Abstract
Marine resources and industry have become one of the most important pillars in economic development all over the world. However, corrosion of materials is always the most serious problem to the infrastructure and equipment served in marine environment. Researchers have found that microbiologically influenced corrosion (MIC) and marine bio-fouling are two main mechanisms of marine corrosions due to the complicated marine environment and marine organisms. This article summarized the latest research progress about these two mechanisms and indicated that both MIC and marine bio-fouling are closely related to the biofilms on material surfaces formed by the marine microorganisms and their metabolites. As a result, to prevent the occurrence of MIC and bio-fouling, it is important to control the microorganisms in biofilms or prevent the adhesion and formation of biofilms. The traditional method of using chemical bactericide or antifoulant faces the problems of pollution and microorganism resistance. This article introduced four research approaches about the new tendency of applying new materials and technologies to cooperate with traditional chemicals to achieve better and longer effects with lower environment pollution through synergistic actions. Finally, some future research tendencies were proposed for whole marine anti-corrosion and anti-fouling areas.
Keywords: Microbiologically influenced corrosion, Marine bio-fouling, Biofilms, Synergistic action, Anti-Corrosion
Abbreviations: MIC, microbiologically influenced corrosion; SRB, sulfate-reducing bacteria; IOB, iron-oxidizing bacteria; EPS, extracellular polymeric substances; BCSR, bio-catalytic cathodic sulfate reaction; EET, extracellular electron transfer; DET, direct electron transfer; MET, mediated electron transfer; SPC, self-polishing copolymers; TBDMSiMA, tert-butyldimethylsilyl methacrylate; MMA, methyl methacrylate; RAFT, reversible addition-fragmentation chain transfer; DSA, Dynamic Surface Antifouling; CL, caprolactone; GA, glycolide
Graphical abstract
Highlights
-
•
Microbiologically influenced corrosion (MIC) and marine bio-fouling are two main mechanisms of marine corrosions.
-
•
Both MIC and bio-fouling are related to the adhesion and formation of biofilms caused by microorganisms in the sea.
-
•
The key to prevent MIC and bio-fouling is to control biofilms in order to prevent their adhesion and formation.
-
•
Traditional method of using chemical bactericide faces the problems of pollution and microorganism resistance.
-
•
Four research approaches to improve or enhance traditional anti-corrosion methods by synergistic action are introduced.
1. Introduction
The ocean covers about 70% of the earth's surface area and the ocean transport supports 90% of freight transportation in the world trade. As a result, marine resources and marine industry have become one of the indispensable pillars in economic development. However, marine environment is an extremely harsh corrosive environment for metals and other materials used in the ocean industry [[1], [2], [3]]. Firstly, seawater itself is an electrolyte with high corrosiveness. Secondly, ocean environment is complicated because marine organisms and their metabolite will influence the materials together to cause corrosion. Corrosion of materials is always the main reason to cause destructions and abandonments of infrastructure and industrial equipment served in the marine environment. It has been recognized all around the world that corrosion losses exceed the total loss of all other nature disasters [4]. In China, the annual loss caused by corrosion is about 3–5% of GDP [4,5]. In order to use marine resources efficiently, it is necessary to study the mechanism of material corrosion in marine environment and the methods to prevent corrosion [6].
Corrosion happened in marine environment is complicated because different kinds of corrosion processes occurred, and the most special one is that there are many kinds of marine organisms in the ocean including microorganisms, plants and animals. A huge part of material corrosions happened in marine environment was related to the interactions between materials and marine organisms. Researchers have found that there are two main mechanisms including microbiologically influenced corrosion (MIC) and marine bio-fouling. This article will summarize some recent research progress about these two mechanisms and introduce some new methods and materials which can improve the protection effects against MIC and marine bio-fouling.
2. Marine corrosion mechanisms
2.1. Microbiologically influenced corrosion (MIC)
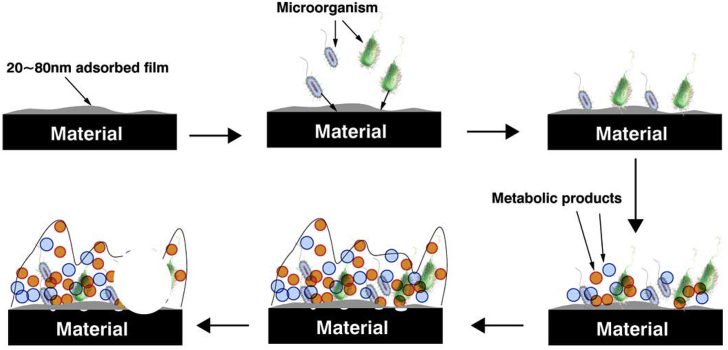
Microbiologically influenced corrosion (MIC) is the corrosion of metal materials which is accelerated directly by the life activities of microorganisms or indirectly by their metabolites [7,8]. A large part of the economic losses in marine industry are caused by MIC. According to statistics, MIC accounts for about 20% of the total economic losses [9,10]. MIC is often produced by a mixture of anaerobic sulfate-reducing bacteria (SRB) and aerobic iron-oxidizing bacteria (IOB) [[11], [12], [13], [14]]. Under actual working conditions, these two micro-organisms accelerate the corrosion of materials through synergistic actions. IOB consumes oxygen in the medium to create an appropriate growth environment for anaerobic SRB and then promote the corrosion of the matrix by SRB [[15], [16], [17]]. During this process, SRB and IOB cooperate together to form biofilms on metal surfaces which are usually composed of sessile cells, extracellular polymeric substances (EPS) and corrosion products from these two bacteria [[18], [19], [20]]. Biofilm plays a very important role in MIC and the development of biofilm theory and analytical techniques have led up to a better understanding of the whole process of MIC [[21], [22], [23]]. Fig. 1 shows the formation and development of biofilm affected by the metabolic activity of microorganisms and the corrosion caused by biofilms [24]. The whole process generally includes 6 steps: (1) an adsorbed film is created on the metal surface; (2) planktonic microorganisms migrate to the material surface attracted by the adsorbed film; (3) planktonic microorganisms attach to the active sites on the material surface and change into sessile microorganisms; (4) the sessile microorganisms grow and produce metabolites to form biofilms; (5) with increase of metabolites and corrosion products, mature and stable biofilms are formed and start causing corrosion; (6) with the pass of time, the stability of biofilms decreases, and then part of them will fall off to create heterogeneous biofilms.
Fig. 1.
Schematic process of formation of biofilm and MIC [24].
