Abstract
Parkinson’s disease (PD) is characterized by dysfunction in frontal cortical and striatal networks that regulate action control. We investigated the pharmacological effect of dopamine agonist replacement therapy on frontal cortical activity and motor inhibition. Using Arterial Spin Labeling MRI, we examined 26 PD patients in the off- and on-dopamine agonist medication states to assess the effect of dopamine agonists on frontal cortical regional cerebral blood flow. Motor inhibition was measured by the Simon task in both medication states. We applied the dual process activation suppression model to dissociate fast response impulses from motor inhibition of incorrect responses. General linear regression model analyses determined the medication effect on regional cerebral blood flow and motor inhibition, and the relationship between regional cerebral blood flow and motor inhibitory proficiency. We show that dopamine agonist administration increases frontal cerebral blood flow, particularly in the pre-supplementary motor area (pre-SMA) and the dorsolateral prefrontal cortex (DLPFC). Higher regional blood flow in the pre-SMA, DLPFC and motor cortex was associated with better inhibitory control, suggesting that treatments which improve frontal cortical activity could ameliorate motor inhibition deficiency in PD patients.
Keywords: Parkinson’s disease, motor inhibition, frontal cortex, dopamine agonist, cerebral blood flow
1. INTRODUCTION
The ability to execute goal-relevant actions and to suppress irrelevant prepotent action impulses is crucial for navigating dynamic action-oriented environments. Cortico-striatal circuits linking the prefrontal cortices to the direct and indirect basal ganglia pathways coordinate, respectively, action selection and suppression of irrelevant action impulses (Alexander et al., 1986; Mink, 1996; Redgrave et al., 1999; Ridderinkhof et al., 2004). Stopping prepotent response tendencies is an important aspect of inhibitory control that is modulated by key frontal cortical areas including the pre-supplementary motor area (pre-SMA), inferior frontal cortex (IFC), and dorsolateral prefrontal cortex (DLPFC) (Aron et al., 2003, 2007; Frank, 2006; Alexander et al., 2007; Forstmann et al., 2008a; Duann et al., 2009; van Gaal et al., 2011; Jahfari et al., 2011; Ridderinkhof et al., 2011; van Belle et al., 2014; Jahanshahi et al., 2015). These cortical-basal ganglia circuits are responsive to dopamine, and thus, diseases that disrupt these networks, like Parkinson’s disease (PD), can be characterized by deficits in inhibitory action control.
Dopamine therapy generally remediates PD-related inhibitory control deficiencies (Wylie et al., 2012; van Wouwe et al., 2016). However, the effect of dopamine therapy on inhibitory control in PD also depends on individual differences in disease duration (Manza et al., 2017), motor severity (Tolleson et al., 2017), and behavioral symptoms that can emerge with dopamine therapy (Wylie et al., 2012; Claassen et al., 2015). While neuroimaging studies comparing PD patients with healthy controls have emphasized functional deficits in regions involved in motor inhibition, including the IFC, pre-SMA, striatum, and thalamus (Fernández-Seara et al., 2012; Herz et al., 2014; Ye et al., 2014; Vriend et al., 2015; Criaud et al., 2016), the effect of dopamine medication on regional neuronal activity in PD patients remains unclear.
This study describes the pharmacological effect of dopamine agonist (DAA) therapy on frontal cerebral blood flow (CBF) in patients with PD, and the relationship between frontal cortical regional CBF (rCBF) and individual differences in inhibitory action control. DAA therapy improves motor symptoms in PD, and can alter the proficiency of inhibiting action impulses (Wylie et al., 2012; Claassen et al., 2015; Yang et al., 2016, 2018). Using non-invasive arterial spin labeling (ASL) MRI (Claassen et al., 2017), we examined a cohort of PD patients in the off- and on-DAA medication states to assess the effect of DAA on rCBF in frontal regions involved in motor inhibition, and the relationship between rCBF and action control performance as measured by a well-established cognitive test, the Simon task (Simon, 1969). Given our previous findings of improved action control in response to DAA therapy, we predicted that DAA administration would improve the suppression of action impulses, and we hypothesized that individual differences in rCBF in frontal regions are associated with proficiency of motor inhibition.
2. METHODS
2.1. Participants
Patients with idiopathic PD meeting UK Brain Bank criteria (Gibb and Lees, 1988) treated with dopaminergic therapy were recruited from the Movement Disorders Clinic at Vanderbilt University Medical Center. The study has been carried out in accordance with The Declaration of Helsinki, and all subjects provided written, informed consent before participating in the study in compliance with the standards of ethical conduct in human investigation regulated by the Vanderbilt Institutional Review Board.
The extent of PD severity was assessed by a board-certified neurologist (DOC). The Movement Disorders Society-United Parkinson’s Disease Rating Scale (MDS-UPDRS) parts II and III were used to assess self-reported quality of life and motor symptom severity, respectively (Goetz et al., 2007). Cognitive screening was performed using the Montreal Cognitive Assessment (MoCA) to rule out patients with frank dementia (Nasreddine et al., 2005), requiring a score of at least 22. Premorbid intelligence was screened using the American version of the National Adult Reading Test (AMNART), and depression symptoms were screened using the Center for Epidemiologic Studies Depression Scale Revised (CESD-R) (Radloff, 1977). During the clinical assessment, participants also completed the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale (QUIP-RS). Patients’ current prescribed dosages of dopaminergic medication, including Levodopa and DAA, were converted to levodopa equivalent daily dose (LEDD) using the conversion factors and formulae reported in (Tomlinson et al., 2010). The DAA medications and doses taken by each subject are listed in the Supplementary material 1.
All participants had normal or corrected-to-normal vision. Patients were excluded if they had (i) history of neurological diseases other than PD, (ii) clinical symptoms of dementia, depression, cerebrovascular, or cardiovascular disease, (iii) an implanted deep brain stimulator, or (iv) implanted hardware that was contraindicated for 3T MRI.
All patients enrolled in the study completed two experimental sessions, each including MRI and Simon task. One session was performed following withdrawal from their dopaminergic medication (Off-DAA), and the other when patients were in their optimal state of dopamine agonist therapy (On-DAA). In the Off-DAA condition, patients refrained from all dopaminergic medications for a total time of 5-half lives of DAA. Practically, this was at least 36 hours for DAA, and 16 hours for Levodopa due to differences in pharmacokinetic properties (Fabbrini et al., 1987; Tompson and Oliver-Willwong, 2009). This period was deemed sufficient to eliminate DAA effects, while minimizing potential patient discomfort. In the On-DAA state, patients were evaluated after taking their prescribed DAA medication, having withheld Levodopa for at least 16 hours. Extended release DAA compounds (taken by 8 patients) were administered 6 hours before MR scanning, whereas non-extended release DAA (taken by 16 patients) were administered 2 hours before scanning. No changes in medication dosages or addition or discontinuation of either drug for clinical purposes were made at any time during study participation. Demographic and clinical features for patients meeting the inclusion criteria are presented in Table 1.
Table 1.
