Breast cancer is the most common malignancy in women and is a major threat to women’s health. Currently, with comprehensive treatment modalities, the outcomes of breast cancer are among the best of solid tumors. A set of Chinese guidelines and recommendations are developed to standardize the clinical practice, to ensure the quality and safety of medical service, and to ultimately improve the survival of breast cancer patients.
1. Screening
Screening refers to the detection of precancerous lesions and early-stage invasive carcinomas in asymptomatic women with effective, convenient and economical methods. The ultimate goal of breast cancer screening is to lower the mortality through early detection and prompt treatment.
There are two kinds of screening: mass screening and opportunistic screening. Mass screening refers to screening organized by the local government for all women living or working in a community. Opportunistic screening refers to screening carried out by health care providers as part of the outpatient consultation. We recommend women to start their first breast cancer screening at the age of 40 years old and earlier for women with higher risks.
1.1 Recommendations for women at normal risk
1.1.1 Age 20−39 years old
(1) Monthly breast self-examination;
(2) Clinical breast examination every 1−3 years.
1.1.2 Age 40−69 years old
(1) Mass screening and opportunistic screening;
(2) Mammography every 1−2 years;
(3) Ultrasound is recommended for women with dense breasts;
(4) Monthly breast self-examination;
(5) Annual clinical breast examination.
1.1.3 Age ≥70 years old
(1) Opportunistic screening (imaging examinations are recommended for symptomatic patients or patients with suspicious physical examination findings);
(2) Monthly breast self-examination;
(3) Annual clinical breast examination.
1.2 Recommendations for women at increased risk
Annual screening should be started earlier (<40 years old) in women at increased risk for breast cancer. Magnetic resonance imaging (MRI) could be considered in addition to mammography.
Increased risk is defined if women meet any of the following criteria: known genetic predisposition to breast cancer (see the criteria for genetic testing); established diagnosis of lobular carcinoma in situ (LCIS) or atypical hyperplasia; or prior history of thoracic irradiation.
Potential hereditary breast-ovarian cancer syndrome is as follows referring to genetic consulting:
(1) Known BRCA1/BRCA2 mutation carrier in the family;
(2) Breast cancer patients meeting any of the following criteria: 1) Early onset breast cancer (<45 years old); 2) Early onset breast cancer (<50 years old) and having one or more close family members diagnosed of early onset breast cancer (<50 years old), and/or having one or more close family members diagnosed of ovarian cancer/fallopian cancer/primary peritoneal cancer; 3) Early onset multiple primary breast cancer with the first diagnosis before the age of 50 years old; 4) Two or more close family members diagnosed of breast cancer and/or ovarian/fallopian/primary peritoneal cancer; 5) Family member with male breast cancer; and 6) History of ovarian/fallopian/primary peritoneal cancer.
(3) History of ovarian/fallopian/primary peritoneal cancer;
(4) History of male breast cancer;
(5) Any first- or second-degree relative meets previously mentioned genetic predispositions or two or more third-degree relatives diagnosed of breast cancer (at least one of them <50 years old) and/or ovarian/fallopian/primary peritoneal cancer.
2. Diagnosis
The diagnosis of breast cancer is based on clinical examination, imaging tests and pathological assessment.
2.1 Clinical manifestations
Early-stage breast cancer is usually asymptomatic and is often diagnosed after suspicious findings during physical examination or screening.
2.1.1 Breast lump
Around 80% of breast cancer patients seek medical consultations for palpable lumps in breasts. The lumps are often accidentally found by patients themselves, often with irregular margins and the surface of the lumps might be uneven. Most of the lumps are painless.
2.1.2 Nipple discharge
Nipple discharge refers to the secretion of unusual fluids such as blood, serum, breast milk or pus in women who are not pregnant or breast-feeding. Possible causes of nipple discharge include intraductal papilloma, fibrocystic breasts, mammary duct ectasia and breast cancer. Ductoscopy may be considered for patients with single-duct bloody nipple discharge.
2.1.3 Skin changes
Skin changes in breast cancer include peau d’orange, dimpling and satellite lesions caused by metastases.
2.1.4 Abnormalities in nipple and areolar area
Both retraction and protrusion of the nipple could be signs of breast cancer. Paget’s disease of the nipple can cause eczema/scaling in the skin around the nipple and areola.
2.1.5 Enlarged axillary lymph nodes
Palpable axillary masses are often the only symptom in patients with occult primary breast cancer. Apart from that, over one third of breast cancer patients have metastases in axillary lymph nodes.
2.2 Breast palpation
Before breast palpation, a detailed medical history should be collected. It is recommended for premenopausal women to receive breast palpation after the period. Make sure to palpate the nipple and areolar regions and the axillae. Look for abnormalities such as partial swelling of the breast, nipple discharge, nipple erosion, nipple retraction, skin dimpling during palpation.
2.3 Imaging tests
2.3.1 Mammography
Mammography is the most important and fundamental tool in breast imaging. It is irreplaceable in detecting calcifications in breast tissues. However, its sensitivity in dense breasts is limited. Mammography also exposes women to radiation, so it is not routinely recommended for young women.
Commonly used positions for mammography are mediolateral oblique and craniocaudal positions. Other additional positions may be considered when breast tissues are not adequately shown. Under certain circumstances, special techniques might be adopted for a clearer imaging.
(1) Indications:
1) Screening in asymptomatic populations;
2) Further investigation of breast abnormalities detected by other methods;
3) Patients with symptoms such as breast mass, partial breast swelling, abnormal nipple discharge, abnormal skin changes and localized breast pains;
4) Follow-up for women with benign breast diseases;
5) Follow-up for breast cancer patients receiving breast-conserving surgeries;
6) Follow-up for patients receiving breast reconstruction;
7) Guided biopsy.
For women under 40 years of age with average risk and no abnormal physical examination finding, mammography is not routinely recommended. Mammography is typically not used in pregnant women.
2.3.2 Breast ultrasound
Breast ultrasound is applicable in women of all ages. Breasts and axillary lymph nodes are usually evaluated at the same time.
Conventional breast ultrasound is sensitive in detecting breast lesions. A series of ultrasound parameters as well as elastic imaging and contrast enhanced imaging can be used to facilitate the differentiation between benign and malignant diseases.
(1) Indications:
1) Patients with symptoms (breast mass, abnormal nipple discharge, abnormal skin changes, etc.);
2) Screening in asymptomatic high-risk patients;
3) Complementary examination to mammography;
4) Follow-up for patients with benign breast diseases, or breast cancer patients after surgery, or postmenopausal women receiving hormone replacement drugs;
5) Guided biopsy.
2.3.3 Breast MRI
Compared with other imaging methods, breast MRI is more sensitive in detecting multifocal/multicentric lesions and bilateral lesions. MRI may provide additional information for evaluating the disease and making surgery plans. However, its disadvantages cannot be neglected either, which include medium specificity, high false-positive rate and inferiority in detecting microcalcifications. In addition, long examination time and high costs also limit its clinical use. So far it has not been recommended as the preferred option for breast evaluation.
(1) Indications:
1) Unspecific results after mammography and breast ultrasound;
2) Preoperative staging and screening for contralateral tumors;
3) Evaluation of tumor response to neoadjuvant therapy;
4) Evaluation of the primary tumor in patients with suspected occult breast cancer;
5) Differential diagnosis between postoperative scar and cancer relapse;
6) Evaluation of residual disease in patients with positive margins after lumpectomy;
7) Evaluation of breast implants;
8) Screening in high-risk women;
9) Guided biopsy.
(2) Contraindications:
1) Implanted pacemaker or defibrillator, metallic clips and other metallic objects that can be heated and/or moved by the magnetic field;
2) Allergy to gadolinium chelates;
3) Claustrophobia;
4) Pregnancy.
2.3.4 Positron emission tomography-computed tomography (PET-CT)
(1) Indications:
1) Pretreatment staging for patients with locally advanced diseases, unfavorable molecular tumor subtypes, or suspected to have distant metastases;
2) Possible relapse or metastases during follow-up.
(2) Relative contraindications:
1) Pregnant or breast-feeding;
2) Severe heart, liver, or kidney dysfunction; allergy to iodine-based contrast materials;
4) Unable to lie flat for at least 15 min; incontinence; claustrophobia;
5) Increased intracranial pressure due to brain metastases.
