Abstract
Background aims.
Human dermal ABCB5-expressing mesenchymal stromal cells (ABCB5+ MSCs) represent a promising candidate for stem cell based therapy of various currently uncurable diseases in several fields of regenerative medicine. We have developed and validated a method to isolate, from human skin samples, and expand ABCB5+ MSCs that meet the guideline criteria of the International Society for Cellular Therapy. We are able to process these cells into a Good Manufacturing Practice conforming, MSC-based advanced-therapy medicinal product.
Methods.
To support the development of ABCB5+ MSCs for potential therapeutic topical, intramuscular and intravenous administration, we have tested our product in a series of Good Laboratory Practice compliant nonclinical in-vivo studies addressing all relevant aspects of biosafety, including potential long-term persistence and proliferation, distribution to nontarget tissues, differentiation into undesired cell types, ectopic tissue formation, tumor formation and local tissue reaction.
Results.
(i) Subcutaneous application of 1 × 107 ABCB5+ MSCs/animal and intravenous application of 2 × 106 ABCB5+ MSCs/animal, respectively, to immunocompromised mice did not result in safety-relevant biodistribution, persistence or proliferation of the cells; (ii) three monthly subcutaneous injections of ABCB5+ MSCs at doses ranging from 1 × 105 to 1 × 107 cells/animal and three biweekly intravenous injections of 2 × 106 ABCB5+ MSCs/animal, respectively, to immunocompromised mice were nontoxic and revealed no tumorigenic potential; and (iii) intramuscular injection of 5 × 106 ABCB5+ MSCs/animal to immunocompromised mice was locally well tolerated.
Discussion.
The present preclinical in vivo data demonstrate the local and systemic safety and tolerability of a novel advanced-therapy medicinal product based on human skin-derived ABCB5+ MSCs.
Keywords: stromal cells, stem cells, MSC, biodistribution, safety, ABCB5, GMP, tumorigenicity, toxicity, persistence
Introduction
Human dermal mesenchymal stromal cells (MSCs) expressing the P-glycoprotein molecule ATP-binding cassette subfamily B member 5 (ABCB5) [1] represent an undifferentiated subpopulation of dermal progenitor cells that fulfill the identity criteria for MSCs proposed by the International Society for Cellular Therapy [2]. In these physiological skin progenitor cells, ABCB5 functions as a determinant of the membrane potential and as such regulates the propensity of the cell to remain undifferentiated or to undergo differentiation [1]. ABCB5+ MSCs exert immunomodulatory and anti-inflammatory effects involving several pathways [3–5], making them a promising novel therapeutic candidate for the treatment of conditions associated with chronic inflammation and tissue degeneration. Thus, ABCB5+ MSCs bear the potential to make a significant contribution toward unmet needs of patients suffering from various currently uncurable diseases. This panorama prompted us to develop and validate a method according to the requirements of Good Manufacturing Practice (GMP) by which human dermal ABCB5+ MSCs can be reliably isolated, expanded on a large scale and manufactured as a homogenous MSC population for future in-human use as an advanced-therapy medicinal product (ATMP).
Even though the vast majority of clinical trials conducted with MSCs derived from various sources has not revealed any significant treatment-related health concerns [6–8], stem cell based products basically may carry specific risks associated with potential donor-to-recipient pathogen transmission and/or undesired effects of the applied cells [9]. Thus, to ensure patient benefit and safety at its best, in addition to rigorous, regulatory-compliant donor screening and testing, a comprehensive set of GMP-compliant quality and safety control measures are carried out during the manufacturing process of our ATMP, testing and securing identity, vitality, viability and potency of the cells as well as purity and stability of the manufactured pharmaceutical product, while precluding the presence of any conceivable bio-burden and other adventitious agents. To further the development of ABCB5+ MSCs as an ATMP for potential topical, intramuscular and intravenous administration, we have tested our final GMP-conform pharmaceutical product in a series of Good Laboratory Practice (GLP)-compliant nonclinical in vivo studies (listed in Table I) addressing all relevant aspects of biosafety, including potential long-term persistence and proliferation of the applied cells in the host organism, distribution of the cells to nontarget tissues, differentiation into undesired cell types, ectopic tissue formation, tumor formation, and local tissue reaction.
Methods
Animals
NOD-scid (NOD/MrkBomTac-Prkdcscid) and NOG (NOD-Shi-scid IL2Rγnull) mice were purchased from Taconic (Ry, Denmark) and NSG (NOD scid gamma; NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice from Charles River (Saint-Germain-Nuelles France). At initiation of treatment, the animals were 7–8 weeks (biodistribution/persistence studies, toxicity/tumorigenicity studies) or 9–10 weeks (local tolerance studies) old. Mice were housed under special hygienic conditions with 12 h/12 h light-dark cycle, fed ad libitum with complete diet and had free access to water. All animal experiments were performed by specialized contract research organizations, meeting the animal protection requirements defined in the European regulations [10] and the applicable national (German and UK) animal welfare legislations. All animal experimentation procedures had been approved by the competent authorities: Regierung Oberbayern, Germany (AZ 55.2–1-54–2532.2–7-13, subcutaneous biodistribution/persistence study; AZ 55.2–1-54–2532.2–13-12, subcutaneous toxicity/tumorigenicity study); United Kingdom Home Office, Animals in Science Regulation Unit (PPL 70/08287, October 2014, intravenous biodistribution/persistence study; PPL 80/02501, January 2012, intravenous toxicity/tumorigenicity study); and Landesamt für Umwelt, Gesundheit und Verbraucherschutz des Landes Brandenburg, Germany (V3–2347-A-13–8-2011, 6 February 2012, local tolerance studies).
Isolation, production and potency testing of human ABCB5+ MSCs
After informed written consent, human ABCB5+ MSCs were derived from skin samples obtained from patients undergoing abdominoplasties or other medical interventions providing leftover skin tissue.
Cell production took place in a clean-room facility complying with GMP grade A D regulations, following a validated protocol. In brief, skin tissue was freed from excess subcutaneous adipose tissue, disinfected, washed, dissected into pieces and enzymatically digested. Cells were centrifuged and expanded after adherence selection in stem cell selecting growth media as unsegregated cell culture. ABCB5+ MSCs were isolated by antibody-coupled magnetic bead sorting using a mouse anti-human ABCB5 monoclonal antibody [1]. Purity of the isolated cells was determined by flow cytometry; a content of ≥90% ABCB5+ cells was set as a release criterion, with the remaining up to 10% (mean: 3,8%) being ABCB5-negative MSCs that also meet the International Society for Cellular Therapy guideline criteria. Corresponding to the main modes of action of MSCs, that is (i) immunomodulation (switch from pro-inflammatory M1 to anti-inflammatory M2 macrophages), (ii) secretion of bioactive molecules (mediation of angiogenesis and neo-vascularization) and (iii) trans-differentiation (into CD31-expressing endothelial cells), the following validated assays were used for potency testing: (i) secretion of interleukin-1 receptor antagonist in a co-culture with M1-polarized macrophages (enzyme-linked immunoassay [ELISA] based screening assay), (ii) secretion of VEGF when cultured under hypoxic conditions (ELISA-based screening assay), and (iii) tube formation assay in extracellular matrix for construction and forming of vessels (trans-differentiation assay, microscopic evaluation). Upon meeting the release specifications, cells were cryo-preserved in CryoStor CS10 freeze medium (BioLife Solutions, Bothell, WA) containing 10% dimethyl sulfoxide and stored in the gas phase of liquid nitrogen.
Reconstitution and injection of human ABCB5+ MSCs
ABCB5+ MSCs were thawed, washed and suspended in HRG solution (Ringer’s lactate solution containing 2.5% human serum albumin and 0.4% glucose) at concentrations ranging from 5 × 105 to 5 × 107 cells/mL, as required. The injection volume (200 μL) was drawn up into a sterile single-use 500-μL or 1-μL syringe. Immediately before injection, the syringes were gently inverted 5–10 times to ensure homogenous suspension of the cells. For injection, mice were isoflurane-anesthetized. Subcutaneous injections (23-G needle) were given at the right flank, intravenous injections (26-G needle) into the left or right caudal vein, and intramuscular injections (30-G needle) split over four injection sites at the left thigh muscles after shaving the left thigh with an electric clipper. Intravenous and intra-muscular injections were given at a rate of 10 μL/5 s.