Demographic and clinical data
| Sex (male/female) | 18/8 |
| Age (years) | 61.2 ± 9.6 |
| Disease Duration (years) | 5.4 ± 3.4 |
| Montreal Cognitive Assessment (MoCA) [min=0, max=30] | 25.6 ± 2.5 |
| American version of the National Adult Reading Test (AMNART) | 117.5 ± 9.3 |
| Center for Epidemiologic Studies Depression Scale (CES-D) [min=0, max=60] | 17.0 ± 9.7 |
| Questionnaire for Impulsive-Compulsive Disorders in PD-Rating Scale (QUIP-RS) [min=0, max=112] | 28.4 ± 13.8 |
| Movement Disorders Society-United Parkinson’s Disease Rating Scale (MDS-UPDRS) | |
| Part II [min=0, max=52] | 23.0 ± 9.1 |
| Part III (Off) [min=0, max=56] | 30.1 ± 12.2 |
| Part III (On) [min=0, max=56] | 21.2 ± 11.1 |
| Dopamine Replacement Therapy | |
| Total Levodopa Equivalent Daily Dose (mg/day) | 565.0 ± 360 |
| Agonist Single Dose Equivalent (mg/day) | 113.2 ± 64.8 |
Data are shown as mean ± standard deviation
2.2. Simon task
The Simon task was administrated on a PC as previously described in the literature (see Wylie et al., 2012; van Wouwe et al., 2016 for details on stimuli and description of individual trials). In brief, participants were instructed to manually respond to the color of a circle that would appear left or right of a fixation point in the center of the screen (e.g., green circle=right thumb press; blue circle=left thumb press). To elicit the Simon effect, two trial types were presented to the patients: 1) Corresponding trials (Cs) in which the circle appeared to the side of fixation that matched the response side signaled by the color of the stimulus (e.g., a green circle calling for a right-hand response appeared to the right side of fixation), and 2) Non-corresponding (Nc) trials, in which the circle appeared on the side of fixation opposite the side of the response signaled by the circle’s color (e.g., a green circle calling for a right-hand response appeared on the left side of fixation). Cs and Nc trial types were presented randomly, but with equal probability, within each block of trials. Reaction times (RT) and accuracy were measured for each trial, and the Simon effect was estimated as Nc trials minus Cs trials for RT and error rates.
2.3. Behavioral analysis
RT latencies for Cs and Nc trials faster than 150 ms (anticipatory reactions) and slower than three standard deviations of the mean within each condition were excluded, but accounted for fewer than 1% of trials across participants (Wylie et al., 2010a). For each level of correspondence (i.e. Cs and Nc), mean RT and log transformed accuracy rates were calculated to analyze mean Simon effect on RT and on accuracy (i.e. the difference in RT or accuracy between Cs and Nc trials).
The dual process activation suppression (DPAS) model provided the conceptual and analytical framework to dissociate fast response impulses and inhibition of incorrect responses by means of distributional analyses (Ridderinkhof, 2002; Wylie et al., 2010a, 2010b). Distributional analyses provide more insight into dynamic control processes that are often masked in global RT or interference measures. Distributional analyses were implemented by rank-ordering single-trial RTs from fastest to slowest and by dividing rank-ordered RTs into 6 equal sized bins (each bin contained the same number of trials).
According to the DPAS model, impulse capture can be measured by plotting the accuracy rates against the RT for each level of correspondence (Conditional Accuracy Function, CAF). The proportion of fast errors on non-corresponding trials (Nc) (i.e. within fastest RT bin) reflects the strength of the incorrect response capture (van den Wildenberg et al., 2010).
The DPAS also allows measurement of the proficiency of inhibitory control by means of delta plots, which shows the size of the Simon effect on RT as a function of the RT. This yields a pattern of increasing interference across fast to intermediate response latencies that is followed by a significant reduction (c.f., Luce, 1986) in interference toward the slow end of the RT distribution (Proctor et al., 2011). According to the DPAS model, when an incorrect response has been triggered by non-corresponding stimulus information, it takes time for inhibitory control to build up. Therefore, inhibitory control is most clearly reflected at the slow end of the RT distribution; the slope of the interference reduction between the final two bins of the delta plot (delta-slope) provides the most sensitive metric of the proficiency of inhibitory control over conflicting motor impulses. This has been supported empirically across several studies using both non-clinical and clinical populations (Burle et al., 2002; Ridderinkhof et al., 2005; Wijnen and Ridderinkhof, 2007; Wylie et al., 2007, 2009, 2010b; for a review, see Ridderinkhof et al., 2011).
2.4. MRI acquisition
Patients were scanned using a 3T MRI scanner (Philips Healthcare, Best, The Netherlands). Anatomical MRI scans, including (i) T1-weighted (MPRAGE; spatial resolution=1×1×1 mm3; TR/TE=8.9/4.6 ms), and (ii) T2-weighted FLAIR (spatial resolution=1×1×1 mm3; TR/TE=4000/120ms; TI=2800 ms), were obtained to exclude coexisting central nervous system disorders. Pseudo-continuous ASL (pCASL) data were acquired using 2D single-shot echo-planar-imaging (field-of-view = 220×220×119 mm3, slices=20; spatial resolution=3.5×3.5×5 mm3; gap = 1 mm, TR/TE=4000/12 ms with post-labeling delay and labeling pulse train length both set to 1500 ms). The field-of-view for the ASL scan was placed perpendicular to the feeding arteries and covered an area between the inferior border of the pons and the superior sagittal sinus.
2.5. Image analysis
All the analyses were performed on each subject’s native space. Image pre-processing was performed using the FMRIB software library (FSL v5.0.2.1, FMRIB, Oxford, UK). This included brain extraction using BET, and affine time-course motion correction using MCFLIRT (Jenkinson et al., 2002). CBF quantification was performed in Matlab (Mathworks, Natick, MA, USA). Motion-corrected images were pair-wise subtracted using the surround subtraction approach (Lu et al., 2006), and the mean across measurements was computed to obtain a mean difference magnetization (ΔM). The difference magnetization was then normalized by the equilibrium magnetization (M0), which was calculated by converting the control image magnetization to equilibrium magnetization by dividing by the term [1-exp(−TR/T1t)], where TR is the repetition time (TR=4000 ms), and the T1t is the 3T T1 of gray matter tissue (T1t=1200 ms). Next, the ΔM/M0 image was converted to absolute CBF (ml/100g/min) using the simplified kinetic model recommended by the ISMRM perfusion study group (Alsop et al., 2015) and accounting for slice-time correction in the 2D EPI readout (duration of EPI readout per slice = 23 ms).
The regions of interest (ROIs) including the medial pre-frontal cortex (MPFC), dorsolateral pre-frontal cortex (DLPFC), ventrolateral pre-frontal cortex (VLPFC), supplementary motor area proper (SMA-p), pre- supplementary motor area (pre-SMA), primary motor cortex (M1), and anterior cingulate cortex (ACC) were selected due to previous evidence of their importance in motor inhibition (Aron et al., 2003, 2007; Frank, 2006; Alexander et al., 2007; Forstmann et al., 2008a; Duann et al., 2009; Jahfari et al., 2011; van Belle et al., 2014), and segmented on each subject’s T1-weighted image using FreeSurfer (Version 5.3.0, http://surfer.nmr.mgh.harvard.edu). The cortical parcellation was performed using the Desikan-Killiany atlas. The cortical labels obtained from the automatic cortical parcellation in surface-space (aparc) was mapped to the automatic segmentation volume (aseg) using the function mri_aparc2aseg from FreeSurfer, which finds each aseg voxel labeled as cortex and assign it the label of the closest cortical vertex.
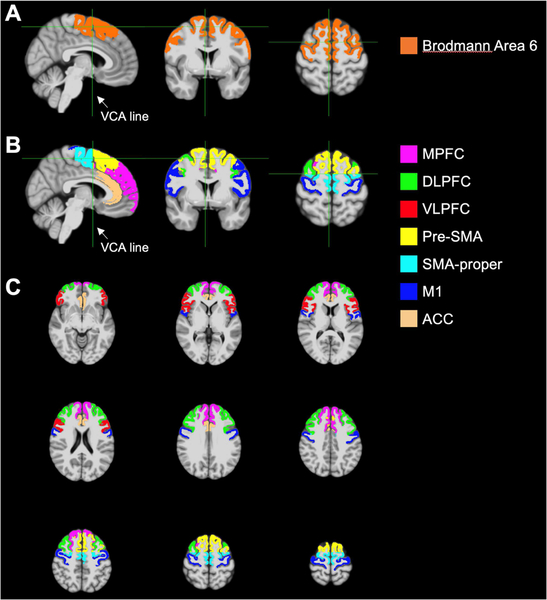
The Brodmann areas atlas was used to parcellate Brodmann area 6 (Fig. 1A), which was used to define the SMA-p and the pre-SMA. We defined the verticofrontal (VCA) line perpendicular to the anterior commissure - posterior commissure (AC-PC) line at the level of the anterior commissure to divide the pre-SMA anteriorly from the SMA-p (Picard and Strick, 1996, 2003; Vergani et al., 2014) (Fig. 1B).
Fig. 1.
