | x | 1 | |||||||
| 29. Planning social support/social | x | 1 | |||||||
| 35. Relapse prevention/coping planning | x | 1 |
BCTs: behaviour change techniques
5. Tier 2 BCTs ‐ trials which had 75% adherence to their specified aerobic exercise prescription.
| BCT | Bourke 2011a | al‐Majid 2015 | Bourke 2014 | Cadmus 2009 | Scott 2013 | Kim 2017 | |
| Frequency of BCTs | |||||||
| Programme set goal | x | x | x | x | x | x | 6 |
| 9. Setting of graded tasks | x | x | x | x | x | 5 | |
| 21. Instruction provided on how to perform behaviour | x | x | x | 3 | |||
| 1. Provide information on consequences of behaviour in general | x | x | 2 | ||||
| 26. Prompt practise | x | x | 2 | ||||
| 8. Barrier identification/problem solving | x | x | 2 | ||||
| 15. Prompt generalisation of a target behaviour | x | x | 2 | ||||
| 5. Goal setting (outcome) | x | 1 | |||||
| 16. Prompt self‐monitoring of behaviour | x | 1 | |||||
| 17. Prompt self‐monitoring of behavioural outcome | x | 1 | |||||
| 20. Information provided on where and when to perform behaviour | x | 1 | |||||
| 29. Planning social support/social | x | 1 | |||||
| 27. Use of follow‐up prompts | x | 1 |
BCTs: behaviour change techniques
Few interventions (Bourke 2014; Cadmus 2009; Daley 2007a; Kim 2006; Perna 2010; Rogers 2015) reported providing information on the consequences of behaviour (BCT #1). All interventions had programme set goals, which we have highlighted as being different for the purpose of this review to goal setting (behaviour) and goal setting (outcome). Only seven studies set exercise goals in conjunction with participants (BCT # 5) (Bourke 2014; Cadmus 2009; Daley 2007a; Perna 2010; Pinto 2005; Pinto 2011; Rogers 2015). These same seven studies also reported problem‐solving with barriers identified (BCT #8) and solutions facilitated. Three interventions (Daley 2007a; Perna 2010; Rogers 2015) which participants had some input into setting of goals were these reviewed (BCT #10). When monitoring did occur (BCT #16) or monitoring of outcome behaviour occurred (BCT #17), feedback on performance (BCT #19) was provided in only five out of 10 (Cadmus 2009; Perna 2010; Pinto 2005; Pinto 2011; Rogers 2015), which is important to note. Fourteen studies (Bourke 2011a; Bourke 2014; Cadmus 2009; Daley 2007a; Drouin 2005; Hayes 2009; Kaltsatou 2011; Kim 2006; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2011; Rogers 2015; Scott 2013) reported providing instruction on how to perform the behaviour (BCT #21), although it may be anticipated that this did occur but just was not reported. In addition, 15 studies prompted practise of the behaviour (BCT #26) (Bourke 2011a; Bourke 2014; Cadmus 2009; Daley 2007a; Drouin 2005; Hayes 2009; Kaltsatou 2011; Kim 2006; McKenzie 2003; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2005; Pinto 2011; Rogers 2015), Only four studies used techniques to increase social support (BCT #29); (Bourke 2014; Cadmus 2009; Daley 2007a; Perna 2010). Other common BCTs included setting of graded tasks (i.e. increased exercise duration or intensity over time) and self‐monitoring of behaviour (exercise) and outcomes of behaviour (e.g. heart rate), although it is not clear for all interventions whether this was done primarily for data collection or as a mechanism of behaviour change.. Only three studies reported relapse prevention (BCT #35) (Daley 2007a; Perna 2010; Rogers 2015).
Measurement of exercise behaviour
Ten studies were identified that attempted to objectively validate independent exercise behaviour with accelerometers or heart rate monitoring (al‐Majid 2015; Bourke 2014; Cadmus 2009; Irwin 2015; Mohamady 2017; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). Seven of these studies attempted to validate self‐reported independent exercise behaviour by using accelerometers or heart rate monitors (al‐Majid 2015; Bourke 2014; Irwin 2015; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013), however in three studies (Pinto 2005; Pinto 2011; Rogers 2015), data either were not supportive of exercise behaviour recorded by participants or were not reported in their entirety.
Excluded studies
Reasons for excluding published studies included the following.
Non‐RCTs (e.g. review manuscripts, comment/editorial articles).
Mixed cancer cohorts or cohorts that included non‐cancer populations.
Studies that failed to describe essential metrics of exercise prescription used in the intervention (e.g. frequency, intensity, duration).
Studies involving active participants at baseline.
Studies involving hospital inpatients.
Interventions that provided follow‐up of less than 6 weeks.
Studies involving participants younger than 18 years of age.
All excluded studies (N = 180) for the 2018 update, are presented in the Characteristics of excluded studies. However for the original review only a subset of excluded studies could be included in the 'Characteristics of excluded studies' section. This is a result of the large volume of studies that had to be full text screened (N = 402) and the high proportion (around 90%) that were excluded. In accordance with editorial advice, we divided this large number (N = 365) into initially unclear studies that required further investigation (N = 76) and those that clearly were not eligible after full text had been retrieved (N = 289). This approach is analogous to the approach adopted in recent reviews (Galway 2012), and is detailed in the existing PRISMA diagram (Figure 1).
For the 2018 update, we sent an additional 101 emails to corresponding authors to request additional information (regarding included studies, excluded studies and studies that we could not access) to determine eligibility and to supplement published data for this review.
Risk of bias in included studies
Only seven studies were judged not to include a high risk of bias (al‐Majid 2015; Bourke 2011a; Cadmus 2009; Cantarero‐Villanueva 2012b; Drouin 2005; Irwin 2015; Scott 2013). Full results of the methodological quality assessment for allocation bias, blinding, incomplete data outcome and selective reporting (along with justifications) are covered in the 'Risk of bias' tables for each study and are illustrated in Figure 2; Figure 3. Twelve studies stated that an intention‐to‐treat analysis was used (Bourke 2011a; Bourke 2014; Cadmus 2009; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Irwin 2015; Perna 2010; Pinto 2005; Rogers 2015; Scott 2013; Thomas 2013).
2.
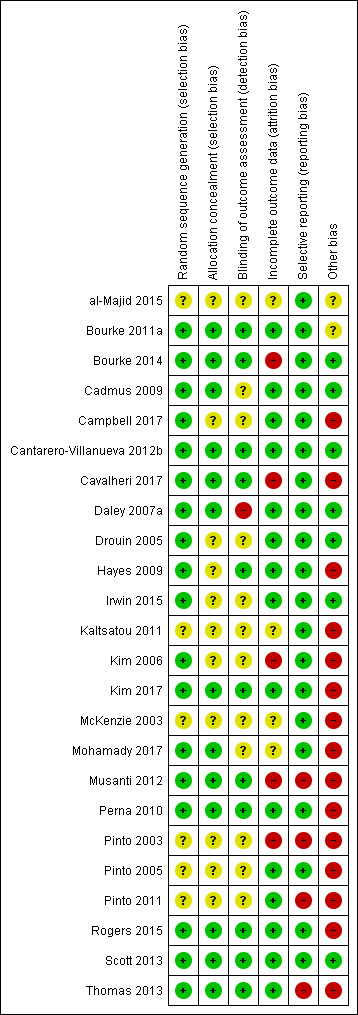
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
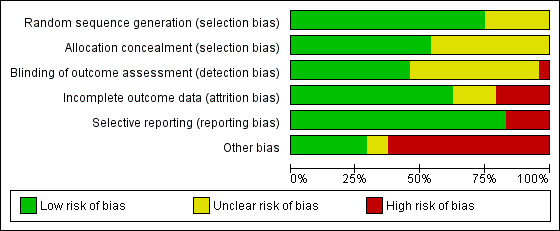
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Eleven studies had an unclear risk in their description of concealment in randomisation allocation. However, no study was judged to have a high risk of bias in this respect.
Blinding
Eleven studies had undertaken the blinding of study assessors (Bourke 2011a; Bourke 2014; Cantarero‐Villanueva 2012b; Cavalheri 2017; Hayes 2009; Kim 2017; Musanti 2012; Perna 2010; Rogers 2015; Scott 2013; Thomas 2013). The remaining studies did not include enough information for the review authors to make a definitive judgement on this criterion.
Incomplete outcome data
Five studies were judged to have been subject to incomplete data outcome bias: Kim 2006 reported data from only 41 of 74 participants randomly assigned; Musanti 2012 reported that 13 women (24%) did not complete their assigned 12‐week programme; and Pinto 2003 did not report control group data for the exercise tolerance test. Bourke 2014 had incomplete outcome data at six months follow‐up. Cavalheri 2017 reported missing patient data in both arms with no reasons given.
Selective reporting
Most studies reported all listed outcomes; however, four studies were judged to omit outcomes from their results reporting. Musanti 2012 did not report waist and upper, mid and lower arm circumference outcomes; Pinto 2003 reported none of the physiological assessments in the control group at 12 weeks of follow‐up; Pinto 2011 did not report data derived from the use of accelerometers; and Thomas 2013 did not report on body fat or lean mass values and no data were given from food frequency questionnaire.
Other potential sources of bias
Other sources of bias found in the included studies that are worth highlighting include adherence data missing or not clear (Cavalheri 2017; Hayes 2009; Kaltsatou 2011; Kim 2017; McKenzie 2003; Mohamady 2017; Musanti 2012); high attrition at follow‐up (Pinto 2003); low recruitment rate (Bourke 2011a; Campbell 2017; Thomas 2013); Significant differences in participants excluded from study analysis/dropouts (Kim 2006; Musanti 2012; Pinto 2003); numbers randomly assigned to study arms with study completion rate unclear (Perna 2010); significant differences in cohorts at baseline (Kim 2017; Musanti 2012; Pinto 2003; Pinto 2005); and inconsistencies between objective and subjective measures of exercise behaviour (Pinto 2005; Pinto 2011; Rogers 2015). Insufficient information was reported to permit a judgement about any single element of bias because of lack of data (al‐Majid 2015; Bourke 2011a; Cadmus 2009; Campbell 2017; Drouin 2005; Hayes 2009; Irwin 2015; Kaltsatou 2011; Kim 2006; McKenzie 2003; Mohamady 2017; Pinto 2003; Pinto 2005; Pinto 2011).
Effects of interventions
See: Table 1
Primary outcome
To assess the effects of interventions designed to promote exercise behaviour in sedentary people living with and beyond cancer
Please see Table 2, 'Summary of included studies'. As it is not meaningful to interpret individually the component metrics of aerobic (frequency, intensity and duration) or resistance exercise (frequency, intensity, type of exercise, sets and repetitions) behaviour, these primary outcomes are presented in the narrative synthesis below of interventions achieving 75% or greater adherence.
In Rogers 2015, adherence to planned intervention components was 98% for supervised exercise sessions, 96% for update sessions, and 91% for discussion group sessions. Only five participants did not receive the allocated intervention (i.e. did not complete 75 % of all intervention components combined). With the intervention group reporting an average of 169 minutes of moderate intensity exercise per week at 12 weeks, and 137 minutes of moderate intensity exercise per week at six months. Although, there is a high risk of bias around how 'in‐active' the recruited participants were at baseline, as baseline accelerometer recordings are incongruent with the inclusion criteria.
Four of the studies included in this review reported that 75% or more of the intervention group met the (Rock 2012) aerobic exercise guidelines at any given follow‐up (Campbell 2017; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015). Four studies reported that 75% or more of the intervention group met the (Rock 2012) resistance exercise guidelines. (Bourke 2011a; Bourke 2014; Kim 2017; Scott 2013). Behaviour change techniques (BCTs) reported in these eight studies are presented in Table 5. Of these studies, only one study explicitly stated it had theoretical basis (Rogers 2015).
Due to of unclear reporting it was not possible to make a judgement on whether some trials achieved adherence of 75% or greater . Reasons for an unclear judgement or unsuccessful adherence are detailed below.
Daley 2007a: judgement unclear; adherence reported as a proportion of participants attending a proportion of set exercise sessions (i.e. 77% of the intervention group attending 70% of sessions).
Drouin 2005: judgement unclear; adherence reported as mean number of days per week when exercise was undertaken, relative to a range within the prescription (i.e. 3.6 days per week, when the prescription was for three to five days per week).
Kaltsatou 2011: judgement unclear; no adherence data reported.
Kim 2006: judgement unclear; high adherence was reported (78%), but in tandem with substantial attrition (i.e. data missing for 45% of the cohort).
Pinto 2003: judgement unclear; high adherence was reported (88%) but in tandem with substantial attrition (i.e. 25% of the intervention group dropped out over the intervention period).
Pinto 2005: judgement unsuccessful; 75% adherence threshold was not met after week four.
Pinto 2011: judgement unsuccessful; three‐day Physical Activity Recall (PAR) questionnaire indicates that 64.7% of the intervention group and 40.9% of controls were adhering to the exercise guidelines at three months.
Hayes 2009: judgement unclear; adherence reported as a proportion of participants attending a proportion of set exercise sessions (i.e. 88% allocated to the intervention group participated in 70% or more of scheduled supervised exercise sessions). Further, adherence from the unsupervised aspect is not reported.
McKenzie 2003: judgement unclear; no adherence data reported.
Musanti 2012: judgement unclear; high adherence reported but only 50% of activity logs returned.
Perna 2010: judgment unclear; women assigned to the structured intervention completed an average of 83% of their scheduled hospital‐based exercise sessions (four weeks in total). Home‐based adherence is not clear.
Mohamady 2017: judgement unclear; no adherence data reported.
Thomas 2013: judgement unsuccessful; the goal of the intervention was for participants to achieve 150 minutes of moderate intensity exercise per week; 33% of the intervention group achieved 150 minutes per week, 56% of the intervention group achieved 120 minutes per week and 75% achieved 90 minutes per week.
Irwin 2015: judgement unsuccessful; resistance exercise ‐ an average of 70% of strength‐training sessions completed.
Cavalheri 2017: judgement unsuccessful; of the nine participants randomised to the exercise intervention, four (44%) adhered to exercise training by completing 15 or more training sessions (i.e.≥60%).
Ideally, a meta‐analysis of objectively verified (e.g. using accelerometers or heart rate monitoring) minutes per week of moderate intensity aerobic exercise achieved in an intervention group, compared with controls, for whom the exercise prescription adherence is at least 75%, would be most informative. However, due to variation in measurement and reporting amongst included studies, this was not possible. Insufficient data were available for a synthesis of evidence to be conducted around free‐living energy expenditure.
Secondary outcomes
Aerobic exercise tolerance
1.1 All cancers: eight to 12 weeks of follow‐up
A meta‐analysis of change in aerobic exercise tolerance was carried out on 10 studies that reported these outcomes and also reported means for final value scores. Standardised mean differences (SMDs) were used to produce effect estimates as variation in how studies assessed this outcome was evident. Standard deviations (SDs) were calculated from 95% confidence intervals (CIs) using the formula in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (i.e. SD = √N * (upper limit‐lower limit)/(t distribution *2), and from standard errors (SEs) using SD = SE*√N, when they were not reported. Length of follow‐up ranged from eight (Cavalheri 2017; Daley 2007a; Kim 2006), to 12 weeks (al‐Majid 2015; Bourke 2011a; Bourke 2014; Musanti 2012; Pinto 2005; Pinto 2011; Rogers 2015) . Aerobic exercise tolerance was significantly better in intervention versus control groups in 604 participants: (SMD 0.54, 95% CI 0.37 to 0.70; 10 studies, 604 participants: low‐certainty evidence; Analysis 1.1). Results were analysed using a fixed‐effect model. Certainty of the evidence assessed using GRADE and was graded as low. As there was some serious concerns over risk of bias and concerns over the number of small studies with positive results (please see Table 1).
1.1. Analysis.
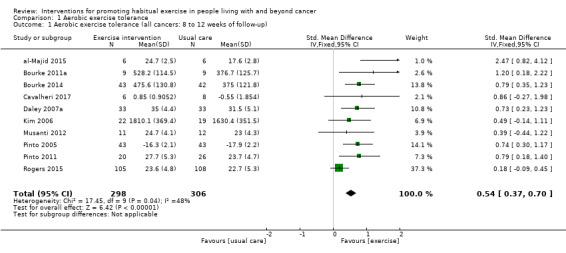
Comparison 1 Aerobic exercise tolerance, Outcome 1 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up).
1.2 All cancers: eight to 12 weeks of follow‐up (sensitivity analysis)
We then removed studies with a high risk of bias relative to this outcome and repeated the analysis with the four remaining studies (al‐Majid 2015; Bourke 2011a; Bourke 2014; Pinto 2005), and aerobic exercise tolerance was better in intervention versus control groups (SMD 0.85, 95% CI 0.56 to 1.14; 4 studies, 201 participants; low‐certainty evidence; Analysis 1.2). The certainty of the evidence was graded as low using GRADE as there were concerns imprecision as there were variations in effect sizes and concerns over the low number of participants in the studies (please see Table 1).
1.2. Analysis.
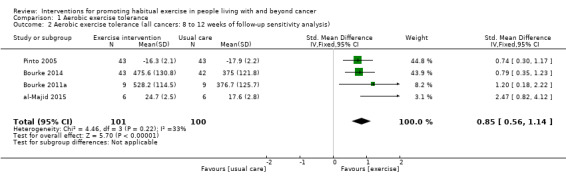
Comparison 1 Aerobic exercise tolerance, Outcome 2 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis).
1.3 All cancers: six months of follow‐up
Seven studies included data from a follow‐up of six months (Bourke 2014; Daley 2007a; Kaltsatou 2011; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013) showing that aerobic exercise tolerance was significantly better at six months in intervention versus control groups (SMD 0.56, 95% CI 0.39 to 0.72; 7 studies; 591 participants: low‐certainty evidence; Analysis 1.3). It should be highlighted that six of these studies have a high risk of bias, which could affect this outcome at six months; specifically, Bourke 2014 had high attrition at six months follow‐up; no adherence data in the Kaltsatou 2011 study; substantial contamination among controls in the Pinto 2011 study; Rogers 2015 objective and subjective measures of exercise results varied greatly and non‐blinded assessors in the Daley 2007a study. Note that in all meta‐analyses, data from Pinto 2005 have been multiplied by ‐1 to control for direction of effect (i.e. lower values in a timed test indicate a better outcome). Brief narrative descriptions of studies not suitable for meta‐analyses include the following: Drouin 2005 VO2 peak data are reported as medians and interquartile ranges; for Pinto 2003, no control group data are presented for the exercise tests; for Campbell 2017, no means or SDs present at baseline and follow‐up. For grading of data please see Table 1.
1.3. Analysis.
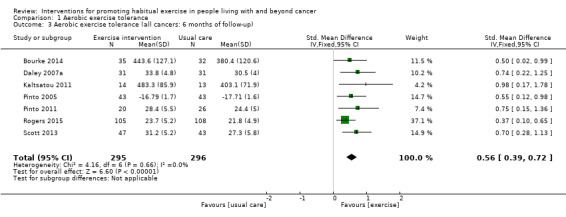
Comparison 1 Aerobic exercise tolerance, Outcome 3 Aerobic exercise tolerance (all cancers: 6 months of follow‐up).
1.4 Breast cancers: eight to 12 weeks of follow‐up
We were able to carry out one subgroup analysis in breast cancer patients This was a meta‐analysis of change in aerobic exercise tolerance carried out on six studies (al‐Majid 2015; Daley 2007a; Kim 2006; Musanti 2012; Pinto 2005; Rogers 2015), showing that aerobic exercise tolerance was significant (SMD 0.57, 95% CI 0.22 to 0.93; 6 studies, 441 participants; very low‐certainty evidence; Analysis 1.4). However, it should be noted that four of the studies were considered to have high risk of bias (Daley 2007a; Kim 2006; Musanti 2012; Rogers 2015). The certainty of the evidence was graded as very low as there was high risk of bias, concerns over the precision of the data as the confidence intervals were wide and there were serious concerns over the heterogeneity of the data (please see Table 1).
1.4. Analysis.
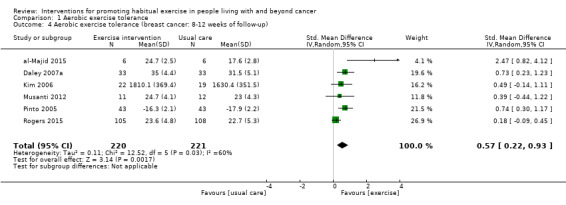
Comparison 1 Aerobic exercise tolerance, Outcome 4 Aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up).
1.5 All cancers: combination of supervised and home‐based exercise: eight to 12 weeks of follow‐up
A meta‐analysis of aerobic exercise tolerance was carried out in the following subgroups: supervised exercise interventions, home‐based interventions, and a combination of both. In a combination of home‐based and supervised exercise interventions (Bourke 2014; Bourke 2011a; Kim 2006; Rogers 2015), aerobic exercise tolerance was better in the intervention than the control : (SMD 0.53, 95% CI 0.01 to 1.04; 4 studies, 357 participants; very low‐certainty evidence; Analysis 1.5). The certainty of the evidence was graded as very low as there were concerns over the precision of the data as the confidence intervals were wide and there were very serious concerns over inconsistency due to the heterogeneity of the data and variations in effect sizes (please see Table 1).
1.5. Analysis.
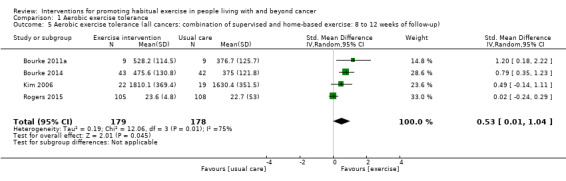
Comparison 1 Aerobic exercise tolerance, Outcome 5 Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up).
1.6 All cancers: home‐based exercise: eight to 12 weeks of follow‐up
In home‐based interventions (Musanti 2012; Pinto 2005; Pinto 2011), aerobic exercise tolerance was better in the intervention than the control (SMD 0.70, 95% CI 0.37 to 1.03: 3 studies, 155 participants; very low‐certainty evidence; Analysis 1.6). The certainty of the evidence was graded as very low due to high risk of bias, low number of participants within the studies and wide confidence intervals (please see Table 1).
1.6. Analysis.
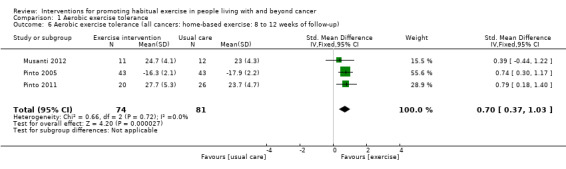
Comparison 1 Aerobic exercise tolerance, Outcome 6 Aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up).
1.7 All cancers: supervised exercise: eight to 12 weeks of follow‐up
In supervised interventions (al‐Majid 2015; Cavalheri 2017; Daley 2007a), aerobic exercise tolerance was better in the intervention group versus control (SMD 1.07, 95% CI 0.26 to 1.89; 3 studies, 92 participants; very low‐certainty evidence; Analysis 1.7). Serious concerns with inconsistency and imprecision were presented due to wide variations in effect sizes and wide confidence intervals. Therefore the certainty of evidence was classed as very low (please see Table 1).
1.7. Analysis.
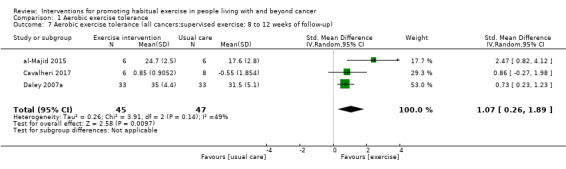
Comparison 1 Aerobic exercise tolerance, Outcome 7 Aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up).
1.8 All cancers: undergoing active treatment: eight to 12 weeks of follow‐up
A meta‐analysis of active treatment and no current treatment for aerobic exercise tolerance was carried out. For participants undergoing active treatment in six studies, (al‐Majid 2015; Bourke 2014; Daley 2007a; Kim 2006; Musanti 2012; Pinto 2005), demonstrated aerobic exercise tolerance was better in the intervention than the control (SMD 0.72, 95% CI 0.49 to 0.95; 6 studies, 313 participants; Analysis 1.8). However, five of these studies had a high risk of bias so interpretation of these results should be done with caution.
