Abstract
The activities of a diverse array of sediment-dwelling fauna are known to mediate carbon remineralisation, biogeochemical cycling and other important properties of marine ecosystems, but the contributions that different seabed communities make to the global inventory have not been established. Here we provide a comprehensive georeferenced database of measured values of bioturbation intensity (Db, n = 1281), burrow ventilation rate (q, n = 765, 47 species) and the mixing depth (L, n = 1780) of marine soft sediments compiled from the scientific literature (1864–2018). These data provide reference information that can be used to inform and parameterise global, habitat specific and/or species level biogeochemical models that will be of value within the fields of geochemistry, ecology, climate, and palaeobiology. We include metadata relating to the source, timing and location of each study, the methodology used, and environmental and experimental information. The dataset presents opportunity to interrogate current ecological theory, refine functional typologies, quantify uncertainty and/or test the relevance and robustness of models used to project ecosystem responses to change.
Subject terms: Element cycles, Ecological modelling, Ecosystem ecology, Behavioural ecology
| Design Type(s) | data integration objective • species comparison design |
| Measurement Type(s) | Publication |
| Technology Type(s) | digital curation |
| Factor Type(s) | temporal_instant • geographic location • depth • Species • season |
| Sample Characteristic(s) | Polychaeta • Earth (Planet) • marine benthic biome • Malacostraca • Bivalvia • Echinoidea • Ophiuroidea |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Background & Summary
Marine sediments are known to harbour significant levels of biodiversity that play a key role in biogeochemical cycling, carbon storage and the regulation of climate active gases1–3, but the geographic contribution of extant benthic communities is not well constrained at large scales4. Quantifying the extent, timing and way in which organisms transport particles and pore water fluids5 has received a considerable amount of attention6, yet few attempts to seek universalities, generalities, and particularities have taken place that can inform the architecture of global biogeochemical models7–10. Variations in the intensity of faunal mediation in relation to changing conditions that alter species interactions11, community structure12 and environmental setting13–15 are well-known and mean that the contributions of individual species and/or definable communities cannot be applied universally16, yet these sources of variation are not generally incorporated into modelling frameworks17. Indeed, most models are parameterised with broad functional descriptors or selected values of bioturbation that oversimplify or misrepresent temporal and spatial variation in the mediating role of biota18–20, largely because comprehensive compilations of such biological information are not readily available21. For these reasons, the treatment of key processes can differ greatly between models such that simulated ecosystem outcomes commonly misalign with ecosystem properties measured at local to regional scales17, frustrating efforts to accurately project the effects and consequences of environmental change22.
Descriptions of how infaunal invertebrates mediate ecosystem properties are common in the literature and have largely become synonymous with particle displacement and burrow ventilation23, although alternative descriptors have been considered and emphasised24. As sediment particle reworking often consists of a series of small particle displacement events, standard practice has been to treat the resulting vertical profile of mixing in an analogous way to that of diffusive heat transport, calculating a biodiffusion coefficient (Db, cm2 year−1) that describes the rate at which the variance of the location of a particle tracer changes over time within the sediment profile25. Similarly, as the active transfer of fluid by infaunal organisms may be orders of magnitude greater (volumetrically) than particle reworking26, the non-diffusive exchange of pore-water solutes with over-lying water is routinely examined27, but these data have not previously been collated in an accessible archive. The combined effect of particulate and fluid transport on sediment biogeochemical processes is reflected in the vertical colour transition (from brown to olive green/black) of the sediment profile28, dictated by the transition from iron (oxyhydr)oxides at the surface to black sulphidic phases at depth29 that correlate with a variety of environmental drivers30. Hence, regions of high reflectance (brown) in an image represent a well-mixed region of sediment and provide a reasonable approximation of the mixing depth31.
Here, motivated by the need to relate changes in ecosystem properties to local heterogeneity rather than global mean conditions17,32, we have collated the extensive repository of information that exists in the primary scientific literature concerning how faunal communities redistribute sediment particles, ventilate their burrows and effect the depth to which mixing typically occurs in relation to their physical location. Our hope is that the inherent spatial and temporal heterogeneity shown within these data will be embraced by modellers, statisticians and ecologists and contribute to the development of next generation biogeochemical models that can better inform conservation and management strategies.