Illustration of the location of ROIs on a T1-weighted template. A) Broadman area 6 was obtained using the Brodmann areas atlas, and the VCA line was used to separate the pre-SMA anteriorly from the SMA-p. B) Sagittal, coronal and axial representations showing all ROIs. C) Axial slices at different levels.
The rest of the ROIs were defined using the cortical parcellations obtained with the Desikan-Killiany atlas. Specifically, M1 corresponded to the precentral gyrus; MPFC was created by merging the superior frontal and frontal pole (excluding the pre-SMA and SMA-p); DLPFC by merging the rostral middle frontal and caudal middle frontal (excluding the pre-SMA and SMA-p), VLPFC by merging the pars opercularis, pars triangularis, and pars orbitalis; and ACC by merging the rostral anterior cingulate and caudal anterior cingulate (Fig. 1C). Finally, the CBF maps were co-registered to the T1-weighted images using FSL’s FLIRT with 6 degrees of freedom, and the median rCBF value (instead of mean) was recorded for each ROI to alleviate the effect of outlying voxels.
Finally, to account for the possible effect of global (whole-brain) CBF, we calculated the CBF for total gray matter for each patient in both of the medication conditions. The total gray matter mask was obtained by merging all the cortical and subcortical structures from FreeSurfer.
2.6. Experimental design and statistical analysis
To assess the effect of DAA on action control, we performed general linear regression model (GLM) analyses using each of the cognitive measures (RT, Simon effect on RT, Simon effect on accuracy, response capture, and delta-slope) as dependent variables, and medication status as independent variable. To ensure that changes in the cognitive performance were not a result of potential confounding factors, we considered age, UPDRS-II, UPDRS-III (Off) and MoCA, and disease duration as possible covariates, i.e., inter-subject variation in those confounding factors. To avoid overfitting, we performed a principal component analysis (PCA) including age, UPDRS-II, UPDRS-III (Off), MoCA, and disease duration, and used the first principal component (PC1), which explained 40% of the variance of the data, as covariate in the GLMs (model 1).
To test the hypothesis that DAA medication has an effect on rCBF in frontal areas, we performed GLM analyses specifying within-ROI rCBF as the dependent variable, and medication condition as the independent variable. To ensure that differences in rCBF were not a result of potential confounding factors, we also included PC1 (as described above) as covariate in the GLMs (model 2). A separate GLM analysis was performed for each of the seven ROIs.
To evaluate if rCBF is related to action control, we performed GLM analyses with each of the Simon task measures as dependent variables, ROI rCBF as independent variable, and PC1 as covariate. These analyses were performed separately for every ROI in each medication condition (model 3).
All analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, 2016). The results were considered significant at the level of false discovery rate (FDR) of 0.10 in concordance with the threshold recommended in the first description of the method (Benjamini and Hochberg, 1995), which has been used in previous neuroimaging studies (Petersen et al., 2018; Stark et al., 2018a, 2018b). The FDR corrections were applied separately to the results from model 1 to account for the multiple cognitive measures, and to the results from models 2 and 3 to account for the multiple ROIs evaluated in the study. In each GLM analysis, we also reported 95% confidence interval (CI) associated with each regression coefficient of interest, using the t-statistic of the coefficient.
3. RESULTS
3.1. Action control performance
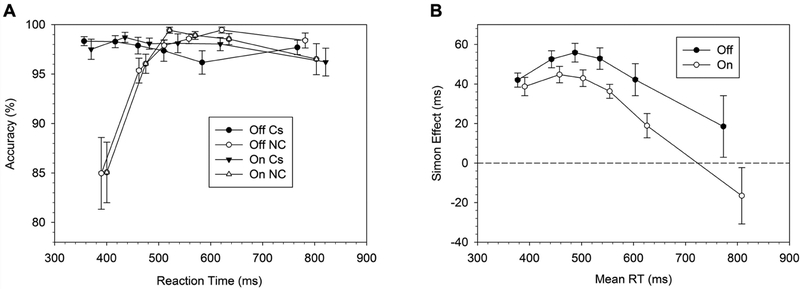
Table 2 summarizes the results for the effect of medication of the cognitive measures. The RT did not differ between medication states. The Simon effect on RT but not on accuracy rates varied by medication state. In the Off-DAA condition, patients showed a Simon effect that was 17 ms larger than in the On-DAA state (P=0.005). The analysis on the response capture rates (accuracy rates in the fastest RT bin of the non-corresponding trials) confirmed that fast impulsive errors on Nc trials were similar across medication states (Fig. 2A). The proficiency of inhibitory control (i.e. delta-slope between the last two bins of the RT distribution) showed a trend for improvement in the On-DAA condition (i.e. more negative slope On- versus Off-DAA), but this finding was not statistically significant (P=0.19) (Fig. 2B, Table 2).
Table 2.
DAA medication effect on action control (Model 1: cognitive measures ~ medication status + PC1)
| Cognitive Measure | t-statistic (DF=25) | 95 % CI | coefficient | P-valuea |
|---|---|---|---|---|
| Overall RT across trials | 1.103 | [−16.180, 53.513] | 18.666 | 0.280 |
| RT for Cs trials | 1.454 | [−11.339, 65.797] | 27.228 | 0.158 |
| RT for Nc trials | 0.655 | [−21.641, 41.844] | 10.101 | 0.518 |
| Simon Effect (RT) | −3.056 | [−28.670, −5.585] | −17.127 | 0.005b |
| Simon Effect (accuracy) | −0.614 | [−0.018, 0.010] | −0.004 | 0.544 |
| Response capture | −0.124 | [−0.591, 0.524] | −0.034 | 0.902 |
| Delta-slope | −1.354 | [−0.271, 0.056] | −0.108 | 0.188 |
Uncorrected p-value
Significant p-value at FDR = 0.1
DF = Degrees of freedom
Negative coefficients represent better performance on action control in the On-DAA condition.
Fig. 2.
A) Conditional Accuracy Function (CAF) for corresponding (Cs) and non-corresponding (Nc) trials. B) Reaction times (RT) delta plots.
3.2. CBF and DAA medication status
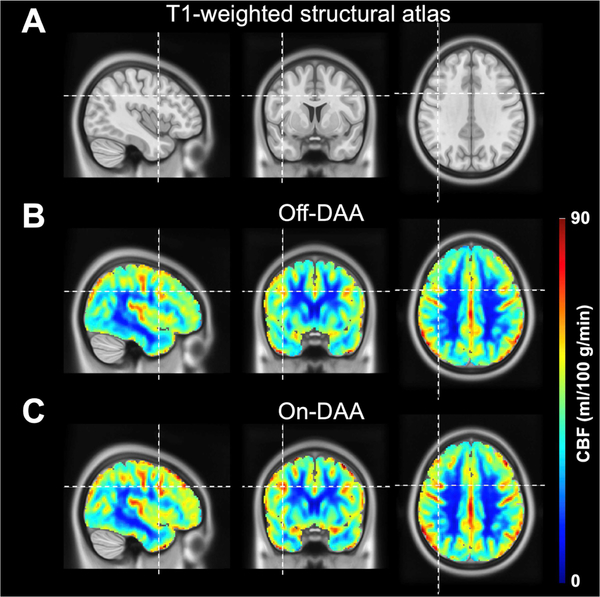
Fig. 3 shows the mean CBF maps for the Off- and On-DAA conditions. When examining the effect of DAA medication status on total gray matter CBF, we did not find significant differences between the Off-DAA (42.53 ± 9.27) and On-DAA (44.68 ± 6.51) states (P=0.12). We observed increases in rCBF in the On-DAA condition. In particular, we found significantly increased rCBF values in the DLPFC (P=0.011) in response to DAA administration. The rCBF in the pre-SMA also showed an increase in the On-DAA condition but did not survive the FDR correction (P=0.041). Table 3 summarizes these results.
Fig. 3.
A) Orthogonal representation of a T1-weighted anatomical atlas, along with mean quantitative CBF maps (ml/100 g/min) across subjects in the (B) Off-DAA and (C) On-DAA states. Increases in rCBF in several regions, including the DLPFC (indicated by the cursor) and pre-SMA, were observed in the On-DAA condition.
