Abstract
Hepatic lipid accumulation, mainly in the form of triglycerides (TGs), is the hallmark of non-alcoholic fatty liver disease (NAFLD). To date, the spatial distribution of individual lipids in NAFLD affected livers is not well characterized. This study aims to map the triglyceride distribution in normal human liver samples and livers with NAFLD and cirrhosis with imaging mass spectrometry (MALDI IMS). Specifically, whether individual triglyceride species differing by fatty acid chain length and degree of saturation correlate with the histopathological features of NAFLD as identified with classical H&E. Using a recently reported sodium doped gold-assisted laser desorption/ionization IMS sample preparation, twenty human liver samples (five normal livers, five samples with simple steatosis, five samples with steatohepatitis, and five samples with cirrhosis) were analyzed at 10 μm lateral resolution. A total of 24 individual lipid species, primarily neutral lipids, were identified (22 TGs and 2 phospholipids). In samples with a low level of steatosis, TGs accumulated around the pericentral zone. In all samples, TGs with different degrees of side-chain saturation and side-chain length demonstrated differential distribution. Furthermore, hepatocytes containing macro lipid droplets were highly enriched in fully saturated triglycerides. This enrichment was also observed in areas of hepatocyte ballooning in samples with steatohepatitis and cirrhosis. In conclusion, macro lipid droplets in NAFLD are enriched in fully saturated triglycerides, indicating a possible increase in de novo lipogenesis that leads to steatohepatitis and cirrhosis.
Keywords: Nonalcoholic Fatty Liver Disease, Triglycerides, Imaging mass spectrometry, Lipids, Fatty acid/Synthesis
Introduction
As the global prevalence of obesity increases, the incidence of metabolic syndrome (abdominal obesity, hypertension, dyslipidemia, and hyperinsulinemia) increases. It is believed that the first step towards developing insulin resistance and metabolic syndrome is associated with the accumulation of lipids in non-adipose tissues (steatosis). In the livers of healthy patients with no significant alcohol consumption, the abnormal accumulation of triacylglycerides (triglycerides, TGs) and diacylglycerides (diglycerides, DAGs) is termed non-alcoholic fatty liver disease (NAFLD). NAFLD is a chronic condition and is estimated to affect one-fourth of the global population [1]. It describes a spectrum of conditions ranging from simple steatosis (SS) to the more severe progressions of nonalcoholic steatohepatitis (NASH) and liver cirrhosis [2]. NASH is now one of the leading causes of liver cirrhosis in adults worldwide, and in the United States, NASH is the second leading etiology of liver disease among adults awaiting liver transplantation [3]. In addition, liver steatosis in donor grafts causes ischemia-reperfusion injury and grafts with high levels of steatosis are often excluded from the donor pool [4].
Histologically, the liver is composed of thousands of hexagonal lobules, the liver’s smallest functional units. At the periphery of each lobule, portal triads supplying the nutrient-rich blood from the portal veins and the oxygen-rich blood from the hepatic arteries join to form small blood vessels called sinusoids that drain into a central hepatic vein. Despite the morphologically homogeneous appearance of the hepatocytes supplied by this vascular structure, the human liver exhibits a functional zonation where hepatocytes in the periportal zone (zone 1), midzone (zone 2) and pericentral zone (zone 3) differ in their metabolic functions and capacities [5]. Liver zonation or hepatic zonation has been described for many metabolic processes including carbohydrate, ammonia, xenobiotic and more recently fatty acid metabolisms [6]. Two patterns of hepatic steatosis are recognized: microvesicular steatosis, where the cytoplasm is replaced by small lipid droplets that do not displace the nucleus; and macrovesicular steatosis, where the cytoplasm is replaced by a large single lipid droplet that displaces the nucleus to the edge of the cell.
TGs are the main lipid component of the liver in NAFLD. Liver TG synthesis utilizes free fatty acids (FFAs) from adipose tissue, diet, and de novo lipogenesis (DNL). Approximately 60% of all fatty acids stored in the fatty liver originate from the adipose tissue [7]. Recent studies have suggested that the formation of lipid droplets and the accumulation of TGs may be protective against lipotoxic lipid species [8]. Saturated fatty acids (SFAs), on the other hand, have been shown to be toxic to many cell types in culture [9–13]. Their toxicity, however, is significantly reduced when co-supplemented with unsaturated fatty acids because the latter promote the channeling of SFAs into triglyceride storage [11]. While adipose tissue is the main source of FFAs, DNL is thought to play a major role in the development of NAFLD. Individuals with NAFLD have an increased rate of DNL, and higher levels of saturated fatty acids when compared to controls [14]. When comparing subjects with high liver fat levels to those with low liver fat, DNL was the only source of FFAs displaying a statistically significant 3-fold increase [14].
The suspected role of different lipid species in the pathogenesis of NAFLD has led to the study of their identity and regional distribution across the spectrum of the disease [15–19]. Previously, Debois et al. utilized cluster TOF-SIMS imaging to study distribution of different lipid species including TGs in normal and fatty human livers. While successful in demonstrating lipid zonation and macrovesicular enrichment of TGs and DAGs, MS lipid identification was obscured by in-source fragmentation [15].