1.8. Analysis.
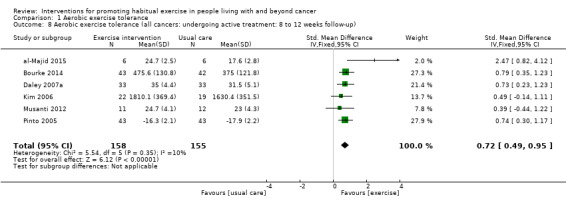
Comparison 1 Aerobic exercise tolerance, Outcome 8 Aerobic exercise tolerance (all cancers: undergoing active treatment: 8 to 12 weeks follow‐up).
1.9 All cancers: no active treatment: eight to 12 weeks of follow‐up
A meta‐analysis of aerobic exercise tolerance in participants not undergoing active treatment was carried out in four studies (Bourke 2011a; Cavalheri 2017; Pinto 2011; Rogers 2015), showing that aerobic exercise tolerance was better in the intervention than the control (SMD 0.61, 95% CI 0.10 to 1.12; 4 studies, 291 participants: Analysis 1.9).
1.9. Analysis.
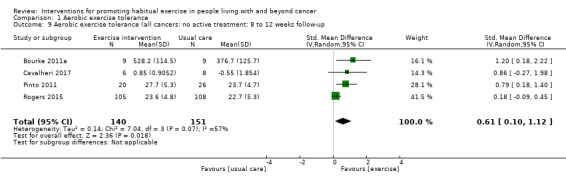
Comparison 1 Aerobic exercise tolerance, Outcome 9 Aerobic exercise tolerance (all cancers: no active treatment: 8 to 12 weeks follow‐up.
Sketal muscle strength
2.1 All cancers: eight to 12 weeks of follow‐up
Four studies that used resistance exercise as a component of the intervention reported changes in lower‐ (Bourke 2011a; Rogers 2015 ) and upper limb (Musanti 2012;Kim 2017) strength. All four studies had reported strength changes at 12 weeks of follow‐up. No significant improvement in strength was found (SMD 0.20, 95% CI ‐0.03 to 0.44; 4 studies, 278 participants: Analysis 2.1).
2.1. Analysis.
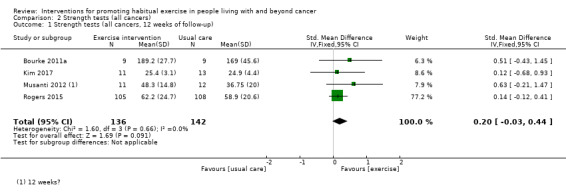
Comparison 2 Strength tests (all cancers), Outcome 1 Strength tests (all cancers, 12 weeks of follow‐up).
2.2 All cancers: eight to 12 weeks of follow‐up
After two studies was removed for high risk of bias (Kim 2017; Musanti 2012), effect estimates remained non‐significant (SMD 0.17, 95% CI ‐0.09 to 0.43; 2 studies, 231 participants; Analysis 2.2).
2.2. Analysis.
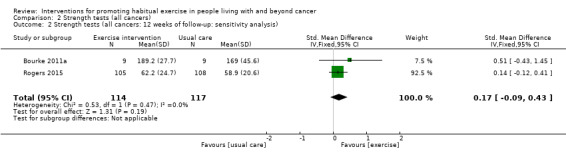
Comparison 2 Strength tests (all cancers), Outcome 2 Strength tests (all cancers: 12 weeks of follow‐up: sensitivity analysis).
Planned subgroup analysis was not possible according to participant age, presence of metastatic disease, theoretical underpinning of interventions or participant body mass index (BMI).
Adverse effects
Thirteen studies reported adverse effects (Bourke 2011a; Bourke 2014; Cadmus 2009; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Irwin 2015; Kim 2006; Musanti 2012; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013); these ranged from minor (e.g. musculoskeletal problems; Musanti 2012; Rogers 2015), to major events (e.g. death; Kim 2006). However, only five studies (Cadmus 2009; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015; Thomas 2013) were explicit as to which of these adverse effects were caused by inclusion of the participant in the intervention group (two instances of plantar fasciitis).
Study recruitment rate
Study recruitment rate ranged from 9.5% (Thomas 2013) to 94% (Thomas 2013, Cantarero‐Villanueva 2012b, respectively). Eleven studies reported a priori sample size estimates (Bourke 2014; Cadmus 2009; Campbell 2017; Daley 2007a; Hayes 2009; Kaltsatou 2011; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2011; Scott 2013), and seven (Bourke 2014; Cadmus 2009; Cantarero‐Villanueva 2012b; Hayes 2009; Perna 2010; Rogers 2015; Scott 2013) met their recruitment target.
Intervention attrition rate
Fifteen studies produced CONSORT diagrams (al‐Majid 2015; Bourke 2011a; Bourke 2014; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Irwin 2015; Kim 2017; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). Intervention attrition rates from the included studies ranged from 0% to 25% (Campbell 2017, Pinto 2003) ,respectively, with seven studies not clearly reporting attrition in the intervention arm (Cavalheri 2017; Kaltsatou 2011; Kim 2006; McKenzie 2003; Mohamady 2017; Musanti 2012; Perna 2010).
Discussion
Summary of main results
In this review update we have found more evidence that there are interventions that meet the Rock 2012 guidelines for aerobic (Campbell 2017; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015) and resistance (Bourke 2011a; Bourke 2014; Kim 2017; Scott 2013) exercise with 75% adherence in previously inactive cancer cohorts. We have identified a hierarchy of the most common behaviour change techniques (BCTs) that feature in these studies (Table 5). The most frequent of these interventions were setting of graded tasks (#BCT 9), programme set goal and instruction of how to perform behaviour (#21). These studies were predominantly exclusively supervised studies or a combination of supervised and home‐based studies. Supervision usually consisted of contact with the exercise professional or research team at least twice weekly. However, from our review of studies at full‐text screening stage, it is still true that adherence to exercise interventions, which is crucial for understanding treatment dose, is frequently either poorly reported or not reported at all in randomised controlled trials (RCTs).
Despite the uncertainty surrounding adherence in many of the included studies, interventions caused improvement in aerobic exercise tolerance at eight to 12 weeks (Analysis 1.1) in intervention participants compared with controls. There is also evidence that this can be sustained at six months of follow‐up, but owing to potential high risk of bias, this should be viewed with caution (Analysis 1.3). We were able to carry out one cancer subgroup analysis in breast cancer patients, showing that aerobic exercise tolerance was significantly improved up to 12 weeks (Analysis 1.4), however caution is again warranted when interpreting the result, due to frequent high risk of bias.
Adverse effects these ranged from minor e.g. musculoskeletal problems ( Musanti 2012; Rogers 2015) to major events e.g. death (Kim 2006). However, only five studies were explicit as to which of these adverse effects were caused by inclusion of the participant in the intervention group (two instances of plantar fasciitis) (Cadmus 2009; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015; Thomas 2013).
Overall completeness and applicability of evidence
We included 23 studies in this systematic review, all of which were RCTs. These studies randomly assigned 1372 participants to exercise or comparison groups. A large majority of these studies included women with breast cancer, two involved colorectal cancer survivors, one involved men with advanced prostate cancer, and one involved lung cancer survivors. As found in the original review (Bourke 2013), although these four primary cancers account for most of the population living with and beyond cancer, other common cancers such as lymphoma do not appear at all in this review and less common cancers also are not represented in the evidence base.
Furthermore, an overwhelming majority of participants were white, and only five studies included an ethnically diverse population. As such, other ethnicities are still substantially underrepresented, as found previously (Bourke 2013). Comorbidities were rarely reported at baseline and only six studies were carried out in obese cohorts. Although we set a limit in this review of 90 minutes per week of moderate‐intensity exercise at baseline as the criterion for categorising participants as 'sedentary' or 'physically inactive', we did not specify any threshold for vigorous exercisers. It is possible that we could have included individuals who were performing as much as 90 minutes per week of vigorous intensity exercise. However, it is important to note that baseline reporting of behaviour in terms of how much 'vigorous' exercise these cohorts were undertaking was rare.
Nineteen of the included studies were conducted in Northern America or Western Europe, and two studies were completed in Australia, one in Egypt and one in North Korea. The majority all are considered high‐income nations according to the World Health Organization (WHO) taxonomy. Very little evidence was derived from developing countries, and it is uncertain whether the resources, infrastructure or both required for some of the interventions included in this review would be applicable in these parts of the world.
Although no single tool for measuring physical activity is infallible (Warren 2010), when possible it is desirable to have self‐reported exercise behaviour supported by objective measurements such as accelerometers or heart rate data. Ten studies were identified that attempted to objectively validate independent exercise behaviour with accelerometers or heart rate monitoring (al‐Majid 2015; Bourke 2014; Cadmus 2009; Irwin 2015; Mohamady 2017; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). Seven studies of these studies attempted to validate self‐reported independent exercise behaviour by using accelerometers or heart rate monitors (al‐Majid 2015; Bourke 2014; Irwin 2015; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013), however in three studies, data either were not supportive of exercise behaviour recorded by participants or were not reported in their entirety (Pinto 2005; Pinto 2011; Rogers 2015). Still a number of studies evaluated non‐supervised exercise behaviour by using self‐report logs or seven‐day physical activity questionnaires. Whilst these tools are relatively non‐complex and affordable for implementation in study design, they are prone to well‐established bias, including difficulties in ascertaining the frequency, duration and intensity of physical activity; social desirability bias; the cognitive demands of recall and overestimation of behaviour, particularly when such data are used to extrapolate MET/hours of exercise per week performed, or kcal/week of energy expenditure.
Analysis by behaviour change techniques as it relates to any given outcome (e.g. aerobic exercise tolerance) was not possible given that few studies stated a theoretical basis for their intervention, and only one study in this update being based on theory i.e. the trans theoretical model in Rogers 2015. It is worthy of note, however, that interventions frequently consisted of little more than telling people how to exercise and providing opportunities for this to occur, with little consideration of the psychological aspects of changing behaviour. A number of interventions were excluded at full‐text screening stage, that had a theoretical basis but did not meet our inclusion criteria. Whilst, the use of theory is variable amongst behaviour change interventions generally, the lack of interventions based on a theoretical model in this review is a concern.
It is also acknowledged that although coding of BCTs was done primarily on the basis of study reports, it is possible that some BCTs may have been implemented but not reported. To overcome this possibility and enhance understanding of the techniques important for changing behaviour in cancer patients, adoption of the CALO‐RE taxonomy or the broader BCT v1 taxonomy is recommended.
We acknowledge that in this review, we have undertaken a synthesis of RCTs that represent a combination of exercise efficacy and behaviour change studies (Courneya 2010), we recognise the distinction and this is reflected in the literature. During the screening process, there were a number of behaviour change studies based on theory that we excluded as they did not meet our inclusion criteria. However, it should be noted by the reader that all eight studies that we judged as successful (i.e. reported 75% or greater adherence over the intervention period to the Rock 2012 guidelines) incorporated intervention elements that were designed to promote independent exercise behaviour and did not place any restrictions on the control group in terms of the exercise they were permitted to undertake during the study.
Finally, we stated in the justification for this review that a better understanding of the types of interventions that could promote long‐term, habitual physical activity (i.e. 12 months or longer) in people living with and beyond cancer was a valuable addition to our knowledge due to the original review not being able to address this issue. Unfortunately, because of limitations in the evidence that we identified, we have not been able to address this issue. As such, this is an area of uncertainty that represents an important research gap. Whilst there is more research available of how to promote exercise behaviour at around eight to 12 weeks up until six months, there is still a lack of long‐term follow‐up of anything beyond this amongst these studies.
Quality of the evidence
Most of the uncertainty in judging study bias came from lack of clarity around randomisation procedures, allocation concealment and blinding of study outcome assessors. Most of the studies in this review were judged to include at least one element of high risk of non‐standard bias, as described in the 'Other potential sources of bias outcome. Of note, we chose to refrain from judging studies according to the performance bias criterion because we considered it not possible to realistically blind intervention participants to 'sham' conditions. Public health guidelines (e.g. the UK CMO report) for aerobic and resistance exercise (which are identical to the Rock 2012 recommendations) are freely available to the public, and given their ease of access via the Internet, the validity of a 'sham' condition is highly dubious. The Table 1 and 'Risk of bias' tables and Figure 2 and Figure 3 provide a summary of the certainty of evidence. Reporting of adherence of exercise behaviour within the studies was infrequent, which impacted upon the certainty of evidence.
We found the certainty of evidence assessed using the GRADE methodology for the majority of the outcomes to be low to very low; this was mainly due to high risk of bias, inconsistency of the results and imprecise results. One of the main reasons for a very low‐certainty of evidence grading was due to the high number of studies presenting a low number of participants in their study. Concerns over inconsistency were present due to variations in effect sizes and heterogeneity. Additionally, the serious concerns were present with the imprecision of the data due to wide confidence intervals and overall low numbers of participants in each study.
Additionally, attrition ranged from 0% to 25%, some studies with longer term follow‐up (post six months) demonstrated poorer attrition rates, but reasons for this were seldom explained. Ensuring reasons for dropout is reported in future studies is important.
Potential biases in the review process
We were not able to translate all non‐English language studies identified through our database, grey literature and snowballing searches, due to not having access to or resources for translation services. However, a huge effort was made to identify all relevant RCTs in this field. To the review authors' knowledge, we have identified and evaluated more RCTs involving exercise interventions in sedentary people living with or beyond cancer than any other systematic review in this field. More than 190 papers were screened at full‐text stage for eligibility for this update, in addition to the 400 papers screen at full text for the original review. For this 2018 update, we sent 112 emails in addition to the 116 emails to request data to inform the screening and data extraction process, so that the conclusions of the review would be as accurate and informative as possible. Dual data extraction was used throughout the review, except for study characteristics.
Agreements and disagreements with other studies or reviews
To the review authors' knowledge, this is still the most comprehensive systematic review of exercise behaviour interventions in sedentary cancer cohorts. A recent systematic review of predictors of adherence to exercise in people living with and beyond cancer found that the trans‐theoretical model of behaviour change and the theory of planned behaviour were significantly associated with better exercise adherence (Husebø 2013). The current review does not explicitly support such conclusions: mainly due to reporting in the included studies.
Howlett 2018 conducted a systematic review and meta‐analysis that aimed to evaluate physical activity interventions for healthy inactive adults. The BCT analysis found that interventions that included 'biofeedback', 'demonstration of the behaviour', 'behaviour practice/rehearsal' and 'setting of graded tasks' showed larger effect sizes for physical activity outcomes than studies that did not include these BCTs. Our review also found 'setting of graded tasks' to be common amongst the studies with higher adherence rates. Additionally, this review reported a number of studies that were judged as having high risk of bias or were judged as being unclear due to the lack of clear reporting. A suggestion was for future studies to use the TIDieR (Template for Intervention Description and Rreplication) framework (Hoffman 2014), particularly around the description of intervention content. Our review findings support this recommendation, as a number of exercise studies appear to display problems with reporting and are often judged with high risk of bias.
Ormel 2017 carried out a recent systematic review of predictors of adherence and identified the issues with low adherence to exercise interventions for people with cancer. Home‐based interventions were found to possibly address the issue of time and travelling to a location, which was identified as a potential barrier. Our review found that interventions that incorporated an element of supervision have better adherence rates to the set exercise target than home‐based interventions. However, as previously stated adherence was infrequently reported amongst the studies.
Authors' conclusions
Implications for practice.
Since the last version of this review none of the new relevant studies have provided additional information to change the conclusions. Service provision to promote exercise in sedentary people living with and beyond cancer could incorporate components of both supervised and independent exercise requirements, with supervision being most important for adherence. The majority of the studies in this review were undertaken in breast cancer cohorts and included mainly white females: these limitations to generalisability that were also present in our 2013 review (Bourke 2013). A number of behaviour change techniques (BCTs) were identified in studies which achieved 75% adherence to the aerobic or resistance guidelines (Rock 2012). Most commonly reported BCTs were goal setting, instruction on how to perform behaviour, and setting graded tasks. In the original review, we argued that expecting the most sedentary survivors to achieve at least 150 minutes per week of aerobic exercise is likely to be unrealistic. This review update has found studies that can achieve these guidelines, but only for limited follow‐up. Exercise interventions were found to significantly improve aerobic exercise tolerance compared to usual care at eight to 12 weeks and six months follow‐up. However, there is low to very low‐certainty evidence according to the GRADE methodology to suggest this is due to issues of high risk of bias, inconsistency and imprecision. So caution is warranted when interpreting these results for future practice. A very small number of serious adverse effects were reported amongst the studies, ensuring researchers, clinicians and guideline developers that these aerobic and resistance exercise studies are safe in cancer survivors. The role of healthcare professionals involved in cancer care is still unclear from the studies we synthesised.
Implications for research.
The majority of cancer survivors are not regularly active. Future research needs to address the following issues.
How to promote and sustain exercise behaviour in other cancer survivorship cohorts who are inactive.
Studies need to improve the standards of reporting adverse effects and if they are related or unrelated to the intervention or study participation.
Studies need to be explicit about baseline exercise behaviour and about how it was assessed.
Studies should attempt to use objective measures of exercise, which may be supported with the use of subjective measures.
Studies should clearly state reasons for drop out.
Studies need to report as standard frequency, intensity and duration of aerobic exercise, as well as repetitions, sets and intensity of resistance exercise used in intervention prescriptions.
There needs to be a standardisation of adherence reporting in clinical studies investigating the effects of exercise in cancer survivors. We still recommend that adherence is reported as a single proportion of the cohort who attended/performed exercise according to the set prescription. If adherence were to be clearly reported, there is a much better chance of understanding which factors improve adherence.
Reporting of BCTS used in such interventions should be standardised. Adoption of the CALO‐RE taxonomy or the broader BCT v1 taxonomy is recommended.
Future interventions should use the TIDieR (Template for Intervention Description and Rreplication) checklist as a guide when designing and when reporting interventions.
By achieving these standards, researchers and clinicians can aim to have an acceptable level of rigour that will demonstrate dose response relationship between exercise and given clinically relevant outcomes. Such rigor can underpin clinical exercise guidelines and so that practitioners are able to communicate achievable exercise recommendations for sedentary people living with and beyond cancer.
