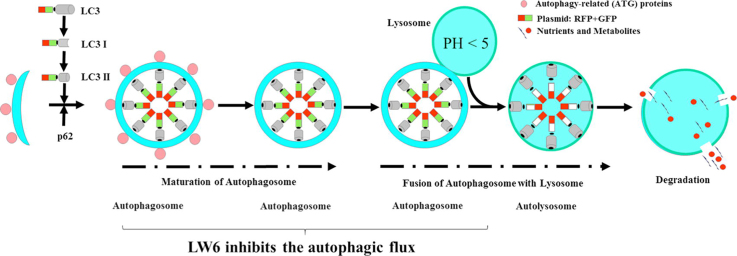
Graphical abstract
Keywords: LW6, Gemcitabine, Pancreatic cancer, Autophagy, Combination therapy
Abbreviations: LC3, microtubule-associated protein 1 light chain 3; p62, p62/SQSTM1; CQ, chloroquine; BrdU, 5-bromo-2′-deoxyuridine; 3-MA, 3-methyladenine; ATCC, American Type Culture Collection
Highlights
-
•
LW6 inhibits proliferation and induces cell death in pancreatic cancer cells.
-
•
LW6 improves the anti-proliferation efficacy of gemcitabine.
-
•
LW6 enhances gemcitabine-induced cell death.
-
•
LW6 in combination with gemcitabine decreases tumor weight.
-
•
LW6 inhibits autophagic flux.
Abstract
The efficacy of gemcitabine therapy is often insufficient for the treatment of pancreatic cancer. The current study demonstrated that LW6, a chemical inhibitor of hypoxia-inducible factor 1α, is a promising drug for enhancing the chemosensitivity to gemcitabine. LW6 monotherapy and the combination therapy of LW6 plus gemcitabine significantly inhibited cell proliferation and enhanced cell death in pancreatic cancer cells. This combination therapy also significantly reduced the tumor weight in a syngeneic orthotopic pancreatic carcinoma model without causing toxic side effects. In addition, this study provides insight into the mechanism of how LW6 interferes with the pathophysiology of pancreatic cancer. The results revealed that LW6 inhibited autophagic flux, which is defined by the accumulation of microtubule-associated protein 1 light chain 3 (LC3) and p62/SQSTM1. Moreover, these results were verified by the analysis of a tandem RFP-GFP-tagged LC3 protein. Thence, for the first time, these data demonstrate that LW6 enhances the anti-tumor effects of gemcitabine and inhibits autophagic flux. This suggests that the combination therapy of LW6 plus gemcitabine may be a novel therapeutic strategy for pancreatic cancer patients.
Introduction
Gemcitabine and gemcitabine-based strategies are the standard chemotherapies for treating advanced tumors that are detected in the breast, lung or pancreas [1], [2], [3]. Unfortunately, in contrast to the overall declining trends of breast and lung cancer, the death rates of pancreatic cancer rose 0.3% per year from 2011 to 2015 [4]. Moreover, for patients with a late stage of pancreatic cancer, the 5-year survival rate is only 3% [4]. Therefore, it is important to develop novel strategies to fight pancreatic cancer.
One promising drug for the treatment of cancer is LW6, which was originally reported to inhibit hypoxia-inducible factor 1α activity [5], [6]. Unfortunately, there is no evidence if this drug is beneficial for the treatment of pancreatic cancer or if this drug can be used in combination with traditional chemotherapeutics such as gemcitabine.
A key process that has been reported to regulate the pathophysiology of cancer and the sensitivity to chemotherapy is macroautophagy (autophagy) [7], [8]. It is recognized as a regulated process that degrades damaged organelles and molecules in order to recycle necessary nutrients. Several studies have demonstrated that upregulated autophagy in pancreatic cancer enhances the tumor progression and has cytoprotective effects when chemotherapeutics, such as gemcitabine and fluorouracil, are applied [7], [9], [10], [11]. Recently, multiple studies have suggested that inhibition of autophagy might be used as an anti-cancer strategy [12], [13], [14].
Thus, the present study evaluated whether the combination therapy of LW6 plus gemcitabine is a promising approach to treat pancreatic cancer. In addition, this study also investigated if LW6 enhances chemosensitivity to gemcitabine via the inhibition of autophagic flux.
Material and methods
Cell culture and reagents
The murine pancreatic adenocarcinoma cell line 6606PDA (a gift from Prof. Tuveson at the University of Cambridge, UK) and the MIA PaCa-2 cell line (human pancreatic cancer cell line purchased from ATCC, Manassas, USA) were cultured in the medium as reported previously [15]. To mimic hypoxic conditions, the cells were cultured in an Innova CO-48 incubator (New Brunswick Scientific Co, Edison, USA) with a 1% oxygen supply [15]. LW6 was purchased from Merck Millipore (Eschborn, Germany, code 400083) and was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 25 mM. Gemcitabine and chloroquine (CQ) were purchased from Sigma-Aldrich (St. Louis, USA, with the codes G6423 and PHR1258, respectively) and were dissolved in phosphate-buffered saline (PBS) to a final concentration of 25 mM or 50 mM, respectively. All these solutions were stored at −20 °C.
Western blotting
For western blots, 2.4 × 105 cells per well were plated in a 6-well plate. After 24 h, the cells were treated with the appropriate drug for distinct time periods. In addition, in experiments analyzing autophagic flux with CQ, the cells were treated for a maximum of 6 h just before the harvest of the cells. The western blots were performed as previously described [16] using the following antibodies: rabbit anti-microtubule-associated protein 1 light chain 3 (LC3, Sigma-Aldrich, St. Louis, USA, code L7543, dilution: 1000×), rabbit anti-p62/SQSTM1 (p62, Abcam, Cambridge, UK, code ab 109012, dilution: 8000×), rabbit anti-cleaved caspase 3 (Cell Signaling, Danvers, USA, code 9661, dilution: 1000×), mouse anti-β-actin antibody (Sigma-Aldrich, St. Louis, USA, code A5441, dilution: 20000×), peroxidase-linked anti-rabbit antibody (Cell Signaling, Danvers, USA, code 7074, dilution: 10000×) or peroxidase-linked anti-mouse antibody (Sigma-Aldrich, St. Louis, USA, code A9044, dilution: 60000×). The ratios of LC3II/β-actin and p62/β-actin were determined using a Chemi-Doc XRS System (Bio-Rad Laboratories, Munich, Germany) and are presented as the mean ± standard deviation (SD).
