Abstract
Background: Attention-deficit hyperactivity disorder (ADHD) is a complex disorder in terms of etiology, clinical presentation, and treatment outcome. Pharmacological and psychological interventions are recommended as primary treatments in ADHD; however, other nonpharmacological intervention such as a dietary supplementation with omega-3 polyunsaturated fatty acids (ω-3 PUFAs) has emerged as an attractive option.
Purpose: The objective of the present study was to assess whether dietary supplementation with highly concentrated ω-3 docosahexaenoic acid (DHA) triglyceride may improve symptoms in ADHD.
Method: A 6-month prospective double-blind placebo-controlled randomized clinical trial was designed in 66 patients with ADHD, aged between 6 and 18 years. Participants in the experimental group received a combination of ω-3 fatty acids (DHA 1,000 mg, eicosapentaenoic acid 90 mg, and docosapentaenoic acid 150 mg). Instruments included d2-test, AULA Nesplora, EDAH scales, and abbreviated Conner’s Rating Scale.
Results: In the cognitive test, between-group differences were not found, but within-group differences were of a greater magnitude in the DHA group. Between-group differences in favor of the DHA arm were observed in behavioral measures, which were already detected after 3 months of treatment. Results were not changed when adjusted by ADHD medication.
Conclusions: This study provides further evidence of the beneficial effect of supplementation with ω-3 DHA in the management of ADHD.
Keywords: omega 3, PUFAs, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), attention-deficit hyperactivity disorder (ADHD)
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a complex disorder in terms of etiology, clinical presentation, and treatment outcome.1,2 Pharmacological and psychological interventions are generally recommended as primary treatments in youths with ADHD,3 but other nonpharmacological strategies have been the subject of growing interest for their potential role as alternative or additive approaches.4–6 Dietary supplementation with omega-3 polyunsaturated fatty acids (ω-3 PUFAs) has emerged as an attractive nonpharmacological option. The rationale for the use of ω-3 PUFAs in ADHD is based on mechanisms supporting the importance of essential fatty acids in neural tissue and evidence from studies showing that ω-3 PUFAs may be relevant to ADHD.7
The ω-3 PUFAs, eicosapentaenoic acid (EPA) and especially docosahexaenoic acid (DHA), play a key role in brain development and function,8,9 but only a small amount is synthesized by the human body. A continued dietary supply of ω-3 fatty acids is needed due to limited storage of these compounds in adipose tissue. The pleiotropic effects of DHA appear to influence many different signaling pathways, receptor systems, enzyme activities, synaptic plasticity, and membrane structure that ultimately lead to better brain functioning.10,11 Experimental depletion of brain DHA in animal studies is associated with reduction of both dopaminergic and serotonergic neurotransmission12 and behavioral impairments.13 Also, low brain DHA content has been shown in some neurodegenerative and neuropsychiatric disorders.14,15
In relation to ADHD, lower levels of PUFAs in blood and red blood cells and higher ω-6/ω-3 ratios have been associated with the severity of ADHD,16–19 and increased DHA and EPA concentrations in erythrocyte membranes resulting from ω-3 PUFAs supplementation appeared to ameliorate symptoms of ADHD.20 Systematic reviews and meta-analyses of randomized controlled trials (RCTs) assessing outcomes of supplementation with PUFAs have shown inconsistent results,16,18,21–27 possibly due to heterogeneity of clinical samples, inclusion of patients with diagnosis other than ADHD, non-parallel designs, or mixed supplementation interventions. However, a recent meta-analysis of seven RCTs with 534 youths with ADHD reported by Chang et al7 showed that ω-3 PUFA supplementation significantly improved parental reports of total symptom score, inattention, and hyperactivity. In two specific measurements, the Conner’s cognition and the Conner’s DSM-IV inattention subscales, ω-3 PUFAs had also a significant effect on both scores.7 Moreover, in three RCTs, ω-3 PUFA supplementation improved cognitive measures associated with attention.7 The findings of this meta-analysis provide further support to the rationale for using ω-3 PUFAs as a treatment option for ADHD.
Interestingly, ω-3 supplements used in previous studies of ADHD were low-dose EPA and DHA combinations, despite being recognized that DHA is a physiologically essential nutrient in the brain where it is required in high concentrations for providing optimal neuronal functioning.28,29 In direct contrast to DHA, EPA is found in near trace amounts in the brain and has a minor contribution to brain functioning.30 Our objective was, therefore, to investigate the effect of dietary supplementation with highly concentrated DHA triglyceride on ADHD symptoms in a randomized double-blind placebo-controlled trial.
Patients and methods
Study design
The study was a randomized double-blind placebo-controlled parallel trial involving parallel treatment for 6 months. The study was approved by the Ethics Committee of the Principality of Asturias (reference: CPMP/ICH/135/95, code: TDAH-Oviedo) and took into account the ethical principles for medical research involving human subjects as stated in the Declaration of Helsinki. Written informed consent was obtained from the parents of all subjects as well as from participants aged 12 years and older. Participants had volunteered to be involved in this study, and they were not given any incentive to take part in it. Once written consent was obtained, the corresponding tests were conducted to verify the diagnosis and to participate in this research. The study was registered in the European Clinical Trials Database (EudraCT trial number 2017–000866-31 for the Sponsor’s Protocol code number TDAH-OVIEDO).
Participants
Boys and girls between 6 and 18 years of age with a DSM-5 diagnosis of ADHD (DSM-5 American Psychiatric Association, 2013) were recruited through the Faculty of Psychology at the University of Oviedo. The clinical diagnosis was confirmed by a trained researcher using the Diagnostic Interview Schedule for Children–Parent Version (DISC-P). Patients with any subtype of ADHD (hyperactive-impulsive, inattentive, combined hyperactive-inattentive) were eligible. The children with ADHD were either medication naïve or using psychostimulant medication. Exclusion criteria were blood coagulation disorders, cognitive impairment or autism spectrum disorder, known intolerance to fish proteins, and treatment with dietary supplements containing ω-6 or ω-3 PUFAs during the month preceding inclusion. Participants with extreme total IQ scores lower than 70 and greater than 130 using the Wechsler Intelligence Scales for Children, fourth edition (WISC-IV)31 were excluded. Study eligibility and pre- and postintervention ratings of study procedures were assessed by psychologists.
Randomization and blinding
Randomization (1:1) was performed according to a computer-generated random sequence, which was used to allocate the participants either to the dietary supplement or the placebo group. Participants, parents, and investigators assessing outcome measures were blind to the intervention condition. Blinding was maintained until data analysis was completed.
Intervention
The DHA studied supplement consisted of a banana-flavored emulsion (4.7 g, 5 mL sachets) (Brudy NEN Emulsión; Brudy Lab, S.L., Barcelona, Spain), registered as a “food for special medical purposes” at the Spanish Agency of Consumption, Food Security, and Nutrition (AECOSAN), and requiring medical prescription and supervision of treatment. This supplement has a high-content DHA triglyceride having a high antioxidant activity patented to prevent cellular oxidative damage.32,33 Each sachet provided a combination of ω-3 fatty acids (DHA 1,000 mg, EPA 90 mg, and docosapentaenoic acid 150 mg), vitamin E (D-alpha-tocopherol) 4.5 mg as an antioxidant, and carbohydrates 0.94 g (fructose 0.46 g). Doses were 1 sachet/day in children weighing ≤32 kg and 2 sachets/day in those weighing >32 kg. The placebo sachets had the same composition and were indistinguishable from the active product. They were composed of the same amount of olive oil with banana flavor to give a similar taste and smell. Treatment duration was 6 months. The use of the current psychostimulant medication was allowed, but other psychotropic medications were not.
