Abstract
The 8th edition of American Joint Committee on Cancer (AJCC 8th) staging manual incorporated depth of invasion (DOI) into pT stage of oral cavity cancer. The aim of this study was to characterize several histological findings that may complicate measurement of DOI in early conventional squamous cell carcinomas (SCC) of the oral tongue: (1) lack of or minimal residual carcinoma following biopsy; (2) positive deep margin; (3) extratumoral perineural invasion (PNI); and (4) lymphatic or vascular invasion. Conventional SCC of the oral tongue (n = 407) with the largest dimension of ≤ 4 cm and with a negative elective cervical lymph node dissection (pN0) were reviewed. A clear plastic ruler was used to measure DOI by dropping a “plumb line” to the deepest point of the invasive tumor from the level of the basement membrane of the normal mucosa closest to the invasive tumor. Examples of identifying reference point on the mucosal surface of oral tongue from which to measure the DOI are illustrated. In the experience of one contributing institution, the residual carcinoma was absent in 14.2% of glossectomies (34/239), while in 4.8% of cases (10/205) there was only minimal residual carcinoma. In 11.5% (21/183) of pT2 cases the deep margin was positive and thus DOI and pT may be underestimated. Of all cases with PNI, extratumoral PNI was identified in 23.1% (31/134) of cases, but represented the deepest point of invasion in only two cases. In one case, lymphatic invasion represented the deepest point of invasion and could have led to upstaging from pT1 to pT2. In conclusion, DOI measurement for SCC of the oral tongue may require re-examination of the diagnostic biopsy in up to 20% of cases due to the absence or only minimal residual carcinoma in glossectomy specimens. In 11.5% of apparently pT2 cases, DOI may be underestimated due to the positive deep margin. Rarely, extratumoral PNI or lymphatic invasion may be the deepest point of invasion. Overall, two issues (absent or minimal residual disease and positive deep margin) may confound DOI measurement in early SCCs of oral tongue.
Keywords: Depth of invasion, Extratumoral perineural invasion, Deep margin, Carcinoma, Oral tongue, AJCC staging
Introduction
The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual incorporated depth of invasion (DOI) into pT stage of oral cavity cancer [1]. DOI may be measured clinically (or using intraoral ultrasonography [1, 2]) and, ultimately, histologically. The histological measurement of DOI requires recognizing the level of the basement membrane of the closest normal mucosa and dropping a “plumb line” to the deepest point of invasion. Ostensibly straightforward, several issues come to the fore, only a few of which have been critically evaluated. For instance, AJCC clearly advocates for the use of DOI rather than tumor thickness and the difference in tumor thickness and DOI may affect pT category in up to 5.7% of patients with oral cancer [3].
The impact on staging of other histological findings such as extratumoral foci of squamous cell carcinoma (SCC) (by extratumoral perineural invasion (PNI) [4], lymphatic or vascular invasion), absent or minimal residual carcinoma on resection following biopsy, and positive deep margin, especially as they apply to oral cavity subsites, was not addressed nor even implied.
Not all extratumoral foci of SCC would increase the DOI sufficiently to change the pT stage. To increase the DOI or pT stage (from pT1 to pT2 and from pT2 to pT3), extratumoral foci of SCC would have to satisfy the following criteria:
Extratumoral focus of SCC would have to represent the deepest point of invasion. Submucosal (“sideways”) foci of SCC, while extratumoral, do not affect DOI.
Extratumoral foci of SCC would have to be far enough from the invasive tumor front (as shown by the bulk of the tumor) to cross the 5 mm or the 10 mm pT thresholds established by the AJCC 8th edition. For instance, if the DOI, as shown by the bulk of the tumor, is 6 mm and the extratumoral focus of SCC is 2 mm from the invasive tumor front (and represents the deepest point of invasion), the overall DOI (6 mm + 2 mm) would still fall between 5 and 10 mm and the staging would remain unchanged (pT2 in this example).
The DOI as shown by the bulk of the tumor has to be less or equal to 10 mm. When the DOI is > 10 mm, additional extratumoral foci of carcinoma do not result in pT upstaging.
