Abstract
Purpose: Effective treatment options for negative symptoms and cognitive impairment in patients with schizophrenia are still to be developed. The present study was to examine potential benefits of repetitive transcranial magnetic stimulation (rTMS) to improve negative symptoms and cognition in this patient population.
Methods: The study was a 4-week, randomized, double-blind sham-controlled trial. Patients with schizophrenia were treated with adjunctive 20-Hz rTMS for 4 weeks or sham condition to the left dorsolateral prefrontal cortex (DLPFC). Negative symptoms were measured using the Scale for the Assessment of Negative Symptoms (SANS) and the Positive and Negative symptom scale (PANSS) negative subscale at baseline and week 4. Cognitive function was measured using the MATRICS Consensus Cognitive Battery (MCCB) at the same two time points. In addition, possible moderators for rTMS treatment efficacy were explored.
Results: Sixty patients (33 in the treatment group, 27 in the sham group) completed the study. There was a significant decrease in negative symptoms after 4-week rTMS treatment as measured by the SANS total score and the PANSS negative symptom subscale score. However, there was no significant improvement in cognition with rTMS treatment. Stepwise multiple linear regression analysis suggested that the baseline severity of positive symptoms may predict poorer improvement in negative symptoms at week 4.
Conclusion: Twenty-Hz rTMS stimulation over left DLPFC as an adjunctive treatment might be beneficial in improving negative symptoms of schizophrenia. Future studies with a longer treatment duration and a larger sample size are needed.
Clinical trial ID: NCT01940939.
Keywords: schizophrenia; negative symptoms; cognitive impairment; repetitive transcranial magnetic stimulation, rTMS; MCCB
Introduction
Schizophrenia is a severe and lifelong neuropsychiatric condition that affects approximately 1% of the world’s population.1,2 Both negative symptoms and cognitive impairment are among the core features of schizophrenia; both types of symptoms are important predictors for real life functioning.3 Despite enormous effort over the past several decades, effective treatment options to improve negative symptoms or cognition are still not available.4–7
Repetitive transcranial magnetic stimulation (rTMS) is a relatively safe and non-invasive method that uses alternating magnetic fields to induce an electric current in the underlying brain tissue.8 Studies have demonstrated that that high-frequency rTMS may be able to increase cortical excitability and modulate dopamine release in certain brain areas, including the prefrontal cortex.9–11 As negative symptoms and cognitive deficits in schizophrenia may be related to a lack of dopamine at the prefrontal cortex and hypofrontality.12,13 rTMS may have beneficial effects to improve these two types of symptoms.9,14
Recently, meta-analyses on rTMS treatment for negative symptoms of schizophrenia suggest an effect size of 0.27–0.53.15–17 In addition, high-frequency rTMS may enhance cognitive function in patients with schizophrenia. An early study found that active high-frequency rTMS applied to the dorsolateral prefrontal cortex (DLPFC) in healthy volunteers significantly increased gamma oscillations generated during the N-back conditions with the greatest cognitive demand.18 Only a few studies focused on the effects of rTMS on cognition impairment of schizophrenia. One study suggested that rTMS could improve working memory in schizophrenia, whereas another study had a negative outcome.19,20
Several important questions remain to be addressed regarding the use of rTMS treatment in patients with schizophrenia: rTMS stimulus frequency, motor threshold (MT), stimulus location, total stimulus strength, duration of stimulus, baseline psychopathology, duration of illness as well as the type of outcome measures used. The present study was a 4-week, randomized, double-blind sham-controlled trial. Patients with schizophrenia were treated with adjunctive 20-Hz rTMS for 4 weeks or sham condition to the left DLPFC. The primary hypothesis was that rTMS treatment can improve both negative symptoms and cognitive impairment at week 4. In addition, possible moderators for rTMS treatment efficacy were explored.
Several important questions remain to be addressed regarding the use of rTMS treatment in patients with schizophrenia: rTMS stimulus frequency, motor threshold (MT), stimulus location, total stimulus strength, duration of stimulus, baseline psychopathology, duration of illness as well as the type of outcome measures used. The present study was a 4-week, randomized, double-blind sham-controlled trial. Patients with schizophrenia were treated with adjunctive 20-Hz rTMS for 4 weeks or sham condition to the left DLPFC. The primary hypothesis was that rTMS treatment can improve both negative symptoms and cognitive impairment at week 4. In addition, possible moderators for rTMS treatment efficacy were explored.
Methods
The study was undertaken in Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine from 2013 to 2014. The trial has been registered at http://www.clinicaltrials.gov (NCT01940939), and the trial protocol has been published. No additional unpublished data are available.
