Abstract
BACKGROUND:
A growing interest had been paid to goal-directed fluid therapy (GDT) in abdominal surgery; however, its impact on the respiratory profile was not well investigated.
AIM:
We evaluated the impact of GDT on postoperative extravascular lung water and oxygenation after prolonged major abdominal surgery.
METHODS:
A randomised, controlled study was conducted in Kasr Alainy hospital from April 2016 till December 2017 including 120 adult patients scheduled for prolonged major abdominal surgery. Patients were randomised into either GDT group (n = 60) who received baseline restricted fluid therapy (2 mL/Kg/hour) which is guided by stroke volume variation, or control group (n = 60) who received standard care. Both study groups were compared according to hemodynamic data, fluid requirements, lung ultrasound score, and PaO2/fraction of inspired oxygen ratio (P/F ratio),
RESULTS:
Intraoperatively, GDT group received less volume of fluids and showed higher intraoperative mean arterial pressure compared to the control group. Postoperatively, lung ultrasound score was lower, and P/F ratio was higher in the GDT group compared to the control group. The number of patients who showed a significant postoperative increase in LUS was higher in the control group 44 (73%) patients versus 14 (23%) patients, P < 0.001).
CONCLUSIONS:
Using stroke volume variation for guiding fluid therapy in prolonged, major abdominal operations were associated with better hemodynamic profile, less intraoperative fluid administration, lower extravascular lung water and better oxygenation compared to standard care.
Keywords: Fluid therapy, Extravascular lung water
Introduction
Major abdominal surgery is characterised by fluid shifts that need meticulous assessment for the volemic status [1], [2]. Inadequate fluid replacement in hypovolemic patients would result in impaired peripheral organ perfusion that might be un-noticed; this would seriously result in postoperative organ dysfunction. Over-infusion of unnecessary fluids in euvolemic patients would result in tissue oedema. Fluid overload would also increase extravascular lung water and impair gas exchange. These unfavourable complications are more likely to happen during lengthy operations. The aim of goal-directed therapy (GDT) is to guide intraoperative fluid replacement using functional hemodynamic targets instead of traditional clinical signs [3], [4]. The impact of GDT on hemodynamic parameters and surgical outcomes was previously investigated; however, its impact on the respiratory profile was only evaluated during thoracotomy [5]. No studies to the best of our knowledge had evaluated the impact of fluid restricted-GDT on extravascular lung water and oxygenation.
Stroke volume variation (SVV) is one of the dynamic parameters used for the evaluation of fluid responsiveness [6]. SVV depends on heart-lung interaction in mechanically ventilated patients. Positive pressure ventilation provokes cyclic changes in stroke volume due to decreased preload (decreased venous return) in addition to increased afterload (increased trans-pulmonary pressure) [6]. SVV is a frequently used target during GDT in the operating room in high-risk patients [7], [8] as well as moderate and low-risk patients [4]. In the operating room, SVV was measured using Vigileo/FloTrac continuous pulse contour monitor [7], [8], [9], [10], trans-esophageal Doppler [11] and recently, using electrical cardiometry [12], [13], [14], [15].
This work aims to evaluate the impact of GDT on extravascular lung water and oxygenation after prolonged major abdominal surgery.
Methods
A randomised controlled trial was conducted in Cairo University hospital after obtaining research ethics committee approval (N-16-2016). The study was registered at clinical.trials.gov registry system (clinical trial identifier: NCT02845310) on 21 July 2016. The study was conducted between September 2016 and June 2017. An online randomiser was used to by a statistician to create patient codes. Closed, sealed, opaque, sequentially-numbered envelopes were used for concealment. The envelopes were opened by a research assistant. The study included 120 adult patients, aged between 18 years and 65 years, scheduled for major abdominal surgery with an anticipated duration of 180 minutes or more. Patients with cardiac arrhythmias, impaired cardiac contractility, patients with body mass index above 40 kg/m2, and patients with neck or chest lesions that impair the application of cardiometry electrodes were excluded from the study.
Management of anaesthesia
Upon arrival to the operating room, patients received midazolam (0.05 mg/Kg) and ranitidine (50 mg), and full monitors were applied (ECG, pulse oximetry, and non-invasive blood pressure monitor were applied before induction of anaesthesia; whilst, invasive blood pressure monitor and capnography were applied after induction of anaesthesia). Electrical cardiometry device (ICON; Cardiotonic, Osypka; Berlin, Germany) was applied to the patient through 4 electrodes at the following sites: (1) Below the left ear; (2) Above the midpoint of the left clavicle; (3) Left mid-axillary line on the horizontal level of the xiphoid process; (4) two inches inferior to the third electrode.
