Abstract
Aflatoxins are secondary metabolites of certain Aspergillus species, that contaminate staple foods, particularly in developing countries. Aflatoxin B1 (AFB1) is the most toxic and common of the major types of aflatoxins. AFB1 is hepatotoxic and has been implicated in increasing the risk of hepatocellular carcinoma (HCC). We have previously shown that subacute exposure to AFB1 for 7 days disrupts hepatic lipids; therefore, this study determined the time-course effects of acute aflatoxin exposure on hepatic mitochondrial lipids and oxidative stress. To achieve this, thirty male albino rats were randomly assigned to six groups. The groups received an oral dose of 1 mg/kg body weight AFB1 or vehicle only (controls) for one, four, or seven days, respectively. Twenty-four hours after the last dose, the animals were sacrificed and liver excised. Mitochondria and cytosolic fractions were obtained from the liver after which lipids (cholesterol, triacylglycerols) were determined in the mitochondria while biomarkers of oxidative stress (glutathione, glutathione transferase (GST), glutathione peroxidase (GPx), glutathione reductase, nitric oxide (NO), malonaldehyde (MDA), thioredoxin reductase (TR), and superoxide dismutase (SOD) were determined spectrophotometrically in the mitochondria and cytosolic fractions. The expression of genes (Nrf2, Acc, Nqo1, and HmgCoa) were determined using quantitative RT-PCR. Results showed that AFB1 significantly increased mitochondrial cholesterol at day seven (treatment vs. control, p = 0.016). It also increased the concentrations of NO and MDA at day one and day seven while the activity of GPx and concentration of GSH were increased at day seven (p = 0.030) and day one (p = 0.025) alone, respectively, compared to control. The activities of cytosolic GR (p = 0.014), TR (p = 0.046) and GST (p = 0.044) were increased at day seven. AFB1 significantly increased the expression of Nrf2 (p = 0.029) and decreased the expression of Acc (p = 0.005) at day one. This study revealed that AFB1 disrupts hepatic mitochondrial lipids and antioxidant capacity. These changes were dependent on the timing of exposure and did not follow a linear time-course trend. These alterations could be part of the hepatic mitochondria response mechanism to acute AFB1 toxicity.
Keywords: aflatoxin B1, liver, mitochondria, lipid, cholesterol, thioredoxin reductase, oxidative stress
Introduction
Aflatoxins are compounds that are produced as secondary metabolites of fungi belonging to the Aspergillus species particularly Aspergillus flavus, parasiticus, and nominus (Mutegi et al., 2018). These fungi thrive well in the Tropical climate where they grow on foodstuffs from farm to table (Gong and Watson, 2016; Mutegi et al., 2018). Hence, aflatoxins contaminate food and feed stuff especially cereals and nuts in sub-Saharan Africa and southeast Asia (Wild and Gong, 2009; Gong and Watson, 2016; Opoku et al., 2018; Rushing and Selim, 2018). There are about 18 types of aflatoxins; however, the major ones are AFB1, AFB2, AFG1, AFG2, AFM1, and AFM2 (Luo et al., 2018). Of these, AFB1 is the most toxic and prevalent and has been named a class 1 carcinogen by the International Agency for Research on Cancer (IARC) (Ostry et al., 2017). AFB1 has been shown to affect several organs and tissues; however, the liver is its target organ, where it has been shown to cause hepatocellular carcinoma (HCC) after chronic exposure (Wogan et al., 2012; Rushing and Selim, 2018). It is also mutagenic, teratogenic, immunotoxic (Bondy and Pestka, 2000; Rushing and Selim, 2018), impairs growth in children (Khlangwiset et al., 2011; Watson et al., 2018) and affects reproductive health (Eze et al., 2018).
AFB1 is usually converted by the microsomal CYP450 family of enzymes to a more toxic epoxide, AFB1-8,9-epoxide (AFBO) (Deng et al., 2018). This highly reactive epoxide is responsible for the majority of the tissue toxicity of AFB1 because it binds to macromolecules such as DNA and proteins (Rushing and Selim, 2018). To be fully detoxified, the epoxide crosses the mitochondria membrane where it goes through phase II metabolism by the detoxification enzymes such as glutathione transferases (GSTs) (Deng et al., 2018). Apart from its detoxification functions, the mitochondrion is also involved in other biological processes such as the oxidation process by the electron transport chain and production of reactive oxygen species (Bhat et al., 2015; Wang et al., 2017). Hence, the critical roles that mitochondria play in the metabolism and detoxification of aflatoxin make it important in the pathogenesis of aflatoxicosis and therefore requires detailed investigation. Previous studies have shown that AFB1 impairs mitochondria function such as mitochondrial permeability (Liu and Wang, 2016; Wang et al., 2017). The permeability of the membrane is due in part to membrane transporters and its lipid composition (Van Meer et al., 2008). We and others have previously shown that AFB1 disrupts lipid metabolism and induces oxidative stress (Lu et al., 2013; El-Nekeety et al., 2014; Rotimi et al., 2016, 2017, 2018; Zhang et al., 2016). The balance of homeostasis within these two biochemical processes is important to the optimal function of mitochondria.
Although acute aflatoxicosis occurs only occasionally when compared with chronic exposure, such acute exposures, which often lead to death, have been reported in Kenya and Tanzania (Ngindu et al., 1982; Azziz-Baumgartner et al., 2005; Lewis et al., 2005; Kamala et al., 2018). Hence, it is important to understand the time-course events associated with acute exposure to AFB1. Due to the essential role of mitochondria in the detoxification of AFB1 and pathogenesis of aflatoxicosis, we hypothesize that mitochondria oxidative stress and dyslipidemia will be associated with it in a time-dependent manner. The aim of this study, therefore, was to investigate the time course effects of subacute AFB1 hepatotoxicity in albino Wistar rats. We achieve this by studying the changes in liver function, mitochondrial lipids and oxidative stress as well as the expression of genes associated with lipid metabolism and oxidative stress.
Materials and Methods
Chemicals
AFB1 and other chemicals including mannitol, sucrose, HEPES, succinate, ethylenediaminetetraacetic acid (EDTA) used in this study were products of Sigma–Aldrich (St. Louis, MO, United States). The RNA extraction kit was a product of Aidlab® Biotechnologies Co., Ltd., (Beijing, China).
Animals
Thirty male inbred albino Wistar rats weighing between 100–120 g were used for this study. They were housed at room temperature and allowed access to food and water ad libitum. The animals were acclimatized to the experimental condition for 2 weeks before the commencement of exposure. The experiment was conducted, and the animals cared for according to the institutional animal care and use committee guidebook and as approved by the Covenant University Ethical committee.
