Abstract
Although sleep habits have long been recognized as a promoter of health, the World Health Organization 2014 report on non-communicable diseases (NCDs) only listed smoking, alcohol intake, diet and physical activity (PA) as key modifiable risk factors that could enhance health and prevent NCDs. Cross-sectional data on 4385 surveys from the 2015 Catalan Health Survey, representative of the 2015 non-institutionalized Catalan population over age 14, were used to assess and compare the independent associations of low PA (International Physical Activity Questionnaire (IPAQ): low activity); poor diet (PREvención con DIeta MEDiterránea questionnaire (PREDIMED): low-adherent); poor sleep health (Satisfaction, Alertness, Timing, Efficiency and Duration scale (SATED): <8); smoking status; and, alcohol intake (high-risk drinker based on standard drink units) with having a poor self-perceived health status. Logistic regression models adjusted by age, gender, education level and number of comorbidities showed that poor sleep health had the strongest independent association with poor self-perceived health status (OR = 1.70; 95%CI: 1.37–2.12), followed by poor diet (OR = 1.37; 95%CI: 1.10–1.72) and low PA (OR = 1.31; 95%CI: 1.01–1.69). This suggests that sleep habits should be included among the important modifiable health risk factors and be considered a key component of a healthy lifestyle.
Subject terms: Epidemiology, Risk factors
Introduction
Having a healthy lifestyle is key to enjoying good health and avoiding non-communicable diseases (NCDs). In this sense, the World Health Organization (WHO) 2014 report on NCDs cited reduction in alcohol, salt/sodium and tobacco use; increased physical activity (PA); and, halting the rise of the hypertension, diabetes and obesity as some of the global targets for the control of NCDs1. Similarly, Ezzati and Riboli listed smoking, alcohol consumption, excess weight and obesity, diet and nutrition, and PA in their summary of behavioural and dietary risk factors for non-communicable diseases published in 20132. Interestingly neither the 280-page long WHO report nor the highly cited article referred to sleep health or sleep habits as a potentially modifiable factor that could enhance health and prevent NCDs.
The interest in healthy sleep as a promoter of health has been documented since the origins of medicine, through the Middle Ages and up to the present. Short sleep duration has specifically been associated with hypertension3,4, cerebrovascular diseases5, coronary heart diseases6,7, cancer5,8, obesity9, diabetes10, and all-cause mortality11,12. Similarly, long sleep duration has been associated with adverse health outcomes10,11,13. Growing evidence suggests that sleep habits beyond sleep duration, such as changes in sleep timing due to shift work14,15 or even subjective sleep quality16,17, could be associated with NCDs, quality of life and overall health status.
In this context, it is reasonable to ask whether sleep health is a neglected pillar of health. Using data from the 2015 Catalan Health Survey (ESCA)18, the authors aimed to assess and compare the relative weights of diet, PA, smoking, alcohol consumption and sleep in relation to self-perceived health status.
Methods
Design, population and data collection
This is an observational cross-sectional study based on the ESCA 2015 survey18. The study population and sampling methodology have been described elsewhere19. Briefly, a multistage probability sampling method was used to obtain a representative sample of the non-institutionalized population in Catalonia in 2015. Up to 5598 surveys were obtained in two waves using computer-assisted interviews. Full details on the interviewing methodology have been described elsewhere19. For the purpose of this manuscript analyses a total of 4385 surveys corresponding to people above 14 years-old and not working night shift were considered.
The survey consisted on almost 500 questions including sociodemographic variables; health status & health-related quality of life; chronic diseases; unintentional injuries; pharmacological treatment; daily life limitations and disability; preventive practices; social support; mental wellbeing; dietary habits; PA and mobility; tobacco; alcohol; cannabis; use of healthcare resources during last 15 days and last year; material deprivation; and, sleep health. A full description of the ESCA 2015 questionnaire can be found elsewhere19 and the complete survey, in Catalan or Spanish, is publicly available from the Catalan Government web site (http://salutweb.gencat.cat/ca/el_departament/estadistiques_sanitaries/enquestes/esca/ [Accessed: August 20th, 2018]).