Participants
Potential subjects were recruited from the inpatient units of Shanghai Mental Health Center. Individuals eligible for the study were adults aged 20–60 years who had a diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) criteria; the diagnosis was further confirmed using the Structured Clinical Interview for DSM-IV-TR, Clinical Trials Version. Patients had to be on a stable dose of antipsychotic medication for at least 1 month before the study enrollment. Benzodiazepines can be used temporarily for no longer than 7 days if patients complained about sleeplessness at night, and stopped 24 h before the cognitive testing and clinical assessment. In addition, patients had to meet the following clinical criteria: the negative symptoms subscale score of the Positive and Negative Syndrome Scale (PANSS) 20 or more.
Exclusion criteria included: a current DSM-IV-TR axis I disorder other than schizophrenia, a history of epilepsy or seizure; significant or unstable neurologic disorder; cardiac pacemaker; previous brain injury or surgery; any metal clips, plates, or other metal items in the head; or substance dependency; or ECT within 3 months. All participants provided written informed consent.
Clinical measures
Clinical measures included: the PANSS21 and the Scale for the Assessment of Negative Symptoms (SANS),22 and the Clinical Global Impressions (CGI) score.23 Cognitive function was assessed using the MATRICS Consensus Cognitive Battery (MCCB) in Chinese Version.24 The MCCB in Chinese Version includes 9 tasks across 7 domains, and a composite score, including Processing Speed (Brief Assessment of Cognition in Schizophrenia Symbol Coding, Animal Fluency, Trails A), Attention (Continuous Performance Test), Working Memory (WMS-III Spatial Span), Verbal Learning (Hopkins Verbal Learning Test – Revised), Visual Learning (Brief Visuospatial Memory Test – Revised), Problem Solving (Neuropsychological Assessment Battery), and Social Cognition (Mayer-Salovey-Caruso Emotional Intelligence Test). The Letter-Number Span Test (LNS) was excluded from the original MCCB because there is no corresponding alphabet in Chinese. Total administration time is 60–90 min. MCCB was used at baseline and within 24 h after rTMS intervention. To avoid practice effects, version A was taken at baseline and version B for retest at the end of intervention.
rTMS protocol
Participants received 20 treatment sessions on consecutive weekdays. Patients were randomly assigned to receive either 20 Hz rTMS applied to the left DLPFC (EEG International 10–20 system, F3-electrode) or the sham condition. High-frequency rTMS has been shown to enhance gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission with increased stimulation frequency, with the maximal inhibitory effect achieved at 20 Hz.25 The F3-position used in our study corresponds to Brodmann areas 8, 9, and 46 in the medial frontal gyrus.26–28 The rTMS treatment had an intensity of 90% of the individual resting motor threshold (MT) and 2,000 stimuli (100 trains with 20 stimuli per train, 9 s intertrain interval) per session. Stimuli were applied using a MagPro X100 stimulator (MagVenture) and a standard butterfly coil (MFC-B65). rTMS was performed in accordance with the recommendations by the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation.29 Sham rTMS stimulation was delivered using the same stimulation parameters and at the same site as in active treatment, but the coil was flipped 180° around its main axis so that the thickness of the integrated cooling system of the coil between the skull and the coil center was 53 mm. This method produces sound and some somatic sensation (eg, contraction of scalp muscles) similar to those of active stimulation, but the resulting increase in spatial distance translated into a reduction in stimulation intensity of 80% (MagVenture).30
Data analysis
Data were analyzed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Baseline differences in demographic and clinical variables between the two treatment groups were examined using independent t-test and chi-squared test. Repeated measures ANOVA was performed for the primary outcome measures (negative symptoms, cognition) with group condition (active treatment vs sham) as the between-subject factor and time (baseline, 4 weeks) as the within-subject factor. Pearson correlation analysis was used to examine the relationship among variables. Stepwise multiple linear regression analysis was used to identify potential predictors for the primary outcome measures.
Ethics statement
The research protocol was approved by the Institutional Review Board of Shanghai Mental Health Center (2013–04). This study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The subjects were assured of the following: their participation was voluntary, they could withdraw at any time without facing any negative consequences, their anonymity would be protected, and the data obtained would not be used for purposes other than the present research. All participants provided their written informed consent. The research purpose and guarantee to protect the privacy of the subjects were explained verbally as well as in written form to the participants by the doctor.