Evaluation of tandem RFP-GFP-targeted LC3 fluorescence
To perform this assay, 1 × 105 6606PDA cells per well or 4 × 105 MIA PaCa-2 cells per well were plated in a glass-bottom dish (NEST, Wuxi, China, code 801001). On the following day, the cells were transfected with the ptfLC3 plasmid (Addgene, Cambridge, UK, code 21074), which was a kind gift from Tamotsu Yoshimori [17], and Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, USA, code L3000001). After 24 h, the cells were treated with medium containing DMSO (Sham) or LW6 (80 µM for MIA PaCa-2, 160 µM for 6606PDA) for another 12 h and then were fixed with 4% formalin. The nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI). The images were acquired by a confocal microscope, Zeiss LSM 780 (Zeiss, Oberkochen, Germany), using the 60× oil objective. Only cells that were transfected with the ptfLC3 plasmid were evaluated. To evaluate the ratio of autophagosomes/cell in each field, the following formula was used: autophagosomes/cell = the number of yellow dots/the number of nuclei. Similarly, the ratio of autolysosomes/cell was defined as the number of red dots divided by the number of nuclei in each field. For each experiment, five fields per treatment were randomly acquired and evaluated.
Analysis of cell proliferation and cell death
For the evaluation of proliferation, 2 × 103 6606PDA cells per well or 4 × 103 MIA PaCa-2 cells per well were seeded into a 96-well plate. After 24 h, the cells were treated with medium containing DMSO (Sham) or with therapeutic agents as indicated in each figure. The cell proliferation was evaluated after 30 h by quantifying the incorporation of 5-bromo-2′-deoxyuridine (BrdU) with the colorimetric Cell Proliferation ELISA (Roche Diagnostics, Mannheim, Germany, code 11647229001) and the Perkin Elmer Victor X3 model 2030 Multilabel Plate Reader platform (PerkinElmer, Waltham, USA).
To analyze cell death, 3 × 104 6606PDA or MIA PaCa-2 cells per well were plated in a 24-well plate. On the following day, these cells were treated for 48 h with medium containing DMSO (Sham) or with the appropriate drug as indicated in each figure. Trypsinized and resuspended cells were stained with a trypan blue staining solution (Thermo Fisher Scientific, Waltham, USA, code 15250-061), and the percentage of dead cells was determined with the help of a Neubauer chamber (Thermo Fisher Scientific, Waltham, USA) in a blinded fashion.
Syngeneic orthotopic carcinoma model
To evaluate the tumor weight, a 5 μl cell suspension that contained 2.5 × 105 6606PDA cells was injected into the pancreas of C57BL/6J mice in accordance with the European Directive (2010/63/EU), and this procedure was approved by the local animal care committee (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern). The details of the syngeneic orthotopic carcinoma model have been described in our previous study [18]. Blood samples for evaluating the concentration of alanine aminotransferase (ALT) and alanine transaminase (AST) were taken before the euthanasia of mice. Then, the ALT and AST activities were analyzed in the blood plasma using the Cobas c111 analyzer (Roche Diagnostics, Mannheim, Germany).
Statistical analysis
In Fig. 1, the data are presented as the mean ± SD, and a two-way analysis of variance (with an inclusive Bonferroni test for post hoc comparison) was used to determine the significant differences in cell proliferation when the cells were cultured in hypoxic and normoxic conditions. In the other figures, the data are presented as the median (25th percentile and 75th percentile), and the Mann-Whitney U test was used to determine the difference between the two groups. In graphs that compare more than two groups, the differences were only considered to be significant when the P-value was lower than 0.05 divided by the number of meaningful comparisons (in order to correct for multiple comparisons using the Bonferroni correction) [19].
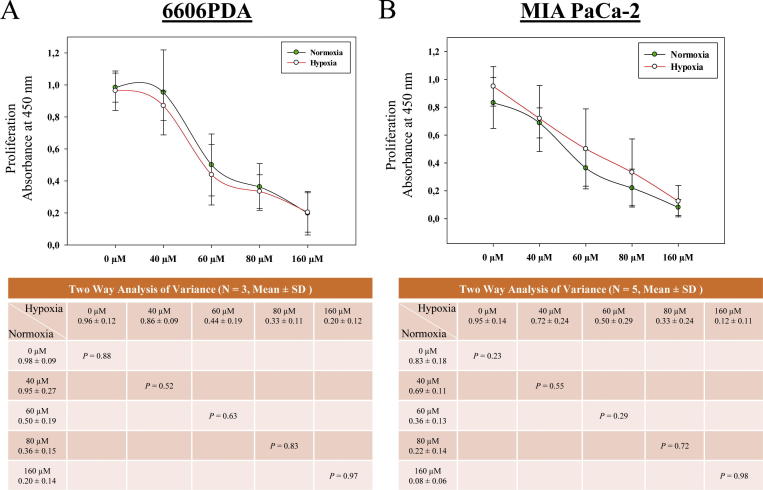
Fig. 1.
LW6 impairs pancreatic cancer cells under normoxic and hypoxic conditions. 6606PDA (A) and MIAPaCa-2 (B) cells were treated with the indicated concentrations of LW6 and were cultured under normoxic and hypoxic conditions for 30 h. The inhibition of cell proliferation by LW6 was not influenced by hypoxic conditions. P < 0.01 indicates a significant difference.
Results
LW6 inhibits proliferation and induces cell death in vitro
To clarify, if hypoxia is necessary to investigate the anti-cancer effects of LW6, 6606PDA and MIA PaCa-2 cells were cultured under normoxic and hypoxic conditions. Surprisingly, the inhibition of cell proliferation by LW6 was not influenced by the oxygen supply (Fig. 1). Thus, the following experiments were performed under normoxic conditions.