Many studies have found that the composition of biofilms is complex, leading to complicated effects on the corrosion of materials, and biofilms formed in different periods also have different effects on the corrosion, and finally heterogeneous biofilms caused by the falling off of unstable biofilms will create localized corrosion of materials to accelerate the corrosion rate [[25], [26], [27], [28]]. The reason why heterogeneous biofilms cause localized corrosion can be explained by the oxygen concentration cell theory [29]. When heterogeneous biofilms occur at material surface, the sites with dense biofilms prevent oxygen to spread to them and the aerobic bacteria in biofilms also exclude the oxygen underneath the biofilms. Both of them result in creating low oxygen concentration sites. Consequently, these sites serve as anodic sites to corrode the material. At the same time, the sites with less dense biofilms or no biofilm having higher oxygen concentrations serve as cathodic sites for oxygen reduction reaction and electron consumption [30].
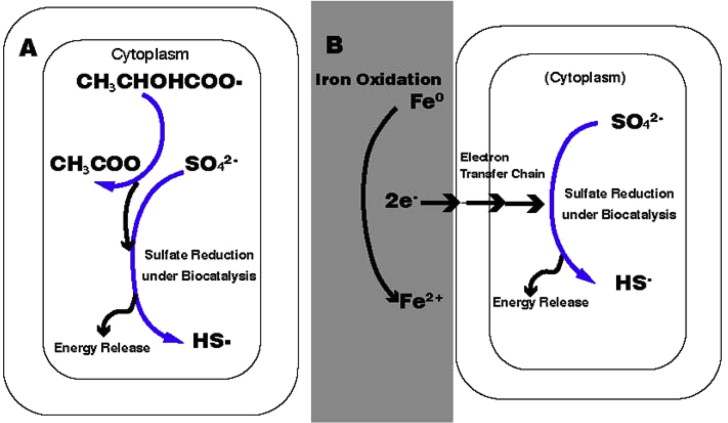
Traditional theories which try to explain the MIC mechanism like the oxygen concentration cell theory above used to consider that microorganisms and biofilms do not participate in corrosion process directly. However, with the research progress of MIC, Gu et al. [31] proposed a new theory called bio-catalytic cathodic sulfate reaction (BCSR) which explains the mechanism of MIC more precisely from the angles of bioenergetics and bio-electrochemistry for the first time in 2009. After that, a lot of literature and studies [27,29,[32], [33], [34], [35], [36], [37]] did researches to explain, prove and complete this BCSR theory. In general situation, microorganisms require an electron donor as energy source and an electron acceptor to provide energy for their metabolism. For the key microorganism which causes MIC, SRB, fatty acids like lactate usually act as organic carbon source and electron donor for its growth and sulfate is used as the terminal electron acceptor to finish the oxidation and reduction reaction process. In BCSR theory, when SRB forms biofilms on the iron surface, biofilms act as mass transfer barriers to prevent carbon source diffusion. When the top layer of a biofilm consume carbon source, the sessile SRB near the metal surface will live in an environment with less carbon source. Since there is no carbon source and electron from outside, starved SRB will use elemental iron as the electron donor to corrode it and produce energy for maintenance. When iron is corroded by SRB, the electrons released by iron oxidation will be transported across the SRB cell wall and finally applied in the sulfate reduction happening in the cytoplasm of SRB. Fig. 2 shows the differences between the situations of organic carbon-sulfate reduction and BCSR with iron as the electron donor [36]. As a result, BCSR answered the first question that the purpose for microorganisms to corrode metal is to obtain energy from the process to maintain their lives due to the lack of carbon source in biofilms.
Fig. 2.
Schematic showing the processes of: (A) organic carbon-sulfate reaction and (B) SRB corrode iron with iron as the electron donor in BCSR [36].
The second question that BCSR tries to answer is how the MIC process happens. As shown in Fig. 2 [36], oxidation of insoluble iron happens outside SRB while sulfate reduction occurs inside SRB. So the electrons released by iron oxidation are needed to be transferred through a form of electron transport chain to across the cell wall and finally into the cytoplasm of SRB to participate the sulfate reduction. A new method, extracellular electron transfer (EET), is introduced to explain how the electrons cross the SRB cell wall [38]. EET has two main types including direct electron transfer (DET) and mediated electron transfer (MET) [39,40]. Fig. 3 illustrates the mechanism of DET and MET [33].
Fig. 3.
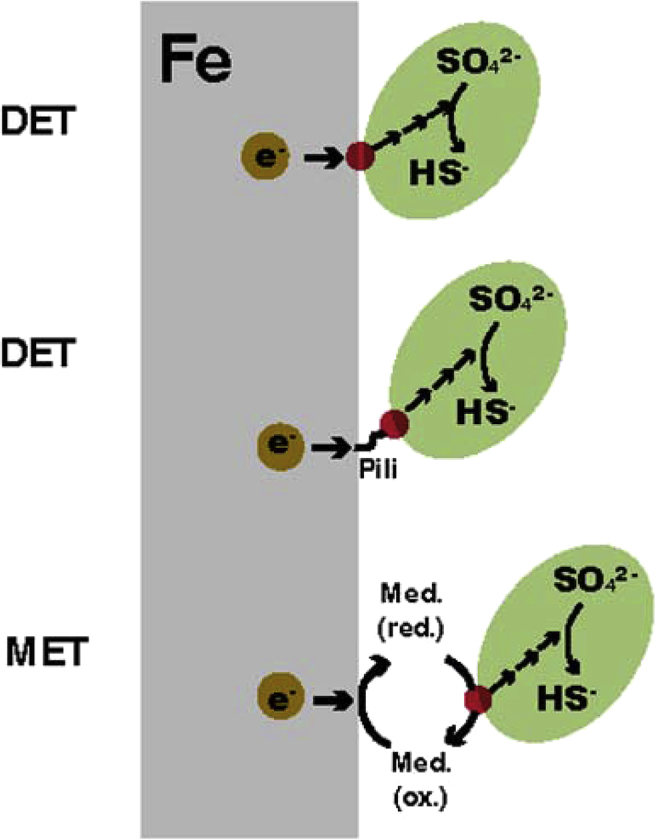
Schematic drawing of mechanisms of DET and MET in EET [33].
As shown in Fig. 3 [33], there are two transfer methods in DET. When sessile cells attach to the iron directly, the c-cytochrome is applied to transfer electrons and when sessile cells are very close to the iron surface, conductive nanowires (pili) will be secreted to link cells to iron surface only when the SRB culture medium is lack of organic carbon. For MET, electron mediators are needed to transfer electrons from iron surface to SRB cells. Zhang et al. [33] and Li et al. [40] demonstrated that two common electron mediators, riboflavin and flavin adenine dinucleotide (FAD), could accelerate the MIC of 304 stainless steel and C1018 carbon steel by the biofilm of SRB Desulfovibrio vulgaris through examining the weight loss and pit depth of metals occurring during corrosion process. Both of these researches proved that EET is a bottleneck for electron transfer in MIC on macro level. Huang et al. [41] went deeper in this area and firstly confirmed that EET is a bottleneck in MIC by Pseudomonas aeruginosa at genetic level. They found that phzH gene in Pseudomonas aeruginosa encodes the enzyme to produce a kind of electron transfer mediator molecules to regulate EET of 2205 DSS.