2.3.5 Bone scan
(1) Pretreatment staging for patients with invasive carcinoma:
1) For patients with clinical stage I−IIB diseases, a bone scan may be ordered in cases of localized bone pain or elevated alkaline phosphatase levels to detect bone metastases.
2) For patients with clinical stage III diseases, a bone scan or a NaF-PET/CT scan may be ordered to detect bone metastases.
3) For patients with cancer relapses or clinical stage IV diseases, a bone scan or a NaF-PET/CT scan may be ordered to detect bone metastases.
(2) Follow-up
For patients with bone pains or elevated alkaline phosphatase levels, bone scans may be used to detect bone metastases. Screening for metastases is not recommended in patients without symptoms or signs indicating relapses.
2.4 Laboratory tests
2.4.1 Blood chemistry: no significant changes in early-stage diseases
2.4.2 Tumor markers
Carbohydrate antigen 15-3 (CA15-3) and carcino-embryonic antigen (CEA) are commonly used for monitoring metastatic breast cancer. However, these tumor markers are low in both sensitivity and specificity, making them inappropriate for breast cancer screening and diagnosis.
3. Pathological assessments
Pathological analysis is fundamental to the diagnosis and treatment of breast cancer. In addition to accurate diagnosis, pathological analysis should also provide makers related to treatment options, prediction of therapeutic effects and prognoses. A clinician is required to provide complete and accurate clinical information, as well as adequate and standardized tissue specimen to the pathologist.
3.1 Specimen type and fixation
3.1.1 Specimen type
Specimens from core needle biopsy, vacuum assisted biopsy and various surgical resections are the common.
3.1.2 Specimen fixation
Core needle biopsy and resection specimens should be immediately fixed (within 1 h) with adequate 4% neutral formaldehyde for 6−48 h. Resection specimen should then be cut at 5 mm intervals. It is advisable to separate the adjacent tissue pieces with gauze or filter paper to ensure sufficient penetration and fixation at 12−72 h.
3.2 Dissection and gross description
(1) Core needle biopsy specimen
1) Gross examination and description: The number of tissue samples, the size of each sample (including diameter and length) should be mentioned;
2) Processing: All tissues are to be processed. Core needle biopsy specimens are not suitable for intraoperative frozen-section examination.
(2) Vacuum assisted biopsy specimen
1) Gross examination and description: The total size of the tissues should be mentioned;
2) Processing: All the tissues are to be processed. Vacuum-assisted biopsy specimens are not suitable for intraoperative frozen-section diagnosis.
(3) Lumpectomy specimen
1) Gross examination and description: Carried out according to the surgeon’s orientation. If the specimen has skin on it, the size of the skin should be measured. Record the location and appearance of the tumor or suspected lesion;
2) Dissection of intraoperative frozen section specimen: The specimen is cut at 5 mm intervals along the long axis. If a clear mass is present, it is directly sectioned. For specimens contain calcifications, preoperative X-ray information or the position of the localization wire should be used to indicate the location of dissection. If no clear mass is present, the suspected lesion is dissected.
Dissection of paraffin embedded specimen: If the maximum diameter of the tumor or suspicious lesion is less than or equal to 5 cm, it should be cut at no more than 1 cm intervals. If the maximum diameter is greater than 5 cm, it should also be cut at 1 cm intervals. If a previous diagnosis of ductal carcinoma in situ exists, it is recommended that all tissues are processed.
(4) Breast conserving surgery (BCS) specimen
1) Gross examination and description
Gross description should be carried out according to the surgeon’s orientation. It is recommended to apply different colored ink to mark the margins of the specimen (anterior, posterior, superior, inferior, medial and lateral). The specimen is cut at 5 mm intervals from the surface to the base, and the orientation and sequence of the sectioned tissues are maintained. The lesion should be carefully looked for and the size of the lesion should be measured in three dimensions. If it is a specimen after chemotherapy, measure the size of the tumor bed and if it is a post-resection specimen, measure the size of the residual cavity and search for residual lesions. Measure the distance between the tumor, the tumor bed or the residual cavity from each margin and observe the nearest margin.
2) Dissection
a. Margin.
There are two main methods for obtaining the margins of a breast conserving specimen: radial sections perpendicular to the margin and shave sections of the margin. Regardless of the methods, it is recommended to apply ink of different colors to the six margins. Margin status should be clearly defined in the pathology report. “Positive margin” refers to the presence of ductal carcinoma in situ or invasive carcinoma on ink. The definition of “negative margin” is not consistent, “no tumor on ink” is used to define a “negative margin” in most guidelines and consensus. It is recommended to report the actual distance between the margin and the tumor.
Radial sections perpendicular to the margin: According to the orientation marked by the surgeon, the specimen is cut into a plurality of slices at 5 mm intervals. Describe the size of the tumor, the location of the tumor, and the distance from the tumor edge to each margin. The margins close to the tumor are sectioned together with the tumor. The advantage of “radial section” is that the distance between the tumor edge and the margin can be accurately measured. The disadvantage of the method is the heavy workload.
Shave sections of the margin: Tissues form all six margins are separated from the specimen and all the margin tissues are to be processed. The advantage of the method is that the amount of tissue is small, and the entire margin can be microscopically observed. The disadvantage is that the distance between the tumor edge and the margin cannot be accurately measured.
b. Tumor and surrounding tissue
The method of dissection is the same as previously described in that of intraoperative frozen section specimens.
c. Additional margins
If the margin is positive, additional margin tissue will be examined. Additional margin tissue is inked and continuously cut perpendicularly to the margin surface. If the specimen is small, all tissues are to be processed.
(5) Mastectomy specimen
1) Gross examination and description
In order to identify the quadrant in which the tumor is located, orientation of the specimen is important. Modified radical mastectomy specimen can be correctly positioned by acknowledging the axillary tissue. The orientation of simple mastectomy specimen is based on the surgeon’s mark. It is recommended that the base of the specimen should be inked.
Measure the size of the entire specimen. Describe the appearance of the skin, the presence of skin abnormalities should be mentioned (surgical incisions, scars, erythema, edema, etc.).
The nipple is cut horizontally to observe the section of the lactiferous duct, and then the nipple is cut perpendicular to the surface. Describe the appearance of the nipple and areola.
The specimen is cut perpendicularly to the base. Look carefully for lesions and record the location and the characteristics of the tumor (texture, color, margin, etc.). If a clear mass is present, measure the size of the mass in three dimensions. If it is a specimen after chemotherapy, measure the size of the tumor bed; if it is a post-resection specimen, measure the size of the residual cavity and search for residual lesions.
After the axillary tissue is removed from the specimen, at least 15 lymph nodes should be found. Count the total number of lymph nodes, measure the maximum diameter of the lymph nodes, and it should be mentioned if lymph nodes become matted or adhered to surrounding tissues.
2) Dissection
The method of dissection of the primary tumor or surgical cavity is the same as previously described in that of intraoperative frozen section specimens. The nipple, the skin closest to the tumor, the basal margin closest to the tumor, and a piece of representative breast tissue from each quadrant should be sectioned as well.
If the lymph nodes are grossly negative, the entire lymph nodes are to be examined. If the lymph nodes are grossly positive, the node is cut along the maximum diameter for examination. The connective tissue around the node should also be examined to identify possible tumor invasion outside the lymph node.
(6) Sentinel lymph node biopsy (SLNB)
SLNB has replaced traditional axillary dissection in some breast cancer patients. Patients with negative SLNBs might avoid axillary dissections.
1) Definitions of SLNB metastases
a. Isolated tumor cells (ITC): The diameter of the tumor in the lymph node ≤0.2 mm or the number of tumor cells on a single slice <200. American Joint Committee on Cancer (AJCC) stages ITC as pN0(i+). At present, most guidelines consider ITC not clinically significant, and it is recommended to treat ITC as negative axilla.
b. Micrometastasis: The maximum diameter of the tumor in the lymph node >0.2 mm but ≤2 mm. AJCC stages micrometastasis as pN1mi. ITC is essentially different from micrometastasis, the former is pN0 and the latter is pN1. It is recommended to cut sentinel lymph nodes at 2 mm intervals to detect possible micrometastasis.
c. Macrometastasis: The maximum diameter of the tumor in the lymph node >2 mm.
