Abstract
Background
Both peripheral arterial thrombolysis and surgery can be used in the management of peripheral arterial ischaemia. Much is known about the indications, risks, and benefits of thrombolysis. However, whether thrombolysis works better than surgery for initial management of acute limb ischaemia remains unknown. This is the second update of the review first published in 2002.
Objectives
To determine whether thrombolysis or surgery is the more effective technique in the initial management of acute limb ischaemia due to thromboembolism.
Search methods
For this update, the Cochrane Vascular Information Specialist (CIS) searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and clinical trials registries up to 7 May 2018.
Selection criteria
All randomised controlled studies comparing thrombolysis and surgery for initial treatment of acute limb ischaemia.
Data collection and analysis
We independently assessed trial quality and extracted data. Agreement was reached by consensus. We performed analyses using odds ratios (ORs) and 95% confidence intervals (CIs).
Main results
We identified no new studies for this update. We included five trials with a total of 1292 participants; agents used for thrombolysis were recombinant tissue plasminogen activator and urokinase. Trials were generally of moderate methodological quality. The quality of evidence according to GRADE was generally low owing to risk of bias (lack of blinding), imprecision in estimates, and heterogeneity.
Trial results showed no clear differences in limb salvage, amputation, or death at 30 days (odds ratio (OR) 1.02, 95% confidence interval (CI) 0.41 to 2.55, 4 studies, 636 participants; OR 0.97, 95% CI 0.51 to 1.85, 3 studies, 616 participants; OR 0.59, 95% CI 0.31 to 1.14, 4 studies, 636 participants, respectively), and we rated the evidence as low, low, and moderate quality, respectively. Trial results show no clear differences for any of the three outcomes at six months or one year between initial surgery and initial thrombolysis. A single study evaluated vessel patency, so no overall association could be determined (OR 0.46, 95% CI 0.08 to 2.76, 20 participants; very low‐quality evidence). Evidence of increased risk of major haemorrhage (OR 3.22, 95% CI 1.79 to 5.78, 4 studies, 1070 participants; low‐quality evidence) and distal embolisation (OR 31.68, 95% CI 6.23 to 161.07, 3 studies, 678 participants; very low‐quality evidence) was associated with thrombolysis treatment at 30 days, and there was no clear difference in stroke (OR 5.33, 95% CI 0.95 to 30.11, 5 studies, 1180 participants; low‐quality evidence). Participants treated by initial thrombolysis had a greater reduction in the level of intervention required, compared with a pre‐intervention prediction, at 30 days (OR 9.06, 95% CI 4.95 to 16.56, 2 studies, 502 participants). None of the included studies evaluated time to thrombolysis as an outcome.
Authors' conclusions
There is currently no evidence in favour of either initial thrombolysis or initial surgery as the preferred option in terms of limb salvage, amputation, or death at 30 days, six months, or one year. Low‐quality evidence suggests that thrombolysis may be associated with higher risk of haemorrhagic complications and ongoing limb ischaemia (distal embolisation). The higher risk of complications must be balanced against risks of surgery in each individual case. Trial results show no statistical difference in stroke, but the confidence interval is very wide, making it difficult to interpret whether this finding is clinically important. We used GRADE criteria to assess the quality of the evidence as generally low. We downgraded quality owing to risk of bias, imprecision, and heterogeneity between included studies.
Plain language summary
Surgery versus thrombolysis for the initial management of acute limb ischaemia
Background
Thrombolysis involves dissolving a blood clot by injecting a chemical agent at the site of the clot. It can be used as an alternative to surgery for managing sudden severely reduced blood flow (acute ischaemia) in the leg. A blood clot (thrombosis) can form in a leg blood vessel that shows severe narrowing (stenosis) in a natural artery or a bypass graft, or it can travel into the leg arteries after forming elsewhere, when it is called an embolus. Major complications of thrombolysis are bleeding and stroke.
Study characteristics and key results
Authors of the review identified five controlled trials with a total of 1292 participants who needed immediate care for reduced blood flow in the leg(s) (current until 7 May 2018). Participants were randomly assigned to one of two groups for initial treatment: (1) non‐surgical thrombolysis, or (2) surgery. The specific agents used to break up clots (thrombolytic agents) were called recombinant tissue plasminogen activator and urokinase. The included studies provided no clear evidence about which treatment ‐ thrombolysis or surgery ‐ was a better option for preventing limb amputation (limb salvage) and no clear evidence about which treatment was better for preventing death or improving amputation rates within one month, six months, or one year after initial treatment. Evidence for these three outcomes at one month was rated between low and very low quality. No conclusion can be made about which treatment was better for keeping vessels unblocked after treatment (vessel patency) because this outcome was not well reported. More major complications, including bleeding (haemorrhage) and continued ischaemia or blockage (distal embolisation), were reported in the group receiving thrombolysis. There was no difference in the occurrence of stroke at one month between the two treatment groups. Although people receiving initial thrombolysis had increased risk of some complications, they showed greater reduction in the level of intervention required compared with that predicted before intervention. The higher risks of complications with thrombolysis have to be weighted against individual risks in surgery.
Quality of the evidence
The quality of the evidence was generally low. We downgraded the quality owing to risk of bias. Bias is a way to describe how researchers, clinicians, or participants might influence results unintentionally. Blinding is a method used to prevent people involved in the trial from knowing what treatment group a participant was in and reducing measurement bias. None of the studies included in this review used methods to stop participants or researchers or outcome assessors from knowing what treatment they were assigned to. Also, there was uncertainty about the true effect of each treatment type. Results show wide differences in outcome measures (effects) between studies (heterogeneity). For example, following surgical treatment, one‐year mortality ranged from 9.8% to 42%. Such a wide range in percentages may indicate that the studies compared were quite different. In addition, both selection criteria (duration of treatment and severity of ischaemia) and method of thrombolysis (agent, dose, and duration) varied between studies, making comparison more difficult.
Conclusion
This review found no evidence of a difference between thrombolysis and surgery for treatment of acute limb ischaemia for our outcomes of interest. Those receiving thrombolysis treatment may be at higher risk of complications such as bleeding. The quality of data generated by the included studies is low.
Summary of findings
Summary of findings for the main comparison. Is surgery or thrombolysis more effective for initial management of acute limb ischaemia?
| Surgery versus thrombolysis for initial management of acute limb ischaemia | ||||||
| Patient or population: patients seeking treatment for initial management of acute limb ischaemia Intervention: thrombolysis Comparison: surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with surgery | Risk with thrombolysis | |||||
| Limb salvage at 30 days | Study population | OR 1.02 (0.41 to 2.55) | 636 (4 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 858 per 1000 | 861 per 1000 (713 to 939) | |||||
| Amputation at 30 days | Study population | OR 0.97 (0.51 to 1.85) | 616 (3 RCTs) | ⊕⊕⊝⊝ LOWa,c | ||
| 69 per 1000 | 68 per 1000 (37 to 121) | |||||
| Death at 30 days | Study population | OR 0.59 (0.31 to 1.14) | 636 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 82 per 1000 | 50 per 1000 (27 to 93) | |||||
| Vessel patency at 30 days | Study population | OR 0.46 (0.08 to 2.76) | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d | ||
| 556 per 1000 | 365 per 1000 (91 to 775) | |||||
| Major haemorrhage at 30 days | Study population | OR 3.22 (1.79 to 5.78) | 1070 (4 RCTs) | ⊕⊕⊝⊝ LOWa,e | ||
| 33 per 1000 | 100 per 1000 (58 to 166) | |||||
| Stroke at 30 days | No events occurred in the surgery group, so it was not possible to estimate the assumed or corresponding risk | OR 5.33 (0.95 to 30.11) | 1180 (5 RCTs) | ⊕⊕⊝⊝ LOWa,e | 0/540 stroke events in the surgery group vs 8/640 in the thrombolysis group | |
| Distal embolisation at 30 days | No events occurred in the surgery group, so it was not possible to estimate the assumed or corresponding risk | OR 31.68 (6.23 to 161.07) | 678 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,e,f | 0/338 events in the surgery group vs 42/340 in the thrombolysis group | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded by one step owing to risk of bias: no evidence of blinding of outcome assessors, participants, or investigators bWe downgraded by one step as evidence showed moderate heterogeneity (I² = 56%) cWe downgraded by one step as evidence showed moderate heterogeneity (I² = 43%) dWe downgraded by one step owing to imprecision: only a single study was included in the analysis with very few reported events eWe downgraded by one step owing to imprecision: very few events were included in the analysis, leading to a wide confidence interval and an imprecise effect estimate fWe downgraded by one step as evidence showed moderate heterogeneity (I² = 33%)
Background
Description of the condition
Acute limb ischaemia (ALI) is defined as “any sudden decrease in limb perfusion causing a potential threat to limb viability” (Norgren 2007). By convention, this usually refers to patients presenting with symptoms for less than two weeks. The spectrum of ALI therefore ranges from the patient with a few hours' history of a painful cold white leg, to the patient with a few days' history of short distance claudication, or to the patient with a sudden increase in ischaemic symptoms on a background of peripheral arterial disease (PAD).
Acute limb ischaemia remains an important cause of morbidity and mortality, with an estimated annual incidence of 10 to 16 per 100,000 population per year (Davies 1997; Norgren 2007). A recent review of Hospital Episode Statistics (HES) data from the UK suggested that the number of admissions for ALI is increasing (von Allmen 2015).
