Abstract
Although modern cochlear implants (CIs) use cathodic-leading symmetrical biphasic pulses to stimulate the auditory nerve, a growing body of evidence suggests that anodic-leading pulses may be more effective. The positive polarity has been shown to produce larger electrically evoked compound action potential (ECAP) amplitudes, steeper slope of the amplitude growth function, and broader spread of excitation (SOE) patterns. Polarity has also been shown to influence pitch perception. It remains unclear how polarity affects the relation between physiological SOE and psychophysical pitch perception. Using a within-subject design, we examined the correlation between performance on a pitch-ranking task and spatial separation between SOE patterns for anodic and cathodic-leading symmetric biphasic pulses for 14 CI ears. Overall, there was no effect of polarity on either ECAP SOE patterns, pitch ranking performance, or the relation between the two. This result is likely due the use of symmetric biphasic pulses, which may have reduced the size of the effect previously observed for pseudomonophasic pulses. Further research is needed to determine if a pseudomonophasic stimulus might further improve the relation between physiology and pitch perception.
Keywords: electrical stimulation, biphasic stimuli, electrically evoked compound action potential
Introduction
Pitch perception for cochlear implant (CI) recipients is influenced by the type of stimulus used (Cohen et al. 1996; Hamzavi and Arnoldner 2006; Townshend et al. 1987). In particular, stimulus polarity has been found to be important for pitch perception. Several studies have shown that the anodic phase contributes most to pitch percepts for CI users, as opposed to the cathodic phase (Carlyon et al. 2013; Macherey and Carlyon 2012; Macherey et al. 2011). Stimulus polarity has also been shown to affect physiological measures in CI users (Hughes et al. 2017; Macherey et al. 2008; Spitzer and Hughes 2017; Undurraga et al. 2010, 2012, 2013). Recently, Spitzer and Hughes (2017) demonstrated that anodic-leading symmetrical biphasic pulses presented in monopolar mode produced larger amplitude, broader electrically evoked compound action potential (ECAP) spread-of-excitation (SOE) patterns than cathodic-leading pulses. SOE patterns have also been shown to be related to pitch perception for pulse trains (Hughes 2008). However, it remains unclear how stimulus polarity affects the relation between pitch perception and SOE, particularly for standard pulse shapes that are used clinically for Cochlear devices (i.e., symmetrical biphasic pulses). It may be that for standard pulse shapes, one leading polarity yields stronger correlations between pitch perception and ECAP SOE patterns than the other. The purpose of the present study was to examine the effects of stimulus polarity on the ECAP SOE patterns, pitch perception, and the relation between these two measures for CI users.
The effect of stimulus polarity on pitch perception has been investigated in a handful of studies. Based on the tonotopic organization of the cochlea, we would expect a lower pitch percept to be associated with a more apical stimulation. In a group of seven subjects, Macherey et al. (2011) presented pseudomonophasic pulses in bipolar (BP + 1) mode with opposite polarities delivered to each electrode in the bipolar pair. These pseudomonophasic pulses consisted of a short-duration high-amplitude phase of one polarity, immediately followed by a long-duration low-amplitude phase of the other polarity. They found that listeners were more likely to judge the apical electrode in the BP + 1 pair as being lower in pitch than the more basal electrode when the apical electrode received the anodic short-high phase. Furthermore, they found that these pseudomonophasic pulses were also perceived as lower in pitch than anodic-first symmetrical biphasic pulses presented in either bipolar or monopolar mode. Carlyon et al. (2013) demonstrated the same effect for quadraphasic pulses in bipolar mode, finding that the apical electrode was more often judged as being lower in pitch when the center (largest amplitude) portion of the pulse was anodic than when it was cathodic. Therefore, the anodic polarity produced a pitch perception that was more in agreement with target place pitch than the cathodic polarity because lower pitch percepts were associated with more apical stimulation for anodic pulses. In general, it appears that the location of the positive phase contributes most to the pitch percept.
Research also suggests that the auditory nerve is sensitive to polarity for physiological measures such as the ECAP and electrically evoked auditory brainstem response (EABR). Using pseudomonophasic pulses, Macherey et al. (2008) showed that ECAP amplitudes were larger and had shorter latencies for anodic than for cathodic stimuli. Similar results were found for other non-clinical pulse shapes such as symmetric pulses with long interphase gaps (Undurraga et al. 2010, 2012), as well as for clinically used symmetrical biphasic pulses (Hughes et al. 2017). These studies support modeling work (Rattay et al. 2001a, b) that demonstrated greater effectiveness of the anodic polarity than the cathodic polarity for stimulating peripherally degenerated neurons. The anodic phase preferentially stimulates the central axons, requiring less current than the cathodic phase to generate an action potential. More recently, we examined the effect of polarity on ECAP SOE patterns obtained with symmetrical biphasic pulses (Spitzer and Hughes 2017). Results showed that ECAP SOE patterns obtained with anodic-leading stimuli were broader and demonstrated less spatial separation between patterns (i.e., more overlap) than SOE patterns obtained with cathodic-leading stimuli, given equal current levels. This finding is in agreement with Undurraga et al. (2012), who suggested that cathodic stimuli produce less effective masking than anodic stimuli. With the forward-masking method used to measure ECAP SOE patterns, cathodic-leading stimuli produce narrower SOE patterns, which might, therefore, underestimate the breadth of the actual SOE. If anodic-leading pulses yield more effective masking than cathodic-leading pulses, then we propose that the SOE patterns obtained with anodic-leading pulses might more accurately reflect the actual patterns of neural excitation that contribute to pitch perception.
A few studies have examined the relation between ECAP SOE and psychophysical pitch ranking. Busby et al. (2008) measured SOE width as the number of electrodes at 75 % of the normalized peak amplitude of the function and found no relation between SOE width and pitch ranking. Hughes and Abbas (2006) also found no significant correlation between SOE width and pitch ranking using a similar width measure. However, a follow-up study (Hughes 2008) re-examined the ECAP data from the Hughes and Abbas (2006) study and quantified the SOE patterns using a novel method. Instead of measuring each SOE function in terms of width at a specific downpoint, pairs of SOE patterns were quantified in terms of spatial overlap. ECAP amplitudes for the entire function were first normalized to that of the probe electrode. Next, the amplitude difference was calculated between functions at each masker location. These differences were then summed together across all masker electrodes yielding an index of spatial separation. The SOE spatial separation index was then related to pitch-ranking performance between those same pairs of electrodes. The results showed that greater spatial separation between SOE patterns was correlated with better performance on a pitch-ranking task. Similarly, Goehring et al. (2014a, b) found a generally positive but not statistically significant result when comparing spatial separation and pitch ranking for virtual-channel stimulation using the same methods.
Given these mixed results, the goal of the present study was to determine if manipulating stimulus polarity affected the correlation between the spatial separation of ECAP SOE patterns and pitch-ranking performance. Because it has been shown that the anodic phase contributes more to place-pitch percepts than the cathodic phase, we hypothesize that CI recipients will score higher on pitch-ranking tasks when the symmetric, biphasic pulses are anodic-leading than when they are cathodic-leading. Results from our recent ECAP work (Spitzer and Hughes 2017) show that cathodic-leading pulses produce narrower SOE patterns than for anodic-leading pulses, presumably due to less effective masking (Undurraga et al. 2012). If anodic-leading stimuli produce more effective masking, we hypothesize that the forward-masked SOE patterns obtained with anodic-leading pulses will more accurately reflect the actual neural activation patterns that contribute to pitch-ranking abilities in CI users. As a result, we expect a significant positive correlation between the spatial separation of ECAP SOE patterns and pitch ranking for anodic-leading stimuli but not necessarily for cathodic-leading stimuli. To test these hypotheses, the present study measured pitch ranking and ECAP SOE patterns for CI recipients using anodic- and cathodic-leading symmetric biphasic pulses and compared the two using a within-subject design.