HeLa cells
HeLa cells (ATCC CCL-2) were cultured in Eagle’s Minimum Essential Medium containing 10% fetal bovine serum, 2 mmol/L glutamine, 1% nonessential amino acids, 1 mmol/L sodium pyruvate, 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were passaged at least twice. On the day of dosing, cells were harvested using trypsin–ethylenediaminetetraacetic acid solution, pelleted by centrifugation and then rinsed once with phosphate-buffered saline (PBS) before being resuspended in PBS at a concentration of 5 × 107 cells/mL (subcutaneous tumorigenicity study) and 5.4 × 106 cells/mL (intravenous tumorigenicity study), respectively. The injection volume (200 μL) was drawn up into a sterile single-use 1-mL syringe and given subcutaneously into the right flank.
Histopathology
After sacrifice of the animals, all organs and tissues designated for histopathological assessment were dissected and preserved in 10% neutral buffered formalin solution containing approximately 4% formaldehyde (testes and eyes: modified Davidson’s fixative). The samples were trimmed, processed, embedded in paraffin wax, cut to an approximate thickness of 2–5 μm, stained with hematoxylin and eosin (H&E), and inspected by conventional light microscopy.
Detection of human-specific DNA
In the biodistribution and persistence studies, the following tissues and organs were collected for detection of human-specific genomic DNA using quantitative polymerase chain reaction (qPCR) according to a validated protocol: blood, injection depot (only after subcutaneous administration and only from animals sacrificed 1 week after cell administration), skin/subcutis and skeletal muscle at the injection site, lymph node draining the injection site, contralateral lymph node, liver, spleen, lungs, brain, femur bone, femur bone marrow, kidneys, thymus, thyroid/para-thyroid gland and ovaries or testes. In the subcutaneous repeated-dose toxicity study, enlarged organs considered to result from spontaneous lymphoma development were collected for human-specific genomic DNA detection.
In the subcutaneous biodistribution/persistence and toxicity/tumorgenicity studies, soft tissues (muscle, lymph nodes, liver, spleen, lung, brain, kidney, thymus, thyroid/parathyroid gland and ovaries or testes) were taken up in lysis/binding buffer (Roche, Mannheim, Germany) and ground with a micropestle. As positive controls, two additional aliquots of liver tissue from one vehicle-treated animal were prepared, and 10 000 human ABCB5+ MSCs in 50 μL saline were added for spiking. Hard tissues (skin, bones) were taken up in lysis/binding buffer (Roche) into homogenization tubes containing ceramic beads (Precellys Ceramic kit 2.8 mm; VWR, Darmstadt, Germany) and disrupted in a MagNA Lyser Instrument (Roche) for 2 × 25 s at 6000 rpm with 90 s cooling between the two cycles. Nucleic acid extraction from tissue homogenates and blood was performed with an automated nucleic acid extraction system (MagnaPure LC, Roche) using the Total Nucleic Acid Isolation kit. For detection of human DNA sequences, forward primer cr17_1a, reverse primer cr17_4b and the FAM-TAMRA-labeled probe TMsatex were used. For detection of mouse DNA sequences, forward primer PTGER2hmf, reverse primer PTGER2r and the VIC-NONE-labeled MGB probe MOUQ01 were used. For sequences, see supplementary Table I. Cycle 40 was set as detection cutoff.
In the intravenous biodistribution and persistence study, tissues were taken up in lysis buffer T1 and proteinase K (NucleoSpin 96 Tissue kit, Macherey-Nagel, Düren, Germany) into homogenization tubes containing ceramic beads (for soft tissues) or steel beads (for bone tissue) and homogenized using the Precellys Evolution homogenizer (Bertin Technologies, Frankfurt, Germany) at 6000 rpm for least two cycles (20 s each) at room temperature with at least 30 s pause between two cycles. When further cycles were needed, samples were cooled in between. Nucleic acid extraction from tissue homogenates and blood was performed using NucleoSpin 96 Tissue kit (Macherey-Nagel). For detection of human DNA sequences, forward primer ACC-25, reverse primer ACC-26 and FAM-labeled probe ACC-27 (Microsynth, Balgach, Switzerland) were used. For detection of mouse DNA sequences, forward primer ACC-28, reverse primer ACC-29 and TAMRA-labeled probe ACC-30 (Microsynth) were used. For sequences, see supplementary Table I. The lower limit of quantification was 125 cells.
Immunohistochemistry
AMA and Ki67 staining
Formalin-fixed, paraffin-embedded lung and skin tissue samples were sectioned at three levels approximately 100 μm apart. At each level three sequential 4- to 5-μm-thick sections were taken and stained with H&E, AMA antibody (ab92824, Abcam, Cambridge, United Kingdom, 2 μg/mL; secondary anti-body: biotinylated rabbit anti-mouse, E0413, Dako, Glostrup, Denmark) and anti-Ki67 antibody (ab16667, Abcam, dilution 1:200; secondary anti-body: biotinylated goat anti-rabbit, E0423, Dako), respectively, according to validated protocols. Sections designated for H&E and AMA staining were stained and examined first. Only if the AMA staining was positive, the corresponding third slide was then stained with the anti-Ki67 antibody.
β2-Microglobulin staining
Staining of 4-μm sections of paraffin-embedded thigh muscle specimens was performed using rabbit anti-β2-microglobulin (polyclonal, immunoglobulin G, Abcam; dilution 1:200) incubated for 60 min at room temperature. Staining was carried out with Leica Bond III fully automated staining system using the horseradish peroxidase (HRP)-linker anti-body conjugate system Bond Polymer Refine Detection kit as secondary reagent (Leica Biosystems, Wetzlar, Germany).
Vimentin staining
Staining of 4-μm sections of paraffin-embedded thigh muscle specimens was performed out using rabbit anti-vimentin (ab137321, polyclonal immunoglobulin G, Abcam; dilution 1:1000) incubated for 60 min at room temperature. Staining was carried out with Leica Bond III fully automated staining system using the HRP-linker antibody conjugate system Bond Polymer Refine Detection kit as secondary reagent (Leica Biosystems).
Statistics
Data acquisition and analysis were carried out using the integrated software system PDS ToxData 3.0 (Pathology Data Systems, Pratteln, Switzerland).
In the subcutaneous biodistribution and persistence study, inferential statistics were based on the results of a Shapiro Wilk test. In the case of normality, homogeneity of medians was assessed by Levene test and analysis of variance. In the case of non-normality and non-homogeneity, a nonparametric Kruskal Wallis test was performed. Multiple comparisons were carried out using a post hoc Dunn’s test.
In the intravenous biodistribution and persistence study, cell concentrations as detected by qPCR were statistically analyzed by non-paired one-way analysis of variance followed by Tukey’s multiple comparison test.
In the subcutaneous repeated-dose toxicity and tumorigenicity study, inferential statistics were performed according to four different decision trees, based on the results of a Shapiro Wilk test, Kolmogorov Smirnov test and Bartlett test, respectively, followed by appropriate post hoc testing procedures.
In the intravenous repeated-dose toxicity and tumorigenicity study, inferential statistics were based on the results of a Bartlett test for variance homogeneity. If the Bartlett test was not significant at the 1% level, a parametric analysis was performed. Groups were compared using t-tests. If the Bartlett test was still significant at the 1% level following both logarithmic and square-root transformations, a nonparametric analysis was performed. Groups were compared using Wilcoxon rank sum tests.
In the local tolerance studies inferential statistics were not performed due to the rather descriptive character of these studies.