Acute limb ischaemia usually results from embolism or thrombosis on a background of pre‐existing PAD or a graft but less commonly can be the result of trauma or obstruction due to other causes such as compression from a tumour (Creager 2012). Mortality and limb loss are high, with mortality rates estimated at 9% to 22% (Aune 1998; Campbell 1998; Eliason 2003; Norgren 2007), limb salvage at 70% to 90% (Campbell 1998; Eliason 2003; Kuukasjarvi 1994; Norgren 2007), and amputation‐free survival at three months at 59%, as reported in the Oxford Vascular (OxVasc) study (Howard 2015).
Description of the intervention
Peripheral arterial thrombolysis consists of intra‐arterial infusion of a lytic agent, for example, urokinase or tissue plasminogen activator, to dissolve the clot and recanalise an occluded artery. Thrombolysis has been used most extensively for treatment of ST segment elevation myocardial infarction (STEMI), which refers to a classic heart attack, although it is now reserved for patients with delayed presentation or for patients for whom percutaneous coronary intervention (PCI) is not available (ACCF/AHA guideline 2013). More recently it has also been used for the treatment of acute ischaemic stroke (NICE 2012), pulmonary embolus (Chaterjee 2014), and acute deep vein thrombosis (Watson 2016).
Thrombolysis may reveal an underlying stenosis, which can be treated by endovascular or surgical means as appropriate. Thrombolysis is usually performed with imaging guidance under local anaesthesia but may be performed as an adjunct to surgery in the theatre (or in a hybrid operating suite). Following commencement of thrombolysis, most patients are cared for in a high‐dependency unit for the duration of the infusion, which may continue for 48 to 72 hours, with check angiograms performed during this period. By its nature, thrombolysis (systemic or catheter‐directed) carries an inherent risk of bleeding complications, including risk of stroke.
How the intervention might work
A clot is formed initially by platelet aggregation. Platelets activate circulating prothrombin to form thrombin, which in turn activates fibrinogen to form fibrin. Thrombolytic agents target fibrin by converting plasminogen to plasmin, which then breaks down fibrinogen and fibrin. Thrombolysis can be delivered systemically (as for acute ischaemic stroke or STEMI) or via a catheter to the target lesion (as for ALI).
Peripheral arterial thrombolysis uses localised catheter‐directed infusion of an enzyme to dissolve the clot, thus increasing the contact area between the lytic agent and the clot. Potentially it allows the preservation of endothelium, the accurate localisation of any underlying aetiological factor causing thrombosis, and its correction either percutaneously (through the skin) or by a more limited directed surgical approach. Therefore peripheral arterial thrombolysis may avoid a more invasive extensive surgical procedure so may be safer for this population of elderly patients.
However, those who undergo thrombolysis may be at risk for a greater incidence of bleeding complications including major bleeding and haemorrhagic stroke. In addition, thrombolysis usually takes longer to relieve ischaemia than surgery, meaning that cases of very severe ischaemia may not be suitable for thrombolysis.
Why it is important to do this review
Direct comparison between initial thrombolysis and initial surgery has now been addressed by several randomised trials. This review is important because it provides available evidence as to which of these techniques is more effective in the initial management of ALI.
Objectives
To determine whether thrombolysis or surgery is the more effective technique in the initial management of acute limb ischaemia due to thromboembolism. Specific hypotheses to be tested were:
advantages in terms of limb salvage and survival are dependent upon whether thrombolysis or surgery is used in the initial management of acute limb ischaemia; and
a reduction in the eventual level of intervention required is dependent upon whether thrombolysis or surgery is used in the initial management of acute limb ischaemia.
Methods
Criteria for considering studies for this review
Types of studies
We included trials in which participants were randomly allocated to receive initial peripheral arterial thrombolysis or initial surgery for immediate or initial management of ALI.
Types of participants
We included participants with acute or acute‐on‐chronic limb ischaemia following a thromboembolic occlusion of a native peripheral artery or a thrombosed lower limb graft. We excluded haemodialysis access grafts. We included participants irrespective of diabetic status, use of aspirin or anticoagulation post thrombolysis, or use of concurrent heparin.
Types of interventions
We included studies that compared peripheral arterial thrombolysis or surgery as initial management for ALI. We noted subsequent interventions required, or performed. We considered all thrombolytic agents.
Types of outcome measures
Primary outcomes
Limb salvage (i.e. avoidance of a major amputation)
Secondary outcomes
Amputation
Death
Vessel patency
Complications including stroke, major haemorrhage, and distal embolisation
Reduction in the level of intervention (level of intervention actually performed compared with level predicted before treatment)
Time to lysis
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials on 7 December 2016.
Cochrane Vascular Specialised Register.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Allied and Complementary Medicine Database (AMED), and through handsearching of relevant journals. The full list of databases, journals, and conference proceedings that have been searched, as well as the search strategies used, is included in the Specialised Register section of the Cochrane Vascular Module in the Cochrane Library (www.cochranelibrary.com).
The CIS also searched the following trial registries for details of ongoing and unpublished studies (7 December 2016).
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
International Standard Randomized Controlled Trials Number (ISRCTN) Register (www.isrctn.com/).
See Appendix 2 for details of the search strategy used.
The CIS performed a supplementary search on 7 May 2018 of the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and clinical trial registries. See Appendix 3 for details of the search strategies used.
Searching other resources
We assessed the reference lists of included studies to look for further relevant studies.
Data collection and analysis
Selection of studies
Two review authors evaluated newly identified studies for inclusion (RD and RF). Criteria for selection of trials were as specified in the above section (Criteria for considering studies for this review).
Data extraction and management
We collected data from each included trial on participant age, sex, and severity of disease as measured by ankle‐brachial index (ABI), the European Consensus definition of critical ischaemia (Consensus Document), the Fontaine classification (Fontaine 1954), and Ad Hoc Committee Recommendations (Reporting Standards). When possible, we recorded limb salvage, death, amputation, vessel or graft patency, complications, and additional procedures.
Assessment of risk of bias in included studies
RD and RF independently assessed the methodological quality of all included studies using the 'Risk of bias' tool, according to Higgins 2011. We assessed the following domains: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias. We classified these domains as low risk, high risk, or unclear risk according to Higgins 2011, and resolved disagreements by discussion.
Measures of treatment effect
We measured odds ratios (ORs) with 95% confidence intervals (CIs) using a fixed‐effect model to compare the dichotomous outcomes of limb salvage, amputation and death, vessel patency, stroke, major haemorrhage, distal embolisation, and degree of intervention. Time to lysis is a continuous variable, so if reported, we would have evaluated this using calculated mean differences (MDs) with 95% CIs, also via a fixed‐effect model.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
Review authors requested missing data from the original investigators, if appropriate. When these could not be obtained, we carried out an intention‐to‐treat (ITT) analysis. For the ITT analysis, we used data on the number of participants with each outcome event by allocated treatment group, irrespective of compliance, and whether or not the participant was later thought to be ineligible or was otherwise excluded from treatment or follow‐up.
Assessment of heterogeneity
We evaluated outcome data for appropriateness for the meta‐analysis on the basis of heterogeneity by using the Chi² test and the I² statistic, both of which describe the percentage of variability in estimates of effect that is due to heterogeneity rather than to chance. If I² was greater than 50%, we evaluated data for heterogeneity using clinical judgement. We used a random‐effects model for meta‐analyses if we found no reason for heterogeneity. We used a fixed‐effect model if I² was lower than 50%.
Assessment of reporting biases
To detect reporting bias, we planned to construct funnel plots for meta‐analyses that included at least 10 studies, as funnel plots with fewer than 10 studies lack the power to distinguish chance from real asymmetry (Sterne 2011).
Data synthesis
We used a pooled, fixed‐effect model meta‐analysis of included trials, unless we noted evidence of heterogeneity, in which case we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We utilised the I² statistic to evaluate for heterogeneity between included studies for each meta‐analysis. If I² was greater than 50% and we could identify no obvious source of heterogeneity, we used a random‐effects model, which makes the assumption that different studies are estimating different intervention effects.
We did not perform subgroup analyses.
Sensitivity analysis
To determine the robustness of calculated estimates, we performed sensitivity analysis by removing any study that was deemed at high risk of bias (more than three domains rated as unclear or high risk of bias). Also, we planned to perform a sensitivity analysis to determine if any study that was highly weighted within an analysis had an overt effect. We did this by removing any study with > 50% weight on any analysis and re‐examining the estimates with confidence intervals. We conducted sensitivity analysis only if at least three studies remained after sensitivity analysis.
'Summary of findings'
We constructed a 'Summary of findings' table for the comparison of surgery versus thrombolysis using GRADEproGDT software to present the main findings of the review (GRADEpro GDT 2015). We included the primary outcome of limb salvage at 30 days and secondary outcomes of amputation, death, vessel patency, and the complications major haemorrhage, stroke, and distal embolisation, all at 30 days post intervention. We calculated assumed risks for each outcome from the mean number of events in the control groups for each included study.
We evaluated the quality of evidence using the GRADE system (developed by Grading of Recommendation, Assessment, Development and Evaluation Working Group), which yields a rating of high, moderate, low, or very low based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Atkins 2004). Please see Table 1.
Results
Description of studies
Results of the search
See Figure 1.
1.
