Abstract
Aim: The prospective, randomized, multicenter Myocardial Ischemia Treated with Percutaneous Coronary Intervention and Plaque Regression by Lipid Lowering & Blood Pressure Controlling assessed by Intravascular Ultrasonography (MILLION) study demonstrated that combined treatment with atorvastatin and amlodipine enhanced coronary artery plaque regression. Although the baseline high-sensitive C-reactive protein (hs-CRP) reportedly plays an important role in atherogenesis, few data exist regarding the relationship between hs-CRP and plaque regression in patients receiving a combined atorvastatin and amlodipine therapy.
Methods: A total of 68 patients (male, 55; mean age, 64.2 years) with baseline and follow-up 3-dimensional intravascular ultrasound examinations in the MILLION study were stratified by baseline hs-CRP level quartiles. The serial measurements of lipid, blood pressure, and percentage changes in the plaque volume were compared between the groups, and the factors associated with the percentage change in the plaque volume were assessed.
Results: There were no significant between-group differences in the extent of change in low-density lipoprotein cholesterol (LDL-C) or systolic and diastolic blood pressure after 18–24 months of treatment. The percentage change in the plaque volume showed a linear association with the baseline hs-CRP (p for trend < 0.05); however, there was no correlation with changes in LDL-C or systolic and diastolic blood pressure. In the multiple regression analysis, the baseline hs-CRP level was independently associated with the percentage change in the plaque volume (β = 0.29, p = 0.022).
Conclusions: Coronary plaque regression was associated with the baseline hs-CRP level in patients treated with a combined lipid- and blood pressure-lowering therapy.
Keywords: High-Sensitivity C-Reactive Protein, Amlodipine, Atorvastatin, Intravascular ultrasound
Introduction
Although the statin-mediated cholesterol-lowering therapy improves clinical outcomes, many treated individuals demonstrate atheroma progression1) and experience subsequent cardiovascular events. Inflammation is a key factor involved in the initiation, progression, and instability of atherosclerotic plaques2, 3), and some consider the prognostic efficacy of the baseline high-sensitivity C-reactive protein (hs-CRP) level to be equivalent to that of serum cholesterol levels4, 5). Although the benefits of statins are attributed to their cholesterollowering effects, both coronary atheroma regression6) and clinical event reductions7) have also been shown to independently correlate with statin-mediated CRP reduction8). Moreover, an amlodipine-based blood pressure (BP)-lowering regimen reduced hs-CRP levels and the incidence of major cardiovascular events in patients with hypertension compared with beta-blocker- and angiotensin-converting enzyme inhibitor-based regimens9, 10). However, few data exist regarding the relationship between changes in hs-CRP levels and plaque regression in the setting of combined atorvastatin and amlodipine therapy.
The Myocardial Ischemia Treated with Percutaneous Coronary Intervention and Plaque Regression by Lipid Lowering & Blood Pressure Controlling assessed by Intravascular Ultrasonography (MILLION) study, a prospective, randomized, multicenter investigation, evaluated the combined effect of aggressive low-density lipoprotein cholesterol (LDL-C) treatment with an atorvastatin-based therapy and BP control with an amlodipine-based therapy on the progression and regression of coronary plaques. That study demonstrated that lipid- and BP-lowering therapies acted synergistically to reduce coronary plaque11).
Aim
This subanalysis of the MILLION study aimed to evaluate the hypothesis that baseline hs-CRP predicts coronary plaque regression and progression in patients receiving combined statin and amlodipine therapy.
Methods
Study Population
Our subanalysis of the MILLION study (the University Hospital Medical Information Network ID: 000002829, https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000003446) was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all enrolled patients at the start of the study. The study design was previously reported12). Briefly, 97 patients with coronary artery disease were randomized to receive either aggressive or standard reduction of both LDL-C and BP by atorvastatin- and amlodipine-based therapies after a successful percutaneous coronary intervention under the intravascular ultrasound (IVUS) guidance. In the aggressive treatment group, the target serum LDL-C level and BP were 70 mg/dL and 120/70 mmHg, respectively. In the standard treatment group, the target serum LDL-C level and BP were 100 mg/dL and 140/90 mmHg, respectively. If the LDL-C or BP did not reach the target value after the administration of the maximal atorvastatin or amlodipine dose, any lipid- or BP-lowering medication could be added. Follow-up IVUS examinations were performed after 18–24 months of statin and calcium channel blocker therapy.
In the present study, patients with 3-dimensional IVUS data at both the baseline and follow-up in the MILLION study were identified and stratified according to the baseline hs-CRP level quartiles (25% quartile = 0.66, 50% quartile = 1.92, and 75% quartile = 8.15 mg/L).
Serial Measurements of Lipid and BP
Serum cholesterol and triglycerides were measured every 2 months during the observation period to regulate atorvastatin and other lipid-lowering medication dosages. Apolipoproteins were analyzed at a central clinical laboratory (SRL, Inc., Tokyo, Japan) at baseline and follow-up. BP was measured using a manual cuff and stethoscope monthly throughout the observation period to regulate amlodipine and other BP-lowering medication dosages.
