Abstract
Objective
To explore the natural history of severe dysplasia/carcinoma in situ (SD/CIS) patients and to evaluate the efficacy of endoscopic treatment to SD/CIS patients.
Methods
Between January 2005 and December 2009, a population-based prospective screening program on esophageal squamous cell carcinoma (ESCC) was performed in Linzhou, China, with endoscopic screening plus iodine staining. All the eligible histologically confirmed SD/CIS patients were followed up through the door-to-door follow-up and local cancer registry. The endpoint was diagnosed as ESCC or the December 31st, 2016. Kaplan-Meier survival analysis and Log-rank test were used to compare the survival rates among treated and untreated patients.
Results
A total of 175 SD/CIS patients were enrolled and grouped by whether they received endoscopic treatment. Eleven-year cumulative incidence rates for untreated and treated SD/CIS patients were 10.7% [95% confidence interval (95% CI): 6.9−16.1] and 3.2% (95% CI: 1.4−7.0), respectively. The ESCC incidence free survival rate, and all-cause incidence and mortality free survival rates were all significantly higher in the treated patientsvs. untreated patients (P=0.043, P=0.008 and P=0.015, respectively). The ESCC mortality free survival rate showed no significant differences between the two groups (P=0.847).
Conclusions
The cumulative incidence rate of SD/CIS patients to ESCC was much lower than previously reported. The Kaplan-Meier survival analysis showed that endoscopic treatment could increase the ESCC and all-cause disease-free survival rates of SD/CIS patients significantly.
Keywords: Endoscopy, esophageal squamous cell carcinoma, precancerous lesions, management, mass screening
Introduction
Esophageal cancer (EC) is one of the most predominant malignancies worldwide. According to GLOBOCAN 2018, EC is the seventh most common cancer, of which 90% are esophageal squamous cell carcinoma (ESCC). ESCC occurs mostly in less developed countries, such as China, Iran and South Africa (1). Malignant progression of ESCC is considered to be a multi-step process, which subsequently develops through basal cell hyperplasia (BCH), mild dysplasia (mD), moderate dysplasia (MD), severe dysplasia/carcinoma in situ (SD/CIS) and ESCC (2,3). The 5-year survival rate is still relatively low associated with atypical early symptoms and late-stage diagnosis (4-6). Therefore, the development of proper mass screening methods needs great efforts to reduce the ESCC mortality.
Since the 1970s, national-wide screening programs have been developed in high-risk areas in China, including occult blood detection (7,8), balloon cytology (9), liquid-based balloon cytology (10,11), and endoscopic examination with Lugol’s iodine staining and biopsy (12-14). These studies showed that endoscopic screening with iodine staining is the best way to detect precancerous lesions of ESCC and has been proved to increase the 5-year survival rate and decrease the cumulative incidence and mortality rates significantly in high-risk areas in China.
Currently, SD/CIS aroused more concern than other dysplasia for its clinical importance and high risks to progress to ESCC (13). Generally, the guideline recommends all the endoscopically discovered patients with SD/CIS should be enrolled in endoscopic treatment, including endoscopic mucosal resection (EMR), multiband mucosectomy (MBM) or endoscopic submucosal dissection (ESD) at the time of diagnosis (15,16). There are, however, fraught uncertainties related to the management of SD/CIS. First, little research has concerned about the progression rate of SD/CIS, and the natural history of SD/CIS is not clear. The only population-based prospective study to evaluate the incidence rate indicated 75% of SD/CIS patients would progress to ESCC, which might amplify the hazard by choosing the dysplasia population screened by cytological method (3,13). Second, no research has reported the efficacy of the endoscopic treatment to SD/CIS patients.
This prospective population-based study aimed to explore the natural history of SD/CIS patients and to evaluate the efficacy of endoscopic treatment to SD/CIS patients by comparing the 11-year ESCC and all-cause cumulative incidence and mortality rates between the treated and untreated groups.