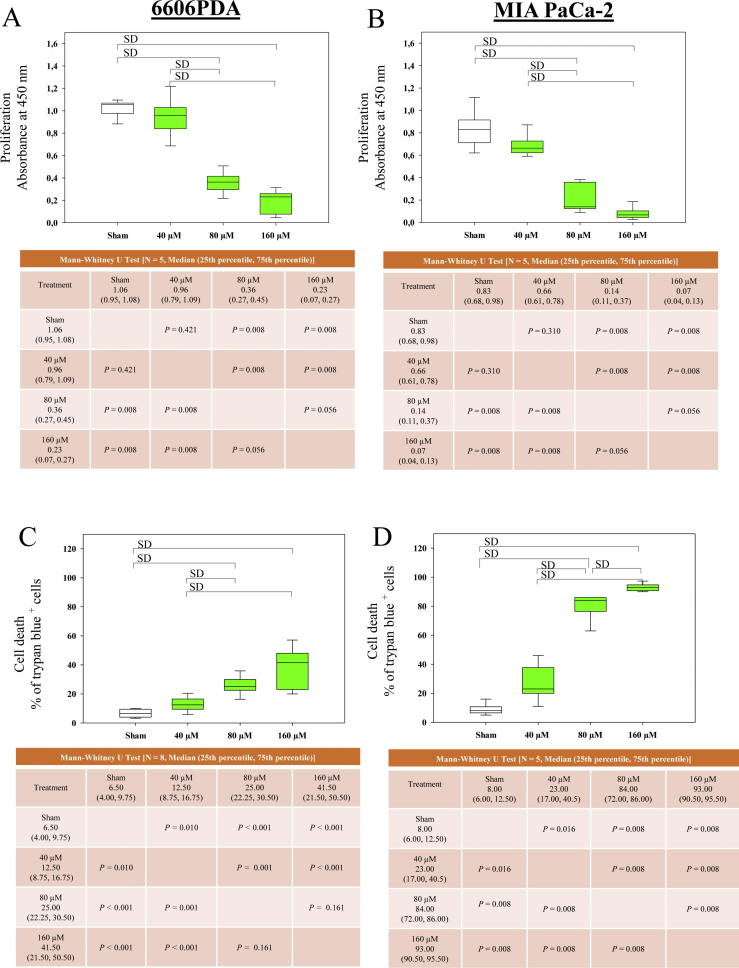
To investigate the anti-cancer effects of LW6, the proliferation and cell death of 6606PDA and MIA PaCa-2 cells were analyzed. In both cell lines, LW6 inhibited proliferation (Fig. 2A and 2B) and induced cell death (Fig. 2C and D) in a dose-dependent manner. In 6606PDA and MIA PaCa-2 cells, the application of 80 µM and 160 µM LW6 significantly inhibited cell proliferation compared to Sham-treated or 40 µM LW6-treated cells, respectively (Fig. 2A and B). In 6606PDA cells, these concentrations of LW6 also significantly increased cell death (Fig. 2C). In addition, LW6 had more efficient cytotoxic effects on MIA PaCa-2 cells than on 6606PDA cells. A dose of 80 µM LW6 killed almost 84% of the MIA PaCa-2 cells within 48 h (Fig. 2D). Thus, in the following experiment, 80 µM LW6 was applied to 6606PDA cells, and 40 µM LW6 was applied to MIA PaCa-2 cells.
Fig. 2.
LW6 inhibits proliferation and induces cell death. The amount of BrdU incorporation was analyzed after treating 6606PDA (A) and MIA PaCa-2 (B) cells with the indicated concentrations of LW6 for 30 h. In addition, 80 µM and 160 µM LW6 significantly inhibited cell proliferation compared to the cell proliferation of the Sham-treated cells or of the 40 µM LW6-treated cells (A and B). The percentage of dead cells was determined by a trypan blue assay after treating 6606PDA (C) and MIA PaCa-2 (D) cells with the indicated concentrations of LW6 for 48 h. The above mentioned concentrations of LW6 also significantly induced cell death compared to that in the Sham-treated cells or that in the 40 µM LW6-treated cells (C and D). P ≤ 0.008 indicates a significant difference (SD).
LW6 enhances chemosensitivity to gemcitabine in vitro
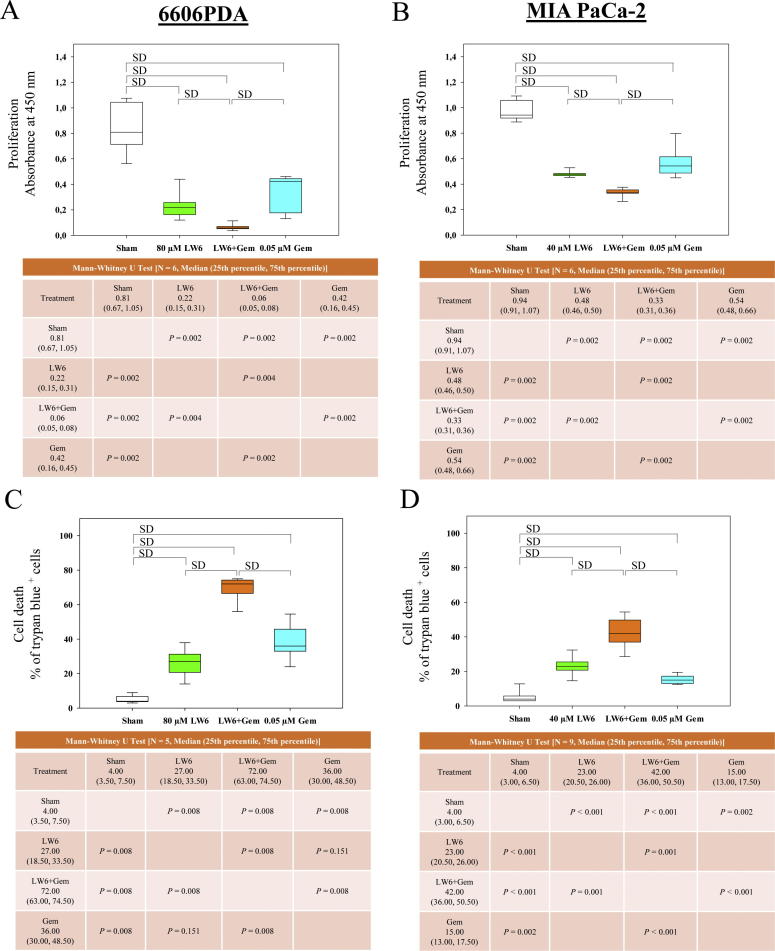
To analyze the feasibility of using LW6 in combination with traditional chemotherapeutics, the cell proliferation and cell death were evaluated after treating distinct pancreatic cancer cell lines with LW6 plus gemcitabine. Both monotherapies, 80 µM LW6 or 0.05 µM gemcitabine, significantly inhibited the proliferation of 6606PDA cells compared to the proliferation of the Sham-treated cells (Fig. 3A). Moreover, LW6 plus gemcitabine significantly inhibited the proliferation of 6606PDA cells compared to the proliferation of the Sham-treated cells or of cells treated separately with each monotherapy (Fig. 3A). Similar results were obtained after treating MIA PaCa-2 cells with 40 µM LW6 plus 0.05 µM gemcitabine (Fig. 3B).
Fig. 3.
LW6 in combination with gemcitabine (Gem) inhibits proliferation and induces cell death. The amount of BrdU incorporation was analyzed after treating 6606PDA (A) and MIA PaCa-2 (B) cells with the indicated concentrations of LW6 or 0.05 µM gemcitabine for 30 h. The combinational therapy, 80 µM LW6 plus 0.05 µM gemcitabine, significantly inhibited the proliferation of 6606PDA cells compared to the proliferation of the Sham-treated cells or that of the monotherapy-treated cells (A). Similar results were obtained after treating MIA PaCa-2 cells with 40 µM LW6 plus 0.05 µM gemcitabine (B). The percentage of dead cells was determined by a trypan blue assay after treating 6606PDA (C) and MIA PaCa-2 (D) cells with the indicated concentrations of LW6 or 0.05 µM gemcitabine for 48 h. LW6 plus gemcitabine treatment also significantly induced cell death compared to the levels of cell death induced in the Sham-treated or the monotherapy-treated cells (C and D). P ≤ 0.008 indicates a significant difference (SD).
