Abstract
Background and Purpose
As little is known about the effect of caffeine, one of the most widely consumed substances worldwide, on intestinal function, we aimed to study its action on intestinal anion secretion and the underlying molecular mechanisms.
Experimental Approach
Anion secretion and channel expression were examined in mouse duodenal epithelium by Ussing chambers and immunocytochemistry. Ca2+ imaging was also performed in intestinal epithelial cells (IECs).
Key Results
Caffeine (10 mM) markedly increased mouse duodenal short‐circuit current (I sc), which was attenuated by a removal of either Cl− or HCO3 −, Ca2+‐free serosal solutions and selective blockers of store‐operated Ca2+ channels (SOC/Ca2+ release‐activated Ca2+ channels), and knockdown of Orai1 channels on the serosal side of duodenal tissues. Caffeine induced SOC entry in IEC, which was inhibited by ruthenium red and selective blockers of SOC. Caffeine‐stimulated duodenal I sc was inhibited by the endoplasmic reticulum Ca2+ chelator (N,N,N′,N′‐tetrakis(2‐pyridylmethyl)ethylenediamine), selective blockers (ruthenium red and dantrolene) of ryanodine receptors (RyR), and of Ca2+‐activated Cl− channels (niflumic acid and T16A). There was synergism between cAMP and Ca2+ signalling, in which cAMP/PKA promoted caffeine/Ca2+‐mediated anion secretion. Expression of STIM1 and Orai1 was detected in mouse duodenal mucosa and human IECs. The Orai1 proteins were primarily co‐located with the basolateral marker Na+, K+‐ATPase.
Conclusions and Implications
Caffeine stimulated intestinal anion secretion mainly through the RyR/Orai1/Ca2+ signalling pathway. There is synergism between cAMP/PKA and caffeine/Ca2+‐mediated anion secretion. Our findings suggest that a caffeine‐mediated RyR/Orai1/Ca2+ pathway could provide novel potential drug targets to control intestinal anion secretion.
Abbreviations
- [Ca2+]cyt
cytosolic Ca2+ concentration
- 2‐APB
2‐aminoethoxydiphenyl borate
- CaCC
Ca2+‐activated Cl− channels
- CFTR
cystic fibrosis transmembrane conductance regulator
- CRAC
Ca2+ release‐activated Ca2+ channels
- DNDS
4,4′‐dinitrostilbene‐2,2′‐disulfonic acid
- ER
endoplasmic reticulum
- GI
gastrointestinal
- IECs
intestinal epithelial cells
- IP3R
inositol 1,4,5‐trisphosphate receptor
- IRBIT
IP3R binding protein released with inositol 1,4,5‐trisphosphate
- Isc
short‐circuit current
- NFA
niflumic acid
- NKA
Na+, K+‐ATPase
- RyR
ryanodine receptor
- SOCE
store‐operated Ca2+ entry
- TPEN
N,N,N′,N′‐tetrakis(2‐pyridylmethyl) ethylenediamine
What is already known
Cell Ca2+ and cAMP signalling play important roles in the regulation of intestinal epithelial ion transports.
What this study adds
Caffeine mediates intestinal epithelial anion secretion through RyR/Orai1/Ca2+ signalling pathway and the crosstalk between Ca2+ and cAMP.
What is the clinical significance
Caffeine‐mediated RyR/Orai1/Ca2+ pathway could provide novel potential drug targets to control intestinal anion secretion.
1. INTRODUCTION
Caffeine is a well‐known constituent of coffee, cacao, and tea, and one of the most widely consumed substances in the world. In 2014, about 85% of American adults ingested, on average, 164‐mg caffeine daily (Mitchell, Knight, Hockenberry, Teplansky, & Hartman, 2014). Caffeine intake induces several physiological effects, including stimulation of nervous and musculoskeletal systems, relaxation of bronchial and vascular smooth muscle, and stimulation of intestinal motility and gastric acid secretion (Liszt et al., 2017; Mitchell et al., 2014; Poleszak et al., 2016). Caffeine also exerts various pharmacological effects with diverse effects on pain, Alzheimer's disease, asthma, cancer, diabetes, and Parkinson's disease (Cano‐Marquina, Tarin, & Cano, 2013). Caffeine has long been known to stimulate small intestinal water secretion in humans (Wald, Back, & Bayless, 1976), but the underlying mechanisms are poorly understood so far, except for its possible activation of intestinal net sodium secretion (Wagner, Mekhjian, Caldwell, & Thomas, 1978). Surprisingly enough, although physiological and pharmacological effects of caffeine intake have been extensively investigated outside the gastrointestinal (GI) tract, very little is known about its effects on intestinal epithelial anion secretion.
Epithelial ion transports are critical physiological processes in the human GI tract. Intestinal epithelium either absorbs electrolytes or secretes anions (such as Cl− and HCO3 −), providing the driving force for fluid transport to maintain fluid homeostasis in the human body (Seidler et al., 2011). Intestinal epithelial anion secretion is under control of several neuro‐humoral factors, such as ACh, PGE2, and 5‐HT. These neuro‐humoral factors mediate intestinal epithelial anion secretion mainly through three cell signalling pathways, dependent on Ca2+‐, cAMP and cGMP (Flemstrom & Garner, 1982). Currently, the physiological roles and molecular mechanisms of cAMP‐ and cGMP‐dependent regulation of epithelial Cl− and HCO3 − secretion are well defined (Rao, Nash, & Field, 1984; Tuo et al., 2009), whereas those mediated by Ca2+ signalling in the epithelia of the small intestine, remain poorly understood. Moreover, although it is known that Ca2+ signalling is critical for intestinal epithelial anion secretion (Jung & Lee, 2014), the underlying detailed mechanisms that control cytosolic Ca2+ concentration ([Ca2+]cyt) homeostasis in small intestinal epithelial cells (IECs) are not fully understood (Xie et al., 2014).
It is generally thought that agonists induce Ca2+ signalling via two major processes in non‐excitable cells: intracellular Ca2+ release mainly from the endoplasmic reticulum (ER) and Ca2+ entry from the extracellular medium (Putney, 2007). The IP3‐sentitive and ryanodine‐sensitive Ca2+ stores have been identified within the ER (Putney, 1986; Putney, 1990). The former is activated by the binding of IP3 to IP3 receptors (IP3R), and the latter is activated by the binding of ryanodine to ryanodine receptors (RyR) to induce ER Ca2+ release into the cytosol (Kocks, Schultheiss, & Diener, 2002). The RyR was first identified in the ER of electrically excitable cells, such as skeletal muscle cells and cardiomycytes, but it was later found to be expressed in non‐excitable cells, such as IEC. Although the roles of IP3R/Ca2+ pathway in the GI epithelial anion secretion have been reported (Berridge, 1993; Prole & Taylor, 2016), little is known about RyR expression and function of the RyR/Ca2+ store in these processes. In GI epithelium, the mRNA expression of RyR was characterized only in native rat colonic epithelium (Prinz & Diener, 2008), where it may be involved in colonic anion secretion (Kocks et al., 2002). However, so far, nothing is known about the functional roles of RyR in the small intestinal epithelium.
Classically, Ca2+ entry in non‐excitable epithelial cells is thought to occur mainly via so‐called capacitative or store‐operated Ca2+ entry (SOCE), whose activation is entirely dependent on the depletion of intracellular Ca2+ stores (Putney, 1990). The channels carrying this Ca2+ current are the Ca2+ release‐activated Ca2+ channels (CRAC) first described in mast cells and Jurkat lymphocytes (Hoth & Penner, 1993), which are formed from the pore forming Ca2+ Orai channels (Mignen, Thompson, Yule, & Shuttleworth, 2005; Molnar et al., 2016). Although there are a few studies on the CRAC/Orai channels in IEC‐6 cell line (Rao et al., 2010) and colonic epithelial cells (Lefkimmiatis et al., 2009; Onodera, Pouokam, & Diener, 2013), little is currently known about their expression and function in the native intestinal epithelium. Furthermore, it is still unclear whether the RyR‐mediated ER Ca2+ release constitutes a critical component of the SOCE or CRAC/Orai channels to regulate anion secretion in epithelial cells of the upper GI tract.
In the present study, we have investigated the effects of caffeine in GI epithelial anion secretion and the underlying molecular mechanisms. Moreover, caffeine is a well‐known RyR activator but it has been shown to inhibit IP3R activity (Brown, Sayers, Kirk, Michell, & Michelangeli, 1992), making it possible to clearly distinguish the function of RyR‐mediated or IP3R‐mediated ER Ca2+ release. We therefore used caffeine to explore the RyR/Ca2+‐mediated native anion secretion in the intestine and also to elucidate the important crosstalk between cell Ca2+ and cAMP signalling in this process.
2. METHODS
2.1. Cell culture
SCBN, a duodenal epithelial cell line of canine origin (Buresi et al., 2001; a kind gift from Dr. André Buret, Univ. of Calgary, Calgary, Canada), was cultured in fresh DMEM supplemented with 10% FBS, l‐glutamine, and streptomycin every 2–3 days. After the cells had grown to confluence, they were replated onto 12‐mm round coverslips (Warner Instruments Inc., Hamden, CT) and incubated for at least 24 hr before use for [Ca2+]cyt measurement.
2.2. Animal study
All animal care and experimental studies complied with the guidelines of the Animal Ethical Committee of Third Military Medical University and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85‐23, revised 1996) and were approved by the Third Military Medical University Committee on Investigations Involving Animal Subjects. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010; McGrath & Lilley, 2015) and with the recommendations made by the British Journal of Pharmacology. All experiments were performed with adult male Harlan C‐57 black mice (6–12 weeks old; 18–22 g; Chongqing Tengxin Biotechnology Co. LTD, Chongqing, China). The mice were housed in a standard animal care room with a 12–12 hr light–dark cycle with free access to water and food pellets. The mice were given an electrolyte solution containing polyethylene glycol 4000 (institutional pharmacy) and fibre‐free chow (diet C1013; Altromin, Beijing, China) to prevent intestinal impaction. Before each experiment, the mice were deprived of food and water for at least 1 hr. After brief narcosis with 100% CO2, the mice were killed by cervical dislocation.
Animal were assigned randomly to different experimental groups for all studies. Data collection and evaluation of all experiments were performed blindly, and the experimenters were unaware of group treatments.
2.3. Ussing chamber experiments
Ussing chamber experiments were performed as previously described (Tuo et al., 2012). The duodenal tissue from each mouse was stripped of seromuscular layers, divided, and examined in four chambers (window area, 0.1 cm2). Experiments were performed under continuous short‐circuited conditions (Voltage–Current Clamp, VCC MC6; Physiologic Instruments, San Diego, CA). The transepithelial short‐circuit currents (I sc) were measured via an automatic voltage clamp, in which μA was used for the original recordings, but μA·cm−2 was used for summary data.
