Abstract
Exposure to indoor air pollution increases the risk of pneumonia in children, accounting for about a million deaths globally. This study investigates the individual effect of solid fuel, carbon monoxide (CO), black carbon (BC) and particulate matter (PM)2.5 on pneumonia in children under 5 in low- and middle-income countries. A systematic review was conducted to identify peer-reviewed and grey full-text documents without restrictions to study design, language or year of publication using nine databases (Embase, PubMed, EBSCO/CINAHL, Scopus, Web of Knowledge, WHO Library Database (WHOLIS), Integrated Regional Information Networks (IRIN), the World Meteorological Organization (WMO)-WHO and Intergovernmental Panel on Climate Change (IPCC). Exposure to solid fuel use showed a significant association to childhood pneumonia. Exposure to CO showed no association to childhood pneumonia. PM2.5 did not show any association when physically measured, whilst eight studies that used solid fuel as a proxy for PM2.5 all reported significant associations. This review highlights the need to standardise measurement of exposure and outcome variables when investigating the effect of air pollution on pneumonia in children under 5. Future studies should account for BC, PM1 and the interaction between indoor and outdoor pollution and its cumulative impact on childhood pneumonia.
Keywords: Indoor air pollution, Black carbon, Particulate matter, Carbon monoxide, Pneumonia, Children under 5, Low- and middle-income countries
Introduction
Pneumonia is an inflammatory disease affecting the lung. It is characterised by an accumulation of fluid in the alveolus, resulting in the obstruction of normal breathing (CDC 2016; Walker et al. 2013). Pneumonia is caused predominantly but not solely by bacteria, viruses and fungi (CDC 2016; Walker et al. 2013). In children, bacterial pneumonia is mostly caused by Streptococcus pneumoniae whilst Haemophilus influenzae type b (Hib) is the next largest cause (CDC 2016; Walker et al. 2013). Respiratory syncytial virus mainly causes viral pneumonia whilst Pneumocystis jirovecii is the major cause of fungal pneumonia in children (Liu et al. 2015). Globally, pneumonia is the major cause of paediatric mortality, especially in children under 5 (McCollum et al. 2015; UNICEF 2016; Walker et al. 2013). A 2015 report by UNICEF has shown that one in six children died from pneumonia before the age of 10 particularly in the poorest parts of low- and middle-income countries (UNICEF 2016). Sub-Saharan Africa and South Asia are the regions with the highest number of pneumonia deaths. The number of deaths in these regions has continued to rise, increasing from 77% in 2000 to 82% in 2015. This staggering number of worldwide pneumonia deaths is concentrated mostly in four countries since 2000: the Democratic Republic of Congo, India, Nigeria and Pakistan (UNICEF 2016).
The high deaths from pneumonia can also be linked to poverty. Looking closely, these deaths are concentrated amongst the poorest populations. Sixty-two per cent of the world’s under-5 population can be found in low- and middle-income countries. However, 90% of the global deaths from pneumonia still originate from these regions.
The very poorest countries carry a disproportionate share of the burden of death: more than 30% of all pneumonia and diarrhoea deaths are concentrated in low-income countries, yet these countries are home to only 15% of the world’s under-5 population (UNICEF 2016). These regions are also characterised by reduced access to quality health care, nutrition and basic environmental hygiene which contributes to exacerbating the disease incidence (UNICEF 2016). Pneumonia is preventable and so is the related morbidity (Chopra et al. 2013; Qazi et al. 2015).
There were over 4.1 million deaths and 106 million daily-adjusted life years (DALYs) lost worldwide due to long-time exposure to particulate matter (PM)2.5 in 2016. Homes that depend on the daily use of solid fuels been exposed to PM2.5 levels six times higher than the least severe WHO Interim Air Quality Target and as high as 20 times higher than the recommended WHO Air Quality Guideline of 10 μg/m3 for PM2.5 (Health Effects Institute 2018). The risk of developing pneumonia in children is doubled following exposure to air pollution, thereby accounting for over 920,000 deaths globally, caused by pneumonia (UNICEF 2016). Pollutants compromise the host’s immune response against invading pathogens in the respiratory tract. The epithelial cells lining the alveolar are specialised in secreting cytokines and radicals in response to foreign evading bodies (Hussey et al. 2017; Smith et al. 2000). These mediate the recruitment of inflammatory cells such as macrophages and phagocytes to the site of evasion, which then engulf and digest these foreign organisms. However, high levels of air pollution can lead to a compromise in this sterilisation and filtration mechanism of the respiratory tract, therefore increasing the risk of the development of acute lower respiratory infections (ALRIs) (Smith et al. 2000).
Furthermore, the mucociliary apparatus and cellular immune defences have also been shown to be significantly reduced by nitrogen dioxide (Carvalho-Oliveira et al. 2015; Caswell 2013; Smith et al. 2000). Most characterised types of pollutants belong to a broad class called “particulate matter” (Smith et al. 2000). PM has been previously reported as the key mediator of inflammation and compromised immune defences that are linked to the pathogenesis of ALRIs (Bauer et al. 2012; Lee et al. 2015; Smith et al. 2000). More recently, black carbon (BC), which is a component of PM, has been reported to be the main mediator of the effects of pollutants (Smith et al. 2000). BC has been reported to significantly affect the behaviour of S. pneumoniae by altering the structure and proteolytic degradation of the biofilm, therefore promoting its tolerance to multiple antibiotics. Finally, BC promotes the spread of bacteria to the lungs and consequently exacerbates the disease occurrence (Baumgartner et al. 2014; Hussey et al. 2017). Although these studies have looked at the pathological relationship between the PM, BC and respiratory diseases, the consensus on disease incidence remains contested.
Indoor pollution is not restricted to habitats of rural settlements and poor people (DD 2013; Hulin et al. 2010; Jung et al. 2012; Ram et al. 2014; Sharma et al. 1998; Shibata et al. 2014). Studies have shown the association between respiratory illness and indoor activities and features such as new painting and wall covers (Emenius et al. 2004; Garrett et al. 1998; Jaakkola et al. 2004), gas appliances (Agabiti et al. 1999; Belanger et al. 2006; Pilotto et al. 1999) or exposure to particles from tobacco smoke or heating system utilising coal (Gonzalez-Barcala et al. 2013; Nandasena et al. 2013).
Children are at higher risk because of their increased resting metabolism and a higher degree of aerobic metabolism relative to their size compared to adults (American Thoracic Society 2004; Chance 2001). Children are continuously undergoing organ development; this coupled with an elevated surface area per unit body weight increases the oxygen demand and respiratory rates (Moya et al. 2004). Furthermore, children have narrower airways compared to adults; therefore, whilst a pollutant may cause a mild irritation in the adult airways, in a child, it can potentially be a more substantial obstruction (Bruce et al. 2013).
Most of the research studies carried out in low- and middle-income countries mainly depend on questionnaires for data collection. These studies are limited because they often depend on self-reported questionnaires to understand exposures related to indoor air pollution and health outcomes, which are subject to several inherent biases (Broor et al. 2001; Sharma et al. 1998; Dhimal et al. 2010; Kelly et al. 2015; Mahalanabis et al. 2002; Karki et al. 2014; PrayGod et al. 2016; Mustapha et al. 2011. In recent years, the emergence of specific instrument allowing the measurement and quantification of indoor air quality has enabled researchers to identify pollutants involved in causing diseases in rural and urban areas in France (Hulin et al. 2010) and low- and middle-income countries such as Eastern Indonesia (Shibata et al. 2014).
We found eight reviews that investigated the impact of indoor air pollution on childhood pneumonia in children under 5. However, most of them investigated indoor air pollution as a single outcome (Bruce et al. 2013; Buchner and Rehfuess 2015; Dherani et al. 2008; Jackson et al. 2013; Rudan et al. 2008; Smith et al. 2000; Sonego et al. 2015; Zar and Ferkol 2014). However, they do not investigate the granularity of exposures. Therefore, this systematic review aims to segregate indoor air pollution into its different constituents and investigate the evidence available on how solid fuel use, PM2.5, carbon monoxide (CO), BC and other risk factors individually affect pneumonia in children under 5 in low- and middle-income countries.