Results
Demographic and clinical characteristics
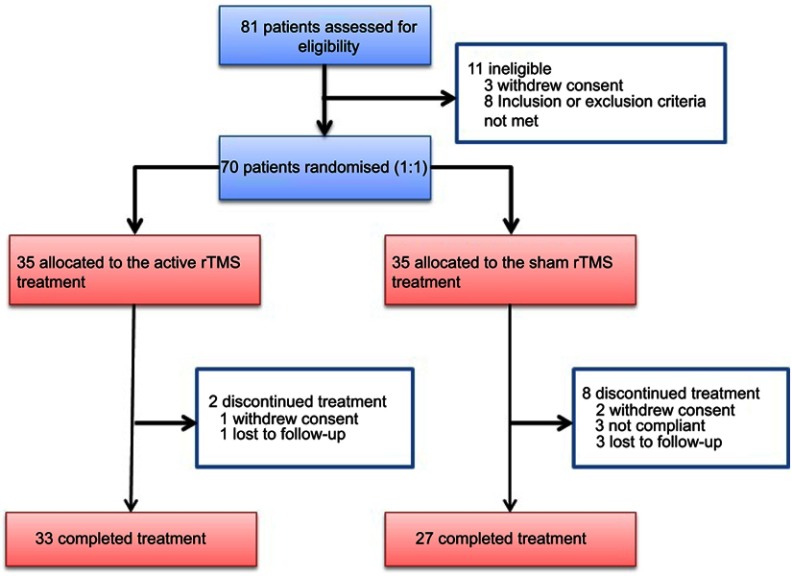
Eighty-one patients were screened, and 70 were randomized to either active treatment or sham condition. Thirty-three patients completed 4-week active treatment, and 27 completed the sham treatment. Seven patients withdrew from the study because of the time commitment whereas three patients dropped out because of a diminished interest in the study (Figure 1). Three patients in the sham group and four in the active group reported a transient headache, and one in the active group reported dizziness in the initial period. After giving comfort and reducing the initial intensity of stimulation, the symptoms were significantly reduced. No other adverse events were observed. Table 1 shows the demographic, clinical and cognitive characteristic of the two groups. The two groups did not differ in demographic, clinical and cognitive characteristics. The numbers of patients on different antipsychotic drugs for the active treatment group versus sham groups are as follows: olanzapine 6/6, risperidone 6/5, paliperidone 12/10, amisulpride 2/2, ziprasidone 3/0, clozapine 1/0, quetiapine 1/1 and aripiprazole 2/3. Five patients also used mood stabilizer (valproate 2/3).
Figure 1.
The study flow chart.
Abbreviation: rTMS, repetitive transcranial magnetic stimulation.
Table 1.
The demographic and clinical characteristic of the two groups
| 20 Hz (n=33) | Sham (n=27) | Group comparison | ||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t/x2 | p | |
| Age (years) | 28.97 (7.40) | 30.63 (8.25) | 0.416 | 0.521 |
| Education (years) | 12.70 (2.54) | 12.41 (2.34) | 0.244 | 0.623 |
| Course of illness (years) | 7.20 (5.46) | 8.11 (5.64) | 0.001 | 0.977 |
| Dose of chlorpromazine equivalents (mg) | 400.48 (252.17) | 421.93 (209.23) | 0.298 | 0.587 |
| IQ | 92.17 (14.25) | 95.72 (14.37) | 0.100 | 0.754 |
| PANSS-total | 68.15 (5.535) | 69.93 (6.528) | 1.824 | 0.182 |
| PANSS-positive | 14.61 (3.381) | 15.26 (2.669) | 0.976 | 0.327 |
| PANSS-negative | 21.70(3.349) | 22.63(3.824) | 1.323 | 0.255 |
| PANSS-general | 31.85(3.022) | 32.04(3.391) | 1.212 | 0.275 |
| SANS | 48.09(11.204) | 50.33(12.551) | 0.405 | 0.527 |
| CGI | 4.88 (0.33) | 4.93 (0.55) | 2.171 | 0.146 |
| Gender | ||||
| Male | 22 | 19 | 0.094 | 0.759 |
| Female | 11 | 8 | ||
Abbreviations: IQ intelligence quotient; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; CGI, clinical global impression.
