Abstract
Cancer is one of the major non-communicable diseases posing substantial challenges in both developing and developed countries. The options available for treatment of different cancer are associated with various limitations, including severe toxicity, drug resistance, poor outcomes and a high risk of relapse. Hence, an increased attention and necessity for screening of various phytochemicals from natural sources for superior and safer alternative has been ongoing for several decades. In recent years, phytochemicals like galantamine, erwinaze, rivastigmine, resveratrol from natural sources have been found to be important therapeutic targets for the treatment of various diseases including cancer, neurodegeneration, diabetes, and cardiovascular effects. Acorus calamus (Sweet flag), and/or its bioactive phytochemical alpha (α)-and beta (β)-asarone, is a well-known drug in the traditional system of medicine which possesses anti-tumor and chemo-preventive activities as evident from numerous pre-clinical studies both in-vitro and in-vivo. In this article, we critically review the current available scientific evidences of A. calamus and/or asarone for cancer chemoprevention based on preclinical in-vitro and in-vivo models. In addition, we also have compiled and discussed the molecular targets of mechanism(s) involved in the anti-cancer activity of A. calamus/asarone. Still, extensive in-vivo studies are necessary using various animal models to understand the molecular mechanism behind the pharmacological activity of the bioactive phytochemicals derived from A. calamus. It is strongly believed that the comprehensive evidence presented in this article could deliver a possible source for researchers to conduct future studies pertaining to A. calamus and/or its bioactive phytochemicals asarone for cancer chemoprevention.
Keywords: Evidence-based medicine, Oncology, Biochemistry, Cancer research, Cell biology, Molecular biology
1. Introduction
The cancer is a major non-communicable disease posing substantial social and economical challenges in both developing and developed countries. The rapid rise in cancer mortality rates accounting together for China, India and Russia is nearly twofold high compared to the UK and the USA. The number of factors contributed for rapid rise in cancer incidence in the growing economies include changes in lifestyles (food habit, decreased physical activity and sedentary lifestyles), low socio-income populations with minimum cancer care facility, different contaminants of the environment, increase in aged populations, and increase in oncogenic communicable infective organisms [1, 2]. According to International Agency for Research on Cancer GLOBOCAN project, the rise in the cancer burden in India will nearly be double in the next 20 years and is predicted to be more than 1.7 million cases by 2035 [2,3].
In the current scenario, the options available for treatment of different cancer are associated with various limitations, including severe toxicity, drug resistance, poor outcomes and a high risk of relapse. Hence, an increased attention and necessity for screening of various phytochemicals from natural sources for superior and safer alternative has been ongoing for several decades. The chemo-preventive agents available from the different parts of plants are used in the form of alternative and evidence-based complementary system of medicine along with the current chemotherapy and/or radiation therapy [4]. The recent research focus has shifted to the molecular mechanisms of these natural phytoconstituents on various signal transduction mechanisms controlling the cell growth and the cell cycle.
Acorus calamus (L.) (Sweet flag), a member of the family Acoraceae, generally used alone or in combination with other herbs in Indian and Chinese traditional medicine has generated great interest and is found to be beneficial [5]. Acorus gramineus (S.) and Acorus tatarinowii (S.) (Acoraceae), the other plants from Acorus species are officially listed in the Chinese Pharmacopoeia [6, 7]. The plant is widely cultivated in different parts of temperate and sub-temperate regions of the world and is native to India, Sri Lanka, Japan, China, Burma, Mongolia, Southern Russia, Europe and Northern USA [8, 9]. The habitat of the herbaceous perennial plant A. calamus, is semi-aquatic and terrestrial with creeping rhizomes. The rhizomes are bitter in taste, highly branched, pinkish or pale green in color and citrus in odor [8, 10]. In this review, we have summarized and discussed the anti-cancer properties of A. calamus and/or its main bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone) and related mechanisms based on in-vitro and in-vivo experimental evidences.
1.1. Chemical constituents of Acorus calamus
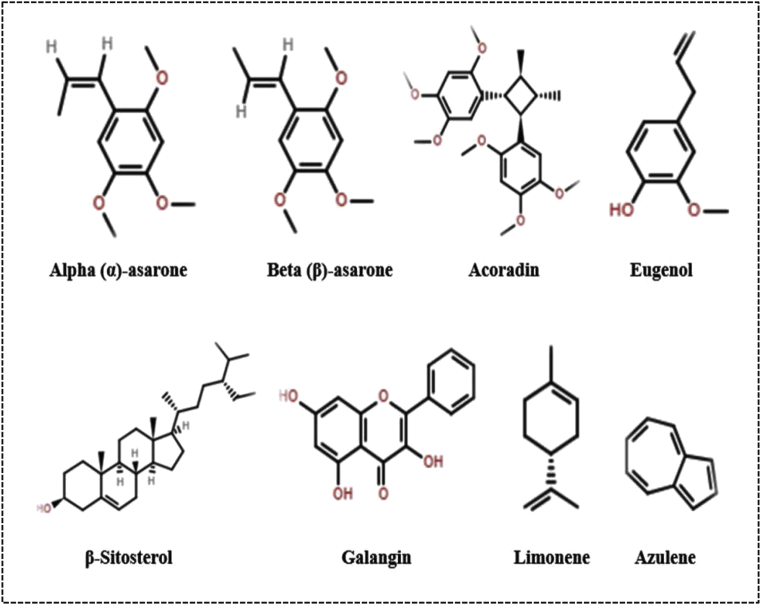
In general, A. calamus consists of various phytoconstituents namely glycosides (xanthone), volatile oil, sesquiterpenes, monoterpenes, flavonoids, steroids, saponins, lignin, tannins, mucilage, alkaloid and polyphenolic compounds [11, 12]. The two main bioactive aromatic constituents isolated from the rhizomes of A. calamus are alpha (α)-asarone (1,2,4-trimethoxy-5-[(E)-prop-1-enyl] benzene) and beta (β)-asarone (1, 2, 4-trimethoxy-5-[(Z)-prop-1-enyl] benzene). Beside these, it also contains the other essential oil such as calamenol, calameon and calamen. The chemical structures of the various constituents of A. calamus are illustrated in Fig. 1 [5,13,14].
Fig. 1.
Chemical structures of various constituents obtained from A. calamus.
1.2. Ethnomedicinal and pharmacological properties of Acorus calamus
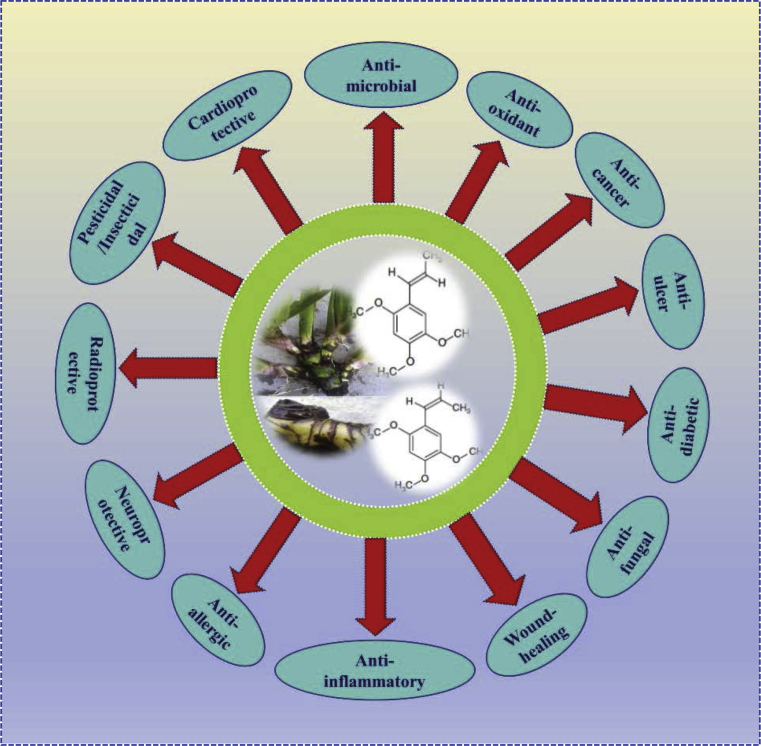
The traditional use of Acorus calamus in Indian Ayurvedic system is widely accepted. The plant has been used to cure several diseases like asthma, fever, cough, epilepsy, hysteria, skin diseases, depression, haemorrhoids, diarrhea, insomnia, dysentery, kidney and liver problems, mental retardation, bronchitis and as a sedative. The external application of the paste of A. calamus on rheumatism, inflamed joints and rheumatic fever improves the pain and swelling in people. The natives of Alberta use A. calamus for prevention of headache, toothache, hangover and as a disinfectant for teeth. Further, in Western herbal medicine, the plant is mainly employed for gastrointestinal related problems like bloating, gas, colic and poor digestive function. In China, it is used by ethnic groups to cure constipation, digestive problems and for decreasing swelling. The plant is also found to reduce the stress-induced suppression of immunity and improves the immunity in experimental rats. The pharmacological studies have established numerous beneficial properties including anti-oxidant, anti-inflammatory, anti-cancer, anti-ulcer, anti-fungal, anti-allergic, anti-diabetic, anti-microbial, wound healing, neuroprotective, radioprotective, pesticidal, insecticidal and cardioprotective effects and others which are depicted in Fig. 2. The plant had a long antiquity and numerous ethnomedicinal, pharmacological and economic applications. Several medicinal practices allow to surpass diverse cultural barriers thereby gaining its widespread usage. Henceforth, considering and incorporating both ecological and socio-economic aspects is important when the demand of the products increased with respect to trade commodities and distant market [10, 11, 15, 16, 17, 18].