Methods
Participants
Fourteen ears from 13 CI recipients were tested for this study (seven males; age range: 18–77 years; mean 58 years). Five participants were bilateral CI users, although only F10/F11 was tested in both ears. For the other four bilateral users (F1, F5, F17, N11), the contralateral ear was not tested because it had either an older device or small/absent ECAPs. All participants were implanted with Cochlear Ltd. (Sydney, New South Wales, Australia) devices with the same internal electronics package. Seven of the 14 ears were implanted with the Nucleus 24RE (CA) array, three with the CI422, three with the CI512, and one with the CI522. Additional demographic information is listed in Table 1. This study was approved by the Boys Town National Research Hospital Institutional Review Board under protocol 03-07-XP. Written informed consent was obtained from all participants. Participants received a small travel stipend and hourly compensation for their time.
Table 1.
Demographic information for study participants. L, left; R, right; IS, initial stimulation; CI, cochlear implant; HL, hearing loss
| Subject | Internal device | Ear | Gender | Age at test (years, months) | Duration of deafness (years, months) | Age at IS (years, months) | Duration of CI use (years, months) | Etiology, onset |
|---|---|---|---|---|---|---|---|---|
| F1 | 24RE(CA) | L | F | 70, 9 | 11, 3 | 60, 7 | 10, 2 | Unknown/progressive |
| F2 | 24RE(CA) | R | F | 69, 3 | 10, 6 | 60, 3 | 9, 0 | Unknown/progressive |
| F5 | 24RE(CA) | R | M | 56, 2 | 7, 7 | 48, 3 | 7, 11 | Unknown/sudden from established HL |
| F10* | 24RE(CA) | R | F | 18, 10 | 8, 3 | 8, 3 | 10, 7 | Waardenburg syndrome/congenital |
| F11* | 24RE(CA) | L | F | 18, 10 | 1, 10 | 1, 10 | 17, 0 | Waardenburg syndrome/congenital |
| F17 | 24RE(CA) | R | M | 53, 3 | 11, 0 | 42, 11 | 10, 4 | Congenital/progressive |
| F27 | 24RE(CA) | L | M | 58, 2 | 10, 6 | 56, 2 | 2, 0 | Otosclerosis/progressive |
| N7 | CI512 | R | M | 75, 5 | 10, 11 | 69, 9 | 5, 8 | Unknown/progressive |
| N11 | CI512 | L | M | 72, 5 | 6, 6 | 67, 5 | 5, 0 | Unknown-familial and noise exposure/progressive |
| N23 | CI512 | R | F | 73, 0 | 1, 10 | 70, 5 | 2, 7 | Meniere’s |
| FS28 | CI422 | R | F | 74, 4 | 3, 7 | 72, 6 | 1, 10 | Unknown/progressive |
| FS31 | CI422 | R | M | 77, 7 | 24, 1 | 76, 4 | 1, 3 | Noise induced/hereditary |
| FS32 | CI422 | L | M | 61, 11 | 8, 3 | 58, 9 | 3, 2 | Unknown/hereditary and progressive |
| NS20 | CI522 | R | F | 32, 5 | 28, 9 | 31, 9 | 0, 8 | Illness/unknown |
*Matched symbols indicate both ears from a participant with bilateral CIs
Stimuli and Procedure—ECAP Recordings
This study utilized a subset of the ECAP SOE data from Spitzer and Hughes (2017). Briefly, ECAPs were recorded using commercially available Custom Sound EP (v. 4.3) software (Cochlear Ltd., Sydney, New South Wales, Australia) using a laboratory Freedom sound processor and programming pod to present stimuli. ECAPs were evoked using symmetric biphasic current pulses presented in monopolar mode (re: MP1). The forward-masking subtraction method was used to remove stimulus artifact (Abbas et al. 1999). SOE patterns were obtained by roving the masker electrode location across the array while the probe and recording electrode locations were fixed. The recording electrode was typically two electrode positions apical to the probe, referenced to the extracochlear electrode MP2. The default gain of 50 dB was used, and the recording delay was optimized individually to minimize artifact. Other relevant stimulus and recording parameters were 400 μs masker-probe interval, 100 averages, and 80-Hz probe rate. Data were collected using the default phase duration of 25 μs for half of the ears (F1, F2, F5, F27, FS31, NS20, and N11) and 50 μs for the remaining ears (F10, F11, F17, N7, N23, FS28, and FS32) due to voltage compliance limitations. SOE patterns were obtained for 14 probe electrodes for both stimulus polarities (probe electrodes were generally 5–18) for a total of 28 SOE patterns per subject. Three regions of the electrode array were delineated: basal (probes 5–9), middle (9–13), and apical (14–18). For each region, the middle electrode of the set was designated as the reference electrode (basal = 7, middle = 11, apical = 16). Electrode pairs were defined between the reference electrode and the other electrodes in that region (for example, the basal comparison pairs were 5–7, 6–7, 8–7, and 9–7).
Both masker and probe stimuli were presented at an “8” of 10 loudness rating (“loud”), which was obtained using an ascending loudness-scaling technique for each electrode. Anodic-leading stimuli were used for the loudness scaling, as this polarity is typically the louder of the two polarities (Macherey et al. 2006, 2008). For two subjects, a lower loudness rating (usually “7” out of 10) was used due to voltage compliance limits. The current levels used for each masker and probe electrode were the same for both polarities.
Stimuli and Procedure—Pitch Ranking
Pitch ranking was tested using a custom program written in Visual Basic that utilized Nucleus Implant Communicator (NIC v. 2.23.0.822) routines (Cochlear Ltd., Sydney, NSW, Australia). For each of the six conditions (three regions: basal, middle, and apical; two polarities: cathodic and anodic), three steps were undertaken. First, the dynamic range for each electrode in a condition was identified by presenting 300-ms pulse trains using MP1 mode ascending from 100 current-level units (CL) in 5-CL steps. Participants were told to provide categorical loudness estimates on the same 10-point scale used for determining stimulation levels for ECAP recordings: when the stimulus was “1” (“just noticeable”), “8” (“loud”), and “9” (“maximum loudness level”). Next, each electrode comparison pair was loudness balanced using an adaptive, two-alternative, forced-choice (2AFC) double-staircase procedure (Jesteadt 1980). For each trial, participants were presented with two sounds sequentially (one on each electrode in the comparison pair) that visually corresponded to two boxes on a touch-screen monitor, and were instructed to click with a mouse or touch the box with the louder sound, regardless of pitch. The two tracks began 5 CL above and below the “8” level, respectively. The tracks were then interleaved at random. Current level was adjusted using a two-up, one-down procedure to find 71 % “correct” on the psychometric function (Levitt 1971). Loudness balancing was terminated after 10 reversals for each track. The final level used for pitch ranking represented the last six reversals from each track averaged together. Step size was two CL for the first two reversals and one CL for the eight remaining reversals.
