1. Overview
Cervical cancer is one of the most common gynecological malignancies worldwide. The incidence of cervical cancer ranks second for female malignancies in China. There are about 500,000 new cases of cervical cancer worldwide every year, and more than 80% of them occur in developing countries. About 130,000 new cases occur annually in China, accounting for 28% of the total number of new cases for cervical cancer worldwide. The peak age for diagnosis is 40−60 years old; however, the age of onset is becoming younger in recent years. The incidence of cervical cancer has regional differences, with the incidence in developing countries being higher compared to developed countries. The occurrence of cervical cancer could be effectively controlled by early examination, diagnosis and treatment of precancerous lesions.
This guideline applies to cervical squamous cell carcinoma, adenocarcinoma and adenosquamous carcinoma, which account for more than 90% of cervical cancer. Several unique pathological types have low incidences, and no consensus has been reached regarding its diagnosis and treatment in China and abroad. Hence, this guideline is not appropriate for cervical cancer with rare pathological types. This guideline references internationally recognized guidelines for the diagnosis and treatment of cervical cancer and is amended according to our past guidelines. In current clinical practice, more attention is paid for comprehensive and individualized treatment. Current treatment strategies are influenced by a combination of hospital equipment, technigue and patient’s condition. With regards to patients with complex cervical cancer, clinicians should follow these guidelines in a rational manner. For patients that fall out of these guidelines, clinical trials should be recommended.
2. Diagnosis
2.1 Etiology
Persistent infection with high-risk types of human papillomavirus (HPV) is the most significant factor for cervical cancer and precancerous lesion manifestation. The common high-risk HPV in China includes HPV 16, 18, 31, 33, 45, 52, and 58. HPV is mainly transmitted through sexual activity. Presently, the HPV vaccine has been on the market in China, and is administered based on appropriate age to prevent cervical precancerous lesions and cervical cancer.
2.2 Clinical manifestation
2.2.1 Symptoms
Precancerous lesions and early cervical cancer are asymptomatic. However, common symptoms include postcoital bleeding, abnormal vaginal discharge, and irregular vaginal bleeding. Late-stage patients may have vaginal hemorrhage, pelvic or lower back pain, lower limb pain, lower limb edema, anemia, fever, oliguria or cachexia and additional clinical manifestations.
2.2.2 Signs
(1) Assessment
Clinical examination and assessment are through direct vulva examination, and examination of the vagina and cervix using a vaginal speculum. Attention should be paid to the location, scope, shape, and volume of cervical tumor and its relationship with surrounding tissues, as well as the extent of vaginal involvement.
(2) Palpation
The texture, infiltration and tumor association to surrounding organs must be determined by palpation. Rectovaginal examination is used to determine whether there is parametrial infiltration, proximity extent between the tumor and pelvic wall, uterosacral ligament disorders, uterine rectal fossa and surrounding organs.
2.3 Auxiliary examination
2.3.1 Cervical/vaginal cytology examination and HPV test
This is the initial screening method used for early diagnosis of cervical cancer and precancerous lesions. Biopsies are obtained from the transitional zone of the cervical epithelium. At present, cervical thinprep cytologic test (TCT) is the method of choice. The combination of TCT and HPV test helps to improve accuracy.
2.3.2 Histological examination
For the diagnosis of cervical intraepithelial neoplasias and cervical cancer, biopsies should be performed and evaluated. If the lesion is not obvious to the naked eye, iodine test should be used. Using a 3% or 5% acetic acid solution, biopsy sites could be observed by the naked eye or colposcopy. Biopsy samples from the cervix should be acquired from areas proximal to the cervical squamocolumnar junction (SCJ) and/or immature squamous metaplasia epithelium to reduce misdiagnosis. Biopsies should include abnormal epithelium adjacent to the ulcer because the center of the ulcer is often necrotic. The number of biopsies depends on the size and severity of the lesion. Multipoint biopsies usually require 2−4 specimens. For patients who are unable to be diagnosed by multiple punch biopsies, the incision method is used to harvest deeper tissues when needed. Endocervical curettage should be performed simultaneously. For patients where the cervical surface biopsy was negative while cytology was positive or when cervical cancer cannot be clinically excluded, or for patients who are diagnosed with cancer but infiltration and the depth of infiltration cannot be determined, cervical conization will be required.
