Abstract
Objective.
Although cough impairment (dystussia) is common in individuals with amyotrophic lateral sclerosis (ALS) and contributes to a reduced physiologic capacity to defend the airway, characteristics of dystussia have not yet been delineated. We therefore aimed to compare voluntary cough spirometry airflow patterns between individuals with ALS and healthy age and gender matched controls.
Methods.
Thirty-two individuals with a diagnosis of probable-definite ALS (El-Escorial Criterion) and 29 healthy age and gender-matched controls underwent voluntary cough spirometry testing. Two blinded raters derived six objective voluntary cough airflow measures including: peak inspiratory phase duration, peak inspiratory flow rate, compression phase duration, peak expiratory rise time, peak expiratory flow rate, and cough volume acceleration. Independent samples t-tests with Cohen’s d effect sizes were performed between Healthy vs. ALS groups for cough metrics (alpha=0.05).
Results.
ALS individuals demonstrated prolonged inspiratory phase and expiratory phase rise time durations, reduced inspiratory and expiratory flow rates, and lower cough volume acceleration during voluntary cough production compared to healthy controls (p< 0.05). No differences in compression phase duration were observed (p>0.05).
Conclusions.
This study compared characteristics of voluntary cough airflow patterns of individuals with ALS to healthy-matched controls. Findings identified impairments in both inspiratory and expiratory voluntary cough airflow, resulting in slower, weaker, and thus less effectiveness voluntary cough production in ALS individuals. These data afford insight into the impaired physiology underlying inadequate airway clearance and secretion management in individuals with ALS.
Keywords: voluntary cough, spirometry, airway protection, amyotrophic lateral sclerosis, dystussia
Introduction.
Cough production is dependent upon a series of finely coordinated events beginning with contraction of the inspiratory muscles (pulling air into the lungs), closure of the glottis (generation of subglottic pressure), and abduction of the vocal folds with an expulsive expiration (forcing the glottis open)(1). This represents the inspiratory, compression and expiratory phases of cough production, respectively. High-velocity shearing forces produced during the expulsive phase of cough serve to maintain pulmonary health by managing secretions and airway safety during swallowing. Impairments in cough production (dystussia) are associated with the development of pneumonia and increased mortality rates across several patient populations, including amyotrophic lateral sclerosis (ALS) (2, 3).
Upper and lower motor neuron degeneration in ALS impacts the ability of respiratory and laryngeal musculature to work in concert during all three phases of cough (inspiratory, compression and expiratory) due to weakness (lower motor neuron) and rigidity (upper motor neuron). This leads to aberrant cough flow and compromised airway clearance abilities in this patient population (4, 5). The underlying pathophysiology of dystussia in ALS involves atrophy of inspiratory muscles responsible for achieving adequate inspired lung volume (creating a mechanical disadvantage during the final expiratory phase of cough); the inability to control, and rapidly abduct and adduct the vocal folds (6); and impaired expiratory muscle contraction to expel material from the airways (7). Rapid movement of high-velocity shearing forces (i.e., high peak expiratory flow rates and short expiratory rise times) is associated with effective airway clearance (5). Therefore, airflow parameters derived during the expulsive phase of cough are thought to serve as a surrogate measure of airway defense physiologic capacity (8, 9). Thus, impairments in respiratory and upper airway function contribute to impaired execution of the cough motor response in ALS, leading to pulmonary sequelae, poor secretion management, respiratory failure, and death (5, 7, 10).
Given the shared anatomical and neural substrates of cough and swallowing function, the relationships between dystussia and dysphagia have been the subject of recent clinical research (4, 11–15). Indeed, the utility of voluntary cough testing to serve as a clinical assay for the identification of dysphagia has been examined within several patient populations, including ALS (4, 11, 16–18). Although it is known that individuals with ALS demonstrate poor airway defense physiologic capacity and secretion management that is attributed, in part, to impaired cough effectiveness (5); no study has comprehensively examined aberrant features of ALS cough airflow patterns in comparison to healthy age and gender-matched individuals. Thus, the precise mechanisms of impaired cough function in ALS are largely unknown. Therefore, the aim of the current study was to determine if differences in voluntary cough function exist between individuals with ALS and healthy age and gender-matched controls. Secondary to identified impairments in ventilation, lung compliance and airway clearance, we hypothesized that individuals with ALS would demonstrate prolonged inspiratory phase duration, reduced inspiratory and expiratory flow rates, prolonged compression phase duration, and reduced cough strength and effectiveness compared to healthy controls.