Materials and methods
Study design and population enrollment
Between January 2005 and December 2009, we performed a population-based prospective cohort study in Linzhou, a high-risk area of ESCC in China and followed them up until December 31st, 2016. The inclusion criteria were: 1) local residents; 2) 40−69 years old; 3) histologically diagnosed as SD/CIS at initial diagnosis; and 4) voluntarily signed the informed consent. The exclusion criteria were: 1) contraindications for endoscopic examinations (e.g., history of allergic reaction to iodine or lidocaine); 2) serious cardiovascular, respiratory, gastrointestinal system diseases, mental impairment and blood coagulation disorders; 3) pregnancy; or 4) progression to ESCC within one year from the time of initial diagnosis.
Endoscopic screening procedures
In the screening site, household registration system at the local police station was used to determine the covered population, which could provide demographic information. Residents were sent to Linzhou Cancer Hospital to 1) explain the study aims; 2) complete a structured questionnaire including demographic information and possible risk factors exposure history; 3) check inclusion and exclusion criteria; and 4) obtain a written consent form from all residents willing to participate in the endoscopic screening.
All epidemiological surveys, endoscopic examinations, histological diagnosis and therapies were conducted by local doctors after training by and under the supervision of experienced doctors from the Cancer Hospital, Chinese Academy of Medical Sciences (CAMS). This study was approved by the Ethics Committee of Cancer Institute and Hospital, CAMS. The study was registered on the Chinese Clinical Trials Register (No. ChiCTR1800017163).
After completion of the informed consent process, participants were provided a local anesthetic (5 mL of 1% lidocaine orally) 2−5 min before the examination. They were placed in the left lateral position, and the entire esophagus and stomach were examined by endoscopy carefully and visually. All visually abnormal areas were described and photographed.
After the initial inspection, Lugol’s iodine (1.2%−1.5%, 20 mL) solution was sprayed to stain the full length of the esophagus, which left suspicious lesions unstained after cleaned up by saline solution. The unstained areas were described and photographed, and targeted for biopsy. The number of biopsies taken depended on the size of the lesion, and ranged from 1 to 3 biopsies. For SD/CIS, EMR, MBM or ESD was strongly recommended as local therapies, unless the treatment was refused by the patients themselves. At the end of the procedure, the stomach was suctioned to remove excess iodine and air. Participants were monitored during recovery and then sent home (12,17). Besides, conventional clinical treatments such as esophagectomy, radiotherapy or chemotherapy were applied immediately to ESCC cases.
Samples processing and histological criteria
Biopsy specimens were laid mucosal side up on filter paper supports, fixed in 10% neutral-buffered formalin, embedded in paraffin, cut in 5-μm sections and stained with hematoxylin and eosin. The biopsy slides were read by two pathologists without knowledge of the visual endoscopic findings. Discrepancies were resolved by more experienced pathologists from National Cancer Center/Cancer Hospital, CAMS. The biopsies were histologically categorized as normal, BCH, mD, MD, SD/CIS and ESCC. Detailed histological criteria were as described previously (3).
Follow-up procedures
The participants were divided into two groups by whether they received the endoscopic treatment (EMR, MBM or ESD). Positive and negative follow-up were both used to make sure the accuracy of the outcomes, namely door-to-door follow-up and local cancer registry. Door-to-door follow-up was carried out once a year. The incidence and mortality status, reasons and dates were recorded by experienced epidemiologists and doctors from Linzhou Cancer Hospital. For the SD/CIS patients who refused the endoscopic treatment in the initial endoscopic examination, we would persuade them to receive the endoscopic treatment during the annual positive follow-up, in case they would like to. The negative follow-up was conducted by comparing the databases of our project and the local cancer registry through the unique identification. The cancer registry would get the all-cause incidence and mortality of the covered population from the local Center for Disease Control and Prevention annually. We used the 10th edition of the International Classification of Diseases (ICD-10) to classify all the diseases both in the initial examination and follow-up. Patients diagnosed with ESCC within the first year of their initial diagnosis were defined as prevalent cases and were excluded. Patients diagnosed with ESCC at least one year after the first endoscopic evaluation with biopsy were defined as incidence cases. The incidence and mortality free time was reported as the time from the baseline endoscopy to the cases or deaths confirmed. The end date of this project was December 31st, 2016.