2.3.3 Cervical colposcopy
Colposcopy is appropriate for patients with abnormal cervical cytology, and is mainly performed to observe the changes to epithelial blood vessels and tissues in cervicovaginal lesions. For patients without visible lesions, or with high-grade squamous intraepithelial lesions or high-grade squamous intraepithelial lesions with HPV 16, 18 infections, colposcopy is used to determine abnormal epithelial changes at the cervical SCJ. Histological examination of localized biopsies from colpscopy helps to improve the accuracy for diagnosis. Endocervical curettage should be performed simultaneously for patients with unsatisfactory colposcopy, postmenopausal women, and patients who have had previous conizations. Satisfactory colposcopy and high-quality pathological examinations are essential for accurate diagnosis and appropriate treatment for cervical precancerous lesions. Patients should be transferred to hospitals capable of performing these procedures.
2.3.4 Cystoscopy and rectoscopy examination
Patients with suspected bladder or rectum involvement should receive endoscopic examination. These patients should be transferred to hospitals capable of performing these procedures.
2.3.5 Imaging examination
The value of imaging examination for cervical cancer is mainly to evaluate the extent of local invasion, lymph node metastasis and distant organ metastasis. Furthermore, it guides clinical decision-making and assesses treatment efficacy. The imaging methods used for cervical cancer include:
(1) Abdominal and pelvic ultrasound: It includes transabdominal and transvaginal (or transrectal) ultrasound. They are mainly used to detect local cervical lesions, pelvic and retroperitoneal lymph node metastasis, hydronephrosis and other abdominal and pelvic organ metastasis.
(2) Pelvic magnetic resonance imaging (MRI): It is a multi-sequencing and multi-parameter imaging modality with excellent soft tissue resolution, has no radiation, and is the best imaging method for cervical cancer detection. Its advantages include: 1) detecting lesions and determining the size and location; 2) defining the extent of lesion invasion, providing an important basis for staging before treatment, revealing the depth of cervical stromal invasion, determining whether the lesion is confined to the cervix, or invaded the parauterine or pelvic wall, and presenting the extent of vaginal lesions and the invasion of the bladder and rectal wall; and 3) detecting lymph node metastasis in the pelvic cavity, retroperitoneal area and inguinal region.
(3) Abdominal and pelvic computed tomography (CT): The advantages of CT are to display lesions during middle and advanced stages, evaluate the relationship between cervical lesions and peripheral organs, lymph node metastasis, and large-scale scanning for metastasis in other abdominal and pelvic organs. For patients with contraindications to magnetic resonance, CT is preferred.
(4) Chest radiography and CT examination: The main purpose is to detect lung metastasis. Chest radiographs should include front and side views, and chest CT should be performed when necessary.
(5) Nuclear medicine imaging examination: Positron emission tomography (PET)-CT is not recommended to evaluate local infiltration of cervical cancer, but is recommended for patients under the following conditions: 1) staging before treatment for patients with stage IB1 or above in International Federation of Gynecology and Obstetrics (FIGO) staging; 2) when a systemic assessment is needed for patients with cervical cancer found unexpectedly at the time of uterectomy; 3) for the delineation of target areas for radiotherapy; 4) for follow-up monitoring of patients with FIGO stage IB2 or above or other high-risk factors 3−6 months after treatment; or 5) for patients with suspected recurrence and metastasis during follow-up, including manifestation of clinical symptoms or elevated tumor markers. Radionuclide bone scans are used only for patients with suspicious bone metastases.
2.3.6 Tumor markers examination
Abnormal elevation of tumor markers could assist in the diagnosis, evaluation of therapeutic efficacy, disease monitoring and follow-up monitoring after treatment. Squamous cell carcinoma antigen (SCC) is an important marker for cervical squamous cell carcinoma, and carcinoembryonic antigen (CEA), carbohydrate antigen (CA)125 or CA19-9 are elevated in patients with adenocarcinoma of the uterine cervix.
2.4 Diagnostic criteria for cervical cancer
2.4.1 Clinical diagnosis
For accurate diagnosis of cervical cancer, the clinical symptoms, signs, and laboratory and imaging examinations are essential: 1) there may be no symptoms and signs during the early stages. However, postcoital bleeding or increased vaginal discharge and odor may occur; 2) there may be vaginal bleeding at late stages which may lead to anemia; tumor confounded with infections may cause fever; there may be renal failure and cachexia; 3) tumor invading the bladder may cause hematuria, rectum invasion may cause bloody stool, and bladder and rectum invasion may cause fistulas; 4) tumor marker SCC has become increasingly significant; and 5) imaging examinations (ultrasonography, MRI, CT) are essential for diagnosis of cervical cancer, and may help to determine parametrial invasion, hydronephrosis, and retroperitoneal lymph node metastasis.