After a 15–30 min measurement of basal parameters, different inhibitors or solutions were added to the mucosal, serosal side, or both sides of the tissues for 10–20 min, followed by addition of caffeine or cabarchol. The mucosal solution used in the Ussing chamber experiments contained the following: 140‐mM Na+, 5.4‐mM K+, 1.2‐mM Ca2+, 1.2‐mM Mg2+, 120‐mM Cl−, 25‐mM gluconate, and 10‐mM mannitol. The serosal solution contained the following: 140‐mM Na+, 5.4‐mM K+, 1.2‐mM Ca2+, 1.2‐mM Mg2+, 120‐mM Cl−, 25‐mM HCO3 −, 2.4‐mM HPO4 2−, 2.4‐mM H2PO4 −, 10‐mM glucose, and 0.1‐μM indomethacin, to inhibit possible endogenous PGE2 production resulting from mucosal injury during experiments which might affect the basal I sc. The osmolalities for both solutions were ∼300 mosmol·kg−1 of H2O.
2.4. Measurement of [Ca2+]cyt by digital Ca2+ imaging
Ca2+ imaging experiments were performed as previously described (Wan et al., 2017). Cells grown on coverslips were loaded with 5‐μM Fura‐2/AM in physiological salt solution, described below, at room temperature (∼22°C) for 50 min and then washed for 30 min. Thereafter, the coverslips with epithelial cells were mounted in a perfusion chamber on a Nikon microscope stage (Nikon Corp., Tokyo, Japan). The ratio of Fura‐2/AM fluorescence with excitation at 340 or 380 nm (F340/380) was followed over time and captured using an intensified charge‐coupled device camera (ICCD200) and a MetaFluor imaging system (Universal Imaging Corp., Downingtown, PA). The physiological salt solution used in digital Ca2+ measurement contained the following: 140‐mM Na+, 5‐mM K+, 2‐mM Ca2+, 147‐mM Cl−, 10‐mM HEPES, and 10‐mM glucose (pH 7.4). For the Ca2+‐free solution, Ca2+ was omitted, but 0.5‐mM EGTA was added. The osmolality for all solutions was ∼300 mosmol·kg−1 of H2O.
2.5. Immunohisto‐cytochemistry
The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology. Immunohisto‐cytochemistry was carried out as described previously (Tuo et al., 2009). Briefly, the slides with duodenal tissues from C‐57 mice or with IECs were incubated with an anti‐STIM1 antibody (1:100 dilution, ab108994, a rabbit mAb) or anti‐Orai1 channels antibody (1:100 dilution, ab86748, a rabbit mAb) or anti‐Na+, K+‐ATPase antibody (NKA; 1:100 dilution, ab2872, a mouse mAb) monoclonal antibody overnight at 4°C. The primary antibodies were detected with biotinylated goat anti‐rabbit IgG or biotinylated goat anti‐mouse IgG (Vector Laboratories, Burlingame, CA) secondary antibody at room temperature for 1 hr. Immunoreactivity was detected using a HRP (3′‐,3′‐diaminobenzidine) kit (BioGenex, San Francisco, CA) followed by counterstaining with haematoxylin, dehydration, and mounting. Slides were then examined with a Nikon Eclipse 800 Research microscope. To demonstrate the STIM1 or Orai1 specificity of the antibody labelling, a control experiment was performed in which the primary antibody was omitted.
2.6. Transfection of siRNA against Orai1 channels
The siRNAs targeted for human Orai1 (si‐Orai1) and the negative control (NC) siRNA were purchased from RiboBio (Guangzhou, China). Caco‐2 cells (RRID:CVCL_0025; Sciencell Research Laboratories, San Diego, CA) were seeded on Transwell polycarbonate membrane (Corning Incorporated, ME, USA) 1 day before siRNA transfection; 2‐ml antibiotic‐free DMEM containing 10% FBS, 0.16‐nM siRNA or NC, and 4‐μl Lipofectamine RNAimax was added to the serosal side of the membrane in each well. Experiments were conducted on 48 hr after transfection.
2.7. Quantitative PCR analysis
The total RNA from Caco‐2 cells homogenized in Trizol reagent was determined by StepOnePlus (ThermoFisher Scientific, USA). The reverse transcription system (20 μl) included the following items: 5 × gDNA Eraser Buffer 2 μl, gDNA Eraser 1 μl, total RNA 1 μg, PrimeScript RT Enzyme Mix I 1 μl, 5 × PrimeScript Buffer 2(for Real Time) 4 μl, RT Primer Mix 4 μl, RNase Free H2O 4 μl. The cDNA synthesized was stored at −20°C. Each 10‐μl PCR system contained 1 μl total cDNA, TB Green Premix Ex Taq (Tli RNaseH Plus; TaKaRa Ex Taq HS, dNTP Mixture, Mg2+, Tli RNaseH, TB Green) 5 μl, ROX Reference Dye 0.2 μl, forward primer and reverse primer 0.5 μl, and nuclease‐free water 3.3 μl. Amplification was performed using reaction cycle at 95°C 10 s, 55°C 10 s, and then 72°C 15 s. The fluorescence signal was detected at the end of each cycle; 18 s rRNA was used as an internal control, and melting curve was used to confirm the specificity of the primers.
Orai1‐F: 5′‐ATGGTGGCAATGGTGGAG‐3′
Orai1‐R: 5′‐CTGATCATGAGCGCAAACAG‐3′
GAPDH‐F: 5′‐GTGGAGTCCACTGGCGTCTT‐3′
GAPDH‐R: 5′‐GTGCAGGAGGCATTGCTGAT‐3′
2.8. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). All results shown are means ± SE. Net peak for duodenal I sc refers to drug‐stimulated maximal peak minus basal level. The rising slope is the ratio of the current difference between the maximum peak of the drug stimulus to the base level and the time difference. The statistical significance of differences in the means of experimental groups was determined using Student's unpaired, two‐tailed t‐test or one‐way ANOVA followed by Dunnett's post test. Post hoc tests were run only if F achieved P < 0.05 (GraphPad Prism 7.0, GraphPad Software, Inc., RRID:SCR_002798), and there was no significant variance inhomogeneity. A probability value P < 0.05 was considered statistically significant.
2.9. Materials
Caffeine, carbachol, CFTRinh‐172, DNDS (4,4′‐dinitrostilbene‐2,2′‐disulfonic acid), flufenamic acid, forskolin, nifedipine, niflumic acid and ruthenium red were purchased from Sigma (Saint Louis, MO, USA). MedChemExpress (MCE; Monmouth Junction, NJ) supplied H‐89, KB‐R7943, mibefradil, RN‐1734, SKF‐96365 and TPEN (N,N,N′,N′‐tetrakis(2‐pyridylmethyl)ethylenediamine). 2‐Aminoethoxydiphenyl borate (2‐APB) was purchased from Tocris Bioscience (Ellisville, MO). Dantrolene and T16Ainh‐A01 were from APExBIO Technology LLC (Houston, TX) and GSK‐7975A was supplied by Glixx Laboratories Inc (Hopkinton, MA). Fura‐2/AM was from Invitrogen (RRID:AB_11156243). Anti‐STIM1 (Abcam Cat# ab108994, RRID:AB_10859115, a rabbit mAb), Orai1 (Abcam Cat# ab86748, RRID:AB_10672921, a rabbit mAb), or NKA (Abcam Cat# ab2872, RRID:AB_2061140, a mouse mAb) monoclonal antibody was from Abcam. The other chemicals were obtained from Fisher Scientific (Santa Clara, CA).
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro, et al., 2017; Alexander, Kelly et al., 2017; Alexander, Peters et al., 2017; Alexander, Striessnig, et al., 2017).
3. RESULTS
3.1. Caffeine induced Ca2+‐dependent duodenal epithelial anion secretion
We performed Ussing chamber experiments to examine the effect of caffeine on duodenal epithelial anion secretion. Caffeine (10 mM) markedly increased I sc, but it s vehicle (H2O) did not (Figure 1a), indicating its specific action. Caffeine induced duodenal I sc in a biophasic manner: the first, a transient and the second, a sustained phase (Figure 1a). Because intestinal epithelium is polarized with the mucosal side facing the lumen and the serosal side facing the serosae, we tested which of these sides was acted on by caffeine. The serosal application of caffeine induced a higher I sc peak (Figure 1a,b) and a faster I sc rising rate (Figure 1a,c) than mucosal application, indicating the major serosal action of caffeine. To further investigate the effect of caffeine on epithelial anion secretion, either HCO3 − or Cl− was removed from both sides of the chambers. As shown in Figure 1d, Cl− and HCO3 − secretion accounted for around 75% of the caffeine‐induced duodenal total I sc. Although removal of either anion significantly attenuated caffeine‐induced I sc, Cl− secretion made a major contribution (around 65%), and HCO3 − secretion made a minor contribution (around 10%) to the total caffeine‐induced I sc (Figure 1d). Caffeine‐induced duodenal I sc was significantly attenuated after Cl− was removed from the mucosal side (Figure 1e). Similarly, the commonly used Ca2+ mobilizer carbachol induced duodenal I sc , which was significantly attenuated by either HCO3 − or Cl− removal (Figure 1f,g). Therefore, like carbachol, caffeine could evoke both HCO3 − and Cl− secretion in duodenal tissue, but the latter was predominant.
Figure 1.
