Abstract
Heart failure (HF) is usually defined by the dominantly affected heart chamber; therefore, termed right or left HF (RHF or LHF). Pulmonologists understand RHF as a complex syndrome characterized by insufficient delivery of blood from the right ventricle associated with elevated systemic venous pressure at rest or exercise. Cardiologists specify LHF by its clinical functional class and the relation to a reduced (HFrEF), preserved (HFpEF) or mid-range ejection fraction (HFmrEF). Pediatric cardiologist, dealing also with patients with a failing single ventricle, define HF as a condition of insufficient systemic oxygen delivery (DO2). Certainly, pediatricians do not think of the right and left heart, or even a single ventricle as an isolated, independently acting entity. Because of the importance of cardiac interactions, the creation of a restrictive atrial communication aims at a palliative approach with the goal to diminish the congestive consequences of a dysfunctional ventricle; further to serve as a pop-off valve in order to prevent syncope and cardiovascular collapse. This review covers the background, the particular indications, the techniques and preliminary results achieved following the creation of a restrictive atrial septum defect (rASD) in different pathophysiological settings. Based on the institutional experience, percutaneous trans-catheter perforation of the atrial septum, followed by gradual balloon dilatation can be performed at any age and location worldwide. Medical institutions in low resource countries can make use of such palliating procedures in the setting of right as well as LHF independent of their pharmacological facilities.
Keywords: Heart failure (HF), pulmonary hypertension, atrioseptostomy, restrictive atrial septum defect (rASD), palliative treatment
Introduction
Heart failure (HF) represents worldwide a leading cause of mortality and morbidity (1,2). Considering that the left and right heart chambers do not work in isolation, but are inextricably linked, left and right heart interactions have to be analyzed together and in either left ventricular (LV) or right ventricular (RV) failure harnessed for therapeutic benefits (3). Given this functional unit, it appears that the well-intentioned definition of right HF (RHF) as a complex syndrome characterized by insufficient delivery of blood from the right ventricle associated with elevated systemic venous pressure at rest or exercise (4) seems to be too narrow; the definition needs to be extended on the systemic oxygen delivery as the most important pathophysiological variable. Oxygen delivery has been defined as the product of cardiac output (CO) and systemic oxygen content (CaO2). While there can be a degree of RHF, even when the systemic CO and DO2 are still preserved; meaning in patients with pulmonary arterial hypertension (PAH)-related RHF, the indication for the creation of an atrial or arterial communication is improvement of DO2 (5). The systemic DO2 is improved by an increase in the CO even at the expense of a lower CaO2. A modeling study showed greater benefits for a reverse Potts-shunt, when compared with those achieved following the creation of an atrial communication (6). Additionally, a restrictive systemic vein fenestration is frequently created when dealing with patients suffering from a failing Fontan circulation; in the case of a systemic vein hypertension that is combined with an increased transpulmonary pressure gradient, a restrictive fenestration can effectively decrease the systemic vein pressure and increase the systemic blood flow while bypassing a small, but effective amount of blood through a high resistance pulmonary circulation (7). The severity of left-HF is assessed by clinical symptoms and characterized as a functional class; the NYHA classification is used for adults and the Ross-classification for infants and young children. From a pathophysiological point of view, LHF is related to a reduced (HFrEF), preserved (HFpEF) or mid-range ejection fraction (HFmrEF) (8), and related to the limitation to increase CO at rest or during exercise.
Our review aims to summarize the present knowledge concerning the creation of a restrictive atrial communication as a therapeutic palliation to improve consequences of RHF and/or LHF, even in the setting of HFpEF or HFrEF.
Why a restrictive atrial septum defect (rASD)?