Assessments
Prespecified outcome measures were changes observed in the study instruments (cognitive and
behavioral variables) after 6 months of treatment in the DHA and placebo groups as compared with baseline in the between-group and within-group comparisons.
The study included a pre-treatment visit (time 0, baseline), an intermediate visit at 3 months of starting dietary supplementation (time 1), and a post-treatment visit at the end of the study (time 2, 6 months). At baseline, participants completed the d2 test of attention and the AULA Nesplora (virtual) test, whereas parents completed the Scale for the Assessment of Attention Deficit Hyperactivity Disorder (EDAH) (as per: EDAH version for families) and the Conner’s Parents Rating Scale. At the intermediate visit, parents completed the EDAH and the Conner’s questionnaires. The same instruments used at baseline were administered to patients and families at the final visit at 6 months.
The investigational product was delivered to the patient at baseline for 90-day treatment and at an intermediate visit at 3 months for a further 90-day treatment. At each visit, participants and their families were carefully interviewed regarding adherence and appearance of adverse events. Compliance with DHA supplementation was assessed by return of supplementation sachet counts and analytical data especially erythrocyte membrane DHA content.
The d2 test measures the ability to concentrate and sustain attention. It was originally described by Brickenkamp and Zillmer34 and a Spanish validated version was used in our study.35 This paper and pencil test asks to cross any letter “d” with two marks (above or below, in any order). The distractors are a “p” with two marks or a “d” with one or three marks. It is made up of 14 lines with 47 characters each for a total of 658 items, and the subject is given 20 s for each line. The results scores are: TR, overall answers, number of elements tried on the 14 lines; TA, number of correct guesses, that is, number of correct relevant elements; O, omitted elements, number of relevant elements tried but not marked; C, commissions, number of irrelevant elements marked; TOT, total test effectiveness, that is TR – (O+C); CON, concentration index, TA-C; and VAR, variation index or difference (TR+)-(TR-), where TR+ is the line with the greater number of tried elements, and TR- is the line with a lower number of elements tried. Higher scores of TA, TOT, and CON indicate better performance, and higher scores of O, C, and VAR indicate worse performance.
The AULA Nesplora test36–38 measures attention, impulsivity, processing speed, and motor activity in a virtual reality environment, which is shown through 3D glasses equipped with motion sensors and headphones. The participant takes the perspective of a student sitting in one of the desks of a classroom and facing the blackboard. The virtual environment and the earphones present a series of visual and auditory stimuli to which the user must respond. AULA comprises two main tasks: a “no-x” (“no-go” paradigm) task in which the participant must press the button whenever the presented stimulus is different from the target stimulus (whenever he/she does not see or hear “apple”) and an “x” (“go” paradigm) task, in which the participant must press the button whenever he/she sees or hears the target stimulus, more specifically when he/she sees or hears “seven”. Both visual and auditory stimuli are presented and, simultaneously, ecological distractors appear progressively, similar to those that may be found in a real-life school classroom (eg, teacher’s walk, paper ball, bell rings, a child passes a note to another student, the teacher’s pen drops, ambulance passes across the street, etc.). The test is completed in about 20 mins and measures the following variables: omissions (O), errors when the participant should respond to a target stimulus but does not do so; commissions (C), errors when the participant clicks the button even if the target stimulus has not been presented; and average response time for correct responses (RT), reaction time in milliseconds. A high number of omissions is related to attention-deficit symptoms, a high number of commissions indicates impulsivity, and TR is a measure of processing speed (the longer response time, the greater the attention deficit [AD]). Measures are differentiated by sensory modality (visual vs auditory), presence/absence of distractors, and task type (“no-go” vs “go”).
The EDAH version for families39 is composed of 20 items assessing the frequency that children and adolescents show behaviors related to AD and/or hyperactivity/impulsivity, rating from 0 (not at all present) to 4 (very much present). This scale provides indicators about ADHD and helps to verify and distinguish between the three ADHD subtypes. It is based on the symptomatology for ADHA listed in the DSM-5 manual. The abbreviated Conners‘ Rating Scale for parents (10-item)40 is a valid instrument assessing only the attention component and is usually used as a screening instrument for the identification of ADHD.
Biochemical analysis
The content of ω-3 DHA in the erythrocyte was measured at baseline and at the end of the study in those participants who gave consent for peripheral blood sampling. The composition of FAs was determined using the method described by Lepage and Roy41 analyzed by gas chromatography–mass spectrometry and identified by comparing the elution pattern and relative retention times of FA methyl esters with a reference FA methyl ester mixture (GLC-44 Nu-Check Prep. Inc, Elysian, MN, USA). The results were expressed in relative amounts (percentage of total FA).
Statistical analysis
Descriptive statistics for the dependent variables were analyzed in each group, paying special attention to kurtosis and skewness values. Kline’s criterion42 was used, according to which the maximum scores accepted for skewness and kurtosis range between 3 and 10. The study variables met this criterion, which allowed performing parametric analyses. Categorical variables are expressed as frequencies and percentages and continuous variables as mean and standard deviation (SD). To investigate treatment effects, all study variables (d2, AULA Nesplora, EDAH for families, and Conners‘ Rating Scale for parents) were fed into analysis of covariance (ANCOVA) models, with medication as a blocking factor. Repeated-measures ANOVA analyses were used to assess within-group differences. Further analyses included
differences related to sex as a blocking factor and the interaction sex ⨰ treatment response, differences related to medication as a blocking factor and the interaction medication ⨰ treatment response, and the interaction time ⨰ treatment. The magnitude of the effect was expressed in Cohen’s d according to which the effect is small when ηp2=0.01 (d=0.20), medium when ηp2=0.059 (d=0.50), and high when ηp2=0.138 (d =0.80). Analyses were performed in the per-protocol (PP) population, that is, all randomized patients who completed the 6-month study period, and in the intention-to-treat population (ITT) of all randomized patients. Statistical significance was set at P<0.05. The SPSS statistical package 24.0 (SPSS, Chicago, IL, USA) was used for the analysis of data.
Results
Between February 1 and March 31, 2017, 98 participants diagnosed with ADHD were screened for eligibility, 95 of which met the inclusion criteria and were randomized, 49 to the DHA group and 46 to placebo. There were 67 boys and 28 girls, aged between 6 and 19 years. The mean IQ score was 100.1 (15.1). Results obtained in the 95 randomized patients (ITT population) are described in the Supplementary material.