Results
Biodistribution and persistence of ABCB5+ MSCs after single subcutaneous administration
Three groups of 10 (5 male, 5 female) NOD-scid mice were subcutaneously injected with 1 × 107 ABCB5+ MSCs/animal. Mice were followed up for 1 week, 3 months and 4 months, respectively, then sacrificed and subjected to necropsy. As control, a fourth group of 10 mice received vehicle without cells and was followed up for 4 months.
Although no spontaneous deaths occurred, two moribund animals, suffering from spontaneously developed lymphoma, which is known to be a frequent occurrence in NOD-scid mice [11], were euthanized during the observation period (days 90 and 69, respectively). In general, clinical findings noted during the observation period as well as macroscopic pathological findings were either associated with spontaneously developing lymphomas or related to shaving, housing or cage mates. There were no clinical or necropsy findings related to administration of ABCB5+ MSCs noted in any of the animals.
In MSC-treated animals, human DNA sequences were detectable by qPCR in all subcutaneous injection depots and two injection site-draining lymph nodes after 1 week and in one skin sample after 3 months, but not in any other tissue and blood examined. A further positive result in one brain sample after 1 week was considered accidental (probably contamination-related), as it was observed only in one of two aliquots tested. In vehicle-treated animals, human DNA sequences could not be detected in either organ/tissue, whereas mouse DNA sequences were detectable in all tissues and at all time points in MSC- and vehicle-treated animals, both of which confirming the reliability of the method.
Biodistribution and persistence of ABCB5+ MSCs after single intravenous administration
To investigate trafficking, homing, engraftment, differentiation and persistence of human ABCB5+ MSCs after single intravenous administration, three groups of ten (five male, five female) NSG mice were intravenously injected with 2 × 106 ABCB5+ MSCs/ animal in 200 μl vehicle solution. After cell injection, mice were followed up for 1, 4, and 13 weeks, respectively, after which the mice were sacrificed. As control, a fourth group of 10 mice received vehicle without cells and was followed up for 13 weeks. During these periods, no case of death and no treatment-related effects on body weight and clinical signs occurred.
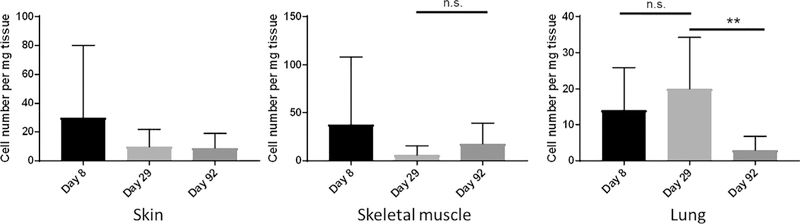
In MSC-treated animals, human-origin cells, as revealed by detection of human DNA sequences using qPCR, were predominantly recorded in the injection site tissues (skin and skeletal muscle) and in the lungs (Table II), where human-origin cells were detectable in individual animals up to the end of the study (day 92). This points to a certain degree of persistence of the administered MSCs in these tissues. Maximum cell concentrations were measured in individual injection-site tissue samples on day 8 and in individual lung tissue samples on day 29, declining thereafter. The mean cell concentration observed in the lungs was, however, not significantly increased in day 29 compared with day 8 samples but was significantly reduced in day 92 samples (Figure 1).
Figure 1.
Concentration of human-origin cells as detected by qPCR in injection-site tissues (skin and skeletal muscle) and in the lung of NSG mice at 8 (black bars), 29 (light gray bars) and 92 days (medium gray bars) after intravenous injection of 2 × 106 ABCB5+ MSCs. Shown are means of 10 animals; error bars indicate SD; **P < 0.01, non-paired one-way analysis of variance followed by Tukey’s multiple comparison test. A separate control group (not shown), comprising the mice injected with cell-free vehicle and sacrificed 92 days thereafter, did not reveal any detectable human-origin cells except for muscle tissue of one animal, which was considered an artifact (see Results).
In the tissues beyond injection site and lungs, human DNA was occasionally detected across the various tissue types (except for reproductive organs and brain) in individual MSC-treated animals. However, quantifiable levels of human cells could only be detected in one kidney (7 cells/mg) and one thymus (151 cells/mg) sample taken on day 29, but not in any other of totally 300 tissue and organ samples beyond injection-site tissues and lungs of the 30 MSC-treated animals. Because these positive signals were singular findings and no quantifiable cell concentrations were recorded in the same tissue types at earlier or later time points, we consider these two positive findings out of 300 samples as incidental, likely resulting from accidental contamination with human cells by the investigators during animal handling and/or tissue sampling or preparation.
Unexpectedly, quantifiable human-origin cell concentrations were detected in four individual samples from 4 out of 10 control animals taken on day 92 (skeletal muscle: 51 cells/mg; thyroids: 82 and 359 cells/mg, respectively; ovaries: 19 cells/mg). However, all other 126 samples analyzed from control animals as well as all assay controls, that is blank (not-spiked mouse matrix) and no-template control (DNAse-free water) samples, tested negative for human cells, which argues against a false-positive background signal of the assay. Instead, given the high sensitivity of our assay detecting as little as 125 cells in 200 μL tissue lysate or 100 μL blood and the high contamination risk associated with qPCR techniques, we deem these findings to have resulted from contamination during sample processing at an indeterminate stage. Because such contamination poses a certain risk to overestimate but in no way underestimate the presence of human MSCs in the examined tissues, we do not consider these findings to be detrimental to the validity or integrity of our data in terms of safety evaluation.
Immunohistochemical staining of injection sites and lungs of the MSC-treated animals with a human-specific antimitochondrial antibody (AMA) and, in case of AMA positivity, with an antibody against the proliferation marker Ki67 was performed on lung tissue samples from all 30 animals and skin samples from 25 animals (for 5 of 10 animals at the 13-week time point, there was no skin tissue available). Positive AMA staining was observed in the subcutaneous injection-site tissue of one animal and within a thrombus in a lung vessel of another animal, both sacrificed at 1 week after MSC application. Cells staining positively for AMA were spindloid-shaped. Only in the lung thrombus was positive staining observed also for Ki67, with the majority of positively staining cells staining positively only for either AMA or Ki67. Most Ki67+ cells appeared as clumsy cells with oval-shaped nuclei, thus morphologically being clearly different from the AM+ (human-origin) cells. Such host-origin Ki67+ cells would be expected within a thrombus due to active reorganization of the thrombus. Nevertheless, in some areas of the thrombus, positive staining for Ki67 and AMA was seen in close association, and as such, it could not be completely ruled out that some cells could have stained positive for both. However, all cells staining positive for AMA were scattered throughout the thrombus and did not show any evidence of clustering together to form a mass. No positive staining for Ki67 was seen at the injection site, indicating that the subcutaneously detected human cells were not actively proliferating. No AMA+ cells were observed at later time points (4 and 13 weeks after MSC application, respectively).
Combined study on subcutaneous repeated-dose toxicity and tumorigenicity
To assess the potential toxicity of human ABCB5+ MSCs, male and female NOD-scid mice were randomly allocated to receive three doses of 1 × 105 (n = 20), 1 × 106 (n = 20) or 1 × 107 (n = 30) ABCB5+ MSCs/animal or vehicle without cells (negative control, n = 30), injected subcutaneously on days 1, 29 and 57. In addition, the tumorigenic potential of human ABCB5+ MSCs was assessed in the high-dose group treated with 1 × 107 cells/animal (as recommended by the World Health Organization [12]). A fifth group of mice receiving a single injection of 1 × 107 HeLa cells on day 1 (n = 10) served as positive control. Full necropsy and subsequent histopathological evaluation were performed after 12 weeks of daily observation, except for HeLa cell treated mice, which were euthanized for animal welfare reasons on day 51. The vehicle and the high-dose group both included additional five male and five female mice undergoing an additional 4-week recovery period (necropsy and subsequent histopathological evaluation after 16 weeks).