Fig. 2.
Schematic representation of various pharmacological activity of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone).
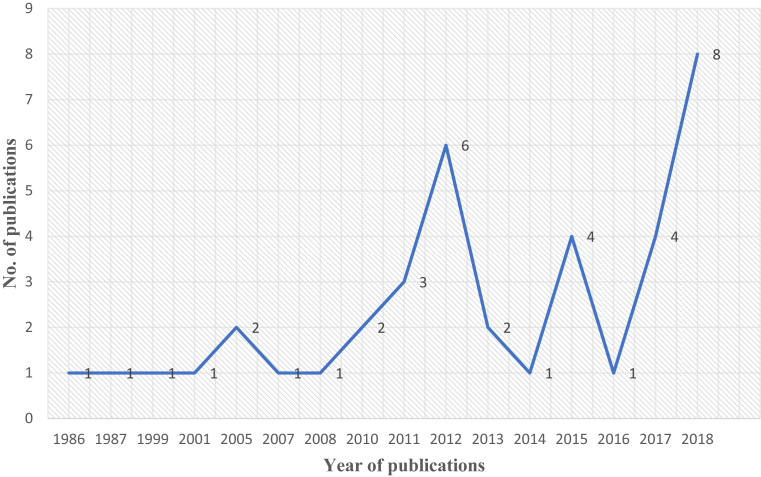
The recent publications indicate the growing interest in the potential of A. calamus and its main constituents as chemo preventive agent/s (Fig. 3). Various experimental investigations of A. calamus and its main constituents with cultured human malignant cell lines and animal models have confirmed antitumor and cancer preventive activities. The subsequent section provides a brief understanding into the anticancer properties of A. calamus and/or its bioactive phytochemicals (α)- and (β)-asarone.
Fig. 3.
Number of publications per year on A. calamus and/or its bioactive phytochemicals asarones. The PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) was searched with the keywords “Acorus calamus and cancer” and “asarone and cancer”.
2. Main text
2.1. In-vitro anti-cancer properties of A. calamus or asarone
2.1.1. Effect of (β)-asarone in human glioblastoma U251 cells
The most common type of primary malignant brain tumor accounts for 82 % cases of malignant gliomas. Glioblastoma is characterized by rapid growth, enhanced angiogenesis and capacity for higher invasion. Although, the current combination therapy has doubled the survival rate in patients with glioblastoma but it remains enormously poor due to severe toxicity, drug resistance and high rates of recurrence [19, 20]. Qi et al. studied the cytotoxic effect of (β)-asarone in human glioblastoma U251 cells. They studied the cell death by fluorescent staining using YO-PRO-1 and propidium iodide (PI), which revealed that the cells treated by (β)-asarone underwent apoptotic and necrotic death. A proteomic based strategy was carried out to characterize the U251 cells treated with or without (β)-asarone to clarify the differential protein targets. In total, seven up-regulated and nine down-regulated proteins were identified by matrix assisted laser desorption/ionization-time of flight (MALDI-TOF/TOF) mass spectrometer. The identified proteins were analyzed by gene ontology (GO) based on cellular components, molecular function and biological processes. Among them, four important proteins namely heterogeneous nuclear ribonucleoprotein H1 (H), isoform CRA_b (hnRNP H1isoform CRA b), heterogeneous nuclear ribonucleo-protein A2/B1, isoform CRA a (hnRNP A2/B1 isoform CRA a), ubiquitin carboxyl-terminal hydrolase isozyme L1 and cathepsin D) were identified to potentially serve as the therapeutic targets. Finally, the changes in the protein alterations were correlated with the mRNA at the transcriptional level by semi-quantitative RT-PCR technique. These results indicated that hnRNP H1 may be involved in HER-2/neu-driven tumor proliferation, invasion and metastasis. Further, authors suggested that the hnRNP A2/B1 could be used as a biomarker for prediction of glioma progression and can act as a novel oncogene in glioblastoma. They concluded that (β)-asarone is effective as anti-tumor agent as it was shown to inhibit the expression of hnRNP H1, hnRNPA2/B1 and cathepsin D against brain tumor [21].
In another study carried by Li et al., the effect and mechanisms of (β)-asarone against tumor invasion and epithelial–mesenchymal transition (EMT) were explored. The inhibitory effects of (β)-asarone on the migration of U251 cells (wound healing assay), suppression of the invasion of U251 cells (Boyden chamber invasion assay) and inhibition of the adhesion of U251 cells (Matrigel) was evaluated. Further the down-regulation of hnRNP A2/B1 oncogenic protein by (β)-asarone supports the study done by Qi et al. In conclusion, the results suggested that (β)-asarone inhibits invasion and EMT in human glioma U251 cells by suppressing splicing factor hnRNP A2/B1 [22]. The above study was further explored by Li et al. for the potential role of hnRNP A2/B1-mediated signaling pathway in the anti-glioma effect of (β)-asarone. They assessed the inhibitory effect of (β)-asarone on the cell viability of human glioma U251 cells by sulforhodamine B (SRB) assay and found it to be effective in a concentration and time dependent manner. Furthermore, the cell apoptosis was characterized with Annexin V/Pi staining by flow cytometry, where (β)-asarone induces cell apoptosis rate and cell cycle arrest at the G1 phase through modulation of p21, p27, cyclin D, cyclin E, CDK2 and Cdc25A. They also demonstrated that the ratio of Bcl-xS/Bcl-xL was enhanced by (β)-asarone in both mRNA and protein level by the inhibition of hnRNP A2/B1-mediated signaling pathway [23]. The P-glycoprotein (P-gp) is a major cause of tumors resistance to chemotherapeutic agents. The relationship between cell resistance and P-gp is crucial as many tumors overexpresses the P-gp gene multi drug resistance-1 (MDR1). Wang et al. suggested that (β)-asarone might contribute to the treatment by promoting Temozolomide's (TMZ) entry into the glioma cells and can inhibit the expression of P-gp and MDR1 mRNA better than single TMZ. This data was further supported by examining the expression of P-gp with different methods and all of the results showed that the expression of P-gp and MDR1 mRNA level decreased in the (β)-asarone, TMZ and co-administration groups, whereas the co-administration group showed better effect [24]. The above study was further continued by Wang et al, focusing on the effect of (β)-asarone induced cell death in U251 cells. They concluded that (β)-asarone can inhibit the progression of glioma U251 cells by arresting the cell cycle in G0/G1 phase and promoting autophagy possibly through P53/Bcl-2/Belin-1 and P53/AMPK/mTOR signal transduction pathway [25] (Table 1; Figs. 4, 5, and 6).
Table 1.