Table 3.
DAA medication effect on rCBF (Model 2: rCBF ~ medication status + PC1)
| ROI | t-statistic (DF=25) | 95 % CI | coefficient | P-valuea |
|---|---|---|---|---|
| Total gray matter | 1.627 | [−0.572, 4.872] | 2.150 | 0.116 |
| ACC | 0.516 | [−3.772, 6.291] | 1.260 | 0.611 |
| MPFC | 1.714 | [−0.539, 5.879] | 2.670 | 0.099 |
| DLPFC | 2.742 | [1.007, 7.087] | 4.047 | 0.011b |
| VLPFC | 2.017 | [−0.061, 5.806] | 2.872 | 0.055 |
| SMA-p | 1.217 | [−2.018, 7.842] | 2.912 | 0.235 |
| Pre-SMA | 2.155 | [0.165, 7.252] | 3.709 | 0.041 |
| M1 | 1.155 | [−1.498, 5.320] | 1.911 | 0.259 |
Uncorrected P-value
Significant P-value at FDR = 0.1
DF = Degrees of freedom
Positive coefficients represent higher rCBF in the On-DAA condition.
3.3. CBF and action control
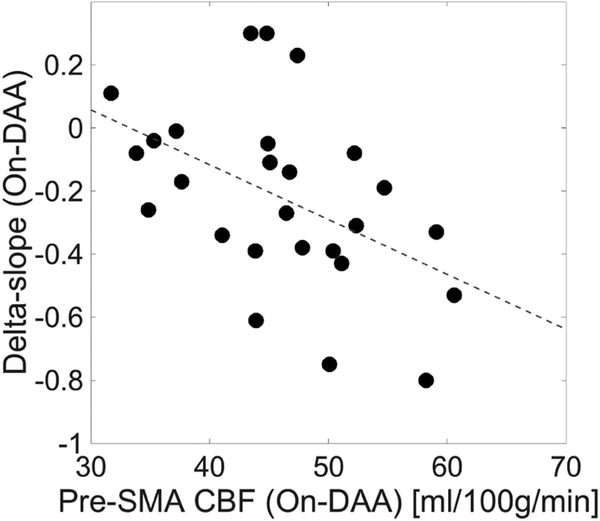
In the On-DAA condition, the delta-slope showed a significant association with the rCBF in the DLPFC (P=0.038), pre-SMA (P=0.006) and M1 (P=0.015) (Table 4), with the strongest relationship in the pre-SMA (Fig. 4). Conversely, no significant relationship was observed between the rCBF and the Simon effect on RT, Simon effect on accuracy, or the response capture in any of the ROIs, regardless of medication state (Table 4).
Table 4.
Relationship between rCBF and action control (Model 3: action control ~ rCBF + PC1)
| Off-DAA | On-DAA | |||||
|---|---|---|---|---|---|---|
| t(DF=23) [95 % CI] | Coeff. | P-valuea | t(DF=23) [95 % CI] | Coeff. | P-valuea | |
| Simon Effect on RT | ||||||
| ACC | 0.954 [−0.551, 1.496] | 0.472 | 0.3497 | −0.441 [−1.322, 0.858] | −0.232 | 0.663 |
| MPFC | 0.235 [−1.185, 1.489] | 0.152 | 0.816 | −0.221 [−1.341, 1.082] | −0.129 | 0.827 |
| DLPFC | −0.209 [−1.209, 0.987] | −0.111 | 0.837 | 0.300 [−0.910, 1.219] | 0.154 | 0.767 |
| VLPFC | 0.208 [−1.176, 1.438] | 0.131 | 0.837 | 0.135 [−1.248, 1.422] | 0.087 | 0.894 |
| SMA-p | −0.721 [−1.384, 0.668] | −0.358 | 0.478 | −0.239 [−1.220, 0.917] | −0.151 | 0.772 |
| Pre-SMA | −0.869 [−1.495, 0.611] | −0.442 | 0.394 | −0.604 [−1.502, 0.823] | −0.339 | 0.552 |
| M1 | −0.585 [−1.432, 0.800] | −0.316 | 0.564 | −0.100 [−1.142, 1.037] | −0.052 | 0.922 |
| Simon Effect on accuracy | ||||||
| ACC | −0.458 [−0.002, 0.001] | −4e-04 | 0.652 | 1.054 [−0.001, 0.003] | 8e-04 | 0.303 |
| MPFC | −0.488 [−0.003, 0.002] | −5e-04 | 0.630 | 1.149 [−0.001, 0.003] | 1e-03 | 0.262 |
| DLPFC | −0.937 [−0.002, 0.001] | −8e-04 | 0.359 | −0.020 [−0.002, 0.002] | 1e-08 | 0.984 |
| VLPFC | −0.550 [−0.003, 0.002] | −5e-04 | 0.588 | 0.542 [−0.001, 0.003] | 5e-04 | 0.593 |
| SMA-p | −0.775 [−0.002, 0.001] | −6e-04 | 0.446 | 0.266 [−0.001, 0.003] | 2e-04 | 0.792 |
| Pre-SMA | 0.675 [−0.001, 0.002] | 5e-04 | 0.506 | 1.165 [−0.001, 0.003] | 1e-03 | 0.256 |
| M1 | −0.622 [−0.002, 0.001] | −5e-04 | 0.540 | 1.161 [−0.001, 0.003] | 9e-04 | 0.258 |
| Response capture | ||||||
| ACC | −0.885 [−0.080, 0.032] | −0.024 | 0.385 | 1.542 [−0.019, 0.127] | 0.054 | 0.137 |
| MPFC | −1.080 [−0.108, 0.034] | −0.037 | 0.291 | 1.583 [−0.019, 0.142] | 0.062 | 0.127 |
| DLPFC | −0.885 [−0.084, 0.034] | −0.025 | 0.385 | 0.775 [−0.046, 0.101] | 0.028 | 0.446 |
| VLPFC | −1.357 [−0.113, 0.024] | −0.045 | 0.188 | 0.906 [−0.052, 0.132] | 0.040 | 0.374 |
| SMA-p | 0.156 [−0.052, 0.061] | 0.004 | 0.877 | 1.251 [−0.029, 0.116] | 0.044 | 0.224 |
| Pre-SMA | 0.175 [−0.053, 0.063] | 0.005 | 0.863 | 1.868 [0.007, 0.146] | 0.069 | 0.075 |
| M1 | −0.355 [−0.071, 0.050] | −0.010 | 0.726 | 1.217 [−0.030, 0.117] | 0.044 | 0.236 |
| Delta-slope | ||||||
| ACC | −1.473 [−0.021, 0.004] | −0.009 | 0.154 | −1.400 [−0.024, 0.005] | −0.010 | 0.175 |
| MPFC | −1.470 [−0.027, 0.005] | −0.011 | 0.155 | −1.835 [−0.029, 0.002] | −0.014 | 0.080 |
| DLPFC | −1.961 [−0.024, 0.001] | −0.012 | 0.062 | −2.158 [−0.027 −0.001] | −0.014 | 0.042b |
| VLPFC | −1.362 [−0.026, 0.005] | −0.010 | 0.187 | −1.881 [−0.032, 0.002] | −0.015 | 0.073 |
| SMA-p | −1.652 [−0.022, 0.003] | −0.009 | 0.112 | −1.794 [−0.025, 0.002] | −0.012 | 0.086 |
| Pre-SMA | −1.925 [−0.024, 0.001] | −0.011 | 0.067 | −2.980 [−0.033, −0.006] | −0.019 | 0.007b |
| M1 | −2.064 [−0.025, 0.000] | −0.013 | 0.050 | −2.678 [−0.029, −0.004] | −0.017 | 0.013b |
Uncorrected p-value
Significant p-value at FDR = 0.1
t(DF) = t-statistic(degrees-of-freedom); Coeff=Coefficient
Fig. 4.
Scatter plot and linear regression showing the relationship between the delta-slope and the rCBF in the pre-SMA in the On-DAA state.