Methods
We searched the Thomson Reuters Web of Science collection (http://www.webofknowledge.com, accessed 07/03/2019) using a ‘General Search’ across all databases with the search term (i) bioturbation, (ii) sediment profile imag*, and (iii) bioirrigation OR burrow ventilation in the titles and key words of all document types, in all languages, for the publication years 1864 to 2018. Citation returns were manually searched for reported values of the sediment mixing depth (L, cm)30,31, the biodiffusion coefficient (Db, cm2 year−1) estimated from models of sediment particle reworking6,25, and the rate of ventilation (q, ml h−1 ind.−1) for named macro-invertebrate species or mixed communities. These data for L and Db supersede records collated elsewhere7–10 and include observations from the older literature (pre-1970) cited by the authors of the returns from our search.
For each unique record, we collated associated environmental metadata (latitude, longitude, water depth, sedimentation rate), information on the methodology used, and details about the timing (year, season, month) and ecoregion (following accepted biogeographical typologies)33,34 from the original publication, personal communication with the corresponding author and/or from third party sources of information. Where specific values were not presented in the original publication and had to be derived, values were extracted from graphical summaries using Web Plot Digitiser (https://automeris.io/WebPlotDigitizer/). When the location of a study was not provided, latitude and longitude coordinates and/or water depth were retrieved from Google Earth (http://earth.google.com/) and manually cross referenced with site descriptions within the source publication. Following standard practice9, the seasonal offset between Northern (NH) and Southern (SH) hemisphere was corrected by attributing a nominal season to each study: Spring, April-June in the NH or October–December in the SH; Summer, July–September in the NH or January–March in the SH; Autumn, October–December in the NH or April–June in the SH; or Winter, January–March in the NH or July–September in the SH. Due to variations in seasonal timing at any given latitude, the scheme is not necessarily representative of geographical clines in forcing. Data collected from multiple months or unspecified periods are also included. The methodology used to generate each record includes 21 techniques for L and Db (reviewed in ref.35) and 18 techniques for q (reviewed in ref.36).
As species ventilation behaviour varies over time37, we distinguish ventilation measurements based solely on active bouts of ventilation (q1, an indication of peak activity) from those estimated over extended periods of time that span rest periods (q2, a more representative indication of species contribution). Similarly, in recognising that experimental configuration24,38 and the geometry of the sediment-water interface39 can influence species behaviour, our database includes information on aquaria dimensions. Given the time span of the studies under consideration, species nomenclature has been standardised in line with the World Register of Marine Species40.
Data Records
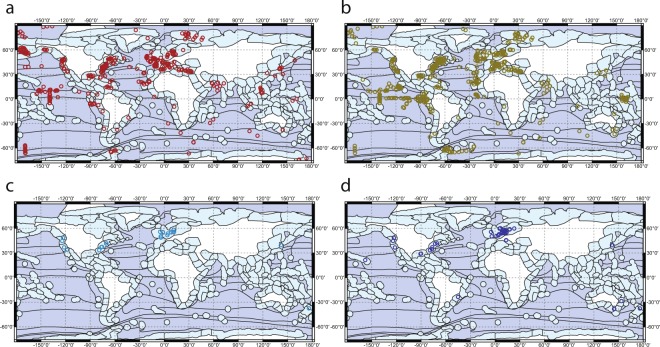
Data records are available via an unrestricted repository hosted by Harvard Dataverse41. Data represent reported values for the biodiffusion coefficient (Db, cm2 year−1; Fig. 1a) and/or the sediment mixing depth (L, cm; Fig. 1b) for specific locations and can be found in solan_etal_DbL.csv. Separately, the data set also includes volumetric ventilation flow rates (q, ml h−1 ind.−1) for named macro-invertebrate species or mixed communities taken during active bouts of ventilation (q1, Fig. 1c) and/or estimated over extended periods of time (q2, Fig. 1d) for specific locations. These can be found in solan_etal_q.csv. The number of records within the dataset are listed to ecoregion (Table 1), method of quantification (Table 2) and by season and water depth (Table 3). Table 3 also includes the number of experimental observations of q1 and q2 listed against taxonomic class. A summary of the definitions for the descriptors (=column headings) used in the Db and L (Descriptor categories S1) and q (Descriptor categories S2) datasets are documented separately in solan_etal_suppl_info_v3.docx41.
Fig. 1.
