Abstract
Introduction:
Few studies have comprehensively characterized toxic chemicals related to waterpipe use and secondhand waterpipe exposure. This cross-sectional study investigated biomarkers of toxicants associated with waterpipe use and passive waterpipe exposure among employees at waterpipe venues.
Method:
We collected urine specimens from employees in waterpipe venues from Istanbul, Turkey and Moscow, Russia, and identified waterpipe and cigarette smoking status based on self-report. The final sample included 110 employees. Biomarkers of exposure to sixty chemicals (metals, volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), nicotine, and heterocyclic aromatic amines (HCAAs)) were quantified in the participants’ urine.
Results:
Participants who reported using waterpipe had higher urinary manganese (geometric mean ratio (GMR): 2.42, 95% confidence interval (CI): 1.16, 5.07) than never/former waterpipe or cigarette smokers. Being exposed to more hours of secondhand smoke from waterpipes was associated with higher concentrations of cobalt (GMR: 1.38, 95% CI: 1.10, 1.75). Participants involved in lighting waterpipes had higher urinary cobalt (GMR: 1.43, 95% CI: 1.10, 1.86), cesium (GMR: 1.21, 95% CI: 1.00, 1.48), molybdenum (GMR: 1.45, 95% CI: 1.08, 1.93), 1-hydroxypyrene (GMR: 1.36, 95% CI: 1.03, 1.80), and several VOC metabolites.
Conclusion:
Waterpipe tobacco users and nonsmoking employees of waterpipe venues had higher urinary concentrations of several toxic metals including manganese and cobalt as well as of VOCs, in a distinct signature compared to cigarette smoke. Employees involved in lighting waterpipes may have higher exposure to multiple toxic chemicals compared to other employees.
Keywords: Waterpipe, Secondhand Smoke, Toxicants, Carcinogen
1. INTRODUCTION
Waterpipes (also known as hookahs, narghile, shisha) have been used to smoke tobacco in the Eastern Mediterranean region and parts of Asia and Africa for centuries (World Health Organization, 2005). In the US and other western countries, the popularity of waterpipe smoking has surged dramatically in recent years particularly among youth (Maziak et al., 2015). The prevalence of waterpipe use among high school students nearly doubled between 2011 and 2014 (Jamal et al., 2017). This increase in prevalence has been related to the perception that waterpipe smoking is less harmful than cigarettes, social culture of waterpipe use, and aggressive marketing (Maziak et al., 2015). Most indoor smoke-free policies are exempt or are not enforced in waterpipe venues (Jawad et al., 2015). Although limited relative to cigarettes, studies indicate that waterpipe smoking is associated with acute and chronic health effects similar to cigarette smoking (El Zaatari et al., 2015). For instance, acute exposure to waterpipe tobacco smoke induces short-term increases in systolic blood pressure and heart rate in humans (Azar et al., 2016). In mice, waterpipe tobacco smoke induces changes in oxidative and inflammatory markers in the lungs (Khabour et al., 2012).
Toxicants in waterpipe smoke originate both from the charcoal and tobacco used in the waterpipe (Jukema et al., 2014). Most studies examining waterpipe secondhand smoke (SHS) constituents have measured markers of indoor air quality (Moon(a) et al. 2015, Al Mulla et al. 2015, Fiala et al. 2012). In a chamber experiment study, the sidestream smoke of a single waterpipe session had four times higher carcinogenic polycyclic aromatic hydrocarbons (PAHs), four times higher volatile organic compounds (VOCs) and 30 times higher carbon monoxide (CO) than a single cigarette (Daher et al., 2010). A case control study reported that, relative to never-smokers (n=40), concentrations of cadmium, lead, and zinc were higher in blood and saliva samples of waterpipe users (n=88) (Khabour et al., 2018). Only a small number of cross-sectional studies have measured biomarkers of exposure among non-smokers (Kumar et al. 2015). In a human subject study measuring VOC metabolites in urine samples of waterpipe users in hookah bars from San Francisco, USA (n=55), 2-cyanoethylmercapturic acid, a metabolite of acrylonitrile, increased 71% and phenylmercapturic acid, a benzene metabolite, increased 91% in urine samples of waterpipe users immediately after a single session of waterpipe smoking (Helen et al. 2014).
No comprehensive studies have determined the chemical profile of waterpipe tobacco smoke in humans compared to cigarette tobacco smoke. In a cross-sectional study in Turkey, Russia and Egypt, we collected urine specimens from workers in waterpipe venues, as part of a multi-site study to characterize exposure to waterpipe smoke exposure (Moon(b) et al., 2018). In an environmental assessment, we found relatively high concentrations of particulate matter (PM2.5), CO, particle bound PAHs, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and nicotine, indicating the presence of relatively high SHS concentrations that could affect the health of venue employees and customers (Moon(a) et al., 2015). In a limited biomarker assessment, we found that nonsmoking employees of waterpipe tobacco venues in Istanbul, Moscow, and Cairo had relatively high concentrations of SHS biomarkers, including two biomarkers of smoke carcinogens (urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), and urine 1-hydroxypyrene glucuronide (1-OHPG)) (Moon(b) et al., 2018).
For the current study, we have comprehensively characterized internal dose and metabolic biomarkers of active and secondhand waterpipe use and exposure, as determined in urine. A total of 64 chemicals were measured, including 13 metals, 25 VOCs, 9 nicotine metabolites, 7 PAHs and 10 heterocyclic aromatic amines (HCAA) many of which are toxic and carcinogenic (International Agency for Research on Cancer, 2004). The study included never-smokers, cigarette smokers, waterpipe smokers, and dual users of both cigarettes and waterpipes, and obtained information on direct waterpipe and cigarette use as well as on secondhand exposure to waterpipe and cigarette smoke. This information thus enabled us to dissect the effects of passive vs. active waterpipe smoking as well as the combined effects of waterpipe and cigarette use.
