1. INTRODUCTION
Prostate cancer is the most common cancer diagnosis made in men today, and while surgery, radiation therapy, and expectant management strategies boast encouraging survival outlooks (with the 10 year risk of death from prostate cancer ranging from 3-18%, depending on risk group), therapeutic advances are still needed, especially for metastatic and hormone refractory patients (1, 2). Accordingly, there is hope that immunotherapy could fill this space—to be an effective therapeutic option for castrate resistant disease—or even become an earlier option, perhaps mitigating the undesired side effects of currently employed therapies. Here, we will review the successes and failures of using immunotherapy to treat prostate cancer. We discuss the mechanisms associated with immunotherapy response in other cancers and why prostate cancer may be more refractory to this approach.
2. LESSONS AND SUCCESSES IN PROSTATE CANCER IMMUNOTHERAPY
Since the FDA approval of Sipuleucel-T for prostate cancer and of immune checkpoint therapies for melanoma, trials of immunotherapies have proliferated across a multitude of tumor types. The greatest success of immune checkpoint therapies was found in densely immune-infiltrated ‘hot’ tumors, such as melanoma (3, 4). However, the trials conducted in immunologically ‘cold’ (i.e. poorly immune infiltrated) tumors, such as prostate cancer, did not match the impressive success of these treatments in other tumor types (5–14). This discrepancy begs the question: why do most prostate tumors respond poorly to immune checkpoint blockade? What is different about tumors that respond more vigorously? Considering these questions, here we review the impact of the tumor immune microenvironment on prostate cancer immunotherapy. We highlight the content and organization of this microenvironment and its critical role in the response to immunotherapy .
2.1. Challenges in Immune Checkpoint Therapy for Prostate Cancer
Having seen great success in many other solid tumors (8–14), immune checkpoint blockade also been investigated in prostate cancer. These efforts have been particularly focused on hormone refractory patients. While early trials showed promising safety and efficacy for immune blockade in prostate cancer, subsequent trials have failed to return impressive results.
In early trials, cytotoxic T-lymphocyte associated protein 4 (CTLA-4) blockade (ipilimumab, tremelimumab) demonstrated safety (15, 16) and indicated some modest clinical activity, particularly when combined with other treatment strategies (5, 6, 15–19). Anti-CTLA-4 treatment in combination with administration of granulocyte-macrophage colony stimulating factor (GM-CSF) was seen to induce prostate specific antigen (PSA) decline of >50% in two patients and to induce expansion of activated, circulating CD25-CD69+ CD8+ T-cells in metastatic castration resistant prostate cancer (mCRPC), particularly in patients receiving higher doses of iHilpilumumab and receiving combination therapy (16, 17). Similarly, phase I/II studies of mCRPC patients receiving ipilimumab in combination with radiotherapy showed evidence of synergistic antitumor activity, as suggested by declining PSA levels of >50%, one patient’s complete response, and six patients’ stabilized disease (18). However, in randomized phase III trials of ipilimumab + radiotherapy in mCRPC patients, no significant difference in overall survival between the ipilimumab and placebo groups was seen, though investigators noted that there were signs of clinical activity which may warrant further investigation, as more patients in the placebo group had early disease progression (before 3 months) than those in the ipilimumab group (6). Similarly, in patients with PSA-recurrent prostate cancer, a phase I trial of tremelimumab in combination with short-term androgen deprivation reported no favorable changes in PSA doubling time soon after completion of treatment, but some patients saw prolonged PSA doubling time at later time points (19). Phase I & II trials of tremelimumab in combination with other therapies are ongoing (NCT02788773, NCT03204812, NCT02616185). In summary, while some patients do respond to CTLA-4 blockade, the inconsistent results seen in prostate cancer have precluded its approval.
Trials of programmed cell death protein 1 (PD-1) blockade (nivolumab, pembrolizumab) and programmed death ligand 1 (PD-L1) blockade (durvalumab, atezolizumab) have seen similar results in prostate cancer (20–28). In a phase I trial, nivolumab was tolerated in CRPC patients, with one patient (of seventeen) having a reduction in measurable lesions (20), and in phase I/II trials, pembrolizumab has shown clinical activity in both heavily pre-treated and less pre-treated prostate cancer patients and in both CRPC and castration sensitive disease (21, 22, 24). In KEYNOTE-028, pembrolizumab was used for advanced prostate cancer and provided an overall response rate of 13%, a stable disease rate of 39%, and a 6-moth progression free survival rate of 39% (21). In another study of men with recurrent or advanced prostate cancer, pembrolizumab treatment returned a response rate of 19% and a stable disease rate of 35% (22). In KEYNOTE-199, pembrolizumab was used specifically in docetaxel-refractory mCRPC patients and initially returned a disease control rate of 26% by RECIST v1.1 criteria across all cohorts, but ultimately returned a disease control rate >6 months of only 11%, demonstrating a lack of durable responses (24). PD-1/PD-L1 blockade has been trialed in combination with other therapies as well, showing some limited activity alongside continued enzalutamide treatment in CRPC patients who had progressed on enzalutamide monotherapy. In this study, three of ten patients achieved a serum PSA response of <0.1 ng/ml (25).
Interestingly, PD-L1 blockade in combination with PARP inhibition (PARPi) has garnered recent attention in the field. A study of enzalutamide/abiaterone pre-treated mCRPC patients with DNA repair defects reported a 59% response rate (ten patients), where response was indicated by PSA decline or radiographic response (29). It is suggested that PARPi may augment response to immunotherapy via activation of the cGAS-STING pathway. PARPi results in increased presence of DNA fragments in the cytoplasm, which can activate the cGAS-STING pathway (30). This pathway results in induction of the interferon response, which assists T-cell activation, ultimately augmenting the anti-tumor T-cell response and the clinical response to immunotherapy (31). Alternative explanations for the success of this combination therapy (PD-L1 blockade + PARPi) could include the notion that PARPi may bolster neoantigen generation, and neoantigen expression and tumor mutational burden have been previously associated with increased immunotherapy response rates in other cancers (32, 33). However, prostate cancers are known to harbor a relatively low mutational burden (34), suggesting cGAS-STING activation may more likely account for the promising results seen with this combination regimen.
Additional trials are arranged and ongoing to combine PD-1/PD-L1 blockade with CTLA-4 blockade (NCT02601014), chemotherapy (NCT03338790, NCT03170960, NCT03016312), radiotherapy (NCT02814669), and therapeutic vaccines (NCT02933255, NCT03024216). As with CTLA-4 blockade, these modest results seen with PD-1/PD-L1 inhibition again suggest that prostate cancer is minimally responsive to immune checkpoint blockade but may be augmented by combination therapy strategies.
2.2. Therapeutic Vaccination Indicates Immune Infiltration into the Prostate Tumor Microenvironment is Possible and Beneficial
While immune checkpoint blockade trials have been largely disappointing in prostate cancer, therapeutic vaccines have been an exciting potential therapeutic strategy in prostate cancer for some time, with the development of various vaccines (e.g. Prostvac and GVAX) and the eventual approval of Provenge in 2011.
Prostvac is a combination virally-based vaccine which includes prostate specific antigen (PSA), intracellular adhesion molecule 1 (ICAM1), a costimulatory molecule for T-cells (CD80/B7-1), and lymphocyte function associated antigen 3 (LFA-3) (35). As the viral vector infects antigen presenting cells, the expression of these molecules promotes interaction with and activation of T-cells, with the goal of cell mediated tumor destruction. Prostvac saw success in Phase II trials—the vaccine was well tolerated and provided improved overall survival (8.5 months) in patients with minimally symptomatic castrate resistant prostate cancer (CRPC) (36–38). However, phase III trials of Prostvac (PROSPECT) report that Prostvac, Prostvac + GM-CSF, and placebo arms resulting in similar median overall survival rates (39). Ongoing trials of this agent in combination with other agents are ongoing.