The geographical location of reported bioturbation parameter values. (a) Db, the biodiffusion coefficient, (b) L, the sediment mixing depth, (c) q1, the ventilation rate for named macro-invertebrate species or for mixed communities taken during active bouts of ventilation and (d) q2, the ventilation rate for named macro-invertebrate species or for mixed communities estimated over extended periods of time. Data points may represent multiple observations at that locality. The boundaries of ecoregion domains and divisions33 (dark blue shading) and provinces34 (light blue shading) are indicated.
Table 1.
Number of observations for bioturbation intensity (Db), mixing depth (L) and ventilation rate (q1 and q2) listed by marine realm.
| Realm | Db | L | q1 | q2 |
|---|---|---|---|---|
| Arctic | 68 | 45 | 0 | 0 |
| Central IndoPacific | 17 | 48 | 0 | 0 |
| Eastern IndoPacific | 29 | 4 | 0 | 7 |
| Not allocated (Polar) | 4 | 4 | 0 | 0 |
| Not allocated (Temperate) | 12 | 17 | 0 | 0 |
| Not allocated (Tropical) | 36 | 21 | 0 | 0 |
| Southern Ocean | 58 | 17 | 0 | 0 |
| Temperate Australasia | 3 | 5 | 3 | 13 |
| Temperate Northern Atlantic | 661 | 1312 | 206 | 553 |
| Temperate Northern Pacific | 272 | 122 | 17 | 45 |
| Temperate South America | 32 | 25 | 0 | 6 |
| Temperate Southern Africa | 1 | 1 | 0 | 0 |
| Tropical Atlantic | 33 | 41 | 0 | 0 |
| Tropical Eastern Pacific | 36 | 88 | 0 | 0 |
| Western IndoPacific | 19 | 30 | 0 | 0 |
| Grand total | 1281 | 1780 | 226 | 624 |
Table 2.
Number of observations for bioturbation intensity (Db), mixing depth (L) and ventilation rate (q1 and q2) categorised against method of quantification.
| Method | Db | L | q1 | q2 |
|---|---|---|---|---|
| 137Cs | 43 | 10 | 0 | 0 |
| 14C | 2 | 59 | 0 | 0 |
| 210Pb | 551 | 429 | 0 | 0 |
| 222Ra | 10 | 10 | 0 | 0 |
| 228Th | 12 | 0 | 0 | 0 |
| 234Th | 423 | 46 | 0 | 0 |
| 235Th | 4 | 1 | 0 | 0 |
| 239240Pu | 34 | 16 | 0 | 0 |
| 32Si | 3 | 3 | 0 | 0 |
| 7Be | 39 | 8 | 0 | 0 |
| calculated | 0 | 71 | 0 | 0 |
| Chla | 71 | 7 | 0 | 0 |
| Bromide | 0 | 0 | 10 | 155 |
| Caesium | 0 | 0 | 0 | 4 |
| clearance | 0 | 0 | 0 | 34 |
| Doppler | 0 | 0 | 14 | 23 |
| dye | 0 | 0 | 19 | 28 |
| Eh | 0 | 109 | 0 | 0 |
| Em | 0 | 0 | 95 | 46 |
| estimate | 0 | 0 | 1 | 0 |
| glassbeads | 19 | 0 | 0 | 0 |
| hydraulic | 0 | 0 | 6 | 6 |
| luminescence | 0 | 2 | 0 | 0 |
| luminophores | 57 | 21 | 0 | 0 |
| model | 0 | 0 | 0 | 6 |
| OrgC | 0 | 1 | 0 | 0 |
| oxygen | 0 | 0 | 6 | 6 |
| permeability | 0 | 0 | 1 | 0 |
| pet | 0 | 0 | 0 | 7 |
| piv | 0 | 0 | 7 | 2 |
| pressure | 0 | 0 | 48 | 265 |
| radon | 0 | 0 | 11 | 0 |
| SPI | 5 | 833 | 0 | 0 |
| TCO2 | 0 | 0 | 0 | 3 |
| tekbeads | 8 | 8 | 0 | 0 |
| thermistor | 0 | 0 | 6 | 38 |
| uranine | 0 | 0 | 2 | 0 |
| visual | 0 | 104 | 0 | 0 |
| xray | 0 | 42 | 0 | 0 |
| Grand total | 1281 | 1780 | 226 | 623 |
Method definitions are listed as Descriptor categories S1 and S2 in in solan_etal_suppl_info_v3.docx41.