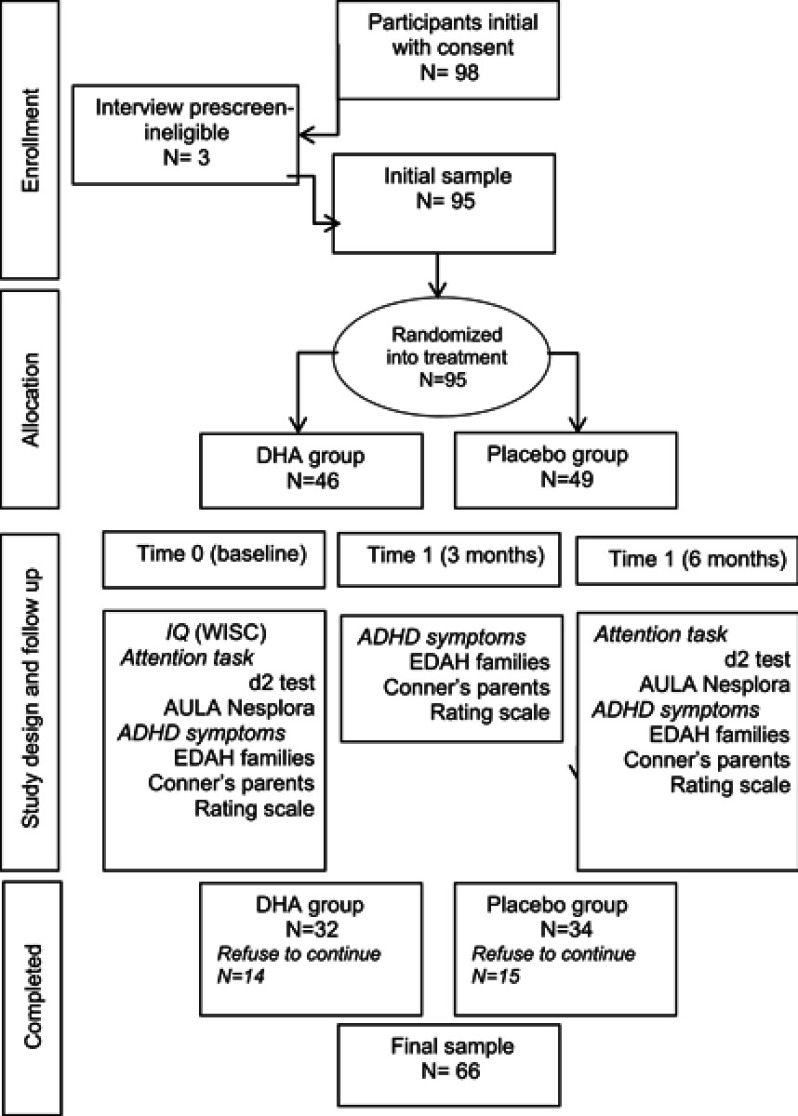
A total of 66 participants (69.5%) completed the study (DHA, n=32, placebo, n=34) and underwent the final evaluation assessment between September 1 and October 31, 2017. The flowchart of participants is shown in Figure 1. Main reasons for not completing the study were refusal to continue and lost to follow-up (change of residence). All participants were Caucasian. There were 47 boys and 19 girls, with a mean age of 11.7 (3.1) years and a mean IQ score of 100.3 (15.5). Twenty-four (75% patients in the DHA group) and 24 (70.6%) in the placebo group received pharmacological medication, mostly stimulants followed by atomoxetine. Also, comorbid conditions were present in 12 and 4 patients in the DHA and placebo groups, respectively, including dyslexia, Tourette’s syndrome, reading difficulties, anxiety, dyscalculia, and psychomotor and behavioral problems. Results here presented were obtained in the PP data set of 66 participants.
Figure 1.
Participants flow from enrollment to final sample.Abbreviations: DHA, decosahexaenoic acid; IQ, intellectual quotient; WISC, Wechsler Intelligence Scale for Children; ADHD, attention-deficit hyperactivity disorder; EDAH, Assessment of Attention Deficit Hyperactivity Disorder Scale.
Cognitive variables
At baseline, there were no significant differences between the two study groups regarding variables of the d2 and AULA Nesplora tests (Tables 1 and 2).
Table 1.
Changes of variables of the d2 test at the end of the study as compared with baseline in the two study groups (within-group comparisons)
| d2 variables | DHA group (n=32) | Placebo group (n=34) | Interaction (time x group) | ||||
|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | At 6 months mean (SD) | P-value (ES) | Baseline mean (SD) | At 6 months mean (SD) | P-value (ES) | P-value (ES) | |
| TOT | 316.1 (91.4) | 373.5 (94.9) | < 0.001 (0.534) | 330.1 (96.0) | 381.8 (11.6) | <0.001 (0.439) | 0.694 (0.002) |
| TR | 329.6 (88.7) | 382.8 (97.0) | < 0.001 (0.507) | 343.6 (93.6) | 394.1 (112.7) | < 0.001 (0.408) | 0.853 (0.001) |
| TA | 130.9 (37.4) | 153.4 (39.3) | < 0.001 (0.508) | 132.8 (40.6) | 154.6 (52.0) | < 0.001 (0.416) | 0.915 (< 0.001) |
| O | 9.2 (12.2) | 8.3 (7.9) | 0.716 (0.004) | 12.3 (13.3) | 9.5 (11.8) | 0.231 (0.043) | 0.566 (0.005) |
| C | 4.8 (6.9) | 1.1 (1.3) | 0.005 (0.224) | 3.1 (5.4) | 1.2 (1.6) | 0.035 (0.127) | 0.258 (0.020) |
| CON | 126.4 (41.4) | 152.1 (39.7) | < 0.001 (0.497) | 129.6 (43.1) | 151.9 (54.9) | < 0.001 (0.427) | 0.604 (0.004) |
| VAR | 12.6 (4.2) | 13.9 (4.8) | 0.291 (0.036) | 13.4 (5.1) | 13.7 (5.3) | 0.831 (0.001) | 0.573 (0.005) |
Abbreviations: ES, effect size; TOT, total test effectiveness; TR, total number of responses; TA, total number of correct answers; O, omissions; C, commissions; CON, concentration index; VAR, variability.
Table 2.
Changes of variables of the AULA Nesplora test at the end of the study as compared with baseline in the two study groups (within-group comparisons)
| AULA variables | DHA group (n=32) | Placebo group (n=34) | Interaction (time x group) | ||||
|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | At 6 months M (SD) | P-value (ES) | Baseline mean (SD) | At 6 months M (SD) | P-value (ES) | P-value (ES) | |
| AULA General Variables | |||||||
| O | 40.6 (38.2) | 36.9 (33.4) | 0.527 (0.013) | 28.3 (26.6) | 26.9 (30.0) | 0.774 (0.003) | 0.759 (0.001) |
| C | 19.1 (29.7) | 13.5 (9.8) | 0.265 (0.040) | 20.5 (23.3) | 15.3 (19.4) | 0.174 (0.055) | 0.948 (<0.001) |
| RT | 885.9 (138.9) | 881.1 (161.2) | 0.789 (0.002) | 850.2 (168.0) | 822.6 (121.7) | 0.240 (0.042) | 0.446 (0.009) |
| Sensory modality | |||||||
| Visual | |||||||
| O | 28.4 (26.9) | 22.0 (21.4) | 0.049 (0.119) | 18.7 (19.2) | 14.2 (16.9) | 0.157 (0.060) | 0.670 (0.003) |
| C | 9.3 (15.3) | 7.3 (5.5) | 0.473 (0.017) | 10.6 (11.1) | 8.6(9.8) | 0.294 (0.033) | 0.986 (<0.001) |
| RT | 787.2 (230.4) | 653.2 (137.2) | < 0.001 (0.480) | 717.9 (179.1) | 610.4 (112.3) | < 0.001 (0.389) | 0.480 (0.008) |
| Auditory | |||||||
| O | 12.2 (18.0) | 14.9 (12.9) | 0.493 (0.015) | 9.6 (15.1) | 12.7 (14.6) | 0.278 (0.038) | 0.926 (<0.001) |
| C | 9.8 (16.6) | 6.2 (5.3) | 0.194 (0.054) | 9.8 (13.1) | 6.7 (9.9) | 0.115 (0.073) | 0.904 (<0.001) |
| RT | 1,009.2 (154.3) | 1,098.4 (184.4) | 0.002 (0.260) | 1,012.7 (232.3) | 1,049.9 (157.2) | 0.303 (0.032) | 0.252 (0.020) |
| Distractors | |||||||
| Presence | |||||||
| O | 14.1 (13.2) | 12.9 (11.4) | 0.561 (0.011) | 11.0 (10.2) | 10.2 (11.4) | 0.698 (0.005) | 0.881 (<0.001) |
| C | 5.8 (9.0) | 4.1 (3.3) | 0.274 (0.038) | 6.5 (6.1) | 5.0 (5.8) | 0.148 (0.062) | 0.884 (<0.001) |