2.4.2 Pathological diagnosis
Pathological examination of cervical biopsies after colposcopy or visual observation is the gold standard for final diagnosis. For difficult or rare pathological types, immunohistochemical examination should be performed to identify tumor or determine differential diagnosis. These types of diagnosis should be performed in specialized hospitals with proper clinical diagnostic laboratories.
2.5 Differential diagnosis
2.5.1 Benign lesions of cervix
Benign lesions such as severe cervical erosion, cervical tuberculosis, cervical polyps, submucous myoma of cervix and other inflammatory ulcer of cervix need to be differentiated from cervical cancer.
2.5.2 Metastatic carcinoma of cervix
Endometrial carcinoma can spread to cervix. The pathological examination of cervical biopsy and immunohistochemical examination help to make diagnosis.
3. Classification and staging of cervical cancer
3.1 Histological classification for cervical cancer (Table 1)
1.
Histological classification of tumors of uterine cervix (WHO, 2014)
| Entities | ICD-O code |
| Epithelial tumors | |
| Squamous cell tumors and precursors | |
| Squamous intraepithelial lesions | |
| Low-grade squamous intraepithelial
lesion |
8,077/0 |
| High-grade squamous intraepithelial
lesion |
8,077/2 |
| Squamous cell carcinoma, NOS | 8,070/3 |
| Keratinizing | 8,071/3 |
| Non-keratinizing | 8,072/3 |
| Papillary | 8,052/3 |
| Basaloid | 8,083/3 |
| Warty | 8,051/3 |
| Verrucous | 8,051/3 |
| Squamotransitional | 8,120/3 |
| Lymphoepithelioma-like | 8,082/3 |
| Benign squamous cell lesions | |
| Squamous metaplasia | |
| Condyloma acuminatum | |
| Squamous papilloma | 8,052/0 |
| Transitional metaplasia | |
| Glandular tumors and precursors | |
| Adenocarcinoma in situ | 8,140/2 |
| Adenocarcinoma | 8,140/3 |
| Endocervical adenocarcinoma, usual type | 8,140/3 |
| Mucinous carcinoma, NOS | 8,480/3 |
| Gastric type | 8,482/3 |
| Intesitinal type | 8,144/3 |
| Signet-ring cell type | 8,490/3 |
| Villoglandular carcinoma | 8,263/3 |
| Endometrioid carcinoma | 8,380/3 |
| Clear cell carcinoma | 8,310/3 |
| Serous carcinoma | 8,441/3 |
| Mesohephric carcinoma | 9,110/3 |
| Adenocarcinoma admixed with
neuroendocrine carcinoma |
8,574/3 |
| Benign glandular tumors and tumor-like lesions | |
| Endocervical polys | |
| Müllerian papilloma | |
| Nabothian cyst | |
| Tunnel clusters | |
| Microglandular hyperplasia | |
| Table 1 (continued) | |
Histological types for cervical cancer include the following: squamous cell carcinoma (keratinization, non-keratinization and verrucous), endometrioid adenocarcinoma, clear cell adenocarcinoma, adenosquamous carcinoma, adenocystic carcinoma, small cell carcinoma and undifferentiated carcinomas.
3.2 Staging of cervical cancer
The clinical staging criteria for cervical cancer were revised in the 2009 meeting of FIGO (Table 2). The revised guidelines are currently used for staging. Rectovaginal examination is essential to determine clinical stage. Clinical staging is usually determined by two experienced gynecological oncologists.
2.
Cervical cancer staging based on International Federation of Gynaecology and Obstetrics (FIGO 2009) guidelines
| Stage | Definition |
| Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus would be disregarded) |
| IA | Invasive carcinoma which can be diagnosed only by microscopy, with the deepest invasion ≤5 mm and the largest extension ≥7 mm |
| IA1: Measured stromal invasion of ≤3.0 mm in depth and extension of ≤7.0 mm | |
| IA2: Measured stromal invasion of >3.0 mm and ≤5.0 mm with an extension of ≤7.0 mm | |
| IB | Clinically visible lesions limited to the cervix uteri or pre-clinical cancers greater than stage IA |
| IB1: Clinically visible lesion ≤4.0 cm in greatest dimension | |
| IB2: Clinically visible lesion >4.0 cm in greatest dimension | |
| Stage II | Cervical carcinoma invades beyond the uterus, but not to the pelvic wall or to the lower third of the vagina |
| IIA | Without parametrial invasion |
| IIA1: Clinically visible lesion ≤4.0 cm in greatest dimension | |
| IIA2: Clinically visible lesion >4.0 cm in greatest dimension | |
| IIB | With obvious parametrial invasion |
| Stage III | The tumor extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or
non-functioning kidney |
| IIIA | Tumor involves lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or non-functioning kidney |
| Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to Stage IV |
| IVA | Spread to adjacent organs |
| IVB | Spread to distant organs |
4. Treatment
4.1 Cervical cancer staging and treatment options
4.1.1 Microscopic diagnosis for invasive carcinoma
Diagnosis of stage IA tumors is based on microscopic examinations. Cervical biopsy specimens do not reveal all lesions present. Hence conization biopsies are required for accurate diagnosis. For the accurate diagnosis of stage IA cervical cancer, careful pathological examination of conization samples with negative margins is required.