What's new
| Date | Event | Description |
|---|---|---|
| 24 September 2018 | Amended | Text amendment. |
History
Protocol first published: Issue 11, 2012 Review first published: Issue 9, 2013
| Date | Event | Description |
|---|---|---|
| 3 May 2018 | New search has been performed | Literature searches updated to 3 May 2018. |
| 3 May 2018 | New citation required but conclusions have not changed | Review updated with the inclusion of 10 additional studies but conclusions remain unchanged. |
Acknowledgements
We thank the editorial team Jo Morrison, Clare Jess, Gail Quinn, Jo Platt and Tracey Harrsion for their kind and helpful support during the review process. We also wish to acknowledge the hard work that went in to the original version of the review by Kate Holmer and Karen Robb.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL 2018 update search
#1 MeSH descriptor Neoplasms explode all trees #2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*) #3 (#1 OR #2) #4 MeSH descriptor Exercise explode all trees #5 MeSH descriptor Exercise Movement Techniques explode all trees #6 MeSH descriptor Exercise Therapy explode all trees #7 MeSH descriptor Physical Fitness, this term only #8 (physical* adj5 (fit* or activ*)) #9 (exercis* or aerobic* or resistance* or strength* or walk* or endurance* or lifestyle* or behav*) #10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 #3 and #10 #12 MeSH descriptor: [Health Behavior] explode all trees #13 MeSH descriptor: [Risk Reduction Behavior] this term only #14 ((promot* or motivat* or advis* or encourag* or assist* or develop* or stimulat* or help* or support* or organis* or aid* or assist* or endors* or prompt* or driv* or inspire* or lead* or inspir* or further* or advocat* or recommend* or endorse* or foster* or champion*) near/5 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*)) #15 #12 or #13 or #14 #16 #11 and #15
CENTRAL 2012 search
#1 MeSH descriptor Neoplasms explode all trees #2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*) #3 (#1 OR #2) #4 MeSH descriptor Exercise explode all trees #5 MeSH descriptor Exercise Movement Techniques explode all trees #6 MeSH descriptor Exercise Therapy explode all trees #7 MeSH descriptor Physical Fitness, this term only #8 (physical* adj5 (fit* or activ*)) #9 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*) #10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 MeSH descriptor Patient Education as Topic, this term only #12 (educat* or inform* or teach* or supervis* or communicat* or leaflet*) #13 MeSH descriptor Survivors, this term only #14 survivor* #15 MeSH descriptor Behavior Therapy explode all trees #16 (behaviour* or behavior* or cognit* or CBT) #17 MeSH descriptor Motivation explode all trees #18 MeSH descriptor Interview, Psychological, this term only #19 (motivat* or interview*) #20 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 (#3 AND #10 AND #20)
Appendix 2. MEDLINE search strategy
MEDLINE 2018 update search
1. exp Neoplasms/ 2. (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ti,ab. 3. 1 or 2 4. exp Exercise/ 5. exp Exercise Movement Techniques/ 6. exp Exercise Therapy/ 7. Physical Fitness/ 8. (physical* adj5 (fit* or activ*)).ti,ab. 9. (exercis* or aerobic* or resistance* or strength* or walk* or endurance* or lifestyle* or behave*).mp. 10. 4 or 5 or 6 or 7 or 8 or 9 11. 3 and 10 12. exp Health Behavior/ 13. risk reduction behavior/ 14. ((promot* or motivat* or advis* or encourag* or assist* or develop* or stimulat* or help* or support* or organis* or aid* or assist* or endors* or prompt* or driv* or inspire* or lead* or inspir* or further* or advocat* or recommend* or endorse* or foster* or champion*) adj5 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*)).ti,ab. 15. 12 or 13 or 14 16. 11 and 15 17. randomized controlled trial.pt. 18. controlled clinical trial.pt. 19. randomized.ab. 20. placebo.ab. 21. clinical trials as topic.sh. 22. randomly.ab. 23. trial.ti. 24. 17 or 18 or 19 or 20 or 21 or 22 or 23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 16 and 26
key:
mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract ti=title sh=subject heading
MEDLINE 2012 search
1. exp Neoplasms/ 2. (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp. 3. 1 or 2 4. exp Exercise/ 5. exp Exercise Movement Techniques/ 6. exp Exercise Therapy/ 7. Physical Fitness/ 8. (physical* adj5 (fit* or activ*)).mp. 9. (exercis* or aerobic* or resistance* or strength* or walk* or endurance*).mp. 10. 4 or 5 or 6 or 7 or 8 or 9 11. Patient Education as Topic/ 12. Patient education handout/ 13. (educat* or inform* or teach* or supervis* or communicat* or leaflet*).mp. 14. Survivors/ or survivor*.mp. 15. exp Behavior Therapy/ 16. (behaviour* or behavior* or cognit* or CBT).mp. 17. exp Motivation/ 18. Interview, Psychological/ 19. (motivat* or interview*).mp. 20. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. 3 and 10 and 20 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. randomized.ab. 25. placebo.ab. 26. clinical trials as topic.sh. 27. randomly.ab. 28. trial.ti. 29. 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 21 and 29 31. exp animals/ not humans.sh. 32. 30 not 31
key: mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract ti=title sh=subject heading
Appendix 3. Embase search strategy
Embase 2018 update search
1. exp neoplasm/ 2. (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ti,ab. 3. 1 or 2 4. exp exercise/ 5. exp kinesiotherapy/ 6. fitness/ 7. (physical* adj5 (fit* or activ*)).ti,ab. 8. (exercis* or aerobic* or resistance* or strength* or walk* or endurance* or lifestyle* or behav*).mp. 9. 4 or 5 or 6 or 7 or 8 10. 3 and 9 11. exp health behavior/ 12. risk reduction/ 13. ((promot* or motivat* or advis* or encourag* or assist* or develop* or stimulat* or help* or support* or organis* or aid* or assist* or endors* or prompt* or driv* or inspire* or lead* or inspir* or further* or advocat* or recommend* or endorse* or foster* or champion*) adj5 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*)).ti,ab. 14. 11 or 12 or 13 15. 10 and 14 16. crossover procedure/ 17. double‐blind procedure/ 18. randomized controlled trial/ 19. single‐blind procedure/ 20. random*.mp. 21. factorial*.mp. 22. (crossover* or cross over* or cross‐over*).mp. 23. placebo*.mp. 24. (double* adj blind*).mp. 25. (singl* adj blind*).mp. 26. assign*.mp. 27. allocat*.mp. 28. volunteer*.mp. 29. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 15 and 29 31. (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 32. 30 not 31
key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Embase 2012 search
1 exp neoplasm/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp. 3 1 or 2 4 exp exercise/ 5 exp kinesiotherapy/ 6 fitness/ 7 (physical* adj5 (fit* or activ*)).mp. 8 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*).mp. 9 4 or 5 or 6 or 7 or 8 10 patient education/ 11 (educat* or inform* or teach* or supervis* or communicat* or leaflet*).mp. 12 survivor/ or survivor*.mp. 13 behavior therapy/ 14 cognitive therapy/ 15 (behaviour* or behavior* or cognit* or CBT).mp. 16 motivation/ 17 interview/ 18 (motivat* or interview*).mp. 19 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20 3 and 9 and 19 21 crossover procedure/ 22 double‐blind procedure/ 23 randomized controlled trial/ 24 single‐blind procedure/ 25 random*.mp. 26 factorial*.mp. 27 (crossover* or cross over* or cross‐over*).mp. 28 placebo*.mp. 29 (double* adj blind*).mp. 30 (singl* adj blind*).mp. 31 assign*.mp. 32 allocat*.mp. 33 volunteer*.mp. 34 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 35 20 and 34 36 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 37 35 not 36
key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 4. AMED search strategy
Amed Ovid 2018 update search
1 exp neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp. 3 1 or 2 4 exp exercise/ 5 exp exercise therapy/ 6 physical fitness/ 7 (physical* adj5 (fit* or activ*)).mp. 8 (exercis* or aerobic* or resistance* or strength* or walk* or endurance* or lifestyle* or behav*).mp. 9 4 or 5 or 6 or 7 or 8 10 exp Health behavior/ 11 ((promot* or motivat* or advis* or encourag* or assist* or develop* or stimulat* or help* or support* or organis* or aid* or assist* or endors* or prompt* or driv* or inspire* or lead* or inspir* or further* or advocat* or recommend* or endorse* or foster* or champion*) adj5 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*)).ti,ab. 12 10 or 11 13 3 and 9 and 12
key: mp=abstract, heading words, title
Amed Ovid 2012 search
1 exp neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp. 3 1 or 2 4 exp exercise/ 5 exp exercise therapy/ 6 physical fitness/ 7 (physical* adj5 (fit* or activ*)).mp. 8 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*).mp. 9 4 or 5 or 6 or 7 or 8 10 exp patient education/ 11 (educat* or inform* or teach* or supervis* or communicat* or leaflet*).mp. 12 survivors/ or survivor*.mp. 13 exp behavior therapy/ 14 (behaviour* or behavior* or cognit* or CBT).mp. 15 exp motivation/ 16 interviews/ 17 (motivat* or interview*).mp. 18 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19 3 and 9 and 18
key: mp=abstract, heading words, title
Appendix 5. CINAHL search strategy
CINAHL 2018 update search
1 exp NEOPLASMS/ 2 (cancer* OR tumor* OR tumour* OR neoplas* OR malignan* OR carcinoma* OR adenocarcinoma* OR choriocarcinoma* OR leukemia* OR leukaemia* OR metastat* OR sarcoma* OR teratoma*).af 3 1 OR 2 4 exp EXERCISE/ 5 exp THERAPEUTIC EXERCISE/ 6 exp PHYSICAL FITNESS/ 7 (physical* AND (fit* OR activ*)).af 8 (exercis* OR aerobic* OR resistance* OR strength* OR walk* OR endurance* or lifestyle* or behave*).af 9 4 OR 5 OR 6 OR 7 OR 8 10 3 and 9 11 exp BEHAVIOR THERAPY/ 12. (risk reduction*) AND (behav*) 13 ((promot* or motivat* or advis* or encourag* or assist* or develop* or stimulat* or help* or support* or organis* or aid* or assist* or endors* or prompt* or driv* or inspire* or lead* or inspir* or further* or advocat* or recommend* or endorse* or foster* or champion*) adj5 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*)).ti,ab. 14 11 or 12 or 13 15 10 AND 14 16 Randomized controlled trials 17 Randomised controlled trials 18 16 or 17 19 15 AND 18
key af=any field
CINAHL 2012 search
1 exp NEOPLASMS/ 2 (cancer* OR tumor* OR tumour* OR neoplas* OR malignan* OR carcinoma* OR adenocarcinoma* OR choriocarcinoma* OR leukemia* OR leukaemia* OR metastat* OR sarcoma* OR teratoma*).af 3 1 OR 2 4 exp EXERCISE/ 5 exp THERAPEUTIC EXERCISE/ 6 exp PHYSICAL FITNESS/ 7 (physical* AND (fit* OR activ*)).af 8 (exercis* OR aerobic* OR resistance* OR strength* OR walk* OR endurance*).af 9 4 OR 5 OR 6 OR 7 OR 8 10 exp PATIENT EDUCATION/ 11 (educat* OR inform* OR teach* OR supervis* OR communicat* OR leaflet*).af 12 CANCER SURVIVORS/ 13 survivor*.af 14 exp BEHAVIOR THERAPY/ 15 (behaviour* OR behavior* OR cognit* OR CBT).af 16 exp MOTIVATION/ 17 MOTIVATIONAL INTERVIEWING/ 18 (motivat* OR interview*).af 19 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 20 3 AND 9 AND 19 21 RANDOMIZED CONTROLLED TRIALS/ 22 20 and 21
Appendix 6. PsycINFO search strategy
PsycINFO 2018 update search
1 neoplasms.af 2 ((cancer* OR tumor* OR tumour* OR neoplas* OR malignan* OR carcinoma* OR adenocarcinoma* OR choriocarcinoma* OR leukemia* OR leukaemia* OR metastat* OR sarcoma* OR teratoma*)).ti,ab 3 exercise.af 4 (physical AND fitness).af 5 ((physical* adj5 (fit* OR activ*))).ti,ab 6 ((exercis* OR aerobic* OR resistance* OR strength* OR walk* OR endurance* OR lifestyle* OR behave*)).af 7 1 OR 2 8 3 OR 4 OR 5 OR 6 9 (health AND behaviour).af 10 (risk AND reduction AND behaviour).af 11 (((promot* OR motivat* OR advis* OR encourag* OR assist* OR develop* OR stimulat* OR help* OR support* OR organis* OR aid* OR assist* OR endors* OR prompt* OR driv* OR inspire* OR lead* OR inspir* OR further* OR advocat* OR recommend* OR endorse* OR foster* OR champion*) adj5 (exercis* OR aerobic* OR resistance* OR strength* OR walk* OR endurance*))).ti,ab 12 9 OR 10 OR 11 13 7 AND 8 AND 12
PsycINFO Ovid 2012 search
1 exp neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp. 3 1 or 2 4 exp exercise/ 5 physical fitness/ 6 (physical* adj5 (fit* or activ*)).mp. 7 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*).mp. 8 4 or 5 or 6 or 7 9 client education/ 10 (educat* or inform* or teach* or supervis* or communicat* or leaflet*).mp. 11 survivors/ or survivor*.mp. 12 exp cognitive behavior therapy/ 13 exp behavior therapy/ 14 (behaviour* or behavior* or cognit* or CBT).mp. 15 exp motivation/ 16 motivational interviewing/ 17 (motivat* or interview*).mp. 18 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19 3 and 8 and 18 20 clinical trials/ 21 (random* or trial* or group* or placebo*).mp. mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures 22 20 or 21 23 19 and 22
key: [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
Appendix 7. PEDro search strategy
PEDro 2012 search
Title and abstract: “cancer”
Therapy: fitness training (selected)
Sub discipline: oncology (selected)
Method: clinical trial (selected)
Appendix 8. SPORTS DISCUS search strategy (EBSCO host)
Sports discus update 2018 search
1. TX cancer* OR tumor* OR tumour* OR neoplas* OR malignan* OR carcinoma* OR adenocarcinoma* OR choriocarcinoma* OR leukemia* OR leukaemia* OR metastat* OR sarcoma* OR teratoma* (26,616)
2. TX randomi*ed controlled trial (12,682)
3. (TX randomi*ed controlled trial) AND (S4 AND S5) (636)
4. Limiters ‐ Published Date: 20120101‐20171231 (411)
Appendix 9. Cochrane Collaboration’s tool for assessing risk of bias
Random sequence generation
• Low risk of bias (e.g. participants assigned to treatments on basis of a computer‐generated random sequence or a table of random numbers) • High risk of bias (e.g. participants assigned to treatments on basis of date of birth, clinic ID number or surname, or no attempt to randomly assign participants) • Unclear risk of bias (e.g. not reported, information not available)
Allocation concealment
• Low risk of bias (e.g. when the allocation sequence could not be foretold) • High risk of bias (e.g. allocation sequence could be foretold by participants, investigators or treatment providers) • Unclear risk of bias (e.g. not reported)
Blinding of participants and personnel
• Low risk of bias, if participants and personnel were adequately blinded • High risk of bias, if participants were not blinded to the intervention that the participant received • Unclear risk of bias, if this was not reported or was unclear
Blinding of outcome assessors
• Low risk of bias, if outcome assessors were adequately blinded • High risk of bias, if outcome assessors were not blinded to the intervention that the participant received • Unclear risk of bias, if this was not reported or was unclear
Incomplete outcome data
We recorded the proportions of participants whose outcomes were not reported at the end of the study. We coded a satisfactory level of loss to follow‐up for each outcome as follows
• Low risk of bias, if fewer than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms • High risk of bias, if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms • Unclear risk of bias, if loss to follow‐up was not reported
Selective reporting of outcomes
• Low risk of bias (e.g. review reports all outcomes specified in the protocol) • High risk of bias (e.g. if it is suspected that outcomes have been selectively reported) • Unclear risk of bias (e.g. if it is unclear whether outcomes were selectively reported)
Other bias
• Low risk of bias, if no other source of bias is suspected and the trial appears to be methodologically sound • High risk of bias, if it is suspected that the trial was prone to an additional bias • Unclear risk of bias, if uncertainty exists about whether an additional bias may have been present
Data and analyses
Comparison 1. Aerobic exercise tolerance.
Comparison 2. Strength tests (all cancers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Strength tests (all cancers, 12 weeks of follow‐up) | 4 | 278 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.03, 0.44] |
| 2 Strength tests (all cancers: 12 weeks of follow‐up: sensitivity analysis) | 2 | 231 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.09, 0.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
al‐Majid 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Participants in the usual‐care group received usual care, which did not involve exercise, and were instructed to document and report any exercise activities they engaged in while on the study. Similarly, the exercise‐group participants to report engagement in non‐protocol exercise activities during the study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | None |
Bourke 2011a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Both groups had access to standard care, which consisted of a holistic nurse‐led colorectal cancer follow‐up service | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by an independent researcher via code numbers using nQuery statistical software |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken by a senior academic who was not directly involved in the recruitment or assessment of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes were assessed by an experienced exercise physiologist, who was blind to the group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was used to compare participants in the groups to which they were randomly assigned, with data carried over from previous visits in cases of participant withdrawal |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Low recruitment rate (18/180) could represent a biased sample |
Bourke 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Men randomised to usual care were followed up in the urology clinic and seen by an oncology nurse specialist and urologist. The treating physicians were informed that the man was participating in a lifestyle intervention study and further information would be available on application. No restrictions were placed on exercise/dietary behaviours over the period of the study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken (1:1) by a senior academic independent of the study, at the patient level using nQuery statistical software. |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken (1:1) by a senior academic independent of the study, at the patient level using nQuery statistical software. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation was undertaken (1:1) by a senior academic independent of the study, at the patient level using nQuery statistical software. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete outcome data at 6 months follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None. |
Cadmus 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
Adherence:
|
|
| Description of usual care | Unclear | |
| Notes | Only YES study included in the review because of the requirement that participants must be sedentary at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme randomly assigned each YES study participant with equal probability to the exercise group or the usual care group |
| Allocation concealment (selection bias) | Low risk | The randomisation code for each participant was obtained by the principal investigator (who was not involved in recruitment or data collection) only after baseline measures for that individual had been completed and staff conducting clinic visits did not have access to the randomisation programme |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were conducted according to the intention‐to‐treat principle. Baseline QOL values were carried forward for the five IMPACT study participants (three exercisers and two controls) and 10 YES study participants (five exercisers and five controls) for whom six‐month data were unavailable |
| Selective reporting (reporting bias) | Low risk | None, all outcomes reported |
| Other bias | Low risk | None |
Campbell 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Participants randomised to the control were asked to maintain usual lifestyle and offered a 12‐week exercise programme upon study completion. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following completion of baseline measures, eligible participants were randomised using permutated blocks of 4 to 6 in a 1:1 ratio to the aerobic exercise intervention group or delayed exercise control. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Low study recruitment rate |
Cantarero‐Villanueva 2012b.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Participants followed usual care recommendations by an oncologist in relation to a healthy lifestyle. Breast cancer survivors received a document printable dossier from the oncologist where they found recommendations related to nutrition, lifestyle behaviours, and exercise. A follow‐up of the physical activity during the control period was used to control bias detected in previous studies with exercise in cancer survivors [35,36]. For that purpose, we used the Spanish version of the Minnesota Leisure Time Physical Activity Questionnaire [37]. Control group were offered the intervention after the 8 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomised, controlled clinical study was conducted. Eligible participants who agreed to participate were randomly assigned into two groups: WATER group who received the water exercise programme or CONTROL group who received the usual care treatment for breast cancer. |
| Allocation concealment (selection bias) | Low risk | We allocated patients to WATER or CONTROL groups into two randomisation cycles using a computer‐generated numbers. The sequence was entered into numbered opaque envelopes by an external member, and they were opened after completion of the baseline assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were assessed 1 week before and after the intervention by an individual blind to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None |
Cavalheri 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Participants in the control group were instructed to continue to perform their usual activities during the period of the study. They received weekly phone calls from a research assistant, which consisted of general conversation as well as standardised questions about their health and well‐being. These phone calls allowed the investigators to maintain contact with those in the control group and optimise their retention in the study and also served to minimise bias resulting from differences in attention provided by the investigators to the participants during the intervention period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated and man‐aged by an independent researcher using a computer. |
| Allocation concealment (selection bias) | Low risk | concealed using sequentially‐numbered opaque envelopes. The sequence was stratified according to the hospital from which the participant was recruited and for the use (or not) of adjuvant chemotherapy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary investigator, who was responsible for the baseline and post‐intervention period assessments, was not aware of whether a participant had been allocated control or intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing patient data in both arms with reasons not given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Poor adherence rates (44%) |
Daley 2007a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | All participants continue to receive usual care from their health team | |
| Notes | Mean and SD data for aerobic exercise tolerance at 8 and 24 weeks provided by authors in response to email request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A telephone randomisation service was provided by an independent studies unit. Randomisation to the three treatment arms was done on a 1:1:1 ratio and was performed using stratified random permuted blocks (with block size of six). Stratification factors were chemotherapy (yes/no) and tamoxifen (yes/no) |
| Allocation concealment (selection bias) | Low risk | Randomisation service was provided by an independent studies unit telephone service |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded to participants’ group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Little’s D test indicated that missing data were missing completely at random (2 88.2; df 1290; P = 0.99). Data were analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Drouin 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Each participant was treated with external beam radiation five days per week for seven weeks. The affected breast and regional lymph nodes received a 4500 to 5000 cGy dose in 200c Gy fractions with a boost of 1000 to 1600 cGy delivered to the primary tumour bed. Treatment dosages were similar between groups | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 23 participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Hayes 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
Qualitative quotes:
|
|
| Description of usual care | Physiotherapy, massage, compression, lymphatic drainage or laser therapy for lymphoedema | |
| Notes | Resistance aspect of this intervention will be excluded from analysis because of unclear exercise metrics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated using a computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All measures were assessed pre‐intervention (time 1; T1), immediately postintervention (time 2; T2) and at 12‐week follow‐up (time 3; T3) and were conducted by the same assessor, who was blinded to participant group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants (n = 32) participated in T1 and T2, whereas data were unavailable for two participants (one in the IG and one in the CG) at T3. To ensure that missing data did not contribute to the results found, data analysis was repeated with these two participants excluded, and no differences in results were observed (data not shown) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Adherence data on home‐based aspect of the intervention not clear |
Irwin 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Women in the usual care group were instructed to continue with their usual activities. Participants in both groups were provided with a breast cancer specific educational booklet developed for the HOPE study, which discussed topics such as lymphoedema and fatigue. This booklet was individually discussed during the exercise training for the exercise group and in a monthly phone call for the usual care group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were grouped according to the intention‐to‐treat principle. Permuted block randomisation (at 1:1 ratio) with random block size was performed, stratified by joint pain before AI therapy and current bisphosphonate use (related to our secondary aim of bone mass). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced across both groups and intention to treat applied to the analysis/ |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None. |
Kaltsatou 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Unclear | |
| Notes | Resistance aspect of this intervention will be excluded from analysis because of unclear exercise metrics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Method of measuring exercise behaviour and adherence not reported |
Kim 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Usual cancer care included general information on the benefits of exercise but did not include specific instructions or further guidance for exercise. Seventy‐eight per cent of women had Stage I and Stage II breast cancer, and chemotherapy was the most common type of adjuvant therapy (48.8%), followed by radiotherapy (34.1%) and a combination of chemotherapy and radiotherapy (17.1%). Regimens of adjuvant therapy most often consisted of Adriamycin 60 mg/m2 and cytoxan 600 mg/m2 every 2 to 3 weeks for 3 doses with or without Taxol 145 mg/m2 every 2 to 3 weeks for 3 to 4 doses. Radiotherapy was typically composed of delivering a total of 45 to 65 Gy over 6 to 7 weeks with booster doses of 20 Gy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on only 41 of 74 randomly assigned participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Women randomly assigned but excluded had higher BMI and more advanced stages of cancer |
Kim 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Instructured to maintain their routine physical activities and not to participate any new exercise programmes during 12 weeks. After the final assessments, they had the option of participation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following baseline assessments, 30 participants were randomly assigned to either an exercise intervention group or a control group using a sealed, computer random number generator with an allocation ratio of 1 to 1 |
| Allocation concealment (selection bias) | Low risk | Following baseline assessments, 30 participants were randomly assigned to either an exercise intervention group or a control group using a sealed, computer random number generator with an allocation ratio of 1 to 1 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Four research staff members who were unaware of group assignment performed all outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts similar in both groups and reasons given. Reasons given for lack of inclusion in final analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Age differences between groups in baseline demographics were present. Adherence data is vague. |
McKenzie 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Unclear | |