Statistical analysis
The χ2 test was used to compare the frequency of categorical variables among treated and untreated SD/CIS patients. Crude rates and 95% confidence interval (95% CI) were adjusted by possible confounding factors. The survival analyses were estimated by the product-limit estimator according to Kaplan-Meier. Log-rank test was used to compare the survival rates among treated and untreated patients. All analyses were done using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA). Statistical significance was accepted as P<0.05 in the current study.
Results
Demographic information
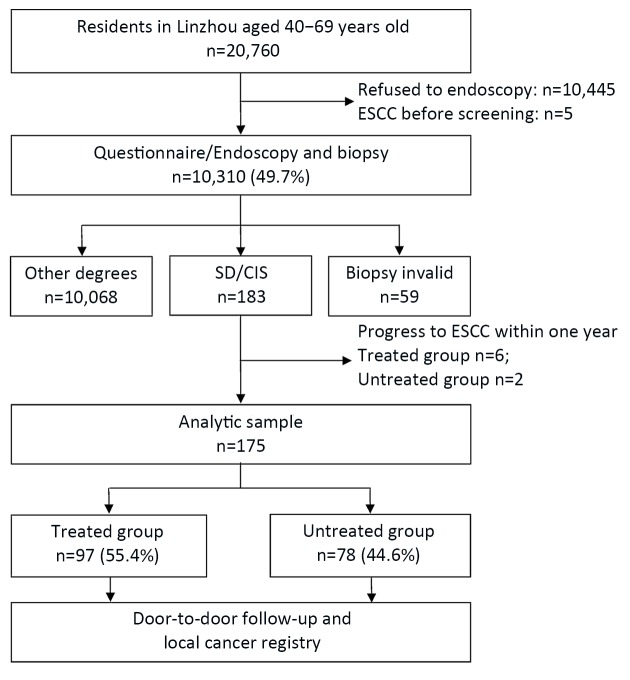
A total of 20,760 local residents aged 40−69 years old were covered in the endoscopic screening project in Linzhou from 2005 to 2009. And 175 patients histologically diagnosed as SD/CIS met the inclusion criteria for this analysis and grouped by whether they received endoscopic treatment. They were followed up until December 31st, 2016. The reasons for exclusions were highlighted inFigure 1 .
1.
Flowchart of severe dysplasia/carcinoma in situ (SD/CIS) patients in this population-based study. SD/CIS patients were divided into two groups by whether they received endoscopic treatments (EMR, MBM or ESD). EMR, endoscopic mucosal resection; MBM, multiband mucosectomy; ESD, endoscopic submucosal dissection; ESCC, esophageal squamous cell carcinoma.
Risk factors listed in Table 1 were compared between the treated and untreated groups. 55.4% (97/175) of the SD/CIS patients received endoscopic treatment and 44.0% (77/175) were males. The mean age of SD/CIS patients at diagnosis was 56.2±7.1 years. Further patient demographic characteristics of the study population were summarized in Table 1 . Education (P=0.022), number of family members (P=0.005) and family history of cancer (P=0.008) were significantly different between the two groups.
1.
