Abstract
From November 2016 to April 2017, a cross-sectional study to determine the sero-prevalence of contagious caprine pleuropneumonia (CCPP) and to investigate its epidemiology was conducted in selected districts of Borana zone in Ethiopia. In addition, the study aimed at identifying Mccp antigens using species specific primer of PCR. A multistage random sampling was implemented to select districts, pastoral associations (villages), and households. A total of 890 serum samples of small ruminants that had not been vaccinated (goats n = 789 and sheep n = 101) were collected and screened for the presence of antibodies against Mycoplasma capricolum subspecies capripneumoniae using a competitive enzyme-linked immunosorbent assay. Lung tissues and pleural fluid samples were collected from 3 sero-positive and clinically suspected goats for isolation of Mycoplasma capricolum subspecies capripneumoniae. Serology showed that overall 31.2% (246/789) of goats and 12.9% (13/101) of sheep were positive with statistically significant differences between districts (p = 0.001). Multivariable logistic regression analysis revealed that goats from Moyale and Yabello districts had higher odds of being positive than goats from Elwoya district with odd ratios of 2.05 and 1.61, respectively. Age of goats was also significantly associated with sero-positivity (OR = 1.47; CI 95% 1.2–1.8). Mycoplasma capricolum subspecies capripneumoniae was identified in 6 (75%) of the tissue samples using species-specific primer of PCR. Besides improving the understanding of the epidemiology of CCPP in the selected districts and demonstrating its wide distribution, the study highly also provides evidence of the possible role of sheep in the maintenance of the disease.
Keywords: CCPP, cELISA, Risk factors, Sero-prevalence
Introduction
Contagious caprine pleuropneumonia (CCPP) caused by Mycoplasma capricolum subspecies capripneumoniae (Mccp) is a severe and devastating respiratory disease with high morbidity and mortality in goats (Sadique et al. 2012; Tsehay et al. 2014), causing considerable economic losses (Asmare et al. 2016). It occurs in many countries in Africa, Asia, and Middle East (Prats-van der Ham et al. 2015) and is a classical trans-boundary animal disease (Shahzad et al. 2016). Moreover, the disease is included in the list of notifiable diseases of the World Organization for Animal Health (OIE 2008) as it threatens a significant number of goat populations throughout the world and has a considerable socioeconomic impact in infected territories (Atim et al. 2016). Though disease is mainly found in goats, subclinical cases were reported in sheep and some wild ruminant species (Asmare et al. 2016).
The classical disease caused by Mycoplasma capricolum subspecies capripneumoniae (Mccp) is predominantly respiratory (Thiaucourt et al. 1996). Typical cases of CCPP are characterized by extreme fever (41–43 °C), and high morbidity and mortality in susceptible herds affecting all ages (AU-IBAR 2015). Associated common clinical signs are anorexia, weakness, emaciation, dullness, exercise intolerance, and respiratory signs such as dyspnea, polypnea, coughing, and nasal discharges (Shahzad et al. 2016). Further, abortion and high mortality rates have been reported (Wazir et al. 2016).
Commonly used serological tests are indirect hemagglutination, complement fixation, and latex agglutination (LAT) to detect the antibody response of goats to Mccp (Samiullah 2013). Recently, a competitive enzyme-linked immunoassay (cELISA) for CCPP has been developed and found highly specific (Peyraud et al. 2014). The introduction of the cELISA for CCPP will permit the implementation of serological studies on a large scale (Younis et al. 2015). In addition to serological tests, molecular detection of Mccp directly in clinical samples was found highly sensitive and specific and should be used for diagnosis of CCPP, especially in outbreaks to confirm the disease for rapid control (Elhassan and Salama 2018).
In Ethiopia, goats play a unique role in the livelihood of pastoral communities, especially for women, as they provide milk and dairy products and are a source of income for the family to cover school fees for children and other family expenses. Despite the presence of a massive goat population and their important socio-economic role, health of small ruminants in general and goats in particular has received little attention so far (Lakew et al. 2014). Only few studies have been carried out in the area, but these showed that CCPP is prevalent and causes considerable mortality in goats. For instance, between 2011 and 2015, 83 outbreaks affecting 23,950 goats were reported (MoLF 2016). Hence, reliable epidemiological information is needed in order to design effective control measures. Specifically, antigen detection of Mccp and the role of sheep in the maintenance of the disease need to be explored. The objectives of the study were to assess the epidemiology of CCPP in the Borana zone and to characterize the causative agent using molecular techniques.
Materials and methods
This study was conducted in the Borana zone that is predominantly inhabited by the Borana community and extends to the Kenyan border in the South; Somali region in the South East; Southern Nation, Nationalities, and People Region (SNNPR) in the West and North; and Guji zone in the North East. Borana rangeland is characterized by a semiarid to arid climate (Kamara et al. 2005; Haile et al. 2011). Geographically, the area is located between from 4 to 6° N latitude and from 36 to 42° E longitude with altitude ranging from 1000 to 1700 m above sea level. The mean annual rainfall of the area ranges from 250 to 700 mm. The annual mean temperature varies from 19 to over 25 °C. Extensive pastoralism is the main means of livelihoods for the Borana people (Gelagay et al. 2007).