Induction of general anaesthesia was achieved using propofol (2 mg/Kg), and fentanyl (2 μg/Kg). The endotracheal tube was inserted by the aid of atracurium (0.5 mg/Kg) after 2-3 minutes of positive pressure ventilation. Anaesthesia was maintained by isoflurane (1-1.5%) and atracurium (10 mg/30min). Morphine (0.1 mg/Kg intravenous bolus) and Ketorolac (30 mg intravenous infusion) were administrated after induction of anaesthesia. Arterial and right internal jugular central venous catheters were inserted. Mechanical ventilation was adjusted at the following settings: Tidal volume 8 mL/Kg, PEEP 5 cm H2O, and respiratory rate titrated to maintain end-tidal CO2 at 30-35 mmHg. By the end of the operation, isoflurane was discontinued, and the residual neuromuscular blocking agent was reversed by neostigmine (0.05 mg/Kg), and atropine (0.02 mg/Kg) and the patient was extubated and transferred to the post-anaesthesia care unit.
Fluid therapy
After induction of anaesthesia, all patients received an initial bolus of 5 mL/Kg lactated Ringer’s solution. Then, patients were randomised into either the GDT group and control group.
GDT group: In this group, fluid therapy was restricted to 2 mL/Kg/hour. SVV was evaluated every 10 minutes, and a fluid bolus of 3 mL/Kg lactated Ringer’s solution was infused to reach a target SVV less than 10%. If the total volume of fluid boluses reached 20 ml/Kg, no additional boluses were infused unless there was evident blood loss or hypotension. If mean arterial pressure (MAP) was not achieved despite reaching 20 mL/Kg infused fluids, norepinephrine infusion was planned to start in the central venous line with an initial dose of 0.01 mcg/kg/min.
Control group: In this group, lactated Ringer’s solution was infused at a rate of 6 mL/Kg/hour. Additional boluses of lactated Ringer’s (200 mL) were infused if MAP was below 65 mmHg and CVP was below 8 mmH2O. Norepinephrine infusion was planned to start at an initial dose of 0.01 mcg/kg/min if MAP was below 65 mmHg and CVP above 8 mmH2O.
In both groups, Packed RBCs were transfused if 1) Estimated blood loss was more than 20% of whole blood volume with MAP lower than 65 mmHg. 2) blood haemoglobin level was lower than 7 g/dL. 3) Continuous blood loss with MAP < 65 mmHg. Bleeding patients who did not meet the criteria for Packed RBCs transfusion were resuscitated by lactated Ringer’s solution at a ratio of lactated Ringer’s solution: blood loss = 3:1).
Lung ultrasound examination
Lung ultrasound was performed by a skilled operator who was blinded to the study group. A Mindray device (DC-N6, with a phased array transducer, model P4-2, 3-6 megahertz) was used for 12-region lung ultrasound examination according to the following protocol [16]. We used the following definitions: 1) B-line: “laser-like vertical hyperechoic artefact which extends between the pleural line and the bottom of the screen, and moves with respiration”. 2) B-7 lines: these lines are characterised by being 7 mm apart, and they denoted the presence of interstitial oedema. 3) B-3 lines: these lines are characterised by being 3 mm apart, and they denoted the presence of alveolar oedema. 4) Lung consolidation: “sub-pleural, hypoechoic, wedge-shaped, tissue-like structure”.
All the 12 spaces were screened vertically, and each hemithorax was sub-divided into 6 areas (2 anterior areas, 2 lateral areas, and 2 posterior areas).
Lung ultrasound score (LUS) was then calculated [16]:
- The B-line score was estimated for each area according to the following protocol: zero: no lines, 1: B-7 lines, 2: B-3 lines, 3: consolidation.
- LUS was further calculated (ranging from 0 to 36) as the sum of B-line score of the 12 zones.
Primary outcome
Our primary outcome was LUS which was evaluated two times: a baseline preoperative measurement and a postoperative measurement which was obtained in the post-anesthesia care unit. The change in LUS delta-LUS was defined as the difference between the two measures: Postoperative LUS-baseline LUS. The number of patients with increased postoperative LUS by 3 or more was also compared between both groups.
Secondary outcomes
Intraoperative fluids: total intraoperative fluid requirements, number of fluid boluses, number of patients requiring vasopressors, and urine output
Hemodynamic data: MAP, heart rate, and central venous pressure (evaluated every 5 minutes starting from the baseline preoperative reading till patient discharge from the post-anesthesia care unit)
Demographic data: age and gender.