Negative symptoms and cognitive function after 4-week treatment
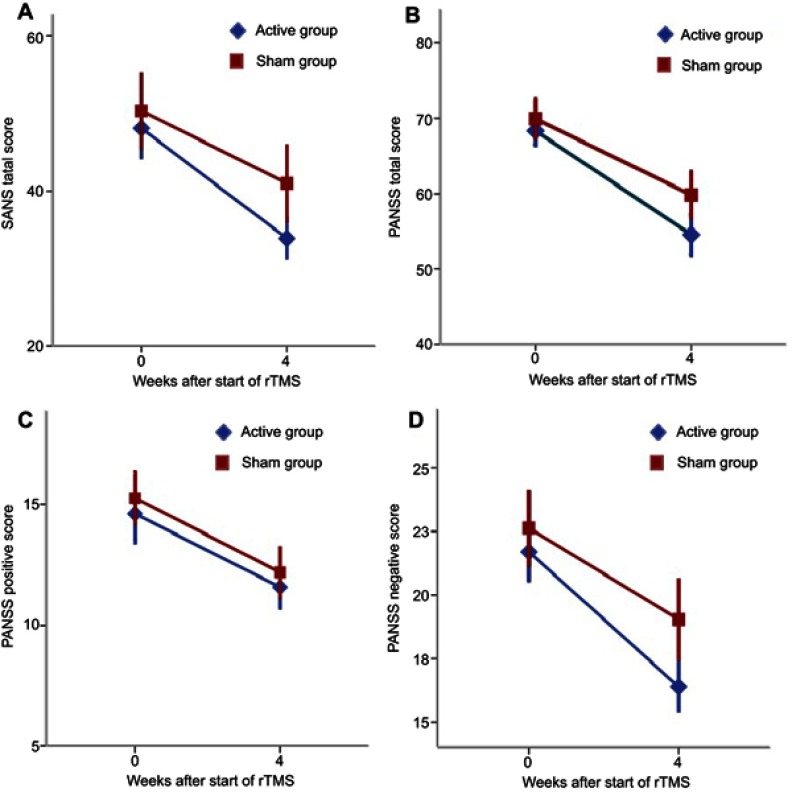
Patients in the active and sham group were classified as having a clinical improvement with baseline CGI, SANS total score, PANSS total score, and the three PANSS subscores (Figure 2). Repeated measures ANOVA revealed a significant group-by-time interaction for SANS total score (F(1, 59)=5.632, p=0.021), PANSS negative subscore (F(1, 59)=8.090, p=0.006), and CGI-S (F(1,59)=4.436, p=0.040). However, no group-by-time interaction was found for PANSS total score, PANSS positive score, and PANSS general score (Table 2).
Figure 2.
Scores for severity of symptoms during the trail. Data appear mean ± SD in blue for active rTMS and in red for sham rTMS.
Notes: Patients in the active rTMS group had a great improvement in SANS total score and PANSS negative subscore after 4-week treatment (A and D). However, there were no significant differences in PANSS total score and positive subscore between the active and sham rTMS group (B and C).
Abbreviation: rTMS, repetitive transcranial magnetic stimulation.
Table 2.
Comparison of the symptoms changes pre- and post-treatment between the two study groups
| 20 Hz (n=33) Mean (SD) |
Sham (n=27) Mean (SD) |
Time | Group | Interaction between group and time of measurement | Effect size2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | F | p | F | p | F | p | ||
| PANSS-total | 68.15 (5.535) | 54.58(7.604) | 69.93 (6.528) | 59.85(7.735) | 171.687 | 0.000* | 5.533 | 0.026* | 3.764 | 0.057 | 0.061 |
| PANSS-positive | 14.61 (3.381) | 11.58(2.424) | 15.26 (2.669) | 12.19(2.573) | 107.405 | 0.000* | 0.901 | 0.346 | 0.006 | 0.941 | 0.000 |
| PANSS-negative | 21.70(3.349) | 16.39(2.806) | 22.63 (3.824) | 19.04(4.100) | 218.809 | 0.000* | 4.328 | 0.042* | 8.090 | 0.006* | 0.122 |
| PANSS-general | 31.85 (3.022) | 26.61(3.824) | 32.04 (3.391) | 28.63(3.387) | 63.894 | 0.000* | 2.470 | 0.121 | 2.876 | 0.095 | 0.047 |
| SANS | 48.09 (11.204) | 33.91(7.776) | 50.33 (12.551) | 41.00(12.713) | 132.484 | 0.000* | 3.013 | 0.088 | 5.632 | 0.021* | 0.089 |
| CGI | 4.88 (0.331) | 3.79 (0.600) | 4.93 (0.550) | 4.11 (0.641) | 211.336 | 0.000* | 2.259 | 0.138 | 4.436 | 0.040* | 0.071 |
Abbreviations: PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms. CGI, Clinical Global Impression.
Though the T scores in some domains (eg, Speed of Processing, Verbal Learning, Visual Learning, Social Cognition, and Composite Score) were improved with baseline after 4-week treatment in both groups (Table 3), no group-by-time interactions in any domains were found between the two groups. Even the repeated measures ANOVA found a trend of group-by-time interactions reflecting those patients in the sham group got more improvement in Composite Score (F(1,59)=3.491, p=0.068) and BACS test (F(1,59)=3.884, p=0.054) than those in the active group.
Table 3.