Demographic information of 175 SD/CIS patients in Linzhou
| Risk factors | Untreated group [n (%)] (N=78) | Treated group [n (%)] (N=97) | P* |
| SD/CIS, severe dysplasia/carcinoma in situ; #, other marital status includes single, divorced and widowed; ##, 12 cases lost the record on the extent of the lesions; *, Pearson χ2 test was used for categorical data; **, P<0.05 was considered statistically significant. SD/CIS patients were divided into two groups by whether they received endoscopic treatments (EMR, MBM or ESD). EMR, endoscopic mucosal resection; MBM, multiband mucosectomy; ESD, endoscopic submucosal dissection. | |||
| Gender | 0.412 | ||
| Male | 37 (47.4) | 40 (41.2) | |
| Female | 41 (52.6) | 57 (58.8) | |
| Age (year) | 0.071 | ||

|
57.7±7.1 | 55.0±6.8 | |
| 40−49 | 7 (9.0) | 18 (18.6) | |
| 50−59 | 34 (43.6) | 47 (48.5) | |
| 60−69 | 37 (47.4) | 32 (33.0) | |
| Marital status | 0.431 | ||
| Married | 66 (84.6) | 86 (88.7) | |
| Others# | 12 (15.4) | 11 (11.3) | |
| Education | 0.022** | ||
| None | 13 (16.7) | 22 (22.7) | |
| Primary school | 41 (52.6) | 31 (32.0) | |
| High school | 24 (30.8) | 44 (45.4) | |
| Number of family members | 0.005** | ||
| ≤3 | 51 (65.4) | 43 (44.3) | |
| >3 | 27 (34.6) | 54 (55.7) | |
| Annual income (yuan) | 0.185 | ||
| ≤5,000 | 40 (51.3) | 40 (41.2) | |
| >5,000 | 38 (48.7) | 57 (58.8) | |
| Drinking water sources | 0.072 | ||
| Cellar, pool, tap, lake, or river | 30 (38.5) | 25 (25.8) | |
| Well | 48 (61.5) | 72 (74.2) | |
| Smoking | 0.986 | ||
| Never | 57 (73.1) | 71 (73.2) | |
| Ever | 21 (26.9) | 26 (26.8) | |
| Alcohol | 0.340 | ||
| Never | 75 (96.2) | 90 (92.8) | |
| Ever | 3 (3.8) | 7 (7.2) | |
| Tea | 0.496 | ||
| Never | 73 (93.6) | 93 (95.9) | |
| Ever | 5 (6.4) | 4 (4.1) | |
| History of digestive diseases | 0.174 | ||
| No | 68 (87.2) | 77 (79.4) | |
| Yes | 10 (12.8) | 20 (20.6) | |
| Family history of cancer | 0.008** | ||
| No | 60 (76.9) | 56 (57.7) | |
| Yes | 18 (23.1) | 41 (42.3) | |
| Extent of the lesion## | 0.480 | ||
| ≤3 | 50 (73.5) | 65 (68.4) | |
| >3 | 18 (26.5) | 30 (31.6) | |
Cumulative incidence and mortality rates of untreated and treated SD/CIS patients
Table 2 showed that eight (10.7 %, 95% CI, 6.9−16.1) and three (3.2%, 95% CI, 1.4−7.0) SD/CIS patients progressed to ESCC, respectively, in untreated and treated group. Similarly, one death case occurred both in the untreated (0.7%, 95% CI, 0.2−3.4) and treated (0.8%, 95% CI, 0.2−3.5) group, respectively. The all-cause cumulative incidence rates were 28.1% (95% CI, 22.0−35.2) and 11.3% (95% CI, 7.4−16.9), while the all-cause cumulative mortality rates were 13.1% (95% CI, 8.9−18.9) and 2.3% (95% CI, 0.9−5.8) in the untreated and treated groups, respectively.
2.