Experimental Design
The animals were randomly divided into six groups of five animals each, and they received an oral gavage dose of AFB1 (1 mg/kg body weight) in olive oil or olive oil only (controls) once daily for one, four, or seven days as shown below;
AFB1 day 1: 1 mg/kg AFB1 for one day.
Control day 1: Olive oil for one day.
AFB1 day 4: 1 mg/kg AFB1 for four days.
Control day 4: Olive oil for four days.
AFB1 day 7: 1 mg/kg AFB1 for seven days.
Control day 7: Olive oil for seven days.
The animals were sacrificed 24 h after the last dose and blood was collected by cardiac puncture into heparinized tubes. A portion of the liver was kept in RNAlater® (Qiagen) for RNA extraction while the remaining part was immediately transferred into the mitochondria isolation buffer. The blood was centrifuged at 3000 rpm for 15 min to obtain the plasma.
Preparation of Liver Mitochondria
Liver mitochondria were isolated as described by Gogvadze et al. (2004). The liver was removed immediately after sacrifice and rinsed in freshly prepared ice-cold (4°C) homogenizing buffer (210 mM mannitol, 70 mM sucrose, 5 mM HEPES, and 1 mM EDTA pH 7.5) to remove blood. A 10% homogenate was then prepared after which the homogenate was centrifuged (600 × g, 8 min, 4°C) to remove nuclei and unbroken cells. The resulting supernatant was centrifuged (5500 × g, 15 min, 4°C) to form the mitochondria pellet while the supernatant represented the cytosolic fraction. The pellet was suspended in about 0.3 ml of the buffer and then diluted with the homogenizing buffer (excluding 1 mM EDTA) after which it was centrifuged again (5500 × g, 15 min, 4°C). The final mitochondria pellet was suspended in about 1 ml of the same buffer and protein concentration was determined according to the method of Lowry et al. (1951). The resulting mitochondrial and cytosolic fractions were used for further analysis.
Biochemical Analysis
Biomarkers of Liver Function
Alkaline phosphatase (ALP), alanine transaminase (AST), and bilirubin were determined spectrophotometrically using Randox kits according to manufacturer’s instructions.
Mitochondrial Cholesterol and Triacylglycerol
Lipid was extracted from the liver mitochondria according to the method of Folch et al. (1957). The extract obtained was used for determining triacylglycerol and cholesterol spectrophotometrically, using commercially available kits, as described by Rotimi et al. (2012).
Mitochondrial and Cytosolic Oxidant/Antioxidant Status
Malondialdehyde (MDA) concentration was determined using the method of Buege and Aust (1978) by measuring the concentration of thiobarbituric acid reactive substances (TBARS). Nitric oxide (NO) concentration was determined using a method described by Yucel et al. (2012). Glutathione (GSH) concentration was determined using the method described by Ellman (1959), while glutathione peroxidase (GPx) activity was determined using the method of Rotruck et al. (1973). Glutathione S-transferase (GST) activity was determined according to the method of Habig et al. (1974) while superoxide dismutase (SOD) activity was determined using the pyrogallol autoxidation method by Marklund and Marklund (1974). Glutathione reductase (GR) was assayed using oxidized glutathione (GSSG) in the method described by Mavis and Stellwagen (1968) while thioredoxin reductase (TR) was determined as described by Holmgren and Bjornstedt (1995).
Mitochondrial ATPase, NADH-Cytochrome C Reductase and Succinate Dehydrogenase
Mitochondrial total ATPase was determined using the Taussky and Shorr, 1953 method. Mitochondrial sample equivalent to 50 μg protein was added to a reaction mixture of 2 M NaCl solution and 24 mM Tris–HCl Buffer containing 0.68 mM EDTA and 6.0 mM MgCl2. The mixture was centrifuged and the supernatant obtained was added to Taussky-Shorr reagent. The mixture which was incubated at 25°C for 5 min after which absorbance was taken at 660 nm.
Succinate dehydrogenase assay was carried out using the method described by Reisch and Elpeleg (2007). Mitochondrial sample equivalent to 10 μg protein was preincubated in 0.1 M phosphate buffer (pH 7.4) with 0.2 M succinate for 20 min at 25°C. After incubation, 0.2 M potassium cyanide, freshly prepared 5 mM 2,6-Dichlorophenolindophenol and 65 mM phenzine methosulfate were added and absorbance read at 660 nm.
NADH – Cytochrome C reductase (complex I-III) was determined as described by Navarro et al. (2004). Mitochondria sample equivalent to 50 μg protein was added to 0.1 M phosphate buffer (pH 7.4) containing 0.2 mM NADH and 1 mM KCN. The reaction was then initiated by the addition of 0.1 mM cytochrome C and absorbance was measured every minute for 3 min at 550 nm.
Extraction of RNA and Real-Time Quantitative Polymerase Reaction(RT-qPCR)
RNA was extracted from the liver as previously described by Rotimi et al. (2017) after which the concentration and purity were checked using a NanoDrop spectrophotometer. 0.5 μg of RNA was reverse transcribed for complementary DNA (cDNA) synthesis using Bio Rad iScript cDNA. The cDNA was diluted 1:10 after which the qPCR was performed on the Bio Rad CFX96 Real-time PCR detection system using iQ SYBR Green Supermix kit and gene-specific primers for Nrf2, Acc, Hmgcoa, and Nqo1 genes (Table 1). The samples were analyzed in triplicates and two housekeeping genes (β-actin and Gapdh) and an internal control were used. The relative expression of the genes was calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
Sequence of gene specific primers for RT-qPCR.
| Gene | Sequence (5′–3′) | References |
|---|---|---|
| Nrf2 | Forward: TAGATGACCATGAGTCGCTT | Sun et al., 2017 |
| Reverse: CTGTAACTCGGGAATGGAAA | ||
| Hmgcr | Forward: CAACCTTCTACCTCAGCAAGC | Su et al., 2016 |
| Reverse: ACAGTGCCACACACAATTCG | ||
| Acc | Forward: TGAGGAGGACCGCATTTATC | Harasiuk et al., 2016 |
| Reverse: CCACAGCAATGGCAGGACTA | ||
| Nqo1 | Forward: GAAGCTTCCTTCGTGACCAG | Sun et al., 2017 |
| Reverse: GGGGGTTAAAGTTCATAGCA | ||
| Gapdh | Forward: GGCAAGTTCAATGGCACAGT | Suzuki et al., 2016 |
| Reverse: TGGTGAAGACGCCAGTAGACTC | ||
| β-Actin | Forward: AGCGTGGCTACAGCTTCACC | Julian et al., 2014 |
| Reverse: AAGTCTAGGGCAACATAGCACAGC | ||
Statistical Analysis
Results were analyzed using SPSS. Two-tailed T-test was used to determine the difference between the control and treatment group at each time point. ANOVA was used to determine the difference between all the groups with post hoc pairwise comparisons by Duncan’s multiple range test.