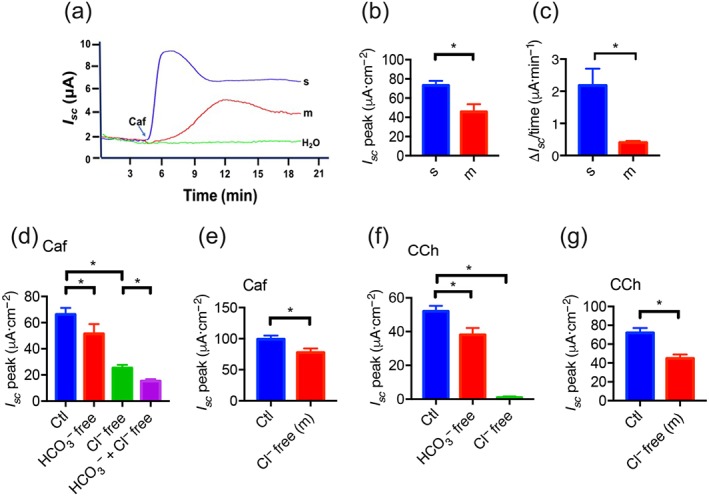
The effect of caffeine on murine duodenal epithelial anion secretion. (a) Representative time courses of caffeine (Caf; 10 mM)‐stimulated I sc or control (H2O) when added to the serosal (s) or mucosal (m) side of duodenal mucosal tissues. (b–c) Summary data of caffeine‐stimulated I sc peak and rising rate when added to the serosal or the mucosal side (n = 6). (d) Summary data of caffeine‐stimulated I sc peak after HCO3 − or Cl− omission from both mucosal and serosal sides. Ctl represents as the control with HCO3 − and Cl− in both mucosal and serosal sides. (e) Summary data of caffeine‐stimulated I sc peak after Cl− omission from the mucosal side (n = 7). (f) Summary data of carbachol (CCh)‐stimulated I sc peak after HCO3 − or Cl− omission from both mucosal and serosal sides. (g) Summary data of carbachol‐stimulated I sc peak after Cl− omission from the mucosal side (n = 6). * P < 0.05, significantly different from the corresponding control
To test if Ca2+ is critical for caffeine‐stimulated anion secretion, the experiments were first compared in the presence or the absence of extracellular Ca2+ on each side of the Ussing chamber. As shown in Figure 2a,b, the caffeine‐stimulated I sc peak was attenuated when extracellular Ca2+ was omitted from the serosal side of duodenal tissues, but not from the mucosal side although the caffeine‐induced second sustained phase declined (Figure 2a). Moreover, the caffeine‐stimulated I sc peak was similar after Ca2+ omission from the serosal side alone and from both sides of the tissues (Figure 2a,b). Second, KN‐93 (10 μM), a selective CaMKII inhibitor, attenuated the caffeine‐stimulated I sc peak when added to the serosal side but not to the mucosal side of duodenal tissues (Figure 2c,d). Therefore, the presence of Ca2+ on the serosal side of duodenal epithelium was critical for caffeine‐induced duodenal I sc.
Figure 2.
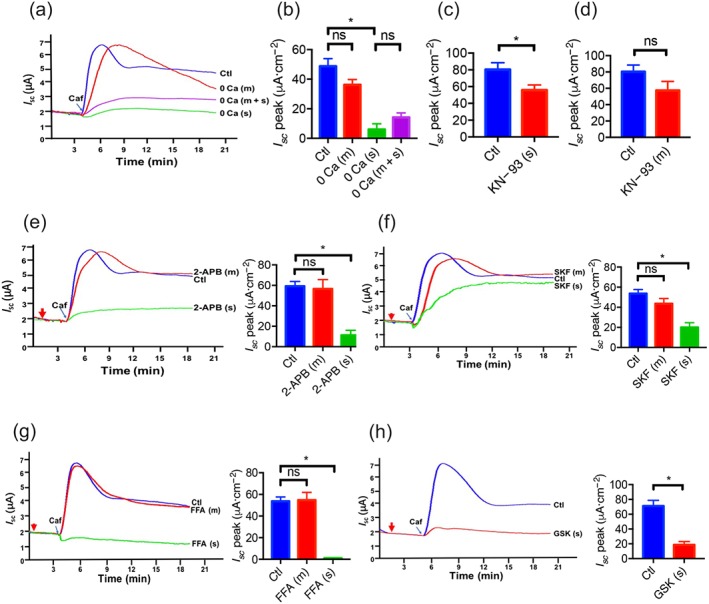
The critical roles of Ca2+ signalling and Ca2+ channels in caffeine‐stimulated duodenal epithelial anion secretion. (a) Representative time courses of caffeine (Caf; 10 mM)‐stimulated I sc after extracellular Ca2+ omission (0 Ca) from mucosal (m), serosal (s) side, or both sides (m + s) of duodenal mucosal tissues. Ctl represents as the control in which 2‐mM extracellular Ca2+ was in both mucosal and serosal sides. (b) Summary data of caffeine‐stimulated I sc peak after Ca2+ omission from mucosal (m, n = 10) side, serosal (s, n = 11) side, or both sides (m + s, n = 8). (c–d) Summary data of caffeine‐stimulated I sc peak after serosal or mucosal addition of KN‐93 (10 μM, n = 6) in the presence of 2‐mM extracellular Ca2+. Ctl represents as the control without drug treatment. (e–g) Representative time courses and summary data of caffeine‐stimulated I sc peak after mucosal or serosal addition of 2‐aminoethoxydiphenyl borate (2‐APB, 50 μM, n = 7), SKF‐96365 (SKF, 30 μM, n = 7), or flufenamic acid (FFA, 100 μM, n = 9). The red arrows indicate the time of inhibitor addition. (h) Representative time courses and summary data of caffeine‐stimulated I sc peak after serosal addition of GSK‐7975A (GSK, 100 μM, n = 8). ns: no significant differences, * P < 0.05, significantly different from the corresponding control
3.2. Caffeine induced the SOCE mechanism on the serosal side of the duodenal epithelium
It is well known that ER Ca2+ release will trigger the SOCE, a critical physiological process in IEC (Mei et al., 2002). To test if this mechanism was involved in caffeine‐induced anion secretion, we used several inhibitors of SOCE. As shown in Figure 2e, mucosal application of 2‐APB (50 μM) did not affect caffeine‐stimulated duodenal I sc, but serosal addition significantly reduced the duodenal I sc. As 2‐APB is a reliable blocker of SOCE but also an inconsistent IP3R inhibitor (Bootman et al., 2002), two other more selective SOCE blockers were applied. Similarly, serosal but not mucosal application of SKF‐96365 (30 μM) or flufenamic acid (100 μM) also significantly reduced or suppressed the duodenal I sc (Figure 2f,g) respectively. Therefore, caffeine induced the SOCE mechanism by acting on the serosal side of the duodenal epithelium.
3.3. CRAC/Orai channels in the regulation of caffeine‐stimulated duodenal I sc
We screened the molecular components of SOCE in duodenal epithelium and identified the CRAC/Orai channels using a specific blocker (Molnar et al., 2016). As shown in Figure 2h, serosal application of GSK‐7975A (100 μM) significantly suppressed caffeine‐stimulated duodenal I sc. However, serosal applications of other selective blockers, such as nifedipine (10 μM) and mibefradil (10 μM) for L‐type and T‐type voltage‐operated Ca2+ channels, RN‐1734 (30 μM) for TRPV4, KB‐R7943 (30 μM) for the Na+/Ca2+ exchanger and TRPV1 knockout mice for TRPV1, excluded these Ca2+‐permeable channels as the molecular components of SOCE (Figure 3a–e). Therefore, CRAC/Orai channels may represent the molecular constituents of the SOCE mechanism involved in the caffeine‐stimulated duodenal I sc.
Figure 3.
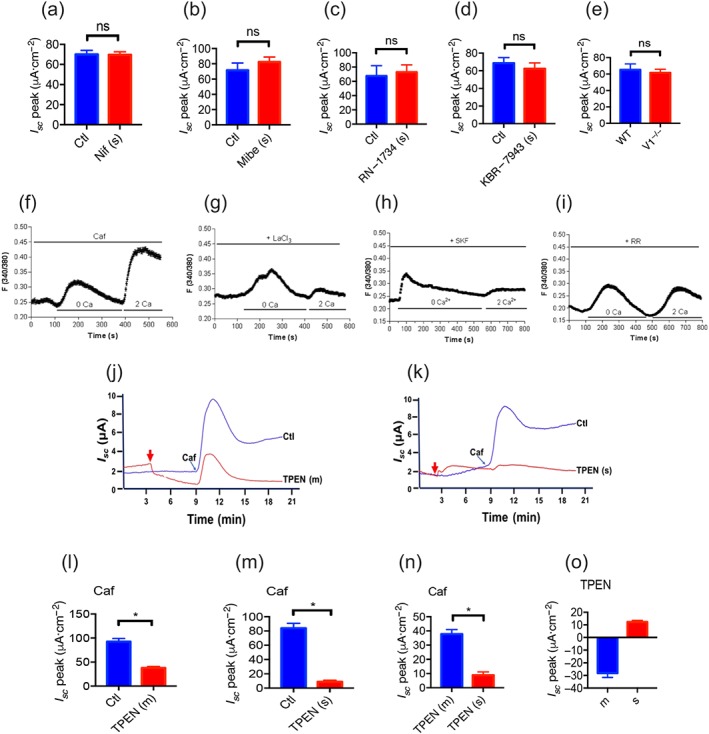
Ca2+ channels and ER Ca2+ in caffeine‐stimulated duodenal anion secretion and caffeine‐mediated SOCE mechanism in small intestinal epithelial cells. (a–d) Summary data of caffeine (Caf; 10 mM)‐stimulated duodenal I sc peak after serosal side (s) addition of nifedipine (Nif, 10 μ, n = 6), mibefradil (Mibe, 10 μ, n = 6), RN‐1734 (30 μM, n = 7), or KBR‐7943 (30 μM, n = 7). Ctl represents as the control without drug treatment. (e) Summary data of caffeine‐stimulated duodenal I sc peak compared between TRPV1 knockout mice (V1−/−, n = 9) and wild‐type mice (WT, n = 8). (f) Summary data showing the time courses of caffeine (10 mM)‐induced Ca2+ signalling in the absence (0 Ca, left) or the presence of extracellular Ca2+ (2 Ca, right) in SCBN cells as a control. (g–i) Summary data showing the effect of LaCl3 (30 μM), SKF‐96365 (SKF, 30 μM), or ruthenium red (RR, 30 μM) on the time courses of caffeine‐induced Ca2+ signalling in the absence or the presence of extracellular Ca2+. Results are presented as mean of 50–60 SCBN cells for each panel. (j–m) Representative time courses and summary data of caffeine‐stimulated duodenal I sc after mucosal (m) or serosal (s) addition of TPEN (1 mM, n = 6). Ctl represents as the control without TPEN treatment. (n) Comparison of caffeine‐stimulated I sc peak after mucosal or serosal addition of TPEN. (o) Summary data on the direct effect of TPEN on basal I sc after mucosal or serosal addition. * P < 0.05, significantly different from the corresponding control or mucosal addition of TPEN
3.4. CRAC/Orai channels in caffeine‐mediated SOCE mechanism
As the SCBN cell line is commonly used as a model of IEC to study anion secretion (Buresi et al., 2001; Dong et al., 2011; Skinn & MacNaughton, 2005), we measured [Ca2+]cyt in these cells to further examine the role of CRAC/Orai channels in the caffeine‐mediated SOCE mechanism. In single SCBN cells superfused with Ca2+‐free solution (0 Ca), caffeine first caused a rapid increase in [Ca2+]cyt due to ER Ca2+ release (Figure 3f). After Ca2+ release from the ER was complete, restoration of extracellular Ca2+ (2 Ca) caused an additional increase in [Ca2+]cyt due to SOCE mechanism. As shown in Figure 3g, LaCl3 (30 μM), a commonly used SOCE blocker and CRAC/Orai channel blocker, significantly inhibited caffeine‐induced SOCE. Similarly, SKF‐96365 (30 μM), another selective SOCE blocker and CRAC/Orai channel blocker, abolished caffeine‐induced SOCE (Figure 3h), further supporting our previous notion that CRAC/Orai channels are the channels involved in SOCE in IEC. Moreover, ruthenium red (30 μM), a RyR antagonist, also inhibited the caffeine‐induced SOCE (Figure 3i), indicating the important role of RyR in this mechanism.