2.2. Marine bio-fouling
Marine bio-fouling comes from the undesirable settlement and accumulation of marine microorganisms, plants and animals on submerged surfaces of materials and it has huge adverse influence on the infrastructure and equipment served in marine industries [[42], [43], [44], [45]]. Marine bio-fouling increases the weight and roughness of ship hulls which increases the frictional resistance and then causes additional fuel consumption. It also initiates or accelerates the corrosion of metal and concrete structures, raising the dangerous of failure of marine equipment and facilities [46]. Bio-fouling will attach on seawater pipelines used in near-sea industries and net cages used in aquaculture industry which will decrease the efficiency of equipment and the production of aquatic products respectively [47,48]. Additional, bio-fouling organisms on ship vessels will migrate to different oceans where they are not naturally belong to and disturb the ecological system [49]. Therefore, marine bio-fouling is a serious issue need to be prevented and solved for both marine economy and marine environment.
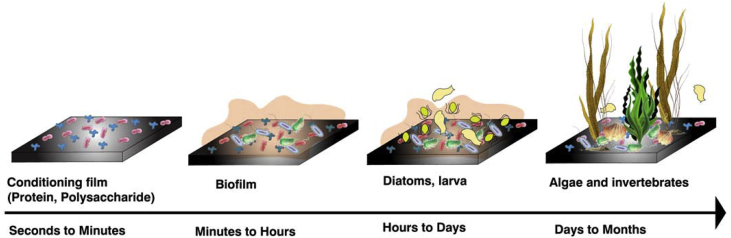
Previous researches have come up with a general consideration about the formation and growth of marine bio-fouling which is related to biofilm as shown in Fig. 4 including the following steps [50]: (1) an absorbed film quickly forms on the submerged surface due to adsorption of proteins, glycoproteins and polysaccharides; (2) bacteria and other microorganisms adhere on the absorbed film and gradually develop into a biofilm by secreting extracellular polymeric substances (EPS) consisting of proteins and polysaccharides to envelope and fix themselves; (3) marine organisms like diatoms, larva and microalgae spores accumulate on the surfaces of materials because biofilm can provide nutrients for them; (4) larvae of marine macro-organisms such as barnacles settle and grow on the surfaces of materials as macro-foulers. This common formation process for most bio-fouling organisms illustrates the relationship between microorganisms and macro-foulers like mussels and barnacles. Macro-foulers are major components and the last results of bio-fouling formation, while microorganisms are the origins of bio-fouling formation because of the proper settle sites and conditions they create, and nutrients they provide to attract new organisms. The activities of microorganisms could regulate the formation of macro-foulers, while the accumulation of macro-foulers could bring some protection for microorganisms and biofilms from being eliminated.
Fig. 4.
The typical growing process of marine bio-fouling [50].
However, this general formation model cannot apply to all marine organisms because there are more than 4000 fouling organisms in the ocean and different marine environments may lead to different dominant fouling organisms and different biological habit. For example, D. Roberts et al. found that the cyprids of barnacle Amphibalanus Amphitrite can settle on the surface of materials without the presence of a biofilm [51].
2.3. Comparison between MIC and marine bio-fouling
Based on the introduction and description of MIC and bio-fouling, some differences and similarities are summarized as below:
The differences between MIC and bio-fouling:
-
1.
MIC is a corrosion process occurring at micro level, while bio-fouling is a settlement and accumulation process occurring at macro level;
-
2.
Organisms related to MIC are only different microorganisms, while organisms related to bio-fouling include different microorganisms, plants and animals;
-
3.
MIC damages the materials directly, while bio-fouling's damage is more wide and complicated at different areas.
The similarities between MIC and bio-fouling include the following points:
-
1.
Both MIC and bio-fouling start from the formation of an absorbed film on the material surface;
-
2.
The formations of MIC and bio-fouling are both closely related to the biofilms created by marine microorganisms;
-
3.
Similar origins of MIC and bio-fouling lead to their similar prevention strategies. If a method could destroy formed biofilms and prevent the formation of new biofilms, it could eliminate or prevent MIC and bio-fouling at the same time.
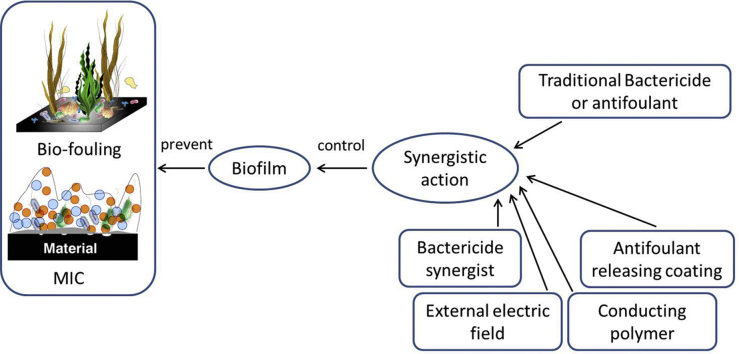
3. Research approaches of marine anti-corrosion and anti-fouling
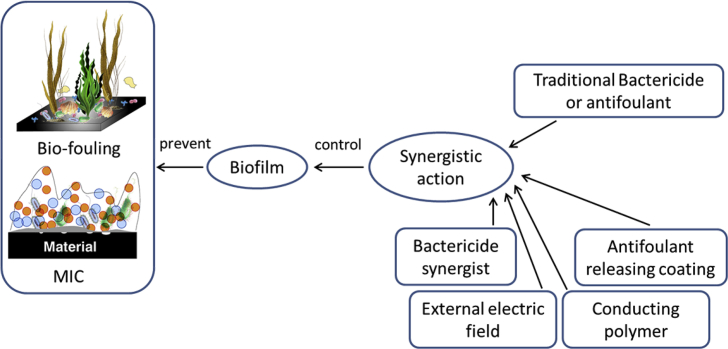
Based on the studies of mechanisms of MIC and marine bio-fouling, it is known that biofilm is the main triggering condition for both of them. For MIC, biofilm is an indispensable key factor which participates into the corrosion process directly according to the newest BCSR theory. For marine bio-fouling, biofilm is the main factor to attract most kinds of fouling organisms to settle and grow on material surface. As a result, to prevent and eliminate MIC or marine bio-fouling on the surface of materials served in marine environment, it is important to control the activity of microorganisms in biofilms or to prevent the adhesion of marine organisms and the formation of biofilms. Nowadays, the mostly applied method in marine industry is to use chemical bactericide or antifoulant by their toxic effects to kill the marine organisms. However, the application of a large number of these chemical reagents will definitely cause secondary pollution to the environment [[52], [53], [54]] and the long time use will also make microorganisms have resistance to these chemicals. Once the microorganism has established an environment suitable for its growth, it is difficult to eliminate it completely by the same chemical reagent [55]. To solve this problem, besides inventing new eco-friendly bactericide and antifoulant, the most popular tendency now is that both chemical bactericide and antifoulant are coupled with some new materials, chemicals and technologies through synergistic action to reduce their dosage and achieve better bactericidal effect at the same time. Fig. 5 is a schematic showing this concept of the article and the following parts of this article summarized four new strategies in line with this concept.