Methods
Five electronic databases for published literature were used to identify peer-reviewed articles. These include Embase, PubMed, EBSCO/CINAHL, Scopus and Web of Knowledge. Additionally, four databases were used to identify grey literature, which included WHO Library Database (WHOLIS), Integrated Regional Information Networks (IRIN), the World Meteorological Organization (WMO)-WHO and Intergovernmental Panel on Climate Change (IPCC). Medical Subject Headings (MeSH), free-text terms and keywords used in the search include pollution (pollution, air pollution, indoor air pollution, carbon monoxide, carbon dioxide, nitrogen monoxide, nitrogen dioxide, particulate matter, sulphur dioxide, ozone, volatile organic compound and black carbon). These were combined with Boolean operators and/or with health (pneumonia, acute lower respiratory infection, respiratory health). The search strategy was customised to each database (Appendix 1). A reference list of selected papers was hand searched for relevant studies. The inclusion criteria were set at full-text articles investigating the effect of indoor air pollution on pneumonia in children under 5 residing in low- and middle-income countries (as defined by the World Bank (The World Bank 2017)), with a sample size above 100. The inclusion was not restricted by study design, language or year of publication.
The electronic search resulted in 21,074 papers. All papers were imported into Endnote, and 164 duplicates were removed automatically. After title and abstract screening by two independent reviewers (EA and WE) 20,838 papers were excluded due to lack of relevance to the current study. Seventy-six articles were selected for full-text review, of which 64 were rejected (17 not in the appropriate age range, 12 not focused on indoor air pollution, 15 measuring an irrelevant outcome, 12 non-full text articles and eight conducted in high-income countries). Eight review papers were considered relevant and included as supporting documents in the final review (Fig. 1). Disagreements throughout the review process were resolved with mutual consent.
Fig. 1.
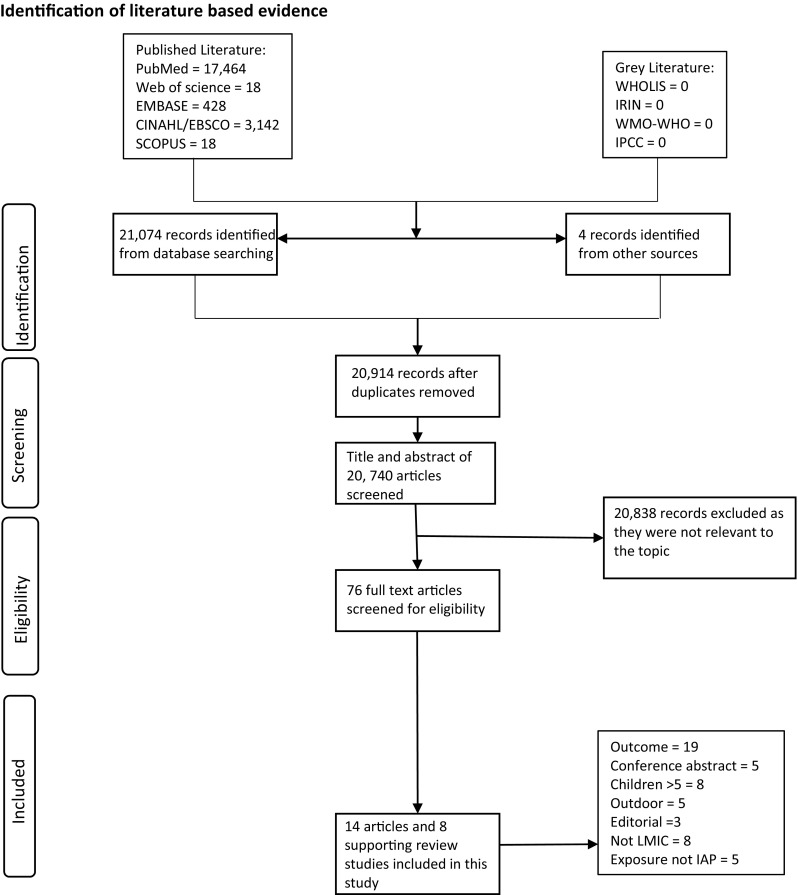
The flow of studies from identification to data extraction from databases based on the PRISMA guidelines
Quality assessment and risk of bias
The PRISMA guidelines were used for the review process (Liberati et al. 2009). The Newcastle-Ottawa Scale (Stang 2010) was used to assess the methodological quality and the risk of bias in individual studies (see Appendixes 5 and 6). The risk of bias for each study was divided into low, medium and high across the following domains: selection of participants (selection bias), sample size justification (selection bias), outcome measurement and confounding adjustment.
Results
The 22 studies included in this review included nine case-control studies (Bassani et al. 2010; Broor et al. 2001; Dionisio et al. 2012; Howie et al. 2016; Karki et al. 2014; Mahalanabis et al. 2002; PrayGod et al. 2016; Ram et al. 2014; Sharma et al. 1998); one cohort study (Kelly et al. 2015); one cross-sectional study (Dhimal et al. 2010); one mixed-method study, using both cross-sectional and case-control methods (Shibata et al. 2014); two randomised trials (Mortimer et al. 2017; Smith et al. 2011); and eight review papers (Bruce et al. 2013; Buchner and Rehfuess 2015; Dherani et al. 2008; Jackson et al. 2013; Rudan et al. 2008; Smith et al. 2000; Sonego et al. 2015; Zar and Ferkol 2014).
The country with the highest number of studies was India with four studies (Bassani et al. 2010; Broor et al. 2001; Mahalanabis et al. 2002; Sharma et al. 1998), followed by Nepal with two studies (Dhimal et al. 2010; Karki et al. 2014), the Gambia with two studies (Dionisio et al. 2012; Howie et al. 2016) and one study each from Bangladesh (Ram et al. 2014), Tanzania (PrayGod et al. 2016), Indonesia (Shibata et al. 2014), Malawi (Mortimer et al. 2017), Guatemala (Smith et al. 2011) and Botswana (Kelly et al. 2015). The studies were conducted between 1994 and 2017 with the majority covering the period 2007–2017.
The sample size and age of children varied in each study with a median size of 522 participants and from age 0 to 60 months, respectively. Nine (64%) studies were carried out in peri-urban cities, with only two studies looking at both urban and rural (Bassani et al. 2010) and peri-urban and rural (Mortimer et al. 2017; Smith et al. 2011; Karki et al. 2014). Thirteen studies collected primary data (Mortimer et al. 2017; Smith et al. 2011; Broor et al. 2001; Dionisio et al. 2012; Howie et al. 2016; Karki et al. 2014; Kelly et al. 2015; Mahalanabis et al. 2002; PrayGod et al. 2016; Ram et al. 2014; Sharma et al. 1998; Shibata et al. 2014), with only two using secondary data (Bassani et al. 2010; Dhimal et al. 2010).
Table 1 provides an overview of the included studies and their main findings. Solid fuel use was found to be significantly associated with childhood pneumonia in a majority of the studies (eight studies, 57%) (Bassani et al. 2010; Broor et al. 2001; Dhimal et al. 2010; Karki et al. 2014; Kelly et al. 2015; Mahalanabis et al. 2002; PrayGod et al. 2016; Sharma et al. 1998). Neither of the two studies investigating exposure to CO reported a significant association with pneumonia as an outcome (Dionisio et al. 2012; Howie et al. 2016). Similarly, exposure to PM2.5 showed no significant association in two studies (17%) that actually measured PM2.5 against using a proxy indicator (Ram et al. 2014; Shibata et al. 2014). Two trials also showed that interventions providing improved cookstoves did not reduce the risk of pneumonia in children (Mortimer et al. 2017; Smith et al. 2011).
Table 1.