Intravascular Ultrasound Examination and Analysis
Details of the IVUS examination and analysis have been documented elsewhere13). Briefly, an Atlantis SR Pro 2, 40 MHz imaging catheter (Boston Scientific, Natick, MA, US) was used to analyze the coronary plaque volume. The baseline and follow-up IVUS images were reviewed side-by-side on a display, and the target segment was selected. The target segment was determined in a non-PCI site (> 5 mm proximal or distal to the PCI site, or the other artery) with a reproducible index such as side branches, calcification, or stent edges. The IVUS catheter pullback was performed automatically at 0.5 mm/s. The plaque volume was assessed by volumetric analysis with the echoPlaque2 system (Indec Systems, Inc., Los Altos, CA, US). Standard 3-dimensional IVUS parameters such as vessel, lumen, and plaque volumes were assessed at both the baseline and follow-up. Volumetric data were standardized by length as the normalized volume, where the volume was multiplied by the median length analyzed in the entire cohort and divided by each observed length to compensate for differences in the segment length between patients. The percentage change in the plaque volume was defined as a change in the plaque volume (follow-up minus baseline plaque volume) divided by the baseline plaque volume.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation if normally distributed, and as the median (interquartile range) if non-normally distributed. Categorical data were compared using the chi-square or Fisher's exact tests. Differences between the groups were evaluated by the analysis of variance for normally distributed continuous variables and by the Kruskal–Wallis tests for non-normally distributed continuous variables. A test of trend in the IVUS parameters was performed across quartiles. The effects of the plaque volume at baseline on IVUS parameters were evaluated by the analysis of covariance with the plaque volume at baseline included as a potentially confounding variable. Since the distribution of the hs-CRP values was skewed to the left, a logarithmic transformation and a natural logarithm [log (hs-CRP)] were employed to achieve approximate normality in further analysis. Univariate and multivariate linear regression analyses were performed to detect factors that influenced percentage changes in the plaque volume. Univariate predictors with a p-value < 0.2 were entered into the multivariate model. A p-value < 0.05 was considered statistically significant. Stat View 5.0 (SAS Institute, Cary, NC, US) was used for data analysis.
Results
Baseline Characteristics
Sixty-eight enrollees of the MILLION study had the baseline and follow-up IVUS data (mean age, 62.9 years; female, 21.2%). Diabetes and acute coronary syndrome were diagnosed in 22 (32.4%) and 43 (63.2%) of these patients, respectively, and plaque regression was observed in 52 (76%). Previous lipid-lowering therapy was administered in 15.4% of patients. There were no significant differences in baseline characteristics in the 4 groups stratified by the baseline hs-CRP level quartile (Table 1).
Table 1. Baseline characteristics in 68 patients receiving atorvastatin and amlodipine.
| Baseline hs-CRP | Baseline hs-CRP | Baseline hs-CRP | Baseline hs-CRP | ||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-value | |
| (n = 17) | (n = 17) | (n = 17) | (n = 17) | ||
| Age (y) | 65.2 ± 9.9 | 62.2 ± 13.9 | 61.9 ± 12.0 | 67.4 ± 8.7 | 0.44 |
| Male (%) | 82.4 | 76.5 | 88.2 | 76.5 | 0.79 |
| BMI | 24.0 ± 2.5 | 24.7 ± 3.4 | 26.4 ± 3.6 | 23.7 ± 3.4 | 0.08 |
| Diabetes mellitus (%) | 35.3 | 35.3 | 29.4 | 29.4 | 0.96 |
| Current smoker (%) | 47.1 | 41.2 | 47.1 | 52.9 | 0.95 |
| ACS (%) | 70.6 | 47.1 | 58.8 | 76.5 | 0.29 |
| Prior lipid therapy (%) | 11.8 | 5.9 | 17.6 | 35.3 | 0.13 |
| Prior blood pressure therapy (%) | 52.9 | 70.6 | 70.6 | 64.7 | 0.67 |
| Concomitant drugs | |||||
| Aspirin | 100 | 100 | 100 | 100 | 0.99 |
| Clopidogrel | 52.9 | 70.6 | 70.6 | 94.1 | 0.06 |
| ACEI or ARB | 41.2 | 52.9 | 41.2 | 41.2 | 0.87 |
| Beta-blocker | 5.9 | 11.8 | 11.8 | 17.6 | 0.77 |
| Warfarin | 0 | 5.9 | 5.9 | 5.9 | 0.79 |
Values are mean ± standard deviation or number (%).
ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein
Lipid and BP Control
In all cohorts, patients achieved very low LDL-C levels (69.1 ± 17.5 mg/dL) and BP (118.6 ± 13.2/70.8 ± 11.9 mmHg) following 18–24 months of atorvastatin and amlodipine therapy. There were no significant differences in LDL-C, high-density lipoprotein cholesterol (HDL-C), triglyceride, systolic pressure, or diastolic pressure at baseline or follow-up between the 4 groups (Table 2). Moreover, there were no significant between-group differences in the extent of change in LDL-C, HDL-C, triglycerides, systolic pressure, or diastolic pressure after treatment (Table 2).
Table 2. Risk factor measurements at baseline and follow-up in 68 patients receiving atorvastatin and amlodipine.
| Baseline hs-CRP | Baseline hs-CRP | Baseline hs-CRP | Baseline hs-CRP | ||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-value | |
| (n = 17) | (n = 17) | (n = 17) | (n = 17) | ||
| LDL-C | |||||
| Baseline, mg/dL | 113.7 ± 27.5 | 126.5 ± 28.2 | 118.6 ± 15.5 | 115.7 ± 24.5 | 0.45 |
| Follow-up, mg/dL | 70.9 ± 18.4 | 65.5 ± 16.5 | 70.1 ± 19.8 | 69.9 ± 15.4 | 0.80 |
| Delta, mg/dL | −46.9 ± 28.8 | −61.0 ± 26.3 | −48.5 ± 18.6 | −45.8 ± 21.0 | 0.23 |
| HDL-C | |||||
| Baseline, mg/dL | 46.2 ± 14.5 | 45.2 ± 10.4 | 43.2 ± 10.5 | 46.8 ± 11.2 | 0.82 |
| Follow-up, mg/dL | 48.2 ± 13.3 | 42.9 ± 6.4 | 43.6 ± 11.3 | 48.6 ± 12.2 | 0.30 |
| Delta, mg/dL | 2.1 ± 7.4 | −2.3 ± 10.7 | 0.4 ± 11.2 | 1.9 ± 10.5 | 0.57 |
| Triglyceride | |||||
| Baseline, mg/dL | 137.0 (97.0; 181.5) | 122.0 (95.0; 138.3) | 138.0 (106.0; 194.8) | 116.0 (83.8; 155.3) | 0.41 |
| Follow-up, mg/dL | 94.0 (75.5; 122.0) | 84.0 (56.0; 99.3) | 116.0 (66.3; 154.0) | 138.0 (68.8; 153.5) | 0.40 |
| Delta, mg/dL | −43.0 (−69.5; −8.3) | −42.0 (−75.8; 3.8) | −23.0 (−41.3; 7.0) | −14.0 (−54.8; 32.5) | 0.39 |
| Systolic blood pressure | |||||
| Baseline, mmHg | 137.9 ± 21.9 | 138.5 ± 23.3 | 139.4 ± 17.2 | 134.6 ± 26.4 | 0.93 |
| Follow-up, mmHg | 114.4 ± 12.4 | 120.6 ± 10.9 | 120.4 ± 15.5 | 119.1 ± 14.1 | 0.50 |
| Delta, mg/dL | −23.5 ± 19.1 | −17.9 ± 25.2 | −26.1 ± 35.5 | −15.5 ± 30.5 | 0.67 |
| Diastolic blood pressure | |||||
| Baseline, mmHg | 78.8 ± 11.2 | 82.2 ± 13.0 | 84.2 ± 11.2 | 77.5 ± 18.4 | 0.47 |
| Follow-up, mmHg | 70.5 ± 10.2 | 69.3 ± 12.7 | 71.9 ± 10.9 | 71.5 ± 13.6 | 0.92 |
| Delta, mg/dL | −8.3 ± 16.2 | −12.9 ± 17.7 | −16.5 ± 22.9 | −6.1 ± 23.8 | 0.90 |
| hs-CRP | |||||
| Baseline, mg/L | 0.52 (0.37; 0.61) | 1.16 (0.87; 1.37) | 2.98 (2.58; 4.60) | 15.10 (10.92; 21.63) | < 0.0001 |
| Follow-up, mg/L | 0.19 (0.09; 0.26) | 0.37 (0.23; 0.65) | 0.77 (0.36; 2.17) | 0.42 (0.35; 0.92) | 0.0011 |
| Delta, mg/L | −0.32 (−0.42; −0.18) | −0.63 (−1.04; −0.36) | −2.33 (−3.82; −1.82) | −13.45 (−17.63; −9.46) | < 0.0001 |
Continuous variables are reported as the mean ± standard deviation if normally distributed and median (interquartile range) if not normally distributed. HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
Intravascular Ultrasound Parameters
There were no significant differences in the vessel volume normalized, lumen volume normalized, or plaque volume normalized across the baseline hs-CRP level quartiles. (Table 3). Furthermore, there were no significant differences in the percentage change in the vessel volume normalized, lumen volume normalized, or plaque volume normalized across the baseline hs-CRP level quartiles. Percentage change in the plaque volume rose progressively with increasing the baseline hs-CRP (p < 0.05 for trend) (Table 3, Fig. 1). In the analysis of covariant with plaque volume at baseline included as a confounding variable, the percentage changes in the vessel volume normalized, lumen volume normalized, or plaque volume normalized were not significantly different among the baseline hs-CRP level quartiles. Also, there was no interaction between the baseline hs-CRP level and the plaque volume at baseline (Table 3).
