Abstract
AS101 is an organotellurium compound with multifaceted immunoregulatory properties that is remarkable for its lack of toxicity. We tested the therapeutic effect of AS101 in experimental autoimmune uveitis (EAU), a model for human autoimmune uveitis. Unexpectedly, treatment with AS101 elicited Treg generation in vivo in otherwise unmanipulated mice. Mice immunized for EAU with the retinal antigen IRBP and treated with AS101 developed attenuated disease, as did AS101-treated recipients of retina-specific T cells activated in vitro. In both settings, eye-infiltrating effector T cells were decreased, whereas regulatory T (Treg) cells in the spleen were increased. Mechanistic studies in vitro revealed that AS101 restricted polarization of retina-specific T cells towards Th1 or Th17 lineage by repressing activation of their respective lineage-specific transcription factors and downstream signals. Retina-specific T cells polarized in vitro towards Th1 or Th17 in the presence of AS101 had impaired ability to induce EAU in naïve recipients. Finally, AS101 promoted differentiation of retina-specific T cells to Tregs in vitro independently of TGF-β. We conclude that AS101 modulates autoimmune T cells by inhibiting acquisition and expression of effector function and by promoting Treg generation, and suggest that AS101 could be useful as a therapeutic approach for autoimmune uveitis.
Keywords: AS101, Regulatory T cell, Th17 cell, Experimental autoimmune uveitis
1. Introduction
The ammonium trichloro(dioxoethylene-O,O') tellurate compound AS101 is a small non-toxic, immunomodulator that has multiple anti-cancer, anti-inflammatory and anti-apoptotic properties [1-4]. Because of its relative lack of toxicity, AS101 has been tested for its beneficial effects in diverse preclinical and clinical studies. Thus far it has demonstrated efficacy orally in AMD and topically in HPV and Psoriasis [5, 6] and in several autoimmune disease models [4, 7-12].
The diverse immunomodulatory functions of AS101 are mediated by its TeIV redox-modulating activities enabling, among other effects, the inactivation of cysteine proteases such as cathepsin B and inhibition of thiol-dependent integrins αvβ3, α4β1 (VLA4) and α4β7 [13-16]. Its diverse functional effects include sensitization of tumor cells to the effects of chemotherapy, inhibition of cell migration and entry into tissues, suppression of Th17 cells and enhancement of regulatory T cells (Treg) [3, 4]. The effects which predominate depend on the disease condition under study.
In experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, treatment with AS101 ameliorated disease by downregulating inflammatory cytokines in the central nervous system (CNS) [10, 12]. In a model of inflammatory bowel disease (IBD), AS101 treatment not only downregulated inflammatory cytokines, but also increased regulatory T cells (Tregs) in colonic lamina propria [8]. AS101 has also been shown to prevent type 2 diabetes (T2D) or a diabetic nephropathy in rat models [7, 11] and our recent work indicates that it can also ameliorate T1D in NOD mice, in part through induction of Tregs (manuscript in revision). These experimental findings strongly support beneficial effects of AS101 in several inflammatory and autoimmune disease models. However, due to the multifaceted, and potentially opposing, biological effects of AS101 that involve cell activation, migration and survival, outcomes in specific disease situations are difficult to predict and must be individually tested and optimized.
Autoimmune uveitis is an intraocular noninfectious inflammatory disease affecting the uvea and retina, which is sight-threatening if not successfully treated. The gradual irreversible damage of photoreceptors caused by autoreactive T cells and recruited leukocytes that secrete inflammatory cytokines eventually leads to visual deficit and blindness [17]. Various immunosuppressive drugs are now approved for the treatment of a wide spectrum of uveitis, but they potentially have serious systemic side effects during long-term treatment [18-20]. Thus, there is a need for development of novel and less toxic medications to treat autoimmune uveitis.
Experimental autoimmune uveitis (EAU) is an animal model of human uveitis and has been widely used as a template for preclinical studies [21]. EAU can be induced by immunization of mice with retinal proteins or their peptides, or by adoptive transfer of activated retina-specific T cells into naïve recipients [22, 23]. Acute disease starts with infiltration of inflammatory cells into the retina [24], recruited by autoreactive retina-specific T cells secreting IFN-γ (Th1) or IL-17A (Th17) that penetrate the blood-retinal barrier and elicit an amplification loop that culminates in inflammation and tissue damage [25, 26]. Th1 and Th17 cells are both pathogenic T cell subsets involved in disease progression [27-30]. The disease is controlled by Tregs which are induced in the periphery, and in part may also arise within the eye [31-33]. Thus, strategies that inhibit transcription factors and signaling pathways utilized by autoreactive Th1 and Th17 cells, or that promote the generation and expansion of cell types with immunosuppressive properties, such as Foxp3+ regulatory T cells (Tregs) would be expected to suppress disease.
In the present study, we observed that systemic administration of AS101 to naïve mice efficiently induced circulating Tregs in vivo. We therefore set out to examine the therapeutic efficacy of AS101 in autoimmune uveitis models and to study the mechanism. We report that treatment with AS101 attenuates EAU by reducing Th1 and Th17 effector cells and by increasing Tregs in the periphery. Mechanistic studies revealed that AS101 restricts polarization of retina-specific autoreactive T cells towards Th1 or Th17 lineages by downregulating lineage-specific transcription factors, T-bet or RORγt, respectively, and that Foxp3+ Treg induction by AS101 is in part independent of, and synergizes with, TGF-β. These data suggest that AS101 directly modulates the immune responses in the setting of autoimmunity and has clinical potential as a therapeutic approach to human autoimmune uveitis.
2. Materials and Methods
2.1. Mice
B10.RIII mice (Stock No. 000457) were obtained from Jackson Laboratories (Bar Harbor, ME). Foxp3-GFP reporter mice obtained from Dr. A. Rudensky (Sloan-Kettering Cancer Center, New York, NY) and CD90.1 congenic mice (Jax) were backcrossed onto the B10.RIII background for >10 generations. IRBP-specific TCR transgenic (R161H) mice were generated on the B10.RIII background in our laboratory as previously described [34] and backcrossed onto CD90.1 congenic background for>10 generations. All mice were kept under a specific pathogen free facility. Animal care and the animal study protocols were approved by the Animal Care and Use Committees of the National Eye Institute (protocol # NEI-581)
2.2. Flow cytometry
Cells were incubated with Fc block (clone 2.4G2, BioXcell) and stained with following antibodies from BioLegend, eBioscience or BD Biosciences: anti-CD3 (clone 145-2C11), anti-CD4 (RM4.5), anti-CD25 (PC61.5), anti-CD45 (30-F11), anti-CD90.1 (OX-7) and anti-CD90.2 (53-2.1). For intracellular cytokine staining, cells were pulsed for 4 h with PMA (50 ng/ml), ionomycin (500 ng/ml), and Brefeldin A (GolgiPlug, BD), followed by 4% paraformaldehyde fixation and 0.05% Triton X-100 permeabilization before intracellular cytokine staining for T-bet (clone 4B10), RORγt (AFKJS-9), IFN-γ (XMG1.2) and IL-17A (TC11-18H10.1). For Foxp3 (FJK-16s) staining, eBioscience Foxp3 transcription factor staining kit was used according to the manufacturer’s protocol. For pSTAT3 (pY705), pSTAT4 (pY693), pAKT (pS473) staining, cells were fixed with Cytofix and permeabilized with Perm III buffer (BD Biosciences) according to manufacturer's instructions. Stained cells were collected on a MACSQuant analyzer (Miltenyi Biotec). Data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
2.3. AS101 treatment and detection of Foxp3-GFP positive cells
Naïve Foxp3-GFP reporter B10.RIII mice were injected with AS101 (27 μg in 200 μl PBS) intraperitoneally for 14 consecutive days. On day 3, 7, 10 and 14, blood samples were obtained from lateral tail vein, and red blood cells were lysed by ACK buffer (Thermo Fisher Scientific). After washing with PBS plus 2% FBS buffer, GFP-positive CD4+ T cells were detected by flow cytometry. Fold increase in Foxp3-GFP expression of CD4+ in blood at each time point was calculated as [% of GFP positive at each time point]/[% of GFP positive at day 0].