3.5. The ER Ca2+ store and RyR in caffeine‐induced epithelial anion secretion
To examine the role of the ER Ca2+ store in caffeine‐induced duodenal I sc, we used N,N,N′,N′‐tetrakis(2‐pyridylmethyl)ethylenediamine (TPEN), a membrane‐permeable ER intraluminal Ca2+ chelator (Caroppo et al., 2003). Because of its low affinity for Ca2+, TPEN can rapidly and reversibly chelate Ca2+ within the ER stores without influencing [Ca2+]cyt. As shown in Figure 3j, either mucosal or serosal application of TPEN (1 mM) significantly reduced caffeine‐stimulated duodenal I sc. However, at the same concentration of TPEN, serosal application suppressed much more caffeine‐stimulated duodenal I sc compared to the effects of mucosal application (Figure 3j–n), indicating a dominant contribution of the ER Ca2+ store close to the serosal side of the tissues. We also noticed that mucosal or serosal application of TPEN itself induced a slight decrease (Figure 3j,o) or increase (Figure 3k,o) in duodenal I sc baseline, respectively, implying its possible different actions on I sc baseline when added to the different sides of epithelium.
It is known that activation of muscarinic receptors stimulates IP3 production, leading to intracellular ER Ca2+ release via IP3R (Lindqvist, Sharp, Johnson, Satoh, & Williams, 1998). LiCl (30 mM) was used to inhibit IP3 production to confirm the specific IP3/Ca2+ pathway in the process of carbachol‐mediated I sc (Figure 4a,b), but LiCl did not alter caffeine‐induced I sc (Figure 4a,c). However, ruthenium red (1 mM), a relatively selective RyR antagonist, did not alter carbachol‐induced I sc (Figure 4d,e) but markedly inhibited caffeine‐induced duodenal I sc (Figure 4d,f). Similarly, serosal application of dantrolene (100 μM), a more selective RyR antagonist, did not alter carbachol‐induced duodenal I sc (Figure 4g) but markedly inhibited caffeine‐induced I sc (Figure 4h), and an increase in the concentration of dantrolene (300 μM and 1 mM) dose‐dependently enhanced the inhibition, indicating a critical role of RyR in caffeine‐induced epithelial anion secretion.
Figure 4.
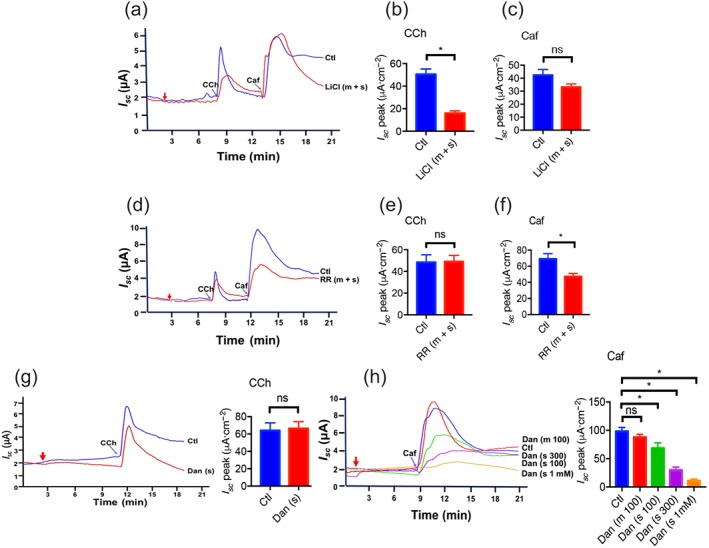
Roles of ryanodine receptors (RyR) and IP3 receptors (IP3R) in caffeine‐stimulated duodenal epithelial anion secretion. (a–c) Representative time courses and summary data showing the effect of LiCl (30 mM, n = 8) on carbachol (CCh)‐ or caffeine (Caf)‐stimulated I sc after mucosal and serosal (m + s) addition. (d–f) Representative time courses and summary data showing the effect of ruthenium red (RR, 1 mM, n = 9) on carbachol‐ or caffeine‐stimulated I sc after mucosal and serosal addition. (g) Representative time courses and summary data showing the effect of dantrolene (Dan, 100 μM, n = 6) on carbachol‐stimulated I sc after serosal (s) addition. (h) Representative time courses and summary data showing the effect of dantrolene (Dan, 100 μM, 300 μM, 1 mM, n = 6 for each) on caffeine‐stimulated I sc after mucosal (m) or serosal (s) addition. ns: no significant differences, * P < 0.05, significantly different from the corresponding control
3.6. The channels and exchangers involved in caffeine‐induced epithelial anion secretion
Firstly, as Ca2+‐activated Cl– channels (CaCC) are critical in epithelial anion secretion stimulated by Ca2+ mobilizing secretagogues (Caputo et al., 2008; Sung et al., 2016), we applied a commonly used CaCC blocker niflumic acid (NFA). At concentrations of 100–300 μM, mucosal application of NFA significantly reduced the caffeine‐stimulated duodenal I sc, (Figure 5a,b). Furthermore, T16Ainh‐A01 (120 μM), a selective CaCC blocker, also reduced caffeine‐induced duodenal I sc (Figure 5c), suggesting that the CaCC is one of the downstream effectors in caffeine‐mediated Ca2+ signalling. Secondly, because the Cl−/HCO3− exchanger is another target of Ca2+ signalling in pancreatic anion secretion (Jung, Kim, Hille, Nguyen, & Koh, 2006), its inhibitor DNDS was also used in the present study. However, mucosal application of DNDS (100 μM) did not alter caffeine‐or carbachol‐induced duodenal I sc (Figure 5d,e), suggesting that the Cl−/HCO3 − exchanger was not the target of caffeine/or carbachol/Ca2+‐mediated intestinal anion secretion.
Figure 5.
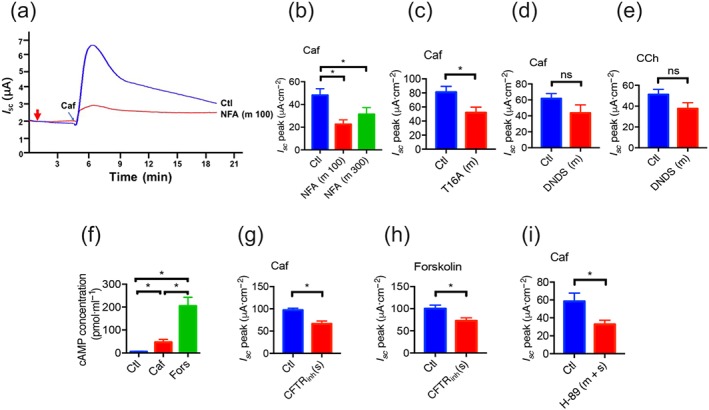
Roles of CaCC and CFTR channels in caffeine‐stimulated duodenal epithelial anion secretion. (a–b) Representative time courses and summary data showing the inhibitory effect of niflumic acid (NFA, 100, n = 8 and 300 μM, n = 6) on caffeine (Caf) ‐stimulated I sc after mucosal (m) addition. Ctl represents as the control without drug treatment. (c–d) Summary data on the effect of T16Ainh‐A01 (T16A, 120 μM, n = 12), or DNDS (100 μM, n = 8) on caffeine‐stimulated I sc peak after their mucosal addition. (e) Summary data on the effect of DNDS (100 μM, n = 9) on carbachol (CCh)‐stimulated duodenal I sc peak after mucosal addition. (f) Summary data on the stimulative effects of caffeine (10 mM, n = 6) or forskolin (10 μM, n = 6) on cAMP concentrations in duodenal mucosae. (g–h) Summary data on the inhibitory effects of CFTRinh‐172 (CFTRinh, 30 μM, n = 6) on caffeine‐or forskolin‐stimulated duodenal I sc peak after serosal (s) addition. (i) Summary data on the inhibitory effect of H‐89 (20 μM, n = 7) on caffeine‐stimulated duodenal I sc peak after mucosal and serosal (m + s) addition. ns: no significant differences, * P < 0.05, significantly different from the corresponding control
Besides acting as a RyR activator, caffeine may also inhibit PDE to induce anion secretion through the cAMP/PKA/cystic fibrosis transmembrane conductance regulator (CFTR) pathway. We first found that caffeine (10 mM) slightly increased, but forskolin (10 μM), an activator of adenylyl cyclase, markedly increased cAMP concentration in duodenal epithelium (Figure 5f). Secondly, CFTRinh‐172 (30 μM), a highly potent and specific CFTR inhibitor (Ma et al., 2002), markedly inhibited both caffeine‐and forskolin‐induced duodenal I sc (Figure 5g,h). Finally, H‐89 (20 μM), a PKA inhibitor, significantly reduced caffeine‐induced duodenal I sc (Figure 5i). Therefore, cAMP/PKA/CFTR pathway may also play a role in the caffeine‐induced duodenal I sc.
3.7. Synergism of Ca2+ and cAMP signalling in intestinal epithelial anion secretion
It is well known that cAMP and Ca2+ signalling pathways synergize to regulate epithelial anion secretion in pancreatic and salivary gland duct (Park et al., 2013), but it has not been explored in intestinal anion secretion. High doses of caffeine (10 mM), carbachol (100 μM), and forskolin (10 μM) induced a maximal duodenal I sc (Figure 6a), but low doses of caffeine (0.67 mM), carbachol (30 μM), and forskolin (0.15 μM) induced a minimally detectable I sc (Figure 6b). However, a combination of carbachol or caffeine and forskolin at low doses induced a marked duodenal I sc response (Figure 6c). Figure 6d,e summarizes the synergistic effects of carbachol or caffeine and forskolin on the net peak of duodenal I sc after they were combined. Therefore, a synergism indeed exists between cAMP and caffeine/Ca2+ (or carbachol/Ca2+) signalling‐mediated intestinal anion secretion.
Figure 6.
