Abstract
Antibacterial Photodynamic therapy (APDT) is a process utilizing light and light sensitive agents (named photosensitizer (PS)) and is usually applied in an oxygen-rich environment.
The energy of the photons is absorbed by the photosensitizer and subsequently transferred to surrounding molecules. Consequently, reactive oxygen species and free radicals are formed. These oxidative molecules can damage bacterial macromolecules such as proteins, lipids and nucleic acids and may result in bacterial killing. Unlike antibiotics, APDT as a novel technique does not lead to the selection of mutant resistant strains, hence it has appealed to researchers in this field.
The type of PS used in APDT is a major determinant regarding outcome. In this review, various types of PS that are used in antimicrobial Photodynamic therapy will be discussed. PSs are classified based on their chemical structure and origin. Synthetic dyes such as methylene blue and toluidine blue are the most commonly used photosensitizers in Antibacterial Photodynamic therapy (APDT). Other photosensitizers including natural PSs (e.g. curcumin and hypericin) and tetra-pyrrole structures like phthalocyanines and porphyrins have also been studied. Furthermore, nanostructures and their probable contribution to APDT will be discussed.
Keywords: Antimicrobial photodynamic therapy, Photosensitizer, Nanostructures, APDT, Nano-structure, Curcumin, Porphyrins, Toluidine blue, methylene blue
A pinch of history
Phototherapy began in ancient Egypt. Ancient Egyptians treated some skin diseases with herbs and sunlight. They applied natural photosensitizers such as psoralens (extracted from particular plants such as Parsley and Stjohn's-wort) for treatment of leprosy lesions 1, 2).
Osar Raab, a medical student who worked in Munich was the first one to notice that dyes like acrydine along with light can kill paramecia. He discovered that the incubation of paramecium with acridine and consequent exposure to light potentially kills paramecium. However, the mere application of acridine without light exposure was not effective 3). In following years, Von Tappeiner coined the term “photodynamic action” and attested that the presence of oxygen is essential in photodynamic action.
The first PDT was performed on a patient with skin carcinoma. It was carried out by T. Appaeiner and H. Jesionek in 1904. They used Eosin as PS along with white light. In recent years, more advances have been made in anticancer photodynamic therapy and different PSs are discovered 1, 2, 4, 5).
Antibacterial Photodynamic Therapy (APDT) was first introduced in 1960. Macmillan used toluidine blue against microorganisms like bacteria, algae, and yeast. It was observed that 99% of bacteria were killed within 30 min of irradiation with 21–30 mW of light at 632 nm from a continuous-wave gas laser. A few years later, researchers found that toluidine acts on cell membrane 6). Other dyes including methylene blue, rose Bengal, eosine Y, neutral red, acridine orange, crystal violet and rhodamine 6G were presented as a photosensitizer and it was established that cationic dyes are more effective against bacteria than anionic PS 7). Cationic molecules carry a positive charge on their functional groups, so they are easily bound to and taken up by bacteria which possess negative charge on the surface. However, the discovery of penicillin and its miraculous bactericidal properties, as well as other antibiotics impeded the progresses made in APDT.
In April 2014, WHO warned that we are on the cusp of a “post-antibiotic era”. The growing resistance to many antibiotics in recent years and emergence of multidrug-resistant bacteria has diverted the attention towards alternative antibacterial therapies such as APDT.
Advantages of APDT over antibiotics
Recent studies strongly uphold the hypothesis that APDT can be a satisfactory alternative since there is a substantial difference in the mode of action of PSs than that of antibiotics. The key benefits of APDT can be outlined as follows:
A broad spectrum of action compared to antibiotics since PS can act on diverse organisms such as bacteria, protozoa, fungi;
Bactericidal effects independent of antibiotic resistance pattern;
More limited adverse effect profile and damage to the host tissue;
No resistance following multiple sessions of therapy.
Mechanism of action
Photodynamic therapy utilizes PS along with visible or ultraviolet light to produce cytotoxic singlet oxygen and free radicals which exert detrimental effects on microorganisms. PSs possess a stable electronic configuration which is set at the lowest or ground state level.
After irradiation at a certain wavelength, the PS is promoted from the ground state to an excited state. In other words, electrons relocate to higher energy orbitals. This singlet state is unstable with a half-life between 10−6 and 10−9. These electrons are liable to lose their excess energy and return to ground state by emitting light (i.e. fluorescence) or heat. Moreover, Changes in electron spins can also shift the molecule to the triplet state. This process is known as “intersystem crossing” 8).
The triplet state PS reacts with the substrate in two different pathways-type Ⅰ and type Ⅱ 9). Type Ⅰ reaction involves electron transfer from triplet state PS to an organic substrate within the cells, leading to the production of free radicals. These free radicals interact with oxygen in molecular level and produce reactive oxygen species (ROS) such as superoxide, hydroxyl radicals and hydrogen peroxide. These oxidizing molecules potentially react with bacterial biomolecules and harm them.
In Type Ⅱ reaction, energy transfer occurs between the excited PS and the ground-state molecular oxygen, producing singlet oxygen that can interact with a large number of molecules in the cell to generate oxidized products (Fig. 1). The ratio of the occurrence between these two types is dependent on the type of PS that is used and the environment in which APDT is applied.
Fig. 1:
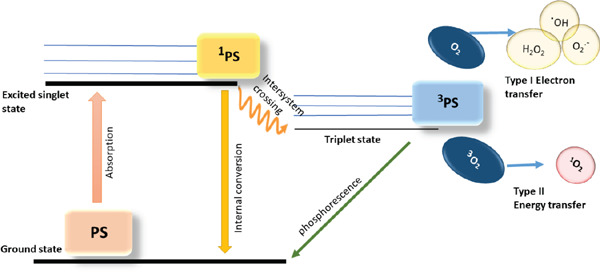
Graphical illustration of type Ⅰ and type Ⅱ photochemical mechanisms of PDT PS, ground state photosensitizer; 1 PS, PS in first excited state; 3 PS, triplet state PS; .OH, hydroxyl radical, O2.-, superoxide anion; 3O2, triplet state oxygen
In this review, we will describe different types of photosensitizers which are used in APDT. PSs used in APDT are classified into four groups based on their structure and origin; synthetic dyes, tetra-pyrrole structures, natural PSs and nano-structures.
Synthetic Dyes
Phenothiazinium is a subgroup of synthetic dyes. The most commonly used phenothiazinium dyes are methylene blue (Mb) and toluidine blue (Tb) (Table 1).