| Notes | Resistance aspect of this intervention will be excluded from analysis because of unclear exercise metrics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Adherence to prescribed exercise not reported |
Mohamady 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | The control group, who performed the usual daily living activities in addition to administration of their medication and nutritional support. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done via random number generator and opening opaque envelopes prepared by an independent individual. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done via random number generator and opening opaque envelopes prepared by an independent individual. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement. |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes. |
| Other bias | High risk | No adherence data. |
Musanti 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation table maintained by office staff in the clinical research office |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physical fitness testing was performed at a hospital‐based fitness centre. The same research assistant, blinded to participant group allocation, performed these measurements at pre‐intervention and postintervention measurement time points |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Thirteen women (24%) did not complete their assigned 12‐week programme |
| Selective reporting (reporting bias) | High risk | Waist, upper and mid and lower arm circumference measures not reported |
| Other bias | High risk |
|
Perna 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified by cancer stage and were randomly assigned to groups |
| Allocation concealment (selection bias) | Low risk | Participant assignment to groups at enrolment was concealed from the project director |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians monitoring graded exercise tests were blinded to participant group assignment. Similarly, a physical therapist or an exercise physiologist, blinded to participant assignment, performed strength assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent‐to‐treat analysis done and multiple imputation used |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | Numbers randomly assigned to intervention and control groups are unclear, as are numbers completing in each arm |
Pinto 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Unclear | |
| Notes | *We estimated study recruitment rate on the basis of numbers randomly assigned of those approached and eligible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exercise tolerance test performed but no control group comparison data reported. 38% lost to follow‐up |
| Selective reporting (reporting bias) | High risk | None of the physiological assessments were performed for the control group at 12 weeks |
| Other bias | High risk | A statistically significant difference was noted between groups for body esteem at baseline (weight concerns and physical condition sub scales) |
Pinto 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Unclear | |
| Notes | *Data from baseline questionnaires indicated that two participants in the intervention group were active at baseline (i.e. a discrepancy was noted between telephone screening and assessment). However, the author has advised that outliers were removed during data analysis of study outcomes. Author advised that accelerometer data should have been reported as kcal/hour) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat approach used and low attrition reported (5%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Significantly more control group participants were receiving hormone treatment: 49% versus 74% in the intervention and control groups, respectively (P = 0.015). Objective accelerometer data do not support the self‐reported physical activity behaviour |
Pinto 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a 'low' or 'high' risk judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% attrition reported |
| Selective reporting (reporting bias) | High risk | Accelerometer data not reported |
| Other bias | High risk | Accelerometer correlation with self‐report questionnaires is weak at follow‐up points when significant differences between groups in physical activity are reported (i.e. r = 0.32 at 3 months). Substantial contamination in the control group |
Rogers 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Usual care participants received printed American Cancer Society materials describing physical activity recommendations for cancer survivors (e.g. Living Smart: The American Cancer Society’s guide to eating healthy and being active). No additional instructions regarding physical activity were given with the materials | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to one of the two study group conditions was completed using computer‐generated numbers in blocks of 4 within each recruiting site to facilitate an even distribution between study conditions during each recruitment wave. |
| Allocation concealment (selection bias) | Low risk | Random assignment was kept in sealed, opaque envelopes which were opened in the order in which participants completed baseline testing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were measured at baseline, 3 months, 6 months and 12 months by blinded members of the team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts similar in both groups with reasons given. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Physical activity reported at baseline, differed between objective and subjective measures. |
Scott 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | The control group received a healthy eating booklet (Eat well), which also included brief advice on keeping active. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using minimisation (on the advice of statistician at the Leeds CTU [we used their distant randomisation service]) to balance the potential confounding variables of chemotherapy, hormone treatment or no hormone treatment. Using this approach, the first participant is allocated a treatment at random. For each subsequent participant a decision has to be made about which treatment would lead to better balance between the groups in the variables of interest. The randomisation ratio was 1:1. |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted by and independent researcher and not revealed until baseline assessment was complete |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A trained technician was blinded to carry out outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up disclosed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None |
Thomas 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Process measures |
|
|
| Compliance |
|
|
| Description of usual care | Women in the usual care group were instructed to continue with their usual activities. If a participant wanted to exercise, she was told she could, but the exercise programme and training materials would not be offered to her until the end of the study. At the end of the study, women in the usual care condition were offered three supervised training sessions, a pedometer, exercise handouts, and the results of their clinical tests. Additionally, all study participants received quarterly newsletters that highlighted issues relevant to breast cancer survivorship. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After completion of all baseline measures, each participant was randomly assigned with equal probability to either the exercise or usual‐care group. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by using a random number generation, and group assignment was placed in a sealed envelope, which was opened by the study coordinator at the time of randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For each participant, the same data that were collected at the baseline visit were collected in a similar manner at 6 months postrandomisation by staff blinded to the participant’s group, *from linked study Irwin 2008* |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were present for 89% of the participants at 6 months. |
| Selective reporting (reporting bias) | High risk | No body fat or lean mass values given. No data given from food frequency questionnaire. |
| Other bias | High risk | Poor recruitment rate (9.5%) |
ADP: androgen‐deprivation therapy; BMI: body mass index; BPI: Brief Pain Inventor; yHR: heart rate; m: metre; MRI: magnetic resonance imaging; NSCLC: non‐small cell lung cancer; PAR: Physical Activity Recall; QoL: quality of life; RPE: Rating of Perceived Exertion; SD: standard deviation; VAS: visual analogue scale;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2016 | Participants were not sedentary at baseline |
| Ahmed 2006 | Sedentary status at baseline is unclear |
| Alibhai 2014 | Participants were not sedentary at baseline |
| Ames 2011 | Exercise prescription metrics are unclear |
| Anderson 2012 | Sedentary status at baseline is unclear |
| Anderson 2013 | Not a homogenous cancer cohort |
| Anderson 2015 | Report ‐ not a full‐text paper |
| Anulika 2015 | Unable to access full text |
| Arbane 2011 | Author advised that baseline sedentary status was not assessed |
| Arbane 2014 | Patients were hospitalised |
| Arikawa 2013 | Not a cancer cohort |
| Banerjee 2013 | Poster |
| Baruth 2015 | Unclear if participants were meeting the baseline moderate exercise sedentary criteria |
| Battaglini 2007 | Author advised that baseline sedentary status was not assessed |
| Battaglini 2008 | Linked to Battaglini 2007 |
| Bloom 2013 | Poster |
| Bracha 2012 | Unclear of duration and intensity of prescribed exercise |
| Brdareski 2012 | No usual care comparison |
| Brown 2012 | Linked to Schmitz 2009 and Schmitz 2010 |
| Bruno 2018 | Participants were not sedentary at baseline |
| Buchan 2016 | No usual care comparison |
| Buffart 2013 | Poster |
| Buffart 2014a | Not a homogenous cancer cohort |
| Buffart 2014b | Not a homogenous cancer cohort |
| Campbell 2005 | Unclear if participants were meeting the baseline moderate exercise sedentary criteria |
| Cantaero‐Villanueva 2013 | Participants were not sedentary |
| Cantaero‐Villanueva 2016 | Unclear if participants were meeting the baseline moderate exercise sedentary criteria |
| Cantarero‐Villanueva 2011 | Intervention exercise prescription metrics unclear |
| Cantarero‐Villanueva 2012a | Linked to Cantarero‐Villanueva 2011 |
| Carmack Taylor 2004 | Linked to Carmack Taylor 2006 |
| Carmack Taylor 2006 | Exercise prescription metrics are unclear |
| Carmack Taylor 2007 | Linked to Carmack Taylor 2006 |
| Carson 2009 | Author advised that baseline sedentary status was not assessed |
| Casla 2015 | Participants were not sedentary at baseline |
| Cerulli 2014 | Unknown exercise prescription |
| Chen 2015 | Baseline exercise activity inclusion criteria is greater then 90 minutes |
| Chen 2016 | Baseline exercise activity inclusion criteria is greater then 90 minutes |
| Cho 2006 | Sedentary status at baseline is unclear |
| Christensen 2014 | Participants not sedentary at baseline |
| Chuang 2017 | Exercise prescription is not clear |
| Coleman 2003 | Exercise prescription metrics are unclear |
| Cornette 2016 | Exercise prescription is not clear |
| Cornie 2013a | Participants not sedentary at baseline |
| Cornie 2013b | Sedentary status at baseline is unclear |
| Cornie 2014 | Protocol paper |
| Cornie 2015 | Participants not sedentary at baseline |
| Courneya 2012 | Participants not sedentary at baseline |
| Courneya 2013 | Participants not sedentary at baseline |
| Courneya 2014a | Author advised us that the participants were not sedentary at baseline |
| Courneya 2014b | Author advised us that the participants were not sedentary at baseline |
| Courneya 2015 | Participants not sedentary at baseline |
| Courneya 2016a | Participants not sedentary at baseline |
| Courneya 2016b | Linked to Courneya 2013 paper |
| Culos Reed 2010 | Exercise prescription metrics are unclear |
| Danhauer 2009 | Sedentary status at baseline is unclear |
| Daubenmier 2006 | Linked to Ornish 2005 |
| De Jesus 2013 | Poster |
| Demark‐Wahnefried 2015 | No usual care comparison |
| DeNysschen 2011 | Sedentary status at baseline is unclear |
| Dieli‐Conwright 2014 | Protocol paper |
| Dieperink 2017 | Authors confirmed that participants were not sedentary at baseline |
| Diepold 2016 | The participants were in palliative care |
| Do 2015 | Cross‐over trial |
| Dolan 2010 | START trial includes non‐sedentary participants |
| Dolan 2014 | Poster |
| Dolan 2016 | Participants not sedentary at baseline |
| Donmez 2017 | Exercise prescription is not clear |
| Donnelly 2011 | Author advised that cohort was not sedentary at baseline |
| Edvarsen 2015 | Sedentary status at baseline is unclear |
| Emslie 2007 | Linked to Mutrie 2007 |
| Eriksen 2017 | Participants were not sedentary at baseline |
| Fan‐Ko 2017 | Exercise prescription is not clear |
| Fernandez‐Lao 2012 | Intervention exercise prescription metrics unclear |
| Fields 2015 | Poster |
| Fields 2017 | Participants were not sedentary at baseline |
| Forbes 2017 | Mixed cancer cohort |
| Frattaroli 2008 | Linked to Ornish 2005 |
| Friedenrich 2016 | Participants were not cancer patients |
| Furzer 2016 | Not a homogenous cancer cohort |
| Galiano‐Castillo 2017 | Unclear whether the participants were sedentary at baseline |
| Galvão 2010 | Sedentary status at baseline is unclear |
| Galvão 2011 | Linked to Galvão 2010 |
| Galvão 2017 | Participants were not sedentary at baseline |
| Gaskin 2016 | Participants were not sedentary at baseline |
| Gehring 2014 | Poster |
| Gehring 2015 | Poster |
| Gehring 2018 | Authors confirmed participants were not sedentary at baseline |
| Gerland 2012 | Abstract |
| Giallauria 2014 | Sedentary behaviour was not assessed |
| Gokal 2016 | Participants were not sedentary at baseline |
| Granger 2013 | Participants were hospitalised |
| Greenlee 2013 | Cross‐over trial |
| Gruenigen 2012a | Participants were not sedentary at baseline |
| Gruenigen 2012b | Linked to Gruenigen 2012a |
| Guinan 2013 | Participants were not sedentary at baseline |
| Guinan 2017 | Authors confirmed participants were not sedentary at baseline. |
| Gómez 2011 | Cohort not sedentary at baseline |
| Haines 2010 | Sedentary status at baseline is unclear |
| Hanssens 2012 | Abstract |
| Hartman 2015 | Protocol paper |
| Hatchett 2013 | Intensity of exercise is unclear |
| Hayes 2011 | Author advised that baseline sedentary status was not assessed |
| Hayes 2012 | Paper not published yet |
| Hayes 2013 | Author clarified that the participants were not sedentary at baseline |
| Hayes 2014 | Trial still ongoing, paper not published yet |
| Headley 2004 | Sedentary status at baseline is unclear |
| Heim 2007 | Sedentary status at baseline is unclear |
| Herbert 2012 | Participants were not sedentary at baseline. |
| Herrero 2006 | Sedentary status at baseline is unclear |
| Ho 2016 | No intensity reported |
| Hoffman 2013 | Poster |
| Hoffman 2017 | Unclear whether the participants were sedentary at baseline |
| Hojan 2016 | Unable to gain copy of paper |
| Hojan 2017 | Unsure whether participants were sedentary at baseline |
| Huang 2015 | Participants were not sedentary at baseline |
| Hubbard 2016 | Participants were not sedentary at baseline |
| Husebo 2014 | Participants were not sedentary at baseline |
| Hwang 2012 | Not all participants were randomised |
| James 2012 | Poster |
| Jarden 2013 | Study protocol |
| Jeffs 2013 | intensity of the exercise is unclear |
| Jensen 2015a | Abstract |
| Jensen 2015b | Participants were hospitalised |
| Jensen 2016 | Length of follow‐up is less than 6 weeks |
| Jones 2014a | Participants were not sedentary at baseline |
| Jones 2014b | Participants were not sedentary at baseline |
| Kalter 2015 | Moderator paper on previous excluded study |
| Kampshoff 2015 | Not homogenous cancer cohort |
| Kampshoff 2016 | Mixed cancer cohort |
| Kanera 2016 | Mixed cancer cohort |
| Kanera 2017 | Mixed cancer cohort |
| Kavanagh 2009 | Sedentary status at baseline is unclear |
| Kilbreath 2006 | Sedentary status at baseline is unclear |
| Kilbreath 2012 | Sedentary status at baseline is unclear |
| Kim 2010 | Sedentary status at baseline is unclear |
| Klepin 2015 | Abstract |
| Klinkhammer‐Schalke 2012 | Sedentary status at baseline is unclear |
| Kwiatkowski 2013 | Participants were not sedentary at baseline |
| Lahart 2016 | Participants were not sedentary at baseline |
| Lai 2017 | Follow‐up is less than 6 weeks |
| Lee 2012a | Study protocol |
| Lee 2012b | Study protocol |
| Lee 2014 | Participants were not sedentary at baseline |
| Leone 2016 | No frequency/duration/intensity of exercise reported |
| Ligibel 2008 | Author advised that exercise intensity was not clear |
| Ligibel 2009 | Linked to Ligibel 2008 |
| Ligibel 2016 | Exercise intensity was unclear |
| Lin 2014 | Not randomised controlled trial |
| Litterini 2013 | Not homogenous cancer cohort |
| Livingston 2015 | Participants were not sedentary at baseline |
| Lynch 2014 | No frequency/duration/intensity data |
| Lyons 2016 | Exercise is carried out for couples |
| MacVicar 1989 | Sedentary status at baseline is unclear |
| Manassero 2007 | Exercise prescription metrics are unclear |
| Martin 2013 | Unclear if the participants were sedentary at baseline |
| Mayo 2014 | Not homogenous cancer cohort |
| McClure 2010 | Sedentary status at baseline is unclear |
| McGowan 2013 | No frequency/duration/intensity data |
| McGuire 2011 | Linked to Waltman 2010 |
| McNeely 2004 | Author advised that cohort was not sedentary |
| Milecki 2013 | Participants were not sedentary at baseline |
| Mina 2013 | No usual care comparison |
| Mock 1994 | Sedentary status at baseline is unclear |
| Mock 1997 | Sedentary status at baseline is unclear |
| Mock 2005 | Sedentary status at baseline is unclear |
| Molassiotis 2015 | Inspiratory muscle training |
| Moller 2015 | Unable to source copy of full‐text paper |
| Monga 2007 | Sedentary status at baseline is unclear |
| Morielli 2018 | Not a randomised controlled trial |
| Mustian 2008 | Exercise prescription metrics are unclear |
| Mustian 2015 | Poster |
| Mutrie 2007 | Author advised that cohort was not sedentary at baseline |
| Naumann 2012 | Not a randomised controlled trial |
| Newton 2014 | Poster |
| Nieman 1995 | Sedentary status at baseline is unclear |
| Nikander 2007 | Sedentary status at baseline is unclear |
| Nikander 2012 | Participants were not sedentary at baseline. |
| Nilsen 2015 | Unclear whether participants were sedentary at baseline or not |
| Nobes 2012 | Poster |
| Nuri 2012 | Unclear on inclusion or exclusion criteria |
| Nuri 2016 | Unclear whether participants were sedentary at baseline or not. |
| Nyrop 2017 | Not sedentary at baseline |
| O'Neil 2015 | Unclear on intensity of prescribed exercise |
| Ohira 2006 | Linked to Schmitz 2005 |
| Ornish 2005 | Sedentary status at baseline is unclear |
| Ornish 2008a | Linked to Ornish 2005 |
| Ornish 2008b | Linked to Ornish 2005 |
| Park 2012 | The interventions were prescribed continence exercises rather than aerobic/resistance exercise |
| Park 2016 | Author confirmed participants were not sedentary at baseline |
| Payne 2008 | Sedentary status at baseline is unclear |
| Philips 2012 | Not a homogenous cancer cohort |
| Pickett 2002 | Sedentary status at baseline is unclear |
| Pinto 2013a | No usual care comparison |
| Pinto 2013b | No usual care comparison |
| Pinto 2015 | No usual care comparison |
| Porserud 2014 | Intensity of prescribed exercise was unclear |
| Portela 2008 | Author advised that baseline sedentary status was not assessed |
| Rabin 2016 | The cancer cohort was not homogenous |
| Rahnama 2010 | Author not able to confirm sedentary status |
| Rao 2012 | Unclear whether participants were sedentary at baseline or not. |
| Reis 2013 | Did not report or measure intensity |
| Rogers 2009 | Author advised that cohort was not sedentary at baseline |
| Rogers 2012 | Author advised that cohort was not sedentary at baseline |
| Rogers 2013a | No usual care comparison |
| Rogers 2013b | Participants were not sedentary at baseline |
| Rogers 2014 | Participants were not sedentary at baseline |
| Rogers 2015b | Linked to Rogers 2014 |
| Saarto 2012a | Participants were not sedentary at baseline |
| Saarto 2012b | Participants were not sedentary at baseline |
| Sajid 2013 | No usual care comparison |
| Samuel 2013 | Control was advised to keep physically active as possible |
| Sandel 2005 | Sedentary status at baseline is unclear |
| Schmidt 2015 | No usual care comparison |
| Schmidt 2017a | Linked to Schmidt 2015 |
| Schmidt 2017b | Unclear whether participants were sedentary or not at baseline |
| Schmitz 2009 | Author advised intensity not assessed |
| Schmitz 2010 | Author advised intensity not assessed |
| Schmitz 2015a | Linked to Schmitz 2015b |
| Schmitz 2015b | Participants were not cancer survivors |
| Schuler 2017 | Not homogenous cancer cohort |
| Schwartz 2015 | Not homogenous cancer cohort |
| Scruggs 2018 | Exercise prescription is not clear |
| Sebio Garcia 2017 | Unclear if the participants were sedentary at baseline |
| Segal 2001 | Author advised exercise behavior not formally assessed at baseline |
| Segal 2003 | Author advised exercise behavior not formally assessed at baseline |
| Segal 2009 | Author advised exercise behavior not formally assessed at baseline |
| Sener 2017 | Intensity of exercise is not clear |
| Sheppard 2016 | Intensity of exercise is not clear |
| Shobeiri 2016 | Participants were not sedentary at baseline |
| Short 2012 | Poster |
| Short 2017a | Participants were not sedentary at baseline |
| Short 2017b | Participants were not sedentary at baseline |
| Singh 2015 | Cross‐over trial |
| Skinner 2016 | Participants were not sedentary at baseline |
| Sohl 2016 | No usual care comparison |
| Spahn 2013 | No usual care comparison |
| Stacey 2016 | Mediator paper reporting on previous unsuitable randomised controlled trial |
| Stefanelli 2013 | Unclear whether the participants were sedentary at baseline or not |
| Stolley 2017 | Unclear whether participants were sedentary at baseline |
| Streckman 2014 | Participants were hospitalised |
| Sturgeon 2017 | Participants were not sedentary at baseline |
| Swisher 2015 | Participants were not sedentary at baseline |
| Taafe 2017 | Usual care participants were active |
| Taleghani 2012 | Participants were not adults |
| Taso 2014 | Intensity not reported |
| Terranova 2017 | Unclear whether participants were sedentary at baseline |
| Tomasello 2017 | Compared with a 'healthy' control |
| Tometich 2017 | Participants were not sedentary at baseline |
| Travier 2015 | Participants were not sedentary at baseline |
| Trinh 2014 | Linked to Mutrie 2007 |
| Uth 2014 | Participants were not sedentary at baseline |
| Uth 2016 | Participants were not sedentary at baseline |
| Van Vulpen 2016 | Not a homogenous cancer cohort |
| van Waart 2015 | Unclear whether participants were sedentary at baseline |
| von Gruenigen 2008 | Author advised that cohort was not sedentary at baseline |
| von Gruenigen 2009 | Linked to von Gruenigen 2008 |
| von Gruenigen 2012 | Author advised that cohort was not sedentary |
| Waltman 2010 | Author advised that cohort was not sedentary |
| Wang 2012 | Sedentary status at baseline is unclear |
| Wasley 2018 | Mixed cancer cohort |
| Wiskemann 2017 | Participants were not sedentary at baseline |
| Xu 2015 | Participants were not sedentary at baseline |
| Yang 2011 | Sedentary status at baseline is unclear |
| Yeo 2012 | Author not able to clarify exercise metrics |
| Yuen 2007 | Author advised that cohort was not sedentary at baseline |
| Yun 2013 | Not homogenous cancer cohort |
| Zhang 2018 | Unclear whether participants were sedentary at baseline |
| Zhao 2016 | Not a randomised controlled trial |
| Zhou 2015 | Conference paper |
| Zimmer 2014 | Compared with a 'healthy control' |
| Zimmer 2016 | Protocol paper |
| Zopf 2012 | Poster |
Characteristics of studies awaiting assessment [ordered by study ID]
Bai 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Bai S‐M, Ma C, Liu Y‐M, Xue W‐P, Luo M, Ou Z‐H. Effects of cognitive behavior intervention and cinesiateics on the quality of life of patients with nasopharyngeal carcinoma after radiotherapy. Chinese Journal of Clinical Rehabilitation 2004;8(29):6312–3. |
Chen 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Chen J, Luo A, He Y. Influence of postoperative rehabilitation exercises on functional recovery of ill limb of breast cancer patients. Chinese Nursing Research 2010;24(4A):875–7. |
Cho 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Cho OH. Effects of a comprehensive rehabilitation programme for mastectomy patients. Taehan Kanho Hakhoe Chi 2004;34(5):809–19. |
Choi 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Still awaiting translation: Choi, J. Y. Kang, H. S. Effects of a home‐based exercise program for patients with stomach cancer receiving oral chemotherapy after surgery. Journal of Korean Academy of Nursing, 2012; 42(1):95‐104 |
Dong 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Dong HY, Wang ZF, Cai L. Correlation between quality of life and rehabilitative guidance education in the postoperative patients with breast cancer. Chinese Journal of Clinical Rehabilitation 2006; 10(42), 28‐30. |
Guo 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Guo Y‐M. Effects of moderate strength and endurance exercise on emotion and quality of sleep in patients with malignant tumor. Chinese Journal of Clinical Rehabilitation 2004;8(35):7896–7. |
Hu 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Still awaiting translation: Hu, H. F. Li, T. C. Liu, L. C. Wu, C. T. Wang, Y. J. Effects of a walking program on fatigue and exercise capacity in post‐surgery breast cancer women, Hu li za zhi [Journal of nursing]. 2013 Oct;60(5):53‐63 |
LeVu 1997.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Le Vu B, Dumortier A, Guillaume MV, Mouriesse H, Barreau‐Pouhaer L. Efficacy of massage and mobilization of the upper limb after surgical treatment of breast cancer. Bulletin du Cancer 1997;80(10):957–61. |
Oliveira 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Oliveira MM, Souza GA, Miranda Mde S, Okubo MA, Amaral MT, Silva MP, Gurgel MS. Upper limb exercises during radiotherapy for breast cancer and quality of life. Revista Brasileira de Ginecologia e Obstetrícia 2010;32(3):133‐8. |
Park 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Park HS, Cho GY, Park KY. The effects of a rehabilitation program on physical health, physiological indicator and quality of life in breast cancer mastectomy patients. Taehan Kanho Hakhoe Chi 2006;36(2):310‐20. |
Wang 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Wang Y;Yao J‐F;Yang J‐Y. Effect of rehabilitation exercises on the recovery outcomes of lung function in postoperative patients with lung cancer. Zhongguo Linchuang Kangfu (Chinese Journal of Clinical Rehabilitation) 2005; 9(39):14‐16. |
Zhang 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Zhang T, Chang XM, He YG, Huang HX, Fan KS. Effects of rehabilitation therapy in relieving pain and improving quality of life in patients with advanced cancer. Zhongguo Linchuang Kangfu (Chinese Journal of Clinical Rehabilitation) 2005;40:59‐61. |
Differences between protocol and review
We have highlighted reasons why we contacted corresponding authors and have quantified how many times we attempted to do this by email (please see Selection of studies; Excluded studies).
We did not examine funnel plots because too few studies were identified (please see Assessment of risk of bias in included studies).
We carried out a GRADE assessment on the quality of our meta‐analysis data and included a 'Summary of findings' table (Table 1) with this information.
We were not able to find any studies describing 'pattern' of resistance exercise (i.e. the period of rest in between sets) and hence did not discount any studies for not reporting this. We judged that it would be more informative to include the studies that we found than to not report on resistance exercise interventions at all.
In the 2018 update, we added contact with healthcare professionals to our secondary objectives. Healthcare professionals have a role to play in the integration of exercise in the cancer care pathway and therefore it would be useful to understand if the exercise studies incorporate healthcare professionals in the role of recruitment or behavioural support during the intervention.
In the 2018 update, we did not search Metaregister (http://www.controlled‐trials.com/rct) website as it is now unavailable.
Contributions of authors
All authors contributed to the design, development and drafting of the protocol for this review. RT, LS, RG and HQ conducted screening and data extraction, with assistance from LB. LS and RT conducted analysis of the studies according to the CALO‐RE taxonomy. MAT, LS, DJR, KAR, SJCT and JMS assisted with interpretation of results and drafting of the final report. RT led the final report.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Rebecca Turner: Liz Steed: None known Helen Quirk: None known Rosa Greasley: None known John Saxton: None known Stephanie Taylor: None known Derek Rosario: None known Mohamed Thaha: None known Liam Bourke: received honoraria for lecturing from Sanofi and Astellas and research funding from the NIHR and CRUK.