The aim of this study was to characterize and assess the prevalence of histological findings that may affect the accuracy of DOI measurement in early conventional SCC of the oral tongue.
Materials and Methods
The studied patients satisfied the following inclusion criteria:
Primary SCC limited to oral/mobile tongue with largest dimension of ≤ 4 cm;
Elective neck dissection without cervical lymph node metastases (pN0);
Conventional SCC morphology. For instance, verrucous and papillary SCC were excluded.
Only cases with residual carcinoma in the resection specimen were included.
No cases which may have extended to or expanded from adjacent sites (such as oropharynx, floor of mouth) were included [5–7].
Glass slides for all cases of oral tongue SCC treated from 1986 to 2015 at contributing institutions were reviewed. The presence of invasive carcinoma at the deep margin was considered a positive margin. A clear plastic ruler was used to measure DOI. DOI was measured by dropping a “plumb line” to the deepest point of invasion from the level of the basement membrane of the adjacent normal mucosa (approximate midpoint of the bulk of the tumor and/or midpoint through the arch-like line connecting normal mucosa on both sides of the tumor was used, especially if adjacent epithelium was undulating or tumor was ulcerated; e.g., Fig. 1). The following parameters were documented: location (peripheral, intra- or extra-tumoral) of lymphatic, vascular, and perineural invasion [4], distance between foci of extra-tumoral PNI and invasive tumor front, DOI, and status of the deep margin (positive or negative). Ultimately, extratumoral PNI, lymphatic and vascular invasion were excluded from assessing the deepest point of invasion (and thus determining pT) with the exception of one case (see below and Fig. 2). For highly infiltrative SCC, small (i.e., < 10–15 cells) clusters of tumor cells within 1 mm of invasive tumor front were not considered as extratumoral foci of SCC. Small clusters of tumor cells > 1 mm from the invasive tumor front were scrutinized for features of extratumoral lymphatic invasion.
Fig. 1.
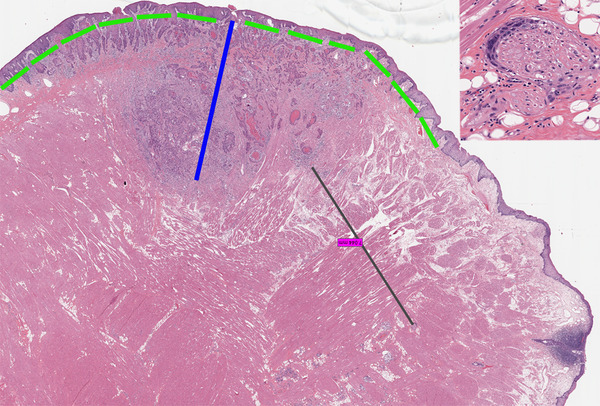
An example of squamous cell carcinoma with extratumoral perineural invasion. The focus of extratumoral perineural invasion was 7 mm from the invasive tumor front; however, the carcinoma was tracking along the nerve running parallel to the overlying normal mucosa (“sideways”) and did not represent the deepest point of invasion. The black line illustrates how the distance between the invasive tumor front and focus of perineural invasion was measured. The blue line shows how the depth of invasion was measured. It may be challenging to select a reference point on the mucosal surface of oral tongue from where to measure the depth of invasion (especially if the tumor is ulcerated or not connected to the mucosa). Theoretically, the reference point may be chosen along the dorsal (superior), lateral, or ventral (inferior, towards floor of mouth) mucosa. Here, an arcuate, rather than straight, line (bright green) connecting normal mucosa on both sides of dysplastic mucosa overlying invasive squamous cell carcinoma was imagined. The “plumb line” was dropped through the middle one-third of the bulk of the tumor with deepest invasion. Higher magnification of the remote focus of perineural invasion is shown in the inset (hematoxylin and eosin, images taken from the scanned whole slide image with original magnification of × 1.2)
Fig. 2.