Provenge (Sipuleucel-T) was the first therapeutic cancer vaccine to be approved by the FDA. Sipuleucel-T is an autologous dendritic cell vaccine, made by harvesting a patient’s monocytes by leukapheresis, pulsing these cells with a granulocyte-macrophage colony stimulating factor-prostatic acid phosphatase (GMCSF-PAP) fusion protein (40). These cells, which then express GMCSF-PAP on their surface, are reinfused to the patient (41, 42). The cells mature and activate, and in turn activate CD8 T-cells to kill tumor cells. Sipuleucel-T has provided an overall survival benefit (+4.1 months) and a 22% relative risk reduction of mortality in metastatic castrate resistant prostate cancer (mCRPC) (43). These results led to Sipuleucel-T’s landmark FDA approval for hormone refractory prostate cancer. Notably, in a phase II study of neoadjuvant Sipuleucel-T in treatment-naïve patients with localized prostate cancer, Sipuleucel-T induced both a circulating T-cell response as well as T-cell infiltration at the tumor interface (44). These infiltrating cells were predominantly CD3+ and CD4+ FoxP3− or CD8+, and most were PD-1+ and Ki67+, indicating their recent activation and proliferation (44). These infiltrating T-cells are the likely mediators of the anti-tumor effects and subsequent survival benefits seen in various trials of Sipuleucel-T. Importantly, this study illustrates that immune infiltration into a stereotypically ‘immunologically cold’ tumor can be achieved. The ability to incite this infiltration is critical, as activated and infiltrating immune cells have been shown to predictive of the response to immune checkpoint blockade in other tumor types (26, 45–49). Specifically, in a study of patients with metastatic melanoma, patients who responded to pembrolizumab treatment had increased CD8, PD-1, and PD-L1 expressing cells at the invasive margin and inside the tumor, and proliferation of intratumoral CD8+ T-cells correlated to radiographic reduction of tumor size (26). Indeed, as Sipulecuel-T incites tumor immune infiltration (44) and as infiltrating immune cells have been shown to be required for the response to immune checkpoint blockade (26), the relatively modest immune infiltration of prostate tumors may account for the discouraging results seen with immune checkpoint blockade in these tumors. As such, moving forward, understanding tumor immune infiltration and the tumor immune microenvironment may be prerequisite to explaining variation in patient and tumor-type response to immunotherapy. In short, to establish the role of immunotherapy in prostate cancer, the tumor immune microenvironment need be investigated.
3. THE COMPOSITION OF THE IMMUNE MICROENVIRONMENT IS PROGNOSTICALLY VALUABLE AND FUNCTIONALLY IMPORTANT
The tumor microenvironment (TME) includes numerous immune cell subsets, which are crucial in the anti-tumor immune response. Depending on the organization, location, and phenotype of the cells, they can either promote or impede the anti-tumor immune response (Fig 1). In order to understand how the immune system is responding to different tumors, the cell populations must be analyzed and studied in order to understand the way these populations interact. The fundamental cell populations in the tumor immune microenvironment are lymphocytes, antigen presenting cells (e.g. dendritic cells and macrophages), and stromal cells.
Figure 1.
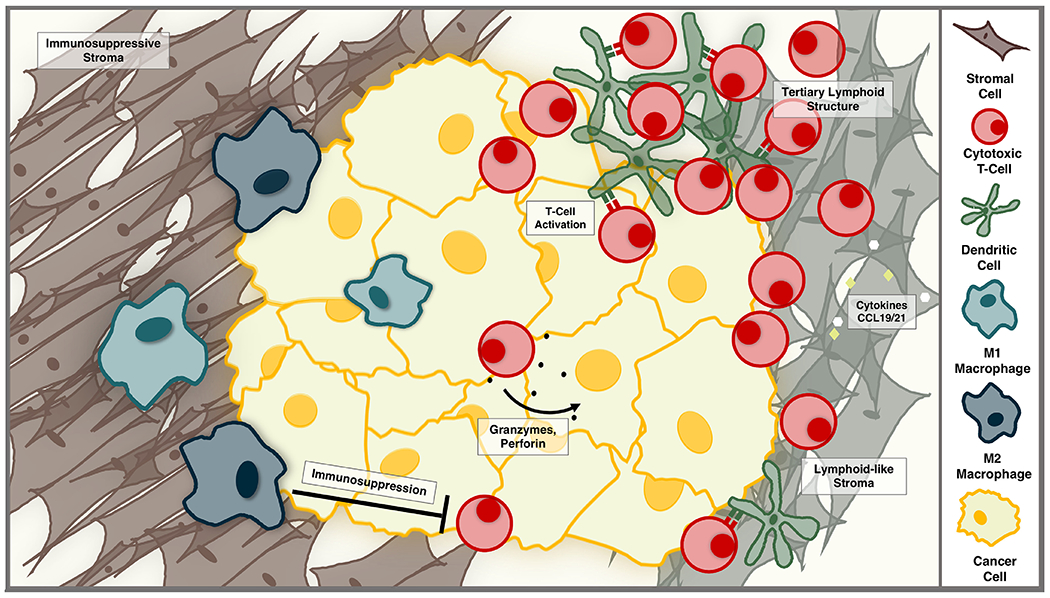
The tumor immune microenvironment can support or thwart the anti-tumor immune response. Infiltrating lymphocytes can kill tumor cells via cytotoxic granules containing granzymes and perforin. Dendritic cells and lymphocytes can organize, with the support of lymphoid-like stromal cells, to form tertiary lymphoid structures—dynamic aggregates that fuel the anti-tumor immune response. Alternatively, a dense, immunosuppressive stroma can limit T-cell activity, and pro-tumorigenic macrophages can support tumor growth and suppress cytotoxic T-cell activity.
3.1. Tumor Infiltrating Lymphocytes are a Positive Prognostic Indicator and a Predictor of Immune Checkpoint Blockade Response
Tumor infiltrating lymphocytes (TILs) are decidedly a positive prognostic factor in many tumor types. This prognostic significance of tumor infiltrating immune cells has been explored in various cancers, and many studies have reported a survival benefit associated with the presence of TILs (50). For example, studies in colorectal cancer have shown that the type, density, and location of TILs in primary colorectal cancer tumors are significant predictors of disease-free and overall survival (DFS, OS) (51). This resulted in the creation of a scoring system—the “Immunoscore”—based on the density of infiltrating CD8+ and CD45RO+ T-cells at the tumor core (CT) and invasive margin (IM), with higher scores reflecting greater lymphocyte invasion (52). Validation of “Immunoscore” reported that patients with higher scores (i.e. higher T-cell densities within primary tumors) had increased DFS and OS compared to low scores, and that the “Immunoscore” was a better predictor of survival than the current TNM staging method (53). Further study has indicated that patients with dense CD8 T-cell infiltration of lung metastases colorectal cancer also have improved overall survival (54). T-cell infiltration is also a positive prognostic indicator in melanoma, breast cancer, head and neck cancer, ovarian cancer, non-small cell lung cancer, esophageal cancer, small cell lung cancer, hepatocellular carcinoma, and renal cell carcinoma (50, 55–58).