4. DISCUSSION
We evaluated the effect of DAA medication on rCBF in frontal cortical areas and examined how action control in PD is informed by rCBF. We found that single-dose DAA administration increases rCBF in the DLPFC and pre-SMA, supporting the hypothesis that DAA administration results in regional brain activity changes to cortical regions involved in motor inhibition. Our results emphasize that greater rCBF in the pre-SMA, DLPFC and M1 reflect better proficiency of inhibitory control. These findings extend our understanding of the cortical localization of dopamine effects on motor inhibition and the source of individual differences in action control.
4.1. Effects of DAA medication on action control
In this study, we find similar motor speed between medication states. However, based on the DPAS model and previous findings of medication on inhibitory control in PD (van Wouwe et al., 2016), we did not expect that medication effects would be purely additive across the RT distribution. Instead, the DPAS model predicts that suppression of interfering information slowly builds up, and becomes most evident at the slow end of the RT distribution where the Simon effect reduces. Based on our previous work that showed that dopamine therapy significantly improves reactive inhibitory control processes engaged to suppress interference from the spontaneously activated impulses (Wylie et al., 2012; van Wouwe et al., 2016), we predicted that this suppression would be more efficient On- compared to Off-DAA, and would be reflected in a sharper decline of the delta slope function in the On-DAA condition (Fig. 3b). Here, DAA significantly improved the Simon effect, and while the GLM analysis did not show significant differences in delta-slope between Off- and On-DAA, the direction of the delta slope is more negative for On-DAA, which is consistent with previous work, and indicates that DAA therapy improves reactive inhibitory control.
4.2. Effects of DAA medication on rCBF
We find that a single-dose of DAA results in rCBF increases in the DLPFC and pre-SMA. Under the common suppression that changes in perfusion are coupled to changes in glucose metabolism and associated neuronal activity, we interpret these results to indicate that DAA administration induces increased neural activity in key cortical regions that subserve motor inhibition and action control. This network effect is likely mediated via reciprocal connections to basal ganglia and thalamic networks. In healthy neurovascular coupling, CBF measured using ASL is a surrogate marker of glucose metabolism and associated neuronal activity (Buxton, 2005; Wolk and Detre, 2012). Our interpretation, that DAA modifies regional CBF, depends on the coupling between rCBF, metabolism, and neuronal activity. Previous animal studies indicate that this coupling is preserved in the presence of non-ergot DAA (McCulloch and Edvisson, 1980; McCulloch et al., 1982). As a supplementary analysis, we evaluated whether global CBF (rather than regional), varied with medication status. In this case, we did not observe a significant DAA effect on global CBF, suggesting that the DAA induced changes in rCBF reflect regional effects, rather than a global hemodynamic change. This supplementary analysis also supports the observation that CBF findings are not being driven by technical variations due to image artifacts, for instance due to variations in labeling efficiency in the pCASL sequence from scan to scan that would manifest as a globally altered CBF measurement.
Previous PET and MRI studies investigating the effects of levodopa, the most commonly used dopamine precursor therapy for PD, indicate that treatment alters rCBF in several regions: the striatum and thalamus (Kobari et al., 1995), nigrostriatal pathway, occipital cortex, and inferior parietal areas (Chen et al., 2015). While both levodopa and DAA augment dopaminergic tone in PD and both appear to increase rCBF in areas associated with PD symptoms, despite the differing mechanisms of action. Pharmacological neuroimaging studies in non-human primates with levodopa (Hershey et al., 2000) and non-ergot DAAs (Black et al., 1997, 2002, 2011) indicate that different dopaminergic agents have unique rCBF responses, suggesting that rCBF changes are mediated by the action as a dopamine precursor (levodopa) or receptor agonist (DAA). Moreover, studies in healthy volunteers investigating the relationship between CBF and activity changes in specific neurotransmitter systems (Donahue et al., 2014; Dukart et al., 2018) showed that CBF reflects specific metabolic demands from diverse underlying neurotransmitter systems, supporting the notion that different pharmacological agents provide unique patters of CBF changes associated to receptor availability, affinity and function. In particular, the CBF changes induced by dopaminergic medications showed a significant positive association with the underlying D1 and D2 receptor densities. These findings are consistent with previous research reporting increased CBF after administration of both dopamine agonist and antagonist (Mu et al., 2007; Fernández-Seara et al., 2011; Handley et al., 2013; Schouw et al., 2013).
In this study, patients were given non-ergoline DAAs (ropinirole, pramipexole, rotigotine) which exert their effects by acting directly on dopamine receptors. These DAAs primarily target D2-like receptors, particularly D3 (Mierau et al., 1995; Piercey, 1998; Millan et al., 2002; Newman-Tancredi et al., 2002; Scheller et al., 2009; Wood et al., 2015), which are localized in greater density to the mesolimbic and mesocortical regions (Sokoloff et al., 1990, 1992; Schwartz et al., 1993; Murray et al., 1994). Previous studies suggest that D3 receptors can influence cognitive function via their inhibitory effect on mesocortical dopaminergic activity (Cole et al., 2012; Gross et al., 2013; Nakajima et al., 2013). We find that DAA produces rCBF increases in the DLPFC and pre-SMA which are important for top-down control of subcortical brain regions that implement selective stopping.
4.3. rCBF and motor inhibition
In the On-DAA condition, higher rCBF in several frontal cortical areas, including the pre-SMA, DLPFC and M1, was associated with better inhibitory control over conflicting action impulses (as measured by the negative slope of the delta plot). These cortical areas play a critical role in selecting task-relevant information during conflict situations (Ullsperger and von Cramon, 2001; Peterson et al., 2002; Mars et al., 2009; Neubert and Klein, 2010; Duque et al., 2012; Soutschek et al., 2013). Previous studies of neural activation patterns associated with response interference trials on conflict tasks indicate the involvement of fronto-parietal and fronto-striatal networks (Ridderinkhof et al., 2004; Nee et al., 2007). The resolution of conflict situations involving two competitive actions, an incorrect prepotent action versus a desired action, may come from diverse sources, including inhibition of motor areas and increased activation of brain areas involved in action selection. When stopping an already-started action, the pre-SMA works together with the right IFC to send a stop command to intercept prepotent response tendencies via the basal-ganglia networks, resulting in the suppression of basal-ganglia output with global inhibitory effects on M1 (Aron and Poldrack, 2006; Badry et al., 2009); when control is needed, the pre-SMA may act in concert with the right IFC to implement stopping.
This is supported by neuroimaging studies that have shown that the network composed of the right IFC, pre-SMA, and STN (i.e. hyperdirect pathway) plays a crucial role in reactive stopping (Aron and Poldrack, 2006; Aron et al., 2007), that individual differences in inhibition are accompanied by differences in brain function in those areas (Forstmann et al., 2008b), and that stimulation of the pre-SMA influences frontal rCBF and has a significant impact on response inhibition (Obeso et al., 2013). The strong connections between the pre-SMA, right IFC and STN (Johansen-Berg et al., 2004) lead to global inhibitory effects of the motor system (Aron et al., 2007; Stinear et al., 2009; Swann et al., 2009). Additionally, this network for reactive stopping can also be prepared in advance, and thus, action control can be proactive (Jahfari et al., 2010).
In this study, we showed a significant association between rCBF in the pre-SMA, DLPFC, and M1 and the ability to suppress irrelevant action impulses, suggesting that higher rCBF in these areas could reflect individual differences in the ability to implement control in conflict situations. Our results are consistent with previous studies (Chikazoe et al., 2009; Jahfari et al., 2010), and indicate that if the stopping networks are preactivated, stopping is more proficient when required. Interestingly, we did not observe an association between rCBF and the mean Simon effect nor with response capture (as reflected by accuracy rates in the fast bin of the RT distribution), which could indicate that individual differences in rCBF may be specifically related to selective response inhibition. The process of selective stopping could be prepared in advance via a top-down influence of the DLPFC over the striatum (Vink et al., 2005; Chikazoe et al., 2009; Zandbelt and Vink, 2010), which takes time to build-up and allows for more selective motor control by targeting particular representations in M1 (Aron and Verbruggen, 2008; Claffey et al., 2010). DLPFC activity seems to be associated with goal-directed activation, resulting in advance preparation of task-relevant areas with subsequent performance benefits (Wylie et al., 2006; Yeung et al., 2006).