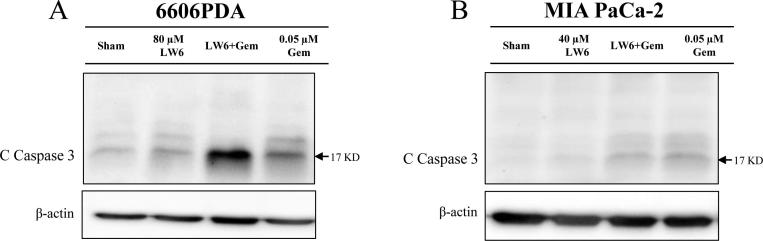
In addition, monotherapy with either 80 µM LW6 or 0.05 µM gemcitabine significantly induced 6606PDA cell death (Fig. 3C) and increased the accumulation of cleaved caspase 3 (Fig. 4A) compared to those of the Sham-treated cells. Moreover, the combined therapy of LW6 plus gemcitabine also significantly induced 6606PDA cell death (Fig. 3C) and increased the accumulation of cleaved caspase 3 (Fig. 4A) compared to those of the Sham-treated cells or those of cells treated with each monotherapy. Very similar results were obtained with MIA PaCa-2 cells when using the trypan blue assay. The application of 40 µM LW6 or 0.05 µM gemcitabine significantly increased the levels of MIA PaCa-2 cell death compared to that of Sham-treated cells (Fig. 3D). In addition, the combination therapy of LW6 plus gemcitabine also significantly induced cell death compared to the cell death induced by either the Sham treatment or treatment with each monotherapy (Fig. 3D). However, LW6 failed to increase the accumulation of cleaved caspase 3 compared to that of the Sham-treated cells, and LW6 reduced gemcitabine-induced cleavage of caspase 3 in MIA PaCa-2 cells (Fig. 4B).
Fig. 4.
Gemcitabine (Gem) increases the accumulation of cleaved caspase 3 (C Caspase 3). Western blots were performed after treating 6606PDA (A) and MIA PaCa-2 (B) cells with gemcitabine, LW6, or the combined treatment for 54 h. Gemcitabine increased the accumulation of cleaved caspase 3 in both 6606PDA (A) and MIA PaCa-2 (B) cells compared with that in Sham-treated cells. A similar result was obtained after treating 6606PDA cells with LW6 (A). However, LW6 failed to increase the level of cleaved caspase 3 in MIA PaCa-2 cells (B). The experiment was repeated three times.
LW6 in combination with gemcitabine impairs pancreatic cancer in vivo
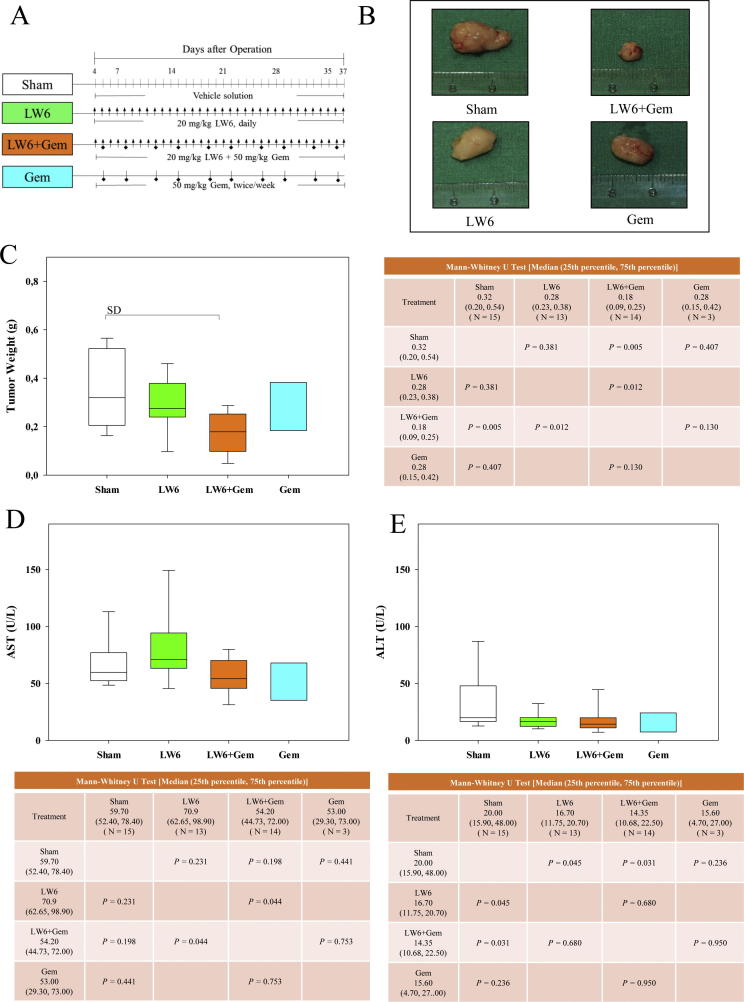
To confirm the in vitro results, the combination therapy of LW6 plus gemcitabine was evaluated using a syngeneic orthotopic pancreatic cancer model (Fig. 5A). Treatment with LW6 plus gemcitabine reduced the tumor volume and weight compared to treatment with LW6 or gemcitabine monotherapy (Fig. 5B and C). This combination therapy also significantly reduced the tumor weight compared to the tumor weight of Sham-treated mice (Fig. 5C). The liver toxicity of each monotherapy as well as the combination therapy was also analyzed. Each therapy failed to significantly increase the activities of AST (Fig. 5D) and ALT (Fig. 5E) in the blood.
Fig. 5.
LW6 in combination with gemcitabine (Gem) decreases the tumor weight. Cohorts of mice were treated i.p. with the vehicle solution (symbol: line), 20 mg/kg LW6 (symbol: arrow), 50 mg/kg gemcitabine (symbol: square), or the combined treatment (symbol: arrow plus square) as indicated in the experimental schema (A). The treatment of mice with 20 mg/kg LW6 plus 50 mg/kg gemcitabine led to an obvious decrease in the tumor size (B). This combination therapy significantly reduced the tumor weight compared to the tumor weight in the Sham-treated mice (C). All indicated therapies did not induce liver toxicity as defined by aspartate transaminase (AST) activity (D) or alanine aminotransferase (ALT) activity (E) in the blood plasma. P ≤ 0.008 indicates a significant difference (SD).