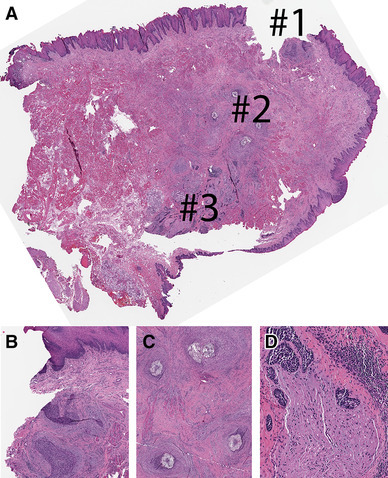
Cross-section through left partial glossectomy with dorsal, lateral, and ventral (towards floor of mouth) mucosa (clockwise, starting from the top). Note, this is a permanent formalin fixed paraffin embedded section of the frozen section remnant shown in Fig. 3. On permanent section, an additional more superficial focus of residual invasive squamous cell carcinoma is seen closer to the mucosa (area #1 in a, b). Also, suture granulomas (area #2 in a, c) were identified. The deepest point of invasion was represented by foci of extratumoral perineural invasion (d). The final “plumb line” to measure the depth of invasion was drawn through areas #1 and #3. Hematoxylin and eosin, permanent formalin fixed paraffin embedded section, images taken from the scanned whole slide image with original magnification of × 1.2
Results
Four hundred and seven cases of conventional SCC involving oral tongue were reviewed, 220 of which were entirely submitted for microscopic examination. The average greatest dimension of SCC was 17.6 mm (range 1–40 mm) with average DOI of 6.8 mm (range < 1–25 mm; including three cases with DOI > 20 mm). The intrinsic tongue musculature was involved in 85.5% (348/407) of patients.
In the experience of one contributing institution, residual carcinoma was absent in 14.2% of glossectomies (34/239) and these cases were excluded from the study. This number of glossectomies without residual SCC is higher than the previously reported 9%, 28/308 [5]. In 4.8% of the remaining cases from this institution (10/205), there was only minimal residual carcinoma in the glossectomy specimen, with the bulk of the tumor removed by diagnostic biopsy (Fig. 4).
Fig. 4.
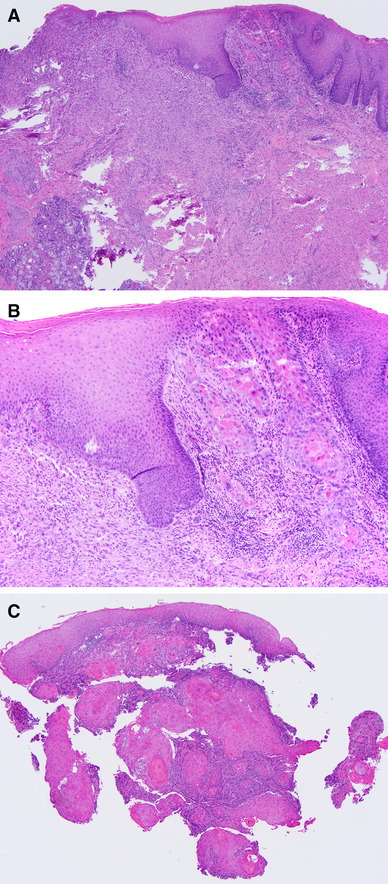
a, b An example of minimal residual squamous cell carcinoma of the oral tongue (hematoxylin and eosin (H&E), original magnification × 40). b A minute focus of residual squamous cell carcinoma, about 1 mm wide and 1 mm deep, with submucosal scar in the left lower corner (H&E, original magnification × 100). c The diagnostic biopsy was represented by five tissue fragments, all of which were smaller than 5 mm in greatest dimension and had invasive squamous cell carcinoma. The exact measurement of the depth of invasion in this case is difficult given the fragmented nature of diagnostic biopsy. Only one biopsy fragment had normal squamous mucosa allowing measurement of the depth of invasion (H&E, original magnification × 40)
The prevalence and distribution of cases with positive deep margin and extratumoral PNI across revised (AJCC 8th) pT stages are summarized in Table 1. The deep margin was positive in 20.5% (14/68) of pT3 cases; though here, underestimating DOI would not affect pT staging. On the other hand, a positive deep margin may result in an under-staging of pT1 and pT2 cases (6.5%, 22/339) (Table 1). More specifically, the deep margin was positive in 11.5% (21/183) of pT2 cases (Fig. 5).