In this study, we utilize imaging mass spectrometry (IMS) [20–22] to image the distribution of TGs in normal and NAFLD human livers. The goal is to study the triglyceride distribution of human liver samples with NAFLD, and whether specific TG species correlate with the histopathological features of NAFLD. Specifically, wither macro and micro lipid droplets have a different TG content. Previous work employing IMS in fatty liver disease studies has focused on phospholipid profiling [18,16], small metabolite imaging [23], and some work showing triglyceride distribution in a mouse model [24]. Few studies have been performed with human samples or targeting TGs specifically. This was due to a lack of MALDI IMS sample preparation method sensitive and/or selective enough to detect a broad range of TGs from tissue sections with resolution fine enough to discriminate relatively small histopathological features (i.e., individual lipid droplets) from tissue. The recently described use of sodium-doped gold-assisted LDI IMS (AuLDI IMS) bridges this gap in triglyceride analysis from tissue sections [25]. This technique specifically favors the detection of sodiated species, greatly enhancing the sensitivity in the detection of TGs in tissue and is capable of high IMS spatial resolution (<15 μm spot-to-spot) [26]. To achieve this higher specificity and sensitivity for the detection of TG molecules, the tissue sections are first treated by depositing a carbonate buffer and sodium acetate solution followed by covering the salt layer with a thin layer of gold to achieve the UV absorption require for TGs desorption, this increases the specificity for TG molecules at the price of fewer MS signals compared to conventional MALDI methods [25].
Materials and Methods
Study sample and tissue procurement
Twenty samples previously scored by a pathologist according to the NAFLD Activity Score were obtained from the Liver Disease Biobank [27]. These samples included four groups: five normal livers, five livers with SS, five livers with NASH, and five livers with cirrhosis. Samples in the normal group were obtained from patients undergoing liver resection for different etiologies. The samples with SS and steatohepatitis were obtained from livers with NAFLD which were deemed unsuitable for transplantation following histopathological examination during organ procurement. Finally, the samples with cirrhosis were obtained from explanted grafts with cirrhosis secondary to NAFLD following liver transplantation. Informed consent was obtained from all patients through the McGill University Health Center Liver Disease Biobank. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics board of the institute. Surgical specimens were procured and released to the Biobank immediately after clearance from the pathologist. Review of charts confirmed no history of excessive alcohol consumption or concurrent liver disease. The specimens were flash frozen within 30 minutes of resection in the normal group and 120 minutes in all the other groups.
Tissue preparation and cryosectioning of liver samples
Four samples, each representing one grade of the disease (n=20 total), were embedded in a block of optimal cutting temperature compound (OCT) in a circular fashion to produce five OCT blocks. This allowed for simultaneous sectioning of more than one sample. Sectioning was accomplished at 20 μm with a Leica CM 3050 cryostat (Leica Microsystems GmbH, Wentzler, Germany) at a sample temperature of −15°C and chamber temperature of −26°C. For IMS, sections were mounted on a chromium-gold-coated glass slides prepared in-house using a Cressington 308R sputter coater (Ted Pella Inc., Redding, CA). Mirrored serial sections were also obtained and mounted on glass slides for hematoxylin and eosin (H&E) staining. Sections were stained following standard H&E protocol and digitally scanned at 20x magnification (corresponding to a 0.454 μm pixel resolution).
Sodium salt deposition and gold sputtering
After desiccation of the IMS slides for 20 minutes in a vacuum pump desiccator, sodium salt was deposited, followed by gold sputtering as detailed in Dufresne et al [25]. Briefly, an aqueous solution of carbonate buffer (pH 10.3) at 85 mM and sodium acetate (NaAc) at 250 mM in a 1:1 ratio was deposited using a first-generation Leap Technologies TM-Sprayer (HTX Technologies, Chapel Hill, NC) to achieve a thickness of 675 μg/cm2. Later, a gold layer of 28 ± 3 nm was sputtered onto the tissue sections using the Cressington 308R sputter coater mentioned above.
AuLDI IMS parameters
IMS data were acquired at 10 μm spatial resolution in the reflectron geometry using a MALDI-TOF/TOF ultrafleXtreme mass spectrometer equipped with a SmartBeam-II Nd:YAG/355-nm laser operating at a repetition rate of 1 kHz using the “minimum” laser setting that is estimated to be 15 μm in diameter (Bruker Daltonics, Billerica, MA). flexControl 3.4 and flexImaging 4.0 were used to perform and display IMS data acquisition and results, respectively (Bruker Daltonics, Billerica, MA). All instrumental parameters (source voltages, laser energy, delayed extraction parameters, etc.) were optimized for maximum mass resolution and signal-to-noise ratio within the 700–900 m/z range, with the acceleration voltage set to 25 kV.