| RT | 897.3 (146.1) | 899.5 (173.7) | 0.922 (<0.001) | 866.9 (209.4) | 865.2 (134.5) | 0.960 (<0.001) | 0.924 (<0.001) |
| Absence | |||||||
| O | 26.5 (25.4) | 24.0 (22.8) | 0.539 (0.012) | 17.3 (17.1) | 16.7 (19.1) | 0.836 (0.001) | 0.708 (0.002) |
| C | 13.2 (21.0) | 9.5 (8.0) | 0.274 (0.038) | 14.0 (18.0) | 10.3 (13.9) | 0.207 (0.048) | 0.977 (<0.001) |
| RT | 880.7 (146.6) | 870.3 (165.5) | 0.613 (0.003) | 841.8 (154.1) | 795.3 (129.7) | 0.025 (0.142) | 0.207 (0.025) |
| “Go” Vs “No Go” tasks | |||||||
| “No Go” tasks | |||||||
| O | 34.3 (33.8) | 27.6 (26.3) | 0.190 (0.055) | 23.2 (22.7) | 19.8 (24.9) | 0.419 (0.020) | 0.124 (0.037) |
| C | 8.6 (6.3) | 8.5 (5.6) | 0.935 (<0.001) | 10.8 (7.0) | 8.9 (6.5) | 0.082 (0.089) | 0.250 (0.021) |
| RT | 859.1 (133.2) | 863.4 (168.2) | 0.863 (0.001) | 841.1 (181.1) | 802.6 (110.7) | 0.151 (0.061) | 0.214 (0.024) |
| “Go” tasks | |||||||
| O | 6.3 (5.7) | 9.3 (9.3) | 0.051 (0.117) | 5.1 (5.8) | 7.1 (7.1) | 0.083 (0.088) | 0.602 (0.004) |
| C | 10.5 (26.3) | 5.0 (6.6) | 0.220 (0.048) | 9.6 (21.4) | 6.4 (15.1) | 0.366 (0.025) | 0.694 (0.002) |
| RT | 973.4 (181.0) | 931.1 (260.3) | 0.307 (0.034) | 898.2 (166.6) | 913.3 (238.9) | 0.622 (0.007) | 0.259 (0.020) |
Abbreviations: ES, effect size; O, ommissions; C, commisions; RT, response time; M, mean; ES, effect size.
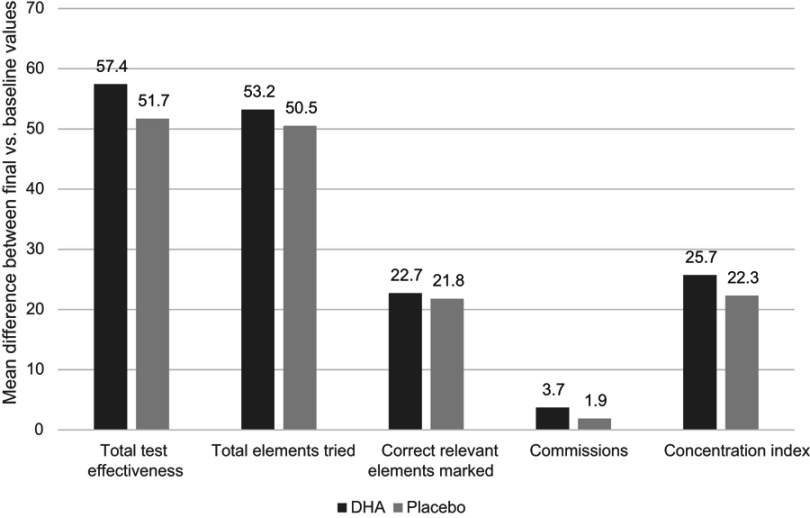
In both study groups, within-group differences in the d2 test were statistically significant regarding improvements in total test effectiveness (TOT), overall answers (TR), correct relevant elements marked (TA), and concentration index (CON), as well as significant decreases in commissions (C) (Table 1). The interaction time ⨰ group was not statistically significant in any case. Between-group differences in any variables of the d2 test were not found, but the magnitudes of within-group differences were greater in the DHA group than in the placebo group (Figure 2), with higher effect sizes in the experimental group. In the ANCOVA models adjusted by presence or absence of ADHD medication, pharmacological treatment had no effect on d2 variables. Similar results were obtained in the analysis of the ITT data set (see Table S1).
Figure 2.
Mean differences between the final visit at 6 months and baseline in d2 variables with statistically significance in within-group comparisons.
Table S1.
Changes of variables of the d2 test at the end of the study as compared with baseline in the two study groups (within-group comparisons). Analysis in the ITT sample
| d2 variables | DHA group (n=46) | Placebo group (n=49) | Interaction (time x group) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | At 6 months mean (SD) | P-value (ES) | Baseline mean (SD) | At 6 months mean (SD) | P-value (ES) | P-value | ES | |
| Total test effectiveness (TOT) | 326.0 (93.5) | 377.45 (102.8) | <0.001 (0.556) | 326.03 (97.2) | 379.37 (109.7) | <0.001 (0.556) | 0.875 | <0.001 |
| Overall answers, total elements tried on 14 lines (TR) | 339.03 (91.3) | 386.66 (105.1) | <0.001 (0.515) | 339.20 (95.2) | 392.54 (109.5) | <0.001 (0.545) | 0.639 | 0.004 |
| Correct guesses, correct relevant elements marked (TA) | 134.34 (38.2) | 155.07 (42.4) | <0.001 (0.529) | 131.49 (40.5) | 152.97 (52.0) | <0.001 (0.458) | 0.891 | <0.001 |
| Omissions, relevant elements not marked (O) | 9.28 (12.8) | 8.34 (8.3) | 0.724 (0.005) | 11.91 (13.3) | 10.51 (12.1) | 0.555 (0.010) | 0.894 | <0.001 |
| Commissions, irrelevant elements marked (C) | 4.61 (6.6) | 1.18 (1.3) | 0.010 (0.220) | 3.09 (5.3) | 1.15 (1.5) | 0.036 (0.127) | 0.322 | 0.016 |
| Concentration index (CON) | 130.17 (41.3) | 153.93 (42.9) | <0.001 (0.537) | 128.40 (42.9) | 150.40 (54.7) | <0.001 (0.472) | 0.763 | <0.001 |
| Variability (VAR) | 12.84 (4.8) | 14.28 (5.4) | 0.362 (0.035) | 13.42 (5.4) | 13.97 (5.3) | 0.703 (0.005) | 0.674 | 0.003 |
Note: There were within-group differences in both groups in all the variables, with the exception of omissions and variability. Both groups improved their performance in the task. Effect sizes were high in all cases, but slightly higher in the case of the DHA group in some variables. Concerning differences between the groups, no statistically significant differences were found neither at the beginning nor the end of the intervention. The effect of the pharmacological treatment was not statistically significant in any case. The interaction time ⨰ treatment did not reach statistical significance.
Abbreviation: ITT, intention-to-treat; DHA, decosahexaenoic acid; SD, standard deviation; ES, effect size.