In-vitro effect of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone) on human cancer cell lines.
| Cell lines | Treatment | Targets/Possible molecular events | Reference |
|---|---|---|---|
| Glioblastoma (U251 cells) | (β)-asarone (240 and 360 μM) |
Apoptosis (YO-PRO-1 and PI staining). Inhibition of the expression of hnRNP H1, hnRNPA2/B1 and cathepsin D. |
[21] |
| Glioblastoma (U251 cells) | (β)-asarone (30 and 60 μM) |
Inhibition of the migration (Wound healing assay). Suppression of the invasion (Boyden chamber invasion assay). Inhibition of adhesion (Matrigel coated). ↓ epithelial–mesenchymal transition (EMT) via upregulation of E-cadherin and down-regulation of vimentin. Inhibition of the expression of hnRNP A2/B1 in a concentration and time-dependent way. ↓ expression of matrix metalloproteinases (MMP-9) and p-STAT3. |
[22] |
| Glioblastoma (U251 cells) | (β)-asarone (60, 120, 240 and 480 μM) |
Apoptosis (Annexin V/Pi staining). Cell cycle arrest at G1 phase. Inhibition of the expression of hnRNP A2/B1 in a concentration and time-dependent way. Ratio of Bcl-xS/Bcl-xL ↑ by (β)-asarone in both mRNA and protein level by the inhibition of hnRNP A2/B1-mediated signaling pathway. Promoted the activation of the death receptor proteins TRAIL and FasL. Cleavage of caspase 8 and caspase 3. ↑ in the expression of cleaved-BID and cellular protein cytochrome C. ↑ in the expression of cell cycle related proteins p21 and p27 and ↓ in expression of cyclin D, cyclin E, Cdc25A and CDK2. |
[23] |
| Glioblastoma (U251 cells) | (β)-asarone (360 μM) |
↓ in the cell proliferation in the medicated groups (CCK-8 assay). ↓ in the expression of P-glycoprotein (P-gp) in the medicated groups. ↓ in the expression of multi drug resistance-1 (MDR1) mRNA expression. |
[24] |
| Glioblastoma (U251 cells) | (β)-asarone (360 μM) |
↓ in the cell proliferation in the medicated groups (CCK-8 assay). Cell cycle arrest at G0/G1 phase. Formation of autophagosome (double-membrane autophagosomes and single-membrane autolysosomes) was observed. ↑ in the expression of autophagic markers like Beclin-1 and LC3-Ⅱ/Ⅰ and ↓ in the expression of p62. ↑ in the expression of P53 mRNA, P53, AMPK and pAMPK and ↓ in the expression of Bcl2, mTOR and pmTOR. |
[25] |
| Colon cancer (LoVo cells) | (β)-asarone (200 and 400 μM) |
↓ in the rate of cell viability (MTT assay). Ultra-structure changes of the LoVo cells indicating apoptosis (Hoechst staining). Apoptosis (Annexin V-FITC/PI staining). Down-regulation of mitochondrial membrane potential (MMP). ↑ proapoptotic proteins (Bax expression) and ↓antiapoptotic regulators (Bcl-2, Bcl-xL and survivin) ↓ in the Bcl-2/Bax ratio and ↑ of the executer apoptosis enzyme caspase-9 and caspase-3 cascades. |
[27] |
| Colorectal cancer (HT29 and SW480 cells) | (β)-asarone (0, 10, 30 and 100 nM) |
↓ in the cell proliferation (MTT assay). Induces cell senescence (SA- β -Gal activity). ↑ in the levels of lamin B1, as well as p53, p21 and p15. |
[28] |
| Colon adenocarcinoma (Caco-2 cells) | (α)- and (β)-asarone (30 μg/ml) |
Enhancement of the vincristine induced cytotoxicity to cells (MTT assay). ↑ in the intracellular accumulation of Rhodamine 123 (Rh123) uptake and inhibited Rh123 efflux in Caco-2 cells. ↓ expression of multi drug resistance-1 (MDR1) gene and P-glycoprotein (P-gp). |
[29] |
| Gastric cancer (SGC-7901, BGC-823 and MKN-28 cells) | (β)-asarone (0.12 and 0.24 mM) |
↓ in the cell viability (MTT assay). Apoptosis (Annexin V-FITC/PI staining). Ultra-structure changes of the cells indicating apoptosis (Hoechst 33342 staining). Inhibited the migration, invasion and adhesion (Wound-healing, transwell and matrix-cell adhesion assay). Activates caspase-3, 8 and 9 levels. ↑ proapoptotic proteins (Bax and Bak expression) and ↓ antiapoptotic regulators (Bcl-2, Bcl-xL and surviving activity). ↑ in the expression of RECK, E-cadherin and ↓ the expression of MMP-2, MMP-9, MMP-14 and N-cadherin. |
[30] |
| Gastric adenocarcinoma (AGS cells) | Alcoholic extracts of A. calamus (15, 30, 60, 120, 240 and 480 μg/ml) | Anti-proliferative effects (MTT assay). Cell cycle arrest at G1 phase. Inhibition of formation of tube-like structures confirming anti-angiogenic property (Tube formation assay). Down-regulation of Oct4 and Nucleostemin. |
[31] |
| Fibroblast (HSkMC cells) | |||
| Prostate cancer (LNCaP cells) | Ethanolic extract of A. calamus (250, 500 and 750 μg/ml) | ↓ in the cell viability (XTT assay). Induces apoptotic cell death (↑ in cleaved poly (ADP-ribose) polymerase/PARP). ↓ in VEGF mRNA expression. |
[33] |
| Prostate cancer (PC-3 cells) | Nitro derivatives of (β)-asarone (1.56–200) μM |
↓ in the cell viability (MTT assay). | [34] |
| Neuroblastoma (IMR-32 cells) | |||
| Cervical cancer (HeLa cells) | |||
| Synovial cancer (SW982 cells) | |||
| Breast cancer (MCF-7 cells) | |||
| Macrophage cancer (P338D1 and J774 cells) | Novel lectins from Acorus species (1.0–10) μg/ml | ↓ in the cell viability (3H-thymidine incorporation). | [35] |
| T cell lymphoma (A20 cells) | |||
| B cell lymphoma (WEHI-279 cells) | |||
| Cervical cancer (HeLa cells) | Green silver nanoparticles synthesized from A. calamus (25, 50, 75, 100, 125, 150, 175 and 200 μg/ml) | Anti-proliferative effect (% inhibition of cell proliferation through MTT assay). Apoptotic cell death (Acridine orange/ethidium bromide (AO/EB) and annexinV-Cy3 staining and TUNEL assay). Nuclear changes as fragmentation and condensation (propidium iodide (PI) and 4′, 6-diamidino-2-phenylindole dilactate (DAPI) staining). |
[36] |
| Adenocarcinoma (A549 cells) | |||
| Breast carcinoma (MDA-MB-435S cells) | Aqueous and methanolic extracts of A. calamus (15.625, 31.25,62.5 and 125 μg/ml) | ↓ in the percentage mitotic index of root tip cells and cell viability (Allium cepa root tip and XTT assay). | [41] |
| Liver carcinoma (Hep3B cells) |
Abbreviations: hnRNP A2/B1: Heterogeneous nuclear ribonucleoproteins; TRAIL: TNF-related apoptosis-inducing ligand; FasL: Fas ligand; CCK-8: Cell counting kit 8; Oct-1, 4: Octamer-binding protein-1,4; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
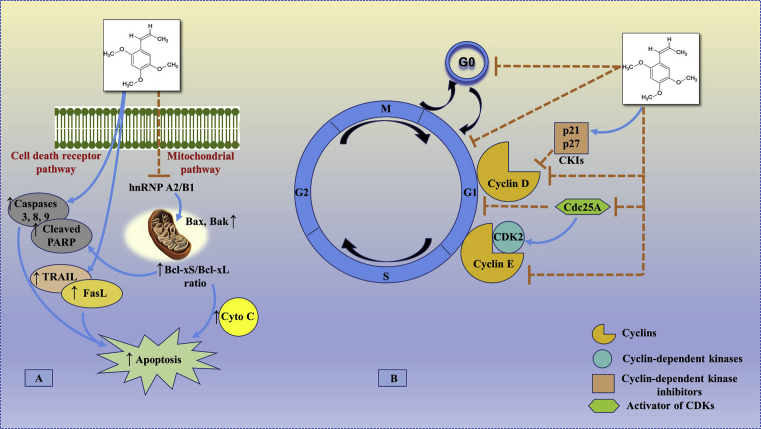
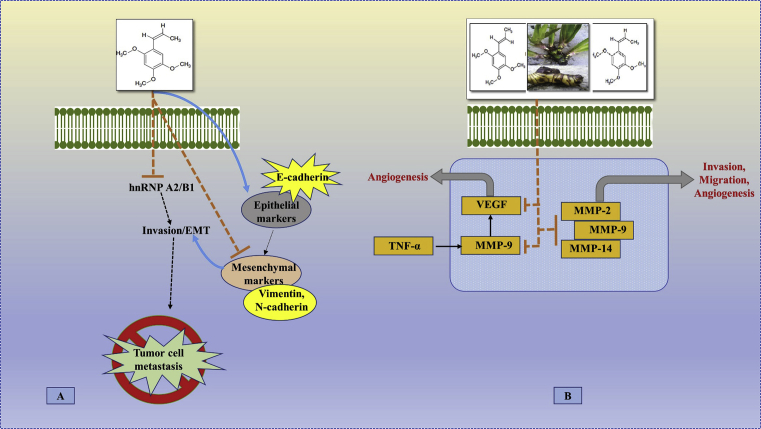
Fig. 4.