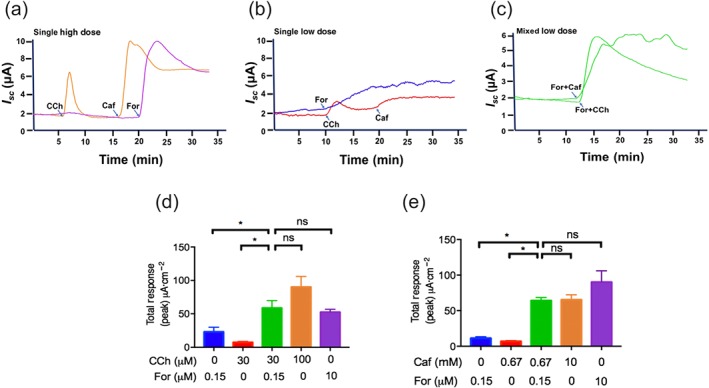
The synergistic effects of forskolin, caffeine, and carbachol in duodenal epithelial anion secretion. (a–b) Representative time courses of duodenal I sc after serosal addition of single high dose or single low dose of carbachol (CCh, 100 or 30 μM, n = 5), caffeine (Caf, 10 or 0.67 mM, n = 5), or forskolin (For, 10 or 0.15 μM, n = 5). (c) Representative time courses of enhanced duodenal I sc after serosal addition of mixed low doses of forskolin (For, 0.15 μM) plus caffeine (Caf, 0.67 mM), or forskolin (For 0.15 μM) plus carbachol (CCh 30 μM). Please note the synergistic effects of forskolin and caffeine (or CCh) after their combinations by comparing Figure b with Figure c. (d–e) Summary data obtained from Figure a–c showing the synergistic effects of forskolin and caffeine (or CCh) in duodenal epithelial anion secretion. * P < 0.05 significantly different from the corresponding control, ns: no significant differences
To further examine if cAMP promotes Ca2+‐mediated secretion, forskolin at a low concentration (0.15 μM) was added firstly and then caffeine at a low dose (0.67 mM) was added. As shown in Figure 7a, both total I sc response (the net peak between baseline and maximal response) and individual I sc response (the net peak between forskolin‐ and caffeine‐induced peak) were obviously enhanced compared to forskolin or caffeine alone. Similarly, a cAMP promotion of carbachol‐mediated secretion was also observed when forskolin at low dose (0.15 μM) was added firstly and then carbachol at low dose (30 μM) was added secondly (Figure 7b). Therefore, there is a cAMP promotion of caffeine/Ca2+‐ or carbachol/Ca2+‐mediated secretion. To further examine if Ca2+ promotes cAMP signalling, caffeine (0.67 mM) or carbachol (30 μM) was added firstly and then forskolin (0.15 μM) was added. As shown in Figure 7c,d, both total I sc response and individual I sc response were not significantly altered compared to caffeine, carbachol, or forskolin alone, indicating there is no caffeine‐or carbachol‐induced Ca2+ promotion of cAMP‐mediated secretion. Therefore, we clearly demonstrate a cAMP promotion of caffeine/Ca2+‐ or carbachol/Ca2+‐mediated secretion, but not vice versa.
Figure 7.
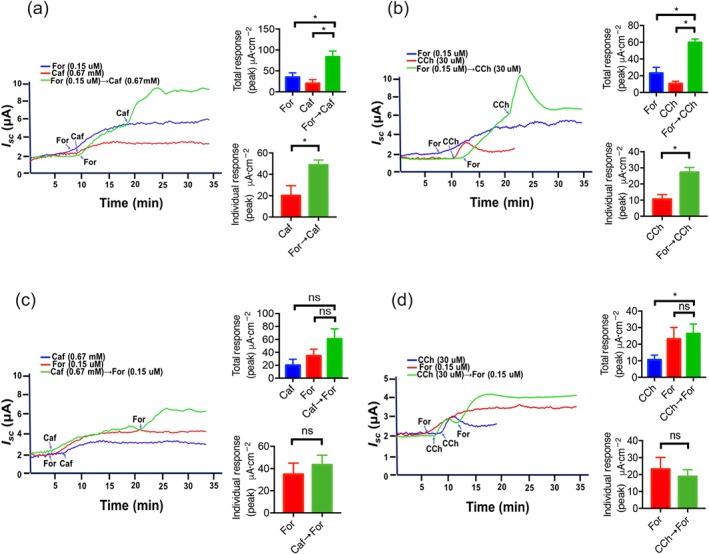
Forskolin‐evoked cAMP promotion of caffeine‐ and carbachol‐evoked Ca2+‐mediated anion secretion, but not vice versa. (a) Representative time courses and summary data showing the effect of low dose forskolin (For; 0.15 μM) or caffeine (Caf; 0.67 mM) addition alone, or the synergistic effect of the same dose of forskolin followed by caffeine in duodenal epithelial anion secretion (n = 5). (b) Representative time courses and summary data showing the effect of low dose forskolin (0.15 μM) or carbachol (30 μM) addition alone, or the synergistic effect of the same dose of forskolin followed by carbachol (n = 5). (c–d) Representative time courses and summary data showing the effect of low dose caffeine (0.67 mM), carbachol (CCh; 30 μM), or forskolin (0.15 μM) addition alone, or same dose caffeine or carbachol addition followed by forskolin (n = 5). * P < 0.05, significantly different from the corresponding control, ns: no significant differences
We further assessed the involvement of the PKA pathway in the synergism of cAMP and Ca2+ signalling for anion secretion in intestinal epithelium, as in salivary gland and pancreatic ducts (Park et al., 2013). As shown in Figure 8a,b, H‐89 (20 μM), a selective PKA inhibitor, suppressed the cAMP promotion of caffeine/Ca2+‐mediated secretion (analysed at both total response and individual response), indicating an important role of PKA in this synergism. However, H‐89 slightly increased but did not significantly alter the cAMP promotion of carbachol/Ca2+‐mediated secretion (Figure 8c,d), suggesting that PKA is not involved in this synergism.
Figure 8.
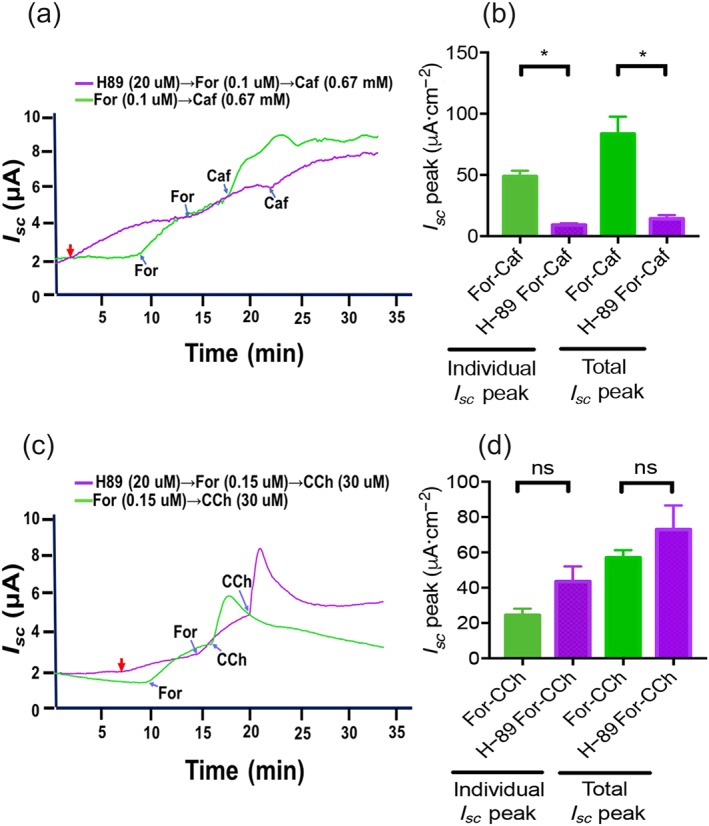
Role of PKA in the synergism of cAMP and Ca2+ signalling for duodenal epithelial anion secretion. (a–b) Representative time courses and summary data showing the synergistic effect of low dose forskolin (For) addition followed by low dose caffeine (Caf) in the absence or the presence of H‐89 (20 μM, n = 6). (c–d) Representative time courses and summary data showing the synergistic effect of low dose forskolin addition followed by low dose carbachol (CCh) in the absence or the presence of H‐89 (20 μM, n = 6). ns: no significant differences, * P < 0.05, significantly different from those without H‐89
3.8. Expression, localization, and function of STIM1/Orai1 in intestinal epithelia
Orai1 and STIM1 proteins were detected in the typical villous cells of mouse duodenal mucosa by immunohischemistry (the middle and the lower panels of Figure 9a); however, the staining was not seen without the primary antibodies against Orai1 and STIM1 (the upper panel of Figure 9a), indicating specific staining on these proteins. The Orai1 was found to primarily locate on the basolateral side of the villous cells and submucosal plexi (the upper right panel of Figure 9b). The NKA that is known as the basolateral marker (the lower left panel of Figure 9b; Jayawardena et al., 2017) was found to co‐localize with the Orai1 on the same side (the lower right panel of Figure 9b).
Figure 9.
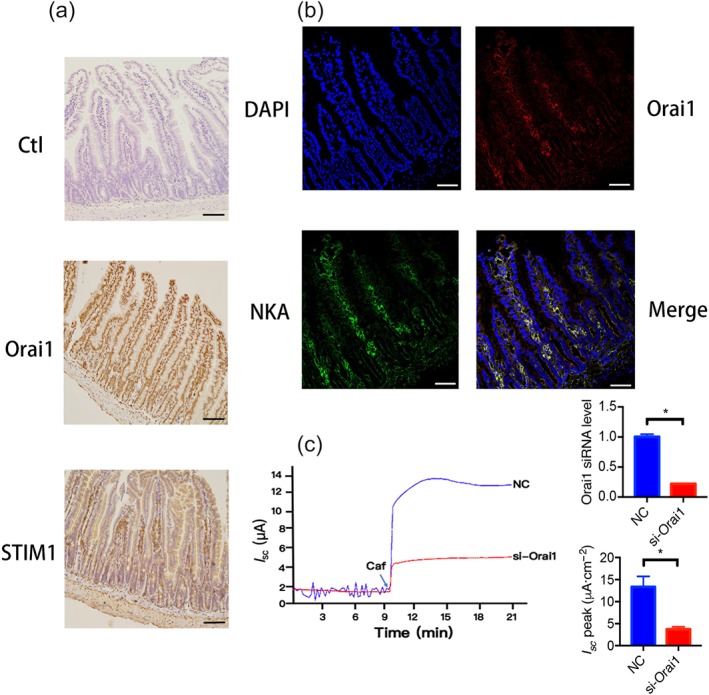
Expression of STIM1/Orai1 and localization of Orai1 in villous cells of mouse duodenal epithelia. (a) The expression of STIM1 and Orai1 in the duodenal epithelia detected by immunohistochemistry. The upper panel showing the typical villous cells of duodenal epithelia without the primary antibodies against Orai1 and STIM1 as a negative control. The middle and lower panels showing the specific brown staining to Orai1 and STIM1 proteins in the villous cells. (b) The localization of Orai1 in the villous cells of duodenal epithelia identified by immunofluorescence staining. The upper left panel showing the nuclei of the villous cells stained with DAPI (blue). The upper right panel showing the specific staining to Orai1 proteins in the villous cells (red). The lower left panel showing the basolateral marker of Na+, K+‐ATPase (NKA) in the villous cells (green) as a positive control. The upper right panel is the merge of the above three image showing the co‐localization of Orai1 and NKA on the basolateral membrane of villous cells (yellow). Each one is the representative of all images taken from the duodenum of three mice with similar results. Scale bar = 50 μm for each image. (c) Representative time courses and summary data showing the inhibitory effect of si‐Orai1 on caffeine (Caf)‐stimulated I sc compared to negative control (NC) after serosal addition to Caco‐2 cells (s, n = 6), and summary data of Orai1 mRNA levels determined by qPCR in Caco‐2 cells (n = 6). * P < 0.05, significantly different from the corresponding control
To examine the functional role of serosal Orai1 channels in caffeine/Ca2+‐stimulated anion secretion, NC siRNA or siRNA against Orai1 channels (si‐Orai1) was added to the serosal side of human intestinal epithelial Caco‐2 cells. While siRNA against Orai1 channels reduced their expression levels in Caco‐2 cells, caffeine‐stimulated I sc in si‐Orai1 group was significantly inhibited compared to NC group (Figure 9c), providing solid evidence for the functional role of serosal Orai1 channels in IEC. We also confirmed STIM1 and Orai1 in Caco‐2 cells (Figure 10a) and observed that STIM1 and Orai1 proteins were predominantly localized in the cytosol and membrane of Caco‐2 cells respectively.