2.4. T cell polarization
Cells isolated from lymph nodes (LN) of R161H mice were cultured (2 million cells/ml) in RPMI+10% fetal bovine serum (FBS) + IRBP161-180 (2 μg/ml). Some cultures contained graded doses of AS101, as specified (0.1– 3 μg/ml). Cytokines and neutralizing antibodies for the desired polarization conditions were as follows. Th17: anti-IFN-γ (10 μg/ml; R4-6A2), anti-IL-4 (10 μg/ml; 11B11), TGF-β (2.5 ng/ml), IL-6 (25 ng/ml), and IL-23 (10 ng/ml); Th1: IFN-γ (10 ng/ml) and IL-12 (10 ng/ml); Treg: TGF-β (5 ng/ml). For testing TGF-β-independent Treg induction, exogenous TGF-β was omitted, and instead, cultures were supplemented with anti-TGF-β (1D11, 10 μg/ml) to neutralize any TGF-β present in FBS. For serum-free condition, CD hybridoma medium (Thermo Fisher Scientific) supplemented with L-glutamine (2 mM) and penicillin/streptomycin (100 U/ml), was used.
2.5. Induction of EAU and treatment with AS101
B10.RIII WT mice were immunized for EAU by subcutaneous injections with 5 μg of IRBP161-180 peptide (SGIPYIISYLHPGNTILHVD) (Anaspec, Fremont, CA) emulsified in an equal volume of Complete Freud’s Adjuvant containing 2.5 mg/ml Mycobacterium tuberculosis strain 37RA (Sigma-Aldrich, St. Louis, MO). Mice were treated with 27 μg of AS101 (or PBS as control) in 200 μl as (i.p.) injections starting two days before immunization. Experiments were harvested and tissues collected for analysis at the peak of disease (between day 14 and 16).
For induction of EAU by adoptive transfer of AS101-treated retina-specific T cells, LN cells from R161H mice were activated with 2 μg/ml IRBP161-180 under the polarization conditions towards Th1 or Th17 cells, as described above, for 3 days in the presence of PBS or AS101 (1 μg/ml) in RPMI+10%FBS media. β-Mercaptoethanol (ME) was omitted from these cultures because it reacts with AS101 and results in toxicity. After purification of live cells by centrifugation over Lympholyte-M (Cedarlane, NC), donor Th1 (1 million/mouse) or Th17 (3 million/mouse) cells were adoptively transferred into congenic B10.RIII recipient mice. For the adoptive transfer experiments with in vivo AS101 treatment, LN cells from R161H mice were activated with 2 μg/ml IRBP161-180 under Th0, Th1 or Th17 conditions for 3 days in DMEM (high glucose) +10%FBS media. Purified live Th0 (10 million/mouse, i.v.), Th1 (2 million/mouse, i.p.) or Th17 (5 million/mouse, i.p.) cells were transferred into B10.RIII congenic mice. The optimal cell numbers to induce disease with 100% incidence were determined by preliminary experiments. Starting from the day of adoptive transfer, the recipient mice were given daily i.p. injections of either AS101 (27 μg/mouse) or PBS for control.
2.6. Assessment of EAU by fundoscopy and histology
For clinical scoring EAU, mice were anesthetized by i.p. injections of ketamine/xylazine and their eyes were evaluated by fundoscopy on a scale of 0–4 according to the following criteria: 0 = no inflammation; 0.5 = trace disease with minimal vasculitis; 1 = mild and active disease with a few retinal folds; 2 = moderately active disease with multiple lesions; 3 = active disease with multiple diffuse lesions and hemorrhages; and 4 = very active inflammation, often with hemorrhage, retinal detachment or atrophy. The precise criteria for scoring and example photographs can be found elsewhere [22]. For histology, eyes were enucleated and immediately fixed for 1 hr. in 4% phosphate-buffered glutaraldehyde and transferred into 10% phosphate-buffered formaldehyde until processing. Fixed and dehydrated tissue was embedded in methacrylate. Sections (4-7 microns) were cut through the pupillary-optic nerve plane and were stained with hematoxylin and eosin. Severity of EAU was scored on a scale of 0–4 using previously published criteria, based on the number, type, and size of lesions[23]. Both fundoscopy and histology score were evaluated by masked observers who were not given information about experimental design or group identity. The same highly experienced investigators score experiments of the same type to keep consistency and comparability among consecutive experiments.
2.7. Isolation of eye-infiltrating cells
Eyes were enucleated and external tissues and lenses were removed. The remaining tissue was minced in HL-1 media (Lonza, Walkersville, MD) and incubated with 1 mg/ml collagenase D treatment (Roche, Indianapolis, IN) for 45 min at 37°C. Cells were then dispersed by trituration, washed, filtered, and resuspended in RPMI + 10% FBS.
2.8. Statistical analysis
Student’s t test (parametric data) or Mann-Whitney U test (non-parametric data) was used for two-group comparisons. A p-value of < 0.05 was considered statistically significant. Data are displayed as mean ± SEM. Experiments were repeated at least twice, and usually three or more times.