Table 1: commonly studied PSs and their photodynamic properties, *: Enterohemorrhagic E. coli.
| class | example | charge | Excitation maximum | Sample (bacteria) | Concentration of PS | Overall efficacy | Ref |
|---|---|---|---|---|---|---|---|
| Phenothiazinium | Methylene blue | cationic | 632nm | Dental plaque samples | 25 µg/ml | 8% | (11) |
| Toluidine blue | cationic | 410nm | S. mutans | 100 mg/l | 2–5 log10 | (77, 78) | |
| E. coli | 35 µM | 0.08 log10 | |||||
| Rose Bengal | anionic | 532nm | E. faecalis | 10 µM | 4 log10 | (79) | |
| P. aeruginosa | 3 log10 | ||||||
| Dimethyl methylene blue | cationic | 635–652nm | A. baumannii | 200 µM | 2 log10 | (80, 81) | |
| New methylene blue | cationic | 635–652nm | A. baumannii | 800 µM | 3.2 log10 | (80, 81) | |
| Natural PSs | Curcumin | neutral | 547nm | S. mutans | 0.75 to 5 g/l | ≤ 3 log10 | (41, 42) |
| L. acidophilus | |||||||
| Hypericin | neutral | 593nm | S. aureus | 100 nM | 4–5 log10 | (82–84) | |
| E. coli | 1 µg/ml | ≤ 0.2 log10 | |||||
| Flavin derivatives | cationic | 450nm | MRSA | 50 µM | 5.1 log10 | (85) | |
| EHEC* | 50 µM | 6.5 log10 | |||||
| Tetra-pyrrole structures | Porphyrin | cationic | 446nm | S. aureus | 10 µM | 1–2 log10 | (32, 86, 87) |
| P. aeruginosa | 225 µM | 4 log10 | |||||
| E. faecalis | 100 µM | No effect | |||||
| Phthalocyanine | Neutral | 670nm | A. hidrophila | 2 mM | ≤ 0.5 log10 | (22) | |
| Zink Pc derivatives | Cationic | 690 nm | S. aureus | 64 ng/ml | 5–6 log10 | (88–90) | |
| P. aeruginosa | 26 µg/ml | 5–6 log10 | |||||
| Chlorine | Neutral | 660nm | S. aureus | 10 µg/ml | 5 log10 | (91) | |
| E. coli | 5 µg/ml | 0.75 log10 | |||||
| Chlorine | Cationic | 532nm | E. coli | 5 µg/ml | 0.77 log10 | (92) | |
| Nano structures | Fullerenes | neutral | 532nm | S. aureus | 1 µg/ml | 3 log10 | (93, 94) |
| E. coli | ≤ 85% | ||||||
| Titanium dioxide | neutral | near-UV light (400) | Water treatment | 1 mg/ml | 77–93% | (95) | |
These dyes were the first generation of PSs that were investigated for anticancer PDT. However, because of their cationic charge, they tend to bind to both gram-negative and gram-positive bacteria with high affinity, thus nowadays they are mainly used in APDT in clinical settings 10).
Many published studies have determined that phenothiazinium PSs such as MB and TB are effective on planktonic bacteria. Furthermore, some studies also tested the efficacy of phenothiazinium against biofilm structures.
Fontana et al. added MB on ex vivo poly-microbial biofilms of dental plaque samples obtained from patients with chronic periodontitis. MB (25–50 µg/ml) and biofilms were incubated for 5 min and then diode laser (665nm) was applied. It was observed that PDT led to the inactivation of 63% of bacteria present in suspension but killed only 32% of bacteria in the biofilm. The conclusion was reached that bacteria in biofilm structure have lower susceptibility than planktonic bacteria because of the low penetration of PS into the biofilm 11).
Phenothiazines possess intrinsic cationic charge that makes them effective against many bacteria. To improve their function, some moieties like methylation can be introduced. Wainwright et al. found that functionalization of methylene blue by adding methyl group led to increasing singlet oxygen production and greater photo-inactivation 12). The effect of additional positive charges on the antimicrobial activity of the photosensitizer has been investigated. For example, Felgentrager et al. showed that functionalization of MB with tertiary ammonium increased both attachment and uptake by Gram-positive and Gram-negative bacterial cells, because these substituents have a greater cationic charge than the secondary ammonium substituents 13).
Other synthetic dyes are Eosin Y, Erythrosine (ERY) and Rose Bengal (RB) which belong to anionic xanthene dyes derived from Fluorescein. All these dyes have an absorption peak in the green wavelength range (480–550 nm). The attachment and uptake of anionic PSs by the bacterial cells are lower than cationic PSs 14).
Kishen et al. compared the efficacy of a cationic PS (MB) and an anionic PS (RB) on inactivate biofilms of E. feacalis. APDT with MB was superior to RB in regard to cytotoxic effects on E. feacalis. They also showed that applying a specific microbial efflux pump inhibitor like verapamil hydrochloride along with MB photodynamic therapy enhances the destruction of biofilm 15).
It has been noted that sometimes bacteria can decrease the effects of PS. The bacterial efflux pumps decrease the concentration of Phenothiazinium dyes in bacterial cells 16, 17). This decreased concentration buys time for the antioxidant machinery of the bacteria to activate, resulting in less inactivation. Tegos et al. have demonstrated that efflux pump inhibitors such as NorA and MexAB inhibitors increase the photo-inactivation of TB in S. aureus and P. aeruginosa 18).
Recently, new derivatives such as dimethyl methylene blue (derived from MB) and EtNBS (N-ethylpropylsulfonamido) have been studied. These dyes possess a high cationic charge that makes them more effective against bacterial cells 19–21).
Tetrapyrrole Structures
Tetra-pyrroles are one of the largest and firstly introduced PSs groups. Tetra-pyrrole structures have been named “pigment of life” because of their abundancy in nature (e.g. in hemoglobin or chlorophyll). There are numerous tetra-pyrrole compounds that are used as PSs in PDT, whereas porphyrins and phthalocyanines are the most frequently used PSs in APDT.
Phthalocyanines
Peak absorption of phthalocyanines is in the red region at 670 nm. Phthalocyanines (Pc) are diverse. Among these agents, Zinc phthalocyanine (ZnPc) is the most studied Pc for APDT.