Comparison of MCCB changes pre- and post-treatment between the two study groups
| MCCB (T score) |
20 Hz (n=33) Mean (SD) |
Sham (n=27) Mean (SD) |
Time | Group | Interaction between group and time of measurement | Effect size2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | F | p | F | p | F | p | ||
| Speed of processing | 46.39(8.909) | 49.91(8.476) | 43.22(9.541) | 48.93(7.908) | 21.305 | 0.000* | 1.045 | 0.311 | 1.201 | 0.278 | 0.005 |
| TMT-A | 46.24 (9.114) | 51.39(9.647) | 44.70 (10.513) | 52.30 (9.067) | 44.767 | 0.000* | 0.019 | 0.890 | 1.642 | 0.205 | 0.011 |
| BACS | 48.03 (6.729) | 47.94 (7.754) | 44.74 (7.739) | 47.85 (8.347) | 3.456 | 0.068 | 0.876 | 0.353 | 3.884 | 0.054 | 0.047 |
| Fluency | 47.21(16.359) | 50.55 (7.934) | 44.22(11.613) | 47.56 (9.116) | 3.694 | 0.060 | 1.392 | 0.243 | 0.000 | 1.000 | 0.017 |
| Attention/vigilance (CPT-IP) | 46.28(8.146) (n=29) |
47.47 (9.677) (n=29) |
42.96(12.545) (n=25) |
46.16 (9.767) (n=25) |
3.704 | 0.060 | 0.728 | 0.397 | 1.171 | 0.284 | 0.017 |
| Working memory (WMS-SS) | 44.88 (10.096) | 44.03 (8.010) | 45.19 (11.007) | 46.22 (11.892) | 0.007 | 0.935 | 0.273 | 0.603 | 0.663 | 0.419 | 0.006 |
| Verbal learning (HVLT-R) | 44.48 (8.382) | 48.48 (7.815) | 43.30 (10.227) | 49.22 (9.040) | 23.224 | 0.000* | 0.012 | 0.913 | 0.874 | 0.354 | 0.002 |
| Visual learning (BVMT-R) | 44.82 (8.206) | 50.06 (10.341) | 45.96 (11.134) | 50.59 (10.976) | 30.190 | 0.000* | 0.115 | 0.736 | 0.116 | 0.734 | 0.015 |
| Reasoning/problem solving (NAB) | 50.79 (8.663) | 51.58 (10.006) | 49.00 (9.307) | 51.81 (8.919) | 3.302 | 0.074 | 0.125 | 0.724 | 1.045 | 0.311 | 0.001 |
| Social cognition (MSCEIT) | 49.30(11.229) (n=32) |
50.03 (11.545) (n=32) |
50.85(10.638) (n=26) |
56.12 (11.147) (n=26) |
4.485 | 0.039* | 2.210 | 0.143 | 2.573 | 0.114 | 0.024 |
| Composite score | 45.07(6.480) | 48.00(7.158) | 43.58(9.259) | 49.04(7.463) | 28.298 | 0.000* | 0.001 | 0.973 | 3.491 | 0.068 | 0.013 |
Note: *Statistically significant.
Abbreviations: TMT-A, trail making test: part A; BACS, brief assessment of cognition in schizophrenia: symbol coding; fluency, category fluency test; CPT-IP, continuous performance test-identical pairs; WMS-SS, wechsler memory scale-third edition (WMS-III): spatial span; HVLT-R, hopkins verbal learning test-revised; BVMT, brief visuospatial memory test-revised; NAB, neuropsychological assessment battery: mazes; MSCEIT, mayer-salovey-caruso emotional intelligence test: managing emotions.
Associations between the reduction of negative symptoms of schizophrenia and clinical and demographic variables
In both groups, the reduction rate of SANS total score, PANSS total score, and the three PANSS subscores were not correlated with demographic variables (age, education, course of illness, dose of antipsychotic drug or IQ). Meanwhile, there were no significant correlations between the T score changes (pre- and post-treatment) of the MCCB with the reduction rate of the SANS, PANSS total score and the three PANSS subscores (Pearson correlation).
Stepwise multiple linear regression analysis of the SANS reduction rate and clinical symptoms showed that a higher PANSS positive symptoms subscore at baseline predicts a lower SANS total score reduction rate at week 4 (Table 4). In addition, a higher SANS total score at baseline seemed to predict a higher SANS total score reduction at week 4. However, these relationships were not observed in the sham group.
Table 4.
Results of the stepwise multiple regression analyses in patients
| Predictor | Beta | p-value | 95% CI | |
|---|---|---|---|---|
| SANS reducation rate R2=0.343; F(2,33)=7.845; p=0.002 |
PANSS positive | −0.402 | 0.021 | −2.807, −0.247 |
| SANS total | 0.283 | 0.096 | −0.061, 0.711 |
Abbreviations: PANSS, positive and negative syndrome scale; SANS, scale for the assessment of negative symptoms.