In the AULA Nesplora test, within-group comparisons showed statistically significant improvements in omissions and response time in the visual modality in the DHA group and response time in the visual sensory modality and in the absence of distractors condition in the placebo group (Table 2). The magnitude of mean changes for omissions in the visual sensory modality was 6.4 in the DHA group and 4.5 in the placebo group, showing a higher effect size in the first group. Also, the mean change in the response time at the end of the study as compared with baseline was 134.0 in the DHA group and 107.5 in the placebo arm. Between-group differences in results of the AULA tests were not observed. ADHD medication as a covariable had no effect on the results obtained. The interaction time ⨰ group was not statistically significant in any of the studied variables. Results obtained in the analysis of the PP data set were replicated in the ITT population (see Table S2).
Table S2.
Changes of variables of the AULA Nesplora test at the end of the study as compared with baseline in the two study groups (within-group comparisons). Analysis in the ITT sample
| AULA variables | DHA group (n=46) | Placebo group (n=49) | Interaction (time x group) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | At 6 months mean (SD) | P-value (ES) | Baseline mean (SD) | At 6 months mean (SD) | P-value (ES) | P-value | ES | |
| Total omissions (O) | 39.4 (38.1) | 35.8 (33.4) | 0.527 (0.013) | 27.8 (26.5) | 28.0 (29.9) | 0.976 (<0.001) | 0.608 | 0.004 |
| Total commissions (C) | 18.6 (29.3) | 13.2 (9.8) | 0.257 (0.040) | 20.3 (23.2) | 16.2 (19.3) | 0.264 (0.037) | 0.826 | 0.001 |
| Total response time (TR) | 878.8 (142.6 | 874.9 (162.4) | 0.826 (0.002) | 853.1 (170.7) | 832.66 (120.6) | 0.369 (0.024) | 0.567 | 0.005 |
| Sensory modality | ||||||||
| Visual | ||||||||
| Omissions (O) | 27.5 (26.8) | 21.3 (21.3) | 0.049 (0.115) | 18.1 (19.1) | 14.4 (16.5) | 0.215 (0.045) | 0.561 | 0.005 |
| Commissions (C) | 9.2 (15.0) | 7.2 (5.4) | 0.466 (0.017) | 10.6 (11.0) | 9.1 (9.7) | 0.430 (0.018) | 0.889 | <0.001 |
| Response time (TR) | 780.9 (229.5) | 647.9 (138.1) | <0.001 (0.483) | 723.7 (183.2) | 614.3 (108.1) | <0.001 (0.402) | 0.483 | 0.007 |
| Auditory | ||||||||
| Omissions (O) | 11.8 (17.8) | 14.4 (12.9) | 0.493 (0.015) | 9.6 (15.1) | 13.5 (14.6) | 0.163 (0.057) | 0.779 | 0.001 |
| Commissions (C) | 9.5 (16.3) | 6.0 (5.3) | 0.186 (0.054) | 9.7 (13.1) | 7.0 (9.8) | 0.171 (0.054) | 0.803 | 0.001 |
| Response time (TR) | 1,000.5 (159.5) | 1,092.0 (185.0) | 0.001 (0.275) | 1,012.9 (232.8) | 1,065.8 (152.7) | 0.135 (0.064) | 0.382 | 0.012 |
| Distractors | ||||||||
| Present | ||||||||
| Omissions (O) | 13.6 (13.1) | 12.5 (11.4) | 0.561 (0.011) | 11.0 (10.3) | 10.7 (11.3) | 0.891 (0.001) | 0.742 | 0.002 |
| Commissions (C) | 5.7 (8.9) | 4.0 (3.2) | 0.274 (0.037) | 6.5 (6.0) | 5.2 (5.8) | 0.177 (0.053) | 0.844 | 0.001 |
| Response time (TR) | 890.0 (149.7) | 893.6 (174.2) | 0.862 (0.001) | 872.1 (210.8) | 875.3 (131.4) | 0.923 (<0.001) | 0.993 | <0.001 |
| Absent | ||||||||
| Omissions (O) | 25.7 (25.4) | 23.2 (22.8) | 0.538 (0.012) | 16.8 (17.0) | 17.2 (19.1) | 0.893 (0.001) | 0.562 | 005 |
| Commissions (C) | 12.9 (20.7) | 9.2 (7.9) | 0.262 (0.039) | 13.7 (17.8) | 10.9 (13.9) | 0.328 (0.028) | 0.825 | 0.001 |
| Response time (TR) | 873.7 (149.8) | 863.9 (166.9) | 0.623 (0.008) | 842.9 (158.0) | 805.4 (130.1) | 0.065 (0.097) | 0.321 | 0.015 |
| Type of task | ||||||||
| “X-no” tasks | ||||||||
| Omissions (O) | 33.2 (33.7) | 26.8 (26.2) | 0.190 (0.053) | 22.7 (22.6) | 20.9 (25.0) | 0.647 (0.006) | 0.464 | 0.005 |
| Commissions (C) | 8.5 (6.2) | 8.3 (5.5) | 0.871 (0.001) | 11.1 (7.2) | 9.25 (6.3) | 0.081 (0.087) | 0.272 | 0.018 |
| Response time (TR) | 852.2 (136.8) | 856.7 (169.9) | 0.829 (0.001) | 845.1 (182.7) | 813.0 (108.9) | 0.217 (0.044) | 0.273 | 0.018 |
| “X” tasks | ||||||||
| Omissions (O) | 6.1 (5.7) | 9.0 (9.2) | 0.51 (0.113) | 5.0 (5.7) | 7.0 (6.8) | 0.064 (0.098) | 0.627 | 0.004 |
| Commissions (C) | 10.1 (25.9) | 4.8 (6.6) | 0.220 (0.012) | 9.2 (21.1) | 6.9 (15.1) | 0.517 (0.012) | 0.585 | 0.005 |
| Response time (TR) | 966.1 (183.0) | 927.8 (256.8) | 0.342 (0.028) | 896.8 (173.1) | 919.0 (239.5) | 0.476 (0.015) | 0.230 | 0.022 |
Notes: Within-group differences showed improvements in the DHA group in omissions and response time in the visual modality of the test, with an increase in the response time in the auditory modality. In the placebo group, significant differences were only observed in the response time in the visual modality. Between-group differences were not observed in any of the variables. Differences in the post-test considering the pharmacological treatment and the pre-test as covariate were not found. The effect of the pharmacological treatment was not statistically significant in any case. The interaction time x treatment did not reach statistical significance.
Abbreviation: ES, effect size.
Behavioral variables
At baseline, statistically significant differences between the study groups in components of the EDAH scale and the Conner’s scale were not found, except for the AD of the EDAH with higher impairment in the intervention group than in the placebo group (Table 3).
Table 3.