Surgical data: surgical duration, blood loss, and type of operation.
Postoperative data: postoperative pH, HCO3, PCO2, P/F ratio (defined as PaO2 / Fraction of inspired oxygen), blood haemoglobin, length of ICU stay, and incidence of surgical complications.
Statistical analysis and sample size calculation
Our primary outcome was LUS in the post-anesthesia care unit. In a pilot study on 10 patients, the mean postoperative LUS in patients undergoing prolonged major abdominal surgery under standard care was 5 ± 0.9. Using MedCalc Software version 14.10.2 (MedCalc Sofware bvba, Ostend, Belgium), we calculated a sample size that would detect a mean difference of 10% (i.e. 0.5) in LUS between both study groups. The minimum number needed to have a study power of 80% and an alpha error of 0.05 was 104 patients (52 patients per group). This number was increased to 120 patients (60 patients per group) to compensate for possible drop-outs.
Statistical package for social science (SPSS) software, version 15 for Microsoft Windows (SPSS inc., Chicago, IL, USA) was used for data analysis. Categorical data were presented as frequency (%) and analysed using the chi-square test. Continuous data were checked for normality using the Shapiro-Wilk test and was presented as mean (standard deviation) or median (interquartile range) as appropriate. Continuous data were analysed using either unpaired t-test or Mann Whitney as appropriate. Repeated measures were analysed using analysis of variance (ANOVA) for repeated measures with post-hoc pairwise comparisons using the Bonferroni test. A P value less than 0.05 was considered statistically significant.
Results
One hundred and twenty patients were available for final analysis (Figure 1). The mean age of our patients was 50 ± 13 years, and the mean surgical duration was 4 ± 0.7 hours. Seventy-four (62%) of our patients were males.
Figure 1.
CONSORT chart showing patient recruitment; GDT: goal-directed therapy
The surgical procedures in our patients were Whipple’s operation (12%), gastrectomy (13%), colorectal resection (45%), common bile duct exploration (25%), and abdominal exploration (4%).
Demographic data (age, gender, and co-morbidities) and baseline measurements were comparable between both study groups (Table 1).
Table 1.
Demographic data and baseline characteristics. Data are presented as mean (standard deviation) and frequency (%)
| GDT group (n = 60) | Control group (n = 60) | P value | |
|---|---|---|---|
| Age (years) | 49 (13) | 50 (12) | 0.25 |
| Male gender | 36 (60%) | 38 (63%) | 0.85 |
| Diabetes | 6 (10%) | 7 (12%) | 0.95 |
| Hypertension | 11 (18%) | 10 (17%) | 0.92 |
| Smoking | 9 (15%) | 11 (18%) | 0.84 |
| Baseline hemodynamic data | |||
| Heart rate (bpm) | 86 (16) | 84 (12) | 0.4 |
| Mean arterial pressure (mmHg) | 93 (13) | 90 (14) | 0.2 |
| Central venous pressure | 7.3 (2.5) | 7.1 (2.2) | 0.61 |
GDT: Goal-directed therapy. * denotes statistical significance.
The GDT group had a slightly shorter surgical duration and lower blood loss compared to the control group (Table 2).
Table 2.
Intraoperative data. Data are presented as mean (standard deviation) and median (quartiles)
| GDT group (n = 60) | Control group (n = 60) | P value | |
|---|---|---|---|
| Surgical duration (hours) | 4.3 (0.5) * | 4.7 (0.7) | < 0.001 |
| Blood loss (mL) | 535 (159)* | 645 (227) | 0.008 |
| Blood loss per hour (mL) | 125 (34) | 135 (37) | 0.08 |
| Urine output (mL) | 400 (400, 500)* | 500 (400, 700) | < 0.001 |
| Urine output per hour (mL) | 100 (125, 150)* | 140 (100, 165) | < 0.001 |
| Total crystalloids (mL) | 1512 (478)* | 3048 (784) | < 0.001 |
| Total crystalloids per hour (mL) | 354 (101) * | 637 (85) | < 0.001 |
| Number of fluid boluses | 1.38 (2.2) | 1.4 (2.2) | 0.97 |
GDT: Goal-directed therapy.
denotes statistical significance.
The GDT group received markedly lower intraoperative fluids and showed higher MAP compared to the control group (Figure 2).
Figure 2.