With regard to the investigated toxicology parameters (i.e., mortality, clinical signs, body weight development, food consumption, ophthalmologic examination, urinalysis, hematology, blood chemistry and coagulation, organ weights, macro-pathological and histopathological findings), the study did not reveal any relevant adverse effects related to ABCB5+ MSCs (for details, see supplementary Tables II IV).
The predominant histopathological findings were systemic lymphoblastic neoplasms, with incidences ranging between 5% in the medium-dose MSC group and 17% in the vehicle group. These neoplasms are known to spontaneously develop in NOD-scid mice [11] and were clearly judged not related to the application of ABCB5+ MSCs (see Discussion). In the positive-control (HeLa cell–treated) group, subcutaneous nodules were palpated in all (10 of 10) animals at the injection site from day 6 (males) and day 8 (females) on. Due to the progressive tumor growth for more than 2 weeks, these animals were euthanized on day 51.
Combined study on intravenous repeated-dose toxicity and tumorigenicity
The potential toxicity and tumorigenicity of human ABCB5+ MSCs after intravenous administration was assessed in male and female NSG mice randomly allocated to receive three intravenous injections of 2 × 106 ABCB5+ MSCs/animal (n = 20) or vehicle without cells (negative control, n = 20) on day 1, 15 and 29. As positive control (i.e., to validate the used mouse strain as being susceptible to tumor formation), 10 mice received 1.08 × 106 HeLa cells injected subcutaneously on day 1. Albeit this dose level was, due to technical reasons, lower than the level recommended by the World Health Organization (1 × 107 cells/mouse [12]), it was considered sufficient in the mouse strain used because all these mice developed tumors (discussed subsequently).
During a 13-week observation period, mortality, clinical condition, palpable masses, body weight development, food consumption, ophthalmologic findings and hematological and blood chemistry parameters (both in the peripheral blood sampled from the retro-orbital sinus under isoflurane anesthesia before sacrifice) were recorded. Thereafter, the mice were sacrificed and subjected to full necropsy, including organ weight measurements and macro-and histopathological examination.
In most cases either no significant changes between vehicle- and MSC-treated animals were observed, or the observed findings were judged unrelated to the treatment with ABCB5+ MSCs, based on incidence and distribution. The deaths of two MSC-treated mice (day 1 and day 15, respectively) were considered related to the injection procedure. Body and organ weight development were not basically altered in the MSC-treated group (Table III). Minor differences between vehicle- and MSC-treated animals were seen for some hematology and clinical chemistry parameters (Table IV). In all (10/10) HeLa cell-treated mice, dorsocranial and/or dorsocaudal masses were palpable on the right side of the body in the region of the injection site. Three mice developed also dorsocranial/dorsocaudal masses on the left side of the body. In contrast, no palpable masses were detectable on vehicle- or MSC-treated animals, and neither macro-pathological nor histopathological examination revealed evidence of tumor forming in these groups (Table V).
To complement and expand the findings of the earlier-reported (single-dose) intravenous biodistribution and persistence study to the repeated-dose setting, lungs and injection-site tissues of all MSC-treated animals were stained with human-specific AMA and an anti-body against the proliferation marker Ki67 to evaluate the presence and proliferation status of the intravenously administered ABCB5+ MSCs. The lungs of four vehicle-treated animals served as negative control, whereas injection-site tissue of a HeLa cell–treated animal was used for assay validation.
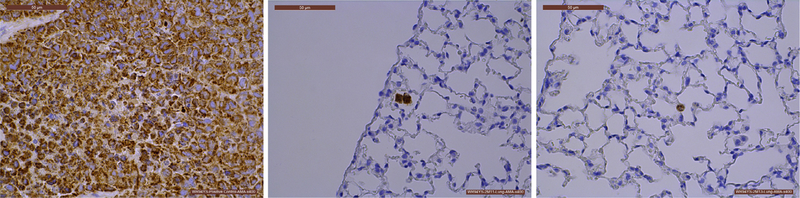
In lung tissue, positive AMA staining was seen in of 7 of the 20 animals treated with human ABCB5+ MSCs, indicating the presence of cells of human origin in the lungs. Staining was exceptionally rare (frequently amounting to no more than 1–2 cells per tissue section), and the positively staining cells were situated within the walls of the alveoli or, occasionally, free within the alveoli (Figure 2). In one animal, a cluster of positively staining cells was seen within the lumen of a lung vessel. This cell cluster was also visible on the corresponding H&E-stained section, although the cell type could not be identified. This appeared to be a thrombus that had detached from the vessel wall during histological processing as a small section of vessel wall adhered to these cells. At the injection sites, there was no positive AMA staining seen in any animal. K-67 staining revealed no positive signals that corresponded to the AMA-positive cells in any tissue section from any of the animals examined.
Figure 2.
Histological evaluation of the persistence of human cells in the lungs of NSG mice treated with three bi-weekly intravenous injections of 2 × 106 ABCB5+ MSCs/mouse. Mice were sacrificed at 13 weeks after the first MSC injection. Tissues were stained with anti-mitochondrial antibody (AMA) (brown) as a marker of human-origin cells. (Left) Skin at the injection site of a mice injected subcutaneously with 1.08 × 106 HeLa cells served as positive control. (Middle and Right) Representative microphotographs of lung sections of MSC-treated mice in which AMA staining was exceptionally rare. Scale bars: 50 μm.
Seven-day local tolerance of intramuscularly injected ABCB5+ MSCs
To evaluate potential local muscular side effects, male NOG mice were intramuscularly injected with 5 × 106 ABCB5+ MSCs/animal in 200 μL vehicle (n = 8) or with 200 μL vehicle without cells (n = 8). One animal in the control group died during application of the vehicle, probably due to intolerance to anesthesia. Mice were followed up for 7 days, during which no effects of the treatment on general condition including body weight development and food consumption became apparent.
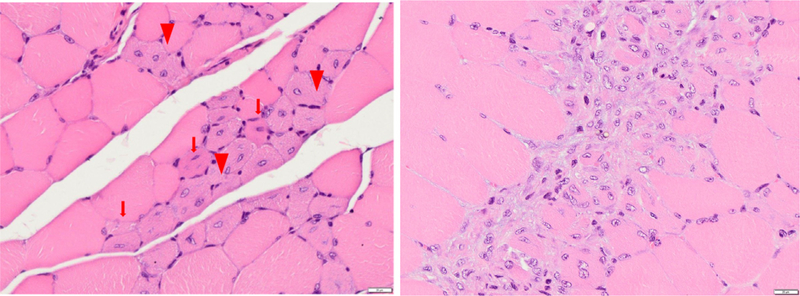
At necropsy all injection muscles appeared macroscopically inconspicuous. Histopathological examination revealed minor degenerative/regenerative myofiber reactions at the injection sites of vehicle-treated animals (Figure 3, left). Myofiber degeneration was manifest in form of focal, small, basophilic fibers. Regenerative large myoblasts were present. These reactions were judged as normal consequence of injection of a volume into muscular tissue. Degenerative and inflammatory processes were quantified as summary score for local cellular and tissue response according to ISO 10993–6:2007 [13], including the criteria polymorphonuclear cells, lymphocytes, plasma cells, macrophages, giant cells, myofiber degeneration, myofiber regeneration, necrosis, neovascularization, fibrosis and fatty infiltrate. MSC-treated animals exhibited a minor increase in the mean summary score for local cellular and tissue response (4.6 compared with 3.7 for vehicle-treated animals). This increase paralleled with the presence of administered MSCs as detected by vimentin and β2-microglobulin mesenchymal cell staining, which formed small strands or foci of mesenchymal cells. These were associated with minimal fibrotic reactions observed in the corresponding H&E-stained sections (Figure 3, right), which demarcated the cell groups from the surrounding tissues. Local intolerance of the applied product was not deduced from these findings.
Figure 3.