Materials and Methods.
Thirty-two individuals with a diagnosis of probable-definite ALS (El-Escorial Criterion), and 29 healthy age and gender-matched controls were evaluated. Demographics of this cohort are provided in Table 1. Diagnosis of ALS was confirmed by a neurologist specializing in neuromuscular disease, and patients were recruited from two multidisciplinary ALS clinics. Inclusion criteria for the ALS group were 1) diagnosis of ALS, and no 2) mechanical ventilation or tracheotomy, or 3) significant concurrent respiratory illness or disease. Exclusion criteria for the healthy control group were: 1) history of smoking, 2) significant concurrent respiratory illness or disease, or 3) history of traumatic brain injury, stroke, radiation therapy, or disease/injury resulting in dysphagia. Healthy age and gender-matched controls were recruited using university approved recruitment portals including Research Match and Health Street. The study was approved by the institutional review board (IRB201501172, 201602098) and conducted in accordance with the amended Declaration of Helsinki.
Table 1.
Demographics for individuals with amyotrophic lateral sclerosis and healthy age and gender-matched controls.
| Demographics | ALS (N=32) | Healthy (N=29) |
|---|---|---|
| Age (years) | 62.16 (+/− 10.75) Range: 36-83 | 57.97 (+/− 13.48) Range: 37-86 |
| Gender | 50% FEMALE (N=16) | 55% FEMALE (N=16) |
| Onset Type | 31% BULBAR (N=10) | N/A |
| Disease Duration (months) | 32.28 (+/− 26.61) Range: 4-96 | N/A |
| Disease Severity (ALSFRS-R) | 36.54 (+/− 6.3) Range: 20 - 46 | N/A |
All participants attended a single testing session to complete voluntary cough testing using the current gold standard voluntary cough spirometry pneumotachograph setup. Voluntary cough spirometry testing permits quantification of aberrant features within all three primary phases of cough production (i.e., inspiratory, compression, expiratory). A three liter syringe was utilized to calibrate an oral pneumotachograph (MLT 1000, ADInstruments, Inc; Colorado Springs, CO), which was connected to a disposable spirometry filter mouthpiece (MQ 304, Vacumed; Ventura, CA) (Figure 1). Participants were seated with nose clips in place, and instructed to hold the spirometry filter comfortably and place their mouth around the mouthpiece prior to starting the task. The clinician held the spirometry filter for participants who were not able to hold the device due to limb immobility. Each participant was provided with a model and the following verbal instructions: “Cough hard, like you have something stuck in your throat” for a total of three trials. Including calibration procedures, voluntary cough testing was completed in 15 minutes. Cough airflow signals were recorded to an iMac desktop computer (Apple, Inc. USA) using Lab Chart software (AD Instruments, Inc. Version 8), and low pass filtered at 60 Hz for subsequent analysis. The first cough of each epoch was analyzed, yielding a total of three coughs per participant. Cough airflow parameters from a single cough for each participant were analyzed by two research assistants with training in cough analysis and LabChart. The following six physiologic parameters of cough motor output were derived for each cough waveform:
Inspiratory phase duration: time from start of inspiration following tidal breathing to the end of inspiration
Peak inspiratory flow rate: from onset of inspiration to the deepest inspiratory flow during the inspiratory phase of cough
Compression phase duration: time from the end of inspiratory phase to the beginning of expulsive phase
Peak expiratory rise time: duration from the beginning of expiration to the greatest peak of the expiratory phase
Peak expiratory flow rate: peak airflow achieved during the expiratory phase of cough
Cough volume acceleration: derived by dividing peak expiratory flow rate by peak expiratory phase rise time
In addition to objective physiological parameters of cough motor output, exploratory visual analysis qualitatively characterizing aberrant waveform airflow characteristics between groups was performed, as previously described(16).