Table 1.
Positive deep margin, extratumoral perineural invasion and AJCC 8th edition pT
| Total | pT1 (n = 156) | pT2 (n = 183) | pT3 (n = 68) | |
|---|---|---|---|---|
| Positive deep margin | 36 | 1 | 21 | 14 |
| Extratumoral perineural invasion | 31 | 5 | 15 | 11 |
Fig. 5.
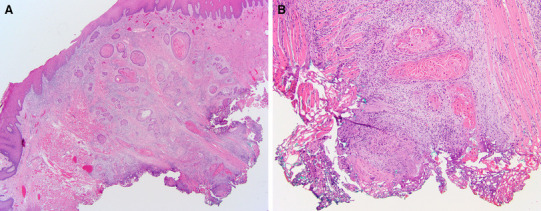
An example of a pT2 squamous cell carcinoma of the oral tongue with positive deep margin, indicating that the depth of invasion may be underestimated. a The apparent depth of invasion is 7 mm; however, the deep margin is involved by carcinoma (hematoxylin and eosin (H&E), original magnification × 20). b Carcinoma at deep margin. Hypothetically, if there is additional 4 mm (along the “plumb line”) of residual carcinoma in the tumor bed, this carcinoma is more appropriately staged as pT3 (H&E, original magnification × 100)
PNI was identified in 33% of cases (134/407). All involved nerves were < 1 mm in largest diameter. Extratumoral PNI was identified in 23.1% (31/134) of cases with any PNI (i.e., including intratumoral and peripheral). Overall, only 7.6% of cases (31/407) had extratumoral PNI and 11 cases were already staged as pT3 based on DOI by the bulk of the tumor. Of the remaining pT1 and pT2 cases with extratumoral PNI (n = 20), only 2 cases showed extratumoral PNI that might alter pT. In other cases extratumoral PNI did not affect pT for two reasons:
The foci of extratumoral PNI had submucosal or “sideways” distribution (Fig. 1).
When the distance from the invasive tumor front to the focus of extratumoral PNI was added to the DOI (as shown by the bulk of the tumor), the overall sum did not cross thresholds of 5 or 10 mm. For instance, two carcinomas showed DOI of 6 mm and a focus of extratumoral PNI was 1 mm away from the invasive tumor front. Even if such foci of extratumoral PNI represented the deepest point of invasion and were added to the DOI, the pT category would remain pT2.
In two pT2 cases, extratumoral PNI was not “sideways”, but represented the deepest point of invasion, and if these foci were taken into account when measuring the DOI, these two cases would be upstaged to pT3. However, only in one such case, the focus of PNI was the only focus of residual carcinoma (Figs. 2, 3). This is the only case in this study with extratumoral PNI determining pT stage. Due to the deep submucosal location and lack of connection between the residual SCC and mucosa, this case also illustrates the challenge of identifying the most appropriate reference point on the mucosal surface of oral tongue from which to measure the DOI (Figs. 2, 3). An alternative approach would be to assign pT based on DOI in diagnostic biopsy.
Fig. 3.