Native ZnPc holds an affinity for gram-positive bacteria while its effectiveness against gram-negative bacteria is debatable (Table 1). This phthalocyanine, if used in conjunction with cationic and anti-membrane agents such as polymyxin or EDTA (ethylenediamine-tetraacetic acid) can become effective against gram-negative bacteria.
Later studies have shown that the functionalization of Pc with cationic groups improves the binding affinity to bacterial cells and there is no need for polymyxin or EDTA 22, 23). Dei D et al. discovered that water-soluble octa-cationic zinc Pc is efficacious against both gram-negative and gram-positive bacteria. Furthermore, the presence of eight positive charges thwarts the aggregation of phthalocyanine, unlike native compounds 24).
Cationic ZnPcs can also eliminate E. coli from blood products, making it advantageous in sterilization 25). No study has been done concerning the use of PC in the clinical setting.
Porphyrin
Advantages like high frequency, high rate of ROS production and easy chemical modification makes Porphyrins one of the most commonly used PSs. Their absorption is in 405–550 nm range. Like other PSs, the presence of cationic charge is a very important factor in APDT 26).
Some bacteria tend to accumulate a large number of porphyrins making them susceptible to killing when irradiated with blue light or UV. Some anaerobic bacteria like Propionibacterium acnes and Bacteroides species and also oral bacteria including Porphyromonas gingivalis, Prevotella spp, and Aggregatibacter actinomycetemcomtans which produce black pigment fall under this category 27, 28, 29). These bacteria with endogenous PS can be killed by mere light irradiation. Thus we can use APDT without PS administration for the treatment of Acne Vulgaris caused by Propionibacterium acnes 30, 31).
Cationic porphyrins like TMPyP (1-methyl-4-piridium-tetra(p-toluensulfonate)) have fourfold positive charge. Collins et al. used TMPyP against Pseudomonas aeruginosa biofilms both wild and mutant strains. Following the irradiation with mercury vapor lamp (400nm) for 10 min, they observed about 4 log10 steps inactivation for both strains 32). In contrast, Fabian C et al. found no reduction of CFU at all when they used TMPyP against biofilms of E. faecalis. It was postulated that this effect might be due to the large molecular structure of TMPyP or strong electrostatic interaction between the fourfold positive charge of cationic porphyrin and negative charge of extracellular polymeric substance (EPS) molecules 33).
Nowadays cationic antimicrobial peptides or cell penetrating peptides are conjugated to porphyrins to improve their efficiency. These conjugated porphyrins show a great cell inactivation during APDT.
Natural PSs
There are many natural compounds extracted from plants and other organisms which act as a photosensitizer and absorb white light or UV-A. Lots of natural PS compounds are yet to be discovered, hence the variety cannot be restricted. However, they hitherto include coumarins, furanocoumarins, benzofurans, anthraquinones and flavin derivatives (Table 1). Hypericin and curcumin are two natural compounds that have been extensively studied as a photosensitizer over the years.
Hypericin
Hypericum perforatum or St John's-wort is a flowering plant which is traditionally known for its healing effects on burns and skin injuries. According to clinical studies, this plant also demonstrates antiviral, antidepressant, antibacterial and antitumor characteristics. Nonetheless, the mechanisms through which these effects are exerted have not been totally understood 34). Hypericin is an anthraquinone derivative isolated from Hypericum perforatum. The best absorption of hypericin occurs at a wavelength of 600 nm which is sensed as orange-colored light.
It has been shown that hypericin-mediated APDT renders gram-positive bacteria including Streptococci mutants, Lactobacilli mutants, and Propionibacterium acnes inactivated 35). Garcia et al. designed a study to determine the photoactivity of hypericin against clinically isolated gram-positive methicillin-sensitive, methicillin-resistant Staphylococcus aureus (MRSA) and E. coli producing gram-negative extended spectrum .-lactamases (ESBL) 36).
The effectiveness of hypericin-mediated APDT on gram-positive MSSA and MRSA was significant, on the other hand, gram-negative E. coli was not susceptible to hypericin. It can be hypothesized that this difference in susceptibility to APDT is due to different cell wall structure that affects hypericin uptake. In fact, anionic and neutral PS tend to bind to gram-positive bacteria rather than gram-negative bacteria. Therefore, development of noble cationic hypericin derivatives will probably enhance the effectiveness of APDT against gram-negative bacteria.
Curcumin
Curcumin is another natural PS isolated from the root of a plant called Curcuma longa. Its optimum absorption is in the range of 405–435 nm. Curcumin executes a series of biological and pharmacological functions of which the following can be numerated: anti-oxidant 37) anti-inflammatory 38) anti-microbial 39) and wound healing 40) properties. Although quite a few studies have addressed these functions, the exact mechanisms are yet to be explored. The anti-inflammatory property of the curcumin makes it a favorable PS for treatment of periodontal diseases.
In all animal studies and a number of cell cultures, it has been established that curcumin is a rather safe compound regarding toxicity. Most studies about curcumin in the past 20 years are done in regard to its anticancer effects. However, recent publications report that curcumin is capable of inhibiting drug-resistant bacterial strain by means of photo-inactivation effect 42). S. aureus is one of the most common resistant bacteria to antibiotic therapy which remains susceptible to curcumin-mediated inactivation 43).
Curcumin has demonstrated some antibacterial properties in absence of irradiation by binding to FtsZ proteins (homologs of eukaryotic cytoskeletal tubulin) and inhibiting the assembly of FtsZ protofilament in Bacillus subtilis 44).
In addition, curcumin seems to inhibit the transcription of mecA gene, leading to a reduced PBP2α expression which in turn causes .-lactam antibiotics act more efficiently. As stated before, curcumin is also considered to be a photosensitizer for PDT as well as these favorable effects 45). Najafi et al. compared the antimicrobial activity of curcumin and chlorhexidine digluconate (CHX)(as the gold standard mouthwash) against Aggregatibacter actinomycetemcomitans (one the main culprit bacteria in periodontal diseases) using curcumin (5 mg/ml), LED (120 J/cm2) and CHX (2%). They concluded that curcumin is an effective substance in preventing the growth of A.actinomycetemcomitans, whose impact is reinforced when used simultaneously with PDT 41).
In terms of photo-killing effects, studies indicate that curcumin is 300 times more effective against the gram-positive S. aureus than the gram-negative E.coli and Salmonella typhimurium 46). It must be noted that curcumin is also photo-labile during its photodynamic action and is rapidly photodegraded.