Figure 1.
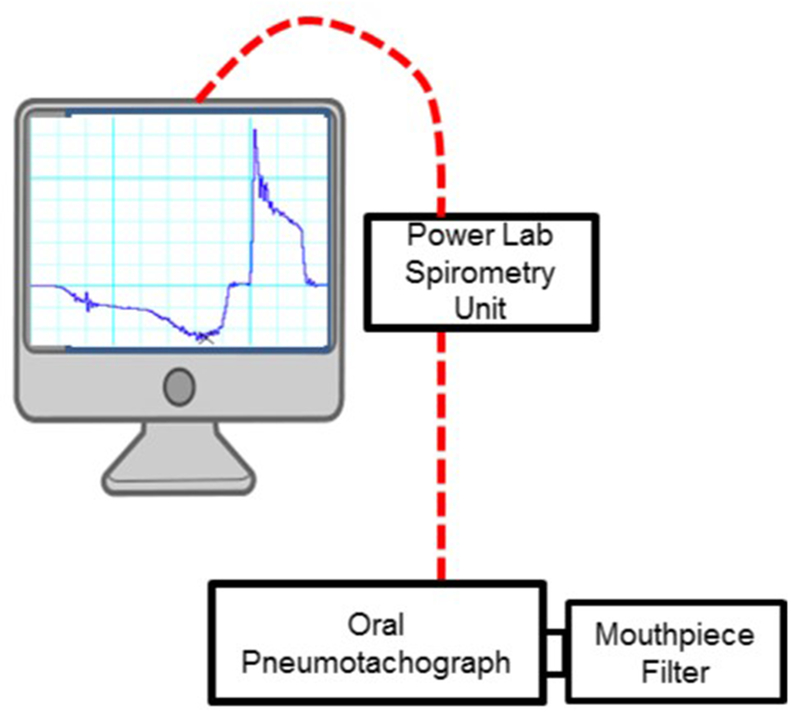
Schematic of the experimental setup for voluntary cough testing. LabChart (ADInstruments, Version 8) interfaced with the power lab and spirometry bridge to record cough motor parameters.
Statistical Analysis.
Inter and intra-rater reliability measurements were conducted on 50% and 25% of the cough dataset respectively. Intraclass correlation coefficients (ICC) were computed using average measure, absolute agreement, two-way random effects model, with ICC of 0.5-0.75 indicating moderate reliability, 0.75-0.90 good reliability and greater than 0.90 excellent reliability (19).
Descriptives were calculated for demographics. All continuous variables that demonstrated a non-normal distribution were transformed using a Blom transformation. Independent sample t-tests were conducted between groups (ALS vs. healthy controls) for the six cough airflow parameters of interest. In case of unequal variances, Welch’s correction was employed. Cohen’s d effect sizes were calculated to determine the magnitude of group differences with d=.2 indicating small magnitude effect, d=.5 a moderate effect and d ≥ .8 indicating a large effect. SPSS (Version 24) was used for all statistical tests and alpha was set at 0.05.
Results
Intra-and inter-rater reliability were acceptable across all cough spirometry ratings (Table 2). The following represent results of the independent samples t-test and Cohen’s d effect size to indicate clinical significance. Compared to healthy age and gender-matched controls, voluntary cough airflow in individuals with ALS demonstrated aberrant cough airflow : 1) longer peak expiratory phase rise time [t (57) = 4.74, p=0.0001, d=.59]; 2) lower peak inspiratory flow rate [t (57) = 4.34, p=0.0001, d=1.14]; 3) reduced cough volume acceleration [t (57) = 3.36, p=.001, , d=.79]; 4) lower peak expiratory phase flow rate [t (57) = 3.03, p=0.004, d=1.03]; and 5) prolonged inspiratory phase duration [t (57) = −2.26, p=0.028, d=.86]. No differences were observed between groups for voluntary cough compression phase duration (p>0.05). Mean group data are presented in Table 3.
Table 2.
