Abstract
Subacute thyroiditis (SAT) is a self-limiting inflammatory condition of the thyroid gland in which multinucleated giant cells constitute a key histological finding. SAT is generally a clinical diagnosis and consequently its histological features are rarely encountered by pathologist. Herein, we present a case that exemplifies the characteristic clinical and pathological features of this entity. In addition, we compare SAT to other thyroid disorders characterized by the presence of multinucleated giant cells.
Keywords: Subacute thyroiditis, Quervain’s thyroiditis, Multinucleated giant cells, Non-neoplastic lesions of the thyroid
Case Presentation
A 39 year old woman was self-referred to an otolaryngologist for the evaluation of a thyroid nodule. The patient had been experiencing throat pain, globus sensation, and dysphagia for 6 weeks. Examination of the thyroid gland revealed a midline normal sized thyroid with tenderness to palpation over the left lobe. Ultrasonography showed a 9 × 7 mm hypoechoic nodule with lobulated borders located in the left upper pole and several benign colloid cysts in the right lobe. A fine-needle aspiration biopsy (FNAB) of the left upper pole nodule was performed and reported as Bethesda category 6. The patient subsequently underwent a hemithyroidectomy of the left lobe.
Macroscopic Findings
Gross examination reveals a 4.2 × 2.6 × 1.3 cm and 9.5 g left thyroid lobe with an intact external surface. The specimen was serially sectioned from superior to inferior to reveal a 0.8 × 0.4 × 0.3 cm ill-defined white nodule located within the superior pole. In addition, a second 1.9 × 1.0 × 0.8 cm firm tan lesion also with ill-defined borders was also identified occupying the in the mid portion of the lobe (Fig. 1). The remainder of the thyroid parenchyma was beefy-red and unremarkable. Sections of both lesions were submitted for histological evaluation.
Fig. 1.
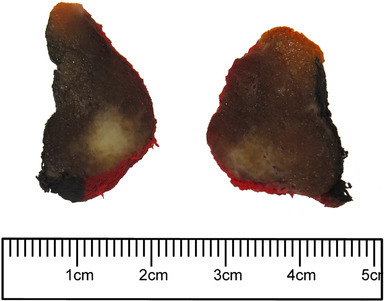
Gross image of the thyroid gland showing a firm tan lesion with ill-defined borders
Microscopic Findings
Histological sections of the upper pole lesion show an infiltrative tumor with papillary architecture. The papillary structures were lined by neoplastic follicular cells showing nuclear features of papillary thyroid carcinoma, including nuclear enlargement, nuclear clearing, irregular nuclear borders, nuclear grooves, and pseudo inclusions. In contrast, the microscopic examination of the second lesion showed a localized area of disrupted follicles infiltrated by inflammatory cells including multinucleated giant cells, lymphocytes, and neutrophils (Fig. 2). The residual colloid appeared to be floating within the multinucleated giant cells and various degrees of intermolecular fibrosis were also appreciated (Fig. 3). Acid-fast bacillus, Fite’s acid-fast, and Grocott’s methenamine silver stains were negative for microorganisms. The overall pathological findings couples with the patient’s symptoms were diagnostic for subacute thyroiditis (SAT).
Fig. 2.
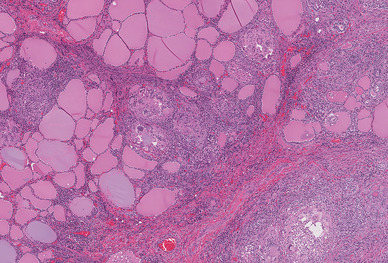
Histopathologic sections of thyroid lesion showing disrupted follicles infiltrated by mixed inflammatory cells, and interfollicular fibrosis (hematoxylin-eosin, original magnification 10 × 40)
Fig. 3.
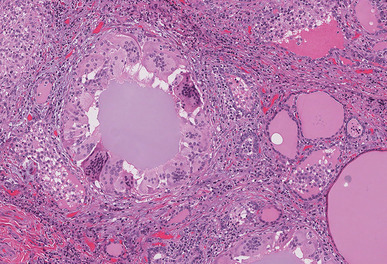
Histopathologic sections of thyroid lesion showing colonization of follicles by multinucleated giant cells (hematoxylin-eosin, original magnification 10 × 20)
Discussion
SAT is a granulomatous inflammatory condition of the thyroid gland, also referred as subacute nonsuppurative thyroiditis, granulomatous/giant cells thyroiditis, painful thyroiditis, and de Quervain’s thyroiditis. It is more common in patients in their 2nd to 6th decades of life, uncommon in children and the elderly, and affects more women than men by 3–7 to 1 ratio [1, 2].