Results
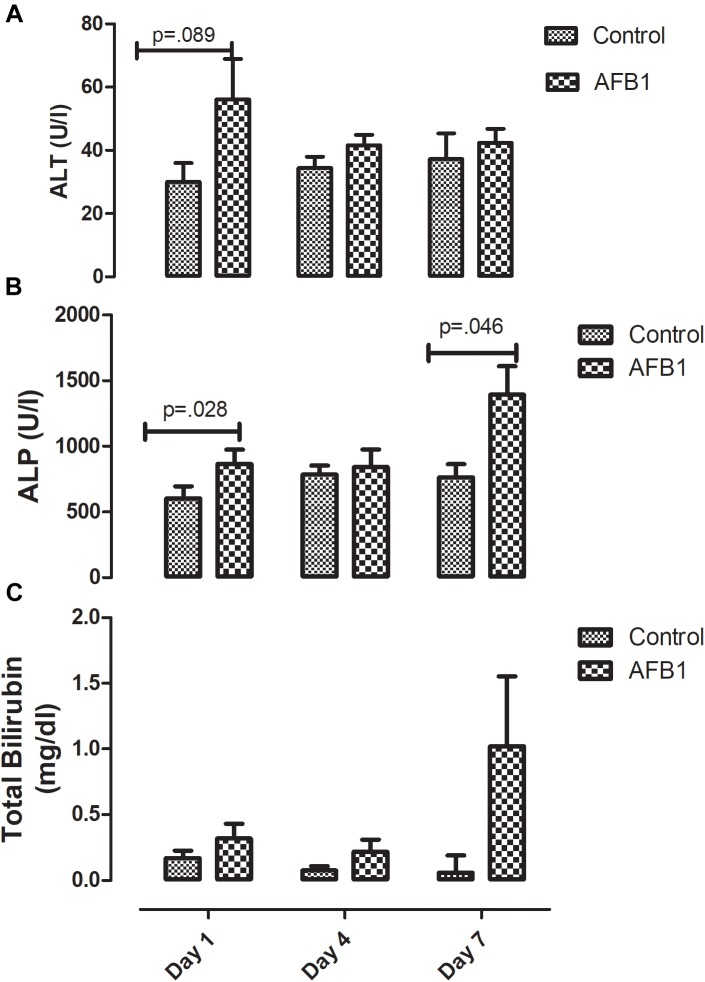
Time course effects of subacute AFB1 exposure significantly increased plasma ALP (Figure 1B) at day 1 (control vs. treatment) (p = 0.028) and day 7 (p = 0.046) while there was no significant difference in ALT (Figure 1A) between the control and treatment groups at all time points. Although there was no statistically significant difference in plasma bilirubin (control vs. treatment) at any time point, bilirubin concentration resulting from AFB1 treatment for 7 days was significantly (p < 0.05) higher than that of day 1 and/or 4 days (Figure 1C).
FIGURE 1.
Time course effects of sub-acute AFB1 exposure on (A) ALT, (B) ALP, and (C) total bilirubin. P-values <0.1 are shown for comparisons between control and AFB1 at a given time point, and p < 0.05 is considered significant. Bars represent mean ± SEM (n = 5).
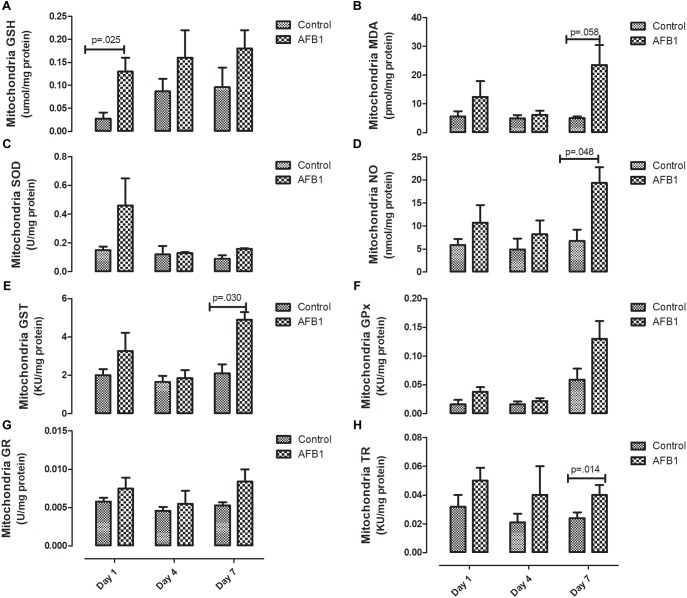
Sub-acute AFB1 exposure disrupts mitochondrial lipids and oxidative stress. AFB1 (1.0 mg/kg) significantly increased mitochondrial cholesterol (p = 0.016) after exposure for 7 days while there was no significant difference in mitochondrial triacylglycerols (Figure 2). The time course effects of subacute AFB1 exposure on mitochondrial oxidant/antioxidant status are depicted in Figure 3. AFB1 exposure significantly increased GSH (p = 0.025) at day 1 while NO (p = 0.048) and MDA (p = 0.058) were increased at day 7. GST (p = 0.030) and TR (0.014) were also significantly increased as a result of AFB1 exposure after 7 days of exposure. NO, MDA, GST, and GPx of the treatment group at day 7 was significantly (p < 0.05) higher than that of day 4. There was no significant difference in mitochondrial SOD on all the days.
FIGURE 2.
Time course effects of sub-acute AFB1 exposure on mitochondrial (A) cholesterol and (B) triacylglycerol. Significant p-values (p < 0.05) for control vs. AFB1 at a given time point are shown. Bars represent mean ± SEM (n = 5).
FIGURE 3.
Time course effects of sub-acute AFB1 exposure on mitochondrial (A) GSH, (B) MDA, (C) SOD, (D) NO, (E) GST, (F) GPx, (G) GR, and (H) TR. P-values <0.1 are shown for comparisons between control and AFB1 at a given time point, and p < 0.05 is considered significant. Bars represent mean ± SEM (n = 5).