Table 3. Intravascular ultrasound analysis at baseline and follow-up in 68 patients receiving atorvastatin and amlodipine.
| Baseline hs-CRP | Baseline hs-CRP | Baseline hs-CRP | Baseline hs-CRP | p-value for trend | p-value§ | p for interaction† | |
|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||
| (n = 17) | (n = 17) | (n = 17) | (n = 17) | ||||
| Vessel volume normalized | |||||||
| Baseline, mm3 | 111.8 ± 60.0 | 128.9 ± 70.3 | 183.9 ± 156.4 | 142.0 ± 116.7 | 0.46 | < 0.0001 | 0.0015 |
| Follow-up, mm3 | 109.1 ± 61.5 | 120.7 ± 65.1 | 169.7 ± 128.8 | 142.6 ± 115.4 | 0.23 | < 0.0001 | < 0.0001 |
| Percentage change | −2.0 ± 14.0 | −5.5 ± 8.4 | −0.3 ± 19.3 | −1.3 ± 9.4 | 0.15 | 0.32 | 0.73 |
| Lumen volume normalized | |||||||
| Baseline, mm3 | 57.5 ± 28.2 | 67.4 ± 45.1 | 89.6 ± 64.5 | 73.6 ± 59.7 | 0.48 | < 0.0001 | 0.0015 |
| Follow-up, mm3 | 61.9 ± 32.1 | 65.2 ± 41.3 | 87.1 ± 53.1 | 76.7 ± 61.3 | 0.34 | < 0.0001 | 0.0002 |
| Percentage change | 8.9 ± 25.8 | 0.5 ± 21.1 | 9.0 ± 34.6 | 7.5 ± 19.3 | 0.78 | 0.51 | 0.95 |
| Plaque volume normalized | |||||||
| Baseline, mm3 | 54.2 ± 34.2 | 61.5 ± 28.3 | 94.4 ± 99.8 | 68.5 ± 59.0 | 0.49 | – | – |
| Follow-up, mm3 | 47.2 ± 32.8 | 55.5 ± 26.3 | 82.6 ± 80.4 | 65.9 ± 58.9 | 0.24 | < 0.0001 | 0.0018 |
| Percentage change | −12.9 ± 12.5 | −9.4 ± 10.5 | −8.7 ± 12.6 | −5.2 ± 11.5 | 0.0466 | 0.73 | 0.69 |
Values are mean ± standard deviation.
Analysis of covariance.
Interaction between the baseline hs-CRP and the plaque volume at baseline. hs-CRP, high-sensitivity C-reactive protein.
Fig. 1.
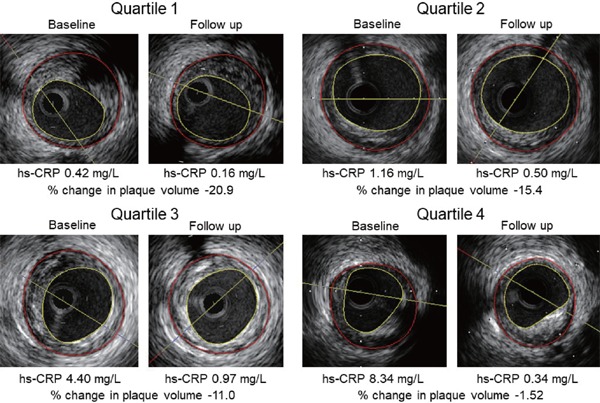
Representative intravascular ultrasound images of coronary plaque regression after lipid- and blood pressure-lowering therapy across the baseline high-sensitivity C-reactive protein (hs-CRP) level quartiles. The percentage change in the plaque volume rose progressively with increasing the baseline hs-CRP.
Factors Associated With Percentage Change in Plaque Volume
A multiple linear regression analysis, including age, male sex, and baseline HbA1c and log hs-CRP as covariates confirmed that the baseline log hs-CRP was independently associated with the percentage change in the plaque volume (β = 0.29, p = 0.022) (Table 4). However, the log hs-CRP at the follow-up and delta log hs- CRP were not associated with this percentage change. The baseline LDL-C level and systolic and diastolic BP were not significantly associated with the percentage change in the plaque volume.