Changes of variables of the EDAH scale for families and Conner’s Rating Scale for parents at the end of the study as compared with baseline in the two study groups (within-group comparisons)
| Instrument | DHA group (n=32) M (SD) | Placebo group (n=34) M (SD) | Between-group P-value (P/ES) |
|---|---|---|---|
| EDAH version for families | |||
| Hyperactivity (H) | |||
| Baseline | 8.4 (3.5) | 8.0 (3.3) | 0.654 (0.003) |
| 3 months | 7.4 (2.8) | 8.0 (2.8) | 0.281 (0.018) |
| 6 months | 7.5 (3.2) | 8.6 (3.3) | 0.027 (0.075) |
| Within-group P-value (P/ES) | 0.132 (0.126) | 0.146 (0.113) | |
| Interaction time x group (P/ES) | 0.083 (0.076) | ||
| Attention deficit (AD) | |||
| Baseline | 10.3 (2.6) | 9.0 (2.8) | 0.047 (0.060) |
| 3 months | 9.1 (2.7) | 9.4 (2.7) | 0.047 (0.061) |
| 6 months | 9.0 (2.7) | 9.6 (2.6) | 0.044 (0.063) |
| Within-group P-value (P/ES) | 0.022 (0.226) | 0.442 (0.050) | |
| Interaction time x group (P/ES) | 0.013 (0.128) | ||
| Attention deficit and hyperactivity (ADHD) | |||
| Baseline | 18.7 (4.7) | 17.0 (4.4) | 0.134 (0.035) |
| 3 months | 16.6 (4.7) | 17.7 (3.8) | 0.067 (0.052) |
| 6 months | 16.5 (4.6) | 18.1 (4.4) | 0.006 (0.133) |
| Within-group P-value (P/ES) | 0.009 (0.270) | 0.345 (0.064) | |
| Interaction time x group (P/ES) | 0.007 (0.145) | ||
| Conner’s Rating Scale for parents, mean (SD) | |||
| Baseline | 25.9 (4.2) | 25.0 (4.4) | 0.420 (0.010) |
| 3 months | 23.9 (3.9) | 23.6 (3.6) | 0.981 (<0.001) |
| 6 months | 24.3 (4.6) | 25.5 (3.8) | 0.059 (0.055) |
| Within-group P-value (P/ES) | 0.032 (0.206) | 0.007 (0.264) | |
| Interaction time x group (P/ES) | 0.091 (0.073) | ||
Abbreviations: ES, effect size; M, mean.
Results of EDAH scale for families and Conner`s Rating Scale for parents are shown in Table 3. In the DHA group, within-group significant differences at follow-up assessments as compared with baseline were observed in AD and attention-deficit hyperactivity (ADHD) components of the EDAH scale, with reduction of symptoms already noted after 3 months of treatment. By contrast, within-group differences were not found in the placebo group in any variables, although the increase in the means indicated deterioration in all the group of symptoms over time. Between-group differences at the end of treatment were observed for all three components of the EDAH scale. In the Conner’s scale, within-group differences were found in both study groups. The DHA group showed a decrease in the symptoms of ADHD from the first to the third moment, while the placebo group showed an increase over time. Between-group differences were not found in this case. The interaction time ⨰ treatment was statistically significant only in the case of AD and ADHD variables from the EDAH scale (see Table 3). As shown in Table S3 of the Supplementary material, similar results were found in the analysis of the ITT population.
Finally, when differences related to sex as a blocking factor or the interaction sex ⨰ treatment response as well as differences related medication as a blocking factor or the interaction medication ⨰ treatment response were analyzed, no statistically significant differences in baseline or postintervention variables (d2, AULA Nesplora, EDAH, and Conner`s) between the two study groups were found (data not shown), with the exception of the variable ADHD from EDAH in the post-test, where the medication groups scored statistically higher than the n onmedication groups, in both the treatment and the placebo conditions.
Adherence and safety
A total of 23 participants (34.8%) (DHA group, n=12; placebo group, n=11) gave consent for blood sampling. At baseline, DHA content of the erythrocyte membrane expressed as % of total FA was 3.4 (0.4) and 3.6 (0.4) in the DHA and placebo groups, respectively. At the end of treatment, however, DHA content of the erythrocyte membrane increased significantly in the DHA group as compared with placebo (5.7 [0.7] vs 3.7 [0.6]; P<0.001). These results confirm DHA treatment adherence and differences between the placebo and DHA groups. None of the participants was excluded due to lack of adherence.
Over the course of the 6 months, no instances of either major or minor adverse events were reported.
Discussion
This study investigated the effects of 6-month supplementation with highly concentrated DHA in the triglyceride form on cognitive and behavioral performance in children and adolescents diagnosed with ADHD. An increased erythrocyte membrane DHA is a reliable indirect indicator of good adherence in the supplemented group with the active product. The ω-3 PUFA DHA is found in large amounts in the phospholipids of neuronal membranes and has been shown to be relevant in brain function due to its involvement in many aspects, including membrane fluidity and interactions with key regulatory proteins, cell growth, gene expression, neural signaling, modulating the synthesis, transport and release of neurotransmitters, endothelial function, and neuronal survival.29,43–45 On the other hand, EPA is not a significant structural component of brain tissue, and DHA is several hundred-fold more abundant than EPA in the brain.30 The key role of DHA to contribute to optimal conditions for brain functioning46 was the rationale for using a highly concentrated DHA in the form of triglyceride as the investigational product. In this respect, this study is the first to investigate the effects of highly concentrated DHA supplementation compared with a placebo formulation on ADHD symptoms.
In relation to the effect of DHA supplementation on cognitive variables, d2, and AULA Nesplora executive function tests, differences between participants supplemented with DHA or placebo were not found. In fact, considerable improvement in attention variables during the 6 months of treatment occurred in both study groups. However, this study population was a clinical sample of ADHD belonging to different associations and clinics of our Principality of Asturias community, which means that all participants were receiving psychopedagogical and/or specific clinical interventions during the duration of the study. Although between-group differences were not observed, within-group improvements of almost all variables of the d2 test (selective attention) and variables related to visual attention (sustain attention tasks) in the AULA Nesplora test were of higher magnitude in the DHA supplementation group. The characteristics of the study sample (receiving clinical and/or psychopedagogical interventions) may explain that cognitive-attention changes may take longer to appear than improvements noted in behavioral features of ADHD. Of note, the greater magnitude of improvement in cognitive domains observed in the DHA-supplemented group opens an encouraging perspective for further studies in larger samples of youths with ADHD.
DHA supplementation for 6 months had a beneficial effect on symptoms of ADHD as shown by scores of the EDAH questionnaire for families. In this case, relevant between-group differences were found, with improvements in ADHD symptoms already evident after 3 months of treatment. Differences in the Conner’s scale were not observed, although there was a trend for improvement in the DHA-supplemented group. The characteristics of the 10-item abbreviated instrument joining hyperactivity/impulsivity and AD symptoms may account for the results obtained, since this instrument is less specific than the EDAH scale in the discrimination of ADHD symptoms.
Findings of the present study pointing toward a beneficial effect of DHA supplementation in the management of ADHD are in line with previous research.7,47,48 In a recent meta-analysis by Chang et al7 of seven RCTs totalling 534 youth with ADHD, the authors concluded that PUFA supplementation can improve both clinical symptoms and cognitive performance in children and adolescents with ADHD, although the number of studies showing significant changes at a behavioral level – clinical symptoms – is greater than those reporting cognitive changes. The authors also concluded that there is an important deficiency in ω-3 PUFAs levels in these populations. In the same line, Bos et al47 examined the effects of dietary ω-3 supplementation on ADHD behavior and cognitive variables in a sample of 40 boys ADHD and 39 controls, aged 8–14 years, included in a 4-month double-blind randomized placebo-controlled trial. The authors found an improvement in attentional symptoms rated by parents, in both study groups. However, changes at a cognitive level, including cognitive control tasks and fMRI measures of brain activity, were not observed. In the present study, covering a 6-month time period, some changes in cognitive variables were found at intrasubject level with generally greater improvements in the DHA group. Interestingly, in a recent 6-month randomized placebo-controlled study in 50 participants aged 7 to 14 years with ADHD, DHA supplementation showed a significant, nonetheless quite small, effect of psychosocial functioning, emotional problems, and focused attention, but failed to be superior as compared to placebo in parent ratings of ADHD behaviors.48 In this study, however, participants assigned to the experimental group received a dose of 500 mg algal DHA, which is half the DHA dose used in our study. The authors concluded that in the light of the absence of adverse events and somewhat positive effects of cognitive difficulties, DHA supplementation could be reasonably followed-up in future intervention studies.48 A recent study based on a Spanish population-based birth cohort provided interesting data on the potential modulating effect of material diet during pregnancy on the risk of developing long-term ADHD symptoms in the offspring.49 In this observational study, the authors measured the ratio of arachidonic acid (ω-6) and DHA and EPA (ω-3) concentrations in 953 cord blood samples and followed 642 children up to 7 years of age. The main finding was that a higher ω-6:ω-3 ratio in cord blood was associated with a higher ADHD index at 7 years using the 27-item Revised Conners’ Parent Rating Scale Short Form, with an increase in ADHD symptom scores about 13% per each ω-6:-3 ratio unit. On the whole, our findings together with previous research would provide support to the rationale for using ω-3 PUFAs as a treatment option for ADHD symptoms, and potentially for their cognitive correlates.