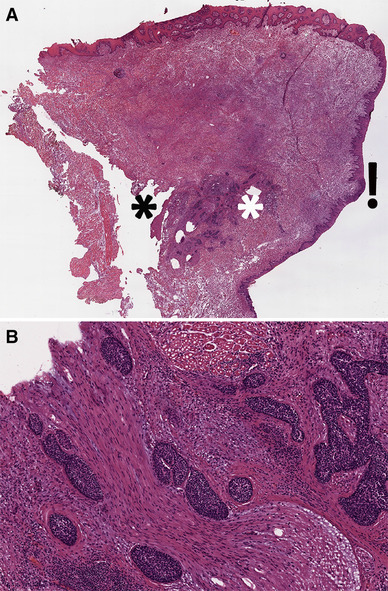
Cross-section through left partial glossectomy with dorsal, lateral, and ventral (towards floor of mouth) mucosa (clockwise, starting from the top). a The focus of residual squamous cell carcinoma (between the white and black asterisks) shows no connection to mucosa. Residual carcinoma is represented by foci of extensive perineural invasion (b). The potential reference points to measure the depth of invasion are along the dorsal, lateral, and ventral mucosa. Measuring depth of invasion from ventral mucosa would result in pT1, while depth of invasion measurement from the dorsal mucosa would result in pT3. On this section (see Fig. 2, also), depth of invasion was measured from the lateral mucosa (next to exclamation mark), because this area showed a focus of moderate to severe dysplasia. Hematoxylin and eosin, frozen section, images taken from the scanned whole slide image with original magnification of × 1.2. Note, this is the same case as in Fig. 2, which is a permanent formalin fixed paraffin embedded section of the frozen section remnant
Lymphatic invasion (LI) was identified in 17.7% (72/407) of cases, of which 27 had DOI > 10 mm (as shown by the bulk of the tumor and regardless of LI). In the remaining cases, LI was intratumoral, submucosal, and did not represent the deepest point of invasion, or was not sufficiently far away from the invasive tumor front to upstage pT. In only one case, two small clusters of carcinoma represented the deepest point of invasion and were deep and far enough away from the invasive tumor front to pass the 5 mm threshold (Fig. 6). The focus of LI was not used to determine the pT stage.
Fig. 6.
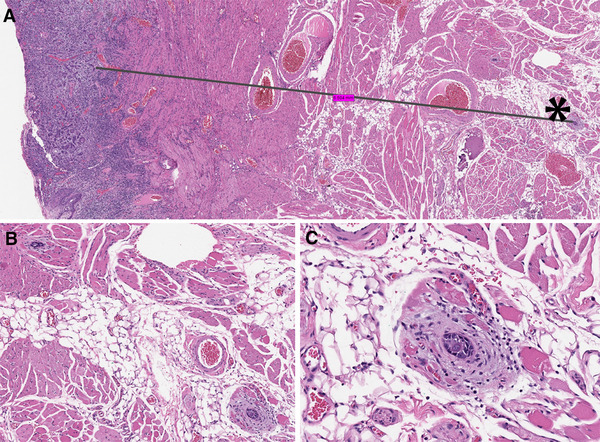
a Two clusters of invasive squamous cell carcinoma (each with about 15 cells, by the black asterisk and in b, c) were 6.5 mm from the bulk of the tumor, suggestive of lymphatic invasion and representing the deepest point of invasion. The black line illustrates the way the distance between the invasive tumor front and remote foci of carcinoma was measured. The pT1 stage was assigned based on the depth of invasion by the bulk of the tumor which was 4.5 mm. b One of the small clusters of carcinoma is in the left upper corner and the second focus of carcinoma is in the right lower corner and in c. Hematoxylin and eosin, images taken from the scanned whole slide image with original magnification of × 1.2
Vascular invasion was identified in 1.7% (7/407) of cases. Four of these seven cases were already pT3 based on DOI > 10 mm. In the remaining three cases, vascular invasion did not represent the deepest point of invasion.
Discussion
The inclusion of DOI in the pT category for oral cavity cancer may improve prognostic performance of AJCC staging by predicting regional control and status of regional lymph nodes [8, 9]. We herein describe some of the challenges of measuring DOI in SCCs of the oral tongue and ≤ 4 cm in largest dimension. These challenges may explain significant variation in DOI measurements by different institutions (with median DOI ranging from 7 to 12 mm) and why a simplified approach to pT stage using DOI alone (without largest tumor dimension) did not correlate with clinical outcome [10]. It is thus appropriate that both DOI and largest tumor size are incorporated into pT stage rather than DOI alone. Largest tumor dimension appears to be a more robust prognosticator and less dependent on histological variables described here (i.e., positive margin, extratumoral foci of SCC). To speculate, this is likely due to the fact that largest tumor dimension measurement is taken grossly. Also, it relies on more generous cut-offs of 2 and 4 cm. In contrast, DOI thresholds were disputed in the literature. Specifically, for oral tongue, cut-offs of 3, 4, 9, 10 mm, and, of course, 5 mm have been suggested [1, 9, 11–14]. Finally, just where to measure DOI from can be difficult to determine in oral tongue (with mucosa on dorsal, lateral, and ventral aspects) and in undulating hyperplastic epithelium, which can create an uneven basement membrane. One has to imagine an arcuate reference line and then drop a “plumb-line” which can be equally as difficult due to variations in normal mucosa and DOI at different tumor section.