The pathogenesis of SAT is thought to be a viral infection or a post-viral infection inflammatory response. Many patients have a history of upper respiratory infection typically 2–8 weeks prior to the onset of thyroiditis and serum studies have associated mumps, measles, influenza, Ebstein-Bar virus, coxsackievirus, adenovirus, and echovirus as possible etiologies. Patients may show anti-thyroid antibodies, however, these antibodies are transient and may be the result of antigens released following thyroid follicles destruction rather than represent a true autoimmune disease. Moreover, a genetic predisposition for SAT has been linked to human leukocyte antigen Bw35 (HLA-Bw35) [3].
The clinical presentation may vary from sudden to gradual onset and 96% of patients present with neck pain localized either to one lobe or to the entire thyroid gland. The pain typically radiates to the jaw, ears, face, and chest. Less commonly, patients can have systemic symptoms, including fever, malaise, fatigue, and myalgia [1]. The thyroid gland may show symmetrical or unilateral enlargement with a firm to hard consistency and tenderness on palpation. There is also a painless form reported, known as atypical subacute thyroiditis, and patients may present with fever of unknown origin [2].
The inflammation in SAT causes damage to thyroid follicles and during its early phase release of thyroglobulin, triiodothyronine (T3), and thyroxine (T4) into circulation occurs. As a consequence, the majority of patients have a mild increase in serum free T4 and T3 concentrations accompanied by decreased serum TSH levels and possible increased erythrocyte sedimentation rate and C reactive protein [4]. This hyperthyroid phase is self-limiting, usually subsiding in 2–8 weeks, and is typically followed by hypothyroidism. The latter phase reflects significant destruction of thyroid gland and absence of iodine uptake and hormone production, with laboratory studies showing decreased serum free T4, T3, and thyroglobulin, and increased TSH [4].
On gross examination, the thyroids affected by SAT are typically asymmetrically enlarged and firm with a tan-white cut surface and ill-defined nodularity [5]. In other occasions, as in our case, SAT can actually mimic a malignant tumor when it presents as a localized firm lesion with ill-defined borders rather than a diffuse parenchymal process [6]. The histological appearance of SAT varies with the phase of the disease. The early phase, which correlates with a hyperthyroid status, is characterized by destruction of follicular epithelial cells with colloid extravasation and colonization of follicles predominately by neutrophils forming microabscesses. As the disease progresses the acute inflammation is gradually replaced by lymphocytes, histiocytes, and characteristically multinucleated giant cells engulfing colloid. Histologically, the late phase is remarkable for increasing amounts of interfollicular fibrosis and corresponds to hypothyroid phase [5, 7].
Even though FNABs are generally not performed in patients with a clinical picture of SAT, its cytological features have been described in the literature. The cellularity on conventional cytology smear varies depending on the stage of the disease and the aspirates typically show an admixture of multinucleated giant cells, epithelioid granulomas and scattered degenerated follicular cells in a dirty mixed inflammatory background with scant colloid [8, 9]. It should be noted that the aforementioned cytological features of SAT are less evident in liquid-based preparations [10].
The presence of multinucleated giant cells is responsible for the term granulomatous / giant cells thyroiditis and has been regarded as the histological hallmark of SAT. However, multinucleated giant cells are also identified in other non-neoplastic conditions restricted to the thyroid, such as palpation thyroiditis; or in systemic processes that can affect the gland, including sarcoidosis and infections (mycobacterial and fungal) [11]. In addition, they also have been recognized as a non-specific feature of papillary thyroid carcinoma [12]. Consequently, the identification of multinucleated giant cells on cytological and surgical specimens of the thyroid gland should always be interpreted within the clinico-pathological context of each patient (Table 1).
Table 1.