Figure 10.
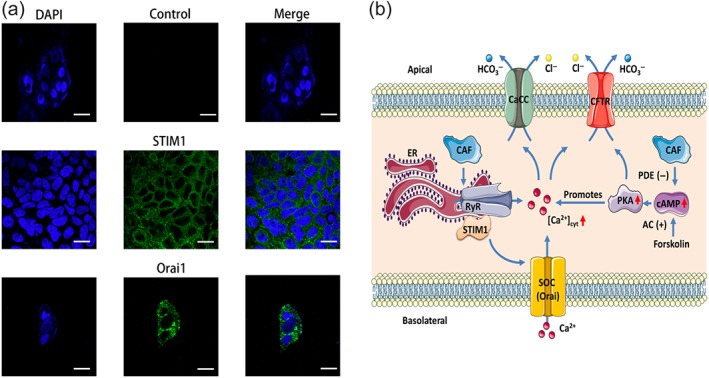
STIM1/Orai1 in human intestinal epithelial cells and schematic diagram depicting the proposed mechanisms of caffeine‐mediated intestinal anion secretion. (a) The left panels showing the nuclei of the Caco‐2 cells stained with DAPI (blue). The middle panel on the first row showing cell image without the primary antibodies against STIM1 and Orai1 as a negative control. The middle panels on the second and third rows showing the specific staining to STIM1 and Orai1 proteins in the cells. The right panels showing the merge of left and middle panels. Each one is the representative of three independent experiments with similar results. Scale bar = 20 μm for each image. (b) The proposed mechanisms. Caffeine (CAF) predominantly activates RyR in the ER to induce Ca2+ signalling probably through STIM/Orai channels and then to stimulate Ca2+‐mediated intestinal anion secretion through CaCC and CFTR channels. Caffeine‐evoked Ca2+ signalling also synergizes the effect of cAMP signalling through activation of PKA. Furthermore, caffeine slightly raises cAMP by inhibiting PDE. AC (+): activation of adenylyl cyclase; CaCC: Ca2+‐activated Cl− channels; Caf: caffeine; CFTR: cystic fibrosis transmembrane conductance regulator; PDE (−): inhibition of PDE; RyR: ryanodine receptor; SOC: store‐operated channels
4. DISCUSSION AND CONCLUSION
In the present study, using native mouse duodenal epithelium with preserved polarity, we found that (a) caffeine stimulated anion secretion by the epithelium of the small intestine mainly through RyR/ER Ca2+ release and the SOCE mechanism; (b) the Orai1 channels serve as an important component of the SOCE mechanism to mediate Ca2+‐dependent intestinal anion secretion; and (c) there is a synergism between cAMP and Ca2+ in caffeine‐mediated intestinal anion secretion, in which cAMP/PKA promoted Ca2+‐mediated secretion, but not vice versa. Therefore, using caffeine as a selective activator of RyR, we not only reveal its critical role in the regulation of native intestinal epithelial anion secretion but also elucidate the underlying molecular mechanisms through the RyR/Orai1/Ca2+ pathway.
As caffeine is widely consumed worldwide in our daily life, its biomedical effects and the mechanisms of action outside the GI tract have been intensively investigated (Davis et al., 2003; Deslandes et al., 2005; Nehlig, Daval, & Debry, 1992). However, it is surprising that we know almost nothing about its action on GI epithelial ion transport, let alone the mechanisms of action. Only a few previous studies showed an effect of caffeine on fluid transport rather than anion secretion in the intestine (Johnston, Clifford, & Morgan, 2003). In the present study, we found that caffeine markedly increased a sustained intestinal I sc, which was significantly attenuated by a removal of either Cl− or HCO3 −, indicating intestinal epithelial anion secretion. Duodenal HCO3 − secretion is critical for normal mucosal protection against various aggressive factors, including gastric acid that is also a well‐recognized physiological stimulator for duodenal HCO3 − secretion (Flemstrom & Isenberg, 2001). Consistent with a recent report that caffeine induced gastric acid secretion in human subjects (Liszt et al., 2017), our study suggests that caffeine may stimulate more duodenal HCO3 − secretion evoked by enhanced gastric acid secretion, which in turn provides mucosal protection, thus yielding evidence for the positive health effect of caffeine on the GI tract.
There have been several known mechanisms of biomedical actions of caffeine. It blocks all four adenosine receptor subtypes (A1, A2A, A2B, and A3) with varying potencies (Froestl, Muhs, & Pfeifer, 2012). The major intestinal epithelial adenosine receptors are of the A2B subtype, activation of which stimulates Cl− secretion (Strohmeier, Reppert, Lencer, & Madara, 1995). However, caffeine should inhibit rather than stimulate intestinal Cl− secretion if it blocks A2B receptors. Caffeine‐mediated adenosine receptor blockade can promote ACh release and inhibit AChE to enhance the activity of ACh. As a well‐known Ca2+ mobilizing agent, ACh (or carbachol) initiates PLC/IP3/IP3R‐mediated ER Ca2+ release to induce SOCE mechanism and eventually stimulates intestinal ion transports (Lindqvist et al., 1998). In contrast, caffeine is a IP3R blocker, which counteracts the caffeine‐mediated actions, via the ACh/IP3 pathway. This may explain why inhibition of IP3 production by LiCl attenuated carbachol‐ but not caffeine‐induced I sc, excluding the involvement of ACh/IP3/IP3R pathway in our study. Our data are consistent with the current notion that caffeine is an RyR activator and IP3R blocker (Brown et al., 1992). Therefore, application of caffeine can clearly distinguish the functional role of RyR from that of IP3R in the regulation of intestinal anion secretion (Yang et al., 2018).
After confirming caffeine as a RyR activator (“Caffeine”(IUPHAR)), we examined if it induced intestinal anion secretion through the RyR‐mediated SOCE mechanism. Indeed, this mechanism played a major role in caffeine‐induced anion secretion. Thus (a) TPEN, an ER Ca2+ chelator, significantly suppressed caffeine‐stimulated duodenal I sc, (b) ruthenium red and dantrolene, two RyR antagonists, selectively inhibited caffeine‐induced duodenal I sc, (c) the caffeine‐stimulated duodenal I sc peak was significantly attenuated when extracellular Ca2+ was omitted from the serosal side of duodenal tissues, but not from the mucosal side, (d) five selective SOCE blockers with different chemical structures also significantly suppressed duodenal I sc from serosal side, (e) caffeine induced RyR‐mediated ER Ca2+ release and SOCE mechanism in IEC, (f) Expression and basolateral localization of STIM1/Orai1, the molecular candidates of SOCE, have been identified in IEC, (g) caffeine‐stimulated I sc was significantly inhibited by siRNA against Orai1 channels in IEC. Although ryanodine inhibited carbachol‐induced colonic I sc (Kocks et al., 2002; Schultheiss, Lan Kocks, & Diener, 2005), whether RyR or IP3R was involved in the carbachol‐induced action is unclear as carbachol is supposed to stimulate IP3R via PLC/IP3 pathway (Lindqvist et al., 1998). Therefore, we demonstrate for the first time that the Ca2+ signalling specifically resulted from the RyR‐mediated ER Ca2+ release and the subsequent Ca2+ entry through Orai channels, namely, the RyR‐initiated SOCE mechanism, plays a critical role in intestinal anion secretion.
Caffeine, like other xanthines, also acts as a competitive, non‐selective PDE inhibitor to raise intracellular cAMP that activates PKA (Ribeiro & Sebastiao, 2010). Indeed, we found that caffeine slightly produced mucosal accumulation of cAMP that induced epithelial anion secretion through PKA/CFTR channels, which may play a minor role compared to the major contribution of caffeine‐induced RyR/SOCE/CaCC channels. Ca2+ and cAMP signalling are two primary second messengers that regulate epithelial ion transport (Ahuja, Jha, Maleth, Park, & Muallem, 2014). Although the cAMP/PKA/CFTR pathway has been extensively studied (Dahan et al., 2001), little is known about the SOCE/Ca2+ signalling pathway. Moreover, mutual regulation of cAMP and Ca2+ signalling is referred to as crosstalk, while integration of their effects can result in an additive or synergistic physiological response (Ahuja et al., 2014). So far, the synergism of cAMP and Ca2+ signalling has been demonstrated for anion secretion in the salivary gland and pancreatic ducts only (Park et al., 2013). We demonstrate for the first time the synergism of cAMP and Ca2+ signalling for anion secretion in the intestine. Importantly, we reveal one way rather than mutual interaction between these second messengers since the cAMP signalling evoked by forskolin promoted Ca2+‐mediated secretion evoked by caffeine and carbachol, but not vice versa. Moreover, cAMP promoted caffeine/Ca2+‐mediated secretion in a PKA‐dependent manner, but cAMP promoted carbachol/Ca2+‐ mediated secretion in a PKA‐independent manner.