Fig. 5.
Schematic of the concept of controlling biofilm to prevent MIC and bio-fouling through the synergistic action between traditional chemicals and some new materials or technologies.
3.1. Bactericide synergist
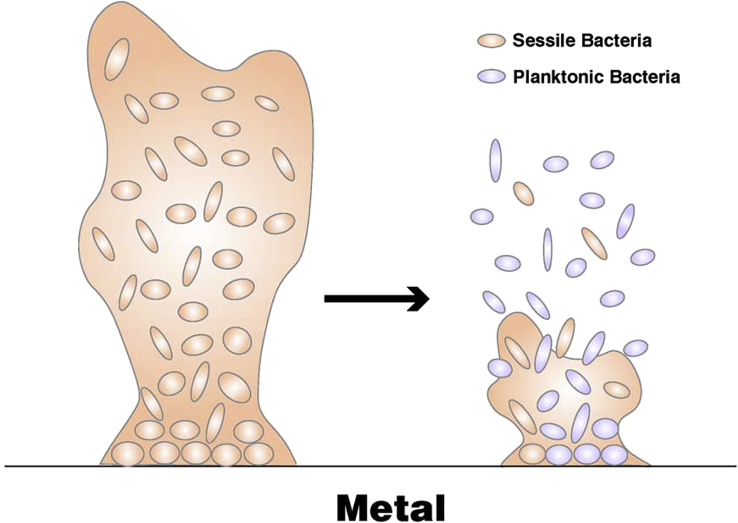
In the area of controlling and preventing MIC caused by SRB and IOB, the bactericide synergist is one of the most common applied chemical agents which can disperse or destroy biofilms to change sessile bacteria into planktonic bacteria and then, the bactericides can reach and kill these bacteria more easily [56,57]. Fig. 6 shows this process schematically.
Fig. 6.
Schematic showing that bactericide synergist could change sessile bacteria in biofilm into planktonic bacteria by dispersing or destroying the biofilm.
Xu D K et al. [58] and Xu H et al. [59] studied the influence of d-amino acid on SRB biofilms and found that although d-Amino acid will not affect the normal metabolic activities, it can obviously decrease the production of EPS in biofilm and the adhesion amount of SRB on the surface of materials which make d-Amino acid a suitable synergist to control SRB biofilms. Xu et al. [60] also found that d-Methionine can enhance the dispersion of biofilm and then the sessile SRB cell body can be transformed into planktonic state which will have proper synergistic effect with bactericide. d-Tyrosine [61] is also considered that it can promote the migration of SRB biofilm together with bactericide to improve the bactericidal effect. These researches on single d-Amino acid found that one kind of acid can only affect limited microorganisms. In order to deal with the complicated biofilms produced by different SRB, Xu et al. [58] also made a d-Amino acid mixture with equimolar of 4 different acids and studied its synergist effect. The results demonstrated that a d-Amino acid mixture can achieve the best synergist effect by cooperating with chelators and bactericides. According to the mechanism of biofilm causing MIC, Xia et al. [26] start to pay attention to the metal material itself and firstly invented a novel Cu-bearing 2205 duplex stainless steel based on the biofilm destroying ability of Cu. This new kind of stainless steel has better antibacterial efficiency than the traditional duplex stainless steel which is 7.75% and 96.92% after one day and seven days, respectively. The experimental results from this work suggest that the copper ions released from 2205-Cu-DSS can inhibit the biofilm and then mitigate MIC.
3.2. Antifoulant releasing coatings with self-polishing copolymers and degradable polymers
For most marine bio-fouling situations, antifoulant is mostly applied to control the activity of microorganisms in biofilms or prevent adhesion of microorganisms and formation of biofilms just like the application of chemical bactericide to prevent MIC. Generally, the antifoulant is carried by different polymer binders to form the so-called antifoulant releasing coatings applied in marine industry, especially on ships. As a result, these polymer binders are virtually the synergists for antifoulant and their performance in marine environment mainly determines the effects of antifoulant. The most popular binder used now is self-polishing copolymers (SPC) [62]. In SPC, the silyl/copper/zinc ester side groups can undergo hydrolysis to generate a hydrophilic surface, which is then polished away by water flow to wash out the bio-fouling adhered and at the same time, the carried antifoulants are released alongside to control the activity of microorganisms in biofilms [63,64]. However, their surface renewal is not quick enough without strong water flow, so most SPC coatings have poor resistance to marine bio-fouling organisms under static conditions. Moreover, slow surface erosion and continuous water absorption may cause the swelling of coatings, which negatively impacts the mechanical and antifouling performance. Several studies have been done to improve the performance of SPC binders to release the antifoulant more stably and efficiently. Bressy et al. [[65], [66], [67], [68], [69]] have developed diblock copolymers of tert-butyldimethylsilyl methacrylate (TBDMSiMA) and methyl methacrylate (MMA) with controlled microstructures by reversible addition-fragmentation chain transfer (RAFT) polymerization for antifouling, which demonstrate better controlled erosion rate and antifouling efficiency than the statistical copolymer coatings [66].
Zhang et al. [62] firstly proposed a concept called Dynamic Surface Antifouling (DSA), where the dynamic surface of coating refers to a changing surface that continuously renews itself in marine environment and thus decreases the adhesion of bio-fouling and improves the antifoulant release as well under both dynamic and static conditions. Based on this innovative concept, Zhang et al. [62] have developed a series of degradable polymer with dynamic surfaces which have tunable renewability, and excellent antifouling and mechanical performance. Degradable polyester based polyurethanes are one kind of these polymers. Ma et al. [70] found that the polyurethane based on a copolymer of caprolactone (CL) and glycolide (GA) demonstrates high degradation rates and the rates increase with GA content. The immersion test in seawater for 3 months for the polyurethane with 10 mol% GA in the soft segment showed best antifouling performance without any antifoulant which indicates that the degradable polymer itself is effective in antifouling. Zhang et al. [62] also summarized that degradable polymer is a better binder for antifoulants than traditional SPC because its constant surface renewal rate in seawater can release out the antifoulant in a more stable manner which can extend the service life of coatings and reduce the pollution for environment. So the cooperation between efficient antifoulant and degradable polymer binder with dynamic surface could be a potential method to prevent marine bio-fouling.