2. METHODS
2.1. Study Population
We conducted a study of waterpipe tobacco venues and their employees in Istanbul, Turkey, Moscow, Russia, and Cairo, Egypt (Moon(a) et al. 2015). The three countries were selected based on high waterpipe consumption data from the Global Adult Tobacco Survey (Morton et al., 2014). Due to administrative hurdles to transport the urine samples from the Cairo participants to the United States for analyses, the current study is restricted to Istanbul and Moscow. Within each city, we identified neighborhoods with a high concentration of waterpipe tobacco venues. Although we initially planned a stratified random sample strategy in each city, we switched to a convenience sample due to a low venue response rate. To be eligible to participate, venue owners/managers had to provide oral informed consent to conduct air sampling in the venue and at least one nonsmoking adult employee (≥18 years of age) had to provide oral informed consent and be willing to provide urine samples. A total of 26 venues (9 in Istanbul and 17 in Moscow) participated (venue response rates were 32% in Istanbul and 34% in Moscow). Data were collected between January and May 2013 in Istanbul and December 2013 to May 2014 in Moscow. Field staff fluent in the local language conducted all communications with venues and participants. Johns Hopkins Bloomberg School of Public Health Institutional Review Board and the ethics committees at the local co-investigators’ institutions approved the study protocol.
2.2. Questionnaire Data Collection
A total of 176 employees (mean six per venue) participated (96% response rate in Istanbul and 95% in Moscow). The questionnaire was administered by the field staffs using face-to-face interview method. Eleven employees did not provide urine samples during the time of the fieldwork. Urine samples were originally collected from 165 participants (94%) and used for an initial biomarker study. For this study, we used urine from 110 participants from 25 venues (9 from Istanbul and 16 from Moscow). The selection of this subset from the original urine samples was based solely on whether adequate volume (3 mL) of urine remained for conducting all the comprehensive laboratory analyses. At each venue, field staff administered a questionnaire to venue employees to assess information on sociodemographic and occupational factors, smoking status, exposure to SHS at work, home, and other places, and involvement with lighting waterpipes at the venue.
We categorized participant smoking status using data on self-reported tobacco use such as never/former smokers, cigarette only users, waterpipe only users, and dual users in order to distinguish the source of chemicals in the urine samples of the tobacco users. Current smokers reported smoking cigarettes or waterpipe within the past three months either “daily”, “less than daily”, or “just a few puffs”. These definitions were adapted from WHO guidelines by adding the question on smoking “just a few puffs” from another study of bar and nightclub employees (Jones et al. 2013). The reason for adding the “just a few puffs” to the questionnaire was to identify all current smokers and appropriately identify them from workers who were truly exclusively exposed to secondhand smoke. In a work environment where smoking is so common as in a waterpipe venue, employees could be smoking occasionally without a full perception that they are current smokers. As a goal was to distinguish between secondhand smoke exposure and active smoking it was important to make sure that even occasional smokers were categorized as smokers rather than as non-smokers. Never-smokers must have either never tried any kind of tobacco, or have smoked fewer than 100 cigarettes and smoked waterpipe for no more than one 20-minute session in their lifetime. Former smokers reported past tobacco use but did not report smoking cigarettes, waterpipes, or other types of tobacco within the past three months. The frequency of use of cigarette and waterpipe are presented in the supplement Table 1.
2.3. Urine Sample Collection
The urine samples (~50 ml first morning void or spot urine sample, whichever was possible) were collected in the morning in the privacy of the workers’ home. Each worker was provided with a set of instructions to collect the urine, as well as the sterile urine cup and an opaque container to put the urine cup inside and bring it to work at the time of their regular shift. The collection of morning urine samples minimized variability in urine dilution. The half-life of many chemicals in the urine are longer than several hours (for example, for cotinine the half-life in urine is around 15 hours, for metals the half-life is days, months, or even years depending on the metal). Therefore, the collection of the urine sample likely at least 8 hours since the last shift is reasonable for the current study. The average time from sampling to incubation in the refrigerator ranged from 2 hours to 6 hours. Samples were placed in a refrigerator until fieldworkers returned to pick up the samples. Because the original focus of the study was not to measure metals, the urine collection cups were not pre-screened for metals content. Samples were stored at −20°C before being shipped on dry ice to Johns Hopkins Bloomberg School of Public Health.
2.4. Laboratory Analysis
Urine samples were subsequently shipped on dry ice to the Centers for Disease Control and Prevention (CDC) Division of Laboratory Sciences for analysis. Urinary tobacco alkaloids (nicotine and its six major metabolites, plus minor tobacco alkaloids anatabine and anabasine) were measured by an isotope dilution high performance liquid chromatography/tandem mass spectrometric (HPLC-MS/MS) method (Wei et al. 2014). The limits of detection (LODs) for these alkaloids ranged from 0.39 to 10.5 ng/mL, depending on the analyte. The 7 PAH metabolites were quantified by online solid phase extraction coupled with high-performance liquid chromatography-isotope dilution tandem mass spectrometry, as previously described (Wang et al., 2014). The LODs for PAHs ranged from 8 to 90 ng/L. Urinary VOC metabolite concentrations were measured using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) according to a published procedure (Alwis et al., 2012). LODs for VOCs ranged from 0.500 to 15.0 ng/mL. Total urine element concentrations of beryllium, cadmium, cobalt, manganese, lead, strontium, thallium, and uranium were measured using inductively coupled plasma mass spectrometry (ICP-MS). The limits of detection for these elements in urine range from 0.002 to 2.34 ng/mL, depending on the analyte (Caldwell et al., 2005, Jarrett et al., 2008). The protocol for urine metal analyses was the same with the protocol used in the U.S. National Health and Nutrition Examination Survey (CDC 2016). Ten heterocyclic aromatic amines (HCAAs) (i.e AαC, MeAC, Trp-P-1, Trp-P-2, Glu-P-1, Glu-P-2, lQ, PhlP, harman and norharman) in urine were measured by an isotope-dilution high-performance liquid chromatography/electrospray ionization tandem mass spectrometry (lD HPLC-ESI MS/MS) using a method of Zhang et al. (Zhang et al., 2016). The limit of detection for urinary HCAA ranged from 0.31 to 0.83 pg/mL, depending on the analyte. Finally, creatinine was measured by a commercial automated, colorimetric enzymatic (creatinase) method implemented on a Roche/Hitachi Cobas 6000 Analyzer. The list of all metabolites with abbreviations and their LOD values are shown in the supplement Table 2. Values below the LOD were replaced with the LOD/square root of two (Maziak et al., 2015).
2.5. Data Analysis
Urinary biomarker concentrations were right-skewed and were log-transformed; medians or geometric means were reported. We examined the correlation between chemicals and cotinine using Spearman rank correlation coefficients. We assessed the median, 25th percentile, and 75th percentile of chemical concentrations overall and by age, sex, country, average work hours per week, smoking status, living with a smoker, average numbers of hours of SHS exposure from cigarettes and from waterpipes per week (separately), and involvement with lighting waterpipes at work. The variables SHS from cigarette and waterpipe were right-skewed and dichotomized by their median values. We divided the concentrations of urine biomarkers by concentrations of urinary creatinine to correct for variability in urine dilution.