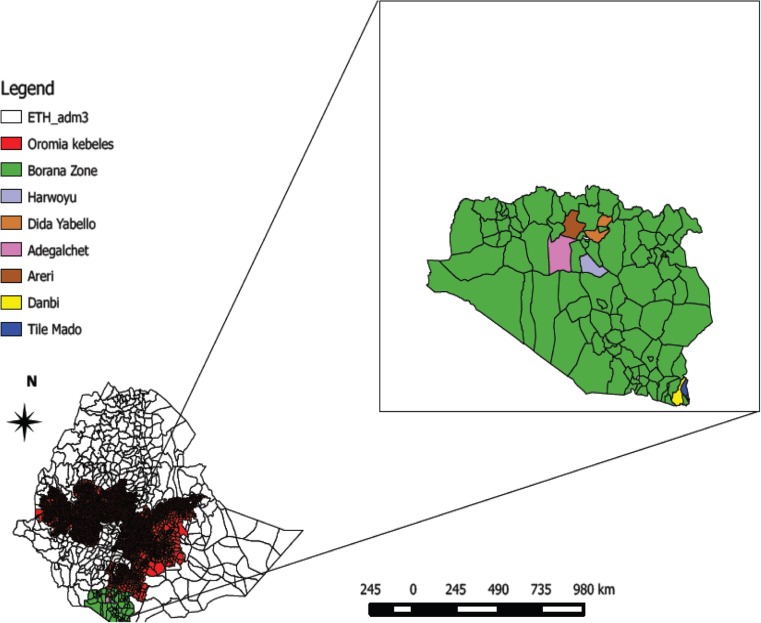
Multistage random sampling was applied to select the study animals. The sampling frame comprised a list of all districts in the zone and pastoral associations (PAs) or villages. Three districts were selected randomly, and in each of them, two PAs where no CCPP vaccination had been conducted for more than 2 years were selected. The resulting six PAs/villages were Areri and Adegalchet from Elwoya, Tile Mado and Dambi from Moyale, and Dida Yabello and Harwoyu from Yabello (Fig. 1).
Fig. 1.
Map of Ethiopia showing study areas
Finally, data were collected from a total of 161 households residing in the study villages. The distribution of households across the villages was 29, 30, 29, 20, 30, and 23 households from Adegalchet, Areri, Dambi, Tile Mado, Dida Yabello, and Harwoyu respectively. A total of 789 goats from 161 households in the selected PAs were sampled. Beside serum sample collection from the districts, randomly selected households (n = 161) who have small ruminants were interviewed using semi-structured questionnaire to capture general information they had on CCPP. If the flock size of a household was greater than five, 4 to 9 goats were selected from each flock whereas all goats per household were sampled if the flock size was less than or equal to five. In addition, 101 in contact sheep were selected purposively. The goats selected were identified with ear tags and information on household profiles and attributes of animals was collected before sampling. The age of animals was estimated using information from owner and dentition. Besides sero-samples, pleural fluids, and lung tissue samples were collected from sero-positive, clinically affected goats for molecular and bacteriological investigations. During sampling, recently introduced animals were excluded to avoid the risk of including vaccinated animals. To categorize flock size into small, medium, and large, five key informants were used from each district.
Sample size estimation
The estimation of sample size for epidemiological investigation using serological assay was done using the formula given by Thrusfield (2005) considering 95% confidence level, expected prevalence of 31.6% (Lakew et al. 2014) and 5% absolute precision.
where,
- n
required sample size
- Pexp
expected prevalence
- d
desired absolute precision (5%)
Accordingly, a minimum of 332 goats was obtained. To account for intra-class correlation at herd, village, and district levels, a design effect of 2 was considered, resulting in a minimum sample size of 664 (calculated with EpiInfo 7.2).
Blood sample collection
Approximately 5–7 mL of blood was collected from jugular vein of apparently healthy goats and sheep not involved in vaccination against CCPP for at least 2 years for serological examination using sterile vacutainer tubes and needles. Samples were then transported in an icebox to the microbiology laboratory of the Yabello Pastoral and Dryland Agriculture Research Center. The sera were separated after centrifugation at 1500 rpm for 10 min. The serum samples were collected into sterile cryogenic tubes and stored at − 20 °C until the samples were transported to the National Animal Health Diagnostic and Investigation Center (NAHDIC), Sebeta, Ethiopia, for analysis.
Collection of tissue samples
Three goats that were positive in the cELISA test or which were suspected to be clinically affected by CCPP after thorough clinical examination were purchased and sacrificed for postmortem examination. Gross pathological lesions were observed and samples of lung at the interface between the consolidated and unconsolidated healthy tissues and pleural fluids were collected and transported to the National Veterinary Institute (NVI), Bishoftu, Ethiopia, for molecular analysis using polymerase chain reaction (PCR) as described by Woubit et al. (2004).
Laboratory analysis of samples
The serum samples were examined for the presence of specific antibodies against Mccp by using a commercial cELISA (Idexx, France), according to the instructions of the manufacturer. The test is characterized by a specificity of 99.9%. At the end of the reactions, ELISA plates were read at 450 nm by BioTek ELx800 ELISA reader to determine the optical density and percentage of inhibition was calculated. Samples with percentage of inhibition greater than or equal to 55% were considered positive (Peyraud et al. 2014).
Polymerase chain reaction
Samples for polymerase chain reaction (PCR) were prepared as described by Woubit et al. (2004). About 1 g samples from each lung tissue and bronchial lymph nodes was taken and chopped with scissors and then grinded by mortar and pestle; mixed with 9 mL phosphate buffer solutions (PBS) and transferred to test tubes. For pleural fluids, 1 mL of pleural fluid was taken and mixed with 9 mL PBS and subjected for DNA extraction. Primers used (Mccp-spe-F, 5′-ATCATTTTTAATCCCTTCAAG-3′ and Mccp-spe-R, 5′-TACTATGAGTAATTATAATA-TATGCAA-3′) amplify a DNA fragment of 316 bp; PCR conditions were set as described by Woubit et al. (2004).
Data analysis
Data collected from the field and laboratory assays were entered and stored in Microsoft Excel spreadsheet, screened for proper coding and errors, and analysis was done. Disease prevalence and odds ratio were calculated using STATA 13.0 (Stata Corp. 1985–2013) statistical software. Logistic regression analysis was used to measure association between potential risk factors and sero-prevalence. Variables with p value of less than 0.05 were included in multivariable analysis and multivariable model was fitted. Finally, odd ratios and 95% confidence interval were calculated and disease-associated risk factors with a p value less than 0.05 considered significant.