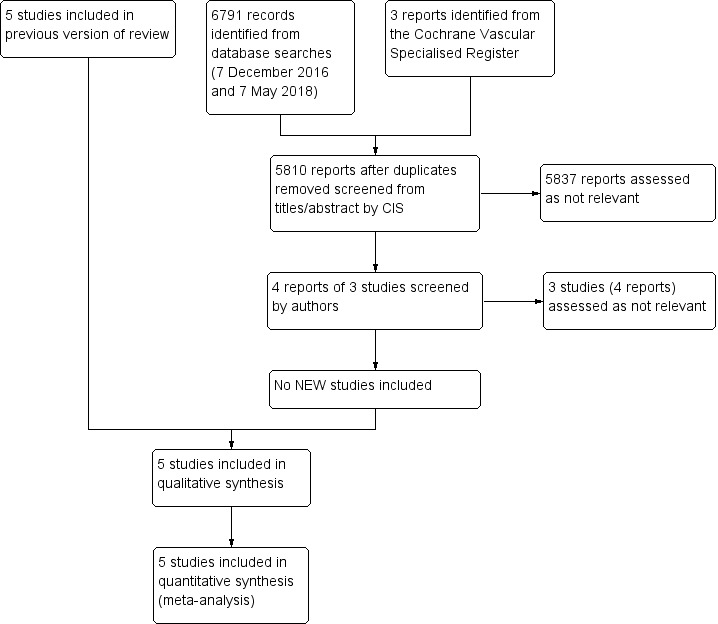
Study flow diagram.
Included studies
See the Characteristics of included studies for further details.
We included five prospective randomised controlled studies comparing initial thrombolysis versus initial surgery in the management of ALI with a total of 1292 randomised participants (Nilsson 1992; Ouriel 1994; Ouriel 1996; Ouriel 1998a; STILE 1994). Study size varied greatly from 20 participants up to 548. Two studies took place at a single centre (Nilsson 1992; Ouriel 1994), and the remaining three were multi‐centre studies ranging from 31 to 113 centres (Ouriel 1996; Ouriel 1998a; STILE 1994).
Lytic agents and dosage differed between studies: Nilsson 1992 administered 30 mg recombinant tissue plasminogen activator (rt‐PA) to participants, and Ouriel 1994, Ouriel 1996, and Ouriel 1998a all performed thrombolysis with urokinase at 4000 IU/min, which was decreased after two to four hours. Ouriel 1996 also used urokinase at 2000 IU/min and 6000 IU/min, but our review reports only on participants who received 4000 IU/min, as this was the dosage that was found most effective and enabled a better comparison with other studies. STILE 1994 left the lytic agent choice up to investigators, who chose from rt‐PA at 0.05 mg/kg/h for 12 hours or a 250,000‐IU bolus of urokinase followed by dose of 4000 IU/min, which decreased after four hours. Follow‐up was variable, from one month in Nilsson 1992 to one year in Ouriel 1994,Ouriel 1996,Ouriel 1998a, and STILE 1994. Major differences in participant demographics require cautious interpretation of meta‐analysis data. Differences include severity of ischaemia, site of occlusion, prosthetic or native vessel, thrombolytic regimen, and agent. It should be noted that STILE 1994 was stopped early because of an excess of complications reported in the thrombolysis group.
Excluded studies
We excluded one study as it was not a randomised controlled trial (Tiek 2009). See Characteristics of excluded studies.
Risk of bias in included studies
We assessed the risk of bias for each included study using Cochrane's 'Risk of bias' tool. We assessed the following domains as low risk, high risk, or unclear risk, according to Higgins 2011.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome data (detection bias).
Incomplete outcome data (attrition bias).
Selective outcomes reporting (reporting bias).
Other bias.
2.
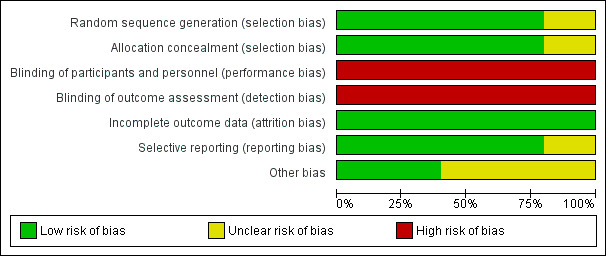
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
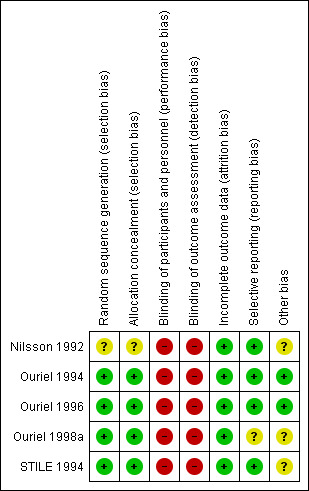
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most included studies had low risk of selection bias, as they used adequate methods of allocation through computer‐generation methods or centralised randomisation centres. We assessed one study as having unclear risk, as researchers did not specify their allocation methods (Nilsson 1992).
Blinding
Most of the included studies did not describe any methods of blinding participants, personnel, or assessors. Only one of the included studies was described as a double‐blind study, but study authors did not provide any description of their blinding techniques (Ouriel 1996). We rated all studies as having high risk of performance and detection bias. However, we have noted that owing to the nature of the procedure, it would be very difficult to blind participants and personnel. Also, the primary outcome measure (limb salvage) and the secondary outcome measures of major amputation and death should be less susceptible to detection bias than more subjective measures.
Incomplete outcome data
All of the included studies had low risk of attrition bias, as all participants were accounted for and dropout explanations were provided and were similar between treatment groups. Four of the five included studies reported utilising an intention‐to‐treat analysis (Ouriel 1994; Ouriel 1996; Ouriel 1998a; STILE 1994).
Selective reporting
We judged most studies to be at low risk of selective outcome reporting. One study described in its methods that the study authors would report on survival free of open surgery in the urokinase treatment group, but this was not done, and we assessed this study as having unclear risk of reporting bias (Ouriel 1998a).
Other potential sources of bias
We rated Nilsson 1992 as having unclear risk of other bias, as we noted differences at baseline between duration of ischaemia, gender, and smoking status between the two groups. Ouriel 1998a also reported differences at baseline between treatment groups, with higher numbers of men, patients with hepatic or renal insufficiency, and patients with rest pain at presentation in the thrombolysis group. Also, STILE 1994 reported that their study required 1000 randomised participants for adequate power but randomised only 393, and study authors noted that they had a significant number of primary outcomes at this point.
Effects of interventions
See: Table 1
Limb salvage
For the outcome limb salvage, we found no clear differences between treatment groups at 30 days, six months, or one year (odds ratio (OR) 1.02, 95% confidence interval (CI) 0.41 to 2.55; 4 studies; 636 participants; P = 0.97; I² = 56%; OR 0.86, 95% CI 0.59 to 1.26; 1 study; 544 participants; OR 0.88, 95% CI 0.62 to 1.23; 2 studies; 654 participants; P = 0.44; I² = 48%), respectively (Analysis 1.1; Analysis 1.2; Analysis 1.3). Analysis 1.1 utilised a random‐effects model owing to increased heterogeneity between studies and results. Evidence for limb salvage at 30 days was rated by GRADE parameters as low quality owing to lack of blinding and heterogeneity between studies.
1.1. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 1 Limb salvage at 30 days.
1.2. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 2 Limb salvage at 6 months.
1.3. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 3 Limb salvage at 1 year.
For sensitivity analysis, we removed Nilsson 1992 from Analysis 1.1 because of increased risk of bias, but the results were not altered.
Amputation
Trial results show no clear differences in 30‐day amputation rate (OR 0.97, 95% CI 0.51 to 1.85; 3 studies; 616 participants; P = 0.93; I² = 43%) (Analysis 1.4). A single study evaluated amputation at six months; therefore we could estimate no overall association (OR 1.16, 95% CI 0.79 to 1.70; 544 participants) (Analysis 1.5). Results show no clear differences in amputation between treatments at one year (OR 1.13, 95% CI 0.82 to 1.55; 3 studies; 768 participants; P = 0.47; I² = 0%) (Analysis 1.6). We rated evidence for 30‐day amputation analysis as low quality owing to lack of blinding and heterogeneity between studies.
1.4. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 4 Amputation at 30 days.
1.5. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 5 Amputation at 6 months.
1.6. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 6 Amputation at 1 year.
Death
Death at 30 days was similar between treatment groups (OR 0.59, 95% CI 0.31 to 1.14; 4 studies; 636 participants; P = 0.12; I² = 0%) (Analysis 1.7). A single study reported on death at six months, so no overall association could be estimated (OR 1.35, 95% CI 0.83 to 2.19; 544 participants) (Analysis 1.8). At one year, results show no clear differences in death between treatment groups, as reported via a random‐effects model (OR 0.67, 95% CI 0.25 to 1.79; 3 studies; 768 participants; P = 0.42; I² = 79%) (Analysis 1.9). Using the GRADE approach, we rated the evidence for death at 30 days as moderate quality and downgraded quality because of lack of blinding.
1.7. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 7 Death at 30 days.
1.8. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 8 Death at 6 months.
1.9. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 9 Death at 1 year.
For sensitivity analysis, we removed Nilsson 1992 from Analysis 1.7 owing to increased risk of bias, but we made no changes to the conclusions.
Vessel patency
A single study reported vessel patency only at 30 days (OR 0.46, 95% CI 0.08 to 2.76; 20 participants) (Analysis 1.10). No overall association could be concluded. We rated the evidence for this outcome as very low quality owing to lack of blinding and extreme paucity of data, with only a single study evaluating the outcome and reporting very few events.
1.10. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 10 Vessel patency at 30 days.