Table 4. Factors associated with the percentage change in the plaque volume by the linear regression analysis in 68 patients receiving atorvastatin and amlodipine.
| Univariate Model |
Multivariate Model* |
|||||
|---|---|---|---|---|---|---|
| Standardized β | Standard Error | p-value | Standardized β | Standard Error | p-value | |
| Age | 0.028 | 0.129 | 0.82 | 0.191 | 0.208 | 0.24 |
| Male | −0.004 | 5.384 | 0.98 | 0.004 | 5.061 | 0.98 |
| Current smoking | −0.005 | 2.901 | 0.97 | |||
| Diabetes mellitus | −0.151 | 3.143 | 0.23 | |||
| Acute coronary syndrome | −0.011 | 3.003 | 0.93 | |||
| Body mass index | 0.121 | 0.434 | 0.33 | |||
| HbA1c at baseline | −0.191 | 1.439 | 0.12 | −0.270 | 1.759 | 0.10 |
| HbA1c at follow-up | −0.128 | 1.758 | 0.31 | |||
| Delta LDL-C | 0.100 | 0.060 | 0.42 | |||
| Delta HDL-C | 0.120 | 0.145 | 0.33 | |||
| (Log) delta triglyceride | 0.089 | 0.021 | 0.47 | |||
| Delta systolic BP | 0.074 | 0.052 | 0.55 | |||
| Delta diastolic BP | −0.024 | 0.072 | 0.84 | |||
| eGFR | −0.121 | 0.072 | 0.84 | |||
| (Log) hs-CRP at baseline | 0.247 | 2.119 | 0.0425 | 0.329 | 2.690 | 0.0449 |
| (Log) hs-CRP at follow-up | 0.119 | 3.255 | 0.36 | |||
| (Log) delta hs-CRP | −0.128 | 2.708 | 0.33 | |||
| Plaque volume at baseline | −0.078 | 0.023 | 0.53 | |||
| Vessel volume at baseline | −0.034 | 0.013 | 0.79 | |||
| Lumen volume at baseline | 0.023 | 0.028 | 0.85 | |||
Adjustment for variables with p < 0.2 in a univariate analysis.
BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; (log) hs-CRP, log-transformed high-sensitive C-reactive protein; LDL, low-density lipoprotein.
Factors Associated With Baseline hs-CRP
Univariate and multivariate linear regression analyses were performed to detect factors that influenced the baseline hs-CRP. Multiple linear regression analysis including age, male sex, baseline white blood cell, and creatinine phosphokinase, and clopidogrel usage at baseline as covariates confirmed that the baseline white blood cell and clopidogrel usage at baseline were independently associated with the baseline log hs-CRP (β = 0.37, p = 0.0013, β = 0.25, p = 0.0245, respectively) (Table 5).
Table 5. Factors associated with the baseline (log) hs-CRP by the linear regression analysis in 68 patients receiving atorvastatin and amlodipine.
| Univariate Model |
Multivariate Model* |
|||||
|---|---|---|---|---|---|---|
| Standardized β | Standard Error | p-value | Standardized β | Standard Error | p-value | |
| Age | 0.072 | 0.007 | 0.56 | 0.153 | 0.007 | 0.18 |
| Male | −0.065 | 0.207 | 0.60 | −0.100 | 0.189 | 0.37 |
| Current smoking | 0.004 | 0.163 | 0.72 | |||
| Diabetes mellitus | 0.018 | 0.177 | 0.88 | |||
| Acute coronary syndrome | 0.106 | 0.168 | 0.39 | |||
| Body mass index | −0.005 | 0.025 | 0.97 | |||
| HbA1c at baseline | 0.099 | 0.081 | 0.43 | |||
| Total cholesterol at baseline | −0.113 | 0.002 | 0.36 | |||
| Triglyceride at baseline | −0.110 | 0.001 | 0.37 | |||
| HDL-C at baseline | 0.048 | 0.007 | 0.70 | |||
| LDL-C at baseline | −0.104 | 0.003 | 0.40 | |||
| Systolic BP at baseline | −0.025 | 0.004 | 0.84 | |||
| Diastolic BP at baseline | −0.040 | 0.006 | 0.75 | |||
| eGFR at baseline | −0.016 | 0.004 | 0.90 | |||
| WBC at baseline | 0.408 | 0.00004 | 0.0006 | 0.371 | 0.00004 | 0.0013 |
| CPK at baseline | 0.261 | 0.001 | 0.0314 | 0.196 | 0.001 | 0.08 |
| Plaque volume at baseline | 0.125 | 0.001 | 0.31 | |||
| Vessel volume at baseline | 0.139 | 0.001 | 0.26 | |||
| Lumen volume at baseline | 0.141 | 0.002 | 0.25 | |||
| Clopidogrel usage at baseline | 0.287 | 0.740 | 0.0176 | 0.251 | 0.161 | 0.0249 |
| ACEI or ARB usage at baseline | −0.026 | 0.164 | 0.84 | |||
| Beta-blocker usage at baseline | 0.129 | 0.251 | 0.29 | |||
| Warfarin usage at baseline | 0.146 | 0.393 | 0.24 | |||
Adjustment for variables with p < 0.2 in a univariate analysis.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CPK, creatinine phosphokinase; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, white blood cell.