Patients in stage IA1 who have no fertility requirements are advised to receive extrafascial hysterectomy. If patients wish to preserve fertility, cervical conization could be performed. Patients with negative margins should be followed up regularly. The lymph node metastasis rate of stage IA1 is <1%, hence there is no need for lymph node resection for stage IA1 patients. However, if the lymphovascular space is invaded, cervical conization (negative incision margins) or modified radical hysterectomy with pelvic lymphadenectomy should be performed.
1.
| Table 1 (continued) | |
| Entities | ICD-O code |
| Lobular endocervical gradular hyperplasia | |
| Diffuse lamina endocervical hyperplasia | |
| Mesonephric remnants and hyerplasia | |
| Arias-Stell reaction | |
| Endocervicosis | |
| Endometriosisi | |
| Tuboendometrioid metaplasia | |
| Ectopic prostate tissue | |
| Other epithelial tumors | |
| Adenosquamous carcinoma | 8,560/3 |
| Glassy cell carcinoma | 8,015/3 |
| Adenoid basal carcinoma | 8,098/3 |
| Adenoid cystic carcinoma | 8,200/3 |
| Undifferentiated carcinoma | 8,020/3 |
| Neuroendocrine tumors | |
| Low-grade neuroendocrine tumor | |
| Carcinoid tumor | 8,240/3 |
| Atypical carcinoid tumor | 8,249/3 |
| High-grade neuroendocrine tumor | |
| Small cell neuroendocrine carcinoma | 8,041/3 |
| Large cell neuroendocrine carcinoma | 8,013/3 |
| Mesenchymal tumors and tumor-like lesions | |
| Benign | |
| Leiomyoma | 8,890/0 |
| Rhabdomyoma | 8,905/0 |
| Others | |
| Malignant | |
| Leiomyosarcoma | 8,890/3 |
| Rhabdomyosarcoma | 8,910/3 |
| Alveolar soft-part sarcoma | 9,581/3 |
| Angiosarcoma | 9,120/3 |
| Malignant peripheral nerve sheath tumor | 9,540/3 |
| Other sarcomas | |
| Liposarcomas | 8,850/3 |
| Undefferentiated endocervical sarcoma | 8,805/3 |
| Ewing sarcoma | 9,364/3 |
| Tumor-like lesions | |
| Postoperative spindle-cell nodule | |
| Lymphoma-like lesion | |
| Mixed epithelial and massenchymal tumors | |
| Adenomyoma | 8,932/0 |
| Table 1 (continued) | |
1.
| Table 1 (continued) | |
| Entities | ICD-O code |
| Adenosarcoma | 8,933/3 |
| Carcimosarcoma | 8,980/3 |
| Melanocytic tumors | |
| Blue naevus | 8,780/0 |
| Malignant melanoma | 8,720/3 |
| Germ cell tumors | |
| Yolk sac tumour | |
| Lymphoid and myeloid tumors | |
| Lymphomas | |
| Myeloid Neoplasms | |
| Secondary tumors | |
The lymph node metastasis rate for stage IA2 cervical cancer is 3%−5%. Subradical hysterectomy (type II modified radical hysterectomy) and pelvic lymphadenectomy may be required. If patients have fertility requirements, cervical conization (negative incision margins) or radical trachelectomy or pelvic lymphadenectomy may be selected. For patients who wish to preserve fertility, radical trachelectomy may be advised.