Processing IMS data, statistical analysis, and imaging output
Raw IMS data were first internally calibrated using gold isotopic peaks using the flexAnalysis Batch Process software (Bruker Dalotnics, Billerica, CA). Next, the calibrated data was exported into the common imzML format using flexImaging 4.1 (Bruker Daltonics, Billerica, CA) then preprocessed and analyzed using the Cardinal package in R [28], which involved peak picking of the spectra with a S/N threshold of 3 and a minimum peak appearance threshold of 5%. Unsupervised clustering was performed with the Cardinal package in R using spatially-aware k-means clustering and the spatial shrunken centroids method [29,30,28].
Results
Study Cohort and Histopathologic Assessment
The 20 samples obtained from the Liver Disease Biobank were scored by a pathologist according to the NAFLD Activity Score (NAS) [27]. Normal livers were obtained from patients undergoing liver resection for various indications (n=5). Livers with simple steatosis (n=5), steatohepatitis (n=5) and cirrhosis (n=5), were obtained from diseased donors with no significant history of alcohol consumption or concurrent liver disease (Table.1).
Table.1.
Study Sample Characteristics.
| NORMAL | SIMPLE STEATOSIS | NASH | CIRRHOSIS | |
|---|---|---|---|---|
| n | 5 | 5 | 5 | 5 |
| STEATOSIS (0–3) | 0 ± 0 | 1.4 ± 0.55 | 3 ± 0 | 0.4 ± 0.55 |
| INFLAMMATION (0–3) | 0.2 ± 0.45 | 1.2 ± 0.45 | 2 ± 0.71 | 2.6 ± 0.55 |
| BALLOONING (0–2) | 0.2 ± 0.45 | 1 ± 0 | 1.4 ± 0.55 | 1.8 ± 0.45 |
| NAFLD ACTIVITY SCORE (0–8) | 0.4 ± 0.55 | 3.6 ± 0.55 | 6.4 ± 1.14 | 4.8 ± 1.3 |
| FIBROSIS (MODE) | 1A | 1A | 2 | 4 |
Data presented as mean ± SD. NASH, Non-alcoholic steatohepatitis. Fibrosis is represented by the mode of individual sample score.
Distribution of Triglycerides Detected by AuLDI IMS in NAFLD Human Liver Sections
Accumulation of triglycerides (TGs) within hepatocytes is the hallmark of NAFLD. Chemically, TGs have a structure of three fatty acyl chains with ester linkages to a glycerol backbone (see Supplementary Figure.1). The diversity of triglyceride species arises from variation of both the length of the fatty acyl chains and the number of unsaturations (C=C double bonds) along the same fatty acid chains. Mass spectrometry detects these changes in fatty acyl chain unsaturation and length as changes in the mass-to-charge (m/z) ratio of the individual TGs. Thus, IMS is well suited to determine the specific TG content of lipid droplets in cells. Utilizing the previously described AuLDI sample preparation for enhanced IMS of triglycerides [25], we have analyzed all samples and identified a total of 24 lipid species (22 TGs and 2 phospholipids, Table.2) from the liver sample set. Typical AuLDI MS spectra of TGs acquired from a NASH samples are observed in Supplementary Figure.2. Observed TG fragment ions obtained by tandem MS (MS/MS) are listed in supplementary Table.1. An example TG MS/MS spectrum (TG 53:3, m/z 879.7) is also presented in Supplementary Figure.1.
Table.2.
List of Lipid Molecular Species Identified by MALDI IMS. TG, Triglyceride. PL, Phospholipid. m/z, mass/charge.
| Lipid | m/z | Ion |
|---|---|---|
| PL | 788.7 | [M+Na]+ |
| TG 46:2 | 797.7 | [M+Na]+ |
| TG 46:1 | 799.7 | [M+Na]+ |
| TG 46:0 | 801.7 | [M+Na]+ |
| PL | 812.7 | [M+Na]+ |
| TG 48:3 | 823.7 | [M+Na]+ |
| TG 48:2 | 825.7 | [M+Na]+ |
| TG 48:1 | 827.7 | [M+Na]+ |
| TG 48:0 | 829.7 | [M+Na]+ |
| TG 50:4 | 849.7 | [M+Na]+ |
| TG 50:3 | 851.7 | [M+Na]+ |
| TG 50:2 | 853.7 | [M+Na]+ |
| TG 50:1 | 855.7 | [M+Na]+ |
| TG 50:0 | 857.7 | [M+Na]+ |
| TG 52:4 | 877.7 | [M+Na]+ |
| TG 52:3 | 879.7 | [M+Na]+ |
| TG 52:2 | 881.7 | [M+Na]+ |
| TG 52:1 | 883.7 | [M+Na]+ |
| TG 52:0 | 885.7 | [M+Na]+ |
| TG 54:4 | 903.7 | [M+Na]+ |
| TG 54:3 | 905.7 | [M+Na]+ |
| TG 54:2 | 907.7 | [M+Na]+ |
| TG 54:1 | 909.7 | [M+Na]+ |
| TG 54:0 | 911.7 | [M+Na]+ |
Triglyceride signals co-localized with both macro and micro lipid droplets and followed a distribution similar to that seen for steatosis when assessing H&E liver sections. In samples with steatosis <5%, triglycerides were accumulating around the pericentral zone (Figure.1).