Studies showing the association between indoor air pollution and childhood pneumonia
| No. | Country | Name/year of study | Setting | Study design | Study period | Primary/secondary data | Age in months | Exposure | Data collected using | Sample size | Outcome parameter | Main result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | India | Broor et al. (2001) | Peri-urban | Case-control | March 1995–February 1997 | Primary | < 60 | Solid fuel use | Questionnaire | 512 | Single disease episode | Cooking with any other type of fuel other than LPG (OR 2.5, 1.51–4.16) |
| 2 | India | Sharma et al. (1998) | Peri-urban | Case-control | November 1994–February 1995 | Primary | < 12 | Fuel used for cooking | Questionnaire | 642 | Multiple disease episode | Pneumonia was the most common illness in both fuel groups used at home for both wood and kerosene |
| 3 | India | Bassani et al. (2010) | Urban/rural | Case-control | February 1998 | Secondary | < 48 | Solid fuel use | Survey data | 616,391 | Mortality and single disease episode | Generally, children (0–4 years) with pneumonia had a higher reported solid fuel use compared to children without pneumonia (boys: PR 1.5, 1–2.4; girls: PR 1.9, 1.1–3.3) |
| 4 | Nepal | Dhimal et al. (2010) | Peri-urban | Cross-sectional | October 2008–January 2009 | Primary and secondary | < 60 | Fuel used for cooking | Questionnaire | 545,777 | Single disease episode | The solid biomass fuel was the primary source of energy for cooking which attributed to about 50% of the burden of pneumonia in children |
| 5 | Botswana | Kelly et al. (2015) | Urban/peri-urban | Cohort study | April 2012–April 2014 | Primary | < 24 | Wood smoke exposure | Questionnaire | 284 | Single disease episode and mortality | The risk of failure to respond to treatment at 48 h was increased in households that used wood as a cooking fuel (RR 1.44, 95% CI 1.09–1.92, P = 0.01). This effect was observed in undernourished children (P = 0.02) |
| 6 | Gambia | Dionisio et al. (2012) | Urban/peri-urban | Case-control | July 2007–January 2011 | Primary | < 60 | Exposure to CO | CO measurement questionnaire | 1181 | Single disease episode | There was an increased risk of pneumonia (OR 4.2, 3.1–5.7) in the rainy season compared to the dry season. Households where firewood or charcoal was purchased exposed children 2.0 (1.2–3.2) or 3.8 (2.1–7.1) times more to indoor air pollution compared to households that collected firewood |
| 7 | Gambia | Howie et al. (2016) | Urban/peri-urban | Case-control | June 2007-September 2010 | Primary | < 60 | Exposure to CO | CO measurement questionnaire | 1581 | Single disease episode | No association was found between CO exposure and childhood pneumonia. However, bed sharing with someone with a cough and severe pneumonia (OR 5.1, 3.2–8.2) and non-severe pneumonia (OR 7.3, 4.1–13.1). Undernutrition was associated with childhood pneumonia (OR 8.7, 4.2–17.8) |
| 8 | Indonesia | Shibata et al. (2014) | Urban | Cross-sectional/case-control | June 2011–June 2012 | Primary | < 60 | Measured PM2.5 and PM10 | PM2.5 measurement questionnaire | 461 | Single disease episode | Hourly sampling showed significant differences in PM2.5 and PM10 concentration between households in which children with pneumonia lived compared with controls |
| 9 | Bangladesh | Ram et al. (2014) | Urban | Case-control | March 2009–March 2010 | Primary | < 60 | Exposure to PM2.5 | PM2.5 measurement questionnaire | 994 | Single disease episode | PM2.5 was not significantly associated with pneumonia. However, crowding, aluminium roofing in living space, households with lower socioeconomic status and being a boy were associated with pneumonia |
| 10 | India | Mahalanabis et al. (2002) | Urban/peri-urban | Case-control | December 1997–November 1998 | Primary | < 35 | Risk factors | Questionnaire | 262 | Single disease episode | Solid fuel use with OR 3.97, 2–7.88, compared to not using any solid fuel for cooking |
| 11 | Nepal | Karki et al. (2014) | Peri-urban and rural | Case-control | June 2012–May 2013 | Primary | < 60 | Risk factors | Questionnaire | 200 | Single disease episode | Using solid fuel with location within living area (OR 3.76, 1.20–11.82) |
| 12 | Tanzania | PrayGod et al. (2016) | Urban/peri-urban | Case-control | May 2013–March 2014 | Primary | < 60 | Behaviour | Questionnaire | 117 | Single disease episode | Cooking indoors increased the risk of developing severe pneumonia (OR 5.5, 1.4–22.1) compared to cooking outdoors |
| 13 | Malawi | Mortimer et al. (2017) | Rural | Randomised control trial | December 2013–February 2016 | Primary | < 60 | Biomass smoke | Questionnaire and exposure measurement | 10,543 | Mortality and single disease | Cleaner burning biomass-fuelled cookstoves did not reduce the risk of pneumonia in young children under 5 in Malawi |
| 14 | Guatemala | Smith et al. (2011) | Rural | Randomised control trial | October 2002–December 2004 | Primary | < 18 | Household air pollution | Questionnaire and exposure measurement | 534 | Mortality and single disease episode | Reduction of wood smoke exposure with chimney stoves did not significantly reduce pneumonia in children under 5 |
Pollution measurements within the studies were compared with the WHO-approved standard (WHO 2017). Measurements were taken over 24 h amongst studies, and repeated measurements were done between 2 and 3 months (Dionisio et al. 2012). Five studies that accounted for seasonal variation matched their study participants according to the season of recruitment (Bassani et al. 2010; Dionisio et al. 2012; Howie et al. 2016; Sharma et al. 1998; Shibata et al. 2014). Given the wide variation in the objectives and designs of the included studies, it was difficult to summarise the findings. We, therefore, describe the results in four subsections according to exposures investigated: solid fuel use, CO, PM2.5 and other general risk factors identified.
Exposure to solid fuel use
Eight (57%) studies investigated the association between solid fuel use and childhood pneumonia. Six studies found positive associations between solid fuel use and pneumonia in children. Broor et al. (2001), from India, reported that any cooking fuel other than liquid petroleum gas was associated with pneumonia in children under 5 (odds ratio (OR) 2.5, 1.51–4.16). Karki et al. (2014), from Nepal, identified a significant association for the type of cooking fuel (chulo) used in the household with increased childhood pneumonia (OR 3.76, 1.20–11.82). Whilst two trials also showed that interventions providing improved cookstoves did not reduce the risk of pneumonia in children (Mortimer et al. 2017; Smith et al. 2011).
Furthermore, Bassani et al. (2010), from India, reported that solid fuel use significantly increased all-cause child mortality for boys (prevalence ratio (PR) 1.30, 1.08–1.56) and girls (PR 1.33, 1.12–1.58); also, association with non-fatal pneumonia was observed for boys (PR 1.54, 1.01–2.35) and girls (PR 1.94, 1.13–3.33). Kelly et al. (2015), from Botswana, found that houses using wood as fuel showed an increased risk of failure to respond to pneumonia treatment at 48 h (RR 1.44, 1.09–1.92). Dhimal et al. (2010), from Nepal, established that a total of 1284 DALYs were lost due to pneumonia and half of it was due to smoke from solid fuels used for cooking indoors. Sharma et al. (1998), from India, stated that pneumonia was common across all study groups using both kerosene and wood as sources of fuel. In contrast, PrayGod et al. (2016), from Tanzania, reported that fuel type was not significantly associated with pneumonia. Shibata et al. (2014), from Indonesia, also confirmed this by reporting that the use of unhealthy cooking fuels was not significantly associated with pneumonia.
Exposure to CO
Two out of the 12 studies investigated the effect of CO on childhood pneumonia. Both studies from the Gambia, Howie et al. (2016) and Dionisio et al. (2012), reported no association between pneumonia and individual CO exposure as a measure of household air pollution.
Exposure to PM2.5
Ten out of the 12 studies investigated the effect of particulate matter with particles less than or equal to 2.5 μm in diameter (PM2.5) on childhood pneumonia. Out of the ten studies that looked at PM2.5, only two directly measured PM2.5 (Ram et al. 2014; Shibata et al. 2014), whilst eight used solid fuel use as a proxy indicator for PM2.5 (Bassani et al. 2010; Broor et al. 2001; Dhimal et al. 2010; Karki et al. 2014; Kelly et al. 2015; Mahalanabis et al. 2002; PrayGod et al. 2016; Sharma et al. 1998). The two studies that directly measured PM2.5 did not find any association, whilst the eight studies that used solid fuel as a proxy all reported associations.
Ram et al. (2014), from Bangladesh, found no association between PM2.5 and childhood pneumonia. However, they found an increased level of exposure to particulate matter in the households of cases than in control households. Particularly looking at cooking spaces in case households, PM2.5 was found to be 1.64 times more likely to be greater than 100 μg/m3 for more than 4 h compared to control households. Similarly, PM2.5 was found to be 1.70 times more likely to be greater than 250 μg/m3 for more than 1 h in cases compared to control households. Furthermore, in living spaces, PM2.5 was found to be 1.65 times more likely to be greater than 250 μg/m3 for more than 30 min in cases compared to control households. Also, increasing the duration of PM2.5 exposure greater than 100 μg/m3 or 250 μg/m3 showed no association with increased pneumonia incidence. In multivariate analysis, no association was found in any of the PM2.5 measures and pneumonia. Shibata et al. (2014), from Indonesia, also confirmed this, as there was no association found between PM2.5 levels when cases were compared with controls.
General risk factors associated with childhood pneumonia
Besides air quality measurements, all 12 studies described general risk factors (Table 2), which included socioeconomic factors, malnutrition, cooking location, bed sharing, the number of people sleeping in a room, the number of siblings, cross-ventilation and season. Howie et al. (2016) found an association between malnutrition and pneumonia (OR 8.7, 4.2–17.8). Karki et al. (2014), from Nepal, found that the risk of pneumonia was increased by up to four times in houses where cooking was done indoors (OR 3.76, 1.20–11.82).