In a separate model for each of the 64 biomarkers (modeled as log-transformed and corrected by urinary creatinine), we estimated their association with smoking status, SHS exposure variables, and lighting of waterpipes (a total of 6 exposure variables) using multivariable linear regression with robust variance and an independent correlation structure within venues. The beta coefficients and 95% confidence intervals from each model were exponentiated to obtain geometric mean ratios (GMRs) and corresponding 95% confidence intervals (CI). All models were adjusted for city, age, sex, and average work hours per week. Models for SHS exposure variables and for lighting of waterpipes were further adjusted for smoking status. In a sensitivity analysis, we also reported the adjusted GMRs (95%CI) for SHS exposure variables and lighting of waterpipes only among never/former smokers.
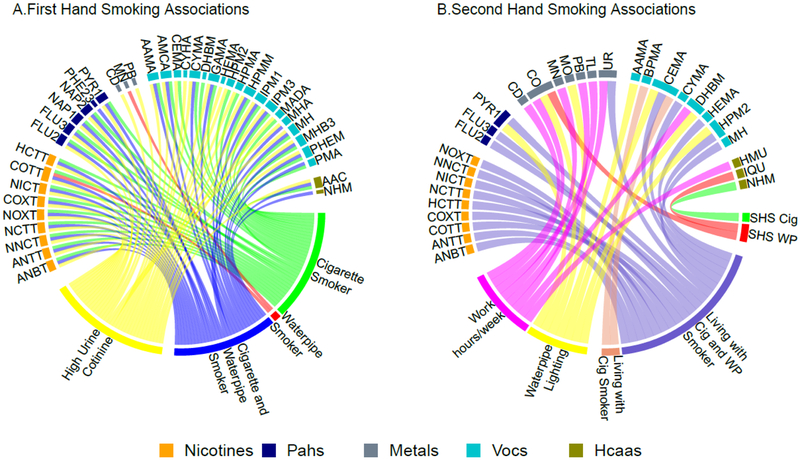
To facilitate the description and assessment of the numerous models run, all statistically significant GMRs at the nominal p-value of 0.05 were graphically displayed in two circle plots (Figure 1). Each line in the circle plot represents a statistically significant GMR between a biomarker and the connected tobacco related exposure variable. The magnitude of the GMR is not shown in the circle plots.
Figure 1: Geometric Mean Ratios (GMR) of nicotine related metabolites, polycyclic aromatic hydrocarbons (PAHs), metals, volatile organic compounds (VOCs) and heterocyclic aromatic amines (HCAAs) by first and secondhand smoking variables.
All lines represent significant association between the biomarkers and first and secondhand smoke variables. The colors of the lines represent the different first hand and secondhand smoke variables.
*Models adjusted for sex, age, average work hour per week and country. SHS: Secondhand Smoke, Cig: Cigarette, WP: Waterpipe
Statistical analyses were performed with Stata Version 13.1 (StataCorp, College Station, TX) and R Version 3.2.2 (R Foundation for Statistical Computing, www.r-project.org, Vienna, Austria). All statistical tests were two-sided and p-values less than 0.05 were considered statistically significant.
3. RESULTS
3.1. Participant Characteristics
The final study sample included 110 total employees, 61 in Istanbul and 49 in Moscow (Table 1). Most employees were men in Turkey, whereas there were equal proportions of men and women in Russia. The majority of employees had less than a high school education. The median age was 30 years. The most common primary job description was bartender/waiter (58%) and a total of 44% of employees reported being involved with lighting waterpipes. Few employees were never smokers (18%) or former smokers (7%). Current use of both cigarette and waterpipe was more common in Istanbul (48%), while only cigarette smoking was more common in Moscow (35%). The prevalence of smoking waterpipe only was 16% (26% in Istanbul and 4% in Moscow). Overall, 47% of employees lived with at least one smoker in their household. Employees reported a median of 48 hours per week of exposure to cigarette SHS and 21 hours per week of exposure to waterpipe SHS (including both working and non-working hours). Waterpipe users were younger and more likely to be men than sole cigarette users or never/former smokers (Supplement Table 1). The median (IQR) number of cigarettes smoked per day was 20 (10, 20) and 17 (10, 20) for sole cigarette users and for dual users, respectively. The median (IQR) number of waterpipes per day was 1.5 (1, 5) and 1.0 (0, 15) for sole waterpipe users and for dual users, respectively.
Table 1:
Characteristics of employees of waterpipe tobacco venues in Istanbul and Moscow in 2013-2014
| Characteristics | Overall (N=110) | Istanbul (N=61) | Moscow (N=49) | p-value (a) |
|---|---|---|---|---|
| Number of employees per venue | 6 (5, 9) | 11(5, 17) | 6 (5, 9) | <0.001 |
| Age, years | 30 (24, 42) | 30 (24, 40) | 29 (23, 48) | 0.97 |
| Male, % | 76% | 93% | 55% | <0.001 |
| Education, % | <0.001 | |||
| Less than high school | 68% | 88% | 43% | |
| High school | 22% | 12% | 35% | |
| College/university | 10% | 0% | 22% | 0.15 |
| Primary job, % | ||||
| Owner/Manager | 14% | 17% | 10% | |
| Bartender/Waiter | 58% | 62% | 55% | |
| Cook/kitchen staff | 11% | 5% | 18% | |
| Other(b) | 17% | 16% | 17% | <0.001 |
| Smoking status, % | ||||
| Never smoker | 18% | 1% | 39% | |
| Former smoker | 7% | 7% | 8% | |
| Current cigarette only smoker | 26% | 18% | 35% | |
| Current waterpipe only smoker | 16% | 26% | 4% | |
| Current cigarette and waterpipe smoker | 33% | 48% | 14% | |
| Average time at work, hours/week | 54(48, 66) | 60 (54, 72) | 48 (36, 48) | 0.001 |
| Lives with a smoker | 0.017 | |||
| No | 48% | 36% | 63% | |
| Yes, Cigarette Smoker Only | 45% | 56% | 31% | |
| Yes, Cigarette and Waterpipe Smoker | 7% | 8% | 6% | |
| Secondhand smoke from cigarettes, hours/week | 48 (32, 70) | 60 (42, 84) | 36 (25, 55) | 0.001 |
| Secondhand smoke from waterpipe, hours/week | 21 (8, 42) | 42 (13, 60) | 10 (4, 24) | 0.001 |
| Involved with lighting waterpipes for customers,% yes | 44% | 57% | 26% | 0.001 |
Notes: Categorical variables are percentages of total sample (N). Continuous variables are median (25th percentile, 75th percentile).