In addition to signaling a positive prognosis, TILs are also predictive of the response to immune checkpoint blockade (26, 45, 48, 49). In a study of melanoma patients, patients who responded to PD-1 inhibition showed proliferation of intratumoral CD8 T-cells in accordance with radiographic reduction in tumor size (26). Pre-treatment analysis of samples from responding patients indicated higher density of CD8, PD-1, and PD-L1 expressing cells in and near the tumor, suggesting that a pre-existing T-cell response predicts the potential for response to immune checkpoint blockade (26). Similarly, in a study of desmoplastic melanoma patients, patients with more CD8 T-cells at the tumor margin were found to gain the most clinical benefit in response to PD-1 blockade (45). Furthermore, while PD-L1 expression status is often suggested to predict the response to immunotherapy (59, 60), classic immunohistochemistry evaluation of expression level fails to comprehensively designate all patients who should receive antiPD-1/PD-L1 therapy (30, 59). Indeed, it has been suggested that TIL presence in the TME plays an additionally critical role predicting immunotherapy response than PD-L1 status (30). Thus, as PD-L1 expression is rare in prostate cancers (61), and as prostate cancers are known to be scarcely T-cell infiltrated (62), these factors may contribute to the low response rate of immunotherapy in prostate cancers. Altogether, these studies suggest that, while TILs are a positive prognostic factor, their presence and the existence of pre-existing anti-tumor immunity may also be a requirement for clinical response to immune checkpoint blockade.
Increased TILs have also been reported to be a positive prognostic indicator in prostate cancer (55, 63–66), though prostate TILs have been reported to be ‘unresponsive’ and terminally differentiated (67). Additionally, prostate tumors are reported to have decreased TILs compared to adjacent normal tissue or prostate intraepithelial neoplasia (58, 62, 67). Higher CD8 T-cell infiltration has been independently associated with improved survival after radical prostatectomy, and of note, this study found no significant differences in age, Gleason score, PSA, or TNM staging between the densely CD8 infiltrated cases and the poorly infiltrated cases (65). Similarly, in a study reporting low numbers of TILs to be a negative predictor of survival, the infiltrating lymphocyte density was independent of prostate tumor differentiation (64). These observations beg the questions: if tumor stage and differentiation do not correlate with lymphocyte infiltration, what factors might predict lymphocyte infiltration instead? And what explains the scarcity of TILs in some patients and some tumor types, but not others? Conceivably, variation in the activation of tumor specific T-cells or in the tumor immune microenvironment may account for the dense lymphocyte infiltration seen in some patients and some tumor types, but not in others. Testing hypotheses such as these will be critical to understanding the mechanisms of immunotherapy and to predicting which patients will respond.
3.2. Antigen Presentation in the Immune Microenvironment
The antigen presenting cell (APC) environment is a crucial part of the TIL response. Without proper antigen presentation and CD8 T-cell activation, there cannot be a CD8 T-cell response (68). Accordingly, the composition of the APC compartment is an essential part of the TME. This APC compartment can include several cells from the myeloid lineage, such as dendritic cells and macrophages. These cells help control how the immune system responds to the tumor and are important for both patient survival and the response to immunotherapies.
Dendritic Cells (DCs) are professional antigen presenting cells that are necessary to activate CD8 T-cells. Importantly, these cells are present in the TME (Fig 1). DCs are vital both at the tumor site (to activate naïve TILs which are recruited to the tumor), as well in the draining lymph nodes (to present tumor antigens to naïve CD8 T-cells). In NSCLC, infiltration of mature DCs was associated with favorable clinical outcomes, and tumors which had fewer infiltrating mature DCs also had fewer infiltrating T-cells (69). Notably, this study illustrates that the number and phenotype of DCs in the TME play a significant role in the CD8 T-cell response to tumors and can impact clinical outcomes.
CD8 T-cells need to recognize antigen presented on MHC-I, and typically, cells afflicted with an intracellular pathogen process and present antigen on MHC-I. Accordingly, DCs that can cross-present exogenous antigens on MHC-I are necessary to mount a CD8 T-cell response to cancer (70). This cross-presenting subset of DCs (known as cDC1 cells) is crucial in presenting tumor associated antigens to activate tumor-specific CD8 T-cells and has been characterized in both humans and mice (71). In a mouse model of melanoma, cross-presenting CD103+ cDC1s have been shown to activate intratumoral CD8 T cells. cDC1 cells were also shown to migrate to draining LN to activate naïve CD8 T cells specific for tumor antigens (72). This study affirms the importance of having a cDC1 population within the TME both to induce a local T-cell response, as well as to migrate to draining LNs for further anti-tumor T-cell activation. Patients with higher levels of cDC1 related transcripts in their tumors (e.g. CD141, IRF8, FLT3) tend to have superior clinical outcomes in 12 different cancers (73). This demonstrates the importance of cross presenting cDC1s in human tumors in addition to in previously described mouse models.
Importantly, studies in a mouse model of melanoma reveal that tumor infiltrating DCs can become less efficient in activating CD8 T-cells, induce less proliferation, express less co-stimulatory molecules, lose the ability to efficiently present antigens, and produce less IL-12 (74). These studies show that the microenvironment can have a suppressive effect on DCs and that the functionality of DCs—rather than merely their presence in the tumor—is fundamental in the anti-tumor immune response. Thus, it is important to understand what differentiates tumors with effective, mature DC responses and those with inefficient or dysfunctional DCs. Undoubtedly, DCs are a crucial part of the TME that both influences CD8 T-cell infiltration and acts as a clinically prognostic factor in the tumor.
Macrophages are another principal aspect of the TME and are particularly important in the immune response to prostate cancer (Fig 1). Due to their highly plastic phenotype, macrophages can be skewed by environmental factors to either a pro-inflammatory phenotype (M1) or to a “wound repair” alternative-activation phenotype (M2). The macrophages that reside in the TME are often referred to as Tumor Associated Macrophages (TAMs) and frequently acquire an M2-like phenotype due to the cytokines present in the TME. These M2-skewed TAMs are thought to promote tumorigenesis. They secrete anti-inflammatory cytokines such as IL-10 and TGF-B, which suppress CD8 T-cell activation. M2 macrophages also secrete growth factors, such as EGF, that can promote tumor growth and metastasis (75). Importantly, they can downregulate CD8 T-cell activity, through anti-inflammatory cytokines, the release of arginase-1, and the expression of checkpoint ligands such as PD-L1/2 (75).
Macrophages in the stroma of lung squamous-cell carcinomas prevent T-cells from migrating in the TME and can prevent their interaction with stimulating APC populations (e.g. DCs) (57). This demonstrates that the localization of macrophages, in addition to their phenotype, can affect the CD8 T-cell response to tumors. In a study that incubated macrophages with conditioned media from prostate cancer cells, the macrophages became M2-like and produced IL-10 (76). This study also showed that the same prostate cancer conditioned media can re-program M1-polarized macrophages to an M2-like phenotype (76). These M2-skewed macrophages act as poor APCs, preventing efficient CD8 T-cell activation and suppressing already activated CD8 T-cells. Together, this suggests that, in prostate cancer, the macrophage population size, phenotype, and localization could deter an effective CD8 T-cell response and suppress activated CD8 T-cells that are present in the microenvironment.