4.1.2 Invasive cervical carcinoma
(1) Stage IB1 and IIA1 both have good prognosis after surgery or radiotherapy. The surgical procedures are type III radical hysterectomy and pelvic lymphadenectomy ± para-aortic lymph node sampling. Patients with postoperative high-risk factors (parametrial invasion, deep stromal invasion or lymph node metastasis) may require concurrent chemoradiation. Patients with moderate-risk factors also require postoperative radiotherapy ± concurrent chemotherapy to reduce pelvic recurrence and improve survival rate. For patients who wish to preserve fertility and the cervical tumor diameter is ≤2 cm, radical trachelectomy and pelvic lymphadenectomy ± para-aortic lymph node sampling could be performed.
(2) Treatment options for stage IB2 and IIA2 (lesion >4 cm) include: 1) concurrent chemoradiotherapy; 2) radical hysterectomy, pelvic lymph node dissection, para-aortic lymph node sampling, and postoperative individualized adjuvant therapy; and 3) adjuvant hysterectomy after primary chemoradiation. The overall 5-year survival rate of stage IB patients is 80%−90%. Among them, the 5-year survival rate of patients with cervical tumor diameter >4 cm and high-risk factors such as lymph node metastasis, parametrial invasion and/or positive incision margins is between 40% and 70%. For selected newly diagnosed patients with early-stage cervical cancer, chemoradiotherapy may be more beneficial for patients with high-risk factors. A large number of studies have demonstrated that radical surgery and postoperative adjuvant radiotherapy have several complications, and pelvic radiotherapy should be avoided after radical surgery. At present, the standard treatment for locally advanced cancer patients is concurrent chemoradiation.
(3) Stage IIB−IVA: Concurrent chemoradiation.
(4) Stage IVB: Systematic treatment is the principal therapy complemented with supportive treatment, with certain patients having palliative surgery or individualized radiotherapy.
4.2 Surgical treatment
Surgical treatment is the main treatment modality for early cervical cancer. Surgeries include hysterectomy and lymphadenectomy. The Piver classification system for the five types of hysterectomy proposed in 1974 is still widely used today (Table 3). Querleu-Morrow classification of hysterectomy published in 2008 is gradually accepted for emphasis on precise anatomy and individualized treatment of surgical resection (Table 4). As pelvic autonomic nerve injury caused by radical hysterectomy results in abnormal bladder function, abnormal colorectal peristalsis and sexual dysfunction, nerve-sparing radical hysterectomy (NSRH) for cervical cancer has been developed. NSRH can be performed through laparotomy, laparoscopy and robotic laparoscopy.
3.
Piver classification system for five types of hysterectomy
| Type | Extent of surgery |
| Type I | Extrafascial hysterectomy |
| Type II | Modified radical hysterectomy. The resection scope includes 1/2 sacrospinous ligament and main ligament and upper 1/3 of the vagina. |
| Type III | Radical hysterectomy. The resection scope includes main ligament adjacent to the pelvic wall, sacrospinous ligament from the sacral attachment, and upper 1/2 of the vagina |
| Type IV | Extended radical hysterectomy |
| Type V | Pelvic exenteration |
4.
Querleu-Morrow classification of radical hysterectomy
| Type | Lateral parametrium | Ventral parametrium | Dorsal parametrium | Vaginectomy |
| A | Halfway between the cervix
and ureter |
Minimal excision | Minimal excision | Less than 1 cm |
| B1 | At the ureter | Partial excision of the vesicouterine ligament | Partial resection of the rectouterine-rectovaginal ligament | Excision of 1 cm |
| B2 | Identical to B1 plus paracervical lymphadenectomy without resection of vascular struction | Identical to B1 | Identical to B1 | Identical to B1 |
| C1 | At the iliac vessels transversally, caudal part is preserved | Excision of the vesicouterine ligament at the bladder (bladder nerves are dissected and spared) | At the rectum (hypogastric nerve is dissected and spared) | Excision of 2 cm or according to demand |
| C2 | At the level of medial aspect of iliac vessels completely (including the caudal part) | At the bladder (bladder nerves are sacrificed) | At the sacrum (hypogastric nerve is sacrificed) | Identical to C1 |
| D1 | At the pelvic wall, including resection of the internal iliac vessels | At the bladder | At the sacrum | According to demand |
| D2 | Identical to D1, including resection of the pelvic sidewall | According to demand | According to demand | According to demand |
Lymph node resection for cervical cancer involves pelvic lymph nodes and para-aortic lymph nodes. Pelvic lymphadenectomy ± para-aortic lymph node sampling should be performed for stage IA1 [combined with lymph-vascular space invasion (LVSI)]−IIA. The postoperative pelvic lymph node metastasis rate for patients with stage I and stage II cervical cancer is not high. Based on the status of sentinel lymph node metastasis, sentinel lymp node biopsy could reduce the incidence of postoperative complications in patients with cervical cancer. Sentinel lymph node biopsy procedure has been incorporated into international guidelines for patients with early-stage cancer; however the indications remain to be determined. Systemic lymphadenectomy and sentinel lymphadenectomy could be performed through laparotomy, laparoscopy and robotic laparoscopy.