Edited (no change to conclusions)
References
References to studies included in this review
al‐Majid 2015 {published data only}
- Al‐Majid S, Wilson LD, Rakovski C, Coburn JW. Effects of exercise on biobehavioral outcomes of fatigue during cancer treatment: results of a feasibility study. Biological Research for Nursing 2015;17:40‐8. [DOI] [PubMed] [Google Scholar]
Bourke 2011a {published data only}
- Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, et al. Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Archives of Physical Medicine & Rehabilitation 2011;92:749‐55. [DOI] [PubMed] [Google Scholar]
Bourke 2014 {published data only}
- Bourke L, Gilbert S, Hooper R, Steed LA, Joshi M, Catto JW, et al. Lifestyle changes for improving disease‐specific quality of life in sedentary men on long‐term androgen‐deprivation therapy for advanced prostate cancer: a randomised controlled trial. European Urology 2014;65:865‐72. [DOI] [PubMed] [Google Scholar]
- Gilbert SE, Tew GA, Fairhurst C, Bourke L, Saxton JM, Winter EM, et al. Effects of a lifestyle intervention on endothelial function in men on long‐term androgen deprivation therapy for prostate cancer. British Journal of Cancer 2016;114:401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadmus 2009 {published data only}
- Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psycho‐Oncology 2009;18:343‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Alvarez‐Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass and bone mass in breast cancer survivors. Obesity (Silver Spring) 2009;17:1534‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Cadmus L, Alvarez‐Reeves M, O'Neil M, Mierzejewski E, Latka R, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: the Yale Exercise and Survivorship Study. Cancer 2008;112:2593‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Varma K, Alvarez‐Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled trial of aerobic exercise on insulin and insulin‐like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiology Biomarkers and Prevention 2009;18:306‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latka RN, Alvarez‐Reeves M, Cadmus L, Irwin ML. Adherence to a randomized controlled trial of aerobic exercise in breast cancer survivors: the Yale exercise and survivorship study. Journal of Cancer Survivorship 2009;3:148‐57. [DOI] [PubMed] [Google Scholar]
Campbell 2017 {published data only}
- Campbell KL, Kam JW, Neil‐Sztramko SE, LIiu Ambrose T, Handy TC, Lim HJ, et al. Effect of aerobic exercise on cancer‐associated cognitive impairment: A proof‐of‐concept RCT. Psycho‐Oncology 2017;27(1):53‐60. [DOI] [PubMed] [Google Scholar]
Cantarero‐Villanueva 2012b {published data only}
- Cantarero‐Villanueva I, Fernandez‐Lao C, Fernandez‐De‐Las‐Penas C, Lopez‐Barajas IB, Del‐Moral‐Avila R, De La‐llave‐Rincon, et al. Effectiveness of water physical therapy on pain, pressure pain sensitivity, and myofascial trigger points in breast cancer survivors: a randomized, controlled clinical trial. Pain Medicine 2012;13:1509‐19. [DOI] [PubMed] [Google Scholar]
Cavalheri 2017 {published data only}
- Cavalheri V, Jenkins S, Cecins N, Gain K, Phillips MJ, Sanders LH, et al. Exercise training for people following curative intent treatment for non‐small cell lung cancer: a randomized controlled trial. Brazilian Journal of Physical Therapy 2017;21:58‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daley 2007a {published and unpublished data}
- Daley AJ, Crank H, Mutrie N, Saxton JM, Coleman R. Determinants of adherence to exercise in women treated for breast cancer. European Journal of Oncology Nursing 2007;11:392‐9. [DOI] [PubMed] [Google Scholar]
- Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. Journal of Clinical Oncology 2007;25:1713‐21. [DOI] [PubMed] [Google Scholar]
Drouin 2005 {published and unpublished data}
- Drouin JS, Armstrong H, Krause S, Orr J, Birk TJ, Hryniuk WM, et al. Effects of aerobic exercise training on peak aerobic capacity, fatigue, and psychological factors during radiation for breast cancer. Rehabilitation Oncology 2005;23:11‐7. [Google Scholar]
- Drouin JS, Birk TJ, Wirth JC. Random control clinical trial on effect of aerobic exercise training on weight management during radiation treatment for breast cancer. Rehabilitation Oncology 2006;24:6‐10. [DOI] [PubMed] [Google Scholar]
- Drouin JS, Young TJ, Beeler J, Byrne K, Birk TJ, Hryniuk WM, et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer 2006;107:2490‐5. [DOI] [PubMed] [Google Scholar]
Hayes 2009 {published and unpublished data}
- Hayes SC, Reul‐Hirche H, Turner J. Exercise and secondary lymphedema: safety, potential benefits, and research issues. Medicine and Science in Sports and Exercise 2009;41:483‐9. [DOI] [PubMed] [Google Scholar]
Irwin 2015 {published data only}
- Irwin ML, Cartmel B, Gross CP, Ercolano E, Li P, Yao X, et al. Randomized exercise trial of aromatase inhibitor‐induced arthralgia in breast cancer survivors. Journal of Clinical Oncology 2015;33(10):1104‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GA, Cartmel B, Harrigan M, Fiellin M, Capozza S, Zhou Y, et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity (Silver spring) 2017;25(2):346‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaltsatou 2011 {published and unpublished data}
- Kaltsatou A, Mameletzi D, Douka S. Physical and psychological benefits of a 24‐week traditional dance program in breast cancer survivors. Journal of Bodywork & Movement Therapies 2011;15:162‐7. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published and unpublished data}
- Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nursing 2006;29:156‐65. [DOI] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim TH, Chang JS, Park KS, Park J, Kim N, Lee JI, et al. Effects of exercise training on circulating levels of Dickkpof‐1 and secreted frizzled‐related protein‐1 in breast cancer survivors: A pilot single‐blind randomized controlled trial. PLOS One 2017;8:e0171771. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2003 {published and unpublished data}
- McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. Journal of Clinical Oncology 2003;21:463‐6. [DOI] [PubMed] [Google Scholar]
Mohamady 2017 {published data only}
- Mohamady HM, Elsisi HF, Aneis YM. Impact of moderate intensity aerobic exercise on chemotherapy‐induced anemia in elderly women with breast cancer: A randomized controlled clinical trial. Journal of Advanced Research 2017;8:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musanti 2012 {published data only}
- Musanti R. A study of exercise modality and physical self‐esteem in breast cancer survivors. Medicine and Science in Sports and Exercise 2012;44:352‐61. [DOI] [PubMed] [Google Scholar]
Perna 2010 {published and unpublished data}
- Perna FM, Craft L, Freund KM, Skrinar G, Stone M, Kachnic L, et al. The effect of a cognitive behavioral exercise intervention on clinical depression in a multiethnic sample of women with breast cancer: a randomized controlled trial. International Journal of Sport and Exercise Psychology 2010;8:36‐47. [Google Scholar]
Pinto 2003 {published data only}
- Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho‐Oncology 2003;12:118‐26. [DOI] [PubMed] [Google Scholar]
Pinto 2005 {published and unpublished data}
- Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home‐based physical activity intervention for breast cancer patients. Journal of Clinical Oncology 2005;23:3577‐87. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Rabin C, Dunsiger S. Home‐based exercise among cancer survivors: adherence and its predictors. Psycho‐Oncology 2009;18:369‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto BM, Rabin C, Papandonatos GD, Frierson GM, Trunzo JJ, Marcus BH. Maintenance of effects of a home‐based physical activity program among breast cancer survivors. Support Care Cancer 2008;16:1279‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto BM, Trunzo JJ, Rabin C, Cady B, Fenton MA, Herman A, et al. Random control clinical trial on effect of aerobic exercise training on weight management during radiation treatment for breast cancer. Journal of Clinical Psychology in Medical Settings 2004;11:171‐8. [Google Scholar]
- Rabin C, Pinto BM, Frierson G. Mediators of a randomized controlled physical activity intervention for breast cancer survivors. Journal of Sport and Exercise Psychology 2006;28:269‐84. [Google Scholar]
Pinto 2011 {published and unpublished data}
Rogers 2015 {published data only}
- Rogers LQ, Courneya KS, Anton PM, Hopkins‐Price P, Verhulst S, Robbs RS, et al. Social cognitive constructs did not mediate the BEAT cancer intervention effects on objective physical activity behavior based on multivariable path analysis. Annals of Behavioral Medicine 2017;51:321‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LQ, Courneya KS, Anton PM, Hopkins‐Price P, Verhulst S, Vicari SK, et al. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Research and Treatment 2015;149:109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LQ, Courneya KS, Carter SJ, Anton PM, Verhulst S, Vicari SK, et al. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Research and Treatment 2016;159:283‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LQ, McAuley E, Anton PM, Courneya KS, Vicari S, Hopkins‐Price P, et al. Better exercise adherence after treatment for cancer (BEATCancer) study: rationale, design, and methods. Contemporary Clinical Trials 2012;33(1):124‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2013 {published data only}
- Saxton JM, Scott EJ, Daley AJ, Woodroofe MN, Mutrie N, Crank H, et al. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic‐pituitary‐adrenal axis regulation and immune function after early‐stage breast cancer: a randomised controlled trial. Breast Cancer Research and Treatment 2014;16(2):R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long‐term prognosis after early‐stage breast cancer: a randomized controlled trial. Cancer Causes & Control 2013;24:181‐91. [DOI] [PubMed] [Google Scholar]
Thomas 2013 {published data only}
- Irwin ML, Cadmus L, Alvarez‐Reeves M, O'Neil M, Mierzejewski E, Latka R, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: the Yale Exercise and Survivorship Study. Cancer 2008;112:2593‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GA, Alvarez‐Reeves M, Lu L, Yu H, Irwin ML. Effect of Exercise on Metabolic Syndrome Variables in Breast Cancer Survivors. International Journal of Endocrinology 2013;Volume 2013:168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2016 {published data only}
- Adams SC, Segal RJ, McKenzie DC, Morielli AR, Mackey JR, Gelmon K, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Research and Treatment 2016 Aug;158(3):497‐507. [DOI] [PubMed] [Google Scholar]
Ahmed 2006 {published data only}
- Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. Journal of Clinical Oncology 2006;24:2765‐72. [DOI] [PubMed] [Google Scholar]
Alibhai 2014 {published data only}
- Alibhai SM, O'Neill S, Fisher‐Schlombs K, Breunis H, Timilshina N, Brandwein JM, et al. A pilot phase II RCT of a home‐based exercise intervention for survivors of AML. Supportive Care in Cancer 2014;22(4):881‐9. [DOI] [PubMed] [Google Scholar]
Ames 2011 {published data only}
- Ames SC, Tan WW, Ames GE, Stone RL, Rizzo TD Jr, Crook JE, et al. A pilot investigation of a multidisciplinary quality of life intervention for men with biochemical recurrence of prostate cancer. Psycho‐Oncology 2011;20:435‐40. [DOI] [PubMed] [Google Scholar]
Anderson 2012 {published data only}
- Anderson RT, Kimmick GG, McCoy TP, Hopkins J, Levine E, Miller G, et al. A randomized trial of exercise on well‐being and function following breast cancer surgery: the RESTORE trial. Journal of Cancer Survivorship 2012;6:172‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2013 {published data only}
- Andersen C, Rørth M, Ejlertsen B, Stage M, Møller T, Midtgaard J, et al. The effects of a six‐week supervised multimodal exercise intervention during chemotherapy on cancer‐related fatigue. European Journal of Oncology Nursing 2013;17(3):331‐9. [DOI] [PubMed] [Google Scholar]
Anderson 2015 {published data only}
- Anderson DJ, Seib C, McCarthy AL, Yates P, Porter‐Steele J, McGuire A, et al. Facilitating lifestyle changes to manage menopausal symptoms in women with breast cancer: A randomized controlled pilot trial of The Pink Women's Wellness Program. Menopause (New York) 2015;22(9):937‐45. [DOI] [PubMed] [Google Scholar]
Anulika 2015 {published data only}
- Anulika Aweto H, Akinbo SRA, Olawale OA. Effects of combined aerobic and stretching exercises on the cardiopulmonary parameters of premenopausal and postmenopausal breast cancer survivors. Nigerian Quarterly Journal of Hospital Medicine 2015;25(3):177‐83. [PubMed] [Google Scholar]
Arbane 2011 {published and unpublished data}
- Arbane G, Tropman D, Jackson D, Garrod R. Evaluation of an early exercise intervention after thoracotomy for non‐small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer 2011;71:229‐34. [DOI] [PubMed] [Google Scholar]
Arbane 2014 {published data only}
- Arbane G, Douiri A, Hart N, Hopkinson NS, Singh S, Speed C, et al. Effect of postoperative physical training on activity after curative surgery for non‐small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy 2014;100(2):100‐7. [DOI] [PubMed] [Google Scholar]
Arikawa 2013 {published data only}
- Arikawa AY, Thomas W, Patel SR, Kurzer MS. No effect of exercise on urinary 6‐sulfatoxymelatonin and catecholamines in young women participating in a 16‐week randomized controlled trial. Cancer Epidemiology, Biomarkers & Prevention 2013;22(9):1634‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2013 {published data only}
- Banerjee S, Manley K, Thomas L, Shaw B, Saxton J, Mills R, et al. Preoperative exercise protocol to aid recovery of radical cystectomy: Results of a feasibility study. European Urology, Supplements 2013;12(6):125‐6. [Google Scholar]
Baruth 2015 {published data only}
- Baruth M, Wilcox S, Ananian C, Heiney S. Effects of home‐based walking on quality of life and fatigue outcomes in early stage breast cancer survivors: a 12‐week pilot study. Journal of Physical Activity & Health 2015;12(1):110‐8. [DOI] [PubMed] [Google Scholar]
Battaglini 2007 {published and unpublished data}
- Battaglini C, Bottaro M, Dennehy C, Rae L, Shields E, Kirk D, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Medical Journal 2007;125:22‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Battaglini 2008 {published and unpublished data}
- Battaglini CL, Mihalik JP, Bottaro M, Dennehy C, Petschauer MA, Hairston LS, et al. Effect of exercise on the caloric intake of breast cancer patients undergoing treatment. Brazilian Journal of Medical and Biological Research 2008;41:709‐15. [DOI] [PubMed] [Google Scholar]
Bloom 2013 {published data only}
- Bloom J, Stewart S, Oakley‐Girvan I, Luce J, Sellmeyer D. Bone health for younger women with breast cancer: effects of a YMCA‐based intervention on exercise, bone density, body mass, and physical and mental health in a multi‐ethnic sample. Psycho‐Oncology 2013;22:81‐2. [Google Scholar]
Bracha 2012 {published data only}
- Bracha J, Katz‐Leurer M. The immediate effect of upper arm exercise compared with lower or combined upper and lower arm exercise on arm volume reduction in women with breast cancer related lymphedema: A randomized preliminary study. Rehabilitation Oncology 2012;30:3‐8. [Google Scholar]
Brdareski 2012 {published data only}
- Brdareski Z, Djurovic A, Susnjar S, Zivotic‐Vanovic M, Ristic A, Konstantinovic L, et al. Effects of a short‐term differently dosed aerobic exercise on maximum aerobic capacity in breast cancer survivors: a pilot study. Vojnosanitetski Pregled [Military Medical and Pharmaceutical Review] 2012;69(3):237‐42. [PubMed] [Google Scholar]
Brown 2012 {published data only}
- Brown JC, Troxel AB, Schmitz K. Safety of weightlifting among women with or at risk for breast cancer‐related lymphedema: musculoskeletal injuries and health care use in a weightlifting rehabilitation trial. Oncologist 2012;17(8):1120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruno 2018 {published data only}
- Bruno E, Roveda E, Vitale J, Montaruli A, Berrino F, Villarini A, et al. Effect of aerobic exercise intervention on markers of insulin resistance in breast cancer women. European Journal of Cancer Care 2018;27(2):e12617. [DOI] [PubMed] [Google Scholar]
Buchan 2016 {published data only}
- Buchan J, Janda M, Box R, Schmitz K, Hayes S. A randomized trial on the effect of exercise mode on breast cancer‐related lymphedema. Medicine and Science in Sports and Exercise 2016;48(10):1866‐74. [DOI] [PubMed] [Google Scholar]
Buffart 2013 {published data only}
- Buffart L, Galvao D, Paw M, Brug CA, Taaffe J, Spry D, et al. How does a resistance and aerobic exercise program improve physical and general health in men undergoing androgen deprivation therapy for prostate cancer?. Psycho‐Oncology 2013;22:28‐9. [Google Scholar]
Buffart 2014a {published data only}
- Buffart LM, Galvão DA, Chinapaw MJ, Brug J, Taaffe DR, Spry Nigel, et al. Mediators of the resistance and aerobic exercise intervention effect on physical and general health in men undergoing androgen deprivation therapy for prostate cancer. Cancer 2014;120:294‐301. [DOI] [PubMed] [Google Scholar]
Buffart 2014b {published data only}
- Buffart LM, Ros WJG, Chinapaw MJ, Brug J, Knol DL, Korstjens I, et al. Mediators of physical exercise for improvement in cancer survivors' quality of life. Psycho‐Oncology 2014;23:330‐8. [DOI] [PubMed] [Google Scholar]
Campbell 2005 {published data only}
- Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. European Journal of Oncology Nursing 2005;9:56‐63. [DOI] [PubMed] [Google Scholar]
Cantaero‐Villanueva 2013 {published data only}
- Cantarero‐Villanueva I, Fernández‐Lao C, Cuesta‐Vargas A, Moral‐Avila R, Fernández‐de‐las‐Peñas C, Arroyo‐Morales M. The effectiveness of a deep water aquatic exercise program in cancer‐related fatigue in breastcancer survivors: a randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2013;94(2):221‐30. [DOI] [PubMed] [Google Scholar]
Cantaero‐Villanueva 2016 {published data only}
- Cantarero‐Villanueva I, Sanchez‐Jimenez A, Galiano‐Castillo N, Diaz‐Rodriguez L, Martin‐Martin L, Arroyo‐Morales M. Effectiveness of lumbopelvic exercise in colon cancer survivors: a randomized controlled clinical. Medicine & Science in Sports & Exercise 2016;48(8):1438‐46. [DOI] [PubMed] [Google Scholar]
Cantarero‐Villanueva 2011 {published and unpublished data}
- Cantarero‐Villanueva I, Fernández‐Lao C, Díaz‐Rodriguez L, Fernández‐de‐las‐Peñas C, Moral‐Avila R, Arroyo‐Morales M. A multimodal exercise program and multimedia support reduce cancer‐related fatigue in breast cancer survivors: a randomised controlled clinical trial. European Journal of Integrative Medicine 2011;3:e189–e200. [Google Scholar]
Cantarero‐Villanueva 2012a {published and unpublished data}
- Cantarero‐Villanueva I, Fernández‐Lao C, Moral‐Avila R, Fernández‐de‐Las‐Peñas C, Feriche‐Fernández‐Castanys MB, Arroyo‐Morales M. Effectiveness of core stability exercises and recovery myofascial release massage on fatigue in breast cancer survivors: a randomized controlled clinical trial. Evidence‐Based Complementary and Alternative Medicine 2012; Vol. 2012:620619. [DOI: 10.1155/2012/620619] [DOI] [PMC free article] [PubMed]
Carmack Taylor 2004 {published data only}
- Carmack Taylor CL, Smith MA, Moor C, Dunn AL, Pettaway C, Sellin R, et al. Quality of life intervention for prostate cancer patients: design and baseline characteristics of the Active for Life After Cancer trial. Controlled Clinical Trials 2004;25:265‐85. [DOI] [PubMed] [Google Scholar]
Carmack Taylor 2006 {published data only}
- Carmack Taylor CL, Demoor C, Smith MA, Dunn AL, Basen‐Engquist K, Nielsen I, et al. Active for Life After Cancer: a randomized trial examining a lifestyle physical activity program for prostate cancer patients. Psycho‐oncology 2006;15:847‐62. [DOI] [PubMed] [Google Scholar]
Carmack Taylor 2007 {published data only}
- Carmack Taylor CL, Moor C, Basen‐Engquist K, Smith MA, Dunn AL, Badr H, et al. Moderator analyses of participants in the Active for Life After Cancer trial: implications for physical activity group intervention studies. Annals of Behavioral Medicine 2007;33:99‐104. [DOI] [PubMed] [Google Scholar]
Carson 2009 {published and unpublished data}
- Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Supportive Care in Cancer 2009;17(10):1301‐9. [DOI] [PubMed] [Google Scholar]
Casla 2015 {published data only}
- Casla S, Lopez‐Tarruella S, Jerez Y, Marquez‐Rodas I, Galvao DA, Newton RU, et al. Supervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: a randomized controlled trial. Breast Cancer Research and Treatment 2015;153(2):371‐82. [DOI] [PubMed] [Google Scholar]
Cerulli 2014 {published data only}
- Cerulli C, Minganti C, Santis C, Tranchita E, Quaranta F, Parisi A. Therapeutic horseback riding in breast cancer survivors: a pilot study. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2014;20(8):632‐9. [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home‐based walking exercise on anxiety, depression and cancer‐related symptoms in patients with lung cancer. British Journal of Cancer 2015;112:438‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. British Journal of Cancer 2016;115(11):1304‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2006 {published data only}
- Cho OH, Yoo YS, Kim NC. Efficacy of comprehensive group rehabilitation for women with early breast cancer in South Korea. Nursing and Health Sciences 2006;8:140‐6. [DOI] [PubMed] [Google Scholar]
Christensen 2014 {published data only}
- Christensen JF, Jones LW, Tolver A, Jørgensen LW, Andersen JL, Adamsen L, et al. Safety and efficacy of resistance training in germ cell cancer patients undergoing chemotherapy: a randomized controlled trial. British Journal of Cancer 2014;111(1):8‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuang 2017 {published data only}
- Chuang TY, Yeh ML, Chung YC. A nurse facilitated mind‐body interactive exercise (Chan‐Chuang qigong) improves the health status of non‐Hodgkin lymphoma patients receiving chemotherapy: randomised controlled trial. International Journal of Nursing Studies 2017;69:25‐33. [DOI] [PubMed] [Google Scholar]
Coleman 2003 {published data only}
- Coleman EA, Hall‐Barrow J, Coon S, Stewart CB. Facilitating exercise adherence for patients with multiple myeloma. Clinical Journal of Oncology Nursing 2003;7:529‐34. [DOI] [PubMed] [Google Scholar]
Cornette 2016 {published data only}
- Cornette T, Vincent F, Mandigout S, Antonini MT, Leobon S, Labrunie A, et al. Effects of home‐based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (SAPA): a randomized controlled trial. European Journal of Physical and Rehabilitation Medicine 2016;52(2):223‐32. [PubMed] [Google Scholar]
Cornie 2013a {published data only}
- Cormie P, Newton R, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer & Prostatic Diseases 2013;16(4):328‐35. [DOI] [PubMed] [Google Scholar]
Cornie 2013b {published data only}
- Cormie P, Newton RU, Taaffe DR, Spry N, Joseph D, Akhlil Hamid M, et al. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer & Prostatic Diseases 2013;16(2):170‐5. [DOI] [PubMed] [Google Scholar]
Cornie 2014 {published data only}
- Cormie P, Chambers SK, Newton RU, Gardiner RA, Spry N, Taaffe DR, et al. Improving sexual health in men with prostate cancer: randomised controlled trial of exercise and psychosexual therapies. BMC Cancer 2014;14(1):14‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornie 2015 {published data only}
- Cormie P, Galvao DA, Spry N, Joseph D, Chee R, Taaffe DR, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen‐deprivation therapy: a randomised controlled trial. BJU International 2015;115(2):256‐66. [DOI] [PubMed] [Google Scholar]
Courneya 2012 {published data only}
- Courneya KS, Sellar CM, Trinh L, Forbes CC, Stevinson C, McNeely ML, et al. A randomized trial of aerobic exercise and sleep quality in lymphoma patients receiving chemotherapy or no treatments. Cancer Epidemiology, Biomarkers & Prevention 2012;21(6):887‐94. [DOI] [PubMed] [Google Scholar]
Courneya 2013 {published data only}
- Courneya KS, Forbes CC, Trinh L, Sellar CM, Friedenreich CM, Reiman T. Patient satisfaction with participation in a randomized exercise trial: effects of randomization and a usual care posttrial exercise program. Clinical Trials (London, England) 2013;10(6):959‐66. [DOI] [PubMed] [Google Scholar]
Courneya 2014a {published data only}
- Courneya K, Segal R, Mackey J, Gelmon K, Friedenreich C, Yasui Y, et al. Effects of exercise dose and type on sleep quality during breast cancer chemotherapy: a multicenter randomized trial. Psycho‐Oncology 2014;23:57‐8. [DOI] [PubMed] [Google Scholar]
Courneya 2014b {published data only}
- Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Medicine and Science in Sports and Exercise 2014;46(9):1744‐51. [DOI] [PubMed] [Google Scholar]
Courneya 2015 {published data only}