The possible site of action of beta (β) asarone in apoptosis, cell proliferation and growth. (A) The (β)-asarone regulates the levels of the key proteins involved in the cell death and mitochondrial apoptosis pathway. (β)-asarone results in enhancement of the ratio of Bcl-xS/Bcl-xL via inhibition of hnRNP A2/B1-mediated signaling pathway, which may be correlated with (β)-asarone-induced apoptosis. On the other hand, the increased expression of cleaved-caspase 3, 8 and 9 along with the activation of the death receptor proteins TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL), it results in the induction of apoptosis. (B) (β)-asarone induced the cell cycle arrest at G0/G1 phase through the up-regulation of cell cycle related proteins as p21 and p27 and down-regulation of cyclin D, cyclin E, Cdc25A and CDK2. [Possible site of action of (β)-asarone as observed in glioblastoma (U251 cells), colon cancer (LoVo cells), colorectal cancer (HT29 and SW480 cells), gastric cancer (SGC-7901, BGC-823 and MKN-28 cells), gastric adenocarcinoma (AGS cells), fibroblast (HSkMC cells) and prostate cancer (LNCaP cells)].
Fig. 5.
The possible site of action of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone) in tumor cell metastasis, invasion, migration and angiogenesis. (A) The (β)-asarone decreases the expression of epithelial-mesenchymal transition (EMT) through upregulation of E-cadherin and down-regulation of vimentin or N-cadherin via inhibition of the expression of hnRNP A2/B1-mediated signaling pathway, thereby suggesting (β)-asarone may block the process of EMT process in cancerous cells. (B) Matrix metalloproteinases (MMPs) are involved in the hnRNP A2/B1-related cancer invasion, migration and metastasis. A. calamus and/or asarone suppresses the expression of matrix metalloproteinases (MMP-2, 9 and 14) and vascular endothelial growth factor (VEGF) thereby underlying the inhibitory effect on invasion, migration and metastasis. [Possible site of action of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone) as observed in glioblastoma (U251 cells), colon cancer (LoVo cells), gastric cancer (SGC-7901, BGC-823 and MKN-28 cells) and prostate cancer (LNCaP cells)].
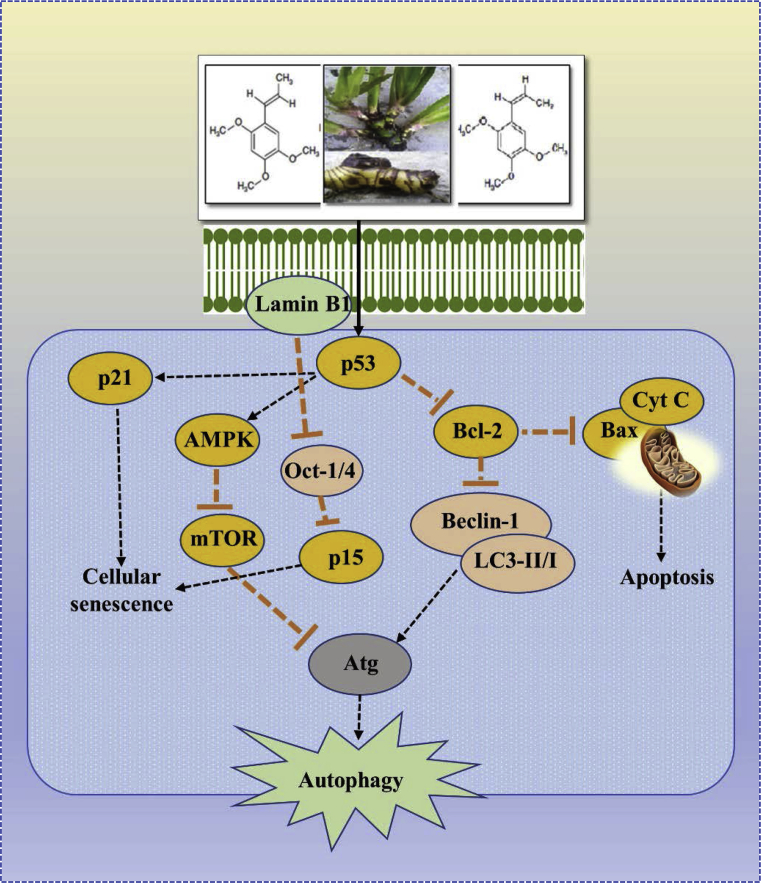
Fig. 6.
The possible site of action of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone) in cellular senescence and autophagy. It inhibits carcinogenesis by inducing cellular senescence through activation of lamin B1. Elevated lamin B1 promotes p53 and p21 expression, and recruits Oct-1 or 4 onto nuclear envelope and prevents binding to the p15 promoter, upregulating p15. On the other hand, activated p53 inhibits Bcl-2, which induces cell apoptosis in the cell cycle. Further autophagy is promoted through up-regulation of autophagy related proteins (Beclin-1 and LC3-II/I) and down-regulation of p53 related proteins (mTOR, Bcl-2). Atg: Autophagy related gene; mTOR: Mammalian target of rapamycin; Oct-1/4: Octamer-binding protein-1,4; LC3: Microtubule-associated proteins 1A/1B light chain 3B. [Possible site of action of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone) as observed in glioblastoma (U251 cells), colorectal cancer (HT29 and SW480 cells), fibroblast (HSkMC cells) and gastric adenocarcinoma (AGS cells)].
2.1.2. Effect of (β)-asarone in human colorectal cancer
The incidence of the colorectal cancer (CRC) is the third most common type of cancer and a leading cause of cancer-related mortality in developed and developing countries [26]. The effectiveness of chemotherapeutic agents is often limited due to high occurrence of severe side effects and drug resistance. Hence, search for new treatment strategies for CRC is ongoing since several decades. In a study undertaken by Zou et al., the anticancer properties by upregulation of caspases activity through the mitochondrial pathway in human colon cancer cells was determined. The (β)-asarone showed dose- and time-dependent cell viability in human LoVo colon cancer cells and induced cell apoptosis by annexin V, which was estimated by fluorescein isothiocyanate/propidium iodide assay by flow cytometry. Further, the relative mRNA expression levels of pro-and anti-apoptotic factors were determined particularly focusing on the change of mitochondrial membrane potential (MMP) via activation of caspase-9, caspase-3 and the ratios of Bcl-2/Bax and Bcl-xL/Bax [27]. The suppression of cellular senescence is one of the features of colorectal cancer and has been linked with aging. One of the factors involved in cellular senescence is the lamin proteins. The lamin B1 gene is suppressed in primary human and murine cell lines when they endure senescence after DNA damage. In another study Liu et al. establish that the (β)-asarone effectively targets cell senescence via lamin protein leading to cell survival. Human colorectal cell lines HT29 and SW480 were treated with (β)-asarone and it had a time- and dose-dependent effect on cell viability. Finally, authors concluded that (β)-asarone inhibits colorectal carcinogenesis by inducing cellular senescence through lamin B1 gene. The increased lamin B1 promotes p53 and p21 expression, and recruits Oct-1 onto nuclear envelope and prevents binding to the p15 promoter, upregulating p15 [28]. Beside that, multiple drug resistance (MDR) is one of the major barriers in cancer chemotherapy. Meng et al. reported that both (α)- and (β)-asarone significantly enhanced the vincristine induced cytotoxicity in human colon adenocarcinoma (Caco-2) cells by enhancing the influx and inhibiting a transmembrane efflux pump (P-Glycoprotein) functions in a concentration dependent manner. Further, it was found that the (α)- and (β)-asarone cause the reduction in P-gp expression and P-gp mRNA in cells [29] (Table 1; Figs. 4, 5, and 6).