For the pitch-ranking task, participants were presented with stimulation on an electrode pair in a similar manner to the loudness-balancing procedure and were asked to indicate which was higher in pitch, regardless of loudness. The stimuli were presented at the CL corresponding to a loudness-balanced “8” (as determined by the loudness balancing procedure). A random level rove equal to 5 % of the dynamic range was applied above and below the “8” level. This was done to help participants avoid making judgments based on loudness instead of pitch and to account for any slight variations in loudness perception throughout the course of the task. No feedback was provided. Pitch ranking was accomplished in blocks of 64 trials, with 16 trials per comparison pair (half with the basal-side electrode presented in interval one) and four comparison pairs per electrode set. All trials were presented in a randomized order with regard to comparison pair and which electrode of the pair was presented first. At least two blocks were completed for each of the six conditions, resulting in a minimum of 32 trials per comparison pair. If the “percent correct” for two blocks differed by approximately 20 % or more, additional blocks were run. Only three participants (F16, F27, and FS31) did not require additional blocks. Additional blocks were completed for a total of 32 of the 84 electrode sets: one additional block was run for 23 electrode sets across eight participants (yielding a total of 48 trials per comparison pair), two additional blocks were run for eight electrode sets across three participants (64 trials/pair), and six additional blocks were run for one electrode set for F11 (96 trials for each of the electrode pairs in the basal cathodic set). Although pitch ranking is a subjective task where there is no truly correct answer for CI recipients, trials where the basal-side electrode was chosen as higher in pitch were deemed correct based on the expected cochlear tonotopic organization. Total percent correct for a comparison pair was calculated by dividing the number of correct trials by the total number of trials for that pair.
Data Analysis—ECAP Recordings
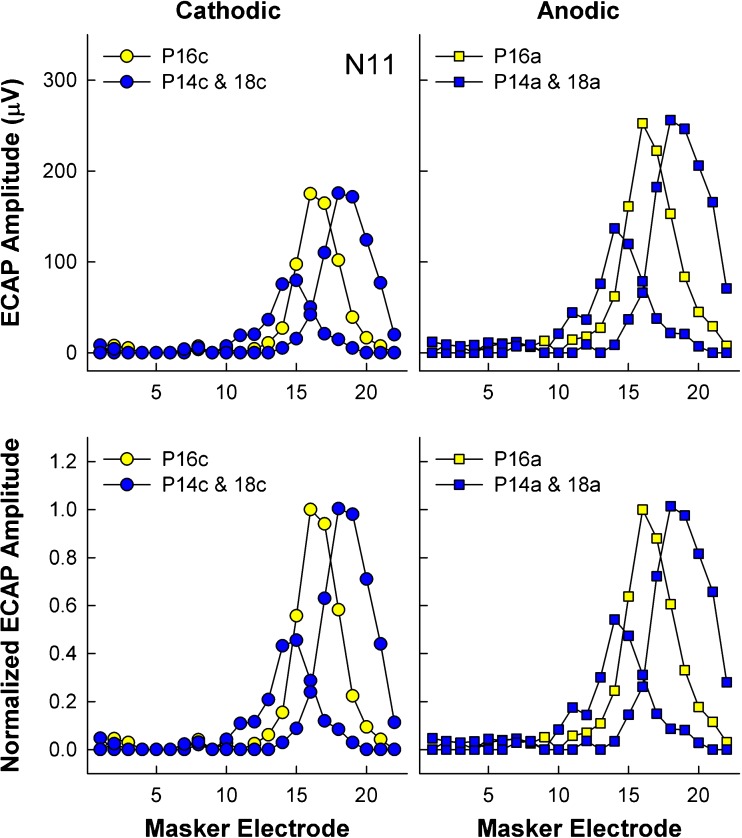
ECAP peaks N1 and P2 were automatically picked by the Custom Sound EP software and manually adjusted as necessary. Amplitude was calculated as the voltage difference between the two peaks and then exported and processed using custom Matlab scripts (Mathworks, Natick, MA). The spatial separation index (Σ; Hughes 2008) was calculated between pairs of SOE functions by normalizing the ECAP amplitude to the peak amplitude of the reference electrode (again, typically electrodes 7, 11, and 16 for basal, middle, and apical regions, respectively). Normalizations were done separately for each region and polarity. This method differs slightly from how the spatial separation index was calculated in Spitzer and Hughes (2017), where SOE functions were normalized to the peak amplitude of each separate comparison pair. Data were normalized as described above to be congruent with the psychophysical pitch ranking data, for which judgments were also made relative to the center/reference electrode in each region. An example of the normalization process is shown in Fig. 1 for apical SOE patterns from participant N11. Raw and normalized ECAP SOE patterns are shown in the top and bottom rows, respectively. Patterns obtained with cathodic-leading and anodic-leading pulses are in the left (circles) and right (squares) columns, respectively. For ease of viewing, only the SOE patterns for the reference electrode (P16) and the most basal (P14) and apical (P18) comparison probes in the set are shown.
Fig. 1.
An individual example of the amplitude normalization process for ECAP SOE patterns for subject N11. Each of the four panels depicts ECAP amplitude as a function of masker electrode for the reference probe electrode (P16, yellow symbols) and the most basal and apical probes in the set (P14 and P18, blue symbols). Left column: SOE patterns for cathodic probes (circles). Right column: SOE patterns for anodic probes (squares). Top row: Raw ECAP amplitudes. Bottom row: ECAP amplitudes normalized to the reference probe electrode (P16)
Next, the absolute difference in normalized amplitude between comparison pairs at each masker electrode location was calculated and summed together to yield the spatial separation index (Hughes 2008). Because ECAPs cannot generally be recorded from the same electrode that delivers the stimulus, the ECAP amplitude had to be estimated for a masker hypothetically delivered to the recording electrode. This was done by averaging the ECAP amplitudes obtained for the two masker electrodes on either side of the recording electrode (linear interpolation). Comparison pairs for anodic-leading stimuli were matched to those for cathodic-leading stimuli to determine if there were differences in the spatial separation index between polarities using a Wilcoxon Signed Rank test (due to non-normal distribution of the data). Although the effect of polarity on spatial separation was examined in our earlier study (Spitzer and Hughes 2017), it was recalculated for this study because the SOE functions were normalized differently (i.e., to the peak of the reference electrode SOE versus the peak of each separate comparison pair).
Data Analysis—Pitch Ranking
Percent-correct scores were transformed into rationalized arcsine units (RAUs) in order to create uniform variance across the percent-correct scale (Studebaker 1985). All statistical analyses were completed using SigmaPlot (Systat Software, San Jose, CA).
Pitch-ranking scores for each electrode pair were then compared between polarities using a Wilcoxon Signed Rank test to determine if performance was better with one polarity or the other. Lastly, pitch-ranking scores were compared to corresponding SOE spatial separation values for each electrode pair within a polarity using Pearson correlations to examine the extent to which the spatial separation among ECAP SOE patterns was correlated with pitch ranking, and whether those relations differed for the two polarities.