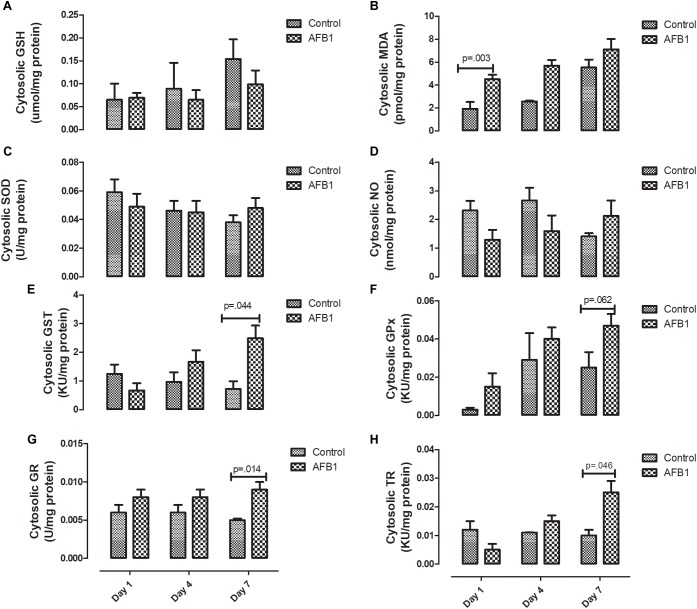
The time course effects of subacute AFB1 exposure on the oxidant/antioxidant status of the cytosolic fractions are depicted in Figure 4. AFB1 significantly increased MDA at only day 1 (p = 0.003) while GST (p = 0.044), GR (0.014), and TR (0.046) were significantly increased at day 7. In the cytosolic fractions, AFB1 exposure at day 7 significantly (p < 0.05) increased MDA, GST, TR, and GPx when compared with AFB1 exposure at day 1. There was no significant difference in cytosolic SOD on all the days.
FIGURE 4.
Time course effects of sub-acute AFB1 exposure on cytosolic (A) GSH, (B) MDA, (C) SOD, (D) NO, (E) GST, (F) GPx, (G) GR, and (H) TR. P-values <0.1 are shown for comparisons between control and AFB1 at a given time point, and p < 0.05 is considered significant. Bars represent mean ± SEM (n = 5).
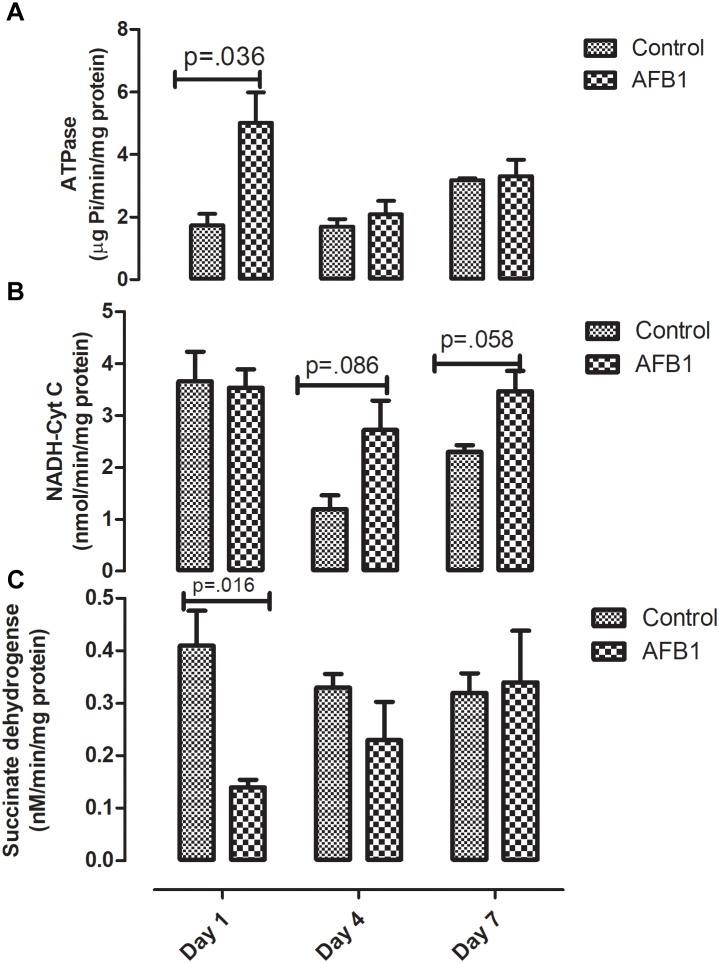
Time course subacute AFB1 exposure on mitochondrial enzymes are shown in Figure 5. AFB1 significantly increased ATPase (p = 0.036) while it decreased succinate dehydrogenase at day 1 (p = 0.016). AFB1 also increased NADH-Cyt C (p = 0.058) at day 7 only.
FIGURE 5.
Time course effects of sub-acute AFB1 exposure on mitochondrial (A) ATPase, (B) NADH-Cyt C, and (C) Succinate dehydrogenase. P-values <0.1 are shown for comparisons between control and AFB1 at a given time point, and p < 0.05 is considered significant. Bars represent mean ± SEM (n = 5).
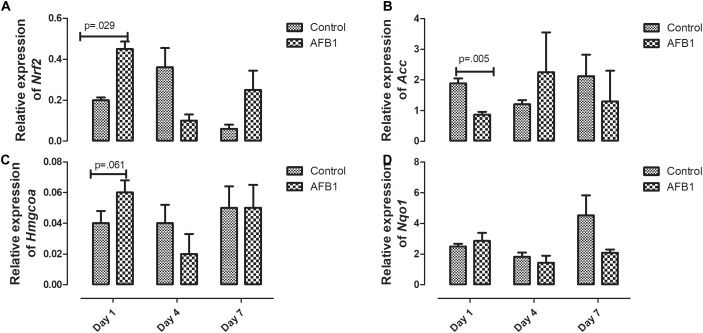
The time course effects of AFB1 on the relative expression of genes associated with oxidative stress are shown in Figure 6. AFB1 increased Nrf2 (p = 0.029) and Hmgcoa (p = 0.061) at day 1 while Acc (0.005) was significantly decreased at day 1.
FIGURE 6.
Time course effects of sub-acute AFB1 exposure on relative expression of (A) Nrf2, (B) Acc, (C) Hmgcoa, and (D) Nqo1 in liver. P-values <0.1 are shown for comparisons between control and AFB1 at a given time point, and p < 0.05 is considered significant. Bars represent mean ± SEM (n = 3).