Another prominent population of immune cells in the TME are myeloid-derived suppressor cells (MDSCs). MDSCs can suppress T-cell activity by inhibiting CD8 T-cell activation and via expression of arginase 1 and reactive oxygen species (ROS) (77). It is currently thought that there are two main subsets of MDSCs: monocytic MDSCS (mMSDCs, originating from monocytes) and polymorphonuclear MDSCs (PMN-MSDCs, arising from a granulocytic PMN precursor) (77). In prostate cancer, PMN-MDSCs have been suggested to promote CRPC, as PMN-MDSCs are enriched CRPC biopsies when compared to castration sensitive prostate cancers (78). Additional investigation into the phenotype of PMN-MDSCs and how to delineate these suppressive cells from traditional PMNs will be necessary to better understand the role of these cells in the TME (77). Similarly, mMDSCs share many markers and characteristics with monocytes and macrophages (77), and further study will be required to distinguish these cell populations and their influence on the TME and on clinical outcomes.
Further study of the APC populations and phenotypes present in prostate cancer may be vital to understanding why some immunotherapy approaches have less effective than in other cancers. The largely M2-skewed macrophages that infiltrate prostate tumors could prevent CD8 T-cells from becoming properly activated, rendering CD8 T-cell focused therapies ineffective (76). When DCs pulsed with a tumor antigen are introduced to the environment, such as with Sipuleucel-T, CD8 T-cell infiltration increases (44), and clinical outcomes are improved (41–43). Altering APC populations in the tumor can directly influence the CD8 T-cell response and is crucial to consider for future immunotherapy approaches. By understanding the APC populations and how they affect the CD8 T-cell responses, future immunotherapies can focus on promoting a more effective CD8 T-cell response.
3.3. Stromal Cells in the Tumor Microenvironment May Support or Limit Antitumor T-cell Activity
Another important player in the TME is the stromal cell (Fig 1). Stromal cells, such as fibroblasts, pericytes, and reticular cells, form a functionally supportive network for various tissues and organs. Stromal cells are typically thought to support cancer growth and progression, but have also been described as supportive in hematopoiesis and inflammatory processes (79, 80). Indeed, stromal cells in lymphoid tissue—known as fibroblastic reticular cells—form conduits to support T-cell trafficking, secrete chemokines that modulate T-cell and dendritic cell migration, and produce cytokines that maintain T-cell homeostasis (79). Accordingly, there is precedent for stromal cells to be either supportive or inhibitory of T-cell activity, and this is certainly true of tumor stromal cells as well. In lung adenocarcinoma, tumor stromal cells have been shown to modulate intratumoral T-cell motility and migration (57). More densely packed fibronectin and collagen containing stroma is thought to inhibit T-cell motility, but more loosely arranged stroma may promote T-cell migration and function (57). Conversely, densely stromal tumors, such as desmoplastic melanoma, harbor dense lymphoid aggregates and can be exceptional responders to immune checkpoint therapy (45). Stromal cells have also been shown to aid in generating and maintaining the immune response and in recruiting T-cells to the tumor site in melanoma, breast, and lung tumors (81–83). In prostate cancer, tumor adjacent stroma is suggested to limit anti-tumor immunity by secreting TGF-β, which induces M2 macrophage polarization and recruits T-regulatory cells (84, 85). Together, these findings indicate that the stromal compartment of the TME may be either immunosuppressive or immunostimulatory, and further investigation is needed to determine how this balance is controlled. Specifically, as the prostate cancer stroma seems to be generally immunosuppressive, investigation into manipulation of this stroma may represent a promising approach to boost response rates to immunotherapy in prostate cancer.
4. ORGANIZATION IN THE IMMUNE MICROENVIRONMENT
The association between tumor T-cell infiltration and improved survival and response to immunotherapy has resulted in the categorization of various tumors as either immunologically ‘hot’ or ‘cold.’ In addition, others have further delineated phenotypic categories of immune infiltration—immune-inflamed, immune-excluded, and immunedesert—in attempts to explain the variation seen clinical behavior (86). Immune-inflamed tumors are generally thought to carry a positive prognosis, while immune-excluded and immune-desert tumors are typically thought to be less favorable and less responsive to immunotherapy (87–93). This suggests that both the organization, as well as the composition, of the TME are critical to building and maintaining a productive antitumor immune response. In short, inflamed, organized ‘hot’ tumors seem to be a favorable environment for anti-tumor T-cell activation and expansion. But what determines whether a tumor is ‘hot’ or ‘cold’—’immune-inflamed’ or ‘immune-excluded’ or ‘immune-desert?’ And what are the functional consequences of these phenotypes?
4.1. Lymphoid Organization Precedes the Productive Immune Response
One variable which may help answer these imminent questions is that of the organization and structural composition of the immune microenvironment. Undoubtedly, the intricate organization of secondary lymphoid organs (e.g. lymph nodes) illustrates that structural components of the microenvironment are important for productive immune responses (94, 95). For example, lymph nodes serve as a crucial depot for blood-circulating lymphocytes to encounter antigens and APCs, which have drained from the peripheral tissues via the lymphatic system. (94, 95). This allows rare populations of antigen specific lymphocytes to encounter their target antigen, which is critical to effective immunosurveillance. Circulating lymphocytes enter the lymph nodes via the high endothelial venules (HEVs), and draining APCs, such as dendritic cells, enter lymph nodes via the lymphatics (94, 95). Once the immune cells arrive in the lymph nodes, stromal cell networks—fibroblastic reticular cells and follicular dendritic cells—assist in cell trafficking (96). Fibroblastic reticular cells (FRCs) serve to recruit T-cells, guide them to the paracortex, and maintain their survival (97). FRCs are known to secrete T-cell chemoattractants (e.g. CCL19 and CCL21), as well as CXCL12, which helps retain T-cells in the paracortex (96, 98, 99). To assist in T-cell survival, FRCs secrete IL-7 (100). The FRC network serves both as a scaffold for T-cell navigation and as support for maintaining HEV integrity (96, 101). Taken together, these studies indicate that both the structural and functional contributions of FRCs are critical to the T-cell response. Indeed, genetic ablation of lymph node FRCs impairs T-cell localization, survival, and priming (97, 101). This central role for FRCs in the T-cell response suggests that in addition to the canonical signals required for T-cell activation (68), a productive immune response requires structural and organizational assistance as well.
4.2. Peripheral Lymphoid Organization in Tertiary Lymphoid Structures Supports the Immune Response
The presence of highly organized immune structures similar to lymphoid tissue in inflamed, infected, and tumoral tissues illustrates the importance of organization in the peripheral immune microenvironment environment (102, 103). Importantly, these inducible, tertiary lymphoid structures (TLS) structures are known to positively alter the clinical course of pulmonary infection but have also been shown to be pathogenic in the setting of autoimmune disease (e.g. Crohn’s disease) and transplant rejection (104–106). While exactly how these structures function in the system immune response is incompletely understood, these clinical associations demonstrate the importance of peripheral lymphoid organization in both therapeutic and pathogenic immune responses. This invites further study into the role of TLS in the systemic immune response.