3.3. External electric field
As summarized above, both MIC and marine bio-fouling are mainly caused by the adhesion and growing of marine microorganisms on the material surfaces, and the formation of biofilms play an important role in inducing these phenomena. Previous studies also found that it is difficult to eradicate biofilms from the material surfaces and the concentrations of bactericides required to kill sessile bacteria growing in the biofilms are much higher than those needed for bacteria in the planktonic state [[71], [72], [73]]. Some early researches have tried to explain this mechanism of biofilms. J. W. Costerton [74] firstly considered that EPS metabolized by microorganisms is responsible for binding bactericides before they reach the target cells because exopolysaccharide contained in EPS is charged and has inherent ion exchange properties. Based on this hypothesis, Sandra A. Blenkinsopp et al. [75] thought that disrupting those charges on EPS could sufficiently allow penetration of bactericides to the target cells by electrifying the system. They also did some initial researches and found that three common industrial bactericides can have enhanced effects against P. aeruginosa biofilms within a low-strength electric field with a low current density. The nature of the mechanisms has not been confirmed but could be the influences of electroporation, electrophoresis, and iontophoresis et al. J. Liu et al. [76] also explored the synergistic effect of electric field and bactericide against SRB biofilms. Their results indicated that extra electric field barely influenced the formation of biofilms but damaged the structure of formed biofilms and helped mass transfer of bactericides through biofilms and desorption of calcium and magnesium ions from biofilms which could enhance the damage effects from electric field. All these mechanisms of external electric field lead to its efficient synergistic effect with bactericides.
Based on the positive effects of external electric field in anti-biofilm, some electro-active materials which can create micro electric field by itself like piezoelectric materials can be considered as potential materials to prevent MIC and bio-fouling. The ability of killing microorganisms from piezoelectric ceramics due to the micro electric field has been proved by some researches and the mechanisms have also been discussed in the biomedical materials area [[77], [78], [79], [80]]. As a result, applying piezoelectric materials in marine anti-corrosion and anti-fouling area could be a new choice for further exploration.
3.4. Conducting polymers
Due to the electrical activity of biofilms on the material surfaces in marine environment, conducting polymers are considered to be potential materials to prevent marine corrosion and bio-fouling because of their special conducting characteristic. Actually, the studies of anti-corrosion of conducting polymer have started since Deberry et al. [81,82] firstly found that electrodeposited polyaniline film on stainless steel can significantly reduce its corrosion rate in sulfuric acid solution. A lot of researchers have explored the corrosion resistance of different conducting polymers like polyaniline, polypyrrole, polythiophene and their derivatives [[83], [84], [85], [86], [87], [88]]. Most of these previous studies focused on the traditional metal corrosion resistance mechanisms like passivation layer formation on metal surface, metal corrosion potential increase and corrosion rate reduction. Recently, more researchers have started to pay attention on the abilities of preventing MIC and marine bio-fouling of conducting polymers. Some concepts and studies have been proposed including (1) Adjusting the pH value of the conducting polymer coating to stabilize it in the acidic range in order to prevent the adhesion and grow of microorganisms and adhesive marine organisms which are suitable for the alkaline seawater on material surface [89]; (2) The conducting polymers can be set as anode and the parts of metals which contact the seawater are set as cathode. When a weak current is applied between these two electrodes, the surface seawater will be electrolyzed into sodium hypochlorite and then forms an ionic membrane which can damage the cell tissues of organisms. Also the concentration of sodium hypochlorite in seawater is low enough and will not pollute the environment [89,90]; (3) Apply conducting polymers with conductivity higher than S/cm as the base matrix of coating without any current [91]. X. H. Wang et al. [92] did these experiments with conducting polyaniline and found that the polymer has special marine antifouling ability without any current applied and it also has good synergetic effects with some kinds of bactericides like cuprous oxide or dichlorodiphenyltrichloroethane. A recently study [93] also found that the conducting polymer polypyrrole can respond to electric signal and then change the hydrophobicity of its surface which indicates that polypyrrole could construct an electric controlled amphiphilic surface in order to prevent the adhesion of marine microorganisms. These previous studies have initially illustrated that conducting polymers could be potential marine anti-corrosion and anti-fouling materials. However their mechanisms are not clear enough and deeper researches should be done in the future.
4. Conclusions
Microbiological corrosion and bio-fouling of materials are two main reasons of marine corrosion to cause the damage and failure of equipment and structures served in marine environment. The microbiologically influenced corrosion (MIC) is caused by SRB, IOB and their biofilms. The new BCSR and EET theories explaining MIC mechanisms are discussed. The formation of bio-fouling is also closely related to the biofilms. As a result, the strategies of preventing MIC and bio-fouling mainly focus on controlling the activity of microorganisms in biofilms, the adhesion of organisms and the formation of biofilms. New materials or technologies are cooperated with traditional bactericide or antifoulant through synergistic action in order to reduce their dosage and achieve better bactericidal effect at the same time.
Four aspects of research approaches of methods based on this concept were summarized including: (1) Bactericide synergist which could disperse biofilms was developed to enhance the bactericidal effect of traditional bactericide. (2) The performance of antifoulant releasing coatings has been improved due to the modified self-polishing copolymers and the new invented degradable polymers. (3) External electric field was applied as a synergistic method with bactericides to damage biofilms and prevent corrosion and bio-fouling. (4) Conducting polymers were introduced to prevent corrosion and bio-fouling due to their conducting characteristics.