Results
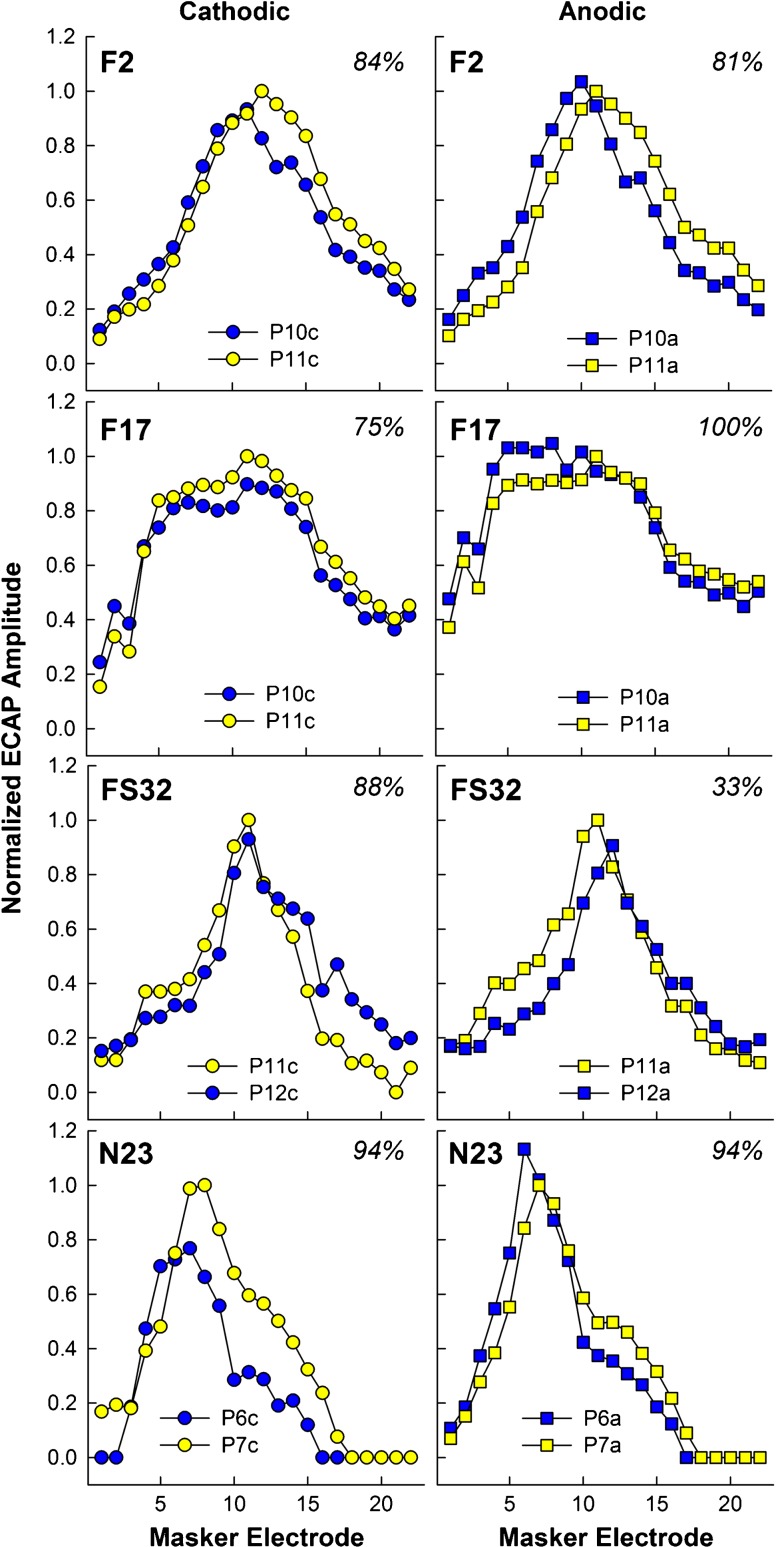
Figure 2 shows individual examples of normalized SOE patterns for a single comparison pair for both polarities (left/circles, cathodic; right/squares, anodic) for four participants (each row). Participant number and percent correct on the pitch-ranking task are indicated at the top of each graph. In each panel, the reference electrode is shown in yellow and the comparison electrode is shown in blue. These examples illustrate how the spatial separation between SOE functions can differ with polarity. For F2 (top row), the basal side of both SOEs overlaps with cathodic-leading pulses (left), whereas there is greater separation between functions on the basal side for the anodic-leading stimuli (right). For F17, the SOE pattern for probe 11 has larger amplitudes overall for the cathodic condition (left), whereas the opposite is true for anodic (right). For FS32, the primary separation between functions obtained with cathodic-leading stimuli occurs across the apical half of the function, with overlap of the basal portions of each function. The opposite trend was observed for the anodic condition. Finally, for N23 (bottom row), the cathodic condition yielded greater separation between functions than the anodic condition.
Fig. 2.
Individual examples of the effect of polarity on the spatial separation between ECAP SOE patterns for four participants. Each row depicts data from a different participant. Normalized ECAP amplitude is plotted as a function of masker electrode. Patterns for cathodic-leading probes (circles) are in the left column and for anodic-leading probes (squares) in the right column. The reference probe electrode is shown in yellow symbols and the comparison probe electrode is shown in blue symbols. The specific reference and comparison probe electrodes are shown in the legend of each panel. Participant number and percent correct for pitch ranking are shown at the top of each panel
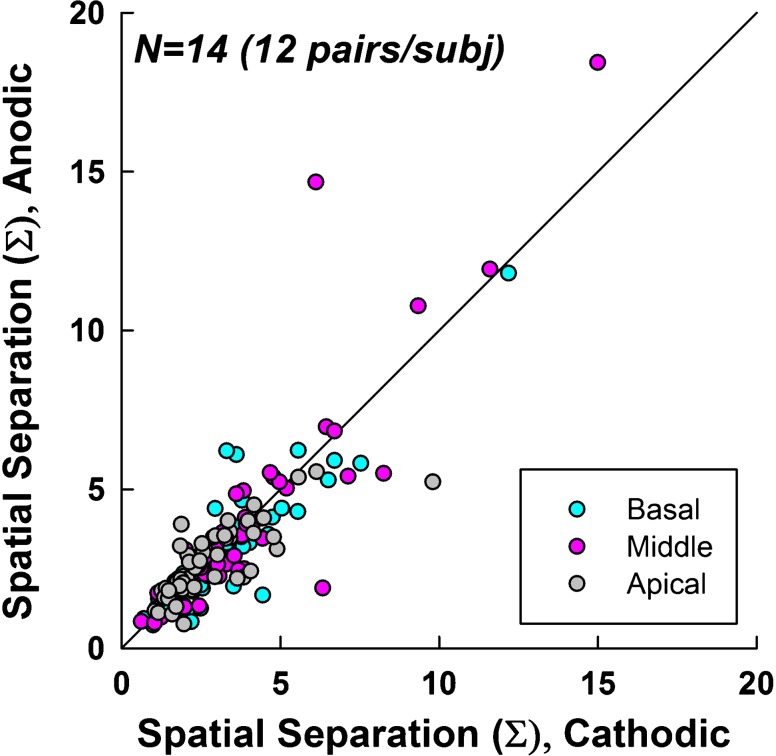
Figure 3 shows the ECAP SOE spatial separation indices for cathodic-leading (abscissa) versus anodic-leading (ordinate) stimuli. Data for all SOE comparison pairs (12 per ear) for all participants/ears are shown. The solid diagonal line represents unity. Data for each electrode region are coded by color, as indicated in the figure legend. A Wilcoxon Signed Rank test (due to non-normal distribution of data) revealed no significant difference (z = − 1.34, p = 0.18) in spatial separation between polarities. The median Σ values were 2.84 and 2.66 for cathodic and anodic conditions, respectively. Mean values were 3.28 and 3.20 for cathodic and anodic conditions, respectively.