Results
Survey result on symptoms of CCPP observed
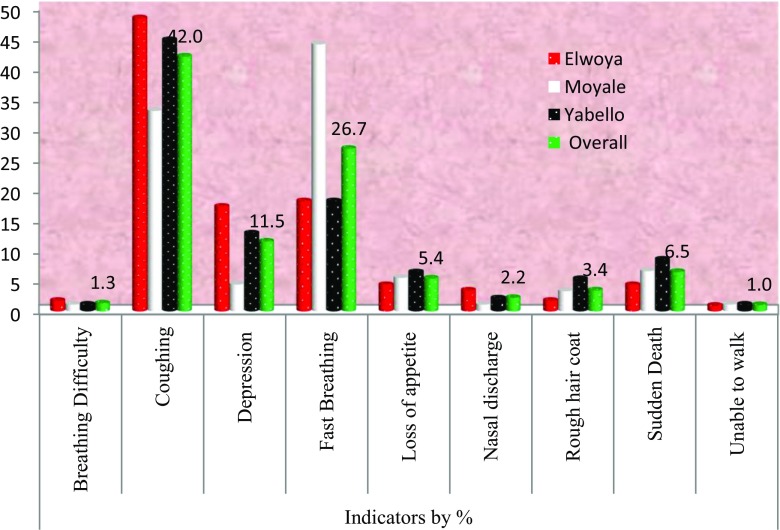
During the current survey, different and common overall symptoms of CCPP mentioned by respondents in the three study districts are coughing, fast breathing, depression, sudden death, inappetance, diarrhea, rough hair coat, nasal discharge difficulty in breathing, and reluctant to walk with 42%, 26.7%, 11.5%, 6.5%, 5.4%, 3.4%, 2.2%, 1.3%, and 1% respectively as indicated in Fig. 2.
Fig. 2.
Clinical symptoms of CCPP as reported by respondents (N = 161)
Sero-prevalence and associated risk factors for CCPP in goats
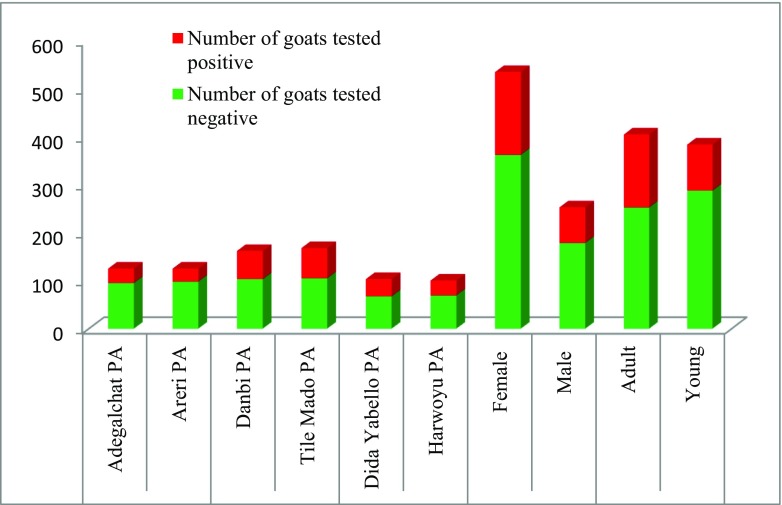
From the 161 households involved in the study, sera of 789 animals were collected. Sero-positivity was detected in all localities surveyed as depicted in Fig. 3. Two hundred forty-six (31.2%) of collected sera tested positive for anti-Mccp antibodies. The highest prevalence (36.70%) was observed in Moyale district, followed by Yabello (32.7%) and Elwoya (22.6%) (Table 1). The difference in sero-prevalence between districts was statistically significant (p = 0.001). There was also a significant difference in the sero-prevalence CCPP between different age groups (p < 0.001) in which adult goats (37.3%) were more likely to test positive than young goats (24.7%). Higher sero-prevalence was recorded in female goats (32.1%) than in males (29.1%) although this difference was not statistically significant. Similarly, sero-prevalence of CCPP was 34.3%, 32.2%, and 28.8% in small, medium, and large flock sizes, respectively. However, the difference in prevalence among various flock sizes was not statistically significant.
Fig. 3.
Proportion of sero-positivity in goats by locality
Table 1.
Results of univariable analysis to identify risk factors of sero-prevalence of CCPP in goats in Borana zone, Oromia, Ethiopia
| Risk factors | Number | Test positive | Prevalence | X 2 | p value |
|---|---|---|---|---|---|
| District | 13.618 | 0.001 | |||
| Elwoya | 252 | 57 | 22.6 | ||
| Moyale | 332 | 122 | 36.7 | ||
| Yabello | 205 | 67 | 32.7 | ||
| Sex | 0.73 | 0.393 | |||
| Female | 535 | 172 | 32.1 | ||
| Male | 254 | 74 | 29.1 | ||
| Age | 14.455 | < 0.001 | |||
| Adult | 405 | 151 | 37.3 | ||
| Young | 384 | 95 | 24.7 | ||
| Flock size | |||||
| Small | 175 | 60 | 34.3 | 1.82 | 0.402 |
| Medium | 267 | 86 | 32.2 | ||
| Large | 347 | 100 | 28.8 | ||
| Overall | 789 | 246 | 31.2 | ||
Fitting a multivariable regression model revealed that among the risk factors considered in the analysis (Table 2), district and age were associated with sero-positivity (p < 0.05), whereas sex and flock size had no statistically significant effect. The results showed that animals in Moyale and Yabello districts had about twice and 1.6 times higher odds of being positive for CCPP, respectively, than those animals reared in Elwoya district. Similarly, the odds of CCPP sero-prevalence was observed to significantly increase by 1.5 times as age of animals increase by 1 year (Table 2).