Discussion
Time-course effects of subacute AFB1 exposure for 1, 4, and 7 days increased biomarkers of liver function, mitochondrial cholesterol, mitochondrial oxidant status (MDA and NO) as well as increased the enzymic and non-enzymic antioxidant status. These changes were associated with upregulation of Nrf2 and downregulation of Acc at day 1. These changes were dependent on the timing of exposure but did not follow a linear time-course trend. Most of the changes observed in this study were at day 1 (initial exposure) or at day 7 (last exposure). The changes at day 1 may be an initial response to toxic AFB1. This initial response was characterized by increases in liver ALP, mitochondrial GSH concentrations, ATPase activity, cytosolic MDA, liver Nrf2 expression, and decreases in mitochondrial succinate dehydrogenase activity and liver Acc expression. The changes collectively, may represent the initial protective response of the mitochondria against the aflatoxin toxicity. At the end of the time course, changes between exposed and controls included increases in ALP and bilirubin, mitochondrial MDA, NO, and GST activity, and cytosolic GST, GR, and TR activity. These outcomes may reflect an overwhelmed response system with increased toxicity manifesting in the liver.
In this study, AFB1 significantly increased the activity of ALP at day 1 and day 7 when compared with controls while the concentration of bilirubin in AFB1 exposed group at day 7 was significantly higher than those of day 1 and day 4. The concentrations of ALT, ALP, and bilirubin are usually increased in the plasma in response to hepatic injury which allows them to leak out into the plasma. Although plasma ALT activity is considered a gold standard for measuring liver injury, ALP, GGT, and total bilirubin are biomarkers used in addition to ALT especially in relation to conventional biliary action. Studies have shown that during biliary cirrhosis, there could be increases in ALP and GGT without corresponding increase in ALT (Ramaiah, 2007; Ozer et al., 2008). Although we did not assay for GGT in this study, the increase in ALP and bilirubin observed in this study suggests that liver injury may be associated with the hepatobiliary action and tends towards cholestatic induction. During the progression of cholestasis, increases in cholestatic induction enzymes such as ALP usually precedes an increase in bilirubin (Ramaiah, 2007). Several studies have reported increases in these biomarkers following AFB1 exposure (El-Nekeety et al., 2014; Rotimi et al., 2016; Eftekhari et al., 2018). Redzwan et al. (2014) found a positive correlation between AFB1-lysine adducts and total bilirubin in Malaysian adults. While ALP is localized within the cell membrane of tissues, ALT could be found in the cytoplasm and mitochondria, and changes in membrane permeability due to lipid accumulation could allow these enzymes to leak out into the cytosol.
AFB1 significantly increased mitochondrial cholesterol at day 7. Of all cellular compartments, the mitochondrion is considered to have low cholesterol concentration. It contains about 0.5–3% of cholesterol found in other organelles (Ikonen, 2008; Van Meer et al., 2008). Cholesterol is an essential component of the mitochondrial membrane, and increased accumulation of cholesterol in the mitochondria decreases its membrane’s fluidity and consequently its permeability (Ikonen, 2008; Van Meer et al., 2008). The increase in mitochondrial cholesterol observed in this study may be one of the mechanisms by which AFB1 affects the mitochondria and induces toxicity. Increase in mitochondrial cholesterol has been suggested to aggravate cholestasis (Nuño-Lámbarri et al., 2016). Apart from cholestasis, recent evidence showed that elevated mitochondria cholesterol might be involved in other diseases associated with the liver such as steatohepatitis and progression of HCC (García-Ruiz et al., 2016). Therefore, elevated mitochondria cholesterol may be one of the events associated with AFB1 induced HCC. Since we report evidence for this after only 7 days of exposure, this needs to be further studied in a chronic exposure experiment beyond 7 days. It is worthy of note that the increase in mitochondrial cholesterol was not associated with increased expression of Hmgcr. Although there was no significant increase in expression of Hmgcr gene at day 7, a nearly significant (p = 0.061) increase was observed at day 1. Vohra et al. (1985) found that acute AFB1 exposure increased mitochondrial cholesterol in rats after a single dose with higher concentration (5 mg/kg) given intraperitoneally.
Elevated mitochondria cholesterol negatively affects several membrane carriers. An example is the GSH transport system responsible for the transport of GSH from the cytosol- where it is synthesized- to the mitochondria through an ATP-dependent process (Fernandez-Checa and Kaplowitz, 2005). In this study, mitochondrial GSH (mGSH) was significantly increased at day 1 while there was no significant difference at day 7. The increase in GSH at day 1 also corresponds with the increase in ATPase observed at day 1. Although the transport of GSH is controlled by mitochondrial cholesterol concentration through an ATP-dependent process, it is worthy of note that the changes in mitochondrial cholesterol, GSH and ATPase did not occur at the same time. It is possible that these changes precede one another, while significant changes in GSH and ATPase were observed at day 1, significant increases in cholesterol were observed at day 7.
GSH together with mitochondrial antioxidant enzymes are responsible for maintaining the oxidant/antioxidant status of the mitochondria considering its role in electron transport chain and the generation of free radicals (reactive oxygen/nitrogen species) (Bhat et al., 2015). In this study AFB1 induced mitochondrial oxidative/nitrosative stress as shown by the increase in mitochondrial MDA and NO at day 7. Surprisingly, the increase in MDA and NO were associated with an increase in mitochondria GST and TR. It is expected that increase in MDA and NO will be associated with a decrease in antioxidant enzymes; however, it seems that the increase in GST and TR observed may be an attempt to counter the increase in oxidative/nitrosative species in this acute exposure. During aflatoxin metabolism, GST catalyzes the conjugation of AFBO with GSH for onward detoxification. The observed increase in GST may be a protective measure in response to subacute AFB1 exposure.
In the same vein, although increase in thioredoxin reductase (TR) seems to play a role in protecting against oxidative stress, other studies show that thioredoxin in reduced form may support the growth of cancer cells by complexing with apoptosis signaling kinase 1 (ASK1), which then prevents apoptosis (Arnér and Holmgren, 2000). Rieswijk et al. (2016) found that repetitive AFB1 (0.3 μM) exposure for 5 days led to persistent hypomethylation and upregulation of TR-1 –which has been shown to be associated with AFB1 and related to the development of HCC- in primary human hepatocytes (Caiment et al., 2014). Our study showed that AFB1 significantly increased the activity of mitochondrial and cytosolic TR at day 7. Considering the similar increase observed in other antioxidants in this study, it seems that the increase in TR activity plays a role in preventing against AFB1-induced oxidative stress.