Fig. 3.
Spatial separation between pairs of ECAP SOE patterns for cathodic- (abscissa) versus anodic-leading (ordinate) pulses. The diagonal line represents equal spatial separation for both polarities. Data points above the line indicate greater spatial separation for anodic-leading stimuli. Each subject (n = 14) contributed 12 data points, 4 in each region (color coded according to the legend) for a total of 168 data points
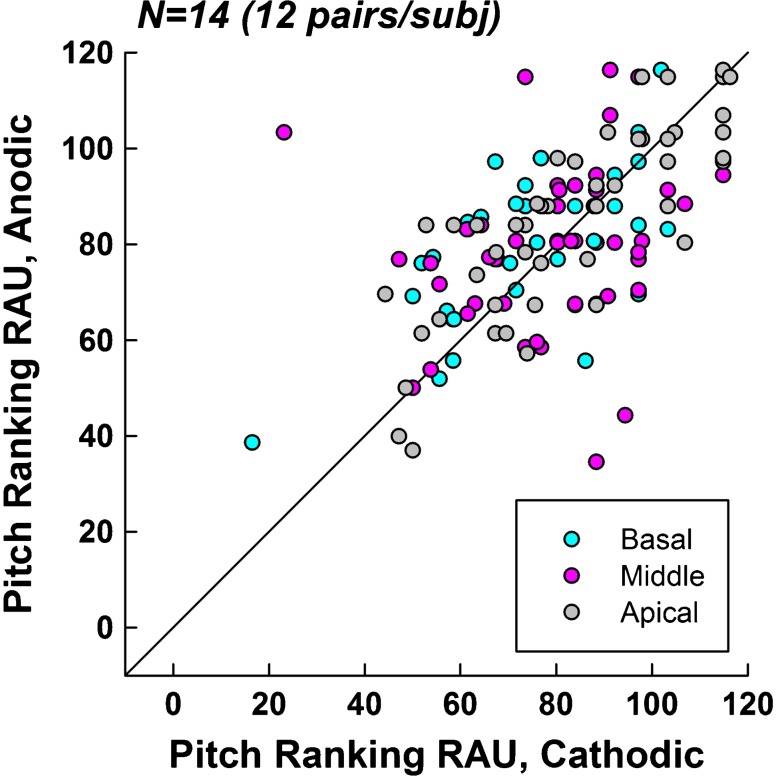
Figure 4 shows pitch-ranking results (in RAU) for cathodic-leading (abscissa) versus anodic-leading (ordinate) stimuli. Data are plotted similar to Fig. 3. A Wilcoxon Signed Rank test (due to non-normal distribution of data) revealed no significant difference (z = 1.01, p = 0.32) in pitch-ranking ability between polarities. The median RAU values were 87.89 for both cathodic and anodic conditions. The mean RAU values were 85.59 and 86.41 for cathodic and anodic, respectively.
Fig. 4.
Pitch ranking scores (in RAU) for cathodic- (abscissa) versus anodic-leading (ordinate) pulses. The diagonal line represents equal pitch-ranking performance for both polarities. Points above the line indicate better performance for anodic-leading stimuli. Each subject (n = 14) contributed 12 data points, 4 in each region (color coded according to the legend) for a total of 168 data points
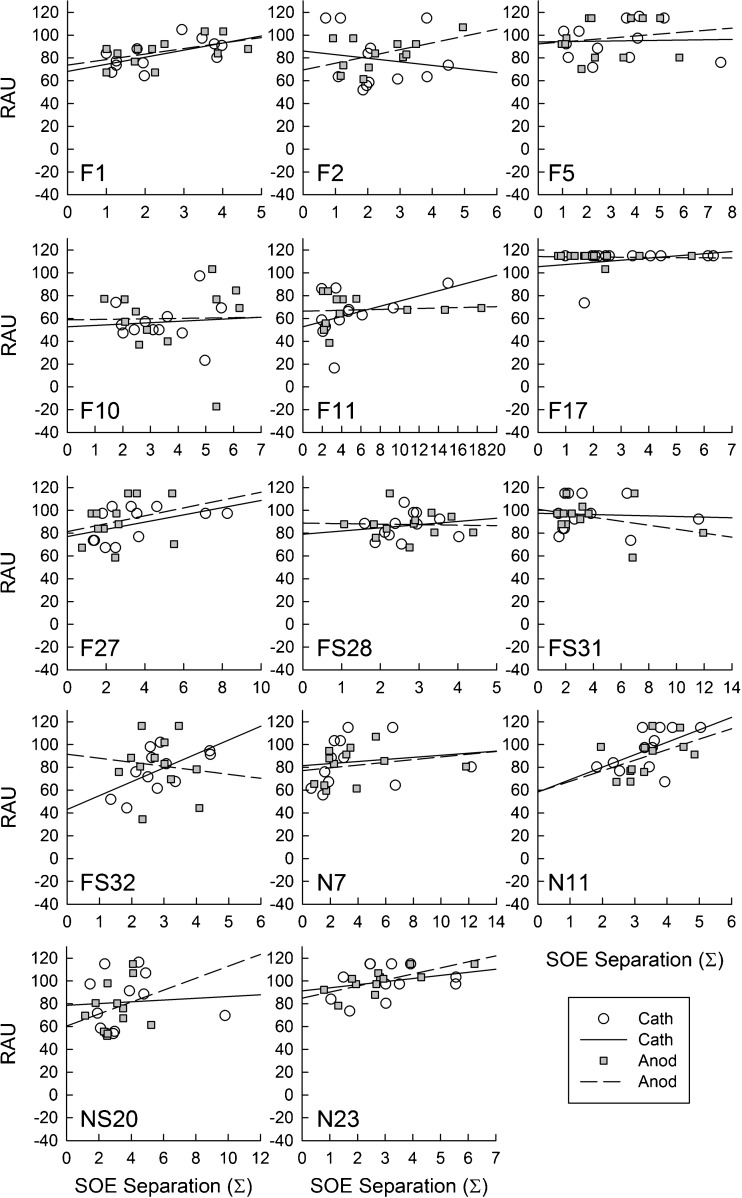
Figure 5 shows individual plots for each participant with the pitch-ranking RAU plotted as a function of ECAP spatial-separation index (Σ) for each comparison pair. Data for cathodic-leading stimuli are shown with open circles and solid regression lines, whereas data for anodic-leading stimuli are shown with gray squares and dashed regression lines. Pearson correlation coefficients (r) and levels of significance (p) are shown for each participant and polarity in Table 2. In general, the expected trend was a positive correlation between performance on the pitch-ranking task and the ECAP spatial separation between electrode pairs (Hughes 2008), especially for anodic-leading stimuli. Not all participants exhibited this trend. For some participants (e.g., F1, F10, F27, N7, and N11), the relation between ECAP and pitch data followed similar trends for both polarities, whereas others had largely divergent trends (e.g., F2, FS32, NS20). Only three participants demonstrated a significant correlation between the ECAP and pitch data: F1, cathodic; FS32, cathodic; and N23, anodic (see Table 2). For two of these participants, phase duration was 50 μs and for the other it was 25 μs, suggesting no clear effect of phase duration. In sum, reversing the stimulus polarity to anodic-leading did not result in improved correlations between the physiological and behavioral measures, which was inconsistent with the hypothesis.