3. Results
3.1. AS101 treatment increases the frequency of regulatory T cells in naïve mice
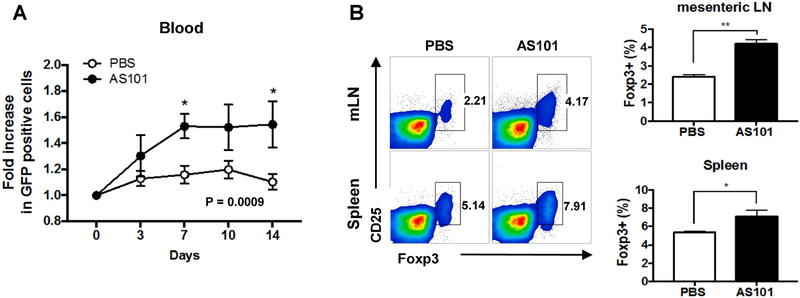
Tregs play a pivotal role in maintaining immune homeostasis and preventing autoimmunity. To examine whether AS101 treatment in naïve mice alters circulating Treg populations in vivo, we intraperitoneally (i.p.) injected AS101 into naïve Foxp3-GFP reporter mice for 14 consecutive days and measured GFP-expressing cells in blood every 3-4 days. As shown in Figure 1A, significant increases of GFP-expressing cells in the blood were detected in AS101-treated Foxp3-GFP reporter mice on day 7 and 14. The increase of Treg cells by AS101 treatment was also confirmed as early as day 5 in the spleen and mesenteric LN, which are the organs immediately exposed to AS101 upon injection (Fig. 1B). However, such increase of Treg cells by AS101 was not observed in the peripheral LN or even in the blood on day 5 (data not shown). These results indicate that prolonged treatment with AS101 increases frequencies of Tregs in nearby lymphoid organs and in circulation.
Fig. 1. Administration of AS101 increases Treg cells in naïve mice.
Naïve Foxp3-GFP reporter mice on the B10.RIII background were injected AS101 (27 μg, i.p.) for 14 consecutive days. (A) On days 3, 7, 10 and 14, blood samples were obtained from lateral tail vein, and GFP-positive cells were detected by flow cytometry. Fold increase in Foxp3-GFP positive cells was calculated as [% of GFP positive at each time point] / [% of GFP positive at day 0]. (B) The percentages of Foxp3+ CD4+ cells in spleen, mesenteric LN (mLN) were assessed by flow cytometry on day 5. Representative data from 2 independent experiments. *p<0.05, ** p<0.01 vs. PBS group.
3.2. AS101 treatment alters effector and regulatory T cell balance and suppresses EAU
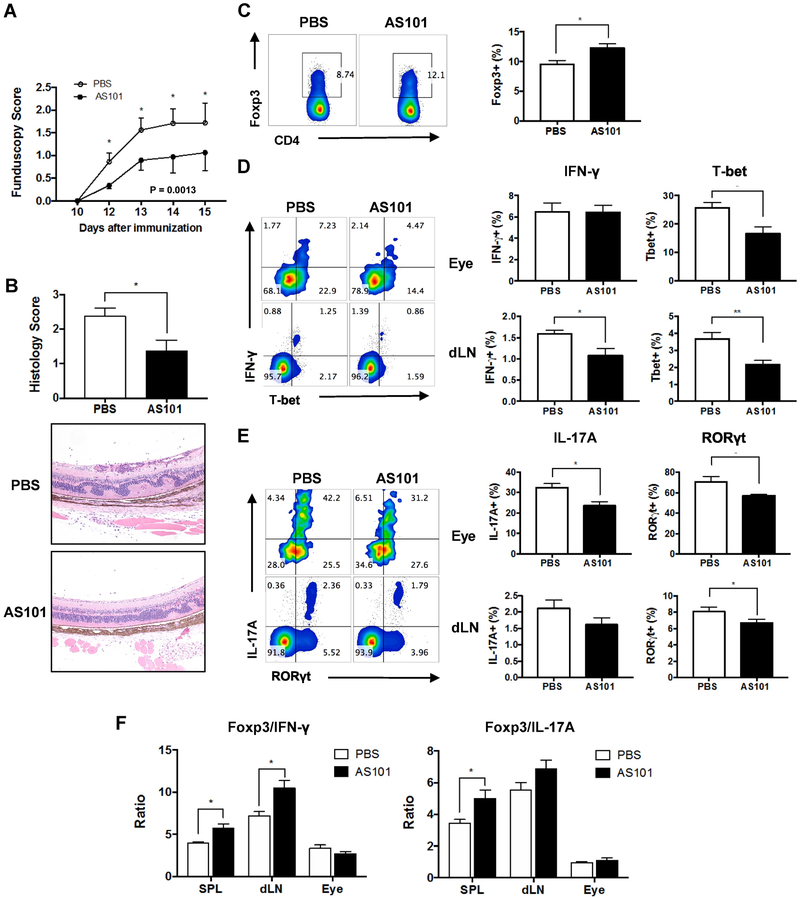
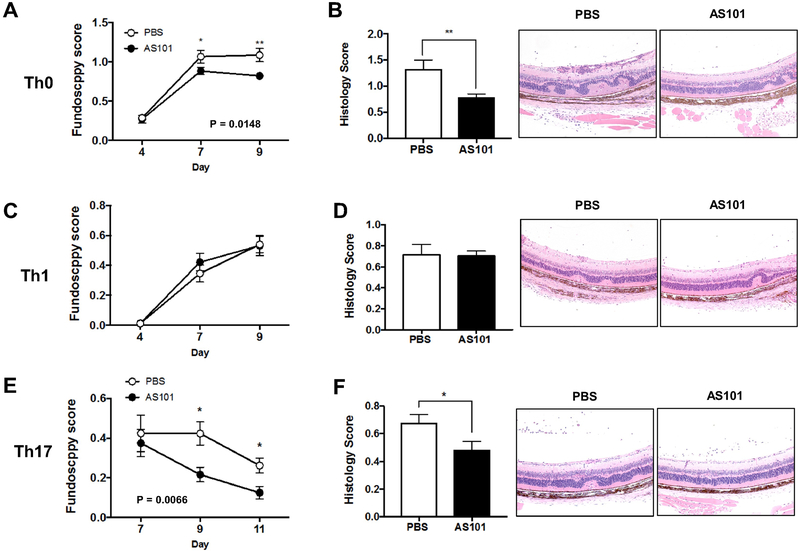
The above finding that higher proportions of Tregs were induced systemically in AS101-treated naïve mice prompted us to investigate the therapeutic ability of AS101 to prevent autoimmune uveitis. We immunized groups of B10.RIII mice with IRBP161-180 and treated them daily with either AS101 or PBS, as the vehicle control. Disease was first observed on day 12 after immunization in both groups, but the progression of EAU was significantly reduced in mice treated with AS101 from day 12 to day 15, as determined by fundus examination (Fig. 2A). Histopathological analysis confirmed that AS101-treated mice had milder disease compared to PBS-treated mice with fewer cellular infiltrates and photoreceptor lesions (Fig. 2B). To address how AS101 treatment may have shaped T cell immune responses and ameliorated EAU, we analyzed Tregs and effector T cells in the eye and LN (inguinal and iliac) draining the immunization sites for lineage-specific markers by intracellular flow cytometry. In AS101-treated mice, there was an increase of CD4+Foxp3+ Treg cells in the spleen (Fig 2C). T-bet and RORγt are the key transcription factors for the differentiation of IFN-γ-producing Th1 or IL-17A-producing Th17 cells, respectively, and were used to identify the corresponding lineages. Treatment of mice with AS101 reduced the frequency of eye-infiltrating CD4+ Th1 and Th17 cells during EAU (Fig. 2D, E). The frequencies of Th1 and Th17 cells were also decreased in the draining LNs of AS101-treated mice (Fig. 2D, E), whereas the ratio between Treg to Th1 or Treg to. Th17 was upregulated (Fig. 2F). These results suggest that systemic AS101 treatment changes the balance of effector and regulatory T cells in the periphery, resulting in suppression of ocular inflammation.