Histopathological evaluation of local reactions in thigh muscle tissue of NOG mice to intramuscular injection of 200 μL HRG solution without (left) and with (right) 5 × 106 ABCB5+ MSCs/mouse, split over four injection sites. (Left) Mice were sacrificed at 7 days after cell injection. Representative microphotograph of a thigh muscle section of a control animal, treated with vehicle only, showing slight focal myofiber degeneration (arrows) and regenerative myoblasts (arrowheads). (Right) Representative microphotograph of a thigh muscle section of an MSC-treated mouse, showing focal accumulation of mesenchymal cells with minimal fibrotic aspect. H&E staining; magnification 20 × (left), 40 × (right); scale bars: 20 μm.
Twenty-eight-day local tolerance of intramuscularly injected ABCB5+ MSCs and of the vehicle
To investigate the effects of both, ABCB5+ MSCs and the vehicle (HRG), on local myofiber degeneration and regeneration over a somewhat longer period, male NOG mice were intramuscularly injected with either 5 × 106 ABCB5+ MSCs/animal in 200 μL vehicle (n = 8), 200 mL vehicle without cells (n = 8) or 200 μL PBS (to detect or rule out possible effects of the HRG vehicle, n = 8). Mice were followed up for 28 days, during which nearly all animals were inconspicuous. Only one PBS-injected animal died 3 days upon application for an unknown reason. The fact that this mouse was considerably more strongly affected by the anesthesia applied for injection than were the other animals may point to an unrecognized pre-existing condition that eventually had led to its premature death. Macroscopic appearance of the thigh muscles during necropsy was inconspicuous. Histopathological evaluation revealed no treatment-related irritation in any of the tested samples. Only mild myofiber degeneration was detected in one MSC- and in one PBS-treated animal. These reactions were considered incidental and judged as a normal consequence of injection of a volume into muscular tissue.
Discussion
In the nonclinical in vivo studies presented here, we investigated and demonstrate the safety and tolerability ABCB5+ MSCs in view of a potential therapeutic in-human use. Basically, the testing strategy followed the recommendations of the European Medicines Agency [14], considering the specific nature of the product and its intended clinical use. Accordingly, it was crucial to demonstrate that the applied cells do not show unwanted homing or even proliferation. Strong attention was paid to possible tumor formation potential and toxicological effects during short and longer (up to 4 months) periods of time. All these aspects were addressed in severely immunocompromised mice, to prevent rejection of the applied cells by the xenogeneic host’s immune system. This should have provided the greatest possible ability to detect any ectopic tissue- or tumor-forming potential of the administered cells. To capture any impact of possible cellular alterations that may occur during the cell expansion and ATMP production process, the final GMP-produced ATMP as intended for use in clinical trials was tested in all studies.
Studying the distribution and persistence behavior after local (subcutaneous) injection, we showed that the applied ABCB5+ MSCs were confined to the injection site and virtually did not migrate to other tissues and organs, including the reproductive organs. Cell concentrations in the injection-site tissues decreased over time and had reached non-detectable levels in all but one sample at 3 months. This is in line with earlier results on placenta-derived MSCs [15], showing that locally administered MSCs do not persist for longer periods but are eradicated even from severely immunocompromised hosts.
Biodistribution and possible persistence and proliferation potential of ABCB5+ MSCs were also investigated after systemic (intravenous) application of the cell product. The first barrier for intravenously administered cells is the capillary bed of the lung, and because culture-expanded MSCs are relatively large cells, obstructive events such as intravascular emboli formation may seem likely during lung passage [16]. Indeed, “first-pass” accumulation of intra-venously delivered MSCs in the lungs has been repeatedly described [17–20]. The risk that such mechanical cell entrapment causes pulmonal embolus formation grows with increasing cell concentrations, which may result in insufficient dilution of the cells in the circulating blood [17]. Thus, it was crucial to test the same cell concentration as that we intend to use in clinical trial (i.e., 1 × 107 MSCs/mL, corresponding to 2 × 106 MSCs/mouse, given the maximal injection volume of 200 μL recommended for intravenous injections to mice [21]). This cell load was well tolerated without clinical signs or mortality. Human-origin cells were predominantly recorded in the injection-site tissues (skin and skeletal muscle) and in the lungs, as expected. The observed kinetics over time suggests a certain degree of persistence of the cells in these tissues (Figure 1). However, the cells did not appear to readily traffic, home or engraft on tissues beyond the immediate area of the dose site and the lung as “first-pass” organ.
For evaluation of these findings in view of the expectable safety of ABCB5+ MSCs given intravenously to humans, it should be considered that the data were obtained in severely immunocompromised (NSG) mice. Culture-expanded MSCs show an inefficient homing potential, which is apparently due to a deficit or even lack of relevant cell adhesion and chemokine receptors responsible for coordinated extravasation and tissue-specific homing [20,22]. In immunocompetent (C57BL/6) mice intravenously injected with human bone marrow-derived MSCs, qPCR revealed a rapid loss of traceable human DNA within the first 24 h after injection. At that time point, only <0.1% of the initially injected cells were detectable in the lungs (maximum: 52.5% at 5 min), and human cells were undetectable in the liver (maximum: 0.05% at 2 h) [23]. Immunodeficiency, as was the case in the present study, seems to have a favoring effect on persistence of exogenous cells, which, however, would not be given in non-immuno-suppressed human patients.
Another major concern regarding the safety of stem cell therapies is a potential tumorigenic risk [24]: first, stem cells present some characteristics that are also seen in cancer cells, including long life span, self-renewal, high proliferation rate and relative resistance to the host’s immune defense mechanisms such as apoptosis [6]. Second, MSCs could aggravate (potentially undiagnosed) pre-existing neoplastic diseases in the host by promoting tumor growth and metastasis through various mechanisms, including the MSCs’ immunomodulatory properties, by which they could suppress the host’s anti-tumor response [25,26], and their pro-angiogenic capacities, by which they may facilitate tumor growth and metastasis [27]. Third, neoplastic transformation of MSCs may result from an accumulation of genetic and/or epigenetic changes, which may occur during the process of in vitro stem cell expansion, isolation and ATMP manufacture [24]. Transformation has been observed in long-term cultures of bone marrow–derived MSCs [28 30], although other investigators have described such events as very rare or even failed to observe any at all [31 33]. Together, a thorough tumor risk assessment after local and systemic application is essential for biosafety evaluation of MSC-based therapies, even if MSC-originating tumors have never been diagnosed in several hundreds of patients treated with MSCs [7,8,34,35].
The tumorigenicity studies were combined with the studies for toxicity assessment, which allowed for keeping animal numbers as low as possible and reduce potential interstudy variance. Doing so seemed reasonable given that toxicity studies on cell-based medicinal products require longer observation periods than conventional single-dose toxicity studies because the applied cells may be effective for longer times than conventional medicinal products, either because they may not immediately be rejected or by affecting resident cells eliciting effects that continue even after the applied cells are no longer present in the tissue [36].
In the subcutaneous study, the predominant findings were systemic lymphoblastic neoplasms seen throughout all groups treated with ABCB5+ MSCs or vehicle, with incidences ranging between 5% in the medium-dose MSC group and 17% in the vehicle group. These tumors were, however, clearly judged not related to the application of ABCB5+ MSCs for three reasons: first, these tumors were observed in both, MSC- and vehicle-treated groups. Second, the obvious hematopoietic/lymphatic (and thus non-mesenchymal) origin of the tumors together with the absence of human DNA sequences in the affected organs as confirmed by qPCR ruled out any involvement of the applied human ABCB5+ MSCs. Third, spontaneously developing lymphomas are known to be a frequent occurrence in NOD-scid mice [11], which were used in this study. In contrast, in the intravenous study, which instead used NSG mice, which exhibit only a very low spontaneous lymphoma incidence [37], neither in the vehicle- nor in the MSC-treated group any signs of gross tumor development, ectopic tissue formation or micrometastases were observed. In the lungs of the MSC-treated animals, a small amount of the administered ABCB5+ MSCs persisted during the observation period. Combined AMA and Ki67 staining demonstrated, however, that these cells were not proliferating.