The development tendency of preventing marine MIC and bio-fouling in the future is to find methods and materials which have highly efficiency, long service life, easy implement process, low cost and environment friendly application under the complicated marine environment. The research approaches introduced in this article brought some new angles, but they may not match all of these requirements by themselves. As a result, the integration and synergistic action of different materials and technologies to avoid the weaknesses of each other will be a very important research direction in marine anti-corrosion and anti-fouling in the future.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC1105304), the National Natural Science Foundation of China (Grant Nos. 51702106), the Natural Science Foundation of Guangdong Province (2016A030308014) and China Postdoctoral Science Foundation (Grant Nos. 2017M622686, 2018T110865).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Wei B.M. Chemical Industry Press; Beijing: 1984. Theory and Application of Metal Corrosion. [Google Scholar]
- 2.Melchers R.E. Microbiological and abiotic processes in modelling longer-term marine corrosion of steel. Bioelectrochemistry. 2014;97:89. doi: 10.1016/j.bioelechem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C.Z., Wei J. Latest research progress of marine anticorrosion coatings. Corros. Sci. Prot. Technol. 2016;3 [Google Scholar]
- 4.Wang L.M. Chemical Industry Press; Beijing: 2008. Marine Materials. [Google Scholar]
- 5.Hou B.R., Li X.G., Ma X.M. The cost of corrosion in China. NPJ Mater. Degradat. 2017;(1):4. [Google Scholar]
- 6.Wang P.G. Chemical Industry Press; Beijing: 2006. Marine Coatings and Coating Technology. [Google Scholar]
- 7.Liu H.W., Gu T.Y., Asif M. The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria. Corros. Sci. 2017;114:102. [Google Scholar]
- 8.Chen B., Qin S., Chen L. Influence of static magnetic field on formation of SRB biofilm. Corros. Sci. Prot. Technol. 2014;26:499. [Google Scholar]
- 9.Castaneda H., Benetton X.D. SRB-biofilm influence in active corrosion sites formed at the steel-electrolyte interface when exposed to artificial seawater conditions. Corros. Sci. 2008;50:1169. [Google Scholar]
- 10.Lin J. Harbin Engineering University; Harbin: 2006. Initial Corrosion Behavior under Biofilms of Metal Material in Sea Water. [Google Scholar]
- 11.Videla H.A., Herrera L.K. Microbiologically influenced corrosion: looking to the future. Int. Microbiol. 2005;8:169–180. [PubMed] [Google Scholar]
- 12.Enning D., Garrelfs J. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl. Environ. Microbiol. 2014;80:1226–1236. doi: 10.1128/AEM.02848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidelberg J.F. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat. Biotechnol. 2004;22:554–559. doi: 10.1038/nbt959. [DOI] [PubMed] [Google Scholar]
- 14.Chamritski I.G., Burns G.R., Webster B.J., Laycock N.J. Effect of iron-oxidizing bacteria on pitting of stainless steel. Corrosion. 2004;60:658–669. [Google Scholar]
- 15.Hamilton W.A. Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling. 2003;19(1):65. doi: 10.1080/0892701021000041078. [DOI] [PubMed] [Google Scholar]
- 16.Xu C.M., Zhang Y.H., Cheng G.X. Localized corrosion behavior of 316L stainless steel in the presence of sulfate- reducing and iron-oxidizing bacteria. Mater. Sci. Eng. 2007;A443(1/2):235. [Google Scholar]
- 17.Wu J.Y., Chai K., Xiao W.L. The single factor of microorganism corrosion on steel in seawater. J. Met. 2010;46(6):755. [Google Scholar]
- 18.Dong Z.H., Liu T., Liu H.F. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling. 2011;27:487–495. doi: 10.1080/08927014.2011.584369. [DOI] [PubMed] [Google Scholar]
- 19.Belkaid S., Ladjouzi M.A., Hamdani S. Effect of biofilm on naval steel corrosion in natural seawater. J. Solid State Electrochem. 2011;15:525–537. [Google Scholar]
- 20.Liu T., Liu H., Hu Y., Zhou L., Zheng B. Growth characteristics of thermophile sulfate-reducing bacteria and its effect on carbon steel. Mater. Corros. 2009;60:218–224. [Google Scholar]
- 21.Jones D., Amy P. A thermodynamic interpretation of microbiologically influenced corrosion. Corrosion. 2002;58:638–645. [Google Scholar]
- 22.Rao T., Sairam T., Viswanathan B., Nair K. Carbon steel corrosion by iron oxidising and sulphate reducing bacteria in a freshwater cooling system. Corros. Sci. 2000;42:1417–1431. [Google Scholar]
- 23.Yuan S., Liang B., Zhao Y., Pehkonen S. Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria. Corros. Sci. 2013;74:353–366. [Google Scholar]
- 24.Liu H.W., Xu D.K., Wu Y.N. Research progress in corrosion of steels induced by sulfate reducing bacteria. Corros. Sci. Prot. Technol. 2015;27:409. [Google Scholar]
- 25.Lenhart T.R., Duncan K.E., Beech I.B. Identification and characterization of microbial biofilm communities associated with corroded oil pipeline surfaces. Biofouling. 2014;30:823. doi: 10.1080/08927014.2014.931379. [DOI] [PubMed] [Google Scholar]
- 26.Xia J., Yang C.G., Xu D.K. Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine Pseudomonas aeruginosa biofilm. Biofouling. 2015;31:481. doi: 10.1080/08927014.2015.1062089. [DOI] [PubMed] [Google Scholar]
- 27.Xu D.K., Li Y.C., Song F.M. Laboratory investigation of microbiologically influenced corrosion (MIC) of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci. 2013;77:385. [Google Scholar]
- 28.Zhang Y.J., Li S., Xu P. Effect of NaClO addition on bacteriostasis of iron bacteria and corrosion control of pipe line steel for water distribution network in urban reclaimed water. Corros. Sci. Prot. Technol. 2015;27:165. [Google Scholar]
- 29.Jia R., Unsal T., Xu D.K., Lekbach Y., Gu T.Y. Microbiologically influenced corrosion and current mitigation strategies: a state of the art review. Int. Biodeterior. Biodegrad. 2019;137:42–58. [Google Scholar]
- 30.Skovhus T.L., Eckert R.B., Rodrigues E. Management and control of microbiologically influenced corrosion (MIC) in the oil and gas industry-overview and a North Sea case study. Biotechnology. 2017;256:31–45. doi: 10.1016/j.jbiotec.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Gu T., Zhao K., Nesic S. NACE International; Atlanta, Georgia: 2009. Corrosion/2009. Paper No. 09390. [Google Scholar]