Right and left atria are separated by a primum and secundum septum, which usually have grown together. From the morphological point of view, a patent foramen ovale (PFO) is not a defect, but rather a functionally open communication; both septal tissues are normally overlapping, but have not grown together. Like a valve, the overlapping septum primum and secundum allows only a right to left shunt, as it is physiological during fetal life. Therefore, any atrial left to right shunt is associated with a defect within the atrial septum. Congenital atrial septum defects can be located anywhere in the septum (9); a secundum defect (ASD-II) has a reduced size primum septum in the region (fossa ovalis) where both septa are usually overlapping; a primum defect (ASD-I) is due to insufficient tissue in the region connected to the atrioventricular valve(s). Therefore, a septum primum defect is oftentimes associated with an inlet ventricular septum defect, described as an atrioventricular septum or cushion defect. Superior or inferior sinus venosus defects (SVD) are usually associated with a partial anomalous pulmonary vein return. Atrial defects can also be based on a multi-perforated fossa ovalis (Chiari network) or even positioned within the coronary sinus. From a pathophysiological point of view, atrial septum defects are described as restrictive or non-restrictive. The extreme variant of a non-restrictive atrium septum defect is a common atrium, in which the secundum and primum septum is completely absent. By definition, a rASD does not allow equalization of left (LAP) and right atrial pressures (RAP), despite the rare condition, that both atrial pressures might have the same level independent of the persistence of an atrial communication. In a structurally normal heart with a right-to-left ventricular free wall thickness ratio of 1:3, a left-to-right shunt with a physiologic interatrial pressure gradient of 2–5 mmHg would be the result. On the other hand, under similar conditions, a non-rASD would be associated with a pulmonary-to-systemic flow ratio (Qp/Qs) of 3:1. Usually, a left-to-right interatrial shunt is considered clinically relevant when the Qp/Qs is >1.5 or when the shunt causes volume and/or pressure overload of the subpulmonary chamber. In case of a restrictive communication, the atrial shunt and consequently the Qp/Qs depend on the size of ASD, the difference of interventricular compliance, atrioventricular valve function and the pressure gradient between the atria. Considering the not so simple pathophysiology of atrial shunts, a rASD may be better defined by morphological criteria such as the ratio of the defect diameter to the total septum length. In congenital atrial defects, a ratio of a defect diameter to total septum length of less than 25% is functionally restrictive, independent of the shunt direction (10). Accordingly, in adults a rASD should have a dimension of less than 14 mm, optimally between 8–10 mm (10). Thus, the direction and the fraction of a shunt at the atrial level are not solely defined by the defect diameter; a common atrium can be associated without any or only a non-significant bi-directional shunt, if the compliance of the right and left ventricle is identical. It follows that a dilated left atrium (LA) in the setting of a left-to-right shunt cannot be associated with a non-restricted ASD, if a structural wall defect is excluded. Irrespective of the underlying cause, left atrial (LA) dilatation due to an increase in LA pressure (LAP) represents the common pathway leading to pulmonary congestion and acute or chronic pulmonary edema that account for more than 90% of admissions in acutely decompensated LV-HF (11). A reduction of the LAP is most likely associated with improved outcome in patients with HFrEF (12), and a significant direct association has been demonstrated between elevated pulmonary capillary wedge pressure (PCWP) and mortality (13). Atrial septostomy with the intent to create an ASD, especially in adult patients with pulmonary hypertension and RHF has become a therapeutic strategy for many years and guidelines recommend an atrial communication for bridging patients to transplant (14,15). The creation of a rASD in order to treat young patients with PAH-related syncope (because of a very “well trained” right ventricle with a hyper-reactive pulmonary vascular system) has rarely been reported (16,17). Additionally, recent data show that the creation of a small (defined as restrictive) ASD determining a pressure-dependent, left-to-right atrial shunt in patients with LV-HF may protect from the impact of LA-congestion and LA-hypertension as well as preventing acute decompensation (18,19).