- Courneya KS, Friedenreich CM, Franco‐Villalobos C, Crawford JJ, Chua N, Basi S, et al. Effects of supervised exercise on progression‐free survival in lymphoma patients: an exploratory follow‐up of the HELP Trial. Cancer Causes & Control 2015;26(2):269‐72. [DOI] [PubMed] [Google Scholar]
Courneya 2016a {published data only}
- Courneya KS, Vardy JL, O'Callaghan CJ, Friedenreich CM, Campbell KL, Prapavessis H, et al. Effects of a structured exercise program on physical activity and fitness in colon cancer survivors: one year feasibility results from the CHALLENGE trial. Cancer Epidemiology, Biomarkers & Prevention 2016;25(6):969‐77. [DOI] [PubMed] [Google Scholar]
Courneya 2016b {published data only}
- Courneya KS, Segal RJ, Vallerand JR, Forbes CC, Crawford JJ, Dolan LB, et al. Motivation for different types and doses of exercise during breast cancer chemotherapy: a randomized controlled trial. Annals of Behavioral Medicine 2016;50(4):554‐63. [DOI] [PubMed] [Google Scholar]
Culos Reed 2010 {published data only}
- Culos‐Reed SN, Robinson JW, Lau H, Stephenson L, Keats M, Norris S, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16‐week intervention. Supportive Care in Cancer 2010;18:591‐9. [DOI] [PubMed] [Google Scholar]
Danhauer 2009 {published data only}
- Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, et al. Restorative yoga for women with breast cancer: finding from a randomized pilot study. Psycho‐Oncology 2009;18:360‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daubenmier 2006 {published data only}
- Daubenmier JJ, Weidner G, Marlin R, Crutchfield L, Dunn‐Emke S, Chi C, et al. Lifestyle and health‐related quality of life of men with prostate cancer managed with active surveillance. Urology 2006;67:125‐30. [DOI] [PubMed] [Google Scholar]
De Jesus 2013 {published data only}
- Jesus S, Fitzgeorge L, Massel D, Prapavessis H, Sanatani M, Suskin, et al. Community‐based exercise intervention for oncology patients suffering from fatigue: effects on symptoms, psychosocial health, aerobic fitness and body composition: a pilot study. Psycho‐Oncology 2013;22:48. [Google Scholar]
Demark‐Wahnefried 2015 {published data only}
- Demark‐Wahnefried W, Colditz GA, Rock CL, Sedjo RL, Liu J, Wolin KY, et al. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good health for You (ENERGY) ‐ randomized weight loss trial among breast cancer survivors. Breast Cancer Research and Treatment 2015;154(2):329‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeNysschen 2011 {published data only}
- DeNysschen CA, Brown JK, Cho MH, Dodd MJ. Nutritional symptom and body composition outcomes of aerobic exercise in women with breast cancer. Clinical Nursing Research 2011;20:29‐46. [DOI] [PubMed] [Google Scholar]
Dieli‐Conwright 2014 {published data only}
- Dieli‐Conwright CM, Mortimer JE, Schroeder ET, Courneya K, Demark‐Wahnefried W, Buchanan TA, et al. Randomized controlled trial to evaluate the effects of combined progressive exercise on metabolic syndrome in breast cancer survivors: rationale, design, and methods. BMC Cancer 2014;14(1):doi: 10.1186/1471‐2407‐14‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dieperink 2017 {published data only}
- Dieperink KB, Johansen C, Hansen S, Wagner LK, Andersen K, Minet LR, et al. Male coping through a long‐term cancer trajectory. Secondary outcomes from a RTC examining the effect of a multidisciplinary rehabilitation program (RePCa) among radiated men with prostate cancer. Acta Oncologica 2017;56(2):254‐61. [DOI] [PubMed] [Google Scholar]
Diepold 2016 {published data only}
- Diepold C, Wiskemann J, Hummler S, Steins M, Thomas M. Physical exercise program in non‐operable lung cancer patients undergoing palliative treatment‐preliminary report of recruitment rates and feasibility of the POSITIVE study (part III). Oncology Research and Treatment 2016;16:10.1186/s12885‐016‐2561‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Do 2015 {published data only}
- Do J, Cho Y, Jeon J. Effects of a 4‐week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. Journal of Breast Cancer 2015;18(1):87‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolan 2010 {published data only}
- Dolan LB, Gelmon K, Courneya KS, Mackey JR, Segal RJ, Lane K, et al. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer Epidemiology Biomarkers & Prevention 2010;19:2826‐32. [DOI] [PubMed] [Google Scholar]
Dolan 2014 {published data only}
- Dolan LB, Campbell K, Neil S, McKenzie D. The influence of exercise intensity on health‐related outcomes in breast cancer survivors. Archives of Physical Medicine and Rehabilitation 2014;95(10):E43‐4. [Google Scholar]
Dolan 2016 {published data only}
- Dolan LB, Campbell K, Gelmon K, Neil‐Sztramko S, Holmes D, McKenzie DC. Interval versus continuous aerobic exercise training in breast cancer survivors ‐ a pilot RCT. Supportive Care in Cancer 2016;24(1):119‐27. [DOI] [PubMed] [Google Scholar]
Donmez 2017 {published data only}
- Donmez A, ArikanKapucu Sevgisun RTY. The effectiveness of a clinical and home‐based physical activity program and simple lymphatic drainage in the prevention of breast cancer‐related lymphedema: a prospective randomized controlled study. European Journal of Oncology Nursing 2017;31:12‐21. [DOI] [PubMed] [Google Scholar]
Donnelly 2011 {published and unpublished data}
- Donnelly CM, Blaney JM, Lowe‐Strong A, Rankin JP, Campbell A, McCrum‐Gardner E, et al. A randomised controlled trial testing the feasibility and efficacy of a physical activity behavioural change intervention in managing fatigue with gynaecological cancer survivors. Gynecologic Oncology 2011;122:618‐24. [DOI] [PubMed] [Google Scholar]
Edvarsen 2015 {published data only}
- Edvardsen E, Skjønsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High‐intensity training following lung cancer surgery: a randomised controlled trial. Thorax 2015;70(3):244‐50. [DOI] [PubMed] [Google Scholar]
Emslie 2007 {published data only}
- Emslie C, Whyte F, Campbell A, Mutrie N, Lee L, Ritchie D, et al. 'I wouldn't have been interested in just sitting round a table talking about cancer'; exploring the experiences of women with breast cancer in a group exercise trial. Health Education Research 2007;22:827‐38. [DOI] [PubMed] [Google Scholar]
Eriksen 2017 {published data only}
- Eriksen AK, Hansen RD, Larsen RG, Jensen JM, Overgaard K, Borre M, et al. A lifestyle intervention among elderly men on active surveillance for non‐aggressive prostate cancer: a randomised feasibility study with whole‐grain rye and exercise. Trials 2017;18(20):doi: 10.1186/s13063‐016‐1734‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan‐Ko 2017 {published data only}
- Fan‐Ko S, Chao‐Ming H, YuChun Y, Chu‐Yun L, Chun‐Ying C. The effects of muscle relaxation and therapeutic walking on depression, suicidal ideation, and quality of life in breast cancer patients receiving chemotherapy. Cancer Nursing 2017;40:E39‐48. [DOI] [PubMed] [Google Scholar]
Fernandez‐Lao 2012 {published and unpublished data}
- Fernández‐Lao C, Cantarero‐Villanueva I, Fernández‐de‐Las‐Peñas C, Moral‐Ávila R, Castro‐Sánchez AM, Arroyo‐Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clinical Journal of Pain 2012;28:113‐21. [DOI] [PubMed] [Google Scholar]
Fields 2015 {published data only}
- Fields J, Richardson A, Fenlon D. Nordic walking as a physical activity intervention for aromatase inhibitor associated arthralgia: a feasibility study. European Journal of Surgical Oncology 2015;41(6):s76‐7. [Google Scholar]
Fields 2017 {published data only}
- Fields J, Richardson A, Hopkinson J, Fenlon D. Nordic walking as an exercise intervention to reduce pain in women with aromatase inhibitor‐associated arthralgia: a feasibility study. Journal of Pain and Symptom Management 2017;52(4):548‐59. [DOI] [PubMed] [Google Scholar]
Forbes 2017 {published data only}
- Forbes CC, Blanchard CM, Mummery K, Courneya K. A pilot study on the motivational effects of an internet‐delivered physical activity behaviour change programme in Nova Scotian cancer survivors. Psychology, Health & Medicine 2017;32(2):234‐52. [DOI] [PubMed] [Google Scholar]
Frattaroli 2008 {published data only}
- Frattaroli J, Weidner G, Dnistrian AM, Kemp C, Daubenmier JJ, Marlin RO, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow‐up. Urology 2008;72:1319‐23. [DOI] [PubMed] [Google Scholar]
Friedenrich 2016 {published data only}
- Friedenreich CM, Pialoux V, Wang Q, Shaw E, Brenner DR, Waltz X, et al. Effects of exercise on markers of oxidative stress: an ancillary analysis of the Alberta Physical Activity and Breast Cancer Prevention trial [with consumer summary]. BMJ Open Sport & Exercise Medicine 2016;2(1):e000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furzer 2016 {published data only}
- Furzer BJ, Ackland TR, Wallman KE, Petterson AS, Gordon SM, Wright KE, et al. A randomised controlled trial comparing the effects of a 12‐week supervised exercise versus usual care on outcomes in haematological cancer patients. Supportive Care in Cancer 2016;24(4):1697‐707. [DOI] [PubMed] [Google Scholar]
Galiano‐Castillo 2017 {published data only}
- Galiano‐Castillo N, Arroyo‐Morales M, Fernandez‐Lao C, Cantarero‐Villanueva I, Lozano‐Lozano M, Martin‐Martin L, et al. Effect of an Internet‐based telehealth system on functional capacity and cognition in breast cancer survivors: a secondary analysis of a randomized controlled trial. Supportive Care in Cancer 2017;25(11):3351‐559. [DOI] [PubMed] [Google Scholar]
Galvão 2010 {published data only}
- Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. Journal of Clinical Oncology 2010;28:340‐7. [DOI] [PubMed] [Google Scholar]
Galvão 2011 {published data only}
- Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Acute versus chronic exposure to androgen suppression for prostate cancer: impact on the exercise response. Journal of Urology 2011;186:1291‐7. [DOI] [PubMed] [Google Scholar]
Galvão 2017 {published data only}
- Galvao DA, Newton RU, Girgis A, Lepore SJ, Stiller A, Mihalopoulos C, et al. Randomized controlled trial of a peer led multimodal intervention for men with prostate cancer to increase exercise participation. Psycho‐Oncology 2017;27(1):199‐207. [DOI] [PubMed] [Google Scholar]
Gaskin 2016 {published data only}
- Gaskin CJ, Fraser SF, Owen PJ, Craike M, Orellana L, Livingston PM. Fitness outcomes from a randomised controlled trial of exercise training for men with prostate cancer: the ENGAGE study. Journal of Cancer Survivorship 2016;10(6):972‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gehring 2014 {published data only}
- Gehring K, Stuiver M, Rutten GJ, Taphoorn M, Aaronson N, Sitskoorn M. A pilot RCT on the efficacy of home‐based exercise to improve cognitive functioning in grade II and III glioma patients. Neuro‐Oncology 2014;16:v185. [Google Scholar]
Gehring 2015 {published data only}
- Gehring K, Stuiver M, Rutten GJ, Taphoorn M, Aaronson N, Sitskoorn M. A pilot RCT on the efficacy of E‐health supported physical exercise to improve cognitive functioning in glioma patients. Neuro‐oncology 2015;17:v146‐7. [Google Scholar]
Gehring 2018 {published data only}
- Gehring K, Kloek C, Aaronson NK, Janssen KW, Jones LW, Sitskoorn MM, et al. Feasibility of a home‐based exercise intervention with remote guidance for patients with stable grade II and III gliomas: a pilot randomized controlled trial. Clinical Rehabilitation 2018;32(8):352‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerland 2012 {published data only}
- Gerland L, Frisse S, Latta S, Bloch W, Harbeck N, Baumann FT. Evaluation of the impact of a 3‐month strength training on strength parameters, EMG and oxidative stress of breast cancer patients during chemotherapy. Journal of Cancer Research and Clinical Oncology 2012;1:133‐8. [Google Scholar]
Giallauria 2014 {published data only}
- Giallauria F, Gentile M, Chiodini P, Mattiello A, Maresca L, Vitelli A, et al. Exercise training reduces high mobility group box‐1 levels in women with breast cancer: the DIANA (diet and androgens)‐5 project. European Journal of Preventive Cardiology 2014;21(1):61‐7. [DOI] [PubMed] [Google Scholar]
Gokal 2016 {published data only}
- Gokal K, Wallis D, Ahmed S, Boiangiu I, Kancherla K, Munir F. Effects of a self‐managed home‐based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: a randomised controlled trial. Supportive Care in Cancer 2016;24(3):1139. [DOI] [PubMed] [Google Scholar]
Gómez 2011 {published data only}
- Gómez AM, Martínez C, Fiuza‐Luces C, Herrero F, Pérez M, Madero L, et al. Exercise training and cytokines in breast cancer survivors. International Journal of Sports Medicine 2011;32:461‐7. [DOI] [PubMed] [Google Scholar]
Granger 2013 {published data only}
- Granger CL, Chao C, McDonald CF, Berney S, Denehy L. Safety and feasibility of an exercise intervention for patients following lung resection: a pilot randomized controlled trial. Integrative Cancer Therapies 2013;12(3):213‐24. [DOI] [PubMed] [Google Scholar]
Greenlee 2013 {published data only}
- Greenlee HA, Crew KD, Mata JM, McKinley PS, Rundle AG, Zhang W, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (19307381) 2013;21(1):65‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruenigen 2012a {published data only}
- Gruenigen V, Frasure H, Kavanagh MB, Janata J, Waggoner S, Rose P, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecologic Oncology 2012;125(3):699‐704. [DOI] [PubMed] [Google Scholar]
Gruenigen 2012b {published data only}
- Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health and Quality of Life Outcomes 2012;7:doi: 10.1186/1477‐7525‐7‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guinan 2013 {published data only}
- Guinan E, Hussey J, Broderick JM, Lithander FE, O'Donnell D, Kennedy MJ, et al. The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors ‐ a pilot study. Supportive Care in Cancer 2013;21(7):1983‐92. [DOI] [PubMed] [Google Scholar]
Guinan 2017 {published data only}
- Guinan E, Doyle SL, O'Neill L, Dunne MR, Foley EK, O'Sullivan J, et al. Effects of a multimodal rehabilitation programme on inflammation and oxidative stress in oesophageal cancer survivors: the ReStOre feasibility study. Supportive Care in Cancer 2017;25(3):749‐56. [DOI] [PubMed] [Google Scholar]
Haines 2010 {published data only}
- Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Research and Treatment 2010;124:163‐75. [DOI] [PubMed] [Google Scholar]
Hanssens 2012 {published data only}
- Hanssens S, Fontaine C, Decoster L, Schallier DCC, Luyten R, Watthy C, et al. The effect of a varied exercise program (VEP) on shoulder function and lymphedema (LE) in breast cancer survivors (BCs): a pilot study. Journal of Clinical Oncology 2012;30:DOI: 10.1200/jco.2012.30.27_suppl.82. [Google Scholar]
Hartman 2015 {published data only}
- Hartman SJ, Natarajan L, Palmer BW, Parker B, Patterson RE, Sears DD. Impact of increasing physical activity on cognitive functioning in breast cancer survivors: Rationale and study design of Memory & Motion. Contemporary Clinical Trials 2015;45:371‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatchett 2013 {published data only}
- Hatchett A, Hallam JS, Ford MA. Evaluation of a social cognitive theory‐based email intervention designed to influence the physical activity of survivors of breast cancer. Psycho‐Oncology 2013;22(4):829‐36. [DOI] [PubMed] [Google Scholar]
Hayes 2011 {published and unpublished data}
- Hayes S, Rye S, Battistutta D, Yates P, Pyke C, Bashford J, et al. Design and implementation of the Exercise for Health trial-a pragmatic exercise intervention for women with breast cancer. Contemporary Clinical Trials 2011;32:577‐85. [DOI] [PubMed] [Google Scholar]
Hayes 2012 {published and unpublished data}
- Hayes S, Battistutta D, Eakin E. Evaluating telephone versus face‐to‐face modes of exercise intervention delivery to women during and following treatment for breast cancer. Asia‐Pacific Journal of Clinical Oncology 2012;8:117‐8. [Google Scholar]
Hayes 2013 {published and unpublished data}
- Hayes SC, Rye S, Disipio T, Yates P, Bashford J, Pyke C, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment‐related side effects following breast cancer. Breast Cancer Research and Treatment 2013;137(1):175‐86. [DOI] [PubMed] [Google Scholar]
Hayes 2014 {published and unpublished data}
- Hayes S, Friedlander M, Obermair A, Mileshkin L, Janda M, Gordon L, et al. Exercise during chemotherapy for ovarian cancer (ECHO): study design features and outcomes of a cancer Australia and cancer council australia funded randomised, controlled trial. International Journal of Gynecological Cancer 2014;24:200‐1. [Google Scholar]
Headley 2004 {published data only}
- Headley JA, Ownby KK, John LD. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncology Nursing Forum 2004;31:977‐83. [DOI] [PubMed] [Google Scholar]
Heim 2007 {published data only}
- Heim ME, v d Malsburg ML, Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor‐related chronic fatigue. Onkologie 2007;30(8‐9):429‐34. [DOI] [PubMed] [Google Scholar]
Herbert 2012 {published data only}
- Hebert JR, Hurley TG, Harmon BE, Heiney S, Hebert CJ, Steck SE. A diet, physical activity, and stress reduction intervention in men with rising prostate‐specific antigen after treatment for prostate cancer. Cancer Epidemiology 2012;36(2):e128‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrero 2006 {published data only}
- Herrero F, San Juan AF, Fleck SJ, Balmer J, Pérez M, Cañete S, et al. Combined aerobic and resistance training in breast cancer survivors: a randomized, controlled pilot trial. International Journal of Sports Medicine 2006;27:573‐80. [DOI] [PubMed] [Google Scholar]
Ho 2016 {published data only}
- Ho RT, Fong TC, Cheung IK, Yip PS, Luk MY. Effects of a short‐term dance movement therapy program on symptoms and stress in patients with breast cancer undergoing radiotherapy: a randomized, controlled, single‐blind trial. Journal of Pain and Symptom Management 2016;51(5):824‐31. [DOI] [PubMed] [Google Scholar]
Hoffman 2013 {published data only}
- Hoffmann R. The impact of exercise on hematopoietic stem cell transplant patients. Biology of Blood and Marrow Transplantation 2013;19:S370‐80. [Google Scholar]
Hoffman 2017 {published data only}
- Hoffman AS, Lowenstein LM, Kamath GR, Housten AJ, Leal VB, Linder SK, et al. An entertainment‐education colorectal cancer screening decision aid for African American patients: a randomized controlled trial. Cancer 2017;123(8):1401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hojan 2016 {published data only}
- Hojan K, Kwiatkowska‐Borowczyk E, Leporowska E, Gorecki M, Ozga‐Majchrzak O, Milecki T, et al. Physical exercise for functional capacity, blood immune function, fatigue, and quality of life in high‐risk prostate cancer patients during radiotherapy: a prospective, randomized clinical study. European Journal of Physical and Rehabilitation Medicine 2016;52(4):489‐501. [PubMed] [Google Scholar]
Hojan 2017 {published data only}
- Hojan K, Kwiatkowska‐Borowczyk E, Leporowska E, Milecki P. Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer: a randomized controlled trial of a 12‐month exercise program. Polskie Archiwum Medycyny Wewnetrznej 2017;127(1):25‐35. [DOI] [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang HP, Wen FH, Tsai JC, Lin YC, Shun SC, Chang HK, et al. Adherence to prescribed exercise time and intensity declines as the exercise program proceeds: findings from women under treatment for breast cancer. Supportive Care in Cancer 2015;23(7):2061‐71. [DOI] [PubMed] [Google Scholar]
Hubbard 2016 {published data only}
- Hubbard G, O'Carroll R, Munro J, Mutrie N, Haw S, Mason H, et al. The feasibility and acceptability of trial procedures for a pragmatic randomised controlled trial of a structured physical activity intervention for people diagnosed with colorectal cancer: findings from a pilot trial of cardiac rehabilitation versus usual care (no rehabilitation) with an embedded qualitative study. Pilot and Feasibility Studies 2016;2(51). [DOI] [PMC free article] [PubMed] [Google Scholar]
Husebo 2014 {published data only}
- Husebo AML, Dyrstad SM, Mjaaland I, Soreide JA, Bru E. Effects of scheduled exercise on cancer‐related fatigue in women with early breast cancer. Scientific World Journal 2014;19((271828)):Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwang 2012 {published data only}
- Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non‐small cell lung cancer receiving targeted therapy. Supportive Care in Cancer 2012;20(12):3169‐77. [DOI] [PubMed] [Google Scholar]
James 2012 {published data only}
- James E, Boyes A, Courbeya K, Lubans D, Stacey F, Morgan P, et al. A home‐based resistance training program for survivors of prostate cancer: a pilot randomized controlled trial. Journal of Science and Medicine in Sport 2012;15:DOI: https://doi.org/10.1016/j.jsams.2012.11.809. [Google Scholar]
Jarden 2013 {published data only}
- Jarden M, Moller T, Kjeldsen L, Birgens H, Christensen JF, Christensen KB, et al. Patient Activation through Counseling and Exercise ‐ Acute Leukemia (PACE‐AL) ‐ a randomized controlled trial. BMC Cancer 2013;13:https://doi.org/10.1186/1471‐2407‐13‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffs 2013 {published data only}
- Jeffs E, Wiseman T. Randomised controlled trial to determine the benefit of daily home‐based exercise in addition to self‐care in the management of breast cancer‐related lymphoedema: a feasibility study. Supportive Care in Cancer 2013;21(4):1013‐23. [DOI] [PubMed] [Google Scholar]
Jensen 2015a {published data only}
- Jensen BT, Jensen JB, Borre M, Laustsen S, Petersen AK. Physical prehabilitation is feasible and effective in patients with advanced bladder cancer. Cancer Nursing 2015;38(4):S5. [Google Scholar]
Jensen 2015b {published data only}
- Jensen BT, Petersen AK, Jensen JB, Laustsen S, Borre M. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scandinavian Journal of Urology and Nephrology 2015;49(2):133‐41. [DOI] [PubMed] [Google Scholar]
Jensen 2016 {published data only}
- Jensen JD, Yale RN, Krakow M, John K, King AJ. Theorizing foreshadowed death narratives: examining the impact of character death on narrative processing and skin self‐exam intentions. Journal of Health Communication 2017;22(1):84‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2014a {published data only}
- Jones LW, Douglas PS, Khouri MG, Mackey JR, Wojdyla D, Kraus WE, et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF‐ACTION randomized trial. Journal of Clinical Oncology 2014;32(23):2496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2014b {published data only}
- Jones LW, Hornsby WE, Freedland SJ, Lane A, West MJ, Moul JW, et al. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. European Urology 2014;65(5):852‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalter 2015 {published data only}
- Kalter J, Buffart LM, Korstjens I, Weert E, Brug J, Verdonck‐de Leeuw IM, et al. Moderators of the effects of group‐based physical exercise on cancer survivors' quality of life. Supportive Care in Cancer 2015;23(9):2623‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kampshoff 2015 {published data only}
- Kampshoff CS, Chinapaw MJ, Brug J, Twisk JW, Schep G, Nijziel MR, et al. Randomized controlled trial of the effects of high intensity and low‐to‐moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Medicine 2015;29(13):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kampshoff 2016 {published data only}
- Kampshoff CS, Mechelen W, Schep G, Nijziel MR, Witlox L, Bosman L, et al. Participation in and adherence to physical exercise after completion of primary cancer treatment. International Journal of Behavioral Nutrition and Physical Activity 2016;13(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanera 2016 {published data only}
- Kanera IM, Bolman CA, Willems RA, Mesters I, Lechner L. Lifestyle‐related effects of the web‐based Kanker Nazorg Wijzer (Cancer Aftercare Guide) intervention for cancer survivors: a randomized controlled trial. Journal of Cancer Survivorship 2016;10(5):883‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanera 2017 {published data only}
- Kanera IM, Willems RA, Bolman CAW, Mesters I, Verboon P, Lechner L. Long‐term effects of a web‐based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2017;14(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2009 {published data only}
- Kavanagh MB, Gruenigen VE, Courneya KS, Gibbons HE, Waggoner SE, Lerner E. Effects of a lifestyle intervention on nutrient intake in overweight/obese endometrial cancer survivors. European e‐Journal of Clinical Nutrition and Metabolism 2009;4:e143‐e147. [Google Scholar]
Kilbreath 2006 {published data only}
- Kilbreath S, Refshauge K, Beith J, Lee M. Resistance and stretching shoulder exercises early following axillary surgery for breast cancer. Rehabilitation Oncology 2006;24:9‐14. [Google Scholar]
Kilbreath 2012 {published data only}
- Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Research and Treatment 2012;133:667‐76. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim do S, Sim YJ, Jeong HJ, Kim GC. Effect of active resistive exercise on breast cancer‐related lymphedema: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2010;91:1844‐8. [DOI] [PubMed] [Google Scholar]
Klepin 2015 {published data only}