Table 2.
Results of multivariate logistic regression analysis of sero-prevalence of CCPP in goats
| Risk factor | Odds ratio | Std. Err. | z | p > |z| | (95% confidence interval) |
|---|---|---|---|---|---|
| District | |||||
| Moyale | 2.050 | 0.3982 | 3.7 | < 0.001 | (1.401–2.999) |
| Yabello | 1.611 | 0.3457 | 2.22 | 0.026 | (1.058–2.453) |
| Sex | |||||
| Male | 0.924 | 0.157 | − 0.47 | 0.64 | (0.662–1.289) |
| Age in year | 1.472 | 0.157 | 3.64 | < 0.001 | (1.195–1.814) |
| Flock size | |||||
| Medium | 1.172 | 0.211 | 0.88 | 0.378 | (0.823–1.669) |
| Small | 1.429 | 0.297 | 1.72 | 0.086 | (0.951–2.146) |
| _cons | 0.137 | 0.036 | − 7.55 | 0.000 | (0.081–0.229) |
Sero-prevalence and associated risk factors of CCPP in sheep
From a total of 101 serum samples collected from apparently healthy sheep and tested by cELISA, 13 (12.9%) were found positive. The differences in sero-prevalence between age groups, sex, and districts examined were not statistically significant (p > 0.05) as presented in Table 3.
Table 3.
Results of multivariable logistic regression analysis of associated risk factors of CCPP in sheep in the study area
| Risk factors | Odds ratio | Std. Err. | z | p > |z| | (95% Conf. interval) |
|---|---|---|---|---|---|
| Age in year | 1.185 | 0.603 | 0.33 | 0.738 | (0.437–3.213) |
| Sex | |||||
| Male | 0.320 | 0.274 | − 1.33 | 0.184 | (0.059–1.715) |
| District | |||||
| Moyale | 1.015 | 0.751 | 0.02 | 0.984 | (0.238–4.329) |
| Yabello | 0.885 | 0.697 | − 0.15 | 0.877 | (0.189–4.138) |
| _cons | 0.150 | 0.163 | − 1.74 | 0.081 | (0.018–1.264) |
Results of gross pathological examination
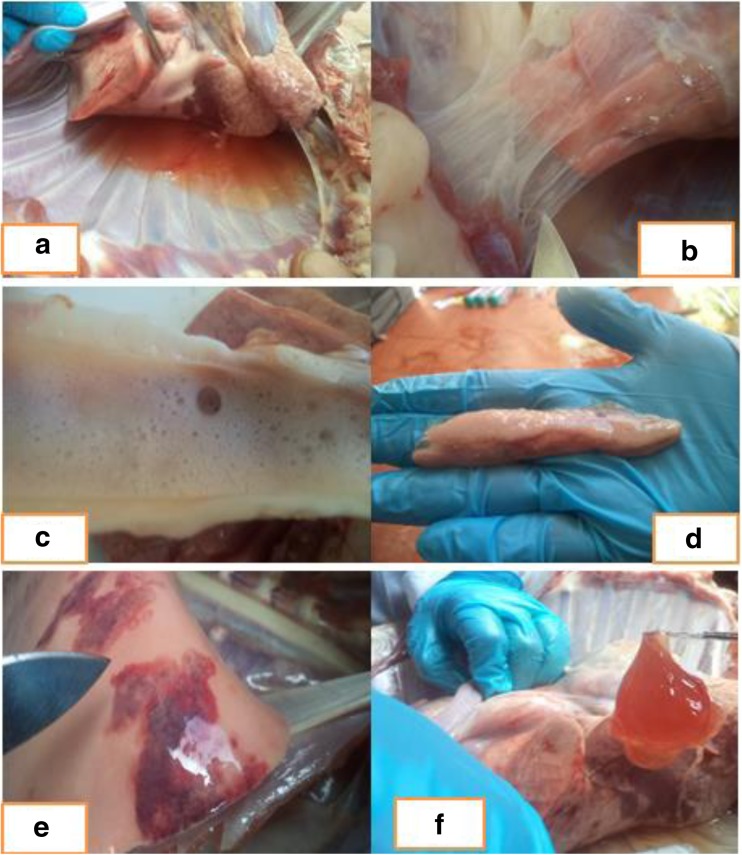
Gross pathological changes observed in three goats showing clinical signs of CCPP include accumulation of fluids in the pleural cavities, adhesion of lungs to the thoracic wall, frothy discharge in the trachea, enlarged bronchial lymph nodes, pneumonic lung tissues, and pleural fluids containing large clots of fibrin (Fig. 4).
Fig. 4.
Postmortem finding of CCPP infected goats. a Accumulation of lung exudate in thorax cavity; b fibrous adhesion of lungs to the chest wall; c froth in the trachea; d enlarged respiratory (mediastinal) lymph nodes; e lung with areas of pneumonia; and f lung exudate containing large clots of fibrin
Mccp detection and confirmation using conventional PCR
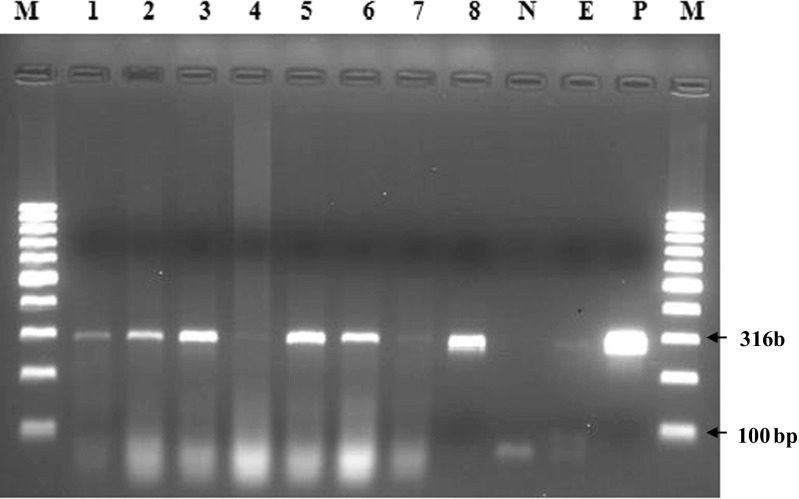
A total of 8 samples (three lung tissues, three pleural fluids, and two bronchial lymph nodes) collected from three clinically affected goats that tested positive in the cELISA were analyzed by conventional PCR. Upon PCR amplification of the genomic DNA from the 8 samples and controls using species-specific Mccp primers, Mccp was detected in 6 (75%) samples.