Complications including major haemorrhage, stroke, and distal embolisation
Major haemorrhage at 30 days was more likely in the thrombolysis treatment group (OR 3.22, 95% CI 1.79 to 5.78; 4 studies; 1070 participants; P < 0.0001; I² = 0%) (Analysis 1.11). For sensitivity analysis, we removed Nilsson 1992 and Ouriel 1998a because of increased risk of bias and > 50% weight, but we noted no changes in the overall association. We rated the evidence for 30‐day major haemorrhage as low quality owing to lack of blinding and few reported events, and this led to wide confidence intervals.
1.11. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 11 Major haemorrhage at 30 days.
Three of the five included studies reported occurrences of stroke; at 30 days, they reported stroke in eight participants receiving thrombolysis and in no participants receiving surgical treatment. Combined data show no clear differences between treatment groups for stroke (OR 5.33, 95% CI 0.95 to 30.11; 5 studies; 1180 participants; P = 0.06; I² = 0%) (Analysis 1.12). These findings were not altered after we removed Nilsson 1992 and Ouriel 1998a for sensitivity analysis. As with major haemorrhage, we rated the evidence for 30‐day stroke as low quality owing to lack of blinding and few included events, which led to wide confidence intervals.
1.12. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 12 Stroke at 30 days.
Distal embolisation was more likely to occur in the thrombolysis treatment group at 30 days (OR 31.68, 95% CI 6.23 to 161.07; 3 studies; 678 participants; P < 0.0001; I² = 33%) (Analysis 1.13). The GRADE evidence rating for this outcome was very low owing to lack of blinding, very few included events, and heterogeneity between studies.
1.13. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 13 Distal embolisation at 30 days.
Reduction in the level of intervention (level of intervention actually performed compared with the level predicted before treatment)
At 30 days participants who were assigned thrombolysis treatment were more likely to have a reduction in the level of surgery required from the level predicted by investigators, when compared with the surgery treatment group (OR 9.06, 95% CI 4.95 to 16.56; 2 studies; 502 participants; P < 0.00001; I² = 17%) (Analysis 1.14). Two studies reported on this outcome. It should be considered that studies use different treatment hierarchies to determine the reduction in the level of intervention. Also this finding is based on a subjective measure of the predicted level of needed intervention.
1.14. Analysis.
Comparison 1 Surgery versus thrombolysis, Outcome 14 Reduction in level of surgery required at 30 days.
Time to lysis
None of the included studies reported on time to lysis.
Discussion
Summary of main results
This systematic review includes five studies with a total of 1292 randomised participants. Meta‐analysis of the included trials showed no clear differences in limb salvage, amputation, or death at 30 days, six months, or one year between initial surgery and initial thrombolysis. The quality of evidence for the outcomes of limb salvage, amputation, and death at 30 days was low, low, and moderate, respectively, owing to no blinding and heterogeneity. Only a single study reported on vessel patency, so no association can be determined at this time, and the quality of evidence was rated as very low because of blinding concerns and imprecision. The complications of major haemorrhage and distal embolisation at 30 days were also more likely within the thrombolysis group. Data showed no clear difference in stroke between groups, but the confidence interval was very wide, making it difficult to interpret the data. We rated the quality of evidence for the outcomes major haemorrhage and stroke as low and for distal embolisation as very low owing to blinding concerns, imprecision, and heterogeneity. We did find a reduction in the level of intervention when compared with pre‐intervention predictions, favouring thrombolysis versus surgery at 30 days. None of the included studies reported on time to lysis.
Overall completeness and applicability of evidence
We included the five randomised controlled trials conducted to answer the question whether surgery or thrombolysis is preferable for initial treatment of people with acute limb ischaemia. These trials include real‐life populations but use selection criteria that are fairly heterogeneous in terms of duration of symptoms, thrombolytic agents and techniques used, and reporting methods applied. Comparison of the demographics of these studies versus those of more recent studies of acute limb ischaemia suggests that the population now presenting with acute limb ischaemia is older and includes greater proportions of octogenarians and women (Baril 2014; Howard 2015). In addition, one would expect more patients to be on "best medical management", particularly antiplatelets, antihypertensives, and statins, although baseline data in the original trials are insufficient to provide clarity on this. This highlights the issue that the most recent study included in this review was published in 1998 (Ouriel 1998a), limiting our ability to comment on these demographic changes in our findings.
No further studies have been done since the latest Ouriel study was published in 1998, although evidence indicates that the proportion of patients with acute limb ischaemia treated with endovascular techniques is increasing, particularly in the United States (von Allmen 2015). Choice of treatment modality will also be influenced by urgency of the intervention (severity of ischaemia) and availability of trained personnel and resources.
Three of the five included studies reported occurrences of stroke, which occurred in eight participants receiving thrombolytic treatment and in no participants receiving surgical treatment. The number of strokes were not found to be statistically greater in the thrombolytic group ‐ this could indicate that it is a clinically relevant outcome, or possibly that study selection criteria were missing certain contraindications that increased the risk of stroke in this treatment group. This is unclear from the limited evidence that we obtained on this outcome.
In addition, the failure rate for catheter placement in STILE 1994 is higher than would be expected for acute limb ischaemia in contemporary endovascular practice. It should be noted that the rate of failure to place a catheter was higher than expected in STILE 1994. The patient group in this trial had symptoms of ischaemia for up to six months and therefore were more likely to have had organised thrombus, which would not allow entry of a catheter to permit direct infusional lysis. This may have affected the conclusions resulting from the 'intention‐to‐treat' analysis, but findings were not substantially different from those of the 'per‐protocol' analysis, indicating that catheter placement failure did not alter the findings drastically.
For STILE 1994 and the Ouriel trials (Ouriel 1996; Ouriel 1998a), the reduction in surgery required after thrombolysis was based on an arbitrary gradation of intervention severity. Although they were not identical, results show close similarities. The level of intervention ranged from no intervention or no medical treatment; through thrombolysis, endarterectomy or graft revision, and new graft placement; and ultimately to major amputation, below‐knee amputation, and then finally above‐knee amputation. The exact positioning of thrombolysis in this list may be considered debatable, but it must be noted that there is no overall difference in limb salvage or death at one year, despite the potentially greater complications of haemorrhage and distal embolisation with thrombolysis. Continuing ischaemia may be dealt with on an elective basis and not necessarily as an emergency procedure.
Quality of the evidence
Overall quality of evidence was low (Table 1). We downgraded all outcomes for risk of bias because none of the included studies provided any detail on measures taken to blind outcomes assessors, participants, or investigators. It would be difficult to blind participants and those performing these procedures because of the nature of the intervention, but outcomes assessors could be blinded, which would improve the quality of the data and reduce risk of bias. Many of the assessed outcomes showed evidence of moderate heterogeneity and suffered from imprecision from lack of events, which led to wide confidence intervals.
It is therefore difficult to draw robust conclusions on the basis of available evidence. However, despite this, results across studies are relatively consistent.
Potential biases in the review process
Every effort was made to limit potential biases in the review process via duplication of study selection, data extraction, and assessment of risk of bias and quality of evidence by two review authors. Disagreements were discussed thoroughly. All possible studies were identified through a stringent search method, and other possible studies from relevant reference lists.
The outcome of 'Reduction of the level of intervention', which was reported by two included studies, is subject to bias, as it is based on comparison of a pre‐intervention prediction versus actual treatments received. Also, the hierarchy of treatments differed between studies. These potential biases should be considered when this outcome is evaluated.
Agreements and disagreements with other studies or reviews
We identified no other similar studies or reviews.
Authors' conclusions
Implications for practice.
There is currently no evidence in favour of either initial thrombolysis or initial surgery as the preferred option in terms of limb salvage, amputation, or death at 30 days, six months, or one year. Evidence indicates a higher incidence of major complications with thrombolysis, including major haemorrhage and distal embolisation, but this finding is balanced against a less invasive intervention. This review found no evidence of a difference in the incidence of stroke, but the confidence interval was very wide, so it is difficult to interpret at this time whether this finding is clinically important.
Thrombolysis must be used only for carefully selected and monitored participants who have been fully informed and have consented. The combined vascular surgical and vascular radiological team looking after the participant should consider available surgical and thrombolytic/endovascular options.
Implications for research.
All trials concerning thrombolysis and surgery must classify and randomise participants according to Ad Hoc Committee reporting standards (Reporting Standards). Researchers have provided no data in support of treating participants with a long duration of symptomatic history. Future trials should look at the use of thrombolysis in patients who traditionally have been considered suitable for peripheral arterial thrombolysis (i.e. those with a duration of ischaemic history up to 14 days). Further research must examine appropriate time frames between onset of symptoms and thrombolytic treatment.
Future studies should also consider the quality of life implications of initial surgery versus initial thrombolysis for management.
All studies included in this review were published during the 1990s. More recently, newer pharmacomechanical techniques such as ultrasound‐enhanced thrombolysis have begun to offer the potential of faster lysis (Schrijver 2015). Such techniques require evaluation against surgery and conventional thrombolysis techniques in future randomised trials.