Overall, the present results corroborate and extend earlier findings: the role of frontal cortical areas, particularly the pre-SMA and DLPFC, in the implementation of selective response inhibition. We found that individual differences in rCBF in the pre-SMA and DLPFC significantly correlate with performance on the proficiency of selective inhibitory control over conflicting motor impulses. When multiple competing actions are possible, the demands on action control are highest, and selecting the correct action may require stronger activation of the pre-SMA and DLPFC (Ridderinkhof et al., 2011; Carbonnell et al., 2013). Finally, we also observed a trend for a correlation between rCBF in the pre-SMA, DLPFC and M1 and delta-slope for the Off-DAA condition, although it was weaker compared to the On-DAA state. This could mean that, even in the absence of a DAA effect, higher rCBF in the pre-SMA is associated with better predisposition to implement action control when needed. However, we hypothesize that DAA administration likely restores a deficient dopamine tone, where increases in rCBF in the pre-SMA and DLPFC reflect stronger frontal-cortical function and more proficient action control (more negative slope).
4.4. Limitations and future directions
In the On-DAA condition we examined patients in their optimal DAA dosage; however, they were still withdrawn from levodopa, and thus, they were not in their optimal ‘On’ state. This design was purposeful as we wished to study agonist-only effects on rCBF. Moreover, in the On-DAA condition, we examined patients after the administration of their routine prescribed DAA, and we did not standardize the acute dose across patients. This was a convenient method, which allowed for us to assess a range of medication doses on behavioral and imaging endpoints. Future studies should take this into consideration in the study design to reduce variability and replicate these results.
Although all imaging and behavioral assessments were performed after dopaminergic washout, we did not address chronic effects of dopamine medication to rCBF and performance in inhibitory control. Future pharmacological neuroimaging and cognitive studies may benefit from study designs that evaluate neuronal function and cognitive performance under the selective influence of dopamine-modifying medications and different time courses (acute vs chronic), and ideally, DAA naive PD patients could be imaged at baseline, and with acute DAA treatment and then followed longitudinally. Additional studies assessing acute DAA response in healthy controls may be useful. Future studies should also investigate rCBF changes during task performance, which would provide a better understanding on how rCBF varies with demands in control.
5. CONCLUSIONS
Our results suggest that DAA medication in PD patients induces rCBF changes in key areas involved in motor inhibition. Increased rCBF in the pre-SMA and DLPFC is linked to improved proficiency of selective inhibitory control over conflicting motor impulses, suggesting that targeted therapies designed to improve frontal cortical function may promote action control.
Supplementary Material
ACKNOWLEDGEMENTS
We offer our sincerest thanks to the volunteers who participated in this study. We would like to acknowledge Kristen Kanoff, Charis Spears, and Carlos Faraco, for their roles in data collection. We also thank Kristen George-Durrett, Leslie McIntosh, Clair Jones, and Christopher Thompson for assistance with the data acquisition.
FUNDING
This study was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01NS097783, K23NS080988); the American Heart Association (14GRNT20150004); and the Clinical and Translational Science Awards award No. UL1TR000445 from the National Center for Advancing Translational Science.
FINANCIAL DISCLOSURES
D.O.C. has received grant support from the National Institutes of Health (NINDS), Michael J. Fox Foundation, as well as AbbVie, Bristol-Myers Squibb, C2N, CHDI, Eli Lilly, Teva, Vaccinex, and Wave Pharmaceuticals. D.C. has received personal fees from AbbVie, Acadia, Huntington Study Group, Lundbeck, Neurocrine, Teva Neuroscience, outside of submitted work. M.J.D. receives research related support from Philips North America and is the CEO of biosight, LLC which provides healthcare and technology consulting services.
ABBREVIATIONS
- ACC
Anterior cingulate cortex
- AMNART
American version of the National Adult Reading Test (AMNART)
- ASL
Arterial spin labeling
- rCBF
Regional Cerebral blood flow
- CESD-R
Center for Epidemiologic Studies Depression Scale Revised
- DAA
Dopamine agonist
- DLPFC
Dorsolateral prefrontal cortex
- IFC
Inferior frontal cortex
- M1
Primary motor cortex
- MDS-UPDRS
Movement Disorders Society-United Parkinson’s Disease Rating Scale
- MoCA
Montreal Cognitive Assessment (MoCA)
- MPFC
Medial prefrontal cortex
- MRI
Magnetic resonance imaging
- PD
Parkinson’s disease
- PET
Positron emission tomography
- Pre-SMA
Pre-supplementary motor area
- QUIP-RS
Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale
- RT
Reaction times
- ROI
Region of interest
- SMA-p
Supplementary motor area proper
- STN
Subthalamic nucleus
- VLPFC
Ventrolateral prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986) Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annu Rev Neurosci 9:357–381 [DOI] [PubMed] [Google Scholar]
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S (2007) Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68:1515–1523 [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007) Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27:3743–3752 A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003) Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116 [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006) Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. J Neurosci 26:2424–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F (2008) Stop the presses: Dissociating a selective from a global mechanism for stopping: Research article. Psychol Sci 19:1146–1153 [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H (2009) Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clin Neurophysiol 120:1717–1723 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Stat Soc 57:289–300 [Google Scholar]
- Black KJ, Gado MH, Perlmutter JS (1997) PET measurement of dopamine D2 receptor-mediated changes in striatopallidal function. J Neurosci 17:3168–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KJ, Hershey T, Gado MH, Perlmutter JS, Koller JM, Campbell MC, Gusnard DA, Stephen I, Videen TO, Mintun MA, Price L, Hallett M (2011) Dopamine D 1 Agonist Activates Temporal Lobe Structures in Primates Dopamine D 1 Agonist Activates Temporal Lobe Structures in Primates. Society 84:549–557 [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS (2002) A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci USA 99:17113–17118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burle B, Possamaï CA, Vidal F, Bonnet M, Hasbroucq T (2002) Executive control in the Simon effect: An electromyographic and distributional analysis. Psychol Res 66:324–336 [DOI] [PubMed] [Google Scholar]
- Buxton RB (2005) Quantifying CBF with arterial spin labeling. J Magn Reson Imaging 22:723–726 [DOI] [PubMed] [Google Scholar]
- Carbonnell L, Ramdani C, Meckler C, Burle B, Hasbroucq T, Vidal F (2013) The N-40: An electrophysiological marker of response selection. Biol Psychol 93:231–236 [DOI] [PubMed] [Google Scholar]
- Chen Y, Pressman P, Simuni T, Parrish TB, Gitelman DR (2015) Effects of acute levodopa challenge on resting cerebral blood flow in Parkinson’s Disease patients assessed using pseudo-continuous arterial spin labeling. PeerJ 3:e1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S (2009) Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci 29:15870–15877 A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen DO, Stark AJ, Spears CA, Petersen KJ, van Wouwe N, Kessler R, Zald D, Donahue M (2017) Mesocorticolimbic hemodynamic response in Parkinson’s disease patients with compulsive behaviors. Mov Disord 32:1574–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen DO, van den Wildenberg WPMM, Harrison MB, van Wouwe NC, Kanoff K, Neimat JS, Wylie SA (2015) Proficient motor impulse control in Parkinson disease patients with impulsive and compulsive behaviors. Pharmacol Biochem Behav 129:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claffey MP, Sheldon S, Stinear CM, Verbruggen F, Aron AR (2010) Having a goal to stop action is associated with advance control of specific motor representations. Neuropsychologia 48:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, Gunn RN, Matthews PM, Rabiner EA, Beaver JD (2012) Orbitofrontal Connectivity with Resting-State Networks Is Associated with Midbrain Dopamine D3 Receptor Availability. Cereb Cortex 22:2784–2793 [DOI] [PubMed] [Google Scholar]