2.1.3. Effect of (β)-asarone/alcoholic extracts of A. calamus in human gastric cancer cells
Gastric or stomach cancer is the fourth most common gastrointestinal malignancy. The primary therapy, surgery combined with chemotherapy may achieve satisfactory results, but is not useful for advanced stage of gastric cancer. A study was undertaken by Wu et al. to explore the impact of (β)-asarone on human gastric cancer cells along with establishment of the possible mechanism. The effect of (β)-asarone was studied on three types of differentiated human gastric cancer cell lines such as SGC-7901, BGC-823 and MKN-28. The (β)-asarone inhibits the proliferation of gastric cancer cells in a dose-dependent manner and induces early apoptosis. Further, transwell and wound-healing assays reveals that (β)-asarone inhibited the migration of BGC-823 cells in a concentration-dependent manner. Finally, mRNA and protein expression changes for studying the mechanism of action on the apoptosis of gastric cancer cells was carried out with western blotting and RT-PCR technique. This study clearly depicts that (β)-asarone inhibited the gastric cancer cell growth by upregulating the expression of caspases, proapoptotic proteins, RECK, E-cadherin and by downregulating the expression of antiapoptotic proteins, matrix metalloproteinases and N-cadherin [30]. Haghighi et al. investigated the anti-cancer and anti-angiogenic activity of the alcoholic extracts and essential oil of A. calamus on gastric cancer cell line (AGS) and compared with primary human fibroblast cell line (HSkMC). The growth inhibitory effects of the extracts and essential oil have a concentration and time dependent significant reduction in the proliferation of AGS cells and cell cycle arrest at G1 phase. Further, the tube formation assay showed anti-angiogenic effects of the extracts and essential oil. It was finally supported by the downregulation of Oct4 and Nucleostemin after treatment of the cells with the extracts as confirmed by flowcytometry and quantitative RT-PCR [31] (Table 1; Figs. 4, 5, and 6).
2.1.4. Effect of (β)-asarone/ethanolic extracts of A. calamus in human prostate cancer cells
Prostate cancer is the most commonly diagnosed cancer and remains the third-leading cause of cancer death in men [32]. The pro-angiogenic factor - vascular endothelial growth factor-A (VEGF-A) is believed to be the single most significant angiogenic factor in prostate cancer for accelerating all stages of angiogenesis making it a desirable target. A recent study suggested that the ethanolic extract of A. calamus root possesses a dose and time dependent anticancer, apoptotic and anti-angiogenic activities on prostate cancer cell culture. Koca et al. examined whether ethanolic extract of A. calamus marks the survival and apoptosis as well as inhibits the angiogenesis. The effect of A. calamus extract on the cell viability showed a dose-response relationship of cell survival. The Poly-(ADP-ribose) polymerase (PARP) cleavage involved in the apoptotic process was found to be at higher expression in A. calamus extract treated cells. It was also supported by the suppression of mRNA expression of the pro-angiogenic factor VEGF-A in LNCaP cells treated with the A. calamus extract [33]. Shenvi et al. reported the anti-cancer assay of nitro derivatives of (β)-asarone using PC-3 (prostate cancer) cell line along with four other different types of human cancer cell lines by using MTT assay. The nitration derivative compounds of (β)-asarone exhibited an increase in activity compared to (β)-asarone confirming its cytotoxic activity in all the cell lines [34] (Table 1; Figs. 4 and 5).
2.1.5. Effect of novel lectins from Acorus species in murine cancer cells
Lectins, the major proteins of many monocotyledonous plants are polyclonal activators towards human lymphocytes. In a study performed by Bains et al, they reported two novel lectins derived from the rhizomes of A. calamus and A. gramineus possessing significant mitogenic activity towards human lymphocytes and inhibitory activity towards murine macrophage cancer cell lines. They employed macrophage cancer cell lines (P338D1 and J774), T cell lymphoma (A20) and B cell lymphoma (WEHI-279) for MTT assay to study the inhibitory activity of the novel lectins. Both the novel lectins (A. calamus and A. gramineus) showed inhibitory activity towards the murine cancer cell lines as measured by 3H-thymidine incorporation confirming its inhibitory potential [35] (Table 1).
2.1.6. Effect of green silver nanoparticles synthesized from A. calamus rhizome extract in human cervical cancer (HeLa) and lung adenocarcinoma (A549) cell lines
The use of the inert metals such as gold, silver, and platinum in the field of nanomedicine is gaining interest for synthesizing metallic nanoparticles having high therapeutic potential for various uses. Green synthesis of silver nanoparticles using various medicinal plants have shown to exhibit in-vitro anticancer activities. Keeping view of the above evidences, Nakkala et al. carried out the in-vitro cytotoxic effects of green silver nanoparticles synthesized from A. calamus rhizome extract (ACAgNPs) in human cervical cancer (HeLa) and lung adenocarcinoma (A549) cell lines. The anti-proliferative effect expressed as % inhibition of cell proliferation through MTT assay were found within the acceptable concentration of 100 mg/L. The apoptotic cell death was confirmed further through acridine orange/ethidium bromide (AO/EB) and annexinV-Cy3 staining techniques being supported by the nuclear changes (fragmentation and condensation) through staining with propidium iodide (PI) and 4′, 6-diamidino-2-phenylindole dilactate (DAPI) and flow cytometry. Collectively, all these findings suggested the cytotoxic activity of green silver nanoparticles synthesized with A. calamus rhizome extract [36] (Table 1).
2.2. In-vivo anti-cancer effect of A. calamus
Beside in-vitro investigation, in-vivo studies provided the evidence supporting that A. calamus and/or its bioactive phytochemicals asarone have potential anti-cancer pharmacological effect. Here, we discussed the detailed evidence about the anti-cancer effect of A. calamus and/or its bioactive phytochemicals asarone.
Zou et al. evaluated the anti-cancer property of (β)-asarone in nude mice through LoVo cancer xenografts model. The experimental animals treated with (β)-asarone at 50 mg/kg/d b.w. concentration suppressed the tumor volume significantly in comparison to placebo controls. Further, to determine that the tumors in the (β)-asarone treated group were inhibited through apoptosis, the isolated tumors was subjected to TUNEL assay. It was confirmed that fragmentation of DNA was observed in the growth-inhibited tumors for the nude mice suggesting the apoptotic changes [27]. Furthermore, another study carried by Liu et al. revealed that (β)-asarone treatment reduced the incidence and number of tumor formation against the genotoxic colonic chemical carcinogen DMH (1, 2-dimethyl hydrazine) in Crj: CD-1 (ICR) mice. Moreover, the reduction in the tumor volume in xenografts model of colorectal cancer in BABL/c nude mice through administration of SW480 or HT-29 cells was observed in the (β)-asarone treated group. It was further supported by data that (β)-asarone induced senescence in human colorectal cancer via activation of lamin B1 by promoting the tumor protein (p53, p21) expressions [28]. Our earlier study reported that (β)-asarone treatment protects the rats from the diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) which was confirmed by the changes in the levels of the liver function markers and cancer bio-marker (alpha-fetoprotein) and supported by histopathological changes [37]. Recently, it was reported that (β)-asarone inhibits tumor growth and induces apoptosis in U251 tumor xenograft nude mice model. The tumor was harvested for apoptosis with a standard in-situ terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay and found that the apoptotic rate in the tumors significantly enhanced in (β)-asarone treatment group when compared with that of vehicle control. Furthermore, the expression of apoptotic proteins in tumor tissues was observed by the increase level of cleaved caspase-3 in the treated group. A dose-dependent decrease of the expression of hnRNP A2/B1 and CKD2, increase ratio of Bcl-xS/Bcl-xL and along with that increase expression of p27 was observed for the treated group when compared with that of the vehicle group. Finally, the results obtained in this study demonstrates that (β)-asarone -induced apoptosis and cell cycle arrest of U251 cells may be linked to the suppression of hnRNPA2/B1-mediated signaling pathway [23] (Table 2).