Fig. 2. AS101 treatment attenuates severity of EAU by reducing effector T cells and increasing Tregs.
EAU was induced in B10.RIII mice by active immunization with 5 μg of IRBP161-180 emulsified in CFA. AS101 (27 μg/mouse, i.p.) was administered daily from 2 days before immunization and treatment continued for the duration of the study. (A) Fundoscopy scores of immunized mice were recorded every 1-3 days after immunization. (B) Histology was performed on eyes collected on day 14. Magnification; X100. (C) The percentage of Foxp3+CD4+ cells in the spleen of immunized mice. Representative data from 3-4 independent experiments, and each group contains at least 5 mice. (D, E) Intracellular cytokine staining for Th1 (IFN-γ and T-bet, D) or Th17 (IL-17A and RORγt. E) markers in eye-infiltrating cells and draining LN (dLN) collected on day 14 and gated on live CD4+ cells after ex vivo stimulation with PMA/ionomycin and brefeldin A for 4 h. (F) Ratios of Tregs (Foxp3+) to Th1 (IFN-γ+) or Th17 (IL-17A+) were calculated. Data shown as mean ± SEM. Significance was determined using Mann-Whitney test (A, B), unpaired t test (C-E), or two-way ANOVA (F). *p<0.05, ** p<0.01 vs. PBS group.
3.3. AS101 inhibits pathways of Th1 and Th17 effector cell differentiation
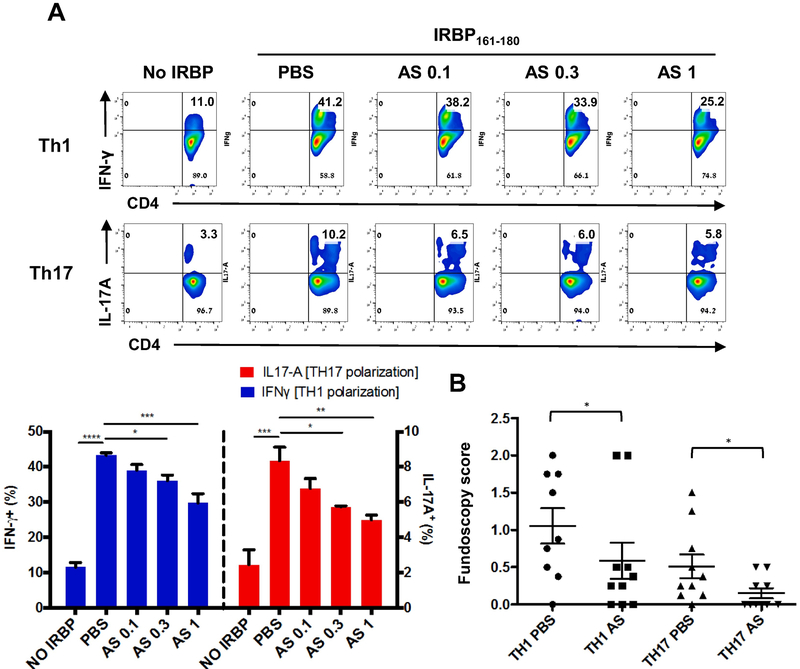
To address the mechanism(s) by which AS101 regulates Th1 and Th17 differentiation of retina-specific T cells, we examined the effects of AS101 on Th1 and Th17 cell differentiation in vitro. IRBP-specific CD4+ T cells were purified from LN of IRBP TCR transgenic (R161H) mice and stimulated with IRBP161-180 peptide under Th1- or Th17-polarizing conditions. Presence of AS101 in the culture inhibited generation of IFN-γ- or IL-17A-producing T cells under Th1 or Th17 conditions, respectively, in a dose-dependent manner (Fig. 3A). We then asked whether AS101 treatment during activation/polarization of R161H T cells affects the pathogenic potential of these autoreactive T cells when they are adoptively transferred into naïve recipient mice. R161H Th1 or Th17 effector cells activated with antigen under the respective polarization condition in the presence of AS101 were less pathogenic than parallel effector cells activated in the absence of AS101 (Fig. 3B). These results indicate that AS101 prevents antigen-specific T cells from becoming autopathogenic effector T cells.
Fig. 3. AS101 inhibits effector T cell polarization and acquisition of pathogenicity in vitro.
R161H LN cells were stimulated with 2 μg/ml IRBP161-180 under Th1 or Th17 polarizing conditions with PBS or various concentrations of AS101 (0.1, 0.3, or 1 μg/ml) for 72 h. (A) After polarization towards Th1 or Th17, the CD3+CD4+ population was assessed for IFN-γ and IL-17A expressing cells, respectively, by flow cytometry. Data shown as mean ± SEM from 3 independent experiments. *p<0.05; **p<0.01; ***p<0.001 vs. PBS. (B) After Th1 or Th17 polarization with PBS or 1 μg/ml of AS101, viable cells were harvested in Lympholyte M, and Th1 cells (1 million cells/mouse) or Th17 cells (3 million cells/mouse) were adoptively transferred i.p. into naïve B10.RIII recipient mice. Graph shows fundoscopy scores of eyes of B10.RIII recipient mice on day 10. Number of animals: Th1 PBS, n=9; Th1 AS, n=10; Th17 PBS, n=10; Th17 AS, n=10. Data shown as mean ± SEM from 3 independent experiments *p<0.05 vs. Th17 PBS group **p<0.01 vs. Th1 PBS group.