Sleep health was assessed using the SATED scale (Satisfaction, Alertness, Timing, Efficiency and Duration)20. Briefly, SATED is the result of a comprehensive review of the literature on sleep dimensions and their association with specific health outcomes in an attempt to create a tool capable of quantifying sleep health. SATED assesses 5 dimensions of sleep health by means of 5 questions: sleep Satisfaction (“Are you satisfied with your sleep?”); Alertness during waking hours (“Do you stay awake all day without dozing?”); Timing of sleep (“Are you asleep, or trying to sleep, between 2:00 a.m. and 4:00 a.m.?”); sleep Efficiency (“Do you spend less than 30 minutes awake at night? This includes the time it takes to fall asleep and awakenings from sleep”); and sleep Duration (“Do you sleep between 6 and 8 hours per day?”). Respondents indicate the frequency with which they experience or engage in each dimension, with answers ranging from 0 to 2 points (0 = “never” or “very rarely”; 1 = “sometimes”; 2 = “often” or “always”). Items on the SATED scale can be totalled to produce a single summary score, ranging from 0 (very poor sleep health) to 10 (excellent sleep health). For the purpose of this study having a SATED score <8 was considered as poor sleep health; this threshold corresponds to the median for this population as published elsewhere19.
The PREvención con DIeta MEDiterránea (PREDIMED) questionnaire21,22, measuring adherence to Mediterranean diet, was used as a measure of the healthiness of participants’ diet. Any score below 9 out of 14 questions was considered as a poor adherence to Mediterranean diet and thus poor diet, whereas scores of 9 or more were considered as a healthy diet. PA was measured using the International Physical Activity Questionnaire (IPAQ)23,24, that classifies subjects’ PA in low, moderate or vigorous. For the purpose of the analyses, we considered moderate and vigorous PA as a healthy PA and low PA as a poor PA. Smoking was assessed by a question on smoking status (never, former, current). Finally, an estimation of each subject’s risk of abusing alcohol was defined based on consumed standard drink units. Any weekly alcohol intake up to 28 standard drinks (“standard drinks” = 10 grams of pure alcohol) for men and 17 for women in the last 12 months was considered as low risk. Intake above these levels was considered high risk. Subjects without any alcohol intake in the last 12 months were considered as non-drinkers25.
Self-rated health status was assessed with the question: “In general, how would you rate your health” with the possible choices being “excellent”, “very good”, “good”, “fair”, or “poor”. For the current analyses, excellent, very good and good ratings were considered as good self-rated health status while fair and poor as a poor self-rated health status.
The ESCA survey is an official statistic of the Catalan Government. It was approved by the Consultants’ Committee of Confidential Information Management (CATIC) from the Catalan Health Department. ESCA was conducted in accordance to the Catalan and Spanish regulatory framework, in agreement with the year 2000 revision of the Helsinki Declaration. All participants in the ESCA survey were adequately informed and provided consent to participate. Data analysed in this study are included in this published article (Supplementary Information Files).
Statistical analysis
Appropriate weighting adjustment was applied to achieve representative frequencies, as less populated territories were oversampled. Continuous variables were summarized as the mean (standard deviation) and categorical variables as percentages. Diet, PA, smoking, alcohol consumption and sleep health were assessed, individually and jointly, as determinants of poor self-rated health using a survey-weighted logistic model26 adjusted for age, gender, education level and number of comorbidities. A modelling of the effect of the number of risk categories on self-perceived health status was used to assess the additive effect of having multiple poor health behaviours. The conferred risks were estimated using odds ratios (OR) and 95% confidence intervals (CI). Differences in the classification accuracy of the models were assessed by comparing the area under the receiver operating characteristic curve (AUC). Goodness of fit was assessed using the Hosmer-Lemeshow calibration test. R statistical software, version 3.4.1, was used for all the analyses. All tests were two tailed, and p-values < 0.05 were considered statistically significant.