Table 3.
Number of observations for bioturbation intensity (Db), mixing depth (L) and ventilation rate (q1 and q2) for season and depth category.
| Db | L | q1 | q2 | |
|---|---|---|---|---|
| Season | ||||
| Spring | 295 | 332 | 81 | 125 |
| Summer | 266 | 607 | 28 | 115 |
| Autumn | 245 | 233 | 3 | 9 |
| Winter | 70 | 31 | 41 | 70 |
| Multiple | 68 | 72 | 1 | 78 |
| Total: | 944 | 1275 | 154 | 397 |
| Depth category | ||||
| 0–50 m | 277 | 956 | 225 | 599 |
| 50–200 m | 281 | 276 | 0 | 16 |
| 200–1000 m | 245 | 145 | 0 | 9 |
| 1000–4000 m | 303 | 251 | 0 | 0 |
| 4000–6000 m | 175 | 152 | 0 | 0 |
| >6000 m | 0 | 0 | 0 | 0 |
| Total: | 1281 | 1780 | 225 | 624 |
| Class | ||||
| Bivalvia | — | — | 9 [4] | 22 [5] |
| Echinoidea | — | — | 5 [2] | 10 [1] |
| Ophiuroidea | — | — | 0 [0] | 9 [1] |
| Malacostraca | — | — | 37 [9] | 89 [11] |
| Polychaeta | — | — | 157 [11] | 427 [21] |
| Community | — | — | 18 [n/a] | 67 [n/a] |
| Total: | 226 | 624 | ||
For q, the number of experimental observations are listed against taxonomic class. The number of species considered within each taxonomic class are indicated in square brackets.
Technical Validation
The data has been collated from the peer-reviewed literature (Data Source S1 in solan_etal_suppl_info_v3.docx)41 and has undergone rigorous quality control prior to publication. Each individual record (unique identification number) in the dataset is traceable to the point of origin (data source identification number)41.
Usage Notes
We have included all reported values from the literature without prejudice or downstream processing steps. Reporting errors and updates of the data will be periodically issued. Users should use the latest version of the data listed (under the ‘versions’ tab) at Harvard Dataverse41 and maintained at Bioturbation Online (http://bioturbation.online). This contribution is based on data release 3.0. There are no limitations on the use of these data.
ISA-Tab metadata file
Acknowledgements
M.S., J.A.G. and R.H. were supported by Work Package 2 of the Shelf Sea Biogeochemistry Programme (NE/K001906/1, 2011–2017), jointly funded by the Natural Environment Research Council (NERC) and the Department for Environment, Food and Rural Affairs (Defra).
Author Contributions
M.S. initiated the database, contributed to data collection and wrote the manuscript. E.R.W. and M.S. co-ordinated data collection, were responsible for quality control and constructed the database. M.S., E.R.W., E.L.W., E.E.H., C.C., J.S., R.H. and J.A.G. contributed to data collection. R.H. and J.A.G. contributed to database design and reviewed and provided critical commentary on the manuscript prior to submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Martin Solan and Ellie R. Ward.
ISA-Tab metadata
is available for this paper at 10.1038/s41597-019-0069-7.