Fig. 5.
ECAP SOE spatial separation (abscissa) versus pitch-ranking performance (ordinate) for individual subjects. Each panel represents one subject (identified in the bottom left corner). Individual data points represent comparison pairs for cathodic (open circles, solid regression line) and anodic (filled squares, dashed regression line) stimuli. Pearson correlation coefficients (r) and levels of significance (p) for each comparison are shown in Table 2
Table 2.
Correlation coefficients (r) and significance levels (p) for pitch ranking (RAU) versus ECAP spatial separation (Σ). Asterisks represent statistically significant correlations (p < 0.05)
| Participant | Pulse phase duration (μs) | Cathodic (r, p) | Anodic (r, p) |
|---|---|---|---|
| F1 | 25 | 0.59, 0.042* | 0.53, 0.078 |
| F2 | 25 | − 0.16, 0.63 | 0.50, 0.10 |
| F5 | 25 | 0.03, 0.92 | 0.17, 0.61 |
| F10 | 50 | 0.09, 0.79 | 0.02, 0.96 |
| F11 | 50 | 0.43, 0.16 | 0.08, 0.81 |
| F17 | 50 | 0.28, 0.39 | − 0.07, 0.82 |
| F27 | 25 | 0.47, 0.12 | 0.28, 0.38 |
| FS28 | 50 | 0.17, 0.59 | − 0.04, 0.91 |
| FS31 | 25 | − 0.06, 0.86 | − 0.36, 0.25 |
| FS32 | 50 | 0.60, 0.038* | − 0.11, 0.73 |
| N7 | 50 | 0.14, 0.66 | 0.23, 0.47 |
| N11 | 25 | 0.54, 0.068 | 0.48, 0.11 |
| NS20 | 25 | 0.07, 0.82 | 0.28, 0.37 |
| N23 | 50 | 0.27, 0.40 | 0.73, 0.007* |
Discussion
The primary goal of this study was to compare the spatial separation between ECAP SOE patterns to pitch ranking for anodic- and cathodic-leading symmetric biphasic pulses to determine whether stimulus polarity had an effect on the relation between the two. Contrary to the hypothesis, there was no effect of polarity on the relation between the physiological and psychophysical measures. Unlike our previous study (Spitzer and Hughes 2017), we did not find a significant effect of polarity on ECAP spatial separation. There was also no effect of polarity on pitch ranking abilities.
The overlap between ECAP SOE patterns obtained with spatially separated probe electrodes is thought to represent the degree to which neurons stimulated by a particular electrode are also stimulated by another electrode. In theory, greater spatial separation between ECAP SOE patterns (i.e., less overlap) indicates that the electrodes activate more distinct populations of neurons. Our previous study (Spitzer and Hughes 2017) demonstrated a significant effect of stimulus polarity on spatial separation, wherein cathodic-leading stimuli yielded greater spatial separation between SOE patterns than anodic-leading stimuli. This was attributed to the cathodic polarity being less effective as a masker when used to derive ECAPs through the forward-masking method. Although the present results are in the same direction as that of our earlier study, the results failed to reach significance despite using the same SOE data from a large subset (14 of 16) of the participants/ears from Spitzer and Hughes (2017).
The primary difference between the two studies is the normalization procedure used to calculate the spatial separation among ECAP SOE functions. In Spitzer and Hughes (2017), ECAPs were normalized to the maximum amplitude of each comparison pair. In the present study, ECAP amplitudes were normalized to the maximum amplitude of the reference/center probe electrode. This change in methodology was necessary to equally compare the pitch-ranking results (which involved comparing several electrodes to a center electrode of a region) and the physiological results. By normalizing the data for all electrodes in the comparison set to a single value, relative amplitude differences and spatial separation differences between pairs in the set are preserved.
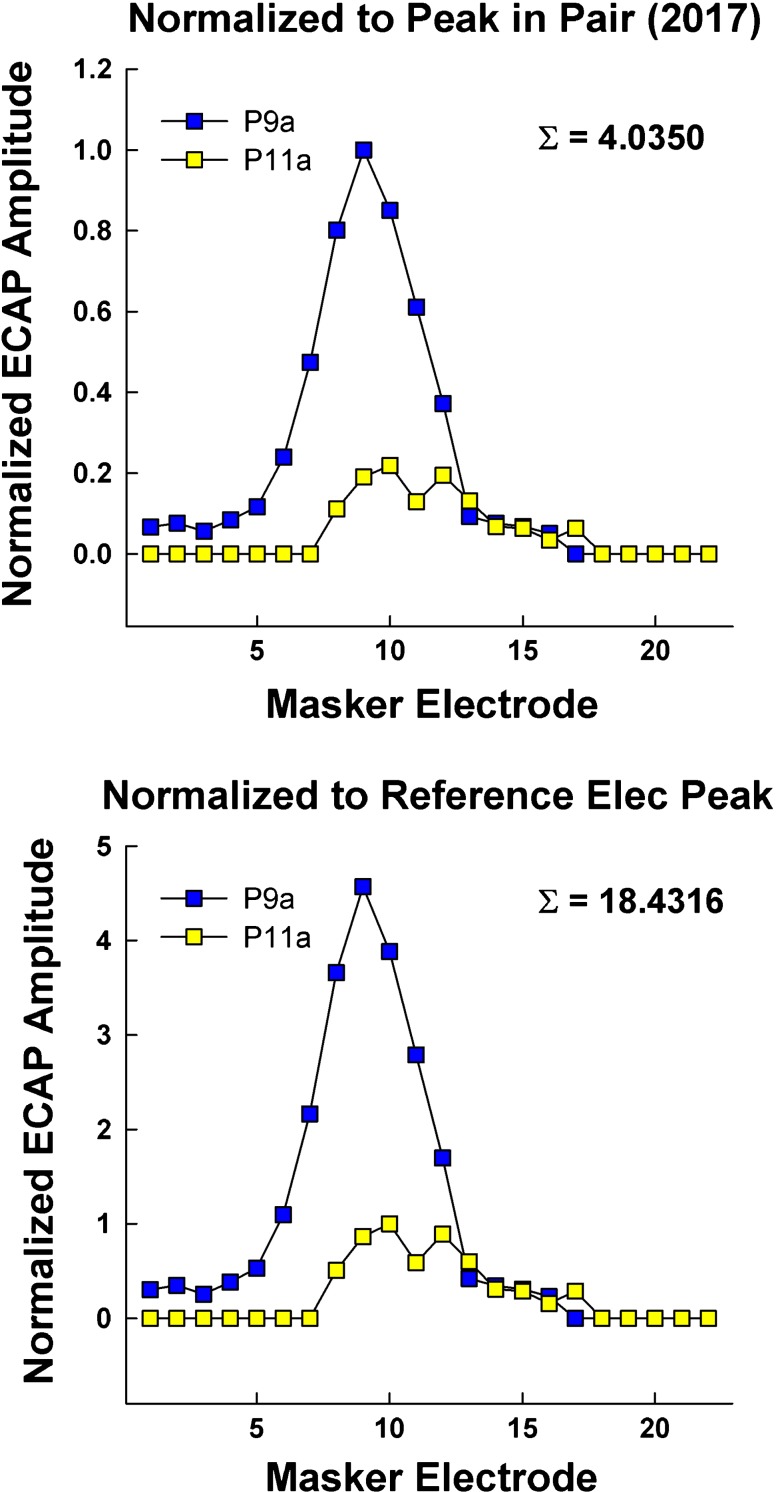
Figure 6 illustrates how differences in the two normalization procedures affect the results. Data are for F11, who had very small ECAP responses for the reference probe electrode (P11; < 50 μV at the peak), compared to most of the other electrodes in the array. The SOE patterns for anodic-leading stimuli that are shown in the top panel were normalized as in Spitzer and Hughes (2017), and data in the bottom panel were normalized using the methods in the present study. When the amplitude for the normalization point (P11 SOE) is small relative to the other function in the pair (P9 SOE), the normalized amplitude values for the other function will exceed 1.0 (see Fig. 6, bottom panel, P9). As a result, the Σ value will also be much larger than if both functions are normalized to the maximum amplitude in the pair (Fig. 6, top panel). In this example for F11, the large Σ values are the two farthest outliers seen in Fig. 3. In Spitzer and Hughes (2017, see Fig. 7 in that publication), the spatial separation between cathodic functions was significantly larger than for the anodic functions (p = 0.002; median Σ values of 2.51 versus 2.43, respectively). The same trend was observed in the present study (median Σ values of 2.84 versus 2.66, respectively). However, because of the larger Σ values that resulted from the current normalization procedure, the standard deviation was larger, which resulted in the current results being underpowered (0.148 versus 0.834 in our previous study). Using the original normalization method (shown in Fig. 6, top panel), we re-analyzed the data from Fig. 7 of Spitzer and Hughes (2017) without the two subjects who did not participate in the present study, and found that there was still a statistically significant difference in spatial separation between SOE patterns for cathodic-leading and anodic-leading stimuli. Therefore, it appears that the lack of a significant difference in the present study was due to the current normalization procedure, rather than having two fewer participants. Although it is important to normalize ECAP data to account for relatively large amplitude differences across and within subjects, the normalization procedure used here yielded larger standard deviations, which in turn reduced the statistical power, thereby making it difficult to see a significant difference in ECAP SOE between the polarities.