2) Intraoperative examination
The main purpose of intraoperative examination of SLNB is to detect metastasis, thereby avoiding performing axillary lymph node dissection (ALND) in a second procedure. However, intraoperative evaluation of SLNBs is controversial.
a. Imprint cytology: Cut the lymph nodes at 2 mm intervals, carefully check for macrometastases on each slice, and imprint cytology is performed for each section. Pap and HE staining are recommended. The advantage of imprint cytology is the preservation of the entire lymph node tissue. It is cheap, simple and time-saving. The major disadvantage is that imprint cytology is difficult to identify scattered cancer cells (such as lobular carcinoma). It has good diagnostic specificity, but the sensitivity is affected by many factors.
b. Intraoperative frozen section: Cut the lymph node at 2 mm intervals, carefully check for macrometastases, then frozen section each slice for pathological evaluation. The advantage of frozen section is good diagnostic specificity and it can avoid unnecessary ALND due to false positive results. The disadvantages include tissue loss, time-consuming, high cost, and technical difficulty in assessing fatty lymph nodes.
3) Paraffin embedded evaluation
Paraffin embedded section is the “gold standard” of diagnosis. However, there is no consensus on how to cut the lymph nodes and what the intervals are, our recommended scheme: (a) Cut the lymph nodes at 2 mm intervals; (b) All tissue slices are paraffin embedded in separated blocks; and (c) Cut at least one slice per block and it is recommended to cut 6 slices at 150−200 μm intervals.
3.3 Classification, grading and staging
3.3.1 Histological classification
Histological classification is mainly based on the World Health Organization (WHO) breast cancer classification and some histological types need to be determined by immunohistochemistry.
Accurate histological classification of invasive breast cancer is crucial to individualized treatment. The National Comprehensive Cancer Network (NCCN) guidelines recommend less intense treatment strategies for tumors with good prognoses (such as tubular carcinoma and mucinous carcinoma). Meanwhile, more intensive treatment strategies are recommended for tumors with poor prognoses (such as inflammatory breast cancer). Medullary carcinoma used to be considered to have a good prognosis, but recent studies have shown that the risk of metastasis is comparable to other invasive breast carcinomas, and the diagnostic reproducibility is poor among different examiners. Therefore, the NCCN guidelines recommend that patients with medullary carcinoma should receive the same treatment as patients with invasive ductal carcinoma. Some special types of breast cancer have special clinical features. For instance, invasive micropapillary carcinoma is more likely to develop lymph node metastasis. For mixed tumor types, it is recommended to report the proportion and molecular biomarkers for each component separately.
3.3.2 Histological grading
(1) Grading of invasive breast cancer
Histological grade is an important prognostic factor. The modified Scarff-Bloom-Richardson grading system is widely used. According to the system, the ratio of duct formation, cell atypia and mitotic count are the three factors each independently assessed and given a score from 1 to 3 points. Invasive breast cancers are divided into high-, intermediate- and low-grade tumors by calculating the sum of the three scores.
Assessment of the ductal formation needs to be assessed at low-power fields. When evaluating tubules and glands, only structures exhibiting clear lumina surrounded by polarized cells are counted.
Nuclear pleomorphism is assessed by referencing to the regularity of nuclear size, shape and nucleolar size of normal mammary epithelial cells. When there is a lack of normal cells, lymphocytes can be used as a reference.
Pathologists only count definite mitotic figures, and hyperchromatic nuclei and nuclear debris are not included. Choose areas with the most mitotic activities for mitotic count, which are usually near the edge of the tumor. If heterogeneity exists, regions exhibiting higher frequency of mitoses should be chosen.
(2) Grading of ductal carcinoma in situ (DCIS)
Low-grade DCIS: Composed of small, monomorphic cells, growing in arcades, micropapillae, cribriform or solid patterns. The nuclei are of uniform size and have inconspicuous nucleoli; mitotic figures are rare.
Intermediate-grade DCIS: Morphological manifestations are between low-grade and high-grade DCIS. It is composed of cells showing mild to moderate variability in size and shape. Coarse chromatin, prominent nucleoli, mitoses and comedo necrosis may be present.
High-grade DCIS: Composed of highly atypical cells most often proliferating in solid, cribriform or micropapillary patterns. Nuclei are pleomorphic, poorly polarized, with irregular contours and distribution, coarse, clumped chromatin and prominent nucleoli. Mitotic figures are common. Abundant necrotic debris often presents in the duct lumina. However, comedo necrosis is not obligatory. Even a single layer of highly atypical cells lining the duct in a clinging fashion is sufficient for the diagnosis of high-grade DCIS.
3.3.3 Staging
Tumor size, the involvement of chest wall or the overlying skin, lymph node metastasis, and distant metastasis are factors that determine the stage of the breast cancer. Correct staging is fundamental to individualized treatment.
The eighth edition AJCC staging manual has described rules for measuring tumor size in details. Only the size of the invasive carcinoma is meaningful in tumor staging. Microscopic measurement is the most accurate, other methods cannot distinguish invasive and intraductal components. In case of in situ carcinoma with microinvasion, the maximum diameter of microinvasion should be measured. If multi-microinvasions are present, it should be mentioned in the report and the size of the largest microinvasion should be measured. If two or more tumors can be grossly identified in the same quadrant, multifocal tumors should be reported by the pathologist and the size of each tumor focus should be measured separately. If more than two tumors can be grossly identified in different quadrants, multicentric tumors should be reported by the pathologist, and the size of each tumor should be measured separately. If the tumor consists entirely of ductal carcinoma in situ, the extent of tumor should be measured as much as possible. Lymph node metastasis is an important factor in determining treatment and prognosis. The number of metastasized lymph nodes should be carefully checked to determine the accurate pN stage.
3.3.4 Immunohistochemistry, molecular pathology tests and quality control
Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER-2) immunohistochemistry tests should be performed for all invasive breast cancers. HER-2 2+ cases should be further tested by in situ hybridization. ER and PR status identify patients who benefit from endocrine therapy, and their positivity also indicates good survival. The staining intensity and the percentage of ER/PR positive cells must be reported. Current definition of ER/PR positivity is >1% of tumor cells are stained positive. HER-2 status is used to identify candidates for HER-2 targeted therapy and to predict prognosis.
HER-2 positivity is defined by more than 10% of cells showing strong membrane staining (3+), and/or in situ hybridization detects HER-2 gene amplification (single copy HER-2 gene >6 or HER-2/CEP17 ratio >2.0).
Ki67 index also plays an important role in selecting treatment options and predicting prognosis. Ki67 should be performed for all invasive carcinomas, and the percentage of Ki67 positive cells should be reported. However, there is currently no consensus on the Ki67 reporting system.
Laboratories that carry out immunohistochemistry and molecular pathology tests should establish complete and effective internal quality controls. Units that do not have adequate facilities or equipment should have the tests done in qualified laboratories.
Qualified laboratories should have the following conditions:
(1) Standard operation procedure (SOP) should be established and strictly followed;
(2) Technicians and pathologists engaged in immunohistochemistry and molecular pathology tests should receive proper trainings and qualification assessments;
(3) External quality control can be achieved by participating in external quality control activities. The recommended frequency of such external quality control activities is 1−2 times per year.
3.3.5 Standardized pathology report
A standardized pathology report includes complete information related to treatment and prognosis of the carcinoma (tumor size, histological type and grade, existence of ductal carcinoma in situ, vascular invasion, margin status and lymph node status, etc.). ER, PR, HER-2, and Ki67 immunohistochemistry results should also be included. For posttreatment specimens, treatment response should be reported. For ductal carcinoma in situ, nuclear grade (low, intermediate or high), necrosis (comedo or spotty necrosis), margin status and ER/PR expression should be reported. Benign lesions adjacent to the cancer should be clearly stated. The pathology report of a BCS specimen should indicate the distance between the edge of the tumor and the nearest margin. Lymphovascular invasion (LVI) needs to be differentiated from the lacunar space caused by tissue contractions. It is more reliable to check for lymphovascular invasion around the tumor.