Eleven-year ESCC and all-cause incidence and mortality rates in SD/CIS patients in Linzhou
| Groups | Cumulative incidence | Cumulative mortality | |||||||||||||
| ESCC | All-cause | ESCC | All-cause | ||||||||||||
| n | Rate (%)* (95% CI) | P** | n | Rate (%)* (95% CI) | P** | n | Rate (%)* (95% CI) | P** | n | Rate (%)* (95% CI) | P** | ||||
| ESCC, esophageal squamous cell carcinoma; SD/CIS, severe dysplasia/carcinoma in situ; 95% CI, 95% confidence interval; *, education, family members and family history of cancer were adjusted for the cumulative incidence and mortality; **, χ2 test was used to compare the rates between the two groups. SD/CIS patients were divided into two groups by whether they received endoscopic treatments (EMR, MBM or ESD). EMR, endoscopic mucosal resection; MBM, multiband mucosectomy; ESD, endoscopic submucosal dissection. | |||||||||||||||
| Untreated group (n=78) | 8 | 10.7 (6.9−16.1) | 0.006 | 20 | 28.1 (22.0−35.2) | <0.001 | 1 | 0.7 (0.2−3.4) | 0.938 | 10 | 13.1 (8.9−18.9) | <0.001 | |||
| Treated group (n=97) | 3 | 3.2 (1.4−7.0) | 10 | 11.3 (7.4−16.9) | 1 | 0.8 (0.2−3.5) | 3 | 2.3 (0.9−5.8) | |||||||
Kaplan-Meier survival analysis of untreated and treated SD/CIS patients
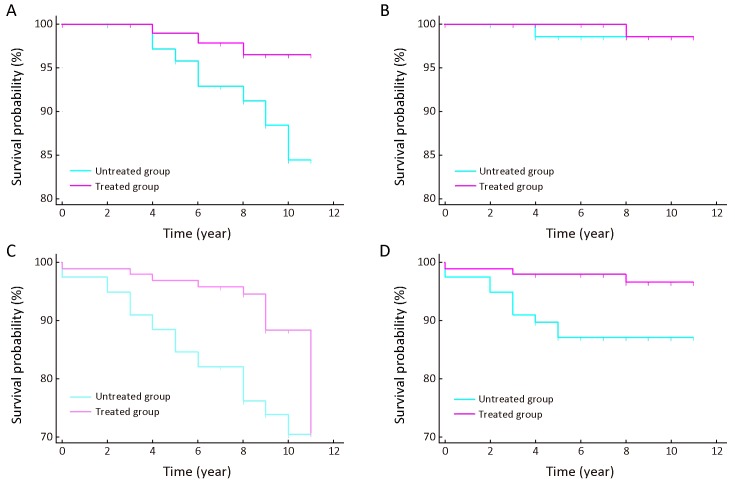
The total follow-up time for the untreated and treated group was 619 person-years and 821 person-years. The mean follow-up time was 7.9±2.6 years and 8.5±1.6 years correspondingly. Figure 2 displayed the Kaplan-Meier survival curve and Log-rank test for SD/CIS patients. The ESCC incidence free survival rate was significantly higher in the treated group than in the untreated group (Figure 2A , Log-rank test P=0.043). The ESCC mortality free survival rate showed no significant differences between the two groups (Figure 2B , Log-rank test P=0.847). We further calculated the all-cause incidence and mortality free survival rates of the SD/CIS patients, which were both significantly higher in the treated patients vs. untreated patients (Figure 2C , Log-rank test P=0.008; Figure 2D , Log-rank test P=0.015).
2.
Kaplan-Meier curves of severe dysplasia/carcinomain situ (SD/CIS) patients. These figures show different survival time between treated and untreated patients with SD/CIS of esophageal squamous cell carcinoma (ESCC) incidence free rate (Log-rank test P=0.043) (A), the ESCC mortality free rate (Log-rank test P=0.847) (B), the all-cause incidence free rate (Log-rank test P=0.008) (C), and the all-cause mortality free rate (Log-rank test P=0.015) (D).