LW6 inhibits autophagic flux and enhances chemosensitivity to gemcitabine
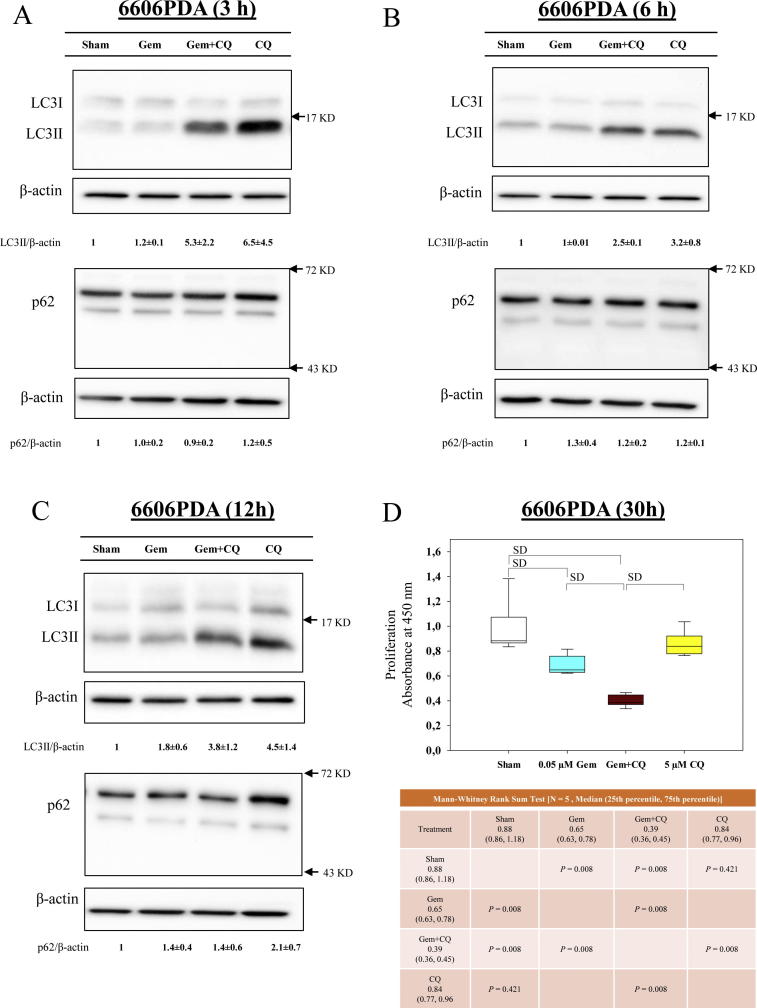
To evaluate if and how autophagy might be involved in the observed synergism between LW6 and gemcitabine, LC3II and p62, two key proteins involved in autophagy, were analyzed. First, the influence of gemcitabine on autophagic flux was assessed. Gemcitabine had no major influence on the accumulation of LC3II and failed to decrease the accumulation of p62, when treating 6606PDA cells for 3 h (Fig. 6A), 6 h (Fig. 6B) and 12 h (Fig. 6C). This suggests that gemcitabine does not induce autophagic flux within 12 h. However, when blocking autophagic flux with CQ, LC3II and p62 accumulated in 6606PDA cells (Fig. 6A–C). Interestingly, inhibition of autophagic flux by CQ (Fig. 6A–C) increased the chemosensitivity of 6606PDA cells to gemcitabine (Fig. 6D) in a similar manner as has been demonstrated with LW6 (Fig. 3A).
Fig. 6.
Blocking autophagic flux enhances the anti-proliferative effect of gemcitabine (Gem). After treating 6606PDA cells by 0.05 µM gemcitabine for 3 h (A), 6 h (B) and 12 h (C), gemcitabine only had minor effects on the accumulation of LC3II and p62. However, blocking autophagy with 5 µM chloroquine (CQ) could enhance the anti-proliferation effect of gemcitabine (D). P ≤ 0.008 indicates a significant difference (SD).
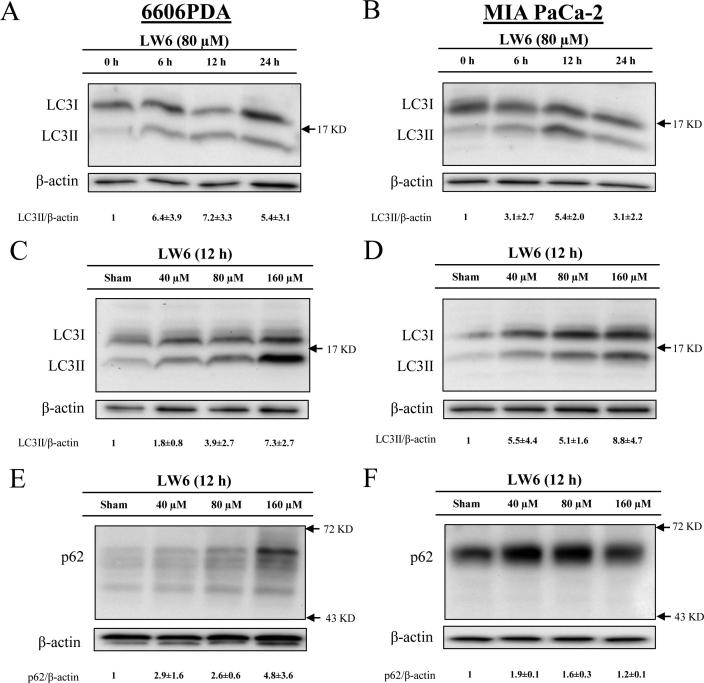
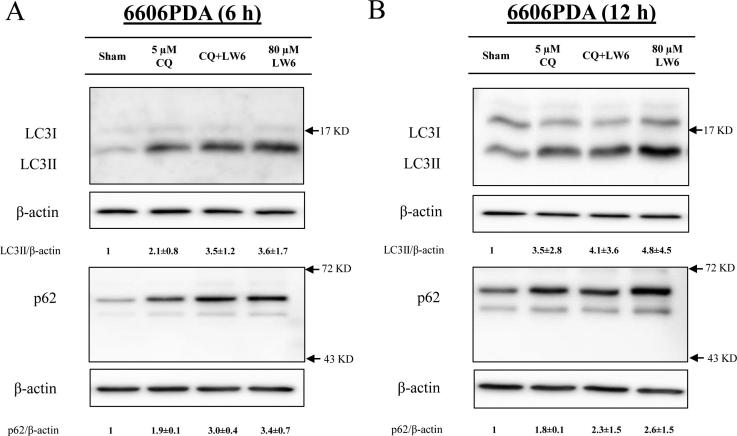
In order to evaluate, if and how LW6 influences the autophagic flux, the accumulation of LC3II and p62 was analyzed. We observed that 80 µM LW6 induced the accumulation of LC3II in a time-dependent manner in 6606PDA cells as well as MIA PaCa-2 cells, with the strongest induction at 12 h (Fig. 7A and B). Moreover, LW6 also induced the accumulation of LC3II in a dose-dependent manner in both cell lines (Fig. 7C and D). Similar to LC3II, p62 also accumulated after treating the cells with LW6 (Fig. 7E and F). LW6 inhibited the accumulation of LC3II and p62 in a similar manner to CQ, a traditional inhibitor of autophagic flux after 6 h (Fig. 8A) as well as 12 h (Fig. 8B). In addition, CQ in combination with LW6 failed to increase the accumulation of LC3II and p62, when compared to cells treated by LW6 monotherapy (Fig. 8). These data demonstrate that 80 µM LW6 completely blocks autophagic flux leading to increased accumulation of LC3II and p62.