Inter and intra-rater reliability with intraclass correlation coefficients compute for voluntary cough spirometry measurements using average measures, absolute agreement, two-way random effects model (SPSS Version 24).
| Average Measures | Inter/Intra-rater Reliability ICC | 95% Confidence Interval | F Test with True Value 0 | |||||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Value | df1 | df2 | Sig. (p=) | |||
| Inspiratory Phase Duration | Inter | .937 | .724 | .981 | 22.419 | 14 | 14 | .000 |
| Intra | . 998 | .962 | .996 | 81.446 | 12 | 12 | .000 | |
| Peak Inspiratory Flow Rate | Inter | .975 | .884 | .993 | 58.112 | 14 | 14 | .000 |
| Intra | .984 | .941 | .995 | 73.623 | 12 | 12 | .000 | |
| Compression Phase Duration | Inter | .707 | .045 | .908 | 4.719 | 13 | 13 | .004 |
| Intra | .994 | .980 | .998 | 158.401 | 12 | 12 | .000 | |
| Peak Expiratory Flow Rise Time | Inter | .777 | .194 | .930 | 6.409 | 13 | 13 | .001 |
| Intra | .755 | .251 | .923 | 4.488 | 12 | 12 | .007 | |
| Peak Expiratory Flow Rate | Inter | .964 | .877 | .988 | 33.617 | 14 | 14 | .000 |
| Intra | .996 | .988 | .999 | 279.769 | 12 | 12 | .000 | |
| Cough Volume Acceleration | Inter | .947 | .815 | .983 | 23.813 | 14 | 14 | .000 |
| Intra | .998 | .994 | .999 | 590.693 | 12 | 12 | .000 | |
Table 3.
Mean (standard deviation) data for the six voluntary cough airflow measures for ALS and healthy groups with associated p-values and Cohen’s d effect size.
| Airflow Measures | ALS (SD) | Healthy (SD) | Sig. (p=) | Cohen’s d |
|---|---|---|---|---|
| Inspiratory Phase Duration (sec) | 1.85 (0.75) | 1.43 (0.68) | .028* | .592 |
| Peak Inspiratory Flow Rate (L/s) | −1.11 (0.74) | −2.17 (1.08) | .000* | 1.14 |
| Compression Phase Duration (sec) | .39 (0.33) | .298 (0.12) | .151 | N/A |
| Peak Expiratory Flow Rate (L/s) | 5.78 (2.5) | 7.44 (1.54) | .004* | .799 |
| Peak Expiratory Rise Time (ms) | .11 (0.08) | .051 (0.03) | .000* | 1.03 |
| Cough Volume Acceleration (L/s/s) | 112.90 (79.1) | 176.53 (68.25) | .001* | .861 |
Alpha set at p<0.05.
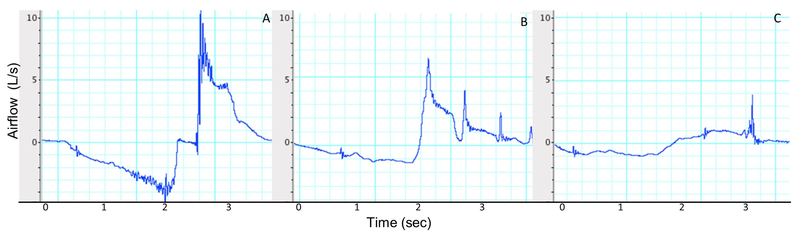
Qualitative visual analysis of generated airflow cough waveforms revealed distinct characteristics within the ALS group including blunted peak expiratory amplitudes, and aberrant compression phase (i.e., absence of compression, airflow occurring during apnoea) compared to healthy controls. Examples of qualitative visual cough waveform characterizations are depicted in Figure 3.
Figure 3.
Voluntary cough spirometry depicting A) cough flow with distinct inspiratory and expiratory parameters in a healthy control, and aberrant cough flow in two individuals with ALS with B) spinal-onset and C) bulbar-onset disease type.