4. Differential diagnosis
Breast cancer needs to be differentiated from hyperplasia, fibroadenoma, cysts, primary mammary lymphoma and metastasis of malignant tumors. Detailed medical history, careful physical examination and imaging examinations are required to establish diagnosis. Then cytological and/or histopathological examinations are required to confirm the diagnosis. About 80% of breast cancers can be found by palpation. The diagnosis can be confirmed by needle biopsy. However, for impalpable tumors, image tests may be necessary to guide needle biopsy. Wire localization may also be necessary to locate biopsy lesions. A small number of breast cancer patients present with nipple discharge need to be differentiated from hyperplasia, ductal dilatation, milk retention, intraductal papilloma and papillomatosis. Smear cytology may be used to find cancer cells. Ductalscopy may be useful in finding intraductal tumors.
5. Treatment
5.1 Principles
A multi-disciplinary approach should be adopted to improve survival and quality of life.
5.1.1 Non-invasive breast cancer
(1) LCIS
Women with LCIS tend to have a slightly higher risk of developing invasive breast cancer. And that is more likely to happen over the long term. A lot of these tumors are multicentric and bilateral.
LCIS usually does not cause symptoms. If calcifications, lumps or tissue distortion is detected by mammography, stereotactic core needle biopsy or wire-localized biopsy may be necessary to confirm the diagnosis. In case core needle biopsy found classic LCIS, regular imaging follow-up may be considered. However, in case core needle biopsy found pleomorphic LCIS or other suspicious finding, a surgical biopsy may be necessary to rule out DCIS and invasive cancer.
For premenopausal women with LCIS, tamoxifen for 5 years is recommended after wide local excision. For postmenopausal women, tamoxifen or raloxifene may be given to lower the risk of breast cancer. Mastectomy may be considered if pleomorphic LCIS cannot be ruled out and breast reconstruction is planned.
(2) DCIS
DCIS is believed to be the precursor to invasive ductal carcinoma. If left untreated, some DCIS might eventually progress to invasive ductal carcinoma. The majority of asymptomatic DCIS is discovered by screening mammography with calcification being the most common finding. A core needle biopsy or wire-localized biopsy may be required to establish diagnosis. However, when the diagnosis of DCIS is made by core needle biopsy, a surgical biopsy may be necessary to rule out invasive ductal carcinoma.
Surgical treatment of DCIS includes wide local excision plus whole breast radiation and mastectomy. The necessity of sentinel lymph node biopsy should be evaluated and the mastectomy patients be offered the choice of breast reconstruction. For patients with pure DCIS, axillary lymph node dissection is not recommended.
For ER-positive DCIS patients after BCS and radiation, tamoxifen for 5 years is indicated to reduce the risk of contralateral breast cancer. For patients after mastectomy, the risks and benefits of tamoxifen must be carefully evaluated.
5.1.2 Invasive breast cancer
(1) BCS and radiation;
(2) Mastectomy with ALND (modified radical mastectomy). Breast reconstruction should be considered;
(3) Mastectomy with SLNB. Breast reconstruction should be considered;
(4) Elderly patients may consider wide local excision or total mastectomy based on patients’ medical conditions. ER and/or PR positive patients should be given endocrine therapy.
5.2 Surgical treatment
5.2.1 Principles
The breast as well as the ipsilateral axilla needs to be considered when planning surgery. Surgery for the breast includes BCS and mastectomy. Surgery for axilla includes SLNB and ALND. The selection of different surgical procedures should take into consideration the TNM stage and the patient's medical condition.
5.2.2 Breast surgery
(1) Mastectomy
Indications include early-stage breast cancer and some locally advanced breast cancer without contraindications; patient have no intention to preserve their breasts or BCS not applicable; some locally advanced breast cancer patients or patients with existing distant metastases who are down-staged by systemic therapy are also candidates for mastectomy.
Usually the fascia overlying the pectoralis major muscle is removed during mastectomy but some believe that the fascia may be kept intact when patients undergo immediate breast reconstructive surgery.
Currently, modified radical mastectomy is being replaced by skin sparing mastectomy plus immediate breast reconstruction which offers similar survival but better cosmetic outcome. Nipple sparing mastectomy is also gaining acceptance but long-term follow-up data are still lacking.
(2) BCS
Indications for BCS should be strictly observed. Institutions that carry out BCS should have the equipment and techniques for histological examination of the BCS specimen in order to obtain negative surgical margins. Facilities for postoperative radiotherapy are also necessary.
The aim of BCS is to remove the tumor entirely without affecting breast appearance. The patient’s wishes should always be considered when deciding treatment. Age alone should not be a determining factor in selecting surgical strategy, however women less than 35 years old may have increased risk of recurrence. Conversely, a woman who has difficulty in complying with six weeks of radiation may be a better candidate for mastectomy.
Absolute contraindications include two or more primary tumors located in different quadrants and diffuse cancerous microcalcifications. Previous breast irradiation history, T4 tumor, tumors that involve the skin or the chest wall, inflammatory breast cancer and repeated positive surgical margins are also contraindications to BCS. Breast irradiation cannot be given during pregnancy, but it may be possible to perform BSC in the third trimester and administer irradiation after delivery.
Most radiation oncologists consider a history of collagen vascular disease a relative contraindication because the poor vasculature in the skin leads to unacceptable cosmetic results. Tumor size is not an absolute contraindication, but the presence of a large tumor in a small breast treated with adequate margins might result in an unwanted cosmetic appearance.
5.2.3 Surgery for axillary lymph nodes
Lymph node status remains one of the strongest predictors of long-term prognosis in early-stage breast cancer, providing information that is important for tailoring treatment. Due to limited sensitivity of the imaging tests, the axillary nodes must be explored surgically.
(1) SLNB. SLNB has replaced ALND as the standard surgical procedure for cN0 early-stage breast cancer. It considerably reduces surgical morbidity without affecting diagnostic accuracy. SLNB is based on the concept that a tumor-free sentinel lymph node (SLN) excludes metastatic involvement of the other axillary nodes. Lymphatic mapping is necessary before SLNB, commonly used tracers include blue dye, radiotracer, and some hybrid tracers which combine with a radioactive and a fluorescent label such as indocyanine green-99mTc-nanocolloid. SLNB can accurately stage the axilla. Patients with negative SLNs can avoid ALND thus reducing edema and other complications of the upper limb. Currently, ALND will be performed if SLN is positive.
(2) ALND. Indications for ALND include fine needle aspiration or core needle biopsy proved axillary lymph node metastases; SLN positive patients who do not meet the ACOSOG Z0011 criteria (women with T1−2 early-stage breast cancer and less than or equal to 2 positive SLNs, who will receive conservative surgery and whole-breast radiotherapy); recent insufficient ALND; failure of SLNB; patients with non-SLN metastases; locally advanced invasive breast cancers (T4) or inflammatory breast cancer patients; patients who cannot perform SLNB; and axillary recurrence after SLNB.
A level I axillary dissection refers to extirpation of tissue lateral to the lateral border of the pectoralis minor muscle. A level II dissection refers to the removal of tissue between the medial and lateral borders of the muscle, and a level III dissection indicates tissue dissection medial to the muscle’s medial border. Most surgeons generally dissect levels I and II of the axillae. If there are palpable lymph nodes lateral to the pectoralis minor muscle, then the pectoralis minor muscle is divided and a level III dissection is performed. The minimum requirement for ALND is to remove 10 lymph nodes, so as to ensure the status of axillary lymph nodes can be accurately reflected.
5.2.4 Oncoplastic and reconstructive surgery
The standard modified radical mastectomy can lead to breast defect and has a typical horizontal scar across the chest wall. In some cases, in order to ensure an oncological safe resection, BCS also may generate unsatisfying cosmetic results. Oncoplastic and reconstructive surgery can reduce anxiety and thereby improve the quality of life, and it has become an indispensable part of the breast cancer treatment. Nowadays, more and more surgeons have accepted the idea and mastered the techniques, the number of reconstructive surgeries is increasing.
The oncological safety of reconstructive surgery in breast cancer patients has been confirmed. The timing and method of reconstructive surgery do not affect survival outcomes.