Fig. 7.
LW6 regulates the accumulation of proteins involved in autophagy. After treating the cells with 80 µM LW6 or vehicle control (Sham) for the indicated time periods, the level of LC3II was increased in 6606PDA (A) and MIA PaCa-2 (B) cells. Treatment with LW6 for 12 h induced the accumulation of LC3II in a dose-dependent manner in both cell lines (C and D). In addition, this treatment also induced the accumulation of p62 in 6606PDA (E) and MIA PaCa-2 (F) cells. All experiments were repeated three times.
Fig. 8.
LW6 blocks autophagic flux. Treatment for 6 h (A) or 12 h (B) with 80 µM LW6 and during the last 6 h with 5 µM chloroquine (CQ) or a combination of both drugs caused accumulation of LC3II and p62. Compared to the LW6-treated cells, the combination therapy failed to increase the accumulation of LC3II and p62. All experiments were repeated three times.
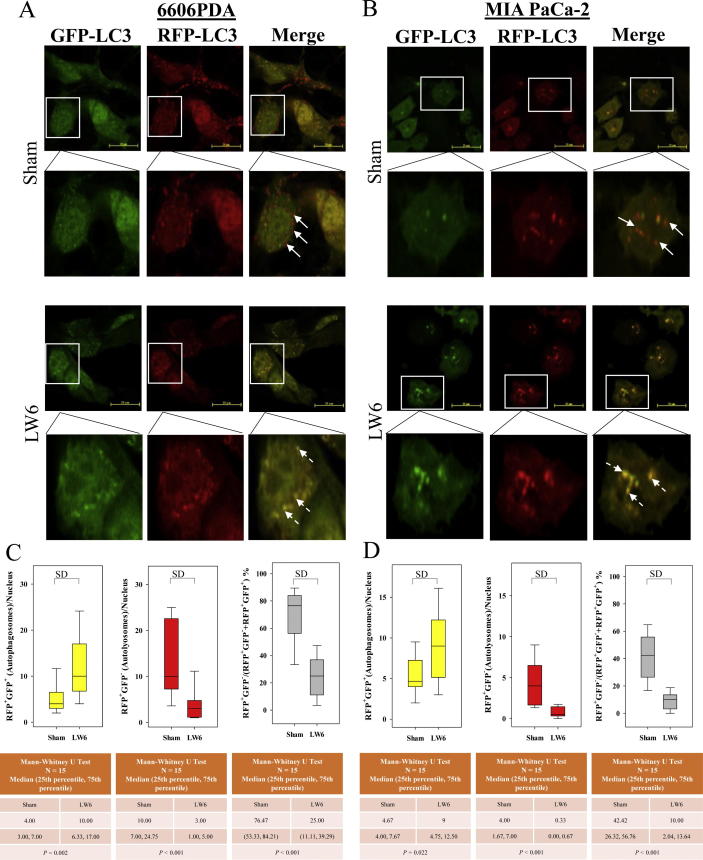
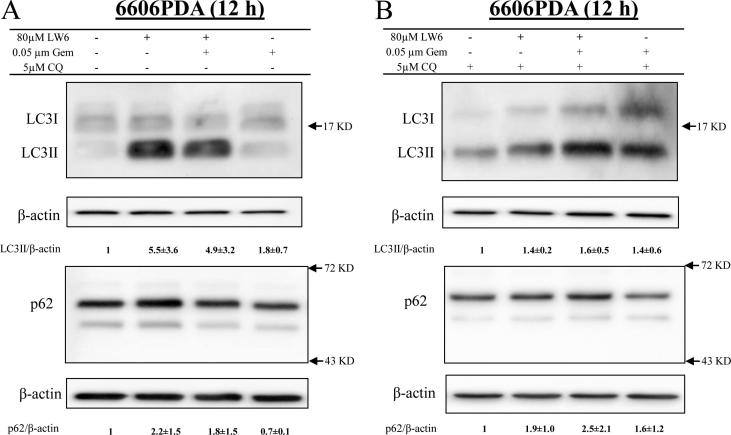
To reaffirm that LW6 inhibits autophagic flux, tandem RFP-GFP-tagged LC3 plasmids were transfected into cancer cells. In transfected 6606PDA and MIA PaCa-2 cells, autophagosomes and autolysosomes were identified based on their yellow or red fluorescence (Fig. 9A and B). Careful quantification demonstrated that LW6 significantly increased the number of autophagosomes and significantly decreased the number and percentage of autolysosomes in both cancer cell lines (Fig. 9C and D). These results confirmed that LW6 inhibited autophagic flux. In addition, the accumulation of LC3II and p62 were measured to determine if LW6 blocked autophagic flux in gemcitabine-treated cells. Indeed, LW6 increased the accumulation of LC3II and p62 in the absence and the presence of gemcitabine (Fig. 10A). Also in the presence of CQ, a minor increase in the accumulation of LC3II and p62 was observed after LW6 treatment (Fig. 10B). All these data suggest that LW6 impaired autophagic flux and enhanced the chemosensitivity to gemcitabine (Fig. 11).
Fig. 9.
LW6 increases the number of autophagosomes and decreases the number of autolysosomes. 6606PDA (A) and MIA PaCa-2 (B) cells were treated with 160 µM LW6 and 80 µM LW6 for 12 h, respectively. LW6 significantly increased the number of autophagosomes (C and D). In addition, LW6 also significantly decreased the number and percentage of autolysosomes in both cancer cell lines (C and D). The scale bar = 20 µm, P ≤ 0.05 indicates a significant difference (SD).
Fig. 10.
LW6 inhibits the autophagic process in gemcitabine-treated cells. LW6 plus gemcitabine caused a major increase in the accumulation of LC3II and p62 in the absence of CQ (A). Only a minor increase was observed when treating the cells with CQ for the last 6 h (B). All experiments were repeated three times.