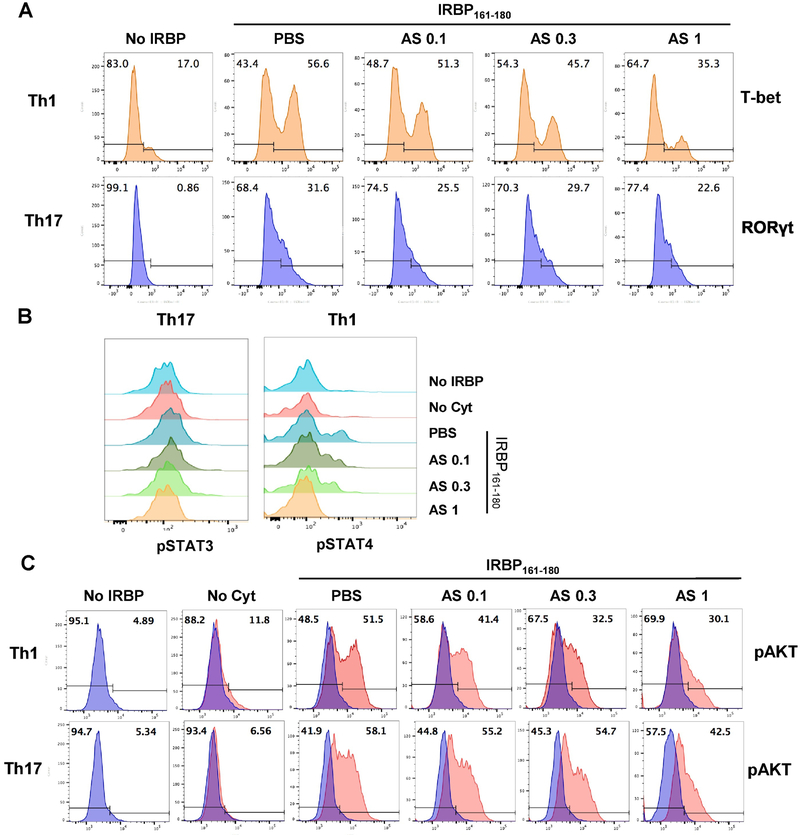
To further investigate the mechanism by which Th differentiation into pathogenic effectors was inhibited by AS101, we examined T-bet and RORγt expression levels in R161H T cells cultured under Th1- or Th17-polarizing conditions in the presence or absence of AS101. Expression levels of these lineage-specific transcription factors were decreased in R161H T cells when AS101 was present in culture (Fig. 4A). Since members of the STAT transcription factor family play a crucial role in the differentiation of Th cells, we examined whether the Jak/STAT intracellular signaling pathway was modulated by AS101. LN cells isolated from R161H mice were stimulated with IRBP161-180 in the presence or absence of AS101 for 72 h. Phosphorylation of STAT3 and STAT4 proteins was induced by antigen stimulation was attenuated in a dose-dependent fashion when AS101 was present in culture (Fig. 4B). Because phosphatidylinositol 3-kinase (PI3K)/Akt signaling has been shown to be important for Th17 differentiation [35, 36], we examined whether AS101 affects PI3K/Akt signaling. The Akt protein was markedly activated by antigen stimulation in R161H T cells under both Th1 and Th17 polarizing conditions, and this activation was effectively inhibited in the presence of AS101, again in a dose-dependent manner (Fig. 4C). Together, these results support the notion that AS101 regulates differentiation of pathogenic T cells by suppressing the expression of master lineage transcription factors and the phosphorylation of downstream signaling molecules.
Fig. 4. AS101 downregulates the expression of Th1 or Th17 lineage-specific transcription factors and the phosphorylation of downstream signaling molecules.
R161H LN cells were stimulated for 72 h with 2 μg/ml IRBP161-180 (IRBP) under Th1 or Th17 polarizing conditions in the absence (PBS) or presence of AS101 (0.1, 0.3, or 1 μg/ml). (A) After Th1 or Th17 polarization, CD3+CD4+ population was assessed for T-bet or RORγt expression, respectively, by flow cytometry. Results represent one of 3 independent experiments. (B) Th1- or Th17-polarized CD3+CD4+ population was assessed for pSTAT4 or pSTAT3, respectively. Results represent one of 2 independent experiments. (C) Th1- or Th17-polarized CD3+CD4+ population was assessed for pAKT expressing cells. No cytokine (Cyt) condition was used as a negative control. Results represent one of 2 independent experiments.
3.4. AS101 treatment in vivo suppresses EAU induction by Th0 or Th17, but not by Th1 cells
As demonstrated above, in vitro treatment with AS101 of retina specific T cells inhibited their lineage commitment and acquisition of pathogenicity (Fig. 3). Next, we explored whether AS101 treatment in vivo can dampen the pathology caused by already activated effector T cells polarized to Th1 or Th17, or non-polarized. To study effects on the effector phase of disease we used an adoptive transfer model, in which disease depends on already activated uveitogenic T cells, but before irreversible tissue destruction occurs that would obscure treatment effects. Recipients of non-polarized Th0 cells treated with vehicle developed severe EAU. In contrast, recipients treated daily with AS101 developed attenuated disease (Figure 5A and 5B). Histological analysis of eyes harvested at the end of the experiment confirmed the clinical observations (Fig. 5B). We next examined the effect of AS101 treatment on the disease induced by adoptively transferred Th1 or Th17 cells (Fig. 5C-F). AS101-treated recipient mice that had received polarized uveitogenic Th1 effector cells developed similar EAU to PBS-treated controls (Fig. 5C and 5D), whereas disease in AS101-treated recipients of Th17 effector cells was significantly ameliorated compared to corresponding PBS-treated controls (Fig. 5E and 5F). These results suggest that AS101 regulates the effector functions of Th1 and Th17 cells differently,
Fig. 5. AS101 suppresses EAU induced by IRBP-specific Th0 or Th17, but not Th1, effector T cells.
Cells were isolated from LN of CD90.1 R161H mice and stimulated with IRBP161-180 peptide under Th0, Th1 or Th17 conditions. After 3 days, viable cells were collected in Lympholyte M and adoptively transferred into CD90.2 B10.RIII WT mice. AS101 (27 ug, i.p.) was injected to the animals daily. (A) Disease was monitored by fundoscopy at days 4, 7 and 9 post-transfer of Th0 cells. Data pooled from 3 experiments. (B) Histology scores and representative images of H&E stained histology slides on day 10. (C) Fundoscopy scores of Th1 cell transferred mice. Data pooled from 6 experiments. (D) Histology scores and representative images of histology slides (H&E) on day 10. (E) Fundoscopy scores of Th17 cell transferred mice. Data pooled from 3 experiments. (F) Histology scores and representative images of histology slides (H&E) on day 11. Magnification; X100. Data shown as mean ± SEM. Significance was determined using Mann-Whitney test. *p<0.05, **p<0.01 vs. PBS group.
3.5. AS101 treatment enhances regulatory T cell generation and/or expansion during EAU
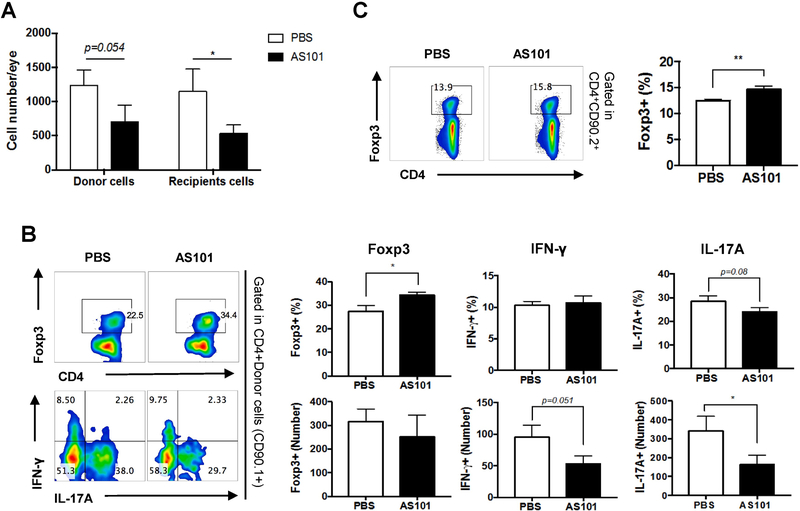
In our adoptive transfer experiments (Figure 5), we used congenic mice that allow to distinguish donor cells (CD90.1+) from recipient cells (CD90.2+). To understand how AS101 modulates the phenotype of the donor-derived effector T cells, as well as recipient-derived immune cells, we examined the numbers and frequencies of eye-infiltrating Th1/Th17/Treg cells on day 10 in the congenic recipient mice that had received Th0 effector cells (Fig. 5A). The absolute numbers of both donor and recipient cells were reduced in uveitic eyes of AS101-treated recipient mice (Fig. 6A). More importantly, AS101 treatment resulted in increased frequency of Foxp3+ Treg cells as well as fewer IFN-γ- and IL-17A-producing donor cells in the eye (Fig. 6B). Of note, frequencies and numbers of Foxp3+ Tregs, IFN-γ- or IL-17A-producing CD4+ T cells of recipient origin in the eyes were not affected by AS101 treatment (data not shown), but splenocytes of AS101-treated recipient mice contained higher proportions of recipient origin Foxp3+ Treg cells than those from PBS-treated recipient mice (Fig. 6C). These results are in line with the findings in naïve AS101 treated mice (Fig 1) as well as in immunization-induced EAU where AS101 treatment enhanced Foxp3+ Treg cells in the spleen (Fig. 2).