The IP3R binding protein released with inositol 1,4,5‐trisphosphate (IRBIT) is a central component to promote the synergy between Ca2+ and cAMP signalling pathways in pancreatic and salivary ducts (Park et al., 2013). In resting cells, IRBIT is sequestered by IP3R in the ER, but activation of cAMP/PKA leads to phosphorylation of IP3R, which increases its affinity for IP3 but reduces its affinity for IRBIT. Subsequently, the IRBIT released from IP3R translocates to CFTR and SLC26a6, resulting in their activation and epithelial anion secretion (Ando et al., 2006; Ando, Kawaai, & Mikoshiba, 2014). However, we found that PKA inhibition significantly attenuated the cAMP‐promoted synergistic effect of caffeine/Ca2+‐induced but not carbachol/Ca2+‐induced anion secretion. Consistently with our previous study (Yang et al., 2018), the regulatory mechanisms of Ca2+ signalling mediated by caffeine/ruthenium red and carbachol/IP3R may be different in intestinal anion secretion. Therefore, further investigation is needed to elucidate the detailed mechanisms underlying the synergism of cAMP and Ca2+ signalling in intestinal anion secretion.
Although cAMP/PKA per se may play a minor role in the present study, it markedly promotes the effect of Ca2+ signalling in caffeine‐mediated intestinal anion secretion. The synergism of cAMP and Ca2+ signalling caused by caffeine may explain the more marked and sustained stimulation of intestinal anion secretion than the single Ca2+ signalling pathway activated by the Ca2+ mobilizing agent carbachol. Because Ca2+ signalling was considered an important regulator of intestinal ion transport (Flemstrom & Isenberg, 2001; Jung & Lee, 2014), there has been less information available about the detailed molecular mechanisms of Ca2+ signalling to mediate anion secretion in native intestinal epithelium. Besides CaCC activation, there are at least three Ca2+‐dependent mechanisms of CFTR activation in epithelia: (a) Ca2+‐activated adenylyl cyclase leading to PKA‐mediated phosphorylation of CFTR, (b) stimulation of Pyk2/Src, which induces CFTR activity by phosphorylating it directly and also by inhibiting its dephosphorylation through inactivation of protein phosphatase type 2A in airway epithelial cells (Billet & Hanrahan, 2013), and (c) increased PI3K/Akt‐dependent phosphorylation of CFTR in intestinal epithelium (Yang et al., 2018). The Ca2+‐activated AC is unlikely to be involved in intestinal epithelium because caffeine slightly increased cAMP concentration compared with marked increase by forskolin, AC activator, in the present study, but carbachol did not alter cAMP concentration in our previous study (Yang et al., 2018). However, further study is needed to test if the Pyk2/Src pathway is also involved in Ca2+‐dependent mechanisms of CFTR activation in intestinal anion secretion.
In conclusion, we demonstrate for the first time that caffeine markedly stimulates intestinal anion secretion mainly through the ER Ca2+ release, and there is synergism between cAMP and Ca2+ in caffeine‐mediated anion secretion, in which cAMP/PKA promoted Ca2+‐mediated secretion, but not vice versa. We also identify the caffeine/RyR/SOCE pathway and elucidate STIM/Orai1 as molecular components of the SOCE to mediate Ca2+‐dependent intestinal anion secretion. A scheme summarizes our findings in Figure 10b. A full understanding of the RyR/Orai1/Ca2+‐mediated intestinal epithelial Cl− and HCO3 − secretion and the precise regulatory mechanisms of this process will greatly enhance our knowledge on GI epithelial anion secretion. Our findings suggest new perspectives for potential drug targets to control intestinal liquid homeostasis through modulating epithelial Cl− secretion and to protect the upper GI tract through promoting epithelial HCO3 − secretion.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
H.D. designed and supervised the project, wrote and finalized the manuscript. F.Z., H.W., and X.Y. conducted most experiments and data analysis. C.L. and J.H. conducted some experiments. S.Y. and B.T. finalized the manuscript. All authors reviewed the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
We thank Dr Hui Ling, an epidemiologist, for his assistance in statistics. These studies were supported by research grants from the National Key Research and Development Program of China (Grant 2016YFC1302200 to H.D.) and the National Natural Science Foundation of China (Grant 81570477 to H.D.).
Zhang F, Wan H, Yang X, et al. Molecular mechanisms of caffeine‐mediated intestinal epithelial ion transports. Br J Pharmacol. 2019;176:1700–1716. 10.1111/bph.14640
REFERENCES
- “Caffeine”(IUPHAR) . International Union of Basic and Clinical Pharmacology. Available at: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=407 (accessed 4/25/2018).
- Ahuja, M. , Jha, A. , Maleth, J. , Park, S. , & Muallem, S. (2014). cAMP and Ca(2)(+) signaling in secretory epithelia: Crosstalk and synergism. Cell Calcium, 55, 385–393. 10.1016/j.ceca.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Other ion channels. British Journal of Pharmacology, 174, S195–S207. 10.1111/bph.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, H. , Kawaai, K. , & Mikoshiba, K. (2014). IRBIT: A regulator of ion channels and ion transporters. Biochimica et Biophysica Acta, 1843, 2195–2204. 10.1016/j.bbamcr.2014.01.031 [DOI] [PubMed] [Google Scholar]
- Ando, H. , Mizutani, A. , Kiefer, H. , Tsuzurugi, D. , Michikawa, T. , & Mikoshiba, K. (2006). IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Molecular Cell, 22, 795–806. 10.1016/j.molcel.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Berridge, M. J. (1993). Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. 10.1038/361315a0 [DOI] [PubMed] [Google Scholar]
- Billet, A. , & Hanrahan, J. W. (2013). The secret life of CFTR as a calcium‐activated chloride channel. The Journal of Physiology, 591, 5273–5278. 10.1113/jphysiol.2013.261909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman, M. D. , Collins, T. J. , Mackenzie, L. , Roderick, H. L. , Berridge, M. J. , & Peppiatt, C. M. (2002). 2‐aminoethoxydiphenyl borate (2‐APB) is a reliable blocker of store‐operated Ca2+ entry but an inconsistent inhibitor of InsP3‐induced Ca2+ release. The FASEB Journal, 16, 1145–1150. 10.1096/fj.02-0037rev [DOI] [PubMed] [Google Scholar]
- Brown, G. R. , Sayers, L. G. , Kirk, C. J. , Michell, R. H. , & Michelangeli, F. (1992). The opening of the inositol 1,4,5‐trisphosphate‐sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. The Biochemical Journal, 282(Pt 2), 309–312. 10.1042/bj2820309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buresi, M. C. , Schleihauf, E. , Vergnolle, N. , Buret, A. , Wallace, J. L. , Hollenberg, M. D. , & MacNaughton, W. K. (2001). Protease‐activated receptor‐1 stimulates Ca(2+)‐dependent Cl(−) secretion in human intestinal epithelial cells. American Journal of Physiology. Gastrointestinal and Liver Physiology, 281, G323–G332. 10.1152/ajpgi.2001.281.2.G323 [DOI] [PubMed] [Google Scholar]
- Cano‐Marquina, A. , Tarin, J. J. , & Cano, A. (2013). The impact of coffee on health. Maturitas, 75, 7–21. 10.1016/j.maturitas.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Caputo, A. , Caci, E. , Ferrera, L. , Pedemonte, N. , Barsanti, C. , Sondo, E. , … Galietta, L. J. V. (2008). TMEM16A, a membrane protein associated with calcium‐dependent chloride channel activity. Science (New York, N.Y.), 322, 590–594. 10.1126/science.1163518 [DOI] [PubMed] [Google Scholar]
- Caroppo, R. , Colella, M. , Colasuonno, A. , DeLuisi, A. , Debellis, L. , Curci, S. , & Hofer, A. M. (2003). A reassessment of the effects of luminal [Ca2+] on inositol 1,4,5‐trisphosphate‐induced Ca2+ release from internal stores. The Journal of Biological Chemistry, 278, 39503–39508. 10.1074/jbc.M305823200 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan, D. , Evagelidis, A. , Hanrahan, J. W. , Hinkson, D. A. , Jia, Y. , Luo, J. , & Zhu, T. (2001). Regulation of the CFTR channel by phosphorylation. Pflügers Archiv, 443(Suppl 1), S92–S96. 10.1007/s004240100652 [DOI] [PubMed] [Google Scholar]
- Davis, J. M. , Zhao, Z. , Stock, H. S. , Mehl, K. A. , Buggy, J. , & Hand, G. A. (2003). Central nervous system effects of caffeine and adenosine on fatigue. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 284, R399–R404. 10.1152/ajpregu.00386.2002 [DOI] [PubMed] [Google Scholar]
- Deslandes, A. C. , Veiga, H. , Cagy, M. , Piedade, R. , Pompeu, F. , & Ribeiro, P. (2005). Effects of caffeine on the electrophysiological, cognitive and motor responses of the central nervous system. Brazilian Journal of Medical and Biological Research, 38, 1077–1086. 10.1590/S0100-879X2005000700011 [DOI] [PubMed] [Google Scholar]
- Dong, X. , Ko, K. H. , Chow, J. , Tuo, B. , Barrett, K. E. , & Dong, H. (2011). Expression of acid‐sensing ion channels in intestinal epithelial cells and their role in the regulation of duodenal mucosal bicarbonate secretion. Acta Physiologica (Oxford, England), 201, 97–107. 10.1111/j.1748-1716.2010.02207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemstrom, G. , & Garner, A. (1982). Gastroduodenal HCO3(−) transport: Characteristics and proposed role in acidity regulation and mucosal protection. The American Journal of Physiology, 242, G183–G193. 10.1152/ajpgi.1982.242.3.G183 [DOI] [PubMed] [Google Scholar]
- Flemstrom, G. , & Isenberg, J. I. (2001). Gastroduodenal mucosal alkaline secretion and mucosal protection. News in Physiological Sciences, 16, 23–28. [DOI] [PubMed] [Google Scholar]