Discussion
The management of SD/CIS patients after screening becomes a notable problem due to limited studies on the natural history of SD/CIS and the efficacy of endoscopic treatment. In this population-based prospective cohort study, we concluded that the 11-year cumulative incidence rate of untreated SD/CIS patients was 10.7% (95% CI, 6.9−16.1), not as high as previously reported (13). The incidence and mortality survival outcomes were significantly better in the treated group than in the untreated group.
The official guideline of ESCC screening in China recommended all the histologically confirmed SD/CIS patients should be enrolled in the endoscopic treatment. However, overtreatment happened since there was little research concentrated on the natural history of SD/CIS patients, and we used to believe that most SD/CIS would soon progress to ESCC. One prospective cohort study indicated that the 13-year cumulative incidence rate was about 75.0% for untreated SD/CIS patients. It led to the common belief that the most majority SD/CIS patients would progress, so the endoscopic treatment was necessary immediately (13). However, in the current study, the 11-year cumulative incidence rate was 10.7% in the untreated SD/CIS patients, much lower than previously reported 75%. Several limitations in the previous study caused the huge disparity between the two studies. Most importantly, all subjects in the previous study had a Chinese cytological diagnosis of dysplasia four years before the endoscopic screening, which meant that the enrolled subjects had a basically higher risk than the ordinary people. Then, in the former study, iodine staining was not applied to highlight the abnormal mucosal areas, and therefore, the reliability of diagnosis could not be guaranteed. Besides, the author did not exclude the prevalence cases. As a result, the previous study overestimated the risk of SD/CIS to ESCC. Opposite to this study, the current study improved the reliability of the results by avoiding the aforementioned weaknesses in a population-based cohort by endoscopic screening with iodine staining. As far as we know, data on the progression rate of SD/CIS to ESCC patients with endoscopic treatment have not been published before. In conclusion, our results of the cumulative incidence question the application of endoscopic treatment to every SD/CIS patient, since overtreatment might happen in about 90% of them and medical resources are greatly wasted. We suggest that endoscopic treatment should be done on high-risk patients. The priority to solve this problem is to stratify the SD/CIS patients by risks to progress in the future.
Our study demonstrated the efficacy of endoscopic treatment to SD/CIS patients after endoscopic screening by comparing the ESCC and all-cause incidence and mortality free survival time between the untreated and treated group. First, the ESCC incidence free survival rate was significantly higher in the treated group than in the untreated group (Figure 2A , P=0.043). The previous study evaluated the efficacy of endoscopic screening on ordinary residents in high-risk areas (14), but no study has focused on the SD/CIS patients. This is the first study to evaluate the efficacy of endoscopic treatment on SD/CIS patients in a community-based study. This result further supports the mass screening of ESCC in high-risk areas in China. Then, no significant differences were found in ESCC mortality free survival rate between the two groups (Figure 2B , P=0.847). Two reasons may explain this result. The mortality rate of ESCC is about 9/100,000 in China (18). Then the mean follow-up time is only 8 years, which hardly covers the whole natural history of the ESCC. As a result, the ESCC death cases are very few in this study. Further studies are needed to amplify the sample size and extend the follow-up time. Moreover, when we focus on the all-cause incidence and mortality, the patients who received endoscopic treatment showed significantly higher survival rates (Figure 2C , P=0.008; Figure 2D , P=0.015). Although the endoscopic treatment only aims at SD/CIS patients who are at high risks to progress to ESCC, the acceptance of the treatment might represent the social status, the education level, the attitudes towards the disease and health, and so on. So, it is reasonable that patients who received the treatment have better survival. In the current study, SD/CIS patients who tended to receive endoscopic treatment were those who had higher education (P=0.022), number of family member >3 (P=0.005), or a family history of cancer (P=0.008) ( Table 1 ).