Discussion
The current analysis investigated the effect of baseline hs-CRP levels on the coronary plaque progression in patients who achieved very low LDL-C levels (on average, 69.1 mg/dL) and BP (on average, 118.6/70.8 mmHg) following 18–24 months of atorvastatin and amlodipine therapy. Our subanalysis of the MILLION study produced 3 main findings in patients receiving potent lipid- and BP-lowering treatment. First, there were no significant differences in the baseline or follow-up LDL-C or systolic and diastolic BP between the 4 groups stratified according to baseline hs-CRP level quartiles. Further, the extent of post-treatment changes in LDL-C and BP showed no significant between-group differences. Second, a linear association was observed between the baseline hs-CRP levels and percentage changes in plaque volume, although changes in LDL-C and systolic and diastolic BP did not correlate with the percentage change in the plaque volume. Third, given these conditions, multiple regression analysis with an adjustment for clinical characteristics and IVUS parameters demonstrated that the baseline hs-CRP level was independently associated with the percentage change in the plaque volume.
Previous trials using IVUS to study the effect of statins on coronary atherosclerosis demonstrated a linear relationship between post-treatment LDL-C levels and the reduction in atheroma burden14–17). On the other hand, although the mean on-treatment LDL-C levels in the Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin (SATURN) were among the lowest achieved in any previously conducted imaging study of statin effects on atherosclerosis, 1 in 3 patients still experienced disease progression14). Similarly, the Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV) trial demonstrated that the mean on-treatment LDL-C level was 36.6 mg/dL after the addition of the PCSK9 inhibitor evolocumab in patients treated with statin therapy, resulting in a favorable effect on coronary atherosclerosis progression measured by IVUS. However, as in the SATURN study, one-third of the patients still experienced disease progression despite achieving very low LDL-C levels18). The results of these trials suggest that other factors, such as inflammation, contributed to disease progression, independently of the cholesterol level in up to one-third of treated patients.
The main findings of our study confirm that hs-CRP might be associated with atherosclerosis progression, independent of the LDL-C level. Further, statin- and amlodipine-treated patients with an inflammation risk, as assessed by the baseline hs-CRP, may have an associated risk of future plaque progression. Recently, in the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS), canakinumab anti-inflammatory therapy targeting the interleukin-1 β innate immunity pathway that reduces CRP and interleukin-6, led to a significantly lower rate of recurrent cardiovascular events than did placebo, independently of lipid-lowering treatment19). This result demonstrated that reducing vascular inflammation reduces the rate of cardiovascular events even in the absence of concomitant lipid-lowering therapy and may support our findings paradoxically.
We found that the baseline hs-CRP level was associated with the percentage change in the plaque volume, but changes in LDL-C and systolic and diastolic BP were not. Our results might have been affected by some of the conditions in the MILLION study. Approximately 15% and 65% of participating patients received lipid- and BP-lowering therapy before enrollment, respectively, resulting in a relatively low cholesterol level and BP even at baseline. Moreover, the percentage changes in LDL-C and BP in the MILLION study were relatively low when compared to those of previous placebo-controlled studies10, 20, 21) because, for ethical reasons, a placebo arm of patients who were not receiving lipid-lowering therapy could not be included. Given these conditions, although the LDL-C concentration and BP have been established as causative risk factors in coronary disease, the effects of a lower LDL-C level and BP on plaque regression might be attenuated causing the impact of inflammatory markers such as hs-CRP on plaque regression to be relatively exaggerated in the current study. Moreover, previous studies suggested that the best clinical outcomes were observed in patients who reached both an LDL-C less than 70 mg/dL and an hs-CRP less than 2.0 mg/L7, 22). The JUPITER trial suggested that the greatest reduction in cardiovascular events was in the treatment group that achieved both LDL-C less than 70 mg/dL and hs-CRP less than 2 mg/L (65% reduction), compared with only a 33% risk reduction in patients that achieved one or neither target23). Furthermore, the CANTOS study confirmed that statin-treated patients with a residual inflammatory risk, as assessed by means of a hs-CRP > 2 mg/L at baseline, had future event rates that were at least as high as those among statin-treated patients with a residual risk due to the LDL-C level19). Amano T, et al. reported that the lipid-rich plaque assessed by the Virtual Histology IVUS and hs-CRP level > 2 mg/L at baseline could predict a coronary event during the follow-up period24). From these results, patients with base- line hs-CRP levels above 2 mg/L were considered as the threshold indicating the presence of an inflammatory condition. In our study, 50% of all patients did not achieve an LDL-C less than 70 mg/dL at the follow-up; however, 91% of all patients achieved an hs-CRP less than 2.0 mg/L at the follow-up, suggesting that our cohort still had residual cholesterol risk. Under these conditions, the baseline hs-CRP level might have greater contribution to the percentage change in the plaque volume as compared with the effects of lower LDL-C level in this study. On the contrary, in some clinical trials including only patients with an hs-CRP below 2 mg/L, any type of LDL-C-lowering treatment did not change the CRP levels in spite of a substantial LDL-C reduction18, 25). These results suggested that the beneficial effect of LDL-C lowering therapies on systemic inflammatory status, as monitored by changes in hs-CRP levels, was evident only in patients presenting with increased inflammatory conditions. In our study, the frequency of hs-CRP less than 2.0 mg/L at baseline was 51.5% of all cohort, those who had only a small change of hs-CRP in spite of statin therapy, indicating that these patients did not present a relevant systemic inflammation. Therefore, the change in the hs-CRP level was not a factor that influenced percentage changes in the plaque volume.