- 32.Sherar B.W.A., Power I.M., Keech P.G. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 2011;53(3):955. [Google Scholar]
- 33.Zhang P., Xu D.K., Li Y. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry. 2015;101:14. doi: 10.1016/j.bioelechem.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Xu D.K., Gu T.Y. Carbon source starvation triggered more aggressive corrosion against carbon steel by the desulfovibrio vulgaris biofilm. Int. Biodeterior. Biodegrad. 2014;91:74. [Google Scholar]
- 35.Xu D.K., Gu T.Y. NACE Corrosion 2011[C]; Houston: 2011. Bioenergetics Explains when and Why More Severe Mic Pitting by SRB Can Occur; p. 11426. [Google Scholar]
- 36.Li Y.C., Xu D.K., Chen C.F., Li X.G., Jia R., Zhang D.W., Sand W., Wang F.H., Gu T.Y. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: a review. J. Mater. Sci. Technol. 2018;34:1713–1718. [Google Scholar]
- 37.Gu T.Y., Jia R., Unsal T., Xu D.K. Toward a better understanding of microbiologically influenced corrosion cause by sulfate reducing bacteria. J. Mater. Sci. Technol. April 2019;35(4):631–636. doi: 10.1016/j.jmst.2018.10.026. [DOI] [Google Scholar]
- 38.Hernandez M.E., Newman D.K. Extracellular electron transfer. Cell. Mol. Life Sci. 2001;58:1562–1571. doi: 10.1007/PL00000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du Z., Li H., Gu T. A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007;25:464–482. doi: 10.1016/j.biotechadv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Li H.B., Xu D.K., Li Y.C., Feng H., Liu Z.Y., Li X.G. Extracellular electron transfer is a bottleneck in the microbiologically influenced corrosion of C1018 carbon steel by the biofilm of sulfate-reducing bacterium desulfovibrio vulgaris. PLoS One. 2015;10(8):e0136183. doi: 10.1371/journal.pone.0136183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y., Zhou E.Z., Jiang C.Y., Jia R., Liu S.J., Xu D.K., Gu T.Y., Wang F.H. Endogenous phenazine-1-carboxamide encoding gene PhzH regulated the extracellular electron transfer in biocorrosion of stainless steel by marine Pseudomonas aeruginosa. Electrochem. Commun. 2018;94:9–13. [Google Scholar]
- 42.Lejars M., Margaillan A., Bressy C. Fouling release coatings: a nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012;112:4347–4390. doi: 10.1021/cr200350v. [DOI] [PubMed] [Google Scholar]
- 43.Selim M.S., Shenashen M.A., El-Safty S.A., Higazy S.A., Selim M.M., Isago H., Elmarakbi A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017;87:1–32. [Google Scholar]
- 44.Grozea C.M., Walker G.C. Approaches in designing non-toxic polymer surfaces to deter marine biofouling. Soft Matter. 2009;5:4088–4100. [Google Scholar]
- 45.Lindholdt A., Dam-Johansen K., Olsen S.M., Yebra D.M., Kiil S. Effects of biofouling development on drag forces of hull coatings for ocean-going ships: a review. J. Coat. Technol. Res. 2015;12:415–444. [Google Scholar]
- 46.Blackwood D.J., Lim C.S., Teo S.L.M., Hu X., Pang J. Macrofouling induced localized corrosion of stainless steel in Singapore seawater. Corros. Sci. 2017;129:152–160. [Google Scholar]
- 47.Rao T.S., Kora A.J., Chandramohan P., Panigrahi B.S., Narasimhan S.V. Biofouling and microbial corrosion problem in the thermo-fluid heat exchanger and cooling water system of a nuclear test reactor. Biofouling. 2009;25:581–591. doi: 10.1080/08927010903016543. [DOI] [PubMed] [Google Scholar]
- 48.Fitridge I., Dempster T., Guenther J., De Nys R. The impact and control of biofouling in marine aquaculture: a review. Biofouling. 2012;28:649–669. doi: 10.1080/08927014.2012.700478. [DOI] [PubMed] [Google Scholar]
- 49.Piola R.F., Dafforn K.A., Johnston E.L. The influence of antifouling practices on marine invasions. Biofouling. 2009;25:633–644. doi: 10.1080/08927010903063065. [DOI] [PubMed] [Google Scholar]
- 50.Yebra D.M., Kiil S., Dam-Johansen K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coating. 2004;50:75–104. [Google Scholar]
- 51.Roberts D., Rittschof D., Holm E., Schmidt A.R. Factors influencing initial larval settlement: temporal, spatial and surface molecular components. J. Exp. Mar. Biol. Ecol. 1991;150:203–221. [Google Scholar]
- 52.Liu H.F., Huang L. Application status and research progress of bactericide of sulfate reducing bacterial. J. Chin. Soc. Corros. Prot. 2009;29(2):154. [Google Scholar]
- 53.Liu H.F., Yang H.X. Study on the synthesis and antibacterial and anticorrosive properties of environment friendly bromine bactericide. Mater. Prot. 2008;41(7):18. [Google Scholar]
- 54.Xu D.K., Wen J., Gu T.Y. Biocide cocktail consisting of glutaraldehyde, ethylene diamine disuccinate (Edds), and methanol for the mitigation of souring and biocorrosion. Corrosion. 2012;68(11):994. [Google Scholar]
- 55.Liu H.F., Xu L.M., Zheng J.S. New bactericide for biocide-resistant sulfate-reducing bacteria. Mater. Perform. 2000;39(4):52. [Google Scholar]
- 56.Schultz T.P., Nicholas D.D. Development of environmentally-benign wood preservatives based on the combination of organic biocides with antioxidants and metal chelators. Phytochemistry. 2002;61(5):555. doi: 10.1016/s0031-9422(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 57.Wen J., Zhao K., Gu T.Y. A green biocide enhancer for the treatment of sulfate- reducing bacteria (Srb) biofilms on carbon steel surfaces using glutaraldehyde. Int. Biodeterior. Biodegrad. 2009;63(8):1102. [Google Scholar]
- 58.Xu D.K., Wen J., Fu W. D-amino acids for the enhancement of a binary biocide cocktail consisting of Thps and Edds against an Srb biofilm. World J. Microbiol. Biotechnol. 2012;28(4):1641. doi: 10.1007/s11274-011-0970-5. [DOI] [PubMed] [Google Scholar]
- 59.Xu H.J., Liu Y. D-amino acid mitigated membrane biofouling and promoted biofilm detachment. J. Membr. Sci. 2011;376(12):266. [Google Scholar]
- 60.Xu D.K., Li Y., Gu T.Y. D-methionine as a biofilm dispersal signaling molecule enhanced tetrakis hydroxymethyl phosphonium sulfate mitigation of desulfovibrio vulgaris biofilm and biocorrosion pitting. Mater. Corros. 2014;65:837. [Google Scholar]