Table 2.
Overview of included studies and summary of variables adjusted for within each study
| No. | Country | Study | Adjusted for | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name/year of study | Definition of air pollution | Pneumonia measurement | Access to care | Immunisation | Malnutrition | Exclusive breastfeeding | LBW | No. of siblings | Marital status | Occupation/SES | Parents smoke | Parent’s education | Religion | Kitchen location | Cooking fuel type | Cook area | No sleeps in room | Ventilation | House material | Source of drinking water | Season | ||
| 1 | India | Broor et al. (2001) | Use of solid fuel | Syndromic | ✔ | ✔† | ✔† | ✔† | ✔ | ✔ | ✔ | ✔ | ✔† | ✔ | |||||||||
| 2 | Botswana | Kelly et al. (2015) | Use of wood as fuel | Syndromic | ✔ | ✔† | ✔ | ✔ | ✔ | ✔† | ✔ | ✔ | |||||||||||
| 3 | India | Sharma et al. (1998) | Types of fuel used at home | Syndromic | ✔ | ✔ | ✔† | ✔ | ✔† | ✔† | ✔ | ✔ | |||||||||||
| 4 | Nepal | Karki et al. (2014) | Condition of home environment | Presumptive | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔† | ✔ | ✔† | ||||||||||
| 5 | India | Mahalanabis et al. (2002) | Condition of home environment | Presumptive | ✔ | ✔ | ✔ | ✔ | ✔† | ✔ | ✔ | ✔† | ✔ | ||||||||||
| 6 | Bangladesh | Ram et al. (2014) | Concentration of PM2.5 | Presumptive | ✔ | ✔† | ✔ | ✔ | ✔† | ✔† | ✔† | ||||||||||||
| 7 | India | Bassani et al. (2010) | Use of solid fuel | ✔† | ✔ | ✔† | ✔ | ✔† | ✔ | ✔† | ✔ | ✔ | |||||||||||
| 8 | Gambia | Dionisio et al. (2012) | Exposure to CO | Presumptive | ✔ | ✔† | ✔ | ✔† | ✔ | ✔† | |||||||||||||
| 9 | Gambia | Howie et al. (2016) | Exposure to CO | Presumptive | ✔ | ✔ | ✔† | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| 10 | Tanzania | PrayGod et al. (2016) | Condition of home environment | Both syndromic and presumptive | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔† | ✔ | |||||||
| 11 | Nepal | Dhimal et al. (2010) | Types of fuel used at home | Both syndromic and presumptive | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||
| 12 | Indonesia | Shibata et al. (2014) | ✔† | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||
| 13 | Malawi | Mortimer et al. (2017) | Types of fuel used at home | Both syndromic and presumptive | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| 14 | Guatemala | Smith et al. (2011) | Exposure from type of fuel used at home | Both syndromic and presumptive | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||
| Total | (15, 79%) | (8, 42%) | (8, 42%) | (10, 53%) | (6, 32%) | (7, 37%) | (2, 11%) | (19, 100%) | (13, 68%) | (14, 74%) | (2, 11%) | (5, 26%) | (15, 79%) | (8, 42%) | (9, 47%) | (2, 11%) | (4, 21%) | (5, 26%) | (8, 42%) | ||||
†Associated variables
Mahalanabis et al. (2002), from India, described a strong association between bed sharing and severe pneumonia (OR 5.1, 3.2–8.2) and non-severe pneumonia (OR 7.3, 4.1–13.1). Howie et al. (2016), from the Gambia, found an association between severe pneumonia (OR 5.1, 3.2–8.2) and non-severe pneumonia (OR 7.3, 4.1–13.1) and bed sharing with someone with a cough.
Ram et al. (2014), from Bangladesh, reported sleeping in a room with four or more people was significantly associated with pneumonia (OR 1.60, 1.18–2.18). However, PrayGod et al. (2016), from Tanzania, reported no significant association between the number of people sleeping in a room and pneumonia (OR 1.1, 0.7–1.7).
Mahalanabis et al. (2002), from India, did not find any association between numbers of siblings and the increase in disease episodes. However, Broor et al. (2001) and Bassani et al. (2010), both from India, reported having four or more siblings was significantly associated with all-cause mortality for both boys (PR 1.44, 1.18–1.76) and girls (PR 1.76, 1.43–2.10).
Ram et al. (2014), from Bangladesh, reported that having a cross-ventilated living area would be protective (OR 0.72, 0.53–0.98). Cross-ventilation in the living spaces of case households was 28% less than that in control households.
Five studies investigated seasonality. Bassani et al. (2010), from India, reported solid fuel use to be higher in children who died compared to living children between ages 1 and 4 in the same geographical area in winter months. Dionisio et al. (2012), from the Gambia, also reported that cooking with biomass fuel indoors or in an enclosed cookhouse rose to 84% during the rainy season compared to the dry season. However, Howie et al. (2016), from the Gambia, only matched participants based on seasons to account for the influence it might have on the study. Finally, Sharma et al. (1998), from India, and Shibata et al. (2014), from Indonesia, only took measurements during the winter and dry seasons, respectively, accepting that the complete seasonal influence within these studies is unknown.
Supporting studies on indoor air pollution and pneumonia
Eight review papers (Bruce et al. 2013; Buchner and Rehfuess 2015; Dherani et al. 2008; Jackson et al. 2013; Rudan et al. 2008; Smith et al. 2000; Sonego et al. 2015; Zar and Ferkol 2014) included six systematic reviews and two meta-analyses. All eight studies tried to understand factors responsible for childhood respiratory infections within low- and middle-income countries. All eight studies (Table 3) reported indoor pollution as an important risk factor for the increase in childhood pneumonia. Sonego et al. (2015) reported OR of 3.02 (2.11–4.31). The study of Zar and Rudans also concluded that improving nutrition, comprehensive immunisation, reduction in household crowding, avoidance of smoking, reduction in exposure to indoor pollutants and tackling of HIV/AIDS incidence in low- and middle-income countries can help in the prevention of pneumonia in children.
Table 3.
Reviews focused on the association between indoor air pollution and childhood pneumonia
| No. | Author (year) | Study design | Country | Number of studies | Exposure | Outcome | Key findings |
|---|---|---|---|---|---|---|---|
| 1 | Jackson et al. (2013) | Systematic review | Developing countries | 36 | Use of biomass fuel for cooking | Pneumonia | Exposure to indoor air pollution (OR 1.57, 1.06–2.31) |
| 2 | Dherani et al. (2008) | Meta-analysis and systematic review | Developing countries | 25 | Behaviour and environment (fuel use) | Pneumonia (ALRI) | Indoor air pollution is associated with pneumonia (OR 1.8, 1.5–2.2) |
| 3 | Smith et al. (2000) | Critical review | Papua New Guinea, Kenya, India, Nepal, China, Gambia | 18 | Indoor air pollution | Pneumonia (ALRI) | Confirms an association between indoor air pollution and childhood pneumonia, particularly in households using biomass fuels |
| 4 | Sonego et al. (2015) | Systematic review | LMIC as defined by the World Bank | 77 | Risk factors | Pneumonia (ALRI) | Confirms an association between indoor air pollution and childhood pneumonia (OR 3.02, 2.11–4.31) |
| 5 | Zar and Ferkol (2014) | Review | LMIC | – | Environmental risk factor, including indoor air pollution | Pneumonia | Improving nutrition, comprehensive immunisation, reduction in household crowding, avoidance of smoking, reduction in exposure to indoor pollutants and tackling of HIV/AIDS incidence in low- and middle-income countries can help in the prevention of pneumonia in children |
| 6 | Bruce et al. (2013) | Systematic review and meta-analysis | Global reports on developing countries | 26 | Solid fuel used for cooking | Pneumonia (ALRI) | Eliminating exposures to indoor air pollution might considerably reduce the risk of pneumonia complications, including fatality |
| 7 | Rudan et al. (2008) | Systematic review | Global reports on developing countries | 28 | Indoor air pollution | Pneumonia (ALRI) | Show an association between indoor air pollution and childhood pneumonia (OR 1.8) |
| 8 | Buchner and Rehfuess (2015) | A cross-sectional multi-country analysis | Benin, Burkina Faso, Cameroon, Ethiopia, Ghana, Guinea, Kenya, Madagascar, Mali, Malawi, Mozambique, Namibia, Niger, Senegal, Tanzania, Uganda, Zambia and Zimbabwe | – | Risk factors (indoor air pollution) | Pneumonia (ALRI) | Show an association between indoor air pollution and childhood pneumonia (OR 2.17, 1.09–4.33) |
Buchner and Rehfuess (2015) found that indoor cooking and season were significantly associated with childhood pneumonia with OR during the rainy season to be 1.80 (1.30–2.50) and OR during the dry season to be 1.51 (1.09–2.10). Bruce et al. (2013) focused on the type of fuel used and exposure to biomass smoke. Jackson et al. (2013) reported that exposure to indoor air pollution from the use of biomass fuels for cooking was associated with childhood pneumonia (OR 1.57, 1.06–2.31). Dherani et al. (2008) conducted a meta-analysis using 25 studies which reported that fuel used at home, living environment and behaviour could influence the quality of indoor air in low- and middle-income countries. Dherani et al. (2008) reported that the use of unprocessed solid fuel was associated with childhood pneumonia (OR 1.8, 1.5–2.2) in low- and middle-income countries. Finally, Smith et al. (2000) confirmed a strong association between household biomass fuel and pneumonia episodes in children (OR 3.02, 2.11–4.31) in low- and middle-income countries.