TLS seem to hold functional similarity to aspects of lymph node biology. TLS are known to harbor T-cell zones with mature dendritic cells, germinal centers with follicular dendritic cells and proliferating B-cells, and HEVs (103, 107–109). TLS and lymph node genesis and maintenance are incited by similar signals—lymphotoxin, TNFa, CCL19/CCL21, CXCL13, and others (103, 110). For example, in a transgenic mouse model, ectopic expression of lymphotoxin induces formation of TLS, which delineated T and B-cell areas, primary and secondary follicles, and HEVs (109). Similarly, in a mouse model of influenza, bronchiole associated lymphoid tissue, which is analogous to TLS (111–113), is induced, but disintegrates when lymphotoxin signaling is inhibited (114). This induced bronchiole associated lymphoid tissue (iBALT) also requires dendritic cell function, as iBALT dissolves when DCs are depleted and antiviral immunity is impaired (114, 115). T-cell areas of iBALT are also known to contain reticular cells, which function as FRCs do in the lymph node (112). Interestingly, these structures are not unique to lung tissue, as TLS have also been noted in chronic gastritis, heliobacter-induced hepatitis, isolated lymphoid follicles in the gut, and chronic transplant rejection (106, 116–120). Taken together, these features of TLS and iBALT demonstrate both the importance of organization in the immune microenvironment and the ability for this organization to take place in an assortment of tissues in response to a variety of stimuli.
4.2. Tertiary Lymphoid Structures are Found in Tumors and are Associated with Favorable Clinical Outcomes
TLS have been noted in a variety of tumor types, and interestingly, the specific organization of infiltrating immune cells has been linked both to increased recruitment and retention of tumor infiltrating lymphocytes and to improved clinical outcomes in non-small cell lung cancer and colorectal cancer (69, 102, 121). This suggests that both immune infiltration and organization may be required for a productive anti-tumor immune response and a strong response to immunotherapy. In other words, the TME of immunologically ‘cold’ tumors may lack sufficient immune cells (44, 89)—or sufficient immune cell organization—to mount a response to existing immunotherapies. Accordingly, it is necessary to further investigate the role of TLS in anti-tumor immunity.
TLS in tumors, as in infection and autoimmunity, harbor T-cell zones with mature DCs, germinal centers with follicular dendritic cells and proliferating B-cells, and HEVs (102, 103, 107, 108, 122). The presence of TLS in human lung tumors are associated with increased expression of chemokines (e.g. CCL19/21, CXCL13), adhesion molecules (e.g. ICAM1, VCAM1), and integrins (102). In a mouse model of melanoma, CCL21 highly expressing tumors were found to contain lymphoid-like reticular stromal networks (123). Taken together, this evidence suggests that tumor TLS represent a similar phenomenon to those seen in the setting of infection and autoimmunity.
Importantly, studying these tumor TLS may further inform currently appreciated observations regarding ‘hot’ and ‘cold’ tumors and their prognostic influence. Tumor TLS are associated with extensive lymphocytic infiltration into tumors and with improved disease-free survival in tumors such as breast and colorectal cancer (54, 103, 124, 125). Specifically, the density of HEVs and mature dendritic cells is found to decrease as breast cancer patients experience progressive disease (125). These findings suggest that dendritic cells are critical to the functional capacity of these structures, and thus to the infiltration and maintenance of anti-tumor T-cells. Similarly, TLS-associated mature dendritic cells are correlated with infiltration of CD8 T-cells and with improved survival in non-small cell lung cancer (69, 103, 121). Furthermore, “lymphoid neogenesis” has also been demonstrated in the lung metastases of melanoma and colorectal cancer patients, indicating that this immune organization can be present at various stages of disease and in various tissue types (54, 108, 122). This demonstrates that regardless of disease stage, grade, or location, the organization of infiltrating immune cells (i.e. TLS) could be a critical step in the generation of the anti-tumor immune response.
TLS have also been documented in prostate cancer. In some prostate tumors these structures seem to be a center of immune suppression, harboring T-regulatory cells and other immunosuppressors (126). Conversely, patients experiencing spontaneous tumor regression are found to undergo a shift in cellular composition of prostate TLS. TLS in these evanescent tumors were found to contain less T-regulatory cells, more Th1-type cells, and more CD8 T cells (126). Interestingly, TLS—termed prostate associated lymphoid tissue (PALT)—have also been documented in healthy prostate (127), raising questions as to whether the immunologically ‘cold’ nature of prostate may be due to the functional or physical disruption of this ‘PALT.’ Could gaining understanding of lymphoid organization in prostate tumors explain the low response rate seen with checkpoint therapy in prostate cancer? Undoubtedly, further investigation is required to understand the role of lymphoid structures in both the benign and malignant prostate, as well as the general role of TLS in anti-tumor immunity.
5. FUTURE PERSPECTIVE
Here we have reviewed the use of immunotherapy in prostate cancer and the mechanisms that support responses to immunotherapy. Additionally, we have specified potential explanations for why immunotherapy has delivered lower response rates than in some other cancers. Based on the evidence presented here, it is clear that immune infiltration and organization in the TME—such as in the formation of TLS—plays an important role in anti-tumor immunity and in the response to immunotherapy. Specifically, this suggests that insufficient immune infiltration (e.g. TILS), suboptimal antigen presentation, lack of adequate immune organization, or some combination of these facts may explain the low response rates to immunotherapy in prostate cancer. Accordingly, understanding the status of immune infiltration and organization in the prostate may hold the key to improving immunotherapy response rates in prostate cancer patients.
Without a doubt, the ideas presented here spur further investigation, as many critical questions remain. How is are TLS formed? What is required for maintenance of TLS? Is stromal density and composition necessarily inhibitory to the anti-tumor immune response? Is tumor stroma plastic enough to mimic a reticular-like network, ultimately facilitating immune organization in the tumor? Both biologically interesting and clinically translatable, seeking answers to these questions holds promise for improving outcomes in both prostate cancer and other tumor types.
HIGHLIGHTS.
Immunotherapy in prostate cancer has seen both challenges and successes.
Therapeutic vaccination reveals beneficial immune infiltration in prostate tumors.
The tumor immune microenvironment is both prognostically and functionally valuable.