Study Limitations
The present study has several limitations. First, it was a retrospective study based on a relatively limited sample size, raising the possibility of selection bias. Even under these conditions, however, we found that the baseline hs-CRP may be independently associated with plaque regression and progression. Second, as designed, our group comparison did not benefit from the original study's randomization; thus, the results could be subject to confounding and should be viewed as associative rather than causal. Further prospective studies with a larger number of patients are necessary to more fully evaluate the impact of baseline hs-CRP on coronary plaque regression. Lastly, other unmeasured variables could have influenced systemic levels of inflammation, raising the possibility of confounding bias.
Conclusion
Our results demonstrate that in patients simultaneously treated with lipid- and BP-lowering therapies, plaque regression and progression were associated with the baseline hs-CRP level. Inflammation may be an important factor that drives the progression of coronary atherosclerosis despite an aggressive lipid- and BPlowering therapy.
Acknowledgments
This work was partly supported by a research grant from Pfizer Ltd.
Conflicts of Interest
Yamagishi received research grant funding from Pfizer Ltd. All other authors have nothing to disclose.
Appendix
The following are the major participants in the MILLION Study Group: Masakazu Yamagishi, Masaaki Kawashiri, Hidekazu Ino, Noboru Fujino, Atsushi Nohara, Katsuharu Uchiyama, Kenshi Hayashi, Kenji Sakata, Tetsuo Konno, Eiichi Masuta, Toshinari Tsubokawa, Hayato Tada, Akihiko Muramoto-Hodatsu, Chiaki Nakanishi, Mika Mori, Syu Takabatake, Toyonobu Tsuda, Ryusuke Yamamoto, Tadatsugu Gamou, Masaya Shimojima, Shohei Yoshida, Division of Cardiology, Kanazawa University Hospital; Honin Kanaya, Takao Matsubara, Toshihiko Yasuda, Kenji Miwa, Masaru Inoue, Ryota Teramoto, Hirofumi Okada, Yohei Yakuta, Takao Matsui, Department of Cardiology, Ishikawa Prefectural Central Hospital; Kosei Ueda, Toshinori Higashikata, Tomoya Kaneda, Mutsuko Takata, Takeshi Inoue, Miho Ohira, Department of Internal Medicine, Komatsu Municipal Hospital; Sumio Mizuno, Kazuo Ohsato, Tatsuaki Murakami, Katsushi Misawa, Hiromasa Kokado, Kensuke Fujioka, Junichiro Yokawa, Department of Cardiology, Fukui Cardiovascular Center; Ichiro Michishita, Taku Iwaki, Tsuyoshi Nozue, Hiromasa Kato, Division of Cardiology, Department of Internal Medicine, Yokohama Sakae Kyosai Hospital; Masanobu Namura, Masatoshi Ikeda, Yuki Horita, Taketsugu Tsuchiya, Hidenobu Terai, Department of Cardiology, Kanazawa Cardiovascular Hospital; Yutaka Nitta, Tomio Taguchi, Bunji Kaku, Shoji Katsuda, Taiji Yoshida, Chikara Fujita, Tohru Aburao, Department of Cardiology, Toyama Red Cross Hospital; Kazuyasu Okeie, Masaru Kiyama, Masayuki Tsuchida, Munenori Ohta, Nobuo Ukawa, Department of Cardiology, Koseiren Takaoka Hospital: Hiroaki Hirase, Tatsuo Haraki, Tomoharu Yoshikawa, Masafumi Hashimoto, Department of Internal Medicine, Takaoka Municipal Hospital.