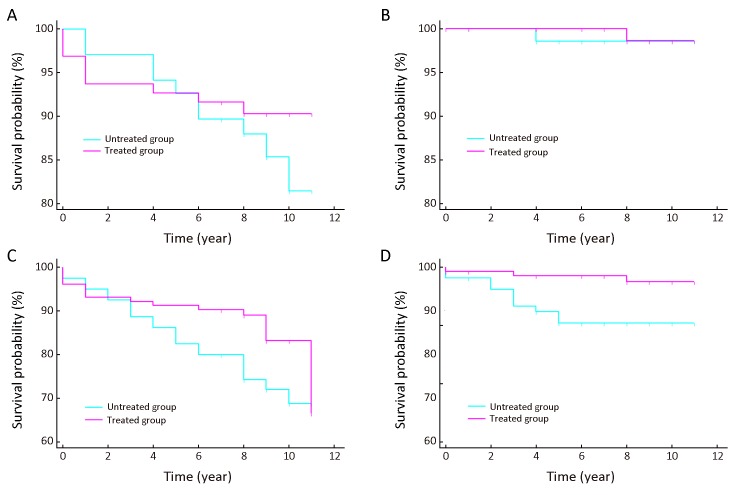
In this study, the new ESCC cases detected within one year after initial endoscopic screening were excluded from analysis. Not all comparable studies have excluded the cases occurred within the first year. But the most recent comments believe that even the golden standard will miss some cases, let alone the screening method. Therefore, they believe these cases most likely represented prevalent cases already presented. In fact, this belief has been widely used in cancer (19-21) and other diseases (22). In the current study, the occurrence rate of ESCC within one year after baseline screening between the untreated and treated groups showed no significant differences (Supplementary Table S1 ). However, the Kaplan-Meier survival analysis displayed that the ESCC incidence free rate and all-cause incidence free survival rates showed no significant differences after the ESCC cases detected within one year were excluded (P=0.371 and P=0.063, respectively, Supplementary Figure S1A , C ). The changes supported that prevalence cases may really exist and cases detected in the first years should be excluded.
S1.
Comparison of cases within and after one year between untreated and treated group
| Groups | Cases within one year [n (%)] | Cases after one year [n (%)] | Total | P* |
| *, Continuity correction χ2 test was used for the comparison. SD/CIS patients were divided into two groups by whether they received endoscopic treatments | ||||
| Untreated Group | 2 (2.5) | 78 (97.5) | 80 | 0.467 |
| Treated Group | 6 (5.8) | 97 (94.2) | 103 | |
| Total | 8 | 175 | 183 | |
S1.
Kaplan-Meier curves of severe dysplasia/carcinoma in situ (SD/CIS) patients. These figures show different survival time between treated and untreated patients with SD/CIS (including the cases detected in the first year) of the esophageal squamous cell carcinoma (ESCC) incidence free rate (Log-rank test P=0.371) (A), the ESCC mortality free rate (Log-rank test P=0.840) (B), the all-cause incidence free rate (Log-rank test P=0.063) (C), and the all-cause mortality free rate (Log-rank test P=0.014) (D).
Our study has various strengths. It is the first prospective population-based study aiming at the natural history of SD/CIS patients. So far, the only prospective cohort to explore the progression of SD/CIS was based on the highly concentrated population, which potentially overestimated the risks (13). The results of this population-based study are more reliable to raise recommendations for ESCC screening. Notably, this study was conducted in a demonstration screening site with experienced physicians, endoscopists, pathologists and epidemiologists, who were trained by experts from National Cancer Center of China, and who had worked on ESCC screening for 40 years, which is the solid foundation of this program.
Several limitations of this study need to be discussed. First, the compliance rate of endoscopic screening is 49.7% (Figure 1 ), which will undoubtedly result in the selection bias. However, it is hard to persuade asymptomatic residents to join an invasive screening program. In a population-based cohort, the compliance rate of 49.7% is already a tough goal to reach. Second, a larger sample size and longer follow-up time are needed to get more accurate conclusions in the future.