Figure.1.
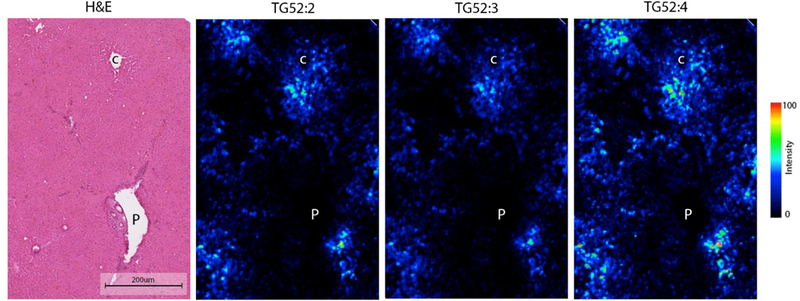
Triglyceride distribution in a normal liver measured by AuLDI IMS. P: Portal triad. C: Central vein. Scale bar: 200 μm.
However, when analyzing a sample with simple steatosis, TGs with different degree of side-chain saturation and side-chain length demonstrated differential distribution. In one liver sample with simple steatosis, the periportal zone hepatocytes were enriched with TG48:2, TG48:3 and TG50:3, while the pericentral zone contained TGs with longer side-chain length including TG52:1, TG54:2 and TG54:3 (Figure.2). Spatially-aware clustering analysis of this individual sample resulted in five clusters that distinctively separated the pericentral, mid and periportal zones (Figure.3, Segments 3, 4 and 5 respectively). The pericentral zone in this sample showed enrichment in TGs with side chain length ranging between 52–54 as compared to 48–52 in the periportal zone (Figure.3). This differential zonation of TG species was observed in each sample individually. However, the zonation differed between samples and no specific TG species was found to be preferentially distributed to a specific zone when accounting for all samples in our dataset.
Figure.2.
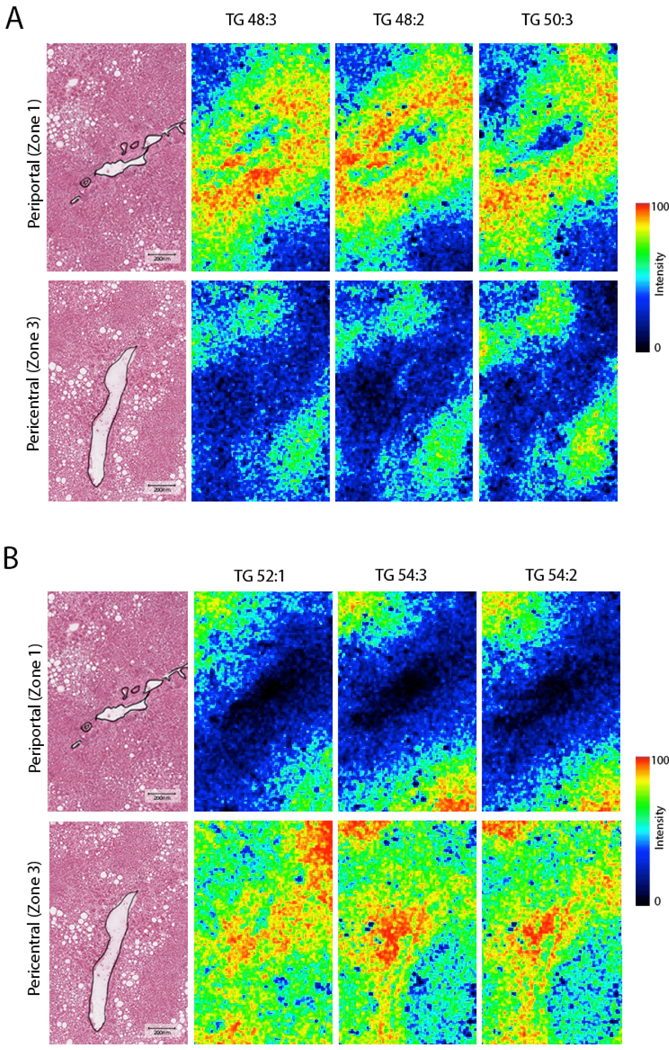
Differential distribution of triglycerides between periportal and pericentral zones in a liver sample with early hepatosteatosis measured by AuLDI IMS. Scale bar: 200 μm.
Figure.3.