P-values are Pearson’s chi-square test of independence for categorical variables and Ranksum for continuous variables.
“Other” includes watchman (N=6) and other miscellaneous primary positions.
The time of data collection were between January and May 2013 in Istanbul and December 2013 to May 2014 in Moscow
3.2. First Hand Smoke Exposure by Employee Characteristics
Participants who reported using waterpipes exclusively had significantly higher concentration of urinary manganese (Table 2) compared to never or former smokers. After adjustment for age, sex, work hours per week and country, the GMR (95%CI) for manganese comparing sole waterpipe users to never/former smokers was 2.60 (1.24, 5.45) (Table 2) (Figure 1A). All PAH metabolite concentrations (Supplemental Table 3), and most VOC metabolite concentrations were higher among dual and sole cigarette users compared to never/former smokers (Supplemental Table 5) (Figure 1A). The concentrations of two HCAA metabolites (2-amino-9H-pyrido[2,3-b] indole (AαC), and 9Hpyrido[3,4-b] indole (norharman)) were significantly higher among dual users than never/former smokers (Supplemental Table 4).
Table 2:
Ratio of geometric means of employee Urine METAL concentrations by employee characteristics in waterpipe tobacco venues in Istanbul and Moscow
| Characteristics | N | Adjusted Geometric Mean Ratios (95% Confidence Intervals) | |||
|---|---|---|---|---|---|
| Smoking Status | Cd | Co | Cs | Mn | |
| Never/Former (Ref) | 28 | 1.00 | 1.00 | 1.00 | 1.00 |
| Current cigarette only | 28 | 1.07 (0.71, 1.63) | 1.07 (0.77, 1.47) | 1.01 (0.80, 1.28) | 1.69 (0.97, 2.94) |
| Current waterpipe only | 18 | 0.92 (0.53, 1.61) | 1.04 (0.68, 1.61) | 0.94 (0.68, 1.29) | 2.60 (1.24, 5.45) |
| Current cigarette and waterpipe | 36 | 1.10 (0.68, 1.76) | 1.11 (0.77, 1.60) | 1.02 (0.78, 1.34) | 1.54 (0.82, 2.89) |
| Urine Cotinine (ng/g) | |||||
| ≤1277.0 (Ref) (tertile 1) | 24 | 1.00 | 1.00 | 1.00 | 1.00 |
| >1277.0 (tertiles 2 and 3) | 86 | 1.57(1.14, 2.17) | 0.99 (0.77, 1.29) | 0.98 (0.82, 1.19) | 0.93 (0.59, 1.45) |
| Work Hours Per Week (per hour) | 109 | 1.01 (1.00, 1.02) | 1.50 (0.96, 2.35) | 1.00 (0.99, 1.01) | 1.02 (1.01, 1.03) |
| Living with a smoker | |||||
| No (Ref) | 53 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes, cigarette smoker only | 49 | 1.07 (0.78, 1.47) | 1.16 (0.91, 1.46) | 0.94 (0.78, 1.13) | 1.34 (0.88, 2.04) |
| Yes, cigarette and waterpipe smoker | 8 | 1.43 (0.80, 2.57) | 1.48 (0.95, 2.29) | 0.90 (0.65, 1.27) | 1.82 (0.84, 3.94) |
| SHS from Cigarettes Per Week (Hours) | |||||
| ≤48 (Ref) | 45 | 1.00 | 1.00 | 1.00 | 1.00 |
| >48 | 65 | 1.05 (0.43, 2.55) | 0.83 (0.43, 1.63) | 0.91 (0.67, 1.23) | 0.71 (0.39, 1.27) |
| SHS from WP Per Week (Hours) | |||||
| ≤21 (Ref) | 49 | 1.00 | 1.00 | 1.00 | 1.00 |
| >21 | 61 | 1.14 (0.83, 1.55) | 1.38 (1.10, 1.75) | 1.14 (0.96, 1.36) | 1.11 (0.73, 1.68) |
| Lighting waterpipes | |||||
| No (Ref) | 62 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 48 | 1.13 (0.79, 1.59) | 1.43 (1.10, 1.86) | 1.21 (1.00, 1.48) | 0.82 (0.51, 1.30) |
| Smoking Status | Mo | Pb | Tl | Ur | |
| Never/Former (Ref) | 28 | 1.00 | 1.00 | 1.00 | 1.00 |
| Current cigarette only | 28 | 1.10 (0.77, 1.56) | 1.10 (0.77, 1.56) | 0.98 (0.74, 1.28) | 1.26 (0.91, 1.77) |
| Current waterpipe only | 18 | 0.96 (0.59, 1.54) | 1.31 (0.94, 1.82) | 0.91 (0.63, 1.31) | 1.11 (0.71, 1.74) |
| Current cigarette and waterpipe | 36 | 1.23 (0.82, 1.84) | 1.23 (0.82, 1.84) | 0.80 (0.59, 1.10) | 1.32 (0.90, 1.92) |
| Urine Cotinine (ng/g) | |||||
| ≤1277.0 (Ref) (tertile 1) | 24 | 1.00 | 1.00 | 1.00 | 1.00 |
| >1277.0 (tertiles 2 and 3) | 86 | 1.19 (0.89, 1.57) | 1.30 (1.07, 1.58) | 1.15 (0.92, 1.42) | 1.02 (0.65, 1.61) |
| Work Hours Per Week (per hour) | 109 | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.01 (1.00, 1.02) | 1.02 (1.01, 1.03) |
| Living with a smoker | |||||
| No (Ref) | 53 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes, cigarette smoker only | 49 | 0.91 (0.69, 1.19) | 0.96 (0.80, 1.16) | 0.93 (0.76, 1.15) | 0.98 (0.76, 1.26) |
| Yes, cigarette and waterpipe smoker | 8 | 1.15 (0.69, 1.89) | 1.40 (0.99, 1.97) | 1.07 (0.73, 1.58) | 1.62 (1.02, 2.56) |
| SHS from Cigarettes Per Week (Hour) | |||||
| ≤48 (Ref) | 45 | 1.00 | 1.00 | 1.00 | 1.00 |
| >48 | 65 | 1.43 (0.83, 2.48) | 0.92 (0.60, 1.41) | 0.81 (0.55, 1.18) | 1.00 (0.54, 1.87) |
| SHS from WP Per Week (Hours) | |||||
| ≤21 (Ref) | 49 | 1.00 | 1.00 | 1.00 | 1.00 |
| >21 | 61 | 1.02 (0.78, 1.33) | 1.02 (0.78, 1.33) | 1.08 (0.88, 1.32) | 0.80 (0.63, 1.03) |
| Lighting waterpipes | |||||
| No (Ref) | 62 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 48 | 1.45 (1.08, 1.93) | 1.08 (0.87, 1.32) | 1.17(0.93, 1.47) | 1.18 (0.89, 1.57) |
The geometric mean ratios for smoking status and work hours per week were adjusted for age, sex, and country.