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2018 | New search has been performed | New updated search run. No new studies identified |
| 7 May 2018 | New citation required but conclusions have not changed | New updated search run. No new studies identified. Text updated to reflect current Cochrane standards. New author joined the review author team. 'Summary of findings' table added. Conclusions not changed |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 10 April 2013 | New search has been performed | Searches were rerun. No new studies included. One additional study excluded |
| 10 April 2013 | New citation required but conclusions have not changed | Searches were rerun. No new studies included. One additional study excluded. Minor copy edits made. Conclusions not changed |
| 11 February 2009 | New search has been performed | Dates of last searches updated. No new trials found New CENTRAL search strategy applied |
| 31 October 2008 | Amended | Converted to new review format |
| 21 February 2007 | New search has been performed | New Plain Language Summary added. Copy edits made throughout text and in analysis graph labels. Acknowledgements and search strategy for CENTRAL updated. Dates of last searches updated. No new trials found; conclusions remain unchanged |
| 23 February 2006 | New search has been performed | No new trials found during most recent literature search. Review updated with minor style guide changes |
| 17 November 2004 | Amended | Review updated by minor change to 'Conflict of interest' section to clarify about payment of consultancy fees |
| 23 August 2004 | Amended | No new trials found. Review updated by minor changes to format to comply with Cochrane Style Guide |
| 21 May 2002 | New search has been performed | Updated review includes extra information from follow‐up trial references |
Notes
This is the first of three reviews conducted to examine different aspects of thrombolysis, all of which are covered by the generic protocol 'Surgery versus thrombolysis for acute limb ischaemia', unique ID 031499080512564323.
The second review is "Infusion techniques for peripheral arterial thrombolysis". The third review is "Fibrinolytic agents for peripheral arterial occlusion".
Acknowledgements
We thank Cochrane Vascular for assistance with the literature searches. We would also like to thank the Cochrane Consumer Network for providing the Plain Language Summary that appeared in the original version of the review.
Appendices
Appendix 1. CENTRAL search strategy, 7 December 2016
| #1 | MESH DESCRIPTOR Arteriosclerosis | 868 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 71 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 619 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 724 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 712 |
| #7 | MESH DESCRIPTOR Ischemia | 789 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2201 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9009 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 7829 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 3327 |
| #12 | (claudic* or IC):TI,AB,KY | 3005 |
| #13 | (isch* or CLI):TI,AB,KY | 23402 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 10 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 94 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 138 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 76 |
| #19 | ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 996 |
| #20 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1107 |
| #21 | MESH DESCRIPTOR Iliac Artery | 144 |
| #22 | MESH DESCRIPTOR Popliteal Artery | 278 |
| #23 | MESH DESCRIPTOR Femoral Artery | 810 |
| #24 | MESH DESCRIPTOR Tibial Arteries | 33 |
| #25 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 1143 |
| #26 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 | 43191 |
| #27 | MESH DESCRIPTOR Thrombolytic Therapy EXPLODE ALL TREES | 1525 |
| #28 | MESH DESCRIPTOR Fibrinolytic Agents EXPLODE ALL TREES | 10829 |
| #29 | MESH DESCRIPTOR Plasminogen Activators EXPLODE ALL TREES | 2219 |
| #30 | (urokinase or streptokinase or streptase or tenecteplase):TI,AB,KY | 2134 |
| #31 | (reteplase or alteplase):TI,AB,KY | 716 |
| #32 | (anistreplase or prourokinase or retavase or rapilysin):TI,AB,KY | 217 |
| #33 | (t‐PA or tPA):TI,AB,KY | 1343 |
| #34 | (r‐PA or rPA):TI,AB,KY | 91 |
| #35 | (lysis or lytic or thromboly*):TI,AB,KY | 5114 |
| #36 | (plasminogen near2 activator):TI,AB,KY | 3537 |
| #37 | (clot near3 (bust* or break* or remov*)):TI,AB,KY | 37 |
| #38 | #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 | 16703 |
| #39 | #26 AND #38 | 4661 |
| #40 | * NOT SR‐PVD:CC AND 28/03/2013 TO 30/11/2016:DL | 298728 |
| #41 | #39 AND #40 | 1208 |
Appendix 2. Trials registries searches, 7 December 2016
ClinicalTrials.gov
94 studies found for: acute limb ischaemia
World Health Organization International Clinical Trials Registry Platform
5 records for 3 trials found for: acute limb ischaemia
ISRCTN Register
17 results for acute limb ischaemia
Appendix 3. Database searches, 7 May 2018
| Source | Search strategy | Hits retrieved |
| 1. VASCULAR REGISTER IN CRSW | #1 ACISCH AND INREGISTER #2 2016 or 2017 or 2018 AND INREGISTER #3 #1 AND #2 |
1 |
| 2. CENTRAL via CRSO | #1 MESH DESCRIPTOR Arteriosclerosis 927 #2 MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES 0 #3 Arteriosclerosis Obliterans 110 #4 MESH DESCRIPTOR Atherosclerosis 963 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 804 #6 MESH DESCRIPTOR Intermittent Claudication 805 #7 MESH DESCRIPTOR Ischemia 1354 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2660 #9 ((atherosclero* or arteriosclero* or PVD or PAOD or PAD )):TI,AB,KY 11622 #10 (((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY 10250 #11 (peripheral near3 dis*) 4641 #12 (claudic* or IC) 5674 #13 (isch* or CLI) 30927 #14 arteriopathic 7 #15 dysvascular* 16 #16 (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 122 #17 (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 209 #18 ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 106 #19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 1491 #20 MESH DESCRIPTOR Leg 2784 #21 MESH DESCRIPTOR Iliac Artery 158 #22 MESH DESCRIPTOR Popliteal Artery 300 #23 MESH DESCRIPTOR Femoral Artery 894 #24 MESH DESCRIPTOR Tibial Arteries 36 #25 ((((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ))):TI,AB,KY 1659 #26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 59473 #27 MESH DESCRIPTOR Thrombolytic Therapy EXPLODE ALL TREES 1585 #28 MESH DESCRIPTOR Fibrinolytic Agents EXPLODE ALL TREES 12349 #29 MESH DESCRIPTOR Plasminogen Activators EXPLODE ALL TREES 2367 #30 (urokinase or streptokinase or streptase or tenecteplase):TI,AB,KY 2291 #31 (reteplase or alteplase):TI,AB,KY 998 #32 (anistreplase or prourokinase or retavase or rapilysin):TI,AB,KY 220 #33 (t‐PA or tPA):TI,AB,KY 1617 #34 (lysis or lytic or thromboly*):TI,AB,KY 6135 #35 (plasminogen near2 activator):TI,AB,KY 4047 #36 (clot near3 (bust* or break* or remov*)):TI,AB,KY 60 #37 (r‐PA or rPA):TI,AB,KY 135 #38 #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 19600 #39 #26 AND #38 5702 #40 07/12/2016 TO 07/05/2018:CD 262199 #41 #39 AND #40 976 |
976 |
| 3. Clinicaltrials.gov | acute limb ischaemia | 7 |
| 4. ICTRP Search Portal | acute limb ischaemia | 0 |
| 5. MEDLINE | 1 ARTERIOSCLEROSIS/ 56440 2 exp ARTERIOLOSCLEROSIS/ 150 3 Arteriosclerosis Obliterans/ 3973 4 ATHEROSCLEROSIS/ 30696 5 Arterial Occlusive Diseases/ 26427 6 Intermittent Claudication/ 7572 7 ISCHEMIA/ 47353 8 exp Peripheral Vascular Diseases/ 49834 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 169961 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 142120 11 (peripheral adj3 dis*).ti,ab. 37468 12 (claudic* or IC).ti,ab. 61651 13 (isch* or CLI).ti,ab. 343234 14 arteriopathic.ti,ab. 162 15 dysvascular*.ti,ab. 214 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 702 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1793 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1471 19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 8326 20 exp LEG/ 61686 21 Iliac Artery/ 13309 22 Popliteal Artery/ 8937 23 Femoral Artery/ 26984 24 Tibial Arteries/ 1472 25 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 9609 26 or/1‐25 820160 27 exp Thrombolytic Therapy/ 22251 28 exp Fibrinolytic Agents/ 161055 29 exp Plasminogen Activators/ 38136 30 (urokinase or streptokinase or streptase or tenecteplase).ti,ab. 20814 31 (reteplase or alteplase).ti,ab. 1852 32 (anistreplase or prourokinase or retavase or rapilysin).ti,ab. 370 33 (t‐PA or tPA).ti,ab. 25894 34 (r‐PA or rPA).ti,ab. 4003 35 (lysis or lytic or thromboly*).ti,ab. 93418 36 (clot adj3 (bust* or break* or remov*)).ti,ab. 668 37 (plasminogen adj2 activator).ti,ab. 34115 38 or/27‐37 283827 39 26 and 38 41162 40 randomized controlled trial.pt. 460304 41 controlled clinical trial.pt. 92384 42 randomized.ab. 410801 43 placebo.ab. 188752 44 drug therapy.fs. 2015202 45 randomly.ab. 289890 46 trial.ab. 427100 47 groups.ab. 1791858 48 or/40‐47 4197643 49 exp animals/ not humans.sh. 4453120 50 48 not 49 3628184 51 39 and 50 20886 52 (2017* or 2018*).ed. 1283142 53 51 and 52 1191 |
1191 |