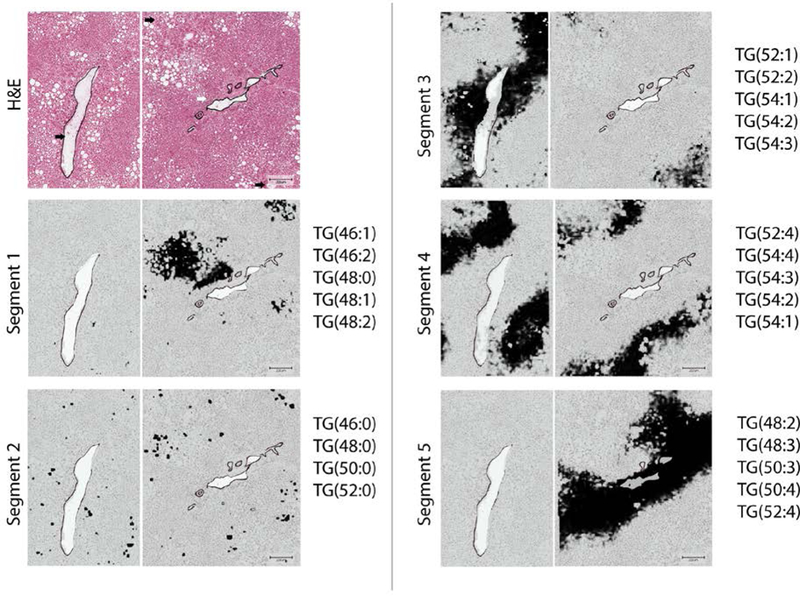
Unsupervised Spatial Clustering of Triglyceride species detected by AuLDI IMS from a liver sample with simple steatosis, resulted in 5 segments. Segment 1 shows hepatocytes surrounding macrovesicular steatosis. Segment 2 shows enrichment of macrovesicular steatosis with saturated Triglycerides. Segments 3–5, represents segments highly correspondent to the pericentral, mid, and periportal zones respectively. Triglycerides contributing to the unsupervised segmentation are listed next to each segment. Scale bar: 200 μm.
Hepatocytes with Macrovesicular Steatosis are Enriched in Saturated Triglycerides
Macrovesicular steatosis with hepatocytes distended by a single lipid droplet that displaces the nucleus to the side is associated with advanced disease and the development of inflammation and fibrosis [31]. It is also associated (when severe) with worse outcomes after liver surgery [4,32].
In all studied samples, hepatocytes containing macro lipid droplets were highly enriched in fully saturated TGs (Figure.4). These clusters of macrovesicular hepatocytes were sometimes surrounded with microvesicular steatosis that were enriched in monounsaturated TGs (Figure.5A; Figure.3 Segments 1&2 and Supplementary Figure.3). The only other histopathological feature associated with enrichment of saturated TGs was hepatocyte ballooning in NASH and cirrhotic samples. In cirrhotic samples, regenerative nodules had very low TG signal with areas of hepatocyte ballooning at the periphery and the center. These hepatocytes with ballooning showed a TG signal rich in saturated TGs similar to that of an area of macrovesicular steatosis in a liver with simple steatosis (Figure.5B). This suggests that areas of hepatocyte ballooning were originally hepatocytes with macrovesicular steatosis.
Figure.4.
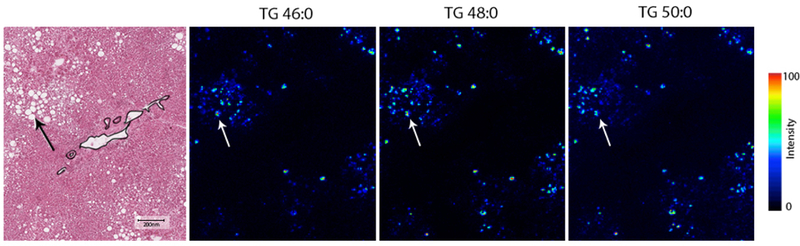
Triglycerides with saturated fatty acids measured by AuLDI IMS from a NASH liver sample are accumulated in hepatocytes with macrovesicular phenotype. Arrow showing area of macrovesicular steatosis. Scale bar: 200 μm.
Figure.5.
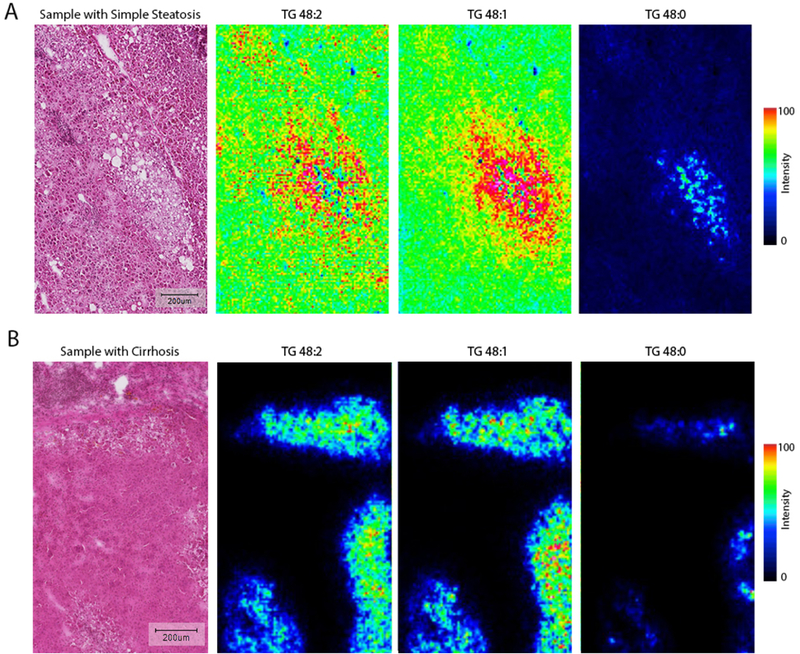
AuLDI IMS measurements from simple steatosis and Cirrhosis liver samples. Areas enriched in saturated triglycerides are surrounded by monounsaturated triglycerides. A: Liver sample with simple steatosis showing area of hepatocyte ballooning enriched in saturated triglycerides surrounded by monounsaturated triglycerides, in panel B, the same distribution is shown in a sample with hepatocyte ballooning and Cirrhosis. Scale bar: 200 μm.