The ovarian metastasis rate for stage I−IIA cervical squamous cell carcinoma is less than 1%. For premenopausal patients who require ovarian preservation, the healthy ovary could be preserved during surgery. At present, the risk of occult ovarian metastasis for cervical adenocarcinoma is high, so preserving the ovary should be carefully considered. The retained ovary could be translocated during surgery (i.e., into the abdominal cavity or at a high position in the retroperitoneal paracolon sulcus) to avoid damage to ovarian function induced by postoperative pelvic radiotherapy.
For younger patients with early-stage cervical cancer without lymph node metastasis and who have fertility requirements, fertility-preserving surgery should be performed. For stage IA1 patients without LVSI, cervical conization with negative margins could be performed. If the lesions are wide, trachelectomy should be performed. For stage IA1 patients with LVSI and stage IA2 patients, cervical conization/trachelectomy (the width for negative margins should be 3 mm) + transabdominal/laparoscopic pelvic lymphadenectomy ± para-abdominal aortic lymph node sampling or transabdominal, transvaginal or laparoscopic radical trachelectomy + pelvic lymphadenectomy ± para-abdominal aortic lymph node sampling should be performed. For stage IB1 patients (<2 cm), radical trachelectomy + pelvic lymphadenectomy ± para- abdominal aortic lymph node sampling should be performed. For stage IA2−IB1 patients with LVSI and stage IB1 patients with tumor diameter >2 cm, there is no consensus for fertility-sparing surgery, which should be carefully considered.
4.3 Radiotherapy
Radiotherapy is suitable for all stages of cervical cancer. It is mainly used in patients with advanced cervical cancer above stage IIB and early cervical cancer patients who are unable to undergo surgical treatment. Radiotherapy includes external beam radiation therapy (EBRT) and brachytherapy or the combined application of the two methods. Studies have shown that concurrent chemoradiation improves the efficacy and reduces the risk of recurrence compared to radiotherapy alone.
4.3.1 Principles of radiotherapy
Radiotherapy is a treatment strategy for malignant tumors. It kills cancer cells to the maximum extent while preserving normal tissues and critical organs. Optimizing the dose and duration of radiotherapy is required for successful treatment. It has been recommended that all EBRT and brachytherapy should be completed within 8 weeks.
If radiotherapy and surgery are combined, preoperative or postoperative radiotherapy should be considered based on the tumor and patients’ condition. Preoperative radiation therapy is intended to reduce tumor size and improve the surgical resection rate. Postoperative radiotherapy is usually considered after review of postoperative pathologic results together with several other adverse prognostic factors. If high-risk factors are present such as positive surgical margins, parametrial invasion and lymph node metastasis, postoperative adjuvant chemoradiation will be necessary. Based on the Sedlis criteria (Table 5) of National Comprehensive Cancer Network (NCCN) guidelines in 2015, postoperative adjuvant pelvic radiotherapy or chemoradiation is required if middle-risk factors such as large tumor size, deep stromal invasion and/or lymphovascular space invasion are observed during or after surgery. This can reduce local recurrence and improve therapeutic efficacy. However, the combination of surgery and radiotherapy also increases complications.
5.
Indications for postoperative pelvic radiotherapy for cervical cancer patients with middle-risk factors
| LVSI | Stromal invasion | Tumor size (cm)
(Determined by clinical palpation) |
| + | Deep 1/3 | any |
| + | Middle 1/3 | ≥2 |
| + | Superficial 1/3 | ≥5 |
| − | Middle or Deep 1/3 | ≥4 |
4.3.2 EBRT
(1) Conventional radiotherapy
Conventional radiotherapy describes the positioning of the simulator or CT simulator. Target volume should generally include the uterus, cervix, parametria and upper 1/2 of the vagina, and pelvic lymphatic drainage areas such as internal iliac, obturator, external iliac and common iliac lymph nodes. Target volume for stage IIIA patients includes all of the vagina and the inguinal region if necessary. Four-field box radiation or isocenter anterior and posterior penetrating radiation is generally used. The EBRT dose is approximately 45 Gy in conventional fractionation of 1.8−2.0 Gy daily, 5 d/week. Stage I−II: 45 Gy/4.5−5 weeks, stage III−IV: 45−50 Gy/5−6 weeks.