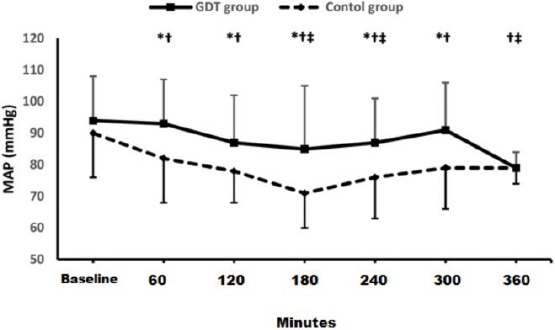
Mean arterial pressure; GDT: goal-directed therapy; MAP: mean arterial pressure; * denotes statistical significance between both groups; † denotes statistical significance within GDT group compared to the baseline reading; ‡ denotes statistical significance within control group compared to the baseline reading
Heart rate and CVP readings were comparable between study groups. None of our patients received vasopressors. Postoperatively, the GDT group had lower LUS and higher P/F ratio compared to the control group (Table 3).
Table 3.
Postoperative data. Data are presented as mean (standard deviation) and median (quartiles)
| GDT group (n = 60) | Control group (n = 60) | P value | |
|---|---|---|---|
| Baseline LUS | 0 (0,2) | 0 (0,2) | 0.95 |
| Post-operative LUS | 2.4 (2.2) * | 4.2 (2.7) | < 0.001 |
| Delta LUS | 1.6 (1.6) * | 3.3 (2) | < 0.001 |
| Post-operative P/F ratio | 368 (37) * | 353 (39) | 0.04 |
| Post-operative pH | 7.36 (7.34, 7.38) | 7.36 (7.33, 7.38) | 0.78 |
| Post-operative Pco2 (mmHg) | 34.4 (3.4) | 34.7 (3.6) | 0.73 |
| Post-operative HCO3 (mg/dL) | 23 (3) | 22 (3) | 0.12 |
| Postoperative hemoglobin (g/dL) | 12.7(1.4) | 12.9(1.6) | 0.44 |
The number of patients who showed significant postoperative increase in LUS was higher in the control group [44/60 (73%)] patients versus 14/60 (23%) patients, P < 0.001). Postoperative arterial blood-gas analysis (HCO3 and pH) was comparable between both groups (Table 3). Length of postoperative ICU-stay and frequency of postoperative complications were also comparable between both groups.
Discussion
We reported that restricted SVV-guided fluid protocol during prolonged major abdominal operations resulted in less fluid administration, higher MAP, less lung congestion, and better oxygenation compared to traditional, standard fluid therapy. We reported favourable respiratory outcomes (lower extravascular lung water and higher P/F ratio) in the GDT therapy group. This is most probably due to the marked reduction in fluid requirements in the GDT group. This is the first study that addresses the impact of GDT on postoperative respiratory profile. Our findings suggest that GDFT is would be beneficial not only in high-risk cardiovascular patients but also in patients with compromised respiratory status. All our patients had prolonged surgery (above 3 hours); this special population would be more sensitive to fluid overload.
Although the patients in the GDT group received less intraoperative fluids, they had higher MAP compared to the control group. This better hemodynamic profile is explained by the accurate evaluation of volume status using SVV. Monitoring of SVV allowed early detection and correction of hypovolemia; and allowed providing the appropriate volume of fluids in the appropriate time. However, we should also clarify that although MAP was lower in the control group, MAP was still in within the acceptable limits.
Our protocol in the study group was based on a restrictive fluid rate (2 mL/Kg/hour) with supplementary boluses to correct hypovolemia. The fluid restriction had been increasingly recommended in the operating room [7], [17] especially in major surgery [18]. In a meta-analysis, school et al. had reported that restrictive fluid therapy is associated with less perioperative complications compared to liberal fluid therapy [17]. We used SVV as an index of volume status. SVV was previously used for guiding fluid therapy in the operating room [4], [7], [8] as well as the intensive care unit [19]. In our study, we chose a special population undergoing prolonged (above 3-hour duration), major abdominal surgery.
We used electrical cardiometry for measurement of SVV. Electrical cardiometry has the advantage of being non-invasive, simple, user-friendly, and does not need expensive disposables. Electrical cardiometry was previously evaluated in the operating room in human patients [15], [20]; as well as animals [12].