The specimens that tested positive include three lung tissues (lane 1–3) and three pleural fluids (lane 5, 6, and 8) whereas the samples of the other bronchial lymph node (lane 4 and lane 7) tested negative. The results of PCR analysis are depicted in Fig. 5. The fragment size of the amplified products was 316 bp.
Fig. 5.
Agarose gel electrophoresis of PCR products (316 bp) amplified with Mccp-specific primers. Lane M: 100 bp DNA molecular weight marker; lane P: positive control; lane N: negative control; lane E: extraction control; lanes 1–8: samples
Discussion
The main objective of this study was to estimate the sero-prevalence and confirm the presence of CCPP in selected districts of Borana zone. The study revealed that CCPP is a major health constraint of goats in Borana pastoral areas. This was confirmed by a general sero-prevalence of 31.2% and by the detection of Mccp in the samples collected from the three suspected cases. The current study indicated the confirmation of the case directly from clinically affected goats for the first time in the study area. It has been shown previously that several outbreaks of CCPP reported in the country were from Oromia, the majority of which were from Borana (MoLF 2016). The previous reports of outbreaks were based on the clinical signs. This study, however, provided confirmation of CCPP cases with molecular techniques and provided reliable information on the presence of Mccp in Borana area. This has important implication for the wellbeing of the pastoral community.
The overall sero-prevalence of 31.2% reported in unvaccinated goats in this study shows that Mccp has been established and is circulating in the area. For unvaccinated population of goats this figure is high and requires attention of the veterinary and livestock authority of the area to minimize the effect CCPP has on livelihoods in the community. The overall prevalence of CCPP in the present study was higher than the national prevalence estimated from pooled sero-prevalence (25.7%) through a systematic review by Asmare et al. (2016) and is largely in agreement with the previous findings from Ethiopia (Lakew et al. 2014) in which 31.6% of goats in Borana were found to be positive to CCPP. Similar observations were also made earlier in goats at an export abattoir at Bishoftu, Ethiopia (Eshetu et al. 2007), and Southern Ethiopia, in Tigray and Afar (Hadush et al. 2009), and in Beetal goats in Pakistan (Sherif et al. 2012; Hussain et al. 2012). Thus, our findings show that little has changed over the years, and the efforts made to control the disease with vaccinations have not resulted in sufficient vaccination coverage to prevent spread or contain the disease. This was also reflected by the fact, that it was easy to find villages in which goats had not been vaccinated against CCPP.
In contrast to our findings, lower prevalence of CCPP has been reported earlier from different parts of Ethiopia (Yousuf et al. 2012; Tesfaye et al. 2011; Mekuria et al. 2008; Mekuria and Asmare 2010; Aklilu et al. 2015; Regassa et al. 2010). Lower CCPP sero-prevalence has also recently described in Pakistan (Shahzad et al. 2016; Wazir et al. 2016). On the other hand, higher sero-prevalence of 44.5%, 47.3%, and 51.8% was reported from Dire Dawa, Afar, and Oromia regions of Ethiopia, respectively, by Gizawu et al. (2009). Hadush et al. (2009) also reported higher prevalence as 38.6% and 43.9% from Afar and Tigray regions of Ethiopia, respectively. In other parts of the world, higher prevalence than our observation has been documented in Beetal, Pakistan (Shahzad et al. 2012), Tanzania (Mbyuzi et al. 2014; Nyanja et al. 2013), Kenya (Kipronoh et al. 2016), Uganda (Atim et al. 2016), and Turkey (Cetinkaya et al. 2009). An international collaborative study done by Peyraud et al. (2014) also reported sero-prevalence of 6 to 90%, 14.6%, 16%, 10.1%, 0%, and (2.7%, 44.2%) from Kenya, Ethiopia, Mauritius, Tajikistan, Afghanistan, and Pakistan, respectively, using monoclonal antibody–based cELISA. The observed variation in sero-prevalence reported from different studies may be due to differences in the husbandry practices, agro-ecology, vaccination history, sampling methods applied, and sample size used.
In our study, the sero-prevalence of CCPP was significantly lower in Elwoya than in Moyale and Yabello. This observation agrees with the reports of Wazir et al. (2016) who reported significantly different prevalence among geographical areas. However, it is contrary to the previous findings (Eshetu et al. 2007; Hadush et al. 2009; Sherif et al. 2012). The higher prevalence in Moyale and Yabello compared to Elwoya observed in this study could be due to differences in frequency of animal movement in the districts. Moyale is a district bordering Kenya. There is free movement of animals between the two countries in search of market and pastures. Pastoralists in the area often cross the border for marketing purposes as well as in search of feed and water mostly during the dry season and during droughts. There is also free movement and contact with animals from neighboring Somali pastoralists in Moyale. Yabello is the center of Borana zone, where animals from surrounding PAs are being moved to for veterinary services and marketing. Therefore, the higher prevalence of CCPP in these two districts is probably due to animal movement for marketing and in search of water and pasture.