Discussion
Our study found a significant improvement of negative symptoms as measured by PANSS negative score and SANS total score after 4 weeks of 20 Hz rTMS over the left DLPFC compared with sham rTMS. Our results are consistent with previous studies,31–34 which found that high-frequency rTMS over the left DLPFC may lead to improvement in the negative symptoms of schizophrenia.
The open-label study of Cohen et al35 first reported that 20 Hz rTMS targeting DLPFC reduced the PANSS negative subscale. However, some follow-up studies did not alleviate negative symptoms despite several methodological improvements (eg, double-blind sham-controlled parallel design and a higher number of pulses). For instance, Novak et al failed to find any significant effect of 20 Hz rTMS (90% MT, 20,000 stimuli) on PANSS negative subscales after a two-week treatment.36 This may be due to the insufficient duration, which was recommended in a meta-analysis to be no less than three weeks.17 Meanwhile, bilateral 20 Hz rTMS (90% MT, 30,000 stimuli) was used in Barr et al’s study, but no significant improvement in negative symptoms or depressive symptoms were found.37 It may be insufficient to detect an improvement in negative symptoms after rTMS treatment because of the relatively small sample size.
Our study also found that patients with less positive symptoms at baseline tended to have a better response in negative symptom improvement even though the positive symptoms did not improve in our study. This suggests that the severity of existing positive symptoms in patients with schizophrenia may have an adverse effect on rTMS in the treatment for negative symptoms. This phenomenon is similar to that of antipsychotic drugs such as clozapine in the treatment of refractory schizophrenia.38 Secondly, we found that patients with more negative symptoms of baseline tended to benefit more from rTMS treatment. Our result is consistent with the findings from a recent meta-analysis.17
In addition, both patients in the active and sham group had clinical improvement with baseline CGI, SANS total score, PANSS total score, and the three PANSS subscores, which suggested that a placebo effect should be taken into account. In Shi et al’s meta-analysis,17 a small placebo effect had been found in the treatment effect of rTMS on negative symptoms in schizophrenia. However, none of the scores in the sham group met the criterion for response (ie a 20% reduction in baseline PANSS negative symptom score and SANS total score) in our study at the end of treatment. Similar results were found in the Mogg et al’s study.39
Dysfunctional oscillations may have a central role in the pathophysiology of schizophrenia, which may be caused by the anomalies in the brain’s rhythm-generating networks of GABA interneurons and cortico-cortical connections. In patients with schizophrenia, the abnormalities of beta and gamma-band activity suggest the cognitive deficits and other symptoms of the disorder.40 High-frequency rTMS (10–20 Hz) can modulate gamma oscillatory activity, which may be a possible avenue for cognitive improvement in this disorder.41 Although Shi et al suggest that 10 Hz stimulation is probably more effective than 20 Hz in the treatment of negative symptoms,17 the optimal rTMS frequency to achieve the maximal therapeutic effect for cognitive impairment is still to be determined. It has been suggested that greater stimulation frequency and a greater number of treatment sessions may result in greater treatment effects.
Several previous studies have examined rTMS treatment and cognitive function in schizophrenia with inconsistent findings. For example, Mogg39 found that 10 Hz high-frequency rTMS led to a significant improvement in verbal learning among patients with schizophrenia, while Schneilder42 did not find any significant change for the Wisconsin Card Sorting Test (WCST) among the three groups of either placebo, 1 Hz low-frequency or 10 Hz high-frequency rTMS at 110% MT over the left DLPFC. These studies only reflected the change in several domains but cannot evaluate the comprehensive and broad improvement of the cognition in schizophrenic patients. Though the T score in some domains were improved after 4-week rTMS treatment, unfortunately, our study found that 20 Hz rTMS had no benefit for cognitive improvement in the active group relative to sham group, while there was a trend towards suggesting those patients in the sham group improved more than those in the active group in BAC test and composite score. The duration of our intervention might be one of the reasons for negative findings, as MCCB was tested before and after rTMS within a timeframe of 4 weeks. This period may be too short to assess the cognitive improvement. An intervention period of at least 6 months is needed to detect possible changes in cognitive assessments in antipsychotic trials, according to a previous meta-analysis.43 Furthermore, the effect of practice needs to be considered despite the high test–retest reliability of the MCCB44 and two different versions were used to assess at baseline and the end of the intervention. The practice-related increase in cognitive performance makes it difficult to distinguish the effect of active rTMS from the reduction of the difference between the active and the sham group.