The present study should be interpreted considering some limitations, particularly the small sample size, thereby decreasing our ability to detect between-group differences in cognitive variables, and the fact that recruitment was based on a convenience (availability) sample. Although analyses were adjusted by ADHD medication, the effect or the intensity of concurrent psychological interventions was not evaluated, nor whether age range had any effect on outcome. On the other hand, doses of the pharmacological medication (stimulants, atomoxetine), intensity of psychological treatment, or eventual intensity of physical activity that could interfere with the results were not registered. A separate analysis for naïve patients and patients taken ADHD medications was not performed because a large majority (more than 70%) of participants was under pharmacological treatment. Cardiovascular data were not evaluated, so that a potential effect of dietary ω-3 supplementation improving cardiovascular risk in stimulant-treated patients was not explored. Biochemical analyses were performed in a relatively small percentage of participants, which prevented a more complete assessment of FA profile as well as to establish a correlation between biochemical and clinical findings. Although compliance with the nutraceutical formulation was checked at study visits by asking the participants to bring the empty boxes, and the investigator insisted on the importance of an adequate daily dosing, the lack of control over dietary intake of the subjects was among the limitations of the study. The fact that all participants had unequivocal diagnosis of ADHD and the randomized double-blind placebo-controlled design are strengths of the study.
Also, the use of the AULA Nesplora is a distinctive methodological feature since this new tool based on virtual reality has not been used in previous RCTs of ω-3 PUFAs supplementation for ADHD. External validity of research findings applies to a target population of Caucasian boys and girls aged 6–18 years diagnosed with ADHD (any type) given a highly concentrated dietary DHA supplement similar to that used in the study. A target population using other ω-3 PUFAs (composition/doses) will produce biased inferences.
Conclusion
The present study adds to evidence suggesting that ω-3 DHA dietary supplementation may be a beneficial complementary therapeutic approach in children and adolescents with ADHD. Further research is warranted to continue to explore the benefits of ω-3 DHA supplementation and the involvement of ω-3 PUFAs in the pathogenetic mechanism of ADHD.
Acknowledgements
The authors thank Jaume Borrás, MD for his coordination, Mayte Sotelo for monitoring of the trial, María Antonia González Álvarez for assistance in blood sampling, and Marta Pulido, MD, PhD, for editing the manuscript and for her editorial assistance.
Data sharing
Study data are available from the authors upon request.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Table S3.
Changes of variables of the EDAH scale for families and Conner’s Rating Scale for parents at the end of the study as compared with baseline in the two study groups (within-group comparisons). Analysis in the ITT sample
| Instrument | DHA group (n=46) | Placebo group (n=49) | Between-group P-value (P/ES) |
|---|---|---|---|
| EDAH version for families. mean (SD) | |||
| Hyperactivity (H) | |||
| Baseline | 8.5 (3.1) | 8.0 (3.2) | 0.272 (0.016) |
| 3 months | 7.3 (3.1) | 7.7 (3.0) | 0.621 (0.005) |
| 6 months | 6.8 (3.2) | 8.5 (3.3) | 0.008 (0.124) |
| Within-group P-value (P-value/ES) | <0.001 (0.455) | 0.072 (183) | |
| Interaction time x group (P-value/ES) | 0.001 (0.242) | ||
| Attention deficit (AD) | |||
| Baseline | 10.4 (2.6) | 8.8 (2.8) | 0.004 (0.108) |
| 3 months | 9.0 (3.0) | 9.2 (3.0) | 0.403 (0.013) |
| 6 months | 8.7 (2.9) | 9.5 (2.5) | 0.171 (0.033) |
| Within-group P-value (P-value/ES) | 0.009 (0.339) | 0.353 (0.072) | |
| Interaction time x group (P-value/ES) | 0.004 (0.190) | ||
| Attention deficit and hyperactivity (ADHD) | |||
| Baseline | 18.8 (5.1) | 16.7 (3.9) | 0.007 (0.094) |
| 3 months | 16.4 (5.3) | 17.1 (4.2) | 0.854 (0.360) |
| 6 months | 15.5 (4.7) | 18.3 (4.1) | 0.017 (0.101) |
| Within-group P-value (P-value/ES) | < 0.001 (0.522) | 0.068 (0.187) | |
| Interaction time x group (P-value/ES) | <0.001 (0.310) | ||
| Conner’s Rating Scale for parents. mean (SD) | |||
| Baseline | 25.6 (4.2) | 25.0 (4.8) | 0.151 (0.027) |
| 3 months | 23.0 (4.6) | 23.7 (4.3) | 0.656 (<0.001) |
| 6 months | 23.6 (4.1) | 24.9 (5.2) | 0.330 (0.020) |
| Within-group P-value (P-value/ES) | 0.032 (0.319) | 0.352 (0.088) | |
| Interaction time x group (P-value/ES) | 0.274 (0.063) | ||
Note: There was a systematic and statistically significant reduction of ADHD in the DHA group, whereas in the placebo group, symptoms appear to be stable or even an increase in mean values was found. The interaction time x treatment was statistically significant in all variables of the EDAH test. In relation to between-group differences, once considering differences in the pre-test and medication an covariables, statistically significant differences in the post-test between the two groups were observed in symptoms of hyperactivity and combined hyperactivity/attention deficit symptoms, in which mean values were higher in the placebo group. Pharmacological treatment did not have a significant effect on this relationship, although differences in the pre-test had an effect in all cases.
Abbreviation: ES, effect size.