Fig. 6. AS101 treatment induces development and/or expansion of regulatory T cells and inhibits migration of IRBP-specific Th17 cells.
Cells were isolated from LNs of CD90.1 R161H mice and stimulated with IRBP161-180 peptide for 3 days (Th0 condition). After the removal of dead cells, 10 million cells were adoptively transferred into CD90.2 B10.RIII WT mice. (A) On day 10, the numbers of CD4+CD90.1+ (donor) and CD4+CD90.2+ (recipient) cells in infiltrating in the eyes of recipient mice were measured. (B) Foxp3, IFN-γ, and IL-17A producing donor cells (CD4+CD90.1+) in the eyes of recipient mice were assessed by flow cytometry after ex vivo stimulation with PMA/ionomycin and brefeldin A for 4 h. (C) The percentage of Foxp3+ cells among CD4+CD90.2+ gated T cells in the spleen of recipient mice. The means and S.E.M. from individual mice from one of four experiments (n=6/group) are shown. * p<0.05, ** p<0.01 Student’s t test.
3.6. AS101 treatment promotes induction of antigen-specific Tregs independently of TGF-β1
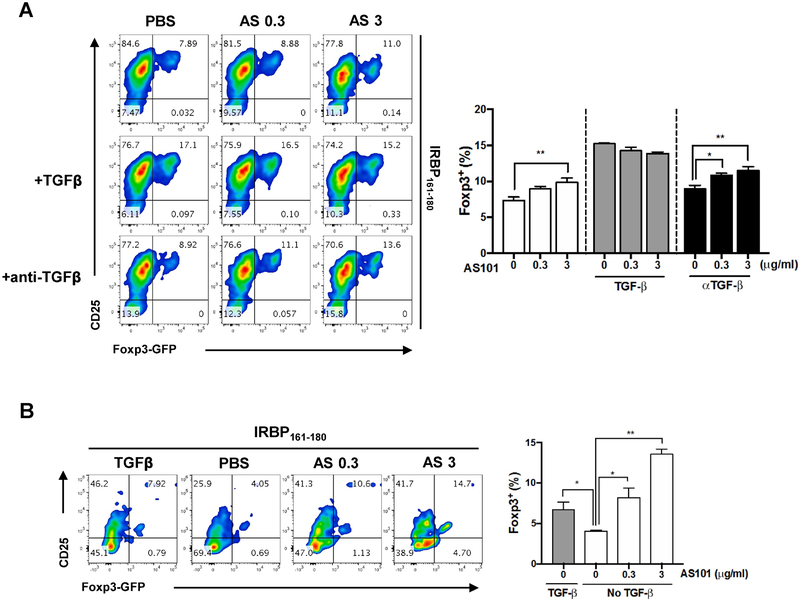
Our findings thus far point suggests that the therapeutic action of AS101 may depend, at least in part, on promoting the generation of polyclonal Tregs in vivo. To examine whether AS101 can support induction of retina-specific Tregs directly, and whether retina-specific T cells can also be differentiated to Tregs, LN cells from R161H mice were subjected to a Treg inducing protocol in vitro in the presence or absence of antigen IRBP161-180 and/or TGF-β1 and AS101 for 96 h. As might be expected TGF-β1 substantially enhanced the number of Foxp3+ T cells. Importantly, however, AS101 could substitute for TGF-β1 in the Treg polarization cultures. If TGF-β1 was omitted, Foxp3+ T cells were still enhanced by graded doses of AS101 in a dose dependent fashion, even when anti-TGF-β antibodies (10 μg/ml) were added to the polarization cultures to neutralize TGF-β that might be present in the serum supplement (Fig. 7A). To address the possibility that TGF-β might not have been neutralized entirely, we repeated the experiment in serum-free medium. Although activation of T cells cultured without serum was not as robust, the results confirmed that AS101 was able to promote T cell to Treg conversion in the absence of TGF-β (Fig. 7B). These results indicate that AS101, similarly to and independently of, TGF-β1, can enhance the differentiation of antigen-specific T cells to Tregs.
Fig. 7. AS101 promotes Treg differentiation independently of TGF-β.
LN cells from R161H-Foxp3-GFP mice were stimulated for 96 h with 2 μg/ml IRBP161-180 in presence of TGFβ1 (5 ng/ml), or in presence of anti-TGFβ (1D11, 10 μg/ml) and graded doses of AS101 in serum-containing (A) or serum-free (B) medium. After polarization, the CD3+CD4+ population was assessed for Foxp3-GFP and CD25 expression by flow cytometry. Results represent one of 2 independent experiments. Significance was determined using ANOVA test. *p<0.05, **p<0.01 vs. PBS group.
4. Discussion
In this study, we investigated the efficacy of a tellurium-based compound, AS101, as a treatment for EAU, a well-established model of human uveitis. Systemic administration of AS101 induced Tregs and downregulated the infiltration of inflammatory cells in the neuroretina in both immunization-induced EAU and adoptive transfer models by affecting differentiation and function of uveitogenic effector and of regulatory T cells, which are central players in elicitation and in control of uveitis. From a mechanistic point of view, much of the biological activity of AS101 is directly related to its chemical redox interactions with vicinal thiols in the exofacial domain of integrins causing their inactivation, and it has also been reported to inactivate cysteine proteases including Cathepsin B [13-16]. It is likely that the complex effects described here on effector and regulatory T cell activation and lineage commitment, combined with effects on integrin-dependent cell extravasation and migration (as reported by others), would reflect an integration of multiple activities, which together translate to attenuation of the disease process.