Discussion
This study represents a novel evaluation of voluntary cough airflow patterns compared between individuals with ALS and a comparative group of healthy age and gender-matched controls. Results revealed differences in voluntary cough production for airflow parameters related to both the inspiratory and expiratory cough components. Specifically, ALS individuals demonstrated a prolonged inspiratory phase duration with a reduced peak inspiratory flow rate, and increased expiratory rise time with lower peak expiratory flow rates. This resulted in a reduced cough volume acceleration compared to healthy controls. Therefore, during voluntary cough production ALS patients inspired a lower volume of air during a prolonged inspiratory phase, followed by a longer time period to generate a lower peak expiratory airflow during the expulsive phase. This indicates a slower and shallower inspiration, likely attributable to LMN degeneration leading to weakness of the diaphragm and external intercostals, and UMN degeneration contributing to reduced range of motion and elasticity of the diaphragm and chest wall. Functionally, the reduction in airflow volume noted in the inspiratory phase of cough could place these individuals at a mechanical disadvantage given that they have a decreased volume of air for the subsequent expulsive phase of cough.
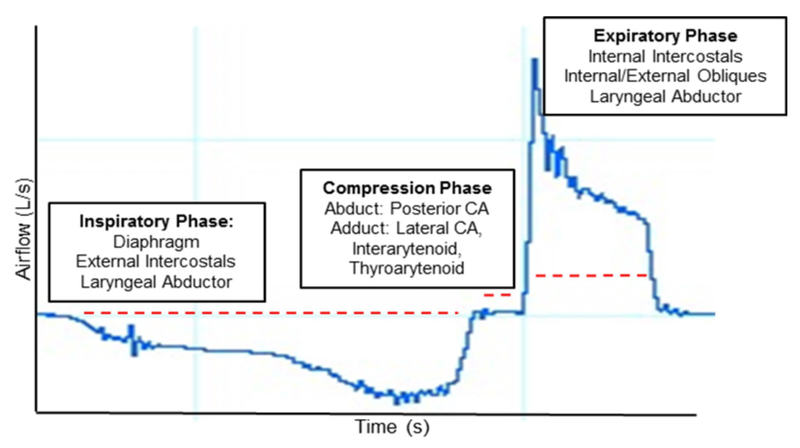
ALS individuals demonstrated expiratory impairments in voluntary cough production characterized by slower or prolonged expiration with resultant lower velocity sheering forces imperative in clearing the airway of aspirate and endogenous material. Similar to impairments in inspiratory phase parameters, expiratory phase flow rate is impacted by UMN and LMN degeneration, resulting in respiratory muscle weakness, namely the internal intercostals and abdominal muscles, as well as reduced lung compliance and elastic recoil(5, 7). Together, a prolonged inspiration and reduced peak expiratory flow rate contributed to reduced cough strength and effectiveness (i.e., cough volume acceleration), which was reduced by 36% in ALS individuals compared to the healthy control group. These data highlight aberrant airflow characteristics, resulting in slower, weaker and thus less effective cough production in individuals with ALS (Figure 2).
Figure 2.
A voluntary cough spirometry waveform of a healthy control depicting the three primary phases of cough: inspiratory compression and expiratory, from which objective temporal airflow parameters are computed.
Interestingly, no differences were noted between groups for compression phase duration. However, measures of timing do not account for previously described aberrant laryngeal kinematics in individuals with motor neuron disease (6). For example, complete laryngeal adduction has been shown to occur during the compression phase of voluntary cough production in individuals with motor neuron disease, suggesting preservation or compensation by the lateral cricoarytenoid and interarytenoid laryngeal muscles involved in adduction (6). However, previous studies identified airflow leak (i.e., aberrant airflow during the apneic period of the compression phase) contributing to weaker expulsive accelerations, and thereby weaker expiratory cough flow (16). Further, muscle fibers of the intrinsic laryngeal muscles, namely the posterior cricoarytenoid responsible for vocal fold abduction, are severely atrophied in ALS individuals likely contributing to impairments in rapid glottic coordination when transitioning from the compression to expulsive phase (6). Thus, these findings may suggest that impairments in the compression phase may be attributable to aberrant laryngeal kinematics rather than the timing of glottic closure.