Fig. 6.
Example ECAP SOE patterns for participant F11 depicting the effect of our previous (top panel) and current (bottom panel) normalization procedures. The comparison pair is probe electrodes 9 and 11 in both panels. Data were obtained with anodic-leading biphasic pulses. Top panel: amplitudes were normalized to the largest amplitude within each pair. Bottom panel: amplitudes were normalized to the peak of the SOE function for the reference electrode (probe 11)
Pitch-ranking ability was assessed in this study for symmetric, biphasic, cathodic-, and anodic-leading pulses. Like the ECAP SOE comparisons, there was no significant difference in performance between the two polarities. Although the mean RAU was slightly larger for anodic-leading pulses, which was a trend in the direction of the hypothesis, the median values were the same for both polarities. This finding differs from previously published work on pitch ranking using unconventional pulse shapes such as asymmetric (pseudomonophasic) or multiphase pulses (Macherey et al. 2011; Carlyon et al. 2013). Macherey et al. (2011) found that pseudomonophasic anodic-leading stimuli produced lower pitch percepts than symmetric biphasic stimuli when the anodic portion of the stimulus was presented to the more apical electrode in a bipolar pair. Similarly, Carlyon et al. (2013) used monopolar stimulation to show that pitch perceptions were lower for symmetric quadraphasic pulses when the two center pulses were anodic than when they were cathodic. These results indicate that the response with these unconventional pulse shapes is largely or exclusively driven by the anodic phase (Macherey et al. 2010). The likely mechanism for this effect is an increase in the duration of the interphase gap (for pseudomonophasic pulses) or the duration of the central phase (for symmetric quadraphasic pulses). The result is that there is more time for neurons to initiate an action potential before the opposite phase can have an opposing effect. Indeed, several studies have shown that longer interphase gaps and longer pulse durations yield lower thresholds, both perceptually (McKay and Henshall 2003; Shannon 1985) and physiologically (e.g., Hughes et al. 2018; Ramekers et al. 2014). The symmetrical biphasic pulse used in the present study has a very short inter-phase gap and equal durations of the cathodic and anodic phases. As a result, the effectiveness of the anodic phase is likely diminished by the equal-amplitude cathodic phase, particularly for short interphase gaps. Biphasic pulses have higher thresholds than monophasic or pseudomonophasic pulses because depolarization induced by the first phase can be obliterated by the second phase before an action potential threshold is reached (Shepherd and Javel 1999). Previous work has shown no effect of polarity on perceptual thresholds when symmetric biphasic pulses are used, in agreement with the results presented here (Macherey et al. 2006). Notably, the present study was the first to examine the effects of polarity on pitch ranking using symmetric biphasic pulses with a short interphase gap.
Finally, a few subjects showed a trend toward a positive correlation between spatial separation and pitch-ranking performance, although the majority did not. Three subjects demonstrated a statistically significant correlation between pitch ranking and spatial separation between SOE patterns for a single polarity, although two were for the cathodic polarity and one was for the anodic polarity. In general, there was large variability among participants. Figure 5 demonstrates the relation between pitch ranking and spatial separation of ECAP SOE patterns for each subject. There are several patterns of performance that emerge. Despite transforming percent correct scores to RAU in order to reduce ceiling effects, several subjects (e.g., F17) were performing extremely well with little variance in their pitch ranking scores. Some other subjects (e.g., F2, F11, FS32, NS20) showed divergent trends in the ECAP-pitch correlation for each polarity. These results differ from those reported in Hughes (2008), where pitch ranking performance was found to positively correlate with ECAP spatial separation for the group as a whole. Because the methods used to calculate pitch-ranking scores and separation between ECAP SOE patterns were similar between the two studies, there is further evidence that the participants in the present study differed or were simply more variable.
Although the participants in Hughes (2008) were also Nucleus users, differences in electrode insertion depth and place of stimulation could be responsible for the lack of a relation between pitch ranking and spread of excitation observed in the present study. The electrodes used in this study ranged in length from 18 to 25 mm. Assuming an average insertion depth (imaging data were not available), our apical electrode region (14–18) would theoretically be inserted at an angle of ~ 220–298° (Landsberger et al. 2015). Therefore, the most apical SOE pattern (probe electrode 18) measured neural activation relatively far from the apex of the cochlea (Dhanasingh and Jolly 2017). Previous work has shown that apical stimuli are generally judged as louder than middle or basal stimuli (Carlyon et al. 2013), suggesting that current spread is greater in the apical region of the cochlea. This conjecture is supported by both animal evidence showing lower physiological thresholds for apical as compared to basal electrodes (Frijns et al. 2001) and modeling work showing greater spread of excitation in the apex than the base of the cochlea (Kalkman et al. 2014). Therefore, it is possible that we may have found a significant relationship between spread of excitation and pitch ranking if a longer electrode array had been used.
In summary, the results from this study showed a lack of an effect of stimulus polarity on the relation between physiological measures of ECAP spatial separation and pitch ranking for symmetric biphasic pulses. These results suggest that at least for stimuli presently used with clinical populations, changing from cathodic-leading to anodic-leading polarity pulses would not seem to improve pitch perception or to systematically improve the relation between pitch and ECAP SOE. Further studies could incorporate more participants or explore the effect of polarity on the relation between ECAP SOE and pitch ranking using pseudomonophasic stimuli.