References
- 1.Matthews M, Nigg JT, Fair DA. Attention deficit hyperactivity disorder. Curr Top Behav Neurosci. 2014;16:235–266. doi: 10.1007/7854_2013_249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez C, Areces D, García T, Cueli M, González-Castro P. Comparison between two continuous performance tests for identifying ADHD: traditional vs. virtual reality. Int J Clin Health Psychol. 2018;18(3):254–263. doi: 10.1016/j.ijchp.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalá-López F, Hutton B, Núñez-Beltrán A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: protocol for a systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2015;4(1):19. doi: 10.1186/s13643-015-0005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García T, Rodríguez C, Rodríguez J, Fernández-Suárez A, Richarte V, Ramos-Quiroga JA. Psychosocial profiles of adults with ADHD: a comparative study of prison and outpatient psychiatric samples. Eur J Psychol Appl L. 2019;11:41–49. doi: 10.5093/ejpalc2018a14 [DOI] [Google Scholar]
- 5.Hodgson K, Hutchinson AD, Denson L. Nonpharmacological treatments for ADHD: a meta-analytic review. J Atten Disord. 2014;18(4):275–282. doi: 10.1177/1087054712444732 [DOI] [PubMed] [Google Scholar]
- 6.Sonuga-Barke EJ, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170(3):275–289. doi: 10.1176/appi.ajp.2012.12070991 [DOI] [PubMed] [Google Scholar]
- 7.Chang JPC, Su KP, Mondelli V, Pariante CM. Omega-3 polyunsaturated fatty acids in youths with attention deficit hyperactivity disorder: a systematic review and meta-analysis of clinical trials and biological studies. Neuropsychopharmacology. 2018;43(3):534–545. doi: 10.1038/npp.2017.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078 [DOI] [PubMed] [Google Scholar]
- 9.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):329–349. doi: 10.1016/j.plefa.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 10.Weiser MJ, Butt CM, Mohajeri H. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. 2016;8(2):99. doi: 10.3390/nu8020099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauritzen L, Brambilla P, Mazzocchi A, Harslof LB, Ciappolino V, Agostoni C. DHA effects in brain development and function. Nutrients. 2016;8:pii E6. doi: 10.3390/nu8010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Fatty Acids. 2006;75(4–5):259–269. doi: 10.1016/j.plefa.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Kitson AP, Metherel AH, Chen CT, et al. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J Nutr Biochem. 2016;33:91–102. doi: 10.1016/j.jnutbio.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 14.Cunnane SC, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P. Docosahexaenoic acid homeostasis, brain aging and Alzheimer‘s disease: can we reconcile the evidence? Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):61–70. doi: 10.1016/j.plefa.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 15.Meyer BJ, Grenyer BF, Crowe T, Owen AJ, Grigonis-Deane EM, Howe PR. Improvement of major depression is associated with increased erythrocyte DHA. Lipids. 2013;48(9):863–868. doi: 10.1007/s11745-013-3801-7 [DOI] [PubMed] [Google Scholar]
- 16.Gillies D, Sinn J, Lad SS, Leach MJ, Ross MJ. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;11:7. doi: 10.1002/14651858.CD007986.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev. 2014;34(6):496–505. doi: 10.1016/j.cpr.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PR. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: a randomized controlled trial. Nutrition. 2012;28(6):670–700. doi: 10.1016/j.nut.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 19.Parletta N, Niyonsenga T, Duff J. Omega-3 and omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS One. 2016;11:e0156432. doi: 10.1371/journal.pone.0156432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Königs A, Kiliaan AJ. Critical appraisal of omega-3 fatty acids in attention-deficit/hyperactivity disorder treatment. Neuropsychiatr Dis Treat. 2016;12:1869–1882. doi: 10.2147/NDT.S68652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramalho R, Pereira AC, Vicente F, Pereira P. Docosahexaenoic acid supplementation for children with attention deficit hyperactivity disorder: a comprehensive review of the evidence. Clin Nutr ESPEN. 2018;25:1–7. doi: 10.1016/j.clnesp.2018.03.126 [DOI] [PubMed] [Google Scholar]
- 22.Cornu C, Mercier C, Ginhoux T, et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur J Adolesc Psychiatry. 2018;27(3):377–384. doi: 10.1007/s00787-017-1058-z [DOI] [PubMed] [Google Scholar]
- 23.Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. Omega-3 polyunsaturated fatty acid supplementation and cognition: a systematic review and meta-analysis. J Psychopharmacol. 2015;29(7):753–763. doi: 10.1177/0269881115587958 [DOI] [PubMed] [Google Scholar]
- 24.Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: a systematic review and meta-analysis. J Affect Disord. 2016;190:474–482. doi: 10.1016/j.jad.2015.09.053 [DOI] [PubMed] [Google Scholar]
- 25.Widenhorn-Müller K, Schwanda S, Scholz E, Spitzer M, Bode H. Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): a randomized placebo-controlled intervention trial. Prostaglandins Leukot Essent Fatty Acids. 2014;91(1–2):49–60. doi: 10.1016/j.plefa.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 26.Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50(10):991–1000. doi: 10.1016/j.jaac.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bélanger SA, Vanasse M, Spahis S, et al. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: a randomized, double-blind, placebo-controlled study. Paediatr Child Health. 2009;14(2):89–98. doi: 10.1093/pch/14.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salem NJR, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36(9):945–959. doi: 10.1007/s11745-001-0805-6 [DOI] [PubMed] [Google Scholar]
- 29.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011;44(2):216–222. doi: 10.1007/s12035-011-8200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Cin Nutr. 2006;86(6):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. The Wechsler Intelligence Scale for Children. 4th ed. London: Pearson Assessment; 2005. [Google Scholar]
- 32.Gasso F, Bogdanov P, Domingo JC. Docosahexaenoic acid improves endogenous antioxidant defense in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2008;49:5932. doi: 10.1167/iovs.07-0624 [DOI] [Google Scholar]
- 33.Results shown in the European Patent EP 1 962 825 B1 (held by BRUDY TECHNOLOGY SL) related to the use of DHA for treating a pathology associated with cellular oxidative damage. European patent granted.2014. April 2.
- 34.Brickenkamp R, Zillmer E. The D2 Test of Attention. Seattle, Washington: Hogrefe & Huber Publishers; 1998. [Google Scholar]
- 35.Seisdedos N. D2, Test de Atención. Adaptación española. Madrid: TEA Ediciones; 2002. [Google Scholar]
- 36.Climent G, Banterla F, Iriarte Y. AULA: Theoretical Manual. San Sebastián, Spain: Nesplora; 2011. [Google Scholar]
- 37.Areces D, Rodríguez C, García T, Cueli M, González-Castro P. Efficacy of a continuous performance test on virtual reality in the diagnosis of ADHD and its clinical presentations. J Atten Disord. 2016;22(11):1081–1091. doi: 10.1177/1087054716629711 [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez C, García T, Areces D. New and future challenges concerning the use of virtual reality tools for assessing ADHD. Curr Dev Disord Res. 2017;4:8–10. doi: 10.1007/s40474-017-0103-4 [DOI] [Google Scholar]
- 39.Farré A, Narbona J. EDAH:Scale for the Assessment of Attention Deficit Hyperactivity Disorder. Madrid: TEA Ediciones; 2001. [Google Scholar]
- 40.Parker JDA, Sitarenios G, Conners CK. Abbreviated Conner’s rating scales revisited: a confirmatory factor analytic study. J Atten Disord. 1996;1(1):55–62. doi: 10.1177/108705479600100105 [DOI] [Google Scholar]
- 41.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27(1):114–120. [PubMed] [Google Scholar]
- 42.Kline RB. Principles and Practice of Structural Equation Modeling. New York (NY): Guilford Press; 2011. [Google Scholar]
- 43.Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000;18(4–5):383–399. doi: 10.1016/S0736-5748(00)00013-7 [DOI] [PubMed] [Google Scholar]
- 44.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102(36):1058–1063. doi: 10.1073/pnas.0502903102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cederholm T, Salem NJR, Palmblad J. ω-3 Fatty acids in the prevention of cognitive decline in humans. Adv Nutr. 2013;4(6):672–676. doi: 10.3945/an.113.004556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LB, Ciappolino V, Agostoni C. DHA effects in brain development and function. Nutrients. 2016;8(1):e6. doi: 10.3390/nu8010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bos DJ, Oranje B, Veerhoek ES, et al. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2015;40(10):2298–2306. doi: 10.1038/npp.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crippa A, Tese A, Sangiorgio F, et al. Behavioral and cognitive effects of docosahexaenoico acid in drug-naïve children with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled clinical trial. Eur Child Adolesc Psychiatry. 2019;28(4):571–583. doi: 10.1007/s00787-018-1223-z [DOI] [PubMed] [Google Scholar]
- 49.López-Vicente M, Ribas Fitó N, Vilor-Tejedor N, et al. Prenatal omega-6: omega-3ratio and attention deficit and hyperactivity disorder symptoms. J Pediatr. 2019;22:pii: S0022-3476(19)30246-X. doi: 10.1016/j.jpeds.2019.02.022 [DOI] [PubMed] [Google Scholar]