In order to overcome the poor water solubility and the rapid decay of the natural curcumin at physiological pH, Winter S et al. examined the applicability of polyvinylpyrrolidone curcumin (PVP-C) at the 50 micro-molar PVP-C (15 or 25 min incubation) and as a result, a complete eradication of S. aureus was achieved 47).
Nanostructures
During the last decade, nanotechnology has had a great impact on PDT. Most of these studies have used nanoparticles to improve the efficacy of anti-cancer PDT while a few of them have been done on the antimicrobial aspects 48).
Nanoparticles are utilized in diagnostic approaches and the delivery of non-water-soluble PSs or anionic PSs. This is done through encapsulation and subsequent improvement in photo-interaction and photo-inactivation.
The results with nanoparticles were more satisfactory than with the PS alone. Distribution of the ROS accounts for this disparity as the ROS produced by PS-nanoparticles was locally concentrated while with the free PS it was uniformly distributed in the medium, hence less efficient. Furthermore, PS bound to a nanoparticle penetrates through the membrane better than free PS.
Some nanostructures such as gold nanoparticles, carbon nanotubes, silica nanoparticles and up-conversions have been used in PDT 49). Fullerenes and some quantum dots belong to another group of nanostructures and they act as a PS themselves 50).
The most commonly used classification of nanostructures and its coupling to PDT is proposed by Chatterjee et al. This classification includes active nanoparticles (nanoparticles applied as PS) and passive nanoparticles which are themselves divided into biodegradable (e.g. liposomes) and non-biodegradable nanoparticles like gold particles 51).
In this review, we describe four different types of interaction between nanoparticles and PS which are used in APDT processes. This classification has been proposed by Stefano Perni et al. and it includes; PS embedded in nanoparticles, PS bound to the surface of nanoparticles, PS-accompanying nanostructures and Nanoparticles as the PS 52).
1. PS embedded in nanoparticles
The majority of nanoparticles have been used as delivery vehicles for PSs such as tetrapyrroles, natural products, and phenothiazinium dyes.
Nanoparticles loaded with PS are primarily based on lipids such as liposomes and micelles, but sometimes carbohydrates like cyclodextrins are used as the basis for nanoparticles.
1-1. Liposome
Lipids exhibit the tendency to spontaneously aggregate in an aqueous environment and form bilayer structures. A well-known structure of this type is a liposome. Liposomes are nanosized vesicles composed of phospholipid and cholesterol and they are frequently used as carriers for PS 53).
There are two ways of incorporating PS into liposomes. First, as for water-soluble hydrophilic PS, it gets suspended in an aqueous environment with other compounds and then locates in the center of the liposome.
Second, non-water soluble hydrophobic PS dissolves in the hydrophobic environment and leads to the production of a liposome that contains the PS within the lipid bilayer. 54)
To optimize the liposome for APDT, cationic lipids like N-[1-(2,3-dioleoyloxy) propyl]-N, N, N-trimethylammonium methylsulfate (DOTAP) or DL-α-dipalmitoyl-phosphatidyl-choline can be affixed to the structure. Cationic liposomes are more effective than anionic or neutral ones in antibacterial photodynamic therapy because they can interact with the negatively-charged bacterial cell wall 55). Furthermore, the encapsulation by liposome prevents PS aggregation which in turns results in an increased photo-inactivation effect 56). In some occasions, an extra layer or another substance is added to modify the liposome charge. For instance, the use of calcium phosphate in the core of liposome leads to an increased effect against P. aeruginosa 57).
Nisnevitch et al. examined the effect of three water-soluble PSs including MB, Neutral Red (NR) and RB in their free form and encapsulated in liposomal formulations on both Gram-positive bacteria such as S. aureus, Sarcina luteaa and S. epidermidis and gram negative bacteria like E. coli, K. pneumonia, P. aeruginosa, Salmonella para B and Shigella flexneri. It was established that MB and NR enclosed in liposomal structures seem to have a greater antimicrobial effect than free PS for both Gram-positive and gram negative bacteria, whereas encapsulation of RB led to no intensification in its activity. Ultimately, it was suggested that encapsulation of PS can increase its deleterious effects on bacteria 58).
1-2. Micelles
Micelles fall under another category of nanoparticles that can incorporate PSs. They have been extensively used to deliver hydrophobic drugs by either entrapping or binding.
Some colloids can spontaneously form these nano-structures under certain conditions (with particle size 5–100 nm). Micelles are smaller in size than liposomes which results in more effective treatment. Besides, they are cheaper and easier to prepare. These unique properties, as well as increased permeability through the biological barriers and good drug bio-distribution, guarantee the widespread use of micelles compared to other nanoparticles 59, 60).
Tsai T et al. tested antimicrobial activity of hematoporphyrin (Hp) enclosed in either liposomes and micelles. The PDT efficacy was evaluated by means of the observed sensitivity of Gram-positive pathogens such as MSSA, MRSA, S. epidermidis and Streptococcus pyogenes. The results indicated that PDT with Hp encapsulated in micelles was more effective than the one encapsulated in liposomes at the same Hp doses 61).
2. PS bound to the surface of nanoparticles
PS bound to the surface of nanoparticles enhances the antimicrobial properties of PS. Several studies have been performed that report different PSs tend to bind to different nanoparticles. For example, porphyrin has a tendency for carbon nanotubes 62) while TB tends to bind to the surface of Au (Aurum) nanoparticles 63) and etc.
In this segment, we are going to explicate TB and its affinity for Au nanoparticles.
Since gold nanoparticle does not have any functional groups on the surface, direct attachment of TB molecules to gold is not possible, so a resurgence of reactive groups on the surface of nanoparticles is essential for absorption and binding of PS to the gold.
Functionalizing the gold with tiopronin is the most common approach to produce TB-Tiopronin-Gold nanoparticles. Jesus et al. compared the effect of TB-Tiopronin- Gold nanoparticles and free TB on the viability of S. aureus. The efficacy of TB-Tiopronin-Gold nanoparticles was four times greater than free TB 63, 64).
A different approach which proposed by Suci et al. is applying of a viral protein cage as a delivery vehicle of PS. Genetic construct of the viral protein cage had two benefits; site-specific targeting (by using Antibodies) and superior inactivation of bacterial cells 65).
3. PS-accompanying nanostructures
Sometimes nanoparticles cannot penetrate the bacterial membrane because of their considerably big size. On these occasions, it is plausible to keep the encased PS next to microbial cells. There are different mechanisms to achieve this goal.
Some studies have employed PSs in the polymer matrices such as silica and the others used nanoparticles like up-conversions 66) or quantum dots 67) in the proximity of PSs.