Normally, breast reconstruction is not in conflict with adjuvant chemotherapy. Chemotherapy will be delayed only when serious complications, such as infection and wound dehiscence occur after immediate reconstruction. Besides this, adjuvant chemotherapy will not increase the incidence of postoperative complications, will not affect the cosmetic outcome of reconstruction, and won’t impair wound healing. However, neoadjuvant chemotherapy might increase the incidence of infection and flap necrosis after reconstruction. Breast reconstruction should not be offered to patients during the course of chemotherapy due to declined immune function and anti-infection ability.
Neither autologous flap reconstruction nor implant reconstruction is a contraindication to radiotherapy, nor will it affect the effect of radiotherapy. However, breast reconstruction does increase the technical difficulty of postoperative radiotherapy. Irradiated reconstructed breasts often show an unsatisfactory aesthetic outcome, which can be evident even after a long time. And the aesthetic appearance and satisfaction were generally worse than in non-irradiated patients.
The principles of breast reconstruction:
1) Oncological safety must be put first. Breast reconstruction should not delay adjuvant therapy;
2) Breast reconstruction is an integral part of the therapeutic regimen. The doctor is obliged to inform the patients that they have the right to choose reconstructive surgery;
3) On the premise of oncological safety, the surgeon should preserve the skin, the subcutaneous tissue and other important aesthetic structures (such as the inframammary fold) as much as possible to allow satisfactory reconstruction of the shape and size of the breasts;
4) The treatment of breast cancer should be carried out by a multidisciplinary team. Preoperative evaluation before breast reconstructive surgery: important factors in assessing whether patients are suitable for breast reconstruction and determining the optimal technique include assessment of a patient’s general health, the body habitus, the size and shape of the ipsilateral and counter lateral breasts and patient’s preference. The ideal surgical option should be the one with minimal trauma, low cost, low rate of complications, and good cosmetic results.
Absolute contradictions to breast reconstruction include stage IV breast cancer, recurrent breast cancer, patient during the course of chemotherapy/radiotherapy, or within six months after radiotherapy.
For patients who have a history of previous radiotherapy or postoperative radiotherapy is planned, the timing and surgical procedure for breast reconstruction should be carefully selected. Severe obesity, smoking history, and peripheral vascular disease are the most important factors associated with postoperative complications.
Breast reconstruction may require a series of surgeries before a desirable cosmetic result can be achieved. Immediate breast reconstruction is an increasingly appealing option offering women the option of waking up after their mastectomy with a reconstructed breast. This has obvious psychological advantages, and patients who choose immediate reconstruction are usually pleased with this decision and the outcomes.
Types of breast reconstructive surgery: breast reconstruction include reconstruction with implant, autologous flap reconstruction, fat grafting, etc. The common techniques used for flap reconstruction include latissimus dorsi flap, transverse rectus abdominis muscle flap, deep inferior epigastric perforator flap, etc.
Follow-up for patients after breast reconstructive surgery: follow-up should start after the surgery and be continued for more than 5 years. Follow-up should be performed regularly according to the type of breast reconstructive surgery. During follow-up, besides regular checks for any possible cancer recurrence, breast shape and symmetry, incision scar, donor site blood supply, prosthetic integrity, capsule contracture and other complications should also be observed. And if possible, changes in patients’ psychological status and quality of life should be observed as well.
5.3 Radiation therapy
5.3.1 Radiation therapy for early-stage breast cancer after BCS
(1) Indications
Generally, all patients underwent BCS require radiation. Under special circumstances (patient over 70, tumor smaller than 2 cm, lymph node negative, or ER positive and endocrine therapy is planned), the omission of radiotherapy may be considered.
(2) Radiation field
1) Preliminary studies of accelerated partial breast irradiation (APBI) suggest that rates of local control in selected patients with early-stage breast cancer may be comparable to those treated with standard whole breast radiation therapy. The criteria and procedure of APBI are mentioned in later chapters.
2) For pN0 patients who underwent BCS, the target area includes the whole breast.
3) For patients with micro-metastases in SLNs, or 1−2 positive SLNs without ALND, high tangent radiation to the whole breast may be considered. If the risk of non-SLN metastasis exceeds 30%, whole breast plus supraclavicular/axillary field radiation should be considered.
4) For patients with 1−3 positive lymph nodes after ALND, the whole breast and the paraclavicular area need to be irradiated. For a subset of these patients with no other high-risk factors (younger than 40 years, grade III, lymphovascular invasion, positive lymph node ratio more than 20%, and HR negative), the paraclavicular area might be omitted.
5) For patients with more than 4 metastatic lymph nodes after ALND, the whole breast and the paraclavicular area should be irradiated.
6) For tumors located in the inner quadrant with 1−3 metastatic lymph nodes, or tumors with more than 4 metastatic lymph nodes, the internal mammary nodes radiation may be considered.
7) For patients with more than 4 metastatic lymph nodes, who didn’t undergo ALND, the whole breast, axilla field, and paraclavicular field should be irradiated.
8) Generally, for patients receiving whole breast radiation, tumor bed boost is recommended, especially for patients with positive surgical margins or younger than 50 years or grade III tumors. For patients with low recurrence risk (older than 70 years, grade I or II tumor, HR positive and adequate negative surgical margins), tumor bed boost might be omitted.
(3) Radiation technology
Radiation therapy after lumpectomy can be performed by three-dimensional conformal radiotherapy, fixed field radiotherapy or volumetric arc radiotherapy. Regardless of the techniques used, it is recommended to contour the target volumes with CT-based treatment planning, and accurately evaluate the dose distribution of the target volumes and the organs at risk.
Compared with two-dimensional radiotherapy, three-dimensional conformal and intensity-modulated radiotherapy can improve dose homogeneity, spare normal tissues, and resolve the problem of the radiation field connection between breast and regional lymph nodes, especially in patients with large breasts or need regional lymph node radiation, at the cost of increased complexity of plan designing.
Tumor bed boost can be delivered using intra-operative radiotherapy, interstitial brachytherapy, electrons or external photons irradiation. Intraoperative marking of the tumor bed with clips is recommended.
(4) Radiation dose and segmentation mode
The recommended dose of whole breast +/− regional lymph nodes is 50 Gy/2 Gy/25 f. External beam radiation of tumor bed can be sequentially applied after whole breast radiation at the dose of 10−16 Gy/2 Gy/5−8 f. Simultaneous radiation of the tumor bed may be considered in experienced institutions. For patients undergoing whole breast radiation only, hypofractionated radiation may be considered.
(5) APBI
Preliminary studies of APBI suggest that local control after APBI in selected breast cancer patients with low recurrence risk may be comparable to those treated with standard whole breast radiation therapy. However, follow-up is limited and studies are ongoing. Patients are encouraged to participate in clinical trials.
If not trial are eligible, patients over 50 years with unifocal T1N0 invasive ducal carcinomas, ER positive, no lymphovascular invasion and adequate negative surgical margins may consider APBI. Patients with small (less than 2 cm) low-grade DCIS and adequate negative surgical margins may also consider APBI.
5.3.2 Radiation therapy after modified radical mastectomy
(1) Indications
Adjuvant radiotherapy should be considered for patients who meet any of the following conditions after modified radical mastectomy.
1) Primary tumor larger than 5 cm; or tumor with direct extension to the chest wall and/or the skin;
2) More than 3 positive axillary lymph nodes; or metastases in ipsilateral supraclavicular lymph nodes or internal mammary lymph nodes;
3) For pT1−2N1 breast cancer patients, radiation therapy is recommended after modified radical mastectomy. However, in patients with no high-risk factors (younger than 50 years, grade III, lymphovascular invasion and HR negative), the omission of adjuvant radiotherapy may be considered.
(2) Radiation field
1) For patients underwent modified radical mastectomy, both the chest wall and paraclavicular field should be radiated.
2) For tumors located in the inner quadrant with 1−3 metastatic lymph nodes, or tumors with more than 4 metastatic lymph nodes, the internal mammary nodes radiation may be considered.
3) After a standard ALND, the axilla is not included in the radiation fields. However, in case of positive SLNs but no ALND or inadequate ALND, the axilla is should be included.