| 6. Embase | 1 arteriosclerosis/ 14657 2 exp arteriolosclerosis/ 486 3 peripheral occlusive artery disease/ 21855 4 atherosclerosis/ 113585 5 peripheral occlusive artery disease/ 21855 6 intermittent claudication/ 6099 7 ischemia/ 59959 8 exp peripheral vascular disease/ 1279245 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 192225 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 144635 11 (peripheral adj3 dis*).ti,ab. 43480 12 (claudic* or IC).ti,ab. 52563 13 (isch* or CLI).ti,ab. 399039 14 arteriopathic.ti,ab. 82 15 dysvascular*.ti,ab. 177 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 684 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2160 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1461 19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 9335 20 exp leg/ 202354 21 iliac artery/ 9837 22 popliteal artery/ 5187 23 femoral artery/ 20853 24 tibial artery/ 2260 25 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10844 26 or/1‐25 1725941 27 exp fibrinolytic therapy/ 19337 28 exp fibrinolytic agent/ 89015 29 exp plasminogen activator/ 52312 30 (urokinase or streptokinase or streptase or tenecteplase).ti,ab. 14763 31 (reteplase or alteplase).ti,ab. 2981 32 (anistreplase or prourokinase or retavase or rapilysin).ti,ab. 207 33 (t‐PA or tPA).ti,ab. 22511 34 (r‐PA or rPA).ti,ab. 4966 35 (lysis or lytic or thromboly*).ti,ab. 86961 36 (clot adj3 (bust* or break* or remov*)).ti,ab. 916 37 (plasminogen adj2 activator).ti,ab. 31933 38 or/27‐37 183599 39 26 and 38 74424 40 randomized controlled trial/ 453537 41 controlled clinical trial/ 415833 42 random$.ti,ab. 1162950 43 randomization/ 69731 44 intermethod comparison/ 224799 45 placebo.ti,ab. 221924 46 placebo.ti,ab. 221924 47 (compare or compared or comparison).ti. 333899 48 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1612523 49 (open adj label).ti,ab. 62493 50 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 157409 51 double blind procedure/ 122856 52 parallel group$1.ti,ab. 19466 53 (crossover or cross over).ti,ab. 71786 54 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 248171 55 (assigned or allocated).ti,ab. 289162 56 (controlled adj7 (study or design or trial)).ti,ab. 260620 57 (volunteer or volunteers).ti,ab. 171553 58 trial.ti. 213453 59 or/40‐58 3464620 60 39 and 59 18832 61 (2017* or 2018*).em. 3390917 62 60 and 61 3144 |
3144 |
| 7. CINAHL | S53 S51 AND S52 149 S52 EM 2017 OR EM 2018 345,743 S51 S36 AND S50 1,497 S50 S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 339,364 S49 (MH "Random Assignment") 37,923 S48 (MH "Random Assignment") 37,923 S47 (MH "Single‐Blind Studies") or (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 32,598 S46 (MH "Crossover Design") 11,148 S45 (MH "Factorial Design") 916 S44 (MH "Placebos") 8,332 S43 (MH "Clinical Trials") 93,200 S42 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,426 S41 TX crossover OR "cross‐over" 14,472 S40 AB placebo* 28,062 S39 TX random* 217,230 S38 TX trial* 248,382 S37 TX "latin square" 141 S36 S24 AND S35 4,262 S35 S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 12,555 S34 TX (clot n3 (bust* or break* or remov*)) 181 S33 TX lysis or lytic or thromboly* 8,527 S32 TX r‐PA or rPA 298 S31 TX t‐PA or tPA 1,289 S30 TX anistreplase or prourokinase or retavase or rapilysin 36 S29 TX reteplase or alteplase 507 S28 TX urokinase or streptokinase or streptase or tenecteplase 1,033 S27 (MH "Plasminogen Activators") 367 S26 (MH "Fibrinolytic Agents") 4,301 S25 (MH "Thrombolytic Therapy") 4,458 S24 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 87,726 S23 TX (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) N3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )) 1,090 S22 (MH "Tibial Arteries") 144 S21 (MH "Femoral Artery") 1,200 S20 (MH "Popliteal Artery") 360 S19 (MH "Iliac Artery") 458 S18 (MH "Leg") 5,360 S17 TX ((iliac or femoral or popliteal or femoro* or fempop* or crural) n3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 934 S16 TX ((lower n3 extrem*) N3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 121 S15 TX (limb n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 272 S14 TX (leg 3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 6 S13 TX dysvascular* 172 S12 TX arteriopathic 10 S11 TX isch* or CLI 39,097 S10 TX claudic* or IC 5,770 S9 TX peripheral n3 dis* 9,175 S8 TX ((arter* or vascular or vein* or veno* or peripher*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 12,540 S7 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD 26,165 S6 (MH "Peripheral Vascular Diseases") 3,114 S5 (MH "Ischemia") 3,349 S4 (MH "Intermittent Claudication") 849 S3 (MH "Arterial Occlusive Diseases") 1,603 S2 (MH "Atherosclerosis") 3,288 S1 (MH "Arteriosclerosis") 4,827 |
149 |
| 8. AMED | 1 ARTERIOSCLEROSIS/ 78 2 ATHEROSCLEROSIS/ 219 3 Intermittent Claudication/ 73 4 ISCHEMIA/ 262 5 exp Peripheral Vascular Diseases/ 0 6 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 802 7 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 458 8 (peripheral adj3 dis*).ti,ab. 435 9 (claudic* or IC).ti,ab. 1024 10 (isch* or CLI).ti,ab. 1663 11 arteriopathic.ti,ab. 1 12 dysvascular*.ti,ab. 57 13 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 21 14 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 32 15 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 25 16 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 54 17 exp LEG/ 11785 18 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 109 19 exp Fibrinolytic Agents/ 7 20 (urokinase or streptokinase or streptase or tenecteplase).ti,ab. 12 21 (reteplase or alteplase).ti,ab. 2 22 (anistreplase or prourokinase or retavase or rapilysin).ti,ab. 1 23 (t‐PA or tPA).ti,ab. 149 24 (r‐PA or rPA).ti,ab. 13 25 (lysis or lytic or thromboly*).ti,ab. 150 26 (clot adj3 (bust* or break* or remov*)).ti,ab. 0 27 (plasminogen adj2 activator).ti,ab. 42 28 or/1‐18 15704 29 or/19‐27 328 30 28 and 29 56 31 exp CLINICAL TRIALS/ 3738 32 RANDOM ALLOCATION/ 314 33 DOUBLE BLIND METHOD/ 653 34 Clinical trial.pt. 1210 35 (clinic* adj trial*).tw. 5364 36 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2816 37 PLACEBOS/ 585 38 placebo*.tw. 3094 39 random*.tw. 17431 40 PROSPECTIVE STUDIES/ 1072 41 or/31‐40 22400 42 30 and 41 12 43 ("2017" or "2018").yr. 1412 44 42 and 43 0 |
0 |
Data and analyses
Comparison 1. Surgery versus thrombolysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Limb salvage at 30 days | 4 | 636 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.41, 2.55] |
| 2 Limb salvage at 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Limb salvage at 1 year | 2 | 654 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.23] |
| 4 Amputation at 30 days | 3 | 616 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.85] |
| 5 Amputation at 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Amputation at 1 year | 3 | 768 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.55] |
| 7 Death at 30 days | 4 | 636 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.31, 1.14] |
| 8 Death at 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Death at 1 year | 3 | 768 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.25, 1.79] |
| 10 Vessel patency at 30 days | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Major haemorrhage at 30 days | 4 | 1070 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.22 [1.79, 5.78] |
| 12 Stroke at 30 days | 5 | 1180 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.33 [0.95, 30.11] |
| 13 Distal embolisation at 30 days | 3 | 678 | Odds Ratio (M‐H, Fixed, 95% CI) | 31.68 [6.23, 161.07] |
| 14 Reduction in level of surgery required at 30 days | 2 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.06 [4.95, 16.56] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Nilsson 1992.
| Methods | Study design: randomised controlled trial Intention‐to‐treat analysis: no Country: Sweden (single centre) |
|
| Participants | Number: total N = 20 (thrombolysis n = 11; surgery n = 9) Exclusions post randomisation: none Losses to follow‐up: 1 Gender: 13 men, 7 women Mean age, years: 74 (range 45 to 91) Inclusion criteria: duration of ischaemia requiring intervention > 24 hours and < 14 days Exclusion criteria: systolic BP higher than 200 mmHg, stroke within past 6 months, surgery within past 3 weeks, history of gastrointestinal bleeding, bleeding diathesis, known active peptic ulcer or current treatment with oral anticoagulants |
|
| Interventions | Thrombolysis: 30 mg rt‐PA over 3 hours with catheter advancement Surgery: balloon thromboembolectomy |
|
| Outcomes | Follow‐up at 30 days Revascularisation, failure of lysis, amputation, ABI | |
| Notes | No use of Fontaine or Rutherford classification of severity of ischaemia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as a randomised controlled trial, but no details of methods of random sequence generation were provided |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Steps taken to blind participants to treatment were not described. Owing to the nature of the treatment, it would be very difficult to blind participants or personnel to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Steps taken to blind personnel to treatment were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for at 30‐day follow‐up (Results of treatment and Table 1, page 191), and dropout explanations were provided |
| Selective reporting (reporting bias) | Low risk | All outcome measures described in the methods were reported |
| Other bias | Unclear risk | Differences between treatment groups at baseline were reported |
Ouriel 1994.