Previous observations have led to the hypothesis that shunting blood between the atria can reduce upstream elevated pressures. Patients with the combination of mitral stenosis and a small ASD are less symptomatic and have better outcomes than patients with pure mitral stenosis. Those patients experience a hemodynamically relevant rise in the pulmonary artery pressure and acute pulmonary edema after closure of their ASD, suggesting that the atrial shunt via the ASD allows LA decompression resulting in a hemodynamic benefit (20). Based on clinical observations and computer models, atrial devices with a fixed diameter or manufactured fenestration have been developed for long-term HF palliation (21,22). Current guidelines recommend pre-interventional testing (balloon occlusion with an assessment of the left ventricular end-diastolic pressure in patients with ASD and systolic or diastolic LV dysfunction prior to the ASD closure (23). Also of importance, the creation of an ASD via balloon atrial septostomy, or placement of a transseptal cannula has been associated with ventricular recovery in patients with severe LV dysfunction who cannot be weaned from extra-corporeal membrane oxygenation because of refractory pulmonary congestion (24). We have shown beneficial effects creating a rASD in children and young adults with dilatative and restrictive or hypertrophic cardiomyopathy (18,19,25); the rASD with a limited left-to-right shunt significantly lowered pulmonary vein pressure, improved congestive symptoms and set in motion a favourable LV reverse remodeling and improved LV-EF. A major issue concerns short- and long-term effects of the left-to-right interatrial shunt on load conditions of the right heart and on forward LV output (26). Noteworthy, the creation of a non-restrictive atrial communication could be fatal in both, left and RHF. In patients with left sided HF-associated high left-to-right atrial pressure gradients a non-restrictive communication may be associated with a hemodynamically relevant, non-tolerable shunt; in the setting of RV-HF a clinically non-tolerable cyanosis may result. As mentioned above, an atrial communication with a diameter of less than 10 mm but above 4 mm is usually restrictive and effective in adult patients with severe HF. However, we learnt in the setting of a decompensated Fontan circulation caused by a systemic venous hypertension of up to 20 mmHg and a high transpulmonary pressure gradient (above 7–10 mmHg), that a fenestration with a diameter of 4 mm—bigger communication might be fatal—is suitable and very effective in reducing the venous pressure and improving the systemic CO. Current PAH guidelines do not recommend the creation of an atrial septum defect in patients with a central venous pressure above 20 mmHg. Here, the size of the ASD needs to be taken into account (14). Based on our own experience, we recommend, particular in patients with a high systemic venous pressure, a palliative atrial communication, as long as the diameter of the ASD does not exceed 4–5 mm.
How to create a rASD?
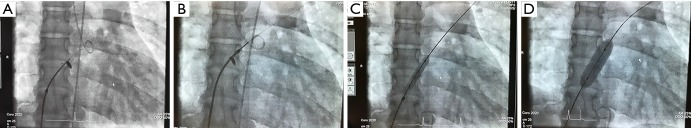
Based on our recently published data (17-19,25), the purpose of generating an effective but restrictive atrial communication is to enrich the palliative repertoire of right (RHF) and left HF (LHF) treatment. Technical details of percutaneous rASD generation have been recently reported (17,18,25). Therefore, here we want only to focus on a few technical aspects. We postulate that the risk of perforation of the intra-atrial septum (IAS) by trans-septal needle technique is reciprocally proportional to the size of the LA. We have translated the Brockenbrough transseptal needle technique applied in critically ill newborns with a hypoplastic left heart syndrome (oftentimes due to an extremely diminished LA) to young adults with PAH-associated LA compression (27,28); in terms of coronary guide-wire advancement immediately after needle perforation of the IAS; the wire is pushed through the transseptal needle and placed in one of the left-sided pulmonary veins before the transseptal instrumental ensemble is advanced to the LA. This technique reduces the risk of LA free-wall perforation and allows even pre-ballooning of the IAS, if necessary to facilitate advancing the transseptal ensemble to the left (Figure 1A,B,C,D). In contrast, the risk of generating a transcatheter rASD is quite a bit lower in LHF with an enlarged LA. Independent of the puncture technique, a high-pressure-balloon should be used for the initial IAS-dilatation, preferably a balloon catheter, which can be advanced through the initially utilized 6 or 8 Fr transseptal sheath. We want to emphasize the process of gradual ballooning of the IAS in order to induce a sustained effectiveness of a solely balloon-dilated IAS, and also to avoid the creation of a non-rASD. Usually, the IAS dilation is complete with a balloon to ASD diameter ratio of 2:1 (5). Safety and efficacy of a balloon-created rASD has been demonstrated, however, some patients need repeat interventions when the atrial communication becomes inefficient. The efficacy of the rASD is achieved when the functional class has been improved and echocardiographic imaging parameters have beneficially changed.
Figure 1.
Depicts the transseptal approach by the Brockenbrough technique. (A) Shows the perforation of the atrial septum after contrast marking of the septal area; (B) demonstrates the trans-needle coronary wire placement within the left upper pulmonary vein; (C) the first balloon dilatation of the septum by an 8 mm × 30 mm Powerflex® balloon; (D) the final dilatation with a 12 mm × 30 mm high-pressure balloon.