Results
4385 surveys representative of the Catalan population were included in the analyses. The main sociodemographic, life-style and health-related characteristics of the population are shown in Table 1. Briefly, 49% of the sample were men, mean age (SD) was 47 (19) years, 58% were never smokers, 34% non-drinkers, 51% had a high adherence to Mediterranean diet and 15% reported vigorous PA. Regarding sleep health, the population had a SATED score of 7.91 (2.17), with a 67% of subjects reporting good sleep health (SATED ≥ 8). Finally, 81.7% of subjects reported themselves as having good self-rated health.
Table 1.
Main characteristics of the population.
| Sociodemographic characteristics | |
| Male gender | 49% |
| Age (years) | 47 (19) |
| Education level | |
| Primary | 24% |
| Secondary | 55% |
| University | 21% |
| Lifestyle habits | |
| Tobacco use | |
| Current smoker | 25% |
| Former smoker | 17% |
| Never smoker | 58% |
| Alcohol | |
| Non-drinker | 34% |
| Drinker (low risk) | 62% |
| Drinker (high risk) | 4% |
| Diet (PREDIMED) | |
| Low adherence to Mediterranean diet | 49% |
| High adherence to Mediterranean diet | 51% |
| Physical activity (IPAQ) | |
| Low | 26% |
| Moderate | 59% |
| Vigorous | 15% |
| Sleep health (SATED) | |
| Poor (SATED < 8) | 33% |
| Good (SATED ≥ 8) | 67% |
| Health status | |
| At least one chronic disease | 72% |
| Good self-rated health status | 82% |
Proportion or mean (SD), as appropriate. PREDIMED: PREvención con DIeta MEDiterránea questionnaire; IPAQ: International Physical Activity Questionnaire; SATED: sleep Satisfaction Alertness Timing Efficiency and Duration scale.
Table 2 shows logistic regression models examining the associations between tobacco use, alcohol consumption, diet, PA and sleep health and poor self-rated health status, adjusted for age, gender and number of comorbidities. Having poor sleep health showed the strongest independent association with a poor self-perceived health status (OR = 1.72; p < 0.001), followed by low adherence to Mediterranean diet (OR = 1.41; p < 0.001) and being a current smoker (OR = 1.38; p = 0.01). The predictive capacity of each of these models according to Receiver operating characteristic (ROC) curves is compared in Fig. 1, and shows that PA (AUC = 0.664) and sleep health were the life-style factors that best identified individuals with poor self-rated health status (AUC = 0.626).
Table 2.
Logistic regression models examining the association between tobacco use, alcohol consumption, diet, physical activity and sleep health and poor self-rated health status.
| OR (95%CI) | p-value | |
|---|---|---|
| Tobacco | ||
| Non-smoker | Ref | |
| Smoker | 1.38 (1.07–1.79) | 0.01 |
| Alcohol | ||
| Low risk drinker | Ref | |
| High risk drinker | 1.25 (0.71–2.20) | 0.43 |
| Diet | ||
| High-moderate adherence to Mediterranean diet | Ref | |
| Low adherence to Mediterranean diet | 1.41 (1.12–1.77) | <0.001 |
| Physical activity | ||
| Vigorous- Moderate | Ref | |
| Low | 1.34 (1.03–1.73) | 0.003 |
| Sleep health | ||
| Good (SATED ≥ 8) | Ref | |
| Poor (SATED < 8) | 1.72 (1.39–2.13) | <0.001 |
Logistic regression models adjusted for age, gender, education level and number of chronic diseases. SATED: sleep Satisfaction Alertness Timing Efficiency and Duration scale.
Figure 1.