On the other hand, this increased activity may be as a result of AFB1-induced hypomethylation and upregulation of TR (Rieswijk et al., 2016) and adequate availability of selenium (a cofactor of TR) whose deficiency has been shown to protect against aflatoxicosis in rats (McLeod et al., 1997). To the best of our knowledge, this study is the first to report the activity of TR in response to subacute AFB1 exposure in rats. Zhang et al. (2016), using RNA-Seq, studied the hepatic transcriptome of ducklings in response to AFB1 after 2 weeks of exposure. They found that TR, GSTs, and Nqo1 were upregulated while Acc was down-regulated. This is similar to the results observed in this study, our results showed that AFB1 downregulated Acc at day 1 only. Acc is associated with the promotion of fatty acid synthesis, and we have previously shown that subacute exposure to AFB1 for seven days increased plasma free fatty acid (FFA) due to downregulation of CPT1 necessary for transporting fatty acids into the mitochondria for ß oxidation (Rotimi et al., 2017).
Nrf2 is a transcription factor usually activated in response to reactive oxygen species. Apart from its role in antioxidant defense, it has also been shown to play a role in hepatic lipid metabolism (Shimpi et al., 2017). In this study, we found that AFB1 upregulated the expression of Nrf2 at day 1 alone. This increase was in response to increased oxidant status and an attempt to maintain the oxidant/antioxidant status balance. Nrf2 does this by upregulating other antioxidant enzymes particularly the phase II detoxification enzymes. Although we did not determine the expression of these enzymes, their activities were increased. The increase in Nrf2 was associated with increased GST both in the mitochondrial and cytosolic compartments. AFB1 (0.5–5 μmol/L) upregulated Nrf2 in broiler hepatocytes and cardiomyocytes after exposure for 24–72 h (Liu and Wang, 2016; Wang et al., 2017). Recently, Muhammad et al. (2018) found that exposure of broilers to AFB1 (5 mg/kg feed) for 28 days significantly downregulated Nrf2. Their result differs from ours, and this could be a result of exposure (subacute vs. subchronic) and species (rat vs. broiler) differences.
In this study AFB1 significantly decreased hepatic succinate dehydrogenase (SDH) at day 1. Our results are similar to other studies such as that of Rastogi et al. (2001) where they observed a 16% decrease in SDH after a single dose of AFB1 (2 mg/kg) in rats. A similar trend was also observed in birds (Quezada et al., 2000). Acute AFB1 exposure of rats to a single dose of AFB1 (7 mg/kg bw) significantly decreased SDH after 12 h of exposure after which the activity of SDH returned to normal (Sajan et al., 1995). Vohra et al. (1985) also observed a significant decrease in the activity of liver SDH 6 to 24 h after a single dose of AFB1 (5 mg/kg) in rats. They observed that the effect of AFB1 on SDH was time-dependent and maximum decrease was observed 24 h after treatment.
Strengths of this study include, time course and the biomarkers used to assess oxidative damage particularly TR activity, which has been reported to play a dual role in protecting against oxidative damage but also in driving cellular carcinogenesis. This study was limited by the sample size (5 in each group) which may have limited our statistical power to detect all true significant differences between groups. Although the dosage used is higher than that reported in human exposure; our previous study established this dosage to be adequate for eliciting toxic response in rats, without causing death. In addition, we analyzed expression of four genes implicated in response to AFB1 out of many possible. Future research should assess the effects of acute AFB1 over several days or weeks on animals of both sexes and multiple ages (adults and young) to determine critical windows in the time course of toxicity that mimic populations exposed to highly contaminated foods.
Conclusion
In conclusion, subacute exposure to AFB1 showed different sets of responses at day 1 and after 7 days of exposure. We showed that AFB1 significantly increased biomarkers of liver functions and oxidative stress which was associated with increased mitochondrial lipids. Interestingly, we also observed an increase in mitochondrial antioxidant enzymes and particularly, we showed for the first time that subacute AFB1 exposure increased thioredoxin reductase activity in both cytosolic and mitochondrial compartments.
Ethics Statement
This study experiment was conducted, and the animals cared for in accordance with the institutional animal care and use committee guidebook. The protocol was approved by the Department of Biological Sciences, Covenant University Ethical committee.
Author Contributions
OR and SR conceived, designed, and supervised the experiments. OR and JG designed and carried out the gene expression assays. IA, EA, and GT carried out the mitochondrial assays. OR drafted the manuscript. All authors contributed to revision and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the publication support of Covenant University Centre for Research, Innovation and Discovery (CUCRID). The authors are grateful to University of Michigan African Presidential Scholars (UMAPS) program which granted OAR the opportunity to carry out the gene expression assays at the Department of Environmental Health Sciences, University of Michigan, and also to Dr. Luke Montrose for his guidance in gene expression analysis.
References
- Arnér E. S., Holmgren A. (2000). Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267 6102–6109. 10.1046/j.1432-1327.2000.01701.x [DOI] [PubMed] [Google Scholar]
- Azziz-Baumgartner E., Lindblade K., Gieseker K., Rogers H. S., Kieszak S., Njapau H., et al. (2005). Case–control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 113 1779–1783. 10.1289/ehp.8384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A. H., Dar K. B., Anees S., Zargar M. A., Masood A., Sofi M. A., et al. (2015). Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 74 101–110. [DOI] [PubMed] [Google Scholar]
- Bondy G. S., Pestka J. J. (2000). Immunomodulation by fungal toxins. J. Toxicol. Environ. Health Part B Critic. Rev. 3 109–143. 10.1080/109374000281113 [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. (1978). “Microsomal lipid peroxidation,” in Methods in Enzymology, eds Sidney F., Lester P. (Amsterdam: Elsevier; ), 302–310. 10.1016/s0076-6879(78)52032-6 [DOI] [PubMed] [Google Scholar]