The collective evidence about the value of GDT in the operating room is controversial; This is most probably to the high heterogeneity in the meta-analyses which investigated GDT. GDT is a term with a broad spectrum of targets such as SV, CO, oxygen delivery, and heart-lung interaction parameters. Specifically, most of the previously reported fluid protocols which optimised dynamic targets (including SVV) showed good outcomes [3], [9], [10]. In a study conducted by Feng et al., they suggested that the use of perioperative GDT might facilitate recovery in patients undergoing noncardiac surgery combined with the application of alpha-1 adrenergic agonists [21]. A meta-analysis had reported that GDT did not improve postoperative outcomes after major abdominal surgery; however, the studies included in this meta-analysis compared GDT to standard care in the context of enhanced recovery after surgery setting [1].
Our study had some limitations. It is a single-centred study. Most of our patients were elective and not emergency patients. Although the lung ultrasound was performed by a blinded physician, we were not able to blind the anaesthetist responsible for patient management.
In conclusion, using SVV for guiding fluid therapy in prolonged, major abdominal operations were associated with higher MAP, less intraoperative fluid administration, lower extravascular lung water and better oxygenation compared to standard care.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
Trial Registration: clinicaltrials.gov: NCT02845310 on 21 July 2016
References
- 1.Rollins KE, Lobo DN. Intraoperative Goal-directed Fluid Therapy in Elective Major Abdominal Surgery:A Meta-analysis of Randomized Controlled Trials. Ann Surg. 2016;263(3):465–76. doi: 10.1097/SLA.0000000000001366. https://doi.org/10.1097/SLA.0000000000001366 PMid:26445470 PMCid:PMC4741406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-Izquierdo JC, Feldman LS, Carli F, Baldini G. Meta-analysis of the effect of goal-directed therapy on bowel function after abdominal surgery. Br J Surg. 2015;102:577–89. doi: 10.1002/bjs.9747. https://doi.org/10.1002/bjs.9747 PMid:25759947. [DOI] [PubMed] [Google Scholar]
- 3.Benes J, Giglio M, Brienza N, Michard F. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome:a meta-analysis of randomized controlled trials. Crit Care. 2014;18:584. doi: 10.1186/s13054-014-0584-z. https://doi.org/10.1186/s13054-014-0584-z PMid:25348900 PMCid:PMC4234857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsingh DS, Sanghvi C, Gamboa J, Cannesson M, Applegate RL. Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients:a randomized controlled trial. J Clin Monit Comput. 2013;27:249–57. doi: 10.1007/s10877-012-9422-5. https://doi.org/10.1007/s10877-012-9422-5 PMid:23264068. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Shu S-H, Wang D, Chai X-Q, Xie Y-H, Zhou W-D. Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation:a randomized controlled trial. J Thorac Dis. 2017;9:2992–3004. doi: 10.21037/jtd.2017.08.98. https://doi.org/10.21037/jtd.2017.08.98 PMid:29221272 PMCid:PMC5708410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasanin A. Fluid responsiveness in acute circulatory failure. J Intensive Care. 2015;3:50. doi: 10.1186/s40560-015-0117-0. https://doi.org/10.1186/s40560-015-0117-0 PMid:26594361 PMCid:PMC4653888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa-Gallego C, Tan KS, Arslan-Carlon V, Gonen M, Denis SC, Langdon-Embry L, et al. Goal-Directed Fluid Therapy Using Stroke Volume Variation for Resuscitation after Low Central Venous Pressure-Assisted Liver Resection:A Randomized Clinical Trial. J Am Coll Surg. 2015;221:591–601. doi: 10.1016/j.jamcollsurg.2015.03.050. https://doi.org/10.1016/j.jamcollsurg.2015.03.050 PMid:26206652 PMCid:PMC4926263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joosten A, Delaporte A, Ickx B, Touihri K, Stany I, Barvais L, et al. Crystalloid versus Colloid for Intraoperative Goal-directed Fluid Therapy Using a Closed-loop System. Anesthesiology. 2018;128:55–66. doi: 10.1097/ALN.0000000000001936. https://doi.org/10.1097/ALN.0000000000001936 PMid:29068831. [DOI] [PubMed] [Google Scholar]