(2) Three-dimensional conformal radiation therapy and intensity-modulated radiation therapy
CT or MRI-based treatment and conformal blocking are considered the standard of care for EBRT. The gross target volume (GTV) is determined based on rectovaginal examination and imaging results, and the clinical target volume (CTV) is determined by direct diffusion of cervical cancer and lymph node metastasis. The target volume for EBRT should include the gross tumor area, parametria, uterosacral ligament, presacral lymph nodes and other potentially involved lymph nodes, and sufficiently long vaginal tissues (the lower margin is at least 3 cm from the tumor). If no positive lymph nodes are found during surgery or by imaging, the radiation range should include the external iliac lymph nodes, internal iliac lymph nodes, obturator lymph nodes and pre-sacral lymph node drainage area. If the risk of lymph node metastasis is high (such as for bulkier tumors, suspected or confirmed low true pelvic lymph node metastasis), radiation range should include the common iliac lymph node areas as well. In patients with documented common iliac and/or para-aortic nodal involvement, extended-field pelvic and para-aortic radiotherapy is recommended, up to the level of the renal vessels (or even more cephalad as directed by involved nodal distribution). If the lesion has invaded the lower 1/3 of the vagina, bilateral inguinal lymph nodes should also be included for radiation therapy. Planning target volume (PTV) is set based on a distance out of CTV (0.5−1.0 cm). The radiotherapy dose is 45−50 Gy/1.8−2 Gy/5−6 weeks. For unresectable gross lesions or lymph nodes of limited size, intensity-modulated radiation therapy (IMRT) could be used to treat lesions with an additional dose of 10−20 Gy.
4.3.3 Brachytherapy
Brachytherapy is an important treatment strategy for radical radiotherapy of cervical cancer. Based on the anatomical characteristics of the patients’ tumor, different vaginal colpostats combined with intrauterine tandem are selected. Frequently-used radioactive sources are listed in Table 6. When combined with EBRT, brachytherapy is usually initiated towards the latter part of treatment. The frequency is usually 1−2 times per week. The weekly dose at point “A” is 5−10 Gy, and the total dose at point “A” is 35−45 Gy. The total dose for EBRT and brachytherapy varies with the different clinical staging and tumor size. The general total dose is 75−90 Gy. The dose at the reference point of the rectum and bladder International Commission on Radiation Units and Measurements (ICRU) should be limited to 60%−70% of the prescription dose at point A, and the maximum dose should not exceed 80%. Image guided brachytherapy could improve the survival rate and reduce side effects.
6.
Radioactive sources of brachytherapy
| Radioactive sources | Radium 226 | Cobalt 60 | Cesium 137 | Iridium 192 |
| Specific activity (Ci/cm3) | 2.1 (3.8 at most) | 1,900 | 27.5 | 9,000 |
| Half-life (year) | 1,590 | 5.3 | 33 | 0.2 (74 d) |
4.3.4 Combination of brachytherapy and EBRT
Except in a few cases for early-stage cervical cancer that only requires brachytherapy, the combination of brachytherapy and EBRT is necessary. Combined irradiation is effective for the target volume of cervical cancer with uniform dose distribution.
4.3.5 Complications of radiotherapy
(1) Acute complications occur during and shortly after treatment, such as infection, vaginitis, vulvitis, dry and wet skin reactions, bone marrow suppression, gastrointestinal reactions, rectal reactions, bladder reactions, mechanical injuries, and others.
(2) Long-term complications include radiation proctitis, radiation cystitis, skin and subcutaneous tissue changes, reproductive organ changes and radiation enteritis. Radiation proctitis is the most common long-term complication and usually occurs 1−1.5 years after radiotherapy. The main manifestations are increased stool frequency, mucous stool, hematochezia, and rectovaginal fistula in severe cases. Radiation cystitis is the second most common late complication and usually occurs 1.5 years after radiotherapy. Its main manifestations are frequent micturition, odynuria, hematuria, dysuria, and vesicovaginal fistula in severe cases.
4.3.6 Normal tissue considerations
Radiotherapy of cervical cancer has potential impacts on surrounding critical organs, such as bladder, rectum, colon, bone, skin, small bowel and ureters. TD5/5 which presenting the incidence of severe complications is less than 5% in 5 years after treatment is used to describe the minimum tolerable dose of radiation. The TD5/5 of different organs is showed in Table 7.