- Caiment F., Tsamou M., Jennen D., Kleinjans J. (2014). Assessing compound carcinogenicity in vitro using connectivity mapping. Carcinogenesis 35 201–207. 10.1093/carcin/bgt278 [DOI] [PubMed] [Google Scholar]
- Deng J., Ling Z., Ni-Ya Z., Karrow N. A., Christopher S. K., De-Sheng Q., et al. (2018). Aflatoxin B1 metabolism: regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. /Rev. Mutat. Res. 778 79–89. 10.1016/j.mrrev.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Eftekhari A., Ahmadian E., Panahi-Azar V., Hosseini H., Tabibiazar M., Maleki Dizaj S. (2018). Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: in vitro/in vivo studies. Artif. cells Nanomed. Biotechnol. 46 411–420. 10.1080/21691401.2017.1315427 [DOI] [PubMed] [Google Scholar]
- Ellman G. L. (1959). Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82 70–77. 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- El-Nekeety A. A., Abdel-Azeim S. H., Hassan A. M., Hassan N. S., Aly S. E., Abdel-Wahhab M. A. (2014). Quercetin inhibits the cytotoxicity and oxidative stress in liver of rats fed aflatoxin-contaminated diet. Toxicol. Rep. 1 319–329. 10.1016/j.toxrep.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze U., Routledge M., Okonofua F., Huntriss J., Gong Y. (2018). Mycotoxin exposure and adverse reproductive health outcomes in Africa: a review. World Mycotoxin J. 11 321–339. 10.3920/wmj2017.2261 [DOI] [Google Scholar]
- Fernandez-Checa J. C., Kaplowitz N. (2005). Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol. Appl. Pharmacol. 204 263–273. 10.1016/j.taap.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226 497–509. [PubMed] [Google Scholar]
- García-Ruiz C., Ribas V., Baulies A., Fernández-Checa J. C. (2016). “Mitochondrial cholesterol and the paradox in cell death,” in Pharmacology of Mitochondria, eds Singh H., Sheu S. S. (Cham: Springer; ), 189–210. 10.1007/164_2016_110 [DOI] [PubMed] [Google Scholar]
- Gogvadze V., Orrenius S., Zhivotovsky B. (2004). Analysis of mitochondrial dysfunction during cell death. Curr. Protoc. Toxicol. 19 18.5.1–18.5.27. 10.1002/0471143030.cb1805s19 [DOI] [PubMed] [Google Scholar]
- Gong Y. Y., Watson S. (2016). Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 4 14–27. 10.14252/foodsafetyfscj.2015026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. (1974). Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249 7130–7139. [PubMed] [Google Scholar]
- Harasiuk D., Baranowski M., Zabielski P., Chabowski A., Górski J. (2016). Liver X receptor agonist TO901317 prevents diacylglycerols accumulation in the heart of streptozotocin-diabetic rats. Cell. Physiol. Biochem. 39 350–359. 10.1159/000445629 [DOI] [PubMed] [Google Scholar]
- Holmgren A., Bjornstedt M. (1995). Thioredoxin and thioredoxin reductase. Methods Enzymol. 25 199–208. [DOI] [PubMed] [Google Scholar]
- Ikonen E. (2008). Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9 125–138. 10.1038/nrm2336 [DOI] [PubMed] [Google Scholar]
- Julian G. S., De Oliveira R. W., Perry J. C., Tufik S., Chagas J. R. (2014). Validation of housekeeping genes in the brains of rats submitted to chronic intermittent hypoxia, a sleep apnea model. PLoS One 9:e109902. 10.1371/journal.pone.0109902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamala A., Shirima C., Jani B., Bakari M., Sillo H., Rusibamayila N., et al. (2018). Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 11 311–320. 10.3920/wmj2018.2344 [DOI] [Google Scholar]
- Khlangwiset P., Shephard G. S., Wu F. (2011). Aflatoxins and growth impairment: a review. Critic. Rev. Toxicol. 41 740–755. 10.3109/10408444.2011.575766 [DOI] [PubMed] [Google Scholar]
- Lewis L., Onsongo M., Njapau H., Schurz-Rogers H., Luber G., Kieszak S., et al. (2005). Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 113 1763–1767. 10.1289/ehp.7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang W. (2016). Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Animal Sci. J. 87 1490–1500. 10.1111/asj.12550 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193 265–275. [PubMed] [Google Scholar]
- Lu X., Hu B., Shao L., Tian Y., Jin T., Jin Y., et al. (2013). Integrated analysis of transcriptomics and metabonomics profiles in aflatoxin B1-induced hepatotoxicity in rat. Food Chem. Toxicol. 55 444–455. 10.1016/j.fct.2013.01.020 [DOI] [PubMed] [Google Scholar]
- Luo Y., Liu X., Li J. (2018). Updating techniques on controlling mycotoxins-A review. Food control 89 123–132. 10.1016/j.foodcont.2018.01.016 [DOI] [Google Scholar]
- Marklund S., Marklund G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47 469–474. 10.1111/j.1432-1033.1974.tb03714.x [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Stellwagen E. (1968). Purification and subunit structure of glutathione reductase from bakers’ yeast. J. Biol. Chem. 243 809–814. [PubMed] [Google Scholar]
- McLeod R., Ellis E. M., Arthur J. R., Neal G. E., Judah D. J., Manson M. M., et al. (1997). Protection conferred by selenium deficiency against aflatoxin B1 in the rat is associated with the hepatic expression of an aldo-keto reductase and a glutathione S-transferase subunit that metabolize the mycotoxin. Cancer Res. 57 4257–4266. [PubMed] [Google Scholar]
- Muhammad I., Wang H., Sun X., Wang X., Han M., Lu Z., et al. (2018). Dual role of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Front. Pharmacol. 9:554. 10.3389/fphar.2018.00554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutegi C., Cotty P., Bandyopadhyay R. (2018). Prevalence and mitigation of aflatoxins in Kenya (1960-to date). World Mycotoxin J. 11 341–357. 10.3920/wmj2018.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A., Gomez C., López-Cepero J. M., Boveris A. (2004). Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286 R505–R511. [DOI] [PubMed] [Google Scholar]
- Ngindu A., Kenya P., Ocheng D., Omondi T., Ngare W., Gatei D., et al. (1982). Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet 319 1346–1348. 10.1016/s0140-6736(82)92411-4 [DOI] [PubMed] [Google Scholar]
- Nuño-Lámbarri N., Domínguez-Pérez M., Baulies-Domenech A., Monte M. J., Marin J. J., Rosales-Cruz P., et al. (2016). Liver cholesterol overload aggravates obstructive cholestasis by inducing oxidative stress and premature death in mice. Oxid. Med. Cell. Longev. 2016:9895176. 10.1155/2016/9895176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opoku N., Achaglinkame M. A., Amagloh F. K. (2018). Aflatoxin content in cereal-legume blends on the Ghanaian market far exceeds the permissible limit. Food Secur. 10 1539–1545. 10.1007/s12571-018-0849-5 [DOI] [Google Scholar]