| Methods | Study design: randomised controlled trial Intention‐to‐treat analysis: yes Country: USA (single centre) |
|
| Participants | Number: total N = 114 (thrombolysis n = 57; surgery n = 57) Exclusions post randomisation: not stated Losses to follow‐up: not stated Gender (M:F): thrombolysis 29:28; surgery 25:32 Mean age ± SD, years : thrombolysis 69 ± 1.7; surgery 71 ± 1.7 Inclusion criteria: limb‐threatening ischaemia of < 7 days' duration (amputation deemed necessary without intervention), 18 years of age and older, embolic or thrombotic native arterial autogenous bypass graft or prosthetic bypass graft Exclusion criteria: mural thrombus found to be the cause of the occlusion (as confirmed by echocardiography), contraindication to thrombolytic therapy, major operative procedure within 14 days, active peptic ulcer disease, intracranial neoplasm, history of cerebrovascular accident, contraindication to operative revascularisation, non‐ambulatory or non‐functional extremity before the ischaemic event, ischaemic process deemed irreversible (Society for Vascular Surgery/International Society for Cardiovascular Surgery Class III), contraindication to arteriography present, including serum creatinine > 2.5 mg/dL or history of significant allergy to contrast agents, positive pregnancy test |
|
| Interventions | Thrombolysis with urokinase: 4000 IU/min; 2000 IU/min after 2 hours; 1000 IU/min after 4 hours Surgical revascularisation or primary amputation if no outflow vessels |
|
| Outcomes | Follow‐up at 12 months Limb salvage, amputation, patency rate, duration of hospitalisation, event‐free survival, time to reperfusion, bleeding complications, death | |
| Notes | Used Rutherford classification of critical ischaemia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomly assigned to receive thrombolytic or operative treatment according to computer‐generated randomisation cards |
| Allocation concealment (selection bias) | Low risk | Study utilised computer‐generated randomisation cards sealed in envelopes that were opened at the time of entry into the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Steps taken to blind participants to treatment were not described. Owing to the nature of the treatment, it would be very difficult to blind participants or personnel to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Steps taken to blind personnel to treatment were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported on and intention‐to‐treat analysis was specified |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported on |
| Other bias | Low risk | No evidence of other bias was found |
Ouriel 1996.
| Methods | Study design: randomised controlled trial Intention‐to‐treat: yes Countries: USA and Canada (79 centres) |
|
| Participants | Number: total N = 217 (thrombolysis 2000 IU/min n = 48; 4000 IU/min n = 52; 6000 IU/min n = 55; surgery n = 58) Exclusions post randomisation: 4 Losses to follow‐up: not stated Gender (% male): thrombolysis 2000 IU/min = 70.7%; 4000 IU/min = 74.4%; 6000 IU/min = 76.1%; surgery = 62.2% Mean age ± SD, years: thrombolysis 2000 IU/min 66.2 ± 1.9; 4000 IU/min 62.2 ± 1.8; 6000 IU/min 62.5 ± 1.8; surgery 66.5 ± 1.8 Inclusion criteria: threatened (Class II) severity of limb ischaemia < 14 days' duration, occlusion confirmed with arteriography, native artery or bypass graft, 18 years of age or older, informed consent by able participant or surrogate, eligible for both operative and thrombolytic interventions Exclusion criteria: profound ischaemia with permanent motor paresis or sensory loss, uncontrolled hypertension (systolic BP > 180, diastolic BP > 110 mmHg), stroke within 6 months, TIA within 2 months, significant internal haemorrhage within 10 days, serious gastrointestinal haemorrhage within 14 days, biopsy of organs, puncture of incompressible vessel within 14 days, severe hepatic dysfunction, life expectancy < 1 year |
|
| Interventions | Thrombolysis with urokinase at 2000 IU/min, or 4000 IU/min, or 6000 IU/min for first 4 hours, followed by 2000 IU/min thereafter for up to 48 hours Surgery including primary amputation |
|
| Outcomes |
Follow‐up at 12 months Arterial recanalisation and extent of clot lysis at 4 hours, amputation‐free survival at 6 and 12 months, composite in‐hospital outcome index (see notes), reduction in severity of predicted intervention, ABI |
|
| Notes | Order of severity of interventions was compared from the initial predicted intervention to the actual intervention ultimately required by the time of initial hospital discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study utilised a centralised randomisation centre that was contacted at the time of patient enrolment via telephone. Participants were stratified by whether the occlusion involved a native artery or a bypass graft |
| Allocation concealment (selection bias) | Low risk | Study utilised a centralised randomisation centre that was contacted by phone at the time of enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was described as double‐blind but steps taken to blind participants to treatment were not described. Owing to the nature of the treatment, it would be very difficult to blind participants or personnel to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Steps taken to blind personnel to treatment were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported on, and intention‐to‐treat analysis was specified |
| Selective reporting (reporting bias) | Low risk | Primary outcomes were accounted for, but it should be noted that haematological parameters were described to be taken at 4 and 48 hours after treatment, although only 4‐hour data were reported |
| Other bias | Low risk | No evidence of other bias was found |
Ouriel 1998a.
| Methods | Study design: randomised controlled trial Intention‐to‐treat analysis: yes Countries: USA and Northern Europe (113 centres) |
|
| Participants | Number randomised: total N = 548 (thrombolysis n = 272; surgery n = 272) Exclusions post randomisation: 4 Losses to follow‐up: thrombolysis 17; surgery 16 Gender M/F: thrombolysis 192/90; surgery 170/102 Mean age ± SD, years: thrombolysis 64.9 ± 0.78; surgery 64.5 ± 0.78 Inclusion criteria: 14 days or less of reversible limb‐threatening ischaemia, over 17 years of age, non‐pregnant, suitable for either open surgical treatment or thrombolysis Exclusion criteria: pregnancy, women of child‐bearing age for whom pregnancy was a possibility |
|
| Interventions | Thrombolysis with urokinase: 4000 IU/min for 2 hours, then 2000 IU/min for a maximum duration of 48 hours' therapy Surgery, including angioplasty and primary amputation |
|
| Outcomes | Follow‐up at 6 and 12 months Amputation‐free survival at 6 and 12 months, survival free of open surgical procedures at 6 months (lysis group), ABI, degree of clot lysis, rates of adverse effects of treatment, including haemorrhagic complications | |
| Notes | Supported by a grant from Abbott | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study utilised a centralised randomisation centre, which was contacted at the time of patient consent via telephone. Participants were stratified by whether occlusion involved native artery or bypass graft |
| Allocation concealment (selection bias) | Low risk | Study utilised a centralised randomisation centre, which was contacted at the time of patient consent via telephone |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Steps taken to blind participants to treatment were not described. Owing to the nature of the treatment, it would be very difficult to blind participants or personnel to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Steps taken to blind personnel to treatment were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported on, and intention‐to‐treat analysis was specified |
| Selective reporting (reporting bias) | Unclear risk | Survival free of open surgery at 6 months in the urokinase treatment group was identified as an outcome in the methods but was not included in the results. All other outcomes were reported on |
| Other bias | Unclear risk | Differences at baseline between treatment groups: larger numbers of men, patients with hepatic or renal insufficiency, and patients with rest pain at presentation in the thrombolysis group |
STILE 1994.
| Methods | Study design: randomised controlled trial Intention‐to‐treat analysis: yes Country: USA (31 centres) |
|
| Participants | Number randomised: total N = 393 (thrombolysis (rt‐PA n = 137; urokinase n = 112); surgery n = 144) Exclusions post randomisation: total N = 28 (thrombolysis n = 12; surgery n = 16) Losses to follow‐up: 4 Gender M/F: thrombolysis: rt‐PA 93/43; urokinase 70/42; surgery 105/39 Mean age, years: thrombolysis: rt‐PA 65.4; urokinase 64.9; surgery 65.3 Inclusion criteria: symptoms of worsening limb ischaemia over past 6 months requiring intervention, angiographically confirmed non‐embolic arterial or bypass graft, 18 to 90 years of age Exclusion criteria: infected peripheral arterial bypass grafts, previous enrolment in this trial, acute embolic occlusion, active internal bleeding, history of any cerebrovascular accident or intracranial bleeding, history of any TIA, intracranial or intraspinal surgery or trauma within past 2 months, any central nervous system neoplasm or aneurysm, known severe bleeding diathesis, severe uncontrolled hypertension (systolic BP > 180 mmHg and diastolic BP > 110 mmHg), known or suspected pregnancy or child‐bearing potential, eye surgery within past 3 months, inability to undergo surgical procedure (e.g. contraindication to general anaesthetic), severe cardiac disease, recent puncture of non‐compressible vessel, participation in another research protocol within the past 30 days; other criteria for which investigators had to exercise good clinical judgement included recent vascular surgery, major non‐vascular surgery within 10 days, significant liver dysfunction, history of internal bleeding or other significant bleeding within past 10 days, high likelihood of left heart thrombus, acute pericarditis or subacute bacterial endocarditis, trauma within past 10 days, asymptomatic cerebrovascular disease, diabetic or haemorrhage retinopathy, haemostatic defects, low platelet count, septic thrombophlebitis or occluded AV cannula at a seriously infected site, any other condition in which bleeding is a significant hazard, and severe ischaemia that requires immediate surgical intervention |
|
| Interventions | Thrombolysis: lytic agent used chosen by investigators ‐ either rt‐PA 0.05 mg/kg/h for up to 12 hours (max dose 200 mg) or urokinase 250,000‐IU bolus followed by 4000 IU/min for 4 hours, then 2000 IU/min for up to 36 hours. In addition, participants in the thrombolysis group received 5000 IU heparin as an intravenous bolus at the time of thrombolysis followed by 1000 U/h titrated to maintain APTT between 1.5 and 2.0 times control, plus 325 mg aspirin at the time of randomisation and daily thereafter Surgical revascularisation including primary amputation |
|
| Outcomes |
Follow‐up at 6 months Composite clinical outcome, ongoing or recurrent ischaemia, death or major amputation (above‐knee or below‐knee), life‐threatening haemorrhage or stroke (hypotension requiring resuscitation), perioperative complications, renal failure requiring dialysis, serious anaesthesia‐related complications, vascular complications, post‐interventional wound complications, clinical improvement and reduction in surgery, patency and perfusion status, duration of ischaemia, length of hospitalisation |
|
| Notes | One of the major criticisms of the STILE publications is that the less than 14 days or more than 14 days analysis was a post hoc arbitrary division ‐ not a stratified part of the original protocol. Over 80% of all participants had a more than 14 days duration of symptoms before intervention. Study was supported by a grant from Genetech, Inc., San Francisco, California, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study utilised a centralised randomisation centre, which was contacted at the time of patient consent via telephone. Participants were stratified by whether occlusion involved native artery or bypass graft |
| Allocation concealment (selection bias) | Low risk | Study utilised a centralised randomisation centre, which was contacted at the time of patient consent via telephone |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Steps taken to blind participants to treatment were not described. Owing to the nature of the treatment, it would be very difficult to blind participants or personnel to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Steps taken to blind personnel to treatment were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study utilised an intention‐to‐treat analysis, but it should be noted that for 1‐year outcomes, results were given in 2 reports: 1 focussing on native artery occlusions, and the other on bypass graft occlusions; the former mentioned report included 261 participants, and the original report included 268 participants, with bypass graft occlusions |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported on |
| Other bias | Unclear risk | Reported that 1000 randomised participants were needed for adequate power, but only 393 were randomised Quote: "Failure of catheter placement occurred in 28% of patients who were randomized to lysis, and thus, were considered treatment failures" |
ABI: ankle‐brachial index. APTT: activated partial thromboplastin time. AV: arteriovenous. BP: blood pressure. h: hour. IU/min: international units per minute. rt‐PA: recombinant tissue plasminogen activator. SD: standard deviation. TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Tiek 2009 | Non‐randomised controlled study |
Differences between protocol and review
For previous versions of this review, each of the three original review authors (DB, DK, IR) independently selected trials for inclusion in previous versions of this review. One review author (DCB) identified all possible trials and sent these to the other review authors (DK/IR).