- Froestl, W. , Muhs, A. , & Pfeifer, A. (2012). Cognitive enhancers (nootropics). Part 1: Drugs interacting with receptors. Journal of Alzheimer's Disease, 32, 793–887. 10.3233/JAD-2012-121186 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth, M. , & Penner, R. (1993). Calcium release‐activated calcium current in rat mast cells. The Journal of Physiology, 465, 359–386. 10.1113/jphysiol.1993.sp019681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena, D. , Guzman, G. , Gill, R. K. , Alrefai, W. A. , Onyuksel, H. , & Dudeja, P. K. (2017). Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. American Journal of Physiology. Gastrointestinal and Liver Physiology, 313, G16–G25. 10.1152/ajpgi.00081.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, K. L. , Clifford, M. N. , & Morgan, L. M. (2003). Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. The American Journal of Clinical Nutrition, 78, 728–733. 10.1093/ajcn/78.4.728 [DOI] [PubMed] [Google Scholar]
- Jung, J. , & Lee, M. G. (2014). Role of calcium signaling in epithelial bicarbonate secretion. Cell Calcium, 55, 376–384. 10.1016/j.ceca.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Jung, S. R. , Kim, K. , Hille, B. , Nguyen, T. D. , & Koh, D. S. (2006). Pattern of Ca2+ increase determines the type of secretory mechanism activated in dog pancreatic duct epithelial cells. The Journal of Physiology, 576, 163–178. 10.1113/jphysiol.2006.114876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks, S. , Schultheiss, G. , & Diener, M. (2002). Ryanodine receptors and the mediation of Ca2+‐dependent anion secretion across rat colon. Pflügers Archiv, 445, 390–397. 10.1007/s00424-002-0947-1 [DOI] [PubMed] [Google Scholar]
- Lefkimmiatis, K. , Srikanthan, M. , Maiellaro, I. , Moyer, M. P. , Curci, S. , & Hofer, A. M. (2009). Store‐operated cyclic AMP signalling mediated by STIM1. Nature Cell Biology, 11, 433–442. 10.1038/ncb1850 [DOI] [PubMed] [Google Scholar]
- Lindqvist, S. M. , Sharp, P. , Johnson, I. T. , Satoh, Y. , & Williams, M. R. (1998). Acetylcholine‐induced calcium signaling along the rat colonic crypt axis. Gastroenterology, 115, 1131–1143. 10.1016/S0016-5085(98)70084-8 [DOI] [PubMed] [Google Scholar]
- Liszt, K. I. , Ley, J. P. , Lieder, B. , Behrens, M. , Stoger, V. , Reiner, A. , … Somoza, V. (2017). Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proceedings of the National Academy of Sciences of the United States of America, 114, E6260–E6269. 10.1073/pnas.1703728114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, T. , Thiagarajah, J. R. , Yang, H. , Sonawane, N. D. , Folli, C. , Galietta, L. J. , & Verkman, A. S. (2002). Thiazolidinone CFTR inhibitor identified by high‐throughput screening blocks cholera toxin‐induced intestinal fluid secretion. The Journal of Clinical Investigation, 110, 1651–1658. 10.1172/JCI0216112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidclines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. British Journal of Pharmacology, 172, 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, F. C. , Qiao, J. , Tsygankova, O. M. , Meinkoth, J. L. , Quilliam, L. A. , & Cheng, X. (2002). Differential signaling of cyclic AMP: Opposing effects of exchange protein directly activated by cyclic AMP and cAMP‐dependent protein kinase on protein kinase B activation. The Journal of Biological Chemistry, 277, 11497–11504. 10.1074/jbc.M110856200 [DOI] [PubMed] [Google Scholar]
- Mignen, O. , Thompson, J. L. , Yule, D. I. , & Shuttleworth, T. J. (2005). Agonist activation of arachidonate‐regulated Ca2+‐selective (ARC) channels in murine parotid and pancreatic acinar cells. The Journal of Physiology, 564, 791–801. 10.1113/jphysiol.2005.085704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D. C. , Knight, C. A. , Hockenberry, J. , Teplansky, R. , & Hartman, T. J. (2014). Beverage caffeine intakes in the U.S. Food and Chemical Toxicology, 63, 136–142. 10.1016/j.fct.2013.10.042 [DOI] [PubMed] [Google Scholar]
- Molnar, T. , Yarishkin, O. , Iuso, A. , Barabas, P. , Jones, B. , Marc, R. E. , … Kri aj, D. (2016). Store‐operated calcium entry in muller glia is controlled by synergistic activation of TRPC and Orai channels. The Journal of Neuroscience, 36, 3184–3198. 10.1523/JNEUROSCI.4069-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig, A. , Daval, J. L. , & Debry, G. (1992). Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Research. Brain Research Reviews, 17, 139–170. 10.1016/0165-0173(92)90012-B [DOI] [PubMed] [Google Scholar]
- Onodera, K. , Pouokam, E. , & Diener, M. (2013). STIM1‐regulated Ca2+ influx across the apical and the basolateral membrane in colonic epithelium. The Journal of Membrane Biology, 246, 271–285. 10.1007/s00232-013-9528-9 [DOI] [PubMed] [Google Scholar]
- Park, S. , Shcheynikov, N. , Hong, J. H. , Zheng, C. , Suh, S. H. , Kawaai, K. , … Muallem, S. (2013). Irbit mediates synergy between Ca(2+) and cAMP signaling pathways during epithelial transport in mice. Gastroenterology, 145, 232–241. 10.1053/j.gastro.2013.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak, E. , Szopa, A. , Wyska, E. , Kukula‐Koch, W. , Serefko, A. , Wosko, S. , … Wlaź, P. (2016). Caffeine augments the antidepressant‐like activity of mianserin and agomelatine in forced swim and tail suspension tests in mice. Pharmacological Reports, 68, 56–61. 10.1016/j.pharep.2015.06.138 [DOI] [PubMed] [Google Scholar]
- Prinz, G. , & Diener, M. (2008). Characterization of ryanodine receptors in rat colonic epithelium. Acta Physiologica (Oxford, England), 193, 151–162. 10.1111/j.1748-1716.2007.01802.x [DOI] [PubMed] [Google Scholar]
- Prole, D. L. , & Taylor, C. W. (2016). Inositol 1,4,5‐trisphosphate receptors and their protein partners as signalling hubs. The Journal of Physiology, 594, 2849–2866. 10.1113/JP271139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney, J. W. Jr. (1986). A model for receptor‐regulated calcium entry. Cell Calcium, 7, 1–12. 10.1016/0143-4160(86)90026-6 [DOI] [PubMed] [Google Scholar]
- Putney, J. W. Jr. (1990). Capacitative calcium entry revisited. Cell Calcium, 11, 611–624. 10.1016/0143-4160(90)90016-N [DOI] [PubMed] [Google Scholar]
- Putney, J. W. Jr. (2007). Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium, 42, 103–110. 10.1016/j.ceca.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, J. N. , Rathor, N. , Zou, T. , Liu, L. , Xiao, L. , Yu, T. X. , … Wang, J. Y. (2010). STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by inducing TRPC1‐mediated Ca2+ signaling after wounding. American Journal of Physiology. Cell Physiology, 299, C579–C588. 10.1152/ajpcell.00066.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M. C. , Nash, N. T. , & Field, M. (1984). Differing effects of cGMP and cAMP on ion transport across flounder intestine. The American Journal of Physiology, 246, C167–C171. 10.1152/ajpcell.1984.246.1.C167 [DOI] [PubMed] [Google Scholar]
- Ribeiro, J. A. , & Sebastiao, A. M. (2010). Caffeine and adenosine. Journal of Alzheimer's Disease, 20(Suppl 1), S3–S15. 10.3233/JAD-2010-1379 [DOI] [PubMed] [Google Scholar]
- Schultheiss, G. , Lan Kocks, S. , & Diener, M. (2005). Stimulation of colonic anion secretion by monochloramine: Action sites. Pflügers Archiv, 449, 553–563. 10.1007/s00424-004-1365-3 [DOI] [PubMed] [Google Scholar]
- Seidler, U. , Song, P. , Xiao, F. , Riederer, B. , Bachmann, O. , & Chen, M. (2011). Recent advances in the molecular and functional characterization of acid/base and electrolyte transporters in the basolateral membranes of gastric and duodenal epithelial cells. Acta Physiologica (Oxford, England), 201, 3–20. 10.1111/j.1748-1716.2010.02107.x [DOI] [PubMed] [Google Scholar]
- Skinn, A. C. , & MacNaughton, W. K. (2005). Nitric oxide inhibits cAMP‐dependent CFTR trafficking in intestinal epithelial cells. American Journal of Physiology. Gastrointestinal and Liver Physiology, 289, G739–G744. 10.1152/ajpgi.00425.2004 [DOI] [PubMed] [Google Scholar]
- Strohmeier, G. R. , Reppert, S. M. , Lencer, W. I. , & Madara, J. L. (1995). The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. The Journal of Biological Chemistry, 270, 2387–2394. 10.1074/jbc.270.5.2387 [DOI] [PubMed] [Google Scholar]
- Sung, T. S. , O'Driscoll, K. , Zheng, H. , Yapp, N. J. , Leblanc, N. , Koh, S. D. , & Sanders, K. M. (2016). Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. American Journal of Physiology. Cell Physiology, 311, C437–C451. 10.1152/ajpcell.00070.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo, B. , Wen, G. , Wang, X. , Xu, J. , Xie, R. , Liu, X. , & Dong, H. (2012). Estrogen potentiates prostaglandin E(2)‐stimulated duodenal mucosal HCO(3)(−) secretion in mice. American Journal of Physiology. Endocrinology and Metabolism, 303, E111–E121. 10.1152/ajpendo.00575.2011 [DOI] [PubMed] [Google Scholar]
- Tuo, B. , Wen, G. , Zhang, Y. , Liu, X. , Wang, X. , Liu, X. , & Dong, H. (2009). Involvement of phosphatidylinositol 3‐kinase in cAMP‐ and cGMP‐induced duodenal epithelial CFTR activation in mice. American Journal of Physiology. Cell Physiology, 297, C503–C515. 10.1152/ajpcell.00460.2008 [DOI] [PubMed] [Google Scholar]
- Wagner, S. M. , Mekhjian, H. S. , Caldwell, J. H. , & Thomas, F. B. (1978). Effects of caffeine and coffee on fluid transport in the small intestine. Gastroenterology, 75, 379–381. [PubMed] [Google Scholar]
- Wald, A. , Back, C. , & Bayless, T. M. (1976). Effect of caffeine on the human small intestine. Gastroenterology, 71, 738–742. [PubMed] [Google Scholar]
- Wan, H. , Xie, R. , Xu, J. , He, J. , Tang, B. , Liu, Q. , … Dong, H. (2017). Anti‐proliferative effects of nucleotides on gastric cancer via a novel P2Y6/SOCE/Ca2+/β‐catenin pathway. Scientific Reports, 7, 2459 10.1038/s41598-017-02562-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, R. , Dong, X. , Wong, C. , Vallon, V. , Tang, B. , Sun, J. , … Dong, H. (2014). Molecular mechanisms of calcium‐sensing receptor‐mediated calcium signaling in the modulation of epithelial ion transport and bicarbonate secretion. The Journal of Biological Chemistry, 289, 34642–34653. 10.1074/jbc.M114.592774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Wen, G. , Tuo, B. , Zhang, F. , Wan, H. , He, J. , … Dong, H. (2018). Molecular mechanisms of calcium signaling in the modulation of small intestinal ion transports and bicarbonate secretion. Oncotarget, 9, 3727–3740. 10.18632/oncotarget.23197 [DOI] [PMC free article] [PubMed] [Google Scholar]