The serological test results showed the presence of anti-Mccp antibodies in all age groups of goats and sheep. However, the results of sero-prevalence study showed that age had significant effect on the occurrence of infection with Mccp in Borana zone, reflecting the fact that older animals have higher chances to be exposed to the pathogen. This observation is in consent with the findings of Aklilu et al. (2015) who reported that adult goats were 1.84 times more likely to be sero-positive than kids. Our findings also agree with the report of Mekuria and Asmare (2010), Bekele et al. (2011), Yousuf et al. (2012), Sherif et al. (2012), Nyanja et al. (2013), and Lakew et al. (2014) who observed the presence of significant variation among age groups. However, the finding of this study contradicts with the works of Gizawu et al. (2009), Nicholas (2002), Eshetu et al. (2007), and Hadush et al. (2009) who observed the absence of association between age and occurrence of CCPP.
In this study, sheep kept along with goats were found to be sero-positive in all PAs except in Areri. That is, sheep in contact with infected goats were sero-positive. In consent with our observation, previous authors showed that sheep were sero-positive from different parts of Ethiopia. For instance, 13% of sheep were found sero-positive by Dawit (1996), 7.14% by Gelagay et al. (2007), and 47.6% by Hadush et al. (2009). In Tanzania, sero-prevalence estimates of 36.7% and 22.9% from sheep serum were reported by Mbyuzi et al. (2014). In addition to this, there are reports describing the isolation of Mcpp from sheep with respiratory disease returning to Eritrea with refugees from Sudan (Houshaymi et al. 2002), from healthy sheep in Kenya that have been in contact with goat herds affected by CCPP (Litamoi et al. 1990), from sick sheep mixed with goats in Uganda (Bolske et al. 1995), and elsewhere in the globe by Cetinkaya et al. (2009) from lung and nasal swab of sheep. This raises questions on the role of sheep as a reservoir and contributing to maintaining transmission of Mccp. The exact role of sheep in the maintenance and spread of Mccp to goats needs to be further investigated.
Our finding of CCPP gross lesions at postmortem which revealed lung exudate containing large clots of fibrin, adhesion of lungs to the thoracic wall, froth in the trachea, enlarged bronchial lymph nodes, and pneumonic lung tissues are similar with those of the previous study of Wesonga et al. (2004) who reported the lesions of classical CCPP caused by Mccp. These observations are also matched with the findings of others (OIE 2008; Sadique et al. 2012).
In conclusion, the present study revealed the prevalence of CCPP in the Borana pastoral area.
The causative agent of CCPP, Mycoplasma capricolum subspecies capripneumoniae, was identified and confirmed by PCR. The study also showed that sheep were infected with Mccp with a sero-prevalence of 12.9%. Based on our and previous studies, it is clear that CCPP represent a priority for goat farming and more coordinated efforts are needed to prevent the disease and mitigate its impact. In addition, further studies on economic impact of the disease on production performance of goats and studies focused on molecular characterization of the circulating strain for both sheep and goats using large sample size should be done.
Acknowledgments
The authors are grateful to National Animal Health Diagnostic and Investigation Center (NAHDIC) Sebeta, as well as to National Veterinary Institute, Bishoftu, for the technical assistance in the laboratory works. We also would like to acknowledge all of the pastoralists who generously participated in this study.
Funding information
This study recieved financing from the International Livestock Research Institute and Oromia Agricultural Research Institute.
Ethical considerations
“Ethical clearance on the use of sheep and goats for this study was obtained from animal research ethics review committee of Addis Ababa University, College of Veterinary Medicine and Agriculture, before the start of this study. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.” The owners of sheep and goats used in this study and the local administration were informed about the study and the owners revealed their consent in the presence of administrative bodies.
Competing interests
The authors declare that they have no competing interests.
References
- Aklilu F, Asfaw Y, Baumann M, Abie G. Epidemiological study of contagious caprine pleuropneumonia (CCPP) in selected districts of Gambella Region, Western Ethiopia. Afr. J. Agric. Res. 2015;10(24):2470–2479. doi: 10.5897/AJAR2014.9264. [DOI] [Google Scholar]
- Asmare K, Abayneh T, Mekuria S, Ayelet G, Sibhat B, Skjerve E, Szonyi B, Wieland B. A meta-analysis of contagious caprine pleuropneumonia (CCPP) in Ethiopia. Act Trop. 2016;158:231–239. doi: 10.1016/j.actatropica.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Atim S, Ayebazibwe C, Mwiine F, Erume J, Tweyongyere R. A Survey for contagious caprine pleuropneumonia in Agago and Otuke districts in Northern Uganda. Open J Vet Med. 2016;6(1):9–14. doi: 10.4236/ojvm.2016.61002. [DOI] [Google Scholar]
- AU-IBAR, 2015. Standard methods and procedures (SMPs): for contagious caprine pleuropneumonia (CCPP) in the Greater Horn of Africa, Nairobi
- Bekele, T., Asfaw, Y., GebreEgziabeher, B. and Getachew Abebe, G. 2011. Sero-prevalence of contagious caprine pleuropneumonia in Borana and Guji lowlands, Southern Ethiopia. Ethiop. Vet. J. 15(2): 69–76.
- Bolske G, Johansson K, Heinonen R, Panvuga P, Twinamasiko E. Contagious caprine pleuropneumonia in Uganda and isolation of Mycoplasma capricolum subsp. capripneumoniae from goats and sheep. Vet. Rec. 1995;137(23):594. [PubMed] [Google Scholar]
- Cetinkaya B, Kalin R, Karahan M, Atil E, Manso-Silván L, Thiaucourt F. Detection of contagious caprine pleuropneumonia in East Turkey. Rev. sci. tech. Off. int. Epiz. 2009;28(3):1037–1044. doi: 10.20506/rst.28.3.1944. [DOI] [PubMed] [Google Scholar]
- Dawit, K. 1996. Serological and microbiological studies of major respiratory mycoplasmoses of goats in Yabello (Range lands of Borana), Ethiopia. Unpublished DVM Thesis, Addis Ababa University, faculty of Veterinary medicine, Debrezeit, Ethiopia. p:1–58.