In addition to objective airflow analysis, visual analysis of cough waveforms has been utilized to identify distinct cough patterns among patient populations. Smith-Hammond and colleagues qualitatively and quantitatively evaluated cough patterns in stroke patients and identified aberrant waveform characteristics in individuals who aspirated (material entered trachea) during swallowing, including a more shallow inspiratory phase and blunted expiratory peak flows (16). Similar findings were reported in individuals with motor neuron disease, characterized by the absence of distinct peak expiratory ‘spikes’, which was associated with reduced cough strength and increased mortality (3). In visually examining the cough waveforms of ALS individuals in the current study, distinct characteristics including blunted peak expiratory amplitudes, and aberrant compression phases (i.e., presence of airflow leak; absent) were observed compared to healthy controls (Figure 3). Although this represents an exploratory qualitative finding of the current study, further investigation to determine the utility of these subjective findings compared to objective airflow parameters is warranted.
Several limitations of this study must be acknowledged. First, voluntary cough spirometry measurements are dependent upon the maximum effort of the participant, thus testing is subject to variability secondary to factors such as fatigue and motivation. The former is a prevalent issue for testing paradigms in ALS. To mitigate this issue, three trials of each testing procedure were performed and the maximum cough production was utilized for analysis. Second, voluntary cough bypasses the sensory system and previous studies indicate that maximum voluntary cough function overestimate reflexive cough function in healthy volunteers; thus generalization to airway defense in the event of aspiration is limited (20). Further, given the clinical heterogeneity of ALS, it would be beneficial to document disease phenotypes, including upper versus lower motor neuron involvement, and slow versus fast progressing to develop more homogenous groups for comparison. Lastly, voluntary cough spirometry testing involves specialized and costly equipment with time consuming data analysis by trained analysists. Thus, future analysis will focus on identification of clinically feasible surrogate measures of voluntary cough strength that would be beneficial for clinical practice.
Conclusions
These data serve to extend our understanding of impairments across airflow parameters necessary for adequate cough production, thus affording insight into the physiologic capacity available for airway protection, clearance, and secretion management in individuals with ALS. This study represents a novel comparison of voluntary cough function between individuals with ALS and healthy controls, controlling for age and gender differences between groups. Results indicate impairments in both inspiratory and expiratory airflow parameters, resulting in slower, weaker, thus less effective cough production in individuals with ALS. These results corroborate previous findings demonstrating impaired respiratory function, including reduced expiratory flow and expiratory pressure generation in individuals with ALS (5, 7, 21). Consistent evaluation of voluntary cough function affords prompt identification of respiratory decline, allowing for timely implementation of cough assist, non-invasive ventilation, and respiratory training prior to significant degradation of function.
Acknowledgements:
LT recruited patients, collected and analyzed data and wrote the final manuscript.
AG analyzed the data and edits the final manuscript.
TV provided statistical expertise for data analysis and edited the final manuscript.
EP assisted in study design, patient recruitment, provided study funding and equipment, and final manuscript write up.
This study was sponsored, in part, by the National Institute of Neurological Disorders and Stroke (1R01 NS100859), the Amyotrophic Lateral Sclerosis Association Clinical Management Grant, and the NIH T32 Neuromuscular Plasticity Training Grant (T32HD043730).
Funding: This work was supported by the National Institute of Neurological Disorders and Stroke (1R01 NS100859), the Amyotrophic Lateral Sclerosis Association Clinical Management Grant, and the NIH Neuromuscular Plasticity Training Grant (T32HD043730).
Biographical Notes:
Lauren C. Tabor-Gray: Dr. Tabor is the Research Director of Speech Pathology at the Phil Smith Neuroscience Institute and ALS Clinic affiliated with Holy Cross Hospital in Fort Lauderdale, Florida.
Terrie Vasilopoulos: Dr. Vasilopoulos is an Assistant Professor in the Department of Anesthesiology in the College of Medicine at the University of Florida.
Alessandra Gallestagui: Ms. Gallestagui is an engineer in the Swallowing Systems Core Laboratory at the University of Florida.
Emily. K Plowman: Dr. Plowman is an Associate Professor in the Department of Speech-Language and Hearing Sciences and Disorders and Co-Director of the Swallowing Systems Core Laboratory at the University of Florida.