Summary of our current experience with the creation of a rASD in HF
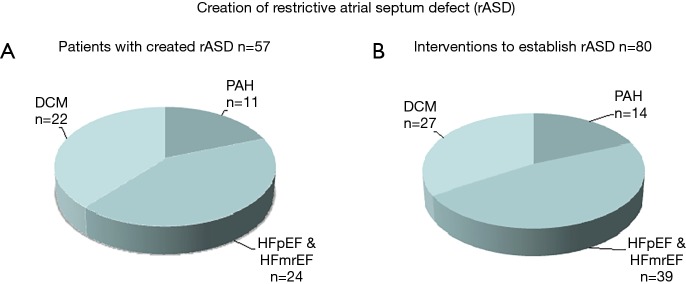
The purpose of a rASD is to palliate congestive symptoms of right or left heart disease and to reduce the risk of PAH-associated syncope. A rASD can be created with a low-risk and almost without mortality at any age from infancy to senior age (Figure 2A), provided that experienced operators perform the procedure. The efficacy of the generated rASD is best shown by immediate and long-lasting improvement of the clinical functional class. When using the technique of balloon dilatation without a device defined fenestration, one should expect that repeat interventions will be necessary; in our case series 30% of the patients required one or more IAS re-manipulations (Figure 2B). The majority of patients had LHF with preserved or reduced ejection fraction; a smaller group was treated because of PAH-associated syncope or manifest RHF.
Figure 2.
Shows patients and interventional procedures to create (A) or to re-establish (B) a restrictive atrial septum defect (rASD). DCM, dilated cardiomyopathy; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; PAH, pulmonary arterial hypertension.
PAH-associated syncope/RH-failure
PAH-related syncope is a rewarding pathophysiological entity to palliate by a rASD when combined with PAH-specific drugs that reduce pulmonary vascular hyper-reactivity. The patients are usually young. They have a muscular RV that is well-adapted to a systemic or suprasystemic pressure-, and they usually have no or insignificant tricuspid valve insufficiency; at rest, the mitral valve allows the passage of a sufficient blood volume with a normal or inverse inflow pattern. The non-dilated inferior cava vein represents normal central venous pressures. Near-syncope or manifest syncope develop acutely during myocardial and cerebral ischemic attacks, the later are frequently misinterpreted as epileptic seizures. The hyper-reactive pulmonary vascular bed prohibits an acute increase in pulmonary blood-flow, thus the LV preload becomes inadequate resulting in a failure of the CO to increase and meet the need of a sufficient cerebral vascular perfusion. An effective, but restrictive atrial communication prevents such ischemic situations. At rest the rASD manifests usually a left-to-right or bi-directional shunt.
Very different is a syncope based on end-stage RHF that results in a systemic low CO at rest. These patients are in functional class IV, demonstrate systemic venous hypertension with a dilated caval vein, liver and bowel congestion peripheral edema, ascites and pericardial effusion. The right ventricular function is marginal, forward flow is insufficient also due to severe tricuspid valve regurgitation, which results from an increased RV volume and RV dilatation. In this “end-stage RV” scenario of severe and progressive PAH syncope attacks can be triggered by bradycardia and be lethal. The therapeutic goal is to unload the failing RV and buy time for a lung transplant, if this treatment is an option. Another option is a reverse Potts-shunt. The creation of a rASD serves as a bridge to transplant or as a life-prolonging palliation. The palliative approach of a rASD, in the setting of an extremely increased systemic venous pressure (up to 20 mmHg), requires that the atrial communication must be truly restrictive with diameters ranging from 3 to maximal 5 mm; if the communication is wider the patient will develop a non-tolerable cyanosis and have an insufficient pulmonary oxygen uptake. In a clinical situation that allows a decision between a rASD or a reverse Potts-shunt, based on a mathematical model, a rPotts appears to be preferable and more effective (6).