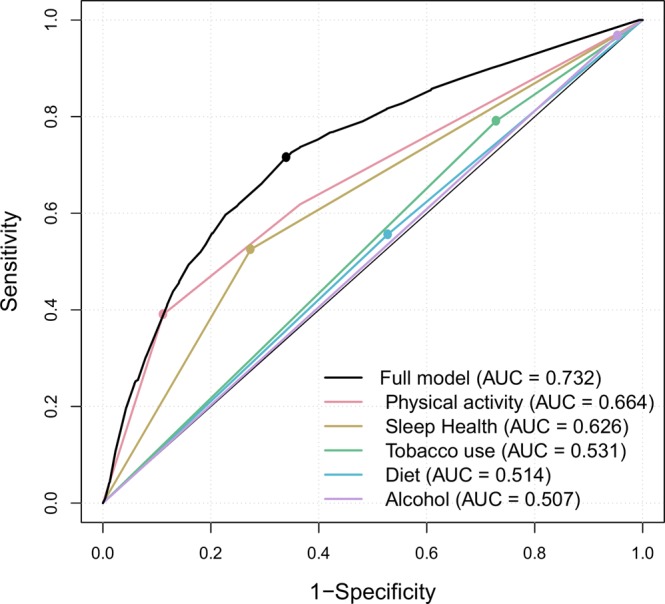
Receiver operating characteristic curves for the associations between life-style habits and self-rated health status. Logistic regression model. AUC: area under the curve.
Table 3 shows the adjusted associations between tobacco use, alcohol consumption, diet, PA and sleep health and poor self-rated health status, in a single model that also adjusts for age, sex, education level and number of comorbidities. In this model, poor sleep health was the factor most strongly associated with poor self-perceived health status (OR = 1.70; p < 0.001). The associations for both smoking status and alcohol intake were not statistically significant.
Table 3.
Logistic regression model examining the mutually adjusted associations between tobacco use, alcohol consumption, diet, physical activity and sleep health and poor self-rated health status.
| OR (95%CI) | p-value | |
|---|---|---|
| Gender: female | 0.96 (0.77–1.20) | 0.71 |
| Age | ||
| <45 years | Ref | |
| 45–64 years | 2.15 (1.63–2.83) | <0.001 |
| 65–74 years | 1.71 (1.25–2.72) | 0.02 |
| ≥75 years | 2.65 (1.35–5.19) | <0.001 |
| Number of chronic diseases | 1.47 (1.41–1.54) | <0.001 |
| Education level | ||
| Primary | Ref | |
| Secondary | 0.74 (0.57–0.95) | 0.02 |
| University | 0.57 (0.39–0.82) | <0.001 |
| Non-smoker | Ref | |
| Smoker | 1.29 (1.00–1.68) | 0.05 |
| Non-drinker/low risk drinker | Ref | |
| High risk drinker | 1.18 (0.66–2.12) | 0.57 |
| High/moderate adherence to Mediterranean diet | Ref | |
| Low adherence to Mediterranean diet | 1.37 (1.10–1.72) | <0.01 |
| Vigorous/Moderate physical activity | Ref | |
| Low physical activity | 1.31 (1.01–1.69) | 0.04 |
| Good sleep health (SATED ≥ 8) | Ref | |
| Poor sleep health (SATED < 8) | 1.70 (1.37–2.12) | <0.001 |
A single logistic regression model adjusted for age, gender, education level, number of chronic diseases and all considered modifiable risk factors at the same time. SATED: sleep Satisfaction Alertness Timing Efficiency and Duration scale.
Finally, a model assessing the additive effect of having multiple poor health behaviours is shown in Table 4. Having multiple poor health behaviours was associated to increased odds of poor self-rated health. This relation was dose-dependent, demonstrating an additive effect of poor health behaviours.
Table 4.
Adjusted logistic regression model examining the additive effect of having multiple poor health behaviours on poor self-rated health status.
| OR (95%CI) | p-value | |
|---|---|---|
| Absence of health risk behaviours | Ref | |
| 1 Health risk behaviour | 1.27 (0.94–1.73) | 0.13 |
| 2 Health risk behaviours | 1.57 (1.13–2.18) | 0.01 |
| 3 Health risk behaviours | 2.66 (1.80–3.92) | <0.001 |
| 4 or 5 Health risk behaviours | 5.18 (2.91–9.24) | <0.001 |
Model adjusted for age, gender, education level and number of chronic diseases.
Discussion
In this study, including data from a representative population sample of 4385 individuals, we assessed and compared the relative weights of diet, PA, smoking, alcohol consumption and sleep health in relation to self-perceived health status. Poor sleep health showed the strongest independent association with a poor self-perceived health status. This association held even when adjusting for the number of comorbidities and when all studied lifestyle factors were simultaneously adjusted.