Identified TG species and their corresponding signal intensities divided according to the study groups are demonstrated in Figure.6.
Figure.6.
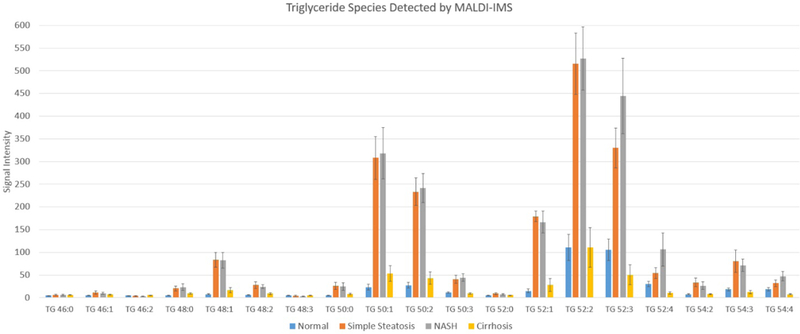
Triglyceride species identified by AuLDI IMS and their intensities divided by study groups. Data in Mean and Standard Error.
Discussion
The goal of this study was to determine the distribution of TGs in normal, steatotic and cirrhotic human liver samples, specifically, if different TG species correlate with histopathological features of NAFLD. First, we determined the distribution of TGs in normal and mildly steatotic tissue (Figures.1 and 2) and further delineated the liver’s zonation in early disease by TG’s distribution (Figure.2) in concert with histopathological examination. Following the novel characterization of TG distribution in normal liver tissue, we demonstrated for the first time that hepatocytes with macrovesicular steatosis are highly enriched in TGs with fully saturated fatty acid side-chains (Figure.3). Macrovesicular steatosis has been associated with worse outcomes following ischemia reperfusion injury (IRI) in liver transplantation and major liver resections [32,33]. In a systematic review of the impact of hepatic steatosis on IRI, transplanted livers with >30% macrosteatosis were associated with liver function derangement and reduced survival while microvesicular steatosis did not influence outcome. While IRI in steatotic livers could be partially contributed to microcirculatory dysfunction, it can also be partially explained by increased sensitivity to reactive oxygen species and impaired endoplasmic reticulum function [34].
TG synthesis utilizes FFAs from the adipose tissue, diet, and through de novo lipogenesis. While the adipose tissue contributes the majority of FFAs in the liver [7], tracer studies on matched controls with or without hepatic steatosis showed no difference in the adipose tissue contribution to the hepatic lipid pool [14]. However, DNL was 3-fold greater in subjects with NAFLD compared to controls [14]. (DNL is the process of which hepatocytes synthesize fatty acids from acetyl-CoA subunits). These are more commonly produced through carbohydrate catabolism. The enrichment of macrovascular hepatocytes with saturated TGs points towards de novo lipogenesis given that the primary products of DNL are saturated fatty acids.
Saturated fatty acids have been shown to be cytotoxic to many cell types in culture [9–13]. They have also been noted to increase the saturation of membrane phospholipids, causing the activation of the unfolded protein response, endoplasmic reticulum stress and apoptosis [35]. SFAs promote reactive oxygen species accumulation and affect mitochondrial metabolism [35]. Further, it has been suggested that insulin resistance in the liver is associated with increased concentration of saturated TGs [36], indicating they induce negative metabolic effects in both free forms and incorporated in larger molecular scaffolds. In our data, saturated TGs were highly concentrated in hepatocytes with macrovesicular steatosis. This might reflect a protective mechanism against high levels of SFAs. This temporary protective measure however doesn’t prevent stored TGs from serving as a source of lipotoxic lipid species when hydrolyzed. Areas of hepatocyte ballooning in our data showed a TG signature enriched in saturated TGs, similar to areas of macrovesicular steatosis (Figure.5). This suggests that macrovesicular steatosis eventually undergoes ballooning, pointing to long term toxicity. The cross-sectional nature of our study prevents a longitudinal look into the role of macrovesicular steatosis and saturated TGs in the development of NASH.