Lymphoid organization is critical to the productive immune response.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017;317(24):2532–42. Epub 2017/06/28. doi: 10.1001/jama.2017.7248.. [DOI] [PubMed] [Google Scholar]
- 2.Daskivich TJ, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Stanford JL, Stroup AM, Litwin MS, Penson DF. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Annals of internal medicine. 2013;158(10):709–17. Epub 2013/05/22. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park CK, Kim SK. Clinicopathological significance of intratumoral and peritumoral lymphocytes and lymphocyte score based on the histologic subtypes of cutaneous melanoma. Oncotarget. 2017;8(9):14759–69. Epub 2017/01/21. doi: 10.18632/oncotarget.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saldanha G, Flatman K, Teo KW, Bamford M. A Novel Numerical Scoring System for Melanoma Tumor-infiltrating Lymphocytes Has Better Prognostic Value Than Standard Scoring. The American journal of surgical pathology. 2017;41(7):906–14. Epub 2017/04/04. doi: 10.1097/pas.0000000000000848.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, Piulats JM, Gonzalez Mella P, Ng SS, Jaeger D, Parnis FX, Franke FA, Puente J, Carvajal R, Sengelov L, McHenry MB, Varma A, van den Eertwegh AJ, Gerritsen W. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017;35(1):40–7. Epub 2016/12/31. doi: 10.1200/jco.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 6.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengel0v L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhrejahani F, Madan RA, Dahut WL, Karzai F, Cordes LM, Schlom J, Gulley JL. Avelumab in metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2017;35(6_suppl):159-. doi: 10.1200/JCO.2017.35.6_suppl.159. [DOI] [Google Scholar]
- 8.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2013;369(2): 122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2017;377(14):1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus Chemotherapy for PD-Ll-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375(19):1823–33. Epub 2016/10/11. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 11.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz H-J, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine J-M, Maglinte GA, Kopetz S, Andre T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. The Lancet Oncology.18(9):1182–91. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overman MJ, Lonardi S, Wong KYM, Lenz H-J, Gelsomino F, Aglietta M, Morse MA, Cutsem EV, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine J-M, Cao ZA, Kamble S, Kopetz S, Andre T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. Journal of Clinical Oncology. 0(0):JCO.2017.76.9901. doi: 10.1200/jco.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 13.Escudier B, Tannir NM, McDermott DF, Frontera OA, Melichar B, Plimack ER, Barthelemy P, George S, Neiman V, Porta C, Choueiri TK, Powles T, Donskov F, Salman P, Kollmannsberger CK, Rini B, Mekan S, McHenry MB, Hammers HJ, Motzer RJ. LBA5CheckMate 214: Efficacy and safety of nivolumab + ipilimumab (N+I) v sunitinib (S) for treatment-naiïve advanced or metastatic renal cell carcinoma (mRCC), including IMDC risk and PD-L1 expression subgroups. Annalsof Oncology. 2017;28(suppl_5):mdx440.029-mdx440.029.doi: 10.1093/annonc/mdx440.029. [DOI] [Google Scholar]
- 14.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbe C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Annals of oncology : official journal of the European Society for Medical Oncology. 2013;24(8):2174–80. Epub 2013/05/15. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvestri I, Cattarino S, Giantulli S, Nazzari C, Collalti G, Sciarra A. A Perspective of Immunotherapy for Prostate Cancer. Cancers (Basel). 2016;8(7). Epub 2016/07/12. doi: 10.3390/cancers8070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small EJ, Tchekmedyian NS, Rini Bl, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 2007;13(6):1810–5. Epub 2007/03/17. doi: 10.1158/1078-0432.Ccr-06-2318. [DOI] [PubMed] [Google Scholar]
- 17.Fong L, Kwek SS, O’Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, Small EJ. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res 2009;69(2):609–15. Epub 2009/01/17. doi: 10.1158/0008-5472.Can-08-3529. [DOI] [PubMed] [Google Scholar]
- 18.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, Scher HI, Chin K, Gagnier P, McHenry MB, Beer TM. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Annals of oncology : official journal of the European Society for Medical Oncology. 2013;24(7):1813–21. Epub 2013/03/29. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, Liu G. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61(7):1137–47. Epub 2012/01/03. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bracarda S, Altavilla A, Hamzaj A, Sisani M, Marrocolo F, Del Buono S, Danielli R. Immunologic checkpoints blockade in renal cell, prostate, and urothelial malignancies. Seminars in oncology. 2015;42(3):495–505. Epub 2015/05/13. doi: 10.1053/j.seminoncol.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Hansen A, Massard C, Ott PA, Haas N, Lopez J, Ejadi S, Wallmark J, Keam B, Delord JP, Aggarwal R, Gould M, Qiu P, Saraf S, Keefe S, Piha-Paul SA. Pembrolizumab for patients with advanced prostate adenocarcinoma: Preliminary results from the KEYNOTE-028 study. Annals of Oncology. 2016;27(suppl_6):725PD-PD. doi: 10.1093/annonc/mdw372.09. [DOI] [PubMed] [Google Scholar]
- 22.Higa J, Wilenius K, Weidhaas JB, Larsen C, Lam RY, Turner J, Scholz MC. Pembrolizumab for recurrent or advanced prostate cancer. Journal of Clinical Oncology. 2018;36(6_suppl):250-. doi: 10.1200/JCO.2018.36.6_suppl.250. [DOI] [Google Scholar]
- 23.Boudadi K, Suzman DL, Luber B, Wang H, Silberstein J, Sullivan R, Dowling D, Harb R, Nirschl T, Dittamore RV, Carducci MA, Eisenberger MA, Haffner M, Meeker A, Eshleman JR, Luo J, Drake CG, Antonarakis ES. Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2017;35(15_suppl):5035-. doi: 10.1200/JCO.2017.35.15_suppl.5035. [DOI] [Google Scholar]
- 24.Bono JSD, Goh JC, Ojamaa K, Rodriguez JMP, Drake CG, Hoimes CJ, Wu H, Poehlein CH, Antonarakis ES. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2018;36(15_suppl):5007-. doi: 10.1200/JCO.2018.36.15_suppl.5007. [DOI] [Google Scholar]
- 25.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, Cetnar JP, Ey FS, Bergan RC, Slottke R, Beer TM. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33):52810–7. Epub 2016/07/19. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. Epub 2014/11/28. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JW, Shaffer DR, Massard C, Powles T, Harshman LC, Braiteh FS, Conkling PR, Sarkar I, Kadel EE, Mariathasan S, O’Hear C, Schiff C, Fasso M, Carroll S, Petrylak DP. A phase la study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2018;36(6_suppl):187-. doi: 10.1200/JCO.2018.36.6_suppl.187. [DOI] [Google Scholar]
- 28.Dallos MC, Drake CG. Blocking PD-1/PD-L1 in Genitourinary Malignancies: To Immunity and Beyond. Cancer journal (Sudbury, Mass). 2018;24(1):20–30. Epub 2018/01/24. doi: 10.1097/ppo.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 29.Karzai F, Madan RA, Owens H, Hankin A, Couvillon A, Cordes LM, Fakhrejahani F, Houston ND, Trepel JB, Chen C, Edelman DC, Meltzer PS, Steinberg SM, Gulley JL, Dahut WL, Lee J-m. Combination of PDL-1 and PARP inhibition in an unselected population with metastatic castrate-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2017;35(15_suppl):5026-. doi: 10.1200/JCO.2017.35.15_suppl.5026. [DOI] [Google Scholar]
- 30.Lee J-M, Gulley JL. Checkpoint and PARP inhibitors, for whom and when. Oncotarget. 2017;8(56):95036–7. doi: 10.18632/oncotarget.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015;36(4):250–6.Epub 2015/03/12. doi: 10.1016/j.it.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280): 1463–9. Epub 2016/03/05. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230): 124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Medicine. 2017;9(1):34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, Feldman J, Poole DJ, Litzinger M, Steinberg SM, Jones E, Chen C, Marte J, Parnes H, Wright J, Dahut W, Schlom J, Gulley JL. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 2007; 178(4 Pt l):1515–20. Epub 2007/08/21. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 36.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28(7):1099–105. Epub 2010/01/27. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulley JL, Arlen PM, Madan RA, Tsang K-Y, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, Cereda V, Vergati M, Steinberg SM, Halabi S, Jones E, Chen C, Parnes H, Wright JJ, Dahut WL, Schlom J. Immunologic and Prognostic Factors Associated with Overall Survival Employing a Poxviral-based PSA Vaccine in Metastatic Castrate-resistant Prostate Cancer. Cancer immunology, immunotherapy : CII. 2010;59(5):663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulley JL, Madan RA, Tsang KY, Jochems C, Marte JL, Farsaci B, Tucker JA, Hodge JW, Liewehr DJ, Steinberg SM, Heery CR, Schlom J. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer immunology research. 2014;2(2):133–41. Epub 2014/04/30. doi: 10.1158/2326-6066.Cir-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J, Shore ND, Chen L, Heery CR, Gerritsen WR, Priou F, Kantoff PW. Results of PROSPECT: A randomized phase 3 trial of PROSTVAC-V/F (PRO) in men with asymptomatic or minimally symptomatic metastatic, castration-resistant prostate cancer. Journal of Clinical Oncology. 2018;36(15_suppl):5006-. doi: 10.1200/JCO.2018.36.15_suppl.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nature Biotechnology. 2009;27:129. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 41.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006;24(19):3089–94. Epub 2006/07/01. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 42.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–9. Epub 2009/06/19. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 43.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363(5):411–22. Epub 2010/09/08. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 44.Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, Amling CL, Stephenson RA, Simko J, Sheikh NA, Sims RB, Frohlich MW, Small EJ. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014;106(11). Epub 2014/09/27. doi: 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, Liniker E, Ben K, Munhoz R, Rapisuwon S, Gherardini PF, Chmielowski B, Wang X, Shintaku IP, Wei C, Sosman JA, Joseph RW, Postow MA, Carlino MS, Hwu W-J, Scolyer RA, Messina J, Cochran AJ, Long GV, Ribas A. High response rate to PD-1 blockade in desmoplastic melanomas. Nature. 2018;553:347.doi:10.1038/nature25187 https://www.nature.com/articles/nature25187#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prat A, Navarro A, Pare L, Reguart N, Galvan P, Pascual T, Martinez A, Nuciforo P, Comerma L, Alos L, Pardo N, Cedres S, Fan C, Parker JS, Gaba L, Victoria I, Vinolas N, Vivancos A, Arance A, Felip E. Immune-Related Gene Expression Profiling After PD-1 Blockade in Non-Small Cell Lung Carcinoma, Head and Neck Squamous Cell Carcinoma, and Melanoma. Cancer Res 2017;77(13):3540–50. Epub 2017/05/11. doi: 10.1158/0008-5472.Can-16-3556. [DOI] [PubMed] [Google Scholar]
- 47.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(40):16723–8. Epub 2011/09/22. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV. SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology. 2013;2(7):e25205. Epub 2013/09/28. doi: 10.4161/onci.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, Algazi AP, Pampaloni MH, Lobach IV, Hwang J, Pierce RH, Gratz IK, Krummel MF, Rosenblum MD. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 2016;126(9):3447–52. Epub 2016/08/16. doi: 10.1172/jci87324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. Epub 2011/06/02. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. Epub 2006/09/30. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 52.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353(25):2654–66. Epub 2005/12/24. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 53.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011;29(6):610–8. Epub 2011/0½0. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 54.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, Flejou JF, Gibault L, Verkarre V, Regnard JF, Pages ON, Oudard S, Mlecnik B, Sautes-Fridman C, Fridman WH, Damotte D. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 2013;19(15):4079–91. Epub 2013/06/21. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 55.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009;29:1093. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 56.Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, Wu C, Jiang J. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cellular Physiology and Biochemistry. 2015;37(4):1560–71. [DOI] [PubMed] [Google Scholar]
- 57.Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J, Ouakrim H, Regnier F, Lupo A, Alifano M, Damotte D, Donnadieu E. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(17):E4041–E50. Epub 2018/04/11. doi: 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen Y-T, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+ / regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maleki Vareki S, Garrigos C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Critical Reviews in Oncology/Hematology. 2017;116:116–24. doi: 10.1016/j.critrevonc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015;14(4):847–56. Epub 2015/02/20. doi: 10.1158/1535-7163.Mct-14-0983. [DOI] [PubMed] [Google Scholar]
- 61.Haffner MC, Guner G, Taheri D, Netto GJ, Palsgrove DN, Zheng Q, Guedes LB, Kim K, Tsai H, Esopi DM, Lotan TL, Sharma R, Meeker AK, Chinnaiyan AM, Nelson WG, Yegnasubramanian S, Luo J, Mehra R, Antonarakis ES, Drake CG, De Marzo AM. Comprehensive Evaluation of Programmed Death-Ligand 1 Expression in Primary and Metastatic Prostate Cancer. The American journal of pathology. 2018;188(6):1478–85. Epub 2018/03/27. doi: 10.1016/j.ajpath.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radestad E, Egevad L, Jorns C, Mattsson J, Sundberg B, Nava S, Ericzon BG, Henningsohn L, Levitsky V, Uhlin M. Characterization of infiltrating lymphocytes in human benign and malignant prostate tissue. Oncotarget. 2017;8(36):60257–69. Epub 2017/09/28. doi: 10.18632/oncotarget.19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nardone V, Botta C, Caraglia M, Martino EC, Ambrosio MR, Carfagno T, Tini P, Semeraro L, Misso G, Grimaldi A, Boccellino M, Facchini G, Berretta M, Vischi G, Rocca BJ, Barone A, Tassone P, Tagliaferri P, Del Vecchio MT, Pirtoli L, Correale P. Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther 2016;17(11):1213–20. Epub 2016/10/30. doi: 10.1080/15384047.2016.1235666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A(12):1797–803. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y, Attwood K, Versaggi C, Omilian A, Bshara W, Xu B, Kauffman E, Mohler J, Guru K, Li Q, George S, Basse P, Morrison C, Papanicolau-Sengos A, Kalinski P, Chatta GS. Association of high CD8+ tumor infiltrating lymphocytes at prostatectomy with improved survival of prostate cancer patients. Journal of Clinical Oncology. 2018;36(15_suppl):5068-. doi: 10.1200/JCO.2018.36.15_suppl.5068. [DOI] [Google Scholar]
- 66.Richardsen E, Uglehus RD, Due J, Busch C, Busund LT. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology. 2008;53(1):30–8. Epub 2008/05/31. doi: 10.1111/j.1365-2559.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 67.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med 2005;201(8):1257–68. Epub 2005/04/13. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature Reviews Immunology. 2013;13:227. doi:10.1038/nri3405 https://www.nature.com/articles/nri3405#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008;26(27):4410–7. Epub 2008/09/20. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 70.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–20. Epub 2002/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JK, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37(1):60–73. Epub 2012/07/17. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF. Critical Role for CD103+/CD141+ Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell. 2016;30(2):324–36. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van’t Veer LJ, Sperling AI, Wolf DM, Krummel MF. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–52. Epub 2014/12/03. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, Ronchese F. Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer Immunol Immunother. 2008;57(11):1665–73. Epub 2008/03/04. doi: 10.1007/s00262-008-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010; 141(1):39–51. Epub 2010/04/08. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33(19):2423–31. Epub 2013/06/04. doi: 10.1038/onc.2013.191. [DOI] [PubMed] [Google Scholar]
- 77.Bronte V, Brandau S, Chen S-H, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nature Communications. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M, Pasquini E, Rinaldi A, Sumanasuriya S, Lambros MB, Neeb A, Luciano R, Bravi CA, Nava-Rodrigues D, Dolling D, Prayer-Galetti T, Ferreira A, Briganti A, Esposito A, Barry S, Yuan W, Sharp A, de Bono J, Alimonti A. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018. Epub 2018/06/29. doi: 10.1038/s41586-018-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Randall TD. Stromal cells put the brakes on T-cell responses. Immunology And Cell Biology. 2011;90:469. doi: 10.1038/icb.2011.106. [DOI] [PubMed] [Google Scholar]