References
- 1.Solan M, et al. Extinction and ecosystem function in the marine benthos. Science. 2004;306:1177–1180. doi: 10.1126/science.1103960. [DOI] [PubMed] [Google Scholar]
- 2.Martin RE. Secular increase in nutrient levels through the Phanerozoic: implications for productivity, biomass and diversity of the marine biosphere. Palaios. 1996;11:209–219. doi: 10.2307/3515230. [DOI] [Google Scholar]
- 3.Covich AP, et al. The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. Bioscience. 2004;54:767–775. doi: 10.1641/0006-3568(2004)054[0767:TROBIT]2.0.CO;2. [DOI] [Google Scholar]
- 4.Wei CL, et al. Global patterns and predictions of seafloor biomass using random forests. Plos One. 2010;5:e15323. doi: 10.1371/journal.pone.0015323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson TH. Functional group ecology in soft-sediment marine benthos: The role of bioturbation. Oceanogr. Mar. Biol. Ann. Rev. 2001;39:233–267. [Google Scholar]
- 6.Meysman FJR, Boudreau B, Middelburg JJ. Relations between local, non-local, discrete and continuous models of bioturbation. J. Mar. Res. 2003;61:391–410. doi: 10.1357/002224003322201241. [DOI] [Google Scholar]
- 7.Boudreau BP. Is burial velocity a master parameter for bioturbation? Geochim. Cosmochim. Acta. 1994;58:1243–1249. doi: 10.1016/0016-7037(94)90378-6. [DOI] [Google Scholar]
- 8.Boudreau BP. Mean mixed depth of sediments: The wherefore and the why. Limnol. Oceanogr. 1998;43:524–526. doi: 10.4319/lo.1998.43.3.0524. [DOI] [Google Scholar]
- 9.Teal LR, Bulling MT, Parker ER, Solan M. Global patterns of bioturbation intensity and mixed depth of marine soft sediments. Aquatic Biol. 2008;2:207–218. doi: 10.3354/ab00052. [DOI] [Google Scholar]
- 10.Lecroart P, et al. Bioturbation, short-lived radioisotopes, and the tracer-dependence of biodiffusion coefficients. Geochim. Cosmochim. Acta. 2010;74:6049–6063. doi: 10.1016/j.gca.2010.06.010. [DOI] [Google Scholar]
- 11.Godbold, J. A. & Solan, M. Long-term effects of warming and ocean acidification are modified by seasonal variation in species responses and environmental conditions. Phil. Trans. R. Soc. B. 368, UNSP 20130186 (2013). [DOI] [PMC free article] [PubMed]
- 12.Wohlgemuth, D., Solan, M. & Godbold, J. A. Specific arrangements of species dominance can be more influential than evenness in maintaining ecosystem process and function. Sci. Rep. 6, 10.1038/srep39325 (2016). [DOI] [PMC free article] [PubMed]
- 13.Berelson WM, et al. Modelling bio-irrigation rates in the sediments of Port Phillip Bay. Mar. Freshwater Res. 1999;50:573–579. doi: 10.1071/MF98076. [DOI] [Google Scholar]
- 14.Morys C, Forster S, Graf G. Variability of bioturbation in various sediment types and on different spatial scales in the southwestern Baltic Sea. Mar. Ecol. Prog. Ser. 2016;557:31–49. doi: 10.3354/meps11837. [DOI] [Google Scholar]
- 15.Wohlgemuth D, Solan M, Godbold JA. Species contributions to ecosystem process and function can be population dependent and modified by biotic and abiotic setting. Proc. R. Soc. B. 2017;284:20162805. doi: 10.1098/rspb.2016.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray F, Douglas A, Solan M. Species that share traits do not necessarily form distinct and universally applicable functional effect groups. Mar. Ecol. Prog. Ser. 2014;516:23–34. doi: 10.3354/meps11020. [DOI] [Google Scholar]
- 17.Snelgrove P, et al. Global carbon cycling on a heterogeneous seafloor. Trends Ecol. Evol. 2018;33:96–105. doi: 10.1016/j.tree.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Butenschoen M, et al. ERSEM 15.06: a generic model for marine biogeochemistry and the ecosystem dynamics of the lower trophic levels. Geosci. Model Dev. 2016;9:1293–1339. doi: 10.5194/gmd-9-1293-2016. [DOI] [Google Scholar]
- 19.Yakushev EV, et al. Bottom RedOx Model (BROM v.1.1): a coupled benthic–pelagic model for simulation of water and sediment biogeochemistry. Geosci. Model Dev. 2017;10:453–482. doi: 10.5194/gmd-10-453-2017. [DOI] [Google Scholar]
- 20.Lessin G, et al. Modelling marine sediment biogeochemistry: Current knowledge gaps, challenges, and some methodological advice for advancement. Front. Mar. Sci. 2018;5:19. doi: 10.3389/fmars.2018.00019. [DOI] [Google Scholar]
- 21.Tyler EHM, et al. Extensive gaps and biases in our knowledge of a well-known fauna: implications for integrating biological traits into macroecology. Glob. Ecol. Biogeogr. 2012;21:922–934. doi: 10.1111/j.1466-8238.2011.00726.x. [DOI] [Google Scholar]
- 22.Sweetman AK, et al. Major impacts of climate change on deep-sea benthic ecosystems. Elem. Sci. Anth. 2017;5:4. doi: 10.1525/elementa.203. [DOI] [Google Scholar]
- 23.Kristensen E, et al. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012;446:285–302. doi: 10.3354/meps09506. [DOI] [Google Scholar]
- 24.Hale, R., Mavrogordato, M. N., Tolhurst, T. J. & Solan, M. Characterizations of how species mediate ecosystem properties require more comprehensive functional effect descriptors. Sci. Rep. 4, 10.1038/srep06463 (2014). [DOI] [PMC free article] [PubMed]
- 25.Crank, J. The mathematics of diffusion (Oxford University Press, 1975).