- Klepin HD, Tooze J, Pardee T, Renee EL, Berenzon D, Howard D, et al. Feasibility of a symptom‐adapted physical activity intervention during induction chemotherapy for older adults with acute myeloid leukemia (AML). Blood 2015;126(23):2012. [Google Scholar]
Klinkhammer‐Schalke 2012 {published data only}
- Klinkhammer‐Schalke M, Koller M, Steinger B, Ehret C, Ernst B, Wyatt JC, et al. Regensburg QoL Study Group. Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer. British Journal of Cancer 2012;106:826‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwiatkowski 2013 {published data only}
- Kwiatkowski F, Mouret‐Reynier MA, Duclos M, Leger‐Enreille A, Bridon F, Hahn T, et al. Long term improved quality of life by a 2‐week group physical and educational intervention shortly after breast cancer chemotherapy completion. Results of the 'Programme of Accompanying women after breast Cancer treatment completion in Thermal resorts' (PACThe) randomised clinical trial of 251 patients. European Journal of Cancer 2013;49(7):1530‐8. [DOI] [PubMed] [Google Scholar]
Lahart 2016 {published data only}
- Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home‐based physical activity intervention in breast cancer survivors. BMC Cancer 2016;17(16):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2017 {published data only}
- Lai Y, Huang J, Yang M, Su J, Liu J, Che G. Seven‐day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. Journal of Surgical Research 2017;209:30‐6. [DOI] [PubMed] [Google Scholar]
Lee 2012a {published data only}
- Lee CE, Kilgour A, Lau YK. Efficacy of walking exercise in promoting cognitive‐psychosocial functions in men with prostate cancer receiving androgen deprivation therapy. BMC Cancer 2012;12:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012b {published data only}
- Lee CE, Leslie WD, Lau YK. A pilot study of exercise in men with prostate cancer receiving androgen deprivation therapy. BMC Cancer 2012;12(103):doi:10.1186/1471‐2407‐12‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee MK, Yun YH, Park HA, Lee ES, Jung KH, Noh DY. A web‐based self‐management exercise and diet intervention for breast cancer survivors: pilot randomized controlled trial [with consumer summary]. International Journal of Nursing Studies 2014;51(12):1557‐67. [DOI] [PubMed] [Google Scholar]
Leone 2016 {published data only}
- Leone LA, Allicock M, Pignone MP, Walsh JF, Johnson L, Armstrong‐Brown J, et al. C Cluster randomized trial of a church‐based peer counselor and tailored newsletter intervention to promote colorectal cancer screening and physical activity among older African Americans. Health Education & Behavior 2016;43(5):568‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ligibel 2008 {published and unpublished data}
- Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. Journal of Clinical Oncology 2008;26:907‐12. [DOI] [PubMed] [Google Scholar]
Ligibel 2009 {published and unpublished data}
- Ligibel JA, Giobbie‐Hurder A, Olenczuk D, Campbell N, Salinardi T, Winer EP, et al. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes & Control 2009;20:1523‐8. [DOI] [PubMed] [Google Scholar]
Ligibel 2016 {published data only}
- Ligibel JA, Giobbie‐Hurder A, Shockro L, Campbell N, Partridge AH, Tolaney S, et al. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer 2016;112(8):1169‐77. [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin KY, Shun SC, Lai YH, Liang JT, Tsauo JY. Comparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapy. Cancer Nursing 2014;37:E21. [DOI] [PubMed] [Google Scholar]
Litterini 2013 {published data only}
- Litterini AJ, Fieler VK, Cavanaugh JT, Lee JQ. Differential effects of cardiovascular and resistance exercise on functional mobility in individuals with advanced cancer: a randomized trial. Archives of Physical Medicine & Rehabilitation 2013;94(12):2329‐35. [DOI] [PubMed] [Google Scholar]
Livingston 2015 {published data only}
- Livingston PM, Craike MJ, Salmon J, Courneya KS, Gaskin CJ, Fraser SF, et al. Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: A multicenter cluster randomized controlled trial (ENGAGE). Cancer 2015;121(15):2646‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 2014 {published data only}
- Lynch BM, Courneya KS, Sethi P, Patrao TA, Hawkes AL. A randomized controlled trial of a multiple health behavior change intervention delivered to colorectal cancer survivors: effects on sedentary behavior. Cancer 2014;120(17):2665‐72. [DOI] [PubMed] [Google Scholar]
Lyons 2016 {published data only}
- Lyons KS, Winters‐Stone KM, Bennett JA, Beer TM. The effects of partnered exercise on physical intimacy in couples coping with prostate cancer. Health Psychology 2016;35(5):509‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacVicar 1989 {published data only}
- MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients' functional capacity. Nursing Research 1989;38:348‐51. [PubMed] [Google Scholar]
Manassero 2007 {published data only}
- Manassero F, Traversi C, Ales V, Pistolesi D, Panicucci E, Valent F, et al. Contribution of early intensive prolonged pelvic floor exercises on urinary continence recovery after bladder neck‐sparing radical prostatectomy: results of a prospective controlled randomized trial. Neurourology and Urodynamics 2007;26:985–9. [DOI] [PubMed] [Google Scholar]
Martin 2013 {published data only}
- Martin E, Battaglini C, Groff D, Naumann F. Improving muscular endurance with the MVe Fitness Chair™ in breast cancer survivors: a feasibility and efficacy. Journal of Science and Medicine in Sport 2013;16(4):372‐6. [DOI] [PubMed] [Google Scholar]
Mayo 2014 {published data only}
- Mayo NE, Moriello C, Scott SC, Dawes D, Auais M, Chasen M. Pedometer‐facilitated walking intervention shows promising effectiveness for reducing cancer fatigue: a pilot randomized trial. Clinical Rehabilitation 2014;28:1198‐209. [DOI] [PubMed] [Google Scholar]
McClure 2010 {published data only}
- McClure MK, McClure RJ, Day R, Brufsky AM. Randomized controlled trial of the Breast Cancer Recovery Program for women with breast cancer‐related lymphedema. American Journal of Occupational Therapy 2010;64:59‐72. [DOI] [PubMed] [Google Scholar]
McGowan 2013 {published data only}
- McGowan EL, North S, Courneya KS. Randomized controlled trial of a behavior change intervention to increase physical activity and quality of life in prostate cancer survivors. Annals of Behavioral Medicine 2013;46(3):382‐93. [DOI] [PubMed] [Google Scholar]
McGuire 2011 {published data only}
- McGuire R, Waltman N, Zimmerman L. Intervention components promoting adherence to strength training exercise in breast cancer survivors with bone loss. Western Journal of Nursing Research 2011;33:671‐89. [DOI] [PubMed] [Google Scholar]
McNeely 2004 {published and unpublished data}
- McNeely ML, Parliament M, Courneya KS, Seikaly H, Jha N, Scrimger R, et al. A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head and Neck 2004;26:518‐30. [DOI] [PubMed] [Google Scholar]
Milecki 2013 {published data only}
- Milecki P, Hojan K, Ozga‐Majchrzak O, Molinska‐Glura M. Exercise tolerance in breast cancer patients during radiotherapy after aerobic training. Wspolczesna Onkologia [Contemporary Oncology] 2013;17(2):205‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mina 2013 {published data only}
- Mina DS, Connor MK, Alibhai SMH, Toren P, Guglietti C, Matthew AG, et al. Exercise effects on adipokines and the IGF axis in men with prostate cancer treated with androgen deprivation: a randomized study. Canadian Urological Association Journal [Journal de l'Association des Urologues du Canada] 2013;7:E692‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mock 1994 {published data only}
- Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney‐Tedder S, et al. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 1994;21:899‐907. [PubMed] [Google Scholar]
Mock 1997 {published data only}
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield‐Wolfe ME, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncology Nursing Forum 1997;24:991‐1000. [PubMed] [Google Scholar]
Mock 2005 {published data only}
- Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psycho‐Oncology 2005;14:464‐77. [DOI] [PubMed] [Google Scholar]
Molassiotis 2015 {published data only}
- Molassiotis A, Charalambous A, Taylor P, Stamataki Z, Summers Y. The effect of resistance inspiratory muscle training in the management of breathlessness in patients with thoracic malignancies: a feasibility randomised trial. Supportive Care in Cancer 2015;23(6):1637‐45. [DOI] [PubMed] [Google Scholar]
Moller 2015 {published data only}
- Moller T, Lillelund C, Andersen C, Bloomquist K, Christensen K, Ejlertsen B, et al. Use of exercise in patients with breast cancer. Supportive Care in Cancer 2015;30:406‐11. [Google Scholar]
Monga 2007 {published data only}
- Monga U, Garber SL, Thornby J, Vallbona C, Kerrigan AJ, Monga TN, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Archives of Physical Medicine and Rehabilitation 2007;88:1416‐22. [DOI] [PubMed] [Google Scholar]
Morielli 2018 {published data only}
- Morielli AR, Boule NG, Usmani N, Kurian J, Tankel K, Severin D, et al. Predictors of adherence to aerobic exercise in rectal cancer patients during and after neoadjuvant chemoradiotherapy. Psychology, Health & Medicine 2018;23(2):224‐31. [DOI] [PubMed] [Google Scholar]
Mustian 2008 {published data only}
- Mustian KM, Palesh OG, Flecksteiner SA. Tai Chi Chuan for breast cancer survivors. Medicine and Sport Science 2008;52:209‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustian 2015 {published data only}
- Mustian K, Janelsins M, Peppone L, Kamen C, Heckler C. Exercise effects on muscular strength, cancer‐related fatigue, and mitochondrial and nuclear gene expression in skeletal muscle among older prostate cancer patients. Supportive Care in Cancer 2015;23:s89‐90. [Google Scholar]
Mutrie 2007 {published and unpublished data}
- Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ 2007;334:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naumann 2012 {published data only}
- Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. Journal of Supportive Oncology 2012;10(5):188‐94. [DOI] [PubMed] [Google Scholar]
Newton 2014 {published data only}
- Newton RU, Galvao DA, Spry N, Cormie P, Chambers SK, Gardiner RA, et al. Musculoskeletal effects of exercise in men with prostate cancer initiating androgen deprivation therapy. Asia‐Pacific Journal of Clinical Oncology 2014;10:206‐7. [Google Scholar]
Nieman 1995 {published data only}
- Nieman DC, Cook VD, Henson DA, Suttles J, Rejeski WJ, Ribisl PM, et al. Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. International Journal of Sports Medicine 1995;16:334‐7. [DOI] [PubMed] [Google Scholar]
Nikander 2007 {published data only}
- Nikander R, Sievänen H, Ojala K, Oivanen T, Kellokumpu‐Lehtinen PL, Saarto T. Effect of a vigorous aerobic regimen on physical performance in breast cancer patients-a randomized controlled pilot trial. Acta Oncologica 2007;46:181‐6. [DOI] [PubMed] [Google Scholar]
Nikander 2012 {published data only}
- Nikander R, Sievanen H, Ojala K, Kellokumpu‐Lehtinen PL, Palva T, Blomqvist C, et al. Effect of exercise on bone structural traits, physical performance and body composition in breast cancer patients ‐ a 12‐month RCT. Journal of Musculoskeletal & Neuronal Interactions 2012;12(3):127‐35. [PubMed] [Google Scholar]
Nilsen 2015 {published data only}
- Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncologica 2015;54(10):1805‐13. [DOI] [PubMed] [Google Scholar]
Nobes 2012 {published data only}
- Nobes JP, Langley SEM, Klopper T, Russell‐Jones D, Laing RW. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy [with consumer summary]. BJU International 2012;109(10):1495‐502. [DOI] [PubMed] [Google Scholar]
Nuri 2012 {published data only}
- Nuri R, Kordi MR, Moghaddasi M, Rahnama N, Damirchi A, Rahmani‐Nia F, et al. Effect of combination exercise training on metabolic syndrome parameters in postmenopausal women with breast cancer. Journal of Cancer Research & Therapeutics 2012;8(2):238‐42. [DOI] [PubMed] [Google Scholar]
Nuri 2016 {published data only}
- Nuri R, Moghaddasi M, Darvishi H, Izadpanah A. Effect of aerobic exercise on leptin and ghrelin in patients with colorectal cancer. Journal of Cancer Research & Therapeutics 2016;12(1):169‐74. [DOI] [PubMed] [Google Scholar]
Nyrop 2017 {published data only}
- Nyrop KA, Callahan LF, Cleveland RJ, Arbeeva LL, Hackney BS, Muss HB. Randomized controlled trial of a home‐basedwalking program to reducemoderate to severe aromatase inhibitor‐associated arthralgia in breast cancer survivors. Oncologist 2017;22(10):1238‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Neil 2015 {published data only}
- O'Neill RF, Haseen F, Murray LJ, O'Sullivan JM, Cantwell MM. A randomised controlled trial to evaluate the efficacy of a 6‐month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. Journal of Cancer Survivorship 2015;9(3):431‐40. [DOI] [PubMed] [Google Scholar]
Ohira 2006 {published data only}
- Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects of weight training on quality of life in recent breast cancer survivors: the Weight Training for Breast Cancer Survivors (WTBS) study. Cancer 2006;106:2076‐83. [DOI] [PubMed] [Google Scholar]
Ornish 2005 {published data only}
- Ornish D, Weidner G, Fair WR, Marlin R, Pettengill EB, Raisin CJ, et al. Intensive lifestyle changes may affect the progression of prostate cancer. Journal of Urology 2005;174:1065‐9. [DOI] [PubMed] [Google Scholar]
Ornish 2008a {published data only}
- Ornish D, Magbanua MJ, Weidner G, Weinberg V, Kemp C, Green C, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proceedings of the National Academy of Sciences in the United States of America 2008a;105:8369‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ornish 2008b {published data only}
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncology 2008;9:1048‐57. [DOI] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park SW, Kim TN, Nam JK, Ha HK, Shin DG, Lee W, et al. Recovery of overall exercise ability, quality of life, and continence after 12‐week combined exercise intervention in elderly patients who underwent radical prostatectomy: a randomized controlled study. Urology 2012;80:299‐305. [DOI] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Park CL, Cho D, Salner A, Dornelas E. A randomized controlled trial of two mail‐based lifestyle interventions for breast cancer survivors. Supportive Care in Cancer 2016;24(7):3037‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Payne 2008 {published data only}
- Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncology Nursing Forum 2008;35:635‐42. [DOI] [PubMed] [Google Scholar]
Philips 2012 {published data only}
- Phillips KM, Jim HS, Small BJ, Tanvetyanon T, Roberts WS, Jacobsen PB. Effects of self‐directed stress management training and home‐based exercise on stress management skills in cancer patients receiving chemotherapy. Stress and Health 2012;25(5):368‐75. [DOI] [PubMed] [Google Scholar]
Pickett 2002 {published data only}
- Pickett M, Mock V, Ropka ME, Cameron L, Coleman M, Podewils L. Adherence to moderate‐intensity exercise during breast cancer therapy. Cancer Practice 2002;10:284‐92. [DOI] [PubMed] [Google Scholar]
Pinto 2013a {published data only}
- Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychology 2013;32:616‐26. [DOI] [PubMed] [Google Scholar]
Pinto 2013b {published data only}
- Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home‐based physical activity intervention for colorectal cancer survivors. Psycho‐Oncology 2013;22(1):54‐64. [DOI] [PubMed] [Google Scholar]
Pinto 2015 {published data only}
- Pinto BM, Stein K, Dunsiger S. Peers promoting physical activity among breast cancer survivors: a randomized controlled trial. Health Psychology 2015;34(5):463‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porserud 2014 {published data only}
- Porserud A, Sherif A, Tollbäck A. The effects of a physical exercise programme after radical cystectomy for urinary bladder cancer. A pilot randomized controlled trial. Clinical Rehabilitation 2014;28(5):451‐9. [DOI] [PubMed] [Google Scholar]
Portela 2008 {published and unpublished data}
- Portela ALM, Santaella CL, Gómez CC, Burch A. Feasibility of an exercise program for Puerto Rican women who are breast cancer survivors. Rehabilitation Oncology 2008;26(2):20‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabin 2016 {published data only}
- Rabin C, Pinto B, Fava J. Randomized trial of a physical activity and meditation intervention for young adult cancer survivors. Journal of Adolescent and Young Adult Oncology 2016;5(1):41‐7. [DOI] [PubMed] [Google Scholar]
Rahnama 2010 {published and unpublished data}
- Rahnama N, Nouri R, Rahmaninia F, Damirchi A, Emami H. The effects of exercise training on maximum aerobic capacity, resting heart rate, blood pressure and anthropometric variables of postmenopausal women with breast cancer. Journal of Research in Medical Sciences 2010;15:78‐83. [PMC free article] [PubMed] [Google Scholar]
Rao 2012 {published data only}
- Rao R, Cruz V, Peng Y, Harker‐Murray A, Haley BB, Zhao H, et al. Bootcamp during neoadjuvant chemotherapy for breast cancer: a randomized pilot trial. Breast Cancer 2012;6(1):39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reis 2013 {published and unpublished data}
- Reis D, Walsh E, Young‐McCaughan S, Jones T. Effects of Nia exercise in women receiving radiation therapy for breast cancer. Oncology Nursing Forum 2013;40(5):E374‐81. [DOI] [PubMed] [Google Scholar]
Rogers 2009 {published and unpublished data}
- Rogers LQ, Hopkins‐Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, et al. A randomized trial to increase physical activity in breast cancer survivors. Medicine and Science in Sports and Exercise 2009;41:935‐46. [DOI] [PubMed] [Google Scholar]
Rogers 2012 {published and unpublished data}
- Rogers LQ, Fogleman A, Trammell R, Hopkins‐Price P, Vicari S, Rao K, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integrative Cancer Therapies 2012;4(2013):323‐35. [DOI: 10.1177/1534735412449687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2013a {published data only}
- Rogers LQ, Anton PM, Fogleman A, Hopkins‐Price P, Verhulst S, Rao K, et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head & Neck 2013;35(8):1178‐88. [DOI] [PubMed] [Google Scholar]
Rogers 2013b {published data only}
- Rogers LQ, Fogleman A, Trammell R, Hopkins‐Price P, Vicari S, Rao K, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integrative Cancer Therapies 2013;12(4):323‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2014 {published data only}
- Rogers LQ, Vicari S, Trammell R, Hopkins‐Price P, Fogleman A, Spenner A, et al. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Medicine & Science in Sports & Exercise 2014;46(6):1077‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2015b {published data only}
- Rogers LQ, Fogleman A, Trammell R, Hopkins‐Price P, Spenner A, Vicari S, et al. Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: pilot randomized controlled trial. Psycho‐Oncology 2015;24(3):302‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saarto 2012a {published data only}
- Saarto T, Penttinen HM, Sievanen H, Kellokumpu‐Lehtinen PL, Hakamies‐Blomqvist L, Nikander R, et al. Effectiveness of a 12‐month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Research 2012;32(9):3875‐84. [PubMed] [Google Scholar]
Saarto 2012b {published data only}
- Saarto T, Sievanen H, Kellokumpu‐Lehtinen P, Nikander R, Vehmanen L, Huovinen R, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12‐month randomised controlled trial. Osteoporosis International 2012;23(5):1601‐12. [DOI] [PubMed] [Google Scholar]
Sajid 2013 {published data only}
- Sajid S, Dale W, Mustian K, Kotwal A, Heckler C, Porto M, et al. Novel physical activity interventions for older patients with prostate cancer on hormone therapy: a pilot randomized study. Journal of Geriatric Oncology 2016;7(2):71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuel 2013 {published data only}
- Samuel SR, Maiya GA, Babu AS, Vidyasagar MS. Effect of exercise training on functional capacity and quality of life in head and neck cancer patients receiving chemoradiotherapy. Indian Journal of Medical Research 2013;137(3):515‐20. [PMC free article] [PubMed] [Google Scholar]
Sandel 2005 {published data only}
- Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality‐of‐life measures in breast cancer survivors. Cancer Nursing 2005;28:301‐9. [DOI] [PubMed] [Google Scholar]
Schmidt 2015 {published data only}
- Shmidt ME, Wiskemann J, Armburst P, Schneeweiss A, Ulrich C, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. International Journal of Cancer 2015;35(10):5623‐9. [DOI] [PubMed] [Google Scholar]
Schmidt 2017a {published data only}
- Schmidt ME, Wiskemann J, Ulrich CM, Schneeweiss A, Steindorf K. Self‐reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow‐up of two randomized exercise intervention trials. Acta Oncologica 2017;56(4):618‐27. [DOI] [PubMed] [Google Scholar]
Schmidt 2017b {published data only}
- Schmidt T, Berner J, Jonat W, Weisser B, Rocken C, Mackelenbergh M, et al. Influence of arm crank ergometry on development of lymphoedema in breast cancer patients after axillary dissection: a randomized controlled trial. Journal of Rehabilitation Medicine 2017;49(1):78‐83. [DOI] [PubMed] [Google Scholar]
Schmitz 2009 {published and unpublished data}
- Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis‐Grant L, et al. Weight lifting in women with breast‐cancer‐related lymphedema. New England Journal of Medicine 2009;361:664‐73. [DOI] [PubMed] [Google Scholar]
Schmitz 2010 {published and unpublished data}
- Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis‐Grant L, Smith R, et al. Weight lifting for women at risk for breast cancer‐related lymphedema: a randomized trial. JAMA 2010;304:2699‐705. [DOI] [PubMed] [Google Scholar]
Schmitz 2015a {published data only}
- Schmitz KH, Williams NI, Kontos D, Domchek S, Morales KH, Hwang WT, et al. Dose‐response effects of aerobic exercise on estrogen among women at high risk for breast cancer: a randomized controlled trial. Breast Cancer Research and Treatment 2015;154(2):309‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2015b {published data only}
- Schmitz KH, Williams NI, Kontos D, Kurzer MS, Schnall M, Domchek S, et al. Women In Steady Exercise Research (WISER) Sister: study design and methods. Contemporary Clinical Trials 2015;41:17‐30. [DOI] [PubMed] [Google Scholar]
Schuler 2017 {published data only}
- Schuler MK, Hentschel L, Kisel W, Kramer M, Lenz F, Hornemann B, et al. Impact of different exercise programs on severe fatigue in patients undergoing anticancer treatment ‐ a randomized controlled trial. Journal of Pain and Symptom Management 2017;53(1):57‐66. [DOI] [PubMed] [Google Scholar]
Schwartz 2015 {published data only}
- Schwartz AL, Biddle‐Newberry M, Heer HD. Randomized trial of exercise and an online recovery tool to improve rehabilitation outcomes of cancer survivors. Physician and Sportsmedicine 2015;43(2):143‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scruggs 2018 {published data only}
- Scruggs S, Mama SK, Carmack CL, Douglas T, Diamond P, Basen‐Engquist K. Randomized trial of a lifestyle physical activity intervention for breast cancer survivors: effects on transtheoretical model variables. Health Promotion Practice 2018;19(1):134‐44. [DOI] [PubMed] [Google Scholar]
Sebio Garcia 2017 {published data only}
- Sebio Garcia R, Yanez‐Brage MI, Gimenez Moolhuyzen E, Salorio Riobo M, Lista Paz A, Borro Mate JM. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single‐blind controlled trial. Clinical Rehabilitation 2017;31(8):1057‐67. [DOI] [PubMed] [Google Scholar]
Segal 2001 {published and unpublished data}
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. Journal of Clinical Oncology 2001;19:657‐65. [DOI] [PubMed] [Google Scholar]
Segal 2003 {published and unpublished data}
- Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. Journal of Clinical Oncology 2003;21:1653‐9. [DOI] [PubMed] [Google Scholar]
Segal 2009 {published and unpublished data}
- Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud'Homme DG, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. Journal of Clinical Oncology 2009;27:344‐51. [DOI] [PubMed] [Google Scholar]
Sener 2017 {published data only}
- Sener HO, Malkoc M, Ergin G, Karadibak D, Yavuzsen T. Effects of clinical pilates exercises on patients developing lymphedema after breast cancer treatment: a randomized clinical trial. Journal of Breast Health 2017;13(1):16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheppard 2016 {published data only}
- Sheppard VB, Hicks J, Makambi K, Hurtado‐de‐Mendoza A, Demark‐Wahnefried W, Adams‐Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: The Stepping STONE study. Contemporary Clinical Trials 2016;46:106‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shobeiri 2016 {published data only}
- Shobeiri F, Masoumi SZ, Nikravesh A, Heidari Moghadam R, Karami M. The impact of aerobic exercise on quality of life in women with breast cancer: a randomized controlled trial [with consumer summary]. Journal of Research in Health Sciences 2016;16(3):127‐32. [PMC free article] [PubMed] [Google Scholar]