7.
TD5/5 of impacted tissues
| Organs | Side effects | TD5/5 | Length or area of radiation |
| Skin | Ulceration, severe fibrosis | 55 | 100 cm2 |
| Bowel | Ulceration, perforation, bleeding | 50 | 100 cm2 |
| Colon | Ulceration, stenosis | 45 | 100 cm2 |
| Rectum | Ulceration, stenosis | 60 | 100 cm2 |
| Kidney | Acute or chronic nephritis | 20 | Kidney |
| Bladder | Contracture | 60 | Entire bladder |
| Ureter | Stenosis | 75 | 5−10 cm |
| Ovary | Permanent sterility | 2−3 | Entire ovary |
| Uterus | Necrosis, perforation | >100 | Entire uterus |
| Vagina | Alceration, fistula | 90 | Entire vaginal |
| Bones | Necrosis, fracture, sclerosis | 60 | Bone or 10 cm2 |
| Spinal marrow | Infarct necrosis | 45 | 10 cm |
| Muscles | Fibrosis | 60 | Muscles |
| Bone marrow | Hypoplasia | 2 | General |
| 30 | Local | ||
| Lymph nodes | Atrophy sclerosis | 50 | Lymph node |
| Fetus | Death | 2 | Fetus |
| Peripheral nervous | Neuritis | 60 | 10 cm2 |
4.4 Chemotherapy
The efficacy of chemotherapy for cervical cancer treatment has attracted increasing attention. It is mainly used with radiotherapy alone or combined with chemotherapy for radiotherapy sensitization. It is also used as a preoperative neoadjuvant chemotherapy as well as palliative treatment for patients with late-stage distant metastasis and recurrence.
4.4.1 Concurrent chemoradiation
Concurrent chemoradiation refers to simultaneous chemotherapy with radiotherapy, also known as sensitization chemotherapy. The regimens for sensitization chemotherapy during radiotherapy that is recommend by current NCCN treatment guidelines are cisplatin + 5-FU, weekly therapy of cisplatin, cisplatin + paclitaxel, and weekly therapy of cisplatin + paclitaxel.
4.4.2 Neoadjuvant chemotherapy
Neoadjuvant chemotherapy refers to 2−3 courses of chemotherapy before surgery. The aim is to reduce the tumor volume and make patients eligible for surgery. Several non-randomized studies have demonstrated that neoadjuvant chemotherapy reduced the probability of intraoperative dissemination and postoperative metastasis. At present, it is mainly used in patients with early-stage locally advanced cervical cancer.
4.4.3 Palliative chemotherapy
It is mainly used in patients with recurrent or metastatic cervical cancer who did not receive surgery or radiotherapy. The first-line chemotherapy regimens recommended by 2018 NCCN guidelines for cervical cancer are cisplatin combined with paclitaxel, cisplatin combined with paclitaxel and bevacizumab, cisplatin and paclitaxel combined with topotecan and bevacizumab, and cisplatin combined with gemcitabine. Patients with recurrent and persistent cervical cancer are encouraged to participate in clinical trials.
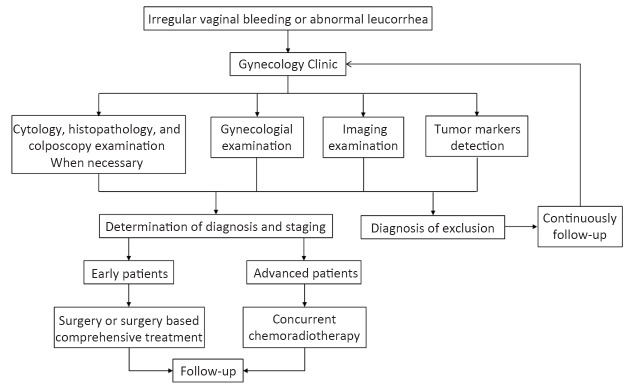
Diagnosis and treatment procedure for cervical cancer is shown in Figure 1.
1.
Diagnosis and treatment procedure for cervical cancer.
5. Follow-up
For newly diagnosed cervical cancer patients, complete medical records and patient characteristics and clinical information should be collected. Regular follow-up after treatment should be monitored. Patients should be followed up every 3 months for the first 2 years after treatment, every 6 months after 3−5 years after treatment, and then once a year after 5 years. The follow-up should be continued based on the patient’s condition after 5 years of continuous follow-up.