- Elhassan MA, Salama A. Clinical and laboratory diagnosis of contagious caprine pleuropneumonia in Qassim region, Saudi Arabia: a comparative study. Trop Biomed. 2018;35(1):67–75. [PubMed] [Google Scholar]
- Eshetu L, Yigezu L, Asfaw Y. A study on Contagious Caprine Pleuropneumonia (CCPP) in goats at an export oriented abattoir, Debrezeit, Ethiopia. Trop Anim Health Prod. 2007;39(6):427–432. doi: 10.1007/s11250-007-9041-1. [DOI] [PubMed] [Google Scholar]
- Gelagay A, Teshale S, Amsalu W, Esayas G. Prevalence of contagious caprine pleuropneumonia in the Borana pastoral areas of Ethiopia. Small Rumin. Res. 2007;70(2):131–135. doi: 10.1016/j.smallrumres.2006.02.001. [DOI] [Google Scholar]
- Gizawu D, GebreEgziabher B, Ayelet G, Asmare K. Investigation of mycoplasma infection in goats slaughtered at ELFORA export abattoir, Ethiopia. Ethiop. Vet. J. 2009;13(1):41–58. [Google Scholar]
- Hadush B, Eshetu L, Mengistu W, Hailesilassie M. Sero-prevalence of contagious caprine pleuropneumonia in Kefta Humera, Alamata (Tigray) and Abaala (Afar), Northern Ethiopia. Trop Anim Health Prod. 2009;41(5):803–806. doi: 10.1007/s11250-008-9255-x. [DOI] [PubMed] [Google Scholar]
- Haile, A., Ayalew, W., Kebede, N., Dessie, T. and Tegegne, A. 2011. Breeding strategy to improve Ethiopian Borana cattle for meat and milk production. In: IPMS (Improving Productivity and Market Success) of Ethiopian Farmers Project Working Paper 26. ILRI, Nairobi, Kenya.
- Houshaymi B, Tekleghiorghis T, Wilsmore AJ, Miles RJ, Nicholas RAJ. Investigations of outbreaks of contagious caprine pleuropneumonia in Eritrea. Trop Anim Health Prod. 2002;34(5):383–389. doi: 10.1023/A:1020087924433. [DOI] [PubMed] [Google Scholar]
- Hussain R, Auon M, Khan A, Khan MZ, Mahmood F, UR-rehman S. Caprine pleuropneumonia in Beetal goats. Trop Anim Health Prod. 2012;44(3):675. doi: 10.1007/s11250-011-0018-8. [DOI] [PubMed] [Google Scholar]
- Kamara A, Kirk M, Swallow B. Property rights and land use change: implications for sustainable resource management in Borana, Southern Ethiopia. J. Sustain. Agric. 2005;25:41–61. doi: 10.1300/J064v25n02_05. [DOI] [Google Scholar]
- Kipronoh K, Ombuib J, Binepald Y, Wesongae H, Gitongae E, Thuranirad E, Kiarac H. Risk factors associated with contagious caprine pleuropneumonia in goats in pastoral areas in the Rift Valley region of Kenya. Prev Vet Med. 2016;132:107–112. doi: 10.1016/j.prevetmed.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Lakew M, Sisay T, Ayelet G, Eshetu E, Dawit G, Tadele T. Sero-prevalence of contagious caprine pleuropneumonia and field performance of inactivated vaccine in Borana pastoral area, southern Ethiopia. Afr. J. Microbiol. Res. 2014;8(24):2344–2351. doi: 10.5897/AJMR2014.6806. [DOI] [Google Scholar]
- Litamoi JK, Wonyangu S, Siman P. Isolation of mycoplasma biotype F38 from sheep in Kenya. Trop. Anim. Health Prod. 1990;22(4):260–262. doi: 10.1007/BF02240409. [DOI] [PubMed] [Google Scholar]
- Mbyuzi A, Erick V, Komba E, Kimera S, Kambarage D. Sero-prevalence and associated risk factors of peste des petits ruminants and contagious caprine pleuropneumonia in goats and sheep in the Southern Zone of Tanzania. Prev Vet Med. 2014;116(1):138–144. doi: 10.1016/j.prevetmed.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Mekuria S, Asmare K. Cross sectional study on Contagious Caprine Pleuro Pneumonia in selected districts of sedentary and pastoral production systems in Southern Ethiopia. Trop Anim Health Prod. 2010;42(1):65–72. doi: 10.1007/s11250-009-9386-8. [DOI] [PubMed] [Google Scholar]
- Mekuria S, Zerihun A, GebreEgziabher B, Tibbo M. Participatory investigation of contagious caprine pleuropneumonia (CCPP) in goats in the Hammer and BennaTsemay Districts of Southern Ethiopia. Trop Anim Health Prod. 2008;40(8):571–582. doi: 10.1007/s11250-008-9136-3. [DOI] [PubMed] [Google Scholar]
- MoLF . Monthly disease outbreak report. Addis Ababa, Ethiopia: Ministry of Livestock and Fishery; 2016. [Google Scholar]
- Nicholas RAJ. Improvements in the diagnosis and control of diseases of small ruminants caused by mycoplasmas. Small Rum Res. 2002;45:145–149. doi: 10.1016/S0921-4488(02)00095-0. [DOI] [Google Scholar]
- Nyanja, P., Kusiluka, L., Mellau, S. 2013. Prevalence of Contagious Caprine Pleuropneumonia in Goats in Musoma District of Mara Region, Tanzania. Tanz. Vet. J.28 (1).