It is well-known that sleep duration, either being too short or too long, is related to a poor health status at all ages27–31. Additionally, although less evidence is available, sleep quality has also been related to health status, showing stronger relations than sleep duration in most studies32,33. However, few studies up to date have tried to assess simultaneously the impact of sleep together with other key lifestyle factors in relation to health status, and only two of them included all the key modifiable risk factors identified by the WHO: smoking, alcohol intake, diet and PA34,35. For instance, Bayán-Bravo et al. prospectively assessed the impact of traditional (non-smoking, being very or moderately active and having healthy diet) and non-traditional (sleeping 7–8 h/d, being seated <8 h/d, and seeing friends every day) health behaviours in relation to health-related quality of life (HRQL) in a cohort of 2,388 subjects aged ≥60. PA, adequate sleep duration and sitting less, were associated with better HRQL in the short-term follow-up (2.5 years), whereas in the long term (8.5 years) only PA showed a significant association with HRQL34. However, this study assumed that health behaviours were stable over time, and did not assess dimensions of sleep health other than duration. Both limitations are relevant to the changes in sleep patterns usually seen with ageing: an increase in sleep duration combined with a decrease in sleep quality19. Duncan et al. assessed the cross-sectional associations of smoking, physical activity, diet, sitting time, sleep duration and sleep quality on self-perceived health among 10,478 individuals. Poor sleep quality, followed by low PA, had the strongest associations with self-perceived health35. Finally, several studies have assessed the combined effects of sleep duration and/or quality and other behavioural risk factors in relation to different health outcomes, all of them identifying significant relationships between sleep health and the studied health outcomes36–40.
The results of this study suggest that having healthy sleep, and thus being satisfied with the way we sleep, is a key factor in the self-perception of health. Individuals describing their sleep health as poor are less likely to report good self-perceived health than individuals engaging in other unhealthy habits such as tobacco smoking or alcohol consumption. This suggests that the link between sleeping well and feeling well is, if anything, tighter than the link with other well-known unhealthy habits. However, these associations do not necessarily imply that that poor sleep habits are the strongest modifiable health risk factor. People’s perceptions of sleep could lead to ratings of poor perceived health via reverse causality: “I perceive that I am not healthy because I don’t feel rested when I get up in the morning” or “I am struggling to get asleep every night thus my health cannot be good”. Therefore, the current results should not be interpreted as a proof of sleep health being the strongest modifiable risk factor for overall health status, but as an indication that sleep health may have been neglected when considering modifiable risk factors related to health.
From a public health perspective, the current study increases the evidence relating sleep health with overall health status. The promotion of healthy sleep habits could, therefore, be considered as a potential strategy to promote not only the overall health status of a given population, but also the self-perception of being healthy. This study also shows that having multiple poor health behaviours is associated to increased odds of poor self-rated health. Therefore, the promotion of healthier sleep habits could have a synergistic effect with other health promotion activities35,41.
The current study has several strengths, including: (i) a large sample size, selected to be representative of the Catalan population; (ii) comprehensive measures of behavioural risk factors; and, (iii) a detailed assessment of sleep health including multiple dimensions. On the other hand, several limitations must be acknowledged: (i) the cross-sectional design did not allow establishment of the direction of the associations and, therefore, whether poor sleep health is a cause or a consequence of a poor self-perceived health status falls beyond the scope of the current analyses; (ii) the SATED scale has yet to be formally validated, although their five dimensions have been consistently associated with health outcomes and no other validated tool is currently available to measure sleep health; (iii) all behavioural risk factors were self-reported and thus potentially subject to some degree of reporting bias and/or social desirability bias; and, (iv) although the dichotomization of the behavioural risk factors allowed for a direct comparison of the magnitude of their effects, it also implied some degree of misclassification. This could be especially relevant in the case of former smokers who quitted because of medical conditions.