The geometric mean ratios for urine cotinine, living with a smoker, SHS from cigarettes per week, and SHS from waterpipe per week and lighting waterpipes were further adjusted for cigarette and waterpipe smoking status.
Cd: Cadmium, Co: Cobalt, Cs: Cesium, Mn, Manganese. Ref: Reference group for regression analysis
The geometric mean ratios for smoking status and work hours per week were adjusted for age, sex, and country.
The geometric mean ratios for urine cotinine, living with a smoker, SHS from cigarettes per week, and SHS from waterpipe per week and lighting waterpipes were further adjusted for cigarette and waterpipe smoking status.
Mo: Molybdenum, Pb: Lead, Tl: Thallium, Ur: Uranium. Ref: Reference group for regression analysis
3.3. Second Hand Smoke Exposure by Employee Characteristics
Being exposed to more hours of SHS from waterpipes was associated with higher concentrations of cobalt. (Figure 1B). After adjustment, the GMR (95%CI) comparing participants who reported more than 21 versus 21 or less hours of SHS exposure from waterpipes per week was 1.38 (1.10, 1.75) for cobalt.
Higher number of work hours per week in a waterpipe venue was associated with higher urinary cadmium, manganese, thallium, and uranium as well as with higher concentrations of 1,3-butadiene (DHBM) and 1-methyl-9h-pyrido-indole (HMU) (Figure 1B). After adjustment for sociodemographic variables and smoking status, each additional hour of work per week in a waterpipe venue was associated with 2% increase in manganese and uranium, 1% increase in cadmium, thallium (Table 2); 1-methyl-9h-pyrido-indole (HMU) (Supplemental Table 4), 1,3-butadiene (DHBM), and acrolein (CEMA) (Supplemental Table 5).
Living with a cigarette and waterpipe smoker was associated with the all nicotine metabolites (Supplemental Table 6), and with several biomarkers of exposure to VOCs (acrolein, acrylonitrile, 34MH (3-methylhippuric acid + 4-methylhippuric acid) (xylene), and propylene oxide) (Figure 1B) (Supplemental Table 5). Living with a cigarette smoker was associated with nicotine 1’N-oxide, a nicotine metabolite, and metabolites of several VOCs (acrolein, acrylonitrile, and styrene) (Supplemental Table 5).
The associations between SHS exposure variables and the different biomarkers remained similar in magnitude but were less likely to be statistically significant in analyses restricted to the subset of participants who were never/former smokers (Supplemental Tables 7, 8, 9, 10, 11).
3.4. Occupational Exposure to First Hand Waterpipe Smoking
Employees involved in lighting waterpipes, compared to those not involved, had higher urine concentrations of cobalt (GMR: 1.43, 95% CI: 1.10, 1.86), cesium (GMR: 1.21, 95% CI: 1.00, 1.48), molybdenum (GMR: 1.45, 95% CI: 1.08, 1.93) (Table 2), 1-hydroxypyrene (GMR: 1.36, 95% CI: 1.03, 1.80) (Supplemental Table 3), acrylamide (AAMA) (GMR: 1.48, 95% CI: 1.07, 2.06), acrolein (CEMA) (GMR: 1.51, 95% CI: 1.07, 2.06), and 1,3-butadiene (DHBM) (GMR: 1.60, 95% CI: 1.17, 2.19) (Supplemental Table 5).
4. DISCUSSION
This is the first study comparing metals, VOCs, PAHs and HCAAs by waterpipe use and secondhand smoke exposure compared to never/former smokers and to cigarette smoking. Sole waterpipe users (no cigarette smoking) had higher manganese concentrations compared to never and former smokers, while manganese concentrations were higher but not statistically significantly different in cigarette smokers, suggesting that waterpipe smoking may be a specific source of manganese. High urine cobalt concentration was significantly associated with high SHS hours from waterpipe and involved in lighting waterpipe. In addition, employees involved in lighting waterpipes had higher urine cobalt, urine cesium, urine molybdenum, urine 1-hydroxypyrene, AAMA (acrylamide), CEMA (acrolein), and DHBM (1,3-butadiene). Cigarette use was more strongly associated with nicotine-derived biomarkers compared to waterpipe use, possibly because of higher daily smoking intensity among cigarette smokers vs. waterpipe smokers. Overall these findings support that waterpipe smoking and occupational and non-occupational exposure to secondhand waterpipe smoke may be an important source of metal exposure and contribute to increased exposure to certain PAHs and VOCs. Waterpipe venue employees and waterpipe users have higher occupational risk of exposure to these potentially toxic chemicals than other persons.
Waterpipe SHS is derived from combustion of both tobacco and the burning source (usually charcoal). To date, over eighty toxicants have been quantified in waterpipe tobacco smoke (Shihadeh(a) et al., 2015). During recent years, smoke machine experiments (Saadawi et al., 2012, Shihadeh(b), 2003, Shihadeh and Saleh, 2005, Schubert et al., 2015, Apsley et al., 2011) showed that mainstream waterpipe smoke delivers phenanthrene, fluoranthene, chrysene (Shihadeh and Saleh, 2005), pyrene and naphthalene (Apsley et al., 2011), benzene, toluene and pyridine (Schubert et al., 2015), and numerous chemical elements such as arsenic, cadmium, chromium, manganese, lead (Saadawi et al., 2012) and copper, zinc, boron, chromium, nickel, and lead (Apsley et al., 2011). Smoke machine studies also suggest that waterpipe SHS contains similar or higher concentrations of many carcinogens and toxic chemicals as compared to cigarette SHS, including carcinogenic PAHs, PM2.5, volatile aldehydes, and CO (Daher et al., 2010, Hammal et al., 2015). We previously reported that in the waterpipe venues where our participants worked, there were high concentrations of indoor air markers of SHS, including PM2.5, CO, PAHs, the tobacco specific nitrosamine NNK, and air nicotine (Moon(a) et al. 2015). In a preliminary biomarker study (Moon(b) et al., 2018), we found that among non-tobacco smokers (including both cigarettes and waterpipes) higher work hours were associated with higher urine cotinine and hair nicotine concentrations, showing that employees in these venues are effectively exposed to secondhand waterpipe smoke.