(3) Arrangement of radiotherapy and systemic therapy
For patients with planned adjuvant chemotherapy, radiotherapy should be given after chemotherapy. And for patients without adjuvant chemotherapy, radiotherapy should be given within 8 weeks after surgery on the premise of good wound healing. Herceptin may be given concurrently with radiotherapy. Before the initiation of radiotherapy, make sure that the left ventricular ejection fraction (LVEF) is over 50%. Radiation dose to the heart should be as low as possible, especially for patients with tumor on the left breast. Endocrine therapy can be given concurrently with radiotherapy.
5.3.3 Radiotherapy under special circumstances
(1) Radiation therapy after neoadjuvant chemotherapy
Indications for radiation therapy and the design of radiation fields should be based on clinical and pathological information both before and after chemotherapy. Age and characteristics of the primary tumor should be taken into consideration. The technique and dose of radiotherapy after neoadjuvant chemotherapy is the same as that used in patients without neoadjuvant chemotherapy.
For patients underwent BCS after neoadjuvant chemotherapy, whole breast irradiation is mandatory. For cN2−3 patients before neoadjuvant chemotherapy or patients with residual positive nodes after neoadjuvant chemotherapy, paraclavicular field and/or internal lymph nodes should be irradiated.
For those underwent modified radical mastectomy after neoadjuvant chemotherapy, if pathology revealed residual positive nodes, adjuvant radiotherapy is necessary. The chest wall, the paraclavicular field and/or internal lymph nodes should be irradiated.
(2) Radiotherapy after breast reconstruction
Radiotherapy increases the rate of complications after breast reconstruction (capsule contracture, implant rupture, fibrosis, etc.), and affects the cosmetic outcomes of both autologous and implant reconstruction. Radiologists should take part in the decision-making process before reconstructive surgery.
If autologous reconstruction is planned, radiotherapy is recommended to be given before reconstruction. And for those implant reconstructions are planned, radiotherapy is recommended to be given after the expander surgery and before the replacement of a permanent implant.
The technique and dose of radiotherapy for patients after breast reconstruction are the same as that used in patients with modified radical mastectomy. Intensity modulated radiation therapy is recommended for patients after breast reconstruction.
(3) Radiotherapy after locoregional recurrence
Most locoregional recurrences are found in the chest wall and supraclavicular region. Generally, patients with unifocal chest wall recurrence should undergo extensive local tumor excision and subsequent radiotherapy. If the tumor is unresectable, radiotherapy is given first. For patients without previous radiotherapy, the chest wall and paraclavicular area should be irradiated. If there is no sign of recurrence in the axilla or internal mammary nodes, prophylactic irradiation is unnecessary.
The treatment for locoregional recurrence must be based on cytological or histological confirmation.
5.4 Chemotherapy
5.4.1 Adjuvant chemotherapy
After a comprehensive analysis of the patient’s medical conditions (age, menstrual status, blood test results, vital organ functions, comorbidities, etc.), tumor characteristics (histological type, tumor grade, lymph node status, HER-2 and hormone receptor status, lymphovascular invasion) and potential treatment strategies (chemotherapy, endocrine therapy, anti-HER-2 therapy, etc.), if the potential benefit of chemotherapy outweighs the risk, adjuvant chemotherapy may be given.
(1) Indications
1) Positive ALNs;
2) Endocrine therapy alone may be considered for postmenopausal patients with 1−3 positive nodes and no other risk factors (HR negative, HER-2 positive, large tumors, high-grade tumors, etc.), or patients who are deemed unfit for chemotherapy;
3) For node negative patients, adjuvant chemotherapy is only indicated for patients with other risk factors (age <35 years old, tumor diameter >2 cm, high-grade tumor, presence of lymphovascular invasion, HER-2 positive, ER/PR negative, etc.).
(2) Relative contraindications
1) Pregnancy (especially during the first and second trimesters);
2) Old or frail patients.
(3) Selection of adjuvant chemotherapy regimens
1) Anthracycline-based polychemotherapy or sequential/concurrent anthracycline-taxane chemotherapy is the preferred combination;
2) For elderly patients, low-risk patients or patients with anthracycline intolerance, polychemotherapy without the anthracyclines may be considered (TC);
3) Generally, the selection of adjuvant chemotherapy regimen is based on the risk of recurrence. For high-risk patients, an anthracycline and taxane containing regimen is recommended. For intermediate-risk patients, an anthracycline or taxane containing regimen is recommended. And for low-risk patients requiring chemotherapy, 4−6 cycles of an anthracycline containing regimen or a non-anthracycline containing regimen (TC) may be considered;
4) For the majority of triple negative breast cancer patients (with the exception of a subset of patients with early-stage tumors and small tumor burden), sequential anthracycline-taxane chemotherapy is recommended. Dose-dense chemotherapy is recommended for patients with better tolerance. Adjuvant chemotherapy is also recommended for most Luminal B cancers and an anthracycline and/or taxane containing regimen is often used. Luminal A breast cancer generally has a poor response to chemotherapy. Luminal A patients with 1−3 positive lymph nodes may consider 4−6 cycles of an anthracycline containing regimen or a TC. For high-risk Luminal A patients with 4 or more positive nodes, a sequential anthracycline-taxane chemotherapy is recommended.
(4) Special considerations
1) The purpose of adjuvant chemotherapy is to seek cure, so emphasis is placed on the standardization of treatment;
2) Attention should be paid to the order of drug administration, as well as infusion time and dose intensity;
3) Chemotherapy regimen selection is based on risk of recurrence, individual tolerability, patients’ will, and existing clinical evidence. Appropriate regimens to prevent and treat nausea/vomit and myelosuppression should be developed;
4) Generally, 4−8 cycles of adjuvant chemotherapy are given. Treatment for patients over 70 years old needs to be individualized;
5) Adjuvant chemotherapy is generally not given simultaneously with endocrine therapy or radiotherapy. Endocrine therapy may start after chemotherapy. Radiotherapy and endocrine therapy may be given sequentially or simultaneously;
6) Chemotherapy should be given according to the recommended dose. If dose reduction is necessary, it is generally recommended that a dose intensity over 85% should be maintained;
7) Ovarian function suppression may be considered in premenopausal patients with hormone receptor negative disease to protect ovarian function during chemotherapy. Ovarian function suppression should start 1 to 2 weeks before chemotherapy, and the last dose should be given 2 weeks after the end of chemotherapy;
8) Anthracyclines are cardiotoxic and LVEF must be monitored, usually once every 3 months;
9) Informed consent is mandatory.
5.4.2 Neoadjuvant chemotherapy
Neoadjuvant chemotherapy is given before local treatment (surgery or radiotherapy). The purpose of neoadjuvant chemotherapy is to down stage tumors, turn inoperable tumors to operable tumors and to in increase the rate of BCS.
(1) Indications
1) Stage IIIA (excluding T3N1M0), IIIB and IIIC tumors that need to be adequately down-staged before surgery;
2) To increase the rate of BCS in stage IIA, IIB, IIIA (T3N1M0 only) tumors.
(2) Contraindications
1) Pathological confirmation of breast cancer or immunohistochemical results (ER, PR, HER-2 and Ki-67) not acquired. Cytology is not the recommended method of establishing diagnosis;
2) Pregnancy (especially during the first and second trimesters);
3) Old or frail patients or patients with other tolerability issues;
4) Diagnosis of invasive carcinoma not established;
5) Clinically unevaluable tumors.
(3) Selection of neoadjuvant chemotherapy regimens
Most adjuvant chemotherapy regimens can be applied to the neoadjuvant stage. Generally, sequential or concurrent anthracycline-taxane containing regimens are recommended. HER2-targeted therapy should be considered in HER-2 positive patients.
(4) Special considerations
1) Before the initiation of chemotherapy, histological diagnosis must be established and immunohistochemical tests must be performed. Suspicious regional lymph nodes may be assessed by needle aspiration cytology;
2) Neoadjuvant chemotherapy is not routinely recommended for stage I breast cancer;
3) Neoadjuvant chemotherapy is usually given for 4−8 cycles. In patients with good responses or stable diseases, it is recommended that all planned cycles are given before surgery;
4) Responses of the primary tumor and ALNs should be evaluated by means of physical examination and imaging tests according to the Response Evaluation Criteria in Solid Tumors (RECIST) or WHO criteria;
5) Switching to surgery, radiation therapy or other systemic treatments (non-cross-resistant chemotherapy regimens or neoadjuvant endocrine therapy) should be considered when tumor shows no response;
6) After neoadjuvant chemotherapy, even if the tumor is no longer clinically detectable, definitive surgery must be performed;
7) The selection of adjuvant chemotherapy should be based on the response to neoadjuvant chemotherapy and residual tumor burden;
8) Tumor stage before chemotherapy is usually used to develop adjuvant radiotherapy plans.