LH-failure with preserved and reduced ejection fraction
In contrast to the established role of atrioseptostomy in PAH patients, the creation of a rASD as a palliation of LHF is less established and has just recently been recommended (29). Indeed, diuretics are used as first-line medication treating congestive LHF with preserved as well as LHF with reduced EF (30). Diuretics are routinely administered and chronically prescribed with little concern regarding the disadvantages of long-term diuretic treatment (31,32). Based on the concept that outcome might be improved by decreasing LA pressure (12), it is worth to discuss the idea of a “first-line” mechanical decompression of the LA, before high-dose diuretic treatment is utilized as a last palliative action (32). Such a strategy which applies the creation of a rASD makes sense particularly in context of the pathophysiology of HF; HFpEF, atrial enlargement and hypertension do not necessarily correlate with symptoms of pulmonary vascular congestion at rest, but likely during exercise. In contrast, patients with HFrEF show early symptoms of LA and pulmonary congestion; LA functional incompetence together with a systolic dysfunctional LV are responsible for a systemic low CO at rest, additional diastolic dysfunction of the LV generates pulmonary congestion. At the time, when diuretic treatment becomes unavoidable in order to reduce the vexing dyspnea, we entertain the option of generating a rASD, rather than starting diuretics that will worsen neuro-humoral activation. Although our experience is still limited, we have observed an improvement of atrioventricular coupling and a reduced occurrence of supra-ventricular tachycardia i.e., atrial fibrillation due to LA decompression. Our follow-up data showed a sustained clinical benefit in adult patients with HFpEF (26).
Conclusions
Generation of a transcatheter interatrial communication by balloon dilatation of the atrial septum is a safe approach for the decompression of the left and right atrium in patients with various cardio-vascular diseases, including patients with a restrictive LV physiology after heart transplantation;
a restrictive atrial communication has no apparent side effects, but achieves age-independent improvements of the clinical functional status;
the rASD provides symptomatic improvement and a bridge towards transplant or to recovery if further regenerative strategies are available;
atrial decompression is a palliative option in the setting of diuretic drug refractory treatment for atrial and pulmonary congestion.
Acknowledgements
Data used within the review are parts of the thesis of cand. med. Anna Bauer.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Towbin JA, Bowles NE. The failing heart. Nature 2002;415:227-33. 10.1038/415227a [DOI] [PubMed] [Google Scholar]
- 2.Maggioni AP, Dahlstrom U, Filippatos G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF pilot). Eur J Heart Fail 2013;15:808-17. 10.1093/eurjhf/hft050 [DOI] [PubMed] [Google Scholar]
- 3.Burns KM, Byrne BJ, Gelb BD, et al. New mechanistic and therapeutic targets for pediatric heart failure: report from a national heart, lung, and blood institute working group. Circulation 2014;130:79-86. 10.1161/CIRCULATIONAHA.113.007980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahm T, Douglas IS, Archer SL, et al. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2018;198:e15-43. 10.1164/rccm.201806-1160ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schranz D, Akintuerk H, Voelkel NF. 'End-stage' heart failure therapy: potential lessons from congenital heart disease: from pulmonary artery banding and interatrial communication to parallel circulation. Heart 2017;103:262-7. 10.1136/heartjnl-2015-309110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delhaas T, Koeken Y, Latus H, et al. Potts shunt to be preferred above atrial septostomy in pediatric pulmonary arterial hypertension patients: a modeling study. Front Physiol 2018;9:1252. 10.3389/fphys.2018.01252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rupp S, Schieke C, Kerst G, et al. Creation of a transcatheter fenestration in children with failure of Fontan circulation: focus on extra-cardiac conduit connection. Catheter Cardiovasc Interv 2015;86:1189-94. 10.1002/ccd.26042 [DOI] [PubMed] [Google Scholar]
- 8.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209-16. 10.1016/j.ahj.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Webb G, Gatzoulis MA, Atrial septal defects in the adults. Circulation 2006;114:1645-53. 10.1161/CIRCULATIONAHA.105.592055 [DOI] [PubMed] [Google Scholar]