In this study, we found that higher urine cobalt concentration was associated with high SHS hours from waterpipe and being involved in lighting waterpipe. Cobalt likely originates from the charcoal and tobacco used in the waterpipe. Indeed, recent studies have detected cobalt in the waterpipe tobacco (Schubert et al., 2015) and charcoal (Schubert et al., 2015) and also in the main stream smoke of waterpipe (Shihadeh(a) et al., 2015, Apsley et al., 2011). Burning charcoal, as a source of cobalt, can release cobalt to environment air (Elsayed et al., 2016). The employees involved in lighting waterpipe might have been exposed to high level of cobalt from burning coal even though they are not a waterpipe user. The employees involved in lighting waterpipe generally prepare the waterpipe tobacco with bare hands, therefore, they might be exposed to the chemicals through oral (hand-mouth) and dermal routes. Another explanation could be that they usually prepare the burned charcoal in non-ventilated areas of the venues and are exposed to the burned charcoal smoke more than other employees. To the best of our knowledge, this is the first study reporting the association between SHS from waterpipe and cobalt biomarkers, supporting that cobalt is an important metal of concern related to occupational waterpipe SHS. Cobalt and cobalt-containing compounds are classified as Group 2B human carcinogens by the International Agency for Research on Cancer (International Agency for Research on Cancer, 2004). High chronic cobalt exposure by inhalation in humans results in effects on the respiratory system (respiratory irritation, wheezing, asthma, decreased lung function, pneumonia, and fibrosis), cardiovascular system (cardiomyopathy, cardiogenic shock, sinus tachycardia, left ventricular failure, and enlarged hearts), and gastrointestinal system (nausea, vomiting, and diarrhea) (United States Environmental Protection Agency 2016).
Participants who reported using waterpipes but not cigarettes had higher concentrations of urinary manganese compared to never/former smokers, and working longer hours per week in a waterpipe venue was also associated with higher urine manganese concentrations (Table 2). Manganese has been previously identified in waterpipe tobacco (Saadawi et al., 2012) and waterpipe charcoal (Elsayed et al., 2016). In another experimental study (Schubert et al., 2015), seventeen metals were analyzed in the waterpipe tobacco and charcoal prior to and after waterpipe smoking. The highest amounts in unburned and burned tobacco and charcoal were aluminum, manganese and barium. These findings in waterpipe tobacco and charcoal, paired with the finding of higher manganese concentrations in the urine samples of waterpipe users and with high working hours in a waterpipe venue confirm that waterpipes are an important source of exposure to manganese. This is a particularly important finding given strong evidence on the impact of chronic manganese exposure on neurodevelopmental and neurodegenerative disorders (Lucchini et al., 2018).
Employees involved in lighting waterpipes had higher urine cobalt, urine cesium, urine molybdenum, urine 1-hydroxypyrene, AAMA (acrylamide), CEMA (acrolein), and DHBM (1,3-butadiene) compared to those not involved. Previously, PAH metabolites in the main stream smoke of waterpipe (Shihadeh and Saleh, 2005, Apsley et al., 2011) and higher 1-hydroxypyrene excretion in the urine samples of waterpipe users after a waterpipe smoking session (Jacob et al., 2013) were reported. Besides, a smoke machine study found that 75–92% of the PAH compounds in the main stream smoke of waterpipe originated in the charcoal and over 95% of the benzo(a)pyrene originated in the charcoal (Monzer et al., 2008). The current study is the first time that PAH biomarkers have been measured in employees involved in lighting waterpipes, finding higher concentrations of 1-hydroxypyrene, a metabolite of pyrene (International Agency for Research on Cancer, 2004). In our preliminary biomarker study, being involved in lighting waterpipes was associated with higher concentrations of urine and saliva cotinine and hair nicotine (Moon(b) et al., 2018). We have substantially expanded the evaluation of real-world exposure to SHS in waterpipe venues and biomarkers of SHS exposure with this study.
The major strength of this study was the application of a comprehensive analytical assessment of harmful and potentially harmful tobacco constituents to biospecimens that have been collected from workers in waterpipe venues and that allowed us to compare non-users with cigarette smokers, as well as active and secondhand waterpipe users. Other strengths included the multicity design, high employee participation rate, and the detailed exposure characterization based on self-reported data and biomarkers of both cigarette and waterpipe smoking status and on exposure to SHS in the home.
This study has several limitations. The sample size was relatively small, in particular for never/former smokers. Our sample of waterpipe tobacco venues in each city was selected by convenience; therefore, these venues may not be representative of all waterpipe venues in each city. Further, fear of regulation by less compliant venues may have played a role in the low venue participation rate (ranging from 32%–34%) in each city. Identifying a threshold to differentiate active versus passive exposure to tobacco is difficult in waterpipe venues in this setting because of substantial occupational SHS exposure. We used whether the employee lived with a smoker as a proxy for secondhand smoke exposure in the home; however, we could not account for SHS exposures in other places and residual confounding is possible. Also, waterpipe secondhand smoke exposure at home always occurred with cigarettes, so we could not isolate exposure to waterpipes at home. This study was conducted among employees of waterpipe venues in a real-world setting, and we may not have been able to account for other important sources of variability that could explain some of the heterogeneity of our findings across biomarkers. The biomarkers measured in this study encompass a variety of different exposure windows depending on the half-lives and concentrations could likely reflect aggregate exposures at work, in public places, or in the home. However, employees reported spending a substantial number of hours at work and therefore, we believe that occupational exposure is likely to be an important source of exposure to these toxicants biomarkers or their precursors. We used never/former smokers as a reference group in order to compare with active waterpipe use. Given every participant in this study had at least some exposure to waterpipe smoke, the effect of active waterpipe use might have been underestimated in the study. The standard plastic urine cups were not airtight; further, an internal standard was not performed on the spot before freezing to be able to calculate later on VOC & PAH loss percentage or extraction efficiency. Therefore, the VOC & PAH results might be underestimated in the current study. The lack of information on food consumption of the study participants is a major limitation as different types of foods can be major sources and act as a potential confounder of many of the chemicals analyzed in this study. We combined the never/former smokers into one group despite the differences in percentages reported by both countries percentage of these group are different between countries (1% Russia, 39% Turkey). Urine concentrations of chemicals between the two cities, however, were not statistically different significant after adjustment for sociodemographic characteristics, supporting that combining the two countries for this group is a reasonable strategy. Urine samples underwent freeze-thaw cycles two times, which might affect the quality of sample. However, for most of the chemicals, e.g. metals, the measures are resistant to the number of freeze-thaw cycles (CDC 2014a). Finally, the effect of passive and occupational exposure to waterpipe was determined mostly by comparing differences in self-reported hours of exposure but a group of participants unexposed to waterpipe SHS was missing.