The question may arise whether a 12-week (subcutaneous) or 13-week (intravenous study) follow-up is actually sufficient for ruling out a tumorigenic potential of the MSCs, considering that in one skin sample in the subcutaneous biodistribution study human cells were detected at 3 months (which could have persisted even longer if the animal was not sacrificed). However, it needs to be considered that the data were obtained in severely immunocompromised mice to prevent degradation of rejection of the MSCs by the host’s immune system and extend cell survival and persistence in the host for as long as possible. Thus, the level of cell survival observed in our studies is far above of what is expected in an immunocompetent organism. In immunocompetent (C57BL/6) mice intravenously injected with human bone marrow–derived MSCs, qPCR revealed a rapid loss of traceable human DNA already within the first 24 h. At that time point, for example, only <0.1% of the initially injected cells were detectable in the lungs (maximum: 52.5% at 5 min) [23].
As with the tumorigenicity parts of the studies, their toxicity parts did not reveal any significant effect of ABCB5+ MSCs in either dose tested on the parameters investigated. In general, either no significant differences between vehicle- and MSC-treated animals were observed, or the observed findings were judged unrelated to the treatment with ABCB5+ MSCs, based on their incidence and distribution.
Taken together, these studies demonstrate that repeated (three monthly) subcutaneous injections of human skin derived ABCB5+ MSCs at doses ranging from 1 × 105 to 1 × 107 cells/mouse and repeated (three biweekly) intravenous injections of human ABCB5+ MSCs at a dose of 2 × 106 cells/mouse, respectively, were non-tumorigenic and nontoxic, with 1 × 107 ABCB5+ MSCs/mouse (for subcutaneous application) and 2 × 106 cells/mouse (for intravenous application), respectively, being considered the “no observed adverse effect level” (NOAEL). As these doses represent the highest doses tested in our studies, the actual NOAEL might be even higher. Although we cannot state with certainty that our studies exactly duplicated the biodistribution condition in humans, the use of immunocompromised mice can be expected to have provided the greatest possible cell survival. This allows for reasonable translation of our animal-derived data to the allogeneic but immunocompetent human setting.
An issue that could not be studied NSG mice, which lack mature T-, B- and natural killer–cell activity, is the potential development of antibodies against foreign cell antigens. On the other hand, studies of human MSCs in immunocompetent mice would reflect the xenogeneic situation and thus provide only limited informative value in view of human (i.e., allogeneic) use. Given the multifaceted immunosuppressive properties of ABCB5+ MSCs [3–5], the generally low immunogenicity of MSCs [2,38] and the observation that occasional anti-donor anti-body formation following repeated intravenous MSC injections was associated with neither clinical consequences nor with loss of therapeutic efficacy of the applied cells [39,40], we do not expect any significant safety concerns.
Finally, to facilitate intramuscular application settings, two studies were specifically designed to investigate short-term (7 days) and longer-term (29 days) intramuscular local tolerance. The latter study involved an additional group that was treated with PBS to detect any potential local side effect of the HRG vehicle. No clinical and macroscopic abnormalities were observed in these studies. Microscopic reactions were for the most part judged as a normal consequence of injection of a volume into muscular tissue. Local intolerance, emanating from either the MSCs or from the vehicle, could not be deduced from the findings of these studies. One may speculate that the immunocompromised state of the animals may have prevented or masked potential local tissue reactions that would otherwise occur. Although such a contribution cannot be definitively ruled out, it may be emphasized that good intramuscular tolerability of MSCs of various sources has also been observed in the immunocompetent organism, as was demonstrated, for example, with intramuscular injections of human bone marrow–derived MSCs to C57BL/6 mice [41] or of adipose tissue–derived MSCs to humans [42].
In conclusion, the present study program covered the specific aspects to be addressed in preclinical safety studies for somatic cell therapy medicinal products [43], including the evaluation of distribution and engrafting after administration, ectopic engraftment and oncogenic transformation. The set of tests and pattern of analysis may be generally applicable to preclinical safety and tolerability evaluation of MSC-based medicinal products. Our data demonstrate the preclinical safety and tolerability of a novel ATMP based on human skin derived ABCB5+ MSCs that may represent a significant contribution toward the unmet medical needs of various degenerative and inflammatory conditions.
Supplementary Material
Table I.
Overview over the preclinical in vivo safety study program of ABCB5+ MSCs.
| Mouse strain | Number of animals | Treatment | Route and time of application | Observation period |
|---|---|---|---|---|
| Biodistribution and persistence studies | ||||
| NOD-scid | 40 (20 M, 20 F) | 1 × 107 ABCB5+ MSCs/animal | SC, day 1 | 1 week (n = 10) |
| 3 months (n = 10) | ||||
| 4 months (n = 10) | ||||
| Vehicle | 4 months (n = 10) | |||
| NSG | 40 (20 M, 20 F) | 2 × 106 ABCB5+ MSCs/animal | IV, day 1 | 1 week (n = 10) |
| 4 weeks (n = 10) | ||||
| 13 weeks (n = 10) | ||||
| Vehicle | 13 weeks (n = 10) | |||
| Repeated-dose toxicity and tumorigenicity studies | ||||
| NOD-scid | 110 (55 M, 55 F) | 1 × 105 ABCB5+ MSCs/animal (n = 20) | SC, days 1, 29, 57 | 12 16 weeks |
| 1 × 106 ABCB5+ MSCs/animal (n = 20) | 12 weeks | |||
| 1 × 107 ABCB5+ MSCs/animal (n = 30) | 12 weeks | |||
| Vehicle (n = 30) | 12 16 weeks | |||
| 1 × 107 HeLa + cells/animal (n = 10) | SC, day 1 | 51 days | ||
| NSG | 50 (25 M, 25 F) | 2 × 106 ABCB5 MSCs/animal (n = 20) | IV, days 1, 15, 29 | 13 weeks |
| Vehicle (n = 20) | ||||
| 1.08 × 106 HeLa cells/animal (n = 10) | SC, day 1 | |||
| Local tolerance studies | ||||
| NOG | 16 males | 5 × 106 ABCB5+ MSCs/animal (n = 8) | IM, day 1 | 7 days |
| Vehicle (n = 8) | ||||
| NOG | 24 males | 5 × 106 ABCB5+ MSCs/animal (n = 8) | IM, day 1 | 28 days |
| Vehicle (n = 8) | ||||
| PBS (n = 8) | ||||
F, female; IM, intramuscular; IV, intravenous; M, male; SC, subcutaneous.
Table II.
Concentration of human-origin cells in injection-site tissues and lungs of NSG mice intravenously injected with 2 × 106 ABCB5+ MSCs.
| Cell concentration (cells/mg tissue) |
|||||
|---|---|---|---|---|---|
| Injection site tissues |
|||||
| Time after cell application | Sex | Animal number | Skin | Skeletal muscle | Lung |
| Day 8 (1 week) | M | 6 | 8 | – | 36 |
| 7 | 162 | Detected | – | ||
| 8 | 59 | 46 | 14 | ||
| 9 | 13 | – | 11 | ||
| 10 | – | – | – | ||
| F | 36 | Detected | 200 | 12 | |
| 37 | Detected | 129 | 7 | ||
| 38 | 16 | Detected | 31 | ||
| 39 | 39 | – | 19 | ||
| 50 | Detected | – | 10 | ||
| Day 29 (4 weeks) | M | 11 | – | – | 15 |
| 12 | 21 | Detected | 15 | ||
| 13 | 18 | – | 12 | ||
| 14 | Detected | Detected | 30 | ||
| 15 | 7 | Detected | 10 | ||
| F | 40 | – | – | – | |
| 41 | 33 | 19 | 44 | ||
| 42 | 18 | 24 | 7 | ||
| 43 | Detected | 15 | 35 | ||
| 51 | – | – | 32 | ||
| Day 92 (13 weeks) | M | 16 | 17 | – | – |
| 17 | – | 57 | 7 | ||
| 18 | – | – | 6 | ||
| 19 | 31 | 46 | – | ||
| 20 | 11 | 35 | 9 | ||
| F | 45 | – | – | – | |
| 46 | Detected | – | – | ||
| 47 | 8 | – | – | ||
| 48 | 13 | 17 | 7 | ||
| 49 | 8 | 18 | – | ||
Animals were sacrificed at 1, 4 or 13 weeks after cell injection. Human-origin cells were determined by detection of human-specific DNA using qPCR. Detected = value < lower limit of quantification but > 2.5 × blank; – = undetected. F, female; M, male.