In human livers with total steatosis <5%, TGs were accumulated around the pericentral zone. This is consistent with the functional properties of the pericentral zone, which exhibits higher levels of lipogenesis compared to periportal zone. In samples with simple steatosis and NASH, different patterns of pericentral, periportal and panacinar distributions were noted. Of interest, many livers had TGs with differential distribution around the central vein or portal triad (Figure.2). We suspect this to be due to alteration in the source of fatty acids in which pericentral hepatocytes having a higher level of de novo synthesized of fatty acids compared to the periportal zone, however this remains to be further investigated.
While our findings are from a small cohort, it provides a new understanding of macrovesicular steatosis, the enrichment of macro lipid droplets with saturated TGs perhaps reflects an increase in de novo lipogenesis and a protective mechanism against SFAs during a time of overabundance. These hepatocytes might represent a metabolic burden when hydrolyzed and an area where apoptosis and inflammation might follow. Further profiling of TG distribution in larger cohorts and studies of the role of macrovesicular steatosis and saturated TGs in the development of NASH are required to confirm these results.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by NCI of the National Institutes of Health under award number R01CA198103. We would also like to acknowledge the support of Stephanie Petrillo and Abdellatif Amri for technical support, the MUHC Liver Disease Biobank for all human specimens and the patients who consented to providing samples to the Biobank, with whose support this study would not have been possible.
Funding: This publication was supported by National Cancer Institute of the National Institutes of Health under award number R01CA198103 and by the Natural Sciences and Engineering Research Council of Canada.
Abbreviation:
- DAG
diglyceride
- TG
triglycerides
- NAFLD
non-alcoholic fatty liver disease
- SS
simple steatosis
- NASH
nonalcoholic steatohepatitis
- FFAs
free fatty acids
- DNL
de novo lipogenesis
- SFAs
saturated fatty acids
- MALDI IMS
matrix assisted laser desorption/ionization imaging mass spectrometry
- AuLDI IMS
gold-assisted laser desorption/ionization imaging mass spectrometry
- OCT
optimal cutting temperature compound
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64 (1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55 (6):2005–2023. doi: 10.1002/hep.25762 [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A (2015) Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148 (3):547–555. doi: 10.1053/j.gastro.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 4.McCormack L, Dutkowski P, El-Badry AM, Clavien P-A (2011) Liver transplantation using fatty livers: Always feasible? Journal of Hepatology 54 (5):1055–1062. doi: 10.1016/j.jhep.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK (2014) Zonation of glucose and fatty acid metabolism in the liver: Mechanism and metabolic consequences. Biochimie 96:121–129. doi: 10.1016/j.biochi.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 6.Schleicher J, Tokarski C, Marbach E, Matz-Soja M, Zellmer S, Gebhardt R, Schuster S (2015) Zonation of hepatic fatty acid metabolism - The diversity of its regulation and the benefit of modeling. Biochimica et biophysica acta 1851 (5):641–656. doi: 10.1016/j.bbalip.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 7.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115 (5):1343–1351. doi: 10.1172/JCI23621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA (2010) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52 (2):774–788. doi: 10.1002/hep.23719 [DOI] [PubMed] [Google Scholar]
- 9.de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M (1997) Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. Journal of Lipid Research 38 (7):1384–1394 [PubMed] [Google Scholar]
- 10.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY (2001) Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes 50 (1):69–76 [DOI] [PubMed] [Google Scholar]
- 11.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr., Ory DS, Schaffer JE (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 100 (6):3077–3082. doi: 10.1073/pnas.0630588100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Wei Y, Pagliassotti MJ (2006) Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147 (2):943–951. doi: 10.1210/en.2005-0570 [DOI] [PubMed] [Google Scholar]
- 13.Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG (2001) Inverse Relationship Between Cytotoxicity of Free Fatty Acids in Pancreatic Islet Cells and Cellular Triglyceride Accumulation. Diabetes 50 (8):1771–1777. doi: 10.2337/diabetes.50.8.1771 [DOI] [PubMed] [Google Scholar]
- 14.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ (2014) Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146 (3):726–735. doi: 10.1053/j.gastro.2013.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debois D, Bralet MP, Le Naour F, Brunelle A, Laprevote O (2009) In situ lipidomic analysis of nonalcoholic fatty liver by cluster TOF-SIMS imaging. Anal Chem 81 (8):2823–2831. doi: 10.1021/ac900045m [DOI] [PubMed] [Google Scholar]