Discussion
Over the past 17 years, an increasing number of studies have investigated the role of indoor air pollution and its effect on childhood pneumonia in children below the age of 5. Twelve of these studies have mainly focused on PM2.5 (Mortimer et al. 2017; Smith et al. 2011; Bassani et al. 2010; Broor et al. 2001; Bruce et al. 2013; Buchner and Rehfuess 2015; Dherani et al. 2008; Dhimal et al. 2010; Dionisio et al. 2012; Howie et al. 2016; Jackson et al. 2013; Karki et al. 2014; Kelly et al. 2015; Mahalanabis et al. 2002; PrayGod et al. 2016; Ram et al. 2014; Rudan et al. 2008; Sharma et al. 1998; Shibata et al. 2014; Smith et al. 2000; Sonego et al. 2015; Zar and Ferkol 2014), solid fuels and general risk factors within low- and middle-income countries. This has led to a greater understanding and better management of the disease over the years. Pneumonia-related deaths are decreasing. In 2000, the mortality rate was 1.7 million but has fallen to 920,000 globally in 2015 (UNICEF 2016). Undeniably, compared to mortality rates in other common paediatric diseases such as measles, HIV and malaria, pneumonia-associated mortality rates have had a significantly slower rate of reduction (UNICEF 2016).
Results from this review confirm the role exposure to indoor air pollution plays in childhood pneumonia. Ninety per cent of all the studies included in this review reported that solid fuel use was significantly associated with an increased incidence of childhood pneumonia. However, 10% reported no significant association between solid fuel use and the risk of childhood pneumonia. These discrepancies in the results might be due to the different study designs and sample sizes used. Also, when indoor air pollution is measured, the association fails to reach a statistical significance. However, when solid fuel is used as a proxy, a larger more significant association is observed. This could be due to equipment used in the measurement of pollution, study power and also lower-quality study designs. Nonetheless, there is evidence suggesting a link between solid fuel use and incidence of pneumonia in children. There is a need to improve the study designs: first, to investigate the solid fuel–meditated pollutant components for each fuel type and then investigate the relationship with pneumonia incidence.
Studies, where CO was used as a proxy for pollution, showed no association on childhood pneumonia. Although solid fuel use has been shown to have a strong association with increased child CO exposure, there is no evidence to suggest the link to disease incidence. However, more studies need to be done to elucidate the validity of CO exposure as a metric for pneumonia incidence in children, especially given the emergence of improved methods of measuring indoor CO exposure (Klasen et al. 2015; Zhang et al. 2013).
Children with higher exposure to particulate matter were at a higher risk of developing pneumonia compared to children with less exposure. It is important to note that this association of PM2.5 and incidence of childhood pneumonia were observed when biomass fuel use was used as a proxy for PM2.5 and not when PM2.5 was directly measured (Karki et al. 2014; Rudan et al. 2008; Sharma et al. 1998; Sonego et al. 2015). PM2.5 as an effective exposure metric in health outcome studies has been contested (Patange et al. 2015). Furthermore, results from this review showed that cooking and living spaces had higher levels of PM2.5 in case houses compared to controls (Karki et al. 2014; PrayGod et al. 2016; Shibata et al. 2014). Crowding was more common in the houses of cases compared to controls (Ram et al. 2014). All these suggest a link between polluted indoor air and pneumonia incidence. However, these studies could not directly link measured PM2.5 with childhood pneumonia after multivariate analysis did not reach a statistical significance. It is, therefore, important to look closely at the components of PM2.5 that might be better exposure metrics to investigate. For example, black carbon (BC), which is produced following the incomplete combustion of solid fuels, has shown a higher association with disease incidences in studies where the direct association between PM2.5 did not show more association (Geng et al. 2013; Wang et al. 2013). So far, no study has looked at BC and childhood pneumonia. Therefore, studies looking at the influence of indoor air pollution and childhood pneumonia in the future should account for individual components of PM2.5 such as PM1, where possible, and BC (Baumgartner et al. 2014).
Other risk factors that showed possible association with childhood pneumonia were season where there was an increased risk of pneumonia (OR 4.2, 3.1–5.7) in the rainy season compared to the dry season (Kelly et al. 2015), and bed sharing with someone with a cough which increased the risk of severe pneumonia (OR 5.1, 3.2–8.2) and non-severe pneumonia (OR 7.3, 4.1–13.1) (Howie et al. 2016). Also, undernutrition was associated with childhood pneumonia (OR 8.7, 4.2–17.8) (Howie et al. 2016). Finally, crowding and building materials showed a significant association with childhood pneumonia (Ram et al. 2014).
Strengths and limitation
This review looked at the individual constituents of indoor air pollution and how they affect pneumonia in children under 5. The search strategy was designed to be extensive and, at all stages, minimise chance and bias by excluding studies with fewer than 100 patients. The review was not restricted by language, study design or year of publication and took into account published and unpublished materials from nine databases. Studies, where secondary data was used, should be interpreted with care because data from national surveys were collected for other objectives. Studies where caregivers or study personnel followed an outcome definition of pneumonia with at least one precise sign of as described by the WHO were included. There was no standard definition of pneumonia, which could mean some of the impacts of indoor air pollution may remain under- or overreported.
A limitation with comparing all of these studies is the lack of standardisation of methods used. The air quality measurements could have been influenced by the equipment and study design used in each study. Not all air quality equipment and study design can give a comprehensive overview of the indoor environment, which will often require continuous measurements with detailed activity monitoring.
Seven of the 12 studies based their diagnosis on a qualified personnel’s objective assessment of pneumonia, with two looking at symptoms. The risk of disease misclassification bias cannot be ruled out given that none of these studies used radiological confirmation of pneumonia. Probably, this could be the reason for some of the findings of the review with regard to association between exposure to pollutants and childhood pneumonia as an outcome. Methodologically, none of these studies was fit to assess causality or dose-effect relationship. Majority of the studies were conducted in rapidly progressing peri-urban areas. Nonetheless, none of them investigated the interaction between outdoor and indoor air quality and their potential combined impacts on childhood pneumonia.
Research gaps identified
Air pollution research and guidelines have exclusively focused on PM2.5 and CO rather than the sources or components including those proposed by the World Health Organization (WHO 2017). Emerging research shows that BC has a higher health-related impact compared to PM2.5 (Baumgartner et al. 2014). PM2.5 is a complex pollutant consisting of components such as sulphates, nitrates and black carbon (US-EPA 2017). Previous studies have shown that an intervention targeted generally at PM reduction does not necessarily lead to a corresponding decrease in all the components of PM (Aung et al. 2016; Kar et al. 2012; Preble et al. 2014). For example, the development of improved cookstoves aimed at reducing PM mass resulted in higher BC concentrations (Aung et al. 2016; Kar et al. 2012; Preble et al. 2014). Propositions have been made to include BC as an outdoor air quality indicator (Baumgartner et al. 2014). BC, as suggested by other researchers, could be significant in the future as an exposure assessment tool for health studies because currently, we might be underestimating the true health effect of indoor air pollution by focusing largely on PM2.5. Also, if BC has a higher health impact than PM2.5, its measurement will involve smaller sample sizes and will lead to more accurate health estimates of the impact of indoor air pollution in children under 5. This will impact on policies and interventions.