Table III.
Body weight (alive) and selected organ weights of NSG mice following three intravenous injections of 2 × 106 ABCB5+ MSCs/animal or vehicle without cells on day 1, 15 and 29.
| Vehicle | 2 × 106 MSCs/animal | |
|---|---|---|
| Males | ||
| Body weight (alive), g | n = 10 | n = 10 |
| Baseline | 27.9 (1.34) | 27.9 (0.94) |
| 13 weeks | 34.4 (1.52) | 32.7 (0.69)* |
| Organ weight, g | n = 10 | n = 9 |
| Brain | 0.4783 (0.0168) | 0.4853 (0.0120) |
| Heart | 0.1800 (0.0132) | 0.1684 (0.0069) |
| Kidneys | 0.5599 (0.0572) | 0.5079 (0.0269) |
| Liver | 1.9112 (0.1416) | 1.6879 (0.0532)** |
| Spleen | 0.0259 (0.0062) | 0.0222 (0.0028) |
| Testes | 0.2059 (0.0122) | 0.2047 (0.0083) |
| Thymus | 0.2059 (0.0122) | 0.2047 (0.0083) |
| Females | ||
| Body weight, g | n = 10 | n = 10 |
| Baseline | 21.8 (1.29) | 22.1 (1.59) |
| 13 weeks | 26.6 (1.23) | 26.9 (2.13) |
| Organ weight, g | n = 10 | n = 10 |
| Brain | 0.4931 (0.0125) | 0.4879 (0.0143) |
| Heart | 0.1395 (0.0111) | 0.1373 (0.0108) |
| Kidneys | 0.3076 (0.0204) | 0.3112 (0.0185) |
| Liver | 1.3087 (0.0888) | 1.2165 (0.1176)* |
| Ovaries | 0.0212 (0.0043) | 0.0210 (0.0044) |
| Spleen | 0.0249 (0.0037) | 0.0243 (0.0033) |
| Thymus | 0.0086 (0.0028) | 0.0069 (0.0030) |
Animals were followed up for 13 weeks. Values are means (SD).
P < 0.05
P < 0.01 versus vehicle group, t-test on log-transformed data (body weight) or after adjustment for body weight (organ weights).
Table IV.
Selected hematology and clinical chemistry parameters of NSG mice after three intravenous injections of 2 × 106 ABCB5+ MSCs/animal or vehicle without cells on day 1, 15 and 29.
| Vehicle | 2 × 106 MSCs/animal | |
|---|---|---|
| Males | n = 10 | n = 9 |
| Hematology | ||
| Hematocrit, L/L | 0.459 (0.0257) | 0.476 (0.0144) |
| Hemoglobin, g/dL | 12.6 (0.63) | 13.0 (0.27) |
| White blood cells,9× 109/L | 0.81 (0.207) | 1.10 (0.574) |
| Neutrophils, × 10 /L | 0.53 (0.153) | 0.65 (0.233) |
| Lymphocytes, × 109/L | 0.22 (0.126) | 0.33 (0.357) |
| Monocytes, × 10 9/L | 0.04 (0.023) | 0.09 (0.039)** |
| Eosinophils, × 10 /L | 0.02 (0.010) | 0.03 (0.011) |
| Clinical chemistry | ||
| AST, U/L | 45 (9.7) | 43 (7.9) |
| ALP, U/L | 46 (3.1) | 45 (2.8) |
| Urea, mmol/L | 8.56 (0.741) | 7.72 (0.433)* |
| Cholesterol, mmol/L | 2.63 (0.127) | 2.38 (0.240)* |
| Triglycerides, mmol/L | 1.02 (0.273) | 0.61 (0.194)** |
| Glucose, mmol/L | 14.09 (1.271) | 15.82 (2.533) |
| Na+, mmol/L | 152 (1.2) | 152 (2.2) |
| K+, mmol/L | 4.3 (0.41) | 4.4 (0.32) |
| Ca2+, mmol/L | 2.39 (0.045) | 2.34 (0.072)* |
| Albumin, g/L | 31 (1.1) | 29 (0.8)** |
| Total protein, g/L | 49 (1.3) | 46 (1.5)** |
| Females | n = 10 | n = 10 |
| Hematology | ||
| Hematocrit, l/L | 0.490 (0.0096) | 0.478 (0.0154)* |
| Hemoglobin, g/dL | 13.6 (0.38) | 13.5 (0.43) |
| White blood cells,9× 109/L | 0.93 (0.256) | 0.80 (0.256) |
| Neutrophils, × 10 /L | 0.62 (0.153) | 0.55 (0.164) |
| Lymphocytes, × 109/L | 0.23 (0.140) | 0.18 (0.137) |
| Monocytes, × 10 9/L | 0.06 (0.038) | 0.05 (0.016) |
| Eosinophils, × 10 /L | 0.02 (0.013) | 0.02 (0.010) |
| Clinical chemistry | ||
| AST, U/L | 48 (10.8) | 43 (8.3) |
| ALP, U/L | 77 (8.1) | 73 (6.0) |
| Urea, mmol/L | 7.37 (0.619) | 6.83 (0.483) |
| Cholesterol, mmol/L | 1.94 (0.143) | 1.81 (0.151) |
| Triglycerides, mmol/L | 0.63 (0.271) | 0.42 (0.220) |
| Glucose | 15.09 (1.147) | 11.93 (1.829)** |
| Na+, mmol/L | 151 (1.3) | 151 (2.9)** |
| K+, mmol/L | 3.7 (0.30) | 4.0 (0.29)* |
| Ca2+, mmol/L | 2.39 (0.046) | 2.34 (0.055)* |
| Albumin, g/L | 31 (0.6) | 31 (1.1) |
| Total protein, g/L | 46 (1.2) | 46 (1.3) |
Animals were followed up for 13 weeks. Values are means (SD)
p<0.05
p<0.01 vs. vehicle group, t-test on log-transformed data (hematology) or t-test or Wilcoxon test (clinical chemistry), as appropriate.
Table V.
Histopathology findings in NSG mice after three intravenous injections of 2 × 106 ABCB5+ MSCs/animal or vehicle without cells on day 1, 15 and 29.
| Number of affected/examined animals |
||
|---|---|---|
| Organ/tissue and findings | Vehicle | 2 × 106 MSCs/animal |
| Males | ||
| Epididymides | ||
| Sperm reduced, luminal, moderate | 1/10 | 0/9 |
| Spermatocele, moderate | 0/10 | 1/9 |
| Kidneys | ||
| Basophilia, tubular, minimal | 0/10 | 1/9 |
| Liver | ||
| Necrosis, hepatocellular, focal, minimal | 0/10 | 1/9 |
| Spleen | ||
| Metaplasia, osseous, slight | 1/10 | 0/9 |
| Testes | ||
| Atrophy, minimal | 3/10 | 3/9 |
| Females | ||
| Kidneys | ||
| Dilatation, tubular, minimal | 2/10 | 0/10 |
| Liver | ||
| Necrosis, hepatocellular, focal, minimal | 0/10 | 1/10 |
| Lungs and bronchi | ||
| Thrombus, minimal | 1/10 | 2/10 |
| Macrophage aggregation, alveolar, minimal | 1/10 | 0/10 |
| Pancreas | ||
| Vacuolation, acinar cells, minimal | 0/10 | 1/10 |
| Spleen | ||
| Metaplasia, osseous, slight | 0/10 | 1/10 |
| Uterus | ||
| Dilatation, glandular, cystic, minimal | 6/10 | 7/10 |
Animals were followed up for 13 weeks.