Lineage commitment to Th1 and Th17 cells involves phosphorylation of STAT4 and STAT3, respectively [37] and for Th17 also PI3K/Akt signaling [35, 36]. From in vitro culture data (Figs 3 and 4), AS101 was clearly able to inhibit commitment of T cells to the Th1 as well as Th17 lineage. This involved suppression of the expression of the master transcription factors for the respective lineages, T-bet for Th1 and RORγt for Th17, and the phosphorylation of downstream signaling molecules, STAT4 for Th1 and STAT3 and PI3K/Akt for Th17. On the other hand, AS101 promoted commitment to the Treg lineage not only by enhancing the effect of TGF-β1 on the process, but also by promoting conversion of T cells to Foxp3+ Tregs even in the absence of added TGF-β1. The downstream signaling events elicited by AS101 in the process of Treg induction remain to be elucidated.
Cytotoxic CD8+ T cells are also reported to play major roles as effectors of autoimmunity. In the EAU model, CD8+ T cells were thought to be either “suppressive” [38, 39] or “dispensable” because their depletion did not affect disease development [40]. However, more recent data suggested that CD8+T cells can also be pathogenic effectors in EAU [33, 41, 42]. It has also been reported that there are few of CD8+ Tregs that produce anti-inflammatory cytokines and have regulatory roles in the LN during EAU [43]. Although the role of CD8+ T cells was not examined in this study, our data do not exclude the possibility that AS101 can induce or upregulate CD8+ Tregs in the spleen (or periphery), similarly to its effect on CD4+ Tregs.
In vivo adoptive transfer studies and analysis of the cells isolated from the treated hosts demonstrated that AS101 is also able to affect lineage stability and function of Th0 and Th17 cells, but not of polarized Th1 cells (Fig 5). Th0 cells are still plastic and can transition to Th17 or Th1 cells. Th17 cells also have been described as having the plasticity to differentiate further, e.g., to Th1-like cells or to Tregs [44-46]. In fact, Hirota et al postulated that Th17 cells must differentiate to Th1-like cells to effect CNS pathology [45]. However, the Th1 phenotype appears to be more stable [44]. Together with the data discussed above, this raises the possibility that AS101 can affect T cells that are still in the process of differentiation and phenotype change, but not stably committed effectors such as Th1. Our data also do not exclude the possibility that AS101, through its action on integrins, also acts by affecting migration to the retina of effector T cells, and of recruited inflammatory leukocytes. This possibility is supported by the recent report in the EAE model where AS101 decreased VLA-4 (α4β1 integrin) activity on immune cells and prevented their migration into the CNS [10], resulting in reduction of inflammation associated IL-6, IL-1β and TNF-α levels and enhancement of anti-inflammatory IL-10 [10]. Other researchers showed that AS101 reduced the IL-17, IFN-γ, GM-CSF, and IL-6 mRNA expression in inflammatory cells from spinal cords in the EAE model [12]. Systemic treatment of AS101 consistently increased Treg in the spleen (Fig. 1B, Fig. 2C, and Fig. 6C), but not in the eye or the eye-draining LN (data not shown). The reason for this compartmentalization of the Treg response under AS101 treatment is unclear and will require further investigation. Treg induction by AS101 in vivo was also seen in an IBD model [8 and Yossipof et al., submitted for publication]. It appears from our results that AS101 drives a Treg response not only in disease but also in steady state in naïve mice and can promote Tregs also without dependence on TGF-β. Taken together, our current study expands and sheds new light on data from these previous reports and provides a mechanistic explanation for some of these observations.
Th17 cells have been implicated in the pathogenesis of human uveitis [47, 48]. The ability of AS101 to modulate disease induced by polarized Th17 cells (a disease reversal paradigm) coupled with the remarkable lack of toxicity of this compound, make this substance a promising therapeutic candidate. Notably, we recently found that a closely related tellurium compound, SAS, can mitigate inflammatory responses of human RPE cells by inhibiting αvβ3 and downregulating inflammatory cytokines [6]. Based on that, AS101 is currently being examined in clinical trials for AMD [49]. We believe that the activity if AS101 in reducing pathogenic Th1 and Th17 immune response and promoting Tregs differentiation during EAU suggest that should also be examined for clinical treatment of uveitis.
Highlights.
AS101 is a novel nontoxic immunomodulator with pleiotropic effects
AS101 promotes regulatory T cells in vivo and in vitro independently of TGF-β
AS101 suppresses Th1 and Th17 effector cell differentiation
Treatment with AS101 attenuates experimental autoimmune uveitis
Acknowledgements
The authors wish to thank the NEI Flow Cytometry Core Facility staff for assistance in conducting flow cytometric analyses and the NEI Histology Core staff for preparation of histological samples. Funding: United States–Israel Binational Science Foundation grant # 2013481 and NEI/NIH Intramural funding, project # EY000184.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brodsky M, Halpert G, Albeck M, Sredni B. The anti-inflammatory effects of the tellurium redox modulating compound, AS101, are associated with regulation of NFkappaB signaling pathway and nitric oxide induction in macrophages. Journal of inflammation (London, England), 2010;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brodsky M, Hirsh S, Albeck M, Sredni B. Resolution of inflammation-related apoptotic processes by the synthetic tellurium compound, AS101 following liver injury. Journal of hepatology, 2009;51:491–503. [DOI] [PubMed] [Google Scholar]
- [3].Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmunity reviews, 2014;13:1230–5. [DOI] [PubMed] [Google Scholar]
- [4].Sredni B Immunomodulating tellurium compounds as anti-cancer agents. Semin Cancer Biol, 2012;22:60–9. [DOI] [PubMed] [Google Scholar]
- [5].Friedman M, Bayer I, Letko I, Duvdevani R, Zavaro-Levy O, Ron B et al. Topical treatment for human papillomavirus-associated genital warts in humans with the novel tellurium immunomodulator AS101: assessment of its safety and efficacy. Br J Dermatol, 2009;160:403–8. [DOI] [PubMed] [Google Scholar]
- [6].Dardik R, Livnat T, Halpert G, Jawad S, Nisgav Y, Azar-Avivi S et al. The small tellurium-based compound SAS suppresses inflammation in human retinal pigment epithelium. Mol Vis, 2016;22:548–62. [PMC free article] [PubMed] [Google Scholar]
- [7].Halperin-Sheinfeld M, Gertler A, Okun E, Sredni B, Cohen HY. The Tellurium compound, AS101, increases SIRT1 level and activity and prevents type 2 diabetes. Aging (Albany NY), 2012;4:436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Halpert G, Eitan T, Voronov E, Apte RN, Rath-Wolfson L, Albeck M et al. Multifunctional activity of a small tellurium redox immunomodulator compound, AS101, on dextran sodium sulfate-induced murine colitis. J Biol Chem, 2014;289:17215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S et al. Cyclophosphamide triggers follicle activation and "burnout"; AS101 prevents follicle loss and preserves fertility. Sci Transl Med, 2013;5:185ra62. [DOI] [PubMed] [Google Scholar]