Conclusion
Findings from this review indicate that measurements and definitions of exposures and outcomes are unclear and so is the current evidence of indoor air pollution and pneumonia in children. The use of proxy indicators such as solid fuel has suggested an association; however, other proxy indicators such as CO and PM2.5 have not shown any associations, and this may be as a result of methods used. We recommend that future studies should have a standardisation of methods to improve the comparability of studies. There were no studies investigating the effect of BC and PM1 on childhood pneumonia. Similarly, the interaction between outdoor and indoor air pollution has not been investigated. Therefore, we propose pilot studies to fill these gaps in regions of high incidence of childhood pneumonia. A stepwise approach starting with hospital cohorts and moving to survey data and then birth cohorts will altogether culminate in filling these knowledge gaps. Preferably, factors influencing pneumonia incidence, disease onset and lasting health outcomes should be ascertained in all cases of respiratory infections in children under age 5. Also, future studies should try to understand how season, bed sharing, undernutrition, crowding and building materials can influence the risk of childhood pneumonia. This may help inform more targeted strategies to reducing the burden of pneumonia in this age group. Studies addressing these research gaps will be important to inform strategies aimed at reducing the incidence of childhood pneumonia in low- and middle-income countries.
Appendix 1. Search terms
PubMed
Indoor air pollution
(((“Air Pollution, Indoor/adverse effects”[Mesh] OR “Air Pollution, Indoor/analysis”[Mesh] OR “Air Pollution, Indoor/statistics and numerical data”[Mesh])) OR “Pollution”[Mesh] “Tobacco Smoke Pollution”[Mesh]) OR “Air Pollution, Indoor”[Mesh]
Pneumonia
“Pneumonia”[Mesh] OR “Pneumonia, Ventilator-Associated”[Mesh] OR “Pneumonia, Bacterial”[Mesh] OR “Pneumonia, Viral”[Mesh] OR “Pneumonia, Staphylococcal”[Mesh] OR “Pneumonia, Rickettsial”[Mesh] OR “Pneumonia, Mycoplasma”[Mesh] OR “Pneumonia, Pneumococcal”[Mesh] OR “Pneumonia, Aspiration”[Mesh] OR “Pulmonary Eosinophilia”[Mesh] OR “Idiopathic Interstitial Pneumonias”[Mesh] OR “Idiopathic Pulmonary Fibrosis”[Mesh] OR “Lung Diseases, Interstitial”[Mesh] OR “Bronchopneumonia”[Mesh]
Children
(“Child, Preschool”[Mesh]) AND “Child”[Mesh]
((((“Air Pollution, Indoor/adverse effects”[Mesh] OR “Pollution”[Mesh] OR “Air Pollution, Indoor/analysis”[Mesh] OR “Air Pollution, Indoor/statistics and numerical data”[Mesh])) OR “Tobacco Smoke Pollution”[Mesh]) OR “Black carbon”[Mesh] OR “Air Pollution, Indoor”[Mesh] AND “Pneumonia”[Mesh] OR “Pneumonia, Ventilator-Associated”[Mesh] OR “Pneumonia, Bacterial”[Mesh] OR “Pneumonia, Viral”[Mesh] OR “Pneumonia, Staphylococcal”[Mesh] OR “Pneumonia, Rickettsial”[Mesh] OR “Pneumonia, Mycoplasma”[Mesh] OR “Pneumonia, Pneumococcal”[Mesh] OR “Pneumonia, Aspiration”[Mesh] OR “Pulmonary Eosinophilia”[Mesh] OR “Idiopathic Interstitial Pneumonias”[Mesh] OR “Idiopathic Pulmonary Fibrosis”[Mesh] OR “Lung Diseases, Interstitial”[Mesh] OR “Bronchopneumonia”[Mesh] AND (“humans”[MeSH Terms] AND ((“infant”[MeSH Terms] OR “child”[MeSH Terms] OR “adolescent”[MeSH Terms]) OR “children nder 5”[Mesh] OR “infant, newborn”[MeSH Terms] OR “infant”[MeSH Terms] OR “infant”[MeSH Terms:noexp] OR “child, preschool”[MeSH Terms])))
Embase search
exp Infant/ or infant$.mp. or infancy.mp. or newborn$.mp. or baby$.mp. or babies.mp. or neonat$.mp. or exp Child/ or child$.mp. or kid.mp. or kids.mp. or toddler$.mp. or exp Adolescent/ or adoles$.mp. or teen$.mp. or boy$.mp. or girl$.mp. or exp Pediatrics/ or pediatric$.mp. or paediatric$.mp. or paediatric$.mp. or young people.mp.
And
Pneu$.mp. or exp Pneumonia
And
Air pollution/ or indoor air pollution/ or indoor air quality.mp
Web of Knowledge
Infant or infant or infancy or newborn or baby or babies or neonat or child or child or kid or kids or toddler or adolescent or adolesc or teen or boy or girl or pediatrics or pediatric or paediatric or paediatric or young people
AND
pneu or pneumonia
AND
Air pollution or indoor air pollution or carbon monoxide or carbon mono or carbon dioxide or carbon diox or nitrogen monoxide or nitrogen dioxide or particulate matter or sulphur dioxide or ozone or volatile organic compounds or black carbon
CINAHL
“(indoor air quality OR indoor air pollution) AND pneumonia AND (children under 5 OR children under five OR children)”
Scopus
Pneumonia AND indoor air pollution AND children under 5
Appendix 2
Table 4.
Pico table of screening criteria for titles and abstracts
| Criterion | Guidance notes | Decision |
|---|---|---|
| 1. Year | No restriction on date | |
| 2. Study design | To include: cross-sectional studies, case-control studies, longitudinal observation studies (prospective and retrospective cohort and longitudinal studies) and systematic reviews (meta-analysis/reviews/reviews of reviews) | |
| 3. Type of participants | Studies involved children aged 0–5 years old | |
| 4. Health outcomes | The study focused on pneumonia as the primary health outcome | |
| 5. Exposure | Does the study look at indoor air pollution as primary exposure |
Appendix 3. Newcastle-Ottawa Quality Assessment Scale case-control studies
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability.
Selection
- Is the case definition adequate?
- Yes, with independent validation
- Yes, e.g. record linkage or based on self-reports
- No description
- Representativeness of the cases
- Consecutive or obviously representative series of cases
- Potential for selection biases or not stated
- Selection of controls
- Community controls
- Hospital controls
- No description
- Definition of controls
- No history of disease (endpoint)
- No description of source
Comparability
- Comparability of cases and controls on the basis of the design or analysis
- Study controls for _______________ (Select the most important factor.)
- Study controls for any additional factor (This criteria could be modified to indicate specific control for a second important factor.)
Exposure
- Ascertainment of exposure
- Secure record (e.g. surgical records)
- Structured interview blinded to case/control status
- Interview not blinded to case/control status
- Written self-report or medical record only
- No description
- Same method of ascertainment for cases and controls
- Yes
- No
- Non-response rate
- Same rate for both groups
- Non-respondents described
- Rate different and no designation
Appendix 4. Newcastle-Ottawa Quality Assessment Scale cohort studies
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability.
Selection
- Representativeness of the exposed cohort
- Truly representative of the average _______________ (describe) in the community
- Somewhat representative of the average ______________ in the community
- Selected group of users, e.g. nurses, volunteers
- No description of the derivation of the cohort
- Selection of the non-exposed cohort
- Drawn from the same community as the exposed cohort
- Drawn from a different source
- No description of the derivation of the non-exposed cohort
- Ascertainment of exposure
- Secure record (e.g. surgical records)
- Structured interview
- Written self-report
- No description
- Demonstration that outcome of interest was not present at the start of study
- Yes
- No
Comparability
- Comparability of cohorts on the basis of the design or analysis
- Study controls for _____________ (select the most important factor)
- Study controls for any additional factor (This criteria could be modified to indicate specific control for a second important factor.)
Outcome
- Assessment of outcome
- Independent blind assessment
- Record linkage
- Self-report
- No description
- Was follow-up long enough for outcomes to occur
- Yes (select an adequate follow-up period for outcome of interest)
- No
- Adequacy of follow-up of cohorts
- Complete follow-up—all subjects accounted for
- Subjects lost to follow-up unlikely to introduce bias—small number lost ≥ 70% (select an adequate %) follow-up, or description provided of those lost
- Follow-up rate < 70% (select an adequate %) and no description of those lost
- No statement
Appendix 5
Table 5.
Risk of bias assessment tool (Newcastle-Ottawa scale)
| Domain (source of bias) | Assessment | Risk of bias |
|---|---|---|
| Selection (representativeness of the sample) | Adequate case definition with independent validation (A) | Low |
| Consecutive or obviously representativeness (B) | Moderate | |
| Selection of community participants (C) | High | |
| No description of sampling strategy (D) | Unclear/high | |
| Selection (sample size) | Justified and satisfactory (A) | Low |
| Not justified (B) | High | |
| Detection (exposure) | Validated measurement tool (A) | Low |
| Tool described but non-validated (B) | High | |
| Tool not described (C) | Unclear/high | |
| Confounding | Adjusted for confounders (A) | Low |
| No adjustment for confounders (B) | High | |
| (Detection) Outcome assessment | Syndromic (A) | – |
| Presumptive (B) | – | |
| Both (C) | – | |
| No description (D) | – |
Appendix 6
Table 6.