Confinement of TB and MB to silica matrix prevents the interaction between PS and microbial cell. During irradiation, produced ROS radicals moved away from the silicon and exerted their effect on neighboring microbial cells. With this approach, there was no need for adding PS again.
Biodegradable matrices such as silica can entrap many different PSs and result in monodisperse distribution and provide antimicrobial activity for a longer period of time. Due to the permeability of matrices to ROS and other types of molecular radicals, these molecules can easily migrate through the matrix, come out and kill bacteria. In addition, PSs inside the matrices are stable in pH changes and are not subject to microbial attack 49, 68).
Quantum dots (QD) such as CdSe and ZnS improve the effectiveness of PS in APDT. These molecules absorb the photons with certain energies (wavelength less than 480nm) and emit photons with longer wavelengths (approximately 642 nm). In this mechanism, the energy of light with appropriate wavelength is transferred to a neighboring PS via QD 69).
Recent studies have suggested that graphene quantum dots (GQD) can be used without PS. graphene is a single layer of carbon atoms forming a hexagonal lattice 67, 70). Wen-Shuo et al. used GQD as the photosensitizer with two-photon absorption on S. aureus as a Gram-positive and E. coli as a Gram-negative bacterium. Both types of bacteria started to decrease during a 10-s irradiation 71).
Up-conversion is a process in which nanoparticle absorbs two or more photons followed by the emission of a shorter wavelength photon. While commonly applied in cancer PDT, this method has not hitherto been used in APDT 66).
4. Nanoparticles themselves act as PS
Fullerenes are acknowledged to be one of the most important nanoparticles that can act as PS. Other nanoparticles in this group are semiconductors 72). Fullerenes display a spheroidal structure composed of pentagonal and hexagonal rings that consist of C60, C70, C84.etc 73). Lipophilic structure and neutral charge of these compounds account for their poor bactericidal effect. Modifications can be made with different cationic compounds.
Studies on E. coli in vitro about APDT showed that cationic fullerene N,N-dimethyl-2-(40-N,N,N-trimethyl-aminophenyl) fulleropyrrodinium iodide (DTC602+) hindered E. coli proliferation about 3.5 log10 after 30 min of irradiation under white light compared to the negligible killing effect of non-charged N-methyl-2-(40-acetamidophenyl) fulleropyrrolidine (MAC60) 74). The high selectivity and efficacy of this PS warrant the need for further investigations.
Alcohol functionalized fullerenes are not effective enough while other cationic fullerenes exhibited dark toxicity 73).
Semiconductors or photocatalysts like Titanium oxide( TiO2) and ZnO are materials with semi-conduction properties. After irradiation with UVA, the electron in the valence band gets excited and shifts to the conductance band. This electron can produce ROS. TiO2 nanoparticles are not used in the medical setting because of their absorption is in the UV range. With sunlight being the light source, TiO2 nanoparticles are dominantly used for the disinfection of water and obtaining hygienic clean water. 75). To make them applicable in clinical practice, researchers have focused their attention on shifting the absorbance spectrum of TiO2 from UV region to visible light through doping with other elements 76).
Conclusions and future
The treatment of infections by means of APDT is a new revolutionary method and faces some challenges which need to be addressed. The most important limitation that must be confronted is the delivery of light and PS to the sites of infection. The use of PDT in infection is restricted to the location of the impaired part of the body on which the light must be administered. Body cavities and skin due to their easily accessible location and localized nature are feasibly treated with light. Therefore, antibacterial PDT is probably more efficient against localized diseases as opposed to systemic infections like sepsis and bacteremia. PS should selectively target microbes and leave out the intact tissue and this is one of the most important challenges which often has been solved by functionalization of PS. Functionalization with cationic moieties also increases the effect of PS on both Gram-positive and Gram-negative bacteria. The advent of nanostructural material, especially those with polymeric or liposomal formulations has been promising regarding some of the challenges like hydrophobic nature of some PSs which diminishes the efficacy of PS applied. The covalent attachment of hydrophilic polymer chain to the PS with low-molecular-weight and the solubilization of PS in liposome carriers has been a great help. Nowadays, we witness a growing yet slow increase in the use of APDT in clinical treatment. Although a scanty number of existing clinical trials about PDT are performed on diseases other than periodontitis, but in the light of recent researches it is plausible to hope that this method can be applied to other infectious diseases as well.
References
- 1: Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochemistry and photobiology. 2001;74(5):656-69. [DOI] [PubMed] [Google Scholar]
- 2: Mitton D, Ackroyd R. History of photodynamic therapy in Great Britain. Photodiagnosis and photodynamic therapy. 2005;2(4):239-46. [DOI] [PubMed] [Google Scholar]
- 3: Raab O. Uber die wirkung fluoreszierender stoffe auf infusorien. Zeitschr Biol. 1900;39:524-46. [Google Scholar]
- 4: Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. JNCI: Journal of the National Cancer Institute. 1998;90(12):889-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5: Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, et al. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers in surgery and medicine. 2006;38(5):468-81. [DOI] [PubMed] [Google Scholar]
- 6: Macmillan JD, Maxwell WA, Chichester C. LETHAL PHOTOSENSITIZATION OF MICROORGANISMS WITH LIGHT FROM A CONTINUOUS-WAVE GAS LASER. Photochemistry and photobiology. 1966;5(7):555-65. [DOI] [PubMed] [Google Scholar]
- 7: Bellin J, Lutwick L, Jonas B. Effects of photodynamic action on E. coli. Archives of biochemistry and biophysics. 1969;132(1):157-64. [DOI] [PubMed] [Google Scholar]