Acknowledgements
The authors gratefully acknowledge expert advice on the study designs from Monika Chabicovsky, PhD, MC Toxicology Consulting GmbH, Vienna, Austria. We also thank the contract research organizations Aurigon Life Science GmbH, Tutzing, Germany; Envigo CRS Limited, Huntingdon, United Kingdom; and Preclinics, Potsdam, Germany, for conducting the subcutaneous, intravenous and intramuscular animal studies, respectively; Accelero Bioanalytics GmbH, Berlin, Germany, for qPCR analysis; and AnaPath GmbH, Oberbuchsiten, Switzerland, for histopathological examinations in the local tolerance studies.
Footnotes
Disclosure of interest: The authors have no commercial, proprietary or financial interest in the products or companies described in this article.
Supplementarymaterial
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcyt.2018.12.005.
References
- [1].Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem 2003;278:47156–65 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- [2].Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–7 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- [3].Schatton T, Yang J, Kleffel S, Uehara M, Barthel SR, Schlapbach C, et al. ABCB5 Identifies Immunoregulatory Dermal Cells. Cell Rep 2015;12:1564–74 10.1016/j.celrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jiang D, Muschhammer J, Qi Y, Kugler A, de Vries JC, Saffarzadeh M, et al. Suppression of neutrophil-mediated tissue damage—a novel skill of mesenchymal stem cells. stem cells 2016;34:2393–406 10.1002/stem.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Webber BR, O’Connor KT, McElmurry RT, Durgin EN, Eide CR, Lees CJ, et al. Rapid generation of Col7a1(−/−) mouse model of recessive dystrophic epidermolysis bullosa and partial rescue via immunosuppressive dermal mesenchymal stem cells. Lab Invest 2017;97:1218–24 10.1038/labinvest.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med 2011;9:29 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS one 2012;7: e47559 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Casiraghi F, Remuzzi G, Abbate M, Perico N. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev 2013;9:65–79 10.1007/s12015-011-9345-4. [DOI] [PubMed] [Google Scholar]
- [9].Basu J, Assaf BT, Bertram TA, Rao M. Preclinical biosafety evaluation of cell-based therapies: emerging global paradigms. Toxicol Pathol 2015;43:115–25 10.1177/0192623314559104. [DOI] [PubMed] [Google Scholar]
- [10].European Parliament and Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union 2010;L276:33–79. [Google Scholar]
- [11].Prochazka M, Gaskins HR, Shultz LD, Leiter EH. The non-obese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci USA 1992;89:3290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].World Health Organization. Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks http://www.who.int/biologicals/Cell_Substrates_clean_version_18_April.pdf. [Accessed November 6, 2019].
- [13].International Organization for Standardization. ISO 10993–6:2007. Biological evaluation of medical devices Part 6: Tests for local effects after implantation https://www.iso.org/standard/44789.html/. [Accessed August 9, 2018].
- [14].Committee for Medicinal Products for Human Use (CHMP) at the European Medicines Agency (EMA). Guideline on human cell-based medicinal products (EMEA/CHMP/ 410869/2006) http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003894.pdf. [Accessed November 6, 2018].
- [15].Ramot Y, Meiron M, Toren A, Steiner M, Nyska A. Safety and biodistribution profile of placental-derived mesenchymal stromal cells (PLX-PAD) following intramuscular delivery. Toxicol Pathol 2009;37:606–16 10.1177/0192623309338383. [DOI] [PubMed] [Google Scholar]
- [16].Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl Med 2018;7:651–63 10.1002/sctm.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int 2013;2013:732742 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sensebe L, Fleury-Cappellesso S. Biodistribution of mesenchymal stem/stromal cells in a preclinical setting. Stem Cells Int 2013;2013:678063 10.1155/2013/678063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang F, Eid S, Dennis JE, Cooke KR, Auletta JJ, Lee Z. Route of delivery influences biodistribution of human bone marrow-derived mesenchymal stromal cells following experimental bone marrow transplantation. J Stem Cells Regen Med 2015;11:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther 2016;7:7–18 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shimizu S Routes of administration. In: Hedrich HJ, Bullock G, eds. The Laboratory Mouse, Amsterdam: Elsevier Academic Press; 2004:527–41. [Google Scholar]
- [22].Karp JM, Teo GSL. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206–16 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [23].Leibacher J, Dauber K, Ehser S, Brixner V, Kollar K, Vogel A, et al. Human mesenchymal stromal cells undergo apoptosis and fragmentation after intravenous application in immune-competent mice. Cytotherapy 2017;19:61–74 10.1016/j.jcyt.2016.09.010. [DOI] [PubMed] [Google Scholar]
- [24].Arango-Rodriguez ML, Ezquer F, Ezquer M, Conget P. Could cancer and infection be adverse effects of mesenchymal stromal cell therapy? World J Stem Cells 2015;7:408–17 10.4252/wjsc.v7.i2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol 2010;184:5885–94 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- [26].Ljujic B, Milovanovic M, Volarevic V, Murray B, Bugarski D, Przyborski S, et al. Human mesenchymal stem cells creating an immunosuppressive environment and promote breast cancer in mice. Sci Rep 2013;3:2298 10.1038/srep02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci 2018;15:36–45 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells 2006;24:1095–103 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- [29].Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 2007;25:371–9 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- [30].Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 2009;69:5331–9 10.1158/0008-5472.Can-08-4630. [DOI] [PubMed] [Google Scholar]
- [31].Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 2007;67:9142–9 10.1158/0008-5472.Can-06-4690. [DOI] [PubMed] [Google Scholar]
- [32].Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 2010;115:1549–53 10.1182/blood-2009-05-219907 [DOI] [PubMed] [Google Scholar]
- [33].Conforti A, Starc N, Biagini S, Tomao L, Pitisci A, Algeri M, et al. Resistance to neoplastic transformation of ex-vivo expanded human mesenchymal stromal cells after exposure to supramaximal physical and chemical stress. Oncotarget 2016;7:77416–29 10.18632/oncotarget.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy 2017;19:1351–82 10.1016/j.jcyt.2017.08.004. [DOI] [PubMed] [Google Scholar]
- [35].Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies—bridging scientific observations and regulatory viewpoints. Cytotherapy 2013;15:753–9 10.1016/j.jcyt.2013.03.005. [DOI] [PubMed] [Google Scholar]
- [36].Zahavi-Goldstein E, Blumenfeld M, Fuchs-Telem D, Pinzur L, Rubin S, Aberman Z, et al. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy 2017;19:1438–46 10.1016/j.jcyt.2017.09.010. [DOI] [PubMed] [Google Scholar]
- [37].Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005;174:6477–89. [DOI] [PubMed] [Google Scholar]
- [38].Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter R. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol 2013;91:40–51 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- [39].Toyserkani NM, Jørgensen MG, Tabatabaeifar S, Jensen CH, Sheikh SP, Sørensen JA. Concise review: a safety assessment of adipose-derived cell therapy in clinical trials: a systematic review of reported adverse events. Stem Cells Transl Med 2017;6:1786–94 10.1002/sctm.17-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis 2017;76:196–202 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- [41].Gothelf Y, Abramov N, Harel A, Offen D. Safety of repeated transplantations of neurotrophic factors-secreting human mesenchymal stromal stem cells. Clin Transl Med 2014;3:21–31 10.1186/2001-1326-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ra JC, Jeong EC, Kang SK, Lee SJ, Choi KH. A prospective, nonrandomized, no placebo-controlled, phase i/ii clinical trial assessing the safety and efficacy of intramuscular injection of autologous adipose tissue-derived mesenchymal stem cells in patients with severe Buerger’s disease. Cell Med 2017;9:87–102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].European Parliament and Council of the European Union. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal of the European Union 2001;L311:67–128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.