- 9.Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients:results of prospective randomized study. Crit Care. 2010;14:R118. doi: 10.1186/cc9070. https://doi.org/10.1186/cc9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheeren TWL, Wiesenack C, Gerlach H, Marx G. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients:a prospective randomized multicentre study. J Clin Monit Comput. 2013;27:225–33. doi: 10.1007/s10877-013-9461-6. https://doi.org/10.1007/s10877-013-9461-6 PMid:23558909. [DOI] [PubMed] [Google Scholar]
- 11.Vallée F, Fourcade O, De Soyres O, Angles O, Sanchez-Verlaan P, Pillard F, et al. Stroke output variations calculated by esophageal Doppler is a reliable predictor of fluid response. Intensive Care Med. 2005;31:1388–93. doi: 10.1007/s00134-005-2768-0. https://doi.org/10.1007/s00134-005-2768-0 PMid:16132887. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, Mutoh T, Mutoh T, Kawashima R, Tsubone H. Electrical velocimetry for noninvasive cardiac output and stroke volume variation measurements in dogs undergoing cardiovascular surgery. Vet Anaesth Analg. 2017;44:7–16. doi: 10.1111/vaa.12380. https://doi.org/10.1111/vaa.12380 PMid:27159382. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki K, Mutoh T, Mutoh T, Taki Y, Kawashima R. Noninvasive stroke volume variation using electrical velocimetry for predicting fluid responsiveness in dogs undergoing cardiac surgery. Vet Anaesth Analg. 2017;44:719–26. doi: 10.1016/j.vaa.2016.11.001. https://doi.org/10.1016/j.vaa.2016.11.001 PMid:28803717. [DOI] [PubMed] [Google Scholar]
- 14.Martin E, Anyikam A, Ballas J, Buono K, Mantell K, Huynh-Covey T, et al. A validation study of electrical cardiometry in pregnant patients using transthoracic echocardiography as the reference standard. J Clin Monit Comput. 2016;30:679–86. doi: 10.1007/s10877-015-9771-y. https://doi.org/10.1007/s10877-015-9771-y PMid:26403606. [DOI] [PubMed] [Google Scholar]
- 15.Hasanin A, Soryal R, Kaddah T, Raouf SA, Abdelwahab Y, Elshafaei K, et al. Hemodynamic effects of lateral tilt before and after spinal anesthesia during cesarean delivery:an observational study. BMC Anesthesiol. 2018;18:8. doi: 10.1186/s12871-018-0473-0. https://doi.org/10.1186/s12871-018-0473-0 PMid:29334907 PMCid:PMC5769501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammad Y, Hasanin A, Elsakka A, Refaie A, Abdelfattah D, Rahman SA, et al. Thoracic fluid content:a novel parameter for detection of pulmonary edema in parturients with preeclampsia. J Clin Monit Comput. 2018 doi: 10.1007/s10877-018-0176-6. https://doi.org/10.1007/s10877-018-0176-6. [DOI] [PubMed] [Google Scholar]
- 17.Schol PBB, Terink IM, Lancé MD, Scheepers HCJ. Liberal or restrictive fluid management during elective surgery:a systematic review and meta-analysis. J Clin Anesth. 2016;35:26–39. doi: 10.1016/j.jclinane.2016.07.010. https://doi.org/10.1016/j.jclinane.2016.07.010 PMid:27871539. [DOI] [PubMed] [Google Scholar]
- 18.Della Rocca G, Vetrugno L, Tripi G, Deana C, Barbariol F, Pompei L. Liberal or restricted fluid administration:are we ready for a proposal of a restricted intraoperative approach? BMC Anesthesiol. 2014;14:62. doi: 10.1186/1471-2253-14-62. https://doi.org/10.1186/1471-2253-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients:a systematic review of the literature. Crit Care Med. 2009;37:2642–7. doi: 10.1097/CCM.0b013e3181a590da. https://doi.org/10.1097/CCM.0b013e3181a590da PMid:19602972. [DOI] [PubMed] [Google Scholar]
- 20.Altamirano-Diaz L, Welisch E, Rauch R, Miller M, Park TS, Norozi K. Does obesity affect the non-invasive measurement of cardiac output performed by electrical cardiometry in children and adolescents? J Clin Monit Comput. 2018;32:45–52. doi: 10.1007/s10877-017-9994-1. https://doi.org/10.1007/s10877-017-9994-1. [DOI] [PubMed] [Google Scholar]
- 21.Feng S, Yang S, Xiao W, Wang X, Yang K, Wang T. Effects of perioperative goal-directed fluid therapy combined with the application of alpha-1 adrenergic agonists on postoperative outcomes:a systematic review and meta-analysis. BMC anesthesiology. 2018;18(1):113. doi: 10.1186/s12871-018-0564-y. https://doi.org/10.1186/s12871-018-0564-y PMid:30119644 PMCid:PMC6098606. [DOI] [PMC free article] [PubMed] [Google Scholar]