Footnotes
The authors have no conflicts of interest to disclose.
Clinicaltrials.gov Registration Numbers: NCT02962050; NCT03122145
References
- 1.Chung KF, Widdicombe JG. Cough: Setting the Scene In: Chung KF, Widdicombe J, editors. Pharmacology and Therapeutics of Cough. Berlin, Heidelberg: Springer Berlin; Heidelberg; 2009. p. 1–21. [DOI] [PubMed] [Google Scholar]
- 2.Niimi A, Matsumoto H, Ueda T, Takemura M, Suzuki K, Tanaka E, et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58(2):152–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudri MB, Liu C, Hubbard R, Jefferson D, Kinnear WJ. Relationship between supramaximal flow during cough and mortality in motor neurone disease. The European respiratory journal. 2002;19(3):434–8. [DOI] [PubMed] [Google Scholar]
- 4.Plowman EK, Watts SA, Robison R, Tabor L, Dion C, Gaziano J, et al. Voluntary Cough Airflow Differentiates Safe Versus Unsafe Swallowing in Amyotrophic Lateral Sclerosis. Dysphagia. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boitano LJ. Management of airway clearance in neuromuscular disease. Respiratory care. 2006;51(8):913–22; discussion 22-4. [PubMed] [Google Scholar]
- 6.Britton D, Benditt JO, Merati AL, Miller RM, Stepp CE, Boitano L, et al. Associations between laryngeal and cough dysfunction in motor neuron disease with bulbar involvement. Dysphagia. 2014;29(6):637–46. [DOI] [PubMed] [Google Scholar]
- 7.Benditt JO. Respiratory complications of amyotrophic lateral sclerosis. Seminars in respiratory and critical care medicine. 2002;23(3):239–47. [DOI] [PubMed] [Google Scholar]
- 8.Vincken W, Elleker G, Cosio MG. Detection of upper airway muscle involvement in neuromuscular disorders using the flow-volume loop. Chest. 1986;90(1):52–7. [DOI] [PubMed] [Google Scholar]
- 9.Hadjikoutis S, Wiles CM. Respiratory complications related to bulbar dysfunction in motor neuron disease. Acta neurologica Scandinavica. 2001;103(4):207–13. [PubMed] [Google Scholar]
- 10.Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: A critical review. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2008:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce BB, Foote KD, Rosenbek J, Sapienza C, Romrell J, Crucian G, et al. Aphasia and thalamotomy: important issues. Stereotactic and functional neurosurgery. 2004;82(4):186–90. [DOI] [PubMed] [Google Scholar]
- 13.Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–31. [DOI] [PubMed] [Google Scholar]
- 14.Silverman EP, Carnaby G, Singletary F, Hoffman-Ruddy B, Yeager J, Sapienza C. Measurement of Voluntary Cough Production and Airway Protection in Parkinson Disease. Archives of physical medicine and rehabilitation. 2016;97(3):413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegland KW, Okun MS, Troche MS. Sequential Voluntary Cough and Aspiration or Aspiration Risk in Parkinson’s Disease. Lung. 2014;192(4):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56(4):502–6. [DOI] [PubMed] [Google Scholar]
- 17.Hegland K, Okun M, Troche M. Sequential Voluntary Cough and Aspiration or Aspiration Risk in Parkinson’s Disease. Lung. 2014;192(4):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutcheson KA, Barrow MP, Warneke CL, Wang Y, Eapen G, Lai SY, et al. Cough strength and expiratory force in aspirating and nonaspirating postradiation head and neck cancer survivors. Laryngoscope. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of Chiropractic Medicine. 2016;15(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandimore AE, Troche MS, Huber JE, Hegland KW. Respiratory kinematic and airflow differences between reflex and voluntary cough in healthy young adults. Front Physiol. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilanus TBM, Groothuis JT, TenBroek-Pastoor JMC, Feuth TB, Heijdra YF, Slenders JPL, et al. The predictive value of respiratory function tests for non-invasive ventilation in amyotrophic lateral sclerosis. Respiratory research. 2017;18(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]