Risk of bias assessment for each study
| No. | Country | Name/year of study | Selection (sampling) | Selection (sample size) | Detection (exposure) | Control confounders | Detection (outcome assessment) |
|---|---|---|---|---|---|---|---|
| 1 | India | Broor et al. (2001) | A | A | B | A | A |
| 2 | Botswana | Kelly et al. (2015) | A | A | B | A | A |
| 3 | India | Sharma et al. (1998) | A | A | A | A | A |
| 4 | Nepal | Karki et al. (2014) | A | A | A | A | B |
| 5 | India | Mahalanabis et al. (2002) | A | A | A | A | B |
| 6 | Bangladesh | Ram et al. (2014) | A | A | A | A | B |
| 7 | India | Bassani et al. (2010) | A | A | A | A | B |
| 8 | Gambia | Dionisio et al. (2012) | A | A | B | A | B |
| 9 | Gambia | Howie et al. (2016) | A | A | A | A | B |
| 10 | Tanzania | PrayGod et al. (2016) | A | A | A | A | B |
| 11 | Nepal | Dhimal et al. (2010) | A | B | A | A | C |
| 12 | Indonesia | Shibata et al. (2014) | A | A | B | A | C |
| 13 | Malawi | Mortimer et al. (2017) | B | A | A | A | A |
| 14 | Guatemala | Smith et al. (2011) | A | B | A | A | A |
Author’s contribution
Enemona Emmanuel Adaji (EA), Dr. Revati Phalkey (RV) and Dr. Michael Clifford (MC) conceptualised the study. All authors (EA and RV) conducted the analysis and confirmed the findings. Winifired Ezekie (WE) was the second reviewer, who independently reviewed the articles for inclusion. All authors contributed for the preparation of manuscript and approved for submission.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Agabiti N, Mallone S, Forastiere F, Corbo GM, Ferro S, Renzoni E, Sestini P, Rusconi F, Ciccone G, Viegi G, Chellini E, Piffer S. The impact of parental smoking on asthma and wheezing. SIDRIA Collaborative Group. Studi Italiani sui Disturbi Respiratori nell’Infanzia e l’Ambiente. Epidemiology. 1999;10:692–698. doi: 10.1097/00001648-199911000-00008. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170:319–343. doi: 10.1164/rccm.200209-1062ST. [DOI] [PubMed] [Google Scholar]
- Aung TW, Jain G, Sethuraman K, Baumgartner J, Reynolds C, Grieshop AP, Marshall JD, Brauer M. Health and climate-relevant pollutant concentrations from a carbon-finance approved cookstove intervention in rural India. Environ Sci Technol. 2016;50:7228–7238. doi: 10.1021/acs.est.5b06208. [DOI] [PubMed] [Google Scholar]
- Bassani DG, Jha P, Dhingra N, Kumar R. Child mortality from solid-fuel use in India: a nationally-representative case-control study. BMC Public Health. 2010;10:279. doi: 10.1186/1471-2458-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain–like receptors. J Allergy Clin Immunol. 2012;129:14–26. doi: 10.1016/j.jaci.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc Natl Acad Sci U S A. 2014;111:13229–13234. doi: 10.1073/pnas.1317176111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Am J Respir Crit Care Med. 2006;173:297–303. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broor S, Pandey RM, Ghosh M, Maitreyi RS, Lodha R, Singhal T, Kabra SK. Risk factors for severe acute lower respiratory tract infection in under-five children. Indian Pediatr. 2001;38:1361–1369. [PubMed] [Google Scholar]
- Bruce NG, Dherani MK, Das JK, Balakrishnan K, Adair-Rohani H, Bhutta ZA, Pope D. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health. 2013;13(Suppl 3):S8. doi: 10.1186/1471-2458-13-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner H, Rehfuess EA. Cooking and season as risk factors for acute lower respiratory infections in African children: a cross-sectional multi-country analysis. PLoS One. 2015;10:e0128933. doi: 10.1371/journal.pone.0128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Oliveira R, Pires-Neto RC, Bustillos JOV, Macchione M, Dolhnikoff M, Saldiva PHN, Garcia MLB. Chemical composition modulates the adverse effects of particles on the mucociliary epithelium. Clinics. 2015;70:706–713. doi: 10.6061/clinics/2015(10)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell JL. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet Pathol. 2013;51:393–409. doi: 10.1177/0300985813502821. [DOI] [PubMed] [Google Scholar]
- CDC (2016) Help prevent pneumonia [Internet]. Centers for Disease Control and Prevention
- Chance GW. Environmental contaminants and children’s health: cause for concern, time for action. Paediatr Child Health. 2001;6:731–743. doi: 10.1093/pch/6.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Mason E, Borrazzo J, Campbell H, Rudan I, Liu L, Black RE, Bhutta ZA. Ending of preventable deaths from pneumonia and diarrhoea: an achievable goal. Lancet (London, England) 2013;381:1499–1506. doi: 10.1016/S0140-6736(13)60319-0. [DOI] [PubMed] [Google Scholar]
- DD O. A predominance of hypertensive heart failure in the Abuja Heart Study cohort of urban Nigerians: a prospective clinical registry of 1515 de novo cases. Eur J Heart Fail. 2013;15:835–842. doi: 10.1093/eurjhf/hft061. [DOI] [PubMed] [Google Scholar]
- Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhimal M, Dhakal P, Shrestha N, Baral K, Maskey M. Environmental burden of acute respiratory infection and pneumonia due to indoor smoke in Dhading. J Nepal Health Res Counc. 2010;8:1–4. [PubMed] [Google Scholar]
- Dionisio KL, Howie SR, Dominici F, Fornace KM, Spengler JD, Donkor S, Chimah O, Oluwalana C, Ideh RC, Ebruke B, Adegbola RA, Ezzati M. The exposure of infants and children to carbon monoxide from biomass fuels in the Gambia: a measurement and modeling study. J Expo Sci Environ Epidemiol. 2012;22:173–181. doi: 10.1038/jes.2011.47. [DOI] [PubMed] [Google Scholar]
- Emenius G, Svartengren M, Korsgaard J, Nordvall L, Pershagen G, Wickman M. Building characteristics, indoor air quality and recurrent wheezing in very young children (BAMSE) Indoor Air. 2004;14:34–42. doi: 10.1046/j.1600-0668.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Garrett MH, Hooper MA, Hooper BM, Abramson MJ. Respiratory symptoms in children and indoor exposure to nitrogen dioxide and gas stoves. Am J Respir Crit Care Med. 1998;158:891–895. doi: 10.1164/ajrccm.158.3.9701084. [DOI] [PubMed] [Google Scholar]
- Geng F, Hua J, Mu Z, Peng L, Xu X, Chen R, Kan H. Differentiating the associations of black carbon and fine particle with daily mortality in a Chinese city. Environ Res. 2013;120:27–32. doi: 10.1016/j.envres.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barcala F-JPS, Sampedro M, Lastres JS, Gonzalez MASJ, Bamonde L, et al. Impact of parental smoking on childhood asthma. Impacto do tabagismo parental sobreaasmainfantil (Portuguese) Original Article. 2013;89:294–299. doi: 10.1016/j.jped.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Health Effects Institute (2018) State of Global Air 2018. Special Report. Health Effects Institute, Boston
- Howie SRC, Schellenberg J, Chimah O, Ideh RC, Ebruke BE, Oluwalana C, Mackenzie G, Jallow M, Njie M, Donkor S, Dionisio KL, Goldberg G, Fornace K, Bottomley C, Hill PC, Grant CC, Corrah T, Prentice AM, Ezzati M, Greenwood BM, Smith PG, Adegbola RA, Mulholland K. Childhood pneumonia and crowding, bed-sharing and nutrition: a case-control study from the Gambia. Int J Tuberc Lung Dis. 2016;20:1405–1415. doi: 10.5588/ijtld.15.0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulin M, Caillaud D, Annesi-Maesano I. Indoor air pollution and childhood asthma: variations between urban and rural areas. Indoor Air. 2010;20:502–514. doi: 10.1111/j.1600-0668.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- Hussey SJK, Purves J, Allcock N, Fernandes VE, Monks PS, Ketley JM, Andrew PW, Morrissey JA. Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonisation. Environ Microbiol. 2017;19:1868–1880. doi: 10.1111/1462-2920.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola JJK, Parise H, Kislitsin V, Lebedeva NI, Spengler JD. Asthma, wheezing, and allergies in Russian schoolchildren in relation to new surface materials in the home. Am J Public Health. 2004;94:560–562. doi: 10.2105/AJPH.94.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Mathews KH, Pulanic D, Falconer R, Rudan I, Campbell H, Nair H. Risk factors for severe acute lower respiratory infections in children: a systematic review and meta-analysis. Croat Med J. 2013;54:110–121. doi: 10.3325/cmj.2013.54.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Hsu S-I, Yan B, Moors K, Chillrud SN, Ross J, Wang S, Perzanowski MS, Kinney PL, Whyatt RM, Perera FP, Miller RL. Childhood exposure to fine particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ Int. 2012;45:44–50. doi: 10.1016/j.envint.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A, Rehman IH, Burney J, Puppala SP, Suresh R, Singh L, Singh VK, Ahmed T, Ramanathan N, Ramanathan V. Real-time assessment of black carbon pollution in Indian households due to traditional and improved biomass cookstoves. Environ Sci Technol. 2012;46:2993–3000. doi: 10.1021/es203388g. [DOI] [PubMed] [Google Scholar]