- 80.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends in Immunology. 2014;35(1):32–7. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E. Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC clinical pathology. 2014;14:38. Epub 2014/09/02. doi: 10.1186/1472-6890-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud JL, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. American journal of respiratory and critical care medicine. 2014;189(7):832–44. Epub 2014/02/04. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 83.Peske JD, Thompson ED, Gemta L, Baylis RA, Fu YX, Engelhard VH. Effector lymphocyte-induced lymph node-like vasculature enables naïve T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun 2015;6:7114. Epub 2015/05/15. doi: 10.1038/ncomms8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L, Pang Y, Moses HL. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends in immunology. 2010;31(6):220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive Stroma in Human Prostate Cancer. Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling. 2002;8(9):2912–23. [PubMed] [Google Scholar]
- 86.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30. Epub 2017/01/20. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 87.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Current opinioninimmunology. 2013;25(2):268–76.Epub2013/04/13. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 88.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Seminars in oncology. 2015;42(4):663–71. Epub 2015/09/01. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gajewski T, Zha Y, Hernandez K, Li Y, Bao R, Alexieff P, Andrade J, Luke JJ, Spranger S. Density of immunogenic antigens and presence or absence of the T cell-inflamed tumor microenvironmentin metastatic melanoma. Journal of Clinical Oncology. 2015;33(15_suppl):3002-. doi: 10.1200/jco.2015.33.15_suppl.3002. [DOI] [Google Scholar]
- 90.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321. doi: 10.1038/nature21349 https://www.nature.eom/articles/nature21349#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 91.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. Epub 2014/11/28. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012;122(3):899–910. Epub 2012/02/02. doi: 10.1172/jci45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. Epub 2015/04/04. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 94.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nature Reviews Immunology. 2003;3:867. doi: 10.1038/nri1222 https://www.nature.eom/articles/nri1222#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 95.Girard J-P, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nature Reviews Immunology. 2012;12:762. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 96.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25(6):989–1001. Epub 2006/11/23. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown FD, Turley SJ. Fibroblastic Reticular Cells: Organization and Regulation of the T Lymphocyte Life Cycle. The Journal of Immunology. 2015;194(4):1389–94. doi: 10.4049/jimmunol.1402520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proceedings of the National Academy of Sciences. 2000;97(23):12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. The Journal of Experimental Medicine. 2007;204(3):489–95. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naïve T cells. Nat Immunol 2007;8(11):1255–65. Epub 2007/09/26. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 101.Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, Ludewig B, Carroll MC, Turley SJ. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol 2014;15(10):973–81. Epub 2014/08/26. doi: 10.1038/ni.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011;71(20):6391–9. Epub 2011/09/09. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 103.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014;35(11):571–80. Epub 2014/12/03. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Hwang JY, Randall TD, Silva-Sanchez A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Frontiers in Immunology. 2016;7:258. doi: 10.3389/fimmu.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McNamee EN, Rivera-Nieves J. Ectopic Tertiary Lymphoid Tissue in Inflammatory Bowel Disease: Protective or Provocateur? Frontiers in Immunology. 2016;7(308). doi: 10.3389/fimmu.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sato M, Hirayama S, Matsuda Y, Wagnetz D, Hwang DM, Guan Z, Liu M, Keshavjee S. Stromal Activation and Formation of Lymphoid-Like Stroma in Chronic Lung Allograft Dysfunction. Transplantation. 2011;91(12):1398–405. doi: 10.1097/TP.0b013e31821b2f7a. [DOI] [PubMed] [Google Scholar]
- 107.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, Validire P, Remark R, Hammond SA, Cremer I, Damotte D, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74(3):705–15. Epub 2013/12/25. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 108.Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology. 2013;2(12):e26836. Epub 2014/02/06. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. The Journal of Experimental Medicine. 1996;183(4):1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol 2003;3(4):292–303. Epub 2003/04/02. doi: 10.1038/nril054. [DOI] [PubMed] [Google Scholar]
- 111.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10577–82. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Randall TD. Chapter 7 - Bronchus-Associated Lymphoid Tissue (BALT): Structure and Function. In: Fagarasan S, Cerutti A, editors. Advances in Immunology: Academic Press; 2010. p. 187–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nature Medicine. 2004;10:927. doi: 10.1038/nm1091 https://www.nature.com/articles/nm1091#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 114.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med 2009;206(11):2339–49. Epub 2009/10/08. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hämmerling G, Garbi N, Sutter G, Worbs T, Förster R. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. The Journal of Experimental Medicine. 2009;206(12):2593–601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winter S, Loddenkemper C, Aebischer A, Räbel K, Hoffmann K, Meyer TF, Lipp M, Höpken UE. The chemokine receptor CXCR5 is pivotal for ectopic mucosa-associated lymphoid tissue neogenesis in chronic Helicobacter pylori-induced inflammation. Journal of Molecular Medicine (Berlin, Germany). 2010;88(11):1169–80. doi: 10.1007/s00109-010-0658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORyt and LTi cells. The Journal of Experimental Medicine. 2011;208(1):125–34. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infection and immunity. 2003;71(6):3572–7. Epub 2003/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thaunat O, Patey N, Caligiuri G, Gautreau C, Mamani-Matsuda M, Mekki Y, Dieu-Nosjean M-C, Eberl G, Ecochard R, Michel J-B, Graff-Dubois S, Nicoletti A. Chronic Rejection Triggers the Development of an Aggressive Intragraft Immune Response through Recapitulation of Lymphoid Organogenesis. The Journal of Immunology. 2010;185(1):717–28. doi: 10.4049/jimmunol.0903589. [DOI] [PubMed] [Google Scholar]
- 120.BF K, NI W, Barbara W, Qi L, RN H, LF G. Lymphoid Neogenesis in Murine Cardiac Allografts Undergoing Chronic Rejection. American Journal of Transplantation. 2005;5(3):510–6. doi: doi: 10.1111/j.1600-6143.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 121.Silina K, Soltermann A, Movahedian Attar F, Casanova R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng PF, Curioni Fontecedro A, Foukas P, Levesque MP, Moch H, Line A, van den Broek M. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res 2017. Epub 2017/12/28. doi: 10.1158/0008-5472.CAN-17-1987. [DOI] [PubMed] [Google Scholar]
- 122.Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012;72(16):3997–4007. Epub 2012/08/02. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 123.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of Lymphoidlike Stroma and Immune Escape by Tumors That Express the Chemokine CCL21. Science. 2010;328(5979):749–52. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 124.Solinas C, Garaud S, De Silva P, Boisson A, Van den Eynden G, de Wind A, Risso P, Rodrigues Vitoria J, Richard F, Migliori E, Noel G, Duvillier H, Craciun L, Veys I, Awada A, Detours V, Larsimont D, Piccart-Gebhart M, Willard-Gallo K. Immune Checkpoint Molecules on Tumor-Infiltrating Lymphocytes and Their Association with Tertiary Lymphoid Structures in Human Breast Cancer. Front Immunol. 2017;8:1412. Epub 2017/1½3. doi: 10.3389/fimmu.2017.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard J-P. High Endothelial Venule Blood Vessels for Tumor-Infiltrating Lymphocytes Are Associated with Lymphotoxin β-Producing Dendritic Cells in Human Breast Cancer. The Journal of Immunology. 2013;191(4):2001–8. doi: 10.4049/jimmunol.1300872. [DOI] [PubMed] [Google Scholar]
- 126.Garcia-Hernandez ML, Uribe-Uribe NO, Espinosa-Gonzalez R, Kast WM, Khader SA, Rangel-Moreno J. A Unique Cellular and Molecular Microenvironment Is Present in Tertiary Lymphoid Organs of Patients with Spontaneous Prostate Cancer Regression. Front Immunol. 2017;8:563. Epub 2017/06/02. doi: 10.3389/fimmu.2017.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Carlo E, Magnasco S, D’Antuono T, Tenaglia R, Sorrentino C. The prostate-associated lymphoid tissue (PALT) is linked to the expression of homing chemokines CXCL13 and CCL21. Prostate. 2007;67(10):1070–80. Epub 2007/05/03. doi: 10.1002/pros.20604. [DOI] [PubMed] [Google Scholar]