In conclusion, our study suggests that sleeping habits are amongst the strongest potentially modifiable risk factors associated with the self-perception of health, independent of age, gender and the number of comorbidities. Although poor sleep health can be a cause or a consequence of a poor self-perceived health status, these findings show that sleep habits should not be neglected when defining a healthy lifestyle.
Supplementary information
Acknowledgements
Partially supported by Ministerio de Economía y Competitividad (COFUND2014-51501), Departament de Salut (PERIS 2016: SLT002/16/00364) and SEPAR (2019). The authors would like to acknowledge the Catalan health department for gently providing all the data required for the current analyses, and express their sincerest gratitude to all professionals involved in ESCA 2015 and all subjects who responded the survey.
Author Contributions
Conceptualization: J.d.B., F.B. and M.S.d.l.T. Data Curation: I.B., O.G.-C. and A.M.-B. Formal Analysis: I.B. Resources: D.J.B., E.S. and J.E. Supervision: J.d.B. and F.B. Interpretation of data: M.D., I.B., E.S.-B., R.E.P., D.J.B., M.S.d.l.T., F.B. and J.d.B. Writing – Original Draft Preparation: M.D., I.B. and J.d.B. Writing – Review & Editing: all. M.D. and I.B. contributed equally to this work and are co-first authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mireia Dalmases and Ivan Benítez contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43873-5.
References
- 1.World Health Organisation (WHO). Report GLOBAL STATUS REPORT on non communicable diseases, 2014 “Attaining the nine global non communicable diseases targets; a shared responsibility”, ISBN 978 92 4 156485 4, WHO Library Cataloguing-in-Publication Data, http://www.who.int/nmh/publications/ncd-status-report-2014/en/ [last accessed 09/05/2018].
- 2.Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 2013;369:954–964. doi: 10.1056/NEJMra1203528. [DOI] [PubMed] [Google Scholar]
- 3.Stranges S, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J. Hypertens. 2010;28:896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- 4.Gangwisch JE, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertens. Dallas Tex. 2006;1979(47):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 5.von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PloS One. 2012;7:e30972. doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayas NT, et al. A prospective study of sleep duration and coronary heart disease in women. Arch. Intern. Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 7.King CR, et al. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakizaki M, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br. J. Cancer. 2008;99:1502–1505. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappuccio FP, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 11.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rod NH, et al. The joint effect of sleep duration and disturbed sleep on cause-specific mortality: results from the Whitehall II cohort study. PloS One. 2014;9:e91965. doi: 10.1371/journal.pone.0091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc. Sci. Med. 2010;1982(71):1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Oishi M, et al. A longitudinal study on the relationship between shift work and the progression of hypertension in male Japanese workers. J. Hypertens. 2005;23:2173–2178. doi: 10.1097/01.hjh.0000189870.55914.b3. [DOI] [PubMed] [Google Scholar]
- 15.Suwazono Y, et al. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. J. Occup. Environ. Med. 2006;48:455–461. doi: 10.1097/01.jom.0000214355.69182.fa. [DOI] [PubMed] [Google Scholar]
- 16.Zamanian Z, Nikeghbal K, Khajehnasiri F. Influence of Sleep on Quality of Life Among Hospital Nurses. Electron. Physician. 2016;8:1811–1816. doi: 10.19082/1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurston, R. C. et al. Sleep Characteristics and Carotid Atherosclerosis Among Midlife Women. Sleep 40 (2017). [DOI] [PMC free article] [PubMed]
- 18.Health survey of Catalonia (ESCA) 2015. Barcelona. Ministry of Health, [http://salutweb.gencat.cat/web/.content/home/el_departament/estadistiques_sanitaries/enquestes/esca_2015.pdf] Accessed: December 21th, 2016 (2016).