- 26.Dornoffer TM, Waldbusser GG, Meile C. Modeling lugworm irrigation behavior effects on sediment nitrogen cycling. Mar. Ecol. Prog. Ser. 2015;534:121–134. doi: 10.3354/meps11381. [DOI] [Google Scholar]
- 27.Riisgård HU, Larsen PS. Water pumping and analysis of flow in burrowing zoobenthos: an overview. Aquatic Ecol. 2005;39:237–258. doi: 10.1007/s10452-004-1916-x. [DOI] [Google Scholar]
- 28.Lyle M. The brown-green color transition in marine sediments: a marker of the Fe(III)–Fe(II) redox boundary. Limnol. Oceanogr. 1983;2:1026–1033. doi: 10.4319/lo.1983.28.5.1026. [DOI] [Google Scholar]
- 29.Statham, P. J. et al. Extending the applications of sediment profile imaging to geochemical interpretations using colour. Cont. Shelf Res., 10.1016/j.csr.2017.12.001 (2017).
- 30.Teal LR, Parker ER, Solan M. Sediment mixed layer as a proxy for benthic ecosystem process and function. Mar. Ecol. Prog. Ser. 2010;414:27–40. doi: 10.3354/meps08736. [DOI] [Google Scholar]
- 31.Germano JD, Rhoads DC, Valente RM, Carey DA, Solan M. The use of Sediment Profile Imaging (SPI) for environmental impact assessments and monitoring studies: lessons learned from the past four decades. Oceanogr. Mar. Biol. Ann. Rev. 2011;49:235–298. [Google Scholar]
- 32.Bates AE, et al. Biologists ignore ocean weather at their peril. Nature. 2018;560:299–301. doi: 10.1038/d41586-018-05869-5. [DOI] [PubMed] [Google Scholar]
- 33.Bailey, R. G. Ecoregions: The ecosystem geography of the oceans and continents (New York: Springer, 1998).
- 34.Spalding MD, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–583. doi: 10.1641/B570707. [DOI] [Google Scholar]
- 35.Maire O, et al. Quantification of sediment reworking rates in bioturbation research: a review. Aquatic Biol. 2008;2:219–238. doi: 10.3354/ab00053. [DOI] [Google Scholar]
- 36.Roskosch A, Hupfer M, Nutzmann G, Lewandowski J. Measurement techniques for quantification of pumping activity of invertebrates in small burrows. Fundam. Appl. Limnol. 2011;178:89–110. doi: 10.1127/1863-9135/2011/0178-0089. [DOI] [Google Scholar]
- 37.Timmerman K, Banta GT, Glud RN. Linking Arenicola marina irrigation behavior to oxygen transport and dynamics in sandy sediments. J. Mar. Res. 2006;64:915–938. doi: 10.1357/002224006779698378. [DOI] [Google Scholar]
- 38.Bulling MT, et al. Species effects on ecosystem processes are modified by faunal responses to habitat composition. Oecologia. 2008;158:511–520. doi: 10.1007/s00442-008-1160-5. [DOI] [PubMed] [Google Scholar]
- 39.Meysman FJR, et al. Quantifying biologically and physically induced flow and tracer dynamics in permeable sediments. Biogeosciences. 2007;4:627–646. doi: 10.5194/bg-4-627-2007. [DOI] [Google Scholar]
- 40.Horton, T. et al. World Register of Marine Species. VLIZ, 10.14284/170 (2017).
- 41.Solan, M. A global database of measured values of benthic invertebrate bioturbation intensity, ventilation rate, and the mixed depth of marine soft sediments. Harvard Dataverse, 10.7910/DVN/GBELFW (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.