5.4.3 Chemotherapy for late-stage breast cancer
The goal of treating late-stage breast cancer is to improve quality of life and prolong survival. Treatment plans for these patients are highly individualized.
Baseline assessments for late-stage breast cancer patients include a complete medical history and physical examination, blood tests, chest X-ray or CT, abdominal ultrasound, bone scan, etc. Radiology confirmation for patients with localized bone pain or suspicious bone scan results is required before a diagnosis of bone metastasis can be established. Abdominal CT or MRI, brain CT or MRI may be necessary for some patients. PET-CT is optional but is not routinely recommended. Biopsy of the metastasis or recurrence site is recommended when possible.
(1) Patients who meet one of the following criteria may consider first-line chemotherapy:
1) Age <35 years old;
2) Rapidly progressing disease;
3) ER/PR negative or low expression;
4) Short disease-free interval (<2 years);
5) Extensive or symptomatic visceral metastases;
6) ER/PR positive but endocrine therapy non-responsive.
(2) Chemotherapy regimens
Commonly used chemotherapy drugs to treat late-stage breast cancer include anthracyclines, taxanes, vinorelbine, capecitabine, gemcitabine, platinum, etc. Individualized treatment plan should be developed based on the extent and the molecular characteristics of the tumor, previous treatment and patient’s will.
1) Single-agent chemotherapy
Single-agent chemotherapy is preferred for patients with low tumor burden and no sign of rapid progression. Single-agent chemotherapy is also preferred for old or frail patients with tolerability issues. Commonly used single-agent chemotherapy drugs include paclitaxel, docetaxel, albumin-bound paclitaxel, capecitabine, vinorelbine, gemcitabine, doxorubicin liposomes, etc.
2) Polychemotherapy
Polychemotherapy is preferred for patients with large tumor burden or rapidly progressing diseases. The selection of polychemotherapy regimen should take into consideration the previous adjuvant chemotherapy regimen, the disease-free interval and other patient factors.
(a) For patients with no previous chemotherapy, sequential or concurrent anthracycline-taxane containing regimens are recommended. In case tumor progressed on an anthracycline-based regimen or the cumulative dose of the anthracycline reaching the upper limit, a taxane-based regimen is preferred. For patients who were given taxanes during adjuvant chemotherapy with disease-free intervals longer than 1 year, taxanes may be used again. Taxanes combined with gemcitabine or capecitabine are commonly used for first-line treatments. Chemotherapy with capecitabine, vinorelbine or gemcitabine may be considered for patients whose diseases progressed on anthracyclines and taxanes.
(b) In case of disease progression after first-line chemotherapy, none cross-resistant single-agent chemotherapy or polychemotherapy is usually needed. Previously effective drugs and drugs with long disease control time may be applied again.
(c) Participation in clinical trials are highly recommended for late-stage patients.
(d) Concurrent HER-2 targeted therapy and chemotherapy are recommended for HER-2 positive patients.
3) Maintenance chemotherapy
After 4−6 cycles of chemotherapy, if treatment is effective and well tolerated, maintenance chemotherapy may be given until disease progression or intolerable toxicity occurs. Regimen used for maintenance chemotherapy may be the original effective regimen or one of the drugs in the original regimen. Patient management should be strengthened during maintenance therapy with efficacy and adverse effects assessed regularly.
Chemotherapy may also be ceased for patients with tolerability issues. Patients should be closely monitored and treatment reinitiated after disease progression. Patients with ER-positive disease may also choose endocrine therapy as maintenance therapy.
5.5 Endocrine therapy
5.5.1 Adjuvant endocrine therapy
(1) Indications
1) ER and/or PR positive invasive breast cancer;
2) Five years of endocrine therapy may be considered for patients with hormone receptor positive carcinoma in situ after BCS or mastectomy (to prevent contralateral breast cancer).
(2) Contraindications
1) History of deep vein thrombosis or pulmonary embolism;
2) Severe liver and/or kidney disfunction;
3) Being pregnant and those with known allergic reaction to endocrine therapy.
(3) Drug selection
1) Tamoxifen is preferred for premenopausal patients;
2) Premenopausal patients with moderate to high recurrence risk (young age, high-grade and lymph node involvement) may consider ovarian function suppression;
3) Postmenopausal patients on tamoxifen may switch to aromatase inhibitors;
4) Upfront third-generation aromatase inhibitor is recommended for postmenopausal patients;
5) Postmenopausal patients who cannot tolerate aromatase inhibitors may choose tamoxifen.
(4) Special considerations
1) Menstrual status should be assessed before the initiation of chemotherapy;
2) Generally, adjuvant endocrine therapy for 5 years is recommended. Extended endocrine therapy needs to be individualized. For high-risk patients, the duration of adjuvant endocrine therapy may be extended to 10 years;
3) Adjuvant endocrine therapy (except for LHRHa) usually starts after adjuvant chemotherapy. Concurrent endocrine therapy and chemotherapy are not recommended. However, adjuvant endocrine therapy may be given together with radiotherapy or HER-2 targeted therapy;
4) ER and PR negative patients are not candidates for adjuvant endocrine therapy;
5) Contraception is needed for patients on tamoxifen. Ultrasound monitoring of the endometrium and routine gynecological examination every 6−12 months are necessary for patients on tamoxifen. For patients on aromatase inhibitors, routine bone mineral density assessment and calcium and vitamin D supplementation are necessary. Severe osteoporosis needs to be treated. Discontinuation of endocrine therapy or switching to other endocrine therapy drugs may be necessary if serious adverse effects occur.
5.5.2 Endocrine therapy for late-stage breast cancer
(1) First-line endocrine therapy is preferred for patients
1) Age >35 years old;
2) Disease-free interval longer than 2 years;
3) Bone and soft tissue metastases;
4) Asymptomatic visceral metastasis;
5) ER and/or PR positive;
6) Patients with unknown or negative hormone receptor status and slow progression diseases may be given experimental endocrine therapy.
(2) Endocrine therapy drugs
1) Fulvestrant, third-generation aromatase inhibitors, tamoxifen or the combination of cyclin-dependent kinase (CDK) 4/6 inhibitor and an aromatase inhibitor may be considered for patients without previous endocrine therapy or those with long disease-free intervals;
2) Tamoxifen with or without ovarian function suppression is preferred for premenopausal patients. Third-generation aromatase inhibitor is preferred for postmenopausal patients. Premenopausal patients with ovarian function suppression may also choose aromatase inhibitors;
3) Postmenopausal patients who have received adjuvant tamoxifen may choose an third-generation aromatase inhibitor or fulvestrant;
4) Patients who have received sequential adjuvant tamoxifen and a non-steroid aromatase inhibitor may choose from fulvestrant, everolimus plus exemestane, progesterone or toremifene, or the combination of CDK 4/6 inhibitor and an aromatase inhibitor.
(3) Special considerations
1) Tumor progression after two consecutive endocrine drugs usually indicates resistance to endocrine therapy, switching to cytotoxic drugs or participation in clinical trials is recommended;
2) Patients should be evaluated every 2−3 months during endocrine therapy. Treatment should be continued for patients with response or stable disease. In case of tumor progression, treatment strategies need to be reevaluated.
5.6 HER-2 targeted therapy
5.6.1 Definition of HER-2 positivity
(1) HER-2 immunohistochemistry 3+, FISH positive or chromogenic in situ hybridization (CISH) positive;
(2) HER-2 immunohistochemistry 2+ case needs FISH or CISH confirmation.