Absent or minimal residual disease and positive deep margin may confound pT staging in up to 30% of pT1 and pT2 (AJCC 8th) SCCs of oral tongue. As suspected by Lydiatt, et al., “generous preoperative biopsies of small tumors may interfere with DOI determination; therefore, it is important that, when pathologists examine the original biopsies, attention should be directed to DOI….” [1]. Indeed, staging of early SCC of oral tongue may require measurement of DOI from diagnostic biopsies in up to 20% of cases. Even when minimal residual carcinoma is identified in glossectomy specimens, one has to consider the possibility that the deepest part of the carcinoma was removed by diagnostic biopsy. Also, exuberant biopsy-induced changes (e.g., scarring, acellular keratin with giant cell reaction, regenerative surface epithelium caught deep in the biopsy site, and atrophic fibers of intrinsic tongue musculature extending deeper than residual carcinoma) may point to the need to review the previous diagnostic biopsy for an DOI measurement. Furthermore, accurate DOI measurement on diagnostic biopsies may be impossible if the biopsy is fragmented or if normal mucosa or intrinsic tongue musculature is absent. A proactive assessment and reporting of DOI on diagnostic biopsies or documentation of factors limiting DOI measurement (e.g., fragmentation, lack of intrinsic tongue musculature, absence of normal mucosa) may minimize the need to re-review the diagnostic biopsy when glossectomy reveals no or minimal residual carcinoma. One of the limitations of this study is the absence of data on glossectomies without residual SCC and lack of data on corresponding diagnostic biopsies. The extent of this issue was realized quite late by the authors and even when one of the contributors volunteered to re-identify glossectomies without residual SCC additional problems emerged: (1) some diagnostic biopsies was performed outside of contributing institutions and were not available for review and (2) some patients had two diagnostic biopsies. A separate study on measuring DOI in diagnostic biopsies is needed.
In about 12% of apparently pT2 carcinomas of the oral tongue, DOI may be underestimated due to positive deep margin. Practically, there is little pathologists can do to correct for this finding, other than to comment on it, especially when the apparent DOI approaches 9–10 mm.
In this study we have highlighted the potential impact on pT staging of extratumoral foci of SCC due to PNI and LI. It must be noted that a large proportion of extratumoral PNI or LI occurs in tumors that are already pT3, thus diminishing their impact on staging. Extratumoral PNI and LI represent a challenge to DOI measurement in isolated cases only. These scenarios are not currently directly addressed in the AJCC 8th edition description of DOI. However, they are covered under a more general TNM principle: when in doubt, the less advanced attribute should be selected (i.e., smaller DOI measurement, not including the extratumoral PNI or LI) [1]. In this study, extratumoral PNI was used to assign pT stage in only one case, because extratumoral PNI represented the only focus of residual carcinoma after the diagnostic biopsy (Figs. 2, 3).
The oral tongue is covered by mucosa on its dorsal, lateral, and ventral aspects and a simple “plumb line” method may be difficult to apply in some cases. When residual carcinoma is small and not connected to the mucosal surface, the reference point from which to measure the DOI is perhaps best represented by mucosa with squamous dysplasia (Figs. 2, 3). In oral tongue, the level of the basement membrane of the closest adjacent normal mucosa is probably better represented by an arcuate rather than a straight line, especially when the line is drawn through two points, i.e., normal mucosa on both sides of carcinoma.
All cases studied here were known to be pN0. It is possible that cN0/pN + SCC, or carcinomas involving other oral cavity subsites, may have different biologic parameters complicating DOI measurement (e.g., higher rates of extratumoral PNI). The issue of tumor thickness vs DOI [3] was not examined here, because carcinomas with exophytic growth (papillary SCC, verrucous carcinoma) were excluded, but this comparison needs further study.