- Criaud M, Poisson A, Thobois S, Metereau E, Redouté J, Ibarrola D, Baraduc P, Broussolle E, Strafella AP, Ballanger B, Boulinguez P (2016) Slowness in movement initiation is associated with proactive inhibitory network dysfunction in Parkinson’s disease. J Parkinsons Dis 6:433–440 A [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Rane S, Hussey E, Mason E, Pradhan S, Waddell KW, Ally BA (2014) γ-Aminobutyric acid (GABA) concentration inversely correlates with basal perfusion in human occipital lobe. J Cereb Blood Flow Metab 34:532–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J-R, Ide JS, Luo X, Li C -s. R (2009) Functional Connectivity Delineates Distinct Roles of the Inferior Frontal Cortex and Presupplementary Motor Area in Stop Signal Inhibition. J Neurosci 29:10171–10179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J et al. (2018) Cerebral blood flow predicts differential neurotransmitter activity. Sci Rep 8:4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB (2012) Dissociating the Role of Prefrontal and Premotor Cortices in Controlling Inhibitory Mechanisms during Motor Preparation. J Neurosci 32:806–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini G, Juncos J, Mouradian MM, Serrati C, Chase TN (1987) Levodopa pharmacokinetic mechanisms and motor fluctuations in Parkinson’s disease. Ann Neurol 21:370–376 [DOI] [PubMed] [Google Scholar]
- Fernández-Seara MA, Aznárez-Sanado M, Mengual E, Irigoyen J, Heukamp F, Pastor MA (2011) Effects on resting cerebral blood flow and functional connectivity induced by metoclopramide: a perfusion MRI study in healthy volunteers. Br J Pharmacol 163:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Seara MA, Mengual E, Vidorreta M, Aznárez-Sanado M, Loayza FR, Villagra F, Irigoyen J, Pastor MA (2012) Cortical hypoperfusion in Parkinson’s disease assessed using arterial spin labeled perfusion MRI. Neuroimage 59:2743–2750 [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers E-J (2008a) Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci 105:17538–17542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WPM, Ridderinkhof KR (2008b) Function and Structure of the Right Inferior Frontal Cortex Predict Individual Differences in Response Inhibition: A Model-Based Approach. J Neurosci 28:9790–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ (2006) Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Networks 19:1120–1136 [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG et al. (2007) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord 22:41–47 [DOI] [PubMed] [Google Scholar]
- Gross G, Wicke K, Drescher KU (2013) Dopamine D3 receptor antagonism—still a therapeutic option for the treatment of schizophrenia. Naunyn Schmiedebergs Arch Pharmacol 386:155–166 [DOI] [PubMed] [Google Scholar]
- Handley R, Zelaya FO, Reinders AATS, Marques TR, Mehta MA, O’Gorman R, Alsop DC, Taylor H, Johnston A, Williams S, McGuire P, Pariante CM, Kapur S, Dazzan P (2013) Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp 34:272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Carl JL, Perlmutter JS (2000) Dopa-Induced Blood Flow Responses in Nonhuman Primates. Exp Neurol 166:342–349 [DOI] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Løkkegaard A, Siebner HR (2014) Functional neuroimaging of motor control in parkinson’s disease: A meta-analysis. Hum Brain Mapp 35:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Rothwell JC, Obeso JA (2015) A fronto–striato–subthalamic–pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci 16:719–732 [DOI] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR (2010) Responding with Restraint: What Are the Neurocognitive Mechanisms? J Cogn Neurosci 22:1479–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WPM, Scholte HS, Ridderinkhof KR, Forstmann BU (2011) Effective Connectivity Reveals Important Roles for Both the Hyperdirect (Fronto-Subthalamic) and the Indirect (Fronto-Striatal-Pallidal) Fronto-Basal Ganglia Pathways during Response Inhibition. J Neurosci 31:6891–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Johansen-Berg H, Behrens TEJ, Behrens TEJ, Robson MD, Robson MD, Drobnjak I, Drobnjak I, Rushworth MFS, Rushworth MFS, Brady JM, Brady JM, Smith SM, Smith SM, Higham DJ, Higham DJ, Matthews PM, Matthews PM (2004) Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101:13335–13340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobari M, Fukuuchi Y, Shinohara T, Obara K, Nogawa S (1995) Levodopa-induced local cerebral blood flow changes in Parkinson’s disease and related disorders. J Neurol Sci 128:212–218 [DOI] [PubMed] [Google Scholar]
- Lu H, Donahue MJ, van Zijl PCM (2006) Detrimental effects of BOLD signal in arterial spin labeling fMRI at high field strength. Magn Reson Med 56:546–552 [DOI] [PubMed] [Google Scholar]
- Luce RD (1986) Response times : their role in inferring elementary mental organization. Oxford University Press. [Google Scholar]
- Mars RB, Klein MC, Neubert F-X, Olivier E, Buch ER, Boorman ED, Rushworth MFS (2009) Short-Latency Influence of Medial Frontal Cortex on Primary Motor Cortex during Action Selection under Conflict. J Neurosci 29:6926–6931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch J, Edvisson L (1980) Cerebral circulatory and metabolic effects of piribedil. Eur J Pharmacol 66:327–337 [DOI] [PubMed] [Google Scholar]
- McCulloch J, Kelly PAT, Ford I (1982) Effect of apomorphine on the relationship between local cerebral glucose utilization and local cerebral blood flow (with an appendix on its statistical analysis). J Cereb Blood Flow Metab 2:487–499 [DOI] [PubMed] [Google Scholar]
- Mierau J, Schneider FJ, Ensinger HA, Chio CL, Lajiness ME, Huff RM (1995) Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors. Eur J Pharmacol 290:29–36 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin J-A, Newman-Tancredi A (2002) Differential Actions of Antiparkinson Agents at Multiple Classes of Monoaminergic Receptor. I. A Multivariate Analysis of the Binding Profiles of 14 Drugs at 21 Native and Cloned Human Receptor Subtypes. J Pharmacol Exp Ther 303:791–804 [DOI] [PubMed] [Google Scholar]
- Mink J (1996) The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425 A [DOI] [PubMed] [Google Scholar]
- Mu Q, Johnson K, Morgan PS, Grenesko EL, Molnar CE, Anderson B, Nahas Z, Kozel FA, Kose S, Knable M, Fernandes P, Nichols DE, Mailman RB, George MS (2007) A single 20 mg dose of the full D1 dopamine agonist dihydrexidine (DAR-0100) increases prefrontal perfusion in schizophrenia. Schizophr Res 94:332–341 A [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN (1994) Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA 91:11271–11275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, Mulsant B, Pollock B, Graff-Guerrero A (2013) The potential role of dopamine D3 receptor neurotransmission in cognition. Eur Neuropsychopharmacol 23:799–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc 53:695–699 [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J (2007) Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7:1–17 [DOI] [PubMed] [Google Scholar]
- Neubert FX, Klein MC (2010) What Is Driving Inhibition-Related Activity in the Frontal Lobe? J Neurosci 30:4830–4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Audinot V, Nicolas J-P, De Ceuninck F, Boutin J-A, Millan MJ (2002) Differential Actions of Antiparkinson Agents at Multiple Classes of Monoaminergic Receptor. II. Agonist and Antagonist Properties at Subtypes of Dopamine D2-Like Receptor and alpha 1/alpha 2-Adrenoceptor. J Pharmacol Exp Ther 303:805–814 [DOI] [PubMed] [Google Scholar]
- Obeso I, Cho SS, Antonelli F, Houle S, Jahanshahi M, Ko JH, Strafella AP (2013) Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul 6:769–776 [DOI] [PubMed] [Google Scholar]
- Petersen K, Van Wouwe N, Stark A, Lin Y-C, Kang H, Trujillo-Diaz P, Kessler R, Zald D, Donahue MJ, Claassen DO (2018) Ventral striatal network connectivity reflects reward learning and behavior in patients with Parkinson’s disease. Hum Brain Mapp 39:509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC (2002) An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cogn Brain Res 13:427–440 [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996) Motor areas of the median wall: a review of their location and functional activation. Cereb Cortex 6:342–353 [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2003) Activation of the supplementary motor area (SMA) during performance of visually guided movements. Cereb Cortex 13:977–986. [DOI] [PubMed] [Google Scholar]