- [10].Lee JH, Halperin-Sheinfeld M, Baatar D, Mughal MR, Tae HJ, Kim JW et al. Tellurium compound AS101 ameliorates experimental autoimmune encephalomyelitis by VLA-4 inhibition and suppression of monocyte and T cell infiltration into the CNS. Neuromolecular Med, 2014;16:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shemesh II, Rozen-Zvi B, Kalechman Y, Gafter U, Sredni B. AS101 prevents diabetic nephropathy progression and mesangial cell dysfunction: regulation of the AKT downstream pathway. PLoS One, 2014;9:e114287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xie L, Chen J, McMickle A, Awar N, Nady S, Sredni B et al. The immunomodulator AS101 suppresses production of inflammatory cytokines and ameliorates the pathogenesis of experimental autoimmune encephalomyelitis. J Neuroimmunol, 2014;273:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yosef S, Brodsky M, Sredni B, Albeck A, Albeck M. Octa-O-bis-(R,R)-Tartarate Ditellurane (SAS) - a novel bioactive organotellurium(IV) compound: synthesis, characterization, and protease inhibitory activity. ChemMedChem, 2007;2:1601–6. [DOI] [PubMed] [Google Scholar]
- [14].Sredni B, Geffen-Aricha R, Duan W, Albeck M, Shalit F, Lander HM et al. Multifunctional tellurium molecule protects and restores dopaminergic neurons in Parkinson's disease models. FASEB J, 2007;21:1870–83. [DOI] [PubMed] [Google Scholar]
- [15].Kalechman Y, Longo DL, Catane R, Shani A, Albeck M, Sredni B. Synergistic anti-tumoral effect of paclitaxel (Taxol)+AS101 in a murine model of B16 melanoma: association with ras-dependent signal-transduction pathways. Int J Cancer, 2000;86:281–8. [DOI] [PubMed] [Google Scholar]
- [16].Layani-Bazar A, Skornick I, Berrebi A, Pauker MH, Noy E, Silberman A et al. Redox modulation of adjacent thiols in VLA-4 by AS101 converts myeloid leukemia cells from a drug-resistant to drug-sensitive state. Cancer Res, 2014;74:3092–103. [DOI] [PubMed] [Google Scholar]
- [17].Luger D, Caspi RR. New perspectives on effector mechanisms in uveitis. Semin Immunopathol, 2008;30:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dunn JP. Review of immunosuppressive drug therapy in uveitis. Curr Opin Ophthalmol, 2004;15:293–8. [DOI] [PubMed] [Google Scholar]
- [19].Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol, 2014;8:1891–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pras E, Neumann R, Zandman-Goddard G, Levy Y, Assia EI, Shoenfeld Y et al. Intraocular inflammation in autoimmune diseases. Semin Arthritis Rheum, 2004;34:602–9. [DOI] [PubMed] [Google Scholar]
- [21].Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol, 1988;140:1490–5. [PubMed] [Google Scholar]
- [22].Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol, 2003;Chapter 15:Unit 15 6. [DOI] [PubMed] [Google Scholar]
- [23].Caspi RR, Silver PB, Luger D, Tang J, Cortes LM, Pennesi G et al. Mouse models of experimental autoimmune uveitis. Ophthalmic Res, 2008;40:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu H, Koch P, Chen M, Lau A, Reid DM, Forrester JV. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res, 2008;87:319–26. [DOI] [PubMed] [Google Scholar]
- [25].Caspi RR. A look at autoimmunity and inflammation in the eye. The Journal of Clinical Investigation, 2010;120:3073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horai R, Caspi RR. Cytokines in autoimmune uveitis. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 2011;31:733–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. The Journal of Experimental Medicine, 2008;205:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ke Y, Liu K, Huang G-Q, Cui Y, Kaplan HJ, Shao H et al. Anti-Inflammatory Role of IL-17 in Experimental Autoimmune Uveitis. Journal of Immunology (Baltimore, Md : 1950), 2009;182:3183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang R, Qian J, Guo J, Yuan YF, Xue K. Suppression of experimental autoimmune uveoretinitis by Anti-IL-17 antibody. Current eye research, 2009;34:297–303. [DOI] [PubMed] [Google Scholar]
- [30].Skurkovich B, Skurkovich S. Anti-interferon-gamma antibodies in the treatment of autoimmune diseases. Current opinion in molecular therapeutics, 2003;5:52–7. [PubMed] [Google Scholar]
- [31].Caspi R Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res, 2008;42:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Silver PB, Horai R, Chen J, Jittayasothorn Y, Chan CC, Villasmil R et al. Retina-specific T regulatory cells bring about resolution and maintain remission of autoimmune uveitis. J Immunol, 2015;194:3011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McPherson SW, Heuss ND, Gregerson DS. Regulation of CD8(+) T Cell Responses to Retinal Antigen by Local FoxP3(+) Regulatory T Cells. Front Immunol, 2012;3:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Horai R, Silver PB, Chen J, Agarwal RK, Chong WP, Jittayasothorn Y et al. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun, 2013;44:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep, 2012;1:360–73. [DOI] [PubMed] [Google Scholar]
- [36].Nagai S, Kurebayashi Y, Koyasu S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann N Y Acad Sci, 2013;1280:30–4. [DOI] [PubMed] [Google Scholar]
- [37].Kitagishi Y, Kobayashi M, Yamashina Y, Matsuda S. Elucidating the regulation of T cell subsets (review). Int J Mol Med, 2012;30:1255–60. [DOI] [PubMed] [Google Scholar]
- [38].Caspi RR, Kuwabara T, Nussenblatt RB. Characterization of a suppressor cell line which downgrades experimental autoimmune uveoretinitis in the rat. J Immunol, 1988;140:2579–84. [PubMed] [Google Scholar]
- [39].McPherson SW, Yang J, Chan C-C, Dou C, Gregerson DS. Resting CD8 T cells recognize beta-galactosidase expressed in the immune-privileged retina and mediate autoimmune disease when activated. Immunology, 2003;110:386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Calder VL, Zhao ZS, Wang Y, Barton K, Lightman SL. Effects of CD8 depletion on retinal soluble antigen induced experimental autoimmune uveoretinitis. Immunology, 1993;79:255–62. [PMC free article] [PubMed] [Google Scholar]
- [41].Cortes LM, Mattapallil MJ, Silver PB, Donoso LA, Liou GI, Zhu W et al. Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice. Invest Ophthalmol Vis Sci, 2008;49:1946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Song L, Le J, Ye F, Shao H, Kaplan HJ, Sun D. Sequence 168 to 177 of interphotoreceptor retinoid-binding protein (IRBP) is an antigenic epitope for autoreactive CD8 T cells in the B10RIII mouse. Journal of neuroimmunology, 2008;193:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yu CR, Dambuza IM, Lee YJ, Frank GM, Egwuagu CE. STAT3 regulates proliferation and survival of CD8+ T cells: enhances effector responses to HSV-1 infection, and inhibits IL-10+ regulatory CD8+ T cells in autoimmune uveitis. Mediators of inflammation, 2013;2013:359674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol, 2008;181:7205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol, 2011;12:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature, 2015;523:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med, 2007;13:711–8. [DOI] [PubMed] [Google Scholar]
- [48].Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H et al. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Invest Ophthalmol Vis Sci, 2008;49:3058–64. [DOI] [PubMed] [Google Scholar]
- [49].https://clinicaltrials.gov/ct2/show/NCT03216538.