- OIE . Contagious caprine pleuropneumonia. Manual of standards for diagnostic tests and vaccines. Paris: Office of International Epizootics; 2008. pp. 1000–1012. [Google Scholar]
- Peyraud A, Poumarat F, Tardy F, Manso-Silván L, Hamroev K, Tilloev T, Amirbekov M, Tounkara K, Bodjo C, Wesonga H, Nkando IG, Jenberie S, Yami M, Cardinale E, Meenowa D, Jaumally MR, Yaqub T, Shabbir MZ, Mukhtar N, Halimi M, Ziay GM, Schauwers W, Noori H, Rajabi AM, Ostrowski S, Thiaucourt F. An international collaborative study to determine the prevalence of contagious caprine pleuropneumonia by monoclonal antibody-based cELISA. BMC Vet. Res. 2014;10:48–57. doi: 10.1186/1746-6148-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats-van der Ham M, Amores Fedela C, Paterna J, Tatay-Dualde A, Gomez-Martin Á. Contagious caprine pleuropneumonia (CCPP) and other emergent mycoplasmal diseases affecting small ruminants in arid lands. J Arid Environ. 2015;119:9–15. doi: 10.1016/j.jaridenv.2015.03.006. [DOI] [Google Scholar]
- Regassa F, Netsere M, Tsertse T. Sero-Prevalence of Contagious Caprine Pleuropneumonia in Goat at Selected Woredas of Afar Region. Ethiop. Vet. J. 2010;14(1):83–89. [Google Scholar]
- Sadique U, Chaudhry ZI, Younas M, Anjum AA, Hassan ZU, Idrees M, Mushtaq M, Sajid A, Sabtain M, Jhang AS, Sciences A. Molecular Characterization of Contagious Caprine Pleuropneumonia (CCPP) in Small Ruminants of Khyber Pakhtunkhwa, Pakistan. J. Anim. Plant Sci. 2012;22(2):33–37. [Google Scholar]
- Samiullah, S. 2013. Contagious caprine pleuropneumonia and its current picture in pakistan: A review’, Vet. Med. 58(8): 389–398.
- Shahzad W, Munir R, Khan MS, Ahmad MUD, Khan MA, Ijaz M, Shakil M, Iqbal M, Ahamd R. Characterization, Molecular Diagnosis and Prevalence of Caprine Mycoplasmosis in Different Areas of Pakistan. Pakistan J. Zool. 2012;44(2):559–568. [Google Scholar]
- Shahzad W, Yaqoob T, Mukhtar N, Munir R, Ahmad R, Khan M, Hussain A. Sero-prevalence of Mycoplasma capricolum subsp. capripneumoniae in goats through cELISA in different districts of Punjab, Pakistan. J. Anim. Plant Sci. 2016;26(4):931–937. [Google Scholar]
- Sherif M, Addis M, Tefera M. Contagious Caprine Pleuropneumonia: Serological survey in selected district of Jigjiga zone Ethiopia. Asian J. Anim. Sci. 2012;6(6):309–315. doi: 10.3923/ajas.2012.309.315. [DOI] [Google Scholar]
- Tesfaye B, Yilikal A, Berhe G, Getachew A. Sero-prevalence of Contagious Caprine Pleuropneumonia in Borana and Guji lowlands, Southern Ethiopia. Ethiop. Vet. J. 2011;15(2):69–76. [Google Scholar]
- Thiaucourt F, Bolske G, Leneguersh B, Smith D, Wesonga H. Diagnosis and control of contagious caprine pleuropneumonia. Rev. Sci. Tech. Off. Int. Epiz. 1996;15(4):1415–1429. doi: 10.20506/rst.15.4.989. [DOI] [PubMed] [Google Scholar]
- Thrusfield M. Veterinary Epidemiology. 3. Black Well Science Ltd: UK; 2005. p. 233. [Google Scholar]
- Tsehay H, Getachew G, Morka A, Tadesse B, Eyob H. Sero-prevalence of brucellosis in small ruminants in pastoral areas of Oromia and Somali regional states, Ethiopia. J. Vet. Med. Anim. Health. 2014;6(11):289–294. doi: 10.5897/JVMAH2014.0331. [DOI] [Google Scholar]
- Wazir I, Hussain I, Khan M, Ali M, Rahman H, Ashraf F, Khan S, Khan B, Ullah S, Ullah Q, Khyber K, Khan A. Sero epidemiological analysis of contagious caprine pleuropneumonia through cELISA in selected districts of Khyber Pakhtunkhwa, Pakistan. ASRJETS. 2016;26(3):274–281. [Google Scholar]
- Wesonga HO, Bolske G, Thiaucourt F, Wanjohi C, Lindberg R. Experimental Contagious Caprine Pleuropneumonia: A Long Term Study on the Course of Infection and Pathology in a Flock of Goats Infected with Mycoplasma capricolum subsp. capripneumoniae. Acta Vet Scand. 2004;45(3):167–179. doi: 10.1186/1751-0147-45-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woubit S, Lorenzon S, Peyraud A, Manso-Silvan L, Thiaucourt F. A specific PCR for the identification of Mycoplasma capricolum subsp. capripneumoniae, the causative agent of contagious caprine pleuropneumonia (CCPP). Vet. Microbiol. 2004;104(1–2):125–132. doi: 10.1016/j.vetmic.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Younis E, Elnaker Y, Awad N, Elshafey D. Sero-prevalence Of Mycoplsma Mycoides Cluster in Small Ruminant Using Monoclonal Antibody Based C Elisa in Dakahilia Province. Assiut Vet. Med. J. 2015;61(146):46–51. [Google Scholar]
- Yousuf E, Melaku A, Bogale B. Sero-prevalence of contagious caprine pleuropneumonia in Dire Dawa provisional administrative council, Eastern Ethiopia. J. Vet. Med. Anim. Health. 2012;4(7):93–96. [Google Scholar]