- 8: Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, Hamblin MR. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers in surgery and medicine. 2012;44(6):490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9: Foote CS. Definition of type I and type II photosensitized oxidation. Photochemistr y and photobiology. 1991;54(5):65-9. [DOI] [PubMed] [Google Scholar]
- 10: Soukos NS, Wilson M, Burns T, Speight PM. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers in surgery and medicine. 1996;18(3):253-9. [DOI] [PubMed] [Google Scholar]
- 11: Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res. 2009;44(6):751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12: Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). The Journal of antimicrobial chemotherapy. 1998;42(1):13-28. [DOI] [PubMed] [Google Scholar]
- 13: Felgentr.ger A, Maisch T, Dobler D, Sp.th A. Hydrogen bond acceptors and additional cationic charges in methylene blue derivatives: photophysics and antimicrobial efficiency. BioMed research international. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14: Pereira CA, Costa AC, Carreira CM, Junqueira JC, Jorge AO. Photodynamic inactivation of Streptococcus mutans and Streptococcus sanguinis biofilms in vitro. Lasers Med Sci. 2013;28(3):859-64. [DOI] [PubMed] [Google Scholar]
- 15: Kishen A, Upadya M, Tegos GP, Hamblin MR. Efflux pump inhibitor potentiates antimicrobial photodynamic inactivation of Enterococcus faecalis biofilm. Photochemistry and photobiology. 2010;86(6):1343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16: Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrobial agents and chemotherapy. 2006;50(1):196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17: Spengler G, Takács D, Horváth á, Szabó áM, Riedl Z, Hajós G, et al. Efflux pump inhibiting properties of racemic phenothiazine derivatives and their enantiomers on the bacterial AcrAB-TolC system. In Vivo. 2014;28(6):1071-5. [PubMed] [Google Scholar]
- 18: Tegos GP, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrobial agents and chemotherapy. 2008;52(9):3202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19: Gollmer A, Felgentr.ger A, B.umler W, Maisch T, Sp.th A. A novel set of symmetric methylene blue derivatives exhibits effective bacteria photokilling-a structure-response study. Photochemical & Photobiological Sciences. 2015;14(2):335-51. [DOI] [PubMed] [Google Scholar]
- 20: Chiniforush N, Pourhajibagher M, Shahabi S, Kosarieh E, Bahador A. Can antimicrobial photodynamic therapy (aPDT) enhance the endodontic treatment? Journal of lasers in medical sciences. 2016;7(2):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21: Hoorijani MN, Rostami H, Pourhajibagher M, Chiniforush N, Heidari M, Pourakbari B, et al. The effect of antimicrobial photodynamic therapy on the expression of novel methicillin resistance markers determined using cDNA-AFLP approach in Staphylococcus aureus. Photodiagnosis and photodynamic therapy. 2017;19:249-55. [DOI] [PubMed] [Google Scholar]
- 22: Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier JE. Photosensitizing activity of water-and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios. 1992;71(286):33-46. [PubMed] [Google Scholar]
- 23: Spesia MB, Durantini EN. Photodynamic inactivation mechanism of Streptococcus mitis sensitized by zinc (II) 2, 9, 16, 23-tetrakis [2-(N, N, N-trimethylamino) ethoxy] phthalocyanine. Journal of Photochemistry and Photobiology B: Biology. 2013;125:179-87. [DOI] [PubMed] [Google Scholar]
- 24: Dei D, Chiti G, De Filippis MP, Fantetti L, Giuliani F, Giuntini F, et al. Phthalocyanines as photodynamic agents for the inactivation of microbial pathogens. Journal of Porphyrins and Phthalocyanines. 2006;10(03):147-59. [Google Scholar]
- 25: Lacey JA, Phillips D. The photosensitisation of Escherichia coli using disulphonated aluminium phthalocyanine. Journal of Photochemistry and Photobiology A: Chemistry. 2001;142(2):145-50. [Google Scholar]
- 26: Alves E, Faustino MA, Neves MG, Cunha A, Tome J, Almeida A. An insight on bacterial cellular targets of photodynamic inactivation. Future. 2014;6(2):141-64. [DOI] [PubMed] [Google Scholar]
- 27: Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, et al. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother. 2005;49(4):1391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28: Lennon AM, Buchalla W, Brune L, Zimmermann O, Gross U, Attin T. The ability of selected oral microorganisms to emit red fluorescence. Caries research. 2006;40(1):2-5. [DOI] [PubMed] [Google Scholar]
- 29: Cieplik F, Spath A, Leibl C, Gollmer A, Regensburger J, Tabenski L, et al. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clinical oral investigations. 2014;18(7):1763-9. [DOI] [PubMed] [Google Scholar]
- 30: Johnsson A, Kjeldstad B, Mel T. Fluorescence from pilosebaceous follicles. Archives of dermatological research. 1987;279(3):190-3. [DOI] [PubMed] [Google Scholar]
- 31: Henry CA, Judy M, Dyer B, Wagner M, Matthews JL. Sensitivity of Porphyromonas and Prevotella species in liquid media to argon laser. Photochemistry and photobiology. 1995;61(4):410-3. [DOI] [PubMed] [Google Scholar]
- 32: Collins TL, Markus EA, Hassett DJ, Robinson JB. The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms. Current microbiology. 2010;61(5):411-6. [DOI] [PubMed] [Google Scholar]
- 33: Cieplik F, Tabenski L, Buchalla W, Maisch T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Frontiers in microbiology. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34: Kubin A, Wierrani F, Burner U, Alth G, Grunberger W. Hypericin-the facts about a controversial agent. Current pharmaceutical design. 2005;11(2):233-53. [DOI] [PubMed] [Google Scholar]
- 35: Lüthi M, Gyenge EB, Engstrüm M, Bredell M, Gr.tz K, Walt H, et al. Hypericin-and mTHPC-mediated photodynamic therapy for the treatment of cariogenic bacteria. Medical Laser Application. 2009;24(4):227-36. [Google Scholar]
- 36: Garcia I, Ballesta S, Gilaberte Y, Rezusta A, Pascual A. Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms. Future microbiology. 2015;10(3):347-56. [DOI] [PubMed] [Google Scholar]
- 37: Satoskar R, Shah S, Shenoy S. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. International journal of clinical pharmacology, therapy, and toxicology. 1986;24(12):651-4. [PubMed] [Google Scholar]
- 38: Masuda T, Jitoe A, Isobe J, Nakatani N, Yonemori S. Anti-oxidative and anti-inflammatory curcumin-related phenolics from rhizomes of Curcuma domestica. Phytochemistry. 1993;32(6):1557-60. [Google Scholar]