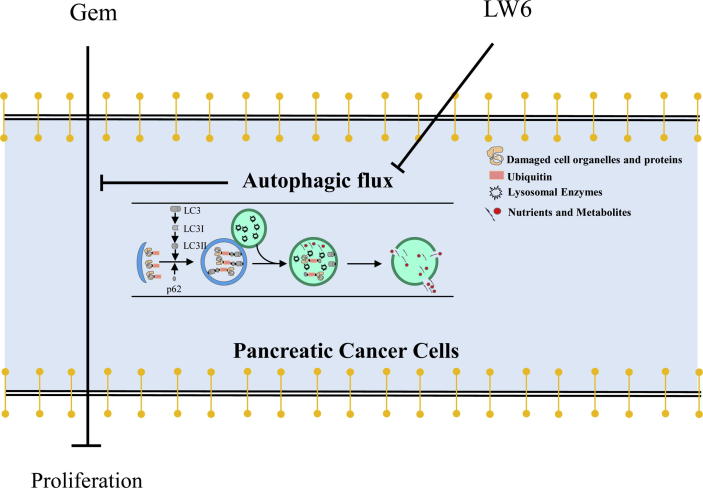
Fig. 11.
Summary. The present study demonstrates that LW6 enhances the chemosensitivity to gemcitabine and inhibits autophagic flux in pancreatic cancer.
Discussion
For the first time, the present study demonstrated that LW6 combined with gemcitabine was beneficial in treating pancreatic cancer. In addition, this study also characterized a completely novel mechanism of action for LW6: the inhibition of autophagic flux.
To evaluate if LW6 regulates autophagic flux, we analyzed LC3II, p62, autolysosomes and autophagosomes in murine and human pancreatic cancer cell lines. The data demonstrated that LW6 caused the accumulation of LC3II, p62 and autophagosomes (Fig. 7, Fig. 8, Fig. 9), whereas it decreases the number of autolysosomes (Fig. 9). All these results demonstrate that LW6 inhibited autophagic flux. In addition, we followed the guideline of monitoring autophagy to evaluate the drugs in combination with traditional inhibitors of autophagy such as CQ [20]. An additive or supra-additive effect in LC3-II levels may indicate that the drug enhances autophagic flux [20]. A drug, which increases the level of LC3-II on its own, but which induces a similar LC3-II level in the presence of CQ, when compared to the drug alone, may completely block autophagy [20]. Such an experiment was executed and the data also support the concept that LW6 inhibits the autophagic process (Fig. 8). Although the experiments were performed following the guidelines for monitoring autophagy [20] and assessed autophagy with these three assays, there were still technical limitations. For example, LC3II is expressed in a tissue- and cell context-dependent manner and may also be associated with the membranes of non-autophagic structures [21], [22], [23]. A relatively stable indicator to determine autophagy is p62. However, its accumulation can sometimes be regulated independent of autophagy [24], [25]. In spite of these minor limitations, the present study demonstrates that LW6 inhibits autophagic flux. LW6 might block autophagic flux between the maturation of autophagosomes and the formation of autolysosomes [26].
In general, many studies agree that gemcitabine can induce autophagic flux [27], [28], [29], [30], although we could not get convincing data that supports such an induction in 6606PDA cells (Fig. 6). However, it is controversial if gemcitabine-induced autophagy is cytoprotective or cytotoxic for pancreatic cancer cells. On the one hand, two studies have demonstrated that gemcitabine can induce cytotoxic autophagy [27], [28]. These studies relied on 3-methyladenine (3-MA) as an autophagic inhibitor, and it is well documented that 3-MA treatment can also induce autophagy [31]. Thus, several studies and the guidelines suggest that caution should be exercised when interpreting data that rely on 3-MA to block autophagy [20], [31], [32]. On the other hand, many studies have demonstrated that gemcitabine-induced autophagy has a cytoprotective effect in pancreatic cancer cells [7], [11], [12], [13], [33]. The results of the present study are consistent with the concept that the inhibition of autophagy enhances the sensitivity to gemcitabine (Fig. 6). A randomized phase II trial that is assessing the inhibition of autophagy in combination with gemcitabine and Abraxane is still ongoing (NCT01978184). This trial might clarify if the inhibition of autophagy is beneficial for cancer patients.
So far, no study has investigated the role of LW6 in pancreatic cancer. The present in vitro data demonstrate that LW6 can inhibit proliferation and can induce cell death in pancreatic cancer cells (Fig. 2). However, LW6 monotherapy leads only to a minor reduction of tumor weight in vivo (Fig. 5C). Interestingly, the combination therapy of LW6 plus gemcitabine did not only impair the proliferation and viability of cancer cells in vitro (Fig. 3) but also significantly reduced the tumor weight in vivo (Fig. 5C). Gemcitabine is still the first-line chemotherapy to treat advanced pancreatic cancer. Unfortunately, pancreatic cancer is often refractory to gemcitabine and, therefore, has a poor prognosis. For the first time, the present study demonstrates that LW6 enhances the chemosensitivity to gemcitabine in vitro and in a syngeneic orthotopic pancreatic carcinoma model. In addition, it suggests that LW6 enhances the chemosensitivity to gemcitabine by inhibiting autophagic flux (Fig. 11). This hypothesis is consistent with several previous studies, which have suggested that blocking autophagy strengthens the tumoricidal effect of gemcitabine [7], [11], [12], [13]. However, it is unlikely that the inhibition of autophagic flux is the only way that LW6 increases the sensitivity to gemcitabine. Regulating other processes, such as tumor immunity [6] and cell metabolism [34], [35], by LW6 might also enhance the anti-cancer effects of gemcitabine [36]. Thus, it was worth to evaluate the anti-cancer effect of LW6 and LW6 plus gemcitabine in vivo since inhibition of several pathways might be superior to an inhibition of only autophagy.
Although several publications have suggested that the inhibition of autophagy in addition to traditional chemotherapy may be a successful strategy [11], [12], the following questions still need to be answered: Does the inhibition of autophagy in addition to traditional chemotherapy truly benefit the patient? How do distinct drugs that inhibit autophagy compare to each other in their efficacy? Are some drugs especially useful because they not only inhibit autophagy but also interfere with other physiological processes that regulate cell survival and proliferation?
Conclusions
In conclusion, this study proposes that LW6 may represent a novel drug to inhibit autophagic flux in cancer cells (Fig. 11). This study also suggests that the combination therapy of LW6 plus gemcitabine might be promising and should, therefore, be evaluated on various cancer entities in preclinical as well as clinical studies.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
Acknowledgments
We thank Eva Lorbeer, Maren Nerowski, Berit Blendow, and Dorothea Frenz (Institute for Experimental Surgery, Rostock University Medical Center) for excellent technical assistance. We thank Prof. Robert Jaster for cooperating with us on the analysis of MiaPaca-2 cells. We also thank Prof. Dr. Barbara Nebe and Dr. rer. hum. Susanne Stählke (Department of Cell Biology, Rostock University Medical Center) for supporting data acquisition with the Zeiss LSM 780 confocal microscope.