Table 2.
In-vivo anti-cancer effect of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone).
| Model/Animal used | Treatment | Targets/Effects/Possible molecular events | Reference |
|---|---|---|---|
| LoVo cancer xenograft model (Nude mice) | (β)-asarone (50 mg/kg/d b.w.; p.o.) |
Suppression of the tumor volume. Apoptosis as confirmed by fragmentation of DNA in the growth-inhibited tumors (TUNEL assay). |
[27] |
| DMH (1, 2-dimethyl hydrazine) induced colorectal cancer (Crj: CD-1 (ICR) mice) |
(β)-asarone (50, 100 and 200 μg/kg/d b.w.; p.o.) |
Reduction in the incidence and number of tumor formation. Induction of senescence in human colorectal cancer via activation of lamin B1 by promoting the tumor protein (p53, p21) expressions. |
[28] |
| Human colorectal cancer (SW480 and HT-29 xenograft model) (BABL/c nude mice) | (β)-asarone (50, 100 and 200 μg/kg/d b.w.; i.v.) |
||
| Diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) (wistar Albino rats) | (β)-asarone (25 μg/kg/d b.w.; p.o.) |
↓ in the levels of serum liver biomarkers (ALT, AST, ALP, TB and DB). ↓ in the levels of cancer biomarkers (DNA, RNA and AFP). |
[37] |
| Glioma U251 tumor xenograft model (Nude mice) | (β)-asarone (25 and 50 mg/kg/d b.w.; p.o.) |
Suppression of the tumor growth. ↑ in the apoptotic rate (TUNEL assay). ↑ level of apoptotic proteins (cleaved caspase-3 and p27). ↓ in the expression of hnRNP A2/B1 and CKD2. ↑ in ratio of Bcl-xS/Bcl-xL. |
[23] |
| Dalton's ascites lymphoma induced tumor (swiss Albino mice) | Methanolic extracts of A. calamus (100 and 200 mg/kg/d b.w.; i.p.) | ↑ in the liver antioxidant enzyme level of SOD, CAT, GPx, GSH, Vitamin C and E. ↑ in the kidney antioxidant enzyme level of SOD, CAT, GPx, GSH, LPO and Vitamin E. ↓ in the tumor volume. ↓ in the levels of serum liver biomarkers (ALT, AST and ALP). |
[42, 43, 44] |
Abbreviations: b.w.: body weight; d: day; p.o.: per oral route; i.v.: intravenous route i.p.: intraperitoneal route.
2.3. Anti-oxidant properties of A. calamus or asarone
Anti-oxidants are known free radical scavengers that allow the cells to rejuvenate from cellular damage by neutralizing the highly reactive oxygen compounds [38]. The anti-oxidant effect of A. calamus or asarone was reported by different in-vitro and in-vivo studies (Table 3). The bioactive compounds of the plant showed up/down regulation of certain endogenous enzymatic and non-enzymatic parameters which ultimately proves their ability to scavenge free radicals. Various reports suggest that the A. calamus or asarone are effective against number of oxidative stress models viz., ischemia-induced brain infarction oxidative stress, γ-radiation induced alterations in oxidative stress, noise stress-induced oxidative stress in brain etc.
Table 3.
In-vitro and in-vivo anti-oxidant effect of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone).
| Model/Animal used/Cell lines | Treatment | Targets/Effects/Possible molecular events | Reference |
|---|---|---|---|
| In-vitro anti-oxidant activity | Essential oils of A. calamus (5–25) μL/mL | The oils isolated from the rhizome and leaves in all the different seasons exhibited antioxidant activity as confirmed by 2, 2-diphenyl picryl hydrazyl (DPPH), reducing power (RP) and chelating properties of Fe2+. | [45] |
| In-vitro anti-oxidant and free radical scavenging activity | Aqueous extracts of A. calamus (25–400) μg/mL | Results showed that the aqueous extracts have a potential free radical scavenging activity as confirmed by DPPH, nitric oxide, superoxide radical, ferrous chelation, RP and phosphomolybdenum assay. | [46] |
| In-vitro anti-oxidant activity | (α)-asarone (10–100) μg/mL |
It exhibited a dose-dependent DPPH radical-scavenging, RP, superoxide radical and hydroxyl radical scavenging activity. | [47] |
| Ischemia-induced brain infarction oxidative stress (wistar rats) | (β)-asarone (10, 20 and 30 mg/kg/d b.w.; p.o.) |
↑ in the levels of reduced glutathione (GSH), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione S transferase (GST) and catalase (CAT) activity in the hippocampus. ↓ in the level of lipid peroxidation (LPO) content in the hippocampus. |
[48] |
| Senescence- accelerated prone 8 (SAMP-8) (Alzheimer's mediated oxidative stress) (mice) | (β)-asarone (34 mg/kg/d b.w.; p.o.) |
(β)-asarone did not affect superoxide dismutase (SOD) activities in brain and malondialdehyde (MDA) level in serum. | [49] |
| High-fat diet (HFD) induced metabolic abnormalities oxidative stress (wistar rats) | (β)-asarone (12.5, 25, and 50 mg/kg/d b.w.; p.o.) |
↑ levels of GSH and ↓ levels of MDA in liver homogenate. | [50] |
| Noise stress-induced oxidative stress in brain (wistar rats) | (α)-asarone (3, 6, and 9 mg/kg/d b.w.; i.p.) |
↓ in the levels of SOD and LPO content in the brain. ↑ in the levels of CAT, GPx, GSH, Vitamin C, E and protein thiols in the brain. |
[51] |
| Brain enzymatic antioxidant activities (Swiss OF1 mice) | (α)-asarone (100 mg/kg/d b.w.; i.p.) |
↑ in the levels of GPx and GR in the three areas of brain (cortex, striatum and hippocampus). SOD activity was unaffected in cortex and ↑ in striatum and hippocampus. |
[52] |
| γ-radiation induced alterations in oxidative stress (swiss Albino mice) | (α)-asarone (50 mg/kg/d b.w.; p.o.) |
↑ in the levels of GSH, SOD, GPx and CAT in brain and kidney homogenate. | [53] |
| Scopolamine induced cognitive deficits mediated oxidative stress (ICR mice) | (α)-asarone (3, 10 and 30 mg/kg/d b.w.; i.p.) |
↓ in the levels of MDA and SOD in both areas of the brain (cerebral cortex and hippocampus). | [54] |
| Noise stress-induced oxidative stress in brain (wistar Albino rats) | (α)-asarone (9 mg/kg/d b.w.; i.p.) |
↓ in the levels of SOD and LPO content in hippocampus. ↑ in the levels of CAT, GPx, GSH, Vitamin C and E in hippocampus. |
[55] |
| Dalton's ascites lymphoma induced tumor (swiss Albino mice) | Methanolic extracts of A. calamus (100 and 200 mg/kg/d b.w.; i.p.) | ↑ in the liver antioxidant enzyme level of SOD, CAT, GPx, GSH, Vitamin C and E. ↑ in the kidney antioxidant enzyme level of SOD, CAT, GPx, GSH, LPO and Vitamin E. |
[42, 43, 44] |
Abbreviations: b.w.: body weight; d: day; p.o.: per oral route; i.v.: intravenous route i.p.: intraperitoneal route.