- 10.Kaye D, Shah SJ, Borlaug BA, et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail 2014;20:212-21. 10.1016/j.cardfail.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 11.Ritzema J, Troughton R, Melton I, et al. on Behalf of the hemodynamically guided home self-therapy in severe heart failure patients (HOMEOSTASIS) Study Group. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010;121:1086-95. 10.1161/CIRCULATIONAHA.108.800490 [DOI] [PubMed] [Google Scholar]
- 12.Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during Exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014;35:3103-12. 10.1093/eurheartj/ehu315 [DOI] [PubMed] [Google Scholar]
- 13.Abbate A, Arena R, Abouzaki N, et al. Heart failure with preserved ejection fraction: refocusing on diastole. Int J Cardiol 2015;179:430-40. 10.1016/j.ijcard.2014.11.106 [DOI] [PubMed] [Google Scholar]
- 14.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 15.Abman SH, Hansmann G, Archer SL, et al. Pediatric Pulmonary Hypertension. Guidelines From the American Heart Association and American Thoracic Society. Circulation 2015;132:2037-99. 10.1161/CIR.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 16.Lammers AE, Haworth SG, Diller GP. Atrial septostomy in patients with pulmonary hypertension: should it be recommended? Expert Rev Respir Med 2011;5:363-76. 10.1586/ers.11.25 [DOI] [PubMed] [Google Scholar]
- 17.Bauer A, Khalil M, Schmidt D, et al. Creation of a restrictive atrial communication in pulmonary arterial hypertension (PAH): effective palliation of syncope and end-stage heart failure. Pulm Circ 2018;8:2045894018776518. 10.1177/2045894018776518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latus H, Yerebakan C, Akintuerk H, et al. Transcatheter interatrial communications for the treatment of left heart disease: application in the pediatric population. J Heart Lung Transplant 2016;35:1274-5. 10.1016/j.healun.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 19.Bauer A, Khalil M, Schmidt D, et al. Transcatheter Left atrial decompression in patients with Dilated Cardiomyopathy: Bridging to Cardiac Transplantation or Recovery. Cardiol Young 2019;29:355-62. 10.1017/S1047951118002433 [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann R, Altiok E, Reith S, et al. Functional Effect of New Atrial Septal Defect After Percutaneous Mitral Valve Repair Using the MitraClip Device. Am J Cardiol 2014;113:1228-33. 10.1016/j.amjcard.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 21.Del Trigo M, Bergeron S, Bernier M, et al. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet 2016;387:1290-7. 10.1016/S0140-6736(16)00585-7 [DOI] [PubMed] [Google Scholar]
- 22.Hasenfuß G, Hayward C, Burkhoff D, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet 2016;387:1298-304. 10.1016/S0140-6736(16)00704-2 [DOI] [PubMed] [Google Scholar]
- 23.Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. 10.1093/eurheartj/ehq249 [DOI] [PubMed] [Google Scholar]
- 24.Seib PM, Faulkner SC, Erickson CC, et al. Blade and balloon atrial septostomy for left heart decompression in patients with severe ventricular dysfunction on extracorporeal membrane oxygenation. Catheter Cardiovasc Interv 1999;46:179-86. [DOI] [PubMed] [Google Scholar]
- 25.Bauer A, Khalil M, Luedemann M, et al. Creation of a restrictive atrial communication in heart failure with preserved and mid-range ejection fraction: effective palliation of left atrial hypertension and pulmonary congestion. Clin Res Cardiol 2018;107:845-57. 10.1007/s00392-018-1255-x [DOI] [PubMed] [Google Scholar]
- 26.Kaye DM, Hasenfuss G, Neuzil P, et al. One-Year Outcomes After Transcatheter Insertion of an Interatrial Shunt Device for the Management of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2016;9(12). 10.1161/CIRCHEARTFAILURE.116.003662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupp S, Michel-Behnke I, Valeske K, et al. Implantation of stents to ensure an adequate interatrial communication in patients with hypoplastic left heart syndrome. Cardiol Young 2007;17:535-40. 10.1017/S104795110700090X [DOI] [PubMed] [Google Scholar]
- 28.Schranz D, Bauer A, Reich B, et al. Fifteen-year single center experience with the ‘‘Giessen Hybrid’’ approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol 2015;36:365-73. 10.1007/s00246-014-1015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 30.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. The Task force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J 2012;33:1787-847. 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 31.Ellison DH, Felker GM. Diuretic Treatment in Heart Failure. N Engl J Med 2017;377:1964-75. 10.1056/NEJMra1703100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schranz D. Diuretic Treatment in Heart Failure. N Engl J Med 2018;378:683. 10.1056/NEJMc1716477 [DOI] [PubMed] [Google Scholar]