5. CONCLUSION
A higher number of work hours per week in a waterpipe venue was associated with higher urinary cadmium, manganese, thallium, and uranium as well as with higher concentrations of DHBM (1,3-butadiene) and HMU (1-methyl-9H-pyrido-indole). Being exposed to SHS from waterpipe in occupational and non-occupational settings and occupational involvement in lighting waterpipe were related to exposure to various metals, PAHs, HCAAs and VOCs. Based on our findings, waterpipe users and nonsmoking employees of waterpipe tobacco venues, and potentially also patrons, may be at an increased risk of health problems as a result of waterpipe use and secondhand exposure. Smoke-free legislation has been successful in reducing exposure to SHS from cigarettes (CDC 2014b). Expanding clean indoor air regulations to waterpipe venues may reduce potentially harmful exposures to workers.
We consider this study as a screening project to identify potentially relevant chemicals related to waterpipe smoking and SHS exposure. Further studies seeking relevant chemicals in more detail related to waterpipe smoking and SHS exposure are needed.
Supplementary Material
HIGHLIGHTS.
Participants who reported using waterpipes exclusively had significantly higher concentration of urinary manganese compared to never or former smokers.
Being exposed to more hours of secondhand smoke from waterpipes was associated with higher urine concentrations of cobalt.
Employees involved in lighting waterpipes, compared to those not involved, had higher urine concentrations of cobalt, cesium, molybdenum, 1-hydroxypyrene, acrylamide, acrolein, and 1,3-butadiene.
Waterpipe tobacco users and nonsmoking employees of waterpipe venues had higher urinary concentrations of several toxic metals including manganese and cobalt as well as of volatile organic compounds.
Acknowledgments
The authors gratefully acknowledge the contributions of our fieldworkers: Bugrahan Cizenmih, Serdar Doruk Avunduk, Deniz Ever, Merve Kayserili, Pelinsu Cagla Batur, Tufan Ayrik, Ortac Ikizler, Didem Ungor, Gizem Kural, Evren Ceylan Morgul in Istanbul and Bogdan Ladan, Nina Slepchenko, Anna Zavelskay in Moscow. Additionally, Victor De Jesus, Li Zhang, and Jun Feng led efforts to measure biomarkers of exposure to VOCs, nicotine, and HCAAs.
Funding: This study was supported by the Institute for Global Tobacco Control at the Johns Hopkins Bloomberg School of Public Health (#119187) with funding from the Bloomberg Initiative to Reduce Tobacco Use and the National Heart, Lung, and Blood Institute (1R01HL134149). Ana Navas-Acien was supported by the National Institute of Environmental Health Sciences (5P30ES009089). Pablo Olmedo was supported by the Alfonso Martin Escudero Foundation (Postdoctoral Fellowship 2014).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no potential conflicts of interest.
Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Defense, Department of the Army, U.S. Medical Department, or the U.S. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the Centers for Disease Control and Prevention. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
6. REFERENCES
- Al Mulla et al. 2015.Al Mulla A, Fanous N, Seidenberg AB, Rees VW. Secondhand smoke emission concentrations in waterpipe cafes in Doha, Qatar. Tob Control 2015;24(e3):227–31. [DOI] [PubMed] [Google Scholar]
- Alwis et al., 2012.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta. 2012;750(42): 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apsley et al., 2011.Apsley A, Galea KS, Sanchez-Jimenez A, Semple S, Wareing H, Van Tongeren M. Assessment of polycyclic aromatic hydrocarbons, carbon monoxide, nicotine, metal contents and particle size distribution of mainstream Shisha smoke. JEHR 2011; 11(2):93–104. [Google Scholar]
- Azar et al., 2016.Azar RR, Frangieh AH, Mroué J, et al. Acute effects of waterpipe smoking on blood pressure and heart rate: a real-life trial. Inhal Toxicol 2016;28(8):339–342. [DOI] [PubMed] [Google Scholar]
- Caldwell et al., 2005.Caldwell KL, Hartel J, Jarrett J, Jones RL. Inductively coupled plasma mass spectrometry to measure multiple toxic elements in urine in NHANES 1999-2000." Atomic Spectroscopy 2005;26(1): 1–7. [Google Scholar]
- CDC 2014.Centers for Disease Control and Prevention 2014. Accessed on March 2019 Laboratory Procedure Manual: Antimony, Arsenic, Barium, Beryllium, Cadmium, Cesium, Cobalt, Lead, Manganese, Molybdenum, Platinum, Strontium, Thallium, Tin, Tungsten, Uranium and Total Arsenic Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/UM_UMS_UTAS_UTASS_H_MET.pdf
- CDC 2014.Centers for Disease Control and Prevention 2014. Accessed on Feb 2017 The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. 2014. Available from: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/. [PubMed]
- CDC 2016.Centers for Diseases Control and Prevention. National Health and Examination Survey. NHANES 2015-2016 Laboratory Data. [Accessed on March 2019] Available from: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBegin Year=2015
- Daher et al., 2010.Daher N, Saleh R, Jaroudi E, et al. Comparison of carcinogen, carbon monoxide, and ultrafine particle emissions from narghile waterpipe and cigarette smoking: Sidestream smoke measurements and assessment of second-hand smoke emission factors. Atmos Environ 2010;44(1): 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed et al., 2016.Elsayed Y, Dalibalta S, Abu-Farha N. Chemical analysis and potential health risks of hookah charcoal. Sci Total Environ 2016;569–570(1):262–268. [DOI] [PubMed] [Google Scholar]
- El Zaatari et al., 2015.El-Zaatari ZM, Chami HA, Zaatari GS. Health effects associated with waterpipe smoking. Tob Control. 2015;24(Suppl 1):i31–i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala et al. 2012.Fiala SC, Morris DS, Pawlak RL. Measuring Indoor Air Quality of Hookah Lounges. Am J Public Health 2012; 102(11):2043–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammal et al., 2015.Hammal F, Chappell A, Wild TC, et al. ‘Herbal’ but potentially hazardous: an analysis of the constituents and smoke emissions of tobacco-free waterpipe products and the air quality in the cafes where they are served. Tob Control. 2015;24(3):290–297. [DOI] [PubMed] [Google Scholar]