2.4. Toxicity profile of A. calamus or asarone
In acute toxicity studies, A. calamus and/or its bioactive phytochemicals asarone were found to be safe at lower doses as summarized in Table 4. An acute toxicity study of (α)-asarone (150, 200, 250, 300 and 350 mg/kg b.w.; i.p.) was undertaken by Morales et al. in male BALB/c mice for 14 days. The most frequent clinical signs observed among the animals were ptosis, ataxia, piloerection and dyspnea [39]. In another study Liu et al. tested the effect of (β)-asarone (0.5, 0.75, 1, 1.25, 1.5, 1.75 and 2 g/kg b.w.; i.v.) on BALB/c mice to evaluate the preliminary toxicity. They reported that the animals that received (β)-asarone did not exhibit any behavior change within 24 h of treatment. Further they performed a long-term toxicity test for 90 days at the dosages of 10, 20 and 50 mg/kg/d. Results showed a dose-dependent toxicology with increase in number of white blood cells (WBC) and decrease in number of red blood cells (RBC) at 10 mg/kg. Additionally, the levels of serum K+ decreased at the dose of 20 mg/kg and Cl− increased in 50 mg/kg treated mice [28].
Table 4.
Acute toxicity studies of A. calamus and/or its bioactive phytochemicals asarone (alpha (α)-and, beta (β)-asarone).
| Animal species/strain/gender | Median lethal dose (LD50) |
Reference | |
|---|---|---|---|
| (α)-asarone | (β)-asarone | ||
| BALB/c mice (male) | 245.2 mg/kg b.w.; i.p. | -- | [39] |
| Rat | -- | 1010 mg/kg b.w.; p.o. | [40] |
| Mice | -- | 184 mg/kg b.w.; i.p. | [40] |
| Swiss mice (male) | >1000 mg/kg b.w.; p.o. | -- | [56] |
| BALB/c mice (both gender) | -- | 1.56 g/kg b.w.; i.v. | [28] |
Abbreviations: b.w.: body weight; p.o.: per oral route; i.v.: intravenous route i.p.: intraperitoneal route.
In sub-acute toxicity study, Sprague Dawley rats treated with (β)-asarone (100 mg/kg/d b.w.; i.p.) exhibited characteristic weight loss and decreased food consumption. Additionally, the weight of heart and thymus were reduced and weight of adrenals was increased. Furthermore, there was no significant changes in haematological and biochemical parameters indicating hepatotoxicity [40]. In a two-year feeding study, Osborne-Mendel fed the male rats with a diet containing either (β)-asarone or calamus oil (400, 800 or 2000 mg/kg). The rats developed an increased incidence of leiomyosarcomas in small intestine. Microscopic analysis revealed cardiac atrophy, fat infiltration and fibrosis in heart of the animals. Further, rats fed with the Jammu oil of calamus (0, 50, 100 or 5000 mg/kg) for two years developed early mortality, liver and heart lesions at a dose of 5000 mg/kg [40].
3. Conclusions
Cancer is one of the growing health problems worldwide, posing a threat to human social and economic conditions. Regardless of the advanced treatment modality available for the treatment of cancer such as chemotherapy, surgical resection, radiotherapy and phototherapy, the survival rate has not been enhanced because of its high recurrence, toxic side-effects and metastasis rate. Hence, on-going efforts in search of anti-cancer agents derived from natural sources with low toxicity and greater enhancement of survival is gaining interest among many researchers. Based on the available literature, it appears that natural phytochemicals obtained from the plants, have been explored for their anti-cancer activity. Acorus calamus, commonly known as the ‘sweet flag’ has a rich history in the Indian and Chinese traditional medicine and found to be a popular remedy with great efficacy for various diseases. The information presented in this review focuses and summarizes the anti-cancer activity of A. calamus and/or its bioactive phytochemicals asarones (alpha (α)-and, beta (β)-asarone) including the underlying molecular mechanisms. Collectively, the mechanism underlying cancer chemo-prevention of A. calamus and/or its bioactive phytochemicals asarone includes variation of all kinds of cancer hallmarks. These includes regulation of cell proliferation and cell cycle arrest, induction of apoptosis, inhibition of angiogenesis as modulated via different signal transduction pathways. Still, extensive in-vivo studies are necessary using various models to understand the molecular mechanism behind the pharmacological activity of the bioactive phytochemicals derived from A. calamus. We believe that the evidence presented in this article could be a possible source for researchers to conduct future studies pertaining to A. calamus and/or its bioactive phytochemical asarone for cancer chemoprevention.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors acknowledge the support of The Principal, KLE College of Pharmacy, Vidyanagar, Hubballi, Karnataka, India for extending the facilities to prepare this article.
References
- 1.Fundytus A. Delivery of global cancer care: an international study of medical oncology workload. J. Glob. Oncol. 2017:1–11. doi: 10.1200/JGO.17.00126. JGO1700126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goss P.E. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15(5):489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 3.Mallath M.K. The growing burden of cancer in India: epidemiology and social context. Lancet Oncol. 2014;15(6):e205–e212. doi: 10.1016/S1470-2045(14)70115-9. [DOI] [PubMed] [Google Scholar]
- 4.Mohan A. Combinations of plant polyphenols and anticancer molecules: a novel treatment strategy for cancer chemotherapy. Anti Cancer Agents Med. Chem. 2013;13(2):281–295. doi: 10.2174/1871520611313020015. [DOI] [PubMed] [Google Scholar]
- 5.Rajput S.B. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomed: Int. J. Phytother. Phytopharm. 2014;21(3):268–276. doi: 10.1016/j.phymed.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z.J. Identification of both GABA-A receptors and voltage-activated Na (+) channels as molecular targets of anticonvulsant alpha-asarone. Front. Pharmacol. 2014;5:40. doi: 10.3389/fphar.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C. Alpha-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology. 2013;65:1–11. doi: 10.1016/j.neuropharm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Balakumbahan R. Acorus calamus: an overview. J. Med. Plants Res. 2010;4(25):2740–2745. Available at: http://www.academicjournals.org/app/webroot/article/article1380712717_Balakumbahan%20et%20al.pdf. [Google Scholar]
- 9.Lansdown R.V. 2014. Acorus calamus. The IUCN Red List of Threatened Species. e. T168639A43116307. Available at: [Google Scholar]
- 10.Sharma V. Acorus calamus (The Healing Plant): a review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014;28(18):1454–1466. doi: 10.1080/14786419.2014.915827. [DOI] [PubMed] [Google Scholar]
- 11.Singh R. Pharmacological properties and ayurvedic value of Indian buch plant. (Acorus calamus): a short review. Adv. Biol. Res. 2011;5(3):145–154. https://pdfs.semanticscholar.org/1083/b32c25ce2afdd8ffbca3d72137b93eb23a89.pdf Available at: [Google Scholar]
- 12.Muthuraman A., Singh N. Acute and sub-acute oral toxicity profile of Acorus calamus (Sweet flag) in rodents. Asian Pac. J. Trop. Biomed. 2012;2(2):S1017–S1023. [Google Scholar]
- 13.Pandit S. Metabolism mediated interaction of alpha-asarone and Acorus calamus with CYP3A4 and CYP2D6. Fitoterapia. 2011;82(3):369–374. doi: 10.1016/j.fitote.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Hanson K.M. Rapid assessment of beta-asarone content of Acorus calamus by micellar electrokinetic capillary chromatography. Electrophoresis. 2005;26(4-5):943–946. doi: 10.1002/elps.200410165. [DOI] [PubMed] [Google Scholar]
- 15.Shu H. Ethnobotany of Acorus in China. Acta Soc. Bot. Pol. 2018;87(2):3585. [Google Scholar]
- 16.Khwairakpam A.D. Acorus calamus: a bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 2018;29(2):107–122. doi: 10.1515/jbcpp-2016-0132. [DOI] [PubMed] [Google Scholar]
- 17.Imam H. Sweet flag (Acorus calamus linn.): an incredible medicinal herb. Int. J. Green Pharm. 2013;7(4):288–296. [Google Scholar]