In conclusion, in up to 12% of apparently pT2 cases DOI may be underestimated due to the positive deep margin. Rarely, extratumoral PNI or LI may be the deepest point of invasion, but it is unlikely to affect pT stage. DOI measurement for early SCC of the oral tongue may require re-examination of the diagnostic biopsy slides in up to 20% of cases due to the absence or only minimal residual carcinoma in glossectomy specimens. A proactive assessment and reporting of DOI on diagnostic biopsies or documentation of factors limiting DOI measurement (e.g., fragmentation, lack of normal mucosa, absence of intrinsic tongue musculature) may minimize the need to re-review the original diagnostic biopsy when the glossectomy reveals no or minimal residual carcinoma.
Conflict of interest
All authors declare that he/she has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (approved by the Total Quality Council, University of Pittsburgh Medical Center and IRB #5968 for SCPMG), which did not require informed consent.
References
- 1.Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 2.Klein Nulent TJW, Noorlag R, Van Cann EM, Pameijer FA, Willems SM, Yesuratnam A, et al. Intraoral ultrasonography to measure tumor thickness of oral cancer: a systematic review and meta-analysis. Oral Oncol. 2018;77:29–36. doi: 10.1016/j.oraloncology.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Dirven R, Ebrahimi A, Moeckelmann N, Palme CE, Gupta R, Clark J. Tumor thickness versus depth of invasion: analysis of the 8th edition American Joint Committee on Cancer Staging for oral cancer. Oral Oncol. 2017;74:30–33. doi: 10.1016/j.oraloncology.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Miller ME, Palla B, Chen Q, Elashoff DA, Abemayor E, St John MA, et al. A novel classification system for perineural invasion in noncutaneous head and neck squamous cell carcinoma: histologic subcategories and patient outcomes. Am J Otolaryngol. 2012;33(2):212–215. doi: 10.1016/j.amjoto.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell JH, Thompson LD, Brandwein-Gensler MS, Weiss BG, Canis M, Purgina B, et al. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1104–1110. doi: 10.1001/jamaoto.2015.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang AM, Kim SW, Duvvuri U, Johnson JT, Myers EN, Ferris RL, et al. Early squamous cell carcinoma of the oral tongue: comparing margins obtained from the glossectomy specimen to margins from the tumor bed. Oral Oncol. 2013;49(11):1077–1082. doi: 10.1016/j.oraloncology.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Prabhu AV, Sturgis CD, Lai C, Maxwell JH, Merzianu M, Hernandez-Prera JC, et al. Improving margin revision: characterization of tumor bed margins in early oral tongue cancer. Oral Oncol. 2017;75:184–188. doi: 10.1016/j.oraloncology.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115(7):1489–1497. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 9.Ganly I, Goldstein D, Carlson DL, Patel SG, O’Sullivan B, Lee N, et al. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119(6):1168–1176. doi: 10.1002/cncr.27872. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimi A, Gil Z, Amit M, Yen TC, Liao CT, Chaturvedi P, et al. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1138–1148. doi: 10.1001/jamaoto.2014.1548. [DOI] [PubMed] [Google Scholar]
- 11.Al-Rajhi N, Khafaga Y, El-Husseiny J, Saleem M, Mourad W, Al-Otieschan A, et al. Early stage carcinoma of oral tongue: prognostic factors for local control and survival. Oral Oncol. 2000;36(6):508–514. doi: 10.1016/S1368-8375(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 12.Ling W, Mijiti A, Moming A. Survival pattern and prognostic factors of patients with squamous cell carcinoma of the tongue: a retrospective analysis of 210 cases. J Oral Maxillofac Surg. 2013;71(4):775–785. doi: 10.1016/j.joms.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Shim SJ, Cha J, Koom WS, Kim GE, Lee CG, Choi EC, et al. Clinical outcomes for T1-2N0-1 oral tongue cancer patients underwent surgery with and without postoperative radiotherapy. Radiat Oncol. 2010;5:43. doi: 10.1186/1748-717X-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuen AP, Lam KY, Wei WI, Lam KY, Ho CM, Chow TL, et al. A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume, and clinicopathological features of oral tongue carcinoma. Am J Surg. 2000;180(2):139–143. doi: 10.1016/S0002-9610(00)00433-5. [DOI] [PubMed] [Google Scholar]