- Ostry V., Malir F., Toman J., Grosse Y. (2017). Mycotoxins as human carcinogens—the IARC Monographs classification. Mycotoxin Res. 33 65–73. 10.1007/s12550-016-0265-7 [DOI] [PubMed] [Google Scholar]
- Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. (2008). The current state of serum biomarkers of hepatotoxicity. Toxicology 245 194–205. 10.1016/j.tox.2007.11.021 [DOI] [PubMed] [Google Scholar]
- Quezada T., Cuellar H., Jaramillo-Juarez F., Valdivia A., Reyes J. (2000). Effects of aflatoxin B1 on the liver and kidney of broiler chickens during development. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 125 265–272. 10.1016/s0742-8413(99)00107-3 [DOI] [PubMed] [Google Scholar]
- Ramaiah S. K. (2007). A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 45 1551–1557. 10.1016/j.fct.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Rastogi R., Srivastava A. K., Rastogi A. K. (2001). Long term effect of aflatoxin B1 on lipid peroxidation in rat liver and kidney: effect of picroliv and silymarin. Phytother. Res. 15 307–310. 10.1002/ptr.722 [DOI] [PubMed] [Google Scholar]
- Redzwan S. M., Rosita J., Sokhini A. M., Nurul‘Aqilah A., Wang J.-S., Kang M.-S., et al. (2014). Detection of serum AFB1-lysine adduct in Malaysia and its association with liver and kidney functions. Int. J. Hygiene Environ. Health 217 443–451. 10.1016/j.ijheh.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Reisch A. S., Elpeleg O. (2007). Biochemical assays for mitochondrial activity: assays of TCA cycle enzymes and PDHc. Methods Cell Biol. 80 199–222. 10.1016/s0091-679x(06)80010-5 [DOI] [PubMed] [Google Scholar]
- Rieswijk L., Claessen S. M., Bekers O., Van Herwijnen M., Theunissen D. H., Jennen D. G., et al. (2016). Aflatoxin B1 induces persistent epigenomic effects in primary human hepatocytes associated with hepatocellular carcinoma. Toxicology 350 31–39. 10.1016/j.tox.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Rotimi O. A., Olayiwola I., Ademuyiwa O., Balogun E. A. (2012). Effects of fibre-enriched diets on tissue lipid profiles of MSG obese rats. Food Chem. Toxicol. 50 4062–4067. 10.1016/j.fct.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Rotimi O. A., Rotimi S. O., Duru C. U., Ebebeinwe O. J., Abiodun A. O., Oyeniyi B. O., et al. (2017). Acute aflatoxin B1–Induced hepatotoxicity alters gene expression and disrupts lipid and lipoprotein metabolism in rats. Toxicology Rep. 4 408–414. 10.1016/j.toxrep.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi O. A., Rotimi S. O., Oluwafemi F., Ademuyiwa O., Balogun E. A. (2016). Coexistence of aflatoxicosis with protein malnutrition worsens hepatic oxidative damage in rats. J. Biochem. Mol. Toxicol. 30 269–276. 10.1002/jbt.21787 [DOI] [PubMed] [Google Scholar]
- Rotimi O. A., Rotimi S. O., Oluwafemi F., Ademuyiwa O., Balogun E. A. (2018). Oxidative stress in extrahepatic tissues of rats co-exposed to aflatoxin B1 and low protein diet. Toxicol. Res. 34 211–220. 10.5487/TR.2018.34.3.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotruck J. T., Pope A. L., Ganther H. E., Swanson A., Hafeman D. G., Hoekstra W. (1973). Selenium: biochemical role as a component of glutathione peroxidase. Science 179 588–590. 10.1126/science.179.4073.588 [DOI] [PubMed] [Google Scholar]
- Rushing B. R., Selim M. I. (2018). Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 124 81–100. 10.1016/j.fct.2018.11.047 [DOI] [PubMed] [Google Scholar]
- Sajan M., Satav J., Bhattacharya R. (1995). Activity of some respiratory enzymes and cytochrome contents in rat hepatic mitochondria following aflatoxin B1 administration. Toxicol. Lett. 80 55–60. 10.1016/0378-4274(95)03256-k [DOI] [PubMed] [Google Scholar]
- Shimpi P. C., More V. R., Paranjpe M., Donepudi A. C., Goodrich J. M., Dolinoy D. C., et al. (2017). Hepatic lipid accumulation and Nrf2 Expression following perinatal and peripubertal exposure to bisphenol a in a mouse model of nonalcoholic liver disease. Environ. Health Perspect. 125:087005. 10.1289/EHP664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Foppen E., Fliers E., Kalsbeek A. (2016). Effects of intracerebroventricular administration of neuropeptide Y on metabolic gene expression and energy metabolism in male rats. Endocrinology 157 3070–3085. 10.1210/en.2016-1083 [DOI] [PubMed] [Google Scholar]
- Sun W., Wang X., Hou C., Yang L., Li H., Guo J., et al. (2017). Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrf2 pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Neuropharmacology 113 556–566. 10.1016/j.neuropharm.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Ito Y., Shinohara M., Yamashita S., Ichinose S., Kishida A., et al. (2016). Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc. Natl. Acad. Sci. 113 7840–7845. 10.1073/pnas.1522054113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussky H. H., Shorr E. (1953). A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 202 675–685. [PubMed] [Google Scholar]
- Van Meer G., Voelker D. R., Feigenson G. W. (2008). Membrane lipids: where they are and how they behave. Nature reviews Molecular cell biology 9 112. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra R., Majumdar S., Malik V., Singh R., Nagpaul J. (1985). Modification of aflatoxin B1-induced changes in certain mitochondrial enzymes and lipids by medroxyprogesterone acetate. Indian J. Physiol. Pharmacol. 29 146–152. [PubMed] [Google Scholar]
- Wang W. J., Xu Z. L., Yu C., Xu X. H. (2017). Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Animal Sci. J. 88 1561–1568. 10.1111/asj.12796 [DOI] [PubMed] [Google Scholar]
- Watson S., Moore S. E., Darboe M. K., Chen G., Tu Y.-K., Huang Y.-T., et al. (2018). Impaired growth in rural Gambian infants exposed to aflatoxin: a prospective cohort study. BMC Public Health 18:1247. 10.1186/s12889-018-6164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C. P., Gong Y. Y. (2009). Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis 31 71–82. 10.1093/carcin/bgp264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan G. N., Kensler T. W., Groopman J. D. (2012). Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A Review. Food Addit. Contam. part A 29 249–257. 10.1080/19440049.2011.563370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel A. A., Gulen S., Dincer S., Yucel A. E., Yetkin G. I. (2012). Comparison of two different applications of the Griess method for nitric oxide measurement. J. Exp. Integr. Med. 2 167–171. [Google Scholar]
- Zhang N.-Y., Qi M., Gao X., Zhao L., Liu J., Gu C.-Q., et al. (2016). Response of the hepatic transcriptome to aflatoxin B1 in ducklings. Toxicon 111 69–76. 10.1016/j.toxicon.2015.12.022 [DOI] [PubMed] [Google Scholar]