References
- 1). Bayturan O, Kapadia S, Nicholls SJ, Tuzcu EM, Shao M, Uno K, Shreevatsa A, Lavoie AJ, Wolski K, Schoenhagen P, Nissen SE. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol, J Am Coll Cardiol. 2010; 55: 2736-2742 [DOI] [PubMed] [Google Scholar]
- 2). Ross R. Atherosclerosis-an inflammatory disease, N Engl J Med. 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 3). Libby P. Inflammation in atherosclerosis, Arterioscler Thromb Vasc Biol. 2012; 32: 2045-2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Björkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB, Sr, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engström G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jørgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012; 367: 1310-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Ridker PM, Kastelein JJ, Genest J, Koenig W. C-reactive protein and cholesterol are equally strong predictors of cardiovascular risk and both are important for quality clinical care, Eur Heart J. 2013; 34: 1258-1261 [DOI] [PubMed] [Google Scholar]
- 6). Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O'Shaughnessy C, Ganz P, Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) Investigators Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease, N Engl J Med. 2005; 352: 29-38 [DOI] [PubMed] [Google Scholar]
- 7). Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E, Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators , C-reactive protein levels and outcomes after statin therapy, N Engl J Med. 2005; 352: 20-28 [DOI] [PubMed] [Google Scholar]
- 8). Razzouk L, Muntner P, Bansilal S, Kini AS, Aneja A, Mozes J, Ivan O, Jakkula M, Sharma S, Farkouh ME. C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention, Am Heart J. 2009; 158: 277-283 [DOI] [PubMed] [Google Scholar]
- 9). Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J, ASCOT Investigators , Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOTBPLA): a multicentre randomised controlled trial, Lancet. 2005; 366: 895-906 [DOI] [PubMed] [Google Scholar]
- 10). Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, Berman L, Shi H, Buebendorf E, Topol EJ, CAMELOT Investigators , Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial, JAMA. 2004; 292; 2217-2225 [DOI] [PubMed] [Google Scholar]
- 11). Kawashiri MA, Sakata K, Hayashi K, Gamou T, Kanaya H, Miwa K, Ueda K, Higashikata T, Mizuno S, Michishita I, Namura M, Nitta Y, Katsuda S, Okeie K, Hirase H, Tada H, Uchiyama K, Konno T, Ino H, Nagase K, Yamagishi M, Behalf of the MILLION Study Group , Impact of combined lipid lowering and blood pressure control on coronary plaque: myocardial ischemia treated by percutaneous coronary intervention and plaque regression by lipid lowering and blood pressure controlling assessed by intravascular ultrasonography (MILLION) study, Heart Vessels. 2017; 32: 539-548 [DOI] [PubMed] [Google Scholar]
- 12). Kawashiri MA, Sakata K, Gamou T, Kanaya H, Miwa K, Ueda K, Higashikata T, Mizuno S, Michishita I, Namura M, Nitta Y, Katsuda S, Okeie K, Hirase H, Tada H, Uchiyama K, Konno T, Hayashi K, Ino H, Nagase K, Terashima M, Yamagishi M. Impact of combined lipid lowering with blood pressure control on coronary plaque regression: rationale and design of MILLION study, Heart Vessels. 2015; 30: 580-586 [DOI] [PubMed] [Google Scholar]
- 13). Gamou T, Sakata K, Tada H, Konno T, Hayashi K, Ino H, Yamagishi M, Kawashiri MA, MILLION Study Group , Effect of Reverse Vessel Remodeling on Regression of Coronary Atherosclerosis in Patients Treated With Aggressive Lipid- and Blood Pressure-Lowering Therapy - Insight From MILLION Study, Circ J. 2017; 81: 1490-1495 [DOI] [PubMed] [Google Scholar]
- 14). Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, Nissen SE. Effect of two intensive statin regimens on progression of coronary disease, N Engl J Med. 2011; 365: 2078-2087 [DOI] [PubMed] [Google Scholar]
- 15). Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis, JAMA. 2007; 297: 499-508 [DOI] [PubMed] [Google Scholar]
- 16). Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM, ASTEROID Investigators , Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial, JAMA. 2006; 295: 1556-1565 [DOI] [PubMed] [Google Scholar]
- 17). Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN, REVERSAL Investigators , Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial, JAMA. 2004; 291: 1071-1080 [DOI] [PubMed] [Google Scholar]
- 18). Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial, JAMA. 2016; 316: 2373-2384 [DOI] [PubMed] [Google Scholar]
- 19). Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group , Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease, N Engl J Med. 2017; 377: 1119-1131 [DOI] [PubMed] [Google Scholar]
- 20). Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, Daida H. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study, Circulation. 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 21). Takashima H, Ozaki Y, Yasukawa T, Waseda K, Asai K, Wakita Y, Kuroda Y, Kosaka T, Kuhara Y, Ito T. Impact of lipid-lowering therapy with pitavastatin, a new HMG-CoA reductase inhibitor, on regression of coronary atherosclerotic plaque, Circ J. 2007; 71: 1678-1684 [DOI] [PubMed] [Google Scholar]
- 22). Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, Rifai N, Califf RM, Braunwald E. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial, Circulation. 2006; 114: 281-288 [DOI] [PubMed] [Google Scholar]
- 23). Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy, N Engl J Med. 2005; 352: 20-28 [DOI] [PubMed] [Google Scholar]
- 24). Amano T, Matsubara T, Uetani T, Kato M, Kato B, Yoshida T, Harada K, Kumagai S, Kunimura A, Shinbo Y, Ishii H, Murohara T. Lipid-rich plaques predict non-target-lesion ischemic events in patients undergoing percutaneous coronary intervention, Circ J. 2011; 75: 157-166 [DOI] [PubMed] [Google Scholar]
- 25). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, FOURIER Steering Committee and Investigators , Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease, N Engl J Med. 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]