- Helen et al. 2014.Helen GS, Benowitz NL, Dains KM, Havel C, Peng M, Jacob P III. Nicotine and carcinogen exposure after water pipe smoking in hookah bars. Cancer Epidemiol Biomarkers Prev 2014;23(6): 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2004. Accessed on Feb 2017 IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 83. Tobacco Smoke and Involuntary Smoking. Available from: http://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf. [PMC free article] [PubMed]
- Jacob et al., 2013.Jacob P 3rd, Abu Raddaha AHA, Dempsey D, Havel C, Peng M, Yu L, et al. Comparison of Nicotine and Carcinogen Exposure with Waterpipe and Cigarette Smoking. Cancer Epidemiol Biomarkers Prev 2013;22 (5):765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal et al., 2017.Jamal A, Gentzke A, Hu SS, et al. Tobacco Use Among Middle and High School Students - United States, 2011-2016. MMWR 2017;66(23):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad et al., 2015.Jawad M, El Kadi L, Mugharbil S, Nakkash R. Waterpipe tobacco smoking legislation and policy enactment: a global analysis. Tob Control 2015;24(Suppl 1): i60–i65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarret et al., 2008.Jarrett JM, Xiao G, Caldwell KL, et al. Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS." Journal of Analytical Atomic Spectrometry 2008;23(7): 962–967. [Google Scholar]
- Jones et al., 2013.Jones MR, Wipfli H, Shahrir S, Avila-Tang E, Samet JM, et al. Secondhand tobacco smoke: an occupational hazard for smoking and non-smoking bar and nightclub employees. Tob Control 2013;22(5):308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukema et al., 2014.Jukema JB, Bagnasco DE, Jukema RA. Waterpipe smoking: not necessarily less hazardous than cigarette smoking. Neth Heart J 2014;22(3):91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour et al., 2012.Khabour OF, Alzoubi KH, Bani-Ahmed M, Eissenberg T, Shihadeh A. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhal Toxicol 2012;24(10): 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour et al., 2018.Khabour OF, Alzoubi KH, Al-Sheyab NA, Azab MA, Massadeh AM, et al. Plasma and saliva levels of three metals in waterpipe smokers: a case control study Inhal Toxicol 2018;30(6): 224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. 2015.Kumar SR, Davies S, Weitzman M, Sherman S. A review of air quality, biological indicators and health effects of second-hand waterpipe smoke exposure. Tob Control 2015;24(Suppl 1):i54–i59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini et al., 2018.Lucchini RG, Aschner M, Landrigan PJ, Cranmer JM. Neurotoxicity of manganese: Indications for future research and public health intervention from the Manganese 2016 conference. NeuroToxicology 2018;64(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak et al., 2015.Maziak W, Taleb ZB, Bahelah R, et al. The global epidemiology of waterpipe smoking. Tob Control 2015;24(Suppl 1):i3–i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzer et al., 2008.Monzer B, Sepetdjian E, Saliba N, Shihadeh A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem Toxicol 2008;46(9):2991–2995. [DOI] [PubMed] [Google Scholar]
- Moon(a) et al. 2015.Moon KA, Magid H, Torrey C, et al. Secondhand smoke in waterpipe tobacco venues in Istanbul, Moscow, and Cairo. Environ Res 2015;142(7):568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon(b) et al., 2018.Moon KA, Rule AM, Magid HS, et al. Biomarkers of Secondhand Smoke Exposure in Waterpipe Tobacco Venue Employees in Istanbul, Moscow, and Cairo. Nicotine & Tobacco Research 2018;20(4):482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton et al., 2014.Morton J, Song Y, Fouad H, et al. Cross-country comparison of waterpipe use: nationally representative data from 13 low and middle-income countries from the Global Adult Tobacco Survey (GATS). Tob Control 2014;23(5):419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert et al., 2015.Schubert J, Müller FD, Schmidt R, Luch A, Schulz TG. Waterpipe smoke: source of toxic and carcinogenic VOCs, phenols and heavy metals? Arch Toxicol 2015;89(11):2129–2139. [DOI] [PubMed] [Google Scholar]
- Shihadeh(a) et al., 2015.Shihadeh A, Schubert J, Klaiany J, El Sabban M, Luch A, Saliba NA. Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob Control 2015;24(Suppl 1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadawi et al., 2012.Saadawi R, Figueroa JAL, Hanley T, Caruso J. The hookah series part 1: total metal analysis in hookah tobacco (narghile, shisha) – an initial study. Anal Methods 2012;4(11):3604–3611. [Google Scholar]
- Shihadeh(b) 2003.Shihadeh A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food and Chemical Toxicology 2003;41(1): 143–152. [DOI] [PubMed] [Google Scholar]
- Shihadeh and Saleh, 2005.Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, tar, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Toxicol 2005;43(5):655–661. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency 2016. Accessed on Feb 2017 Cobalt Compounds. United States Environmental Protection Agency. Available from: https://www.epa.gov/sites/production/files/2016-09/documents/cobalt-compounds.pdf. [Google Scholar]
- Wei et al. 2014.Wei B, Feng J, Rehmani IJ, et al. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin Chim Acta. 2014;436(10):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2014.Wang Y, Meng L, Pittman EN, et al. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409(4):931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2005. World Health Organization Study Group on Tobacco Product Regulation. [Accessed on Jan 2017] Water pipe tobacco smoking: health effects, research needs and recommended actions by regulators. Available from: http://www.who.int/tobacco/global_interaction/tobreg/Waterpipe%20recommendation_Final.pdf. [Google Scholar]
- Zhang et al., 2016.Zhang L, Xia Y, Xia B, et al. High-throughput and sensitive analysis of urinary heterocyclic aromatic amines using isotope-dilution liquid chromatography–tandem mass spectrometry and robotic sample preparation system. Anal Bioanal Chem 2016;408(28):8149–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.