- 61.Xu D.K., Li Y., Gu T.Y. A synergistic D-tyrosine and tetrakis hydroxymethyl phosphonium sulfate biocide combination for the mitigation of an Srb biofilm. World J. Microbiol. Biotechnol. 2012;28(10):3067. doi: 10.1007/s11274-012-1116-0. [DOI] [PubMed] [Google Scholar]
- 62.Xie Q.Y., Pan J.S., Ma C.F., Zhang G.Z. Dynamic surface antifouling: mechanism and systems. Soft Matter. 2018 doi: 10.1039/c8sm01853g. [DOI] [PubMed] [Google Scholar]
- 63.Omae I. General aspects of tin-free antifouling paints. Chem. Rev. 2003;103:3431–3448. doi: 10.1021/cr030669z. [DOI] [PubMed] [Google Scholar]
- 64.Bressy C., Nguyen M.N., Tanguy B., Ngo V.G., Margaillan A. Poly(trialkylsilyl methacrylate)s: a family of hydrolysable polymers with tuneable erosion profiles. Polym. Degrad. Stabil. 2010;95:1260–1268. [Google Scholar]
- 65.Nguyen M.N., Bressy C., Margaillan A., Polym J. Controlled radical polymerization of a trialkylsilyl methacrylate by reversible addition-fragmentation chain transfer polymerization. Sci., Part A: Polym. Chem. 2005;43:5680–5689. [Google Scholar]
- 66.Nguyen M.N., Bressy C., Margaillan A. Synthesis of novel random and block copolymers of tert-butyldimethylsilyl methacrylate and methyl methacrylate by RAFT polymerization. Polymer. 2009;50:3086–3094. [Google Scholar]
- 67.Ngo V.G., Bressy C., Leroux C., Margaillan A. Synthesis of hybrid TiO2 nanoparticles with well-defined poly(methylmethacrylate) and poly(tert-butyldimethylsilyl methacrylate) via the RAFT process. Polymer. 2009;50:3095–3102. [Google Scholar]
- 68.Lejars M., Margaillan A., Bressy C. Well-defined graft copolymers of tert-butyldimethylsilyl methacrylate and poly(dimethylsiloxane) macromonomers synthesized by RAFT polymerization. Polym. Chem. 2013;4:3282. [Google Scholar]
- 69.Lejars M., Margaillan A., Bressy C. Synthesis and characterization of diblock and statistical copolymers based on hydrolysable siloxy silylester methacrylate monomers. Polym. Chem. 2014;5:2109–2117. [Google Scholar]
- 70.Ma C.F., Xu L.G., Xu W.T., Zhang G.Z. Degradable polyurethane for marine anti-biofouling. J. Mater. Chem. B. 2013;1:3099. doi: 10.1039/c3tb20454e. [DOI] [PubMed] [Google Scholar]
- 71.Liu H.F. Study on drug resistance of sulfate reducing bacteria. Microbiology China. 1997;24(6):334. [Google Scholar]
- 72.LeChevallier M.W., Cawthon C.D., Lee R.G. Inactivation of biofilm bacteria. Appl. Environ. Microbiol. 1988;54:2492–2499. doi: 10.1128/aem.54.10.2492-2499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wright J.B., Ruseska I., Athar M.A., Corbett S., Costerton J.W. Legionella pneumophila grows adherent to surfaces in vitro and in situ. Infect. Control Hosp. Epidemiol. 1989;10:408–415. doi: 10.1086/646062. [DOI] [PubMed] [Google Scholar]
- 74.Costerton J.W. The role of bacterial exopolysaccharides in nature and disease. Dev. Ind. Microbiol. 1985;26:249–261. [Google Scholar]
- 75.Blenkinsopp S.A., Costerton J.W. Electrical enhancement of biocide efficacy against pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 1992;58(11):3770. doi: 10.1128/aem.58.11.3770-3773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J., Zheng J.S., Xu L.M. Synergistic effect of electric field and bactericide against sulfate- reducing bacteria biofilms. Corros. Sci. Prot. Technol. 2002;14(1):23–26. [Google Scholar]
- 77.Yao T.T., Chen J.Q. The antibacterial effect of potassium-sodium niobate ceramics based on controlling piezoelectric properties. Colloids Surfaces B Biointerfaces. 2019;175:463–468. doi: 10.1016/j.colsurfb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 78.Tan G.X., Wang S.Y. Surface-selective preferential production of reactive oxygen species on piezoelectric ceramics for bacterial killing. Appl. Mater. Interfaces. 2016;8:24306–24309. doi: 10.1021/acsami.6b07440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X.Q., Tian X.Z., Shin I., Yoon J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2011;40(9):4783–4804. doi: 10.1039/c1cs15037e. [DOI] [PubMed] [Google Scholar]
- 80.Murphy M.P., Holmgren A. Unraveling the biological roles of reactive oxygen species. Cell Metabol. 2011;13(4):361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Debrry D.W. Corrosion behavior of polyaniline-modified stainlesssteels. J. Electrochem. Soc. 1984;131(8):302. [Google Scholar]
- 82.Debrry D.W. Modification of the electrochemical and corrosion behavior of stainless- steels with an electroactive coating. J. Electrochem. Soc. 1985;132:1022. [Google Scholar]
- 83.Armelin E., Oliver R., Liesa F. Marine paint fomulations: conducting polymers as anticorrosive additives. Prog. Org. Coating. 2007;59:46. [Google Scholar]
- 84.Ocampo C., Armelin E., Liesa F. Application of a polythiophene derivative as anticorrosive additive for paints. Prog. Org. Coating. 2005;53:217. [Google Scholar]
- 85.Wessling Bernhard, Posdorfer Joerg. Corrosion prevention with all organic metal(polyaniline) corrosion test results. Electrochim. Acta. 1999;44:2139–2140. [Google Scholar]
- 86.Zhong L., Xiao S.H. Application of polyaniline to galvanic anodie protection on stainless steel in H2S04 solutions. Corros. Sci. 2006;48:3960–3968. [Google Scholar]
- 87.Ocdn P., Cristobal A.B., Herrasti P., Fatas E. Corrosion performance of conducting polymer coatings applied on mild steel. Corros. Sci. 2005;47:649–660. [Google Scholar]
- 88.Tuken T., Yazici B., Erbil M. The use of polythiophene for mild steel protection. Prog. Org. Coating. 2004;51:205–212. [Google Scholar]
- 89.Zheng H., Ye Y. The application of conducting polyaniline in corrosion prevention and antifouling of marine equipment. Corrosion Science and Protection Technology. Sept. 2013;25(5):429–432. [Google Scholar]
- 90.Xie Z.P., Wang J.J., Huang C.S., Ye Z.J. The development of the non-toxic antifouling technology. Material Development and Application. Feb. 2011:85–88. [Google Scholar]
- 91.Zhou C.L. History, current situation and future of antifouling coatings for ships. Chinese Coatings. 1998;(6):9–10. [Google Scholar]
- 92.Wang X.H. Polyaniline as marine antifouling and corrosion- prevention agent. Syntetie Metal. 1999;102:1377–1380. [Google Scholar]
- 93.Zhou Z.N., Li W.P. Controllable protein adsorption and bacterial adhesion on polypyrrole nanocone arrays. J. Mater. Sci. Technol. 2016;32:950–955. [Google Scholar]