Short 2012 {published data only}
- Short C, James E, Girgis A, McElduff P, Plotnikoff R. The efficacy of two theoretically‐based print interventions for promoting PA behaviour among post‐treatment breast cancer survivors: a nationally‐based 3‐arm RCT. Journal of Science and Medicine in Sport 2012;15:S175‐6. [Google Scholar]
Short 2017a {published data only}
- Short CE, James EL, Girgis A, D'Souza MI, Plotnikoff RC. Main outcomes of the Move More for Life Trial: A randomised controlled trial examining the effects of tailored‐print and targeted‐print materials for promoting physical activity among post‐treatment breast cancer survivors. Psycho‐Oncology 2017;24(7):771‐8. [DOI] [PubMed] [Google Scholar]
Short 2017b {published data only}
- Short CE, Rebar A, James EL, Duncan MJ, Courneya KS, Plotnikoff RC, et al. How do different delivery schedules of tailored web‐based physical activity advice for breast cancer survivors influence intervention use and efficacy?. Journal of Cancer Survivorship 2017;11(1):80‐91. [DOI] [PubMed] [Google Scholar]
Singh 2015 {published data only}
- Singh B, Newton RU, Cormie P, Galvao DA, Cornish B, Reul‐Hirche H, et al. Effects of compression on lymphedema during resistance exercise in women with breast cancer‐related lymphedema: A randomized, cross‐over trial. Lymphology 2015;48(2):80‐92. [PubMed] [Google Scholar]
Skinner 2016 {published data only}
- Skinner TL, Peeters G, Croci I, Bell KR, Burton NW, Chambers SK, et al. Impact of a brief exercise program on the physical and psychosocial health of prostate cancer survivors: a pilot study. Asia‐Pacific Journal of Clinical Oncology 2016;12(3):225‐34. [DOI] [PubMed] [Google Scholar]
Sohl 2016 {published data only}
- Sohl SJ, Danhauer SC, Birdee GS, Nicklas BJ, Yacoub G, Aklilu M, et al. A brief yoga intervention implemented during chemotherapy: a randomized controlled pilot study. Complementary Therapies in Medicine 2016;25:139‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spahn 2013 {published data only}
- Spahn G, Choi KE, Kennemann C, Ludtke R, Franken U, Langhorst J, et al. Can a multimodal mind‐body program enhance the treatment effects of physical activity in breast cancer survivors with chronic tumor‐associated fatigue? A randomized controlled trial. Integrative Cancer Therapies 2013;12(4):291‐300. [DOI] [PubMed] [Google Scholar]
Stacey 2016 {published data only}
- Stacey FG, James EL, Chapman K, Lubans DR. Social cognitive theory mediators of physical activity in a lifestyle program for cancer survivors and carers: findings from the ENRICH randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2016;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stefanelli 2013 {published data only}
- Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, et al. High‐intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non‐small‐cell lung cancer undergoing lobectomy. European Journal of Cardio‐thoracic Surgery 2013;44(4):e260‐5. [DOI] [PubMed] [Google Scholar]
Stolley 2017 {published data only}
- Stolley M, Sheean PM, Gerber B, Arroyo C, Schiffer L, Banerjee A, et al. Efficacy of a weight loss intervention for African American breast cancer survivors. Journal of Clinical Oncology 2017;35(24):2820‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Streckman 2014 {published data only}
- Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihorst G, et al. Exercise program improves therapy‐related side‐effects and quality of life in lymphoma patients undergoing therapy. Annals of Oncology 2014;25(2):493‐9. [DOI] [PubMed] [Google Scholar]
Sturgeon 2017 {published data only}
- Sturgeon KM, Dean LT, Heroux M, Kane J, Bauer T, Palmer E, et al. Commercially available lifestyle modification program: randomized controlled trial addressing heart and bone health in BRCA1/2+ breast cancer survivors after risk‐reducing salpingo‐oophorectomy. Journal of Cancer Survivorship 2017;11(2):246‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swisher 2015 {published data only}
- Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple‐negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Supportive Care in Cancer 2015;23(10):2995‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taafe 2017 {published data only}
- Taaffe DR, Newton RU, Spry N, Joseph D, Chambers SK, Gardiner RA, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year‐long randomised controlled trial. European Urology 2017;72(2):293‐9. [DOI] [PubMed] [Google Scholar]
Taleghani 2012 {published data only}
- Taleghani F, Karimain J, Babazadeh S, Mokarian F, Tabatabaiyan M, Samimi MA, et al. The effect of combined aerobic and resistance exercises on quality of life of women surviving breast cancer. Iranian Journal of Nursing and Midwifery Research 2012;17(1):47‐51. [PMC free article] [PubMed] [Google Scholar]
Taso 2014 {published data only}
- Taso CJ, Lin HS, Lin WL, Chen SM, Huang WT, Chen SW. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: a randomized controlled trial. Journal of Nursing Research 2014;22(3):155‐64. [DOI] [PubMed] [Google Scholar]
Terranova 2017 {published data only}
- Terranova CO, Lawler SP, Spathonis K, Eakin EG, Reeves MM. Breast cancer survivors' experience of making weight, dietary and physical activity changes during participation in a weight loss intervention. Supportive Care in Cancer 2017;25(5):1455‐63. [DOI] [PubMed] [Google Scholar]
Tomasello 2017 {published data only}
- Tomasello B, Malfa GA, Strazzanti A, Gangi S, Giacomo C, Basile F, et al. Effects of physical activity on systemic oxidative/DNA status in breast cancer survivors. Oncology Letters 2017;13(1):441‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tometich 2017 {published data only}
- Tometich DB, Mosher CE, Winger JG, Badr HJ, Snyder DC, Sloane RJ, et al. Effects of diet and exercise on weight‐related outcomes for breast cancer survivors and their adult daughters: an analysis of the DAMES trial. Supportive Care in Cancer 2017;25(8):2559‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Travier 2015 {published data only}
- Travier N, Velthuis MJ, Steins Bisschop CN, Buijs B, Monninkhof EM, Backx F, et al. Effects of an 18‐week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Medicine 2015;13(121):Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trinh 2014 {published data only}
- Trinh L, Mutrie N, Campbell AM, Crawford JJ, Courneya KS. Effects of supervised exercise on motivational outcomes in breast cancer survivors at 5‐year follow‐up. European Journal of Oncology Nursing 18;6:557‐63. [DOI] [PubMed] [Google Scholar]
Uth 2014 {published data only}
- Uth J, Hornstrup T, Schmidt JF, Christensen JF, Frandsen C, Christensen KB, et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scandinavian Journal of Medicine & Science in Sports 2014;24:105‐12. [DOI] [PubMed] [Google Scholar]
Uth 2016 {published data only}
- Uth J, Hornstrup T, Christensen JF, Christensen KB, Jørgensen NR, Schmidt JF, et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32‐week follow‐up of the FC prostate randomised controlled trial. Osteoporosis International 2016;27(4):1507‐18. [DOI] [PubMed] [Google Scholar]
Van Vulpen 2016 {published data only}
- Vulpen JK, Velthuis MJ, Steins Bisschop CN, Travier N, Buijs BJ, Backx FJ, et al. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Medicine & Science in Sports & Exercise 2016;48(5):767‐75. [DOI] [PubMed] [Google Scholar]
van Waart 2015 {published data only}
- Waart H, Stuiver MM, Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. Journal of Clinical Oncology 2015;33(17):1918‐27. [DOI] [PubMed] [Google Scholar]
von Gruenigen 2008 {published and unpublished data}
- Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecologic Oncology 2008;109:19‐26. [DOI] [PubMed] [Google Scholar]
von Gruenigen 2009 {published and unpublished data}
- Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health and Quality of Life Outcomes 2009;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Gruenigen 2012 {published and unpublished data}
- Gruenigen V, Frasure H, Kavanagh MB, Janata J, Waggoner S, Rose P, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecologic Oncology 2012;125:699‐704. [DOI] [PubMed] [Google Scholar]
Waltman 2010 {published and unpublished data}
- Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, et al. The effect of weight training on bone mineral density and bone turnover in post menopausal breast cancer survivors with bone loss: a 24‐month randomized controlled trial. Osteoporosis International 2010;21:1361‐9. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang Q, Suo J, Jiang J, Wang C, Zhao YQ, Cao X. Effectiveness of fast‐track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Disease 2012;14:1009‐13. [DOI] [PubMed] [Google Scholar]
Wasley 2018 {published data only}
- Wasley D, Gale N, Roberts S, Backx K, Nelson A, Deursen R, et al. Patients with established cancer cachexia lack the motivation and self‐efficacy to undertake regular structured exercise. Psycho‐Oncology 2018;27(2):458‐64. [DOI] [PubMed] [Google Scholar]
Wiskemann 2017 {published data only}
- Wiskemann J, Schmidt M, Klassen O, Debus J, Ulrich CM, Potthoff K, et al. Effects of 12‐week resistance training during radiotherapy in breast cancer patients. Scandinavian Journal of Medicine & Science in Sports 2017;27(11):1500‐10. [DOI] [PubMed] [Google Scholar]
Xu 2015 {published data only}
- Xu Y, Cheng C. Effects of a walk‐and‐eat intervention for patients with esophageal cancer undergoing neoadjuvant chemoradiation. Supportive Care in Cancer 2015;23:1216‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2011 {published data only}
- Yang CY, Tsai JC, Huang YC, Lin CC. Effects of a home‐based walking program on perceived symptom and mood status in postoperative breast cancer women receiving adjuvant chemotherapy. Journal of Advanced Nursing 2011;67:158‐68. [DOI] [PubMed] [Google Scholar]
Yeo 2012 {published and unpublished data}
- Yeo TP, Burrell SA, Sauter PK, Kennedy EP, Lavu H, Leiby BE, et al. A progressive postresection walking program significantly improves fatigue and health‐related quality of life in pancreas and periampullary cancer patients. Journal of the American College of Surgeons 2012;214:463‐75. [DOI] [PubMed] [Google Scholar]
Yuen 2007 {published and unpublished data}
- Yuen HK, Sword D. Home‐based exercise to alleviate fatigue and improve functional capacity among breast cancer survivors. Journal of Allied Health 2007;36:e257‐75. [PubMed] [Google Scholar]
Yun 2013 {published data only}
- Yun YH, Lee MK, Bae Y, Shon EJ, Shin BR, Ko H, et al. Efficacy of a training program for long‐term disease‐ free cancer survivors as health partners: a randomized controlled trial in Korea. Asian Pacific Journal of Cancer Prevention 2013;14(12):7229‐35. [DOI] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang Q, Li F, Zhang H, Yu X, Cong Y. Effects of nurse‐led home‐based exercise & cognitive behavioral therapy on reducing cancer‐related fatigue in patients with ovarian cancer during and after chemotherapy: a randomized controlled trial. International Journal of Nursing Studies 2018;78:52‐60. [DOI] [PubMed] [Google Scholar]
Zhao 2016 {published data only}
- Zhao SG, Alexander NB, Djuric Z, Zhou J, Tao Y, Schipper M, et al. Maintaining physical activity during head and neck cancer treatment: results of a pilot controlled trial. Head & Neck 2016;38 (supp 1):E1086‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2015 {published data only}
- Zhou Y, Gottlieb L, Cartmel B, Li F, Ercolano EA, Harrigan M, et al. Randomized trial of exercise on quality of life and fatigue in women diagnosed with ovarian cancer: the women's activity and lifestyle study in connecticut (WALC). Journal of Clinical Oncology 2015;33:9505. [Google Scholar]
Zimmer 2014 {published data only}
- Zimmer P, Baumann FT, Bloch W, Schenk A, Koliamitra C, Jensen P, et al. Impact of exercise on pro inflammatory cytokine levels and epigenetic modulations of tumor‐competitive lymphocytes in Non‐Hodgkin‐Lymphoma patients‐randomized controlled trial. European Journal of Haematology 2014;93(6):527‐32. [DOI] [PubMed] [Google Scholar]
Zimmer 2016 {published data only}
- Zimmer P, Oberste M, Bloch W, Schenk A, Joisten N, Hartig P, et al. Impact of aerobic exercise training during chemotherapy on cancer related cognitive impairments in patients suffering from acute myeloid leukemia or myelodysplastic syndrome ‐ study protocol of a randomized placebo‐controlled trial. Contemporary Clinical Trials 2016;49:1‐5. [DOI] [PubMed] [Google Scholar]
Zopf 2012 {published data only}
- Zopf EM, Braun M, Machtens S, Zumbe J, Bloch W, Baumann F. Effects of a 15‐month rehabilitative exercise program in prostate cancer patients following a radical prostatectomy‐first results of the ProRehab Study. Journal of Cancer Research and Clinical Oncology 2012;138:409‐18. [Google Scholar]
References to studies awaiting assessment
Bai 2004 {published data only}
- Bai S‐M, Ma C, Liu Y‐M, Xue W‐P, Luo M, Ou Z‐H. Effects of cognitive behavior intervention and cinesiateics on the quality of life of patients with nasopharyngeal carcinoma after radiotherapy. Chinese Journal of Clinical Rehabilitation 2004;8(29):6312‐3. [Google Scholar]
Chen 2010 {published data only}
- Chen J, Luo A, He Y. Influence of postoperative rehabilitation exercises on functional recovery of ill limb of breast cancer patients. Chinese Nursing Research 2010;24(4A):875‐7. [Google Scholar]
Cho 2004 {published data only}
- Cho OH. Effects of a comprehensive rehabilitation programme for mastectomy patients. Taehan Kanho Hakhoe Chi 2004;34(5):809‐19. [DOI] [PubMed] [Google Scholar]
Choi 2012 {published data only}
- Choi J Y, Kang H S. Effects of a home‐based exercise program for patients with stomach cancer receiving oral chemotherapy after surgery. Journal of Korean Academy of Nursing 2012;42(1):95‐104. [DOI] [PubMed] [Google Scholar]
Dong 2006 {published data only}
- Dong HY, Wang ZF, Cai L. Correlation between quality of life and rehabilitative guidance education in the postoperative patients with breast cancer. Chinese Journal of Clinical Rehabilitation 2006;10(42):28‐30. [Google Scholar]
Guo 2004 {published data only}
- Guo Y‐M. Effects of moderate strength and endurance exercise on emotion and quality of sleep in patients with malignant tumor. Chinese Journal of Clinical Rehabilitation 2004;8(35):7896‐7. [Google Scholar]
Hu 2013 {published data only}
- Hu HF, Li TC, Liu LC, Wu CT, Wang Y J. Effects of a walking program on fatigue and exercise capacity in post‐surgery breast cancer women. Hu li za zhi [Journal of nursing] 2013;60(5):53‐63. [DOI] [PubMed] [Google Scholar]
LeVu 1997 {published data only}
- Vu B, Dumortier A, Guillaume MV, Mouriesse H, Barreau‐Pouhaer L. Efficacy of massage and mobilization of the upper limb after surgical treatment of breast cancer. Bulletin du Cancer 1997;80(10):957‐61. [PubMed] [Google Scholar]
Oliveira 2010 {published data only}
- Oliveira MM, Souza GA, Miranda Mde S, Okubo MA, Amaral MT, Silva MP, Gurgel MS. Upper limb exercises during radiotherapy for breast cancer and quality of life. Revista Brasileira de Ginecologia e Obstetrícia 2010;32(3):133‐8. [DOI] [PubMed] [Google Scholar]
Park 2006 {published data only}
- Park HS, Cho GY, Park KY. The effects of a rehabilitation program on physical health, physiological indicator and quality of life in breast cancer mastectomy patients. Taehan Kanho Hakhoe Chi 2006;36(2):310‐20. [DOI] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang Y, Yao J‐F, Yang J‐Y. Effect of rehabilitation exercises on the recovery outcomes of lung function in postoperative patients with lung cancer. Zhongguo Linchuang Kangfu (Chinese Journal of Clinical Rehabilitation) 2005;9(39):14‐6. [Google Scholar]
Zhang 2005 {published data only}
- Effects of rehabilitation therapy in relieving pain and improving quality of life in patients with advanced cancer. Zhongguo Linchuang Kangfu (Chinese Journal of Clinical Rehabilitation) 2005, (40):59‐61. [Google Scholar]
Additional references
Bandura 2000
- Bandura A. Exercise of human agency through collective efficacy. Current Directions in Psychological Science 2000;9(3):75‐8. [Google Scholar]
Bandura 2002
- Bandura A. Social cognitive theory in cultural context. Applied Psychology: An International Review 2002;51:269‐90. [Google Scholar]
Borg 1982
- Borg GA. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise 1982;14(5):377‐81. [PubMed] [Google Scholar]
Bourke 2012a
- Bourke L, Rosario D, Copeland R, Taylor S. Physical activity for cancer survivors. BMJ 2012;344:d7998. [DOI] [PubMed] [Google Scholar]
Carver 1982
- Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality‐social, clinical, and health psychology. Psychological Bulletin 1982;92:111‐35. [PubMed] [Google Scholar]
Corner 2013
- Corner J, Wagland R, Glaser A, Richards SM. Qualitative analysis of patients’ feedback from a PROMs survey of cancer patients in England. BMJ Open 2013;3(4):e002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Courneya 2003
- Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. European Journal of Cancer Care 2003;12(4):347‐57. [DOI] [PubMed] [Google Scholar]
Courneya 2010
- Courneya KS. Efficacy, effectiveness, and behavior change trials in exercise research. International Journal of Behavioural Nutrition and Physical Activity 2010;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2012
- Das P, Horton R. Rethinking our approach to physical activity. Lancet 2012;380(9838):189‐90. [DOI] [PubMed] [Google Scholar]
Department of Health 2011
- Department of Health Physical Activity Health Improvement and Protection. Start Active, Stay Active: A report on physical activity from the four home countries’ Chief Medical Officers. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_128210.pdf [accessed 4 January 2012].
Dittus 2017
- Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: A systematic review. Preventive Medicine 2017;104:124‐32. [DOI] [PubMed] [Google Scholar]
Elliott 2011
- Elliott J, Fallows A, Staetsky L, Smith PWF, Foster CL, Maher EJ, et al. The health and well‐being of cancer survivors in the UK: findings from a population‐based survey. British Journal of Cancer 2011;105(Supp 1):S11‐S20. doi:10.1038/bjc.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galway 2012
- Galway K, Black A, Cantwell M, Cardwell CR, Mills M, Donnelly M. Psychosocial interventions to improve quality of life and emotional well being for recently diagnosed cancer patients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007064.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2014
- Garcia DO, Thomson CA. Physical activity and cancer survivorship. Nutrition in Clinical Practice 2014;29(6):768‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2011
- Gardner B, Bruijn GJ, Lally P. A systematic review and meta‐analysis of applications of the Self‐Report Habit Index to nutrition and physical activity behaviours. Annals of Behavioral Medicine 2011;42:174‐87. [DOI] [PubMed] [Google Scholar]
Global Burden of Disease Cancer Collaboration 2017
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncology 2017;3(4):524‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Godin 1986
- Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self‐report: a concurrent validity study. Canadian Journal of Public Health 1986;77:359‐62. [PubMed] [Google Scholar]
Hashim 2016
- Hashim D, Boffetta P, Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Annals of Oncology 2016;27(5):926‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handboook.org.
Hoffman 2014
- Hoffmann TC, Glasziou P, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. British Journal of Medicine 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Howlett 2018
- Howlett N, Trivedi D, Troop NA, Chater AM. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta‐analysis. Translational Behavioural Medicine 2018;28:iby010, https://doi.org/10.1093/tbm/iby010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Husebø 2013
- Husebø AM, Dyrstad SM, Søreide JA, Bru E. Predicting exercise adherence in cancer patients and survivors: a systematic review and meta‐analysis of motivational and behavioural factors. Journal of Clinical Nursing 2013;22:4‐21. [DOI] [PubMed] [Google Scholar]
Independent Cancer Taskforce
- Independent Cancer Taskforce. ACHIEVING WORLD‐CLASSCANCER OUTCOMESA STRATEGY FOR ENGLAND 2015‐2020. https://www.cancerresearchuk.org/sites/default/files/achieving_world‐class_cancer_outcomes_‐_a_strategy_for_england_2015‐2020.pdf.
Kampshoff 2014
- Kampshoff CS, Jansen F, Mechelen W, May AM, Brug J, Chinapaw MJ, et al. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. International Journal of Behavioral Nutrition and Physical Activity 2014;11(1):doi:10.1186/1479‐5868‐11‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwasnica 2016
- Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychology Review 2016;10(3):277–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2016
- Li T, Wei S, Yun S, Pang S, Qin Q, Yin J, et al. The dose–response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. British Journal of Sports Medicine 2016;50:339. [DOI] [PubMed] [Google Scholar]
Low 2014
- Low CA, Beckjord E, Bovbjerg DH, Dew MA, Posluszny DM, Schmidt JE, et al. Correlates of positive health behaviors in cancer survivors: results from the 2010 LIVESTRONGsurvey. Journal of Psychosocial Oncology 2014;32(6):678‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macmillan Cancer Support 2011
- Macmillan Cancer Support. Move More. http://www.macmillan.org.uk/Cancerinformation/Livingwithandaftercancer/Physicalactivity/Physicalactivity.aspx (accessed 4 January 2012).
Meneses‐Echavez 2015
- Meneses‐Echavez JF, Gonzalez‐Jimenez E, Ramirez‐Velez R. Supervised exercise reduces cancer‐related fatigue: a systematic review. Journal of Physiotherapy 2015;61(1):3‐9. [DOI] [PubMed] [Google Scholar]
Michie 2011
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO‐RE taxonomy. Psychology & Health 2011;26(11):1479‐98. [DOI] [PubMed] [Google Scholar]
Mishra 2012a
- Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health‐related quality of life for people with cancer during active treatment. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD008465.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2012b
- Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health‐related quality of life for cancer survivors. Cochrane Database of Systematic Reviews 2012;8:CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2014
- Mishra SI, Scherer RW, Snyder C, Geigle P, Gotay C. Are exercise programs effective for improving health‐related quality of life among cancer survivors? A systematic review and meta‐analysis. Oncology Nursing Forum 2014;41(6):E326‐42. doi:10.1188/14.ONF. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakash 2014
- Nakash O, Levav I, Aguilar‐Gaxiola S, Alonso J, Andrade L, Angermeyer M, et al. Comorbidity of common mental disorders with cancer and their treatment gap: findings from the World Mental Health Surveys. Psycho‐Oncology 2014;23:40‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2007
- National Institute for Health and Clinical Excellence (NICE). NICE public health guidance 6. Behaviour change at population, community and individual levels. October 2007. http://www.nice.org.uk/PH6 (accessed 4 January 2012).
Ormel 2017
- Ormel HL, Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psycho‐Oncology 2017;27(3):713‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rock 2012
- Rock CL, Doyle C, Demark‐Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians 2012;62(4):242‐74. [DOI] [PubMed] [Google Scholar]
Schmitz 2010a
- Schmitz KH, Courneya KS, Matthews C, Demark‐Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine Science Sports Exercise 2010;43(1):195. [DOI] [PubMed] [Google Scholar]
Stout 2017
- Stout NL, Baima J, Swisher AK, Winters‐Stone KM, Welsh J. A systematic review of exercise systematic reviews in the cancer literature (2005‐2017). PM & R: the Journal of Injury, Function, and Rehabilitation 2017;9(9 (supp 2)):S347‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verplanken 2009
- Verplanken B, Melkelvik O. Predicting habit: the case of physical exercise. Psychology of Sport and Exercise 2009;9:15‐26. [Google Scholar]
Warren 2010
- Warren JM, Ekelund U, Besson H, Mezzani A, Geladas N, Vanhees L, Experts Panel. Assessment of physical activity-a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. European Journal of Cardiovascular Prevention & Rehabilitation 2010;17:127‐39. [DOI] [PubMed] [Google Scholar]
Winter 2009
- Winter EM, Fowler N. Exercise defined and quantified according to the Systeme International d'Unites. Journal of Sports Sciences 2009;27(5):447‐60. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bourke 2012b
- Bourke L, Homer KE, Thaha MA, Steed L, Rosario D, Robb KA, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010192] [DOI] [PubMed] [Google Scholar]
Bourke 2013
- Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010192.pub2] [DOI] [PubMed] [Google Scholar]