Contributions of authors
RD selected trials for inclusion; assessed risk of bias and revised the 2017 version of the review with GRADE ratings. DCB identified all possible trials; selected trials for inclusion; assessed quality of trials; and extracted data. DK selected trials for inclusion; assessed quality of trials; and extracted data. IR selected trials for inclusion; assessed quality of trials; and extracted data. RF selected trials for inclusion; assessed risk of bias and GRADE ratings and updated the review.
Sources of support
Internal sources
St James's University NHS Hospital, UK.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
Declarations of interest
RD: none known. DCB: none known. DK: none known. IR: none known. RF: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Nilsson 1992 {published data only}
- Nilsson L, Albrechtsson U, Jonung T, Ribbe E, Thorvinger B, Thorne J, et al. Surgical treatment versus thrombolysis in acute arterial occlusion: a randomised controlled study. European Journal of Vascular Surgery 1992;6(2):189‐93. [DOI] [PubMed] [Google Scholar]
Ouriel 1994 {published data only}
- Ouriel K, Shortell CK, DeWeese JA, Green RM, Francis CW, Azodo MV, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. Journal of Vascular Surgery 1994;19(6):1021‐30. [DOI] [PubMed] [Google Scholar]
Ouriel 1996 {published data only}
- Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. Journal of Vascular Surgery 1996;23(1):64‐73: discussion 74‐5. [DOI] [PubMed] [Google Scholar]
Ouriel 1998a {published data only}
- Ouriel K, Veith FJ. Acute lower limb ischemia: determinants of outcome. Surgery 1998;124(2):336‐41. [PubMed] [Google Scholar]
- Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis Or Peripheral Arterial Surgery (TOPAS Investigators). New England Journal of Medicine 1998;338(16):1105‐11. [DOI] [PubMed] [Google Scholar]
- Patel ST, Haser PB, Bush HL Jr, Kent KC. Is thrombolysis of lower extremity acute arterial occlusion cost‐effective?. Journal of Surgical Research 1999;83(2):106‐12. [DOI] [PubMed] [Google Scholar]
STILE 1994 {published data only}
- Anonymous. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Annals of Surgery 1994;220(3):251‐66; discussion 266‐8. [MEDLINE: ; 8092895 (PubMed)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerota AJ, Weaver FA, Hosking JD, Froehlich J, Folander H, Sussman B, et al. Results of a prospective, randomized trial of surgery versus thrombolysis for occluded lower extremity bypass grafts. American Journal of Surgery 1996;172(2):105‐12. [DOI] [PubMed] [Google Scholar]
- Weaver FA, Comerota AJ, Youngblood M, Froehlich J, Hosking JD, Papanicolaou G. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: results of a prospective randomized trial. The STILE Investigators. Surgery versus Thrombolysis for Ischemia of the Lower Extremity. Journal Vascular Surgery 1996;24(4):513‐21; discussion 521‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Tiek 2009 {published data only}
- Tiek J, Fourneau I, Daenens K, Nevelsteen A. The role of thrombolysis in acute infrainguinal bypass occlusion: a prospective nonrandomized controlled study. Annals of Vascular Surgery 2009;23:179‐85. [DOI] [PubMed] [Google Scholar]
Additional references
ACCF/AHA guideline 2013
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127(4):e362‐e425. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aune 1998
- Aune S, Trippestad A. Operative mortality and long‐term survival of patients operated on for acute lower limb ischaemia. European Journal of Vascular and Endovascular Surgery 1998;15:143‐6. [DOI] [PubMed] [Google Scholar]
Baril 2014
- Baril DT, Ghosh K, Rosen AB. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. Journal of Vascular Surgery 2014;60:669‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1998
- Campbell W, Ridler B, Szymanska T. Current management of acute leg ischaemia: results of an audit by the Vascular Surgical Society of Great Britain and Ireland. British Journal of Surgery 1998;85(11):1498‐503. [DOI] [PubMed] [Google Scholar]
Chaterjee 2014
- Chaterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all‐cause mortality, major bleeding, and intracranial haemorrhage. JAMA 2014;311(23):2414‐21. [DOI] [PubMed] [Google Scholar]
Consensus Document
- Anonymous. Second European Consensus Document on chronic critical leg ischemia. Circulation 1991;84(4 Suppl):IV1‐26. [PubMed] [Google Scholar]
Creager 2012
- Creager MA, Kaufman JA, Conte MS. Acute limb ischemia. New England Journal of Medicine 2012;366:2198‐296. [DOI] [PubMed] [Google Scholar]
Davies 1997
- Davies B, Braithwaite B, Birch P, Poskitt K, Heather BP, Earnshaw JJ. Acute leg ischaemia in Gloucestershire. British Journal of Surgery 1997;84(4):504‐8. [PubMed] [Google Scholar]
Eliason 2003
- Eliason J, Wainess R, Proctor M, Dimick J, Cowan J, Upchurch G, et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischaemia. Annals of Surgery 2003;238(3):382‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;5/6:499‐533. [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc). GRADEpro GuidelineDevelopment Tool (GRADEpro GDT). Hamilton (ON): McMaster University (developed by Evidence Prime, Inc), 2015.
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howard 2015
- Howard DPJ, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM, on behalf of the Oxford Vascular Study. A population‐based study of incidence, risk factors, outcome and prognosis of ischaemic peripheral arterial events: implications for prevention. Circulation 2015;132(19):1805‐15. [DOI: 10.1161/CIRCULATIONAHA.115.016424] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuukasjarvi 1994
- Kuukasjarvi P, Salenius J, Finnvasc Study Group. Perioperative outcome of acute lower limb ischaemia on the basis of the National Vascular Registry. European Journal of Vascular and Endovascular Surgery 1994;8:578‐83. [DOI] [PubMed] [Google Scholar]
NICE 2012
- Alteplase for treating acute ischaemic stroke. NICE technology appraisal guidance TA264 2012. [DOI] [PMC free article] [PubMed]
Norgren 2007
- Norgren L, Hiatt W, Dormandy JA, Nehler M, Harris K, Fowkes F. Inter‐Society Consensus for the Management of Peripheral Arterial Disease (TASC II). European Journal of Vascular and Endovascular Surgery 2007;33(Suppl 1):S1‐S75. [DOI] [PubMed] [Google Scholar]
Reporting Standards
- Anonymous. Suggested standards for reports dealing with lower extremity ischemia. Prepared by the Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. Journal of Vascular Surgery 1986;4(1):80‐94. [PubMed] [Google Scholar]
Schrijver 2015
- Schrijver AM, Leersum M, Fioole B, Reijnen MM, Hoksbergen AW, Vahl AC, et al. Dutch randomized trial comparing standard catheter‐directed thrombolysis and ultrasound‐accelerated thrombolysis for arterial thromboembolic infrainguinal disease (DUET). Journal of Endovascular Therapy 2015;22(1):87‐95. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
von Allmen 2015
- Allmen R, Anjum A, Powell JT, Earnshaw JJ. Hospital trends of admissions and procedures for acute limb ischaemia in England, 2000‐2011. Annals of the Royal College of Surgeons of England 2015;97:59‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2016
- Watson L, Broderick C, Armon PA. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD002783.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Berridge 2002
- Berridge DC, Kessel DO, Robertson I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002784] [DOI] [PubMed] [Google Scholar]
Berridge 2013
- Berridge DC, Kessel DO, Robertson I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD002784.pub2] [DOI] [PubMed] [Google Scholar]