Funding
Xianbin Zhang was supported by the China Scholarship Council (grant number: 201608080159). The study was supported by the Deutsche Forschungsgemeinschaft (DFG research group FOR 2591, grant number: 321137804, ZE 712/1-1 and VO 450/15-1).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Hu X.C., Zhang J., Xu B.H., Cai L., Ragaz J., Wang Z.H. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–446. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 2.Thatcher N., Hirsch F.R., Luft A.V., Szczesna A., Ciuleanu T.E., Dediu M. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.1, Kang J.E., Park S.K., Jin Y., Chung K.S., Kim H.M. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem Pharmacol. 2010;80:982–989. doi: 10.1016/j.bcp.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Eleftheriadis T., Pissas G., Antoniadi G., Liakopoulos V., Stefanidis I. Malate dehydrogenase-2 inhibitor LW6 promotes metabolic adaptations and reduces proliferation and apoptosis in activated human T-cells. Exp Ther Med. 2015;10:1959–1966. doi: 10.3892/etm.2015.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto D., Blauer M., Hirota M., Ikonen N.H., Sand J., Laukkarinen J. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. Eur J Cancer. 2014;50:1382–1390. doi: 10.1016/j.ejca.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeldt M.T., O'Prey J., Morton J.P., Nixon C., MacKay G., Mrowinska A. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 9.Gukovsky I., Li N., Todoric J., Gukovskaya A., Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(1199–209) doi: 10.1053/j.gastro.2013.02.007. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang A., Rajeshkumar N.V., Wang X., Yabuuchi S., Alexander B.M., Chu G.C. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. CancerDiscov. 2014;4:905–913. doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M.C., Wang H.C., Hou Y.C., Tung H.L., Chiu T.J., Shan Y.S. Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol Cancer. 2015;14:179. doi: 10.1186/s12943-015-0449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleger A., Perkhofer L., Seufferlein T. Smarter drugs emerging in pancreatic cancer therapy. Ann Oncol. 2014;25:1260–1270. doi: 10.1093/annonc/mdu013. [DOI] [PubMed] [Google Scholar]
- 13.Verbaanderd C., Maes H., Schaaf M.B., Sukhatme V.P., Pantziarka P., Sukhatme V. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience. 2017;11:781. doi: 10.3332/ecancer.2017.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor M.A., Das B.C., Ray S.K. Targeting autophagy for combating chemoresistance and radioresistance in glioblastoma. Apoptosis. 2018;23:563–575. doi: 10.1007/s10495-018-1480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zechner D., Burtin F., Albert A.C., Zhang X., Kumstel S., Schonrogge M. Intratumoral heterogeneity of the therapeutical response to gemcitabine and metformin. Oncotarget. 2016;7:56395–56407. doi: 10.18632/oncotarget.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zechner D., Albert A.C., Burtin F., Vollmar B. Metformin inhibits gemcitabine induced apoptosis in pancreatic cancer cell lines. J Cancer. 2017;8:1744–1749. doi: 10.7150/jca.17972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura S., Noda T., Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 18.Zechner D., Radecke T., Amme J., Burtin F., Albert A.C., Partecke L.I. Impact of diabetes type II and chronic inflammation on pancreatic cancer. BMC Cancer. 2015;15:51. doi: 10.1186/s12885-015-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 20.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanida I., Minematsu-Ikeguchi N., Ueno T., Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima N., Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 24.Bardag-Gorce F., Francis T., Nan L., Li J., He Lue Y., French B.A. Modifications in P62 occur due to proteasome inhibition in alcoholic liver disease. Life Sci. 2005;77:2594–2602. doi: 10.1016/j.lfs.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Nakaso K., Yoshimoto Y., Nakano T., Takeshima T., Fukuhara Y., Yasui K. Transcriptional activation of p62/A170/ZIP during the formation of the aggregates: possible mechanisms and the role in Lewy body formation in Parkinson's disease. Brain Res. 2004;1012:42–51. doi: 10.1016/j.brainres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Reggiori F., Ungermann C. Autophagosome maturation and fusion. J Mol Biol. 2017;429:486–496. doi: 10.1016/j.jmb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Mukubou H., Tsujimura T., Sasaki R., Ku Y. The role of autophagy in the treatment of pancreatic cancer with gemcitabine and ionizing radiation. Int J Oncol. 2010;37:821–828. doi: 10.3892/ijo_00000732. [DOI] [PubMed] [Google Scholar]
- 28.Pardo R., Lo Re A., Archange C., Ropolo A., Papademetrio D.L., Gonzalez C.D. Gemcitabine induces the VMP1-mediated autophagy pathway to promote apoptotic death in human pancreatic cancer cells. Pancreatology. 2010;10:19–26. doi: 10.1159/000264680. [DOI] [PubMed] [Google Scholar]
- 29.Ropolo A., Bagnes C.I., Molejon M.I., Lo Re A., Boggio V., Gonzalez C.D. Chemotherapy and autophagy-mediated cell death in pancreatic cancer cells. Pancreatology. 2012;12:1–7. doi: 10.1016/j.pan.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Song B., Bian Q., Zhang Y.J., Shao C.H., Li G., Liu A.A. Downregulation of ASPP2 in pancreatic cancer cells contributes to increased resistance to gemcitabine through autophagy activation. Mol Cancer. 2015;14:177. doi: 10.1186/s12943-015-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y.T., Tan H.L., Shui G., Bauvy C., Huang Q., Wenk M.R. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samaras P., Tusup M., Nguyen-Kim T.D.L., Seifert B., Bachmann H., von Moos R. Phase I study of a chloroquine-gemcitabine combination in patients with metastatic or unresectable pancreatic cancer. Cancer Chemother Pharmacol. 2017;80:1005–1012. doi: 10.1007/s00280-017-3446-y. [DOI] [PubMed] [Google Scholar]
- 34.Eleftheriadis T., Pissas G., Mavropoulos A., Liakopoulos V., Stefanidis I. Comparison of the effect of the aerobic glycolysis inhibitor dichloroacetate and of the Krebs cycle inhibitor LW6 on cellular and humoral alloimmunity. Biomed Rep. 2017;7:439–444. doi: 10.3892/br.2017.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naik R., Han S., Lee K. Chemical biology approach for the development of hypoxia inducible factor (HIF) inhibitor LW6 as a potential anticancer agent. Arch Pharm Res. 2015;38:1563–1574. doi: 10.1007/s12272-015-0632-5. [DOI] [PubMed] [Google Scholar]
- 36.Shukla S.K., Purohit V., Mehla K., Gunda V., Chaika N.V., Vernucci E. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart femcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32:71–87. doi: 10.1016/j.ccell.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.