- 39: Negi P, Jayaprakasha G, Jagan Mohan Rao L, Sakariah K. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. Journal of agricultural and food chemistry. 1999;47(10):4297-300. [DOI] [PubMed] [Google Scholar]
- 40: Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Molecular and cellular biochemistry. 2006;290(1):87-96. [DOI] [PubMed] [Google Scholar]
- 41: Najafi S, Khayamzadeh M, Paknejad M, Poursepanj G, Fard MJK, Bahador A. An In vitro comparison of antimicrobial effects of curcumin-based photodynamic therapy and chlorhexidine, on aggregatibacter actinomycetemcomitans. Journal of lasers in medical sciences. 2016;7(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42: Dahl TA, McGowan WM, Shand MA, Srinivasan VS. Photokilling of bacteria by the natural dye curcumin. Archives of microbiology. 1989;151(2):183-5. [DOI] [PubMed] [Google Scholar]
- 43: Ribeiro APD, Pavarina AC, Dovigo LN, Brunetti IL, Bagnato VS, Vergani CE, et al. Phototoxic effect of curcumin on methicillin-resistant Staphylococcus aureus and L929 fibroblasts. Lasers in medical science. 2013;28(2):391-8. [DOI] [PubMed] [Google Scholar]
- 44: Rai D, Singh JK, Roy N, Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochemical Journal. 2008;410(1):147-55. [DOI] [PubMed] [Google Scholar]
- 45: Teow S-Y, Liew K, Ali SA, Khoo AS-B, Peh S-C. Antibacterial action of curcumin against Staphylococcus aureus: a brief review. Journal of tropical medicine. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46: Parvathy K, Negi P, Srinivas P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chemistry. 2009;115(1):265-71. [Google Scholar]
- 47: Winter S, Tortik N, Kubin A, Krammer B, Plaetzer K. Back to the roots: photodynamic inactivation of bacteria based on water-soluble curcumin bound to polyvinylpyrrolidone as a photosensitizer. Photochemical & Photobiological Sciences. 2013;12(10):1795-802. [DOI] [PubMed] [Google Scholar]
- 48: Hamblin MR, Chiang LY, Lakshmanan S, Huang Y-Y, Garcia-Diaz M, Karimi M, et al. Nanotechnology for photodynamic therapy: a perspective from the Laboratory of Dr. Michael R. Hamblin in the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School. Nanotechnology reviews. 2015;4(4):359-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49: Huang Y-Y, Sharma SK, Dai T, Chung H, Yaroslavsky A, Garcia-Diaz M, et al. Can nanotechnology potentiate photodynamic therapy? Nanotechnology reviews. 2012;1(2):111-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50: Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photodynamic therapy with fullerenes. Photochemical & Photobiological Sciences. 2007;6(11):1139-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51: Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Advanced drug delivery reviews. 2008;60(15):1627-37. [DOI] [PubMed] [Google Scholar]
- 52: Perni S, Prokopovich P, Pratten J, Parkin IP, Wilson M. Nanoparticles: their potential use in antibacterial photodynamic therapy. Photochemical & Photobiological Sciences. 2011;10(5):712-20. [DOI] [PubMed] [Google Scholar]
- 53: Vemuri S, Rhodes C. Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharmaceutica Acta Helvetiae. 1995;70(2):95-111. [DOI] [PubMed] [Google Scholar]
- 54: Thompson DH, Anderson VC. Liposomal delivery system with photoactivatable triggered release. Google Patents; 1994. [Google Scholar]
- 55: Ferro S, Ricchelli F, Mancini G, Tognon G, Jori G. Inactivation of methicillin-resistant Staphylococcus aureus (MRSA) by liposome-delivered photosensitising agents. Journal of Photochemistry and Photobiology B: Biology. 2006;83(2):98-104. [DOI] [PubMed] [Google Scholar]
- 56: Merchat M, Spikes J, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. Journal of Photochemistry and Photobiology B: Biology. 1996;35(3):149-57. [DOI] [PubMed] [Google Scholar]
- 57: Schwiertz J, Wiehe A, Gr.fe S, Gitter B, Epple M. Calcium phosphate nanoparticles as efficient carriers for photodynamic therapy against cells and bacteria. Biomaterials. 2009;30(19):3324-31. [DOI] [PubMed] [Google Scholar]
- 58: Nisnevitch M, Nakonechny F, Nitzan Y. Photodynamic antimicrobial chemotherapy by liposome-encapsulated water-soluble photosensitizers. Russian Journal of Bioorganic Chemistry. 2010;36(3):363-9. [DOI] [PubMed] [Google Scholar]
- 59: van Nostrum CF. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Advanced drug delivery reviews. 2004;56(1):9-16. [DOI] [PubMed] [Google Scholar]
- 60: Torchilin V. Targeted polymeric micelles for delivery of poorly soluble drugs. Cellular and molecular life sciences. 2004;61(19):2549-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61: Tsai T, Yang YT, Wang TH, Chien HF, Chen CT. Improved photodynamic inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers in surgery and medicine. 2009;41(4):316-22. [DOI] [PubMed] [Google Scholar]
- 62: Banerjee I, Mondal D, Martin J, Kane RS. Photoactivated Antimicrobial Activity of Carbon Nanotube- Porphyrin Conjugates. Langmuir. 2010;26(22):17369-74. [DOI] [PubMed] [Google Scholar]
- 63: Gil-Tomás J, Tubby S, Parkin IP, Narband N, Dekker L, Nair SP, et al. Lethal photosensitisation of Staphylococcus aureus using a toluidine blue O-tiopronin-gold nanoparticle conjugate. Journal of materials chemistry. 2007;17(35):3739-46. [Google Scholar]
- 64: Narband N, Tubby S, Parkin IP, Gil-Tomás J, Ready D, Nair SP, et al. Gold nanoparticles enhance the toluidine blue-induced lethal photosensitisation of Staphylococcus aureus. Current Nanoscience. 2008;4(4):409-14. [Google Scholar]
- 65: Suci PA, Varpness Z, Gillitzer E, Douglas T, Young M. Targeting and photodynamic killing of a microbial pathogen using protein cage architectures functionalized with a photosensitizer. Langmuir. 2007;23(24):12280-6. [DOI] [PubMed] [Google Scholar]
- 66: Ai F, Ju Q, Zhang X, Chen X, Wang F, Zhu G. A core-shell-shell nanoplatform upconverting near-infrared light at 808 nm for luminescence imaging and photodynamic therapy of cancer. Scientific reports. 2015;5:srep10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67: Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nature communications. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68: Couleaud P, Morosini V, Frochot C, Richeter S, Raehm L, Durand J-O. Silica-based nanoparticles for photodynamic therapy applications. Nanoscale. 2010;2(7):1083-95. [DOI] [PubMed] [Google Scholar]