- Piercey MF (1998) Pharmacology of pramipexole, a dopamine D3-preferring agonist useful in treating Parkinson’s disease. Clin Neuropharmacol 21:141–151 [PubMed] [Google Scholar]
- Proctor RW, Miles JD, Baroni G (2011) Reaction time distribution analysis of spatial correspondence effects. [DOI] [PubMed]
- Radloff LS (1977) The CES-D Scale. Appl Psychol Meas 1:385–401 [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K (1999) The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89:1009–1023 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR (2002) Micro- and macro-adjustments of task set: Activation and suppression in conflict tasks. Psychol Res 66:312–323 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Scheres A, Oosterlaan J, Sergeant JA (2005) Delta Plots in the Study of Individual Differences: New Tools Reveal Response Inhibition Deficits in AD/HD That Are Eliminated by Methylphenidate Treatment. J Abnorm Psychol 114:197–215 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science (80- ) 306:443–447 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof RK, Forstmann BU, Wylie SA, Burle B, van den Wildenberg WPM (2011) Neurocognitive mechanisms of action control: Resisting the call of the Sirens. Wiley Interdiscip Rev Cogn Sci 2:174–192 [DOI] [PubMed] [Google Scholar]
- Scheller D, Ullmer C, Berkels R, Gwarek M, Lübbert H (2009) The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol 379:73–86 [DOI] [PubMed] [Google Scholar]
- Schouw MLJ, Kaag AM, Caan MWA, Heijtel DFR, Majoie CBLM, Nederveen AJ, Booij J, Reneman L (2013) Mapping the hemodynamic response in human subjects to a dopaminergic challenge with dextroamphetamine using ASL-based pharmacological MRI. Neuroimage 72:1–9 [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Levesque D, Martres MP, Sokoloff P (1993) Dopamine D3 receptor: basic and clinical aspects. Clin Neuropharmacol 16:295–314 [DOI] [PubMed] [Google Scholar]
- Simon JR (1969) Reactions toward the source of stimulation. J Exp Psychol 81:174–176 [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151 [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Andrieux M, Besancon R, Pilon C, Bouthenet ML, Souil E, Schwartz JC (1992) Localization and function of the D3 dopamine receptor. Arzneimittelforschung 42:224–230 [PubMed] [Google Scholar]
- Soutschek A, Taylor PCJ, Muller HJ, Schubert T (2013) Dissociable Networks Control Conflict during Perception and Response Selection: A Transcranial Magnetic Stimulation Study. J Neurosci 33:5647–5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AJ, Smith CT, Lin Y-C, Petersen KJ, Trujillo P, van Wouwe NC, Kang H, Donahue MJ, Kessler RM, Zald DH, Claassen DO (2018a) Nigrostriatal and Mesolimbic D2/3 Receptor Expression in Parkinson’s Disease Patients with Compulsive Reward-Driven Behaviors. J Neurosci 38:3230–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AJ, Smith CT, Petersen KJ, Trujillo P, van Wouwe NC, Donahue MJ, Kessler RM, Deutch AY, Zald DH, Claassen DO (2018b) [18F]fallypride characterization of striatal and extrastriatal D2/3 receptors in Parkinson’s disease. NeuroImage Clin 18:433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Coxon JP, Byblow WD (2009) Primary motor cortex and movement prevention: Where Stop meets Go. Neurosci Biobehav Rev 33:662–673 A [DOI] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR (2009) Intracranial EEG Reveals a Time- and Frequency-Specific Role for the Right Inferior Frontal Gyrus and Primary Motor Cortex in Stopping Initiated Responses. J Neurosci 29:12675–12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653 [DOI] [PubMed] [Google Scholar]
- Tompson D, Oliver-Willwong R (2009) Pharmacokinetic and pharmacodynamic comparison of ropinirole 24-hour prolonged release and ropinirole immediate release in patients with Parkinson’s disease. Clin Neuropharmacol 32:140–148 [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2001) Subprocesses of Performance Monitoring: A Dissociation of Error Processing and Response Competition Revealed by Event-Related fMRI and ERPs. Neuroimage 14:1387–1401 [DOI] [PubMed] [Google Scholar]
- van Belle J, Vink M, Durston S, Zandbelt BB (2014) Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. Neuroimage 103:65–74 [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WPMM, Wylie SA, Forstmann BU, Burle B, Hasbroucq T, Ridderinkhof KR (2010) To Head or to Heed? Beyond the Surface of Selective Action Inhibition: A Review. Front Hum Neurosci 4:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaal S, Scholte HS, Lamme VA, Fahrenfort JJ, Ridderinkhof KR (2011) Pre-SMA graymatter density predicts individual differences in action selection in the face of conscious and unconscious response conflict. J Cogn Neurosci 23:382–390 [DOI] [PubMed] [Google Scholar]
- van Wouwe NC, Kanoff KE, Claassen DO, Spears CA, Neimat J, van den Wildenberg WPM, Wylie SA (2016) Dissociable Effects of Dopamine on the Initial Capture and the Reactive Inhibition of Impulsive Actions in Parkinson’s Disease. J Cogn Neurosci 28:710–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani F, Lacerda L, Martino J, Attems J, Morris C, Mitchell P, De Schotten MT, Dell’Acqua F (2014) White matter connections of the supplementary motor area in humans. J Neurol Neurosurg Psychiatry 85:1377–1385. [DOI] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF (2005) Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp 25:336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend C, Gerrits NJHM, Berendse HW, Veltman DJ, van den Heuvel OA, van der Werf YD (2015) Failure of stop and go in de novo Parkinson’s disease--a functional magnetic resonance imaging study. Neurobiol Aging 36:470–475 [DOI] [PubMed] [Google Scholar]
- Wijnen JG, Ridderinkhof KR (2007) Response inhibition in motor and oculomotor conflict tasks: Different mechanisms, different dynamics? Brain Cogn 63:260–270 [DOI] [PubMed] [Google Scholar]
- Wolk DA, Detre JA (2012) Arterial spin labeling Mri: an emerging biomarker for Alzheimer’. Curr Opin Neurol 25:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M, Dubois V, Scheller D, Gillard M (2015) Rotigotine is a potent agonist at dopamine D1 receptors as well as at dopamine D2 and D3 receptors. Br J Pharmacol 172:1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ (2006) Jumping the gun: Is effective preparation contingent upon anticipatory activation in task-relevant neural circuitry? Cereb Cortex 16:394–404 [DOI] [PubMed] [Google Scholar]
- Wylie SA, Claassen DO, Huizenga HM, Schewel KD, Ridderinkhof KR, Bashore TR, van den Wildenberg WPM (2012) Dopamine agonists and the suppression of impulsive motor actions in Parkinson disease. J Cogn Neurosci 24:1709–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Bashore TR, van den Wildenberg WPM (2010a) The effect of Parkinson’s disease on the dynamics of on-line and proactive cognitive control during action selection. J Cogn Neurosci 22:2058–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Eckerle MK, Manning CA (2007) Inefficient response inhibition in individuals with mild cognitive impairment. Neuropsychologia 45:1408–1419 [DOI] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WPM (2010b) Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson’s disease. Brain 133:3611–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WPM, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, Wooten GF (2009) The effect of speed-accuracy strategy on response interference control in Parkinson’s disease. Neuropsychologia 47:1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XQ, Glizer D, Vo A, Seergobin KN, MacDonald PA (2016) Pramipexole Increases Go Timeouts but Not No-go Errors in Healthy Volunteers. Front Hum Neurosci 10:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XQ, Lauzon B, Seergobin KN, MacDonald PA (2018) Dopaminergic Therapy Increases Go Timeouts in the Go/No-Go Task in Patients with Parkinson’s Disease. Front Hum Neurosci 11:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, Huddleston C, Rae CL, Regenthal R, Sahakian BJ, Barker RA, Robbins TW, Rowe JB (2014) Selective serotonin reuptake inhibition modulates response inhibition in Parkinson’s disease. Brain 137:1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson J a, Cohen JD (2006) Between-task competition and cognitive control in task switching. J Neurosci 26:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M (2010) On the role of the striatum in response inhibition Rodriguez-Fornells A, ed. PLoS One 5:e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.