- 18.Sarjan H.N. The protective effect of the vacha rhizome extract on chronic stress-induced immunodeficiency in rat. Pharm. Biol. 2017;55(1):1358–1367. doi: 10.1080/13880209.2017.1301495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omuro A., DeAngelis L.M. Glioblastoma and other malignant gliomas: a clinical review. J. Am. Med. Assoc. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 20.Woodworth G.F. Emerging insights into barriers to effective brain tumor therapeutics. Front. Oncol. 2014;4:126. doi: 10.3389/fonc.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi H. Proteomic analysis of beta-asarone induced cytotoxicity in human glioblastoma U251 cells. J. Pharm. Biomed. Anal. 2015;115:292–299. doi: 10.1016/j.jpba.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Li L. β-Asarone inhibits invasion and EMT in human glioma U251 cells by suppressing splicing factor HnRNP A2/B1. Molecules. 2018;23(3):E671. doi: 10.3390/molecules23030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L. β-Asarone induces apoptosis and cell cycle arrest of human glioma U251 cells via suppression of HnRNP A2/B1-mediated pathway in vitro and in vivo. Molecules. 2018;23(5):E1072. doi: 10.3390/molecules23051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang N. β-Asarone promotes Temozolomide's entry into glioma cells and decreases the expression of P-glycoprotein and MDR1. Biomed. Pharmacother. 2017;90:368–374. doi: 10.1016/j.biopha.2017.03.083. [DOI] [PubMed] [Google Scholar]
- 25.Wang N. β-asarone inhibited cell growth and promoted autophagy via P53/Bcl-2/Bclin-1 and P53/AMPK/mTOR pathways in human glioma U251 cells. J. Cell. Physiol. 2018;233(3):2434–2443. doi: 10.1002/jcp.26118. [DOI] [PubMed] [Google Scholar]
- 26.Siegel R.L. Cancer statistics. CA. Cancer J. Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 27.Zou X. Beta-asarone induces LoVo colon cancer cell apoptosis by up-regulation of caspases through a mitochondrial pathway in vitro and in vivo. Asian Pac. J. Cancer Prev. 2012;13(10):5291–5298. doi: 10.7314/apjcp.2012.13.10.5291. [DOI] [PubMed] [Google Scholar]
- 28.Liu L. β-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine. 2013;20(6):512–520. doi: 10.1016/j.phymed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Meng X. Reversing P-glycoprotein-mediated multidrug resistance in vitro by α-asarone and β-asarone, bioactive cis-trans isomers from Acorus tatarinowii. Biotechnol. Lett. 2014;36(4):685–691. doi: 10.1007/s10529-013-1419-8. [DOI] [PubMed] [Google Scholar]
- 30.Wu J. β-Asarone inhibits gastric cancer cell proliferation. Oncol. Rep. 2015;34(6):3043–3050. doi: 10.3892/or.2015.4316. [DOI] [PubMed] [Google Scholar]
- 31.Haghighi S.R. Anti-carcinogenic and anti-angiogenic properties of the extracts of Acorus calamus on gastric cancer cells. Avicenna J. Phytomed. 2017;7(2):145–156. [PMC free article] [PubMed] [Google Scholar]
- 32.Litwin M.S., Tan H.J. The diagnosis and treatment of prostate cancer: a review. J. Am. Med. Assoc. 2017;317(24):2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 33.Koca H.B. Effects of Acorus calamus plant extract on prostate cancer cell culture. Anatolian J. Bot. 2018;2(1):46–51. [Google Scholar]
- 34.Shenvi S. Nitro derivatives of naturally occurring β-Asarone and their anticancer activity. Int. J. Med. Chem. 2014;2014:835485. doi: 10.1155/2014/835485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bains J.S. Novel lectins from rhizomes of two Acorus species with mitogenic activity and inhibitory potential towards murine cancer cell lines. Int. Immunopharmacol. 2005;5(9):1470–1478. doi: 10.1016/j.intimp.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Nakkala J.R. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur. J. Med. Chem. 2014;85:784–794. doi: 10.1016/j.ejmech.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Roy S.R., Gadad P.C. Effect of β-asarone on diethylnitrosamine-induced hepatocellular carcinoma in rats. Indian J. Health Sci. Biomed. Res. 2016;9(1):82–88. [Google Scholar]
- 38.Lu J.M. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell Mol. Med. 2009;14(4):840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales-Ramirez P. Sister-chromatid exchange induction produced by in vivo and in vitro exposure to alpha-asarone. Mutat. Res. 1992;279(4):269–273. doi: 10.1016/0165-1218(92)90243-s. [DOI] [PubMed] [Google Scholar]
- 40.European-commission . 2002. Opinion of the Scientific Committee on Food on the Presence of β-asarone in Flavourings and Other Food Ingredients with Flavouring Properties, Scientific Committee on Food; pp. 1–15.https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out111_en.pdf Available at: [Google Scholar]
- 41.Rajkumar V. Evaluation of cytotoxic potential of Acorus calamus rhizome. Ethnobotanical Leafl. 2009;13:832–839. https://opensiuc.lib.siu.edu/ebl/vol2009/iss7/2 Available at: [Google Scholar]
- 42.Sreejaya S.B., Santhy K.S. Antineoplastic and antioxidant activities of Acorus calamus L on swiss albino mice bearing Dalton’s ascites lymphoma. Asian J. Pharmaceut. Clin. Res. 2015;8(6):97–100. https://innovareacademics.in/journals/index.php/ajpcr/article/view/7723/3346 Available at: [Google Scholar]
- 43.Sreejaya S.B., Santhy K.S. Evaluation of antitumor properties of rhizome of Acorus calamus using Dalton’s ascites lymphoma bearing swiss albino mice. Int. J. Pharm. Bio. Sci. 2014;5(4):119–125. https://ijpbs.net/abstract.php?article=MzYwMQ== Available at: [Google Scholar]
- 44.Sreejaya S.B. Biochemical changes in the serum of experimental animals treated with Acorus calamus rhizome. Stud. Ethno-Med. 2017;11(3):216–220. [Google Scholar]
- 45.Parki A. Seasonal variation in essential oil compositions and antioxidant properties of Acorus calamus L. Access., Med. (Basel). 2017;4(4):E81. doi: 10.3390/medicines4040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subathraa K., Poonguzhali T.V. In vitro studies on antioxidant and free radical scavenging activities of aqueous extract of Acorus calamus L. Int. J. Curr. Sci. 2012;1:169–173. http://www.currentsciencejournal.info/issuespdf/Subatra-Poonguzhali.pdf Available at: [Google Scholar]
- 47.Asha Devi S. Antioxidant properties of alpha asarone. Asian J. Biochem. 2014;9(2):107–113. [Google Scholar]
- 48.Yang Y.X. Beta-asarone, a major component of Acorus tatarinowii Schott, attenuates focal cerebral ischemia induced by middle cerebral artery occlusion in rats. BMC Complement Altern. Med. 2013;13:236. doi: 10.1186/1472-6882-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y. β-Asarone prevents autophagy and synaptic loss by reducing ROCK expression in asenescence-accelerated prone 8 mice. Brain Res. 2014;1552:41–54. doi: 10.1016/j.brainres.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Thakare M.M., Surana S.J. β-Asarone modulate adipokines and attenuates high fat diet-induced metabolic abnormalities in Wistar rats. Pharmacol. Res. 2016;103:227–235. doi: 10.1016/j.phrs.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Manikandan S., Devi R.S. Antioxidant property of alpha-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol. Res. 2005;52(6):467–474. doi: 10.1016/j.phrs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Pages N. Activities of α-asarone in various animal seizure models and in biochemical assays might be essentially accounted for by antioxidant properties. Neurosci. Res. 2010;68(4):337–344. doi: 10.1016/j.neures.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Sandeep D., Nair C.K. Radioprotection by α-asarone: prevention of genotoxicity and hematopoietic injury in mammalian organism. Mutat. Res. 2011;722(1):62–68. doi: 10.1016/j.mrgentox.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Kumar H. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 2012;76(8):1518–1522. doi: 10.1271/bbb.120247. [DOI] [PubMed] [Google Scholar]
- 55.Sundaramahalingam M. Role of Acorus calamus and alpha-asarone on hippocampal dependent memory in noise stress exposed rats. Pakistan J. Biol. Sci. 2013;16(16):770–778. doi: 10.3923/pjbs.2013.770.778. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q.X. Anticonvulsant activity of acute and chronic treatment with a-asarone from Acorus gramineus in seizure models. Biol. Pharm. Bull. 2013;36(1):23–30. doi: 10.1248/bpb.b12-00376. [DOI] [PubMed] [Google Scholar]