- Karki S, Fitzpatrick AL, Shrestha S. Risk factors for pneumonia in children under 5 years in a teaching hospital in Nepal. Kathmandu Univ Med J. 2014;12:247–252. doi: 10.3126/kumj.v12i4.13729. [DOI] [PubMed] [Google Scholar]
- Kelly MS, Wirth KE, Madrigano J, Feemster KA, Cunningham CK, Arscott-Mills T, Boiditswe S, Shah SS, Finalle R, Steenhoff AP. The effect of exposure to wood smoke on outcomes of childhood pneumonia in Botswana. Int J Tuberc Lung Dis. 2015;19:349–355. doi: 10.5588/ijtld.14.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasen EM, Wills B, Naithani N, Gilman RH, Tielsch JM, Chiang M, Khatry S, Breysse PN, Menya D, Apaka C, Carter EJ, Sherman CB, Miranda JJ, Checkley W. Low correlation between household carbon monoxide and particulate matter concentrations from biomass-related pollution in three resource-poor settings. Environ Res. 2015;142:424–431. doi: 10.1016/j.envres.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Kinney P, Chillrud S, Jack D. A systematic review of innate immunomodulatory effects of household air pollution secondary to the burning of biomass fuels. Ann Glob Health. 2015;81:368–374. doi: 10.1016/j.aogh.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- Mahalanabis D, Gupta S, Paul D, Gupta A, Lahiri M, Khaled MA. Risk factors for pneumonia in infants and young children and the role of solid fuel for cooking: a case-control study. Epidemiol Infect. 2002;129:65–71. doi: 10.1017/S0950268802006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum ED, King C, Hollowell R, Zhou J, Colbourn T, Nambiar B, Mukanga D, Burgess DCH. Predictors of treatment failure for non-severe childhood pneumonia in developing countries—systematic literature review and expert survey—the first step towards a community focused mHealth risk-assessment tool? BMC Pediatr. 2015;15:547. doi: 10.1186/s12887-015-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer K, Ndamala C, Naunje A, Malava J, Katundu C, Weston W, Havens D, Pope D, Bruce N, Nyirenda M, Wang D, Crampin A, Grigg J, Balmes J, Gordon S. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet. 2017;389(10065):167–175. doi: 10.1016/S0140-6736(16)32507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya J, Bearer CF, Etzel RA. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics. 2004;113:996–1006. [PubMed] [Google Scholar]
- Mustapha BA, Blangiardo M, Briggs DJ, Hansell AL. Traffic air pollution and other risk factors for respiratory illness in schoolchildren in the Niger-Delta region of Nigeria. Environ Health Perspect. 2011;119:1478–1482. doi: 10.1289/ehp.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandasena S, Wickremasinghe AR, Sathiakumar N. Indoor air pollution and respiratory health of children in the developing world. World J Clin Pediatrics. 2013;2:6–15. doi: 10.5409/wjcp.v2.i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patange OS, Ramanathan N, Rehman IH, Tripathi SN, Misra A, Kar A, Graham E, Singh L, Bahadur R, Ramanathan V. Reductions in indoor black carbon concentrations from improved biomass stoves in rural India. Environ Sci Technol. 2015;49:4749–4756. doi: 10.1021/es506208x. [DOI] [PubMed] [Google Scholar]
- Pilotto LS, Smith BJ, Nitschke M, Ruffin RE, Mitchell R. Industry, air quality, cigarette smoke and rates of respiratory illness in Port Adelaide. Aust N Z J Public Health. 1999;23:657–660. doi: 10.1111/j.1467-842X.1999.tb01556.x. [DOI] [PubMed] [Google Scholar]
- PrayGod G, Mukerebe C, Magawa R, Jeremiah K, Török ME. Indoor air pollution and delayed measles vaccination increase the risk of severe pneumonia in children: results from a case-control study in Mwanza, Tanzania. PLoS One. 2016;11:e0160804. doi: 10.1371/journal.pone.0160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble CV, Hadley OL, Gadgil AJ, Kirchstetter TW. Emissions and climate-relevant optical properties of pollutants emitted from a three-stone fire and the Berkeley-Darfur stove tested under laboratory conditions. Environ Sci Technol. 2014;48:6484–6491. doi: 10.1021/es5002715. [DOI] [PubMed] [Google Scholar]
- Qazi SAS, MacLean R, Fontaine O, Mantel C, Goodman T, et al. Ending preventable child deaths from pneumonia and diarrhoea by 2025. Development of the integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea. Arch Dis Child. 2015;100(Suppl 1):S23–S28. doi: 10.1136/archdischild-2013-305429. [DOI] [PubMed] [Google Scholar]
- Ram PK, Dutt D, Silk BJ, Doshi S, Rudra CB, Abedin J, Goswami D, Fry AM, Brooks WA, Luby SP, Cohen AL. Household air quality risk factors associated with childhood pneumonia in urban Dhaka, Bangladesh. Am J Trop Med Hyg. 2014;90:968–975. doi: 10.4269/ajtmh.13-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Sethi GR, Rohtagi A, Chaudhary A, Shankar R, Bapna JS, Joshi V, Sapir DG. Indoor air quality and acute lower respiratory infection in Indian urban slums. Environ Health Perspect. 1998;106:291–297. doi: 10.1289/ehp.98106291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Wilson J, Watson L, LeDuc A, Meng C, Ansariadi EY, La Ane R, Manyullei S, Maidin A. Childhood acute respiratory infections and household environment in an eastern Indonesian urban setting. Int J Environ Res Public Health. 2014;11:12190–12203. doi: 10.3390/ijerph111212190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55:518–532. doi: 10.1136/thorax.55.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, McCracken J, Weber M, Hubbard A, Jenny A, Thompson L, Balmes J, Diaz A, Arana B, Bruce N. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378(9804):1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- Sonego M, Pellegrin MC, Becker G, Lazzerini M, Sankoh OA. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: a systematic review and meta-analysis of observational studies. PLoS One. 2015;10:e0116380. doi: 10.1371/journal.pone.0116380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- The World Bank (2017) World Bank Country and Lending Groups—World Bank Data Help Desk, Datahelpdesk.worldbank.org
- UNICEF (2016): One is too many: ending child deaths from pneumonia and diarrhoea. UNICEF DATA [Internet]. UNICEF DATA
- US-EPA (2017) Particle pollution designations. https://www3.epa.gov/pmdesignations/2012standards/docs/pm2.5_chemical_composition.pdf. Accessed 31 May 2017
- Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet (London, England) 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen R, Meng X, Geng F, Wang C, Kan H. Associations between fine particle, coarse particle, black carbon and hospital visits in a Chinese city. Sci Total Environ. 2013;458:1–6. doi: 10.1016/j.scitotenv.2013.04.008. [DOI] [PubMed] [Google Scholar]
- WHO (2017): WHO indoor air quality guidelines: household fuel combustion. WHO, pp. Who.int [PubMed]
- Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014;49:430–434. doi: 10.1002/ppul.23030. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li L, Smith M, Guo Y, Whitlock G, Bian Z, Kurmi O, Collins R, Chen J, Lv S, Pang Z, Chen C, Chen N, Xiong Y, Peto R, Chen Z. Exhaled carbon monoxide and its associations with smoking, indoor household air pollution and chronic respiratory diseases among 512 000 Chinese adults. Int J Epidemiol. 2013;42:1464–1475. doi: 10.1093/ije/dyt158. [DOI] [PMC free article] [PubMed] [Google Scholar]