Conclusions
Based on the results of our 11-year ESCC endoscopic screening program, we highlighted the natural history of SD/CIS patients in both treated and untreated groups and the efficacy of endoscopic treatment. Because the majority of the patients are free of ESCC in the long run, treatment and surveillance decisions should be made individually based on the risk stratification. With the expansion of the screening program, more cases will be recruited. Development of a risk-stratification model that combines the clinical and biomarker co-variables to improve individualized follow-up guidelines should be a priority in the future.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2016YFC0901404); the Youth Research Fund by Peking Union Medical College (No. 2017310044); the National Natural Science Foundation of China (No. 81573224); and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2017PT32001 and 2016ZX310178).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Dawsey SM, Lewin KJ, Liu FS, et al Esophageal morphology from Linxian, China. Squamous histologic findings in 754 patients. Cancer. 1994;73:2027–37. doi: 10.1002/1097-0142(19940415)73:8<2027::aid-cncr2820730803>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Dawsey SM, Lewin KJ, Wang GQ, et al Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686–92. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Okines AF, Ashley S Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858–9. doi: 10.1056/NEJMc0911925. [DOI] [PubMed] [Google Scholar]
- 5.Brock MV, Gou M, Akiyama Y, et al Prognostic importance of promoter hypermethylation of multiple genes in esophageal adenocarcinoma. Clin Cancer Res. 2003;9:2912–9. [PubMed] [Google Scholar]
- 6.Zeng H, Chen W, Zheng R, et al Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555–e67. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 7.Qin D, Wang G, Zuo J, et al Screening of esophageal and gastric cancer by occult blood bead detector. Cancer. 1993;71:216–8. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 8.Qin D, Wang G, Yuan F, et al Screening for upper digestive tract cancer with an occult blood bead detector. Investigation of a normal north China population. Cancer. 1988;62:1030–4. doi: 10.1002/1097-0142(19880901)62:5<1030::aid-cncr2820620533>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Roth MJ, Liu SF, Dawsey SM, et al Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer. 1997;80:2047–59. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 10.Pan Q, Roth MJ, Guo H, et al Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Linxian, China. Acta Cytol. 2008;52:14–23. doi: 10.1159/000325430. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Hao C, Ma Q, et al DNA image cytometry test for primary screening of esophageal cancer: a population-based multi-center study in high-risk areas in China. Chin J Cancer Res. 2016;28:404–12. doi: 10.21147/j.issn.1000-9604.2016.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawsey SM, Fleischer DE, Wang GQ, et al Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–31. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 13.Wang GQ, Abnet CC, Shen Q, et al Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–92. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei WQ, Chen ZF, He YT, et al Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33:1951–7. doi: 10.1200/JCO.2014.58.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Ma S, Guan C, et al The National Cohort of Esophageal Cancer-Prospective Cohort Study of Esophageal Cancer and Precancerous Lesions based on High-Risk Population in China (NCEC-HRP): study protocol. BMJ Open. 2019;9:e027360. doi: 10.1136/bmjopen-2018-027360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Zeng H, Chen R, et al Evaluating efficacy of screening for upper gastrointestinal cancer in China: a study protocol for a randomized controlled trial. Chin J Cancer Res. 2017;29:294–302. doi: 10.21147/j.issn.1000-9604.2017.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muto M, Minashi K, Yano T, et al Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–72. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Bautista MC, Jiang SF, et al Risks and predictors of gastric adenocarcinoma in patients with gastric intestinal metaplasia and dysplasia: A population-based study. Am J Gastroenterol. 2016;111:1104–13. doi: 10.1038/ajg.2016.188. [DOI] [PubMed] [Google Scholar]
- 20.Hvid-Jensen F, Pedersen L, Drewes AM, et al Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge PJ, van Blankenstein M, Looman CW, et al Risk of malignant progression in patients with Barrett’s oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–6. doi: 10.1136/gut.2009.176701. [DOI] [PubMed] [Google Scholar]
- 22.Global BMI Mortality Collaboration Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–86. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]