- 69: Narband N, Mubarak M, Ready D, Parkin I, Nair S, Green M, et al. Quantum dots as enhancers of the efficacy of bacterial lethalphotosensitization. Nanotechnology. 2008;19(44):445102. [DOI] [PubMed] [Google Scholar]
- 70: Akbari T, Pourhajibagher M, Hosseini F, Chiniforush N, Gholibegloo E, Khoobi M, et al. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagnosis and photodynamic therapy. 2017;20:148-53. [DOI] [PubMed] [Google Scholar]
- 71: Kuo W-S, Chang C-Y, Chen H-H, Hsu C-LL, Wang J-Y, Kao H-F, et al. Two-photon photoexcited photodynamic therapy and contrast agent with antimicrobial graphene quantum dots. ACS applied materials & interfaces. 2016;8(44):30467- 74. [DOI] [PubMed] [Google Scholar]
- 72: Tutt LW, Boggess TF. A review of optical limiting mechanisms and devices using organics, fullerenes, semiconductors and other materials. Progress in quantum electronics. 1993;17(4):299-338. [Google Scholar]
- 73: Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, et al. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-· versus 1O2. Journal of the American Chemical Society. 2003;125(42):12803-9. [DOI] [PubMed] [Google Scholar]
- 74: Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chemistry & biology. 2005;12(10):1127-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75: Thandu M, Comuzzi C, Goi D. Phototreatment of water by organic photosensitizers and comparison with inorganic semiconductors. International Journal of Photoenergy. 2015;2015. [Google Scholar]
- 76: Wang W, Shang Q, Zheng W, Yu H, Feng X, Wang Z, et al. A novel near-infrared antibacterial material depending on the upconverting property of Er3+-Yb3+-Fe3+ tridoped TiO2 nanopowder. The Journal of Physical Chemistry C. 2010;114(32):13663-9. [Google Scholar]
- 77: Zanin IC, Lobo MM, Rodrigues LK, Pimenta LA, Hofling JF, Goncalves RB. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. European journal of oral sciences. 2006;114(1):64-9. [DOI] [PubMed] [Google Scholar]
- 78: Fekrazad R, Zare H, Vand SM. Photodynamic therapy effect on cell growth inhibition induced by Radachlorin and toluidine blue O on Staphylococcus aureus and Escherichia coli: An in vitro study. Photodiagnosis Photodyn Ther. 2016;15:213-7. [DOI] [PubMed] [Google Scholar]
- 79: Shrestha A, Kishen A. Polycationic chitosan-conjugated photosensitizer for antibacterial photodynamic therapy. Photochem Photobiol. 2012;88(3):577-83. [DOI] [PubMed] [Google Scholar]
- 80: Ragàs X, Dai T, Tegos GP, Agut M, Nonell S, Hamblin MR. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in vitro and in vivo studies. Lasers in surgery and medicine. 2010;42(5):384-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81: Phoenix DA, Harris F. Phenothiazinium-based photosensitizers: antibacterials of the future? Trends in molecular medicine. 2003;9(7):283-5. [DOI] [PubMed] [Google Scholar]
- 82: García I, Ballesta S, Gilaberte Y, Rezusta A, Pascual á. Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms. Future microbiology. 2015;10(3):347-56. [DOI] [PubMed] [Google Scholar]
- 83: Engelhardt V, Krammer B, Plaetzer K. Antibacterial photodynamic therapy using water-soluble formulations of hypericin or mTHPC is effective in inactivation of Staphylococcus aureus. Photochemical & Photobiological Sciences. 2010;9(3):365-9. [DOI] [PubMed] [Google Scholar]
- 84: Yow C, Tang HM, Chu ES, Huang Z. Hypericin-mediated Photodynamic Antimicrobial Effect on Clinically Isolated Pathogens. Photochemistr y and photobiology. 2012;88(3):626-32. [DOI] [PubMed] [Google Scholar]
- 85: Maisch T, Eichner A, Sp.th A, Gollmer A, K.nig B, Regensburger J, et al. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PloS one. 2014;9(12):e111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86: Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials. 2009;30(18):3158-66. [DOI] [PubMed] [Google Scholar]
- 87: Cieplik F, Spath A, Regensburger J, Gollmer A, Tabenski L, Hiller KA, et al. Photodynamic biofilm inactivation by SAPYR-- an exclusive singlet oxygen photosensitizer. Free radical biology & medicine. 2013;65:477-87. [DOI] [PubMed] [Google Scholar]
- 88: Strakhovskaya M, Antonenko YN, Pashkovskaya A, Kotova E, Kireev V, Zhukhovitsky V, et al. Electrostatic binding of substituted metal phthalocyanines to enterobacterial cells: its role in photodynamic inactivation. Biochemistry (Moscow). 2009;74(12):1305-14. [DOI] [PubMed] [Google Scholar]
- 89: Segalla A, Borsarelli CD, Braslavsky SE, Spikes JD, Roncucci G, Dei D, et al. Photophysical, photochemical and antibacterial photosensitizing properties of a novel octacationic Zn (II)-phthalocyanine. Photochemical & Photobiological Sciences. 2002;1(9):641-8. [DOI] [PubMed] [Google Scholar]
- 90: Simonetti O, Cirioni O, Orlando F, Alongi C, Lucarini G, Silvestri C, et al. Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068/Cl in an experimental model of Staphylococcus aureus wound infection. British Journal of Dermatology. 2011;164(5):987-95. [DOI] [PubMed] [Google Scholar]
- 91: Karygianni L, Ruf S, Follo M, Hellwig E, Bucher M, Anderson A, et al. Novel broad-spectrum antimicrobial photoinactivation of in situ oral biofilms by visible light plus water-filtered infrared A. Applied and environmental microbiology. 2014;80(23):7324-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92: Mesquita MQ, Menezes JC, Neves MG, Tomé AC, Cavaleiro JA, Cunha Â, et al. Photodynamic inactivation of bioluminescent Escherichia coli by neutral and cationic pyrrolidine- fused chlorins and isobacteriochlorins. Bioorganic & medicinal chemistry letters. 2014;24(3):808-12. [DOI] [PubMed] [Google Scholar]
- 93: Wang M, Huang L, Sharma SK, Jeon S, Thota S, Sperandio FF, et al. Synthesis and photodynamic effect of new highly photostable decacationically armed [60]-and [70] fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. Journal of medicinal chemistry. 2012;55(9):4274-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94: Spesia MB, Milanesio ME, Durantini EN. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C 60 derivatives. European journal of medicinal chemistry. 2008;43(4):853-61. [DOI] [PubMed] [Google Scholar]
- 95: Maness P-C, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Applied and environmental microbiology. 1999;65(9):4094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