- 16.Wattacheril J, Seeley EH, Angel P, Chen H, Bowen BP, Lanciault C, Caprioli RM, Abumrad N, Flynn CR (2013) Differential intrahepatic phospholipid zonation in simple steatosis and nonalcoholic steatohepatitis. PLoS One 8 (2):e57165. doi: 10.1371/journal.pone.0057165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ (2007) A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46 (4):1081–1090. doi: 10.1002/hep.21763 [DOI] [PubMed] [Google Scholar]
- 18.Hall Z, Bond NJ, Ashmore T, Sanders F, Ament Z, Wang X, Murray AJ, Bellafante E, Virtue S, Vidal‐Puig A, Allison M, Davies SE, Koulman A, Vacca M, Griffin JL (2017) Lipid zonation and phospholipid remodeling in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 65 (4):1165–1180. doi: 10.1002/hep.28953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall Z, Chu Y, Griffin JL (2017) Liquid Extraction Surface Analysis Mass Spectrometry Method for Identifying the Presence and Severity of Nonalcoholic Fatty Liver Disease. Anal Chem 89 (9):5161–5170. doi: 10.1021/acs.analchem.7b01097 [DOI] [PubMed] [Google Scholar]
- 20.Norris JL, Caprioli RM (2013) Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev 113 (4):2309–2342. doi: 10.1021/cr3004295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaurand P (2012) Imaging mass spectrometry of thin tissue sections: a decade of collective efforts. J Proteomics 75 (16):4883–4892. doi: 10.1016/j.jprot.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 22.McDonnell LA, Heeren RM (2007) Imaging mass spectrometry. Mass Spectrom Rev 26 (4):606–643. doi: 10.1002/mas.20124 [DOI] [PubMed] [Google Scholar]
- 23.Scupakova K, Soons Z, Ertaylan G, Pierzchalski KA, Eijkel GB, Ellis SR, Greve JW, Driessen A, Verheij J, De Kok TM, Olde Damink SWM, Rensen SS, Heeren RMA (2018) Spatial Systems Lipidomics Reveals Nonalcoholic Fatty Liver Disease Heterogeneity. Anal Chem. doi: 10.1021/acs.analchem.7b05215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa K, Hashimoto M, Itoh Y, Hiroi S, Kusai A, Hirata F, Sakamoto T, Iwaya K (2014) Detection of changes in the structure and distribution map of triacylglycerol in fatty liver model by MALDI-SpiralTOF. FEBS Open Bio 4:179–184. doi: 10.1016/j.fob.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufresne M, Masson JF, Chaurand P (2016) Sodium-Doped Gold-Assisted Laser Desorption Ionization for Enhanced Imaging Mass Spectrometry of Triacylglycerols from Thin Tissue Sections. Anal Chem 88 (11):6018–6025. doi: 10.1021/acs.analchem.6b01141 [DOI] [PubMed] [Google Scholar]
- 26.Hamilton LK, Dufresne M, Joppe SE, Petryszyn S, Aumont A, Calon F, Barnabe-Heider F, Furtos A, Parent M, Chaurand P, Fernandes KJ (2015) Aberrant Lipid Metabolism in the Forebrain Niche Suppresses Adult Neural Stem Cell Proliferation in an Animal Model of Alzheimer’s Disease. Cell Stem Cell 17 (4):397–411. doi: 10.1016/j.stem.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 27.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research N (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41 (6):1313–1321. doi: 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 28.Bemis KD, Harry A, Eberlin LS, Ferreira C, van de Ven SM, Mallick P, Stolowitz M, Vitek O (2015) Cardinal: an R package for statistical analysis of mass spectrometry-based imaging experiments. Bioinformatics (Oxford, England) 31 (14):2418–2420. doi: 10.1093/bioinformatics/btv146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandrov T, Kobarg JH (2011) Efficient spatial segmentation of large imaging mass spectrometry datasets with spatially aware clustering. Bioinformatics (Oxford, England) 27 (13):i230–238. doi: 10.1093/bioinformatics/btr246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tibshirani R, Hastie T, Narasimhan B, Chu G (2002) Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A 99 (10):6567–6572. doi: 10.1073/pnas.082099299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A, Network NCR (2008) Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. Journal of Hepatology 48 (5):829–834. doi: 10.1016/j.jhep.2008.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien P-A (2007) Hepatic Steatosis Is a Risk Factor for Postoperative Complications After Major Hepatectomy: A Matched Case-Control Study. Annals of Surgery 245 (6):923–930. doi: 10.1097/01.sla.0000251747.80025.b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu MJ, Dare AJ, Phillips AR, Bartlett AS (2015) Donor Hepatic Steatosis and Outcome After Liver Transplantation: a Systematic Review. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 19 (9):1713–1724. doi: 10.1007/s11605-015-2832-1 [DOI] [PubMed] [Google Scholar]
- 34.Peralta C, Jiménez-Castro MB, Gracia-Sancho J (2013) Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. Journal of Hepatology 59 (5):1094–1106. doi: 10.1016/j.jhep.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 35.Leamy AK, Egnatchik RA, Young JD (2013) Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res 52 (1):165–174. doi: 10.1016/j.plipres.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luukkonen PK, Zhou Y, Sädevirta S, Leivonen M, Arola J, Orešič M, Hyötyläinen T, Yki-Järvinen H (2016) Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. Journal of Hepatology 64 (5):1167–1175. doi: 10.1016/j.jhep.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 37.Hsu F-F, Turk J (1999) Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. Journal of the American Society for Mass Spectrometry 10 (7):587–599. doi: 10.1016/S1044-0305(99)00035-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