Thyroid disorders with multinucleated giant cells
| Condition | Clinical | Macroscopic | Microscopic |
|---|---|---|---|
| Subacute thyroiditis | Painful thyroid Variable systemic symptoms Hyperthyroid phase followed by hypothyroid phase |
Diffuse ill-defined white-tan nodularity Localized firm white- tan lesion with ill-defined borders |
Early phase: destruction of follicles and neutrophilic infiltration Later phase: Colonization of follicles by lymphocytes, histiocytes, and multinucleated giant cells Floating colloid Variable degree of interfollicular fibrosis |
| Palpation thyroiditis | Incidental finding in surgically excised thyroid | No specific gross appearance | Multifocal process Chronic inflammatory cells, predominately giant cells and macrophages, within follicles |
| Sarcoidosis | Systemic disease No thyroid-specific symptomatology |
No specific gross appearance | Non-necrotizing granulomas in a interstitial distribution Colloid colonization and follicles destruction absent |
| Infections (mycobacterial and fungal) | Immunosuppressed or immunocompromised patients Painful thyroid Systemic symptoms |
Multifocal caseating areas Abscess formation |
Necrotizing granulomas Associated acute inflammation Acid-fast bacillus or Grocott’s methenamine silver may identified microorganisms |
| Papillary thyroid carcinoma | Painless thyroid mass with or without enlargement of cervical lymph nodes Cold nodule |
Well-defined to invasive lesion White-tan, firm, gritty cut surface Cystic tumors with papillary structures |
Papillary and/or follicular architecture Cells with nuclear features (i.e. enlargement, grooves, optical clearing, pseudo inclusions) Multinucleated giant cells within neoplastic follicles or adjacent to papillae |
Treatment of SAT is based upon observational studies and clinical experience, with treatment directed towards relieving pain and hyperthyroid symptoms. Nonsteroidal anti-inflammatory medications have shown positive outcomes and corticosteroids have a role in severe cases [13]. Complete recovery from SAT is nearly always, however in one study 15% of patients developed permanent hypothyroidism, requiring hormonal replacement therapy, and less than 5% of patients had recurrent disease, 6–21 years after the initial presentation [1].
References
- 1.Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metabol. 2003;88(5):2100–2105. doi: 10.1210/jc.2002-021799. [DOI] [PubMed] [Google Scholar]
- 2.Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Int Med (Tokyo Japan) 2008;47(8):725–729. doi: 10.2169/internalmedicine.47.0740. [DOI] [PubMed] [Google Scholar]
- 3.Ohsako N, Tamai H, Sudo T, Mukuta T, Tanaka H, Kuma K, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metabol. 1995;80(12):3653–3656. doi: 10.1210/jcem.80.12.8530615. [DOI] [PubMed] [Google Scholar]
- 4.Weihl AC, Daniels GH, Ridgway EC, Maloof F. Thyroid function tests during the early phase of subacute thyroiditis. J Clin Endocrinol Metabol. 1977;44(6):1107–1114. doi: 10.1210/jcem-44-6-1107. [DOI] [PubMed] [Google Scholar]
- 5.Wenig BM, Heffes CS, Adair CF. Atlas of endocrine pathology. Philadelphia: W.B. Sauders Company; 1997. Nonautoimmune thyroiditides; pp. 53–55. [Google Scholar]
- 6.Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, Tallini G. Tumor-Like conditions of the thyroid gland tumors of the thyroid and parathyroid gland. 21. 4th ed. American Registry of Pathology; 2014. p. 342.
- 7.Wenig BM. Atlas of head and neck pathology. 3. Amsterdam: Elsevier; 2016. Non-neoplastic diseases of the thyroid gland; pp. 1240–1243. [Google Scholar]
- 8.Vural C, Paksoy N, Gok ND, Yazal K. Subacute granulomatous (De Quervain’s) thyroiditis: fine-needle aspiration cytology and ultrasonographic characteristics of 21 cases. Cyto Journal. 2015;12:9. doi: 10.4103/1742-6413.157479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsheikh TM, Cochand-Priollet B, Hong SW, Benign SMK. In: The bethesda system for reporting thyroid cytopathology. 2. Ali S, Cibas E, editors. New York: Springer; 2018. pp. 39–40. [Google Scholar]
- 10.Rosa M. Cytologic features of subacute granulomatous thyroiditis can mimic malignancy in liquid-based preparations. Diagn Cytopathol. 2016;44(8):682–684. doi: 10.1002/dc.23495. [DOI] [PubMed] [Google Scholar]
- 11.Harach HR, Williams ED. The pathology of granulomatous diseases of the thyroid gland. Sarcoidosis. 1990;7(1):19–27. [PubMed] [Google Scholar]
- 12.Guiter GE, DeLellis RA. Multinucleate giant cells in papillary thyroid carcinoma. A morphologic and immunohistochemical study. Am J Clin Pathol. 1996;106(6):765–768. doi: 10.1093/ajcp/106.6.765. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney LB, Stewart C, Gaitonde DY. Thyroiditis: an integrated approach. Am Family Phys. 2014;90(6):389–96. [PubMed] [Google Scholar]