- 19.Dalmases, M. et al. Assessing sleep health in a European population: Results of the Catalan Health Survey 2015. PLoS ONE 13 (2018). [DOI] [PMC free article] [PubMed]
- 20.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-González MA, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PloS One. 2012;7:e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schröder H, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011;141:1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 23.Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 24.Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) Med. Sci. Sports Exerc. 2004;36:556. doi: 10.1249/01.MSS.0000117161.66394.07. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Martos Dauer A, Gual Solé A, Llopis Llácer JJ. The ‘standard drink unit’ as a simplified record of alcoholic drink consumption and its measurement in Spain. Med. Clin. (Barc.) 1999;112:446–450. [PubMed] [Google Scholar]
- 26.Lumley T, Scott A. Fitting Regression Models to Survey Data. Stat. Sci. 2017;32:265–278. doi: 10.1214/16-STS605. [DOI] [Google Scholar]
- 27.Chaput J-P, et al. Systematic review of the relationships between sleep duration and health indicators in the early years (0–4 years) BMC Public Health. 2017;17:855. doi: 10.1186/s12889-017-4850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltzer K, Pengpid S. Sleep duration and health correlates among university students in 26 countries. Psychol. Health Med. 2016;21:208–220. doi: 10.1080/13548506.2014.998687. [DOI] [PubMed] [Google Scholar]
- 29.Frange C, de Queiroz SS, da Silva Prado JM, Tufik S, de Mello MT. The impact of sleep duration on self-rated health. Sleep Sci. Sao Paulo Braz. 2014;7:107–113. doi: 10.1016/j.slsci.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cepeda MS, Stang P, Blacketer C, Kent JM, Wittenberg GM. Clinical Relevance of Sleep Duration: Results from a Cross-Sectional Analysis Using NHANES. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016;12:813–819. doi: 10.5664/jcsm.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama E, et al. Association between subjective well-being and sleep among the elderly in Japan. Sleep Med. 2008;9:157–164. doi: 10.1016/j.sleep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Gadie A, Shafto M, Leng Y, Kievit RA, & Cam-CAN. How are age-related differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open. 2017;7:e014920. doi: 10.1136/bmjopen-2016-014920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basnet S, et al. Seasonality, morningness-eveningness, and sleep in common non - communicable medical conditions and chronic diseases in a population. Sleep Sci. Sao Paulo Braz. 2018;11:85–91. doi: 10.5935/1984-0063.20180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayán-Bravo A, et al. Combined Impact of Traditional and Non-Traditional Healthy Behaviors on Health-Related Quality of Life: A Prospective Study in Older Adults. PloS One. 2017;12:e0170513. doi: 10.1371/journal.pone.0170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan MJ, et al. Cross-sectional associations between multiple lifestyle behaviors and health-related quality of life in the 10,000 Steps cohort. PloS One. 2014;9:e94184. doi: 10.1371/journal.pone.0094184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding D, et al. Revisiting lifestyle risk index assessment in a large Australian sample: should sedentary behavior and sleep be included as additional risk factors? Prev. Med. 2014;60:102–106. doi: 10.1016/j.ypmed.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Hoevenaar-Blom MP, Spijkerman AMW, Kromhout D, Verschuren WMM. Sufficient sleep duration contributes to lower cardiovascular disease risk in addition to four traditional lifestyle factors: the MORGEN study. Eur. J. Prev. Cardiol. 2014;21:1367–1375. doi: 10.1177/2047487313493057. [DOI] [PubMed] [Google Scholar]
- 38.Martínez-Gómez D, Guallar-Castillón P, León-Muñoz LM, López-García E, Rodríguez-Artalejo F. Combined impact of traditional and non-traditional health behaviors on mortality: a national prospective cohort study in Spanish older adults. BMC Med. 2013;11:47. doi: 10.1186/1741-7015-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eguchi E, et al. Healthy lifestyle behaviours and cardiovascular mortality among Japanese men and women: the Japan collaborative cohort study. Eur. Heart J. 2012;33:467–477. doi: 10.1093/eurheartj/ehr429. [DOI] [PubMed] [Google Scholar]
- 40.Odegaard AO, Koh W-P, Gross MD, Yuan J-M, Pereira MA. Combined lifestyle factors and cardiovascular disease mortality in Chinese men and women: the Singapore Chinese health study. Circulation. 2011;124:2847–2854. doi: 10.1161/CIRCULATIONAHA.111.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Prev. Med. 2008;46:181–188. doi: 10.1016/j.ypmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