The present study has several limitations. First, the patients in this study were chronic and medicated. It is possible that antipsychotic medication might affect cognitive dysfunction. Further, given the relatively small sample size and a high rate of shedding, the negative findings in cognition might be due to low statistical power. Secondly, depressive symptoms were not measured or controlled in our study. High-frequency rTMS over the left DLPFC is able to produce antidepressant effects, which may confound its beneficial effect on negative symptoms of schizophrenia. Taken together, early episode patients, longer rTMS periods, different stimulation frequency or location may have led to different results.45
Conclusion
In summary, high-frequency 20 Hz rTMS stimulation over the left DLPFC at a high stimulation intensity and a sufficient number of applied stimulating pulses may represent an effective augmentation to antipsychotics to treat the negative symptoms of schizophrenia. Existing positive symptoms may be an important predictor factor of the efficacy of rTMS treatment. Moreover, the results suggested that rTMS may have differential effects on negative symptoms and cognitive impairment in schizophrenia. The stimulation parameters for the treatment of negative and cognitive dysfunction may be different. Optimal rTMS parameters still need to be explored to achieve improvement in cognitive function in this patient population.
Acknowledgments
The authors would like to acknowledge those patients taking part in this study and the support of the funding sources. This work was sponsored by Key program of Multidisciplinary Cross Research Foundation of Shanghai Jiao Tong University (YG2017ZD13), Multidisciplinary Cross Research Foundation of Shanghai Jiao Tong University (YG2017MS43), Natural Science Foundation of Shanghai (15ZR1435600), Medical guidance project of Science and Technology Commission Shanghai Municipality (14411961400, 15411964400), Shanghai Municipal Commission of Health and Family Planning Foundations (2014ZYJB0002), Shanghai Municipal Hospital Appropriate Technology Program (SHDC12014214), Shanghai Excellent Academic & Technology Leaders Program (16XD1402400), Young Doctor Training Program (20141058), and National Natural Science Foundation of China (81371479). It was also supported by Shanghai Key Laboratory of Psychotic Disorders (14K03), Shanghai Clinical Center for Mental Disorders (2014) and Early Psychosis Program of Shanghai Mental Health Center (2013-YJTSZK-05). These funding agents had no role in the study design, collection, analysis, and interpretation of the data, writing of the manuscript, or decision to submit the paper for publication.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi: 10.1038/nature09552 [DOI] [PubMed] [Google Scholar]
- 2.Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351–356. doi: 10.1176/ajp.151.3.351 [DOI] [PubMed] [Google Scholar]
- 3.Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495 [DOI] [PubMed] [Google Scholar]
- 4.Wykes T, Huddy V, Cellard C, Mcgurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2008;164(12):1791–1802. [DOI] [PubMed] [Google Scholar]
- 5.Erhart SM, Marder SR, Carpenter WT. Treatment of schizophrenia negative symptoms: future prospects. Schizophr Bull. 2006;32(2):234–237. doi: 10.1093/schbul/sbj055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychoph. 2005;8(3):457. doi: 10.1017/S146114570500516X [DOI] [PubMed] [Google Scholar]
- 7.Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633 [DOI] [PubMed] [Google Scholar]
- 8.Loo CK, McFarquhar TF, Mitchell PB. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int J Neuropsychopharmacol. 2008;11(1):131–147. doi: 10.1017/S1461145707007717 [DOI] [PubMed] [Google Scholar]
- 9.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenegger C, Treyer V, Fehr E, Knoch D. Time-course of “off-line” prefrontal rTMS effects–a PET study. NeuroImage. 2008;42(1):379–384. doi: 10.1016/j.neuroimage.2008.04.172 [DOI] [PubMed] [Google Scholar]
- 11.Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93(1):59–98. doi: 10.1016/j.pneurobio.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110(4):243–256. doi: 10.1111/j.1600-0447.2004.00376.x [DOI] [PubMed] [Google Scholar]
- 13.Remington G, Agid O, Foussias G. Schizophrenia as a disorder of too little dopamine: implications for symptoms and treatment. Expert Rev Neurother. 2011;11(4):589–607. doi: 10.1586/ern.10.191 [DOI] [PubMed] [Google Scholar]
- 14.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4(8):e6725. doi: 10.1371/journal.pone.0006725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1–3):11–24. doi: 10.1016/j.schres.2008.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dlabac-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71(4):411–418. doi: 10.4088/JCP.08r04808yel [DOI] [PubMed] [Google Scholar]
- 17.Shi C, Yu X, Cheung EF, Shum DH, Chan RC. Revisiting the therapeutic effect of rTMS on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Res. 2014;215(3):505–513. doi: 10.1016/j.psychres.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr MS, Farzan F, Rusjan PM, Chen R, Fitzgerald PB, Daskalakis ZJ. Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacol. 2009;34(11):2359–2367. doi: 10.1038/npp.2009.79 [DOI] [PubMed] [Google Scholar]