5.6.2 Special considerations
(1) HER-2 positivity must be established before the initiation of targeted therapy;
(2) Trastuzumab 6 mg/kg (loading dose 8 mg/kg) every 3 weeks, or 2 mg/kg (loading dose 4 mg/kg) weekly;
(3) Patient should be monitored for 4−8 h after the first dose;
(4) Trastuzumab is generally not given concurrently with the anthracyclines;
(5) Trastuzumab may be given concurrently with non-anthracycline chemotherapy, endocrine therapy and radiation therapy;
(6) LVEF should be evaluated prior to the initiation of trastuzumab and regularly monitored at 3-month intervals during treatment.
5.6.3 Adjuvant HER-2 targeted therapy
(1) Indications
1) HER-2 positive primary invasive carcinoma >1 cm (T1c and above);
2) HER-2 positive primary invasive carcinoma 0.6−1 cm (T1b) or smaller but with lymph node micrometastasis (pN1mi);
3) Trastuzumab is generally not recommended for patients with HER-2 positive, lymph node negative, and primary invasive carcinoma <0.5 cm (T1a). However, for patients with high-risk factors (hormone receptor negative, high-grade, high Ki-67), trastuzumab may be considered.
(2) Relative contraindications:
1) LVEF<50%;
2) Ongoing chemotherapy using anthracyclines.
(3) Treatment regimens
1) TCH and AC-TH are generally recommended. AC-TH is preferred for high-risk patients, especially those with 4 or more positive nodes. TCH may offer better cardiac safety profile;
2) Weekly paclitaxel with trastuzumab (wPH) is recommended for patients with small tumors (less than 1 cm);
3) The addition of pertuzumab to trastuzumab may be considered.
(4) Special considerations
1) Trastuzumab may be given concurrently with taxanes, but concurrent trastuzumab with an anthracycline should be avoided;
2) Trastuzumab is given for total of 1 year;
3) Trastuzumab may be given concurrently with radiotherapy and endocrine therapy.
5.6.4 Neoadjuvant HER-2 targeted therapy
HER-2 targeted therapy should be included in neoadjuvant treatment regimens for HER-2 positive patients.
(1) Principles
1) Trastuzumab is currently the mainstay of neoadjuvant HER-2 targeted therapy;
2) Trastuzumab-containing adjuvant chemotherapy regimens may be adopted for neoadjuvant chemotherapy;
3) The addition of pertuzumab to trastuzumab increases pCR rate and may be considered for neoadjuvant chemotherapy;
4) Anthracycline-free regimens such as TCH offer better cardiac safety profiles and may be considered for patients with contraindications to anthracyclines.
(2) Special considerations
1) Efficacy should be closely monitored during neoadjuvant therapy. Response of the primary tumor and/or lymph nodes should be evaluated according to the RECIST or WHO criteria. Patients who progressed during chemotherapy and trastuzumab may consider switching to another non-cross-resistant chemotherapy regimen but trastuzumab may be continued;
2) The total duration of trastuzumab is 1 year (neoadjuvant and adjuvant targeted therapy);
3) The use of neoadjuvant HER-2 targeted therapy alone or in combination with neoadjuvant endocrine therapy should be limited to clinical trials.
5.6.5 HER-2 targeted therapy for late-stage HER2-positive breast cancer
(1) First-line treatment for late-stage HER2-positive breast cancer
1) The combination of taxane and trastuzumab is preferred for patients progressed on an anthracycline-containing regimen;
2) Trastuzumab can be combined with vinorelbine, capecitabine, gemcitabine or other chemotherapy drugs to treat HER2 positive patients who progressed on taxane- containing regimens;
3) Taxanes in combination with trastuzumab and pertuzumab may further prolong survival;
4) HER-2 targeted therapy is still recommended for patients received previous adjuvant trastuzumab. In case of disease-free interval longer than 12 months, trastuzumab is preferred. In case of short disease-free interval, second-line HER-2 targeted therapy drugs may be considered;
5) Generally, trastuzumab combined with chemotherapy is recommended for first-line treatment. Lapatinib is not recommended for first-line treatment;
6) For patients with slow progressing HER-2 positive and HR positive diseases, HER-2 targeted therapy combined with endocrine therapy may be considered as first-line treatment. This combination may also be considered for HER-2 positive and HR positive patients who are unfit for chemotherapy.
(2) Treatment options after disease progression on trastuzumab
1) HER-2 targeted therapy should be continued after disease progression on trastuzumab;
2) The following options may be considered:
(a) Continue trastuzumab and switch to other non-cross-resistant chemotherapy regimens;
(b) Switch to new combinations of HER-2 targeted drug and chemotherapy regimen;
(c) T-DM1 is currently the preferred option for second-line treatment after trastuzumab failure.
(3) Special considerations
1) Trastuzumab in combination with chemotherapy is recommended for late-stage HER-2 positive patients;
2) For hormone receptor positive and HER-2 positive late-stage breast cancer:
(a) HER-2 targeted therapy combined with chemotherapy is generally preferred;
(b) However, HER-2 targeted therapy combined with an aromatase inhibitor may also be considered as first-line treatment for highly selective postmenopausal patients (slow progressing tumors, unfit for chemotherapy, bone and soft tissue metastases only);
(c) Generally, endocrine therapy alone is not used as maintenance treatment, it should be combined with HER-2 targeted therapy;
(d) The cessation of HER-2 targeted therapy may be considered for patients with long complete remission after treatment.
6. Flowchart of diagnosis and treatment
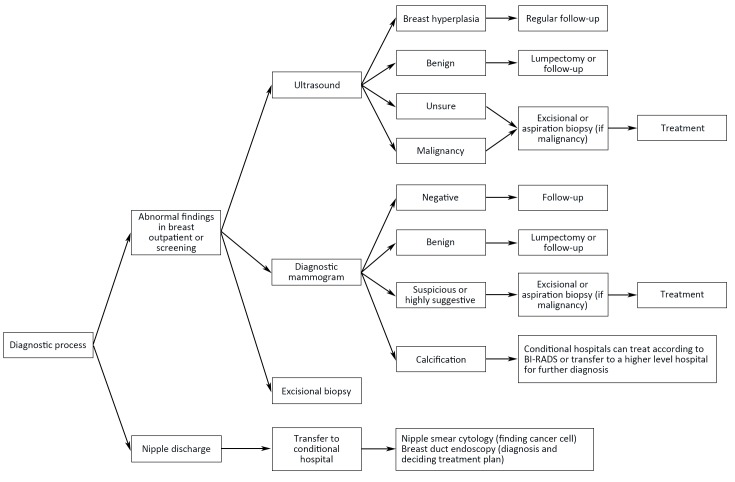
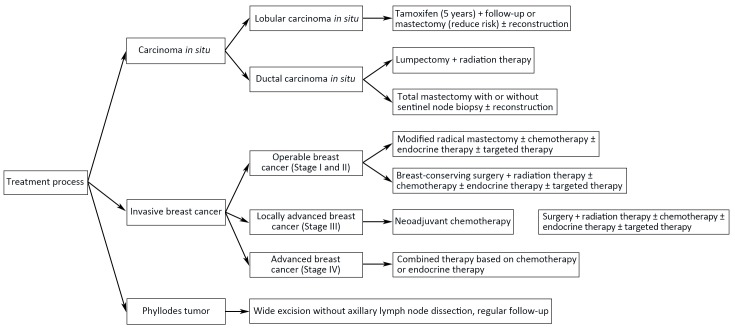
Flowcharts of diagnosis and treatment for breast cancer are shown in Figure 1 ,2 .
1.
Diagnosis procedure for breast cancer. BI-RADS, Breast Imaging Reporting and Data System.
2.
Treatment procedure for breast cancer.
7. Follow-up
7.1 Physical examination
Every 4−6 months for the first 2 years, then every 6 months between 3rd and 5th year, and annually after 5 years.
7.2 Breast ultrasonography
Every 6 months.
7.3 Mammogram
Annually.
7.4 Chest imaging (X-ray or CT)
Annually.
7.5 Abdominal ultrasonography
Every 6 months for the first 3 years, then annually after 3 years.
7.6 Bone scan
Annually for the first 5 years, then every 2 years after 5 years for patients with risk factors (such as 4 or more positive lymph nodes).
7.7 Blood tests and tumor markers
Every 6 months for the first 3 years, then annually after 3 years.
7.8 Annual pelvic examination
It is recommended for patients on tamoxifen.