Funding Information
This study was funded by the National Institutes of Health. This research was supported by NIH/NIDCD grants R01 DC009595 and T35 DC008757 and NIGMS P20 GM109023. The content of this project is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
This study was approved by the Boys Town National Research Hospital Institutional Review Board under protocol 03-07-XP. Written informed consent was obtained from all participants.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emily R. Spitzer, Email: emily.spitzer@nyulangone.org
Sangsook Choi, Email: sangsook_choi@hotmail.com.
Michelle L. Hughes, Phone: 402-472-3921, Email: mhughes3@unl.edu
References
- Abbas PJ, Brown CJ, Shallop JK, Firszt JB, Hughes ML, Hong SH, Staller SJ. Summary of results using the nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear. 1999;20:45–59. doi: 10.1097/00003446-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Busby PA, Battmer RD, Pesch J. Electrophysiological spread of excitation and pitch perception for dual and single electrodes using the Nucleus Freedom cochlear implant. Ear Hear. 2008;29:853–864. doi: 10.1097/AUD.0b013e318181a878. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Deeks JM, Macherey O. Polarity effects on place pitch and loudness for three cochlear-implant designs and at different cochlear sites. J Acoust Soc Am. 2013;134:503–509. doi: 10.1121/1.4807900. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Busby PA, Whitford LA, Clark GM. Cochlear implant place psychophysics 1. Pitch estimation with deeply inserted electrodes. Audiol Neuro-Otol. 1996;1:265–277. doi: 10.1159/000259210. [DOI] [PubMed] [Google Scholar]
- Dhanasingh A, Jolly C. An overview of cochlear implant electrode array designs. Hear Res. 2017;356:93–103. doi: 10.1016/j.heares.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, Briaire JJ, Grote JJ. The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otol Neurotol. 2001;22:340–349. doi: 10.1097/00129492-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Goehring JL, Neff DL, Baudhuin JL, Hughes ML. Pitch ranking, electrode discrimination, and physiological spread-of-excitation using Cochlear’s dual-electrode mode. J Acoust Soc Am. 2014;136:715–727. doi: 10.1121/1.4884881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring JL, Neff DL, Baudhuin JL, Hughes ML. Pitch ranking, electrode discrimination, and physiological spread of excitation using current steering in cochlear implants. J Acoust Soc Am. 2014;136:3159–3171. doi: 10.1121/1.4900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzavi J, Arnoldner C. Effect of deep insertion of the cochlear implant electrode array on pitch estimation and speech perception. Acta Otolaryngol. 2006;126:1182–1187. doi: 10.1080/00016480600672683. [DOI] [PubMed] [Google Scholar]
- Hughes ML. A re-evaluation of the relation between physiological channel interaction and electrode pitch ranking in cochlear implants. J Acoust Soc Am. 2008;124:2711–2714. doi: 10.1121/1.2990710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ML, Abbas PJ. The relation between electrophysiologic channel interaction and electrode pitch ranking in cochlear implant recipients. J Acoust Soc Am. 2006;119:1527–1537. doi: 10.1121/1.2163273. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Goehring JL, Baudhuin JL. Effects of stimulus polarity and artifact reduction method on the electrically evoked compound action potential. Ear Hear. 2017;38:332–343. doi: 10.1097/AUD.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ML, Choi S, Glickman E. What can stimulus polarity and interphase gap tell us about auditory nerve function in cochlear-implant recipients? Hear Res. 2018;359:50–63. doi: 10.1016/j.heares.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesteadt W. An adaptive procedure for subjective judgments. Percept Psychophys. 1980;28:85–88. doi: 10.3758/BF03204321. [DOI] [PubMed] [Google Scholar]
- Kalkman RK, Briaire JJ, Dekker DMT, Frijns JHM. Place pitch versus electrode location in a realistic computational model of the implanted human cochlea. Hear Res. 2014;315:10–24. doi: 10.1016/j.heares.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Svrakic S, Roland JT, Svirsky M. The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear Hear. 2015;36:e207–e213. doi: 10.1097/AUD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP. Place-pitch manipulations with cochlear implants. J Acoust Soc Am. 2012;131:2225–2236. doi: 10.1121/1.3677260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, van Wieringen A, Carlyon RP, Deeks JM, Wouters J. Asymmetric pulses in cochlear implants: effects of pulse shape, polarity, and rate. J Assoc Res Otolaryngol. 2006;7:253–266. doi: 10.1007/s10162-006-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP, van Wieringen A, Deeks JM, Wouters J. Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol. 2008;9:241–251. doi: 10.1007/s10162-008-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, van Wieringen A, Carlyon RP, Dhooge I, Wouters J. Forward-masking patterns produced by symmetric and asymmetric pulse shapes in electric hearing. J Acoust Soc Am. 2010;127:326–338. doi: 10.1121/1.3257231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Deeks JM, Carlyon RP. Extending the limits of place and temporal pitch perception in cochlear implant users. J Assoc Res Otolaryngol. 2011;12:233–251. doi: 10.1007/s10162-010-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, Henshall KR. The perceptual effects of interphase gap duration in cochlear implant stimulation. Hear Res. 2003;181:94–99. doi: 10.1016/S0378-5955(03)00177-1. [DOI] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SFL, Grolman W. Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol. 2014;15:187–202. doi: 10.1007/s10162-013-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattay F, Lutter P, Felix H. A model of the electrically excited human cochlear neuron: I. Contribution of neural substructures to the generation and propagation of spikes. Hear Res. 2001;153:43–63. doi: 10.1016/S0378-5955(00)00256-2. [DOI] [PubMed] [Google Scholar]
- Rattay F, Leao RN, Felix H. A model of the electrically excited human cochlear neuron. II. Influence of the three-dimensional cochlear structure on neural excitability. Hear Res. 2001;153:64–79. doi: 10.1016/S0378-5955(00)00257-4. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18:135–143. doi: 10.1016/0378-5955(85)90005-X. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res. 1999;130:171–188. doi: 10.1016/S0378-5955(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Spitzer ER, Hughes ML. Effect of stimulus polarity on physiological spread of excitation in cochlear implants. J Am Acad Audiol. 2017;28:786–798. doi: 10.3766/jaaa.16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker GA. A rationalized arcsine transform. J Speech Hear Res. 1985;28:455–462. doi: 10.1044/jshr.2803.455. [DOI] [PubMed] [Google Scholar]
- Townshend B, Cotter N, Van Compernolle D, White RL. Pitch perception by cochlear implant subjects. J Acoust Soc Am. 1987;82:106–115. doi: 10.1121/1.395554. [DOI] [PubMed] [Google Scholar]
- Undurraga JA, van Wieringen A, Carlyon RP, Macherey O, Wouters J. Polarity effects on neural responses of the electrically stimulated auditory nerve at different cochlear sites. Hear Res. 2010;269:146–161. doi: 10.1016/j.heares.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Undurraga JA, Carlyon RP, Macherey O, Wouters J, van Wieringen A. Spread of excitation varies for different electrical pulse shapes and stimulation modes in cochlear implants. Hear Res. 2012;290:21–36. doi: 10.1016/j.heares.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Undurraga JA, Carlyon RP, Wouters J, van Wieringen A. The polarity sensitivity of the electrically stimulated human auditory nerve measured at the level of the brainstem. J Assoc Res Otolaryngol. 2013;14:359–377. doi: 10.1007/s10162-013-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]