- 19.Barr MS, Farzan F, Rajji TK, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73(6):510–517. doi: 10.1016/j.biopsych.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 20.Hasan A, Guse B, Cordes J, et al. Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: results from a multicenter randomized sham-controlled trial. Schizophr Bull. 2016;42(3):608–618. doi: 10.1093/schbul/sbv142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39(7):784–788. [DOI] [PubMed] [Google Scholar]
- 23.Guy W. Early Clinical Drug Evaluation (ECDEU) Assessment Manual. Rockville: National Institute Mental Health; 1976. [Google Scholar]
- 24.Shi C, Kang L, Yao S, et al. The MATRICS Consensus Cognitive Battery (MCCB): Co-norming and standardization in China. Schizophr Res. 2015;169(1–3):109. doi: 10.1016/j.schres.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daskalakis ZJ, Möller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174(3):403–412. doi: 10.1007/s00221-006-0472-0 [DOI] [PubMed] [Google Scholar]
- 26.Homan RW, Herman J, Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987;66(4):376–382. [DOI] [PubMed] [Google Scholar]
- 27.Herwig U, Padberg F, Unger J, Spitzer M, Schonfeldt-Lecuona C. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry. 2001;50(1):58–61. [DOI] [PubMed] [Google Scholar]
- 28.Herwig U, Satrapi P, Schönfeldtlecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16(2):95–99. [DOI] [PubMed] [Google Scholar]
- 29.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. [DOI] [PubMed] [Google Scholar]
- 30.Bilek E, Schafer A, Ochs E, et al. Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. J Neurosci. 2013;33(16):7050–7056. doi: 10.1523/JNEUROSCI.3081-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prikryl R, Kasparek T, Skotakova S, Ustohal L, Kucerova H, Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. 2007;95(1–3):151–157. doi: 10.1016/j.schres.2007.06.019 [DOI] [PubMed] [Google Scholar]
- 32.Prikryl R, Ustohal L, Prikrylova Kucerova H, et al. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double-blind trial. Schizophr Res. 2013;149(1–3):167–173. doi: 10.1016/j.schres.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 33.Quan WX, Zhu XL, Qiao H, et al. The effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia and the follow-up study. Neurosci Lett. 2015;584:197–201. doi: 10.1016/j.neulet.2014.10.029 [DOI] [PubMed] [Google Scholar]
- 34.Zhao S, Kong J, Li S, Tong Z, Yang C, Zhong H. Randomized controlled trial of four protocols of repetitive transcranial magnetic stimulation for treating the negative symptoms of schizophrenia. Shanghai Arch Psychiatry. 2014;26(1):15–21. doi: 10.3969/j.issn.1002-0829.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen E, Bernardo M, Masana J, et al. Repetitive transcranial magnetic stimulation in the treatment of chronic negative schizophrenia: a pilot study. J Neurol Neurosurg Psychiatry. 1999;67(1):129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak T, Horacek J, Mohr P, et al. The double-blind sham-controlled study of high-frequency rTMS (20Hz) for negative symptoms in schizophrenia: negative results. Neuroendocrinol Lett. 2006;27(1–2):209–213. [PubMed] [Google Scholar]
- 37.Barr MS, Farzan F, Tran LC, Fitzgerald PB, Daskalakis ZJ. A randomized controlled trial of sequentially bilateral prefrontal cortex repetitive transcranial magnetic stimulation in the treatment of negative symptoms in schizophrenia. Brain Stimul. 2012;5(3):337–346. doi: 10.1016/j.brs.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 38.Umbricht DS, Wirshing WC, Wirshing DA, et al. Clinical predictors of response to clozapine treatment in ambulatory patients with schizophrenia. J Clin Psychiatry. 2002;63(5):420–424. [DOI] [PubMed] [Google Scholar]
- 39.Mogg A, Purvis R, Eranti S, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93(1–3):221–228. doi: 10.1016/j.schres.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 40.Uhlhaas PJ, Wolf S. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774 [DOI] [PubMed] [Google Scholar]
- 41.Barr MS, Farzan F, Arenovich T, Chen R, Fitzgerald PB, Daskalakis ZJ. The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS One. 2011;6(7):e22627. doi: 10.1371/journal.pone.0022627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider AL, Schneider TL, Stark H. Repetitive transcranial magnetic stimulation (rTMS) as an augmentation treatment for the negative symptoms of schizophrenia: a 4-week randomized placebo controlled study. Brain Stimul. 2008;1(2):106–111. doi: 10.1016/j.brs.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 43.Desamericq G, Schurhoff F, Meary A, et al. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol. 2014;70(2):127–134. doi: 10.1007/s00228-013-1600-y [DOI] [PubMed] [Google Scholar]
- 44.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- 45.Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979–988. doi: 10.1016/j.biopsych.2014.10.009 [DOI] [PubMed] [Google Scholar]